Update on Drug-Drug Interactions A Scientific Perspective药物间相互作用的科学的角度,更新
药物英语期末试题及答案

药物英语期末试题及答案一、选择题(每题2分,共20分)1. Which of the following is the correct English term for "抗生素"?A. AntibioticB. AntisepticC. AntitoxinD. Antivenom2. The term "药物代谢" in English is translated as:A. Drug metabolismB. Drug synthesisC. Drug absorptionD. Drug distribution3. The abbreviation "FDA" stands for:A. Federal Drug AdministrationB. Food and Drug AdministrationC. Federal Dietary AdministrationD. Food and Dietary Administration4. The process of "药物吸收" is known in English as:A. AbsorptionB. MetabolismC. ExcretionD. Distribution5. The term "药物相互作用" is translated into English as:A. Drug interactionB. Drug reactionC. Drug combinationD. Drug synergy6. Which of the following is the correct translation for "药物副作用"?A. Drug side effectB. Drug adverse effectC. Drug secondary effectD. Drug negative effect7. The abbreviation "OTC" refers to:A. Over The CounterB. On The CounterC. Out The CounterD. Off The Counter8. The term "药物耐受性" in English is:A. Drug toleranceB. Drug resistanceC. Drug dependenceD. Drug sensitivity9. The process of "药物排泄" is known in English as:A. ExcretionB. EliminationC. SecretionD. Ejection10. The term "药物剂量" is translated into English as:A. Drug dosageB. Drug amountC. Drug quantityD. Drug volume二、填空题(每空2分,共20分)11. The English term for "药物制剂" is __________.Answer: Pharmaceutical formulation12. The abbreviation "NDC" stands for __________.Answer: National Drug Code13. "药物过敏反应" is translated into English as __________. Answer: Drug allergy reaction14. The process of "药物作用机制" is known in English as__________.Answer: Mechanism of drug action15. The term "药物依赖性" is translated into English as__________.Answer: Drug dependence16. The abbreviation "IV" in medical terms refers to__________.Answer: Intravenous17. "药物处方" in English is __________.Answer: Drug prescription18. The process of "药物筛选" is known in English as__________.Answer: Drug screening19. The term "药物不良反应" is translated into English as__________.Answer: Adverse drug reaction20. The abbreviation "BID" stands for __________.Answer: Twice a day三、简答题(每题10分,共20分)21. Explain the difference between "Drug metabolism" and "Drug elimination".Answer: Drug metabolism refers to the process by whichthe body breaks down and modifies a drug into more easily excretable forms. Drug elimination, on the other hand, is the process by which the body removes the drug or its metabolites from the body, typically through the kidneys, liver, or lungs.22. What is the significance of "Drug-drug interactions" in clinical practice?Answer: Drug-drug interactions occur when two or more drugs affect each other's action or effectiveness. These interactions can lead to increased or decreased effectiveness, increased side effects, or even toxicity, which is why theyare significant in clinical practice to ensure patient safety and the effectiveness of treatment.四、论述题(每题15分,共40分)23. Discuss the importance of understanding "Drug resistance" in the context of antimicrobial therapy.Answer: Understanding drug resistance is crucial in antimicrobial therapy as it helps in the appropriate selection of antibiotics to prevent the development of resistant strains. It also guides the development of new antimicrobial agents and informs treatment strategies to combat infections caused by resistant pathogens.24. Elaborate on the role of "Pharmacovigilance" in ensuring patient safety.Answer: Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. It plays a vital role in ensuring patient safety by monitoring the safety profile of marketed drugs, identifying risks, and taking appropriate regulatory actions to minimize harm to patients.五、翻译题(每题5分,共20分)25. Translate the following sentence into English: "药物的剂量应根据患者的具体情况来调整。
药学英语翻译

1.每一种药物都有其固有的药理作用特点。
如果给药剂量,给药次数,给药途径恰当,大多数病人可以产生预期的药理效应。
但对具体的病人来说,药理效应可有一定的甚至是非常明显的差异。
病人体质,药物质量,病原微生物,以及各种环境条件都可能影响药物作用。
它们可以使药物效应减弱或增强。
产生个体差异的主要原因是药物的吸收,分布,生物转化和排泄的差异。
要保证每个病人都能达到最大疗效、最小不良反应的治疗目的,单纯根据药理作用选药和用药显然是不够的,还必须掌握影响各种药物的因素,结合病人具体情况,决定适当的治疗方案,并在用药过程中不断根据变化及时适当地作出调整,直至病人痊愈。
Each medicine has its inherent the pharmacological function characteristics. If the administration dosage, to therapy, delivery methods appropriate, most patients can produce the desired pharmacological effect. But to specific patients for, pharmacological effect can have certain even very clear differences. Patients constitution, drug quality, pathogenic microbes, and various environmental conditions may affect drug interactions. They can make the effects of drugs increase or decrease. Produce individual difference is the main reason of the drug absorption, distribution, biological transformation and excretion of differences. To ensure that every patient can achieve maximum efficacy, minimum adverse reaction of therapeutic purposes, pure according to choose medicine and pharmacology drug it is not enough, still must master the influence of the factors of different drugs, patients with specific situations, to determine the appropriate treatment, and in the process of drug use in time according to change constantly adjust properly, until the patient recover.2、构成的解决方案干燥固体颗粒从这些正是构成方案准备注射熊的标题形式(药品注射。
药物相互作用

苯妥英钠 利福平 氨基比林 灰黄霉素
一些例子: 一些例子: 癫痫患儿长期服用苯巴比妥与苯妥英钠易出现佝偻病 癫痫患儿长期服用苯巴比妥与苯妥英钠易出现佝偻病,因 佝偻病, 为二药均有酶诱导作用,提高维生素D的代谢率, 为二药均有酶诱导作用,提高维生素D的代谢率,影响钙 的吸收,因此应注意补充维生素D 的吸收,因此应注意补充维生素D。 服用泼尼松已经控制哮喘发作的患者, 服用泼尼松已经控制哮喘发作的患者,在加服苯巴比妥之 哮喘发作次数增加, 后,哮喘发作次数增加,可能是苯巴比妥增加泼尼松的代 降低其浓度使疗效降低。 谢,降低其浓度使疗效降低。 器官移植患者应用免疫抑制剂环孢菌素和泼尼松, 器官移植患者应用免疫抑制剂环孢菌素和泼尼松,利福平 的酶诱导作用增加上述二药的代谢灭活,使机体出现排斥 的酶诱导作用增加上述二药的代谢灭活,使机体出现排斥 反应; 反应; 合用利福平可使口服避孕药失效 避孕药失效。 合用利福平可使口服避孕药失效。 在个别情况下,药物被代谢转化为毒性代谢物, 在个别情况下,药物被代谢转化为毒性代谢物,如异烟肼 产生肝毒性代谢物,若与卡马西平合用, 产生肝毒性代谢物,若与卡马西平合用,后者酶诱导作用 将加重异烟肼的肝毒性 毒性。 将加重异烟肼的肝毒性。 日常生活中的一些化学物质,如有机氯、多氯联苯、 日常生活中的一些化学物质,如有机氯、多氯联苯、油炸 食品等也具有药物代谢酶的诱导作用,若与药物同用时, 食品等也具有药物代谢酶的诱导作用,若与药物同用时, 可加速药物的代谢。 可加速药物的代谢。
(一) pH对药物吸收的影响 pH对药物吸收的影响 - pH与解离度 、脂溶性的关系 pH与解离度 - 弱酸性药物水杨酸类、磺胺类、巴比 弱酸性药物水杨酸类、磺胺类、 妥类等与抗酸药同时服用, 妥类等与抗酸药同时服用,就会增加 弱酸性药物的解离度,减少吸收, 弱酸性药物的解离度,减少吸收,使 血药浓度降低,药物的疗效下降。 血药浓度降低,药物的疗效下降。
药理学与药学专业SCI杂志影响因子排序

2 ANNU REV PHARMA COL 22.4683 PHARMA COL REV 174 DRUG RESIST UPDATE 12.5815 ADV DRUG DELIVE R REV 11.9576 TRENDS PHARMA COL SCI 9.0647 PHARMA COL THERAP EUT 8.8978 MED RES REV 8.6569 CURR OPIN PHARMACOL 7.25910 NEUROP SYCHO PHARM ACOL 6.99311 CLIN PHARMACOL THER 6.96112 DRUG DISCOV TODAY 6.6313 REV PHYSIO L BIOCHP 6.2514 J CONTRO L RELEASE 5.94915 DRUG METAB REV 5.43916 MOL PHARMA CEUT 5.40817 NEUROT HERAP EUTIC S 5.38118 CARDIO VASCDRUG REV 5.20819 BRIT J PHARMACOL 5.20420 J CLIN PSYCHO PHARM 5.09221 CNS DRUG REV 4.92322 CURR OPIN DRUG DISC 4.90423 INT J NEUROP SYCHO PH 4.87424 ANTIMI CROBAGENTS CH 4.80225 DRUGS 4.73226 CURR MED CHEM 4.70827 CLIN PHARMA COKIN ET 4.5628 MOL PHARMA COL 4.53129 CURR PHARMDESIGN 4.41430 PHARMA COGEN OMICS J 4.39831 ALIMEN T PHARMTHER 4.35732 J ANTIMI CROBCHEMOT H 4.35233 ANTIVI R THER 4.32234 BIOCHE M PHARMA COL 4.25435 EXPERT OPIN INV DRUG 4.21836 PSYCHO PHARM ACOLO GY 4.10337 J PHARMA COL EXP THER 4.09338 PHARMA COGEN ET GENOM 3.99139 CURR DRUG METAB 3.98940 PHARMRES-DORDR 3.93342 PHARMA COL RES 3.92943 NEUROP HARMA COLOG Y 3.90944 PHARMA COGEN OMICS 3.89345 CNS DRUGS 3.87947 EXPERT OPIN THER TAR 3.71348 EUR NEUROP SYCHO PHARM 3.68449 J PSYCHO PHARM ACOL 3.64750 ANTIVI R RES 3.61251 CNS NEUROL DISORD-DR 3.57152 CURR OPIN INVEST DR 3.54953 AAPS J 3.5454 DRUG SAFETY 3.52255 ALTERN MED REV 3.51556 BIODRU GS 3.50657 REV BRAS FARMAC OGN 3.46258 J CLIN PHARMA COL 3.44259 CURR PHARMBIOTEC HNO 3.40460 TOXICO L APPL PHARM 3.35961 EXPERT OPIN DRUG DEL 3.345 61 INT CLIN PSYCHO PHARM 3.34563 J NEUROI MMUNE PHARM 3.31964 CRIT REV THER DRUG 3.30865 CLIN THER 3.2566 BRIT J CLIN PHARMACO 3.24667 TOXICO LOGY 3.24168 CHEMME DCHEM 3.23269 J NAT PROD 3.15970 EUR J PHARM*** 3.15171 EXPERT OPIN DRUG MET 3.07672 INVEST NEW DRUG 3.07273 INT J IMMUNO PATHPH 3.06174 INT J ANTIMI CROBAG 3.03275 QSAR COMB SCI 3.02776 CURR VASC PHARMA COL 2.9777 PHARMA COL BIOCHE M BE 2.96778 INT J PHARMA CEUT 2.96279 NEUROT OXICO LOGY 2.91880 J PHARMSCI-US 2.90681 J DRUG TARGET 2.88582 EXPERT REV ANTI-INFE 2.85783 BEHAVPHARMACOL 2.85484 J CARDIO VASCPHARM 2.82685 PROG NEURO-PSYCHO PH 2.82386 RECENT PAT ANTI-CANC 2.82287 XENOBI OTICA 2.79988 EUR J CLIN PHARMA COL 2.74389 CARDIO VASCTHER 2.74191 ASSAYDRUG DEV TECHN 2.71391 EXP CLIN PSYCHO PHARM 2.71393 PEPTID ES 2.70594 CNS NEUROSCI THER 2.6995 CHIRAL ITY 2.67796 CANCER CHEMOT H PHARM 2.65497 N-S ARCH PHARMA COL 2.63198 INT J NANOME D 2.61298 PHARMA COECO NOMIC S 2.612 100 EUR J PHARMSCI 2.608 101 J CHILDADOL PSYCHOP 2.59102 EUR J PHARMA COL 2.585 103 LIFE SCI 2.56104 DRUG METABPHARMACOK 2.544 105 PHARMA COEPI DEM DR S 2.527 106 CARDIO VASCDRUG THER 2.515 107 EXPERT OPIN DRUG SAF 2.496 107 HUM PSYCHO PHARM CLIN 2.496 109 COMB CHEM HIGH T SCR 2.464 110 CHEM-BIOL INTERA CT 2.457111 ANN PHARMA COTHE R 2.453 111 J PHARMA CEUTBIOMED 2.453 113 THER DRUG MONIT 2.429 114 J MANAGE CARE PHARM 2.412 115 ALCOHO L 2.407 115 EXPERT OPIN EMERGDR 2.407 117 FUND CLIN PHARMA COL 2.372 118 CLIN NEUROP HARMA COL 2.349 119 J ETHNOP HARMA COL 2.322120 PHARMA COPSY CHIAT RY 2.317 121 BASICCLIN PHARMA COL 2.308 122 BIOMED PHARMA COTHE R 2.238 123 ANTI-CANCER DRUG 2.23124 INT IMMUNO PHARM ACOL 2.214 125 DRUG AGING 2.209126 J PHARMA COL SCI 2.176 127 PHYTOM EDICI NE 2.174 128 MOL DIAGNTHER 2.167 129 TOXICO N 2.128130 SKIN PHARMA COL PHYS 2.117 131 DRUG NEWS PERSPE CT 2.101 132 AM J HEALTH-SYST PH 2.097133 PHARMA COL REP 2.086135 VASC PHARMA COL 2.044 136 PLANTA MED 2.037137 CHEMOT HERAPY 2.028 138 PULM PHARMACOL THER 2.024 139 EXPERT OPIN PHARMACO 2.018 140 MICROB DRUG RESIST 1.989 141 AM J CARDIO VASCDRUG 1.964 142 PHARMSTAT 1.957143 CLIN EXP PHARMA COL P 1.936 144 IDRUGS 1.932145 J MICROE NCAPS UL 1.89 146 J CARDIO VASCPHARMT 1.871 147 PHARMA COLOG Y 1.833 148 BIOL PHARMBULL 1.81149 REGULTOXICO L PHARM 1.798 150 J LIPOSO ME RES 1.792 151 ARCH PHARM 1.785152 ACTA PHARMA COL SIN 1.783 153 PHYTOT HER RES 1.746 154 J PHARMPHARMA COL 1.742 155 CURR NEUROP HARMA COL 1.731 156 CHEM PHARMBULL 1.698 157 J CLIN PHARMTHER 1.671158 BIOMED CHROMA TOGR 1.639 159 J ANTIBI OT 1.628160 HIV CLIN TRIALS 1.626 161 J PHARMPHARMSCI 1.619162 DRUG TODAY 1.588163 ANNU REP MED CHEM 1.526 164 CONTEM P CLIN TRIALS 1.506 165 J CLIN LIPIDOL 1.462166 J OCUL PHARMA COL TH 1.457 167 CANCER BIOTHE R RADIO 1.443 168 CLIN DRUG INVEST 1.414169 DRUG DELIV 1.413170 J VET PHARMA COL THER 1.408 171 BIOLOG ICALS 1.381171 INT J CLIN PHARMTH 1.381173 FITOTE RAPIA 1.363174 DRUGS R。
《药剂学专业英语》PPT课件

被动语态的广泛应用
优::The work was carried out on the Imperial College gas atomizer,which was described in detail elsewhere . 劣:We carried out this work on the Imperial College gas atomizer,which was described in detail elsewhere.
பைடு நூலகம்
指标词的运用
This article reviews recent advances in our understanding of the structure,drug interaction mechanism…….. In view of its wide localization ….. of P-gp,and its ATP-dependent outward-oriented transport, P-gp actively participates in intestinal secretion,bloodtissue barriers,and …………... Moreover,the importance of P-gpmediated drug interactions in clinical practice can hardly be underestimated,since it may result in severe side effects,such as digitalis drug interaction. Polymorphism or single nucleotide polymorphism (SNP)associated with P-gp may exert a significant effect on the pharmacokinetic behavior of its substrates,a fact ……..In addition,dietary components and pharmaceutical excipients may modulate P-gp activity,and as a result affect in vivo drug disposition and therapeutic efficacy;examples include grapefruit juice,Pluronic P85,PEG 300,etc..In summary,it should be emphasized that P-gp is an integral component in the process of drug discovery,development strategy,and clinical therapy.
SCI收录的药学期刊

