chapter thirteen American government
美国宪法-英文原版带中文翻译

主条目:美国宪法第七章
宪法第七章规定了这部宪法本身得以生效的表决程序。起初美国宪法 作为邦联条例的修正形式,需要获得全部 13 个州的批准方能成立。 然而宪法第七章只要求获得 9 个州以上的批准就可以使宪法生效。为 此,许多学者认为一旦只有 9 个州批准了这部宪法草案,那么将从原 有的邦联中脱离出来,成立一个新的联邦体国家。而不批准的其余州 将留在旧邦联体制内。事实上,这种理论并没有得到实践的印证,因 为 13 个州最终全部批准了这部宪法。
1786年9 月,5 个州的行政长官在安那波利斯举行会议,讨论如何修 改邦联条例以促进各州之间的通商往来。会后他们邀请各州的代表来 到费城进一步讨论发展联邦政府的事宜。在激烈的辩论之后,邦联国 会在1787年2 月 21 日批准了修订邦联条约的方案。除罗德岛州之外 的 12 个州都接受了邀请,并派代表参加 1787 年 5 月在费城举行的会 议。最初的决议案写明了这次会议的目的是起草邦联条例的修正案, 但是会议最终决定重新起草一部宪法。费城制宪会议代表投票同意采 用秘密会议的方式,并且同意新的法案需要获得 13 个州中的 9 个州 的批准才能生效。有人批评说这是对会议权限和现行法律的逾越。但 是对于邦联体制下的政府极度不满的会议代表全体一致同意将宪法 草案交付各州表决。1787 年9 月 17日,宪法在费城正式完稿,此后 经过数个州立法机构伴随激烈辩论的批准过程,1788 年 6 月 21 日新 罕布什尔州成为第九个接纳宪法州。邦联议会随即设置了宪法运作的 时间表,在宪法框架内运作的联邦政府终于在1789年3 月 4日成立。
其中译文如下:
我们合众国人民,为建立更完善的联邦,树立正义,保障国内安宁, 提供共同防务,促进公共福利,并使我们自己和后代得享自由的幸福, 特为美利坚合众国制定本宪法。
英语国家概况:Chapter 10 Government(美国政府)

V . Foreign Policy 1. Neutrality 2. Containment and Intervention
Ⅰ. Constitution
ernment
CONTENTS
01
The Fcial system & state judicial system
Difference between federal court
and state court
Ⅲ. Political Parties
2022
Election
Ⅴ.Foreign Policy
Review Questions
foreign policy?
Thank you!
03 The Executive
3.1 Departments
3.2 Functions of The President
3.3 Qualifications of The President
3.4 White House
3.1 Departments
The executive branch consists of 15 departments and many independent agencies; Cabinet: major source of advice and assistance to the president President: the chief of the executive branch. First citizen & First Lady
T
III. Political Parties 1. Two-party system
美利坚独立宣言英文版

美利坚独立宣言英文版The Declaration of Independence was the first formal statement by a nation's people asserting their right to choose their own government. It was adopted by the Continental Congress on July 4, 1776, and declared the 13 American colonies independent from British rule.The declaration is based on the idea that all men are created equal and have the unalienable rights of life, liberty, and the pursuit of happiness. It also asserts that governments exist to protect these rights, and that if a government fails to do so, the people have the right to alter or abolish it.The declaration details a long list of grievances against King George III, accusing him of violating the colonists' rights and liberties. It also emphasizes the colonists'repeated attempts to peacefully address their concerns and the King's failure to respond.The document concludes with a formal declaration of independence, asserting the colonies' right to be free and independent states, with full power to make war, conclude peace, contract alliances, establish commerce, and to do all other acts and things which independent states may of right do.The Declaration of Independence was a significant step towards establishing the United States as a free and independent nation. It has since become a symbol of freedom and democracy around the world, inspiring other movements for independence and self-determination.。
Chapter 9 American government

Foreign Language Teaching and Research Press
3. Executive Branch President is chief of the executive. first citizen his wife—First Lady Cabinet is formed by department heads— secretaries; ; source of advice and assistance to the president
Foreign Language Teaching and Research Press
4. Legislative Branch— Congress
Law-making and the supreme legislative body; Two houses: • the Senate • the House of Representatives The Capitol is no longer opened to the public after the terror event on Sept. 11th, 2001.
Constitution—basic instrument law, drawn up in 1787, came into effect in 1789.
Foreign Language Teaching and Rest
The Federal Government—the central government. Three equal and separate branches: : The Executive branch The Legislative branch The Judicial branch They are checked and balanced by one another.
THE UNANIMOUS DECLARATION OF THE THIRTEEN UNITED STATES OF AMERICA(中英文对照)

The Declaration of Independence英属北美殖民地人民宣布独立的纲领性文件。
1775年北美殖民地爆发了独立战争。
1776年7月4日第2届大陆会议通过了《独立宣言》,是日定为美国国庆日。
资产阶级民主派T.杰斐逊是宣言的主要起草人。
宣言继承和发展了天赋人权和社会契约理论,阐述了殖民地人民争取独立的理论根据。
宣言宣布,一切人生而平等,上帝赋予他们诸如生存、自由和追求幸福等不可让与的权利。
指出,为保障上述权利,人们才建立政府,任何政府一旦损害这些权利,人们就有权改换它或废除它,建立新政府。
宣言列举和痛斥了英王对殖民地实施的暴政,向全世界庄严宣告北美殖民地脱离英国,由独立的美利坚合众国正式成立。
《独立宣言》第一次以政治纲领形式确立了资产阶级的革命原则——人权原则。
The Declaration,which explained why the Colonies(now States)declared their independence,was adopted by the Continental Congress July4,1776.The leading draftsman was Thomas Jefferson,assisted by John Adams,Benjamin Franklin, Robert R.Livingston,and Roger Sherman.]The text follows below.The UnanimousDeclaration of theThirteen UnitedStates of America美利坚合众国13个州的一致宣言WHEN in the Course of human Events,it becomes necessary for one People to dissolve the Political Bands which have connected them with another,and to assume among the Powers of the Earth,the separate and equal Station to which the Laws of Nature and of Nature's God entitle them,a decent Respect to the Opinions of Mankind requires that they should declare the causes which impel them to the Separation.在有关人类事务的发展过程中,当一个民族必须解除其和另一个民族之间的政治联系并在世界各国之间依照自然法则和上帝的意旨,接受独立和平等的地位时,出於对人类舆论的尊重,必须把他们不得不独立的原因予以宣布。
