Maya2010下载地址
Maya2009下载地址

Autodesk? Maya? 2009中有很多的改进,它可以帮助你更有生产力,包括Maya的Assets(资产),预选高亮(Pre-Selection highlighting),热图显示(Heat Map Display),属性传递(Transfer Attribute)以及强大用户界面更新。此外,您还可以发现Maya 2009中显著的性能改善,在许多领域,包括支持多线程的工作和算法,而且它相比之前版本的速度更快,加速模拟和渲染的表现,甚至可以支持更多巨大的场景。
在SIGGRAPH 2008会议上,作为对Maya十周年的贺礼,Autodesk 宣布Autodesk Maya 2009 3D动画和视觉特效软件的发行。Maya 2009包括了许多在建模、动画、渲染和特效方面的改进,这些改进使得工作效率和工作流程得到最大的提升和优化,并且它提供了新的创作空间。Maya 2009将会在SIGGRAPH 2008的Autodesk展区(展区号501,洛杉矶会议中心,8月12-14号)陈列。
5 网友:yfz 2009年06月21日 星期日 上午 11:51 | 回复 你好!我出现第二个问题,d3dx9_36.dll文件已下载,但不知道要放在那个目录下,才能启动maya....能否告知?谢谢!
6 匿名网友 2009年06月25日 星期四 下午 03:06 | 回复 感谢感谢。。。。。。
Maya 2009新功能:
模型:模型师和纹理绘制艺术家将会使工作变得更加有效,由于范围广泛的新特点和工作流程的改进,在Autodesk? Maya? 2009中,真正的软选择,调整模式,快速修改,新的UV布局和展开选项,合并顶点等新功能,都会让设计师们随心所欲的进行创作。
动画:在Autodesk? Maya? 2009中,您可以找到一个强大的新动画分层技术,它是建立在Autodesk? MotionBuilder? 的基础上开发的. 此功能可以让艺术家们在非破坏性原则上创造出多层次的动画。这是一套灵活的体系结构,使得工程可以在任何属性之间游刃有余;动画层可以融合,合并,组合归类,并重新排序动画,以及可以设置动画的优先级。
ps,ae,pr,3dmax,c4d,maya软件下载
Adobe photoshop(简称PS):Adobe公司旗下最为出名的图像处理软件之一,最为人熟知的大神软件之一,也是最受业余爱好者喜爱的。
很多不懂的人拿PS和毁图秀秀(美图秀秀)作比较,觉得ps还不如毁图秀秀方便。
这种观念是很荒谬的,学习过你就会发现ps绝对甩了毁图秀秀好几条街。
如果把毁图秀秀比喻为搞装修的,那么ps就是搞建筑一条龙,没了房子毁图就没用,而PS却可以平地起高楼,总之,PS的强大绝对超乎你的想象。
上手难度:一般超神难度:难下载地址:Adobe photoshop CC : /Lwdqg9注册机+破解补丁:/KXDitQAdobe最新版本即为CC版本,破解教程视频有空给录制一个。
先可以自己百度研究研究。
Adobe After Effects(简称AE):Adobe公司开发的一个影视后期特效合成及设计软件,用于高端视频特效系统的专业特效合成非线性编辑软件。
这是一款很专业高端的软件,在许多电视剧和电影中的特效都是应用它做成的,不过如今已经平民化,也是大受业余爱好者喜爱的,即使你是业余者也能学的很好。
上手难度:难超神难度:困难下载地址:AE CC : /MyUowx从AE CC版本开始有官方中文版了,对于天朝子民来说是个巨大的福音。
Adobe Premiere(简称PR):提到AE不得不说的一款软件,与会声会影相同,是一款视频编辑软件,大多与AE配合使用。
Premiere Pro是视频编辑爱好者和专业人士准备的必不可少的编辑工具。
它可以提升您的创作能力和创作自由度,它是易学、高效、精确的视频剪辑软件。
Premiere提供了采集、剪辑、调色、美化音频、字幕添加、输出、DVD刻录的一整套流程,并和其他Adobe软件高效集成,使您足以完成在编辑、制作、工作流上遇到的所有挑战,满足您创建高质量作品的要求。
上手难度:中超神难度:难下载地址:/K7dsDB说了这三个二维软件(当然AE可以安装3D插件),再说几个正儿八经高端大气上档次的,当然学起来也不是很简单了。
3dmax2010下载,安装,注册,激活(或破解)教学

3DMAX2010/2011下載,安裝,註冊,激活(或破解)教學安装准备官方网址:先下载软件(是电驴下载地址,右键选择迅雷下载或者直接把地址复制粘贴到迅雷里就可以下载):(只有加精才下得快,浏览下载的多,速度才会上来;资源的名字更改正确,方便别人找资源国外网盘一堆确不能保障稳定性)3ds Max 2011官方英文原版安装包(这个iso包括两个版本,装的时候讓你选择32bit or 64bit)Max版:ed2k://|file|[%E6%AC%A7%E7%89%B9%E5%85%8B3Ds.MAX.2010%E8%8B%B1%E6%96%87%E6%AD%A3%E5%BC %8F%E7%89%88].Autodesk.3Ds.Max.v2010-ISO.nfo|1080|34A8422E488BFAC263E4997D5254AD5B|h=ESUBRTVYR6XDY2D H5EKLJHK4N4T6MHGA|/Design版:ed2k://|file|%5B%E5%81%B6%E7%89%B9%E5%85%8BMAX.Design.2010%E8%8B%B1%E6%96%87%E6%AD%A3%E5 %BC%8F%E7%89%88%5D.Autodesk.3Ds.MAX.Design.v2010-ISO.iso|3644981248|3a3a7ebcb01d184201b46ae9297d5c50|/ design是给建筑师用的. 另一个版本是给动画,影视,游戏开发用的.design没有SDK.但默认比3DSMAX多了一种灯光. 3DSMAX也有那个灯光,但得花时间调出来.俩版本唯一的区别就是design没有SDK.为什么3ds max 2010完整版(大概3 4个G左右)和精简版(大概500M左右)和再精简版(大概100M左右)有这么大的体积差距!!原因是:完整版本里面有视频教学文件----按F1可以打开帮助----可以连接到视频教程(当然是英文语音的);精简版本就是把视频文件给剪掉了;再精简版就是把视频和帮助文件都一起给剪掉了(按F1没反应)!!!!max精到不能再精的版本只要50多M——这是max的核心引擎程序。
Maya2010插件开发环境搭建

1.开发Maya2010插件,与之相匹配的编译器是vs2005,由于本项目涉及到数
据库,使用sql2005(数据库的版本没有限制),这里使用的是32位的Maya,编译器以及maya都安装在C盘。
2.本文档由Maya帮助文档整理而来,C:\Program
Files\Autodesk\Maya2010\docs\Maya2010\en_US\index.html,下的Developer Resources / Setting up your build environment / 。
有关maya的二次开发都在Developer Resources里。
3.默认的maya的插件目录是:C:\Program
Files\Autodesk\maya2010\devkit\plug-ins,Maya插件开发向导的目录:C:\Program Files\Autodesk\Maya2010\devkit\pluginwizard
4.在插件向导目录里寻找MayaPluginWizard2.0.zip,并解压可得到下面的文件:
5.把
MayaPluginWizard.vsdir
MayaPluginWizard.vsz
MayaPluginWizard.ico
把上面3个文件复制到C:\Program Files\Microsoft Visual Studio 8\VC\vcprojects目录内。
6.把剩下的那个MayaPluginWizard文件夹复制到C:\Program Files\Microsoft
Visual Studio 8\VC\VCWizards,
7.到此,配置结束,打开vs2005,新建项目,就会看到Maya向导。
Maya教程与视频教程类专题资料免费下载整理合集

Maya教程与视频教程类专题资料免费下载整理合集《3ds Max、zbrush、Maya、Revit Architecture书籍光盘合集》【82.2GB】/jiaocheng/shipin/28205.html《Autodesk Maya影视动画设计师标准培训教材》随书光盘【8.2GB】/jiaocheng/shipin/24808.html《Autodesk Maya 2013_官方中文版》【1.5GB】/ruanjian/duomeiti/31004.html《Maya角色建模与渲染完全攻略》随书光盘【6.6 GB】/jiaocheng/shipin/28352.html《神话-MaYa 动画制作实战技法》随书光盘【2.1GB】/jiaocheng/shipin/28350.html《Maya广告特效经典案例解析》随书光盘【3.4GB】/jiaocheng/shipin/28324.html《中科院新科海影视包装系列视频教程》(After Effects CS3 3ds 3D Max Maya AE Premiere )【17.5GB】/jiaocheng/shipin/24164.html《maya2008诺宝教程(210小时)》【27.4GB】/jiaocheng/shipin/29741.html《Maya总动员Fluid流体特效篇》(maya fluid effect)【12.7GB】/jiaocheng/shipin/25142.html《完美视界Maya+After Effects CS4 影视包装技术精粹DVD》随书光盘【3.7GB】/jiaocheng/shipin/25396.html《卡能教育游戏设计系列教程_内容涉及maya 3dsmax zbrush photoshop cinama 4d》【5.3GB】/jiaocheng/shipin/31798.html《MAYA渲染的艺术》(MAYA)随书光盘【6.8 GB】/jiaocheng/shipin/25684.html《Maya深入精髓随书光盘》原版【138.5MB】/jiaocheng/shipin/27905.html《欧特克2011-2013数字娱乐创作解决方案》(Autodesk Digital Entertainment Creation Solutions 2011.2013)Win & Mac OSX【3.2GB】/ruanjian/hangye/31008.html《使用Maya和ZBrush创建游戏道具教程》(Digital Tutors Creating Game Weapons in Maya and ZBrush )【758.4MB】/jiaocheng/shipin/25846.html《maya总动员插件篇》【18.9GB】/jiaocheng/shipin/25544.html《Maya2008从入门到精通》随书光盘【4.4GB】/jiaocheng/shipin/25524.html《使用Maya进行超酷摩托车建模技术教程》(Digital Tutors Motorcycle Modeling Techniques in Maya )更新完毕/共4CD【2.3 GB】/jiaocheng/shipin/25878.html《Maya印象:角色绑定与动画规律》随书光盘【4.2GB】/jiaocheng/shipin/25530.html《MAYA动画实例教程》【2.1GB】/jiaocheng/shipin/26106.html《动画游戏角色制作教程》(Digital Tutors Animating Next Gen Characters In Maya)【2.3GB】/jiaocheng/shipin/22536.html《maya总动员动画编程篇》【7.9 GB】/jiaocheng/shipin/26440.html《Maya极速引擎:特效篇》(Maya)随书光盘【675.5MB】/jiaocheng/shipin/25673.html《Digital Tutor公司maya教程合集》(Digital Tutor Maya Collection August 2009)【30.7GB】/jiaocheng/shipin/25086.html《卡能教育游戏设计系列教程_内容涉及maya 3dsmax zbrush photoshop cinama 4d 等》(Cone Education Center)【5GB】/jiaocheng/shipin/25091.html《maya总动员_animation角色动画篇》(maya_animation)【14.4 