Nalidixic acid_389-08-2_DataSheet_MedChemExpress
Naglazyme(galsulfase)(抗生物)商品说明书

Naglazyme® (galsulfase)(Intravenous)Document Number: MH-0084 Last Review Date: 02/01/2022Date of Origin: 11/28/2011Dates Reviewed: 12/2011, 02/2013, 02/2014, 12/2014, 10/2015, 10/2016, 10/2017, 10/2018, 02/2019,02/2020, 02/2021, 02/2022I.Length of AuthorizationCoverage will be provided for 12 months and may be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:•Naglazyme 5 mg vial: 23 vials per 7 daysB.Max Units (per dose and over time) [HCPCS Unit]:•115 billable units every 7 daysIII.Initial Approval Criteria 1Coverage is provided in the following conditions:•Patient is at least 5 years of age; AND•Documented baseline 12-minute walk test (12-MWT), 3-minute stair climb test (3-MSCT), and/or pulmonary function tests (e.g., FEV1, etc.); AND•Documented baseline value for urinary glycosaminoglycan (uGAG); ANDMucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome) † Ф1,4,5•Patient has a definitive diagnosis of MPS VI as confirmed by the following:o Detection of pathogenic mutations in the ARSB gene by molecular genetic testing; ORo Arylsulfatase B (ASB) enzyme activity of <10% of the lower limit of normal in cultured fibroblasts or isolated leukocytes; AND▪Patient has normal enzyme activity of a different sulfatase (excluding patients with Multiple Sulfatase Deficiency [MSD]); AND▪Patient has an elevated urinary glycosaminoglycan (uGAG) level (i.e. dermatan sulfate or chondroitin sulfate) defined as being above the upper limit of normal bythe reference laboratory†FDA-approved indication(s); ‡Compendia recommended indication(s); ФOrphan DrugIV.Renewal Criteria 1,4,5Coverage can be renewed based on the following criteria:•Patient continues to meet indication-specific relevant criteria such as concomitant therapy requirements (not including prerequisite therapy), performance status, etc. identified insection III; AND•Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: anaphylaxis and hypersensitivity reactions, immune-mediated reactions, acute respiratorycomplications associated with administration, acute cardiorespiratory failure, severeinfusion reactions, spinal or cervical cord compression, etc.; AND•Disease response with treatment as defined by improvement or stability from pre-treatment baseline by the following:o Reduction in uGAG levels; AND▪Improvement in or stability of 12-minute walk test compared (12-MWT); OR▪Improvement in or stability of 3-minute stair climb test (3-MSCT); OR▪Improvement in or stability of pulmonary function testing (e.g., FEV1, etc.)V.Dosage/Administration 1Indication DoseMucopolysaccharidosis VI(MPS VI, Maroteaux-Lamy Syndrome) 1 mg/kg administered as an intravenous (IV) infusion oncea weekVI.Billing Code/Availability InformationHCPCS Code:•J1458 – Injection, galsulfase, 1 mg; 1 billable unit = 1 mgNDC:•Naglazyme 5 mg per 5 mL solution; single-use vial: 68135-0020-xxVII.References1.Naglazyme [package insert]. Novato, CA; BioMarin Pharmaceutical Inc.; December 2019.Accessed January 2022.2.Giugliani R, Harmatz P, Wraith JE. Management guidelines for mucopolysaccharidosis VI.Pediatrics. 2007 Aug;120(2):405-18.3.Giugliani R, Federhen A, Rojas MV, et al. Mucopolysaccharidosis I, II, and VI: Brief reviewand guidelines for treatment. Genet Mol Biol. 2010 Oct;33(4):589-604. Epub 2010 Dec 1.4.Vairo F, Federhen A, Baldo G, et al. Diagnostic and treatment strategies inmucopolysaccharidosis VI. Appl Clin Genet. 2015 Oct 30;8:245-55.5.Valaannopoulos V, Nicely H, Harmatz P, et al. Mucopolysaccharidosis VI. Orphanet J RareDis. 2010; 5: 5.6.Harmatz P, Giugliani R, Schwartz I, et al. Enzyme replacement therapy formucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled,multinational study of recombinant human N-acetylgalactosamine 4-sulfatase(recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. JPediatr. 2006 Apr;148(4):533-539.Appendix 1 – Covered Diagnosis CodesICD-10 ICD-10 DescriptionE76.29 Other mucopolysaccharidosesAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National CoverageDetermination (NCD), Local Coverage Determinations (LCDs), and Local Coverage Articles (LCAs) may exist and compliance with these policies is required where applicable. They can be found at: https:///medicare-coverage-database/search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD/LCA): N/AMedicare Part B Administrative Contractor (MAC) JurisdictionsJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
MedBio_CAS号210830-32-3 N-Me-D-Val-OH·HCl相关物理参数

≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11116
Fmoc-(2-氯苄氧基羰基)赖氨酸
