【材料研究方法】光谱分析(英文)
光谱分析法简介(UV AAS FTIR NMR)..

式中,A ——吸光度
K ——常数 ——意义 该式是原子吸收光谱定量分析的基本关系式:吸光度 (absorbance)A与样品中某元素的含量 C呈线性关系。通过一组已 知浓度的标准样品,做出A与C之间的工作曲线。在同样条件下,测 量未知物的吸光度后,利用工作曲线就可求得未知物的浓度Cx
2018/10/8
转变成原子蒸气,是原子吸收光谱分析法中的关键部件之一。有 火焰原子化器和无焰原子化器两类
●分光系统单色器 ●检测系统
作用是把要测量的吸收谱线同其他谱线
分开。分光部件有棱镜和光栅两种类型
作用是接受光信号,并把光信号转换成电信号 ,经放大和运算处理,给出分析结果。主要由检测器、放大器、 读数和记录系统等组成
——摩尔吸收系数 在A=lg(I0/It)=Kbc中,当浓度c用mol· L-1, 液层b厚度用cm表示,则K用摩尔吸光系数代之
A= bc
的意义 物质的量浓度为1mol· L-1,液层厚度为1cm时溶液的吸
光度。它反映了吸光物质对光吸收的能力,一定条件下为常数; 同一物质用不同显色剂时,有不同的值
——同一波长的光称单色光,由不同波长组成的光称复合光。物质 有色是因其分子对不同波长的光选择性吸收而产生。下页表列出了 颜色与吸收光之间的关系。其中对应颜色的光称互补色光 ——测量物质对不同波长单色光的吸收程度,以波长为纵坐标,吸 光度为横坐标,得一条吸收光谱曲线,它清楚地描述物质对光的吸 收情况。图片分光UV-1图1为MnO4-和Cr2O72-的选择性吸收曲线 。可见, MnO4-在可见光范围内对525nm附近的绿光有最大吸收 max=525nm。浓度不同光吸收曲线形状相同,吸光度大小不同 UV-11
( 6)灵敏度高
(7)样品损坏少
第三章光谱分析-SEM

4、扫描电镜图像和衬度
背散射电子像
1. 成分衬度
样品表面上平均原子序数Z大 的部位形成较亮的区域;
平均原子序数较低的部位形 成较暗的区域。
背散射电子像
2. 形貌衬度
分辨率远比二次电子低。 背反射电子是来自一个较大 的作用体积; 背向检测器的样品表面检测 器无法收集到背反射电子。
背散射电子探头采集的成分像(左图)和形貌像(右图)
6、试样的制备 生物医学材料试样制备
生物样品制备的一般原则: 防止样品污染、损伤,保持原有形貌、微细结构 去除样品内的水分-避免样品体积变小、表面收缩 增加样品的导电性能 注意辨认和保护观察面
3. 扫描电镜衬度像 二次电子像 背散射电子像
衬度:明暗程度
4、扫描电镜图像和衬度 二次电子像
KUST
6、试样的制备 非金属材料试样制备
镀膜材料的选择:Au、C、Ag、Cr、Pt等 熔点较低、易蒸发 与常用的钨丝加热器不发生任何作用 二次电子、背散射电子发射效率高 化学性能稳定
镀膜厚度 导电膜应均匀、连续,厚度200-300Å 不能太薄,否则导电膜显著不均、易破裂,甚至部分表 面未蒸镀上导电膜 不能太厚,否则导电膜易产生龟裂,掩盖试样表面结构 细节 先蒸发一层很薄的炭,然后再蒸镀金属层可以获得比较 好的效果。
制约因素主要有: 入射电子束束斑直径 入射电子束在样品中的扩展效应 信噪比
4、扫描电镜的主要性能和特点
景深D大
景深: 透镜对高低不平的试样 各部位能同时聚焦成像的一个能力 范围。
图像立体感强,适合粗糙不平 的断口样品观察。
扫描电镜的景深比透射电镜的 景深大10 倍。
4、扫描电镜的主要性能和特点
2、扫描电镜的结构原理和构造
光谱分析法导论(中英文版)

2. 联用技术
电感耦合高频等离子体(ICP)—质谱
激光质谱:灵敏度达10-20 g
3. 新材料
光导纤维传导,损耗少;抗干扰能力强;
04:01:03
4. 交叉
电致发光分析;光导纤维电化学传感器
5. 检测器的发展
电荷耦合阵列检测器光谱范围宽、量子效率高、线性范 围宽、多道同时数据采集、三维谱图,将取代光电倍增管; 光二极激光器代替空心阴极灯,使原子吸收可进行多元素 同时测定;
04:01:03
原子的能级通常用光谱项符号表示:nMLJ
n:主量子数;M:谱线多重性符号; L:总角量子数; J :内量子数
钠原子的光谱项符号 32S1/2;
表示钠原子的电子处于n=3,M =2(S = 1/2),L =0,
J = 1/2 的能级状态(基态能级);
04:01:03
电子能级跃迁的选择定则
5. 分子磷光分析法
处于第一最低单重激发态分子以无辐射弛豫方式进入第 一三重激发态,再跃迁返回基态发出磷光。测定磷光强度进 行定量分析的方法。
6. X射线荧光分析法
原子受高能辐射,其内层电子发生能级跃迁,发射出特征
X射线( X射线荧光),测定其强度可进行定量分析。
7. 化学发光分析法
利用化学反应提供能量,使待测分子被激发,返回基态 时发出一定波长的光,依据其强度与待测物浓度之间的线性 关系进行定量分析的方法。
1.光谱项符号
原子外层有一个电子时,其能级可由四个量子数决定: 主量子数 n;角量子数 l;磁量子数 m;自旋量子数 s; 原子外层有多个电子时,其运动状态用总角量子数L;总 自旋量子数S;内量子数J 描述;
04:01:03
总角量子数
L=∑ l 外层价电子角量子数的矢量和,(2 L +1)个 L=| l 1+ l2 | , | l 1+ l2 -1|,· · · · · · , | l 1 - l2 | 分别用S,P,D,F · · · · · · ,表示:
【材料研究方法】光谱分析(英文)