美国《联邦规章典集》(CFR)第21篇“食品与药品”总目
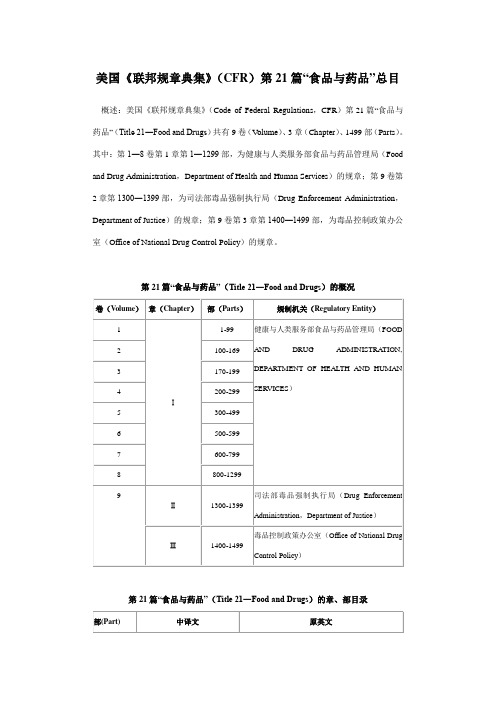
美国《联邦规章典集》(CFR)第21篇“食品与药品”总目概述:美国《联邦规章典集》(Code of Federal Regulations,CFR)第21篇“食品与药品”(Title 21―Food and Drugs)共有9卷(V olume)、3章(Chapter)、1499部(Parts)。
其中:第1―8卷第1章第1―1299部,为健康与人类服务部食品与药品管理局(Food and Drug Administration,Department of Health and Human Services)的规章;第9卷第2章第1300―1399部,为司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)的规章;第9卷第3章第1400―1499部,为毒品控制政策办公室(Office of National Drug Control Policy)的规章。
第21篇“食品与药品”(Title 21―Food and Drugs)的概况卷(Volume)章(Chapter)部(Parts)规制机关(Regulatory Entity)1Ⅰ1-99健康与人类服务部食品与药品管理局(FOODAND DRUG ADMINISTRA TION,DEPARTMENT OF HEALTH AND HUMANSERVICES)2100-1693170-1994200-2995300-4996500-5997600-7998800-12999Ⅱ1300-1399司法部毒品强制执行局(Drug EnforcementAdministration,Department of Justice)Ⅲ1400-1499毒品控制政策办公室(Office of National DrugControl Policy)第21篇“食品与药品”(Title 21―Food and Drugs)的章、部目录部(Part)中译文原英文第Ⅰ章―健康与人类服务部食品与药品管理局(CHAPTERADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)第A分章―总则(SUBCHAPTER A―GENERAL)1一般强制执行规章GENERAL ENFORCEMENT REGULA TIONS2一般行政规则与决定GENERAL ADMINISTRA TIVE RULINGS ANDDECISIONS3产品管辖权PRODUCT JURISDICTION5组织ORGANIZA TION7强制执行政策ENFORCEMENT POLICY10行政规范与程序ADMINISTRA TIVE PRACTICES ANDPROCEDURES11电子化记录;电子化签名ELECTRONIC RECORDS; ELECTRONICSIGNA TURES12正式证据的公众听证FORMAL EVIDENTIARY PUBLIC HEARING13在公众质询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC BOARD OF INQUIRY14在公众咨询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC ADVISORY COMMITTEE15在FDA局长前的公众听证PUBLIC HEARING BEFORE THECOMMISSIONER16在FDA前的规制性听证REGULA TORY HEARING BEFORE THE FOODAND DRUG ADMINISTRA TION17行政罚款听证CIVIL MONEY PENALTIES HEARINGS19行为标准与利益冲突STANDARDS OF CONDUCT AND CONFLICTSOF INTEREST20公共信息PUBLIC INFORMA TION21隐私保护PROTECTION OF PRIVACY25环境影响考虑ENVIRONMENTAL IMPACT CONSIDERA TIONS26药品良好制造规范报告、医疗器械质量体系核查报告以及某些医疗器械产品评价报告的互认:美国与欧共体MUTUAL RECOGNITION OFPHARMACEUTICAL GOOD MANUFACTURINGPRACTICE REPORTS, MEDICAL DEVICEQUALITY SYSTEM AUDIT REPORTS, ANDCERTAIN MEDICAL DEVICE PRODUCTEV ALUA TION REPORTS: UNITED STA TES ANDTHE EUROPEAN COMMUNITY50人类受试者的保护PROTECTION OF HUMAN SUBJECTS54临床试验者的财务公开FINANCIAL DISCLOSURE BY CLINICALINVESTIGA TORS56机构审查委员会INSTITUTIONAL REVIEW BOARDS58对非临床实验室研究的良好实验室规范GOOD LABORA TORY PRACTICE FOR NONCLINICAL LABORA TORY STUDIES60专利期恢复PA TENT TERM RESTORA TION70色素添加剂COLOR ADDITIVES71色素添加剂申请COLOR ADDITIVE PETITIONS73免除认证的色素添加剂的列表LISTING OF COLOR ADDITIVES EXEMPTFROM CERTIFICA TION74适用认证的色素添加剂的列表LISTING OF COLOR ADDITIVES SUBJECT TOCERTIFICA TION80色素添加剂认证COLOR ADDITIVE CERTIFICA TION81用于食品、药品和化妆品的临时性色素添加剂的一般规范和一般限制GENERAL SPECIFICA TIONS AND GENERALRESTRICTIONS FOR PROVISIONAL COLORADDITIVES FOR USE IN FOODS, DRUGS, ANDCOSMETICS82经认证的临时性列表的色素和规范的列表LISTING OF CERTIFIED PROVISIONALL Y LISTED COLORS AND SPECIFICA TIONS83-98[预留的][Reserved]99已上市的药品、生物制品和器械的未经批准的/新的用途的信息的发布DISSEMINA TION OF INFORMA TION ONUNAPPROVED/NEW USES FOR MARKETEDDRUGS, BIOLOGICS, AND DEVICES第B分章―用于人类消费的食品(SUBCHAPTER B―FOOD FOR HUMAN CONSUMPTION)100总则GENERAL101食品标识FOOD LABELING102非标准化食品的普通的或者通常的名称COMMON OR USUAL NAME FOR NONSTANDARDIZED FOODS104食品的营养质量指南NUTRITIONAL QUALITY GUIDELINES FORFOODS105特殊膳食用途的食品FOODS FOR SPECIAL DIETARY USE106婴儿配方母乳替代食品质量控制程序INFANT FORMULA QUALITY CONTROL PROCEDURES107婴儿配方母乳替代食品INFANT FORMULA108紧急许可控制EMERGENCY PERMIT CONTROL109在人类食品与食品-包装材料中的不可避免的污染物UNA VOIDABLE CONTAMINANTS IN FOODFOR HUMAN CONSUMPTION ANDFOOD-PACKAGING MA TERIAL110在制造、包装或者保存人类食品中的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE IN MANUFACTURING, PACKING, ORHOLDING HUMAN FOOD113装在密封容器中的热加工低酸食品THERMALL Y PROCESSED LOW-ACID FOODSPACKAGED IN HERMETICALL Y SEALEDCONTAINERS114酸化食品ACIDIFIED FOODS 115带壳蛋SHELL EGGS119存在显著或者不合理风险的膳食补充剂DIETARY SUPPLEMENTS THA T PRESENT A SIGNIFICANT OR UNREASONABLE RISK120危害分析与关键控制点(HACCP)体系HAZARD ANALYSIS AND CRITICAL CONTROL POINT (HACCP) SYSTEMS123鱼与渔业产品FISH AND FISHERY PRODUCTS129饮用水加工与装瓶PROCESSING AND BOTTLING OF BOTTLEDDRINKING WA TER130食品标准:总则FOOD STANDARDS: GENERAL131乳与奶油MILK AND CREAM133乳酪与相关乳酪产品CHEESES AND RELA TED CHEESE PRODUCTS 135冷冻点心FROZEN DESSERTS136烘焙产品BAKERY PRODUCTS137谷物粉与相关产品CEREAL FLOURS AND RELA TED PRODUCTS 139通心粉与面条产品MACARONI AND NOODLE PRODUCTS145罐装水果CANNED FRUITS146罐装水果汁CANNED FRUIT JUICES150水果黄油、果冻、防腐剂以及相关产品FRUIT BUTTERS, JELLIES, PRESERVES, AND RELA TED PRODUCTS152水果馅饼FRUIT PIES155罐装蔬菜CANNED VEGETABLES156蔬菜汁VEGETABLE JUICES158冷冻蔬菜FROZEN VEGETABLES160蛋与蛋制品EGGS AND EGG PRODUCTS161鱼与有壳的水生动物FISH AND SHELLFISH163可可制品CACAO PRODUCTS164树坚果与花生制品TREE NUT AND PEANUT PRODUCTS 165饮料BEVERAGES166人造黄油MARGARINE168增甜剂与餐桌糖浆SWEETENERS AND TABLE SIRUPS169食品敷料与调味料FOOD DRESSINGS AND FLA VORINGS 170食品添加剂FOOD ADDITIVES171食品添加剂申请FOOD ADDITIVE PETITIONS172允许直接加入用于人类消费食品的食品添加剂FOOD ADDITIVES PERMITTED FOR DIRECTADDITION TO FOOD FOR HUMANCONSUMPTION173在用于人类消费的食品中允许的次直接的食品添加剂SECONDARY DIRECT FOOD ADDITIVESPERMITTED IN FOOD FOR HUMANCONSUMPTION174间接食品添加剂:总则INDIRECT FOOD ADDITIVES: GENERAL175间接食品添加剂:胶粘剂与涂层的组分INDIRECT FOOD ADDITIVES: ADHESIVES AND COMPONENTS OF COA TINGS176间接食品添加剂:纸与纸板组分INDIRECT FOOD ADDITIVES: PAPER ANDPAPERBOARD COMPONENTS177间接食品添加剂:聚合体INDIRECT FOOD ADDITIVES: POL YMERS178间接食品添加剂:辅剂、生产助剂和消毒剂INDIRECT FOOD ADDITIVES: ADJUVANTS, PRODUCTION AIDS, AND SANITIZERS179在食品生产、加工和处理中的辐照IRRADIA TION IN THE PRODUCTION, PROCESSING AND HANDLING OF FOOD180在额外试验期间临时在食品或者在与食品接触中被允许的食品添加剂FOOD ADDITIVES PERMITTED IN FOOD OR INCONTACT WITH FOOD ON AN INTERIM BASISPENDING ADDITIONAL STUDY181先前核准的食品配料PRIOR-SANCTIONED FOOD INGREDIENTS182一般认为安全的物质SUBSTANCES GENERALL Y RECOGNIZED ASSAFE184被确认为一般认为安全的直接食品物质DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALL Y RECOGNIZED AS SAFE186被确认为一般认为安全的间接INDIRECT FOOD SUBSTANCES AFFIRMED AS食品物质GENERALL Y RECOGNIZED AS SAFE 189禁止用于人类食品的物质SUBSTANCES PROHIBITED FROM USE INHUMAN FOOD190膳食补充剂DIETARY SUPPLEMENTS191-199[预留的][Reserved]第C分章―药品:总则(SUBCHAPTER C―DRUGS: GENERAL)200总则GENERAL201标识LABELING202处方药广告PRESCRIPTION DRUG ADVERTISING203处方药销售PRESCRIPTION DRUG MARKETING205对批发处方药销售商颁发州执照的指南GUIDELINES FOR STA TE LICENSING OFWHOLESALE PRESCRIPTION DRUGDISTRIBUTORS206人用固体口服剂型药品的印码IMPRINTING OF SOLID ORAL DOSAGE FORMDRUG PRODUCTS FOR HUMAN USE207药品生产者的登记与商业销售的药品的列表REGISTRA TION OF PRODUCERS OF DRUGSAND LISTING OF DRUGS IN COMMERCIALDISTRIBUTION208处方药的药物治疗指导MEDICA TION GUIDES FOR PRESCRIPTIONDRUG PRODUCTS210制造、加工、包装或者保存药品的现行良好制造规范;总则CURRENT GOOD MANUFACTURINGPRACTICE IN MANUFACTURING, PROCESSING,PACKING, OR HOLDING OF DRUGS; GENERAL211对完成的药品的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR FINISHEDPHARMACEUTICALS216药房配药PHARMACY COMPOUNDING225对含药饲料的现行良好制造规CURRENT GOOD MANUFACTURING范PRACTICE FOR MEDICA TED FEEDS226对A型含药物品的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR TYPE A MEDICA TEDARTICLES250对特殊人用药品的特殊要求SPECIAL REQUIREMENTS FOR SPECIFICHUMAN DRUGS290管制的药品CONTROLLED DRUGS299药品;正式名称与已确定的名称DRUGS; OFFICIAL NAMES AND ESTABLISHEDNAMES第D分章―人用药品(SUBCHAPTER D―DRUGS FOR HUMAN USE)300总则GENERAL310新药NEW DRUGS312试验用新药申请INVESTIGA TIONAL NEW DRUG APPLICA TION 314为FDA批准上市新药的申请APPLICA TIONS FOR FDA APPROV AL TOMARKET A NEW DRUG315诊断用放射性药品DIAGNOSTIC RADIOPHARMACEUTICALS316罕见病药ORPHAN DRUGS320生物利用度与生物等效性要求BIOA VAILABILITY AND BIOEQUIVALENCEREQUIREMENTS328含有酒精的预期用于口部摄入的非处方药品OVER-THE-COUNTER DRUG PRODUCTSINTENDED FOR ORAL INGESTION THA TCONTAIN ALCOHOL330一般认为安全与有效以及不错误标识的非处方人用药品OVER-THE-COUNTER (OTC) HUMAN DRUGSWHICH ARE GENERALL Y RECOGNIZED ASSAFE AND EFFECTIVE AND NOTMISBRANDED331用于非处方的人类使用的抗酸产品ANTACID PRODUCTS FOR OVER-THE-COUNTER (OTC) HUMAN USE332用于非处方的人类使用的抗胃肠气胀产品ANTIFLA TULENT PRODUCTS FOR OVER-THE-COUNTER HUMAN USE333用于非处方的人类使用的局部抗菌药品TOPICAL ANTIMICROBIAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE335用于非处方的人类使用的止泻药品ANTIDIARRHEAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE336用于非处方的人类使用的止吐药品ANTIEMETIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE338用于非处方的人类使用的帮助夜间睡眠的药品NIGHTTIME SLEEP-AID DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE340用于非处方的人类使用的兴奋药品STIMULANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE341用于非处方的人类使用的感冒、咳嗽、过敏症药、支气管扩张以及平喘药品COLD, COUGH, ALLERGY, BRONCHODILA TOR,AND ANTIASTHMA TIC DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE343用于非处方的人类使用的内服的止痛、退热以及抗风湿药品INTERNAL ANALGESIC, ANTIPYRETIC, ANDANTIRHEUMA TIC DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE344用于非处方的人类使用的局部的耳部药品TOPICAL OTIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE346用于非处方的人类使用的肛肠药品ANORECTAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE347用于非处方的人类使用的皮肤保护药品SKIN PROTECTANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE348用于非处方的人类使用的外部的止痛药品EXTERNAL ANALGESIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE349用于非处方的人类使用的眼科药品OPHTHALMIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE350用于非处方的人类使用的止汗药品ANTIPERSPIRANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE352用于非处方的人类使用的遮光药品SUNSCREEN DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE [STAYEDINDEFINITEL Y]355用于非处方的人类使用的防龋药品ANTICARIES DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE357用于非处方的人类使用的其他内服药品MISCELLANEOUS INTERNAL DRUGPRODUCTS FOR OVER-THE-COUNTERHUMAN USE358用于非处方的人类使用的其他外用药品MISCELLANEOUS EXTERNAL DRUGPRODUCTS FOR OVER-THE-COUNTERHUMAN USE361一般认为安全与有效以及不错误标识的处方人用药品:用于研究的药品PRESCRIPTION DRUGS FOR HUMAN USEGENERALL Y RECOGNIZED AS SAFE ANDEFFECTIVE AND NOT MISBRANDED: DRUGSUSED IN RESEARCH369在用于非处方销售的药品与器械上关于警告的解释性声明INTERPRETA TIVE STA TEMENTS REW ARNINGS ON DRUGS AND DEVICES FOROVER-THE-COUNTER SALE370-499[预留的][Reserved]第E分章―动物药品、饮料和相关产品(SUBCHAPTER E―ANIMAL DRUGS, FEEDS, AND RELA TED PRODUCTS)500总则GENERAL501动物食品标识ANIMAL FOOD LABELING502非标准化的动物食品的普通的或通常的名称COMMON OR USUAL NAMES FOR NONSTANDARDIZED ANIMAL FOODS509在动物食品与食品-包装材料中UNA VOIDABLE CONTAMINANTS IN ANIMAL的不可避免的污染物FOOD AND FOOD-PACKAGING MA TERIAL510新动物药NEW ANIMAL DRUGS511作为试验用途的新动物药NEW ANIMAL DRUGS FOR INVESTIGA TIONALUSE514新动物药申请NEW ANIMAL DRUG APPLICA TIONS515含药饲料厂执照MEDICA TED FEED MILL LICENSE520口服剂型的新动物药ORAL DOSAGE FORM NEW ANIMAL DRUGS 522植入或者注射剂型的新动物药IMPLANTA TION OR