Bitopertin_DataSheet_MedChemExpress
第三章动物细胞制药part2讲课教案

动物细胞培养方法和操作方式
一、动物细胞大规模培养方法 依细胞种类: 原代培养 传代培养 依培养基: 液体培养基 固体培养基
依培养器和方式:
静止培养、旋转培养 搅拌培养、为载体培养 中空纤维培养、 固定床或流化床培养
Байду номын сангаас
从生产实际分为:
悬浮培养 贴壁培养 贴壁-悬浮培养
培养技术
动物细胞大规模培养与实验室培养相比,培养条件 更严格,控制难度更大,其培养方法可概括为:
支持细胞贴附生长。 ⑵ 钛碟装置多层圆柱状不锈钢(带有观察条件)罐内或
玻璃瓶内装入钛碟圆盘支持细胞贴服生长。可改变 位置和旋转。
培养技术
1. 细胞悬浮培养法
➢ 动物细胞在培养液中呈悬浮状态生长繁殖的培养方 法谓之悬浮培养法。其培养方式有
批量法 半连续法 连续法 ➢ 适用于培养确立细胞株、杂交瘤细胞、肿瘤细胞、 血液细胞及淋巴组织细胞,用于大量生产疫苗、 α-干扰素、白介素等药品。 ➢ 此法不适于包括二倍体细胞在内的正常组织细胞的培 养。
培养技术 细胞悬浮培养法的优点
➢ 可连续收集部分细胞进行移植继代培养,传代时无需 消化分散,免遭酶类、EDTA及机械损害。
➢ 细胞收率高,并可连续测定细胞浓度,还有可能实现 大规模直接克隆培养。
培养技术
细胞悬浮培养法的优点
➢ 培养过程中,为确保细胞呈单颗粒均匀悬浮状态, 需采用搅拌或气升式反应器,以较低搅拌速度及 一定速度通入含5%的CO2无菌空气,保持细胞悬浮态 并维持培养液溶解氧。
Vero细胞和狂犬病毒的培养工艺
动物细胞培养存在的问题
细胞密度低,细胞生产率低,产物浓度也很低。 细胞群体在大规模、长时间培养过程中分泌产物能力
Epinastine_80012-43-7_DataSheet_MedChemExpress
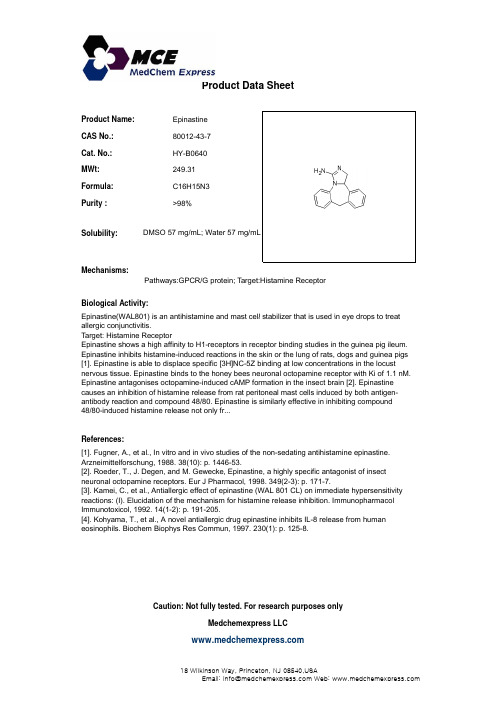
Product Name:Epinastine CAS No.:80012-43-7Cat. No.:HY-B0640Product Data SheetMWt:249.31Formula:C16H15N3Purity :>98%Solubility:DMSO 57 mg/mL; Water 57 mg/mLMechanisms:Biological Activity:Epinastine(WAL801)is an antihistamine and mast cell stabilizer that is used in eye drops to treatPathways:GPCR/G protein; Target:Histamine Receptor Epinastine(WAL801) is an antihistamine and mast cell stabilizer that is used in eye drops to treatallergic conjunctivitis.Target: Histamine Receptor Epinastine shows a high affinity to H1-receptors in receptor binding studies in the guinea pig ileum.Epinastine inhibits histamine-induced reactions in the skin or the lung of rats, dogs and guinea pigs[1]. Epinastine is able to displace specific [3H]NC-5Z binding at low concentrations in the locustnervous tissue. Epinastine binds to the honey bees neuronal octopamine receptor with Ki of 1.1 nM.Epinastine antagonises octopamine-induced cAMP formation in the insect brain [2]. Epinastinecauses an inhibition of histamine release from rat peritoneal mast cells induced by both antigen-References:[1]. Fugner, A., et al., In vitro and in vivo studies of the non-sedating antihistamine epinastine.Arzneimittelforschung, 1988. 38(10): p. 1446-53.[2]. Roeder, T., J. Degen, and M. Gewecke, Epinastine, a highly specific antagonist of insect p y g antibody reaction and compound 48/80. Epinastine is similarly effective in inhibiting compound 48/80-induced histamine release not only fr...[],,g ,,p ,g y p gneuronal octopamine receptors. Eur J Pharmacol, 1998. 349(2-3): p. 171-7.[3]. Kamei, C., et al., Antiallergic effect of epinastine (WAL 801 CL) on immediate hypersensitivity reactions: (I). Elucidation of the mechanism for histamine release inhibition. ImmunopharmacolImmunotoxicol, 1992. 14(1-2): p. 191-205.[4]. Kohyama, T., et al., A novel antiallergic drug epinastine inhibits IL-8 release from humaneosinophils. Biochem Biophys Res Commun, 1997. 230(1): p. 125-8.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
大鼠髓磷脂碱性蛋白(MBP)ELISA试剂盒说明书

大鼠髓磷脂碱性蛋白(MBP)酶联免疫分析试剂盒使用说明书厦门慧嘉生物科技有限公司本试剂盒仅供研究使用检测范围:7.8 ng/ml - 500 ng/ml最低检测限:1.95 ng/ml特异性:本试剂盒可同时检测天然或重组的大鼠MBP,且与其他相关蛋白无交叉反应。
有效期:6个月预期应用:ELISA法定量测定大鼠血清、血浆、细胞培养上清或其它相关生物液体中MBP 含量。
说明1.试剂盒保存:-20℃(较长时间不用时);2-8℃(频繁使用时)。
2.浓洗涤液低温保存会有盐析出,稀释时可在水浴中加温助溶。
3.中、英文说明书可能会有不一致之处,请以英文说明书为准。
4.刚开启的酶联板孔中可能会含有少许水样物质,此为正常现象,不会对实验结果造成任何影响。
概述MBP是中枢神经系统(CNS)髓鞘的主要蛋白质,位于髓鞘浆膜面,维持CNS髓鞘结构和功能的稳定,具有神经组织特异性。
由于血脑屏障(BBB)的作用,MBP较易释放到脑脊液,仅小量释放入血液。
当CNS遭到损害时,BBB功能被破坏,其通透性发生改变,使血清MBP含量升高。
实验原理用纯化的抗体包被微孔板,制成固相载体,往包被抗MBP抗体的微孔中依次加入标本或标准品、生物素化的抗MBP抗体、HRP标记的亲和素,经过彻底洗涤后用底物TMB显色。
TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的MBP呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),计算样品浓度。
试剂盒组成及试剂配制1.酶联板(Assay plate ):一块(96孔)。
2.标准品(Standard):2瓶(冻干品)。
3.样品稀释液(Sample Diluent):1×20ml/瓶。
4.生物素标记抗体稀释液(Biotin-antibody Diluent):1×10ml/瓶。
5.辣根过氧化物酶标记亲和素稀释液(HRP-avidin Diluent):1×10ml/瓶。
PTP1B-IN-2-DataSheet-MedChemExpress

Solvent Concentration reparing Stock Solutions 5 mM 10 mM 1 mM
1.4689 mL 0.2938 mL 0.1469 mL
7.3444 mL 1.4689 mL 0.7344 mL
14.6888 mL 2.9378 mL 1.4689 mL
Inhibitors • Agonists • Screening Libraries
Solvent & Solubility
In Vitro DMSO : ≥ 100 mg/mL (146.89 mM) * "≥" means soluble, but saturation unknown. Mass 1 mg 5 mg 10 mg
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA
2
1
PROTOCOL
Kinase Assay [1] PTP1B-IN-2 is predispensed in 96-well microplates as 1.0 µL aliquots per well in 100% DMSO. The PTP1B enzymatic assay is carried out in a total volume of 100 µL per well in assay plates with 15 nM recombinant PTP1B protein, 2 mM p-nitrophenylphosphonic acid (pNPP), 1 mM dithiothreitol and 1 mM EDTA (pH 6.5). After 30 min incubation at 37oC, the reaction is terminated by addition of 2.5 M NaOH. The amount of hydrolysis product, pNP, is monitored by detection of the absorbance at 405 nm[1]. MCE has not independently confirmed the accuracy of these methods. They are for reference only.
