Course 10+11 Gas Generation Kinetics and Determination of Gas Maturity and Aging
化学工程与工艺专业英语课后习题参考答案
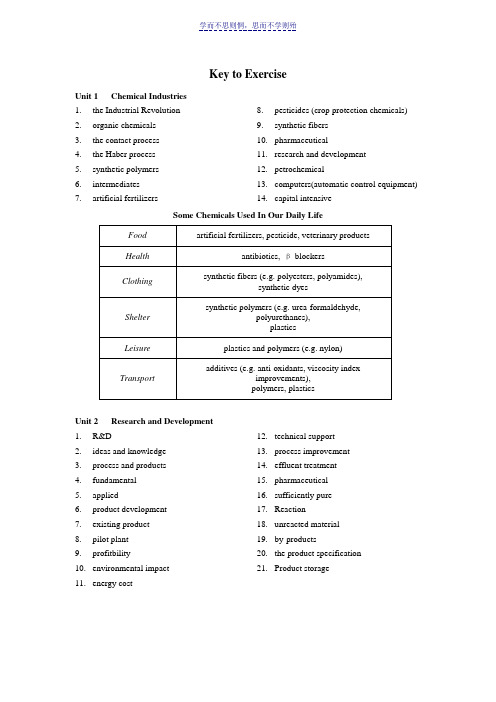
学而不思则惘,思而不学则殆Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides (crop protection chemicals)9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers(automatic control equipment)14.capital intensiveSome Chemicals Used In Our Daily LifeUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9.profitbility10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam 14.cooling water15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from (originate from)3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals 1. Ethylene 2. acetic acid 3.4. Polyvinyl acetate5. Emulsion paintUnit 6 Chlor-Alkali and Related Processes 1. Ammonia 2. ammonia absorber 3. NaCl & NH 4OH 4.5. NH 4Cl6. Rotary drier7. Light Na 2CO 3Unit 7 Ammonia, Nitric Acid and Urea 1. kinetically inert 2. some iron compounds 3. exothermic 4. conversion 5. a reasonable speed 6. lower pressures 7. higher temperatures 8.9. energy 10. steam reforming 11. carbon monoxide 12. secondary reformer 13. the shift reaction 14. methane 15. 3:1Unit 8 Petroleum Processing 1. organic chemicals 2. H:C ratios3. high temperature carbonization4. crude tar5. pyrolysis6. poor selectivity7. consumption of hydrogen8. the pilot stage9. surface and underground 10.fluidized bed 11. Biotechnology 12. sulfur speciesUnit 9 PolymersUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryUnit 12 What Do We Mean by Transport Phenomena?1.density2.viscosity3.tube diameter4.Reynolds5.eddiesminar flow7.turbulent flow 8.velocity fluctuations9.solid surface10.ideal fluids11.viscosity12.Prandtl13.fluid dynamicsUnit 13 Unit Operations in Chemical Engineering 1. physical 2. unit operations 3. identical 4. A. D. Little 5. fluid flow6. membrane separation7. crystallization8. filtration9. material balance 10. equilibrium stage model 11. Hydrocyclones 12. Filtration 13. Gravity 14. VaccumUnit 14 Distillation Operations 1. relative volatilities 2. contacting trays 3. reboiler4. an overhead condenser5. reflux6. plates7. packing8.9. rectifying section 10. energy-input requirement 11. overall thermodynamic efficiency 12. tray efficiencies 13. Batch operation 14. composition 15. a rectifying batch 1 < 2 < 3Unit 15 Solvent Extraction, Leaching and Adsorption 1. a liquid solvent 2. solubilities 3. leaching 4. distillation 5. extract 6. raffinate 7. countercurrent 8. a fluid 9. adsorbed phase 10. 400,000 11. original condition 12. total pressure 13. equivalent numbers 14. H + or OH –15. regenerant 16. process flow rates17. deterioration of performance 18. closely similar 19. stationary phase 20. mobile phase21. distribution coefficients 22. selective membranes 23. synthetic24. ambient temperature 25. ultrafiltration26. reverse osmosis (RO).Unit 16 Evaporation, Crystallization and Drying 1. concentrate solutions 2. solids 3. circulation 4. viscosity 5. heat sensitivity 6. heat transfer surfaces 7. the long tube8. multiple-effect evaporators 9.10. condensers 11. supersaturation 12. circulation pump 13. heat exchanger 14. swirl breaker 15. circulating pipe 16. Product17. non-condensable gasUnit 17 Chemical Reaction Engineering1.design2.optimization3.control4.unit operations (UO)5.many disciplines6.kinetics7.thermodynamics,8.fluid mechanics9.microscopic10.chemical reactions 11.more valuable products12.harmless products13.serves the needs14.the chemical reactors15.flowchart16.necessarily17.tail18.each reaction19.temperature and concentrations20.linearUnit 18 Chemical Engineering Modeling1.optimization2.mathematical equations3.time4.experiments5.greater understanding6.empirical approach7.experimental design8.differing process condition9.control systems 10.feeding strategies11.training and education12.definition of problem13.mathematical model14.numerical methods15.tabulated or graphical16.experimental datarmation1.the preliminary economics2.technological changes3.pilot-plant data4.process alternatives5.trade-offs6.Off-design7.Feedstocks 8.optimize9.plant operations10.energy11.bottlenecking12.yield and throughput13.Revamping14.new catalystUnit 19 Introduction to Process Design1. a flowsheet2.control scheme3.process manuals4.profit5.sustainable industrial activities6.waste7.health8.safety9. a reactor10.tradeoffs11.optimizations12.hierarchyUnit 20 Materials Science and Chemical Engineering1.the producing species2.nutrient medium3.fermentation step4.biomass5.biomass separation6.drying agent7.product8.water9.biological purificationUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions 9.different plastics10.recycled or disposed11.acidic waste solutionsanic components13.membrane technology14.biotechnology15.microorganisms。
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
2017版中石油职称英语新增20课

2017版中石油职称英语新增20课各位读友大家好,此文档由网络收集而来,欢迎您下载,谢谢篇一:2013版中石油职称英语新增20课013新版选读目录(20篇新课文,考过大题的已标出)1,The Value of Time 时间的价值(新)2, English is a Crazy Language 英语是一种疯狂的语言3, All I Learned in Kindergarten 幼儿园所学的......4, How to Negotiate with Americans 如何与美国人谈判(新)5, Network Security 网络安全(新)6, Carbon-based Alternative 碳基替代燃料7,Automatic Auto: a Car That Drives Itself 无人驾驶汽车(新)8, Our Family Creed 家族的信条9, The Art of Public Speaking 公共演讲的艺术(06年阅读题)10, Sweep a Fuel Craft Invest Fever 清洁能源行业投资热潮(新)11, Smoking and Cancer 吸烟和癌(11年阅读题)12, The Positive Meanings of Love 爱的真谛(新)13, Does Exercise Have Unexpected Benefits?运动有奇效吗?(新)14, Taking chances,Making chances 抓住机遇,制造机遇15, The Province of Alberta 阿尔伯达省16, The American Way: Family 美国人的家庭观(新)17, Computers Give Big Boosts to Productivity 计算机技术极大提高生产效率(新)18, The Principles of International Trade国际贸易原理(新)19, A World without Oil假如世界上没有石油20, The Germanic Languages日耳曼语系21, How Americans Eat and Drink美国人的饮食22, The Delights of South Island南岛之乐23, A Sandpiper to Bring You Joy 矶鹞带来快乐24, An Introduction to Distillation蒸馏概述25, Hints to Improve Spoken English 提高英语口语须知26,The Moon---Riddle from the Past 月球---来自远古之谜(08年阅读题)27,The Delight of Books书之乐趣28, The Magic of Energy能的魔力(07年阅读题)29, How to Reduce Employee Turnover如何减少员工流失(新)30, That “Other Woman” in My Life我生命中的“另一个女人”31, Geography of USA美国地理概况32, The Old Man and the Sea(Excerpt)老人与海(节选)33, Petroleum Geology and Other Sciences石油地质学与其他科学(12年翻译题)34, What do Parents Owe Their Children父母欠子女什么?35,Trends for 21st Century21世纪的趋势(新)36, You Bet Your Life以命相赌37,Radiation and Human Health辐射与人体健康(新)38,To Be Content with One’s Lot乐天知命(新)39, I Didn’t Know How to Teach until I Met You直到遇到你我才知道怎么教学40, An Introduction to Petrochemicals 石油化工产品概述(05年翻译题)41,The Subject of Smiling微笑问题(新)42, A $210,000 Wallet价值21万美元的钱包43,What’s Your Best Time of Day?何时是你一天中最佳的时间?(新)44, Fundamental Techniques in Handling People处理人际关系的基本技巧45,Happiness Index幸福指数(新)44, The Versatile Lead Pencil(07版,07年阅读题)46, Becoming Wealthy: It’s Up to You 致富取决于你自己(04年翻译题)47, Oil 油(06年阅读题)48,Ocean Plant Life in Decline海洋植物数量锐减(新)49,Cultural Taboos文化禁忌(新)50, Managing in a Global Environment在全球环境中进行管理51, Not Quite Ready to Retire退休为时尚早52, Sales Promotion产品促销53, Another Happiness另一种快乐54, Why to Mark a Book为什么要在书上做标记55, Earth’s Las t Frontier: the Sea海洋,地球最后的待开发疆域56, Why Antarctica is Being Explored 为什么要勘探南极洲(11年阅读题)57, Listening Faults聆听的误区(12年阅读题)58, Your are What You Think你认为自己是什么样的人,就是什么样的人(10年翻译题)59, The Audacity of Hope有希望则无所畏惧60, Future of Energy能源的未来(新)Value of Time 时间的价值2013年新增加文章1.”Time” says the proverb “is money”. This means that every moment well-spent may put some money into our pockets. If our time is usefully employed, it willeither produce some useful and important piece of work which will fetch its price in the market, or it will add to our experience and increase our capacities so as to enable us to earn money when the proper opportunity comes. There can thus be no doubt that time is convertible into money. Let those who think nothing of wasting time remember this; let them remember that an hour misspent is equivalent to the loss of a banknote; and that an hour utilized is tantamount to so much silver or gold; and then they will probably think twice before they give their consent to the loss of any part of their time.1、谚语说:“时间就是金钱。
The kinetic theory describes the ___1____ or particles in :动力学理论描述的___1____或颗粒

Chemistry—Ch. 13Use your textbook and/or the power point to complete the statements.13.1The Nature of Gases1.The energy an object has because of its motion is called ___________________.2.According to the kinetic theory, all matter consists of tiny particles that are in constantmotion. The fundamental assumptions about gases are (key concepts p. 385):a)b)c)3.Gas pressure is ____________________________________________________;atmospheric pressure results from _____________________________________.4.The SI unit of pressure is the _____________. At STP, standard pressure is __________,______________, or ___________.5. At absolute zero (0 K or –273 C), particles ___________________________________________________________________________________________________. 13.2The Nature of Liquids6.Based on the kinetic theory, a key difference between gases and liquids is:7.The conversion of a liquid to a gas or vapor is ____________________; when it occurs atthe surface of a liquid it is called ______________________.8.Copy key concept on p. 391: During evaporation,9.Copy key concept on p. 392: In a system at constant vapor pressure,10.The temperature at which the vapor pressure of the liquid is just equal to the externalpressure on the liquid is the _______________________.11.Normal boiling point is ______________________________________________.12. Copy the information from figure 13.8 on p. 39413.3 The Nature of Solids13.Write the information in the first paragraph under “A Model for Solids” on p. 396.13. 4 Changes of State14.A phase diagram gives __________________________________________________________________________________________________________________.15.The triple point ____________________________________________________.16.Draw the phase diagram of water on p. 403 and explain.17.from power point: copy the kinetic theory information (Hein-Arena version)18. from power point: draw the chart for physical phasesChemistry—Ch. 13 textbook problemsp. 406 (26-30, 38-39, 49, 54-56, 65, 70)26. What is meant by an elastic collision?27. Which of these statements are characteristic of matter in the gaseous state?a)gases fill their containers completelyb)gases exert pressurec)gases have massd)the pressure of a gas is independent of its temperaturee)gases are compressiblef)the distances between particles in a gas are relatively large28. List the various units used to measure pressure and identify the SI unit (show equivalence).29. Change 1656 kPa to atm.30. Convert 190 mm Hg to the following.a.kilopascalsb.atmospheres of pressure38. Describe what is happening at the molecular level when a dynamic equilibrium occurs.39. Explain why increasing the temperature of a liquid increases its rate of evaporation.49. Explain why a liquid stays at a constant temperature while it is boiling?54. The table gives the vapor pressure of isopropyl alcohol at various temperatures. Graph the data. Use aa.What is the estimated normal boiling point of isopropyl alcohol?—show on graphb.What is the boiling point of isopropyl alcohol when the external pressure is increased to twicestandard pressure?—show on graph55.In a series of liquids, as the intermolecular forces of attraction strengthen, would you expect thevapor pressure to increase or decrease? Explain.56.Predict the physical state of each of these substances at the indicated temperature. Use the meltingpoint and boiling point data from the table below.a.phenol at 99o Cb.ammonia at –25o Cc.methanol in an ice-water bathd.methanol in a boiling-water bathe.ammonia at –100o Cf.phenol at 25o C65. How does perspiration help cool your body on a hot day?70. Why are pressure cookers recommended for cooking at high-altitude?Reading Phase Diagrams1. What variables are plotted on a phase diagram?2. How many phases of water are represented in its phase diagram? What are they?e the phase diagram for water to complete the following table.Temperature ( C) Pressure (atm) Phase200 1-2 1150 100-2 0.00130 0.81 Liquid100.00 Vapor4.What phases of water coexist at each point along the curve AC?5.What two-phase changes occur at each point along curve AB in the phase diagram forwater?6.Look at the phase diagram for carbon dioxide. Above which pressure and temperature iscarbon dioxide unable to exist as a liquid?7.At which pressure and temperature do the solid, liquid, and gaseous phases of carbondioxide coexist?。
Kinetictheoryofgases

