BD-Application of BCRP, BSEP and MRP2 Transporter Vesicles in Drug Development Studies
美国cGMP-中英文对照
PART 210 CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING,PROCESSING,PACKING, OR HOLDING OF DRUGS;GENERAL210部分有关于生产、加工、包装和药品的储存的现行GMP;一般准则Sec. 210.1 Status of current goodmanufacturing practice regulations。
210.1 cGMP的法规地位。
(a)The regulations set forth in this part and in parts 211 through 226 of this chapter contain the minimum current good manufacturing practice for methods to be used in, and the facilities or controls to be used for, the manufacture,processing, packing, or holding of a drug to assure that such drug meets the requirements of the act as to safety, and has the identity and strength and meets the quality and purity characteristics that it purports or is represented to possess. (a)在本部分及本章第211-226部分中所陈述的法规,为现行GMP最低要求,适用于药品制造、加工、包装或贮存中所采用的方法及所使用的设施或控制手段,以保证该药品符合《联邦食品、药品及化妆品法案》(以下简称法案)对安全性的要求,具有均一性和效价(或含量)并符合或代表其生产过程的质量及纯度等特征。
甲磺酸艾立布林注射液(海乐卫)中文说明书

甲磺酸艾立布林注射液(海乐卫)中文说明书甲磺酸艾立布林注射液 海乐卫∙Ha1aven®Eribu1inMesi1ateInjectionJiahuangsuanAi1ibu1inZhusheye【成份】本品主要成份为甲磺酸艾立布林。
化学名称:11,15:18,21:24,28-三环氧基-7,9-桥亚乙基-12,15-桥亚甲基-9H,15H-峡喃并[3,2-i 映喃并⑵3:5,6]毗喃并[4,3-b][1,4]二氧杂环二十五烷-5(4H)-酮,2-[(2S)3氨基2羟丙基]二十六氢3甲氧基-26-甲基-20,27-二(亚甲基),(2R,3R,3aS,7R,8aS,9S,10aR,11S,12R,13aR,13bS,15S,18S,21S,24S,26R,28R,29aS)-,甲磺酸盐 化学结构式:【适应证】本品适用于既往接受过至少两种化疗方案的局部晚期或转移性乳腺癌患者。
既往的化疗方案应包含一种慈环类和一种紫杉烷类药物。
【规格】2m1:1mg (以甲磺酸盐计) 【用法用量】推荐剂量本品的推荐剂量为14mg∕m2,2-5分钟内静脉推注,21天为一个周期,每个周期第1天和第8天给药一次。
肝功能损害患者肝转移导致的肝功能受损:轻度肝功能损害(Chi1d-PUghA)患者中,本品推荐剂量为1.1mg∕m 2,2-5分钟内静脉推注,21天为一个周期,每个周期第1天和第8天给药一次。
中度肝功能损害(ChiId-PughB)患者中,本品推荐剂量为0∙7mg∕m2,2-5分钟内静脉推注,21天为一个周期,每个周期第1天和第8天给药一次。
尚未对重度肝功能损害(Chi1d-PughO 进行研窕,但是如果这些患者使用艾立布林,估计使用剂量需要更为明显的降低。
肝硬化导致的肝功能受损•.尚未对这组患者进行研究。
上述剂量可以用于轻度和中度肝功能损害患者,但建议对其进行密切监测,因为可能需要重新调整剂量。
肾脏损害患者中度或重度肾脏损害(肌肝清除率(C1Cr )15-49m1∕min )患者中,本品推荐剂量为11mg"?,2-5分钟内静脉推注,21天为一个周期,每个周期第1天和第8天给药一次。
药物基因组学与个体化给药

CYP2C18 氟西汀,丙咪嗪, 吡罗昔康,利福平 CYP2C19 氟西汀,丙咪嗪,异烟肼,去甲替林,苯妥英,利福平, 华法林 CYP2D6 CYP2E1 UTG2 氟西汀,地尔硫卓, 丙咪嗪,美托洛尔,去甲替林,茶碱 氟西汀,异烟肼,茶碱,异搏定 布洛芬,萘普生
NAT2
异烟肼
重庆医科大学药学院 秧茂盛
LDG
疾病基因研究室/药物基因组研究中心/生命科学研究院
药物基因组学与个体化给药
重庆医科大学药学院
秧茂盛
LDG
疾病基因研究室/药物基因组研究中心/生命科学研究院
疗效好
药物
无效 不良反应
重庆医科大学药学院
秧茂盛
LDG
疾病基因研究室/药物基因组研究中心/生命科学研究院 年龄
遗传背景
性别
治疗效果
并发症
LDG
疾病基因研究室/药物基因组研究中心/生命科学研究院
疗效好
TPMT+
致死性的骨髓抑制
TPMT-
疗效差
六巯基嘌吟 TPMT:硫嘌呤甲基转移酶
重庆医科大学药学院 秧茂盛
LDG
疾病基因研究室/药物基因组研究中心/生命科学研究院
药物作用的多基因本质
疾病的病源基因 治疗作用 不良反应
宿主易感基因
药物代谢和转运基因
疾病基因研究室/药物基因组研究中心/生命
Alleles C>T C>A A>G C>G C>T C>T G >C G>C G>A C>T G >C 2 4A 4B 10A 10B 10C 17 188 188 188 188 188 1062 1072 1085 1062 1072 1085 1127 1127 1749 1749 1934 1934 1749 1749 1749 2938 4268 4268 4268 4268 4268 4268 2938 4268
ISPE指南目录

ISPE指南按系列分类的目录清单:GAMP?5GAMP?Good Practice Guides• A Risk-Based Approach to Calibration Management (Second Edition)•基于风险的校正管理方法(第二版)• A Risk-Based Approach to Electronic Records and Signatures•基于风险的电子记录和签名方法• A Risk-Based Approach to GxP Compliant Laboratory Computerized Systems (Second Edition)•基于风险的GXP符合性实验室计算机化系统方法(第二版)• A Risk-Based Approach to GxP Process Control Systems (Second Edition)•基于风险的GXP工艺控制体系方法(第二版)• A Risk-Based Approach to Operation of GxP Computerized Systems - A Companion Volume to GAMP 5•基于风险的GXP计算机系统操作方法---GAMP 5姊妹篇• A Risk-Based Approach to Regulated Mobile Applications•基于风险的移动APP管理方法• A Risk-Based Approach to Testing of GxP Systems (Second Edition)•基于风险的GXP系统检测方法(第二版)•Electronic Data Archiving•电子数据归档•Global Information Systems Control and Compliance•全球信息系统控制和符合性•IT Infrastructure Control and Compliance•IT基础设施控制和符合性•Legacy Systems•遗留系统•Manufacturing Execution Systems – A Strategic and Program Management Approach•生产执行系统—策略和编程管理方法•GAMP Good Practice Guides Under Development•制订中的GAMP GPGISPE Baseline?Pharmaceutical Engineering Guides for New and Renovated FacilitiesISPE基准:新设施和创新型设施药品工程指南•Volume 1: Active Pharmaceutical Ingredients (Second Edition) - Revision to Bulk Pharmaceutical Chemicals•卷1:活性药物成分(第二版)---对散装药用化学品的修订•Volume 2: Oral Solid Dosage Forms (Second Edition)•卷2:口服固体制剂(第二版)•Volume 3: Sterile Product Manufacturing Facilities (Second Edition)•卷3:无菌药品生产设施(第二版)•Volume 4: Water and Steam Systems (Second Edition)•卷4:水和蒸汽系统(第二版)•Volume 5: Commissioning and Qualification•卷5:调试和确认•Volume 6: Biopharmaceutical Manufacturing Facilities (Second Edition)•卷6:生物药品生产设施(第二版)•Volume 7: Risk-Based Manufacture of Pharmaceutical Products (Risk-MaPP)•卷7:基于风险的药品生产(风险MAPP)•Baseline Guides Under Development•制订中的基准指南ISPE Guides•ISPE Guide: Science and Risk-Based Approach for the Delivery of Facilities, Systems, and Equipment•ISPE指南:基于风险的设施、系统和设备传送科学方法•ISPE Guide: Biopharmaceutical Process Development and Manufacturing•ISPE指南:生物药品工艺开发和生产(新出版)•ISPE Guides Under Development•在制订中的ISPE指南ISPE Good Practice Guides 优良规范指南•ISPE Good Practice Guide: Applied Risk Management for Commissioning and Qualification•ISPE GPG:在调试和确认中应用风险管理•ISPE Good Practice Guide: Approaches to Commissioning and Qualification of Pharmaceutical Water and Steam Systems (Second Edition)•ISPE GPG:药用水和蒸汽系统调试和确认方法(第二版)(新出)•ISPE Good Practice Guide: Assessing the Particulate Containment Performance of Pharmaceutical Equipment (Second Edition)•ISPE GPG:制药设备颗粒密闭性能的评估(第二版)•ISPE Good Practice Guide: Booklet Labels•ISPE GPG:书册标签•ISPE Good Practice Guide: Clinical Supply Systems•ISPE GPG:临床补给系统(新出)•ISPE Good Practice Guide: Cold Chain Management•ISPE GPG:冷链管理•ISPE Good Practice Guide: Comparator Management•ISPE GPG:对照组管理•ISPE Good Practice Guide: Development of Investigational Therapeutic Biological Products•ISPE GPG:临床前治疗用生物产品开发•ISPE Good Practice Guide: Good Engineering Practice•ISPE GPG:优良工程规范•ISPE Good Practice Guide: Harmonizing the Definition and Use of Non-Investigational Medicinal Products (NIMPs)•ISPE GPG:协调非临床前药品的定义和使用•ISPE Good Practice Guide: Heating, Ventilation, and Air Conditioning (HVAC)•ISPE GPG:HVAC•ISPE Good Practice Guide: Interactive Response Technology•ISPE GPG:互动反馈技术•ISPE Good Practice Guide: Maintenance•ISPE GPG:维护•ISPE Good Practice Guide: Ozone Sanitization of Pharmaceutical Water System•ISPE GPG:制药用水系统的臭氧消毒•ISPE Good Practice Guide: Packaging, Labeling, and Warehousing Facilities•ISPE GPG:包装、贴标和仓储设计•ISPE Good Practice Guide: Process Gases•ISPE GPG:工艺用气•ISPE Good Practice Guide: Project Management for the Pharmaceutical Industry•ISPE GPG:制药行业的项目管理•ISPE Good Practice Guide: Quality Laboratory Facilities•ISPE GPG:质量化验室设施•ISPE Good Practice Guide: Technology Transfer (Second Edition)•ISPE GPG:技术转移(第二版)(新出)•ISPE Good Practice Guides Under Development•制订中的ISPE GPGPQLI?Guides 药品质量生命周期实施指南•PQLI Overview Good Practice Guide•PQLI概览GPG•Product Quality Lifecycle Implementation (PQLI) from Concept to Continual ImprovementPart 1: Product Realization using QbD, Concepts and Principles •从概念到持续改进的药品质量生命周期实施(PQLI)第一部分:利用质量源于设计(QbD)实现实现,概念和原则欢迎您的下载,资料仅供参考!致力为企业和个人提供合同协议,策划案计划书,学习资料等等打造全网一站式需求。
20100731 欧盟API GMP 中英文对照 CX 20110112
EUROPEAN COMMISSION 欧盟委员会ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL 企业与工业管理局Consumer goods 消费品Pharmaceuticals 药品Brussels, 03 February 2010 布鲁塞尔2010.02.03ENTR/F/2/AM/an D(2010) 3374EudraLex(European Union Law On drug regulatory affairs)欧盟药品法规The Rules Governing Medicinal Products in the European Union欧盟医药产品管理规则Volume 4卷4Good Manufacturing Practice良好生产规范Medicinal Products for Human and Veterinary Use人用和兽用医药产品Part II: Basic Requirements for Active Substances used as Starting Materials 第二部分:作为起始物料的原料药的基本要求Table of Contents目录1 Introduction1简介1.1 Objective1.1目的1.2 Regulatory Applicability1.2法规适用性1.3 Scope1.3范围2 Quality Management2质量管理2.1 Principles2.1原则2.2 Quality Risk Management2.2质量风险管理2.3 Responsibilities of the Quality Unit(s) 2.3质量部门的职责2.4 Responsibility for Production Activities 2.4生产活动的职责2.5 Internal Audits (Self-Inspection)2.5内部审计(自检)2.6 Product Quality Review2.6产品质量回顾3 Personnel3 人员3.1 Personnel Qualifications3.1 人员资质3.2 Personnel Hygiene3.2 人员卫生3.3 Consultants3.3 顾问4 Buildings and Facilities4 厂房设施4.1 Design and Construction4.1 设计和建造4.2 Utilities4.2 公用工程4.3 Water4.3 水4.4 Containment4.4 限制4.5 Lighting4.5 照明4.6 Sewage and Refuse4.6 废水废物4.7 Sanitation and Maintenance4.7 公共卫生及保养5 Process Equipment5 工艺设备5.1 Design and Construction5.1 设计和建造5.2 Equipment Maintenance and Cleaning5.2 设备的保养和清洁5.3 Calibration5.3 校验5.4 Computerized Systems5.4 计算机系统6 Documentation and Records6 文件和记录6.1 Documentation System and Specifications6.1 文件系统与规格标准6.2 Equipment Cleaning and Use Record6.2 设备清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labelling and Packaging Materials 6.3 原料、中间产品、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)6.4 生产指令(生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)6.5批生产记录(批生产和控制记录)6.6 Laboratory Control Records6.6 实验室控制记录(批检验记录)6.7 Batch Production Record Review6.7批生产记录审核7 Materials Management7 物料管理7.1 General Controls7.1 控制通则7.2 Receipt and Quarantine7.2 接受和待检7.3 Sampling and Testing of Incoming Production Materials7.3 到货物料的取样和检测7.4 Storage7.4 贮存7.5 Re-evaluation7.5 再评估8 Production and In-Process Controls8 生产和过程控制8.1 Production Operations8.1 生产操作8.2 Time Limits8.2 时间限制8.3 In-process Sampling and Controls8.3 中控取样和控制8.4 Blending Batches of Intermediates or APIs8.4 中间产品和原料药的混批8.5 Contamination Control8.5 污染控制9 Packaging and Identification Labelling of APIs and Intermediates 9 中间产品和原料药的包装和贴签9.1 General9.1 总则9.2 Packaging Materials9.2 包装材料9.3 Label Issuance and Control9.3 标签放行和控制9.4 Packaging and Labelling Operations9.4 包装和贴签操作10 Storage and Distribution10 贮存和销售10.1 Warehousing Procedures10.1 入库程序10.2 Distribution Procedures10.2 销售程序11 Laboratory Controls11 实验室控制11.1 General Controls11.1 控制通则11.2 Testing of Intermediates and APIs11.2 中间产品和原料药的检测11.3 Validation of Analytical Procedures11.3 分析方法的验证11.4 Certificates of Analysis11.4 分析报告11.5 Stability Monitoring of APIs11.5 原料药的稳定性监测11.6 Expiry and Retest Dating11.6 失效和复检日期11.7 Reserve/Retention Samples11.7 留样12 Validation12 验证12.1 Validation Policy12.1 验证方针12.2 Validation Documentation12.2 验证文件12.3 Qualification12.3 确认12.4 Approaches to Process Validation12.4 工艺验证方法12.5 Process Validation Program12.5 工艺验证计划12.6 Periodic Review of Validated Systems12.6 验证系统的定期审核12.7 Cleaning Validation12.7 清洁验证12.8 Validation of Analytical Methods12.8 分析方法验证13 Change Control13 变更控制14 Rejection and Reuse of Materials14 物料的拒收和再利用14.1 Rejection14.1 拒收14.2 Reprocessing14.2 返工14.3 Reworking14.3 重新加工14.4 Recovery of Materials and Solvents14.4 物料和溶剂的回收利用14.5 Returns14.5 退回15 Complaints and Recalls15 投诉和召回16 Contract Manufacturers (including Laboratories)16 合同生产企业(包含实验室)17 Agents, Brokers, Traders, Distributors, Repackers, and Relabellers 17 代理商、经纪商、贸易商、经销商、重新包装商和重新贴签商17.1 Applicability17.1 适用性17.2 Traceability of Distributed APIs and Intermediates17.2 已销售中间产品和原料药的追踪17.3 Quality Management17.3 质量管理17.4 Repackaging, Relabelling and Holding of APIs and Intermediates 17.4 中间产品和原料药的重新包装、重新贴签和处理17.5 Stability17.5 稳定性17.6 Transfer of Information17.6 信息的传输17.7 Handling of Complaints and Recalls17.7 投诉和召回的处理17.8 Handling of Returns17.8 退货的处理18 Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18 用于细胞培养/发酵而得原料药的特殊指南18.1 General18.1 总则18.2 Cell Bank Maintenance and Recordkeeping18.2 细胞库的维护和记录保存18.3 Cell Culture/Fermentation18.3 细胞培养/发酵18.4 Harvesting, Isolation, and Purification18.4 收获、分离和精制18.5 Viral Removal/Inactivation Steps18.5 病毒除去/灭火步骤19 APIs for Use in Clinical Trials19 用于临床试验的原料药19.1 General19.1 总则19.2 Quality19.2 质量19.3 Equipment and Facilities19.3 设备设施19.4 Control of Raw Materials19.4 原料的控制19.5 Production19.5 生产19.6 Validation19.6 验证19.7 Changes19.7 变更19.8 Laboratory Controls19.8 实验室控制19.9 Documentation19.9 文件20 Glossary20 词汇表1 Introduction1 介绍This guideline was published in November 2000 as Annex 18 to the GMP Guide reflecting the EU’s agreement to ICH Q7A and has been used by manufacturers and GMP inspectorates on a voluntary basis. Article 46 (f) of Directive 2001/83/EC and Article 50 (f) of Directive 2001/82/EC; as amended by Directives 2004/27/EC and 2004/28/EC respectively, place new obligations on manufacturing authorisation holders to use only active substances that have been manufactured in accordance with Good Manufacturing Practice for starting materials. The directives go on to say that the principles of Good Manufacturing Practice for active substances are to be adopted as detailed guidelines. Member States have agreed that the text of former Annex 18 should form the basis of the detailed guidelines to create Part II of the GMP Guide.本指南已经在2000年11月以GMP指南附录18的形式公布过,它反应了欧盟对ICH Q7A的认可以,该指南已经被生产商和GMP检查员在自愿的原则下所使用。
肝脏的药物转运体及其临床意义的研究进展

