电化学基础 单元检测
陕西省西安中学2017-2018年高二第二学期《电化学单元检测》试题 无答案-精选文档

电化学基础单元检测题一、选择题(每小题2分,共50分,每小题都只有一个选项符合题意)1.关于右图所示的原电池,下列说法正确的是A .电子从铜电极通过检流计流向锌电极B .盐桥中的阳离子向硫酸铜溶液中迁移C .锌电极发生还原反应,铜电极发生氧化反应D .铜电极上发生的电极反应是↑=+-+222H e H2.观察下列几个装置示意图,有关叙述正确的是A .装置①中阳极上析出红色固体(两电极均为石墨电极)B .装置②的待镀铁制品应与电源正极相连C .装置③闭合电键后,外电路电子由a 极流向b 极D .装置④的离子交换膜允许阳离子、阴离子、水分子自由通过(提示:图中离子交换膜为阳离子交换膜)3.如图所示,a 、b 、c 均为石墨电极,d 为碳钢电极,通电进行电解。
假设在电解过程中产生的气体全部逸出,下列说法正确的是A .甲、乙两烧杯中溶液的pH 均保持不变B .甲烧杯中a 的电极反应式为:4OH -―4e -=O 2↑+2H 2OC .当电解一段时间后,将甲、乙两溶液混合,一定会产生蓝色沉淀D .当b 极增重3.2g 时,d 极产生的气体为2.24L (标况)4.下列反应中,不能设计成原电池的是A .2KMnO 4+16HCl=2KCl+2MnCl 2+ 5Cl 2+8H 2OB .Ba (OH )2·8H 2O+2NH 4Cl=BaCl 2+2NH 3↑+10H 2OC . 2CO+O 2=2CO 2D .2FeCl 2+Cl 2=2FeCl 35.将AsO -34+2I -+2H+ AsO -33+I 2+H 2O 设计成右图所示的电化学装置,其中C 1、C 2均为碳棒。
甲、乙两组同学分别进行下述操作:甲组:向B烧杯中逐滴加入浓盐酸乙组:向B烧杯中逐滴滴加入40%NaOH溶液下列描述中,正确的是A.甲组操作过程中,C2做负极B.乙组操作过程中,C1上发生的电极反应为:2I--2e-=I2C.两次操作过程中,微安表(G)指针的偏转方向相反D.甲组操作时该装置为原电池,乙组操作时该装置为电解池6.某同学按右图所示的装置进行电解实验。
电化学基础阶段质量检测答案

(满分100分时间90分钟)一、选择题(本题包括16小题,每小题3分,共48分)1.解析:干电池将化学能转化为电能;电解是将电能转化为化学能;水力发电是将势能转化为电能;太阳能热水器是将太阳能转化为热能。
答案:D2.解析:铜锌原电池工作时,电子由负极(锌)沿外电路流向正极(铜),B错误。
答案:B3.解析:此原电池放电时,反应消耗硫酸,使溶液的酸性降低。
答案:B4.解析:A、B中形成原电池都是铁作负极,加速铁的腐蚀;D中铸铁管作阳极,加速腐蚀;C中锌比铁活泼,铁作正极,受到保护。
答案:C5.解析:铅蓄电池放电时铅电极失去电子发生氧化反应,A错误;电解饱和食盐水,阳极上得到氯气,阴极上得到氢气,B错误;电镀时电镀液采用镀层金属的可溶性盐溶液,C正确;食盐水为中性溶液,所以应发生吸氧腐蚀,D错误。
答案:C6.解析:由图知,烧杯b中的Zn棒失去电子,发生氧化反应,电子转移到Fe棒上,烧杯a中通入的氧气在Fe棒表面得电子生成氢氧根离子,使a中溶液的pH升高。
所以正确的为A、B。
答案:AB7.解析:在氢氧燃料电池的负极上反应的是氢气;粗铜精炼时,纯铜与电源的负极相连,钢铁腐蚀的负极反应是Fe-2e-===Fe2+。
答案:A8.解析:水电解时阴极为2H++2e-===H2↑,C项错误;燃料电池放电时负极为H2+2OH--2e-===2H2O,故D项错误;A项中H2O可以循环利用,错误;B项正确。
答案:B9.解析:根据原电池工作原理,电子由负极(Cu)沿导线传递给正极(Ag),电解质溶液中阴离子沿盐桥从乙流向甲;正极(Ag):2Ag++2e-===2Ag,负极(Cu):Cu-2e-===Cu2+,总反应:Cu+2Ag+===Cu2++2Ag,与将Cu片浸入AgNO3溶液中发生的化学反应相同。
故选D。
答案:D10.解析:电解时阳极不能选用活泼的金属材料,故A错;电解饱和食盐水时阴极产物为还原产物H2,故B错;电解时阳极产物为氧化产物氯气,故C正确;电解饱和食盐水除生成氢气和氯气外,在阴极还有NaOH产生,所以电解实验结束后,搅拌溶液,溶液中有NaOH,显碱性,故D错。
化学单元测考试试题-电化学基础-A卷-附答案.doc

单元训练金卷■高三■化学卷(A )第十二单元电化学基础注意事项:1. 答题前,先将自己的姓名、准考证号填写在试题卷和答题卡上,并将准考证号条 形码粘贴在答题卡上的指定位罝。
2. 选择题的作答:每小题选出答案后,用2B 铅笔把答题卡上对应题0的答案标号涂 黑,写在试题卷、草稿纸和答题卡上的非答题区域均无效。
3. 非选择题的作答:用签字笔直接答在答题卡上对应的答题区域内。
写在试题卷、 草稿纸和答题卡上的非答题区域均无效。
4. 考试结束后,请将本试题卷和答题卡一并上交。
可能用到的相对原子质量:HJ C:12 N:14 0:16 Al:27 Cu:64 Zn:65一、选择题(每小题3分,共48分)1. 课堂学习中,同学们利用铝条、锌片、铜片、导线、电流计、橙汁、烧杯等用品探究原租 池的组成。
下列结论错误的是A. 原电池是将化学能转化成电能的装置B. 原电池巾电极、电解质溶液和导线等组成C. 图屮a 极为铝条、b 极为锌片时,导线屮会产生电流D. 图中a 极为锌片、b 极为铜片时,电子由铜片通过导线流向锌片2. 如图,在盛有稀H 2SO 4的烧杯中放入用导线连接的电极X 、Y,外电路中电子流向如图所 示,关于该装置的下列说法正确的是稀 H 2SO 4 fOlT — ---- ------------- --- —) 电极a%,电极b■ ■ ■ —*y 橙汁 -A.外电路的电流方向为X->外电路一>YB.若两电极分别为铁和碳棒,则X为碳棒,Y为铁C.X极上发生的是还原反应,Y极上发生的是氧化反应D.若两电极都是金属,则它们的活动性强弱为X〉Y3.下列有关电化学装賈完全正确的是4.烧杯A中盛放0.1 mol/L的H2SO4溶液,烧杯B中盛放0.1 mol/L的CuCl2溶液(两种溶液均足量),组成的装置如图所示。
下列说法不正确的是■ MB .•• •» •• •» •稀硫酸氯化铜溶液A BA.A为原电池,B为电解池B.A为电解池,B力原电池C.当A烧杯屮产生0.1 mol气体时,B烧杯中产生气体的物质的呈也为0.1 molD.经过一段时间,B烧杯中溶液溶质的浓度减小5.锌空气燃料电池可用作电动车动力电源,电池的电解质溶液为KOH溶液,反应为2Zn +O2+4OH_+2H2O===2Zn(OH)f。
强烈推荐电化学测试题(含答案)