Vibrational spectroscopy6.3.7 Absorbant intensity and IR spectroscopyt r a n s m i t t a n c e Fig. 6-18 IR spectrum. s (strong )、m (medium )、w(weak )、vw (very weak )a b s o r b a n c eVibrational spectroscopy3334 cm -1–OH stretch. Normal range: 3350±150 cm -1. This is a verycharacteristic group frequency. All of the peaks due to the OH group are broad due to hydrogen bonding.Vibrational spectroscopy 3390 cm-1–NH2antisymmetric stretch.Normal range: 3300±100 cm-1. Muchweaker adsorption than the OH stretchin hexanol.Vibrational spectroscopy 3290cm-1–NH2symmetric stretch. 2°amines have only one NH stretch, and3°amines have none.Vibrational spectroscopy Spectral interpretation always starts at the high end, because there are the best group frequencies and they are the easiest to interpret. No peaks appear above 3000 cm-1, the cut-off for unsaturated C-H. the four peaks below 3000 cm-1 are saturated C-H stretching modes.Vibrational spectroscopy 3050 ±50 cm-1 corresponds to the aromatic orunsaturated C(sp2)-H stretch. Always above3000 cm-1. These bands are not assigned tospecific vibrational modes.Vibrational spectroscopy 3080cm-1=CH3080 cm1 2 antisymmetric stretch. An absorption above 3000 cm-1 indicates the presence of an unsaturation (double or triple bond or an aromatic ring).Vibrational spectroscopy 2247 cm-1 C≡N stretch. Normal range:2250±10 cm-1 , lowered 10-20 cm-1when conjugated. Compare to the C ≡Cstretch in 1-heptyne (3000 cm-1).Vibrational spectroscopy 1742 cm-1, -C=O stretch. In small ring esters, this vibration is shifted to higher frequency by coupling to the stretch ofthe adjacent of O-C and C-C bonds. The amount of coupling depends on the O-C(O)-C angle. As with other carbonyl groups, conjugation lowers the frequency.Vibrational spectroscopy 1642 cm-1, C=C stretch. Normalrange:164020cm-1for cis andrange: 1640±20 cm for cis andvinyl, 1670±10 cm-1for trans, tri andtetra substituted. Trans-2-hexene(overlay menu) has only a very weakabsorption, because there is verylittle dipole change when an internaldouble bond stretches (it is nearlysymmetric).Vibrational spectroscopyThe broad peak at approximately 1460 cm-1is actually two overlapping peaks. At1640±10 cm-1, the antisymmetric bend ofthe CH3group absorbs. This is a degenerage bend (one shown).Vibrational spectroscopy At 1375 ±10cm-1, the CH3 symmetric bend (also called the“umbrella” bend) absorbs. This peak is very useful becauseit is isolated from the other peaks. Compare the spectrum ofcyclohexane. The most prominent difference between thetwo spectra is the absence of a CH3 symmetric bend in thecyclohexane spectrum./cm-11715 1800 1828 1928。
光谱分析法导论(中英文版)

13:52:22
辐射能的特性:
(1) 吸收 (2) 发射 (3) 散射 物质选择性吸收特定频率的辐射能,并从低能 将吸收的能量以光的形式释放出; 丁铎尔散射和分子散射;
级跃迁到高能级;
(4) 折射
(5) 反射 (6) 干涉
折射是光在两种介质中的传播速度不同;
干涉现象;
(7) 衍射
(8) 偏振 光。
光分析法导论
an introduction to optical analysis
13:52:22
第一节 光分析基础
fundamental of optical analysis
13:52:22
一、光分析及其特点 optical analysis and its feature 二、电磁辐射的基本性质 properties of electromagnetic radiation 三、光分析法的分类 classification of optical analysis 四、各种光分析法简介 a brief introduction of optical analysis 五、光分析的进展 development of optical analysis
13:52:22
一、光分析法及其特点
optical analysis and its characteristics
光分析法:基于电磁辐射能量与待测物质相互作用后 所产生的辐射信号与物质组成及结构关系所建立起来的分析 方法;
电磁辐射范围:射线~无线电波所有范围;
相互作用方式:发射、吸收、反射、折射、散射பைடு நூலகம்干 涉、衍射等;
原子光谱(线性光谱):最常见的三种
基于原子外层电子跃迁的原子吸收光谱(AAS); 原子发射光谱(AES)、原子荧光光谱(AFS); 基于原子内层电子跃迁的 X射线荧光光谱(XFS); 基于原子核与射线作用的穆斯堡谱;
常用材料分析方法中英文对照