肝脏的药物转运体及其临床意义的研究进展傅超;刘洋【摘要】To introduce the function,distribution,and substrate characteristics of drug transporters in the liver and the effect on the treatment of drug in human body disposal process. Based on the liver drug delivery system,and the disposal of the influence in the body to classifyand summarize. Liver as an important organ for the metabolism and excretion of endoge-nous and exogenous(drug),except for hepatic enzymes,played an role in the process of metabolism and excretion of the drugs. The efficacy and safety of the drugs would be changed when the function of the transporters was affected.%介绍肝脏的药物转运体的功能、分布、底物特征及其对药物的体内处置过程的影响。
按照肝脏药物转运体系统、体内处置的影响进行分类归纳总结。
肝脏作为机体对内源物和外源物(药物)的代谢和排泄的重要器官,除了药物代谢酶外,肝脏转运体在其中也发挥着一定的作用。
当药物转运体的功能受到影响时,往往会使其底物性药物的有效性和安全性发生改变。
【期刊名称】《药学研究》【年(卷),期】2015(000)012【总页数】4页(P731-733,743)【关键词】转运体;肝脏;药物相互作用【作者】傅超;刘洋【作者单位】青岛市食品药品检验研究院,山东青岛 266071;昆泰企业管理上海有限公司,上海 200032【正文语种】中文【中图分类】R969.1肝脏是参与药物代谢和排泄最重要的器官。
药物转运体蛋白的靶点作用

Distribution
Circulating blood
Metabolism
Excretion
OCT1
MRP3
OATP1A2 OATP2B1
P-gp BCRP MRP4 MRP5
OATP1B1 OATP2B1 OAT2,OCT1 NTCP
MRP3 MRP4 MRP6
OCT2,OAT1 OAT2,OAT3
④ 肿瘤耐药蛋白模型
a) P-gp (P-糖蛋白) b) MRP2 (多剂耐药蛋白) c) BCRP (乳腺癌耐药蛋白)
① 钠-葡萄糖协同转运体(SGLT2)模型
1. SGLT1 和 SGLT2 的比较
Data from: Lee, Y. J. & Han, H. J. Regulatory mechanisms of Na+/ glucose cotransporters in renal proximal tubule cells. Kidney Int. Suppl. 106, S27–S35 (2007).
ABC transporter
Breast cancer resistant protein (BCRP) Multidrug resistance-associated protein 4 (MRP4) Multidrug resistance-associated protein 5 (MRP5) P-gp/MDR1
Urine
Excretion
3)癌组织
Cell membrane
SLC transporter
L-type amino acid transporter 1 (LAT1)
ABC transporter
药物转运体

Uptake (% of control)
Uptake (% of control)
100 75 50 25 PAH 0
PCG
100 75 50 25 0
Uptake (% of control)
Schematic diagram of renal drug transport systems
Hasegawa M, et al J Pharmacol Exp Ther, 305: 1087-97 (2003). Deguchi T, et al Kidney Int, 65: 162-74 (2004).
Brain side
kDa 175 83
1 2 3 4
OAT 3
Cl-, HCO3-(?) Glutarate
Oatp 4
Oatp1c1
62 48 antigen absorption
X
Phase II enzymes SULT, UGT, GST
X-Sul X-Glu X-S-G
ATP ADP
2.09±0.43mM 11.4±3.1 μM 17.0±4.4 μM
10 0 10 0 10 0 0 10 00 00 00
1 10 10 0 10 0 10 0 0 10 00 00 00
1 10
PAH concentration (µM) Uptake (% of control) 125 75 50 25 0
Brain choroid plexus T/M ratio
In vitro :
time
CSF side
原料药GMP指南中英文对照

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System and Specifications 6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, 6.3 原料、中间体、原料药的标签和包装材料API Labeling and Packaging Materials 的记录6.4 生产工艺规程(主生产和控制记录)6.4 Master Production Instructions (MasterProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 进厂物料的取样与测试7.3 Sampling and Testing of IncomingProduction Materials7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. 生产和过程控制8. PRODUCTION AND IN-PROCESSCONTROLS8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates or APIs 8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. 原料药和中间体的包装和贴签9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. VALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIsQ7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
原料药GMP指南(中英文对照)

Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介1.1 Objective 1.1目的1.2 Regulatory Applicability 1.2法规的适用性1.3 Scope 1.3范围2. QUALITY MANAGEMENT 2.质量管理2.1 Principles 2.1总则2.2 Responsibilities of the Quality Unit(s) 2.2质量部门的责任2.3 Responsibility for Production Activities 2.3生产作业的职责2.4 Internal Audits (Self Inspection) 2.4内部审计(自检)2.5 Product Quality Review 2.5产品质量审核3. PERSONNEL 3. 人员3.1 Personnel Qualifications 3.人员的资质3.2 Personnel Hygiene 3.2 人员卫生3.3 Consultants 3.3 顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施4.1 Design and Construction 4.1 设计和结构4.2 Utilities 4.2 公用设施4.3 Water 4.3 水4.4 Containment 4.4 限制4.5 Lighting 4.5 照明4.6 Sewage and Refuse 4.6 排污和垃圾4.7 Sanitation and Maintenance 4.7 卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备5.1 Design and Construction 5.1 设计和结构5.2 Equipment Maintenance and Cleaning 5.2 设备保养和清洁5.3 Calibration 5.3 校验5.4 Computerized Systems 5.4 计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录6.1 Documentation System andSpecifications6.1 文件系统和质量标准6.2 Equipment cleaning and Use Record 6.2 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 6.3 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (MasterProduction and Control Records)6.4 生产工艺规程(主生产和控制记录)6.5 Batch Production Records (BatchProduction and Control Records)6.5 批生产记录(批生产和控制记录)6.6 Laboratory Control Records 6.6 实验室控制记录6.7 Batch Production Record Review 6.7批生产记录审核7. MATERIALS MANAGEMENT 7. 物料管理7.1 General Controls 7.1 控制通则7.2 Receipt and Quarantine 7.2接收和待验7.3 Sampling and Testing of IncomingProduction Materials7.3 进厂物料的取样与测试7.4 Storage 7.4储存7.5 Re-evaluation 7.5复验8. PRODUCTION AND IN-PROCESSCONTROLS8. 生产和过程控制8.1 Production Operations 8.1 生产操作8.2 Time Limits 8.2 时限8.3 In-process Sampling and Controls 8.3 工序取样和控制8.4 Blending Batches of Intermediates orAPIs8.4 中间体或原料药的混批8.5 Contamination Control 8.5 污染控制9. PACKAGING AND IDENTIFICATIONLABELING OF APIs ANDINTERMEDIATES9. 原料药和中间体的包装和贴签9.1 General 9.1 总则9.2 Packaging Materials 9.2 包装材料9.3 Label Issuance and Control 9.3 标签发放与控制9.4 Packaging and Labeling Operations 9.4 包装和贴签操作10. STORAGE AND DISTRIBUTION 10.储存和分发10.1 Warehousing Procedures 10.1 入库程序10.2 Distribution Procedures 10.2 分发程序11. LABORATORY CONTROLS 11.实验室控制11.1 General Controls 11.1 控制通则11.2 Testing of Intermediates and APIs 11.2 中间体和原料药的测试11.3 Validation of Analytical Procedures 11.3 分析方法的验证11.4 Certificates of Analysis 11.4 分析报告单11.5 Stability Monitoring of APIs 11.5 原料药的稳定性监测11.6 Expiry and Retest Dating 11.6 有效期和复验期11.7 Reserve/Retention Samples 11.7 留样12. V ALIDATION 12.验证12.1 Validation Policy 12.1 验证方针12.2 Validation Documentation 12.2 验证文件12.3 Qualification 12.3 确认12.4 Approaches to Process Validation 12.4 工艺验证的方法12.5 Process Validation Program 12.5 工艺验证的程序12.6 Periodic Review of Validated Systems 12.6验证系统的定期审核12.7 Cleaning Validation 12.7 清洗验证12.8 Validation of Analytical Methods 12.8 分析方法的验证13. CHANGE CONTROL 13.变更的控制14. REJECTION AND RE-USE OFMATERIALS14.拒收和物料的再利用14.1 Rejection 14.1 拒收14.2 Reprocessing 14.2 返工14.3 Reworking 14.3 重新加工14.4 Recovery of Materials and Solvents 14.4 物料与溶剂的回收14.5 Returns 14.5 退货15. COMPLAINTS AND RECALLS 15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 17.1适用性17.2 Traceability of Distributed APIs andIntermediates17.2已分发的原料药和中间体的可追溯性17.3 Quality Management 17.3质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5 Stability 17.5稳定性17.6 Transfer of Information 17.6 信息的传达17.7 Handling of Complaints and Recalls 17.7 投诉和召回的处理17.8 Handling of Returns 17.8 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 18.1 总则18.2 Cell Bank Maintenance and RecordKeeping18.2细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 18.3细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 18.4收取、分离和精制18.5 Viral Removal/Inactivation steps 18.5 病毒的去除/灭活步骤19.APIs for Use in Clinical Trials 19.用于临床研究的原料药19.1 General 19.1 总则19.2 Quality 19.2 质量19.3 Equipment and Facilities 19.3 设备和设施19.4 Control of Raw Materials 19.4 原料的控制19.5 Production 19.5 生产19.6 Validation 19.6 验证19.7 Changes 19.7 变更19.8 Laboratory Controls 19.8 实验室控制19.9 Documentation 19.9 文件20. Glossary 20. 术语Q7a GMP Guidance for APIs Q7a原料药的GMP指南1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
生物药剂学第六章 药物排泄