GB】/ziliao/25165.html《使用Maya 2010制作灾后建筑摧毁效果教程》(Digital Tutors Modeling ArchitecturalDestruction in Maya 2010)【689.7MB】/jiaocheng/shipin/25861.html《maya总动员Dynamics动力学篇》7CD(maya_cynamics)【22.2 GB】/ziliao/25155.html《Maya Vray 渲染插件》(Vray Adv)Vray for Autodesk Maya 7.0-2012 更新2.00.04 For Maya 2011/2012版本【712.6 MB】/ruanjian/duomeiti/13584.html《Maya 2011和After Effects CS4镜头跟踪与合成路径视频教程》(Digital Tutors Match Moving and Compositing Pipeline in Maya 2011 and After Effects CS4)【3.5GB】/jiaocheng/shipin/26089.html《Maya 2011 结合RealFlow 5设计流程教学》(Digital Tutors Pipeline Integration with Maya 2011 and RealFlow 5 )【6GB】/jiaocheng/shipin/26057.html《Maya创建渲染网络教程》(Dover Studios Maya Shading Networks)【220.3 MB】/jiaocheng/shipin/20924.html《Maya 2011:角色建模教程》(Maya 2011: Modeling a Character)【598.8 MB】/jiaocheng/shipin/26011.html《maya表面材质节点参考》【378.8MB】/ziliao/28202.html《聚光数字maya metalray极致表现(角色篇)》随书光盘【3.7GB】/jiaocheng/shipin/26458.html《完美动力MAYA案例教程-影视包装篇》【2.6 GB】/jiaocheng/shipin/25153.html《maya通用节点参考》【579.2MB】/jiaocheng/shipin/28210.html《利用Maya和HDR Light Studio 进行专业级汽车渲染教程》(Digital Tutors Automotive Rendering with HDR Light Studio and Maya )【308.8MB】/jiaocheng/shipin/25877.html《Maya Shader Recipes:衍射光栅教程》(Digital Tutors Shader Recipes: Diffraction Grating in Maya )【122.9MB】/jiaocheng/shipin/25867.html《火星Maya栏目包装班公开课》火星时代内部资料,采用DV机拍摄【27.9MB】/jiaocheng/shipin/20165.html《Maya 2008完美风暴》随书光盘【3.7GB】/jiaocheng/shipin/25516.html《Maya四足动物动画教程》(Digital-Tutors Animating Quadrupeds in Maya)2CDs 【900.7 MB】/jiaocheng/shipin/22244.html《卡能教育MAYA系列教程_涉及MAYA骨骼绑定_动画调节_蒙皮_表情动画_MAYA建模》【2.8GB】/jiaocheng/shipin/25575.html《3D人体建模与动画》(3D Human Modeling and Animation)CD Book, 2nd Edition 【780.2MB】/ziliao/20926.html《Maya 2011 Mental Ray光影渲染教程》(Maya 2011 Lighting and Rendering In Mental Ray )【1.2GB】/jiaocheng/shipin/25759.html《玛雅创建纹理和材料视频教程》( Maya Essentials 4 Creating Textures and Materials)【436.2MB】/jiaocheng/shipin/32215.html《高级异形创作教程》(Gnomonology The Making Of Fume)附Maya梅子制作教程【1.2 GB】/jiaocheng/shipin/22474.html《照明与渲染原理1:光线与色彩理论》(CG ACADEMY LIGHTING AND RENDERING FUNDAMENTALS 1 LIGHT AND COLOUR THEORY)【3.3 GB】/jiaocheng/shipin/24451.html《Alias 迪斯尼动画大师教程》(Alias Maya Techniques | SuperToon Animation)【947.1MB】/jiaocheng/shipin/20398.html《中科院新科海_CG游戏_魔兽世界场景制作》(CG 3d max maya game course)【374.8 MB】/ziliao/24179.html《水晶石技法MAYA 2008模型制作》【11.5GB】/jiaocheng/shipin/25190.html《MAYA中级建模教程》(Digital Tutors Maya | Maya Intermediate: Female Android Modeling)【2.2 GB】/jiaocheng/shipin/20132.html《Maya 最新光照权威教程》(Learning Autodesk Maya Lighting)Windows AG 教程【794.4MB】/jiaocheng/shipin/20404.html《Maya 2010:MatchMover, Toxik和Backburner入门教学》(Maya 2010: Getting Started with MatchMover, Toxik, and Backburner)【1.9 GB】/jiaocheng/shipin/25996.html《动画场景镜头教程-maya与shake联合教程》(Gnomon Camera Projection Techniques in Maya)【3.8GB】/jiaocheng/shipin/20584.html《Maya角色动画规律及设定Alpha MEDIA》随书光盘(MAYA Animation &Ring)3【660.3MB】/ziliao/24745.html《关键帧动画教程》(GNOMONOLOGY INTRO TO KEYFRAME ANIMATION INMAYA)QuicktimeMov【542 MB】/jiaocheng/shipin/22334.html《Maya直观动画教程》(Gnomon Digital Training Intuitive Animation)【878.3 MB】/jiaocheng/shipin/24181.html《Autodesk Maya 2011_2012_x86_X64_中英双语补丁、软件大集合》(Autodesk Maya 2011_2012_x86_X64)Autodesk Maya 2011_2012【1.4GB】/ruanjian/hangye/16150.html《Autodesk Maya2011性能与功能中文视频教程》【562.1 MB】/jiaocheng/shipin/25490.html。
3D动画设计优秀资源学习下载地址大全

3D动画设计优秀资源教程目录:3D动画设计优秀资源学习下载汇总!(不断更新)3D动画设计主流软件下载:Autodesk 3dsMax 2010 安装下载(x86 x64版3.5G)3ds max 2010 + 3ds max desigh 2010 32bit 简体中文版下载(附注册机)欧特克 Maya 09终极版(Autodesk Maya Unlimited v2009 SP1-添加Mac /Linux 版本共1.4G)Autodesk AutoCAD Inventor Suite 2010 Win32 CHS 安装下载(简体中文10G)3ds Max、Maya等视频及相关教程下载:3D Max视频教程小而清晰的Flash版视频3dsMax9 超级手册(视频教程10G下载)MAYA 2009入门教学3DSMax 大师班教程3Ds Max 9 宝典3D Max 8 宝典三维制作完美风暴:3ds Max 2009完全学习手册(中文版)3ds max 2009基础教程(免费提供下载)火星人3D MAX 8白金手册 PDF文字版[特别推荐]用多媒体学Maya 6.0 视频教程(全4CD) 精品推荐3dsmax2009从入门到精通-建筑效果表现DVD3D材质、插件及源文件下载:后期处理素材库(不断更新!)精品无缝贴图系列(打包下载)材质大集合(下载)3dsmax 9.0最新插件合集3dsmax插件(261个)3DS MAX 2010插件小全(压缩包)3DSMAX插件和部分下载地址其他3D动画优秀资源:史上最强3Dsmax室内设计家庭装修(实例视频教)美女的制作流程渲染——MentalRay质感的艺术(12种材质详解)模神3dsmax时尚家具高级建模宝典3ds max毛发大师Hairtrix插件秘笈之人物短发发型3D贴图材质制作的提示和秘籍3DMAX中英文对照大全-3dmax命令大全绝对强悍的后期教程!老外制作的建筑后期VRay打造清新样板间-特色材质进阶篇。
Maya教程与视频教程类专题资料免费下载整理合集

Maya教程与视频教程类专题资料免费下载整理合集近年来,Maya(玛雅)软件在电影、动画、游戏等行业中得到广泛应用,成为了制作高质量图形和动画的首选工具。
为了帮助初学者快速入门Maya软件,本文整理了一系列免费的Maya教程与视频教程资料,供大家学习和下载。
一、Maya软件简介Maya是由Autodesk公司开发的一款三维计算机图形软件,目前已经成为了电影、游戏和广告等行业的标准工具。
Maya软件提供了丰富的功能和工具集,可以实现从模型创建、动画、渲染到特效制作等多个方面的需求。
通过学习Maya软件,你可以打造出逼真的场景、精美的角色和令人惊叹的动画效果。
二、Maya教程资料免费下载在互联网上,有很多网站提供了免费的Maya教程资料下载,下面介绍几个值得推荐的网站:1. Autodesk官方网站Autodesk官方网站提供了大量的免费教程资料,包括视频教程、文档教程和案例分析等。
你可以在官网上直接搜索Maya相关的教程资料,并进行免费下载。
此外,官网还有Maya用户社区,你可以在社区中与其他Maya用户交流和分享学习心得。
2. YouTubeYouTube是一个充满资源的视频分享网站,在YouTube上搜索Maya教程,你会找到许多优质的视频教学资源。
许多专业的Maya用户也在YouTube上分享了他们的经验和技巧,你可以跟随他们的教学视频一步步学习Maya的使用方法。
3. CGSocietyCGSociety是一个聚集了全球最优秀的数码艺术家和3D动画师的社区平台。
在CGSociety上,你可以找到大量的Maya教程和案例分析,学习到各种不同领域的Maya技术应用。
4. Digital-TutorsDigital-Tutors是一个专业的在线教育平台,提供了多种Maya教程和视频教学课程。
虽然其中一部分课程需要付费,但平台也提供一些免费的Maya教程资源,供用户免费下载。
以上推荐的网站仅是提供Maya教程资料的一部分,你可以根据自身需求和学习进度,选择合适的网站进行查找和下载。
官方maya2014下载链接+安装破解图文教程
maya下载链接:64bit下载(右击复制超级连接,到迅雷新建下载。
)autodesk2014注册机:64bit
安装说明:下面的安装不需要断网。
2,
3,
4,序列号:667-98989898 密钥:657F1 其它的通用序列号:666-69696969, 400-45454545 , 066-66666666
5,
6,
7,
8,打开maya
9,
10,点激活
11,下面的这个提示是正常的,关闭这个界面,返回到上面那步的那个界面,再次点激活12,勾选下图中的“我具有Autodesk提供的激活码”
12,打开注册机,注意,windows注册机是分32位和64位的,你自己安装多少位的maya,就选择对应位数的注册机,win7操作系统的,右键注册机,使用管理员身分打开。
13,打开注册机后,先单击左边的按钮Patch,链接成功后,会弹出对话框,点确定就OK
14,复制下图中的申请号,粘贴到注册上面的框,再点中间的按钮Generate,下框会生成激活码,复制到激活代码框,如下图:
15,单击一下步,激活成功!
