欧洲食品安全局
欧洲食品安全局(EFSA)批准新的木糖醇口香糖防龋功能声称

其以科学为基础的事实可以用如下文字描述 ,木糖醇 口香糖降低儿童致龋风险” “ 。
20 06年欧洲食 品安全局 ( F A) E S 提议 , 在整个 欧洲建立 一个协调 营养 和健康声称 的新法 规 ( eu t n( C)N 9 4 R gli E ao o12 / 20 ) 06 。为 了能将产品/ 配料包含在此法规 的框架 中, 针对于该 产品/ 配料 的科学论证是极为重要的。食 品配料 和食 品生产商 都纷纷递交 了拟加入新法规 的声称和相应的数据。这些数据通过专家委员会的评估作出评价 , 然后存档和发表 。 ( 杨海燕 )
月 桂 酰 肌 氨 酸钠
{ 8 、\{ 虿 0 2 5
\
\
、 \
— 、
\
— — —
—
~
3 2 急性 皮肤 刺激性试验 ( 3 . 图 )
5 0 7 5 lO O
() %
图 5 发泡 剂 复 配 的眼 刺 激 值 和质 量 分 数的 关 系
图 5中显 示采 用 十二 醇硫 酸 钠/ 油酰 甘氨 酸 椰
的 、 有 力 度 的声 称 。 更
其科学评价基于如下几点 :
膳食产 品、 营养 和过敏症专 家委员会是根据详实的木糖醇可 以降低致 龋风险的科学论证来发表对 于其健康声称 的观点
的, 而且该观点基于非常严格的论证标准。 专家委员会认为 ,食用 10 “ 0 %木糖醇为甜味剂的 口香糖与降低儿童的致龋风险之间存 在因果关系” 。 科学论证是指餐后食用 2— 3克 10 木糖醇为甜味剂的 口香糖 , 0% 每天至少 3次并在餐后食用。
表2
小 属 急 性 经 口毒 性 ( D L ∞) L 卯( / g D mgk )
各国食品安全体系

各国食品安全体系在美国,食品安全体系主要由美国食品药品管理局(FDA)和美国农业部(USDA)负责管理。
FDA负责监督食品、药品、化妆品和医疗器械的安全,而USDA则负责农产品的安全和检验。
美国的食品安全体系非常注重食品标签和包装的规范,以确保消费者可以清楚地了解食品的成分和营养价值。
在欧洲,食品安全主要由欧洲食品安全局和各个成员国的国家食品安全局共同管理。
欧洲食品安全局致力于评估食品安全相关的科学数据,为欧盟委员会提供食品安全方面的建议。
此外,各个成员国也有自己的食品安全体系,根据欧盟的法律法规设立国家食品安全局,负责管理国内的食品安全事务。
在中国,食品安全体系主要由国家食品药品监督管理局负责监督。
中国的食品安全体系相对较新,但政府在近年来加大了对食品安全的监管力度。
同时,中国也实行了严格的食品安全监测和检验制度,以保障消费者的权益。
总的来说,各国的食品安全体系都是为了保障公众的健康和安全而设立的。
尽管存在差异,但都致力于建立完善的监管体系,规范食品生产和销售活动,为消费者提供安全可靠的食品。
随着全球化的发展,各国也在加强食品安全相关信息的交流和合作,共同应对食品安全挑战,保障全球食品供应链的安全和稳定。
食品安全是全球性的挑战,其影响范围涉及到了全人类的生活与健康。
因此,各国政府和国际组织都在加强合作,共同制定和实施食品安全相关的标准和政策。
在加强国际合作方面,各国之间开展了广泛的信息交流和技术合作。
例如,成员国可以通过国际组织如世界卫生组织(WHO)和联合国粮农组织(FAO)等渠道共享食品安全信息,学习和借鉴其他国家的食品安全管理经验,共同推进食品安全事业的发展。
此外,各国也在制定和执行食品安全标准上进行了合作。
国际标准化组织(ISO)制定了一系列的食品安全相关标准,如ISO 22000食品安全管理体系标准和ISO 22004食品安全管理体系指导标准等,各国可以根据这些国际标准自行制定适合本国国情的食品安全标准,并根据实际情况对其进行执行和监督。
efsa认证申请-定义说明解析

efsa认证申请-概述说明以及解释1.引言1.1 概述概述:EFSA认证申请是指向欧洲食品安全局(European Food Safety Authority,简称EFSA)提交的一项申请,旨在获得EFSA的认可和审核。
EFSA是欧洲境内主管食品安全的官方机构,负责评估和监管欧洲市场上的食品及其相关成分的安全性。
因此,获得EFSA认证对于从事食品生产、销售或进口的企业来说具有重要的意义和价值。
EFSA认证申请涉及多项程序和要求,包括提供详细的产品信息、生产工艺、食品安全控制措施等。
此外,申请者还需提交相关的实验数据和科学研究结果,以证明所申请的食品或成分的安全性和合规性。
EFSA会对申请材料进行严格的审查和评估,确保所申请的食品或成分符合欧洲食品安全标准。
获得EFSA认证将为企业带来诸多好处。
首先,EFSA认证是食品市场上的一项重要资质标志,可以增强企业在消费者心中的信任度和品牌形象。
其次,EFSA认证可以提供法律和商业保护,减少企业面临的风险和法律诉讼的可能性。
此外,EFSA认证还能够拓展企业的市场份额,因为许多批发商和零售商在选择供应商时更倾向于选择已获得EFSA认证的产品。
尽管EFSA认证申请过程可能相对繁琐和时间消耗较长,但其带来的好处和影响是不可忽视的。
随着欧洲食品安全意识的增强和监管力度的加强,EFSA认证将会成为企业进入欧洲市场的一项重要前提和竞争优势。
相信随着时间的推移,越来越多的企业将会意识到并努力获得EFSA认证,为消费者提供更安全、更合规的食品产品。
1.2文章结构文章结构的设计是非常重要的,可以帮助读者更好地理解和组织文章的内容。
以下是本文的文章结构。
首先是引言部分,它包括概述、文章结构、目的和总结。
概述部分将简要介绍EFSA认证申请的背景和重要性,引起读者的兴趣。
文章结构部分将详细介绍整篇文章的框架和组织方式,帮助读者理清思路和逻辑。
目的部分将明确本文的写作目的和意义,为读者提供一个明确的方向。
欧盟食品安全局的使命与任务

关键词 :食品安 全;风险分析 ;风险评 估 ;风 险管理 ;E S FA 欧 盟 食 品 安 全 局 ( u o e n FO d S f t E r P a O aey Auh r y F A)是在欧洲发生 了疯牛病 ( E)、 toi ,E S t BS
3 %。 9
与欧盟成 员国进 行紧密合 作 ,与欧盟 委员会 、欧洲议
会和成员 国分 享该领域 的风险交 流 ,提 供食 品安全意
见和建议。
2 欧盟食 品安全局的结构
欧盟食 品安全局 由执行局 长领 导 ,管理 部 门有 局 长及管理 委员会办 公室、特别顾 问 、战略规划 、质量 管理 /审计部 门,还设 有学术委 员会 ,主要领 导和保
-
障风险评估 、科研合作 和支撑两个 业务部 门的工作 。 2 0 年计划编 制人 员4 0 。 08 2名
 ̄X ( ix n — I p S 英 d o i )和其它食 品危机 的背景下 ,于2 0 02 年成立 的 ,总部 设 在意 大利 帕尔 玛 ( ama 。在 当 Pr )
2 1 风险评估理事会 .
摘
要 :欧盟食品安 全局是欧盟委 员会设立的、独立的从 事食品安 全风 险评估的科 学机构 ,为欧盟委 员会和成 员
国提供食品安全风险管理所需的科 学支撑。本文根据现场考察、双边交流和相 关资料 ,介绍 了该机构的组织结构、主
要任务、优 先领域 、合作机制和具体工作 。 了解和分析该机构的性质和工作特点,对建 设中国的食品安 全风险评估机
S ft, 0 ) ;2 0 年通过 了执行法律( lme t g aey 0 0 2 02 I e ni mp n
食品中的激素残留限量规定

