化学专业英语第三章
化学化工专业英语.ppt

词
• 单词是构成句子的基本要素之一,因此, 单词的翻译直接关系到句子3.1.1 可数名词复数前没有数量词时,一般要 把复数含义翻译出来,即在名词前加译“一 些,这些,许多”等词,例如: • The teacher may be asked questions. 可以向老师提一些问题。 • Our first electronic computers were made in 1958. 我国首批电子计算机是1958年制成的。 • we are students of Henan University. 我们是河南大学的学生。
3.1.3 a(an)+名词(可数,不可数)单数表示“一类” 时,不能译出不定冠词“a(an)”
Salts may also be found by the replacement of hydrogen from an acid with a metal. 盐也能通过用金属置换酸中的氢而获得。
An acid was once defined as a substance that would form hydrogen ions(H+) in water solution and a base as one that would form hydroxide ions(OH-) in the same. 人们曾把酸定义为在水溶液中能产生氢离子的 物质,而碱则是在同样溶液中会产生氢氧根离子 的物质。
• 注:对于billion及其以上的数字,英语和美语的 译法不同,翻译时应视此句出于何种国家的刊物 而定。 英语 美语 million 106 106 billion 1012(万亿) 109(十亿) trillion 1018(百万兆) 1012(兆,百万个百万) quadrillion 1024(百万的四次方)1015(一千的五次方) quintillion 1030(百万的五次方) 1018(百万之三次方)
化学专业基础英语

potentiometric method hazardous equipment purport responsibility prior to
referenced document 参考文献 ASTM(American Society for Testing and Materials) 美国材料实验协会 aqueous solution 水溶液 glass electrode 玻璃电极 preparation of specimen 样品的制备
portion increment end point inflection point plot… against… automatic titrator
alkali 碱,碱性的 dehydrate (使)脱水 anhydrous solvent medium 无 anhydrous solvent medium
Standard Test Methods for Acid Number of Rosin
课堂教学内容安排
第一节课 教学要求说明 词汇预习 课文阅读理解 第二节课 课文阅读理解(续) 答疑 布置课后作业 词汇和短文翻译(书面练习)
一、教学要求
掌握:课文中有关酸碱滴定方面的专业词
汇;
熟悉:松香酸度的标准测试方法 的原理; 了解:通过本课文的学习,加深对内指示剂 法尤其是电位法酸碱滴定终点确定的理 解,并能够借助词典阅读相关的英文文 献及书籍。
二、 词汇(New Words and Expressions)
designation 名称 acid number 酸度, 酸值 standard 标准 rosin 松香 scope 范围 intend 想要, 打算 internal indicator method 内指示剂法
化学专业英语

化学专业英语分析术语英语翻译玻璃漏斗 Glass funnel long stem试管 test tube test tube brush test tube holder test tube rack 蒸发皿 evaporating dish small烧杯 beaker锥形瓶 Erlenmeyer量筒 grad cylinder洗瓶 plastic wash bottle勺皿 casserole ,smallstoppered flask分液漏斗 separalory funnelwater bath/oil bathstrring barmagnetic stirrer冷凝器 condenserBallast bottle圆颈烧瓶 Round-buttom flask试剂瓶 reagent bottles托盘天平 platform balance 台秤0.1g 托盘pan 指针刻度表pointer and scalecrossbeams and sliding weights 游码分析天平 two-pan/single-pan analytical balance滴定管 burette glass bead(basic) nozzle移液管 pipette 胖肚 elongated glass bulb洗耳球 rubber suction bulb玻棒 glass rod玻璃活塞 stopcock容量瓶 pyknowmeter flasks比重瓶 (one-mark)volumetric flasks胖肚吸管 one-mark pipette刻度吸管 graduated pipettes实验仪器清单1、柜子中四、抽屉中:锥形瓶(conical flask) 250ml×4 药匙(medicine spoon)×1 (Erlenmeyer flask) 100 ml×3 滴管(drip tube;dropper)×2烧杯(beaker) 500 ml×1 玻棒(Glass stic)×2250 ml×3 木试管夹(test tube clamp;test tube holder)×1100 ml×3 胖肚吸管(straws) 25 ml×150 ml×2 10 ml×1容量瓶(volumetric flask) 100 ml×2 乳钵(morta)×150 ml×4 洗耳球(ear wadhing bulb)碘量瓶 (iodin numoe flask;iodineflask) 500 ml×3试剂瓶 (reagent bottle) (无色)×2(棕色)×2 配洗液:量筒(cylinder) 100 ml×1 K2Cr2O72g+5ml水→65mlH2SO4(graduated cylinder)10ml×1 边加边搅拌(stir)。
分析化学专业英语词汇总结

