常见标准热力学数据
标准热力学数据
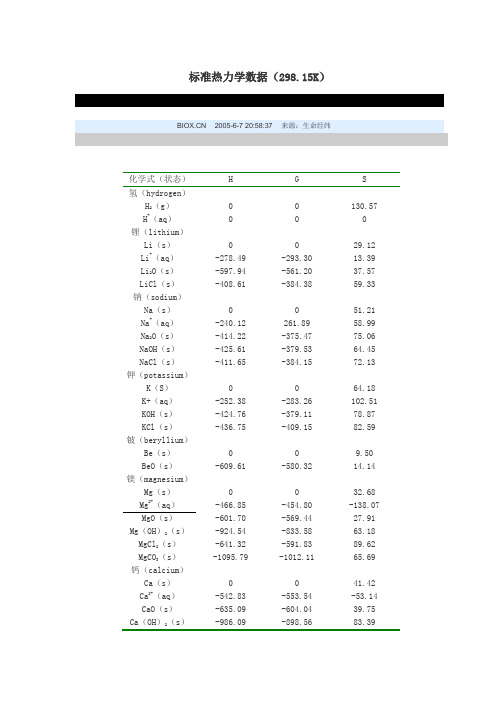
标准热力学数据(298.15K) 2005-6-7 20:58:37 来源:生命经纬化学式(状态)H G S氢(hydrogen)H2(g)0 0 130.57 H+(aq)0 0 0锂(lithium)Li(s)0 0 29.12 Li+(aq)-278.49 -293.30 13.39 Li2O(s)-597.94 -561.20 37.57 LiCl(s)-408.61 -384.38 59.33 钠(sodium)Na(s)0 0 51.21 Na+(aq)-240.12 261.89 58.99 Na2O(s)-414.22 -375.47 75.06 NaOH(s)-425.61 -379.53 64.45 NaCl(s)-411.65 -384.15 72.13 钾(potassium)K(S)0 0 64.18 K+(aq)-252.38 -283.26 102.51 KOH(s)-424.76 -379.11 78.87 KCl(s)-436.75 -409.15 82.59 铍(beryllium)Be(s)0 0 9.50 BeO(s)-609.61 -580.32 14.14 镁(magnesium)Mg(s)0 0 32.68 Mg2+(aq)-466.85 -454.80 -138.07 MgO(s)-601.70 -569.44 27.91 Mg(OH)2(s)-924.54 -833.58 63.18 MgCl2(s)-641.32 -591.83 89.62 MgCO3(s)-1095.79 -1012.11 65.69 钙(calcium)Ca(s)0 0 41.42 Ca2+(aq)-542.83 -553.54 -53.14 CaO(s)-635.09 -604.04 39.75 Ca(OH)2(s)-986.09 -898.56 83.39续表CaSO4(s)-1434.11 -1326.88 106.69CaCO3(方解石,s)-1206.92 -1128.84 92.88锶(strontium)Sr(s)0 0 52.30Sr2+(aq)-545.80 -599.44 -32.64SrCO3(s)-1220.05 -1140.14 97.07钡(barium)Ba(s)0 0 62.76Ba2+(aq)-537.64 -560.74 9.62BaCl2(s)-858.56 -810.44 123.68BaSO4(s)-1469.42 -1362.31 132.21硼(boron)B(s)0 0 5.86H3BO3(s)-1094.33 -969.01 88.83BF3(g)-1137.00 -1120.35 254.01BN(s)-254.39 -228.45 14.81铝(aluminum)Al(s)0 0 28.33Al(OH)3(无定形)-1276.12 - -Al2O3(s,刚玉)-1675.69 -1582.39 50.92碳(carbon)C(石墨)0 0 5.74C(金刚石) 1.897 2.900 2.377CO(g)-110.525 -137.15 197.56CO2(g)-393.51 -394.36 213.64硅(silicon)Si(s)0 0 18.83SiO2(石英,s)-910.94 -856.67 41.84SiCl4(g)-657.01 -617.01 330.62SiC(s,β)-65.27 -62.76 16.61Si3N4(s,α)-743.50 -642.66 101.25锡(tin)Sn(s,白)0 0 51.55Sn(s,灰)-2.09 0.126 44.14SnO2(s)-580.74 -519.65 52.3铅(lead)Pb(s)0 0 64.81PbO(s,红)-218.99 -188.95 66.73PbO(s,黄)-215.33 -187.90 68.70PbS(s)-100.42 -98.74 91.21续表氮(nitrogen)N2(g)0 0 191.50 NO(g)90.25 86.57 210.65 NO2(g)33.18 51.30 39.65 NO-3(aq)-207.36 -111.34 146.44 NH+4(aq)-132.51 -79.37 113.39 NH3(aq)-80.29 -26.57 111.29 NH3(g)-46.11 -16.48 192.34 磷(phosphorus)P(s,白)0 0 41.09 P(s,红)-17.5 -12.13 22.80 P4O10(s)-2984.03 -2697.84 228.86 PH3(g) 5.44 13.39 210.12 PCl3(g)-287.02 -267.78 311.67 氧(oxygen)O2(g)0 0 205.03 O3(g)142.67 163.18 238.82 H2O(l)-285.83 -237.18 69.91 H2O(g)-241.82 -228.59 188.72 OH-(aq)-229.99 -157.29 -10.75 H2O2(l)-187.78 -120.42 -硫(sulfur)S(s,斜方)0 0 31.80 S(s,单斜)0.33 - - SO2(g)-297.04 -300.19 248.11 SO3(g)-395.72 -371.08 256.65 H2S(g)-20.63 -33.56 205.69 氟(fluorine)F2(g)0 0 202.67 HF(g)-271.12 -273.22 -173.67 F-(aq)-332.63 -278.82 -13.81 氯(chlorine)Cl2(g)0 0 222.96 HCl(g)-92.31 -95.30 186.80 Cl-(aq)-167.16 -131.26 56.48 ClO-(aq)-107.11 -36.82 41.84 溴(bromine)Br2(l)0 0 152.23 Br2(g)30.91 3.14 245.35 HBr(g)-36.40 -53.43 198.59 Br-(aq)-121.55 -103.97 82.42续表碘(iodine)I2(s)0 0 116.14 I2(g)62.44 19.36 260.58 HI(g)26.48 1.72 206.48 I-(aq)-55.19 -51.59 111.29 钪(scandium)Sc(s)0 0 34.64 钛(titanium)Ti(s)0 0 30.54 TiO2(s,金红石)-939.73 -884.50 49.92 钒(vanadium)V(s)0 0 28.91 V2O5(s)-1550.59 -1419.63 130.96 铬(chromium)Cr(s)0 0 23.77 Cr2O3(s)-1139.72 -1058.13 81.17-881.19 -727.85 50.21 CrO(aq)-1490.34 -1301.22 261.92 Cr2O(aq)锰(manganese)Mn(s,α)0 0 32.01 Mn2+(aq)-220.75 -228.03 -73.64 MnO2(s)-520.03 -465.18 53.05 铁(iron)Fe(s)0 0 27.28 Fe2+(aq)-89.12 -78.87 -137.65 Fe3+(aq)-48.53 -4.60 -315.89 Fe(OH)2(s)-569.02 -486.60 87.86 Fe(OH)3(s)-822.99 -696.64 -106.69 FeS(s,α)-95.06 -97.57 67.4 Fe2O3(s)-824.25 -742.24 87.40 Fe3O4(s)-1118.38 -1015.46 146.44 钴(cobalt)Co(s,α)0 0 30.04 Co2+(aq)-58.16 -54.39 -112.97 镍(nickel)Ni(s)0 0 29.87 Ni2+(aq)-53.97 -45.61 -128.87 铜(copper)Cu(s)0 0 33.15Cu2+(aq)64.77 65.52 -99.58 Cu(OH)2(s)-449.78 - -续表CuO(s)-157.32 -129.70 48.63CuSO4(s)-771.36 -661.91 108.78 CuSO4·5H2O(s)-2279.65 -1880.06 300.41 银(silver)Ag(s)0 0 42.55Ag+(aq)105.58 77.12 72.68Ag2O(s)-31.05 -11.21 121.34 Ag2S(s,α)-32.59 -40.67 144.01AgCl(s)-127.07 -109.80 96.23AgBr(s)100.37 -96.90 107.11AgI(s)-61.84 -66.19 115.48 Ag(NH3)+2(aq)-111.89 -17.24 245.18 金(gold)Au(s)0 0 47.40 [Au(CN)2]-(aq)242.25 285.77 171.54 [AuCl4]-(aq)-322.17 -235.22 266.94 锌(zinc)Zn(s)0 0 41.63Zn2+(aq)-153.89 -147.03 -112.13ZnO(s)-348.28 -318.32 43.64 镉(cadmium)Cd(s,γ)0 0 51.76Cd2+(aq)-75.90 -77.58 -73.22CdS(s)-161.92 -156.48 64.85 汞(mercury)Hg(l)0 0 76.02Hg(g)61.32 31.85 174.85Hg2Cl2(s)-265.22 -210.78 192.46CH4(g)-74.85 -50.6 186.27C2H6(g)-83.68 -31.80 229.12(l)48.99 124.35 173.26C2H4(g)52.30 68.24 219.20C2H2(g)226.73 209.20 200.83CH3OH(l)-239.03 -166.82 127.24C2H5OH(l)-277.98 -174.18 161.04 C6H5COOH(s)-385.05 -245.27 167.57C12H22O11(s)-2225.5 -1544.6 360.2。
热力学基础数据查询类

氧气
Oxygen
在PUREDATA中查找物质序号,填入C6中, 并将相应的温度,压力填入F6,I6中, 按"F9"键重新计算即可 47 查PureData 氧气 31.999 温度 英文名 常压沸点 临界压力 Rackett Zra 修正偏心因子 生成焓 A 25 298.00 Oxygen 90.20 5,075.13 0.2908 C K K Kpa 临界体积 分子体积 0.04977 0.002871 m /Kmol m /Kmol
纯物质热力学计算表
使用说明: 物质序号 中文名 分子量 临界性质 临界温度 临界压缩因子 偏心因子 热性质 常压沸点汽化热 理想气体热容 等压热容 蒸汽压 6.1604E+04 液相表面张力 VAPOR!! 液相密度 首选 6.8184E+03 KJ/kmol 系数 2.9499E+01 KJ/kmol.K Antoine Kpa Tension N/M 回归法系数 VAPOR!! Kmol/m^3 154.58 0.1965 0.0190 定义值 K
3 3
压力 分子式
0 O2
Kpa
0.0190 SRK, PR方程用 0.0000E+00 B KJ/kmol C 2.5874E-05 C -4.176 608.13 atmA Gibbs自由能 D -1.4147E-08 0.0000E+00 E KJ/kmol F
29.26061662 -0.00558608 A 15.699 A 0.038066 A 4.356 B 780.26 B 1.214 B 0.3025
C 7.928 C 0.599 EpsDivK 0.0 28.9 1.21255
D -0.03168 D -0.189 MolDia 0 4.83 4.6368
标准热力学数据

标准热力学数据
首先,让我们来了解一下常用的热力学数据包括哪些内容。
常见的标准热力学
数据包括物质的热容、热膨胀系数、热导率、相变潜热等。
这些数据在热力学计算和实验研究中起着至关重要的作用。
热容是物质在吸热或放热过程中温度变化的敏感程度的量度。
它是描述物质热
力学性质的重要参数之一。
热容的大小与物质的种类、温度等因素有关。
在工程实践中,我们经常需要使用不同物质的热容数据来进行热力学计算和分析。
热膨胀系数是描述物质在温度变化时体积变化的比例系数。
它是描述物质热力
学性质的重要参数之一。
热膨胀系数的大小与物质的种类、温度等因素有关。
在工程实践中,我们经常需要使用不同物质的热膨胀系数数据来进行热力学计算和分析。
热导率是描述物质传热性能的参数。
它是描述物质热力学性质的重要参数之一。
热导率的大小与物质的种类、温度等因素有关。
在工程实践中,我们经常需要使用不同物质的热导率数据来进行热力学计算和分析。
相变潜热是描述物质在相变过程中吸热或放热的热量。
它是描述物质热力学性
质的重要参数之一。
相变潜热的大小与物质的种类、相变类型等因素有关。
在工程实践中,我们经常需要使用不同物质的相变潜热数据来进行热力学计算和分析。
总之,标准热力学数据在热力学研究和工程实践中具有重要的作用。
通过了解
和应用这些数据,我们可以更好地进行热力学计算和分析,为科学研究和工程设计提供有力的支持。
希望本文所介绍的内容能对大家有所帮助,谢谢阅读!。
纯物质热化学数据手册

纯物质热化学数据手册纯物质热化学数据手册是一份包含各种物质的热力学数据和热力学性质的参考手册。
这些数据是在标准状态下(常温常压)测定的,对于热化学计算和工程设计非常有用。