食品中的激素残留限量规定随着人们对食品安全和健康的关注度日益提高,对食品中激素残留的问题也变得越来越重要。
激素在食品中的残留可能对人体健康造成潜在风险,因此,各国纷纷制定了激素残留限量规定,以确保食品的安全性。
本文将介绍一些国家对食品中激素残留的限量规定,并分析其对食品行业和消费者的影响。
一、美国食品药品监督管理局(FDA)激素残留限量规定美国食品药品监督管理局(FDA)对激素残留设立了一系列严格的限量规定,以确保食品的安全性。
根据FDA的规定,禽类产品中激素的使用是禁止的,而对于其他肉类和乳制品,则规定了严格的激素残留限量。
例如,牛肉中的雌激素总量限制为每公斤0.3纳克,而奶制品中的黄体酮总量限制为每公斤10纳克。
此外,FDA还规定了其他一些激素的残留限量,例如在动物饲料中使用的人工生长激素的使用和残留也受到限制。
二、欧洲食品安全局(EFSA)激素残留限量规定欧洲食品安全局(EFSA)是欧盟成员国的食品安全监管机构,负责制定激素残留的限量规定。
根据EFSA的规定,禽类产品、肉类和乳制品中的激素残留限量非常严格,且禁止使用任何生长激素。
例如,牛肉中的雌激素总量限制为每公斤4纳克,而奶制品中的黄体酮总量限制为每公斤1纳克。
此外,EFSA还对其他激素的残留限量进行了规定,并使用严格的监测和检测机制来确保食品的合规性。
三、中国食品安全国家标准中国也制定了一系列食品安全国家标准,限制食品中激素的残留量。
例如,根据《食品安全国家标准餐桌盐中激素限量》(GB 5009.65-2010)的规定,食用盐中的激素总量限制为每公斤5毫克。
此外,根据《食品安全国家标准牛肉中瘦肉精的限量》(GB 2760-2014)的规定,在牛肉中瘦肉精的残留限量为每公斤2毫克。
这些限量规定是为了保护消费者的健康,确保食品的合格和安全。
四、激素残留限量规定对食品行业和消费者的影响激素残留限量规定对食品行业和消费者都有着重要的影响。
对于食品行业来说,合规是一项重要任务。
全球食品安全标准

全球食品安全标准
全球食品安全标准是指在全球范围内用于确保食品安全的一系列准则和规定。
以下是一些常见的全球食品安全标准:
1. 联合国粮食及农业组织(FAO)和世界卫生组织(WHO)制定的《食品法典》:这是一个国际参考标准,涵盖了食品的生产、加工、储存、运输和销售等各个环节。
2. 国际食品法典委员会(CAC)制定的标准:CAC 是由 FAO 和 WHO 共同成立的机构,负责制定和协调全球食品安全标准。
3. 美国食品药品监督管理局(FDA)制定的标准:FDA 是美国负责监管食品安全的机构,其标准在全球范围内具有很高的影响力。
4. 欧洲食品安全局(EFSA)制定的标准:EFSA 是欧盟负责食品安全的机构,其标准在欧洲和其他地区也有广泛的应用。
5. 中国国家食品药品监督管理总局(CFDA)制定的标准:CFDA 是中国负责监管食品安全的机构,其标准在中国和其他地区也有一定的影响力。
这些标准的制定和实施旨在确保食品的安全、卫生和质量,保护消费者的健康和权益。
不同国家和地区可能会根据自身的情况和需求,采用不同的食品安全标准。
欧洲进口茶叶法规

欧洲进口茶叶法规
欧洲对茶叶的进口设有一系列法规和标准,以确保茶叶的质量和安全。
以下是一些重要的欧洲茶叶进口法规:
1. 欧洲食品安全局(EFSA):EFSA负责对食品安全进行评
估和监管。
茶叶进口必须符合EFSA的要求,并通过食品安全评估。
2. 残留物限制:欧盟对茶叶中残留农药和其他化学物质的数量设有严格的限制。
进口茶叶必须符合这些限制,并通过检测,确认其符合欧洲标准。
3. 农药残留最大限量值:欧洲对不同农药在茶叶中的最大限量值有具体规定。
进口茶叶必须符合这些限值,以确保人们饮用的茶叶安全。
4. 标签要求:欧洲要求进口茶叶的包装上必须标明茶叶的产地、成分、保质期等信息。
必须确保这些标签信息真实准确。
5. 有害物质限制:欧洲规定茶叶中的重金属、农药和其他有害物质的含量必须低于一定的限量值。
进口茶叶必须进行测试,以确保这些限值不被超过。
6. 原产地证书:进口商必须提供茶叶的原产地证书,证明茶叶确实来自标注的原产地。
原产地证书可以确保茶叶的质量和真实性。
这些法规和标准旨在保障欧洲消费者对茶叶品质和安全的需求,也促进了茶叶贸易的正规化和发展。
进口商和生产商需要了解这些法规和标准,并确保他们的茶叶符合欧洲的要求。
食品添加剂安全法规与监管措施

食品添加剂安全法规与监管措施食品安全一直备受社会关注,合理使用食品添加剂成为保障食品安全和卫生的重要措施之一。
为了确保食品添加剂的使用不会对人体健康产生负面影响,各国纷纷制定了一系列的法规和监管措施。
本文将探讨食品添加剂的安全法规,并介绍各国的监管措施。
一、食品添加剂的定义与分类食品添加剂是指用于改善食品质量、保持食品特性以及加工和制造食品时使用的物质。
它既可以是天然来源,也可以是合成的化学物质。
根据其作用及用途的不同,食品添加剂可分为增加剂、防腐剂、稳定剂、乳化剂等多个类别。
二、食品添加剂的安全法规为了确保食品添加剂在合理使用的前提下不对人体健康造成危害,各国制定了相应的法规来对食品添加剂进行管理和监管。
以下是一些国际上常见的食品添加剂的安全法规:1. 欧洲食品安全局(EFSA)欧洲食品安全局是欧洲联盟负责食品安全的机构,制定了一系列的食品添加剂的安全标准与法规。
EFSA根据科学评估,制定了对不同食品添加剂的摄入限量。
同时,EFSA也对新的食品添加剂进行审查和评估,确保其安全性。
2. 美国食品药品监督管理局(FDA)美国FDA对食品添加剂的安全使用制定了一系列的规章和指南。
根据FDA的规定,所有的食品添加剂必须经过严格的审查和评估,确保其对人体健康无害。
3. 中国国家食品药品监督管理局(CFDA)中国国家食品药品监督管理局负责对食品添加剂的管理和监督。
根据中国的相关法律法规,食品添加剂必须符合国家标准,并经过审批才能使用。
CFDA还定期对食品添加剂进行抽检,确保其质量和安全性。
三、食品添加剂的监管措施为了更好地管理和监督食品添加剂的使用,各国采取了一系列的监管措施。
以下是几个常见的监管措施:1. 颁发许可证各国针对使用食品添加剂的企业进行许可管理,要求企业必须符合一定的条件和标准,才能获得食品添加剂的使用许可证。
2. 制定标准各国制定了一系列食品添加剂的标准,明确了使用范围、用量限制、生产或进口要求等,以确保食品添加剂的使用符合安全规定。
欧洲食品安全局简介

作者: 无
作者机构:
出版物刊名: 中国标准化
页码: NULL-NULL页
主题词: NULL
摘要:一、欧洲食品安全局的任务为《食品法》规定基本原则和要求及成立欧洲食品安全局(EFSA)的法规于 2 0 0 2年 1月 2 8日正式采用。
法规中确定的食品安全局的主要任务是 ,为成员国类似的机构提供独立的科学建议和支持 ,并建立一个紧密的合作网络。
此外 ,食品安全局应对与食品链相关的危害进行评估 ,并将有关食品危害的信息告知广大公众。
食品安全局的主要任务有六项 :(1 )按照欧洲委员会、欧洲议会 (EP)和成员国的要求 ,对食品安全问题和其他相关事宜 ,如动物健康/福利、植物健康、GMO(转基因 )和营养等方面提供独立的科学建议 ,并将此建议作为风险管理决策的基础 ;(2 )对食品问题提出技术建议 ,以促进与食品链相关的政策和法规的制定;(3)为监测欧盟内整个食品链的安全性 ,对有关食品的数据及其与任何潜在危害相关的必要信息进行收集和分析 ;(4)对紧急危害进行识别和早期报警 ;(5)在关键时刻支持欧洲委员会的工作 ;(6)对其权限范围内的所有事宜向公众征求意见。
法规的采用为 2 0 0 2年欧洲食品安全局的成立和运行铺平了道路。
二、食品安全局的法律地位和工作范围食品安全局是一个独立的法人实体 ,与...。
专访欧洲食品安全局局长

