如何在中国开展临床试验_二_一个临床研究者的经验分享
临床试验的流程

临床试验的流程临床试验是一项重要的科学研究方法,用于评估新药物、治疗方法以及医疗器械的疗效和安全性。
临床试验的流程非常严格,下面将详细介绍其流程和各个环节。
1. 研究立项在进行临床试验之前,需要进行研究立项。
这包括制定研究目的、研究方案、确定研究群体等等。
研究者需要详细规划整个试验过程,并提交相关机构进行审批。
2. 招募研究对象在开始试验之前,研究者需要招募符合试验条件的研究对象。
这些对象通常是患有特定疾病或症状的人群,需要经过严格的筛选和评估。
3. 伦理审查和知情同意进行临床试验前,需要得到伦理委员会的批准。
伦理委员会会对试验方案进行评估,确保试验符合伦理道德标准。
同时,研究对象还需要签署知情同意书,明确知晓试验的目的、过程和可能的风险。
4. 随机分组和盲法为了保证试验的科学性和客观性,研究对象会被随机分为实验组和对照组。
同时,为了避免主观干扰,试验中通常采用盲法,使得研究对象和研究人员在试验中对实验组和对照组的分组情况不知晓。
5. 实施试验实施试验的过程包括药物或治疗的给予、数据的收集、结果的分析等等。
研究人员需要按照试验方案的要求,严格操作,确保数据的准确性和可靠性。
6. 试验结果分析和报告试验结束后,研究人员需要对数据进行统计分析,并根据分析结果撰写试验结果报告。
试验结果报告需要准确反映试验结果,包括药物或治疗的疗效、安全性以及可能存在的不良反应。
7. 伦理审查和批准试验结果报告需要提交给伦理委员会进行审查。
伦理委员会会重新审查试验的过程和结果,确保试验符合伦理和法律标准。
8. 结论和推广研究人员要根据试验结果得出结论,并针对结论进行推广。
结论的推广可以是进一步的研究,也可以是药物或治疗方法的应用指南等。
总结起来,临床试验的流程包括研究立项、研究对象招募、伦理审查和知情同意、随机分组和盲法、实施试验、试验结果分析和报告、伦理审查和批准以及结论和推广。
临床试验的严格流程确保了试验的科学性和可靠性,为新药物和治疗方法的安全推广提供了重要的依据。
如何在中国开展临床试验一个临床研究者的经验分享

如何在中国开展临床试验一个临床研究者的经验分享作为一个临床研究者,我有幸在中国开展了多个临床试验。
在这篇文章中,我将分享我在中国开展临床试验的经验,并提供一些建议。
首先,开展临床试验需要遵循中国国家食品药品监督管理局(CFDA)的相关法规和指南。
在准备阶段,研究者需要编写一份研究计划,并交由CFDA进行审批。
这个过程通常需要一些时间和耐心,因为CFDA对研究计划的严格要求。
因此,建议研究者尽早开始准备,并与CFDA保持密切的沟通。
其次,临床试验的受试者招募也是一个重要的环节。
中国是一个人口众多的国家,这为研究者提供了一个广阔的受试者基础。
然而,由于中国人口的地域分布和文化差异,受试者招募可能会面临一些挑战。
为了提高受试者的招募率,建议研究者与医院和研究机构建立良好的合作关系,并制定详细的招募计划和策略。
在实施过程中,研究者需要与中国的医疗机构合作。
中国的医疗机构通常由大型综合医院和较小的社区医院组成。
为了成功开展临床试验,研究者需要选择合适的医疗机构,并与医院管理层和临床医生进行沟通。
此外,研究者还需要了解中国的医疗流程和标准,并确保临床试验的操作符合中国的实践要求。
此外,临床试验的监督与管理也是至关重要的。
中国的临床试验监管部门要求研究者建立完善的试验监控机制,并提交监督报告。
为了确保试验的质量和可靠性,研究者需要招聘有丰富临床试验经验的监测员,并在试验期间进行定期监测和数据审核。
最后,研究结果的回顾和分析对于临床试验的成功和推广至关重要。
建议研究者在临床试验结束后,与团队共同分析试验数据,并根据结果撰写研究报告。
此外,研究者还可以与学术界和医药企业合作,进行进一步的数据分析和论文发表。
同时,及时将研究结果向CFDA和中国医疗界进行推广,有助于提高临床试验的知名度和质量,促进临床研究的发展。
总之,在中国开展临床试验需要遵循CFDA的相关法规和指南,与医院和研究机构合作,建立完善的试验监控机制,并在试验结束后及时回顾和分析结果。
临床试验参与者管理研究

