五氟碘乙烷五氟戊醇
全氟碘烷的概况

全氟碘烷的概况
1.1 全氟碘烷的概况
全氟碘烷别名:全氟碘代烷;全氟烷基碘;C6~12全氟碘代烷;
英文名:1-iodoperfluoro-C 6~12-alkanes;perfluoroalkyl(C6~12)iodide
分子式:I(CF2)nF(n = 2, 4, 6, 8 ……);
CAS:25398-32-7;
图1.1 全氟碘烷结构式
全氟碘烷化合物是一类氢原子被氟原子完全取代的单碘代全氟烷化合物,是重要的有机氟中间体。
目前具有商业价值的是碳数在6~12的全氟碘烷,其中8碳含量最为重要,其相应的产品具有极高的表面活性,是生产含氟表面活性剂、织物整理剂和其他精细化学品的关键中间体。
含氟表面活性剂和织物整理作为各自领域的精英产品倍受国内外关注,具有极好的市场空间和发展前景,因此全氟碘烷化合物作为基础原料具有良好开发前景。
用于全氟碘烷的现代合成技术主要有:全氟羧酸衍生物法、全氟烯烃法、亲电碘氟化、亲核碘氟化、以及调聚法等等。
目前全氟碘烷主要由五氟碘乙烷和全氟烯烃以调聚法合成,工艺复杂,链长难以控制。
国内以前无全氟碘烷的生产,严重制约了有机氟精细化学品的发展。
1.2 全氟碘烷的理化性质
全氟碘烷为紫色至粉红色透明的蜡状液体或半固体,沸点(常态下)115~
240℃,密度(35℃)1.8g/mL,微溶于水。
表1.1 全氟碘烷的理化性质表
全氟烷基碘易与乙烯反应,生成全氟烷基乙基碘化物,是生产含氟表面活性剂和低表面能涂料的关键中间体。
内容摘自六鉴化工咨询()发布《全氟碘烷技术与市场调研报告》。
专家基本情况一览表-飞源化工

附件2:推荐淄博市有突出贡献的中青年专家基本情况一览表推荐单位(盖章):工作单位:淄博飞源化工有限公司 2017年 4月 15日姓名肖玉岭性别男出生日期1967年7月 4日推荐顺序(位次/人数)1/1 学历学位硕士研究生现聘专业技术职务高级工程师党政职务无主要业绩获奖或专利情况发表或出版的主要论文、著作、作品等肖玉岭自大学毕业后一直从事技术工作,曾任车间副主任、设计室主任、技术发展中心主任,因研发成功四氟丙醇引进外商投资6000万元,与中方合计投资1亿元成立山东中氟化工科技有限公司,任副总经理兼总工程师。
擅长成果转化,即根据小试技术可设计工业化生产装置。
主要个人业绩:1、参加2001年度山东省科技厅科技计划项目《新型氟树脂及氟涂料的研制与开发》工作, 2003年1月8日通过省科技厅鉴定。
2.主持《四氟丙醇联产八氟戊醇的研制与开发》项目于2004年7月顺利通过省科技厅组织的鉴定,主持设计《1000吨/年四氟丙醇联产八氟戊醇生产装置》一次开车成功,其生产技术和产品质量均达到国际先进水平。
3、2004年主持并成功开发了绿色农药——四氟丙酸钠、含氟医药中间体—四氟磺酸钠。
4、主持2005年度济南市科技计划项目《五氟乙烷的研制与开发》,于2006年通过济南市科技局的验收及山东省科技厅组织的科技成果鉴定。
并建成年产2000吨五氟乙烷生产装置。
5、2005至2006年开发成功五氟碘乙烷(PFEI)生产技术,并成功建成国内第一条年产1000吨/年五氟碘乙烷、1500吨/年全氟烷基碘、1500吨/年全氟烷基乙基碘、1000吨/年全氟烷基乙醇、1000吨/年全氟烷基丙烯酸酯生产装置,填补国内空白,为国内领先水平。
6.2016年12月,所主持项目300吨/年高效、低毒、低残留功能性含氟农药中间体被淄博市列为市科学技术发展计划。
获奖项目名称获得时间获奖类别等级位次/人数题目出版或发表时间SCI\EI收录或出版社名称或发表刊物名称位次/人数1.新型含氟聚合物及氟化学材料含氟醇的合成与应用开发2.基于四氟乙烯开发氟涂料树脂系列高端氟材料的关键技术及产业化3.新型含氟聚合物、氟涂料及氟化学材料含氟醇的研制与应用开发4.四氟丙醇(TFP)联产八氟戊醇(OFP)生产技术5.新型含氟聚合物、氟涂料及氟化学材料含氟醇的研制与应用开发6.新型氟涂料、氟树脂及氟化学材料四氟丙醇的研制开发1.2006/9/82.2010/10/153.2008/4/14.2008/125.2008/2/186.2006/11/151.社会科学优秀成果奖2.科技进步奖3.科技进步奖4.科技进步奖5.科技进步奖6.科技进步奖1.国家级2.一等奖3.省级4.市级5.市级6.二等奖1.第贰完成者2.第壹位3.第贰位4.第壹位5.第贰位6.第壹位1.SiO_2填充型聚氨酯IPN绝缘冷浇注树脂的研究2.三甲醇丙烷蓖麻油聚氨酯IPN的合成及性质3.我国氟化氢工业发展的现状及对策4.无水氟化氢反应转炉的腐蚀与防护5.减轻无水氟化氢反应转炉腐蚀的途径6.氟磺酸对无水氟化氢生产的影响7.无水氟化氢冷凝器腐蚀原因分析8.合理使用降膜式列管吸收器提高盐酸浓度9 .无水氟化氢生产中洗涤塔结构改进10.废触媒再生利用的关键设备设计11.填充型弹性聚氨酯IPN绝缘冷浇注树脂的合成及性能研究12.Sio-2填充型聚氨酯IPN绝缘冷浇注树脂的研究13.