Fmoc-Lys(2-Cl-Z)-OH
133970-31-7
25g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11078
Fmoc-N-甲基-D-亮氨酸
Fmoc-D-N-Me-Leu-OH
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11079
N-叔丁氧羰基-N-甲基-D-苯丙氨酸二环己胺盐
Boc-D-N-Me-Phe.DCHA
102185-45-5
25g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11002
2-(叔-丁氧基碳酰胺)-2-苯乙腈
Boc-ON
58632-95-4
25g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11120
N,N'-双芴甲氧羰基-L-赖氨酸
Fmoc-Lys(Fmoc)-OH
78081-87-5
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11110
Fmoc-L-异亮氨酸
Fmoc-Ile-OH
【精品】INCI命名表

【精品】INCI命名表INCI名称——C——中文名称 CAS号C18-36 ACID C18-36 酸C20-40 ACIDC20-40 酸C29-70 ACID C29-70 酸C30-50 ACID C30-50 酸C40-60 ACID C40-60 酸C16-22 ACID AMIDE MEA C16-22 酸酰胺 MEAC14-18 ACID GLYCOL ESTER C14-18 酸甘醇酯C18-30 ACID GLYCOL ESTER C18-30 酸甘醇酯C18-36 ACID GLYCOL ESTER C18-36 酸甘醇酯C12-20 ACID PEG-8 ESTER C12-20 酸 PEG-8 酯C23-43 ACID PENTAERYTHRITOL TETRAESTER C23-43 酸季戊四醇四酯95465-86-4C8-12 ACID TRIGLYCERIDE C8-12 酸甘油三酯C12-18 ACID TRIGLYCERIDE C12-18 酸甘油三酯C18-36 ACID TRIGLYCERIDE C18-36 酸甘油三酯 91052-08-3 苏木(CAESALPINIA SAPPAN)皮提CAESALPINIA SAPPAN BARK EXTRACT 取物苏木(CAESALPINIA SAPPAN)茎粉CAESALPINIA SAPPAN STEM POWDER 末刺云实(CAESALPINIA SPINOSA)CAESALPINIA SPINOSA FRUIT EXTRACT 果提取物刺云实(CAESALPINIA SPINOSA)CAESALPINIA SPINOSA GUM 39300-88-4 胶CAESALPINIA SPINOSA 刺云实羟丙基三甲基氯化铵HYDROXYPROPYLTRIMONIUM CHLORIDECAFFEIC ACID 咖啡酸331-39-5CAFFEINE 咖啡因 58-08-2CAFFEINE BENZOATE 咖啡因苯甲酸盐 5743-17-9CAFFEINE CARBOXYLIC ACID 咖啡因羧酸 652-37-9CAJANUS CAJAN LEAF EXTRACT 木豆(CAJANUS CAJAN)叶提取物滨海卡克勒(CAKILE MARITIMA)CAKILE MARITIMA EXTRACT 84929-60-2 提取顲ALAMINE 炉甘-96-9 药用新风轮菜(CALAMINTHACALAMINTHA OFFICINALIS 石 8011EXTRACT OFFICINALIS)提取物虾脊兰(CALANTHE DISCOLOR)提CALANTHE DISCOLOR EXTRACT 取物CALCITE 方解石 13397-26-7CALCIUM ACETATE 乙酸钙62-54-4CALCIUM ALGINATE 海藻酸钙 9005-35-0CALCIUM ALUMINUM BOROSILICATE 硼硅酸铝钙 65997-17-3CALCIUM ASCORBATE 抗坏血酸钙 5743-27-1CALCIUM ASPARTATE 天冬氨酸钙 21059-46-1CALCIUM BEHENATE 山嵛酸钙 3578-72-1CALCIUM BENZOATE 苯甲酸钙2090-05-3CALCIUM CARBONATE 碳酸钙 471-34-1CALCIUMCARBOXYMETHYL CELLULOSE 羧甲基纤维素钙 9050-4-8CALCIUM CARRAGEENAN 角叉菜钙 9049-05-2CALCIUM CASEINATE 酪蛋白钙9005-43-0CALCIUM CERIUM OXIDE 氧化钙铈CALCIUM CHLORIDE 氯化钙10043-52-4CALCIUM CITRATE 柠檬酸钙 5785-44-4CALCIUM CYCLAMATE 环己氨基磺酸钙 103-06-0CALCIUM DIHYDROGEN PHOSPHATE 磷酸二氢钙7758-23-8CALCIUM DISODIUM EDTA EDTA二钠钙 62-33-9CALCIUM DNA DNA 钙CALCIUM DODECYLBENZENESULFONATE 十二烷基苯磺酸钙26264-06-2CALCIUM FERRITE 铁酸钙 12013-33-1CALCIUM FLUORIDE 氟化钙7789-75-5CALCIUM FRUCTOBORATE 果糖硼酸钙CALCIUMFRUCTOHEPTONATE 果庚糖酸钙CALCIUM GLUCOHEPTONATE 葡庚糖酸钙CALCIUM GLUCONATE 葡糖酸钙 299-28-5 126-95-41336-00-127214-CALCIUM GLYCEROPHOSPHATE 甘油磷酸钙 00-258409-70-4CALCIUM HYDROXIDE 氢氧化钙 1305-62-0CALCIUM LACTATE 乳酸钙 814-80-25743-47-5CALCIUM LAURATE 月桂酸钙 4696-56-4CALCIUM LAUROYL TAURATE 月桂酰牛磺酸钙 138705-25-6CALCIUM LIGNOSULFONATE 木素磺酸钙 8061-52-7CALCIUMMONOFLUOROPHOSPHATE 单氟磷酸钙 7789-74-4CALCIUM MONTANATE 褐煤酸钙68308-22-552258-47-6CALCIUM MYRISTATE 肉豆蔻酸钙15284-51-2CALCIUM OXIDE 氧化钙 1305-78-8CALCIUM PANTETHEINE SULFONATE 泛酰巯基乙胺磺酸钙 9007-3-8CALCIUM PANTOTHENATE 泛酸钙 137-08-6CALCIUM PARABEN 羟苯甲酸钙 69959-44-0CALCIUM PCA PCA 钙31377-05-6CALCIUM PEROXIDE 过氧化钙 1305-79-9CALCIUM PHOSPHATE 磷酸钙 7758-23-810103-46-5CALCIUM POTASSIUM CARBOMER 卡波姆钙钾CALCIUM PROPIONATE 丙酸钙 4075-81-4CALCIUM PYROPHOSPHATE 焦磷酸钙 7790-76-3CALCIUM RNA RNA 钙CALCIUM SACCHARIN 糖精钙6485-34-3CALCIUM SALICYLATE 水杨酸钙 824-35-1CALCIUM SILICATE 硅酸钙1344-95-2CALCIUM BETA-SITOSTERYL SULFATE β-谷甾醇硫酸酯钙CALCIUM SODIUM BOROSILICATE 硼硅酸钠钙CALCIUM SODIUM PHOSPHOSILICATE 磷硅酸钠钙CALCIUM/SODIUM PVM/MA COPOLYMER62386-95-2CALCIUM SORBATE 山梨酸钙 PVM/MA共聚物钙/钠7492-55-9CALCIUM STARCH ISODODECENYLSUCCINATE 异十二碳烯基琥珀酸淀粉钙 194810-88-3CALCIUM STARCH OCTENYLSUCCINATE 辛烯基琥珀酸淀粉钙CALCIUM STEARATE 硬脂酸钙 1592-23-0CALCIUM STEAROYL LACTYLATE 硬脂酰乳酰乳酸钙 5793-94-2CALCIUM SULFATE 硫酸钙-4CALCIUM SULFIDE 硫化钙 20548-54-3CALCIUM TARTRATE 酒石酸10101-41 钙 3164-34-9CALCIUM THIOGLYCOLATE 巯基乙酸钙 814-71-1CALCIUM TITANATE 钛酸钙 12049-50-2CALCIUM UNDECYLENATE 十一碳烯酸钙1322-14-1CALCIUM XYLENESULFONATE 二甲苯磺酸钙 28088-63-3C9-11 ALCOHOLS C9-11 醇 68551-08-666455-17-2C12-13 ALCOHOLS C12-13 醇75782-86-4C12-15 ALCOHOLS C12-15 醇 63393-82-8C12-16 ALCOHOLS C12-16 醇 68855-56-1C14-15 ALCOHOLS C14-15 醇C14-22 ALCOHOLS C14-22 醇C20-22ALCOHOLS C20-22 醇 90604-34-5C20-40 ALCOHOLS C20-40 醇C30-50 ALCOHOLS C30-50 醇C40-60 ALCOHOLS C40-60 醇金盏花(CALENDULACALENDULA OFFICINALIS FLOWER OFFICINALIS)花金盏花(CALENDULACALENDULA OFFICINALIS FLOWER EXTRACT 84776-23-8 OFFICINALIS)花提取物金盏花(CALENDULACALENDULA OFFICINALIS FLOWER OIL 70892-20-5 OFFICINALIS)花油CALF BLOOD EXTRACT 牛犊血提取物CALF SERUM 牛犊血清CALF SKIN EXTRACT 牛犊皮提取物C9-12ALKANE C9-12 烷C10-13 ALKANE C10-13 烷 129813-66-7C13-14 ALKANE C13-14 烷C13-15 ALKANE