药物研发与技术审评沟通交流管理办法-EN
药物研发与技术审评沟通交流管理办法

药物研发与技术审评沟通交流管理办法(试行)(修订稿)第一章总则第一条为规范申请人与国家食品药品监督管理总局药品审评中心(以下简称药审中心)之间的沟通交流,根据《国务院关于改革药品医疗器械审评审批制度的意见》(国发〔2015〕44号)和《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》(厅字〔2017〕42号)等有关规定,制定本办法。
第二条本办法所指的沟通交流,系指在药物研发过程中,经申请人提出,由药审中心项目管理人员(以下简称项目管理人员)与申请人指定的药品注册专员共同商议,并经药审中心适应症团队同意,就现行药物研发与评价指南不能涵盖的关键技术等问题所进行的沟通交流。
第三条沟通交流的形式包括:面对面会议、视频会议、电话会议或书面回复。
鼓励申请人与审评机构通过电话会议沟通。
沟通交流的提出、商议、进行,以及相关会议的准备、召开、记录和纪要等均应遵守本办法。
第四条本办法规定的沟通交流会议适用于创新药物、改良型新药、生物类似药、复杂仿制药以及一致性评价品种等研发过程和注册申请中的沟通交流。
第五条沟通交流是申请人与审评人员就技术问题互动的过程,双方在沟通交流过程中可就讨论问题充分阐述各自观点,最终形成的共识可作为研发和评价的重要依据。
第二章沟通交流会议类型第六条沟通交流会议分为Ⅰ类、Ⅱ类和Ⅲ类会议。
(一)Ⅰ类会议,系指为解决药物临床试验过程中遇到的重大安全性问题和突破性治疗药物研发过程中的重大技术问题而召开的会议。
(二)Ⅱ类会议,系指为创新药物在研发关键阶段而召开的会议,主要包括下列情形:1.临床试验申请前会议。
为解决首次递交临床试验申请前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否支持拟开展的临床试验;临床试验受试者风险是否可控等。
对新机制新结构药物全球首次申报进行临床试验的,申请人应与审评机构进行沟通,明确申报技术要求。
2.Ⅱ期临床试验结束/Ⅲ期临床试验启动前会议。
为解决Ⅱ期临床试验结束后和关键的Ⅲ期临床试验开展之前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否充分支持拟开展的Ⅲ期临床试验;对Ⅲ期临床试验方案等进行评估。
中药新药研究过程中沟通交流会议药学资料要求的指导原则

中药新药研究过程中沟通交流会议药学资料要求的指导原则(征求意见稿)2020年07月目录一、概述 (1)二、基本要求 (1)三、沟通交流会议药学资料要求 (2)(一)药物临床试验申请前会议 (2)1. 药物研究概况和药学研究资料 (2)2. 拟讨论问题 (3)(二)药物Ⅱ期临床试验结束/Ⅲ期临床试验启动前会议 (3)1.药物研究概况和药学研究资料 (4)2.拟讨论问题 (4)(三)药品上市许可申请前会议 (5)1.药物研究概况和药学研究资料 (5)2.拟讨论问题 (5)(四)其他会议 (6)四、参考文献 (6)五、著者 (7)中药新药研究过程中沟通交流会议药学资料要求的指导原则(征求意见稿)一、概述1沟通交流是申请人与国家药品审评机构解决研发问题2的有效方式,有利于解决中药新药研究及审评中存在的问题,3加速新药研发进程,促进中药传承创新。
为规范沟通交流会4议的药学资料,提高沟通交流的质量和效率,根据中药特点、5中药新药研发规律和沟通交流制度的相关规定,制定本指导6原则。
7本指导原则适用于中药新药研发过程中沟通交流会议8的药学资料,重点针对药物临床试验申请前、药物Ⅱ期临床9试验结束/Ⅲ期临床试验启动前、药品上市许可申请前的沟通10交流会议。
其他沟通交流会议的中药药学资料要求可参考。
11沟通交流会议的程序等参照国家药品审评机构相关会12议要求。
中药新药药学研究内容可参考相关指导原则和技术13要求。
14二、基本要求15坚持以问题为导向的基本原则,明确拟讨论问题。
申请16人应根据现行指导原则和技术要求等进行深入思考和充分17研究,围绕问题提供相关的背景信息、详实的研究资料和拟18解决方案,提供的资料同时应符合相关会议资料要求,以便19提高沟通交流的质量和效率,达到沟通交流会议的预期目的。
20申请人应基于不同研发阶段,提供相应的药学研究资料,21以便更好地评估已有研究数据是否支持拟开展的各期临床22试验、临床试验受试者安全风险是否可控、是否支持药品上23市许可的技术要求等。
药品注册审评一般性技术问题咨询管理管理规范

药品注册审评一般性技术问题咨询管理规范第一章总则 .................................................................. - 1 - 第二章工作流程............................................................... - 2 - 第三章工作要求............................................................... - 3 - 第四章附则 .................................................................. - 4 -第一章总则第一条为贯彻《国务院关于改革药品医疗器械审评审批制度的意见》(国发〔2015〕44号)精神,落实国家食品药品监督管理总局《药物研发与技术审评沟通交流管理办法(试行)》(2016年第94号)要求,进一步加强药品技术审评咨询工作,优化技术咨询程序,提高药品审评技术咨询服务质量和效率,制定本规范。
第二条本规范所指的一般性技术问题咨询(以下简称“咨询问题”)是指:申请人和药品审评中心(以下简称“药审中心”)之间通过药审中心官网()的“申请人之窗”就一般性技术问题进行交流咨询。
该沟通交流方式通常不就技术审评过程中的重大决策性问题进行讨论。
咨询问题包括与中心职能相关的技术审评问题及相关管理问题,涉及保密的问题不属于一般性技术问题。
一般性技术问题咨询的反馈意见基于当前政策法律法规规章等规范性文件和技术要求做出,可能会随政策法律法规规章等规范性文件或技术要求的变化而变化。
如果总局就相关问题做出解释或解答,以总局的解释或解答为准。
第三条业务管理处负责咨询问题的接收、分配、意见形成及反馈的总体协调管理,业务管理处项目管理人根据任务分工开展具体协调管理工作,中心其他相关部门配合完成此项工作。
国家药品监督管理局关于调整药物临床试验审评审批程序的公告【模板】

