GE Healthcare
GE Healthcare Dash 监测系列产品说明书

GE HealthcareBedside flexibility and adaptabilityThe Dash® monitoring family is a portable monitoring system that is flexible and easy to use. Dash allows the acuity of any bed to be modified to meet changing patient needs.Gold standard algorithms and technologyDash monitors revolutionize patient care and assessment by combining the most complete selection of gold standard patient monitoring parameters with leading-edge cardiac technology.Enterprise networkingHard-wired and wireless network connectivity – including access to CIS, CVIS, PACS, RIS, HIS and more than 350 beds without central station support – contributes to the Dash monitors’ unprecedented ability to adapt to changing patient acuity demands.Dash 3000, 4000 & 5000 Flexible acuity monitoringSize Dash 3000 – 8.4 in., Dash 4000 – 10.4 in., Dash 5000 – 12.1 in.Type Active-matrix color LCDResolution Dash 3000 and 4000: 640 by 480 dpi, Dash 5000: 800 by 600 dpiNumber of traces 7 (maximum)Number of seconds/trace 4.9 at 25 mm/secSweep speed 6.25, 12.5, 25 mm/sec (with erase bar)TrimKnob® controlFive hard keys Standard Silence Alarm, Print, NBP Go/Stop, Zero All and Power On/Off. Dash 5000 addsTrends, NBP Auto, Admit/Discharge, Standby and Main ViewRemote control option AvailableCategories Patient status and system statusPriority 4 levels – Crisis, Warning, Advisory and MessageNotification Audible and visualSetting Default and individualSilencing 1 minute, current alarm onlyPause 5 minutes in Adult ICU mode, 3 minutes in Neonatal ICU mode and 5 minute,15 minute, or permanent pause in OR modeVolume Default 70 dB measured at 1 meterNumber of channels 1 to 4 (optional)Transducer sites Arterial, femoral artery, pulmonary arterial, central venous, right atrial, left atrial,intracranial and specialTransducer requirements Excitation voltage: 5 V dc ± 0.1%Transducer output 5 µV/V/mmHgRange -25 mmHg to 300 mmHgOffset ± 150 mmHgFrequency response dc to 50 Hz (-3 dB)Zero balance range ± 150 mmHgZero balance accuracy ± 1 mmHgZero balance drift ± 1 mmHg over 24 hoursAccuracy ± 2% or ± 1 mmHg, whichever is greater(exclusive of transducer)Alarms User-selectable upper and lower limits for systolic, diastolic and mean pressuresStandard leads available I, II, III, V, aVR, aVL and aVF3 leadwire I, II, or III5 leadwire I, II, III, V, AVR, AVL, and AVF10 leadwire I, II, III, AVR, AVL, AVF, VI, V2, V3, V4, V5 and V6Leads analyzed simultaneously I, II, III and V (multi-lead mode)Lead fail Identifies failed leadAlarms User-selectable upper and lower heart rate limitsVoltage range ± 0.5 mV to ± 5 mVSignal width 40 ms to 120 ms (Q to S)Heart rate range 30 to 300 bpmInput impedance Common mode > 10 MΩ at 50/60 HzDifferential > 2.5 MΩ from dc to 60 HzCommon mode rejection 90 dB minimum at 50 or 60 HzImpulse response For an impulse of 3 mV applied for 100 msDisplacement following impulse < 0.1 mVSlope following impulse < 0.3 mV/sFrequency response R esponse of non-permanent displays is limited by resolution to 40 Hz (-3 dB)@25 mm/s. Specified upper frequency limits may vary by ± 2 Hz.Diagnostic mode 0.67 Hz (+0.4 dB) to 100 Hz (-3 dB)For compliance with China 1.0 Hz (+0.4dB) to 75 Hz (-3 dB)National StandardMonitoring mode 0.67 (+0.4 dB) to 40 Hz (-3 dB)Moderate mode 0.67 (+0.4 dB) to 25 Hz (-3 dB)Maximum mode 5.0 Hz (-0.3 dB) to 25 Hz (-3 dB)Noise < 30 µV (referred to input)Input voltage range ± 2 mV to ± 700 mVInput pulse width 0.1 ms to 2 msRise time 10 µs to 100 µsOver/under shoot 2 mV (max)Baseline drift < 0.5 mV per hour with a ± 700 mV, 2 msPacemaker pulse AppliedMeasurement technique Impedance variation detectionRange 0-200 breaths per minute for variations of 1.0 – 10.0 ΩRespiration rate 0-200 breaths per minuteBase impedance 100-1000 Ω at 52.6 kHzDetection sensitivity 0.4 to 10 Ω variationWaveform display bandwidth 0.1 to 1.8 Hz (-3 dB)Alarms User-selectable upper and lower respiration rate limits, and user-selectable apnea limitNumber of channels 2Probe type YSI Series 400 or 700 (determined by input cable)Temperature range 0°C to 45°C (32°F to 113°F)Resolution ± 0.1°CParameters displayed T1, T2Accuracy (independent of source) ± 0.1°C for YSI series 400; ± 0.3°C for YSI series 700 probes Alarms User-selectable upper and lower limits for T1, T2Probe type In-line or bath probeCatheter size 5F, 6F, 7F, 7.5F and 8FInjectate volume 3, 5 or 10 ccParameters displayed Cardiac output, blood temperature, injectate temperature and trial numberCardiac output 0.2-15 (liters per minute)Blood temperature 30-42°CInjectate temperature 0-30°CCardiac output ± 5%Blood temperature ± 0.2°CInjectate temperature ± 0.3°CFrequency response dc to 15 Hz ± 2 HzParameters monitored Arterial oxygen saturation (SpO2) and peripheral pulse rate (PPR)SpO2 range Nellcor 1-100%; Masimo 30-100%; GE Ohmeda 30-100%PPR range Nellcor 20-300 BPM; Masimo 25-240 BPM; GE Ohmeda 30-250Accuracy Actual accuracy depends on probe. Please referencemanufacturer’s specifications.Nellcor SpO2 ± 2 digits (70-100% SpO2)Masimo SpO2 ± 2% Adults/Pediatric (70-100% SpO2)GE Ohmeda SpO2 ± 2% (70-100% SpO2), SpO2 ± 3% Neonates, ≤ 69% unspecifiedPPR ± 3 beats per minuteAlarms User-selectable upper and lower limits for SpO2 and PPRTechnology DINAMAP® classic and SuperSTAT™ (SuperSTAT onlyavailable with Masimo and Nellcor SpO2)Measurement technique OscillometricDisplayed parameters Systolic, diastolic and mean pressures, time oflast measurementMeasurement modes Adult ICU and OR modes; manual, auto and stat,neonatal mode; manual and autoAdult 30-285 mmHgPediatric 30-235 mmHgNeonate 30-140 mmHgAdult 20-260 mmHgPediatric 20-220 mmHgNeonate 20-125 mmHgAdult 10-220 mmHgPediatric 10-210 mmHgNeonate 10-110 mmHgAdult 30-200 bpmPediatric 30-200 bpmNeonate 30-200 bpmOverall system accuracy Meets or exceeds SP 10-1992 AAMI standards Automatic cycle times 0-4 hoursTubing length 12 feet adult, 8 feet neonatalAutomatic cuff deflation Cycle time exceeding 3 minutes (90 seconds neonatal),French mode – Cycle time exceeding 2 minutes (60 secondsneonatal), power off, or cuff pressure exceeds 294 mmHg(± 6 mmHg) adult, 250 (± 5 mmHg) pediatric,147 (± 3 mmHg) neonatalCuff sizes Thigh, large adult, adult, small adult, child, infant andneonatal, sizes #5 - #1 and assorted long sizesAlarms User-selectable upper and lower limits for systolic,diastolic and mean pressures2Supports Novametrix CapnoStat (mainstream) and CapnoFlex LF (low-flow sidestream) CO2 technologiesPrinciple of operation Non-dispersive infrared (NDIR) single beam optics,dual wavelength and no moving partsWarm-up time 2 minutes warm-up time to meet accuracy specifications; waveform immediateupon power up, calculated end tidal after two breathsCable length (mainstream) 8 foot (2.4 m)Sample line length (low-flow sidestream) 7 foot (2.1 m)Inspired and expired CO2 concentrations in %, mmHg or kPa; respiratory rate, continuous CO2 waveform0-100 mmHg, 0-13%, 0-12.5 kPaPiCO2 /FiCO20-50 mmHg, 0-6.5%, 0-6.25 kPaRespiration rate range Low-flow SS 0-150 breaths/minMainstream 0-120 breaths/minMS ±2 mmHg or 5%, whichever is greaterSS 0-40 mmHg ± 2 mmHg; 41-70 mmHg ± 5% of reading;71-100 mmHg ± 8% of reading; all specifications ± 12%of actual from 80-150 BrPMDisplay resolution 1 mmHgRise time Less than 200 ms (low-flow sidestream); less than 60 ms(mainstream adult reusable or SPU); less than 50 ms(mainstream infant reusable or SPU)Respiration rate accuracy ± 1 breath/minAutomatic barometric pressure ± 25 mmHg from 530-785 mmHgOperator-selectable O2/N2O compensationMainstream No routine user calibration required. 15 second airway adapter zero performedwhen changing to a different style of airway adapter.Low-flow sidestream No routine user calibration requiredTypes Adult reusable (standard), adult disposable, infantDeadspace Adult reusable/disposable < 5 ccInfant disposable < 1 ccTaper meets ISO 5356-1Types Adult reusable (standard), adult disposable, infantDeadspace Adult reusable/disposable < 7.3 cc infant disposable < 1 ccAdult, pediatric and infant Nasal CO2 and nasal CO2/O2A dult and pediatric Nasal/oral CO2 and nasal/oral CO2/O2CO2High inspired CO2; high/low expired CO2Respiratory rate Adjustable high and lowMethod Thermal dot arrayHorizontal resolution 480 dots/in. @25 mm/sec.Vertical resolution 200 dots/in.Number of waveform channels fourPaper width 50 mm (1.97 in.)Paper length 30 m (100 ft.)Paper speed 0.1, 0.5, 1, 5, 10, 12.5, 25 and 50 mm/sec. (± 2%)Gain 1 V/mV ± 10%DC offset ± 100 mV (max)Noise < 5 mV peak to peak 0-300 HzFrequency response Refer to frequency response section under ECGGain 10 mV/mmHg ± 2%DC offset ± 20 mV (max)Noise < 5 mV peak to peak 0-300 HzFrequency response dc to 50 Hz-0/+2 HzOperating frequency 2.4 to 2.5 GHzTransmit power 100 mWData rate (802.11) 1Mbps and 2Mbps per channel; (802.11b) 1, 2, 5.5, 11 MbpsRadio technology Frequency-hopping spread spectrumCommunication protocol IEEE 802.11 or IEEEE 802.11bUL 1950 Listed (ITE 9B97), CE Mark RF StandardUS/CAN FCC Part 15 Class B, RSS-210, Europe: ETSI EN 300 328, Japan: RCD STD-33RBattery type Exchangeable Lithium-IonMaximum number of batteries 2Voltage 11.1 V (nominal)Capacity ≥ 3.45 Ah (varies with manufacturers)Charge time Less than 4 hours eachRun time 4 to 5 hoursBattery life 500 cycles to 50% capacityPower requirements 90-132 VAC 50/60 Hz 2.0A190-264 VAC 50/60 Hz 1.0APower consumption 75 W (fully loaded)Cooling ConvectionHeat dissipation 240 Btu/hr. (maxGE imagination at workGE HealthcareP.O. Box 900, FIN-00031 GE, FinlandTel. +358 10 394 11 • Fax +358 9 146 ©2006 General Electric Company – All rights reserved.GE and GE Monogram are trademarks of General Electric Company.Masimo and SET are registered trademarks of Masimo Corporation.Nellcor is a registered trademark of Nellcor Puritan Bennett, Inc.Dash, TrimKnob, DINAMAP and SuperSTAT are registered trademarks of General Electric Company.GE Healthcare Oy., doing business as GE Healthcare.CertificationUL2601-1 classifiedIEC 60601-1 certifiedCE Marking for the 93/42/EEC UL Classified for CAN/CSA Medical Device Directive C22.2 No. 601.12028034-002-2006.03-V2.0Do not exceed Maximum 70°C (158°F) at 95% relative humidity Minimum -40°C (-40°F)CO 2 sensor -30 to 65°C (-22 to 149°F)Batteries-20 to 60°C (-4 to 140°F)Ambient temperature 0-40°C (32-104°F) (Nellcor 0-35°C (32-95°F))While charging batteries 0-35°C (35-95°F)CO 2 sensor 10-40°C (50-104°F)Relative humidity 5-95% @40°CVibration MIL-STD 810E, Method 514.4, Category 1Altitude-273 m to 2,943 m (-896 to 9,655 ft.)WarrantyStandard warranty is one year.。
GE Healthcare B125患者监测器简单、灵活、可靠说明书

