罗氏TUNEL使用说明书(中文)
TUNEL 凋亡染色-罗氏公司

TUNEL 凋亡染色
参照罗氏公司的TUNEL凋亡试剂盒说明书进行并适当改进
1、石蜡切片常规二甲苯脱蜡,梯度酒精脱水;
2、蒸馏水缓慢冲洗后室温下干燥;
3、切片置于250ml柠檬酸(0.1M,pH6.0,10.505g C6H8O7.H2O+500ml H2O)中微波加热至沸腾,静置5min后加入100ml ddH2O;
4、15min后取出切片,标本上滴上10%山羊血清(0.5%BSA),于37°C封闭15min(室温封闭45min);
5、TUNEL试剂1和试剂2按照1:9比例充分混匀,离心,滴加至标本(试剂覆盖组织即可),37°C孵育1h;
6、PBS清洗3次,每次10min,抗荧光淬灭剂封片,荧光显微镜摄像。
(5、6需避光操作)
7、去掉盖玻片,PBS清洗3次,每次5min;
8、滴加POD至标本,37°C孵育30min;
9、PBS清洗3次,每次5min,DAB显色;
10、苏木素复染,光学显微镜摄像。
凋亡指数(AI)计算:采用IPP随机选取5个高倍(X400)视野,计数至少1000个细胞,阳性细胞占细胞总数的百分比即为AI。
数据采用均数+/-标准差表示,采用SPSS17.0统计软件,多个样本均数比较采用ANVOA分析,以SNK-q检验进行均数间多重比较,以p<0.05有差异统计学意义。
TUNEL-roche使用流程

In situ cell death detection kit-POD法一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是脱氧核糖核苷酸末端转移酶(TdT Enzyme)把荧光素(fluorescein)标记的dUTP(三磷酸脱氧尿甘)连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,HRP又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
TUNEL细胞凋亡试剂盒内容及操作步骤

TUNEL细胞凋亡试剂盒内容及操作步骤--------------------------------------------------------------------------------凋亡细胞的原位末断转移酶标记法(TUNEL法)细胞凋亡的多步骤机制作用的最终环节是;细胞内源性核酸内切酶的激活而导致核染色质DNA双链的断裂。
大量DNA片段暴露出的3 羟基在末断转移酶(terminal deoxynucleotidyl transferase, TdT)或DNA多聚酶的作用下,与生物素或地高辛标记的核苷酸结合,最终借助与卵白素或抗地高辛抗体结合的荧光素或HRP,使凋亡细胞被特异性地标记和显示出来。
TUNEL细胞凋亡试剂盒由美国罗氏公司提供试剂1:酶浓缩溶液(Enzyme Solution)试剂2:标记溶液(Lable Solution)试剂3:转化剂-POD(Converter-POD)酶标记抗荧光素抗体(即用型)试验所需其它试剂:非石蜡切片:·冲洗缓冲液:磷酸盐缓冲液(PBS)·阻断溶液:0.3%H2O2甲醇溶液·固定溶液:4%多聚甲醛(溶剂pH7.4新鲜配制的PBS溶液)·渗透液:0.1%Triton甔-100(溶于新鲜配制的0.1%枸橼酸钠溶液)Triton X-100(聚乙二醇辛基苯基醚)名:曲拉通X-100,乳化剂OP分子式:C34H62O11石蜡切片:·二甲苯和乙醇(100%、95%、90%、80%、70%用蒸馏水稀释)·冲洗缓冲液:磷酸盐缓冲液(PBS)·蛋白酶K 工作液10~20mg/ml溶于10mM Tris/HCl(pH7.4~7.8)根据需要选择:·渗透液:0.1%TritonX-100(溶于新鲜配制的0.1%枸橼酸钠溶液)·胃酶溶液(0.25%-0.5%溶于HCl ,pH2)或胰酶·0.1M枸橼酸缓冲液,pH6,微波修复材料:微波炉,微波输出功率850W-2000W2 .选用中性甲醇固定的活检及实验动物标本,常规脱水,二甲苯透明,石蜡包埋。
罗氏公司TUNEL细胞凋亡检测程序

罗氏公司TUNEL细胞凋亡检测程序作者:Roche发表于:2009-03-0614:54来源:丁香园(In situ cell death detection kit-POD法)一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT10×、荧光素标记的dUTP1×、标记荧光素抗体的H RP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5μl20×DAB+1μL30%H2O2+94μl PBS)、Proteinase K工作液(10-20μg/ml in10mM Tris/HCl,pH7.4-8)或细胞通透液(0.1%Triton X-100in0.1%sodium citrate,临用前配制)、苏木素或甲基绿、DNase1(3000U/ml–3U/ml in50mM Tris-HCl,pH7.5,10mM MgCl2,1mg/ml BSA)等。
自己翻译的罗氏tunel检测细胞凋亡试剂盒说明书

罗氏tunel检测细胞凋亡试剂盒说明书注意:Label溶液含有甲次砷酸盐和二氯化钴,严禁吸入和食入。
反应悬浮物收集于密闭、不易碎、有明确标识的容器中,按有毒废物处理。
除上表所列试剂外,还需准备以下溶液。
下表列出每步所需物品概览:特异性:TUNEL反应优先标记凋亡产生的DNA链断裂,从而辨别凋亡与坏死、以及由抑制细胞生长的药物或放射线产生的primary DNA链断裂实验干扰:假阴性:在某些型式的凋亡细胞中DNA链断裂可能缺失或不完全。
空间位阻,如细胞外元件可能阻止TdT到达DNA断裂处。
两种情况均能产生假阴性。
假阳性:在坏死晚期,可能产生大量的DNA片段DNA链断裂也可能在具有高增殖和代谢活动的细胞中出现。
两种情况均能产生假阳性。
为确认细胞死亡的凋亡型式,应认真进行每种细胞的形态学检查凋亡过程中产生的形态学改变尤其特征形式,因此,对于可以结果进行解释时,细胞形态评估是一项重要的参数样本:细胞离心涂片和细胞涂片在chamber slides上培养的黏附细胞冰冻或福尔马林固定、石蜡包埋样本分析时间:2-3小时,除外培养、固定和渗透检测次数:一个试剂盒50T试剂盒存储/稳定性:未开封试剂盒储存于-15~-25℃可稳定至标签上标明的效期。
1 流程图:2 样品准备2.1 黏附细胞、细胞涂片和细胞离心涂片需准备的其他试剂:Washing buffer:磷酸盐缓冲液(PBS)Blocking buffer封闭溶液:甲醇稀释的3% H2O2Fixation solution固定溶液:PBS配制的4%多聚甲醛,ph 7.4,新鲜配制Permeabilisation solution 渗透液:0.1%Triton1)X-100溶于0.1%柠檬酸钠溶液中,新鲜配制步骤:下表描述了细胞固定、内源性过氧化物酶封闭和细胞渗透过程。
2.2 组织部分2.2.1 福尔马林-包埋组织福尔马林包埋组织的预处理:可按4种不同的方式预处理。
翻译好的罗氏公司Tunel试剂盒操作说明书

罗氏 (Roche)公司 Tunel 试剂盒操作说明书(In situ cell death detection kit-POD法)一、原理:TUNEL (TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA 的断裂情况。
其原理是荧光素( fluorescein)标记的 dUTP 在脱氧核糖核苷酸末端转移酶( TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂 DNA 的3’-OH 末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与 HRP 底物二氨基联苯胺(DAB )反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有 DNA 断裂,因而没有 3‘-OH 形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含:1 号(蓝盖) Enzyme Solution 酶溶液: TdT 10×、2号(紫盖) Label Solution 标记液:荧光素标记的 dUTP 1×、3号(棕瓶) Converter-POD:标记荧光素抗体的 HRP;自备试剂: PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB 工作液(临用前配制, 5 μl 20 ×DAB+1 μL 30%H2O2+94 μl PBS)、Proteinase K工作液( 10-20 μg/ml in 10 mM Tris/HCl ,pH 7.4-8)或细胞通透液(0.1% Triton X-100 溶于 0.1% 柠檬酸钠,临用前配制)、苏木素或甲基绿、 DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl ,pH 7.5, 10 mM MgCl2 ,1 mg/ml BSA )等。
翻译好的 罗氏公司Tunel试剂盒操作说明书