电化学单元测试题可能用到的相对原子质量 H 1 C 12 N 14 O 16 Cu 64 Zn 65 第Ⅰ卷(选择题 共48分)一、选择题(本题包括16小题,每题3分,共48分。
每小题只有一个选项符合题意。
) 1、实验室中欲快速制取氢气,最好的方法应该是A .纯锌与稀硫酸 B .纯锌与浓硫酸 C .粗锌与稀硫酸D .粗锌与稀硝酸 2、金属防护的方法不正确的是A .对健身器材涂油漆以防止生锈B .将炊具制成铁铜合金而不用纯铁制品C .用牺牲锌块的方法来保护船身D .自行车的钢圈上镀上一层铬防锈 3、关于如右图所示装置的叙述,正确的是A .铜是阳极,铜片上有气泡产生B .铜片质量逐渐减少C .电流从锌片经导线流向铜片D .氢离子在铜片表面被还原4、下列关于原电池的叙述中,正确的是A .原电池中,正极就是阳极,负极就是阴极B .电子从负极经电解质溶液流向正极C .原电池工作时,溶液中的阳离子向负极移动D .形成原电池时,在负极上发生氧化反应5、下列关于原电池、电解池、电镀的说法中正确的是A .原电池中负极金属材料一定失电子被氧化B .用惰性电极电解Na 2SO 4溶液本质是电解水C .在铁片表面镀锌,将锌作阳极,铁做阴极D .在电镀池中电解质溶液浓度和溶液pH 都不变6、化学用语是学习化学的重要工具,下列用来表示物质变化的化学用语中,正确..的是 A .电解饱和食盐水时,阳极的电极反应式为:2Cl - -2e -=Cl 2 ↑B .氢氧燃料电池的负极反应式:O 2 + 2H 2O + 4e - =4OH -C .粗铜精炼时,与电源正极相连的是纯铜,电极反应式为:Cu-2e - = Cu 2+D .钢铁发生电化学腐蚀的正极反应式:Fe-2e - = Fe 2+7、对于金属冶炼的工业方法。
下列有关说法中正确的是A .可用电解饱和的MgCl 2溶液的方法获得金属镁B .工业上常用电解CuSO 4溶液的方法冶炼金属铜C .电解熔融Al 2O 3方法冶炼金属铝时,同时要加入冰晶石作熔剂D .工业上常采用活泼金属还原法冶炼金属银8、若某电能与化学能的转化装置(电解池或原电池)中发生反应的总反应离子方程式是:Cu+2H +=Cu 2++H 2↑,则关于该装置的有关说法正确的是A .该装置可能是原电池,也可能是电解池B .该装置只能是原电池,且电解质溶液为硝酸C .该装置只能是电解池,电解质溶液不可能是盐酸D .该装置只能是电解池,且金属铜为该电解池的阳极9、用铂电极电解CuSO 4溶液,当阴极端发现有气体产生时,继续再通电一会,则加入下面C K 1 ° ° ° ° K 2NaCl )Li 接,浸入硫酸铜溶液中,发现A 棒从加是(向(序为(填写字母)。
人教版高中化学选修4第4章 电化学基础 测试题
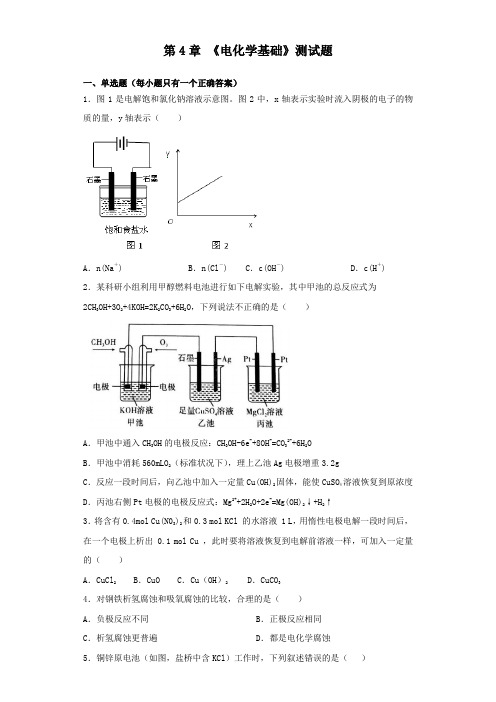
第4章《电化学基础》测试题一、单选题(每小题只有一个正确答案)1.图1是电解饱和氯化钠溶液示意图。
图2中,x轴表示实验时流入阴极的电子的物质的量,y轴表示()A.n(Na+) B.n(Cl-) C.c(OH-) D.c(H+) 2.某科研小组利用甲醇燃料电池进行如下电解实验,其中甲池的总反应式为2CH3OH+3O2+4KOH=2K2CO3+6H2O,下列说法不正确的是()A.甲池中通入CH3OH的电极反应:CH3OH-6e-+8OH-=CO32-+6H2OB.甲池中消耗560mLO2(标准状况下),理上乙池Ag电极增重3.2gC.反应一段时间后,向乙池中加入一定量Cu(OH)2固体,能使CuSO4溶液恢复到原浓度D.丙池右侧Pt电极的电极反应式:Mg2++2H2O+2e-=Mg(OH)2↓+H2↑3.将含有0.4mol Cu(N03)2和0.3 mol KCl 的水溶液 1 L,用惰性电极电解一段时间后,在一个电极上析出 0.1 mol Cu ,此时要将溶液恢复到电解前溶液一样,可加入一定量的()A.CuCl2 B.CuO C.Cu(OH)2 D.CuCO34.对钢铁析氢腐蚀和吸氧腐蚀的比较,合理的是()A.负极反应不同B.正极反应相同C.析氢腐蚀更普遍D.都是电化学腐蚀5.铜锌原电池(如图,盐桥中含KCl)工作时,下列叙述错误的是()A.正极反应为:Cu2++2e–=Cu B.电池反应为:Zn+Cu2+=Zn2+ +CuC.在外电路中,电子从负极流向正极 D.盐桥中的K+移向ZnSO4溶液6.下列有关电化学的说法正确的是()A.锌锰干电池工作一段时间后碳棒变细B.在海轮外壳上镶入锌块可减缓船体的腐蚀,是采用了牺牲阳极的阴极保护法C.铜锌原电池工作时,电子沿外电路从铜电极流向锌电极D.电解MgCl2饱和溶液,可制得金属镁7.某同学用如图所示的电化学装置电解硫酸铜溶液,有一个电极为Al,其它三个电极均为Cu,则下列说法正确的是()A.电子方向:电极Ⅳ→→电极ⅠB.电极Ⅰ发生还原反应C.电极Ⅱ逐渐溶解D.电极Ⅲ的电极反应:Cu-2e-═Cu2+ 8.下列事实不能用电化学原理解释的是( )A.铝片不用特殊方法保护B.轮船水线下的船体上装一定数量的锌块C.纯锌与稀硫酸反应时,滴入少量CuSO4溶液后速率增大D.镀锌铁比较耐用9.在盛有稀H2SO4的烧杯中放入用导线连接锌片和铜片,下列叙述正确的是()A.正极附近的SO42―离子浓度逐渐增大 B.电子通过导线由铜片流向锌片C.正极有O2逸出 D.铜片上有H2逸出10.某原电池装置如图所示,电池总反应为2Ag+Cl2=2AgCl。
2021届高三化学——电化学基础单元检测(有答案和详细解析)

2021届高三化学——电化学基础单元检测(有答案和详细解析)考生注意:
1.本试卷共4页。
2.答卷前,考生务必用蓝、黑色字迹的钢笔或圆珠笔将自己的姓名、班级、学号填写在相应位置上。
3.本次考试时间90分钟,满分100分。
4.请在密封线内作答,保持试卷清洁完整。
一、选择题(本题共12小题,每小题2分,共24分。
每小题只有一个选项符合题意。
)
1.某同学做了如下实验:
装置
现象电流表指针未发生偏转电流表指针发生偏转
下列说法中正确的是()
A.加热铁片Ⅰ所在烧杯,电流表指针会发生偏转
B.用KSCN溶液检验铁片Ⅲ、Ⅳ附近溶液,可判断电池的正、负极
C.铁片Ⅰ、Ⅲ的腐蚀速率相等
D.“电流表指针未发生偏转”,说明铁片Ⅰ、铁片Ⅱ均未被腐蚀
乙醇燃料电池中采用磺酸类质子溶剂,在200 ℃左右时供电,电池总反应式为C2H5OH+3O2===2CO2+3H2O,电池示意图如图所示,下列说法中正确的是()
A.电池工作时,质子向电池的负极迁移
B.电池工作时,电流由b极沿导线流向a极
C.a极上发生的电极反应是C2H5OH+3H2O+12e-===2CO2+12H+
D.b极上发生的电极反应是2H2O+O2+4e-===4OH-
3. 化学家正在研究尿素动力燃料电池,尿液也能发电。
用这种电池直接去除城市废水中的尿素,既能产生净化的水,又能发电,尿素燃料电池结构如图所示,下列有关描述正确的是()。
人教选修四第四章《电化学基础》单元检测题(含答案)

《电化学基础》单元检测题一、单选题1.下列关于如图所示原电池装置的叙述中,正确的是A.铜片是负极 B.铜片质量逐渐减少C.电流从锌片经导线流向铜片 D.氢离子在铜片表面被还原2.下列说法正确的是 ( )A.在原电池中,电子由正极流向负极B.在电解池中,物质在阴极发生氧化反应C.在原电池中,物质在负极发生氧化反应D.在电解池中,与电源正极相连的电极是阴极3.有两只串联的电解池(Pt为电极),甲池盛有足量的CuSO4溶液,乙池盛有足量的某硝酸盐的稀溶液。
电解时当甲池电极析出6.4gCu时,乙池电极析出21.6g金属,则乙池的溶质可能是A.NaNO3B.Cu(NO3)2C.Al(NO3)3D.AgNO34.如图所示电化学装置,X可能为“锌棒”或“碳棒”,下列叙述错误的是A.X为锌棒,仅闭合K1,Fe电极上发生还原反应B.X为锌棒,仅闭合K1,产生微量电流方向:Fe→XC.X为碳棒,仅闭合K2,该电化学保护法称为“牺牲阳极阴极保护法”D.若X为碳棒,仅闭合K1,铁电极的极反应为:Fe -2e-→ Fe2+5.燃料电池的优点是化学能直接转化为电能,而不经过热能这一中间环节,能量利用率高。
氢氧燃料电池可同时供应电和水蒸气,所需燃料为H2,电解质为熔融K2CO3。
已知该电池的正极反应为O2+2CO2+4e-2CO32-。
下列叙述正确的是( )A.放电时CO32-向正极移动B.随着反应的进行,CO32-在不断消耗C.负极反应为H2+CO32--2e-H2O+CO2D.当该电池产生的水蒸气折算成标准状况下的体积为22.4 L时,转移电子4 mol6.如图所示的装置,两烧杯中均为相应的水溶液,通电一段时间后,测得甲池中某电极质量增加2.16g,乙池中某电极上析出0.64g某金属,下列说法正确的是A.甲池是b极上析出金属银,乙池是c极上析出某金属B.甲池是a极上析出金属银,乙池是d极上析出某金属C.某盐溶液可能是CuSO4溶液D.某盐溶液可能是Mg(NO3)2溶液7.下列说法正确的是( )A.实验时酸或碱溅到眼中,应立即用水冲洗,并不断眨眼,不能用手搓揉眼睛B.检验硫酸亚铁铵溶液中Fe2+的方法是:先滴加新制氨水后滴加KSCN溶液C.证明钢铁吸收氧腐蚀的方法是:在镀锌铁皮上滴1~3滴含酚酞的饱和食盐水,静置1~2min,观察现象D.因为氧化铁是一种碱性氧化物,所以常用作红色油漆和涂料8.下列冶炼金属的原理不正确的是()A.电解饱和食盐水制备金属钠B.加热分解Ag2O制备金属银C.Fe2O3与CO高温下反应制备金属铁D.Cu2S与O2高温下反应制备金属铜9.下列各组的电极材料和电解液,不能组成原电池的是A.铜片、铜片、稀硫酸 B.铜片、石墨棒、硝酸银溶液C.锌片、铜片、稀硫酸 D.铜片、银片、FeCl3溶液10.下图中的电化学装置以甲醇(CH3OH)为主要原料合成碳酸二甲酯[(CH3O)2CO],相关说法错误的是A.B是直流电源的负极B.碳酸二甲酯中碳均为+4价C.阳极附近溶液pH降低D.每当有2molH+通过离子交换膜,消耗氧气的体积在标准状况下为11.2L 二、填空题11.某原电池的装置如图所示,看到b极上有红色金属析出,回答下列问题:①若a、b是两种活动性不同的金属,则活动性a____b(填>、<或=);②电路中的电子从____经导线流向_____(填a或b);③溶液中的SO42-向________极移动(填a或b);④若两电极分别是Al和C,则负极的电极反应式为_________________。
《电化学基础》测试卷.doc