专业英语词汇-----分析化学第一章绪论分析化学:analytical chemistry定性分析:qualitative analysis定量分析:quantitative analysis物理分析:physical analysis物理化学分析:physico-chemical analysis仪器分析法:instrumental analysis流动注射分析法:flow injection analysis;FIA顺序注射分析法:sequentical injection analysis;SIA化学计量学:chemometrics第二章误差的分析数据处理绝对误差:absolute error相对误差:relative error系统误差:systematic error可定误差:determinate error随机误差:accidental error不可定误差:indeterminate error准确度:accuracy精确度:precision偏差:debiation,d平均偏差:average debiation相对平均偏差:relative average debiation标准偏差〔标准差〕:standerd deviation;S相对平均偏差:relatibe standard deviation;RSD变异系数:coefficient of variation误差传递:propagation of error有效数字:significant figure置信水平:confidence level显著性水平:level of significance合并标准偏差〔组合标准差〕:pooled standard debiation 舍弃商:rejection quotient ;Q化学定量分析第三章滴定分析概论滴定分析法:titrametric analysis滴定:titration容量分析法:volumetric analysis化学计量点:stoichiometric point等当点:equivalent point电荷平衡:charge balance电荷平衡式:charge balance equation质量平衡:mass balance物料平衡:material balance 质量平衡式:mass balance equation第四章酸碱滴定法酸碱滴定法:acid-base titrations质子自递反响:auto protolysis reaction质子自递常数:autoprotolysis constant质子条件式:proton balance equation酸碱指示剂:acid-base indicator指示剂常数:indicator constant变色范围:colour change interval混合指示剂:mixed indicator双指示剂滴定法:double indicator titration第五章非水滴定法非水滴定法:nonaqueous titrations质子溶剂:protonic solvent酸性溶剂:acid solvent碱性溶剂:basic solvent两性溶剂:amphototeric solvent无质子溶剂:aprotic solvent均化效应:differentiatin g effect区分性溶剂:differentiating solvent离子化:ionization离解:dissociation结晶紫:crystal violet萘酚苯甲醇: α-naphthalphenol benzyl alcohol奎哪啶红:quinadinered百里酚蓝:thymol blue偶氮紫:azo violet溴酚蓝:bromophenol blue第六章配位滴定法配位滴定法:compleximetry乙二胺四乙酸:ethylenediamine tetraacetic acid,EDTA 螯合物:chelate compound金属指示剂:metal lochrome indcator第七章氧化复原滴定法氧化复原滴定法:oxidation-reduction titration碘量法:iodimetry溴量法:bromimetry ]溴量法:bromine method铈量法:cerimetry高锰酸钾法:potassium permanganate method条件电位:conditional potential溴酸钾法:potassium bromate method硫酸铈法:cerium sulphate method偏高碘酸:metaperiodic acid高碘酸盐:periodate亚硝酸钠法:sodium nitrite method重氮化反响:diazotization reaction重氮化滴定法:diazotization titration亚硝基化反响:nitrozation reaction亚硝基化滴定法:nitrozation titration外指示剂:external indicator外指示剂:outside indicator重铬酸钾法:potassium dichromate method 第八章沉淀滴定法沉淀滴定法:precipitation titration容量滴定法:volumetric precipitation method 银量法:argentometric method第九章重量分析法重量分析法:gravimetric analysis挥发法:volatilization method引湿水〔湿存水〕:water of hydroscopicity 包埋(藏)水:occluded water吸入水:water of imbibition结晶水:water of crystallization组成水:water of composition液-液萃取法:liquid-liquid extration溶剂萃取法:solvent extration反萃取:counter extraction分配系数:partition coefficient分配比:distribution ratio离子对〔离子缔合物〕:ion pair沉淀形式:precipitation forms称量形式:weighing forms仪器分析概述物理分析:physical analysis物理化学分析:physicochemical analysis仪器分析:instrumental analysis第十章电位法及永停滴定法电化学分析:electrochemical analysis电解法:electrolytic analysis method电重量法:electrogravimetry库仑法:coulo metry库仑滴定法:coulo metric titration电导法:conductometry电导分析法:conductometric analysis电导滴定法:conductometric titration 电位法:potentiometry直接电位法:dirext potentiometry电位滴定法:potentiometric titration伏安法:voltammetry极谱法:polarography溶出法:stripping method电流滴定法:amperometric titration化学双电层:chemical double layer相界电位:phase boundary potential金属电极电位:electrode potential化学电池:chemical cell液接界面:liquid junction boundary原电池:galvanic cell电解池:electrolytic cell负极:cathode正极:anode电池电动势:eletromotive force指示电极:indicator electrode参比电极:reference electroade标准氢电极:standard hydrogen electrode一级参比电极:primary reference electrode饱和甘汞电极:saturated calomel electrode银-氯化银电极:silver silver-chloride electrode液接界面:liquid junction boundary不对称电位:asymmetry potential表观PH值:apparent PH复合PH电极:combination PH electrode离子选择电极:ion selective electrode敏感器:sensor晶体电极:crystalline electrodes均相膜电极:homogeneous membrance electrodes非均相膜电极:heterogeneous membrance electrodes非晶体电极:non- crystalline electrodes刚性基质电极:rigid matrix electrode流流体载动电极:electrode with a mobile carrier气敏电极:gas sensing electrodes酶电极:enzyme electrodes金属氧化物半导体场效应晶体管:MOSFET离子选择场效应管:ISFET总离子强度调节缓冲剂:total ion strength adjustment buffer,TISAB永停滴定法:dead-stop titration双电流滴定法〔双安培滴定法〕:double amperometric titration 第十一章光谱分析法概论普朗克常数:Plank constant电磁波谱:electromagnetic spectrum光谱:spectrum光谱分析法:spectroscopic analysis原子发射光谱法:atomic emission spectroscopy质量谱:mass spectrum质谱法:mass spectroscopy,MS第十二章紫外-可见分光光度法紫外-可见分光光度法:ultraviolet and visible spectrophotometry;UV-vis肩峰:shoulder peak末端吸收:end absorbtion生色团:chromophore助色团:auxochrome红移:red shift长移:bathochromic shift短移:hypsochromic shift蓝〔紫〕移:blue shift增色效应〔浓色效应〕:hyperchromic effect减色效应〔淡色效应〕:hypochromic effect强带:strong band弱带:weak band吸收带:absorption band透光率:transmitance,T吸光度:absorbance谱带宽度:band width杂散光:stray light噪声:noise暗噪声:dark noise散粒噪声:signal shot noise闪耀光栅:blazed grating全息光栅:holographic grating光二极管阵列检测器:photodiode array detector偏最小二乘法:partial least squares method ,PLS褶合光谱法:convolution spectrometry褶合变换:convolution transform,CT离散小波变换:wavelet transform,WT多尺度细化分析:multiscale analysis供电子取代基:electron donating group吸电子取代基:electron with-drawing group第十三章荧光分析法荧光:fluorescence荧光分析法:fluorometryX-射线荧光分析法:X-ray fluorometry原子荧光分析法:atomic fluorometry分子荧光分析法:molecular fluorometry振动弛豫:vibrational relaxation内转换:internal conversion 外转换:external conversion体系间跨越:intersystem crossing激发光谱:excitation spectrum荧光光谱:fluorescence spectrum斯托克斯位移:Stokes shift荧光寿命:fluorescence life time荧光效率:fluorescence efficiency荧光量子产率:fluorescence quantum yield荧光熄灭法:fluorescence quenching method散射光:scattering light瑞利光:R a yleith scattering light拉曼光:Raman scattering lightAbbe refractometer 阿贝折射仪absorbance 吸收度absorbance ratio 吸收度比值absorption 吸收absorption curve 吸收曲线absorption spectrum 吸收光谱absorptivity 吸收系数accuracy 准确度acid-dye colorimetry 酸性染料比色法acidimetry 酸量法acid-insoluble ash 酸不溶性灰分acidity 酸度activity 活度第十四章色谱法additive 添加剂additivity 加和性adjusted retention time 调整保存时间adsorbent 吸附剂adsorption 吸附affinity chromatography 亲和色谱法aliquot 〔一〕份alkalinity 碱度alumina 氧化铝ambient temperature 室温ammonium thiocyanate 硫氰酸铵analytical quality control〔AQC〕分析质量控制anhydrous substance 枯燥品anionic surfactant titration 阴离子外表活性剂滴定法antibiotics-microbial test 抗生素微生物检定法antioxidant 抗氧剂appendix 附录application of sample 点样area normalization method 面积归一化法argentimetry 银量法arsenic 砷arsenic stain 砷斑ascending development 上行展开ash-free filter paper 无灰滤纸〔定量滤纸〕assay 含量测定assay tolerance 含量限度atmospheric pressure ionization(API) 大气压离子化attenuation 衰减back extraction 反萃取back titration 回滴法bacterial endotoxins test 细菌内毒素检查法band absorption 谱带吸收baseline correction 基线校正baseline drift 基线漂移batch, lot 批batch(lot) number 批号Benttendorff method 白田道夫〔检砷〕法between day (day to day, inter-day) precision 日间精密度between run (inter-run) precision 批间精密度biotransformation 生物转化bioavailability test 生物利用度试验bioequivalence test 生物等效试验biopharmaceutical analysis 体内药物分析,生物药物分析blank test 空白试验boiling range 沸程British Pharmacopeia (BP) 英国药典bromate titration 溴酸盐滴定法bromimetry 溴量法bromocresol green 溴甲酚绿bromocresol purple 溴甲酚紫bromophenol blue 溴酚蓝bromothymol blue 溴麝香草酚蓝bulk drug, pharmaceutical product 原料药buret 滴定管by-product 副产物calibration curve 校正曲线calomel electrode 甘汞电极calorimetry 量热分析capacity factor 容量因子capillary zone electrophoresis (CZE) 毛细管区带电泳capillary gas chromatography 毛细管气相色谱法carrier gas 载气cation-exchange resin 阳离子交换树脂ceri(o)metry 铈量法characteristics, description 性状check valve 单向阀chemical shift 化学位移chelate compound 鳌合物chemically bonded phase 化学键合相chemical equivalent 化学当量Chinese Pharmacopeia (ChP) 中国药典Chinese material medicine 中成药Chinese materia medica 中药学Chinese materia medica preparation 中药制剂Chinese Pharmaceutical Association (CPA) 中国药学会chiral 手性的chiral stationary phase (CSP) 手性固定相chiral separation 手性别离chirality 手性chiral carbon atom 手性碳原子chromatogram 色谱图chromatography 色谱法chromatographic column 色谱柱chromatographic condition 色谱条件chromatographic data processor 色谱数据处理机chromatographic work station 色谱工作站clarity 澄清度clathrate, inclusion compound 包合物clearance 去除率clinical pharmacy 临床药学coefficient of distribution 分配系数coefficient of variation 变异系数color change interval 〔指示剂〕变色范围color reaction 显色反响colorimetric analysis 比色分析colorimetry 比色法column capacity 柱容量column dead volume 柱死体积column efficiency 柱效column interstitial volume 柱隙体积column outlet pressure 柱出口压column temperature 柱温column pressure 柱压column volume 柱体积column overload 柱超载column switching 柱切换committee of drug evaluation 药品审评委员会comparative test 比较试验completeness of solution 溶液的澄清度compound medicines 复方药computer-aided pharmaceutical analysis 计算机辅助药物分析concentration-time curve 浓度-时间曲线confidence interval 置信区间confidence level 置信水平confidence limit 置信限congealing point 凝点congo red 刚果红〔指示剂〕content uniformity 装量差异controlled trial 对照试验correlation coefficient 相关系数contrast test 对照试验counter ion 反离子〔平衡离子〕cresol red 甲酚红〔指示剂〕crucible 坩埚crude drug 生药crystal violet 结晶紫〔指示剂〕cuvette, cell 比色池cyanide 氰化物cyclodextrin 环糊精cylinder, graduate cylinder, measuring cylinder 量筒cylinder-plate assay 管碟测定法daughter ion 〔质谱〕子离子dead space 死体积dead-stop titration 永停滴定法dead time 死时间decolorization 脱色decomposition point 分解点deflection 偏差deflection point 拐点degassing 脱气deionized water 去离子水deliquescence 潮解depressor substances test 降压物质检查法derivative spectrophotometry 导数分光光度法derivatization 衍生化descending development 下行展开desiccant 枯燥剂detection 检查detector 检测器developer, developing reagent 展开剂developing chamber 展开室deviation 偏差dextrose 右旋糖,葡萄糖diastereoisomer 非对映异构体diazotization 重氮化2,6-dichlorindophenol titration 2,6-二氯靛酚滴定法differential scanning calorimetry (DSC) 差示扫描热量法differential spectrophotometry 差示分光光度法differential thermal analysis (DTA) 差示热分析differentiating solvent 区分性溶剂diffusion 扩散digestion 消化diphastic titration 双相滴定disintegration test 崩解试验dispersion 分散度dissolubility 溶解度dissolution test 溶出度检查distilling range 馏程distribution chromatography 分配色谱distribution coefficient 分配系数dose 剂量drug control institutions 药检机构drug quality control 药品质量控制drug release 药物释放度drug standard 药品标准drying to constant weight 枯燥至恒重dual wavelength spectrophotometry 双波长分光光度法duplicate test 重复试验effective constituent 有效成分effective plate number 有效板数efficiency of column 柱效electron capture detector 电子捕获检测器electron impact ionization 电子轰击离子化electrophoresis 电泳electrospray interface 电喷雾接口electromigration injection 电迁移进样elimination 消除eluate 洗脱液elution 洗脱emission spectrochemical analysis 发射光谱分析enantiomer 对映体end absorption 末端吸收end point correction 终点校正endogenous substances 内源性物质enzyme immunoassay(EIA) 酶免疫分析enzyme drug 酶类药物enzyme induction 酶诱导enzyme inhibition 酶抑制eosin sodium 曙红钠〔指示剂〕epimer 差向异构体equilibrium constant 平衡常数equivalence point 等当点error in volumetric analysis 容量分析误差excitation spectrum 激发光谱exclusion chromatography 排阻色谱法expiration date 失效期external standard method 外标法extract 提取物extraction gravimetry 提取重量法extraction titration 提取容量法extrapolated method 外插法,外推法factor 系数,因数,因子feature 特征Fehling’s reaction 费林反响field disorption ionization 场解吸离子化field ionization 场致离子化filter 过滤,滤光片filtration 过滤fineness of the particles 颗粒细度flame ionization detector(FID) 火焰离子化检测器flame emission spectrum 火焰发射光谱flask 烧瓶flow cell 流通池flow injection analysis 流动注射分析flow rate 流速fluorescamine 荧胺fluorescence immunoassay(FIA) 荧光免疫分析fluorescence polarization immunoassay(FPIA) 荧光偏振免疫分析fluorescent agent 荧光剂fluorescence spectrophotometry 荧光分光光度法fluorescence detection 荧光检测器fluorimetyr 荧光分析法foreign odor 异臭foreign pigment 有色杂质formulary 处方集fraction 馏分freezing test 结冻试验funnel 漏斗fused peaks, overlapped peaks 重叠峰fused silica 熔融石英gas chromatography(GC) 气相色谱法gas-liquid chromatography(GLC) 气液色谱法gas purifier 气体净化器gel filtration chromatography 凝胶过滤色谱法gel permeation chromatography 凝胶渗透色谱法general identification test 一般鉴别试验general notices 〔药典〕凡例general requirements 〔药典〕通那么good clinical practices(GCP) 药品临床管理标准good laboratory practices(GLP) 药品实验室管理标准good manufacturing practices(GMP) 药品生产质量管理标准good supply practices(GSP) 药品供应管理标准gradient elution 梯度洗脱grating 光栅gravimetric method 重量法Gutzeit test 古蔡〔检砷〕法half peak width 半峰宽[halide] disk method, wafer method, pellet method 压片法head-space concentrating injector 顶空浓缩进样器heavy metal 重金属heat conductivity 热导率height equivalent to a theoretical plate 理论塔板高度height of an effective plate 有效塔板高度high-performance liquid chromatography (HPLC) 高效液相色谱法high-performance thin-layer chromatography (HPTLC) 高效薄层色谱法hydrate 水合物hydrolysis 水解hydrophilicity 亲水性hydrophobicity 疏水性hydroscopic 吸湿的hydroxyl value 羟值hyperchromic effect 浓色效应hypochromic effect 淡色效应identification 鉴别ignition to constant weight 灼烧至恒重immobile phase 固定相immunoassay 免疫测定impurity 杂质inactivation 失活index 索引indicator 指示剂indicator electrode 指示电极inhibitor 抑制剂injecting septum 进样隔膜胶垫injection valve 进样阀instrumental analysis 仪器分析insulin assay 胰岛素生物检定法integrator 积分仪intercept 截距interface 接口interference filter 干预滤光片intermediate 中间体internal standard substance 内标物质international unit(IU) 国际单位in vitro 体外in vivo 体内iodide 碘化物iodoform reaction 碘仿反响iodometry 碘量法ion-exchange cellulose 离子交换纤维素ion pair chromatography 离子对色谱ion suppression 离子抑制ionic strength 离子强度ion-pairing agent 离子对试剂ionization 电离,离子化ionization region 离子化区irreversible indicator 不可逆指示剂irreversible potential 不可逆电位isoabsorptive point 等吸收点isocratic elution 等溶剂组成洗脱isoelectric point 等电点isoosmotic solution 等渗溶液isotherm 等温线Karl Fischer titration 卡尔·费歇尔滴定kinematic viscosity 运动黏度Kjeldahl method for nitrogen 凯氏定氮法Kober reagent 科伯试剂Kovats retention index 科瓦茨保存指数labelled amount 标示量leading peak 前延峰least square method 最小二乘法leveling effect 均化效应licensed pharmacist 执业药师limit control 限量控制limit of detection(LOD) 检测限limit of quantitation(LOQ) 定量限limit test 〔杂质〕限度〔或限量〕试验limutus amebocyte lysate(LAL) 鲎试验linearity and range 线性及范围linearity scanning 线性扫描liquid chromatograph/mass spectrometer (LC/MS) 液质联用仪litmus paper 石蕊试纸loss on drying 枯燥失重low pressure gradient pump 低压梯度泵luminescence 发光lyophilization 冷冻枯燥main constituent 主成分make-up gas 尾吹气maltol reaction 麦牙酚试验Marquis test 马奎斯试验mass analyzer detector 质量分析检测器mass spectrometric analysis 质谱分析mass spectrum 质谱图mean deviation 平均偏差measuring flask, volumetric flask 量瓶measuring pipet(te) 刻度吸量管medicinal herb 草药melting point 熔点melting range 熔距metabolite 代谢物metastable ion 亚稳离子methyl orange 甲基橙methyl red 甲基红micellar chromatography 胶束色谱法micellar electrokinetic capillary chromatography(MECC, MEKC) 胶束电动毛细管色谱法micelle 胶束microanalysis 微量分析microcrystal 微晶microdialysis 微透析micropacked column 微型填充柱microsome 微粒体microsyringe 微量注射器migration time 迁移时间millipore filtration 微孔过滤minimum fill 最低装量mobile phase 流动相modifier 改性剂,调节剂molecular formula 分子式monitor 检测,监测monochromator 单色器monographs 正文mortar 研钵moving belt interface 传送带接口multidimensional detection 多维检测multiple linear regression 多元线性回归multivariate calibration 多元校正natural product 天然产物Nessler glasses(tube) 奈斯勒比色管Nessler’s reagent 碱性碘化汞钾试液neutralization 中和nitrogen content 总氮量nonaqueous acid-base titration 非水酸碱滴定nonprescription drug, over the counter drugs (OTC drugs) 非处方药nonproprietary name, generic name 非专有名nonspecific impurity 一般杂质non-volatile matter 不挥发物normal phase 正相normalization 归一化法notice 凡例nujol mull method 石蜡糊法octadecylsilane chemically bonded silica 十八烷基硅烷键合硅胶octylsilane 辛〔烷〕基硅烷odorless 无臭official name 法定名official specifications 法定标准official test 法定试验on-column detector 柱上检测器on-column injection 柱头进样on-line degasser 在线脱气设备on the dried basis 按枯燥品计opalescence 乳浊open tubular column 开管色谱柱optical activity 光学活性optical isomerism 旋光异构optical purity 光学纯度optimization function 优化函数organic volatile impurities 有机挥发性杂质orthogonal function spectrophotometry 正交函数分光光度法orthogonal test 正交试验orthophenanthroline 邻二氮菲outlier 可疑数据,逸出值overtones 倍频峰,泛频峰oxidation-reduction titration 氧化复原滴定oxygen flask combustion 氧瓶燃烧packed column 填充柱packing material 色谱柱填料palladium ion colorimetry 钯离子比色法parallel analysis 平行分析parent ion 母离子particulate matter 不溶性微粒partition coefficient 分配系数parts per million (ppm) 百万分之几pattern recognition 模式识别peak symmetry 峰不对称性peak valley 峰谷peak width at half height 半峰宽percent transmittance 透光百分率pH indicator absorbance ratio method? pH指示剂吸光度比值法pharmaceutical analysis 药物分析pharmacopeia 药典pharmacy 药学phenolphthalein 酚酞photodiode array detector(DAD) 光电二极管阵列检测器photometer 光度计pipeclay triangle 泥三角pipet(te) 吸移管,精密量取planar chromatography 平板色谱法plate storage rack 薄层板贮箱polarimeter 旋光计polarimetry 旋光测定法polarity 极性polyacrylamide gel 聚丙酰胺凝胶polydextran gel 葡聚糖凝胶polystyrene gel 聚苯乙烯凝胶polystyrene film 聚苯乙烯薄膜porous polymer beads 高分子多孔小球post-column derivatization 柱后衍生化potentiometer 电位计potentiometric titration 电位滴定法precipitation form 沉淀形式precision 精密度pre-column derivatization 柱前衍生化preparation 制剂prescription drug 处方药pretreatment 预处理primary standard 基准物质principal component analysis 主成分分析programmed temperature gas chromatography 程序升温气相色谱法prototype drug 原型药物provisions for new drug approval 新药审批方法purification 纯化purity 纯度pyrogen 热原pycnometric method 比重瓶法quality control(QC) 质量控制quality evaluation 质量评价quality standard 质量标准quantitative determination 定量测定quantitative analysis 定量分析quasi-molecular ion 准分子离子racemization 消旋化radioimmunoassay 放射免疫分析法random sampling 随机抽样rational use of drug 合理用药readily carbonizable substance 易炭化物reagent sprayer 试剂喷雾器recovery 回收率reference electrode 参比电极refractive index 折光指数related substance 有关物质relative density 相对密度relative intensity 相对强度repeatability 重复性replicate determination 平行测定reproducibility 重现性residual basic hydrolysis method 剩余碱水解法residual liquid junction potential 剩余液接电位residual titration 剩余滴定residue on ignition 炽灼残渣resolution 分辨率,别离度response time 响应时间retention 保存reversed phase chromatography 反相色谱法reverse osmosis 反渗透rider peak 驼峰rinse 清洗,淋洗robustness 可靠性,稳定性routine analysis 常规分析round 修约〔数字〕ruggedness 耐用性safety 平安性Sakaguchi test 坂口试验salt bridge 盐桥salting out 盐析sample applicator 点样器sample application 点样sample on-line pretreatment 试样在线预处理sampling 取样saponification value 皂化值saturated calomel electrode(SCE) 饱和甘汞电极selectivity 选择性separatory funnel 分液漏斗shoulder peak 肩峰signal to noise ratio 信噪比significant difference 显著性差异significant figure 有效数字significant level 显著性水平significant testing 显著性检验silanophilic interaction 亲硅羟基作用silica gel 硅胶silver chloride electrode 氯化银电极similarity 相似性simultaneous equations method 解线性方程组法size exclusion chromatography(SEC) 空间排阻色谱法sodium dodecylsulfate, SDS 十二烷基硫酸钠sodium hexanesulfonate 己烷磺酸钠sodium taurocholate 牛璜胆酸钠sodium tetraphenylborate 四苯硼钠sodium thiosulphate 硫代硫酸钠solid-phase extraction 固相萃取solubility 溶解度solvent front 溶剂前沿solvophobic interaction 疏溶剂作用specific absorbance 吸收系数specification 规格specificity 专属性specific rotation 比旋度specific weight 比重spiked 参加标准的split injection 分流进样splitless injection 无分流进样spray reagent 〔平板色谱中的〕显色剂spreader 铺板机stability 稳定性standard color solution 标准比色液standard deviation 标准差standardization 标定standard operating procedure(SOP) 标准操作规程standard substance 标准品stationary phase coating 固定相涂布starch indicator 淀粉指示剂statistical error 统计误差sterility test 无菌试验stirring bar 搅拌棒stock solution 储藏液stoichiometric point 化学计量点storage 贮藏stray light 杂散光substituent 取代基substrate 底物sulfate 硫酸盐sulphated ash 硫酸盐灰分supercritical fluid chromatography(SFC) 超临界流体色谱法support 载体〔担体〕suspension 悬浊液swelling degree 膨胀度symmetry factor 对称因子syringe pump 注射泵systematic error 系统误差system model 系统模型system suitability 系统适用性tablet 片剂tailing factor 拖尾因子tailing peak 拖尾峰tailing-suppressing reagent 扫尾剂test of hypothesis 假设检验test solution(TS) 试液tetrazolium colorimetry 四氮唑比色法therapeutic drug monitoring(TDM) 治疗药物监测thermal analysis 热分析法thermal conductivity detector 热导检测器thermocouple detector 热电偶检测器thermogravimetric analysis(TGA) 热重分析法thermospray interface 热喷雾接口The United States Pharmacopoeia(USP) 美国药典The Pharmacopoeia of Japan(JP) 日本药局方thin layer chromatography(TLC) 薄层色谱法thiochrome reaction 硫色素反响three-dimensional chromatogram 三维色谱图thymol 百里酚〔麝香草酚〕〔指示剂〕thymolphthalein 百里酚酞〔麝香草酚酞〕〔指示剂〕thymolsulfonphthalein ( thymol blue) 百里酚蓝〔麝香草酚蓝〕〔指示剂〕titer, titre 滴定度time-resolved fluoroimmunoassay 时间分辨荧光免疫法titrant 滴定剂titration error 滴定误差titrimetric analysis 滴定分析法tolerance 容许限toluene distillation method 甲苯蒸馏法toluidine blue 甲苯胺蓝〔指示剂〕total ash 总灰分total quality control(TQC) 全面质量控制traditional drugs 传统药traditional Chinese medicine 中药transfer pipet 移液管turbidance 混浊turbidimetric assay 浊度测定法turbidimetry 比浊法turbidity 浊度ultracentrifugation 超速离心ultrasonic mixer 超生混合器ultraviolet irradiation 紫外线照射undue toxicity 异常毒性uniform design 均匀设计uniformity of dosage units 含量均匀度uniformity of volume 装量均匀性〔装量差异〕uniformity of weight 重量均匀性〔片重差异〕validity 可靠性variance 方差versus …对…,…与…的关系曲线viscosity 粘度volatile oil determination apparatus 挥发油测定器volatilization 挥发法volumetric analysis 容量分析volumetric solution(VS) 滴定液vortex mixer 涡旋混合器watch glass 外表皿wave length 波长wave number 波数weighing bottle 称量瓶weighing form 称量形式weights 砝码well-closed container 密闭容器xylene cyanol blue FF 二甲苯蓝FF〔指示剂〕xylenol orange 二甲酚橙〔指示剂〕zigzag scanning 锯齿扫描zone electrophoresis 区带电泳zwitterions 两性离子zymolysis 酶解作用簡體書目錄Chapter 1 Introduction 緒論1.1 The nature of analytical chemistry 分析化學的性質1.2 The role of analytical chemistry 分析化學的作用1.3 The classification of analytical chemistry分析化學的分類1.4 The total analytical process分析全過程Terms to understand重點內容概述Chapter 2 Errors and Data Treatment in Quantitative Analysis 定量分析中的誤差及數據處理2.1 Fundamental terms of errors誤差的根本術語2.2 Types of errors in experimental data實驗數據中的誤差類型2.2.1 Systematic errors 系統誤差2.2.2 Random errors偶然誤差2.3 Evaluation of analytical data分析數據的評價2.3.1 Tests of significance顯著性檢驗2.3.2 Rejecting data可疑值取捨2.4 Significant figures有效數字ProblemsTerms to understand重點內容概述Chapter 3 Titrimetric Analysis滴定分析法3.1 General principles根本原理3.1.1 Relevant terms of titrimetric analysis滴定分析相關術語3.1.2 The preparation of standard solution and the expression of concentration 標準溶液的配製與濃度表示方法3.1.3 The types of titrimetric reactions滴定反應類型3.2 Acid-base titration酸鹼滴定3.2.1 Acid-base equilibria 酸鹼平衡3.2.2 Titration curves滴定曲線3.2.3 Acid-base indicators酸鹼指示劑3.2.4 Applications of acid-base titration酸鹼滴定的應用3.3 Complexometric titration配位滴定3.3.1 Metal-chelate complexes金屬螯合物3.3.2 EDTA 乙二胺四乙酸3.3.3 EDTA titration curves EDTA滴定曲線3.3.4 Metal Ion indicators金屬離子指示劑3.3.5 Applications of EDTA titration techniques EDTA滴定方法的應用3.4 Oxidation-reduction titration氧化還原滴定3.4.1 Redox reactions氧化還原反應3.4.2 Rate of redox reactions氧化還原反應的速率3.4.3 Titration curves滴定曲線3.4.4 Redox indicators氧化還原指示劑3.4.5 Applications of redox titrations氧化還原滴定的應用3.5 Precipitation titration沉澱滴定3.5.1 Precipitation reactions沉澱滴定反應3.5.2 Titration curves滴定曲線3.5.3 End-point detection終點檢測ProblemsTerms to understand重點內容概述Chapter 4 Potentiometry 電位分析法4.1 Introduction簡介4.1.1 Classes and characteristics分類及性質4.1.2 Definition定義4.2 Types of potentiometric electrodes電極種類4.2.1 Reference electrodes 參比電極4.2.2 Indicator electrodes指示電極4.2.3 Electrode response and selectivity電極響應及選擇性4.3 Potentiometric methods and application電位法及應用4.3.1 Direct potentiometric measurement 直接電位法4.3.2 Potentiometric titrations電位滴定4.3.3 Applications of potentiometry 電位法應用ProblemsTerlns to understand重點內容概述Chapter 5 Chromatography色譜法5.1 An introduction to chromatographic methods色譜法概述5.2 Fundamental theory of gas chromatography氣相色譜根本原理5.2.1 Plate theory塔板理論5.2.2 Kinetic theory(rate theory) 速率理論5.2.3 The resolution Rs as a measure of peak separation 分離度5.3 Gas chromatography 氣相色譜5.3.1 Components of a gas chromatograph 氣相色譜儀的組成5.3.2 Stationary phases for gas-liquid chromatography 氣液色譜固定相5.3.3 Applications of gas-liquid chromatography 氣液色譜的應用5.3.4 Adsorption chromatography 吸附色譜5.4 High performance liquid chromatography 高效液相色譜5.4.1 Instrumentation 儀器組成5.4.2 High-performance partition chromatography 高效分配色譜5.5 Miscellaneous separation methods 其他分離方法5.5.1 High-performance ion-exchange chromatography 高效離子交換色譜5.5.2 Capillary electrophoresis 毛細管電泳5.5.3 Planar chromatography 平板色譜ProblemsTerms to understand重點內容概述Chapter 6 Atomic Absorption Spectrometry原子吸收光譜分析法6.1 Introduction 概述6.2 Principles 原理.6.2.1 The process of AAS,resonance line and absorption line 原子吸收光譜法的過程,共振線及吸收線6.2.2 The number of ground atom and the temperature of flame 基態原子數與光焰溫度6.2.3 Quantitative analysis of AAS原子吸收光譜定量分析6.3 Instrumentation 儀器6.3.1 Primary radiation sources 光源6.3.2 Atomizer 原子儀器6.3.3 Optical dispersive systems 分光系統6.3.4 Detectors 檢測器6.3.5 Signal measurements 信號測量6.4 Quantitative measurements and interferences 定量測定及干擾6.4.1 Quantitative measurements 定量測定6.4.2 Interferences 干擾6.4.3 Sensitivity6.5 Applications of AAS原子吸收光譜法的應用ProblemsTerms to understand重點內容概述Chapter 7 Ultraviolet and Visible Spectrophotometry 紫外-可見分光光度法7.1 Introduction簡介7.2 Ultraviolet and visible absorption spectroscopy 紫外-可見吸收光譜7.2.1 Introduction for radiant energy 輻射能簡介7.2.2 Selective absorption of radiation and absorbance spectrum 物質對光的選擇性吸收和吸收光譜7.2.3 Absorbing species and electron transition 吸收物質與電子躍遷7.3 Law of absorption吸收定律7.3.1 Lambert-Beer's law朗伯-比爾定律7.3.2 Absorptivity吸光係數7.3.3 Apparent deviations from Beer's law對比爾定律的明顯偏離7.4 Instruments儀器7.5 General types of spectrophotometer分光光度計種類7.6 Application of UV-Vis absorption spectroscopy 紫外-可見吸收光譜的應用7.6.1 Application of absorption measurement to qualitative analysis 光吸收測定在定性分析上的應用7.6.2 Quantitative analysis by absorption measurements 光吸收測量定量分析法7.6.3 Derivative spectrophotometry 導數分光光度法ProblemsTerms to understand重點內容概述Chapter 8 Infrared Absorption Spectroscopy紅外吸收光譜8.1 Theory of infrared absorption紅外吸收根本原理8.1.1 Dipole changes during vibrations and rotations 振轉運動中的偶極距變化8.1.2 Mechanical model of stretching vibrations 伸縮振動機械模型8.1.3 Quantum treatment of vibrations 振動的量子力學處理、8.1.4 Types of molecular vibrations分子振動形式8.2 Infrared instrument components紅外儀器組成8.2.1 Wavelength selection波長選擇8.2.2 Sampling techniques 採樣技術8.2.3 Infrared spectrophotometers for qualitative analysis 定性分析用紅外分光光度計8.2.4 Other techniques其他技術8.3 The group frequencies of functional groups in organiccompounds 有機化合物官能團的特徵頻率8.4 The factors affecting group frequencies 影響基團特徵吸收頻率的因素8.4.1 Adjacent groups 鄰近基團的影響8.4.2 Hydrogen bonding 氫鍵8.5 Qualitative applications to structural analysis 結構分析的定性應用ProblemsTerms to understand重點內容概述Chapter 9 Nuclear Magnetic Resonance Spectroscopy 核磁共振波譜法9.1 Theory of nuclear magnetic resonance 核磁共振理論9.1.1 Quantum description of NMR NMR 的量子描述9.1.2 Classical description of NMR NMR 的經典描述9.2 Experimental methods of NMR spectroscopy NMR波譜的實驗方法9.3 The chemical shift of protons in organic compounds 有機化合物中質子的化學位移9.3.1 Souroe of the chemical shift化學位移產生原9.3.3 Environmental effects on the chemical shift of protonNMR spectra 影響NMR波譜中質子化學位移的環境因素9.4 Spin-Spin coupling 自旋-自旋耦合9.4.1 Source of Spin-Spin coupling and splitting 自旋-自旋耦合與裂分的產生原因9.4.2 Coupling constant耦合常數9.4.3 Rule8 governing the interpretation of spectra光譜解析規則9.5 Qualitative applications of proton NMR質子NMR波譜的定性應用.。
化学化工专业英语(课本内容)

