左西替利嗪说明书
盐酸左西替利嗪片说明书
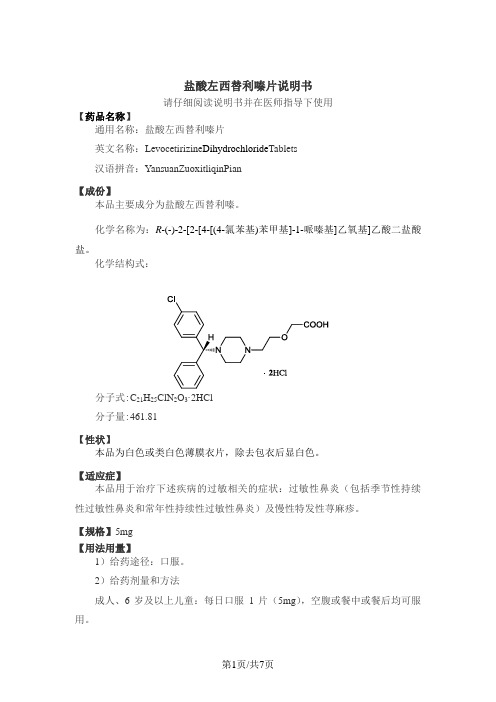
盐酸左西替利嗪片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:盐酸左西替利嗪片英文名称:Levocetirizine Dihydrochloride Tablets汉语拼音:YansuanZuoxitliqinPian【成份】本品主要成分为盐酸左西替利嗪。
化学名称为:R-(-)-2-[2-[4-[(4-氯苯基)苯甲基]-1-哌嗪基]乙氧基]乙酸二盐酸盐。
化学结构式:分子式:C21H25ClN2O3·2HCl分子量:461.81【性状】本品为白色或类白色薄膜衣片,除去包衣后显白色。
【适应症】本品用于治疗下述疾病的过敏相关的症状:过敏性鼻炎(包括季节性持续性过敏性鼻炎和常年性持续性过敏性鼻炎)及慢性特发性荨麻疹。
【规格】5mg【用法用量】1)给药途径:口服。
2)给药剂量和方法成人、6岁及以上儿童:每日口服1片(5mg),空腹或餐中或餐后均可服用。
肾功能损害的患者:轻度肾功能损害患者无需调整剂量,中重度肾功能损害患者用法用量根据下表调整:肾病晚期-采用透析疗法的患者<10 禁忌症a.肝功能损害患者:仅有肝功能损害的患者,无需调整给药剂量;如伴有肾功能损害的患者,请参照“肾功能损害患者”的用法用量。
老年患者:对于中度和重度肾功能损害的老年病人,建议调整给药剂量,请参照“肾功能损害患者”的用法用量。
【不良反应】儿科患者原研产品在6-11月龄和1-6岁儿科患者中完成两项安慰剂对照研究,159例受试者暴露的暴露量为左西替利嗪每日1.25mg,共给药2周,每日2次,每次1.25mg。
在安慰剂对照组或左西替利嗪治疗组中,下述药物不良反应的发生率为1%或1%以上。
露给药时间不等(1周-13周)的5mg左西替利嗪。
在安慰剂对照组或左西替利嗪治疗组中,下述药物不良反应的发生率为1%或1%以上。
成人和12岁以上的儿童原研产品在12-71岁男女患者完成的治疗性研究5mg左西替利嗪治疗组和安慰剂对照组中,分别有15.1%患者,11.3%患者发生至少1例次药物不良反应。
盐酸左西替利嗪片(优泽)的说明书

盐酸左西替利嗪片(优泽)的说明书皮肤病是一种具有传染性的疾病,如果不及时治疗的话对于身边的人都会有一定的影响。
在治疗皮肤病时,药物治疗是首选,目前治疗皮肤病的药物当中盐酸左西替利嗪片(优泽)就是其中最好的一种。
它能应对各种皮肤病人群,对于不同的皮肤都具有相当好的疗效,下面我们来看看对于盐酸左西替利嗪片(优泽)的介绍吧。
【药品名称】通用名称:盐酸左西替利嗪片商品名称:盐酸左西替利嗪片(优泽)英文名称:Levocetirizine Dihydrochloride Tablets拼音全码:YanSuanQinXiTiLiZuoPian(YouZe)【主要成份】本品主要成份为盐酸左西替利嗪。
【成份】分子式:C21H25ClN2O3·2HCl分子量: 461.8【性状】本品为白色薄膜衣异形片,除去包衣后显白色。
【适应症/功能主治】治疗下述疾病的过敏相关症状,如季节性过敏性鼻炎、常年性过敏性鼻炎、慢性特发性荨麻疹。
【规格型号】5mg*7s【用法用量】1.给药途径:口服。
2.给药剂量和方法成人、6岁及以上儿童:每日口服1片(5mg),空腹或餐中或餐后均可服用。
【不良反应】过敏反应、呼吸困难、恶心、血管性水肿、搔痒、荨麻疹、皮疹和体重增加。
【禁忌】1、禁用于对本品任何成分过敏者或者对哌嗪类衍生物过敏者。
2、禁用于肌酐清除率3、禁用于伴有特殊遗传性疾病(包括患有罕见的半乳糖不耐受症、原发性乳糖酶缺乏(lapp lactase)或葡萄糖-半乳糖吸收不良)的患者。
【注意事项】1.由于目前本品无法减半使用,不建议6岁以下儿童使用本品。
2.虽然目前暂无研究资料,但当某些敏感的病人同时服用左西替利嗪和酒精或中枢神经系统抑制剂时可能会对其中枢神经系统产生影响。
3.对驾驶和操作机械能力的影响:对照临床试验证实,左西替利嗪在推荐剂量下不会削弱人的警戒性、反应和驾驶的能力。
如果患者需要驾驶、从事有潜在危险性的活动或操作机械时,切勿过量服用并考虑其对本品的反应;合并服用酒精或其他中枢神经系统抑制剂可能导致其警戒性降低和操作能力削弱。
左西替利嗪说明书

左西替利嗪说明书【药品名称】通用名称:盐酸左西替利嗪片英文名称:Levocetirizine Hydrochoride Tablets商品名称:迪皿【成份】本品主要成分为盐酸左西替利嗪。
【适应症】荨麻疹、过敏性鼻炎、湿疹、皮炎、皮肤瘙痒症等。
【用法用量】口服,成人及6岁以上儿童用量为每日一次,每次一片。
2~6岁儿童,每日一次,每次半片。
【不良反应】本品耐受性良好,不良反应轻微且多可自愈,常见不良反应有嗜睡、口干、头痛、乏力等。
【禁忌】对本品及其它辅料过敏者禁用。
【注意事项】1.有肝功能障碍或障碍史者慎用。
2.高空作业、驾驶或操纵机器期间慎用。
3.避免与镇静剂同服。
4.酒后避免使用本品。
5.肾功能减损患者使用本品适当减量。
【药物相互作用】尚无使用本品进行药物相互作用的研究。
消旋西替利嗪与伪麻黄碱、西咪替丁、酮康唑、红霉素、阿奇霉素、格列吡嗪和安定间无相互作用。
【毒理研究】遗传毒性本品Ames试验、人淋巴细胞染色体畸变试验、小鼠淋巴瘤试验和大鼠微核试验结果均为阴性。
生殖毒性小鼠生育力和一般生殖毒性试验结果提示,西替利嗪经口给药剂量达64mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的25倍)时,对生育力无损伤。
小鼠、大鼠和兔经口给药剂量分别达96、225和135mg/kg(按体表面积折算,分别约相当于成人临床推荐最大日口服剂量的40、180和220倍)时,均未见致畸作用。
但目前尚无充分和严格控制的孕妇临床研究资料。
由于动物生殖研究并不总能预测药物对人的影响,所以只有当确实需要时,才可以在怀孕期间服用本品。
哺乳期小鼠(母鼠)经口给药剂量达96mg/kg(按体表面积折算,约相当于成人临床推荐剂量大日口服剂量的40倍)时,可引起仔鼠体重增长延迟。
Beagie犬的研究表明,给药量的大约3%经乳汁排泄。
致癌性大鼠连续2年经口给药的致癌性试验中,剂量达20mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的15倍,或儿童临床推荐最大日剂量的10倍)时,未见致癌性。
盐酸左西替利嗪口服溶液(迪皿)的说明书

