天津大学无机化学课后习题参考答案
天津大学无机化学第五版习题答案

第1章 化学反应中的质量关系和能量关系 习题参考答案1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g 。
3.解:一瓶氧气可用天数33111-1222()(13.210-1.0110)kPa 32L9.6d 101.325kPa 400L d n p p V n p V -⨯⨯⨯===⨯⨯4.解:pV MpVT nR mR== = 318 K 44.9=℃ 5.解:根据道尔顿分压定律ii n p p n=p (N 2) = 7.6⨯104 Pap (O 2) = 2.0⨯104 Pa p (Ar) =1⨯103 Pa6.解:(1)2(CO )n = 0.114mol; 2(CO )p = 42.87 10 Pa ⨯(2)222(N )(O )(CO )p p p p =--43.7910Pa =⨯ (3)4224(O )(CO ) 2.6710Pa0.2869.3310Pan p n p ⨯===⨯ 7.解:(1)p (H 2) =95.43 kPa (2)m (H 2) =pVMRT= 0.194 g 8.解:(1)ξ = 5.0 mol(2)ξ = 2.5 mol结论: 反应进度(ξ)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:∆U = Q p - p ∆V = 0.771 kJ 10.解: (1)V 1 = 38.3⨯10-3m 3= 38.3L(2) T 2 =nRpV 2= 320 K (3)-W = - (-p ∆V ) = -502 J (4) ∆U = Q + W = -758 J (5) ∆H = Q p = -1260 J11.解:NH 3(g) +45O 2(g) 298.15K−−−−→标准态NO(g) + 23H 2O(g) m r H ∆= - 226.2 kJ ·mol -1 12.解:m r H ∆= Q p = -89.5 kJ m r U ∆= m r H ∆- ∆nRT= -96.9 kJ13.解:(1)C (s) + O 2 (g) → CO 2 (g)m r H ∆ = m f H ∆(CO 2, g) = -393.509 kJ ·mol -121CO 2(g) + 21C(s) → CO(g)m r H ∆ = 86.229 kJ ·mol -1CO(g) +31Fe 2O 3(s) → 32Fe(s) + CO 2(g)m r H ∆ = -8.3 kJ ·mol -1各反应 m r H ∆之和m r H ∆= -315.6 kJ ·mol -1。
天津大学无机化学教研室《无机化学》(第4版)课后习题(原子结构与元素周期性)【圣才出品】

价层电子构型为 为相似。
所以它属于第七周期、ⅣA 族,可能与已知元素 Pb 的性质最
5 / 13
圣才电子书
十万种考研考证电子书、题库视频学习平
台
(2)电子最先填充在第一个 59 轨道上的元素的原子序数可能是 121。
推测:根据电子填充轨道的次序为
可知出
现第一个 59 电子的元素的电子分布式应为
8.(1)试写出 S 区、P 区、d 区及 ds 区元素的价层电子构型。 (2)具有下列价层电子构型的元素位于周期表中哪一个区?它们各是金属还是非金 属?
答:(1)
表 5-2
(2)
表 5-3
3 / 13
圣才电子书
十万种考研考证电子书、题库视频学习平 台
9.已知某副族元素的 A 原子,电子最后填入 3d,最高氧化数为+4;元素 B 的原子, 电子最后填入 4p,最高氧化数为+5。回答下列问题:
所以该元素原子序数是 121。
(3)118。第七周期最后一种元素的价层构型应为
其电子分布式为
所以第七周期最后一种元素的原子序数应为 118。
(4)50。第八周期的元素种数应该是第八能级组可填充的电子数,即
,所以第八周期应该包括(2+18+14+10+6)=50 种元素。
(二)习题 1.在下列各组量子数中,恰当填入尚缺的量子数。 (1) (2) (3) (4) 解:(1)n≥3 正整数; (2)l=1;
(1)写出 A、B 元素原子的电子分布式; (2)根据电子分布,指出它们在周期表中的位置(周期、区、族)。 答:(1)A: B: (2)A:四周期、d 区、ⅣB 族元素;B:四周期、p 区、ⅤA 族元素。
10.不参看周期表,试推测下列每一对原子中哪一个原子具有较高的第一电离能和较 大的电负性值?