GE HealthcareB125 Patient MonitorSimple. Flexible. Reliable.1 Impedance respiration is intended for use with only adult and pediatric patients inUnited States, Argentina, Guam, Puerto Rico, Saint Croix and Saint Thomas.CO 2 measurement through E-miniC Module is intended for use with patients weighing over 5kg (11 lb) only.The B125 patient monitor from GE Healthcare delivers clinical performance you can trust. It is simple and intuitive for busy caregivers, flexible for diverse care areas and acuity needs, and reliable for challenging work environments.Parameter excellenceThe B125 Monitor is designed with advanced parameter technologies for accurate and reliable patient monitoring: •EK-Pro arrhythmia analysis•DINAMAP™ SuperSTAT non-invasive blood pressure •TruSignal™ enhanced SpO 2 saturation monitoring. Also available with Nellcor ® OxiMax ® SpO 2 or Masimo ® SET ® SpO 2 algorithms •GE EtCO 2 sidestream measurement•Comprehensive package for neonatal 1 measurementsSimple & intuitive user interfaceThe B125 Monitor makes it easy to acquire, monitor and interpret patient data to support timely decision-making: •Seven pre-configured workflow settings•Choice of numerical or continuous waveform monitoring •Big numeric view capability •Capacitive touch screenFlexible for diverse care areas & acuity needsDesigned with advanced technologies to support connectivity & operations across hospital enterprise.•Connectivity to GE CARESCAPE™ networks and Mobile Care Web Viewer •Flexibility to share parameter modules and accessories across other GE monitors •Advanced applications such as ST Segment and Full ArrhythmiaRobust & reliableIts rugged & robust design stands up to harsh environments and the everyday wear-and-tear of busy care areas. •Upgraded cyber security•Stable performance in tough environmental conditions •75 cm height drop test for main unit •Three hour batteryTechnical specificationsDisplaySize 12.1 in (diagonal)Resolution: 1280 x 800 pixels (WVGA) Number of waveforms Up to 6Display layout and colors User-configurableControls Capacitive Touch screen & TrimKnob™ control and hard keys(standard)Parameters and modulesECGLeads available 3-lead configuration: I, II, III5-lead configuration: I, II, III, aVR,aVL, aVF and VSweep speed 12.5, 25 or 50 mm/sGain range 0.5x, 1x, 2x and 4xHeart rate accuracy 30 to 300 bpm, ±5% or ±5 bpm,whichever is greaterBandwidth50/60 Hz power supply Monitor: 0.5 to 40 HzST: 0.05 to 40 HzDiagnostic: 0.05 to 145 Hz Pacemaker detection Range: 2 to 700 mVPulse width: 0.5 to 2 ms Arrhythmia analysis Asystole, V Fib / V Tach, V Tach, VT>2R on T, V Brady, Couplet, Bigeminy,Accelerated Ventricular arrhythmia,Multifocal PVCs, A Fib, Missing beat,Pause, Tachy, Brady, Trigeminy ST segment analysis Numeric range: -9 to +9 mm(-0.9 to +0.9 mV)Accuracy: ±0.2 mm or ±10%,whichever is greater, within themeasurement range of -8 to 8 mmNumeric resolution: 0.1 mm(0.01 mV)ST Trends: Up to 168h Impedance respiration3Range Adult/pediatric: 4 to 120 resp/minNeonate3: 4 to 180 resp/min Accuracy ±5% or ±5 resp/min, whichever isgreaterGain range 0.1 to 5 cm/OhmSpO2TruSignal SpO2Measurement rangePulse oximetry 1 to 100%Pulse rate 30 to 250 bpmMeasurement accuracySaturation Without motion-adult/pediatricFinger sensor: 70 to 100% ±2%Without motion-neonate3:70 to 100% ±3%With motion-adult/pediatric/neonate3: 70 to 100% ±3%Low perfusion-adult/pediatric:70 to 100% ±3%(1~69% unspecified)Pulse Rate Without motion: ±2 bpm(Adult/Pediatric/Neonatal3)2 Refer to B125 User’s Manual for more information.3Impedance respiration is intended for use with only adult and pediatric patients in United States, Argentina, Guam, Puerto Rico, Saint Croix and Saint Thomas.CO2 measurement through E-miniC Module is intended for use with patients weighing over 5kg (11 lb) only.Nellcor OxiMaxMeasurement rangePulse oximetry 1 to 100%Pulse rate 20 to 250 bpm Measurement accuracySaturation Adult: 70 to 100% ±2%Neo: 70 to 100% ±3%Low perfusion: 70 to 100% ±2%0~69% unspecifiedPulse Rate ±3 bpmMasimo SETMeasurement rangePulse oximetry 1 to 100%Pulse rate 25 to 240 bpm Measurement accuracySaturation Without motion-adult/pediatric:70 to 100% ±2%Without motion-neonate4:70 to 100% ±3%With motion-adult/pediatric/neonate6: 70 to 100% ±3%Low perfusion: 70 to 100% ±2%(0~69% unspecified)Pulse rate Without motion: ±3 bpmWith motion: ±5 bpmNIBPMeasurement technique Oscillometric with step deflation Modes Manual, automatic and stat NIBP Measurement rangesSystolic Adult/Pediatric: 30 to 290 mmHgNeonate4: 30 to 140 mmHg MAP Adult/Pediatric: 20 to 260 mmHgNeonate4: 20 to 125 mmHg Diastolic Adult/Pediatric: 10 to 220 mmHgNeonate4: 10 to 110 mmHg Accuracy Meets AAMI ISO81060-2 andIEC 80601-2-30Default initialinflation pressure Adult/Pediatric: 135 ±15 mmHgNeonate4: 100 ±15 mmHg Maximumdetermination time Adult/Pediatric: 2 minNeonate4: 85 s Over pressure monitor Adult/Pediatric: 294 ±6 to330 mmHgNeonate4: 147 ±3 to 165 mmHg Invasive blood pressureMeasurement range -40 to 320 mmHg (-5.3 to 42.7 kPa) Measurement accuracy ±5% or ±2 mmHg, whichever isgreaterFrequency response 4 to 22 HzTransducer sensitivity 5 μV/V/mmHgTemperatureNumerical display T1, T2, T2-T1Measurement range 10 to 45°C (50 to 113°F) Measurement accuracy ±0.1°C without probeDisplay resolution 0.1°CProbe YSI probes recommended byGE HealthcareNetworkingCompatibility CARESCAPE NetworkWi-Fi connectivity5IEEE 802.11a/b/g/nWi-Fi security5 WPA-Personal;WPA2-Personal;WPA-Enterprise;WPA2-EnterpriseConnectivity to EMR HL7® outbound protocol directthrough the CARESCAPE Gateway I/O connectorsRS-232 computer serial output, Defibrillation synch, Nurse call, USB port, additional display connectorMountingGCX compatibleIntegrated carrying handle4Impedance respiration is intended for use with only adult and pediatric patients in United States, Argentina, Guam, Puerto Rico, Saint Croix and Saint Thomas.CO2 measurement through E-miniC Module is intended for use with patients weighing over 5kg (11 lb) only.5 Available in registered regions only.Paper RecorderMethod Thermal dot arrayHorizontal resolutions 24 dots/mm (600 dpi)Vertical resolution 8 dots/mm (200 dpi) Waveforms Selectable 1, 2, or 3 waveforms Numerics HR, SpO2, NIBP, IBP1, IBP2,EtCO2/FiCO2, T1, T2, Resp Graphical trend printout HR, ST, IBP1, IBP2, NIBP, SpO2, Pleth,CO2, Resp, T1+T2Paper width 50 mm, printing width 48 mm Paper speed 6.25, 12.5, 25 mm/sPerformance specificationsAlarmsPriority High, Medium, Low and Message Notification Audible and visualSetting Default and individualVisual alarm notification Red, yellow, cyanAudio silence messageGeneral alarm messageAudio pause 2 minAdjustment Adjustment pageTrendsGraphical All parameters, selectable timescales from 20 min to 168h Numerical All parameters, sampling accordingto time setting or after NIBPdeterminationSnapshot Up to 200 snapshots Manual oralarm triggeredOCRG trend6Real time or snapshot Neonatemode onlyTrend cursor In graphical trend Environmental specificationsOperating conditionsTemperature 5 to 40°C (41 to 104°F)Relative humidity 20 to 90% noncondensing Atmospheric pressure 700 to 1060 hPa (525 to 795 mmHg) Storage and transport conditionsTemperature -20 to 60°C (-4 to 140°F) Relative humidity 10 to 90% noncondensing Atmospheric pressure 700 to 1060 hPa (525 to 795 mmHg)Power specificationsAC input 100 to 240V ±10%, 50/60 Hz, 150VA Protection Class IBattery Exchangeable lithium-ion, 1 pcsmaxCharging time < 4 h to 90% capacityRun time > 3 hPhysical specificationsDimensions (H x W x D) Without extension rack:280 x 317 x 150 mm(11.0 x 12.5 x 5.9 in)Weight 4.3 kg (9.5 lb) w batteryIngress protection IPX1CertificationsIEC 60601-1 passedCE marking according to Council Directive 93/42/EEC concerning medical devices amended by 2007/47/EC6Neonate Resp is not available for USA and 510k required countries.Imagination at workProduct may not be available in all countries and regions. Full product technical specification is available upon request. Contact a GE Healthcare Representative for more information. Please visit/promotional-locations.Data subject to change.© 2017 General Electric Company.GE, the GE Monogram, Imagination at work, CARESCAPE, DINAMAP, Trim Knob and TruSignal are trademarks of General Electric Company.Masimo and SET are trademarks of Masimo Corporation. Nellcor and OxiMax are trademarks of a Medtronic company. HL7 is a registered trademark of Health Level Seven (HL7), Inc. All other third-party trademarks are the property of their respective owners. Reproduction in any form is forbidden without prior written permission from GE. Nothing in this material should be used to diagnose or treat any disease or condition. Readers must consult a healthcare professional.DOC1937971 Rev. 3 4/17。
GE Healthcare LOGIQ P9 产品说明书

GE HealthcareL OGIQ P9M ake it easy. Make it your own.When we asked ultrasound users like you to describe the ideal general imaging system, they had three words for us. Make it easy.The LOGIQ ™ P9 is easy to use and enables fast exams through customizable user workflow and productivity tools. It’s the choice for a Personalized , Patient-centric and Practical ultrasound system.Make it easyMake it easy to operate the system. To acquire diagnostic images. To access features and applications. To take on a busy schedule. To receive support. And, make it easy to afford.Introducing the LOGIQ P9 ultrasound system from GE Healthcare – a general imaging system designed for quick diagnostic exploration and full schedules.Whether the need is immediate triage or a comprehensive exam, you can rely on the budget friendly LOGIQ P9 system to provide the consistent image quality, comprehensive application coverage, and ease of use that enable clinicians to make timely, confident decisions.Innovative My Page provides simplified user controls:• Personalize to user preference • Large 10.4 inch touchpanel32%Faster exam time 144% Keystroke reduction 180%Fewer hard keys 1You asked for simplicity.Personalized...to help you customize your workflowThe key to the system’s simplicity is intuitive console controls and a personalized digital user interface called My Page. This innovative GE Healthcare feature enables you to customize workflow preferences and populate the large touch screen with use case presets you designate.With My Page, there are no extra controls to slow you down. Simply log in, select the appropriate exam type, and you are ready to begin scanning.Patient-centric...to help you provide excellent patient careThe LOGIQ P9 supports your diagnostic confidence across a broad range of patient exams.Enhance diagnostic confidence• Excellent image quality• Intuitive image optimization requiring few keystrokes• Superb B-Mode spatial and contrast resolution Address various clinical needs• Wide selection of probes for excellent exam coverage including abdominal, small parts, vascular, cardiac, musculoskeletal and OB/GYN imagingApply advanced imaging tools• Utilize advanced tools available as purchased options when you need them:– B-Flow™ to directly visualize blood flow without the limitationsof Doppler– 3D/4D enables you to acquire and construct volumetric images in real time– Elastography to evaluate tissue stiffness– Integrated Smart Stress Echo Package– Tissue Velocity Imaging (TVI), a cardiac tool that measures themyocardial velocities longitudinally, evaluating systolic anddiastolic function– Tissue Velocity Doppler (TVD) measures segmental velocity of the myocardium longitudinally Practical...a smart choice for usability and investment valueThe LOGIQ P9 system easily adjusts to your working style, preferences and variable needs throughout your workday. Easy access to support• My Trainer, on-board training modules, help accelerate operational confidence• LOGIQ Club website offers educational resources to help optimize system utilizationDesigned for user comfort and easy workflow • Windows® 7 operating system• Large 21.5 inch monitor and accessible 10.4 inch touchscreen • Fully articulating arm, with up/down/swivel• Compact, lightweight, enhanced mobility including wireless LAN and Power Assistant battery operationEnhance exam speed and efficiency• Automated tools help provide fast exams including:– Measure Assistant for measurement intensive studies, with userguidance, the technology generates automatic measurementsthat can be easily edited or accepted.– Compare Assistant helps streamline comparison studies by allowing you to view a patient’s prior and current studies side-by-side on the ultrasound monitor during an exam– Scan Assistant helps reduce keystrokes1 and exam time with the simple touch of a button– Productivity Packages for thyroid and breast studiesImagination at work©2015 General Electric Company – All rights reserved.General Electric Company reserves the right to make changes in specifications and features shown herein, or discontinue the product described at any time without notice or obligation. Contact your GE Representative for the most current information.GE, GE monogram, LOGIQ, and B-Flow are trademarks of General Electric Company or one of its subsidiaries.Windows is a registered trademark of Microsoft Corporation. Third party trademarks are the property of their respective owners.GE Medical Systems Ultrasound & Primary Care Diagnostics, LLC, a General Electric company, doing business as GE Healthcare.GE Healthcare9900 Innovation Drive Wauwatosa, WI 53226 U.S.A.1Internal GE engineering study using standardized protocols for an abdominal exam compared with prior version GE LOGIQ P6ultrasound system.Image uniformity in liver scanning with C1-5-RS probe Patient-centric ImagingRendered fetal face using RAB2-6-RS probe for enhanced clinical confidenceClear definition of breast cyst using ML6-15-RS probe Thyroid elastography with 12L-RS probe Apical 4-chamber view of the heart with 3Sc-RS probeCommon carotid artery with 9L-RS probeB-Flow, Advanced 3D/4D, Elastography, Stress Echo, TVI/TVD, Auto IMT, AutoEF, Measure Assistant, Compare Assistant and Scan Assistant are all purchasable options.To learn more, visit /ultrasound and call your GE sales representative at (866) 281-7545.June 2015JB31709US。
GE Healthcare Treadmill系统说明书