专访欧洲食品安全局局长:食品安全关乎政治,但科学必须独立。
有时候科学家传播的内容,监管部门并不爱听,因为还没有找到解决方法。
这时候科学家会说:对不起,我们必须发表出来。
重建消费者对食品安全的信任并不容易,ESFA是欧盟最开放的机构之一,民众可以随时旁听会议。
只有公开、透明才能重建信任。
南方周末记者郭丝露发自意大利帕尔马市编辑|蒋昕捷在帕尔马这座以火腿闻名的小城里,隐藏着欧洲食品安全体系的“两座大山”之一——欧洲食品安全局(European Food Safety Authority,以下简称ESFA)。
如果说欧盟委员会是欧洲食品安全体系中的管理者,那么ESFA就是冷静的观察者和科学家。
在“科学评估”和“行政管理”分而治之的“欧洲模式”中,二者平起平坐。
国人在新闻中看到的“油炸食品和烟草含相同致癌物丙烯酰胺”“甲基汞含量较高的鱼类要少吃”等警示信息,就源自这里。
如何保持ESFA的“独立性”,排除行政管理者的牵制,这也是2014年7月上任的欧洲食品安全局局长伯恩哈特.乌尔(Bernhard Url)所面临的难题。
乌尔来自奥地利,在欧洲食品安全体系中工作十余年,历任ESFA科研小组科学家、ESFA 管委会成员。
2015年7月7日,在接受南方周末记者专访时,乌尔反复强调“风险评估必须独立”。
“科学与政治无关。
”他说。
“风险评估和风险管理最好分开”南方周末:食品安全领域有一种说法,只有爆发大规模的食品安全危机,才能驱动监管体制改革。
你认同这种说法吗?乌尔:我并不同意一定需要有食品安全危机,才会让政府转变。
不过我也认同一个事实,在上世纪90年代,欧洲面临最严重的食品安全危机是疯牛病,加上前些年马肉冒充牛肉等事件,这让欧盟领导人觉得食品安全管理体制需要改变,ESFA正是这种转变的产物。
管理者从疯牛病事件中学到最重要的一点,就是风险管理和风险评估最好分开。
2002年,欧盟委员会决定设立一个独立的风险评估部门,这个部门层级要够高,ESFA由此而来。
美日欧食品质量监督管理体系

美日欧食品质量监督管理体系食品安全是一个得不到忽视的问题。
全球的食品市场对于消费者来说是一个无形的挑战,而且组成了经济的重要组成部分,特别是在美国、日本、和欧洲地区。
由于小时候吃的什么塑化剂和农药毒素等问题,导致很多人长大后深信不疑其实没有什么安全存在。
因此,对食品生产者质量监管成为一个重要的问题。
本文将重点探讨美日欧三国的食品质量监督管理体系。
美国美国是一个采用自由市场经济的新兴国家。
美国食品和药物管理局(FDA)是美国负责食品质量和药品的监管机构。
其主要职责是监督以上生产商,确保他们符合规定的质量标准,并限制某些有害物质出现在食品中。
FDA 列出的食品添加剂包括增味剂、香料、颜料、脱脂乳、结构类成分、膳食辅助剂等。
与此同时,FDA 每年都有关于警告、产品召回、以及批准新药的报告和发布。
为了确保食品安全,有一组规定负责者需要遵守,即美国食品法规(FSMA)。
根据该法规,美国食品生产企业必须在其生产和配送过程中建立并实施食品安全计划。
美国国家标准促进组织协助FDA 管理和颁布相关的标准。
此外,还有许多监管组织,诸如联邦监管机构(FSIS)和美国环境保护局(EPA),分别对肉、禽、鱼、奶、蔬菜和水等产品尽力维持高质量监管水准。
日本日本是一个政府机构在经济和其他领域的重要性远远超过食品行业的国家。
在日本,食品安全是一个不可忽视的问题。
日本基本法食品卫生法(Food Sanitation Law)作为主要立法,确保了食品的安全性,并由卫生省监管。
以日本为代表的民间组织,日本饮食文化协会(JFCA)制定了很多行业标准。
JFCA 颁布了最严格的国家标准,确保每个厨师的手的干净程度、产生食物的健康性和每天开放到自制和称呼的食物标准。
此外,日本专业机构将大量技术发布到消费市场,以减少有害物质的量,包括作物上的农药等。
欧洲欧洲食品安全局(EFSA)负责对欧盟范围内的食品进行评估,并对可能对人体健康造成危害的有害物质进行风险评估。
欧洲食品安全局(EFSA)批准新的木糖醇口香糖防龋功能声称

图 6阳离子对 S P 膜水蒸气透过系数的影响 I
F g6Efe t f a i n o h W VPv l eo S If m i . f c o c t s nt e o au f P i l
F o E gn ei g 2 0 , 0 2 521 od n ie r , 0 5 7 : 0 - n 0
sypo i i le [ .oma f i eh o g, 05 10 o r n s a s J Ju l o Bo cn l y2 0 ,2 : e t o t 】 t o
26 37 9 - 0
[] 熊犍, 4 冯凌凌, 叶君, S C溶解性 的研究[ . 等. P J食品工业科技, 】
a d O 0 r p ris f M o i e S y r oen Io ae n C lr P o e t o e df d o P ti s lt i
V m [ .o ra o r u ua F o hmir, 00 4 : a s ] Junl f i l r o dC e sy 20 , 8 J Ag c t l t
空 白
氯化 钠 氯化锌 氯化铁 阳离子
4 3 -9 1 9 74 4
[ O . WagY, agS .e a R l o f acai i 2 ] uSY, n Tn . ,t . oe f e l dn Z 1 ni c
pe rr  ̄ geilfmsf m ypo i i l [ .oma o be l r s rtn s a J Ju l f d i o o e ot ] e
增加 3.%,MC增加 7 %;对 E 22 . 4 B影 响较 小 ; ( ) n+JS I 的 影 响较 小 , B降低 3 . 4 Z 2) P 膜  ̄ - E O 4
欧洲食品安全局的风险交流机制及启示

一
、
欧洲 食 品安全 局 的设 立 2 0 0 0 年1 月, 欧 盟 发布食 品安 全 白皮 书 , 提 出成立 欧 洲食 品 安全 局( E F S A )
注 。2 0 世 纪9 0 年 代 后期 , 欧洲 发 生 了一 连 串的食 品 安 全 事件 , 使 欧 洲 的食 品 安 全 亮 起 了红 灯 , 如
比利 时戴 奥 辛 污染 食 用 油事 件 , 英 国疯 牛 病 和 口
饲 料 的方 面 的信 息 给欧盟 及成 员 国的相关单 位 。 欧盟 食 品安 全 风 险 交 流 的 参 与 者 包 括 欧 盟
食品是人 类赖 以生存 的物 质基础 , 食 品安全 不仅关 系到人类 的身体健康 和 生命安 全 , 也关 系到 国家及社会 的稳定 与发展 。食 品安全风 险交流是 指在食 品 安全风 险分析 的过程 中 , 社会各 方对危 害 、 风险, 以及 风险相关 因素 和风险认 知 信息看 法 的互动 与交 流 。… 食 品安全 风险交 流是 培养 公众 风险感 知 、 衔 接 风险 评估与形成食 品政策 的关键 因素 , 正成为各 国政府和 学者关注 的重要 议题 。 依 托 欧 洲 食 品 安全 局( E u r o p e a n F o o d S a f e t y A u t h o r i t y , 简称 E F S A 1 , 欧 盟 已经 形 成 了高效 、 科学 、 灵 活 的食 品安 全 监督 管理 体 系 , 其风 险 交流 机 制有 效
国 际视 野
随 着E F S A的成 立 , 欧 盟基 本 形 成 以欧洲 议 会 , 欧
食品安全工作的监管机构与法规体系