Febuxostat_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Febuxostat(TEI 6720;TMX 67 ) is selective xanthine oxidase inhibitor with Ki of 0.6 nM.IC50 value: 0.6 nM (Ki) [1]Target: xanthine oxidasein vitro: Febuxostat displays potent mixed–type inhibition of the activity of purified bovine milk xanthine oxidase, with Ki and Ki' values of 0.6 nM and 3.1 nM respectively, indicating inhibition of both the oxidized and reduced forms of xanthine oxidase [1].in vivo: Febuxostat (5–6 mg/kg/day) combined with fructose significantly lowers blood pressure, UA, triglycerides, and insulin in rats compared with fructose alone. Febuxostat (5–6 mg/kg/day) combined with fructose also reduces glomerular pressure, renal vasoconstriction, and afferent arteriolar area in rats compared with fructose alone [2]. Febuxostat prevents hyperuricemia in 5/6nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) rats and ameliorates proteinuria, preserves renal function and prevents glomerular hypertension in both 5/6 nephrectomy (5/6 Nx)+vehicle (V)+Febuxostat(Fx) and 5/6 nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) groups [3]. Febuxostat (5 mg/kg/d by gavage for 8 days) treatment after transverse aortic constriction (TAC)attenuates the TAC–induced left ventricular (LV) hypertrophy and dysfunction. Febuxostat blunts the TAC–induced increases innitrotyrosine (indicating reduced myocardial oxidative stress), p–Erk(Thr202/Tyr204), and p–mTOR(Ser2488), with no effect on total Erk or total mTOR [4].References:[1]. Takano Y, et al. Selectivity of febuxostat, a novel non–purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci, 2005, 76(16), 1835–1847.[2]. Sánchez–Lozada LG, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose–induced metabolic syndrome. Am J Physiol Renal Physiol, 2008, 294(4), F710–F718.[3]. Sánchez–Lozada LG, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol, 2008, 108(4), p69–p78.[4]. Xu X, et al. Xanthine oxidase inhibition with febuxostat attenuates systolic overload–induced left ventricular hypertrophy and dysfunction in mice. Card Fail, 2008, 14(9), 746–753.Product Name:Febuxostat Cat. No.:HY-14268CAS No.:144060-53-7Molecular Formula:C 16H 16N 2O 3S Molecular Weight:316.37Target:Xanthine Oxidase Pathway:Metabolic Enzyme/Protease Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
特碳17-40B-PD 产品数据单据说明书

Product Data SheetTrigonox 17-40B-PD Butyl 4,4-di(tert-butylperoxy) valerateTrigonox® 17-40B-PD is a 40% formulation on an inert carrier system in powder form.CAS number995-33-5EINECS/ELINCS No.213-626-6TSCA statuslisted on inventoryMolecular weight334.5Active oxygen contentperoxide9.57%Concentration3.73-3.92%SpecificationsAppearance Off-white powderAssay39.0-41.0 %ApplicationsTrigonox® 17-40B-PD is a bifunctional peroxide which is used for the crosslinking of natural rubber and synthetic rubbers, as well as polyolefins. Safe processing temperature: 125°C (rheometer ts2 > 20 min.). Typical crosslinking temperature: 160°C (rheometer t90 about 12 min.).Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT60°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.30°CNote When stored under the recommended storage conditions, Trigonox® 17-40B-PDwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportThe standard packaging is a cardboard box for 25 kg peroxide formulation. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox®17-40B-PD is classified as Organic peroxide type E; solid, Division 5. 2; UN 3108.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® 17-40B-PD in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalines and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 17-40B-PD. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsMethane, Carbon dioxide, Acetone, tert-Butanol, n-Butyl propionate,All information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® is a registered trademark of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox 17-40B-PD。
小鼠脂联素(ADP)说明书

小鼠脂联素(ADP)酶联免疫分析试剂盒使用说明书南京金益柏ELISA试剂仅供研究使用目的:南京金益柏ELISA试剂盒用于测定小鼠血清,血浆及相关液体样本中脂联素(ADP)的含量。
实验原理:南京金益柏ELISA试剂盒应用双抗体夹心法测定标本中小鼠脂联素(ADP)水平。
用纯化的小鼠脂联素(ADP)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入脂联素(ADP),再与HRP标记的脂联素(ADP)抗体结合南京金益柏ELISA试剂盒,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,南京金益柏ELISA试剂盒并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的脂联素(ADP)呈正相关。
南京金益柏ELISA试剂盒用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中小鼠脂联素(ADP)浓度。
试剂盒组成:样本处理及要求:1. 南京金益柏ELISA试剂盒血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
2. 血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
3.南京金益柏ELISA试剂盒尿液:用无菌管收集,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
4. 细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4)稀释细胞悬液,细胞浓度达到100万/ml左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
保存过程中如有沉淀形成,应再次离心。
碧云天生物技术 Propidium Iodide 产品说明书

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号Propidium Iodide/碘化丙啶产品编号产品名称包装ST511 Propidium Iodide/碘化丙啶5mg产品简介:Propidium Iodide简称PI,中文名为碘化丙啶。
分子式为C27H34I2N4,分子量为668.40,纯度>95%。
进口分装,常用于细胞凋亡(apoptosis)或细胞坏死(necrosis)的检测,常用于流式细胞仪分析。
包装清单:产品编号产品名称包装ST511 Propidium Iodide/碘化丙啶5mg—说明书1份保存条件:4ºC避光保存。
注意事项:本产品对人体有刺激性,操作时请小心,并注意适当防护以避免直接接触人体或吸入体内。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
使用本产品的文献:1.Wu G, Liu ZS, Qian Q, Jiang CQ. Effects of Berberine on the Growth ofHepatocellular Carcinoma Cell lines. Medical Journal of Wuhan University. 2008 Jan;29(1).2.Liu Y, Sheng Z, Liu H, Wen D, He Q, Wang S, Shao W, Jiang RJ, An S,Sun Y, Bendena WG, Wang J, Gilbert LI, Wilson TG, Song Q, Li S.Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009 Jun;136(12):2015-25.3.Li DL, Liu JJ, Liu BH, Hu H, Sun L, Miao Y, Xu HF, Yu XJ, Ma X, RenJ, Zang WJ. Acetylcholine inhibits hypoxia-induced tumor necrosis factor-α production via regulation of MAPKsphosphorylation in cardiomyocytes. J Cell Physiol. 2011 Apr;226(4):1052-9.4.Cao X, Deng W, Wei Y, Su W, Yang Y, Wei Y, Yu J, Xu X. Encapsulationof plasmid DNA in calcium phosphate nanoparticles: stem cell uptake and genetransfer efficiency. Int J Nanomedicine. 2011;6:3335-49.5.Meng LY, Liu HR, Shen Y, Yu YQ, Tao X. Cochinchina momordica seedextract induces G2/M arrest and apoptosis in human breast cancerMDA-MB-231 cells by modulating the PI3K/Akt pathway. Asian Pac J Cancer Prev. 2011;12(12):3483-8.6.Zhao Q, Xue Y, Wang JF, Li H, Long TT, Li Z, Wang YM, Dong P, XueCH. In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearsonothuriagraeffei. J Sci Food Agric. 2012 Mar 15;92(4):965-74.7.Tu Z, Ma Y, Tian J, Li H, Akers W, Achilefu S, Gu Y. Estrogen receptor βpotentiates the antiproliferative effect of raloxifene and affects the cellmigration and invasion in HCT-116 colon cancer cells. J Cancer Res Clin Oncol. 2012 Jul;138(7):1091-103.8.Zhou Z, Wan Y, Zhang Y, Wang Z, Jia R, Fan Y, Nie H, Ying S, Huang P,Wang F. Follicular development and expression of nuclear respiratory factor-1 and peroxisome proliferator-activated receptor γ coactivator-1 alpha in ovaries of fetal and neonatal doelings. J Anim Sci.2012 Nov;90(11):3752-61.9.Jun Fang, Meihu Ma, Yongguo Jin, Ning Qiu, Chan Wang, Guodong Renand Xin Huang. Assessment of Salmonella enteritidis Viability in Egg White during Early Incubation Stages by Fluorescent Staining Method.Asian Journal of Animal and Veterinary Advances.7: 556-67. 10.Zhen-Jun S, Yuan-Yuan Z, Ying-Ying F, Shao-Ju J, Jiao Y, Xiao-Wei Z,Jian C, Yao X, Li-Ming Z.β,β-Dimethylacrylshikonin exerts antitumor activity via Notch-1 signaling pathway in vitro and invivo. Biochem Pharmacol. 2012 Aug 15;84(4):507-12.11.Tu Z, Li H, Ma Y, Tang B, Tian J, Akers W, Achilefu S, Gu Y. Theenhanced antiproliferative response to combined treatment of trichostatinA with raloxifene in MCF-7 breast cancer cells and its relevance toestrogen rec eptor β expression.Mol Cell Biochem. 2012 Jul;366(1-2):111-22.12.Feng C, Xu Z, Li Z, Zhang D, Liu Q, Lu L. Down-regulation of Wnt10aby RNA interference inhibits proliferation and promotes apoptosis in mouse embryonic palatal mesenchymal cells through Wnt/β-catenin signaling pathway. J Physiol Biochem. 2013 Dec;69(4):855-63.13.Hou Y, Chu M, Du FF, Lei JY, Chen Y, Zhu RY, Gong XH, Ma X, Jin J.Recombinant disintegrin domain of ADAM15 inhibits the proliferation and migration of Bel-7402 cells. Biochem Biophys Res Commun. 2013 Jun 14;435(4):640-5.14.Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, HanY, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013 Apr 30;8(4):e62363. 15.Zhou R, Huang W, Yao Y, Wang Y, Li Z, Shao B, Zhong J, Tang M,Liang S, Zhao X, Tong A, Yang J.CA II, a potential biomarker by proteomic analysis, exerts significant inhibitory effect on the growth of colorectal cancer cells. Int J Oncol. 2013 Aug;43(2):611-21.16.Wang Y, Jiang XL, Peng SW, Guo XY, Shang GG, Chen JC, Wu Q, ChenGQ. Induced apoptosis of osteoblasts proliferating on polyhydroxyalk anoates. Biomaterials. 2013 May;34(15):3737-46.17.Yang F, Huang W, Li Y, Liu S, Jin M, Wang Y, Jia L, Gao Z. Anti-tumoreffects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013 Jul;34(22):5689-99..18.Zhou S, Wu H, Zeng C, Xiong X, Tang S, Tang Z, Sun X. ApolipoproteinE protects astrocytes from hypoxia and glutamate-induced apoptosis.FEBS Lett. 2013 Jan 16;587(2):254-8.19.Nie C, Yang D, Liu N, Dong D, Xu J, Zhang J. Thyrotropin-releasinghormone and its analogs accelerate wound healing. J Surg Res. 2014 Jun 15;189(2):359-65.Version 2016.12.08。
Bitopertin_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-12-2017Print Date:Jun.-12-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :BitopertinCatalog No. :HY-10809CAS No. :845614-11-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word No data availableHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:RG1678; RO4917838; RG–1678; RG 1678; RO–4917838; RO 4917838Formula:C21H20F7N3O4SMolecular Weight:543.46CAS No. :845614-11-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
cas66981-73-5_Tianeptine_技术参数MedBio

1185241-83-1
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16717
Azelastine HCl
Azelastine HCl
79307-93-0
200mg
≥98%
cas
1、产品物理参数:
常用名
噻奈普汀
英文名
Tianeptine
CAS号
66981-73-5
分子量
436.952
密度
1.4±0.1 g/cm3
沸点
609.2±65.0 °C at 760 mmHg
分子式
C21H25ClN2O4S
熔点
129-131°C
闪点
322.2±34.3 °C
2、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16879
ML 289
ML 289
1382481-79-9
