Cell-wall carbohydrates and their modification as a resource for biofuels
Plantcellwall(Lecture12)
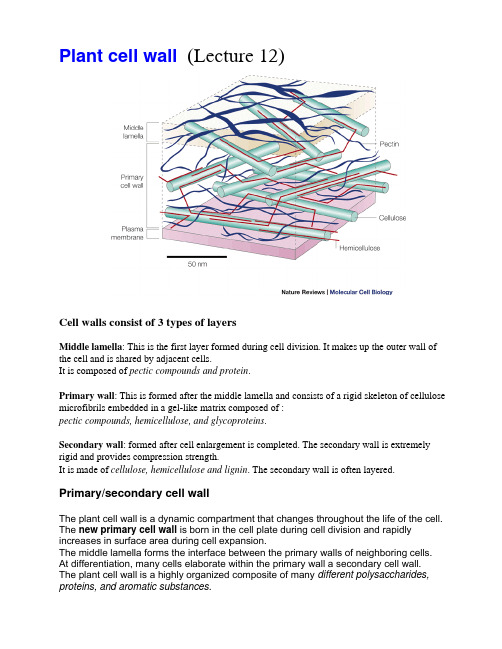
Cell walls also contain functional proteins. Enzymatic activities in cell walls include: •Oxidative enzymes - peroxidases•Hydrolytic enzymes - pectinases, cellulases•"Expansins" - enzymes that catalyze cell wall "creep" activity General functions of cell wall enzymes include:
galacturonic acid
Pectic acid with salt bridges
4. Pectin - polymer of around 200 galacturonic acid molecules - many of the carboxyl groups are methylated (COOCH3) - less hydrated then pectic acid but soluble in hot water - another major component of middle lamella but also found in primary walls
carbohydrate polymers影响因子

carbohydrate polymers影响因子Carbohydrate polymers, commonly referred to as “starch,” is an important macronutrient found in plants. The macromolecules of carbohydrate polymers play a vital role in human health and nutrition as an energy source and as a structural component of plant cells. Additionally, carbohydrate polymers are also utilized in various industries for a variety of uses.The primary factor that affects the macromolecular structure of carbohydrate polymers is the source of the raw material. Starch can be derived from a variety of plant sources such as corn, wheat, potatoes, and rice and the macromolecular structure of the starch molecules differs accordingly. The size, shape, and arrangement of the macromolecules are of distinct importance since they are intimately associated with the biochemical and nutraceutical properties of starch molecules.In general, starch molecules are primarily based on two distinct types of macromolecules, amylose and amylopectin. Amylose is composed of straight-chain glucose molecules, while amylopectin is composed of branched glucose molecules. The ratio of amylose to amylopectin can vary significantly between different sources which may have an influence on properties such as solubility, viscosity, swelling, and water absorption and retention capacities.Another factor that affects the macromolecular structure of carbohydrate polymers is the chemical processing methods employed. Starch molecules can be modified by enzymatic processing and other chemical treatments resulting in different physical characteristics such as increased solubility and stability, improved sensitivity to water and lower viscosity. Chemical processing can also be used to produce a gelling agent when carbohydrates are treated with anionic detergents.Lastly, the environment in which starch molecules are processed can also have an impact on their macromolecular structure. For instance, changes in temperature and pH levels can alter the structure of the molecules. In addition, other environmental conditions such as the presence of light, oxygen, and ultraviolet radiation can also affect the conformation of the molecules.In conclusion, there are a variety of different factors which can influence the macromolecular structure of carbohydrate polymers. Depending on the source of starch, the chemical processing methods employed and the environment in which it is processed, the physical properties and properties associated with nutritional and functional features ofstarch can be altered. Therefore, it is important to understand the interactions between these factors in order to develop novel products with appropriate qualities.。
植物细胞的基本构造

植物细胞的基本构造英文回答:Plant Cell Basic Structure.Plant cells are eukaryotic cells, meaning they have a true nucleus and other membrane-bound organelles. They are also unique in their possession of a cell wall, chloroplasts, and a large central vacuole.Cell Wall.The cell wall is a rigid structure that surrounds the cell membrane and provides support and protection. It is composed of cellulose, hemicellulose, and pectin. The cell wall also contains pores that allow water and nutrients to enter and exit the cell.Cell Membrane.The cell membrane is a thin, flexible layer that surrounds the cytoplasm. It controls the movement of substances into and out of the cell. The cell membrane is composed of a phospholipid bilayer with embedded proteins.Cytoplasm.The cytoplasm is the gel-like substance that fills the cell. It contains all of the cell's organelles, including the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and chloroplasts.Nucleus.The nucleus is a membrane-bound organelle that contains the cell's DNA. DNA is the genetic material that controls all of the cell's activities. The nucleus also contains the nucleolus, which is a small structure that produces ribosomes.Endoplasmic Reticulum.The endoplasmic reticulum is a network of membranesthat folds and transports proteins. The rough endoplasmic reticulum is studded with ribosomes, which are small structures that assemble proteins. The smooth endoplasmic reticulum is involved in the synthesis of lipids and carbohydrates.Golgi Apparatus.The Golgi apparatus is a stack of flattened membranes that modifies and packages proteins. Proteins are transported from the endoplasmic reticulum to the Golgi apparatus, where they are sorted and packaged into vesicles. The vesicles then transport the proteins to their final destination.Mitochondria.Mitochondria are small, bean-shaped organelles that produce energy for the cell. They are often called the "powerhouses of the cell." Mitochondria contain their own DNA, which is different from the DNA in the nucleus.Chloroplasts.Chloroplasts are green organelles that contain chlorophyll, a pigment that absorbs light energy from the sun. Chloroplasts use the light energy to convert carbon dioxide and water into glucose, a sugar molecule that provides energy for the cell.Vacuole.The vacuole is a large, membrane-bound organelle that stores water, salts, and other molecules. The vacuole also helps to maintain the cell's shape and turgor pressure.中文回答:植物细胞的基本结构。
动植物细胞比较练习英文版 compare animal and plants cell

Comparing Plant And Animal Cells/video?v=Hmwvj9X4GNYPlant Cellsshape - most plant cells are squarish or rectangular in shape.amyloplast (starch storage organelle)- an organelle in some plant cells that stores starch. Amyloplasts are found in starchy plants like tubers and fruits.cell membrane - the thin layer of protein and fat that surrounds the cell, but is inside the cell wall. The cell membrane is semipermeable, allowing some substances to pass into the cell and blocking others.cell wall - a thick, rigid membrane that surrounds a plant cell. This layer of cellulose fiber gives the cell most of its support and structure. The cell wall also bonds with other cell walls to form the structure of the plant.chloroplast - an elongated or disc-shaped organelle containing chlorophyll. Photosynthesis (in which energy from sunlight is converted into chemical energy - food) takes place in the chloroplasts. chlorophyll - chlorophyll is a molecule that can use light energy from sunlight to turn water and carbon dioxide gas into glucose and oxygen (i.e. photosynthesis). Chlorophyll is green.cytoplasm - the jellylike material outside the cell nucleus in which the organelles are located.Golgi body - (or the golgi apparatus or golgi complex) a flattened, layered, sac-like organelle that looks like a stack of pancakes and is located near the nucleus. The golgi body modifies, processes and packages proteins, lipids and carbohydrates into membrane-bound vesicles for "export" from the cell. lysosome - vesicles containing digestive enzymes. Where the digestion of cell nutrients takes place. mitochondrion - spherical to rod-shaped organelles with a double membrane. The inner membrane is infolded many times, forming a series of projections (called cristae). The mitochondrion converts the energy stored in glucose into ATP (adenosine triphosphate), a high energy molecule, for use by cell. nuclear membrane - the membrane that surrounds the nucleus.nucleolus - an organelle within the nucleus. Ribosomal RNA is produced here, then exported into the cytoplasm where it forms the ribosomes.nucleus - The nucleus is a spherical body surrounded by the nuclear membrane. It contains mostly DNA in chromosomes. The nucleus controls many of the functions of the cell (by controlling protein synthesis). The nucleolus is found within the nucleus.ribosome - small organelles composed of RNA. Are sites of protein synthesis.rough endoplasmic reticulum - (rough ER) a vast system of interconnected, membranous, infolded and convoluted sacks that are located in the cell's cytoplasm (the ER is continuous with the outer nuclear membrane). Rough ER is covered with ribosomes that give it a rough appearance. Rough ER transport materials and produces proteins (which are sent to the Golgi body, or inserted into the cell membrane).smooth endoplasmic reticulum - (smooth ER) a vast system of interconnected, membranous, infolded and convoluted tubes that are located in the cell's cytoplasm (the ER is continuous with the outer nuclear membrane). It produces lipids (fats) and membrane proteins; smooth ER buds off from rough ER, moving the newly-made proteins and lipids to the Golgi body for further processing and to the membranes.vacuole - a large, membrane-bound space within a plant cell that is filled with fluid, mostly water. Most plant cells have a single vacuole that takes up much of the cell. It helps maintain water balance and the shape of the cell.vesicle - a small, membrane-bound space that helps to transport material in/out or within the cell. Some are storage vessels. eg. proteins produced in the rough endoplasmic reticulum are transported by vesicles produced at the tips of the rough ER, to the golgi body, for processing.Animal Cellshape - most animals cells are roundish or irregular in shape.cell membrane - the thin layer of protein and fat that surrounds the cell. The cell membrane is semipermeable, allowing some substances to pass into the cell and blocking others.centriole - during cell division in animal cells, two pairs of centrioles form from microtubules at each end of the cell. The two centrioles are arranged perpendicular to each other. Microtubules