国家药品监督管理局关于调整药物临床试验审评审批程序的公告(2018年第50号)2018年07月27日发布为鼓励创新,加快新药创制,满足公众用药需求,落实申请人研发主体责任,依据中共中央办公厅、国务院办公厅《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》(厅字〔2017〕42号),对药物临床试验审评审批的有关事项作出调整:在我国申报药物临床试验的,自申请受理并缴费之日起60日内,申请人未收到国家食品药品监督管理总局药品审评中心(以下简称药审中心)否定或质疑意见的,可按照提交的方案开展药物临床试验。
现就具体事宜公告如下:一、沟通交流会议的准备与申请(一)申请人在提出新药首次药物临床试验申请之前,应向药审中心提出沟通交流会议申请,并在确保受试者安全的基础上,确定临床试验申请资料的完整性、实施临床试验的可行性。
(二)申请人准备的沟通交流会议资料应包括临床试验方案或草案、对已有的药学和非临床研究数据及其他研究数据的完整总结资料。
申请人应自行评估现有的研究是否符合申报拟实施临床试验的基本条件,并明确拟与药审中心讨论的问题。
(三)申请人应按照《药物研发与技术审评沟通管理办法(试行)》(以下简称《沟通交流办法》)要求,提交沟通交流会议申请表(附件1)。
药审中心应及时通知申请人是否召开沟通交流会议,并与申请人商议会议时间。
申请人应按沟通交流相关要求按时提交完整的沟通交流会议资料(附件2)。
药审中心对沟通交流会议资料进行初步审评,在沟通交流会议召开至少2日前,通过“申请人之窗”将初步审评意见和对申请人所提出问题的解答意见告知申请人。
申请人在收到初步审评意见和解答意见后,应尽快反馈问题是否已经得到解决。
申请人认为问题已经解决不需要召开沟通交流会议的,应通过药审中心网站“申请人之窗”告知药审中心取消沟通交流会议申请;申请人认为申请沟通交流的问题仍未得到解决的,按原定计划继续组织会议召开。
二、沟通交流会议的召开(四)会议由药审中心工作人员主持,双方围绕药物临床试验方案就申请人提出的关键技术问题,以及已有资料和数据是否支持实施临床试验开展和受试者安全风险是否可控进行讨论,并为后续研究提出要求和建议。
临床研究进程中沟通交流会的药学资料准备要求[1]
![临床研究进程中沟通交流会的药学资料准备要求[1]](https://img.taocdn.com/s3/m/024afdfe81c758f5f61f67b1.png)
临床研究进程中沟通交流会的药学资料准备要求2001年5月 美国FDA发布2009年6月 药审中心组织翻译德国奈科明有限公司翻译北核协会审核药审中心最终核准目 录Ⅰ. 前言 (1)Ⅱ. 一般原则 (1)A. 会议目的 (1)B. 申请会议 (2)C. 信息包 (2)D. 会议的形式 (2)1.多学科会议 (2)2.药学方面专门会议 (3)E. 会议重点 (3)Ⅲ. 新药申报前的会议 (3)A. 会议目的 (3)B. 申请会议、信息包和形式 (3)C. 会议重点 (3)Ⅳ. Ⅱ期临床试验结束会议 (4)A. 会议目的 (4)B. 申请会议、信息包和形式 (5)C. 会议重点 (5)1.所有药品 (5)2.rDNA蛋白生物技术药物 (6)3.常规生物制品 (6)D. 后续会议 (7)Ⅴ. 申请上市前的会议 (7)A. 会议目的 (7)B. 申请会议、信息包和形式 (7)C. 会议重点 (7)临床研究进程中沟通交流会的药学资料准备要求Ⅰ. 前言本指南为制药企业提供有关新药研发申请(INDs)申办者与药物审评和研究中心(CDER)或生物制品审评和研究中心(CBER)之间关于药学方面(CMC)信息的正式会议的指南。
本指南适用于人用药物和生物制品(统称为药品)的INDs。
本指南覆盖了可能在申办者和审评机构之间举行的三种会议:(1)新药临床申报前(pre-IND),(2)Ⅱ期临床试验结束(EOP2),(3)新药上市申报(pre-NDA)或生物制品许可证申请前(pre-BLA)。
这些会议可在申办者的要求下召开,以便论述在临床研究过程中出现的重要问题和科学问题,帮助解决问题并促进药品的审评。
(我们)建议, 但并不强制性地要求举办该会议,这些会议常与药品开发和/或注册过程的重要时间点相吻合。
如有必要,也可在其它时间申请附加会议。
本指导原则的目的在于当会议讨论CMC信息时,通过提供关于(1)目的,(2)申请会议,(3)信息包,(4)形式和(5)会议的重点等信息,帮助促使这些会议更高效。
创新药物研发中的CMC要求学习笔记

创新药物研发中的CMC要求学习笔记一、背景1、2015年11月11日原食药监局发布2015年第230号文指出:第三条、优化临床试验申请的审评审批对新药的临床试验申请,实行一次性批准,不再采取分期申报、分期审评审批的方式;审评时重点审查临床试验方案的科学性和对安全性风险的控制,保障受试者的安全。
加强临床试验申请前及过程中审评人员与申请人的沟通交流,及时解决注册申请和临床试验过程中的问题。
申请人需按要求及时补报最新研究资料。
在Ⅰ期、Ⅱ期临床试验完成后,申请人应及时提交试验结果及下一期临床试验方案。
未发现安全性问题的,可在与药审中心沟通后转入下一期临床试验。
申请人应如实报告临床试验中发生的严重不良事件,按时提交研究年度报告;对不能控制临床试验安全性风险的,应立即停止临床试验。
药审中心与申请人当面沟通,应当场形成会议纪要列明议定事项。
并将创新药物申请纳入优先审评之中。
2、2016年6月,国家食药监总局发布《药物研发与技术审评技术沟通交流管理办法(试行)》,并于2018年3月发布修改稿向公众征求意见:办法指出,创新药物可以在临床各期试验前提交Ⅱ类会议申请,即临床试验申请前(Ⅰ期临床前)、Ⅱ期结束/Ⅲ期临床试验前以及提交上市申请前提交会议申请。
3、2018年7月27日,国家药监局发布2018年第50号文,就沟通交流会给出了具体安排,并强调了开展Ⅱ期结束/Ⅲ期临床试验前沟通交流会的重要性。
(十一)对于技术指南明确、药物临床试验有成熟研究经验,申请人能够保障申报资料质量的,或国际同步研发的国际多中心临床试验申请,在监管体系完善的国家和地区已经获准实施临床试验的,申请人可不经沟通交流直接提出临床试验申请。
(十二)已获准开展新药临床试验的,在完成Ⅰ期、Ⅱ期临床试验后、开展Ⅲ期临床试验之前,申请人应向药审中心提出沟通交流会议申请,就包括Ⅲ期临床试验方案设计在内的关键技术问题与药审中心进行讨论。
申请人也可在临床研发不同阶段就关键技术问题提出沟通交流申请。
药品注册审评一般性技术问题咨询管理管理规范

