进口饲料和饲料添加剂申请材料一览表 (中英对照) (1)
进口饲料和饲料添加剂产品登记证目录

养殖动物
All species or categories of animals
西班牙皮特鲁巴公司
ANDRéS PINTALUBA,S.A.Spain
2017.05-
2022.05
新办
(2017)外饲准字215号
混合型饲料添加剂
维生素
Feed Additive Mixture Vitamin
法国普乐维美公司
PROVIMI France
2017.05-
2022.05
新办
(2017)外饲准字218号
混合型饲料添加剂
酸度调节剂香味剂
Feed Additives Mixture
Acidity regulators flavors
普乐吉吉酸
biacid
混合型饲料添加剂
Feed Additives Mixture
2017.05-
2022.05
新办
(2017)外饲准字217号
混合型饲料添加剂
酸度调节剂香味剂
Feed Additives Mixture Acidity regulators flavors
普乐吉吉酸500
biacid 500
混合型饲料添加剂
Feed Additives Mixture
家禽
Poultry
丙三醇
Feed Additives Mixture
Glycerine
能量宝
MANNA-C
混合型饲料添加剂
Feed Additives Mixture
猪,鸡
Swine、Chicken
韩国索玛公司
SOMA Inc., Korea
新饲料添加剂申请表(示范)

新饲料添加剂申请表
(示范文本)
通用名称:
产品类别:
申请类型: 申请新饲料添加剂证书 申请扩大饲料添加剂适用范围□申请生产含量规格低于《饲料添加剂安全使用规范》等规范性文件要求的饲料添加剂品种 申请生产工艺发生重大变化的饲料添加剂
申请进口含有我国尚未批准使用的饲料添加剂的产品
农业农村部规定的其他情形
申请人名称:(公章)法定代表人:
申请人注册地址:
邮政编码:
申请人通讯地址:
邮政编码:
联系人:传真:
固定电话:手机:
电子邮件:
申报日期:年月日
中华人民共和国农业农村部制
二〇年。
进口饲料和饲料添加剂登记管理目录2010-01
Nuklospray K26
蛋白质饲料
Protein Feed
仔猪
Piglet
荷兰司劳特公司
Sloten B.V., TheNetherlands
2010.01-
2015.01
(2010)外饲准字017号
乳清粉、大豆粉及植物油
Whey Powder, Soybean Flour and Vegetable Oil
2010.01-
2015.01
(2010)外饲准字005号
水合硅铝酸钠钙
Hydrated Sodium Calcium Aluminosilicate
好力盾
Meri-Bond Xtra
饲料添加剂
Feed additive
养殖动物
All species or categories of animals
非反刍动物Animal except Ruminant
智利苟尔贝斯卡股份有限公司Arica工厂
CorpescaS.A.AricaPlant,Chile
2010.01-
2015.01
(2010)外饲准字013号
鱼粉
Fishmeal
Corpesca牌红鱼粉(特级)
Corpesca Red Fishmeal (Superfine)
萨那
SanaHZ-P
添加剂预混合饲料
Additive Premix
猪
Swine
日本Sana Co., Ltd.株式会社埼玉工厂
Sana Co., Ltd.Saitama Factory,Japan
2010.01-
2015.01
(2010)外饲准字008号
鱼粉
换发进口饲料和饲料添加剂产品登记证目录(2020–07)
意大利贝科瑞化工大药厂
Bioscreen Technologies Srl, Italy
百尔康(意大利)大药厂
Balchem Italia S.r.l., Italy
(2019)外饲准字192号
艾多乳
Aldosperse O-20 KFG
混合型饲料添加剂聚氧乙烯20山梨醇酐单油酸酯单硬脂酸甘油酯
混合型饲料添加剂氯化胆碱
Feed Additives Mixture Choline Chloride
申请企业名称
意大利贝科瑞化工大药厂
Bioscreen Technologies Srl, Italy
百尔康(意大利)大药厂
Balchem Italia S.r.l., Italy
生产厂家名称
意大利贝科瑞化工大药厂
附件2
换发进口饲料和饲料添加剂产品登记证目录(2020–7)
登记证号
商品名称
通用名称
变更内容
原名称
变更名称
(2019)外饲准字122号
饲料级DL-蛋氨酸
DL-Methionine Feed Grade
饲料添加剂DL-蛋氨酸
Feed Additive DL-Methionine
申请企业名称
赢创营养与消费化学品有限责任公司
Lonza Inc., USA
美国龙沙有限责任公司
Lonza LLC, USA
(2020)外饲准字423号
利补舒
Liposorb
混合型饲料添加剂卵磷脂聚乙二醇甘油蓖麻酸酯
Feed Additives Mixture Lecithin Glyceryl Polyethylenglycol Ricinoleate
进口饲料、饲料添加剂申报指南

进口饲料、饲料添加剂申报指南Application Guidelines for Registration of Imported Feeds and FeedAdditivesNational Feed Industry OfficeApril, 2004Address: National Feed Industry Office, Ministry of AgricultureNo.11, Nongzhanguan Nanli, Beijing, P.R.of China. 100026Tel: 8610-64192831Fax: 8610-64192869IntroductionFeeds and Feed Additives Administration Regulation and Administrative Measures for the Registration of Imported Feeds and Feed Additives specify that foreign enterprises shall apply to the Ministry of Agriculture of the People's Republic of China (MOA) to obtain product registration license for their feed and feed additive products if they are to be place on the market for the first time within the territory of the People's Republic of China. For those feeds and feed additives with no registration license, they are not allowed to be sold or used in the territory of China. In the practice of application, we found that some producers and distributors are unfamiliar with China’s feed regulations, hence resulting in materials submitted in the applications being inadequate or presentations unclear. This Application Guidelines was then compiled to hopefully promote smooth registration procedures for imported feeds and feed additives.CONTENTS1 Scope and Coverage for Registration2 Common Terminology for Feed Industry3 Procedures of Application for licenses of imported feeds and feed additives3.1 Asking for Application Form and Category of Required Materials3.2 Application3.3 Appraisal of the Application Materials3.4 Product Inspection3.5 License Issuing3.6 Inquiries of registration License4 Application Form and Required Materials4.1 Format Requirement for Application Materials4.2 Filling in Application Form4.3 Contents of Required Materials4.4 Additional Information for Pet Food and Aquaculture Feed4.5 Related Regulations and Interpretation for License Application5 Sample Requirements6 Application for Re-registration6.1 Asking for Re-registration Form6.2 Required Materials for Re-registration6.3 Related Regulations for Re-registrationAppendix:1. Application Form for Registration of Imported Feeds and Feed Additives2. A Sample of the Application Form3. Flowchart of Appraisal of imported Feeds and Feed Additives4. A Sample of Chinese Label5. Product Classification6. Application Materials for Appraisal of Imported Products7. Re-registration Form for Imported Feeds and Feed AdditivesApplication Guidelines forRegistration of Imported Feeds and Feed Additives1 Scope and Coverage for RegistrationFeeds and feed additives to be imported to the P.R. of China.2 Common Terminology for Feed Industry (See GB10647- 89)2.1 Feedstuff (Single Feed)Feedstuff originated from only one kind of animal, plant, microbial or mineral source.2.1.1 Energy FeedFeedstuff with less than 18% crude fiber and less than 20% crude protein on dry matter basis.2.1.2 Protein FeedFeedstuff with less than 18% crude fiber and 20% or more crude protein on dry matter basis. 2.2 Complete FeedCompound feed which can meet the nutrition requirements from raised animals (except of water).2.3 Concentrate FeedMixture of protein ingredients, trace minerals, vitamins and non-nutritional feed additives according to a certain formula.2.4 Concentrate SupplementCompound feed mixed at a certain formula with many kinds of feed ingredients to supplement nutrition for herbivorous animals fed on roughage (dry, fresh or silage).2.5 Additive PremixPremix of more than two kinds of feed additives with carriers or diluents at a certain formula. 2.6 Compound PremixPremix of two or more kinds of feed additives in the category of trace minerals, vitamins, amino acids and non-nutritional feed additives with carriers or diluents at a certain formula.2.7 Feed AdditiveMinor or trace ingredients added during feed processing, production or utilization. They were categorized into nutritive and non-nutritive feed additives.2.7.1 Nutritive AdditiveTrace or minor substance to supplement nutrition in feed2.7.2 Non-nutritive AdditiveMinor or trace substance added in the feed to ensure or improve feed quality, promote animal production, ensure animal health and improve feed utilization efficiency.a. Anti-oxidant:Additive added to prevent or delay oxidation or damage of some active ingredients in the feed.b. Preservative:Additive added to prevent or inhibit fermentation or decaying of feed.c. Mould inhibitor:Additives added to prevent mold growth in the feed.d. Flavor enhancement:Additives added in the feed to improve feed palatability and animal appetite.e. Color pigment:Additives added to improve color of animal products or feed.f. Binder:Additive added in the feed to improve molding capacity of mash feed or anti-shape damaging capacity of pellet feed.2.7.3 See Appendix 5 for examples of different products.3 Procedures of Application for Licenses of Imported Feeds and Feed Additives (See Appendix 3 for Flow Chart)3.1Asking for Application Form and Category of Required Materials3.1.1 These documents can be acquired from National Feed Industry Office (NFIO).3.1.2 Downloaded from (Application Documents at left bottom section on home page).3.2 ApplicationAn Applicant shall submit original copy of application materials to NFIO. Application materials include Application Form for Registration of Imported Feeds and Feed Additives(see Appendix 1) and Materials Required for Submission.3.3 Appraisal of the Application MaterialsNFIO shall complete the appraisal of the application materials within 15 working days and inform the applicant of the appraisal result.a. If application materials do not meet requirements, NFIO shall inform the applicant via fax to make supplements or corrections. Application shall not be accepted unless required materials are supplemented or corrected in line with