formed in the centriole grow into spindle fibers which then attach to replicated chromosomes and assist in separating them during mitosis.cytoplasm - the jellylike material outside the cell nucleus in which the organelles are located.Golgi body - (also called the golgi apparatus or golgi complex) a flattened, layered, sac-like organelle that looks like a stack of pancakes and is located near the nucleus. The golgi bodymodifies, processes and packages proteins, lipids and carbohydrates into membrane-bound vesicles for "export" from the cell.lysosome - vesicles containing digestive enzymes. Where the digestion of cell nutrients takes place. mitochondrion - spherical to rod-shaped organelles with a double membrane. The inner membrane is infolded many times, forming a series of projections (called cristae). The mitochondrion converts the energy stored in glucose into ATP (adenosine triphosphate), a high energy molecule, for use by the cell.nuclear membrane - the membrane that surrounds the nucleus.nucleolus - an organelle within the nucleus. Ribosomal RNA is produced here, then exported into the cytoplasm where it forms the ribosomes.nucleus - The nucleus is a spherical body surrounded by the nuclear membrane. It contains mostly DNA in chromosomes. The nucleus controls many of the functions of the cell (by controlling protein synthesis). The nucleolus is found within the nucleus.ribosome - small organelles composed of RNA. Are sites of protein synthesis.rough endoplasmic reticulum - (rough ER) a vast system of interconnected, membranous, infolded and convoluted sacks that are located in the cell's cytoplasm (the ER is continuous with the outer nuclear membrane). Rough ER is covered with ribosomes that give it a rough appearance. Rough ER transport materials and produces proteins (which are sent to the Golgi body, or inserted into the cell membrane).smooth endoplasmic reticulum - (smooth ER) a vast system of interconnected, membranous, infolded and convoluted tubes that are located in the cell's cytoplasm (the ER is continuous with the outer nuclear membrane). It produces lipids (fats) and membrane proteins; smooth ER buds off from rough ER, moving the newly-made proteins and lipids to the Golgi body for further processing and to the membranes.vacuole - fluid-filled, membrane-surrounded cavities inside a cell. The vacuole fills with food being digested and waste material that is on its way out of the cell. Smaller than plant cell vacuole. vesicle - a small, membrane-bound space that helps to transport material in/out or within the cell. Some are storage vessels. eg. proteins produced in the rough endoplasmic reticulum are transported by vesicles produced at the tips of the rough ER, to the golgi body, for processing.Name________________Comparing Plant And Animal Cells VENN DiagramDirections: Fill in the VENN Diagram to compare PLANT CELLS to ANIMAL CELLS. Use the words in the word box. Add descriptions to show the differencescell membrane cell wall chloroplast cytoplasm shape nucleus ribosome vacuole centriole mitochondriaPLANT CELL ANIMAL CELL。
医学英语新教程 王兰英 第一单元 课文原文+翻译

第一课细胞结构及细胞转运机制1 所有的生物都是由细胞和细胞外基质构成的。
这种的简单的论述叫做细胞理论,是150年前第一次提出来的。
我们可以把这种理论叫做猜测或者假设,有时候也确实如此,但是证据证实了细胞理论的正确性。
All living organisms are made of cells and cell products. This simple statement, called the Cell Theory, was first proposed over 150 years ago. You may think of a theory as a guess or hypothesis, and sometimes this is so. But a theory is actually the best explanation of all the available evidence. All of the evidence science has gathered so far supports the validity of the Cell Theory.2 细胞是多细胞生物最小活的亚单位,比如人。
细胞是复杂的化学排列;是活体;并且进行着特殊的活动。
微生物如变形虫、细菌是单细胞生物,其细胞有着独立功能。
然而,人类细胞必须相互依赖,共同作用。
内环境稳定取决于所有不同类型细胞的作用。
Cells are the smallest living subunits of a multicellular organism such as a human being. A cell is a complex arrangement of the chemicals; is living; and carries out specific activities. Microorganisms, such as amoebas and bacteria, are single cells which function independently. Human cells, however, must work together, and function interdependently. Homeostasis depends upon the contributions of all of the different kinds of cells.3人类细胞在大小、形状和功能上有所不同。
桉木木素含量与结构

Lignin Composition and Structure in Young versus Adult Eucalyptus globulus Plants1Jorge Rencoret,Ana Gutie´rrez,Lidia Nieto,J.Jime´nez-Barbero,Craig B.Faulds,Hoon Kim,John Ralph,A´ngel T.Martı´nez,and Jose´C.del Rı´o*Instituto de Recursos Naturales y Agrobiologı´a de Sevilla,Consejo Superior de Investigaciones Cientı´ficas, E–41080Seville,Spain(J.Rencoret,A.G.,J.C.d.R.);Centro de Investigaciones Biolo´gicas,Consejo Superior de Investigaciones Cientı´ficas,E–28040Madrid,Spain(L.N.,J.J.-B.,C.B.F.,A.T.M.);and Departments of Biochemistry and Biological Systems Engineering and Department of Energy Great Lakes Bioenergy Research Center,University of Wisconsin,Madison,Wisconsin53706(J.Rencoret,H.K.,J.Ralph)Lignin changes during plant growth were investigated in a selected Eucalyptus globulus clone.The lignin composition and structure were studied in situ by a new procedure enabling the acquisition of two-dimensional nuclear magnetic resonance (2D-NMR)spectra on wood gels formed in the NMR tube as well as by analytical pyrolysis-gas chromatography-mass spectrometry.In addition,milled-wood lignins were isolated and analyzed by2D-NMR,pyrolysis-gas chromatography-mass spectrometry,and thioacidolysis.The data indicated that p-hydroxyphenyl and guaiacyl units are deposited at the earlier stages,whereas the woods are enriched in syringyl(S)lignin during late lignification.Wood2D-NMR showed that b-O-4#and resinol linkages were predominant in the eucalypt lignin,whereas other substructures were present in much lower amounts. Interestingly,open b-1#structures could be detected in the isolated lignins.Phenylcoumarans and cinnamyl end groups were depleted with age,spirodienone abundance increased,and the main substructures(b-O-4#and resinols)were scarcely modified.Thioacidolysis revealed a higher predominance of S units in the ether-linked lignin than in the total lignin and,in agreement with NMR,also indicated that resinols are the most important nonether linkages.Dimer analysis showed that most of the resinol-type structures comprised two S units(syringaresinol),the crossed guaiacyl-S resinol appearing as a minor substructure and pinoresinol being totally absent.Changes in hemicelluloses were also shown by the2D-NMR spectra of the wood gels without polysaccharide isolation.These include decreases of methyl galacturonosyl,arabinosyl,and galactosyl (anomeric)signals,assigned to pectin and related neutral polysaccharides,and increases of xylosyl(which are approximately 50%acetylated)and4-O-methylglucuronosyl signals.Plant cell walls are composed mainly of three struc-tural polymers,the carbohydrates cellulose and the hemi-celluloses and the aromatic polymer lignin.The lignin polymer provides mechanical support to the plant.In addition,it waterproofs the cell wall,enabling trans-port of water and solutes through the vascular system, and plays a role in protecting plants against patho-gens.Lignin is a complex polymer synthesized mainly from three hydroxycinnamyl alcohols differing in their degree of methoxylation:p-coumaryl,coniferyl,and sinapyl alcohols(Higuchi,1997;Boerjan et al.,2003;Ralph et al.,2004a).Each of these monolignols gives rise to a different type of lignin unit called p-hydroxy-phenyl(H),guaiacyl(G),and syringyl(S)units,re-spectively,when incorporated into the polymer.The amount and composition of lignins vary among taxa, cell types,and individual cell wall layers and also with environmental conditions.Softwood lignin con-sists almost exclusively of G-type lignin,while hard-wood lignin also consists of S units(H units being minor components).After their synthesis,the lignin monomers are transported to the cell wall,where they are polymerized in a combinatorial fashion by free radical coupling mechanisms in a reaction mediated by peroxidases and/or laccases,generating a variety of structures and linkages within the polymer(Boerjan et al.,2003;Ralph et al.,2004a).Wood(secondary xylem)is produced seasonally at the periphery of the trunk by the vascular cambium(De´jardin et al.,2010). Lignin deposition is one of thefinal stages of xylem cell differentiation and mainly takes place during secondary thickening of the cell wall.Lignification starts in the middle lamella and cell corners and proceeds toward the lumen,filling up pores in the al-ready deposited polysaccharide network(Donaldson, 2001;Boerjan et al.,2003).The relative abundance of the different linkages formed depends on the relative1This study was supported by the Spanish project AGL2005–01748,the Consejo Superior de Investigaciones Cientı´ficas(project nos.200640I039and201040E075),the European Union projects BIORENEW(grant no.NMP2–CT–2006–026456),WALLESTER (grant no.PIEF–GA–2009–235938),and LIGNODECO(grant no. KBBE–244362),the Department of Energy Great Lakes Bioenergy Research Center(grant no.BER DE–FC02–07ER64494),and the Spanish Ministry of Education(postdoctoral fellowship to J. Rencoret).