一、生理因素
(二)胆汁流量: ➢ 高蛋白和高脂肪的食物能引起胆汁的大量分泌和排出,而
碳水化合物类食物的作用较小。 ➢ 进食之后,迷走神经兴奋,是胆汁大量流入十二指肠 ➢ 胆囊收缩素引起胆囊的强烈收缩和括约肌的扩展 ➢ 促进激素刺激肝细胞分泌胆汁。 ➢ 胆汁量增加时,随其进入肠道内的药物量均增加。
一、生理因素
因素:药物的脂溶性、pKa、蛋白结合率、唾液pH等 ➢ 以唾液代替血浆样品,进行药物动力学研究。 ➢ 主动转运:锂。
三、药物从肺部的排泄
➢ 共同特性:分子量较小,沸点较低 ➢ 影响因素:肺部的血流量、呼吸的频率、挥
发性药物的溶解性等。
四、药物从汗腺和毛发的排泄
➢ 汗腺排泄主要依赖于药物分子型的被动扩散 ➢ 毛发中只有微量的药物排泄:汞和砷
➢ 也可以采用离体法:离体肾灌流技术
第二节 药物的胆汁排泄
第二节 药物的胆汁排泄
➢ 胆汁排泄是肾之外排泄中最主要的途径 ➢ 维生素A、D、E、B12、性激素、甲状腺素及这些
物质的代谢产物胆汁中排泄非常显著。 ➢ 高胆汁清除的药物具有的特点:能主动分泌;药物
是极性物质;相对分子量超过300
一、药物胆汁排泄的过程与特征
该药肾清除率等于肾小球的滤过率,值为125mL/min。 ➢ 若肾清除率低于fuxGFR,则表示从肾小球滤过后有一定的
肾小管重吸收。 ➢ 若高于该值,则表示除肾小球滤过外,分泌>重吸收
五、研究药物肾排泄的方法
➢ 多采用在体外法或体内法,对象是人或动物,通常 是在给药后不同时间收集尿样,记录尿量,测定尿 量浓度,计算累计排泄量,直至排泄完成。
第六章 药物排泄
Excretion
学习目标
1、熟悉药物排泄的特点 2、掌握药物肾排泄的机制和影响肾排泄的主要因素 3、掌握药物胆汁排泄的过程和影响药物胆汁排泄的因素 4、熟悉肝肠循环概念及对药物作用的影响 5、了解药物的其他排泄途径
公司管理中的英文缩写