C13-15 烷C14-17 ALKANE C14-17 烷C14-19 ALKANE C14-19 烷C15-19 ALKANE C15-19 烷C15-23 ALKANE C15-23 烷C18-21 ALKANE C18-21 烷C8-9 ALKANE/CYCLOALKANE C8-9 烷/环烷64742-49-0C9-10 ALKANE/CYCLOALKANE C9-10 烷/环烷 64742-49-0C9-11 ALKANE/CYCLOALKANE C9-11 烷/环烷 64742-49-0C9-16ALKANE/CYCLOALKANE C9-16 烷/环烷C10-12 ALKANE/CYCLOALKANE C10-12 烷/环烷 64742-48-9C11-14 ALKANE/CYCLOALKANE C11-14 烷/环烷C11-15 ALKANE/CYCLOALKANE C11-15 烷/环烷C12-13ALKANE/CYCLOALKANE C12-13 烷/环烷C8-10ALKANE/CYCLOALKANE/AROMATIC C8-10 烷/环烷/芳烃类64742-82-1HYDROCARBONSC12-15 ALKANE/CYCLOALKANE/AROMATICC12-15 烷/环烷/芳烃类 64742-94-5HYDROCARBONSC5-6ALKANE/CYCLOALKANE/TERPENE C5-6 烷/环烷/萜烯共聚物COPOLYMERC18-28 ALKYL ACETATE C18-28 醇乙酸酯C14-30 ALKYL BEESWAXC14-30 醇蜂蜡酸酯C18-38 ALKYL BEESWAX C18-38 醇蜂蜡酸酯C30-50 ALKYL BEESWAX C30-50 醇蜂蜡酸酯C20-40 ALKYL BEHENATE C20-40醇山嵛酸酯C10-14 ALKYL BENZENESULFONIC ACID C10-14 烷基苯磺酸C12-15 ALKYL BENZOATE C12-15 醇苯甲酸酯 68411-27-8C18-38 ALKYL C24-54 ACID ESTER C18-38 醇C24-54 酸酯C6-8 ALKYL C3-6 ALKYL GLUCOSIDE C6-8 烷基C3-6 烷基葡糖苷聚二DIMETHICONE 甲基硅氧烷C30-45 ALKYL CETEARYL DIMETHICONE C30-45 烷基鲸蜡硬脂基聚二甲基CROSSPOLYMER 硅氧烷交联聚合物C20-24 ALKYL DIMETHICONE C20-24 烷基聚二甲基硅氧烷200074-76-6C24-28 ALKYL DIMETHICONE C24-28 烷基聚二甲基硅氧烷192230-29-8C30-45 ALKYL DIMETHICONE C30-45 烷基聚二甲基硅氧烷C30-45 -45 烷基聚二甲基硅氧烷/聚环DIMETHICONE/POLYCYCLOHEXENE ALKYL C30OXIDE 330809-27-3;389082-70-6 己烯氧化物交联聚合物CROSSPOLYMERC12-13 ALKYL ETHYLHEXANOATE C12-13 醇乙基己酸酯 90411-66-8C12-15 ALKYL ETHYLHEXANOATE C12-15 醇乙基己酸酯 90411-66-8C14-18 ALKYL ETHYLHEXANOATE C14-18 醇乙基己酸酯C8-10 ALKYL ETHYL PHOSPHATE C8-10 醇乙醇磷酸酯68412-60-2C1-5 ALKYL GALACTOMANNAN C1-5 烷基半乳甘露聚糖C12-20 ALKYL GLUCOSIDE C12-20 烷基葡糖苷C18-38 ALKYL HYDROXYSTEAROYL STEARATE C18-38 醇羟基硬脂酰硬脂酸酯C12-13 ALKYL LACTATE C12-13 醇乳酸酯 93925-36-1C12-15 ALKYL LACTATE C12-15 醇乳酸酯 93925-36-1C20-24 ALKYL METHICONE C20-24 烷基聚甲基硅氧烷200074-77-7C24-28 ALKYL METHICONE C24-28 烷基聚甲基硅氧烷C30-45ALKYL METHICONE C30-45 烷基聚甲基硅氧烷C9-15 ALKYL PHOSPHATE C9-15醇磷酸酯C12-15 ALKYL SALICYLATE C12-15 醇水杨酸酯C16-36 ALKYL STEARATE C16-36 醇硬脂酸酯C20-40 ALKYL STEARATE C20-40 醇硬脂酸酯C30-50 ALKYL STEARATE C30-50 醇硬脂酸酯C40-60 ALKYL STEARATE C40-60 醇硬脂酸酯C1-8 ALKYL TETRAHYDROXYCYCLOHEXANOATE C1-8 醇四羟基环己酸酯澳洲蓝柏(CALLITRISCALLITRIS INTROTROPICA WOOD OIL 180287-43-8 INTROTROPICA)木油方苞非洲柏(CALLITRISCALLITRIS QUADRIVALVIS GUM 9000-57-1 QUADRIVALVIS)胶胡桐(CALLOPHYLLUMCALLOPHYLLUM INOPHYLLUM SEED OIL INOPHYLLUM)籽油帚石楠(CALLUNA VULGARIS)提取CALLUNA VULGARIS EXTRACT 84603-54-3 物CALLUNA VULGARIS FLOWER WATER 帚石楠(CALLUNA VULGARIS)花水好望角美树(CALODENDRUMCALODENDRUM CAPENSE NUT OIL CAPENSE)坚果油洋胡桐(CALOPHYLLUMCALOPHYLLUM TACAMAHACA SEED OIL TACAMAHACA)籽油云杉光皮树CALYCOPHYLLUMCALYCOPHYLLUM SPRUCEANUM BARK EXTRACT SPRUCEANUM树皮提取物CAMELINA SATIVA SEED OIL 亚麻荠(CAMELINA SATIVA)籽油山茶(CAMELLIA JAPONICA)叶提CAMELLIA JAPONICA LEAF EXTRACT 取物山茶(CAMELLIA JAPONICA)籽提CAMELLIA JAPONICA SEED EXTRACT 取物CAMELLIA JAPONICA SEED OIL 山茶(CAMELLIA JAPONICA)籽油CAMELLIA KISSI SEED OIL 落瓣油茶(CAMELLIA KISSI)籽油CAMELLIA OLEIFERA LEAF 油茶(CAMELLIA OLEIFERA)叶油茶(CAMELLIA OLEIFERA)叶提CAMELLIA OLEIFERA LEAF EXTRACT 取物CAMELLIA OLEIFERA LEAF POWDER 油茶(CAMELLIA OLEIFERA)叶粉油茶(CAMELLIA OLEIFERA)籽提CAMELLIA OLEIFERA SEED EXTRACT 取物CAMELLIA OLEIFERA SEED OIL 油茶(CAMELLIA OLEIFERA)籽油茶(CAMELLIA SINENSIS)儿茶素CAMELLIA SINENSIS CATECHINS 类茶(CAMELLIA SINENSIS)叶提取CAMELLIA SINENSIS LEAF EXTRACT 84650-60-2 物CAMELLIA SINENSIS LEAF OIL 茶(CAMELLIA SINENSIS)叶油68916-73-4CAMELLIA SINENSIS LEAF POWDER 茶(CAMELLIA SINENSIS)叶粉CAMELLIA SINENSIS LEAF WATER 茶(CAMELLIA SINENSIS)叶水-00-479-92-5CAMPHOR 樟脑 76-22-2464-49-3CAMPHOR CAMPHENE 莰烯 565 BENZALKONIUM METHOSULFATE 樟脑苯扎铵甲基硫酸盐CAMPHYLCYCLOHEXANOL 莰基环已醇 68877-29-2CANADIAN COLLOIDAL CLAY 加拿大皂土依兰(CANANGA ODORATA)花提取CANANGA ODORATA FLOWER EXTRACT 物CANANGA ODORATA FLOWER OIL 依兰(CANANGA-3CANANGA ODORATA FLOWER WAX 依兰ODORATA)花油 8006-81(CANANGA ODORATA)花蜡爪哇橄榄(CANARIUM COMMUNE)胶CANARIUM COMMUNE GUM OIL 97675-63-3 油.。
高效液相色谱法测定复方磺胺甲恶唑片中磺胺甲恶唑和甲氧苄啶的含量_熊久林

续表1序号保留时间t /min化合物名称分子式分子量相对含量(%)79.3213-octadecenoic acid(Z)(Z)-13-十八碳烯酸C 18H 34O 228221.6289.73octadecan oic acid 十八烷酸C 18H 36O 228433.46910.6210,13-octadecadienoic acid 10,13-十八碳二烯酸C 18H 32O 22800.131010.7910-noadecen oic acid 10-十九烯酸C 19H 36O 22960.091111.26noadecanoic caid 十九烷酸C 19H 38O 22980.281212.7211-eicosenoic acid 11-二十碳烯酸C 20H 38O 2310 2.101313.23eicosanoic acid 二十碳烷酸C 20H 40O 2312 1.771417.42docosanoic acid 二十二碳烷酸C 22H 44O 23400.18由表1可以看出,从中药材木鳖子中共鉴定出14种脂肪酸,占脂肪酸总含量的89.23%,其中饱和脂肪酸7种,占总脂肪酸含量的47.32%不饱和脂肪酸7种,占脂肪酸总量的41.91%。
3 讨论木鳖子中不饱和脂肪酸以亚油酸(19.85%)、(Z)-13-十八(碳)烯酸(21.62%)、11-二十(碳)烯酸(2.10%)为主。
近年来的研究表明不饱和脂肪酸对人体有降低血脂、胆固醇和血压,抗血栓、抗动脉硬化,预防心血管疾病,增强记忆力,预防老年痴呆症,防癌等多种作用[3]。
木鳖子是一种常见的中药,对中药木鳖子的研究已较深入,但对其脂肪酸的研究还未见报道。
本实验方法具有简单、准确、脂肪酸检出率高等优点,其结果将对木鳖子的深层次的开发和应用提供科学依据。