食品安全工作的监管机构与法规体系食品安全工作的重要性在现代社会日益凸显,不仅关乎公众的健康和生命安全,也对社会经济稳定和可持续发展产生着重要影响。
为了保障食品安全,各国普遍设立了专门的监管机构,并建立了完善的法规体系。
本文将介绍一些常见的食品安全监管机构和法律法规,旨在提高公众对食品安全问题的认识和了解。
一、食品安全监管机构1. 国家食品药品监督管理总局(FDA)国家食品药品监督管理总局(以下简称FDA)是美国负责食品和药品监管的最高机构。
其职责包括制定食品安全标准、执行相关法律法规、监督食品生产和销售企业的合规性等。
FDA通过对食品安全问题进行风险评估,制定相应的监管措施,以保障公众的食品安全。
2. 欧洲食品安全局(EFSA)欧洲食品安全局(以下简称EFSA)是欧盟负责食品安全评估和风险管理的机构。
其主要任务是提供科学依据,评估潜在食品风险并制定相应的食品安全措施。
EFSA在欧盟的食品管制决策中发挥着重要的作用,保障了欧洲市民的食品安全。
3. 国家食品药品监督管理局(CFDA)国家食品药品监督管理局(以下简称CFDA)是中国负责食品和药品监管的官方机构。
其主要职责包括食品安全标准的制定、食品生产许可的发放、食品安全监测和抽检等。
CFDA通过加强对食品生产企业的监管,推动食品安全管理工作,确保国民的食品安全。
二、食品安全法规体系1. 食品安全法食品安全法是各国制定的基本法律,目的是确保食品安全、保护公众健康。
食品安全法规定了食品生产、加工、储存、运输和销售等各个环节的管理要求,并规定了追溯制度、食品标签标识等内容。
此外,食品安全法还设立了食品安全监管机构,明确了其职责和权力。
2. 食品安全标准食品安全标准是针对不同食品类别和成分制定的指导性文件,用于规范食品的质量和安全要求。
食品安全标准包括限量标准、残留农药和兽药标准、添加剂使用标准等。
各国在制定食品安全标准时,一般参考国际组织的相关标准,以确保食品的全球贸易公平和顺畅。
efsa认证申请

efsa认证申请全文共四篇示例,供读者参考第一篇示例:EFSA(European Food Safety Authority)是欧洲食品安全局的缩写,成立于2002年,是欧洲联盟的一个独立机构,负责对食品、饮料、饲料及动植物保健产品进行风险评估,并向欧盟委员会和成员国提供建议。
EFSA认证是欧洲食品安全局对食品、饮料和营养保健产品进行的认证,其主要目的是保障消费者的健康和安全。
EFSA的认证流程相对复杂,需要经历多个步骤。
申请者需要向EFSA提交申请表格,详细描述产品的成分、生产过程、安全性等信息。
接着,EFSA将对申请进行初步审查,确定是否符合认证标准。
如果初步审查通过,EFSA将进行更加详细的评估,包括对产品成分、安全性、食用指导等方面的审核。
在评估过程中,EFSA可能会要求申请者提供额外的信息或进行必要的实验。
EFSA的认证标准非常严格,申请者需要确保其产品符合欧盟相关法规和标准。
申请者必须提供充分的科学证据证明产品的安全性和有效性,包括临床研究、毒理学实验等。
申请者还需要确保产品的标签、广告等内容符合欧盟法规,不得误导消费者。
获得EFSA认证对申请者来说有很多好处。
EFSA认证是欧盟市场准入的重要条件之一,可以帮助申请者开拓欧盟市场。
EFSA认证可以提高消费者对产品的信任度,增加产品的竞争力。
EFSA认证还可以为申请者提供权威的科学支持,帮助其提高产品质量和安全性。
获得EFSA认证并不容易,需要申请者投入大量时间和精力。
申请者需要配合EFSA进行详细的评估和审核,提供充分的证据证明产品的安全性和有效性。
申请者还需要支付一定的费用用于评估和认证过程。
EFSA认证是欧盟食品安全管理体系中的重要环节,是保障消费者健康和安全的重要手段。
获得EFSA认证可以帮助申请者提高产品的市场竞争力,增加消费者信任度。
尽管认证过程较为繁琐,但对于追求高质量和安全的企业来说,获得EFSA认证无疑是值得的投资。
希望更多的企业能够关注EFSA认证,提高产品质量,为消费者提供更加安全、健康的食品。
欧盟食品安全的管理体系

欧盟食品安全的管理体系引言食品安全是生命安全和健康的基本保障,对于欧盟来说,确保食品安全是非常重要的任务。
欧盟通过建立完善的食品安全管理体系,确保欧洲市场上的食品都符合严格的安全标准。
本文将介绍欧盟食品安全的管理体系,并探讨其运作机制及其对食品安全的重要性。
欧盟食品安全管理体系的构成欧盟的食品安全管理体系由多个组成部分构成,包括立法、监管机构、风险评估和风险管理等。
下面将对这些部分逐一进行介绍。
1. 立法欧盟食品安全管理体系的基础是其严格的法律法规。
欧盟制定了一系列食品安全法律法规,以确保食品生产、加工、运输和销售过程中的食品安全。
这些法律法规旨在保护消费者的权益,并规定了食品生产商和供应商必须遵守的规则。
2. 监管机构欧盟设立了专门的食品安全监管机构,如欧洲食品安全局(EFSA)。
这些机构负责对食品安全进行监督和管理,进行风险评估和风险管理工作。
监管机构的职责是确保食品安全标准得以有效执行,并对食品进行监测和检测。
3. 风险评估风险评估是欧盟食品安全管理体系的重要环节。
风险评估是指对食品中可能存在的危险物质和微生物进行评估,以确定其可能对人体健康产生的风险。
欧洲食品安全局负责进行风险评估工作,通过科学研究和数据分析来评估各种食品中可能存在的风险。
4. 风险管理风险管理是指根据风险评估的结果,制定并实施相应的食品安全管理措施。
风险管理的目标是减少食品中的危险物质和微生物,以及食品相关疾病的发生和传播。
欧盟在制定食品安全管理措施时,通常基于科学证据,并与相关利益相关者进行广泛的磋商和协商。
欧盟食品安全管理体系的运作机制欧盟食品安全管理体系的运作机制主要包括风险评估、风险管理和风险沟通三个方面。
1. 风险评估风险评估的过程主要包括以下几个步骤:•收集和分析相关数据和信息;•评估食品中可能存在的危险物质和微生物的风险;•判断危险物质和微生物对人体健康的潜在危害;•计算风险的可能性和程度;•根据风险评估的结果,提出相应的管理建议。
国外餐饮安全监管制度内容

国外餐饮安全监管制度内容欧洲欧洲食品安全局(EFSA)是欧洲联盟的一个机构,负责评估食品安全和风险,为欧盟成员国的决策提供科学依据。
欧盟也制定了严格的食品安全法律,包括《食品卫生标准法规》、《食品信息法规》、《食品添加剂法规》等。
此外,欧盟要求食品生产和销售企业必须满足HACCP(危害分析与关键控制点)制度,确保产品符合安全、卫生、质量等标准。
在欧洲各国中,英国是其中一个比较注重食品安全的国家。
英国政府机构Food Standards Agency(FSA)负责制定食品政策,该机构除了关注食品质量和卫生,还负责监管动物饲养、宰杀和屠宰。
FSA并不支持使用转基因食品,推动消费者对于食品的了解和食品安全问题的公开。
美国美国食品药品监督管理局(FDA)是美国联邦政府的一个机构,负责确保食品、药品、医疗器械、化妆品和烟草制品等产品的安全。
FDA制定了大量的法规、标准和指导,并且不断更新和调整,以适应快速变化的市场和科技环境。
此外,美国还推行GMP(良好的制造实践)和HACCP制度,确保产品的卫生和质量。
美国各州和县市也会发布自己的食品安全法规。
纽约市的公共卫生部门颁布了医院和餐厅规定(HHC Rules and Regulations)和城市卫生代码(New York City Health Code),监督食品企业的卫生条件,要求对于食品加工、储存和销售过程进行全程追溯。
日本日本的食品安全监管系统是基于国家标准和技术标准的,制定了零售食品安全法、食品及添加剂安全法、动物卫生管理法等一系列法规。
此外,日本在食品安全标签的规定上,比如限制表示有『药理作用』、『医药功效』的字眼,以避免不良商业行为。
在餐饮业方面,日本采用了用餐坐位查询系统和原料追溯系统来保障食品供应链、追溯食品来源等问题。
此外,日本的食品生产者都必须遵守HACCP制度,确保生产出的食品质量和安全性。
结论不同国家致力于保障餐饮安全的食品监管制度有所差别,但都秉持着同样的原则——食品安全和卫生优先。
食品安全管理组织机构及食品安全管理制度