蓖麻油型双组份聚氨酯绝缘冷浇注树脂的合成及性能研究1.1995年6月2.1999年3月3.2001年1月4.1999年5月5.1998年4月6.2000年1月7.2000年4月8.2001年1月9.1997年3月10.2000年4月11.1995年12月12.1993年12月13.2002年1.《绝缘材料》2.《高分子材料科学与工程》3.《有机氟化物》4.《化工设备与防腐蚀》5.《有机氟工业》6.《有机氟工业》7.《有机氟工业》8.《有机氟工业》9.出版社:中国化工机械动力技术协会中国化工信息中心,《化工机械与动力技术》10.出版社;化学工业出版社《化工设备与防腐蚀》11.《绝缘材料》12.《塑料工业》13.第五期《济南大学学报》,国外发行:中国国际图书贸易1.3人3位2.4人3位3.独立4.4人1位5.2人1位6.2人2位7.3人2位8.3人2位9.2人2位10.3人3位11.4人3位12.3人3位13.2人1位专利名称(是否授权)获得时间专利类型或专利奖名称等级位次/人数1.双氧水法合成四氟丙酸钠的方法2.一种全氟烷基乙烯的制备方法3.一种含氟烷基醇钠的制备方法4.一步法制备全氟烷基碘的方法5.一种提高含氟烷基乙醇收率的方法6.一种清洁生产含氟烷基丙烯酸酯的方法7.一种一锅法生产全氟烷基乙基碘化物的方法1.2009/5/272.2011/12/143.2011/12/284.2012/5/305.2012/5/306.2012/6/67.2012/6/61.发明专利2.发明专利3.发明专利4.发明专利5.发明专利6.发明专利7.发明专利1.中国2.中国3.中国4.中国5.中国6.中国7.中国1.2人1位2.3人1位3.2人1位4.4人1位5.4人2位6.4人2位7.4人2位注:1.此表由单位人事部门填写,由各推荐部门(单位)一并报送市人力资源和社会保障局专业技术人员管理处;2.表中“类别”系指获国家及省(部)级自然科学奖、技术发明奖、科学技术进步奖、优秀教学成果奖、社会科学优秀成果奖等奖励,“专利类型”是指技术发明、实用新型和外观设计专利;3.表中“空白项目”填“无”。
五氟碘乙烷合成工艺

五氟碘乙烷合成工艺
嘿,今天咱来聊聊五氟碘乙烷合成工艺这超酷的事儿!五氟碘乙烷啊,就像是化学世界里的一颗璀璨明珠。
你知道吗,合成五氟碘乙烷就像是一场奇妙的冒险!这过程可不简单呐,需要各种巧妙的步骤和精确的控制。
就好比是搭积木,每一块都要放对位置才能搭出完美的造型。
先来说说原料的选择吧,这可太重要啦!就像做菜选食材一样,得精挑细选。
然后是反应条件,温度啦、压力啦,都得拿捏得恰到好处,稍有偏差可能就前功尽弃啦!这多像走钢丝啊,得小心翼翼保持平衡。
在反应过程中,各种化学物质相互作用,发生着神奇的变化,就如同一场精彩的魔术表演。
看着它们一点点转化,最终变成我们想要的五氟碘乙烷,那感觉,哇塞,真的太有成就感了!
而且啊,这个合成工艺的发展就像是一棵不断成长的大树,不断地开枝散叶,越来越完善。
科研人员们就像辛勤的园丁,不断地浇灌、修剪,让它变得更加茂盛。
想想看,如果没有这么精湛的合成工艺,我们的生活得失去多少便利和精彩呀!五氟碘乙烷在好多领域都有着重要的作用呢,难道不是吗?所以说,这个合成工艺真的太了不起啦!它是化学智慧的结晶,是推动科技进步的重要力量。
我们真应该为它点赞,为那些致力于研究它的科学家们鼓掌!这就是我对五氟碘乙烷合成工艺的看法,它真的超级棒!。
五氟乙烷化学性质
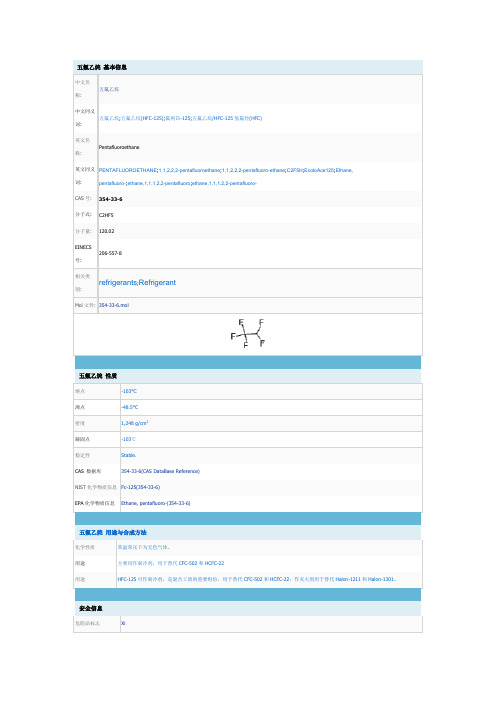
五氟乙烷 基本信息
中文名 五氟乙烷 称: 中文同义 五氟乙烷;五氟乙烷(HFC-125);氟利昂-125;五氟乙烷/HFC-125 氢氟烃(HFC) 词: 英文名 Pentafluoroethane 称: 英文同义 PENTAFLUOROETHANE;1,1,2,2,2-pentafluoroethane;1,1,2,2,2-pentafluoro-ethane;C2F5H;EcoloAce125;Ethane, 词: CAS 号: 分子式: 分子量: EINECS 206-557-8 号: 相关类 别: pentafluoro-;ethane,1,1,1,2,2-pentafluoro;ethane,1,1,1,2,2-pentafluoro354-33-6 C2HF5 120.02
危险品标志 Xi
安全说明 危险品运输编号 RTECS 号 Hazard Note TSCA HazardClass 毒害物质数据
23-38 3220 KI6365000 Irritant T 2.2 354-33-6(Hazardous Substances Data)
五氟乙烷 用途与合成方法