*Corresponding author;e-mail delrio@irnase.csic.es.The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors()is: Jose´C.del Rı´o(delrio@irnase.csic.es)./cgi/doi/10.1104/pp.110.167254contribution of the particular monomers to the po-lymerization process as well as on steric hindrances and chemical interactions in the growing wall.There-fore,the differences in the timing of monolignol de-position and the changes in cell wall ultrastructure during growth would regulate lignin composition and structure during lignification.A main challenge in elucidating the structure of lignins is in obtaining high-yield isolation from wood in a chemically unaltered form(the same applies to hemicellulose polysaccharides).Several lignin isola-tion procedures have been developed,but it is well recognized that the different procedures,including the reference milled-wood lignin(MWL),yield only a part of the native lignin in wood and may not be represen-tative of the whole lignin.Indeed,it has also been demonstrated that MWL can undergo some structural modifications during isolation,especially during the milling process,and often contains some amount of “contaminating”compounds(such as lignin-linked carbohydrates;Fujimoto et al.,2005;Guerra et al.,2006; Hu et al.,2006;Balakshin et al.,2008).Because lignin is intimately interpenetrating the other major compo-nents(cellulose and hemicelluloses),it is obvious that its truly native form can only be studied by analytical methods applicable directly on the whole plant mate-rial.For this purpose,in this paper,the wood samples were analyzed in situ by two-dimensional(2D)-NMR spectroscopy and pyrolysis-gas chromatography-mass spectrometry(Py-GC-MS).The use of these techniques avoids isolation procedures that may lead to partial or modified polymer extraction.For in situ NMR analy-ses,a recent approach has been developed that con-sists of swellingfinely ground plant material in deuterated dimethyl sulfoxide(DMSO-d6;Kim et al., 2008;Rencoret et al.,2009)or DMSO-d6:pyridine-d5 (4:1;Kim and Ralph,2010)and forming a gel directly in the NMR tube,which is readily amenable to NMR analysis.Heteronuclear single quantum correlation (HSQC)NMR of these gels has been shown to be an efficient method for the rapid in situ analysis of lignin in plants without the need of prior isolation.The method requires only low amounts of sample and can be used for rapid characterization of the major struc-tural features of plant lignins(i.e.interunit linkages and H-G-S composition),also providing information on the hemicellulose polysaccharides.Py-GC-MS is another powerful tool for the in situ characterization of plant constituents,especially lignin(Ralph and Hatfield,1991;Rodrigues et al.,1999;del Rı´o et al., 2005;Rencoret et al.,2007).Wood lignin is pyrolyzed to produce a mixture of relatively simple phenols,which result from cleavage of ether and certain carbon-carbon linkages.These phenols retain their substitu-tion patterns from the lignin polymer,and it is thus possible to identify compounds from the H,G,and S lignin units.The aim of this paper is to elucidate the changes produced in the composition and structure of the lignin in eucalypt wood with maturation and includes analyses of young plants and adult trees.This knowl-edge is important not only for providing additional insight into the mechanisms of lignin deposition but also for the industrial processing of wood for pulp, chemical,or biofuel production,as the lignin compo-sition and structure greatly influence the delignifica-tion reactions(Gonza´lez-Vila et al.,1999;del Rı´o et al., 2005).For this purpose,samples of Eucalyptus globulus wood from the same clone(to avoid genetic variations within species)were collected at different stages of growth(1month,18months,and9years)and the composition and structure of their lignins were thor-oughly investigated.A combination of the above-mentioned2D-NMR and Py-GC-MS of whole wood samples was used for the in situ study of lignin changes.In order to obtain further insights into their structures and compare with the results from the in situ analyses,MWL was also isolated from the differ-ent woods and analyzed by NMR,pyrolysis,and thioacidolysis.As far as we know,this is thefirst report describing in situ structural analyses of wood lignin during tree growth using a combination of2D-NMR and other techniques.RESULTS AND DISCUSSIONAfter a general analysis of wood composition in E. globulus plants of different ages(young and adult trees from a clonal plantation),the changes in lignin(and hemicellulose)during growth were analyzed in situ by a combination of Py-GC-MS and2D-NMR of whole wood,and the results were compared(and comple-mented)with those obtained from lignins(MWL) isolated from the same samples.Wood Composition during Eucalypt GrowthThe contents of the main wood constituents(i.e.ace-tone extractives,water-soluble material,Klason lignin, acid-soluble lignin,crystalline cellulose,amorphous glucan,xylan,arabinan,galactan,mannan,rhamnan, fucan,total uronic acids,and ash)in the selected E. globulus clone at different stages of growth are sum-marized in Table I.The total lignin content(Klason lignin plus acid-soluble lignin)increased during growth(from16%in the1-month-old sample to25% in the9-year-old wood),whereas the content of other constituents(namely acetone extractives,water-soluble material,and ash)decreased with maturity.Interest-ingly,there is also a great variation in the composition of polysaccharides(from neutral sugar analysis)dur-ing maturation,with a depletion of Ara,Gal,and Man and a progressive enrichment of Xyl.The amount of crystalline cellulose has the highest content(37%)after 18months,while that of amorphous glucan was lower and showed a progressive increase during growth. Finally,the uronic acid content was the highest after 1month(7%)and showed only a moderate decrease during growth.Variations in the uronic acid natureRencoret et al.during growth are discussed after the NMR analyses below.Py-GC-MS of Whole Woods and Their Isolated Lignins Py-GC-MS,although not a fully quantitative tech-nique,has been successfully used to analyze the relative H-G-S composition of lignin in different hard-woods,including eucalypt wood(Rodrigues et al., 1999;Yokoi et al.,1999,2001;del Rı´o et al.,2005; Rencoret et al.,2007,2008).Pyrograms from the euca-lypt wood samples after different growth periods and their corresponding MWLs are shown in Figures1and 2,and the identities and relative molar abundances of the released lignin-derived compounds are listed in Table II.The pyrolysis of the different eucalypt woods re-leased both carbohydrate-and lignin-derived com-pounds.Among the latter,guaiacol-and syringol-type phenols,derived from the G and S lignin units,were identified,including guaiacol(compound2),4-vinyl-guaiacol(8),syringol(11),4-methylsyringol(14),4-vinyl-syringol(22),4-allylsyringol(25),trans-4-propenylsyringol (32),syringaldehyde(34),and trans-sinapaldehyde (49).In addition,significant amounts of compounds derived from H lignin units,such as phenol(1), methylphenols(3and4),and dimethylphenol(6), could be detected after pyrolysis of the youngest wood,although some of them can also derive from polysaccharides(Ralph and Hatfield,1991).The H-G-S composition of the lignin in the different woods, obtained from the molar areas of all the lignin-derived compounds,is shown in Table II.In all samples,the S-type phenols were released in higher abundances than the respective G-type phenols,with a S-G ratio ranging from1.4in the youngest wood to3.8in the oldest wood.The amount of H-type compounds from the youngest wood(9%)decreases during maturation(to only2%in the oldest wood).This indicates that H units are depositedfirst,followed by G and then S units,in agreement with previous microautoradiogra-phy and microspectroscopy studies in other plants (Terashima et al.,1986).An increase of lignin S-G ratio with plant maturity has also been reported after Py-GC-MS of nonwoodyfibers(Mazumder et al.,2005). This difference in timing of monolignol deposition would also be responsible for the within-tree variation of the S-G ratio observed in Eucalyptus camaldulensis wood(Ona et al.,1997;Yokoi et al.,1999).Pyrolysis of the MWLs isolated from the different E. globulus woods(Fig.2)released a similar distribution of lignin-derived compounds as from their respective woods,although the content of H units was lower (Table II).This is especially evident in the case of the MWL isolated from the1-month-old wood.However, we must note that MWL may reflect only the most accessible part of the native lignin in the plant,which may be depleted in highly condensed H lignin units. In any case,the same trend observed in the pyrolysis of woods,which indicates an increase of S lignin units and a decrease of H and G lignin units with maturity, was also observed in the pyrolysis products of MWL, supporting the in situ analysis and confirming a monolignol deposition order of H,G,and then S during E.globulus lignification.2D-NMR of Wood Gels and Their Isolated LigninsThe eucalypt wood samples from different growing periods were analyzed by2D-NMR(in the gel state)to overcome the drawbacks associated to polymer isola-tion,namely low yield and artifact formation,and the spectra were compared with those from the lignins (MWL)isolated from the same woods.The HSQC spectra of the different woods,and their MWLs,are shown in Figures3and4.Carbohydrate signals were predominant in the spectra of the whole wood.They included correlations in the range d C/d H 60to85/2.5to5.5,which partially overlapped with lignin signals,and the well-resolved anomeric corre-lations in the range d C/d H90to110/3.5to6.0.How-ever,lignin signals were also clearly observed in the HSQC spectra,including that of the youngest wood with the lowest lignin content.On the other hand,the spectra of the MWL presented mostly lignin signals that,in general terms,matched those observed in the HSQC spectra of the woods.Lignin and carbohydrate contours in the HSQC spectra were assigned by comparison with the litera-ture(A¨mma¨lahti et al.,1998;Ralph et al.,1999,2004b; Capanema et al.,2001,2004,2005;Balakshin et al.,2003, 2005;Liitia¨et al.,2003;Ha et al.,2005;Golovchenko et al.,2007;Ibarra et al.,2007a,2007b;del Rı´o et al., 2008,2009;Kim et al.,2008;Rencoret et al.,2008,2009; C¸etinkol et al.,2010;Kim and Ralph,2010;Ralph and Landucci,2010).The main lignin correlation assign-ments are listed in Table III,and the main lignin substructures found in the different eucalypt woods are depicted in Figure5.The assignments of the main carbohydrate signals are listed in Table IV.Table I.Abundances(%)of the main constituents of E.globuluswood at different growth stagesConstituents1Month18Months9YearsAcetone extractives8.60.50.6Water-soluble extracts 6.6 1.4 2.2Klason lignin13.017.519.8Acid-soluble lignin 2.7 5.2 4.7Cellulose(crystalline)25.036.729.9Glucan(amorphous)11.415.016.2Xylan12.214.017.1Arabinan 3.80.90.8Galactan 2.7 1.2 1.5Mannan0.90.40.4Rhamnan0.70.40.5Fucan0.30.10.1Uronic acids7.4 5.9 5.8Ash 