ch1-9 0318
EN 1999-45-EC

Avis juridique important31999L0045Directive 1999/45/EC of the European Parliament and of the Council of 31 May 1999 concerning the approximation of the laws, regulations and administrative provisions ofthe Member States relating to the classification, packaging and labelling of dangerous preparationsOfficial Journal L 200 , 30/07/1999 P. 0001 - 0068DIRECTIVE 1999/45/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof 31 May 1999concerning the approximation of the laws, regulations and administrative provisions of theMember States relating to the classification, packaging and labelling of dangerouspreparationsTHE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,Having regard to the Treaty establishing the European Community, and in particular Article95 thereof,Having regard to the proposal of the Commission(1),Having regard to the opinion of the Economic and Social Committee(2),Acting in accordance with the procedure laid down in Article 251 of the Treaty(3),(1) Whereas Council Directive 88/379/EEC of 7 June 1988 on the approximation of the laws,regulations and administrative provisions of the Member States relating to the classification,packaging and labelling of dangerous preparations(4) has been amended on severaloccasions; whereas on the occasion of further amendments, the said Directive should, forreasons of clarity, be recast;(2) Whereas, in spite of Community provisions, the rules applying to certain dangerouspreparations in the Member States exhibit considerable differences as regards classification,packaging and labelling; whereas these differences constitute a barrier to trade, createunequal competition conditions and directly affect the functioning of the internal market;whereas it is therefore necessary to remove this barrier to trade by approximating therelevant legislation existing in the Member States;(3) Whereas measures for the approximation of the provisions of the Member States affectingthe establishment and functioning of the internal market must, in so far as they concernhealth, safety and protection of man and the environment, adopt a high level of protection asa basis; whereas this Directive must, at the same time, ensure protection for the generalpublic, and, in particular, persons who come into contact with dangerous preparations in thecourse of their work or in the pursuit of a hobby, protection for consumers and for theenvironment;(4) Whereas containers containing certain categories of dangerous preparations offered orsold to the general public must be fitted with child-resistant fastenings and/or carry a tactilewarning of danger; whereas certain preparations not falling within these categories of dangermay nevertheless, owing to their composition, present a danger for children; whereas thepackaging of such preparations should therefore be equipped with child-resistant fastenings;(5) Whereas it is necessary to provide concentration limits expressed as a volume/volumepercentage in the case of preparations marketed in gaseous form;(6) Whereas this Directive contains special labelling provisions applicable to certainpreparations; whereas, to ensure an adequate level of protection for man and theenvironment, special labelling provisions must also be introduced for certain preparationswhich, although not dangerous within the meaning of this Directive, may neverthelesspresent a danger to the user;(7) Whereas on 30 April 1992 the Council adopted Directive 92/32/EEC amending for the seventh time Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances(5); whereas on 27 April 1993 the Commission adopted Directive 93/21/EEC(6) adapting to technical progress for the 18th time Council Directive 67/548/EEC; whereas new criteria developed for classifying and labelling substances dangerous for the environment were introduced by those Directives, together with the appropriate symbols, indications of danger, risk phrases and safety advice required to appear on labelling; whereas provisions should be adopted at Community level on the classification and labelling of preparations to take account of their effects on the environment and whereas it is therefore necessary to introduce a method for assessing the hazards of a given preparation for the environment either by a calculation method, or by determining the ecotoxicological properties by test methods under certain conditions;(8) Whereas the number of animals used for experiments should be reduced to a minimum, in accordance with the provisions of Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes(7); whereas Article 7(2) of that Directive stipulates that an experiment shall not be performed if another scientifically satisfactory method of obtaining the results sought, not entailing the use of an animal, is reasonably and practically available; whereas, therefore, this Directive makes use of the results of assessments of toxicological and ecotoxicological properties only when these are already known and entails no obligation to conduct further experiments on animals;(9) Whereas it is necessary to define what human experience might be considered for the evaluation of the health hazards of a preparation; whereas, if clinical studies may be accepted, it is taken as given that such studies comply with the Helsinki Declaration and OECD Guidelines for Good Clinical Practice;(10) Whereas the characteristics of alloys are such that it may not be possible accurately to determine their properties using currently available conventional methods; whereas it is therefore necessary to develop a specific method of classification which takes into account their particular chemical properties; whereas the Commission, in consultation with Member States, will examine this need and submit a proposal, if appropriate, before the implementation date of this Directive;(11) Whereas classification, packaging and labelling of plant protection products covered by Council Directive 78/631/EEC of 26 June 1978 on the approximation of the laws of the Member States relating to the classification, packaging and labelling of dangerous preparations (pesticides)(8) need to be revised taking into account technical and scientific developments as well as regulatory developments following implementation of Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market(9);(12) Whereas Directive 91/414/EEC and Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market (10), in contrast to the provisions applicable to chemical preparations covered by this Directive, provide for an authorisation procedure for each product on the basis of a dossier presented by the applicant and an assessment carried out by the competent authority in each Member State; whereas furthermore that authorisation procedure includes a control relating specifically to the classification, packaging and labelling of each product before it is placed on the market; whereas it is appropriate, as part of a clear and transparent information process, to classify and label plant protection products according to the provisions of this Directive, and also to provide instructions for use in accordance with the results of the evaluation carried out in the framework of Directive 91/414/EEC and to ensure that the labelling satisfies the high level of protection sought by both this Directive and Directive 91/414/EEC; whereas, in addition, a safety data sheet has to be established for plant protectioon products in accordance with this Directive;(13) Whereas it is appropriate to provide, in relation to environmental labelling, that specific exemptions or specific provisions may be decided upon in specific cases where it can be demonstrated that the overall environmental impact of the product types in question is lower than that of corresponding product types;(14) Whereas, although munitions are not covered by this Directive, explosives marketed to produce an explosive or pyrotechnic effect may, through their chemical composition, present dangers to health; whereas it is therefore necessary as part of a transparent informationprocess to classify them and assign to them a safety data sheet in accordance with the provisions of this Directive and also to label them in accordance with the international rules used for the transport of dangerous goods;(15) Whereas, in order to take account of certain preparations which, although they are not considered dangerous under this Directive, may nevertheless present a danger for users, it is necessary to extend certain provisions of this Directive to cover such preparations;(16) Whereas the label constitutes a basic tool for users of the dangerous preparations in so far as it provides them with the initial essential concise information; whereas it nevertheless needs to be supplemented by a two-fold system of more detailed information, consisting firstly of the safety data sheet, intended for professional users as defined by Commission Directive 91/155/EEC of 5 March 1991 defining and laying down the detailed arrangements for the system of specific information relating to dangerous preparations in implementation of Article 10 of Directive 88/379/EEC(11) and secondly of the bodies appointed by the Member States which are responsible for the provision of information solely for medical purposes, both preventive and curative;(17) Whereas, on the basis of information to be supplied by the Member States and the various parties concerned, the Commission will submit a report to the European Parliament and the Council within two years of the entry into force of this Directive on experience with the present overall approach to labelling of dangerous preparations and in particular on its understanding and application by users, experience with publicity campaigns and educational and training programmes; whereas, on the basis of this report, the Commission will, if appropriate, submit the necessary proposals;(18) Whereas it is necessary to require safety data sheets providing proportionate information on the dangers to man and the environment arising from preparations not classified as dangerous within the meaning of this Directive but containing substances classified as dangerous or having a Community exposure limit; whereas the Commission, on the basis of information submitted by Member States, will review Directive 91/155/EEC and submit proposals, if appropriate, before the expiry of the date for implementation of this Directive; (19) Whereas, in the case of preparations classified as dangerous within the meaning of this Directive, it is appropriate to permit Member States to allow certain derogations with respect to labelling where the packaging is too small, or otherwise unsuitable for labelling, or where such small packaging or such small quantities are involved that there is no reason to fear any danger to man or the environment; whereas in such cases appropriate consideration should also be given to the approximation of the relevant provisions at Community level; whereas the Commission will therefore examine the needs for harmonisation and, if appropriate, submit proposals;(20) Whereas the confidentiality of certain substances contained in the preparations should be guaranteed and whereas it is therefore necessary to