临床试验参与者管理研究江世雄;孙耀志;高松;谢佳佳【摘要】开展临床试验的主要目的是验证药品的安全性和有效性,是药品研究工作的重要环节,也是整个药品研究工作中花费较大的环节.目前,国内的临床试验水平还没有达到国际先进水平,如何在现有条件下快速、高效地进行临床试验,最大程度节约试验成本,是必须解决的问题.该研究从临床试验参与者的角度进行分析,给临床试验中各参与者的管理提供一些建议.【期刊名称】《中国卫生产业》【年(卷),期】2015(012)035【总页数】2页(P165-166)【关键词】临床试验;参与者;管理【作者】江世雄;孙耀志;高松;谢佳佳【作者单位】河南中医学院,河南郑州 450000;仲景宛西制药股份有限公司,河南南阳 474550;仲景宛西制药股份有限公司,河南南阳 474550;河南中医学院,河南郑州450000【正文语种】中文【中图分类】R969.4临床试验是指在人体(患者和健康志愿者)进行药物的系统性研究,以证实或揭示试验药物的作用、不良反应及/或试验用药品的吸收、分布、代谢和排泄,目的是确定试验用药品或其他干预措施的疗效与安全性[1]。
临床试验质量关系到药品的质量安全以及受试者、志愿者的生命健康,必须对试验全过程进行质量控制。
申办者、合同研究组织、伦理委员及伦理委员会、研究者、临床试验协调研究者、受试者及志愿者是临床试验的主要参与者,贯穿临床试验的全过程。
我国已建立起符合国际通行标准的临床试验管理法规,但是在标准化操作规程(SOP)等方面与发达国家仍有不小差距。
为保证临床试验质量,需要参与临床试验的所有人员、组织机构严格遵循临床试验方案、GCP、标准操作规程等规定。
该研究从临床试验参与者的角度进行分析,归纳出存在的问题,最后提出一些解决问题的方法和建议。
1各参与者的职责及出现的问题1.1申办者申办方是临床试验的发起者,对整个试验的启动、管理、财务和监查负责。
审办方也可以聘请对自己负责的监查员,对试验进展情况进行监查和报告并对数据进行核实。
研究者发起临床研究的运行管理制度和流程
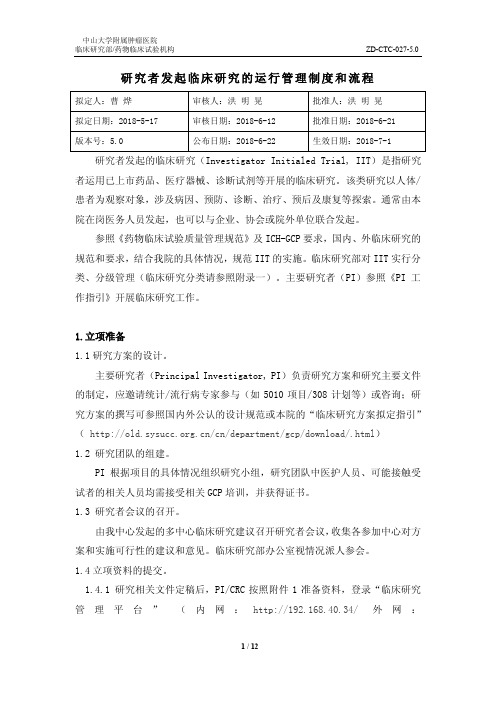
研究者发起临床研究的运行管理制度和流程研究者发起的临床研究(Investigator Initialed Trial, IIT)是指研究者运用已上市药品、医疗器械、诊断试剂等开展的临床研究。
该类研究以人体/患者为观察对象,涉及病因、预防、诊断、治疗、预后及康复等探索。
通常由本院在岗医务人员发起,也可以与企业、协会或院外单位联合发起。
参照《药物临床试验质量管理规范》及ICH-GCP要求,国内、外临床研究的规范和要求,结合我院的具体情况,规范IIT的实施。
临床研究部对IIT实行分类、分级管理(临床研究分类请参照附录一)。
主要研究者(PI)参照《PI工作指引》开展临床研究工作。
1.立项准备1.1研究方案的设计。
主要研究者(Principal Investigator, PI)负责研究方案和研究主要文件的制定,应邀请统计/流行病专家参与(如5010项目/308计划等)或咨询;研究方案的撰写可参照国内外公认的设计规范或本院的“临床研究方案拟定指引”(/cn/department/gcp/download/.html)1.2 研究团队的组建。
PI根据项目的具体情况组织研究小组,研究团队中医护人员、可能接触受试者的相关人员均需接受相关GCP培训,并获得证书。
1.3 研究者会议的召开。
由我中心发起的多中心临床研究建议召开研究者会议,收集各参加中心对方案和实施可行性的建议和意见。
临床研究部办公室视情况派人参会。
1.4立项资料的提交。
1.4.1 研究相关文件定稿后,PI/CRC按照附件1准备资料,登录“临床研究管理平台”(内网:http://192.168.40.34/外网:/)申请账号及提交送审资料(其中附件2、附件3、附件5、PI简历需签字后扫描原件上传)1.4.2资料的形式审查。
临床研究部办公室秘书进行形式审查,审查合格后生成“机构受理号”。
1.4.3 纸质资料的上交。
待系统通知形式审查通过后,在附件2中填写好“机构受理号”,递交纸质版材料1套至临床研究部办公室,供上会讨论。
如何开展临床研究及需要注意的问题

可 行 性 研 究 主 要 涉 及 到 : 一 , 研 究 的 内 第 要
容、 方法 、 对象是 否为本专业 的范 围, 如果需 同其
他 专 科 合 作 才 能 进 行 该 项 研 究 , 无 可 能 ?第 二 , 有 该 研 究 有无 临 床 意 义 ? 第 三 , 研 究 的疾 病 的 发 要
如某 项研 究入 选平 均 收缩 压为 (6 ̄ 0 m H 10 21 g m
的患者 ,现在 研究某 降压药是否会 降低 收缩 压 。 如果研究者 以平均 降低 1 m H 为 目标 ,那么 0m g 均 数差/ 准差 = 02 = . , 照表 1 则 每组 样 标 1/0 05 参 0 ,
维普资讯
中华肾脏病 杂志 2 0 年 3 07 月第 2 卷第 3 3 期 C i J e ho M r 07V l 3 N . h N p r , a h2 0 ,o 2 , n l c D 3
・
专 家讲 座 ・
如 何 开 展 临 床 研 究 及 需 要 注 意 的 问题
顾 勇 李铭 新 临床 科 室 在 各 类 临 床 研 究 中扮 演 的是 研 究 者
Hale Waihona Puke 估算 出来 的肾小球 滤过率 不完全相 同。系统性红 斑 狼疮 的活动 指数也有数 种计算 方法 , 必须予 以 限定 。
的角色 , 而临床研究 中最常见 的是 新药 的临床研 究 。按 照 国家 食 品药 品监 督 管 理 局 (F A) 定 , SD 规 注 册不 同类别 的新 药 , 要完成不 同要求 的临床试 验 。本文主要述及 药物 临床研究 , 其它类 型研究 也 具 有 共 同点 。 般 临床试验 的过程可 以分为 准备 、启 动 、 正 式 运行 、 析 总结 诸 阶 段 。本 文 将 重 点 介 绍 临 分
临床科研的一般流程(二)