参考文献:[1] 宋立人,洪 恂,丁绪亮,等.现代中药大辞典,上册[M ].北京:人民出版社:349-351.[2] S.R..Heller ,G.W.A.M iline.EPA/NIH M ass Spectral date[M ].U .S.Washington D.C.,1978:1-4.[3] 周永红.火麻仁油中脂肪酸的GC-M S 分析[J ].中国油脂,2004,29(3):72-72.收稿日期:2004-11-12; 修订日期:2005-02-12作者简介:熊久林(1956-),男(汉族),湖北黄梅人,现任湖北省黄石市药品检验所副主任药师,主要从事药品检验工作.高效液相色谱法测定复方磺胺甲唑片中磺胺甲唑和甲氧苄啶的含量熊久林,孙仲葆,黎 源,马锦星,运 委,张 晶(湖北省黄石市药品检验所 435000)摘要:目的:建立同时测定复方磺胺甲唑片中磺胺甲唑和甲氧苄啶含量的高效液相色谱法。
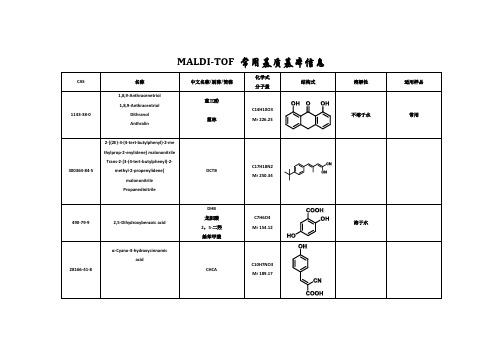
MALDI-TOF 常用基质基本信息
盐酸利多卡因注射剂遗传毒性杂质研究_NormalPdf

Journal of China Pharmaceutical University2020,51(4):466-471学报盐酸利多卡因注射剂遗传毒性杂质研究冼芷然1,孙春萌2,骆雪芳1*,钟文英1**(1中国药科大学理学院药物质量研究中心,南京211198;2中国药科大学药学院,南京211198)摘要确定2,6-二甲基苯胺为盐酸利多卡因注射液中遗传毒性杂质,N-氯乙酰-2,6-二甲基苯胺为潜在遗传毒性杂质,建立LC-MS/MS方法,用色谱柱Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm)对原料、自制制剂及原研制剂进行遗传毒性杂质研究。
研究结果表明自制制剂中杂质2,6-二甲基苯胺与N-氯乙酰-2,6-二甲基苯胺除由原料引入外,可能分别由氧化条件或碱性条件下降解引入,为盐酸利多卡因注射液的遗传毒性风险评估和工艺优化提供参考与指导。
关键词盐酸利多卡因注射液;遗传毒性杂质;LC-MS/MS中图分类号R917文献标志码A文章编号1000-5048(2020)04-0466-06doi:10.11665/j.issn.1000-5048.20200412引用本文冼芷然,孙春萌,骆雪芳,等.盐酸利多卡因注射剂遗传毒性杂质研究[J].中国药科大学学报,2020,51(4):466–471.Cite this article as:XIAN Zhiran,SUN Chunmeng,LUO Xuefang,et al.Profiling of genotoxic impurities in a lidocaine hydrochloride injec‐tion[J].J China Pharm Univ,2020,51(4):466–471.Profiling of genotoxic impurities in a lidocaine hydrochloride injection XIAN Zhiran1,SUN Chunmeng2,LUO Xuefang1*,ZHONG Wenying1**1Drug Quality Research Center,College of Science,China Pharmaceutical University;2School of Pharmacy,China Pharmaceutical University,ChinaAbstract2,6-dimethylbenzenamine was determined as a genotoxic impurity in lidocaine hydrochloride injec‐tion,and2-chloro-N-(2,6-dimethylphenyl)acetamide was determined as potential genotoxic impurity.An LC-MS/ MS method was established to research the profiling of genotoxic impurities in active pharmaceutical ingredients (API),homemade preparation and reference preparation on column Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm).The results show that in the homemade preparation the2,6-dimethylbenzenamine and the 2-chloro-N-(2,6-dimethylphenyl)acetamide may be degraded under oxidation condition and alkaline condition in addition to the introduction from API preparation process.This study provides guidance for genotoxic risk assess‐ment and prescription process optimization of lidocaine hydrochloride.Key words lidocaine hydrochloride injection;genotoxic impurities;LC-MS/MS盐酸利多卡因(lidocaine hydrochloride)为临床上常制成盐酸利多卡因注射剂应用于局部麻醉药[1]和抗心律失常药物等[2-3]。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
依地酸二钠美国药典34版标准

Edetate Disodium(ed' e tate dye soe' dee um).C10H14N2Na2O8·2H2O 372.24Glycine, N,N¢-1,2-ethanediylbis[N-(carboxymethyl)-, disodium salt, dihydrate.Disodium (ethylenedinitrilo)tetraacetate dihydrate [6381-92-6].Anhydrous 336.21 [139-33-3].» Edetate Disodium contains not less than 99.0 percent and not more than 101.0 percent of C10H14N2Na2O8, calculated on the dried basis.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Edetate Disodium RSIdentification—A: Infrared Absorption 197K: undried.B: To 5 mL of water in a test tube add 2 drops of ammonium thiocyanate TS and 2 drops of ferric chloride TS, and mix. To the deep red solution add about 50 mg of Edetate Disodium, and mix: the red color is discharged, leaving a yellowish solution.C: It responds to the flame test for Sodium 191.pH 791: between 4.0 and 6.0, in a solution (1 in 20).Loss on drying 731— Dry it at 150 for 6 hours: it loses not less than 8.7% and not more than 11.4% of its weight.Calc ium—To a solution (1 in 20) add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide. Add 3 N hydrochloric acid dropwise until the solution is just acid, and then add 1 mL of ammonium oxalate TS: no precipitate is formed.Heavy metals, Method II 231: 0.005%.Limit of nitrilotriacetic acid—Mobile phase— Add 10 mL of 1.0 M tetrabutylammonium hydroxide in methanol to 200 mL of water, and adjust with 1 M phosphoric acid to a pH of 7.5 ±0.1. Transfer the solution so obtained to a 1000-mL volumetric flask, add 90 mL of methanol, dilute with water to volume, mix, pass through a filter having a 0.5-µm or finer porosity, and degas.Cupric nitrate solution—Prepare a solution containing about 10 mg of cupric nitrate(Cu(NO3)2) per mL.Standard stock solution— Transfer about 100 mg of nitrilotriacetic acid, accurately weighed, to a 10-mL volumetric flask, add 0.5 mL of ammonium hydroxide, and mix. Dilute with water to volume, and mix.Resolution solution—Transfer 10 mg of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Standard solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Test solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Chromatographic system (see Chromatography 621)— The chromatograph is equipped witha 254-nm detector and a 4.6-mm × 15-cm column that contains packing L7. The flow rate is about2 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.35 for nitrilotriacetic acid, 0.65 for copper, and 1.0 for edetate; and the resolution, R, between nitrilotriacetic acid and copper is not less than 3. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The response of the nitrilotriacetic acid peak obtained from the Test solution does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard solution and the Test solution: not more than 0.1% of nitrilotriacetic acid is found.Assay—Assay preparation— Dissolve about 5 g of Edetate Disodium, accurately weighed, in about 100 mL of water contained in a 250-mL volumetric flask, add water to volume, and mix.Procedure— Place about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours, cooled in a desiccator, and accurately weighed, in a 400-mL beaker, add 10 mL of water, and swirl to form a slurry. Cover the beaker with a watch glass, and without removingthe latter, add 2 mL of 3 N hydrochloric acid from a pipet. Swirl the contents of the beaker, and dissolve the calcium carbonate. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring the solution, preferably with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 0.30 g of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in mg, of C10H14N2Na2O8 in the portion of Edetate Disodium taken by the formula:(336.21/100.09)W(VT /V)in which 336.21 and 100.09 are the molecular weights of edetate disodium and calcium carbonate, respectively; W is the weight, in mg, of calcium carbonate; VT is the volume, in mL, of the Assay preparation; and V is the volume, in mL, of the Assay preparation consumed in the titration.Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Elena Gonikberg, Ph.D.Principal Scientific Liaison1-301-816-8251 (SM32010) Monographs - Small Molecules 3Reference Standards RS Technical Services1-301-816-8129**************USP34–NF29 Page 2663Pharmacopeial Forum: V olume No. 32(4) Page 1070。
高效液相色谱法测定注射用美罗培南的有关物质

第35卷第3期 长治医学院学报2021 年 6 月JOURNAL OF CHANGZHI MEDICAI COLLEGE167Vol. 35 No. 3Jun. 2021高效液相色谱法测定注射用美罗培南的有关物质李金格禹玉洪**作者单位山西医科大学药学院药剂教研室(030001)* 通信作者(E-mail :3024546064@ qq. com)摘要目的:探讨优化注射用美罗培南杂质A 、B 的测定方法。
方法:运用高效液相色谱法(HPLC) 进行检测,色谱柱以十八烷基硅烷键合硅胶为填充剂;流动相A :20. 0 mmol-L'1磷酸二氢钠-甲醇(89 :11, V/V),流动相B :甲醇,流速1.0 mL-min 1,检测波长220 nm,柱温30 P 。
结果:主成分峰与杂质峰可实现基线分离,杂质A 检测限和定量限分别为1. 62,5.15 ng,杂质B 检测限和定量限分别为0. 85,2. 51 ng ;1.2~24.0 ixg-mL -1的杂质A 具有良好的线性关系(r=0. 999 9) ,0.7-14.0 ixg-mL 1的杂质B 具有良好的线性 关系(r=0. 999 9);杂质A 平均加样回收率为101.2%(RSD= 1.38%,“ = 9),杂质B 平均加样回收率为100.2%(RSD=1.29%,n = 9)o 经破坏性试验,美罗培南可能的降解杂质A 、B 均不干扰美罗培南主峰的测定。
结论:检测限及定量限、精密度、稳定性、耐用性试验结果均符合HPLC 有关物质测定的方法学验证要求。
本HPLC 法专属性良好,可用于美罗培南的主要杂质A 、B 的定量控制。
关键词美罗培南;有关物质;高效液相色谱法中图分类号R97&1文献标识码 A 文章编号1006(2021)03-167-05Determination of Related Substances of Meropenem for Injection by High Performance Liquid ChromatographyLI Jinge , YU YuhongDepartment of Pharmacy , School of Pharmacy , Shanxi Medical UniversityAbstract Objective : To explore and optimize the determination method of impulity A andB of meropenem for injection. Meth ods :Using the high performance liquid chromatography ( HPLC ) to detection , Octadecylsilane-bonded silica gel was used as the fi ler ; The mobile phase A : 20. 0 mmol * L -1 sodium dihydrogen phosphate-methanol ( 89 : 11, V/V) . The mobile phase B : methanol ,the flow rate was 1. 0 mL *m in _1 and the detection wavelength was set at 220 nm. The column temperature was set at 30 % . Re sults :The principal component peak and impurity peak could achieve baseline separation. The detection limit and quantitative limit of impurity A were 1. 62 ng and 5. 15 ng respectively , and the detection limit and quantitative lim 让 of impurity B were 0. 85 ng and 2. 51 ng respectively. There was A good linear relationship between impurity A (r = 0. 999 9) and impurity B ( r= 0. 999 9 ) in therange of 1. 2-24. 0 |xg *m L _1 and 0. 7 ~ 14. 0 jig * mL -1. The average recovery of impurity A was 101. 2% ( RSD = 1. 38% , n = 9),and that of impurity B was 100. 2% ( RSD = 1. 29% , n= 9). After stressing test, both of impurities A and B of meropenem didn * tinterfere w 让h the determination of meropenem main peak. Conclusion : The test results of detection lim 让 and quant N ative lim 让,pre cision ,stabil 让y and durability all meet the methodological verification requirements of HPLC related substance determination. The HPLC method has good specificity and can be used for the quant N ative control of major impurities A and B.Key words meropenem ; related substances ; HPLC注射用美罗培南(Meropenem, C ”H25弘0申) 是由日本住友制药公司与英国ICI 制药公司共同 开发的第二代碳青霉烯抗生素,通过干扰细菌细胞壁的合成发挥杀菌作用,具有广谱耐酶的特 点[1_4]o 在美罗培南原料中常检测出杂质A 及杂质B,杂质A(C 17H 27N 3O 6S)为美罗培南四元内酰 胺环结构发生水解反应而形成,系美罗培南的降 解产物;杂质B(C 34H 50N 6O 10S 2)为美罗培南与杂质A 发生聚合反应而形成,系美罗培南的二聚体X 。