药品注册审评一般性技术问题咨询管理规范第一章总则 .................................................................. - 1 - 第二章工作流程............................................................... - 2 - 第三章工作要求............................................................... - 3 - 第四章附则 .................................................................. - 4 -第一章总则第一条为贯彻《国务院关于改革药品医疗器械审评审批制度的意见》(国发〔2015〕44号)精神,落实国家食品药品监督管理总局《药物研发与技术审评沟通交流管理办法(试行)》(2016年第94号)要求,进一步加强药品技术审评咨询工作,优化技术咨询程序,提高药品审评技术咨询服务质量和效率,制定本规范。
第二条本规范所指的一般性技术问题咨询(以下简称“咨询问题”)是指:申请人和药品审评中心(以下简称“药审中心”)之间通过药审中心官网()的“申请人之窗”就一般性技术问题进行交流咨询。
该沟通交流方式通常不就技术审评过程中的重大决策性问题进行讨论。
咨询问题包括与中心职能相关的技术审评问题及相关管理问题,涉及保密的问题不属于一般性技术问题。
一般性技术问题咨询的反馈意见基于当前政策法律法规规章等规范性文件和技术要求做出,可能会随政策法律法规规章等规范性文件或技术要求的变化而变化。
如果总局就相关问题做出解释或解答,以总局的解释或解答为准。
第三条业务管理处负责咨询问题的接收、分配、意见形成及反馈的总体协调管理,业务管理处项目管理人根据任务分工开展具体协调管理工作,中心其他相关部门配合完成此项工作。
180724关于调整药物临床试验审评审批程序的公告(2018年第50号)

关于调整药物临床试验审评审批程序的公告(2018年第50号)颁布时间:20180724为鼓励创新,加快新药创制,满足公众用药需求,落实申请人研发主体责任,依据中共中央办公厅、国务院办公厅《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》(厅字〔2017〕42号),对药物临床试验审评审批的有关事项作出调整:在我国申报药物临床试验的,自申请受理并缴费之日起60日内,申请人未收到国家食品药品监督管理总局药品审评中心(以下简称药审中心)否定或质疑意见的,可按照提交的方案开展药物临床试验。
现就具体事宜公告如下:一、沟通交流会议的准备与申请(一)申请人在提出新药首次药物临床试验申请之前,应向药审中心提出沟通交流会议申请,并在确保受试者安全的基础上,确定临床试验申请资料的完整性、实施临床试验的可行性。
(二)申请人准备的沟通交流会议资料应包括临床试验方案或草案、对已有的药学和非临床研究数据及其他研究数据的完整总结资料。
申请人应自行评估现有的研究是否符合申报拟实施临床试验的基本条件,并明确拟与药审中心讨论的问题。
(三)申请人应按照《药物研发与技术审评沟通管理办法(试行)》(以下简称《沟通交流办法》)要求,提交沟通交流会议申请表(附件1)。
药审中心应及时通知申请人是否召开沟通交流会议,并与申请人商议会议时间。
申请人应按沟通交流相关要求按时提交完整的沟通交流会议资料(附件2)。
药审中心对沟通交流会议资料进行初步审评,在沟通交流会议召开至少2日前,通过“申请人之窗”将初步审评意见和对申请人所提出问题的解答意见告知申请人。
申请人在收到初步审评意见和解答意见后,应尽快反馈问题是否已经得到解决。
申请人认为问题已经解决不需要召开沟通交流会议的,应通过药审中心网站“申请人之窗”告知药审中心取消沟通交流会议申请;申请人认为申请沟通交流的问题仍未得到解决的,按原定计划继续组织会议召开。
二、沟通交流会议的召开(四)会议由药审中心工作人员主持,双方围绕药物临床试验方案就申请人提出的关键技术问题,以及已有资料和数据是否支持实施临床试验开展和受试者安全风险是否可控进行讨论,并为后续研究提出要求和建议。
药审中心 三类会议沟通要求

药审中心三类会议沟通要求
药审中心的三类会议分别是:内部会议、外部会议和跨部门会议。
1. 内部会议的沟通要求:
- 持续更新信息:确保会议中发布的信息是最新的,包括药物审批进展、政策变化等。
- 有效沟通:在会议中确保交流的有效性,鼓励成员之间相互倾听和理解,确保信息传递准确。
- 保密管理:内部会议通常涉及敏感信息,要求会议成员严守保密,不得泄露会议内容。
2. 外部会议的沟通要求:
- 提前准备资料:确保与外部参会者交流时准备充分,提供准确的数据和资料。
- 有效传递信息:与外部参会者进行有效的信息沟通,确保对外部专家和机构的意见和建议能
够被充分理解。
- 决策透明:外部会议通常涉及到决策和政策制定,要求对参会者做出的决策进行透明度管理,确保公正和客观。
3. 跨部门会议的沟通要求:
- 协同工作:不同部门间的联席会议要求各部门之间能够有效协同工作,合理分工,确保任务
顺利推进。
- 沟通平台:在沟通中使用统一的沟通平台,确保信息的同步和共享。
- 目标一致:确保各部门在会议中对目标的理解一致,避免产生混淆和误解。
总的来说,药审中心的会议沟通要求包括持续更新信息、有效沟通、保密管理、提前准备资料、有效传递信息、决策透明、协同工作、统一沟通平台和目标一致等方面。
这些要求有助于保证会议的高效性和信息的准确性,促进各方合作和决策的公正性。
药物研发与技术审评沟通交流管理办法

附件药物研发与技术审评沟通交流管理办法第一章总则第一条为规范申请人与国家药品监督管理局药品审评中心(以下简称药审中心)之间的沟通交流,根据中共中央办公厅、国务院办公厅《关于深化审评审批制度改革鼓励药品医疗器械创新的意见》(厅字〔2017〕42号)和《国务院关于改革药品医疗器械审评审批制度的意见》(国发〔2015〕44号)等有关规定,制定本办法。
第二条本办法所指的沟通交流,系指在药物研发过程中,经申请人提出,由药审中心项目管理人员(以下简称项目管理人员)与申请人指定的药品注册专员共同商议,并经药审中心适应症团队同意,就现行药物研发与评价指南不能涵盖的关键技术等问题所进行的沟通交流。
第三条沟通交流的形式包括:面对面会议、视频会议、电话会议或书面回复。
鼓励申请人与审评机构通过电话会议沟通。
沟通交流的提出、商议、进行,以及相关会议的准备、召开、记录和纪要等均应遵守本办法。
第四条本办法规定的沟通交流会议适用于创新药物、改良型新药、生物类似药、复杂仿制药以及一致性评价品种等研发过程和注册申请中的沟通交流。
第五条沟通交流是申请人与审评人员就技术问题互动的过程,双方在沟通交流过程中可就讨论问题充分阐述各自观点,最终形成的共识可作为研发和评价的重要依据。
第二章沟通交流会议类型第六条沟通交流会议分为Ⅰ类、Ⅱ类和Ⅲ类会议。
(一)Ⅰ类会议,系指为解决药物临床试验过程中遇到的重大安全性问题和突破性治疗药物研发过程中的重大技术问题而召开的会议。
(二)Ⅱ类会议,系指为药物在研发关键阶段而召开的会议,主要包括下列情形:1.新药临床试验申请前会议。
为解决首次递交临床试验申请前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否支持拟开展的临床试验;临床试验受试者风险是否可控等。
对新机制新结构药物全球首次申报进行临床试验的,申请人应与审评机构进行沟通,明确申报技术要求。
2.新药Ⅱ期临床试验结束/Ⅲ期临床试验启动前会议。
药物研发与技术审评沟通交流管理办法(试行)

