湖南省长沙市长郡中学2015届高三第一次月考化学试卷(新解析版)
湖南省长郡中学2015届高三月考(一)地理试题(WORD版)
湖南省长郡中学2015届高三月考(一)地理试卷本试题卷分选择题和非选择题两部分,共8页。
时量90分钟,满分100分。
第Ⅰ卷选择题(共50分)一、选择题(本大题共25小题,每小题2分,共50分。
在每小题给出的四个选项中,只有一项是符合题目要求的。
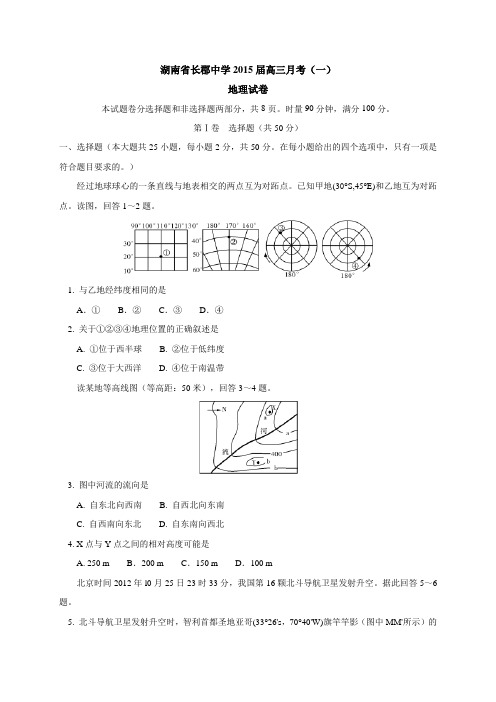
)经过地球球心的一条直线与地表相交的两点互为对跖点。
已知甲地(30°S,45°E)和乙地互为对跖点。
读图,回答1~2题。
1. 与乙地经纬度相同的是A.①B.②C.③D.④2. 关于①②③④地理位置的正确叙述是A. ①位于西半球B. ②位于低纬度C. ③位于大西洋D. ④位于南温带读某地等高线图(等高距:50米),回答3~4题。
3. 图中河流的流向是A. 自东北向西南B. 自西北向东南C. 自西南向东北D. 自东南向西北4. X点与Y点之间的相对高度可能是A. 250 m B.200 m C.150 m D.100 m北京时间2012年l0月25日23时33分,我国第16颗北斗导航卫星发射升空。
据此回答5~6题。
5. 北斗导航卫星发射升空时,智利首都圣地亚哥(33°26's,70°40'W)旗竿竿影(图中MM'所示)的朝向为A B C D6. 自北斗导航卫星发射之日起一个月内湖南长沙A. 正午太阳高度渐大B. 日出方位为东北方C. 昼短夜长且昼渐长D. 白昼时间比北京长下图为日本山河分布示意图以及富山市气温和降水季节分布示意图。
读图完成7~8题。
7. 关于富山市降水特征及其成因的叙述,正确的是A. 冬季降水丰富——处于冬季风迎风坡B. 降水季节分配较均匀——常年受西风带影响C. 夏季降水较少——与气压带、风带季节移动有关D. 降水总量丰富——常年受赤道低气压带控制8. 日本多山且河流众多,其河流A. 径流量丰富,利于航运B. 冬季多封冻C. 径流量季节变化明显,冬季断流D. 水能丰富,有利于发电下图为赤道上六大板块的分布示意图,①板块主要位于经度10°W~60°E之间。
最新长郡中学高三第一次月考化学(答案)

错误 解析 由氯化银 溴化银和碘化银的溶度积常数可知 其溶解度依次减小 " # / ,! . 错误将硫酸银溶解于水 后 向其中加入少量硫化钠固体 由于硫化银的溶度积常数远远小于硫酸银 所以可以得到黑色沉淀 -错
: ' : !的 : % : !的 误在'@ 8 加入!滴 8 8! C ' F ! # @ A B ; < , B溶液中 !@ 8 约" #滴 ! C # F ! # @ A B . ; 4 & G : ' : % C ' F ! # F ' C # F ! # F # / # ' : ! : ! 5 ! : ! 溶液 混 合 溶 液 中 , B ? @ A B 8 . ? @ A B 8 H? G ' 5 # / # ' ' 5 # / # ' : ' : % F ' ! F # / # ' ! / ' F ! # / # F ! # : ! ! 所以不能观察到白色沉淀 由硫酸银的 I ! C ' F ! # , 正确 F & $I > > ' 5 # / # ' ' 5 # / # ' & 5 : " : ! : ! 错误 数据可知 硫酸银的饱和溶液中 8 8 . ? "槡 @ A B # C "@ A B + & / 'F ! # $ G
为< 当B 若同时微热两种溶液 二者 4=: , 正确 7: 物质的量增多 J: 物质的量不变 $K G ?& 时 # 7: 溶液的体积相等 所以 比值增大 + 错误 J:
湖南省长沙长郡中学2015届高三上学期第四次月考化学试题(word版)

湖南省长沙长郡中学2015届高三上学期第四次月考化学试题(word版)时量:90分钟满分:100分可能用到的相对原子质量:H~1C-12 O~16 S~32 Cr~52 Fe~56 Cu~64 Ba~137第I卷选择题(共48分)一、选择题(本题共16小题,每小题3分,共48分,每小题只有一个选项符合题意)1.化学与生产生活、环境保护、资利用、能开发等密切相关。
下列说法错误的是A.煤炭经气化、液化和干馏等过程,可以转化为清洁能B.利用二氧化碳制造全降解塑料,可以缓解温室效应C.利用生物方法脱除生活污水中的氮和磷,防止水体富营养化D.高纯硅广泛应用于太阳能电池、计算机芯片和光导纤维2.在汽车尾气净化装置中,气体在催化剂表面吸附与解吸作用的过程如图所示。
下列说法错误的是A.NO2是红棕色气体B.NO和O2必须在催化剂表面才能反应C.汽车尾气的主要污染成分包括CO和NOD.催化转化总反应为:3.常温离子液体(Ionic Liquid)也称常温熔融盐。
硝酸乙基铵[(C2H5)NH3NO3]是人类发现的第一种常温离子液体,其熔点为12℃。
已知C2H5NH2结合质子的能力比NH3略强,下列有关硝酸乙基铵的说法正确的是A.可用作电池的电解质B.水溶液呈碱性C.是共价化合物D.结构和性质类似于硝酸乙酯4.已知:下列关于上述反应焓变的判断正确的是A.△H1>O,△H3<0 B.△H2<0,△H4>0C.△H2=△H1-△H3D.△H3 =△H4+△H15.以下实验方案可以从海洋生物中提取具有抗肿瘤活性的天然产物:下列说法错误的是A.步骤(1)需要用到玻璃棒B.步骤(2)需要用到分液漏斗C.步骤(3)需要用到坩埚D.步骤(4)需要用到温度计6.用下列实验装置或操作进行相应实验,能达到实验目的的是A.用图1装置验证化学能转化为电能B.用图2装置检验溴乙烷与NaOH醇溶液共热产生的乙烯C.用图3装置制备氢氧化亚铁D.用图4装置证明酸性:CH3COOH>H2CO3>苯酚7.下列表示对应化学反应的离子方程式正确的是A.向FeCl3溶液中加入Mg(OH)2:3Mg(OH)2+2Fe3+2Fe(OH)3 +3 Mg2+B.草酸使酸性KMnO4溶液褪色:C.往NaHS04溶液中滴加Ba(OH)2至溶液呈中性:O + D.向NaCl0溶液中通人少量的CO2:CO2 +2C1O-+H2O C232 HC1O已知Ka1(H2 CO3)>K a(HC1O)>Ka2(H2 CO3)]8.制备纳米Fe3O4的过程如下:下列有关叙述不合理的是A.纳米Fe3O4分散在适当溶剂中,当强光照射时,会产生丁达尔现象B.反应①的反应类型为消去反应C.反应②中,环丙胺的作用可能是促进氯化铁水解D.反应③的化学方程式为:6FeOOH+CO 2Fe3 O4+3H2O+CO29.A、B、C、D、E五种短周期元素的原子序数依次增大,A原子为半径最小的原子,C原子最外层电子数与A、B原子最外层电子数之和相等,D原子和B原子最外层电子数之和为C原子最外层电子数的2倍,D原子的最外层电子数为电子层数的3倍,A、E同主族。
长郡中学高一化学第一次月考试卷及解析

长郡中学高一年级学情调查化学试题可能用到的相对原子质量:C(12)N(14)O(16)Na(23)Mg(24)Al(27)S(32)Cl(35.5)K(39)Fe(56)Zn(65)Cu(64)Ag(118)Ba(137)第Ⅰ卷一、选择题(本题包括10个小题,每小题2分,共20分。
每小题均只有一个选项符合题意)1.人类未来最理想的燃料是A、煤B、石油C、天然气D、氢气2.下列物质中,几乎不具有导电性的是A、熔融NaOHB、石墨棒C、氯化氢水溶液D、KCl晶体3.下列反应中,盐酸作氧化剂的是:A、MgO + 2HCl == MgCl2 + H2OB、Fe + 2HCl == FeCl2 + H2↑C、MnO2 + 4HCl == MnCl2 + Cl2↑+ 2H2OD、NaOH + HCl == NaCl + H2O4.根据下列物质中硫的化合价判断,下列说法中错误..的是:A、H2SO3具有氧化性B、H2SO3具有还原性C、H2SO4具有氧化性D、H2SO4具有还原性5.下列物质的水溶液中存在溶质分子的是A、氯化氢B、醋酸C、氢氧化钠D、碳酸钠6.下列说法中不正确...的是A、凡是在加热条件下进行的反应都是吸热反应B、所有的燃烧反应都是放热反应C、铝和盐酸的反应是放热反应D、Ba(OH)2·8H2O晶体与NH4Cl晶体混合研磨会吸收热量7.符合H++OH—=H2O 这一离子方程式的是A、稀硫酸与Ba(OH)2溶液B、氢氧化钡和稀盐酸C、氢氧化铜与盐酸D、烧碱溶液与醋酸8.下列物质不能做还原剂的是A、SO2B、Na+C、H2D、HCl9.下列变化中,发生了氧化反应的是A、HCl→H2B、HCl→Cl2C、CuSO4→CuD、Fe3+→Fe2+10.在电解质溶液的导电性实验(装置如图所示)中,若向某一电解质溶液中逐滴加入另一溶液时,则灯泡由亮变暗,至熄灭后又逐渐变亮的是ArrayA、盐酸中逐滴加入食盐溶液B、硫酸中逐滴加入氢氧化钠溶液C、硫酸中逐滴加入氢氧化钡溶液D、醋酸中逐滴加入氨水二、选择题(本题包括10个小题,每小题3分,共30分。
高考一轮复习湖南省长郡中学上学期高三第一次月考化学试题.docx

高中化学学习材料唐玲出品长郡中学2016届高三月考试卷(一)化学本试题卷分选择题和非选择题两部分,共8页。
时量90分钟,满分100分。
可能用到的相对原子质量:0-16 Cu:-64一、选择题(本题有20个小题,每小题2分,共40分。
每小题只有一个选项符合题意)1.设N A为阿伏加德罗常数的数值,下列说法正确的是A.1 L 0.1 mol/L的氨水中有N A个B. 常温常压下,8 gO2含有4N A个电子C.标准状况下,22.4 L三氯甲烷含有N A个分子D.1 mol Na被完全氧化生成,失去2N A电子2.不能用来鉴别两种白色固体的实验操作是A.分别加热这两种固体物质,并将生成的气体通人澄清的石灰水中B.分别在这两种物质的溶液中,加入CaCl2溶液C.分别在这两种固体中,加入同浓度的稀盐酸D.分别在这两种物质的溶液中,加入少量澄清的石灰水3.①氧化钠②氢氧化钠③过氧化钠④亚硫酸钠,1 mol上述固体物质长期放置于空气中,下列说法正确的是A.上述物质都有发生了氧化还原反应B.过氧化钠在反应中转移了2 mol电子C.质量增加的情况是①>③>④>②D.质量增加的情况是②>①>③>①4.某溶液中含有等四种阴离子,向其中加入足量的固体后,假设溶液体积无变化,溶液中离子浓度基本保持不变的是5.由铝屑与盐酸、氢氧化钠溶液反应制取1,最少需要消耗HC1和NaOH的物质的量为A.3 mol、3 molB.1 mol、1 molC.0. 75 mol、0.75 molD.0.25 mol、0.25 mol6.下列除去杂质的方法不正确的是A.镁粉中混有少量铝粉:加入过量烧碱溶液充分反应,过滤、洗涤、干燥 B.用过量氨水除去Fe抖溶液中的少量Als+C.加入新制的生石灰,再加热蒸馏,可以除去乙醇中的少量水D.Mg(OH)2中混有少量Al(OH).3,加入足量烧碱溶液,充分反应,过滤,向滤液中通入过量C02后过滤7.一定量Fe和Fe2 03的混合物投入250 mL 2 mol/L HN03溶液中,固体全部溶解后,生成1.12 L(标准状况)NO(HNO3的还原产物假定仅此一种)。
湖南省长郡中学2015届高三月考(一)英语试题及答案

长郡中学2015届高三月考(一)英语试卷本试卷分为四个部分,包括听力、语言知识运用、阅读和书面表达。
时量120分钟。
满分150分。
Part I Listening Comprehension (30 marks)Section A (22.5 marks)Directions: In this section, you will hear six conversations between two speakers. For each conversation, there are several questions and each question is followed by three choices marked A, B and C. Listen carefully and then choose the best answer for each question.You will hear each conversation TWICE.Conversation 11. What will the speakers do tomorrow night?A. Have dinner together.B. Visit neighbors.C. Go for a walk near the man’s house.2. How will the man probably go to the woman’s house?A. On foot.B. By bus.C. By taxi.Conversation 23. What did the woman ask the man to do?A. Help her organize her paper.B. Check her essay.C. Choose a topic for her.4. What’s the man’s opinion about the topic?A. It’s too broad.B. It’s very narrow.C. It’s perfect.Conversation 35. Why is the woman unwilling to apply for the position?A. Because the pay is too low.B. Because it’s in a different field.C. Because she wants to learn more in her current job.6. What’s the man’s attitude toward the woman?A. He is worried.C. He is bossy.Conversation 47. Why does Sally like her shirt?A. Because it was given by a friend.B. Because she is trying to be fashionable.C. Because she bought it in Paris.8. What does the man think of Sally’s clothes?A. They’re not decent.B. They’re in fashion.C. She should get more new dresses.9. What can we learn from the conversation?A. Sally loves arguing with her dad.B. Teens have their own ideas about how to dress.C. Sally’s dad doesn’t want her to spend too much money on clothes. Conversation 510. What does the boy’s mother like when she w as young?A. She was very naughty.B. She was eager to know things around.C. She was always in trouble.11. What did the boy’s mother do to know more about hippos?A. She read books.B. She watched films in the library.C. She asked a lot of people.12. What can we learn about the woman?A. She was very strict.B. She used to know a lot about hippos.C. She supported her daughter’s curiosity.Conversation 613. How long has the man’s company been in business?A. For 5 years.B. For 3 years.C. For 2 years.14. What does the woman imply about her company?A. They offer the lowest price.B. They have grown very fast.C. They design satisfying ads.15. What will the woman give the man next?B. Some designs.C. A contract.Section B (7.5 marks)Directions: In this section, you will hear a short passage. Listen carefully and then fill in the numbered blanks with the information you’ve got. Fill in each blank with NO MORE THAN 3 WORDS.You will hear the short passage TWICE.A Wedding CDPart ⅡLanguage Knowledge(45 marks)Section A (15 marks)Directions: Beneath each of the following sentences there are four choices marked A, B, C and D. Choose the one answer that best completes the sentence.21. On 4 July 1776, the Americans declared that they would no longer submit to British rule, __________ led to war, and to America __________ its independence.A. which; gainingB. this; gainedC. this; gainingD. which; gained22. The radiation amount of a mobile phone for two half-hour periods per day is equal to __________ to an X-ray for ten seconds.A. exposeB. exposingC. exposedD. being exposed23. I like __________ in the autumn when the weather is clear and bright.A. thisB. eitherC. itD. one24. An ecosystem consists of the living and nonliving things in an area __________ interact with one another.A. thatB. whereC. whoD. what25. It __________ we had stayed together for a couple of weeks __________ I found we had a lot in common.A. was until; whenB. was until; thatC. wasn’t until; whenD. wasn’t until; that26. __________ you eat the correct foods __________ be able to keep fit and stay healthy.A. Only if; will youB. Only if; you willC. Unless; will youD. Unless; you will27. —Who should be responsible for the accident?