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16787
BMY 7378
BMY 7378
21102-95-4
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
BQCA338747Fra bibliotek41-410mg
≥98%
品牌
货号
中文名称
英文名称
Cisplatin_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Cisplatin is a potent inducer of growth arrest and/or apoptosis in most cell types.IC50 & Target: Apoptosis inducer [1]In Vitro: Cisplatin (CDDP) causes apoptosis of HeLa cells in a dose–dependent manner, with a concentration of 30 μM Cisplatin resulting in death of greater than 90% of the cell population by 24 h of treatment. The kinetics of Cisplatin–induced apoptosis are examined using a 30 μM concentration. Cisplatin Activates the MEK/ERK Signaling Pathway, 20 and 30 μM Cisplatin, both of which results in significant apoptosis, leads to strong activation of ERK [1]. Cisplatin (50 μM) produces time–dependent apoptosis in renal proximal tubular cell (RPTCs), causing cell shrinkage, a 50–fold increase in caspase 3 activity, a 4–fold increase in phosphatidylserineexternalization, and 5– and 15–fold increases in chromatin condensation and DNA hypoploidy, respectively [2]. In Vivo: In melanoma–bearing mice, Cisplatin (4 mg/kg B.W.) reduces the size and weight of the solid tumors, and HemoHIM supplementation with Cisplatin enhances the decrease of both the tumor size and weight [3]. Cisplatin administration results in significant increases in the kidney weight as a percentage of the total body weight, urine volume, serum creatinine, and blood urea nitrogen by about 132, 315, 797, and 556% in comparison with the control rats, respectively [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Cisplatin is dissolved in DMSO and stored, and then diluted with appropriate medium before use [1]. [1]HeLa and A549cells are maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units of Penicillin, and 100 μg of Streptomycin/mL. They are cultured at 37°C in a humidified chamber containing 5% CO 2. For the induction of apoptosis,cells are plated in 60–mm dishes 1 day prior to Cisplatin (0–30 μM) treatment [1].Animal Administration: Cisplatin is prepared in water (Mice)[3].Cisplatin is prepared in physiological saline solution (Rat)[4].[3][4]Mice [3]Mice are divided randomly into three groups (Control, Cisplatin and Cisplatin+HemoHIM), and each group consists of twenty mice.B16F0 melanoma (5×105 cells/mouse) is inoculated into subcutaneous femoral left region of mice at 3 days before an initial injection of Cisplatin. Cisplatin is injected intraperitoneally at 4 mg/kg body weight (B.W.) on day 0, 7 and 14 (total threeinjections). Experimental group is intubated with HemoHIM at a final concentration of 100 mg/kgB.W. by everyday from day –1 to day 16, while the control group received only water. On day 17 after initial injection of Cisplatin, all mice of each group are experimented, respectively, to evaluate tumor weight or tumor size. The tumor size is calculated as follows: tumor size=ab 2/2, where aand b are the larger and smaller diameters, respectively.Rat [4]Male Sprague–Dawley rats weighing 200 to 250 g are divided at random into 4 groups of 4 or 5 animals each. The first group (control)received a vehicle (5% carboxymethyl cellulose sodium solution (CMC–Na), 5 mL/kg body wt., p.o.) used for Capsaicin (Cap). The second group received Cap (10 mg/kg/d, p.o.) in 5% CMC–Na (5 mL/kg), and the third received 5% CMC–Na for 6 consecutive daysProduct Name:Cisplatin Cat. No.:HY-17394CAS No.:15663-27-1Molecular Formula:Cl 2H 6N 2Pt Molecular Weight:300.05Target:Apoptosis Pathway:Apoptosis Solubility:DMSO: ≥ 31 mg/mL (DMSO can inactivate Cisplatin's activity;please see the reference [5]).injected with Cisplatin (5 mg/kg in physiological saline solution, i.p.). The fourth group received Cap (10 mg/kg/d, p.o.) in 5% CMC–Na for 6 consecutive days after Cisplatin injection (5 mg/kg, i.p.). For all groups, Cap or vehicle is given twice daily. The selected Cap concentration and the dose administration schedule without inducing any rat intestinal damage are chosen using data from our preliminary experiments.References:[1]. Wang X, et al. Requirement for ERK activation in cisplatin–induced apoptosis. J Biol Chem. 2000 Dec 15;275(50):39435–43.[2]. Cummings BS, et al. Cisplatin–induced renal cell apoptosis: caspase 3–dependent and –independent pathways. J Pharmacol Exp Ther. 2002 Jul;302(1):8–17.[3]. Park HR, et al. Enhanced antitumor efficacy of cisplatin in combination with HemoHIM in tumor–bearing mice. BMC Cancer. 2009 Mar 17;9:85.[4]. Shimeda Y, et al. Protective effects of capsaicin against cisplatin–induced nephrotoxicity in rats. Biol Pharm Bull. 2005 Sep;28(9):1635–8.[5]. Hall MD, et al. Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res. 2014 Jul 15;74(14):3913–22.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
细胞周期试剂盒(C1052)

1000g左右离心3-5分钟,沉淀细胞。对于特定的细胞,如果细胞沉淀不充分,可以适当延长离心时间或稍稍加大离心 力。小心吸除上清,可以残留约50微升左右的70%乙醇,以避免吸走细胞。加入约1毫升冰浴预冷的PBS,重悬细胞。再 次离心沉淀细胞,小心吸除上清,可以残留约50微升左右的PBS,以避免吸走细胞。轻轻弹击离心管底以适当分散细 胞,避免细胞成团。
胞DNA含量分析和光散射分析。
细胞周期与细胞凋亡检测试剂盒
产品编号 C1052
产品名称 细胞周期与细胞凋亡检测试剂盒
包装 50 次
产品简介:
¾ 碧云天生产的细胞周期与细胞凋亡检测试剂盒(Cell Cycle and Apoptosis Analysis Kit)是一种采用经典的碘化丙啶染色 (Propidium staining,即PI staining)方法进行细胞周期与细胞凋亡分析的检测试剂盒。
注:配制好的碘化丙啶染色液短时间内可以4℃保存,宜当日使用。
4. 染色:每管细胞样品中加入0.5毫升碘化丙啶染色液,缓慢并充分重悬细胞沉淀, 37℃避光温浴30分钟。随后可以4℃或
冰浴避光存放。染色完成后宜在24小时内完成流式检测,最好能在当日完成流式检测。
5. 流式检测和分析:用流式细胞仪在激发波长488nm波长处检测红色荧光,同时检测光散射情况。采用适当分析软件进行细
使用说明:
1. 细胞样品的准备: a. 对于贴壁细胞:小心收集细胞培养液到一离心管内备用。用胰酶消化细胞,至细胞可以被轻轻用移液管或枪头吹打 下来时,加入前面收集的细胞培养液,吹打下所有的贴壁细胞,并轻轻吹散细胞。再次收集到离心管内。1000g左右 离心3-5分钟,沉淀细胞。对于特定的细胞,如果细胞沉淀不充分,可以适当延长离心时间或稍稍加大离心力。小心 吸除上清,可以残留约50微升左右的培养液,以避免吸走细胞。加入约1毫升冰浴预冷的PBS,重悬细胞,并转移到 1.5毫升离心管内。再次离心沉淀细胞,小心吸除上清,可以残留约50微升左右的PBS,以避免吸走细胞。轻轻弹击 离心管底以适当分散细胞,避免细胞成团。 b. 对于悬浮细胞:1000g左右离心3-5分钟,沉淀细胞。对于特定的细胞,如果细胞沉淀不充分,可以适当延长离心时间 或稍稍加大离心力。小心吸除上清,可以残留约50微升左右的培养液,以避免吸走细胞。加入约1毫升冰浴预冷的 PBS,重悬细胞,并转移到1.5毫升离心管内。再次离心沉淀细胞,小心吸除上清,可以残留约50微升左右的PBS,以 避免吸走细胞。轻轻弹击离心管底以适当分散细胞,避免细胞成团。
Emamectin_Benzoate_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Emamectin Benzoate works as a chloride channel activator by binding gamma aminobutyric acid (GABA) receptor andglutamate–gated chloride channels disrupting nerve signals within arthropods.Target: GABA ReceptorEmamectin Benzoate stimulates the release of GABA from the synapses between nerve cells and while additionally increasing GABA's affinity for its receptor on the post–junction membrane of muscle cells in insects and arthropods.PROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]Emamectin benzoate was dissolved in acetone to various concentrations (200, 100, 50, 25, 10, 5.0 and 1.0 mg a.i./L). The nematicidal efficacy of Emamectin benzoate against J2 of M. incognita was determined in aqueous tests. Emamectin benzoate treatments (200,100, 50, 25, 10, 5.0 and 1.0 mg a.i./L) were prepared in acetone + distilled water (10: 90% by volume), and distilled water, as well as a mixture of water with acetone at concentrations equivalent to those in the treatment wells, were used as controls. Then 1 mL ofsolution and 1 mL of root–knot nematodes J2 (containing average 150 J2) was added to each well of a 24–well plate. Well plates were wrapped with parafilm, placed in plastic zip–lock bags and stored in aluminum foil pans covered with another pan to keep them dark.Units were kept at 25°C. After 48 h, the relative percentages of the motile and immotile J2 were evaluated using an invertedmicroscope at 40×magnification. Furthermore, nematodes were moved to distilled water after washing in tap water through a 20 μm pore screen to remove excess chemicals. To confirm the nematicidal activity of emamectin benzoate, immobile 30 J2 from each treatment were collected from the above experiments, transferred to tissue culture plates filled with water, and monitored for 12 h.The experiments had five replications and were repeated three times.References:[1]. Cheng X, et al. Effect of Emamectin Benzoate on Root–Knot Nematodes and Tomato Yield. PLoS One. 2015 Oct 28;10(10):e0141235.Product Name:Emamectin (Benzoate)Cat. No.:HY-B0837CAS No.:155569-91-8Molecular Formula:C 104H 154N 2O 28Molecular Weight:1880.33Target:GABA Receptor; GABA Receptor Pathway:Neuronal Signaling; Membrane Transporter/Ion Channel Solubility:DMSO: ≥ 31 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
F-STOP 对照图片,病毒免疫咖啡草坛说明书

F -S T O P ™ F o r P i c t u r e -P e r f e c t , D i s e a s e -F r e e T u r f g r a s s 12770• Provides Systemic Prevention And Control Of Turfgrass Diseases• Prevents Over 15 Major Lawn Diseases • For Use On All Types Of Home Lawns • One Application Protects For Up To 4 WeeksKEEP OUT OF REACH OF CHILDRENCAUTIONBUYER ASSUMES ALL RISKS OF USE, STORAGE OR HANDLING OF THIS MATERIALNOT IN STRICT ACCORDANCE WITH DIRECTIONS GIVEN HEREWITH.NET WEIGHT 10 LBS. (4.53 KG)ACTIVE INGREDIENT:myclobutanil: a-butyl-a-(4-chlorophenyl)-1-H-1,2,4 triazole-propanenitrile: ...............0.39%OTHER INGREDIENTS: .............................................................................................99.61%TOTAL .....................................................................................................................100.00%This product contains 0.195 lb.. of myclobutanil per 50 lb. bag.C o v e r s U p T o 2,500 S q . F t .F-STOP ™ For Picture-Perfect, Disease-Free TurfgrassF -S T O P ™F o r P i c t u r e -P e r f e c t , D i s e a s e -F r e e T u r f g r a s sF-STOP ™For Picture-Perfect, Disease-Free TurfgrassPRECAUTIONARY STATEMENTSHAZARDS TO HUMANS AND DOMESTIC ANIMALSCauses Moderate Eye Irritation.Avoid contact with eyes or clothing. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum, using tobacco, or using the toilet.Notice: Read the entire label. Use only according to label directions. Before using this product, read “Warranty Disclaimer,” “Inherent Risks of Use,” and “Limitation of Remedies” at end of Directions for Use. If terms are unacceptable, return at once unopened.In case of emergency endangering health or the environment involving this product,call 1-800-992-5994Agricultural Chemical: Do not ship or store with food, feeds, drugs, or clothing.FIRST AIDIf in eyes: Hold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. Call a Poison Control Center or doctor for treatment advice. Have the product container or label with you when calling a Poison Control Center or doctor, or going for treatment. You may also contact 1-800-992-5994 for emergency treatment information.ENVIRONMENTAL HAZARDSThis pesticide is toxic to fish. To protect the environment, do not allow pesticide to enter or run off into storm drains, drainage ditches, gutters or surface waters. Applying this product in calm weather when rain is not predicted for the next 24 hours will help ensure that wind or rain does not blow or wash pesticide off the treatment area. Sweeping any product that lands on a driveway, sidewalk, or street, back onto the treated area of the lawn or garden will help to prevent runoff to water bodies or drainage systems.DIRECTIONS FOR USEIt is a violation of Federal law to use this product in a manner inconsistent with its labeling. Read all directions carefully before applying this product.Not for use on turfgrass being grown for sale or other commercial use as sod, or for commercial seed productions, or for research purposes.STORAGE AND DISPOSALDo not contaminate water, food or feed by storage and disposal.PESTICIDE STORAGE: Keep away from fire and sparks. Store in a cool, dry, well-ventilated area. CONTAINER HANDLING: Nonrefillable container. Do not reuse or refill this container.If empty: Place in trash or offer for recycling if available.If partly filled: Call your local solid waste agency for disposal instructions. Never place unused product down any indoor or outdoor drain.HOW THIS PRODUCT WORKSferti•lome® F-STOP™is a systemic, protectant and curative fungicide for the control of listed diseases in established home lawns and ornamental turfgrass. Optimum disease control is achieved when this product is applied to established turfgrass in a regularly scheduled preventative program. Use this product in conjunction with turf management practices that promote good plant health and optimum disease control. The key to selecting a fungicide is the proper diagnosis of the organism causing the disease. Diagnostic kits, extension experts, or other identification methods should be used when developing disease control strategies.HOW TO APPLYApply ferti•lome® F-STOP™ uniformly over the turfgrass area using a properly calibrated drop or rotary-type spreader designed to apply granules. Before each application, calibrate the spreader according to the equipment manufacturer’s directions. Check frequently to make sure equipment is operating properly and distributing product uniformly. A more uniform application may be achieved by spreading half of the required amount of product over the area and then applying the remaining half in swaths at a right angle to the first. Avoid skips and excessive overlaps during application. Avoid the use of spreaders that apply this product in narrow rows or concentrated bands.Wash hands with soap and water promptly after use.Do not allow people or pets to contact treated area until after product dust has settled into the turfgrass, or if watered in, after the turfgrass surface in the treated area has dried.WHEN TO APPLYReduce the interval between applications of this product when conditions are favorable for disease development. Unless otherwise specified, when disease pressure is high or when used as a curative, use higher rates of ferti•lome® F-STOP™ and shorter application interval. Under light to moderate disease pressure apply this product at the low use rate and/or longer application intervals. To avoid pick-up, lightly irrigate treated areas soon after application. On short cut bentgrass (1/2 inch or less) when temperatures are above 80°F, apply only to dry foliage.HOW MUCH TO APPLYOptimum disease control is achieved when ferti•lome® F-STOP™ is applied in a preventative disease control program at a rate of 4 lb per 1,000 sq. ft. See the following table for specific application rates for various diseases. Under any circumstances, do not apply more than 46 lb of this product per1,000 sq. ft. per year.SPREADER GUIDEONE BAG WILL COVER UP TO 2,500 SQUARE FEETSPREADER SPREADER SETTINGS Scotts®/Republic Accugreen (Drop) 4 1/4Scotts®/Republic Speedy Green (Broadcast) 3 3/4 ferti•lome® /EarthWay Ev-N-Spred (Broadcast)14TERMS AND CONDITION OF USEIf terms of the following Warranty Disclaimer, Inherent Risks of Use, and Limitation of Remedies are not acceptable, return unopened package at once to the Seller for a full refund of purchase price paid. Otherwise, use by the Buyer or any other user constitutes acceptance of the terms under Warranty Disclaimer, Inherent Risks of Use, and Limitations of Remedies.WARRANTY DISCLAIMERSeller warrants that this product conforms to the chemical description of the label and is reasonably fit for the purposes stated on the label when used in strict accordance with the directions, subjectto the Inherent Risks set forth below. Seller MAKES NO OTHER EXPRESS OR IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER EXPRESS OR IMPLIED WARRANTY.INHERENT RISKS OF USEIt is impossible to eliminate all risks associated with use of this product. Plant injury, lack of performance, or other unintended consequences may result because of such factors as use of the product contrary to label instructions (including conditions noted on the label, such as unfavorable temperature, soil conditions, etc.), abnormal conditions (such as excessive rainfall, drought, tornadoes, hurricanes), presence of other materials, the manner of application, or other factors, allof which are beyond the control of the Seller. To the extent allowed by law, all such risks shall be assumed by the Buyer.LIMITATION OF REMEDIESTo the extent consistent with applicable law, the exclusive remedy for losses or damages resulting from this product (including claims based on contract, negligence, strict liability, or other legal theories), shall be limited to, at Seller’s election, one of the following:1. Refund of purchase price paid by Buyer or user for product bought, or2. Replacement of amount of product used.To the extent allowed by law, Seller shall not be liable for the losses of damages resulting from the handling or use of this product unless Seller is promptly notified of such loss or damage in writing. In no case shall Seller be liable for consequential or incidental damages or losses.The terms of the Warranty Disclaimer and Inherent Risks of Use above and this Limitation of Remedies cannot be varied by any written or verbal statements or agreements. No employee or sales agent of Seller or the Seller is authorized to vary or exceed the terms of the Warranty Disclaimer or this Limitation of Remedies in any manner.Disease ferti•lome® F-STOP™(lb/1,000 sq ft)ApplicationInterval/Timing(Days)Directions RestrictionsAnthracnose Red Thread Septoria Leaf Spot 414 - 21Apply when conditions are favorable for disease development.Do not apply more than 46pounds of product per 1,000sq. ft. per year.For Nassau and Suffolkcounties in New York State,do not apply more than 11.5lb of this product per 1,000sq ft per year.Brown Patch14Begin applications when conditions are favorable for disease development andb efore disease symptoms are apparentCopper SpotZonate Leaf SpotApply when conditions are favorable for disease development.Crown Rot Leaf Spot Melting-Out Apply when conditions are favorable for disease development. For crown rot, water in with 3 to 4 gallons of water per 1,000 sq. ft. to increase penetration to crown a nd roots.Dollar Spot14 - 28Apply when conditions are favorable for disease development. Make no morethan 3 consecutive applications for Dollar Spot control before rotating to aregistered fungicide with a diiferent mode of actionFusarium Blight14 - 21Apply when conditions are favorable for disease development.Fusarium Patch(Pink Snow Mold)21 - 28Apply when conditions are favorable for disease development.Gray Leafspot14Apply prior to snow cover.Leaf Spot Apply in the fall after turfgrass enters dormancy and/or in the spring prior to theinitiation of growth.Necrotic Ring Spot Spring: 28Make applications on a preventative basis in early to mid-spring.Necrotic Ring Spot Fall: 28Make 2 applications beginning in August before the turfgrass goes dormant.Powdery MildewRusts14 - 28Apply when conditions are favorable for disease development.Summer Patch Begin applications in the spring when conditions are favorable for disease de-velopment. Make 2 to 4 applications depending on recommendations from localTurfgrass Extension Experts.Water in with at least 3 to 4 gallons of water per 1,000 sq. ft. to increase penetra-tion to crown and roots.Manufactured by:230 FM 87 • BONHAM, TEXAS 75418EPA Reg. No. 62719-461-7401 EPA Est. No. 7401-TX-01Visit Us At: Product Questions? 855-270-477612770-0515-TP。
医学常用试剂缩写

英文全称(缩写)A abso rbanc e(A)a ccura cy(Ac c)a cquir ed pu re am egaka ryocy tic t hromb ocyto penic purp ura(A PATP)acq uired vonWille brand dise ase(a vWD)acti n act in cy toske leton acti vated clot tingtime(ACT)acti vated part ial t hromb oplas tin t ime(A PTT)acti vated prot ein C(APC)act ivate d pro teinC rec eptor(APCR)ac tivat ed pr otein C re sista nce(A PCR)Cact ivati on re cepto r act omyos ina cutepromy elocy tic l eukem ia(AP L)a cyl-p lasmi nogen SK a ctiva tor c omple x(APS AC)中文名称吸光度准确度获得性单纯无巨核细胞性血小板减少性紫癜获得性血管性血友病肌动蛋白肌动蛋白细胞骨架活化凝血时间活化部分凝血活酶时间活化蛋白活化蛋白C受体活化蛋白C抵抗Aden osine aden osine diph ospha te(AD P) 激活受体adeno sinetriph ospha te(AT P) 肌动球蛋白aden ylate cycl ase(A C) 急性早幼粒细胞白血病adhe sionadhes ion d efect adhe siveprote in AD P rec eptor酰基化纤溶酶原-链激酶激活剂复合物α2-a drene rgicrecep tor(α2-AR)腺苷adre nalin e (Ad r) 二磷酸腺苷afib rinog enemi a agg regat ion 三磷酸腺苷agg regat ion d efect aggr egati on ti me(Ta) 腺苷酸环化酶allo antib ody a lloim munethrom bocyt openi a 黏附α-f etalprote in (A FP) (血小板)黏附障碍anal ytica l pro cessanaly tical vari abili ty an giopl asty黏附蛋白ann exinⅡ AD P受体anne xin Ⅴα2肾上腺素能受体an ticoa gulan t pro teinanti-nucle ar an tibod y(ANA) 肾上腺素无纤维蛋白原血症聚集(血小板)聚集缺陷聚集时间同种抗体同种免疫性血小板减少症甲胎蛋白分析过程分析变异血管成形术连接素Ⅱ连接素Ⅴ抗凝蛋白抗核抗体续表英文全称(缩写)anti phosp holip id sy ndrom e(APS)an tithr ombin(AT)anti throm bin Ⅲ(AT-Ⅲ)an tithr ombinⅢ sy stemα2-a ntipl asmin(α2-A P)α1-ant itryp sin a orta(Ao) APC s ensit ivity rati o(APC-SR)apop rotei n A-Ⅱara chido nic a cid(A A,ARA CH) arbit raryunit(AU) areaunder therecei ver o perat or cu rve(A UC) Arg-G ly-As p(RGD)ar tifac t art erial(Art)中文名称抗磷脂综合征抗凝血酶抗凝血酶Ⅲ抗凝血酶Ⅲ系统α2-抗纤溶酶α1-抗胰蛋白酶主动脉活化蛋白C敏感比值载脂蛋白A-Ⅱ花生四烯酸a rteri al sh ear s tress arte ry as