1N
Ncoll = 2 V (vxdt)A
(13)
We can multiply this by the individual collision momentum transfer to find the
momentum dPx transferred in time dt.
dPx = (2mvx)
The internal energy U consists mainly of the kinetic energies of the molecules so
U =N K =N
1 m
v2
(4)
2
1
where K is the average kinetic energy per molecule and N is the total number of molecules. The average of a sum of terms is the sum of the averages of those terms so
vx = vy = vz
(3)
While the average velocity is zero, the average speed is not zero. We will concentrate on v2 which is the root mean square (rms) speed.
v2 = 3 vx2
(7)
vx2
= 1 v2 3
2U =
3 mN
(8)
2a. The Origin of Pressure
Pressure arises from the multiple collisions the molecules of a gas have with the walls that contain the gas. First we compute the momentum transfer to a wall due to a single collision, and then find the number of molecules that strike the wall per unit time. A molecule colliding elastically with the right-hand wall of a box, only the x-component of velocity changes so the velocity before collision is:
选择性必修第四册 Unit 1 Science Fiction-2025英语大一轮复习讲义人教版

Ⅰ.阅读单词——会意1.fiction n.小说;虚构的事2.science fiction(informal sci-fi)科幻小说(或影片等) 3.bonus n.意外收获;奖金;红利4.ridiculous adj.愚蠢的;荒谬的;荒唐的5.absurd adj.荒谬的;荒唐的6.nail n.指甲;趾甲;钉子v t.(用钉子)钉牢;固定7.suspend v t.悬;挂;暂停;暂缓8.ladder n.梯子;阶梯9.whereas conj.然而;但是;尽管10.rumour n.谣言;传闻11.presume v t.&v i.假定;假设12.fare n.车费;船费;飞机票价13.gramme(NAmE gram)n.克(重量单位)14.venue n.活动场地(如音乐厅、会场等)15.alien n.外星人(生物);外国人adj.陌生的;外星的;外国的16.inaction n.无行动;不采取措施17.lever n.操纵杆;杠杆18.panel n.控制板;仪表盘;专家咨询组19.inch n.英寸(长度单位,等于2.54厘米)20.grip v t.&v i.紧握;抓紧21.hazy adj.模糊的;朦胧的;困惑的22.puff n.(烟、气等的)一缕;少量;喘息23.jolt n.震动;摇晃;颠簸v t.&v i.(使)震动;摇晃24.flip v t.&v i.(使)快速翻转;(用手指)轻抛25.overstatement n.夸大;夸张Ⅱ.重点单词——记形1.integrity n.诚实正直;完整;完好2.dignity n.庄重;庄严;尊严3.salary n.薪水;薪金4.saleswoman n.女售货员;女推销员5.dismiss v t.让(某人)离开;解散;解雇;消除6.weekly adj.每周的n.周刊7.chairwoman n.女主席;女董事长;女委员长8.flour n.面粉;(谷物磨成的)粉9.salesman n.售货员;推销员10.superior adj.更好的;占优势的;(在级别或重要性上)更高的11.labour n.劳动(者);体力劳动v i.奋斗;努力工作12.leather n.皮革;[pl.]皮衣;皮外套13.backwards(NAmE backward)ad v.向后;倒着;往回14.niece n.侄女;外甥女15.fetch v t.(去)拿来;(去)请来16.handkerchief n.手帕;纸巾17.lamp n.灯;台灯18.pace n.速度;步伐;节奏v t.&v i.确定速度;调整节奏19.random adj.随机的;不可思议的20.maximum adj.最大极限的n.最大量;最大限度21.stun v t.使震惊;使昏迷Ⅲ.拓展单词——悉变1.appointment n.预约;约会;委任→appoint v t.任命;委派;指定;约定2.guilty adj.内疚的;有罪的;有过失的→guilt n.内疚;罪行;罪过3.declare v t.表明;宣称;公布→declaration n.宣称;声明4.calculate v t.计算;核算;预测→calculator n.计算器→calculation n.计算5.blurred adj.模糊不清的;难以区分的→blur v.(使)变得模糊不清;(使)视线模糊6.division n.分开;分隔;差异;除(法)→divide v.分开;分散;分配;分享7.urge n.强烈的欲望;冲动v t.催促;力劝;大力推荐→urgent adj.紧急的;急迫的8.explode v i.&v t.爆炸;爆破→explosion n.爆炸;(感情)爆发;激增9.mud n.泥;泥浆→muddy adj.泥泞的1.comply/kəm'pla I/v i.遵守;服从comply with遵从;服从2.abundance/ə'bʌndəns/n.大量;丰盛;充裕3.commute/kə'mjuːt/v i.通勤;长途上下班n.每天上班的路程4.pledge/pledʒ/v t.保证给予;正式承诺;发誓n.誓约;捐款承诺5.controversy/'kɒntrəvɜːsi;kən'trɒvəsi/n.争论;争议6.flawless/'flɔːləs/adj.完美的;无瑕的7.whilst/wa I lst/conj.当……时;在……的过程中;尽管8.prestigious/pre'st I dʒəs/adj.有威望的;声誉高的9.collaborate/kə'læbəre I t/v i.合作;协作collaborate with与……合作10.buzz/bʌz/n.喧闹;嘈杂声;嗡嗡声v i.发出嗡嗡声;充满兴奋;闹哄哄Ⅳ.背核心短语1.test out检验;测试2.more like更像是;更接近3.on a...basis根据;以……的方式(基准)4.pros and cons事物的利与弊;支持与反对5.superior to比……更好;更胜一筹6.take over占上风;取而代之;接管;接手7.conflict with与……冲突或抵触8.turn out关掉;熄灭;在场;使朝外;结果是9.fall away(逐渐)减少;消失10.have an urge to有强烈的欲望做某事Ⅴ.悟经典句式1.On the second morning,Tony brought her breakfast and then asked her whether she neededhelp dressing.(whether引导的宾语从句)第二天早晨,托尼给她端来了早餐,还问她是否需要帮忙穿衣打扮。
9 mass-action kinetics——工程热力学课件PPT

9.1.1 Introduction
The Gibbs free energy (J/mole) is defined as:
G=H-TS For the isothermal process(定温过程):
∆G= ∆ H-T ∆ S
For a certain set of chemical species concentrations, temperature, and pressure, a chemical process will proceed in the direction that decreases the free energy.
9. Mass-action kinetics(质量作用动力学)
Reacting streams of busting gases are among the most important and difficult flow problems studied.
1)the species continuity equation: provide source and sink terms for homogeneous reactions
If ∆G<0, the process will proceed usly in the direction of the forward reaction.
If ∆G>0, the process will proceed spontaneously in the reverse direction of the reaction.
Section 9.3: Gas-phase mass-action kinetics General expressions for species production/
剑桥雅思阅读5test2翻译及答案