罗氏(Roche)公司Tunel试剂盒操作说明书(In situ cell death detection kit-POD法)一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含:1号(蓝盖)Enzyme Solution 酶溶液:TdT 10×、2号(紫盖)Label Solution标记液:荧光素标记的dUTP 1×、3号(棕瓶)Converter-POD:标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl,pH 7.4-8)或细胞通透液(0.1% Triton X-100 溶于0.1% 柠檬酸钠,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml–3 U/ml in 50 mM Tris-HCl,pH 7.5,10 mM MgCl2,1 mg/ml BSA)等。
罗氏 荧光 tunel (11684795910)说明书

In Situ Cell Death Detection Kit, Fluorescein
y Version 17
Content version: November 2012
Preface .....................................................................................................................................................2 Table of contents ................................................................................................................................................................. 2 Kit contents ............................................................................................................................................................................ 3 Introduction ............................................................................................................................................5 Product overview .................................................................................................................................................................. 5 Background information ................................................................................................................................................... 8 Procedures and required materials .................................................................................................10 Flow chart .............................................................................................................................................................................10 Preparation of sample material ....................................................................................................................................11 Cell suspension ..................................................................................................................................................................11 Adherent cells, cell smears, and cytospin preparations .....................................................................................12 Tissue sections ....................................................................................................................................................................12 Treatment of paraffin-embedded tissue ...................................................................................................................12 Treatment of cryopreserved tissue ..............................................................................................................................14 Labeling protocol ...............................................................................................................................................................15 Before you begin ................................................................................................................................................................15 Labeling protocol for cell suspensions ......................................................................................................................16 Labeling protocol for adherent cells, cell smears, cytospin preparations and tissues ...........................................................................................................................................................................17 Labeling protocol for difficult tissue ...........................................................................................................................18 Typical results ......................................................................................................................................19 Appendix ................................................................................................................................................20 Troubleshooting .................................................................................................................................................................20 References ............................................................................................................................................................................23 Related products ................................................................................................................................................................24
TUNEL SOP2012.9.24

TUNEL Standard Operating Procedure一、试剂盒来源美国罗氏(Roche)公司二、实验原理T UNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3’-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
图1 TUNEL 实验原理示意图PROTOCOL OF APOPTOSISPrinciple: The TUNEL (Terminal deoxynucleotidyl Transferase fluorescein-dUTP Nick End Labeling) method identifies apoptotic cells in situ by using terminal deoxynucleotidyl transferase (TdT) to transfer fluorescein-dUTP to the free 3'-OH of cleaved DNA. Then Detect the incorporated fluorescein with an anti-fluorescein antibody conjugating AP(alkaline phosphatase).Procedure:1. Paraffin embedding tissue sections2. Dewax, rehydrate sections by standard methods:Xylene and ethanol(absolute, 95%,90%,80,%70%,diluted in double distilled water)3. Optional: Inactivate endogenous POD/AP activity4. Wash slides with PBS(0.01M, PH7.2~7.4) 3 times, 3~5min/time5. Add protease and incubate (30 min, 37°C, protetase K, working solution:10~20ug/ml in 10mM Tris/HCl, PH7.4~8.0)6. Wash slides with PBS 3 times, 3~5min /time7. Permeabilize sections (2 min, on ice, permeabilisation solution0.1%TritonX-100, 0.1% sodium citrate, freshly prepared.8. Add TUNEL-reaction mixture and incubate (60 min, 37°C)9. Wash slides with PBS 3 times, 3~5min /time10. Optional: Analyze by fluorescence microscopy11. Add Anti-Fluorescein-AP or -POD and incubate (30 min, 37°C)12. Wash slides with PBS 3 times, 3~5min /time13. Add substrate and incubate (5–20 min, RT)14. Analyze by light-microscopy三、实验器材1. 光学器材(可选其一):①正置光学显微镜及其成像系统(带荧光)②正置光学显微镜(Olympus)+数码相机(小镜头,易伸入显微镜头中拍照)2. 上行脱水缸:75%乙醇溶液、90%乙醇溶液、95%乙醇溶液、100%乙醇溶液、100%乙醇溶液、二甲苯(各1缸,共6个染色缸)3. 下行脱蜡与水化缸:二甲苯2缸(必要时3个)、100%乙醇溶液2缸、95%乙醇1缸、90%乙醇溶液1缸、75%乙醇溶液1缸、三蒸水1缸(8~9个)4.小型染色缸(PBS用):3个5. 免疫组化笔或唇膏:1支6. 湿盒(带纱布)或自制湿盒:1~2个7. 盖玻片:若干片和若干规格(需处理后用)8. 吸管:若干支9. 加样器:200μL~1000μL、40μL~200μL、2μL~20μL10.枪头:200μL~1000μL、40μL~200μL、2μL~20μL11. 50.0mL螺口血清瓶(高压、消毒):若干个12. 1.5 mL EP管、2.0 mL 与5.0 mL 冻存管(高压、消毒):若干个13. EP管与冻存管架:若干个14. 苏木素染缸:1个15. 分色缸:1个16. PBS大缸:1个17.针式滤器(0.6μm)与10mL、50mL注射器:若干个18. 500mL容量瓶或盐水瓶:2个19. 有齿镊、小弯镊、小竹签(滴中性树胶用):各1把(支)20. 清洁盖玻片储存盒:1个或清洁盖玻片储存缸1个21. 培养箱或温箱:37℃孵育用22. 烤箱:60℃考片用23.量筒:250mL、500mL、1000 mL、2000 mL,各1个24. 烧杯:50 mL、250 mL、500mL、1000 mL、2000 mL,各1个25. 玻棒:2支26. 磁力搅拌器:1个27. 50 mL 容量瓶1个28. 10mL试管(配DAB专用)若干支四、试剂1. 试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;2. 自备试剂(1)PBS(0.01M,PH 7.4):中国福州迈新公司(2)单蒸水、三蒸水或超纯水(3)二甲苯(4)梯度乙醇溶液(100%、95%、90%、75%):采用95%与无水乙醇配制(5)DAB显色试剂盒(含20×DAB、30%H2O2、PBS):中国福州迈新公司(6)蛋白酶K(Proteinase K):(MERCK公司)①母液(1mg/mL): -20℃保存;②工作液:10-20 μg/mL in 10 mM Tris/HCL,pH 7.4-8,4℃保存,以1月内使用为宜。
翻译好的罗氏公司Tunel试剂盒操作说明方案

