72898-Control de Vibraciones y sus medidas correctoras
基于转铁蛋白受体(TfR1)的肿瘤与脑部疾病靶向治疗研究进展

基于转铁蛋白受体(TfR1)的肿瘤与脑部疾病靶向治疗研究进展人转铁蛋白受体(TfR1)在不同组织器官中普遍表达,其主要功能是协助转铁蛋白在细胞和血脑屏障内外转运,维持细胞铁平衡。
在肿瘤细胞中以及血脑屏障中,TfR1的表达水平明显高于正常细胞组织,因此,TfR1被认为是肿瘤靶向治疗和脑部疾病靶向治疗的重要靶点。
基于TfR1靶向治疗的药物载体主要有转铁蛋白(Tf)、抗TfR1抗体、TfR1结合肽,这些生物大分子能与TfR1特异性结合,结合之后可以通过受体介导的跨胞转运机制进入细胞或穿过血脑屏障。
将小分子药与这些载体偶联可以促进许多亲水性的化疗药物或神经治疗药物进入肿瘤细胞或血脑屏障,而许多中枢神经治疗性大分子则主要通过融合蛋白的方式与抗TfR1抗体连接转运进入中枢神经系统。
Abstract:Human TfR1 was universally expressed in different tissues. The major function of TfR1 was to facilitate delivery of transferrin across cells and blood-brain barrier(BBB). As a result, iron homo-stasis was maintained. TfR1 was recognised as a critical target for tumor and brain disease therapy due to its over expression in tumor cells and BBB. In recent years, drug carriers based on TfR1 recognition were developed such as Transferrin (Tf), anti-TfR1 antibody and TfR1 binding peptide. These carriers bind to TfR1 specifically and enter into cell or BBB through receptor mediated endocytosis. Chemicals conjugated with these carriers can be facilitated to enter into tumor cells and brain tissue. Therapeutic proteins can be engineered to fused with anti-TfR1 antibody and transported across BBB.Key words:TfR1; Tumor target therapy;Brain directed delivery1轉铁蛋白受体(TfR1)简介转铁蛋白受体(TfR1)是一种在不同组织和细胞系中普遍表达的糖蛋白。
恩曲替尼化学式-概述说明以及解释

恩曲替尼化学式-概述说明以及解释1.引言1.1 概述概述恩曲替尼(英文名称:Entrectinib)是一种靶向抗癌药物,属于酪氨酸激酶抑制剂。
它通过抑制肿瘤细胞中的激酶信号通路,发挥抗肿瘤的作用。
恩曲替尼被广泛应用于非小细胞肺癌、神经母细胞瘤和其他肿瘤的治疗。
该药物的化学性质使其具备出色的抗肿瘤效果。
恩曲替尼的分子式为C31H34Cl2N5O3,分子量为602.54克/摩尔。
其分子结构复杂,由多个不同原子组成的编织网状结构构成。
这种特殊的结构赋予了恩曲替尼优异的特性,包括其强大的抑制肿瘤生长能力和独特的靶向治疗机制。
除了化学性质外,恩曲替尼还具有一系列独特的物理性质。
该药物为白色或类白色结晶粉末,具有极高的纯度要求。
其熔点为210-215,在这个温度范围内可以保持稳定。
此外,恩曲替尼在常温下可溶于一些有机溶剂,如二氯甲烷和二甲基亚砜,但不溶于水。
在药理作用方面,恩曲替尼主要表现出针对肿瘤细胞的抗增殖和抗转移能力。
它通过干扰肿瘤细胞的激酶信号通路,阻止肿瘤细胞的分裂和生长。
此外,恩曲替尼还具有特异性靶向治疗作用,能够选择性地抑制特定的激酶,从而实现精确的治疗效果。
然而,恩曲替尼也存在一些副作用,如恶心、呕吐、疲劳和食欲不振等,这些副作用需在使用时留意并及时处理。
综上所述,恩曲替尼作为一种靶向抗肿瘤药物,具有复杂的化学性质、独特的物理性质以及较广泛的药理作用。
进一步的研究和应用将有助于更好地发掘恩曲替尼的潜力,为肿瘤治疗提供新的突破和可能性。
1.2 文章结构文章结构部分的内容如下:文章结构部分主要介绍了整篇文章的组织结构和内容安排。
本文的目录分为引言、正文和结论三个部分。
引言部分主要是对整篇文章的背景和目的进行概述,并对恩曲替尼的化学式进行引入。
接着,文章结构部分将详细介绍恩曲替尼的化学性质、物理性质和药理作用。
最后,结论部分将对恩曲替尼的化学性质、物理性质和药理作用进行总结。
在正文部分,恩曲替尼的化学性质将包括分子式、分子量和结构式的介绍。
信迪利单抗结合改良DCF方案在胃部恶性肿瘤患者治疗中的应用效果研究

信迪利单抗结合改良DCF方案在胃部恶性肿瘤患者治疗中的应用效果研究吴小珍,胡晓波,戴盈英苏州市第九人民医院药学部,江苏苏州215299[摘要]目的研究在胃部恶性肿瘤患者接受治疗的过程中选择信迪利单抗结合改良DCF方案(多西他赛+顺铂+氟尿嘧啶)方案取得的临床效果。
方法选取2020年3月—2023年3月苏州市第九人民医院收治的72例胃部恶性肿瘤患者作为研究对象,采用随机数表法平均分组,对照组中36例患者接受改良DCF方案来开展治疗,观察组中36例患者在接受改良DCF方案(多西他赛+顺铂+氟尿嘧啶)治疗的同时,应用信迪利单抗治疗。
对比两组治疗效果。
结果治疗后,观察组客观有效率为77.78%,疾病控制率为91.67%,优于对照组的52.78%、72.22%,差异有统计学意义(χ2=4.963、4.560,P<0.05);同时观察组癌胚抗原、糖类抗原、干扰素、白细胞介素等指标更优,差异有统计学意义(P<0.05)。
结论对于胃部恶性肿瘤患者开展治疗期间,信迪利单抗结合改良DCF方案具有良好的治疗效果。
[关键词]信迪利单抗;改良DCF方案;胃部恶性肿瘤;应用效果[中图分类号]R4 [文献标识码]A [文章编号]2096-1782(2023)09(b)-0009-04Efficacy of Sintilimab Combined with Modified DCF Regimen in the Treat⁃ment of Gastric Malignant TumorsWU Xiaozhen, HU Xiaobo, DAI YingyingDepartment of Pharmacy, Suzhou Ninth People's Hospital, Suzhou, Jiangsu Province, 215299 China[Abstract] Objective To study the clinical effect of Sintilimab combined with modified DCF regimen (docetaxel + cis⁃platin + fluorouracil) during the treatment of patients with gastric malignant tumors. Methods A total of seventy-two patients with gastric malignancies treated in Suzhou Ninth People's Hospital from March 2020 to March 2023 were se⁃lected as the study subjects. They were grouped averagely according to random number table method, thirty-six pa⁃tients in the control group received the modified DCF regimen, and thirty-six patients in the observation group re⁃ceived the modified DCF regimen (docetaxel + cisplatin + fluorouracil) at the same time received Sintilimab. The treat⁃ment effects of the two groups were compared. Results After the treatment, the objective effective rate and disease con⁃trol rate of the observation group were 77.78% and 91.67%, which were better than those of the control group (52.78% and 72.22%), and the difference was statistically significant (χ2=4.963, 4.560, P<0.05). The indexes of carcinoembry⁃onic antigen, carbohydrate antigen, interferon and interleukin in the observation group were better, and the differences were statistically significant (P<0.05).Conclusion During the treatment of gastric malignant tumor patients, Sintilimab combined with improved DCF regimen not only has good therapeutic effect.[Key words] Sintilimab; Improved DCF program; Gastric malignancy; Application effect胃部恶性肿瘤是胃肠道恶性肿瘤中占比较高的一种,由于胃部恶性肿瘤的发病相对比较隐匿,因此患者在发病初期很难及时发现病变,在确诊时往往已经处于晚期[1]。
08版依替巴肽注射液国外说明书
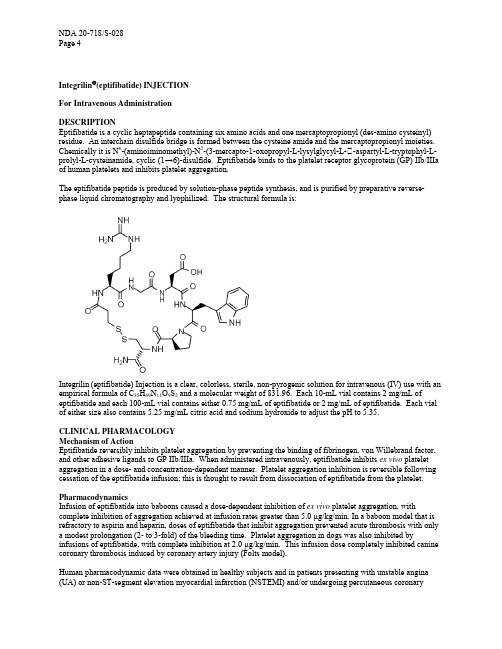
Integrilin®(eptifibatide) INJECTIONFor Intravenous AdministrationDESCRIPTIONEptifibatide is a cyclic heptapeptide containing six amino acids and one mercaptopropionyl (des-amino cysteinyl) residue. An interchain disulfide bridge is formed between the cysteine amide and the mercaptopropionyl moieties. Chemically it is N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl-L-lysylglycyl-L-�-aspartyl-L-tryptophyl-Lprolyl-L-cysteinamide, cyclic (1→6)-disulfide. Eptifibatide binds to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets and inhibits platelet aggregation.The eptifibatide peptide is produced by solution-phase peptide synthesis, and is purified by preparative reverse-phase liquid chromatography and lyophilized. The structural formula is:Integrilin (eptifibatide) Injection is a clear, colorless, sterile, non-pyrogenic solution for intravenous (IV) use with an empirical formula of C35H49N11O9S2 and a molecular weight of 831.96. Each 10-mL vial contains 2 mg/mL of eptifibatide and each 100-mL vial contains either 0.75 mg/mL of eptifibatide or 2 mg/mL of eptifibatide. Each vial of either size also contains 5.25 mg/mL citric acid and sodium hydroxide to adjust the pH to 5.35.CLINICAL PHARMACOLOGYMechanism of ActionEptifibatide reversibly inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor, and other adhesive ligands to GP IIb/IIIa. When administered intravenously, eptifibatide inhibits ex vivo platelet aggregation in a dose- and concentration-dependent manner. Platelet aggregation inhibition is reversible following cessation of the eptifibatide infusion; this is thought to result from dissociation of eptifibatide from the platelet. PharmacodynamicsInfusion of eptifibatide into baboons caused a dose-dependent inhibition of ex vivo platelet aggregation, with complete inhibition of aggregation achieved at infusion rates greater than 5.0 µg/kg/min. In a baboon model that is refractory to aspirin and heparin, doses of eptifibatide that inhibit aggregation prevented acute thrombosis with only a modest prolongation (2- to 3-fold) of the bleeding time. Platelet aggregation in dogs was also inhibited by infusions of eptifibatide, with complete inhibition at 2.0 µg/kg/min. This infusion dose completely inhibited canine coronary thrombosis induced by coronary artery injury (Folts model).Human pharmacodynamic data were obtained in healthy subjects and in patients presenting with unstable angina (UA) or non-ST-segment elevation myocardial infarction (NSTEMI) and/or undergoing percutaneous coronaryinterventions. Studies in healthy subjects enrolled only males; patient studies enrolled approximately one-third women. In these studies, eptifibatide inhibited ex vivo platelet aggregation induced by adenosine diphosphate (ADP) and other agonists in a dose- and concentration-dependent manner. The effect of eptifibatide was observed immediately after administration of a 180-µg/kg intravenous bolus. Table 1 shows the effects of dosing regimens of eptifibatide used in the IMPACT II and PURSUIT studies on ex vivo platelet aggregation induced by 20 µM ADP in PPACK-anticoagulated platelet-rich plasma and on bleeding time. The effects of the dosing regimen used in ESPRIT on platelet aggregation have not been studied.Table 1Platelet Inhibition and Bleeding TimeIMPACT II PURSUIT135/0.5* 180/2.0** Inhibition of platelet aggregation 15 min after bolus 69% 84% Inhibition of platelet aggregation at steady state 40-50% >90% Bleeding-time prolongation at steady state <5x <5x Inhibition of platelet aggregation 4h after infusion discontinuation <30% <50% Bleeding-time prolongation 6h after infusion discontinuation 1x 1.4x* 135-µg/kg bolus followed by a continuous infusion of 0.5 µg/kg/min.** 180-µg/kg bolus followed by a continuous infusion of 2.0 µg/kg/min.The eptifibatide dosing regimen used in the ESPRIT study included two 180-µg/kg bolus doses given 10 minutes apart combined with a continuous 2.0 µg/kg/min infusion.When administered alone, eptifibatide has no measurable effect on prothrombin time (PT) or activated partial thromboplastin time (aPTT) (see also PRECAUTIONS, Drug Interactions section).There were no important differences between men and women or between age groups in the pharmacodynamic properties of eptifibatide. Differences among ethnic groups have not been assessed.PharmacokineticsThe pharmacokinetics of eptifibatide are linear and dose-proportional for bolus doses ranging from 90 to 250 µg/kg and infusion rates from 0.5 to 3.0 µg/kg/min. Plasma elimination half-life is approximately 2.5 hours. Administration of a single 180-µg/kg bolus combined with an infusion produces an early peak level, followed by a small decline prior to attaining steady state (within 4-6 hours). This decline can be prevented by administering a second 180-µg/kg bolus 10 minutes after the first. The extent of eptifibatide binding to human plasma protein is about 25%. Clearance in patients with coronary artery disease is about 55 mL/kg/h. In healthy subjects, renal clearance accounts for approximately 50% of total body clearance, with the majority of the drug excreted in the urine as eptifibatide, deaminated eptifibatide, and other, more polar metabolites. No major metabolites have been detected in human plasma.In patients with moderate to severe renal insufficiency (creatinine clearance <50 mL/min using the Cockcroft-Gault equation), the clearance of eptifibatide is reduced by approximately 50% and steady-state plasma levels approximately doubled (see WARNINGS and DOSAGE AND ADMINISTRATION).Special PopulationsPatients in clinical studies were older (range 20-94 years) than those in the clinical pharmacology studies. Elderly patients with coronary artery disease demonstrated higher plasma levels and lower total body clearance of eptifibatide when given the same dose as younger patients. Limited data are available on lighter weight (<50 kg) patients over 75 years of age.No studies have been conducted in patients with hepatic impairment.Males and females have not demonstrated any clinically significant differences in the pharmacokinetics of eptifibatide.CLINICAL STUDIESEptifibatide was studied in three placebo-controlled, randomized studies. PURSUIT evaluated patients with acute coronary syndromes: unstable angina (UA) or non-ST-segment elevation MI (NSTEMI). Two other studies, ESPRIT and IMPACT II, evaluated patients about to undergo a percutaneous coronary intervention (PCI). Patients underwent primarily balloon angioplasty in IMPACT II and intracoronary stent placement, with or without angioplasty, in ESPRIT.Non-ST-segment Elevation Acute Coronary SyndromeNon-ST-segment elevation acute coronary syndrome is defined as prolonged (≥10 minutes) symptoms of cardiac ischemia within the previous 24 hours associated with either ST-segment changes (elevation between 0.6 mm and 1 mm or depression >0.5 mm), T-wave inversion (>1 mm), or positive CK-MB. This definition includes "unstable angina" and "NSTEMI" but excludes myocardial infarction that is associated with Q waves or greater degrees ofST-segment elevation.PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy)PURSUIT was a 726-center, 27-country, double-blind, randomized, placebo-controlled study in 10,948 patients presenting with UA or NSTEMI. Patients could be enrolled only if they had experienced cardiac ischemia at rest (≥10 minutes) within the previous 24 hours and had either ST-segment changes (elevations between 0.6 mm and 1 mm or depression >0.5 mm), T-wave inversion (>1 mm), or increased CK-MB. Important exclusion criteria included a history