无机化学(下)_天津大学中国大学mooc课后章节答案期末考试题库2023年

无机化学(下)_天津大学中国大学mooc课后章节答案期末考试题库2023年1.下列不属于真正矾类的是。
参考答案:FeSO4·7H2O2.金属锡与浓硝酸反应所得到的产物有_________。
参考答案:H2SnO3(β)和NO23.列硝酸盐中,热分解产物之一为金属单质的是______。
参考答案:Hg(NO3)24.用于说明铋酸钠具有强氧化性的是。
参考答案:惰性电子对效应5.下列物质不是一元酸的是_______。
参考答案:偏硅酸6.下列化合物中不属于缺电子化合物的是_____。
参考答案:Na[BF4]7.下列各组离子中每种离子分别与过量NaOH溶液反应时,都不生成沉淀的是。
参考答案:Be2+、Al3+、Sb3+8.过氧化钠常作融矿剂,使既不溶于水又不溶于酸的矿石被氧化分解为可溶于水的化合物。
参考答案:正确9.下列物质可与二氧化碳反应生成氧气的是________。
参考答案:KO210.在所有的金属中,熔点最高的是副族元素,熔点最低的是主族元素。
参考答案:错误11.碳酸氢钠和碳酸钠可以通过分别在其溶液中加入CaCl2观察是否生成沉淀来进行鉴别。
参考答案:错误12.氢气能使粉红色的PdCl2水溶液迅速变黑,可利用这一反应检出氢气。
参考答案:正确13.第一个稀有气体化合物是XeF2,打破了过去长时间以来人们一直认为稀有气体的化学性质是“惰性”错误认识。
参考答案:错误14.通常,同一元素不同氧化态的氧化物的水合物,该元素的氧化数越高,酸性越强。
参考答案:正确15.液氢是超低温制冷剂,可将除氦外的所有气体冷冻成固体。
参考答案:正确16.氢与钙元素形成的二元化合物为金属型氢化物。
参考答案:错误17.我国古代炼丹术是化学的雏形,如采用朱砂氧化法制备得到金属汞。
参考答案:正确18.碱金属离子因其电荷少,半价大,所以不会形成配合物。
参考答案:错误19.治理土壤的碱性常用的物质为________。
参考答案:石膏20.下列物质在水中溶解度最大的是________。
无机化学第四版习题答案

无机化学第四版习题答案【篇一:天大第4版无机化学_课后习题参考答案】>第1章化学反应中的质量关系和能量关系习题参考答案3.解:一瓶氧气可用天数n1(p?p1)v1(13.2?103-1.01?103)kpa?32l???9.6dn2p2v2101.325kpa?400l ? d-14.解:t?pvmpv?= 318 k ?44.9℃5.解:根据道尔顿分压定律pi?p(n2) = 7.6?104 pap(o2) = 2.0?104 pa p(ar) =1?103 panip n6.解:(1)n(co2)? 0.114mol; p(co2)? 2.87 ? 104 pa(2)p(n2)?p?p(o2)?p(co2)?3.79?104pa (3)n(o2)p(co2)4???0.286 np9.33?104pa7.解:(1)p(h2) =95.43 kpa(2)m(h2) =pvm= 0.194 g 8.解:(1)? = 5.0 mol(2)? = 2.5 mol结论: 反应进度(?)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:?u = qp ? p?v = 0.771 kj 10.解:(1)v1 = 38.3?10-3 m3= 38.3l(2) t2 =pv2= 320 k nr(3)?w = ? (?p?v) = ?502 j (4) ?u = q + w = -758 j (5) ?h = qp = -1260 j11.解:nh3(g) +5o(g) ???3?298.15k4212.解:?rhm= qp = ?89.5 kj ?rum= ?rhm? ?nrt= ?96.9 kj13.解:(1)c (s) + o2 (g) → co2 (g)1co(g) + 1c(s) → co(g)222?co(g) +1feo(s) → 2fe(s) + co(g)23233?(2)总反应方程式为3c(s) + o(g) + 1feo(s) → 3co(g) + 2fe(s)22322323?由上看出:(1)与(2)计算结果基本相等。
天津大学无机化学 课后习题参考答案

� 9.6d
��解� T
�
pV nR
�
MpV mR
= 318 K � 44.9 ℃
��解�根据道尔顿分压定律
p(N2) = 7.6�104 Pa p(O2) = 2.0�104 Pa p(Ar) =1�103 Pa
pi
�
ni n
p
��解��1� n(CO2 ) � 0.114mol; p(CO2 ) � 2.87 � 104 Pa �2� p(N 2 ) � p � p(O2 ) � p(CO2 ) � 3.79 �104 Pa
�
r
H
� m
(298.15
K)
�1573.15
�
r
S
� m
(298.15
K)