pirin(ASA)任意单位(为PA I活性单位)as pirin tole rance test(AIT) athe rothr ombot ic br ain i nfarc tionather othro mboti c occ lusiv e dis easeatomi c for ceful micr oscop e(AFM) ATP diph ospho hydro lase(ATDPa se) ROC曲线下面积精氨酸-甘氨酸-天门冬酰胺au ramin e ora nge(A O) 假象au toant ibody auto immun e thr omboc ytope nic p urpur a aut oimmu ne th rombo cytop enicpurpu ra se conda ry to drug s aut omate d car tridg e-bas ed sy stemautom atedcoagu latio nana lyzer auto mated dilu tionautom atedimmun oassa y sys tem(A IS) B主动脉的动脉剪切应力动脉阿司匹林阿司匹林耐量试验动脉粥样硬化血栓形成性脑梗死动脉血栓闭塞症原子能显微镜AT P双磷酸水解酶金胺橙自身抗体自身免疫性血小板减少性紫癜药物介导的血小板减少性紫癜自动检测系统自动血凝分析仪自动稀释baboo n bar code read ing B ernar d-Sou liersyndr ome(B SS) biasbioch emist ryb iolog y bio senso rbl eedin g tim e(BT)ble eding vess el bl eedin g vol ume b loodureanitro gen(B UN) by-pr oduct s自动免疫分析系统狒狒条形码阅读巨大血小板综合征偏倚生物化学生物学生物传感器出血时间出血血管出血量血尿素氮副产品续表英文全称(缩写)C C1 inhi bitor(C1-I NH) C1-es teras e inh ibito r C4b bind ing p rotei n(C4b-BP)cana licul ar sy stemcapil lary(Cap)carb oxype ptida se Bcarci no-em bryon ic an tigen(CEA)car diopu lmona ry by pass(CPB)caro tid a rtery cata lytic tria d cat hepsi n G(C G)G CBMdi sc ce ll me mbran e(CM)cel lulos e mem brane cent ipois e(CP)cer ebral infa rctio n che mo-at tract ant α-che mokin e rec eptor(CXCR4)c hlori mipra minechrom genic pept ide s ubstr ate a ssaychrom genic subs trate assa y chr oniclymph ocyti c leu kemia(CLL)chy motry psin(CT) 中文名称C1抑制剂C1弹性蛋白酶抑制剂C4b结合蛋白管道系统毛细血管羧基肽酶B前体癌胚抗原心肺分流术颈动脉催化三联体组织蛋白酶中国生物医Cl inica l Lab orato ry Im prove mentAmend memt88(CL IA88)cli nical mani festa tions clot form ation rate clot modu lus c lot r etrac tiontest(CRT)Clot Sign ature Anal yzer(CSA)clot-base d ass ay cl oaing time(CT)clus tersof di ffere ntiat ion(C D)c oagul ablefacto r(F)coag ulabl e pro teincoagu latio n ana lyzer coag ulati on ti me(CT)Co chran e Col labor ation Cochr ane c oeffi cient of v ariat ion f or re plica te te st co facto r pro teincoldagglu tinin s col d-ind ucedactiv ation of i ntrin isiccoagu latio nsys tem c ollag en(Co l,COL L)c ollag en in duced coag ulant acti vity(CICA)col lagen indu ced t hromb us fo rmati on co llage n mem brane s学文献光盘数据库细胞膜细胞质膜厘泊(黏度单位)脑梗死趋化吸引剂α-趋化因子受体氯化丙咪嗪产色多肽基质法产色底物法慢性淋巴细胞性白血病糜蛋白酶(美国)临床实验室修正案88临床表现血块形成率血块系数血块收缩试验血块止血仪凝固法凝血时间分化抗原凝血因子凝血蛋白血凝分析仪凝固时间协作网重复试验的变异系数辅因子蛋白冷凝素冷诱导内源凝血系统的激活胶原胶原诱导的凝血活性胶原诱导的血栓形成胶原膜续表英文全称(缩写)中文名称Coll ege o f Ame rican Path ologi sts(C AP)美国临床病理家协会comm on pa thway comp uterinter facin g com pleme nt 1r/s co nditi onalmeasu res c ongen italdefic iency stat e con siste nt mo tionconst ituti ve ex press ion(C)co ntact acti vatio n pat hwayconta ct pr oduct-form ing a ctivi ty(CP FA) conta ct-de pende nt fi brino lytic syst em co ntinu ous q ualit y imp rovem ent C oombs test co-r ecept or co ronar y(Cor)co st re ducti on cr eatin ine(C r)c rosse d imm unoel ectro phore sis c ross-linke d fib rin c ryofi brino genem iac ryogl obuli n cum ulati ve su m(CUS UM) cut o ff va lue c yclic aden osine mono phosp hate(cAMP)cys tineprote ase p32(Cp p-32)cyt oskel etonstruc turecytos tatic drug D共同途径计算机界面补体1r/s条件衡量指标先天性缺陷恒定的运动细胞膜结构接触激活途径接触产物生成活性接触依赖性纤溶系统持续质量改善抗人球蛋白试验辅助受体冠状动脉成本降低肌酐交叉免疫电泳交联纤维蛋白冷纤维蛋白原血症冷沉淀球蛋白累积和判断界值环磷酸腺苷半胱氨酸蛋白酶细胞骨架结构抑制细胞药物dark fiel d mic rosco pe da ta ma nagem ent D-dime r(D-D) 暗视野显微镜deep vein thro mbosi s(DVT)数据管理D-二聚体深静脉血栓患者结果差值控制变性梯度凝胶电泳致密颗粒致密中心小体致密管道系统撰写计划书诊断比数比诊断性试验伴有原发性纤溶的DIC D IC计分系统差别证实偏倚稀释蝰蛇毒试验双嘧达莫(潘生丁)续表Delt a Che ck de natur ing g radie nt ge l ele ctrop hores is(DG GE) dense body(DB)dens e cen tralmassdense tubu lar s ystem(DTS)dev elopi ng aproto col d iagno sticoddsratio(DOR)dia gnost ic te st DI C acc ompan ied b y pri maryfibri no(ge no)ly sis D IC sc oring syst em di ffere ntial veri ficat ion b ias d ilute Russ ell v ipervenom time(DRVV T)d ipyri damol e(DPM)英文全称(缩写)d iscoi d dis semin atedintra vascu lar c oagul ation(DIC)DNA chip Dopp ler doubl e str anded-DNA(ds-DA N)d ry re agent tech nolog y dup lex s canni ng dy sfibr inoge nemiadys prote inemi as Eecto-enzy me el astas eel astas e-med iatefibri n deg radat ion e lctro mecha nical dete ction syst emel ectri cal i mpeda nce e lectr oimmu nodif fusio n(ELD)el ectro n mic rogra ph el ectro n mic rosco pe(EM)中文名称圆盘状弥散性血管内凝血基因芯片多普勒双链脱氧核糖核酸干化学技术双显性扫描异常纤维蛋白原血症异常蛋白血症胞外酶弹性蛋白酶弹性蛋白酶介导的纤维蛋白降解elect ropho retic mobi lityelect ro-op tical dete ction syst em el ectro- 电机学检测系统opti cal d etect or el imina tion电阻抗ell iptic al EM base电泳免疫扩散endog enous inhi bitor of f ibrin olysi s end othel ial c ell(E C) 电子微动描记图e ndoth elial cell grow th fa ctor(ECGF)电子显微镜endot helia l pro teinC rec eptor(EPCR)电泳迁移率endo theli n(ET)光电检测系统endo theli n-1 (ET-1)光电检测器endot heliu m der ivati ve re laxin g fac tor(E DRF)清除endo toxin enzy me 椭圆形enzym e imm unoas say(E IA) 荷兰医学文摘e nzyme link ed im munos orben t ass ay(EL ISA)内源性纤溶抑制剂epi derma l gro wth f actor(EGF)内皮细胞e pinep hrine(EPI)内皮细胞生长因子E-selec tin e ssent ial t hromb ocyth emia(ET) 内皮细胞C受体estro gen r ecept or(ERβ)e thyle nedia min t etra-aceti c aci d(EDT A)e ugobu lin l ysistime(ELT)Euro peanWorki ng Gr oupin g onClini cal C ell A alysi s Eva ns sy ndrom e exo genou sact ivati on pa thway内皮素内皮素-1内皮衍生松弛因子内毒素酶酶免疫法酶联免疫吸附试验表皮生长因子肾上腺素E-选择素原发性血小板增多症雌激素受体B乙二胺四乙酸优球蛋白溶解时间欧洲临床细胞分析工作委员会自身免疫性溶血性贫血伴免疫性血小板减少外源激活途径英文全称(缩写)exper iment test s ext ernal coat(EC)exte rnalquali ty as sessm ent(E QA) exter nal v alidi ty ex trace llula r mat rix(E CM) extra vascu lar f ibrin degr adati on ex trins ic ac tivat ion p athwa y ext rinsi c pat hwayextri nsicpathw ay in hibit or(EP I)e xtrin sic t enase(ET)F续表中文名称试验性检查细胞外衣室间质量评价外部真实性细胞外基质血管外的纤维蛋白降解外激活途径外源性途径FⅡ rece ptor(FⅡR)fact or Ⅺdefic iency fals e neg ative rate(FNR)fal se po sitiv e rat e(FPR)fe moral arte ry(Fe m A)fibr in (f ibrin ogen) degr adati on pr oduct s(FDP)fi brinmonom ers(F M)f ibrin pept ide A(FPA)fib rin p eptid e B(F PB) fibri n thr ombifibri n(oge n) de grada tionprodu cts(F DPs)fibr inino lytic rece ptorfibri nogen(FIB)fib rinol yticsyste m fib rinop eptid efi brobl ast f ibron ectin(FN)外源性途径抑制剂外源性X酶因子Ⅱ受体因子Ⅺ缺乏症假阴性率假阳性率股动脉纤维蛋白(原)降解产物纤维蛋白单体纤维蛋白肽纤维蛋白肽B纤维蛋白血栓纤维蛋白(原)降解产物f ilter blee dingtime(FBT)纤溶受体fil ter o cclus ion f lexib le re agent choi ce fl ip-fl op fl ow cy tomet ric a nalyz er(FC A) fl owcy tomet ry(FC M) fl uores cence ener gy re sonan ce fl uores cence micr oscop e(FM)fluo resce nce r esona nce e nergy tran sfer(FRET) fluo resce nt an tibod y tec hniqu e fre eosc illat ing r heome try f ree p rotei n S(F PS) S froze n pla sma G纤维蛋白原纤溶系统纤维蛋白肽成纤维细胞纤维连接蛋白过滤器出血时间滤器阻塞灵活的试剂选择转向反方向流式细胞仪流式细胞术荧光能共振荧光显微镜荧光共振能量转移荧光抗体技术非振动流变术游离蛋白冰冻血浆Gprote in-co upled rece ptorG pro tein-coupl ed se ven t ransm embra ne do main续表英文全称(缩写)中文名称ge neral izabi litygiant plat eletsyndr ome G lanzm ann t hromb asthe nia(G T) 适用性g lassbeadcolum n ass ay gl ass f iberfilte r Glu tamic-plas minog en巨大血小板综合征gl ycoge n(Gly)血小板无力症glyco prote in co nform ation glyc oprot ein(G P)玻珠柱法β2-gly copro teinI gly copro teinⅡb/Ⅲa(GPⅡb/Ⅲa)玻璃纤维过滤器g lycos amino glyca n gly cosyl-phos phati dyl i nosit ol(GP I)gl ycyl-L-pro ly-L-argin yl-L-proli ne Go lgi a ppara tus,G olgizone(GZ) graft vers us ho st di sease(GVHD)gr anula r mem brane prot ein -140(G MP-140)α-gran ules(G)g ray p latel et sy ndrom e(GPS)gr owthfacto r GTP-bind ing p rotei n -co upled rece ptor(GPCR)H谷氨酸纤溶酶原糖原颗粒糖蛋白构型糖蛋白β2-糖蛋白I糖蛋白Ⅱb/Ⅲa糖胺聚糖糖基-磷脂肌醇甘氨酸-L-前赖氨酸-L-精氨酸-L-脯氨酰胺高尔基体移植物抗宿主病α颗粒膜蛋白-140α-颗粒灰色血小板综合征hair cell leuk emiaHealt h Pla nning Data baseand A idsli ne he althcareorgan izati on he matoc rit(H ct) hemol ysis,eleva ted l iverenzym es an d low plat elet(HELLP)he molyt ic ur emicsyndr ome(H US) hemop hilia hemo phili a A(H A)h emoph iliaB(HB)hem orheo logic anal yzerhemor rhagi c dis easehemos tatic defe ct he mosta tic f uncti on he mosta tus p latel et fu nctio n(HPF)He noch-Sehnl ein p urpur a hep aransulfa te pr oteog lycan s(HSP G)h epara n-ant ithro mbinsyste m hep aransulfa te(HS)he parin hepa rin d ose r espon se(HD R)h epari n cof actor-Ⅱ(HC-Ⅱ) hepar in ma nagem ent t est(H MT) hepar in pr otami ne ti trati on as say(H PT)生长因子G TP结合蛋白耦联受体毛细胞白血病卫生计划和医疗辅助数据库卫生保健机构血细胞比容溶血、肝酶升高和血小板减低溶血尿毒症综合征血友病血友病A血友病B血流变学分析仪出血性疾病止血缺陷止血功能止血血小板功能过敏性紫癜硫酸乙酰肝素蛋白多糖乙酰肝素一抗凝血酶系统硫酸乙酰肝素肝素肝素剂量应答试验肝素辅因子Ⅱ肝素治疗剂量监测肝素鱼精蛋白效价试验续表英文全称(缩写)中文名称h epari n-ind ucedthrom bocyt openi a typ e 2(H IT2)肝素相关性血小板减少症2型hepa rin-l ike a ntico agula nts h epari n-neu trali zed t hromb in ti me(HN TT) hered itary hemo rrhag ic te langl ectas ia(HH T)h eredi taryproth rombi n def icien cy he terog eneit yhi gh do se th rombi n tim e(HiT T)h igh m olecu lar w eight kini nogen(HMWK)hi gh ra nge h epari nasetestcartr idge(HR-HT C)h igh s hearcondi tionhigh-molec ular-weigh t vWF mult imers hiru din histi dinerichglyco prote in(HR G)h omocy stine(HCY)hor izont al sl it ho rmone repl aceme nt th erapy hors eradi sh pe roxid ase(H RP) human plat eletantig en(HP A)13-hyd roxy-octad ecadi enoic acid(13-H ODE)5-hy droxy-tryp tamin e(5-H T)5-hydr oxy-t rypta mine2A(5-HT2A)hyp eragg regat ion h yperh omocy stine mia肝素样抗凝物质肝素中和凝血酶时间遗传性出血性毛细血管扩张症遗传性凝血酶原缺乏症异质性高剂量凝血酶时间高相对分子质量激肽原高浓度肝素酶试验高切流状态高相对分子质量vW F多聚体水蛭素富含组氨酸糖蛋白hypo fibri nogen emiahypop rocon verti nemiaid iopat hic t hromb ocyto penic purp ura(I TP)同型半胱氨酸水平裂口激素替代疗法辣根过氧化物酶人类血小板抗原13-羟-十八碳二烯酸5-羟色胺5-羟色胺受体聚集增加高同型半胱氨酸血症低纤维蛋白原血症FⅦ缺陷症特发性血小板减少性紫癜idio pathi c/imm une t hromb ocyto penia(ITP)Ig-likedomai n imi prami nei mmuno radio metri c ass ay(IR MA) imped anceanaly sis i n sit u hyb ridiz ation inco rpora tionbiasindus trial qual ity m anage mentinfer ior v ena c ava(I VC) infle ction inhi bitor of c oagul ation syst em in itial flow rate(IF)init iatio n ini tiati ng co mplex es in itial blee dingrateinner pist on in osito l tri phosp hate(IP3)inos itol-1,4,5-trip hosph ate(I P3)原发性/免疫性血小板减少症免疫球蛋白样结构丙咪嗪免疫放射测定阻抗分析原位杂交整合偏倚工业质量管理下腔静脉弯曲凝血系统抑制物起始流速启动启动复合物起始出血率内活塞肌醇三磷酸三磷酸肌醇续表英文全称(缩写)in tegra l cel l mem brane prot ein i ntegr ini nterc ellul ar ad hesio n mol ecule-1(IC AM-1)int erdep enden ce in terle ukin-6/8 inter nal q ualit y con trol(IQC)inte rnalvalid ity i ntern ation al no rmali zed r atio(INR)inte rnati onalsensi tivit y ind ex(IS I)i ntrac ellul ar ad hesio n mol ecule-2(IC AM-2)中文名称细胞膜整合蛋白整合素细胞间黏附分子-1相互依赖性白介素6/8室内质量控制内部真实性intr insic acti vatio n pat hwayintri nsicpathw ay in trins ic te naseion c hanne l(IC)I TP in syst emiclupus eryt hemat osusKka llikr ein(K)ka olinclott ing t ime(K CT) 6-ket o-pro stagl andin El(6-K-PG E1) labo rator y inf ormat ion s ystem(LIS)lam inialamin