常用制药GMP英文词汇
GMP词汇常规翻译

GMP词汇一更衣室 Changing Room一更 First Changing Room手消室 Hands Disinfection Room气闸室 Airlock Room洁具室 Cleaning Tools Room清洗室 Cleaning Room模具室 Dies Room内包装室Immediate Package Room安全门 Emergency Door外包清室Outer Package Removing Room 存料间 Storage Room of Raw Materials 粉碎室 Pulverizing Room备料室 Materials Preparing Room硬胶室 Hard Capsules Filling Room软胶室 Soft Capsules Room制粒干燥室 Granulating and Drying Room 总混间 Blending Room中间站 Intermediate Station压片室 Tablets Room Compression Room 包衣室 Coating Room配浆间 Coating Mixture Preparing Room 铝塑包装间 Packing Room传递窗 Transferring Window外包装室Outer Packing Room蒸馏水室Water Purifying Room质检室 Quality Control Room浓配室 Concentrated Solution Room稀配室 Diluted Solution Room BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部药品制备preparation of drug products针对 pertain to人用生物制品 biological products for human use补充 supplement代替条例supersede the requlation提议免除proposed exemption一般销售和消费 ordinarily marketed and consumed美德,优点,效力 virtue联邦注册表 FR/federal register人用物品 human foods用于鉴别,测定 shall be applied in determining生产,加工,包装,贮存 manufacture,process,pack,hold,职责和权力responsibility and authority批准和拒收 approve or reject/withhold复查生产记录review production records对qc来说可以获得的shall be available to QC适当的 adequate效价和含量 strength提供证明文件 shall be documented符合 conform to制订完善 sound每装货量 each shipment变质的is subject to deteriorationaccommodation (车,船,飞机等的)预定铺位批号 batch有害微生物 objectionable microorganism联合批号 the number of units准确性,灵敏性,特异性,重复性accuracy, sensitivity, specificity, and reproducibility特征,属性attribute配伍reconstitution设计 project顺势治疗药品 homeopathic drug products可配伍性compatibility标明 purporting to应成文并遵循shall be in writing and shall be followed.可检出水平 detectable levels通过参考文献具体化 incorporated by reference副作用 adeverse/side effect类型 type混放 mixups签收 receipt处理 disposition正压下过滤 be filtered under positive pressure层流或非层流laminar or nonlaminar;无菌环境aseptic conditions照明 lighting通风、空气过滤、空气加热与冷却 Ventilation, air filtration, air heating and cooling.空气输送系统 Air-handling systems附近建筑物immediate premises专用毛巾 single-service towels 进料 Charge-in标示量或规定量labeled or established amount实际产量 Actual yields理论产量 theoretical yields生产周期 the production of a batch of a drug product药品的一致性和完整性uniformity and integrity of drug products崩解时间 Disintegration time溶液的澄明度、溶解完全性及pH值 Clarity, completeness, or pH of solutions稳定性评估 variability estimates装卸 handlingNIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROVAL:加速批准FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION ):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所及的设备、生产过程或物品。
制药行业GMP英文词汇

Approve 批准Artwork 药品标签Authorized Person,AQ WHO关于质量受权人Bacteriostatic Water for Injection 抑菌注射用水Batch-based production 按批次生产Blending 混合Blending batches 混批Calibration 校验Calibration 校准Campaign-based production 阶段性生产Checked 校验Cleanance or site cleaning 清场Cleaning 清洁Cleaning Validation 清洁验证Clinical Trials 临床研究Contamination 污染Contamination Control 污染控制Continuous production 连续生产Contract manufacturing 委托生产Contract Analysis 委托检验Cool Storage 阴凉储存Critical Deviation 关键偏差Critical Process Parameter 关键工艺参数Critical Processing Step 关键操作步骤Cross contamination 交叉污染Design qualification, DQ 设计确认Deviation 偏差Drinking Water 饮用水Dry Place 干燥储存education 个人学历Equipment logbook 设备使用日志Excessive heat 过热Expected Yield, expected 预期收率experience 工作经验Expiry Date 有效期Factory Acceptance Test,FAT 供应商工厂的验收测试Freezer Storage 冷冻储存Holding Time 贮存期I:Implemente 执行Impurity profile 杂质概况In-process Controls 过程控制In-process Sampling 过程取样Installation qualification, IQ 安装确认Intermediate 中间体Logbook 使用日志Maintenance Basic Practice 维护基本实践Maintenance Best Practice 维护最佳实践Maintenance Good Practice 维护良好实践Maintenance Plan 维护计划Maintenance Program 维护管理程序Manufacture 制造Master Cell Bank , MCB 主细胞库mix-ups 混淆Non-conformance 不合格Operation qualification, OQ 运行确认Out of Specification , OOS 超标Performance qualification, PQ 性能确认Preliminary Cell Bank ,PCB 原始细胞库Preventive Maintenance 预防性维护Production 生产Production Operations 生产操作Purified Water 纯化水Qaultiy Assurance,QA 质量保证Qualification 确认Qualified Person,QP 质量受权人Quality Agreement 质量协议Quality Control,QC 质量控制Quality Management,QM 质量管理Quality review 质量审核Quality Unit,QU/Quality Operations,QO质量管理部门Responsible 负责Rechecked 复验Reconciliation 物料平衡Refrigerator Storage 冷藏储存Reject 拒收Retest dates 复验期Risk Assessment 风险评估Room Temperature Storage 室温储存Safety Environment Health, EHS 环境、健康及安全Semi-continuous production 半连续生产Site Acceptance Test,SAT 用户工厂的验收测试Specification 质量标准Stability 稳定性Sterile Purified Water 灭菌纯化水Sterile Water for Inhalation 灭菌吸入用水Sterile Water for Injection 灭菌注射用水Sterile Water for Irrigation 灭菌冲洗用水Subdividing Operation 分装操作Tamper Evidence 防篡改封签Time Limits 生产时限training 培训Update Batch Production Record, BPR 批记录User Requirement Specification, URS 用户需求标准Validation 验证Validation master plan 验证主计划Verification 复核Verification 检定Water for Injection 注射用水Working Cell Bank , WCB 工作细胞库Worst Case 最差情况Yield 收率Yield , actual 实际收率Signature (signed) 签名CIP 在线清洗SIP 在线灭菌消毒MAINTENANCE 维护保养。
GMP常见英文缩写

GMP常见英文缩写AQAI(Automated Quality Assurance Inspection Equipment):在线自动质量保证检查设备API(Active Pharmaceutical Ingredient):活性药物物质,即原料药ANDA(Abbreviated New Drug Application):简化新药申请ADR(Adverse Drug Reaction):不良反应BSE(Bovine Spongiform Encephalopathy):疯牛病BPCS(Business Planning and Control System):业务计划及控制系统BIA(Business impact assessment):商业影响评估cGMP(current Good Manufacturing Practice):现行药品生产质量管理规范CCCD(China Certification Committee for Drugs):中国药品认证委员会CIP(Cleaning In Place):在线清洁CV(Concurrent Validation):同步验证CDER(Center for Drug Evaluation and Research):药品研究与评价中心COA(Certificate Of Analysis):分析报告单CFR(Code of Federal Regulation):(美国)联邦法规CDC(Centers for Disease Control and Prevention):疾病预防控制中心COS/CEP(Certificate of Suitability for European Pharmacopeia):欧洲药典适用性证书CCD(Certification Committee for Drugs):药品认证管理中心CPMP(Committee for Proprietary Medicinal Products):欧洲专利药品委员会CTD(Common Technical Document):通用技术文件CDC(Centers for Disease Control and Prevention):疾病预防控制中心GMP(Good Manufacturing Practice):药品生产质量管理规范ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药品注册技术要求国际协调会EU(European Union):欧洲联盟EFPIA(European Federation of Pharmaceutical Industries Associations):欧洲制药工业协会联合会MHW(Ministry of Health and Welfare,Japan):日本厚生省JPMA(Japan Pharmaceutical Manufacturers Association):日本制药工业协会FDA(US Food and Drug Adminiistration):美国食品与药品管理局PRMA(Pharmaceutical Research and Manufacturers of America):美国药物研究和生产联合会WHO(World Health Organization):世界卫生组织IFPMA(International Federation of Pharmaceutical Manufacturers Associations):国际制药工业协会联合会TQC(Total Quality Control),TQM(Total Quality Management):全面质量管理PDCA(Plan,Do,Check,Action):计划,执行,检查,处理QA(Quality Assurance):质量保证QC(Quality Control):质量控制QS(Quality System):质量体系QM(Quality Management):质量管理SOP(Standard Operating Procedure):标准操作规程SMP(Standard Management Procedure):标准管理程序SOR(Standard Operating Record):标准操作记录GEP(Good Engineering Practice):工程设计规范HV AC(Heating Ventilation and Air Conditioning):空调净化系统DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认OOS(Out-Of-Specification):检验不合格;超标PFDS(Process Flow Diagrams):工艺流程图MRA(cMutual Reognition Agreements):现场检查多边认同协议DMF(Drug Master File):EDMF(European Drug Master File)欧盟药物主文件EDQM(European Directorate for Quality Medicines):欧洲药品质量管理局ORA(Office of Regulatory Affairs):药政事务办公室GGPs(Good Guidance Practices):优良指南规范MOA(Method Of Analysis):分析方法VMP(Validation Master Plan):验证主计划VP(Validation Protocol):验证方案MSDS(Material Safety Data Sheet):物料安全技术说明书NDA(New Drug Application):新药申请OTC(Over-the-counter):非处方INN(International Nonproprietary Name)国际非专有名称USP(the united state pharmacopeia):美国药典NF(National Formulary):(美国)国家药品集GAP(Good Agricultural Practice):中药材种植管理规范GCP(Good Clinical Practice):药物临床试验质量管理规范GLP(Good Laboratory Practice):药物实验室管理规范GSP(Good Supply Practice):药品经营质量管理规范GUP(Good Use Practice):药品使用质量管理规范SM(Starting Material):起始物料PMF(Plant Master File);SMF(Site Master File):工厂主文件EDL(List of Essential Drugs):基本药物目录PI(Package Insert):说明书PCT(Patent Cooperation Treaty):专利合作条约PPAC(Patent Protection Association of China):中国专利保护协会PIC(Person In Charge):负责人PDS(Pharmaceutical Development Services):整体新药研发机构SPC(Summary of Product Characteristics):产品特性摘要。
制药行业GMP英文词汇