附件药物研发与技术审评沟通交流管理办法(试行)第一章总则第一条为规范申请人与国家食品药品监督管理总局药品审评中心(以下简称药审中心)之间的沟通交流,根据《国务院关于改革药品医疗器械审评审批制度的意见》(国发〔2015〕44号)等有关规定,制定本办法。
第二条本办法所指的沟通交流,系指在药物研发过程中,经申请人提出,由药审中心项目管理人员(以下简称项目管理人员)与申请人指定的药品注册专员共同商议,并经药审中心技术审评项目组长同意,就现行药物研发与评价指南不能涵盖的关键技术等问题所进行的沟通交流。
第三条沟通交流的提出、商议、进行,以及相关会议的准备、召开、记录和纪要等均应遵守本办法。
第四条本办法规定的沟通交流会议优先适用于创新药物、采用先进制剂技术药物,以及临床急需药物研发的注册申请中的沟通交流。
第二章沟通交流会议类型第五条沟通交流会议分为Ⅰ类、Ⅱ类和Ⅲ类会议。
(一)Ⅰ类会议,系指为解决药物临床试验过程中遇到的重大安全性问题和突破性治疗药物研发过程中的重大技术问题而召开的会议。
(二)Ⅱ类会议,系指为创新药物在研发关键阶段而召开的会议,主要包括下列情形:1.I期临床试验申请前会议。
为解决首次递交临床试验申请前重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否支持拟开展I期临床试验;临床试验受试者风险是否可控等。
对研究数据支持开展I期临床试验且受试者风险可控达成一致的,申请人在会后方可向国家食品药品监督管理总局递交临床试验申请。
2.Ⅱ期临床试验结束/Ⅲ期临床试验启动前会议。
为解决Ⅱ期临床试验结束后和关键的Ⅲ期临床试验开展之前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否充分支持拟开展的Ⅲ期临床试验;对Ⅲ期临床试验计划和临床试验方案进行评估。
3.提交新药上市申请前会议。
为探讨现有研究数据是否满足新药上市审查所需资料要求,对包括但不限于下述问题进行讨论:现有研究数据是否充分支持新药上市申请审查所需资料要求。
药品审评中心与注册申请人沟通交流质量管理规范(试行)

药品审评中心与注册申请人沟通交流质量管理规范(试行)第一章总则一、为进一步加强药品审评中心(以下简称药审中心)与注册申请人(以下简称申请人)的沟通交流,不断加大公开透明力度,规范相关工作管理,提高药品技术审评管理与决策的水平和质量,制定本规范。
二、药审中心与申请人的沟通交流一般可分为以下几种类型:(一)双向预约式沟通交流;(二)查询式沟通交流;(三)问询式沟通交流;(四)开放式沟通交流。
三、本规范是在梳理总结药审中心和申请人既往沟通交流经验和相关规范化文件的基础上,结合当前我国药品技术审评和药品研发面临的现状,本着提升沟通交流质量和效率,服务于药品研发创新和技术审评的原则而制定。
四、本规范对药审中心与申请人之间沟通交流的分类、组织程序和要求等进行了明确,对于既往药审中心相关规范化文件与本规范表述等不尽一致之处,参照本规范执行。
第二章双向预约式沟通交流五、双向预约式沟通交流系指药审中心和申请人中的一方提前一定时间以特定的方式提出预约后进行的一种沟通交流.六、双向预约式沟通交流为基于鼓励创新和解决临床急需用药的沟通交流。
其适用于申请人在药品研发的关键阶段遇到关键或重大技术问题时,需要和药审中心进行沟通交流的情形;也适用于药审中心在药品技术审评过程中,为提升决策质量和效率,降低决策风险,需要与申请人进行沟通交流的情形.七、为保证沟通交流的质量和效率,提出预约的一方应提前足够的时间提出预约,以便对方做好充分的前期调研、信息采集、分析、评估等工作.八、双向预约式沟通交流可通过药审中心网站相应栏目、公函等途径提出。
九、双向预约式沟通交流一般以会议方式进行,具体包括面对面会议、视频会议和电话会议等.十、双向预约式沟通交流申请,根据品种研究进程和注册申请的不同阶段可分为以下五种类型.1。
临床前(Pre-IND)申请-基本完成非临床试验研究,但尚未提出临床研究申请阶段的申请;2。
临床(IND)申请——已提出临床研究申请阶段的申请;3.完成I期临床后(End of phase I)申请—-已批准进行临床研究,并已经完成I期临床研究阶段的申请;4.完成Ⅱ期临床后(End of phase Ⅱ)申请——已批准进行临床研究,并已完成Ⅱ期临床研究阶段的申请;5.生产(NDA)申请-—完成临床研究,并提出生产注册申请阶段的申请。
国家药品监督管理局关于调整药物临床试验审评审批程序的公告

国家药品监督管理局关于调整药物临床试验审评审批程序的公告⽂号:国家药品监督管理局公告2018年第50号颁布⽇期:2018-07-24执⾏⽇期:2018-07-24时效性:现⾏有效效⼒级别:部门规章为⿎励创新,加快新药创制,满⾜公众⽤药需求,落实申请⼈研发主体责任,依据中共中央办公厅、国务院办公厅《关于深化审评审批制度改⾰⿎励药品医疗器械创新的意见》(厅字〔2017〕42号),对药物临床试验审评审批的有关事项作出调整:在我国申报药物临床试验的,⾃申请受理并缴费之⽇起60⽇内,申请⼈未收到国家⾷品药品监督管理总局药品审评中⼼(以下简称药审中⼼)否定或质疑意见的,可按照提交的⽅案开展药物临床试验。
现就具体事宜公告如下:⼀、沟通交流会议的准备与申请(⼀)申请⼈在提出新药⾸次药物临床试验申请之前,应向药审中⼼提出沟通交流会议申请,并在确保受试者安全的基础上,确定临床试验申请资料的完整性、实施临床试验的可⾏性。
(⼆)申请⼈准备的沟通交流会议资料应包括临床试验⽅案或草案、对已有的药学和⾮临床研究数据及其他研究数据的完整总结资料。
申请⼈应⾃⾏评估现有的研究是否符合申报拟实施临床试验的基本条件,并明确拟与药审中⼼讨论的问题。
(三)申请⼈应按照《药物研发与技术审评沟通管理办法(试⾏)》(以下简称《沟通交流办法》)要求,提交沟通交流会议申请表(附件1)。
药审中⼼应及时通知申请⼈是否召开沟通交流会议,并与申请⼈商议会议时间。
申请⼈应按沟通交流相关要求按时提交完整的沟通交流会议资料(附件2)。
药审中⼼对沟通交流会议资料进⾏初步审评,在沟通交流会议召开⾄少2⽇前,通过“申请⼈之窗”将初步审评意见和对申请⼈所提出问题的解答意见告知申请⼈。
申请⼈在收到初步审评意见和解答意见后,应尽快反馈问题是否已经得到解决。
申请⼈认为问题已经解决不需要召开沟通交流会议的,应通过药审中⼼⽹站“申请⼈之窗”告知药审中⼼取消沟通交流会议申请;申请⼈认为申请沟通交流的问题仍未得到解决的,按原定计划继续组织会议召开。
研发项目(药学研究)阶段性技术评审管理规程