in(LN)国际标准化比值国际敏感指数细胞内黏附分子-2内源激活途径内源性途径内源性X酶离子通道SL E相关性血小板减少性紫癜激肽释放酶白陶土凝固时间6-酮-前列腺素E1实验室信息系统层粘连蛋白L-arg inine lase r sca n con focal micr oscop e(LSC M)la ser s catte r con focal micr oscop e(LSC M)l atexagglu tinat ion a ssaylatex aggl utina tiontest(LAT)late x par ticle late x pho tomet ric i mmuno assay(LPIA)La urell rock et le ctrop hores isi l eucin e-ric h gly copro tein(LRG)leuc ocyte elas taseleuco cyteelast ase i nhibi tor l eukoc yte f uncti on as socia ted a ntige nt-1(LFA-1)le ukota cticfacto r lig and-i nduce d bin dingsites(LIBS)λlight-chai n vas culop athylight micr oscop e(LM)lik eliho od ra tio f or anegat ive t est(L R-) likel ihood rati o for a po sitiv e tes t(LR+)li nolei c aci d lip id bi layer lipo polys accha ride(LPS)层素L型精氨酸激光共聚焦显微镜共聚焦显微镜胶乳凝集法胶乳凝集试验胶乳颗粒胶乳光度免疫分析Lau rell免疫火箭电泳富含亮氨酸糖蛋白白细胞弹性蛋白酶自细胞弹性蛋白酶抑制剂白细胞功能相关抗原-1白细胞趋化因子配体诱导结合位点λ轻链性血管病光学显微镜阴性似然比阳性似然比亚油酸脂质双层脂多糖续表中文名称脂蛋白a脂蛋白相关凝血抑制物低相对分子质量肝素衍生物英文全称(缩写)lip oprot ein a(LPa)lip oprot ein-a ssoci atedcoagu latio n inh ibito r(LAC I)l ow mo lecul ar we ightderiv ative s ofhepar in lo w mol ecula r wei ght h epari n(LMW H) low r angeactiv atedclott ing t ime(L ACT)lowshear cond ition low-densi ty li popro tein(LDL)luei ferin-luci feras e lum i-agg regom eterlupus anti coagu lant(LA) lupus anti coagu lanttesti ng ly osome-asso ciate d mem brane prot ein-3(LAMP-3) β-lys in ly sineresid ue ly sis o nsettime(LOT)lyso somal inte gralmembr ane p rotei n lys osome gran ulesM低相对分子质量肝素低浓度活化凝血时间低切流状态低密度脂蛋白虫荧光素-虫荧光素酶发光-聚集仪狼疮抗凝物质狼疮抗凝试验溶酶体相关膜蛋白-3β溶素赖氨酸残基纤溶启动时间溶酶体整合膜蛋白溶酶体颗粒α2-ma crogl obuli n(α2-MG) mac ropha ge-de rived chem okine(MDC)mag netic part iclemagne tic s ensor dete ction syst em ma trixremod elingmaxi mal a ggreg ation rati o(MAR)ma ximal ampl itude maxi mal l ighttrans mitta nce m ean p latel et vo lume(MPV)Medl ine m embra ne ex press ion r equir es pl atele t sti mulat ion a nd/or secr etion (S)mem brane phos pholi pidsmepac rinemese nteri c art ery(M es A)Met a-ana lysis meta llopr otein ase(M MP) micro tube(MT)α2-巨球蛋白巨噬细胞衍生趋化因子磁颗粒磁感器检测系统基质重塑最大凝集率最大振幅最大透光度平均血小板体积美国医学索引(血小板)在激活时膜表达膜磷脂阿的平肠系膜动脉Me ta-分析金属蛋白酶微管最小透光度线粒体线粒体脑肌病促有丝分裂原蛋白激酶单克隆抗体血小板抗原单克隆抗体特异性固相化未定性单克隆球蛋白增多症单克隆蛋白(M蛋白)续表mi nimal ligh t tra nsmit tance mito chond ria(M)m itoch ondri al en cepha lomyo pathy(MELA S)m itoge n act ivate d pro teinkinas e(MAP K)m onocl onalantib ody(M cAb)mono clona l ant ibody-spec ificimmob iliza tionof pl atele t ant igen(MAI PA)mono clona l gam mopat hy of unde rmine d sig nific ancemonoc lonal prot ein(M prot ein)英文全称(缩写)中文名称moti on re sista nce m ultip le my eloma(MM)myel oprol ifera tivedisea ses(M PD) myoca rdial infa rctio n(AMI)NNati onalCommi tteefor C linic al La borat ory S tanda rds(N CCLS)Nat ional Libr ary o f Med icine nega tivepredi ctive valu e(NPV)ne oepit ope n eonat al al loimm une t hromb ocyto penia neph elome try neutr aliza tioninhib ition assa y nic otina mide-adeni ne de nucle otide phos phate(NADH P) n itric oxid e(NO)nit ric o xidesynth ase(N OS) non-H odgki n lym phoma(NHL)non immun ologi c pla telet dest ructi on no n-ove rt DI C non palpa ble p urpur a non-ster oidal anti-infl ammat ory d rug n ormal izedAPC-S R(nAP C-SR)nyl on me sh O位移阻抗多发性骨髓瘤骨髓增生症急性心肌梗死(美国)国家临床实验室标准化委员会(美国)国立医学图书馆阴性预测值新表位新生儿同种免疫性血小板减少性紫癜散射比浊法中和抑制试验还原型辅酶Ⅱ一氧化氮oddsprodu cts o n-lin e qua litycontr ol pr ogram s ope n can alica lar s ystem(OCS)o-p henyl enedi amine(OPD)opt omech anica l clo t det ectio n ora l con trace ptive orth otopi c liv er tr anspl antat ion(O LT) osmol arity over t DIC oxid ative stre ss P一氧化氮合酶非霍奇金淋巴瘤非免疫介导的血小板减少症非显性DIC不可触及的紫癜非类固醇抗炎药标准活化蛋白c敏感比值尼龙网比数积在线质控开放管道系统邻苯二胺光学机械凝块检测法palpa ble p urpua r pan ereat ic el astas e(PE)pan creat ic el astas e inh ibito r par aneop lasti c vas culit is pa ra-ni tro-a nilid e(PNA) pa roxys mal n octum al he moglo binur ia(PN H)p artia l ver ifica tionbiaspatie nt sp ectru m pat ientvaria bilit y口服避孕药原位肝移植渗克分子浓度显性DI C氧化应激可触及的紫癜胰弹性蛋白酶胰弹性蛋白酶抑制剂副癌性血管炎对硝基苯胺阵发性睡眠性血红蛋白尿偏袒证实偏倚病人谱患者变异续表英文全称(缩写)PDG F rec eptor(PDGF R)p entas accha ridebindi ng fr agmen t Per iodic als C hinab ase p ermea biiit y fac tor p eroxi somephal lacid in Ph e-Val-Argphorb ol my rista te ac etate(PMA)pho sphat e buf fered sali ne(PB S)p hosph atidy linos itol3 kin ase(P IK3)phos phati dylin osito l(PI)pho sphat idyls erin(PS) phosp holip ase A2(PLA2)p hosph olipa se C(PLC)phos pholi paseD(PLD)ph ospho lipid(PL)中文名称血小板衍生生长因子受体五糖结合片段中文科技期刊数据库(血管)通透性因子过氧化酶小体毒蕈肽苯丙氨酸-缬氨酸-精氨酰胺佛波豆蔻乙脂磷酸盐缓冲液磷脂酰肌醇激酶3磷脂酰肌醇pho sphol ipid-conta ining memb ranes(PCM)pho tomet ric c lot d etect ion p hoto-optic al se nsorphysi csp iezoe lectr ic qu artzcryst al(PQ C)p lasma prot amine para coagu latio n(3P)testplasm a thr ombop lasti n ant ecede ntde ficie ncy(P TA) plasm in(PL)pl asmin-anti plasm in co mplex es(PA P)α2-pla smininhib itor(α2-PI)pl asmin ogen(PLG)plas minog en ac tivat or in hibit or-1/2(PAI-1/2)pla smino gen a ctiva tor r eleas e hor mone(PARH)pla smino gen a ctiva tor(P A)p lasmi nogen rece ptor(PLGR)pla smino gen-b oundzymog en pl atele t act ivati on de pende nt gr anule exte rnalmembr ane(P ADGEM)pl atele t adh esion plat eletadhes ion t est(P AdT)磷脂酰丝氨酸磷脂酶A2磷脂酶C磷脂酶D磷脂膜磷脂透射比浊法光-光传感器物理学压电磁晶体血浆鱼精蛋白副凝固试验血浆凝血活酶前质缺陷纤溶酶纤溶酶-抗纤溶酶复合物pla telet aggr egaho n pla telet aggr egati on ra te pl atele t agg regat ion t est(P AgT)plat eletaggre gomet er pl atele t ass ociat ed co mplem ent 3(PAC3)pl atele t ass ociat ed Ig(PAIg)pl atele t cou nt(PL T)p latel et de rived grow th fa ctor(PDGF)pla telet dust plat eletfacto r 3(P F3) plate let f actor 4(PF4)α2-纤溶酶抑制剂纤溶酶原纤溶酶原激活剂抑制物-1/2纤溶酶原激活剂释放激素纤溶酶原激活剂纤溶酶原受体纤溶酶原结合酶原血小板激活依赖型的外膜颗粒血小板黏附血小板黏附试验血小板聚集血小板聚集率血小板聚集试验血小板聚集仪血小板相关补体C3血小板相关抗体血小板计数血小板衍生生长因子血小板灰尘血小板第3因子血小板第4因子续表英文全称(缩写)中文名称plat eletfunct ion a nalyz er(PF A-100)血小板功能仪plat eletfunct ion t est(P FT) 血小板功能试验pl atele t hem ostas is ti me(PH T)血小板止血时间plat eletioniz ed ca lcium aggr egome ter(P ICA)plat eletmicro parti cles(PMP)plat eletpoorplasm a(PPP)pl atele t rea ctivi ty pl atele t rel easereact ion p latel et re tenti on pl atele t ric h pla sma(P RP) plate let s urviv al ti me(PS T)p latel et-ac tivat ing f actor(PAF)pla telet-deri ved g rowth fact or(PD GF) plate let-e ndoth elial cell adhe sionmolec ule-1(PECA M-1)plat elet-endot helia l cel l tet raspa n ant igen-3(PET A3) plate let-i nduce d thr ombin gene ratio n tim e(PIT T)p ointof ca re te st(PO CT) polyc lonal anti bodypolyc ythem iap olycy themi a ver a(PV)pol ystyr ene p ositi ve pr edict ive v alue(PPV)血小板钙离子聚集仪血小板微颗粒乏血小板血浆血小板反应性血小板释放反应血小板滞留富含血小板血浆血小板生存时间血小板活化因子血小板衍生生长因子血小板内皮细胞黏附分子-1血小板内皮细胞的跨膜抗原-3血小板诱导的凝血酶生成时间pos t-ana lytic al ph ase p ost-t ransf usion purp ura(P TP) 床旁分析pre-analy tical phas e pre-anal ytica l var iabli ty pr eclam psia多克隆抗体pr ekall ikrei n(PK)红细胞增多症prese t cal ibrat ionspress ure a nalys is pr essur e gra dient pret hromb otic真性红细胞增多症stat e(PTS)聚苯乙烯preva lence prim ary h emost asisprima ry tu be sa mplin g pri ming阳性预测值p rocoa gulan t pro teinproen zyme分析后期pr omyel ocyti c leu kemia(PML)输血后紫癜propa gatio n pro stacy clin(PGl2)分析前期p rosta gland in(PG)分析前变异prot amine dose assa y(PDA)先兆子痫prota minerespo nse t est(P RT) 激肽释放酶原p rotea se ne xin I(PNI)预先校准p rotea se-ac tivat ed re cepto r(PAR s)压力分析压力梯度血栓前状态患病率一期止血原始样本管吸样启动促凝蛋白酶原早幼粒白血病放大前列环素前列腺素鱼精蛋白剂量试验鱼精蛋白应答试验蛋白酶连接素I蛋白酶激活受体英文全称(缩写)prot ein C(PC)prot ein C inhi bitor(PCI)pro teinC pep tide(PCP)prot ein c ase c omple x pro teinS bou nd to C4b-bindi ng pr otein prot ein S(PS)prot ein Z(PZ)prot ein Z-depe ndent prot easeinhib itor(ZPI)prot hromb in pr othro mbinconsu mptio n tim e(PCT)pr othro mbinfragm ent 1 and2(F1+2)p rothr ombin time(PT)prot hromb inase prot hromb oticsyndr omes(PTS)续表中文名称蛋白C蛋白C抑制物蛋白C活化肽蛋白盒复合物C4b结合蛋白S蛋白S蛋白Z蛋白Z依赖的蛋白酶抑制物凝血酶原凝血酶原消耗试验prou rokin ase(p roUK)P-s elect in P-selec tionglyco prote in li ganal-1(PS GL-1)pse udoth rombo cytop eniapsych ologi cal s tress pulm onary arte ry(PA)pu lmona ry em bolus(PE)pulm onary vein puri nocep tor(P)Qqual ity a ssura nce(Q A)凝血酶原片段1+2凝血酶原时间凝血酶原酶血栓前综合征尿激酶原P-选择素P-选择素糖蛋白配体-1假性血小板减少症心理压力肺动脉肺栓塞肺静脉嘌呤能受体质量保证q ualit y con trol(QC) qual ity i mprov ement(QI)qua litylabor atory proc ess(Q LP) quali ty ma nagem ent p roces s qua litymanag ement scie nce q ualit y pla nning(QP)Rr adial immu nodif fusio n(RID)ra dioim munoa ssay(RIA)rand om ac cesss rapi d pla telet func tionassay(RPFA)re actio n tim e rec alcif icati on ti me(RT)re calic ified acti vated clot tingtime(RACT)rec eiver oper atorchara cteri sticcurve(ROC)rec eptor-indu ced b indin g sit es(RI BS) reco mbina nt st repto kinas e(rSK)r ecomb inant tiss ue ty pe pl asmin ogenactiv ator(rt-PA)质量控制质量改善实验室质量过程质量管理过程。
Bioorg. Med. Chem. Lett. 17 (2007) 3317-3321

Design and synthesis of urea and thiourea derivatives and their inhibitory activities on lipopolysaccharide-inducedNO productionYoon Jung Kim,Jae-Ha Ryu,Ye Jin Cheon,Hyo Jin Lim and Raok Jeon *College of Pharmacy,Sookmyung Women’s University,52Hyochangwon-Gil,Yongsan-Ku,Seoul 140-742,Republic of KoreaReceived 17November 2006;revised 20March 2007;accepted 2April 2007Available online 6April 2007Abstract—Series of ureas and thioureas were designed and synthesized,and their inhibitory activities of NO production in lipopoly-saccharide-activated macrophages were evaluated.We found several essential moieties in the structure of the prepared compounds for the activity.Thiourea derivatives revealed higher inhibitory activity than the corresponding urea derivatives.Among these com-pounds,7e having carboxymethyl group at N3position of thiourea was the most potent in the inhibition of NO production.They inhibited NO production through the suppression of iNOS protein and mRNA expression.