4.60.70.4Lignin in Young and Adult Eucalypt PlantsSide Chain Region of the HSQC Spectra:Analysis of Interunit Linkages in LigninThe side chain region of the spectra gave useful information about the interunit linkages present in lignin.All the spectra showed prominent signals corresponding to b-O-4#ether units(substructure A). The C a-H a correlations in b-O-4#substructures were observed at d C/d H71to72/4.7to4.9ppm,while the C b-H b correlations were observed at d C/d H84/4.3and 86/4.1ppm for substructures linked to G and S units, respectively.The C g-H g correlations in b-O-4#sub-structures were observed at d C/d H59/3.4and3.7ppm, partially overlapped with other signals.In addition, strong signals for resinol(b-b#)substructures(B)were observed in all spectra,with their C a-H a,C b-H b,and the double C g-H g correlations at d C/d H85/4.7,54/3.1, and71/3.8and4.2,respectively.Phenylcoumaran (b-5#)substructures(C)were also found,although in lower amounts,the signals for their C a-H a and C b-H b correlations being observed at d C/d H87/5.5and54/ 3.5,respectively,and that of the C g-H g correlation overlapping with other signals around d C/d H63/3.7. Finally,small signals corresponding to spirodienone (b-1#/a-O-a’)substructures(D)could also be ob-served in the spectra,their C a-H a,C a#-H a#,C b-H b, and C b#-H b#correlations being at d C/d H82/5.1,87/4.4, 60/2.8,and79/4.1,respectively.Other small signals observed in the side chain region of the HSQC spectra corresponded to C b-H b correlations(at d C/d H84/5.2) of b-O-4#substructures bearing a C a carbonyl group (F)and the C g-H g correlation(at d C/d H62/4.1)as-signed to p-hydroxycinnamyl alcohol end groups(I). The HSQC spectra of the isolated MWL also reflected the same side chain signals observed in the spectra of the whole woods,although they were better resolved and some new signals were observed.These included small signals corresponding to C b-H b correlations(atdC/d H55/2.8)of conventional open b-1#substructures (E;Lundquist,1987)that were observed only in the MWL spectra.Some aliphatic(nonoxygenated)cross-signals appeared in the d C/d H10to40/0.5to2ppm region(not included in Fig.4),which were especially abundant in the1-month sample and couldincludecutin-like material(Deshmukh et al.,2005)or other polymethylenic structures.Aromatic Region of the HSQC Spectra:Analysis ofLignin UnitsThe main cross-signals in the aromatic region of the HSQC spectra corresponded to the aromatic rings of the different lignin units.Correlations from S,G,and H lignin units could be observed in the spectra of whole wood and their MWLs.The S lignin units showed a prominent signal for the C2,6-H2,6correlation at d C/d H 104/6.7,while the G units showed different correlations for C2-H2(d C/d H111/7.0),C5-H5(d C/d H115/6.7and 7.0),and C6-H6(d C/d H119/6.8).Signals corresponding to C2,6-H2,6correlations in C a-oxidized S lignin units(S#) were observed at d C/d H107/7.3and107/7.2.Signals of H lignin units at d C/d H115/6.7and128/7.2for C3,5-H3,5 and C2,6-H2,6respectively,were only detected in the HSQC spectra of the youngest wood sample(1month), in agreement with the higher presence of H units shown by Py-GC-MS.An extra and well-resolved sig-nal was also detected at d C/d H109/7.1in this sample (in both wood and MWL)that was tentatively assigned to a G-type structure.Olefinic cross-signals of fatty acid structures with one/two double bonds,similar to those from oleic acid(d C/d H130/5.3)and linoleic acid(d C/d H 128/5.3and130/5.3),were also identified(Fig.4).They probably originate from the cutin-like structures men-tioned in the previous section.The cross-signal of pyridine used to form the wood gels was also observed (d C/d H around124/7.3).Summary of Changes in Lignin Structure as Revealedby2D-NMRThe relative abundances of the H,G,and S lignin units,and those of the main interunit linkages(re-ferred to as per100aromatic units and as a percentage of the total side chains),calculated from the HSQC spectra of the whole woods and of their respective MWLs,are shown in Table V.The H-G-S composition and the S-G ratio(ranging from1.2in the youngest wood to3.3in the oldest one)are in closeagreementwith the data obtained by Py-GC-MS,indicating a decrease of H and G units and an increase of S lignin units during lignification.The content of H lignin in the isolated MWL was lower than in the respective wood samples,as already observed by Py-GC-MS.With respect to the different linkage types,all the lignins showed a predominance of b-O-4#units(A and F;69%–72%of total side chains)followed by b-b# resinol-type units(B;16%–19%)and lower amounts of b-5#phenylcoumaran-type(C;1%–5%)and b-1#spiro-Table II.Identification and relative molar abundance(%)of the lignin-derived compounds identified in the Py-GC-MS of E.globulus wood at the different growth stages and from their isolated MWLspounds1Month18Months9Years Wood MWL Wood MWL Wood MWL1Phenol 5.5 1.00.80.20.70.32Guaiacol8.78.4 4.0 3.6 3.5 3.83Methylphenol0.90.50.30.10.30.24Methylphenol 2.70.50.40.10.40.254-Methylguaiacol 2.97.3 1.7 3.5 2.2 3.06Dimethylphenol0.30.60.40.20.50.174-Ethylguaiacol 1.9 2.60.60.80.50.884-Vinylguaiacol9.710.0 4.5 3.9 4.9 3.39Eugenol0.90.50.60.60.60.610Propylguaiacol0.50.20.10.10.10.111Syringol11.813.414.110.711.413.112cis-Isoeugenol0.70.60.50.70.40.613trans-Isoeugenol 5.4 2.3 3.1 2.5 2.7 2.5144-Methylsyringol 3.99.07.99.09.68.515Vanillin0.9 2.60.7 2.40.8 1.916Propynylguaiacol0.40.40.5 1.00.40.417Propynylguaiacol0.40.50.6 1.10.40.518Homovanillin0.00.20.30.90.50.9194-Ethylsyringol 2.9 3.2 2.3 1.90.2 2.120Vanillic acid methyl ester0.00.30.00.30.00.321Acetoguaiacone0.6 1.60.8 1.30.6 1.3224-Vinylsyringol12.68.714.6 6.612.3 6.923Guaiacylacetone0.8 1.20.30.50.40.424Propylsyringol0.00.60.00.70.00.825Allylsyringol 2.40.4 3.4 1.6 3.5 1.726Propiovanillone0.10.40.10.30.10.327Guaiacylvinylketone0.00.40.0 1.10.0 1.028cis-Propenylsyringol 1.9 1.0 2.1 1.9 1.9 2.029Propynylsyringol0.50.6 1.8 1.7 2.4 1.130Propynylsyringol0.30.40.9 1.2 1.10.731Vanillic acid0.00.50.00.20.00.132trans-Propenylsyringol 6.4 3.011.2 6.511.47.133Dihydroconiferyl alcohol0.70.50.90.30.70.334Syringaldehyde 1.8 5.5 4.610.4 5.29.135Homosyringaldehyde0.00.00.7 2.3 3.2 3.136Syringic acid methyl ester0.10.30.20.60.20.537Acetosyringone 1.4 2.6 2.6 4.2 3.5 4.338trans-Coniferyl alcohol 3.00.00.80.50.30.839Coniferaldehyde0.5 1.30.8 1.6 1.1 1.440Syringylacetone 2.2 2.3 2.3 1.4 3.0 1.541Propiosyringone0.70.90.7 1.10.9 1.042Syringyl-3-oxo-propanal0.00.60.00.60.00.743Syringylvinylketone0.10.10.2 1.20.3 1.144Syringic acid0.00.70.00.70.00.545Dihydrosinapyl alcohol0.60.2 1.10.4 1.20.546cis-Sinapyl alcohol0.50.00.60.50.40.747cis-Sinapaldehyde0.00.10.10.10.10.148trans-Sinapyl alcohol 1.30.00.60.70.3 1.849trans-Sinapaldehyde0.7 2.0 4.8 6.0 5.7 5.7Total H9.4 2.6 1.90.7 1.90.8Total G38.542.021.227.520.424.3Total S52.155.476.971.877.674.9 Rencoret et al.dienone-type (D;1%–5%)units.The conventional open b -1#structures (E;Lundquist,1987),which were ob-served only in the MWL samples,ranged from 1%to 2%.Some interesting information could be obtained from the wood NMR data.First,it is clear that the changes in monolignol availability during growth influence not only the unit composition but also af-fect the abundances of some interunit linkages.For example,despite the relative percentage of the b -O -4#linkages remaining relatively constant with growth,their abundances per aromatic unit slightly increases (from 46to 50linkages per 100aromatic units),and the same happens with the b -b #resinol-type structures (which increase from 10to 12linkages per 100aro-matic units),probably as a consequence of the increase of S units.Interestingly,the ratio between theabun-Figure 3.HSQC NMR spectra (d C /d H 45–135/2.5-8.0ppm)of the E.globulus wood samples at different growth stages after forming a gel in DMSO-d 6:pyridine-d 5(4:1).See Table III for lignin signal assignment and Figure 5for the main lignin structures identified.The assignments of the carbohydrate signals are listed in Table IV.Figure 4.HSQC NMR spectra (d C /d H 45–135/2.5-8.0ppm)of the MWLs isolated from the E.globulus wood samples at different growth stages.See Table III for lignin signal assignment and Figure 5for the main lignin structures identified.Olefinic cross-signals of unsaturated fatty acid structures (U F )were also identified.Lignin in Young and Adult Eucalypt Plantsdances of b-O-4#and b-b#resinol-type structures seems to remain more or less constant along lignifica-tion.The spirodienone-b-1#ratio also increased during growth(from0.8to3.2).In contrast,the abundance of phenylcoumaran structures decreases with lignifica-tion,which is most probably related to the decrease in G lignin observed.On the other hand,a small but continuous oxidation of the C a of the lignin side chain (from one to four C a oxidized b-O-4#linkages per100 aromatic units)occurs during lignification,probably as a result of wood aging.Finally,the abundance of cinnamyl alcohol end groups decreases with lignifica-tion,as also observed by Py-GC-MS.Hemicellulose PolysaccharidesThe HSQC spectra also reveal differences in the carbohydrates present in eucalypt wood after the different growth periods,which are observed in two differentiated regions of the spectra:the aliphatic-oxygenated region and the region corresponding to the anomeric correlations(Fig.3).The aliphatic-oxy-genated region shows strong signals from carbohy-drates,including naturally acetylated hemicelluloses. Among them,signals from O-acetylated xylans(3-O-acetyl-b-D-xylopyranoside[X#3]and2-O-acetyl-b-D-xylopyranoside[X#2])and,at the earlier stages of growth,O-acetylated mannans(2-O-acetyl-b-D-man-nopyranoside[M#2])were observed.Other signals in this region correspond to C2-H2,C3-H3,C4-H4,and C5-H5correlations of xylans(b-D-xylopyranoside[X2,X3, X4,and X5]),which overlap with unassigned cross-signals of other pentose and hexose polysaccharide units(note that crystalline cellulose is practically“in-visible”in the HSQC spectra of the wood gels due to its reduced mobility),and the C4-H4correlation for 4-O-methyl-a-D-GlcUA(U4).However,the