食品安全管理组织机构及食品安全管理制度为了保障公众的食品安全,各国纷纷建立了相应的食品安全管理机构和制度。
本文将探讨食品安全管理组织机构的职责和食品安全管理制度的重要性。
一、食品安全管理组织机构食品安全管理组织机构是负责食品安全监管和管理的机构,其职责包括食品安全规划制定、监督检查、风险评估、紧急处置等。
以下是常见的食品安全管理组织机构:1. 国家食品药品监督管理总局国家食品药品监督管理总局是中国负责食品和药品监管的部门,其职责包括制定相关法规、监督食品安全工作、处理食品安全事故等。
2. 美国食品药品监督管理局美国食品药品监督管理局(FDA)负责监督美国的食品安全工作,确保食品的质量和安全性,并管理食品添加剂、食品标签等事宜。
3. 欧洲食品安全局欧洲食品安全局(EFSA)是欧盟的食品安全管理机构,负责评估食品安全风险和提供相关的科学意见,以确保欧洲消费者的食品安全。
二、食品安全管理制度食品安全管理制度是针对食品安全问题制定的一系列规定和制度,旨在确保食品的质量和安全性。
以下是食品安全管理制度的重要性以及相关措施:1. 食品安全法律法规的制定和完善各国制定了一系列食品安全法律法规,明确了食品安全的基本要求和标准。
通过法律法规的制定和完善,可以对食品生产、流通、销售等环节进行监管和管理。
2. 食品安全标准的制定和执行食品安全标准是保障食品质量和安全的重要依据。
制定科学合理的食品安全标准,并严格执行,可以确保食品的质量和安全性。
3. 食品安全检测和监测食品安全检测和监测是食品安全管理的重要环节。
通过对食品中的有害物质、微生物等进行检测和监测,可以及时发现和处理食品安全问题,保障公众的健康。
4. 食品安全风险评估和预警食品安全风险评估和预警是保障食品安全的重要手段。
通过科学的风险评估和及时的预警机制,可以降低食品安全风险,并采取相应的措施进行处理。
5. 食品安全教育和宣传加强食品安全教育和宣传是提高公众食品安全意识的重要途径。
食品安全领域组织机构
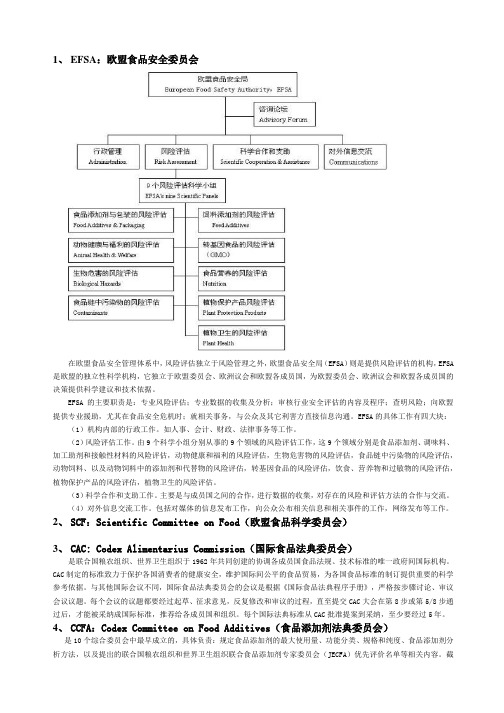
1、EFSA:欧盟食品安全委员会在欧盟食品安全管理体系中,风险评估独立于风险管理之外,欧盟食品安全局(EFSA)则是提供风险评估的机构,EFSA 是欧盟的独立性科学机构,它独立于欧盟委员会、欧洲议会和欧盟各成员国,为欧盟委员会、欧洲议会和欧盟各成员国的决策提供科学建议和技术依据。
EFSA的主要职责是:专业风险评估;专业数据的收集及分析;审核行业安全评估的内容及程序;查明风险;向欧盟提供专业援助,尤其在食品安全危机时;就相关事务,与公众及其它利害方直接信息沟通。
EFSA的具体工作有四大块:(1)机构内部的行政工作。
如人事、会计、财政、法律事务等工作。
(2)风险评估工作。
由9个科学小组分别从事的9个领域的风险评估工作,这9个领域分别是食品添加剂、调味料、加工助剂和接触性材料的风险评估,动物健康和福利的风险评估,生物危害物的风险评估,食品链中污染物的风险评估,动物饲料、以及动物饲料中的添加剂和代替物的风险评估,转基因食品的风险评估,饮食、营养物和过敏物的风险评估,植物保护产品的风险评估,植物卫生的风险评估。
(3)科学合作和支助工作。
主要是与成员国之间的合作,进行数据的收集,对存在的风险和评估方法的合作与交流。
(4)对外信息交流工作。
包括对媒体的信息发布工作,向公众公布相关信息和相关事件的工作,网络发布等工作。
2、SCF:Scientific Committee on Food(欧盟食品科学委员会)3、CAC: Codex Alimentarius Commission(国际食品法典委员会)是联合国粮农组织、世界卫生组织于1962年共同创建的协调各成员国食品法规、技术标准的唯一政府间国际机构。
CAC制定的标准致力于保护各国消费者的健康安全,维护国际间公平的食品贸易,为各国食品标准的制订提供重要的科学参考依据。
与其他国际会议不同,国际食品法典委员会的会议是根据《国际食品法典程序手册》,严格按步骤讨论、审议会议议题。
食品生产法规与标准