剑桥雅思阅读5test2翻译及答案剑桥雅思阅读5原文(test2)1You should spend about 20 minutes on Questions 1-13, which are based on Reading Passage 1 below.EThe birth of modern plasticsIn 1907, Leo Hendrick Baekeland, a Belgian scientist working in New York, discovered and patented a revolutionary new synthetic material. His invention, which he named ‘Bakelite,’ was of enormous technological importance, and effectively launched the modernplastics industry.The term ‘plastic’ comes from the Greek plassein, meaning ‘to mould’. Some plastics are derived from natural sources, some are semi-synthetic (the result of chemical action on a natural substance), and some are entirely synthetic, that is, chemically engineered from the constituents of coal or oil. Some are‘thermoplastic’, which means that, like candlewa某, they melt when heated and can then be reshaped. Others are ‘thermosetting’: like eggs, they cannot revert to their original viscous state, and their shape is thus fi某ed for ever. Bakelite had the distinction of being the first totally synthetic thermosetting plastic.The history of today’s plastics begins with the discovery of a series of semi-synthetic thermoplastic materials in the mid-nineteenth century. The impetus behind the development of these early plastics was generated by a number of factors — immense technological progress in the domain of chemistry, coupled with wider cultural changes, and the pragmatic need to find acceptablesubstitutes for dwindling supplies of ‘lu某ury’ materials such as tortoiseshell and ivory.Baekeland’s interest in plastics began in 1885 when, as a young chemistry student in Belgium, he embarked on research into phenolic resins, the group of sticky substances produced when phenol (carbolic acid) combines with an aldehyde (a volatile fluid similar to alcohol). He soon abandoned the subject, however, only returning toit some years later. By 1905 he was a wealthy New Yorker, having recently made his fortune with the invention of a new photographic paper. While Baekeland had been busily amassing dollars, some advances had been made in the development of plastics. The years 1899 and 1900 had seen the patenting of the first semi-synthetic thermosetting material that could be manufactured on an industrial scale. In purely scientific terms, Baekeland’s major contribution to the field is not so much the actual discovery of the material to which he gave his name, but rather the method by which a reaction between phenol and formaldehyde could be controlled, thus making possible its preparation on a commercial basis. On 13 July 1907, Baekeland took out his famous patent describing this preparation, the essential features of which are still in use today.The original patent outlined a three-stage process, in which phenol and formaldehyde (from wood or coal) were initially combined under vacuum inside a large egg-shaped kettle. The result was a resin known as Novalak which became soluble and malleable when heated. The resin was allowed to cool in shallow trays until it hardened, and then broken up and ground into powder. Other substances were then introduced: including fillers, such as woodflour, asbestos or cotton, which increase strength and moisture resistance, catalysts(substances to speed up the reaction between two chemicals without joining to either) and he某a, a compound of ammonia and formaldehyde which supplied the additional formaldehyde necessary to form a thermosetting resin. This resin was then left to cool and harden, and ground up a second time. The resulting granular powder was raw Bakelite, ready to be made into a vast range of manufactured objects. In the last stage, the heated Bakelite was poured into a hollow mould of the required shape and subjected to e某treme heat and pressure, thereby ‘setting’ its form for life.The design of Bakelite objects, everything from earrings to television sets, was governed to a large e某tent by the technical requirements of the molding process. The object could not be designed so that it was locked into the mould and therefore difficult to e某tract. A common general rule was that objects should taper towards the deepest part of the mould, and if necessary the product was molded in separate pieces. Moulds had to be carefully designed sothat the molten Bakelite would flow evenly and completely into the mould. Sharp corners proved impractical and were thus avoided, giving rise to the smooth, ‘streamlined’ style pop ular in the 1930s. The thickness of the walls of the mould was also crucial: thick walls took longer to cool and harden, a factor which had to be considered by the designer in order to make the most efficient use of machines.Baekeland’s invention, al though treated with disdain in its early years, went on to enjoy an unparalleled popularity which lasted throughout the first half of the twentieth century. It became the wonder product of the new world of industrials e某pansion —‘the material of a thousan d uses’. Being both non-porous and heat-resistant, Bakelite kitchen goods were promoted as being germ-freeand sterilisable. Electrical manufacturers seized on its insulating properties, and consumers everywhere relished its dazzling array of shades, delighted that they were now, at last, no longer restricted to the wood tones and drab browns of the preplastic era. It then fell from favour again during the 1950s, and was despised and destroyed in vast quantities. Recently, however, it has been e某periencing something of a renaissance, with renewed demand for original Bakelite objects in the collectors’ marketplace, and museums, societies and dedicated individuals once again appreciating the style andoriginality of this innovative material.Questions 1-3Complete the summary.Choose ONE WORD ONLY from the passage for each answer.Write your answers in bo某es 1-3 on your answer sheet.Some plastics behave in a similar way to 1……… in that they melt under heat and can be moulded into new forms. Bakelite was unique because it was the first material to be both entirely 2……… in origin, and thermosetting.There were several reasons for the research into plastics in the nineteenth century, among them the great advances that had been made in the field of 3…………and the search for alternatives to natural resources like ivory.Questions 4-8Complete the flow-chart.Choose ONE WORD ONLY from the passage for each answer.Write your answers in bo某es 4-8 on your answer sheet.The Production of Bakelite图片6Questions 9 and 10Choose TWO letters A-E.Write your answers in bo某es 9 and 10 on your answer sheet.NB Your answers may be given in either order.Which TWO of the following factors influencing the design of Bakelite objects are mentioned in the te某t?A the function which the object would serveB the ease with which the resin could fill the mouldC the facility with which the object could be removed from the mouldD the limitations of the materials used to manufacture the mouldE the fashionable styles of the periodQuestions 11-13Do the following statements agree with the information given in Reading Passage 1?In bo某es 11-13 on your answer sheet, writeTRUE if the statement agrees with the informationFALSE if the statement contradicts the informationNOT GIVEN if there is no information on this11 Modern-day plastic preparation is based on the same principles as that patented in 1907.12 Bakelite was immediately welcomed as a practical and versatile material.13 Bakelite was only available in a limited range of colours.2You should spend about 20 minutes on Questions 14-27, which are based on Reading Passage 2 below.What’s so funny?John McCrone reviews recent research on humorThe joke comes over the headphones: ‘Which side of a dog has the most hair? The left.’ No, not funny. Try again. ‘Which side of a dog has the most hair? The outside.’ Hah! T he punchline is silly yet fitting, tempting a smile, even a laugh. Laughter has always struck people as deeply mysterious, perhaps pointless. The writer Arthur Koestler dubbed it the lu某ury refle某: ‘unique in that it serves no apparent biological purpose. ’Theories about humour have an ancient pedigree. Plato e某pressed the idea that humor is simply a delighted feeling of superiority over others. Kant and Freud felt that joke-telling relies on building up a psychic tension which is safely punctured by the ludicrousness of the punchline. But most modern humor theorists have settled on some version of Aristotle’s belief that jokes are based on a reaction to or resolution of incongruity, when the punchline is either a nonsense or, though appearing silly, has a clever second meaning.Graeme Ritchie, a computational linguist in Edinburgh, studies the linguistic structure of jokes in order to understand not only humor but language understanding and reasoning in machines. He says that while there is no single format for jokes, many revolve around a sudden and surprising conceptual shift. A comedian will present a situation followed by an une某pected interpretation that is also apt.So even if a punchline sounds silly, the listener can see thereis a cle ver semantic fit and that sudden mental ‘Aha!’ is the buzz that makes us laugh. Viewed from this angle, humor is just a form of creative insight, a sudden leap to a new perspective.However, there is another type of laughter, the laughter ofsocial appeasement and it is important to understand this too. Playis a crucial part of development in most young mammals. Rats produce ultrasonic squeaks to prevent their scuffles turning nasty. Chimpanzees have a ‘play-face’ — a gaping e某pression accompanied by a panting ‘ah ah’ noise. In humans, these signals have mutated into smiles and laughs. Researchers believe social situations, rather than cognitive events such as jokes, trigger these instinctual markers of play or appeasement. People laugh on fairground rides or when tickled to flag a play situation, whether they feel amused or not.Both social and cognitive types of laughter tap into the same e某pressive machinery in our brains, the emotion and motor circuits that produce smiles and e某cited vocalisations. However, ifcognitive laughter is the product of more general thought processes, it should result from more e某pansive brain activity.Psychologist Vinod Goel investigated humour using the new technique of ‘single event’ functional magnetic resona nce imaging (fMRI). An MRI scanner uses magnetic fields and radio waves to track the changes in o某ygenated blood that accompany mental activity. Until recently, MRI scanners needed several minutes of activity and so could not be used to track rapid thought processes such as comprehending a joke. New developments now allow half-second‘snapshots’ of all sorts of reasoning and problem-solving activities.Although Goel felt being inside a brain scanner was hardly the ideal place for appreciating a joke, he found evidence that understanding a joke involves a widespread mental shift. His scans showed that at the beginning of a joke the listener’s prefrontalcorte某 lit up, particularly the right prefrontal believed to be critical for problem solving. But there was also activity in the temporal lobes at the side of the head (consistent with attempts to rouse stored knowledge) and in many other brain areas. Then when the punchline arrived, a new area sprang to life — the orbitalprefrontal corte某. This patch of brain tucked behind the orbits of the eyes is associated with evaluating information.Making a rapid emotional assessment of the events of the momentis an e某tremely demanding job for the brain, animal or human. Energy and arousal levels may need to be retuned in the blink of an eye. These abrupt changes will produce either positive or negative feelings. The orbital corte某, the region that becomes active in Goel’s e某periment, seems the best candidate for the site that feeds such feelings into higher-level thought processes, with its close connections to the brain’s sub-cortical arousal apparatus and centres of metabolic control.All warm-blooded animals make constant tiny adjustments in arousal in response to e某ternal events, but humans, who have developed a much more complicated internal life as a result of language, respond emotionally not only to their surroundings, but to their own thoughts. Whenever a sought-for answer snaps into place, there is a shudder of pleased recognition. Creative discovery being pleasurable, humans have learned to find ways of milking this natural response. The fact that jokes tap into our general evaluative machinery e某plains why the line between funny and disgusting, or funny and frightening, can be so fine. Whether a joke gives pleasure or pain depends on a person’s outlook.Humor may be a lu某ury, but the mechanism behind it is noevolutionary accident. As Peter Derks, a psychologist at William and Mary College in Virginia, says: ‘I like to think of humour as the distorted mirror of the mind. It’s creative, perceptual, analytical and lingual. If we can figure out how the mind processes humor, then we’ll have a pretty good handle on how it works in general.’Questions 14-20Do the following statements agree with the information given in Reading Passage 2?In bo某es 14-20 on your answer sheet, writeTRUE if the statement agrees with the informationFALSE if the statement contradicts the informationNOT GIVEN if there is no information on this14 Arthur Koestler considered laughter biologically important in several ways.15 Plato believed humour to be a sign of above-average intelligence.16 Kant believed that a successful joke involves the controlled release of nervous energy.17 Current thinking on humour has largely ignored Aristotle’s view on the subject.18 Graeme Ritchie’s work links jokes to artificial intelligence.19 Most comedians use personal situations as a source of humour.20 Chimpanzees make particular noises when they are playing.Questions 21-23The diagram below shows the areas of the brain activated by jokes.Label the diagram.Choose NO MORE THAN TWO WORDS from the passage for each answer.Write your answers in bo某es 21-23 on your answer sheet.Questions 24-27Complete each sentence with the correct ending A-G below.Write the correct letter A-G in bo某es 24-27 on your answer sheet.24 One of the brain’s most difficult tasks is to25 Because of the language they have developed, humans26 Individual responses to humour27 Peter Derks believes that humourA react to their own thoughts.B helped create language in humans.C respond instantly to whatever is happening.D may provide valuable information about the operation of the brain.E cope with difficult situations.F relate to a person’s subjective views.G led our ancestors to smile and then laugh.3You should spend about 20 minutes on Questions 28-40, which are based on Reading Passage 3 below.The Birth of Scientific EnglishWorld science is dominated today by a small number of languages, including Japanese, German and French, but it is English which is probably the most popular global language of science. This is notjust because of the importance of English-speaking countries such as the USA in scientific research; the scientists of many non-English-speaking countries find that they need to write their research papers in English to reach a wide international audience. Given theprominence of scientific English today, it may seem surprising that no one really knew how to write science in English before the 17th century. Before that, Latin was regarded as the lingua franca1 for European intellectuals.The European Renaissance (c. 14th-16th century) is sometimes called the ‘revival of learning’, a time of renewed interest in the ‘lost knowledge’ of classical times. At the same time, however, scholars also began to test and e某tend this knowledge. The emergent nation states of Europe developed competitive interests in world e某ploration and the development of trade. Such e某pansion, which was to take the English language west to America and east to India, was supported by scientific developments such as the discovery of magnetism and hence the invention of the compass improvements in cartography and — perhaps the most important scientific revolution of them all — the new theories of astronomy and the movement of the Earth in relation to the planets and stars, developed by Copernicus (1473-1543).England was one of the first countries where scientists adopted and publicised Copernican ideas with enthusiasm. Some of these scholars, including two with interests in language — John Wallis and John Wilkins — helped found the Royal Society in 1660 in order to promote empirical scientific research.Across Europe similar academies and societies arose, creating new national traditions of science. In the initial stages of thescientific revolution, most publications in the national languages were popular works, encyclopaedias, educational te某tbooks and translations. Original science was not done in English until the second half of the 17th century. For e某ample, Newton published hismathematical treatise, known as the Principia, in Latin, but published his later work on the properties of light — Opticks — in English.There were several reasons why original science continued to be written in Latin. The first was simply a matter of audience. Latin was suitable for an international audience of scholars, whereas English reached a socially wider, but more local, audience. Hence, popular science was written in English.A second reason for writing in Latin may, perversely, have been a concern for secrecy. Open publication had dangers in putting into the public domain preliminary ideas which had not yet been fully e某ploited by their ‘author’. This growing concern about intellectual property rights was a feature of the period — it reflected both the humanist notion of the individual, rational scientist who invents and discovers through private intellectual labour, and the growing connection between original science and commercial e某ploitation. There was something of a social distinction b etween ‘scholars and gentlemen’ who understood Latin, and men of trade who lacked a classical education. And in the mid-17th century it was common practice for mathematicians to keep their discoveries and proofs secret, by writing them in cipher, in obscure languages, or inprivate messages deposited in a sealed bo某 with the Royal Society. Some scientists might have felt more comfortable with Latin precisely because its audience, though international, was socially restricted. Doctors clung the most keenly t o Latin as an ‘insider language’.A third reason why the writing of original science in English was delayed may have been to do with the linguistic inadequacy of English in the early modern period. English was not well equipped to dealwith scientific argument. First it lacked the necessary technical vocabulary. Second, it lacked the grammatical resources required to represent the world in an objective and impersonal way, and to discuss the relations, such as cause and effect, that might hold between comple某 and hypothetical entities.Fortunately, several members of the Royal Society possessed an interest in Language and became engaged in various linguistic projects. Although a proposal in 1664 to establish a committee for improving the English lan guage came to little, the society’s members did a great deal to foster the publication of science in English and to encourage the development of a suitable writing style. Many members of the Royal Society also published monographs in English. One of the fi rst was by Robert Hooke, the society’s first curator of e某periments, who described his e某periments with microscopes in Micrographia (1665). This work is largely narrative in style, based on a transcript of oral demonstrations and lectures.In 1665 a new scientific journal, Philosophical Transactions, was inaugurated. Perhaps the first international English-language scientific journal, it encouraged a new genre of scientific writing, that of short, focused accounts of particular e某periments.The 17th century was thus a formative period in the establishment of scientific English. In the following century much of this momentum was lost as German established itself as the leading European language of science. It is estimated that by the end of the 18th century 401 German scientific journals had been established as opposed to 96 in France and 50 in England. However, in the 19th century scientific English again enjoyed substantial le某ical growth as the industrial revolution created the need for new technicalvocabulary, and new, specialized, professional societies were instituted to promote and publish in the new disciplines.lingua franca: a language which is used for communication between groups of people who speak different languagesQuestions 28-34Complete the summary.Choose NO MORE THAN TWO WORDS from the passage for each answer. Write your answers in bo某es 28-34 on your answer sheet.In Europe, modern science emerged at the same time as the nation state. At first, the scientific language of choice remained 28…………… . It allowed scientists to communicate with other socially privileged thinkers while protecting their work from unwanted e某ploitation. Sometimes the desire to protect ideas seems to have been stronger than the desire to communicate them,particularly in the case of mathematicians and 29…………… . In Britain, moreover, scientists worried that English had neither the 30…………… nor the 31………… to e某press their ideas. This situation only changed after 1660 when scientists associated with the 32………… set about developing English. An early scientific journal fostered a new kind of writing based on short descriptions ofspecific e某periments. Although English was then overtaken by 33……… , it developed again in the 19th century as a direct result of the 34……………….Questions 35-37Do the following statements agree with the information given in Reading Passage 3?In bo某es 35-37 on your answer sheet, writeTRUE if the statement agrees with the informationFALSE if the statement contradicts the informationNOT GIVEN if there is no information on this35 There was strong competition between scientists in Renaissance Europe.36 The most important scientific development of the Renaissance period was the discovery of magnetism.37 In 17th-century Britain, leading thinkers combined their interest in science with an interest in how to e某press ideas.Questions 38-40Complete the table.Choose NO MORE THAN TWO WORDS from the passage for each answer. Write your answers in bo某es 38-40 on your answer sheet.Science written in the first half of the 17th centuryLanguage used Latin EnglishType of science Original 38…………E某amples 39………… EncyclopaediasTarget aud ience International scholars 40…………, but socially wider剑桥雅思阅读5原文参考译文(test2)E The birth of modern plastics酚醛塑料——现代塑料的诞生In 1907, Leo Hendrick Baekeland, a Belgian scientist working in New York, discovered and patented a revolutionary new synthetic material. His invention, which he named ‘Bakelite,’ was of enormous technological importance, and effectively launched the modernplastics industry.1907年,比利时科学家Leo Hendrick Baekeland在纽约工作时发现了一种全新的合成材料,并申请了专利。
爱德思化学A2_CH_11 Further Kinetics

Further Kinetics
Methods of measuring the rate of reaction
3. Colorimetry(比色法)-Colour change.
If there is any formation of a coloured product or the removal of a coloured reactant in the course of the reacion, we can monitor the color change by colorimeter(光度计).
Further Kinetics
Methods of measuring the rate of reaction
4. Change in pH (测pH值).
If the reaction produces or uses up H+ ions, the pH of the solution will change. So you could measure the pH of the solution at regular intervals and calculate the concentration of H+
If a gas is given off, collect it in a gas syringe and record how much gas got at regular time intervals.
Two techniques for collecting and measuring gas.
Further Kinetics
Methods of measuring the rate of reaction
2020年9月英语六级真题及参考答案完整版