main differences are observed in the carbohydrate anomeric region of the spectra,which have been depicted in detail in Figure6.The main C1-H1correlation signals in this region,which are listed in Table IV,were assigned according to Kim and Ralph (2010),together with some additional references for pectin(Ha et al.,2005;Golovchenko et al.,2007, Hedenstro¨m et al.,2008).Cross-signals from arabinans (Ar1and Ar1(T)),mannans(M1),galactans(Ga1),xylans (X1,a X1(R),and b X1(R)),and glucans including non-crystalline cellulose(Gl1),as well as signals from O-acetylated mannans and xylans(M#1and X#1)and from the4-O-methyl-a-D-glucuronic(U1)and galact-uronic(UGA1)acids(the latter forming part of pectin as the methyl ester)are readily apparent and well resolved in this region of the spectra.A small signal ofa-Rha(R1)units was also observed,especially in the 18-month-old wood.Interestingly,the signals of arab-inans,mannans,and galactans,which are observed inTable III.Assignments of the lignin13C-1H correlation signals in the HSQC spectra of E.globuluswood at the different growth stages and their isolated MWLsLabels d C/d H AssignmentppmC b53.5/3.46C b-H b in phenylcoumaran substructures(C)B b53.5/3.06C b-H b in resinol substructures(B)E b55.0/2.75C b-H b in b-1#substructures(E)-OMe55.6/3.73C-H in methoxylsA g59.4/3.40and3.72C g H g in b-O-4#substructures(A)D b59.6/2.75C b-H b in spirodienone substructures(D)I g61.3/4.09C g-H g in cinnamyl(sinapyl/coniferyl)alcohol endgroups(I)C g62.5/3.72C g-H g in phenylcoumaran substructures(C)B g71.0/3.83and4.19C g-H g in resinol substructures(B)A a71.7/4.86C a-H a in b-O-4#substructures(A)D b#79.3/4.11C b#-H b#in spirodienone substructures(D)D a81.2/5.09C a H a in spirodienone substructures(D)A b(G)83.5/4.28C b-H b in b-O-4#linked to a G unit(A)F b83.8/5.23C b-H b in oxidized(C a=O)b-O-4#substructures(F)B a84.8/4.67C a-H a in resinol substructures(B)D a#84.8/4.75C a#H a#in spirodienone substructures(D)A b(S)85.8/4.11C b-H b in b-O-4#linked to a S unit(A)C a86.8/5.46C a-H a in phenylcoumaran substructures(C)S2,6103.8/6.69C2,6-H2,6in etherified syringyl units(S)S#2,6106.6/7.32and7.19C2,6-H2,6in oxidized(C a=O)phenolic syringyl units(S#)G2110.9/6.99C2-H2in guaiacyl units(G)D2#113.2/6.27C2#H2#in spirodienone substructures(D)H3,5114.9/6.74C3,5-H3,5in p-hydroxyphenyl units(H)G5/G6114.9/6.72and6.94;118.7/6.77C5-H5and C6-H6in guaiacyl units(G)D6#118.9/6.09C6#H6#in spirodienone substructures(D)H2,6128.0/7.23C2,6-H2,6in p-hydroxyphenyl units(H)Rencoret et al.。
细菌真菌的区别与联系

相同点:结构上都有细胞膜、细胞壁(成分不同)、细胞质、核糖体,组成上都有DNA、RNA、蛋白质、水、糖类、脂类等等The same point: the structure has a cell membrane, cell wall (different components), cytoplasm, ribosomes, the composition of both DNA, RNA, protein, water, carbohydrates, lipids, etc.不同点:细胞和真菌的不同点:生物类型、结构、大小、增殖方式和名称上都有不同:different points Biological type, structure, size, proliferation, and the name has a different way一、生物类型First, no bacteria membrane surrounding the nucleus formation, are prokaryotes; fungi membrane surrounding formation nucleus, are eukaryotes.一是就有无成形的细胞核来看:细菌没有核膜包围形成的细胞核,属于原核生物;真菌有核膜包围形成的细胞核,属于真核生物。
二是就组成生物的细胞数目来看:细菌全部是由单个细胞构成,为单细胞型生物;真菌既有由单个细胞构成的单细胞型生物(如酵母菌),也有由多个细胞构成的多细胞型生物(如食用菌、霉菌等)。
The second is the number of cells on the formation of biological point of view: All bacteria constituted by a single cell, single-cell organisms; fungi both constituted by a single cell, single-cell organisms (such as yeast), there are also constituted by a plurality of cells multi-cell organisms (such as fungi, molds, etc.).二、细胞结构细菌和真菌都具有细胞结构,属于细胞型生物,在它们的细胞结构中都具有细胞壁、细胞膜、细胞质,但却存在诸多不同,具体表现在:一是细胞壁的成分不同:细菌细胞壁的主要成分是肽聚糖,而真菌细胞壁的主要成分是几丁质。
植物细胞壁组成物质

植物细胞壁组成物质The composition of the plant cell wall is a complex and fascinating topic that plays a crucial role in thestructure and function of plants. Composed primarily of cellulose, hemicellulose, and lignin, the plant cell wall provides strength, support, and protection to plant cells. This intricate network of molecules also contributes to various physiological processes, such as cell expansion, cell-to-cell communication, and defense against pathogens. Understanding the composition of the plant cell wall is essential for comprehending the biology of plants and their interactions with the environment.Cellulose, the main component of the plant cell wall,is a long-chain polymer made up of glucose units. It forms microfibrils that are embedded in a matrix of hemicellulose and pectin. Cellulose provides structural integrity to the cell wall and gives plants their rigidity. It is a remarkable molecule that can withstand tremendous mechanical stress, allowing plants to grow upright andresist external forces. The arrangement and orientation of cellulose microfibrils determine the mechanical properties of the cell wall, making it adaptable to different tissues and plant species.Hemicellulose, another major component of the plant cell wall, is a heterogeneous group of polysaccharides. It surrounds and interacts with cellulose microfibrils, providing cross-linking and stability to the cell wall structure. Hemicellulose also plays a role in regulating cell expansion and plant growth. Different types of hemicellulose can be found in various plant tissues, reflecting their specific functions and requirements. For example, xyloglucans are prevalent in primary cell walls and are involved in cell expansion, while xylans are abundant in secondary cell walls and contribute to their strength and rigidity.Lignin, a complex phenolic polymer, is a crucial component of the secondary cell wall. It provides additional strength and water impermeability to the cell wall, allowing plants to withstand mechanical stresses andresist microbial attack. Lignin also contributes to the woody nature of plant tissues, enabling the formation of sturdy structures like tree trunks. However, lignin poses challenges in the utilization of plant biomass for various industrial applications, such as biofuel production, due to its recalcitrant nature and resistance to degradation.Besides cellulose, hemicellulose, and lignin, the plant cell wall also contains other components such as pectin, proteins, and various minor polysaccharides. Pectin is a complex polysaccharide that acts as a glue, binding cells together and providing flexibility to the cell wall. Proteins are crucial for cell wall synthesis, remodeling, and signaling. They contribute to the structural integrity of the cell wall and participate in various physiological processes. Minor polysaccharides, such as arabinogalactans and arabinans, are involved in cell wall assembly and modification.The composition of the plant cell wall is not static but can change in response to developmental and environmental cues. For example, during cell expansion, thecomposition and arrangement of cellulose, hemicellulose, and pectin may be modified to accommodate the growth of the cell. Similarly, in response to pathogen attack, the cell wall can undergo structural changes to strengthen its defense mechanisms. Understanding these dynamic changes in the composition of the plant cell wall is crucial for developing strategies to enhance plant growth, improve crop yield, and protect plants against diseases and pests.In conclusion, the plant cell wall is a complex and dynamic structure composed of cellulose, hemicellulose, lignin, pectin, proteins, and minor polysaccharides. This intricate network of molecules provides strength, support, and protection to plant cells, allowing them to grow upright and resist external forces. The composition of the cell wall can change in response to developmental and environmental cues, reflecting the adaptability and resilience of plants. By unraveling the composition and functions of the plant cell wall, scientists can gain valuable insights into plant biology and develop innovative strategies to enhance plant growth and protect crops.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
HARNESSING PLANT BIOMASS FOR BIOFUELS AND BIOMATERIALSCell-wall carbohydrates and their modification as a resource for biofuelsMarkus Pauly *and Kenneth KeegstraDepartment of Energy Plant Research Laboratory and Department of Biochemistry and Molecular Biology,Michigan State University,East Lansing,MI 48824,USAReceived 1December 2007;revised 5February 2008;accepted 8February 2008.*For correspondence (fax +15173539168;e-mail paulymar@).SummaryPlant cell walls represent the most abundant renewable resource on this planet.Despite their great abundance,only 2%of this resource is currently used by humans.Hence,research into the feasibility of using plant cell walls in the production of cost-effective biofuels is desirable.The main bottleneck for using wall materials is the recalcitrance of walls to efficient degradation into fermentable sugars.Manipulation of the wall polysaccharide biosynthetic machinery or addition of wall structure-altering agents should make it possible to tailor wall composition and architecture to enhance sugar yields upon wall digestion for biofuel fermentation.Study of the biosynthetic machinery and its regulation is still in its infancy and represents a major scientific and technical research challenge.Of course,any change in wall structure to accommodate cost-efficient biofuel production may have detrimental effects on plant growth and development due to the diverse roles of walls in the life of a plant.However,the diversity and abundance of wall structures present in the plant kingdom gives hope that this challenge can be met.Keywords:cell walls,polysaccharide biosynthesis,hemicellulose,biofuel.Plant cell walls are the most abundant renewable resource on this planetIt has been estimated that the net CO 2fixation by land plants per year is approximately 56·109tonnes (Table 1)(Field et al.,1998)and that the worldwide biomass production by land plants is 170–200·109tonnes (Lieth,1975).Of this amount,70%is estimated to represent plant cell walls (Duchesne and Larson,1989;Poorter and Villar,1997).Humans use these wall materials mainly in the form of wood for heat production (Table 2),and as a building material (timber),in the pulp