祝你学习进步,工作顺利!
MAYA界面的英文或中文的切换方法:
以Windows 7系统为例,右键计算机,属性,高级系统设置,环境变量,新建一个环境变量,变量名输入MAYA_UI_LANGUAGE ,变量值输入zh_CN就是启用中文界面,变量名输入en_US就是启用英文界面,根据你的需要进行修改。
Maya2010注册方法
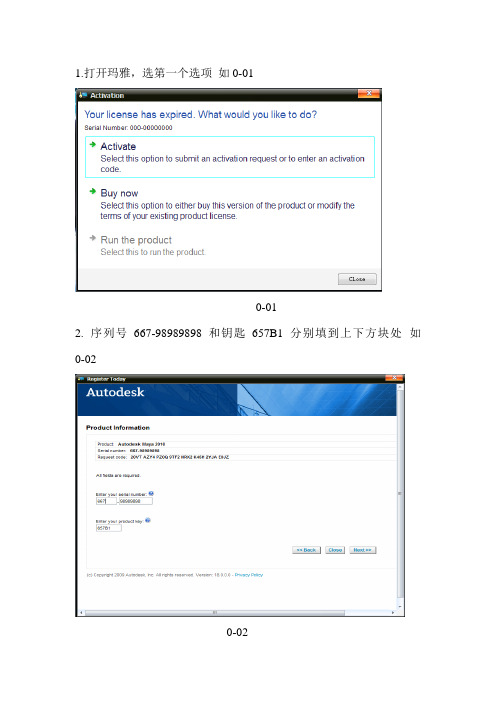
1.打开玛雅,选第一个选项如0-01
0-01
2. 序列号667-98989898和钥匙657B1分别填到上下方块处如0-02
0-02
3.在网上下载maya2010注册机解压打开如0-03
0-03
4. 将注册界面上(Request code)后的一串字母Ctrl+c 复制
Ctrl+v粘贴到注册机上Request的一栏出(上面已有的字母删掉)点击(Mem Patch)测试
0-04
如果出现如0-05那么你需要重新下载一个新的注册机
0-05
5.在单击(Generate)后在Activation一栏处出现一串字母如0-06
0-06
6.单击注册界面的Next 出现下一个界面
选(Enter an activation code)
下方出现一个空白框
将注册机里Activation一栏后的字母复制到空白框内如0-07
0-07
7.然后点击Next
最后点击close完成!
注意:
在断网的情况下注册。
maya2012下载以及破解安装

Maya2012下载[size=1em]地址(直接复制地址到下载工具就可以了):32位maya2012下载:/47549/503/4656503/Autodesk_Maya_2012_English_Japanese_Win_32bit.exe
64位maya2012注册机:欧特克2012-Windows.x64全系列注册机.rar (195.21 KB)
Maya2012的安装方法[size=1em]:[p=20, null, left]首先下载Maya2012安装包,解压,然后里面有一个setup.exe,双击运行,然后点击Install进入安装。出现许可协议,点击I Accept,然后下一步,进入产品信息界面,选择I have my product information,输入序列号(serial mumber):666-86868686 产品密钥(product Key):657D1 ,点击下一步。根据需要选择安装组件,下面可以选择安装路径,然后点击install安装,这里可能有点慢,等待...等安装完成以后,点击Finish完成安装。然后运行Maya2012,这时候会出现激活画面,点击Activate激活按钮然后选中界面中的复选框,点击Continue继续。这里要注意一下,需要断开网络才能成功激活。进入激活界面,选择第二个,然后点next继续ProductRegistration界面中,选择中国,然后下一步然后出现用户信息界面,按照格式填写好以后,点击下一步就可以了。这个时候maya就记录了你的信息,点击关闭,maya2012会自动运行,虽然这次能运行maya,但是这时候还没有成功激活,关闭maya2012,再点击桌面的哦maya2012图标重新进入,这时候依然要保持断网状态。重启之后,又会出现激活界面,重复之前的步骤,继续。这时候就出现下面这个界面,然后复制Request code一串代码。然后打开maya2012注册机,先按一下Men Patch按钮,然后再第一项输入开始复制的代码,然后点击Generat按钮,下面就会生成Activation激活码,复制这一串激活码!然后把复制的激活码,直接在第一个框中粘贴就可以了,点击下一步,恭喜你,Maya2012激活完成!安装上面的方法我成功安装了maya2012,
Maya相关资源

Maya相关资源Autodesk Maya Developer Area:Maya API Help:<maya_root>/devkit/plug-insVideo Tutorials:03 Python Scripting in MayaCGCircuit - Introduction to the Maya APIcmiVFX - PyQt4 UI Development for MayaDigital Tutors - Creating Custom User Interfaces in Maya and Qt DesignerDigital Tutors - Getting Started with Python Scripting in MayaEat 3D - Autodesk Maya Scripting - An Introduction and ApplicationGnomon_MEL 101_Fundamentals_repackedMaya Programming Pack 12 Trainings For MEL Python API ( the real deal)Apply Maya NodeIdsMaya plugin 下载(Bouns tools,....)Maya Help (doc)根⽬录在线⽂档其他论坛Blogs:plugincg相关论坛cg ftpqq群: (68118301)教程melt deformer for maya 1.2.0Simulating Musculature in Mayamuscle systemmaya视频教程插件供应商⾻骼插件 Advanced Skeletonv视频教程The Ultimate Collection Of Maya 3D Tutorials /2009/08/27/the-ultimate-collection-of-maya-3d-tutorials/ EnvNode /showthread.php?t=848065/tag/maya-animation/Dover Studios Maya Shading NetworksDOVER STUDIOS MAYA: Hypershade WorkflowDOVER STUDIOS MAYA: Surface Materials电⼦书教程展UV的插件开源插件、项⽬:liquid ( ), , ,- A file format and data structures for storing 3D voxel and simulation data., googlecode, blog,等作者的其他项⽬is a python framework based on wrapping the Maya API to speed updevelopment and ease of use within pipelines using Autodesk Maya. it a tools set for translating Maya scenes into Renderman and Gelato scene descriptions.is a Public character/animation pipeline tools for Autodesk's Maya.Audits MSI packages for basic issues.Maya lighting tool.on github (, ....)Ptex (, , , )batchRenderhttps:///eliang/mayadummyrenderer//44694.htmllip-sync/p/papagayo//p/stretchmesh/ website:/tools/Visualize complex shaders with GLSL code without having to write a any c++ node, just point the Shader node at the shader files and have your attributes and uniforms work with coral as native data types.shatter/p/proceduralshatter/UsefulResourcesForumsThis is the one I use the most to ask for help or search for answers towrite my scripts. Very active community, your question will (almost)always be addressed by someone!Also very active community.: Google group. You must be a member to read and post. Quite activeScript repositories and tutorials and wikisHuge collection of scripts for Maya, both in Python and MEL.MEL Database from the TU Delft.Wiki set up by our former scripting tutor Tobias Schwimm, with some tutorials and scripts to download.mel editor"The Proxy.wiki is a platform of sharing and discussion forcomputational techniques in architecture. Scripts and tutorials ofgeneral interest can be found here, as well as student codes and otherresources."InspirationBlog written by people from ZH's research lab.Alisa AndrasekDesign Explorer"Daniel Shiffman works as an Assistant Arts Professor at theInteractive Telecommunications Program at NYU’s Tisch School of theArts.""Marc Fornes, Architect DPLG, is the founder of THEVERYMANY,, a design studio and collaborative research forumengaging the field of architecture via encoded and explicit processes.""Generator.x is a conference and exhibition examining the current roleof software and generative strategies in art and design.""kokkugia is an international design practice based in london and newyork that operates through design, research, experimentation andteaching. "-------------------API Dev--------------------Free rigs/~jdoubl20/rigsScripts.html/archive/index.php/t-397196.html/vbforum/showthread.php?137361-Free-Maya-Character-Rigs/forums/showthread.php?t=647/2010/03/22-of-the-most-fun-rigs-for-maya/animation/model download/?fromuid=117330/maya/downloads/3d-motion-data-files/c/?/maya/downloads/character-rigs/c/?free shaders mayasoftware/mentalray。
Maya各版本软件下载链接

Maya各版本版本下载链接-SK整理为了方便大家对maya软件的学习,在这给出maya各个版本软件对应的下载链接: (迅雷新建任务粘贴链接地址即可)MAYA-2012:-Maya2012安装指引:ed2k://|file|autodesk.2012.安装说明(产品密匙&序列号).txt|1507|e91aa7eae1d1a5b5f65664138ff9dde2|h=exxolwgw77it7cbkmu7jpmwnl2lamjaw|/ -Maya2012(32位):ed2k://|file|%5BAutodesk%E4%B8%89%E7%BB%B4%E7%89%B9%E6%95%88%E8 %BD%AF%E4%BB%B6Maya.2011%5D.TLF-SOFT-AUTODESK.MAYA.V2011.SP1.WI N32-ISO.iso|1550354432|5dcdb3d9a83073d272b6786a4246cf9f|h=4z3yoesof5okwrvjh6jhiofdlxpwngj3|/-Maya2012(64位):ed2k://|file|%5BAutodesk%E4%B8%89%E7%BB%B4%E7%89%B9%E6%95%88%E8 %BD%AF%E4%BB%B6Maya.2011%5D.TLF-SOFT-AUTODESK.MAYA.V2011.SP1.WI N64-ISO.iso|1811849216|f11d1b352b7340d49e843ec6af390e47|h=bxbudozrhgkmbxunjpxdyhe6btriwsiy|/-Maya2012(32/64位):ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.TLF-SOF T-AUTODESK.MAYA.V2012.WIN32-ISO.iso|1553752064|2461cf07816ab94a6b022e112442a19b|h=osau4k54bdls6ynporvep2ea4tm6kvx5|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.TLF-SOF T-AUTODESK.MAYA.V2012.WIN32-ISO.nfo|1220|926c6abe545df02fbe3c44e5fc287582|h=264bivft6itjlfsiard3xwjsfsaggt73|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.TLF-SOF T-AUTODESK.MAYA.V2012.WIN64-ISO.iso|1630885888|2aa4fb2b57cea59ad7ab2d2eeeaa3935|h=3dr2vy53yoyrxxh44dek23xe4esaidnb|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8%E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.TLF-SOF T-AUTODESK.MAYA.V2012.WIN64-ISO.nfo|1220|a8d5e7236eb088b71559c5d714215443|h=tickelhf2gfeudwpuelp6mw3yegdqvc3|/ored2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.AUTODE SK.MAYA.V2012.SP1.WIN32-ISO.iso|1625896960|b409b5b99fddbd497a0f188d2acd59b3|h=gydup3gb6d2jrgezpiafbjdxvp3kwyzb|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.AUTODE SK.MAYA.V2012.SP1.WIN32-ISO.nfo|1226|b44ad01dc9dcb950d48346553bff6fb6|h=xlcemunh36sjemyla2wab7owgdqvody6|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.AUTODE SK.MAYA.V2012.SP1.WIN64-ISO.iso|1689147392|d4be474672f5c6a8e2b210f2fc33a2d7|h=nu2wgivmkkexqnibhyzvfwxcv6t4s7qo|/ed2k://|file|%5B%E4%B8%89%E7%BB%B4%E8%A7%92%E8%89%B2%E5%8A%A8 %E7%94%BB%E5%88%B6%E4%BD%9C%E5%B7%A5%E5%85%B7%5D.AUTODE