公司管理中的英文缩写收藏6•分类: 英语学习•发布时间: 2011/9/19 12:52:16•阅读(5084) | 评论(0)推荐1. MM --- Materials Management: 物料管理2. CMM --- Component Module Move: 零组件 (乡村包围城市); 系统组装 (火车头火车头工业驱动供应链); 整合供应链 (运筹物流, ERP, VMI, SFC … )3. ECMMS$ --- Engineering Component Module Move Service Dollars: 工程 (研究开发); 零组件 (乡村包围城市); 系统组装(火车头火车头工业驱动供应链); 整合供应链 (运筹物流, ERP, VMI, SFC … ) ; 服务, 代收钱4. Forecast --- 客户需求预测5. WO --- Work Order = PO --- Production Order: 生产工令6. MRP --- Material Requirement Planning: 物料需求计划7. VPO --- Vendor Purchase Order: 供货商采购订单8. MAWB --- Master Air Waybill: 空运主提单9. HAWB --- House Air Waybill: 小提单10. B / L --- Bill of Loading: 海运提单11. Consignee: 收货者12. ETD --- Estimated to Departure: 预计出发13. MIN / MAX --- Minimum and Maximum: 最小量与最大量14. VPO Burning: 向供货商采购货的平衡量15. VMI --- Vendor Management Inventory: 供货商免费存放, 在距离客户组装地2小时车程内, 3天到2周之库存16. VDPS --- Vendor Daily Planning Schedule: 供货商日生产排配17. ETA --- Estimated to Arrival: 预计到达时间18. Stock Level: 库存水准19. WO / PO Consumption --- Work Order / Production Order Consumption: 工令消耗20. BO Replenish --- Back Order Replenish: 订单欠交补货21. VMSA Burning --- Vendor Managed Stock Area Burning: 供货商管理库存平衡22. Pull Back: 由后往前拉23. Pipeline: 物流供应链中的库存24. ERP – SAP --- Enterprise Resource Planning / System Application Product in Process: 企业资源规划及生产应用管制操作系统25. SFC --- Shop Floor Control: 现场车间管制操作系统26. MOQ --- Minimum Order Quantity: 最小订购量27. MSQ --- Maximum Supply Quantity: 最大供应量28. Where Use Report: 零件共同使用报表28. Where Use Report: 零件共同使用报表29. EXW-EX Works: 工厂交货价30. RTV- Return to vendor: 退货31.RMA-Return Material Approval 退货验收32.ETA-Estimated Time of Arrival 预计到港时间33.ETD-Estimated Time of Departure 预计离港时间ERP专业词汇1 ABM Activity-based Management 基于作业活动管理2 AO Application Outsourcing 应用程序外包3 APICS American Production and Inventory Control Society,Inc 美国生产与库存管理协会4 APICS Applied Manufacturing Education Series 实用制造管理系列培训教材5 APO Advanced Planning and Optimization 先进计划及优化技术6 APS Advanced Planning and Scheduling 高级计划与排程技术7 ASP Application Service/Software Provider 应用服务/软件供应商8 ATO Assemble To Order 定货组装9 ATP Available To Promise 可供销售量(可签约量)10 B2B Business to Business 企业对企业(电子商务)11 B2C Business to Consumer 企业对消费者(电子商务)12 B2G Business to Government 企业对政府(电子商务)13 B2R Business to Retailer 企业对经销商(电子商务)14 BIS Business Intelligence System 商业智能系统15 BOM Bill Of Materials 物料清单16 BOR Bill Of Resource 资源清单17 BPR Business Process Reengineering 业务/企业流程重组18 BPM Business Process Management 业务/企业流程管理19 BPS Business Process Standard 业务/企业流程标准20 C/S Client/Server(C/S)\Browser/Server(B/S) 客户机/服务器\浏览器/服务器21 CAD Computer-Aided Design 计算机辅助设计22 CAID Computer-Aided Industrial Design 计算机辅助工艺设计23 CAM Computer-Aided Manufacturing 计算机辅助制造24 CAPP Computer-Aided Process Planning 计算机辅助工艺设计25 CASE Computer-Aided Software Engineering 计算机辅助软件工程26 CC Collaborative Commerce 协同商务27 CIMS Computer Integrated Manufacturing System 计算机集成制造系统28 CMM Capability Maturity Model 能力成熟度模型29 COMMS Customer Oriented Manufacturing Management System 面向客户制造管理系统30 CORBA Common Object Request Broker Architecture 通用对象请求代理结构31 CPC Collaborative Product Commerce 协同产品商务32 CPIM Certified Production and Inventory Management 生产与库存管理认证资格33 CPM Critical Path Method 关键线路法34 CRM Customer Relationship Management 客户关系管理35 CRP capacity requirements planning 能力需求计划36 CTI Computer Telephony Integration 电脑电话集成(呼叫中心)37 CTP Capable to Promise 可承诺的能力38 DCOM Distributed Component Object Model 分布式组件对象模型39 DCS Distributed Control System 分布式控制系统40 DMRP Distributed MRP 分布式MRP41 DRP Distribution Resource Planning 分销资源计划42 DSS Decision Support System 决策支持系统43 DTF Demand Time Fence 需求时界44 DTP Delivery to Promise 可承诺的交货时间45 EAI Enterprise Application Integration 企业应用集成46 EAM Enterprise Assets Management 企业资源管理47 ECM Enterprise Commerce Management 企业商务管理48 ECO Engineering Change Order 工程变更订单49 EDI Electronic Data Interchange 电子数据交换50 EDP Electronic Data Processing 电子数据处理51 EEA Extended Enterprise Applications 扩展企业应用系统52 EIP Enterprise Information Portal 企业信息门户53 EIS Executive Information System 高层领导信息系统54 EOI Economic Order Interval 经济定货周期55 EOQ Economic Order Quantity 经济订货批量(经济批量法)56 EPA Enterprise Proficiency Analysis 企业绩效分析57 ERP Enterprise Resource Planning 企业资源计划58 ERM Enterprise Resource Management 企业资源管理59 ETO Engineer To Order 专项设计,按订单设计60 FAS Final Assembly Schedule 最终装配计划61 FCS Finite Capacity Scheduling 有限能力计划62 FMS Flexible Manufacturing System 柔性制造系统63 FOQ Fixed Order Quantity 固定定货批量法64 GL General Ledger 总账65 GUI Graphical User Interface 图形用户界面66 HRM Human Resource Management 人力资源管理67 HRP Human Resource Planning 人力资源计划68 IE Industry Engineering/Internet Exploration 工业工程/浏览器69 ISO International Standard Organization 国际标准化组织70 ISP Internet Service Provider 互联网服务提供商71 ISPE International Society for Productivity Enhancement 国际生产力促进会72 IT/GT Information/Group Technology 信息/成组技术73 JIT Just In Time 准时制造/准时制生产74 KPA Key Process Areas 关键过程域75 KPI Key Performance Indicators 关键业绩指标76 LP Lean Production 精益生产77 MES Manufacturing Executive System 制造执行系统78 MIS Management Information System 管理信息系统79 MPS Master Production Schedule 主生产计划80 MRP Material Requirements Planning 物料需求计划81 MRPII Manufacturing Resource Planning 制造资源计划82 MTO Make To Order 定货(订货)生产83 MTS Make To Stock 现货(备货)生产84 OA Office Automation 办公自动化85 OEM Original Equipment Manufacturing 原始设备制造商86 OPT Optimized Production Technology 最优生产技术87 OPT Optimized Production Timetable 最优生产时刻表88 PADIS Production And Decision Information System 生产和决策管理信息系统89 PDM Product Data Management 产品数据管理90 PERT Program Evaluation Research Technology 计划评审技术91 PLM Production Lifecycle Management 产品生命周期管理92 PM Project Management 项目管理93 POQ Period Order Quantity 周期定量法94 PRM Partner Relationship Management 合作伙伴关系管理95 PTF Planned Time Fence 计划时界96 PTX Private Trade Exchange 自用交易网站97 RCCP Rough-Cut Capacity Planning 粗能力计划98 RDBM Relational Data Base Management 关系数据库管理99 RPM Rapid Prototype Manufacturing 快速原形制造100 RRP Resource Requirements Planning 资源需求计划101 SCM Supply Chain Management 供应链管理102 SCP Supply Chain Partnership 供应链合作伙伴关系103 SFA Sales Force Automation 销售自动化104 SMED Single-Minute Exchange Of Dies 快速换模法105 SOP Sales And Operation Planning 销售与运作规划106 SQL Structure Query Language 结构化查询语言107 TCO Total Cost Ownership 总体运营成本108 TEI Total Enterprise Integration 全面企业集成109 TOC Theory Of Constraints/Constraints managemant 约束理论/约束管理110 TPM Total Productive Maintenance 全员生产力维护111 TQC Total Quality Control 全面质量控制112 TQM Total Quality Management 全面质量管理113 WBS Work Breakdown System 工作分解系统114 XML eXtensible Markup Language 可扩展标记语言115 ABC Classification(Activity Based Classification) ABC分类法116 ABC costing 作业成本法117 ABC inventory control ABC 库存控制118 abnormal demand 反常需求119 acquisition cost ,ordering cost 定货费120 action message 行为/活动(措施)信息121 action report flag 活动报告标志122 activity cost pool 作业成本集123 activity-based costing(ABC) 作业基准成本法/业务成本法124 actual capacity 实际能力125 adjust on hand 调整现有库存量126 advanced manufacturing technology 先进制造技术127 advanced pricing 高级定价系统128 AM Agile Manufacturing 敏捷制造129 alternative routing 替代工序(工艺路线)130 Anticipated Delay Report 拖期预报131 anticipation inventory 预期储备132 apportionment code 分摊码133 assembly parts list 装配零件表134 automated storage/retrieval system 自动仓储/检索系统135 Automatic Rescheduling 计划自动重排136 available inventory 可达到库存137 available material 可用物料138 available stock 达到库存139 available work 可利用工时140 average inventory 平均库存141 back order 欠交(脱期)订单142 back scheduling 倒排(序)计划/倒序排产?143 base currency 本位币144 batch number 批号145 batch process 批流程146 batch production 批量生产147 benchmarking 标杆瞄准(管理)148 bill of labor 工时清单149 bill of lading 提货单150 branch warehouse 分库151 bucketless system 无时段系统152 business framework 业务框架153 business plan 经营规划154 capacity level 能力利用水平155 capacity load 能力负荷156 capacity management 能力管理157 carrying cost 保管费158 carrying cost rate 保管费率159 cellular manufacturing 单元式制造160 change route 修改工序161 change structure 修改产品结构162 check point 检查点163 closed loop MRP 闭环MRP164 Common Route Code(ID) 通用工序标识165 component-based development 组件(构件)开发技术166 concurrent engineering 并行(同步)工程167 conference room pilot 会议室模拟168 configuration code 配置代码169 continuous improvement 进取不懈170 continuous process 连续流程171 cost driver 作业成本发生因素172 cost driver rate 作业成本发生因素单位费用173 cost of stockout 短缺损失174 cost roll-up 成本滚动计算法175 crew size 班组规模176 critical part 急需零件177 critical ratio 紧迫系数178 critical work center 关键工作中心179 CLT Cumulative Lead Time 累计提前期180 current run hour 现有运转工时181 current run quantity 现有运转数量182 customer care 客户关怀183 customer deliver lead time 客户交货提前期184 customer loyalty 客户忠诚度185 customer order number 客户订单号186 customer satisfaction 客户满意度187 customer status 客户状况188 cycle counting 周期盘点189 DM Data Mining 数据挖掘190 Data Warehouse 数据仓库191 days offset 偏置天数192 dead load 空负荷193 demand cycle 需求周期194 demand forecasting 需求预测195 demand management 需求管理196 Deming circle 戴明环197 demonstrated capacity 实际能力198 discrete manufacturing 离散型生产199 dispatch to 调度200 DRP Distribution Requirements Planning 分销需求计划201 drop shipment 直运202 dunning letter 催款信203 ECO workbench ECO工作台204 employee enrolled 在册员工205 employee tax id 员工税号206 end item 最终产品207 engineering change mode flag 工程变更方式标志208 engineering change notice 工程变更通知209 equipment distribution 设备分配210 equipment management 设备管理211 exception control 例外控制212 excess material analysis 呆滞物料分析213 expedite code 急送代码214 external integration 外部集成215 fabrication order 加工订单216 factory order 工厂订单217 fast path method 快速路径法218 fill backorder 补足欠交219 final assembly lead time 总装提前期220 final goods 成品221 finite forward scheduling 有限顺排计划222 finite loading 有限排负荷223 firm planned order 确认的计划订单224 firm planned time fence 确认计划需求时界225 FPR Fixed Period Requirements 定期用量法226 fixed quantity 固定数量法227 fixed time 固定时间法228 floor stock 作业现场库存229 flow shop 流水车间230 focus forecasting 调焦预测231 forward scheduling 顺排计划232 freeze code 冻结码233 freeze space 冷冻区234 frozen order 冻结订单235 gross requirements 毛需求236 hedge inventory 囤积库存237 in process inventory 在制品库存238 in stock 在库239 incrementing 增值240 indirect cost 间接成本241 indirect labor 间接人工242 infinite loading 无限排负荷243 input/output control 投入/产出控制244 inspection ID 检验标识245 integrity 完整性246 inter companies 公司内部间247 interplant demands 厂际需求量248 inventory carry rate 库存周转率249 inventory cycle time 库存周期250 inventory issue 库存发放251 inventory location type 仓库库位类型252 inventory scrap 库存报废量253 inventory transfers 库存转移254 inventory turns/turnover 库存(资金)周转次数255 invoice address 发票地址256 invoice amount gross 发票金额257 invoice schedule 发票清单258 issue cycle 发放周期259 issue order 发送订单260 issue parts 发放零件261 issue policy 发放策略262 item availability 项目可供量263 item description 项目说明264 item number 项目编号265 item record 项目记录266 item remark 项目备注267 item status 项目状态268 job shop 加工车间269 job step 作业步骤270 kit item 配套件项目271 labor hour 人工工时272 late days 延迟天数273 lead time 提前期274 lead time level 提前期水平275 lead time offset days 提前期偏置(补偿)天数276 least slack per operation 最小单个工序平均时差277 line item 单项产品278 live pilot 应用模拟279 load leveling 负荷量280 load report 负荷报告281 location code 仓位代码282 location remarks 仓位备注283 location status 仓位状况284 lot for lot 按需定货(因需定量法/缺补法)285 lot ID 批量标识286 lot number 批量编号287 lot number traceability 批号跟踪288 lot size 批量289 lot size inventory 批量库存290 lot sizing 批量规划291 low level code 低层(位)码292 machine capacity 机器能力293 machine hours 机时294 machine loading 机器加载295 maintenance ,repair,and operating supplies 维护修理操作物料296 make or buy decision 外购或自制决策297 management by exception 例外管理法298 manufacturing cycle time 制造周期时间299 manufacturing lead time 制造提前期300 manufacturing standards 制造标准301 master scheduler 主生产计划员302 material 物料303 material available 物料可用量304 material cost 物料成本305 material issues and receipts 物料发放和接收306 material management 物料管理307 material manager 物料经理308 material master,item master 物料主文件309 material review board 物料核定机构310 measure of velocity 生产速率水平311 memory-based processing speed 基于存储的处理速度312 minimum balance 最小库存余量313 Modern Materials Handling 现代物料搬运314 month to date 月累计315 move time , transit time 传递时间316 MSP book flag MPS登录标志317 multi-currency 多币制318 multi-facility 多场所319 multi-level 多级320 multi-plant management 多工厂管理321 multiple location 多重仓位322 net change 净改变法323 net change MRP 净改变式MRP324 net requirements 净需求325 new location 新仓位326 new parent 新组件327 new warehouse 新仓库328 next code 后续编码329 next number 后续编号330 No action report 不活动报告331 non-nettable 不可动用量332 on demand 急需的333 on-hand balance 现有库存量334 on hold 挂起335 on time 准时336 open amount 未清金额337 open order 未结订单/开放订单338 order activity rules 订单活动规则339 order address 订单地址340 order entry 订单输入341 order point 定货点342 order point system 定货点法343 order policy 定货策略344 order promising 定货承诺345 order remarks 定货备注346 ordered by 定货者347 overflow location 超量库位348 overhead apportionment/allocation 间接费分配349 overhead rate,burden factor,absorption rate 间接费率350 owner's equity 所有者权益351 parent item 母件352 part bills 零件清单353 part lot 零件批次354 part number 零件编号355 people involvement 全员参治356 performance measurement 业绩评价357 physical inventory 实际库存358 picking 领料/提货359 planned capacity 计划能力360 planned order 计划订单361 planned order receipts 计划产出量362 planned order releases 计划投入量363 planning horizon 计划期/计划展望期364 point of use 使用点365 Policy and procedure 工作准则与工作规程366 price adjustments 价格调整367 price invoice 发票价格368 price level 物价水平369 price purchase order 采购订单价格370 priority planning 优先计划371 processing manufacturing 流程制造372 product control 产品控制373 product family 产品系列374 product mix 产品搭配组合375 production activity control 生产作业控制376 production cycle 生产周期377 production line 产品线378 production rate 产品率379 production tree 产品结构树380 PAB Projected Available Balance 预计可用库存(量) 381 purchase order tracking 采购订单跟踪382 quantity allocation 已分配量383 quantity at location 仓位数量384 quantity backorder 欠交数量385 quantity completion 完成数量386 quantity demand 需求量387 quantity gross 毛需求量388 quantity in 进货数量389 quantity on hand 现有数量390 quantity scrapped 废品数量391 quantity shipped 发货数量392 queue time 排队时间393 rated capacity 额定能力394 receipt document 收款单据395 reference number 参考号396 regenerated MRP 重生成式MRP397 released order 下达订单398 reorder point 再订购点399 repetitive manufacturing 重复式生产(制造)400 replacement parts 替换零件401 required capacity 需求能力402 requisition orders 请购单403 rescheduling assumption 重排假设404 resupply order 补库单405 rework bill 返工单406 roll up 上滚407 rough cut resource planning 粗资源计划408 rounding amount 舍入金额409 run time 加工(运行)时间410 safety lead time 安全提前期411 safety stock 安全库存412 safety time 保险期413 sales order 销售订单414 scheduled receipts 计划接收量(预计入库量/预期到货量) 415 seasonal stock 季节储备416 send part 发送零件417 service and support 服务和支持418 service parts 维修件419 set up time 准备时间420 ship address 发运地址421 ship contact 发运单联系人422 ship order 发货单423 shop calendar 工厂日历(车间日历)424 shop floor control 车间作业管理(控制)425 shop order , work order 车间订单426 shrink factor 损耗因子(系数)427 single level where used 单层物料反查表428 standard cost system 标准成本体系429 standard hours 标准工时430 standard product cost 标准产品成本431 standard set up hour 标准机器设置工时432 standard unit run hour 标准单位运转工时433 standard wage rate 标准工资率434 status code 状态代码435 stores control 库存控制436 suggested work order 建议工作单437 supply chain 供应链438 synchronous manufacturing 同步制造/同期生产439 time bucket 时段(时间段)440 time fence 时界441 time zone 时区442 top management commitment 领导承诺443 total lead time 总提前期444 transportation inventory 在途库存445 unfavorable variance, adverse 不利差异446 unit cost 单位成本447 unit of measure 计量单位448 value chain 价值链449 value-added chain 增值链450 variance in quantity 量差451 vendor scheduler,supplier scheduler 采购计划员/供方计划员452 vendor scheduling 采购计划法453 Virtual Enterprise(VE)/ Organization 虚拟企业/公司454 volume variance 产量差异455 wait time 等待时间456 where-used list 反查用物料单457 work center capacity 工作中心能力458 workflow 工作流459 work order 工作令460 work order tracking 工作令跟踪461 work scheduling 工作进度安排462 world class manufacturing excellence 国际优秀制造业463 zero inventories 零库存464465 Call/Contact/Work/Cost center 呼叫/联络/工作/成本中心466 Co/By-product 联/副产品467 E-Commerce/E-Business/E-Marketing 电子商务/电子商务/电子集市468 E-sales/E-procuement/E-partner 电子销售/电子采购/电子伙伴469 independent/dependent demand 独立需求/相关需求件470 informal/formal system 非/规范化管理系统471 Internet/Intranet/Extranet 互联网/企业内部网/企业外联网472 middle/hard/soft/share/firm/group ware 中间/硬/软/共享/固/群件473 pegging/kitting/netting/nettable 追溯(反查)/配套出售件/净需求计算474 picking/dispatch/disbursement list 领料单(或提货单)/派工单/发料单475 preflush/backflush/super backflush 预冲/倒冲法/完全反冲476 yield/scrap/shrinkage (rate) 成品率/废品率/缩减率477 scrap/shrinkage factor 残料率(废品系数)/损耗系数478479 costed BOM 成本物料清单480 engineering BOM 设计物料清单481 indented BOM 缩排式物料清单482 manufacturing BOM 制造物料清单483 modular BOM 模块化物料清单484 planning BOM 计划物料清单485 single level BOM 单层物料清单486 summarized BOM 汇总物料清单487488 account balance 账户余额489 account code 账户代码490 account ledger 分类账491 account period 会计期间492 accounts payable 应付账款493 accounts receivable 应收账款494 actual cost 实际成本495 aging 账龄496 balance due 到期余额497 balance in hand 现有余额498 balance sheet 资产负债表499 beginning balance 期初余额500 cash basis 现金收付制501 cash on bank 银行存款502 cash on hand 现金503 cash out to 支付给504 catalog 目录505 category code 分类码506 check out 结帐507 collection 催款508 cost simulation 成本模拟509 costing 成本核算510 current assets 流动资产511 current liabilities 流动负债512 current standard cost 现行标准成本513 detail 明细514 draft remittance 汇票汇出515 end of year 年末516 ending availables 期末可供量517 ending balance 期末余额518 exchange rate 汇率519 expense 费用520 financial accounting 财务会计521 financial entity 财务实体522 financial reports 财务报告523 financial statements 财务报表524 fiscal period 财务期间525 fiscal year 财政年度526 fixed assets 固定资产527 foreign amount 外币金额528 gains and loss 损益529 in balance 平衡530 income statement 损益表531 intangible assets 无形资产532 journal entry 分录533 management accounting 管理会计534 manual reconciliation 手工调账535 notes payable 应付票据536 notes receivable 应收票据537 other receivables 其他应收款538 pay aging 付款账龄539 pay check 工资支票540 pay in 缴款541 pay item 付款项目542 pay point 支付点543 pay status 支付状态544 payment instrument 付款方式545 payment reminder 催款单546 payment status 付款状态547 payment terms 付款期限548 period 期间549 post 过账550 proposed cost 建议成本551 simulated cost 模拟成本552 spending variance,expenditure variance 开支差异553 subsidiary 明细账554 summary 汇总555 tax code 税码556 tax rate 税率557 value added tax 增值税558559 as of date , stop date 截止日期560 change lot date 修改批量日期561 clear date 结清日期562 date adjust 调整日期563 date available 有效日期564 date changed 修改日期565 date closed 结束日期566 date due 截止日期567 date in produced 生产日期568 date inventory adjust 库存调整日期569 date obsolete 作废日期570 date received 收到日期571 date released 交付日期572 date required 需求日期573 date to pull 发货日期574 earliest due date 最早订单完成日期575 effective date 生效日期576 engineering change effect date 工程变更生效日期577 engineering stop date 工程停止日期578 expired date 失效日期,报废日期579 from date 起始日期580 last shipment date 最后运输日期581 need date 需求日期582 new date 新日期583 pay through date 付款截止日期584 receipt date 收到日期585 ship date 发运日期586587 allocation 已分配量588 alphanumeric 字母数字589 approver 批准者590 assembly 装配(件)591 backlog 未结订单/拖欠订单592 billing 开单593 bill-to 发票寄往地594 bottleneck 瓶颈资源595 bulk 散装596 buyer 采购员597 component 子件/组件598 customer 客户599 delivery 交货600 demand 需求601 description 说明602 discrete 离散603 ergonomics 工效学(人类工程学) 604 facility 设备、功能605 feature 基本组件/特征件606 forecast 预测607 freight 运费608 holidays 例假日609 implement 实施610 ingredient 配料、成分611 inquire 查询612 inventory 库存613 item 物料项目614 job 作业615 Kanban 看板616 level 层次(级)617 load 负荷618 locate 定位619 logistics 后勤保障体系;物流管理620 lot 批次621 option 可选件622 outstanding 逾期未付623 overhead 制造费用624 override 覆盖625 overtime 加班626 parent 双亲(文件)627 part 零件628 phantom 虚拟件629 plant 工厂,场所630 preference 优先权631 priority 优先权(级)632 procurement 采购633 prototyping 原形测试634 queue 队列635 quota 任务额,报价636 receipt 收款、收据637 regeneration 全重排法638 remittance 汇款639 requisition 请购单640 returned 退货641 roll 滚动642 routing 工艺线路643 schedule 计划表644 shipment 发运量645 ship-to 交货地646 shortage 短缺647 shrink 损耗648 spread 分摊649 statement 报表650 subassembly 子装配件651 supplier 供应商652 transaction 事务处理653 what-if 如果怎样-将会怎样654655 post-deduct inventory transaction processing 后减库存处理法656 pre-deduct inventory transaction processing 前减库存处理法657 generally accepted manufacturing practices 通用生产管理原则658 direct-deduct inventory transaction processing 直接增减库存处理法659 Pareto Principle 帕拉图原理660 Drum-buffer-rope 鼓点-缓冲-绳子661663 Open Database Connectivity 开放数据库互连664 Production Planning 生产规划编制665 Work in Process 在制品666 accelerated cost recovery system 快速成本回收制度667 accounting information system 会计信息系统668 acceptable quality kevel 可接受质量水平669 constant purchasing power accounting 不买够买力会计670 break-even analysis 保本分析671 book value 帐面价值672 cost-benefit analysis 成本效益分析673 chief financial office 财务总监674 degree of financial leverage 财务杠杆系数675 degree of operating leverage 经济杠杆系数676 first-in , first-out 先进先出法677 economic lot size 经济批量678 first-in ,still-here 后进先出法679 full pegging 完全跟踪680 linear programming 线性规划681 management by objective 目标管理682 value engineering 价值工程683 zero based budgeting 零基预算684 CAQ computer aided quality assurance 计算机辅助质量保证685 DBMS database management system 数据库管理系统686 IP Internet Protocol 网际协议687 TCP Transmission Control Protocol 传输控制协议689690 API Advanced Process Industry 高级流程工业691 A2A Application to Application 应用到应用(集成)692 article 物品693 article reserves 物品存储694 assembly order 装配订单695 balance-on-hand-inventory 现有库存余额696 bar code 条形码697 boned warehouse 保税仓库698 CPA Capacity Requirements Planning 能力需求计划699 change management 变革管理700 chill space 冷藏区701 combined transport 联合运输702 commodity inspection 进出口商品检验703 competitive edge 竞争优势704 container 集装箱705 container transport 集装箱运输706 CRP Continuous Replenishment Program 连续补充系数707 core competence 核心才能708 cross docking 直接换装709 CLV Customer Lifetime Value 客户生命周期价值710 CReM Customer Relationship Marketing 客户关系营销711 CSS Customer Service and Support 客户服务和支持712 Customer Service Representative 客户服务代表713 customized logistics 定制物流714 customs declaration 报关715 cycle stock 经常库存716 data cleansing 数据整理717 Data Knowledge and Decision Support 数据知识和决策支持718 data level integration 数据层集成719 data transformation 数据转换720 desktop conferencing 桌面会议721 distribution 配送722 distribution and logistics 分销和后勤723 distribution center 配送中心724 distribution logistics 销售物流725 distribution processing 流通加工726 distribution requirements 分销量727 DRP distribution resource planning 配送/分销资源计划728 door-to-door 门到门729 drop and pull transport 甩挂运输730 DEM Dynamic Enterprise Module 动态企业建模技术731 ECR Efficient Consumer Response 有效顾客反应732 e-Government Affairs 电子政务733 EC Electronic Commerce 电子商务734 Electronic Display Boards 电子公告板735 EOS Electronic order system 电子订货系统736 ESD Electronic Software Distribution 电子软件分发737 embedding 插入738 employee category 员工分类739 empowerment 授权740 engineering change effect work order 工程变更生效单741 environmental logistics 绿色物流742 experiential marketing 直效行销(又称体验行销)743 export supervised warehouse 出口监管仓库744 ERP Extended Resource Planning 扩展资源计划745 field sales/cross sale/cross sell 现场销售/交叉销售/连带销售746 franchising 加盟连销权747 FCL Full Container Load 整箱货748 Global Logistics Management 全球运筹管理749 goods collection 集货750 goods shed 料棚751 goods shelf 货架752 goods stack 货垛753 goods yard 货场754 handing/carrying 搬运755 high performance organization 高绩效组织756 inland container depot 公路集装箱中转站757 inside sales 内部销售758 inspection 检验759 intangible loss 无形消耗760 internal logistics 企业物流761 international freight forwarding agent 国际货运代理762 international logistics 国际物流763 invasive integration 侵入性集成764 joint distribution 共同配送765 just-in-time logistics 准时制物流766 KM Knowledge Management 知识管理767 lead (customer) management 潜在客户管理768 learning organization 学习型组织769 LCL less than container load 拼装货770 load balancing 负载平衡771 loading and unloading 装载772 logistics activity 物流活动773 logistics alliance 物流联盟774 logistics center 物流中心775 logistics cost 物流成本776 logistics cost control 物流成本管理777 logistics documents 物流单证778 logistics enterprise 物流企业779 logistics information 物流信息780 logistics management 物流管理781 logistics modulus 物流模数782 logistics network 物流网络783 logistics operation 物流作业784 LRP Logistics Resource Planning 物流资源计划785 logistics strategy 物流战略786 logistics strategy management 物流战略管理787 logistics technology 物流技术788 MES Manufacture Execute System 制造执行系统789 mass customization 大规模定制790 NPV Net Present Value 净现值791 neutral packing 中性包装792 OLAP On-line Analysis Processing 联机/在线分析系统793 OAG Open Application Group 开放应用集成794 order picking 拣选795 outsourcing 外包796 package/packaging 包装797 packing of nominated brand 定牌包装798 palletizing 托盘包装799 PDA Personal Digital Assistant 个人数据助理800 personalization 个性化801 PTF Planning time fence 计划时界802 POS Point Of Sells 电子收款机803 priority queuing 优先排队804 PBX Private Branch Exchange 专用分组交换机805 production logistics 生产物流806 publish/subscribe 发布/订阅807 quality of working life 工作生活品质808 Quick Response 快速反映809 receiving space 收货区810 REPs Representatives 代表或业务员811 return logistics 回收物流812 ROI Return On Investment 投资回报率813 RM Risk Management 风险管理814 sales package 销售包装815 scalability 可扩充性816 shipping space 发货区817 situational leadership 情境领导818 six sigma 六个标准差819 sorting/stacking 分拣/堆拣820 stereoscopic warehouse 立体仓库821 storage 保管822 stored procedure 存储过程823 storehouse 库房824 storing 储存825 SRM Supplier Relationship Management 供应商关系管理826 tangible loss 有形消耗827 team building 团队建立。
开启片剂完整性的窗户(中英文对照)
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
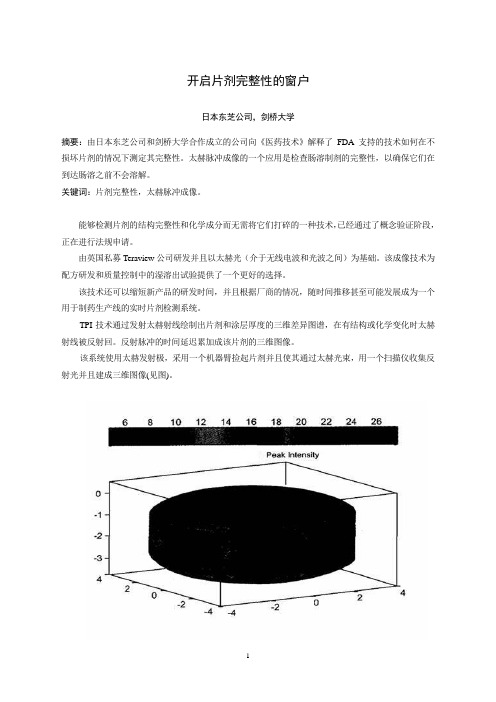
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
欧洲药品GMP检查指南及附件(中英文)