of bleeding diathesis, evidence of abnormal bleeding within the previous 30 days, uncontrolled hypertension, major surgery within the previous 6 weeks, stroke within the previous 30 days, any history of3 hemorrhagic stroke, serum creatinine >2.0 mg/dL, dependency on renal dialysis, or platelet count <100,000/mm.Patients were randomized to either placebo, eptifibatide 180-µg/kg bolus followed by a-2.0 µg/kg/min infusion (180/2.0), or eptifibatide 180-µg/kg bolus followed by a 1.3-µg/kg/min infusion (180/1.3). The infusion was continued for 72 hours, until hospital discharge, or until the time of coronary artery bypass grafting (CABG), whichever occurred first, except that if PCI was performed, the eptifibatide infusion was continued for 24 hours after the procedure, allowing for a duration of infusion up to 96 hours.The lower-infusion-rate arm was stopped after the first interim analysis when the two active-treatment arms appeared to have the same incidence of bleeding.Patient age ranged from 20 to 94 (mean 63) years, and 65% were male. The patients were 89% Caucasian, 6% Hispanic, and 5% Black, recruited in the United States and Canada (40%), Western Europe (39%), Eastern Europe (16%), and Latin America (5%).This was a "real world" study; each patient was managed according to the usual standards of the investigational site; frequencies of angiography, PCI, and CABG therefore differed widely from site to site and from country to country. Of the patients in PURSUIT, 13% were managed with PCI during drug infusion, of whom 50% received intracoronary stents; 87% were managed medically (without PCI during drug infusion).The majority of patients received aspirin (75-325 mg once daily). Heparin was administered intravenously or subcutaneously, at the physician's discretion, most commonly as an intravenous bolus of 5000 U followed by a continuous infusion of 1000 U/h. For patients weighing less than 70 kg, the recommended heparin bolus dose was 60 U/kg followed by a continuous infusion of 12 U/kg/h. A target aPTT of 50 to 70 seconds was recommended. A total of 1250 patients underwent PCI within 72 hours after randomization, in which case they received intravenous heparin to maintain an activated clotting time (ACT) of 300 to 350 seconds.The primary endpoint of the study was the occurrence of death from any cause or new myocardial infarction (MI) (evaluated by a blinded Clinical Endpoints Committee) within 30 days of randomization.Compared to placebo, eptifibatide administered as a 180-µg/kg bolus followed by a 2.0-µg/kg/min infusion significantly (P=0.042) reduced the incidence of endpoint events (see Table 2). The reduction in the incidence of endpoint events in patients receiving eptifibatide was evident early during treatment, and this reduction was maintained through at least 30 days (see Figure 1). Table 2 also shows the incidence of the components of the primary endpoint, death (whether or not preceded by an MI) and new MI in surviving patients at 30 days.Table 2Clinical Events In The PURSUIT StudyPlacebo Eptifibatide (180/2.0) P-value(n = 4739) (n = 4722)Death or MI n (%)7 days 552 (11.6%) 477 (10.1%) 0.01630 daysDeath or MI (Primary Endpoint) 745 (15.7%) 672 (14.2%) 0.042 Death 177 (3.7%) 165 (3.5%)Nonfatal MI 568 (12.0%) 507 (10.7%)Figure 1: Kaplan-Meier Plot of Time to Death or Myocardial Infarction Within 30 Days of RandomizationTreatment with eptifibatide prior to determination of patient management strategy reduced clinical events regardless of whether patients ultimately underwent diagnostic catheterization, revascularization (i.e., PCI or CABG surgery) or continued to receive medical management alone. Table 3 shows the incidence of death or MI within 72 hours.Table 3Clinical Events (Death or MI) in the PURSUIT Study Within 72 Hours of Randomization180/2.0Overall Patient Population n =4739 n =4722– At 72 hours7.6%5.9%Patients undergoing early PCI n =631 n =619– Pre-procedure (nonfatal MI only) 5.5% 1.8% – At 72 hours14.4%9.0%Patients not undergoing early PCI n =4108 n =4103– At 72 hours6.5%5.4%All of the effect of eptifibatide was established within 72 hours (during the period of drug infusion), regardless ofmanagement strategy. Moreover, for patients undergoing early PCI, a reduction in events was evident prior to theprocedure. An analysis of the results by sex suggests that women who would not routinely be expected to undergo percutaneouscoronary intervention (PCI) receive less benefit from eptifibatide (95% confidence limits for relative risk of 0.94 to 1.28) than do men (0.72 to 0.90). This difference may be a true treatment difference, the effect of other differencesin these subgroups, or a statistical anomaly. No differential outcomes were seen between male and female patientsundergoing PCI(see results for ESPRIT). Follow-up data were available through 165 days for 10,611 patients enrolled in the PURSUIT trial (96.9 percent of the initial enrollment). This follow-up included 4566 patients who received eptifibatide at the 180/2.0 dose. As reported by the investigators, the occurrence of death from any cause or new myocardial infarction for patients followed for at least 165 days was reduced from 13.6 percent with placebo to 12.1 percent with eptifibatide 180/2.0. Percutaneous Coronary InterventionIMPACT II (Integrilin to Minimize Platelet Aggregation and Prevent Coronary Thrombosis II) IMPACT II was a multicenter, double-blind, randomized, placebo-controlled study conducted in the United States in 4010 patients undergoing PCI. Major exclusion criteria included a history of bleeding diathesis, major surgery within 6 weeks of treatment, gastrointestinal bleeding within 30 days, any stroke or structural CNS abnormality, 3uncontrolled hypertension, PT >1.2 times control, hematocrit <30%, platelet count <100,000/mm , and pregnancy.Patient age ranged from 24 to 89 (mean 60) years, and 75% were male. The patients were 92% Caucasian, 5% Black, and 3% Hispanic. Forty-one percent of the patients underwent PCI for ongoing ACS. Patients were randomly assigned to one of three treatment regimens, each incorporating a bolus dose initiated immediately prior to PCI followed by a continuous infusion lasting 20 to 24 hours: 1) 135-µg/kg bolus followed by a continuous infusion of 0.5 µg/kg/min of eptifibatide (135/0.5); 2) 135-µg/kg bolus followed by a continuous infusion of 0.75-µg/kg/min of eptifibatide (135/0.75); or 3) a matching placebo bolus followed by a matching placebo continuous infusion.Each patient received aspirin and an intravenous heparin bolus of 100 U/kg, with additional bolus infusions of up to2000 additional units of heparin every 15 minutes to maintain an activated clotting time (ACT) of 300 to 350seconds.The primary endpoint was the composite of death, MI, or urgent revascularization, analyzed at 30 days afterrandomization in all patients who received at least one dose of study drug.As shown in Table 4, each eptifibatide regimen reduced the rate of death, MI, or urgent intervention, although at 30days, this finding was statistically significant only in the lower-dose eptifibatide group.As in the PURSUIT study, the effects of eptifibatide were seen early and persisted throughout the 30-day period.Table 4Clinical Events in the IMPACT II StudyEptifibatide EptifibatidePlacebo(135/0.5) (135/0.75)n (%) n (%) n (%)Abrupt(3.3%)Closure 65 (5.1%) 36 (2.8%) 43 P-value vs placebo 0.003 0.030Death, MI, or Urgent Intervention24 hours 123 (9.6%) 86 (6.6%) 89 (6.9%)P-value vs placebo 0.006 0.01448 hours 131 (10.2%) 99 (7.6%) 102 (7.9%)P-value vs placebo 0.021 0.04530 days (primary endpoint) 149 (11.6%) 118 (9.1%) 128 (10.0%)P-value vs placebo 0.035 0.179Death or MI30 days 110 (8.6%) 89 (6.8%) 95 (7.4%)P-value vs placebo 0.102 0.272(10.3%)*6 months 151 (11.9%)* 136 (10.6%)* 130P-value vs placebo 0.297 0.182* Kaplan-Meier estimate of event rate.ESPRIT (Enhanced Suppression of the Platelet IIb/IIIa Receptor with Integrilin Therapy)The ESPRIT study was a multicenter, double-blind, randomized, placebo-controlled study conducted in the United States and Canada that enrolled 2064 patients undergoing elective or urgent PCI with intended intracoronary stent placement. Exclusion criteria included MI within the previous 24 hours, ongoing chest pain, administration of any oral anti-platelet or oral anticoagulant other than aspirin within 30 days of PCI (although loading doses of thienopyridine on the day of PCI were encouraged), planned PCI of a saphenous vein graft or subsequent “staged” PCI, prior stent placement in the target lesion, PCI within the previous 90 days, a history of bleeding diathesis, major surgery within 6 weeks of treatment, gastrointestinal bleeding within 30 days, any stroke or structural CNS3 abnormality, uncontrolled hypertension, PT >1.2 times control, hematocrit <30%, platelet count <100,000/mm, and pregnancy.Patient age ranged from 24 to 93 (mean 62) years and 73% of patients were male. The study enrolled 90% Caucasian, 5% African American, 2% Hispanic and 1% Asian patients. Patients received a wide variety of stents. Patients were randomized either to placebo or eptifibatide administered as an intravenous bolus of 180 µg/kg followed immediately by a continuous infusion of 2.0 µg/kg/min, and a second bolus of 180 µg/kg administered 10 minutes later (180/2.0/180). Eptifibatide infusion was continued for 18 to 24 hours after PCI or until hospital discharge, whichever came first. Each patient received at least one dose of aspirin (162-325 mg) and 60 U/kg of heparin as a bolus (not to exceed 6000 Units) if not already receiving a heparin infusion. Additional boluses of heparin (10-40 U/kg) could be administered in order to reach a target ACT between 200 and 300 seconds.The primary endpoint of the ESPRIT study was the composite of death, MI, urgent target vessel revascularization (UTVR) and “bailout” to open label eptifibatide due to a thrombotic complication of PCI (TBO) (e.g., visible thrombus, “no reflow,” or abrupt closure) at 48 hours. MI, UTVR and TBO were evaluated by a blinded Clinical Events Committee.As shown in Table 5, the incidence of the primary endpoint and selected secondary endpoints was significantly reduced in patients who received eptifibatide. A treatment benefit in patients who received eptifibatide was seen by 48 hours and at the end of the 30-day observation period.Table 5Clinical Events in the ESPRIT StudyPlacebo(n=1024) Death, MI, Urgent Target Vessel Revascularization, or Thrombotic “Bailout”48 Hours (primary endpoint) 30 Days 108 (10.5%) 120 (11.7%)Death, MI, or Urgent Target Vessel Revascularization Eptifibatide180/2.0/180(n=1040)69 (6.6%)78 (7.5%)Relative Risk(95% CI)0.629 (0.471,0.840)0.640 (0.488,0.840)P-Value0.00150.001148 Hours95 (9.3%) 62 (6.0%) 0.643 (0.472, 0.004530 Days (key secondary 107 (10.4%) 71 (6.8%) 0.875) 0.0034 endpoint) 0.653 (0.490,0.871)Death or MI48 Hours94 (9.2%) 57 (5.5%) 0.597 (0.435, 0.001330 Days 104 (10.2%) 66 (6.3%) 0.820) 0.00160.625 ( 0.465,0.840)The need for thrombotic “bailout” was significantly reduced with eptifibatide at 48 hours (2.1% for placebo, 1.0% for eptifibatide; P=0.029). Consistent with previous studies of GP IIb/IIIa inhibitors, most of the benefit achieved acutely with eptifibatide was in the reduction of MI. Eptifibatide reduced the occurrence of MI at 48 hours from 9.0% for placebo to 5.4% (P=0.0015) and maintained that effect with significance at 30 days.There was no treatment difference with respect to sex in ESPRIT. Eptifibatide reduced the incidence of the primary endpoint in both men (95% confidence limits for relative risk: 0.54, 1.07) and women (0.24, 0.72) at 48 hours. Follow-up (12-month) mortality data were available for 2024 patients (1017 on eptifibatide) enrolled in the ESPRIT trial (98.1% of the initial enrollment). Twelve-month clinical event data were available for 1964 patients (988 on eptifibatide) representing 95.2% of the initial enrollment. As shown in Table 6, the treatment effect of eptifibatide seen at 48 hours and30 days appeared preserved at 6 months and 1 year. Most of the benefit was in reduction of MI.Table 6Clinical Events at 6 months and 1 year in the ESPRIT StudyPlacebo Eptifibatide Hazard Ratio180/2.0/180(n=1024) (n=1040) (95% CI)Death, MI, or Target Vessel Revascularization6 Months187 (18.5%) 146 (14.3%) 0.744 (0.599, 0.924)1 Year 222 (22.1%) 178 (17.5%) 0.762 (0.626, 0.929)Death, MI6 Months117 (11.5%) 77 (7.4%) 0.631 (0.473, 0.841)1 Year 126 (12.4%) 83 (8.0%) 0.630 (0.478, 0.832) Percentages are Kaplan-Meier event rates.INDICATIONS AND USAGEIntegrilin is indicated:• For the treatment of patients with acute coronary syndrome (unstable angina/non-ST- segment elevation myocardial infarction), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI). In this setting, Integrilin has been shown to decrease the rate of a combinedendpoint of death or new myocardial infarction.