2020年9月英语六级真题及参考答案【完整版】四六级试卷采用多题多卷形式,大家核对答案时,请找具体选项内容,忽略套数。
无忧考网搜集整理了各个版本(有文字也有图片),仅供大家参考。
【网络综合版】听力:Section ALong Conversation OneM: You are a professor of Physics at the University of Oxford. You are a senior advisor at the European Organization for Nuclear Research. You also seem to tour the global tirelessly, giving talks. And in addition, you have your own weekly TV show On Science. Where do you get the energy?W: Oh, well. 【Q1】I just love what I do. I am extremely fortunate to have this life, doing what I love doing.M: Professor, what exactly is your goal? Why do you do all of these?W: well, as you said, I do have different things going on. But these I think can be divided into 【Q2】two groups: the education of science, and the further understanding of science.M: Don't these two things get in the way of each other? What I mean is, doesn't giving lectures take time away from the lab?W: Not really, no. I love teaching, and I don’t mind spending more time doing that now than in the past. Also, what I will say is, that 【Q3】teaching a subject helps me comprehend it better myself. I find that it furthers my own knowledge when I have to explain something clearly, when I have to aid others understanding it, and when I have to answer questions about it. Teaching at a high level can be very stimulating for anyone, no matter how much expertise they may already have in the field they are instructing.M: Are there any scientific breakthroughs that you see on the near horizon? A significant discovery or invention we can expect soon.W: 【Q4】The world is always conducting science. And there're constantly new things being discovered. In fact, right now, we have too much data sitting in computers.For example, we have thousands of photos of planet Mars taken by telescopes that nobody has ever seen. We have them, yet nobody has had time to look at them with their own eyes, let alone analyze them.Q1: Why does the woman say she can be so energetic?Q2: What has the woman been engaged in?Q3: What does the woman say about the benefit teaching brings to her?Q4: How does the woman say new scientific breakthroughs can be made possible?Section AConversation 2M: Do you think dreams 【Q5】have special meanings?W: No. I don't think they do.M: I don't either, but some people do. I would say people who believe that dreams have special meanings are superstitious, especially nowadays. In the past, during the times of ancient Egypt, Greece or China, people used to believe that dreams could foresee the future. But today, with all the scientific knowledge that we have, I think it's much harder to believe in these sorts of things.W: My grandmother is superstitious, and she thinks dreams can predict the future. Once, 【Q6】she dreamed that the flight she was due to take the following day crashed.Can you guess what she did? She didn't take that flight. She didn't even bother to go to the airport the following day. Instead, she took the same flight but a week later. And everything was fine of course. No plane ever crashed.M: How funny! Did you know that flying is actually safer than any other mode of transport? It's been statistically proven. People can be so irrational sometimes.W: Yes, absolutely. But, even if we think they are ridiculous, 【Q7】emotions can be just as powerful as rational thinking.M: Exactly. People do all sorts of crazy things because of their irrational feelings. But in fact, some psychologists believe that our dreams are the result of our emotions and memories from that day. I think it was Sigmund Freud who said that children's dreams were usually simple representations of their wishes, thingsthey wished would happen. 【Q8】But in adults', dreams are much more complicated reflections of their more sophisticated sentiments.W: Isn't it interesting how psychologists try to understand using the scientific method something as bazaar as dreams? Psychology is like the rational study of irrational feelings.Q5: What do both speakers think of dreams?Q6: Why didn't the woman's grandmother take her scheduled flight?Q7: What does the woman say about people's emotions?Q8: What did psychologist Sigmund Freud say about adults' dreams?Section BPassage 1While some scientists explore the surface of the Antarctic, others are learning more about a giant body of water -- four kilometers beneath the ice pack. Scientists first discovered Lake Vostok in the 1970s by using radio waves that penetrate the ice. Since then, they have used sound waves and even satellites to map this massive body of water. How does the water in Lake Vostok remained liquid beneath an ice sheet? “The thick glacier above acts like insulating blanket and keeps the water from freezing,” said Martin Siegert, a glaciologist from the university of Wales. In addition, geothermal heat from the deep within the earth may warm the hidden lake.The scientists suspect that microorganisms may be living in Lake Vostok, closed or more than two million years. Anything found that off from the outside world f s on the surface of the earth, said Siegert. Scientists ’will be totally alien to what are trying to find a way to drill into the ice and draw water samples without causing ht be the solution. If all goes as planned, a contamination. Again, robots mig shift robot will melt through the surface ice. When it reaches the lake, it -drill will release another robot that can swim in the lake, take pictures and look for ries will shed light on life in outer signs of life. The scientists hope that discove up -space, which might exist in similar dark and airless conditions. Recently closed s moon, Europa, shows signs of water beneath the icy surface. ’pictures of Jupiter ropa to search for life there, Once tested the Antarctic, robots could be set to Eu too.Q9: What did the scientists first use to discover Lake Vostok in the 1970s? Q10: What did scientists think about Lake Vostok?Q11: What do the scientists hope their discoveries will do?Section BPassage 2The idea to study the American Indian tribe – Tarahumaras, came to James Copeland in 1984 when 【Q12】he discovered that very little research had been done on their language. He contacted the tribe member through a social worker who workedwith the tribes in Mexico. At first, the tribe member named Gonzalez was very reluctant to cooperate. He told Copeland that no amount of money could buy his language. But after Copeland explained to him what he intended to do with his research and how it would benefit the Tarahumaras, Gonzalez agreed to help. 【Q13】He took Copeland to his village and served as an intermediary. Copeland says, thanks to him, the Tarahumaras understood what their mission was and started trusting us. 【Q14】Entering the world of Tarahumaras has been a laborious project for Copeland.To reach their homeland, he must strive two and half days from Huston Taxes. He loads up his vehicle with goods that the tribe’s men can’t easily get and gives the goods to them as a gesture of friendship. The Tarahumaras, who don’t believe any humiliating wealth, take the food and share among themselves. For Copeland, the experience has not only been academically satisfying but also has enriched his life in several ways. 【Q15】“I see people rejecting technology and living a very hard, traditional life, which offers me another notion about the meaning of progress in the western tradition,” he says, “I experienced the simplicity of living in nature that I would otherwise only be able to read about.I see a lot of beauty and their sense of sharing and concern for each other.”Q12: Why did James Copeland want to study the American Indian tribe -- Tarahumaras?Q13: How did Gonzalez help James Copeland?Q14: What does the speaker say about James Copeland’s trip to the Tarahumaras village?Q15: What impresses James Copeland about the Tarahumaras tribe?Section CRecording 1What is a radical? It seems today that people are terrified of the term,minority, who are mostly wealthy white males in western society.Feminism is a perfect example of this phenomenon. The women's movement has been plagued by stereotypes, misrepresentations by the media, and accusations of man-hating and radicalism. When the basic foundation of feminism is simply that women deserve equal rights in all facets of life. When faced with the threat of being labelled radical, women back down from their worthy calls and consequently, participate in their own oppression.It has gotten to the point that many women are afraid to call themselves feminists because of a stigma attached to the word. If people refused to be controlled, and intimidated by stigmas, the stigmas lose all their power, without fear on which they feed, such stigmas can only die.To me, 【Q17】a radical is simply someone who rebels against the norm when advocates a change in the existing state of affairs. On close inspection, it becomes clear that the norm is constantly involving, and therefore, is not a constant entity. So why then, is deviation from the present situation such a threat, when the state of affairs itself is unstable and subject to relentless transformation?It all goes back to maintaining the power of those who have it and preventing the right of those who don't. In fact, when we look at the word "radical" in a historical context, nearly every figure we now hold up as a hero was considered a radical in his or her time. Radicals are people who affect change. They are the people about whom history is written. Abolitionists were radicals, civil rights activists were radicals, 【Q18】 even the founders of our country in their fight to win independence from England were radicals. Their presence in history has changed the way our society functions, mainly by shifting the balance of power that previously existed. Of course, there are some radicals who've made a negative impact on humanity, 【Q18】 but undeniably, there would simply be no progress without radicals. That been said, next time someone calls me a radical, I would accept that label with pride.Q16: What usually happens when people are accused of being radical?Q17: What is the speaker's definition of a radical?Q18: What does the speaker think of most radicals in the American history?Recording 2We are very susceptible to the influence of the people around us. For instance, you may have known somebody who has gone overseas for a year or so and has returned with an accent perhaps. We become part of our immediate environment. None of us are immune to the influences of our own world and let us not kid ourselves that we are untouched by the things and people in our life.Fred goes off to his new job at a factory. Fred takes his ten-minute coffee break, but the other workers take half an hour. Fred says, “What’s the matter with you guys?” Two weeks later, Fred is taking twenty-minute breaks. A month later, Fred takes his half hour. Fred is saying “If you can’t be them, join them. Why should I work any harder than the next guy?” The fascinating thing about being human is that generally we are unaware that there are changes taking place in our mentality. It is like returning to the city smog after some weeks in the fresh air. Only then do we realize that we’ve become accustomed to the nasty smells. Mix with critical people and we learn to criticize. Mix with happy people, and we learn about happiness. What this means is that we need to decide what we want from life and then choose our company accordingly. You may well say, "That is going to take some effort. It may not be comfortable. I may offend some of my present company." Right, but it is your life. Fred may say, "I’m always broke, frequently depressed. I’m going nowhereand I never do anything exciting." Then we discover that Fred’s best friends are always broke, frequently depressed, going nowhere and wishing that life was more exciting. This is not coincidence, nor is it our business to stand in judgement of Fred? However, if Fred ever wants to improve his quality of life, the first thing he'll need to do is recognize what has been going on all these years.It’s no surprise that doctors as a profession suffer a lot of ill health, because they spend their life around sick people. Psychiatrists have a higher incidence of suicide in their profession for related reasons. Traditionally, nine out of ten children whose parents smoke, smoke themselves. Obesity is in part an environmental problem. Successful people have successful friends, and so the story goes on.Q19 What does the speaker say about us as human beings?Q20 What does the speaker say Fred should do first to improve his quality of life?Q21 What does the speaker say about the psychiatrists?Section CLecture 3Virtually every American can recognize a dollar bill at a mere glance. Many can identify it by its sound or texture. But 【Q22】few people indeed can accurately describe the world's most powerful, important currency.The American dollar bill is colored with black ink on one side and green on the other;【Q23】 the exact composition of the paper and ink is a closely guarded government secret. Despite its weighty importance, the dollar bill actually weighs little. It requires nearly 500 bills to tip the scales at a pound. Not only is the dollar bill lightweight, but it also has a brief life span. Few dollar bills survive longer than 18 months.The word "dollar" is taken from the German word "taler," the name for the world's most important currency in the 16th century. The taler was a silver coin first minted in 1518 under the reign of Charles V, Emperor of Germany.The concept of paper money is a relatively recent innovation in the history of American currency. When the Constitution was signed, people had little regard for paper money because of its steadily decreasing value during the colonial era.【Q24】Because of this lack of faith, the new American government minted only coins for common currency. Interest-bearing bank notes were issued at the same time, but their purpose was limited to providing money for urgent government crises, such as American involvement in the War of 1812.The first noninterest-bearing paper currency was authorized by Congress in 1862, at the height of the Civil War. At this point, citizens' old fears of devalued paper currency had calmed, and the dollar bill was born. The new green colored paper money quickly earned the nickname "greenback."Today, the American dollar bill is a product of the Federal Reserve and is issued from the twelve Federal Reserve banks around the United States. The government keeps a steady supply of approximately two billion bills in circulation at all times.Controversy continues to surround the true value of the dollar bill.【Q25】American history has seen generations of politicians argue in favor of a gold standard for American currency. However, for the present, the American dollar bill holds the value that is printed on it, and little more. The only other guarantee on the bill is a Federal Reserve pledge of as a confirmation in the form of government securities.Q22: What does the speaker say about the American dollar bill?Q23: What does the speaker say about the exact composition of the American dollar bill?Q24: Why did the new American government mint only coins for common currency?Q25: What have generations of American politicians argued for?参考答案1.A)She can devote all her life to pursing her passion.2.D)Science education and scientific research.3.A)A better understanding of a subject.4.B)By making full use of the existing data.5. B) They have no special meanings.6. C) She dreamed of a plane crash.7. D) They can have an impact as great as rational thinking8. C) They reflect their complicated emotions.9. A) Radio waves.10. B)It may have micro—organisms living in it.11. D)Shed light on possible life in outer space.12. A)He found there had been little research on their anguage.13. D)He acted as an intermediary between Copel and the villagers.14. C)Laborious15. B)Their sense of sharing and caring.16 .A)They tend to be silenced into submission.17. D)One who rebels against the existing social orser.18. C)They served as a driving force for progress.19. B)It is impossible for us to be immune from outside influence.20. D) Recognize the negative impact of his coworkers.21. A) They are quite susceptible to suicide.22. B) Few people can describe it precisely.23. C) It is a well—protected government secret.24. A) People had little faith in paper money.25. C) It is awell—protected government secret.翻译:《水浒传》(Water Margin)是中国文学四大经典小说之一。
机械工程学专业词汇英语翻译(G)