引言概述:临床科研的一般流程是指临床医生在开展科学研究过程中的一系列操作和步骤。
这些步骤包括确定研究目标、设计研究方案、采集数据、数据分析和结果解读等。
正确认识和掌握临床科研的一般流程对于提高研究的质量和效果至关重要。
本文将通过对临床科研的一般流程进行详细阐述,帮助读者全面了解临床科研的步骤和重要环节。
正文内容:一、确定研究目标1.分析临床现状:首先需要对所研究的领域进行全面的调研和分析,了解目前的临床现状和存在的问题。
2.设定研究问题:在分析临床现状的基础上,确定自己的研究问题,明确所要解决的具体问题。
3.确定研究目标:根据所设定的问题,明确研究目标,即研究的具体目的和预期成果。
二、设计研究方案1.研究设计类型选择:根据研究目标和问题的性质,选择合适的研究设计类型,如前瞻性研究、回顾性研究、实验研究等。
2.受试者选择和样本容量计算:根据研究设计类型和研究目标,确定合适的受试者选择方法,并进行样本容量的计算。
3.方法选择:根据研究目标和问题的性质,选择合适的研究方法,如问卷调查、实验观察、临床试验等。
4.数据采集工具和流程设计:确定数据采集工具和流程,确保数据的准确性和完整性。
5.计划研究时间安排:根据研究方案的复杂程度和资源限制,制定合理的研究时间表,确保研究进度的合理性。
三、采集数据1.数据采集过程:按照设计好的方案,对受试者进行数据采集。
包括问卷调查、实验观察、临床试验等。
2.数据管理和存储:建立数据管理和存储系统,确保数据的安全、完整性和可用性。
3.数据质量控制:通过数据校验、数据清洗等手段对数据进行质量控制,排除异常数据和噪声。
4.数据的匿名处理:对于涉及隐私的数据,进行匿名处理,保护受试者的隐私权。
四、数据分析和结果解读1.数据统计分析:采用合适的统计方法对数据进行分析,如描述统计、相关分析、回归分析等。
2.结果呈现和解读:将数据分析的结果进行呈现,如制作图表和报告,对结果进行解读和分析。
中国药物临床试验项目管理经验小结

中国药物临床试验项目管理经验小结编辑整理:尊敬的读者朋友们:这里是精品文档编辑中心,本文档内容是由我和我的同事精心编辑整理后发布的,发布之前我们对文中内容进行仔细校对,但是难免会有疏漏的地方,但是任然希望(中国药物临床试验项目管理经验小结)的内容能够给您的工作和学习带来便利。
同时也真诚的希望收到您的建议和反馈,这将是我们进步的源泉,前进的动力。
本文可编辑可修改,如果觉得对您有帮助请收藏以便随时查阅,最后祝您生活愉快业绩进步,以下为中国药物临床试验项目管理经验小结的全部内容。
临床试验项目管理实施要点临床试验的项目管理,是对临床试验项目的实施、质量、人员、成本和时间的管理。
临床试验虽在医药健康这个特殊领域,有着相对独立的项目内涵、各国相关法律法规的约束和伦理、科学方面的考虑,但目前没有特定为临床试验的项目管理而产生的理论体系。
新技术的运用比如CTMS和互联网技术对此做了有效补充并加入新的内容。
基于临床试验项目管理的特殊性,国内的项目经理一般都是有着丰富的临床监察经验,一般医药教育背景。
与之不同的是国外以项目管理为背景(如MBA)的项目经理不少见,一般只要求自然科学背景.国内很多项目经理对于监察和研究中心的管理了如指掌。
而过度到对一个临床项目的管理,存在的挑战还是很大。
首先是成本管理,包括项目预算的计划阶段、执行阶段和结束阶段,难点在于计划和控制。
影响成本的关键因素包括:患者入组数、参与国家的选择、研究中心数、监察频率、是否同时做PK试验,对照药品的采购包装运输、项目是否外包、是否选用中心实验室和SMO、试验系统的配置(EDC/IVRS/IWRS/e—diary),等等.这要求在立案阶段,项目经理需要对整个项目有充分的了解,对风险有足够洞悉,并提前进行部署,制定计划包括可行性研究,与相关人员一起核查计划.尽可能多的参与到项目立项研究和方案设计环节,有助于预算计划的完成并降低后期风险。
人力资源的管理是多数从监察岗位转到项目管理岗位的项目经理所需要面对的新课题。
中国临床研究

中国临床研究中国临床研究是指在人体中进行的医学研究,是医学科学发展的重要组成部分。
随着中国医学科学的不断发展和进步,临床研究在中国也取得了显著的成就。
下面将简要介绍中国临床研究的现状和发展趋势。
中国临床研究的现状可以概括为规模庞大、研究领域广泛、发展水平较高的特点。
首先,中国临床研究的规模庞大。
中国是人口最多的国家之一,具有庞大的临床研究人群,可以提供丰富的样本资源。
这为临床研究提供了广阔的空间,使得研究结果具有很高的代表性。
其次,中国临床研究的研究领域广泛。
中国拥有世界上最完善的医疗保健体系之一,涵盖了多个领域的医学研究,包括肿瘤学、心血管病学、神经科学、消化学等。
同时,中国还不断涌现出新的疾病和研究领域,如新冠肺炎疫情的爆发和应对,进一步提高了中国在临床研究领域的影响力。
第三,中国临床研究的发展水平较高。
中国的医学科学发展经验丰富,临床研究领域的专家和研究人员众多,科研机构设施完善,方法技术先进,研究水平和研究成果受到国际同行的广泛关注。
近年来,中国在临床试验设计、数据分析和研究规范等方面的经验积累和改进,使得其临床研究的质量和可信度得到了显著提高。
然而,中国临床研究仍面临一些挑战和问题。
首先,临床研究的质量和可信度仍有待进一步提高。
虽然中国的临床研究水平已相对较高,但与发达国家相比,仍存在一定差距。
一些研究方法和技术仍需要进一步改进和完善,以提高研究的可靠性和准确性。
其次,中国临床研究的资源和人才分布不均衡。
一些发达的城市和地区在临床研究领域拥有更多的资源和专业人才,而农村地区和欠发达地区则严重缺乏相关研究支持和人才培养。
这导致了临床研究的不平衡,影响了全国范围内研究成果的全面推广和应用。
最后,临床研究的伦理和法律问题也是亟需解决的难题。
临床研究涉及到大量的人体实验和人体样本,必须严格遵守伦理规范和法律法规。
然而,一些不规范的临床研究行为仍存在,导致了对研究结果的质疑和争议。
为了进一步促进中国临床研究的发展,可以采取以下措施:首先,加强临床研究的规范和管理。
临床试验带教经验分享