• For the treatment of patients undergoing PCI, including those undergoing intracoronary stenting. In this setting, Integrilin has been shown to decrease the rate of a combined endpoint of death, new myocardial infarction, or need for urgent intervention.In the IMPACT II, PURSUIT and ESPRIT studies of eptifibatide, most patients received heparin and aspirin (see CLINICAL STUDIES).CONTRAINDICATIONSTreatment with eptifibatide is contraindicated in patients with:• A history of bleeding diathesis, or evidence of active abnormal bleeding within the previous 30 days.• Severe hypertension (systolic blood pressure >200 mm Hg or diastolic blood pressure >110 mm Hg) not adequately controlled on antihypertensive therapy.• Major surgery within the preceding 6 weeks.• History of stroke within 30 days or any history of hemorrhagic stroke.• Current or planned administration of another parenteral GP IIb/IIIa inhibitor.• Dependency on renal dialysis.• Known hypersensitivity to any component of the product.WARNINGSBleedingBleeding is the most common complication encountered during eptifibatide therapy. Administration of eptifibatide is associated with an increase in major and minor bleeding, as classified by the criteria of the Thrombolysis in Myocardial Infarction Study group (TIMI) (see ADVERSE REACTIONS). Most major bleeding associated with eptifibatide has been at the arterial access site for cardiac catheterization or from the gastrointestinal or genitourinary tract.In patients undergoing percutaneous coronary interventions, patients receiving eptifibatide experience an increased incidence of major bleeding compared to those receiving placebo without a significant increase in transfusion requirement. Special care should be employed to minimize the risk of bleeding among these patients (see PRECAUTIONS). If bleeding cannot be controlled with pressure, infusion of eptifibatide and concomitant heparin should be stopped immediately.Renal InsufficiencyApproximately 50% of eptifibatide is cleared by the kidney in patients with normal renal function. Total drug clearance is decreased by approximately 50% and steady-state plasma eptifibatide concentrations are doubled in patients with an estimated creatinine clearance <50 mL/min (using the Cockcroft-Gault equation). Therefore, the infusion dose should be reduced to 1 µg/kg/min in such patients (see DOSAGE AND ADMINISTRATION). There has been no clinical experience in patients dependent on dialysis.Platelet Count <100,000/mm3Because it is an inhibitor of platelet aggregation, caution should be exercised when administering eptifibatide to patients with a platelet count <100,000/mm3; there has been no clinical experience with eptifibatide initiated in patients with a platelet count <100,000/mm3.PRECAUTIONSBleeding PrecautionsCare of the Femoral Artery Access Site in Patients Undergoing Percutaneous Coronary Intervention (PCI)In patients undergoing PCI, treatment with eptifibatide is associated with an increase in major and minor bleeding at the site of arterial sheath placement. After PCI, eptifibatide infusion should be continued until hospital discharge or up to 18 to 24 hours, whichever comes first. Heparin use is discouraged after the PCI procedure. Early sheath removal is encouraged while eptifibatide is being infused. Prior to removing the sheath, it is recommended thatheparin be discontinued for 3 to 4 hours and an aPTT of <45 seconds or ACT <150 seconds be achieved. In any case, both heparin and eptifibatide should be discontinued and sheath hemostasis should be achieved at least 2 to 4 hours before hospital discharge.Use of Thrombolytics, Anticoagulants, and Other Antiplatelet AgentsIn the IMPACT II, PURSUIT, and ESPRIT studies, eptifibatide was used concomitantly with unfractionated heparin and aspirin (see CLINICAL STUDIES). In the ESPRIT study, clopidogrel or ticlopidine were used routinely starting the day of PCI. Because eptifibatide inhibits platelet aggregation, caution should be employed when it is used with other drugs that affect hemostasis, including thrombolytics, oral anticoagulants, nonsteroidal anti-inflammatory drugs, and dipyridamole. To avoid potentially additive pharmacologic effects, concomitant treatment with other inhibitors of platelet receptor GP IIb/IIIa should be avoided.There is only a small experience with concomitant use of eptifibatide and thrombolytics. In a study of 180 patients with acute myocardial infarction (AMI), eptifibatide (in regimens up to a bolus of 180 µg/kg followed by a continuous infusion of 0.75 µg/kg/min for 24 hours) was administered concomitantly with the approved "accelerated" regimen of alteplase, a thrombolytic agent. The studied regimens of eptifibatide did not increase the incidence of major bleeding or transfusion compared to the incidence seen when alteplase was given alone.In the IMPACT II study, 15 patients received a thrombolytic agent in conjunction with the 135/0.5 dosing regimen, 2 of whom experienced a major bleed. In the PURSUIT study, 40 patients who received eptifibatide at the 180/2.0 dosing regimen received a thrombolytic agent, 10 of whom experienced a major bleed.In another AMI study involving 181 patients, eptifibatide (in regimens up to a bolus of 180 µg/kg followed by a continuous infusion of up to 2.0 µg/kg/min for up to 72 hours) was administered concomitantly with streptokinase (1.5 million units over 60 minutes), another thrombolytic agent. At the highest studied infusion rates (1.3 µg/kg/min and 2.0 µg/kg/min), eptifibatide was associated with an increase in the incidence of bleeding and transfusions compared to the incidence seen when streptokinase was given alone.These limited data on the use of eptifibatide in patients receiving thrombolytic agents do not allow an estimate of the bleeding risk associated with concomitant use of thrombolytics. Systemic thrombolytic therapy should be used with caution in patients who have received eptifibatide.Minimization of Vascular and Other TraumaArterial and venous punctures, intramuscular injections, and the use of urinary catheters, nasotracheal intubation, and nasogastric tubes should be minimized. When obtaining intravenous access, noncompressible sites (e.g., subclavian or jugular veins) should be avoided.Laboratory TestsBefore infusion of eptifibatide, the following laboratory tests should be performed to identify preexisting hemostatic abnormalities: hematocrit or hemoglobin, platelet count, serum creatinine, and PT/aPTT. In patients undergoing PCI, the activated clotting time (ACT) should also be measured.Maintaining Target aPTT and ACTThe aPTT should be maintained between 50 and 70 seconds unless PCI is to be performed. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT. Table 7 displays the risk of major bleeding according to the maximum aPTT attained within 72 hours in the PURSUIT study.。
DERSIMELAGON 产品说明书

488 Scientific AbstractsSystemic sclerosis, myositis and related syndromes - aetiology, pathogenesis and animal modelsPOS0467 DERSIMELAGON, A NOVEL ORAL MELANOCORTIN1 RECEPTOR AGONIST, DEMONSTRATES DISEASE-MODIFYING EFFECTS IN PRECLINICAL MODELS OFSYSTEMIC SCLEROSISM. Kondo1, T. Suzuki1, Y. Kawano1, S. Kojima2, M. Miyashiro1, A. Matsumoto1, G. Kania3, P. Blyszczuk3, R. Ross4, P. Mulipa4, F. Del Galdo4, Y. Zhang5, J. H. W. Distler5. 1Mitsubishi T anabe Pharma Corporation, Research Unit/Immunology & Inflammation, Souyaku Innovative Research Division, Y okohama, Japan;2Mitsubishi T anabe Pharma Corporation, Discovery T echnology Laboratories, Souyaku Innovative Research Division, Y okohama, Japan;3University Hospital Zurich, University of Zurich, Center of Experimental Rheumatology, Department of Rheumatology, Schlieren, Switzerland;4University of Leeds, Leeds Instituteof Rheumatic and Musculoskeletal Medicine, Faculty of Medicine and Health, Leeds, United Kingdom;5Friedrich-Alexander-University Erlangen-Nürnberg (FAU) and University Hospital Erlangen, Department of Internal Medicine 3—Rheumatology and Immunology, Erlangen, GermanyBackground: Activation of melanocortin 1 receptor (MC1R) is known to have broad anti-inflammatory and anti-fibrotic effects. The bleomycin (BLM)-induced skin fibrosis murine model is well-established for systemic sclerosis (SSc). α-mel-anocyte-stimulating hormone, an endogenous ligand of MC1R, inhibits skin fibro-sis and MC1R knock-out enhances skin fibrosis in this model. These pieces of evidence suggest that MC1R agonism has potential in the treatment of SSc. Objectives: Dersimelagon phosphate (MT-7117) is an investigational small molecule that is an orally administered, selective agonist for MC1R. The purpose of this study is to investigate the potential of MT-7117 as a therapeutic agent for SSc by evaluat-ing its efficacy and mechanism of action in complementary preclinical models. The expression and distribution of MC1R in the skin of SSc patients was investigated. Methods: The effects of MT-7117 on skin fibrosis and lung inflammation were eval-uated in BLM-induced SSc murine models that were optimized for prophylactic and therapeutic evaluation. Microarray-based gene expression analysis and serum pro-tein profiling were performed to investigate the mechanism of action of MT-7117 in the BLM-induced SSc models. The effect of MT-7117 on TGF-β-induced activation of human dermal fibroblasts was evaluated in vitro. Immunohistochemical analyses of MC1R expression in skin samples from SSc patients were performed. Results: Prophylactic treatment with MT-7117 (≥0.3 mg/kg/day p.o.) significantly inhibited the increase in collagen content of the skin, the serum level of sur-factant protein D, and the weight of the lungs from BLM-induced skin fibrosis and lung inflammation model. Therapeutic treatment with MT-7117 (≥3 mg/kg/ day p.o.) significantly suppressed skin thickening and the numbers of myofi-broblasts in pre-established BLM-induced skin fibrosis model. Gene array anal-ysis using the BLM-induced SSc model demonstrated changes in numerous categories related to macrophages, monocytes, and neutrophils, followed by endothelial cell-related categories after treatment with MT-7117. In the analy-sis that focused on biological functions, categories of inflammatory response, activation of antigen-presenting cells, angiogenesis, atherosclerosis, vascu-logenesis, and vaso-occlusion were suppressed by MT-7117. In the analysis that focused on molecular signaling pathways, triggering receptor expressed on myeloid cells-1, IL-6, and oncostatin M involved in inflammation, and perox-isome proliferator-activated receptor that is related to fibrosis were all affected by MT-7117. Serum protein profiling using BLM-induced SSc model revealed that multiple SSc-related biomarkers including P-selectin, osteoprotegerin, cys-tatin C, growth and differentiation factor-15 and S100A9 were suppressed by MT-7117. MT-7117 inhibited the activation of human dermal fibroblasts by sup-pressing TGF-β-induced ACTA2 (encoding α-smooth muscle actin) mRNA ele-vation in vitro. Immunohistochemical analyses showed that MC1R positivity was observed in 40 of 50 diffuse cutaneous SSc patients. MC1R was expressed by monocytes/macrophages, neutrophils, blood vessels (endothelial cells), fibro-blasts, and epidermis (keratinocytes) in the skin of SSc patients. Conclusion: MT-7117 demonstrates disease-modifying effects in preclinical mod-els of SSc. Investigations of its mechanism of action and target expression anal-yses indicate that MT-7117 