institute a system which allows the person responsible for placing the preparation on the market to request confidentiality for such substances;(21) Whereas the provisions of this Directive will have regard to the commitment entered into by the Community and its Member States, in accordance with the goals for sustainable development set under Agenda 21, Chapter 19, at the UNCED conference held in June 1992 in Rio de Janeiro, to strive for the future harmonisation of systems for the classification of dangerous substances and preparations;(22) Whereas the Commission should be given the powers necessary to adapt all the Annexes to this Directive to technical progress;(23) Whereas the adoption of this Directive should not affect the obligations of the Member States concerning the deadlines for transposition into national law and for application of the Directives indicated in Annex VIII;(24) Whereas the Directives indicated in Annex VIII should be repealed, subject to certain conditions; whereas the conditions for repealing the Directives indicated in Annex VIII should be specified for Austria, Finland and Sweden in order to take account of the present level of their legislation, in particular as regards the protection of health and the protection of the environment,HAVE ADOPTED THIS DIRECTIVE:Article 1Objectives and scope1. This Directive aims at the approximation of the laws, regulations and administrative provisions of the Member States relating to:- the classification, packaging and labelling of dangerous preparations, and to- the approximation of specific provisions for certain preparations which may present hazards, whether or not they are classified as dangerous within the meaning of this Directive,when such preparations are placed on the market of the Member States.2. This Directive shall apply to preparations which:- contain at least one dangerous substance within the meaning of Article 2,and- are considered dangerous within the meaning of Article 5, 6 or 7.3. The specific provisions set out:- in Article 9 and defined in Annex IV,- in Article 10 and defined in Annex V, and- in Article 14shall also apply to preparations which are not considered dangerous within the meaning of Articles 5, 6 or 7 but may nevertheless present a specific hazard.4. Without prejudice to Directive 91/414/EEC, the articles on classification, packaging, labelling and safety data sheets of this Directive shall apply to plant protection products.5. This Directive shall not apply to the following preparations in the finished state, intended for the final user:(a) medicinal products for human or veterinary use, as defined in Directive 65/65/EEC(12);(b) cosmetic products as defined in Directive 76/768/EEC(13);(c) mixtures of substances which, in the form of waste, are covered by Directives 75/442/EEC(14) and 78/319/EEC(15);(d) foodstuffs;(e) animal feedingstuffs;(f) preparations containing radioactive substances as defined by Directive 80/836/Euratom(16);(g) medical devices which are invasive or used in direct physical contact with the human body in so far as Community measures lay down provisions for the classification and labelling of dangerous substances and preparations which ensure the same level of information provision and protection as this Directive.6. This Directive shall not apply to:- the carriage of dangerous preparations by rail, road, inland waterway, sea or air,- preparations in transit which are under customs supervision, provided they do not undergo any treatment or processing.Article 2Definitions1. For the purposes of this Directive:(a) "substances" means chemical elements and their compounds in the natural state or obtained by any production process, including any additive necessary to preserve the stability of the products and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition;(b) "preparations" means mixtures or solutions composed of two or more substances;(c) "polymer" means a substance consisting of molecules characterised by the sequence of one or more types of monomer units and comprising a simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactant and consists of less than a simple weight majority of molecules of the same molecular weight. Such molecules must be distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units. In the context of this definition a "monomerunit" means the reacted form of a monomer in a polymer;(d) (...);(e) "placing on the market" means making available to third parties. Importation into the Community customs territory shall be deemed to be placing on the market for the purposes of this Directive;(f) "scientific research and development" means scientific experimentation, analysis or chemical research carried out under controlled conditions; it includes the determination of intrinsic properties, performance and efficacy as well as scientific investigation related to product development;(g) "process-orientated research and development" means the further development of a substance in the course of which pilot plant or production trials are used to test the fields of application of the substance;(h) "Einecs" means the European Inventory of Existing Commercial Chemical Substances. This inventory contains the definitive list of all chemical substances deemed to be on the Community market on 18 September 1981.2. The following are "dangerous" within the meaning of this Directive:(a) explosive substances and preparations: solid, liquid, pasty or gelatinous substances and preparations which may also react exothermically without atmospheric oxygen thereby quickly evolving gases, and which, under defined test conditions, detonate, quickly deflagrate or upon heating explode when partially confined;(b) oxidising substances and preparations: substances and preparations which give rise to a highly exothermic reaction in contact with other substances, particularly flammable substances;(c) extremely flammable substances and preparations: liquid substances and preparations having an extremely low flash-point and a low boiling-point and gaseous substances and preparations which are flammable in contact with air at ambient temperature and pressure; (d) highly flammable substances and preparations:- substances and preparations which may become hot and finally catch fire in contact with air at ambient temperature without any application of energy, or- solid substances and preparations which may readily catch fire after brief contact with a source of ignition and which continue to burn or to be consumed after removal of the source of ignition, or- liquid substances and preparations having a very low flash-point, or- substances and preparations which, in contact with water or damp air, evolve extremely flammable gases in dangerous quantities;(e) flammable substances and preparations: liquid substances and preparations having a low flash-point;(f) very toxic substances and preparations: substances and preparations which in very low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;(g) toxic substances and preparations: substances and preparations which in low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;(h) harmful substances and preparations: substances and preparations which may cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;(i) corrosive substances and preparations: substances and preparations which may, on contact with living tissues, destroy them;(j) irritant substances and preparations: non-corrosive substances and preparations which, through immediate, prolonged or repeated contact with the skin or mucous membrane, may cause inflammation;(k) sensitising substances and preparations: substances and preparations which, if they are inhaled or if they penetrate the skin, are capable of eliciting a reaction of hypersensitisation such that on further exposure to the substance of preparation, characteristic adverse effects are produced;(l) carcinogenic substances and preparations: substances or preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce cancer or increase its incidence; (m) mutagenic substances and preparations: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce heritable genetic defects or increase their incidence;(n) substances and preparations which are toxic for reproduction: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may produce, or increase the incidence of, non-heritable adverse effects in the progeny and/or an impairment of male or female reproductive functions or capacity;(o) substances and preparations which are dangerous for the environment: substances and preparations which, were they to enter the environment, would or could present an immediate or delayed danger for one or more components of the environment.Article 3Determination of dangerous properties of preparations1. The evaluation of the hazards of a preparation shall be based on the determination of:- physico-chemical properties,- properties affecting health,- environmental properties.These different properties shall be determined in accordance with the provisions laid down in Articles 5, 6 and 7.Where laboratory tests are conducted, they shall be carried out on the preparation as placed on the market.2. Where the determination of dangerous properties is carried out in accordance with Articles 5, 6 and 7, all dangerous substances within the meaning of Article 2 and in particular those which:- are listed in Annex I to Directive 67/548/EEC,- are listed in Elincs in accordance with Article 21 of Directive 67/548/EEC,- are classified and labelled provisionally by the person responsible for the placing on the market in accordance with Article 6 of Directive 67/548/EEC,- are classified and labelled in accordance with Article 7 of Directive 67/548/EEC and are not yet included in Elincs,- are covered by Article 8 of Directive 67/548/EEC,- are classified and labelled in accordance with Article 13 of Directive 67/548/EEC,shall be taken into consideration in accordance with the provisions laid down in the method used.3. For preparations covered by this Directive, dangerous substances as referred to in paragraph 2 which are classified as dangerous on the basis of their health and/or environmental effects, whether they are present as impurities or additives, shall be taken into consideration when their concentrations are equal to, or greater than, those defined in the following table unless lower values are given in Annex I to Directive 67/548/EEC, or in Part B of Annex II to this Directive or in Part B of Annex III thereto, unless otherwise specified in Annex V to this Directive.