化学化工专业英语(课本内容)第二章科技英语构词法词是构成句子的要素,对词意理解的好坏直接关系到翻译的质量。
所谓构词法即词的构成方法,即词在结构上的规律。
科技英语构词特点是外来语多(很多来自希腊语和拉丁语);第二个特点是构词方法多,除了非科技英语中常用的三种构词法—转化、派生及合成法外,还普遍采用压缩法、混成法、符号法和字母象形法。
2.1转化法(Conversion)由一种词类转化成另一种词类,叫转化法。
例如:water(n.水)→water(v.浇水)charge(n.电荷) →charge(v.充电)yield(n.产率) →yield(v.生成)dry(a.干的) →dry(v.烘干)slow(a.慢的) →slow(v.减慢)back(ad.在后、向后) →back(v.使后退、倒车)square(n.正方形) →square(a.正方形的)2.2派生法(Derivation)通过加前、后缀构成一新词。
派生法是化工类科技英语中最常用的构词法。
例如“烷烃”就是用前缀(如拉丁或希腊前缀)表示分子中碳原子数再加上“-ane”作词尾构成的。
若将词尾变成“-ane”、“-yne”、“-ol”、“-al”、“-yl”,则分别表示“烯”、“炔”、“醇”、“醛”、“基”、等。
依此类推,从而构成千成种化学物质名词。
常遇到这样的情况,许多化学化工名词在字典上查不到,全若掌握这种构词法,能过其前、后缀分别代表的意思,合在一起即是该词的意义。
下面通过表1举例说明。
需要注意的是,表中物质的数目词头除前四个另有名称外,其它均为表上的数目词头。
本书附录为化学化工专业常用词根及前后缀。
此外还可参阅《英汉化学化工词汇》(第三版)附录中的“英汉对照有机基名表”、“西文化学名词中常用的数止词头”及“英汉对照有机词尾表”。
据估计,知道一个前缀可帮助人们认识450个英语单词。
一名科技工作者至少要知道近50个前缀和30个后缀。
这对扩大科技词汇量,增强自由阅读能力,提高翻译质量和加快翻译速度都是大有裨益的。
化学化工专业英语电子版课本