2006年在美国科学出版社(Science Press,USA)出版的中国科学与技术进展(The proceedings of the China association for science and technology)中发表《输液反应与预防措施研究》论文正2009年12月9日,王辰、李兴旺教授等代表中国甲型流感(H1N1)临床专家组于《新英格兰医学杂志》在线发表了《中国2009大流行甲型流感(H1N1)病毒感染最初病例的临床特征》一文。
SCI收录的药学期刊SCI收录的药学期刊(1) CHEMISTRY, MEDICINAL (26) 1. ANNUAL REPORTS IN MEDICINAL CHEMIS-TRY Annual ISSN:0065-7743 ACADEMIC PRESS INC, 525 B STREET,SUITE 1900, SAN DIEGO, CA, 921-1-4495 2. ANTI-CANCER DRUG DESIGN Bimonthly ISSN: 0266-9536 OXFORD UNIV PRESS, GREAT CLARENDON ST, OXFORD, ENGLAND, OX2 6DP 3. ARCHIV DER PHARMAZIE Monthly ISSN:0365-6233 WILEY-V C H VERLAG GMBH, MUHLENS-TRASSE 33-34, BERLIN, GERMANY, D-13187 4. ARZNEIMITTEL-FORSCHUNG-DRUG RESEARCH Monthly ISSN: 0004-4172 ECV-EDITIO CANTOR VERLAG MEDIZIN NA TURWISSENSCHAFTEN, BANDELSTOCKWEG 2-,POSTFACH 1255, AULENDORF, GERMANY, D-88322 5. BIOORGANIC & MEDICINAL CHEMISTRY Bimonthly ISSN: 0968-0896 PERGAMON-ELSEVIER SCIENCE LTD, THE BOULEV ARD, LANGFORD LANE,KIDLINGTON, OXFORD, ENGLAND, OX5 1GB 6. BIOORGANIC & MEDICINAL CHEMISTRY LETTERS Semimonthly ISSN: 096-0894X PERGAMON-ELSEVIER SCIENCE LTD, THE BOULEV ARD, LANGFORD LANE,KIDLINGTON, OXFORD, ENGLAND, OX5 1GB 7. CHEMICAL & PHARMACEUTICAL BULLETIN Monthly ISSN: 0009-2363 PHARMACEUTICAL SOC JAPAN, 2-12-15-2-1 SHIBUY A,SHIBUY A-KU, TOKYO, JAPAN, 150 8. CHEMICAL RESEARCH IN TOXICOLOGY Monthly ISSN: 0893-228X AMER CHEMICAL SOC, 1155 16TH ST, NW, WASHINGTON, DC, 20036 9. CHEMICO-BIOLOGICAL INTERACTIONS Monthly ISSN: 0009-2797 ELSEVIER SCI IRELAND LTD, CUSTOMER RELATIONS MANAGER,BAY 15, SHANNON INDUSTRIALESTA TE COCLARE, IRELAND SCI收录的药学期刊(1) CHEMISTRY, MEDICINAL (26) 10. CHIRALITY Bimonthly ISSN: 0899-0042 WILEY-LISS, DIV JOHN WILEY & SONS INC, 6-5 THIRD A VE, NEW YORK, NY, 10158-0012 11. CURRENT MEDICINAL CHEMISTRY Monthly ISSN: 0929-8673 BENTHAM SCIENCE PUBL BV, PO BOX 1673, HILVERSUM, NETHERLANDS, 1200 BR 12. DRUG DEVELOPMENT AND INDUSTRIAL PHARMACY Monthly ISSN: 0363-9-45 MARCEL DEKKER INC, 270 MADISON A VE, NEW YORK, NY, 10016 13. DRUG DEVELOPMENT RESEARCH Monthly ISSN: 0272-4391 WILEY-LISS, DIV JOHN WILEY & SONS INC,6-5 THIRD AVE, NEW YORK, NY, 10158-0012 14. EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY Monthly ISSN: 0223-5234 EDITIONS SCIENTIFIQUES MEDICALES ELSEVIER, 23 RUE LINOIS, PARIS CEDEX 15, FRANCE, 75724 15. JOURNAL OF COMBINATORIAL CHEMISTRY Bimonthly ISSN: 1520-4766 AMER CHEMICAL SOC, 1155 16TH ST, NW, WASHINGTON, DC, 20036 16. JOURNAL OF LABELLED COMPOUNDS & RADIOPHARMACEUTICALS Monthly ISSN: 0362-4803 JOHN WILEY& SONS LTD, BAFFINS LANE, CHICHESTER, W SUSSEX, ENGLAND, PO19 1UD 17. JOURNAL OF MEDICINAL CHEMISTRY Semimonthly ISSN: 0022-2623 AMER CHEMICAL SOC, 1155 16TH ST, NW, W ASHINGTON, DC, 20036 SCI收录的药学期刊(1) CHEMISTRY, MEDICINAL (26) 18. JOURNAL OF NATURAL PRODUCTS Monthly ISSN: 0163-3864 AMER CHEMICAL SOC, 1155 16TH ST, NW, W ASHINGTON, DC, 20036 19. JOURNAL OF PHARMACEUTICAL SCIENCES Monthly ISSN: 0022-3549 AMER PHARMACEUTICAL ASSN, 2215 CONSTITUTION A VE NW, WASHINGTON, DC, 20037 20. MEDICINAL RESEARCH REVIEWS Quarterly ISSN: 0198-6325 JOHN WILEY & SONS INC, 605 THIRD A VE, NEW YORK, NY, 10158-0012 21. NA TURAL PRODUCT REPORTS Bimonthly ISSN: 0265-0568 ROYAL SOC CHEMISTRY, THOMAS GRAHAM HOUSE,SCIENCE PARK,MILTON RD,CAMBRIDGE, ENGLAND, CB4 -WF 22. PERSPECTIVES IN DRUG DISCOVERY AND DESIGN Triennial ISSN: 0928-2866 KLUWER ACADEMIC PUBL, SPUIBOU-LEVARD 50,PO BOX 17, DORDRECHT, NETHERLANDS, 3300 AA 23. PHARMAZIE Monthly ISSN: 0031-7144 GOVI-VERLAG GMBH, PHARMAZEU-TISCHER VERLAG, GINNHEIMER STRASSE 26, ESCHBORN, GERMANY, D-65760 24. PHYTOTHERAPY RESEARCH Bimonthly ISSN: 0951-418X JOHN WILEY & SONS LTD, BAFFINS LANE, CHICHESTER, W SUSSEX, ENGLAND, PO19 1UD 25. PLANTA MEDICA Bimonthly ISSN: 0032-0943 GEORG THIEME VERLAG, P O BOX 30 11 20, STUTTGART, GERMANY, D-70451 26. QUANTITATIVE STRUCTURE-ACTIVITY RELATIONSHIPS Quarterly ISSN: 0931-8771 WILEY-V C H VERLAG GMBH, MUHLENSTRASSE 33-34, BERLIN, GERMANY, D-13187。
泊沙康唑 美国说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NOXAFIL ORAL SUSPENSION safely and effectively. See full prescribing information for NOXAFIL ORAL SUSPENSION. NOXAFIL® (Posaconazole) ORAL SUSPENSION 40 mg/mLInitial U.S. Approval: 2006---------------------------INDICATIONS AND USAGE----------------------------- NOXAFIL is a triazole antifungal agent indicated for: •prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk ofdeveloping these infections due to being severelyimmunocompromised, such as HSCT recipients with GVHD orthose with hematologic malignancies with prolonged neutropenia from chemotherapy. (1.1)•the treatment of oropharyngeal candidiasis (OPC), including OPC refractory (rOPC) to itraconazole and/or fluconazole. (1.2)-------------------------DOSAGE AND ADMINISTRATION---------------------- Indication Dose and Duration of therapyProphylaxis of Invasive Fungal Infections 200 mg (5 mL) three times a day. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.1)Oropharyngeal Candidiasis (OPC) Loading dose of 100 mg (2.5 mL) twice a day on the first day, then 100 mg (2.5 mL) once a day for 13 days. (2.1)OPC Refractory(rOPC) to Itraconazole and/or Fluconazole 400 mg (10 mL) twice a day. Duration of therapy should be based on the severity of the patient’s underlying disease and clinical response. (2.1)-----------------------DOSAGE FORMS AND STRENGTHS-------------------- NOXAFIL Oral Suspension 40 mg per mL (3)-----------------------------CONTRAINDICATIONS--------------------------------- • Do not administer to persons with known hypersensitivity to posaconazole, any component of NOXAFIL, or other azoleantifungal agents (4.1)• Do not coadminister NOXAFIL with the following drugs;NOXAFIL increases concentrations of:o Sirolimus: can result in sirolimus toxicity (4.2, 7.1)o CYP3A4 substrates (pimozide, quinidine): can result inQTc interval prolongation and rare occurrences of TdP(4.3, 7.2)o Simvastatin: can result in rhabdomyolysis (4.4,7.3)o Ergot alkaloids: can result in ergotism (4.5, 7.4)-----------------------WARNINGS AND PRECAUTIONS------------------------ •Calcineurin Inhibitor Toxicity: NOXAFIL increasesconcentrations of cyclosporine or tacrolimus; reduce dose ofcyclosporine and tacrolimus and monitor concentrationsfrequently. (5.1)•Arrhythmias and QTc Prolongation: NOXAFIL has beenshown to prolong the QTc interval and cause rareoccurrences of TdP. Administer with caution to patients withpotentially proarrhythmic conditions. Do not administer withdrugs known to prolong QTc interval and metabolizedthrough CYP3A4. Correct K+, Mg++, and Ca++ before startingNOXAFIL. (5.2)•Hepatic Toxicity: elevations in LFTs (generally reversible ondiscontinuation) may occur. Discontinuation should beconsidered in patients who develop abnormal LFTs ormonitor LFTs during treatment. (5.3)● Midazolam: NOXAFIL can prolong hypnotic/sedative effects.Monitor patients and benzodiazepine receptor antagonistsshould be available. (5.4, 7.5)-------------------------------ADVERSE REACTIONS------------------------------•Common treatment-emergent adverse reactions (>30%) inprophylaxis studies are fever, diarrhea and nausea.(6.2)• Common treatment-emergent adverse reactions (>5%) incontrolled OPC pool are diarrhea, nausea, headache, andvomiting. Common adverse reactions (>20%) in the refractoryOPC pool are fever, diarrhea, nausea, and vomiting (6.2).To report SUSPECTED ADVERSE REACTIONS, contact ScheringCorporation, a subsidiary of Merck & Co., Inc., at 1-800-526-4099or FDA at 1-800-FDA-1088 or /medwatch.-------------------------------DRUG INTERACTIONS------------------------------Interaction Drug InteractionRifabutin, phenytoin, efavirenz,cimetidine, esomeprazoleAvoid co-administration unlessthe benefit outweighs the risks(7.6, 7.7, 7.8, 7.9)Other drugs metabolized byCYP3A4 (tacrolimus,cyclosporine, vinca alkaloids,calcium channel blockers)Consider dosage adjustment andmonitor for adverse effects andtoxicity (7.1,7.10, 7.11)Digoxin Monitor digoxin plasmaconcentrations (7.12)Metoclopramide Monitor for breakthrough fungalinfections (7.13)--------------------------USE IN SPECIFIC POPULATIONS---------------------• Pregnancy: Based on animal data, may cause fetal harm. (8.1)• Nursing Mothers: Discontinue drug or nursing, taking in toconsideration the importance of drug to the mother. (8.3)• Severe renal impairment: Monitor closely for breakthrough fungalInfections. (8.6)See 17 for PATIENT COUNSELING INFORMATION.Revised:10/2011FULL PRESCRIBING INFORMATION: CONTENTS*1.INDICATIONS AND USAGE1.1 Prophylaxis of Invasive Aspergillus and Candida Infections1.2 Treatment of Oropharyngeal Candidiasis IncludingOropharyngeal Candidiasis Refractory to Itraconazoleand/or Fluconazole2.DOSAGE AND ADMINISTRATION2.1 Dosage2.2 Administration Instructions3.DOSAGE FORMS AND STRENGTHS4.CONTRAINDICATIONS4.2 Use With Sirolimus4.3 QT Prolongation With Concomitant Use With CYP3A4Substrates4.4 Use With Simvastatin4.5 Use With Ergot Alkaloids5.WARNINGS AND PRECAUTIONS5.1 Calcineurin-Inhibitor Drug Interactions5.2Arrhythmias and QT Prolongation5.3 Hepatic Toxicity5.4 Use With Midazolam6.ADVERSE REACTIONS6.1Serious and Otherwise Important Adverse Reactions6.2Clinical Trials Experience6.3 Postmarketing Experience7.DRUG INTERACTIONS7.1 Immunosuppressants Metabolized by CYP3A47.2 CYP3A4 Substrates7.3 HMG-CoA reductase Inhibitors (Statins) Metabolized ThroughCYP3A47.4 Ergot Alkaloids7.5 Benzodiazepines Metabolized by CYP3A47.6 Anti-HIV Drugs7.7 Rifabutin7.8 Phenytoin7.9 Gastric Acid Suppressors/Neutralizers7.10 Vinca Alkaloids7.11 Calcium Channel Blockers Metabolized by CYP3A47.12 Digoxin7.13 Gastrointestinal Motility Agents7.14 GlipizideE IN SPECIFIC POPULATIONS8.1Pregnancy8.3Nursing Mothers8.4Pediatric Use8.5Geriatric Use8.6Renal Insufficiency8.7Hepatic Insufficiency8.8Gender8.9Race10.OVERDOSAGE11.DESCRIPTION12.CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics12.4 Microbiology13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2Animal Toxicology and/or Pharmacology14. CLINICAL STUDIES14.1Prophylaxis of Aspergillus and Candida Infections14.2Treatment of Oropharyngeal Candidiasis14.3Treatment of Oropharyngeal Candidiasis Refractory toTreatment With Fluconazole or Itraconazole16.HOW SUPPLIED/STORAGE AND HANDLING17.PATIENT COUNSELING INFORMATION17.1 Administration With Food17.2 Drug Interactions17.3 Serious and Potentially Serious Adverse Reactions17.4See Accompanying FDA-Approved Patient Labeling* Sections or subsections omitted from the full prescribing information are not listed.________________________________________________________FULL PRESCRIBING INFORMATION1. INDICATIONS AND USAGE1.1 Prophylaxis of Invasive Aspergillus and Candida InfectionsNOXAFIL Oral Suspension is indicated for prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy.1.2 Treatment of Oropharyngeal Candidiasis Including Oropharyngeal Candidiasis Refractory to Itraconazole and/or FluconazoleNOXAFIL is indicated for the treatment of oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole.2. DOSAGE AND ADMINISTRATION2.1DosageIndication Dose and Duration of TherapyProphylaxis of Invasive Fungal Infections 200 mg (5 mL) three times a day. The duration of therapy is based on recovery from neutropenia or immunosuppression.Oropharyngeal Candidiasis Loading dose of 100 mg (2.5 mL) twice a day on the first day, then100 mg (2.5 mL) once a day for 13 days.Oropharyngeal Candidiasis Refractory to itraconazole and/or fluconazole 400 mg (10 mL) twice a day. Duration of therapy should be based on the severity of the patient’s underlying disease and clinical response.2.2Administration InstructionsShake NOXAFIL Oral Suspension well before use.Figure 1: A measured dosing spoon is provided, marked for doses of 2.5 mL and 5 mL.It is recommended that the spoon is rinsed with water after each administration and before storage.Each dose of NOXAFIL should be administered with a full meal or with a liquid nutritional supplement or an acidic carbonated beverage (e.g. ginger ale) in patients who cannot eat a full meal.To enhance the oral absorption of posaconazole and optimize plasma concentrations:•Each dose of NOXAFIL should be administered during or immediately (i.e. within 20 minutes) following a full meal. In patients who cannot eata full meal, each dose of NOXAFIL should be administered with a liquid nutritional supplement or an acidic carbonated beverage. For patientswho cannot eat a full meal or tolerate an oral nutritional supplement or an acidic carbonated beverage, alternative antifungal therapy should be considered or patients should be monitored closely for breakthrough fungal infections.•Patients who have severe diarrhea or vomiting should be monitored closely for breakthrough fungal infections.•Co-administration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections [see Drug Interactions(7.6, 7.7, 7.8, 7.9, 7.13)].3. DOSAGE FORMS AND STRENGTHSNOXAFIL Oral Suspension is available in 4-ounce (123 mL) amber glass bottles with child-resistant closures (NDC 0085-1328-01) containing105 mL of suspension (40 mg of posaconazole per mL).4. CONTRAINDICATIONS4.1HypersensitivityNOXAFIL is contraindicated in persons with known hypersensitivity to posaconazole, any component of NOXAFIL, or other azole antifungal agents.4.2 Use With SirolimusNOXAFIL is contraindicated with sirolimus. Concomitant administration of NOXAFIL with sirolimus increases the sirolimus blood concentrations by approximately 9 fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].4.3 QT Prolongation With Concomitant Use With CYP3A4 SubstratesNOXAFIL is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of NOXAFIL with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and rare occurrences of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].4.4 Use With SimvastatinConcomitant administration of NOXAFIL with simvastatin increases the simvastatin plasma concentrations by approximately 10 fold. Increased plasma statin concentrations can be associated with rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].4.5 Use With Ergot AlkaloidsPosaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)].5. WARNINGS AND PRECAUTIONS5.1 Calcineurin-Inhibitor Drug InteractionsConcomitant administration of NOXAFIL with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including isolated deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus or cyclosporine dose adjusted accordingly.5.2 Arrhythmias and QT ProlongationSome azoles, including posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, rare cases of torsades de pointes have been reported in patients taking posaconazole.Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18-85 years of age) administered posaconazole 400 mg BID with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc (F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc (F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered posaconazole had a QTc (F) interval ≥500 msec or an increase ≥60 msec in their QTc (F) interval from baseline.Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)]. Rigorous attempts to correct potassium, magnesium, and calcium should be made before starting posaconazole.5.3 Hepatic ToxicityHepatic reactions (e.g.,mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver function tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption and rarely required drug discontinuation. Isolated cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with posaconazole. These severe hepatic reactions were seen primarily in subjects receiving the 800 mg daily (400 mg BID or 200 mg QID) in clinical trials.Liver function tests should be evaluated at the start of and during the course of posaconazole therapy. Patients who develop abnormal liver function tests during posaconazole therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver function tests and bilirubin). Discontinuation of posaconazole must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to posaconazole.5.4 Use With MidazolamConcomitant administration of NOXAFIL with midazolam increases the midazolam plasma concentrations by approximately 5 fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].6. ADVERSE REACTIONS6.1 Serious and Otherwise Important Adverse ReactionsThe following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling: • Hypersensitivity[see Contraindications (4.1)]•Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)][see Warnings and Precautions (5.3)]• HepaticToxicity6.2 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of NOXAFIL cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.The safety of posaconazole therapy has been assessed in 1844 patients in clinical trials. This includes 605 patients in the active-controlled prophylaxis studies, 557 patients in the active-controlled OPC studies, 239 patients in refractory OPC studies, and 443 patients from other indications. Thisrepresents a heterogeneous population, including immunocompromised patients, e.g., patients with hematological malignancy, neutropenia post-chemotherapy, graft vs. host disease post hematopoietic stem cell transplant, and HIV infection, as well as non-neutropenic patients. This patient population was 71% male, had a mean age of 42 years (range 8-84 years, 6% of patients were ≥65 years of age and 1% was <18 years of age), and were 64% white, 16% Hispanic, and 36% non-white (including 14% black). Posaconazole therapy was given to 171 patients for ≥6 months, with 58 patients receiving posaconazole therapy for ≥12 months. Table 1 presents treatment-emergent adverse reactions observed at an incidence of >10% in posaconazole prophylaxis studies. Table 2 presents treatment-emergent adverse reactions observed at an incidence of at least 10% in the OPC/rOPC studies.Prophylaxis of Aspergillus and Candida: In the 2 randomized, comparative prophylaxis studies, the safety of posaconazole 200 mg threetimes a day was compared to fluconazole 400 mg once daily or itraconazole 200 mg twice a day in severely immunocompromised patients.The most frequently reported adverse reactions (>30%) in the prophylaxis clinical trials were fever, diarrhea and nausea.The most common adverse reactions leading to discontinuation of posaconazole in the prophylaxis studies were associated with GI disorders,specifically, nausea (2%), vomiting (2%), and hepatic enzymes increased (2%).TABLE 1: Study 1 and Study 2. Number (%) of Randomized Subjects Reporting Treatment-Emergent Adverse Reactions: Frequency of at Least 10% in the Posaconazole or Fluconazole Treatment Groups (Pooled Prophylaxis Safety Analysis)Body SystemPreferred Term Posaconazole(n=605)Fluconazole(n=539)Itraconazole(n=58)Subjects Reporting any Adverse Reaction 595 (98) 531 (99) 58 (100) Body as a Whole - General DisordersFever 274 (45) 254 (47) 32 (55) Headache 171 (28) 141 (26) 23 (40) Rigors 122 (20) 87 (16) 17 (29) Fatigue 101 (17) 98 (18) 5 (9) Edema Legs 93 (15) 67 (12) 11 (19) Anorexia 92 (15) 94 (17) 16 (28) Dizziness 64 (11) 56 (10) 5 (9) Edema 54 (9) 68 (13) 8 (14) Weakness 51 (8) 52 (10) 2 (3) Cardiovascular Disorders, GeneralHypertension 106 (18) 88 (16) 3 (5) Hypotension 83 (14) 79 (15) 10 (17) Disorders of Blood and Lymphatic SystemAnemia 149 (25) 124 (23) 16 (28) Neutropenia 141 (23) 122 (23) 23 (40) Febrile Neutropenia 118 (20) 85 (16) 23 (40) Disorders of the Reproductive System and BreastVaginal Hemorrhage* 24 (10) 20 (9) 3 (12) Gastrointestinal System DisordersDiarrhea 256 (42) 212 (39) 35 (60) Nausea 232 (38) 198 (37) 30 (52) Vomiting 174 (29) 173 (32) 24 (41) Abdominal Pain 161 (27) 147 (27) 21 (36) Constipation 126 (21) 94 (17) 10 (17) Mucositis NOS 105 (17) 68 (13) 15 (26) Dyspepsia 61 (10) 50 (9) 6 (10) Heart Rate and Rhythm DisordersTachycardia 72 (12) 75 (14) 3 (5) Infection and InfestationsBacteremia 107 (18) 98 (18) 16 (28) Herpes Simplex 88 (15) 61 (11) 10 (17) Cytomegalovirus Infection 82 (14) 69 (13) 0Pharyngitis 71 (12) 60 (11) 12 (21)Upper Respiratory Tract Infection 44 (7) 54 (10) 5 (9)Liver and Biliary System DisordersBilirubinemia 59 (10) 51 (9) 11 (19)Metabolic and Nutritional DisordersHypokalemia 181 (30) 142 (26) 30 (52)Hypomagnesemia 110 (18) 84 (16) 11 (19)Hyperglycemia 68 (11) 76 (14) 2 (3)Hypocalcemia 56 (9) 55 (10) 5 (9)Musculoskeletal System DisordersMusculoskeletal Pain 95 (16) 82 (15) 9 (16)Arthralgia 69 (11) 67 (12) 5 (9)Back Pain 63 (10) 66 (12) 4 (7)Platelet, Bleeding and Clotting DisordersThrombocytopenia 175 (29) 146 (27) 20 (34)Petechiae 64 (11) 54 (10) 9 (16)Psychiatric DisordersInsomnia 103 (17) 92 (17) 11 (19)Anxiety 52 (9) 61 (11) 9 (16)Respiratory System DisordersCoughing 146 (24) 130 (24) 14 (24)Dyspnea 121 (20) 116 (22) 15 (26)Epistaxis 82 (14) 73 (14) 12 (21)Skin and Subcutaneous Tissue DisordersRash 113 (19) 96 (18) 25 (43)Pruritus 69 (11) 62 (12) 11 (19)* Percentages of sex-specific adverse reactions are based on the number of males/females.NOS = not otherwise specified.HIV Infected Subjects With OPC: In 2 randomized comparative studies in OPC, the safety of posaconazole at a dose of ≤400 mg QD in 557 HIV-infected patients was compared to the safety of fluconazole in 262 HIV-infected patients at a dose of 100 mg QD.An additional 239 HIV-infected patients with refractory OPC received posaconazole in 2 non-comparative trials for refractory OPC (rOPC).Of these subjects, 149 received the 800-mg/day dose and the remainder received the ≤400-mg QD dose.In the OPC/rOPC studies, the most common adverse reactions were fever, diarrhea, nausea, headache, and vomiting.The most common adverse reactions that led to treatment discontinuation of posaconazole in the Controlled OPC Pool included respiratory insufficiency (1%) and pneumonia (1%). In the refractory OPC pool, the most common adverse reactions that led to treatment discontinuation of posaconazole were AIDS (7%) and respiratory insufficiency (3%).TABLE 2: Treatment-Emergent Adverse Reactions With Frequency of at Least 10% in OPC Studies (Treated Population)Number (%) of SubjectsControlled OPC Pool Refractory OPC PoolPosaconazole Fluconazole PosaconazoleBody SystemPreferred Term n=557 n=262 n=239356 (64) 175 (67) 221 (92)Subjects Reporting any AdverseReaction*Body as a Whole – General DisordersFever 34 (6) 22 (8) 82 (34)Headache 44 (8) 23 (9) 47 (20)Anorexia 10 (2) 4 (2) 46 (19)Fatigue 18 (3) 12 (5) 31 (13)Asthenia 9 (2) 5 (2) 31 (13)Rigors 2 (<1) 4 (2) 29 (12)Pain 4 (1) 2 (1) 27 (11)Disorders of Blood and Lymphatic SystemNeutropenia 21 (4) 8 (3) 39 (16)Anemia 11 (2) 5 (2) 34 (14)Gastrointestinal System DisordersDiarrhea 58 (10) 34 (13) 70 (29)Nausea 48 (9) 30 (11) 70 (29)Vomiting 37 (7) 18 (7) 67 (28)Abdominal Pain 27 (5) 17 (6) 43 (18)Infection and InfestationsCandidiasis, Oral 3 (1) 1 (<1) 28 (12)Herpes Simplex 16 (3) 8 (3) 26 (11)Pneumonia 17 (3) 6 (2) 25 (10)Metabolic and Nutritional DisordersWeight Decrease 4 (1) 2 (1) 33 (14)Dehydration 4 (1) 7 (3) 27 (11)Psychiatric DisordersInsomnia 8 (1) 3 (1) 39 (16)Respiratory System DisordersCoughing 18 (3) 11 (4) 60 (25)Dyspnea 8 (1) 8 (3) 28 (12)Skin and Subcutaneous Tissue DisordersRash 15 (3) 10 (4) 36 (15)Sweating Increased 13 (2) 5 (2) 23 (10)OPC=oropharyngeal candidiasis; SGOT=serum glutamic oxaloacetic transaminase (same as AST);SGPT=serum glutamic pyruvic transaminase (same as ALT).* Number of subjects reporting treatment-emergent adverse reactions at least once during the study,without regard to relationship to treatment. Subjects may have reported more than 1 event.Adverse reactions were reported more frequently in the pool of patients with refractory OPC. Among these highly immunocompromised patients with advanced HIV disease, serious adverse reactions (SARs) were reported in 55% (132/239). The most commonly reported SARs were fever (13%) and neutropenia (10%).Less Common Adverse Reactions: Clinically significant adverse reactions reported during clinical trials in prophylaxis, OPC/rOPC or other trials with posaconazole which occurred in less than 5% of patients are listed below:•Blood and lymphatic system disorders: hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, neutropenia aggravated •Endocrine disorders: adrenal insufficiency•Nervous system disorders: paresthesia•Immune system disorders: allergic reaction [see Contraindications (4.1)]•Cardiac disorders: Torsades de pointes [see Warnings and Precautions (5.2)]•Vascular disorders: pulmonary embolism•Liver and Biliary System Disorders: bilirubinemia, hepatic enzymes increased, hepatic function abnormal, hepatitis, hepatomegaly, jaundice, SGOT Increased, SGPT Increased•Metabolic and Nutritional Disorders: Hypokalemia•Platelet, Bleeding, and Clotting Disorders: Thrombocytopenia•Renal & Urinary System Disorders: Renal Failure AcuteClinical Laboratory Values: In healthy volunteers and patients, elevation of liver function test values did not appear to be associated with higher plasma concentrations of posaconazole. The majority of abnormal liver function tests were minor, transient, and did not lead to discontinuation of therapy.For the prophylaxis studies, the number of patients with changes in liver function tests from Common Toxicity Criteria (CTC) Grade 0, 1, or 2 at baseline to Grade 3 or 4 during the study is presented in Table 3.TABLE 3: Study 1 and Study 2. Changes in Liver Function Test Results From CTC Grade 0, 1, or 2 at Baseline to Grade 3 or 4Number (%) of Patients With Change*Study 1Laboratory Parameter Posaconazolen=301Fluconazolen=299AST 11/266 (4) 13/266 (5)Number (%) of Patients With Change*ALT 47/271 (17) 39/272 (14) Bilirubin 24/271 (9) 20/275 (7) Alkaline Phosphatase 9/271 (3) 8/271 (3)Study 2Laboratory Parameter Posaconazole(n=304)Fluconazole/Itraconazole(n=298)AST 9/286 (3) 5/280 (2)ALT 18/289 (6) 13/284 (5)Bilirubin 20/290 (7) 25/285 (9)Alkaline Phosphatase 4/281 (1) 1/276 (<1)* Change from Grade 0 to 2 at baseline to Grade 3 or 4 during the study.These data are presented in the form X/Y, where X represents the numberof patients who met the criterion as indicated, and Y represents the numberof patients who had a baseline observation and at least one post-baselineobservation.CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase;ALT= Alanine Aminotransferase.The number of patients treated for OPC with clinically significant liver function test (LFT) abnormalities at any time during the studies is provided in Table 4. (LFT abnormalities were present in some of these patients prior to initiation of the study drug).TABLE 4: Clinically Significant Laboratory Test Abnormalities Without Regard to Baseline ValueControlled RefractoryPosaconazole Fluconazole PosaconazoleLaboratory Test n= 557(%) n=262(%) n=239(%)ALT > 3.0 x ULN 16/537 (3) 13/254 (5) 25/226 (11)AST > 3.0 x ULN 33/537 (6) 26/254(10)39/223 (17)Total Bilirubin > 1.5 x ULN 15/536 (3) 5/254 (2) 9/197 (5)Alkaline Phosphatase > 3.0 x ULN 17/535 (3) 15/253 (6) 24/190 (13)ALT= Alanine Aminotransferase; AST= Aspartate Aminotransferase.6.3 Postmarketing ExperienceNo clinically significant postmarketing adverse reactions were identified that have not previously been reported during clinical trials experience.7. DRUG INTERACTIONSPosaconazole is primarily metabolized via UDP glucuronidation and is a substrate of p-glycoprotein efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)].7.1 Immunosuppressants Metabolized by CYP3A4Sirolimus: Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9 fold and can result in sirolimus toxicity. Therefore, posaconazole is contraindicated with sirolimus [see Contraindications (4.2) and Clinical Pharmacology (12.3)].Tacrolimus: Posaconazole has been shown to significantly increase the C max and AUC of tacrolimus. At initiation of posaconazole treatment, reduce the tacrolimus dose to approximately one-third of the original dose. Frequent monitoring of tacrolimus whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].Cyclosporine: Posaconazole has been shown to increase cyclosporine whole blood concentrations in heart transplant patients upon initiation of posaconazole treatment. It is recommended to reduce cyclosporine dose to approximately three-fourths of the original dose upon initiation of posaconazole treatment. Frequent monitoring of cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the cyclosporine dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].7.2 CYP3A4 SubstratesConcomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and rare occurrences of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs. [see Contraindications (4.3), and Warnings and Precautions (5.2)].。
决奈达隆片说明书