1. Elemental Analysis 元素分析Atomic absorption spectroscopy 原子吸收光谱Auger electron spectroscopy (AES) 俄歇电子能谱Electron probe microanalysis (EPMA) 电子探针微分析Electron spectroscopy for chemical analysis (ESCA) 化学分析电子能谱Energy dispersive spectroscopy (EDS) 能量色散谱Flame photometry 火焰光度法Wavelength dispersive spectroscopy (WDS)X-ray fluorescence X射线荧光2. Molecular and Solid State Analysis 分子与固态分析Chromatography [gas chromatography (GC), size exclusion chromatography (SEC)]色谱[气相色谱,体积排除色谱]Electron diffraction 电子衍射Electron microscopy [scanning electron microscopy (SEM),transmission electron microscopy (TEM),scanning TEM (STEM)] 电子显微镜Electron spin resonance (ESR) 电子自旋共振Infrared spectroscopy (IR) 红外光谱Mass spectrometry 质谱Mercury porosimetry 压汞法Mossbauer spectroscopy 穆斯堡尔谱Nuclear magnetic resonance (NMR) 核磁共振Neutron diffraction 中子衍射Optical microscopy 光学显微镜Optical rotatory dispersion (ORD) 旋光色散Raman spectroscopy 拉曼光谱Rutherford back scattering (RBS) 卢瑟福背散射Small angle x-ray scattering (SAXS) 小角X射线散射Thermal analysis [differential scanning calorimetry (DSC),thermal gravimetric analysis (TGA),differential thermal analysis (DTA) temperature desorption spectroscopy (TDS),thermomechanical analysis (TMA)]热分析[差示扫描量热计法,热-重分析,微分热分析,升温脱附,热机械分析]UV spectroscopy 紫外光谱X-ray techniques [x-ray photoelectron spectroscopy (XPS), x-ray diffraction (XRD), x-ray emission,x-ray absorption] X射线技术[x射线光电子能谱,x射线衍射,x射线发射,x射线吸收]3. Surface Characterization Techniques 表面表征技术Electron energy loss spectroscopy (EELS) 电子能量损失谱Ellipsometry 椭圆偏振术Extended x-ray absorption fine structure (EXAFS) 扩展X射线吸收精细结构Helium (or atom) diffractionLateral (or frictional) force microscopy (LFM) 横向(摩擦)力显微镜Low-energy electron diffraction (LEED) 低能电子衍射Magnetic force microscopy (MFM) 磁力显微镜Near-edge x-ray adsorption fine structure (NEXAFS) 近边X射线吸收精细结构Near field scanning 近场扫描Reflection high-energy electron diffraction (RHEED) 反射高能电子衍射Scanning tunneling microscopy (STM) 扫描隧道显微镜Scanning force microscopy (SFM) 扫描力显微镜Secondary ion mass spectroscopy (SIMS) 二次离子质谱Surface enhanced raman spectroscopy (SERS) 表面增强拉曼光谱Surface extended x-ray adsorption fine structure (SEXAFS) 表面扩展X射线吸收精细结构Surface force apparatus 表面力仪器。
光谱分析法导论(中英文版)

5. 分子磷光分析法
处于第一最低单重激发态分子以无辐射弛豫方式进入第 一三重激发态,再跃迁返回基态发出磷光。测定磷光强度进 行定量分析的方法。
6. X射线荧光分析法
原子受高能辐射,其内层电子发生能级跃迁,发射出特征 X射线( X射线荧光),测定其强度可进行定量分析。
7. 化学发光分析法
(2) 发射 将吸收的能量以光的形式释放出; (3) 散射 丁铎尔散射和分子散射; (4) 折射 折射是光在两种介质中的传播速度不同; (5) 反射 (6) 干涉 干涉现象; (7) 衍射 光绕过物体而弯曲地向他后面传播的现象; (8) 偏振 只在一个固定方向有振动的光称为平面偏振 光。
21:49:51
21:49:51
光分析法导论
an introduction to optical analysis
第一节 光分析基础
fundamental of optical analysis
21:49:51
一、光分析及其特点 optical analysis and its feature 二、电磁辐射的基本性质 properties of electromagnetic radiation 三、光分析法的分类 classification of optical analysis 四、各种光分析法简介 a brief introduction of optical analysis 五、光分析的进展 development of optical analysis
法法法
原 原原 X
子
子子
射 线
发 吸荧 荧
射 收光 光
原子光谱法
吸收光谱法
原紫红核 子外外磁 吸可可共 收见见振
材料现代研究方法第八章 分子光谱分析法-红外和拉曼光谱法_1