制药行业GMP英文词汇Approve 批准Artwork 药品标签Authorized Person,AQ WHO关于质量受权人Bacteriostatic Water for Injection 抑菌注射用水Batch-based production 按批次生产Blending 混合Blending batches 混批Calibration 校验Calibration 校准Campaign-based production 阶段性生产Checked 校验Cleanance or site cleaning 清场Cleaning 清洁Cleaning Validation 清洁验证Clinical Trials 临床研究Contamination 污染Contamination Control 污染控制Continuous production 连续生产Contract manufacturing 委托生产Contract Analysis 委托检验Cool Storage 阴凉储存Critical Deviation 关键偏差Critical Process Parameter 关键工艺参数Critical Processing Step 关键操作步骤Cross contamination 交叉污染Design qualification, DQ 设计确认Deviation 偏差Drinking Water 饮用水Dry Place 干燥储存education 个人学历Equipment logbook 设备使用日志Excessive heat 过热Expected Yield, expected 预期收率experience 工作经验Expiry Date 有效期Factory Acceptance Test,FAT 供应商工厂的验收测试Freezer Storage 冷冻储存Holding Time 贮存期I:Implemente 执行Impurity profile 杂质概况In-process Controls 过程控制In-process Sampling 过程取样Installation qualification, IQ 安装确认Intermediate 中间体Logbook 使用日志Maintenance Basic Practice 维护基本实践Maintenance Best Practice 维护最佳实践Maintenance Good Practice 维护良好实践Maintenance Plan 维护计划Maintenance Program 维护管理程序Manufacture 制造Master Cell Bank , MCB 主细胞库mix-ups 混淆Non-conformance 不合格Operation qualification, OQ 运行确认Out of Specification , OOS 超标Performance qualification, PQ 性能确认Preliminary Cell Bank ,PCB 原始细胞库Preventive Maintenance 预防性维护Production 生产Production Operations 生产操作Purified Water 纯化水Qaultiy Assurance,QA 质量保证Qualification 确认Qualified Person,QP 质量受权人Quality Agreement 质量协议Quality Control,QC 质量控制Quality Management,QM 质量管理Quality review 质量审核Quality Unit,QU/Quality Operations,QO质量管理部门Responsible 负责Rechecked 复验Reconciliation 物料平衡Refrigerator Storage 冷藏储存Reject 拒收Retest dates 复验期Risk Assessment 风险评估Room Temperature Storage 室温储存Safety Environment Health, EHS 环境、健康及安全Semi-continuous production 半连续生产Site Acceptance Test,SAT 用户工厂的验收测试Specification 质量标准Stability 稳定性Sterile Purified Water 灭菌纯化水Sterile Water for Inhalation 灭菌吸入用水Sterile Water for Injection 灭菌注射用水Sterile Water for Irrigation 灭菌冲洗用水Subdividing Operation 分装操作Tamper Evidence 防篡改封签Time Limits 生产时限training 培训Update Batch Production Record, BPR 批记录User Requirement Specification, URS 用户需求标准Validation 验证Validation master plan 验证主计划Verification 复核Verification 检定Water for Injection 注射用水Working Cell Bank , WCB 工作细胞库Worst Case 最差情况Yield 收率Yield , actual 实际收率Signature (signed) 签名CIP 在线清洗SIP 在线灭菌消毒MAINTENANCE 维护保养。
常用制药及GMP英文缩写

ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW(Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(Certificate of Suitability to the monographs of European Pharmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4 :药典ICH-Q4A :药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7 :原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8 :药物研发指南ICH-Q9 :质量风险管理ICH- Q10(PQS):药物质量体系QA(Quality Assurance):质量保证QC(Quality Control):质量控制QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SAL(Sterility Assurance Level):无菌保证水平灭菌后微生物的存活概率的负对数,要求≥6D值:杀灭90%的微生物所需要的时间,D值越大,微生物死亡越难,D值与细菌的耐热性成正比Z值:指灭菌时间减少到原来的10%所需要升高的温度或是相同的灭菌时间内杀死99%的微生物所需要提高的温度F值:为一定温度下,给定Z值所产生的灭局效果与参比温度T0下给定Z值所产生的灭菌效果相同时所相当的时间F值用于干热灭菌F0值:为一定温度下,Z值为10℃产生的灭菌效果与120℃,Z值为10℃时产生的灭菌效果相当的时间,t分钟内的灭菌效果相当于120℃下灭菌F0分钟的效果F0被称为标准灭菌时间,用于热压灭菌LRV:除菌过滤的对数下降值LRV=lgN0-lgNSOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药。
常用GMP英文词汇

国际组织ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human U se):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构) PDA(Parenteral Drug Association):美国注射剂协会 EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW (Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门 D&B(Dun & Bradstreet):邓白氏公司DUNS(DataUniversal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到ATCC(American Type Culture Collection):美国模式培养物集存库 ASTM(American Society for Testing Materials):美国材料与试验协会法规GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的 GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范 GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范 GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范 GDP (Good Documentation Practice):良好文件管理 GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范 USP(united states pharmacopeia):美国药典 EP(European Pharmacopeia):欧洲药典 JP(Japanese Pharmacopoeia):日本药典 CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分 CEP/COS (Certificate of Suitability to the monographs of European Pharmacopoeia):欧洲药典适应性认证证书CEP认证,COS 证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件 EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点 REACH (REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH法规ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8:药物研发指南ICH-Q9:质量风险管理ICH- Q10(PQS):药物质量体系ICH-Q11:原料药研发与生产常见术语QA(Quality Assurance):质量保证QC(Quality Control):质量控制CQA(Critical Quality Attribute):关键质量属性QRM(Quality Risk Management):质量风险管理IPC(InproceicsQuality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FAT(Factory Acceptance Test):工厂验收测试SAT(Site Acceptance Test):现场验收测试FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality byDesign):质量源于设计PMC(Product Material Control):生产物料控制PC生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):年度质量回顾CNC(Controlled Non-Classified Area):受控非洁净区应用技术APS(Aseptic Processing Simulation):培养基模拟灌装CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌BFS(Blowing Filling and Sealing):吹-灌-封PA T(Process Analytical Technology):过程分析技术PLC(Programmable Logic Controller):可编程逻辑控制EDI(Electrodeionization):一种制备纯化水的离子交换技术MAC(Minimum Acceptable Cycle):最低可接受程序SAM(Steam-Air Mixture):蒸汽空气混合气体灭菌程序WIT(Water IntrusionTest):水侵入测试(东富龙疏水性滤器的在线进行完整性测试的方法) BP(Bubble Point Test):起跑点试验FF(Forward Flow/Diffusive Flow):前进流、扩散流试验HPLC(High Performance Liquid Chromatography):高效液相色谱GC(Gas Chromatography):气相色谱FTIR(Fourier Transform Infrared spectroscopy):傅氏转换红外线光谱分析仪MS(Mass Spectroscopy):质谱LC/MS:液质联用GC/MS:气质联用TOC(Total Organic Carbon):总有机碳NVR(NonvolatileResidue):不挥发残留物RFS(Ready for Sterilization):免洗胶塞RFU(Ready for Use):即用胶塞物品名称SVP(Small V olume Parenteral):小容量注射剂 LVP(Large Volume Parenteral):大容量注射剂 APA (Aseptic Processing Area):无菌区P&ID(Piping and Instrument Diagram):工艺管道仪表流程图 PFD(Process Flow Diagram):工艺流程图 UFD (Utility Flow Diagram):公用工程流程图HV AC(Heating Ventilation Air Conditioning):供热空气调节净化系统 HEPA(High Efficiency Particulate Air Filter):高效过滤器 FFU(Fan Filter Units):风机滤器单元 AHU(Air Handling Unit):空气处理单元COA(Certificate of Analysis):分析证书/检验报告书/检验报告单 BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药 WFI(Water for Injection):注射用水DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶 PAO:聚-α-烯烃,HEPA检漏用的气溶胶 IBC (IntermediateBulkContainer):中型散装容器 FBD(Fluid Bed Dryer):流化床IRTD(IntelligentResistance Temperature Detector):智能热电阻温度探头,标准温度探头 SV(Solenoid Valve):电磁阀FV:气动阀P/HG(Porous/Hard Goods Loads):多孔/坚硬装载,包括过滤器、胶塞、软管、拖把、工作服、塞子、清洁器具或设备的更换部件。
GMP英语词汇

GMP英语词汇GMP英语词汇2009-09-11 16:13GMP英语PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸 Authorized Person 授权人 Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品。
FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药GMP文件常见缩写ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPAEDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products)欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory Affairs European Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)GCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority (HSA)HSA’s Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for Harmonisation IDMC Independent Data-Monitoring Committee IEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary Name International Conference on Harmonisation (ICH) IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相認證同意MRFG Mutual Recognition Facilitation GroupMRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE)标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典。
GMP中英文单词对照表
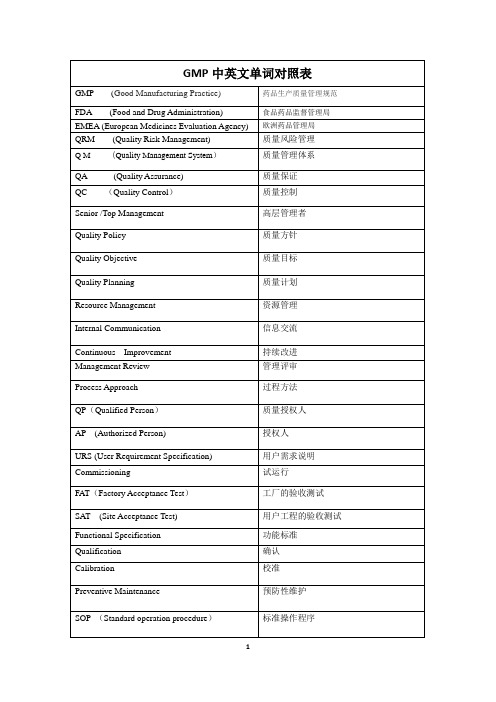
功能标准
Qualification
确认
Calibration
校准
Preventive Maintenance
预防性维护
SOP(Standard operation procedure)
标准操作程序
Retirement Management
退役管理
EquipmentLogbook
Remedial Action
矫正措施
OOS (Out of Specification)
偏差调查和实验室超标结果
PAR(Annual Product review)
产品年度回顾
Risk Identification
风险识别
Collect and Organize Information
收集和组织信息
可编程逻辑控制器
Metrology Confirmation
计量确认
Drinking water
饮用水
Purified water
纯化水
Sterile Purified water
灭菌纯化水
FDS(Functional Design Specification)
功能设计技术说明书
DDS(Detailed Design Specification)
质量目标
Quality Planning
质量计划
Resource Management
资源管理
Internal Communication
信息交流
Continuous Improvement
持续改进
Management Review
常用制药及GMP英文缩写

ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW (Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(C ertificate o f S uitability to the monographs of E uropean P harmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8:药物研发指南ICH-Q9:质量风险管理ICH- Q10(PQS):药物质量体系QA(Quality Assurance):质量保证QC(Quality Control):质量控制QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SAL(Sterility Assurance Level):无菌保证水平灭菌后微生物的存活概率的负−lgN0对数,要求≥6SAL=−lg存活率=F0DD值:杀灭90%的微生物所需要的时间,D值越大,微生物死亡越难,D值与细菌的耐热性成正比Z值:指灭菌时间减少到原来的10%所需要升高的温度或是相同的灭菌时间内杀死99%的微生物所需要提高的温度F值:为一定温度下,给定Z值所产生的灭局效果与参比温度T0下给定Z值所产生的灭菌效果相同时所相当的时间F值用于干热灭菌F0值:为一定温度下,Z值为10℃产生的灭菌效果与120℃,Z值为10℃时产生的灭菌效果相当的时间,t分钟内的灭菌效果相当于120℃下灭菌F0分钟的效果F0被称为标准灭菌时间,用于热压灭菌LRV:除菌过滤的对数下降值LRV=lgN0-lgNSOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药PMC(Product Material Control):生产物料控制PC生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):年度质量回顾KPI(Key Performance Indicators):关键业绩指标P&ID(Piping and Instrument Diagram):工艺管道仪表流程图PFD(Process Flow Diagram):工艺流程图UFD(Utility Flow Diagram):公用工程流程图CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌WFI(Water for Injection):注射用水HVAC(Heating Ventilation Air Conditioning):供热空气调节净化系统HEPA(High Efficiency Particulate Air Filter):高效过滤器DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶PAO:聚-α-烯烃,HEPA检漏用的气溶胶IBC(I ntermediate Bulk Container):中型散装容器BFS(Blowing Filling and Sealing):吹-灌-封PAT(Process Analytical Technology):过程分析技术PLC(Programmable Logic Controller):可编程逻辑控制CPP(Critical Process Parameters):关键工艺参数FBD(Fluid Bed Dryer):流化床AHU(Air Handling Unit):空气处理单元SAT(Site Acceptance Test):现场验收测试FAT(Factory Acceptance Test):工厂验收测试。
GMP常用名词-中英文对照

GMP常用名词-中英文对照Lot Number –批号见批号(Batch Number)Manufacture –制造物料的接收、原料药的生产、包装、重新包装、贴签、重新贴签、质量控制、放行、贮存和分发以及相关控制的所有操作。
Material –物料原料(起始物料,试剂,溶剂),工艺辅助用品,中间体,原料药,和包装及贴签材料的统称。
Mother Liquor –母液结晶或分离后剩下的残留液。
母液可能含有未反应的物料、中间体、不同级别的原料药和/或杂质。
它可用于进一步加工。
Packaging Material –包装材料在储运过程中保护中间体或原料药的任何物料。
Procedure –程序对要进行的操作、要采取的预防措施以及与原料药或中间体生产直接或间接相关的方法的描述文件。
Process Aids –工艺辅料除溶剂外,在原料药或中间体生产中起辅助作用、本身不参与化学或生物学反应的物料(例如,助滤剂、活性炭)。
Process Control –工艺控制见中间控制在原料药制备过程中,从接收原料,到工艺加工和原料药包装所涉及的所有操作。
Qualification –确认证明设备或辅助系统,安装正确、工作正常、确实产生预期的结果,并以文件佐证的行为。
确认是验证的一部分,但单独的确认步骤不构成工艺验证。
Quality Assurance (QA) –质量保证以确保所有原料药达到其应用所要求的质量,并维持质量体系为目的的全部组织安排的总和。
Quality Control –质量控制是否符合质量规格的检查或测试。
Quality Unit(s) –质量部门独立于生产部门的履行质量保证和质量控制职责的组织机构。
按照组织机构的大小和结构,可以是单独的QA 和QC部门,或个人,或小组。
Quarantine –待验在实物上或以其它有效方式将物料隔离,等待对其随后的批准或拒收做出决定的状态。
Raw Material –原料用来表示中间体或原料药的生产中要用的起始物料、试剂和溶剂的通用专业名词。
GMP制药专业英语词汇