• The final report must be configurable with a logo or image for display and printing
• The system must allow configurable color schemes to display real-time data
supine ergometers • The system must provide a detachable patient ECG acquisition module
with replaceable patient leadwires • The system must provide detachable, exchangeable connectors from
GE Healthcare
CASE* Tender Specifications**
Hardware
• The system must operate on Intel 64-bit architecture with a clock cycle ≥ 1.8 GHz
• The system must support laser printer and thermal chart recorders in both US letter and A4 paper sizes
• The system must be able to operate in a shared database and stand-alone mode
• The system must allow for database synchronization when reconnected to a shared database configuration
GE Healthcare LOGIQ 9 超声医学设备说明书
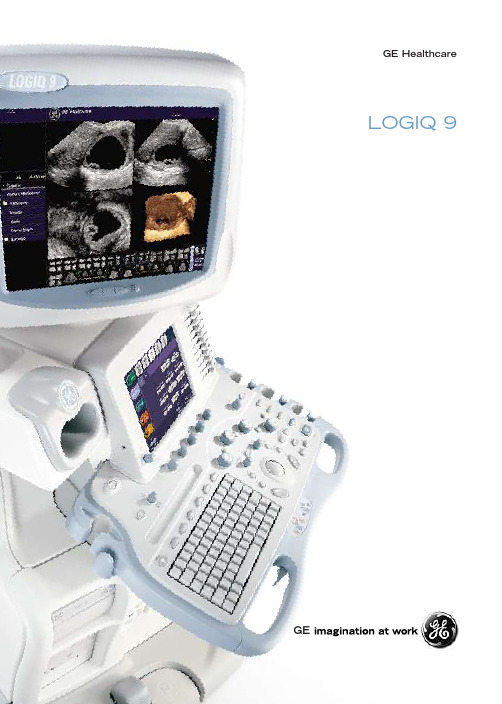
LOGIQ 9GE H e a l t h ca reGEA t t he le a dingedge of he a l t h ca re E r gonomi c sI m a g e Q u a l i tyR a w D a taP r od u c t i v i t y F o r mo r e t h a n a c en tury ,GE H e a l t h ca r e h a s b een in v en t ing medi ca l t e c hnologie s .I n u l tr a s o u nd ,o ur c on t in u o us str e a m of b r e a k t h r o u gh s h a v e r edefined t he st a nd a r d s fo r im a ge q u a li ty ,acc ele r a t ed t he de v elopmen t of ne w a ppli ca t ion s a nd in c r e a s ed c lini ca l effic ien c y fo r us e rs w o r ld w ide.A s w e s ee i t , t he f utur e of u l tr a s o u nd look s e v en mo r e e x c i t ing.GE H e a l t h ca r e i s e v ol v ing.A pionee r in di a gno st i c im a ging a nd info r m a t ion t e c hnologie s ,w e a r e no w a t t he fo r ef r on t of mole c u l a r a nd gene t i c medi c ine a s w ell.T he s e ca p ab ili t ie s w ill help s h a pe a ne w a ge of he a l t h ca r e in w hi c h di s e a s e i s de t e c t ed e a r lie r ,di a gno s ed mo r e p r e c i s el y a nd tr e a t ed le ss in v a s i v el y .U l tr a s o u nd w ill b e a t t he he a rt of t hi s tr a n s fo r m a t ion.A nd s o w ill y o u .T he sys t e m of c hoi c e fo r gene r a l i m a gingI m a gine a le a ding-edge u l tr a s o u nd syst ems o v e rs a t ile t h a t i t ca n mee t t he dem a nd sof v i rtu a ll y a n y c lini ca l s e tt ing.W i t h t heLO GI Q®9, y o u’ll h a v e a high-pe r fo r m a n c esyst em ca p ab le of m u l t i-dimen s ion a l im a gingfo r a f u ll r a nge of c lini ca l a ppli ca t ion s –f r omab domin a l t o b r e a st t o v a s c u l a r im a ging.A nda n e r gonomi c de s ign t h a t imp r o v e s s ca nningc omfo rt a nd c lini ca l w o r kflo w.N o w,im a gine w h a t LO GI Q9c o u lddo fo r y o u a nd y o ur p a t ien ts.LO GI Q9i s b u il t on t he ind ustry’s mo sta d v a n c ed a nd p r o v en syst em a r c hi t e c tur e,T ru S ca n.A s of tw a r e-d r i v en syst em pl a t fo r m,T ru S ca n p r o v ide s u np r e c eden t ed im a ge q u a li tya nd f u n c t ion a li ty, w hile offe r ing a c le a r u pg r a dep a t h fo r b r e a k t h r o u gh s t o c ome.T od a y, t he r e a r et ho us a nd s of LO GI Q syst em s in us e w o r ld w idepo w e r ed b y GE’s p a t en t ed T ru S ca n a r c hi t e c tur e.L e a de r s hi p inu l tr a s o u nd t e c hnolog yB r e a k t h r o u g h a f t e r b r e a k t h r o u g hA t GE H e a l t h ca r e, w e a r e c ommi tt ed t oinno v a t ion t h a t op t imi z e s e v e ry st ep of t hedi a gno st i c p r o c e ss.W e fo c us o ur r e s e a r c ha nd de v elopmen t effo rts on t e c hnologie s t h a toffe r t he g r e a t e st c lini ca l v a l u e ac r o ss a b r o a dr a nge of a ppli ca t ion s.T he LO GI Q9 syst eme x emplifie s t hi s str a t eg y b y deli v e r ing:•I nd ustry-le a ding im a ge q u a li ty fo r c l a r i ty•A n a l yt i ca l t ool s t o in c r e a s e di a gno st i cc onfiden c e•O utst a nding de s ign fo r e r gonomi c s ca nning•A ut om a t i c a ppli ca t ion s t o str e a mlinec lini ca l w o r kflo wW i t h a LO GI Q9, y o u ca n a dd t he l a t e st,mo st a d v a n c ed fe a tur e s a v a il ab le t o en sur et h a t y o u’r e a l w a ys a t t he fo r ef r on t in p a t ien tca r e.A nd t h a t m a ke s t he LO GI Q9a r eli ab let e c hnolog y in v e st men t fo r t od a y –a nd fo rt he f utur e.Av irtu a l c yst o s c opi c v ie w of ur e t e r o c ele N od u l a r i ty a nd s m a ll s i z e of c i rr ho t i c li v e rT od a y ,GE i s defining a ne w a ge of u l tr a s o u nd.W e ca ll i t V ol u me U l tr a s o u nd.T he LO GI Q 9syst em a nd ne w 4D tr a n s d u c e rs en ab le r e a l -t ime t e c hniq u e s fo r ac q u i r ing ,op t imi z ing a ndn a v ig a t ing v ol u me tr i c im a ge s s o t h a t y o u ca nm a ke c lini ca l de c i s ion s w i t h u np r e c eden t edc onfiden c e.B y a dding 4D im a ging ca p ab ili t ie st o t he LO GI Q 9’s o utst a nding 2D im a ge q u a li ty ,t he syst em a llo ws y o u t o m a ke m u l t i -pl a n a rim a ging p a rt of y o ur c lini ca l r o ut ine.T u r ning u p t he v ol u m e in u l tr a s o u nd W i t h t he LO GI Q 9, y o u ca n no w ac q u i r e a nd c on stru c t v ol u me tr i c im a ge s in st a n t a neo us l y –u p t o 30 v ol u me s pe r s e c ond.W i t h V ol u me U l tr a s o u nd , y o u ’ll e x pe r ien c e c lini ca l a nd p r od u c t i v i ty b enefi ts in c l u ding :•M o r e c omp r ehen s i v e a nd r eli ab le e x a m d a t a •G r e a t e r di a gno st i c c onfiden c e •Fa st e r p a t ien t e x a m s •I mp r o v ed p a t ien t c omfo rtC od e d U l tr a s o u nd T e c h no l og y GE ’s e x c l us i v e c oded u l tr a s o u nd t e c hnolog y us e s a d v a n c ed en c oding a nd de c oding a lgo r i t hm s a nd t e c hniq u e s t o imp r o v e im a ge q u a li ty a nd ne w a ppli ca t ion s .•U l tr a C oded Ha r moni c s•B -F lo w•C oded E x c i t a t ion•C oded C on tr a st 1,C oded P h a s e I n v e rs ion ,C oded Ha r moni c A ngioH ig he r p e r f o r m a n c e i s r ig h tin y o u r h a nd sT r a n s d u c e rs a r e a c r i t i ca l elemen t in im a geq u a li ty a nd p r od u c t i v i ty .GE offe rs M a tr i xA rr a y tr a n s d u c e rs w i t h m u l t iple r o ws ofelemen ts t o ac hie v e u nifo r m r e s ol ut iont h r o u gho ut t he field of v ie w , w hi c h r ed u c e s v ol u me a v e r a ging a nd imp r o v e s o v e r a llim a ge c on s i st en c y .T u r ning i m a ge s in t o a n s w e r s I m a ge q u a li ty i s t he c o r ne rst one of t he LO GI Q 9.T he syst em c om b ine s a ll of GE ’s a d v a n c emen ts in s p a t i a l c ompo u nding ,s pe c kle r ed u c t ion ,c oded ac q u i s i t ion t e c hniq u e s a nd w o r ld -c l a ss tr a n s d u c e rs t h a t a llo w c lini c i a n s t o tur n im a ge s in t oa n sw e rs fo r t hei r p a t ien ts .C r o ss X B e a m S p a t i a l C om p o u nding C r o ss X B e a m i s a r e a l -t ime me t hod t h a t r e su l ts in enh a n c edb o r de r defini t ion ,r ed uc ed ac o ust i c a rt if ac t a nd imp r o v edc on tr a st r e s ol ut ion.C r o ss X B e a m p r o v ide s :•L i v e , s ide -b y -s ide di s pl a ys •C oded Ha r moni c s in c l u ding high f r eq u en c ie s •V i su a li z a t ion of u p t o nine a ngle s S p e c k le R e d u c t ion I m a ging (S RI )S R I i s a n a d a p t i v e , r e a l -t ime s of tw a r e a lgo r i t hm th a t r ed u c e s t he s pe c kle a rt if ac t inhe r en t t o u l tr a s o u nd im a ging b y :•S u pp r e ss ing s pe c kle a rt if ac t w he r e nob o r de rs o r edge s a r e p r e s en t•P r e s e rv ing b o r de rs w he r e e c hogeni c i tydiffe r en c e s o cc ur•A v oiding stru c tur e c r e a t ionO r igin a l L o w M edi u m H igh O p t i calI m a g e Q u a l i t yT u r ning i m a ge s in t o a n s w e rs S pe c tr a l a nd c olo r D opple r im a g e s ho w in g t o rtu o us a rt e r i a l flo wLEV v a r i c ie s ut ili z in g LOGIQV ie w w i t h C odedH a r moni cs 4D im a g e of g a ll b l a dde r a nd ca l c u liH i g h -r e s ol ut ion im a g e of t h yr oid le s ion s ut ili z in gC oded H a r moni c s ,SRI a nd C r o ss XB e a mD emon str a t ion of p u l s a t ile po rt a l v ein flow B -F lo w w i t h B -st ee r acc ur a t el y depi c t in g CC Ast eno s isF e t a l t h y m us g l a nd w i t ho ut SRI (lef t )a nd w i t h SRI (r i g h t)E a r l y fe t a l im a g e fe a tur in g C oded H a r moni c s w i t h C r o ss XB e a m ut ili z in g a n endo v a g in a l tr a n s d u c e rS im u l t a neo us mode w i t ho ut SRI (lef t )a nd w i t h SRI (r i g h t)S u pe r fic i a l b r e a st le s ion ut ili z in g SRI,C r o ss XB e a m a nd C oded H a r moni c s M u l t i -pl a n a r v ie w of a n o v a r i a n c yst depi c t in ge x c ellen t r e s ol ut ion in a ll pl a nesE a sy 3D C o r on a l v ie w of b r e a st s ho w in g d u c t a l m a ss a nd dil a t a tionR a w D a t aE n ab led b y GE 's T ru S ca n a r c hi t e c tur e , t he LO GI Q 9 st o r e s r a w im a ge d a t a e a r l y in t he im a ging c h a in fo r op t im u m fle x i b ili ty d ur ing po st p r o c e ss ing a nd a n a l ys i s .W i t h acc e ss t o r a w im a ge d a t a,c lini c i a n s a r e ab le t o c ompen s a t e fo r v a r i a t ion s in im a ge ac q u i s i t iont o in c r e a s e t hei r di a gno st i c c onfiden c e , w hiler ed u c ing t he n u m b e r of p a t ien t r e s ca n s .GE 's u niq u e r a w d a t a a nd a ppli ca t ion s t ool s e ta llo ws c lini c i a n s t o r e c on stru c t im a ge s us inga v a r ie ty of t e c hniq u e s in c l u ding :•A d j ust ing t ime g a in c on tr ol s•Modif y ing B -M ode g a in ,c olo r g a in ,a nd d y n a mi c r a nge•A n a l y z ing a nd m a nip u l a t ing v ol u me d a t a•C on stru c t ing 3D v ol u me im a ge s f r oma c ine loop•C h a nging ba s eline s hif t , sw eep s peeda nd D opple r g a in•A ppl y ing S R IT he p l a t fo r m t h a t onl y G E ca n deli v e rE x c e p t ion a l e r gono m i c s .M a x i m u m c o m fo rt .I n u l tr a s o u nd , t he c lini c i a n a nd syst em f u n c t ion a s one d ur ing a n e x a m.T h a t ’s w h y w e de s igned t he LO GI Q 9 t o op t imi z e c omfo rt ,c on v enien c e a nd p r od u c t i v i ty .T he syst em ’s e r gonomi c de s ign i s fo c us ed on p r o v iding a n e x c ep t ion a l s ca nning e x pe r ien c e.•F lo a t ing c on s ole t h a t ele v a t e s ,r o t a t e s a nd e xt end s•17-in c h ,p r og r e ss i v e s ca n ,high -r e s ol ut ion c olo r di s pl a y•C olo r t o u c h L CD s c r een w i t hp r og r a mm ab le ke ys•F u ll -s i z e ,bac k -li t ke y b o a r d•G el w a r me rs•F o ur sw i v el w heel s w i t ha ut om a t i c tw o lo c king w heel sV oi c e S ca nT he l a t e st in w i r ele ss a nd s pee c h r e c ogni t iont e c hnologie s ,V oi c e S ca n a llo ws h a nd s -f r eev oi c e c omm a nd a nd tr ac k ba ll c on tr ol oft he LO GI Q 9 syst em.V oi c e S ca n en ab le sc lini c i a n s t o us e in tu i t i v e w o rd s a nd ph r a se st o ac t i v a t e mo r e t h a n 150f u n c t ion s w i t he x c ep t ion a l acc ur ac y –p r o v iding t hef r eedomt o pe r fo r m m u l t iple t a s k s s im u l t a neo us l ya nd imp r o v e s ca nning t e c hniq u e a ndb od y mec h a ni c s.E r gonomi c sB u il t fo r s p eed T he LO GI Q 9i s de s igned w i t h y o ur p r od u c t i v i ty in mind ,offe r ing a u niq u e s e t of fe a tur e s t o str e a mline c lini ca l w o r k flo w f r om st a rt t o fini s h.F r om t he s peed a nd pe r fo r m a n c e of t he T ru S ca n pl a t fo r m t o v e ry s pe c ific a ut om a t edfe a tur e s ,o ur c omple t e syst em a ndw o r k st a t ion s ol ut ion s w ill help y o uw o r k mo r e effic ien t l y a nd imp r o v ep a t ien t t h r o u ghp ut .A u t om a t i c O p t imi z a t ionW i t h a s e t of ne w a lgo r i t hm s ,A ut om a t i cO p t imi z a t ion h a r ne ss e s t he e xt en s i v ep r o c e ss ing po w e r of t he LO GI Q 9t o a ut om a t i ca ll y a ppl y t ime g a inc ompen s a t ion ,B -M ode ,c olo r g a ina nd s pe c tr a l D opple r op t imi z a t ion –in c l u ding ba s eline a nd p u l s er epe t i t ion f r eq u en c y.P r od u c t i v i t y W o r k s t a t ion s o l u t ion s d e s ign e d f o r y o u B y c om b ining t he e x pe rt i s e of t he w o r ld ’s le a ding p r o v ide r of he a l t h ca r e IT a nd u l tr a s o u nd syst em s ,GE i s u niq u el y su i t ed t o p r o v ide y o u w i t h c omp r ehen s i v e u l tr a s o u nd IT s ol ut ion s t h a t ca n :•E n ab le V ol u me U l tr a s o u nd t e c hniq u e s •S tr e a mline c lini ca l w o r k flo w •I mp r o v e di a gno st i c c onfiden c e •C onne c t w i t h m u l t i -v endo r u l tr a s o u nd syst em s ,P A C S ,R I S ,a nd HI S LOGIQ w o r k s i s a po w e r f u l w o r k st a t ion t h a t in t eg r a t e s GE ’s e x c l us i v e r a w d a t a p r o c e ss ing a nd p r o v en C en tr i c i ty m u l t i -mod a li ty w o r k st a t ion t o p r o v ide high -pe r fo r m a n c e p r o c e ss ing a nd im a ge r e v ie w .A s ca le ab le s ol ut ion ba s ed on D I C O M st a nd a r d s ,LO GI Q w o r k s i s v e rs a t ile eno u gh t o su ppo rt a n y ty pe of c lini ca l s e tt ing –f r om a n im a ging dep a rt men t t o a f u ll y ne tw o r kedhe a l t h ca r e en t e r p r i s e.U s ing a d v a n c ed c lini ca la ppli ca t ion s su c h a s q u i c k o r g a n r e v ie w ,m u l t i -pl a n a r me a sur emen t t ool s a nd v ol u me a n a l ys i s ,y o u 'll h a v e t he po w e r t o di a gno s e f a st e r a ndmo r e p r e c i s el y .G ene r a l E le c tr i c C omp a n y r e s e rv e s t he r igh t t o m a ke c h a nge s in s pe c ifi ca t ion s a nd fe a tur e s s ho w n he r ein,o r di s c on t in u e t he p r od u c t de s c r i b ed a t a n y t ime w i t ho ut no t i c e o r o b lig a t ion.C on t ac t y o ur GE R ep r e s en t a t i v e fo r t he mo st c urr en t info r m a t ion.©C op yr igh t 2004G ene r a l E le c tr i c C omp a n yGE H e a l t h ca r e,a di v i s ion of G ene r a l E le c tr i c C omp a n y.300-05-U003E-P r in t ed in A ustr i a F o r mo r e t h a n100 y e a rs, s c ien t i stsa nd ind ustry le a de rs h a v e r elied onG ene r a l E le c tr i c fo r t e c hnolog ys e rv i c e s a nd p r od u c t i v i ty s ol ut ion s.S o no m a tt e r w h a t c h a llenge s y o urhe a l t h ca r e syst em f ac e s – y o u ca na l w a ys c o u n t on GE t o help y o u deli v e r t he highe st q u a li ty he a l t h ca r e.F o r de t a il s,ple a s e c on t ac t y o urGE r ep r e s en t a t i v e t od a y.1T he LO GI Q9i s de s igned fo r c omp a t i b ili ty w i t h c omme r c i a ll ya v a il ab le u l tr a s o u ndc on tr a st a gen ts.B e ca us e t he a v a il ab ili ty oft he s e a gen ts i s su b j e c t t o go v e r nmen t r eg u l a t ion a nd a pp r o v a l,p r od u c t fe a tur e s in t ended fo r us e w i t h t he s e a gen ts m a y no t b ec omme r c i a ll y m a r ke t ed no r m a de a v a il ab le b efo r e t he c on tr a sta gen t i s c le a r ed fo r us e.C on tr a st r el a t ed p r od u c t fe a tur e s a r een ab led onl y on syst em s fo r deli v e ry t o a n a ut ho r i z ed c o u n tryo r r egion of us e.A v a il ab le o uts ide t he U ni t ed S t a t e s.LO GI Q i s a r egi st e r ed tr a dem a r k of GEGE U l tr a s c h a ll D e uts c hl a nd G m bH&C o.K GB ee t ho v en str.239,D-42655S olingenFa x:(+49)212-280228,T el.:(+49)212-2802-0GE M edi ca l S yst em s U l tr a s o u nd/U ni t ed K ingdomFa x:(+44)1234266261,T el.:(+44)1234340881GE M edi ca l S yst em s /A me r i ca:M il w a u kee,W I,U S A–Fa x:(+1)262544-3384GE M edi ca l S yst em s /A s i a:T ok y o,J a p a n/Fa x:(+81)3-3223-8524S h a ngh a i,C hin a/Fa x:(+86)21-52080582。
GE Healthcare OEC 9900 Elite产品说明书

GE HealthcareDiscover the clear difference.All the special features you’ve come toassociate with OEC C-arms are residenton this latest generation C-arm, butwe’re taking the 9900 Elite to new heights.We’ve given you new features that canpositively impact your outcomes andenhance your productivity, while providingbetter return on your investment. Justwhat you’d expect from a recognizedmarket leader in mobile imagingtechnology for over 30 years.Come with us on a journey to discover thenew 9900 Elite’s superb imaging features.For over three decades,GE OEC has been amarket leader inmobile C-arm surgicalimaging. Now, theOEC 9900 Elite raisesthe mobile C-armstandard with PrecisionImaging Technologyusing DRM –DynamicRange Management.vascular productivity.Streamline eveStandard non-motorized C-arm offers superb mechanical design usingPremium 1k 2 High Resolution ImagingDigital Subtraction Roadmapping Iris Collimation Dual-Leaf CollimationSpine image withoutcollimator and Smart Window Image with Smart Window Metal Introduced Smart Metal EnabledNormal Digital Zoom Realtime Edge EnhancementImage I.Q.During live fl uoro, invisible AutoTraksampling window automatically moveswith the anatomy, ensuring superbimages no matter the position ofcollimation or centering.AutoTrakMoveable 18”Flat Panel Monitors• DICOM 3.0 internalinterface with Query andRetrieve standard.*• On-board DVD/CD Readand Write capability.• On-board Paper or HardCopy Film Option.Easy Archiving &DocumentationOEC ®9900 Elite....the cle • Twice the resolution.• Four times the information.21k 2 High ResolutionImaging • Standard High ResolutionBlack and White brightmonitors for superbfl uoroscopic viewing.• 700 Cd/m 2 maximumbrightness.• Optional right hand colormonitor for side-by-sideendoscopic and Black andWhite fl uoro images.• Advanced articulationmakes monitors visiblefrom all four sides.* DICOM options vary outside the United States.Physician Controlled X-ray Footswitch & Handswitch1k 2High ResolutionImaging Chain ear difference.C-arm Controls• Operation from eitherside of the system.Collision DetectionBumper forPatient Safetyon MD confi guration• High Resolution 9” (23 cm)or 12” (31 cm) Tri-ModeIntensifi er.- 9”, 6”, 4.5” (23, 15, 11 cm)- 12”, 9”, 6” (31, 23, 15 cm)15 kW Generator forPulsed CineBolus Chasing• 15 and 30 ppswith up to 150 mAfor high poweredpulse mode tostop motion orfreeze motion.• Generator isseparated fromthe X-ray tubeto provide highpower withoutincreasing X-raytube housingheat, improvingcooling effi ciency.High Power Rotating Anode X-ray Tube • High heat capacity X-ray tube with cooling system for long fl uoro on-times.• Fluoro• HLF High Level Fluoro• Digital Spot• Roadmap• Digital Subtraction• Low Dose ModePrecision Imaging...The DRM AdvantageProfi le Conventional OEC Image OEC 9900 Elite Image GeneralOrthopedicSpineVascularBolus ChaseCardiacvery procedure.working for you and your patients.training that can be shared with yourOur dedicated team of more than 200Field Service Engineers is made up offocused experts dedicated to servicingrst timerst time).Having engineers in all 50 states and a30-minute call-back commitment meanswe’re there for you when you need us.OEC systems maintained and running atpeak performance are better for you andperformance, choose the service offering*ts your needs. Our full-service,Primary Care coverage boasts a 97%preventative maintenance and offersconnectivity and unlimited parts andlabor features. Limited Service coverageis also available and provides technicalphone support, back-up on-site support* Service options may vary outside the United States.LR-990018-01©2008 General Electric Company – All rights reserved.General Electric Company reserves the right to make changes in specifi cations and features shown herein, or discontinuethe product described at any time without notice or obligation. Contact your GE Representative for the most current information.GE, GE Monogram and OEC 9900 Elite are trademarks of General Electric Company. GE OEC Medical Systems, Inc.Healthcare Re-imaginedGE is dedicated to helping you transformhealthcare delivery by driving criticalbreakthroughs in biology and technology. Our expertise in medical imaging and information technologies, medical diagnostics, patientmonitoring systems, drug discovery, andbiopharmaceutical manufacturing technologies is enabling healthcare professionals around the world discover new ways to predict, diagnose and treat disease earlier. We call this model of care “Early Health.” The goal: to help clinicians detect disease earlier, access more information and interveneearlier with more targeted treatments, so they can help their patients live their lives to the fullest. Re-think, Re-discover, Re-invent, Re-imagine.imagination at workg GE Healthcare,Surgery — Americas:Phone 801-328-9300Fax 801-328-4300GE Healthcare — Europe: Paris, FranceFax 33-1-30-70-94-35GE Healthcare — Asia:Tokyo, Japan —Fax: +81-452-85-5490Hong Kong —Fax: +852-2559-3588。
GE Healthcare S 5 Avance Carestation产品说明书