SK.MAYA.V2012.SP1.WIN64-ISO.nfo|1225|b93e5b1764e5de530450b31652d49aa7|h=5xvuga5rt6onr5ph2ims4gbyedekupgy|/-Maya2012注册机(32/64位):ed2k://|file|Maya2012注册机_x86_x64.rar|36063484|f01d7ade720aa1d4f48e02a8c902daf8|/ed2k://|file|欧特克2012-Windows.x32全系列注册机.rar|198691|4ce3d3ff51c60f9ef421da7b356129bc|/ed2k://|file|欧特克2012-Windows.x64全系列注册机.rar|199896|5c5b95697fd56779d90068d46a635a7c|/-Maya2012官方汉化包(32位):/adsk/files/autodesk_maya2012_hotfix3_win_32bit.mspored2k://|file|maya2012官方汉化包_autodesk_maya2012_hotfix3_win_32bit.msp|80429056|92e37434626730f76d93c7b79748cdc0|/-Maya2012官方汉化包(64位):ed2k://|file|[欧特克2011-2012数字娱乐创作解决方案].autodesk_maya2012_hotfix3_win_64bit.msp|96407552|e1de82c70818d5e581b5cc395c5adbcb|h=siltkqdd4f6dh7bbpqxfnqjvcksv4igi|/-Maya2012官方中文帮助文档:ed2k://|file|[欧特克2011-2012数字娱乐创作解决方案].maya2012_help_chs_win.exe|218257627|cad739a064740b0d6d15df04735de9e3|h=h7rnkzuvxjwa72rra7pitppcvac35vzb|/MAYA-2011:-Maya2011安装指引:ed2k://|file|欧特克2011数字娱乐创作解决方案.install.for.maya.txt|1229|4f3ca2a47d8613e0f233c9ab6c10ff53|h=ykw6caq6rvsyo2v6v25dcej3kfomfymh|/-Maya2011(32位):ed2k://|file|[Autodesk三维特效软件Maya.2011].TLF-SOFT-AUTODESK.MAYA.V2011.SP1.WIN32-ISO.iso|1550354432|5dcdb3 d9a83073d272b6786a4246cf9f|h=4z3yoesof5okwrvjh6jhiofdlxpwngj3|/-Maya2011(64位):ed2k://|file|[Autodesk三维特效软件Maya.2011].TLF-SOFT-AUTODESK.MAYA.V2011.SP1.WIN64-ISO.iso|1811849216|f11d1b 352b7340d49e843ec6af390e47|h=bxbudozrhgkmbxunjpxdyhe6btriwsiy|/-Maya2011注册机(32/64位):ed2k://|file|Maya2011.x86_x64注册机.rar|153902|89a6349bad485c62bdce8cc9fdbb9b92|/ed2k://|file|欧特克2011-Windows.x32全系列注册机.rar|76496|2f4fc2380c13affdb403e8c8d11ecf46|h=umki5zbtypxiyz7co6og5ersx2jk2wel|/ed2k://|file|欧特克2011-Windows.x64全系列注册机.rar|77427|3b5ee6205d225436fd1c8731ed5f4a8d|h=k5lbafnkwuhy6m7auvp7smg3g3iruorq|/-Maya2011中英双语补丁(32位):ed2k://|file|Autodesk.Maya.2011.中英双语补丁.rar|22573609|e6b182c4f3bb4efcdab1795a9c380be7|/MAYA-2010:-Maya2010(32位):ed2k://|file|[欧特克.maya2010.无限正式版].maya2010-Win-64.rar|376401182|ffd5e6042b46542fde913e4d20b6d1fa|h=ugneiolfw7lomqtncgq2k5bimqono57z|/-Maya2010(64位):ed2k://|file|[Autodesk三维特效软件Maya.2011].TLF-SOFT-AUTODESK.MAYA.V2011.SP1.WIN64-ISO.iso|1811849216|f11d1b 352b7340d49e843ec6af390e47|h=bxbudozrhgkmbxunjpxdyhe6btriwsiy|/-Maya2010注册机:ed2k://|file|maya2010注册机-32bits.rar|76418|7e58eb2f0d4e6c6ae8e65cbf3cb6dae7|h=bc624bohh3ee3wm7zu7i7wpwstowy5mo|/MAYA-2009:-Maya2009(完全破解版):thunder://QUFlZDJrOi8vfGZpbGV8JTVCJUU2JUFDJUE3JUU3JTg5JUI5JUU1JTg1JTh CJUU3JThFJTlCJUU5JTlCJTg1MjAwOURWRCVFNSVBRSU4QyVFNSU4NSVBOCVF NyU4OSU4OCU1RC5BVVRPREVTSy5NQVlBLjIwMDkuaXNvfDMwMzA0NjY1NjB8MT g2M2NkZGNhZDgxOTUzNzgzNmQ2NzZmMGE3ZTQzNGN8aD01VTNKUVJONFVKW TdFU0pQQVk2R0ZUQTdUWTRVSExJN3wvWlo=-Maya2009(终极版32位):ed2k://|file|[歐特克Maya.09終極版].Autodesk.Maya.Unlimited.v2009.SP1.Win32-XFORCE.rar|378896903|4632e46e3ec7b07f 08b15654cb026bf7|h=zxeyoepc4x5vigidmm4rh5d7g5josphe|/-Maya2009(终极版64位):ed2k://|file|[歐特克Maya.09終極版].Autodesk.Maya.Unlimited.v2009.SP1.Win64-XFORCE.rar|401029476|bc8f8d6d6bd3168 f5fa02c090178c5b3|h=luy2wkidiqmplrev6t3xqdwbtpn7hrcf|/-Maya2009注册机:/down/maya2009注册机.rar-Maya2009汉化补丁:ed2k://|file|Maya2009.汉化补丁(无广告)小怪物整理.rar|25502833|5b99b5acc450196a139cef007a29711e|h=272jhvwrf4vlzevtqngj6y2cwtzir74t|/其他一些较老的版本就不罗列出来了,也不推荐现阶段的初学者学习。
1-2007_-_Y_F_Han_-_PreparationofnanosizedMn3O4SBA15catalystforcomplet[retrieved-2016-11-15]
![1-2007_-_Y_F_Han_-_PreparationofnanosizedMn3O4SBA15catalystforcomplet[retrieved-2016-11-15]](https://img.taocdn.com/s3/m/9d89122483c4bb4cf7ecd13a.png)
Preparation of nanosized Mn 3O 4/SBA-15catalyst for complete oxidation of low concentration EtOH in aqueous solution with H 2O 2Yi-Fan Han *,Fengxi Chen,Kanaparthi Ramesh,Ziyi Zhong,Effendi Widjaja,Luwei ChenInstitute of Chemical and Engineering Sciences,1Pesek Road,Jurong Island 627833,Singapore Received 11May 2006;received in revised form 18December 2006;accepted 29May 2007Available online 2June 2007AbstractA new heterogeneous Fenton-like system consisting of nano-composite Mn 3O 4/SBA-15catalyst has been developed for the complete oxidation of low concentration ethanol (100ppm)by H 2O 2in aqueous solution.A novel preparation method has been developed to synthesize nanoparticles of Mn 3O 4by thermolysis of manganese (II)acetylacetonate on SBA-15.Mn 3O 4/SBA-15was characterized by various techniques like TEM,XRD,Raman spectroscopy and N 2adsorption isotherms.TEM images demonstrate that Mn 3O 4nanocrystals located mainly inside the SBA-15pores.The reaction rate for ethanol oxidation can be strongly affected by several factors,including reaction temperature,pH value,catalyst/solution ratio and concentration of ethanol.A plausible reaction mechanism has been proposed in order to explain the kinetic data.The rate for the reaction is supposed to associate with the concentration of intermediates (radicals: OH,O 2Àand HO 2)that are derived from the decomposition of H 2O 2during reaction.The complete oxidation of ethanol can be remarkably improved only under the circumstances:(i)the intermediates are stabilized,such as stronger acidic conditions and high temperature or (ii)scavenging those radicals is reduced,such as less amount of catalyst and high concentration of reactant.Nevertheless,the reactivity of the presented catalytic system is still lower comparing to the conventional homogenous Fenton process,Fe 2+/H 2O 2.A possible reason is that the concentration of intermediates in the latter is relatively high.#2007Elsevier B.V .All rights reserved.Keywords:Hydrogen peroxide;Fenton catalyst;Complete oxidation of ethanol;Mn 3O 4/SBA-151.IntroductionRemediation of wastewater containing organic constitutes is of great importance because organic substances,such as benzene,phenol and other alcohols may impose toxic effects on human and animal anic effluents from pharmaceu-tical,chemical and petrochemical industry usually contaminate water system by dissolving into groundwater.Up to date,several processes have been developed for treating wastewater that contains toxic organic compounds,such as wet oxidation with or without solid catalysts [1–4],biological oxidation,supercritical oxidation and adsorption [5,6],etc.Among them,catalytic oxidation is a promising alternative,since it avoids the problem of the adsorbent regeneration in the adsorption process,decreases significantly the temperature and pressure in non-catalytic oxidation techniques [7].Generally,the disposalof wastewater containing low concentration organic pollutants (e.g.<100ppm)can be more costly through all aforementioned processes.Thus,catalytic oxidation found to be the most economical way for this purpose with considering its low cost and high efficiency.Currently,a Fenton reagent that consists of homogenous iron ions (Fe 2+)and hydrogen peroxide (H 2O 2)is an effective oxidant and widely applied for treating industrial effluents,especially at low concentrations in the range of 10À2to 10À3M organic compounds [8].However,several problems raised by the homogenous Fenton system are still unsolved,e.g.disposing the iron-containing waste sludge,limiting the pH range (2.0–5.0)of the aqueous solution,and importantly irreversible loss of activity of the reagent.To overcome these drawbacks raised from the homogenous Fenton system,since 1995,a heterogeneous Fenton reagent using metal ions exchanged zeolites,i.e.Fe/ZSM-5has proved to be an interesting alternative catalytic system for treating wastewater,and showed a comparable activity with the homogenous Fenton system [9].However,most reported heterogeneous Fenton reagents still need UV radiation during/locate/apcatbApplied Catalysis B:Environmental 76(2007)227–234*Corresponding author.Tel.:+6567963806.E-mail address:han_yi_fan@.sg (Y .