目录1、product ['prɑdʌkt] (9)2、process [proˈsɛs;(for n.)ˈprɑsɛs] (10)3、manufacture ['mænjə'fæktʃɚ] (10)4、quality ['kwɑləti] (11)5、material [mə'tɪrɪəl] (12)6、medicinal[mɪ'dɪsənəl] (12)7、test [tɛst] (13)8、batch [bætʃ] (13)9、control [kən'trol] (14)10、record [(for v.) rɪˈkɔrd; (for n.) ˈrekərd] (15)11、system ['sɪstəm] (15)12、appropriate [əˈproprɪət;(for v.) əˈproprɪet] (16)13、sample ['sæmpl] (16)14、drug [drʌg] (16)15、equipment [ɪ'kwɪpmənt] (17)16、procedure [prə'sidʒɚ] (17)17、container [kən'tenɚ] (18)18、package ['pækɪdʒ] (18)19、requirement [rɪ'kwaɪɚmənt] (19)20、area ['ɛrɪə] (19)21、risk [rɪsk] (20)22、directive [daɪ'rɛktɪv] (20)23、operation [,ɑpə'reʃən] (21)24、contamination [kən,tæmɪ'neɪʃən] (22)25、part [pɑrt] (22)26、label [ˈlebəl] (23)27、validation [,vælə'deʃən] (23)28、cell [sɛl] (24)29、person ['pɝsn] (24)30、ensure [ɪn'ʃʊr] (25)31、release [rɪ'lis] (25)32、specification ['spɛsəfə'keʃən] (25)33、condition [kən'dɪʃən] (26)34、follow ['fɑlo] (26)35、storage ['stɔrɪdʒ] (26)36、provide [prə'vaɪd] (27)37、take [tek] (27)38、relevant ['rɛləvənt] (27)39、annex [əˈnɛks; æˈnɛks; (for n.) ˈænˌɛks] (28)40、fill [fɪl] (28)41、market ['mɑrkɪt] (29)42、perform [pɚ'fɔrm] (29)43、define [dɪ'faɪn] (29)44、number ['nʌmbɚ] (30)45、monitor ['mɔnɪtɚ] (30)46、component [kəm'ponənt] (31)47、design [dɪ'zaɪn] (31)48、write [raɪt] (32)49、change [tʃendʒ] (32)50、finish ['fɪnɪʃ] (33)51、reference ['rɛfrəns] (33)52、substance ['sʌbstəns] (34)53、authorisation [,ɔ:θəraɪ'zeɪʃən] (34)55、data ['detə] (35)56、accordance [ə'kɔrdns] (36)57、principle ['prɪnsəpl] (36)58、chapter ['tʃæptɚ] (36)59、maintain [men'ten] (36)60、date [det] (37)61、measure ['mɛʒɚ] (38)62、establish [ɪˈstæblɪʃ] (38)63、biology [baɪ'ɑlədʒi] (39)64、practice ['præktɪs] (39)65、manage ['mænɪdʒ] (40)66、article ['ɑrtɪkl] (41)67、active ['æktɪv] (41)68、site [saɪt] (42)69、standard ['stændɚd] (42)70、particular [pɚ'tɪkjəlɚ] (43)71、air [ɛr] (43)72、apply [ə'plaɪ] (44)73、case [kes] (44)74、trial ['traɪəl] (45)75、check [tʃɛk] (45)76、available [əˈveləbəl] (45)77、different [ˈdɪfrənt] (46)78、document [ˈdɑ:kjumənt] (46)79、environment [ɛnˈvaɪrənmənt] (47)80、base [bes] (48)81、limit [ˈlɪmɪt] (48)82、blood [blʌd] (49)83、describe [dɪˈskraɪb] (49)84、consist [kənˈsɪst] (50)85、place [ples] (50)86、little ['lɪtl] (51)87、subject [ˈsʌbdʒɪkt;(for adj.)ˈsʌbdʒɪkt;(for v.)səbˈdʒɛkt] (51)88、clean [klin] (52)89、information ['ɪnfɚ'meʃən] (53)90、section ['sɛkʃən] (53)91、approve [ə'prʊv] (54)92、facility [fə'sɪləti] (54)93、prevent [pri'vɛnt] (55)94、guide [gaɪd] (55)95、electronic [ɪˌlɛkˈtrɑnɪk] (56)96、source [sɔrs] (56)97、unit ['junɪt] (57)98、method ['mɛθəd] (57)99、gas [gæs] (58)100、sterile ['stɛrəl] (59)101、order ['ɔrdɚ] (59)102、organism ['ɔrɡənɪzəm] (60)103、stability [stə'bɪləti] (60)104、investigation [ɪn,vɛstɪ'ɡeʃən] (61)105、review [rɪ'vju] (62)106、code [kod] (62)107、plasma ['plæzmə] (63)108、handle ['hændl] (63)109、responsible [rɪ'spɑnsəbl] (63)110、transfer [træns'fɝ] (64)111、veterinary ['vɛtərənɛri] (64)112、qualify [ˈkwɑləˌfaɪ] (65)113、retention [rɪ'tɛnʃən] (65)114、stage [stedʒ] (66)115、grade [ɡred] (66)116、type [taɪp] (67)117、identify [aɪ'dɛntɪfaɪ] (67)118、intend [ɪn'tɛnd] (68)119、lot [lɑt] (69)120、action ['ækʃən] (69)121、certification [,sɝtɪfɪ'keʃən] (70)122、commission [kə'mɪʃən] (70)123、step [stɛp] (71)124、compliance [kəm'plaɪəns] (71)125、cross [krɔs] (72)126、possible ['pɑsəbl] (72)127、adequate [ˈædɪkwət] (72)128、plant [plænt] (73)129、cylinder [ˈsɪləndɚ] (73)130、level [ˈlɛvəl] (74)132、agent [ˈedʒənt] (75)133、involve [ɪnˈvɑ:lv] (75)134、report [rɪˈpɔ:rt] (75)135、instruction [ɪnˈstrʌkʃən] (76)136、justify [ˈdʒʌstəˌfaɪ] (76)137、laboratory [ˈlæbrətɔ:ri] (77)138、period [ˈpɪriəd] (77)139、closure [ˈkloʊʒə(r)] (78)140、train [tren] (78)141、determine [dɪˈtɜ:rmɪn] (79)142、clinical [ˈklɪnɪkəl] (79)143、mean [min] (80)144、assessment [əˈsɛsmənt] (80)145、clear [klɪr] (81)146、premise [ˈpremɪs] (81)147、regulation [ˌrɛɡjəˈleʃən] (82)148、set [sɛt] (82)149、distribution [ˌdɪstrəˈbjuʃən] (83)150、retain [rɪˈten] (83)151、account [əˈkaʊnt] (83)152、meet [mit] (84)153、pharmaceutical [ˌfɑ:rməˈsu:tɪkl] (84)154、herbal [ˈɜ:rbl] (85)155、individual [ˌɪndəˈvɪdʒuəl] (85)156、preparation [ˌprɛpəˈreʃən] (86)157、assure [əˈʃʊr] (86)158、criteria [kraɪˈtɪrɪə] (86)159、critical [ˈkrɪtɪkəl] (87)160、pressure [ˈprɛʃɚ] (87)161、competent [ˈkɑ:mpɪtənt] (88)162、size [saɪz] (88)163、supplier [səˈplaɪər] (89)164、volume [ˈvɑ:lju:m] (89)165、state [stet] (90)166、amend [əˈmɛnd] (90)167、programme [ˈproˌɡræm] (91)168、consideration [kənˌsɪdəˈreʃən] (91)169、deviation [ˌdiviˈeʃən] (92)170、vessel ['vɛsl] (92)171、parameter [pə'ræmɪtɚ] (93)172、intermediate [,ɪntɚ'midɪət] (93)173、sufficient [səˈfɪʃənt] (93)174、separate [(for v.) sɛpəˌret; (for adj.) sɛprɪt] (94)176、fraction ['frækʃən] (95)177、tissue ['tɪʃu] (96)178、recall ['rikɔl] (96)179、final ['faɪnl] (97)180、acceptance [ək'sɛptəns] (97)181、media ['miːdɪə] (98)182、cryogenic [,kraɪə'dʒɛnɪk] (98)183、detail [dɪˈtel] (99)184、prior ['praɪɚ] (99)185、status [ˈstetəs] (100)186、technical ['tɛknɪkl] (100)187、temperature [ˈtempərtʃʊər; tɛmpɚ-ˌtʃʊr] (101)188、collection [kə'lɛkʃən] (102)189、inspection [ɪn'spɛkʃən] (102)190、line [laɪn] (102)191、obtain [əb'ten] (103)192、study ['stʌdi] (103)193、affect [ə'fɛkt] (104)194、evaluation [ɪ,væljʊ'eʃən] (104)195、example [ɪg'zæmpl] (105)196、return [rɪ'tɝn] (105)197、significant [sɪɡ'nɪfɪkənt] (106)198、suitable [ˈsudəb(ə)l] (106)199、trace [tres] (107)200、concern [kən'sɝn] (107)201、culture ['kʌltʃɚ] (107)202、primary ['praɪmɛri] (108)203、regard [rɪ'ɡɑrd] (108)204、sponsor ['spɑnsɚ] (109)205、additional [ə'dɪʃənl] (109)206、aseptic [,e'sɛptɪk] (110)207、audit ['ɔdɪt] (111)208、conduct [kən'dʌkt] (111)209、filter ['fɪltɚ] (112)210、mix [mɪks] (112)211、normal ['nɔrml] (113)212、radiopharmaceutical [,redio,fɑrmə'sʊtɪkəl] (113)213、approach [ə'protʃ] (114)214、avoid [ə'vɔɪd] (114)215、confirm [kən'fɝm] (114)216、contract ['kɑntrækt] (115)217、derived [dɪ'raɪv] (115)218、modify ['mɑdɪfaɪ] (115)220、content ['kɑntɛnt] (116)221、equivalent [ɪ'kwɪvələnt] (117)222、health [hɛlθ] (117)223、quantity ['kwɑntəti] (118)224、room [rum] (118)225、organize ['ɔrgə,naɪz] (119)226、result [rɪ'zʌlt] (119)227、agree [ə'ɡri] (120)228、certain ['sɝtn] (120)229、impact [ɪm'pækt] (120)230、indicate ['ɪndɪket] (121)231、minimise ['minimaiz] (121)232、receive [rɪ'siv] (122)233、reject [rɪ'dʒɛkt] (122)234、arrangement [ə'rendʒmənt] (123)235、complete [kəm'plit] (123)236、correct [kə'rɛkt] (123)237、manner ['mænɚ] (124)238、point [pɔɪnt] (124)239、expiry [ɪk'spaɪəri] (125)240、ingredient [ɪn'ɡridɪənt] (125)241、defect [‘dɪfɛkt] (125)242、integrity [ɪn'tɛɡrəti] (126)243、water [ˈwɔtɚ] (126)244、dedicate ['dɛdɪket] (127)245、hold [hold] (127)246、adverse [ædˈvɚs, ˈædˌvɚs] (128)247、virus ['vaɪrəs] (128)248、animal ['ænɪml] (129)249、safe [sef] (129)250、dose [dos] (130)251、bulk [bʌlk] (130)252、contact ['kɑntækt] (131)253、microbiological ['maɪkrobaɪo'lɑdʒɪkl] (131)254、particle ['pɑrtɪkl] (132)255、verification [,vɛrɪfɪ'keʃən] (132)256、life [laɪf] (133)257、master ['mæstɚ] (133)258、present [prɪˈzɛnt;(for n.)ˈprɛznt] (134)259、protocol ['protə'kɔl] (134)260、radiation [,redɪ'eʃən] (134)261、remain [rɪ'men] (135)262、continuous [kən'tɪnjʊəs] (135)264、transport ['trænspɔrt] (136)265、valve [vælv] (137)266、analysis [ə'næləsɪs] (137)267、development [dɪ'vɛləpmənt] (138)268、device [dɪ'vaɪs] (139)269、initial [ɪ'nɪʃəl] (139)270、permit [pɚ'mɪt] (139)271、potential [pə'tɛnʃl] (140)272、seal [sil] (140)273、examination [ɪg'zæmə'neʃən] (141)274、file [faɪl] (141)275、nature ['netʃɚ] (142)276、plan [plæn] (142)277、address [əˈdrɛs;(for n)ˈædres; ædrɛs] (142)278、associate [ə'soʃɪet] (143)279、complaint [kəm'plent] (143)280、live [laɪv;lɪv] (144)281、routine [rʊ'tin] (144)282、seed [sid] (145)283、work [wɝk] (145)284、represent [,rɛprɪ'zɛnt] (146)285、cause [kɔz] (146)286、raw [rɔ] (147)287、party ['pɑrti] (147)288、shelf [ʃɛlf] (148)289、heat [hit] (148)290、cycle ['saɪkl] (148)291、vector ['vɛktɚ] (149)292、quarantine ['kwɔrən'tin] (149)293、maximum [ˈmæksəməm] (150)294、chemical ['kɛmɪkl] (150)295、stock [stɑk] (151)296、purity ['pjʊrəti] (151)297、computerise [kəm'pju:təraiz] (152)298、trend [trɛnd] (152)299、treatment ['tritmənt] (153)300、spatial [ˈspeʃəl] (153)301、department [dɪ'pɑrtmənt] (153)302、surface ['sɝfɪs] (154)303、physical ['fɪzɪkl] (154)304、load [lod] (155)305、head [hɛd] (155)306、connection [kə'nɛkʃən] (156)308、solution [sə'luʃən] (157)309、failure [eljɚ] (157)310、cloth [klɔθ] (158)311、chain [tʃen] (158)312、attribute [ə'trɪbjut] (159)313、key [kiː] (159)314、vial ['vaɪəl] (160)315、reserve [rɪ'zɝv] (160)316、space [spes] (161)317、particulate [pɚ'tɪkjə,let] (161)318、dry [draɪ] (162)319、visual ['vɪʒʊəl] (162)320、liquid ['lɪkwɪd] (163)321、indicator ['ɪndɪketɚ] (163)322、freeze [friz] (164)323、error ['ɛrɚ] (164)324、alternative [ɔl'tɝnətɪv] (165)325、wash [wɔʃ] (165)326、terminal [tɜ:mənl] (166)327、positive ['pɑzətɪv] (166)328、version ['vɝʒn] (166)329、formulation [,fɔrmjə'leʃən] (167)330、installation ['ɪnstə'leʃən] (168)331、waste [west] (168)332、reaction [rɪ'ækʃən] (169)333、bioburden [,baɪəʊ'bɜːdən] (169)334、humidity [hju'mɪdəti] (170)335、extraction [ɪk'strækʃən] (170)1、product ['prɑdʌkt]n. 产品;结果;[数] 乘积;作品production [prə'dʌkʃən]n. 成果;产品;生产;作品GMP applies to the lifecycle stages from the manufacture of investigational medicinal products, technology transfer, commercial manufacturing through to product discontinuation.(欧盟GMP附录第一章1.2)GMP的应用贯穿于生产临床研究用药品、技术转移、商业化生产直至产品退市的整个生命周期中。
GMP常用英语单词