related regulations.Documents that fail to meet requirements will not be accepted.b. If application materials meet requirements, NFIO shall inform the applicant in a Note of Acceptance to send a copy of application documents, samples, Note of Acceptance and test fees (charged by test items) to MOA Feed Quality Inspection Center (FQIC) specified in the Note for product quality inspection.3.4 Product InspectionFQIC shall submit test results to NFIO.a. If a product is qualified, FQIC shall prepare and submit a test report to NFIO.b. If the product is not qualified, FQIC shall inform the applicant to submit samples for a second test. And if the second test still fail, this product shall not be registered in the P. R. of China.FQIC shall not release test report to the applicant and the applicant shall not request the report or its copies.3.5 License IssuingQualified Feeds and Feed additives shall be submitted to MOA for Appraisal. Once approved by MOA, a license will be issued.3.5.1 NFIO will inform the applicant about the date for releasing the license or failure of any product to be registered.3.5.2 The applicant shall pay registration fee of RMB 8300 yuan (remittance, cash or check is acceptable except for USD cash) to Department of Finance of MOA upon obtaining the license. 3.6 Inquiries of registration LicenseThe approval of registration will be announced on MOA Bulletin and also on the web site: . An applicant can inquire about commodity name, manufacturer and registration number of any approved product.4 Application Form and Required Materials Required4.1 Format requirement for application materialsa. One set of original application materials and a copy of it shall be submitted for each product.b. The application materials shall be in both Chinese and English, with Chinese version coming first.c. The English version shall be printed on formal stationery paper heading with manufacturer’s name and with signature or seals.d. The Chinese translation of the application materials shall be the same with the English version, and unrelated information about the products shall not be submitted.e. If materials submitted by the manufacturer are not in English, they shall be translated intoEnglish and Chinese.f. Application materials in Chinese shall be printed with A4 paper, in #4 or small#4 fonts.There’s no specific limitations for the English version.g. Application Form shall be on the first page of the whole set of application materials, whichshall be bounded in durable document folders.4.2 Filling in Application Form (Please see Appendix 2 for the Sample)4.2.1 Commodity nameImported feed and feed additives marketed in the P. R. of China shall have a Chinese name, which shall be defined according to GB/T 10647 Standard. A product shall be named after its real nature, instead of a simple translation of the English pronunciation.4.2.2 Common nameIt means a name that can reflect the nature of a feed and feed additive.4.2.3 Product classification (See Appendix 5)Refer to Common Terminology In Feed Industry (See Section 2).4.2.4 AppearanceIt means color, shape (mash, pellet, crystal and block) and physical properties (e.g. odor, solidity or liquidity)4.2.5 Major and minor ingredients and their contentsSpecification for key nutrition should be filled in the blank under “key ingredient and content”.Other items such as specification of hygiene and heavy metal shall be filled in under “other ingredient and content”.4.2.6 Manufacturer’s name and addressDetailed name and address of the manufacturer shall be filled in as exactly the same as indicated in the free sale certificate, except thata. An applicant is a group company:Name of the producer shall be given, though the group company, to which the producer is affiliated, shall be registered as the applicant. If the group company produces a same product in different countries, this product shall be registered by each of the producers in these countries before being exported to China. If the group company has several plants in different regions in one country, then this product shall also be registered by each of these plants, i.e., one license for one plant or one product. If there’s no specific name for a plant, it can be specified as “xxx plant” under “xxx group company”.b. An applicant places an order with another manufacturer for processing. The actual manufacturer should be filled in “manufacturer column” (co-manufacturer), and the consigner shall be filled in “applicant column”. Co-manufacturing contract or verification letter to the consigner by the co-manufacturer (printed on letterhead paper of the co-manufacturer, and signed by responsible person or properly stamped) is required to verify that the product is co-manufactured with the requirement from the consigner. Co-manufacturer is responsible for the production and the consigner for the sales of the product.c. An applicant is registering for fish meal. When the manufacturer applies, themanufacturer’s name and address shall be given under the columns of “manufacturer” and “applicant”. In this circumstance, distributor has nothing to do with the application.When the first distributor applies, the name and address of the first distributor shall be filled in the column of “applicant”. Under such circumstances, names and addresses of all theproducers shall be given in the one of “manufacturer”. Extra pages can be used if the space is not enough. (Distributors shall submit a list of producers with whom it has relations in fish meal trade, including producers’ name s and addresses). An original copy of application materials is needed only from one of the producers (see 4.3.1-4.3.9).4.2.7 Name and address of applicanta. For most products, manufacturers are the applicants.b. When a group company is an applicant, the plant manufacturing the product shall submit a letter (original and in English) to explain its relationship with the group company.c. When the first distributor is the applicant (only for fish meal), the plant shall submit a letter to explain that the distributor has been authorized to market the fish meal in the P. R. of China.4.2.8 AgencyName, telephone and fax numbers, as well address of the agency shall be provided.4.2.9 Signature and dateDocuments shall be signed by a person authorized by the applicant.4.3 Contents of required materials4.3.1 Certificate of Free Sale (CFS) approved by country or region of origin and materials for registration in other countriesCFS is a certificate that proves production and sales are permitted in a country of origin. CFS is issued by an official department that is in charge of manufacturing of feed or the product itself to certify that the producer is legal in manufacturing the product. CFS also certifies that the product can be safely used as feed or feed additive, and free to sell and export without limitations in and from the country of origin. Name and detailed address of the producer shall be provided in the certificate.If a country of origin is not English speaking, CFS, health certificate or official stamp, which is not in English, shall be notarized by the Chinese embassies in the country. Other application materials are not required for notarization.Chambers of commerce, associations or official notarization departments have no authorities to issue CFS for any products other than fish meal from Peru and Chile.It is operational to provide a copy of an import license once obtained in other countries.4.3.2 Origin, composition and manufacturing method4.3.2.1 OriginInformation about the origin shall be provided, either of animal, plant or basic chemicals for the synthetic process.4.3.2.2 Ingredient compositionComposition or effective ingredients shall be explained, including chemical structure, if there’s any, and detailed name of raw material. For plants and microbes, their names shall be detailed down to genus and strains.4.3.2.3 Manufacturing methodIt includes processing flowchart and literal explanation.Processing flowchart should be logical in describing technical conditions and methods for the key