update where exists 和update where in 使用案例(关联表更新)

update where exists 和update where in 使用案例(关联表更新)使用where in 或where exists更新数据当使用exists 语句时,如下update table1 a set a.col1 ='test' where exists (select 1 from table2 b where a.col2=b.col2 )以上语句exist() 表示是否查出记录,只要记录存在,即不为0 ,则更新,其中的1无关紧要,换成* 也可,所以语句效率较高in 查询表示完全匹配才能执行更新,效率较低,如下例:update table1 a set a.clo1='test' where a.col2 in (select b.col3 from table2 b where b.col4='condition')-- 下面讲述根据药品名称和药品库存更新药品价表的例子。
要求是只要药品名称中含有vv字符并且库存为0的药品一律停价,药品名称如‘vv青霉素针’,具体步骤-- 查询原始数据select a.* from drug_price_list a where (a.drug_code,a.drug_spec,a.units,a.firm_id) in( select distinct c.drug_code,c.drug_spec,c.units,d.firm_id from drug_price_list b,drug_dict c,drug_stock d where b.drug_code=c.drug_code and b.drug_code=d.drug_code and b.drug_spec=c.drug_spec andb.drug_spec=d.drug_spec and b.units=c.units and b.units=d.units and b.firm_id=d.firm_id andc.drug_name like 'vv%' andd.quantity <=0 and b.stop_date is null ) and a.stop_date is null ;select * from drug_dict a where (a.drug_code,a.drug_spec,a.units) in( select distinct c.drug_code,c.drug_spec,c.units from drug_price_list b,drug_dict c,drug_stock d where b.drug_code=c.drug_code and b.drug_code=d.drug_code and b.drug_spec=c.drug_spec andb.drug_spec=d.drug_spec and b.units=c.units and b.units=d.units and b.firm_id=d.firm_id andc.drug_name like 'vv%' andd.quantity <=0 and b.stop_date is null ) ;select * from drug_stock a where (a.drug_code,a.drug_spec,a.units,a.firm_id) in( select distinct c.drug_code,c.drug_spec,c.units,d.firm_id from drug_price_list b,drug_dict c,drug_stock d where b.drug_code=c.drug_code and b.drug_code=d.drug_code and b.drug_spec=c.drug_spec andb.drug_spec=d.drug_spec and b.units=c.units and b.units=d.units and b.firm_id=d.firm_id andc.drug_name like 'vv%' andd.quantity <=0 and b.stop_date is null ) ;*//*select * from price_list a where a.item_name like 'vv%' and stop_date is null ;*/-- 备份原始数据/*create table price_list_20140930 as select * from price_list;*/-- 比较备份数据/*select count(*) from price_list_20140930;select count(*) from price_list ;*/-- 更新药品价格数据/*update drug_price_list b set b.stop_date = to_date('2014-09-30','yyyy-mm-dd') where(b.drug_code,b.drug_spec,b.units,b.firm_id) in( select c.drug_code,c.drug_spec,c.units,d.firm_id from drug_dict c,drug_stock d whereb.drug_code=c.drug_code and b.drug_code=d.drug_code and b.drug_spec=c.drug_spec andb.drug_spec=d.drug_spec and b.units=c.units and b.units=d.units and b.firm_id=d.firm_id andc.drug_name like 'vv%' andd.quantity <=0 )and b.stop_date is null;*/-- 注意一定要要查看更新行数和前面的查询数据一致才可提交,否则会造成严重后果。
药物相互作用

第一节 药动学的相互作用
药动学方面的相互作用-类别
根据发生部位和机制不同:
1. 影响药物吸收的相互作用; 2. 影响药物分布的相互作用; 3. 影响药物代谢的相互作用; 4. 影响药物排泄的相互作用。
第一节 药动学的相互作用
二、影响药物分布的相互作用
1. 竞争血浆蛋白结合部位
2. 改变组织分布量
A
受变药 甲
靶 位
B
更高血浆蛋 白结合率
促变药 乙
游离药物
游离药物
血 浆
白蛋白
A
药物竞争蛋白结合部位 单独给甲药 B 甲药+乙药
血浆蛋白结合率变化的后果
血浆蛋白结合率大于85%的 不良反应后果严重 血浆蛋白结合率小于85%的 不良反应后果相对较轻 分布容积小,引起严重不良反应的 几率高,如华法林 分布容积大,引起严重不良反应的 几率相对较小,如苯妥因钠
• 胃排空、肠蠕动速率→药物到达小肠吸收 部位和小肠内滞留时间→起效和持效时间 • 影响胃肠运动的药物可引发DI。
第一节 药动学的相互作用
影响药物吸收的相互作用
胃肠运动的影响
促变药 受变药 增加胃肠道运动药 联用药物 甲氧氯普胺 西沙必利 泻药 减弱胃肠道运动药 联用药物 抗胆碱药(阿托
品、溴丙胺太林等)
相互作用
加快胃排空,缩短 小肠滞留时间; 起效快,吸收不全
起效慢,吸收完全
去甲丙咪嗪
第一节 药动学的相互作用
影响药物吸收的相互作用-方式
1. 离子作用;
2. 吸附作用;
3. pH的影响; 4. 食物的影响; 5. 胃肠道运动的影响; 6. 肠吸收功能; 7. 其他。
第一节 药动学的相互作用
药物代谢性相互作用

U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
药物代谢性相互作用
Metabolic Drug-Drug Interaction
1
问题的提出
复方制剂,都是选择作用彼此增 强、相互抵销或减少不良反应的原则 配伍组成。
现代治疗很少使用单一药物、几 乎都是少则2~3种,多则6~7种同时 应用,难免发生药物相互作用。
2
近几年,致死性药物相互作用时有报道, 三唑仑与阿米替林,氟西汀与氯氮平,喷
被置换出的药物的分布容积小于015lkg2525june2019june强力结合药被置换药结果降血糖药保泰松水杨酸类香豆素抗凝血药凝血时间延苯妥英钠长出血乙胺嘧啶奎宁奎宁毒性增强速尿磺胺类甲氨喋呤毒性增强水杨酸类2626june2019june20193代谢性药物相互作用代谢性药物相互作用metabolicdruginteraction是指两种或两种以上药物在同时或前后序贯用药时在代谢环节产生作用的干扰结果使疗效增强甚至产生毒副作用或疗效减弱甚至治疗失败
June 1, 2019
14
二、药效学的相互作用
Pharmacodynamic Drug Interactions Additive, synergistic, or antagonistic effects from co-administration of two or more drugs
临床上常见的药品交互作用(Drug-DrugInteractionsDDIs)
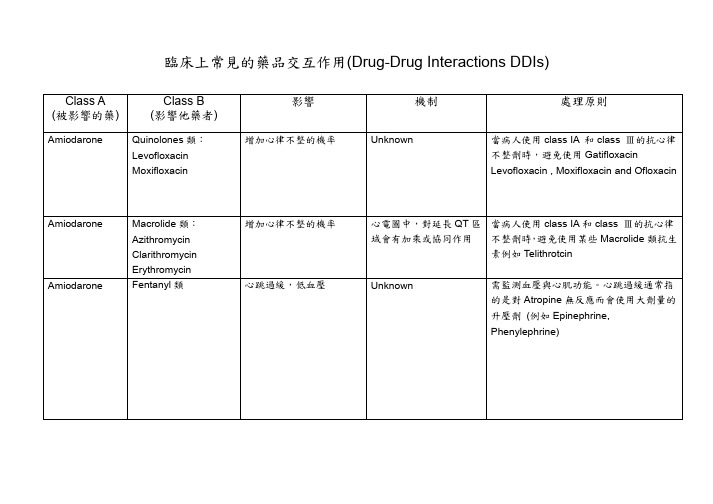
Digoxin Digoxin Digoxin
Digoxin
Paroxetine
Propafenone Tetracyclines: Minocycline Tetracycline
Verapamil
增加 Digoxin 濃度和 Digoxin 的毒性風險
增加 Digoxin 濃度和 Digoxin 的毒性風險
血小板活性會降低
經由 CYP2C19 代謝成活 Ranitidine)或許是個較安全的替代藥品
性代謝物
增加 Digoxin 濃度和 Digoxin 的毒性風險,尤其 以 Erythromycin 的影響時 間可以持續好幾週
Macrolide 類抗生素可以 抑制腎小管 P-glycoprotein 分泌 Digoxin(會受基因的變異 影響)
素例如 Telithrotcin
Unknown
需監測血壓與心肌功能。心跳過緩通常指 的是對 Atropine 無反應而會使用大劑量的 升壓劑 (例如 Epinephrine, Phenylephrine)
Carbamazepine Clopidogrel
Digoxin Digoxin
Macrolide Antibiotic 類: Clarithromycin Erythromycin PPI (Proton pump Inhibitor)類: Omeprazole Esomeprazole Lansoprazole Pantoprazole Raerprazole
Macrolide Antibiotic 類: Azithromycin Clarithromycin Erythromycin Thiazide Diuretics: Trichlormethiazide
adversedrugreaction药学英语

2 Type D-delayed reactions (alkylating agents leading to carcinogenesis, or retinoid-associated teratogenesis )
3 Type E-end-of-use reactions such as adrenocortical insufficiency following withdrawal of corticosteroids, or withdrawal syndromes following discontinuation of treatment with clonidine, benzodiazepines, tricyclic antidepressants or beta-adrenoreceptor antagonists.
11
3 Factors involved in the Etiology of Adverse Drug Reactions Can Be Classified as Follows
1 patient factors Intrinsic: Age-neonatal, infant and elderly Sex-hormonal environment Genetic abnormalities Previous adverse drug reactions, allergy, atopy Presence of organ dysfunction-disease Personality and habits-alcoholic, drug addict, nicotine, compliance .
药物相互作用的基本形式

药物相互作用的基本形式药物相互作用(Drug-drug interactions,DDIs)是指当一个药物与另一个药物一起使用时,相互干扰或改变彼此的药物效应的现象。
药物相互作用可能导致许多不良反应、治疗失败,甚至严重的药物中毒和死亡。
因此,了解药物相互作用的基本形式至关重要。
1. 药物-药物相互作用(Drug-drug interactions):不同药物同时应用时,可能产生相互作用。
其中最常见的类型是药物在体内的代谢相互影响。
例如,一些药物可以干扰其他药物的代谢途径,导致药物浓度升高或降低。
此外,药物还可以相互竞争结合蛋白质结构,影响它们的吸收、分布、代谢和排泄等过程。
2. 药物-食物相互作用(Drug-food interactions):并非所有药物都可以与食物一起安全使用。
一些食物可以改变药物的吸收率、分布和代谢,从而影响药物的效果。
例如,一些药物在与葡萄柚汁一起摄入时会发生药物浓度升高的现象,因为葡萄柚汁中的一些成分可以抑制药物的代谢酶。
3. 药物-饮料相互作用(Drug-beverage interactions):与食物相似,一些饮料也可能与药物相互作用。
例如,咖啡因可以增强一些药物(如退烧药)的效果,而酒精则可能与许多药物产生严重的相互作用,包括增加药物的毒性和加速药物的代谢。
4. 药物-疾病相互作用(Drug-disease interactions):一些疾病状态可能影响药物的代谢和排泄,从而降低或增强药物的效果。
此外,一些药物也可能对疾病产生不良影响,导致病情加重或恶化。
5. 药物-化学物质相互作用(Drug-chemical interactions):包括药物与化学物质如植物、草药、化妆品等相互作用。
这些化学物质和药物可能共享相同的代谢途径,从而干扰药物的药代动力学。
了解药物相互作用的基本形式并做好合理的药物管理对患者的治疗非常重要。
以下是几个预防药物相互作用的建议:1.了解药物信息:2.增加监测频率:对于同时使用多种药物的患者,定期监测药物血浆浓度和相关的生理参数,以优化药物治疗效果,并及时发现并处理可能的药物相互作用。
药物相互作用及其对药物疗效的影响

药物相互作用及其对药物疗效的影响药物相互作用(Drug-Drug Interactions)是指两种或更多药物在体内同时存在时可能发生的互相影响的现象。
这种相互作用可能导致药物治疗效果的改变,包括增强或降低治疗效果,甚至产生毒性效应。
了解药物相互作用对于临床用药和患者安全至关重要。
一、药物相互作用的类型药物相互作用可分为药物-药物相互作用、药物-食物相互作用、药物-饮酒相互作用等多种类型。
其中,药物-药物相互作用最为常见。
下面将依次介绍不同类型的药物相互作用。
1. 药物-药物相互作用在患者同时使用两种或更多种药物时,这些药物可能会相互影响,从而改变其药物疗效。
例如,某些药物可能通过竞争性结合蛋白,影响其他药物的吸附。
也有可能一种药物能够激活或抑制另一种药物的药代动力学或药效学,导致不良疗效甚至毒性反应的发生。
2. 药物-食物相互作用饮食与药物的相互作用也是一种重要的药物相互作用形式。
有些食物可以影响药物的吸收、分布、代谢和排泄过程,从而改变药物在体内的浓度和药物疗效。
例如,某些食物可以影响药物在胃肠道内的吸收,减慢药物的进入血液循环,降低药物的疗效。
3. 药物-饮酒相互作用饮酒与药物的相互作用是一种常见而容易被忽视的药物相互作用。
饮酒可能影响药物的代谢过程,增加药物在体内的浓度,从而导致药物的副作用加重或药效减弱。
尤其是一些镇静催眠类药物,与酒精同时使用会产生相加效应,增加中枢神经系统抑制作用,导致呼吸抑制、昏迷等不良反应。
二、药物相互作用对疗效的影响药物相互作用可能产生不同的影响。
一方面,药物相互作用可能显著改变药物的疗效,使其治疗效果增强或减弱;另一方面,药物相互作用也可能导致不良反应和毒性。
以下是药物相互作用可能对药物疗效产生的影响:1. 增效作用某些药物相互作用可能导致药物的治疗效果增强。
例如,两种药物同时使用可以协同作用,提高疗效。
这种情况下,医生可以根据患者的病情和药物特性,选择合适的药物组合,以期达到更好的治疗效果。
人工智能在医疗方面的应用英语作文