—The boss, not the workers. They just carried out the order __________.A. as toldB. as are toldC. as tellingD. as they told28. If you open up any medicine cupboard in the world, there is a high probability __________ you will find aspirin and penicillin.A. whichB. whatC. thatD. when29. __________ and I’ll get the work finished.A. Have one more hourB. One more hourC. Given one more hourD. If I have one more hour30. Many a man __________ come to help us.A. haveB. hasC. isD. are31. W hat he said is __________ but practical since __________ depends on “if”.A. anything; everythingB. nothing; everythingC. everything; anythingD. none; everything32. He __________ have forgotten to pay before he was stopped by the store keeper.A. shouldB. couldC. mustD. would33. You never imagine what great trouble I have __________ this paper in my house.A. foundB. for findingC. to findD. finding34. He __________ football regularly for many years when he was young.A. was playingB. playedC. has playedD. had played35. __________ tomorrow, he would get there by Friday.A. Were he to leaveB. If he had leftC. Did he to leaveD. Had he leftSection B (18 marks)Directions: For each blank in the following passage there are four words or phrases marked A, B, C and D. Fill in each blank with the word or phrase that best fits the context.There are about fifteen hundred languages in the world. But 36 a few of them are very important. English is one of these. Many, many people use it, not only in England and the US, but in other parts of theworld. About 200, 000, 000 people speak it as their own language. It is difficult to say how many people are learning it as a 37 language. Many millions are trying to do so.Is it easy or difficult to learn English? Different people may have different 38 . Have you ever noticed the ads of this kind in newspapers or magazines?“Learn English in six month, or your 39 back...” “Easy and funny? Our records and tapes 40 you master your English in a month. From the first day your pronunciation will be excellent. Just send...” Of course, it never 41 quite like this.The only language that seems easy to learn is the mother tongue. We should remember that we all learned our own language well when we were children. If we could learn English in the same way, it would not seem so difficult. 42 what a small child does. He listens to what people say. He tries what he hears. When he is using the language, talking in it, and 43 in it all the time, just imagine how much 44 that he gets!So it is 45 to say that learning English is easy, because a good command of English 46 upon a lot of practice. And practice needs great effort and takes much time. Good teachers, records, tapes, books, and dictionaries will 47 . But they cannot do the student’s work of him.36. A. not B. quite C. only D. very37. A. native B. foreign C. useful D. mother38. A. questions B. problems C. ideas D. answers39. A. knowledge B. time C. money D. English40. A. force B. help C. get D. allow41. A. happens B. knows C. seems D. feels42. A. Get B. Mind C. Do D. Think of43. A. using B. thinking C. trying D. practicing44. A. time B. money C. language D. practice45. A. hard B. easy C. funny D. silly46. A. depends B. tries C. has D. takes47. A. do B. go C. help D. masterSection C (12 marks)Directions: Complete the following passage by filling in each blank with one word that best fits the context.Maria is the founder of a chain of coffee bars. She comes from Singapore, but later moved to London with her family. Her father, who was a factory manager, filled 48 with a belief in hard work. Maria studied political science at university. 49 leaving university, she became a teacher. When her father passed away, she went to New York for 50 break. Every morning she’d go to a coffee bar. When she got 51 to London, she realized that 52 was nothing like those coffee bars. 53 she decidedto give up her job and do something about it. She opened her 54 coffee bar in the year 1995 and her success was quick. She opened another ten the following year. Now she is proud of her success and plans to open 80 more 55 the 85 she already has. Just think Maria is only 35!Part ⅢReading Comprehension (30 marks)Directions: Read the following three passages. Each passage is followed by several questions or unfinished statements. For each of them there are four choices marked A, B, C and D. Choose the one that fits best according to the information given in the passage.AA report, published in last week’s Journal of the American Medical Association, offers a picture of how risky it is to get a lift from a teenage driver. Indeed, a 16-year-old driver with three or more passengers is three times as likely to have a fatal (致命) accident as a teenager driving alone. By contrast, the risk of death for drivers between 30 and 59 decreases with each additional passenger.The author also found that the death rates for teenage drivers increased dramatically after 10 p. m., and especially after midnight. With passengers in the car, the driver was even more likely to die in a late-night accident.Robert Foss, a scientist at the University of North Carolina Highway Safety Research Center, says the higher death rates for teenage drivers have less to do with “really stupid behavior” than with just a lack of driving experience. “The basic thing,” he says, “is that a dults who are responsible for issuing (发放) licenses fail to recognize how skilled a task driving is.”Both he and the author of the study believe that the way to reduce the harm is to have so-called graduated licensing systems, in which getting a license is a slower process. A graduated license requires that a teenager first prove himself capable of driving in the presence of an adult, followed by a period of driving at night with a limited number of passengers before graduating to get a full driving license.Graduated licensing systems have reduced teenage driver crashes, according to recent studies. About half of the states now have some sort of graduated licensing system in place, but only 10 of those states have number limitation on passengers. California is the strictest, with a new driver prohibited from carrying any passenger under 20 for the first six months.56. Which of the following situations is most dangerous according to the passage?A. Adults giving a lift to teenagers on the highway after 10 p. m.B. A teenager driving after midnight with passengers in the car.C. Adults driving with three or more teenage passengers late at night.D. A teenager getting a lift from a stranger on the highway at midnight.57. According to Robert Foss, the high death rate of teenage drivers is mainly due to __________.A. their frequent driving at nightB. their lack of driving experienceC. their improper way of drivingD. their driving with passengers58. Which of the following statements is true according to Paragraph 3?A. Teenagers should spend more time learning to drive.B. Driving is a skill too difficult for teenagers to learn.C. Teenagers should be limited in taking driving lessons.D. People issuing license are partly responsible for the accidents.59. A suggested measure to be taken to reduce teenagers’ driving accidents is that __________.A. driving in the presence of an adult should be made a ruleB. they should be forbidden to take on passengersC. they should not be allowed to drive after 10 p. m.D. the licensing systems should be improved60. The present situation in about half of the states is that the graduated licensing system __________.A. is under discussionB. is about to be set upC. has been put into effectD. has been perfectedB“Confidence”is probably one of the most noticeable traits (特点) in the Americans. They show confidence in the way they talk, the way they smile, the way they dress and the way they walk. Living and competing with all these confident American students, I find it extremely important to be confident as an international student and a foreign instructor. As a student, being confident means you should never hesitate to raise your hand whenever a question or a point comes to your mind. Don’t mind if it sounds simple or silly. Otherwise you will never get a chance to speak in class at all. What’s worse, the professors may think you are not prepared for the discussion or you do not have your own opinion on the issue—this is the last comment any graduate would like to receive.Being confident for me as a foreign instructor means calmly asking the student to repeat what he or she has said if I did not get it. Pretending to understand what you actually do not may just bring yourself embarrassment or even disgrace. But the time I most need to be confident is when my students come to myoffice and bargain about the grades I have given for their speeches (The course I’m teaching here is Public Speaking). Modesty is a trait highly valued in China, but it won’t be of much help here if you want to survive and succeed in a good American graduate program.61. To compete with American students, it’s very important to __________.A. be quite confidentB. be polite and friendlyC. have more discussions with themD. understand what they think about62. A professor will have the worst opinion of a student who __________.A. gives a silly or simple answerB. tries to seize any chance to speak in classC. shows no interest in the courseD. is considered to have no opinion of his own63. The author is most likely to feel embarrassed if __________.A. he asks a