研发项目(药学研究)阶段性技术评审管理规程一、总则1.目的:为了加强研发项目的评审管理,规范研发项目评审行为,提高研发效率,特制定本规定。
2.原则:客观公正、效益为先。
二、职责1.对研发项目的立项报告进行可行性、应用前景、技术和市场风险及预期价值评审。
2.对研发项目的实施方案进行可实施性评审,并对实施方案中存在的问题提出修改和完善建议。
3.对研发项目的成果进行技术水平和经济价值评审。
三、评审组的组成1.评审组由公司领导、专家、有关部门负责人组成,总人数为7-15人,其中专家人数不少于三分之二。
2.可根据公司实际情况外聘专家。
3.被评审项目组的成员不得参加本项目的评审组。
四、评审程序1.立项评审项目组提交立项报告→项目申请发起人陈述→评审组成员提问、项目组回答→评审组讨论→表决→形成评审意见→宣读评审结论→报CEO或授权人审批。
2.实施方案评审项目组提交研发项目实施方案→项目经理陈述→评审组成员提问、项目组回答→评审组讨论→表决→形成评审意见→宣读评审结论→报CEO或授权人审批。
3.成果评估项目组提交研发成果报告→项目经理陈述→评审组成员提问、项目组回答→评审组讨论→表决→形成评审意见→宣读评审结论→报CEO审批。
五、评审要求1.项目组保证提交的评审资料的真实性、合法性,不得提供虚假材料。
2.评审组要求核实和取证时,项目组不得拒绝。
3.评审组须在规定的期限内完成评审并出具评审结论。
4.评审表决以三分之二多数通过。
5.项目立项和实施方案的评审结论是明确和唯一的,即:同意、修改或不同意。
6.成果评估的结论包括两个方面,即:技术指标和经济价值。
7.评审组成员可以充分发表个人意见,可以拒绝在评审结论上签字,但须书面说明不签字理由。
8.建立完整的项目评审档案,准确、真实地记录项目的评审情况。
药物研发与技术审评沟通交流管理办法

药物研发与技术审评沟通交流管理办法第一章总则第一条为加强对药物研发与技术审评沟通交流工作的管理,规范申请人与国家药品监督管理局药品审评中心(以下简称药审中心)之间的沟通交流,根据《中华人民共和国药品管理法》、《药品注册管理办法》等有关规定,制定本办法。
第二条本办法所指的沟通交流,系指在药物研发与注册申请技术审评过程中,申请人与药审中心审评团队就现行药物研发与评价指南不能涵盖的关键技术等问题所进行的沟通交流。
沟通交流会议经申请人提出,由药审中心项目管理人员(以下简称项目管理人员)与申请人指定的药品注册专员共同商议,并经药审中心审评团队同意后召开。
第三条沟通交流的形式包括:面对面会议、视频会议、电话会议或书面回复。
鼓励申请人与药审中心通过电话会议沟通。
沟通交流的提出、商议、进行,以及相关会议的准备、召开、记录和纪要等均应遵守本办法。
第四条本办法规定的沟通交流会议适用于中药、化学药和生物制品研发过程和注册申请技术审评中的沟通交流。
第五条申请人与审评团队在沟通交流过程中可就讨论问题充分阐述各自观点,形成的共识可作为研发和评价的重要参考。
第二章沟通交流会议类型第六条沟通交流会议分为Ⅰ类、Ⅱ类和Ⅲ类会议,就关键阶段重大问题进行沟通交流。
(一)Ⅰ类会议,系指为解决药物临床试验过程中遇到的重大安全性问题和突破性治疗药物研发过程中的重大技术问题,或其他规定情形,而召开的会议。
(二)Ⅱ类会议,系指为药物在研发关键阶段而召开的会议,主要包括下列情形:1. 新药临床试验申请前会议。
为解决首次递交临床试验申请前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否支持拟开展的临床试验;临床试验受试者风险是否可控等。
申请人准备的沟通交流会议资料应包括临床试验方案或草案、已有的药学和非临床研究数据及其他研究数据的完整资料。
2.药物Ⅱ期临床试验结束/Ⅲ期临床试验启动前会议。
为解决Ⅱ期临床试验结束后和关键的Ⅲ期临床试验开展之前的重大技术问题,对包括但不限于下述问题进行讨论:现有研究数据是否充分支持拟开展的Ⅲ期临床试验;对Ⅲ期临床试验方案等进行评估。
生物制药技术中的团队沟通和合作技巧

生物制药技术中的团队沟通和合作技巧在当今的生物制药领域中,团队沟通和合作是取得成功的关键。
由于生物制药研发工作的复杂性和多样性,一个高效的团队需要良好的沟通和协作能力。
本文将讨论一些生物制药技术中的团队沟通和合作技巧,以帮助团队成员更好地协同工作并取得良好的研究成果。
首先,团队沟通和合作的关键是建立良好的沟通渠道。
团队成员之间应该保持定期的沟通交流,确保每个人都了解他们的贡献和进展。
这可以通过定期开会、分享工作进展和发展共享平台来实现。
通过分享信息和讨论困难,团队成员可以共同解决问题,并达成一致的目标。
另外,团队合作的关键是建立互相信任和尊重的文化。
团队成员应该珍惜彼此的贡献,并相信每个人都在为团队的成功做出努力。
鼓励团队成员相互支持和扶持,尊重彼此的专业能力和经验。
在这样的环境中,每个人都愿意分享并接受反馈,从而改善个人和团队的表现。
此外,明确的角色定义和任务分配也是团队沟通和合作的关键要素。
团队成员应该清楚自己的职责,并了解其他成员的职责和任务。
这样可以避免任务的重复和遗漏,并确保整个团队的工作有条不紊。
定期进行任务分配和进展检查,有助于团队成员了解每个人的进展情况,并及时调整工作计划。
除了以上几点,团队领导者在团队沟通和合作中起着至关重要的作用。
一个优秀的领导者应该有明确的愿景和目标,并能够激励团队成员积极投入工作。
领导者应该倾听团队成员的意见和建议,并鼓励开放的讨论和思维碰撞。
此外,领导者还可以通过设立明确的目标和奖励措施,激励团队成员达到更高的绩效。
最后,团队成员之间的积极互动和信息共享也是团队沟通和合作的重要因素。
鼓励团队成员积极参与科学讨论和思维碰撞,分享自己的实验和科研成果。
通过共享信息和经验,团队成员可以从彼此的经验中学习,促进团队整体的进步。
此外,团队成员应该积极参与团队活动和培训,加强彼此之间的交流和了解。
总而言之,一个成功的生物制药团队需要良好的沟通和合作能力。
团队沟通和合作的关键在于建立良好的沟通渠道,建立互相信任和尊重的文化,明确的角色定义和任务分配,优秀的领导者的作用,积极互动和信息共享。
药物管理中的团队合作与沟通策略