临床试验带教经验分享临床试验带教是培养临床研究人才的重要途径,以下是一些临床试验带教经验分享:1. 制定明确的教学计划:在带教之前,制定详细的教学计划,包括教学目标、教学内容、教学方法和时间安排等。
确保教学内容系统、全面,能够帮助学生逐步掌握临床试验的相关知识和技能。
2. 结合实际案例教学:通过实际的临床试验案例,让学生了解临床试验的整个过程,包括试验设计、伦理审查、受试者招募、试验实施、数据管理和统计分析等。
这样可以使学生更加直观地理解和掌握知识。
3. 强调伦理和法规:在临床试验带教过程中,要强调临床试验的伦理和法规要求,让学生了解并遵守相关规定,确保试验的合法性和合理性。
4. 培养团队合作精神:临床试验需要多学科团队的合作,因此要培养学生的团队合作精神。
可以通过小组讨论、团队项目等方式,让学生在实践中体会团队合作的重要性。
5. 引导学生主动学习:在带教过程中,要引导学生主动学习,培养他们的自主学习能力。
可以布置一些相关的阅读材料,让学生自行学习,然后进行讨论和交流。
6. 提供实践机会:给学生提供参与临床试验的实践机会,让他们在实际操作中积累经验。
可以安排学生参与试验的部分工作,如受试者招募、数据录入等。
7. 定期评估和反馈:定期对学生的学习情况进行评估,及时给予反馈和指导。
根据学生的表现和需求,调整教学内容和方法,以提高教学效果。
8. 自身不断学习:作为带教老师,要不断学习和更新自己的知识,了解最新的临床试验进展和相关法规,以便更好地指导学生。
总之,临床试验带教需要有系统的教学计划,注重实践操作,强调伦理和法规,培养学生的团队合作精神和自主学习能力。
通过不断的学习和实践,学生可以逐步掌握临床试验的相关知识和技能,为今后从事临床研究工作打下坚实的基础。
中国临床试验设计的方法和要求

中国临床试验设计的方法和要求主要包括以下几个方面:
1. 随机化:临床试验应采用随机分组的方法,即将参与试验的患者随机分配到不同的治疗组和对照组,以减少选择偏倚的影响。
2. 对照组设计:临床试验应设立对照组,即将一部分患者接受新的治疗方法,另一部分患者接受已有的标准治疗方法或安慰剂,以评估新的治疗方法的疗效。
3. 盲法:临床试验应采用盲法,即试验参与者和研究人员在试验过程中对治疗方法的情况保持不知情,以减少主观因素的干扰。
4. 样本容量计算:临床试验应根据研究目的和预期效应大小进行样本容量计算,以保证试验结果的统计学意义。
5. 治疗方案和观察指标:临床试验应明确治疗方案和观察指标,包括治疗剂量、用药途径、观察时间点等,以确保试验的可比性和结果的准确性。
6. 伦理审查:临床试验应经过伦理委员会的审查和批准,确保试验的合理性和安全性。
7. 数据分析和结果解读:临床试验应采用适当的统计方法进行数据分析,并对结果进行客观、科学的解读。
总的来说,中国临床试验设计的方法和要求主要是为了保证试验的科学性、可靠性和伦理性,以提供有效的临床证据,指导临床实践和药物注册。
药物临床试验项目经验分享

药物临床试验项目经验分享一、项目背景与目标药物临床试验是药物研发过程中至关重要的一环,旨在评估药物在人体内的安全性和有效性。
我们团队负责了一项针对治疗某罕见疾病的创新药物的临床试验项目。
该项目旨在验证新药在治疗该疾病方面的疗效及安全性,为药物上市提供科学依据。
二、项目执行过程1. 立项与研究设计在立项阶段,我们对市场上的竞品进行了深入调研,明确了研究目标。
根据目标,我们制定了详细的研究方案,包括试验设计、样本量计算、随机分组、给药方案等。
同时,我们还建立了数据采集、整理和分析的标准化操作流程。
2. 招募受试者招募受试者是临床试验的关键环节。
我们通过医院、社区和在线平台等多渠道进行广泛招募。
在招募过程中,我们向受试者充分解释了试验目的、风险与获益,并签订知情同意书。
3. 试验实施在试验实施阶段,我们严格按照试验方案进行操作,确保数据的准确性和可靠性。
同时,我们还建立了安全监测机制,密切关注受试者的不良反应情况,及时调整给药方案。
4. 数据管理与统计分析我们采用了电子数据采集系统,确保数据的质量和完整性。
在统计分析阶段,我们采用了多种统计方法对数据进行分析,评估新药的疗效和安全性。
三、项目成果与经验总结1. 试验结果经过严谨的试验设计和实施,我们得到了丰富的数据。
分析结果显示,新药在治疗该罕见疾病方面取得了显著疗效,且未发生严重不良反应。
这一结果为新药的上市提供了有力支持。
2. 经验总结通过本次临床试验项目,我们总结了以下经验:a. 充分的前期调研和方案设计至关重要;b. 招募受试者需要多渠道、多角度宣传;c. 严格遵守试验方案和规范操作是保证数据质量的关键;d. 数据管理与统计分析的标准化流程有助于得出准确结论;e. 与受试者保持良好的沟通与互动可以提高试验的依从性。
3. 未来展望尽管本次临床试验取得了成功,但仍需继续开展后续研究,以进一步验证新药在更大范围人群中的疗效和安全性。
同时,我们也将关注该疾病的最新研究进展,以便及时调整治疗方案,为更多患者带来福音。
如何做好一项临床试验