Mechanic Model as a Stretching Vibration
in a Diatomic Molecule 双原子分子伸缩振动的力学模型
Hooke’s law 胡克定律
F ky
k: force Constance for the bond 化学键的力常数
Mechanic Model as a Stretching Vibration in a Diatomic Molecule双原子分子伸缩振动的力学模型
CHCl3
Calculated* Measured
C-H stretching
3002
3020
C-H bending
1120
1219
C-Cl stretching
701
773
C-Cl bending
418
671
* Spartan ’02 AM1 minimization
CDCl3
Measured
2256 912 737 652
– Linear Molecule 线形分子: 3N-5
Instrumentation for IR Measurement 用于红外范围测量的仪器
• Dispersive Infrared Spectrometers色散型红外光谱仪
The same as UV-vis spectrophotometer with the light source, the dispersive elements and the detector adequately designed for IR Drawbacks:缺点 Slow scan speed, low sensitivity and low resolution 扫速慢 灵敏度低 光谱分辨率低
【材料研究方法】光谱分析(英文)
Vibrational spectroscopy6.3.1 Fundamentals of vibrational spectroscopyDefinition Vibrational spectroscopy:is concerned with the d t ti f t iti detection of transitions between energy levels in molecules that result from stretching and bending vibrations of the interatomic bonds.asymmetricalVibrational spectroscopyKinds of vibrational spectroscopy ¾Infra-red spectroscopy(more sensitive to polarized group)6.3.1 Fundamentals of vibrational spectroscopysymmetrical¾Raman spectroscopy (moresensitive to non-polarized)Both methods are concerned with vibrations in molecules , they differ in the manner in which interaction with the exciting radiation occurs .Linear PE: (a) IR, (b)RamanFig. 6-14 Dipole moment of HClVibrational spectroscopyVibrating of Disulfide carbonSymmetrical stretchingInfrared inactive 6.3.2 Infrared spectroscopyAsymmetrical stretchingBendingInfrared activeInfrared inactive Fig. 6-15 Vibration of Disulfide carbonm1lowHigh/cm-1High/cm-1lowVibrational spectroscopy Methylbenzene(甲苯)2005.2 S. Guv =0 represents the ground state v =l the excited vibrational state6.3.3 Raman spectroscopy(1)(2)(3)Vibrational spectroscopy ¾The essential prerequisite for Raman scattering is a change in the polarizability of the bond when vibrations occur.Polarizability may be thought of as a measure of 6.3.3 Raman spectroscopy¾Polarizability may be thought of as a measure of theFig. 6-16 Motion state of linear molecules Degrees of freedom (H2O) : 3×3−6 = 3Vibraitonal modes (methylene group):2926cm-1(s)asνAsymmetricalsν: 2853 cm Symmetricalδ:1468 cm-1(m) δr:720 cm-1(CH1306~1303cm-1(w)γt :1250cmscissoring rocking waggingHexaneFour peakspSpectral interpretation always starts at the high end, because there are the best group frequencies and they are the easiest to interpret. No peaks appear above 3000 cm-1, the cut-off for unsaturated C-H. the four peaks below 3000 cm-1 are saturated C-H stretching modes.HexaneThe peak at 2962 cm-1 isassigned to the antisymmetricassigned to the antisymmetricstretch of the CH3group. Thisvibration is always found inthe range 2962±10 cm-1. thereare actually two degenerateantisymmetric stretchingmodes (only one shown).HexaneAt 2926cm-1, the CH2antisymmetric stretchabsorbs.Normal range:2926±10 cm-1.HexaneAt 2872cm-1, the CH3symmetric stretchabsorbs.Normal range:2872±10 cm-1.HexaneAt2853-1,the CHAt 2853cm, the CH2symmetric stretchabsorbs.Normal range:2853±10 cm-1.Vibrational spectroscopy Hexane1470cm-1This is the C-H bendingregion, expanded to show thenearly overlapping peaks forthe CH3and CH2bends.Vibrational spectroscopyHexanerocking When four or more CH2groups arein a chain, a vibration at 720±10cm-1corresponds to concertedrocking of all of the CH2’s.Vibrational spectroscopyHexanol3334 cm-1–OH stretch. Normal range: 3350±150 cm-1.This is a very characteristic group frequency. All of thepeaks due to the OH group are broad due to hydrogenbonding.Vibrational spectroscopy Hexanol 1430 cm -1–OH bend . Normal range: 1400±100 cm -1. This broad peak is buried under the CH bending modes.Vibrational spectroscopyHexanol660 cm -1–OH wag. While not a group frequency, this is another band due to the OH.Vibrational spectroscopy Aromatic ring expansion (Methylbenzene )At 1601 cm -1, thesymmetric ring strethch absorbs. Normal range: 1590±10 cm -1. This ib ti h di lOnly notsymmetrically substituted.vibration has a dipole change (and absords in IR) only when notsymmetrically substituted. The intensity of this band also varies with thesubstituent. Compare to p-xylene from the overlay menu.Vibrational spectroscopyAromatic ring expansion (Methylbenzene )At 1500cm -1, a different ring stretch absorbs. Range: 1500±10cm -1. Variable intensityVibrational spectroscopy 6.3.6 Comparing of IR and Raman SpectroscopyasymmetricalsymmetricalFig. 6-17 Linear PE: (a) IR, (b) Raman。
光谱分析仪分析流程