C linician inspired perioperative solutionsG E’s Avance Carestation® was developed using Datex-Ohmeda’s unique approach to perioperative solutions – close and continuous collaboration with clinicians. With you as our guide, we designed a compact, integrated anesthesia carestation that combines our highly advanced anesthesia delivery, the very best in anesthesia patient monitoring, and care information management. By combining these care elements with our supplies and services, we deliver an essential component of your integrated perioperative solution.S imply the best anesthesia ventilationY ou asked for sophisticated ventilation capabilities thathelp you meet the needs of the full patient range. TheDatex-Ohmeda Avance satisfi es your request with the7900 SmartVent™ ventilator. Ventilation capabilities include:Volume Control, Pressure Control, PSVPro, SIMV (Volume andPressure), and manual ventilation.The SmartVent uses a similar gas delivery system to thatfound in most critical care ventilators, yet has been adaptedspecifi cally for anesthesia applications and is easily control-led via our intuitive Datex-Ohmeda user interface.The SmartVent’s latest modes, Synchronized IntermittentMandatory Ventilation (SIMV) with Pressure Support and PSV-Pro® (Pressure Support with Apnea backup mode), expand theclinical capabilities of the Avance to help meet the needs ofyour patients. With an adjustable fl ow trigger, electronic PEEPand an apnea backup mode, the SIMV and PSVPro modeshelp simplify the work of caring for your spontaneouslybreathing patients. Pediatric patients, patients with laryngealmask airways (LMAs) and those that cannot tolerate certainanesthetic agents are examples of persons that will benefi tfrom the use of these modes.S etting the standard for electronic gas mixing Incredible response time and accuracy provides you with the ultimate in fresh gas control and effi ciency.• Low fl ow anesthesia supported:fl ow of 150 mL/min.gasminimum• 500 millisecond mixer response time –even for dramatic fl ow changes. Thisallows you to deliver exactly what youwant, exactly when you want it.• Since the mixer delivers fresh gasdirectly to the inspiratory port on yourcommand, there is no fresh gas oragent wasted to “charge the circuit”.This facilitates low fl ow clinicalpractices – even when changing fromfl ows to very low fl ows.highvery• Intuitive and fast setting of fresh gas fl ow mixture makes using our state-of-the-artgas mixer easy.• Dual fl ow sensing technology helpsensure safe operation. Gas fl ow ischecked 200 times per second to insurethe carestation is delivering the properblend based on your setting.• Electronic cylinder pressure sensingtechnology alerts you when cylinderslow.are• Alternate O2 control provides an inde-pendent fresh gas source and fl ow metercontrol when required – helping you tosupport your patient under unforeseenconditions.A dvanced Breathing System (ABS)The Avance comes equipped with our Advanced Breathing System, the ABS TM. Its design, based on your needs and our expertise, is fully integrated into the carestation.• Fewer parts and connections reducethe potential for leaks and misconnects,helping to provide greater patient safety.• Our Multi Absorber canister facilitatesfast, easy removal and replacement.• Fully autoclavable.• Choice of gas scavenging options helps provide compatibility with your existingwaste gas system.• Easy on/off capability and no toolsdisassembly of the ABS facilitates easiercleaning and reduces maintenance time.E nhancing your productivityS pecial features of the Avance make it easy to use and enable you tofocus more attention on your patient.• System checkout is fast and intuitive, with full-color photoimages to illustrate each step and confi rmation tones wheneach test is complete. A real-time clock displays time tocomplete each test and the date and time it passed.• Patient trends can be displayed in three views: measured(numerical), settings, and graphical. Measured and settingsdata is saved every fi ve minutes for the most recent 24 hours.Graphical data is saved every minute for the most recent 24 hours.• Quick Keys let you easily change O2 and total fl ow settings.You can use Vent Setup keys to enter and change multiplesettings and one button to confi rm them.• You can press any Gas or Vent key to take the machine outof standby and initiate gas fl ow to start the case, essential forcases.emergency• An 8-second power-down delay protects against accidentalshutdown during a case.P atient Spirometry TM measures airway pressures, fl ow, vol-umes, compliance and airway resistance breath by breath. On the Avance ventilation screen, the spirometry information is displayed as graphical loops, which may help you detect leaks or obstructions in the airway and adjust optimal ven-tilator settings. Because the spirometry loops are saveable, Patient Spirometry off ers you an intuitive tool for detecting changes in the patient’s ventilatory status.•Optional Datex-Ohmeda CompactAirway module can be physicallyintegrated into Avance for PatientSpirometry• Airway gases CO2, O2 and N2O andanesthetic agent measurement withidentifi cationautomatic• Patient Spirometry measured atthe patient’s airway as shown onthe Avance ventilation screen• A complete and integrated pictureof your patient’s ventilatory statusSubcortical ComponentsCortical Components AntinociceptionImmobilityAutonomic StabilityUnconsciousnessAmnesiaBrain FunctionEEG Airway GasesCO O Oxygen ReturnSvO 79MetabolismVO 340VCO 290EE 2400RQ 0.85Lung MechanicsCardiac FunctionECG RelaxationNMTLevel of HypnosisRE 42SE 40BIS 40TemperatureGastric PerfusionPgCO 45CirculationOxygen DeliverySpO 98Haemodynamic monitoring• Standard haemodynamic measurement of ECG, NIBP, up to six invasive blood pressure channels, temperature, SpO2 and respiration rate, C.O. and SpO2 technologies with oxygenation and• SvOhaemodynamic calculations give a thorough view of your patient’s haemodynamic statusECG and ST analysis• Up to 12-lead ECG with multi-lead arrhythmia analysis• Viewing, printing and adjustable ST alarm settings for ante- rior, inferior and lateral lead groups• Sophisticated Ischemic Burden view for easy and accurate visualization of patient’s ischemic eventsLevel of hypnosis• Entropy® monitors the eff ects of certain anaesthetics on the patient’s central nervous system• Response Entropy shows the reactivity of the patient’s,State Entropy the hypnotic state of cortex• Helps to avoid unnecessarily deep anaesthesia and to prevent unexpected recoveryNeuromuscular transmission• The NMT module measures the patient’s individual re- sponse to nerve stimulation and regional block• Continuous hands-free measurement with automatic trending and recovery note• All simulation modes (TOF, DBS, ST, PTC) to optimize thepatient’s level of neuromuscular blockEEG• A sensitive tool for monitoring the neurophysiological sta- tus of the perioperative patient• Up to four channels of continuous measurement and spec- tral EEG with trending• Auditory Evoked Potential (AEP) measurement for brain- stem and mid-latency responsesI ntegrated solutions designed to enhance your careO ur constant cooperation and interaction with clinicians al-lows us to continuously refi ne and improve our user interfacecapabilities and identify valuable integration benefi ts. Werecognize that making our carestations easy to use is abso-lutely one of the most important aspects we can provide toyou and your practice.Unique ergonomic advantages• Heralded Datex-Ohmeda user interface shared with allcomponents of the carestation• Consistent menus, quick key actions and alarm management help minimize the need for training and reducecomplexity during critical and non-critical events• One switch power up for the entire carestation• Flexibility with diff erent display options for monitored data• Extra large work surface area – space to meet your needs• LED light strip provides bi-level work surface illumination• Mains electrical surge protection and battery backupprovide operation capability even under abnormal powerconditionsSupport in decision making• By combining the set and measured inspiratory and ex-piratory gas values on the same full-color, 12 inch venti-lation screen helps make the administration and controlof gases delivered to the patient logical and easy to use• Sophisticated, yet simple alarm management providesyou with intelligent information when you need it most• Help screens provide you with immediate assistance• Strong commitment to enhancing the Avance’s abilityto provide decision support through intelligent parameterinteractionAnesthesia documentation• Centricity Anesthesia is an open, modular anaesthesiasolution providing a continuum of patient data throughoutthe anaesthesia workfl ow.• Deio Anesthesia shares the Datex-Ohmeda anaesthesiamonitoring user interface and hardware platform. Ittherefore provides an excellent solution for customerswho already own Datex-Ohmeda monitors and arefamiliar with their operation. Like Centricity Anesthesia,Deio Anesthesia provides tools covering the entireprocess.anaesthesiaInformation at the point of needThe iCentral suite of applications off ers scalable networking, viewing, storing and exporting of all patient data. It provides real-time waveforms, numerical data, alarms, vital signs, trends, event history with snapshots and full disclosure – all logically organized into cardiac, haemodynamic, ventilation and neurological views. Using iCentral, consultation and remote surveillance are easier when the same, complete information is available simultaneously in all departments, offi ces and other care locations.iConnect solutions include iCollect, a valuable research tool for professionals that provides an easy and efficient way to collect all data from iMM monitors; iDIS (Device Interfacing Solution) allowing interfacing of external medical devices to iMM monitoring; and iGate, a communication platform to hospital information systems supporting standards developed specifi cally for the healthcare domain, such as HL7.iPON solutions help clinicians capture and share vital information wheneverand wherever they need it. iPOC – information at the point of care – makes it easy to navigate a wealth of clinical information, giving care providers bedside access to various information sources like laboratory results, patient demograph-ic data and image access, while also offering the possibility to run otherclinical information systems applications. Complementing iPOC are Cellular Viewer, Pocket Viewer and Web Viewer, which allow real-time information access from both within and outside of the hospital.For more than 100 years, scientists and industry leaders have relied on General Electric for technology, services and productivity solutions. So no matter what challenges your healthcare system faces – you can always count on GE to help you deliver the highest quality services and support.For details, please contact your GE Healthcare represen-tative today.General Electric Company reserves the right to make changes in speci fi cations and features shown herein, or discontinue the pro-duct described at any time without notice or obligation. Contact your GE representative for the most current information.© 2005 General Electric Company GE Healthcare Finland Oy, a General Electric Company, going to market as GE Healthcare Printed in Finland GE HealthcareGE Healthcare Finland OyP.O. Box 900,FIN-00031 GE, FinlandTel. +358 10 394 11Fax +358 9 146 3310M1040439/0105 © 2005 The decisive di ff erenceSupplies and accessories are not born equal, whether in design, quality or price. Our original Supplies & Accessories are designed and validated speci fi cally for our equipment. They make a decisive di ff erence to your operation by ensuring:• Constant availability and lifetime support• Compatibility• High quality – tested and valitated• Accurate measurements• Ease-of-use – true ergonomic design• Excellent value。
GE Healthcare

方法 使用以下步骤对膜蛋白进行裂解和溶解: 1. 4℃下 8000×g 离心 10 min 或 1000 – 1500×g
离心 30 min 收集培养液中的细胞。 2. 弃上清。将细菌沉淀置于冰上。 3. 每 1 克湿细胞用 5 至 10 ml 裂解缓冲液进行
细胞裂解和膜蛋白溶解
膜蛋白富集
材料 在以下缓冲液中对大肠杆菌进行化学和反复冻融, 使其达到溶解和细胞破裂:裂解缓冲液:20 mM 磷 酸钠, 100 mM 氯化钠, 20 mM 咪唑, 0.5 mM 磷酸 三(2-氯乙基)酯, 5 U/ml 核酸酶, 1 mg/ml 溶菌酶, 不含EDTA的蛋白酶抑制剂混合物, 1%到2% 待筛查 的去污剂, pH 7.4
11. 重复一次(或直到所有未结合样品清除,高纯度 时,280 nm检测值[A280]应小于0.1)。
12. 加入200 µl洗脱液/孔,混合1 min。500×g 将平 板离心2 min,收集液体。重复两次(总共3次洗 脱)或直至所有目标蛋白清除。如有必要,每次 洗脱之间可更换收集板(避免目标蛋白不必要的 稀释)。
浓度分析
由于His MultiTrap FF的富集作用,使得目标膜蛋白 的浓度可通过SDS-PAGE检测。对于每种待筛查的去 污剂,收集His MultiTrap FF板上富集蛋白的前两次 洗脱液进行分析(图2)。若需要更加敏感或特异的方 法,可采用Anti-His Antibody(GE Healthcare 编号: 27-4710-01)进行定量蛋白免疫印迹或点印迹分析 (图3)。需要了解GE Healthcare ECL Western blotting 产品,参见参考文献1。
1.当膜蛋白去稳定后容易发生寡聚和聚集的现象,因此大 小均一性可作为稳定性的有用的指征。
GE Healthcare Vscan Air 肺部超声扫描仪数据册说明书

GE Healthcare Vscan Air™Data SheetVscan Air is a battery-operated general-purpose diagnostic ultrasound imaging system for use by qualified and trained healthcare professionals or practitioners. It enables ultrasound imaging guidance, visualization and measurement of anatomical structures and fluid.Vscan Air consists of a dual-headed probe, which integrates both curved and linear array transducers, and an app that can be installed on Android™ or iOS® mobile devices.Its pocket-sized portability and simplified user interface enables integration into training sessions and examinations in professional healthcare facilities (ex. hospital, clinic, medical office, home environment, road/air ambulance and in other environments described in the product user manual).The information can be used for basic/focused assessments and adjunctively with other medical data for clinical diagnosis purposes during routine, periodic follow-up, and triage assessments for adult, pediatric and neonatal patients. Vscan Air can also be useful for interventional guidance.Vscan Air customers have access to the Vscan web portal, including online access to product and product usage information for selected clinical scenarios.Probe CharacteristicsCurved array transducer for deep scanningSpecific clinical applications and exam types include: abdominal, fetal/obstetrics, gynecological, urology, thoracic/lung, cardiac (adult and pediatric, 40 kg and above), vascular/peripheral vascular, musculoskeletal (conventional), pediatrics, interventional guidance (includes free hand needle/catheter placement, fluid Specific clinical applications and exam types include: vascular/peripheral vascular, musculoskeletal(conventional and superficial), small organs, thoracic/lung, ophthalmic, pediatrics, neonatal cephalic, interventional guidance (includes free hand needle/catheter placement, fluid drainage, nerve block, vascular access and biopsy) Broad-bandwidth linear array: from 3 - 12 MHz with center frequency of 7.7 MHz Number of elements: 192Footprint: 40 mm x 7 mm (lens)Depth: up to 8 cmUser InterfaceThe Vscan Air offers ultrasound imaging with a minimized number of keys and intuitive thumb-controllable touchscreen user interface. The Vscan Air app supports portrait as well as landscape mode to optimize image size and ergonomics for different use scenarios.Single key/gesture to control freeze/unfreeze, store, color on/off, gain and depth control2 steps to change preset with appropriate transducer 2 steps to start reviewing images from an examPresets with optimized settings for imaging different organs. User-selectable default preset for immediate use after starting the app.Measurements: distance, ellipseDevice configuration and management tools in easy reach through swiping in menu:• Enablement of TGC controls, preview mode, storage of binary images• Setting Auto Freeze Time, video duration• Configuration of probe button function (Freeze or Store)• Download user manual in selectable language to Vscan Air app• Diagnostics in Vscan Air app with ability to upload log files to GE server• Direct access to customer support information • Link to cloud-based educational materials• Information about software status of probe and app with ability to un- and re-registerData for up to 500 exams can be stored on mobile device Data is stored in generic formats: jpg for still frames, mpg for videosComplementing storage of binary image data can be selected. Such data could be useful for further image analytics in collaboration with GE.Data is organized as individual examinations with collection of images and can be linked with patient identificationAll stored data can be recalled for review Anonymized images and videos can be shared with other apps available on smart deviceImages, video clips or exams with or without patientinformation can be wirelessly exported in generic formats (jpg, mp4) to shared network foldersImages, video clips or exams with patient information can be wirelessly exported in DICOM format to DICOM PACS VerifyModality Worklist StoreStorage Commitment Secure DICOM (TLS)Data StoragePatient data identification:• Manual data entry of patient information for an exam • Select from DICOM Modality Worklist on request.Such worklist supports consistent labeling of images, video clips and exams before export to DICOM PACS.Standard ConfigurationThe following items are included in the standard Vscan Air offering:Secured data at rest:• Vscan Air app starts only after confirmation ofmobile device protection with user authentication • Images and other patient information data arestored in private space of device with no access from other apps on mobile device• Images are stored on device without embeddedpatient identification and linked with encrypted patient database• FIPS 140-2 compliant database encryption(AES-256 bit encryption)• User selectable, additional PIN protected access topatient data on Vscan Air app• Wiping off exam data after 10 attempts withincorrect PIN Secured data on the move:• Images are anonymized before being shared withother apps on the mobile device• Support of enterprise-grade wireless encryptionstandards including EAP and WPA2 (PSK)• TLS encryption with optional peer authentication tosupport secure DICOM transfer• Configurable time period for image removal on thedevice after export to a DICOM PACS serverSupported Mobile Platforms iiiAndroid phones and tablets with OS version 9, 10 or 11, device with 0x64 ARM based CPU architecture and 64-bit Kernel, Android open GL ES 3.0, and compatibility withScreen requirements Size: from 5 to 20 inchesSecurity requirements WPA2™Connectivity requirements IEEE 802.11nPeer-to-peer connectivity (Android only)Bluetooth BLE 4.0Internal memory requirements 8GB or moreAvailable AccessoriesHardcopy user manual in different languages Additional protective carrying case Additional wireless charger pad International AC adapters iiVerified/ Validated mobile devicesThe list of the verified and validated mobile devices can found on Vscan family web portal.Ultrasound education solutions ivTo help users get familiar with common point-of-careapplications and improve ultrasound skills and knowledge, two digital education solutions are available via our partners.Point of Care Ultrasound FocusClass by 123 Sonography• This course includes access to five hours ofhigh-quality video content, easy-to-follow hands-on demos, practical clinical examples and proven didactive principles to help increase competence and confidence. This program is designed for primary care covering a variety of ultrasound exam types including cardiac, OB, abdominal, lung and joints SonoSim ® 365 for GE Healthcare• SonoSim 365 for GE Healthcare provides convenientultrasound education through integrated didactic instruction, hands-on training, and knowledge assessment. A portable, virtual ultrasound training experience utilizing real patient cases with a broad spectrum of normal and pathologic conditions. This offering includes a SonoSim probe, SonoSim drive, and your choice of five modules immediately accessible online – choose from a wide selection of modules including anatomy, physiology, and clinical procedures.User Support ToolsOnline services to enhance the Vscan Air experience by providing resources, from product information to clinicalSafety ConformanceVscan Air CL probe is classified as internally powered medical electrical equipment with type BF applied parts according to IEC 60601-1vVscan Air CL probe is CE-marked according to MDD(93/43/EEC), RED (2014/53/EU), RoHS (2011/65/EU), and is compliant to 2012/19/EU (WEEE)Vscan Air for Android and Vscan Air for iOS are CE-marked according to MDD (93/42/EEC)Vscan Air CL probe is NRTL Certified to CAN/CSA-C22.2 No. 60601-1 and ANSI/AAMI ES60601-1.Wireless charger pad of Vscan Air is certified according to IEC/EN62368-1 and/or IEC/UL/cUL60950-1Vscan Air conforms to applicable clauses of the following safety standards:IEC 60601-1vi Medical electrical equipment –Part 1: General requirements for basicsafety and essential performanceIEC 60601-1-2iv Medical electrical equipment –Part 1-2: General requirements for basicsafety and essential performance –Collateral Standard: Electromagneticdisturbances – Requirements and tests.(Group One, Class B per CISPR 11 / EN55011)IEC 60601-2-37Medical electrical equipment –Part 2-37: Particular requirementsfor the basic safety and essentialperformance of ultrasonic medicaldiagnostic and monitoring equipment IEC 60601-1-11Medical electrical equipment –Part 1-11: General requirements forbasic safety and essential performance –Collateral Standard: Requirementsfor medical electrical equipment andmedical electrical systems used in thehome healthcare environmentIEC 60601-1-12Medical electrical equipment –Part 1-12: General requirements forbasic safety and essential performance –Collateral Standard: Requirementsfor medical electrical equipment andmedical electrical systems intended foruse in the emergency medical servicesenvironmentEN 13718-1Medical vehicles and their equipment –Air ambulancesPart 1: Requirements for medical devicesused in air ambulancesEN 1789Medical vehicles and their equipment –Road ambulancesISO 10993-1vii Biological evaluation of medical devicesPart 1: Evaluation and testing within arisk management processIEC62304Medical device software – Software lifecycle processes.IEC62366-1Medical devices – Part 1: Application ofusability engineering to medical devices© 2020 General Electric Company – All rights reserved.GE Healthcare reserves the right to make changes in specifications and features shown herein, or discontinue the productdescribed at any time without notice or obligation. Contact your GE Healthcare representative for the most current information. GE, the GE Monogram, imagination at work, Vscan Air and Vscan are trademarks of General Electric Company. GE Healthcare, a division of General Electric Company. Google, Android and Google Play are registered trademarks of Google LLC. DICOM is the registered trademark of the National Electrical Manufacturers Association for its standards publications relating to digital communications of medical information. App Store is a trademark of Apple Inc., registered in the U.S. and other countries. IOS is a trademark or registered trademark of Cisco in the U.S. and other countries and is used under license. The Bluetooth word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. WPA and WPA2 are registered trademarks of Wi-Fi Alliance. SonoSim is a registered trademark of SonoSim, Inc. GE Medical Systems, Inc., doing business as GE Healthcare.January 2021DOC2229649iThe Vscan Air app can be downloaded via App Store or Google Play, accordingly. It converts after confirmed by e-mail registration into a medical device. Before converting, it can be used for preview purposes as non-medical device.iiIn accordance to IEC classification for power plugs, one AC adapter with either an A, C, G, or I connector will be part of standard configuration.iii Using the Vscan Air app with a mobile device which does not meet the minimum requirements may result in low-quality images, unexpected results and possible misdiagnosis. The Vscan Air app may not work in all devices. A recommended step in testing a particular device compatibility is the download, installation and first use of the Vscan Air app in preview mode.iv Not available in every country vWhen not charging using the wireless charger.viIncluding national deviations.viiIncludes compliance to relevant sub-parts of ISO 10993 as per the intended use of Vscan Air.。
GE Healthcare Mixer M-925 用户手册说明书