>TABLE>Article 4General principles of classification and labelling1. The classification of dangerous preparations according to the degree and specific nature of the hazards involved shall be based on the definitions of categories of danger laid down in Article2.2. The general principles of the classification and labelling of preparations shall be applied in accordance with the criteria laid down in Annex VI to Directive 67/548/EEC, save where alternative criteria referred to in Article 5, 6, 7 or 10 and the relevant Annexes of this Directive are applied.Article 5Evaluation of the hazards deriving from physico-chemical properties1. The hazards of a preparation deriving from its physico-chemical properties shall be assessed by determining, by means of the methods specified in Part A of Annex V to Directive 67/548/EEC, the physico-chemical properties of the preparation necessary for appropriate classification and labelling in accordance with the criteria laid down in Annex VI to that Directive.2. By way of derogation from paragraph 1:the determination of the explosive, oxidising, extremely flammable, highly flammable, or flammable properties is not necessary provided that:- none of the constituents possesses such properties and that, on the basis of the information available to the manufacturer, the preparation is unlikely to present hazards of this kind,- in the event of a change in the composition of a preparation of known composition, scientific evidence indicates that a reassessment of the hazards will not lead to a change in classification,- preparations placed on the market in the form of aerosols satisfy the provisions of Article 9a of Directive 75/324/EEC(17).3. For certain cases for which the methods laid down in Part A of Annex V to Directive67/548/EEC are not appropriate, alternative calculation methods are laid down in Part B of Annex I to this Directive.4. Certain exemptions from the application of the methods laid down in Part A of Annex V to Directive 67/548/EEC are referred to in Part A of Annex I to this Directive.5. The hazards deriving from the physico-chemical properties of a preparation covered by Directive 91/414/EEC shall be assessed by determining the physico-chemical properties of the preparation necessary for appropriate classification in accordance with the criteria set out in Annex VI to Directive 67/548/EEC. These properties shall be determined by means of the methods laid down in Part A of Annex V to Directive 67/548/EEC unless other internationally recognised methods are acceptable in accordance with the provisions of Annexes II and III to Directive 91/414/EEC.Article 6Evaluation of health hazards1. The health hazards of a preparation shall be assessed by one or more of the following procedures:(a) by a conventional method described in Annex II;(b) by determining the toxicological properties of the preparation necessary for appropriate classification in accordance with the criteria in Annex VI to Directive 67/548/EEC. These properties shall be determined by means of the methods laid down in Part B of Annex V to Directive 67/548/EEC, unless, in the case of plant protection products, other internationally recognised methods are acceptable in accordance with the provisions of Annexes II and III to Directive 91/414/EEC.2. Without prejudice to the requirements of Directive 91/414/EEC, only where it can be scientifically demonstrated by the person responsible for placing the preparation on the market that the toxicological properties of the preparation cannot correctly be determined by the method outlined in paragraph 1(a), or on the basis of existing test results on animals, the methods outlined in paragraph 1(b) may be used, provided they are justified or specifically authorised under Article 12 of Directive 86/609/EEC.When a toxicological property is established by the methods outlined in paragraph 1(b) to obtain new data, the test shall be conducted in compliance with the principles of good laboratory practice provided for in Council Directive 87/18/EEC of 18 December 1986 on the harmonisation of laws, regulations and administrative provisions relating to the application of the principles of good laboratory practice and the verification of their applications for tests on chemical substances(18) and the provisions of Directive 86/609/EEC, in particular Articles 7 and 12 thereof.Subject to the provisions of paragraph 3, where a toxicological property has been established on the basis of both the methods outlined in paragraphs 1(a) and (b), the results from the methods outlined in paragraph 1(b) shall be used for classifying the preparation, except in the case of carcinogenic, mutagenic or toxic effects for reproduction for which only the method。
DERSIMELAGON 产品说明书

488 Scientific AbstractsSystemic sclerosis, myositis and related syndromes - aetiology, pathogenesis and animal modelsPOS0467 DERSIMELAGON, A NOVEL ORAL MELANOCORTIN1 RECEPTOR AGONIST, DEMONSTRATES DISEASE-MODIFYING EFFECTS IN PRECLINICAL MODELS OFSYSTEMIC SCLEROSISM. Kondo1, T. Suzuki1, Y. Kawano1, S. Kojima2, M. Miyashiro1, A. Matsumoto1, G. Kania3, P. Blyszczuk3, R. Ross4, P. Mulipa4, F. Del Galdo4, Y. Zhang5, J. H. W. Distler5. 1Mitsubishi T anabe Pharma Corporation, Research Unit/Immunology & Inflammation, Souyaku Innovative Research Division, Y okohama, Japan;2Mitsubishi T anabe Pharma Corporation, Discovery T echnology Laboratories, Souyaku Innovative Research Division, Y okohama, Japan;3University Hospital Zurich, University of Zurich, Center of Experimental Rheumatology, Department of Rheumatology, Schlieren, Switzerland;4University of Leeds, Leeds Instituteof Rheumatic and Musculoskeletal Medicine, Faculty of Medicine and Health, Leeds, United Kingdom;5Friedrich-Alexander-University Erlangen-Nürnberg (FAU) and University Hospital Erlangen, Department of Internal Medicine 3—Rheumatology and Immunology, Erlangen, GermanyBackground: Activation of melanocortin 1 receptor (MC1R) is known to have broad anti-inflammatory and anti-fibrotic effects. The bleomycin (BLM)-induced skin fibrosis murine model is well-established for systemic sclerosis (SSc). α-mel-anocyte-stimulating hormone, an endogenous ligand of MC1R, inhibits skin fibro-sis and MC1R knock-out enhances skin fibrosis in this model. These pieces of evidence suggest that MC1R agonism has potential in the treatment of SSc. Objectives: Dersimelagon phosphate (MT-7117) is an investigational small molecule that is an orally administered, selective agonist for MC1R. The purpose of this study is to investigate the potential of MT-7117 as a therapeutic agent for SSc by evaluat-ing its efficacy and mechanism of action in complementary preclinical models. The expression and distribution of MC1R in the skin of SSc patients was investigated. Methods: The effects of MT-7117 on skin fibrosis and lung inflammation were eval-uated in BLM-induced SSc murine models that were optimized for prophylactic and therapeutic evaluation. Microarray-based gene expression analysis and serum pro-tein profiling were performed to investigate the mechanism of action of MT-7117 in the BLM-induced SSc models. The effect of MT-7117 on TGF-β-induced activation of human dermal fibroblasts was evaluated in vitro. Immunohistochemical analyses of MC1R expression in skin samples from SSc patients were performed. Results: Prophylactic treatment with MT-7117 (≥0.3 mg/kg/day p.o.) significantly inhibited the increase in collagen content of the skin, the serum level of sur-factant protein D, and the weight of the lungs from BLM-induced skin fibrosis and lung inflammation model. Therapeutic treatment with MT-7117 (≥3 mg/kg/ day p.o.) significantly suppressed skin thickening and the numbers of myofi-broblasts in pre-established BLM-induced skin fibrosis model. Gene array anal-ysis using the BLM-induced SSc model demonstrated changes in numerous categories related to macrophages, monocytes, and neutrophils, followed by endothelial cell-related categories after treatment with MT-7117. In the analy-sis that focused on biological functions, categories of inflammatory response, activation of antigen-presenting cells, angiogenesis, atherosclerosis, vascu-logenesis, and vaso-occlusion were suppressed by MT-7117. In the analysis that focused on molecular signaling pathways, triggering receptor expressed on myeloid cells-1, IL-6, and oncostatin M involved in inflammation, and perox-isome proliferator-activated receptor that is related to fibrosis were all affected by MT-7117. Serum protein profiling using BLM-induced SSc model revealed that multiple SSc-related biomarkers including P-selectin, osteoprotegerin, cys-tatin C, growth and differentiation factor-15 and S100A9 were suppressed by MT-7117. MT-7117 inhibited the activation of human dermal fibroblasts by sup-pressing TGF-β-induced ACTA2 (encoding α-smooth muscle actin) mRNA ele-vation in vitro. Immunohistochemical analyses showed that MC1R positivity was observed in 40 of 50 diffuse cutaneous SSc patients. MC1R was expressed by monocytes/macrophages, neutrophils, blood vessels (endothelial cells), fibro-blasts, and epidermis (keratinocytes) in the skin of SSc patients. Conclusion: MT-7117 demonstrates disease-modifying effects in preclinical mod-els of SSc. Investigations of its mechanism of action and target expression anal-yses indicate that MT-7117 exerts its positive effects by affecting the pathologies of inflammation, vascular dysfunction, and fibrosis through inflammatory cells, endothelial cells, and fibroblasts. In view of its potent beneficial impact on all these three main pathologies of SSc, MT-7117 is a potential therapeutic agent for the treatment of clinically challenging SSc, which has diverse and difficult to treat symp-toms. A phase 2 clinical trial investigating the efficacy and tolerability of MT-7117 in patients with early, progressive diffuse cutaneous SSc is currently in progress. Disclosure of Interests: Masahiro Kondo Employee of: Mitsubishi Tanabe Pharma Corporation, Tsuyoshi Suzuki Employee of: Mitsubishi Tanabe Pharma Corporation, Yuko Kawano Employee of: Mitsubishi Tanabe Pharma Corpora-tion, Shinji Kojima Employee of: Mitsubishi Tanabe Pharma Corporation, Masa-hiko Miyashiro Employee of: Mitsubishi Tanabe Pharma Corporation, Atsuhiro Matsumoto Employee of: Mitsubishi Tanabe Pharma Corporation, Gabriela Kania: None declared, Przemyslaw Blyszczuk: None declared, rebecca ross:None declared, Panji Mulipa: None declared, Francesco Del Galdo Grant/ research support from: Prof. F. Del Galdo received fees and research supportfrom Abbvie, AstraZeneca, Boehringer-Ingelheim, Capella, Chemomab, Kymab, Janssen and Mitsubishi-Tanabe., Yun Zhang: None declared, Jörg H.W. DistlerGrant/research support from: Prof. J.H.W. Distler received consulting fees, lec-ture fees, and/or honoraria from Actelion, Active Biotech, Anamar, ARXX, aTyr,Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GSK, Inventiva, JB Therapeutics, Medac, Pfizer, Sanofi-Aventis, RedX, RuiYi and UCB. J. H. W.Distler is stock owner of 4D Science and Scientific head of FibroCure.DOI: 10.1136/annrheumdis-2022-eular.29POS0468 EXTRACELLULAR VESICLES FROM SERUM OFMYOSITIS PATIENTS AS CIRCULATING BIOMARKERSAND DISEASE MEDIATORSS. Kivity1,2, H. Kravitz3, C. Cohen3, D. Margoulis3, M. Amar3, G. Kazimirsky3,D. Ozeri4, A. Dori5, C. Brodie3. 