药物管理中的团队合作与沟通策略药物管理是医疗机构中一个极其重要的环节,它涉及到药品的采购、配送、存储、发放以及患者用药等多个环节。
在这个过程中,团队合作和有效沟通是确保药物管理安全高效运行的关键因素。
本文将探讨药物管理中的团队合作和沟通策略,以提高医疗机构的药物管理水平。
一、团队合作策略团队合作是指不同领域、不同职能的人员共同协作,通过共享信息和资源,达到既定目标的过程。
在药物管理中,团队合作的策略如下:1.明确分工和职责在药物管理团队中,需要明确每个成员的分工和职责。
例如,药剂师负责药品采购和配送,医护人员负责患者用药指导等。
明确分工可以保证每个人都专注于自己的领域,提高工作效率。
2.建立跨部门协作机制药物管理需要涉及不同部门的协作,如医护部门、财务部门和行政部门等。
建立跨部门的协作机制,可以确保信息的及时传递和资源的有效利用,提高团队整体协作效果。
3.定期召开团队会议定期召开团队会议是促进团队合作的有效方式。
会议可以用来分享工作进展、解决问题和协调合作。
通过会议,可以确保团队成员之间的交流和合作,及时解决工作中的矛盾和困难。
二、沟通策略沟通是团队合作的基础,良好的沟通可以减少工作失误和误解,提高工作效率。
在药物管理中,沟通策略如下:1.及时共享信息药物管理涉及到药品的进销存情况、患者用药情况等多个方面的信息。
团队成员需要及时将相关信息进行共享,避免信息滞后和遗漏。
2.使用明确的沟通工具团队成员可以通过电子邮件、即时通讯工具或者内部平台等明确的方式进行沟通。
这些工具可以记录沟通内容,方便查阅和跟踪问题,同时也能减少沟通中的误解。
3.建立跨部门协作的联络人在多个部门协作的情况下,可以在团队中指定特定的人员作为联络人。
这些联络人负责协调部门之间的沟通和信息共享,确保信息传递的高效和准确。
三、团队合作与沟通的重要性和意义团队合作和沟通对于药物管理非常重要。
首先,团队合作可以整合团队成员的专长和资源,提高工作效率和结果质量。
CDE沟通交流会的申报策略与定量药理学的应用
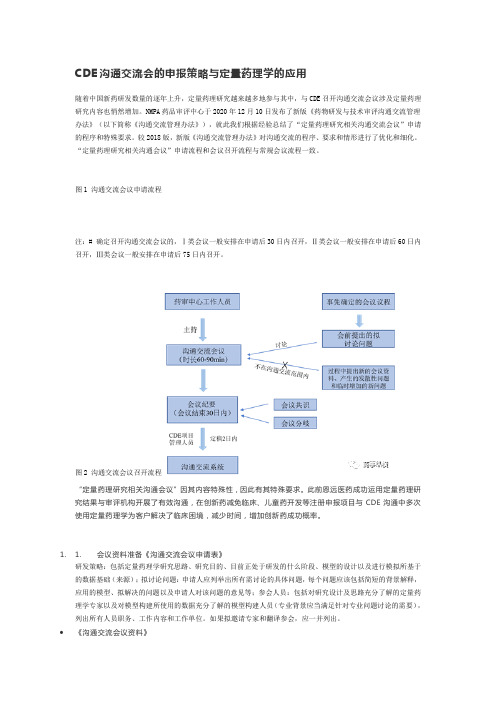
CDE沟通交流会的申报策略与定量药理学的应用随着中国新药研发数量的逐年上升,定量药理研究越来越多地参与其中,与CDE召开沟通交流会议涉及定量药理研究内容也悄然增加。
NMPA药品审评中心于2020年12月10日发布了新版《药物研发与技术审评沟通交流管理办法》(以下简称《沟通交流管理办法》),就此我们根据经验总结了“定量药理研究相关沟通交流会议”申请的程序和特殊要求。
较2018版,新版《沟通交流管理办法》对沟通交流的程序、要求和情形进行了优化和细化。
“定量药理研究相关沟通会议”申请流程和会议召开流程与常规会议流程一致。
图1 沟通交流会议申请流程注:# 确定召开沟通交流会议的,Ⅰ类会议一般安排在申请后30日内召开,Ⅱ类会议一般安排在申请后60日内召开,Ⅲ类会议一般安排在申请后75日内召开。
图2 沟通交流会议召开流程“定量药理研究相关沟通会议”因其内容特殊性,因此有其特殊要求。
此前恩远医药成功运用定量药理研究结果与审评机构开展了有效沟通,在创新药减免临床、儿童药开发等注册申报项目与CDE沟通中多次使用定量药理学为客户解决了临床困境,减少时间,增加创新药成功概率。
1. 1. 会议资料准备《沟通交流会议申请表》研发策略:包括定量药理学研究思路、研究目的、目前正处于研发的什么阶段、模型的设计以及进行模拟所基于的数据基础(来源);拟讨论问题:申请人应列举出所有需讨论的具体问题,每个问题应该包括简短的背景解释,应用的模型、拟解决的问题以及申请人对该问题的意见等;参会人员:包括对研究设计及思路充分了解的定量药理学专家以及对模型构建所使用的数据充分了解的模型构建人员(专业背景应当满足针对专业问题讨论的需要),列出所有人员职务、工作内容和工作单位。
如果拟邀请专家和翻译参会,应一并列出。
•《沟通交流会议资料》讨论问题清单:最终确定的讨论问题列表,每个问题应该包括简短的背景解释,应用的模型、拟解决的问题以及申请人对该问题的意见等;支持性数据:数据的逻辑层次应当切题,所采用的数据应当能够对相关研究、结果和结论提供支持,避免使用空话。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Administrative Regulation on the Communication for Drug R&D Activities andTechnical Review(Draft for Comment)Chapter I General ProvisionsArticle 1This regulation is formulated for the purpose of good performance of communication and standardization of the communication between the applicants and the Center for Drug Evaluation of NMPA (hereinafter referred to as CDE), by following the principles of the Pharmaceutical Administration Law of the People's Republic of China, and Measures for the Administration of Drug Registration.Article 2The communication, described in this regulation, is defined as the consultation and discussion applied by the applicants during the drug research and development process, through negotiation between the project management staff of CDE (hereinafter referred to as the “Project Manager”) and the drug registration specialist designated by the applicant, and approved by the review team of CDE, discussing the key technical issues that are not covered by the current drug R&D and review guidelines.Article 3The forms of communication include: face-to-facemeeting, video conference, teleconference and written correspondence. Applicants are encouraged to communicate with CDE through teleconference. The proposal, consultation and conduct of the communication, as well as the preparation, convening, recording and minutes of relevant meetings, shall comply with this regulation.Article 4The communication meetings stipulated in this regulation shall be applicable to the communication and consultation for the innovative drugs, modified new drugs, biosimilar drugs, Chinese herbal medicine compound preparation based on traditional classic prescription, drugs with the same name and prescription, complex generic drugs, and equivalency assessment drugs during their R&D process and registration applications.Article 5Communication is an interaction process dealing with the technical issues between the applicant and the review team, during which both parties may fully present their respective viewpoints with regard to such issues.Chapter II Types of Communication MeetingsArticle 6Communication Meetings are divided into Class I, Class II and Class III meetings, at which the communication takes place in respect to major issues at key stages.(I)Class I meeting is defined as the meeting held on thepurpose to address the major safety issues encountered during the clinical trials of drugs, and the major technical issues in the R&D process of the breakthrough therapeutic drugs, or held in other prescribed situations.(II)Class II meeting is defined as the meeting held at the key stages of drug R&D process, which mainly include the following situations:1.Pre-Clinical Trial Application meeting. To address majortechnical issues before the first submission of the clinical trial application, the following issues can be discussed, including but not limited to: whether the available research data supports the proposed clinical trial; whether the risk of clinical trial subjects is controllable. The communication meeting materials prepared by the applicant shall include complete information on the clinical trial protocols or drafts, existing pharmaceutical and non-clinical study data, and other research data. For the clinical trial applications of the first global applications of the drugs with new mechanism and new structure, the applicant shall initiate the communication with CDE, and the technical requirements on the registration shall be established clearly.2.End of Phase II clinical trial of drugs/Phase III pre-clinicaltrial meeting. To address major technical issues encounteredat the end of Phase II clinical trial and prior to the initiation of pivotal Phase III clinical trial, the following issues can be discussed, including but not limited to: whether the available research data can fully support the proposed Phase III clinical trial; make assessment on Phase III clinical trial protocol.3.Pre-meeting for drug marketing authorization. To explorewhether the available research data meets the technical requirements for the application for drug marketing authorization, the following issues can be discussed, including but not limited to: whether the available research data supports the technical requirements for the application for drug marketing authorization. The applicant for conditional approval and/or for priority review approval procedures shall communicate with the CDE before submitting the application for drug marketing authorization to NMPA.4.Risk assessment and control meeting. This meeting is tohave a discussion before NDA approval on the adequacy and controllability of the post-marketing risk control in order to assess and control the post-marketing risk.5.Other circumstances of Class II meetings as prescribed. (III)Class III meeting is defined as the meetings other than ClassI and Class II meetings.Article 7Applicants who have fallen into one of the following circumstances may propose the corresponding communication application based on the proposed studies or submissions in accordance with the provisions of the three classes of meetings mentioned above.(I)For clinical trial application of adding new indication, theapplicant shall carry out the research on the basis of available data in combination with the characteristics of the new indication, and make communication with CDE if necessary.(II)For the key technical issues found during the R&D process of drugs for urgent clinical needs or treatment of rare diseases, the applicant may make the communication application.