GUIDE TO GOOD MANUFACTURINGPRACTICE FOR MEDICINAL PRODUCTS药品GMP检查指南.PIC/S July 2004Reproduction prohibited for commercial purposes.Reproduction for internal use is authorised,provided that the source is acknowledged.Editor: PIC/S SecretariatP.O. Box 5695CH-1211 Geneva 11e-mail: daniel.brunner@web site: :// 1 July 2004 PE 009-2TABLE OF CONTENT目录INTRODUCTION介绍 (1)CHAPTER 1 QUALITY MANAGEMENT 质量管理 (4)PRINCIPLE 原则 (4)QUALITY ASSURANCE 质量保证 (4)GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS (GMP) 药品GMP (6)QUALITY CONTROL 质量控制 (7)CHAPTER 2 PERSONNEL 人员 (10)PRINCIPLE 原则 (10)GENERAL 通则 (10)KEY PERSONNEL 关键人员 (10)TRAINING 培训 (13)PERSONAL HYGIENE 个人卫生 (14)CHAPTER 3 PREMISES AND EQUIPMENT 厂房和设备 (16)PRINCIPLE 原则 (16)PREMISES General总则 (16)Production Area 生产区域 (17)Storage Areas 储存区域 (19)Quality Control Areas 质量控制区域 (20)Ancillary Areas 辅助区域 (20)EQUIPMENT 设备 (21)CHAPTER 4 DOCUMENTATION 文件 (23)PRINCIPLE 原则 (23)GENERAL 总则 (23)DOCUMENTS REQUIRED 必需的文件 (25)MANUFACTURING FORMULA AND PROCESSING INSTRUCTIONS 生产方法和加工指示 (27)PACKAGING INSTRUCTIONS 包装指示 (28)BA TCH PROCESSING RECORDS 批加工记录 (29)BA TCH PACKAGING RECORDS 批包装记录 (30)PROCEDURES AND RECORDS 程序和记录 (32)CHAPTER 5 PRODUCTION 生产 (36)PRINCIPLE 原则 (36)GENERAL 通则 (36)PREVENTION OF CROSS-CONTAMINATION IN PRODUCTION 生产过程中防止交叉污染 (38)V ALIDATION 验证 (39)STARTING MA TERIALS 起始物料 (40)PROCESSING OPERA TIONS - INTERMEDIATE AND BULK PRODUCTS 加工操作:中间体和散装产品 (42)PACKAGING MATERIALS 包装材料 (42)PACKAGING OPERATIONS 包装操作 (43)FINISHED PRODUCTS 最终成品 (45)REJECTED, RECOVERED AND RETURNED MATERIALS 拒绝的,回收的和退回的物料46CHAPTER 6 QUALITY CONTROL 质量控制 (48)PRINCIPLE 原则 (48)GENERAL 通则 (48)GOOD QUALITY CONTROL LABORATORY PRACTICE 优良质量控制实验室实践 (49)DOCUMENTATION 文件 (49)SAMPLING 取样 (50)TESTING 检测 (52)CHAPTER 7 CONTRACT MANUFACTURE AND ANAL YSIS 合同加工和分析 (55)PRINCIPLE 原则 (55)GENERAL 通则 (55)THE CONTRACT GIVER 合同提供人 (55)THE CONTRACT ACCEPTOR 合同接受人 (56)THE CONTRACT 合同 (57)CHAPTER 8 COMPLAINTS AND PRODUCT RECALL 抱怨和产品召回 (59)PRINCIPLE 原则 (59)COMPLAINTS 抱怨 (59)RECALLS 召回 (60)CHAPTER 9 SELF INSPECTION 自检 (61)PRINCIPLE 原则 (61)ANNEX 1 MANUFACTURE OF STERILE MEDICINAL PRODUCTS无菌药品的生产 (63)PRINCIPLE (63)GENERAL (63)BLOW/FILL/SEAL TECHNOLOGY (67)TERMINALL Y STERILISED PRODUCTS (67)ASEPTIC PREPARA TION (68)PERSONNEL (68)PREMISES (70)EQUIPMENT (71)SANITATION (71)PROCESSING (71)STERILISATION (73)STERILISATION BY HEA T (74)MOIST HEAT (75)DRY HEAT (75)STERILISATION BY RADIATION (75)STERILISATION WITH ETHYLENE OXIDE (76)FILTRATION OF MEDICINAL PRODUCTS WHICH CANNOT BE STERILISED IN THEIR FINAL CONTAINER (77)FINISHING OF STERILE PRODUCTS (77)QUALITY CONTROL (78)ANNEX 2 MANUFACTURE OF BIOLOGICAL MEDICINAL PRODUCTS FOR HUMAN USE人用生物药品的生产 (79)SCOPE (79)PRINCIPLE (79)PERSONNEL (80)PREMISES AND EQUIPMENT (81)ANIMAL QUARTERS AND CARE (82)DOCUMENTATION (82)PRODUCTION (83)QUALITY CONTROL (84)ANNEX 3 MANUFACTURE OF RADIOPHARMACEUTICALS 放射性药品的生产 (85)PRINCIPLE (85)PERSONNEL (85)PREMISES AND EQUIPMENT (85)PRODUCTION (86)QUALITY CONTROL (86)DISTRIBUTION AND RECALLS (86)ANNEX 4 MANUFACTURE OF VETERINARY MEDICINAL PRODUCTS OTHER THAN IMMUNOLOGICALS MANUFACTURE OF PREMIXES FOR MEDICATED FEEDING STUFFS 除为预混合加药饲料原料生产的免疫产品以外的,兽药产品的生产 (87)THE MANUFACTURE OF ECTOPARASITICIDES (88)THE MANUFACTURE OF VETERINARY MEDICINAL PRODUCTS CONTAINING PENICILLINS (88)RETENTION OF SAMPLES (point 1.4. viii and point 6.14.) (88)STERILE VETERINARY MEDICINAL PRODUCTS (88)ANNEX 5 MANUFACTURE OF IMMUNOLOGICAL VETERINARY MEDICAL PRODUCTS免疫兽药产品的生产 (89)PRINCIPLE (89)PERSONNEL (89)PREMISES (90)EQUIPMENT (93)ANIMALS AND ANIMAL HOUSES (94)DISINFECTION - WASTE DISPOSAL (94)PRODUCTION (95)STARTING MA TERIALS (95)QUALITY CONTROL (98)ANNEX 6 MANUFACTURE OF MEDICINAL GASES药用气体的生产 (99)1. PRINCIPLE (99)2. PERSONNEL (99)3. PREMISES AND EQUIPMENT (99)4. DOCUMENTA TION (100)5. PRODUCTION (101)6. QUALITY CONTROL (104)7. STORAGE AND RELEASE (105)ANNEX 7 MANUFACTURE OF HERBAL MEDICINAL PRODUCTS草药产品的生产 (108)PRINCIPLE (108)PREMISES (108)DOCUMENTATION (108)SAMPLING (109)QUALITY CONTROL (110)ANNEX 8 SAMPLING OF STARTING AND PACKAGING MA TERIALS起始物料和包装材料的取样 (111)PRINCIPLE (111)PERSONNEL (111)STARTING MA TERIALS (111)PACKAGING MATERIAL (112)ANNEX 9 MANUFACTURE OF LIQUIDS, CREAMS AND OINTMENTS流体,霜体和膏体药品的生产 (113)PRINCIPLE (113)PRODUCTION (113)ANNEX 10 MANUFACTURE OF PRESSURISED METERED DOSE AEROSOL PREPARATIONS FOR INHALATION吸入式剂量仪的气雾剂的生产 (115)PRINCIPLE (115)GENERAL (115)PREMISES AND EQUIPMENT (115)PRODUCTION AND QUALITY CONTROL (116)ANNEX 11 COMPUTERISED SYSTEMS 计算机化系统 (117)PRINCIPLE (117)PERSONNEL (117)V ALIDATION (117)ANNEX 12 USE OF IONISING RADIATION IN THE MANUFACTURE OF MEDICINAL PRODUCTS使用离子放射生产药品 (120)INTRODUCTION (120)RESPONSIBILITIES (120)DOSIMETRY (121)V ALIDATION OF THE PROCESS (121)COMMISSIONING OF THE PLANT (122)PREMISES (124)PROCESSING (124)DOCUMENTATION (126)MICROBIOLOGICAL MONITORING (126)ANNEX 13 MANUFACTURE OF INVESTIGA TIONAL MEDICINAL PRODUCTS观察期药品的生产 (127)PRINCIPLE (127)GLOSSARY (128)QUALITY MANAGEMENT (130)PERSONNEL (130)PREMISES AND EQUIPMENT (130)DOCUMENT A TION (131)PRODUCTION (132)QUALITY CONTROL (136)RELEASE OF BATCHES (137)SHIPPING (139)COMPLAINTS (139)RECALLS AND RETURNS (139)DESTRUCTION (140)ANNEX 14 MANUFACTURE OF PRODUCTS DERIVED FROM HUMAN BLOOD OR HUMAN PLASMA生产自人类血液或人体组织分离的产品 (143)PRINCIPLE (143)GLOSSARY (144)QUALITY MANAGEMENT (144)PREMISES AND EQUIPMENT (145)BLOOD AND PLASMA COLLECTION (145)TRACEABILITY AND POST COLLECTION MEASURES (146)PRODUCTION AND QUALITY CONTROL (147)RETENTION OF SAMPLES (148)DISPOSAL OF REJECTED BLOOD, PLASMA OR INTERMEDIATES (148)ANNEX 15 QUALIFICATION AND V ALIDATION 确认和验证 (149)PRINCIPLE (149)PLANNING FOR V ALIDATION (149)DOCUMENTATION (150)QUALIFICATION (150)PROCESS V ALIDATION (151)CLEANING VALIDATION (153)CHANGE CONTROL (154)REV ALIDATION (154)GLOSSARY (154)[ANNEX 16] [QUALIFIED PERSON AND BA TCH RELEASE]*经授权的人员和批放行 (157)ANNEX 17 PARAMETRIC RELEASE参数放行 (158)1. PRINCIPLE (158)2. PARAMETRIC RELEASE (158)3. PARAMETRIC RELEASE FOR STERILE PRODUCTS (158)4. GLOSSARY (160)[ANNEX 18] [GMP GUIDE FOR ACTIVE PHARMACEUTICAL INGREDIENTS] 17原料药GMP 指南 (161)GLOSSARY术语表 (162)GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS药品GMP指南INTRODUCTION介绍为进一步消除药品贸易壁垒,促进许可证的一致性,以及确保整个欧洲在研发,生产和控制药品中保持高标准的质量保证,根据药品检查协会(PIC)同意,药品检查使用一致的GMP原则,和药品检查合作计划表中的欧洲药品GMP及其附录。
GMP文件常见缩写