盐酸左西替利嗪口服溶液(迪皿)的说明书关于《盐酸左西替利嗪口服溶液(迪皿)的说明书》,是我们特意为大家整理的,希望对大家有所帮助。
医治五官的各种各样病症需要我们仔细认真,五官是一个较为大致的通称,其实分到较为细。
说白了的五官病症指的便是耳鼻喉、双眼、呼吸系统、脖子以上的脸部这些。
这一类的疾病治疗起來并不是很艰难,可是还要尽快医治。
用药治疗就能非常好的痊愈,现如今,我们为您强烈推荐了一种称为盐酸左西替利嗪内服水溶液(迪皿)的药品,它能合理医治各种各样五官病症。
生产药品名称疫苗通用性名字:盐酸左西替利嗪内服水溶液产品名称:盐酸左西替利嗪内服水溶液(迪皿)英文名字:Levocetirizine Hydrochloride Oral Solution拼音字母全码:YanSuanZuoXiTiLiZuoKouFuRongYe(DiMin)生产关键成分疫苗本产品关键成分为盐酸左西替利嗪,其化学名称为(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]甲酸·二盐酸盐。
生产成份疫苗化学名:(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]甲酸·二盐酸盐生产性状疫苗本产品为没有颜色、回应、浓稠液體,味甜。
生产适用范围/功效与作用疫苗医治以下病症的皮肤过敏有关病症,如周期性过敏性鼻炎、常年性过敏性鼻炎、漫性难治性荨麻疹。
生产型号规格疫苗10Ml*6支生产使用方法使用量疫苗使用量:内服,成年人及6岁以上少年儿童,一日一次,每一次10Ml(1支)。
2~6岁少年儿童,一日一次,每一次5ml(0.5支)。
生产副作用疫苗本产品可能会使某些病人造成头痛、总想睡觉、口干舌燥、疲惫、衰微、腹痛等副作用。
生产禁忌疫苗 1.禁止使用于肌酐清除率<10Ml/分鐘的肾病末期病人。
2.禁止使用于伴随独特遗传疾病(身患少见的半乳糖不耐症症、原发性肠乳糖酶欠缺(Lapp lactase)或葡萄糖-乳清蛋白消化吸收欠佳)的病人。
盐酸左西替利嗪片的使用说明书

盐酸左西替利嗪片的使用说明书一、药品名称盐酸左西替利嗪片二、主要成分盐酸左西替利嗪三、适应症盐酸左西替利嗪片适用于治疗以下症状或疾病:1. 抑郁症:用作抗抑郁药物,对轻、中度抑郁症病人有效;2. 强迫症:用作治疗强迫症的辅助药物。
四、用法用量1. 建议成人口服剂量为每日1片,早餐后服用。
2. 药物使用剂量和疗程应根据病情和医生建议进行调整。
五、不良反应使用盐酸左西替利嗪片可能引起以下不良反应:1. 常见不良反应包括嗜睡、头晕、口干、便秘、胃部不适、视物模糊等;2. 长期大剂量使用可能引起心动过速、心悸等。
六、注意事项在使用盐酸左西替利嗪片时,请注意以下事项:1. 严格按照医生建议用药,不得超量或长期滥用;2. 可能出现嗜睡、头晕等副作用,患者在驾驶、操作机器等需要警觉的情况下应谨慎使用;3. 使用过程中如出现过敏反应,应立即停药并就医;4. 孕妇、哺乳期妇女、儿童和老年患者慎用,必要时遵医嘱;5. 盐酸左西替利嗪片与某些药物可能存在相互作用,患者在使用其他药物时应告知医生。
七、贮藏方法1. 盐酸左西替利嗪片应置于阴凉、干燥处,避免阳光直射;2. 小孩无法触及的地方储存。
八、包装规格盐酸左西替利嗪片通常以每盒10片/盒的规格进行包装。
九、生产企业请在药品包装上查看具体生产企业信息。
十、有效期请在药品包装上查看有效期信息。
十一、批准文号请在药品包装上查看批准文号信息。
使用说明书仅供参考,请在医生指导下正确使用,如有其他疑问请咨询医生或药师。
以上所述为盐酸左西替利嗪片的使用说明书,如有变更,请以医生或药师的指导为准。
盐酸左西替利嗪缓解过敏症状的说明书

盐酸左西替利嗪缓解过敏症状的说明书盐酸左西替利嗪是一种常见的抗过敏药物,广泛应用于缓解过敏症状。
本说明书将为您详细介绍盐酸左西替利嗪的相关信息,包括用途、用量、不良反应等内容,帮助您正确使用该药物。
一、药物名称盐酸左西替利嗪二、药物成分盐酸左西替利嗪的化学名称为(R)-2-[(2-异丙氨基-1-甲基乙基)氨基]-4-乙基氮杂环[5,4-β]苯丙脒盐酸盐。
其化学结构式为:请在此处插入盐酸左西替利嗪的化学结构式三、适应症盐酸左西替利嗪主要用于缓解过敏症状,包括但不限于以下疾病:1. 过敏性鼻炎:主要症状包括鼻塞、流涕、打喷嚏等。
2. 荨麻疹:表现为皮肤瘙痒,出现红色肿块或丘疹。
3. 过敏性皮炎:皮肤发红、瘙痒,并可能出现疱疹、脱屑等症状。
其他过敏相关疾病亦可考虑使用盐酸左西替利嗪,具体用药需在医生指导下进行。
四、用法用量请在使用盐酸左西替利嗪前咨询医生或药师,并按照其建议使用。
1. 成人用量:每次口服25mg,每日2次,可根据病情调整用量。
2. 儿童用量:请咨询医生或药师,根据儿童年龄、体重等因素确定用药剂量。
请勿自行调整用药剂量,严格按照医生或药师的指导使用。
五、不良反应在使用盐酸左西替利嗪过程中,可能会出现以下不良反应:1. 嗜睡、乏力:部分患者在用药后出现嗜睡、乏力等症状。
2. 干口、口干症状:少数患者可能会出现口干、喉咙干燥等不适感。
3. 恶心、呕吐:个别患者可能会出现恶心、呕吐等胃肠道反应。
如果出现以上不适症状,请立即停止使用盐酸左西替利嗪,并咨询医生或药师的意见。
六、注意事项在使用盐酸左西替利嗪时,请注意以下事项:1. 避免饮酒:在用药期间应避免饮酒,以免增加不良反应风险。
2. 注意驾驶安全:由于盐酸左西替利嗪可能引起嗜睡、乏力等症状,使用期间请勿驾驶车辆或操纵机械。
3. 儿童使用:儿童使用盐酸左西替利嗪需在医生指导下进行,用药剂量需根据儿童年龄、体重等因素确定。
4. 孕妇和哺乳期妇女:请在医生指导下使用,尽量避免使用该药物。
盐酸左西替利嗪片说明书

盐酸左西替利嗪片【药品名称】通用名称:盐酸左西替利嗪片商品名称:盐酸左西替利嗪片(希替宁)英文名称:Levocetirizine Hydrochloride Tablets拼音全码:YanSuanZuoXiTiLiZuoPian(XiTiNing)【主要成份】盐酸左西替利嗪。
【成份】化学名:R-(-)- 2-[2-[4-[(4-氯苯基)苯甲基]-1-哌嗪基]乙氧基]乙酸二盐酸盐分子量:C21H25ClN2O3·2HCl【性状】本品为类白色片。
【适应症/功能主治】荨麻疹、过敏性鼻炎、湿疹、皮炎、皮肤瘙痒症等。
【规格型号】 5mg*7s【用法用量】口服,成人及6岁以上儿童用量为每日一次,每次1片。
2-6岁儿童,每日一次,每次半片。
【不良反应】本品可能会使个别患者产生头痛、嗜睡、口干、疲倦、衰弱、腹痛等不良反应。
【禁忌】 1.禁用于肌酐清除率<10ml/分钟的肾病晚期患者。
2.禁用于伴有特殊遗传性疾病(患有罕见的半乳糖不耐受症、原发性肠乳糖酶缺乏(Lapp lactase)或葡萄糖-乳糖吸收不良)的患者。
【注意事项】 1、有肝功能障碍或障碍史者慎用;2、高空作业,驾驶或操作机器期间慎用;3、避免与镇静剂同服;4、酒后避免使用本品;5、肾功能减损患者使用本品适当减量;6、妊期及哺乳期妇女禁用本品;7、2周岁以下儿童用药的安全性尚未确定;8、通常老年人生理机能衰退,需慎用本品。
请仔细阅读说明书并遵医嘱使用。
【儿童用药】 2周岁以下儿童用药的安全性尚未确定。
【老年患者用药】通常老年人生理机能衰退,需慎用本品。
【孕妇及哺乳期妇女用药】妊期及哺乳期妇女禁用本品。
【药物相互作用】尚无左西替利嗪与其他药物相互作用的相关研究资料,至今未有西替利嗪与其他药物相互作用的报导。
【药物过量】 1、症状:成人为嗜睡,儿童为起初兴奋,随后嗜睡。
2、处理:尚无特效的解毒剂。
过量服用本品后,建议采取对症治疗及支持性治疗;如刚服用可考虑洗胃;血液透析对去除本品无效。
盐酸左西替利嗪口服滴剂使用说明