《电化学基础》测试卷The electrochemical base test volume(1) basic practiceA,multiple choiceThe following processes need to be electrified to do so: ionization;(2) the electrolysis; (3) plating; (4) electrophoresis; The electrochemical corrosion;A,(1) (2) (3); B, (2) (3) (4); C, (2) (4) (5); D, all;At room temperature, the PH of 0. 1 molar of acid is:A, 1; B, > 1; C, < 1; D, can,t be sure;3, 1 liter contains NaOH under normal temperature of saturated salt water, the PH = 10, with platinum electrodes during electrolysis, when the cathode has a 11. 2 liter gases (standard conditions), the PH of the solution is close to (set after electrolytic solution volume is still 1 liter):A., 0; B, 12; C, 13; D, 14;The basic structure of the newly developed bromine-zinc battery in foreign countries is to use carbon rods as the poles, and the electrolyte is the zinc bromide solution. There are four electrode reactions:N - 2e = Zn = Zn + 2e 二ZnThe anode reaction and discharge when charged are:A, (4) (1); B, (2) (3); C, (3) (1); D, (2) (4);The colbay reaction is 2RC00K + 2H2OR -r + H2 + 2K0H, and the following statement is true (C)The products ofB. the products of hydrogen are produced in the cathode.C. the products of carbon are produced in the anode.D. The products of carbon are produced in the cathode.hydrogen are produced in the anode.Use 0.01 mo/litre H2S04 titrate 0. 01 mo/liter NaOH solution, neutralize and add water to 100m 1. If there is an error in the end, the ratio of 1 drop of H2S04 to 1 drop of H2S04 (1 drop to 0. 05 ml), and the ratio of C (H +) is:A: ten b. fifty c. fiveIn a beaker of saturated sodium carbonate, insert inert electrodes, keep the temperature constant, and after a certain amount of time:The PH of the solution will increaseB.The ratio of sodium ions to carbonate ions will be smallerC.The concentration of the solution gradually increases, and a certain amount of crystal is precipitated outThe concentration of the solution stays the same8, room temperature, the pH = 1 hydrochloride average into 2 portions, 1 add right amount water, the other one to join with the mo1ar concentration of hydrochloric acid after the same amount of NaOH solution, pH is increased by 1, belong to the water and the volume of NaOH solution is as follows:A.10 c. 11 d. 129, there are five bottles of solution are respectively (1) 10 ml of 0. 60 mo/NaOH aqueous solution (2) 20 ml mo / 0. 50 litres of sulfuric acid aqueous solution (3) 30 ml of 0. 40 the 40 ml/1 HC1 solution (4) the 0. 30 / d) aqueous solution (5) 50 ml mo / 0. 20 litres of sucrose solution. The order of the total number of ions and molecules in each of these bottles is:A, B, >, >, >, >, >C, >, >, >, >, >, >To connect Al and Cu slices to a wire, a group of HN03 is inserted into a dilute NaOH solution, which forms the original cel 1. In the two original batteries, the two are:A, Al, Al pills; B, Cu, Al pills; C, Al, Cu pills; D, Cu and Cu pills;11, the mass fraction of 0. 052 (5. 2%) of the NaOlI solution 1 liter (density of 1.06 g/ml) with a platinum electrode electrolysis, when the mass fraction of the NaOH solution changed 0. 010 stops electrolysis(1. 0%), should comply with the relationships of the solution is: The mass fraction of NaOHThe mass of the anode (grams)・ The mass of the cathode deposition.19152B0. 062 (6. 2%)152 a.0. 062 (6. 2%)0. 042 (4. 2%)1.29.4D0. 042 (4. 2%)9.41.212, a team of extracurricular activities, will cut a piece of galvanized iron in the conical flask, and drops into a small amount of salt water to soak, addend phenolphthalein try drops again, according to the figure device experiment, observation, a few minutes later the following phenomenon is impossible:A,B, the air bubble in the ducts;B,B, a water column formed in the trachea;C,metal shears red; A BZinc is corroded;13, a new type of fuel cell, it is porous nickel plate as the electrode in KOH solution, then access to polar ethane and oxygen respectively, the overall reaction is: 2 c2h6 o2 + 7 + 8 KOH k2co3 + 10 二4 h2o; The correct inference about the batteryis:A, the negative reaction is: 14H20 + 702 + 28e -.B: after a period of discharge, the PH around the negative pole rises. C, 1 molc2h6, and 14 moles of electrons transferred on the circuit.D,the concentration of KOH in the process of discharge is essentially the same;14,contains two kinds of solute in a solution of NaCl and - H2S04, the amount of substance ratio of 3:1, the mixed solution with graphite electrode electrolysis, according to the electrode product, can be clearly divided into three stages・ The following statements are incorrect:A, the cathode starts from the beginning and only comes out of H2; B, the anode comes out of the C12, and then it comes out of 02.The final stage of C and electrolysis is electrolytic water; D, the PH of the solution goes up, up to 7.The anode is made of iron, and copper is an electrolyte of the sufficient amount of NaOH solution, and after a period of time, two moles of Fe (OH) 3 solid are obtained, and the water is consumed in this room:A, , 3 mol. B, 4 mol. C, 5 mol; D, 6 mol;16, 100 ml, 2 mo/liter of NaOH solution, 100 ml, 2 mo/1 - H2S04 solution and mix a certain amount of ammonia, the solution obtained make phenolphthalein solution show pale red, the relationship between ion concentration in the solution is correct:A, C (S042 —)二C (Na +) > C (NH4 +) >・B, C (Na +) > C (S042 -)> C (NH4 +)・C, C (NH4 +), >, C (S042 —)二 C (Na +), >, C (OH C(H +)・C (H +) + C (NH4 +) + C (Na +)二C (OH -)+ 2C (S042 -);The PH value of the acid and acetic acid are diluted to the original n times m, and the PH of the two solutions is still the same, and the relationship between n and m isA, m = n B, m > n C, m < n D, m? nThe volume of hydrochloric acid with the same volume, the same pH, NaOH, and NH3 H20 is VI, V2, and V3, and the three relationships are:A, VI, >, V2 bl, V3, B, V3, V2, > VI, C, V3, >, V2, V2, VI, V2, VI, V2, VI, V2, VI, V2, all the way to V2Second, fills up the topicHydrogen peroxide (H202) and water are extremely weak electrolytes, but H202 is more acidic than H20.(1)if you view H202 as a di-acid, write the ionization equation in the water:(2)because of the weak acidity of H202, it can form positive salt with strong base, and also form acid salt under certain conditions・ Write the chemical equation of the salt in the form of H202 and Ba (OH) 2:(3)hydroelectricity is dissociated from the generation of H30 + and OH 一called water・Like water, hydrogen peroxide has a very weak since I ionization, it accidentally ionization equation is as follows:;The industrial wastewater containing cr2o72-acid industrial waste water is used in the following way: the appropriate amount of NaCl is added to the industrial waste water, and the mixing is uniform・Using Fe to electrolyte the electrode, over a period of time there are Cr (OH) 3 and Fe (OH) 3 deposition; The waste water reaches the discharge standard. Try to answer:(1)electrode reaction in electrolysis: anode cathode(2)write an ion reaction equation that changes Cr2072 to Cr3 + •■(3)how does the precipitation of the Cr (OH) 3, Fe (OH) 3 deposit occur in the electrolysis process?It is known that the ionizing degree of ammonia is equivalent to the degree of ionization of acetic acid, which is equal to the same temperature, and the solution of ammonium chloride is acidic・ Now, in a small amount of Mg (OH) 2 suspension, add the right amount of saturated ammonium chloride solution, and the solid is completely dissolved. A classmate,s explanation is:It's Mg (OH) 2.(2) NH4 + + H20NH3? H20 + H +; H + OH 二H20;Because NH4 + hydrolysis is acidic, H + and OH - react to form water, causing the reaction to balance right and dissolve・The interpretation of classmate b is:It's Mg (OH) 2. NH4 plus OH minus NH3? H20.Because NH4 cl is ionized by an NH4 plus the OH, which is ionized by Mg (OH) 2, which produces a weak electrolyte NH3? H20, which causes the equilibrium between the reaction, right, and Mg (OH) 2 is dissolved ・(1) c student can,t sure which students reasonable explanation, then choose one of the following reagents, to prove that a, b two classmates explain only a right, he chooses reagent is: (fill in the number)A,NH4N03; B, CH3C00NH4; C, Na2C03; D, NH3? H20.(2)please explain why the classmate made the choice・(3) in the suspension of Mg (OH) 2, the selected reagent is put into the suspension of Mg (OH) 2. As a result of this, the interpretation of the classmate is more reasonable (the or 〃b〃);Completing the NH4C1 saturation solution makes the ion equation for Mg (OH) 2 dissolved:.22. From H +, Na +, Cu2 +, Ba2 +, Cl 一,S042 一ion, choose appropriate ion of an electrolyte, electrolyte solution according to the following requirements to electrolysis (are inert electrode):(1)the electrolyte is reduced in electrolyte and the amount of water is constant・(2)the quality of the electrolyte is kept constant while electrolyte, the amount of water is reduced, and the electrolyte used is:(3)the quality of electrolytes and water changes when electrolysis, and the electrolyte used is;At room temperature, 0. 01 molCH3C00Na and 0. 004 mollICl dissolve in water and make 0. 5 L mixed solution. Judge:There is a particle in the solution;The amount of matter in the solution that has two particles must be equal to 0.01 moles, and they're sum;In this case, it's n (CH3COO 一) + n (OH -) -n (H +)二mol.Three, calculation problem24.Under a certain temperature to a certain volume density of 1. 15 g/ml of sodium chloride solution with inert electrodes electrolytic, set exactly the electrolysis of sodium chloride and no other reaction occurs, the oxygen mass fraction of 80% in the solution, try to calculate:(1)the ratio of the amount of solute and solvent in an electrolyte solution.(2)the concentration of the substance of the original sodium chloride solution・25.To 8 grams of divalent metal oxide solid dilute sulphuric acid is added to make it completely dissolved, known to the consumption of sulfuric acid volume is 100 ml, insert the platinum electrode in the solution of electrolysis, electricity after a certain period of time, collected on an electrode 224 ml (standard conditions) of oxygen, in another 1. 28 grams of the metal electrode・(1)the name of the metal oxide is determined by calculation.(2)the concentration of the substance in the solution of sulfuric acid (the volume of solution) is calculated by 100 ml.(2) ability testingA,multiple choiceAs people's quality of life improves, the problem that the battery must be focused on is raised to the agenda, which is the primary reasone the metal material of the battery caseB.prevent the contamination of soil and water by heavy metal ions such as mercury, cadmium and leadC.not to corrode other substances from the electrolyte that leaks inthe batteryRecycle the graphite electrodesThe following statement is trueZinc reacts with dilute sulphuric acid to make hydrogen,Adding a small amount of copper sulfate can accelerate the reaction. When the coating is broken, the white iron (galvanized iron) is more corrosive than the tin plate・ When plating, it is necessary to place the plated in the cathode of the electrolyte・When smelting aluminum, the aluminum oxide is added to the liquid crystal and it becomes the molten body and then the surface of the steel surface is easily corroded to produce Fe203 nh20A. in b. by c. with d. inThe right image is an electrolytic CuC12 solution, where c and d are graphite electrodes・ The following are the right onesA. a is negative, b is positive B・a is anode and b is cathodeIn the process of electrolysis, d electrode mass increases d. In the process of electrolysis, the concentration of chloride ions remains constantElectrolysis with inert electrodes・The following statement is correctA. electrolytic dilute sulphuric acid solution, which is essentiallyelectrolytic water, so the solution p H remains unchangedThe solution of sodium hydroxide is to consume OH -,so the pH of the solution decreasesC. electrolysis of sodium sulphate, which is a ratio of 1 to 2 in the cathode and anodeD.Electrolytic chlorinated copper solution, the ratio of the amount of matter in the cathode and anode is 1:1The correct description of the device in the right picture is correct A: this is an original batteryThis is an electrolytic NaOH solutionC. P is positive, and the electrode reaction is 2H + + 2e -二H2 arrowThe d. f. e is negative, and its electrodes react to it: 40H 一二2H20 + 02What happens when you get the reaction Cu + 2H20 二Cu (OH) 2 and the H2 arrowA.the copper is the anode of the original battery, the carbon is the anode of the original battery, and the sodium chloride is the electrolyte solutionB.copper zinc alloy is chemically corroded in damp aire copper to make Yin, electropositive, and electrolyte sodium sulphate solutione copper to make Yin, electropositive, electrolysis of copper sulfate solution 7. Hydrogen fuel cell is a kind of high performance battery, total reaction of 112 + 02 二2 1120, electrolyte solution of KOH solution, in the following concerning the describe of the battery is not correctA.H 2 is negative, 02 is positiveB. The PH of electrolyte solution is increasing at workThe negative reaction: 2h2-4e - + 40H - 二4H20When iron rods are connected with graphite rods, they are immersed in 0. 01 mol/L of salt solution, which may occurAn oh~b in the vicinity of an iron bar is corrodedRelease the C12 D. On the graphite rodUse the inert electrode electrolyte to electrolyte the Na2C03 solution, and if the temperature remains constant, it will be over a period of timeThe ratio of the pH of the solution to the c (Na +) and c (C032 -)is greaterC. The concentration of the solution is large, and the crystal is precipitated out・ The concentration of the solution is constant10. With a platinum electrode electrochemical a certain concentration of aqueous solution of the following materia1. After the electrolysis, add right amount of water to the rest of the electrolyte, can make the solution and electrolyticbefore is the sameA. gnoc3b・ cA, B, and C are three kinds of metals, in which case A and B are immersed in A dilute H2S04. (2) the amount of electrolytic material concentration in the same A and C of the mixed solution of salt, on the cathode precipitation C first, (using inert electrode), determineB, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, C, B, B, B, B, B, B, B, C, B, B, B, B, C, B, B, C, their reducing strength for sequenceB, B, B 12. With graphite electrode, electrolytic silver nitrate solution in the anode collected 0. 4 g of oxygen, and electrolytic acid generated 250 ml of a sodium hydroxide solution, is the concentration of a solution of sodium hydroxideA. 0. 20 moles of IT. 0. 15 moles of 1-1 c. 10 moles of 1-1. 0. 05 moles of 1~113.The solid-oxide fuel cell was developed by Westinghouse・ It is an electrolyte from zirconium oxide, a solid electrolyte that allows oxygen ions (02 -) to pass through during high temperatures・ The battery,s working principle is shown in the diagram, where the porous electrode a and b are not involved in the electrode reaction・ The following is trueA.there's A very strong battery in the reaction of 02B.there is an H2 participating in the positive electrode of the BThe corresponding electrode for c a is 02 + 2H20The total reaction equation of the battery is 2H2 + 02 二2H2014. With a platinum electrode electrolysis of NaCl and the mixture of CuS04 solution, when circuit through the 4 mol, electronic power, Yin and Yang both produce 1. 4 mol, gas, after electrolytic solution volume is 4 1, the final pH value of electrolyte nearestA. four b. two c. 13 d. 14There is a button microcell with an electrode of Ag20 and Zn. Electrolytic cell is KOH solution, so commonly known as silver-zinc battery, the battery electrode reaction to zinc + OH - 2 + 2 二e zinc (OH) 2, Ag20 + H20 + 2 二2 e ag + OH - the following claims, the right is for (1) zinc anode, Ag20 (2) for the positive discharge, pH near the anode rise (3) discharging, near the cathode solution pH value lower (4) anion in the solution to the positive direction, cation to negative directionA. in b. by c. in d. inWhen using graphite as an electrode for electrolysis of 3 mol/LNaCl dnd 0.4 molar 2 (S04) 3, the following curve is correct ()Second, fills up the topic17. Molten salt fuel cells with high power generation efficiency, therefore, available Li2CO3, Na2C03 and molten salt mixture of electrolyte, CO as the anode gas, a mixture of air and C02 gas for cathode to gas and work under 650 °C of the f uel cell system, complete the relevant battery reactive: reactive: battery anode reaction: 2 CO + 2 co32 — > 4 C02 + 4 e -・The cathode reaction:, total reaction: battery.Add the Na2S04 solution to the u-tube, add a few drops of litmus test liquid, insert the inert electrode, and turn on the power suppl y.(1)the observable phenomenon is observed after a period of time.(2)to cut off the power supply, remove the electrodes will be in the u-tube solution in a small beaker, stirring, observable phenomenon is that the reason for this is that・If (3) to switch to the device electrolysis with a few drops of phenolphthalein try drops Na2C03 saturated solution of anode was observed after a period of time, the cathode・ 19. A research study to explore the influencing factors of steel rust, design the following solutions: A, B, C, D four little flask respectively into dry chicken wire, soaked in salt water of fine wire, fine wire soaked in water, salt water and fine wire, and make the wire completely immersed in salt water, and then to assemble as shown in the four sets of devices, every once in A while measuring the height of the catheter in the water rise, result in the following table data (listed in the table for the catheter in the height of water rise/cm)・The rise of water at different timesTime/hour0. 51.01.52.02.53.0A bottle (dry wire)B (iron wire with salt water)0.41.23.45.67.69.8C (the wire with water in it)(the wire that is fully immersed in the salt water)If you are the group leader, please answer the following questions: (1)why the water in the catheter rises・(2)in these experiments,The speed of wire rust is in the order of large to smal1.(3)the factors that affect the rust of steel are mainly.There are two different points of view about the "pH change in the electrolytic CuC12 solution":The idea is that the "theorist" thinks that the pH of the solution after electrolytic CuC12 is elevated・Point 2 it is, "experimental" after repeated several times, precise experimental determination, prove that the electrolytic CuC12 solution pH change as shown in the curve relationship with・ Please answer the following questions:(1)of CuC12 before electrolytic solution pH value at A point location is (with ion equation)・(2)the theoretical basis of the theory is that it isIt is.(3)^experimental^ experimental conclusion is that they are "precise experiment" is described through the accurate determination of the pH of the solution.(4)what do you think? Your point of view of the reason is that (briefly) through chemistry.A battery is a device that can charge and discharge repeatedly, with a battery that occurs when charging and dischargingThe reaction is: Ni02 + Fe + 2H20 Fe (OH) 2 + Ni (OH) 2Use this battery for electrolytic M (N03) x solution:(1)the anode (inert) of the electrolytic pool shall be connected (the serial number)・A.NIf the battery works for a period of time, it consumes 0. 36 grams of water・(2)the calculation of the relative atomic mass of M (N03) x when the M (N03) x solution increases to mg(using m and x).(3)this battery electrolysis contains 0. 01 moles of CuS04 and 0.01 moles of NaCl, and the anode produces gasL (standard condition), the solution of electrolysis after electrolysis is released to IL, the pH of the solution is.Three, calculation problem22.To 8 g of divalent metal oxide solid adding rare - 1I2S04, make its just completely dissolved, known by sulfuric acid volume is 100ml, insert the platinum electrode in electrolysis in the solution obtained, electricity after a certain period of time, collected on an electrode 224 ml (standard conditions) of oxygen, in another electrode on the metal precipitation 1. 28 g.(1)the name of the metal oxidation is determined by calculation.(2)the concentration of the substance in the solution of sulfuric acid (the volume of solution) is calculated by lOOmL・The basic chemistry foundation of chapter four(1)basic practiceone23456co DDB9 001415161718CBa.CDCCDBC(1)H02 - H++ 022 -(2)H202 + Ba (OH) 2 = Ba 02 + 2 H20.2H202 + Ba (OH) 2 = Ba (H02) 2+2 H20;(3) 2 H202 H02 - + H302 +(1)the anode・ Cathode: 2H + + 2e -二H2 arrow(2)Cr2072 - + 6Fe2 + + 14H + 二2Cr3 + + + 7H20(3)as the reaction progresses, the H + concentration decreases, the oh-concentration increases, and eventually the Cr (OH) 3, Fe (OH) 3 precipitates・21,(1) B; (2) the CH3C00NH4 solution appears neutral, whichcan prove which classmate is correct・⑶ b;22,(1) CuC12, HC1; (2) H2S04, Na2S04 (3) NaCl, BaC12, CuS0423,7 (1) ; (2) CH3COOH + CH3COO 二0. 01 moles; 0. 006 mol, (3);24,(1) 1:10 (2) 4.48 molar(1)the concentration of copper oxide (2) sulphuric acid is 0. 2 molar ・[please note: 224 ml (standard condition) oxygen is less than 8 grams with 1.28 grams・This shows that MS04 is not fully electrolytic!(2)ability testing oneC 広 DCO O02 + 2C02 + 4e - 二2C032-2C0 + 02 二2C02(1)the area is red near the anode and blue near the cathode・ The solution is also purple, and by electron conservation, the amount of H + and OH minus of the Yin and Yang is exactly the same, while the Na2S04 solution is not hydrolyzed and is neutra1.(2)the red is getting lighter; The red gradually deepens・(1)when iron rusts, it reacts with oxygen in the air, depleting oxygen and reducing the gas pressure in a small beaker.(2) B > C > A = D・(3)contact with oxygen; The presence of water; There is an electrolyte (or a salt) that is present, and at the same time the iron rusts the fastest (or from the condition of the battery that makes up the original battery)・(1)Cu2 + hydrolysis, the solution is acidic, Cu2 + + 2H20 Cu (OH) 2 + 2H +・(2)when electrolysis, the Cu2 + is separated by the cathode, and because the concentration of Cu2 + decreases, the hydrolysis equilibrium moves to the left, so the H + concentration decreases and the pH increases・(3)the pH of the solution is lower, pH.(4)the "experimentalisls" are correct because, while electrolysis, the anode is dialyse and C12 is dissolved in water to form hydrochloric acid・(1) A (2) 50mx (3) 0. 16& 222.(1)the metal is M, n (02)二0.01 mol, and the amount of matter measured by M is 0. 02 moles, according to the mo 1 ar mass of M, and the metal oxide is Cu0・(2)in accordance with the Cu ~ - H2S04,N of H2S04 is equal to 0. 02 moles・ one。
高中化学选修四《电化学基础》单元检测(整理含答案)