1Meir Medical Center, Rheumatology Unit, KefarSava, Israel;2T el Aviv University, Sackler faculty of Medicine, T el Aviv-Y afo, Israel;3Bar-Ilan University, The Mina and Everard Goodman Faculty of Life Sciences,Ramat Gan, Israel;4T el-HaShomer The Sheba Medical Center, ZabludowiczCenter for Autoimmune Disease, Ramat Gan, Israel;5T el-HaShomer The ShebaMedical Center, Department of Neurology, T alpiot Medical Leadership Program,Sackler Faculty of Medicine, T el Aviv University, Ramat Gan, IsraelBackground: Inflammatory myopathies (IM) are a heterogeneous group of disor-ders characterized by autoimmune inflammatory destruction of skeletal muscles.It is many times associated with lung, skin and joint involvement. Identifying bio-markers that can differentiate IM from other muscle disorders may elucidate the pathophysiology of IM, guide novel therapies, monitor disease activity/responseto treatments and predict prognosis. Exosomes are membrane-bound nanove-sicles with diameters of 30-150 nm that contain multiple proteins, nucleic acid,lipids and other molecules in a tissue- and cell-specific manner. Exosomes are secreted by a large variety of cells, play major roles in cell-cell interactions, andhave recently emerged as circulating biomarkers in a variety of pathological con-ditions, including several autoimmune diseases.Objectives: To characterize exosomes from serum of IM patients, analyze pro-tein expression and study their potential mediators of disease pathologies.Methods: Serum was collected from patients suffering from IM(n=5) and from patients suffering from Becker (BMD) and Duchenne (DMD) muscular dystro-phies (n=6). Exosomes were isolated by Exoquick precipitation and analyzedfor size distribution and by nanoparticle tracking analysis (NTA) and by Westernblot for exosome markers. The effects of the isolated EVs on human satellitecell proliferation and differentiation and macrophage activation were examined. Results: Exosomes from IM patients decreased human satellite cell proliferation (51%, P<0.01) and inhibited their myogenic differentiation as indicated by lower fusionindex (24% inhibition, P<0.01) and expression of myosin heavy chain (72% inhibi-tion, P<0.001). Similar results were obtained also with exosomes derived from DMDand BMD patients; however, their inhibitory effect were more pronounced on MyoG expression. T reatment of macrophages with exosomes from IM patients significantly increased the expression of IL-10 (3-fold, P<0.001), compared to exosomes of healthy controls and DMD patients. Another significant difference was in the expression of sig-naling molecules: Thus, exosomes from BMD patients increased the phosphorylationof Erk and p38, whereas a smaller effect was induced by IM exosomes.Conclusion: Exosomes from IM patients decrease satellite cell proliferationand myogenic differentiation compared to healthy exosomes. In addition, these exosomes increased the expression of IL-10 in macrophages. These effects areunique to exosomes of IM patients compared to muscular dystrophies. These promising results suggest that serum exosomes should be further investigatedas a novel biomarker with potential therapeutic implications.Disclosure of Interests: Shaye Kivity Speakers bureau: BI, Abbvie, Lilly, Pfizer, Janssen, Neopharm, Grant/research support from: Sobi, Haya Kravitz: None declared, Coral Cohen: None declared, Darya Margoulis: None declared, MosheAmar: None declared, Gila Kazimirsky: None declared, David Ozeri Speakers bureau: Neopharm, Consultant of: Abbvie, Amir Dori Grant/research supportfrom: Biogen, Chaya Brodie Grant/research support from: Biogen.DOI: 10.1136/annrheumdis-2022-eular.63POS0469 ENDOTHELIAL TO MESENCHYMAL TRANSITIONAND SENESCENCE ARE PART OF THE FIBROTICPATHOGENESIS IN SYSTEMIC SCLEROSISY. H. Chiu1,2, J. Spierings1, J. M. Van Laar1, J. De Vries-Bouwstra3, M. VanDijk4, R. Goldschmeding4. 1University Medical Center Utrecht, Departmentof Rheumatology and Clinical Immunology, Utrecht, Netherlands;2T ri-ServiceGeneral Hospital, Division of Rheumatology/Immunology/Allergy, T aipei, T aiwan, Republic of China;3Leiden University Medical Center, The Department of on December 24, 2023 by guest. Protected by copyright./ Ann Rheum Dis: first published as 10.1136/annrheumdis-2022-eular.29 on 23 May 2022. Downloaded from。
1-乙基-(3-二甲基氨基丙基)碳酰二亚胺盐酸盐质量规范-2023最新

1-乙基-(3-二甲基氨基丙基)碳酰二亚胺盐酸盐质量规范1范围本文件规定了1-乙基-(3-二甲基氨基丙基)碳酰二亚胺盐酸盐质量规范的要求、试验方法、检验规则、标志、包装、运输、贮存和保质期。
本文件适用于1-乙基-(3-二甲基氨基丙基)碳酰二亚胺盐酸盐的生产、检验、采购活动。
分子式:C8H17N3·HCl相对分子量:191.70(按2019年国际相对原子质量)结构式:2规范性引用文件下列文件中的内容通过文中的规范性引用而构成本文件必不可少的条款。
其中,注日期的引用文件,仅该日期对应的版本适用于本文件;不注日期的引用文件,其最新版本(包括所有的修改单)适用于本文件。
GB/T191包装储运图示标志GB/T601化学试剂标准滴定溶液的制备GB/T603化学试剂试验方法中所用制剂及制品的制备GB/T6679固体化工产品采样通则GB/T6682—2008分析实验室用水规格和试验方法GB/T8170数值修约规则与极限数值的表示和判定JJF1070定量包装商品净含量计量检验规则JJG701熔点测定仪检定规程《定量包装商品计量监督管理办法》国家质量监督检验检疫总局令第75号(2005)3术语和定义本文件没有需要界定的术语和定义。
4要求4.1感官要求感官要求应符合表1的规定。
表1感官要求项目要求性状结晶或结晶性粉末色泽白色杂质无肉眼可见外来杂质4.2理化指标理化指标应符合表2的规定。
表2理化指标项目指标水分/%≤0.5熔点/℃108~114炽灼残渣/%≤0.1含量/%98.0~102.04.3净含量应符合国家质量监督检验检疫总局第75号令《定量包装商品计量监督管理办法》的规定。
5试验方法5.1一般规定除非另有说明,仅使用确认为分析纯的试剂和符合GB/T6682—2008规定的三级水。
试验中所用标准滴定溶液、制剂及制品,在没有注明其他要求时,均按GB/T601和GB/T603的规定制备与标定。
5.2感官要求将供试品平铺于无色透明的PE袋内,置于白色A4纸或其他白色背景上,在光线充足的环境下,从上至下目测检验。
氢化-1-癸烯的均聚物的hs编码

氢化-1-癸烯的均聚物的hs编码全文共四篇示例,供读者参考第一篇示例:氢化-1-癸烯是一种重要的化学原料,广泛应用于聚合物的制备过程中。
氢化-1-癸烯的聚合物是一种高分子化合物,具有优异的物理性能和化学性质,被广泛用于塑料、橡胶、涂料等领域。
在国际贸易中,每种商品都会被赋予一个独特的编码,以便于跨国贸易和海关检查。
对于氢化-1-癸烯的均聚物,其在国际贸易中的编码为HS编码3901909000。
下面我们来详细了解一下这个编码的含义以及氢化-1-癸烯的均聚物在国际贸易中的应用。
让我们来解读一下这个HS编码。
HS编码由世界关务组织(WCO)制定,全称为“危险品和物品分类标准(Harmoized Commodity Description and Coding System)”,是国际上通用的商品分类系统。
该系统采用六位数编码,用于统一对各种商品的分类。
在HS编码3901909000中,前两位数字39代表塑料及其制品,具体到氢化-1-癸烯的均聚物,其被分类为塑料制品的一种。
接着的四位数字19表示其他聚合物,最后两位000则是具体到氢化-1-癸烯的均聚物这种商品的分类编码。
氢化-1-癸烯的均聚物在国际贸易中的应用十分广泛,其HS编码3901909000为其在国际贸易中的唯一标识。
随着科技的发展和经济的全球化,氢化-1-癸烯的均聚物的需求将会不断增长,其在各个领域的应用也将会持续扩大。
希望通过本文的介绍,您对氢化-1-癸烯的均聚物有了更深入的了解,并能更好地了解其在国际贸易中的地位和作用。
【字数:565】第二篇示例:氢化-1-癸烯,又称为氢化癸烯或环己基巴龙,是一种重要的有机化合物,化学式为C10H20。
它是一种无色液体,具有类似石油的刺激性气味,主要用作溶剂和原料。
氢化-1-癸烯可以通过氢化反应制得,主要用途是作为合成橡胶的原料,也用于制作润滑油和油漆。
氢化-1-癸烯还可以用于制备聚合物,其中最重要的产物就是氢化-1-癸烯的均聚物。
FOR-311有害物质一览表(1)

NO.Restricted for Use限用物質Short Name物質縮寫Controlled Applications限制範圍Unit/PPMNotice Lead in Plastic Parts or EnclosureLead in Paint, Ink, Lacquer, or otherCoatingsLead in Product Packaging MaterialLead in Metal Parts (except RoHSexemptions)Lead in Cable Jacket, Lead wireLead in Battery CellLead in Electronic Components andInterconnectsLead in Solder or PCB AssemblyLead in Other Applications2Cadmium and its Compounds(鎘及其化合物)Cd Cadmium in All applications75 3Mercury and its Compounds(汞及其化合物)Hg Mercury in all applications10004Hexavalent Chromium and its Compounds (六價鉻及其化合物)Cr6+Hexavalent Chromium in Allapplications10005Polybrominated Biphenyls(多溴聯苯)PBBs PBBs in All applications10006Polybrominated Diphenyl Ethers(多溴聯苯醚)PBDEs PBDEs in All applications10007Polyvinyl Chloride (聚氯乙烯)PVC All applications except cable jacket,lead wire8CFCs(氟氯碳化物)CFCs All applications09Halons(海龍)Halons All applications010HCFCs(氟氯烴化合物)HCFCs All applications011C10-C13 Chlorinated paraffins (氯化石臘)C10-C13ChlorinatedparaffinsAll applications012Medium-Chained Chlorinated Paraffins (MCCP)(中鏈氯化石蠟) (C14-C17)MCCP All applications1000 131,1,1-trichloroethane(1,1,1-三氯乙烷)C2H3Cl3All applications014Carbon tetrachloride(四氯化碳)CCl4All applications015Polychlorinated biphenyl(多氯聯苯)PCB All applications016Polychlorinated triphenyl(多氯三聯苯)PCT All applications017Halogenated flame retardants (鹵素耐燃物質)Halogenated flameretardantsAll applications except cables,connectors, electronic components,fans, power supplies, and printedcircuit boards100018Nickel and its Compounds(鎳及其化合物)Ni Only External Chassis/ Case!!100019Asbestos(石棉)Asbestos All applications020Azo compounds (colorants)(有機氮化合物)AZO All applications021Formaldehyde(甲醛)CH2O All applications010010010001Lead and its Compounds(鉛及其化合物)PbNO.Restricted for Use 限用物質Short Name 物質縮寫Controlled Applications 限制範圍Unit/PP M Notice22Polychlorinated Naphthalenes (多氯化荼) Cl>=3PCNs All applications 023Radioactive Substances (放射性物質)Radioactive Substances All applications 024Organic Tin compounds(有機錫化合物)-Tributyl Tin (TBT)-Triphenyl Tin (TPT)-Tributyl Tin Oxide (TBTO)Organic Tin All applications 025Substitute for Halon (氟溴烴化合物含量)HBFCs All applications 026Chlorobromomethane/Methyl bromide CH 2BrCl/ CH 3Br All applications 027Dioxins (戴奧辛)Dioxin All applications 028All applications 029Antimony and its Compounds(銻及其化合物)Sb All applications 1000PCB不在管控之内30Arsenic and its Compounds(砷及其化合物)As All applications 0PCB不在管控之内31Beryllium and its Compounds(鈹及其化合物)Be All applications 032Bismuth and its Compounds(鉍及其化合物)Bi All applications 1000PCB不在管控之内33Selenium and its Compounds(硒及其化合物)Se All applications 100034Magnesium(鎂)Mg All applications 100035Phthalates (邻苯二甲酸鹽類)(DINP, DEHP, DBP, DIDP, DNOP BBP)Phthalates (DBP, DEHP)All applications 100036Cooper and its Compounds(銅及其化合物)Cu All applications NA 37Gold and its Compounds(金及其化合物)Au All applications 1000PCB不在管控之内38Palladium and its Compounds(鈀及其化合物)Pd All applications 1000PCB不在管控之内39Silver and its Compounds(銀及其化合物)Ag All applications NA 40Chromium compounds(鉻化合物)Cr All applications 1000PCB不在管控之内41Cobalt and its Compounds(鈷及其化合物)Co All applications NA 42Tellurium and its Compounds(碲及其化合物)Te All applications NA 43Thallium and its Compounds(鉈及其化合物)Tl All applications NA 44非特定臭素系難燃剤(非特定溴系難燃燒劑)All applications NA 45Cyanides(氰基化物)CN All applications NA 46Ozone depleting substancesHFCs/PFCs/CH3All applications 0Specific organic compounds(有機化合物)C2H2Cl4/C2H3Cl3/C2H2Cl2/C2H4Cl2/C3H4Cl2/C18H30O/C12H11N/C12H9NO2/C12H8O4/DDT/C10H7NH2/Aldrin/C2H4O/Endrin/C8H24N4O3P2/Chlordane/C2H3Cl/CHCl3/C2H5ClO/CH2Cl2/C2H2Cl2/Dieldrin/C2Cl4/C10H10Cl8/C2HCl3/C2H4Cl2NO.Restricted for Use限用物質Short Name物質縮寫Controlled Applications限制範圍Unit/PPMNotice47Expanded Polystyrene(冒泡聚苯乙烯)EPS All applications048Biphenyl Oxides(聯苯基氧化物)PBDO All applications0 49Chlorinated Hydrocarbons(氯化烴)CHC All applications050Benzene and Toluene Solvents(苯及甲苯溶劑)All applications100051Polycyclic-aromatic hydrocarbons (多環芳香族烴)[Benzo(a)pyrene (苯駢(a)芘)]PAHs All applications10[1]52Brominated Flame Retardants(other than PBBs or PBDEs)(溴化難燃燒劑)BFRs All applications100053Halogenated biphenyls, terphenyls,naphthalenes(>3 Cl atoms)(鹵化聯苯, terphenyls, naphthalenes(>3 Cl原子)All applications554Halogenated dioxins and furans(鹵化二氧芑和furans)All applications0 55Halogen Content Test (Br)(鹵素含量測試 :溴)Br All applications90056Halogen Content Test (Cl)(鹵素含量測試 :氯)Cl All applications90057Halogen Content Test (Cl+Br)(鹵素含量測試 ,氯+溴)All applications<150058Other Brominated organic compounds(其他的溴化有機化合物)All applications059Other Chlorinated organic compounds(其他的氯化有機化合物)All applications060Persistent organic pollutants(餘留性有機污染物質)POPs All applications061N,N'-ditolyl-p-phenylenediamin(), N-tolyl-N'-xylyl-p-phenylenediamine(),N,N'-dixylyl-p-phenylenediamine()PDA-T2PDA-TXPDA-X2All applications062Ethylene glycol All applications5 63Yellow Phosphorus(黄磷)All applications0in preparations5All applications1000 65Perfluorooctyl acid (PFOA)(全氟辛酸銨)PFOA All applications5066Hexabromocyclododecane (HBCDD) (六溴環十二烷)HBCDD All applications100067Tetrabromobisphenol A-bis(TBB-A-bis) (Tetrabromobisphenol A (TBBPA) (四溴雙酚-A) )BPATBBPAAll applications100068Musk xylene (二甲苯麝香)All applications500 69Musk ketone (酮麝香)All applications50070DTDMAC(二牛油基二甲基氯化銨)All applicationsIn total1000pp PFOSPerfluorooctane sulfonates (PFOS) (全氟辛烷磺酸)64NO.Restricted for Use限用物質Short Name物質縮寫Controlled Applications限制範圍Unit/PPMNotice71DODMAC/DSDMAC(双十八烷基二甲基氯化銨)All applications72DHTDMAC(二(硬化牛油)二甲基氯化銨)All applications73Pentachlorophenol (PCP) (五氯酚)PCP All applications100074Triclosan (三氯沙)All applications10Note:1、当斯丹达的标准比客户的要求宽时,以客户的要求为准。
LOCTITEHysol1C产品数据说明书
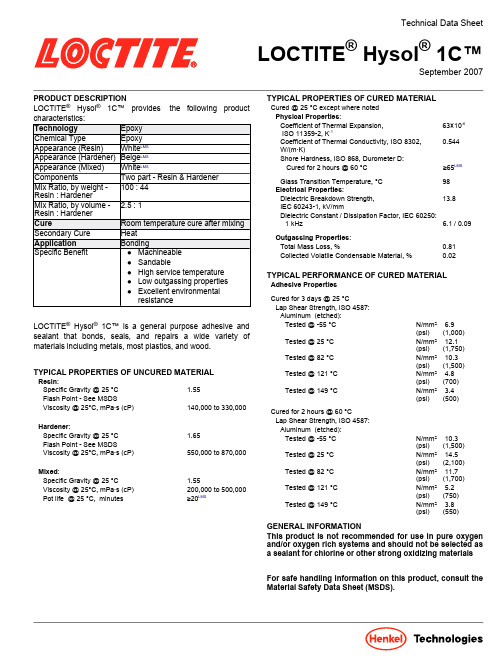