= 70759 J ·mol�1
lg K � (1573.15 K) = �2.349, K � (1573.15 K) = 4.48�10�3
10. 解� 平衡分压�kPa
H2(g) + I2(g)
2HI(g)
2905.74 �χ 2905.74 �χ
平衡分压/kPa
x
x
� �� � K � = p (NH 3 ) / p� p (H 2S) / p� = 0.070
则 x = 0.26�100 kPa = 26 kPa
平衡时该气体混合物的总压为 52 kPa
�2�T 不变� K � 不变。
NH4HS(s) � NH3(g) + H2S(g)
平衡分压/kPa
�2� K c
=
�c
(N
2
)�
1 2
�c
(H 2 )
�3 2
天津大学无机化学课后习题答案第五章 原子结构与元素周期性

第5章原子结构与元素周期性习题参考答案1.解:(1)n≥3正整数;
(2)l=1;
(3)m s= +½(或 ½);
(4)m=0。
2.解:(1)不符合能量最低原理;
(2)不符合能量最低原理和洪德规则;
(3)不符合洪德规则;
(4)不符合泡利不相容原理;
(5)正确。
3.解:(1)2p x、2p y、2p z为等价轨道;
(2)第四电子层共有四个亚层,最多能容纳32个电子。
亚层轨道数容纳电子数
s 1 2
p 3 6
d 5 10
f 7 14
32
4.解:(2)P(Z=15)
(3)1s22s22p63s23p64s2
(4)Cr [Ar]
(5)Cu
(6)[Ar]3d104s24p6
5.解:(1)[Rn] 5f146d107s27p2, 第7周期,ⅣA族元素,与Pb的性质最相似。
(2)[Rn] 5f146d107s27p6,原子序数为118。
6.解:离子电子分布式
S2-1s22s22p63s23p6
K+1s22s22p63s23p6
Pb2+ [Xe]4f145d106s2
Ag+ [Kr]4d10
Mn2+ 1s22s22p63s23p63d5
Co2+1s22s22p63s23p63d7
7.解:
8.解:
9.解:(1)A、B;
(2)C-、A+;
(3)A;
(4)离子化合物,BC2。
10.解:(1)有三种,原子序数分别为19、24、29;(2)
11.解:
12.解:
(1)原子半径大小(2)第一电离能小大(3)电负性小大(4)金属性强弱。
天大无机化学第四版思考题和习题答案

第八章配位化合物思考题1. 以下配合物中心离子的配位数为6,假定它们的浓度均为0.001mol·L-1,指出溶液导电能力的顺序,并把配离子写在方括号内。
(1) Pt(NH3)6C14(2) Cr(NH3)4Cl3(3) Co(NH3)6Cl3 (4) K2PtCl6解:溶液导电能力从大到小的顺序为[Pt(NH3)6]C14>[Co(NH3)]6Cl3>K2[PtCl6]>[Cr(NH3)4Cl2]Cl2. PtCl4和氨水反应,生成化合物的化学式为Pt(NH3)4Cl4。
将1mol此化合物用AgN03处理,得到2molAgCl。
试推断配合物内界和外界的组分,并写出其结构式。
解:内界为:[PtCl2(NH3)4]2+、外界为:2Cl-、[PtCl2(NH3)4]Cl23.下列说法哪些不正确? 说明理由。
(1) 配合物由内界和外界两部分组成。
不正确,有的配合物不存在外界。
(2) 只有金属离子才能作为配合物的形成体。
不正确,有少数非金属的高氧化态离子也可以作形成体、中性的原子也可以成为形成体。
(3) 配位体的数目就是形成体的配位数。
不正确,在多齿配位体中配位体的数目不等于配位数。
(4) 配离子的电荷数等于中心离子的电荷数。
不正确,配离子电荷是形成体和配体电荷的代数和。
(5) 配离子的几何构型取决于中心离子所采用的杂化轨道类型。
正确4.实验测得下列配合物的磁矩数据(B.M.)如下: 试判断它们的几何构型,并指出哪个属于内轨型、哪个属于外轨型配合物。
5.下列配离子中哪个磁矩最大?[Fe(CN)6]3-[Fe(CN)6]4-[Co(CN)6]3-[Ni(CN)4]2-[Mn(CN)6]3-可见[Mn(CN)6]4的磁矩最大6.下列配离子(或中性配合物)中,哪个为平面正方形构型?哪个为正八面体构型? 哪个为正四面体构型?*7. 用价键理论和晶体场理论分别描述下列配离子的中心离子的价层电子分布。
天津大学无机化学教研室《无机化学》(第4版)课后习题(酸碱反应和沉淀反应)【圣才出品】

第3章 酸碱反应和沉淀反应(一)思考题1.阐述下列化学名词、概念的含义。
解离常数,解离度,分步解离,水解常数,水解度,分步水解,水的离子积,缓冲溶液,溶度积,溶度积规则,分步沉淀,沉淀完全,沉淀转化答:(1)解离常数:在溶液中存在着已解离的弱电解质的组分离子和未解离的弱电解质分子之间的平衡,该平衡的平衡常数称为解离常数。
(2)解离度:弱电解质在溶剂中解离达平衡后,已解离的弱电解质分子百分数称为解离度。