-F.Han).0926-3373/$–see front matter #2007Elsevier B.V .All rights reserved.doi:10.1016/j.apcatb.2007.05.031oxidation of organic compounds.This might limit the application of homogeneous Fenton system.Exploring other heterogeneous catalytic system considering the above disadvantages,is still desirable for this purpose.Here,we present an alternative catalytic system for the complete oxidation of organic com-pounds in aqueous solution using supported manganese oxide as catalyst under mild conditions,which has rarely been addressed.Mn-containing oxide catalysts have been found to be very active for the catalytic wet oxidation of organic effluents (CWO)[10–14],which is operated at high air pressures(1–22MPa)and at high temperatures(423–643K)[15].On the other hand,manganese oxide,e.g.MnO2[16],is well known to be active for the decomposition of H2O2in aqueous solution to produce hydroxyl radical( OH),which is considered to be the most robust oxidant so far.The organic constitutes can be deeply oxidized by those radicals rapidly[17].The only by-product is H2O from decomposing H2O2.Therefore,H2O2is a suitable oxidant for treating the wastewater containing organic compounds.Due to the recent progress in the synthesis of H2O2 directly from H2and O2[18,19],H2O2is believed to be produced through more economical process in the coming future.So,the heterogeneous Fenton system is economically acceptable.In this study,nano-crystalline Mn3O4highly dispersed inside the mesoporous silica,SBA-15,has been prepared by thermolysis of organic manganese(II)acetylacetonate in air. We expect the unique mesoporous structure may provide add-itional function(confinement effect)to the catalytic reaction, i.e.occluding/entrapping large organic molecules inside pores. The catalyst as prepared has been examined for the complete oxidation of ethanol in aqueous solution with H2O2,or to say, wet peroxide oxidation.Ethanol was selected as a model organic compound because(i)it is one of the simplest organic compounds and can be easily analyzed,(ii)it has high solu-bility in water due to its strong hydrogen bond with water molecule and(iii)the structure of ethanol is quite stable and only changed through catalytic reaction.Presently,for thefirst time by using the Mn3O4/SBA-15catalyst,we investigated the peroxide ethanol oxidation affected by factors such as temperature,pH value,ratio of catalyst(g)and volume of solution(L),and concentration of ethanol in aqueous solution. In addition,plausible reaction mechanisms are established to explain the peroxidation of ethanol determined by the H2O2 decomposition.2.Experimental2.1.Preparation and characterization of Mn3O4/SBA-15 catalystSynthesis of SBA-15is similar to the previous reported method[20]by using Pluronic P123(BASF)surfactant as template and tetraethyl orthosilicate(TEOS,98%)as silica source.Manganese(II)acetylacetonate([CH3COCH C(O)CH3]2Mn,Aldrich)by a ratio of2.5mmol/gram(SBA-15)werefirst dissolved in acetone(C.P.)at room temperature, corresponding to ca.13wt.%of Mn3O4with respect to SBA-15.The preparation method in detail can be seen in our recent publications[21,22].X-ray diffraction profiles were obtained with a Bruker D8 diffractometer using Cu K a radiation(l=1.540589A˚).The diffraction pattern was taken in the Bragg angle(2u)range at low angles from0.68to58and at high angles from308to608at room temperature.The XRD patterns were obtained by scanning overnight with a step size:0.028per step,8s per step.The dispersive Raman microscope employed in this study was a JY Horiba LabRAM HR equipped with three laser sources(UV,visible and NIR),a confocal microscope,and a liquid nitrogen cooled charge-coupled device(CCD)multi-channel detector(256pixelsÂ1024pixels).The visible 514.5nm argon ion laser was selected to excite the Raman scattering.The laser power from the source is around20MW, but when it reached the samples,the laser output was reduced to around6–7MW after passing throughfiltering optics and microscope objective.A100Âobjective lens was used and the acquisition time for each Raman spectrum was approximately 60–120s depending on the sample.The Raman shift range acquired was in the range of50–1200cmÀ1with spectral resolution1.7–2cmÀ1.Adsorption and desorption isotherms were collected on Autosorb-6at77K.Prior to the measurement,all samples were degassed at573K until a stable vacuum of ca.5m Torr was reached.The pore size distribution curves were calculated from the adsorption branch using Barrett–Joyner–Halenda(BJH) method.The specific surface area was assessed using the BET method from adsorption data in a relative pressure range from 0.06to0.10.The total pore volume,V t,was assessed from the adsorbed amount of nitrogen at a relative pressure of0.99by converting it to the corresponding volume of liquid adsorbate. The conversion factor between the volume of gas and liquid adsorbate is0.0,015,468for N2at77K when they are expressed in cm3/g and cm3STP/g,respectively.The measurements of transmission electron microscopy (TEM)were performed at Tecnai TF20S-twin with Lorentz Lens.The samples were ultrasonically dispersed in ethanol solvent,and then dried over a carbon grid.2.2.Kinetic measurement and analysisThe experiment for the wet peroxide oxidation of ethanol was carried out in a glass batch reactor connected to a condenser with continuous stirring(400rpm).Typically,20ml of aqueous ethanol solution(initial concentration of ethanol: 100ppm)wasfirst taken in the round bottomflask(reactor) together with5mg of catalyst,corresponding to ca.1(g Mn)/30 (L)ratio of catalyst/solution.Then,1ml of30%H2O2solution was introduced into the reactor at different time intervals (0.5ml at$0min,0.25ml at32min and0.25ml at62min). The total molar ratio of H2O2/ethanol is about400/1. Hydrochloric acid(HCl,0.01M)was used to acidify the solution if necessary.NH4OH(0.1M)solution was used to adjust pH to9.0when investigating the effect of pH.The pH for the deionized water is ca.7.0(Oakton pH meter)and decreased to 6.7after adding ethanol.All the measurements wereY.-F.Han et al./Applied Catalysis B:Environmental76(2007)227–234 228performed under the similar conditions described above if without any special mention.For comparison,the reaction was also carried out with a typical homogenous Fenton reagent[17], FeSO4(5ppm)–H2O2,under the similar reaction conditions.The conversion of ethanol during reaction was detected using gas chromatography(GC:Agilent Technologies,6890N), equipped with HP-5capillary column connecting to a thermal conductive detector(TCD).There is no other species but ethanol determined in the reaction system as evidenced by the GC–MS. Ethanol is supposed to be completely oxidized into CO2and H2O.The variation of H2O2concentration during reaction was analyzed colorimetrically using a UV–vis spectrophotometer (Epp2000,StellarNet Inc.)after complexation with a TiOSO4/ H2SO4reagent[18].Note that there was almost no measurable leaching of Mn ion during reaction analyzed by ICP(Vista-Mpx, Varian).3.Results and discussion3.1.Characterization of Mn3O4/SBA-15catalystThe structure of as-synthesized Mn3O4inside SBA-15has beenfirst investigated with powder XRD(PXRD),and the profiles are shown in Fig.1.The profile at low angles(Fig.1a) suggests that SBA-15still has a high degree of hexagonal mesoscopic organization even after forming Mn3O4nanocrys-tals[23].Several peaks at high angles of XRD(Fig.1b)indicate the formation of a well-crystallized Mn3O4.All the major diffraction peaks can be assigned to hausmannite Mn3O4 structure(JCPDS80-0382).By N2adsorption measurements shown in Fig.2,the pore volume and specific surface areas(S BET)decrease from 1.27cm3/g and937m2/g for bare SBA-15to0.49cm3/g and 299m2/g for the Mn3O4/SBA-15,respectively.About7.7nm of mesoporous diameter for SBA-15decreases to ca.6.3nm for Mn3O4/SBA-15.The decrease of the mesopore dimension suggests the uniform coating of Mn3O4on the inner walls of SBA-15.This nano-composite was further characterized by TEM. Obviously,the SBA-15employed has typical p6mm hex-agonal morphology with the well-ordered1D array(Fig.3a). The average pore size of SBA-15is ca.8.0nm,which is very close to the value(ca.7.7nm)determined by N2adsorption. Along[001]orientation,Fig.3b shows that the some pores arefilled with Mn3O4nanocrystals.From the pore A to D marked in Fig.3b correspond to the pores from empty to partially and fullyfilled;while the features for the SBA-15 nanostructure remains even after forming Mn3O4nanocrys-tals.Nevertheless,further evidences for the location of Mn3O4inside the SBA-15channels are still undergoing in our group.Raman spectra obtained for Mn3O4/SBA-15is presented in Fig.4a.For comparison the Raman spectrum was also recorded for the bulk Mn3O4(97.0%,Aldrich)under the similar conditions(Fig.4b).For the bulk Mn3O4,the bands at310,365, 472and655cmÀ1correspond to the bending modes of Mn3O4, asymmetric stretch of Mn–O–Mn,symmetric stretch of Mn3O4Fig.1.XRD patterns of the bare SBA-15and the Mn3O4/SBA-15nano-composite catalyst.