人工智能在医疗方面的应用英语作文全文共3篇示例,供读者参考篇1The Role of Artificial Intelligence in Modern HealthcareArtificial intelligence (AI) has rapidly emerged as a transformative force across numerous industries, and the healthcare sector is no exception. As a student passionate about the intersection of technology and medicine, I am fascinated by the vast potential of AI to revolutionize healthcare delivery, enhance patient outcomes, and unlock new frontiers in medical research.At its core, AI encompasses a broad range of technologies that enable machines to perceive, learn, reason, and assist humans in decision-making processes. In the context of healthcare, AI has already demonstrated its remarkable capabilities in areas such as medical imaging analysis, drug discovery, and predictive modeling.One of the most promising applications of AI in healthcare is its ability to augment medical imaging analysis. Radiology, for instance, relies heavily on the interpretation of complex medicalimages, such as X-rays, CT scans, and MRI scans. AI algorithms can be trained to detect patterns and anomalies within these images with unprecedented accuracy, helping radiologists make more informed diagnoses and catch potential issues earlier.AI is also playing a pivotal role in drug discovery and development. Traditionally, the process of identifying and testing new drug candidates has been time-consuming and resource-intensive. However, AI-powered computational models can rapidly screen vast chemical libraries, predict potential drug-target interactions, and optimize drug design. This accelerated approach has the potential to significantly reduce the time and costs associated with drug development, ultimately bringing life-saving therapies to patients more quickly.Furthermore, AI has emerged as a powerful tool for predictive modeling in healthcare. By analyzing vast amounts of patient data, including medical records, genetic information, and lifestyle factors, AI algorithms can identify patterns and make predictions about disease risk, disease progression, and potential treatment outcomes. This predictive capability can aid in early intervention, personalized treatment plans, and more effective resource allocation within healthcare systems.Beyond these specific applications, AI is also being leveraged in areas such as virtual assistants, robotic surgery, and clinical decision support systems. Virtual assistants powered by natural language processing can provide patients with personalized health information, answer medical queries, and even assist in monitoring chronic conditions. Robotic surgical systems, guided by AI algorithms, offer greater precision and control during complex procedures, potentially reducing risks and improving outcomes. Additionally, clinical decision support systems can integrate AI-driven insights with electronic health records, providing healthcare professionals with real-time guidance and recommendations for diagnosis and treatment.Despite the immense potential of AI in healthcare, there are legitimate concerns and ethical considerations that must be addressed. Privacy and data security are paramount, as AI systems rely on vast amounts of sensitive patient data for training and analysis. Robust safeguards and ethical frameworks must be in place to protect patient privacy and ensure the responsible use of AI in healthcare.Moreover, there is a risk of bias and discrimination if AI algorithms are trained on biased or incomplete data sets. This could lead to perpetuating existing healthcare disparities ormaking inaccurate predictions for certain demographics. Addressing these biases through diverse and representative data, as well as rigorous testing and auditing of AI systems, is crucial.Additionally, the transparency and interpretability of AI models in healthcare decision-making processes are essential. Healthcare professionals and patients alike need to understand the reasoning behind AI-generated recommendations, and there should be clear accountability measures in place.As a student, I am excited by the prospect of witnessing and contributing to the ongoing integration of AI into healthcare during my future career. However, I recognize that this process must be approached with caution, ethical vigilance, and a deep commitment to putting patient well-being first.In conclusion, artificial intelligence is poised to transform the healthcare landscape in profound ways. From enhancing medical imaging analysis and accelerating drug discovery to enabling predictive modeling and powering clinical decision support systems, AI holds immense promise for improving patient outcomes and advancing medical research. However, as we embrace these technological advancements, it is imperative that we address the ethical and practical challenges surrounding privacy, bias, transparency, and accountability. By thoughtfullynavigating these complexities, we can harness the full potential of AI to create a more efficient, personalized, and equitable healthcare system for all.篇2The Role of Artificial Intelligence in Modern HealthcareOver the past few decades, artificial intelligence (AI) has rapidly evolved from a futuristic concept to an integral part of our daily lives. From smart assistants like Siri and Alexa toself-driving cars, AI is transforming the way we live, work, and interact with technology. However, one of the most promising and impactful applications of AI lies in the field of healthcare, where it has the potential to revolutionize diagnosis, treatment, and disease management.As a student pursuing a degree in computer science with a keen interest in healthcare technology, I have been fascinated by the intersection of these two fields. The integration of AI into healthcare practices has the potential to improve patient outcomes, enhance efficiency, and ultimately save lives. In this essay, I will explore the various ways in which AI is being applied in the medical domain, highlighting its current and potential impact.Medical Imaging and DiagnosticsOne of the most significant applications of AI in healthcare is in the realm of medical imaging and diagnostics. Advancements in machine learning algorithms and computer vision have enabled AI systems to analyze medical images, such as X-rays, CT scans, and MRI images, with unprecedented accuracy and efficiency.AI-powered image analysis can help detect and diagnose various conditions, including cancers, brain disorders, and cardiovascular diseases, with greater precision than human radiologists. These systems can identify subtle patterns and abnormalities that may be difficult for the human eye to discern, leading to earlier and more accurate diagnoses.Furthermore, AI can assist in streamlining the diagnostic process by providing near-real-time analysis, reducing the time and resources required for manual image interpretation. This not only enhances patient care but also alleviates the workload on healthcare professionals, allowing them to focus on more complex cases and patient interactions.Drug Discovery and DevelopmentThe process of developing new drugs is notoriouslytime-consuming, expensive, and fraught with challenges. However, AI has the potential to revolutionize drug discovery and development by accelerating the process and increasing the success rate.AI algorithms can analyze vast amounts of data, including genomic information, molecular structures, and patient records, to identify potential drug targets and predict the efficacy and safety of candidate compounds. This data-driven approach can significantly reduce the time and resources required for traditional drug development methods.Additionally, AI can assist in optimizing drug formulations, dosages, and delivery methods, ensuring more effective and personalized treatments for patients. By streamlining the drug development process, AI can accelerate the delivery of lifesaving therapies to those in need.Personalized Medicine and Precision HealthcareOne of the most promising applications of AI in healthcare is the advancement of personalized medicine and precision healthcare. Traditional medical practices have often employed a "one-size-fits-all" approach, which may not be effective for all patients due to genetic, environmental, and lifestyle factors.AI can analyze an individual's genetic profile, medical history, lifestyle data, and other relevant factors to develop tailored treatment plans and preventive strategies. By leveraging machine learning algorithms and predictive analytics, AI can identify patterns and correlations that may not be apparent to human clinicians, enabling more accurate risk assessments and personalized interventions.Personalized medicine facilitated by AI has the potential to improve treatment outcomes, reduce adverse effects, and enhance patient adherence to prescribed regimens. This approach aligns with the growing trend towardspatient-centered care and empowers individuals to take an active role in managing their health.Clinical Decision Support SystemsIn the fast-paced and complex world of healthcare, clinicians are often faced with overwhelming amounts of data and information to process, which can lead to decision fatigue and potential errors. AI-powered clinical decision support systems (CDSS) can assist healthcare professionals in making more informed and accurate decisions by providing real-time guidance and recommendations.CDSS can integrate patient data, medical knowledge bases, and evidence-based guidelines to provide diagnostic suggestions, treatment recommendations, and risk assessments. These systems can also alert clinicians to potential drug interactions, contraindications, or abnormal test results, reducing the likelihood of medical errors and improving patient safety.By leveraging the vast computational power and data processing capabilities of AI, CDSS can serve as valuable decision-making tools, complementing human expertise and enhancing the quality of care provided to patients.Remote Monitoring and Virtual AssistantsAI is also playing a significant role in enabling remote monitoring and virtual assistants in healthcare. With the advent of wearable devices and smart home sensors, AI can continuously monitor and analyze patient data, such as vital signs, activity levels, and sleep patterns.This real-time monitoring capability enables early detection of potential health issues and timely interventions, reducing the need for hospital visits and promoting proactive disease management. AI-powered virtual assistants can also provide personalized guidance, reminders, and support to patients,empowering them to take an active role in maintaining their well-being.Furthermore, AI-enabled telemedicine platforms allow for remote consultations and virtual care, bridging geographical barriers and increasing access to quality healthcare services, particularly in underserved areas or during times of crisis, such as the COVID-19 pandemic.Ethical Considerations and ChallengesWhile the potential benefits of AI in healthcare are vast, it is crucial to address the ethical considerations and challenges associated with its implementation. Privacy and data security are paramount concerns, as AI systems often rely on sensitive patient information for training and decision-making.Robust data governance frameworks and stringent privacy protocols must be in place to ensure the responsible and ethical use of patient data. Additionally, addressing issues of bias and fairness in AI algorithms is essential to prevent unintended discrimination and ensure equitable access to healthcare services.Furthermore, the integration of AI into healthcare practices raises questions about liability, accountability, and thehuman-machine dynamic. Clear guidelines and regulatory frameworks must be established to ensure the safe and responsible deployment of AI technologies in healthcare settings.ConclusionThe applications of artificial intelligence in healthcare are vast and transformative, offering the potential to improve patient outcomes, enhance efficiency, and advance the frontiers of medical science. From medical imaging and diagnostics to drug discovery, personalized medicine, and remote monitoring, AI is revolutionizing the way we approach healthcare delivery.However, as we embrace these technological advancements, it is crucial to address the ethical considerations and challenges associated with the implementation of AI in healthcare. By fostering collaboration between technology experts, healthcare professionals, policymakers, and the public, we can harness the power of AI to create a more accessible, personalized, and effective healthcare system for all.篇3The Role of AI in Transforming HealthcareThe field of artificial intelligence (AI) has been rapidly evolving and finding its way into various industries, including healthcare. As a student studying computer science and with a keen interest in emerging technologies, I find the integration of AI into the medical realm particularly fascinating. This technological advancement holds the potential to revolutionize the way we approach healthcare, offering numerous benefits that could improve patient outcomes, streamline processes, and ultimately save lives.One of the most promising applications of AI in healthcare is its ability to assist in disease diagnosis. Traditional diagnostic methods often rely heavily on the expertise and experience of medical professionals, which can be subjective and susceptible to human error. AI algorithms, on the other hand, can analyze vast amounts of medical data, including patient history, symptoms, and test results, to identify patterns and make accurate diagnictions. This technology has already shown remarkable success in detecting various conditions, such as cancers, cardiovascular diseases, and neurological disorders, often outperforming human experts.Furthermore, AI can play a crucial role in medical imaging analysis. Radiology is a field that heavily relies on theinterpretation of complex images, such as X-rays, CT scans, and MRI scans. AI systems can be trained to recognize subtle abnormalities or patterns that may be difficult for the human eye to detect, leading to earlier and more accurate diagnoses. This technology has the potential to significantly improve the efficiency and accuracy of diagnostic procedures, reducing the risk of misdiagnosis and enabling prompt treatment.Another area where AI is making significant strides is in drug discovery and development. The process of identifying potential drug candidates and testing their efficacy is oftentime-consuming and resource-intensive. AI algorithms can assist in virtual screening of vast chemical libraries, predicting the potential interactions between drugs and target molecules, and simulating the behavior of these interactions. This can accelerate the drug development process, reducing the time and cost associated with traditional methods, ultimately bringinglife-saving treatments to patients more quickly.In addition to its diagnostic and research applications, AI can also play a role in personalized medicine. By analyzing an individual's genetic information, medical history, and lifestyle factors, AI systems can help tailor treatment plans and medication regimens to each patient's unique needs. Thisapproach has the potential to improve treatment outcomes, reduce the risk of adverse reactions, and ensure that patients receive the most effective care possible.Despite the numerous benefits of AI in healthcare, there are also legitimate concerns and challenges that need to be addressed. One of the primary concerns is the issue of data privacy and security. Medical data is highly sensitive, and the mishandling or misuse of this information could have severe consequences for patients. It is crucial that AI systems are designed with robust security measures and comply with strict data protection regulations.Another challenge is the potential for AI systems to perpetuate or amplify biases present in the data they are trained on. If the training data is skewed or incomplete, the AI algorithms may learn and reinforce these biases, leading to unfair or discriminatory outcomes. Addressing these biases and ensuring that AI systems are fair and equitable is an ongoing challenge that requires careful consideration and responsible development practices.Furthermore, the integration of AI into healthcare raises ethical questions regarding the role of human medical professionals. While AI can augment and support humandecision-making, it should not entirely replace the expertise and human touch that healthcare professionals provide. Striking the right balance between leveraging AI's capabilities and maintaining human oversight and control is crucial for ensuring patient trust and preserving the human aspect of healthcare.As a student, I am excited about the potential of AI in healthcare, but I also recognize the importance of addressing these challenges. It is essential that the development and implementation of AI technologies in this field are guided by ethical principles, informed by diverse perspectives, and subject to rigorous testing and validation.In conclusion, the applications of AI in healthcare are vast and promising. From assisting in disease diagnosis and medical imaging analysis to accelerating drug discovery and enabling personalized medicine, AI has the potential to revolutionize the way we approach healthcare. However, it is crucial that we address the challenges of data privacy, algorithmic bias, and the ethical implications of this technology. By embracing AI responsibly and thoughtfully, we can unlock its full potential and pave the way for a more efficient, accurate, and patient-centered healthcare system.。
医疗质量下降英语

Title: The Impact of Declining Medical QualityIntroduction:The healthcare system is an essential component of any society, aiming to provide optimal medical services to its citizens. However, various factors are leading to a decline in medical quality, posing significant challenges to the system's effectiveness and patient well-being. This document highlights the key aspects of this decline, analyzing its impact on various facets of medical care.1. Diagnostic Accuracy Decline:The initial step in effective treatment is accurate diagnosis. However, recent studies indicate a consistent decline in diagnostic accuracy, possibly due to increased workload, human error, or a lack of updated diagnostic tools and techniques. This inaccuracy can lead to inappropriate treatment, increased patient discomfort, and even worse outcomes.2. Therapeutic Efficacy Reduction:Several reports have shown a decrease in the overall effectiveness of medical treatments over time. This could be due to drug resistance, the emergence of new diseases or variants, or the lack of new and effective treatment methods. The consequences are higher rates of treatment failure, disease progression, and patient mortality.3. Decline in Nursing Service Quality:Nurses play a crucial role in providing patient care, yet there are signs of a decline in the quality of their services. This may be due to increased workloads, staff shortages, and a lack of training and support. The result is compromised patient care, leading to poorer outcomes and patient dissatisfaction.4. Rise in Hospital Infection Rates:Hospital-acquired infections are on the rise, possibly due to issues with hygiene, poor sterilization techniques, or inadequate infection control measures. This not only affects patient recovery but also poses a significant threat to public health.5. Patient Satisfaction Decrease:Patient satisfaction is a key indicator of the quality of healthcare delivery. Multiple studies have shown a consistent decline in patient satisfaction over the years. This could be due to longer waiting times, less personalized care, or a lack of patient education and communication from healthcare providers.6. Increase in Medical Malpractice Incidents:Medical errors and malpractices are on the rise, possibly due to medical negligence, a lack of accountability, or overwhelmed healthcare workers. These incidents result in increased pain and suffering for patients, along with potential long-term health effects and high compensation payouts to victims.7. Drug Adverse Events Increase:Drug safety has become a major concern with an increasing number of reports on drug-related adverse events. This could be due to drug interactions, side effects, or patients not being informed about potential risks. The consequences are unnecessary patient suffering and potential long-term health issues.8. Equipment Failure Rate Heighten:Equipment failure is becoming more common, possibly due to inadequate maintenance, ageing equipment, or a lack of updated technology. This not only affects patient care but also poses a significant risk to healthcare workers and potentially spreads diseases or infections.9. Medical Record Quality Decline:The quality of medical records has been on a downward trend, possibly due to staff workload, poor record-keeping practices, or a lack of digitalization. This results in incomplete or inaccurate patient records, leading to potential medical errors and 阻碍有效的治疗。
大学英语四级模拟卷一