Technical Data SheetLOCTITE®Hysol®1C™September2007 PRODUCT DESCRIPTIONLOCTITE®Hysol®1C™provides the following productLOCTITE®Hysol®1C™is a general purpose adhesive andsealant that bonds,seals,and repairs a wide variety ofmaterials including metals,most plastics,and wood.TYPICAL PROPERTIES OF UNCURED MATERIALResin:Specific Gravity@ 25°C 1.55Flash Point-See MSDSViscosity@25°C,mPa·s(cP)140,000 to330,000Hardener:Specific Gravity@ 25°C 1.65Flash Point-See MSDSViscosity@25°C,mPa·s(cP)550,000 to870,000Mixed:Specific Gravity@ 25°C 1.55Viscosity@25°C,mPa·s(cP)200,000 to500,000Pot life @25°C, minutes≥20LMSTYPICAL PROPERTIES OF CURED MATERIALCured @ 25°C except where notedPhysical Properties:Coefficient of Thermal Expansion,ISO11359-2,K-163×10-6Coefficient of Thermal Conductivity, ISO8302,W/(m·K)0.544Shore Hardness, ISO868,Durometer D:Cured for2 hours @60°C≥65LMSGlass Transition Temperature,°C98Electrical Properties:Dielectric Breakdown Strength,IEC60243-1,kV/mm13.8Dielectric Constant/Dissipation Factor,IEC60250:1kHz 6.1/0.09Outgassing Properties:Total Mass Loss,%0.81Collected Volatile Condensable Material,%0.02TYPICAL PERFORMANCE OF CURED MATERIALAdhesive PropertiesCured for 3 days @25°CLap Shear Strength,ISO4587:Aluminum (etched):Tested@-55°C N/mm² 6.9(psi) (1,000)Tested@25°C N/mm² 12.1(psi) (1,750)Tested@82°C N/mm² 10.3(psi) (1,500)Tested@121°C N/mm² 4.8(psi) (700)Tested@149°C N/mm² 3.4(psi) (500)Cured for 2 hours @60°CLap Shear Strength,ISO4587:Aluminum (etched):Tested@-55°C N/mm² 10.3(psi) (1,500)Tested@25°C N/mm² 14.5(psi) (2,100)Tested@82°C N/mm² 11.7(psi) (1,700)Tested@121°C N/mm² 5.2(psi) (750)Tested@149°C N/mm² 3.8(psi) (550)GENERAL INFORMATIONThis product is not recommended for use in pure oxygenand/or oxygen rich systems and should not be selected asa sealant for chlorine or other strong oxidizing materialsFor safe handling information on this product,consult theMaterial Safety Data Sheet(MSDS).TDS LOCTITE®Hysol®1C™, September2007Directions for useMixing:1.When mixing by hand,combine Part A(Resin)and PartB(Hardener)in the correct ratio and mix thoroughly until the color and consistency are uniform.EPOXI-PATCH®Tube Kits have been designed so that squeezing EQUAL LENGTH BEADS of Part A&Part B will give the proper ratio.2.Mixing the adhesive just prior to use is recommended.The temperature of the separate components prior to mixing is not critical,but they should be close to room temperature.3.Heat buildup during and after mixing is normal.To reducethe likelihood of exothermic reaction or excessive heat buildup,mix less than 900grams at a time.Mixing smaller amounts will minimize heat buildup.Applying1.Bonding surfaces should be clean,dry,and free ofcontamination.2.Once the adhesive is applied,the bonded parts should beheld in contact until the part has developed handling strength(3 to4 hours @25°C)note:this can vary with different bond configurations.It is not necessary to clamp the parts unless movement during cure is likely.Cureplete cure is obtained after72 hours @25°C.LOCTITE®Hysol®1C™can also be fully cured with heat such as;2 hours at a maximum temperature of60°C.2.After24hours,approximately90%of full cure propertiesare attained at room temperature.3.This product can also be cured for1 hour@82°C or20 to30 minutes@121°C.4.Heat cures can be modified to achieve a desired degreeof cure from handling strength to full cure.Clean up1.It is important to clean up excess adhesive from the workarea and application equipment before it hardens.2.Denatured alcohol and many common industrial solventsare suitable for removing uncured adhesive.Loctite Material Specification LMSLMS dated April27,2005. Test reports for each batch are available for the indicated properties. LMS test reports include selected QC test parameters considered appropriate to specifications for customer use. Additionally,comprehensive controls are in place to assure product quality and consistency. Special customer specification requirements may be coordinated through Henkel Quality.StorageStore product in the unopened container in a dry location. Storage information may be indicated on the product container labeling.Optimal Storage: 8°C to21°C. Storage below8°C or greater than28°C can adversely affect product properties. Material removed from containers may be contaminated during use. Do not return product to the original container. Henkel Corporation cannot assume responsibility for product which has been contaminated or stored under conditions other than those previously indicated.If additional information is required, please contact your local Technical Service Center or Customer Service Representative.Conversions(°C x1.8)+32=°FkV/mm x25.4=V/milmm/25.4=inchesµm/25.4=milN x0.225=lbN/mm x5.71=lb/inN/mm²x145=psiMPa x145=psiN·m x 8.851= lb·inN·m x0.738=lb·ftN·mm x0.142=oz·inmPa·s=cPNoteThe data contained herein are furnished for information only and are believed to be reliable. We cannot assume responsibility for the results obtained by others over whose methods we have no control. It is the user's responsibility to determine suitability for the user's purpose of any production methods mentioned herein and to adopt such precautions as may be advisable for the protection of property and of persons against any hazards that may be involved in the handling and use thereof.In light of the foregoing,Henkel Corporation specifically disclaims all warranties expressed or implied, including warranties of merchantability or fitness for a particular purpose,arising from sale or use of Henkel Corporation’s products. Henkel Corporation specifically disclaims any liability for consequential or incidental damages of any kind,including lost profits.The discussion herein of various processes or compositions is not to be interpreted as representation that they are free from domination of patents owned by others or as a license under any Henkel Corporation patents that may cover such processes or compositions. We recommend that each prospective user test his proposed application before repetitive use,using this data as a guide. This product may be covered by one or more United States or foreign patents or patent applications.Trademark usageExcept as otherwise noted,all trademarks in this document are trademarks of Henkel Corporation in the U.S.and elsewhere. ®denotes a trademark registered in the U.S. Patent and Trademark Office.Reference0.1Henkel Loctite Americas +860.571.5100Henkel Loctite Europe+49.89.320800.1800Henkel Loctite Asia Pacific+86.21.2891.8863For the most direct access to local sales and technical support visit:。
HI98319 Waterproof Salinity Tester 使用说明书

HI98319Waterproof Salinity TesterWarrantyThis meter is warranted for a period of one year against defects in workmanship and materials when used for its intended purpose and maintained according to instructions. This warranty is limited to repair or replacement free of charge. Damage due to accidents, misuse, tampering or lack of prescribed maintenance is not covered. If service is required, contact your local Hanna Instruments Office. If under warranty, report the model number, date of purchase, serial number and the nature of the problem. If the repair is not covered by the warranty, you will be notified of the charges incurred. If the instrument is to be returned to Hanna Instruments, first obtain a Returned Goods Authorization (RGA) number from the Technical Service department and then send it with shipping costs prepaid. When shipping any instrument, make sure it is properly packaged for complete protection.SolutionCodeDescriptionHI70024P35.00 ppt calibration solution, 20 mL sachet (25 pcs.)AccessoriesIST98319 10/18Recommendations for UsersBefore using Hanna Instruments products, make sure that they are entirely suitable for your specific application and for the environment in which they are used. Any variation introduced by the user to the supplied equipment may degrade the meter’s performance. For yours and the instrument safety do not use or store the instrument in hazardous environments.Operational GuideTurn the meter ON and check the battery statusPress the ON/OFF button to turn the meter on. At start-up, all the LCD segments are displayed for 1 second, then the percent indication of the remaining battery life is displayed for another second. The meter then enters the normal measuring mode using the last selected unit, that is displayed on the secondary LCD for 3 seconds.Note : Keeping the ON/OFF button pressed while turning the meter on will display all LCD segments as long as the button is pressed.Enter calibration modePress the CAL button. “CAL “ tag is displayed.Enter setup modeRemove the battery cover and press the Setup button located on the side of the battery.Meter SetupWhile in measurement mode, remove the battery cover. Press the Setup button located on the side of the battery, in the battery compartment. The meter will enter in setup mode. Press the ON/OFF button to move through setup parameters. Change option by pressing CAL button. The default settings are: Salinity measure unit - “PPt “, Temperature unit - “°C “, Auto OFF - 8 min.Select the salinity unit (PPt/PSU/S.G.)To select the salinity unit when Unit is displayed press the CAL button to change between PPt , PSU , S.G.Setup ButtonCare and MaintenanceTo obtain the highest accuracy for measurements it is important to follow these tips:• Calibration is only as good as the solution being used. The calibration solution values change over time. Fresh solution should be used for each calibration.• The probe should be rinsed with purified water each time before placingin calibration solution or sample to be tested.Battery ReplacementThe meter features a low battery indicator. When the battery is running low (under 10 %), the battery indicator will blink on the LCD. When the battery is discharged “dEAd bAtt” will be displayed on the LCD for 2 seconds and the meter will turn off.To change the CR2032 Li-ion battery, turn the battery cover located on the back of the meter counterclockwise to unlock. Remove cover and replace with new battery “+“ sign facing up.Note : Batteries should only be replaced in a safe area using the battery type specified in this instruction manual. Old batteries should be disposed in accordance with local regulations.Clear CalibrationPress the CAL button and the meter enters calibration mode. Press ON/OFF button and the “CLR ” message is displayed. The meter will now be at default calibration.Error MessagesDuring user calibration, if the reading is out of the accepted range, the meter will display the “--- Err “ message. During measurement mode if the reading is out of range, for example in ppt the “70.0“ value will blink on the LCD.In measurement mode if the measured temperature is higher than 50.0 ºC or lower than 0.0 ºC, the 50.0 ºC or 0.0 ºC temperature value will blink on the LCD.All rights are reserved. Reproduction in whole or in part is prohibited without the written consent of the copyright owner.Hanna Instruments reserves the right to modify the design, construction or appearanceof its products without advance notice.Select the temperature unit (°C/°F)To select the temperature unit when SET t is displayed press the CAL button to change between °C or °F .Select the Auto Off time (8/60/---)To select the AUTO OFF when “AOFF ” is displayed press the CAL button to change between 8 min, 60 min or ---(disabled).Return to measurement modePress the ON/OFF button.CertificationAll Hanna Instruments products conform to CE European Directives .Disposal of Electrical & Electronic Equipment. The product should not be treated as household waste. Instead hand it over to the appropriate collection point for the recycling of electrical and electronic equipment which will conserve natural resources.Disposal of waste batteries. This product contains batteries, do notdispose of them with other household waste. Hand them over to the appropriate collection point for recycling.Ensuring proper product and battery disposal prevents potential negative consequences for the environment and human health. For more information, contact your city, your local household waste disposal service, the place of purchase or go to .Measurement and CalibrationFor better accuracy calibration of the meter is recommended at least once a month. In addition, the meter must be recalibrated whenever high accuracy is required.MeasurementPress the ON/OFF button to turn the meter ON. Place the probe in the desired solution. The salinity value, automatically compensated for temperature variations, will be displayed on the LCD.The measured temperature will be displayed on the secondary LCD.Note : Before taking any measurements make sure the meter has been calibrated.Calibration Procedure• Press the CAL button to enter calibration mode from measurement mode.