光谱分析仪分析流程英文回答:Spectroscopy is a technique used to analyze the interaction between matter and electromagnetic radiation.It provides valuable information about the composition, structure, and properties of materials. Spectroscopy instruments, known as spectrometers, are used to measure and analyze the intensity and wavelength of electromagnetic radiation.The analysis process using a spectrometer typically involves several steps. Here is a general outline of the spectroscopy analysis workflow:1. Sample Preparation: The first step is to prepare the sample for analysis. This may involve cleaning, grinding, or diluting the sample depending on its nature. It is essential to ensure that the sample is representative and homogeneous.2. Instrument Calibration: Before starting the analysis, the spectrometer needs to be calibrated. Calibrationinvolves measuring known reference samples to establish a baseline for accurate measurements. This step ensures that the instrument is properly adjusted and provides reliable results.3. Measurement: Once the sample is prepared and the instrument is calibrated, the measurement can begin. The sample is placed in the spectrometer, and the instrument measures the intensity of the radiation at different wavelengths. The resulting data is called a spectrum.4. Data Analysis: After obtaining the spectrum, thenext step is to analyze the data. This involvesinterpreting the peaks, patterns, and intensities in the spectrum. Various mathematical and statistical techniques can be applied to extract meaningful information from the data.5. Identification and Quantification: Based on theanalysis of the spectrum, the next step is to identify and quantify the components present in the sample. This can be done by comparing the obtained spectrum with reference spectra or using spectral databases. Quantification involves determining the concentration or amount of each component.6. Data Reporting: Finally, the analysis results are documented and reported. This includes summarizing the findings, presenting the spectra and their interpretations, and providing any additional relevant information. The report may also include recommendations or further analysis suggestions.Spectroscopy analysis can be performed using various techniques such as UV-Vis spectroscopy, infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance spectroscopy, among others. Each technique hasits specific principles and applications, but the general analysis workflow remains similar.中文回答:光谱分析仪是一种用于分析物质与电磁辐射相互作用的技术。
中英文对照(材料研究方法)

中英⽂对照(材料研究⽅法) Optical microscopy 光学显微镜 XRD (X-ray diffraction) X射线衍射 TEM Transmission Electron Microscopy 透射电⼦显微镜 SEM Scanning Electron Microscopy 扫描电⼦显微镜注:扫描电⼦显微镜和透射电⼦显微镜的成像原理完全不同,它是使⽤从样品表⾯激发出的各种物理信号来调制成像的。
resolution 分辨率 focal point 焦点 focal distance 焦距 Depth of field 景深 depth of focus 焦长 Aberrations 偏差,差错 Astigmatism 像散 Spherical aberration 球差 Chromatic aberration ⾊差 x射线和γ射线区别:Gamma rays are now usually distinguished by their origin: X-rays are emitted by definition by electrons outside the nucleus, while gamma rays are emitted by the nucleus. Spectroscopy 光谱 Residual strain/stress 参与应⼒ Neutron beam 中⼦束 Laue pattern 劳埃衍射花样 Debye pattern 德拜衍射花样 The structure of crystalline 晶体结构 Primitive cell 原胞 smallest possible unit cell Unit cell 单胞 arbitrarily chosen but in practical always highest possible symmetry, so as many right angles as possible Crystal structure = lattice + basis 晶体结构 = 点阵 + 基元 7 crystal systems(7个晶系): according to symmetry(对称性) 7 lattice systems (7个点阵系统): according to lattice cubic ⽴⽅ hexagonal 六⽅ tetragonal 四⽅ trigonal 三 orthorhombic 正交晶系 monoclinic 单斜 triclinic 三斜 HCP 密排六⽅ Diffraction intensity 衍射强度。
材料现代分析测试技术-光谱分析

弧层边缘的温度较低,因而这里处于基态的同类原子较多。 这些低能态的同类原子能吸收高能态原子发射出来的光而 产生吸收光谱。原子在高温时被激发,发射某一波长的谱 线,而处于低温状态的同类原子又能吸收这一波长的辐射, 这种现象称为自吸现象。
光电直读光谱仪
在原子发射光谱法中, 一般多采用摄谱法(spectrography)。
摄谱法是用感光板记录光谱。将光谱感光板置于摄谱仪 焦面上,接受被分析试样的光谱作用而感光,再经过 显影、定影等过程后,制得光谱底片,其上有许多黑 度不同的光谱线。然后用影谱仪观察谱线位置及大致 强度,进行光谱定性及半定量分析。
(6)谱线的自吸与自蚀
三、谱线的自吸与自蚀(self-absorption and selfreversal of spectral lines)
在实际工作中,发射光谱是通过物质的蒸发、激发、 迁移和射出弧层而得到的。首先,物质在光源中蒸发形成 气体,由于运动粒子发生相互碰撞和激发,使气体中产生
大量的分子、原子、离子、电子等粒子,这种电离的气 体在宏观上是中性的,称为等离子体。在一般光源中, 是在弧焰中产生的,弧焰具有一定的厚度,如下图:
4. Atomic fluorimetry
气态自由原子吸收特征波长的辐射后,原子的外层 电子 从基态或低能态跃迁到较高能态,约经10-8 s,又跃
迁至基态或低能态,同时发射出与原激发波长相同(共 振荧光)或不同的辐射(非共振荧光—直跃线荧光、阶 跃线荧光、阶跃激发荧光、敏化荧光等),称为原子荧 光。波长在紫外和可见光区。在与激发光源成一定角度 (通常为90)的方向测量荧光的强度,可以进行定量分 析。
光谱学英语