化学性质 用途 用途 常温常压下为无色气体。 主要用作制冷剂,用于替代 CFC-502 和 HCFC-22 HFC-125 可作制冷剂,是混合工质的重要组份,用于替代 CFC-502 和 HCFC-22;作灭火剂用于替代 Halon-1211 和 Halon-1301。
安全信息
refrigerants;Refrigerant
Mol 文件: 354-33-6.mol
五氟乙烷 性质
五氟碘乙烷的合成方法

五氟碘乙烷的合成方法
嘿!你知道五氟碘乙烷是怎么合成的吗?哈哈,让我来给你讲讲这超有趣的过程哟!
比如说吧,你想象一下,就像是搭积木一样,要把各种“小零件”巧妙地组合起来。
那合成五氟碘乙烷也差不多是这样啦!
有一种常见的方法呢,就是从一些特定的原料开始,哎呀,这些原料就像是神奇的魔法材料一样!然后呢,通过一系列复杂又精细的反应步骤,哇塞,就像一场奇妙的魔法变身一样,逐渐转化成五氟碘乙烷。
就好比你精心培育一朵花,看着它一点点绽放出美丽的样子!这过程简直太神奇啦,不是吗?
还有哦,在整个合成过程中,就像一场刺激的冒险,每个环节都得小心翼翼,稍微有一点差错可能就前功尽弃啦!但当最终成功得到五氟碘乙烷的时候,那种成就感简直无与伦比,就跟你终于爬上了山顶,看到了最美的风景一样!
总之呢,五氟碘乙烷的合成方法真的超级神奇,超级有趣!我觉得这就是化学的魅力所在呀,能创造出这么神奇的东西!。
五氟碘乙烷合成反应

五氟碘乙烷合成反应嘿,朋友们,今天咱们来聊聊五氟碘乙烷的合成反应,这就像是一场超级酷炫的化学魔术表演呢!你看啊,那些参与反应的原料,就像是一群性格各异的小演员。
有的原料特别活泼,活像一群调皮的小猴子,上蹿下跳,迫不及待地要参与到这个“大舞台”的表演当中。
而这个合成反应的条件呢,就像是导演给演员们制定的规则,稍微有点偏差,这场戏可就演砸喽。
在反应开始的时候,分子们就像是接到了紧急集合的命令,迅速开始排列组合。
这就好比是一群乱哄哄的小朋友,突然被老师要求站成整齐的队列,只不过这里的小朋友是超级微小的分子罢了。
五氟碘乙烷的合成过程中,化学键的断裂和形成就像是在搭积木。
旧的化学键断开,就像是把搭错的积木块拆下来,还发出一种“嘿呀”的小声响,当然这声响我们人类的耳朵可听不到,那得用超级灵敏的“化学耳朵”才行。
那些反应中的催化剂呀,简直就是化学世界里的魔法棒。
它轻轻一挥,整个反应就像被施了魔法一样,速度加快了不少。
如果没有这个催化剂,这反应就像是一辆慢吞吞的牛车,有了它,瞬间就变成了火箭。
反应的中间产物就像是走在钢丝上的杂技演员,一不小心就可能掉下去变成其他的东西。
它们得小心翼翼地按照化学规律这个“剧本”来走,才能最终变成我们想要的五氟碘乙烷。
这个合成反应的容器就像是一个小小的化学王国,各种分子在里面忙忙碌碌地进行着自己的工作。
它们有时候碰撞一下,就像是在互相打招呼:“嘿,兄弟,咱们一起合成五氟碘乙烷吧!”当反应接近尾声的时候,就像一场盛大的狂欢派对即将结束。
五氟碘乙烷分子们慢慢成形,它们就像是在这个派对上诞生的新星,闪耀着独特的化学光芒。
而且哦,整个合成反应的过程,从宏观上看,那些仪器设备就像是一群沉默的守护者。
它们默默地看着这些微观世界里的精彩表演,就像我们在看一场精彩的电影一样。
要是在这个合成过程中出现了杂质,那就像是一场美妙的音乐会里突然闯进了几个跑调的家伙,特别煞风景。
所以化学家们得像精明的侦探一样,把这些杂质找出来并且赶走。
全氟碘烷应用技术与市场开发前景

全氟碘烷应用技术与市场开发前景江镇海【摘要】简要介绍了全氟碘烷在生物活性物质、含氟织物整理剂、含氟表面活性剂等方面的应用,并对其合成技术和市场情况作了简单的介绍。
【期刊名称】《有机氟工业》【年(卷),期】2010(000)002【总页数】3页(P45-47)【关键词】全氟碘烷;含氟表面活性剂;含氟织物整理剂;生物活性物质;市场开发【作者】江镇海【作者单位】不详【正文语种】中文【中图分类】TQ225.24全氟碘烷化合物是一类氢原子被氟原子完全取代的单碘代全氟烷化合物。
目前具有商业价值的是碳数在 6~12的全氟碘烷,其中 8碳含量最为重要,其相应的产品具有极高的表面活性,是生产含氟表面活性剂、织物整理剂和其他精细化学品的关键中间体。
全氟碘烷易与乙烯发生加成反应,生成全氟烷基碘乙烯加成物。
在生成产物中,碘原子不再与全氟烷基相连,因此特别容易发生取代反应,可以转变成相应的醇、硫醇和磺酰氯等各种含氟中间体,再进一步合成各种含氟表面活性剂、织物整理剂等。
它们具有高表面活性、高耐热稳定性、高化学稳定性和憎水憎油等优良独特的技术性能。
在许多工业领域中有着重要的应用。
全氟碘烷在合成生物活性化合物方面具有特别重要的地位。
CF31和其某些同系物与有机基质的相互作用可以获得具有各种生物活性的环、芳香和杂环的全氟烷基化的有机精细化工中间体。
在合成生物活性化合物时经常使用碘三氟甲烷,主要原因是以其为原料制备的化合物活性最好。
三氟甲基化已经成为目前新型农药和医药新产品开发和生产的重要手段之一。