化学化工专业英语电子版课本————————————————————————————————作者:————————————————————————————————日期:ContentPART 1 Introduction to Materials Science &Engineering 1 Unit 1 Materials Science and Engineering 1 Unit 2 Classification of Materials 9 Unit 3 Properties of Materials 17 Unit 4 Materials Science and Engineering: What does the Future Hold? 25 Part ⅡMETALLIC MATERLALS AND ALLOYS 33 Unit 5 An Introduction to Metallic Materials 33 Unit 6 Metal Manufacturing Methods 47 Unit 7 Structure of Metallic Materials 57 Unit 8 Metal-Matrix Composites 68 PartⅢCeramics 81 Unit 9 Introduction to Ceramics 81 Unit 10 Ceramic Structures —Crystalline and Noncrystalline 88 Unit 11 Ceramic Processing Methods 97 Unit 12 Advanced ceramic materials –Functional Ceramics 105 PARTⅣNANOMATERIALS 112 Unit 13 Introduction to Nanostructured Materials 112 Unit14 Preparation of Nanomaterials 117 Unit 15 Recent Scientific Advances 126 Unit 16 The Future of Nanostructure Science and Technology 130 Part ⅤPOLYMERS 136Unit17 A Brief Review in the Development of Synthetic Polymers 136 Unit18 Polymer synthesis: Polyethylene synthesis 146 Unit19 Polymer synthesis:Nylon synthesis 154 Unit 20 Processing and Properties Polymer Materials 165 PART VI POLYMERIC COMPOSITES 172 Unit21 Introduction to Polymeric Composite Materials 172Unit22 Composition, Structure and Morphology of Polymeric Composites 178 Unit23 Manufacture of Polymer Composites 185 Unit24 Epoxy Resin Composites 191 Part 7 Biomaterial 196 Unit 25 Introduction to Biomaterials 196 Unit 26 Biocompatibility 205 Unit 27 Polymers as Biomaterials 213 Unit 28 Future of Biomaterials 224 PARTⅧMaterials and Environment 237 Unit29 Environmental Pollution & Control Related Materials 237 Unit30 Bio-degradable Polymer Materials 241 Unit 31 Environmental Friendly Inorganic Materials 248 Unit 32 A Perspective on the Future: Challenges and Opportunities 256 附录一科技英语构词法263 附录二科技英语语法及翻译简介269 附录三:聚合物英缩写、全名、中文名对照表280 附录四:练习题参考答案284PART 1 Introduction to Materials Science &EngineeringUnit 1Materials Science and Engineering Historical PerspectiveMaterials are probably more deep-seated in our culture than most of us realize. Transportation, housing, clothing, communication, recreation, and food production —virtually every segment of our everyday lives is influenced to one degree or another by materials. Historically, the development and advancement of societies have been intimately tied to the members’ ability to produce and manipulate materi- als to fill their needs. In fact, early civilizations have been designated by the level of their materials development (Stone Age, Bronze Age, Iron Age).The earliest humans had access to only a very limited number of materials, those that occur naturally: stone, wood, clay, skins, and so on. With time they discovered techniques for producing materials that had properties superior to those of the natural ones; these new materials included pottery and various metals. Furthermore, it was discovered that the properties of a material could be altered by heat treatments and by the addition of other substances. At this point, materials utilization was totally a selection process that involved deciding from a given, rather limited set of materials the one best suited for an application by virtue of its characteristics.①It was not until relatively recent times that scientists came to understand the relationships between the structural elements of materials and their properties. This knowledge, acquired over approximately the past 100 years, has empowered them to fashion, to a large degree, the characteristics of materials. Thus, tens of thousands of different materials have evolved with rather specialized charac- teristics that meet the needs of our modern and complex society; these include metals, plastics, glasses, and fibers. deep-seated根深蒂固的, 深层的pottery / ☐♦☯❒♓/ ⏹ 陶器structural elements结构成分;property / ☐❒☐☜♦♓/⏹.性能The development of many technologies that make our existence so comfortable has been intimately associated with the accessibility of suitable materials. An advancement in the understanding of a material type is often the forerunner to the stepwise progression of a technology. For example, automobiles would not havebeen possibl- e without the availability of inexpensive steel or some other comparable substitute. In our contemporary era, sophisticated electronic devices rely on components that are made from what are called semiconducting materials. Materials Science and EngineeringThe discipline of materials science involves investigating the relationships that exist between the structures and properties of materials. In contrast, materials engineering is, on the basis of these structure–property correlations, designing or engineering the structure of a material to produce a predetermined set of properties.“Structure’’ is at this point a nebulous term that deserves some explanation. In brief, the structure of a material usually relates to the arrangement of its internal components. Subatomic structure involves electrons within the individual atoms and interactions with their nuclei. On an atomic level, structure encompasses the organization of atoms or molecules relative to one another. The next larger structural realm, which contains large groups of atoms that are normally agglomerated together, is termed ‘‘microscopic,’’ meaning that which is subject to direct observation using some type of microscope. Finally, structural elements that may be vie wed with the naked eye are termed ‘‘macroscopic.’’The notion of ‘‘property’’ deserves elaboration. While in service use, all materials are exposed to external stimuli that evoke some type of response. For example, a specimen subjected to forces will experience deformation; or a polished metal surface will reflect light. Property is a material trait in terms of the kind and magnitude of response to a specific imposed stimulus. Generally, definitions of properties are made independent of material shape and size.Virtually all important properties of solid materials may be grouped into six different categories: mechanical, electrical, thermal, magnetic, optical, and stepwise / ♦♦♏☐♦♋♓/ ♎逐步的sophisticated/♦☯♐♓♦♦♓ ♏♓♦♓♎/ ♎精制的,复杂的;semiconducting materials 半导体材料nebulous/ ⏹♏♌✞●☯♦/♎ 含糊的,有歧义的subatomic/ ♦✈♌☯♦❍/♎ 亚原子的microscopic/❍♓❒☯♦☐♓/ ♎微观的❍♋♍❒☐♦♍☐☐♓♍/❍✌ ❒☯✞♦☐♓/♎宏观的deteriorative. For each there is a characteristic type of stimulus capable of provokingdifferent responses. Mechanical properties relate deformation to an applied load or force; examples include elastic modulus and strength. For electrical properties, such as electrical conductivity and dielectric constant, the stimulus is an electric field. The thermal behavior of solids can be represented in terms of heat capacity and thermal conductivity. Magnetic properties demonstrate the response of a material to the application of a magnetic field. For optical properties, the stimulus is electro- magnetic or light radiation; index of refraction and reflectivity are representative optical properties. Finally, deteriorative characteristics indicate the chemical reactivity of materials.In addition to structure and properties, two other important components are involved in the science and engineering of materials, viz. ‘‘processing’’ and ‘‘performance.’’ With regard to the relationships of these four components, the structure of a material will depend on how it is processed. Furthermore, a material’s performance will be a function of its properties.Fig. 1.1 Photograph showing the light transmittance of three aluminum oxide specimens. From left to right: single crystal material (sapphire), which is transparent;a polycrystalline and fully dense (nonporous) material, which is translucent; and a polycrystalline material that contains approximately 5% porosity, which is opaque. (Specimen preparation, P. A. Lessing; photography by J. Telford.)We now present an example of these processing-structure-properties-perfor- mance principles with Figure 1.1, a photograph showing three thin disk specimens placed over some printed matter. It is obvious that the optical properties (i.e., the deformation/ ♎♓♐ ❍♏♓☞☯⏹/ ⏹变形deteriorative/♎♓♦♓☯❒♓☯❒♏♓♦♓❖/ ⏹破坏(老化的)elastic modulus 弹性模量strength /♦♦❒♏⏹♑/ ⏹强度;dielectric constant介电常数;heat capacity 热容量refraction/❒♓♐❒✌☞☯⏹/ ⏹折射率;reflectivity/ ❒♓♐●♏ ♦♓❖♓♦♓/ ⏹反射率processing/☐❒☯◆♦♏♦♓☠/ ⏹加工light transmittance) of each of the three materials are different; the one on the left is transparent (i.e., virtually all of the reflected light passes through it), whereas the disks in the center and on the right are, respectively, translucent and opaque.All of these specimens are of the same material, aluminum oxide, but the leftmost one is what we call a single crystal—that is, it is highly perfect—which gives rise to its transparency. The center one is composed of numerous and verysmall single crystals that are all connected; the boundaries between these small crystals scatter a portion of the light reflected from the printed page, which makes this material optically translucent.②And finally, the specimen on the right is composed not only of many small, interconnected crystals, but also of a large number of very small pores or void spaces. These pores also effectively scatter the reflected light and render this material opaque.Thus, the structures of these three specimens are different in terms of crystal boundaries and pores, which affect the optical transmittance properties. Furthermore, each material was produced using a different processing technique. And, of course, if optical transmittance is an important parameter relative to the ultimate in-service application, the performance of each material will be different.Why Study Materials science and Engineering?Why do we study materials? Many an applied scientist or engineer, whether mechanical, civil, chemical, or electrical, will at one time or another be exposed to a design problem involving materials. Examples might include a transmission gear, the superstructure for a building, an oil refinery component, or an integrated circuit chip. Of course, materials scientists and engineers are specialists who are totally involved in the investigation and design of materials.Many times, a materials problem is one of selecting the right material from the many thousands that are available. There are several criteria on which the final decision is normally based. First of all, the in-service conditions must be charac- terized, for these will dictate the properties required of the material. On only rare occasions does a material possess the maximum or ideal combination of properties. transmittance/♦❒✌⏹❍♓♦☜⏹♦/ ⏹. 透射性sapphire /♦✌♐♓☯/ ⏹蓝宝石transparent/♦❒✌⏹♦☐☪☯❒☯⏹♦/ ♎透明的;polycrystalline/ ☐●♓❒♓♦♦☯●♓⏹/ ⏹多晶体;translucent/♦❒✌⏹●✞♦⏹♦/♎ 半透明的;opaque☯✞☐♏♓♎不透明的single crystal 单晶体Thus, it may be necessary to trade off one characteristic for another. The classic example involves strength and ductility; normally, a material having a high strength will have only a limited ductility. In such cases a reasonable compromise between two or more properties may be necessary.A second selection consideration is any deterioration of material properties that may occur during service operation. For example, significant reductions in mecha- nical strength may result from exposure to elevated temperatures or corrosive envir- onments.Finally, probably the overriding consideration is that of economics: What will the finished product cost? A material may be found that has the ideal set of proper- ties but is prohibitively expensive. Here again, some compromise is inevitable.The cost of a finished piece also includes any expense incurred during fabrication to produce the desired shape. The more familiar an engineer or scientist is with the various characteristics and structure–property relationships, as well as processing techniques of materials, the more proficient and confident he or she will be to make judicious materials choices based on these criteria.③Reference:William D. Callister,Materials science and engineering :anintroduction, Press:JohnWiley & Sons, Inc.,2007;2-5 transmission gear传动齿轮dictate/♎♓♦♏♓♦/ ❖ 决定trade off 权衡;折衷ductility♎✈♦♓●♓♦♓⏹延展性overriding/ ☯✞❖☯❒♋♓♎♓☠/♎最主要的judicious/♎✞✞♎♓☞☯♦/♎明智的Notes1.At this point, materials utilization was totally a selection process that involved deciding froma given, rather limited set of materials the one best suited for an application by virtue of itscharacteristics由此看来,材料的使用完全就是一个选择过程,且此过程又是根据材料的性质从许多的而不是非有限的材料中选择一种最适于某种用途的材料。
大学化学专业英语Lesson 3_organic