盐酸左西替利嗪口服滴剂【用法用量】推荐成年人和1岁以上儿童使用。
滴剂使用时,先按瓶盖上图示打开瓶盖,然后瓶口垂直向下,药液即会滴出。
1.成年人:(1)在大多数正常情况下,推荐剂量为每日1ml(10mg,约20滴),一次口服。
(2)从本品治疗适应症来看,建议在晚餐期间用少量液体送服此药。
(3)若病人对不良反应敏感,可每日早晚两次服用,每次0.5ml(5mg,约10滴)。
2.儿童:(1)6岁以上儿童:早上和晚上各服用0.5ml(5mg,约10滴)或每天一次1ml(10mg,约20滴)。
(2)2-6岁儿童:早上和晚上各服用0.25ml(2.5mg,约5滴)或每天一次0.5ml(5mg,约10滴)。
(3)1-2岁儿童:早上和晚上各服用0.25ml(2.5mg,约5滴)(4)1岁以下儿童:虽然有6个月以上到1岁婴儿服用西替利嗪的临床数据,但相关评估尚未完全结束,如需使用,请遵医嘱,谨慎使用。
3.老年患者:(1)肾功能正常的老年患者,参照成人推荐剂量。
(2)肾功能不全的老年患者,参见肾功能不全患者推荐剂量。
(3)肾功能不全的患者:建议减半服用推荐剂量。
4.即:(1)成年或老年患者:推荐剂量为每日0.5ml(5mg,约10滴),一次口服。
(2)6岁以上儿童:早上和晚上各服用0.25ml(2.5mg,约5滴)或每天一次0.5ml(5mg,约10滴)(3)1-6岁的儿童:每天一次0.25ml(2.5mg,约5滴)(4)肝功能不全患者,如没有同时患有肾功能不全症状,无须调整给药剂量。
5.口服滴剂:(1)20滴(1ml)=10mg(2)10滴(0.5ml)=5mg(3)5滴(0.25ml)=2.5mg【不良反应】尚不明确。
【禁忌】尚不明确。
【药物相互作用】如与其他药物同时使用可能会发生,详情请咨询医师或药师。
【包装】-【类型】处方药【医保】非【剂型】-说明:以上信息仅供参考,具体请以商品说明书为准。
盐酸左西替利嗪说明书

盐酸左西替利嗪说明书盐酸左西替利嗪说明书一、药品名称盐酸左西替利嗪二、药品成分本品主要成分为盐酸左西替利嗪。
三、适应症盐酸左西替利嗪用于治疗各种抑郁症,如不同程度的抑郁、心境低落以及其他相关症状的病人。
四、规格和用法- 规格:每片含盐酸左西替利嗪x毫克。
- 用法:口服,一般每天一次,每次x毫克。
具体用量和用药频率应由医生根据患者具体情况来确定。
五、禁忌症以下情况患者禁用本药品:- 对盐酸左西替利嗪过敏的患者;- 正在使用单胺氧化酶抑制剂(MAOI)的患者;- 患有严重心脏病、肝功能障碍、肾功能障碍、癫痫等疾病的患者。
六、注意事项1. 使用本药品时应根据医嘱的剂量进行用药,不得超量使用。
2. 在使用本药品期间,如果出现皮疹、过敏等不良反应,请立即停药并就医。
3. 此药品可能引起一些副作用,包括头痛、恶心、失眠等,如果症状严重或持续时间较长,请及时就医。
4. 长期使用左西替利嗪时,应定期监测肝功能,以确保药物使用的安全性。
5. 本药品会对反应能力产生一定影响,患者在用药期间应注意驾驶和操作机械的安全。
七、药物相互作用1. 盐酸左西替利嗪与单胺氧化酶抑制剂(MAOI)合用会增加不良反应的风险,使用本品前请告知医生是否正在使用MAOI。
2. 与其它中枢神经系统抑制剂(如镇静剂、催眠药)合用时,会增加镇静和催眠的效果。
八、孕妇和哺乳期妇女使用的安全性孕妇和哺乳期妇女使用本药品时需谨慎,并在医生指导下使用。
九、不良反应使用本药品可能会出现以下不良反应:1. 常见的不良反应包括头痛、恶心、失眠等,大多数症状都是轻度的,并且会在治疗一段时间后逐渐消失。
2. 罕见但严重的副作用可能包括:过敏反应(皮疹、荨麻疹等)、严重心律失常、肝损害等,如出现请立即停药并就医。
十、药物储存请将本药品存放在阴凉、干燥的地方,避免阳光直射。
请将本药品放在儿童无法触及的地方。
十一、药物有效期请参考药品包装上的有效期,过期的药物请勿使用。
甲磺酸左西替利嗪说明书

甲磺酸左西替利嗪说明书甲磺酸左西替利嗪(化学名:Levocetirizine Dihydrochloride)是一种抗过敏药物,具有抗组胺和抗过敏反应的作用。
以下为甲磺酸左西替利嗪的说明书,包括药品的成分、适应症、用法用量、不良反应、禁忌症和注意事项等内容。
一、药品成分每片甲磺酸左西替利嗪含有10毫克左西替利嗪盐酸盐。
辅料包括:微晶纤维素、乳糖、玉米淀粉、长链糊精、聚乙酸乙烯酯、硬脂酸镁。
二、适应症甲磺酸左西替利嗪适用于下列过敏性疾病的治疗:1. 季节性及常年性过敏性鼻炎;2. 慢性荨麻疹。
三、用法用量成人和12岁以上的儿童:口服,每日一次,每次一片(10毫克)。
6至11岁的儿童:口服,每日一次,每次半片(5毫克)。
2至5岁的儿童:口服,每日一次,每次四分之一片(2.5毫克)。
四、不良反应使用甲磺酸左西替利嗪可能会出现以下不良反应:1. 最常见的副作用是嗜睡,尤其是在刚开始使用时。
此外,还可能出现头痛、乏力等症状。
2. 儿童可能会出现焦躁、紧张等行为异常。
3. 鲜见严重过敏反应,如荨麻疹、皮疹、瘙痒、呼吸困难、喉头水肿和头晕等。
如出现上述症状,请立即停药并求医治疗。
五、禁忌症对甲磺酸左西替利嗪及其任何成分过敏者禁用本品。
六、注意事项1. 甲磺酸左西替利嗪只限于医生处方,请严格按照医嘱使用。
2. 需告知医生有关的过敏史和目前正在使用的药物。
3. 如患者有肝功能损害,应减少用药剂量或延长用药间隔时间。
4. 过量使用可能会出现嗜睡、头晕、口干等症状,如出现过量使用症状应立即停药并就医。
5. 孕妇和哺乳期妇女应在医生指导下使用此药。
七、贮藏请将甲磺酸左西替利嗪放置在儿童无法触及的地方。
避免高温潮湿环境,储存在干燥处。
八、其他信息如需了解更多关于甲磺酸左西替利嗪的信息,请咨询医生或药师。
以上为甲磺酸左西替利嗪的说明书,如需使用,请仔细阅读说明,并遵照医生的嘱咐使用。
如出现不良反应或疑问,请及时就医。
西可新盐酸左西替利嗪片说明书

核准日期:2006年09月04日修改日期:2014年09月26日盐酸左西替利嗪片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名:盐酸左西替利嗪片商品名称:西可新®英文名:Levocetirizine Dihydrochloride Tablets 汉语拼音:Yansuan Zuoxitiliqin Pian 【成份】本品活性成份为盐酸左西替利嗪。
化学名称:[2-[4-[(R)(4-氯-苯基)-苯甲基]-1-哌嗪基]乙氧基]乙酸二盐酸盐。
化学结构式:NNC H ClCH 2CH 2OCH 2COOH. 2HCl分子式:C 21H 25ClN 2O 3•2HCl 分子量:461.8【性状】本品为薄膜衣片,除去包衣后显白色或类白色。
【适应症】治疗下述疾病的过敏相关症状,如季节性过敏性鼻炎、常年性过敏性鼻炎、慢性特发性荨麻疹。
【规格】5mg 【用法用量】成人、6岁及以上儿童:每日口服1片(5mg ),空腹或餐中或餐后均可服用。
肾功能损害的患者:轻度肾功能损害患者无需调整剂量,中重度肾功能损害患者用法用量根据下表调整:病人肾功能状态肌酐清除率(ml/min)a剂量和服药次数中毒肾功能损害30~49每2日一次,5mg 重度肾功能损害<30每3日一次,5mg 肾病晚期—采用透析疗法的患者<10禁忌症a.CLcr=体重({0.85女性患者系数}肝功能损害患者:仅有肝功能损害的患者,无需调整给药剂量;如伴有肾功能损害的患者,请参照“肾功能损害患者”的用法用量。
老年患者:对于中度和重度肾功能损害的老年病人,建议调整给药剂量,请参照“肾功能损害患者”的用法用量。
【不良反应】原研产品临床研究儿科患者:原研产品在6-11月龄和1~6岁儿科患者中完成两项安慰剂对照研究,159例受试者暴露的暴露量为左西替利嗪每日1.25mg,共给药2周,每日2次,每次1.25mg。
在安慰剂对照组或左西替利嗪治疗组中,下述药物不良反应的发生率为1%或1%以上。
盐酸左西替利嗪口服溶液(迪皿)的说明书