罗氏(R o c h e)公司T u n e l试剂盒操作说明书(Insitucelldeathdetectionkit-POD法)一、原理:TUNEL(TdT-mediateddUTPnickendlabeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdTEnzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radishperoxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含:1号(蓝盖)EnzymeSolution酶溶液:TdT10×、2号(紫盖)LabelSolution标记液:荧光素标记的dUTP1×、3号(棕瓶)Converter-POD:标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5μl20×DAB+1μL30%H2O2+94μlPBS)、ProteinaseK工作液(10-20μg/mlin10mMTris/HCl,pH7.4-8)或细胞通透液(0.1%TritonX-100溶于0.1%柠檬酸钠,临用前配制)、苏木素或甲基绿、DNase1(3000U/ml–3U/mlin50mMTris-HCl,pH7.5,10mMMgCl2,1mg/mlBSA)等。
TUNEL染色
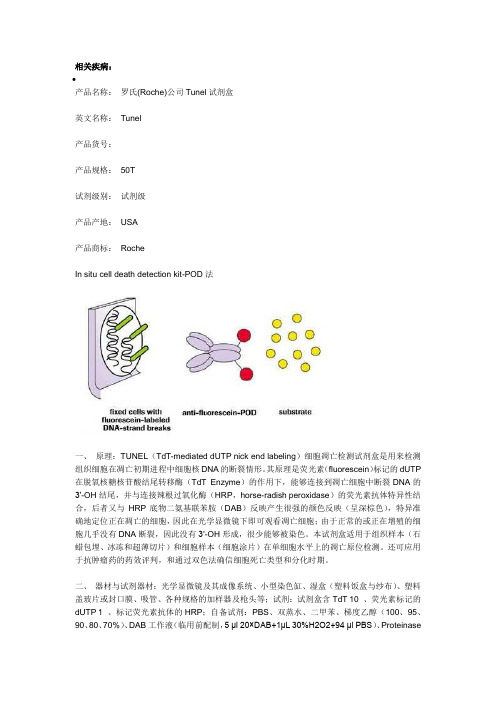
相关疾病:•产品名称:罗氏(Roche)公司Tunel试剂盒英文名称:Tunel产品货号:产品规格:50T试剂级别:试剂级产品产地:USA产品商标:RocheIn situ cell death detection kit-POD法一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡初期进程中细胞核DNA的断裂情形。
其原理是荧光素(fluorescein)标记的dUTP 在脱氧核糖核苷酸结尾转移酶(TdT Enzyme)的作用下,能够连接到凋亡细胞中断裂DNA的3’-OH结尾,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反映产生很强的颜色反映(呈深棕色),特异准确地定位正在凋亡的细胞,因此在光学显微镜下即可观看凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因此没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评判,和通过双色法确信细胞死亡类型和分化时期。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、ProteinaseK工作液(10-20 μg/ml in 10 mM Tris/HCl,pH )或细胞通透液(% Triton X-100 in % sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH ,10 mM MgCl2,1 mg/ml BSA)等。
TUNEL说明书

TUNEL说明书1 介绍TUNEL是提供单细胞水平细胞凋亡的稳定系统,能够迅速、快捷、精确的检测出凋亡细胞。
该试剂盒可以通过测定核DNA片段检测组织切片和培养细胞的凋亡细胞。
多数高等真核生物的细胞都通过启动自身的细胞自杀程序实现程序性死亡或细胞凋亡。
凋亡在发育、内环境稳定和一些疾病中具有重要作用。
凋亡具有某些特定的形态学特征,包括细胞膜起泡,细胞核和细胞质固缩,染色质浓缩,并且不发生局部炎症反应。
细胞死亡与此相反,它的特点是细胞肿胀,染色质絮凝,细胞膜完整性破坏,细胞溶解和产生及局部炎症反应。
凋亡过程中,内源性Ca2+、Mg2+依赖性核酸内切酶被激活,DNA被降解而形成末端为3’-OH、含180~200碱基对的不同倍数的核苷酸片段。
TUNEL 可用于多种细胞凋亡的检测,已经经过验证的应用范围: Vibratome® 神经组织切片, Jurkat 细胞, HL-60细胞这本技术小册子包括检测组织切片和茴香霉素诱导的HL-60细胞的细胞凋亡。
检测原理: DeadEnd™ Colorimetric TUNEL 系统使用改良的TUNEL (TdT-mediated dUTP Nick-End Labeling)对凋亡细胞的断裂DNA进行末端标记。
使用末端脱氧核苷转移酶(TdT)将生物素标记的核苷被掺入到DNA的3′-OH末端。
然后,辣根过氧化物酶标记的链霉亲和素(Streptavidin HRP)结合在上述生物素标记的核苷上,可以通过过氧化物酶的底物——过氧化氢和稳定的显色剂氨基联苯胺(DAB)检测到。
用这种程序,凋亡细胞的核被染成深棕色。
2 产品内容G7361平衡缓冲液(G327B)——4.8ml末端脱氧核苷酰酶酸转移酶(M828B)——20ul抗生物素蛋白链菌素辣根过氧化物酶(G714A)——40ul生物素化的核苷混合物(G715A)——20ul蛋白酶K(V302A)——10mgG7362塑料盖玻片(G326B)——2020X SSC(G329B)——20mlDAB 20X 色原体(G716A)——200ul20X DAB底物缓冲液(G717A)——200ul20X过氧化氢(G718A)——200ul储存条件: 将平衡缓冲液, TdT酶, 生物素标记的核苷混合物和蛋白酶K 储存于–20°C。
TUNEL说明书

不适用于诊断,仅供生命科学实验使用。
仅供体外使用。
TUNEL细胞凋亡原位检测试剂盒(标记POD)本试剂盒可标记DNA链末端,即TUNEL技术。
用于单个细胞水平凋亡(细胞程序性死亡)的免疫组织化学检测和定量分析。
光镜检测。
批号:11684817910一盒可用50次储存:-15—-25℃操作指南2006.01.1.2 试剂盒组成注意事项本品标记溶液含有二甲基砷酸盐,容易被吸入而产生毒性和导致呕吐;也含有二氯化钴,吸入后容易致癌。
因此应避免接触并遵循相关的操作说明。
使用过程中不能吃、喝和抽烟。
如果接触到皮肤,应立即用大量水冲洗干净。
如果感觉不适或者突发其它情况,应立即就医。
酶标反应应在致密的、无损坏的容器中进行,收集上清液后应标明成分。
垃圾应作为有毒废物进行处理。
注意:与先前的试剂盒/管不同,次试剂盒的酶溶解液不再含有毒的二甲砷基酸盐,因此瓶1没有毒性。
试剂盒组成请参照下表对比试剂盒组成试剂盒外自备仪器和试剂出上表所列试剂外,实验者需制备系列溶液。
下表列出了在不同实验步骤中所需要的试剂。
在每步操作前都给出了详细的说明。
2 引言2.1 产品描述实验原理在细胞凋亡过程中,基因组DNA 会断裂产生双链、低分子量的DNA 片段和高分子量的单链DNA 断端(缺口),这些DNA 链缺口可以利用酶标记核苷酸3’末端方法来识别。
应用原位细胞凋亡检测试剂盒具有准确、快速、简单、非辐射等特点,可以用来对细胞或者组织中的单个细胞进行检测并定量,因而次法被用在很多分析系统中,例如:●在基础研究和日常病理中检测冰冻和福尔马林固定的组织切片。
●在肿瘤研究和临床癌基因研究中确定某些恶性肿瘤对某种药物的敏感性。
●通过双染色操作,确定经历死亡的异常增生细胞的分型。
专一性TUNEL反应可以更好的标记通过凋亡产生的DNA链末端,因此就可以区分出凋亡与坏死,以及由抗肿瘤药物或者放射诱发的初始DNA链末端。
试验干扰假阴性:在某些形式的细胞凋亡中,DNA逃逸酶切或者不完全(37)。
TUNEL说明书