常用中译英 系统 物料平衡 批 批号 批生产记录 文件 标准操作规程 生产工艺规程 工艺用水 纯化水 注射用水 状态标志 中间产品 理论产量 物料 待验 起始原料 洁净室(区) 待包品 成品 灭菌 控制点 质量监督 生产过程控制 退货 拒收 交叉污染 放行 质量要求 可追溯性 计量确认 人员净化室 物料净化室 悬浮粒子 洁净度 净化 传递箱 洁净服 洁净工作台 静态 动态 粗效过滤器 中效过滤器 高效过滤器 安装确认 运行确认 性能确认 工艺验证
GMP english words Abbreviated New drug Accelerated approval Adverse effcet Adverse reaction Agency ANDA(Abbreviated New drug application) Animal trial Archival copy Batch production records Batch production CFR(Code of federal regulation) Clinical trial COS/CEP Dietary supplement DMF(Drug master file) Drug substance Generic name ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human
第 2 页,共 2 页
有关物质 放行 残留溶剂 保留期限 留样 保留时间 回顾性验证 再验证 审核并批准 给药途径 环境卫生 报产报告 严重GMP缺陷 在线灭菌 氢氧化钠 比旋度 标准 稳定性数据 稳定性监控计划 状态 无菌原料药 消毒 连续批号 供应商 技术转化 微生物总数 可追踪的 验证文件集 验证总计划 验证报告
GMP常见英文缩写

GMP常见英文缩写AQAI(Automated?Quality?Assurance?Inspection?Equipment):在线自动质量保证检查设备API(Active?Pharmaceutical?Ingredient):活性药物物质,即原料药ANDA?(Abbreviated?New?Drug?Application):简化新药申请ADR(Adverse?Drug?Reaction):不良反应BSE(Bovine?Spongiform?Encephalopathy):疯牛病BPCS(Business?Planning?and?Control?System):业务计划及控制系统BIA(Business?impact?assessment):?商业影响评估cGMP(current?Good?Manufacturing?Practice):现行药品生产质量管理规范CCCD(China?Certification?Committee?for?Drugs):中国药品认证委员会CIP(Cleaning?In?Place):在线清洁CV(Concurrent?Validation):同步验证CDER(?Center?for?Drug?Evaluation?and?Research):?药品研究与评价中心COA(Certificate?Of?Analysis):分析报告单CFR(Code?of?Federal?Regulation):(美国)联邦法规?CDC(Centers?for?Disease?Control?and?Prevention):疾病预防控制中心COS/?CEP(?Certificate?of?Suitability?for?European?Pharmacopeia?):欧洲药典适用性证书CCD?(Certification?Committee?for?Drugs):药品认证管理中心CPMP(Committee?for?Proprietary?Medicinal?Products):?欧洲专利药品委员会CTD(Common?Technical?Document):通用技术文件CDC(?Centers?for?Disease?Control?and?Prevention):?疾病预防控制中心GMP(Good?Manufacturing?Practice):药品生产质量管理规范ICH(International?Conference?on?Harmonization?of?Technical?Requirements?for?Registrat ion?of?Pharmaceuticals?for?Human?Use):人用药品注册技术要求国际协调会EU(European?Union):欧洲联盟EFPIA(European?Federation?of?Pharmaceutical Industries?Associations):欧洲制药工业协会联合会MHW(Ministry?of?Health?and?Welfare,Japan):日本厚生省JPMA(Japan?Pharmaceutical?Manufacturers?Association):日本制药工业协会FDA(US?Food?and?Drug?Adminiistration):美国食品与药品管理局PRMA(Pharmaceutical?Research?and?Manufacturers?of?America):美国药物研究和生产联合会WHO(World?Health?Organization):世界卫生组织IFPMA(International? Federation? of? Pharmaceutical? Manufacturers?Associations):国际制药工业协会联合会TQC(Total?Quality?Control),TQM(Total?Quality?Management):?全面质量管理PDCA(Plan,Do,Check,Action):计划,执行,检查,处理QA(Quality?Assurance):质量保证QC?(Quality?Control):质量控制QS(Quality?System):质量体系QM(Quality?Management):?质量管理SOP(Standard?Operating?Procedure):?标准操作规程SMP(Standard?Management?Procedure):标准管理程序SOR(Standard?Operating?Record):?标准操作记录GEP(Good?Engineering?Practice):工程设计规范HV AC(Heating?Ventilation?and?Air?Conditioning):空调净化系统DQ(Design?Qualification):设计确认IQ(Installation?Qualification):安装确认OQ(Operational?Qualification):运行确认PQ(Performance?Qualification):性能确认OOS(Out-Of-Specification):检验不合格;超标PFDS(Process?Flow?Diagrams):工艺流程图MRA(cMutual?Reognition?Agreements):?现场检查多边认同协议DMF(?Drug?Master?File):EDMF(European?Drug?Master?File)欧盟药物主文件EDQM(European?Directorate?for?Quality?Medicines):?欧洲药品质量管理局ORA(Office?of?Regulatory?Affairs):药政事务办公室GGPs(?Good?Guidance?Practices):?优良指南规范MOA(Method?Of?Analysis):分析方法VMP(Validation?Master?Plan):验证主计划VP(Validation?Protocol):验证方案MSDS(Material?Safety?Data?Sheet):物料安全技术说明书NDA?(New?Drug?Application):新药申请OTC(Over-the-counter):非处方INN(International?Nonproprietary?Name)国际非专有名称USP(the?united?state?pharmacopeia):?美国药典NF(National?Formulary):(美国)国家药品集GAP(Good?Agricultural?Practice):中药材种植管理规范GCP(Good?Clinical?Practice):药物临床试验质量管理规范GLP(Good?Laboratory?Practice):药物实验室管理规范GSP(Good?Supply?Practice):药品经营质量管理规范GUP(Good?Use?Practice):药品使用质量管理规范SM(Starting?Material):起始物料PMF(Plant?Master?File);?SMF(Site?Master?File):工厂主文件EDL(List?of?Essential?Drugs?)?:?基本药物目录PI(Package?Insert):说明书PCT(?Patent?Cooperation?Treaty):?专利合作条约PPAC(Patent? Protection? Association ?of? China):中国专利保护协会PIC(?Person ?In? Charge)?:负责人PDS(Pharmaceutical? Development? Services):?整体新药研发机构SPC(Summary ?of? Product? Characteristics):产品特性摘要。
制药行业GMP英文词汇

制药行业G M P英文词汇标准化管理处编码[BBX968T-XBB8968-NNJ668-MM9N]Approve 批准Artwork 药品标签Authorized Person,AQ WHO关于质量受权人Bacteriostatic Water for Injection 抑菌注射用水Batch-based production 按批次生产Blending 混合Blending batches 混批Calibration 校验Calibration 校准Campaign-based production 阶段性生产Checked 校验Cleanance or site cleaning 清场Cleaning 清洁Cleaning Validation 清洁验证Clinical Trials 临床研究Contamination 污染Contamination Control 污染控制Continuous production 连续生产Contract manufacturing 委托生产Contract Analysis 委托检验Cool Storage 阴凉储存Critical Deviation 关键偏差Critical Process Parameter 关键工艺参数Critical Processing Step 关键操作步骤Cross contamination 交叉污染Design qualification, DQ 设计确认Deviation 偏差Drinking Water 饮用水Dry Place 干燥储存education 个人学历Equipment logbook 设备使用日志Excessive heat 过热Expected Yield, expected 预期收率experience 工作经验Expiry Date 有效期Factory Acceptance Test,FAT 供应商工厂的验收测试Freezer Storage 冷冻储存Holding Time 贮存期I:Implemente 执行Impurity profile 杂质概况In-process Controls 过程控制In-process Sampling 过程取样Installation qualification, IQ 安装确认Intermediate 中间体Logbook 使用日志Maintenance Basic Practice 维护基本实践Maintenance Best Practice 维护最佳实践Maintenance Good Practice 维护良好实践Maintenance Plan 维护计划Maintenance Program 维护管理程序Manufacture 制造Master Cell Bank , MCB 主细胞库mix-ups 混淆Non-conformance 不合格Operation qualification, OQ 运行确认Out of Specification , OOS 超标Performance qualification, PQ 性能确认Preliminary Cell Bank ,PCB 原始细胞库Preventive Maintenance 预防性维护Production 生产Production Operations 生产操作Purified Water 纯化水Qaultiy Assurance,QA 质量保证Qualification 确认Qualified Person,QP 质量受权人Quality Agreement 质量协议Quality Control,QC 质量控制Quality Management,QM 质量管理Quality review 质量审核Quality Unit,QU/Quality Operations,QO质量管理部门Responsible 负责Rechecked 复验Reconciliation 物料平衡Refrigerator Storage 冷藏储存Reject 拒收Retest dates 复验期Risk Assessment 风险评估Room Temperature Storage 室温储存Safety Environment Health, EHS 环境、健康及安全Semi-continuous production 半连续生产Site Acceptance Test,SAT 用户工厂的验收测试Specification 质量标准Stability 稳定性Sterile Purified Water 灭菌纯化水Sterile Water for Inhalation 灭菌吸入用水Sterile Water for Injection 灭菌注射用水Sterile Water for Irrigation 灭菌冲洗用水Subdividing Operation 分装操作Tamper Evidence 防篡改封签Time Limits 生产时限training 培训Update Batch Production Record, BPR 批记录User Requirement Specification, URS 用户需求标准Validation 验证Validation master plan 验证主计划Verification 复核Verification 检定Water for Injection 注射用水Working Cell Bank , WCB 工作细胞库Worst Case 最差情况Yield 收率Yield , actual 实际收率Signature (signed) 签名CIP 在线清洗SIP 在线灭菌消毒MAINTENANCE 维护保养。
GMP英文单词

GMP英文单词
6GP:GMP、GSP、GLP、GCP、GAP、GUP GMP:药品生产质量管理规范
GSP:药品经营质量管理规范
GLP:药品非临床研究质量管理规范GCP: 药品临床试验管理规范
GAP:中药材生产质量管理规范
GUP:医疗机构药剂质量管理规范
ICH:国际协调协商会议
AIP:原料药(活性药物成分)
cGMP:动态药品生产管理规范
Q7A:原料药的优良制造规范指南
QA:质量保证
QC:质量控制
QM:质量管理
QP:质量授权人
CAPA:纠正和预防
DO:溶氧量
SOP:标准操作规程
Change Control:变更控制
Deviation:偏差
OOS:检验结果偏差
OOT:超趋势结果OOE:非期望结果
HV AC:空气调节系统HEPA:高效空气过滤器PW:纯化水
WFI:注射用水
SIP:在线灭菌
CIP:在线清洗Validation:验证Qualification:确认DQ:设计确认
IQ:安装确认
OQ:运行确认
PQ:性能确认
PV:产品验证
STP标准技术规程SMP:标准管理规程BPR:批生产记录Batch No:批号
MFG date:生产日期self inspection:自检EXP date:失效期。
317个制药行业的英语词汇