• The meter will enter the calibration mode, displaying “35.00 ppt USE ” message, with CAL tag blinking.1. Pour 2” (5 cm) of standard calibration solution into a beaker.2. Place the probe in standard calibration solution. The probe tip should be centered in the beaker and submersed 1.18” (3 cm). The meter will automatically recognize the solution.3. If the standard calibration solution is not recognized or is out of the accepted range the “--- Err “ message is displayed.4. If the standard calibration solution is recognized the “REC “ message is displayed until the reading is stable and the calibration is accepted.• After acceptance, the “Stor “ message is displayed and the meter returns to measurement mode.PreparationThe probe is shipped dry. Before using the electrode, remove the protective cap. Then follow the calibration procedure.• Turn the tester on by pressing ON/OFF button.• Remove the protective cap and immerse the tip of the probe in the sample to be tested.• Stir gently and wait for the stability tag to disappear.• The electrode automatically compensates for temperature variations.• The reading on display is directly expressed in: ppt, PSU or S.G. depending on the last selected unit.• For best results, recalibrate periodically.• After use rinse the probe with water.• Always replace the protective cap after each use.SpecificationsLiquid Crystal DisplayOperation*****************************************************.************************************Preliminary ExaminationRemove the meter and accessories from the packaging and examine it carefully to make sure that no damage has occurred during shipping. Notify your nearest Hanna Customer Service Center if damage is observed.Each meter is supplied with:• CR2032 battery (inside the meter)• Storage / Protection sleeve • Instruction manual • Quality certificate• 35.00 ppt calibration standard sachet (4 pcs.)Note : Save all packing material until you are sure that the meter works correctly. Any damaged or defective items must be returned in their original packing material together with the supplied accessories.Intended UseThe meter is waterproof and is designed for the measurement of industrial, domestic, field or aquariums salinity.Salinity should be checked regularly in the aquariums. Oceans are very stable environments, where there is not a lot of day to day change in the water parameters. Fish can respond negatively to even small changes in salinity.RangepptPSU S.G.Temperature 0.0 to 70.0 ppt (g/L)0.0 to 70.0 PSU1.000-1.0410.0 to 50.0 °C / 32.0 to 122.0 °F Resolutionppt PSU S.G.Temperature0.1 ppt (g/L)0.1 PSU0.0010.1 °C / 1°FAccuracyppt±1.0 ppt for 0.0 - 40.0 ppt±2.0 ppt for 40.0 - 70.0 ppt PSU ±1.0 PSU for 0.0 - 40.0 PSU±2.0 PSU for 40.0 - 70.0 PSUS.G.±0.001Temperature ±0.5 °C (±1.0 °F)Method ppt International Oceanographic Tables, 1966PSU Standard Methods for the Examinationof Water and Wastewater, 2520 B,Electrical Conductivity MethodS.G.Standard Methods for the Examinationof Water and Wastewater, 2520 C,Density MethodCalibration Solution HI70024 (35.00 ppt)Calibration automatic, single point 35.00 ppt Temperature Compensation automatic from 5 to 50.0 ºC Environment 0 to 50 °C (32 °C to 122 °F); RH max 100%Battery Type CR2032 3V Li-Ion (1 pc.)Battery Life approximately 100 hours of continuous use Weight 68 g (2.4 oz.) without battery。
4-(甲氧基羰基)环己基-1-羧酸安全技术说明书

4-(甲氧基羰基)环己基-1-羧酸安全技术说明书
一、化学信息
4-(甲氧基羰基)环己基-1-羧酸(简称MCA)是一种有机化合物,化学式为C9H16O4。
该化合物广泛应用于医药、农药和化工领域。
在此,我们主要关注其安全技术方面的问题。
二、危害特性
MCA具有较强的腐蚀性和刺激性。
接触皮肤和眼睛时,可导致化学灼伤。
吸入气体或粉尘可引起呼吸道刺激,严重时可能导致肺水肿。
误食可引起口腔、食道和胃肠道灼伤。
三、操作安全措施
1.佩戴个人防护装备,如口罩、防护眼镜、手套和防护服。
2.避免与皮肤和眼睛直接接触,操作过程中切勿进食和使用化妆品。
3.保持良好的通风,降低空气中气体浓度。
4.避免长时间连续操作,定期休息。
四、事故应对与处理
1.如不慎接触皮肤,立即用大量清水冲洗,并寻求医疗救助。
2.如接触到眼睛,立即用大量清水冲洗,并就医。
3.如吸入气体,立即撤离现场,到通风良好的地方,如症状严重,就医。
4.如误食,立即催吐,并就医。
五、储存与运输
1.储存于阴凉、干燥、通风良好的地方,远离火源、热源和阳光直射。
2.避免与易燃、易爆、强酸、强碱等危险品同储。
3.运输过程中,遵循相关法规,确保安全。
六、暴露预防
1.加强培训,提高员工安全意识。
2.遵循操作规程,确保安全操作。
3.定期检查个人防护装备,确保完好。
七、参考文献
[略]
请注意,本文为简化版本,实际应用时,请根据具体情况进行详细阐述和扩展。
天门冬氨酸甲酯盐酸盐检验标准

天门冬氨酸甲酯盐酸盐检验标准天门冬氨酸甲酯盐酸盐(简称天冬氨酸)是一种重要的氨基酸,在生物体内具有多种重要的生理功能。
为了确保天冬氨酸的质量和使用安全,需要进行检验和评估。
本文将从多个方面来探讨天门冬氨酸甲酯盐酸盐的检验标准,旨在为您全面、深入地理解这一主题。
1. 天门冬氨酸甲酯盐酸盐的基本特性天冬氨酸,化学式C5H11NO4,是一种具有酸性的氨基酸盐酸盐。
它以白色结晶固体的形式存在,在水中可溶解。
2. 天门冬氨酸甲酯盐酸盐的用途和重要性天冬氨酸在生物体内发挥着重要的生理功能,如参与蛋白质合成、能量代谢和神经递质转运等。
天冬氨酸甲酯盐酸盐也被广泛应用于医药领域,如用于治疗肝病、肌肉萎缩和神经系统疾病等。
3. 天门冬氨酸甲酯盐酸盐检验的重要性天冬氨酸甲酯盐酸盐的质量和纯度对其应用效果和安全性至关重要。
对天冬氨酸甲酯盐酸盐进行检验和评估,可以确保其质量符合相关标准和要求。
4. 天门冬氨酸甲酯盐酸盐的检验方法常用的天冬氨酸甲酯盐酸盐检验方法包括质量检测、纯度分析和安全性评估。
其中,质量检测可以通过物理性质、化学性质和毒理学性质等方面来评估。
纯度分析则可以通过比色法、高效液相色谱(HPLC)和质谱分析等技术来实施。
安全性评估则需要结合相关法规和标准,考察天冬氨酸甲酯盐酸盐的毒性和不良反应等方面。
5. 天门冬氨酸甲酯盐酸盐检验标准的制定天冬氨酸甲酯盐酸盐的检验标准通常由相关的国家标准化机构和医药监督部门制定。
这些标准会涵盖天冬氨酸甲酯盐酸盐的纯度、质量、安全性和合理用药等方面内容。
通过遵循这些标准,可以确保天冬氨酸甲酯盐酸盐的质量和使用安全。
总结回顾:在本文中,我们全面评估了天门冬氨酸甲酯盐酸盐的检验标准。
我们首先介绍了天门冬氨酸甲酯盐酸盐的基本特性和重要性,然后强调了它的用途和应用领域。
我们详细探讨了天冬氨酸甲酯盐酸盐的检验方法,包括质量检测、纯度分析和安全性评估。
我们提到了天门冬氨酸甲酯盐酸盐检验标准的制定和重要性。
DOT 科学公司 Dextran Sulfate 产品说明书

SECTION 1 - CHEMICAL IDENTIFICATIONPRODUCT NAME: Dextran SulfateCATALOG #: DSD20020SUPPLIER’S NAME: DOT SCIENTIFIC INC.SUPPLIERS ADDRESS: 4165 Lippincott, Burton, MI 48519EMERGENCY CONTACT: 1-800-424-9300(Reference Customer Number: 18739) OTHER INFORMATION: 1-800-878-1785OTHER INFORMATION: 800-878-1785SECTION 2 - HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)H315Skin corrosion/irritation Category 2 H319Serious eye damage/eye irritation Category 2 2.2 GHS Label elements, including Hazard and Precautionary Statement(s)PictogramSignal word: WarningHazard statement(s)H315Causes skin irritationH319Eye irritationPrevention, Response, Storage and Disposal Precautionary Statement(s)P264Wash skin thoroughly after handlingP280Wear protective gloves/protective clothing/eye protection/face protection.P302 + P352IF ON SKIN: Wash with plenty of soap and water.P305 + P351 + P338IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.P321Specific treatment (see. on this label).P332 + P313If skin irritation occurs: Get medical advice/attention.P337+P313If eye irritation persists: Get medical advice/attention.P362 + P364Take off contaminated clothing and wash before reuse.SECTION 3- COMPOSITION/INFORMATION ON INGREDIENTSCAS Number: 9011-18-1SECTION 4 - FIRST-AID MEASURES4.1 Description of first aid measuresGeneral advice: Consult a doctor and show this safety data sheet.i.If inhaled: Remove to fresh air and monitor breathing. If breathing becomes difficult, give oxygen. If breathingstops, give artificial respiration. Consult a doctor.SAFETY DATA SHEETii.In case of skin contact: Immediately wash skin with copious amounts of soap and water for at least 15 minutes.Remove contaminated clothing and shoes and wash before reuse. Consult a doctor.iii.In case of eye contact: Flush with copious amounts of water for at least 15 minutes. Consult a doctor.iv.If swallowed: Rinse mouth with water. Do not induce vomiting unless directed to do so by medical personnel.Never give anything by mouth to an unconscious person. Consult a doctor.4.2 Most important symptoms and effects, both acute and delayed: To the best of our knowledge, the chemical, physical and toxicological properties have not been thoroughly investigated.4.3 Indication of immediate medical attention and special treatment needed: Show this safety data sheet to the doctor in attendance. Immediate medical attention is required.SECTION 5 - FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing media: Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide5.2 Special hazards arising from the substance or mixture: Carbon oxides. Sulfur oxides.5.3 Precautions for fire-fighters: Wear suitable protective clothing to prevent contact with skin and eyes and self-contained breathing apparatus.5.4 Further information: No data available.SECTION 6 - ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency procedures: Do not take action without suitable protective clothing - see section 8 of SDS. Evacuate personnel to safe areas. Ensure adequate ventilation. Avoid breathing vapors, mist, dust or gas.6.2 Environmental precautions: Do not let product enter drains.6.3 Methods and materials for containment and cleaning up: Cover spillage with suitable absorbent material. Using non-spark tools, sweep up material and place in an appropriate container. Decontaminate spill site with 10% caustic solution and ventilate area until after disposal is complete. Hold all material for appropriate disposal as described under section 13 of SDS.6.4 Reference to other sections: For required PPE see section 8. For disposal see section 13.SECTION 7 - HANDLING AND STORAGE7.1 Precautions for safe handling: Use in a chemical fume hood, with air supplied by an independent system. Avoid inhalation, contact with eyes, skin and clothing. Avoid the formation of dust and aerosols. Use in a well-ventilated area. Keep away from sources of ignition. Avoid prolonged or repeated exposure.7.2Conditions for safe storage, including any incompatibilities: Store in cool, well-ventilated area. Keep away from direct sunlight. Keep container tightly sealed until ready for use. Keep in a dry place.7.3 Specific end use(s): Use in a laboratory fume hood where possible. Refer to employer's COSHH risk assessment.SECTION 8 - EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parameters: Contains no substances with occupational exposure limit values.8.2 Exposure Controlsi.Appropriate engineering controls: Use in a fume hood where applicable. Ensure all engineering measuresdescribed under section 7 of SDS are in place. Ensure laboratory is equipped with a safety shower and eye wash station.8.3 Personal protective equipmenti.Eye/face protection: Face shield and safety glasses Use equipment for eye protection tested and approved underappropriate government standards such as NIOSH (US) or EN 