不适用于诊断,仅供生命科学实验使用。
仅供体外使用。
TUNEL细胞凋亡原位检测试剂盒(标记POD)本试剂盒可标记DNA链末端,即TUNEL技术。
用于单个细胞水平凋亡(细胞程序性死亡)的免疫组织化学检测和定量分析。
光镜检测。
批号:11684817910一盒可用50次储存:-15—-25℃操作指南2006.01.1.2 试剂盒组成注意事项本品标记溶液含有二甲基砷酸盐,容易被吸入而产生毒性和导致呕吐;也含有二氯化钴,吸入后容易致癌。
因此应避免接触并遵循相关的操作说明。
使用过程中不能吃、喝和抽烟。
如果接触到皮肤,应立即用大量水冲洗干净。
如果感觉不适或者突发其它情况,应立即就医。
酶标反应应在致密的、无损坏的容器中进行,收集上清液后应标明成分。
垃圾应作为有毒废物进行处理。
注意:与先前的试剂盒/管不同,次试剂盒的酶溶解液不再含有毒的二甲砷基酸盐,因此瓶1没有毒性。
试剂盒组成请参照下表对比试剂盒组成试剂盒外自备仪器和试剂出上表所列试剂外,实验者需制备系列溶液。
下表列出了在不同实验步骤中所需要的试剂。
在每步操作前都给出了详细的说明。
2 引言2.1 产品描述实验原理在细胞凋亡过程中,基因组DNA 会断裂产生双链、低分子量的DNA 片段和高分子量的单链DNA 断端(缺口),这些DNA 链缺口可以利用酶标记核苷酸3’末端方法来识别。
应用原位细胞凋亡检测试剂盒具有准确、快速、简单、非辐射等特点,可以用来对细胞或者组织中的单个细胞进行检测并定量,因而次法被用在很多分析系统中,例如:●在基础研究和日常病理中检测冰冻和福尔马林固定的组织切片。
●在肿瘤研究和临床癌基因研究中确定某些恶性肿瘤对某种药物的敏感性。
●通过双染色操作,确定经历死亡的异常增生细胞的分型。
专一性TUNEL反应可以更好的标记通过凋亡产生的DNA链末端,因此就可以区分出凋亡与坏死,以及由抗肿瘤药物或者放射诱发的初始DNA链末端。
试验干扰假阴性:在某些形式的细胞凋亡中,DNA逃逸酶切或者不完全(37)。
罗氏TUNEL使用说明书中文

罗氏公司TUNEL细胞凋亡检测程序(In situ cell death detection kit-POD法)一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3'-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 µl 20×DAB+1μL30%H2O2+94 µl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
TUNEL-roche使用流程

In situ cell death detection kit-POD法一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是脱氧核糖核苷酸末端转移酶(TdT Enzyme)把荧光素(fluorescein)标记的dUTP(三磷酸脱氧尿甘)连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,HRP又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
TUNEL 染色罗氏操作步骤protocol