steps. Explanation shall focus on the control indicators for major steps and the whole process of quality control.For microbial products, name, origin and culture media ingredients of the strain shall be additionally provided.4.3.3 Quality Standard, Testing Method, Quality Testing Report and Health Certificate4.3.3.1 Quality StandardA producer shall apply with national standard, industrial standard or standard within the company.The quality standard covers appearance, sense perception, physi-chemical and hygiene indexes.Appearance and sense perception: color, smell and physical appearance.Physi-chemical index: Specification for key nutrition and processing quality of the product shall be provided with ceiling and bottom levels.Hygiene index: limitation for safe usage of toxins, harmful substances or pathogenic microbes of natural, secondary and exogenous sources. These include heavy metals (Pb, As, Hg, Cd), fluorine, nitrite (for fish meal only, calculated as NaNO2) and pathogenic microbes (salmonella, total bacteria count, molds and aflatoxin B1)4.3.3.2 Testing MethodsTesting methods shall be provided for key nutrition and hygienic conditions. Standard code is needed if the testing methods follow the international practice. For example, with national, AOAC and ISO standards, only standard code is needed for such nutrition as crude protein, crude fat, moisture, crude fiber and crude ash. Otherwise, some more information shall be provided like detailed steps of testing, reagents and concentrations, instruments and calculation methods.4.3.3.3 Quality testing reporta.For animal origin protein feed (such as fish meal, meat and bone meal, whey, etc.), a genuine and legal testing report shall be issued by department concerned in the country of origin.b. For other feed and feed additives, a testing reports issued by the producer is acceptable. It is required that the sample used for testing shall be in the same batch with the one to be registered. Moreover, all quality indicators shall be included in the testing report.4.3.3.4 Health certificatea. For fish meal, it shall be certified that no animal origin protein or fat other than fish meal is contained in the product.b. For meat and bone meal (MBM), it is to certify that what origin it is from (such as MBM originated from cattle, pig, or chicken) and that all raw materials are from healthy animals from non-epidemic areas.c. For whey, it is to certify that the fresh milk is supplied by healthy animals and no animal origin protein or fat other than dairy products is contained in the product.d. For fat type product, it is to certify that no dioxin exists in the product.e. Health certificate is a must for a product originated from epidemic areas and suspected of animal origin.f. In other cases, no health certificate is required.4.3.4 Label and trademark4.3.4.1 A label in original and Chinese is required.a. Original label includes the picture and literal explanation on an imported package. A Photo of the label is also acceptable.b. For feed and feed additives to be marketed within the territory of China, a label in Chinese shall be required according to Feed Label Standard (GB 10648-1999). (See appendix 4)4.3.4.2 TrademarkA trademark is necessary, only if the product has been registered in China.4.3.5 Application scope, method and dosageIt means coverage of animals, dosage and notices for using different kinds of feed products. 4.3.6 Package, storage conditions and shelf lifePackaging materials and net weight for each package shall be specified.Storage requirements cover location, conditions and ways of storage.Shelf life means the valid period since the date of production.4.3.7 Providing reports on safety evaluation and stability testing when necessaryThey are only necessary for the feed additives marked with “*” in No. 318 MOA Announcement and those products not listed in this Announcement.4.3.8 Report on feed efficiency and status of extensiona. Report on feed efficiency (i.e. trial feeding reports) and status of extension in other countries or regions are required for imported feed additives to be registered.b. It is not necessary for animal origin feeds permitted by China, such as fish meal, meat and bone meal, whey, fish oil, pet food and aquaculture feed.4.3.9 Letter of authorizationAn applicant shall submit a letter from the producer authorizing the applicant to register the product. No such document is required if the producer register by itself.4.4 Additional information about pet food, aquaculture feed and feed for ornamental fishes4.4.1 The application materials shall be separated respectively for cat food and dog food, and also for dry food and canned food. It means one set of application materials shall be prepared for each categories of dry cat food, canned cat food, dry dog food, and canned dog food. It also is the same case with the registration licenses.4.4.2 For ornamental fish (cat and dog) feed, application materials shall be submitted based on different breeds and ages. For instance, the materials shall be listed in the order of seedling carp, parr carp and matured carp; and in the case of golden fish, it shall be in the order of seedling golden fish, parr golden fish and matured golden fish.4.4.3 For the items mentioned in Section4.3.1-4.3.9. , the materials provided shall be different for the compound feeds of different varieties, with different tastes or for animals at different ages. Of course, some of the items are exempt from that, such as the free sale certificate, manufacturing method and testing methods.4.4.4 For the compound feeds with the same ingredients and of the same quality, but with different pellet size, it is required to describe in the manuals the reasonable sizes for animals at different ages.4.4.5 For the compound feeds of the same quality, but with different colors and tastes, it is required to specify the names of pigments and flavors added in the products.4.4.6 An sample for testing shall be of the same type with the one submitted for registration.A certain amount of testing fee will be collected based on different series of products. Additional 200 USD will be charged for one more taste or shape of the same series.4.5Related regulations and interpretations for license application4.5.1 There are 191 kinds of feed and feed additives that have been approved for use by China. Please see No. 318 MOA Announcement for details.4.5.2 There’s no requirements for a producer of any approved animal origin feed to submit the materials mentioned in Section 4.3.7 and 4.3.8. For instance, protein feeds like fish meal, meat and bone meal, compound feeds like aquaculture feed and pet food, and energy feeds like fish oil and animal fat.4.5.3 For feeds and feed additives that have not been approved or registered by their countries or origins of origin, trial feeding and safety evaluation must be conducted when applied for registration in China. The applicants will cover all the expenditures for the trials and evaluation. (Please see Article 8 of Administrative Measures for the Registration of Imported Feeds and Feed Additives)4.5.4 For feeds and feed additives that have been approved by exporting countries, while not approved for use in China, trial feeding and, if necessary, safety evaluation shall be conductedwhen they are imported by China. Both plans and implementer of the trial or evaluation shall be examined and approved by MOA. The applicants will cover all the expenditures for the trials and evaluation. (Please see Article 9 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).4.5.5 For an imported feed or feed additive with complete application materials and qualified testing results, a license will be issued once upon the approval by the MOA. A product subjective to Article 8 and 9 will receive a license from the MOA after the results of trial feeding and safety evaluation are submitted for consideration of the China National Feed Appraisal Committee. (Please see Article 11 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).4.5.6 For a feed or feed additive that has