高中化学选修四《电化学基础》单元检测时间:90分钟,总分:100分一、选择题(本题包括17个小题,每小题3分,共51分)1.化学用语是学习化学的重要工具,下列用来表示物质变化的化学用语中,正确的是()A.惰性电极电解饱和食盐水时,阳极的电极反应式为:2Cl--2e-===Cl2↑B.氢氧燃料电池的负极反应式为:O2+2H2O+4e-===4OH-C.粗铜精炼时与电源正极相连的是纯铜电极反应式为:Cu-2e-===Cu2+D.钢铁发生电化学腐蚀的正极反应式为:Fe-2e-===Fe2+2.下列叙述中,正确的是()①电解池是将化学能转变成电能的装置②原电池是将电能转变成化学能的装置③金属和石墨导电均为物理变化,电解质溶液导电是化学变化④不能自发进行的氧化还原反应,通过电解的原理有可能实现⑤电镀过程相当于金属的“迁移”,可视为物理变化A.①②③④B.③④C.③④⑤ D.④3.下列各装置中铜电极上能产生气泡的是()4.下列说法不正确的是()A.充电电池充电时,发生电解反应;放电时,发生原电池反应B.电镀时,应将镀层金属与电源正极相连C.电解饱和NaCl溶液时,阳极上放出黄绿色气体的同时还产生大量的氢氧化钠D.利用电化学原理保护金属主要有两种方法,分别是牺牲阳极的阴极保护法和外加电流的阴极保护法5.如图所示,铜片、锌片和石墨棒用导线连接后插入番茄里,电流表中有电流通过,则下列说法正确的是()A.锌片是负极B.两个铜片上都发生氧化反应C.石墨是阴极D.两个番茄都形成原电池6.在理论上不能用于设计成原电池的化学反应是()A. 4Fe(OH)2(s)+2H2O(l)+O2(g)===4Fe(OH)3(s)ΔH<0B.CH3CH2OH(l)+3O2(g)===2CO2 (g)+3H2O(l)ΔH<0C.Al(OH)3(s)+NaOH(aq)===NaAlO2(aq)+2H2O(l)ΔH<0D.H2(g)+Cl2(g)===2HCl(g)ΔH<07.下列事实与电化学腐蚀无关的是()A.钢铁制品生锈后用盐酸处理B.黄铜(Cu、Zn合金)制的铜锣不易产生铜绿C.铜、铝电线一般不连接起来作导线D.生铁比熟铁(几乎是纯铁)容易生锈8.下列过程需要通电才能进行的是()①电离②电解③电镀④电泳⑤电化学腐蚀A.①②③B.②④⑤C.②③④D.全部9.对外加电流的金属保护中,下列叙述正确的是()A,被保护的金属与电源的正极相连B.被保护的金属与电源的负极相连C.在被保护的金属表面上发生氧化反应D.被保护的金属为阴极,其表面上不发生氧化反应,而发生还原反应10.我国某大城市今年夏季多次降下酸雨。
电化学基础专题测试