M-925
Chamber lock
Unpack the mixer and check the items against the packing list. Inspect the items for obvious damage which may have occurred during transportation.
GE Healthcare
Mixer M-925
Instructions
Ion
All users must read this entire manual to fully understand the safe use of Mixer M-925.
9
2 Installation
-connected to other products recommended or described in this manual, and
-used in the same state as it was delivered from GE Healthcare except for alterations described in this manual.
Mixer M-925 Instructions 56-8101-00 Edition AG
Introduction 1
1 Introduction
Mixer M-925 is a dynamic, single chamber mixer with interchangeable mixer volumes. The mixer is used in stand-alone applications with ÄKTAdesign™ Pump P-900 series and in ÄKTAdesign chromatography systems. Features: • 2-step mixing for optimum results. • Flow rates up to 100 ml/min. • Interchangeable mixing chambers with volumes of 90 µl, 0.2, 0.6, 2,
GE Healthcare Vivid E9 XDclear 实时超声检查仪说明书
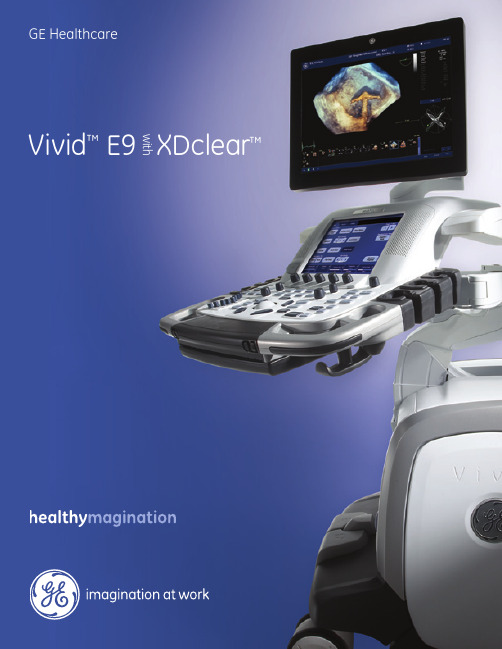
Accelerated Volume ArchitectureGE’s exclusive and patented beam-forming technology, the Accelerated Volume Architecture, provides several times the power of traditional GE ultrasound systems with increased volume size for full volume single beat, as well as for high-volume rate multi-beat 4D acquisition. Using both coherent and harmonic image processing, the system provides computational power, ease of imaging, workflow flexibility and product upgradeability.Raw data formatThe GE Vivid product line has always acquired and storedits data in a specific raw data format, which enables onboard, as well as after-the-fact post-processing capabilities. This flexible and innovative format and storage of pre-scan converted data has enabled development of utilities with high clinical value.This has resulted in an increase in the ability to perform additional advanced algorithms for all of the stepsin the data processing chain, culminating in the releaseof the Vivid E9 with XDclear.XDclear. High speed and image quality to help make your work life easier.An advanced, intuitive ultrasound leadership system, Vivid E9 with XDclear provides extraordinary image quality for the following imaging modes: 2D, color flow, Doppler, and 4D. It’s well-suited for serving your cardiovascular and shared services needs.Five new imaging transducers—two designed with GE Healthcare’s XDclear transducer technology—complementan already robust portfolio of adult, pediatric, vascular, and abdominal transducers.Vivid E9 with XDclear helps strengthen 4D display and visualization, as well as 2D and 4D quantification. It offers excellent workflow and portability—designed into a powerful, versatile platform built for current and future innovations. Advanced transducer technologyThanks to recent advances in transducer technology, XDclear transducers are GE Healthcare‘s highest-performing transducers. XDclear transducers are designed to deliver more powerful and efficient sound waves, with higher bandwidth and efficiency than traditional GE transducers. XDclear technology is built on a combination of three innovations in acoustic engineering:• S ingle Crystal provides a method to “grow” advanced piezoelectric material with the polarity of the molecules aligned to deliver a high-quality acoustic signal with enhanced bandwidth and efficiency.• A coustic Amplifier design acoustically insulates the core transducer structure from the mechanical housing to capture and redirect unused energy that passes through the crystal. This enhances power efficiency bandwidth and helps reduce noise and heat dissipation.• C ool Stack helps optimize energy use by relieving inherent heat generation that can otherwise reduce sensitivityand penetration.The result: Impressive penetration and/or high sensitivity—while still maintaining high spatial resolution. At your serviceFrom your adult and pediatric echo labs, to your interventional suite and OR, Vivid E9 with XDclear can help deliver improved image quality in 2D, 4D, color, and Doppler, for enhanced diagnostic confidence and to help shorten exam times. Adult echoBy providing superb image quality even on difficult-to-scan patients, Vivid E9 with XDclear helps increase diagnostic confidence and productivity in adult echocardiography. Interventional/ORWith the introduction of the visualization tools PolarVision and Depth Illumination, Vivid E9 with XDclear offers extraordinary, clear 3D depth perception of cardiac structures during image-guided device placements, as well as during other transthoracic or transesophageal procedures. PediatricsFor your pediatric patient population, the echo imaging capability of the Vivid E9 with XDclear can provide lifetime follow-up – from the fetal heart, through pediatrics, to adult congenital heart issues – for excellent patient care.Shared servicesWith a comprehensive portfolio of transducers that deliver extraordinary image quality on a broad spectrum of body types, Vivid E9 with XDclear is well-suited for a variety of shared services uses, including cardiac, peripheral vascular, abdominal, and OB/Gyn.for every dayXDclear TechnologyCrisp imaging. Focused workflow. Clear quantification.Vivid E9 makes short work of your routine 2D exams.2D image qualityDesigned with GE’s proprietary XDclear technology, the M5Sc transducer delivers excellent endocardial definition and texture. Together with controls like UD Clarity and HD imaging, it helps provide crisp valves and borders across a wide rangeof patients. Similar image quality is achieved with pediatrics, vascular, abdominal, and TEE transducers.Vascular imagingnotice the clarity and excellent definition of layers and vessel walls in this carotid image acquired with the new 9L-D Carotid_A preset. Fetal heart imagingFetal heart C2-9-D color Doppler image combining high temporal and spatial resolution.Adult echo color DopplerThe XDclear transducer technology provides the color sensitivity needed for visualization of pulmonary vein inflow.Abdominal imagingC1-5-D abdominal image in harmonics for excellent spatial and contrast resolution.i mage qualityAutomated Function Imaging (AFI)This software tool assesses andquantifies left ventricular wall motion at rest. It calculates a large set of parameters to describe the function of the left ventricular walls. AFI specifically calculates peak systolic longitudinal strain (both segmental and global) and presents the results as parametric images.As part of its healthymagination validation, a study has shown that AFI offers potential in predicting mortality in patients with suspected LV impairment compared to Ejection Fraction and Wall Motion.1Based on extensive feedback from clinicians just like you, TTE and TEE on the Vivid E9 are all about making imaging simple, intuitive, and quantifiable to help make your workeasy and efficient.4D StrainAs an extension to the 4D LV Mass tool, both global and regional strain values are calculated based upon a spatial speckle-tracking algorithm. The end result is presented in a Strain Bull’s-eye plot accompanied by time-strain curves and cut planes for enhanced visualtracking assessment.AFIBull’s-eye as well as segmental traces showingreduced longitudinal strain, likely due to right coronary flow obstruction.4D Auto LVQA mesh-based surface-tracking model, the 4D Auto LVQ quantification tool provides you with a graphical output of pure 4D volume data. Utilizing temporal data, it delivers reproducible results for automatic volumes and ejection fractions.4D LV MassUsing the above-mentioned mesh-based surface-trackingmodel, by adding the epicardial border, an LV Mass and an LV Mass Index is derived from the same data set.1Stanton et al, ‘Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall-motion scoring’, Circulation: Cardiovascular Imaging, 2009; 2: 356-364quantificationMV AssessmentThe semi-automated MV Assessment tool, now also available on Vivid E9 for TEE and TTE, provides theability to include quantitative results for the mitral valve apparatus, into the patient exam.Extraordinary workflow4D Views4D Views provides you with “one-touch” options to view images such as 4-chamber, 2-chamber, APLAX, mitral valve, septum, and aortic valve. After a rapid alignment, it takes the full volume acquisition data set and, with the touch of a button, automatically crops away the volume to instantly deliver the view you want. 4D Views helps reduce the manual cropping and cutting of conventional 3D workflow—a time-consuming process that’s difficult to teach and learn.4D Virtual StoreThis innovative feature helps reduce the size of patient studies by using image pointers to refer back to the original full-volume data set, rather than saving multiple large data sets for every new crop view or measurement.Advanced 4D User Tool BoxFor 4D Auto LVQ and 4D Views, an automated tool, Auto Align, helps simplify and speed up the process of aligning the left ventricle. Multi-Slice Imaging provides you with a live imaging mode where you can chose between viewing 5, 7, 9, or 12 slices, for simultaneous acquisition and assessment. Dynamic Multi-Slice and Dynamic Crop enable continuous display of the same structures throughout the cardiac cycle, compensating for out-of-plane motion in short-axis views, potentially improving the accuracy of wall-motion scoring.4D StressHelping improve workflow for stress echo procedures, 4D Stress is an innovative first step in helping you integrate 4D into the routine of your day-to-day clinical practice. Acquire full volume, then using 4D Stress, the Vivid E9 cuts thatvolumetric view into three planes for short-axis analysis and three planes for long-axis analysis, so you can view images more easily. With the Vivid E9, you can now visualize short-axis slices of the entire ventricle under stress. See views you’ve never seen before with conventional echo, which only shows you one slice in the short-axis view.Scan AssistWith Scan Assist, you can quickly customize the system for your departmental protocols for CRT optimization, and let the system guide you to the next view, mode, and measurement. There are also templates for both exercise and pharmacologic stress, all customizable using Scan Assist. Also available for 4D TTE and TEE.Scan Assist ProWith Scan Assist Pro, you can customize the system for your standard echo, vascular, and abdominal exams. The protocols assist you throughout each step of an exam by automatically setting up modes and measurements, as well as annotations, helping enhance image acquisition consistency and helping reduce the number of keystrokes. Also available for 4D TTE and TEE.The Vivid E9 helps make 4D imaging as easy as 2D imaging. It can bring remarkable enhancements to your entire 4D workflow process, thanks to fast, consistent reproducibility.4D ViewsSAX 4D Stress ViewsFlexiZoomSee the mitral valve in 4D TEE with one push of a button.Visualizing the mitral valve in live mode typically requires extensive cropping, rotation, and translation. FlexiZoom provides a fast, efficient way to see a surgeon’s view of the mitral valve to help increase flexibility and reduce keystrokes. There’s no need to turn the volume toward you, manually crop into it, or rotate it to position the aortic valve above the mitral valve. FlexiZoom’s intuitive user interface enables flexible, quick and easy visualization of the structures of interest. you don’t need to adjust gains to optimize the image. Just push a button, and FlexiZoom does it all for you.FlexiSliceEasily switch from volumes to slices and back in live or replay mode.Extracting 2D slices from 4D volumes can be a complicated process. FlexiSlice is an intuitive, interactive tool forobtaining many 2D or render views in either live or replaymode. With FlexiSlice, you can slice in any direction, and easily select a slice view to be projected as a render image.Image shown is a left ventricle outflow track.Polar VisionThe Polar Vision option is designed to enhancecommunication in the cath lab and OR, introducing a new stereo vision technology combining polarized stereo withdepth rendering displayed on a dedicated 3D monitor.2-Click CropClick and drag. See any 4D view in live mode or replay.Obtaining standard or non-standard views during live scanning or replay can be difficult. A simple, quick, extremely intuitive live crop tool, 2-Click Crop lets you crop from the inside out, starting with two extracted scan planes.Image shown is a 4D TTE view of the mitral valve.Multi-sliceMulti-slice imaging, available in live or replay, helps the user extract conventional long-axis and short-axis views from 4D volume data sets.Image shown is from a 4D TTE volume loop, extracting the three conventional long-axis views and four short-axis slices simultaneously.Depth IlluminationA new depth color map enables illumination of structures by an imaginary movable light source, casting a shadow to help improve depth/distance perception. Shown above is an ASD closure device.imagination at workExtraordinary ergonomicsFrom its slim, lightweight, maneuverable design, to its adjustable electronic keyboard, the Vivid E9 system is ergonomically designed to be easy to handle and operate.Adjustable LCD displayThe 17-inch, all-digital high-definition LCD display tilts and swivels to a comfortable viewing angle.Highly mobile40% smaller and 30% lighter than console-based ultrasound systems,* the highly mobile Vivid E9 is ready to roll right to the er adaptableUsing one-touch ergonomics, the Vivid E9’s keyboard position, LCD display angle and touch panel interface can be easily configured to your preferences.Front and rear handles Handles on both the front and rear of the Vivid E9 make it easy to transport the system.Accessible touch panel controls The Vivid E9’s touch panel uses fewer hard keys, making the keyboard smaller and the keys larger for easier access.* Expanded 4D imaging controls are organized for an easy 4D workflow with flexibility.Adjustable floating keyboardWith one touch, you can easily adjust the height and position of the Vivid E9 keyboard. Once comfortably positioned, just lock it in place to help prevent accidental shifting.Easy keyboard storageThe keyboard stores conveniently out of the way in a drawer when not in use.Convenient data management Data management options are conveniently located with multiple USB ports and a DVR recorder.ULTC-0266-09.13-En-USDOC1440959©2013 General Electric Company – All rights reserved.General Electric Company reserves the right to make changes in specifications andfeatures shown herein, or discontinue the product or service described at any time, without notice or obligation. This information does not constitute a representation or warranty or documentation regarding the product or service featured. Timing and availability remain at GE’s discretion and are subject to change and applicable regulatory approvals. Contact your GE representative for the most current information.GE, the GE Monogram, imagination at work, Vivid, and XDclear are trademarks of General Electric Company.GE Medical Systems Ultrasound & Primary Care Diagnostics, LLC, a General Electric company, doing business as GE Healthcare. *As compared to previous GE scanners.GE Healthcare9900 Innovation Drive Wauwatosa, WI 53226 USA。
GE Healthcare Dash 2500 监测仪说明书