由于碘三氟甲烷的稳定性最大,在化学反应过程中不会因为反应条件的变化而引起全氟烷基取代基遭到破坏的副反应,因而在农药和医药工业领域的新产品开发和生产过程中发挥着重要的作用。
含氟织物整理剂具有以往任何织物整理剂都无法比拟的功效,成为当今织物整理剂的发展主流。
通常使用的主要是由含氟丙烯酸酯和其他不饱和单体进行的乳液共聚,而含氟丙烯酸酯则由全氟烷基碘化物反应制备的含氟醇和丙烯酸发生酯化反应而得。
五氟乙烷原料

五氟乙烷原料
五氟乙烷的原料主要包括乙烯和氟化氢。
乙烯是一种无色气体,它是五氟乙烷的主要原料之一。
乙烯可以通过石油或天然气的裂解来获得,也可以通过乙烷的脱氢反应得到。
乙烯是化工行业中非常重要的原料之一,广泛应用于合成塑料、橡胶、纤维等。
氟化氢是五氟乙烷的另一个重要原料。
氟化氢可以由氟化铝和硫酸反应得到,也可以通过氢氟酸和硫酸钙的反应制备。
氟化氢是一种无色气体,具有强烈的刺激性和腐蚀性。
它主要应用于金属表面的蚀刻、制备氟化物化合物以及作为催化剂等。
以上是五氟乙烷的主要原料,通过乙烯和氟化氢的反应可以合成五氟乙烷。
五氟乙烷是一种无色气体,具有较低的沸点和较高的溶解度,被广泛应用于医药、塑料加工、灭火剂等领域。
全氟碘烷合成技术进展

全氟碘烷合成技术进展1.概述全氟碘烷是一类重要的有机氟中间体,是生产含氟表面活性剂、含氟织物整理剂和其他含氟精细化学品的主要原料,含氟表面活性剂和织物整理作为各自领域的精英产品倍受国内外关注,具有极好的市场空间和发展前景,因此全氟碘烷化合物作为基础原料具有良好开发前景。
2.合成技术全氟碘烷化合物是一类氢原子被氟原子完全取代的单碘代全氟烷化合物,目前具有商业价值的是碳数在6-12的全氟碘烷,其中8碳含量最为重要,其相应的产品具有极高的表面活性,是生产含氟表面活性剂、织物整理剂和其他精细化学品的关键中间体。
全氟碘烷文献报道合成路线众多,但是具有工业化意义和前景的合成技术主要有以下几条。
1.1全氟羧酸衍生物法把带有轻微过量的碘的无水全氟烷基羧酸银慢慢加热进行反应,可以得到产率较高的相应的全氟碘烷。
反应是多种的,不仅能合成脂族,还能合成环族和全氟烷氧基碘化物,当使用二元酸盐情况下,也能合成全氟二碘烷。
一般认为该反应过程中经过酰基次碘酸盐的生产过程。
研究表明全氟碘烷也能从碱金属盐或者汞盐制取,通常反应在非质子极性溶剂中进行,有的研究者采用更具有极性一氯化碘时,反应收率没有增加,由于反应过程分两步,中间盐需要很长时间的蒸煮和干燥,能耗消耗比较大,限制了较大规模生产中的应用。
当其他全氟羧酸衍生物分解时候也能生成全氟碘烷,如三氟乙酰基次碘酸盐的电解或在室温下当六甲基二锡烷存在时三氟乙酰基碘化物的分解导致生成低产率的碘三氟甲烷。
研究表明用相应的酸和碘及苯酰过氧化物相互作用合成高级全氟碘烷,反应带有原子团的特性和发生在非极性溶剂中,溶剂可以选择二溴或者二氯乙烷、二溴丙烷等。
1.2全氟烯烃法由于全氟烯烃、碘三氟甲烷和碘氟化剂越来越容易获得,因此以全氟烯烃为原料合成全氟碘烷具有现实意义,目前以全氟烯烃为原料合成技术向三个方向发展。
1.2.1亲电子碘氟化以全氟烯烃为原料,采用碘或者五氟化碘为碘氟化剂,将碘向烯烃加成时经过生成碳阳离子进行反应,此反应适用于不同结构的烯烃,包括全氟异丁烯和全氟甲基乙烯基醚,产率较高。
五氟化碘安全技术说明书

化学品中化学品英技术说明生产企生有五危险侵健环燃皮中文名称: 五英文名称:p 明书编码:2企业名称:地址: 生效日期: 有害物成分 五氟化碘险性类别: 侵入途径:健康危害:对性环境危害:燃爆危险: 本皮肤接触: 立五五氟化碘 pentafluoroio 2842对皮肤、眼睛性烟雾,吸入本品助燃,有立即脱去污染五氟化碘第一部dine 第二部第三部睛和粘膜有强入会中毒。
遇有毒,具腐蚀第四染的衣着,用碘安全技部分:化学品部分:成分/组含部分:危险性强烈的刺激性遇热分解释出蚀性、强刺激四部分:急救用大量流动清术说明书品名称组成信息含量性概述性和腐蚀性,出高毒的氟、激性,可致人救措施清水冲洗至少书化学品俗名英文名称CAS No.与水或潮湿碘烟雾。
人体灼伤。
少15分钟。
就名: 称: .: 7783-66C 77湿空气剧烈反就医。
6-6CAS No. 783-66-6反应,放出剧毒毒和腐蚀眼危有害燃灭应操作注储存注眼睛接触: 立吸入:迅行食入: 用危险特性:强接燃烧产物: 氟灭火方法:应急处理: 迅式下至燥注意事项: 密过衣不备注意事项: 储与的立即提起眼睑迅速脱离现场行人工呼吸。
用水漱口,给强氧化剂。
与接触酸或酸气氟化氢、碘化迅速撤离泄漏式呼吸器,穿下水道、排洪至槽车或专用燥、洁净、有密闭操作,提过专门培训,衣式胶布防毒不能进行焊接备相应品种和储存于阴凉、与易(可)燃的收容材料。
睑,用大量流场至空气新鲜就医。
给饮牛奶或蛋第五与易燃物、有气能产生有毒化氢。
第六部漏污染区人员穿防腐防毒服洪沟等限制性用收集器内,有盖的容器中第七部分提供充分的局严格遵守操毒衣,戴橡胶接、切割等作和数量的消防干燥、通风燃物、食用化流动清水或生鲜处。