西那卡塞杂质汇总
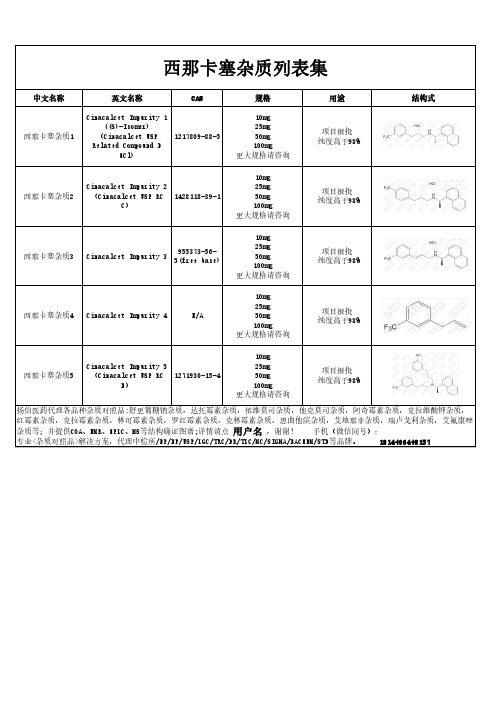
中文名称
英文名称
CAS
规格
西那卡塞杂质1
Cinacalcet Impurity 1 ((S)-Isomer)
(Cinacalcet USP 1217809-88-5 Related Compound D
HCl)
10mg 25mg 50mg 100mg 更大规格请咨询
用途
项目报批 纯度高于98%
B)
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
扬信医药代理各品种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质,他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质, 红霉素杂质,克拉霉素杂质,林可霉素杂质,罗红霉素杂质,克林霉素杂质,恩曲他滨杂质,艾地那非杂质,瑞卢戈利杂质,艾氟康唑
杂质等;并提供COA、NMR、HPLC、MS等结构确证图谱;详情请点 用户名 ,谢谢! 手机(微信同号):
专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌。 1814+064+3157
结构式
西那卡塞杂质2
Cinacalcet Impurity 2 (Cinacalcet USP RC 1428118-39-1
C)
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
955373-56西那卡塞杂质3 Cinacalcet Impurity 3
5(free base)
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
西那卡塞杂质4 Cinacalcet Impurity 4
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
GLP Bio产品说明书:Nystatin (Fungicidin) (GC10090)

Product Data Sheet Product Name:Nystatin (Fungicidin)Cat. No.:GC10090Chemical PropertiesCas No.1400-61-9化学名(4E,6E,8E,10E,14E,16E,18S,19R,20R,21S,35S)-3-[(2S,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,14,16-hexaene-38-carboxylic acidCanonical SMILES CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O )O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O分子式C47H75NO17分子量926.09溶解度≥ 30.45 mg/mL in DMSO储存条件-20°C, sealed storage, away from moisture and light,unstable in solution, ready to use.General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProtocolCell experiment [1]:Cell lines Oral Candida species and human buccal epithelial cellsPreparation method The solubility of this compound in DMSO is > 30.5 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months.Reacting condition 1 hrApplications The minimal inhibitory concentrations (μg/mL) of Nystatin for C. albicans, C. tropicalis, C. krusei, C. parapsilosis, C. glabrata and C. guilliermondii in RPMI broth were 0.78 ~ 1.56, 1.56 ~ 3.12, 3.12, 1.56 ~ 3.12, 0.78 ~ 1.56 and 0.39 ~ 0.78, respectively. Compared with the control group, Nystatin significantly reduced adhesion of 6 Candida species to buccal epithelial cells. However, the adhesion of C. albicans isolates was least affected by Nystatin treatment, which was significantly different from that of the non-albicans species.Animal experiment [2]:Animal models Aspergillus-infected, neutropenic mice Dosage form2, 4, 6 and 8 mg/kg/day; i.v.Product Data SheetApplications At a dose as low as 2 mg/kg/day, Liposomal Nystatin significantly protected neutropenic mice from Aspergillus-induced death compared to either the no-treatment, the saline or the empty-liposome group. Liposomal Nystatin-treated mice showed no evidence of Aspergillusinfection either at day 5 in all of the treatment groups or at day 52 in the 8 mg/kg/dayliposomal-Nystatin treatment group.Other notesPlease test the solubility of all compounds indoor, and the actual solubility may slightly differwith the theoretical value. This is caused by an experimental system error and it is normal.References:[1]. Ellepola AN, Panagoda GJ, Samaranayake LP. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol Immunol. 1999 Dec;14(6):358-63.[2]. Wallace TL, Paetznick V, Cossum PA, Lopez-Berestein G, Rex JH, Anaissie E. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob Agents Chemother. 1997Oct;41(10):2238-43.BackgroundNystatin (Fungicidin) is a polyene antifungal antibiotic [1].Antifungal antibiotic is a pharmaceutical fungicide used to treat and prevent mycoses.Nystatin is a polyene antifungal antibiotic that is effective against yeast and mycoplasma [1]. In liquid media,Nystatin inhibited C. albicans at concentrations of 5-20 U/ml[2].In a 200 clinical isolates, which comprised of 113 Candida albicans, 54 Candida glabrata, 11 Candida parapsilosis, 11Candida tropicalis and 11 Candida krusei. Nystatin exhibited MIC90 value of 4 mg/L against C. albicans isolates and all non-albicans Candida species tested. The results confirmed C. Albicans was most frequently susceptible andNystatin could be used to treat vulvovaginal candidiasis caused by non-albicans Candida species. Nystatin would be an important choice for women affected by non-albicans Candida species which present higher resistance to the imidazole-based treatments [3].制霉菌素(Fungicidin )是一种多烯类抗真菌抗生素[1]。