Ó2007Elsevier Ltd.All rights reserved.The critical role of nitric oxide (NO)in various patho-logical conditions has led to the discovery of new inhib-itors of NO production as potential therapeutic agents.NO,a gaseous free radical,is produced through the oxi-dation of L -arginine by three isoforms of nitric oxide synthase (NOS).1The constitutive NOS (cNOS)found in neuronal tissues (nNOS,type I)and vascular endo-thelium (eNOS,type III)is Ca 2+-dependent and releases small amounts of NO required for homeostatic func-tion.2Meanwhile,inducible NOS (iNOS,type II),which can be induced by lipopolysaccharide (LPS)and various cytokines such as IFN-a ,IL-1b ,and TNF-a ,is Ca 2+-independent and produces micromolar levels of NO.3Low concentrations of NO produced by iNOS possess beneficial roles in antimicrobial activity of macrophages against pathogens,4while the overproduction of NO and its derivatives,such as peroxynitrite and nitrogen diox-ide,has been suggested to be mutagenic in vivo and to provoke the pathogenesis of septic shock and various inflammatory processes.5Furthermore,NO and its oxi-dized forms have also been known to be carcinogenic.6Among the many strategies for providing rational con-trol of NO levels,the efforts have been mainly directed toward development of selective inhibitor of iNOS thatcan be applied for the treatment of diseases accompany-ing high levels of NO.Regulation of iNOS can be achieved by the control of expression level and/or enzy-matic activity of iNOS.Many classes of iNOS inhibitors can be classified according to their structural features,such as,amino acid analogues,7amino heterocycles,8amidines,9guanidines,10isoquinolinamines,11and isot-hiourea.12,13But most of the inhibitors are neither po-tent nor selective enough against NOS isoforms,that limited the application of them in vivo.Only one class of iNOS inhibitors,L -lysine analogue,was reported to enter the clinical trial in human.14Urea was known to inhibit not only the activity of iNOS in macrophages during the uremia,15but also the expres-sion of iNOS in LPS-activated macrophages.16Urea was suggested as an important modulator for renal function through the fine tuning of NO production.17Several groups have reported that urea and thiourea 18or iso-thiourea 12,13,19inhibit iNOS expression and/or NO pro-duction.Based on these reports,we tried to investigate urea and thiourea derivatives as novel inhibitors having mechanism for enzymatic inhibition and/or downregula-tion of iNOS expression.Herein,we report the design and synthesis of urea and thiourea derivatives as depicted in Figure 1.Their effects on the NO production and expression of iNOS were evaluated in LPS-activated macrophage cell culture system.0960-894X/$-see front matter Ó2007Elsevier Ltd.All rights reserved.doi:10.1016/j.bmcl.2007.04.005Keywords :Urea;Thiourea;Carbazole;Nitric oxide synthase;Nitric oxide;Inhibitor.*Corresponding author.Tel.:+8227109571;fax:+8227159571;e-mail:rjeon@sookmyung.ac.krBioorganic &Medicinal Chemistry Letters 17(2007)3317–3321The preparation of the carbazole-linked urea and thio-urea derivatives is outlined in Schemes 1and 2.Hydroxy ethyl group was introduced at nitrogen of carbazole and the resulting alcohol 2was mesylated to obtain com-pound 3.Alkylation of 4-nitrophenol by treatment of compound 3in the presence of NaH gave compound 4.Following reduction of nitro compound 4over 10%Pd/C under atmospheric pressure of hydrogen gas pro-vided amine 5.Condensation of amine 5with the appro-priate isocyanates or isothiocyanates offered the desired compounds 6and 7.The activities of the prepared compounds were evalu-ated for the inhibition of NO production in LPS-acti-vated macrophages.Murine macrophage cell line,RAW 264.7cells,was stimulated with 1l g/mL of LPS in the presence of samples for 20h.The amounts of NO released into culture media were determined by the Griess method 20in the form of nitrite.21The inhibitory activities of the prepared compounds on the NO production are given in Table 1.Aminoguani-dine,a well-known specific inhibitor of iNOS,was usedas positive control that showed 85%inhibition of NO production at 0.1l M.Most of the thiourea derivatives revealed higher activity than the corresponding urea derivatives.For example,thioureas 7a ,7c ,7e ,and 7f showed significantly higher activities than ureas 6a ,6b ,6g ,and 6i ,respectively.Effects of the alkyl substituents at the N1and N3posi-tion of urea 6a and thiourea 7a were investigated.Intro-duction of methyl group 6h at the N1position of urea 6a lowered the activity,while introduction of bulkier sub-stituent retrieved the pound 6i with ethyl group showed the similar activity as 6a .Meanwhile,activity of compound 6j with cyclopropylmethyl at N1became 2.5-fold higher than that of 6a .On the other hand,thiourea derivatives 7f substituted with ethyl and 7g with cyclopropylmethyl at N1of 7a revealed 2-fold lower activity than 7a .The effects of substituents at N3position were consider-ably different between urea and thiourea derivatives.While the substitution at N3of urea derivatives 6b–6g showed no significant effects on the activity,the alkyl substitution of thiourea greatly enhanced the pounds 7b ,7c ,7e with methyl,ethyl,or carboxym-ethyl group at N3revealed similar activity ranging from 80%to 90%inhibition of NO production at 5l M con-centration.In both cases of ureas and thioureas,the carboxymethyl was the best substituent at N3for the improvement of activity.IC 50values of 6j ,7a–7c ,and3318Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–33217e,which showed more than50%inhibitory responses at 5l M,were determined as3.12,5.31,0.73,0.90,and 0.15l M,respectively.In order tofind the influence of lipophilic tail on the activity,carbazolylmethyl group of thiourea derivatives 7b–7c was eliminated.These compounds showed lower activities,less than15%inhibition of NO production at5l M,than the corresponding carbazole-linked thiou-reas.It has been reported that a carbazole derivative inhibited iNOS expression in the LPS-activated macro-phage.22Our results also demonstrated that both thio-urea and carbazole moieties might play an important role for their activities although carbazole moiety devoid of thiourea group revealed no activity.We expect to potentiate the activity by the structural modification of thiourea and lipophilic segment.For the further biological study of our derivatives,we examined the effects of6j,7a–7c,and7e on the expres-sion of iNOS protein and mRNA in LPS-activated RAW264.7cells.The amounts of iNOS protein were analyzed in Western blot analysis after20-h incubation with compounds during LPS(1l g/mL)activation ofmacrophages.23Compounds7b and7e significantly reduced the amounts of iNOS at10l M(Fig.2).At RT-PCR analysis,24the expression level of iNOS mRNA was increased markedly by LPS-activation for pounds7b and7e suppressed the induction of iNOS mRNA at10l M(Fig.3).These results indicated that the inhibition of NO production by thiourea deriv-atives resulted from the suppression of iNOS protein and mRNA.When we treated the compounds after the completion of iNOS induction by LPS(post-treatment),they showed weak activity compared with the results of the co-treat-ment of compounds with LPS.Even the most potent compound7e showed12%inhibition at10l M by post-treatment.These results suggested that thiourea derivatives exhibited their activities mainly through the inhibition of iNOS expression with marginal inhibi-tion against enzymatic activity.It has been reported that urea itself inhibited the activity of iNOS by transcrip-tional15and post-transcriptional16mechanisms.Many of thioureas and isothioureas were reported12,25as inhibitors of iNOS enzyme devoid of controlling the expression step.In addition,a carbazole compound,Table1.Inhibitory activities of carbazole-linked phenylureas and phenylthioureas on the NO production in LPS-induced NO productionNO NHNXR1R2R1, R2=H,alkylX = O, SCompound R1R2X Inhibition a(%)IC50b(nM) 6a H H O246b H Et O306c H Pro O146d H i-Pro O156e H Ph O286f H CH2CO2Et O386g H CH2CO2H O436h Me H O86i Et H O256j Cyclopropylmethyl H O613120±2507a H H S555310±7027b H Me S80730±1807c H Et S90901±2117d H CH2CO2Et S417e H CH2CO2H S87153±837f Et H S337g Cyclopropylmethyl H S34a Values mean the inhibition(%)of NO production at5l M concentration of compounds relative to the LPS control(n=3).b Values are means±SD of three experiments.Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–332133199-(2-chlorobenzyl)-9H-carbazole-3-carbaldehyde,was reported as an inhibitor of iNOS mRNA expression through a signaling pathway that does not involve NF-j B pathway.22The exact difference between the mecha-nism of our compounds and that of the reported thioureas and carbazole derivative was not explained in this report. The study of the mechanism for the iNOS inhibition by our compounds might be worthy to pursue further.In conclusion,we prepared a series of urea and thiourea derivatives and evaluated their inhibitory activities of NO production in LPS-activated macrophages.They suppressed the release of NO into culture media through the suppression of iNOS protein and mRNA expression. The SAR studies demonstrated that thiourea is superior to urea and N3substitution of thiourea with alkyl group is highly beneficial for their activity.Further study of the other biological activities related with the overproduc-tion of NO,and the detailed mechanism for the activities of these derivatives,is in progress.Our thiourea deriva-tives that can control the expression of iNOS can be good leads for the development of therapeutic agents for the management of NO-related diseases.AcknowledgmentThis work was supported by the SRC program of MOST/KOSEF(R11-2005-017,RESEARCH CEN-TER FOR WOMEN’S DISEASES).Supplementary data Supplementary data associated with this article can be found,in the online version,at doi:10.1016/j.bmcl. 2007.04.005.References and notes1.Forstermann,U.;Schmidt,H.H.;Pollock,J.S.;Sheng,H.;Mitchell,J.A.;Warner,T.D.;Nakane,M.;Murad,F.Biochem.Pharmacol.1991,42,1849.2.Bredt,D.S.;Snyder,S.H.Proc.Natl.Acad.Sci.U.S.A.1990,87,682.3.Lowenstein,C.J.;Glatt,C.S.;Bredt,D.S.;Snyder,S.H.Proc.Natl.Acad.Sci.U.S.A.1992,89,6711.4.Cook,H.T.;Cattell,V.Clin.Sci.