如何做好一项临床试验临床试验是医学研究中非常重要的环节,通过对人类进行观察、实验和评估,找到新的治疗方案和药物,从而提高医疗技术和服务质量。
然而,一项成功的临床试验需要严格的计划、执行和分析,下面将从设计、伦理、数据收集、分析和结果等方面介绍如何做好一项临床试验。
一、设计与计划在进行临床试验之前,首先需要制定详细的研究计划和试验设计。
这包括确定研究目的、研究对象、研究方法和试验组织等。
同时,还需要进行相关文献的综述和现有临床实践的分析,以确保试验的科学性和可行性。
二、伦理审查和知情同意在进行任何人类试验前,必须获得伦理委员会的批准,并且确保所有参与者完全理解试验的目的、过程、风险和福利,并自愿参与。
通过签署知情同意书,确保试验的道德合规性和合法性。
三、数据收集和管理临床试验需要精确和可靠的数据来支持科学分析和结论。
设计合理的数据收集工具和观察指标,并培训研究人员以确保数据的准确性和一致性。
同时,建立科学的数据管理系统,包括数据录入、存储、校验和备份,以确保数据的安全性和可追溯性。
四、试验执行和监督在试验过程中,需要严格按照研究计划和试验设计的要求进行试验执行,包括药物使用、治疗方案、随访等。
同时,建立专业的监督机制和数据监测委员会,定期审核和评估试验的进展和质量,确保试验的可靠性和有效性。
五、数据分析和结果解读在试验结束后,需要对收集到的数据进行统计分析和结果解读。
通过合适的统计方法,评估试验的效果和安全性,并给出可靠的结论和建议。
同时,将试验结果发布和共享,促进科学进步和临床实践的改进。
六、讨论和总结在完成一项临床试验后,还需要进行讨论和总结,包括试验结果的意义、局限性和启示,以及对进一步研究的建议和展望。
与其他研究者和专家进行交流和合作,进一步提高临床试验的质量和水平。
综上所述,要做好一项临床试验,需要从设计与计划、伦理审查、数据收集和管理、试验执行和监督、数据分析和结果解读、讨论和总结等多个方面进行全面的考虑和操作。
如何在中国开展临床试验:一个临床研究者的经验分享

如何在中国开展临床试验:一个临床研究者的经验分享随着循证医学的发展,越来越多的临床试验在我国开展。
如何高质量地完成临床试验,成为有志于临床科研的医生的重要课题。
临床科研种类繁多。
凡涉及人群研究的大凡有两种:一种是临床调查,如某种疾病的临床特点观察、注册研究等,一般对治疗不予干预;另一种是对新的诊断方法和新的治疗手段的评价(包括药物、器械、治疗手段等),一般称为临床试验。
作为药物试验,可以分为Ⅰ~Ⅳ期,每一期都有不同的目的、试验人群和试验方法。
目前我国参加的大规模临床试验多为Ⅲ期或Ⅳ期试验,以临床疗效或安全性为主要试验目的。
本文重点介绍临床试验。
在我国,临床试验可以是国家项目,包括政府部门和学术组织发起的项目。
也可以是医药器械厂家、研究单位自行或联合开展的项目。
所谓研究者发起的科研项目应该挂靠在一个研究单位进行。
近年来随着国际学术交往的增加,国际多中心大规模临床试验不断进入我国,使我们有了参与国际合作和介入世界顶级临床科研项目的机会。
无论试验的种类如何,有一个共同的原则必须遵守:临床试验管理规范(GCP)。
这是临床试验的精髓。
国际上实行临床试验的GCP管理已经数十年。
我国也在上世纪90年代颁布了自己的规范,目前实施的是2003年的版本。
GCP内容十分丰富,但归结起来就是两个基本点:保证试验的科学性;保证受试者的权益和安全。
一切措施和规定都要从这两个“保证”出发。
既往一些试验受到批评,除了在学术上存在问题外,多数与没有完全遵从GCP有关。
特别是试验涉及新药或新器械批准上市的问题,国内外各级药监当局都十分重视试验进程中是否遵循GCP,这也成为稽查的主要内容。
试验前的准备一项临床试验在实施之前最重要的是确定试验的目的、方法和方案。
如果是多中心试验,由牵头单位(国际或国内)提出试验设想和初步方案,由试验指导委员会会议进行全面讨论,确定方案。
在试验准备阶段,可以对方案提出修改意见,包括各中心的课题负责人。
但一旦经伦理委员会批准确定后,任何人和单位都无权自行修改。
如何在中国开展临床试验(二)——一个临床研究者的经验分享

2 入选 策略 :临床 试验 的入 选是课题 正式 实施 的 .
3正 确随机 :随机分 配入 选 患者是现代 临床 试验 .
1 中国处方药 ']J a]3 OJ 2 O ON 0 0
上 l 性的基础 ,多数 试验 采用 双盲试验 。每个 试 几 方法可 能有所 不 同,为保证 随机 的质量 ,现
心 随 机 。 国 际 多 中心 试 验 一 般 采 用 全 球 电话
些是从 试验 的科 学性 考虑 ( 如不要使 用具有相 同作 用 的 药物 ),有 些则是 考虑 受试者 的安全性 ,特别是 药
物 的协 同副作 用 。 所有 试 验都 要 求严 格 记 录 伴 随用 药 。有 时伴 随用 药 的 变化 可 能反 映 了不 良事 件 的发 生,这是 必须注 意的 。患者有 时 因其他 疾病去 试验 中 心以外 的医院或 医生就诊 ,此 时特 别容 易发生 问题 。
可 以发 动更多 的医生推荐 患者,但入选 最好 集 中在 了
解 方 案 的 少 数 医生 , 而 且 一 定要 随 时能 联 系 到 。 在 入
选排 除标 准方 面出现似 是 而非的情况 时要请 示。不要 “ 请示”监 查 员,而应 首先请 示本单位P 。若在 是否 I
入 选 方 面 一 时 无 法 确 定 ,应 以 患者 病 情 处 理 为 首要 任
宣讲 ,并要 有 书面材料放 在相应位 置 ,急诊 室和重症
病 房 的 一 线 医生 的配 合 最 为 重 要 。课 题 入 选 人 员要 随 叫 随 到 ,有 些 医院 将 课 题 交 给 心 内科 总住 院 医 师 , 这
或 被发 现 有 方 案 违 背 ,应 积极 主 动 向 上级 汇报 , 由试 验
如何在中国开展临床试验三——一个临床研究者的经验分享