and paper industry (Fenning and Gershenzon,2002),and as raw material in the textile industry, e.g.cotton fibers (/site/567/default.aspx;parameter settings:production quantity,cot-ton lint,world,2006).Taken together,only 2%of the plant cell-wall-based biomass is currently utilized by humans (Table 2).It is thus not surprising that interest in using this resource as a material for biofuels has increased in recent years (Schubert,2006).Important advantages of wall materials as feedstocks for biofuel production are their greatabundance and the fact that they do not serve as food for animals and humans as starch does,for example.All cell walls of higher plants contain cellulose,a homo-polymer of b -1,4-linked glucose units,mainly in the form of crystalline microfibrils as well as in an amorphous form (Carpita and McCann,2000).Walls also contain hemicellu-loses (Figure 1),such as substituted glucans,xylans and/or mannans,and anionic components such as the galacturonic acid-containing pectic polysaccharides.Walls may also contain polyphenols such as lignins,and,to a minor extent,structural proteins.The prevalence of polysaccharides in the wall is particularly advantageous for plants,as they are generated directly from the products of photosynthesis without the utilization of large amounts of nitrogen or phos-phorus,two macronutrients that frequently limit plant growth.Not all cell walls have the same polysaccharide composition When considering wall materials for the production of bio-fuels,one should be aware that walls from higher plantsª2008The Authors559Journal compilation ª2008Blackwell Publishing LtdThe Plant Journal (2008)54,559–568doi:10.1111/j.1365-313X.2008.03463.xdiffer quite substantially in content,both qualitatively and quantitatively.While the cell is still elongating,a primary wall is formed.Primary walls contain cellulose and a hydrated (65%water)matrix consisting of hemicelluloses and pectins,with some structural proteins (Brett and Waldron,1996).Based on their polysaccharide composition,primary cell walls are usually classified as type I or type II.Type I walls are present in dicots and non-commelinoid monocots;in addition to cellulose,they generally contain xyloglucan as the main hemicellulose and abundant amounts of pectic polysaccharides (Carpita and Gibeaut,1993).In type II walls,the walls of the Poales,such as the grasses,arabinoxylan is the major hemicellulose.In addition,type II walls contain a higher percentage of cellulose and only negligible amounts of pectins and proteins (Carpita,1996).Secondary walls,deposited once cell elongation ceases,are usually thicker than primary walls and may be deposited in a number of layers.Sec-ondary walls contain cellulose and arabinoxylan and/or glucomannans as the major hemicellulose (Brett and Waldron,1996).More importantly,in secondary walls,water is largely replaced by lignin,making them nearly impenetrable to solutes and enzymes.At the single-plant level,nearly all of the approximately 35different cell types can be distinguished based on their varying wall structures,as observed by microscopy (Carpita and McCann,2000),chemical composition analysis (Richmond and Somerville,2001)and labeling of wall polymers with specific antibodies (Willats et al.,2000).Hence,it is notTable 2Annual human utilization of plant cell walls Material ProductTonnes per year Wood Energy1.05·109WoodTimber,pulp and paper0.95·109Table 1Annual production of plant cell walls Production SourceTonnesAssimilated CO 2Land plants,net primary production 56·109Plant biomass Land,worldwide 170–200·109Cell wallsLand,worldwide150–170·109560Markus Pauly and Kenneth Keegstraª2008The AuthorsJournal compilation ª2008Blackwell Publishing Ltd,The Plant Journal ,(2008),54,559–568surprising that plant-wall feedstocks that could be used for biofuel production can differ quite significantly in their composition,even though the materials derive mainly from secondary walls(Figure2).In Table3,some of the prominent plant materials that could be used for biofuel production are listed.Although the numbers cannot be compared with each other because different methods were used to establish them,some general features become evident.The most dominant polysaccharide in these walls is cellulose,making up40.6–51.2%of the wall material.The next largest fraction comprises the hemi-celluloses,representing28.5–37.2%of the walls.Lignin occurs in these walls at a lower percentage(13.6–28.1%), with a more than twofold difference between walls of switchgrass,for example,and those of softwood.Viewed in greater detail,composition is seen to vary widely with regard to the hemicelluloses(Table3,and references cited therein;for structures,see Figure1).The grasses contain mainly arabinoxylan,but the degree of arabinosylation can vary greatly.Wheat straw contains the lowest degree of substitution,whereas sorghum xylans have an excep-tionally high degree of substitution(Verbruggen et al., 1995).Hardwood(from angiosperm trees)also contains mainly xylan,but with a negligible degree of arabinosy-lation.The xylans here are mainly substituted with glucuronic acid or4-O-methyl-glucuronic acid residues (Ebringerova and Heinze,2000).In contrast,softwood (from conifers)contains mannans such as O-acetylated galactoglucomannans(Capek et al.,2002)as their main hemicellulose,although ample amounts of xylans are also present.Most importantly,the differences and ranges of wall components and theirfine structures are also a result of the differences in tissues from which the feedstocks are derived(e.g.corn stover;Chundawat et al., 2007).Bottlenecks in utilizing cell-wall materials for biofuelsPlant cell-wall materials can be converted in a number of ways.One way is the combustion and gasification of plant material.The resulting CO and hydrogen gas(also called syngas)can be converted to hydrocarbons of various lengths via a catalyzed chemical reaction(Fischer–Tropsch process).Hydrocracking of the large hydrocarbons can be used to produce diesel fuels(Tijmensen et al.,2002).Here, the main objective from a cell-wall perspective is simplyan Table3Comparison of biomass feedstocksFeedstock a Cellulose Hemi cellulose Lignin Ash Protein Solubles ReferenceCorn stover b39.433.114.9ND 3.78.9Chundawat et al.(2007) Wheat straw34.922.521.39.4ND11.9Lynd et al.(1999)Rice straw41.631.512.514.4a ND ND Jin and Chen(2007) Miscanthus41.926.613.3 3.2ND15.0Magid et al.(2004) Sorghum(whole sorghumpith and bark)15.012.3 5.80.4ND66.5b Billa et al.(1997)Switchgrass(late cut)46.132.212.3 4.7 4.6ND Lynd et al.(1999)Sugar cane48.631.119.1 1.2ND ND Sanjuan et al.(2001) Hardwood(beech;Fagus sylvatica)43.331.824.40.5ND ND Fengel and Wegener(1989) Softwood(spruce;Picia abies)40.431.128.00.5ND ND Fengel and Wegener(1989)Values have been adjusted to a percentage basis(dry weight).a Mainly silicate.b Mainly sucrose.ND,not determined in these studies.Cell-wall carbohydrates561ª2008The AuthorsJournal compilationª2008Blackwell Publishing Ltd,The Plant Journal,(2008),54,559–568increase in the production of biomass(cell walls)per hectare,irrespective of its wall composition,although a low water and ash content is desirable(Tijmensen et al., 2002).A more sophisticated approach in biofuel produc-tion involves the degradation of wall materials to mono-saccharides and subsequent fermentation to liquid fuels such as bioethanol(Schubert,2006).However,plants have evolved wall structures to accommodate their needs in completing their lifecycle,not to suit mankind’s desire to exploit this resource for the production of biofuels.As a result,cell walls are naturally resistant to breakdown by mechanical and microbial forces,which are precisely the processes needed for the cost-effective and efficient pro-duction of monosaccharides.Hence,one major objective is to make walls more accessible to degradation(Himmel et al.,2007;Houghton et al.,2006).We could achieve this goal by increasing water solubility and hence access of enzymes to polysaccharides.One way to do this would be to add de novo synthesized,water-soluble polysaccharides to existing cells,leading to a greater abundance of these polysaccharides in the wall.One alternative would be a shift in the ratio of less soluble polysaccharides to soluble ones.This objective would require:(1)for cellulose,an increase in the abundance of amorphous glucan chains rather than crystalline microfibrils;(2)for hemicelluloses, addition of side chains to decrease hydrogen bonding with cellulose microfibrils;and(3)for lignins,a general reduc-tion in their amount or amendment to a more easily degradable form(Akin,2007),for example by introduction of specific monolignols(Boerjan et al.,2003;Chen and Dixon,2007)and/or decrease of the existing lignin–hemi-cellulose linkages(Grabber et al.,2002).Another consid-eration is the fermentability of the wall degradation products,the resulting monosaccharides.Currently,the sugars most easily fermentable by yeasts are the hexoses, such as glucose and mannose,rather than the pentoses, although yeast and bacterial strains have been developed that can efficiently ferment pentoses(Chu and Lee,2007). Hence,an increased production of hexose-containing polymers such as cellulose,glucomannans and to some extent xyloglucan is more desirable than an increase in arabinoxylans,for example(see hexose and pentose annotation in Figure1).Depending on the fermentation-or catalyst-based chemical process(Huber et al.,2005)used to produce fuels,monosaccharide fermentation-inhibiting components,such as aliphatic acids(e.g.acetic acid)or phenolic compounds(Larsson et al.,1999)are present to varying degrees in the degraded biomass.One goal should be to reduce the abundance of such compounds to a minimum.All of the above-mentioned changes could be accom-plished by either manipulation of the plant biosynthetic pathways for the respective polymers and/or post-deposi-tion metabolism alterations in planta.Manipulation of the biosynthetic pathwaysThe natural variability in wall compositional quantity and quality(Figure2)suggests that there is an opportunity for altering the abundance