For life science research only. Not for use in diagnostic procedures.In Situ Cell Death Detection Kit, TMR redKit for detection and quantification of apoptosis (programmed cell death) at single cell level, based on labeling of DNA strand breaks (TUNEL technology): Analysis by fluorescence microscopy or flow cytometry.Cat. No. 12 156 792 910 1 Kit (50 tests)Store at Ϫ15 to Ϫ25°Cy Version 12Content version: March 20161. Preface1.1 Table of Contents (2)1. Preface1.1 Table of Contents 21.2 Kit Contents 3 (5)2. Introduction2.1Product Overview 52.2 Background Information 83. Procedures and Required Materials (10)3.1Flow Chart 103.2Preparation of Sample Material 113.2.1Cell Suspension 113.2.2 Adherent Cells, Cell Smears, and Cytospin Preparations 123.2.3Tissue Sections 133.2.3.1 Treatment of Paraffin-Embedded Tissue 133.2.3.2 Treatment of Cryopreserved Tissue 153.3Labeling Protocol 163.3.1 Before you Begin 163.3.2 Labeling Protocol for Cell Suspensions 173.3.3Labeling Protocol for Adherent Cells, Cell Smears, Cytospin Preparations,and Tissues 183.3.4Labeling Protocol for Difficult Tissue 194. Typical results (20)5.Appendix (21)5.1Troubleshooting215.2 References 24Information 255.3 OrderingChanges to previous version26Regulatory Disclaimer26Disclaimer of License261.2 KitContentsCaution The Label Solution contains cacodylate, toxic when inhaled or swal-lowed and cobalt dichloride, which may cause cancer by inhalation.Avoid exposure and obtain special instructions before use.When using the solution do not eat, drink or smoke. After contact withskin, wash immediately with plenty of water. In case of accident or ifyou feel unwell seek medical advice immediately (show label wherepossible).Collect the supernatants from the labeling reactions in a tightly closed,non-breakable container and indicate contents. Discard as regulatedfor toxic waste.Kit Contents Please refer to the following table for the contents of the kit.Vial/CapLabel Contents1 blue Enzyme Solution•Terminal deoxynucleotidyl transferasefrom calf thymus (EC 2.7.7.31), recom-binant in E. coli, in storage buffer•10× conc.• 5 × 50 l2 red Label Solution•Nucleotide mixture in reaction buffer•1×conc.• 5 × 550 lAdditional Solutions Required In addition to the reagents listed above, you have to prepare several solutions. In the table you will find an overview about the equipment which is needed for the different procedures.Detailed information is given before each procedure.Procedure Equipment Reagents Preparation of sample material (section 3.2)•Cell suspension (section 3.2.1)•Adherent cells, cell smears and cytospin preparations (section 3.2.2.)•Cryopreserved tissue (section 3.2.3.2)•Shaker•V-bot-tomed 96-well micro-plate•Washing buffer: Phosphate bufferedsaline (PBS*)•Fixation solution: 4% Paraformaldehyde inPBS, pH 7.4, freshly prepared•Permeabilisation solution: 0.1% Triton X-100 in 0.1% sodium citrate, freshly pre-pared (6)Paraffin-embedded tissue (section 3.2.3.1)•Xylene and ethanol (absolute, 95%, 90%, 80%, 70%, diluted in double-distilled water)•Washing buffer: PBS*•Proteinase K*, PCR Grade, working solu-tion: [10 –20 g/ml in 10 mM Tris/HCl, pH 7.4–8]Alternative treatments •Permeabilisation solution: (0.1% Triton1) X–100, 0.1% sodium citrate), freshly pre-pared•Pepsin* (0.25%–0.5% in HCl, pH 2) or trypsin*, 0.01 N HCl•0.1 M Citrate buffer, pH 6 for microwave irradiationLabeling protocol (section 3.3)Positive control (section 3.3.1)•Micrococcal nuclease or •DNase I recombinant*•Cell suspensions (section 3.3.2)•Adherent cells (section 3.3.3)•Parafilm orcoverslips•HumidifiedchamberWashing buffer: PBS*Difficult tissue (section 3.3.4)•Plastic jar•Microwave•Humidifiedchamber•Citrate buffer, 0.1 M, pH 6.0.•Washing buffer: PBS*•Tris-HCl, 0.1 M pH 7.5, containing 3%BSA* and 20% normal bovine serum1.2 KitContents,continued2. Introduction2.1Product OverviewTest Principle Cleavage of genomic DNA during apoptosis may yield double-stranded, low molecular weight DNA fragments (mono- and oligonu-cleosomes) as well as single strand breaks (“nicks“) in high molecularweight DNA.Those DNA strand breaks can be identified by labeling free 3’-OH ter-mini with modified nucleotides in an enzymatic reaction.Application The In Situ Cell Death Detection Kit is designed as a precise, fast and simple, non-radioactive technique to detect and quantify apoptotic celldeath at single cell level in cells and tissues. Thus, the In Situ CellDeath Detection Kit can be used in many different assay systems.Examples are:•Detection of individual apoptotic cells in frozen and formalin-fixedtissue sections in basic research.•Determination of sensitivity of malignant cells to drug-induced apop-tosis in cancer research.•Typing of cells undergoing cell death in heterogeneous populationsby double staining procedures (6, 7).Specificity The TUNEL reaction preferentially labels DNA strand breaks generated during apoptosis. This allows discrimination of apoptosis from necrosisand from primary DNA strand breaks induced by cytostatic drugs orirradiation (3, 4).Test Interference False negative results: DNA cleavage can be absent or incomplete in some forms of apoptotic cell death (37). Sterical hindrance such asextracellular matrix components can prevent access of TdT to DNAstrand breaks. In either case false negative results may be obtained.False positive results: Extensive DNA fragmentation may occur in cer-tain forms of necrosis (38).DNA strand breaks may also be prominent in cell populations withhigh proliferative or metabolic activity. In either case false positiveresults may be obtained.To confirm the apoptotic mode of cell death, the morphology ofrespective cells should be examined very carefully. Morphologicalchanges during apoptosis have a characteristic pattern. Thereforeevaluation of cell morphology is an important parameter in situationswhere there is any ambiguity regarding interpretation of results. Sample Material•Cell suspensions from•permanent cell lines (2, 27, 35),•lymphocytes and leukemic cells from peripheral blood (4),•thymocytes(1,6),•bone marrow cells•fine needle biopsies (5)•Cytospins and cell smear preparations•Adherent cells cultured on chamber slides (31)•Frozen or formalin-fixed, paraffin-embedded tissue sections (1, 25,26, 29, 30, 32–34, 36, 39)Assay Time1-2 hours, excluding culture, fixation and permeabilisation of cells and preparation of tissue sections.Number of Tests The kit is designed for 50 tests.Kit Storage/ Stability The unopened kit is stable at Ϫ15 to Ϫ25°C until the expiration date printed on the label.Note: The TUNEL reaction mixture should be prepared immediately before use and should not be stored. Keep the TUNEL reaction mixture on ice until use.Advantage Please refer to the following table.Benefit FeatureSensitive Detection of apoptotic cell death at single celllevel via fluorescence microscopy and at cell pop-ulations via FACS analysis at very early stages (1,2, 6).Specific Preferential labeling of apoptosis versus necrosis(3, 4).Fast Short assay time (1– 2 h).Convenient•No secondary detection system required.•One incubation and one washing step only.•Reagents are provided in stable, optimizedform.•No dilution steps required.•Application in combination with fluoresceinlabel possibleFlexible•Suitable for fixed cells and tissue. This allowsaccumulation, storage and transport of sam-ples (2, 5).•Double staining enables identification of typeand differentiation state of cells undergoingapoptosis (6).Function-tested Every lot is function-tested on apoptotic cells incomparison to a master lot.2.2 BackgroundInformationCell Death Two distinct modes of cell death, apoptosis and necrosis, can be dis-tinguished based on differences in morphological, biochemical andmolecular changes of dying cells.Programmed cell death or apoptosis is the most common form ofeukaryotic cell death. It is a physiological suicide mechanism that pre-serves homeostasis, in which cell death naturally occurs during normaltissue turnover (8, 9). In general, cells undergoing apoptosis display acharacteristic pattern of structural changes in nucleus and cytoplasm,including rapid blebbing of plasma membrane and nuclear disintegra-tion. The nuclear collapse is associated with extensive damage tochromatin and DNA-cleavage into oligonucleosomal length DNA frag-ments after activation of a calcium-dependent endogenous endonu-clease (10, 11). However, very rare exceptions have been describedwhere morphological features of apoptosis are not accompanied witholigonucleosomal DNA cleavage (37).Apoptosis Apoptosis is essential in many physiological processes, includingmaturation and effector mechanisms of the immune system (12, 13),embryonic development of tissue, organs and limbs (14), developmentof the nervous system (15, 16) and hormone-dependent tissueremodeling (17).Identification of Apoptosis Several methods have been described to identify apoptotic cells (22– 24). Endonucleolysis is considered as the key biochemical event of apoptosis, resulting in cleavage of nuclear DNA into oligonucleosome-sized fragments. Therefore, this process is commonly used for detec-tion of apoptosis by the typical “DNA ladder” on agarose gels during electrophoresis. This method, however, can not provide information regarding apoptosis in individual cells nor relate cellular apoptosis to histological localization or cell differentiation. This can be done by enzymatic in situ labeling of apoptosis-induced DNA strand breaks. DNA polymerase as well as terminal deoxynucleotidyl transferase (TdT) (1– 6, 25– 36) have been used for the incorporation of labeled nucleotides to DNA strand breaks in situ. The tailing reaction using TdT, which was also described as ISEL (in situ end labeling) (5, 35) or TUNEL (TdT-mediated dUTP nick end labeling) (1, 6, 31, 33) tech-nique, has several advantages in comparison to the in situ nick trans-lation (ISNT) using DNA polymerase:•Label intensity of apoptotic cells is higher with TUNEL compared to ISNT, resulting in an increased sensitivity (2, 4).•Kinetics of nucleotide incorporation is very rapid with TUNEL com-pared to the ISNT (2, 4).•TUNEL preferentially labels apoptosis in comparison to necrosis, thereby discriminating apoptosis from necrosis and from primary DNA strand breaks induced by antitumor drugs or radiation (3, 4).2.2 BackgroundInformation,continued3. ProceduresandR equired M aterialsThe working procedure described below was published by R. Sgoncand colleagues (6). The main advantage of this kit is the use of tetra-methyl- rhodamine- dUTP to directly label DNA strand breaks with redfluorescence. This allows the direct detection of DNA fragmentationin the red channel and secondary labeling with fluorescein in thegreen channel by flow cytometry or fluorescence microscopy.3.1Flow ChartAssay Procedure The assay procedure is explained in the following flow chart.Cell suspensionAdherentcells, cellsmears, andcytospinprepara-tionsCryopre-served tis-sue sectionsParaffin-embeddedtissue sections↓↓↓↓FixationDewaxation Rehydration Protease treat-ment↓↓↓Permeabilisation↓Labeling reactionwith TUNEL reaction mixture↓↓Analyze byflow cyto-metry or byfluores-cencemicroscopyAnalyze by fluorescence microscopy3.2Preparation of Sample Material3.2.1Cell SuspensionPrelabeling For dual parameter flow cytometry with fluorescein-conjugated anti-bodies, incubate the cells prior to fixation with the cell surface marker.Additional Buf-fers and Equip-ment Required •Washing buffer: Phosphate buffered saline (PBS)•Fixation solution: Paraformaldehyde (4% in PBS, pH 7.4), freshly prepared•Permeabilisation solution: 0.1% Triton X–100 in 0.1% sodium citrate, freshly prepared•Shaker•V-bottomed 96-well microplateNote:Use of a V-bottomed 96-well microplate minimize cell loss during fixation, permeabilisation and labeling and allows simultaneous preparation of multiple samples.Procedure Please find in the following protocol the procedure for cell fixation and permeabilisation.Note: Fix and permeabilisate two additional cells for the negative andpositive labeling controls.Step Action1Wash test sample 3 times in PBS and adjust to 2 × 107cells/ml.2Transfer 100 l/well cell suspension into a V-bottomed 96-wellmicroplate.3Add 100 l/well of a freshly prepared Fixation solution to cellsuspension (final concentration 2% PFA).4Resuspend well and incubate 60 min at +15 to +25°C.Note:To avoid extensive clumping of cells, the microplateshould be incubated on a shaker during fixation.5Centrifuge the microplate at 300 g for 10 min and remove fixa-tive by flicking off or suction.6Wash cells once with 200 l/well PBS.7Centrifuge the microplate at 300 × g for 10 min and removePBS by flicking off or suction.8Resuspend cells in 100 l/well Permeabilisation solution for2 min on ice (+2 to +8°C).9Proceed as described under 3.3.3.2.2 Adherent Cells, Cell Smears, and Cytospin PreparationsAdditional Solutions Required •Washing buffer: Phosphate buffered saline (PBS)•Fixation solution: 4% Paraformaldehyde in PBS, pH 7.4, freshly pre-pared•Permeabilisation solution: 0.1% Triton X-100 in 0.1% sodium citrate, freshly prepared (6)Procedure The following table describes preparations of adherent cells, cellsmears and cytospin.Note: Fix and permeabilisate two additional cell samples for the nega-tive and positive labeling controls.Step Action1Fix air dried cell samples with a freshly prepared Fixationsolution for 1 h at +15 to +25°C2Rinse slides with PBS.3Incubate in Permeabilisation solution for 2 min on ice (+2to +8°C).4Proceed as described under 3.3.3.2.3Tissue Sections3.2.3.1 Treatment of Paraffin-Embedded TissuePretreatment of Paraffin Embed-ded Tissue Tissue sections can be pretreated in 4 different ways. If you use Pro-teinase K the concentration, incubation time and temperature have to be optimized for each type of tissue (1, 29, 33, 36, 40, 41).Note: Use Proteinase K tested for absence of nucleases which might lead to false-positive results!The other 3 alternative procedures are also described in the following table (step 2).Additional Solutions Required •Xylene and ethanol (absolute, 95%, 90%, 80%, 70%, diluted in dou-ble distilled water)•Washing buffer: PBS•Proteinase K, PCR Grade*, working solution: [10-20 g/ml in 10 mM Tris/HCl, pH 7.4-8]Alternative treatments•Permeabilisation solution: 0.1% Triton X–100, 0.1% sodium citrate, freshly prepared•Pepsin* (0.25% - 0.5% in HCl, pH 2) or trypsin*, 0.01 N HCl•0.1 M Citrate buffer, pH 6 for the microwave irradiationProcedure In the following table the pretreatment of paraffin-embedded tissuewith Proteinase K treatment and 3 alternative procedures aredescribed.Note: Add additional tissue sections for the negative and positivelabeling controls.Step Action1Dewax and rehydrate tissue section according to standardprotocols (e.g., by heating at +60°C followed by washing inxylene and rehydration through a graded series of ethanol anddouble dist. water) (1, 33, 36).2Incubate tissue section for 15 –30 min at +21 to +37°C withProteinase K working solution.Alternatives:Treatment:1. Permeabilisa-tion solutionIncubate slides for 8 min.2. Pepsin* (30, 40)or trypsin15–60 min at +37°C.3. Microwave irradiation •Place the slide(s) in a plastic jar containing 200 ml 0.1 M Citrate buffer, pH 6.0.