盐酸左西替利嗪口服溶液(迪皿)的说明书治疗五官的各种疾病需要我们认真仔细,五官是一个比较大至的统称,实则分得比较细。
所谓的五官疾病指的就是耳鼻喉、眼睛、呼吸道、脖颈以上的面部等等。
这一类的疾病治疗起来不是很困难,但是也要尽早治疗。
药物治疗就能很好的治愈,如今,我们为您推荐了一种叫做盐酸左西替利嗪口服溶液(迪皿)的药物,它能有效治疗各种五官疾病。
【药品名称】通用名称:盐酸左西替利嗪口服溶液商品名称:盐酸左西替利嗪口服溶液(迪皿)英文名称:Levocetirizine Hydrochloride Oral Solution 拼音全码:YanSuanZuoXiTiLiZuoKouFuRongYe(DiMin)【主要成份】本品主要成份为盐酸左西替利嗪,其化学名称为(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]乙酸·二盐酸盐。
【成份】化学名:(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]乙酸·二盐酸盐【性状】本品为无色、澄清、粘稠液体,味甜。
【适应症/功能主治】治疗下述疾病的过敏相关症状,如季节性过敏性鼻炎、常年性过敏性鼻炎、慢性特发性荨麻疹。
【规格型号】10ml*6支【用法用量】用量:口服,成人及6岁以上儿童,一日一次,每次10ml(1支)。
2~6岁儿童,一日一次,每次5ml(0.5支)。
【不良反应】本品可能会使个别患者产生头痛、嗜睡、口干、疲倦、衰弱、腹痛等不良反应。
【禁忌】1.禁用于肌酐清除率<10ml/分钟的肾病晚期患者。
2.禁用于伴有特殊遗传性疾病(患有罕见的半乳糖不耐受症、原发性肠乳糖酶缺乏(Lapp lactase)或葡萄糖-乳糖吸收不良)的患者。
【注意事项】1.不建议6岁以下儿童使用本品,由于目前可使用的该产品的分散片仍无法允许调整剂量。
2.虽然目前暂无研究资料,但当某些敏感的病人同时服用左西替利嗪和酒精或中枢神经系统抑制剂时可能会对其中枢神经系统产生影响。
盐酸左西替利嗪口服滴剂使用说明

盐酸左西替利嗪口服滴剂使用说明哎呀,小伙伴们,今天咱们来聊聊一个非常实用的东西——盐酸左西替利嗪口服滴剂使用说明哦!这个东西可是个好东西,能帮助我们解决很多身体上的小毛病呢!不过,别看它简单,用起来还是有一些讲究的。
咱们就一起来聊聊吧!
咱们要明白,盐酸左西替利嗪口服滴剂是一种治疗过敏性鼻炎、荨麻疹等疾病的药物。
它的主要作用就是抑制组胺的释放,从而减轻过敏症状。
所以,当我们感觉到鼻子痒、流鼻涕、打喷嚏,或者皮肤红肿、瘙痒的时候,就可以用这个药来缓解症状啦!
那么,怎么用这个药呢?其实很简单,只需要按照说明书上的要求来就可以了。
咱们要先把药瓶摇匀,然后取出适量的药液。
一般来说,每次使用一滴就可以了。
具体用量还是要根据医生的建议来定。
接下来,咱们要把药液滴在舌头下面,然后再喝一口水。
这样可以让药液更好地吸收。
用了这个药之后,有的小伙伴可能会觉得头晕、口干等不适症状。
这时候,不用太担心,这些都是正常的反应。
如果症状比较严重,可以暂时停药,等到症状消失后再继续使用。
如果症状持续不改善,还是要去医院看看医生哦!
在使用盐酸左西替利嗪口服滴剂的过程中,还有一些注意事项要注意哦。
咱们要按照规定的时间服用药物,不能随意增减剂量。
这个药不能和酒精一起服用,否则会引起不良反应。
如果正在使用其他药物,一定要告诉医生,以免发生药物相互作用。
盐酸左西替利嗪口服滴剂虽然看起来简单,但是用起来还是有很多讲究的。
只有按照说明书上的要求来使用,才能发挥出它的最佳效果哦!希望小伙伴们都能健康快乐地度过每一天!。
甲磺酸左西替利嗪胶囊说明书

甲磺酸左西替利嗪胶囊说明书【药品名称】通用名称:甲磺酸左西替利嗪胶囊汉语拼音:Jia Suan Juan Zuo Xi Ti Li Qian Jiao Nang【成分】本药品的成分为甲磺酸左西替利嗪。
【性状】本品为固体胶囊剂,外观为硬壳胶囊,胶囊内容物为白色到类白色颗粒。
【适应症】甲磺酸左西替利嗪胶囊适用于治疗以下病症:1. 精神分裂症:用于缓解精神分裂症引起的阳性症状和阴性症状。
2. 双相障碍:用于预防和缓解双相障碍的发作。
3. 抑郁症:用于治疗抑郁症的症状。
【用法用量】请在医生的指导下使用本药品。
一般情况下,成人的推荐初始剂量为12.5毫克,每晚一次口服。
随后,根据病情和耐受性逐渐调整剂量。
剂量的最大上限为50毫克每晚一次口服。
【禁忌症】下列情况患者禁止使用本药品:1. 对甲磺酸左西替利嗪或类似药物过敏的患者。
2. 心脏疾病、心律失常或血压异常的患者。
3. 曾经出现过癫痫发作的患者。
【不良反应】使用甲磺酸左西替利嗪胶囊可能会导致以下不良反应:1. 颤抖或肌肉僵硬2. 眼部运动障碍,如眨眼和眼球运动不协调3. 恶心、呕吐和便秘4. 焦虑、头晕和嗜睡【注意事项】在使用本药品时,请注意以下事项:1. 不要饮酒或同时使用其他中枢神经系统抑制剂。
2. 不要开车或操作机器,因为本药品可能会导致嗜睡和注意力不集中。
3. 长期使用本药品时,应注意定期进行眼科检查,以及血压和心脏监测。
4. 在使用本药品时,避免进行过多的剧烈运动。
【药物相互作用】请告知医生您正在使用的其他药物,包括出售的非处方药。
甲磺酸左西替利嗪可能与其他药物相互作用,例如:1. 中枢神经系统抑制剂,如安眠药、镇静剂和酒精。
2. 某些抗高血压药物。
【贮藏】请将本药品放置在干燥、阴凉的地方,避免阳光直射。
【生产企业】XX制药有限公司【批准日期】XXXX年XX月XX日【批准文号】国药准字HXXXXXX【附:贴签】-------------------------------------------------------------------------------------------------------------------------------【本说明书仅供参考,使用时请遵循医生的指导,并详细阅读药品说明书】。
盐酸左西替利嗪口服滴剂使用说明

盐酸左西替利嗪口服滴剂使用说明嘿,亲们!今天小生给大家普及一下关于盐酸左西替利嗪口服滴剂的使用说明,让大家吃得开心,吃得健康!话说这可是咱们老百姓家庭药箱里的一把好手呢!
让我们来了解一下盐酸左西替利嗪口服滴剂的基本信息。
它是一种抗组胺药,主要用于治疗过敏性疾病,如过敏性鼻炎、荨麻疹等。
它的成分主要是盐酸左西替利嗪,作用是抑制组胺的释放,从而减轻过敏症状。
那么,怎么使用这款神奇的药物呢?别着急,小生这就给大家细细道来。
我们需要准备一瓶盐酸左西替利嗪口服滴剂,还有一根滴管。
接下来,我们要按照医生的建议来服用药物。
一般来说,成人每次口服10-20毫升,每日2-3次;儿童的用量则根据年
龄和体重来定。
记得要按时按量服用哦,不要随意增减剂量。
在使用过程中,我们还需要注意一些事项。
盐酸左西替利嗪口服滴剂不适用于孕妇、哺乳期妇女和儿童。
患有严重肝肾功能不全的患者应慎重使用。
如果服用过程中出现过敏反应、心悸、头晕等症状,应立即停药并就医。
好了,现在我们已经了解了盐酸左西替利嗪口服滴剂的基本用法和注意事项。
接下来,我们来说说如何正确保存这款药物吧。
我们要将药物放在阴凉干燥的地方,避免阳光直射和潮湿。
我们要将药物放在儿童拿不到的地方,以免误食。
我们要定期检查药物的有效期,过期的药物要及时丢弃。
盐酸左西替利嗪口服滴剂是一款非常实用的药物,可以帮助我们缓解过敏症状,让我们的生活更加美好。
但是,我们在使用过程中一定要遵循医生的建议,注意药物的用法和注意事项,确保药物的安全有效。
希望各位亲们都能吃得开心,吃得健康!下次再见啦!。
FDA左西替利嗪说明书2013