GE HealthcareDash 2500The standard of excellence for sub-acuity monitoringThe Dash® 2500 monitor from GE Healthcare allows youto deliver a new standard of clinical excellence to patients in sub-acuity settings. Because it leverages the powerful capabilities of the clinically-advanced family of Dash monitors, there’s no need to sacrifice performance in the interest of cost. The Dash 2500 monitor is a reliable, affordable bedside monitor that gives you the clinical intelligence you need to assess and treat your patients with speed, accuracy and precision.Clinically advancedThe Dash 2500 monitor includes sophisticated clinical parameters to capture vital patient measurements and exceptional cardiac monitoring to help accurately detect arrhythmias.• GE EK-Pro™ arrhythmia program• GE DINAMAP® SuperSTAT™ non-invasive blood pressure • Masimo® SET® or Nellcor® OxiMax® SpO2• Alaris® Turbo Temp®Rugged, ergonomic designThe Dash 2500 monitor is designed for demanding clinical environments. Built with outstanding durability, it is also totally portable and easy to use.• M eets the same rigorous environmental requirements as all Dash monitors• B uilt with chemically-resistant LEXAN® plastic for excellent resilience and enduring performance• E asily configurable display to suit your viewing preferences and information requirements• C ompact size and long battery life allow patient transport without data gaps or interruption• C ompatible with CARESCAPE™ CIC Pro for centralized viewingProduct specificationsPhysical specificationsHeight 22.0 cm (8.7 in)Width 35.8 cm (14.1 in)Depth 17.0 cm (6.7 in)Weight 5.5 kg (12 lb)EnvironmentalOperating temperature 5°C to 40°C (41°F to 104°F) Storage temperature -20°C to 60°C (-4°F to 140°F) Operating humidity 5 to 95% non-condensing Storage humidity 5 to 95% non-condensing Operating atmospheric 700 to 1060 hPapressureStorage atmospheric 500 to 1060 hPapressureElectricalAC input voltage 100 to 240 VAC input frequency 50/60 HZAC input power 120 VAInternal battery 8.4 V nickel metal-hydride (NiMH) Power supply T he Dash 2500 Patient Monitorcan be powered from the internalbattery or AC powerBatteryCapacity 8.4 V; 7.0 amp-hrBattery life G reater than 180 minutes usingfully charged internal battery (NIBP:five min auto cycle with adult cuff.ECG, RESP, SpO2: Active. TEMP:predictive mode. Printer: printingtwo waveforms for one min every20 min at 25 mm/sec)Charge time 4 hours maximum with theMonitor switched OFF8 hours maximum with theMonitor switched ONHeart Rate/PulseElectrocardiography (ECG)Heart rate accuracy 30 to 300 bpm, ± 3 bpm or 3%of reading, whichever is greater Nellcor SpO2Range 20 to 250 bpmAccuracy and tolerance 20 to 250 bpm ± 3 digitsLow perfusion 20 to 250 bpm ± 3 digits Masimo SpO2Range 25 to 240 bpmAccuracy and motion toleranceWithout motion 25 to 240 bpm ± 3 digitsWith motion normal physiologic range,25 to 240 bpm ± 5 digitsNon-invasive blood pressure (NIBP)Adult/pediatric range 30 to 200 bpmNeonate range 30 to 220 bpmAccuracy ± 3.5% or 3bpm, whichever is greater ECGLeads available3-electrode I, II, IIIconfiguration5-electrode I, II, III, aVR, aVL, aVF, and VA configurationHeart rate accuracy 30 to 300 bpm, ± 3 bpm or 3% ofreading, whichever is greater Heart rate resolution 1 bpmBandwidth 0.5 to 40 Hz +1/-6 dB0.05 to 40 Hz +1/-6 dB0.05 to 100 Hz +1/-6 dB Standardizing voltage 1 mV markerCommon mode rejection 1 mV RTI or 10 mm p-p maxdisplayed noise allowed with 20Vrms, 50-60 Hz inputInput impedanceCommon mode > 2.5 MΩ at 10 HzDifferential > 2.5 MΩ from DC to 60 Hz60 Hz tolerance up to 10 mVPacemaker detection/rejectionInput voltage range ± 2 to ± 700 mVLead off sensing current < 0.1 μA DC signal leads,< 1 μA DC driven lead RespiratoryECG-Derived respiration rateLeads available I or IIRange 6 to 120 breaths/min (adult/pediatric)6 to 180 breaths/min (neonate) Accuracy ± 2 breaths/min or ± 3% of reading,whichever is greaterResolution 1 breath/minBase impedance 100 to 2000 ΩDetection sensitivity 0.2 Ω at 30 breath/min with500 Ω baseline impedanceNIBPMethod Oscillometric with step deflation Modes Manual, automatic, statBP Measurement rangesSystolic 30 to 290 mmHg (adult/pediatric)4.0 to 38.7 kPa (adult/pediatric)30 to 140 mmHg (neonate)4.0 to 18.7 kPa (neonate)MAP 20 to 260 mmHg (adult/pediatric)2.7 to 34.7 kPa (adult/pediatric)20 to 125 mmHg (neonate)2.7 to 16.7 kPa (neonate) Diastolic 10 to 220 mmHg (adult/pediatric)1.3 to 29.3 kPa (adult/pediatric)10 to 110 mmHg (neonate)1.3 to 14.7 kPa (neonate) Resolution 1 mmHgAccuracy M eets AAMI/ANSI standardSP10:2002Initial cuff 135 ± 15 mmHg default;inflation pressure user selectable (adult/pediatric)100 ± 15 mmHg default;user selectable (neonate) Maximum determination 120s (adult/pediatric)time 85s (neonate)Over pressure monitor 300 to 330 mmHg (adult/pediatric)150 to 165 mmHg (neonate)Hose/cuff interface Compatible with current Dash hoses Pulse rate W hen NIBP is the source, HR valuesare derived from the pulse rate thatis determined by the oscillometrictechnique of measuring bloodpressure. The rate source field islabeled NIBP.Adult/pediatric range 30 to 200 bpm (± 3.5% or 3 bpm) Neonate range 30 to 220 bpm (± 3.5% or 3 bpm)Nellcor OxiMax SpO2Measurement rangeSpO21 to 100%Pulse rate 20 to 250 bpmAccuracySaturationAdult 70 to 100% ±2 digitsNeonate 70 to 100% ±3 digitsLow perfusion 70 to 100% ±2 digitsPulse RateAdult and neonate 20 to 250 bpm ±3 digitsLow perfusion 20 to 250 bpm ±3 digits Masimo SET SpO2Measurement rangeSpO21 to 100%Pulse rate 25 to 240 bpmAccuracy and motion toleranceSaturationWithout motion– 70 to 100% ±2 digitsadult/pediatricWithout motion– 70 to 100% ±3 digitsneonateWith motion– 70 to 100% ±3 digitsadult/pediatric/neoLow perfusion 70 to 100% ±2 digits,0 to 69% unspecifiedPulse RateWithout motion 25 to 240 bpm ±3 digitsWith motion n ormal physiologic range25 to 240 bpm ±5 digitsAlaris Turbo TempScale °Fahrenheit (F)°Celsius (C)Predictive modeRange 35.6°C to 41.1°C (96.0°F to 106.0°F) Resolution 0.1°C (0.1°F)Monitor modeRange 26.7°C to 42.2°C (80.0°F to 108.0° F) Accuracy ± 0.1°C (± 0.2°F) (when tested in acalibrated liquid bath; meets ASTME1112, Table 1, in range specified) Resolution 0.1°C (0.1°F)Probes U se only Alaris Turbo Temp probesand probe covers. The size, shape,and thermal characteristics of theprobe covers can affect the perfor-mance of the instrument. Inaccuratereadings or retention problems mayoccur unless Alaris Turbo Tempprobes and probe covers are used. Determination time Approximately 10 seconds, typical© 2008 General Electric Company – All rights reserved.General Electric Company reserves the right to make changes inspecifcations and features shown herein, or discontinue the productdescribed at any time without notice or obligation. Contact your GERepresentative for the most current information.GE and GE Monogram are trademarks of General Electric company.Masimo and SET are trademarks of Masimo Corporation.Nellcor and OxiMax are trademarks of Nellcor Puritan Bennett, Inc.Alaris and Turbo Temp are trademarks of Cardinal Health, Inc.LEXAN is a trademark of SABIC Innovative Plastics IP BV.Dash, CARESCAPE, DINAMAP, EK-PRO and SuperSTAT are trademarksof GE Medical Systems, Information Technologies Inc.GE Medical Systems Information Technologies, Inc.,a General Electric company, doing business as GE Healthcare.Healthcare Re-imaginedGE is dedicated to helping you transform healthcaredelivery by driving critical breakthroughs in biologyand technology. Our expertise in medical imagingand information technologies, medical diagnostics,patient monitoring systems, drug discovery, andbiopharmaceutical manufacturing technologies isenabling healthcare professionals around the worldto discover new ways to predict, diagnose and treatdisease earlier. We call this model of care “Early Health.”The goal: to help clinicians detect disease earlier,access more information and intervene earlier withmore targeted treatments, so they can help theirpatients live their lives to the fullest. Re-think,Re-discover, Re-invent, Re-imagine.GE HealthcareP.O. Box 900, FIN-00031 GE, FinlandTel. +358 10 394 11 • Fax +358 9 146 3310EMEA M1123620-1/0409Global code DOC0509914。
GE Healthcare Senographe Pristina 用户手册说明书

GE HealthcareWomen’s HealthcareSenographePristinaGE imagination at workThe Senographe* Pristina is a full field digital mammography system designed to offer an extensive breast care solution with screening and diagnostic capabilities, focused on an ergonomic design for the technologist and patient comfort. Senographe Pristina features a 24 x 29 cm detector, designed to offer full breast coverage in a single image. Smaller breasts can also be imaged in any view with paddles that can slide to both sides of the detector.The Senographe Pristina does not require daily calibration. Ergonomics for technologists•Re-imagined user interface• Park Positioning during patient positioning• One touch access to preset rotation for positioning• Variable speed motorized gantry movements• Sliding compression paddles can move to the side of the detector for compressionErgonomics and design for patient comfort•Designed for Patient comfort•Wheelchair access, MITA compliant•Thinner Bucky than previous platform•Rounded edges detector for patient comfortImage quality• Automatic Optimization of Parameters (AOP), selects all exposure parameters based on breast radiological properties • Three AOP modes + 1 Automatic mode for implants• eContrast is an image processing feature that makes automatic adjustments of brightness and contrast• DQE at IEC 62220-2-3 equivalent spectrum, at 75µGy: 70% (+/-3) at 0.5lp/mm and 64% (+/-3) at 2lp/mm Smooth digital workflow connectivity• Automated Quality Control• Integrated Repeat and Reject AnalysisTechnical SpecificationsDetector• Detector ready to use right after system boot• Detector size: 24 x 29 cm• Pixel size (pitch): 100 μm• Acquisition dynamic range: 14 bits• Bucky front cover thickness: 49mm• Optimized room for positioning due to the bucky depth: 470mm • Image size:– LFOV image size - approx. 13 MB per image– Regular image size - approx. 9 MB per image• Patented needle structure CsI scintillator, single piece construction• Breast support with rounded edge• Air coolingTube technology• X-Ray tube type: Artemis• Anode target materials - Dual track: Molybdenum (Mo) enriched with Vanadium, and Rhodium (Rh)• Four focal spots: 0.1 and 0.3 IEC on each target• Target angle: 0 degree• Maximal high voltage: 49 kV• Tube current:– Molybdenum target:• 100 mA from 25 to 30 kV on large focal spot• 40 mA from 25 to 30 kV on small focal spot– Rhodium target:• 62 mA from 25 to 30 kV on large focal spot• 35 mA from 25 to 30 kV on small focal spot• Anode size (tracks diameter): 100 mm• Anode heat storage capacity: 250kJ (340 kHU)• Anode maximum dissipation: 500 W (40 kHU/min)• Max casing con tinuous dissipation:150 W (12 kHU/min) at 40 °C• Permanent filtration: 0.69 mm Beryllium• Weight: 7 kg• X-ray tube assembly: self-encased X-ray tube, oil-free, lead-free, air-cooled head• Tube protection: software monitoring of tube loadGrid/breast support•Universal grid compatible with 2D Conventional Mammography and DBT•Ergonomic breast support designed for patient comfort and cleanability• Motorized lock of the grid and breast support• Breast support material: carbon fiber composite• Optimized grid motion ensuring no grid structure visible in the image• Detector to breast support edge-to-edge distance ≤ 5 mm• It is always possible for the technologist to takecompression control even if the patient has started self-compression• PAC is inhibited during acquisition, the patient cannot interfere with the examinationPositioner• Isocentric arm with motorized rotation and vertical movement• Source to image receptor distance: 660 mm• Floor to image receptor distance: from 65 cm to 150 cm • Rotation angle: -180/+180 degrees• Ergonomic hand -rest: one at each side of the tube arm and two additional behindSafety features• Gantry motions locked when compression force appliedUser interface• Four sets of single speed switches for rotation , angulation and lift movements, with an accelerating speed profile• Four sets of preset position switches for positioning in CC and MLO• Automatic sto p at +/- 90 degrees for lateral positions • Collimation buttons on the tube head for field of view size and location• Parameters display– Tube arm support rotation angle– Compressed breast thickness (in mm) – Compression force (in daN)• Ergonomic control console– Controls exposure– Provides information on system status– Gives access to advanced parameters for system set-up• Patented automatic view names marking based on breast laterality• View name can be edited while the exam is performedAcquisition workstation• Time to display processed image (average): 10 seconds • Time between exposures (typical): 12 seconds• Dose calculated and displayed on the image after every exposure (Entrance Skin Dose and Average Glandular Dose) • Quad core Intel i5 workstation:– Memory: 32GB– Hard disk: 1 internal 250GB disk for the system – Hard disk: 1TB for image storage – Ports: 4 Gigabit Ethernet port – DVI Display and port connector• 2 types of display available – 1MP LCD Monitor• 48 cm (19”) medical grade • 1280 x 1024 pixels (landscape) • High luminance - up to 300 Cd/m2 • Contrast ratio: 2000:1• Viewing angle: 170 degrees• Mounted on a rotating arm for in -room accessAutomatic exposureAutomatic Optimization of Parameters (AOP) Fully automatic mode• AOP is an automatic exposure system that selects all exposure parameters based on radiological density of the breast: - track (Mo or Rh) - filter (Mo or Ag) - kV - mAs• The system identifies the densest part of the breast to select the appropriate exposure parameters • Three AOP modes are available:– "Standard + ”: dose to patient comparable to screen/film Mammography– “Dose -”: priority is given to dose reduction– “Standard”: balances low noise and dose reduction • Automatic acquisition mode for implants Manual mode• Manual selection of all parameters: track, filter, kV and mAsCollimator• Filters: Molybdenum: 0.030 mm; Silver: 0.030 mm • Field of View (FOV) in detector plane, in cm:– For standard contact views: 24 x 29 maximum FOV or 19 x 23 regular FOV, automatic adjustment depending on paddle used, breast support and gantry rotation angle• Field of View (FOV) selection: automatic and manual• FOV size: selected automatically based on the paddle or geometric magnification platform used, can be modified manually by using the collimation size switch on the tube head • FOV location (left, right, center): selected automatically based on the tube arm angle, can be modified manually by using the collimation position switch on the tube head • Compression and exposure are prevented if the FOV and compression paddle sizes or locations are not consistent • Light centering device: a light automatically switches on when a preset position is reached, at compression start or at paddle insertion; can be turned on with the collimation switches buttons located on the tube head or on the acquisition consoleCompression• Compression modes:– Motor driven compression up to 20 daN – Manual compression up to 27 daN• Dual foot-pedals for column height and compression adjustments• User defined motorized compression force limit: 4 to 20 daN • Min force for AOP: 3 daN• Compression speed: 3 speed levels• Selectable automatic decompression after exposure, to minimize patient time under compressionPatient Assisted Compression (PAC)*Commercialized as Pristina Dueta in some countries• Wireless and ergonomic -designed device that allows the patient to continue the compression after the technologist has positioned correctly and reached a threshold of compression• Designed to minimize patients' perceived pain and discomfort• Intended to be available for every patient positioning• PAC’s speed profile is similar to the technologist-controlled one-Nio Color 3MP (MDNC-3421) – Barco:• High performance color IPS-TFT Color LCD• 54cm (21.3”)• 2048 x 1536 pixels (landscape)• Brightness: 900 cd/m2• Contrast ratio: 1400:1• Viewing angle: 178°• Mounted on a rotating arm for in-room access • Image PresentationeContrast allows you to choose among 6 levels to better adapt to breast morphology and radiologist display preferences:–eContrast 1 provides a “film-like” aspect withimproved visibility of the skin line– eContrast 2 to 4 provide increasing steps of imagesharpness and contrast– eContrast 5 provides a high level of sharpnessand contrast, with a very high level of tissuepenetration– eContrast 6 is adapted to very dense breast orimplants– Automatic windowing (window level and windowwidth)– Other features: zoom, roaming, inversion, flip,rotation of images, window width and level setting,annotations and measurements• In case of power failure, an Uninterruptible Power Supp ly (UPS) allows to close the examination without loss of informationConnectivity• DICOM** 3.0 platform:– Modality Worklist User– Storage Provider– Storage Commitment User– Query/Retrieve User– Basic Grayscale Print User– Verification Provider– DICOM-compliant CD, DVD-R/-RW and USB DataInterchange• Connectivity features: customizable Autopush to multiple DICOM databases, Autoprint, Autodelete based on Storage Commitment• Modality Perform Procedure Step User• Connectivity to GE Service for remote diagnostic capability • IHE Profiles: Scheduled workflow, Mammography image, Tomosynthesis profile, Portable data for imaging, Consistent time integrationQuality assurance• Complete quality control program• Automation of quality control tests: Flat Field, MTF, AOP, SNR• Test history and results can be reviewed• Data can be exported for data tracking• Automated Repeat and Reject Analysis Radiation shield• Choice between two radiation shields:– Integrated to the control console– StandaloneHigh voltage generator• Generator Integrated into the gantry for room saving • Generator type: high frequency single-phase power supply• Ripple: < 4% from peak to peak• Power: 5 kW max• Generator max rating:• 2 to 600 mAs (depending on track, filter and kV)•22 to 49 kV, in 1 kV steps depending on track • Generator protection: software monitoring tube load Standard configuration• Motorized isocentric gantry• X-ray tube with rotating Mo/Rh anode• 24 x 29 cm flat panel detector• Acquisition workstation– CD,DVD-R/-RW– 1MP or 3MP display– Control console– UPS• Pair of dual foot-pedals• Standard Face shield• 24 x 29 cm bucky with grid• 24 x 29 cm paddle• Quality control toolkit• User manual and technical documentationOptions• 1.5 and 1.8 magnification stands• Additional 24 x 29 cm paddle• 19 x 23 cm sliding paddle• 24 x 29 cm Flexible compression paddle• 19 x 23 cm Flexible & sliding compression paddle• 10x23 Sliding Implant/Small breast compression paddle • Square spot sliding compression paddle• Round spot sliding paddle• 2D Localization 19x23 Swiss Cheese sliding compression paddle• 2D Localization 19x23 sliding standard compressionpaddle•2D crosshair device• X-Ray protective shield• Bar code reader• Printers compatibility: AGFA DRYSTAR AXYS• Upgradable to Senographe Pristina 3D and/or SenoBright HD• X-ray remote control hand switch• X-ray footswitchSenographe Pristina 3DSenographe Pristina 3D is a three-dimensional imaging technology that uses a low dose short X-ray sweep around a compressed breast. The acquired projection images are processed electronically in order to reconstruct a 3D representation of the entire breast. This imaging technique is designed to separate the tissues and to reduce the overlapping of structures, which represents a limiting factor in standard 2D mammography.The 3D option is available for the Senographe Pristina platform that generates 3D and 2D images. Senographe Pristina 3D Technology• Sweep angle is 25° with 9 projections at any rotation angle between -160°/+160°• The “Step and Shoot” tube motion stops for each exposure to avoid image blur• Mo and Rh tube tracks create narrow x-ray spectra,exactly where the dose efficiency is for thin (Mo) andmedium and thick breasts (Rh)• Detector: 100 microns with no binning, high DQE in 3Dmode (IEC 62220-2-3, equivalent spectrum at 5µGy):65% (+/-2) at 0.5lp/mm and 57% (+/-2) at 2lp/mm• Automatic reconstruction of the images by using ASIR DBT iterative algorithms• The dose of a DBT (Digital Breast Tomosynthesis) view isdesigned to be equivalent to the dose of a 2D standardacquisition of the same view• Capability to reconstruct 0.5mm or 1mm distancebetween tomo-planes• 3D+2D mode allows the user to acquire in a single action a 3D sequence followed by 2D image for a given view,without releasing the compressionSenoBright HDThe SenoBright HD (Contrast Enhanced Spectral Mammography CESM) application shall enable contrast heightened breast imaging using a dual energy technique. This imaging technique can be used as an adjunct following mammography and ultrasound exams to localize a known or suspected lesion.Patient Comfort• As with previous generation GE mammography systems, patients lying in a recumbent position can be examined with SenoBright HDErgonomics designed for technologist• User can switch between standard mammography and Spectral Mammography mode during the same exam session• SenoBright HD provides a timer function to both monitor and record time after injection, which is displayed as an annotated field in the images• SenoBright HD offers Automatic Optimization of Parameters (AOP) and manual exposure modes for the dual-energy exam• S enoBright HD will automatically acquire the Spectral Mammography images for each view with a single action of the x-ray exposure control TechnologySenoBright HD chooses filtering materials depending on the operating mode and the exposure levels necessary. For the high-energy acquisition, a proprietary multi-layer filter is used to shape the resulting energies of the x-ray spectrum to those required to best highlight iodine.Energy LevelsThe energy levels may vary depending on breast thickness • 26-34 KVp for lower energy acquisition• 49 KVp for higher energy acquisition.System Power supply• Input frequency: 50Hz/60Hz• Input voltage: single-phase 200-240 V~• EATON UPS 5P650 650VASystem Weight• Gantry: 420 kg• Control Station without monitors: 160 kg Environmental conditions• Temperature range: 15° to 30°C• Humidity range: 10% to 80%• Atmospheric pressure range: 70 kPa to 106kPa(0 to 3000m altitude)Screening ProtocolFor reference, in the US a DBT screening examination may consist of one of the following combinations (CC: craniocaudal, MLO: mediolateral oblique):- a 2D CC view and a 3D DBT MLO view, or- a 3D DBT image set consisting of CC and MLO views, and a 2D synthesized image set consisting of CC and MLO V-Preview images.V-Preview is the 2D synthesized image generated by GE SenoIris mammography software from GE DBT images.Note: Breast cancer screening may be regulated by country specific rules. Please refer to competent Healthcare Authorities for guidanceWireless Footswitch OptionThe wireless footswitch is available on the Senographe Pristina platform. The footswitch includes pedals to activate lift up, lift down, compression and decompression functionalities. The communication range between the footswitch and the receiver shall be located within 1.5m radius from the gantry.Mobile OptionA Mobile Mounting Device is available for Senographe Pristina 2D and 3D to allow its installation and transportation in a mobile unitWorkflow OptionsThe Senographe Pristina is compatible with iCAD Second Look (2D CAD), iCAD Tomo Detection 1.0 (3D CAD) and iCAD ProFound AI for Tomo (3D CAD)Senographe PristinaNOTE:- Weights and dimensions may vary slightly depending on equipment configuration.Senographe Pristina, PAC, Wireless footswitch, Mobiles and iCAD are not available in all countries. Please refer to your GE Healthcare sales representative.1885m775368Maximum HeightGantry BasePlateM ax imM ax im uData subject to change.Marketing Communications GE Medical SystemsSociété en Commandite Simple au capital de 85.418.040 Euros 283, rue de la Minière, 78530 Buc France RCS Versailles B 315 013 359A General Electric company, doing business as GE HealthcareUK: 0800 0329201Spain: 0900 993620 Germany************France: 0800 908719Austria: 0800 291888 SwitzerlandItaly: 0800 786947 German: 0800 837279French: 0800 837279GE, the GE Monogram, and imagination at work are trademarks of the General Electric Company*Trademark of General Electric Company** DICOM is a trademark of National Electrical Manufacturers Association.All other trademarks, service marks, company names and product names are the property of their respective owners© 2016-2019 Copyright GE Healthcare。
GE Healthcare Panda 产后婴儿保温暖器说明书