以下是一些常见物质的热力学数据:1. 水(H2O)标准状态下,水的热力学性质如下:- 摩尔质量:18.01528 g/mol- 摩尔熵(S°):69.91 J/(mol K)- 摩尔焓(H°):-285.83 kJ/mol- 摩尔自由能(G°):-237.13 kJ/mol- 热容(Cp):75.29 J/(mol K)2. 氨(NH3)标准状态下,氨的热力学性质如下:- 摩尔质量:17.03052 g/mol- 摩尔熵(S°):193.60 J/(mol K)- 摩尔焓(H°):-45.92 kJ/mol- 摩尔自由能(G°):-16.45 kJ/mol- 热容(Cp):35.63 J/(mol K)3. 二氧化碳(CO2)标准状态下,二氧化碳的热力学性质如下:- 摩尔质量:44.0095 g/mol- 摩尔熵(S°):213.79 J/(mol K)- 摩尔焓(H°):-393.52 kJ/mol- 摩尔自由能(G°):-394.36 kJ/mol- 热容(Cp):37.13 J/(mol K)4. 乙醇(C2H5OH)标准状态下,乙醇的热力学性质如下:- 摩尔质量:46.06844 g/mol- 摩尔熵(S°):160.70 J/(mol K)- 摩尔焓(H°):-277.69 kJ/mol- 摩尔自由能(G°):-174.76 kJ/mol- 热容(Cp):112.97 J/(mol K)以上仅是几个常见物质的热力学数据,不同温度和压力下的数据会有所不同,具体数据可在相关手册或数据库中查找。
热力学基础数据查询表

-1.2715E-11 3.83149E-15
atmAKmol Cavet法估算 m^3/Kmol Rickett法估算 m^3/Kmol 临界压缩因子法估算 Vis Pa.S A 656.25 A 52634 A 57608000 StielF 0.023 B 283.160 B 241.19 B 0.6964 PolarP 1
C -0.85085 C -0.7797 EpsDivK 775.0 775.0 0.51641
D 0.001 D 0.47678 MolDia 2.52 2.52 1.9118
E 0
2.1093E-02 mPa.S (CP) INT Omega
水
Water
在PUREDATA中查找物质序号,填入C6中, 并将相应的温度,压力填入F6,I6中, 按"F9"键重新计算即可 62 查PureData 水 18.0152 温度 英文名 常压沸点 临界压力 Rackett Zra 修正偏心因子 生成焓 A 251.3 524.30 Water 373.15 22,112.78 0.0000 C K K Kpa 临界体积 分子体积 0.063494 0.002552 m /Kmol m /Kmol
3 3
压力 分子式
4000 H2O
Kpa
0.3480 SRK, PR方程用 -2.4178E+05 B KJ/kmol C Gibbs自由能 -2.2856E+05 D E KJ/kmol F
33.36212264 -0.00287942 1.16315E-05 8.02439E-09 A 18.304 A 0.1386 A 4.6137 B 3816.4 B 1.687 B 0.26214 C 647.29 868.3226 #VALUE! 654.7122 D 0.23072 Kg/m^3 Kg/m^3 Kg/m^3 C -46.13 40.03
热力学数据

附录Ⅳ若干种热力学数据表1.单质和无机物物质适用温度范围/KAg0 042.71225.48 23.975.284-0.25293~1234-506.14 -437.09 167.36-30.56 -10.82 121.71 65.57Al(s)0 028.31524.35 20.67 12.38273~931.7Al(g)313.80 273.2 164.553-1669.8 -2213.160.98679.0 92.3837.535-26.86127~1937-3434.98 -3728.53 239.3 259.4 368.57 61.92 -113.47298~1100111.88482.396175.02130.71 3.109 245.455 35.99 37.200.690-1.188300~1500C(金刚石) 1.896 2.8662.4396.079.1213.22 -6.19 ~1200C(石墨)0 05.6948.6617.154.27-8.79298~2300CO(g)-110.525 -137.285 198.01629.14227.6 5.0290~2500-393.511 -394.38 213.7637.1244.149.04-8.54298~2500Ca(s)0 0 41.63 26.27 21.92 14.64 