GMP英语1.ABBREVIATED(NEW)DRUG:简化申请的新药2.AirLock 气闸3.ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请4.API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分5.Authorized Person 授权人6.Batch Number/Lot-Number 批号7.Batch Numbering System 批次编码系统8.BATCH PRODUCTION RECORDS:生产批号记录9.BATCH PRODUCTION:批量生产;分批生产10.Batch Records 批记录11.Batch/Lot 批次12.Bulk Product 待包装品13.Calibration 校正14.CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规15.Clean area 净区16.Consignmecnt(Delivery)托销药品17.DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)18.FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局19.HOLDER:DMF持有者20.IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)RMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)22.NDA(NEW DRUG APPLICATION):新药申请23.OTC DRUG(OVER—THE—COUNTER DRUG):非处方药24.PANEL:专家小组25.PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical InspectionCooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S 为医药审查会议/合作计划(PIC/S)26.PIC的权威翻译:药品生产检查相互承认公约27.POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督28.PRESCRIPTION DRUG:处方药29.TREATMENT IND:研究中的新药用于治疗GMP文件常见缩写ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member State 每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPA EDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products)欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory Affairs European Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)GCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority (HSA)HSA’s Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for Harmonisation IDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary Name International Conference on Harmonisation (ICH) IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相認證同意MRFG Mutual Recognition Facilitation Group MRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE)标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典。
药物转运及转运体

ABC转运体 MRD1/P-gp BSEP MDR3 ABCA1 MRP1 MRP2 MRP3 MRP4 MRP5 MRP6
多药耐药基因/P-糖蛋白 胆盐分泌蛋白 多药耐药基因/P-糖蛋白
多药耐药相关蛋白1 多药耐药相关蛋白2 多药耐药相关蛋白3 多药耐药相关蛋白4 多药耐药相关蛋白5 多药耐药相关蛋白6
简单扩散的规律:
1.弱酸药在酸性体液中,或弱碱药在碱性体液中的解离度小,药物 易通过生物膜扩散转运; 2.当生物膜两侧pH值不等时,弱酸药易由较酸侧进入较碱侧,弱碱 性药则易由较碱侧进入较酸侧。
弱酸药(巴比妥类、阿司匹林)可由胃中转运到较碱的血浆中去 ,而弱碱药(吗啡、利血平)则很少自胃中吸收。
简单扩散的规律:
简单扩散的条件:脂溶性、解离度、浓度差。 绝大多数药物为弱酸性或弱碱性,均有解离型与非解离型,
后者脂溶性高。
弱酸或弱碱药物的解离
Handerson-Hasselbalch公式
以弱酸药物为例
HA
Ka
H+ + A-
pKa = pH
log
[H+] [A-]
Ka =
[HA]
[A-] [HA]
10pH-pKa
药物
组织分布
细胞内浓度
MRP3
OCT2 OAT1 OAT2 OAT3
O-2ABT1P
OATP -1A2
OCT4
MDR1 MRP2 MRP4
肾小管细胞
肝脏摄入
小肠吸收
胆汁分泌
药物转运体在体内的分布
肾脏排泌
(一)转运体分类
根据对底物的转运方向,主要可以将转运体分为摄入转运体和外 排转运体: l 摄入转运体负责将外源性物质摄入细胞内,包括有机阴离子转 运多肽家族(OATP)、有机阴离子转运体家族(OAT)、有机 阳离子转运体家族(OCT)。 l 外排型转运体主要是P-gp蛋白,多药耐药蛋白(MDR)、多药 耐药相关蛋白(MRP)、乳腺癌耐药相关蛋白(BCRP)以及胆 盐分泌蛋白(BSEP)等。
安立生坦片