•Apply 350 W microwave irradia-tion for 5 min.3Rinse slide(s) twice with PBS.4Proceed as described under 3.3.3.2.3.1 Treatment of Paraffin-Embedded Tissue, continued3.2.3.2 Treatment of Cryopreserved TissueAdditional Solutions required •Fixation solution: 4% Paraformaldehyde in PBS, pH 7.4, freshly pre-pared•Washing buffer: PBS•Permeabilisation solution: 0.1% Triton X–100, 0.1% sodium citrate, freshly preparedCryopreserved Tissue In the following table the pretreatment of Cryopreserved tissue is described.Note: Fix and permeabilisate two additional samples for the negative and positive labeling controls.Step Action1Fix tissue section with Fixation solution for 20 min at +15 to +25°C.2Wash 30 min with PBS.Note:For storage, dehydrate fixed tissue sections 2 min inabsolute ethanol and store at Ϫ15 to Ϫ25°C.3Incubate slides in Permeabilisation solution for 2 min on ice (+2 to +8°C).4Proceed as described under 3.3.3.3Labeling Protocol 3.3.1 Before You BeginPreparation of TUNEL Reaction Mixture One pair of tubes (vial 1: Enzyme Solution, and vial 2: Label Solution) is sufficient for staining 10 samples by using 50 l TUNEL reaction mix-ture per sample and 2 negative controls by using 50 l Label Solution per control.Note:The TUNEL reaction mixture should be prepared immediately before use and should not be stored. Keep TUNEL reaction mixture on ice until use.Step Action1Remove 100 l Label Solution (vial 2) for two negative con-trols.2Add total volume (50 l) of Enzyme Solution (vial 1) to the remaining 450 l Label Solution in vial 2 to obtain 500 lTUNEL reaction mixture.3Mix well to equilibrate components.Additional Reagents Required •Micrococcal nuclease or •DNase I recombinant *Controls Two negative controls and a positive control should be included in each experimental setup.Negative control:Incubate fixed and permeabilized cells in 50 l/well Label Solution (without terminal transferase) instead of TUNEL reaction mixture.Positive control:Incubate fixed and permeabilized cells with micro-coccal nuclease or DNase I recombinant (3000 U/ml– 3 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA) for 10 min at +15 to +25°C to induce DNA strand breaks, prior to labeling procedures.* available from Roche Diagnostics3.3.2 Labeling Protocol for Cell SuspensionsAdditional Equipment and Reagents Required •Washing buffer: PBS •Humidified chamberProcedure Please refer to the following table.Step Action1Wash cells twice with PBS (200 l/well).2Resuspend in 50 l/well TUNEL reaction mixture.Note: For the negative control add 50 l Label solution.3Add lid and incubate for 60 min at +37°C in a humidifiedatmosphere in the dark.4Wash samples twice in PBS.5Transfer cells in a tube to a final volume of 250-500 l in PBS.6Samples can directly be analyzed by flow cytometry or fluores-cence microscopy. For evaluation by fluorescence microscopyuse an excitation wavelength in the range of 520-560 nm(maximum 540 nm; green) and detection in the range of 570-620 nm (maximum 580 nm, red).3.3.3Labeling Protocol for Adherent Cells, Cell Smears, Cytospin Preparations,and TissuesAdditional Equipment and Reagents Required •Washing buffer: PBS •Parafilm or coverslips •Humidified chamberProcedure Please refer to the following table.Step Action1Rinse slides twice with PBS.2Dry area around sample.3Add 50 l TUNEL reaction mixture on sample.Note:For the negative control add 50 l Label solution each.To ensure a homogeneous spread of TUNEL reaction mixtureacross cell monolayer and to avoid evaporative loss, samplesshould be covered with parafilm or coverslip during incuba-tion.4Incubate slide in a humidified atmosphere for 60 min at +37°Cin the dark.5Rinse slide 3× with PBS.6Samples can directly be analysed under a fluorescence micro-scope or embedded with antifade prior to analysis. Use anexcitation wavelength in the range of 520-560 nm (maximum540 nm; green) and detection in the range of 570-620 nm(maximum 580 nm, red).3.3.4Labeling Protocol for Difficult TissueAdditional Equipment and Solutions Required •Citrate buffer, 0.1 M, pH 6.0.•Washing buffer: PBS•Tris-HCl, 0.1 M pH 7.5, containing 3% BSA and 20% normal bovine serum•Plastic jar•MicrowaveProcedure Please refer to the following table.Step Action1Dewax paraformaldehyde- or formalin-fixed tissue sectionsaccording to standard procedures.2Place the slide(s) in a plastic jar containing 200 ml 0.1 MCitrate buffer, pH 6.0.3•Apply 750 W (high) microwave irradiation for 1 min.•Cool rapidly by immediately adding 80 ml double dist.water (+20 to +25°C).•Transfer the slide(s) into PBS (+20 to +25°C).DO NOT perform a Proteinase K treatment!4Immerse the slide(s) for 30 min at +15 to +25°C in Tris-HCl,0.1 M pH 7.5, containing 3% BSA and 20% normalbovine serum.5•Rinse the slide(s) twice with PBS at +15 to +25°C.•Let excess fluid drain off.6Add 50 l of TUNEL reaction mixture on the section.Note: For the negative control add 50 l Label solution.7Incubate for 60 min at +37°C in a humidified atmosphere inthe dark.8•Rinse slide(s) three times in PBS for 5 min each.•Evaluate the section under a fluorescence microscope.results4. TypicalAssay Procedures•Incubate U937 cells at a density of 106 cells/ml in the presence ofcamptothecin (2 g/ml, 4 h at +37°C) to induce apoptosis.•As control for non-apoptotic population, an aliquot of the cells isincubated in normal culture medium without camptothecin.•Harvest cells and proceed as described under 3.2.1.Fig. 2: Analysis of camptothecin induced apoptosis in U937 cell by flow cytometryDotted line: Cells cultured in the absence of camptothecin.Solid line: Cells cultured in the presence of camptothecin (2 g/ml, 4 h).Cells analyzed under marker M1 are apoptotic (TUNEL positive)5.Appendix5.1TroubleshootingThis table describes various troubleshooting parameters. Problem Step/Reagent ofProcedurePossible Cause RecommendationNonspecific labeling Embedding oftissueUV-irradiation forpolymerization ofembedding material(e.g., methacrylate)leads to DNAstrand breaksTry different embedding material ordifferent polymerization reagent.Fixation Acidic fixatives(e.g., methacarn,Carnoy’s fixative)•Try 4% buffered paraformaldehyde.•Try formalin or glutaraldehyde.TUNELreactionTdT concentrationtoo highReduce concentration of TdT by dilu-ting it 1:2 up to 1:10 with TUNEL Dilu-tion Buffer*.Nucleases,PolymerasesSome tissues (e.g.,smooth muscles)show DNA strandbreaks very soonafter tissue prepa-ration.•Fix tissue immediately after organpreparation.•Perfuse fixative through liver vein.Some enzymes arestill active.Block with a solution containing ddUTPand dATP.High back-ground Sample Mycoplasma con-taminationMycoplasma Detection Kit*Highly proliferatingcellsDouble staining, e.g., with Annexin-V-Fluos*.Note: Measuring via microplate readernot possible because of too high back-ground.Fixation Formalin fixationleads to a yellowishstaining of cellscontaining melaninprecursors.Try methanol for fixation but take intoaccount that this might lead toreduced sensitivity.TUNEL reac-tionConcentration oflabeling mix is toohigh for research inmamma carcinoma.Reduce concentration of labeling mixto 50% by diluting with TUNEL DilutionBuffer.continued on next page* available from Roche DiagnosticsLow labeling Fixation Ethanol and metha-nol can lead to lowlabeling (nucleo-somes are notcross-linked withproteins during fix-ation and are lostduring the proce-dure steps)•Try 4% buffered paraformaldehyde.•Try formalin or glutaraldehyde.Extensive fixation leads to excessive cross-linking of proteins •Reduce fixation time.•Try 2% buffered paraformaldehyde.Permeabilisa-tion Permeabilisationtoo short so thatreagents can’treach their targetmolecules•Increase incubation time.•Incubate at higher temperature(e.g., +15 to +25°C).•Try Proteinase K (concentration andtime has to be optimized for eachtype of tissue).•Try 0.1 M sodium citrate at +70°Cfor 30 min.Paraffin-embedding Accessibility forreagents is too low•Treat tissue sections after dewax-ing with Proteinase K (concentra-tion, time and temperature have tobe optimized for each type of tis-sue).•Try microwave irradiation at 370 W(low) for 5 min in 200 ml 0.1 MCitrate buffer pH 6.0 (has to be opti-mized for each type of tissue).continued on next pageProblem Step/Reagent ofProcedurePossible Cause RecommendationNo signal on positive con-trol DNasetreatmentConcentration ofDNase is too low•For cryosections apply 3 U/mlDNase I recombinant.•For paraffin-embedded tissue sec-tions apply 1,500 U/ml DNase Irecombinant.•In general, use 1 U/ml DNase Irecombinant, dissolved in 10 mMTris-HCl, pH 7.4 containing 10 mMNaCl, 5 mM MnCl2, 0.1 mM CaCl2,25 mM KCl and incubate 30 min at+37°C.•Alternative buffer: Tris- HCl pH 7.5containing 1 mM MgCl2 and 1 mg/ml BSA.Counter-staining diminishes TUNEL staining DNA stain Too high concen-trations of DNA dyeUse 0.1–1 g/ml BOBO–1 from Molec-ular Probes for counterstaining.Equivocal signals DoublestainingEarlier stage ofapoptosis thanstage detected byTUNEL reactionFor additional measurement of apopto-sis: M30 CytoDEATH* is suitable orAnnexin V – Fluos*.Problems with inter-pretation of results FACS Analysis Positive and nega-tive peaks are notdistinguishable,because too manyapoptotic bodieswere acquired,apoptosis is too faradvancedChange apoptosis inducing procedure:2-3 Clusters should be visible in theFSC/SSC histogram:1. debris and apoptotic bodies2. whole cells3. shrinked cells gate should deleteclearly separated peaks.No signal for apop-tosisTime depends on cell line and inducingagents and should be optimized.Problem Step/Reagent ofProcedurePossible Cause Recommendation* available from Roche Diagnostics5.2 References1Gavrieli, Y., Sherman, Y. & Ben-Sasson, S. A. (1992) J. Cell Biol.119, 493-501.2Gorczyca, W., Gong, J. & Darzynkiewicz, Z. (1993) Cancer Res. 53,1945-1951.3Gorczyca, W. et al. (1993) Leukemia 7, 659-670.4Gold, R. et al. (1994) Lab. Invest. 71, 2195Gorczyca, W. et al. (1994) Cytometry 15, 169-175.6Sgonc, R. et al. (1994) Ternds Genetics 10, 41-42.7Schmied, M. et al. (1993) Am. J. Pathol. 143, 446-452.8Wyllie, A. H. et al. (1980) Int. Rev. Cytol. 68, 251.9Kerr, J. F. R. et al. (1972) Br. J. Cancer 26, 239-257.10Duvall, E. & Wyllie, A. H. (1986) Immunol. Today 7, 115.11Compton, M. M. (1992) Canc. Metastasis Rev.11, 105-119.12Allen, P. D., Bustin, S. A. & Newland, A. C. (1993) Blood Reviews 7,63-73.13Cohen, J. J. & Duke, R. C. (1992) Annu. Rev. Immunol. 10, 267-293.14Clarke, P. G. H. (1990) Anat. Embryol. 181, 195-213.15Johnson, E. M. & Deckwerth, T. L. (1993) Annu. Rev. Neurosci. 16,31-46.16Batistatou, A. & Greene, L. A. (1993) J. Cell Biol. 122, 523-532.17Strange, R. et al. (1992) Development 115, 49-58.18Carson, D. A. & Ribeiro, J. M. (1993) Lancet 341, 1251-1254.19Edgington, S. M. (1993) Biotechnology 11, 787-792.20Gougeon. M.-L. & Montagnier, L. (1993) Science 260, 1269-1270.21Hickman, J. A. (1992) Cancer Metastasis Rev. 11, 121-139.22Afanasyev, V. N. et al. (1993) Cytometry 14, 603-609.23Bryson, G. J., Harmon, B. V. & Collins, R. J. (1994) Immunol. CellBiology 72, 35-41.24Darzynkiewicz, Z. et al. (1992) Cytometry 13, 795-808.25Ando, K. et al. (1994) J. Immunol. 152, 3245-3253.26Berges, R. R. et al. (1993) Proc. Natl. Acad. Sci. USA 90, 8910-8914.27Gorczyca, W. et al. (1992) Int. J. Oncol. 1, 639-648.28Gorczyca, W. et al. (1993) Exp. Cell Res. 207, 202-205.29Billig, H., Furuta, I. & Hsueh, A. J. W. (1994) Endocrinology 134,245-252.30MacManus, J. P. et al. (1993) Neurosci. Lett. 164, 89-92.31Mochizuki, H. et al. (1994) Neurosci. Lett. 170, 191-194.32Oberhammer, F. et al. (1993) Hepatology 18, 1238-1246.33Portera-Cailliau, C. (1994) Proc. Natl. Acad. Sci. USA 91, 974-978.34Preston, G. A. et al. (1994) Cancer Res. 54, 4214-4223.35Weller, M. et al. (1994) Eur. J. Immunol. 24, 1293-1300.36Zager, R.A. et al. (1994) J. Am. Soc. Nephrol. 4, 1588-1597.37Cohen, G. M. et al. (1992) Biochem. J. 286, 331-334.38Collins, R. J. et al. (1992) Int. J. Rad. Biol. 61, 451-453.39Sei, Y. et al. (1994) Neurosci. Lett. 171, 179-182.40Ansari, B. et al. (1993) J. Pathol. 170, 1-8.41Negoescu, A. et.al. (1998) Biochemica3, 34-41。
罗氏公司TUNEL细胞凋亡检测程序