student to repeat what he has saidB. the students bargain with himC. he pretends to know what he doesn’tD. he has to give a speech64. We learn from the second paragraph that __________.A. we should also remain modest in AmericaB. modesty doesn’t help you much in AmericaC. Americans also like modest peopleD. modesty can help you get through an American graduate program65. The passage is mainly developed by __________.A. providing examplesB. making comparisonsC. giving different figuresD. telling personal experiencesCDo you want to live with a strong sense of peacefulness, happiness, goodness, and self-respect? The collection of happiness actions broadly categorized(把……归类) as “honor”help you create this life of good feelings.Here’s an example to show how honorable actions create happiness.Say a store clerk fails to charge us for an item. If we keep silent, and profit from the clerk’s mistake, we would drive home with a sense of sneaky (暗中的) excitement. Later we might tell our family or friendsabout our good fortune. On the other hand, if we tell the clerk about the uncharged item, the clerk would be grateful and thank us for our honesty. We would leave the store with a quiet sense of honor that we might never share with another soul.Then, what is it to do with our sense of happiness?In the first case, where we don’t tell the clerk, a couple of things would happen. Deep down inside we would know ourselves as a type of thief. In the process, we would lose some peace of mind and self-respect. We would also demonstrate that we cannot be trusted, since we advertise our dishonor by telling our family and friends. We damage our own reputations by telling others. In contrast, bringing the error to the clerk’s attention causes different things to happen. Immediately the clerk knows us to be honorable. Upon leaving the store, we feel honorable and our self-respect is increased. Whenever we take honorable actions we gain the deep internal rewards of goodness and a sense of nobility.There is a beautiful positive cycle that is created by living a life of honorable actions.Honorable thoughts lead to honorable actions. Honorable actions lead us to a happier existence. And it’s easy to think and act honorably again when we’re happy. While the positive cycle can be difficult to start, once it’s started, it’s easy to continue. Keeping on doing good deeds brings us peace of mind, which is important for our happiness.66. According to the passage, the positive action in the example contributes to our __________.A. self-respectB. financial rewardsC. advertising abilityD. friendly relationship67. The author thinks that keeping silent about the uncharged item is equal to __________.A. lyingB. stealingC. cheatingD. advertising68. The underlined phrase “bringing the error to the clerk’s attention” means __________.A. telling the truth to the clerkB. offering advice to the clerkC. asking the clerk to be more attentiveD. reminding the clerk of the charged items69. How will we feel if we let the clerk know the mistake?A. We’ll be very excited.B. We’ll feel unfortunate.C. We’ll have a sense of honor.D. We’ll feel sorry for the clerk.70. Which of the following can be the best title of this passage?A. How to Live TruthfullyB. Importance of PeacefulnessC. Ways of Gaining Self-respectD. Happiness through Honorable ActionsPart ⅣWriting (45 marks)Section A (10 marks)Directions: Read the following passage. Fill in the numbered blanks by using the information from the passage.Write NO MORE THAN 3 WORDS for each answer.No one should be forced to wear a uniform under any circumstance. Uniforms are demanding to the human spirit and totally unnecessary in a democratic(民主的) society. Uniforms tell the world that the person who wears one has no value as an individual but only lives to function as a part of a whole. The individual in a uniform loses all self-worth.There’re those who say that wearing a uniform gives a person a sense of identification with a larger, more important concept. What could be more important than the individual himself? If an organization is so weak that it must rely on cloth and buttons to inspire its members, that organization has no right to continue its existence. Others say that the practice of making persons wear uniforms, say in a school, removes all envy and competition in the matter of dress, such that a poor person who can’t afford good quality clothing needn’t be looked down upon by a wealthy person who wears expensive, quality clothing. Those persons conveniently ignore such vital concepts as freedom of choice, motivation and individuality. If all persons were to wear the same clothing, why would anyone struggle to be better? It’s only a short step from forcing everyone to wear the same clothing to forcing everyone to drive the same car, have the same type of house, and eat the same type of food. When this happens, all inspiration to improve one’s life is removed. Why would parents bother to work hard so that their children are going to be forced to have exactly the same life that they had?Uniforms also hurt the economy. Right now, billions of dollars are spent on the fashion industry yearly. Thousands of persons are employed in designing, creating and marketing different types of clothing. If everyone were forced to wear uniforms, artistic personnel would be unnecessary. Sales persons would be more than enough as well; why bother to sell the only items that are available? The wearing of uniforms would destroy the fashion industry which in turn would have a chain effect on such industries as advertising and promotion. Without advertising, newspapers, magazines and television wouldn’t be able to remain in business. The entire information and entertainment industries would crash.Title: 71 effects of forcing people to wear the uniformⅠ. Main view* 72 have more disadvantages than advantagesⅡ. 73 :(1) Making the individual totally 74(2) Letting people ignore 75 of the individual, freedom of choice, 76 and individuality(3) Hurting the economy:*Causing artistic personnel to lose 77*Making sales persons 78*Destroying the industries like 79 , advertising, promotion, 80 and entertainmentSection B (10 marks)Directions: Read the following passage. Answer the questions according to the information given in the passage and the required words limit. Write your answers on your answer sheet.The young man heading out of the convenience store wanted to do the right thing. He really did.But Zagros Bigvand was in a hurry that day last summer, and the elderly woman with the cane (拐杖) “was walking really, really slow”. So he let go of the door he’d been holding open for the stranger—just as she neared it. “I held the door for that lady for five minutes,” said Bigvand, of Dallas, Texas. “But finally I had to go. So I let it go and she looked at me and I looked at her. I felt bad about it all day. And I said I wish I could apologize to that woman.” So he did. His online apology became the beginning for apology, one of a growing number of so-called confession(忏悔) websites aimed at allowing people anonymously(匿名地) to admit to, and apologize for, anything—from being a shopaholic(购物狂) to cheating on a partner. “Human beings have a ne ed to confess,” said James Campbell Quick, a fellow with the American Psychological Association. “We don’t always want people to know the depths of our souls. Anonymity lets us flush that stuff out there and walk away.” Confession websites have become popular over the past few years. Almost all online confession sites promise anonymity. Website operators say there is no way to know which claims are factual and which are not. And there is no way to track participants either. Though it is commonly believed that confession is therapeutic(有治疗功能的), Timothy Wolff, an associate professor of psychiatry at the University of Texas, said he doesn’t see online confession as the best way to remove guilt or handle a crisis. “There’s an element(元素) of separation, physically, that allows people to do it without fear of reprisal(报复),” Wolff said. “But if someone does it and the response is not quite so bad, then they might put it out there in reality.”81. Why did Zagros Bigvand want to apologize? (No more than 20 words)_________________________________________________________________82. What was special about Zagros Bigvand’s apology? (No more than 5 words)_________________________________________________________________83. Why has online confession become common? (No more than 10 words)_________________________________________________________________84. What does Timothy Wolff think of online confession? (No more than 16 words)_________________________________________________________________Section C (25 marks)Directions: Write an English composition according to the instructions given below in Chinese.请以Car Explosion in China为题,描述我国近10年来私人拥有小汽车情况,说明人们生活水平的变化和你的看法。
2015-2016学年湖南省长沙市长郡中学高三(上)第一次月考数学试卷(理科)

离心率相同,且点( ,1)在椭圆 C1 上. (Ⅰ)求椭圆 C1 的方程; (Ⅱ)设 P 为椭圆 C2 上一点,过点 P 作直线交椭圆 C1 于 A、C 两点,且 P 恰为弦 AC 的中 点.求证:无论点 P 怎样变化,△AOC 的面积为常数,并求出此常数.