(3)分步解离:多元弱酸在水溶液中的解离是分步(或分级)进行的,平衡时每一级都有一个相应的解离平衡常数。
(4)水解常数:对于强碱弱酸盐、强酸弱碱盐、弱酸弱碱盐,盐的组分离子与水解离出来的H+或OH-结合成弱电解质的反应的平衡常数称为水解常数。
(5)水解度:盐水解部分的物质的量或浓度与始态盐的物质的量或浓度的比值称为水解度。
(6)分步水解:与多元弱酸(或多元弱碱)的分步解离相对应,多元弱酸盐(或多元弱碱盐)的水解也是分步进行的。
(7)水的离子积:水的解离平衡常数称为水的离子积。
(8)缓冲溶液:弱酸与弱酸盐、弱碱与弱碱盐等混合液保持pH相对稳定作用的溶液称为缓冲溶液。
(9)溶度积:难溶强电解质在水中虽然难溶,但仍有一定数量的构晶离子离开晶体表面而进入水中,当溶解与沉淀的速率相等时,晶体和溶液相应的离子之间达到动态的多相离子平衡,该溶解平衡常数称为溶度积。
(10)溶度积规则:,该规律称为溶度积规则。
(11)分步沉淀:体系中同时含有多种离子,离子可能与加入的某一沉淀剂均会发生沉淀反应,生成难溶电解质,则离子积首先超过溶度积的难溶电解质先沉出,这种在混合溶液中多种离子发生先后沉淀的现象称为分步沉淀。
(12)沉淀完全:在进行沉淀反应时,当离子浓度小于10-5时,可以认为沉淀基本完全。
(13)沉淀转化:借助于某一试剂的作用,把一种难溶电解质转化为另一难溶电解质的过程称为沉淀转化。
2.在氨水中加入下列物质时,的解离度和溶液的pH 将如何变化?(1)加(2)加(3)加答:(1)使的解离度下降,溶液的pH 减小;(2)使的解离度下降,溶液的pH 升高;(3)使的解离度增大,溶液的pH 减小;(4)使的解离度增大,溶液的pH 的变化与加水量的多少有关。
天津大学无机化学第五版习题答案解析

第1章 化学反应中的质量关系和能量关系 习题参考答案1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g 。
3.解:一瓶氧气可用天数 4.解:pV MpVT nR mR== = 318 K 44.9=℃ 5.解:根据道尔顿分压定律p (N 2) = 7.6⨯104 Pa p (O 2) = 2.0⨯104 Pa p (Ar) =1⨯103 Pa6.解:(1)2(CO )n = 0.114mol; 2(CO )p = 42.87 10 Pa ⨯(2)222(N )(O )(CO )p p p p =--43.7910Pa =⨯ (3)4224(O )(CO ) 2.6710Pa0.2869.3310Pan p n p ⨯===⨯7.解:(1)p (H 2) =95.43 kPa (2)m (H 2) =pVMRT= 0.194 g 8.解:(1)ξ = 5.0 mol(2)ξ = 2.5 mol结论: 反应进度(ξ)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:∆U = Q p - p ∆V = 0.771 kJ 10.解: (1)V 1 = 38.3⨯10-3m 3= 38.3L(2) T 2 =nRpV 2= 320 K (3)-W = - (-p ∆V ) = -502 J (4) ∆U = Q + W = -758 J (5) ∆H = Q p = -1260 J11.解:NH 3(g) +45O 2(g) 298.15K−−−−→标准态NO(g) + 23H 2O(g) m r H ∆= - 226.2 kJ ·mol -1 12.解:m r H ∆= Q p = -89.5 kJ m r U ∆= m r H ∆- ∆nRT= -96.9 kJ13.解:(1)C (s) + O 2 (g) → CO 2 (g)m r H ∆ =m f H ∆(CO 2, g) = -393.509 kJ ·mol -121CO 2(g) + 21C(s) → CO(g)m r H ∆ = 86.229 kJ ·mol -1CO(g) +31Fe 2O 3(s) → 32Fe(s) + CO 2(g)m r H ∆ = -8.3 kJ ·mol -1各反应 m r H ∆之和m r H ∆= -315.6 kJ ·mol -1。
天大无机化学第四版 思考题和习题答案要点

天大无机化学第四版思考题和习题答案要点
第八章配位化合物
思考题
1. 以下配合物中心离子的配位数为6,假定它们的浓度均为0.001mol·L-1,指出溶液导电能力的顺序,并把配离子写在方括号内。
(1) Pt(NH3)6C14 (2) Cr(NH3)4Cl3 (3) Co(NH3)6Cl3 (4) K2PtCl6
解:溶液导电能力从大到小的顺序为3- 磁矩/B.M. 4.5 n 杂化类型几何构型 4 sp3d2 2 sp3 0 dsp2 0 d2sp3 3 d2sp3 1 d2sp3 正八面体正四面体正八面体正八面体正八面体轨型外外内内内4- 1.8 平面正方形内
5.下列配离子中哪个磁矩最大?