电化学基础专题测试一、选择题1、综合下图判断,下列正确的说法是A. 装置I 和装置II 中负极反应均是B. 装置I 和装置II 中正极反应均是C. 装置I 和装置II 中盐桥中的阳离子均向右侧烧杯移动D. 放电过程中,装置I 左侧烧杯和装置II 右侧烧杯中溶液的pH 均增大2、如图所示,将铁棒和石墨棒插入饱和食盐水中。
下列说法正确的是( )A .甲中铁被保护不会腐蚀B .甲中正极反应式为4OH --4e - =2H 2O +O 2↑C .乙中铁电极上发生氧化反应D .乙中石墨电极附近滴几滴碘化钾淀粉溶液变蓝色3、右图所示装置中,已知电子由b 极沿导线流向锌。
下列判断正确的是( )A 、该装置中Cu 极为阴极B 、一段时间后锌片质量减少C 、b 极反应的电极反应式为:H 2-2e -+20H -=2H 2OD 、当铜极的质量变化为32g 时,a 极上消耗的O2的体积为5.6L4、用铅蓄电池电解甲、乙电解池中的溶液。
己知铅蓄电池的总反应为:Pb+PbO 2+2H 2SO 4放电充电2PbSO 4十2H 2O 电解一段时间后向c 极和d 极附近分别滴加酚酞试剂,c 极附近溶液变红,下列说法正确的是( )A .d 极为阴极B .放电时铅蓄电池负极的电极反应式为:PbO 2+4H +十SO 42-+4e -=PbSO 4+2H 2OC .若利用甲池精炼铜,b 极应为粗铜D .若四个电极材料均为石墨,当析出6.4 gCu 时,两池中共产生标准状况下的气体3.36L5、电渗析法是一种利用离子交换膜进行海水淡化的方法,其原理如图所示。
已知海水中含Na +、Cl -、Ca 2+、Mg 2+、SO 42-等离子,电极为惰性电极。
下列叙述中正确的是 ( )A .A 膜是阳离子交换膜B .通电后,海水中阴离子往b 电极处运动C .通电后,a 电极的电极反应式为 4OH --4e -=O 2↑ +2H 2OD .通电后,b 电极上产生无色气体,溶液中出现少量白色沉淀6、氯碱工业常利用阳离子交换膜电解食盐水,下列说法不正确的是 ( )A .随着电解的进行,c (NaCl)降低,需不断补充饱和食盐水B .电解过程中采用增大阳极区溶液pH 的方法,可以减少Cl 2在水中的溶解量C .阳离子交换膜的作用是阻止OH -移向阳极,以使氢氧化钠在阴极区富集D .阳极表面用钛氧化物涂层处理,目的是降低电解产物Cl 2对电极的腐蚀7、锂钒氧化物凝胶电池能量密度高,成本低,便于大量推广。
2020—2021学年高中化学人教版化学反应原理第四章《电化学基础》单元检测题