exerts its positive effects by affecting the pathologies of inflammation, vascular dysfunction, and fibrosis through inflammatory cells, endothelial cells, and fibroblasts. In view of its potent beneficial impact on all these three main pathologies of SSc, MT-7117 is a potential therapeutic agent for the treatment of clinically challenging SSc, which has diverse and difficult to treat symp-toms. A phase 2 clinical trial investigating the efficacy and tolerability of MT-7117 in patients with early, progressive diffuse cutaneous SSc is currently in progress. Disclosure of Interests: Masahiro Kondo Employee of: Mitsubishi Tanabe Pharma Corporation, Tsuyoshi Suzuki Employee of: Mitsubishi Tanabe Pharma Corporation, Yuko Kawano Employee of: Mitsubishi Tanabe Pharma Corpora-tion, Shinji Kojima Employee of: Mitsubishi Tanabe Pharma Corporation, Masa-hiko Miyashiro Employee of: Mitsubishi Tanabe Pharma Corporation, Atsuhiro Matsumoto Employee of: Mitsubishi Tanabe Pharma Corporation, Gabriela Kania: None declared, Przemyslaw Blyszczuk: None declared, rebecca ross:None declared, Panji Mulipa: None declared, Francesco Del Galdo Grant/ research support from: Prof. F. Del Galdo received fees and research supportfrom Abbvie, AstraZeneca, Boehringer-Ingelheim, Capella, Chemomab, Kymab, Janssen and Mitsubishi-Tanabe., Yun Zhang: None declared, Jörg H.W. DistlerGrant/research support from: Prof. J.H.W. Distler received consulting fees, lec-ture fees, and/or honoraria from Actelion, Active Biotech, Anamar, ARXX, aTyr,Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GSK, Inventiva, JB Therapeutics, Medac, Pfizer, Sanofi-Aventis, RedX, RuiYi and UCB. J. H. W.Distler is stock owner of 4D Science and Scientific head of FibroCure.DOI: 10.1136/annrheumdis-2022-eular.29POS0468 EXTRACELLULAR VESICLES FROM SERUM OFMYOSITIS PATIENTS AS CIRCULATING BIOMARKERSAND DISEASE MEDIATORSS. Kivity1,2, H. Kravitz3, C. Cohen3, D. Margoulis3, M. Amar3, G. Kazimirsky3,D. Ozeri4, A. Dori5, C. Brodie3. 1Meir Medical Center, Rheumatology Unit, KefarSava, Israel;2T el Aviv University, Sackler faculty of Medicine, T el Aviv-Y afo, Israel;3Bar-Ilan University, The Mina and Everard Goodman Faculty of Life Sciences,Ramat Gan, Israel;4T el-HaShomer The Sheba Medical Center, ZabludowiczCenter for Autoimmune Disease, Ramat Gan, Israel;5T el-HaShomer The ShebaMedical Center, Department of Neurology, T alpiot Medical Leadership Program,Sackler Faculty of Medicine, T el Aviv University, Ramat Gan, IsraelBackground: Inflammatory myopathies (IM) are a heterogeneous group of disor-ders characterized by autoimmune inflammatory destruction of skeletal muscles.It is many times associated with lung, skin and joint involvement. Identifying bio-markers that can differentiate IM from other muscle disorders may elucidate the pathophysiology of IM, guide novel therapies, monitor disease activity/responseto treatments and predict prognosis. Exosomes are membrane-bound nanove-sicles with diameters of 30-150 nm that contain multiple proteins, nucleic acid,lipids and other molecules in a tissue- and cell-specific manner. Exosomes are secreted by a large variety of cells, play major roles in cell-cell interactions, andhave recently emerged as circulating biomarkers in a variety of pathological con-ditions, including several autoimmune diseases.Objectives: To characterize exosomes from serum of IM patients, analyze pro-tein expression and study their potential mediators of disease pathologies.Methods: Serum was collected from patients suffering from IM(n=5) and from patients suffering from Becker (BMD) and Duchenne (DMD) muscular dystro-phies (n=6). Exosomes were isolated by Exoquick precipitation and analyzedfor size distribution and by nanoparticle tracking analysis (NTA) and by Westernblot for exosome markers. The effects of the isolated EVs on human satellitecell proliferation and differentiation and macrophage activation were examined. Results: Exosomes from IM patients decreased human satellite cell proliferation (51%, P<0.01) and inhibited their myogenic differentiation as indicated by lower fusionindex (24% inhibition, P<0.01) and expression of myosin heavy chain (72% inhibi-tion, P<0.001). Similar results were obtained also with exosomes derived from DMDand BMD patients; however, their inhibitory effect were more pronounced on MyoG expression. T reatment of macrophages with exosomes from IM patients significantly increased the expression of IL-10 (3-fold, P<0.001), compared to exosomes of healthy controls and DMD patients. Another significant difference was in the expression of sig-naling molecules: Thus, exosomes from BMD patients increased the phosphorylationof Erk and p38, whereas a smaller effect was induced by IM exosomes.Conclusion: Exosomes from IM patients decrease satellite cell proliferationand myogenic differentiation compared to healthy exosomes. In addition, these exosomes increased the expression of IL-10 in macrophages. These effects areunique to exosomes of IM patients compared to muscular dystrophies. These promising results suggest that serum exosomes should be further investigatedas a novel biomarker with potential therapeutic implications.Disclosure of Interests: Shaye Kivity Speakers bureau: BI, Abbvie, Lilly, Pfizer, Janssen, Neopharm, Grant/research support from: Sobi, Haya Kravitz: None declared, Coral Cohen: None declared, Darya Margoulis: None declared, MosheAmar: None declared, Gila Kazimirsky: None declared, David Ozeri Speakers bureau: Neopharm, Consultant of: Abbvie, Amir Dori Grant/research supportfrom: Biogen, Chaya Brodie Grant/research support from: Biogen.DOI: 10.1136/annrheumdis-2022-eular.63POS0469 ENDOTHELIAL TO MESENCHYMAL TRANSITIONAND SENESCENCE ARE PART OF THE FIBROTICPATHOGENESIS IN SYSTEMIC SCLEROSISY. H. Chiu1,2, J. Spierings1, J. M. Van Laar1, J. De Vries-Bouwstra3, M. VanDijk4, R. Goldschmeding4. 1University Medical Center Utrecht, Departmentof Rheumatology and Clinical Immunology, Utrecht, Netherlands;2T ri-ServiceGeneral Hospital, Division of Rheumatology/Immunology/Allergy, T aipei, T aiwan, Republic of China;3Leiden University Medical Center, The Department of on December 24, 2023 by guest. Protected by copyright./ Ann Rheum Dis: first published as 10.1136/annrheumdis-2022-eular.29 on 23 May 2022. Downloaded from。
血清同型半胱氨酸及心肌酶水平与精神分裂症的相关性研究

血清同型半胱氨酸及心肌酶水平与精神分裂症的相关性研究发布时间:2022-07-29T12:28:18.817Z 来源:《中国医学人文》2022年3月3期作者:孔培超[导读]血清同型半胱氨酸及心肌酶水平与精神分裂症的相关性研究孔培超(佛山市第三人民医院;广东佛山 528041)摘要:目的探讨精神分裂症患者血清同型半胱氨酸(HCY)及心肌酶水平的相关性,为临床对于精神分裂症诊治和病情观察提供参考价值。
方法选取2020年7月到2021年6月在我院住院治疗的50例精神分裂症患者作为观察组,另选50例同期在我院健康体检者作为对照组,所有研究对象均进行HCY及心肌酶水平检测,并用阳性及阴性症状量表(PANSS)对观察组患者进行病情评估。
结果观察组HCY、天冬氨酸氨基转移酶(AST)、乳酸脱氢酶(LDH)、肌酸磷酸激酶(CK)、肌酸激酶同工酶(CK-MB)水平均显著高于对照组,差异具有统计学意义(P<0.05);观察组患者血清HCY、CK、CK-MB水平与PANSS评分均呈正相关(P<0.05)。
结论精神分裂症患者血清HCY、AST、LDH、CK、CK-MB水平升高,HCY、CK、CK-MB水平与精神分裂症病情严重程度呈正相关。
关键词:精神分裂症;同型半胱氨酸;心肌酶;相关性研究Correlation between levels of serum homocysteine and myocardial enzyme and schizophrenia Abstract: Objective To explore the correlation of serum homocysteine (HCY) and myocardial enzyme levels in schizophrenia patients, and to provide a reference value for the clinical diagnosis and treatment and observation of schizophrenia. Methods 50 patients with schizophrenia hospitalized in our hospital from July 2020 to June 2021 were selected as the observation group, and 50 patients were examined in our hospital as the control group. All study subjects were tested for HCY and myocardial enzyme level, and the observation group were evaluated with the positive and negative symptom scale (PANSS).Results The levels of Hcy, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine phosphokinase (CK) and creatine kinase isoenzyme (CK-MB) in the observation group were significantly higher than those in the control group (P<0.05). he levels of serum Hcy, CK and CK-MB in the observation group were positively correlated with PANSS score (P<0.05).Conclusion The serum levels of HCY, AST, LDH, CK, and CK-MB were increased, and the HCY, CK, and CK-MB levels were positively associated with the severity of schizophrenia. Key words: schizophrenia; homocysteine; myocardial enzyme; correlation study精神分裂症是目前临床尚未明确病因、发病机制的一种常见精神疾病,青壮年患者较为常见,临床症状主要为意识、情感、智能、知觉等方面出现功能障碍,个体差异大、病情反复[1]。
Trodelvy产品说明书

Trodelvy® (sacituzumab govitecan‐hziy)(Intravenous)Document Number: MH‐0532 Last Review Date: 03/01/2022Date of Origin: 06/02/2020Dates Reviewed: 06/2020, 09/2020, 01/2021, 05/2021, 03/2022I.Length of AuthorizationCoverage will be provided for six months and may be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:∙Trodelvy 180 mg single-dose vial:12 vials every 21 daysB.Max Units (per dose and over time) [HCPCS Unit]:∙432 billable units weekly for two doses every 21 daysIII.Initial Approval Criteria 1Coverage is provided in the following conditions:Submission of medical records (chart notes) related to the medical necessity criteriais REQUIRED on all requests for authorizations. Records will be reviewed at thetime of submission. Please provide documentation related to diagnosis, steptherapy, and clinical markers (i.e. genetic and mutational testing) supportinginitiation when applicable. Medical records may be submitted via direct uploadthrough the PA web portal or by fax.∙Patient at least 18 years of age; ANDUniversal Criteria 1∙Therapy will NOT be substituted for or used in combination with irinotecan; AND∙Patients that are homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele will be closely monitored for adverse reactions; AND∙Therapy will not be used in combination with UGT1A1 inhibitors (e.g., nilotinib, regorafenib, etc.) or inducers (e.g., phenytoin, carbamazepine, etc.); AND∙Used as single agent therapy; ANDBreast Cancer † ‡ 1-3∙Patient has unequivocal triple-negative disease [TNBC] (i.e., estrogen, progesterone, and HER2-negative)*; AND∙Patient was previously treated with at least two systemic therapies, at least one of them for metastatic disease; ANDo Patient has recurrent unresectable, locally advanced, or metastatic disease; ORo Patient has inflammatory breast cancer with no response to preoperative systemic therapyUrothelial Cancer (Bladder Cancer)† ‡ 1,2,10∙Patient has one of the following diagnoses:o Locally advanced or metastatic urothelial carcinoma; ORo Muscle invasive bladder cancer with local recurrence or persistent disease in a preserved bladder ‡; ORo Metastatic or local bladder cancer recurrence post-cystectomy ‡; ORo Primary carcinoma of the urethra ‡; ANDUsed for recurrent (excluding recurrence of stage T3-4 disease or palpable inguinal lymph nodes) or metastatic disease; ORo Metastatic upper genitourinary (GU) tract tumors ‡; ORo Metastatic urothelial carcinoma of the prostate ‡; AND∙Patient was previously treated with platinum-containing chemotherapy and programmed death (PD-1 or PD-L1)-directed therapy (e.g., avelumab, nivolumab, atezolizumab,durvalumab, etc.)† FDA approved indication(s); ‡ Compendia Recommended Indication(s); Ф Orphan Drug∙Immunohistochemistry (IHC) assay is 0 or 1+; OR∙Dual-probe in situ hybridization (ISH) assay indicating (Group 5) HER2/CEP17 ratio <2.0 AND average HER2 copy number <4.0 signals/cell; OR∙Concurrent dual-probe ISH and IHC assay results indicating one of the following: o(Group 2) HER2/CEP17 ratio ≥2.0 AND average HER2 copy number <4.0 signals/cell and concurrent IHC 0-1+ or 2+; ORo(Group 3) HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥6.0 signals/cell and concurrent IHC 0-1+; ORo(Group 4) HER2/CEP17 ratio <2.0 AND average HER2 copy number ≥4.0 and <6.0 signals/cell and concurrent IHC 0-1+ or 2+9∙Immunohistochemistry (IHC) assay: Sample is considered ER/PR negative if the percentage of cancer cells staining on evaluation is <1% OR 0% of tumor cell nuclei are immunoreactiveNote: A sample may be deemed uninterpretable for ER or PR if the sample is inadequate(insufficient cancer or severe artifacts present, as determined at the discretion of thepathologist), if external and internal controls (if present) do not stain appropriately, or ifpre-analytic variables have interfered with the assay’s accuracy.IV.Renewal Criteria 1Coverage can be renewed based upon the following criteria:∙Patient continues to meet universal and other indication-specific relevant criteria such as concomitant therapy requirements (not including prerequisite therapy), performancestatus, etc. identified in section III; AND∙Disease response with treatment as defined by stabilization of disease or decrease in size of tumor or tumor spread; AND∙Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: severe hypersensitivity and infusion-related reactions, severe nausea/vomiting, severeneutropenia/febrile neutropenia, severe anemia, severe diarrhea, etc.V.Dosage/Administration 1Breast Cancer/ Bladder Cancer Administer 10 mg/kg as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles. Continue treatment until disease progression or unacceptable toxicity. Do not administer doses greater than 10 mg/kg.VI.Billing Code/Availability InformationHCPCS Code:∙J9317 – Injection, sacituzumab govitecan-hziy, 2.5 mg; 1 billable unit = 2.5 mgNDC:∙Trodelvy 180 mg lyophilized powder in a single-dose vial: 55135-0132-xxVII.References1.Trodelvy [package insert]. Morris Plains, NJ; Immunomedics, Inc; October 2021. AccessedFebruary 2022.2.