保持呼蛋清。
就医。
五部分:消防有机物接触易毒气体。
部分:泄漏应员至安全区,服。
不要直接性空间。
用砂回收或运至中。
分:操作处置局部排风。
防操作规程。
建胶手套。
远离作业。
远离易防器材及泄漏风良好的库房化学品等分开生理盐水彻底呼吸道通畅。
4,4,5,5,5-五氟戊醇的合成

STUDY o N SURFACE PRoPERTIES oF SUCCINATE SULFoNATE ASYM M ETRIC GEM INI SURFACTANT
Y u H ongm ei。Zhu X ia,H ua Ping
(School of Chemistry and Chemical Engineering,Nantong University, N antong 226019,Jiangsu,China)
戊 醇 ];2)五 氟碘 乙烷 先 与炔 丙 醇 合 成 2碘一4, 4,5,5,5-五 氟 一2一戊 烯 基 一1一醇 ,再 经 还 原 得 到 五 氟
收 稿 日期 :2018—0l一14;修 改 稿 收 到 日 期 :2Ol8一O4—25。 作 者 简介 :杜 友 兴 (1964一),硕 士 ,工 程 师 ,主 要 从 事 有 机 中 间体 及 农 药 的研 究工 作 。E—mail:yxdu—student@ sina.coin。
34
精 细 石 油 化 工
SPECIALITY PETROCHEM ICALS
第 35卷 第 3期 201 8年 5月
4,4,5,5,5-7.氟 戊 醇 的 合 成
杜 友 兴 ,何 立
(上 海 威 耳化 工科 技 有 限 公 司 ,上 海 200331)
摘 要 :报 道 了 以 五 氟 丙 酸 乙 酯 和 乙 酸 乙酯 为 起 始 原 料 ,经 克 莱 森 缩 合 、氯 化 、氢 化 及 还 原 等 4步 反 应 合 成 4,4, 5,5,5-五氟 戊 醇 的新 工艺 。通 过 正 交 实 验 优 化 了 克 莱 森 缩 合 反 应 工 艺 条 件 ,并 对 其 他 三 步 反 应 工 艺 条 件 进 行 了考 察 。优 化 的工 艺条 件 为 :克 莱 森 反 应 温 度 5O ℃ ,反 应 时 间 2 h,n(五 氟 丙 酸 乙 酯 ):”(乙 酸 乙 酯 ):” (NaH)一 1:1.3:1.3;氯 化 亚 砜 在 5o℃ 反 应 ;雷 尼 镍 和 三 乙胺 在 室 温 反 应 ;硼 氢化 钠 和 三氟 化 硼 四氢 呋 哺 溶 液 在 8~ 12℃ 反 应 。在 此 条 件 下 ,4步 反 应 总 收 率 为 75.5 ,纯 度 98.5 。 关 键 词 :氟 维 司群 4,4,5,5,5-五 氟 戊 醇 五 氟 丙 酸 乙酯 中 间体 合 成 中图 分 类 号 :0623.43 文 献 标 识 码 :A
五氟碘乙烷合成方法

五氟碘乙烷合成方法小伙伴们!今天咱们就来唠唠五氟碘乙烷的合成方法哈。
这玩意儿在化学领域可是有不少用处呢。
一、气相氟化法。
这种方法呀,就是让反应物在气相状态下进行氟化反应来合成五氟碘乙烷。
一般来说,得先准备好合适的原料,常见的原料像一氯五氟乙烷之类的。
然后把这些原料和氟化剂一起通入到反应釜中。
氟化剂呢,通常会选用像氟化氢这种。
在一定的温度和压力条件下,它们就会发生反应啦。
温度大概得控制在一个合适的范围,比如说200 400摄氏度左右吧,压力也得恰到好处,不然反应可能就不太理想。
在这个过程中,原料分子里的一些原子就会被氟原子替换掉,慢慢就生成五氟碘乙烷啦。
不过这个过程得好好控制反应条件,要是温度太高或者压力太大,可能会产生一些副产物,那就麻烦咯。
二、液相氟化法。
液相氟化法和气相氟化法不太一样哈。
它是让反应在液相状态下进行的。
一般是把原料溶解在某种溶剂里,然后加入氟化剂。
常用的溶剂有一些有机溶剂,像二氯甲烷之类的。
把原料和氟化剂在溶剂里混合均匀后,在一定的温度下搅拌反应。
这个温度相对气相氟化法可能会低一些,大概在100 200摄氏度左右吧。
在液相环境里,分子之间的接触会更充分一些,反应也能更平稳地进行。
反应结束后,再通过一些分离提纯的方法,把生成的五氟碘乙烷从反应混合物里分离出来。
比如说可以用蒸馏的方法,根据不同物质沸点的不同,把五氟碘乙烷蒸出来,然后再收集起来。
三、电化学氟化法。
电化学氟化法就比较有意思啦。
它是利用电化学的原理来进行氟化反应。
简单来说,就是在一个电解槽里进行反应。
把原料和电解质溶液放在电解槽里,然后通上直流电。
在电流的作用下,电解质溶液里的一些离子就会发生迁移和反应,从而让原料分子得到氟化。
这个方法的优点呢,就是可以比较精确地控制反应的进行,而且相对来说比较环保一些,不会产生太多的废弃物。
不过呢,它对设备的要求会高一些,毕竟涉及到电化学的装置嘛。