⼤学英语四级模拟卷⼀⼤学英语四级模拟卷⼀Part I WritingDirections: For this part, you are allowed 30 minutes to write a short essay about a college association that influences you most. You should state the reasons and write at least 120 words but no more than 180 words.Part II Listen ComprehensionSection AQuestions 1 and 2 will be based on the following news item.1. A. The growth of new teeth. C. A natural process of tooth repair.B. The decay of bad teeth. D. A medical effect on tooth repair.2. A) Early-stage cavities. B. Late-stage cavities. C. A headache. D. A stomachache. Questions 3 and 4 will be based on the following news items.3. A. The UK Open badminton tournament. C. The US Open badminton tournament.B. The UK Open tennis tournament. D. The US Open tennis tournament.4. A. Thirty-three players. B. Three players. C. Twenty-two players. D. Twenty-four players. Question 5 to 7 will be based on the following news item.5. A. The ocean. B. The forest. C. The grassland. D. The farmland.6. A. To make the ocean cleaner. C. To make fishing more sustainable.B. To make the fishermen richer. D. To make fishing more competitive.7. A. The weather gets worse than before. C. Many fishermen switch to other business.B. There are more mechanical problems with boats. D. Many fishermen lose their business.Section BConversation One Questions 8 to 11 are based on the conversation you have just heard.8. A. Close friends who talk about almost everything. C. Colleagues working in the same office.B. Dating service agent and customer. D. Interviewer and interviewee in a survey.9. A. Men who like donkeys. C. Men who have a sense of humor.B. Men who love to laugh loudly. D. Men who are emotional.10. A. Books and cooking. B. Books and movies. C. Movies and cleaning. D. Politics and exercising.11. A. The man invites her out to have dinner. C. They go out to enjoy a jazz concert.B. The man comes to have dinner at her home. D. They have a good conversation over coffee. Conversation Two Questions12 to 15 are based on the conversation you have just heard.12. A. It’s the easiest way to communicate with other users over network.B. It’s printed on every card for people to exchange with others.C. Everybody is talking about it nowadays.D. If a person doesn’t have one, he will be out of time.13. A. It cannot contain any commercial information.B. It may not be of a high level of security.C. One can only use the free e-mail account at home.D. It is difficult to get access to the website with such service.14. A. A specific program for e-mail. C. IE and Windows.B. It may not be of a high level of security. D. Additional software.15. A. Print an e-mail address on her card. C. Pay the ISP for an e-mail account.B. Check her hardware and software. D. Try to get a free e-mail account.Section CPassage One Questions 16 to 18 are based on the passage you have just heard.16. A. In 1604. B. Around 1700. C. In 1750. D. In 1755.17. A. Robert Cawdrey. B. John Kersey. C. Samuel Johnson. D. Daniel Webster.18. A. It has a complete list of difficult words. C. It is a 20-volume work.B. It has sentences includes as examples. D. It includes the presentation of word histories. Passage Two Questions 19 to 22 are based on the passage you have just heard.19. A. About 7 000. B. About 70 000. C. About 4 million. D. About 40 million.20. A. American doctors would pay the medical bills. C. The patients should pay for the doctors service.B. American doctors are paid by the government. D. The government would pay for the medical bills.21. A. Health insurance plans, government help and individual payment.B. The federal government of the US.C. The wise investment of their money.D. The companies they work for.22. A. Only individuals pay for their health insurance.B. Health insurance can greatly lower the cost of individuals.C. Health insurance covers everything on the bills.D. There is no time limit for health insurance.Passage Three Questions 23 to 25 are based on the passage you have just heard.23. A. She worked in a clothing shop not far from home.B. She did experiments in a lab not far from her home.C. She stayed at home and designed clothes.D. She became a partner of an old lady.24. A. After she designed the clothes women wanted. C. After her former boss of the shop retired.B. When her former boss of the shop died. D. Soon after she worked in the shop.25. A. Cheap hotels were not comfortable. C. She wanted to show her wealth.B. She was wearing good clothes. D. The best hotels could bring more customers.Part III Reading ComprehensionSection A Questions 26 to 35 are based on the following passage.Thanks giving is America’s national holiday for giving thanks to God. Thanksgiving Day has s special 26 for Americans because it is traced back to that group of people who were among the first to come to the New World in search of freedom.In 1620, 102 sea weary(疲倦的) Pilgrims landed on the peninsula of Cape Cod. Their ship, the Mayflower, had 27 to go to Virginia, but it made its landfall far to the north. After some weeks of 28 they decided not to make the trip to Virginia but to remain where they were. But when they stepped ashore in this 29 alien world, they were totally isolated from any outside 30 and knew no means of livelihood. And the greater trouble is that in the woods live Indians, some of whom were 31 . This added to the hardship of daily life. But the vast 32 of forests gave them a hope. In this way, the nation’s forefathers not only 33 the first severe winter, but also saw the first harvest of crops in the next autumn. Their Indian friends were also invited to join their festival.This story is told every year to young children in schools as Thanksgiving Day (the fourth Thursday of November) 34 . Today, in US Thanksgiving Day is celebrated by many Americas whose roots do not stem from Britain. Now it is marked by families gathering together to enjoy a 35 dinner for roast turkey, and to tell the things for which they are thankful.Section BWireless Health CareA.Is it possible that among all the advertisements about Apple’s iPad, one potential use has beenoverlooked? Larry Nathanson, head of emergency-medicine “informatics”at one of Harvard Medical School’s hospitals, has experimented with using the device in the patients’ rooms. He writes that “initial tests with our clinical applications went amazingly well...the EKG s (⼼电图) look better on screen than on paper. It was great having all of the clinical information right at the bedside to discuss with the patient.”B.Dr. Nathanson’s enthusiasm hints at the potential of wireless instruments to improve health care, and toensure more personalized treatment in particular. Experts have long predicted that advances in genetics will bring in a golden age of individually tailored therapies. But in fact it is much lower-tech wireless devices and Internet-based health software that are speeding up the mass personalization of health care, and creating entirely new business models in the process.C.Wireless health is “becoming universal”in hospitals, according to Kalorama Information, amarket-research firm. It estimates that the market for such devices and services in America alone will grow from $2.7 billion in 2007 to $9.6 billion in 2012. Don Jones of Qualcomm, a maker of networking technology, argues that the trend speeds diagnosis and tr eatment, and saves doctors’ and nurses’ time. GE, an industrial giant, and Sprint, an American mobile operator, have joined forces to offer hospitals such services. GE’s software allows the secure monitoring of patients’ health via mobile phones, as does rival software from Airstrip.D.Doctors are an obvious early target for wireless health. A forthcoming report by the California HealthCare Foundation (CHCF), a think tank, estimates that two-thirds of American physicians already have smart-phones. Over one-third of American doctors use Epocrates, a program for mobiles and laptops which offers instant information on drug-to-drug interactions, treatment recommendations and so on.The software will soon be able to access electronic health records (EHRs) via mobiles—which the author of the CHCF’s report thinks could be “the killer application” of wireless health.E.The hope is that nimble new technologies, from smart-phones to EHRs to health-monitoring devices,will give more power to patients and doctors, and thus improve outcomes while cutting costs. The popularization of mobile phones is the chief reason to think this optimistic thought may come true.Patients with smart-phones can certainly benefit from interactive “wellness” applications that track diet, exercise and important signs.F.But Carolyn Buck-Luce of Ernst & Young, a consultancy, points out that “Mobile Health”istransforming health care in poor countries as well as rich ones. Medical Home, a Mexican team that provides medicalconsultations by mobile, already has millions of customers. Paul Meyer of V oxiva, anAmerican technology firm that has set up Mobile Health systems in Rwanda and Peru, among other places, says that such schemes have been so successful in the developing world that they are now being adopted in the rich world, too. His firm has helped the American government with its recent launch of “Information Baby”, a public-health movement to educate pregnant mothers (they receive free text messages with medical advice) that will soon become the biggest such effort in the world.G.What is more, mobile phones are but one part of a broader wireless trend in health care that McKinsey, a consultancy, estimates may soon be worth up to $60 billion globally. Many companies are coming up with “homehealth”devices based on the wireless technology. Some are obviously used in clinical.Medtronic, a devices giant, is developing a bedside monitor that wirelessly tracks the blood sugar levels in diabetic children sleeping nearby. GE has come up with “body sensor networks”, tiny wireless devices that track the vital signs of those who wear them.H.The most successful devices may be, as Eric Dishman of Intel puts it, “secret”. His firm, a big chipmaker,is investing in devices to track the health of the elderly, such as “magic carpets” that sense erratic (不稳定的) movements and thus can predict a fall. Continua, an industry group, is developing shared standards so that blood-pressure monitors and scales can wirelessly trans fer readings to doctors’ offices or personal EHR services like Google Health.I.All these devices and services do not just allow doctors to make more accurate diagnoses, prescribe moreeffective treatments and keep better track of patients’ conditions. They also allow health services to tailor treatments depending on patients’ personal preferences and behavioral shortcomings. Studies show, for example, that although some patients with long-standing diseases are not caring about taking pills properly, others are careless or forgetful. Some prefer efficient electronic reminders, whereas others respond best when a nurse calls home. A global consumer survey released on April 6th by PricewaterhouseCoopers (PWC), a consultant company, finds that the elderly prefer high-quality care with lots of personal attention, whereas younger types prefer low-cost care and health schemes.J.Many health systems, PWC’s report finds, are beginning to divide customers into different categories to arrange treatments accordingly. For example, Discovery Health, a South African insurer, uses a variety of different methods to get patients with diseases to follow through on their treatments, from text messages reminding them to take their pills to rewards for good behavior.K. A similar scheme run by Health Media, a health firm owned by Johnson & Johnson, a big drugs firm, uses online tools (it calls them “digital health coaches”) to help patients manage diabetes and lose weight.Its studies suggest that half of the digitally directed people do lose weight. And the improved health of those with chronic conditions is worth $1000 a year to their employers. Virgin Health Miles, an American rival, has taken the same idea a step further, using online social networks, through which co-workers or family members can cheer on or remind patients electronically, in order to encourage exercise or weight loss. Patients seem to like this kind of thing: one patient who suffers from heart disease, for example, has created a forum for fellow sufferers that can be accessed through an iPhone application. L.All these measures are particularly promising because they help bring about behavioral change, normally the hardest element of any treatment. Patients often ignore doctors’ lectures, but are more inclined to listen to supportive friends and family. By the same token, doctors and nurses are not always on hand to encourage healthy behavior, but mobile phones and other wireless gadgets can be. That is something that even personalized genetic therapies could not offer.36. According to PWC, the elderly prefer high-quality care while the young prefer low-cost care.37. Patients often ignore doctors’ lecture, but are more likely to follow the advice of supportive friends andfamily.38. The main aim of “magic carpets” is to predict when the elderly will fall.39. According to PWC’s report, many health systems are starting to divide customers into different categoriesso that they can arrange treatments accordingly.40. People believe nimble new technologies will help patients and doctors greatly because mobile phones arewidely used.41. According to Don Jones, the wide use of wireless health devices in hospitals quickens diagnosis andtreatment.42. According to Larry Nathanson, iPad can be used in clinical applications.43. At the beginning, wireless health aims at doctors in the hospital.44. The scheme run by Health Media suggests that half of the people who use its online tools do lose weight.45. The lower-tech wireless devices and Internet-based health software promote the mass personalization ofhealth care.Section CPassage One Questions 46 to 50 are based on the following passage.Academic qualification’s value in the workplace is a big issue for students, policymakers and taxpayers, especially as the rising numbers of students in higher education make them less distinctive. In the latest annual report on education by the OECD (Organization for Economic Cooperation and Development), a rich-country think-tank, the answer is clear: the pay-off from tertiary education(⾼等教育) is still good, both for the individual and the economy. Most graduates take jobs fitting their qualifications, earn more than non-graduates, and thus tend to pay more in taxes.The workforce is smartening up. In the OECD 35% of the 25- to 34-year-old workforce has completed tertiary education, compared with 20% of the cohort approaching retirement. Countries such as Japan and South Korea have invested so heavily in educating their young that more than half now hold post-school qualifications. Norway, Denmark, Sweden and the Netherlands are close behind. Andreas Schleicher, the OECD’s chief of education research, reckons that these countries may well become more competitive as a result.The OECD’s compendium (概要) also shows that graduate jobs fared better during the global recession. Data show those who had completed tertiary education were more likely to be employed, and less likely to be unemployed in 2008. Earnings data are from the middle of the decade, so it is not yet clear how the downturn has hit graduate pay.The “education is good” mantra does not work everywhere. In some countries many students have to be content with the intellectual rewards of study. In Spain, for example, 44% of college- and university-educated youngsters are working in low-skill jobs. America, Canada and Britain also have high shares of graduates working in jobs for which they are overqualified. In lucky Luxembourg hardly any graduates end up in menial jobs.Salaries vary sharply too. Poland has fewer graduates in non-graduate jobs than America, but the gross earnings of 25- to 34-year-olds with tertiary qualifications in that country is $11,800 compared with $56,200 in the land of the free. Hardly surprising therefore that Polish graduates hanker after jobs in America and that American companies like investing in places such as Poland and Hungary, where they can hire highly qualified labour for far less money than at home.46. Why does academic qualifications’ value become an important issue?A. People can find a good job without a good qualification.B. More and more access to colleges and universities.C. An increasing number of students lose their own characteristic.D. Most graduates can find good jobs and pay much more in taxes.47. Why does the author say the workforce is smartening up?A. There are many old people approaching retirement.B. A lot of money is put on the basic education.C. Some countries are very competitive in education.D. More people have finished higher education.48. What is unclear in the third higher education?A. Whether the earning data are accurate. C. How graduate pay has been affected.B. How graduates look for jobs. D. Whether more graduates lose their jobs.49. Why does not the “education is good” mantra work everywhere?A. In some countries some graduates often lose their jobs.B. In some countries some graduates cannot find suitable jobs.C. In some countries some graduates are underqualified.D. In some countries some graduates can be highly paid.50. What is hardly surprising according to the author?A. Graduates in Poland earn more money than those in America.B. Graduates from Poland would like to look for a job in America.C. Graduates in Poland are more qualified than those in America.D. Graduates from Poland always do the low-skilled jobs in America.Passage Two Questions 51 to 55 are based on the following passage.The two economists call their paper “Mental Retirement,” and their argument has aroused the interest of behavioral researchers. Data from the United States, England and 11 other European countries suggest that the earlier people retire, the more quickly their memories decline.“It’s incredibly interesting and exciting,” said Laura Carstensen, director of the Center on Longevity at Stanford University. “It suggests work actually provides an important component of the environment that keeps people functioning optimally (最佳地).”While not everyone is convinced by the new analysis, published recently in T he Journal of Economic Perspectives, a number of leading researchers say the study is, at least, a bit of evidence for a hypothesis that is widely believed but surprisingly difficult to demonstrate.Researchers repeatedly find that retired people as a group tend to do less well on cognitive (认知的) tests than people who are still working. But, they note, that could be because people whose memories and thinking skills are declining may be more likely to retire than people whose cognitive skills remain sharp.And research has failed to support the premise that mastering activities like memory exercises, crossword puzzles and games like Sudoku carry over into real life, improve overall functioning.“If you do crossword puzzles, you get better at crossword puzzles,” said Lisa Berkman, director of the Center for Population and Development Studies at Harvard University. “If you do Sudoku, you get better at Sudoku. You get better at one narrow task. But you don’t get better at cognitive behavior in life.”The study was possible, explains one of its authors, Robert Willis, a professor of economics at the University of Michigan, because the National Institute on Aging began a large study in the United Statesnearly 20 years ago. Called the Health and Retirement Study, it surveys more than 22,000 Americans over age 50 every two years, and administers memory tests.51. According to the data from America and some European countries, retired people ___________.A. have aroused the interest of many psychologistsB. are more forgetful than they were at workC. don’t have a functioning mind any moreD. can have much better cognitive skills52. In Laura L. Carstensen’s opinion, what is the relationship between work and mental function?A. Work has nothing to do with people’s mental function.B. Work has a positive effect on people’s mental function.C. People’s mental function decreases gradually after work.D. People’s mental function has no influence on people’s work.53. Lisa Berkman claimed that Sudoku could ____________.A. improve man’s overall functioning greatly C. help develop man’s cognitive skillsB. make people good at this narrow task D. help people live much longer54. What can we learn about the Health and Retirement Study?A. It has been carried out for about 20 years.B. It surveys Americans under the age of 50.C. It is led by Robert Willis in the National Institute.D. It gets support from the University of Michigan.55. According to the passage, what does “mental Retirement mean”?A. People are reluctant to retire at an early age.B. People have to retire earlier than expected.C. People’s mental functions will decline even though they are still working.D. People’s memories and reasoning abilities decline if they are not working.Part IV Translation⾃驾游(self-driving tour)属于⾃助旅游的⼀种,是近年来我国新兴的旅游⽅式。
SCI英文期刊缩写对照