如何在中国开展临床试验(三)保证试驻质量%床试验竹质量是其成败的*键伴Ⅱ试啦晴量,A 验十-o起着m定*的作月。
“试验进行十”日各《自客部置镕*试口质量∞措《,在此{*赞4(一)#∞枉§耙t§埔量∞—十t要律、Ⅸ是自∞报告袁(C it F)”填写.《药蜥*康试☆质量管理规范》十研究者∞职责第二十t素规赶:研克者应镕证蒋敷#真实准确充垫,厦目、§*№栽^璃自女蒋自拒告私根*《样—素规定+高质量的0啦镕具有ⅡT备件:1_教据∞T目#-B.这是保证试验科学性日i妻}求.对于%J茕诲§.舟i L的敷#幕£自∞和原*检查报告有女试验为Ti便,自制7十i¨资料粟,相g十批R F自译成十文.这#材料可“作自十月媒舟日}式使月,但{■作为原*赍料。
在稽查或女律层自*于承沁目此,十分重要日一.£是,c盯上日自喜在#自±一£要能壹到重要*涉A伦gn自客&一定兽在#自±有M记录,如L经#得患者善半日知情目毒韦8一^{太睿易做到,目*#床试☆要求∞自客自—&自目要求是{一#的我目有些E&∞n诊惠者没有镕存^∞,4自数据∞TⅫ*性&成7根t*麻Ⅻ.解4∞#女之一是自Ⅸ%#章管理部f1联§,为t±拳∞1占A试n自々患者特““£符夸自棠要求日$m.并保日在E№女#,在‰康试验十,要§m#牲假#列晰的修&2cR F的标堆操作规程元论i《质∞c1/F还是t}的el l F(包括目±CIt F和1D at arax)都*《根据G cP的要求规£书目或i^速在任何一十魁束A验∞B自"桩都是m须**的.*果健月纸质应镕十g清楚t规岳2.如果发生错误,接月规范进行傍&井§丰.&¨修燕E l期无论*何修&,都R&使月最原*的一*表格,绝*能重抄.现在使月目上#d报告表时试☆越束越,,但除7记录和传递式与‰质袁格}目外,其他、9织‘j矿原Ⅲ都是一样∞目上病“报告表提高7I作效}.# #提杖目j d就&E馈质t#“}统(ocl,Tm在短H目自£成整十i螗.3厦目性:在目蹙试者进n7八《№机或H访£.&镕尽快Z自啦"最好在3十I作日自完成*提女."目抱得越长,越客易牲错*,《误-缸4一£"目5.*爱试者套被列^可能女#的2单.$库试验—般都有∞,对L提交的c盯谢t质控,#e发日质控掘告收j惦要尽快按要求恬陡(&意鍪字和日期},#目再女提交.很} *床试嘧时cⅡ进行£期∞质量姊,甜疏成并提主c”的时间cl t t:酌一女正日丰.C l IF的O c敷量oc垮&情况覆擞据的§前t目率等(二}监圭和稽查:*药物%床试啦质量管4规范*中研究者B责第=十^条微“研克者应接量十办者镕遣∞监查自或稽查自的监查和镕查噩药sm督管4∞1的#查榭嘛自保#庳试验的质量”监壶I作女;自中办者自秆或委托进行,在我目也有,c0委托t行的情况,但{得自试验的参*^自宾秆监查是镕证试验质量的|要措施,自监查i按M#$操#规程时试验∞每一十自节m行检查研£者&E}监查,在&查5仔自目读监查i提☆∞报},并且*对其十的目艇t”&进值得*意的是.监壹自R m责有*课题进行十自#学&十伦理j自的监壹,{自B#*患者.{《代*研i者进行八连或随访,}能参与惠者∞%*i毒∞《括自±m{R事件自≈理.在《验十∞职责*据{R《题有* {目.但}能要求其解№m有目题稽查可自中^者进行,也■自地i目家甚i目外∞药;管理-5局进目稽查∞目∞&括帮m提高试§日质量,在批准根据A验%£申报*t&证前自严格捡壹,m 丑对试验¨真实*进行拴查等,对这#稽圭,自然要认真准备、但平目试蜥量的好#0经女定7稽查的镕^在稽圭十,应镕实事求是,{要对女现的月《做过多时解释,£{能造韫.在矗5一位爱试者完成置e一女随访e,%床试謦的进行阡R就完成T.但这并非意味着整个*床*验的镕束,还有很}事要靛.1受试者∞.女排患者完成最e一女随访e,就进八T常规E疗状态.如H从试嘧;}疗精接到常规镕疗,有H 并{喜易.卑分试嘧在此剥需要奎雌揭言,“便安排f一步%疗.但;擞试验是}揭盲自从斌§%疗苴*进^常规%疗.}素—艇台对衔接}女做自建议,研觅者要根据速g建Ⅸ和-每位患者的情况为其安排%疗i棠.对f晟事件∞m麝有时奢超☆最5随访的日期—段时间,甚至敷月有些试验喜g长月药的现釉,速宾Rt是开*7*—十试鞋.需要&i圭伦理垂i喜∞批准,患者要重新§暑自情目毒书.2完成试鞋托据包括cRF厦其婶,缚£事件和支持材料,d覆其他需要提交古々材料.自f目前口床试骑也存在竞争,需要在镕柬后尽快发表#果,所d对敷据∞收集在速一阶段往往进^冲剌状态.套制定一十时问表,规£在何时锁定敷据库作为试验中o,应谊积极配§运一i作.3完成试验报告:根据《药枷#床试验质量管理规范》研充者的职青年=十备,“*床斌蹬完成后,研究者*gg自尊%报告,鉴0并i明日期后送十女者。
临床试验方案1