23.(13 分)(2015•咸阳一模)已知函数
第 1 页(共 46 页)
A.1 B.e+l C.3 D.e+3
8.(5 分)(2015•上海模拟)设 θ 为两个非零向量 , 的夹角,已知对任意实数 t,| +t | 的最小值为 1.( )
A.若 θ 确定,则| |唯一确定 B.若 θ 确定,则| |唯一确定
C.若| |确定,则 θ 唯一确定 D.若| |确定,则 θ 唯一确定 9.(5 分)(2015•上海模拟)已知函数 f(x)=sinπx 的图象的一部分如左图,则右图的函数 图象所对应的函数解析式为( )
6.(5 分)(2009•临沂一模)使奇函数 f(x)=sin(2x+θ)+ cos(2x+θ)在[ ,0]上
为减函数的 θ 值为( )
A. B. C. D.
7.(5 分)(2015•南昌校级二模)设 x∈R,若函数 f(x)为单调递增函数,且对任意实数 x, 都有 f[f(x)ex]=e+1(e 是自然对数的底数),则 f(ln2)的值等于( )
,
上的一个动点,则 • 的取值范围是( )
A.[1,0] B.[0,1] C.[0,2] D.[1,2] 【考点】简单线性规划的应用;平面向量数量积的运算.
菁优网版权所有
【专题】数形结合.
【分析】先画出满足约束条件
的平面区域,求出平面区域的角点后,逐一代入 •
【名师解析】湖南省长沙市长郡中学2015届高三上学期第一次月考政治试题 Word版含解析

炎德·英才大联考长郡中学2015届高三月考试卷(一)政治长郡中学高三月考政治备课组组稿得分:本试题卷分选择题和非选择题两部分。
时量90分钟。
满分100分。
第I卷选择题(共50分)【试卷综析】本套试卷是一套综合性试卷,题目的选择针对性很强,主要针对学生容易出错的一些知识点,如:货币贬值率、物价上涨率的区分,汇率的换算,基层群众自治机关的性质、政协的职能、联系的特点等;材料题选择的材料十分新颖和贴切,如对过年年味与环境的问题的探讨,新生代农民工的问题等,设问也都很严谨,试卷质量很高。
一、选择题(本大题共25小题,每小题2分,共计50分。
在每小题列出的四个选项中,只有一个选项是符合题目要求的【题文】1、比特币(注:跟腾讯公司的Q币类似,可以在网上购买虚拟装备,也可以购买现实生活中的物品)作为一种金融创新产品其价格一路攀升,2013年12月5日,中国人民银行发布《通知》说:“比待币是一种待定的虚拟商品,不具有与货币等同的法律地位。
但是,作为一种互联网上的商品买卖行为,普通民众在自担风险的前提下拥有参与的自由。
”这说明①金融创新必须以制定规则为前提②比特币在特定的情况下具有有货币的某些职能③比特币只能在我国国内流通使用④比特币的使用是公民个人的经济行为A、①③B、②③C、②④D、③④【知识点】A1本题考查货币的知识【答案解析】C解析:①说法错误,金融创新应该以遵循经济发展的规律为前提;③材料中没有体现;故选C【思路点拔】比待币可以在网上购买虚拟装备,也可以购买现实生活中的物品说明比特币在特定的情况下具有有货币的某些职能;普通民众在自担风险的前提下拥有参与的自由说明了比特币的使用是公民个人的经济行为。
【题文】2、2012年某国待售商品200亿件,平均每件商品售价15元,该年度货币流通次数为3次。
受金融危机的冲击。
该国政府多发行了250亿元该国货币,在其他条件不变的情况下,该国当年货币贬值和物价上涨的幅度分别是A、25% 25%B、30% 35%C、80% 75%D、20% 25%【知识点】A1本题考查货币的知识【答案解析】D 解析:该国流通中实际需要的货币量是200*15/3=1000亿货币贬值率=(流通中实际发行的货币量-流通中实际需要的货币量)/流通中实际发行的货币量故该国货币贬值率为(1250-1000)/1250=20%;物价上涨率=纸币发行量/流通中所需要的货币量-1,故物价上涨的幅度是:1250/1000-1=25%;故选D【思路点拔】货币贬值率=(流通中实际发行的货币量-流通中实际需要的货币量)/流通中实际发行的货币量; 物价上涨率=纸币发行量/流通中所需要的货币量-1【题文】3、人民币外汇牌价(人民币/美元)材料中人民币汇率的变化是A、美元汇率下降,人民币币值上升,我国出口美国的商品竞争力下降B、人民币汇率下降,美元币值下降我国出口美国的商品竞争力提高C、美元汇率升高,人民币币值降低,不利于我国对美投资D、人民币汇率升高,美元币值上升,有利于我国对美投资【知识点】A1本题考查汇率的知识【答案解析】A 解析:从图表可以看出,美元兑换的人民币变少了,即美元汇率下降,人民币在升值,我国出口到美国的商品需要的美元变多了,竞争力减小了,故选A【思路点拔】美元的汇率即美元的汇价,即用人民币表示的美元的价格③该市经济总体呈高增长低通胀运行态势④经济发展水平对财政收入的影响是基础性的A.①②B.③④C.②④D.①③【知识点】C2本题考查财政的知识【答案解析】B 解析:①说法错误,该市经济增长由投资和消费拉动的;②与材料无关,材料中没有体现收入差距;故选B【思路点拔】此题用排除法即可【题文】5、北京时问2013年9月10日凌晨,苹果公司举行新品发布会,正式发布其新一代产品iPhone 5s和iPhone 5c,关于产品的设计,“苹果之父”乔布斯曾说:“根据大众的需要去设计产品其实是非常难的。
湖南长沙长郡中学高三第一次月考化学试卷.doc

湖南长沙长郡中学20XX届高三第一次月考化学试卷时量:90min 满分:110分命题:姚建民老师可能用到的相对原子质量:H~1,C~12,N~14,O~16,Na~23,S~32,Cl~35.5,K~39,Ca~40,Fe~56,Cu~64,Zn~65。
第I卷(选择题共48分)一、选择题(本大题包括16个小题,共48分。
每小题只有一个选项符合题意)1.下列说法正确的是A.NaCl溶液、MgCl2溶液、KNO3溶液、Al2(SO4)3溶液可以用一种试剂加以区别B.合成氨工业与接触法制硫酸中SO2催化氧化的过程中都采用了高压的生产条件C.在医院中为酸中毒病人输液不应采用0.9%氯化铵溶液D.汽车排放的尾气中含有氮氧化物的主要原因是汽油燃烧不充分引起的2.接触法生产H2SO4过程,对废气、废水、废渣、“废热”的处理正确的是①尾气用氨水处理;②污水用石灰乳处理;③废渣用来造水泥,炼铁;④设置“废热”锅炉产生蒸气,供热或发电A.只有①②B.只有①③④C.只有①②③D.全部3.用惰性材料作电极,分别电解下列物质,当通过相同电量时,下列指定的电极上析出气体质量最大的是A.NaOH溶液(阴极)B.NaCl溶液(阴极)C.熔融的NaCl(阳极)D.Na2SO4溶液(阳极)4.常温下,下列各组物质不能用一种试剂通过化学反应区别的是A.MnO2CuO FeO B.Na2CO3NaHCO3K2CO3C.AgNO3KNO3Na2CO3 D.(NH4)2SO4K2SO4NH4Cl5.在盛有饱和Na2CO3溶液的烧杯中,插入惰性电极,保持温度不变,通电一定的时间后,下列判断正确的是A.溶液的pH将增大B.Na+数和CO32-数的比值将变小C.溶液浓度不变,有晶体析出D.溶液浓度逐渐增大并有晶体析出6.金属镍有广泛的用途。
粗镍中含有少量Fe、Zn、Cu、Pt等杂质,可用电解法制备高纯度的镍(已知:氧化性Fe2+<Ni2+<Cu2+),下列叙述正确的是A.阳极发生还原反应,其电极反应式:Ni2+ + 2e-== NiB.电解过程中,阳极质量的减少与阴极质量的增加相等C.电解后,溶液中存在的金属阳离子只有Fe2+ 和Zn2+D.电解后,电解槽底部的阳极泥中只有Cu和Pt7.用惰性电极电解一段时间后(溶质都有剩余),甲、乙两池串联且甲乙两池中溶液的pH 变化趋势相同,且两阳极、两阴极的反应产物的物质的量分别相等的是8.某溶液中可能存在Br-、CO32-、SO32-、Al3+、I-、Mg2+、Na+等7种离子中的几种。
湖南省师大附中2015届高三第一次月考化学试题 Word版

湖南师大附中高三化学备课组组稿本试题卷分选择题和非选择题两部分,共6页。
时量90分钟,满分100分。
可能用到的相对原子质量:H~1B~11C~12O~16Na~23第Ⅰ卷选择题(共42分)【试卷综析】本试卷是高三开学第一次月考试卷,主要是考查学生的基础知识。
在注重考查基础知识的同时,突出考查考纲要求的基本能力,重视学生科学素养的考查。
以基础知识和基本技能为载体,以能力测试为主导。
本试卷主要考查到了金属及化合物的性质,离子方程式的书写和判断,化学反应速率和化学平衡的基础知识,元素周期表和元素周期律的基本应用,,原电池和电解池原理的应用,有机化学基础知识,实验方案的设计和评价等知识点,本试卷题量较大,综合性较强。
注重常见化学方法,应用化学思想,体现了学科的基本要求。
一、选择题(本题为单项选择,每小题3分,共42分)【题文】1.日常生活中一些事例常涉及到化学知识,下列分析不正确...的是( ) A.硅酸钠的水溶液俗称水玻璃,是制备硅胶和木材防火剂的原料B.用灼烧并闻气味的方法区别纯棉织物和纯毛织物C.某雨水样品采集后放置一段时间,pH由4.68变为4.28,是因为水中溶解了较多的CO2D.蒙古牧民喜欢用银器盛放鲜牛奶有其科学道理:用银器盛放鲜牛奶,溶入的极微量的银离子,可杀死牛奶中的细菌,防止牛奶变质【知识点】化学与生活M2 D1 D3【答案解析】C 解析:C.正常雨水是因为水中溶解了一定量的CO2而使雨水的pH值大约为5.6,二氧化硫和水反应生成亚硫酸,会导致雨水的pH值小于5.6,亚硫酸易被空气中的氧气氧化生成硫酸,使溶液的pH值变得更小,故错误。
【思路点拨】本题考查了化学与生活的联系,知识点较多,但难度不大,根据元素化合物的性质分析解答即可,注意正常雨水的pH值5.6,为学生解答的易错点。