1. The parent chain is numbered so that the multiple bonds have the lowest numbers (double and triple bonds have priority over alkyl and halo substituents).
3. When both double and triple bonds are present, the -en suffix follows the parent chain directly and the -yne suffix follows the -en suffix (notice that the e is left off, -en instead of -ene). The location of the double bond(s) is(are) indicated before the parent name as before, and the location of the triple bond(s) is(are) indicated between the -en and -yne suffixes. See below for examples.
CH3 CH H2C CH2
methylcyclopropane
CH3
CH3
CH3 CH CH2 CH2 CH CH2 CH CH2 CH3
CH CH3
CH2 CH3
5-sec-butyl-2,7-dimethylnonane
CH3 CH2 CH CH CH2 CH3 CH3 CH2 CH3
(完整版)化学专业英语
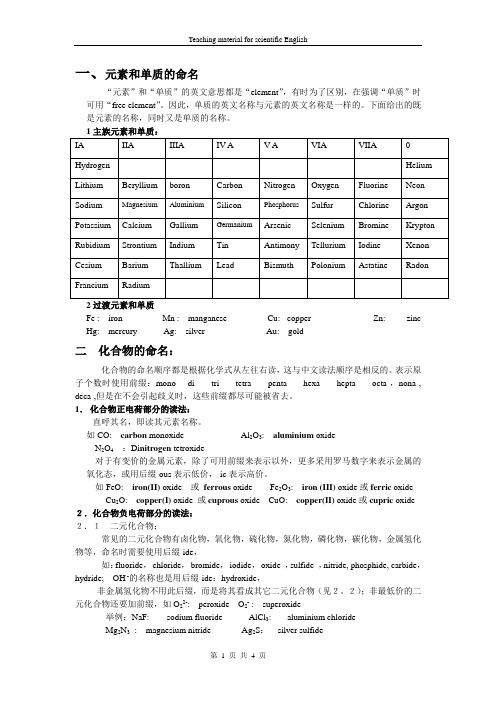
一、元素和单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold二化合物的命名:化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxideN2O4:Di nitrogen tetroxide对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:2.1二元化合物:常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。
《化学工程与工艺专业英语》课文翻译-Unit 3 Typical Activities of Che