第四章《电化学基础》单元检测题一、单选题(共15小题)1.下列装置中,属于原电池的是()A.答案AB.答案BC.答案CD.答案D2.Mg-AgCl电池是一种能被海水激活的一次性贮备电池,电池反应方程式:2AgCl+Mg === Mg2++2Ag+2Cl-。
有关该电池的说法正确的是()A. Mg为电池的正极,发生反应:Mg2++2e-===MgB.负极反应为AgCl+e-===Ag+Cl-C.不能被KCl溶液激活D.可用于海上应急照明供电3.(2011·新课标全国卷,11)铁镍蓄电池又称爱迪生电池,放电时的总反应为Fe+Ni2O3+3H2O===Fe(OH)2+2Ni(OH)2下列有关该电池的说法不正确的是()A.电池的电解液为碱性溶液,正极为Ni2O3、负极为FeB.电池放电时,负极反应为Fe+2OH--2e-===Fe(OH)2C.电池充电过程中,阴极附近溶液的pH降低D.电池充电时,阳极反应为2Ni(OH)2+2OH--2e-===Ni2O3+3H2O4.如图所示的装置中,在产生电流时,以下说法不正确的是()A. Fe是负极,C是正极B.负极反应式为Fe-3e-===Fe3+C.内电路中阴离子移向FeCl2溶液D.电流由石墨电极流向Fe电极5.关于钢铁腐蚀与防护的说法不正确的是()A.钢铁的吸氧腐蚀和析氢腐蚀的负极反应式均为Fe-2e-===Fe2+B.钢铁发生吸氧腐蚀时,正极反应式为O2+2H2O+4e-===4OH-C.地下钢管连接镁块是采用牺牲阳极的阴极保护法D.用外加电流的阴极保护法防止钢铁腐蚀时,钢铁接电源的正极6.下列装置能够组成原电池的是()A.答案AB.答案BC.答案CD.答案D7.MgAgCl电池是一种以海水为电解质溶液的水激活电池。
下列叙述错误的是() A.负极反应式为Mg-2e-===Mg2+B.正极反应式为Ag++e-===AgC.电池放电时Cl-由正极向负极迁移D.负极会发生副反应Mg+2H2O===Mg(OH)2+H2↑8.按下图所示实验装置的K闭合,下列判断正确的是()A. Cu电极上发生还原反应B.电子沿Zn→a→b→Cu路径流动C.片刻后甲池中c(SO42−)增大D.片刻后可观察到滤纸b点变红色9.下列事实与电化学腐蚀无关的是()A.光亮的自行车钢圈不易生锈B.黄铜(Cu、Zn合金)制的铜锣不易生锈C.铜、铝电线一般不连接起来作导线D.生铁比熟铁(几乎是纯铁)容易生锈10.在某电解质溶液里,用M和N作电极,通电一段时间后,发现M极质量减小,N极质量增大,符合这一情况的是()A.电解质溶液是稀硫酸B.金属M是阳极,金属N是阴极C. M和N是石墨电极D. M是阴极,N是阳极11.将纯锌片和纯铜片按图示方式插入同浓度的稀硫酸中一段时间,以下叙述正确的是()A.两烧杯中铜片表面均无气泡产生B.甲中铜片是正极,乙中铜片是负极C.两烧杯中溶液的酸性均减弱D.产生气泡的速率甲比乙慢12.在盛有稀硫酸的烧杯中放入用导线连接的锌片和铜片,下列叙述正确的是( )A.正极附近的硫酸根离子浓度逐渐增大B.正极有氧气逸出C.电子通过导线由铜片流向锌片D.铜片上有氢气逸出13.流动电池是一种新型电池,其主要特点是可以通过电解质溶液的循环流动,在电池外部调节电解质溶液,以保持电池内部电极周围溶液浓度的稳定。
化学选修4人教新课标第4章 电化学基础 章末综合检测

章末综合检测(90分钟,100分)一、选择题(本题包括18个小题,每小题3分,共54分)1.下列过程需要通电后才可以发生或进行的是()①电离②电泳③电解④电镀⑤电化学腐蚀A.①②B.②③④C.②③④⑤D.全部解析:电解质溶于水即可发生电离,不需要通电;电化学腐蚀的实质是发生原电池反应,是化学能转变为电能的自发反应,也不需要通电。
答案:B2.下列叙述中,正确的是()①电解池是将化学能转变成电能的装置②原电池是将电能转变成化学能的装置③金属和石墨导电均为物理变化,电解质溶液导电是化学变化④不能自发进行的氧化还原反应,通过电解的原理有可能实现⑤电镀过程相当于金属的“迁移”,可视为物理变化A.①②③④B.③④C.③④⑤D.④解析:①、②正好相反了,③、④正确,⑤中电镀是特殊形式的电解,仍然是化学变化。
答案:B3.用石墨棒作电极,电解下列物质的水溶液,实质上与电解水一致的是()A.NaOH B.NaClC.CuSO4D.CuCl2解析:B项中电解NaCl溶液生成NaOH、H2、Cl2。
C项中电解CuSO4溶液生成Cu、O2、H2SO4。
D项中电解CuCl2溶液生成Cu和Cl2。
答案:A4.某同学为了使反应2HCl+2Ag===2AgCl+H2↑能进行,设计了如下所示的四个实验方案,你认为可行的方案是()解析:由题意知,以盐酸作电解质溶液,Ag作阳极,设计成电解池可实现该反应。
答案:C5.选用下列试剂和电极:稀HSO4、Fe2(SO4)3溶液、Fe、Cu、Zn,组成右图所示的原电池装置(只有两个电极),观察到电流计Ⓖ的指针均明显转偏,则其可能的组合共有()A.3种B.4种C.5种D.6种解析:电极的组合有三种:Fe—Cu、Zn—Fe、Zn—Cu,三种组合都能与两种电解质溶液发生自发的氧化还原反应,选D。
答案:D6.(2014·三明一中高二期中)在铁制品上镀上一定厚度的铜层,以下电镀方案中正确的是() A.铜作阳极,铁制品作阴极,溶液中含Fe2+B.铜作阴极,铁制品作阳极,溶液中含Cu2+C.铜作阴极,铁制品作阳极,溶液中含Fe3+D.铜作阳极,铁制品作阴极,溶液中含Cu2+解析:在铁上镀铜,则要求铜在阳极,铁在阴极,电解质溶液含Cu2+,D项符合题意。
人教版选修4电化学基础单元测试