制药行业的英语词汇制药行业常用英语词汇(中英对照)1、药品生产质量管理规范GMP:Good ManufacturingPractice2、国家食品与药品监督管理局State Food andDrug Administration3、总则GeneralProvisions4、《中华人民共和国药品管理法》the DrugAdministration Law of the People's Republic of China5、制剂Preparation6、原料药API: Active PharmaceuticalIngredient7、成品finished goods8、工序process9、机构与人员organization and personnel10、专业知识professional knowledge11、生产经验production experience12、组织能力organizational skill13、技术人员technical staff14、实施implementation15、药品生产pharmaceutical manufacturing 16、质量管理quality management17、质量检验quality inspection18、专业技术培训professional and technicaltraining 19、基础理论知识basic theoreticalknowledge20、实际操作技能practical operationskills 21、高生物活性highly potent22、高毒性high toxicity23、污染contamination24、考核评估assessment25、厂房与设施buildings and facilities 26、生产环境production environment 27、空气洁净级别clean air level28、昆虫insect29、洁净室(区)clean room(area)30、光滑smooth31、无裂缝no cracks32、无颗粒物脱落no particle shedding 33、耐受endure34、消毒disinfection35、无菌sterile36、交界处junction, joint37、弧形arc38、灰尘积聚dues accumulation 39、储存区store area40、生产规模production scale 41、设备equipment42、物料material43、中间产品intermediate product 44、待验品quarantined material 45、交叉污染cross-contamination 46、管道pipeline, ductwork47、风口tuber48、公用设施, 公用工程utilities of publicservice 49、照明lighting50、照度illumination。
常用制药GMP英文词汇

国际组织ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW(Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到ATCC(American Type Culture Collection):美国模式培养物集存库ASTM(American Society for Testing Materials):美国材料与试验协会法规GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(C ertificate o f S uitability to the monographs of E uropean P harmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH法规ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8:药物研发指南ICH-Q9:质量风险管理ICH- Q10(PQS):药物质量体系ICH-Q11:原料药研发与生产常见术语QA(Quality Assurance):质量保证QC(Quality Control):质量控制CQA(Critical Quality Attribute):关键质量属性QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FAT(Factory Acceptance Test):工厂验收测试SAT(Site Acceptance Test):现场验收测试FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计PMC(Product Material Control):生产物料控制PC生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):年度质量回顾CNC(Controlled Non-Classified Area):受控非洁净区应用技术APS(Aseptic Processing Simulation):培养基模拟灌装CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌BFS(Blowing Filling and Sealing):吹-灌-封PAT(Process Analytical Technology):过程分析技术PLC(Programmable Logic Controller):可编程逻辑控制EDI(Electrodeionization):一种制备纯化水的离子交换技术MAC(Minimum Acceptable Cycle):最低可接受程序SAM(Steam-Air Mixture):蒸汽空气混合气体灭菌程序WIT(Water Intrusion Test):水侵入测试(东富龙疏水性滤器的在线进行完整性测试的方法)BP(Bubble Point Test):起跑点试验FF(Forward Flow/Diffusive Flow):前进流、扩散流试验HPLC(High Performance Liquid Chromatography):高效液相色谱GC(Gas Chromatography):气相色谱FTIR(Fourier Transform Infrared spectroscopy):傅氏转换红外线光谱分析仪MS(Mass Spectroscopy):质谱LC/MS:液质联用GC/MS:气质联用TOC(Total Organic Carbon):总有机碳NVR(Nonvolatile Residue):不挥发残留物RFS(Ready for Sterilization):免洗胶塞RFU(Ready for Use):即用胶塞物品名称SVP(Small Volume Parenteral):小容量注射剂LVP(Large Volume Parenteral):大容量注射剂APA(Aseptic Processing Area):无菌区P&ID(Piping and Instrument Diagram):工艺管道仪表流程图PFD(Process Flow Diagram):工艺流程图UFD(Utility Flow Diagram):公用工程流程图HVAC(Heating Ventilation Air Conditioning):供热空气调节净化系统HEPA(High Efficiency Particulate Air Filter):高效过滤器FFU(Fan Filter Units):风机滤器单元AHU(Air Handling Unit):空气处理单元COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药WFI(Water for Injection):注射用水DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶PAO:聚-α-烯烃,HEPA检漏用的气溶胶IBC(I ntermediate Bulk Container):中型散装容器FBD(Fluid Bed Dryer):流化床IRTD(Intelligent Resistance Temperature Detector):智能热电阻温度探头,标准温度探头SV(Solenoid Valve):电磁阀FV:气动阀P/HG(Porous/Hard Goods Loads):多孔/坚硬装载,包括过滤器、胶塞、软管、拖把、工作服、塞子、清洁器具或设备的更换部件。
GMP常见英文缩写

GMP常见英文缩写AQAI(Automated Quality Assurance Inspection Equipment):在线自动质量保证检查设备API(Active Pharmaceutical Ingredient):活性药物物质,即原料药ANDA (Abbreviated New Drug Application):简化新药申请ADR(Adverse Drug Reaction):不良反应BSE(Bovine Spongiform Encephalopathy):疯牛病BPCS(Business Planning and Control System):业务计划及控制系统BIA(Business impact assessment): 商业影响评估cGMP(current Good Manufacturing Practice):现行药品生产质量管理规范CCCD(China Certification Committee for Drugs):中国药品认证委员会CIP(Cleaning In Place):在线清洁CV(Concurrent Validation):同步验证CDER( Center for Drug Evaluation and Research): 药品研究与评价中心COA(Certificate Of Analysis):分析报告单CFR(Code of Federal Regulation):(美国)联邦法规CDC(Centers for Disease Control and Prevention):疾病预防控制中心COS/ CEP( Certificate of Suitability for European Pharmacopeia ):欧洲药典适用性证书CCD (Certification Committee for Drugs):药品认证管理中心CPMP(Committee for Proprietary Medicinal Products): 欧洲专利药品委员会CTD(Common Technical Document):通用技术文件CDC( Centers for Disease Control and Prevention): 疾病预防控制中心GMP(Good Manufacturing Practice):药品生产质量管理规范ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药品注册技术要求国际协调会EU(European Union):欧洲联盟EFPIA(European Federation of Pharmaceutical Industries Associations):欧洲制药工业协会联合会MHW(Ministry of Health and Welfare,Japan):日本厚生省JPMA(Japan Pharmaceutical Manufacturers Association):日本制药工业协会FDA(US Food and Drug Adminiistration):美国食品与药品管理局PRMA(Pharmaceutical Research and Manufacturers of America):美国药物研究和生产联合会WHO(World Health Organization):世界卫生组织IFPMA(International Federation of Pharmaceutical Manufacturers Associations):国际制药工业协会联合会TQC(Total Quality Control),TQM(Total Quality Management): 全面质量管理PDCA(Plan,Do,Check,Action):计划,执行,检查,处理QA(Quality Assurance):质量保证QC (Quality Control):质量控制QS(Quality System):质量体系QM(Quality Management): 质量管理SOP(Standard Operating Procedure): 标准操作规程SMP(Standard Management Procedure):标准管理程序SOR(Standard Operating Record): 标准操作记录GEP(Good Engineering Practice):工程设计规范HV AC(Heating Ventilation and Air Conditioning):空调净化系统DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认OOS(Out-Of-Specification):检验不合格;超标PFDS(Process Flow Diagrams):工艺流程图MRA(cMutual Reognition Agreements): 现场检查多边认同协议DMF( Drug Master File):EDMF(European Drug Master File)欧盟药物主文件EDQM(European Directorate for Quality Medicines): 欧洲药品质量管理局ORA(Office of Regulatory Affairs):药政事务办公室GGPs( Good Guidance Practices): 优良指南规范MOA(Method Of Analysis):分析方法VMP(Validation Master Plan):验证主计划VP(Validation Protocol):验证方案MSDS(Material Safety Data Sheet):物料安全技术说明书NDA (New Drug Application):新药申请OTC(Over-the-counter):非处方INN(International Nonproprietary Name)国际非专有名称USP(the united state pharmacopeia): 美国药典NF(National Formulary):(美国)国家药品集GAP(Good Agricultural Practice):中药材种植管理规范GCP(Good Clinical Practice):药物临床试验质量管理规范GLP(Good Laboratory Practice):药物实验室管理规范GSP(Good Supply Practice):药品经营质量管理规范GUP(Good Use Practice):药品使用质量管理规范SM(Starting Material):起始物料PMF(Plant Master File); SMF(Site Master File):工厂主文件EDL(List of Essential Drugs ) : 基本药物目录PI(Package Insert):说明书PCT( Patent Cooperation Treaty): 专利合作条约PPAC(Patent Protection Association of China):中国专利保护协会PIC( Person In Charge) :负责人PDS(Pharmaceutical Development Services): 整体新药研发机构SPC(Summary of Product Characteristics):产品特性摘要。
制药工程常用英文缩写,缩略语