而且反应的速度可能没有前面两种方法那么快,需要一定的时间来完成反应。
五氟乙烷操作规程(3篇)

第1篇一、概述五氟乙烷(R125)是一种常用的制冷剂,具有低毒、低压力、低臭氧消耗潜值等优点。
为保障操作人员的人身安全和设备的正常运行,特制定本操作规程。
二、适用范围本规程适用于公司生产、储存、使用五氟乙烷的操作人员。
三、操作前的准备1. 确认五氟乙烷的质量符合国家标准。
2. 检查设备是否完好,阀门是否灵活,管道是否畅通。
3. 熟悉五氟乙烷的物理、化学性质及安全操作规程。
4. 准备好必要的防护用品,如防毒面具、防护手套、防护服等。
5. 确认操作区域通风良好,无火源、易燃易爆物品。
四、操作步骤1. 打开储罐阀门,缓慢释放五氟乙烷至制冷设备。
2. 观察设备运行状态,确保制冷效果达到预期。
3. 定期检查设备压力、温度等参数,确保在正常范围内。
4. 若发现设备异常,立即停止操作,查明原因并采取措施。
5. 操作过程中,严禁将五氟乙烷直接接触皮肤、眼睛等部位。
6. 五氟乙烷泄漏时,立即关闭泄漏阀门,切断泄漏源。
7. 使用五氟乙烷灭火器进行灭火时,确保灭火器处于正常状态。
五、注意事项1. 五氟乙烷易燃易爆,操作过程中严禁吸烟、动火。
2. 操作人员应熟悉五氟乙烷的物理、化学性质,掌握安全操作规程。
3. 操作人员应穿戴防护用品,确保自身安全。
4. 严禁将五氟乙烷用于非制冷设备。
5. 储存五氟乙烷的场所应通风良好,远离火源、易燃易爆物品。
六、应急处理1. 五氟乙烷泄漏:立即关闭泄漏阀门,切断泄漏源。
使用干粉灭火器或二氧化碳灭火器进行灭火。
2. 五氟乙烷接触皮肤:立即用大量清水冲洗,并就医。
3. 五氟乙烷接触眼睛:立即用大量清水冲洗,并就医。
4. 五氟乙烷吸入:迅速离开现场,吸入新鲜空气,并就医。
七、培训与考核1. 操作人员需接受五氟乙烷操作规程培训,掌握安全操作技能。
2. 培训结束后,进行考核,考核合格后方可上岗操作。
3. 定期对操作人员进行安全教育和技能培训,提高安全意识。
本规程自发布之日起实施,如有未尽事宜,由相关部门负责解释。
五氟化碘处理方法

五氟化碘处理方法
五氟化碘处理方法:
五氟化碘(IF5)是一种具有强氧化性和腐蚀性的化合物,因此在处理和使用时需要采取适当的安全措施。
以下是五氟化碘的处理方法:
1. 个人防护:在处理五氟化碘时,必须佩戴适当的个人防护装备,包括眼镜、实验室外套、手套和防护面具。
这些装备能够保护皮肤、眼睛和呼吸系统免受五氟化碘的侵害。
2. 通风措施:在使用五氟化碘的实验室中必须保持良好的通风系统,并确保室内空气流通。
这可以减少五氟化碘对人体及环境的影响。
3. 储存:五氟化碘必须储存在密封的容器中,远离可燃物和易燃物品。
同时,储存区域应干燥、阴凉,并与酸性化合物分开存放。
4. 液体处理:五氟化碘是液体状态下使用的,因此在处理时需要小心。
如果泼洒在皮肤上,应立即用大量清水冲洗。
如果不慎吸入或进入眼睛,应立即寻求医疗救助。
5. 废弃物处理:五氟化碘废弃物需要按照相关法规进行处理。
应将其置于特定的容器中,并与其他废物分开储存。
最好将五氟化碘废弃物交给专业的废物处理单位进行处理,以确保环境和人员安全。
6. 灭火方法:在发生火灾时,不应使用水来灭火,因为五氟化碘和水反应会产生有毒气体。
最好使用干粉灭火器或二氧化碳灭火器进行灭火。
总之,处理五氟化碘需要谨慎,遵循相关安全规定,并在必要时寻求专业人士的帮助。
确保正确处理五氟化碘可以降低对人员和环境的潜在风险。
五氟碘乙烷催化合成新工艺研究的开题报告

五氟碘乙烷催化合成新工艺研究的开题报告一、研究背景五氟碘乙烷是一种重要的芳香族化合物,具有广泛的应用前景。
目前,五氟碘乙烷的主要生产路线是氟硼酸二甲酯催化重氮化的方法,但该方法存在着反应条件严苛、产物纯度低、废气处理困难等问题,因此需要研发出更加高效、环保的催化合成新工艺。
二、研究目的本研究旨在探究五氟碘乙烷的催化合成新工艺,通过避免或减少有害物的排放,提高产物纯度和产量,从而实现资源利用和环境保护的双重目的。
三、研究内容1.筛选催化剂通过实验室实验,比较不同催化剂在五氟碘乙烷催化合成中的活性和选择性,以及产物纯度和产量等性能表现,选择出最佳的催化剂。
2.优化反应条件在最佳催化剂情况下,进一步优化反应条件,探究温度、压力、时间等因素对催化合成的影响,确定最佳反应条件。
3.确定催化机理通过表征催化剂的物理化学性质,以及实验室实验得到的数据,分析五氟碘乙烷的催化合成机理。
四、研究意义1.提高五氟碘乙烷的生产效率和产量,降低生产成本。
2.减少有害物的排放,实现环境保护和资源利用。
3.为其他类似化合物的催化合成提供借鉴和指导,具有广阔的应用前景。
五、研究方法通过实验室实验,采取多因素综合实验设计和响应面法等方法,探究影响五氟碘乙烷催化合成的关键因素,并优化反应条件。
六、研究计划第一年:1.收集五氟碘乙烷的催化合成相关文献,建立催化合成实验系统。