食品生产法规与标准食品生产法规与标准随着全球化的不断加深以及食品安全问题的日益凸显,各国纷纷制定了一系列的食品生产法规与标准,以保障公众健康和食品安全。
下面就详细介绍一些常见的食品生产法规与标准。
1. 食品药品管理局食品药品管理局(FDA)是美国国家级机构,主要负责管理和监督相关食品和药品的审批和监管工作。
FDA发布了各种和食品安全和营养相关的规章制度和标准,包括营养标签的要求、儿童营养补充剂的标准、各种医学设备和产品的安全标准等。
2. 国际标准化组织国际标准化组织(ISO)是一个泛国际性组织,致力于制定各种标准并推广其普及,旨在提高产品、服务和系统的质量和效率。
与食品相关的ISO标准主要包括ISO22000食品安全管理体系标准和ISO9001质量管理体系标准。
3. 环境保护署环境保护署(EPA)是美国联邦政府机构,主要负责监管和管理国家水环境、空气和土壤的质量和污染物排放控制。
与食品相关的EPA标准包括食品安全和水质标准的要求等。
4. 国际食品法典委员会国际食品法典委员会(Codex Alimentarius Commission,CAC)是联合国粮食及农业组织(FAO)和世界卫生组织(WHO)下属的附属机构,主要负责编制和制定全球食品标准、准则和指南。
其旨在保障人们的健康,促进公平贸易和保护消费者利益,提高食品安全和营养质量,并推广食品质量的全球一致性,降低非关税壁垒。
5. 欧洲食品安全局欧洲食品安全局(EFSA)是一个欧盟机构,主要负责确保欧盟市场内食品的安全和营养品质。
通过对欧盟内各种食品成分、物质和添加剂的风险评估和监测,为政策制定者提供重要的科学食品安全建议。
6. 中国食品安全法食品安全法是2009年5月1日颁布实施的全国性法律,用于保障公众健康和食品安全。
该法规定了食品生产、加工、储存、销售和使用的监管制度、责任和违法行为处罚等,旨在确保食品质量维持在安全和卫生水平。
7. 食品添加剂食品添加剂是指向食品添加的物质,包括了增艳剂、防腐剂、稳定剂等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The EFSA Journal (2009) 1089, 1-15SCIENTIFIC OPINIONCopper(II) oxide as a source of copper added for nutritional purposesto food supplements1Scientific Opinion of the Panel on Food Additives and NutrientSources added to Food(Questions No EFSA-Q-2005-156, EFSA-Q-2006-219, EFSA-Q-2006-286,EFSA-Q-2006-287)Adopted on 14 May 2009P ANEL M EMBERSF. Aguilar, U.R Charrondiere, B. Dusemund, P. Galtier, J. Gilbert, D.M. Gott, S. Grilli, R. Guertler,G.E.N. Kass, J. Koenig, C. Lambré, J-C. Larsen, J-C. Leblanc, A. Mortensen, D. Parent-Massin, I. Pratt, I.M.C.M. Rietjens, I. Stankovic, P. Tobback, T. Verguieva, R.A. Woutersen.S UMMARYFollowing a request from the European Commission to the European Food Safety Authority, the Scientific Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to provide a scientific opinion on the safety of copper oxide added for nutritional purposes as a source of copper in food supplements and on the bioavailability of copper from this source. The Panel notes, that even under the assumption that copper exposure from drinking water is unlikely to occur at the levels of the permitted maximum water copper concentration, exposure from foods at the 97.5th percentile is in the range of 1.2-4.2 mg copper/day, and that the exposure to copper from copper(II) oxide at the use levels proposed by the petitioners would lead to an additional copper exposure of 2-2.5 mg/day, resulting in total mean copper exposure of 3.6-4.7 mg/day for adults and 3.7-6.7 mg/day at the 97.5th percentile. For children the mean copper exposure is estimated to be at 3.4-3.6 mg/day and 4.1-4.9 mg/day at the 97.5th percentile assuming that children take the adult dose of the supplement. At the higher 1For citation purposes: Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food on copper(II) oxide as a source of copper added for nutritional purposes to food supplements following a request from the European Commission. The EFSA Journal (2009) 1089, 1-15.end this exposure may in some cases exceed the Tolerable Upper Intake Level of 5 mg/day established by the SCF. An additional exposure from water could increase the total exposure significantly.Copper salts have moderate acute toxicity, with soluble salts being more toxic than insoluble ones. In short-term (2 weeks) repeated dose toxicity tests in rats and mice, copper salts are associated with adverse effects such as gastro-intestinal irritation and liver and kidney toxicity. Reported No-Observed-Adverse-Effect-Levels are in the range of 23-104 mg/kg bw/day copper, but kidney effects have been shown in male rats at levels as low as 10 mg/kg bw/day.In a human study twenty subjects presented gastrointestinal disturbances at least once during the study, suffering diarrhoea (with or without abdominal pain and vomiting), and the other eleven subjects reported abdominal pain, nausea, or vomiting.Copper(II) oxide shows lower bioavailability compared to other inorganic sources of copper(II), due to its low solubility. The Panel notes that copper from copper(II) oxide is expected to be bioavailable to some extent.The Panel concludes that, provided that the Tolerable Upper Intake Level is not exceeded, the use of copper(II) oxide as a source of copper at the proposed use levels is not of safety concern.Key words:Food supplements, copper oxide, CAS Registry Number 1317-38-0T ABLE OF C ONTENTSPanel Members (1)Summary (1)Table of Contents (3)Background as provided by the European Commission (4)Terms of reference as provided by the European Commission (4)Acknowledgements (4)Assessment (5)1.Introduction (5)2.Technical data (5)2.1.Chemistry (5)2.2.Specifications (5)2.3.Manufacturing process (6)2.4.Methods of analysis in food (6)2.5.Reaction and fate in foods to which the source is added (6)2.6.Case of need and proposed uses (6)rmation on existing authorisations and evaluations (7)2.8.Exposure (7)3.Biological and toxicological data (9)3.1.Bioavailability (9)3.2.Toxicological data (9)3.2.1.Animal toxicity data (10)3.2.2.Human toxicity data (10)4.Discussion (10)Conclusions (11)Documentation provided to EFSA (12)References (12)B ACKGROUND AS PROVIDED BY THE E UROPEANC OMMISSIONThe European Community legislation lists nutritional substances that may be used for nutritional purposes in certain categories of foods as sources of certain nutrients.The Commission has received a request for the evaluation of copper oxide added for nutritional purposes to food supplements. The relevant Community legislative measure is: •Directive 2002/46/EC of the European Parliament and of the Council on the approximation of the laws of the Member States relating to food supplements2.T ERMS OF REFERENCE AS PROVIDED BY THE E UROPEAN C OMMISSIONIn accordance with Article 29 (1) (a) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to provide a scientific opinion, based on its consideration of the safety and bioavailability of copper oxide added for nutritional purposes in food supplements.A CKNOWLEDGEMENTSThe European Food Safety Authority wishes to thank the members of the Working Group B on Food Additives and Nutrient Sources added to Food for the preparation of this opinion: D. Boskou, U.R. Charrondiere, B. Dusemund, D. Gott, T. Hallas-Møller, K.F.A.M. Hulshof, J. König, C. Le Donne, D. Parent-Massin, I.M.C.M. Rietjens, G.J.A. Speijers, P. Tobback, T. Verguieva, R.A. Woutersen.2 OJ L 183, 12.7.2002, p.51.A SSESSMENT1. IntroductionThe present opinion deals only with the safety of copper(II) oxide as a particular source of copper and with the bioavailability of copper from copper(II) oxide. The safety of copper itself, in terms of amounts that may be consumed, is outside the remit of this Panel.2. Technical data2.1. ChemistryCopper(II) oxide is a dark grey to black odourless amorphous powder, practically insoluble inethanol and water, but soluble in weak and strong acids and slowly soluble in ammonia. Its chemical formula is CuO having a molecular weight of 79.54 g/mol and the CAS Registry Number is 1317-38-0.Synonyms of copper oxide are copper(II) oxide, cupric oxide and black copper oxide.2.2. SpecificationsThe chemical and microbial specifications of copper(II) oxide as proposed by the petitioners are listed in Table 1.Table 1. Chemical and microbial specifications for copper(II) oxide as proposed by thepetitioners Technical Dossier 2005a Technical Dossier 2005b Technical Dossier 2005c TechnicalDossier 2005dChemicalPurity > 99.9% 99.0-101% 99% > 99%Arsenic < 3 mg/kg < 3 mg/kgHeavy metals (as Pb) 10 mg/kg*Lead < 5 mg/kg < 50 mg/kg 100 mg/kg < 50 mg/kgCadmium < 20 mg/kg Mercury < 1 mg/kg < 1 mg/kgIron < 50 mg/kg 500 mg/kgChloride < 50 mg/kg 50 mg/kgTotal sulphur (as sulphate) < 100 mg/kg 200 mg/kgTotal nitrogen < 20 mg/kg 20 mg/kgCarbon < 1000 mg/kg 100 mg/kgPotassium 200 mg/kg Sodium 500 mg/kg MicrobialTotal plate count 1000 cfu/gYeast and mold 100 cfu/gE. coli negative Salmonella negative * The petitioner indicates a heavy metal concentration (as Pb) of 0.001 without any unit for this value; all other values provided in the chemical specification table of the dossiers are given in %, hence it was assumed that this is also true forheavy metals.The Panel notes that according to Commission Regulation (EC) No 629/2008 the maximum levels of lead, mercury and cadmium in food supplements as sold should be 3.0 mg/kg, 0.1 mg/kg and 1 mg/kg, respectively (EC, 2008).2.3.Manufacturing processThe manufacturing process as described by one of the petitioners indicates the preparation by pyrolysis of copper(II) nitrate or copper(II) carbonate providing copper(II) oxide and either NO2 (in case of copper(II) nitrate) or CO2 (in case of copper(II) carbonate).The preparation of copper(II) oxide by the addition of alkali hydroxide to a copper(II) salt solution and dehydration of the bulky blue slurry of copper(II) hydroxide to give copper(II) oxide is also mentioned.2.4.Methods of analysis in foodTypical methods for the analysis of copper in food are Atomic Absorption Spectrometry (AAS) and Inductively Coupled Plasma spectrometry (ICP) following appropriate extraction and preparation of the samples. There is no specific method for copper(II) oxide as such, as indicated by the petitioner.2.5.Reaction and fate in foods to which the source is addedAs there is no specific method for copper(II) oxide as such, the reaction and fate of copper(II) oxide in foods to which the source is added is monitored by the copper content of