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) sacituzumab govitecan. National Comprehensive Cancer Network, 2022.The NCCN Compendium® is a derivative work of the NCCN Guidelines®. NATIONALCOMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCN GUIDELINES® aretrademarks owned by the National Comprehensive Cancer Network, Inc. To view the mostrecent and complete version of the Compendium, go online to . AccessedFebruary 2022.3.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology(NCCN Guidelines®) for Breast Cancer 2.2022. National Comprehensive Cancer Network, 2022. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCNGUIDELINES® are trademarks owned by the National Comprehensive Cancer Network, Inc. To view the most recent and complete version of the Guidelines, go online to .Accessed February 2022.4.Fahrenbruch R, Kintzel P, Bott AM, et al. Dose Rounding of Biologic and CytotoxicAnticancer Agents: A Position Statement of the Hematology/Oncology PharmacyAssociation. J Oncol Pract. 2018 Mar;14(3):e130-e136.5.Hematology/Oncology Pharmacy Association (2019). Intravenous Cancer Drug Waste IssueBrief. Retrieved from /images/hopa/advocacy/Issue-Briefs/Drug_Waste_2019.pdf6.Bach PB, Conti RM, Muller RJ, et al. Overspending driven by oversized single dose vials ofcancer drugs. BMJ. 2016 Feb 29;352:i788.7.Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in RefractoryMetastatic Triple-Negative Breast Cancer. N Engl J Med. 2019 Feb 21;380(8):741-751. doi:10.1056/NEJMoa1814213.8.Wolff AC, Hammond EH, Allison KH, et al. Human epidermal growth factor receptor 2testing in breast cancer: American Society of Clinical Oncology/College of AmericanPathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-2122.9.Allison KH, Hammond EH, Dowsett M, et al. Estrogen and Progesterone Receptor Testingin Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 38:1346-1366.10.Tagawa S, Balar A, Petrylak, et al. TROPHY-U-01: A Phase II Open-Label Study ofSacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma ProgressingAfter Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021 Aug1;39(22):2474-2485. doi: 10.1200/JCO.20.03489. Epub 2021 Apr 30.Appendix 1 – Covered Diagnosis Codes1010C50.011 Malignant neoplasm of nipple and areola, right female breastC50.012 Malignant neoplasm of nipple and areola, left female breastC50.019 Malignant neoplasm of nipple and areola, unspecified female breastC50.021 Malignant neoplasm of nipple and areola, right male breastC50.022 Malignant neoplasm of nipple and areola, left male breastC50.029 Malignant neoplasm of nipple and areola, unspecified male breastC50.111 Malignant neoplasm of central portion of right female breastC50.112 Malignant neoplasm of central portion of left female breastC50.119 Malignant neoplasm of central portion of unspecified female breastC50.121 Malignant neoplasm of central portion of right male breastC50.122 Malignant neoplasm of central portion of left male breastC50.129 Malignant neoplasm of central portion of unspecified male breastC50.211 Malignant neoplasm of upper-inner quadrant of right female breastC50.212 Malignant neoplasm of upper-inner quadrant of left female breastC50.219 Malignant neoplasm of upper-inner quadrant of unspecified female breast C50.221 Malignant neoplasm of upper-inner quadrant of right male breastC50.222 Malignant neoplasm of upper-inner quadrant of left male breastC50.229 Malignant neoplasm of upper-inner quadrant of unspecified male breast C50.311 Malignant neoplasm of lower-inner quadrant of right female breastC50.312 Malignant neoplasm of lower-inner quadrant of left female breastC50.319 Malignant neoplasm of lower-inner quadrant of unspecified female breast C50.321 Malignant neoplasm of lower-inner quadrant of right male breastC50.322 Malignant neoplasm of lower-inner quadrant of left male breastC50.329 Malignant neoplasm of lower-inner quadrant of unspecified male breastC50.411 Malignant neoplasm of upper-outer quadrant of right female breastC50.412 Malignant neoplasm of upper-outer quadrant of left female breastC50.419 Malignant neoplasm of upper-outer quadrant of unspecified female breast C50.421 Malignant neoplasm of upper-outer quadrant of right male breastC50.422 Malignant neoplasm of upper-outer quadrant of left male breastC50.429 Malignant neoplasm of upper-outer quadrant of unspecified male breast C50.511 Malignant neoplasm of lower-outer quadrant of right female breastC50.512 Malignant neoplasm of lower-outer quadrant of left female breastC50.519 Malignant neoplasm of lower-outer quadrant of unspecified female breast C50.521 Malignant neoplasm of lower-outer quadrant of right male breastC50.522 Malignant neoplasm of lower-outer quadrant of left male breastC50.529 Malignant neoplasm of lower-outer quadrant of unspecified male breastC50.611 Malignant neoplasm of axillary tail of right female breastC50.612 Malignant neoplasm of axillary tail of left female breastC50.619 Malignant neoplasm of axillary tail of unspecified female breastC50.621 Malignant neoplasm of axillary tail of right male breastC50.622 Malignant neoplasm of axillary tail of left male breastC50.629 Malignant neoplasm of axillary tail of unspecified male breastC50.811 Malignant neoplasm of overlapping sites of right female breastC50.812 Malignant neoplasm of overlapping sites of left female breastC50.819 Malignant neoplasm of overlapping sites of unspecified female breastC50.821 Malignant neoplasm of overlapping sites of right male breastC50.822 Malignant neoplasm of overlapping sites of left male breastC50.829 Malignant neoplasm of overlapping sites of unspecified male breastC50.911 Malignant neoplasm of unspecified site of right female breastC50.912 Malignant neoplasm of unspecified site of left female breastC50.919 Malignant neoplasm of unspecified site of unspecified female breastC50.921 Malignant neoplasm of unspecified site of right male breastC50.922 Malignant neoplasm of unspecified site of left male breastC50.929 Malignant neoplasm of unspecified site of unspecified male breastC61 Malignant neoplasm of prostateC65.1 Malignant neoplasm of right renal pelvisC65.2 Malignant neoplasm of left renal pelvisC65.9 Malignant neoplasm of unspecified renal pelvisC66.1 Malignant neoplasm of right ureterC66.2 Malignant neoplasm of left ureterC66.9 Malignant neoplasm of unspecified ureterC67.0 Malignant neoplasm of trigone of bladderC67.1 Malignant neoplasm of dome of bladderC67.2 Malignant neoplasm of lateral wall of bladderC67.3 Malignant neoplasm of anterior wall of bladderC67.4 Malignant neoplasm of posterior wall of bladderC67.5 Malignant neoplasm of bladder neckC67.6 Malignant neoplasm of ureteric orificeC67.7 Malignant neoplasm of urachusC67.8 Malignant neoplasm of overlapping sites of bladderC67.9 Malignant neoplasm of bladder, unspecifiedC68.0 Malignant neoplasm of urethraD09.0 Carcinoma in situ of bladderZ85.51 Personal history of malignant neoplasm of bladderZ85.59 Personal history of malignant neoplasm of other urinary tract organAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD), Local Coverage Determinations (LCDs), and Local Coverage Articles (LCAs) may exist and compliance with these policies is required where applicable. They can be found at:https:///medicare-coverage-database/search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD/LCA): N/AJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
盐酸苯海索片联合盐酸司来吉兰片治疗帕金森病的效果

DOI:10.16662/ki.1674-0742.2023.06.118盐酸苯海索片联合盐酸司来吉兰片治疗帕金森病的效果赵臣松,应国民,秦保健单县中心医院神经内科,山东菏泽274300[摘要]目的对帕金森病患者采用盐酸苯海索片联合盐酸司来吉兰片治疗的效果进行探究。
方法随机选择2020年1月—2021年6月单县中心医院收治的90例帕金森病患者,根据患者所接受的治疗方案不同平均分。
甲组(45例)接受盐酸司来吉兰片治疗,乙组(45例)则在甲组的基础之上接受盐酸苯海索片治疗。
对比两组的治疗效果、SCOPA-AUT评分、MMSE评分、UPDRS评分、氧化应激指标。
结果乙组的总有效率(97.78%)高于甲组(80.00%),差异有统计学意义(χ2=7.200,P<0.05)。
治疗后,乙组的SCOPA-AUT评分、UPDRS评分、MMSE评分低于甲组,NOS、SOD、PON1、CGP等氧化应激指标高于甲组,差异有统计学意义(P< 0.05)。
结论在治疗帕金森患者时,联合应用盐酸苯海索及盐酸司来吉兰能够达到很好的治疗效果,可以快速缓解各项症状,提高神经功能及认知功能,还可以改善机体抗氧化应激能力。
[关键词]帕金森;盐酸苯海索片;盐酸司来吉兰片;神经功能;认知功能;抗氧化应激能力[中图分类号]R246.1 [文献标识码]A [文章编号]1674-0742(2023)02(c)-0118-05Effect of Trihexyphenidyl Hydrochloride Tablets Combined with Selegiline Hydrochloride Tablets in the Treatment of Parkinson's DiseaseZHAO Chensong, YING Guomin, QIN BaojianDepartment of Neurology, Shanxian Central Hospital, Heze, Shandong Province, 274300 China[Abstract] Objective To investigate the therapeutic effect of benhexol hydrochloride combined with selegiline hydro‐chloride tablets on Parkinson's disease patients. Methods A total of 90 Parkinson's disease patients admitted to the Single County Central Hospital from January 2020 to June 2021 were selected randomly, and they were evenly divided according to the different treatment plans they received. Group A (45 cases) received treatment with selegiline hydro‐chloride tablets, while group B (45 cases) received treatment with benhexol hydrochloride tablets on top of group A. Compared the treatment efficacy, SCOPA-AUT score, MMSE score, UPDRS score, and oxidative stress indicators be‐tween the two groups. Results The total effective rate of group B (97.78%) was higher than that of group A (80.00%), and the difference was statistically significant (χ2=7.200, P<0.05). After treatment, the SCOPA-AUT score, UPDRS score, and MMSE score of group B were lower than those of group A, while oxidative stress indicators such as NOS, SOD, PON1, CGP were higher than those of group A, and the difference was statistically significant (P<0.05). Conclu⁃sion When treating Parkinson's patients, the combination of benhexol hydrochloride and selegiline hydrochloride can achieve good therapeutic effects, quickly alleviate various symptoms, improve neurological and cognitive functions, and also improve the body's antioxidant stress capacity.[Key words] Parkinson's disease; Trihexyphenidyl hydrochloride tablets; Selegiline hydrochloride tablets; Neurological function; Cognitive function; Antioxidant stress capacity帕金森病的主要发病群体为老年人群,该病具有比较复杂的发病机制,主要是由于中脑黑质多巴[作者简介] 赵臣松(1986-),男,本科,主治医师,研究方向为神经病学。
恩格列净联合西格列汀治疗老年2_型糖尿病患者的临床疗效分析

·药物与临床·糖尿病新世界 2023年3月DOI:10.16658/ki.1672-4062.2023.05.059恩格列净联合西格列汀治疗老年2型糖尿病患者的临床疗效分析臧道军,龚红燕江苏省常州市德安医院老年内科,江苏常州213000[摘要]目的探讨老年2型糖尿病患者使用恩格列净+西格列汀治疗的临床效果。
方法选取2020年1月—2021年12月常州市德安医院接诊的100例老年2型糖尿病患者作为研究对象,根据不同用药方式分为对照组与研究组,各50例,对照组接受西格列汀治疗,研究组接受恩格列净+西格列汀治疗,就两组患者血糖指标、炎性指标、胱抑素C(Cys-C)、血尿素氮(BUN)、血同型半胱氨酸(Hcy)指标进行比较。
结果治疗前两组血糖指标相比,差异无统计学意义(P>0.05),治疗后,研究组HbA1c、FPG及2 hPG明显低于对照组,差异有统计学意义(P<0.05);治疗前两组炎性指标比较,差异无统计学意义(P>0.05),治疗后,研究组IL-4、IL-6及TNF-α明显低于对照组,差异有统计学意义(P<0.05);治疗前两组Cys-C、BUN及Hcy相比,差异无统计学意义(P>0.05),治疗后,研究组患者Cys-C、BUN及Hcy明显低于对照组,差异有统计学意义(P<0.05)。
结论对于老年2型糖尿病患者开展恩格列净+西格列汀治疗能有效改善血糖指标,降低Hcy,提升肾功能,治疗效果显著。