of specific wall components without compromising the life cycle of a plant.Such a feat could be accomplished by manipulation of the biosynthesis of specific wall polysaccharides.The two most abundant polysaccharides of plant cell walls,cellulose and hemicellulose,are synthesized in different compartments by significantly different processes. Cellulose,generally the most abundant component in secondary cell walls(see Figure1),is synthesized at the plasma membrane by a complex machinery that we are just beginning to understand(Somerville,2006).Whereas the glucosyl residues come from UDP-glucose molecules that are present in the cytosol,the cellulose microfibrils are deposited into the extracellular wall matrix at a location adjacent to the plasma membrane(Somerville,2006).On the other hand,the hemicellulosic polysaccharides present are synthesized in the Golgi and packaged into secretory vesicles before delivery to the cell surface and incorporation into the wall matrix.The assembly events that combine these components into the composite that exists in the wall matrix are still poorly understood.The CesA proteins are thought to be the catalytic subunits of the cellulose synthase complexes(Somerville,2006). These proteins are encoded by a family of CesA genes that are found throughout the plant kingdom(Hazen et al.,2002; Richmond and Somerville,2000).Genetic studies have led to the conclusion that three CesA genes are needed for cellulose biosynthesis in primary cell walls(Persson et al., 2007b),and another set of three CesA genes is required for cellulose synthesis in secondary cell walls(Somerville, 2006).The three different CesA proteins are thought to cluster into a higher-order structure,which forms the rosette structure observed in the plasma membrane(Somerville, 2006).The rosettes containing multiple CesA proteins are thought to move in the plasma membrane in a direction that is defined by cortical microtubules,thereby producing cellulose microfibrils outside the plasma membrane.These microfibrils are deposited in a pattern that reflects the orientation of the cortical microtubules present on the cytosolic side of the plasma membrane(Paredez et al., 2006).Despite this emerging outline of how cellulose is deposited,many important issues remain unresolved. Genetic experiments provide evidence that additional pro-teins are involved in cellulose deposition(Lane et al.,2001; Pagant et al.,2002).One of these proteins has been shown to be a membrane-bound endoglucanase/cellulase and is thought to act as an editing/repairing protein during cellulose biosynthesis(Mølhoj et al.,2002).However,the precise roles of this and the other proteins are still not clear.562Markus Pauly and Kenneth Keegstraª2008The AuthorsJournal compilationª2008Blackwell Publishing Ltd,The Plant Journal,(2008),54,559–568Whether the encoded proteins are part of the rosette structure or whether they have other roles in cellulose deposition remain to be determined.As more is learned about the details of cellulose biosyn-thesis,it may be possible to alter these processes in ways that would render the walls more easily digestible during processing to biofuels.For example,if one understood the details of how the glucan chains come together to form crystalline cellulose,it might be possible to modify this process such that cellulose microfibrils would have larger amorphous regions.The biosynthesis of hemicellulosic polysaccharides in the Golgi apparatus differs significantly from cellulose biosyn-thesis.In the case of the mannans,the backbone is synthe-sized by CslA proteins(Liepman et al.,2007)that have been identified in a number of species(Dhugga et al.,2004; Liepman et al.,2005,2007;Suzuki et al.,2006).Each plant species for which the complete genome is available has a small family of CslA genes that are part of the CesA superfamily.The CslA proteins produced in heterologous systems not only have the ability to synthesize mannan when GDP-mannose is present,they also have the ability to synthesize glucomannan when a mixture of GDP-mannose and GDP-glucose is present(Liepman et al.,2005,2007; Suzuki et al.,2006).Thus,the same protein is able to incorporate both sugars into the backbone in vitro,and it is likely that the same proteins produce both mannans and glucomannans in vivo.The degree of galactosylation of the mannan backbone has implications for mannan solubility.Mannans with a low degree of galactosyl substitution have limited solubility in water,whereas polymers with a high degree of substitution have important properties as emulsifiers(Reid et al.,1988). The galactosyltransferase enzymes that add side chains to the mannan and glucomannan backbones have been iden-tified and characterized(Edwards et al.,1999,2002).The levels of these enzymes appear to control the degree of substitution of the backbone,with the side chains being added in patterns that are described by hidden Markov models(Edwards et al.,2004).Altering the degree of side-chain substitutions will be vital in engineering more soluble mannans.The mannans are attractive candidates for enhancing wall composition with the aim of creating improved biofuel crops for several reasons.First,the genes and proteins needed for mannan biosynthesis have been identified.Second,the genes needed for mannan biosynthesis appear to be present in all land plants,although their expression levels are such that few mannans are present in the walls of most angio-sperms.However,mannans accumulate to high levels in the seeds of many plants,where they serve as storage carbo-hydrates(Meier and Reid,1982).During germination,seed-lings have the ability to rapidly degrade the mannans and use the resulting sugars as a source of carbon for early seedling development.Because the released sugars are all hexoses,they can easily speed up the central metabolism of the developing seedling.Given these circumstances,it may be possible to enhance mannan levels in vegetative tissues such that the polymers could be easily degraded after harvest to yield hexoses,which could be converted to biofuels more efficiently than the pentoses released from the more abundant xylans.Although xyloglucan is found mainly in the primary cell walls of many plants(Hayashi,1989),its biosynthesis is relevant to our general understanding of hemicellulose biosynthesis and therefore will be briefly summarized here. All the glycosyltransferases involved in synthesis of its side chains have been tentatively identified(see reviews by Scheible and Pauly,2004;Lerouxel et al.,2006).However,it remains unclear how the enzymes achieve the structural side-chain diversity found in this polymer.Cocuron et al.(2007)recently presented evidence that the glucan synthase required for making the backbone of xyloglucan is encoded by a CslC gene.When CslC genes were expressed in Pichia pastoris cells,the cells accumu-lated significant quantities of oligosaccharides containing b-1,4-linked glucosyl residues.When one of the xyloglucan xylosyltransferase genes,which is responsible for substitut-ing the glucan backbone with xylosyl residues(Faik et al., 2002),was co-expressed with the CslC gene,the cells produced large quantities of unsubstituted b-1,4-glucan (Cocuron et al.,2007).These observations provide evidence that these xylosyltransferase and glucan synthase enzymes interact to form a complex that has an impact on the nature of the resulting product,even though one of them does not exhibit any activity.Further work is needed to confirm this interesting hypothesis,as protein complexes involved in hemicellulose biosynthesis have yet to be discovered. Given that xylans are the most abundant hemicellulose present in the secondary walls of plants being considered for use in biofuel production(see Figure1),it is unfortunate that we know so little about their biosynthesis.Recently,several groups have begun to make progress in this difficult area. One of the most interesting observations comes from the work of Pen˜a et al.(2007),who examined the xylan polysac-charides present in two mutant lines of Arabidopsis that have irregular xylem phenotypes.First,these authors redis-covered an older,but little noticed,observation that xylan polysaccharides often have an unusual oligosaccharide at the reducing end.This oligosaccharide contains the glycosyl sequence4-b-D-Xyl-(1,4)-b-D-Xyl-(1,3)-a-L-Rha-(1,2)-a-D-GalA-(1,4)-D-Xyl.Because it is at the reducing end of the polysac-charide,it is possible that this oligosaccharide serves as the primer for chain initiation,if chain elongation occurs from the reducing end toward the non-reducing end,as is commonly hypothesized.Both of the mutants have reduced levels of xylan in the secondary walls of xylem elements, leading to the irregular xylem phenotype.Cell-wall carbohydrates563ª2008The AuthorsJournal compilationª2008Blackwell Publishing Ltd,The Plant Journal,(2008),54,559–568One of the mutants,irx9,has increased levels of the unusual oligosaccharide,but the chains containing it are shorter than in wild-type plants,suggesting that the IRX9 gene is involved in elongating the xylan chains.On the other hand,the other mutant,irx8,has little of the unusual oligosaccharide and lower quantities of xylan(Persson et al.,2007a);the xylan that is present is longer and more heterodisperse in size.These observations suggest that IRX8 may be involved in synthesizing the unusual oligosaccha-ride,which may serve as a primer in wild-type plants.These observations highlight the complexity of xylan biosynthesis, but offer some hope that these new observations can lead to an improved understanding of this important cell-wall polymer.As illustrated above,much is to be learned about the biosynthetic machinery of polysaccharides.We are just beginning to understand the carbonflux into the specific wall polysaccharides(Sharples and Fry,2007)and regulation of polysaccharide biosynthesis.Post-deposition wall changesAnother way to make wall structures more enzyme-acces-sible is to add‘loosening agents’through transgenic approaches.Such agents include the expansins,plant pro-teins that have been