2.筛选不同催化剂,并比较其催化活性和选择性。
3.确定最佳催化剂,优化反应条件。
第二年:1.在最佳催化剂情况下,进一步优化反应条件。
2.实验室实验得到数据,并分析催化机理。
第三年:1.总结分析实验结果,撰写论文,完成毕业设计。
七、预期成果本研究将探究五氟碘乙烷的催化合成新工艺,探究各适宜的催化剂和反应条件,实现更加环保高效的催化合成,为类似芳香族化合物的催化合成提供新的研究思路,具有重要的科学意义和实际应用价值。
8危险化学品五氟乙烷的理化性质及危险特性表MSDS
本品不燃。切断气源。喷水冷却容器,可能的话将容器从火场移至空旷处。
燃烧爆炸危险性
燃烧性
不燃
闪点(℃)
/
爆炸上限%(v%):
/
自燃温度(℃)
/
爆炸下限%(v%):
/
危险特性
若遇高热,容器内压增大,有开裂和爆炸的危险。
建规火险分级
戊
稳定性
稳定
聚合危害
不聚合
禁忌物
强氧化剂、易燃或可燃物。
储运条件
与泄漏处理
储运条件:①储存注意事项:储存于阴凉、通风的库房。远离火种、热源。库温不不应过高。应与易(可)燃物、氧化剂分开存放,切忌混储。储区应备有泄漏应急处理设备。②运输注意事项:采用刚瓶运输时必须戴好钢瓶上的安全帽。钢瓶一般平放,并应将瓶口朝同一方向,不可交叉;高度不得超过车辆的防护栏板,并用三角木垫卡牢,防止滚动。严禁与易燃物或可燃物、氧化剂等混装混运。夏季应早晚运输,防止日光曝晒。铁路运输时要禁止溜放。泄漏处理:迅速撤离泄漏污染区人员至上风处,并进行隔离,严格限制出入。建议应急处理人员戴自给正压式呼吸器,穿一般作业工作服。尽可能切断泄漏源。合理通风,加速扩散。如有可能,即时使用。漏气容器要妥善处理,修复、检验后再用。
五氟乙烷的理化性质及危险特性
标识
中文名:五氟乙烷
危险货物编号:/
英文名:1,1,1,2,2-Pentafluoroethane; Hydrofluorocarbon
UN编号:3220
分子式:C2HF5
分子量:120.022
CAS号:/
理化性质
外观与性状
无色气体,有微芳香味。
熔点(℃)
/
相对密度(水=1)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
added followed by a mixtureof sodium hydrosulfite (30.44 g. 174.9mmol) and sodium hydrogen
carbonate (14.7 & 174.9mmol). Afcee:the add&o% the mixturewas stirredat Ooc for 5 h. The
occured. Two equivalents of trietbylamine gave the best result (95%). The thiol 1 from the alcohol 3 was
prepared by first converting to thioleater4 followed by basic hydrolysis. Thus, the reaction of tltiolacetic scid
a 4.4,5,5,5-Pentafluoropentan-I-thiol
(1) moiety in the side chain of estradiol significantly increased its
antiestrogenic potency. The antiestrogens RU 58 6881 and ICI 182.7802 are good examples of the above
reactionmixtunewrs~~~water(3oomL)andexaac~withether(3XXmL).The
combined organic layer was wasbed with a sat. NaCl solution and then dried (MgSO4). Removal of
leactionvessel+ wyl
iodide (43&g, 174.9mmol) was bubbkd into a stirringmixtureof
acetonihile (350 mL) and water (300 mL) +MO=‘C. Ropa%yl alcohol (9.81 g. 174.9mmol) was
2. Wakeling. AX.; Dudes, hi.; Bowler, J. Cancer Research 1991,5I, 3867-373. 3. Kitazume,T.; Ishikawa, N. J. Am Chem Sot. 1985,iO7, 5186-5191. 4. Sun, W.-C.; Ng, C.-S.; Prestwich, G. D. . Chem. 1992.57. 132-137.