临床试验方案1一、研究背景和目的目前,随着医学科技的不断进步,临床试验在医学研究中扮演着至关重要的角色。
本研究旨在探索一种新型药物对特定疾病的疗效和安全性,为该疾病的治疗提供科学依据。
二、研究对象和方法1. 研究对象:本研究将招募符合以下条件的病患作为研究对象:- 年龄在18岁至60岁之间;- 疾病确诊时间超过3个月;- 具有该特定疾病的明确症状。
2. 研究方法:本研究将采用随机分组的双盲试验方法,将被选中的研究对象随机分为两组:实验组和对照组。
实验组将接受新药物治疗,而对照组将接受安慰剂。
3. 实施步骤:- 首先,将符合入选标准的病患随机分组,并告知实验组和对照组的用药方案;- 针对实验组,按照给药方案进行药物治疗,并记录下每次用药的剂量和频率;- 针对对照组,按照相同的用药方案进行安慰剂治疗,确保双盲试验的严密性;- 在整个治疗期间,对研究对象的疾病症状进行定期观察和评估,并记录下来;- 在试验结束后,对实验组和对照组的治疗效果进行统计学分析。
三、预期结果本研究预期可以得出以下结果:- 新药物治疗组与安慰剂治疗组相比,在改善特定疾病症状上具有显著差异;- 新药物治疗组的不良反应发生率较低,安全性较高;- 新药物治疗组在生活质量、身体功能等方面的改善效果优于安慰剂治疗组。
四、伦理与安全性考虑1. 伦理考虑:本研究将遵循医学伦理原则,研究对象的存在与利益将得到充分的尊重和保护。
研究过程中将获得研究对象的知情同意,并明确告知研究的目的、方法、可能的风险和潜在好处,确保研究过程的透明性和公正性。
2. 安全性考虑:针对新药物治疗组,严密监测研究对象的身体反应和用药过程中的不良反应,及时采取必要的安全措施。
研究进行期间,研究团队将与医院和监管机构保持密切合作,及时上报任何重大不良事件。
五、数据分析和结果呈现1. 数据分析:本研究将采用统计学方法对研究结果进行数据分析,使用适当的统计指标来评估治疗效果和安全性。
药品临床试验管理的国际合作与经验分享

药品临床试验管理的国际合作与经验分享在全球范围内,药品的研发和临床试验管理是医药行业的重要环节。
为了确保试验结果的准确性和可靠性,各国之间进行了广泛的合作与经验分享。
本文将探讨药品临床试验管理的国际合作形式以及各国的经验分享。
一、国际合作形式1. 药品知识共享平台为促进国际间的知识交流,一些国际组织和机构设立了药品知识共享平台。
该平台收集和整合各国的药品信息和临床试验数据,为研究人员提供参考和借鉴的资源。
例如,世界卫生组织(WHO)的国际药品审评合作项目就提供了一个平台,让各国合作共享药品的监管经验和专业知识。
2. 跨国临床试验合作跨国临床试验合作是药品临床试验管理中的重要形式之一。
各国研究机构和制药公司可以共同进行跨国试验,以扩大样本量、增加多样性和提高试验结果的可靠性。
这种合作形式有助于加速药物研发过程,并且更好地满足不同国家和地区的特殊需求。
3. 数据共享和互认数据共享和互认是国际合作中的关键环节。
各国可以共享试验数据,以提高药品研发的效率和保证试验结果的可靠性。
同时,各国可以互认临床试验结果,减少重复试验的需要,并加速药物的上市和推广。
二、国际合作的意义和优势1. 提高研发效率国际合作可以加速研发进程,减少资源和时间的浪费。
合作伙伴之间共享资源、经验和数据,有效减少重复工作,提高研发效率。
2. 扩大试验样本范围国际合作可以扩大试验样本的范围,包括不同地理区域、不同年龄段和不同人群。
这有助于更准确地评估药物的安全性和有效性,并更好地适应全球人口的需求。
3. 共同面对挑战国际合作使各国能够共同面对药物研发和临床试验管理中的挑战。
合作伙伴可以共同制定标准和指南,共享最佳实践,以提高试验质量和保证试验的伦理和法律合规性。
三、各国经验分享1. 美国的经验美国在药品临床试验管理方面具有丰富的经验。
美国食品药品监督管理局(FDA)制定了严格的法规和指南,确保临床试验符合伦理和法律要求。
此外,美国还通过建立数据库和信息共享平台,促进了科学知识的交流与传播。
年度临床研究成果总结