INJECTABLE DOSAGEFORM NEW ANIMAL DRUGS524眼科和局部剂型的新动物药OPHTHALMIC AND TOPICAL DOSAGE FORMNEW ANIMAL DRUGS526乳房内的剂型INTRAMAMMARY DOSAGE FORMS529某些其他剂型的新动物药CERTAIN OTHER DOSAGE FORM NEWANIMAL DRUGS530在动物中的特别标签药品使用EXTRALABEL DRUG USE IN ANIMALS556在食品中新动物药残留的容许量TOLERANCES FOR RESIDUES OF NEW ANIMAL DRUGS IN FOOD558用于动物饲料的新动物药NEW ANIMAL DRUGS FOR USE IN ANIMALFEEDS564[预留的][Reserved]570食品添加剂FOOD ADDITIVES571食品添加剂申请FOOD ADDITIVE PETITIONS573在动物饲料与饮用水中允许的食品添加剂FOOD ADDITIVES PERMITTED IN FEED AND DRINKING WA TER OF ANIMALS579在动物饲料和宠物食品的生产、加工和处理中的辐照IRRADIA TION IN THE PRODUCTION,PROCESSING, AND HANDLING OF ANIMALFEED AND PET FOOD582一般认为安全的物质SUBSTANCES GENERALL Y RECOGNIZED ASSAFE584在动物饲料与饮用水中被确认为一般认为安全的食品物质FOOD SUBSTANCES AFFIRMED ASGENERALL Y RECOGNIZED AS SAFE IN FEEDAND DRINKING WA TER OF ANIMALS589禁止用于动物食品或者饲料的物质SUBSTANCES PROHIBITED FROM USE IN ANIMAL FOOD OR FEED590-599[预留的][Reserved]第F分章―生物制品(SUBCHAPTER F―BIOLOGICS)600生物制品:总则BIOLOGICAL PRODUCTS: GENERAL 601颁发执照LICENSING606对血液与血液组分的现行良好制造规范CURRENT GOOD MANUFACTURINGPRACTICE FOR BLOOD AND BLOODCOMPONENTS607对人类血液与血液制品的制造者的机构登记与产品列表ESTABLISHMENT REGISTRA TION ANDPRODUCT LISTING FOR MANUFACTURERS OFHUMAN BLOOD AND BLOOD PRODUCTS610普通生物制品标准GENERAL BIOLOGICAL PRODUCTSSTANDARDS630对血液、血液组分和血液衍生物的一般要求GENERAL REQUIREMENTS FOR BLOOD,BLOOD COMPONENTS, AND BLOODDERIVA TIVES640对人类血液和血液制品的附加标准ADDITIONAL STANDARDS FOR HUMAN BLOOD AND BLOOD PRODUCTS660对用于实验室检测的诊断物质的附加标准ADDITIONAL STANDARDS FOR DIAGNOSTIC SUBSTANCES FOR LABORATORY TESTS680对其他产品的附加标准ADDITIONAL STANDARDS FORMISCELLANEOUS PRODUCTS第G分章―化妆品(SUBCHAPTER G―COSMETICS)700总则GENERAL701化妆品标识COSMETIC LABELING710化妆品机构的自愿登记VOLUNTARY REGISTRA TION OF COSMETICPRODUCT ESTABLISHMENTS720化妆品配料构成声明的自愿存档VOLUNTARY FILING OF COSMETIC PRODUCT INGREDIENT COMPOSITION STA TEMENTS740化妆品警告声明COSMETIC PRODUCT WARNINGSTA TEMENTS741-799[预留的][Reserved]第H分章―医疗器械(SUBCHAPTER H―MEDICAL DEVICES)800总则GENERAL801标识LABELING803医疗器械报告MEDICAL DEVICE REPORTING806医疗器械;改正与移动的报告MEDICAL DEVICES; REPORTS OFCORRECTIONS AND REMOV ALS807对器械的制造者与首次进口者的机构登记与器械列表ESTABLISHMENT REGISTRA TION ANDDEVICE LISTING FOR MANUFACTURERS ANDINITIAL IMPORTERS OF DEVICES808对州和地方医疗器械要求的联邦优先权的豁免EXEMPTIONS FROM FEDERAL PREEMPTIONOF STA TE AND LOCAL MEDICAL DEVICEREQUIREMENTS809人用体外诊断产品IN VITRO DIAGNOSTIC PRODUCTS FORHUMAN USE810医疗器械召回权MEDICAL DEVICE RECALL AUTHORITY812试验用器械豁免INVESTIGA TIONAL DEVICE EXEMPTIONS813[预留的][Reserved]814医疗器械的上市前批准PREMARKET APPROVAL OF MEDICALDEVICES820质量体系规章QUALITY SYSTEM REGULA TION医疗器械跟踪要求MEDICAL DEVICE TRACKING 821REQUIREMENTS822上市后监视POSTMARKET SURVEILLANCE医疗器械分类程序MEDICAL DEVICE CLASSIFICA TION 860PROCEDURES性能标准制定程序PROCEDURES FOR PERFORMANCE 861STANDARDS DEVELOPMENT临床化学与临床毒理学器械CLINICAL CHEMISTRY AND CLINICAL862TOXICOLOGY DEVICES864血液学与病理学器械HEMA TOLOGY AND PA THOLOGY DEVICES 免疫学与微生物学器械IMMUNOLOGY AND MICROBIOLOGY866DEVICES868麻醉学器械ANESTHESIOLOGY DEVICES870心血管器械CARDIOVASCULAR DEVICES872牙科器械DENTAL DEVICES874耳、鼻和咽器械EAR, NOSE, AND THROA T DEVICES876胃肠病学-泌尿学器械GASTROENTEROLOGY-UROLOGY DEVICES878普通与整形外科器械GENERAL AND PLASTIC SURGERY DEVICES普通医院与个人使用器械GENERAL HOSPITAL AND PERSONAL USE880DEVICES882神经学器械NEUROLOGICAL DEVICES产科与妇科学器械OBSTETRICAL AND GYNECOLOGICAL 884DEVICES886眼科器械OPHTHALMIC DEVICES888矫形外科器械ORTHOPEDIC DEVICES890内科学器械PHYSICAL MEDICINE DEVICES892放射学器械RADIOLOGY DEVICES895禁止的器械BANNED DEVICES898电极铅线与患者电缆的性能标准PERFORMANCE STANDARD FOR ELECTRODE LEAD WIRES AND PA TIENT CABLES第I分章―乳房造影质量标准法(SUBCHAPTER I―MAMMOGRAPHY QUALITY STANDARDS ACT)900乳房造影法MAMMOGRAPHY第J分章―放射学的健康(SUBCHA PTER J―RADIOLOGICAL HEALTH)1000总则GENERAL1002记录与报告RECORDS AND REPORTS1003缺陷与未能守法的通报NOTIFICA TION OF DEFECTS OR FAILURE TOCOMPL Y1004电子产品的回购、修理或者置换REPURCHASE, REPAIRS, OR REPLACEMENTOF ELECTRONIC PRODUCTS1005电子产品的进口IMPORTA TION OF ELECTRONIC PRODUCTS1010电子产品的性能标准:总则PERFORMANCE STANDARDS FORELECTRONIC PRODUCTS: GENERAL 1020电离辐射发生产品的性能标准PERFORMANCE STANDARDS FOR IONIZINGRADIA TION EMITTING PRODUCTS1030微波与射电频率发生产品的性能标准PERFORMANCE STANDARDS FORMICROWA VE AND RADIO FREQUENCYEMITTING PRODUCTS1040发光产品的性能标准PERFORMANCE STANDARDS FORLIGHT-EMITTING PRODUCTS1050声波、次声波和超声波发生产品的性能标准PERFORMANCE STANDARDS FOR SONIC,INFRASONIC, AND ULTRASONICRADIA TION-EMITTING PRODUCTS第K分章―[预留的](SUBCHAPTER K―[RESERVED])第L分章―根据由食品与药品管理局行政执行的某些其他法的规章(SUBCHAPTERL―REGULA TIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRA TION)1210根据《联邦进口乳法》的规章REGULA TIONS UNDER THE FEDERAL IMPORTMILK ACT1230根据《联邦腐蚀性毒物法》的规章REGULA TIONS UNDER THE FEDERAL CAUSTIC POISON ACT1240传染病的控制CONTROL OF COMMUNICABLE DISEASES1250州际运输卫生INTERSTA TE CONVEYANCE SANITA TION1251-1269[预留的][Reserved]1270预期用于移植的人体组织HUMAN TISSUE INTENDED FORTRANSPLANTA TION1271人体细胞、组织以及细胞的和基于组织的产品HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS1272-1299[预留的][Reserved]第Ⅱ章―司法部毒品强制执行局(CHAPTER ADMINISTRATION, DEPARTMENT OF JUSTICE)1300定义DEFINITIONS1301管制物质的制造者、分销者和调剂者的登记REGISTRA TION OF MANUFACTURERS,DISTRIBUTORS, AND DISPENSERS OFCONTROLLED SUBSTANCES1302对管制物质的标识与包装要求LABELING AND PACKAGING REQUIREMENTSFOR CONTROLLED SUBSTANCES1303定额QUOTAS1304登记者的记录与报告RECORDS AND REPORTS OF