光谱学英语一、单词1. spectrum(复数:spectra)- 英语释义:A band of colors, as seen in a rainbow, produced by separation of theponents of light by their different degrees of refraction according to wavelength.- 用法:可以用作名词,如“The spectrum of light includes colors f rom red to violet.”(光谱包括从红色到紫色的颜色。
) - 双语例句:The visible spectrum is just a small part of the electromagnetic spectrum.(可见光谱只是电磁光谱的一小部分。
)2. spectroscopy- 英语释义:The study of the interaction between matter and radiated energy, especially in terms of the frequencies present in a spectrum of the radiation.- 用法:作名词,例如“Spectroscopy is widely used in chemical analysis.”(光谱学在化学分析中被广泛应用。
)- 双语例句:Infrared spectroscopy can be used to identify different chemicalpounds.(红外光谱学可用于识别不同的化合物。
)3. wavelength- 英语释义:The distance between successive crests of a wave, especially points in a sound wave or electromagnetic wave.- 用法:名词,如“Each color has a different wavelength.”(每种颜色都有不同的波长。
光谱分析法概论

顺磁共振波谱分析法——在外磁场的作用下,电子的自旋 磁矩与磁场相互作用而裂分为磁量子数不同的磁能级,测定 其对微波辐射的吸取来进展的构造分析 。
图 电磁波谱
其次节 电磁辐射与物质的相互作用
reciprocity of electromagnetic radiation and matter
电磁辐射与物质的相互作用是简单的物理现象。
(1) 吸取 物质选择性吸取特定频率的辐射能,并从基 态跃迁到激发态的过程。
(2) 放射 物质从激发态返回至基态,并以光的形式释 放出吸取能量的过程。
n=1
4
3
2
4
1
3
2
1
分子能级示意图
电子能级的跃迁不行避开的要产生振动能级和转动能级 的跃迁。假设电子能级的跃迁需要5eV,振动能级的跃迁需要 0.1eV,转动能级的跃迁需要0.005eV,那么,电子能级跃迁 产生的吸取光谱波长为248nm,而振动能级和转动能级跃迁 并不产生一条波长为248nm的线,而是产生一系列的线,其 波长间隔为5nm和0.25nm。
v1 / 1 = v2 / 2 = = c / =
二、电磁波谱
电磁辐射依据波长(或频率、波数、能量)大小的挨次排列 就得到电磁波谱。
电磁波谱的排列具有明显的规律性:
1.波长渐渐增大,频率和光子能量渐渐减小
2.各种电磁辐射不仅波长不同,产生的机理也不同:
高能辐射区
光学光谱区
波谱区
表 电磁波谱
其次章 光谱分析法概论
光学分析方法〔optical analysis〕
基于物质放射的电磁辐射(electro-mageneticradiation ) 或“辐射与物质相互作用”之后产生的辐射信号或发生的信号 变化等光学特性,进展物质的定性和定量分析的方法。
材料研究方法--光谱分析

元素轻则高波数,元素重则低波数; 高价则高波数,低价则低波数。
谱带的强度 ③ 谱带的强度
峰相对强度 与分子振动时偶极矩的变化率有关,与 样品的厚度、种类及其含量有关。 IR可对某一基团定量分析。
压片法: 是固体样品红外测定的标准方法。 将固体样品0.5-1.0mg与150mg左右的 KBr一起粉碎,用压片机压成薄片。 薄片应透明均匀。压片模具及压片机因 生产厂家不同而异。
压片法
固体样品
调糊法: 又称重烃油法,Nujol法。 将固体样品(5-10mg)放入研钵中充分 研细,滴1-2滴重烃油调成糊状,涂在盐 片上用组合窗板组装后测定。 重烃油可用六氯丁二烯替代。
谱带的形状 ④ 谱带的形状
峰形状,包括峰是否有分裂和峰的宽窄。 与结晶程度及相对含量有关。 结晶差说明晶体结构中键长与键角有差 别,引起振动频率有一定变化范围,每 一谱带形状就不稳定。 用半高宽表示 WHFM
width at half full maximum
(2)红外吸收峰的强度
决定于振动时偶极矩变化ε大小。 ε愈大, 吸收强度愈大; ε愈小,吸收强度愈小; 没有偶极矩变化,不产生红外吸收。 吸收强度的表示: vs (ε> 200) s (ε= 75~200) m (ε= 25~75) w (ε= 5~25) vw (ε< 5)
能量在4000~ 400cm-1的红外光可以使 样品产生振动能级与转动能级的跃迁。 分子在振动和转动过程中只有伴随偶极 矩变化的键才有红外活性。
2.2 红外吸收光谱的特点
优点: 特征性高。极少有不同化合物有相同的 红外光谱。 无机、有机、高分子等气、液、固均可 测定。 所需样品少,几毫克到几微克。 操作方便、速度快、重复性好。 已有的标准图谱较多,便于查阅。
分析化学专业英语词汇