年度临床研究成果总结近年来,临床研究在改善医疗质量、提高患者生活质量方面发挥了重要作用。
本文将总结我院在过去一年中所取得的临床研究成果,以期为医学界提供有价值的参考和经验分享。
一、研究背景临床研究是为了解决医学领域中的问题和提升临床实践的有效性而进行的系统研究。
本次年度总结主要关注于我院在各个科室开展的研究工作,旨在为医疗团队提供更好的诊疗方案和治疗方法。
二、研究成果1. 临床试验研究成果在过去的一年中,我院开展了多项临床试验研究,涉及癌症治疗、心血管疾病管理、神经科学等多个领域。
其中,我们对某种新型药物的疗效和安全性进行了评估,获得了积极的结果。
此外,我们还探索了一种新的手术治疗方式,有效地提高了手术成功率和患者的生活质量。
2. 临床观察研究成果除了临床试验研究外,我院还重点关注了临床观察研究。
我们通过长期随访和数据分析,对某种慢性疾病的发病机制和治疗方法进行深入研究。
通过收集患者的临床数据和生活方式数据,我们发现某种疾病的预防和控制与患者的饮食结构和运动习惯密切相关。
这一发现为疾病预防和康复治疗提供了有益的参考。
3. 数字医疗研究成果随着数字医疗技术的不断发展,我院积极开展了相关研究工作。
我们通过搭建远程医疗平台,实现了医患之间的在线交流和医学影像的远程诊断。
这种创新的医疗方式不仅提高了患者就医的便利性和效率,还有效降低了医疗资源的浪费。
4. 医学教育研究成果作为一所医学院附属医院,我院一直注重医学教育的研究和改进。
在过去的一年中,我们通过问卷调查和课堂观察等方式,对医学生的学习效果和教学方法进行了评估。
在我们的努力下,医学生的综合能力和专业素养得到了显著提高。
三、成果应用与前景展望我们的研究成果已经得到了一定的应用和推广。
某项新药物的研究结果已被药物监管部门认可,并纳入了临床常用治疗方案。
我们的数字医疗平台已在多个科室得到应用,为大量患者提供了及时高效的医疗服务。
此外,我们的成果还被用于学术交流和研讨会上,取得了良好的反响。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
开始。
一般来讲是按照所谓连续入选的原则进行,即凡是符合标准的患者都应进入入选的程序进行筛选。
入选患者一定要严格遵从方案的入选排除标准,过松会增加不符方案的患者进入,过严实际上是增加了新的排除标准,最终变成所谓“超选”。
两种情况都P32RAS,联合控制心肾损害的靶标心脏和肾脏各自独立,却又紧密相联。
多种危险因素可同时作用于心肾,造成两者联合损害。
肾素-血管紧张素系统(RAS)是两器官联系的“纽带”,其激活是心血管疾病发生发展的关键。
因此,抑制RAS,是心血管疾病防治的“重头戏”。
ABSTRACT重点导读P26对超说明书用药多种意义上的完善思考缺乏医院批准和行政许可的临床试验和实验性临床医疗程序而所采取的“超说明书用药”,本身既不科学,也不合法。
“科学性”、“社会性”、“为患者好”必须建立在符合法律程序之上才具有正当性。
P10如何在中国开展临床试验(二)临床试验进行中的方案执行尤为重要,关乎整个试验的成功与否。
临床试验团队应对方案执行中的每一细节力求按计划进行,如发生方案偏倚或违背时,要及时发现并积极处理,尽可能令方案始终按预定的基线进行。
另外,试验进行中首先考虑的是患者的安全性,一切以患者为中心。
P70浅析临床试验中的偏离或违反方案问题偏离及违反方案的情况及发生率尽管不可避免,但是要尽一切努力,并采取积极措施,减少其发生,因为严重的违反方案会直接影响试验数据的质量,进而影响试验的结果,甚至左右该新药能否通过政府药政主管机构的核决。
P28健康中国,掀起“心”革命——第21届长城国际心脏病学会议报道心血管疾病已成为全世界的“头号杀手”,并仍持续增长,其中大约有一半的病例发生在亚太地区。
而且与西方国家相比,亚洲地区的心血管疾病患者更趋年轻化。
因此,对心血管的防治战线必须前移。
P38ACEI,阻断RAS系统的领跑者自第一个肾素血管紧张素转化酶抑制剂(ACEI)问世以来,ACEI便以卓越的疗效活跃于降压药物的舞台,拥有大量的循证医学证据,其适应证也在不断扩展。
即便在RAS系统抑制剂的其他成员的挑战下,其光环依旧,王者地位尚不可动摇。
P50氨磷汀在血液系统疾病中的应用细胞保护剂已成为抗肿瘤药物研究与开发领域的“新星”,氨磷汀就是其中的一名佼佼者,在抗肿瘤、逆转耐药和保护正常细胞方面具有潜在的应用价值,目前已被广泛用于临床多种血液系统疾病。
英文摘要Journal of China Prescription Drug2010.10VOL.103SummarySpecial ReportOff-label Drug Use, Pros and Consfrom the V iew of Regulation and Science?Off-label use is the practice of prescribing pharmaceuticals for an unapproved indication; off-label use may include irrational use and evidence-based treatments required by the patients’ conditions. The former one is due to the irresponsibility of physicians, and the latter one is needed by the patients’ conditions. Although the latter condition may be supported by clinical evidence, off-label use implicates that both the physicians and patients will face more extra risks. The phenomenon of off-label will continue to exist, how to regulate and avoid the possible risk should be considered the most importantly.H o w t o R e g u l a t e O f f -l a b e lD r u g U s e ,a M u l t i -p e r s p e c t i v e ExplorationWithout hospital approval and administrative licensing, any off-label use in the name of clinical trial and experimental clinical procedure is neither rational nor legal. Only rationality, sociality, and benefits to the patients that comply with legal proceedings are legitimate.ColumnH o w t o C o n d u c t C l i n i c a l Tr i a l s i n China? (II)The implementation of clinical trial protocol is particularly important, which is related to whether the whole clinical trial can succeed or not. Clinical trial team should ensure that any detail in the protocol should be implemented according to the plan, if any bias or violation to the original portal occurs, the protocol should be implemented according to the planned baseline as strict as possible. In addition, safety of the participating patients should be the priority to be considered in clinical trial, and patients should be focused as the center.InsightDevelopment of Diet Pills Becomes a Hot PotatoFaced with the huge market of diet pills, why some many pharmaceutical manufacturers stay away? Is it due to dif fi culty in drug development, strict drug approval, or lawsuit and huge losses due to post-marketing adverse effects? The paradox is worth pondering!ConferencesHealthy Heart Revolution in Healthy China—Report from the 21st Great Wall International Congress of Cardiology (GW-ICC)Cardiovascular disease has become the world's No. 1 killer, and still growing, with about half of the cases occurred in the Asia Paci fi c region. And compared with the western countries, the patients with cardiovascular diseases in Asia tend to be younger. Therefore, the front for the prevention and treatment of cardiovascular diseases in Asia should be moved forward.Cardiovascular DiseaseRAS:the Target for Combined Controlof Heart and Kidney ImpairmentHeart and kidney are independent, but closely linked. Multiple rick factors may act on heart andkidney simultaneously, causing combined damage to heart and kidney. The renin-angiotensin system (RAS) is a hormone system that regulates blood pressure and fl uid balance, and the activation of RAS is the key of pathogenesis and development of cardiovascular disease. Therefore, inhibition of RAS is the key to the prevention and treatment of cardiovascular disease.ACEI, the Primary Blocker of RASSince the marketing of the first angiotensin-converting enzyme inhibitor (ACEI), ACEIs have been used primarily in treatment of hypertension and congestive heart failure with the excellent efficacies that are supported by lots of clinical evidences, and the indications have been expanded. Even challenged by other RAS inhibitors, the efficacies of ACIEs remain to be excellent, and the preferred position cannot be shaken.JCPD•CSCO Clinical OncologyAmifostine in the Treatment of Blood System DiseasesCell protective agents have become a new star in R&D of antineoplastic agents, and amifostine is one of the leaders with potential antineoplastic, tolerance-reversing, and normal cell protecting values, which has been widely used in a variety of blood system diseases in clinical practices.Fan DachaoAnalysis of Bias or Violation inClinical TrialsAlthough bias and violation to the clinical trial protocol cannot be avoided, any effort should be exerted and positive measures should be taken to reduce its occurrence, because serious violation of the clinical trial protocol can directly affect the data quality of clinical trial, thereby affecting the trial result, and even can in fl uence the veri fi cation and approval of a new drug by the regulators.(Editor Yao Lixin)。