been approved for production and use in its country of origin, but not in China, its applicant is required to submit application materials according to the requirements from the Category of Application Materials for Appraisal of Imported Products(See Appendix 6). The National Feed Appraisal Committee will organize experts concerned to review these materials.5 Sample RequirementsThe registration of each product requires three samples originated from different batches of raw materials. Each sample shall weigh no less than 200 grams in solidity or 200 ml in liquidity. A testing report for the sample shall be enclosed in the application materials.6 Re-registration6.1 Asking for application form for Re-registration (See Appendix 7)6.1.1 The form is available from NFIO.6.1.2 It can also be downloaded from (See Application Materials at the bottom to the left of the home page)6.2 Requirements for the application materialsTwo copies of the previous license;Two copies of application forms for re-registration (printed in both Chinese and English);Two copies of quality standard specification and manuals;One original copy of authorization letter;and re-registration fee of RMB 4150 yuan for each product.6.2.1 Letter of authorizationA letter shall be submitted by a producer to authorize its distributor, partner or representative to re-register its product on behalf of the producer.No letter of authorization is required in case that a producer or its branch or representative office in China conducts the procedures of re-registration.6.3 Regulations for re-registration6.3.1 The license of registration for imported feeds and feed additives is valid for five years. If a product continues to be marketed within the territory of China after its license expires, re-registration shall be applied within 6 months before the expiry date of the previous license. (See Article 14 of Administrative Measures for the Registration of Imported Feeds and Feed Additives)6.3.2 For a feed or feed additive that are not re-registered on time or not qualified in the sample test for once, its samples shall be submitted for reexamination. A producer whose license has been suspended, however, has no chance for re-registration. (See Article 16 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).6.3.3 Re-registration is not available for any feed or feed additive that has been suspended for production or use in its country or region of origin, or that has failed to pass through the sample test for twice successively in China. (See Article 17 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).6.3.4 A feed or feed additive that has been changed in its producer’s address, quality standard, formula or application scope, shall be re-registered in time. (See Article 18 of Administrative Measures for the Registration of Imported Feeds and Feed Additives).*Applicant: Companies or individuals that apply for registration in MOA. They can be branch company, representative office, distributor, partner or authorized individuals of the producer or applying companies.Appendix 1进口饲料和饲料添加剂申请表Application Form for Registration of Imported Feeds and Feed AdditivesAppendix 2: A Sample of Application Form进口饲料和饲料添加剂申请表Application Form for Registration of Imported Feeds and Feed AdditivesAppendix 3进口饲料和饲料添加剂审批流程图Flowchart of Appraisal of Imported Feeds and Feed AdditivesAppendix 4 A sample of Chinese Labels进口饲料级乳清粉Imported Feed-Grade Whey许可证号:外饲准字200( )号 License Number: Foreign Registration 200 ( )1、本产品符合饲料卫生标准This product is in compliance with feed hygienic standard2、产品成分分析保证值 Product guaranteed analysis specification3Ingredients composition:This product is spray dried with pure whey4、产品标准编号:本产品符合加拿大XXXX 标准。
进口饲料和饲料添加剂产品登记证目录(2019—07)
Pioneer®1152 Silage Inoculant
混合型饲料添加剂
Feed Additives Mixture
青贮饲料
Silage
美国科汉森有限公司威斯康辛州工厂
Chr. Hansen Inc., Plant WI, USA
2019.11—
2024.11
新办
(2019)外饲准字392号
2019.11—
2024.11
新办
(2019)外饲准字382号
混合型饲料添加剂酿酒酵母
Feed Additives MixtureSaccharomyces cerevisiae
威灵赐壮
Wellingstrong
混合型饲料添加剂
Feed Additives Mixture
畜禽、水产养殖动物
Livestock, Poultry, Aquaculture animals
勃林格殷格翰动物保健(丹麦)公司
Boehringer Ingelheim Animal Health Denmark A/S, Denmark
2019.11—
2024.11
新办
(2019)外饲准字397号
奶牛维生素预混合饲料
Vitamin Premix for Cow
好奶牛
BIOCOW PREMIX
2019.11—
2024.11
新办
(2019)外饲准字394号
混合型饲料添加剂香味物质
Feed Additives Mixture Flavouring Substances
爱乐吸粉剂
Aromax Dry
混合型饲料添加剂
Feed Additives Mixture
进口药用辅料注册资料列表-英文版-带详细说明

进口药用辅料注册资料列表-英文版-带详细说明CFDA Statement No.61 Application Information Requirements of Excipients which issued in 2005, the following items should be submitted.(I) Summary1. Name of the excipient.Include the official name of the excipient, chemical name, English name and Chinese Phonetics name. The Nomenclature basis of the excipient.2. Certified Documents.(1) Certified Documents, notarized document for the free sale certificate (FSC) issued from the competent authorities of the local country or region where the manufacturer is located, and the GMP Certificate of the manufacturer, and the Chinese translation.(2) When the registration of a foreign excipient manufacturer is conducted by manufacturer’s office in China, copies of Registration Certificate for Resident Office of Foreign Enterprise should be provided.(3) When a foreign excipient manufacturer authorizes domestic agent to conduct the registration, copies of the authorization document, notarized document and the Chinese translation, as well as the Business License of the domestic agent should be provided.(4) Documents and explanations to evidence the patent status and ownership of the excipient, the formula of the excipient, the production technology and process of the excipient, as well as letters of guarantee stating that the excipient will not infringe upon the patent rights of others.(5) Notes: ① Certified Documents, notarized document for the FSC and GMP Certificate of the manufacturer should comply with the recommended format by World Health Organization (WHO). The document in other format must be legalized by the Chinese embassy in the original country.② In the event that the excipients not yet approved to sale in the manufacturing country or region, the Certified Documents from the other country where the products being marketed and documents of DMF could be provided. And the notarized document and the Chinese translation should also be provided. All the documents should be recognized by CFDA. But the certified documents of the GMP for the excipients should be issued by the competent authorities of the local country or region where the manufacturer is located.③ For application of imported pharmaceutical vacant capsules, gelatin for capsules, pharmaceutical grade gelatin which all are bovine original, the related documents and certificates of the source and category of the raw materials of preparing gelatin which is the main raw material for preparing capsules should be provided. The certificates which issued by the government for demonstrating the raw materials of preparing gelatin were not come from the BSE Country.3. Objectives and basis for R & D.Include R&D, market status, related literatures, manufacture, summary about the excipient application in formulations.4. Summary of main study work.Include summary for the main research results which had been done by the applicant, overall evaluation of the excipient on safety, effectiveness, quality controllability and etc.5. Draft of packaging insert, note to the draft, and latestliteratureInclude instruction for usage of the excipient, includes name of the excipient, chemical structure or molecular formula, usage, cautions, package (strength, content), shelf life, the “Pharmaceutical Excipient” should be labeled.6. Design of packaging and labelingSamples of designed package and label(II) Pharmaceutical data7. Summary of Pharmaceutical StudyInclude the synthetic technics, formula selection, structure identification, quality study and establishing the quality standard, the summary of experiment and global literature of stability study.8. Research information and relevant literature of the production