罗氏公司TUNEL细胞凋亡检测程序细胞凋亡是生物体内一个自然而常见的程序性死亡过程,它在许多不同的情况下发生,如细胞发育、成熟和肿瘤。
目前发现的细胞凋亡方式有三种:凋亡小体途径、线粒体途径和内质网途径,它们的共同特点是在细胞死亡的过程中释放出很多旁泉酯酶(DNase)和酶联蛋白(ELP),并且同时伴随着核酸的降解、膜表面的翻转和蛋白酶的活化等,这些对于细胞凋亡的检测提供了许多途径和方法。
TUNEL细胞凋亡检测程序是细胞凋亡的常用检测技术之一,采用基于末端脱氧核糖核酸(TUNEL)的特异性核酸检测方法来检测细胞凋亡。
该方法是在细胞凋亡过程中DNA断裂,出现大量自由3’OH末端引导核酸聚合酶独特标记的文库和浓缩。
在该检测程序下,使用了可以不依赖载体的TDT(终端脱氧核糖核酸转移酶)作为核苷酸的生物素化酶,使断裂的DNA末端和蛋白质之间牢固地结合,从而得到了一种可以从细胞中清除的标记DNA分子。
标记DNA可经过染色和流式细胞术检测。
TUNEL技术的优点在于,可以很方便地用来评估细胞凋亡的水平,同时TUNEL技术也具有很高的灵敏度和特异性,可以检测到细胞凋亡的早期阶段。
此外,与许多其他的技术相比,TUNEL技术非常简单、可靠、灵活、快速以及有用。
因此,TUNEL技术被广泛应用于肿瘤、免疫、结构、神经系统和其他疾病等方面的研究。
罗氏公司TUNEL细胞凋亡检测程序采用随机扫描的激光共聚焦显微镜技术,可以在单个细胞水平上直接观察细胞凋亡的过程以及细胞内部的一些分子结构的可视化。
除此之外,该检测程序所采用的标记分子还可以被使用于定量荧光显微镜、流式细胞术和凝胶电泳等其他多种检测技术中。
总的来说,罗氏公司TUNEL细胞凋亡检测程序是一个性能非常高的细胞凋亡检测技术,主要应用于医学和生命科学领域的研究中。
在很多疾病治疗和药物研发方面,TUNEL技术可以为研究人员提供极为重要的信息,因此该技术的发展前景仍然非常广阔。
翻译好的 罗氏公司Tunel试剂盒操作说明方案 (2)