(III)For the major R&D issues in the complex generic drugs and equivalency assessment or re-assessment drugs (e.g.selection of the reference preparation, evaluation criteria for bioequivalence, etc.), the applicant may make the communication application.(IV)For the protocol designed for the complicated important non-clinical studies (e.g. carcinogenicity studies), the applicant may make the communication application. (V)Where there is technical disputation in the review process after the applicant has received the inquiry consultation andsupplementary notification, the applicant may make the communication application if the applicant still has any difference regarding the comprehensive assessment results. (VI)Application for communications can be made in the R&D process of drugs on the frontier technology field.Chapter III Proposal and Discussion of CommunicationMeetingsArticle 8The convening of a communication meeting shall meet the following basic conditions:(I)The “Application Form for Communication Meeting”(Annex 1) and “Communication Meeting Materials”(Annex 2) submitted shall comply with the requirements of the regulation;(II)"Communication Meetings Materials” and the "Application Form for Communication Meetings" shall be submitted at the same time;(III)The professional background of personnel participating in the communication meeting shall be qualified for the discussion on professional issues.Article 9Where the above communication requirements are met, the applicant shall submit the Application Form for Communication Meeting and Communication MeetingsMaterials through the “window of applicant” of the website of CDE, specifying the type of communication at application. Article 10Within 2 days of receiving the “Application Form for Communication Meeting”, the Project Manager shall complete the preliminary review according to requirements above. Where the materials are found not complete or to have fallen into other cases of nonconformity, the application shall be ended. Those meeting the requirements shall be further submitted to relevant professional review team.Within 18 days after the application, the review team shall determine how to carry out the communication: communication meeting (face-to-face meeting, video conference, teleconference) or written feedback. If the review team finds that any data is incomplete or inconsistent, the application shall be terminated directly.Article 11Within 20 days after the application, the Project Manager will notify the applicant the conclusion confirmed by the review team.For the communication meeting application approved, the Project Manager will notify the meeting agenda to the applicant through the “window of applicant” within 5 days after the confirmation of meeting date, including the date, place, matters needing attention and materials to be further discussed at the meeting.Article 12Communication meeting application may not beapproved for the following circumstances:(I)Additional data shall be provided before satisfying thecommunication requirements for the issues to be discussed; (II)The professional background of attendees is not qualified for the discussion of the technical issues and not satisfactory to the need for communication;(III)Other circumstances that do not guarantee the effective convening of the meeting.If the communication meeting cannot be convened, the Project Manager shall provide the specific reasons through the “window of applicant”. After finishing relevant works, the applicant can make a new communication application.Article 13If the communication meeting is approved, in general, Class I meeting will be held within 30 days after the application, Class II meeting will be held within 60 days after the application, and Class III meeting, within 75 days after the application.If the communication meeting is confirmed not to be held, the feedback shall be completed within the above-mentioned time limit according to the meeting type, in principle.Chapter IV Preparation for Communication MeetingsArticle 14The applicant shall submit the electronic version ofCommunication Meeting Materials in accordance with the requirements of the “Communication Meeting Materials” through the "Window of Applicant".Article 15To ensure the quality and efficiency of communication meetings, the drug registration specialist shall make comprehensive discussion with Project Manager before the meeting, and confirm the date, time, place and agenda of the meeting.Chapter V Convening of Communication MeetingsArticle 16The communication meeting will be chaired by the CDE staff, and conducted according to the pre-determined meeting agenda. Discussion shall be made on proposed issues put forward before the meeting. In principle, it is not within the scope of communication to raise new meeting materials, generate divergent issues and to temporarily add new issues. In general, the communication meeting duration shall be within 45 minutes, but not exceed 60 minutes.Article 17The minutes of meeting shall be prepared in accordance with the "Template of Communication Meeting Minutes" (Annex 3). Where both parties have reached an agreement, viewpoints in common shall be specified. If the parties fail to reach an agreement, their viewpoints shall be statedrespectively. The applicant is encouraged to make minutes of meeting on the spot, for the purpose of which the applicant can make a draft of the minutes before the meeting on the basis of the preliminary feedback from CDE and make it clear after discussion. If the minutes cannot be made on the spot for special reasons, the applicant shall generally submit the draft of minutes within one week after the meeting is completed. Minutes of meeting shall be finalized within 30 days upon the completion of the meeting. The Project Manager shall upload the minutes within 2 days upon finalization, to the communication system which can be accessed by the applicant through the “Window of Applicant”. Meeting minutes shall be archived as an important document. Article 18If necessary, CDE will make audio or video recordings of whole procedure of the meeting, and archive them as working files for future reference. The applicant and other attendees are not allowed to make any audio, video or photo recordings without permission. Where the applicant’s trade secrets are involved, CDE shall keep such trade secrets confidential in accordance with applicable laws.Chapter VI Postponement or Cancellation ofCommunication MeetingsArticle 19The meeting will be postponed if any of the following circumstances occurs:(I)The communication conditions are met but additionalinformation shall be supplemented;(II)The review team considers that there are other important issues beyond the meeting topic that need further discussion; (III)There are too many meeting materials for the review team to review in a limited period;(IV)The important attendees cannot attend the meeting on time; (V)Other force majeure factors.In general, the applicant shall be notified of the decision to postpone a meeting, at least 5 days prior to the meeting. The postponed meeting will be discussed by the Project Manager and the drug registration specialist. In general, the delay time shall not exceed 2 months. It will be considered that the meeting cannot be held if the