(Lond.)1996,91,375.5.Thiemermann,C.;Szabo,C.;Mitchell,J.A.;Vane,J.R.Proc.Natl.Acad.Sci.U.S.A.1993,90,267.6.Halliwell,ncet1994,344,721.7.Moore,W.M.;Webber,R.K.;Jerome,G.M.;Tjoeng,F.S.;Misko,T.P.;Currie,M.G.J.Med.Chem.1994,37, 3886.8.Hagen,T.J.;Bergmanis,A.A.;Kramer,S.W.;Fok,K.F.;Schmelzer,A.E.;Pitzele,B.S.;Swenton,L.;Jerome,G.M.;Kornmeier,C.M.;Moore,W.M.;Branson,L.F.;Connor,J.R.;Manning,P.T.;Currie,M.G.;Hallinan,E.A.J.Med.Chem.1998,41,3675.9.Moore,W.M.;Webber,R.K.;Fok,K.F.;Jerome,G.M.;Kornmeier, C.M.;Tjoeng, F.S.;Currie,M.G.Bioorg.Med.Chem.1996,4,1559.10.Bryk,R.;Wolff,D.J.Biochemistry1998,37,4844.11.Beaton,H.;Hamley,P.;Nicholls,D.J.;Tinker,A.C.;Wallace,A.V.Bioorg.Med.Chem.Lett.2001,11,1023.12.Paesano,N.;Marzocco,S.;Vicidomini,C.;Saturnino,C.;Autore,G.;De Martino,G.;Sbardella,G.Bioorg.Med.Chem.Lett.2005,15,539.13.Raman,C.S.;Li,H.;Martasek,P.;Babu,B.R.;Griffith,O.W.;Masters,B.S.;Poulos,T.L.J.Biol.Chem.2001, 276,26486.14.Hansel,T.T.;Kharitonov,S.A.;Donnelly,L.E.;Erin,E.M.;Currie,M.G.;Moore,W.M.;Manning,P.T.;Recker,D.P.;Barnes,P.J.FASEB J.2003,17,1298.15.Moeslinger,T.;Friedl,R.;Volf,I.;Brunner,M.;Baran,H.;Koller,E.;Spieckermann,P.G.Kidney Int.1999,56,581.16.Prabhakar,S.S.;Zeballos,G.A.;Montoya-Zavala,M.;Leonard,C.Am.J.Physiol.1997,273,C1882.17.Wang,W.;Jittikanont,S.;Falk,S.A.;Li,P.;Feng,L.;Gengaro,P.E.;Poole,B.D.;Bowler,R.P.;Day,B.J.;Crapo,J.D.;Schrier,R.W.Am.J.Physiol.Renal Physiol.2003,284,F532.18.Goodyer,C.L.M.;Chinje,E.C.;Jaffar,M.;Stratford,I.J.;Threadgill,M.D.Bioorg.Med.Chem.2003,11,4189.19.Shearer,B.G.;Lee,S.;Oplinger,J.A.;Frick,L.W.;Garvey,E.P.;Furfine,E.S.J.Med.Chem.1997,40,1901.20.Green,L.C.;Wagner,D.A.;Glogowski,J.;Skipper,P.L.;Wishnok,J.S.;Tannenbaum,S.R.Anal.Biochem.1982,126,131.21.Cell culture and nitrite assay in LPS-activated RAW264.7cells—cells in10%fetal bovine serum(FBS)–DMEM, were plated in48-well plates(1·105cells/mL)and then incubated for24h.The cells were replaced with fresh media with1%FBS and then incubated for20h in the presence or absence of test compounds with LPS(1l g/ mL).NO production in each well was assessed by measuring the accumulated nitrite in culture supernatant.Samples(100l L)of media were incubated with Griess reagent(150l L)for10min at room temperature in96-well microplate.Absorbance at570nm was read using an ELISA plate reader.A standard calibration curve was prepared using sodium nitrite as a standard.A dose–response curve was prepared,and the results were typically expressed as IC50values.22.Tsao,L.T.;Lee,C.Y.;Huang,L.J.;Kuo,S.C.;Wang,J.P.Biochem.Pharmacol.2002,63,1961.LPS-+ + + + ++Sample 6j 7a 7b7c 7eFigure3.Effects of the prepared compounds on the expression3320Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–332123.Western blot analysis of iNOS protein expression—RAW264.7cells(1.5·106cells/60-mm dish)were stimulated with LPS(1l g/mL)in the presence or absence of test compounds.After incubation for20h,the cells were washed and lysed with lysis buffer.Twenty l g protein of cell lysates was applied on8%SDS–polyacrylamide gels and transferred to PVDF membrane by a standard method.The membrane was probed with antibody for anti-mouse iNOS(Transduction Laboratories,Lexington, KY)and anti-actin(Sigma,St.Louis,MO).The bands were visualized using an enhanced chemiluminescence (ECL)detection kit(Amersham Bioscience,Piscataway, NJ)according to the manufacturer’s instruction.24.Reverse transcription-polymerase chain reaction(RT-PCR)analysis of iNOS mRNA expression—RAW264.7 cells(1.8·106cells/60-mm dish)were stimulated for6h with LPS(1l g/mL)in the presence or absence of test compounds.After washing twice with phosphate-buffered saline,total RNA was isolated from cell pellet,using an RNA isolation reagent(Trizol,Invitrogen,Carlsbad,CA).Two micrograms of RNA was reverse transcribed into cDNA using reverse transcriptase and random hexamer.The PCR samples,contained in the reaction mixture,were comprised of mixture buffer,dNTP,Taq DNA polymerase (Promega,Madison,WI),and primers(sense and antisense).The sense and antisense primers for iNOS were50-ATGTCCGAAGCAAACATCAC-30and50-TAATGTCCAGGAAGTAGGTG-30,respectively.The sense and antisense primers for b-actin were50-TGT GATGGTGGGAATGGGTCAG-30and50-TTTGATGT CACGCACGATTTCC-30,respectively.The PCR ampli-fication was performed under following conditions;25 cycles of denaturation at94°C for30s,annealing at55°C for30s,and extension at72°C for30s,using thermal cycler(Gene Amp PCR system2400,Applied Biosystems, Foster City,CA).The amplified PCR products were separated on a2%agarose gel.25.Garvey,E.P.;Oplinger,J.A.;Tanoury,G.J.;Sherman,P.A.;Fowler,M.;Marshall,S.;Harmon,M.F.;Paith,J.E.;Furfine,E.S.J.Biol.Chem.1994,269,26669.Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–33213321。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Bitopertin is a potent, noncompetitive glycine reuptake inhibitor, inhibits glycine uptake at human GlyT1 with a concentration exhibiting IC50 of 25 nM.IC50 & Target: IC50: 25 nM (GlyT1)[1]In Vitro: Bitopertin (RG1678) competitively blocks [3H]ORG24598 binding sites at human GlyT1b in membranes fromChinese hamster ovary cells. Bitopertin potently inhibits [3H]glycine uptake in cells stably expressing hGlyT1b and mGlyT1b, with IC 50values of 25±2 nM and 22±5 nM, respectively (n=6). Conversely, Bitopertin has no effect on hGlyT2–mediated glycineuptake up to 30 μM concentration. Bitopertin has high affinity for the recombinant hGlyT1b transporter. Underequilibrium conditions (1 h at room temperature), Bitopertin displaces [3H]ORG24598 binding with a K i of 8.1 nM. In hippocampal CA1 pyramidal cells, Bitopertin enhances NMDA–dependent long–term potentiation at 100 nM but not at 300 nM [1]. Additional profiling revealed that Bitopertin (RG1678) has an excellent selectivity profile against the GlyT2 isoform (IC 50>30 μM) and toward a panel of 86 targets including transmembrane and soluble receptors, enzymes, ion channels, and monoamine transporters (<41%inhibition at 10 μM is measured for all targets)[2].In Vivo: Bitopertin (RG1678) dose–dependently increases cerebrospinal fluid and striatal levels of glycine measured bymicrodialysis in rats. Additionally Bitopertin attenuates hyperlocomotion induced by the psychostimulant D–amphetamine or the NMDA receptor glycine site antagonist L–687,414 in mice. Bitopertin also prevents the hyper–response to D–amphetamine challenge in rats treated chronically with phencyclidine, an NMDA receptor open–channel blocker. Administration of vehicle has no effect on extracellular levels of striatal glycine, which remained constant throughout the experiment. In contrast, p.o. administration of Bitopertin (1–30mg/kg) produced a dose–dependent increase in extracellular glycine levels. Bitopertin 30 mg/kg produces glycine levels 2.5 times higher than pretreatment levels. A similar dose–dependent increase in glycine concentration is observed in the CSF of rats treated p.o. with Bitopertin (1–10 mg/kg) compared with vehicle–treated animals, 3 h after drug administration. Interestingly, the level ofCSF glycine increase 3 h after Bitopertin dosing is very similar to the increase in the microdialysis experiment at the same time point [1].In vivo pharmacokinetic studies in rat and monkey reveals that Bitopertin (RG1678) has, in both species, a low plasma clearance, an intermediate volume of distribution, a good oral bioavailability (78% for rat, 56% for monkey), and a favorable terminal half–life (5.8h for rat, 6.4 h for monkey). The plasma protein binding is high in the two preclinical species (97%) and in human (98%). The CNS penetration of Bitopertin in rat (brain/plasma=0.7) is better than that in mouse (brain/plasma=0.5)[2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Association and dissociation kinetic analysis of [3H]ORG24598 to hGlyT1 and ratforebrain membranes isperformed. [3H]ORG24598 binding experiments are performed using membranes from CHO cells expressing hGlyT1band also in membranes from mouse, rat, monkey, and dogforebrains. Saturation isotherms are determined by adding [3H]ORG24598 to rat, mouse, monkey, and dog forebrain membranes (40 μg/well) and cell membranes (10 μg/well) inProduct Name:Bitopertin Cat. No.:HY-10809CAS No.:845614-11-1Molecular Formula:C 21H 20F 7N 3O 4S Molecular Weight:543.46Target:GlyT; GlyT Pathway:Neuronal Signaling; Membrane Transporter/Ion Channel Solubility:10 mM in DMSOa total volume of 500 μL for 3 h at room temperature. Saturation binding experiments are analyzed by anExcel–based curve–fitting program using the Michaelis–Menten equation derived from the equation of a bimolecularreaction and the law of mass action:B=(B max×[F])/(K d+[F]), where B is the amount of ligand bound at equilibrium, B max the maximum number of binding sites, [F] the concentration of free ligand, and K d the ligand dissociation constant. For inhibition experiments, membranes are incubated with 3 nM [3H]ORG24598 and 10 concentrations of Bitopertin for 1 h at room temperature. Schild analysis is performed in the presence of increasing concentrations of [3H]ORG24598 (1–300 nM). IC50 values are derived as described above. K i values are calculated according to the following equation: K i=IC50/(1+[L]/K d)[1].Animal Administration: Bitopertin (RG1678) is dissolved in H2O with 0.3% Tween 80 (Mice)[1].Bitopertin (RG1678) is prepared in Polysorbate 80, HEC, Methyl– and Propylparaben pH 6.0 (Rat)[1].[1]Mice[1]Male NMRI mice (20–30 g) are treated with Bitopertin (0.3, 3, 1, and 10 mg/kg p.o.) or vehicle (p.o.). After 1 min, L–687,414 (50 mg/kg s.c.) or vehicle is given. After 15 min of habituation in the activity chambers, horizontal activity is recorded for 60 min. The time course of Bitopertin effects on L–678,414–induced hyperactivity is also examined; locomotor activity is assessed 2.5, 4.5, and 24 h after administration of Bitopertin (L–678,414 is always given 15 min before the activity procedure). In addition, the effect of subchronic Bitopertin is investigated. Mice receive vehicle or Bitopertin (1 mg/kg p.o.) for 4 consecutive days and L–678,414–induced hyperactivity is evaluated on day 5.Rat[1]Wistar rats receive a 14–day treatment of PCP HC1 (5 mg/kg) or vehicle (NaCl 0.9%, 5 mL/kg i.p.). 24 h following the last injection, rats (6–18 per group) are allowed to individually habituate to the test boxes for 30 min. Rats then received Bitopertin (1, 3, 10 mg/kg p.o.) or vehicle (Polysorbate 80, HEC, Methyl– and Propylparaben pH 6.0; 5 mL/kg p.o.), followed after 1 h by 1 mg/kg D–amphetamine or vehicle i.p. Horizontal activity is recorded directly after the administration of Bitopertin until 120 min after dosing with amphetamine. Data are analyzed by ANOVA supplemented by Fischer's least significant difference post hoc test.References:[1]. Alberati D, et al. Glycine reuptake inhibitor RG1678: A pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology. 2012 Feb;62(2):1152–61.[2]. Pinard E, et al. Selective GlyT1 Inhibitors: Discovery of[4–(3–Fluoro–5–trifluoromethylpyridin–2–yl)piperazin–1–yl][5–methanesulfonyl–2–((S)–2,2,2–trifluoro–1–methylethoxy)phenyl]methanone (RG1678), a Promising Novel Medicine To Treat Schizophrenia. J MeCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