罗氏(R o c h e)公司T u n e l试剂盒操作说明书(Insitucelldeathdetectionkit-POD法)一、原理:TUNEL(TdT-mediateddUTPnickendlabeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdTEnzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radishperoxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含:1号(蓝盖)EnzymeSolution酶溶液:TdT10×、2号(紫盖)LabelSolution标记液:荧光素标记的dUTP1×、3号(棕瓶)Converter-POD:标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5μl20×DAB+1μL30%H2O2+94μlPBS)、ProteinaseK工作液(10-20μg/mlin10mMTris/HCl,pH7.4-8)或细胞通透液(0.1%TritonX-100溶于0.1%柠檬酸钠,临用前配制)、苏木素或甲基绿、DNase1(3000U/ml–3U/mlin50mMTris-HCl,pH7.5,10mMMgCl2,1mg/mlBSA)等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
罗氏公司TUNEL细胞凋亡检测程序
(In situ cell death detection kit-POD法)
一、原理:
TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是荧光素(fluorescein)标记的dUTP在脱氧核糖核苷酸末端转移酶(TdT Enzyme)的作用下,可以连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,后者又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3'-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂
器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;
试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 µl 20×DAB+1μL
30%H2O2+94 µl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
三、实验步骤
操作流程图:制作石蜡切片→脱蜡、水合→细胞通透→加TUNEL 反应液→加converter-POD→与底物DAB反应显色→光学显微镜计数并拍照。
具体操作步骤:
1.用二甲苯浸洗2次,每次5min;
2.用梯度乙醇(100、95、90、80、70%)各浸洗1次,每次3min;
3.PBS漂洗2次;
4.用Proteinase K工作液处理组织15-30 min 在21–37°C(温度、时间、浓度均需摸索)或者加细胞通透液8min;
5.PBS漂洗2次;
6.制备TUNEL反应混合液,处理组用50μl TdT+450μl 荧光素标记的dUTP液混匀;而阴性对照组仅加50μl 荧光素标记的dUTP 液,阳性对照组先加入100μl DNase 1,反应在15~25℃×10min,后面步骤同处理组。
7.玻片干后,加50μl TUNEL反应混合液(阴性对照组仅加50μl 荧光素标记的dUTP液)于标本上,加盖玻片或封口膜在暗湿盒中反
应37℃×1h。
8.PBS漂洗3次;
9.可以加1滴PBS在荧光显微镜下计数凋亡细胞(激发光波长为450~500nm,检测波长为515~565nm);
10.玻片干后加50μl converter-POD于标本上,加盖玻片或封口膜在暗湿盒中反应37℃×30min。
11.PBS漂洗3次;
12.在组织处加50~100μlDAB底物,反应15~25℃×10min;
13.PBS漂洗3次;
14.拍照后再用苏木素或甲基绿复染,几秒后立即用自来水冲洗。
梯度酒精脱水、二甲苯透明、中性树胶封片。
15.加一滴PBS或甘油在视野下,用光学显微镜观察凋亡细胞(共计200~500个细胞)并拍照。
可结合凋亡细胞形态特征来综合判断(未染色细胞变小,胞膜完整但出现发泡现象,晚期出现凋亡小体,贴壁细胞出现邹缩、变圆、脱落;而染色细胞呈现染色质浓缩、边缘化,核膜裂解,染色质分割成块状/凋亡小体)
四、注意事项
1.进行PBS 清洗时,每次清洗5 min。
2.PBS清洗后,为了各种反应的有效进行,请尽量除去PBS 溶液后再进行下一步反应。
3.在载玻片上的样本上加上实验用反应液后,请盖上盖玻片或保鲜膜,或在湿盒中进行,这样可以使反应液均匀分布于样本整体,又
可以防止反应液干燥造成实验失败。
4.TUNEL反应液临用前配制,短时间在冰上保存。
不宜长期保存,长期保存会导酶活性的失活。
5.如果20×DAB 溶液颜色变深成为紫色,则不可使用,需重新配制。
6.用甲基绿(Methyl Green)染液(3-5%甲基绿溶于0.1M 醋酸巴比妥PH4.0)染色后,请用灭菌蒸馏水清洗多余的甲基绿。
然后进行洗净(100%乙醇)、脱水(二甲苯)透明、封片后通过光学显微镜观察操作。
如果此时使用80~90%的乙醇洗净时,甲基绿比较容易脱色,注意快速进行脱水操作。
7.荧光素标记的dUTP液含甲次砷酸盐和二氯钴等致癌物,可通过吸入、口服等途径进入机体,注意防护。
8.试剂保存;未打开的试剂盒贮存在-20℃(-15~25℃);converter -POD液一旦解冻,以后就保存在4℃(2~8℃)下,至少在6 m内稳定,避免再次冻存;TUNEL反应液临用前配好后,放至冰上直至使用。
9.结果分析时注意:在坏死的晚期阶段或在高度增殖/代谢的组织细胞中可产生大量DNA片断,从而引起假阳性结果;而有些类型的凋亡性细胞死亡缺乏DNA断裂或DNA裂解不完全,以及细胞外的矩阵成分阻止TdT进入胞内反应,进而产生假阴性结果。