4的磁矩最大
6.下列配离子(或中性配合物)中,哪个为平面正方形构型? 哪个为正八面体构型? 哪个为正四面体构型?
配合物2+ (kJ·mol-1)
=-96.8 kJ·mol. (kJ·mol-1)
=-156 kJ·mol-1. (kJ·mol-1) =-49.6 kJ·mol-1.
10. 试解释下列事实:
(1) 用王水可溶解Pt,Au等惰性较大的贵金属,但单独用硝酸或盐酸则不能溶解。
(2) [Fe(CN)6]4-为反磁性,而[Fe(CN)6]3-为顺磁性。
*(3) [Fe(CN)6]3-,为低自旋,而[FeF6]3-为高自旋。
(4) [Co(H2O)6]3+的稳定性比[Co(NH3)6]3+差得多。
解:(1)由于王水是由浓硝酸和浓盐酸组成的,浓硝酸将Pt和Au氧化形成的金属离子可与浓盐酸提供的高浓度的
-1。
天津大学无机化学教研室《无机化学》(第4版)(上册)-课后习题-第5~8章【圣才出品】

第5章原子结构与元素周期性(一)思考题1.量子力学的轨道概念与波尔原子模型的轨道有什么区别和联系?答:(1)量子力学的轨道与波尔原子模型的轨道的联系:二者均是用于描述电子、种子、质子等微观粒子的运动。
(2)量子力学的轨道与波尔原子模型的轨道的区别:波尔原子模型的轨道概念是1913年由N.Bohr提出,该模型是建立在牛顿的经典力学理论基础上的,认为微观粒子遵循经典力学的运动规律,电子在原子核外某个确定的原形轨道,但实际上粒子微小、运动速度又极快且在极小的原子体积内运动,根本不遵循经典力学的运动规律。
所以它只能解释单电子原子(离子)光谱的一般现象,不能解释多电子原子光谱,具有一定的局限性;量子力学的轨道概念是1923年薛定谔提出,该模型是建立在波粒二象性的基础上,认为微观粒子不仅具有粒子性,也具有波动性,电子在原子核外某个空间范围内运动,原子中个别电子运动的轨迹是无法确定,没有确定的轨道。
但是电子的运动呈现一定的规律性,可用量子力学理论的电子云进行描述。
2.量子力学原子模型是如何描述核外电子运动状态的?答:用四个量子数描述核外电子运动状态,它们分别是:主量子数-描述原子轨道的能级;副量子数-描述原子轨道的形状;磁量子数-描述原子轨道的伸展方向;自旋量子数-描述电子的自旋方向。
3.下列各组量子数哪些是不合理的?为什么?表5-1答:(2)、(3)不合理。
当n=2时,l只能是0.1,而(2)中的l=2;当l=0时,m只能是0,而(3)中的m却为+1。
4.为什么任何原子的最外层最多只能有8个电子,次外层最多只能有18个电子?答:由于有能级交错的现象,使得轨道的能级次序发生变化,当电子层数(n)较大时,电子填充到轨道的次序为:可见,最外层为nsnp轨道,最多只能填充8个电子;而次外层最多只能填充轨道,即最多有18个电子。
5.为什么周期表中各周期的元素数目并不一定等于原子中相应电子层的电子最大容量数()?答:由于能级交错的原因。
无机化学(上)_天津大学中国大学mooc课后章节答案期末考试题库2023年

无机化学(上)_天津大学中国大学mooc课后章节答案期末考试题库2023年1.关于原子轨道的说法正确的是参考答案:sp3杂化轨道是由同一个原子中能量相近的s轨道和p轨道混合起来形成的一组能量相近的新轨道2.已知K2[ Ni (CN)4]与Ni(CO)4均呈反磁性,所以这两种配合物的空间构型均为平面正方形。
参考答案:错误3.所有金属离子的氨配合物在水中都能稳定存在。
参考答案:错误4.磁矩大的配合物,其稳定性强。
.参考答案:错误5.Cu[SiF6]的名称为六氟合硅(IV)化铜。
参考答案:错误6.配位数为4的杂化轨道类型有两种:dsp2和sp3。
参考答案:正确7.在[FeF6]3-中,仍有5个未成对电子,与自由Fe3+的未成对电子数相同,说明Fe3+以某杂化轨道与配位原子(F)形成轨配键。
参考答案:外8.由实验测得K3[Fe(CN)6]的磁矩为2.0B.M.,此数值与具有一个未成对电子的磁矩理论值1.73B.M.很接近,表明在成键过程中,中心离子的未成对d 电子数减少,d电子重新分布,腾出2个空d轨道,而以某杂化轨道与配位原子(C)形成轨配键。
参考答案:内9.在含有Zn2+和Al3+的溶液中加入过量(两个汉字),可达到Zn2+与Al3+分离的目的。
参考答案:氨水10.[Cr(OH)(C2O4) (H2O)(en)]的配位数是。
(阿拉伯数字)参考答案:611.下列有关分子轨道的叙述错误的是参考答案:原子轨道能级相近即可组合成分子轨道12.在生产中,化学平衡原理应用于 ________。
参考答案:处于平衡状态的反应13.体系从环境吸收60 kJ热,对环境做功70 kJ,根据热力学第一定律,体系热力学能的改变量为______kJ参考答案:-1014.室温下,下列正向反应熵变数值最大的是参考答案:2NH3(g) → 3H2(g) + N2(g)15.下列陈述正确的是参考答案:反应速率常数k是反应物为单位浓度时的反应速率。