第四单元电化学基础测评(四)B本试卷分第Ⅰ卷(选择题)和第Ⅱ卷(非选择题)两部分。
第Ⅰ卷54分,第Ⅱ卷46分,共100分,考试时间90分钟。
第Ⅰ卷(选择题共54分)一、选择题(本大题共18小题,每小题3分,共54分。
在每小题给出的四个选项中,只有一项是符合题目要求的)1.如图所示的原电池装置,X、Y为两电极,电解质溶液为稀硫酸,外电路中的电子流向如图所示,对此装置的下列说法正确的是:A.外电路的电流方向为X→外电路→YB.若两电极分别为Zn和碳棒,则X为碳棒,Y为ZnC.若两电极都是金属,则它们的活动性为X>YD.X极上发生的是还原反应,Y极上发生的是氧化反应2.关于镀铜和电解精炼铜,下列说法中正确的是:A.都用粗铜作阳极、纯铜作阴极B.电解液的成分都保持不变C.阳极反应都只有Cu-2e-===Cu2+D.阴极反应都只有Cu2++2e-===Cu3.如图所示,烧杯内盛有浓HNO3,在烧杯中放入用铜线相连的铁、铅两个电极,已知原电池停止工作时,Fe、Pb都有剩余。
下列有关说法正确的是:A.Fe比Pb活泼,始终作负极B.Fe在浓HNO3中钝化,始终不会溶解C.电池停止工作时,烧杯中生成了Fe(NO3)3D.利用浓HNO3作电解质溶液不符合“绿色化学”思想4.下列关于下图实验装置的说法正确的是:图CB4-3A.该装置电流表中没有电流通过C.总反应为4Al+3O2+6H2O===4Al(OH)3B.铝箔是正极D.电子从铝箔流出,经电流表、活性炭、滤纸回到铝箔5.下列有关钢铁腐蚀与防护的说法正确的是:A.钢管与电源正极连接,钢管可被保护B.铁遇冷浓硝酸表面钝化,可保护内部不被腐蚀C.钢管与铜管露天堆放在一起时,钢管不易被腐蚀D.钢铁发生析氢腐蚀时,负极反应是Fe-3e-===Fe3+6.把a、b、c、d四块金属片浸入稀硫酸中,用导线两两相连可以组成几个原电池。
若a、b 相连时a为负极;c、d相连时电流由d到c;a、c相连时c上产生大量气泡;b、d相连时b上有大量气泡产生,则四种金属的活动性顺序由强到弱为:A.a>c>d>b B.a>b>c>d C.c>a>b>d D.b>d>c>a7.微型纽扣电池在现代生活中有广泛应用,有一种银锌电池,其电极分别是Ag2O和Zn,电解质溶液为KOH溶液,电极反应式为Zn+2OH--2e-===ZnO+H2O ,Ag2O+H2O+2e-===2Ag+2OH-总反应式为Ag2O+Zn===ZnO+2Ag根据上述反应式,判断下列叙述中正确的是:A.在使用过程中,电池负极区溶液的pH增大B.在使用过程中,电子由Ag2O经外电路流向Zn极C.Zn是负极,Ag2O是正极D.Zn极发生还原反应,Ag2O极发生氧化反应8.MgH2O2电池可用于驱动无人驾驶的潜航器。
电化学基础单元测试(A卷)高二化学同步单元双基双测“AB”卷(解析版)

2017~2018学年同步课堂系列之单元测试AB卷第四章电化学基础单元测试(A卷)(测试时间:90分钟满分:100分)班级姓名学号分数第Ⅰ卷(选择题,48分)选择题(每题只有一个正确答案,每题3分,共48分)1.下列在理论上可设计成原电池的化学反应是( )A.C(s)+H2O(g)=CO(g)+H2(g)B.Ba(OH)2•8H2O(s)+2NH4C1(s)=BaCl2(aq)+2NH3•H2O(l)+8H2O(l)C.C(s)+CO2(g)=2CO(g)D.CH4(g)+2O2(g)→CO2(g)+2H2O(l)【答案】D点睛:明确原电池的工作原理是解答的关键,注意理论上凡是能自发进行的放热的氧化还原反应均可以设计为原电池,解答时注意结合选项灵活应用.2.锌−空气燃料电池可用作电动车动力电源,电池的电解质溶液为KOH溶液,反应为2Zn+O2+4OH–+2H2O=2Zn(OH)42-。
下列说法正确的是( )A.充电时,电解质溶液中K+向阳极移动B.充电时,电解质溶液中C(OH—)逐渐减小C.放电时,负极反应为:Zn+4OH–—2e–=Zn(OH)42—D.放电时,电路中通过2mol电子,消耗氧气22。
4L(标准状况)【答案】C【解析】A.充电时阳离子向阴极移动,故A错误;B.充电时,电池反应为Zn(OH)42-+2e—═Zn+4OH-,电解质溶液中c(OH-)逐渐增大,故B错误;C.放电时,负极反应式为Zn+4OH—-2e—═Zn (OH)42—,故C正确;D.放电时,每消耗标况下22。
4L氧气,转移电子4mol,故D错误;故选C。
点晴:正确判断正负极、阴阳极,注意电极反应式的书写及电子转移的计算,正确判断化合价的变化为解答该题的关键,根据2Zn+O2+4OH-+2H2O═2Zn(OH)42—可知,O2中元素的化合价降低,被还原,应为原电池正极,Zn元素化合价升高,被氧化,应为原电池负极,电极反应式为Zn+4OH-—2e-═Zn(OH)42-,充电时阳离子向阴极移动,以此解答该题。
高中化学 专题04 电化学基础单元双基双测(B卷)(含解析)新人教版选修4

电化学基础(测试时间:90分钟满分:100分)班级姓名学号分数第Ⅰ卷(选择题,48分)每题只有一个正确答案,每题3分,共48分1.【杭州地区(含周边)重点中学2014-2015学年上学期期末考】2014年6月5日为世界环境日,主题为“提高你的呼声而不是海平面”,提倡节能减排,以下措施中不能体现这一思想的是()A.在电解铝工业中添加冰晶石 B.大力发展火电,缓解用电紧张C.开发太阳能、风能、氢能等清洁能源 D.研制出性能优良的催化剂,降低反应所需温度【答案】B考点:化学与环境。
2.【太原五中2014-2015学年上学期期末】下列有关钢铁腐蚀与防护的说法正确的是()A.钢管与电源正极连接,钢管可被保护B.铁遇冷浓硝酸表面钝化,可保护内部不被腐蚀C.钢管与铜管露天堆放在一起时,钢管不易被腐蚀D.钢铁发生析氢腐蚀时,负极反应是Fe-3e-===Fe3+【答案】B【解析】试题分析:A、钢管和电源正极相连,做阳极,容易腐蚀,所以不能被保护,不选A;B、铁在常温下在浓硝酸钝化,形成致密的氧化膜,保护内部不被腐蚀,选B;C、钢管和铜管放在一起,容易形成原电池,铁做负极,容易被腐蚀,不选C;D、钢铁腐蚀时,负极的反应是铁失去电子生成亚铁离子,不选D。
考点:钢铁腐蚀和防护。
3.【聊城市(茌平、东昌府、东阿)三县2014-2015学年上学期期末】某同学根据离子反应方程式2Fe 3++Fe=3Fe 2+来设计原电池。
下列设计方案中可行的是( ) A .电极材料为铁和锌,电解质溶液为FeCl 3溶液 B .电极材料为铁,电解质溶液为 Fe(NO 3)3溶液 C .电极材料为铁和铜,电解质溶液为FeCl 3 溶液 D .电极材料为石墨,电解质溶液为 FeCl 3溶液 【答案】C 【解析】试题分析:根据2Fe 3++Fe=3Fe 2+,铁失电子,作原电池的负极,正极选择碳棒或比铁不活泼的金属作正极,电解质溶液采用可溶性的铁盐。
人教版高中化学选修四§4 电化学基础单元检测.docx

高中化学学习材料§4 电化学基础单元检测一.选择题(每题6分,共42分)1.下列各变化中属于原电池反应的是()A.空气中金属铝表面迅速氧化形成保护层B.镀锌铁表面有划损时,也能阻止铁被氧化C.红热的铁丝与冷水接触,表面形成蓝黑色保护层D.浓硝酸比稀硝酸更易氧化金属铜2.铁棒与石墨棒用导线连接后浸入0.01mol•L-1的食盐溶液中,可能出现的现象是()A.铁棒附近产生OH- B.铁棒逐渐被腐蚀C.石墨棒上放出Cl2 D.石墨棒上放出O23.对外加电流的保护中,下列叙述正确的是()A.被保护的金属与电源的正极相连 B.被保护的金属与电源的负极相连C.在被保护的金属表面上发生氧化反应D.被保护的金属表面上不发生氧化反应,也不发生还原反应4.(2014·高考天津卷)已知:锂离子电池的总反应为Li x C+Li1-x CoO2放电充电C+LiCoO2锂硫电池的总反应为2Li+S 放电充电Li2S,有关上述两种电池说法正确的是( )A.锂离子电池放电时,Li+向负极迁移B.锂硫电池充电时,锂电极发生还原反应C.理论上两种电池的比能量相同 D.上图表示用锂离子电池给锂硫电池充电5.下列有关说法正确的是()A.CaCO3(s)=CaO(s)+CO2(g)室温下不能自发进行,说明该反应的△H<0黄铜矿 冰铜(mCu 2S∙nFeS) 气体A 泡铜(Cu 2O 、Cu) 熔渣B Al 高温粗铜精铜 电解精炼 石英砂石英砂 空气 空气焙烧 焙烧 B.镀铜铁制品镀层受损后,铁制品比受损前更容易生锈C.N 2(g)+3H 2(g)2NH 3(g) △H <0,其他条件不变时升高温度,反应速率V(H 2)和氢气的平衡转化率均增大D.水的离子积常数Kw 随着温度的升高而增大,说明水的电离是放热反应6.某兴趣小组设计如下微型实验装置。
实验时,先断开K 2 ,闭合K 1 ,两极均有气泡产生;一段时间后,断开K 1 ,闭合K 2 ,发现电流计A 指针偏转。
化学选修iv人教新课标4电化学基础测试题(一)单元测试解读