药物过量
目前没有关于凡瑞克超量给药的经验。健康志愿者中应用的凡瑞克最高单剂量为100mg,而肺动脉高压患者 中为10mg每日一次。在健康志愿者中,50mg和100mg单剂量(最大推荐剂量的5至10倍)会伴随出现头痛、面部发 红、眩晕、恶心、和鼻充血。严重超剂量可能会导致需要治疗干预的低血压。
临床试验
药物相互作用
体外研究 用人类肝脏组织进行的研究表明,安立生坦由CYP 3A、CYP 2C19、5 -二磷酸葡萄糖基转移酶(UGTs)、 1A9S、2B7S以及1A3S进行代谢。体外实验提示,安立生坦是器官阴离子转运蛋白(OATP)的底物,同时也是Pgp的底物(而非抑制剂)。 体内研究 安立生坦与下述药物联合应用不会导致有临床意义的安立生坦暴露量改变 : ·酮康唑 ·奥美拉唑 ·昔多芬 ·他达拉非 联合应用安立生坦不会导致下述药物暴露量的改变: ·华法令
贮藏
遮光,密封保存。
包装
包装材料和容器:铝塑泡罩 包装规格:10片,30片/盒
肺动脉高压
两项为期12周的随机、双盲、安慰剂对照、多中心研究在393名肺动脉高压(WHO组1)患者中开展。除了凡 瑞克的剂量和研究中心的地理区域之外,两项研究的设计是相同的。ARIES-1研究将每日一次5 mg和10 mg凡瑞克 与安慰剂进行比较,而ARIES-2研究则将每日一次2.5 mg和5 mg凡瑞克与安慰剂进行比较。在两项研究中,都是 在目前治疗(可以包括抗凝剂、利尿剂、钙通道阻滞剂、或地高辛,但不包括依前列醇、曲罗尼尔、伊洛前列素、 波生坦、或西地那非)的基础上添加凡瑞克或安慰剂。研究的主要终点事件是6分钟步行距离。此外,也对临床恶 化、WHO功能分级、呼吸困难、和SF-36健康调查进行评估。
安立生坦片
介绍
GMP常见英文缩写(本站推荐)

GMP常见英文缩写(本站推荐)第一篇:GMP常见英文缩写(本站推荐)GMP常见英文缩写AQAI(Automated Quality Assurance Inspection Equipment):在线自动质量保证检查设备 API(Active Pharmaceutical Ingredient):活性药物物质,即原料药 ANDA(Abbreviated New Drug Application):简化新药申请ADR(Adverse Drug Reaction):不良反应BSE(Bovine Spongiform Encephalopathy):疯牛病BPCS(Business Planning and Control System):业务计划及控制系统 BIA(Business impact assessment): 商业影响评估cGMP(current Good Manufacturing Practice):现行药品生产质量管理规范 CCCD(China Certification Committee for Drugs):中国药品认证委员会CIP(Cleaning In Place):在线清洁CV(Concurrent Validation):同步验证CDER(Center for Drug Evaluation and Research): 药品研究与评价中心COA(Certificate Of Analysis):分析报告单CFR(Code of Federal Regulation):(美国)联邦法规CDC(Centers for Disease Control and Prevention):疾病预防控制中心COS/ CEP(Certificate of Suitability for European Pharmacopeia):欧洲药典适用性证书 CCD(Certification Committee for Drugs):药品认证管理中心CPMP(Committee for Proprietary Medicinal Products): 欧洲专利药品委员会 CTD(Common Technical Document):通用技术文件CDC(Centers for Disease Control and Prevention): 疾病预防控制中心 GMP(Good Manufacturing Practice):药品生产质量管理规范ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药品注册技术要求国际协调会 EU(European Union):欧洲联盟EFPIA(European Federation of PharmaceuticalIndustries Associations):欧洲制药工业协会联合会MHW(Ministry of Health and Welfare,Japan):日本厚生省JPMA(Japan Pharmaceutical Manufacturers Association):日本制药工业协会 FDA(US Food and Drug Adminiistration):美国食品与药品管理局PRMA(Pharmaceutical Research and Manufacturers of America):美国药物研究和生产联合会WHO(World Health Organization):世界卫生组织IFPMA(International Federation of Pharmaceutical Manufacturers Associations): 国际制药工业协会联合会TQC(Total Quality Control),TQM(Total Quality Management): 全面质量管理PDCA(Plan,Do,Check,Action):计划,执行,检查,处理QA(Quality Assurance):质量保证QC(Quality Control):质量控制QS(Quality System):质量体系 QM(Quality Management): 质量管理SOP(Standard Operating Procedure): 标准操作规程SMP(Standard Management Procedure):标准管理程序SOR(Standard Operating Record): 标准操作记录GEP(Good Engineering Practice):工程设计规范HVAC(Heating Ventilation and Air Conditioning):空调净化系统DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认OOS(Out-Of-Specification):检验不合格;超标 PFDS(Process Flow Diagrams):工艺流程图MRA(cMutual Reognition Agreements): 现场检查多边认同协议 DMF(Drug Master File): EDMF(European Drug Master File)欧盟药物主文件EDQM(European Directorate for Quality Medicines): 欧洲药品质量管理局 ORA(Office of Regulatory Affairs):药政事务办公室GGPs(Good Guidance Practices): 优良指南规范MOA(Method Of Analysis):分析方法VMP(Validation Master Plan):验证主计划VP(Validation Protocol):验证方案MSDS(Material Safety Data Sheet):物料安全技术说明书NDA(New Drug Application):新药申请OTC(Over-the-counter):非处方INN(International Nonproprietary Name)国际非专有名称USP(the united state pharmacopeia): 美国药典NF(National Formulary):(美国)国家药品集GAP(Good Agricultural Practice):中药材种植管理规范GCP(Good Clinical Practice):药物临床试验质量管理规范 GLP(Good Laboratory Practice):药物实验室管理规范GSP(Good Supply Practice):药品经营质量管理规范 GUP(Good Use Practice):药品使用质量管理规范 SM(Starting Material):起始物料PMF(Plant Master File);SMF(Site Master File):工厂主文件EDL(List of Essential Drugs): 基本药物目录 PI(Package Insert):说明书PCT(Patent Cooperation Treaty): 专利合作条约PPAC(Patent Protection Association of China):中国专利保护协会 PIC(Person In Charge):负责人PDS(Pharmaceutical Development Services): 整体新药研发机构 SPC(Summary of Product Characteristics):产品特性摘要第二篇:GMP英文缩写1.AQAI(Automated Quality Assurance Inspection Equipment):在线自动质量保证检查设备2.API(Active Pharmaceutical Ingredient):活性药物物质即原料药3.ANDA(Abbreviated New Drug Application):简化新药申请4.ADR(Adverse Drug Reaction):不良反应5.BSE(Bovine Spongiform Encephalopathy):疯牛病6.BPCS(Business Planning and Control System):业务计划及控制系统7.BIA(Business impact assessment): 商业影响评估8.cGMP(current Good Manufacturing Practice):现行药品生产质量管理规范 CD(China Certification Committee for Drugs):中国药品认证委员会10.CIP(Cleaning In Place):在线清洁11.CV(Concurrent Validation):同步验证12.CDER(Center for Drug Evaluation and Research): 药品研究与评价中心13.COA(Certificate Of Analysis):分析报告单14.CFR(Code of Federal Regulation):(美国)联邦法规15.CDC(Centers for Disease Control and Prevention):疾病预防控制中心16.COS / CEP(Certificate of Suitability for European Pharmacopeia):欧洲药典适用性证书D(Certification Committee for Drugs):药品认证管理中心18.CPMP(Committee for Proprietary Medicinal Products): 欧洲专利药品委员会19.CTD(Common Technical Document):通用技术文件20.CDC(Centers for Disease Control and Prevention): 疾病预防控制中心21.GMP(Good Manufacturing Practice):药品生产质量管理规范22.ICH(International Conference on Harmonization of Technical Requirements for Registration ofPharmaceuticals for Human Use):人用药品注册技术要求国际协调会 23.EU(European Union):欧洲联盟24.EFPIA(European Federation of Pharmaceutical Industries Associations):欧洲制药工业协会联合会25.MHW(Ministry of Health and Welfare,Japan):日本厚生省26.JPMA(Japan Pharmaceutical Manufacturers Association):日本制药工业协会27.FDA(US Food and Drug Adminiistration):美国食品与药品管理局28.PRMA(Pharmaceutical Research and Manufacturers of America):美国药物研究和生产联会29.WHO(World Health Organization):世界卫生组织30.IFPMA(International Federation of Pharmaceutical Manufacturers Associations):国际制药工业协会联合会31.TQC(Tota lQuality Control),TQM(Total Quality Management): 全面质量管理32.PDCA(Plan,Do,Check,Action):计划执行检查处理33.QA(Quality Assurance):质量保证 34.QC(Quality Control):质量控制 35.QS(Quality System):质量体系36.QM(Quality Management): 质量管理37.SOP(Standard Operating Procedure): 标准操作规程38.SMP(Standard Management Procedure):标准管理程序39.SOR(Standard Operating Record): 标准操作记录 40.GEP(Good Engineering Practice):工程设计规范41.HVAC(Heating Ventilation and Air Conditioning):空调净化系统42.DQ(Design Qualification):设计确认43.IQ(Installation Qualification):安装确认44.OQ(Operational Qualification):运行确认 45.PQ(Performance Qualification):性能确认46.OOS(Out-Of-Specification):检验结果偏差,有别于偏差47.PFDS(Process Flow Diagrams):工艺流程图48.MRA(cMutual Reognition Agreements): 现场检查多边认同协议 49.DMF(Drug Master File):药物主文件50.EDMF(European Drug Master File)欧盟药物主文件51.EDQM(European Directorate for Quality Medicines): 欧洲药品质量管理局 52.ORA(Office of Regulatory Affairs):药政事务办公室53.GGPs(Good Guidance Practices): 优良指南规范54.MOA(Method Of Analysis):分析方法 55.VMP(Validation Master Plan):验证主计划 56.VP(Validation Protocol):验证方案57.MSDS(Material Safety Data Sheet):物料安全技术说明书58.NDA(New Drug Application):新药申请59.OTC(Over-the-counter):非处方60.INN(International Nonproprietary Name):国际非专有名称P(the united state pharmacopeia): 美国药典62.NF(National Formulary):(美国)国家药品集63.GAP(Good Agricultural Practice):中药材种植管理规范64.GCP(Good Clinical Practice):药物临床试验质量管理规范65.GLP(Good Laboratory Practice):药物实验室管理规范66.GSP(Good Supply Practice):药品经营质量管理规范67.GUP(Good Use Practice):药品使用质量管理规范 68.SM(Starting Material):起始物料69.PMF(Plant Master File);SMF(Site Master File):工厂主文件70.EDL(List of Essential Drugs): 基本药物目录 71.PI(Package Insert):说明书72.PCT(Patent Cooperation Treaty): 专利合作条约73.PPAC(Patent Protection Association of China):中国专利保护协会 74.PIC(Person In Charge):负责人75.PDS(Pharmaceutical Development Services):整体新药研发机构 76.SPC(Summary of Product Characteristics):产品特性摘要第三篇:常用制药及GMP英文缩写ISO(International Organization for Standardization):国际标准化组织日常办事机构是中央秘书处,设在瑞士日内瓦WHO(World Health Organization):世界卫生组织是联合国属下的专门机构,国际最大的公共卫生组织,总部设于瑞士日内瓦PIC/S(Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme):国际医药品稽查协约组织由欧洲自由贸易区(EFTA)组建ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use):人用药物注册技术要求国际协调会由欧盟(EU)、欧洲制药工业协会联合会(EFPIA)、日本厚生省(MHW)、日本制药工业协会(JPMA)、美国FDA、美国药物研究生产联合会(PRMA)等机构组成WHO、EFTA、加拿大卫生保健局(CHPB)为观察员ISPE(International Society for Pharmaceutical Engineering):国际制药工程协会是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一,在美国坦帕州设有全球总部,在布鲁塞尔设有欧洲总部,亚洲总部在新加坡HHS(United States Department of Health and Human Services):美国卫生及公共服务部(美国卫生部)FDA(Food and Drug Administration):美国食品药品监督管理局(HHS下属机构)PDA(Parenteral Drug Association):美国注射剂协会EPA (Environmental Protection Agency):美国国家环境保护局CDER(Center for Drug Evaluation and Research):FDA药物评价与研究中心EMEA(The European Agency for the Evaluation of Medicinal Products):欧洲药物评审组织MHW(Ministry of Health and Welfare):日本厚生省,现改为厚生劳动省MHLW(Ministry of Health, Labor and Welfare),负责医疗卫生和社会保障的主要部门 D&B(Dun & Bradstreet):邓白氏公司DUNS(DataUniversal Numbering System):邓白氏公司提供的唯一的公司代号,用于信用评级等在SMF文件中会用到GMP(Good Manufacturing Practice):药品良好生产规范cGMP(Current Good Manufacture Practices):动态药品生产管理规范,即现行的GLP(Good Laboratory Practice):药物非临床研究质量管理规范,及优良实验室规范GSP(Good Supplying Practice):药品经营质量管理规范,及良好的药品供应规范GAP(Good Agricultural Practice for Chinese Crude Drugs):中药材生产质量管理规范GDP(Good Documentation Practice):良好文件管理GEP (Good Engineering Practice):工程设计规范GAMP(Good Automated Manufacturing Practice):优良自动化生产规范USP(united states pharmacopeia):美国药典EP (European Pharmacopeia):欧洲药典JP(Japanese Pharmacopoeia):日本药典CFR(Code of Federal Regulations):美国联邦法律CFR 21 Part 11(Code of Federal Registry Part11):联邦法规法律标题21第11部分CEP/COS(Certificate of Suitability to the monographs of European Pharmacopoeia):欧洲药典适应性认证证书CEP认证,COS证书CTD(Common Technical Document):国际注册用常规技术文件CTD文件是国际公认的文件编写格式,用来制作一个向药品注册机构递交的结构完善的注册申请文件EHS(Environment、Health、Safety):环境-健康-安全管理体系HACCP(Hazard Analysis and Critical Control Point):(保健食品)危害分析和关键控制点REACH(REGULATION concerning the Registration, Evaluation, Authorization and Restriction of Chemicals):欧盟规章《化学品注册、评估、许可和限制》,欧盟建立的,并于2007年6月1日起实施的化学品监管体系 ICH-Q1A:新原料药和制剂的稳定性试验ICH-Q1B:稳定性试验:新原料药和制剂的光稳定性试验ICH-Q1C:稳定性试验:新剂型的要求ICH-Q1D:新原料药和制剂的稳定性试验的括号法和矩阵法设计ICH-Q1E:稳定性数据的评价ICH-Q1F:气候带Ⅲ和Ⅳ注册申请的稳定性数据 ICH-Q2A:分析步骤验证:正文ICH-Q2B:分析步骤验证:方法学ICH-Q3A:原料药中的杂质 ICH-Q3B:新制剂中的杂质ICH-Q3C:杂质;残留溶剂的指导原则 ICH-Q4:药典ICH-Q4A:药典的同一化ICH-Q4B:各地区使用的药典正文评估和建议ICH-Q5A:来源于人或动物细胞系的生物技术产品的病毒安全性评价ICH-Q5B:生物技术产品的质量:rDNA衍生蛋白质产品生产细胞的表达构建体分析ICH-Q5C:生物技术产品的质量:生物制品/生物技术产品的稳定性试验ICH-Q5D:用于生物技术产品及生物制品生产的细胞基质的来源和鉴定 ICH-Q5E:生物技术产品/生物制品在工艺变更时的可比性ICH-Q6A:质量标准新原料药和制剂的检测以及可接受标准:化学物质ICH-Q6B:质量标准:生物技术产品及生物制品的检测方法和可接受标准ICH-Q7:原料药良好制造规范(ICH-Q7A的新版)ICH-Q7A:原料药的GMP规范 ICH-Q8:药物研发指南 ICH-Q9:质量风险管理ICH-Q10(PQS):药物质量体系QA(Quality Assurance):质量保证QC(Quality Control):质量控制QRM(Quality Risk Management):质量风险管理IPC (InproceicsQuality Control):制程品质控制/中控OOS(Out of Specification):检验结果超标 OOT(Out of Trend):超趋势结果OOL(Out of Limit):超出极限的结果,如温湿度等OOE (Out of Expectation):超期望结果SAL(SterilityAssuranceLevel):无菌保证水平灭菌后微生物的存活概率的负对数,要求≥6SAL=−lg存活率=F0D−lgN0D值:杀灭90%的微生物所需要的时间,D值越大,微生物死亡越难,D值与细菌的耐热性成正比Z值:指灭菌时间减少到原来的10%所需要升高的温度或是相同的灭菌时间内杀死99%的微生物所需要提高的温度F值:为一定温度下,给定Z值所产生的灭局效果与参比温度T0下给定Z值所产生的灭菌效果相同时所相当的时间F值用于干热灭菌F0值:为一定温度下,Z值为10℃产生的灭菌效果与120℃,Z 值为10℃时产生的灭菌效果相当的时间,t分钟内的灭菌效果相当于120℃下灭菌F0分钟的效果F0被称为标准灭菌时间,用于热压灭菌LRV:除菌过滤的对数下降值LRV=lgN0-lgN SOP(Standard Operation Procedure):标准操作规程 DMF(Drug Master File):药品主文件 SMF(Site Master File):工厂主文件URS(User Requirement Specification):用户需求标准FS (Functional Specification):功能标准DS(Design Specification):设计标准 DQ(Design Qualification):设计确认IQ(Installation Qualification):安装确认OQ(Operational Qualification):运行确认PQ(Performance Qualification):性能确认 RQ(Requalification):再确认CAPA(Corrective Action & Preventive Action):纠正预防系统,Q10的四大要素之一QbD(Quality byDesign):质量源于设计COA(Certificate of Analysis):分析证书/检验报告书/检验报告单 BPR(Batch Production Record):批生产记录API(Active Pharmaceutical Ingredients):药物活性成分,通常指的原料药 PMC(Product Material Control):生产物料控制PC 生产控制;MC物料控制CMC(Chemistry and manufacture control):生产和化学控制APR(Annual Products Review):质量回顾 KPI(Key Performance Indicators):关键业绩指标P&ID(Piping and Instrument Diagram):工艺管道仪表流程图 PFD(Process Flow Diagram):工艺流程图 UFD(Utility Flow Diagram):公用工程流程图CIP(Cleaning in Place):原位清洗(全自动,如针剂配制系统)WIP(Washing in Place):在线清洁(半自动,需要手动的拆卸,如流化床)SIP(Sterilization in Place):在线灭菌WFI (Water for Injection):注射用水HVAC(Heating Ventilation Air Conditioning):供热空气调节净化系统 HEPA(High Efficiency Particulate Air Filter):高效过滤器DOP:为邻苯二甲酸二辛酯,HEPA检漏用的气溶胶PAO:聚-α-烯烃,HEPA检漏用的气溶胶 IBC(IntermediateBulkContainer):中型散装容器BFS(Blowing Filling and Sealing):吹-灌-封PAT (Process Analytical Technology):过程分析技术PLC (Programmable Logic Controller):可编程逻辑控制CPP (Critical Process Parameters):关键工艺参数FBD(Fluid Bed Dryer):流化床AHU(Air Handling Unit):空气处理单元SAT (SiteAcceptance T est):现场验收测试 FAT(Factory Acceptance Test):工厂验收测试第四篇:常见电力英文缩写B-MCR:锅炉的最大连续工况,B-ECR:锅炉的最经济连续工况,THA:汽轮机热耗率验收工况,TRL:铭牌功率,VWO:调节阀全开工况,AMS:asset management system 设备管理系统说明:为西屋公司OVATION系统的一个软件包,实现对智能变送器、智能执行器的诊断、调校、故障预测、定期校验管理等功能,采用该系统能节省大量的设备调试时间及维护量。
《临床药理学》10第十章 遗传药理学与临床合理用药