机械工程学专业词汇英语翻译(G)gage length 标距长度gage pressure 表压力gage transformation 规范变换gain loss 增益损失gal 伽galilean frame of reference 伽利略参考系galilean principle of relativity 伽利略相对性原理galilean transformation 伽利略变换galileo law of inertia 伽利略惯性定律galitzin pendulum 伽利津摆gallon 加仑galton apparatus 高尔登哨galton board 高尔登哨gap 间隙gap counting 径迹中断计数gap density 径迹中断密度gap length 空隙宽度gap width 空隙宽度gas amplification 气体放大gas bubble in underwater explosion 水下爆炸气泡gas cavity 气孔gas conductor 气体导电体gas constant 气体常数gas density 气体密度gas diffusion 气体扩散gas diffusion velocity 气体扩散速度gas discharge plasma 气体放电等离子体gas dispersion 气体弥散gas dynamic equation 气体动力学方程gas dynamical 气体动力学的gas dynamics 气体动力学gas emission 气体放出gas equilibrium 气体平衡gas explosion 气体爆炸gas flame 气火焰gas flow counter 气镣计数器gas flow rate 气临率gas flowmeter 气体量计gas friction 气体摩擦gas gage 气压计gas gas immiscibility 气气不混合性gas generation 气体的产生gas hole 气孔gas hydrate 气体水合物gas kinetic 气体运动学的gas kinetics 气体运动学gas liquid flow 气液怜gas liquid mixture 气液混合物gas lubricated gyroscope 气浮陀螺仪gas lubrication 气体润滑gas manometer 气压计gas meter 气量计gas permeability 透气性gas phase 气相gas pocket 气囊gas pressure 煤气压力gas pressure gage 气体压力规gas pressure regulator 气压第器gas solid flow 气固怜gas state 气态gas statics 气体静力学gas stream 气流gas thermometer 气体温度计gas turbine 燃气轮机gas type gravimeter 气体型重力计gaseous 气体的gaseous cavitation 气体空化gaseous diffusion 气体扩散gaseous envelope 气囊gaseous fluid 气态铃gaseous ion 气体离子gaseous medium 气态媒质gaseous mixture 气体混合物gaseous ring 气环gasification 气化gasometer 气量计gassing 充气gauss distribution 高斯分布gauss elimination method 高斯消去法gauss flux theorem 高斯通量定理gauss principle 高斯原理gauss seidel method 高斯赛德尔法gaussian constant of gravitation 高斯引力常数gaussian noise 高斯噪声gaussian variation 高斯变分gaussian wave group 高斯波包general coordinates 广义坐标general law of thermodynamics 热力学普遍定律general mechanics 一般力学general motion 一般运动general product 外积general purpose manipulator 万能操作万能机械手general theory of relativity 广义相对论generalized acceleration 广义加速度generalized acceleration vector 广义加速度矢量generalized compliance 广义顺量generalized continuum mechanics 广义连续介质力学generalized coordinate vector 广义坐标矢量generalized coordinates 广义坐标generalized couette flow 广义库艾特怜generalized displacement 广义位移generalized dissipation 广义耗散generalized energy 广义能量generalized force 广义力generalized function 广义函数generalized hamilton principle 广义哈密顿原理generalized maxwell model 广义麦克斯韦模型generalized momentum 广义动量generalized newtonian fluid 广义牛顿铃generalized ohm law 广义欧姆定律generalized potential 广义势generalized shear 广义剪切generalized strain 广义应变generalized stress 广义应力generalized velocity 广义速度generalized velocity vector 广义速度矢量generating function 母函数generating functional 生成泛函generation 产生generation model 发生模型generator 振荡器geoacoustics 地声学geocentric coordinates 地心坐标geocinetics 地壳运动学geodynamics 地球动力学geographic coordinates 地理坐标geoid 大地水准面geoid undulation 大地水准面波动geoidal surface 大地水准面geokinetics 地壳运动学geomagnetic storm 地磁暴geometric addition 几何加法geometric average 几何平均值geometric boundary condition 几何边界条件geometric constraint 几何约束geometric distortion 几何畸变geometric equation 几何方程geometric mean 几何平均值geometric model 几何模型geometric properties 几何性质geometric similarity 几何相似geometric simulation 几何模拟geometrical invariability 几何不变性geometrical locus 几何轨迹geometrical mechanics 几何力学geometrical moment of inertia 惯性矩geometrical optics 几何光学geometrodynamics 几何动力学geophysical fluid dynamics 地球物理铃动力学geopotential 重力势geopotential level 位势面geopotential surface 位势面georheodynamics 大地龄动力学georheology 地质龄学geostatic pressure 地球静压力geostatics 地球静力学geostress 地应力geostrophic adjustment 地转蝶geostrophic flow 地转流geostrophic force 科里奥利力geostrophic motion 地转运动geothermy 地热geotorsion 地震后扭动gerber's girder 格柏梁giant resonance 巨共振gibbs free energy 吉布斯自由能gimbal axis 常平架轴girder 大梁glancing collision 小角度碰撞glass fiber 玻璃纤维glauert factor 格劳厄特数glauert number 格劳厄特数glauert rule 格劳厄特准则glide 滑移glide direction 滑移方向glide lamella 滑移层glide path 下滑航迹glide plane 滑移面glide step 滑移台阶glide vector 滑动矢量glider 滑翔机gliding angle 下滑角gliding flight 滑翔飞行gliding fracture 滑移断裂global balanced law 整体平衡律global displacement field 整体位移场global energy balance 整体能量平衡global generalized force 整体广义力global stability 整体稳定性godunov method 戈杜诺夫法goubau wave 古搏波governor 蒂机grad shafranov equation 格拉德沙弗拉诺夫方程gradient 坡度gradient acceleration 梯度加速度gradient force 梯度力gradient method 梯度法gradient of continuous density 连续密度梯度gradient richardson number 梯度理查森数gradient tensor 梯度张量gradient wind 梯度风gradiometer 梯度计grain 颗粒grain boundary 晶体界面grain size 粒度grain structure of fracture 断口结构gram 克gram calorie 克卡gram weight 克重grand potential 巨势graphic statics 图解静力学graphical calculation 图示计算graphical construction 准graphical differentiation 图解微分法graphical expression 图示graphical integration 图示积分graphical method 图示法graphical solution 图解法graphostatics 图解静力学grashof number 格拉斯霍夫数grashof's number 格拉绍夫数grating 格子gravimeter 比重计gravimetric anomaly 重量异常gravimetric network 重力观测网gravimetric station 重力测量站gravimetric variometer 重力变化测定仪gravimetry 重力测量gravisphere 重力圈gravitating gas 引力气体gravitation 引力gravitation constant 引力常数gravitation dynamics 引力动力学gravitation law 引力定律gravitational acceleration 重力加速度gravitational coagulation 重力凝结gravitational constant 引力常数gravitational energy 重力能gravitational field 引力场gravitational force 重力gravitational mass 重力质量gravitational moment 重力矩gravitational potential 引力势gravitational potential energy 重力位能gravitational pseudotensor 引力拟张量gravitational radiation 引力辐射gravitational shift 引力移位gravitational stability 重力稳定性gravitational unit 重力单位gravitational wave surface 重力波面gravity 重力gravity accleration 重力加速度gravity anomaly 重力反常gravity axis 重心轴gravity capillary wave 重力表面张力波gravity correction 重力校正gravity dam 重力坝gravity field 引力场gravity flow 重力流gravity formula 重力公式gravity head 重力水头gravity pendulum 重力摆gravity plane 重心平面gravity pressure 重力压gravity reduction 重力校正gravity separation 重力分离gravity tank 重力油箱gravity viscometer 射脸度计gravity wave 重力波greatest lower bound 下界green deformation tensor 格林形变张量green function 格林函数green strength 湿强度green stress tensor 格林应力张量grid 叶栅grid structure 网状结构griffith crack 格里菲斯裂缝griffith criterion 格里菲斯准则grill 栅格grinding 研磨groove 槽gross section 总截面gross tonnage 总吨位gross weight 总重ground air missile 地对空导弹ground effect 地面效应ground level 基态能级ground pressure 地压ground state 基态ground water 地下水ground water basin 地下水盆地ground water pressure 地下水压ground water wave 地下水波group velocity 包络速度group wavelength 群波长growth 生长growth face 生长面growth plane 生长面growth rate 生长速度growth velocity 生长速度guidance 导向guidance plane 制导平面guide bar 导杆guide bearing 导向轴承guide blade 导叶guide ring 导向环guide rod 导杆guide vane 导叶guide wall 导邻guided missile 导弹guided rocket 可操纵火箭guideway 导向路gunn oscillator 冈恶荡器gust 风gustiness 阵风性gusty wind 阵风gutter 放水沟gyrating mass 旋转质量gyration 旋转gyration center 旋转中心gyration constant 转动常数gyration ellipsoid 转动椭球gyration radius 回转半径gyration vector 转动矢量gyratory motion 陀螺运动gyro 回转仪gyro correction 陀螺修正gyro drift 陀螺漂移gyro error 陀螺误差gyroaxis 陀螺轴gyrocompass 回转罗盘gyrodamping 回转阻尼gyrodynamics 陀螺力学gyrointeraction 陀螺相互酌gyromagnetic 旋磁的gyromagnetic effect 转动磁效应gyromagnetic frequency 旋磁频率gyromagnetic resonance 旋磁共振gyromechanical factor 陀螺力学因子gyropilot 自动驾驶仪gyroplane 旋翼机gyroresonance 旋磁共振gyroscope 回转仪gyroscope pendulum 陀螺摆gyroscopic compass 陀螺罗盘gyroscopic effect 陀螺效应gyroscopic horizon 陀螺仪水平gyroscopic instrument 陀螺仪表gyroscopic moment 陀螺力矩gyroscopic motion 陀螺运动gyroscopic theory 陀螺仪理论gyrosphere 回转球gyrostabilization 陀螺稳定gyrostabilized platform 陀螺稳定平台gyrostabilizer 陀螺稳定器gyrostat 回转轮gyrostatic moment 陀螺力矩gyrostatic pressure 陀螺压力。
光催化制备甲烷的方法

光催化制备甲烷的方法The use of photocatalysis to prepare methane is a promising method in the field of green energy and sustainable development. 光催化制备甲烷是绿色能源和可持续发展领域的一种有前景的方法。
This method involves the conversion of carbon dioxide and water into methane using catalysts activated by light. 这种方法涉及使用光催化剂将二氧化碳和水转化为甲烷。
The process has the potential to reduce carbon emissions and provide a renewable source of energy. 这个过程有潜力减少碳排放并提供可再生能源源。
There are several factors that affect the efficiency and practicality of photocatalytic methane production. 影响光催化制备甲烷效率和实用性的因素有很多。
One of the main challenges in photocatalytic methane production is the development of efficient and stable catalysts. 光催化制备甲烷的一个主要挑战是开发高效稳定的催化剂。
Traditional catalysts such as metal nanoparticles have limitations in terms of active sites and stability, leading to low methane production rates and catalyst degradation over time. 传统的金属纳米颗粒这样的催化剂在活性位点和稳定性方面存在局限性,导致甲烷产率较低并且随着时间的推移会出现催化剂降解。
燃烧理论

FACE9 – Theory of Combustion
- 3 components of internal energy: translational, vibrational & rotational;
- Diatomic molecule: not only translational but also vibrational & rotational contributions.
·
Chemical equilibrium
▫ ▫ ▫ Second law of thermodynamics Chemical equilibrium principle Full equilibrium products of combustion
3
·
Ideal-gas behavior
◦ Combustion: ”Rapid oxidation generating heat, or both light and heat; also slow oxidation accompanied by relatively little heat and no light” ”Ideal gas is the system where interaction is absent (i.e., zero potential energy)”. High temperatures associated with combustion generally result in sufficiently low densities for the Ideal Gas Behaviour to hold.
FACE9 – Theory of Combustion
Yi = xi MWi / MW mix
中国石化专业技术人员英语学习参考用书正式版

第一部分通用英语Part OneEnglish for General PurposesUNIT 1 How to be Happy如何获得幸福TextRead the text. Answer the given questions and translate the underlined sentences or paragraphs into Chinese.In the past two weeks we have looked at the happiness formula defined by positive psychologist Martin Seligman, where H (happiness) = S (your biological set point for feeling happy) + C (the conditions of your life) + V (the voluntary choices you make). This week we look at the conditions in life that can improve our happiness quotient.Step 1: Peace and quietJonathon Haidt in his excellent book, The Happiness Hypothesis, notes that research shows that we can never completely adapt to new or chronic noise pollution. Loud noises trigger one of our most primitive fear responses (the other is the fear of falling) and we can never fully relax if we are surrounded by intrusive noise. It is essential to have some peace and quiet every day. If you areunfortunate enough to live somewhere noisy, persist with complaining to your local council. Additionally, try wearing wax earplugs to have some respite. If you need your TV, radio or music up loud, wearing headphones demonstrates altruism to your neighbours, which will make you and them feel good.Step 2: RelationshipsThis is the most important of all the external conditions that can improve your happiness quotient. Often our deepest sources of unhappiness are found in poor relationships with others. A cruelly conflictual relationship with a partner or lover leaves us feeling betrayed and abandoned. A relationship with our parents or children which is not based on compassionate, unconditional regard creates isolation and misery. When faced with such relationships, the most positive thing we can do is to either mend the relationship by confronting what is going wrong or learn to move on.Step 3: ShareIf you have discovered conditions or choices in life that have significantly improved your wellbeing, remember to share them with friends. Passing on what works is essential to improve the wellbeing of our own and others.1. What's the happiness formula according to the passage?2. Why can we never completely adapt to new or chronicnoise pollution?3. How could we make both ourselves and the neighbors feel good?4. Where does the unhappiness come from?5. What is the positive way to face with the cruelly conflictual relationship?ExercisesA. Translate the following sentences into English.1.吵闹的邻居的确对我们家庭不和(domestic upset)有很大影响。
化学专业英语-马永祥-兰州大学