《化学工程与工艺专业英语》课文翻译-Unit3 Typical Activities of ChemicalEngineers本文将介绍化学工程师在日常工作中的一些典型活动。
化学工程师旨在将化学与工程学的原理应用于产业中,解决生产过程中的各种问题并开发新产品。
化学工程师随着技术的发展和市场的变化而发展和变化。
但以下是其典型活动:第一,设计新的工艺流程。
化学工程师需要了解化学反应原理、物质转化和热量传递方程式,以及流体力学和系统分析等方面的知识,创造出新的工艺流程。
在此基础上,他们可以设计出化学反应器、分离装置和加工设备等。
而在设计之前,化学工程师会根据产品需求和工厂生产线等因素,制定经济可行性和技术可行性分析,制定整个流程方案。
第二,工厂的设计和规划。
这些安装要求使用化学工程师的技能和知识。
工程师需要考虑由工厂所需的供电、供水和废物处理等各个方面。
他们需要选择合适的材料和设备,也需要在设计中采用节能和环境保护技术。
他们也必须为紧密的安全要求和法律法规做好准备。
第三,处理产品的生产和质量控制。
生产线上的任何错误都有可能导致生产过程劣化和生产失败。
化学工程师通过控制生产过程的不同参数和调整生产中的机器设备、材料流程和成品质量保障等,确保产品的质量和产品的性能。
如果有任何生产上的问题,他们都需要快速响应,并及时寻找解决方法。
第四,进行研究和开发。
在工业生产中,研究和开发是至关重要的。
化学工程师必须熟悉当前和未来的技术发展。
他们需要收集和分析大量的数据和材料,以探索和开发新的技术和产品,面对多种工艺流程,为生产线输入新的元素。
第五,进行销售和市场分析。
销售和市场分析也是化学工程师需要了解的另一个方面。
他们需要熟悉市场需求和市场潜力。
工程师必须在市场竞争激烈的环境中发挥创新、争取同行的业务和合作伙伴,为他们的产品寻找最佳销售渠道。
第六,维护和管理设备。
对于工厂设备的维护和管理是保证生产线平稳运转的必要条件。
化学化工专业英语免费课件