REGISTRANTS 1305令的格式ORDER FORMS1306处方PRESCRIPTIONS1307杂项MISCELLANEOUS1308管制物质的表SCHEDULES OF CONTROLLED SUBSTANCES1309表I化学品的制造者、分销者、进口者和出口者的登记REGISTRA TION OF MANUFACTURERS,DISTRIBUTORS, IMPORTERS AND EXPORTERSOF LIST I CHEMICALS1310列入表的化学品和某些机器的记录与报告RECORDS AND REPORTS OF LISTED CHEMICALS AND CERTAIN MACHINES1311[预留的][Reserved]1312管制物质的进口与出口IMPORTA TION AND EXPORTA TION OFCONTROLLED SUBSTANCES1313前体与必要化学品的进口与出口IMPORTA TION AND EXPORTA TION OF PRECURSORS AND ESSENTIAL CHEMICALS1314-1315[预留的][Reserved]1316行政职能、规范和程序ADMINISTRA TIVE FUNCTIONS, PRACTICES,AND PROCEDURES第Ⅲ章―毒品控制政策办公室(CHAPTER Ⅲ―Off)1400[预留的][Reserved]1401信息的公众可及性PUBLIC A VAILABILITY OF INFORMA TION 1402强制性解密审查MANDATORY DECLASSIFICA TION REVIEW1403对给予州和地方政府资金和合作协议的统一行政要求UNIFORM ADMINISTRA TIVE REQUIREMENTSFOR GRANTS AND COOPERA TIVEAGREEMENTS TO STA TE AND LOCALGOVERNMENTS1404政府范围的排除与暂停(非获得)GOVERNMENTWIDE DEBARMENT AND SUSPENSION (NONPROCUREMENT)1405对无毒品工作场所的政府范围的要求(财政援助)GOVERNMENTWIDE REQUIREMENTS FORDRUG-FREE WORKPLACE (FINANCIALASSISTANCE)1406-1499[预留的][Reserved]。
研究生公共英语教材阅读B第3、4、10、11、14课文原文及翻译

Unite 3 Doctor’s Dilemma: Treat or Let Die?Abigail Trafford1. Medical advances in wonder drugs, daring surgical procedures, radiation therapies, and intensive-care units have brought new life to thousands of people. Yet to many of them, modern medicine has become a double-edged sword.2. Doctor’s power to treat with an array of space-age techniques has outstripped the body’s capacity to heal. More medical problems can be treated, but for many patients, there is little hope of recovery. Even the fundamental distinction between life and death has been blurred.3. Many Americans are caught in medical limbo, as was the South Korean boxer Duk Koo Kim, who was kept alive by artificial means after he had been knocked unconscious in a fight and his brain ceased to function. With the permission of his family, doctors in Las Vegas disconnected the life-support machines and death quickly followed.4. In the wake of technology’s advances in medicine, a heated debate is taking place in hospitals and nursing homes across the country --- over whether survival or quality of life is the paramount goal of medicine.5. “It gets down to what medicine is all about, ” says Daniel Callahan, director of the Institute of Society, Ethics, and the Life Sciences in Hastings-on-Hudson, New York. “Is it really to save a life? Or is the larger goal the welfare of the patient?”6. Doctors, patients, relatives, and often the courts are being forced to make hard choices in medicine. Most often it is at the two extremes of life that these difficultyethical questions arise --- at the beginning for the very sick newborn and at the end for the dying patient.7. The dilemma posed by modern medical technology has created the growing new discipline or bioethics. Many of the country’s 127 medical s chools now offer courses in medical ethics, a field virtually ignored only a decade ago. Many hospitals have chaplains, philosophers, psychiatrists, and social workers on the staff to help patients make crucial decisions, and one in twenty institutions has a special ethics committee to resolve difficult cases.Death and Dying8. Of all the patients in intensive-care units who are at risk of dying, some 20 percent present difficult ethical choices --- whether to keep trying to save the life or to pull back and let the patient die. In many units, decisions regarding life-sustaining care are made about three times a week.9. Even the definition of death has been changed. Now that the heart-lung machine can take over the functions of breathing and pumping blood, death no longer always comes with the patient’s “last gasp” or when the heart stops beating. Thirty-one states and the District of Columbia have passed brain-death statutes that identify death as when the whole brain ceases to function.10. More than a do zen states recognize “living wills” in which the patients leave instructions to doctors not to prolong life by feeding them intravenously or by other methods if their illness becomes hopeless. A survey of California doctors showed that 20 to 30 percent were following instructions of such wills. Meanwhile, the hospicemovement, which its emphasis on providing comfort --- not cure --- to the dying patient, has gained momentum in many areas.11. Despite progress in society’s understanding of death and dying, t heory issues remain. Example: A woman, 87, afflicted by the nervous-system disorder of Parkinson’s disease, has a massive stroke and is found unconscious by her family. Their choices are to put her in a nursing home until she dies or to send her to a medical center for diagnosis and possible treatment. The family opts for a teaching hospital in New York city. Tests show the woman’s stroke resulted from a blood clot that is curable with surgery. After the operation, she says to her family: “Why did you bring me back to this agony?” Her health continues to worsen, and two years later she dies.12. On