processInclude process flow and chemical equations, the starting material and organic solvent, reaction conditions (temperature, pressure, time, catalysts and etc.) and operating steps, refining methods, the main physicochemical constants and periodically accumulated results of the data, the amount of the raw materials, yields and the impurities or other intermediates which was produced orintroduced in the manufacture process, the specifications of the involved chemical materials, the source and the scientific name of the animal, botanical or mineral materials. The reasons for the modification of manufacture process if the manufacture process was different with the literatures.9. Study information and relevant literature for chemical structure or components identification of the excipient Include documents for chemical structure or components identification of the excipient10. Study information and literature for quality specificationInclude physicochemical constants, purity analysis test, content analysis test and methods validation, periodically accumulated results of the data of the excipient11. Study information and literature for compatibility between API and excipients12. Draft of quality specification and notes to the draft, and provide reference substance or standard substanceThe format of the quality specification should comply with current ChP and using ChP terminology and measurement unit. The used categories of reagents, solutions, buffer solution and titrants should be listed in ChP and with the same concentration, it should be demonstrated detailed if there were different in concentration or categories. The documents for source, physicochemical constants, purity, content analysis test and its analysis method and results data of the reference substance or standard substance should be provided.13. Test reports of continuing 3 batches of the samples (the samples should be same as application usage)14. Stability study and relevant literaturesAlso include the stability test of the excipient which packed in the primary package.15. Selection basis and quality specification of immediate packing material and container(III) Pharmacology and toxicology study information16. Summary of pharmacology and toxicology study17. Study information and literature for whether excipients affect API’s pharmacological effect or not18. General Pharmacology study and literature19. Acute/single dose toxicity study and literature20. Repeated dose toxicity study and literature21. Special safety study and literature of hypersensitive (topical, systemic and photo-toxicity), hemolytic and topical irritative (blood vessel, skin, mucous membrane, and muscle) reaction related to topical and systemic use of the drugs22. Study and literature of mutagenicity test23. Study and literature of reproductive toxicity24. Study and literature of carcinogenicity test(IV) Clinical Study Information25. Summary of global clinical study information26. Clinical study protocol27. Investigator’s Brochure28. Draft of Informed Consent Form, approval of the Ethics Committee29. Clinical study report。
饲料添加剂公告

饲料添加剂公告附件1:进口饲料和饲料添加剂登记材料要求一、登记范围由外国企业生产的、首次在中华人民共和国境内销售和使用的饲料和饲料添加剂。
我国香港、澳门特别行政区和台湾省生产的饲料和饲料添加剂产品参照本要求登记。
《饲料和饲料添加剂管理条例》所称饲料,是指经工业化加工、制作的供动物食用的饲料,包括单一饲料、添加剂预混合饲料、浓缩饲料、配合饲料和精料补充料。
《饲料和饲料添加剂管理条例》所称饲料添加剂,是指饲料加工、制作、使用过程中添加的少量或者微量物质,包括营养性饲料添加剂和一般饲料添加剂。
二、登记材料的格式要求(一)登记材料包括《进口饲料和饲料添加剂登记申请表》(见表1,以下简称《申请表》)和申请材料(具体内容见表2《进口饲料和饲料添加剂申请材料一览表》,以下简称《申请材料一览表》),如果登记产品为中华人民共和国尚未允许使用的饲料或饲料添加剂,需要经过全国饲料评审委员会组织专家进行评审通过后方可进行登记。
(二)登记材料要求中英文对照,中文在前,英文在后。
如生产国为非英语语种国家,还应附具本语种登记材料。
所有登记材料一式两份,原件和复印件各一份。
(三)外文登记材料原件要求用生产厂家的信头纸出具,并由负责人签字或者加盖公章。
(四)中文登记材料应使用A4规格纸、小四号宋体打印。
(五)登记材料中不应夹带与申报产品无关的信息。
(六)登记材料应按《申请材料一览表》的顺序装订成册并标注页码,《申请表》置于登记材料的首页。
三、《申请表》的填写《申请表》的填写应中、英文对照。
(一)通用名称:能够反映饲料和饲料添加剂产品的真实属性。
(二)商品名称:在中国销售和使用时拟采用的名称,不得完全用外文字母、符号、汉语拼音或数字表示。
(三)产品类别:应与生产国批准的产品类别一致,同时应符合我国《饲料工业通用术语》(GB/T 10647)的要求。
(四)外观:说明产品的颜色、气味、形状(粉末、颗粒、结晶、块状)和状态(固态、液态)。
进口饲料和饲料添加剂产品登记证目录 (2020–05)
Livestock, Poultry, Aquaculture animals (Not including ruminant)
智利苟尔贝斯卡股份有限公司,Arica南厂(注册号:1095)
Corpesca S.A., Arica South Plant(Register No.1095), Chile
单一饲料
Single Feed
养殖动物
All species or categories of animals
阿根廷CARGILL SOCIEDAD ANONIMA COMERCIAL E INDUSTRIAL(SENASA登记号:9454/A/E)
CARGILL SOCIEDAD ANONIMA COMERCIAL E INDUSTRIAL (SENASA No.:9454/A/E), Argentina
Corpesca S.A., Iquique East Plant(Register No.1101), Chile
2020.06—
2025.06
续展
(2020)外饲准字774号
鱼粉
Fishmeal
红鱼粉(三级至特级)
Red Fishmeal (III to Superfine)
单一饲料
Single Feed
颗粒状胍基乙酸
Creamino
混合型饲料添加剂
Feed Additives Mixture
肉仔鸡、生长育肥猪
Broiler, Growing-finishing pigs
德国阿兹肯化工股份有限公司
AlzChem Trostberg GmbH, Germany
2020.06—
1-进口饲料和饲料添加剂产品登记证目录(2014-09)

附件1进口饲料和饲料添加剂产品登记证目录(2014-09)登记证号通用名称商品名称使用范围生产厂家有效期限备注(2014)外饲准字324号饲料添加剂L-蛋氨酸Feed AdditiveL-MethionineBestAmino L-蛋氨酸BestAminoL-Methionine猪Swine家禽Poultry希杰生化马来西亚有限公司CJ Bio Malaysia Sdn. Bhd., Malaysia2014.12-2019.12(2014)外饲准字325号饲料添加剂β-葡聚糖酶(产自棘孢曲霉)Feed Additiveβ-Glucanase (SourceAspergillus aculeatus)乐多仙®VP(包被颗粒)Ronozyme® VP(CT)仔猪Piglet育肥鸡Chickenfor Fattening丹麦诺维信公司Novozymes A/S, Denmark2014.12-2019.12(2014)外饲准字326号饲料添加剂β-1,3-D-葡聚糖Feed Additiveβ-1,3-D-Glucan迈得佳Macorgard®水产动物Aquaculture巴西库塔糖业公司(Quata工厂)Acucareira Quata, S.A. (Quata Plant),Brazil2014.12-2019.12(2014)外饲准字327号饲料添加剂壳寡糖Feed Additive ChitosanOligosaccharide泰和素THCOS猪Swine鸡Chicken鸭Duck虹鳟鱼Rainbow Trout韩国Amicogen株式会社Amicogen, Inc., Korea2014.12-2019.12(2014)外饲准字328号饲料添加剂植物乳杆菌Feed AdditiveLactobacillus plantarum易可食Ecosyl青贮饲料Silage英国弗莱克国际有限公司Volac International Ltd., UK2014.12-2019.12(2014)外饲准字329号饲料添加剂嗜酸乳杆菌Feed AdditiveLactobacillus acidophilus祖莱克益生菌Zoolac FeedgradePremix养殖动物All species orcategories ofanimals丹麦ChemVet dk A/S 公司ChemVet dk A/S, Denmark2014.12-2019.12(2014)外饲准字330号混合型饲料添加剂香味物质Feed Additives MixtureFlavouring Substances普乐葡萄Grape PP Concentrate养殖动物All species orcategories ofanimals荷兰普乐维美公司Provimi B.V., the Netherlands2014.12-2019.12(2014)外饲准字331号混合型饲料添加剂香味物质Feed Additives MixtureFlavouring Substances新吉吉Biacid Nucleus家禽Poultry法国普乐维美公司PROVIMI France, France2014.12-2019.12(2014)外饲准字332号混合型饲料添加剂磷酸氢钙维生素D3嗜酸乳杆菌Feed Additives MixtureDibasic CalciumPhosphate Vitamin D3Lactobacillus acidophilus钙胃能(小、中型犬用)SINGENCareCalcium Supplement(for Small & MediumDog)狗Dog台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字333号混合型饲料添加剂磷酸氢钙维生素D3 嗜酸乳杆菌Feed Additives MixtureDibasic CalciumPhosphate Vitamin D3Lactobacillus acidophilus钙胃能(大、巨型犬用)SINGENCareCalcium Supplement(for Large & GiantDog)狗Dog台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字334号混合型饲料添加剂磷酸氢钙维生素D3嗜酸乳杆菌Feed Additives MixtureDibasic CalciumPhosphate Vitamin D3Lactobacillus acidophilus钙胃能(猫用)SINGENCareCalcium Supplement(for Cat)猫Cat台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字335号混合型饲料添加剂维生素嗜酸乳杆菌Feed Additives MixtureVitamins Lactobacillusacidophilus发育宝-S 整肠配方(离乳犬用)SINGENCareGastrointestinalFormula (forWeanling Puppy)狗Dog台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字336号混合型饲料添加剂维生素嗜酸乳杆菌Feed Additives MixtureVitamins Lactobacillusacidophilus发育宝-S 整肠配方(小、中型犬用)SINGENCareGastrointestinalFormula (for Small &Medium Dog)狗Dog台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字337号混合型饲料添加剂维生素嗜酸乳杆菌Feed Additives MixtureVitamins Lactobacillusacidophilus发育宝-S 整肠配方(大、巨型犬用)SINGENCareGastrointestinalFormula (for Large &Giant Dog)狗Dog台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字338号混合型饲料添加剂维生素嗜酸乳杆菌Feed Additives MixtureVitamins Lactobacillusacidophilus发育宝-S 整肠配方(猫用)SINGENCareGastrointestinalFormula (for Cat)猫Cat台湾信元制药股份有限公司(中科厂)Singen Animal Health Industry Co., Ltd.2014.12-2019.12(2014)外饲准字339号混合型饲料添加剂抗氧化剂抗结块剂柠檬酸Feed Additives MixtureAntioxidants AnticakingCitric Acid纽埃特AQ抗氧化剂OXY-NIL® AQ Dry养殖动物All species orcategories ofanimals比利时纽埃特国际营养公司Beveren-Waas工厂NUTRI-AD International N.V., Plant inBeveren-Waas, Belgium2014.12-2019.12(2014)外饲准字340号猫配合饲料Cat Compound Feed宠物健康幼猫猫粮健育配方Wellness KittenHealth Recipe猫Cat比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字341号猫配合饲料Cat Compound Feed宠物健康猫粮室内健体配方Wellness IndoorHealth Recipe猫Cat比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字342号猫配合饲料Cat Compound Feed宠物健康猫粮控制毛球配方Wellness HairballControl Recipe猫Cat比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字343号犬配合饲料Dog Compound Feed宠物健康大型犬幼犬犬粮(鸡肉配方)Wellness Large BreedPuppy Health withChicken Meal狗Dog比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字344号犬配合饲料Dog Compound Feed宠物健康大型犬成犬犬粮(鸡肉配方)Wellness Large BreedAdult Health withChicken Meal狗Dog比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字345号犬配合饲料Dog Compound Feed宠物健康小型犬幼犬犬粮(鸡肉配方)Wellness Small BreedPuppy Health withChicken Meal狗Dog比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字346号犬配合饲料Dog Compound Feed宠物健康小型犬成犬犬粮(鸡肉配方)Wellness Small BreedAdult Health withChicken Meal狗Dog比利时联合宠物食品公司United Petfood Producers NV, Belgium2014.12-2019.12(2014)外饲准字347号水产苗液体配合饲料Aquaculture LarvalLiquid Compound Feed艾比科-PLEPILITE-PL虾Shrimp美国Epicore Networks (USA), Inc.