(a)At low angles:(A)Mn3O4/SBA-15,(B)SBA-15;and (b)at high angles of Mn3O4/SBA-15.Fig.2.N2adsorption–desorption isotherms:(!)SBA-15,(~)Mn3O4/SBA-15.Y.-F.Han et al./Applied Catalysis B:Environmental76(2007)227–234229groups,respectively [24–26].However,a downward shift ($D n 7cm À1)of the peaks accompanying with a broadening of the bands was observed for Mn 3O 4/SBA-15.For instance,the distinct feature at 655cm À1for the bulk Mn 3O 4shifted to 648cm À1for the nanocrystals.The Raman bands broadened and shifted were observed for the nanocrystals due to the effect of phonon confinement as suggested previously in the literature [27,28].Furthermore,a weak band at 940cm À1,which should associate with the stretch of terminal Mn O,is an indicative of the existence of the isolated Mn 3O 4group [26].The assignment of this unique band has been discussed in our previous publication [22].3.2.Kinetic study3.2.1.Blank testsUnder a typical reaction conditions,that is,20ml of 100ppm ethanol aqueous solution (pH 6.7)mixed with 1ml of 30%H 2O 2,at 343K,there is no conversion of ethanol was observed after running for 120min in the absence of catalyst or in the presence of bare SBA-15(5mg).Also,under the similar conditions in H 2O 2-free solution,ethanol was not converted for all blank tests even with Mn 3O 4/SBA-15catalyst (5mg)in the reactor.It suggests that a trace amount of oxygen dissolved in water or potential dissociation of adsorbed ethanol does not have any contribution to the conversion of ethanol under reaction conditions.To study the effect of low temperature evaporation of ethanol during reaction,we further examined the concentration of ethanol (100ppm)versus time at different temperatures in the absence of catalyst and H 2O 2.Loss of ca.5%ethanol was observed only at 363K after running for 120min.Hence,to avoid the loss of ethanol through evaporation at high temperatures,which may lead to a higher conversion of ethanol than the real value,the kinetic experiments in this study were performed at or below 343K.The results from blank tests confirm clearly that ethanol can be transformed only by catalytic oxidation during reaction.3.2.2.Effect of amount of catalystThe effect of amount of catalyst on ethanol oxidation is presented in Fig.5.Different amounts of catalyst ranging from 2to 10mg were taken for the same concentration of ethanol (100ppm)in aqueous solution under the standard conditions.It can be observed that the conversion of ethanol increases monotonically within 120min,reaching 15,20and 12%for 2,5and 10mg catalysts,respectively.On the other hand,Fig.5shows that the relative reaction rates (30min)decreased from 0.7to ca 0.1mmol/g Mn min with the rise of catalyst amount from 2to 10mg.Apparently,more catalyst in the system may decrease the rate for ethanol peroxidation,and a proper ratio of catalyst (g)/solution (L)is required for acquiring a balance between the overall conversion of ethanol and reaction rate.In order to investigate the effects from other factors,5mg (catalyst)/20ml (solution),corresponding to 1(g Mn )/30(L)ratio of catalyst/solution,has been selected for the followedexperiments.Fig.4.Raman spectroscopy of the Mn 3O 4/SBA-15(a)and bulk Mn 3O 4(b).Fig.3.TEM images recorded along the [001]of SBA-15(a),Mn 3O 4/SBA-15(b):pore A unfilled with hexagonal structure,pores B and C partially filled and pore D completely filled.Y.-F .Han et al./Applied Catalysis B:Environmental 76(2007)227–2342303.2.3.Effect of temperatureAs shown in Fig.6,the reaction rate increases with increasing the reaction temperature.After 120min,the conversion of ethanol increases from 12.5to 20%when varying the temp-erature from 298to 343K.Further increasing the temperature was not performed in order to avoid the loss of ethanol by evaporation.Interestingly,the relative reaction rate increased with time within initial 60min at 298and 313K,but upward tendency was observed above 333K.3.2.4.Effect of pHIn the pH range from 2.0to 9.0,as illustrated in Fig.7,the reaction rate drops down with the rise of pH.It indicates that acidic environment,or to say,proton concentration ([H +])in the solution is essential for this reaction.With considering our target for this study:purifying water,pH approaching to 7.0in the reaction system is preferred.Because acidifying the solution with organic/inorganic acids may potentially causea second time pollution and result in surplus cost.Actually,there is almost no effect on ethanol conversion with changing pH from 5.5to 6.7in this system.It is really a merit comparing with the conventional homogenous Fenton system,by which the catalyst works only in the pH range of 2.0–5.0.3.2.5.Effect of ethanol concentrationThe investigation of the effect of ethanol concentration on the reaction rate was carried out in the ethanol ranging from 50to 500ppm.The results in Fig.8show that the relative reaction rate increased from 0.07to 2.37mmol/g Mn min after 120min with increasing the concentration of ethanol from 50to 500ppm.It is worth to note that the pH value of the solution slightly decreased from 6.7to 6.5when raising the ethanol concentration from 100to 500ppm.paring to a typical homogenous Fenton reagent For comparison,under the similar reaction conditions ethanol oxidation was performed using aconventionalFig.5.The ethanol oxidation as a function of time with different amount of catalyst.Conversion of ethanol vs.time (solid line)on 2mg (&),5mg (*)and 10mg (~)Mn 3O 4/SBA-15catalyst,the relative reaction rate vs.time (dash line)on 2mg (&),5mg (*)and 10mg (~)Mn 3O 4/SBA-15catalyst.Rest conditions:20ml of ethanol (100ppm),1ml of 30%H 2O 2,708C and pH of6.7.Fig.6.The ethanol oxidation as a function of temperature.Conversion of ethanol vs.time (solid line)at 258C (&),408C (*),608C (~)and 708C (!),the relative reaction rate vs.time (dash line)at 258C (&),408C (*),608C (~)and 708C (5).Rest conditions:20ml of ethanol (100ppm),1ml of 30%H 2O 2,pH of 6.7,5mg ofcatalyst.Fig.7.The ethanol oxidation as a function of pH value.Conversion of ethanol vs.time (solid line)at pH value of 2.0(&),3.5(*),4.5(~),5.5(!),6.7(^)and 9.0("),the relative reaction rate vs.time (dash line)at pH value of 2.0(&),3.5(*),4.5(~),5.5(5),6.7(^)and 9.0(").Rest conditions:20ml of ethanol (100ppm),1ml of 30%H 2O 2,708C,5mg ofcatalyst.Fig.8.The ethanol oxidation as a function of ethanol concentration.Conver-sion of ethanol vs.time (solid line)for ethanol concentration (ppm)of 50(&),100(*),300(~),500(!),the relative reaction rate vs.time (dash line)for ethanol concentration (ppm)of 50(&),100(*),300(~),500(5).Condi-tions:20ml of ethanol,pH of 6.7,1ml of 30%H 2O 2,708C,5mg of catalyst.Y.