micro- 微 micrometer侧微计; microampere微安培;microbe微生物
poly- 多聚
polymer聚合物;
polyfunctional多功能的;polyacid多酸的
4.表示方位的前缀
par(a)- 对位
Paradichlorobenzene对二氯苯
ortho- 邻位
4.名词+动名词(n.+v.ing)
paper-making 造纸 ship-building 造船 Machine-shaping ?
5.其他构成方式
By-product 副产品(介词+名词) Make-up 化妆品(动词+副词) Out-of-door 户外 (副词+介词+名词) Pick-me-up 兴奋剂 (动词+代词+副词)
➢ Rust resistance = ability to resist rust 防锈 (表示动作对象)
➢ Water vapor = vapor of water 水蒸气 (表示所属关系)
➢ Pollution problem = problem concerning pollution 污染问题(表示有关方面)
propane butane
propyl butyl
pentane pentyl
propene butene
propyne propanol propyl aldehyde butyne butanol butyl aldehyde
pentene pentyne pentanol
pentanal
six hex(a)- hexane hexyl hexene hexyne hexanol
(完整版)化学专业英语

(完整版)化学专业英语一、基础词汇篇1. 原子与分子Atom(原子):物质的基本单位,由质子、中子和电子组成。
2. 化学反应Reactant(反应物):参与化学反应的物质。
Product(物):化学反应后的物质。
Catalyst(催化剂):能改变化学反应速率而本身不发生永久变化的物质。
3. 物质状态Solid(固体):具有一定形状和体积的物质。
Liquid(液体):具有一定体积,无固定形状的物质。
Gas(气体):无固定形状和体积的物质。
4. 酸碱盐Acid(酸):在水溶液中能电离出氢离子的物质。
Base(碱):在水溶液中能电离出氢氧根离子的物质。
Salt(盐):由酸的阴离子和碱的阳离子组成的化合物。
5. 溶液与浓度Solution(溶液):由溶剂和溶质组成的均匀混合物。
Solvent(溶剂):能溶解其他物质的物质。
Solute(溶质):被溶解的物质。
Concentration(浓度):溶液中溶质含量的度量。
二、专业术语篇1. 有机化学Organic Chemistry(有机化学):研究碳化合物及其衍生物的化学分支。
Functional Group(官能团):决定有机化合物化学性质的原子或原子团。
Polymer(聚合物):由许多重复单元组成的大分子化合物。
2. 无机化学Inorganic Chemistry(无机化学):研究不含碳的化合物及其性质的化学分支。
Crystal(晶体):具有规则排列的原子、离子或分子的固体。
OxidationReduction Reaction(氧化还原反应):涉及电子转移的化学反应。
3. 物理化学Physical Chemistry(物理化学):研究化学现象与物理现象之间关系的化学分支。
Chemical Bond(化学键):原子间相互作用力,使原子结合成分子。
Thermodynamics(热力学):研究能量转换和物质性质的科学。
4. 分析化学Analytical Chemistry(分析化学):研究物质的组成、结构和性质的科学。
《化学工程与工艺专业英语》课文翻译-Unit 3 Typical Activities of Chemical Engineers

Unit 3 Typical Activities of Chemical Engineers化学工程师的例行工作The classical role of the chemical engineer is to take the discoveries made by the chemist in the laboratory and develop them into money--making, commercial-scale chemical processes. The chemist works in test tubes and Parr bombs with very small quantities of reactants and products (e.g., 100 ml), usually running “batch”, constant-temperature experiments. Reactants are placed in a small container in a constant temperature bath. A catalyst is added and the reactions proceed with time. Samples are taken at appropriate intervals to follow the consumption of the reactants and the production of products as time progresses.化学工程师经典的角色是把化学家在实验室里的发现拿来并发展成为能赚钱的、商业规模的化学过程。
化学家用少量的反应物在试管和派式氧弹中反应相应得到少量的生成物,所进行的通常是间歇性的恒温下的实验,反应物放在很小的置于恒温水槽的容器中,加点催化剂,反应继续进行,随时间推移,反应物被消耗,并有生成物产生,产物在合适的间歇时间获得。
石油化学化工专业英语第三单元翻译

Part 2 Unit 1. 元素、化合物和物质元素是用一般的化学方法无法使之分解为更简单物质的纯物质。
总的来说, 到2008年, 已经发现的元素有117种, 其中94种是地球上天然存在的元素。
人们熟悉的一些常见元素有碳、氢、氧、氮、磷、钾、硫、铝、铁、铜和金。
这些元素是物质组成的基本单元, 就像数字但是由0 - 9组成一样。
众所周知, 整个宇宙也同样是由这些已经在地球上发现的元素所构成。
这些自然界中存在的元素和其他元素结合在一起形成了矿物质、植物或水和二氧化碳等物质。
铜、银、金和其它20多种元素可以以高纯度形式存在于自然界中。
还有16种元素是自然界中没有发现的,这些元素是在核爆炸和核研究中所产生的少量物质。
因而,他们都是人造元素。
由两个或两个以上元素组成的纯物质称为化合物。
因为化合物含有两种或更多的元素, 因而其与元素的性质不同, 他们可以通过化学变化分解成更简单的物质。
化合物经化学分解最终产生的物质正是组成化合物的元素。
组成化合物的元素的原子以整数比例,而非以分数比,结合在一起。
(组成化合物的元素各原子间以整数比例而非分数比结合在一起)原子间相互结合形成的化合物以分子或离子状态存在。
一个化合物的分子是由两个或两个以上的原子组成的,化合物的分子体积很小,而且不带电(呈电中性).如果我们把一滴水细分成越来越小的微粒, 我们最终可以得到一个水的单体,即一个水分子。
这个水分子是由两个氢原子和一个氧原子键合在一起。
人们通常无法进一步分解分子单元, 只有破坏分子结构才能将其分解成所组成的元素。
因此, 水分子是水这种化合物的最小单元。
离子是带正电的或负电的原子或元素簇。
一个化合物中的离子依靠它们正电和负电基团间的相互引力结合在一起, 形成晶体结构。
由离子组成的化合物中不存在分子。
氯化钠是一种典型的非分子结构的化合物。
这类化合物包含大量的正、负离子, 其分子式如分子结构的化合物那样,通常采用组成化合物的原子间以简单比例式方式来表达。
化学化工专业英语-references