电化学基础测试题(一)重庆蔡中华一、选择题(每小题只有一个选项符合题意)1. 关于原电池的叙述中正确的是A. 构成原电池的两极必须是两种不同金属B. 原电池是将化学能转化为电能的装置C. 原电池工作时总是负极溶解,正极上有物质析出D. 原电池的正极是还原剂,总是溶液中的阳离子在此被还原1. B2. 如图所示,电流表指针发生偏转,同时A极质量减少,B极上有气泡产生,C为电解质溶液,下列说法错误的是A.B极为原电池的正极A n n BB. A、B、C可能分别为Zn、Cu、稀盐酸W芝謬-匚C•中阳离子向A极移动D.A极发生氧化反应2. C【解析】A为原电池的负极,B为原电池的正极,负极失去电子发生氧化反应,C中的阳离子带有正电荷,应该向着富集有大量负电荷的B极移动。
3•下列四组原电池,其中放电后,电解质溶液质量增加,且在正极有单质生成的是()A . Cu、Ag、AgNO 3 溶液B. Zn、Cu、稀H2SO4C. Fe、Zn、ZnSO4溶液D. Fe、C、Fe2(SO4)3 溶液3. B4•下列有关金属的说法正确的是A •银器在空气中变暗后一定条件下被还原又会变光亮B •当镀锌铁制品的镀层破损时,镀层不能对铁制品起保护作用C. 不锈钢不生锈是因为表面有保护膜D •可将地下输油钢管与外加直流电源的正极相连以保护它不受腐蚀4. A【解析】A项,当银的化合物又变成单质银时可以变光亮。
B项,锌比铁活泼,所以镀层破损后仍与铁构成原电池作负极,铁被保护。
C项,不锈钢不生锈是因为内部结构原因而具有强的耐腐蚀能力。
D项,要使地下输油钢管不生锈,应要与外加电源的负极相连。
5. 下列装置中能组成原电池的是()5. B6. 分析下图所示的四个原电池装置,结论正确的是()A .⑴(2)中Mg作负极,⑶(4)中Fe作负极B. (2)中Mg作正极,电极反应式为:6H2O + 6e「===6OH 「+ 3出匸—2+C. (3)中Fe作负极,电极反应式为:Fe—2e ===FeD. ⑷中Cu 作正极,电极反应式为:2H* + 2e 「===H 2f 6. B 【解析】⑴中Mg 作负极;⑵中Al 作负极,发生的反应为2AI + 2NaOH + 2H 2O===2NaAIO 2 + 3H 2f, Mg 不与NaOH 反应;(3)中铁遇浓 HNO 3钝化,Cu 与浓HNO 3反应,Cu + 4HNO 3===C U (NO 3)2+ 2NO 2T+ 2H 2O ; (4)中铁作负极,为中性环境,发生吸氧腐蚀。
高中化学 第四章 电化学基础综合能力检测(含解析)4

促敦市安顿阳光实验学校第四章电化学基础综合能力检测4时间:90分钟分值:100分第Ⅰ卷(选择题,共48分)一、选择题(每小题3分,共48分)1.暖宝宝是市场上常见的热敷、止痛、消肿贴剂。
它由原料层、明层、无纺布袋层三组成,其料是由铁、石墨、活性炭、无机盐合成的聚合物,构成了原电池,其中铁作( )A.正极B.负极C.阴极D.阳极答案B解析由题意知Fe、石墨、无机盐形成了原电池,铁在原电池中作负极。
2.对外加电流的金属保护中,下列叙述正确的是( )A.被保护的金属与电源的正极相连B.被保护的金属与电源的负极相连C.在被保护的金属表面上发生氧化反D.被保护的金属为阴极,其表面上发生氧化反答案B解析外加电流的金属保护法依据的是电解池的原理,被保护的金属与电源的负极相连,作阴极,发生还原反,故只有B项正确。
3.已知蓄电池在充电时作电解池,放电时作原电池。
铅蓄电池上有两个接线柱,一个接线柱旁标有“+”,另一个接线柱旁标有“-”。
关于标有“+”的接线柱,下列说法中正确的是( )A.充电时作阳极,放电时作正极B.充电时作阳极,放电时作负极C.充电时作阴极,放电时作负极D.充电时作阴极,放电时作正极答案A解析铅蓄电池是常见的二次电池,充电时电极反是放电时电极反的逆过程。
放电时正极得到电子发生还原反作正极,充电时则失去电子发生氧化反作阳极。
4.在电解质溶液中插入M、N电极,并连接直流电源进行电解,可以看到两极上均有气泡产生,电解后测电解质溶液,其H+浓度无变化。
符合这些条件的是( )A.两个电极均为铁片,M是阴极,电解质溶液是0.4%的NaOH溶液B.两个电极均为石墨,M是阳极,电解质溶液是0.4%的H2SO4溶液C.两个电极均为石墨,M是阳极,电解质溶液是0.4%的KOH溶液D.m是铁片,作阴极,N是石墨,电解质溶液是0.4%的KNO3溶液答案D解析由电解原理可知,A项中Fe作阳极时失去电子,不会产生气体,不符合题意;B项中电解水,H2SO4浓度增加,不符合题意;C项电解KOH时也是电解水,KOH浓度增大即c(OH-)增大,故c(H+)减小,不符合题意;D项中虽电解水,但溶液中c(H+)=c(OH-),c(H+)不发生变化。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
《第四章电化学基础》单元检测
一、选择题(本题包括18小题,每小题3分,共54分;每小题只有一个选项符合题意)
1、将镁条和铝条平行插入一定浓度的氢氧化钠溶液中,用导线连接形成原电池。
下列有关该装置的叙述正确的是( )
A.因镁比铝活泼,故镁是原电池的负极,铝为正极
B.铝条表面虽有氧化膜,但可不必处理
C.该电池的内、外电路中,电流均由电子定向移动形成
D.铝是电池的负极,工作时溶液中会立即有白色沉淀生成
答案 B
2关于下图所示的原电池,下列说法正确的是( )
A.电子从锌电极通过电流表流向铜电极
B.盐桥中的阴离子向硫酸铜溶液中迁移
C.铜电极发生还原反应,其电极反应是2H++2e-===H2↑
D.取出盐桥后,电流表仍会偏转,铜电极在反应前后质量不变
答案 A
3下列事实与电化学腐蚀无关的是( )
A.光亮的自行车钢圈不易生锈
B.黄铜(Cu、Zn合金)制的铜锣不易生锈
C.铜、铝电线一般不连接起来作导线
D.生铁比熟铁(几乎是纯铁)容易生锈
答案 A
4、如图装置中,小试管内为红墨水,具支试管内盛有pH=4的雨水和生铁片。
观察到:开始导管内液面下降,一段时间后导管内液面回升,略高于小试管液面。
以下有关解释合理的是( )
A.生铁片中的碳是原电池的负极,发生还原反应
B.雨水酸性较强,生铁片仅发生析氢腐蚀
C.墨水回升时,碳极反应式为O2+2H2O+4e-===4OH-
D.具支试管中溶液pH逐渐减小
答案 C
5下列关于如图所示的实验装置的判断中错误的是( )
A.若X为碳棒,开关K置于A处可减缓铁的腐蚀
B.若X为锌棒,开关K置于A或B处都可减缓铁的腐蚀
C.若X为锌棒,开关K置于B处时,为牺牲阳极的阴极保护法
D.若X为碳棒,开关K置于B处时,铁电极上发生的反应为2H++2e-===H2↑
答案 D
6如图是工业电解饱和食盐水的装置示意图,下列有关说法中不正确的是( )
A.装置中出口①处的物质是氯气,出口②处的物质是氢气
B.该离子交换膜只能让阳离子通过,不能让阴离子通过
C.装置中发生反应的离子方程式为Cl -+2H +=====通电Cl 2↑+H 2↑
D.该装置是将电能转化为化学能
答案 C
7、(2019·腾冲县期末)锂空气电池放电时的工作原理如图所示。
下列叙述正确的是(
)
A.放电时Li +由B 极向A 极移动
B.电池放电反应为4Li +O 2+2H 2O===4LiOH
C.B 电极反应式为O 2+4H ++4e -===2H 2O
D.电解液a 可以为氯化锂溶液
答案 B
8某电池以K2FeO4和锌为电极材料,氢氧化钾溶液为电解质溶液。
下列说法不正确的是( )
A.锌为电池的负极
B.正极反应式为2FeO2-4+10H++6e-===Fe2O3+5H2O
C.该电池放电过程中电解质溶液浓度增大
D.电池工作时OH-向负极迁移
答案 B
9金属镍有广泛的用途。
粗镍中含有少量铁、锌、铜、铂等杂质,可用电解法制备高纯度的镍,下列叙述正确的是(已知:氧化性Fe2+<Ni2+<Cu2+)( )
A.阳极发生还原反应,其电极反应式:Ni2++2e-===Ni
B.电解过程中,阳极质量的减少与阴极质量的增加相等
C.电解后,溶液中存在的金属阳离子只有Fe2+和Zn2+
D.电解后,电解槽底部的阳极泥中有铜和铂,没有锌、铁、镍
答案 D
10某原电池构造如图所示,下列叙述正确的是( )
A.在外电路中,电子由银电极流向铜电极
B.取出盐桥后,电流表的指针仍发生偏转
C.外电路中每通过0.1 mol电子,铜的质量理论上减小6.4 g
D.原电池的总反应式为Cu+2AgNO3===2Ag+Cu(NO3)2
答案 D
11关于下图所示①②两个装置的叙述,正确的是( )
A.装置名称:①是原电池,②是电解池
B.硫酸浓度变化:①增大,②减小
C.电极反应式:①中阳极:4OH--4e-===2H2O+O2↑,②中正极:Zn-2e-===Zn2+
D.离子移动方向:①中H+向阴极方向移动,②中H+向负极方向移动
答案 B
12.(2020·内江市高三质检)以SO2为原料,通过下列工艺可制备化工原料H2SO4和清洁能源H2。
下列说法中不正确的是( )
A.该生产工艺中Br2被循环利用
B.在电解过程中,电解槽阴极附近溶液的pH变大
C.原电池中负极发生的反应为SO2+2H2O-2e-===SO2-4+4H+
D.该工艺总反应的化学方程式表示为SO2+Br2+2H2O===2HBr+H2SO4。