115 V ± 10%; 6.6 Amps • 220-240 VAC, 50/60 Hz Models:
230 V ± 10%; 3.3 Amps*
• Nominal Power Consumption: 520 W at max. heater power, max. lighting
GE Medical Systems, a General Electric company doing business as GE Healthcare.
MIC-0271-10.07-EN-US
• Pulse Rate Accuracy: No motion: +/- 3 bpm Motion: +/- 5 bpm
GE Healthcare 8880 Gorman Road Laurel, MD 20723 USA
Integrated Scale Characteristics • Accuracy: +/- 10 g (0.35 oz) • Range: 300 g to 8 kg (0.66 lbs to 17.6 lbs)
• Complies with IEC 60601-1 for electrical safety
Integrated Resuscitation Characteristics • Input pressure: 40-75 psi (275-517 kPa) • Minimum Input Flow: 7ห้องสมุดไป่ตู้ lpm
Flowmeters • Range: 0-15 lpm • Accuracy:
GE Healthcare清洁和消毒FAQ说明书

GE Healthcare FAQ forCleaning and DisinfectionVersion 3-24-20; 11:30amIt is important to follow specific facility/site local infection control protocols, the information below does not supersede or replace health care facility infection control protocols.1.Does GE Healthcare have a website for information on cleaning and disinfection?Yes, it can be found here for most devices: https:///Ultrasound probes can be found here or by linking to this site from the site above:https:///products/ultrasound/ultrasound-transducersNote – you may need to clear your cache in your web browser if you have accessed this website previously to ensure you have the most up to date information.2.What does GE Healthcare mean when it says it has evaluated cleaning agents?Cleaning agents listed in GE Healthcare product user manuals and on the GE Healthcare website are those for which GE Healthcare has performed testing to demonstrate that they do not damage our products after repeated cleanings.3.What would happen if I use a cleaning agent that has not been evaluated or have not passed materialcompatibility testing?The list of cleaning agents identifies which agents have been evaluated for cleaning compatibility with the materials used in our products. Cleaning agents that have not been evaluated or have not passed material compatibility testing could have an unknown or harmful impact if used on device surfaces. Impacts may include degrading cosmetic or functional performance, damaging device surfaces or labels, causing immediate equipment failure or even causing longer term latent failures.4.Has GE Healthcare evaluated cleaning agents for effectiveness for SARS-CoV-2?GE Healthcare has not tested any cleaning agents for disinfecting effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that causes COVID-19. The U.S. Environmental Protection Agency (EPA) has released a list of disinfectants that claim to be effective for use against SARS-CoV-2. GE Healthcare has cross referenced the disinfectants from the EPA list with the cleaning agents identified in our product user manuals, which have undergone compatibility testing with our products. The cross-referenced list is posted on our website https:///. Users should follow instructions provided by the cleaning agent manufacturer when using those disinfectants to clean GE Healthcare products.5.Where should I go for more information related to cleaning and disinfection for SARS-CoV-2? Please follow your user manual for instructions regarding cleaning GE Healthcare products, and consult GE Healthcare’s cleaner compatibility website at https://. The situation regarding SARS-CoV-2 and COVID-19 is rapidly evolving. New information is being gathered daily, you may be able to find additional information on the following websites:•WHO:https://www.who.int/health-topics/coronavirushttps://www.who.int/infection-prevention/publications/decontamination/en/•US CDC:https:///coronavirus/2019-ncov/index.html?CDC_AA_refVal=https%3A%2F%%2Fcoronavirus%2Fnovel-coronavirus-2019.htmlhttps:///coronavirus/2019-ncov/community/organizations/cleaning-disinfection.htmlhttps:///coronavirus/2019-ncov/infection-control/control-recommendations.html•US EPA:https:///sites/production/files/2020-03/documents/sars-cov-2-list_03-03-2020.pdf•European Centre for Disease Prevention and Control (ECDC):https://www.ecdc.europa.eu/en/novel-coronavirus-china6.How do I find the correct product user manual?You can access user manual information at the following website:https:///#/cdp/dashboard7.My GE Healthcare device isn’t listed on GE Healthcare’s cleaner compatibility website. What shouldI do?The GE Healthcare website is being updated routinely. Additionally, your product user manual includes the cleaning agents that GE Healthcare has evaluated for material compatibility for your device. We recommend you also consult the user manual for cleaning instructions.8.How can I clean and disinfect accessories to my GE Healthcare device?You should only clean and disinfect accessories that are intended for multiple uses. GE Healthcare recommends consulting the manufacturer’s user manual or contacting the accessory manufacturer for cleaning and disinfection instructions.9.There are no disinfectants that the EPA has identified as being effective against SARS-CoV-2 for mydevice listed on your website, what should I do?Your product user manual includes the cleaning agents that GE Healthcare has evaluated for material compatibility for your device. We recommend you also consult the user manual for cleaning instructions.GE Healthcare cautions against using cleaning agents that have not been evaluated. Cleaning agents that have not been evaluated or not passed material compatibility testing could have an unknown or harmful impact on device surfaces. The use of cleaning agents that have not been evaluated could degrade cosmetic or functional performance, damage device surfaces or labels, cause immediate equipment failure or even cause longer term latent failures.GE Healthcare is monitoring the EPA website as well as other external resources related to effectiveness of cleaning agents on SARS-CoV-2 and will update the GE Healthcare cleaner compatibility website as more information becomes available.10.What if I live outside the United States and do not have any of the disinfectants that the EPA hasidentified as being effective against SARS-CoV-2 available in my country?At this time, GE Healthcare is not aware of any international or country level government agency that has published a list similar to the one published by the U.S. EPA. GE Healthcare is monitoring the EPA website as well as other external resources related to effectiveness of cleaning agents on SARS-CoV-2 and will update the GE Healthcare cleaner compatibility website as more information becomes available.There are also additional references available, for example the U.S. Centers for Disease Control and Prevention (CDC) provides surface cleaning instructions for use during this COVID-19 pandemic:https:///coronavirus/2019-ncov/community/organizations/cleaning-disinfection.htmlNote - these are surface cleaning instructions only, for Anesthesia and Respiratory devices you should follow your device’s user manual related to internal cleani ng and disinfection.Your product user manual includes the cleaning agents that GE Healthcare has evaluated for material compatibility for your device. We recommend you also consult the user manual for cleaning instructions. GE Healthcare cautions against using cleaning agents that have not been evaluated. Cleaning agents that have not been evaluated or not passed material compatibility testing could have an unknown or harmful impact on device surfaces. The use of cleaning agents that have not been evaluated could degrade cosmetic or functional performance, damage device surfaces or labels, cause immediate equipment failure or even cause longer term latent failures.11.What if the supply of disinfectants that the EPA has identified as being effective against SARS-CoV-2has been exhausted?Your product user manual includes the cleaning agents that GE Healthcare has evaluated for material compatibility for your device. We recommend you also consult the user manual for cleaning instructions. There are also additional references available, for example the U.S. Centers for Disease Control and Prevention (CDC) provides surface cleaning instructions for use during this COVID-19 pandemic:https:///coronavirus/2019-ncov/community/organizations/cleaning-disinfection.htmlNote - these are surface cleaning instructions only, for Anesthesia and Respiratory devices you should follow your device’s user manual related to internal clea ning and disinfection.GE Healthcare cautions against using cleaning agents that have not been evaluated. Cleaning agents that have not been evaluated or not passed material compatibility testing could have an unknown or harmful impact on device surfaces. The use of cleaning agents that have not been evaluated could degrade cosmetic or functional performance, damage device surfaces or labels, cause immediate equipment failure or even cause longer term latent failures.GE Healthcare is monitoring the EPA website as well as other external resources related to effectiveness of cleaning agents on SARS-CoV-2 and will update the GE Healthcare cleaning and disinfection website as more information becomes available.12.Can medical device fans spread SARS-CoV-2?Most electronic devices use fans to pull air into the system to cool electronic components. Many electronic devices also use filters to prevent dust, lint, and similar particles from entering the system, but those filters may not be intended to block any specific pathogen. There is no data regarding the spread of SARS-CoV-2 based on fans or air movement.13.How long does the novel coronavirus survive on surfaces?Please refer to the latest publications from scientific bodies regarding this data given that new information is emerging often. For example, the U.S. National Institutes of Health (NIH) and World Health Organization (WHO) have published some information on survivability of SARS-CoV-2 on surfaces.14.Can a GE Healthcare device (e.g. ultrasound scanner and probes, mobile x-ray etc...) be disinfected tosafe levels after exposure to SARS-CoV-2?GE Healthcare has not tested any cleaning agents for disinfecting effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that causes COVID-19. The U.S. Environmental Protection Agency (EPA) has released a list of disinfectants that claim to be effective for use against SARS-CoV-2. GE Healthcare has cross referenced the disinfectants from the EPA list with the cleaning agents identified in our product user manuals, which have undergone compatibility testing with our products. The cross-referenced list is posted on our website https:///. Users should follow instructions provided by the cleaning agent manufacturer when using those disinfectants to clean GE Healthcare products.15.Can pathogens get inside a medical device and can they get out?Some devices are sealed and may prevent ingress of pathogens. However, the majority of electronic devices have cooling pathways which may include fans. Therefore, pathogens could get inside the device and get out. Dust filters are present on a majority of electronic devices and are designed for the sole purpose of filtering out dust particles.16.Can I clean and reuse an air filter on a medical device?Your product user manual should be consulted for filter service instructions. If a new filter is available, the best option is to dispose of a potentially contaminated filter when it becomes clogged and replace with a new filter. 17.Should a HEPA filter be cut to size and placed over the dust filter to reduce medical devicecontamination?No. It is highly likely that doing this would reduce sufficient airflow and cause the device to overheat, which may lead to loss of function (e.g. imaging during a procedure). The majority of electronic devices do not have airtight intake channels.18.Can I wrap the medical device in plastic to reduce possible contamination?No. The plastic will trap air and reduce airflow and possibly lead to overheating of electronic devices, which may lead to loss of function (e.g. imaging during a procedure). Some devices (e.g. ultrasound systems, patient tables etc.) could be wrapped in semi-permeable gowns or drapes which could provide added protection against large droplets or contamination.19. Should I use disposable probe covers to prevent SARS-CoV-2 transmission?Using disposable covers can improve infection control. Always follow the cleaning instructions in your device’s user manual.20.I did not see any MR products on your website what information can you provide?The GE Healthcare website is being updated routinely. Additionally, your product user manual includes the cleaning agents that GE Healthcare has evaluated for material compatibility for your device. We recommend you also consult the user manual for cleaning instructions. Below is some information related to cleaning agents that have been evaluated for material compatibility for GE Healthcare MR products.Data subject to change.© 2020 General Electric Company – All rights reserved.GE Healthcare reserves the right to make changes in specifications and features shown herein, ordiscontinue the product described at any time without notice or obligation. Contact your GE Healthcarerepresentative for the most current information. GE and the GE Monogram are trademarks of GeneralElectric Company. GE Healthcare, a division of General Electric Company. GE Medical Systems, Inc., doingbusiness as GE Healthcare.21. Is there any additional PPE for infection control that I should consider?It is important to follow the specific facilities/site local infection control protocols. The CDC has also published some Environmental Cleaning and Disinfection Recommendations that can be found here:https:///coronavirus/2019-ncov/community/organizations/cleaning-disinfection.htmlNote – refer to Material Safety Data Sheet from the cleaning agent manufacturer for any PPE related to that cleaning agent.DeviceCleaner MR Surface Coil10%/90% bleach water solution MR Dielectric Pad – AbdomenMR Dielectric Pad – NeckFlex Coil PositionerPediatric Positioner Pad 5432778• Disinfect with commercially available wipes that contain 0.525% minimum sodium hypochlorite as the only active ingredient following the manufacturer’s instructions. If commercially available wipes are not available, then follow these instructions. • Disinfect with a lint free cloth, a 1:10 dilution of commonly available bleach containing a recommended minimum sodium hypochlorite of 5.25%. Dilute the bleach with tap water. • For general disinfection or disinfection following cleaning of blood and/or body fluids, 5 minutes contact time is recommended. •Refer to internal procedures or refer to publications such as “CDC Guideline for Disinfection and Sterilization in Healthcare Facilities,” 2008 or latest revision, for guidance. Disinfectant may need to be reapplied to ensure surfaces remain wet for the duration of the selected contact time. • After you have cleaned and disinfected with bleach(sodium hypochlorite), wipe surfaces with a disposablelint free wipe that has been dampened with purified waterto remove any remaining bleach residue.Discovery MR750 3.0T, 37654Discovery MR450 1.5T, 376543.0T Signa HDxt, 37654SIGNA Premier, 37654SIGNA PET / MRDiscovery MR750w 3.0T, 37654Optima MR450w 1.5T, 376541.5T Signa HDxt, 37654SIGNA Architect, 37654SIGNA Pioneer, 37654SIGNA VoyagerSIGNA CreatorSIGNA ExplorerBrivo MR355 Optima MR360。