[关键词] 老年人群;恩格列净;西格列汀;2型糖尿病[中图分类号] R4 [文献标识码] A [文章编号] 1672-4062(2023)03(a)-0059-04Clinical Efficacy Analysis of Empagliflozin Combined with Sitagliptin in the Treatment of Elderly Patients with Type 2 Diabetes MellitusZANG Daojun, GONG HongyanDepartment of Geriatric Medicine, Changzhou De'an Hospital, Changzhou, Jiangsu Province, 213000 China[Abstract] Objective To investigate the clinical effect of treatment with empagliflozin + sitagliptin in elderly patients with type 2 diabetes mellitus.Methods A total of 100 elderly patients with type 2 diabetes mellitus admitted to Chang⁃zhou De'an Hospital from January 2020 to December 2021 were selected as study subjects. The cases were divided into control group and study group according to different medication administration, fifty cases in each. The control group received sitagliptin treatment and the study group received empagliflozin + sitagliptin treatment. The blood glu⁃cose index, inflammatory index, cystatin C (Cys-C), blood urea nitrogen (BUN), and blood homocysteine (Hcy) index were compared between the two groups.Results There was no statistically significant difference in blood glucose in⁃dexes between the two groups before treatment (P>0.05). After treatment, HbA1c, FPG and 2 hPG of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05). There was no statistically significant difference in inflammatory indexes between the two groups before treatment (P>0.05). After treatment, IL-4, IL-6 and TNF-α in the study group were significantly lower than those in the control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the Cys-C, BUN and Hcy between the two groups before treatment (P>0.05). After treatment, the Cys-C, BUN and Hcy of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05).Conclusion For elderly patients with type 2 diabetes mellitus, treatment with empagliflozin + sitagliptin can effec⁃tively improve blood glucose index, reduce Hcy and enhance renal function, with significant therapeutic effects.[作者简介]臧道军(1974-),男,本科,副主任医师,研究方向为老年内科。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
心内科5:中国经皮冠状动脉介入治疗指南(2016)

中国经皮冠状动脉介入治疗指南(2016)中华心血管病杂志,第44卷第5期第382页-第400页自"中国经皮冠状动脉介入治疗指南2012(简本)" [1]更新以来,在经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)及其相关领域又积累了众多临床证据。
为此,中华医学会心血管病学分会介入心脏病学组、中国医师协会心血管内科医师分会血栓防治专业委员会、中华心血管病杂志编辑委员会组织专家组,在2009和2012年中国PCI 指南[1,2]的基础上,根据最新临床研究成果、特别是结合中国人群的大型随机临床试验结果,参考最新美国心脏病学学院/美国心脏协会(ACC/AHA)以及欧洲心脏病学学会(ESC)等组织发布的相关指南[3,4,5,6,7,8,9]、并结合我国国情及临床实践,对PCI治疗领域的热点和焦点问题进行了全面讨论并达成一致共识,在此基础上编写了本指南。
为便于读者了解PCI对某一适应证的价值或意义,本指南对推荐类别的表述沿用国际通用的方式。
Ⅰ类:指已证实和(或)一致公认有益、有用和有效的操作或治疗,推荐使用。
Ⅱ类:指有用和(或)有效的证据尚有矛盾或存在不同观点的操作或治疗。
Ⅱa类:有关证据/观点倾向于有用和(或)有效,应用这些操作或治疗是合理的。
Ⅱb类:有关证据/观点尚不能被充分证明有用和(或)有效,可考虑应用。
Ⅲ类:指已证实和(或)一致公认无用和(或)无效,并对一些病例可能有害的操作或治疗,不推荐使用。
对证据来源的水平表达如下。
证据水平A:资料来源于多项随机临床试验或荟萃分析。
证据水平B:资料来源于单项随机临床试验或多项非随机对照研究。
证据水平C:仅为专家共识意见和(或)小规模研究、回顾性研究和注册研究。
概述一、建立质量控制体系对于每一个开展PCI的中心,应建立质量控制体系(Ⅰ,C),包括:(1)回顾分析整个中心的介入治疗结局和质量;(2)回顾分析每个术者的介入治疗结局和质量;(3)引入风险调控措施;(4)对复杂病例进行同行评议;(5)随机抽取病例作回顾分析。
维格列汀与常见降糖药联合治疗2型糖尿病的研究进展

2020年12月 第17卷 第24期2型糖尿病(Type 2 Diabetes Mellitus,T2DM)以β细胞功能障碍[1-3]、较高的体质指数[4-6]、胰岛素抵抗指数[7-9]等为主要的表型特征。
在T2DM 的治疗指南中,建议将维格列汀等二肽基肽酶4抑制剂作为二线或三线药物[10]。
与其他降糖药相比,维格列汀等具有良好的药物耐受性和安全性。
那么,维格列汀与常见降糖药联合治疗T2DM的疗效与安全性如何呢?本文将综述相关研究成果。
1 维格列汀+二甲双胍联合治疗的相关研究1.1 维格列汀+二甲双胍联合用药与其他联合疗法的比较Peng等[11]以二甲双胍为基础,通过随机对照试验验证了格列美脲、吡格列酮、艾塞那肽、格列本脲、罗格列酮和维格列汀6种降血糖药物在T2DM中的不同疗效。
网络荟萃分析结果表明,这6种药物均能降低HbA1c水平。
与其他方案相比,艾塞那肽+二甲双胍联合治疗降低空腹血糖(Fasting Plasma Glucose,FPG)、空腹血浆胰岛素(Fasting Plasma Insulin,FPI)、总胆固醇(Total Cholesterol,TC)和稳态模型评估胰岛素抵抗指数(Homeostasis Model Assessment Insulin Resistance Index,HOMA-IR),且可提高高密度脂蛋白胆固醇(High-density Lipoprotein Cholesterol,HDL-C)水平;维格列汀+二甲双胍联合用药可降低FPI和低密度脂蛋白胆固醇(Low-density Lipoprotein Cholesterol,LDL-C)水平;格列本脲+二甲双胍联合用药可降低FPG水平,且提高HDL-C水平;格列美脲+二甲双胍联合用药可降低TC水平;罗格列酮+二甲双胍联合用药可降低LDL-C水平。
研究结果表明,艾塞那肽+二甲双胍和维格列汀+二甲双胍联合用药对T2DM有较好的疗效,二者均能改善胰岛素敏感性。
PICS认证的成员

A. PARTICIPATING AUTHORITIES :Argentinian National Institute of DrugsI nstituto Na cional de Me dicamentos (INAME)Inspection DepartmentAvda. Caseros 2161 – 1er PisoAR - C1264AAB Buenos AiresArgentinaAustralian T herapeutic G oods A dministration(TGA)Departement of HealthOffice of Manufacturing QualityPO Box 100AU - Woden Act 2606AustraliaAustrian Medicines and Medical Devices AgencyÖsterreichische A gentur für G esundheit undE rnährungs-s icherheit (AGES)Traisengasse 5,AT - 1200 ViennaAustriaBelgian Federal Agency for Medicines and HealthProductsA gence Fédérale des Médicaments et des P roduits deS anté (AFMPS)F ederaal A gentschap voorG eneesmiddelen enG ezondheidsproducten (FAGG)EUROSTATION Building, block 2Place Victor Horta, 40/40BE - 1060 BruxellesBelgiumCanadian H ealth P roducts and F ood B ranchI nspectorate (HPFBI)Health CanadaGraham Spry Building, 7th Floor, Room 710250 Lanark Avenue, AL 2007BCA - Ottawa, Ontario, K1A 0K9CanadaT aiwan F ood and D rug A dministration (TFDA)Department of HealthNo. 161-2, Kunyang St.Nangang DistrictTW - 115-61 Taipei CityChinese TaipeiAs from 1 January 2016Agency for Medicinal Products and Medical Devices of CroatiaAgencija za lijekove i medicinske proizvode (HALMED) Ksaverska cesta 4HR - 10 000 ZagrebCroatiaCy priot Ph armaceutical S ervices (CyPHS)Ministry of Health1475 Lefkosia (Nicosia)CyprusCzech State Institute for Drug ControlS tátníÚstav pro K ontrolu Léčiv (SÚKL)Srobárova 48CZ - 100 41 Prague 10Czech RepublicCzech I nstitute for S tate C ontrol of V eterinaryB iologicalsand M edicines (ISCVBM)Hudcova 56ACZ - 621 00 BrnoCzech RepublicD anish H ealth and M edicines A uthority(DHMA)1 Axel Heides GadeDK - 2300 Copenhagen SDenmarkEstonian S tate A gency of M edicines (SAM)1 Nooruse Str.EE - 50411 TartuEstoniaFi nnish Me dicines A gency (FIMEA)Mannerheimintie 103bP.O. Box 55FI - 00034 FIMEA HelsinkiFinlandFrench National Agency for Medicines and HealthProducts SafetyA gence n ationale de sécuritédu médicament et desproduits de santé (ANSM)143-145 Boulevard Anatole FranceFR - 93285 Saint DenisFranceFrench Agency for Food, Environmental &Occupational Health SafetyAgence nationale de sécuritésanitaire del'alimentation, de l'environnement et du travail(ANSES)14, rue Pierre et Marie CurieFR - 94701 Maisons-Alfort cedexFranceGerman Federal Ministry of Health *B undes m inisterium für G esundheit (BMG)Rochusstr. 1DE – 53123 BonnGermanyCentral Authority of the Laender for HealthProtection regarding Medicinal Products and MedicalDevices *Z entralstelle der Länder für G esundheitsschutz beiArzneimitteln und Medizinprodukten (ZLG)Heinrich-Boll-Ring 10DE - 53119 BonnGermany* the German Ministry of Health (BMG) and the German Central Authority of the Laender (ZLG) count as one PIC/S Participating Authority. All German Medicinal Authorities, which are listed on the ZLG web site, are considered as PIC/S Participating Authorities and are represented in PIC/S by ZLG.Greek National Organisation for MedicinesΕθνικός Οργανισμός Φαρμάκων (EOF)Messoghion Ave 284GR - Holargos 155 62 (ELLAS)Greece香港藥劑業及毒藥管理局Pharmacy and Poisons Board of Hong Kong As from 1 January 2016P harmacy and P oisons B oard of H ong K ong (PPBHK)1/F Shun Feng International Centre182 Queen's Road EastWan ChaiHong KongHungarian N ational I nstitute of P harmacy andN utrition (NIPN)Zrínyi u. 3. 1372 P.O. Box 450HU - 1051 BudapestHungaryI celandic M edicines A gency (IMA)Vínlandsleið 14IS - 113 ReykjavikIcelandIndonesian N ational A gency for D rug and F ood C ontrol(NADFC)(Badan Pengawas Obat dan Makanan Republik Indonesia)Jl. Percetakan Negara No.23ID - Jakarta 10560IndonesiaH ealth P roducts R egulatory A uthority (HPRA) Kevin O'Mally HouseEarlsfort CentreEarlsfort TerraceIE - Dublin 2IrelandIsraeli I nstitute for S tandardization and C ontrolof P harmaceuticals (ISCP)Eliav St. 9,P.O. Box 34410IL - Jerusalem 91342IsraelItalian Medicines AgencyA genzia I taliana del Fa rmaco (AIFA)Via del Tritone, 181IT - 00187 RomeItalyJapanese M inistry of H ealth,L abour and W elfare(MHLW) *1-2-2 Kasumigaseki Chiyoda-kuJP - 100-8916 TokyoJapanJapanese P harmaceuticals and M edical D evicesA gency (PMDA) *Shin-Kasumigaseki Building3-3-2 Kasumigaseki Chiyoda-kuJP - 100-0013 TokyoJapan* Japan's Ministry of Health, Labour and Welfare (MHLW) and Japan's Pharmeuticals and Medical Devices Agency (PMDA) count as one PIC/S Participating Authority. The Japanese Prefectures are represented by MHLW.Korea (Republic of) M inistry of F ood and D rugS afety (MFDS)Osong Health Technology AdministrationComplex187 Osongsaengmyeong2(i)-roOsong-eup, Cheongwon-gun, ChungbukKR – 363-700KoreaLatvian State Agency of MedicinesZāļu v alsts aģentūra(ZVA)15, Jersikas St.LV - 1003 RigaLatviaLiechtenstein's Office of HealthcareA mt für G esundheit (AG)Äulestrasse 51Postfach 684FL - 9490 VaduzLiechtensteinLithuanian S tate M edicines C ontrol A gency (SMCA) Žirmūnų str. 139ALT –09120 VilniusLithuaniaMalaysian N ational P harmaceutical C ontrol B ureau (NPCB)Ministry of Health MalaysiaJalan UniversitiPO Box 31946730 Petaling JayaMY – SELANGORMalaysiaMaltese Medicines Authority (MAM)198, Rue D'ArgensMT – GZIRA GZR 1368MaltaDutch Health Care Inspectorate*I nspectie voor de G ezondheids z org (IGZ)P.O. Box 2680NL - 3500 GR UtrechtNetherlands* The competence for GMP/GDP inspections in the Netherlands is allocated to the central authority, Dutch Healthcare Inspectorate (IGZ). IGZ is the PIC/S Participating Authority representing GMP/GDP for human as well as veterinary medicinal products. IGZ performs national and international GMP/GDP inspections representing the Health Care Inspectorate - Pharmaceutical Affairs and Medical Technology as well as the Medicines Evaluation Board - Veterinary Medicinal Products Unit, which is mandated to issue GMP certificates on behalf of the Ministry of Economic Affairs.New Zealand's Medicines and Medical DevicesSafety Authority (Medsafe)P.O. Box 5013NZ - 6011 WellingtonNew ZealandNo rwegian M edicines A gency (NOMA)Postboks 63, KalbakkenNO - 0901 OsloNorwayPolish M ain P harmaceutical I nspectorate (MPI)12 Senatorska StreetPL - 00-082 WarsawPolandPortuguese National Authority of Medicines andHealth Products, IPAutoridade Nacional do Medicamento e Produtos deSaúde IP (INFARMED IP )Avenida do Brasil, no 53Pavilhão 21-APT - 1700 LisbonPortugalRomanian N ational A gency for M edicines and M edical D evices (NAMMD)Strada Maior Aviator Sanatescu 48Sectorul IRO - BucharestZIPCODE 011478RomaniSingapore's H ealth S ciences A uthority (HSA)150 Cantonment Road, Cantonment Centre,Blk A # 01-02SG – Singapore 089762Slovak S tate I nstitute for D rug C ontrol (SIDC)Kvetná 11SK - 825 08 Bratislava 26Slovak RepublicSlovenian Agency for Medicinal Products andMedical DevicesJavna agencija Republike Slovenije zazdravila in medicinske pripomočke (JAZMP)Ptujska ulica 21SI-1000 LjubljanaSlovenijaSouth African M edicines C ontrol C ouncil (MCC)Private Bag X 828ZA – 0001 PretoriaSouth AfricaSpanish Agency of Medicines andMedical Devices *A gencia E spañola de M edicamentosy P roductos S anitarios (AEMPS)Departamento de Inspección yControl de MedicamentosC/ Campezo, 1ES - 28022 MadridSpain* The competence for GMP/GDP inspections in Spain is shared between the central authority, Spanish Agency for Medicines and Medical Devices (AEMPS), and the Spanish regional authorities, which count as one PIC/S Participating Authority. All Spanish Medicinal Authorities, which are listed on the AEMPS web site, are considered as PIC/S Participating Authorities and are represented in PIC/S by the AEMPS.Swedish M edical P roducts A gency (MPA)Box 26SE - 751 03 UppsalaSwedenSwiss Agency for Therapeutic Products (Swissmedic)Hallerstrasse 7PostfachCH - 3000 Bern 9SwitzerlandUkrainian State Administration on MedicinalProducts(SAUMP)120, Peremogy av.UA - 03115 KyivUkraineUnited Kingdom's M edicines and H ealthcareProducts R egulatory A gency (MHRA)151 Buckingham Palace RoadVictoriaGB - London SW1W 9SZUnited KingdomUnited Kingdom's V eterinary M edicine D irectorate(VMD)Woodham Lane, New HawAddlestoneGB - Surr ey KT15 3LSUnited KingdomU.S. F ood and D rug A dministration (US FDA)10903 New Hampshire AvenueBuilding 31, Room 3502Silver Spring, Maryland 20993USAB. PARTNERS TO PIC/S.EDQMEuropean Directorate for the Quality of Medicines &HealthCare7 allée KastnerCS 30026FR - 67081 StrasbourgFranceEMAEuropean Medicines Agency30 Churchill PlaceCanary WharfUK - London E14 5EUUnited KingdomUNICEFUnited Nations International Children's EmergencyFundSupply DivisionOceanvej 10-12DK - 2100 Copenhagen ØDenmarkWHOWorld Health OrganizationAvenue Appia, 20CH - 1211 Geneva 27Switzerland.。
Ligusticumwallichii