【题文】2.下列热化学方程式或离子方程式正确的是( )A.已知H2的标准燃烧热ΔH=-285.8 kJ·mol-1,则用热化学方程式可表示为:H2(g)+1/2O2(g)===H2O(g)ΔH=-285.8 kJ·mol-1B.NaClO溶液与FeCl2溶液混合:Fe2++2ClO-+2H2O===Fe(OH)2↓+2HClOC .NH 4HSO 3溶液与足量NaOH 溶液共热:NH +4+H ++2OH -=====△NH 3↑+2H 2OD .用足量KMnO 4溶液吸收SO 2气体:2MnO -4+5SO 2+2H 2O===2Mn 2++5SO 2-4+4H + 【知识点】离子方程式的书写和判断 B1【答案解析】D 解析:A .标准燃烧热指的是1mol 可燃物燃烧生成稳定的产物所放出的热量,故H 2燃烧的热化学方程式可表示为: H 2(g)+1/2O 2(g)===H 2O(l) ΔH =-285.8 kJ ·mol -1,错误; B . ClO —具有氧化性,能把Fe 2+氧化,错误; C . HSO 3—是弱酸的酸式酸根离子,在离子方程式中不拆,错误; D .用足量KMnO 4溶液吸收SO 2气体:2MnO -4+5SO 2+2H 2O===2Mn 2++5SO 2-4+4H +,正确。
2025届湖南省长沙市长郡中学高三上学期月考化学试题(一)(含答案)

大联考长郡中学2025届高三月考试卷(一)化学1.各式各样的材料与生活息息相关,下列说法正确的是(时量75分钟,满分100分。
可能用到的相对原子质量:H~1Li~7C~12N~14O~16Ti~48Cu~64一、选择题(本题共14小题,每小题3分,共42分。
在每小题给出的四个选项中,只有一项是符合题目要求的。
))A .大型天线所使用的碳纤维是一种有机高分子材料B .2SiO 具有导电性,所以可以用于制作光导纤维C .橡胶使用时间过长容易被氧化导致性能下降D .新型陶瓷的主要成分是硅酸盐2.下列化学用语表述正确的是()A .基态2Fe +的价电子排布图:B .HCl 分子中σ键的形成:C .聚丙烯的链节:222CH CH CH ----D .4CF的电子式:★3.下列实验操作规范且能达到实验目的的是()A .图甲:验证碳酸钠和碳酸氢钠的稳定性B .图乙:稀释浓硫酸C .图丙:萃取分离碘水中的碘D .图丁:测定醋酸溶液浓度4.下列有关物质结构或性质的说法错误的是()A .乙醇的质谱图中,相对丰度最高的峰归属于32CH CH O H+B .乳酸的结构简式为,则乳酸分子是手性分子C .邻二氯苯只有一种结构,说明苯环中并不存在单、双键相间的结构D .苯环与羟基的相互作用使酚羟基中的氢原子比醇羟基中的氢原子更活泼5.下列过程中,对应的反应方程式错误的是()A .AgCl 沉淀在氨水中溶解:()332AgCl 2NH Ag NH Cl⎡⎤+⎣⎦B .钢铁在溶有氧气的中性溶液中发生吸氧腐蚀:()2222Fe O 2H O2Fe OH ++C .2SO 通入漂白粉溶液中:2223SO H O Ca2Cl 2HClO+-+++↓+D .以熔融金属氧化物作介质的氢氧燃料电池负极反应:222H 2e OH O---+★6.核酸检测为确认病毒感染提供了关键的支持性证据,某核糖核酸(RNA )的结构片段示意图如图,它在蛋白酶的催化作用下能完全水解生成戊糖、碱基和某酸,下列说法错误的是()A .核酸是由许多核苷酸单体形成的聚合物B .该核糖核酸催化水解所使用的蛋白酶是蛋白质,其催化活性与温度有关C .该核糖核酸完全水解生成的酸是34H PO D .该核糖核酸完全水解生成的产物中不含氮元素★7.选用下图所示仪器中的两个或几个(内含物质)组装成实验装置,以验证木炭可被浓硫酸氧化成2CO ,下列说法正确的是()A .按气流从左向右流向,连接装置的正确顺序是A →F →E →C →D →B B .丁中溶液褪色,乙中溶液变浑浊,说明甲中生成2COC .丙中品红溶液褪色,乙中溶液变浑浊,说明甲中生成2COD .丁和丙中溶液都褪色,乙中溶液变浑浊,说明甲中有2CO 生成8.下列实验操作、现象和推论都正确的是()选项实验操作及现象推论A 向4CuSO 溶液中通入2H S 气体,出现黑色沉淀酸性:224H S H SO >B 取少量丙烯醛于试管中,滴加酸性高锰酸钾溶液,酸性高锰酸钾溶液褪色丙烯醛中含有碳碳双键C 用干净的玻璃棒蘸取少量未知无色溶液于酒精灯上灼烧,火焰呈黄色该未知无色溶液中一定含有Na+D向()3Fe SCN 溶液中加入少量铁粉,溶液红色变浅3Fe +与SCN -的反应是可逆反应9.绿矾()42O FeSO 7H ⋅的结构示意图如下图所示,下列说法正确的是()A .24SO -中S 的杂化方式为2sp 杂化B .电负性:O S Fe >>C .2H O 的键角大于24SO -的键角D .绿矾中存在的化学键类型有共价键、离子键、氢键和配位键★10.双极膜可用于电解葡萄糖()6126C H O 溶液的同时制备山梨醇()6146C H O 和葡萄糖酸()6127C H O 。
长郡中学2015届高三月考理科数学试卷(一)
长郡中学2015届高三月考试卷(一)数 学(理科)总分:150分 时量:120分钟一、选择题:本大题共10小题,每小题5分,共50分.在每小题给出的四个选项中,只有一项是符合题目要求的,请将所选答案填在答题卡中对应位置.1.如果复数212bii-+(其中i 为虚数单位,b 为实数)的实部和虚部互为相反数,那么b = ( )A. B. 23 C. 23- D. 2【解】选由222(4)125bi b b i i ---+=+,依题有2240b b ---=,即23b =-. 2.用简单随机抽样的方法从含有100个个体的总体中依次抽取一个容量为5的样本,则个体m 被抽到的概率为( )A.1100 B.120 C.199D.150 【解】选 由抽样的公平性可知,每个个体入样的概率均为5110020P ==. 3.设偶函数满足()24(0)xf x x =-≥,则{|()0}x f x >=( ) A. {|24}x x x <->或 B. {|04}x x x <>或 C. {|22}x x x <->或D. {|06}x x x <>或【解】选 当0x ≥时,由()240x f x =->,得2x >,由图象对称性可知选C. 4.若21()n x x-展开式中的所有二项式系数之和为512,则该开式中常数项为( )A. 84-B. 84C. 36-D. 36 【解】选 由二项式系数之和为2512n =,即9n =,又18319(1),r r r r T C x -+=- 令1830r -=,则6r =故常数项为784T =.5.设条件:|2|3p x -<,条件:0q x a <<,其中a 为正常数.若p 是q 的必要不充分条件,则a 的取值范围是( ) A. (0,5]B. (0,5)C.[5,+∞)D. (5,+∞) 【解】选 由条件p 对应的集合为(1,5)A =-, 条件q 对应(0,)(0)B a a =>.且依题意A B =≠=⊃,可知5a ≤,又0a >,故05a <≤.6.按照如图所示的程序运行,已知输入的x 的值为21log 3+, 则输出y 的值为( )A. 112B. 38C. 712D. 1124【解】选由于输入的初始值为21log 34+<,故221log 312log 3x =++=+,即2log 3211111()()224312y =⨯=⨯=.故选A.7.已知一个几何体的三视图及有关数据如图所示, 则该几何体的体积为( )A.B.C.D.【解】选由该几何体的为直观图如右所示,(由简到繁)由俯视图→侧视图→正视图→直观图, 其为四棱锥P ABCD -,所以13P ABCD ABCD V S -==矩,选B.8.设2(),0,()1,0x a x f x x a x x -≤⎧⎪=⎨++>⎪⎩,若(0)f 是()f x 的最小值,则a 的取值范围为( ) A. [-1,2]B. [-1,0]C. [1,2]D. [0,2]【解】选 当0a <时,显然(0)f 不是()f x 的最小值,当0a ≥时,可知0x ≤时,2()(0)f x f a ≥=,而当0x >时,1()2f x x a a x=++≥+,依题意22a a +≥,得12a -≤≤,所以02a ≤≤即求.9.已知锐角A 是ABC ∆的一个内角,,,a b c 是三角形中各角的对应边,若221sin cos 2A A -=,则下列各式正确的是( ) A. 2b c a += B. 2b c a +< C. 2b c a +≤ D. 2b c a +≥【解】选 由221sin cos 2A A -=得,1cos22A =-,又A 为锐角,故02A π<<,于是223A π=,即3A π=.于是由余弦定理有2222()3a b c bc b c bc =+-=+-,即22223()()()44b c a b c b c +≥+-+=,解得2a b c ≥+,选C.【一点开心】事实上在ABC ∆中,如果三边,,a b c 成等差或等比数列,即22b a c b ac =+=或, 那么我们都可以结合重要不等式知识得到60B ≤.10.如图,圆O 的半径为1,A 是圆上的定点,P 是圆上的动点,角x 的始边为射线OA ,终边为射线OP ,过点P 作直线 OA 的垂线,垂足为M ,将点M 到直线OP 的距离表示 为x 的函数()f x ,则()y f x =在[0,]π上的图象大致为( )正视图 1 1222 2 侧视图 俯视图【解】选由OP HM PM OM ⋅=⋅,于是HM PM OM =⋅,由三角函数线有, 1|s i n ||c o s ||s i n 2|2H M x x x =⋅=,于是1()|sin2|2f x x =的最大值为1,22T π=,故选C.二、填空题:本大题共5小题,共25分,把答案填在答题卡中对应题号后的横线上. 11.已知直线的极坐标方程为sin()4πρθ+=则极点到直线的距离为 .由sin()4πρθ+=1x y +=,于是极点 (0,0)O 到该直线的距离为d ==即求. 12.设,,x y z 均为正数,满足230x y z -+=,则2y xz的最小值是 .由230x y z -+=可化为23y x z =+,得224(3)43y x z x z =+≥⋅, 其中运用了重要不等式的变形式2()4,,a b ab a b R +≥∈,故23y xz≥(当3x z =时取等号). 