公司Epicore Networks (USA), Inc., USA2014.12-2019.12(2014)外饲准字348号水产苗液体配合饲料Aquaculture LarvalLiquid Compound Feed艾比科-MEPILITE-M虾Shrimp美国Epicore Networks (USA), Inc.公司Epicore Networks (USA), Inc., USA2014.12-2019.12(2014)外饲准字349号水产苗液体配合饲料Aquaculture LarvalLiquid Compound Feed艾比科-ZEPILITE-Z虾Shrimp美国Epicore Networks (USA), Inc.公司Epicore Networks (USA), Inc., USA2014.12-2019.12(2014)外饲准字350号含可溶物干玉米酒糟DDGSPOET马里昂DDGSPOET Marion DDGS猪Swine牛Cattle家禽Poultry美国POET生物提炼公司-马里昂POTE Biorefining- Marion, USA2014.12-2019.12(2014)外饲准字351号含可溶物干玉米酒糟DDGSPOET莱比锡DDGSPOET Leipsic DDGS猪Swine牛Cattle家禽Poultry美国POET生物提炼公司-莱比锡POET Biorefining- Leipsic, USA2014.12-2019.12(2014)外饲准字352号含可溶物干玉米酒糟DDGSPOET雷克托DDGSPOET Lake CrystalDDGS猪Swine牛Cattle家禽Poultry美国POET生物提炼公司-雷克托POET Biorefining - Lake Crystal, USA2014.12-2019.12(2014)外饲准字353号菜籽粕Rapeseed Meal菜籽粕Rapeseed Meal鱼Fish猪Swine家禽Poultry巴基斯坦R&R Agro Industries (Private)Ltd.公司R&R Agro Industries (Private) Ltd.,Pakistan2014.12-2019.12(2014)外饲准字354号菜籽粕Canola Meal菜籽粕(菜籽来源加拿大)Canola Meal(Canadian Seeds)鱼Fish猪Swine家禽Poultry巴基斯坦Sharif Solvent Plant(私人)有限公司Sharif Solvent Plant (Private) Limited,Pakistan2014.12-2019.12(2014)外饲准字355号牛肉骨粉Bovine Meat and BoneMeal牛肉骨粉Bovine Meat andBone Meal猪Swine家禽Poultry鱼Fish阿根廷LOGROS公司LOGROS S.A., Argentina2014.12-2019.12(2014)外饲准字356号鱼粉Fishmeal丹麦红鱼粉(三级)Danish Red Fishmeal(Ⅲ)畜禽Livestockand Poultry水产动物Aquaculture丹麦三九鱼蛋白有限公司Esbjerg工厂TripleNine Fish Protein A/S, EsbjergPlant, Denmark2014.12-2019.12(2014)外饲准字357号鱼油Fish Oil智利鱼油(饲料级)Chilean Fish Oil-FeedGrade水产动物Aquaculture猪Swine智利Camanchaca Pesca Sur S.A.渔业公司Talcahuano 工厂(No.08130)Camanchaca Pesca Sur S.A., TalcahuanoPlant (No.08130), Chile2014.12-2019.122014)外饲准字358号饲料添加剂维生素B2Feed Additive Vitamin B2罗维素®B2 80-SDRovimix ®B2 80-SD养殖动物All Species orCategories ofAnimals帝斯曼营养产品德国有限公司DSM Nutritional Products GmbH,Germany2014.12-2019.12续展登记(2014)外饲准字359号饲料添加剂维生素A乙酸酯Feed Additive Vitamin AAcetate罗维素®A 500 WSRovimix®A 500 WS养殖动物All species orcategories ofanimals帝斯曼营养产品法国有限公司DSM Nutritional Products France SAS,France2014.12-2019.12续展登记(2014)外饲准字360号饲料添加剂dl-α-生育酚乙酸酯Feed Additivedl-alpha-TocopherolAcetate罗维素®E 50 SDRovimix® E 50 SD养殖动物All species orcategories ofanimals帝斯曼营养产品法国有限公司DSM Nutritional Products France SAS ,France2014.12-2019.12续展登记(2014)外饲准字361号饲料添加剂L-抗坏血酸-2-磷酸酯Feed AdditiveL-Ascorbyl-2-Phosphate罗维素安定-C 35Rovimix® Stay-C35养殖动物All species orcategories ofanimals帝斯曼营养产品法国有限公司DSM Nutritional Products France SAS ,France2014.12-2019.12续展登记(2014)外饲准字362号饲料添加剂核黄素(维生素B2)Feed Additive Riboflavin(Vitamin B2)露他维B2 80Lutavit B2 SG80养殖动物All Species orCategories ofAnimals韩国巴斯夫公司BASF Company Ltd., Korea2014.12-2019.12续展登记(2014)外饲准字363号饲料添加剂蛋白酶(源自枯草芽孢杆菌)Feed Additive Protease(by Bacillus subtilis)康畜宝70One-Q® kangxubao猪Swine牛Cattle家禽Poultry水产动物Aquaculture韩国英赛特生物技术公司Insect Biotech Co., Ltd., Korea2014.12-2019.12续展登记(2014)外饲准字364号饲料添加剂枯草芽孢杆菌Feed Additive Bacillussubtilis班克Sporezyme猪Swine韩国Woogene株式会社Woogene B&G Co., Ltd., Korea2014.12-2019.12续展登记(2014)外饲准字365号混合型饲料添加剂甜味剂Feed Additives MixtureSweeteners甜味素Sugarcap仔猪Piglet西班牙埃特亚公司Industrial Tecnica Pecuaria, Spain2014.12-2019.12续展登记(2014)外饲准字366号混合型饲料添加剂甜味剂Feed Additives MixtureSweeteners特佳甜Optisweet® T仔猪Piglet英国纽埃特有限公司Nutriad Ltd., UK2014.12-2019.12续展登记(2014)外饲准字367号混合型饲料添加剂低聚壳聚糖Feed Additives MixtureLow-Molecular-WeightChitosan福德安Bestfude猪Swine家禽Poultry水产动物Aquaculture中国派斯德股份有限公司China Bestar Laboratories Ltd.2014.12-2019.12续展登记(2014)外饲准字368号复合预混合饲料Premix产得乐RindavitalEnergietrunk奶牛Dairy Cow德国威廉绍曼爱尔斯雷本有限责任公司H.W. Schaumann Eilsleben GmbH,Germany2014.12-2019.12续展登记(2014)外饲准字369号鱼粉Fishmeal秘鲁红鱼粉(三级)Peruvian RedFishmeal (Ⅲ)畜禽Livestockand Poultry水产动物Aquaculture秘鲁Tecnologica De Alimentos S.A.公司Parachique工厂Tecnologica De Alimentos S.A.,Parachique Plant, Peru2014.12-2019.12续展登记。
进口饲料和饲料添加剂产品登记证目录(2012-13)
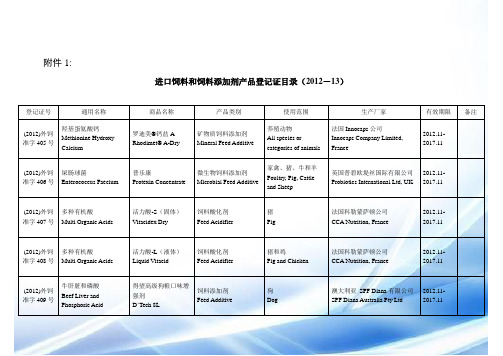
附件1:进口饲料和饲料添加剂产品登记证目录(2012-13)登记证号通用名称商品名称产品类别使用范围生产厂家有效期限备注(2012)外饲准字405号羟基蛋氨酸钙Methionine HydroxyCalcium罗迪美®钙盐ARhodimet® A-Dry矿物质饲料添加剂Mineral Feed Additive养殖动物All species orcategories of animals法国Innocaps公司Innocaps Company Limited,France2012.11-2017.11(2012)外饲准字406号屎肠球菌Enterococcus Faecium普乐康Protexin Concentrate微生物饲料添加剂Microbial Feed Additive家禽、猪、牛和羊Poultry, Pig, Cattleand Sheep英国普碧欧堤丝国际有限公司Probiotics International Ltd, UK2012.11-2017.11(2012)外饲准字407号多种有机酸Multi Organic Acids活力酸-S(固体)Vitacidex Dry饲料酸化剂Feed Acidifier猪Pig法国科勒蒙萨顿公司CCA Nutrition, France2012.11-2017.11(2012)外饲准字408号多种有机酸Multi Organic Acids活力酸-L(液体)Liquid Vitacid饲料酸化剂Feed Acidifier猪和鸡Pig and Chicken法国科勒蒙萨顿公司CCA Nutrition, France2012.11-2017.11(2012)外饲准字409号牛肝脏和磷酸Beef Liver andPhosphoric Acid得望高级狗粮口味增强剂D’Tech 8L饲料添加剂Feed Additive狗Dog澳大利亚SPF Diana有限公司SPF Diana Australia Pty Ltd2012.11-2017.11(2012)外饲准字410号天然类固醇萨洒皂角苷(源自丝兰)YUCCA (YuccaSchidigera Exact)丝兰宝Biopowder饲料添加剂Feed Additive家禽、猪、牛和宠物Poultry, Pig, Cattleand Pet墨西哥BAJA Agro International,S.A. de C.V.公司BAJA Agro International, S.A. deC.V., Mexico2012.11-2017.11(2012)外饲准字411号木质纤维素Lignocelluloses万利纤Opticell饲料添加剂Feed Additive猪、鸡、兔子、小牛和宠物Pig, Chicken , Rabbit,Calf and Pet奥地利艾吉美公司Agromed Austria GmbH2012.11-2017.11(2012)外饲准字412号麦麸和碳酸钙Wheat Flour andCalcium Carbonate育幼保Baby Guard饲料添加剂Feed Additive家禽Poultry台湾信逢股份有限公司New Well Powder Co., Ltd.2012.11-2017.11(2012)外饲准字413号水解植物油Hydrolyzed VegetableOil朋洛弥Palomys能量饲料Energy Feed家禽和猪Poultry and Pig美国哈迪动物营养公司Hardy Animal Nutrition, USA2012.11-2017.11(2012)外饲准字414号鱼油Fish Oil鱼油(饲料级)Fish Oil(Feed Grade)能量饲料Energy Feed养殖动物All species orcategories of animals墨西哥Maz Industrial S.A. deC.V.公司Maz Industrial S.A. de C.V.,Mexico2012.11-2017.11(2012)外饲准字415号发酵豆粕Fermentation of DefattedSoybean meal速益泰Soytide蛋白质饲料Protein Feed猪、家禽、水产、反刍动物Swine, Poultry,AquacultureRuminant希杰第一制糖仁川2工厂CJ Cheiljedang Corporation,Incheon 2 Plant, Korea2012.11-2017.11(2012)外饲准字416号含可溶物干玉米酒糟Dried Corn DistillersGrains With Solubles玛吉斯DDGSMarquis DDGS蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture玛吉斯能源有限公司Marquis Energy LLC, USA2012.11-2017.11(2012)外饲准字417号肉骨粉Meat and Bone Meal牛羊肉骨粉Bovine Ovine Meatand Bone Meal蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture乌拉圭Yarus S.A.公司Yarus S.A., Uruguay2012.11-2017.11(2012)外饲准字418号肉骨粉Meat and Bone Meal鸡肉粉Poultry By-ProductMeal蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture美国温泽世家公司G.A.Wintzer & Son Co., USA2012.11-2017.11(2012)外饲准字419号肉骨粉Meat and Bone Meal羽毛粉Wapak Feather Meal蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture美国温泽世家公司G.A.Wintzer & Son Co., USA2012.11-2017.11(2012)外饲准字420号鱼骨粉Fish Bone Meal鱼骨粉Fish Bone Meal蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture美国Westward Seafoods Inc.公司Westward Seafoods Inc., USA2012.11-2017.11(2012)外饲准字421号红鱼粉Red Fishmeal红鱼粉(三级)Red Fishmeal (Ш)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture毛里塔尼亚ALFA ServicesLimited 公司ALFA Services Limited,Mauritania2012.11-2017.11(2012)外饲准字422号红鱼粉Red Fishmeal智利红鱼粉(一级)Chilean Red Fishmeal(І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture智利Orizon S.A.