分析化学专业英语词汇引言分析化学作为化学领域的重要分支之一,在实验室中发挥关键作用。
掌握与分析化学相关的英语词汇对于专业人士至关重要。
本文将介绍一些常见的分析化学专业英语词汇,帮助读者更好地理解和应用这些术语。
基本概念1.分析化学 (Analytical Chemistry): 一门通过利用实验方法和仪器设备来揭示物质组成和特性的科学。
2.样品 (Sample): 被选中用于分析或实验的物质。
3.分析 (Analysis): 通过实验手段对样品进行检验和测量以获取定性和定量的信息。
4.示性 (Indicator): 溶液中使用的化学物质,能够通过改变颜色、产生沉淀等方式指示物质之间发生的反应。
5.仪器 (Instrument): 用于进行化学分析的设备或仪器,如光谱仪、质谱仪等。
常见分析方法1.光谱分析 (Spectroscopic Analysis): 利用与物质相互作用的电磁辐射进行分析的方法,如紫外可见光谱、红外光谱等。
2.色谱分析 (Chromatographic Analysis): 通过物质在固定相和移动相之间的分配系数差异实现分离和分析的方法,如气相色谱、液相色谱等。
3.质谱分析 (Mass Spectrometry): 通过测量物质离子质量-电荷比来进行分析的方法,可用于物质的结构鉴定和定量分析。
4.电化学分析 (Electrochemical Analysis): 利用电化学技术进行分析的方法,如电位滴定、电化学计量等。
5.化学计量法 (Volumetric Analysis): 通过加滴定液或碰撞试剂进行浓度确定的分析方法,如酸碱滴定、氧化还原滴定等。
分析仪器和设备1.气相色谱仪 (Gas Chromatograph): 一种用于气相色谱分析的设备,能够对复杂混合物进行定性和定量分析。
2.液相色谱仪(Liquid Chromatograph): 一种用于液相色谱分析的设备,广泛应用于药学、环境和化学领域。
光谱分析检验程序(中英)

Content目录1 Purpose目的2 Reference编制依据3 Application scope适用范围:4 The requirement of qualification of personnel for spectrum analysis光谱分析人员资质的要求:5 The inspection and testing procedure and requirements检验程序及要求:6 To sign and issue the inspection and testing report and management of the information and document.检验报告签发及资料管理。
7 The flow chart of spectrum analysis inspection procedure1 Purpose目的To assure the inspection result of spectrum analysis is accurate and real and to meet the requirement of relevant regulations issued by the State during the project installation.为保证工程安装中光谱分析检验结果准确、可靠,满足国家有关规范的要求。
2 Reference编制依据2.1 GB50235-97《Code for Construction and acceptance of industrial metallic piping》GB50235-97《工业金属管道工程施工及验收规范》2.2 DL5007-92《Technical regulation of construction and acceptance of power project》DL5007-92《电力建设施工及验收技术规范》2.3 《Manual of metal material for boiler& pressure vessel》《锅炉压力容器金属材料手册》2.4 《Physics identification of metal》《金属物理鉴别》2.5 《Specification of spectrometer and AC arc generator》《看谱镜及交流电弧发生器使用说明书》3 Application scope适用范围3 .1 Physics and chemical inspection and testing are used by the method of spectrum analysis for the manufacturing and installation in Nanhai project .适用于南海项目制造、安装工程,采用光谱分析方法的理化检验。
第六章 光谱分析