13.数列{}a 的前n 项和为n S ,若*111,3,n n a a S n N +==∈,则2014a = .若填为201234⋅形式则视为错误,得分为0.*3,n S n N =∈……①,可推出,21133,3,2n n a a a S n -===≥……②①-②式得,14,2n n a a n +=≥,于是224n n a a -=⨯,2n ≥,故2012201434a =⨯.注意定义域了吗?14.若,x y 满足约束条件0,22,2y x y ≥⎧⎪≥-⎨⎪≤,且z kx y =+取得最小值的点有无数个,则k = .首先作出可行域如右图: 0k -≠,所以①当0k ->,即0k <时,依题意有目标直线//l BC 时,当其运动至与BC 重合时,最优解有无数个,符合题意,即2k -=,即2k =-; ②同理当0k -<,即0k >时,必有//l AB ,即1k -=-,即1k =, 综上①②可知,1k =或 2-为所求.15.已知椭圆22221(0)x y ab a b +=>>过椭圆上一点M 作直线MA MB 、分别交椭圆于A B 、两点,且斜率为12k k 、,若点A B 、【解】填13- 由222619b e a =-=,得2213b a =,如右图所示 取BM 中点D ,连结OD ,,2213O D B Mb k k a ⋅=-=-,又//OD AM ,故1OD k k =,即1213k k ⋅=- 【一点开心】显然,本题有一般性结论,即过椭圆2222:1(x ya bΓ+= l 交椭圆Γ于A B 、两点,P 是椭圆Γ上异于A B 、的任意一点,且当PA PB k k 、都存在时,则有22PA PB b k k a⋅=-.三、解答题:本大题共6小题,共75分.解答应写出文字说明,证明过程或演算步骤. 16.(本小题满分12分)2014年巴西世界杯的志愿者中有这样一组志愿者:有几个人只通晓英语,还有几个人只通晓俄语,剩下的人只通晓法语,已知从中任抽一人恰是通晓英语的概率为12,恰是通晓俄语的人的概率为310,且通晓法语的人数不超过3人.(Ⅰ)求这组志愿者的人数; (Ⅱ)现从这组志愿者中选出通晓英语、俄语和法语的志愿者各1人,若甲通晓俄语,乙通晓法语,求甲和乙不全被选中的概率; (Ⅲ)现从这组志愿者中抽取3人,求3人所会的语种数X 的分布列. 【解】(Ⅰ)设通晓英语、俄语、法语人分别有,,x y z 人,且*,,,3x y z N z ∈≤;则依题意有1,23,10x x y z y x y z ⎧=⎪++⎪⎨⎪=⎪++⎩,即,733,x y z y x z =+⎧⎨=+⎩…………………………………………2分消去x 得,*32zy N =∈,当且仅当2z =时,3y =符合正整数条件,所以5x =,也即这组志愿者有10人;………………………………………………………3分 (Ⅱ)记事件A 为“甲、乙不全被选中”,则A 的对立事件A 表示“甲、乙全被选中”,于是1155()1()15326P A P A ⨯⨯=-=-=⨯⨯;…………………………………………………7分(Ⅲ)随机变量X 的可能取值为1,2,3,且由古典概型知33212121535537283310101179(1),(2)120120C C C C C C C C P X P X C C +++====== 11153231030(3)120C C C P X C ===.………………………………………………………………11分:. ……………………………………………………………12分 17.(本小题满分12分)如图,点A 是单位圆与x 轴的正半轴的交点,点1(2B -. (Ⅰ)若AOB α∠=,求sin 2α; (Ⅱ)设点P 为单位圆上的动点,点Q 满足,OQ OA OP =+2(),62AOP ππθθ∠=≤≤()f OB OQ θ=⋅,求()f θ的取值范围.【解】(Ⅰ)由三角函数定义可知31sin ,cos 22y x r r αα====-, 所以313sin 22sin cos 2()222ααα==⨯⨯-=-,即求…………………………………5分 (Ⅱ)由三角函数定义知(cos2,sin 2)P θθ,所以(1cos2,sin2),OQ OA OP θθ=+=+所以131()(1cos 2)sin 2sin(2)2262f OB OQ πθθθθ=⋅=-++=--, 又因62ππθ≤≤,故52666πππθ≤-≤,即1sin(2)126πθ≤-≤,于是10()2f θ≤≤,所以()f θ的取值范围是1[0,]2.……………………………………12分18.(本小题满分12分)直三棱柱111ABC A B C -中,5,4,3,AB AC BC ===14AA =,点D 在AB 上. (Ⅰ)若D 是AB 中点,求证:1//AC 平面1B CD ;(Ⅱ)当13BD AB =时,求二面角1B CD B --的余弦值.【解】(Ⅰ)连接1BC 交1B C 于点E ,连接DE , 因为直三棱柱中侧面11BCC B 为矩形,所以 E 为1BC 的中点,又D 是AB 中点,于是1//DE AC ,且D E ⊂面1B CD ,1AC ⊄面1B CD , 所以1//AC 平面1B CD ;…………………………6分 (Ⅱ)由5,4,3,AB AC BC ===知90ACB ∠=,即AC CB ⊥, 又直三棱柱中1AA ⊥面ABC ,于是以C 为原点建立空间 直角坐标系C xyz -如右图所示,于是1(3,0,0),(3,0,4)B B 又13BD AB =,由平面几何易知4(2,,0)3D ,显然平面BCD 的一个法向量为1(0,0,1)=n ,又设平面1B CD 的一个法向量为2(,,)x y z =n ,则由212(3,0,4),4(2,,0),3CB CD ⎧⊥=⎪⎨⊥=⎪⎩n n ,得340,4203x x y +=⎧⎪⎨+=⎪⎩, 解得4,23x y =-=,取1z =,则24(,2,1)3=-n ,设二面角1B CD B --的平面角为θ,则1212||3361|cos |||||61θ⋅===⨯n n n n ,又由图知θ为锐角, 361…………………………………………………………………12分A C DB C 1 A 1B 1AC D C 1 A 1B 1 E y x z A CDB C 1 A 1B 119.(本小题满分13分)在数列{}n a 中,已知*111,21,n n a a a n n N +=-=-+∈. (Ⅰ)求证:{}n a n -是等比数列;(Ⅱ)令,2nn n na b S =为数列{}n b 的前n 项和,求n S 的表达式. 【解】(Ⅰ)证明:由*111,21,n n a a a n n N +=-=-+∈ 可得11(1)2(),120n n a n a n a +-+=--=-≠所以数列{}n a n -以是-2为首项,以2为公比的等比数列………………………………6分(Ⅱ) 由(Ⅰ)得:1222n n n a n --=-⨯=-,所以2n n a n =-,12n n nb =-所以12221212(1)(1)(1)()222222n n n n n nS b b b n =+++=-+-++-=+++-令212222n n n T =+++,则2311122222n n n T +=+++,两式相减得2311111111122222222n n n n n n nT ++=+++-=--, 所以222n n n T +=-,即222n n n S n +=--…………………………………………………13分20.(本小题满分13分)已知椭圆的一个顶点为(0,1)A -,焦点在x 轴上.若右焦点到直线0x y -+的距离为3. (Ⅰ)求椭圆的方程; (Ⅱ)设椭圆与直线(0)y kx m k =+≠相交于不同的两点M N 、.当||||AM AN =时,求m 的取值范围.【解】(Ⅰ)依题意可设椭圆方程为2221x y a+=,右焦点22(,0),1F c c a =-,3=,得c 故2213a c =+=;故椭圆的方程为2213x y +=………5分化简得2231m k =+1>,得12m >,又代入②式得,22m m <,解得02m <<, 综上可得122m <<,即为所求...…………………………………………………………13分 21.(本小题满分13分)已知函数()ln 3()f x a x ax a R =--∈. (Ⅰ)求函数()f x 的单调区间;(Ⅱ)若函数()y f x =的图象在点(2,(2))f 处的切线的倾斜角为45,对于任意的[1,2]t ∈,函数32()[()]2mg x x x f x '=++在区间(,3)t 上总不是单调函数,求m 的取值范围; (Ⅲ)求证:*ln2ln3ln4ln 1(2,)234n n N n n⨯⨯⨯⨯<≥∈. 【解】(Ⅰ)由(1)()(0)a x f x x x-'=>,.………………………………………………………1分①当0a >时,显然01x <<时,()0f x '>,当1x >时,()0f x '<, 所以此时()f x 的单调递增区间为(0,1),递减区间为(1,)+∞,②同理当0a <时, ()f x 的单调递增区间为(1,)+∞,递减区间为(0,1),③当0a =时,()3f x =-不是单调函数;.……………………………………………………4分(Ⅱ)由题知,(2)12af '=-=,得2a =-,所以()2ln 23f x x x =-+-.所以32()(2)2,02mg x x x x x =++->,且2()3(4)2,0g x x m x x '=++->,……………6分令()0g x '=时,可知2(4)240m ∆=++>恒成立,即()0g x '=一定有两个不等实根12,x x ,且注意到12203x x =-<,所以不妨设120x x <<,又0x >,于是可知20x x <<时,()0g x '<,又2x x >时,()0g x '>即()g x 在2(0,)x 上递减,在2(,)x +∞上递增,依题意可知2(,3)x t ∈,于是只须2()03(4)20(3)03370g t t m t g m '<++-<⎧⎧⇔⎨⎨'>+>⎩⎩,…………………………………………7分 又以上事实对[1,2]t ∈恒成立.故(1)50(2)21803370g m g m m '=+<⎧⎪'=+<⎨⎪+>⎩,得3793m -<<-;……………9分(Ⅲ)分析:要证*ln2ln3ln4ln 1(2,)234n n N n n⨯⨯⨯⨯<≥∈成立, 即证ln 2ln3ln 4ln 123(1),2n n n ⨯⨯⨯⨯<⨯⨯⨯⨯-≥,ln 1,n n n <-≥2成立,下面用综合法证明. 由(Ⅰ)知当1a =-时,()f x =-在上递增,所以)ln 3(1)2ln 1,1x x f x x x =-+->=-⇔<->………………………………11分 也所以在上式中分别令2,3,4,,x n =得, ln 21,ln32,ln 43,,ln 1,2n n n <<<<-≥,ln 2ln3ln 4ln 123(1),2n n n ⨯⨯⨯⨯<⨯⨯⨯⨯-≥两边同除以!n 得,*ln2ln3ln4ln 1(2,)234n n N n n⋅⋅⨯⨯<≥∈,即证.…………………13分。