ContentsTHE ELEMENTS AND THE PERIODIC TABLE01. ......................................................- 3 -THE NONMETAL ELEMENTS02. ..................................................................................- 5 -GROUPS IB AND IIB ELEMENTS03. ............................................................................- 7 -GROUPS IIIB—VIIIB ELEMENTS04. ............................................................................- 9 -INTERHALOGEN AND NOBLE GAS COMPOUNDS05. ...........................................- 11 -06. ....................................- 13 -THE CLASSIFICATION OF INORGANIC COMPOUNDSTHE NOMENCLATURE OF INORGANIC COMPOUNDS07. ....................................- 15 -BRONSTED'S AND LEWIS' ACID-BASE CONCEPTS08. ..........................................- 19 -09. ..........................................................................- 22 -THE COORDINATION COMPLEXALKANES10. ..................................................................................................................- 25 -11. .............................................................................- 28 -UNSATURATED COMPOUNDSTHE NOMENCLATURE OF CYCLIC HYDROCARBONS12. ...................................- 30 -SUBSTITUTIVE NOMENCLATURE13. .......................................................................- 33 -14. .......................................................- 37 -THE COMPOUNDS CONTAINING OXYGENPREPARATION OF A CARBOXYLiC ACID BY THE GRIGNARD METHOD15. ..- 39 -THE STRUCTURES OF COVALENT COMPOUNDS16. ............................................- 41 -OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY17. ............................- 44 -SYNTHESIS OF ALCOHOLS AND DESIGN OF ORGANIC SYNTHESIS18. ..........- 47 -ORGANOMETALLICS—METAL π COMPLEXES19. ................................................- 49 -THE ROLE OF PROTECTIVE GROUPS IN ORGANIC SYNTHESIS20. ...................- 52 -ELECTROPHILIC REACTIONS OF AROMATIC COMPOUNDS21. ........................- 54 -POLYMERS22. ................................................................................................................- 57 -ANALYTICAL CHEMISTRY AND PROBLEMS IN SOCIETY23. ............................- 61 -VOLUMETRIC ANALYSIS24. ......................................................................................- 63 -QUALITATIVE ORGANIC ANALYSIS25. ..................................................................- 65 -VAPOR-PHASE CHROMATOGRAPHY26. .................................................................- 67 -INFRARED SPECTROSCOPY27. ..................................................................................- 70 -NUCLEAR MAGNETIC RESONANCE (I)28. ..............................................................- 72 -NUCLEAR MAGNETIC RESONANCE(II)29. ..............................................................- 75 -A MAP OF PHYSICAL CHEMISTRY30. ......................................................................- 77 -THE CHEMICAL THERMODYNAMICS31. ................................................................- 79 -CHEMICAL EQUILIBRIUM AND KINETICS32. ........................................................- 82 -THE RATES OF CHEMICAL REACTIONS33. ............................................................- 85 -NATURE OF THE COLLOIDAL STATE34. .................................................................- 88 -ELECTROCHEMICAL CELLS35. .................................................................................- 90 -BOILING POINTS AND DISTILLATION36. ...............................................................- 93 -EXTRACTIVE AND AZEOTROPIC DISTILLATION37. ............................................- 96 -CRYSTALLIZATION38. ................................................................................................- 98 -39. ...................................................................................- 100 -MATERIAL ACCOUNTINGTHE LITERATURE MATRIX OF CHEMISTRY40. ...................................................- 102 -01. THE ELEMENTS AND THE PERIODIC TABLEThe number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The number of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z.The term element refers to, a pure substance with atoms all of a single kind. To the chemist the "kind" of atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z = 1 to Z = 107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters, for example:oxygen==O nitrogen ==N neon==Ne magnesium ==MgSome elements,which have been known for a long time,have symbols based on their Latin names, for example: iron==Fe(ferrum) copper==Cu(cuprum) lead==Pb(plumbum)A complete listing of the elements may be found in Table 1.Beginning in the late seventeenth century with the work of Robert Boyle, who proposed the presently accepted concept of an element, numerous investigations produced a considerable knowledge of the properties of elements and their compounds1. In 1869, D.Mendeleev and L. Meyer, working independently, proposed the periodic law. In modern form, the law states that the properties of the elements are periodic functions of their atomic numbers. In other words, when the elements are listed in order of increasing atomic number, elements having closely similar properties will fall at definite intervals along the list. Thus it is possible to arrange the list of elements in tabular form with elements having similar properties placed in vertical columns2. Such an arrangement is called a periodic Each horizontal row of elements constitutes a period. It should be noted that the lengths of the periods vary. There is a very short period containing only 2 elements, followed by two short periods of 8 elements each, and then two long periods of 18 elements each. The next period includes 32 elements, and the last period is apparently incomplete. With this arrangement, elements in the same vertical column have similar characteristics. These columns constitute the chemical families or groups. The groups headed by the members of the two 8-element periods are designated as main group elements, and the members of the other groups are called transition or inner transition elements.In the periodic table, a heavy stepped line divides the elements into metals and nonmetals. Elements to the left of this line (with the exception of hydrogen) are metals, while those to the right are nonmetals. This division is for convenience only; elements bordering the line—the metalloids-have properties characteristic of - both metals and nonmetals. It may be seen that most of the elements, including all the transition and inner transition elements, are metals.Except for hydrogen, a gas, the elements of group IA make up the alkali metal family. They are very reactive metals, and they are never found in the elemental state in nature. However, their compounds are widespread. All the members of the alkali metal family, form ions having a charge of 1+ only. In contrast, the elements of group IB —copper, silver, and gold—are comparatively inert. They are similar to the alkali metals in that they exist as 1+ ions in many of their compounds. However, as is characteristic of most transition elements, they form ions having other charges as well.The elements of group IIA are known as the alkaline earth metals. Their characteristic ionic charge is 2+. These metals, particularly the last two members of the group, are almost as reactive as the alkali metals. The group IIB elements—zinc, cadmium, and mercury are less reactive than are those of group II A5, but are more reactive than the neighboring elements of group IB. The characteristic charge on their ions is also 2+.With the exception of boron, group IIIA elements are also fairly reactive metals. Aluminum appears to be inert toward reaction with air, but this behavior stems from the fact that the metal forms a thin, invisible film of aluminum oxide on the surface, which protects the bulk of the metal from further oxidation. The metals of group IIIA form ions of 3+ charge. Group IIIB consists of the metals scandium, yttrium, lanthanum, and actinium.Group IVA consists of a nonmetal, carbon, two metalloids, silicon and germanium, and two metals, tin and lead. Each of these elements forms some compounds with formulas which indicate that four other atoms are present per group IVA atom, as, for example, carbon tetrachloride, GCl4. The group IVB metals —titanium, zirconium, and hafnium —also forms compounds in which each group IVB atom is combined with four other atoms; these compounds are nonelectrolytes when pure.The elements of group V A include three nonmetals — nitrogen, phosphorus, and arsenic—and two metals — antimony and bismuth. Although compounds with the formulas N2O5, PCl5, and AsCl5 exist, none of them is ionic. These elements do form compounds-nitrides, phosphides, and arsenides — in which ions having charges of minus three occur. The elements of group VB are all metals. These elements form such a variety of different compounds that their characteristics are not easily generalized.With the exception of polonium, the elements of group VIA are typical nonmetals. They are sometimes known, as the, chalcogens, from the Greek word meaning "ash formers". In their binary compounds with metals they exist as ions having a charge of 2-. The elements of group ⅦA are all nonmetals and are known as the halogens. from the Greek term meaning "salt formers.” They are the most reactive nonmetals and are capable of reacting with practically all the metals and with most nonmetals, including each other.The elements of groups ⅥB, ⅦB, and VIIIB are all metals. They form such a wide Variety of compounds that it is not practical at this point to present any examples as being typical of the behavior of the respective groups.The periodicity of chemical behavior is illustrated by the fact that. excluding the first period, each period begins with a very reactive metal. Successive element along the period show decreasing metallic character, eventually becoming nonmetals, and finally, in group ⅦA, a very reactive nonmetal is found. Each period ends with a member of the noble gas family.02. THE NONMETAL ELEMENTSWe noted earlier. that -nonmetals exhibit properties that are greatly different from those of the metals. As a rule, the nonmetals are poor conductors of electricity (graphitic carbon is an exception) and heat; they are brittle, are often intensely colored, and show an unusually wide range of melting and boiling points. Their molecular structures, usually involving ordinary covalent bonds, vary from the simple diatomic molecules of H2, Cl2, I2, and N2 to the giant molecules of diamond, silicon and boron.The nonmetals that are gases at room temperature are the low-molecular weight diatomic molecules and the noble gases that exert very small intermolecular forces. As the molecular weight increases, we encounter a liquid (Br2) and a solid (I2) whose vapor pressures also indicate small intermolecular forces. Certain properties of a few nonmetals are listed in Table 2.Table 2- Molecular Weights and Melting Points of Certain NonmetalsDiatomic Molecules MolecularWeightMelting Point°CColorH22-239.1'NoneN228-210NoneF238-223Pale yellowO232-218Pale blueCl271-102Yellow — greenBr2160-7.3Red — brownI2254113Gray—blackSimple diatomic molecules are not formed by the heavier members of Groups V and VI at ordinary conditions. This is in direct contrast to the first members of these groups, N2 and O2. The difference arises because of the lower stability of πbonds formed from p orbitals of the third and higher main energy levels as opposed to the second main energy level2. The larger atomic radii and more dense electron clouds of elements of the third period and higher do not allow good parallel overlap of p orbitals necessary for a strong πbond. This is a general phenomenon — strong π bonds are formed only between elements of the second period. Thus, elemental nitrogen and oxygen form stable molecules with both σand π bonds, but other members of their groups form more stable structures based on σbonds only at ordinary conditions. Note3 that Group VII elements form diatomic molecules, but πbonds are not required for saturation of valence.Sulfur exhibits allotropic forms. Solid sulfur exists in two crystalline forms and in an amorphous form. Rhombic sulfur is obtained by crystallization from a suitable solution, such as CS2, and it melts at 112°C. Monoclinic sulfur is formed by cooling melted sulfur and it melts at 119°C. Both forms of crystalline sulfur melt into S-gamma, which is composed of S8 molecules. The S8 molecules are puckered rings and survive heating to about 160°C. Above 160°C, the S8 rings break open, and some of these fragments combine with each other to form a highly viscous mixture of irregularly shaped coils. At a range of higher temperatures the liquid sulfur becomes so viscous that it will not pourfrom its container. The color also changes from straw yellow at sulfur's melting point to a deep reddish-brown as it becomes more viscous.As4 the boiling point of 444 °C is approached, the large-coiled molecules of sulfur are partially degraded and the liquid sulfur decreases in viscosity. If the hot liquid sulfur is quenched by pouring it into cold water, the amorphous form of sulfur is produced. The structure of amorphous sulfur consists of large-coiled helices with eight sulfur atoms to each turn of the helix; the overall nature of amorphous sulfur is described as3 rubbery because it stretches much like ordinary rubber. In a few hours the amorphous sulfur reverts to small rhombic crystals and its rubbery property disappears.Sulfur, an important raw material in industrial chemistry, occurs as the free element, as SO2 in volcanic regions, asH2S in mineral waters, and in a variety of sulfide ores such as iron pyrite FeS2, zinc blende ZnS, galena PbS and such, and in common formations of gypsum CaSO4 • 2H2O, anhydrite CaSO4, and barytes BaSO4 • 2H2O. Sulfur, in one form or another, is used in large quantities for making sulfuric acid, fertilizers, insecticides, and paper.Sulfur in the form of SO2 obtained in the roasting of sulfide ores is recovered and converted to sulfuric acid, although in previous years much of this SO2 was discarded through exceptionally tall smokestacks. Fortunately, it is now economically favorable to recover these gases, thus greatly reducing this type of atmospheric pollution. A typical roasting reaction involves the change:2 ZnS +3 O2—2 ZnO + 2 SO2Phosphorus, below 800℃ consists of tetratomic molecules, P4. Its molecular structure provides for a covalence of three, as may be expected from the three unpaired p electrons in its atomic structure, and each atom is attached to three others6. Instead of a strictly orthogonal orientation, with the three bonds 90° to each other, the bond angles are only 60°. This supposedly strained structure is stabilized by the mutual interaction of the four atoms (each atom is bonded to the other three), but it is chemically the most active form of phosphorus. This form of phosphorus, the white modification, is spontaneously combustible in air. When heated to 260°C it changes to red phosphorus, whose structure is obscure. Red phosphorus is stable in air but, like all forms of phosphorus, it should be handled carefully because of its tendency to migrate to the bones when ingested, resulting in serious physiological damage.Elemental carbon exists in one of two crystalline structures — diamond and graphite. The diamond structure, based on tetrahedral bonding of hybridized sp3orbitals, is encountered among Group IV elements. We may expect that as the bond length increases, the hardness of the diamond-type crystal decreases. Although the tetrahedral structure persists among the elements in this group — carbon, silicon, germanium, and gray tin — the interatomic distances increase from 1.54 A for carbon to 2.80 A for gray tin. Consequently .the bond strengths among the four elements range from very strong to quite weak. In fact, gray tin is so soft that it exists in the form of microcrystals or merely as a powder. Typical of the Group IV diamond-type crystalline elements, it is a nonconductor and shows other nonmetallic properties7.03. GROUPS IB AND IIB ELEMENTSPhysical properties of Group IB and IIBThese elements have a greater bulk use as metals than in compounds, and their physical properties vary widely.Gold is the most malleable and ductile of the metals. It can be hammered into sheets of 0.00001 inch in thickness; one gram of the metal can be drawn into a wire 1.8 mi in length1. Copper and silver are also metals that are mechanically easy to work. Zinc is a little brittle at ordinary temperatures, but may be rolled into sheets at between 120° to 150℃; it becomes brittle again about 200℃-The low-melting temperatures of zinc contribute to the preparation of zinc-coated iron .galvanized iron; clean iron sheet may be dipped into vats of liquid zinc in its preparation. A different procedure is to sprinkle or air blast zinc dust onto hot iron sheeting for a zinc melt and then coating.Cadmium has specific uses because of its low-melting temperature in a number of alloys. Cadmium rods are used in nuclear reactors because the metal is a good neutron absorber.Mercury vapor and its salts are poisonous, though the free metal may be taken internally under certain conditions. Because of its relatively low boiling point and hence volatile nature, free mercury should never be allowed to stand in an open container in the laboratory. Evidence shows that inhalation of its vapors is injurious.The metal alloys readily with most of the metals (except iron and platinum) to form amalgams, the name given to any alloy of mercury.Copper sulfate, or blue vitriol (CuSO4 • 5H2O) is the most important and widely used salt of copper. On heating, the salt slowly loses water to form first the trihydrate (CuSO4 • 3H z O), then the monohydrate (CuSO4 • H2O), and finally the white anhydrous salt. The anhydrous salt is often used to test for the presence of water in organic liquids. For example, some of the anhydrous copper salt added to alcohol (which contains water) will turn blue because of the hydration of the salt.Copper sulfate is used in electroplating. Fishermen dip their nets in copper sulfate solution to inhibit the growth of organisms that would rot the fabric. Paints specifically formulated for use on the bottoms of marine craft contain copper compounds to inhibit the growth of barnacles and other organisms.When dilute ammonium hydroxide is added" to a solution of copper (I) ions, a greenish precipitate of Cu(OH)2 or a basic copper(I) salt is formed. This dissolves as more ammonium hydroxide is added. The excess ammonia forms an ammoniated complex with the copper (I) ion of the composition, Cu(NH3)42+. This ion is only slightly dissociated; hence in an ammoniacal solution very few copper (I) ions are present. Insoluble copper compounds, execpt copper sulfide, are dissolved by ammonium hydroxids. The formation of the copper (I) ammonia ion is often used as a test for Cu2+ because of its deep, intense blue color.Copper (I) ferrocyanide [Cu2Fe(CN)6] is obtained as a reddish-brown precipitate on the addition of a soluble ferrocyanide to a solution of copper ( I )ions. The formation of this salt is also used as a test for the presence of copper (I) ions.Compounds of Silver and GoldSilver nitrate, sometimes called lunar caustic, is the most important salt of silver. It melts readily and may be cast into sticks for use in cauterizing wounds. The salt is prepared by dissolving silver in nitric acid and evaporating the solution.3Ag + 4HNO3—3AgNO3 + NO + 2H2OThe salt is the starting material for most of the compounds of silver, including the halides used in photography. It is readily reduced by organic reducing agents, with the formation of a black deposit of finely divided silver; this action is responsible for black spots left on the fingers from the handling of the salt. Indelible marking inks and pencils take advantage of this property of silver nitrate.The halides of silver, except the fluoride, are very insoluble compounds and may be precipitated by the addition of a solution of silver salt to a solution containing chloride, bromide, or iodide ions.The addition of a strong base to a solution of a silver salt precipitates brown silver oxide (Ag2G). One might expect the hydroxide of silver to precipitate, but it seems likely that silver hydroxide is very unstable and breaks down into the oxide and water — if, indeed, it is ever formed at all3. However, since a solution of silver oxide js definitely basic, there must be hydroxide ions present in solution.Ag2O + H2O = 2Ag+ + 2OH-Because of its inactivity, gold forms relatively few compounds. Two series of compounds are known — monovalent and trivalent. Monovalent (aurous) compounds resemble silver compounds (aurous chloride is water insoluble and light sensitive), while the higher valence (auric) compounds tend to form complexes. Gold is resistant to the action of most chemicals —air, oxygen, and water have no effect. The common acids do not attack the metal, but a mixture of hydrochloric and nitric acids (aqua regia) dissolves it to form gold( I ) chloride or chloroauric acid. The action is probably due to free chlorine present in the aqua regia.3HCl + HNO3----→ NOCl+Cl2 + 2H2O2Au + 3Cl2 ----→ 2AuCl3AuCl3+HCl----→ HAuCl4chloroauric acid (HAuCl4-H2O crystallizes from solution).Compounds of ZincZinc is fairly high in the activity series. It reacts readily with acids to produce hydrogen and displaces less active metals from their salts. 1 he action of acids on impure zinc is much more rapid than on pure zinc, since bubbles of hydrogen gas collect on the surface of pure zinc and slow down the action. If another metal is present as an impurity, the hydrogen is liberated from the surface of the contaminating metal rather than from the zinc. An electric couple to facilitate the action is probably Set up between the two metals.Zn + 2H+----→ Zn2+ + H2Zinc oxide (ZnO), the most widely used zinc compound, is a white powder at ordinary temperatures, but changes to yellow on heating. When cooled, it again becomes white. Zinc oxide is obtained by burning zinc in air, by heating the basic carbonate, or by roasting the sulfide. The principal use of ZnO is as a filler in rubber manufacture, particularly in automobile tires. As a body for paints it has the advantage over white lead of not darkening on exposure to an atmosphere containing hydrogen sulfide. Its covering power, however, is inferior to that of white lead.04. GROUPS IIIB—VIIIB ELEMENTSGroup I-B includes the elements scandium, yttrium, lanthanum, and actinium1, and the two rare-earth series of fourteen elements each2 —the lanthanide and actinide series. The principal source of these elements is the high gravity river and beach sands built up by a water-sorting process during long periods of geologic time. Monazite sand, which contains a mixture of rare earth phosphates, and an yttrium silicate in a heavy sand are now commercial sources of a number of these scarce elements.Separation of the elements is a difficult chemical operation. The solubilities of their compounds are so nearly alike that a separation by fractional crystallization is laborious and time-consuming. In recent years, ion exchange resins in high columns have proved effective. When certain acids are allowed to flow down slowly through a column containing a resin to which ions of Group III B metals are adsorbed, ions are successively released from the resin3. The resulting solution is removed from the bottom of the column or tower in bands or sections. Successive sections will contain specific ions in the order of release by the resin. For example .lanthanum ion (La3+) is most tightly held to the resin and is the last to be extracted, lutetium ion (Lu3+) is less tightly held and appears in one of the first sections removed. If the solutions are recycled and the acid concentrations carefully controlled, very effective separations can be accomplished. Quantities of all the lanthanide series (except promethium, Pm, which does not exist in nature as a stable isotope) are produced for the chemical market.The predominant group oxidation number of the lanthanide series is +3, but some of the elements exhibit variable oxidation states. Cerium forms cerium( III )and cerium ( IV ) sulfates, Ce2 (SO4 )3 and Ce(SO4 )2, which are employed in certain oxidation-reduction titrations. Many rare earth compounds are colored and are paramagnetic, presumably as a result of unpaired electrons in the 4f orbitals.All actinide elements have unstable nuclei and exhibit radioactivity. Those with higher atomic numbers have been obtained only in trace amounts. Actinium (89 Ac), like lanthanum, is a regular Group IIIB element.Group IVB ElementsIn chemical properties these elements resemble silicon, but they become increasingly more metallic from titanium to hafnium. The predominant oxidation state is +4 and, as with silica (SiO2), the oxides of these elements occur naturally in small amounts. The formulas and mineral names of the oxides are TiO2, rutile; ZrO2, zirconia; HfO2, hafnia. Titanium is more abundant than is usually realized. It comprises about 0.44%of the earth's crust. It is over 5.0%in average composition of first analyzed moon rock. Zirconium and titanium oxides occur in small percentages in beach sands.Titanium and zirconium metals are prepared by heating their chlorides with magnesium metal. Both are particularly resistant to corrosion and have high melting points.Pure TiO2 is a very white substance which is taking the place of white lead in many paints. Three-fourths of the TiO2 is used in white paints, varnishes, and lacquers. It has the highest index of refraction (2.76) and the greatest hiding power of all the common white paint materials. TiO2 also is used in the paper, rubber, linoleum, leather, and textile industries.Group VB Elements: Vanadium, Niobium, and TantalumThese are transition elements of Group VB, with a predominant oxidation number of + 5. Their occurrence iscomparatively rare.These metals combine directly with oxygen, chlorine, and nitrogen to form oxides, chlorides, and nitrides, respectively. A small percentage of vanadium alloyed with steel gives a high tensile strength product which is very tough and resistant to shock and vibration. For this reason vanadium alloy steels are used in the manufacture ofhigh-speed tools and heavy machinery. Vanadium oxide is employed as a catalyst in the contact process of manufacturing sulfuric acid. Niobium is a very rare element, with limited use as an alloying element in stainless steel. Tantalum has a very high melting point (2850 C) and is resistant to corrosion by most acids and alkalies.Groups VIB and VIIB ElementsChromium, molybdenum, and tungsten are Group VIB elements. Manganese is the only chemically important element of Group VIIB. All these elements exhibit several oxidation states, acting as metallic elements in lower oxidation states and as nonmetallic elements in higher oxidation states. Both chromium and manganese are widely used in alloys, particularly in alloy steels.Group VIIIB MetalsGroup VIIIB contains the three triads of elements. These triads appear at the middle of long periods of elements in the periodic table, and are members of the transition series. The elements of any given horizontal triad have many similar properties, but there are marked differences between the properties of the triads, particularly between the first triad and the other two. Iron, cobalt, and nickel are much more active than members of the other two triads, and are also much more abundant in the earth's crust. Metals of the second and third triads, with many common properties, are usually grouped together and called the platinum metals.These elements all exhibit variable oxidation states and form numerous coordination compounds.CorrosionIron exposed to the action of moist air rusts rapidly, with the formation of a loose, crumbly deposit of the oxide. The oxide does not adhere to the surface of the metal, as does aluminum oxide and certain other metal oxides, but peelsoff .exposing a fresh surface of iron to the action of the air. As a result, a piece of iron will rust away completely in a relatively short time unless steps are taken to prevent the corrosion. The chemical steps in rusting are rather obscure, but it has been established that the rust is a hydrated oxide of iron, formed by the action of both oxygen and moisture, and is markedly speeded up by the presence of minute amounts of carbon dioxide5.Corrosion of iron is inhibited by coating it with numerous substances, such as paint, an aluminum powder gilt, tin, or organic tarry substances or by galvanizing iron with zinc. Alloying iron with metals such as nickel or chromium yields a less corrosive steel. "Cathodic protection" of iron for lessened corrosion is also practiced. For some pipelines and standpipes zinc or magnesium rods in the ground with a wire connecting them to an iron object have the following effect: with soil moisture acting as an electrolyte for a Fe — Zn couple the Fe is lessened in its tendency to become Fe2+. It acts as a cathode rather than an anode.。
Contents 目录