273~673-62.8 -67.8 70.2 62.34 68.6 11.88 -8.66 298~720-1206.87 -1128.70 92.8 81.83 104.52 21.92 -25.94 298~1200-795.0 -750.2 113.8 72.63 71.88 12.72 -2.51 298~1055CaO(s)-635.6 -604.2 39.7 48.53 43.834.52-6.52298~1800-986.5 -896.89 76.1 84.5(硬石膏)-1432.68 -1320.24 106.7 97.65 77.49 91.92 -6.561273~1373 -167.456 -131.168 55.10Cu(s)0 0 33.32 24.47 24.564.18-1.201 ~1357CuO(s)-155.2 -127.1 43.51 44.4 38.79 20.08 298~1250-166.69 -146.33 100.8 69.8 62.34 23.85 298~12000 0 203.5 31.46 34.691.84-3.35273~2000 0 27.15 25.23 17.28 26.69 273~1041-747.68 -673.84 92.8 82.13 48.66 112.1 298~885FeO(s)-266.52 -244.3 54.0 51.1 52.806.242-3.188273~1173-822.1 -741.0 90.0 104.6 97.74 17.13 -12.887298~1100-117.1 -1014.1 146.4 143.42 167.03 78.91 -14.88 298~1100(续表)物质适用温度4 00 0130.69528.8329.08-0.842.0300~15000 0144.88429.228.5770.8791.958298~1500HBr(g)-36.24 -53.22 198.6029.1226.155.861.09298~1600HBr(aq)-120.92 -102.8080.7 1HCl(g)-92.311 -95.265186.78629.1226.534.61.90298~2000HCl(aq)-167.44 -131.1755.1 0-698.7 -623.37 191.2Hl(g)-25.94 -1.32 206.4229.1226.325.940.92298~1000-241.825 -228.577188.82333.57130.1211.30273~2000-285.838 -237.14269.94075.296-291.850 (-234.03) (39.4)-187.61 -118.04 102.2682.2 9-20.146 -33.040 205.7533.9729.2915.69273~1300-811.35 (-866.4) 156.85 137.57 -811.32-885.75 -752.99 126.8662.242 19.34 260.6036.8 70 0191.59829.1226.874.27273~2500-46.19 -16.603 192.6135.6529.7925.48-1.665273~1400NO(g)89.860 90.37210.30929.86129.583.85-0.59273~150033.85 51.86 240.5737.942.938.54-6.7481.55 103.62 220.1038.745.698.62-8.54273~5009.660 98.39 304.42 79.083.8930.7514.92.51 110.5 342.4 108.0O(g)247.521 230.095161.06321.930 0205.13829.3731.463.39-3.77273~2000142.3 163.45 237.738.1 5-229.940 -157.297-10.53 9S(单斜)0.29 0.09632.5523.6414.929.08368.6~392S(斜方)0 0 31.922.614.9826.11273~368.6(g)124.94 76.08 227.7632.5536.111.09273~2000S(g)222.80 182.27167.825-3.51-395.18 -370.40 256.3450.757.3226.86 -13.05273~900-907.51 -741.90 17.2 2.