Acc. Chem. Res.Accounts of Chemical ResearchACH - Models Chem.ACH - Models in ChemistryACI Mater. J.ACI Materials JournalACS Symp. Ser.ACS Symposium SeriesActa Biochim. Pol.Acta Biochimica PolonicaActa Biotechnol.Acta BiotechnologicaActa Chem. Scand.Acta Chemica ScandinavicaActa Chim. SinicaActa Chimica SinicaActa Cienc. Indica, Chem.Acta Cienceia Indica ChemistryActa Cienc. Indica, Phys.Acta Ciencia Indica PhyicsActa Crystallogr., Sect. A: Found. Crystallogr.Acta Crystallographica Section A: FoundationsActa Crystallogr., Sect. B: Struct. SciActa Crystallographica Section B: Structural ScienceActa Crystallogr., Sect. C: Cryst. Struct. Commun.Acta Crystallographica Section C: Crystal Structure CommunicationsActa Crystallogr., Sect D: Biol. Crystallogr.Acta Crystallographica Section D: Biological CrystallographyActa Crystallogr. Sect. E: Struct. Rep. OnlineActa Crystallographica Section E Structure Reports OnlineActa Hydroch. Hydrob.Acta Hydrochimica et HydrobiologicaActa Mater.Acta MaterialiaActa Metall.Acta MetallurgicaActa Phys. Pol., AActa Physica Polonica AActa Phys. Pol., BActa Physica Polonica BActa Polym.Acta PolymericaActa Polytech. Scand., Chem. Technol. SerActa Polytechnica Scandinavica - Chemical Technology SeriesAdhes. AgeAdhesives AgeAdsorpt. Sci. Technol.Adsorption Science and TechnologyAdv. Appl. Microbiol.Advances in Applied MicrobiologyAdv. At. Mol. Opt. Phy.Advances in Atomic Molecular and Optical PhysicsAdv. Biochem. Eng./Biotechnol.Advances in Biochemical Engineering / BiotechnologyAdv. Carbohydr. Chem. Biochem.Advances in Carbohydrate Chemistry and BiochemistryAdv. Chem. Phys.Advances in Chemical PhysicsAdv. Chem. Ser.Advances in Chemistry SeriesAdv. Chromatogr.Advances in ChromatographyAdv. Colloid Interface Sci.Advances in Colloid and Interface ScienceAdv. Compos. MaterAdvanced Composite MaterialsAdv. Cryog. Eng.Advances in Cryogenic EngineeringAdv. Eng. Mater.Advanced Engineering MaterialsAdv. Enzyme Regul.Advances in Enzyme RegulationAdv. Enzymol. Relat. Areas Mol. Biol.Advances in Enzymology and Related Areas of Molecular BiologyAdv. Filtr. Sep. Technol.Advances in Filtration and Separation TechnologyAdv. Funct. Mater.Advanced Functional MaterialsAdv. Heterocycl. Chem.Advances in Heterocyclic ChemistryAdv. Inorg. Chem.Advances in Inorganic ChemistryAdv. Mass Spectrom.Advances in Mass SpectrometryAdv. Synth. Catal.Advanced Synthesis and CatalysisAdv. Mater.Advanced MaterialsAdv. Mater. Opt. Electron.Advanced Materials for Optics and ElectronicsAdv. Mater. ProcessesAdvanced Materials and ProcessesAdv. Mater. Res.Advances in Materials ResearchAdv. Organomet. Chem.Advances in Organometallic ChemistryAdv. Phys. Org. Chem.Advances in Physical Organic ChemistryAdv. Polym. Sci.Advances in Polymer ScienceAdv. Polym. Tech.Advances in Polymer TechnologyAdv. Powder Technol.Advanced Powder TechnologyAdv. Powder. Metall. Part. Mater.Advances in Powder Metallurgy and Particulate MaterialsAdv. Protein Chem.Advances in Protein ChemistryAdv. Quantum Chem.Advances in Quantum ChemistryAdv. Second Messenger Phosphoprotein Res.Advances in Second Messenger and Phosphoprotein ResearchAdv. Space Res.Advances in Space ResearchAdv. X-Ray Anal.Advances in X-Ray AnalysisAdverse Drug React. Toxicol. Rev.Adverse Drug Reactions and Toxicological ReviewsAerosol Sci. Technol.Aerosol Science and TechnologyAlChE J.AIChE JournalAlChE Symp. Ser.AIChE Symposium SeriesAm. Ceram. Soc. Bull.American Ceramic Society BulletinAm. Ind. Hyg. Assoc. J.American Industrial Hygiene Association JournalAm. J. Respir. Cell Mol. Biol.American Journal of Respiratory Cell and Molecular BiologyAm. Lab.American LaboratoryAm. Mineral.American MineralogistAmmonia Plant Saf. Relat. FacilAmmonia Plant Safety and Related FacilitiesAn. Asoc. Quim. Argent.Anales de la Asociacion Quimica ArgentinaAn. Quim.Anales de QuimicaAnal. Biochem.Analytical BiochemistryAnal. Chem.Analytical ChemistryAnal. Chim.Annali di ChimicaAnal. Chim. ActaAnalytica Chimica ActaAnal. Commun.Analytical CommunicationsAnal. Lett.Analytical LettersAnal. Sci.Analytical SciencesAngew. Chem. Int. Ed.Angewandte Chemie International EditionAngew. Makromol. Chem.Angewandte Makromolekulare ChemieAnn. Chim. (Rome)Annali di ChimicaAnn. Chim. - Sci. Mat.Annales de Chimie - Science des MateriauxAnn. Clin. Biochem.Annals of Clinical BiochemistryAnn. N.Y. Acad. Sci.Annals of the New York Academy of SciencesAnnu. Rep. Med. Chem.Annual Reports in Medicinal ChemistryAnnu. Rep. Prog. Chem. Sect. A: Inorg. Chem.Annual Reports on the Progress of Chemistry, Section A: Inorganic ChemistryAnnu. Rep. Prog. Chem. Sect. B: Org. Chem.Annual Reports on the Progress of Chemistry, Section B: Organic ChemistryAnnu. Rep. Prog. Chem. Sect. C: Phys. Chem.Annual Reports on the Progress of Chemistry, Section C: Physical ChemistryAnnu. Rev. BiochemAnnual Review of BiochemistryAnnu. Rev. Biophys. Biomol. Struct.Annual Review of Biophysics and Biomolecular StructureAnnu. Rev. Cell Dev. Biol.Annual Review of Cell and Developmental BiologyAnnu. Rev. Energy Env.Annual Review of Energy and the EnvironmentAnnu. Rev. Mater. Sci.Annual Review of Materials ScienceAnnu. Rev. Pharmacool. Toxicol.Annual Review of Pharmacology and ToxicologyAnnu. Rev. Phys. Chem.Annual Review of Physical ChemistryAnti-Cancer Drug Des.Anti-Cancer Drug DesignAnticancer Res.Anticancer ResearchAntimicrob. Agents Chemother.Antimicrobial Agents and ChemotherapyAntisense Nucleic Acid Drug Dev.Antisense and Nucleic Acid Drug DevelopmentAntiviral Chem. Chemother.Antiviral Chemistry and ChemotherapyAppita J.Appita JournalAppl. Biochem. Biotechnol.Applied Biochemistry and BiotechnologyAppl. Catal., AApplied Catalysis AAppl. Catal., BApplied Catalysis BAppl. Compos. Mater.Applied Composite MaterialsAppl. Environ. Microbiol.Applied and Environment MicrobiologyAppl. Geochem.Applied GeochemistryAppl. Magn. Reson.Applied Magnetic ResonanceAppl. Microbiol. Biotechnol.Applied Microbiology and BiotechnologyAppl. Opt.Applied OpticsAppl. Organomet. Chem.Applied Organometallic ChemistryAppl. Phys. AApplied Physics AAppl. Phys. BApplied Physics BAppl. Phys. Lett.Applied Physics LettersAppl. Radiat. Isot.Applied Radiation and IsotopesAppl. Sci. Res.Applied Scientific ResearchAppl. Spectrosc.Applied SpectroscopyAppl. Supercond.Applied SuperconductivityAppl. Surf. Sci.Applied Surface ScienceAppl. Therm. Eng.Applied Thermal EngineeringAquat. Toxicol.Aquatic ToxicologyArch. Biochem. Biophys.Archives of Biochemistry and BiophysicsArch. Environ. Contam. Toxicol.Archives of Environment Contamination and ToxicologyArch. Environ. HealthArchives of Environment HealthArch. Insect Biochem. Physiol.Archives of Insect Biochemistry and PhysiologyArch. Microbiol.Archives of MicrobiologyArch. Pharm.Archiv der PharmazieArch. Pharmacal Res.Archives of Pharmacal ResearchArch. Physiol. Biochem.Archives of Physiology and BiochemistryArch. Toxicol.Archives of ToxicologyArch. VirolArchives of VirologyArtif. Cells, Blood Substitues, Immobilization Biotechnol.Artificial Cells Blood Substitutes and Immobilization BiotechnologyArzneim.-Forsch.Arzneimittel-Forschung/Drug ResearchAsian J. Chem.Asian Journal of ChemistryAsian J. Spectro.Asian Journal of SpectroscopyASTM Spec. Tech. Publ.ASTM Specical Technical PublicationAstron. Astrophys.Astronomy and AstrophysicsAstron. J.Astronomy JournalAstrophys J.Astrophysics JournalAt. Data Nucl. Data TablesAtomic Data and Nuclear Data TablesAt. Energ.Atomic EnergyAt. Spectrosc.Atomic SpectroscopyAtmos. Environ.Atmospheric EnvironmentAtomization SpraysAtomization and SpraysAust. J. Chem.Australian Journal of ChemistryBehav. Pharmacol.Behavioural PharmacologyBer.Chemische BerichteBer. Bunsen-Ges. Phys. ChemBerichte der Bunsen-Gesellschaft Physical Chemistry Chemical PhysicsBiocatal. Biotransform.Biocatalysis and BiotransformationBiochem. Arch.Biochemical ArchivesBiochem. Biophys. Res. Commun.Biochemical and Biophysical Research CommunicationsBiochem. Cell Biol.Biochemistry and Cell Biology-Biochimie et Biologie CellulaireBiochem. Eng. J.Biochemical Engineering JournalBiochem. Genet.Biochemical GeneticsBiochem. JBiochemical JournalBiochem. Med. Metab. Biol.Biochemical Medicine and Metabolic BiologyBiochem. Mol. Biol. Int.Biochemistry and Molecular Biology InternationalBiochem. Mol. Med.Biochemical and Molecular MedicineBiochem. Pharmacol.Biochemical PharmacologyBiochem. Soc. Symp.Biochemical Society SymposiumBiochem. Soc. Trans.Biochemical Society TransactionsBiochem. Syst. Ecol.Biochemical Systematics and EcologyBiochim. Biophys. ActaBiochimica et Biophysica ActaBioconjugate Chem.Bioconjugate ChemistryBioelectrochem. Bioenerg. Bioelectrochemistry and BioenergeticsBiog. AminesBiogenic AminesBiol. Chem.Biological ChemistryBiol. Chem. Hoppe-SeylerBiological Chemistry Hoppe-SeylerBiol. Membr.Biological MembranesBiol. Pharm. Bull.Biological and Pharmaceutical BulletinBiol. Trace Elem. Res.Biological Trace Element ResearchBiomass BioenergyBiomass and BioenergyBiomed. Chromatogr.Biomedical ChromatographyBio-Med. Mater. Eng.Bio-Medical Materials and EngineeringBiomed. MicrodevicesBiomedical MicrodevicesBiomol. EngBiomolecular EngineeringBioorg. Chem.Bioorganic ChemistryBioorg. KhimBioorganicheskaya Khimiya (Russian Journal of Bioorganic Chemistry)Bioorg. Med. Chem.Bioorganic and Medicinal ChemistryBioorg. Med. Chem. Lett.Bioorganic and Medicinal Chemistry LettersBiopharm. Drug Dispos.Biopharmaceutics and Drug DispositionBiophys. Chem.Biophysical ChemistryBiophys. J .Biophysical JournalBioprocess. Eng.Bioprocess EngineeringBiorem. J.Bioremediation JournalBioresour. Technol.Bioresource TechnologyBiosci. Rep.Bioscience ReportsBiosci., Biotechnol., Biochem.Bioscience Biotechnology and BiochemistryBiosens. Bioelectron.Biosensors and BioelectronicsBiotech. Histochem.Biotechnic and HistochemistryBiotechnol. Adv.Biotechnology AdvancesBiotechnol. Appl. Biochem.Biotechnology and Applied BiochemistryBiotechnol. Bioeng.Biotechnology and BioengineeringBiotechnol. Biotechnol. Equip. Biotechnology and Biotechnological EquipmentBiotechnol. LettBiotechnology LettersBiotechnol. Progr.Biotechnology ProgressBiotechnol. Tech.Biotechnology TechniquesBol. Soc. Chil. Quim.Boletin de la Sociedad Chilena de QuimicaBr. Ceram. Trans.British Ceramic TransactionsBr. Corros. J.British Corrosion JournalBr. J. Pharmacol.British Jornal of PharmacologyBrennst.-Warme-KraftBrennstoff-Warme-KraftBull. Chem. Soc. Jpn.Bulletin of the Chemical Society of JapanBull. Electrochem.Bulletin of ElectrochemistryBull. Environ. Contam. Toxicol.Bulletin of Environment Contamination and ToxicologyBull. Hist. Chem.Bulletin for the History of ChemistryBull. Exp. Biol. Med.Bull. Korean Chem. Soc.Bulletin of the Korean Chemical SocietyBull. Mater. Sci.Bulletin of Materials ScienceBull. Pol. Acad. Sci., Chem.Bulletin of the Polish Academy of Sciences ChemistryBull. Soc. Chim. Belg.Bulletin des Societes Chimiques BelgesBull. Soc. Chim. Fr.Bulletin de la Societe Chimique de FranceC.R. Acad. Sci., Ser. IIIComptes Rendus de l' Academie des Sciences Serie III: Sciences de la VieC.R. Acad. Sci., Ser. IIa: Sci. Terre PlanetsComptes Rendus de l' Academie des Sciences Serie IIa:Sciences de la Terre et des PlanetsC.R. Acad. Sci., Ser. IIb: Mec., Phys., Chim., Astron.Comptes Rendus de l' Academie des Sciences Serie IIb:Mecanique Physique Chimie AstronomieC.R. Acad. Sci., Ser. IIc: Chim.Comptes Rendus de l' Academie des Sciences Serie IIc:ChemieCah. Inf. Tech./Rev MetallCahiers d'Informations Techniques / Revue de MetallurgieCalphadCalphad - Computer Coupling of Phase Diagrams and ThermochemistryCan. Ceram. Q.Canadian Ceramics QuarterlyCan. J. Anal. Sci. Spectros.Canadian Journal of Analytical Sciences and SpectroscopyCan. J. Biochem.Canadian Journal of BiochemistryCan. J. Chem.Canadian Journal of ChemistryCan. J. Chem. Eng.Canadian Journal of Chemical EngineeringCan. J. Microbiol.Canadian Journal of MicrobiologyCan. J. Phys.Canadian Journal of PhysicsCan. J. Physiol. Pharmacol.Canadian Journal of Physiology and PharmacologyCan. Metall. Q.Canadian Metallurgical QuarterlyCan. Min. J.Canadian Mining JournalCan. Mineral.Canadian MineralogistCarbohydr. Chem.Carbohydrate ChemistryCarbohydr. Lett.Carbohydrate LettersCarbohydr. Polym.Carbohydrate PolymersCarbohydr. Res.Carbohydrate ResearchCat. Rev. - Sci. Eng.Catalysis Reviews - Science and EngineeringCatal. Commun.Catalysis CommunicationsCatal. Lett.Catalysis LettersCatal. TodayCatalysis TodayCell Biochem. Funct.Cell Biochemistry and FunctionCell. Physiol. Biochem.Cellular Physiology and BiochemistryCell. Polym.Cellular PolymersCellul. Chem. Technol.Cellulose Chemistry and TechnologyCem. Concr. Compos.Cement and Concrete CompositesCem. Concr. Res.Cement and Concrete ResearchCeram. Int.Ceramics InternationalCeram. Silik.Ceramics - SilikatyCeram. Trans.Ceramic TransactionsCereal Chem.Cereal ChemistryChem. -Anlagen VerfahrenChemie Anlagen und VerfahrenChem. Anal. (Warsaw)Chemia AnalitycznaChem. Aust.Chemistry in AustraliaChem. Ber.Chemische BerichteChem. Ber. Recl.Chemische Berichte-RecueilChem. Biochem. Eng. Q.Chemical and Biochemical Engineering QuarterlyChem. Biol. Interact.Chemico-Biological InteractionsChem. Br.Chemistry in BritainChem. Commun.Chemical CommunicationsChem. Eng. (London)Chemical Engineer (London)Chem. Eng. (New York)Chemical Engineering (New York)Chem. Eng. Commun.Chemical Engineering CommunicationsChem. Eng. J.Chemical Engineering JournalChem. Eng. NewsChemical and Engineering NewsChemical Engineering and ProcessingChem. Eng. Prog.Chemical Engineering ProgressChem. Eng. Res. Des.Chemical Engineering Research and DesignChem. Eng. Sci.Chemical Engineering ScienceChem. Eng. Technol.Chemical Engineering and TechnologyChem. Eur. J.Chemistry- A European JournalChem. ExpressChemistry ExpressChem. Fibers Int.Chemical Fibers InternationalChem. Eur. J.Chemistry A European JournalChem. Geol.Chemical GeologyChem. Heterocycl. Compd.Chemistry of Heterocyclic CompoundsChem. Ind. (london)Chemistry and IndustryChem. Ing. Tech.Chemie Ingenieur TechnikChem. Lett.Chemistry LettersChem. ListyChemicke ListyChem. Mater.Chemistry of MaterialsChem. Nat. Compd.Chemistry of Natural