大学无机化学第二版答案

大学无机化学第二版答案大学无机化学第二版答案【篇一:无机化学(天津大学第四版答案)】质量关系和能量关系习题参考答案n1(p?p1)v1(13.2?103-1.01?103)kpa?32l9.6dn2p2v2101.325kpa?400l ? d-14.解:t?pvmpvnrmr= 318 k ?44.9℃5.解:根据道尔顿分压定律pi?p(n2) = 7.6?104 pap(o2) = 2.0?104 pa p(ar) =1?103 panip 6.解:(1)n(co2)? 0.114mol; p(co2)? 2.87 ? 104 pa(2)p(n2)?p?p(o2)?p(co2)?3.79?104pa (3)n(o2)p(co2)2.67?104pa0.286 np9.33?104pa7.解:(1)p(h2) =95.43 kpa(2)m(h2) =pvm= 0.194 g rt8.解:(1)? = 5.0 mol(2)? = 2.5 mol结论: 反应进度(?)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:?u = qp ? p?v = 0.771 kj 10.解:(1)v1 = 38.3?10-3 m3= 38.3l(2) t2 =pv2= 320 k nr(3)?w = ? (?p?v) = ?502 j (4) ?u = q + w = -758 j (5) ?h = qp = -1260 j11.解:nh3(g) +5o(g) 3?298.15k4212.解:?rhm= qp = ?89.5 kj ?rum= ?rhm? ?nrt= ?96.9 kj13.解:(1)c (s) + o2 (g) → co2 (g)1co(g) + 1c(s) → co(g)222co(g) +1feo(s) → 2fe(s) + co(g)23233(2)总反应方程式为3c(s) + o(g) + 1feo(s) → 3co(g) + 2fe(s)22322323由上看出:(1)与(2)计算结果基本相等。
《无机化学》答案天津大学无机化学教研室编写高等教育出版社出版第8单元
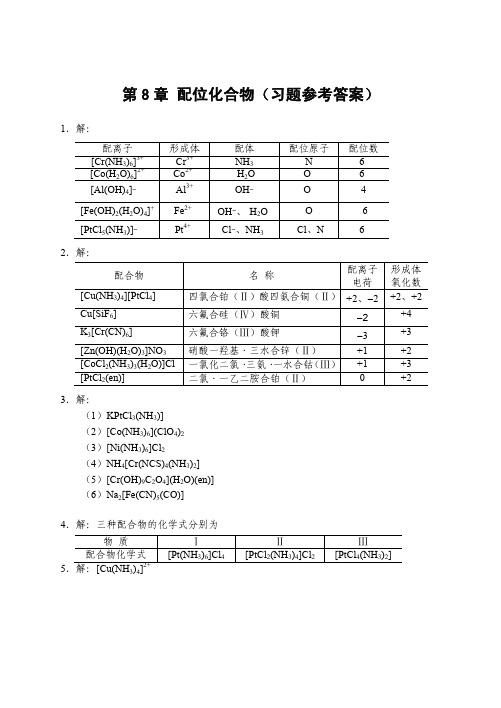
[Ag(CN)2] − y
+ I− y
\ (AgI) = (1.26×1021) × (8.52×10−17) = 1.07×105 K \ = K f\ ( [Ag (CN) 2 ] − ) · K sp
y = 0.49
可见 KCN 可溶解较多的 AgI。
10.解:设 1.0 L 1.0 mol·L−1 氨水可溶解 x mol AgBr,并设溶解达平衡时 c([Ag(NH3)2]+) = x mol·L−1(严格讲应略小于 x mol·L−1)c(Br− ) = x mol·L−1 AgBr(s) + 2NH3·H2O 平衡浓度/(mol·L ) 6.0 − 2 x
配体 NH3 -H2O OH― OH―、-H2OCl―、NH3-
配位原子 N OOO -Cl、N
配位数 664 -6 6-
四氯合铂(Ⅱ)酸四氨合铜(Ⅱ) +2、―2 六氟合硅(Ⅳ)酸铜 六氟合铬(Ⅲ)酸钾 硝酸一羟基·三水合锌(Ⅱ) 一氯化二氯· 三氨· 一水合钴 (Ⅲ) 二氯·一乙二胺合铂(Ⅱ)
[CoF6]3-
8. 解:混合后未反应前: c(Cu2+) = 0.050 mol·L−1 c(NH3) = 3.0 mol·L−1 Cu2+ + 4NH3·H2O
达平衡时: 平衡浓度/(mol·L−1)
2+ 3
[Cu(NH3)4]2+ + 4H2O 0.050 − x
K f\ =
{c ([Cu (NH ) ] )} {c (Cu )} { c (NH ) }
−1
[Ag(NH3)2]+ + Br− + 2H2O x x
\ K \ = K f\ ( [Ag (NH 3 ) 2 ]+ ) · K sp (AgBr) = 5.99×10−6
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第1章 化学反应中的质量关系和能量关系 习题参考答案1.解:1.00吨氨气可制取2.47吨硝酸。
2.解:氯气质量为2.9×103g 。