B
Quantitative free radical addition of perfluoroethyl iodide to ptopargyl alcohol in the presence of
sodium hydrosulfite gave En-2-iodo-4,4,5~~-~~u~Z-~~-l~~,
(2) 3. CH&QW
THF
OYi, 0.5 h; 25oC, 3 h
I
65%
Scheme 1
0
-HP0
SK
CH3 ,ooec 3 h
’
65%
Selective deiodination of the En isomers via catalytic hydrogenation over Raney Ni or PdlC in the
obtained (Bnc+ N.O.3. muorinc Utem. 19@2,20.313-327).
7. Rolf, B.; Hermam~. K; Hans-~, M.-S.; Yukishige, N.; Martin, S. Deutsche Putent, DE 4132
182 A 1,1993.
which were converted to 4,4,5,5,5-
pentafluoropentan-l-01 in one step in excellent yield by catalytic hydrogenation over platinum oxide in the
presence of triethylamine. 4.4,5,5.5-Penufluoropentan-1-thiol
with alcohol 3 under modified hfitsunobu conditionso gave thiol ester 4 which, without much purification. wss
treatedwith sodium hydroxide to give thiol 1 at a 65% yield.10
0040-4039(94)02056-6
Tetmhedht Letters,Vol. 3% NO. 49, pp. 9141-9144, 1944 Jhevicr Science Ltd
Printed in Great Britain cKlw4039l94 s7.alto.oo
Lablbratory scale Prepu8uan of ~4$#s~-PeBtdhmmpemtan-l-thio~: An Important Cbdn of Anti-Breast Cancer As-b
8. Albano. E-L.; Hotton, D. 1. Org. C&m 1969.34.3519-3522.
9. Volante, RP. Tavahcdron Lea 1981.22.3119-3122.
10. (a) -on
of @2)-2-iodo4,4~5.5-pent8fluoro-2-penten- lzols (w2b): In and Shankar M. Sit@* Medi&alChcmi&bomtoryofMolecularEndoctinology
CHUL Research Center, @%ec City. Qu&ec GlV 4G2. Canada
in&aledapplication.
RU 58 688
ICI 182,780
Despite the interest of this chain, there is a lack of general methods to prepam thiol 1. Herein, we describe a
In conclusion. the present approach, while beiig very practical and eftkient, provides an opportunity to
prepare the thiol 1 in gram quantities. AU steps can be carried out on a bench top without much extra
precautions. AU reaction conditions am mild”
1. Van de Velde. P.; Nique, F.; Bouchoux, F.; Bmmaud. J.; Hameau. MC.; Lucas. D.; Moratille. C.; Viet, S.; Philibert, D.; Teutsch. G. J. Steroid Biochem MO&C. Biol. 1994.48, 187-196.
pmsence of a base (NaGH, KGH or KqAc) gave a mixtutu of partial d&d&&a products, sllyl alcohols and
4,4.5.5,!5-pentafluoropentan-l-ol(3)P When hydrogenation over platinum oxide 07
was cartied out in the
pmsence of triethylan& (Et@ in ethyl acetate whem the salt (&NH+I-) could selectively be precipitatedout
alcohol 3 was obtained in one step in excellent yield. Deiodinationalong with reduction of the double bond also
was obtained in good yield via modiied
Mitsunobu maction of the alcohoL
Importance of fluorhmmd compounds as thempeutic agents is well documented. Recently, introduction of
simple and relatively straight forward synthesis of 4,4,5,5,EP~~uoropentan-1-thiol
from perfluoroethyl
iodide.
Scheme1 outlines our approaclu the key steps of this approach are the pmparation of allylic alcohols 2a and 2b followed by their reduction to provide alcohol 3. Ultrasound-promoted addition3 of perfluorcethyl
9143
5. (a) Tang. X.-Q.; Hu. C.-M. J. t&m. Sot. chcm Conuu~. 1994.631-632. (b) Huang. W.-Y.;