Alternative Medicine Review Monographs Page 249C o p y r i g h t © 2002 T h o r n e R e s e a r c h , I n c . A l l r i g h t s r e s e r v e d . A l t e r n a t i v e M e d i c i n e R e v i e w M o n o g r a p h sLigusticum wallichiiDescriptionA member of the Umbelliferae family, Ligusticum wallichii is used in Chinese medicine for a variety of hematological disorders, including ischemia and thrombosis. When combined with Astragalus, Ligusticum has demonstrated a notable immunopotentiating effect. Included in many classic Chinese formulations, it is also part of the Japanese and Korean herbal formu-laries. Classically, it is prescribed for headaches, abdominal pain, arthralgias, and menstrual disorders due to blood stasis.1 Ligusticum’s active ingredients include tetramethylpyrazine, ferulic acid, chrysophanol, sedanoic acid, and 1-2 percent essential oils.Clinical Indications IschemiaOne-hundred-and-fifty-eight subjects with transient ischemic attacks were randomly divided into a Ligusticum group (111 cases) and an aspirin group (47 cases). The total effec-tive rate in the Ligusticum group was 89.2 percent, compared to 61.7 percent in the aspirin group (P<0.01). Ligusticum increased cerebral blood flow, accelerated the velocity of blood flow, dilated the spastic artery, and decreased peripheral arterial resistance.2 In another study, Ligusticum was evaluated in the treatment of ischemic stroke. Injectable preparations were shown to improve brain microcirculation through inhibiting thrombus formation, decreasing platelet aggregation, and improving blood viscosity. The effect of Ligusticum was the same or better than controls using papaverine, dextran, and aspirin-persantin.3Antibacterial/AntifungalLigusticum has demonstrated in vitro antibacterial activity against several strains of pathogenic bacteria including Pseudomonas aeruginosa, Shigella sonnei, Salmonella typhi and Vibrio cholera , as well as many dermatomycoses .4InflammationWhen given to guinea pigs with histamine/acetylcholine-induced bronchospasm, Ligusti-cum decreased plasma levels of thromboxane B2, relaxed tracheal muscle, increased the forced expiratory volume, and inhibited synthesis and release of thromboxane A2, with no adverse side effects. The total effective rate was 92 percent, compared with 62 percent in the control group (p <0.01).5 In a Japanese study, the active ingredients in Ligusticum, tetramethylpyrazine and ferulic acid, were found to have both significant anti-inflammatory and analgesic effects.6Page 250 Alternative Medicine Review MonographsC o p y r i g h t © 2002 T h o r n e R e s e a r c h , I n c . A l l r i g h t s r e s e r v e d . A l t e r n a t i v e M e d i c i n e R e v i e w M o n o g r a p h sDosage and ToxicityLigusticum is prescribed in traditional Chinese decoctions at dosages up to 9 grams, administered over several days. Overdose symptoms may include vomiting and dizziness.1References1. Hong YH. Oriental Materia Medica: A Concise Guide. Long Beach, CA: Oriental Healing Arts Institute; 1986.2. Chen DR. Clinical and experimental study of Ligusticum wallichii and aspirin in the treatment of transient ischemic attack. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992;12:672-674. [article in Chinese]3. Chen KJ, Chen K. Ischemic stroke treated with Ligusticum chuanxiong . Chin Med J (Engl) 1992;105:870-873.4. Bensky D, Gamble A. Chinese Herbal Medicine : Materia Medica, Revised Edition. Seattle, WA: Eastland Press; 1993.5. Shao CR, Chen FM, Tang YX. Clinical and experimental study on Ligusticum wallichi mixture in prevent-ing and treating bronchial asthma. Zhongguo Zhong Xi Yi Jie He Za Zhi 1994;14:465-468. [article in Chinese]6.Ozaki Y . Anti-inflammatory effect of tetramethylpyrazine and ferulic acid. Chem Pharm Bull (Tokyo) 1992;40:954-956.。
美国阿格迪agdia公司转基因检测产品
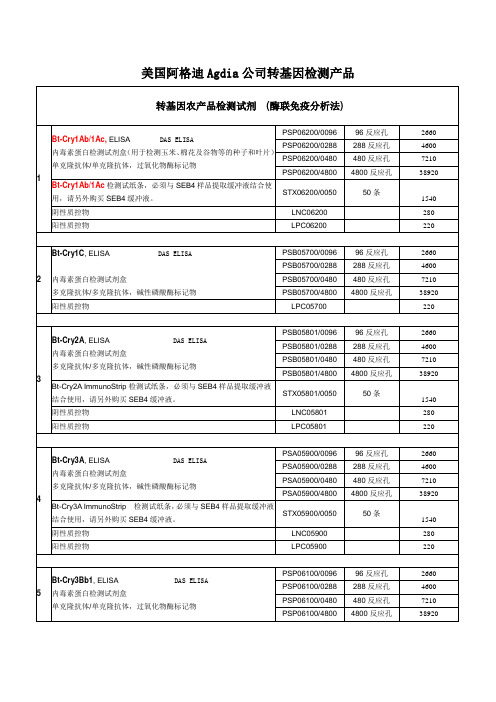
STX74000/0050
50条
2450
阳性质控物
LPC73000
220
8Байду номын сангаас
Bt-Cry1F, ELISA DAS ELISA
内毒素蛋白检测试剂盒
过氧化物酶标记物
PSP10301/0096
96反应孔
2660
PSP10301/0288
288反应孔
4600
PSP10301/0480
480反应孔
6.Bt-Cry1F and Bt-Cry34Ab1货号:STX10900/0050规格:50条报价:2240元
7.Bt-Cry2A货号:STX05801/0050规格:50条报价:1540元
8.Bt-Cry34Ab1货号:STX04500/0050规格:50条报价:1540元
9.Bt-Cry3Bb1货号:STX06100/0050规格:50条报价:1540元
多克隆抗体/多克隆抗体,碱性磷酸酶标记物
PSA05900/0096
96反应孔
2660
PSA05900/0288
288反应孔
4600
PSA05900/0480
480反应孔
7210
PSA05900/4800
4800反应孔
38920
Bt-Cry3AImmunoStrip检测试纸条,必须与SEB4样品提取缓冲液结合使用,请另外购买SEB4缓冲液。
10.mBt-Cry3A货号:STX06700/0050规格:50条报价:1540元
11.neomycin phosphotransferase II货号:STX73000/0050规格:50条报价:2450元
替雷利珠单抗联合白蛋白结合型紫杉醇

[收稿日期]2021-12-30 [修回日期]2023-03-16[基金项目]安徽省高校自然科学研究重点项目(KJ2018A0997)[作者简介]向俊馨(1993-),女,硕士研究生.[通信作者]李殿明,硕士研究生导师,主任医师,教授.E⁃mail:listar1588@[文章编号]1000⁃2200(2024)03⁃0302⁃05㊃临床医学㊃替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗晚期肺鳞癌病人的近期疗效分析向俊馨1,2,李小利1,刘 莹1,刘 允1,王 璐1,李殿明1(1.蚌埠医科大学第一附属医院呼吸与危重症医学科,分子诊断中心,呼吸系病临床基础安徽省重点实验室,安徽蚌埠233004;2.重庆市綦江区人民医院呼吸与危重症医学科,401420)[摘要]目的:探讨替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗晚期肺鳞癌的近期疗效及不良反应㊂方法:选取驱动基因表皮生长因子受体㊁间变性淋巴瘤激酶㊁C⁃ROS 原癌基因阴性的晚期肺鳞癌病人80例,随机分为单纯化疗组和免疫联合化疗组,各40例,分别给予白蛋白结合型紫杉醇/奈达铂和替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗4周期,比较2组病人的近期疗效及主要不良反应㊂结果:免疫联合化疗组完全缓解(CR)1例,部分缓解(PR)28例,稳定(SD)9例,进展(PD)2例,客观缓解率(ORR)72.5%(29/40);疾病控制率(DCR)95%(38/40)㊂单纯化疗组CR 0例,PR 13例,SD 22例,PD5例,ORR 37.5%(13/40),DCR 87.5%(35/40)㊂免疫联合化疗组ORR 明显高于单纯化疗组(P <0.01),2组DCR 差异无统计学意义(P >0.05)㊂2组病人白细胞计数减少㊁中性粒细胞计数减少㊁血小板计数减少㊁贫血㊁低蛋白血症㊁转氨酶升高㊁乏力㊁脱发㊁恶心㊁四肢疼痛等不良反应发生率差异均无统计学意义(P >0.05)㊂结论:雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗晚期肺鳞癌较单纯化疗具有更佳的近期疗效,不会增加病人治疗期间的不良反应,安全性好,可以作为驱动基因阴性的晚期肺鳞癌病人的一线治疗方案㊂[关键词]肺鳞癌;替雷利珠单抗;紫杉醇;奈达铂[中图法分类号]R 734.2 [文献标志码]A DOI :10.13898/ki.issn.1000⁃2200.2024.03.005Short⁃term efficacy analysis of tislelizumab combined with nab⁃paclitaxel /nedaplatinin the treatment of patients with advanced lung squamous cell carcinomaXIANG Junxin 1,2,LI Xiaoli 1,LIU Ying 1,LIU Yun 1,WANG Lu 1,LI Dianming 1(1.Department of Respiratory and Critical Medicine ,Molecular Diagnosis Center ,Clinical Basis of Respiratory Diseases Key Laboratory of Anhui Province ,The First Affiliated Hospital of Bengbu Medical University ,Bengbu Anhui 233004;2.Department of Respiratory and Critical Medicine ,Qijiang District People′s Hospital ,Chongqing 401420,China )[Abstract ]Objective :To investigate the short⁃term efficacy and adverse reactions of tislelizumab combined with nab⁃paclitaxel /nedaplatin in the treatment advanced lung squamous cell carcinoma.Methods :Eighty advanced lung squamous cell carcinoma patientswith negative driver gene including epidermal growth factor receptor,anaplastic lymphoma kinase,and C⁃ROS oncogene 1were selected and randomly divided into a simple chemotherapy group and an immunotherapy combined with chemotherapy group,with 40cases in each group,which were treated with nab⁃paclitaxel /nedaplatin and tislelizumab combined with nab⁃paclitaxel /nedaplatin for 4cycles,respectively.The short⁃term efficacy and major adverse reactions of patients in the two groups were compared.Results :In the immunotherapy combined with chemotherapy group,there was 1case with complete remission (CR),28cases with partial remission (PR),9cases with stable disease (SD),and 2progressive disease (PD),and the objective remission rate (ORR)was 72.5%(29/40),the disease control rate (DCR)was 95%(38/40).In the simple chemotherapy group,there was 0case with CR,13cases with PR,22cases with SD,5cases with PD,and the ORR was 37.5%(13/40),DCR was 87.5%(35/40).The ORR in the immunotherapy combined with chemotherapy group was significantly higher than that in the simple chemotherapy group (P <0.01),but there was no statistically significant difference in DCR between the two groups (P >0.05).There was no statistically significant difference in the incidence of adverse reactions such as decreased white blood cell count,decreased neutrophil count,decreased plateletcount,anemia,hypoalbuminemia,elevated transaminases,fatigue,hair loss,nausea,and limb pain of patients between the two groups(P>0.05).Conclusions:Tislelizumab combined with nab⁃paclitaxel/nedaplatin in the treatment of advanced lung squamous cell carcinoma has better short⁃term efficacy than simple chemotherapy,does not increase incidence of adverse reactions in patients during the treatment period,and has good safety,which may be used as the first⁃line treatment for advanced lung squamous cell carcinoma patients with negative driver gene.[Key words]lung squamous cell carcinoma;tislelizumab;nab⁃paclitaxel;nedaplatin 肺癌是全球发病率最高的肿瘤,同时也是全球肿瘤相关死亡的第一死因㊂根据世界卫生组织统计,2018年全球肺癌死亡病人达176万[1],在我国肺癌也是最常见的恶性肿瘤[2]㊂肺癌主要有非小细胞肺癌(non⁃small⁃cell lung cancer,NSCLC)和小细胞肺癌(small⁃cell lung cancer,SCLC),其中NSCLC约占85%,SCLC约占15%[3]㊂在过去十多年中,由于表皮生长因子受体(epidermal growth factor receptor,EGFR)突变㊁间变性淋巴瘤激酶(anaplastic lymphoma kinase,ALK)重排和C⁃ROS原癌基因(recombinant C⁃Ros oncogene1,ROS1)重排的发现,开创了肺癌靶向治疗的时代㊂肺腺癌病人由于其较高的突变率而受益,而肺鳞癌由于突变率较低,对于经基因检测EGFR㊁ALK和ROS1驱动基因阴性的晚期肺鳞癌病人采取含铂两药方案化疗[4],病人的总生存期很低㊂随着程序性死亡受体1(PD⁃1)/程序性死亡配体1(PD⁃L1)免疫检查点抑制剂的出现打破了这一局面,近年免疫治疗大量用于NSCLC的一线㊁二线治疗[6],晚期肺鳞癌病人也拥有了更多的选择[7],这些药物通过刺激病人T细胞免疫反应以识别和破坏癌细胞,免疫疗法的毒性特征与化学疗法不同,而且总体上耐受性更好,病人获益更大[8]㊂替雷利珠单抗2021年在中国NMPA 首次获批联合紫杉醇或白蛋白结合型紫杉醇/铂类作为驱动基因阴性的晚期肺鳞癌一线治疗方案[9],但在研究设计中铂类多采用卡铂或顺铂,而奈达铂是一种专门为提高耐受性而设计的铂类衍生物,研究[10]表明在晚期NSCLC中,以奈达铂为基础的方案的呕吐和肾脏毒性低于顺铂,由于其在晚期或复发的鳞状NSCLC中较好的疗效而获指南推荐[10]㊂目前关于替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗晚期肺鳞癌尚未见报道㊂因此,本研究探讨替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗驱动基因阴性的晚期肺鳞癌病人在真实世界中的近期疗效及不良反应㊂1 资料与方法1.1 一般资料 选取2021年1月至2022年6月在蚌埠医科大学第一附属医院经基因检测EGFR㊁ALK和ROS1驱动基因阴性的晚期肺鳞癌病人80例,随机分为免疫联合化疗组和单纯化疗组,各40例㊂免疫联合化疗组男36例,女4例,年龄36~76岁;单纯化疗组男32例,女8例,年龄47~79岁㊂2组性别㊁年龄㊁临床分期等差异均无统计学意义(P>0.05)(见表1),具有可比性㊂本研究获得我院伦理委员会批准㊂纳入标准:(1)经细胞学或病理组织学及免疫组化确诊为肺鳞癌病人;(2)肿瘤分期Ⅲb~Ⅳ期病人;(3)ECOG PS评分0~1分;(3)连续接受4周期及以上替雷利珠单抗联合化疗或单纯化疗;(4)影像学至少存在一个可测量的病灶;(5)临床资料完整;(6)同意参加本研究,并签署知情同意书㊂排除标准:(1)ECOG PS评分2~3分;(2)中途终止治疗或疗程未达4个周期病人;(3)合并有其他系统肿瘤病人;(4)临床资料缺失或不完整㊂表1 一般资料比较(n)临床特征免疫联合化疗组(n=40)单纯化疗组(n=40)χ2P 性别 男 女364328 1.57>0.05年龄/岁 >60 ≤601228832 1.07>0.05是否吸烟 是 否132710300.46>0.05临床分期 Ⅲ期 Ⅳ期172320200.45>0.05淋巴结转移 有 无3462812 2.58>0.05初诊肺内转移 有 无832634 2.58>0.05初诊胸腔积液 有 无8326340.35>0.05续表1临床特征免疫联合化疗组(n=40)单纯化疗组(n=40)χ2P初诊肝转移 有 无33740 1.39△>0.05初诊大血管侵犯 有 无3374360.00△>0.05初诊骨转移 有 无9307330.38>0.05初诊脑转移(无症状) 有 无2382380.26△>0.05 △示校正χ2值1.2 治疗方法 (1)免疫联合化疗组:第1天,替雷利珠单抗200mg+100mL0.9%氯化钠溶液静脉滴注,时间大于30min;第2天,白蛋白结合型紫杉醇260mg/m2+ 100mL0.9%氯化钠溶液静脉滴注,奈达铂80mg/ m2+500mL0.9%氯化钠溶液静脉滴注㊂(2)单纯化疗组:白蛋白结合型紫杉醇260mg/m2+100mL 0.9%氯化钠溶液静脉滴注,奈达铂80mg/m2+ 500mL0.9%氯化钠溶液静脉滴注㊂2组均给予4周期治疗,每21d为1周期,治疗期间常规予以护胃㊁止吐㊁水化㊁护肝㊁保肾等治疗㊂1.3 临床疗效评价 疗效评价按照实体肿瘤疗效判定标准RESIST1.1[11]进行评定,通过影像学观测,将其分为:(1)完全缓解(CR),所有靶病灶完全消失,持续4周以上;(2)部分缓解(PR),所有靶病灶长径总和缩小≥30%;(3)稳定(SD),所有靶病灶长径总和缩小<30%或增大≤20%;(4)进展(PD),所有靶病灶长径总和增大>20%或出现新病灶㊂并计算客观缓解率(ORR)和疾病控制率(DCR),ORR=(CR例数+PR例数)/总例数×100%,DCR=(CR例数+PR例数+SD例数)/总例数×100%㊂1.4 不良反应评价 根据美国国立癌症研究所制定的不良事件通用术语标准(NCI⁃CTCAE v5.0)[12]进行不良反应评级㊂1.5 统计学方法 采用χ2检验和Fisher′s确切概率法㊂2 结果2.1 2组病人临床疗效比较 免疫联合化疗组ORR明显高于单纯化疗组(P<0.01),2组DCR差异无统计学意义(P> 0.05)(见表2)㊂表2 2组病人疗效比较[n;百分率(%)]分组n CR PR SD PD ORR DCR免疫联合化疗组401(2.5)28(70.0)9(22.5)2(5.0)29(72.5)38(95.0)单纯化疗组400(0)13(32.5)22(55.0)5(12.5)13(32.5)35(87.5)χ2 12.830.63P <0.01>0.05 2.2 2组病人不良反应发生率比较 2组病人白细胞计数减少㊁中性粒细胞计数减少㊁血小板计数减少㊁贫血㊁低蛋白血症㊁转氨酶升高㊁乏力㊁脱发㊁恶心㊁四肢疼痛等不良反应发生率差异均无统计学意义(P>0.05)(见表3)㊂表3 2组病人不良反应比较(n)类别免疫联合化疗组 Ⅰ Ⅱ Ⅲ 发生率/% 单纯化疗组 Ⅰ Ⅱ Ⅲ 发生率/% χ2P白细胞计数减少81022.5 52120.0 0.07>0.05中性粒细胞计数降低40010.051015.00.46>0.05血小板计数减少32012.560015.00.11▲>0.05贫血232062.5230057.50.21>0.05低蛋白血症110027.5140035.00.52>0.05转氨酶升高100 2.5100 2.5 >0.05△乏力90022.560015.00.74>0.05脱发200 5.0200 5.00.26▲>0.05恶心100025.060015.0 1.25>0.05四肢疼痛70017.560015.00.09>0.05 △示Fisher′s确切概率法;▲示校正χ2值3 讨论 替雷利珠单抗是一种抗PD⁃1单克隆抗体,可结合并阻断激活的免疫细胞如T淋巴细胞上表达的PD⁃1,PD⁃1与其配体PD⁃L1之间的相互作用导致T 细胞耗竭,从而减弱抗癌免疫反应㊂经过专门设计,可最大限度地减少FcγR与巨噬细胞的结合,从而消除抗体依赖性吞噬作用,与帕博利珠单抗和纳武利尤单抗相比,替雷利珠单抗对PD⁃1的亲和力更高,该药物于2019年12月首次在中国获批用于二线化疗后复发或难治的经典霍奇金淋巴瘤病人[9,13]㊂目前国产研发的替雷利珠单抗已加入中国国家医保[14],成为更具性价比的治疗选择[15],为晚期肺鳞癌病人带来了福音㊂白蛋白结合型紫杉醇是一种与人血清白蛋白结合的紫杉醇纳米粒制剂㊂传统紫杉烷类(如紫杉醇和多西紫杉醇)会破坏微管的稳定性,从而阻止细胞增殖,是最广泛使用的化疗药物之一㊂由于白蛋白结合型紫杉醇不含溶剂,例如聚氧乙烯蓖麻油,因此不需要大剂量的皮质类固醇和抗组胺药来预防过敏反应[16]㊂白蛋白结合型紫杉醇通常被认为是一种相对温和的化疗药物,与包括NSCLC在内的各种实体瘤的其他化疗方案相比,具有良好的耐受性㊂临床试验表明,白蛋白结合型紫杉醇比传统紫杉醇具有更好的疗效和安全性[17-18]㊂奈达铂是顺铂的衍生物,WJOG5208L[19]指出它具有与顺铂相同的胺载体配体,但具有不同的基团,其中乙醇酸以双齿配体的形式与铂离子结合,是为了减少顺铂引起的胃肠道和肾毒性反应而研制的,与传统的顺铂或卡铂没有交叉耐药性㊂该研究纳入355例晚期肺鳞癌病人,随机分为奈达铂+多西紫杉醇组和顺铂+多西紫杉醇组,结果显示奈达铂+多西紫杉醇组总生存期(OS)为13.6个月,明显长于顺铂+多西紫杉醇组OS的11.4个月,一些重要的非血液学不良事件(恶心㊁厌食和疲劳)在奈达铂+多西紫杉醇组的发生率比顺铂+多西紫杉醇组低,且程度较轻,而且可以接受三线化疗的病人数量明显增多㊂一项Ⅱ期临床试验[20]表明,白蛋白结合型紫杉醇联合奈达铂双周化疗方案可用于治疗晚期鳞状细胞肺癌,且具有更高的安全性和临床耐受性,可能成为晚期鳞状细胞肺癌病人新的一线治疗选择㊂一项替雷利珠单抗联合铂类化疗一线治疗中国晚期肺癌病人的Ⅱ期研究[9]表明,在中国晚期肺癌病人中,化疗联合替雷利珠单抗通常具有良好的耐受性,并表现出持久的抗肿瘤活性㊂该试验共纳入54例病人,其中肺鳞癌组(替雷利珠单抗+紫杉醇+顺铂/卡铂)ORR为80%,非鳞NSCLC组(培美曲塞+顺铂/卡铂)ORR为94%,2组ORR差异有统计学意义,该研究出现最常见的与替雷利珠单抗相关的不良反应是乏力和甲状腺功能减退㊂RATIONALE307[21]研究纳入360例病人,分为替雷利珠单抗+紫杉醇+卡铂组㊁替雷利珠单抗+白蛋白结合型紫杉醇+卡铂组㊁紫杉醇+卡铂组,包含替雷利珠单抗的2组中位无进展生存期和ORR明显增高,但OS数据不成熟㊂该试验中最常见的不良反应是贫血,最常见的Ⅲ级或以上不良反应是中性粒细胞水平降低㊂在接受含替雷利珠单抗治疗的病人中,高血糖㊁甲状腺功能减退和肺炎是最常见的潜在免疫介导不良反应㊂这两项研究结果表明在中国晚期肺癌病人中,替雷利珠单抗可显著降低晚期肺鳞癌病人的进展或死亡风险,但都缺乏OS数据㊂本研究探讨替雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂对比单纯化疗方案治疗晚期肺鳞癌的短期治疗效果,免疫联合化疗组ORR为72.5%,单纯化疗组ORR为37.5%,与以上临床试验数据基本符合,替雷利珠单抗联合两药化疗相比与单纯化疗效果评价更加侧重于ORR,而非DCR,本研究最常见的不良反应为贫血㊂2组共同不良反应差异均无统计学意义,与以上两项研究[9,21]结果相符㊂综上所述,与单纯化疗相比,雷利珠单抗联合白蛋白结合型紫杉醇/奈达铂治疗晚期肺鳞癌具有更佳的近期疗效,不会增加病人治疗期间的不良反应,安全性好,可以作为晚期驱动基因阴性的晚期肺鳞癌病人的一线治疗㊂[参考文献][1] BADE BC,DELA CRUZ CS.Lung cancer2020epidemiology,etiology,and prevention[J].Clin Chest Med,2020,41(1):1.