-F .Han et al./Applied Catalysis B:Environmental 76(2007)227–234231homogenous reagent,Fe 2+(5ppm)–H 2O 2(1ml)at pH of 5.0.It has been reported to be an optimum condition for this system [17].As shown in Fig.9,the reaction in both catalytic systems exhibits a similar behavior,that is,the conversion of ethanol increases with extending the reaction time.Varying reaction temperature from 298to 343K seems not to impact the conversion of ethanol when using the homogenous Fenton reagent.Furthermore,the conversion of ethanol (defining at 120min)in the system of Mn 3O 4/SBA-15–H 2O 2is about 60%of that obtained from the conventional Fenton reagent.There are no other organic compounds observed in the reaction mixture other than ethanol suggesting that ethanol directly decomposing to CO 2and H 2O.3.2.7.Decomposition of H 2O 2In the aqueous solution,the capability of metal ions such as Fe 2+and Mn 2+has long been evidenced to be effective on the decomposition of H 2O 2to produce the hydroxyl radical ( OH),which is oxidant for the complete oxidation/degrading of organic compounds [9,17].Therefore,ethanol oxidation is supposed to be associated with H 2O 2decomposition.The investigation of H 2O 2decomposition has been performed under the reaction conditions (in an ethanol-free solution)with different amounts of catalyst.H 2O 2was introduced into the reaction system by three steps,initially 0.5ml followed by twice 0.25ml at 32and 62min,the pH of 6.7is set for all experiments except pH of 5.0for Fe 2+.As shown in Fig.10,H 2O 2was not converted in the absence of catalyst or presence of bare SBA-15(5mg);in contrast,by using the Mn 3O 4/SBA-15catalyst we observed that ca.Ninety percent of total H 2O 2was decomposed in the whole experiment.It can be concluded that that dissociation of H 2O 2is mainly caused by Mn 3O paratively,the rate of H 2O 2decomposition is relatively low with the homogenous Fenton reagent,total conversion of H 2O 2,was ca.50%after runningfor 120min.Considering the fact that H 2O 2decomposition can be significantly enhanced with the rise of Fe 2+concentration,however,it seems not to have the influence on the reaction rate for ethanol oxidation simultaneously.The similar behavior of H 2O 2decomposition was also observed during ethanol oxidation.The rate for ethanol oxidation is lower for Mn 3O 4/SBA-15comparing to the conventional Fenton reagent.The possible reasons will be discussed in the proceeding section.3.3.Plausible reaction mechanism for ethanol oxidation with H 2O 2In general,the wet peroxide oxidation of organic constitutes has been suggested to proceed via four steps [15]:activation of H 2O 2to produce OH,oxidation of organic compounds withOH,recombination of OH to form O 2and wet oxidation of organic compounds with O 2.It can be further described by Eqs.(1)–(4):H 2O 2À!Catalyst =temperture 2OH(1)OH þorganic compoundsÀ!Temperatureproduct(2)2 OHÀ!Temperature 12O 2þH 2O(3)O 2þorganic compoundsÀ!Temperature =pressureproduct(4)The reactive intermediates produced from step 1(Eq.(1))participate in the oxidation through step 2(Eq.(2)).In fact,several kinds of radical including OH,perhydroxyl radicals ( HO 2)and superoxide anions (O 2À)may be created during reaction.Previous studies [29–33]suggested that the process for producing radicals could be expressed by Eqs.(5)–(7)when H 2O 2was catalytically decomposed by metal ions,such asFeparison of ethanol oxidation in systems of typical homogenous Fenton catalyst (5ppm of Fe 2+,20ml of ethanol (100ppm),1ml of 30%H 2O 2,pH of 5.0acidified with HCl)at room temperature (~)and 708C (!),and Mn 3O 4/SBA-15catalyst (&)under conditions of 20ml of ethanol (100ppm),pH of 6.7,1ml of 30%H 2O 2,708C,5mg ofcatalyst.Fig.10.An investigation of H 2O 2decomposition under different conditions.One milliliter of 30%H 2O 2was dropped into the 20ml deionized water by three intervals,initial 0.5ml followed by twice 0.25ml at 32and 62min.H 2O 2concentration vs.time:by calculation (&),without catalyst (*),SBA-15(~),5ppm of Fe 2+(!)and Mn 3O 4/SBA-15(^).Rest conditions:5mg of solid catalyst,pH of 7.0(5.0for Fe 2+),708C.Y.-F .Han et al./Applied Catalysis B:Environmental 76(2007)227–234232and Mn,S þH 2O 2!S þþOH Àþ OH (5)S þþH 2O 2!S þ HO 2þH þ(6)H 2O $H þþO 2À(7)where S and S +represent reduced and oxidized metal ions,both the HO 2and O 2Àare not stable and react further with H 2O 2to form OH through Eqs.(8)and (9):HO 2þH 2O 2! OH þH 2O þO 2(8)O 2ÀþH 2O 2! OH þOH ÀþO 2(9)Presently, OH radical has been suggested to be the main intermediate responsible for oxidation/degradation of organic compounds.Therefore,the rate for ethanol oxidation in the studied system is supposed to be dependent on the concentra-tion of OH.Note that the oxidation may proceed via step four (Eq.(4))in the presence of high pressure O 2,which is so-called ‘‘wet oxidation’’and usually occurs at air pressures (1–22MPa)and at high temperatures (423–643K)[15].However,it is unlikely to happen in the present reaction conditions.According to Wolfenden’s study [34],we envisaged that the complete oxidation of ethanol may proceed through a route like Eq.(10):C 2H 5OH þ OH À!ÀH 2OC 2H 4O À! OHCO 2þH 2O(10)Whereby,it is believed that organic radicals containing hydroxy-groups a and b to carbon radicals centre can eliminate water to form oxidizing species.With the degrading of organic intermediates step by step as the way described in Eq.(10),the final products should be CO 2and H 2O.However,no other species but ethanol was detected by GC and GC–MS in the present study possibly due to the rapid of the reaction that leads to unstable intermediate.Fig.5indicates that a proper ratio of catalyst/solution is a necessary factor to attain the high conversion of ethanol.It can be understood that over exposure of H 2O 2to catalyst will increase the rate of H 2O 2decomposition;but on the other hand,more OH radical produced may be scavenged by catalyst with increasing the amount of catalyst and transformed into O 2and H 2O as expressed in Eq.(3),instead of participating the oxidation reaction.In terms of Eq.(10),stoichiometric ethanol/H 2O 2should be 1/6for the complete oxidation of ethanol;however,in the present system the total molar ratio is 1/400.In other words,most intermediates were extinguished through scavenging during reaction.This may explain well that the decrease of reaction rate with the rise of ratio of catalyst/solution in the system.The same reason may also explain the decrease of reaction rate with prolonging the time.Actually,H 2O 2decomposition (ca.90%)may be completed within a few minutes over the Mn 3O 4/SBA-15catalyst as illustrated in Fig.10,irrespective of amount of catalyst (not shown for the sake of brevity);in contrast,the rate for H 2O 2decomposition became dawdling for Fe 2+catalyst.As a result,presumably,the homogenous system has relatively high concentration ofradicals.It may explain the superior reactivity of the conventional Fenton reagent to the presented system as depicted in Fig.9.Therefore,how to reduce scavenging,especially in the heterogeneous Fenton system [29],is crucial for enhancing the reaction rate.C 2H 5OH þ6H 2O 2!2CO 2þ9H 2O(11)On the other hand,as illustrated by Eqs.(1)–(4),all steps in the oxidation process are affected by the reaction temperature.Fig.6demonstrates that increasing temperature remarkably boosts the reactivity of ethanol oxidation in the system of Mn 3O 4/SBA-15–H 2O 2possibly,due to the improvement of the reactions in Eqs.(2)and (4)at elevated temperatures.In terms of Eqs.