the other hand, doctors say prognosis is often uncertain and that patients, just because they are old and disabled, should not be denied life-saving therapy. Ethicists also fear that under the guise of medical decision not to treat certain patients, death may become too easy, pushing the country toward the acceptance of euthanasia.13. For some people, the agony of watching high-technology dying is too great. Earlier this year, Woodrow Wilson Collums, a retired dairyman from Poteet, Texas, was put on probation for the mercy killing of his older brother Jim, who lay hopeless in his bed at a nursing home, a victim of severe senility resul ting from Alzheimer’s disease. After the killing, the victim’s widow said: “I think God, Jim’s out of his misery. I hate to think it had to be done the way it was done, but I understand it. ”Crisis in Newborn Care14. At the other end of the life span, technology has so revolutionized newborn carethat it is no longer clear when human life is viable outside the womb. Newborn care has got huge progress, so it is absolutely clear that human being can survive independently outside the womb. Twenty-five years ago, infants weighting less than three and one-half pounds rarely survived. The current survival rate is 70 percent, and doctors are “salvaging” some babies that weigh only one and one-half pounds. Tremendous progress has been made in treating birth deformities such as spina bifida. Just ten years ago, only 5 percent of infants with transposition of the great arteries --- the congenital heart defect most commonly found in newborns --- survived. Today, 50 percent live.15. Yet, for many infants who owe their lives to new medical advances, survival has come at a price. A significant number emerge with permanent physical and mental handicaps.16. “The question of treatment and nontreatment of seriously ill newborns is not a single one,”says Thomas Murray of the Hastings Center. “But I feel strongly that retardation or the fact that someone is going to be less than perfect is not good grounds for allowing an infant to die.”17. For many parents, however, the experience of having a sick newborn becomes a lingering nightmare. Two years ago, an Atlanta mother gave birth to a baby suffering from Down’s Syndrome, a form of mental retardation; the child also had blocked intestines. The doctors rejected the parents’ plea not to operate, and today the child, severely retarded, still suffers intestinal problems.18. “Every time Melanie has a bowel movement, she cries,” explains her mother.“She’s not able to take care of herself, and we won’t live forever. I wanted to save her from sorrow, pain, and suffering. I don’t understand the emphasis on life at all costs, and I’m very angry at the doctors and the hospital. Who will take care of Melanie after we’re gone? Where will you doctors be then?”Changing Standards19. The choices posed by modern technology have profoundly changed the practice of medicine. Until now, most doctors have been activists, trained to use all the tools in their medical arsenals to treat disease. The current trend is toward nontreatment as doctors grapple with questions not just of who should get care but when to take therapy away.20. Always in the background is the threat of legal action. In August, two California doctors were charged with murdering a comatose patient by allegedly disconnecting the respirator and cutting off food and water. In 1981, a Massachusetts nurse was charged with murdering a cancer patient with massive doses of morphine but was subsequently acquitted.21. Between lawsuits, government regulations, and patients’ rights, many doctors feel they are under siege. Modern technology actually has limited their ability to make choices. More recently, these actions are resolved by committees.Public Policy22. In recent years, the debate on medical ethics has moved to the level of national policy. “It’s just beginning to hit us that we don’t have unlimited resources,” says Washington Hospital Center’s Dr. Lynch. “You can’t talk about ethics without talkingethics without talking about money.”23. Since 1972. Americans have enjoyed unlimited access to a taxpayer-supported, kidney dialysis program that offers life-prolonging therapy to all patients with kidney failure. To a number of police analysts, the program has grown out of control --- to a $1.4billion operation supporting 61,000 patients. The majority are over 50, and about a quarter have other illness, such as cancer or heart disease, conditions that could exclude them from dialysis in other countries.24. Some hospitals are pulling back from certain lifesaving treatment. Massachusetts General Hospital, for example, has decided not perform heart transplants on the ground that the high costs of providing such surgery help too few patients. Burn units --- through extremely effective --- also provide very expensive therapy for very few patients.25. As medical scientists push back the frontiers of therapy, the moral dilemma will continue to grow for doctors and patients alike, making the choice of to treat the basic question in modern medicine.1. 在特效药、风险性手术进程、放疗法以及特护病房方面的医学进展已为数千人带来新生。