水落坡药品基本信息
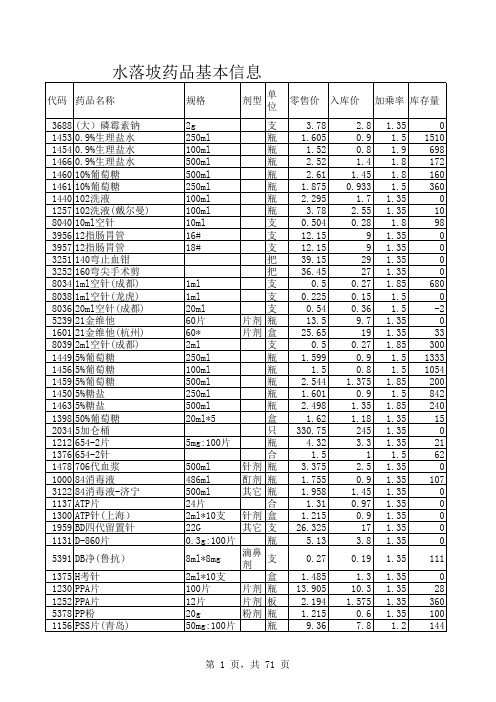
0.38 31.185 5.4 4.455 7.56 1.148 1.58 1.32 6.75 1.431 1.89 3.105 3.915 3.713 21.195 15.525 3.105 2.768 2.7 15.755 4.72 3.375 4.59 8.37 1.89 9.18 3.105 3.375 2.16 10.598 5.4 14.85 19.575 3.78 3.105 2.835 6.075 4.05 4.995 3.51 6.075 1.823 1.98 1.08 1.013 2.025 2.565
10支 1ml*10mg 0.25g*12t 20李*0.25g 0.25g*10粒 250mg*10粒 0.25g*10粒
0.25g*10s*2b
针剂 片剂 胶囊 胶囊 胶囊
颗粒 125mg*12代 0.6 0.6g-海口 0.6g 0.2285g6袋 20t 1g 1g 0.5g*12s 100片 6t*0.25g 0.1g*6 0.1g*4d 0.25g 0.1g*3包 0.25g*6t
粉剂
片剂 片剂 粉剂
针剂 粉剂 片剂
6粒* 0.25g*6 0.25G*10粒 0.1g*18d 0.1G*6D 0.1g*6包 粉剂 0.1g*6d 0.25g 25mg*6 0.25g*6t 250 0.25g 0.25g 25mg*100片 25mg 25mg*60t
片剂 片剂 针剂
片剂
g 盒 盒 盒 盒 板 板 板 盒 板 盒 合 支 支 支 盒 合 支 支 盒 瓶 盒 盒 盒 包 支 合 盒 盒 盒 盒 盒 盒 盒 盒 盒 盒 支 盒 合 瓶 支 支 瓶 瓶 瓶 瓶
3.78 3.78 3.78 1.215 1.62 1.62 1.02 1.208 1.17 0.81 1.2 0.945 1.971 1.08 0.878 2.16 0.9 0.945 0.975 2.1 1.013 1.92 2.025 1.89 4.32 2.16 2.295 26.865 8.64 2.12 4.32 1.688 1.89 2.4 2.805 2.43 1.188 8.033 2.295 10.125 6.75 7.425 2.538 1.418 5.13 607.5 297
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
大肠埃希氏菌鉴定试剂盒

大肠埃希氏菌干制生化鉴定试剂盒E.coli Dehydration Biochemical Identification Kit大肠埃希氏菌干制生化鉴定试剂盒组成:干制生化鉴定试剂:4种/盒×10 盒;0.5麦氏浊度比浊管:1支;配套试剂:Kovacs氏靛基质试剂1瓶、甲基红试剂1瓶、V-P甲、乙液试剂各1瓶; 使用说明书:1份实验操作步骤: 1. 从铝箔袋中取出试剂盒,打开盒盖,在试剂盒的长条槽中加入0.5mL无菌水;2. 用接种环从平板上挑取新鲜培养的单个纯菌落至适量无菌水中,制成0.5麦氏浊度的均一菌悬液;3. 接种菌悬液分别至试剂盒的4个圆孔中,每孔接种量0.2mL,盖上盒盖,36℃±1℃培养24h。
结果判定:保存条件和保质期:室温避光保存1年。
其他相关试剂盒:名称用途及用法大肠埃希氏菌干制生化鉴定试剂盒E.coli Dehydration Biochemical Identification Kit 用于大肠杆菌的IMVC 生化系统鉴定(GB4789.38-2012),包括蛋白胨水、MR、VP和西蒙氏枸橼酸盐共 4 种生化鉴定试验和配套试剂(靛基质、甲基红、VP甲乙液)阪崎肠杆菌干制生化鉴定试剂盒Enterobacter sakazakii Dehydration Biochemical Identification Kit 用于阪崎肠杆菌的生化鉴定(GB4789.40-2010),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、精氨酸双水解酶、西蒙氏柠檬酸盐、D-山梨醇、L-鼠李糖、D-蜜二糖、D-蔗糖和苦杏仁苷共10种生化鉴定试验和配套试剂(无菌石蜡)志贺氏菌干制生化鉴定试剂盒Shigella Dehydration Biochemical Identification Kit 用于志贺氏菌的生化鉴定(GB4789.5-2012),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、靛基质、尿素、水杨苷、七叶苷、甘露醇、棉子糖、ONPG、甘油、葡萄糖铵、西蒙氏柠檬酸盐、粘液酸对照、粘液酸、三糖铁、半固体共17 种生化鉴定试验和配套试剂(无菌石蜡、靛基质)单增李斯特氏菌干制生化鉴定试剂盒Listeria Monocytogenes Dehydration Biochemical Identification Kit 用于单增李斯特氏菌的生化鉴定(GB4789.30-2010),包括MR、VP、葡萄糖、麦芽糖、鼠李糖、木糖、甘露醇、七叶苷共8 种生化鉴定试验和配套试剂(糖发酵添加剂、甲基红、VP 甲乙液)沙门氏菌干制生化鉴定试剂盒Salmonella Dehydration Biochemical Identification Kit 用于沙门氏菌的生化鉴定(GB4789.4-2010),包括氨基酸对照、赖氨酸脱羧酶、氰化钾对照、氰化钾、靛基质、尿素、甘露醇、山梨醇、ONPG、三糖铁共10 种生化鉴定试验和配套试剂(无菌石蜡、靛基质)大肠埃希氏菌O157:H7干制生化鉴定试剂盒E.coli O157:H7 Dehydration Biochemical Identification Kit 用于大肠埃希氏菌O157:H7/NM的生化鉴定(GB/T4789.36-2008),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、MR、VP、靛基质、西蒙氏柠檬酸盐、纤维二糖、棉子糖、三糖铁、半固体共11种生化鉴定试验和配套试剂(无菌石蜡、靛基质、甲基红、VP甲乙液)蜡样芽孢杆菌干制生化鉴定试剂盒Bacillus cereus Dehydration Biochemical Identification Kit 用于蜡样芽孢杆菌的生化鉴定(GB 4789.14-2014),包括葡萄糖、VP、硝酸盐、明胶、动力培养基(蜡样)、甘露醇、西蒙氏柠檬酸盐、溶菌酶肉汤共8种生化鉴定试验和配套试剂(无菌石蜡、硝酸盐还原甲乙液、VP 甲乙液)副溶血性弧菌干制生化鉴定试剂盒Vibrio parahaemolyticus Dehydration Biochemical 用于副溶血性弧菌的生化鉴定(GB 4789.7-2013),包括无盐胨水、6% 盐胨水、8% 盐胨水、10% 盐胨水、葡萄糖、蔗糖、乳糖、Identification Kit 甘露醇、氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、精氨酸双水解酶、VP、ONPG、3%NaCl三糖铁、3%NaCl半固体共16种生化鉴定试验和配套试剂(无菌石蜡、糖发酵添加剂、VP甲乙液)致泻大肠埃希氏菌干制生化鉴定试剂盒Diarrheogenic E.coli Dehydration Biochemical Identification Kit 用于致泻大肠埃希氏菌的生化鉴定(GB/T4789.6- 2003),包括氨基酸对照、赖氨酸脱羧酶、氰化钾对照、氰化钾、靛基质、尿素、三糖铁、半固体共8种生化鉴定试验和配套试剂(无菌石蜡、靛基质)小肠结肠炎耶尔森氏菌干制生化鉴定试剂盒Yersinia enterocolitica Dehydration Biochemical Identification Kit 用于小肠结肠炎耶尔森氏菌的生化鉴定(GB/T 4789.8-2008),包括氨基酸对照、鸟氨酸脱羧酶、VP、蔗糖、鼠李糖、棉子糖、甘露醇、山梨醇、尿素、半固体共10 种生化鉴定试验和配套试剂(无菌石蜡、糖发酵添加剂、VP 甲乙液)。
二氟尼柳美国药典

Diflunisal Tablets» Diflunisal Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of C13H8F2O3.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Diflunisal RS.Identification—A: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation, obtained as directed in the Assay.B: Transfer a quantity of finely ground Tablets, equivalent to about 100 mg of diflunisal, to a 10-mL volumetric flask, add 2 mL of water, and sonicate for 5 minutes. Dilute with methanol to volume, sonicate for an additional 5 minutes, mix, and filter. Separately apply 10 µL each of the filtrate and a Standard solution of USP Diflunisal RS in methanol solution (4 in 5) containing 10 mg per mL to a thin-layer chromatographic plate (see Chromatography 62) coated with a 0.25-mm layer of chromatographic silica gel mixture.Develop the chromatogram in a solvent system consisting of n-hexane, glacial acetic acid, and chloroform (17:3:2) until the solvent front has moved aboutthree-fourths of the length of the plate. Remove the plate from the chamber, air-dry, and examine under