shown to induce the extensibility of plant tissues under stress(Cosgrove,2000).The precise mechanism of expansin action is unknown,but it is thought that they act at the interface of hemicellulose polymers and cellulose microfibrils(Cosgrove,2000).Proteins with a similar mode of action are the fungal swollenins,proteins that consist of a cellulose-binding domain and an expansin-like domain.They are thought to disrupt cellulose micro-fibrils without hydrolytic activity,i.e.the release of reducing sugars(Saloheimo et al.,2002).Adding expansins to wall materials can double the yield of sugars released by fungal cellulases(Cosgrove,2001a,b),and it is expected that swollenin might have a similar effect.Another class of proteins that could be used to make the wall more accessible is glycanases,in particular endoglucanases.Expression of a poplar endoglucanase in Arabidopsis leads to increased cell elongation and subsequent plant growth(Park et al.,2003).A similar effect was found when a fungal xyloglucanase(a xylo-glucan-specific endoglucanase;Pauly et al.,1999)was expressed in poplar(Park et al.,2004).This is not surprising,as this enzyme is the only protein other than expansin that is known to induce wall extension(Yuan et al.,2001).One effect in the transgenic poplar material is an observed increase in cellulose,which leads to material with higher hexose content.A side-effect of the enhanced growth of these transgenic plants is an increase in the photosynthetic canopy,potentially allowing more biomass to accumulate.Other agents that work on the hemicellulose–cellulose network are xyloglucan transglycosylases/hydrolases.This class of enzymes is thought to be involved in either incorporating newly synthesized hemicelluloses and/or remodeling existing hemicelluloses present in the wall by loosening/re-ligating xyloglucan(Fry et al.,1992;Nishitani, 1997).It has been demonstrated that xyloglucan transgly-cosylases/hydrolases are active in cell elongation and act at the cellulose/xyloglucan interface(Vissenberg et al.,2000, 2005).Manipulating the levels of this agent thus has the potential to loosen cell-wall structures.Other examples of glycanases that have been expressed in plants are pectin-degrading enzymes.Expression of a galactanase in potato tubers led to significant wall alteration (i.e.reduction of galactans),but had no effect on plant or tuber development(Sørensen et al.,2000).Interestingly,the tubers exhibited a marked change in physical tissue prop-erties(Ulvskov et al.,2005).In particular,the water-binding capacity was changed,indicating that removing pectin side chains would probably render such wall material less degradable.Agents that work on the hemicellulose–lignin interface, i.e.that break the covalent bonds between the polymers,can lead to more easily degradable wall materials.For example, expression of phenolic esterases improves the release of fermentable sugar(Akin,2007).Modification of plant cell walls will challengetheir biological functionAny strategy to improve wall materials in planta as feed-stocks for biofuels needs to take into account the possibility of functional‘failure’of the cell wall,which could be detri-mental to plant growth,leading to a concomitant reduction in wall biomass and ultimately threatening the very survival of the plant.Cell walls are important for structural integrity of the cell and indeed the whole plant.Through the evolutionary introduction of polyphenol incorporation into the wall, land plants were able to increase their sunlight-harvesting capacity by increasing the plant canopy,not only in terms of width,but also in terms of height,enabling the plant to compete with other plants for sunlight.A number of examples have demonstrated that altering walls can lead to structural concessions such as dwarfism(Desprez et al., 2007)and even to lethality(Goubet et al.,2003).Cell walls determine the shape of the cell,so altering them can lead to changes in morphology,such as an irregular xylem (Turner et al.,2007),that may be disadvantageous to water transport.Walls,in particular the pectinaceous middle lamellae,ensure attachment of the cells.A num-ber of mutants with altered pectic polysaccharides have been shown to have reduced cell adhesion(Bouton et al., 2002;Iwai et al.,2002;Krupkova et al.,2007),probably564Markus Pauly and Kenneth Keegstraª2008The AuthorsJournal compilationª2008Blackwell Publishing Ltd,The Plant Journal,(2008),54,559–568limiting the plant’s ability to withstand certain mechanical stresses(such as wind).On the other hand,wall materials from such plants might be more accessible to wall-degrading enzymes,making processing of these plants more rapid,easier,and thus more cost-effective.Another function of the wall is keeping plant pathogens such as bacteria and fungi away from the nutritious cytosolic content of the cells.In addition to a simple mechanical line of defense,walls contain signaling molecules that allow the plant cell to recognize a pathogen attack and to respond with various lines of defense(Cote and Hahn, 1994;Vorwerk et al.,2004).Alterations in wall composi-tion and architecture thus also introduce the possibility of increased susceptibility to pathogens,or endogenous release of wall-derived oligosaccharides might lead to disease symptoms.For example,expression of a fungal arabinanase in potato tubers led to a severely stressed plant morphology(Skjot et al.,2002),presumably through the release of apoplastic arabinan-oligosaccharides.This morphology was overcome when the enzyme was tar-geted to the Golgi apparatus instead of the apoplast. Targeting and retaining the arabinanase in the Golgi led to plants and tubers with unaltered appearance but with a significant decrease in pectic arabinans.In a few cases where inhibition of polysaccharide biosynthesis through genetic engineering of the glycan synthases was achieved,these alterations did not result in diminishment of pathogen resistance(Jacobs et al.,2003), and in some cases even increased resistance(Hernandez-Blanco et al.,2007).The plant cell wall is a dynamic entity that undergoes delicate metabolic changes during cell elongation and differentiation.Throughout the elongation process,the cell must balance loosening the wall with maintaining turgor pressure and cohesiveness of the wall structure.It is thought that metabolism of the hemicellu-loses interlacing cellulose microfibrils with wall-loosening enzymes such as endoglucanases,xyloglucantransglycos-ylases and expansins allows slippage of cellulose micro-fibrils and thus controlled cell elongation(Cosgrove, 2001a,b).As cells elongate,new cell-wall material is deposited(Refregier et al.,2004),probably leading to strengthening of the wall.Consequently,changing the abundance or structure of wall polymers may stiffen the wall to the extent that the cell cannot enlarge effectively, or may lead to mechanical failure and hence bursting of the cell during the elongation process.It has become clear that the plant cell has a hitherto unknown mecha-nism for monitoring wall integrity and compensating for change(Humphrey et al.,2007;Pilling and Ho¨fte,2003). Candidates for such a monitoring activity are plasma membrane-localized,wall-associated kinases(Wagner and Kohorn,2001),which that have been shown to bind to the pectin matrix in the apoplast(Kohorn et al.,2006). Recently,another plasma membrane-localized receptor has been identified that may also act as such a wall sensor(Hematy et al.,2007).Manipulating putative sens-ing mechanisms has the potential to overcome unex-pected wall structural changes,even though they might be beneficial,such as decreasing lignin content but increasing the relative content of cellulose(Hu et al., 1999).Concluding remarksOwing to the abundance of cell-wall material generated by plants,cell walls could play a prominent role in our quest for the reduced utilization of carbon dioxide-emit-ting fossil fuels.The scientific and technical challenges inherent in realizing this goal are enormous.The pro-duction of walls with tailored polysaccharide composition and structures is still in its infancy due to our lack of knowledge of polysaccharide biosynthesis and its regula-tion.Despite the above-mentioned difficulty of making the wall polysaccharides more degradable,the current recal-citrance of wall materials brings with it the advantage that harvested wall materials,unlike grains and fruits,can be stored relatively easily for extended periods without loss of yield prior to factory processing.Also,identification of specific bioenergy crop species with high biomass yields grown in various climatic regions has just begun,as have breeding programs for the increased production of biomass.ReferencesAkin,D.E.(2007)Grass lignocellulose–strategies to overcome recalcitrance.Appl.Biochem.Biotechnol.137,3–15.Billa,E.,Koullas,D.P.,Monties,B.and Koukios,E.G.(1997)Struc-ture and composition of sweet sorghum stalk components. Indust.Crops Prod.6,297–302.Boerjan,W.,Ralph,J.and Baucher,M.(2003)Lignin biosynthesis. Annu.Rev.Plant Biol.54,519–546.Bouton,S.,Leboeuf,E.,Mouille,G.,Leydecker,M.T.,Talbotec,J., Granier,F.,Lahaye,M.,Ho¨fte,H.and Truong,H.N.(2002)Quasi-modo1encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Ara-bidopsis.Plant Cell,14,2577–2590.Brett,C.and Waldron,K.(1996)Physiology and Biochemistry of Plant Cell Walls.Topics in Plant Functional Biology.London: Chapman and Hall.Capek,P.,Alfoldi,J.and Liskova,D.(2002)An acetylated galacto-glucomannan from Picea abies L.Karst.Carbohydr.Res.337, 1033–1037.Carpita,N.C.(1996)Structure and biogenesis of the cell walls of grasses.Annu.Rev.Plant Physiol.Plant Mol.Biol.47,445–476.Carpita,N.C.and Gibeaut,D.M.(1993)Structural models of primary cell walls inflowering plants:consistency of molecular structure with the physical properties of the walls during growth.Plant J.3, 1–30.Carpita,N.and McCann,M.(2000)The cell wall.In Biochemistry and Molecular Biology of Plants(Buchanan,B.B.,Gruissem,W.andCell-wall carbohydrates565ª2008The AuthorsJournal compilationª2008Blackwell Publishing Ltd,The Plant Journal,(2008),54,559–568。