delay time is more than 2 months due to the applicant, in which case the applicant is required to submit a new communication application after supplementing additional materials.Article 20 A meeting will be cancelled if any of the following situations occurs:(I)The meeting materials are not submitted within thespecified date;(II)The submitted meeting materials do not meet the requirements of this regulation;(III)The applicant makes the proposal of canceling the meeting which is approved by CDE;(IV)The issue from the applicant has been resolved or has been given written correspondence.In general, the decision to cancel a meeting shall be informed to the applicant 5 days prior to the meeting. After cancellation of the meeting, the applicant may submit a separate communication application according to this regulation.Chapter VII Supplementary ProvisionsArticle 21For the verification or consultation of general technical issues, the applicant may communicate with the Project Manager through the general technical issue consultation platform in "Window of Applicant", telephone, fax, E-mail, or by other means. Consultations on general technical issues will not include the key technical issues in drug R&D and technical review processes.Article 22When making submission of the drug registration dossier, the applicant shall appoint 1 or 2 drug registrationspecialists, and provide the specific information of the drug registration specialist such as the name and telephone number. The drug registration specialist shall be responsible for drug registration. The applicant shall make communication with CDE through the drug registration specialist, and the Project Manager shall only contact the drug registration specialist designated by the applicant. If there is any change of the drug registration specialist, the applicant shall make updates through the “Window of Applicant” in a timely manner.Article 23During the review process, CDE may make a proposal on communication as required, which will be discussed by Project Manager with the drug registration specialist to determine the, time, agenda, and information requirements for the communication meeting.Article 24The meeting materials used for communication shall be included in the applied registration documents (which may be compiled into an individual volume) as the basis for review. Important meeting materials prior to the submission of a drug registration application, e.g. Communication Meetings Materials, Application Form for Communication Meeting and meeting minutes, should be submitted by incorporating into the dossiers. The meeting materials generated during the review should be included into the dossiers by CDE.Article 25CDE staff shall strictly follow this regulation, andmust not contact the applicant privately in any way other than those provided in this regulation. The special case shall be approved by CDE.Article 26This regulation shall come into force as of the date of promulgation.Annexes:1.Application Form for Communication Meetingmunication Meeting Materials3.Template of Communication Meeting MinutesAnnex 1Application Form for Communication MeetingI.Basic information of drug research and development1.Applicant2.Drug name3.Acceptance number (if available)4.Chemical name and structure (or prescription, for ChineseTraditional Medicine)5.Proposed indications (or major functions)6.Dosage form, administration route and regimen (medicationfrequency and treatment cycle)7.Drug R&D strategies, including drug R&D backgroundinformation, drug development plans, brief description of the R&D process, key events, and current development status. II.Specific contents of meeting application1.Meeting types: Class I, Class II, or Class III.2.Classification of meeting: such as Pre-Clinical trialapplication meeting, End of Phase II clinical trial/Phase III pre-clinical trial meeting, Pre-meeting for drug marketing authorization or risk evaluation and control meeting.3.Meeting forms: face-to-face meeting, video conference,teleconference or written correspondences.4.Meeting objective: brief description.5.Proposed meeting date and time: please provide 3 alternatives.6.Proposed meeting agenda: including estimated duration foreach topic to be discussed (in general, the discussion duration of all topics should be controlled within 60 minutes.)7.List of attendees of applicant: the list shall set forth the namesof attendees, including the positions, job contents and work units. If the applicant plans to invite experts and interpreters to attend the meeting, they shall also be involved in the list.8.List of proposed issues under discussion: it is recommendedthat the applicant shall make the list of issues by discipline, including but not limited to the issues of the pharmaceutical, pharmacology & toxicology, and clinical practice. Each issue should include a brief description of background, the purpose of proposed issues and the applicant’s opinions of issues. In general, the number of issues to be discussed at a meeting shall not exceed 10.Annex 2Communication Meeting Materials1.List of issues under discussion: a list of issues that applicanthas finalized. It is recommended that the applicant shall make the list of issues by discipline, including but not limited to the issues of the pharmaceutical, pharmacology & toxicology, and clinical practice. Each issue shall include a brief description of background and the purpose of issues proposed.2.Supporting data summary: summary of supporting data bydiscipline and the order of issues.In terms of the summary of supporting data, relevant studies, results and conclusions shall be explained by data. Taking end of Phase II clinical trial meeting as an example, the clinical professional summary shall include the following: (1) a brief summary of completed clinical trials, including data, results and conclusions. Important dose-effect relationship information shall also be included. In general, a complete clinical study report does not need to be provided; (2) a detailed introduction of the proposed Phase III clinical trial protocol to confirm the main characteristics of clinical trials, such as clinical trial subject population, the key inclusion and exclusion criteria, clinical trialdesign (e.g., randomization, blinding, control selection, justification of non-inferiority threshold if non-inferiority trial used), dose selection, primary and secondary efficacy endpoints, and primary analysis methods (including planned interim analysis, characteristics of adaptation studies, and major safety concerns).Annex 3Template of Communication Meeting MinutesMeeting types: Class I, Class II, or Class III.Classification of meeting: such as Pre-clinical trial application meeting, End of Phase II clinical trial/Phase III pre-clinical trial meeting, Pre-meeting for drug marketing authorization or risk evaluation and control meeting.Date and time of the meeting:Meeting place:Acceptance number (if available):Drug Name:Proposed indications (or major functions):Applicant:Chairman:Recorder:Attendees: a list of all attendees of the applicant and CDE.Text:1.Purpose of the meeting:2.Meeting background:3.Issues discussed at the meeting and results:(1)Issue 1: XXXXXXXXXDo both sides reach consensus?□ Yes.Common opinion: XXXXXXXXX □ No.Applicant's comments:CDE's comments:(2)Issue 2: XXXXXXXXX…。