普罗帕酮血浆浓度和CYP2D6基因多态性的关系
血浆普罗帕酮(ng/ml)
1600
剂量: 3 x 150 mg / day
1400 房颤: 安慰剂:
33 %
普罗帕酮: 16 %
1200
1000
ß-阻滞 CNS副反应
1080
800
695
600
15 % Afib
400
200
0
33
127
18 % Afib
16
遗传药理学的研究方法
分子生 物学研
究
双生 子法
组织与 细胞水 平研究
群体 研究
系谱 研究
17
第2节 药物代谢酶的基因多态性
18
药物反应差异的生物学基础:单核苷酸多态性(SNP)
细胞
细胞核
染色 体 基因
---- 占人类遗传变异的 90% 最常见的遗传变异 基因突变发生频率超过1%
第430bp
-
4
4,*5 *10,*17
中国(黄种)人(PM) 白人(PM) 黑人(PM) 导致极快代谢的突变
-
-
-
-
-
-
-
-
-
-
-
-
-
<1% 5%~20% 1%~2%
-
-
-
<1% 2%~6% 5%~10% -
-
-
<1% 2%~7% 0%~19% -
-
-
-
-
*2N
-
-
(N=2,3,4,
5,13)
中国(黄种)人(UM)
EM
EM
IM
PM
野生/野生 野生/突变 低活性突变/ 无活性突变/
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
7
ABC Transporter Membrane Vesicles Available from BD
BD GentestTM ABC Transporter Membrane Vesicles
ABC: ATP-Binding Cassette Family of Transporters
8
Uptake Assay in ABC Transporter Vesicles
•
Assessment of additional transporter-dependent drug disposition and drug interactions is now routinely expected by regulatory agencies Recent consensus by the USFDA and industry representatives has outlined guidance for assessment of ABC and SLC transporter-mediated interactions during drug development
3
Role of Transporters in PK/PD
• Efflux of drug from various tissues
– BCRP (rosuvastatin) – BSEP (vinblastine)
• Vectorial transport of drugs into and out of hepatocytes
Disadvantages
• • Potential that extremely high permeability compounds could generate high background (in control condition) Typically requires radiolabeled test articles for substrate ID (non-labeled compounds can be assayed as potential inhibitors of radiolabeled probe substrates)
Pravastatin Portal vein Uptake by unconfirmed transporter Liver Uptake by OATP1B1 Bile Efflux by MRP2
4
Importance of In Vitro Transporter Assays in Drug Development
•
Giacomini, Huang, Tweedie et al, Nature Reviews Drug Discovery (2010) 9:215-236
6
Guidance and Position Papers
• Tucker et al. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential – toward a consensus. Basel Conference 2001, Sponsored by FDA, EUFEPS, AAPS:
Application of BCRP, BSEP and MRP2 Transporter Vesicles in Drug Development Studies
Lisa Fox
June 2, 2010
Topics
• Drug-transporter interaction in drug development • ABC Transporter membrane vesicles • Uptake and inhibition kinetics in vesicles • Drug development study designs • Other transporter applications
– – – – Clin Pharmacol Ther (2001), 70:103-14, Br J Clin Pharmacol (2001), 52:107-17, Eur J Pharm Sci (2001), 13:417-28, Pharm Res (2001), 18:1071-80
• • • • • •
5
Regulatory Expectations and Guidance
• FDA Guidance (2006)* outlines recommendations for assessing drug interactions with human MDR1 (ABCB1)
* FDA Draft Guidance for Industry (Sept 2006) Drug Interaction Studies – Study Design, Data Analysis and Implications for Dosing and Labeling (/cder/guidance/index.htm)
ATP-dependent uptake of drugs into vesicles via “inside-out” efflux transporters
Inside Out
ATP
Drug
ADP + P
Vesicles prepared from baculovirus/Sf9 insect cell expression system Efflux transporter is in an “inside-out” or reverse orientation on the sphere Orientation allows substrates to be taken up by the transporter into the vesicles 96-well reaction plate filter plate via cell harvester/vacuum manifold LSC Measures uptake activity of test articles in the absence and presence of known inhibitors
Zhang et al. (2006) Scientific Perspectives on Drug Transporters and Their Role in Drug Interactions. Mol Pharm 3:62-69 FDA DRAFT Guidance for Industry (Sept 2006) Drug Interaction Studies – Study Design, Data Analysis and Implications for Dosing and Labeling (/cder/guidance/index.htm) Huang et al. (2008) New Era in Drug Interaction Evaluation: US FDA Update on CYP Enzymes, Transporters, and the Guidance Process. J Clin Pharmacol 48:662-670 Zhang et al. (2008) A Regulatory Viewpoint on Transporter-based Drug Interactions. Xenobiotica 38:709-724 Giacomini, et. al. Membrane Transporters in Drug Development – The International Transporter Consortium Nature Reviews: Drug Discovery (Mar 2010) 9:215-236 EMEA DRAFT: European Medicines Agency Guideline on the Investigation of Drug Interactions, (Apr 2010) CPMP/EWP/560/95/Rev. 1 – Corr.
– SLC Transporters
2
Importance of BCRP, BSEP and MRP2 in Drug Development
Consensus of the International Transporter Consortium: Giacomini, Huang, Tweedie et al, Nature Reviews Drug Discovery (2010) 9:215-236
10
Time- and Temperature-Dependence
Characterization data with human MRP2 membrane vesicles demonstrating linear time-dependence and effect of temperature on transport activity
• Understand function of transporters in the absorption, distribution and elimination of drug candidates • Evaluate and improve drug bioavailability (transporter-SAR) • Predict and avoid transporter involved DDIs • Explain/predict pharmacokinetic data (e.g. non-proportional PK, excretion of unchanged drug) • To provide information in support of IND or NDA filings addressing potential transporter-mediated drugVesicle Model