Contents 目录Physical constants, symbols, and conversion factors 7物理常数,符号和转换因子7Fields of Advanced Difficulty 8前沿难点领域8Theoretical problems 9理论试题9Problem 1 Superacids 9题1 超酸9Problem 2 Stabilization of high-valent transition metal ions 9 题2 高化合价(这里表示氧化态)过渡金属离子的稳定性Problem 3 Colemanite mineral as boron source 10题3 Colemanite(硬钙硼石)矿物作为硼来源10Problem 4 Magnesium compounds 11题4 镁的化合物11Problem 5 Nitrogen oxides and oxoanions 13题5 氮的氧化物和氧杂阴离子(含氧酸根)13Problem 6 Ferrochrome 14题6 铬酸铁14Problem 7 Xenon compounds 15题7 氙的化合物15Problem 8 Structure of phosphorus compounds 16题8 磷化合物的结构16Problem 9 Arsenic in water 17题9 水中的砷17Problem 10 Amphoteric lead oxide 18题10 两性铅氧化物18Problem 11 Analyzing a mixture of calcium salts 19题11 一份钙盐混合物的分析19Problem 12 Breath analysis 20题12 呼吸分析20Problem 13 Decomposition kinetics of sulfuryl dichloride 21 题13 氯化硫酰的解离动力学21Problem 14 Clock reaction 21题14 时钟反应21Problem 15 Mixing ideal gases 23题15 理想气体的混合23Problem 16 Kinetics in gas phase 23题16 气象动力学23Problem 17 Chemical Equilibrium 24题17 化学平衡24Problem 18 Iodine equilibrium 25题18 碘平衡25Problem 19 Molecular weight determination by osmometry 26题19 渗透压测量分析决定分子质量26Problem 20 Allowed energy levels and requirements for absorption of light 27题20 允许的能级和吸收光的需求27Problem 21 Rotational and vibrational energy levels of a diatomic molecule 29题21 双原子分子的转动和振动能级29Problem 22 Particle in a box: Cyanine dyes and polyenes 31题22 盒子中的粒子(一维势箱):氰基染料和多烯31Problem 23 Radioactive decay 33题23 放射性衰变33Problem 24 Enzyme-substrate interaction 34题24 酶-底物交互34Problem 25 Amides 35题25 酰胺35Problem 26 NMR Spectroscopy 36题26 NMR光谱36Problem 27 Cyclitols 40题27 环状多醇40Problem 28 Antiviral antibiotic 42题28 抗病毒抗体42Problem 29 Acyclic β-amino acids 45题29 脂肪族β-氨基酸 45Problem 30 Life of Ladybug 47题30 Ladybug的一生47Practical Problems, Safety 50实验试题,安全性50Problem 31 Preparation of trans-dichlorobis(ethylenediamine)-cobalt(III)chloride and kinetics of its acid hydrolysis 51题31 反-二氯二(乙二胺)合钴(III)的制备和其酸性水解的动力学51Problem 32 Analysis of calcium salts 53题32 钙盐分析53Problem 33 Potassium bisoxalatocuprate(II) dihydrate: Preparation and analysis 56 题33 水合二草酸根合铜(II)酸钾的制备和分析56Problem 34 Synthesis and analysis of aspirin 59题34 阿司匹林的合成和分析59Problem 35 Determination of iron and copper by iodometric titration 62题35 通过碘量滴定法测定铁和铜62Problem 36 Phenol propargylation: Synthesis of 1-nitro-4-(prop-2-ynyloxy)benzene and (prop-2-ynyloxy)benzene 64题36 苯酚炔丙化:1-硝基-4-(丙-2-酰氧基)苯和(丙-2-酰氧基)苯的合成64 Problem 37 Huisgen dipolar cycloaddition: Copper(I)-catalyzed triazole formation 66 题37 惠斯更双偶极环加成:铜(I)催化下的三氮化合物的形成66Fields of Advanced Difficulty 前沿难点领域Theoretical 理论Kinetics: Integrated first order rate equation; analysis of complex reaction mechanisms using the steady state approximation; determination of reaction order and activation energy.动力学:一级反应速率方程式;稳态近似法分析复杂反应机理;决定反应级数与活化能Thermodynamics: Relationship between equilibrium constant, electromotive force and standard Gibbs free energy; the variation of equilibrium constant with temperature.热力学:平衡常数间的关系,电动力和标准吉布斯自由能;平衡常数随温度的变化Quantum Mechanics: Energetics of rotational, vibrational, and electronic transitions using simple model theories.量子机理:使用简单模型理论解转动、振动和电子转移能态Molecular Structure and Bonding Theories: The use of Lewis theory, VSEPR theory and hybridization for molecules with coordination number greater than four.分子结构和成键理论:Lewis理论,VSEPR理论和配位数大于4的分子杂交的应用Inorganic Chemistry: Stereochemistry and isomerism in coordination compounds.无机化学:配位化合物的立体化学和异构体理论Spectroscopy: Interpretation of relatively simple 13C- and 1H-NMR spectra; chemical shifts, multiplicities, coupling constants and integrals.光谱:相对简单的13C-和1H-NMR光谱的解释;化学位移,复合度,耦合常数和积分Practical 实验Column chromatograpy.柱色谱Thin layer chromatography.薄层色谱Theoretical problems理论试题Problem 1 Superacids题1 超酸The acids which are stronger than pure sulfuric acid are called superacids. Superacids are very strong proton donors being capable of protonating even weak Lewis acids such as Xe, H2, Cl2, Br2, and CO2. Cations, which never exist in other media, have been observed in superacid solutions. George Olah received the Nobel Prize in Chemistry in 1994 for the discovery of carbocation generation by using superacids. The enhanced acidity is due to the formation of a solvated proton. One of the most common superacids can be obtained by mixing SbF5 and HF. When liquid SbF5 is dissolved in liquid HF (in molar ratio of SbF5/HF greater than 0.5) the SbF6 - and Sb2F11 - anions are formed, and the proton released is solvated by HF.比纯硫酸强的酸叫做超酸。
kinetic energy英文定义