湖南省长郡中学2015届高三月考(一)化学试题及答案

湖南长郡中学2015届高三月考(一)化学试卷时量:90分钟 满分:100分可能用到的相对原子质量:H ~l C ~12 O ~16 Na ~23 S ~32 Cl ~35.5 Ca ~40Fe ~56 Cu ~64 Ba ~137一、选择题(本题共16小题,每小题3分,共48分) 1. 用N A 表示阿伏伽德罗常数的值。
下列说法正确的是 A. 17 g 羟基中含有的电子数为10 N AB. 12.0 g 熔融的NaHSO 4中含有的阳离子数为0.2 N AC. 常温常压下,30g 乙酸与30g 葡萄糖含有的氧原子数为N AD. 常温下,5.6g Fe 投入到足量浓硝酸中,转移电子数为0.3 N A2. 在a L Al 2(SO 4)3和(NH 4)2SO 4的混合溶液中加入b mol BaCl 2,恰好使溶液中的SO -24完全沉淀;如加入足量强碱并加热可得到c mol NH 3,则原溶液中的Al+3物质的量浓度(mol ·L 1-)为A.a cb 22- B. ac b -2 C. a c b 32- D. acb 62- 3. 为除去括号内的杂质,下列各选项中所选用的试剂或方法不正确的是A. Na 2CO 3溶液(NaHCO 3):选用适量的NaOH 溶液B. NaHCO 3溶液(Na 2CO 3):通入过量的CO 2气体C. Na 2O 2粉末(Na 2O):将混合物在O 2中加热D. Na 2CO 3溶液(Na 2SO 4):加入适量的Ba(OH)2溶液,过滤4. 水溶液X 中只可能含有K +、Mg 2+、A13+、Al -2O 、Si -23O 、S -23O 、C -23O 、S -24O 中的若干种离子。
某同学对该溶液进行了如下实验:下列判断正确的是A. 气体甲一定是纯净物B. 沉淀甲是硅酸和硅酸镁的混合物C. K +、Al -2O 和Si -23O 一定存在于溶液X 中 D. C -23O 和S -24O 一定不存在于溶液X 中5. 下图所示是验证氯气性质的微型实验,a 、b 、d 、e 是浸有相关溶液的滤纸。
湖南省长沙市长郡中学高三化学上学期第二次月考试题(

长郡中学2015届高三月考试卷(二)化学时量:90分钟满分:100分第I卷选择题(共48分)【试卷综析】本试卷是高三年级化学月考试卷,在注重考查学科核心知识的同时,突出考查考纲要求的基本能力,注重主干知识,兼顾覆盖面。
以基础知识和基本技能为载体,以能力测试为主导。
重点考查:氧化还原、离子反应、元素化合物知识、物质的性质及检验、化学基本概念、元素周期表和元素周期律、有机化学等主干知识,考查了较多的知识点。
注重常见化学方法,应用化学思想,体现学科基本要求,试题难度不大。
一、选择题(本题共16小题,每小题3分,共48分)【题文】1.下列叙述正确的是A.向含有CaCO3沉淀的水中通入CO2至沉淀恰好溶解,再向溶液中加入NaHCO3饱和溶液,又有CaCO3沉淀生成B.向Na2 CO3溶液中逐滴加入含等物质的量HCl的稀盐酸,生成的CO2与原Na2 CO3的物质的量之比为1:2C.等质量的Na HCO3和Na2 CO3分别与足量盐酸反应,在同温同压下,生成的CO2体积相同D.向Na2 CO3饱和溶液中通入CO2,有Na HCO3结晶析出【知识点】钠及其化合物的性质C1【答案解析】D 解析:A、向含有CaCO3沉淀的水中通入CO2至沉淀恰好溶解得到Ca(HCO3)2溶液,再向溶液中加入NaHCO3饱和溶液,二者不反应无有CaCO3沉淀生成,故A错误;B、向Na2 CO3溶液中逐滴加入含等物质的量HCl的稀盐酸,得到NaHCO3和NaCl混合溶液,无CO2生成,故B错误;C、等物质的量的NaHCO3和Na2 CO3分别与足量盐酸反应,在同温同压下,生成的CO2体积相同,而等质量的NaHCO3和Na2 CO3分别与足量盐酸反应,在同温同压下,生成的CO2体积不相同,故C错误;D、向Na2 CO3饱和溶液中通入CO2,得到NaHCO3的质量比Na2 CO3大,且NaHCO3溶解度比Na2 CO3小,所以会有Na HCO3结晶析出,故D正确。
2015年长郡中学高一上学期第一次月考化学试卷

长郡中学化学第一次月考一、选择题(50分)1.1998年诺贝尔化学奖授予科恩(美)和波普尔(英)以表彰他们在理论化学领域作出的重大贡献.他们的工作使实验和理论能够共同协力探讨分子体系的性质,引起整个化学领域正在经历一场革命的变化.下列说法正确的是()A.化学不做实验,就什么都不知道 B.化学不再需要实验C.化学不再是纯实验科学 D.未来化学的方向是经验2.下列实验基本操作(或实验注意事项)中,主要是处于实验安全考虑的是()A.实验剩余的药品不能放回原试剂瓶B.可燃性气体的的验纯C.气体实验装置在实验前进行气密性检查D.滴管不能交叉使用3.进行化学实验必须注意安全,下列说法不正确的是()A.不慎将酸溅到眼中,应立即用水冲洗,边洗边眨眼睛B.不慎将浓碱溶液沾到皮肤上,要立即用大量水冲洗,然后涂上硼酸溶液C.配制硫酸溶液时,可先在量筒中加入一定体积的水,再在搅拌下加入浓硫酸D. 上述说法均不正确4.下列实验仪器不宜直接用来加热的是()A.试管 B.坩埚 C.蒸发皿 D.烧杯5.在盛放浓硫酸的试剂瓶的标签上应印有下列警示标记的是()A. B. C. D.6.下列混合物的分离和提纯方法中,主要是从溶解性的角度考虑的是()A.蒸发B.蒸馏C.升华D.萃取7. 有关化学实验的下列操作中,一般情况下不能相互接触的是()A.过滤操作中,玻璃棒与三层滤纸B.过滤操作中,漏斗颈与烧杯内壁C.分液操作中,分液漏斗颈与烧杯内壁D.用胶头滴管向试管滴液体时,滴管尖端与试管内壁8.下列事故处理的方法中不正确的是()A.电器起火,立即用水扑灭B.炒菜时油锅着火,立即盖上锅盖C.厨房煤气管道漏气,立即关闭阀门并开窗通风D.图书管内图书着火,立即用液态二氧化碳灭火器扑灭9.现有三组溶液:①汽油和氯化钠溶液②39%的乙醇溶液③碘水溶液,分离以上各混合液的正确方法依次是()A.分液、萃取、蒸馏B.萃取、蒸馏、分液C.分液、蒸馏、萃取D.蒸馏、萃取、分液10.下列说法正确的是()A.摩尔是物质的数量单位,含有6.02×1023个微粒的物质叫做1摩尔B.1mol氧含有6.02×1023个氧原子C.1mol气体的体积随压强增大和温度降低而变小D.在标准状况下,任何物质的摩尔体积约22.4L11.配制溶质质量分数一定的氯化钠溶液涉及的操作有:①称量②溶解③计算,其正确的操作顺序为()A.②①③B.②③①C.③①②D.③②①12. 下列关于容量瓶及其使用方法的叙述,正确的是()①是配制一定物质的量浓度溶液的专用仪器②使用前要先检查容量瓶是否漏液③容量瓶可以用来加热④不能用容量瓶贮存配制好的溶液⑤一定要用500ml容量瓶配制250ml溶液A.①③B.①②④C.①②④⑤D.①②③④13.下列都是生活中常见的物质,其中属于纯净物的是()A.葡萄酒B.冰水C.碘盐D.食醋14.“厦钨股份”正在研究和利用的白钨矿,其有效成分CaWO4中钨元素(w)的化合价为()A.+2B.+3C.+6D.+715. 下列事故处理的方法中不正确的是()A.电器起火,立即用水扑灭B.炒菜时油锅着火,立即盖上锅盖C.厨房煤气管道漏气,立即关闭阀门并开窗通风D.图书管内图书着火,立即用液态二氧化碳灭火器扑灭16.下列对于摩尔的理解正确的是()A.摩尔是国际科学界建议采用的一种物理量B.摩尔是物质的量的单位,简称摩,符号为molC.摩尔可以把物质的宏观数量与微观粒子的数量联系起来D.国际上规定,0.012kg碳原子所含有的碳原子数目为1摩17.下列有关阿伏伽德罗常数(NA)的说法错误的是()A.82克O2所含有的原子数目为NA2O含有的原子数目为1.5NAC.1molH2O含有的H2O分子数目为NA A个氯气分子的物质的量是0.5mol18.下列有关气体摩尔体积的描述中正确的是()A.单位物质的量的气体所占的体积叫做气体摩尔体积B.通常状况下的气体摩尔体积约为22.4L/molC.标准状况下的气体体积约22.4LD.相同物质的量的气体,其体积相同19.2molCl2和2molCO2相比较下列叙述中正确的是()A.分子数相等B.原子数相等C.体积相等D.质量相等20.下列物质中氧原子数目与11.7gNa2O2中氧原子数一定相等的是()A.6.72L COB.6.6g CO2 C.8g SO2D.9.6g H2SO421. 检验某未知溶液中是否含有SO42-,下列操作最合理的是 ( )A.加入稀硝酸酸化的Ba(NO3)2溶液B.加入盐酸酸化的BaCl2溶液C.先加稀硝酸酸化,再加Ba(NO3)2溶液D.先加盐酸酸化,再加BaCl2溶液22.下列叙述正确的是()A.同温同压下,相同体积的物质,其物质的量一定相等B.任何条件下,等物质的量的甲烷和一氧化碳所含的原子数一定相等C.1L一氧化碳气体一定比1L氧气的质量小D.相同条件下的一氧化碳气体和氮气,若体积相等,则质量一定相等23.质量是14.2g,体积是4.48升(标准状况下),该气体的摩尔质量是()A.28.4B.28.4g·mol-1C.71D.71g·mol-124.实验室里需用480ml0.1mol·L-1的硫酸铜溶液,现选取500ml容量瓶进行配制,以下操作正确的是()A.称取7.68g硫酸铜,加入500ml水B.称取12.0g胆矾配成500ml溶液C.称取8.0g硫酸铜,加入500水D.称取12.5g胆矾配成500ml溶液25.某溶液中含有较大量的Cl-、CO32-、OH- 3种阴离子,如果只取一次该溶液就能够分别将3种阴离子依次检验出来,下列实验操作顺序正确的是 ( )①滴加Mg(NO3)2溶液②过滤③滴加AgNO3溶液④滴加Ba(NO3)2溶液A①②④②③ B④②①②③ C①②③②④ D④②③②①二、填空题(44分)26.(3分)19g某二价金属的氯化物RCl2中含有0.4mol的Cl- 离子,则R的相对原子质量27.(12分)现有m g某气体,它由双原子分子构成,它的摩尔质量为M g·mol-1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