公司Orizon S.A., Chile2012.11-2017.11(2012)外饲准字423号白鱼粉White Fishmeal白鱼粉(一级)White Fishmeal (І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture列宁集体渔庄(工船加工Seroglazka CH-036)Lenin Kolkhoz Fishing Company(Produced on Board SeroglazkaCH-036)2012.11-2017.11(2012)外饲准字424号白鱼粉White Fishmeal白鱼粉(一级)White Fishmeal (І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture列宁集体渔庄(工船加工SergeyNovosyolov CH-038)Lenin Kolkhoz Fishing Company(Produced on Board SerggeyNovosyolov CH-038)2012.11-2017.11(2012)外饲准字425号白鱼粉White Fishmeal白鱼粉(一级)White Fishmeal (І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture列宁集体渔庄(工船加工MikhailStaritsyn CH-037)Lenin Kolkhoz Fishing Company(Produced on Board MikhailStaritsyn CH-037)2012.11-2017.11(2012)外饲准字426号白鱼粉White Fishmeal白鱼粉(一级)White Fishmeal (І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture列宁集体渔庄(工船加工UMSVictor Gavrilov CH-106)Lenin Kolkhoz Fishing Company(Produced on Board UMS VictorGavrilov CH-106)2012.11-2017.11(2012)外饲准字427号白鱼粉White Fishmeal白鱼粉(三级)White Fishmeal (Ш)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture塔里斯集团有限公司PH384渔船Talley’s Group Limited, Product onV essel, No.PH3842012.11-2017.11(2012)外饲准字428号白鱼粉White Fishmeal白鱼粉(三级)White Fishmeal (Ш)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture塔里斯集团有限公司PH622渔船Talley’s Group Limited, Product onV essel, No.PH6222012.11-2017.11(2012)外饲准字429号白鱼粉White Fishmeal白鱼粉(三级)White Fishmeal (Ш)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture塔里斯集团有限公司PH475渔船Talley’s Group Limited, Product onV essel, No.PH4752012.11-2017.11(2012)外饲准字430号狗干粮Dog Dry Food优卡小型犬成犬犬粮Eukanuba Adult SmallBreed配合饲料Compound Feed狗Dog宝洁阿根廷有限公司Procter Gamble ArgentinaS.R.L., Argentina2012.11-2017.11(2012)外饲准字431号狗干粮Dog Dry Food优卡迷你雪纳瑞犬专用犬粮Eukanuba MiniatureSchnauzer配合饲料Compound Feed狗Dog宝洁阿根廷有限公司Procter Gamble ArgentinaS.R.L., Argentina2012.11-2017.11(2012)外饲准字432号狗干粮Dog Dry Food优卡小型犬体重控制犬粮Eukanuba WeightControl Small Breed配合饲料Compound Feed狗Dog宝洁阿根廷有限公司Procter Gamble ArgentinaS.R.L., Argentina2012.11-2017.11(2012)外饲准字433号狗干粮Dog Dry Food优卡中型犬体重控制犬粮Eukanuba WeightControl MediumBreed配合饲料Compound Feed狗Dog宝洁阿根廷有限公司Procter Gamble ArgentinaS.R.L., Argentina2012.11-2017.11(2012)外饲准字434号狗干粮Dog Dry Food幼犬用软性饲料Dr. Soft Food (Puppy)配合饲料Compound Feed狗Dog韩国巴乌哇呜公司BOWWOW, Korea2012.11-2017.11(2012)外饲准字435号丙酸、甲酸、乙酸和丙酸铵Propionic Acid, FormicAcid, Acetic Acid andAmmonium Propionate菲乐斯(液体)FYLAX®-Liquid饲料防霉剂Feed Mould Inhibitor养殖动物All species orcategories of animals荷兰赛尔可公司Selko B.V., the Netherlands2012.11-2017.11续展(2012)外饲准字436号丙酸、甲酸、乙酸和甲酸铵Propionic Acid, FormicAcid, Acetic Acid andAmmonium Formate肥酸宝Selacid®-Dry饲料酸化剂Feed Acidifier养殖动物All species orcategories of animals荷兰赛尔可公司Selko B.V., the Netherlands2012.11-2017.11续展(2012)外饲准字437号维生素D3VD3罗维素®D3 500Rovimix®D3 500饲料级维生素Vitamin Feed Grade养殖动物All species orcategories of animals帝斯曼营养产品法国有限公司DSM Nutritional Products FranceSAS, France2012.11-2017.11续展(2012)外饲准字438号维生素A乙酸酯Vitamin A Acetate露他维A500SLutavit A500S饲料级维生素Vitamin Feed Grade养殖动物All species orcategories of animals巴斯夫欧洲公司BASF SE, Germany2012.11-2017.11续展(2012)外饲准字439号维生素E乙酸酯Vitamin A Acetate露他维E50SLutavit E50S饲料级维生素Vitamin Feed Grade养殖动物All species orcategories of animals巴斯夫欧洲公司BASF SE, Germany2012.11-2017.11续展(2012)外饲准字440号98.5%L-赖氨酸盐酸盐L-LysineMonohydrochloride98.5%饲料级98.5%L-赖氨酸盐酸盐L-LysineMonohydrochloride98.5% Feed Grade饲料级氨基酸Amino Acid Feed Grade养殖动物All species orcategories of animals味之素(泰国)有限公司Ajinomoto Co., (Thailand)Ltd.2012.11-2017.11续展(2012)外饲准字441号维生素EVitamin E维生素E®混合型50Microvit® E Promix饲料级维生素Vitamin Feed Grade养殖动物All species orcategories of animals安迪苏法国公司Rue Marcel Lingot, France2012.11-2017.11续展(2012)外饲准字442号蛋氨酸羟基类似物Methionine HydroxyAnalogue粉状美斯特®蛋氨酸羟基类似物MetaSmart®饲料级氨基酸Amino Acid Feed Grade奶牛Cow安迪苏法国公司Rue Marcel Lingot, France2012.11-2017.11续展(2012)外饲准字443号灭活酿酒酵母InactivatedSaccharomycescerevisiae莱克素Biolex®MB40饲料添加剂Feed Additive养殖动物All species orcategories of animals德国莱博有限公司Leiber GmbH,Germany2012.11-2017.11续展(2012)外饲准字444号水合硅铝酸钠钙HydratedSodium-CalciumAluminosilicate克毒宝Fintox饲料添加剂Feed Additive养殖动物All species orcategories of animals西班牙Lipidos Toledo有限公司Lipidos Toledo S.A.C., Spain2012.11-2017.11续展(2012)外饲准字445号多种维生素、氨基酸、大豆蛋白Multi Vitamin, AminoAcid, Soybean Protein爱胺补Arcavit Amino添加剂预混合饲料Feed Additive Premix畜禽Livestock and Poultry意大利阿卡公司Prodotti Arca S.R.L.,Italia2012.11-2017.11续展(2012)外饲准字446号多种维生素、氨基酸、矿物元素Multi Vitamin, AminoAcid, Minerals爱固壮Arcavit WP添加剂预混合饲料Feed Additive Premix家禽和猪Swine and Poultry意大利阿卡公司Prodotti Arca S.R.L.,Italia2012.11-2017.11续展(2012)外饲准字447号多种维生素、氨基酸、矿物元素Multi Vitamin, AminoAcid, Minerals爱金维Arcavit Forte添加剂预混合饲料Feed Additive Premix家禽和猪Swine and Poultry意大利阿卡公司Prodotti Arca S.R.L.,Italia2012.11-2017.11续展(2012)外饲准字448号白鱼粉White FishmealRamoen牌白鱼粉(一级)Ramoen Brand WhiteFishmeal(І)蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture挪威沃达海产品公司(工船加工F/T Ramoen)Vartdal Seafood AS, Produced InFactory Trawler F/T RamoenNorway2012.11-2017.11续展(2012)外饲准字449号鱼油Fish Oil鱼油(饲料级)Fish Oil(Feed Grade)能量饲料Energy Feed家禽、猪和水产Poultry, Swine andAquaculture厄瓜多尔Fortidex S.A.公司Data de Posorja工厂Fortidex S.A., Data de PosorjaPlant2012.11-2017.11续展(2012)外饲准字450号白鱼粉White FishmealICICLE®白鱼粉(特级)ICICLE Brand WhiteFishmeal蛋白质饲料Protein Feed家禽、猪和水产Poultry, Swine andAquaculture美国ICICLE 海鲜公司(工船加工:M/V NorthernVictor,工船编号4078)ICICLE Seafoods, Inc., Producton Vessel M/V Northern Victor,No.40782012.11-2017.11续展(2012)外饲准字451号乳清粉Whey Permeate Powder饲料级乳清粉Feed Grade WheyPermeate Powder能量饲料Energy Feed家畜、仔猪和犊牛Livestock,Piglet andCattle美国国际生物营养有限公司Bio-Nutrirtiong International,Inc., USA2012.11-2017.11(2012)外饲准字452号乳清粉Whey Permeate Powder加士能低蛋白乳清粉Milk Permeate Powder能量饲料Energy Feed猪Pig美国绿草地乳制品公司Grassland Dairy Products Inc.,USA2012.11-2017.11(2012)外饲准字453号美国栗树叶提取物Chestnut Leaves Extract福美酚Farmatan LE饲料香味剂Feed FlavoringEnhancement养殖动物All species orcategories of animals斯洛文尼亚天菱有限公司Tanin Sevnica D.D., Slovenija2012.11-2017.11(2012)外饲准字454号蛋白酶(源自米曲霉)Protease(byAspergillusniger oryzae)六畜安®(粉末)Toxi-end® (Powder)饲料酶制剂Feed Enzymes畜禽Livestock and Poultry台湾生百兴业有限公司Life Rainbow Biotech Co., Ltd2012.11-2017.11。
进口饲料和饲料添加剂产品登记证目录

进口饲料和饲料添加剂产品登记证目录(
登记证号
通用名称
商品名称
产品类别
使用范围
生产厂家
有效期限
备注
(2018)外饲 准字458号
混合型饲料添加剂 低聚壳聚糖
Feed Additives
Mixture
Low-molecular-weight
Chitosan
太仆体键素
GD
混合型饲料添 加剂
Feed Additives
美国BioMatrix国际公司
BioMatrix International, USA
2018.10
2023.10
新办
(2018)外饲 准字468号
混合型饲料添加剂 香味物质
Feed Additives Mixture Flavouring Substances
喀泰宝20
CARVOTHYME
20
混合型饲料添 加剂
2023.10
新办
(2018)外饲 准字479号
酿酒酵母培养物Saccharomyces CerevisiaeYeast
Culture
益康XP(有机)
XP Green
单一饲料
Single Feed
养殖动物
All species or categories of animals
美国达农威公司
Diamond V Mills, Inc., USA
Calf protector
添加剂预混合 饲料
Premix
犊牛、羔羊、 小山羊Calves, Lamb, Kid
德国Biochem添加剂贸易和生产 有限公司
Biochem Zusatzstoffe Handels-und Produktionsgesellschaft mbH, Germany
新饲料和新饲料添加剂申报材料要求

新饲料和新饲料添加剂申报材料要求一、审定范围新饲料,是指我国尚未批准使用的新研制开发的饲料。
新饲料添加剂,是指在我国境内研究、创制的未经农业部审定公布的饲料添加剂品种。
新饲料和新饲料添加剂的主要类别:(一)在我国境内研制的创新型单一饲料和饲料添加剂。
(二)其他国家己批准生产、销售,我国尚未批准生产,在我国境内研制的单一饲料和饲料添加剂。
(三)我国境内其他行业使用,首次应用于动物养殖的单一饲料和饲料添加剂。
(四)我国已批准使用,但工艺有重大改进或扩大适用范围的饲料添加剂和新剂型。
(五)其他符合《新饲料和新饲料添加剂管理办法》规定的单一饲料和饲料添加剂。
二、申报材料的格式要求(一)申报材料包括《新饲料和新饲料添加剂审定申请表》(见表1,以下简称《申请表》)和申请材料(具体内容见表2《新饲料和新饲料添加剂申请材料一览表》,以下简称《申请材料一览表》)。
(二)申报材料使用A4规格纸,正文小四号宋体打印。
材料中所有试验报告应加盖试验承担单位公章。
(三)申报材料按《申请材料一览表》的顺序装订成册并标注页码,《申请表》应置于申报材料的首页。
(四)申报材料一式三份,原件一份,复印件两份。
三、《申请表》的填写(一)通用名称:能够反映饲料和饲料添加剂产品真实属性的名称,并与申请材料中的通用名称一致。
(二)商品名称:产品在市场上销售时拟采用的名称,没有的可以不填写。
(三)产品类别:按饲料工业通用术语(GB/T 10647)分类。
(四)外观:说明产品的颜色、气味、形状(粉末、颗粒、结晶、块状)和状态(固态、液态)。
(五)有效成分及含量:有效成分名称及含量保证值。
(六)其他成分及含量:其他成分名称及控制指标。
(七)研制单位:研制该产品的单位名称、地址和邮编,并加盖研制单位公章。
(八)生产企业:生产该产品的企业名称、地址和邮编,并加盖生产企业公章。
(九)联系人:申报单位经办人的姓名、联系电话、传真及电子邮箱。
(十)签字:生产企业(研制单位)法人代表签字。
进口饲料和饲料添加剂申请表(示范文本)
名称美国×××有限公司(生产厂家名称中文译名)
Name×××Co., Ltd., USA
地址工船加工,工船名×××,工船号×××(生产厂家地址中文译名;工船加工的鱼粉,填写工船名称及编号)
AddressProduced on Board at Vessel:×××, Official No.×××
公章(Seal)
境内代理机构负责人签字:
Signature of Domestic Agent
公章(Seal)
1.境内代理机构应当如实向农业部提交有关材料,对翻译材料的准确性负责。
2.境外企业、境内代理机构隐瞒有关情况或者提供虚假材料的,按照《进口饲料和饲料添加剂登记管理办法》第二十九条规定承担相应的法律责任。
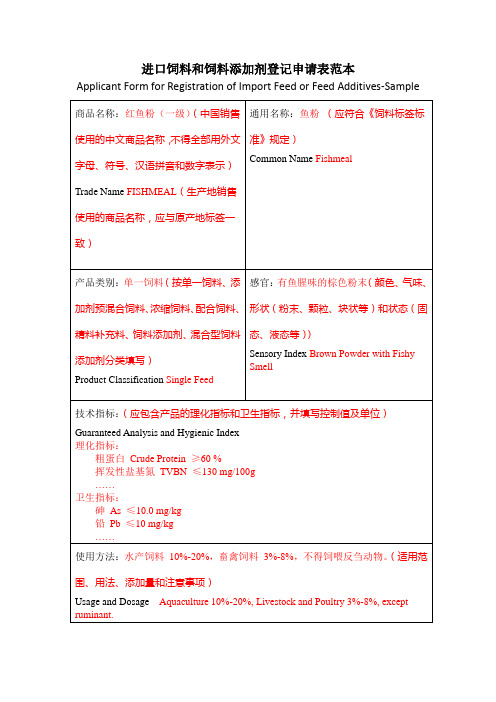
进口饲料和饲料添加剂登记申请表范本
ApplicantForm for Registration of Import Feed or Feed Additives-Sample
商品名称:红鱼粉(一级)(中国销售使用的中文商品名称,不得全部用外文字母、符号、汉语拼音和数字表示)
Trade NameFISHMEAL(生产地销售使用的商品名称,应与原产地标签一致)
申请企业:Applicant Company
名称美国×××有限公司(一般与生产厂家一致,也可填写总公司名称;工船加工的鱼粉,填写总公司名称)
Name×××Co., Ltd., USA
地址美国×××,邮编×××(申请企业地址中文译名)
Address×××,(zip code)×××, USA
境内代理机构:
1.The domestic agent should submit the genuine documents to the MOA and take full responsibility for the accuracy of the translations.