大致说来,理解阶段包括一下几个方面:
1.领略全文大意
通读一遍,不同的词,词组甚至是句子在不 同的语境中可能有不同的意思。任何一篇文章或 一段文字都是一个有机整体,词与词,词与句子, 句子与段落甚至整个篇章之间,都有着必然的内 在联系。这就要求译者在动手翻译之前,务必通 读全文,领略大意,切忌一开始就把注意力集中 在一词一词的推敲上,看一句译一句。
第一 注意表达的规范性
科技英语语体讲究论证的逻辑性,语言要规 范。我们翻译的是科技文章,自然就必须用符合 科技语体规范的汉语来表达。 例如:
The rotation of the earth on its own axis causes the change from day to night. 地球绕轴自转,造成昼夜的更替。
3.广泛使用缩写词,并且缩写词的词义专 一,使用频率高。
4.前后缀出现频率高
英语的构词法主要有:合成、转化和派生, 其中派生法的核心是依靠添加前缀或后缀来构成新 词,这就导致了前后缀使用频率高。
例如:
bio-
biochemistry; biotechnology; biocatalyst; biodegradable能生物降解的; bioengineering, etc.
表达的好坏取决于理解原文的确切程度和对 汉语的掌握程度。如果译文仅仅是意思对,但不 能用通顺流畅的汉语表达,仍不是一篇好译文。
例句:
The homologs of benzene are those containing an alkyl group or alkyl groups in place of one or more hydrogen atoms.
该句话易于理解,但却难于表达。若译作:苯的 同系物就是那些被一个或多个烷基取代一个或多 个氢原子所形成的产物。则该译文尽管意思差不 多,但令人感到啰唆费解。
化学专业基础英语教案

化学专业基础英语教案第一章:Introduction to Chemical Bonding1.1 Types of Chemical Bonds1.2 Ionic Bonding1.3 Covalent Bonding1.4 Metallic Bonding1.5 Polarization and Hydrogen Bonding第二章:Atoms and Molecules2.1 Atomic Structure2.2 Elements and Periodic Table2.3 Molecular Structure2.4 Chemical Formulas and Stoichiometry2.5 Isomers第三章:Reactions and Equilibria3.1 Chemical Reactions3.2 Balancing Chemical Equations3.3 Rate Laws and Reaction Mechanisms3.4 Equilibrium Constants and Le Chatelier's Principle 3.5 Acids and Bases第四章:Chemical Thermodynamics4.1 Thermodynamic Laws4.2 Enthalpy and Energy Changes4.3 Entropy and Randomness4.4 Free Energy and Reaction Favorability4.5 Thermochemical Equations第五章:Chemical Kinetics5.1 Reaction Rates5.2 Rate Laws5.3 Integrated Rate Laws5.4 Reaction Mechanisms5.5 Catalysis第六章:Chemical Instrumentation6.1 Types of Chemical Analyzers6.2 Spectroscopy6.3 Chromatography6.4 Thermogravimetric Analysis (TGA)6.5 X-ray Diffraction (XRD)第七章:Chemical Reactions and Equipotential Surfaces 7.1 Activation Energy and Transition State7.2 Equipotential Surfaces and reaction Coordinate 7.3 Transition State Theory7.4 Catalysis and Activation Energy7.5 Reaction机理and Mechanics第八章:Thermodynamics of Reactions8.1 Enthalpy Changes in Reactions8.2 Entropy Changes in Reactions8.3 Free Energy Changes in Reactions8.4 Equilibrium Constants and Reaction favorability8.5 Phase Transitions and Thermodynamics第九章:Electrochemistry9.1 Redox Reactions9.2 Electrochemical Series9.3 Galvanic Cells and电池9.4 Electrolysis9.5 Corrosion and Electrochemical Protection第十章:Chemistry of the Elements10.1 Periodic Table and Block Classification10.2 s-block Elements10.3 d-block Elements10.4 p-block Elements10.5 f-block Elements10.6 Transition Metals and Their Compounds这些后续的章节涵盖了化学领域的其他重要主题,如仪器分析、化学反应动力学、热力学反应、电化学和元素化学等。
化学专业英语3

30
⑧被动意义译成主动意思
In the reaction both the acid and the base are neutralized forming water and salt.
反应中,酸与碱被中和而形成水和盐。
反应中,酸与碱中和而形成水和盐。
七、被动语态的译法法 31
The kinds of activities which the organic chemist engage may be grouped in the following way. 有机化学工作者所从事的活动可以按照下列方法 归类。
七、被动语态的译法法 27
Hydrochloric acid (HCl) was added to the solution to enhance acidity. 将盐酸加到溶液中以增强酸性。
七、被动语态的译法法
28
⑦将被动语态译成“是……的 、对…进行”
• The solution is filtered to recover the precipitation.
….be done as….格式 翻译时加上“被”等
It is known as… 被称为、叫做…… It is spoken of as… 被说成是…… It is considered as… 被认为是……;被看为…… ----经常使用
如果原子的一个或多个电子被除去,那么该原子 就被说成是带正电荷。
如果原子失去了一个或多个电子,我们就说这个 原子带正电荷。
七、被动语态的译法法
22
④用英语句中的动作者作汉语 中的主语
• The complicated problem will be solved by them. 这个复杂的问题会被他们解决。 他们将会/会解决这个复杂的问题。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
(1)When compounds containing nitrates are heated,they not readily release all of the oxygen atoms in the compound. They decompose to give a compound that is a nitrite along with oxygen gas.
8
二、歧化反应句型
常用词汇是disproportionation 和disproportionate
例如:
(1)Mn3+ is unstable;its disproportionation is spontaneous. (2)Manganate ion is also unstable in acidic solution;its disproportionation gives permanganate and manganese (II)ion.
9
三、中和反应句型 常用词汇有名词neutralization和动词neutralize, 例如: (1)CaCl2 is a salt formed during the neutralization reaction of hydrochloric acid with calcium hydroxide. (2)A salt and water are formed when sulfuric acid neutralizes sodium hydroxide. (3) A neutralization reaction involves the combination of hydrogen ions and hydroxide ions to form water.
5
Oxi• • • • • • • Oxygen:n. 氧气 Oxide: n. 氧化物 Oxidation: n.氧化作用 Oxidize: v. 使氧化 Oxidant: n. 氧化剂 Oxidizer: n. 氧化剂 Oxidable:adj. 可氧化的
6
无机化合物典型的化学反应常用句型表达方式 一、分解反应句型 分解反应的词为decompose或decomposition,常用句型 如下:
12
Reaction Types :
1. Direct Combination ( Synthesis) Reaction 2. Decomposition ( Analysis) Reaction 3. Single Displacement ( Substitution) Reaction 4. Metathesis ( Double Displacement) Reaction 调换,交换 5. Acid-base Reaction
11
(4)Concentrated H2SO4 is a sufficiently strong oxidizing agent to oxidize Br– to Br2 and I– to I2. (5)Ammonia and CuO can undergo oxidation -reduction to form nitrogen.
2
Neutron
3.3 Periodicity of Atomic Properties
• atomic radius • ionization energy • electron affinity • electronegativity
3
3.6 Chemical Reactions reactants
4
products
6. Oxidation-reduction (Redox) Reaction 7. Combustion Reaction 8. IsБайду номын сангаасmerization Reaction 异构化反应 9. Disproportionation Reaction 歧化反应 水解反应 [ 10. Hydrolysis Reaction
10
四、氧化还原反应句型
常用词汇:oxide、oxidation、reduction和reduce ( 1 ) After purification , the tin ( IV ) oxide is reduced with carbon to produce tin metal. (2)Calcium,strontium,and barium are obtained by the reduction of their oxides with aluminum. ( 3 ) Pb(IV)compounds tend to undergo reduction to com pounds of Pb(II)and therefore good oxidizing agents.
7
(2)When compounds containing chlorates are heated, decompose to give the metal chloride and oxygen gas.
(3)Hydrogen peroxide undergoes decomposition in the presence of catalyst to produce oxygen gas and water. The iodide ion or MnO2 catalyzes this reaction. (4)Carbonic acid is a very unstable substance and decomposes to give carbon dioxide and water.
Chapter 3
• law of mass conservation:质量守恒定律 质量守恒定律 • law of definite proportions:定比定律 : • law of multiple proportions:倍比定律 :
1
Proton Atomic nucleus Atom Electron Atomic number = the number of proton Mass number = the number of proton +neutron