美国宪法全文(中、英文版)

美国宪法全文(中、英文版)篇一:美国宪法美利坚合众国宪法(1787年9月17日)序言我们合众国人民,为了建立一个更完善的联邦,树立正义,确保内部安宁,提供共同防御,增进公共福利,并保证我们自身和子孙后代永享自由的幸福,特制定美利坚合众国宪法。
第一条第一款本宪法所授予的全部立法权均属于由参议院和众议院组成的合众国国会。
第二款众议院由各州人民每两年选举产生的议员组成。
每个州的选举人应具备该州州议会人数最多一院的选举人所需具备的资格。
凡年龄不满25岁,成为合众国公民未满7年以及当选时非其选出州居民者,不得为众议院议员。
众议员人数和直接税税额均应按本联邦所辖各州的人口比例分配于各州。
各州人口数是指自由人总数加上所有其它人口的3/5.自由人总数包括必须在一定年限内服劳役的人,但不包括未被征税的印第安人。
人口的实际统计应于合众国国会第一次会议后3年内和此后每10年内,依法律规定的方式进行。
众议员人数以每3万人选出1人为限,但每州至少应有众议员1人;在实行此种人口统计前,新罕布什尔州可选出3人,马萨诸塞州8人,罗得岛州和普罗维登斯种植地1人,康涅狄格州5人,纽约州6人,新泽西州4人,宾夕法尼亚州8人,特拉华州1人,马里兰州6人,弗吉尼亚州10人,北卡罗来纳州5人,南卡罗来纳州5人,佐治亚州3人。
任何一州所选众议员中出现缺额时,该州行政长官应发布选举令以补足此项缺额。
众议院应选举本院议长和其它官员,并独自享有弹劾权。
第三款合众国参议院由每州州议会选出的两名参议员组成,参议员任期6年,每名参议员有一票表决权。
参议员在第一次选举后集会时,应即尽可能平均分为三组:第一组参议员应于第二年年终改选,第二组参议员应于第四年年终改选,第三组参议员应于第六年年终改选,以便每两年改选参议员总数的l/3.在任何一州州议会休会期间,如因辞职或其它原因而出现参议员缺额,该州行政长官在州议会召开下次会议补足缺额之前,任命临时参议员。
凡年龄未满30岁,为合众国公民末满9年以及当选时非其选出州居民者,不得为参议员。
《英语国家概况》课后题参考答案

《英语国家概况》课后题参考答案Chapter Thirteen Geography1. How many states are there in the United States? Which one is the largest and which one is the smallest?There are 50 states in the United States. The largest one in area is Alaska and Rhode Island the smallest.2. Why does the United States have an ideal location for trade?The United States has an ideal location for trade. Its Atlantic coast faces the developed countries of Western Europe and its Pacific coast and Hawaii give the nation an approach to the Far East and Australasia. So the United States is well connected to the rest of the world.3. Look at a physical map of the United States and find out and name the main mountain ranges, rivers and lakes in the United States.There are two main mountain ranges in the United States. They are the Appalachian mountains and the Rocky mountains. Many important rivers in the United States include the Mississippi River and its two tributaries- the Missouri and the Ohio, two great rivers on the Pacific coast the Colorado and the Columbia, the Rio Grande River, the Hudson River and the Potomac. The most important lakes in the United States are the Great Lakes including Lake Superior, Lake Michigan, Lake Huron, Lake Erie and Lake Ontario. They are located between Canada and the United States except Lake Michigan.4. What are the benefits of the lakes, rivers and seacoasts of the United States?The Lakes and rivers form a complete system of water ways which provides cheap transportation for materials. Many swift rivers provide good sources of hydroelectric power. The long and irregular seacoasts provide many excellent harbors.5. What are the factors which influence the climate of the United States?The most important factors are the Atlantic and the Pacific oceans, the Gulf of Mexico and the Great Lakes. The western mountain ranges have an important effect on the climate of the Far West.6. How many types of climate can be found in the United States? What are they?Six types of climate can be found in the United States. They are the humid continental climate in the northeastern part of the country, the humid subtropical climate in the southeastern United States, the continental steppe climate of the Great Plains, the continental desert climate of the intermountain region, the maritime climate in the Pacific northwest and the Mediterranean climate in the southern part of the Pacific coast.7. How many geographical regions can be found in the United States? What are they?Traditionally from the east to west the United States can be divided into seven geographical region. They areNew England, the Middle Atlantic States, the Midwest, the South, the Great Plains, Rockies and Intermountain region or the American West, the Pacific Coast and the New States.8. What are the major economic activities of the Midwest and the South of the United States?The Midwest has the most developed agriculture. It is also a major manufacturing region and the nation’s leading centre of heavy industry. The South is rich in mineral resources and has light as well as heavy industries. It produces over half of the petroleum. It contains 90% of the American textile industry. It has a large agriculture. 9. Why is the tourist trade so important in the American West?Because much of the Rocky mountain area is too mountainous for grazing, and very little of it is usable farmland. And the government has set aside large areas of land as national parks.10. List three geographical differences between Alaska and Hawaii.The two states have many contrasts. Alaska occupies the north-western corner of North America. It extends northward into the Arctic Circle. Hawaii is located in the Pacific Ocean in the tropic. Alaska has the largest land area of all the states, and Hawaii has one of the smallest land areas.。
劳动法(英文)

Article 15 It is forbidden for employers to employ persons under the age of sixteen.