CompoundsChem. Pap. - Chem. ZvestiChemical Papers - Chemicke ZvestiChem. Pharm. Bull.Chemical and Pharmaceutical BulletinChem. Phys.Chemical PhysicsChem. Phys. CarbonChemistry and Physics of CarbonChem. Phys. Lett.Chemical Physics LettersChem. Phys. LipidsChemistry and Physics of LipidsChem. Process.Chemical ProcessingChem. Res. Toxicol.Chemical Research in ToxicologyChem. Rev.Chemical ReviewsChem. SensesChemical SensesChem. Soc. Rev.Chemical Society ReviewsChem. Speciation Bioavailability Chemical Speciation and BioavailabilityChem. Tech.ChemTechChem. Tech. (Leipzig)Chemische TechnikChem. Technol. Fuels OilsChemistry and Technology of Fuels and OilsChem. Vap. DepositionChemical Vapor DepositionChem. WeekChemical WeekChem. unserer ZeitChemie in unserer ZeitChemom. Intell. Lab. Syst.Chemometrics and Intelligent Laborary SystemsChin. Chem. Lett.Chinese Chemical LettersChin. J. Chem .Chinese Journal of ChemistryChin. J. Chem. Eng.Chinese Journal of Chemical EngineeringChin. J. Nucl. Phys.Chinese Journal of Nuclear PhysicsChin. J. Polym. Sci.Chinese Journal of Polymer ScienceChin. Sci. Bull.Chinese Science BulletinChromatogr. Sci. Ser.Chromatographic Science SeriesCIM Bull.CIM BulletinClays Clay Miner.Clays and Clay MineralsClin. Biochem.Clinical BiochemistryClin. Chem.Clinical ChemistryClin. Chim. ActaClinica Chimica ActaCollect. Czech. Chem. Commun.Collection of Czechoslovak Chemical CommunicationsColloid J.Colloid JournalColloid. Polym. Sci.Colloid and Polymer ScienceColloids Surf., AColloids and Surfaces AColloids Surf., BColloids and Surfaces BComb. Chem. High Throughput ScreeningCombinatorial Chemistry and High Throughput ScreeningCombust. FlameCombustion and FlameCombust. Sci. Technol.Combustion Science and TechnologyComments Inorg. Chem.Comments on Inorganic ChemistryComp. Biochem. Physiol. A: Physiol.Comparative Biochemistry and Physiology A: PhysiologyComp. Biochem. Physiol. B: Biochem. Mol. Biol.Comparative Biochemistry and Physiology B: Biochemistry and Molecular BiologyComp. Biochem. Physiol. C: Pharmacol. Toxicol.Comparative Biochemistry and Physiology C: Pharmacology Toxicology and EndocrinologyCompos. Eng.Composites EngineeringCompos. InterfacesComposite InterfacesCompos. Sci. Technol.Composites Science and TechnologyCompos. Struct.Composite StructuresComposites Part AComposites Part A Applied Science and ManufacturingComposites Part BComposites Part B EngineeringComput. Chem. (Oxford)Computers and ChemistryComput. Chem. Eng.Computers and Chemical EngineeringComput. Mater. Sci.Computation Materials ScienceComput. Phys. Commun.Computer Physics CommunicationsComput. Theor. Polym. Sci.Computational and Theoretical Polymer ScienceConcepts Magn. Reson.Concepts in Magenetic ResonanceConcr. Eng. Int.Concrete Engineering InternationalConcr. Int.Concrete InternationalConcr. Sci. Eng.Concrete Science and EngineeringCondens. Matter Phys.Condensed Matter PhysicsCondens. Matter Theor.Condensed Matter TheoriesContinuum Mech. Thermodyn.Continuum Mechanics and ThermodynamicsContrib. Mineral. Petrol.Contributions to Mineralogy and PetrologyCoord. Chem. Rev.Coordination Chemistry ReviewsCorros. Rev.Corrosion ReviewsCorros. Sci.Corrosion ScienceCPP Chem. Plants and Process.CPP Chemical Plants and ProcessingCrit. Rev. Anal. Chem.Critical Reviews in Analytical ChemistryCrit. Rev. Biochem. Mol. Biol.Critical Reviews in Biochemistry and Molecular BiologyCrit. Rev. Biotechnol.Critical Reviews in BiotechnologyCrit. Rev. Env. Sci. Technol.Critical Reviews in Environment Science and TechnologyCrit. Rev. Solid State Mater. Sci.Critical Reviews in Solid State and Materials SciencesCrit. Rev. Toxicol.Critical Reviews in ToxicologyCroat. Chem. ActaCroatica Chemica ActaCryst. Eng.Crystal EngineeringCryst. Growth Des.Crystal Growth and DesignCryst. Res. Technol.Crystal Research and TechnologyCurr. Opin. Biotechnol.Current Opinion in BiotechnologyCurr. Opin. Chem. Biol.Current Opinion in Chemical BiologyCurr. Opin. Colloid Interface Sci.Current Opinion in Colloid and Interface ScienceCurr. Opin. Pharmacol.Current Opinion in PharmacologyCurr. Opin. Solid State Mater. Sci.Current Opinion in Solid State and Materials ScienceCurr. Org. Chem.Current Organic ChemistryCurr. Plant Sci. Biotechnol. Agric.Current Plant Science in Biotechnology and AgricultureCurr. Top. Membr.Current Topics in MembranesCurr. Top. Med. Chem.Current Topics in Medicinal ChemistryDent. Mater.Dental MaterialsDiamond Films Technol.Diamond Films and TechnologyDiamond Relat. Mater.Diamond and Related MaterialsDokl. Akad. NaukDoklady Akademii NaukDrug Chem. Toxicol.Drug and Chemical ToxicologyDrug Dev. Ind. Pharm.Drug Development and Industrial PharmacyDrug Dev. Res.Drug Development ResearchDrug Discovery TodayDrug Discovery TodayDrying Technol.Drying TechnologyEEarth. Planet. Sci. Lett.Earth and Planetary Science LettersEcol. Eng.Ecological EngineeringEcon. Geol.Economic Geology and the Bulletin of the Society of Economic GeologistsEcotoxicol. Environ. Saf.Ecotoxicology and Environment SafetyEduc. Chem.Education in ChemistryElectro- Magnetobiol.Electro- and MagnetobiologyElectrochem. Commun.Electrochemistry CommunicationsElectrochem. Soc. InterfaceElectrochemical Society InterfaceElectrochem. Solid-State Lett.Electrochemical and Solid-State LettersElectrochim. ActaElectrochimica ActaElectron. LettElectronics LettersElectron Technol.Electronic TechnologyEMBO J.EMBO JournalEnergy Convers. Manage.Energy Conversion and ManagementEnergy FuelsEnergy and FuelsEng. Min. J.Engineering and Mining JournalEnviron. Carcinog. Ecotoxicol. Rev.Environment Carcinogenesis and Ecotoxicology ReviewsEnviron. Geochem. HealthEnvironmental Geochemistry and HealthEnviron. Geol.Environmental GeologyEnviron. Health Perspect.Environmental Health PerspectivesEnviron. Microbiol.Environmental MicrobiologyEnviron. Monit. Assess.Environmental Monitoring and AssessmentEnviron. Pollut.Environmental PollutionEnviron. Prog.Environmental ProgressEnviron. Res.Environmental ResearchEnviron. Sci. Technol.Environmental Science and TechnologyEnviron. Technol.Environmental TechnologyEnviron. Toxicol. Chem.Environmental Toxicology and ChemistryEnviron. Toxicol. Pharmacol.Environmental Toxicology and PharmacologyEnviron. Toxicol. Water Qual.Environmental Toxicology and Water QualityEnzyme Microb. Technol.Enzyme and Microbial TechnologyEnzyme ProteinEnzyme and ProteinErdol and Kohle Erdgas, Petrochem.Erdol and Kohle Erdgas, PetrochemieEur. J. Biochem.European Journal of BiochemistryEur. J. Clin. Chem. Clin. Biochem.European Journal of Clinical Chemistry and Clinical BiochemistryEur. J. Inorg. Chem.European Journal of Inorganic ChemistryEur. J. Lipid Sci. Technol.European Journal of Lipid Science and TechnologyEur. J. Mass Spectrom.European Journal of Mass SpectrometryEur. J. Med. Chem.European Journal of Medical ChemistryEur. J. Mineral.European Journal of MineralogyEur. J. Org. Chem.European Journal of Organic ChemistryEur. J. Pharmacol.European Journal of PharmacologyEur. J. Solid State Inorg. Chem.European Journal of Solid State and Inorganic ChemistryEur. Mass Spectrom.European Mass SpectrometryEur. Polym. J.European Polymer JournalEurophys. Lett.Europhysics LettersExp. FluidsExperiments in FluidsExp. Therm Fluid Sci.Experimental Thermal and Fluid ScienceExplor. Min. Geol.Exploration and Mining GeologyFaraday Discuss.Faraday DiscussionsFASEB J.FASEB JournalFatigue Fract. Eng. Mater. Struct.Fatigue and Fracture of Engineering Materials and StructuresFEBS Lett.FEBS LettersFEMS Immunol. Med. Microbiol.FEMS Immunology And Medical MicrobiologyFEMS Microbiol. Ecol.FEMS Microbiology EcologyFEMS Microbiol. Lett.FEMS Microbiology LettersFEMS Microbiol. Rev.FEMS Microbiology ReviewFerroelectr. Rev.Ferroelectrics ReviewFerroelectr. Lett.Ferroelectrics LettersFett - LipidFett - LipidFiber Integr. Opt.Fiber and Integrated OpticsField Anal. Chem. Technol.Field Analytical Chemistry and Technology.Filtr. Sep.Filtration and SeparationFiz. Met. Metalloved.Fizika Metallov i MetallovedenieFluid/Part. Sep. J.Fluid/Particle Separation JournalFluid Phase Equilib.Fluid Phase EquilibriaFold Des.Folding and DesignFood Addit. Contam.Food Additives and ContaminantsFood Biotechnol.Food BiotechnologyFood Chem.Food ChemistryFood Chem. Toxicol.Food and Chemical ToxicologyFood Sci. Technol. Int.Food Science and Technology InternationalFree Radical Biol. Med.Free Radical Biology and MedicineFree Radical Res.Free Radical ResearchFresenius Environ. Bull.Fresenius Environment bulletinFresenius J. Anal. Chem.Fresenius Journal of Analytical ChemistryFront Sci. Ser.Frontier Science SeriesFuel Process. Technol.Fuel Processing TechnologyFuel Sci. Technol. Int.Fuel Science and Technology InternationalFullerene Sci. Technol.Fullerene Science and TechnologyFunct. Integr. GenomicsFunctional and Integrative GenomicsFundam. Appl. Toxicol.Fundamental and Applied ToxicologyFusion Eng. Des.Fusion Engineering and DesignFusion Technol.Fusion TechnologyGGalvanotechnikGalvanotechnikGas Sep. Purif.Gas Separation and PurificationGazz. Chim. Ital.Gazzetta Chimica ItalianaGefahrstoffe - Reinhalt. LuftGefahrstoffe Reinhaltung der LuftGenet. Anal. - Biomol. Eng.Genetic Analysis - Biomolecular EngineeringGeochem. Geophys. Geosyst.Geochemistry, Geophysics, GeosystemsGeochem. J.Geochemical JournalGeochem. Trans.Geochemical TransactionsGeochem.: Explor. Environ., Anal. Geochemistry: Exploration, Environment, AnalysisGeochim. Cosmochim. ActaGeochimica et Cosmochimica ActaGeol GeofizGeologiya i GeofizikaGeomicrobiol. J.Geomicrobiology JournalGlass Ceram.Glass and CeramicsGlass Phys. ChemGlass Physics and ChemistryGlass Res.Glass ResearchGlass Sci. Technol.Glass Science and TechnologyGlass Technol.Glass TechnologyGlobal Biogeochem. CyclesGlobal Biogeochemical CyclesGlobal J. Pure Appl. Sci.Global Journal of Pure and Applied SciencesGlycoconjugate J.Glycoconjugate JournalGreen Chem.Greem ChemistryGround Water Monit. Rem.Ground Water Monitoring and RemediationHandb. Exp. Pharmacol.Handbook of Experimental Pharmacology。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
- hPXR assay - Primary culture of human hepatocytes
Enzyme activity mRNA
• Clearly very helpful in selection of drug candidates • But is quantitative in vitro/in vivo prediction possible?
impaired clinical conditions)
6
Induction
• Three major concerns for induction:
- Reduction in therapeutic efficacy (most common) - Autoinduction (time-dependent PK) - Imbalance between toxification/detoxification
induction
9
Some hypothetical examples
Drug A
5-day Mouse
hPXR (EC50, uM)
>10 uM
mRNA (% of Rif1)
30-50%
Enzyme Act. (% of Rif1)
30-50%
Cmax Conc.2
15 uM
B
5 uM
50-60%
Concentration response info often primarily for efficacy
For probe substrates, still not much consensus (though much progress for CYP3A4 inhibition)
Induction, in particular, has been problematic to interpret
November 3, 2019
1
Outline of this Presentation
• Approach to assessing drug interactions? • Areas of Agreement with the “concept paper” • Areas for further discussion:
- In vivo variability
Rifampin fold-decreases on AUC of midazolam in humans: 11-55
• Effect of plasma protein binding on CYP induction • Involvement of multiple mechanisms in CYP
- But:
There are areas where the science is evolving… Necessary tools (e.g. probes) are still lacking…
3
Approach to Assessing Drug Interactions (2)
• How do we use the data to answer the primary question?
• Dose- and time- dependent • Dependent also on clearance and route of administration • A concern both with initiation and discontinuation of
interacting drug
- CYP Induction - Transporters - Multiple Inhibitors/Multiple Impaired (QTc)
• Not covered today:
- Specific comments on the concept paper
Study designs Tables
12
Induction of CYP3A4: effect on oral midazolam AUC
% B aselin e E xp o su re
80
60
40
20
0
Rifam pin Anticonvulsants SJW
G lucocorticoids
13
Induction of CYP3A4: effect on oral midazolam AUC
- Additional clinical data sometimes help:
Evidence of autoinduction, or evidence of less accumulation than expected
• How do we interpret an in vivo study?
• Which DDI to study and how to answer?
- We are moving from the “past” where choice was largely determined by the likelihood of co-administration/clinical consequences of an interaction
- Goal: science-driven approach (where feasible)
Preclinical in vitro studies to determine in vivo studies to conduct in vivo studies using probe substrates Robust study designs are critical
• Use of PK in poor metabolizers (where appropriate) • Robust study designs • Useful and consistent labeling language
- Still some discussion needed on how to label “moderate inhibitors” and how to define “sensitive” CYP substrates
- Pre-specified criteria to compare the PK (or PD) measures for the substrate drug in the presence of the interacting drug Based on the safety and efficacy profile of the substrate drug (e.g., therapeutic index), clinical context of the use of the drug, and concentration-response data for the substrate Often not clearly “positive” or “negative” For the NCE, often data is limited
- The “devil is in the details”….
5
Science-driven approach: where we are today with in vitro predictions of in vivo DDI’s?
• Ready for “prime-time”:
- CYP inhibition (1A2, 2C9, 2C19, 2D6, 3A4)
70-80%
2 uM
C
2.6 uM
30-50%
~40%
<200 nM
D
E
1 uM N/A
60-70% N/A
~0%
<400 nM
(also a CYP inhibitor)
<30%
<400 nM
1 Percent of 10 uM rifampin control with drug tested at 1 and 10 uM; 2 Cmax in humans at clinical dose
8
Factors that complicate the in vitro/in vivo extrapolation • Interindividual variability
- In vitro variability
Rifampin fold-increases on CYP3A4 protein: 3-24
• Almost ready for “prime time”:
- P-glycoprotein (P-gp)? - UGT1A1 (and other UGTs) - CYP2B6 and CYP2C8 - CYP induction
• Not ready for “prime time”:
- Most transporters - Multiple paths of inhibition (interacting drugs/ genetics/
7
How to assess for the potential for CYP induction?
• Animal models
- Previously, short-term, high dose studies in rodents - Poor predictor? Species differences in induction?
4
Areas of Agreement
• Integrated and scientific approach • Clarity on CYP interactions – very useful