166(EU).ii.Skin protection: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166iii.Body Protection: Wear appropriate protective clothing. Complete suit protecting against chemicals, The type ofprotective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace.iv.Respiratory Protection: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.v.Control of environmental exposure: Do not let product enter drainsSECTION 9 - PHYSICAL AND CHEMICAL PROPERTIESInformation on basic physical and chemical propertiesVapor Pressure No Data Available Appearance White To Off-WhiteSolidOdor No Data Available Vapor Density No Data Available Odor Threshold No Data Available Relative Density No Data AvailablepH No Data Available Water Solubility No Data Available Melting / Freezing Point No Data Available Partition Coefficient No Data Available Initial Boiling Point Range No Data Available Auto-Ignition Temperature No Data Available Flash Point No Data Available Decomposition Temperature No Data Available Evaporation Rate No Data Available Viscosity No Data Available Flammability (Solid, Gas)No Data Available Explosive Properties No Data Available Upper / Lower FlammabilityNo Data Available Oxidizing Properties No Data AvailableOr Explosive LimitsSECTION 10 - STABILITY AND REACTIVITYStability: Stable under recommended storage conditions.Hazardous Decomposition Products: None under normal use condition.Conditions to avoid: Light.Incompatibilities: strong oxidizing agent.SECTION 11 - TOXICOLOGICAL INFORMATIONAcute ToxicityLD50 Oral LD50 Dermal LD50 Inhalation LD50 Intraperitoneal 20,600 mg/kg (Rat)Not Listed Not Listed Not ListedSECTION 12 - ECOLOGICAL INFORMATIONEco Toxicity: No data availablePersistence and degradability/ Bioaccumlative potential/ Mobility in soil: No data availableResults of PBT and vPvB assessment: No data availableOther adverse effects: No data availableChronic Toxicity: There are no known carcinogenic chemicals in this productSECTION 13 - DISPOSAL CONSIDERATIONSWhatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirements.SECTION 14 - TRANSPORT INFORMATIONDOT TDG IATA IMDG/IMO Not regulated Not regulated Not regulated Not regulatedSECTION 15 - REGULATORY INFORMATIONINTERNATIONAL INVENTORIESTSCA DSL NDSL ELINCS NLP PICCS ENCS AICS CHINA KECL Listed Listed----Listed Listed Listed ListedUSA FEDERAL REGULATIONTSCA 12(b)/ OSHA/ CERCLA: Not ApplicableSARA 313: Not ApplicableClean Water Act/Clean Air Act: Not ApplicableSARA 311/312 HAZARDOUS CATEGORIZATIONAcute Health Hazard Chronic HealthHazardFire Hazard Sudden Release OfPressure HazardReactiveHazardNo Yes No No NoCalifornia Proposition 65: This product does not contain any Proposition 65 chemicals.HMIS RatingHealth hazard Chronic Health Hazard Flammability Physical Hazard 0000NFPA RatingHealth hazard Chronic Health Hazard Flammability Reactivity Hazard 0000STATE RIGHT TO KNOWMassachusetts New Jersey Pennsylvania Illinois Rhode Island -Listed Listed--US DEPARTMENT OF TRANSPORTATIONREPORTABLE QUANTITY (RQ)DOT MARINE POLLUTANT DOT SEVER MARINE POLLUTANT No No NoU.S. DEPARTMENT OF HOMELAND SECURITY: This product does not contain any DHS chemicals.Canada: This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations (CPR) and the SDS contains all the information required by the CPR.This SDS complies with the requirements of Regulation (EC).SECTION 16 - OTHER INFORMATIONThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. DOT SCIENTIFIC INC. shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. This product is sold for laboratory research and development purposes use only. REV October 2015。
A693[1]
![A693[1]](https://img.taocdn.com/s3/m/4e07c4d276a20029bd642d83.png)
D1This specification is under the jurisdiction of ASTM Committee A-1on Steel, Stainless Steel and Related Alloys,and is the direct responsibility of SubcommitteeA01.17on Flat Stainless Steel Products.Current edition approved July15,1993.Published September1993.Originally published as A693–st previous edition A693–92a.2For ASME Boiler and Pressure Vessel Code applications see related Specifi-cation SA-693in Section II of that Code.3Annual Book of ASTM Standards,V ol01.03.4Annual Book of ASTM Standards,V ol01.01.5Available from SAE,400Commonwealth Drive,Warrendale,PA15096. Copyright©ASTM,100Barr Harbor Drive,West Conshohocken,PA19428-2959,United States.19.Heat Treatment of Test Specimens9.1Samples cut from the plate,sheet,or strip shall conformto the mechanical properties of Table5when precipitationhardened as specified in Table2and Table3.TABLE1Chemical Requirements AComposition,%UNS Desig-nation B Type CarbonManga-nesePhos-phorusSulfur Silicon Chromium Nickel AluminumMolyb-denumTitanium CopperOtherElements CS174006300.07 1.000.0400.030 1.0015.00–17.50 3.00–5.00......... 3.00–5.00D S177006310.09 1.000.0400.030 1.0016.00–18.00 6.50–7.750.75–1.50............ S157006320.09 1.000.0400.030 1.0014.00–16.00 6.50–7.750.75–1.50 2.00–3.00......... S350006330.07–0.110.50–1.250.0400.0300.5016.00–17.00 4.00–5.00... 2.50–3.25......E S355006340.10–0.150.50–1.250.0400.0300.5015.00–16.00 4.00–5.00... 2.50–3.25......F S176006350.08 1.000.0400.030 1.0016.00–17.50 6.00–7.500.40...0.40–1.20...... S36200XM-90.050.500.0300.0300.3014.00–14.50 6.25–7.000.100.300.60–0.90...... S15500XM-120.07 1.000.0400.030 1.0014.00–15.50 3.50–5.50......... 2.50–4.50D S13800XM-130.050.200.0100.0080.1012.25–13.257.50–8.500.90–1.35 2.00–2.50......G S45500XM-160.050.500.0400.0300.5011.00–12.507.50–9.50...0.500.80–1.40 1.50–2.50F S45000XM-250.05 1.000.0300.030 1.0014.00–16.00 5.00–7.00...0.50–1.00... 1.25–1.75HA Limits are in percent maximum unless shown as a range or stated otherwise.B New designation established in accordance with Practice E527and SAE J1086.C The terms Columbium(Cb)and Niobium(Nb)both relate to the same element.D Columbium plus tantalum0.15–0.45.E Nitrogen0.07–0.13.F Columbium plus tantalum0.10–0.50.G Nitrogen0.01.H Columbium8times carbon minimum.TABLE2Heat Treatment,°FUNSDesig-nationType Solution Treatment Precipitation Hardening Treatment AS174006301925650°F(cool as required)900615°F,1h,air cool.925615°F,4h,air cool.1025615°F,4h,air cool.1075615°F,4h,air cool.1100615°F,4h,air cool.1150615°F,4h,air cool.(1400615°F,2h,air cool+1150615°F,4h,air cool).S177006311950625°F(cool as required)1750615°F,hold10min,cool rapidly to room temperature.Coolwithin24h,to−100610°F,hold not less than8h.Warm in air toroom temperature.Heat to950610°F,hold1h,air cool.Alternative Treatment:1400625°F,hold90min,cool to5565°F within1h.Hold not less than30min,heat to1050610°F,hold for90min,air cool.S157006321950625°F(cool as required)Same as Type631S350006331710625°F(water quench),hold not less than3h at−100°F or lower.850615°F,3h,air cool. 1000615°F,3h,air cool.S3*******B1900625°F(quench),hold not less than3h at−100°F orlower.1750−10°F for not less than10min,but not more than1h,water quench.Cool to not higher than−100°F,hold for not less than3h. Temper at1000625°F,holding for not less than3h.S176006351900625°F(air cool)950615°F,30min,air cool.1000615°F,30min,air cool.1050615°F,30min,air cool.S36200XM-91550625°F(air cool)900610°F,8h,air cool.S15500XM-121900625°F(cool as required)Same as Type630S13800XM-131700625°F(cool as required to below60°F)950610°F,4h,air cool.1000610°F,4h,air cool.S45500XM-161525625°F(water quench)900610°F,4h,air cool.or950610°F,4h,air cool. S45000XM-251900625°F(cool rapidly)900615°F,4h,air cool.1000615°F,4h,air cool.1150615°F,4h,air cool.A Times refer to time material is at temperature.B Equalization and over-tempering treatment:1425650°F for not less than3h,cool to room temperature,heat to1075625°F for not less than3h.TABLE3Heat Treatment,°CUNSDesignationType Solution Treatment Precipitation Hardening Treatment AS174006301050625°C(cool as required)48268°C,1h,air cool.49668°C,4h,air cool.55268°C,4h,air cool.57968°C,4h,air cool.59368°C,4h,air cool.62168°C,4h,air cool.(76068°C,2h,air cool+62168°C,4h,air cool).S177006311065615°C(water quench)95468°C,hold10min,cool rapidly to room temperature.Cool within24h to−73°C66°C,hold not less than8h.Warm in air to roomtemperature.Heat to51066°C,hold1h,air cool.Alternative Treatment760615°C,hold90min,cool to1563°C within1h.Hold not less than30min,heat to56666°C,hold for90min,air cool.S157006321038615°C(water quench)Same as Type631S35000633930615°C(water quench),hold not less than3h at−75°C or lower.45568°C,3h,air cool. 54068°C,3h,air cool.S3*******B1038615°C(quench),hold not less than3h at−73°Cor lower.95466°C for not less than10min,but not more than1h,water quench.Cool to not higher than−73°C,hold for not less than3h. Temper at538615°C,holding for not less than3h.S176006351038615°C(air cool)51068°C,30min,air cool.53868°C,30min,air cool.56668°C,30min,air cool.S36200XM-9843615°C(air cool)48268°C,8h,air cool.S15500XM-121038615°C(cool as required)Same as Type630S13800XM-13927615°C(cool as required to below60°C)51066°C,4h,air cool.53866°C,4h,air cool.S45500XM-16829615°C(water quench)48266°C,4h,air cool.or51066°C,4h,air cool.S45000XM-251038615°C(cool rapidly)48268°C,4h,air cool.53868°C,4h,air cool.62168°C,4h,air cool.A Times refer to time material is at temperature.B Equalization and over-tempering treatment:774625°C for not less than3h,cool to room temperature,heat to579615°C for not less than3h.TABLE4Mechanical Test Requirements in Solution-Treated ConditionType Tensile Strength,max Yield Strength,max Elongation in2in.or50mm,min,%Hardness,max ksi MPa ksi MPa Rockwell Brinell6300.015to4.0in.(0.38to102mm)185125516011053C383636310.010in.(0.25mm)and underOver0.010to4.0in.(0.25to102mm)150150103510356555450380...20...B92......6320.0015to4.0in.(0.038to102mm)15010356545025B100... 6330.001to0.0015in.(0.03to0.038mm),excl2001380906208C30...0.0015to0.002in.(0.03to0.05mm),excl2001380886058C30...0.002to0.005in.(0.05to0.13mm),excl2001380865958C30...0.005to0.010in.(0.13to0.25mm),excl2001380855858C30...Over0.010in.(0.254mm)20013808558512C30... 634A Plate...............C40...6350.030in.(0.76mm)and underOver0.030to0.060in.(0.76to1.52mm)Over0.060in.(1.52mm)120120120825825825757575515515515345C32C32C32.........XM-9Over0.010in.(0.25mm)15010351258604C28... XM-120.0015to4.00in.(0.038to101.6mm)...............C38363 XM-130.0015to4.00in.(0.038to101.6mm)...............C38363 XM-160.010in.(0.25mm)and greater175120516011053C36331 XM-25B0.010in.(0.25mm)and greater165120515010354C33311A Solution-treated,equalized,and over-tempered plate only.B XM-25also furnished to the following minimum properties:130895906204C25255TABLE 5Mechanical Test Requirements After Precipitation Hardening TreatmentGradeHardening or Precipitation Treatment or both,°F(°C)Thickness,in.