from a 5 mg once daily dose in adults. (12.3).See 17 for PATIENT COUNSELING INFORMATION.Revised: 11/2013 FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE1.1 Seasonal Allergic Rhinitis1.2 Perennial Allergic Rhinitis1.3 Chronic Idiopathic Urticaria2 DOSAGE AND ADMINISTRATION2.1 Adults and Children 12 Years of Age and Older2.2 Children 6 to 11 Years of Age2.3 Children 6 months to 5 Years of Age2.4 Dose Adjustment for Renal and Hepatic Impairment3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS4.1 Patients with known hypersensitivity4.2 Patients with end-stage renal disease4.3 Pediatric patients with impaired renal function5 WARNINGS AND PRECAUTIONS5.1 Somnolence5.2 Urinary Retention6 ADVERSE REACTIONS6.1 Clinical Trials Experience6.2 Post-Marketing Experience7 DRUG INTERACTIONS7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine7.2 Ritonavir8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Renal Impairment8.7 Hepatic Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2 Animal Toxicology14 CLINICAL STUDIES14.1 Seasonal and Perennial Allergic Rhinitis14.2 Chronic Idiopathic Urticaria16 HOW SUPPLIED/STORAGE AND HANDLING6.1 Clinical Trials ExperienceThe safety data described below reflect exposure to XYZAL in 2708 patients with seasonal orperennial allergic rhinitis or chronic idiopathic urticaria in 14 controlled clinical trials of 1 week to 6months duration.The short-term (exposure up to 6 weeks) safety data for adults and adolescents are based upon eight clinical trials in which 1896 patients (825 males and 1071 females aged 12 years and older) were treated with XYZAL 2.5, 5, or 10 mg once daily in the evening.The short-term safety data from pediatric patients are based upon two clinical trials in which 243children with seasonal or perennial allergic rhinitis (162 males and 81 females 6 to 12 years of age)were treated with XYZAL 5 mg once daily for 4 to 6 weeks, one clinical trial in which 114 children (65males and 49 females 1 to 5 years of age) with allergic rhinitis or chronic idiopathic urticaria were treated with XYZAL 1.25 mg twice daily for 2 weeks, and one clinical trial in which 45 children (28males and 17 females 6 to 11 months of age) with symptoms of allergic rhinitis or chronic urticaria were treated with XYZAL 1.25 mg once daily for 2 weeks.The long-term (exposure of 4 or 6 months) safety data in adults and adolescents are based upon two clinical trials in which 428 patients (190 males and 238 females) with allergic rhinitis were exposed to treatment with XYZAL 5 mg once daily. Long term safety data are also available from an 18-month trial in 255 XYZAL-treated subjects 12-24 months of age.Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.Adults and Adolescents 12 years of Age and OlderIn studies up to 6 weeks in duration, the mean age of the adult and adolescent patients was 32 years, 44%of the patients were men and 56% were women, and the large majority (more than 90%) was Caucasian.In these trials 43% and 42% of the subjects in the XYZAL 2.5 mg and 5 mg groups, respectively, had at least one adverse event compared to 43% in the placebo group.In placebo-controlled trials of 1-6 weeks in duration, the most common adverse reactions weresomnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis, and most were mild to moderate in intensity. Somnolence with XYZAL showed dose ordering between tested doses of 2.5, 5 and 10 mg and was the most common adverse reaction leading to discontinuation (0.5%).Table 1 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 12years and older exposed to XYZAL 2.5 mg or 5 mg in eight placebo-controlled clinical trials and that were more common with XYZAL than placebo.Table 1 Adverse Reactions Reported in ≥ 2% of Subjects Aged 12 Years andOlder Exposed to XYZAL 2.5 mg or 5 mg Once Daily in Placebo-ControlledClinical Trials 1-6 Weeks in DurationAdverse ReactionsXYZAL 2.5 mg (n = 421)XYZAL 5 mg (n = 1070)Placebo (n = 912)*Somnolence22 (5%)61 (6%)16 (2%)Nasopharyngitis25 (6%)40 (4%)28 (3%)Fatigue5 (1%)46 (4%)20 (2%)Dry Mouth12 (3%)26 (2%)11 (1%)Pharyngitis 10 (2%)12 (1%)9 (1%)*Rounded to the closest unit percentageAdditional adverse reactions of medical significance observed at a higher incidence than in placebo in adults and adolescents aged 12 years and older exposed to XYZAL are syncope (0.2%) and weight increased (0.5%).Pediatric Patients 6 to 12 Years of AgeA total of 243 pediatric patients 6 to 12 years of age received XYZAL 5 mg once daily in two short-term placebo controlled double-blind trials. The mean age of the patients was 9.8 years, 79 (32%) were 6 to 8 years of age, and 50% were Caucasian. Table 2 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 6 to 12 years exposed to XYZAL 5 mg in placebo-controlled clinical trials and that were more common with XYZAL than placebo.Table 2 Adverse Reactions Reported in ≥2% of Subjects Aged 6-12 YearsExposed to XYZAL 5 mg Once Daily in Placebo-Controlled Clinical Trials 4and 6 Weeks in DurationAdverse ReactionsXYZAL 5 mg (n = 243)Placebo (n = 240)*Pyrexia10 (4%) 5 (2%)Cough8 (3%) 2 (<1%)Somnolence7 (3%) 1 (<1%)Epistaxis6 (2%) 1 (<1%)Pediatric Patients 1 to 5 Years of AgeA total of 114 pediatric patients 1 to 5 years of age received XYZAL 1.25 mg twice daily in a two week placebo-controlled double-blind safety trial. The mean age of the patients was 3.8 years, 32% were 1 to 2 years of age, 71% were Caucasian and 18% were Black. Table 3 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 1 to 5 years exposed to XYZAL 1.25 mg twice daily in the placebo-controlled safety trial and that were more common with XYZAL than placebo.Table 3 Adverse Reactions Reported in ≥2% of Subjects Aged 1-5 YearsExposed to XYZAL 1.25 mg Twice Daily in a 2-Week Placebo-ControlledClinical TrialAdverse ReactionsXYZAL 1.25 mg Twice Daily (n = 114)Placebo (n = 59)*Pyrexia5 (4%) 1 (2%)Diarrhea4 (4%) 2 (3%)Vomiting4 (4%) 2 (3%)Otitis Media3 (3%)0 (0%)Pediatric Patients 6 to 11 Months of AgeA total of 45 pediatric patients 6 to 11 months of age received XYZAL 1.25 mg once daily in a two week placebo-controlled double-blind safety trial. The mean age of the patients was 9 months, 51%were Caucasian and 31% were Black. Adverse reactions that were reported in more than 1 subject (i.e.greater than or equal to 3% of subjects) aged 6 to 11 months exposed to XYZAL 1.25 mg once daily in the placebo-controlled safety trial and that were more common with XYZAL than placebo included diarrhea and constipation which were reported in 6 (13%) and 1 (4%) and 3 (7%) and 1 (4%) children in the XYZAL and placebo-treated groups, respectively.*Rounded to the closest unit percentage *Rounded to the closest unit percentageLong-Term Clinical Trials ExperienceIn two controlled clinical trials, 428 patients (190 males and 238 females) aged 12 years and older were treated with XYZAL 5 mg once daily for 4 or 6 months. The patient characteristics and the safety profile were similar to that seen in the short-term studies. Ten (2.3%) patients treated with XYZAL discontinued because of somnolence, fatigue or asthenia compared to 2 (<1%) in the placebo group. There are no long term clinical trials in children below 12 years of age with allergic rhinitis or chronic idiopathic urticaria.Laboratory Test AbnormalitiesElevations of blood bilirubin and transaminases were reported in <1% of patients in the clinical trials. The elevations were transient and did not lead to discontinuation in any patient.6.2 Post-Marketing ExperienceIn addition to the adverse reactions reported during clinical trials and listed above, adverse events have also been identified during post-approval use of XYZAL. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse events of hypersensitivity and anaphylaxis, increased appetite, angioedema, fixed drug eruption, pruritus, rash and urticaria, convulsion, paraesthesia, dizziness, tremor, dysgeusia, vertigo, movement disorders (including dystonia and oculogyric crisis), aggression and agitation, hallucinations, depression, insomnia, suicidal ideation, visual disturbances, blurred vision, palpitations, tachycardia, dyspnea, nausea, vomiting, hepatitis, dysuria, urinary retention, myalgia, and edema have been reported.Besides these events reported under treatment with XYZAL, other potentially severe adverse events have been reported from the post-marketing experience with cetirizine. Since levocetirizine is the principal pharmacologically active component of cetirizine, one should take into account the fact that the following adverse events could also potentially occur under treatment with XYZAL: orofacial dyskinesia, severe hypotension, cholestasis, glomerulonephritis, still birth, tic, myoclonus, and extrapyramidal symptoms.7 DRUG INTERACTIONSIn vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction studies have been performed with levocetirizine. Drug interaction studies have been performed with racemic cetirizine.7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and PseudoephedrinePharmacokinetic interaction studies performed with racemic cetirizine demonstrated that cetirizine did not interact with antipyrine, pseudoephedrine, erythromycin, azithromycin, ketoconazole, and cimetidine. There was a small decrease (~16%) in the clearance of cetirizine caused by a 400 mg dose of theophylline. It is possible that higher theophylline doses could have a greater effect.7.2 RitonavirRitonavir increased the plasma AUC of cetirizine by about 42% accompanied by an increase in half-life (53%) and a decrease in clearance (29%) of cetirizine. The disposition of ritonavir was not altered by concomitant cetirizine administration.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category BThere are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, XYZAL should be used during pregnancy only if clearly needed.Teratogenic Effects:In rats and rabbits, levocetirizine was not teratogenic at oral doses approximately 320 and 390,respectively, times the maximum recommended daily oral dose in adults on a mg/m basis.8.3 Nursing MothersNo peri- and post-natal animal studies have been conducted with levocetirizine. In mice, cetirizinecaused retarded pup weight gain during lactation at an oral dose in dams that was approximately 40 times the maximum recommended daily oral dose in adults on a mg/m basis. Studies in beagle dogs indicated that approximately 3% of the dose of cetirizine was excreted in milk. Cetirizine has been reported to be excreted in human breast milk. Because levocetirizine is also expected to be excreted in human milk,use of XYZAL in nursing mothers is not recommended.8.4 Pediatric UseThe recommended dose of XYZAL for the treatment of the uncomplicated skin manifestations ofchronic idiopathic urticaria in patients 6 months to 17 years of age is based on extrapolation of efficacy from adults 18 years of age and older [see Clinical Studies (14)].The recommended dose of XYZAL in patients 6 months to 11 years of age for the treatment of the symptoms of perennial allergic rhinitis and chronic idiopathic urticaria and in patients 2 to 11 years of age for the treatment of symptoms of seasonal allergic rhinitis is based on cross-study comparisons of the systemic exposure of XYZAL in adults and pediatric patients and on the safety profile of XYZAL in both adult and pediatric patients at doses equal to or higher than the recommended dose for patients 6months to 11 years of age.The safety of XYZAL 5 mg once daily was evaluated in 243 pediatric patients 6 to 12 years of age in two placebo-controlled clinical trials lasting 4 and 6 weeks. The safety of XYZAL 1.25 mg twice daily was evaluated in one 2-week clinical trial in 114 pediatric patients 1 to 5 years of age and the safety of XYZAL 1.25 mg once daily was evaluated in one 2-week clinical trial in 45 pediatric patients 6 to 11months of age [see Adverse Reactions (6.1)].The effectiveness of XYZAL 1.25 mg once daily (6 months to 5 years of age) and 2.5 mg once daily (6to 11 years of age) for the treatment of the symptoms of seasonal and perennial allergic rhinitis and chronic idiopathic urticaria is supported by the extrapolation of demonstrated efficacy of XYZAL 5 mg once daily in patients 12 years of age and older based on the pharmacokinetic comparison between adults and children.Cross-study comparisons indicate that administration of a 5 mg dose of XYZAL to 6 to 12 year old pediatric seasonal allergic rhinitis patients resulted in about 2-fold the systemic exposure (AUC)observed when 5 mg of XYZAL was administered to healthy adults. Therefore, in children 6 to 11 years of age the recommended dose of 2.5 mg once daily should not be exceeded. In a populationpharmacokinetics study the administration of 1.25 mg once daily in children 6 months to 5 years of age resulted in systemic exposure comparable to 5 mg once daily in adults. [see Dosage and Administration (2.2); Clinical Studies (14); and Clinical Pharmacology (12.3)].8.5 Geriatric UseClinical studies of XYZAL for each approved indication did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other 22reported clinical experience has not identified differences in responses between the elderly andyounger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.8.6 Renal ImpairmentXYZAL is known to be substantially excreted by the kidneys and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function [see Dosage and Administration (2) and Clinical Pharmacology (12.3)].8.7 Hepatic ImpairmentAs levocetirizine is mainly excreted unchanged by the kidneys, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Clinical Pharmacology (12.3)].10 OVERDOSAGEOverdosage has been reported with XYZAL.Symptoms of overdose may include drowsiness in adults and initially agitation and restlessness,followed by drowsiness in children. There is no known specific antidote to XYZAL. Should overdose occur, symptomatic or supportive treatment is recommended. XYZAL is not effectively removed by dialysis, and dialysis will be ineffective unless a dialyzable agent has been concomitantly ingested.The acute maximal non-lethal oral dose of levocetirizine was 240 mg/kg in mice (approximately 190times the maximum recommended daily oral dose in adults, approximately 230 times the maximum recommended daily oral dose in children 6 to 11 years of age, and approximately 180 times themaximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m basis). In rats the maximal non-lethal oral dose was 240 mg/kg (approximately 390 times the maximum recommended daily oral dose in adults, approximately 460 times the maximum recommended daily oral dose inchildren 6 to 11 years of age, and approximately 370 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m basis).11 DESCRIPTIONLevocetirizine dihydrochloride, the active component of XYZAL tablets and oral solution, is an orally active H -receptor antagonist. The chemical name is (R)-[2-[4-[(4-chlorophenyl) phenylmethyl]-1-piperazinyl] ethoxy] acetic acid dihydrochloride. Levocetirizine dihydrochloride is the R enantiomer of cetirizine hydrochloride, a racemic compound with antihistaminic properties. The empirical formula of levocetirizine dihydrochloride is C H ClN O •2HCl. The molecular weight is 461.82 and the chemical structure is shown below:221212523Levocetirizine dihydrochloride is a white, crystalline powder and is water soluble.XYZAL 5 mg tablets are formulated as immediate release, white, film-coated, oval-shaped scoredtablets for oral administration. The tablets are imprinted on both halves of the scored line with the letter Y in red (Opacode Red). Inactive ingredients are: microcrystalline cellulose, lactose monohydrate,colloidal anhydrous silica, and magnesium stearate. The film coating contains hypromellose, titanium dioxide, and macrogol 400.XYZAL 0.5 mg/mL oral solution is formulated as an immediate release, clear, colorless liquid. Inactive ingredients are: sodium acetate trihydrate, glacial acetic acid, maltitol solution, glycerin, methylparaben,propylparaben, saccharin, flavoring (consisting of triacetin, natural & artificial flavors, dl-alpha-tocopherol), purified water.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionLevocetirizine, the active enantiomer of cetirizine, is an anti-histamine; its principal effects are mediated via selective inhibition of H receptors. The antihistaminic activity of levocetirizine has beendocumented in a variety of animal and human models. In vitro binding studies revealed that levocetirizine has an affinity for the human H -receptor 2-fold higher than that of cetirizine (Ki = 3 nmol/L vs. 6nmol/L, respectively). The clinical relevance of this finding is unknown.12.2 PharmacodynamicsStudies in adult healthy subjects showed that levocetirizine at doses of 2.5 mg and 5 mg inhibited the skin wheal and flare caused by the intradermal injection of histamine. In contrast, dextrocetirizineexhibited no clear change in the inhibition of the wheal and flare reaction. Levocetirizine at a dose of 5mg inhibited the wheal and flare caused by intradermal injection of histamine in 14 pediatric subjects (aged 6 to 11 years) and the activity persisted for at least 24 hours. The clinical relevance of histamine wheal skin testing is unknown.A QT/QTc study using a single dose of 30 mg of levocetirizine did not demonstrate an effect on the QTc interval. While a single dose of levocetirizine had no effect, the effects of levocetirizine may not be at steady state following single dose. The effect of levocetirizine on the QTc interval following ®11XYZAL has not been studied in patients with hepatic impairment. The non-renal clearance (indicative of hepatic contribution) was found to constitute about 28% of the total body clearance in healthy adult subjects after oral administration.As levocetirizine is mainly excreted unchanged by the kidney, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Dosage and Administration (2)].13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityNo carcinogenicity studies have been performed with levocetirizine. However, evaluation of cetirizine carcinogenicity studies are relevant for determination of the carcinogenic potential of levocetirizine. In a 2-year carcinogenicity study, in rats, cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 15 times the maximum recommended daily oral dose in adults, approximately 10 times the maximum recommended daily oral dose in children 6 to 11 years of age and approximately 15 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign hepatic tumors in males at a dietary dose of 16 mg/kg (approximately 6 times the maximum recommended daily oral dose in adults, approximately 4 times the maximum recommended daily oral dose in children 6 to 11years of age, and approximately 6 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m basis). No increased incidence of benign tumors was observed at a dietary dose of 4 mg/kg (approximately 2 times the maximum recommended daily oral dose in adults, equivalent to the maximum recommended daily oral dose in children 6 to 11 years of age and approximately 2 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m basis).The clinical significance of these findings during long-term use of XYZAL is not known.Levocetirizine was not mutagenic in the Ames test, and not clastogenic in the human lymphocyte assay,the mouse lymphoma assay, and in vivo micronucleus test in mice.In a fertility and general reproductive performance study in mice, cetirizine did not impair fertility at an oral dose of 64 mg/kg (approximately 25 times the recommended daily oral dose in adults on a mg/m basis).13.2 Animal ToxicologyReproductive Toxicology StudiesIn rats and rabbits, levocetirizine was not teratogenic at oral doses up to 200 and 120 mg/kg,respectively, (approximately 320 and 390, respectively, times the maximum recommended daily oral dose in adults on a mg/m basis).In mice, cetirizine caused retarded pup weight gain during lactation at an oral dose in dams of 96 mg/kg (approximately 40 times the maximum recommended daily oral dose in adults on a mg/m basis).14 CLINICAL STUDIES14.1 Seasonal and Perennial Allergic RhinitisAdults and Adolescents 12 Years of Age and OlderThe efficacy of XYZAL was evaluated in six randomized, placebo-controlled, double-blind clinical trials in adult and adolescent patients 12 years and older with symptoms of seasonal allergic rhinitis or perennial allergic rhinitis. The six clinical trials include three dose-ranging trials of 2 to 4 weeks duration, one 2-week efficacy trial in patients with seasonal allergic rhinitis, and two efficacy trials 222222(one 6-week and one 6-month) in patients with perennial allergic rhinitis.These trials included a total of 2412 patients (1068 males and 1344 females) of whom 265 were adolescents 12 to 17 years of age. Efficacy was assessed using a total symptom score from patient recording of 4 symptoms (sneezing, rhinorrhea, nasal pruritus, and ocular pruritus) in five studies and 5symptoms (sneezing, rhinorrhea, nasal pruritus, ocular pruritus, and nasal congestion) in one study.Patients recorded symptoms using a 0-3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3= severe) once daily in the evening reflective of the 24 hour treatment period. In one study, patients also recorded these symptoms in an instantaneous (1 hour before the next dose) manner. The primary endpoint was the mean total symptom score averaged over the first week and over 2 weeks for seasonal allergic rhinitis trials, and 4 weeks for perennial allergic rhinitis trials.The three dose-ranging trials were conducted to evaluate the efficacy of XYZAL 2.5, 5, and 10 mg once daily in the evening. One trial was 2 weeks in duration conducted in patients with seasonal allergic rhinitis, and two trials were 4 weeks in duration conducted in patients with perennial allergic rhinitis. In these trials, each of the three doses of XYZAL demonstrated greater decrease in the reflective total symptom score than placebo and the difference was statistically significant for all three doses in two of the studies. Results for two of these trials are shown in Table 4.Table 4 Mean Reflective Total Symptom Score in Allergic Rhinitis Dose-Ranging TrialsTreatment N Baseline On Treatment Adjusted MeanDifference from PlaceboEstimate 95% CI p-value *Seasonal Allergic Rhinitis Trial – Reflective total symptom scoreXYZAL 2.5 mg1167.83 4.270.91(0.37, 1.45)0.001XYZAL 5 mg1157.45 4.06 1.11(0.57, 1.65)<0.001XYZAL 10 mg1187.15 3.57 1.61(1.07, 2.15)<0.001Placebo 1187.94 5.17Perennial Allergic Rhinitis Trial – Reflective total symptom scoreXYZAL 2.5 mg1337.14 4.12 1.17(0.71, 1.63)<0.001XYZAL 5 mg1277.18 4.07 1.22(0.76, 1.69)<0.001XYZAL 10 mg1297.58 4.19 1.10(0.64, 1.57)<0.001Placebo 1287.22 5.29One clinical trial was designed to evaluate the efficacy of XYZAL 5 mg once daily in the eveningcompared with placebo in patients with seasonal allergic rhinitis over a 2-week treatment period. In this trial, XYZAL 5 mg demonstrated a greater decrease from baseline in the reflective and instantaneous total symptom score than placebo, and the difference was statistically significant (see Table 5). The results of the instantaneous total symptom score support efficacy at the end of the dosing interval.One clinical trial evaluated the efficacy of XYZAL 5 mg once daily in the evening compared to placebo in patients with perennial allergic rhinitis over a 6-week treatment period. Another trial conducted over a 6-month treatment period assessed efficacy at 4 weeks. XYZAL 5 mg demonstrated a greater decrease *Total symptom score is the sum of individual symptoms of sneezing, rhinorrhea, nasal pruritus, and ocular pruritus as assessed by patients on a 0-3 categorical severity scale.from baseline in the reflective total symptom score than placebo and the difference from placebo was statistically significant. Results of one of these trials are shown in Table 5.Table 5 Mean Reflective Total Symptom Score and Instantaneous Total Symptom Score inAllergic Rhinitis TrialsTreatment N Baseline On Treatment Adjusted MeanDifference from PlaceboEstimate 95% CI p-value *Seasonal Allergic Rhinitis Trial – Reflective total symptom scoreXYZAL 5 mg1188.40 5.200.89(0.30, 1.47)0.003Placebo 1178.50 6.09Seasonal Allergic Rhinitis Trial – Instantaneous total symptom scoreXYZAL 5 mg1187.24 4.580.73(0.17, 1.28)0.011Placebo 1177.48 5.30Perennial Allergic Rhinitis Trial – Reflective total symptom scoreXYZAL 5 mg1507.69 3.93 1.17(0.70, 1.64)<0.001Placebo 1427.44 5.10Onset of action was evaluated in two environmental exposure unit studies in allergic rhinitis patients with a single dose of XYZAL 2.5 or 5 mg. XYZAL 5 mg was found to have an onset of action 1 hour after oral intake. Onset of action was also assessed from the daily recording of symptoms in the evening before dosing in the seasonal and perennial allergic rhinitis trials. In these trials, onset of effect was seen after 1 day of dosing.Pediatric Patients Less than 12 Years of AgeThere are no clinical efficacy trials with XYZAL 2.5 mg once daily in pediatric patients under 12 years of age, and no clinical efficacy trials with XYZAL 1.25 mg once daily in pediatric patients 6 months to 5years of age. The clinical efficacy of XYZAL in pediatric patients under 12 years of age has been extrapolated from adult clinical efficacy trials based on pharmacokinetic comparisons [see Use in Specific Populations (8.4)].14.2 Chronic Idiopathic UrticariaAdult Patients 18 Years of Age and OlderThe efficacy of XYZAL for the treatment of the uncomplicated skin manifestations of chronicidiopathic urticaria was evaluated in two multi-center, randomized, placebo-controlled, double-blind clinical trials of 4 weeks duration in adult patients 18 to 85 years of age with chronic idiopathic urticaria. The two trials included one 4-week dose-ranging trial and one 4-week single-dose level efficacy trial. These trials included 423 patients (139 males and 284 females). Most patients (>90%)were Caucasian and the mean age was 41. Of these patients, 146 received XYZAL 5 mg once daily in the evening. Efficacy was assessed based on patient recording of pruritus severity on a severity score of 0–3 (0 = none to 3 = severe). The primary efficacy endpoint was the mean reflective pruritus severity score over the first week and over the entire treatment period. Additional efficacy variables were the instantaneous pruritus severity score, the number and size of wheals, and duration of pruritus.The dose-ranging trial was conducted to evaluate the efficacy of XYZAL 2.5, 5, and 10 mg once daily *Total symptom score is the sum of individual symptoms of sneezing, rhinorrhea, nasal pruritus, and ocular pruritus as assessed by patients on a 0-3 categorical severity scale.。
盐酸左西替利嗪颗粒说明书