3.解:一瓶氧气可用天数33111-1222()(13.210-1.0110)kPa 32L9.6d 101.325kPa 400L d n p p V n p V -⨯⨯⨯===⨯⨯4.解:pV MpVT nR mR== = 318 K 44.9=℃ 5.解:根据道尔顿分压定律ii n p p n=p (N 2) = 7.6⨯104 Pap (O 2) = 2.0⨯104 Pa p (Ar) =1⨯103 Pa6.解:(1)2(CO )n = 0.114mol; 2(CO )p = 42.87 10 Pa ⨯(2)222(N )(O )(CO )p p p p =--43.7910Pa =⨯ (3)4224(O )(CO ) 2.6710Pa0.2869.3310Pan p n p ⨯===⨯ 7.解:(1)p (H 2) =95.43 kPa (2)m (H 2) =pVMRT= 0.194 g 8.解:(1)ξ = 5.0 mol(2)ξ = 2.5 mol结论: 反应进度(ξ)的值与选用反应式中的哪个物质的量的变化来进行计算无关,但与反应式的写法有关。
9.解:∆U = Q p - p ∆V = 0.771 kJ 10.解: (1)V 1 = 38.3⨯10-3 m 3= 38.3L(2) T 2 =nRpV 2= 320 K (3)-W = - (-p ∆V ) = -502 J (4) ∆U = Q + W = -758 J (5) ∆H = Q p = -1260 J11.解:NH 3(g) +45O 2(g) 298.15K−−−−→标准态NO(g) + 23H 2O(g) m r H ∆= - 226.2 kJ·mol -112.解:m r H ∆= Q p = -89.5 kJ m r U ∆= m r H ∆- ∆nRT= -96.9 kJ13.解:(1)C (s) + O 2 (g) → CO 2 (g)m r H ∆ =m f H ∆(CO 2, g) = -393.509 kJ·mol -121CO 2(g) + 21C(s) → CO(g) m r H ∆ = 86.229 kJ·mol -1CO(g) +31Fe 2O 3(s) → 32Fe(s) + CO 2(g)m r H ∆ = -8.3 kJ·mol -1各反应m r H ∆之和m r H ∆= -315.6 kJ·mol -1。
(2)总反应方程式为23C(s) + O 2(g) + 31Fe 2O 3(s) → 23CO 2(g) + 32Fe(s) m r H ∆ = -315.5 kJ·mol -1由上看出:(1)与(2)计算结果基本相等。
所以可得出如下结论:反应的热效应只与反应的始、终态有关,而与反应的途径无关。
14.解:m r H ∆(3)=m r H ∆(2)×3-m r H ∆(1)×2=-1266.47 kJ·mol -115.解:(1)Q p = m r H ∆== 4 m f H ∆(Al 2O 3, s) -3m f H ∆(Fe 3O 4, s) =-3347.6 kJ·mol -1(2)Q = -4141 kJ·mol -116.解:(1) m r H ∆ =151.1 kJ·mol -1 (2) m r H ∆ = -905.47 kJ·mol -1(3) m r H ∆ =-71.7kJ·mol -117.解: m r H ∆=2 m f H ∆(AgCl, s)+ m f H ∆(H 2O, l)- m f H ∆(Ag 2O, s)-2 m f H ∆(HCl, g) m f H ∆(AgCl, s) = -127.3 kJ·mol -118.解:CH 4(g) + 2O 2(g) → CO 2(g) + 2H 2O(l)m r H ∆ = m f H ∆(CO 2, g) + 2 m f H ∆(H 2O, l) - m f H ∆(CH 4, g)= -890.36 kJ·mo -1 Q p = -3.69⨯104kJ第2章 化学反应的方向、速率和限度 习题参考答案1.解: m r H ∆ = -3347.6 kJ·mol -1;m r S ∆ = -216.64 J·mol -1·K -1;m r G ∆ = -3283.0kJ·mol -1 < 0该反应在298.15K 及标准态下可自发向右进行。
2.解:m r G ∆ = 113.4 kJ·mol -1 > 0该反应在常温(298.15 K)、标准态下不能自发进行。
(2) m r H ∆ = 146.0 kJ·mol -1;m r S ∆ = 110.45 J·mol -1·K -1;m r G ∆ = 68.7 kJ·mol -1 > 0该反应在700 K 、标准态下不能自发进行。