GE Healthcare Vivid S5和S6心血管超声仪探头指南说明书
GE HealthcareVivid S5 and S6 cardiovascular ultrasound Transducer guideComplete cardiovascular scanning.Vivid™ S5 and S6 feature a wide range of applications thatincrease the system’s versatility. The system’s probes featurea ComfortScan design and the miniaturized RS connector,with ergonomics that maximize ease of use and patientcomfort. A lightweight transducer cable minimizes strainfor the user, facilitating transducer placement.Transesophageal6T-RS6Tc-RS9T-RS6T*9T*3S-RS**M4S-RS*5S-RS6S-RS7S-RS10S-RSSpecialConvex8L-RS9L-RS12L-RS4C-RS8C-RSH40402LSE8C-RSi12L-RSP2DP6DULTC-0243-08.09-EN-USGE Healthcare9900 Innovation Drive Wauwatosa, WI 53226 U.S.A.imagination at work©2009 General Electric Company – All rights reserved.General Electric Company reserves the right to make changes in specifications and features shown herein, or discontinue the product described at any time without notice or obligation.GE and GE Monogram are trademarks of General Electric Company.Vivid and EchoPAC are trademarks of GE Medical Systems Ultrasound & Primary Care Diagnostics, LLC.Schering is a trademark of Bayer Healthcare AG.GE Medical Systems Ultrasound & Primary Care Diagnostics, LLC,a General Electric Company, doing business as GE Healthcare.*Only for Vivid S6 **Only for Vivid S5***SI/SRI and TSI are also available, but only on the Vivid S6Note: Some applications depend on the availability of certain options.†Harmonic imaging for supporting contrast agent imaging was developed by Schering.™。
GE Healthcare Panda Warmers 说明书
Multiple Solutions foradvanced technologies with innovative featuresto deliver state-of-the-art thermoregulationwhile promoting family-centered care. And nowavailable in three designs—Bedded, Freestandingand Wall Mount—to meet the specific needs ofPanda Wall Mount WarmerThe Panda Wall Mount Warmer provides a stationarymounted solution to work with your bassinet, withoutcompromising the thermal needs of your newborns. Theunique design plans of your L&D require equipment thatcan be mounted in the most esthetically pleasing way.If you are using custom cabinetry or standard cabinetryto enclose the warmer, the Panda Wall Mount Warmer isintended to fit your specific design needs while providingthe clinical features you require.Panda Freestanding Warmer The Panda Freestanding Warmer provides all the features of a Bedded Warmer, but is designed to be used with your own bed or bassinet. Offering the clinical and workflow advantages of a Bedded Warmer with the freedom to move the thermal support of the Panda technology where and when you need it, the Panda Freestanding Warmer will complete your L&D and nursery warmer needs.Panda Bedded WarmerThe Panda Bedded Warmer offers the full capabilities found across the Panda Warmer portfolio. It can be configured with full resuscitation capability and SpO2 monitoring, meeting clinical standards in a singleconfiguration. It is intended to provide a complete warming environment with monitoring of critical newborn parameters.Integrated Scale DisplayParameter Masimo Rainbow SET ®Nellcor Oximax ™Measurement Range SpO 230–100%1–100%Pulse Rate 25–240 bpm 20–300 bpm SpO 2 Accuracy From 70–100%± 3 digits for neonates ± 3 digits for neonatesBelow 69%Unspecified UnspecifiedPulse Rate AccuracyNo Motion ± 3 bpm ± 3 bpmMotion ± 5 bpm N/A MechanicalPanda Bedded Warmer• Height: 193–218 cm• Width: 64 cm• Depth: 119 cm• Weight: 100 kg• Mattress Size: 66 x 48 x 2 cm• Bed Capacity: 14 kg• Bed Tilt: ± 12° continuous tilt• Maximum patient weight: 40 kg (88 lbs)Panda Freestanding Warmer• Height: 195 cm• Width: 85 cm• Depth: 77 cm• Weight: 43 kgPanda Wall Mount Warmer• Height: 69 cm• Width: 40 cm• Depth: 77 cm• Weight: 15 kgAccessories – Bedded Only• S torage drawer package: 6.8 kg max load• Instrument shelf: 3.6 kg max loadOperating Environment• Temperature: 18°C to 30°C• H umidity: 5% to 75% non-condensing relative humidity• Pressure: 70–106 kPa• Air Velocity: up to 0.3 m/sec • Water Ingress: IPXO Electrical Power Requirements • 5.25 A @ 100v ~, 50/60 Hz • 4.57 A @ 115v ~, 50/60 Hz • 2.39 A @ 200v ~, 50/60 Hz • 2.28 A @ 230v ~, 50/60 Hz • 2.19 A @ 240v ~, 50/60 Hz Integrated Resuscitation Characteristics • Input pressure: 40-75 psi (275–517 kPa)• Minimum Input Flow: 70 lpm • Vacuum Range: 0–150 mmHg • Vacuum Accuracy: ± 5% of full scale • Flow Range: 0–15 lpm • Air/O 2 Blender Range: 21–100% O 2• Blender Accuracy: ± 5% O 2Adjustable PIP (For T-Piece Resuscitation System only)• Maximum PIP: 45 ± 5 cm H 2O • PIP Override: > 30 ± 4 cm H 2O • Flow Capacity: 15 lpm Integrated SpO 2 Characteristics System Performance • W armer expected: Approx. 8 years service life • Heater Element: 360 Watts • P atient temperature: ± 0.3°C @ 30°C measurement accuracy: to 42°C • O bservation Light: 2 dimmable 35W halogen bulbs: est. life 3000 hrs • P rocedure Light: Avg. 2000 lux (at nominal voltage); est. life 3000 hrs User control settings • P atient Control: 34–37.5°C in 0.1° temperature increments • R adiant heat power: 0–100% in 5% increments Irradiance • B edded Warmer 100% Heater Power: 31 mW/cm 2• N on-Bedded Warmer (at Highest Mattress Height Position): 100% Heater Power: 31 mW/cm 2• Heater Warmup Time at 100% Power: less than 3 minutes Weight scale performance • Functional range: 300 g to 8 kg • Accuracy: ±10 gTechnical Specifications© 2013 General Electric Company. All rights reserved.General Electric Company reserves the right to make changes in specifications and features shown herein, or discontinue the product described at any time without notice or obligation. Contact your GE Representative for the most current information.GE and GE Monogram are trademarks ofGeneral Electric Company.*Panda is a trademark of General Electric Company.Datex-Ohmeda, Inc., a General Electric company,doing business as GE Healthcare.MIC-0270-10.12-EN-Asia Pacific Printed in China March 2013DOC1279910_r12GEA30498 (01/2013)0086About GE Healthcare GE Healthcare provides transformational medical technologies and services that are shaping a new age of patient care. Our broad expertise in medical imaging and information technologies, medical diagnostics, patient monitoring systems, drug discovery, biopharmaceutical manufacturing technologies, performance improvement and performance solutions services help our customers deliver better care to more people around the world at a lower cost. In addition, we partner with healthcare leaders, striving to leverage the global policy change necessary to implement a successful shift to sustainable healthcare systems.Our “healthymagination” vision for the future invites the world to join us on our journey as we continuously develop innovations focused on reducing costs, increasing access, and improving quality around the world.Headquartered in the United Kingdom, GE Healthcare is a unit of General Electric Company (NYSE: GE). Worldwide, GE Healthcare employees are committed to serving healthcare professionals and their patients in more than 100 countries. For more information about GE Healthcare, visit our website at .GE Healthcare 8880 Gorman Road Laurel, MD 20723 GE Healthcare Asia PacificJapan4-7-127, Asahigaoka Hino-shi, Tokyo 191-8503 Japan Tel: +81 42 585 5111ANZBuilding 4B, 21 South St Rydalmere NSW 2116Australia Tel: 1300 722 2298 Tangihua StreetAuckland 1010New ZealandTel: 0800 434 325ASEAN1 Maritime Square #13-01HarbourFront CentreSingapore 099253Tel: +65 6291 8528Korea8F, POBA Gangnam Tower343, Hakdong-ro, Gangnam-guSeoul 135-820, KoreaTel: +82 2 6201 3114。
GEHealthcare 心脏后处理 快速说明书

心脏扫描快速操作 4、心脏螺旋扫描 a. 扫描程序设定: 造影剂用量:100~120ml,4ml/sec
• • • • • • • • 扫描类型 – Cardiac Helical 螺旋时间 – 0.5 sec 扫描范围 – 心底部~心尖 层厚: 0.625/1.25mm 无重叠重建 扫描野– Large 显示野– 25cm kV – 120 mA – 300-400
mA:270mA—300mA—320mA
前门控设置: 触发延迟60%
图像间间隔时间:50ms 第 5 页
Smartscore快速操作
三、启动SmartScore软件
• 将扫描得到的图像传输至AW 上;把记录心电图的软盘插入 AW上的软驱内,AW将自动 读取心电图。 • 在AW的浏览窗中选定欲进行 SmartScore软件分析的图像序 列;点击 菜单(图4)中选取 [SmartScore],计算机运行后 弹出右侧界面(图5)。 • • 按界面中的提示逐项输入病人 的一般及特殊信息。 输完信息后,可点击[Analysis], 进入分析阶段。 ,从下拉
图11 图12
第 10 页
Smartscore快速操作
五、选择设置
• • 点击[Preference]目录下的[Category]和[Guide]键分别出现下列界面(图13、14)。在其 中输入数据库后,计算机会自动对病人的积分进行分析,得出结论打印在病人报告中。 点击[Quit]键,可以退出SmartScore软件。
箭 头 指 示 键
第 8 页
Smartscore快速操作
四、报告积分
• 图像分析结束后,点击其下方的[Report]键, 进入报告阶段。图9显示为在[AJ130]理论模 式的计算下所得的冠状动脉的钙化积分。 • 点击下方的[Output]键,出现右侧下拉菜单 (图10)。点击[Save Screens]、[Send To Film]、[Save To Diskette]等键可以对图像 及报告进行存储、照相 。 选择图像和(或)报告 后点击[Save Screens] 可将其作为一个新的序 列进行存储
GE Healthcare Dash 2500 Patient Monitor快速指南说明书

You can discharge a patient at any time during monitoring. Discharging a patient automatically saves the patient vious patient.
1
Quick guide-new.indd 2
Downloaded from manuals search engine
12-7-17 9:33
Start Monitoring
• Turn on the power by pushing either the Power button or the Trim Knob when plugged into AC power
Discharging a patient
Suspend all monitoring and alarms for ECG. Choose primary lead.
Choose secondary lead.
Increase or decrease the size of all the ECG waveforms displayed on the screen.
Freeze - captures up to 16.8 seconds of waveforms on the screen. The number of seconds varies depending on the selected sweep speed.
Trend - enters and exits trends (view patient trends data). This hardkey can be configured through the configuration mode to display two different views: mini trends or full trends.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Our Vision: Healthcare Re-imagined
We are striving to transform the delivery of healthcare. Our Purpose is to help predict, diagnose, treat and monitor disease earlier so people can live life to the fullest
GE Healthcare global presence
USA 21,277 / 46%
Employees
Americas 2,168 / 5% Employees L America 1,504 Canada 664
5 Yr V%
~50%
Europe & MEA
14,970 / 32% Employees
From
"Late Disease"
Symptom-based Incomplete data Managing illness Standardized treatment
To
"Early Health"
Prevention/prediction Detailed patient Info Early diagnosis Targeted therapies
Global HC Spend Profile
Screen
Inform
Localize Characterize Intervene
Feedback
Today Predict Diagnose
5%
15%
Treat 70%
Future Predict 9%
Diagnose 19%
Treat 60%
$250B+ diagnostic spending shift over the
Meeting healthcare needs • Transparency of cost • Quality of healthcare • Early health, earlier diagnosis • Increasing productive lifespan • Equity in healthcare access
Creating new opportunities, serving healthcare customers, positioned for growth
Page 3 GE Healthcare European MedTech and Healthcare Conference September 5, 2007
8
Japan: "Enterprise" driving portfolio Emerging markets: Big opportunities
$3.2
Japan 5
"Company to Country" and Rural Health in developing world
Europe 16
UK
2,776
France
2,399
Sweden
1,887
Germany
1,657
Norway
1,410
Finland
931
MEA
259
Eastern Europe 478
~100+%
Strong global commitment
Asia
7,994 / 17% Employees
China India Japan Korea ANZ SEA
Only GE Healthcare
Information Technology & Services
• Electronic med records • PACS • Performance solutions • Multi-vendor services
Life Sciences • Discovery systems & tools • Protein and cell sciences
Healthcare Environment
+
+ Strong global revenues
-
– U.S. Market – reimbursement cuts
+ Emerging markets infrastructure spend
+ Excellent Ultrasound and Devices growth
2,950 2,122 1,859
394 382 287
~50%
Page 8 GE Healthcare European MedTech and Healthcare Conference September 5, 2007
Growth in developed and developing markets
Unmatched global, product
Clinical Systems and services reach
Medical
World-class execution and
$7
Diagnostics Service
process rigor
Growth through disease focus
+ Life Sciences growing double digit
– Regulatory pressure
– Japan weakness
– Increased scrutiny on contrast agent “appropriateness criteria”
+ Demographics
+ Privatization
+ Hospital digitization
Dynamic market…many opportunities to grow
Page 2 GE Healthcare European MedTech and Healthcare Conference September 5, 2007
GE Healthcare
Revenue ($ in billions)
New technologies
+
= growth
market understanding
How we win
~$17
Industry-leading technology
Life Sciences
$13
Healthcare IT
next decade
Monitor 10%
Monitor 12%
Key drivers … why now?
• Technology … advances and costs Enable it
• Economics … healthcare systems Require it
• Demographics … baby boomers Demand it
Well-positioned for China rebound
2000
2007E
Localization in MEA driving 25% growth
$4.5B growth in 7 years
Page 7 GE Healthcare European MedTech and Healthcare Conference September 5, 2007
Broad-based Diagnostics
Diagnostic Imaging
CT, PET/CT MR, XR
Medical Diagnostics
Contrast agents Molecular diagnostics
Clinical Systems
Ultrasound Critical care
Diagnostic Imaging
Success in eቤተ መጻሕፍቲ ባይዱerging markets
2000
2004
2007E
Delivering clinical efficacy and efficiency
Positioned for continued growth
Page 6 GE Healthcare European MedTech and Healthcare Conference September 5, 2007
GE Healthcare
Katy McCarthy Executive Vice-President and CFO
European Medtech and Healthcare Conference
September 5, 2007
“This document contains "forward-looking statements" - that is, statements related to future, not past, events. In this context, forward-looking statements often address our expected future business and financial performance, and often contain words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," or "will." Forwardlooking statements by their nature address matters that are, to different degrees, uncertain. For us, particular uncertainties which could adversely or positively affect our future results include: the behavior of financial markets, including fluctuations in interest rates and commodity prices; strategic actions, including dispositions; future integration of acquired businesses; future financial performance of major industries which we serve, including, without limitation, the air and rail transportation, energy generation, media, real estate and healthcare industries; unanticipated loss development in our insurance businesses; and numerous other matters of national, regional and global scale, including those of a political, economic, business, competitive and regulatory nature. These uncertainties may cause our actual future results to be materially different than those expressed in our forwardlooking statements. We do not undertake to update our forward-looking statements.