long-wavelength UV light: the RF value of the principal spot in the chromatogram of the test solution corresponds to that obtained from the Standard solution.Dissolution 71—pH 7.20, 0.1 M Tris buffer— Dissolve 121 g of tris (hydroxymethyl) aminome thane (THAM) in 9 liters of water. Adjust the solution with a 7 in 100 solution of anhydrous citric acid in water to a pH of 7.45, at 25. Dliters, equilibrate to 37, a H of 7.20, if necessary.Medium: pH 7.20, 0.1 M Tris buffer; 900 mL.Apparatus 2: 50 rpm.Time: 30 minutes.Procedure— Determine the amount of C13H8F2O3 dissolved from UV absorbances at the wavelength of maximum absorbance at about 306 nm of filtered portions of the solution under test, suitably diluted with pH 7.20, 0.1 M Tris buffer, in comparison with a Standard solution having a known concentration of USP Diflunisal RS in the same Medium.Tolerances— Not less than 80% (Q) of the labeled amount of C13H8F2O3 is dissolved in 30 minutes.Uniformity of dosage units 90: mProcedure for content uniformity—Transfer 1 finely powdered Tablet to a200-mL volumetric flask, add 50 mL of water, shake by mechanical means for 30 minutes, and sonicate for 2 minutes. Add 100 mL of alcohol to the flask, shake by mechanical means for 15 minutes, and sonicate for 2 minutes. Dilute with alcohol to volume, mix, and centrifuge a portion of the solution. Quantitatively dilute an accurately measured volume of the resultant clear supernatant with alcohol, if necessary, to obtain a test solution containing about 1.25 mg per mL. Transfer about 125 mg of USP Diflunisal RS, accurately weighed, to a 100-mL volumetric flask, add 75 mL of alcohol to dissolve, dilute with water to volume, and mix to obtain the Standard solution. Transfer 3.0 mL each of the Standard solution and the test solution to separate 50-mL volumetric flasks. To each flask add 5.0 mL of a solution containing 1 g of ferric nitrate in 100 mL of 0.08 N nitric acid, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions at the wavelength of maximum absorbance at about 550 nm, with a suitable spectrophotometer, using water as the blank. Calculate the quantity, in mg, of C13H8F2O3 in the Tablet by the formula:(TC / D)(AU / AS)in which T is the labeled quantity, in mg, of diflunisal in the Tablet; C is the concentration, in µg per mL, of USP Diflunisal RS in the Standard solution; D is the concentration, in µg per mL, of diflunisal in the test solution, based uponthe labeled quantity per Tablet and the extent of dilution; and AU and AS are the absorbances of the solutions from the test solution and the Standard solution, respectively.Assay—Mobile phase—Prepare a suitable degassed mixture of water, methanol, acetonitrile, and glacial acetic acid (45:40:17:6) such that the retention time of diflunisal is about 8 minutes.Standard preparation— Dissolve a suitable quantity of USP Diflunisal RS in a mixture of acetonitrile and water (60:40) to obtain a solution having a known concentration of about 1.0 mg per mL.Assay preparation—Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 100 mg of diflunisal, to a 100-mL volumetric flask containing about 5 mL of water. Sonicate for 5 minutes, add 60.0 mL of acetonitrile, sonicate for an additional 5 minutes, dilute with water to volume, mix, and filter.Chromatographic system (see Chromatography 62)—The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1.The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor for the analyte peak is not more than 2.0, and the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of diflunisal (C13H8F2O3) in the portion of Tablets taken by the formula:100C(rU / rS)in which C is the concentration, in mg per mL, of USP Diflunisal RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.。
化妆品中地氯雷他定等51种原料的检验方法2023年

附件17化妆品中地氯雷他定等51种原料的检验方法Determination of desloratadine and other50kinds of components in cosmetics1范围本方法规定了采用高效液相色谱-质谱法测定化妆品中的地氯雷他定等51种原料,包括定性与定量。
本方法适用于膏霜乳液类、液态水基类、液态油基类、凝胶类、面膜类、粉类、蜡基类等化妆品中地氯雷他定等51种原料的定性与定量。
2方法提要样品以10mmol/L乙酸铵甲醇为溶剂提取,采用高效液相色谱仪分离,质谱检测器检测,根据保留时间和特征离子对的相对丰度比定性,定量离子对峰面积定量,以标准曲线法计算含量。
本方法对地氯雷他定等51种原料的检出限均为1ng/mL,定量下限均为2ng/mL,如以取样品0.2g,定容体积50mL计,检出浓度均为0.250μg/g,最低定量浓度均为0.500μg/g。
3试剂和材料除另有规定外,本方法所用试剂均为分析纯或以上规格,水为GB/T6682规定的一级水。
3.1甲醇,色谱纯。
3.2乙酸铵,色谱纯。
3.310mmol/L乙酸铵溶液:称取乙酸铵(3.2)0.77g,加水1000mL溶解,用0.22μm滤膜过滤。
3.410mmol/L乙酸铵甲醇溶液:称取乙酸铵(3.2)0.77g,加甲醇(3.1)1000mL溶解,摇匀。
3.5标准储备溶液分别称取地氯雷他定等51种原料的标准品(附表1)10mg(精确到0.00001g)置于10mL棕色容量瓶中,加甲醇(3.1)使溶解并定容至刻度,摇匀,即得质量浓度为1.0mg/mL 的标准储备溶液。
置于-18℃冰箱中贮存。
3.6混合标准储备溶液分别精密移取各待测组分标准储备溶液(3.5)1.0mL于100mL棕色容量瓶中,用甲醇(3.1)稀释并定容至刻度,作为混合标准储备液。
置于-18℃冰箱中贮存。
4仪器和设备4.1液相色谱-三重四极杆质谱联用仪。
4.2分析天平。