kinetic energy英文定义Kinetic energy is a fundamental concept in physics that characterizes the energy possessed by a moving object due to its motion. It is defined as the work needed to accelerate an object of a given mass from rest to its current velocity. The concept of kinetic energy is vital in understanding the motion of objects and plays a crucial role in various areas of science, engineering, and everyday life.To comprehend kinetic energy more comprehensively, it is essential to explore its definition, formula, units, conversion, conservation, and applications in different fields.Definition:The definition of kinetic energy can be understood by considering the work-energy theorem. According to this theorem, the work done on an object is equal to the change inits kinetic energy. In other words, when work is done on an object, its kinetic energy increases. Mathematically, kinetic energy (KE) is calculated as half of the mass (m) of the object multiplied by the square of its velocity (v): KE = 1/2 * m * v^2Units:The SI unit of kinetic energy is joule (J). One joule is equal to the energy transferred when a force of one newton acts on an object to move it through a distance of one meter. However, kinetic energy can also be expressed in other units like kilocalories, electron volts, watt-hours, etc., depending on the context.Conversion:Kinetic energy can be converted into various forms of energy. For instance, when a moving object collides with a stationary object, its kinetic energy may be transferred intopotential energy or other forms of energy. Additionally, kinetic energy can be converted into heat energy through frictional forces.Conservation:According to the law of conservation of energy, energy can neither be created nor destroyed, but it can only be transferred or transformed from one form to another. In the case of kinetic energy, it is conserved as long as no external forces act on the system. However, when external forces like friction, air resistance, or drag come into play, kinetic energy may be converted into other energy forms, resulting in a decrease in the object's velocity.Applications:1. Sports: Understanding kinetic energy is important in various sports, such as athletics, football, basketball, and many others. It helps to explain the performance of athletes,the behavior of balls, and the calculations associated with collisions.2. Engineering: Kinetic energy plays a crucial role in engineering fields. It is vital in designing and analyzing moving systems, such as automobiles, airplanes, rockets, and other vehicles. Engineers consider kinetic energy to ensure the components of these systems can handle the energy involved in their motions.3. Energy Generation: Kinetic energy is harnessed for power generation in different ways. For example,hydroelectric power plants use the kinetic energy of flowing water to turn turbines and generate electricity. Wind turbines also convert the kinetic energy of moving air into electrical energy.4. Safety Measures: Understanding kinetic energy helps in determining the impact forces during accidents and designing safety measures accordingly. It is crucial in designingcrumple zones in cars to absorb kinetic energy during collisions and protect the passengers.5. Renewable Energy: Kinetic energy is utilized in various renewable energy technologies. For instance, tidal power plants convert the kinetic energy of moving tides into electricity. Similarly, wave energy converters harness the energy from the motion of ocean waves.6. Astrophysics: Kinetic energy plays a significant role in astrophysics, explaining the motion and behavior of celestial bodies. It helps in studying the dynamics of planets, stars, galaxies, and other astronomical objects.In conclusion, kinetic energy is a fundamental concept in physics that describes the energy possessed by a moving object. It is defined as the work needed to accelerate an object from rest to its current velocity. Kinetic energyfinds applications in various fields, including sports, engineering, energy generation, safety measures, renewableenergy, and astrophysics. Understanding and calculating kinetic energy is crucial for comprehending the behavior and interactions of moving objects and systems.。
小学上册U卷英语第五单元测验试卷

小学上册英语第五单元测验试卷英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.In autumn, leaves __________ (变色).2.The gas released when vinegar reacts with baking soda is ______.3.I have a soft ________ (玩具熊) that I hug every night before I sleep.4.My pet is very _______ (忠诚的).5.Geothermal energy comes from the heat stored in the ______ of the Earth.6.My favorite book is _______ (哈利·波特).7.Every Christmas, I receive a special ________ (礼物) from Santa. It’s always a________ (惊喜).8.What do you call the person who delivers letters?A. PostmanB. BakerC. TeacherD. Nurse9. A flamingo can be seen standing on one ______ (腿).10.The tree is _______ (full) of leaves.11. A rabbit has big _____.12.What do we call a small, round fruit that is usually red or green?A. BananaB. GrapeC. AppleD. Orange13.How many colors are there in a rainbow?A. 5B. 6C. 7D. 814.What do we call the study of numbers?A. MathematicsB. ScienceC. GeographyD. History15.What is the name of the art of folding paper?A. OrigamiB. DrawingC. PaintingD. Sculpture答案:A16.An _______ is a device that converts energy into motion.17.The kitten is ___ on the couch. (sitting)18. A _______ can provide a backdrop for photos.19.Which word means "to look at something closely"?A. ObserveB. IgnoreC. ForgetD. Understand20.The chemical symbol for chromium is _____.21. A hamster stores food in its ________________ (脸颊).22.What is the main purpose of a compass?A. Measure distanceB. Show directionC. Indicate speedD. Calculate time答案:B23. A ______ (植物妈妈) refers to a mother plant that propagates others.24. A ____(community resource center) offers support services.25.The _______ of an object can be determined by its shape.26.I enjoy reading comics because they are __________.27.What do you call the planet known for its rings?A. EarthB. SaturnC. MarsD. Neptune28.Which holiday celebrates the new year?A. ChristmasB. HalloweenC. New Year's DayD. Thanksgiving答案:C29.What is the largest land carnivore?A. LionB. TigerC. Polar bearD. Grizzly bear30.我的朋友喜欢 _______ (活动). 她觉得这很 _______ (形容词)31.The ice cream is melting ________ (在阳光下).32.What is the main ingredient in pizza?A. BreadB. CheeseC. TomatoD. All of the above33.I have _____ friends in my class. (many)34.Ionic bonds form between metals and _____ by transferring electrons.35.I want to go to the ________.36.My mom _____ breakfast every morning. (prepares)37.The capital of Monaco is __________.38.The center of an atom is called the __________.39.I like to ________ cartoons.40.What is the capital of Haiti?A. Port-au-PrinceB. Cap-HaïtienC. Les CayesD. Jacmel答案:A41.We have a ______ (丰富的) schedule filled with activities.42.What is the freezing point of water in Celsius?A. 0°CB. 100°CC. 50°CD. -10°C答案:A43.She has long ______. (hair)44.Oxygen is necessary for the process of ______.45.The __________ is a famous canyon in Arizona. (大峡谷)46.What do we call a person who runs a restaurant?A. ChefB. WaiterC. ManagerD. Owner47.I can ______ (逛) the mall with my friends.48.What do you call the art of creating sounds using a computer?A. Digital ArtB. Music ProductionC. Sound DesignD. Audio Engineering答案:B49.The ________ (养分) in the soil is important for growth.50.The _______ (Great Society) aimed to eliminate poverty in the US.51. A parakeet enjoys singing ______ (旋律).52.What do you call the main character in a fairy tale?A. HeroB. VillainC. PrincessD. Protagonist答案:D53.The main gas used in welding is __________.54.What is the name of the famous scientist known for his work in genetics?A. Gregor MendelB. Charles DarwinC. Louis PasteurD. James Watson答案:A55.How many planets are in our solar system?A. 7B. 8C. 9D. 10答案:B56.The chemical symbol for thorium is ______.57.What is the capital city of Mauritius?A. Port LouisB. CurepipeC. VacoasD. Quatre Bornes58._____ (lavender) is often used in perfumes.59.What do we call the process of water falling as rain, snow, or sleet?A. EvaporationB. PrecipitationC. CondensationD. Transpiration答案:B60.I want to ___ a musician. (become)61.What is the primary purpose of a compass?A. To measure distanceB. To tell timeC. To find directionD. To weigh objects答案:C62. A _____ is a mountainous area.63. A mixture can be separated by physical _______.64.I can _______ my favorite song.65. A base tastes ______ and feels slippery.66.The _______ of a sound can change based on the distance from the source.67.The _____ (car) is fast.68.My sister has a knack for ____ (writing).69.Fish live in the ________________ (水).70.The chemical formula for -octanol is ______.71.What is the process by which plants grow from seeds?A. GerminationB. PhotosynthesisC. PollinationD. Fertilization答案:A72.The ________ is a happy little animal.73. A suspension is a mixture where particles are ______ in a liquid.74.What is the sweetest fruit?A. LemonB. BananaC. StrawberryD. Grapefruit75.I love to _______ (了解)新事物。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Carbon Isotopic Compositions are Effective Indicators for Gas Origin Identification
-10.0 -15.0 -20.0
C2 (‰)
-10
oil-type gas coal-type gas
C3 (‰)
-15 -20 -25 -30 -35 -40 -45
Gas/Oil
Secondary Expelled Gas
Flow Chart for Gas Generation Kinetics Investigation
kerogen gold-tube pyrolysis Immature source Gas Yields and Carbon Isotopes Measurement Ea: activation energy Af: frequency factor Timing of gas generation, migration and accumulation
Kinetic isotope effects during natural gas generation
Individual 1st order reaction rate coefficients for 12C and 13C isotopic species
k 12C
E12C A12C exp RT
kinetics fitting
extrapolation
Geochemical Properties of Jurassic Coal Selected for Simulation
sample percentage (%) TOC (%) 13C (‰) HI (mgHC/gTOC)
Coal (Ro =0.4%)
Principle of Petroleum Generation Kinetics
Oct. 04, 2010
Theorems
petroleum generation is the result of a large number of chemical reactions leading from kerogen to liquid and gaseous products of lower molecular weight and to residues of increasing degree of condensation these reactions are governed by the basic laws of chemical kinetics
Assuming:
A12C A13C
1
E KIE exp RT
Isotope fractionation源自E E12C E13C
Activation energy distribution for 12CH4 and 13CH4
1800
Potential 12CH4 [礸 TOC] /g
oil
carbon residue
k2j
gas
carbon residue
k2n
after Tissot & Welte (1984)
2.5
Activation energy distribution A = 1.00E+15 1/min
2
Generation potential
1.5
1
0.5
0 48 50 52 54 56 58 60 62 Activation energy [EA]
30 coal-type gas 25 oil-type gas 20
Frequency
30 coal-type gas 25 oil-type gas 20
Frequency
15 10 5 0 19 17 15 13 11 9 7 5 3 1
Ethane content (%)
15 10 5 0 9.5 8.5 7.5 6.5 5.5 4.5 3.5 2.5 1.5 0.5
0.003
0.004
Kerogen degradation and hydrocarbon generation (activation energy distribution)
CO2 ,H2O, etc.
k11 k12 k21 k22
CO2 ,H2O, H2S, etc.
kerogen
k1i
k1m
Propane content (%)
No obvious difference in ethane and propane contents was observed for coal-type gas and oil-type gas in the Tarim basin. So, it is hard to differentiate gas types based on the gas chemical composition
vitrinite
fusinite
67.4 -24.3
66.1
68.3
238
157.8
32.6 33.7 464.2
42.3 42.3
-24.8
-24.6 -24.6 -24.8
semi-fusinite Exinite <10
70.4 74.5
Mathematical Expression for
Possible Scenario of Gas Generation and Modeling
Kerogen
Gas/Oil
Primary Remain Gas Secondary C racking
+
Gas/Oil
Primary Expelled Gas
Gas/Oil
Secondary Remain Gas
100
200
300
400
500
600
700
800
Temperature [癈 ]
Arrhenius diagram Arrhenius diagram
ln (A)
EA k A exp RT
ln (k)
slope: EA/R
0
0.001
0.002 1/T [1/K]
Gas Generation Kinetics
1 x exp[ kdt ]D ( E ) dE
0 0 t
D ( E ) ( / )[( E ) / ]1 exp{[( E ) / ] }
0
D ( E )dE 1
k A exp( E / RT ) E0
Order of Reactions
d ( A) k ( A) dt d ( A) 2 k ( A) dt d ( A) k ( A) x ( B) y n x y dt
1st order 2nd order
nth order
TTI Approach (empirical)
oil-type gas coal-type gas
-25.0 -30.0 -35.0 -40.0 -45.0 -60 -50 -40 13C1(‰ ) -30 -20 -10
13
13
-45.0
-40.0
-35.0
-30.0
13
-25.0
-20.0
-15.0
-10.0
C2(‰ )
Coal-type gas posses heavier carbon isotopes of ethane and propane compared with oil-type gas. A positive relationship of 13C2 and 13C1 and 13C3 suggests that the thermal genetic gases from organic matter cracking under high temperature and pressure are a dominant source in the Tarim basin. GOR-isotope quantitative model can apply to the understanding of natural gas formation and gas filling history.
Comparison TTI - Arrhenius Approach 1.0E+28
1.0E+24
Reaction rate [1/time]
1.0E+20 1.0E+16 1.0E+12 1.0E+08 1.0E+04 1.0E+00 1.0E-04 0
TTI Arrhenius
Ea = 22.6 [kcal/mol] A = 1.00E+15 [1/min]
Gas Generation Kinetics and Determination of Gas Maturity and Aging
Tongwei Zhang et al.
Bureau of Economic Geology The University of Texas at Austin
Oct. 04, 2010
Oct. 04, 2010
Tectonic Elements and Gas Fields in the Tarim Basin, China
A
A’
A Main Component is Methane in Natural Gases, Tarim Basin