(mm)Tensile Strength,minYield Strength,minElonga-tion in 2in.or 50mm,min,%AReduc-tion of Area,min,%A Hardness,minImpact CharpyV,min B ksiMPa ksi MPa Rockwell,min/max Brinell,min/max ft·lbf J 630and 900(482)Under 0.1875(4.762)190131017011705...C40/C48.........XM-120.1875to 0.625(4.762to 15.88)19013101701170825C40/C48388/477......0.626to 4.0(15.90to 102)190131017011701030C40/C48388/477......925(496)Under 0.1875(4.762)170117015510705...C38/C46.........0.1875to 0.625(4.762to 15.88)17011701551070825C38/C47375/477......0.626to 4.0(15.90to 102)170117015510701030C38/C47375/477......1025(552)Under 0.1875(4.762)155107014510005...C35/C43.........0.1875to 0.625(4.762to 15.88)15510701451000830C33/C42321/41510140.626to 4.0(15.90to 102)155107014510001235C33/C42321/41515201075(579)Under 0.1875(4.762)14510001258605...C31/C40.........0.1875to 0.625(4.762to 15.88)1451000125860930C29/C38293/37515200.626to 4.0(15.88to 102)14510001258601335C29/C38293/37520271100(593)Under 0.1875(4.762)1409651157905...C31/C40.........0.1875to 0.625(4.762to 15.88)1409651157901030C29/C38293/37515200.626to 4.0(15.88to 102)1409651157901435C29/C38293/37520271150(621)Under 0.1875(4.762)1359301057258...C28/C38.........0.1875to 0.625(4.762to 15.88)1359301057251035C26/C36269/35225340.626to 4.0(15.88to 102)1359301057251640C26/C36269/35230411400+1150Under 0.1875(4.762)115790755159...C26/C36255/331......(760+621)0.1875to 0.625(4.762to 15.88)115790755151140C24/C34248/32155750.626to 4.0(15.88to 102)115790755151845C24/C34248/32155756311400(760)+0.0015to 0.0049(0.038to 0.124)180124015010353...C38.........plus 55(15)+0.0050to 0.0099(0.127to 0.251)180124015010354...C38.........1050(566)0.010to 0.0199(0.25to 0.505)180124015010355...C38.........0.020to 0.1874(0.51to 4.760)180124015010356...C38.........0.1875to 0.625(4.762to 15.88)1701170140965720C38352......1750(954)+0.0015to 0.0049(0.038to 0.124)210145019013101...C44.........minus 100(73)0.0050to 0.0099(0.127to 0.251)210145019013102...C44.........+950(510)0.010to 0.0199(0.25to 0.505)210145019013103...C44.........0.020to 0.1874(0.51to 4.760)210145019013104...C44.........0.1875to 0.625(4.762to 15.88)20013801801240620C43401......Cold rolled at mill 0.0015to 0.050(0.038to 1.27)200138017512051...C41.........Cold rolled at mill +900(482)0.0015to 0.050(0.038to 1.27)240165523015801...C46.........6321400(760)+0.0015to 0.0049(0.038to 0.124)190131017011702...C40.........plus 55(15)+0.0050to 0.0099(0.127to 0.251)190131017011703...C40.........1050(566)0.010to 0.0199(0.25to 0.505)190131017011704...C40.........0.020to 0.1874(0.51to 4.760)190131017011705...C40.........0.1875to 0.625(4.762to 15.88)19013101701170420C40375......1750(954)+0.0015to 0.0049(0.038to 0.124)225155020013801...C46.........minus 100(73)0.0050to 0.0099(0.127to 0.251)225155020013802...C46.........+950(510)0.010to 0.0199(0.25to 0.505)225155020013803...C46.........0.020to 0.1874(0.51to 4.760)225155020013804...C46.........0.1875to 0.625(4.762to 15.88)22515502001380420C45429......Cold rolled at mill 0.0015to 0.050(0.038to 0.13)200138017512051...C41.........Cold rolled at mill +900(482)0.0015to 0.050(0.038to 0.13)240165523015851...C46.........633850(455)0.0005to 0.0015(0.022to 0.038)185127515010352...C42.........0.0015to 0.0020(0.038to 0.041)185127515010354...C42.........0.0020to 0.0100(0.041to 0.254)185127515010356...C42.........0.0100to 0.1875(0.254to 4.762)185127515010358...C42.........1000(540)0.0005to 0.0015(0.022to 0.038)165114014510002...C36.........0.0015to 0.0020(0.038to 0.041)165114014510004...C36.........GradeHardening or Precipitation Treatment or both,°F(°C)Thickness,in.(mm)Tensile Strength,minYield Strength,minElonga-tion in 2in.or 50mm,min,%AReduc-tion of Area,min,%A Hardness,minImpact CharpyV,min B ksiMPa ksi MPa Rockwell,min/max Brinell,min/max ft·lbf J 0.0020to 0.0100(0.041to 0.254)165114014510006...C36.........0.0100to 0.1875(0.254to 4.762)165114014510008...C36 (634)850(455)1901310165114010...............1000(540)1701170150103512...C37 (635)950(510)0.030(0.76)and under0.030to 0.060(0.76to 1.52)190190131013101701701170117034......C39C39..................Over 0.060(1.52)Plate190190131013101701701170117058...25C39C39...363............1000(540)0.030(0.76)and under180124016011053...C37.........0.030to 0.060(0.76to 1.52)180124016011054...C37.........Over 0.060(1.52)180124016011055...C37.........Plate18012401601105830C38352......1050(565)0.030(0.76)and under170117015010353...C35.........0.030to 0.060(0.76to 1.52)170117015010354...C35.........Over 0.060(1.52)170117015010355...C35.........Plate17011701501035830C36331......XM-13950(510)Under 0.020(0.51)220151*********...C45.........0.020to 0.1874(0.51to 4.760)220151*********...C45.........0.1875to 0.625(4.760to 15.88)2201515205141010...C45.........0.626to 4.0(15.90to 102)2201515205141010...C45429......1000(538)Under 0.020(0.51)200138019013106...C43.........0.020to 0.1874(0.51to 4.760)200138019013108...C43.........0.1875to 0.625(4.760to 15.88)2001380190131010...C43.........0.626to 4.0(15.90to 102)2001380190131010...C43401......XM-16950(510)Up to 0.020(0.51)Over 0.020to 0.062(0.51to 1.57)Over 0.062(1.57)222222222152515251525205205205141014101410...34.........C44C44C44...........................XM-25900(482)Up to 0.020(0.51)Over 0.020to 0.062(0.51to 1.57)Over 0.062(1.57)180180180124012401240170170170117011701170345.........C40C40C40...........................1000(538)Up to 0.020(0.51)Over 0.020to 0.062(0.51to 1.57)Over 0.062(1.57)160160160110511051105150150150103510351035567.........C36C36C36...........................1150(621)Up to 0.020(0.51)Over 0.020to 0.062(0.51to 1.57)Over 0.062(1.57)1251251258608608607575755155155158910.........C26C26C26...........................XM-9900(482)Over 0.010(0.25)180124016011053...C38.........A Applicable to tests in the long transverse direction.Transverse to the direction of rolling and parallel to the product surface.BImpact test is not required unless specified on the purchase order.TABLE 6Bend Test Requirements in Solution-Treated ConditionType Size,in.(mm)Cold Bend DegreesBend Test Mandrel630none required6310.187(4.76)and underOver 0.187to 0.275(4.76to 6.98)1801801T A 3T 6320.187(4.76)and underOver 0.187to 0.275(4.76to 6.98)1801801T 3T 633Under 0.1875(4.762)1802T 6340.187to 0.249(4.76to 6.32)Over 0.249to 0.750(6.32to 19.08)130903T 3T 635none requiredType Size,in.(mm)Cold BendDegrees Bend Test MandrelXM-90.109(2.77)and under1809T XM-12none requiredXM-13none requiredXM-16Under0.1875(4.762)1806T XM-25Under0.1875(4.762)1806T A T5thickness of sheet being tested.The American Society for Testing and Materials takes no position respecting the validity of any patent rights asserted in connection with any item mentioned in this ers of this standard are expressly advised that determination of the validity of any suchpatent rights,and the risk of infringement of such rights,are entirely their own responsibility.This standard is subject to revision at any time by the responsible technical committee and must be reviewed everyfive years and if not revised,either reapproved or withdrawn.Your comments are invited either for revision of this standard or for additional standardsand should be addressed to ASTM Headquarters.Your comments will receive careful consideration at a meeting of the responsibletechnical committee,which you may attend.If you feel that your comments have not received a fair hearing you should make yourviews known to the ASTM Committee on Standards,100Barr Harbor Drive,West Conshohocken,PA19428.This standard is copyrighted by ASTM,100Barr Harbor Drive,West Conshohocken,PA19428-2959,United States.Individual reprints(single or multiple copies)of this standard may be obtained by contacting ASTM at the above address or at610-832-9585(phone),610-832-9555(fax),or service@(e-mail);or through the ASTM website().。
001x7 氢型 树脂 国标

001x7 氢型树脂国标
001x7 氢型树脂是一种符合国际标准的新型高分子材料。
本文将详细介绍001x7 氢型树脂的特点、应用领域以及制备方法。
001x7 氢型树脂是一种具有优异性能的高分子材料。
首先,它具有较高的热稳定性,能够在高温环境下保持良好的物理性能。
其次,001x7 氢型树脂具有良好的耐化学性,能够抵抗多种有机溶剂的侵蚀。
此外,它还具有优异的电绝缘性能,适用于电子元器件的封装和绝缘材料的制备。
001x7 氢型树脂广泛应用于多个领域。
在航空航天领域,它被用作航天器的结构材料,能够承受极端的温度和压力。
在汽车制造领域,001x7 氢型树脂可用于制作车身部件和发动机罩,具有轻质、高强度和抗冲击的特点。
此外,它还可以用于电子电气领域,如电路板的封装和绝缘材料的制备。
001x7 氢型树脂的制备方法主要有以下几种。
首先是聚合法,通过将合适的单体进行聚合反应,得到001x7 氢型树脂。
其次是交联法,通过加入交联剂使树脂分子交联形成三维空间网络结构,提高其热稳定性和机械性能。
还有溶液聚合法、悬浮聚合法等多种制备方法可供选择。
001x7 氢型树脂是一种具有优异性能的高分子材料,广泛应用于航空航天、汽车制造和电子电气领域。
通过选择合适的制备方法,可
以获得不同性能的001x7 氢型树脂,满足不同领域的需求。
未来,随着科学技术的不断进步,相信001x7 氢型树脂将在更多领域展现其潜力和价值。
甘氨酸盐酸盐

甘氨酸盐酸盐
基甘氨酸盐酸盐,呈白色固体,CAS号是6939-23-7,分子式是C6H14ClNO2,分子量是167.63.熔点是223-224℃,一般在室温下储存。
N-叔丁基甘氨酸盐酸盐分子中同时具有酸性和碱性官能团,在水中可电离,具有很强的亲水性,但属于非极性氨基酸,溶于极性溶剂,而难溶于非极性溶剂,而且具有较高的沸点和熔点,通过水溶液酸碱性的调节以使N-叔丁基甘氨酸盐酸盐呈现不同的分子形态。
甘氨酸盐酸盐产品广泛应用于医药制药、染料、纺织、橡胶、建筑、生物、杀菌、电子、石油、合憃化工、日用化工、堕料等行业。
6-羰基-8-羟基辛酸乙酯

6-羰基-8-羟基辛酸乙酯
摘要:
1.6-羰基-8-羟基辛酸乙酯的概述
2.6-羰基-8-羟基辛酸乙酯的化学性质
3.6-羰基-8-羟基辛酸乙酯的用途
4.6-羰基-8-羟基辛酸乙酯的安全性
正文:
6-羰基-8-羟基辛酸乙酯,是一种有机化合物,具有独特的化学结构和性质。
它的化学式为C13H22O4,是一种无色至淡黄色的油状液体,不溶于水,但可溶于许多有机溶剂,如醇、醚和酮等。
6-羰基-8-羟基辛酸乙酯具有许多化学性质。
它是一种酸酐,可以在碱性条件下水解为对应的酸,即8-羟基辛酸。
同时,它也是一种醇酯,可以在酸性条件下水解为对应的醇,即辛醇。
此外,它还具有酮的性质,可以与许多有机化合物发生反应,如亲核加成、亲电取代等。
6-羰基-8-羟基辛酸乙酯在许多领域都有广泛的应用。
在化工领域,它可以用于合成其他化学品,如塑料、树脂和涂料等。
在医药领域,它可以用于合成药物,如非甾体抗炎药和抗肿瘤药等。
在食品工业中,它可以用作香料和调味剂,如烘焙食品和糖果等。
然而,6-羰基-8-羟基辛酸乙酯也具有一定的安全性问题。
它对皮肤和眼睛有刺激性,长期接触可能会引起过敏反应。
此外,它也可能对肝脏和肾脏造成损害,长期接触可能会对人体健康产生不利影响。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
r
1 1 nO 1 r1 r2
2 Φ nO 1 r
n s f s n s f s
若n n
s s
n 1 n
作图求像法
(1) 平行于主光轴的入射光线,折射后通过像方焦点;
(2) 通过物方焦点的入射光线,折射后平行于主光轴; (3) 通过光心的光线,折射后不改变方向。
入射到平面镜上的平行光束,反射后仍为平行光束。即反射平面右 方无限远处的物经反射后在面镜左方无穷远处成像。
第三步 求平面镜反射回来的光线经凹面镜成像。
注意:现在光线是从右向左入射,右方无限远为凹面镜的物。
n3 1.5 ,n3 1,r3 5cm,s3
n3 1 s3 r3 ( 5 ) 10cm n3 n3 1 1. 5 n3 s3 n1 s1 1 2 3 1 1 n1 s1 n3 s3
' 2
最终成虚像与O2点左侧10cm处。
'
-s1
s1’
-s2
n' n n' n 由折射成像公式 ' s1 s1 r1
' 得 : s1 16cm
-s’2
第一次成实像与O1点右侧16cm处。
2. P1′作为实物P2经球面O2折射成像
s2 20 16 4cm ,r2 2cm ,n 1.6 ,n' 1 n' s ' = 10cm n n n r2 s2
s1
C
s
s
解:物点经平面折射所成的像S’1在凹面镜反射后再 经平面折射生成最后的像。 按照光路可逆原理,仅当物点S经平面镜折射所 成的像S’1在凹面镜的曲率中心C处时,通过凹面镜 反射的光线沿原路返回再经平面折射最后成像S’才 能在物点S处。
s 150mm, s1 2 125 7 243mm , n 1 ns' s 243 1 n n 1.62 可用逐次成像 n' s s 150 法验证
1
Q
M1
s1 s2 s 2
d12
u1 y1
s3
d 23
Qy
3
M3
4
y1
u1
P
Q
P
y2
u2 u2
M2
y 2,
3
P
y 3 y 4
Q'
2
P
P'
Q
各球面的物像距公式 k 个
n1 n1 n1 n1 s1 s1 r1 nk nk nk nk sk sk rk
解:第一步 求物体经凸球面折射所成的像:
n1 n1 n1 n1 s1 s1 r1
s1 10 ,r 5 ,n 1,n' 1.5
s1 成像于无穷远处
表明:物体位于球面镜的物方焦面上,物体上各点发出的光经球面镜折 射后以平行光照射到平面镜上。 第二步 求平面镜的反射成的像:
(a) 实 物 成 实 像
(b) 虚 物 成 实 像
n
Q
n'
2
1
3
F
P
Q
P
F
C
5
4
1
n
5
n
2
C
3
F
Q
Q
F
5
4
P
P
2
n n no n n no s s r1 r2
高斯公式
薄透镜傍轴成像物像距公式 牛顿公式
f f 1 s s
n
xx ff
f
有
ni1 ni ,ui1 ui , yi1 yi 于是 n1 y1 u1 n1 y1 u1 n2 y2 u2 ... nk yk uk n1 y1 u1 nk yk uk
系统成像时物和像空间的nyu是一个常量
例题1. 有一玻璃半球,折射率为1.5,球面半径为5.0cm,平面镀 银。在球面顶点前方10cm处有一小物,用逐次成像法求这个系统 最后的成像。
P
F
O
F'
P'
光线作图法: (物点在轴上或轴外不远处):近轴条件下,利用两个焦平面 和副光轴,一面一轴。
§ 1.9
—共轴球面系统。
共轴球面系统的逐次成像法
主光轴
具有多个折射 (反射)球面,所有球面的中心都在一条直线上—
逐次成像法
两种方法: 基点成像法
---k个球面共轴折射系统
PQ—PQ’—PQ’’—…. 各个球面独立,上一级成的像作为自身的物成 像——最后系统成像。
通过物方焦点的入射光线,折射后平行于主光轴; 斜入射的平行光束聚焦于像方焦平面上一点;
轴上物点成像 常用规律
M
1
4
3
2
N
Q
P
F
F
P
轴上物点成像
(1) 平行于主光轴的入射光线,折射后通过像方焦点; (2) 通过物方焦点的入射光线,折射后平行于主光轴;
(3) 通过曲面曲率中心的光线,折射后不改变方向。
1 2 3 k
即:总的垂轴放大率是各球面垂轴放大率的乘积
每一个折射球面的成像规律必须遵守拉—亥公式
n1 y1u1 n1 y1u1 ,n2 y2 u2 n2 y2 u2 , ... nk yk uk nk yk uk
s1 , s3 0 物像虚实相同
<0 成倒像
结论:
物体经系统最终成像为:在原处的等大倒立实像。
例题2. 一凹面镜焦距为125mm,水平放置,凹面向上,并在凹面上注以 CS2 液 体 , 液 体 中 心 厚 度 为 7mm , 当 一 个 发 光 点 放 在 光 轴 上 距 液 面 150mm时,其像点与物点重合。试求CS2的折射率。
nO n n nO r1 r2
n f nO n n nO r1 r2
1 2
n n 1
ff 1 1 1 nO 1 r r 1 2
若 n = n' = 1;r1 r2 r
ff
2 nO 1
P
F
f1 s1
O
f1 '
F'
P'
s1 '
P'
f2
F F'
O
F' F
P
f2 s2 s2
L
D
D
F
实物DD经透镜L成虚像D’D’
F
D
D
L
D D
F
D
虚像D’D’经透镜L成实物DD
F
D
单球面折射作图求像法
——物点发出的 ——任意 ,经球面折射后都将通过(会聚)其像点; 经球面折射后的光线(延长线)会聚点即为像点。
例题3.一个折射率为1.6的玻璃哑铃,长20cm,两端的曲率半径为2cm。 若在离哑铃左端5cm处的轴上有一物点,试求像的位置和性质。 [解]:两次折射成像问题。 1. P1为实物,经球面O1折射成像P1′ n n′ P O1 P’2
P2 P’1
n
O2
已知 : s1 5cm ,r1 2cm , n 1,n 1.6
相邻球面距离
d12 s1 s2 , d 23 s2 s3 d k 1 k sk 1 s k
各球面半径及球面两侧的折射率已知,对已知 s1,方程数和未知
数相等,完全可解像距s’k。 垂轴放大率为
yky3 , ... yk y1 y2 y3 ...yk 1 2 3 k y1 y1 y2 y3 ...yk