3.解: m r H ∆ = -70.81 kJ·mol -1 ;m r S ∆ = -43.2 J·mol -1·K -1; m r G ∆ = -43.9 kJ·mol -1(2)由以上计算可知:m r H ∆(298.15 K) = -70.81 kJ·mol -1; m r S ∆(298.15 K) = -43.2 J·mol -1·K -1m r G ∆ = m r H ∆ - T ·m r S ∆ ≤ 0 T ≥K)(298.15K) (298.15m r m rS H ∆∆ = 1639 K4.解:(1)c K = {}O)H ( )(CH )(H (CO) 2432c c c c p K = {}O)H ( )(CH )(H (CO) 2432p p p pK = {}{}{}{}p p p p p p p p / O)H ( /)(CH / )(H / (CO) 2432(2)c K ={}{})(NH )(H )(N 3232212c c c p K ={}{})(NH )(H )(N 3232212p p pK ={}{}pp p p p p / )(NH/)(H/)(N3232212(3)c K =)(CO 2c p K =)(CO 2p K = p p /)(CO 2 (4)c K ={}{}3232 )(H O)(H c c p K ={}{}3232 )(H O)(H p pK ={}{}3232 /)(H/O)(Hpp p p5.解:设 m r H ∆、m r S ∆基本上不随温度变化。
m r G ∆ = m r H ∆ - T · m r S ∆m r G ∆(298.15 K) = -233.60 kJ·mol -1 m r G ∆(298.15 K) = -243.03 kJ·mol -1K lg (298.15 K) = 40.92, 故 K (298.15 K) = 8.3⨯1040 K lg (373.15 K) = 34.02,故 K (373.15 K) = 1.0⨯10346.解:(1) m r G ∆=2m f G ∆(NH 3, g) = -32.90 kJ·mol -1 <0该反应在298.15 K 、标准态下能自发进行。
(2) K lg (298.15 K) = 5.76, K (298.15 K) = 5.8⨯1057. 解:(1) m r G ∆(l) = 2m f G ∆(NO, g) = 173.1 kJ·mol -11lgK =RTG 303.2)1(m f∆- = -30.32, 故1K = 4.8⨯10-31(2) m r G ∆(2) = 2m f G ∆(N 2O, g) =208.4 kJ·mol -12lgK =RTG 303.2)2(m f∆- = -36.50, 故2K = 3.2⨯10-37(3) m r G ∆(3) = 2m f G ∆(NH 3, g) = -32.90 kJ·mol -1 3lg K = 5.76, 故 3K = 5.8⨯105由以上计算看出:选择合成氨固氮反应最好。
8.解: m r G ∆ = m f G ∆(CO 2, g) - m f G ∆(CO, g)-m f G ∆(NO, g)= -343.94 kJ·mol -1< 0,所以该反应从理论上讲是可行的。
9.解: m r H ∆(298.15 K) = m f H ∆(NO, g) = 90.25 kJ·mol -1 m r S ∆(298.15 K) = 12.39 J·mol -1·K -1m r G ∆(1573.15K)≈ m r H ∆(298.15 K) -1573.15 m r S ∆(298.15 K)= 70759 J ·mol -1K lg (1573.15 K) = -2.349, K (1573.15 K) = 4.48⨯10-3 10. 解: H 2(g) + I 2(g)2HI(g)平衡分压/kPa 2905.74 -χ 2905.74 -χ 2χ22)74.2905()2(x x -= 55.3 χ= 2290.12p (HI) = 2χkPa = 4580.24 kPan =pV RT= 3.15 mol11.解:p (CO) = 1.01⨯105 Pa, p (H 2O) = 2.02⨯105 Pa p (CO 2) = 1.01⨯105 Pa, p (H 2) = 0.34⨯105 PaCO(g) + H 2O(g) → CO 2(g) + H 2(g) 起始分压/105 Pa 1.01 2.02 1.01 0.34 J = 0.168, p K = 1>0.168 = J,故反应正向进行。
12.解:(1) NH 4HS(s) → NH 3(g) + H 2S(g)平衡分压/kPa x xK ={}{}/ S)(H / )(NH 23 p p p p = 0.070则 x = 0.26⨯100 kPa = 26 kPa 平衡时该气体混合物的总压为52 kPa(2)T 不变, K 不变。