盐酸左西替利嗪颗粒以下内容仅供参考,请以药品包装盒中的说明书为准。
妊娠:禁用哺乳期:禁用盐酸左西替利嗪颗粒说明书请仔细阅读说明书并在医师指导下使用。
【药品名称】通用名:盐酸左西替利嗪颗粒英文名:Levocetirizine Hydrochloride Granules汉语拼音:YansuanZuoxitiliqin Keli【成份】本品主要成份:本品主要成份为盐酸左西替利嗪。
【性状】本品为白色或类白色的可溶颗粒;味甜。
【适应症】荨麻疹、过敏性鼻炎、湿疹、皮炎、皮肤瘙痒症等。
【规格】2.5mg【用法用量】口服,成人及6 岁以上儿童用量为每日一次,每次两袋。
2~6 岁儿童,每日一次,每次一袋。
【不良反应】本品耐受性良好,不良反应轻微且多可自愈,常见不良反应有嗜睡、口干、头痛、乏力等。
【禁忌】对本品及其它辅料过敏者禁用。
【注意事项】1、有肝功能障碍或障碍史者慎用。
2、高空作业、驾驶或操纵机器期间慎用。
3、避免与镇静剂同服。
4、酒后避免使用本品。
5、肾功能减损的患者使用本品时应适当减量。
【孕妇及哺乳期妇女用药】孕期及哺乳期妇女禁用本品。
【儿童用药】2 周岁以下儿童用药的安全性尚未确定。
【老年患者用药】通常老年人生理机能衰退,需慎用本品。
【药物相互作用】目前尚无关于左西替利嗪的药物相互作用的研究(包括没有CYP3A4诱导剂的研究);此前对于西替利嗪消旋体的研究显示其没有临床相关的药物间不良反应(与伪麻黄碱、西米替丁、酮康唑、红霉素、阿齐霉素、格列吡嗪、地西泮)。
在一项多剂量西替利嗪合并使用茶碱(400mg/日)的研究中发现,西替利嗪的清除率下降了16%,而茶碱的清除率并未因为合并使用西替利嗪而改变。
进食可能会导致左西替利嗪的吸收速率下降,吸收程度不会降低。
【药物过量】症状:成人为嗜睡,儿童为起初兴奋,随后嗜睡。
处理:尚无特效的解毒剂。
过量服用本品后,建议采取对症治疗及支持性治疗。
如刚服用可考虑洗胃;血液透析对去除本品无效。
盐酸左西替利嗪口服滴剂使用说明