200nm
400nm
800nm
CCZU
光谱分析
一.紫外吸收光谱的产生
价电子种类
σ 键电子、π 键电子、未成对的孤对n电子。
价电子能级跃迁方式
-*、n-*、
σ* π* △ E n π
-*和n-*
σ
2014/6/21 - 8
CCZU
光谱分析
-*跃迁 • 饱和烃的C-C键是键,产生跃迁的能量大。 • 吸收波长小于 150nm的光子,电子光谱都在真空紫 外区。
2014/6/21 - 18
CCZU
光谱分析
(3)B吸收带 芳香化合物及杂芳香化合 物的特征谱带。
溶剂的极性,酸碱性等对
精细结构的影响较大。
图6-4 苯和甲苯的B吸收带 (在环己烷中)(实线为苯,虚线为甲苯)
2014/6/21 - 19
CCZU
光谱分析
苯酚在非极性溶剂 庚烷中的 B 吸收带呈精 细结构,而在极性溶剂
乙醇中观察不到精细结
构,如图6-5所示。
图6-5 苯酚的B吸收带 1-庚烷溶液 2-乙醇溶液
2014/6/21 - 20
CCZU
光谱分析
(4)E吸收带
也是芳香族化合物的特
征谱带之一。
吸收强度大,为2 000~
14 000。
吸收波长偏向紫外的低
波长部分,有的在真空
紫外区。
2014/6/21 - 21
2014/6/21 - 13
CCZU
光谱分析
基本术语
生色团: 产生紫外或可见吸收的不饱和基团,如C=C、C=O、 NO2等。 助色团 其本身是饱和基团(常含杂原子),它连到生色团上时,能 使后者吸收波长变长或(和)吸收强度增加,如-OH、NH2、Cl等。 蓝移(blue shift) 吸收峰向短波长方向移动 红移(red shift) 吸收峰向长波长方向移动
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
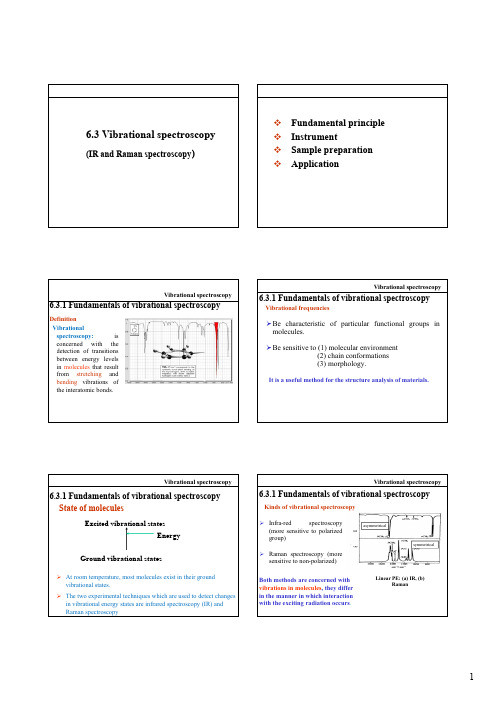
Vibrational spectroscopy
6.3.1 Fundamentals of vibrational spectroscopy
Definition Vibrational spectroscopy:is concerned with the d t ti f t iti detection of transitions between energy levels in molecules that result from stretching and bending vibrations of the interatomic bonds.
asymmetrical
Vibrational spectroscopy
Kinds of vibrational spectroscopy ¾Infra-red spectroscopy
(more sensitive to polarized group)6.3.1 Fundamentals of vibrational spectroscopy
symmetrical
¾Raman spectroscopy (more
sensitive to non-polarized)Both methods are concerned with vibrations in molecules , they differ in the manner in which interaction with the exciting radiation occurs .
Linear PE: (a) IR, (b)
Raman
Fig. 6-14 Dipole moment of HCl
Vibrational spectroscopy
Vibrating of Disulfide carbon
Symmetrical stretching
Infrared inactive 6.3.2 Infrared spectroscopy
Asymmetrical stretching
Bending
Infrared active
Infrared inactive Fig. 6-15 Vibration of Disulfide carbon
m1
low
High/cm-1
High/cm-1
low
Vibrational spectroscopy Methylbenzene(甲苯)
2005.2 S. Gu
v =0 represents the ground state v =l the excited vibrational state
6.3.3 Raman spectroscopy
(1)
(2)
(3)
Vibrational spectroscopy ¾The essential prerequisite for Raman scattering is a change in the polarizability of the bond when vibrations occur.
Polarizability may be thought of as a measure of 6.3.3 Raman spectroscopy
¾Polarizability may be thought of as a measure of the
Fig. 6-16 Motion state of linear molecules Degrees of freedom (H2O) : 3×3−6 = 3
Vibraitonal modes (methylene group)
:2926cm-1(s)as
ν
Asymmetrical
s
ν: 2853 cm Symmetrical
δ:1468 cm-1(m) δr:720 cm-1(CH
1306~1303cm-1(w)γ
t :1250cm
scissoring rocking wagging
Hexane
Four peaks
p
Spectral interpretation always starts at the high end, because there are the best group frequencies and they are the easiest to interpret. No peaks appear above 3000 cm-1, the cut-off for unsaturated C-H. the four peaks below 3000 cm-1 are saturated C-H stretching modes.
Hexane
The peak at 2962 cm-1 is
assigned to the antisymmetric
assigned to the antisymmetric
stretch of the CH3group. This
vibration is always found in
the range 2962±10 cm-1. there
are actually two degenerate
antisymmetric stretching
modes (only one shown).Hexane
At 2926cm-1, the CH2
antisymmetric stretch
absorbs.Normal range:
2926±10 cm-1.
Hexane
At 2872cm-1, the CH3
symmetric stretch
absorbs.Normal range:
2872±10 cm-1.Hexane
At2853-1,the CH
At 2853cm, the CH2
symmetric stretch
absorbs.Normal range:
2853±10 cm-1.
Vibrational spectroscopy Hexane
1470cm-1This is the C-H bending
region, expanded to show the
nearly overlapping peaks for
the CH3and CH2bends.
Vibrational spectroscopy
Hexane
rocking When four or more CH2groups are
in a chain, a vibration at 720±10
cm-1corresponds to concerted
rocking of all of the CH2’s.
Vibrational spectroscopy
Hexanol
3334 cm-1–OH stretch. Normal range: 3350±150 cm-1.
This is a very characteristic group frequency. All of the
peaks due to the OH group are broad due to hydrogen
bonding.
Vibrational spectroscopy Hexanol 1430 cm -1–OH bend . Normal range: 1400±100 cm -1. This broad peak is buried under the CH bending modes.
Vibrational spectroscopy
Hexanol
660 cm -1–OH wag. While not a group frequency, this is another band due to the OH.
Vibrational spectroscopy Aromatic ring expansion (Methylbenzene )
At 1601 cm -1, the
symmetric ring strethch absorbs. Normal range: 1590±10 cm -1. This ib ti h di l
Only not
symmetrically substituted.
vibration has a dipole change (and absords in IR) only when not
symmetrically substituted. The intensity of this band also varies with the
substituent. Compare to p-xylene from the overlay menu.
Vibrational spectroscopy
Aromatic ring expansion (Methylbenzene )
At 1500cm -1, a different ring stretch absorbs. Range: 1500±10cm -1. Variable intensity
Vibrational spectroscopy 6.3.6 Comparing of IR and Raman Spectroscopy
asymmetrical
symmetrical
Fig. 6-17 Linear PE: (a) IR, (b) Raman。