[2] CAO W,CHEN HD,YU YW,et al.Changing profiles of cancerburden worldwide and in China:a secondary analysis of the globalcancer statistics2020[J].Chin Med J,2021,134(7):783. [3] DUMA N,SANTANA⁃DAVILA R,MOLINA J.Non⁃small celllung cancer:epidemiology,screening,diagnosis,and treatment[J].Mayo Clin Proc,2019,94(8):1623.[4] 仲佳.‘CSCO非小细胞肺癌诊疗指南2021“更新要点解读[J].实用肿瘤杂志,2022,37(1):8.[5] MAO G,YANG D,LIU B,et al.Deciphering a cell death⁃associated signature for predicting prognosis and response toimmunotherapy in lung squamous cell carcinoma[J].RespirRes,2023,24(1):176.[6] 徐晓婉,唐淑慧,张锚,等.非小细胞肺癌病人常见免疫治疗不良反应的影响因素分析[J].蚌埠医学院学报,2022,47(8):1096.[7] 于江泳,武晓楠,马俊玲,等.中国人群肺鳞癌患者免疫治疗疗效及不良反应观察[J].中国肺癌杂志,2022,25(7):546.[8] DAABOUL N,NICHOLAS G,LAURIE S.Algorithm for thetreatment of advanced or metastatic squamous non⁃small⁃cell lungcancer:an evidence⁃based overview[J].Curr Oncol,2018,25:S77.[9] WANG Z,ZHAO J,MA Z,et al.A phase2study of tislelizumabin combination with platinum⁃based chemotherapy as first⁃linetreatment for advanced lung cancer in Chinese patients[J].LungCancer,2020,147:259.[10] WU Y,SPICER J.Nedaplatin:a new platinum for squamous lungcancer?[J].Lancet Oncol,2015,16(16):1573. [11] EISENHAUER E,THERASSE P,BOGAERTS J,et al.Newresponse evaluation criteria in solid tumours:revised RECISTguideline(version1.1)[J].Eur J Cancer,2009,45(2):228.[12] BASCH E,REEVE B,MITCHELL S,et al.Development of theNational Cancer Institute′s patient⁃reported outcomes version ofthe common terminology criteria for adverse events(PRO⁃CTCAE)[J].J Natl Cancer Inst,2014,106(9):dju244. [13] LEE A,KEAM S.Tislelizumab:first approval[J].Drugs,2020,80(6):617.[14] 姚欣凯,穆新林,张海英.4种准入中国医保的PD⁃1抑制剂药学特性及临床应用评价[J].中国现代应用药学,2022,39(7):952.[15] GONG J,SU D,SHANG J,et al.Cost⁃effectiveness of tislelizumabversus docetaxel for previously treated advanced non⁃small⁃celllung cancer in China[J].Front Pharmacol,2022,13:830380.[16] WANG S,LIANG Q,CHI Y,et al.Retrospective analysis of theeffectiveness and tolerability of nab⁃paclitaxel in Chinese elderlypatients with advanced non⁃small⁃cell lung carcinoma[J].ThoracCancer,2020,11(5):1149.[17] DECHOW T,RIERA⁃KNORRENSCHILD J,HACKANSON B,etal.First⁃line nab⁃paclitaxel plus carboplatin for patients withadvanced non⁃small cell lung cancer:results of the NEPTUNstudy[J].Cancer Med,2021,10(22):8127.[18] SHITARA K,TAKASHIMA A,FUJITANI K,et al.Nab⁃paclitaxelversus solvent⁃based paclitaxel in patients with previously treatedadvanced gastric cancer(ABSOLUTE):an open⁃label,randomised,non⁃inferiority,phase3trial[J].Lancet GastroenterolHepatol,2017,2(4):277.[19] SHUKUYA T,YAMANAKA T,SETO T,et al.Nedaplatin plusdocetaxel versus cisplatin plus docetaxel for advanced or relapsedsquamous cell carcinoma of the lung(WJOG5208L):arandomised,open⁃label,phase3trial[J].Lancet Oncol,2015,16(16):1630.[20] LV WZ,LIN Z,WANG SY,et al.PhaseⅡstudy of a Bi⁃weeklychemotherapy regimen of combined liposomal paclitaxel andnedaplatin for the treatment of advanced squamous cell lungcancer[J].Transl Oncol,2019,12(4):656.[21] WANG J,LU S,YU X,et al.Tislelizumab plus chemotherapy vschemotherapy alone as first⁃line treatment for advanced squamousnon⁃small⁃cell lung cancer:a phase3randomized clinical trial[J].JAMA Oncol,2021,7(5):709.(本文编辑 赵素容)(上接第301页)[8] AMIR LH.ABM clinical protocol#4:mastitis,revised March2014[J].Breastfeed Med,2014,9(5):239.[9] MARTÍNEZ⁃LÓPEZ M,IBORRA S,CONDE⁃GARROSA R,et al.Microbiota sensing by mincle⁃Syk axis in dendritic cells regulatesinterleukin⁃17and⁃22production and promotes intestinal barrierintegrity[J].Immunity,2019,50(2):446.[10] GAO JR,SHI MM,JIANG H,et al.MicroRNA⁃339⁃5p inhibitslipopolysaccharide⁃induced rat mesangial cells by regulating theSyk/Ras/c⁃Fos pathway[J].Naunyn Schmiedebergs ArchPharmacol,2022,395(9):1075.[11] DING N,LI P,LI H,et al.The ROCK⁃ezrin signaling pathwaymediates LPS⁃induced cytokine production in pulmonary alveolarepithelial cells[J].Cell Commun Signal,2022,20(1):65. [12] 王振杰,陈硬,宋琦,等.乌司他丁通过miR⁃146a调节TLR4/NF⁃κB信号通路减轻失血性休克大鼠肾炎性损伤研究[J].蚌埠医学院学报,2020,45(3):286.[13] 李磊,徐志鹏,夏群,等.醋酸钠林格液复苏联合乳酸菌对创伤失血性休克大鼠肠黏膜屏障的保护作用[J].蚌埠医学院学报,2021,46(6):708.[14] LIU L,LUCAS RM,NANSON JD,et al.The transmembraneadapter SCIMP recruits tyrosine kinase Syk to phosphorylate Toll⁃like receptors to mediate selective inflammatory outputs[J].JBiol Chem,2022,298(5):101857.[15] TAKASHIMA M,LALONDE C,OLSZANSKI LA,et al.Localizedand systemic inflammatory mediators in a murine acute mastitismodel[J].J Inflamm Res,2021,14:4053.[16] WANG X,LIU M,GENG N,et al.Staphylococcus aureus mediatespyroptosis in bovine mammary epithelial cell via activation ofNLRP3inflammasome[J].Vet Res,2022,53(1):10. [17] KOBAYASHI K,MATSUNAGA K,TSUGAMI Y,et al.IL⁃1βis akey inflammatory cytokine that weakens lactation⁃specific tightjunctions of mammary epithelial cells[J].Exp Cell Res,2021,409(2):112938.[18] ZHAO W,DENG Z,BARKEMA HW,et al.Nrf2and NF⁃κB/NLRP3inflammasome pathways are involved in Prototheca bovisinfections of mouse mammary gland tissue and mammaryepithelial cells[J].Free Radic Biol Med,2022,184:148. [19] WANG H,HAO W,YANG L,et al.Preconditioning withprocyanidin B2protects MAC⁃T cells against heat exposure⁃induced mitochondrial dysfunction and inflammation[J].MolImmunol,2022,147:126.(本文编辑 卢玉清)。
Vyleesi(布莫拉尼德)产品说明说明书

UnitedHealthcare PharmacyClinical Pharmacy ProgramsProgram Number 2022 P 2178-3Program Prior Authorization/Medical NecessityMedication Vyleesi (bremelanotide)P&T Approval Date 11/2019, 8/2021, 9/2022Effective Date 12/1/2022;Oxford only: 12/1/20221.Background:Vyleesi is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not due to a co-existing medical orpsychiatric condition, problems within the relationship, or the effects of a medication or other drug substance. Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occursregardless of the type of stimulation, situation or partner. Vyleesi is not indicated for the treatment of HSDD in postmenopausal women or in men and is not indicated to enhance sexual performance.a13.Additional Clinical Rules:•Supply limits may be in place•Notwithstanding Coverage Criteria, UnitedHealthcare may approve initial and re-authorization based solely on previous claim/medication history, diagnosis codes (ICD-10) and/or claim logic. Use of automated approval and re-approval processes varies byprogram and/or therapeutic class.© 2022 UnitedHealthcare Services Inc.24. References:1.Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc; June 2019.2.Sexual dysfunctions. In: Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,American Psychiatric Association, Arlington, Virginia 2013.3.Overview of sexual dysfunction in women: Management. UpToDate. Updated April 4,2022. Last accessed August 9,2022.Program Prior Authorization/Medical Necessity – VyleesiChange ControlDate Change11/2019 New program.8/2021 Updated references.9/2022 Updated references.© 2022 UnitedHealthcare Services Inc.3。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Expertos en Ruido y Vibraciones
El control de vibraciones es una materia de gran importancia especialmente cuando se producen en áreas con presencia de viviendas. A diferencia del ruido aéreo, las vibraciones se transmiten por las estructuras de las construcciones, alcanzando una mayor velocidad de transmisión dependiendo de su naturaleza.
Expertos en Ruido y Vibraciones
¿Qué son las vibraciones? Vibración es el movimiento oscilante que realiza una partícula alrededor de un punto fijo
Expertos en Ruido y Vibraciones
En la ejecución de medidas orientadas a evitar la transmisión de vibraciones, es fundamental poder llevarlas a cabo con anterioridad a la ubicación de las instalaciones ruidosas, pues la ejecución de medidas correctoras, una vez puesta en funcionamiento la actividad, conlleva una mayor complejidad debido a la imprevisión de estas medidas en el origen, y por tanto, debido a la falta de espacio y de otras necesidades.
Expertos en Ruido y Vibraciones
El control de las vibraciones ha de ser tenido en cuenta tanto en los efectos producidos sobre las personas, como en los producidos en los materiales a ellas expuestos.
Modos propios de vibración
Cada frecuencia de resonancia tiene asociado lo que se llama un modo propio de vibración, es decir, una forma particular de moverse que depende de las propiedades del objeto (geometría, masa, estado de carga, condiciones de contorno, etc). Hay puntos donde la amplitud de la vibración es máxima y otros puntos donde ésta se anula (nodos).
Expertos en Ruido y Vibraciones
RESONANCIA
El período de un péndulo depende únicamente de la longitud del hilo del que pende la masa.
Experimento: en una cuerda tensa colguemos tres péndulos, dos de igual longitud y uno más largo. El período de los dos primeros es el mismo, pero distinto del tercero. Coloquemos al más largo entre los cortos. A continuación, separemos de su posición de equilibrio uno de los péndulos cortos.
MEJORAS TÉCNICAS DISPONIBLES EN LA GESTIÓN ACÚSTICA Y VIBRACIONES
Control de Vibraciones y sus medidas correctoras
Ignacio González Svantek España, S.L.
Donde: awx, awy, awz son los valores de las aceleraciones de cada uno de los tres ejes ortogonales x, y, z. kx, ky, kz son las constantes de multiplicación cuyos valores dependen de la aplicación de la medición
Expertos en Ruido y Vibraciones
UNIDADES DE MEDIDA DE VIBRACIÓN
G’s m/s2
1 G = 9,80665 m/s2
Decibelio dB
a (dB) = 20 log a (m/s2) / 10-6 (m/s2)
Expertos en Ruido y Vibraciones
RESONANCIA
Al cabo de unos instantes, comprobaremos que el otro péndulo corto se empieza a mover y en pocos segundos lo hace con tanta intensidad como el primero, de manera que no podríamos distinguir cuál de los dos ha empezado a oscilar. Por el contrario, el péndulo largo no se ve afectado por la oscilación de los otros y permanece quieto .
Expertos en Ruido y Vibraciones
Importancia de la resonancia Ingeniería civil
Movimientos sísmicos
Expertos en Ruido y Vibraciones
Expertos en Ruido y Vibrቤተ መጻሕፍቲ ባይዱciones
¿A qué se debe este fenómeno?
Los péndulos de igual longitud tienen períodos de oscilación iguales. El primero provoca la oscilación del segundo transmitiéndole energía a través del hilo que los une. El tercer péndulo asiste al fenómeno del "paso" de energía de un péndulo al otro, pero no puede participar en él porque su período de oscilación es distinto. A este tipo fenómenos se le llama de resonancia y ocurre con mucha frecuencia en la naturaleza. Cuando una causa provoca una oscilación y cerca de ella se encuentra un objeto con la misma frecuencia propia que la de la oscilación, éste empieza a vibrar.
MOVIMIENTO ONDULATORIO
Expertos en Ruido y Vibraciones
MOVIMIENTO ONDULATORIO
Tenemos un muelle en su posición de equilibrio (XO). Si tiramos de él y lo separamos de su posición de equilibrio una distancia Xm, al soltarlo, el muelle volverá a la posición de equilibrio y la rebasará una distancia Xm igual a la distancia hasta la que fue alargado al principio. Si no hay rozamiento, este movimiento, llamado oscilatorio, se repetirá indefinidamente.
Expertos en Ruido y Vibraciones
MTVV
Máximo valor de la vibración transitoria: El valor máximo de la aceleración eficaz móvil cuando el tiempo de integración es igual a 1 segundo (Slow)