制药工程常用英文缩写,缩略语1GMP Good Manufacturing Practices药品生产质量管理规范2GxP各种药品规范的统称3GCP Good Clinical Practice药物临床试验质量管理规范4GLP Good Laboratory Practice药物非临床试验(实验室)质量管理规范5GSPGDPGood Supplg practiceGood Distribute Practice(美)药品经营质量管理规范6GDP Good Dossier practice申报资料质量管理规范7GPP Good Pharmacy practice药房质量管理规范8GQP Good Quality Practice 药品质量管理规范9GRP Good Rearch Practice药品研究质量管理规范10GUPGPPGood Use PracticeGood Preparation Practice(欧美)药品使用质量管理规范11GVP Good Validation Practice验证管理规范12GAP Good Agricultural Practice中药材生产质量管理规范13GEP Good Engineering Practice工程管理规范14GWP Good Warehousing Practice药品仓储规范15GMPC Good Manufacture Practice of Cosmetic Products 化学品生产质量管理规范16cGMP Current Good Manufacturing Practice现行药品生产质量管理规范17EU-GMP European –Good Manufacturing Practice欧洲GMP18CFR Code of Federal Regulations美国联邦法规19ChP Chinese Pharmacopoeia中国药典20USP United States Pharmacopoeia美国药典21EP European Pharmacopoeia欧洲药典22JP Japanese Pharmacopoeia日本药典23BP British Pharmacopoeia英国药典24IP Indian Pharmacopoeia印度药典25EN European Norm欧洲规范,欧洲标准26ANSI American National Standards Institute美国国家标准学会27ASME American Society of Mechanical Engineers美国机械工程师学会28ASTM American Society for Testing and Materials美国材料实验学会29ISPE International Society for Pharmaceutical Engineering 国际制药工程学会30WHO World Health Organization世界卫生组织31ISO International Standards Organization国际标准组织32EEC European Economic Community欧洲共同体、欧共体33EU European Union欧盟34ES European Commission欧洲委员会35CFDA China Food and Drug Administration中国食品和药品监督管理局36FDA Food and Drug Administration(美国)食品和药品管理局37MHRA Medicines & Healthcare Products Regulatory Agency(英国)药品和健康产品管理局38EHX Environment Health Safety环境、职业健康、安全管理体系39BPE Bioprocessing Equipment生物处理设备403A美国卫生行业协会、美国卫生论证标识41NBST National Bureau of Standards and Technology美国国家标准研究院42EMA European Medicines Agency欧洲药监局43EMEA European Agency for the evaluationof medicinal欧洲药品评价局44EDQM European Directorate for the Quality of Medicines 欧洲药品理事会45EQDM European Directorate for the Quality of Medicines & Healthcare欧洲药品与健康理事会46EHEDG European Hygienic Equipment Design Group欧洲卫生设备设计组织47ICH International Conference on Harmonization of TechnicalRequirements for Registration of Pharmaceuticals for Human 人用药物注册技术要求国际协调会议48IEC International Electrotechnical Commission国际电工委员会通用及组织49NEMA National Electrical Manufacturers Association美国电器制造商协会50CEP Certificate of Suitability for European Pharmacopeia欧洲药典适用性证书51CE Conformite Europeenne 欧洲电气安全论证52PIC/S Pharmaceutical Inspection ConventionPharmaceutical Inspection Cooperation Scheme国际医药品稽查协约组织53HHS United States Department of Health and Human Services美国卫生及公共服务部、美国卫生部54PDA Parenteral Drug Association(美国)注射剂协会55EPA Environmental Protection Agency(美国国家)环境保护局56CDER Center for Drug Evaluation and Research药物评价与研究中心57MHWMHLWMinistry of Health and WelfareMinistry of Health, Labor and Welfare(日本)厚生省(日本)厚生劳动省5821 CFR Title 21―Food and Drugs美国联邦法规,第21篇,食品与药品59Part11Electronic Records; Electronic Signatures第11节,电子记录与电子签名60Part210Current Good Manufacturing Practice in Manufacturing,Processing,Packing,or Holding of Drugs;General第210节,药品生产、加工、包装、储存质量规范部分61Part211Current Good Manufacturing Practice for Finished 第211节,制剂药物生产质量规范部分62Part314Applications for FDA Approval to Market a New Drug第314节,新药上市申请部分63Part320Bioavailability and Bioequivalence Requirements 第320节,生物利用度和等效性要求1QMS Quality Management System质量管理体系2QRS Quality Regulation System质量控制体系3QA Quality Assurance质量保证4QC Quality Control质量控制5QM Quality Management质量管理6QI Quality Inspection质量检验7QP Quality Plan质量计划8QRM Quality Risk Management质量风险管理9URS User Requirement Specification用户需求10DQ Design Qualification设计确认11IQ Installation Qualification安装确认12OQ Operational Qualification操作确认13PQ Performance Qualification性能确认14VIT Vendor Internal Test供应商内部测试15FAT Factory Acceptance Test工厂验收测试16SAT Site Acceptance Test现场验收测试17SOP Standard of Operation标准操作规程18FDS Functional and Design Specifications功能设计说明、功能设计规范19FS Functional Specifications功能说明20DS Design Specifications设计说明21TS Technical Specification技术说明、技术规范22RTM(TM)Requirement Traceability Matrix需求追溯矩阵23ITR Inspection T est Reports检查测试报告24QOR Quality Observation Report质量检查报告25QR Quality Requirements质量要求26QR Quality Records质量记录27RA Risk Assessment风险评估28SIA System Impact Assessment系统影响性评估29CCA CriticalComponents Assessment部件关键性评估30PV Process Validation工艺验证31CV Cleaning Validation清洁验证32CSV Computer System Validation计算机验证33VMP Validation Master Plan 验证主计划质量、验证34VP Validation Plan 验证计划35VP Validation Protocol验证方案36VR Validation Report验证报告37PVP Project Validation Plan项目验证计划38PVR Project Validation Report项目验证报告39QbD Quality by Design质量源于设计40DMF Drug Master File药品主文件、药物管理档案41FMEA Failure Mode and Effects Analysis失效模式和效果分析42SST System Suitability Test系统适应性测试43CAL Calibration校验、校准44CAPA Corrective Action and Preventive Action纠正预防措施45RCA Root Cause Analysis根本原因分析46ERES Electronic Record and Electronic Signature电子记录与电子签名47AQL Acceptable Quality Level可接受质量水平48CQA Critical Quality Attribut关键质量属性49CPP Critical Process Pararneter关键工艺参数50CTD Common T echnical Document通用技术文件51IA Impact Assessment影响评估52PQR Procut Quality Review产品质量回顾53COA Certification of Analysis分析合格证书、检验报告54BPR Batch Production Records批生产记录55BR Batch Records批记录56CC Change Control变更控制57DR Deviation Records偏差记录58COM Commissioning试车59BAR Batch Analysis Record批检验记录60PP Process Procedure工艺规程61OOS Out of Specification超出标准(限度)62LAL Limulus Smoebocyte Lysate鲎试剂63AQL Acceptable Quality Level可接受质量水平64SMF Site Master File工厂主文件65PM Preventive Maintenance预防性维修66QP Qualified Person质量授权人67R&D Research and Development研发部门68NDA New Drug Application新药申请电气及自控1GAMP Good Automated Manufacturing Practices设备自动化生产管理规范2HMI Human Machine Interface人机界面3OIT Operator Interface Terminals操作员界面终端4OIP Operator Interface Panel操作员界面面板5PLC Programmable Logic Controller可编程序控制器6PCS Process Control System过程控制系统7DCS Distributed Control System集散控制系统8PCS Process Control System工艺控制系统9DDC Direct Digital Controller直接数字控制器10IPC Industrial Personal Computer工业控制计算机,工控机11PAC Programmable Automation Controller可编程自动化控制器12PCC programmable computer controller可编程计算机控制器13MCU Microcontroller Unit单片机14CPU Central Process Unit中央处理器15PC Personal Computer个人电脑16SCADA Supervisory Control And Data Acquisition监控及数据采集17SDS Software design specification软件设计说明18HDS Hardware Design Specification硬件设计说明19FL Functional Logic功能逻辑说明20I/O Input / Output输入/输出21AI Analog Input模拟量输入22AO Analog Output模拟量输出23DI Digital Input数字量输入24DO Digital Output数字量输出25RTD Resistance Temperature Detector热电阻26T/C Thermocouple热电偶27RTU Remote Terminal Unit远程终端单元28ARS Automation Requirement Specification自动化需求规范29VFD Variable Frequency Drive变频驱动30EMC Electromagnetic Compatibility电磁兼容31UPS Uninterrupted Power supply不间断电源32EPS Emergency Power supply应急电源33FL Functional Logic功能逻辑说明34ER and Electronic Signature电子记录35ES Electronic Signature电子签名36AT Audit Trail审计踪迹37NO Normally Open常开38NC Normally Close常关39FO Fault Open故障开40FC Fault Close故障关41AC Alternating Current交流42DC Direct Current直流43PID Proportional Integral Derivative比例积分微分44LED Light Emitting Diode发光二极管45LCD Liquid Crystal Display液晶显示器46LIMS Laboratory Information Management System实验室信息管理系统 47LECP Laboratory Equipment Calibration Program 实验室仪器校准程序48WMS Warehouse Management System仓库管理系统49MES Manufacturing Execution System制造执行系统50ERP Enterprise Resource Planning企业资源计划其它1N/A Not Applicable不适用2NLT Not Less Than不少于3NMT Not More Than不多于4NB Nominal Bore公称管径5PED Pressure Equipment Directive压力设备指令(欧洲) 6PW Purified Water纯化水7WFI Water for Injections注射用水8PS Pure Steam纯蒸汽发生器9PWG PW Generator Unit纯化水制备机组10WFIG WFI Generator注射用水制备机组11MEWD Multi-effect Water Distillator 多效蒸馏水机12PSG PS Generator纯蒸汽发生器13PAC Poly Alumina Chlorine聚合氯化铝14DW Demineralized Water脱盐水,去离子水15MF Micro-Filter微滤16UF Ultra-Filter 超滤17NF Nano-Filter纳滤18MMF Multi-Media Filter多介质过滤器19ACF Activated Carbon Filter活性炭过滤器20SF Softener软化器21DG Degasifier脱气塔22RO Reverse Osmosis 反渗透23EDI Electrodeionization电法去离子24MB Mixed Bed混床25MDG Membrane Degasifier膜脱气26COP Clean out Place离线清洗27CEB Chemical Enhanced Backwash化学增强反冲洗28CIP Clean In Place在线清洗29SIP Sterilization in Place在线灭菌30POU Point Of Use使用点31PH