盐酸左西替利嗪口服滴剂使用说明嘿,伙计们!今天我们来聊聊一个非常实用的东西——盐酸左西替利嗪口服滴剂。
别看它只是一瓶小小的药水,但是它可是能让你的生活变得更美好的哦!下面就让我来给大家详细介绍一下这个神奇的药物吧!
让我们来了解一下盐酸左西替利嗪口服滴剂的基本信息。
它是一种抗组胺药,主要用于治疗过敏性鼻炎、荨麻疹等过敏性疾病。
它的成分非常安全,不会对你的身体造成任何伤害。
而且,它的味道也非常好喝,就像一杯甜甜的果汁一样。
所以,你完全不用担心会喝不下去哦!
接下来,我们来说说如何正确使用盐酸左西替利嗪口服滴剂。
你需要打开药瓶,然后轻轻摇晃几下,让药液充分混合。
接着,你只需要将药液滴入鼻孔中,每次1-2滴即可。
如果你觉得药液滴得太多,可以用纸巾轻轻擤掉多余的药液。
你需要将药瓶盖紧,存放在阴凉干燥的地方。
使用盐酸左西替利嗪口服滴剂也有一些需要注意的地方。
你不能把它当成普通的饮料来喝。
虽然它的味道很好,但是你还是要按照医生的建议来服用。
你不能把它跟其他药物一起服用。
如果你需要同时服用其他药物,一定要告诉医生或药师,以免发生不良反应。
如果你在使用过程中出现了不适的症状,比如头晕、恶心等,一定要立刻停止使用,并及时就医。
盐酸左西替利嗪口服滴剂是一种非常实用的药物,可以帮助你摆脱过敏性疾病的困扰。
只要你按照正确的方法来使用它,相信它一定会给你带来满意的效果。
那么,赶快行动起来吧!让盐酸左西替利嗪口服滴剂成为你生活中的小助手,让你的生活更加美好!。
盐酸左西替利嗪溶口服液说明书