有机化合物在指定温度范围内恒压热容可用下式计算物质适用温度范围/K烃类-74.847 50.827 186.3035.71517.45160.461.117-7.205298~1500226.748 209.200200.92843.92823.4685.768-58.34215.870298~150052.283 68.157 219.56 43.564.197154.59-81.09016.815298~1500-84.667 -32.821 229.6052.654.936182.259-74.85610.799298~150020.414 62.783 267.05 63.893.305235.86-117.60022.677298~1500-103.847 -23.391 270.02 73.51-4.799307.311-160.15932.748298~1500-0.13 71.60 305.71 85.652.540344.929-191.28441.664~1500-6.99 65.96 300.94 78.918.774342.448-197.32234.271298~1500-11.17 63.07 296.59 87.828.381307.541-148.25627.284298~1500-16.90 58.17 293.70 89.127.084321.632-166.07133.497298~1500-126.15 -17.02 310.23 97.450.469385.376-198.88239.996298~1500-134.52 -20.79 294.75 96.82-6.841409.643-220.54745.739298~150082.927 129.723 269.31 81.67 -33.899471.872-298.34470.835298~150049.028 124.597 172.35 135.77 59.50 255.01 281~353-123.14 31.92 298.51 106.27 -67.664679.452-380.76178.006298~1500-167.19 -0.09 388.85 143.093.084565.786-300.36962.061298~1500-198.82 -4.08 295.89 194.9349.999 122.388 319.86 103.76 -33.882557.045-342.37379.873298~150018.995 122.207 352.86 133.26 -14.811591.136-339.59074.697~1500-24.439 110.495 246.48 187.917.238 118.977 357.80 127.57 -27.384620.87-363.89581.379298~1500-25.418 107.817 252.17 183.317.949 121.266 352.53 126.86 -25.92460.670-350.56176.877298~1500(续表)物质适用温度范围/K -24.426 110.244 247.36 183.7含氧化合物-115.90 -110.0 220.2 35.3618.8258.379-15.606291~1500-362.63 -335.69 251.1 54.4 30.67 89.20-34.539300~700-201.17 -161.83 237.8 49.4 20.42 103.68-24.64~700-238.57 -166.15 126.8 81.6-166.36 -133.67 265.8 62.831.054121.457-36.577298~1500-487.0 -392.4 159.8 123.4 54.81 230-436.4 -381.5 293.4 72.4 21.76 193.09 -76.78 300~700-277.63 -174.36 160.7 111.46 106.52 165.7 575.3 283~348-235.31 -168.54 282.1 71.120.694-205.38-99.809300~1500-248.283 -155.33 200.0 124.73 55.61 232.2 298~320-216.69 -152.2 296.00 75.322.472201.78-63.521298~1500-273.2 -116.47 253.1 170.7 290-384.55 -245.5 170.7 155.2 卤代烃-82.0 -58.6 234.29 40.7914.90396.2-31.552273~800-88 -59 270.62 51.38 33.47 65.3 273~800-131.8 -71.4 202.9 116.3-100 -67 296.48 65.8129.506148.942-90.713273~800-139.3 -68.5 214.43 131.75 97.99 111.71 273~330-106.7 -64.0 309.41 85.51116.3 -198.2 197.5 145.6含氮化合物(待续)见网页(fulu4b2s2.html)(续表)物质适用温度范围/K78.87 159.9 179.1 140.2 29335.31 153.35 191.6 199.6 338.28 -1068.6 2022.1 278~34815.90 146.36 244.3 185.4 293本附录数据主要取自Handbook of Chemistry and Physics ,70 th Ed.,1990;Editor John A.Dean ,Lange's Handbook of Chemistry ,1967。
化工计算 第二章化工基础数据 第二节常用热力学物性数据

⑴蒸发热(或冷凝热):当温度和压力不变,1mol液体蒸发时所需的热量 (或气体冷凝时放出的热量),H用V 表示。 ⑵熔融热(或凝固热):当温度和压力不变,1mol固体熔融所需的热量(或 液体凝固时放出的热量),用Hm 表示。 ⑶升华热(或凝华热):当温度和压力不变,1mol固体升华时所需的热量 (或气体凝华时放出的热量),H用s 表示。
解:(1)用(2-16)式计算,C1 取88,则
HV C1Tb 88 307.6 27068.8J mol 1
(2)用(2-17)式计算,由 TC 466 .7K 、pc 3.637 106 Pa 、Tb 307 .6K
Tbr
Tb Tc
307.6 466.7
0.66
H v
1.093RTcTbt
H U pV
式中 H——体系的焓,J;
U——体系的内能,J;
p——体系的压力,Pa;
V——体系的体积,m3
2.计算
H 2
H1
n
T2 T1
C
p,m
dT
高职高专“十一五”规划教材《化工计算》 军
k
k
k
k
Cp,m nkak nkbkT nkckT 2 nkdkT 3
k 1
k 1
k 1
k 1
式中各参数查取见教材
适合:理想气体
3.举例:略(见教材)
高职高专“十一五”规划教材《化工计算》 军
第二节 常用热力学物性数据
二、相变热
1.定义
任何物质都有三种相态,气相、液相和固相,在化工生产过程中,因为反 应条件的变化和化学反应的影响,常有物质会从一种相态变到另一种相态,出 现蒸发、冷凝、结晶、升华等相变过程,在相变发生的过程中伴随着热量的产 生,称为相变热。
热力学知识:热力学中的热力学计算和热力图表

热力学知识:热力学中的热力学计算和热力图表热力学是一门能够描述物质能量转化和传递的物理学科,它研究的是热量、功、能量和熵等基本的物理量之间的相互关系。
在实际应用中,热力学常常被用来计算各种体系的热力学性质,例如热容、热力学势函数、化学反应平衡等等。
在热力学计算中,热力图表也是非常常用的工具,它可以直观地描述各种体系的物理和化学性质,便于我们进行分析和研究。
一、热力学计算1、热力学基本量在热力学的计算中,热量、功、能量和熵是最基本的物理量,它们之间的关系可以表示为:ΔU=Q-W其中,ΔU表示系统内能的变化,Q表示系统所吸收的热量,W表示系统所做的功。
另外,在热力学中还有几个重要的热力学量,例如热容、热力学势函数、化学反应平衡等等。
这些量的计算也是非常重要的,它们可以反映出体系的热力学性质和稳定性。
2、热力学循环在热力学循环中,热量和功的转化可以循环进行,从而实现能量的连续转化和利用。
例如,蒸汽动力发电机系统中,水被加热蒸发,蒸汽驱动涡轮机运转产生功,然后通过冷凝器进行冷却回到水箱中,从而循环再次加热。
在这个过程中,热量和功的变化可以通过热力学计算进行分析和优化,以达到最高的能量转化效率。
3、化学反应化学反应是热力学计算中的一个重要应用,它可以研究各种化学反应的平衡状态和反应能量。
在化学反应中,物质的热化学态函数是计算反应热量和反应平衡的重要工具。
例如,在S→SO2的氧化反应中,热化学态函数可以表示为:ΔH=-297 kJ/mol(S)+0 kJ/mol(O2)-395 kJ/mol(SO2)其中,ΔH表示反应热量,单位为kJ/mol。
二、热力图表1、热力图热力图是一种图形化表示热力学性质的图表,它直观地展示了各种变量之间的相互关系。
在热力学中,常用的热力图有比热容图、热力学势函数图、化学反应热图等等。
这些图表可以帮助我们更好地理解和分析热力学性质,以便制定更好的计算和实验方案。
2、比热容图比热容图是一种描述物质热力学性质的图表,它可以直观地显示出不同物质在不同温度下的比热容变化。