the product. The petitioners indicated that a change of the copper content cannot be expected and results are provided by one petitioner on the long-term stability of copper in a typical multi-vitamin and multi-mineral product (chewable tablet). In three different batches the copper content did not change after 36 months.2.6.Case of need and proposed usesCopper(II) oxide is to be used by food supplement manufacturers as an ingredient to tablets, caplets, capsules, chewable tablets, effervescent powders and liquids. The method of incorporation is determined by the different petitioners as appropriate for the particular type of finished product.Copper(II) oxide is to be included in food supplements as a source of copper providing a maximum of 2 mg copper/day for adults, which is equivalent to 2.5 mg of copper(II) oxide. One petitioner indicated that a content of 2.5 mg copper, corresponding to 3 mg copper(II) oxide is also present on the market.rmation on existing authorisations and evaluationsIn an earlier evaluation the Joint FAO/WHO Committee on Food Additives (JECFA) reaffirmed a previous tentative evaluation of a maximum daily load of 0.5 mg/kg body weight as a Provisional Maximal Tolerable Intake (PMTDI) for man from all sources, which amounts to 30 mg/day for a 60 kg person (JECFA, 1982). The Scientific Committee on Food (SCF) has established a Population Reference Intake (PRI) of 1.1 mg copper/day (SCF, 1993). The Tolerable Upper Intake Level (UL) for copper set by the SCF is 5 mg/day for adults (SCF, 2003), which would correspond to 6.34 mg/day of copper(II) oxide and 1-4 mg/day for children, depending on age. The UL takes into account copper intake from all food sources including supplements. Copper(II) oxide has not been evaluated for food use by either the SCF or JECFA, whereas copper as cupric sulphate has been evaluated several times by JECFA. Cupric sulphate was also evaluated by the SCF for food additive use as a colour stabilizer of canned green beans and cucumber salad (SCF, 1991). The SCF considered the possible intake from this use to be unlikely to contribute significantly to the total dietary intake of copper and would lie well below the PMTDI of 0.5 mg/kg bw calculated as Cu from all sources, established by JECFA. Therefore, the SCF considered the continued use of cupric sulphate for this purpose as toxicologically acceptable. Copper complexes of chlorophyll (E141i) and chlorophyllin (E141ii) are permitted food colours.The International Programme on Chemical Safety (IPCS) has issued an extensive evaluation of copper and its salts (WHO, 1998).2.8.ExposureFood is the major source of copper intake; in particular, shellfish and organ meats (e.g. liver) provide rich sources of copper, but drinking water can make an important contribution in some circumstances. Copper concentrations in foods may vary widely between countries due to growing conditions, e.g. copper containing fertilizers and fungicides, and type of processing (WHO, 1998).The SCF reported copper intakes from foods on average in the range from 1.1-2.2 mg/day and in the range from 1.2-4.2 mg/day at the 97.5th percentile (SCF, 2003) as shown in Table 2. Table 2.Summary information on copper intake and anticipated exposure to copper from copper(II) oxideNutrient: Copper Intake (mg/day) ReferencesRecommended intake/ adults 1.1 SCF, 1993 Recommended intake/ children 0.4-0.7 (up to 7 years)0.8-1.0 (children older than 7 yearsand adolescents)SCF, 1993Tolerable Upper Intake Level/ adults (excluding pregnant and lactating women) 5 SCF,2003Tolerable Upper Intake Level/ children 1 (1-3 years),2 (4-6 years),3 (7-10 years),4 (11-17 years)SCF, 2003Average intake (mg/day) High intake (97.5th) (mg/day)Intake range from food in Europe for adults 1.1-2.2 1.2-4.2 SCF, 2003Intake range from food in Europe for children (3-17 years) 0.9-1.1 1.6-2.4 Leblancet al.,2005Amount of copper added as copper(II) oxide as indicated by the petitioner 2.5 TechnicaldossierTotal anticipated exposure to copper from supplement and food intake1 for adults. 3.6-4.7 3.7-6.7 CalculationbyPanelTotal anticipated exposure to copper from supplement and food intake2 for children (3-17 years). 3.4-3.6 4.1-4.9 CalculationbyPanel1 Calculation based on the proposed use level of 2.5 mg/day plus average dietary intake of 1.1-2.2 mg/day and high dietary intake of 1.2-4.2 mg/day for adults.2 Calculation based on the proposed use level of 2.5 mg/day plus average dietary intake of 0.9-1.1 mg/day and high dietary intake of 1.6-2.4 mg/day for children.Other sources of copper exposure are emissions from mines, smelters and foundries, from the burning of coal for power generation and from municipal water incinerators. The contribution of airborne copper to total daily intake is considered as negligible. However, copper exposure can also result from copper plumbing, depending on the hardness, pH and quality of the water. Dissolved copper levels are higher in acidic waters, especially where pipe runs are long and water can stagnate. Thus, drinking water can make an important contribution to copper exposure in some circumstances. The Expert Group on Vitamins and Minerals (EVM) estimated the maximum copper exposure to be 11 mg/day with intake levels of 3 mg/day at the 97.5th percentile coming from foods, up to 2 mg/day from food supplements and up to 6 mg/day from drinking water (assuming 2 L/day consumption and the maximum permitted water copper concentration of 3 mg/L) (EVM, 2003). However EVM stated that there is no evidence that copper intakes from water (in the UK) present any risk to health, since in practice copper levels in UK drinking water are much lower and this level of exposure is unlikely to occur.The Panel notes, that even under the assumption that copper exposure from drinking water is unlikely to occur at the levels of the permitted maximum water copper concentration, exposure from foods at the 97.5th percentile is in the range of 1.2-4.2 mg/day. Moreover, the exposure to copper from copper(II) oxide at the use levels proposed by the petitioners would lead to an additional copper exposure of 2-2.5 mg/day, resulting in total mean copper exposure of 3.6-4.7 mg/day for adults and 3.7-6.7 mg/day at the 97.5th percentile. For children the mean copper exposure is estimated to be at 3.4-3.6 mg/day and 4.1-4.9 mg/day at the 97.5th percentile assuming that children take the adult dose of the supplement. An additional exposure from water could increase the total exposure significantly.3.Biological and toxicological data3.1.BioavailabilityThe bioavailability of copper from copper(II) oxide is mainly dependent on the solubilisation of the oxide in the gastrointestinal system. Copper(II) oxide dissolves in acids such as hydrochloric acid forming copper(II) chloride:CuO + 2 HCl → CuCl2 + H2OCopper(II) chloride is soluble in water (75.7 g/100 mL at 25° C) (Lide, 2009).No specific data on the bioavailability of copper from copper(II) oxide were provided by the petitioners. One petitioner indicated, that it can be expected that, due to the acidic conditions in the stomach, copper(II) oxide will dissociate generating copper(II) ions and hydroxyl ions or water (depending on pH), rendering copper bioavailable from the source. However, the petitioner also stated that copper absorption is generally affected by a number of factors including the chemical form. Absorption of copper from various sources, mostly soluble salts such as copper sulphate, has been found to vary between 25-60% (JECFA, 1982), and the petitioner mentioned that it can be anticipated that the less soluble copper(II) salts will be in the lower end of that range.In a series of human studies it has been convincingly demonstrated that the rate of copper absorption varies inversely with copper intake and can be as low as 12% with very high copper intakes. A theoretical maximum absorptive capacity of 63-67% has been estimated (Turnlund et al., 1989).The ingestion of copper as a mineral salt is relevant in humans only when taken as a nutritional supplement (Wapnir, 1998); in this situation, the potential of interactions with other mineral elements may have nutritional significance. The bioavailability of copper ranges between approximately 35% from normal diets and 60% from low copper diets (Winge and Mehra, 1990). Animal protein and fructose enhance copper absorption whilst vegetable protein, zinc, iron and calcium impede it (Hughes and Buttriss, 2000).3.2.Toxicological dataThe petitioner did not provide any specific data on the toxicity of copper(II) oxide but there are a lot of data on the toxicity of copper (ATDSR, 1990; EVM, 2003). Copper salts have moderate acute toxicity, with soluble salts being more toxic than insoluble ones. Consequently, the acute toxicity of copper(II) oxide is assumed to be rather low. Although the data are limited, rats appear to be more tolerant to acute copper toxicity than other species. In short- term (2 weeks) repeated dose toxicity tests in rats and mice, copper salts are associated with adverse effects such as gastro-intestinal irritation and liver and kidney toxicity. Reported No-Observed-Adverse-Effect-Levels (NOAELs) are in the range of 23-104 mg/kg bw/day copper, but kidney effects have been shown in male rats at levels as low as 10 mg/kg bw/day (ATSDR, 1990).3.2.1.Animal