Potential of Hydrogen酸碱度32TOC Total Organic Carbon总有机碳33ORP Oxidation-Reduction Potential氧化还原电位34COD Chemical Oxygen Demand化学耗氧量35BOD Biological Oxygen Demand生物耗氧量36SDI Silt Density Index污染密度指数37TUB Turbidity浊度38TSS Suspended Solid总悬浮固体39DO Dissoved Cxygn溶解氧40TDS Total dissolved solids总溶解固体41TH Total Hardness总硬度42PAT Process Analytical & Measurement T echnology过程分析技术43IRS Installation Requirement Specification安装要求说明44OEM Original Equipment Manufacturer 原始设备制造商45GDS General Design Specification总体设计说明46DDS Detailed Design Specification详细设计说明47PCP Preparation of Construction Plan施工组织设计48WMS Work Method Statement施工方案49BOQ Bill of Quantities工程量清单50BOM Bill of Material材料清单51P&ID Process and Instrumentation Diagram工艺与仪表流程图52PFD Process Flow Diagram工艺流程示意图53ANDA Abbreviation New Drug Application仿制药或仿制新药申请54OPQ Operational Personnel Qualification操作人员资格鉴定55MBT Microbiologic Test微生物测定56ADR Adverse Drug Reaction药物副作用报告,药品不良报告57OMM Operating and Maintenance Manual操作和维护保养手册58HACCP Hazard Analysis and Critical Control Point危害分析及关键环节控制点59CCP Critical Control Point关键环节控制点60IPC In Process Control过程控制61IPC Intermediate Production Control中间生产控制62CIPC Critical In-Process Control关键中间控制点63MBR Master Batch Record主生产批记录64PPM Parts Per Million百万分之一65OC Organizational Charts组织结构图66FIT Filter Integrity Test过滤器完整性测试67WIT Water Intergrity Test水侵入测试68GA General Arrangement总平面图69RPM Rotations per minute转/分70PD Prescription Drug处方药71Rx Receptor x处方药72NPD Nonprescription Drug非处方药73OTC Over The Counter非处方药74API Active Pharmaceutical Ingredient原料药、活性药75BPC Bulk Pharmaceutical Chemical原料药(原简称)76DS Drug Substance原料药77DP Drug Product成品药78RO Restriction orifice限流孔板79SG Sight Glass视镜80LG Lamp Glass,Light Glass灯镜81RD Rupture Disk爆破片材料1MOC Material Of Construction建造材质2SS Stainless Steel不锈钢3CI Cast iron铸铁4NCI Nodular east iron球墨铸铁5CS Carbon Steel碳钢6 C.Stl Cast Steel铸钢7 F.Stl Freezing Steel锻钢8PA Polyamide聚酰胺9PB Polybutylene聚丁烯10PC Polycarbonate聚碳酸酯11PE Polyethylene聚乙烯12PEX Cross-linked PolyEthylene交联聚乙烯13HDPE High-density polyethylene plastics高密度聚乙烯14MDPE Medium-density polyethylene plastics中密度聚乙烯15PO Polyolefin聚烯烃16PP Polypropylens聚丙烯17FRPP Polypropylens玻纤增强聚丙烯18PPR Polypropyla无规共聚聚丙烯19PPS PolyPhenylene Sulfide聚苯硫醚20PS Polystrene聚苯乙烯21PU Polyurethane,或者缩写为PUR聚氨酯22POM PolyOxyMethylene or Polyacetal聚甲醛,聚氧化亚甲基23HIPS High impact polystyrene高抗冲聚苯乙烯26PFA Polyfluoroalkoxy四氟乙烯—全氟烷氧基乙烯基醚共聚物27PTFE Polytetrafluoroethylene聚四氟乙烯28PVDF Poly vinylidene fluofide聚偏二氟乙烯29PVC Polyvinyl chloride聚氯乙烯30UPVC Unplasticised Polyvinyl Chloride硬聚氯乙烯,增强聚氯乙烯31CPVC Chlorinated polyvinyl chloride,或者缩写为PVCC氯化聚氯乙烯32PA Nylon,Polyamide尼龙,聚酰胺33PES PolyEtherSulfone聚醚砜,聚酯34AAS Acrylonirile butadiene styrene丙烯腈-丙烯酸酌-苯乙烯35ABS Acrylonitrile-Butadiene-Styrene丙烯腈-丁二烯-苯乙烯共聚物36ACS Acrylonitrile Chlorinated polyethylene Styrene丙烯胯-氯化聚乙烯-苯乙烯37ASB Asbestos石棉38PMMA Polymethel methacrylate聚甲基丙烯酸甲酯39SR Styrene-rubber苯乙烯橡胶24EPDM Ethylene Propylene Diene Monomer三元乙丙橡胶25EPM Ethylene Propylene Methylene乙丙橡胶,乙烯/丙烯共聚物40SR Silicone rubber硅橡胶40HTV High Temperature Vulcanization高温硫化(硅橡胶)40RTV Room Temperature Vulcanization室温硫化(硅橡胶)40MQ Silicone rubber甲基硅橡胶40VMQ Silicone rubber甲基乙烯基硅橡胶40PVMQ Silicone rubber甲基乙烯基苯基硅橡胶41FPMFKMFluororubberFluorocarbon Rubber氟橡胶42NBR Vulcanized nitrile rubber丁腈橡胶43FRP Glass Fibre Reinforced Plastic玻璃钢,玻璃纤维增强塑料1HVAC Heating Ventilation and Conditioning供热通风空调2AC Air Conditioner空调3AHU Air Handling Unit空气处理单元4BMS Building Monitoring System建筑管理系统、楼宇检测系统5CFU Colony Forming Unit菌落形成单位6CNC Controlled Non-Classified控制但未分级7FFU Fan Filter Unit风机过滤单元8FMS Factory Monitoring System车间监控系统9HEPA High Efficiency Particulate Air高效空气过滤器10LAF Laminar Air Flow层流、单向流11UDF Unidirectional Flow单向流12RABS Restricted Access Barrier Systems限制通过隔离系统13DP Differential Pressure压差14SDP Static Differential Pressure静压差15RH Relative Humidity相对湿度16CHWs Chilled Water (Supply)冷冻水(供给)17CWr Cooling Water (Return)冷却水(回流)18HW Hot Water热水19FS Factory Steam工厂蒸汽20SC Steam Condensate蒸汽冷凝水21WD Waste Drain废水排放22PWW Process Wastewater工艺污水23CA Compressed Air压缩空气24PA Process Air工艺压缩空气25IA Instrument Air仪表压缩空气26RW Raw Water原水27SW Soft Water软水28MW Middle Water中水29DW Domestic Water生活用水30CW City Water市政供水、自来水31DK Drinking Wat 饮用水32LPG Liquefied Petroleum Gas液化石油气33LNG Liquefied Natural Gas液化天然气34CNG Compressed natural gas压缩天然气35VE Visual Examination外观检查36UT Ultrasonic inspection Test超声探伤37RT Radiographic inspection Test射线探伤38MT Magnetic particle inspection Test磁粉探伤39PT liquid Penterant inspection Test液体渗透探伤40AutoclaveSterilizer灭菌柜公用工程41FBD Fluid Bed Dryer流化床42BFS Blowing Filling and Sealing吹灌封43HPLC High Pressure Liquid Chromatograph高效液相色谱44TLC Thin Layer Chromatograph薄层色谱45GC Gas Chromatograph气相色谱46UV Ultra-Violet紫外线47IR InfraRed红外线48RFQ Request for Quotations报价征询书49NPT American standard taper pipe thread美国标准锥管螺纹50NPS American standard straight pipe thread美国标准直管螺纹51NF American national fine thread美国标准细牙螺纹52NC American national coarse thread美国标准粗牙螺纹53Union Union 活接头,由宁。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
国际组织ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA(Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW(Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门D&B(Dun & Bradstreet):邓白氏公司DUNS(Data Universal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到ATCC(American Type Culture Collection):美国模式培养物集存库ASTM(American Society for Testing Materials):美国材料与试验协会法规GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP(Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP(European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(C ertificate o f S uitability to the monographs of E uropean P harmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系ICH法规ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范ICH-Q8:药物研发指南ICH-Q9:质量风险管理ICH- Q10(PQS):药物质量体系ICH-Q11:原料药研发与生产常见术语QA(Quality Assurance):质量保证QC(Quality Control):质量控制CQA(Critical Quality Attribute):关键质量属性QRM(Quality Risk Management):质量风险管理IPC(Inproceics Quality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE(Out of Expectation):超期望结果SOP(Standard Operation Procedure):标准操作规程DMF(Drug Master File):药品主文件SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FAT(Factory Acceptance Test):工厂验收测试SAT(Site Acceptance Test):现场验收测试FS(Functional Specification):功能标准DS(Design Specification):设计标准DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality by Design):质量源于设计PMC(Product Material Control):生产物料控制PC生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):年度质量回顾CNC(Controlled Non-Classified Area):受控非洁净区应用技术APS(Aseptic Processing Simulation):培养基模拟灌装CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌BFS(Blowing Filling and Sealing):吹-灌-封PAT(Process Analytical Technology):过程分析技术PLC(Programmable Logic Controller):可编程逻辑控制EDI(Electrodeionization):一种制备纯化水的离子交换技术MAC(Minimum Acceptable Cycle):最低可接受程序SAM(Steam-Air Mixture):蒸汽空气混合气体灭菌程序WIT(Water Intrusion Test):水侵入测试(东富龙疏水性滤器的在线进行完整性测试的方法)BP(Bubble Point Test):起跑点试验FF(Forward Flow/Diffusive Flow):前进流、扩散流试验HPLC(High Performance Liquid Chromatography):高效液相色谱GC(Gas Chromatography):气相色谱FTIR(Fourier Transform Infrared spectroscopy):傅氏转换红外线光谱分析仪MS(Mass Spectroscopy):质谱LC/MS:液质联用GC/MS:气质联用TOC(Total Organic Carbon):总有机碳NVR(Nonvolatile Residue):不挥发残留物RFS(Ready for Sterilization):免洗胶塞RFU(Ready for Use):即用胶塞物品名称SVP(Small Volume Parenteral):小容量注射剂LVP(Large Volume Parenteral):大容量注射剂APA(Aseptic Processing Area):无菌区P&ID(Piping and Instrument Diagram):工艺管道仪表流程图PFD(Process Flow Diagram):工艺流程图UFD(Utility Flow Diagram):公用工程流程图HVAC(Heating Ventilation Air Conditioning):供热空气调节净化系统HEPA(High Efficiency Particulate Air Filter):高效过滤器FFU(Fan Filter Units):风机滤器单元AHU(Air Handling Unit):空气处理单元COA(Certificate of Analysis):分析证书/检验报告书/检验报告单BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药WFI(Water for Injection):注射用水DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶PAO:聚-α-烯烃,HEPA检漏用的气溶胶IBC(I ntermediate Bulk Container):中型散装容器FBD(Fluid Bed Dryer):流化床IRTD(Intelligent Resistance Temperature Detector):智能热电阻温度探头,标准温度探头SV(Solenoid Valve):电磁阀FV:气动阀P/HG(Porous/Hard Goods Loads):多孔/坚硬装载,包括过滤器、胶塞、软管、拖把、工作服、塞子、清洁器具或设备的更换部件。