饲料级添加剂申请书

饲料级添加剂申请书英文回答:Feeding additive application form.Introduction:Hello, my name is [Your Name] and I am submitting this application for the approval of a new feeding additive. I believe that this additive will greatly benefit the animal feed industry and improve the overall health and productivity of livestock. In this application, I will provide detailed information about the additive, its benefits, and the necessary safety measures.Product Details:The feeding additive I am proposing is a blend of natural herbs and minerals that have been scientifically proven to enhance the digestion and nutrient absorption ofanimals. This additive will be available in the form of a powder, which can be easily mixed with animal feed. It is specifically designed for use in poultry and swine diets.Benefits:The use of this feeding additive will result in several benefits for animals and farmers alike. Firstly, it will improve the digestibility of feed, leading to better nutrient absorption and utilization. This will result in healthier animals with improved growth rates and feed conversion ratios. Additionally, the additive will support gut health by promoting the growth of beneficial bacteria and reducing the risk of digestive disorders. This will ultimately lead to reduced mortality rates and increased profitability for farmers.Safety Measures:Ensuring the safety of animals and consumers is of utmost importance. Therefore, extensive safety testing has been conducted to ensure that the additive meets allregulatory requirements. The additive has been proven to be non-toxic and free from any harmful residues. Furthermore, it does not have any negative impact on the taste orquality of animal products.Examples:To illustrate the effectiveness of this feeding additive, let me share a couple of examples. Farmer John, who operates a poultry farm, started using the additive in his chicken feed. He noticed a significant improvement in the overall health and weight gain of his chickens. The mortality rate also decreased, resulting in higher profits for John. Another example is Mary, a pig farmer, who added the feeding additive to her pig's diet. She observed that her pigs had better digestion and were less prone to diarrhea. This not only saved her money on veterinary expenses but also improved the quality of her pork.中文回答:饲料级添加剂申请书。
农业部进出口饲料和饲料添加剂登记管理办法-

农业部进出口饲料和饲料添加剂登记管理办法正文:---------------------------------------------------------------------------------------------------------------------------------------------------- 农业部进出口饲料和饲料添加剂登记管理办法(2002年2月20日)第一条为加强进出饲料、饲料添加剂监督管理,保证养殖动物的安全生产,根据《饲料和饲料添加管理条例》的规定,制定本办法。
第二条本办法所称饲料的指经工业化加工制作的供动物食用的饲料,包括单一饲料、添加剂预混合饲料、浓缩饲料配合饲料和精料补充料。
本办法所称饲料添加剂是指饲料加工、制作、使用过程中添加的少量或者微量物质,包括营养性饲料添加剂和一般饲料添加剂。
第三条外国企业生产的饲料和饲料添加剂首次在中华人同共和国国境内销售的,应当向中华人民共和国农业部申请登记,取得产品登记证;未取得产品登记证的饲料、饲料添加剂不得在中国境内销售、使用。
第四条进口的饲料、饲料添加剂应当符合安全、有效和污染环境的原则。
生产国(地区)已淘汰或禁止生产、销售、使用的饲料和饲料添加剂,不予登记。
第五条外国厂商或其代理人申请进口饲料和饲料添加剂产品登记证,应当向中华人民共和国农业部提交下列资料和产品样品:(一)进口饲料或饲料添加剂登记申请表(一式二份,中英文填写)。
(二)代理人需提交生产企业委托登记授权书。
(三)提交申请(中英文一式二份),包括下列内容:1、产品名称(通用名称、商品名称);2、生产国(地区)批准在本国允许生产、销售的证明和在其他国家的登记资料;3、产品来源、组成成分和制造方法;4、质量标准和检验方法;5、标签式样、使用说明书和商标;6、适用范围和使用方法或添加量;7、包装规格、贮存注意事项及保质期;8、必要时提供安全性评价试验报告和稳定性试验报告;9、饲喂试验资料及推广应用情况;10、其他相关资料。
进口饲料和饲料添加剂产品登记证目录(2014-02)

2014.02-
2019.02
(2014)外饲准字064号
含可溶物玉米酒糟粕
DDGS
王牌DDGS
Ace DDGS
单一饲料
Single Feed
猪Swine
牛Cattle
家禽Poultry
水产动物
Aquaculture
美国王牌乙醇公司——斯坦利
AceEthanol-Stanley,USA
2014.02-
2019.02
(2014)外饲准字067号
含可溶物玉米酒糟粕
DDGS
POET艾什顿DDGS
POET Ashton DDGS
单一饲料
Single Feed
猪Swine
牛Cattle
家禽Poultry
水产动物
Aquaculture
美国POET生物提炼公司-艾什顿
POETBiorefining-Ashton,USA
单一饲料
Single Feed
猪Swine
牛Cattle
家禽Poultry
水产动物
Aquaculture
加拿大理查森油籽有限公司Yorkton工厂
Richardson Oilseed Ltd,YorktonPlant,Canada
2014.02-
2019.02
(2014)外饲准字060号
血粉
Blood Meal
牛羊血粉
Bovine Ovine
Blood Meal
单一饲料
Single Feed
猪Swine
家禽Poultry
水产动物
Aquaculture
宠物Pet
进口饲料和饲料添加剂产品登记证目录(XXXX-10)

(2021)外饲准字267号
鱼粉
Fishmeal
红鱼粉〔二级〕
RedFishmeal (Ⅱ)
蛋白质饲料
Protein Feed
非反刍动物
Animal except Ruminant
厄瓜多尔Tadel
TadelS.A.,Ecuador
2021.09
(2021)外饲准字268号
鱼粉
Fishmeal
2021.09
(2021)外饲准字280号
犬罐头
Canned dog food
希尔思科学方案-美味鸡肉餐〔熟龄犬7岁+〕
SciencePlan Savory Chicken Mature Adult7+
配合饲料
Compound Feed
熟龄犬
Mature adult 7+
美国希尔思宠物营养食品〔堪萨斯州工厂〕
母猪和仔猪
Sow andPiglet
奥地利拉曼公司
LallemandGmbH,Austria
2021.09
(2021)外饲准字256号
沸石粉和酿酒酵母
Zeolite andsaccharomyces cerevisiae
艾可肥去霉益生素
AlquerfeedAntitox Plus
饲料添加剂
FeedAdditive
2021.09
(2021)外饲准字277号
猫罐头
Canned Cat Food
希尔思科学方案-美味鸡肉餐〔熟龄猫7岁+〕
SciencePlanwithChicken Mature Adult7+
配合饲料
Compound Feed
幼猫
Kitten
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
进口饲料和饲料添加剂申请材料一览表Application Material List for Registration of Import Feed or Feed Additives
序号No.
申请材料Application Material
1进口饲料和饲料添加剂登记申请表
Application Form for Registration of Import Feed or Feed Additives
2境内代理机构《企业法人营业执照》复印件Domestic Agent Business License
3委托书
Power of Attorney
4生产地批准生产、使用的说明
Production or using licences of the local country
(1)产品及其主要成分在生产地允许作为饲料或饲料添加剂生产、使用的证Documents prove the product or ingredients are allowed to be used as feed or feed in the local country.
(2)生产地官方机构出具的允许生产的许可证明性文件;
The applicant company’s registration certificate or feed manufacturing business reg (3)生产地官方机构出具的自由销售证明;
Free sale certificate provided by official institutions in the local country.
5产品理化性质(如产品颜色,气味,形状,粒度,混合均匀度,溶解性,密Product Physicochemical Properties (e.g. color, odour, taste, appearance, particle mixing uniformity, solubleness, density, etc.)
6产品来源、组成成分
Source of the product & composition
7制造方法Manufacturing Method
8质量标准和检测方法
Product Standard and Test Method
9生产地使用的标签、中文标签式样和商标Labels used in the local country and in China, brand
10使用目的、适用范围和使用方法
Product function, Range of Application and Usage and dosage
11包装材料、包装规格、保质期和贮存条件Packing and Storage。