toxicity dataThere is a wide range of LD50 values for different copper salts, and the more soluble copper salts tend to be more acutely toxic than those with lower solubility. In addition, although data are limited, rats appear to be less susceptible to the acute effects of copper than other animals (EVM, 2002).3.2.2.Human toxicity dataAcute toxicity of copper in humans is rare. Copper ions (originated from any copper salts or oxide) have an irritant effect on mucosal membranes and daily intakes ranging from 2 to 32 mg in drinking water have been reported to cause symptoms of general gastric irritation (EPA, 1987). A study in Wisconsin also suggested that high levels of copper in the water supply may increase the rate of gastrointestinal upsets (Knobeloch et al., 1998). A study on the acute gastrointestinal effects of drinking water containing graded levels of added copper (Pizarro et al., 1999) showed that an average daily consumption of 1.64 litres of drinking water containing 3 mg/L ionised copper(II) was associated with nausea, abdominal pain or vomiting. A study to determine the tolerance of chronic exposure to copper in drinking water in infants aged 3-12 months (Olivares et al., 1998) concluded that no acute or chronic adverse consequences were detected in the first year of life in infants by consuming water with a copper content of 2 mg/L.In a human study both copper sulphate (a soluble compound) and copper(II) oxide (an insoluble compound) showed comparable effects, implying that the ionic copper present in the stomach is responsible for the induction of gastrointestinal manifestations. People with the condition of achlorhydria or elderly may be at increased risk for copper deficiency, as is the case for subjects consuming antacids, because copper absorption requires that the metal is in the ionized form.Twenty subjects presented gastrointestinal disturbances at least once during the study, suffering diarrhoea (with or without abdominal pain and vomiting), and the other eleven subjects reported abdominal pain, nausea, or vomiting. No differences were found in incidence of abdominal pain, nausea, vomiting, and diarrhoea at different ratios of copper sulphate to copper(II) oxide. Both copper sulphate (a soluble compound) and copper(II) oxide (an insoluble compound) have comparable effects on the induction of gastrointestinal manifestations, implying similar levels of ionic copper were present in the stomach. Dose levels of copper(II) salts in this study were 5 mg/L drinking water and an average water consumption of 1.5/day (Pizarro, 2001).4.DiscussionThe Panel notes that the bioavailability of copper from copper(II) oxide is mainly dependent on the solubilisation of the oxide in the gastrointestinal system. It can be therefore expected that, due to the acidic conditions in the stomach, copper(II) oxide will dissociate into copper(II) ions and hydroxyl ions or water (depending on pH), rendering copper absorbable from the source in the lower end of the range that has been found for other inorganic sources of copper(II) of 25-60%.The Panel notes, that even under the assumption that copper exposure from drinking water is unlikely to occur at the levels of the permitted maximum water copper concentration, exposure from foods at the 97.5th percentile is in the range of 1.2-4.2 mg copper/day, and that the exposure to copper from copper(II) oxide at the use levels proposed by the petitioners would lead to an additional copper exposure of 2-2.5 mg/day resulting in a worst case calculation for total copper exposure of 3.2-6.7 mg/day and that at the higher end this exposure may in some cases exceed the Tolerable Upper Intake Level of 5 mg/day established by the SCF.Copper salts have moderate acute toxicity, with soluble salts being more toxic than insoluble ones. In short- term (2 weeks) repeated dose toxicity tests in rats and mice, copper salts are associated with adverse effects such as gastro-intestinal irritation and liver and kidney toxicity. Reported NOAELs are in the range of 23-104 mg/kg bw/day copper, but kidney effects have been shown in male rats at levels as low as 10 copper mg/kg bw/day.Acute toxicity of copper in humans is rare. An average daily consumption of 1.64 litres of drinking water containing 3 mg/L ionised copper(II) was associated with nausea, abdominal pain or vomiting.In a human study both copper sulphate (a soluble compound) and copper(II) oxide (an insoluble compound) showed comparable effects, implying that the ionic copper present in the stomach is responsible for the induction of gastrointestinal manifestations. Twenty subjects presented gastrointestinal disturbances at least once during the study, suffering diarrhoea (with or without abdominal pain and vomiting), and the other eleven subjects reported abdominal pain, nausea, or vomiting.C ONCLUSIONSThe present opinion deals with the safety of copper(II) oxide as a source for copper added for nutritional purposes to food supplements and with the bioavailability of copper from this source. The safety of copper itself, in term of amounts that may be consumed, is outside the remit of this Panel.Copper(II) oxide shows lower bioavailability compared to other inorganic sources of copper(II) due to its low solubility. However, the Panel notes that copper from copper(II) oxide is expected to be bioavailable to some extent.The Panel concludes that, provided that the Tolerable Upper Intake Level is not exceeded, the use of copper(II) oxide as a source of copper at the proposed use levels is not of safety concern.D OCUMENTATION PROVIDED TO EFSA1.Technical dossier, 2005a. Dossier on Copper Oxide Proposed for the Addition to Annex IIof Directive 2002/46/EC of the European Parliament and of the Council Relating to Food Supplements. May 2005. Submitted by Heath Food Manufacturers Association.2.Technical dossier, 2005b. Dossier for Safety Evaluation of Copper (II) Oxide. April 2005.Submitted by Béres Pharmaceuticals Ltd.3.Technical dossier, 2005c. Dossier on Copper Oxide. Submitted by Kabco PharmaceuticalLtd.4.Technical dossier, 2005d. Dossier on Copper Oxide. July 2005. Submitted by the DanishFood and Veterinary Administration.R EFERENCESATSDR (Agency for Toxic Substances and Disease Registry), 1990. Toxicological profile for copper. Atlanta, December.EC, 2008. Commission Regulation (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs.EPA (Environmental Protection Agency), 1987. Drinking water criteria document for copper.Cincinnati, Ohio.EVM, 2002. Expert Group on Vitamins and Minerals. Review of copper. London.EVM, 2003. Expert Group on Vitamins and Minerals. Report on safe upper levels for vitamins and minerals. London. May 2003.Hughes J, Buttriss J, 2000. An update on copper: contribution of MAFF-funded research.Nutrition Bulletin 25, 271-280.JECFA (Joint FAO/WHO Expert Committee of Food Additives), 1982. Copper. In: Evaluation of certain food additives and contaminants. WHO Food Additives Series No.17. Twenty-sixth Report of the Joint FAO/WHO Expert Committee of Food Additives.World Health Organisation. Geneva.Knobeloch L, Schubert C, Hayes J, Clark J, Fitzgerald C, Fraundorff A, 1998.Gastrointestinal upsets and new copper plumbing - Is there a connection? Wisconsin Medical Journal 44-48.Leblanc JC, Guerin T, Noel L, Calamassi-Tran G, Volatier JL, Verger P, 2005. Dietary exposure estimates of 18 elements from the 1st French Total Diet Study. Food Additives and Contaminants 22, 624-641.Lide DR (ed), 2009. Properties of the Elements and Inorganic Compounds. CRC Handbook of Chemistry and Physics, 89th Edition, CRC Press/Taylor and Francis, Boca Raton, FL, (Internet Version).Olivares M, Pizarro F, Speisky H, Lönnerdal B, Uauy R, 1998. Copper in Infant Nutrition.Safety of World Health Organisation Provisional Guideline Value for copper content of drinking water. Journal of Pediatric Gastroenterology and Nutrition 26, 251-257.Pizarro F, Olivares M, Uauy R, Contreras P, Rebelo A, Gidi V, 1999. Acute Gastrointestinal Effects of Graded Levels of Copper in Drinking Water. Environmental Health Perspectives 107, 117-121.Pizarro F, Olivares M, Araya M, Gidi V, Uauy R, 2001. Gastrointestinal Effects Associated with Soluble and Insoluble Copper in Drinking Water. Environmental Health Perspectives 109, 949-952.SCF (Scientific Committee for Food), 1991. Reports of the Scientific Committee for Food of the European Community. Twenty-fifth series. First series of food additives of various technological functions. Commission of the European Countries. Luxembourg. May 1991.SCF (Scientific Committee for Food), 1993. Reports of the Scientific Committee for Food of the European Community. Thirty-first series. Nutrient and energy intakes for the European Community. Commission of the European Countries. Luxembourg.SCF (Scientific Committee for Food), 2003. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Copper. Commission of the European Countries.http://ec.europa.eu/food/fs/sc/scf/out176_en.pdf.Turnlund JR, Keyes WR, Anderson HL, Acord LL, 1989. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr 49, 870–878.Wapnir RA, 1998. Copper absorption and bioavailability. Am J Clin Nutr 67, 1054S-1060S.WHO, 1998. Copper. Environmental Health Criteria 200. International Programme on Chemical Safety (IPCS). World Health Organisation. Geneva.。