(6)and (7),acidic conditions may delay the H 2O 2decomposition but enhance the formation of OH (Eqs.(5),(8)and (9)).This ‘‘delay’’is supposed to reduce the chance of the scavenging of radicals and improve the efficiency of H 2O 2in the reaction.The protons are believed to have capability for stabilizing H 2O 2,which has been elucidated well previously [18,19].Consequently,it is understandable that the reaction is favored in the strong acidic environment.Fig.7shows a maximum reactivity at pH of 2.0and the lowest at pH of 9.0.As depicted in Fig.8,the reaction rate for ethanol oxidation is proportional to the concentration of ethanol in the range of 50–500ppm.It suggests that at low concentration of ethanol (100ppm)most of the radicals might not take part in the reaction before scavenged by catalyst.With increasing the ethanol concentration,the possibility of the collision between ethanol and radicals can be increased significantly.As a result,the rate of scavenging radicals is reduced relatively.Thus,it is reasonable for the faster rate observed at higher concentration of ethanol.Finally,it is noteworthy that as compared to the bulk Mn 3O 4(Aldrich,98.0%of purity),the reactivity of the nano-crystalline Mn 3O 4on SBA-15is increased by factor of 20under the same typical reaction conditions.Obviously,Mn 3O 4nanocrystal is an effective alternative for this catalytic system.The present study has evidenced that the unique structure of SBA-15can act as a special ‘‘nanoreactor’’for synthesizing Mn 3O 4nanocrystals.Interestingly,a latest study has revealed that iron oxide nanoparticles could be immobilized on alumina coated SBA-15,which also showed excellent performance as a Fenton catalyst [35].However,the role of the pore structure of SBA-15in this reaction is still unclear.We do expect that during reaction SBA-15may have additional function to trap larger organic molecules by adsorption.Thus,it may broaden its application in this field.So,relevant study on the structure of nano-composites of various MnO x and its role in the Fenton-like reaction for remediation of organic compounds in aqueous solution is undergoing in our group.4.ConclusionsIn the present study,we have addressed a new catalytic system suitable for remediation of trivial organic compound from contaminated water through a Fenton-like reaction withY.-F .Han et al./Applied Catalysis B:Environmental 76(2007)227–234233。
MAYA软件安装方法

MAYA软件安装方法Maya 是一款非常流行的三维动画与视觉效果软件,广泛应用于影视、游戏、建筑等领域。
本文将介绍如何安装 Maya 软件。
步骤一:下载 Maya 软件首先,您需要从官方网站上下载 Maya 软件的安装包。
请确保您下载的是最新版本的 Maya 软件,并且与您的操作系统兼容。
步骤二:运行安装程序一旦下载完成,您可以找到下载文件并双击运行安装程序。
在开始安装之前,您可能需要管理员权限。
步骤三:选择安装选项在安装程序开始运行后,您将看到一系列的安装选项。
根据您的需求选择以下选项:•安装类型:根据您的需求选择“Typical”(典型)或“Custom”(自定义)安装。
典型安装会安装 Maya 软件的常用组件,而自定义安装则允许您选择要安装的组件。
•安装路径:选择您希望安装 Maya 软件的目标路径。
您可以使用默认路径,也可以选择其他路径。
•附加工具:根据您的需求选择是否安装附加工具,如 Maya API 开发工具包等。
步骤四:接受许可协议在继续安装之前,您需要阅读并接受 Maya 软件的许可协议。
仔细阅读许可协议,并在同意之后点击“同意”按钮。
步骤五:安装进程安装程序将开始复制文件并设置 Maya 软件。
这个过程可能需要一些时间,取决于您的计算机性能和所选择的安装选项。
步骤六:完成安装安装程序完成后,您将看到一个安装成功的消息。
请点击“完成”按钮退出安装程序。
步骤七:激活 Maya 软件安装完成后,您需要激活 Maya 软件才能正常使用。
打开 Maya 软件,并按照首次使用时的提示进行激活。
通常您需要提供序列号或许可证文件以完成激活过程。
根据您的购买方式,可能需要在线激活或离线激活。
注意事项•在下载和安装 Maya 软件之前,确保您的计算机符合软件的最低系统要求。
这样可以确保软件能够正常运行并发挥其功能。
•在安装过程中,确保您的计算机连接到稳定的互联网,这样可以确保安装程序能够顺利下载所需的文件。
Maya2010新功能讲解

Maya2010新功能讲解 Maya2010面世了,今天我们和飞特网的朋友们一起来看看MAYA2010的一些新功能,和界面!安装拿到软件首先是要安装,不装不知道,一装吓一跳,安装路径选择完以后是资源包的选择,本次Maya的安装包竟然高达1.2G,包含了5个软件!其中有:Autodesk Maya 2010,Autodesk Maya 2010的说明文档(这个超级重要!),Autodesk MatchMover 2010,Autodesk Toxik 2010,Autodesk Backburner 2008。
MatchMover是一款高级追踪软件,可以通过实拍镜头反求三维相机,从而再导入Maya中渲染三维物体合成到电影或胶片实拍视频中。
Toxik是一款高级节点式电影合成软件,这个在我以前的教学文章中有详细的说明。
Mocap动捕数据预置在Visor窗口中新加入了一个 Mocap 标签, (Window > General Editors > Visor) 其中预置了一些人类常用的动作捕捉数据,方便进行各种测试(如布料测试)。
新增nParticle样例在Visor窗口中的nParticle样例增加了几种预置,方便初学者进行临摹学习。
其它功能方面并没有更新。
渲染新功能加入新的立体相机工作流立体相机在2009版本中得以增强,大纲中的调节更加方便。
Global illumination增强官方对这个功能只做了简单的介绍,只是说这个版本中mia_material_x_passes材质变得更好用了。
Autodesk Toxik and Backburner可以被Maya使用Backburner是一个后台任务工具,通过Backburner可以让Toxik、MatchMover及Maya进行协同作业,提高工作效率。
Maya vector 渲染器现在只提供给 Windows 64-bit和Mac OS X 32-bit这个没有太多可说的内容,用过的都知道。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Autodesk 媒体及娱乐部高级副主席Marc Petit说:“我们庆贺在过去的十年中,Maya被运用在电影、电视、游戏、工业设计和建筑领域,并且给那些艺术家和创作家的工作带来了成功。我们设计的Maya 2009给创作想象和生产效率带来了新的贡献。新版的Maya为艺术家们清除了障碍,使得他们可以创作出更多焕然一新的CG作品。
最近读者: 登录后,您就出现在这里。
网友评论: 发表评论:姓 名: 注册 | 登录 *姓名最长为50字节
网址或邮箱: (选填)
内 容: 插入表情 ▼ 闪光字
验证码: 请点击后输入四位验证码,字母不区分大小写
10.总体上来看,Maya2010就是2009两个版本的集合体,没有什么大的改动,运行速度比较快。安装包300M左右,安装完毕之后900M。
论坛已经发布....
请用迅雷或者其他下载软件下载
(限制单线程下载,否则会下载失败)
下载地址:
/maya/maya2010.rar
Maya 是目前世界上最为优秀的三维动画的制作软件之一,主要是为了影视应用而研发的,所以在出世后不久就在《精灵鼠小弟》、《恐龙》等这些大片中一展身手。除了影视方面的应用外Maya在三维动画制作,影视广告设计,多媒体制作甚至游戏制作领域都有很出色的表现。初识Maya 是目前世界上最为优秀的三维动画的制作软件之一,它是Alias|Wavefront公司在1998年才推出的三维制作软件。虽然相对于其他老牌三维制作软件来说Maya还是一个新生儿,但Maya凭借其强大的功能,友好的用户界面和丰富的视觉效果,一经推出就引起了动画和影视界的广泛关注,成为顶级的三维动画制作软件。
本次, 欧特克还推出了包括全新的数字娱乐创作套件,包括 Autodesk MotionBuilder 2010和Autodesk Maya 2010的实时动画套件Real-Time Animation Suite以及包括Autodesk MotionBuilder 2010、Autodesk Mudbox 2010 和Autodesk Maya 2010的数字娱乐创作套件 Entertainment Creation Suite。这些套件使艺术家和数字工作室获得了一系列功能强大的创作工具,其费用也较单独购买每款产品更为便宜。综合使用这两款套件产品中的各种软件可帮助美术师最大限度地发挥其创造力并提高工作效率。
看不清?2010 的新功能包括:强大的基于 Autodesk Toxik 软件的高度动态合成系统Autodesk Maya Composite(一种、先进的三维跟踪和匹配移动系统Autodesk MatchMover、5个 mental ray Batch 渲染节点以及 Autodesk Backburner 网络渲染序列管理器等。
Crytek的高级动画师兼MotionBuilder beta版的测试者表示,Autodesk MotionBuilder 一直以来都是市场上最强大和可靠的角色动画制作工具之一,无论是在关键帧方面还是动作捕捉制作方面。“作为一名动画师,我喜欢2009版本中的新的刚体物理学和布娃娃处理器。这些功能完善了集体动作的制作,而原来的实景捕捉方法是件非常痛苦的工作。技术指导也会喜欢看到新的Python 代码编写器和用它生成新的用户界面。这样使得他们可以把MotionBuilder高度地集成到整个流程当中。”
3.我们当然要选择第一项:Activate.
4.进入注册画面之后,将Crack文件夹中的压缩包xf-maya2010-32bits.rar解压,根据你自己的系统位数来选择解密文件。
5.首先打开注册机,然后点击Mem Patch,你会看到提示:successfully patched.
6.把你在注册画面里看到的request code(也就是那几组英文字母)复制并粘贴到注册ē.﹖ヅ|▍╋:滾妳媽的天長地久.去妳媽的長相厮守゛, 主页博客相册|个人档案 |好友 查看文章
Maya2010下载,Maya 2010破解版,Maya2010注册机(官方原版+注册机)2010-03-18 12:58
maya2010破解,maya2010注册码,maya2010绿色
类别:默认分类 | | 添加到搜藏 | 分享到i贴吧 | 浏览(3) | 评论 (0) 上一篇:幽岛正品鞋折扣店--只卖正品! 下一篇:Maya2010注册机,破解文件,注册... 相关文章:? maya 2010下载;Maya 2010注册机... ? maya 2010下载;Maya 2010注册机...
程序语言: 英文
文件大小: 330M
maya2010软件免费下载,Autodesk Maya 2010 下载
Maya2010下载 Maya 2010 发布 (无限正式版)
Autodesk Maya 2010 Maya动画资源网 发布
Maya 2010融合了Autodesk Maya Complete 2009 和 Autodesk Maya Unlimited 2009 的功能,将相匹配的移动、合成和渲染功能统一整合进一款价廉物美的产品之中。从照片级真实感视觉效果到真实逼真的三维角色,Autodesk Maya 2010可以帮助美术师、设计师和CG爱好者们更加轻松地创作出极具吸引力的娱乐体验,设计出更加激动人心的数字图像。Windows、Linux 和 Mac OS 操作系统均可支持新版本Autodesk Maya 2010。
Autodesk Maya 2010 拥有Autodesk Maya Unlimited 2009 和Autodesk Maya Complete 2009 的全部功能,包括先进的模拟工具:Autodesk Maya Nucleus Unified Simulation Framework、Autodesk Maya nCloth、Autodesk Maya nParticles、Autodesk Maya Fluid Effects、Autodesk Maya Hair、Autodesk Maya Fur;另外还拥有广泛的建模、纹理和动画工具、基于画刷的三维技术、完整的立体工作流程、卡通渲染 (Toon Shading)、渲染、一个广泛的 Maya 应用程序界面/软件开发工具包以及 Python 和 MEL 脚本功能。
或点此下载
本文欢迎各大网站 个人 及机构转载 本站会员转载奖励积分
以下信息可能对您有帮助:
Maya2010注册机tml
Maya2008+破解文件+安装和破解教程(本地下载!)请点击此处
666-69696969(已使用),667-98989898,400-45454545(已使用)356-72378422……或者其他的一组数字。
9.有些用户可能在出现激活码之后不知道往什么位置粘贴,在serial code框的下方有一个单选项,默认的是上方选项,把它改为下方选项,然后会出现填写激活码的空白框区域,这时候把注册机里得到的激活码粘贴进去点击 Next就可以了,如果提示正确注册信息就证明成功了!
有人说maya2010要注册码,其实maya2010的安装和之前的版本不一样了,maya2010的安装方式变的和max的安装一样了。
1.下载Maya2010并安装Maya2010
2.全部安装完毕之后双击打开Autodesk Maya2010程序,这时会弹出一个对话框,有两个选项:一个是Activate(注册),另一个是试用30天
7.这时候注册机里出现activation code(激活码),把这一大串的字母都复制粘贴到注册画面的下方空白框中,但是不要点击Next,因为你会发现上方有一个serial code没有填写,格式是XXX-XXXXXXXX!
8.注册机里没有直接提示这组数字应该填写什么,你可以在以下的注册码中测试:
自2001年以来所有获得奥斯卡“最佳视觉效果奖”的影片以及业界顶尖的20大游戏发行商都使用了Autodesk Maya。在过去十年中,Autodesk Maya 已经成为了许多全球顶级制片公司的首选创意工具,并被用来帮助全球的数字艺术家们来创造独特和创新的娱乐体验,包括Chris Landreth 的《The Spine》等独立生产@电影以及2009奥斯卡最佳特效影片《返老还童》(The Curious Case of Benjamin Button)等创新的视效大片都使用了 Autodesk Maya。