湖南省长沙市长郡中学2015届高三第一次月考化学试卷
一、选择题(本题共16小题,每小题3分,共48分)
羟基的物质的量为:=1mol 酸氢钠的物质的量为:
的量为:=1mol
2.(3分)(2011•信阳一模)在a L Al2(SO4)3和(NH4)2SO4的混合溶液中加入b mol BaCl2,恰好使溶液中的SO42﹣离子完全沉淀;如加入足量强碱并加热可得到c mol NH3,则原溶液3+
B
NH
=mol/L
NH
=
3+mol/L
x=
4.水溶液X中只可能溶有K+、Mg2+、Al3+、AlO2﹣、SiO32﹣、SO32﹣、CO32﹣、SO42﹣中的若干种离子.某同学对该溶液进行了如图所示实验:下列判断正确的是()
、和
5.图所示是验证氯气性质的微型实验,a、b、d、e是浸有相关溶液的滤纸.向KMnO4晶体滴加一滴浓盐酸后,立即用另一培养皿扣在上面.已知:
2KMnO4+16HCl→2KCl+5Cl2↑+2MnCl2+8H2O
对实验现象的“解释或结论”正确的是()
7.(3分)下列说法不正确的是()
①将BaSO4放入水中不能导电,所以BaSO4是非电解质
②氨溶于水得到的氨水能导电,所以氨水是电解质
③固态共价化合物不导电,熔融态的共价化合物可以导电
④固态的离子化合物不导电,熔融态的离子化合物也不导电
8.某溶液中可能含有H+、NH4+、Mg2+、Al3+、Fe3+、CO32﹣、SO42﹣、NO3﹣中的几种.①若加入锌粒,产生无色无味的气体;②若加入NaOH溶液,产生白色沉淀,且产生的沉淀量与加入NaOH的物质的量之间的关系如图所示.则下列说法正确的是()
10.某溶液可能含有Cl﹣、SO42﹣、CO32﹣、NH4+、Fe3+、Al3+和K+.取该溶液100mL,加入过量NaOH溶液,加热,得到0.02mol气体,同时产生红褐色沉淀;过滤,洗涤,灼烧,得到1.6g固体;向上述滤液中加足量BaCl2溶液,得到4.66g不溶于盐酸的沉淀.由此可知
12.(3分)要从苯酚的乙醇溶液中回收苯酚,有下列操作;①蒸馏;②过滤;③静置分液;④加入足量金属钠;⑤通入过量CO2;⑥加入足量NaOH溶液;⑦加入足量FeCl3
13.(3分)鉴别苯酚溶液,己烷,己烯,乙酸溶液和乙醇5种无色液体,可选用是最佳试
14.(3分)乙醇分子结构中的各种化学键如图所示,下列关于乙醇在各种反应中断键的说法正确的是()
15.有关下图所示化合物的说法不正确的是()
16.向27.2gCu和Cu2O的混合物中加入某浓度的稀硝酸0.5L,固体物质完全反应,生成NO和Cu(NO3)2.在所得溶液中加入1.0mol/L 的NaOH溶液1.0L,此时溶液呈中性,金
=0.4mol
c=
=0.4mol
所以原硝酸溶液的浓度为
二、非选择题(本题包括5个小题,共52分)
17.(8分)我国政府为了消除碘缺乏病,规定在食盐中必须加入适量的碘酸钾.检验食盐中是否加碘,可利用如下反应:1KIO3+5KI+3H2SO4═3K2SO4+3I2+ 3H2O.
(1)将上述氧化还原反应的化学方程式配平.
(2)该反应中,氧化剂和还原剂的物质的量之比为1:5.
(3)如果反应中转移0.2mol电子,则生成I2的物质的量为0.12mol.
(4)利用上述反应检验食盐中是否加碘,所需试剂是②③④(填序号).
①碘水②KI溶液③淀粉溶液④稀硫酸⑤AgNO3溶液.
18.(6分)有下列化学仪器:①托盘天平,②玻璃棒,③药匙,④烧杯,⑤量筒,⑥容量瓶,⑦胶头滴管,⑧细口试剂瓶,⑨标签纸.
(1)现需要配制500mL 1mol/L硫酸溶液,需用质量分数为98%、密度为1.84g/cm3的浓硫酸27.2mL.
(2)从上述仪器中,按实验使用仪器的先后顺序.其编号排列是⑤④②⑥⑦⑧⑨.(3)若实验遇到下列情况,所配硫酸溶液的物质的量浓度偏小的有①③⑤(填序号).
①用以稀释硫酸的烧杯未洗涤;
②未经冷却趁热将溶液注入容量瓶中;
③摇匀后发现液面低于刻度线再加水;
④容量瓶中原有少量蒸馏水;
⑤定容时仰视观察液面.
分析操作对溶质的物质的量或对溶液的体积的影响判断.
19.(14分)某化学课外兴趣小组探究铜与浓硫酸的反应情况.甲.乙.丙三位同学进行了下列实验:取12.8g铜片和20ml.18mol/L的浓硫酸放在圆底烧瓶中共热,直至反应完毕,最后发现烧瓶中还有铜片剩余外,同时根据所学的知识认为还有较多的硫酸剩余.
(1)请写出铜跟浓硫酸反应的化学方程式:Cu+2H2SO4(浓)
CuSO4+2H2O+SO2↑,试问:为什么较多的余酸不再与铜片继续反应?简述理由:
铜片过量,硫酸反应后溶液变稀不支持反应.
可以证明有余酸的实验方案是ad(填写字母,错选或多选扣分)
(a)再加入铁粉(b)再滴入BaCl2溶液(c)再加入银(d)再滴入Na2CO3溶液
(2)甲学生设计求余酸浓度的实验方案是测定产生气体的量.其方法有多种,请问下列方案中不可行的是ac(填写字母,错选或多选扣分)
(a)将产生的气体缓缓通过预先称量盛有碱石灰的干燥管,结束反应后再次称量.
(b)将产生的气体缓缓通入酸性高锰酸钾溶液,再加入足量BaCl2溶液,过滤、洗涤、干燥、称量沉淀.
(c)用排水法测定其产生气体的体积(折算成标准状况).
(d)用排饱和NaHSO3溶液的方法测出其产生气体的体积(折算成标准状况).
(3)乙同学设计测定余酸浓度的实验方案是:测定反应后的混合液中Cu2+的量.在反应后的溶液中加蒸馏水稀释至100ml,加入足量Na2S溶液,充分反应后,过滤.洗涤.干燥、称量沉淀.请写出生成沉淀的离子方程式:
Cu2++S2﹣═CuS↓.
(4)根据甲、乙两同学的实验方案,除测算产生气体的物质的量或反应掉的铜的物质的量外,尚缺少的测量数据是反应后溶液的体积.
(5)丙同学提出甲、乙两同学的实验方案设计的复杂,为此他设计了下列较为简易的实验方案:取出反应后的铜片,进行洗涤.干燥.称量.若称得剩余铜片的质量为Wg,测得反
应后溶液的体积为Vml,请计算剩余硫酸的物质的量浓度=
mol/L(用含W.V的代数式表示)
(浓)CuSO
,反应掉的硫酸的物质的量是铜的二倍,
,用总的硫酸的物质的量减去反应的就是剩余的,为
,然后根据物质的量浓度等于溶液中溶质的物质的量物质的量除以溶
.
20.(12分)氯气是一种重要的工业原料.
Ⅰ.实验室可用二氧化锰和浓盐酸反应制取氯气,反应的化学方程式是MnO2+4HCl(浓)
MnCl2+Cl2↑+2H2O.
Ⅱ.某研究性学习小组查阅资料得知,漂白粉与硫酸反应可制取氯气,化学方程式为:
Ca(ClO)2+CaCl2+2H2SO42CaSO4+2Cl2↑+2H2O
他们利用该反应设计如下制取氯气并验证其性质的实验.
回答下列问题:
(1)该实验中A部分的装置是b(填标号).
(2)请你帮助他们设计一个实验,证明洗气瓶C中的Na2SO3已被氧化(简述实验步骤):取少量溶液置于洁净的试管中,向其中滴加稀盐酸至不再产生气体,再向其中滴入氯化钡溶液,若产生白色沉淀,证明亚硫酸钠被氧化.
(3)写出D装置中发生反应的离子方程式Cl2+H2O═H++Cl﹣+HClO;H++HCO3﹣
═CO2↑+H2O.
(4)该实验存在明显的缺陷,请你提出改进的方法应将尾气通入NaOH溶液中.(5)该小组又进行了如下实验:称取漂白粉2.0g,研磨后溶解,配制成250mL溶液,取出25mL加入到锥形瓶中,再加入过量的KI溶液和过量的H2SO4溶液,静置.待完全反应后,用0.1mol•L﹣1的Na2S2O3溶液作标准液滴定反应生成的碘,已知反应方程式为:
2Na2S2O3+I2═Na2S4O6+2NaI,共用去Na2S2O3溶液20.0mL.则该漂白粉中Ca(ClO)2的质量分数为35.75%.
(浓)
(浓)
气
n
×
%=
21.以乙炔为原料通过以下流程能合成有机物中间体D.
请回答下列问题:
(1)化合物D的分子式为C8H12O4,写出D中一种官能团的名称羟基或醛基.(2)写出生成A的化学反应方程式:HC≡CH+2HCHO→HOCH2C≡CCH2OH.
(3)化合物B在浓硫酸催化下,加热与HOOCCOOH反应生成环状酯的化学方程式为:
(注明条件).
(4)化合物C的结构简式为:OHCCH2CH2CHO.
(5)已知1mol HCHO和1mol CH3CH2CHO发生类似已知(2)的反应,生成1molE.以下关于E的说法正确的是AC.
a、E能与H2发生加成反应,也能使酸性高锰酸钾溶液褪色.
b、E属于酯类物质.
c、1molE完全燃烧消耗5molO2.
d、生成E的反应属于取代反应.
方程式为
故答案为:
﹣。