盐酸左西替利嗪口服溶液【药品名称】通用名称:盐酸左西替利嗪口服溶液商品名称:盐酸左西替利嗪口服溶液(迪皿)英文名称:Levocetirizine Hydrochloride Oral Solution拼音全码:YanSuanQinXiTiLiZuoKouFuRongYe(DiMin)【主要成份】本品主要成份为盐酸左西替利嗪,其化学名称为(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]乙酸·二盐酸盐。
【成份】化学名:(R)-(-)-2-[2-(4-(4-氯苯基)苯甲基)-1-哌嗪基)乙氧基]乙酸·二盐酸盐【性状】本品为无色、澄清、粘稠液体,味甜。
【适应症/功能主治】荨麻疹、过敏性鼻炎、湿疹、皮炎、皮肤瘙痒症等。
【规格型号】 10ml*6支【用法用量】用量:口服,成人及6岁以上儿童,一日一次,每次10ml(1支)。
2~6岁儿童,一日一次,每次5ml(0.5支)。
使用方法:于餐前半小时服用。
【不良反应】本品耐受性良好,不良反应轻微且多可自愈,常见不良反应有嗜睡、口干、头痛、乏力等。
【禁忌】对本品及其他辅料过敏者禁用。
【注意事项】 1.有肝功能障碍或障碍史者慎用。
2.高空作业、驾驶或操纵机器期间慎用。
3.避免与镇静剂同服。
4.酒后避免使用本品。
5.肾功能减损患者使用本品适当减量。
请仔细阅读说明书并遵医嘱使用。
【儿童用药】 2周岁以下儿童用药的安全性尚未确定。
【老年患者用药】通常老年人生理机能衰退,需慎用本品。
【孕妇及哺乳期妇女用药】孕期及哺乳期妇女禁用本品。
【药物相互作用】尚无使用本品进行药物相互作用的研究,消旋西替利嗪与伪麻黄碱、西咪替丁、酮康唑、红霉素、阿奇霉素、格列吡嗪和安定间无相互作用。
【药物过量】本品无特效拮抗剂,严重超量服用本品应立即洗胃。
【药理毒理】药理作用: 本品为口服选择性组胺H1受体拮抗剂。
无明显抗胆碱和抗5-羟色胺作用,中枢抑制作用较小。
左西替利嗪滴丸说明书

左西替利嗪滴丸说明书尊敬的患者:感谢您选择使用左西替利嗪滴丸。
为了确保您能够正确合理地使用该药品,我们特别为您提供详细的说明书。
在使用前,请仔细阅读以下内容,并按照说明进行使用。
一、药品名称及规格药品名称:左西替利嗪滴丸(Generic Name: Zotepine Capsules)药品规格:每粒含有50毫克左西替利嗪。
二、药品适应症左西替利嗪滴丸是一种新型的抗精神病药物,主要适用于以下疾病的治疗:1. 急性或慢性精神分裂症;2. 带有明显痴呆状综合征的老年性精神病。
左西替利嗪滴丸通过改善症状,如幻觉、妄想等,有助于减轻患者的不适感,改善患者的心理状态。
三、禁忌症以下人群禁止使用左西替利嗪滴丸:1. 对本品或其中任何成分过敏的患者;2. 曾有心脏疾病、心律不齐、长QT综合征或阿斯匹林过敏;3. 孕妇、哺乳期妇女,以及准备怀孕的妇女;4. 严重的中枢神经系统抑制,如昏迷等。
对于其他由于特殊原因不能使用左西替利嗪滴丸的患者,请在使用前咨询医生的指导。
四、用法用量请根据医生的指导进行用法用量,不要超过建议剂量。
通常,每日用量为100-200毫克,分两次服用。
如果患者服用过程中出现任何不良反应或副作用,请及时告知医生,以便调整用量或采取其他治疗措施。
五、不良反应左西替利嗪滴丸可能引起以下不良反应:1. 患者可能会出现乏力、头晕、口干、便秘等常见的轻微不良反应;2. 根据个体差异,也有可能出现心悸、体重增加、泌乳减少等症状;3. 对极少数人群而言,还可能引发严重的皮肤过敏反应,如荨麻疹、瘙痒等。
如果出现上述不良反应,请立即停用药物,并告知医生。
医生会根据具体情况作出进一步的治疗调整。
六、注意事项在使用左西替利嗪滴丸时,请注意以下事项:1. 该药物可能使您产生嗜睡、注意力不集中等不良反应。
因此,在服药期间,应避免驾驶机动车辆或从事危险操作,以免发生意外;2. 酒精与该药物有相互作用,可能增加其镇静作用。
因此,患者在使用左西替利嗪滴丸期间应避免饮酒;3. 请按医嘱定期复诊,以便医生对您的病情进行监测和调整治疗方案;4. 请将该药品放在儿童无法接触的地方,以免发生意外。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
左西替利嗪说明书
【药品名称】
通用名称:盐酸左西替利嗪片
英文名称:Levocetirizine Hydrochoride Tablets
商品名称:迪皿
【成份】
本品主要成分为盐酸左西替利嗪。
【适应症】
荨麻疹、过敏性鼻炎、湿疹、皮炎、皮肤瘙痒症等。
【用法用量】
口服,成人及6岁以上儿童用量为每日一次,每次一片。
2~6岁儿童,每日一次,每次半片。
【不良反应】
本品耐受性良好,不良反应轻微且多可自愈,常见不良反应有嗜睡、口干、头痛、乏力等。
【禁忌】
对本品及其它辅料过敏者禁用。
【注意事项】
1.有肝功能障碍或障碍史者慎用。
2.高空作业、驾驶或操纵机器期间慎用。
3.避免与镇静剂同服。
4.酒后避免使用本品。
5.肾功能减损患者使用本品适当减量。
【药物相互作用】
尚无使用本品进行药物相互作用的研究。
消旋西替利嗪与伪麻黄碱、西咪替丁、酮康唑、红霉素、阿奇霉素、格列吡嗪和安定间无相互作用。
【毒理研究】
遗传毒性
本品Ames试验、人淋巴细胞染色体畸变试验、小鼠淋巴瘤试验和大鼠微核试验结果均为阴性。
生殖毒性
小鼠生育力和一般生殖毒性试验结果提示,西替利嗪经口给药剂量达64mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的25倍)时,对生育力无损伤。
小鼠、大鼠和兔经口给药剂量分别达96、225和135mg/kg(按体表面积折算,分别约相当于成人临床推荐最大日口服剂量的40、180和220倍)时,均未见致畸作用。
但目前尚无充分和严格控制的孕妇临床研究资料。
由于动物生殖研究并不总能预测药物对人的影响,所以只有当确实需要时,才可以在怀孕期间服用本品。
哺乳期小鼠(母鼠)经口给药剂量达96mg/kg(按体表面积折算,约相当于成人临床推荐剂量大日口服剂量的40倍)时,可引起仔鼠体重增长延迟。
Beagie犬的研究表明,给药量的大约3%经乳汁排泄。
致癌性
大鼠连续2年经口给药的致癌性试验中,剂量达20mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的15倍,或儿童临床推荐最大日剂量的10倍)时,未见致癌
性。
小鼠连续2年经口给药的致癌性试验中,剂量达16mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的6倍,或儿童临床推荐最大日剂量的4倍)时,可引起雄性动物良性肝肿瘤的发生率增加;剂量为4mg/kg(按体表面积折算,约相当于成人临床推荐最大日口服剂量的2倍,或儿童临床推荐最大日剂量)时,未见肝肿瘤发生率的增加。
上述发现的临床意义尚不清楚。
【批准文号】
国药准字H
【生产企业】
重庆华邦制药股份有限公司
【药物分类】
抗组胺和抗过敏药。