Whenever an unit in culture and arts, sports and special arts and crafts needs to employ young persons under the age of sixteen, examination and approval procedures-shall be undertaken according to relevant regulations of the State and the employees thereof should be ensured the right of receiving compulsory education.
CHAPTER TWO PROMOTION OF EMPLOYMENT
Article 10 The State shall create employment conditions and expand employment opportunities by way of promoting economic and social development.
CHAPTER FIVE WAGE
CHAPTER SIX SAFETY AND HEALTH CARE
CHAPTER SEVEN SPECIAL PROTECTION TO WOMEN WORKERS AND UNDERAGE WORKERS
CHAPTER EIGHT JOB TRAINING
Article 9 The labour administrative department of the State Council is responsible for labour work in the whole country.
《哈利波特与阿兹卡班囚徒》第13章《格兰芬多对立拉文克劳》中英文对照学习版

中英文对照学习版Harry Potter and the Prisoner of Azkaban《哈利波特与阿兹卡班囚徒》Chapter ThirteenGryffindor versus Ravenclaw第13章格兰芬多对立拉文克劳It l ooked like the end of Ron and Hermione's friendship. Each was so angry with the other that Harry coul dn't see how they'd ever make it up.看起来,罗恩和赫敏的友谊到此结束了。
两人彼此都恨得牙痒痒,哈利不知道他们怎么才能言归于好。
Ron was enraged that Hermione had never taken Crookshanks's attempts to eat Scabbers seriously, hadn't bothered to keep a cl ose enough watch on him and was still trying to pretend that Crookshanks was innocent by suggesting Ron l ook for Scabbers und er all the boys' beds. Hermione, meanwhil e, maintained fiercely that Ron had no proof that Crookshanks had eaten Scabbers, that the ginger hairs might have been there since Christmas, and that Ron had been prejudiced against her cat ever since Crookshanks had land ed on Ron's head in the Magical Menagerie.罗恩生气,因为赫敏从来没有认真对待过克鲁克山想吃斑斑这件事,没有费心好好看住克鲁克山,而且到现在还想诡称它是无辜的,并建议罗恩到所有男生的床底下去寻找斑斑。
专业英语四级(听写听力)-试卷174

专业英语四级(听写听力)-试卷174(总分:12.00,做题时间:90分钟)一、 DICTATION(总题数:6,分数:12.00)1.PART I DICTATIONDirections: Listen to the following passage. Altogether the passage will be read to you four times. During the first reading, which will be done at normal speed, listen and try to understand the meaning. For the second and third readings, the passage will be read sentence by sentence, or phrase by phrase, with intervals of 15 seconds. The last reading will be done at normal speed again and during this time you should check your work.(分数:2.00)__________________________________________________________________________________________ 解析:2.(分数:2.00)__________________________________________________________________________________________ 正确答案:(正确答案:Black Friday Black Friday is the beginning of the Christmas shopping season in America. / It falls the day after Thanksgiving celebration / and has become a discount shopping day / when millions of US shoppers are tempted by massive savings. / There are two explanations for the day's name. / Some claim it was coined to illustrate the point / at which retailers start to make a profit, or go "into the black". / Others believe it was first coined by the Philadelphia police department / to describe the crowded pavements and traffic jams on the Friday after Thanksgiving. /)解析:3.(分数:2.00)__________________________________________________________________________________________ 正确答案:(正确答案: Buying a Used Car There are several ways to buy used cars. / Places that sell new cars usually have used cars for sale as well. / The car dealerships get these used cars from people / who trade them in for new cars. / These cars might cost more than other used cars, / but they are more likely to work well. / Some people buy a used car directly from the owner. / Car owners advertise these cars for sale over the Internet or in the newspaper. / It may cost less to buy a car from the owner, / but it can also be a bigger risk. /)解析:4.(分数:2.00)__________________________________________________________________________________________ 正确答案:(正确答案: Bankruptcy Bankruptcy is a legal process for people and businesses that are in debt. / In America, two kinds of bankruptcy are most common for individuals. / These are called chapter seven and chapter thirteen. /Chapter thirteen bankruptcy requires people to have a plan to repay their creditors. / They make payments for three to five years / until most of their debt is paid off. / In chapter seven bankruptcy, people must give up some of their property. / The property is then sold to pay their creditors. / But the people are able to keep the money they earn / after they bring their bankruptcy case. /)解析:5.(分数:2.00)__________________________________________________________________________________________ 正确答案:(正确答案: English New Words New words enter the English language all the time. / In recent years more and more words and phrases have entered the language. / But where do all these words come from? / Words come out of the culture they represent and describe. / So if you've got a new development in medicine, for example, bird flu, / then you'll get the new word coming out of that. / If there's a military conflict, / that may well bring all sorts of new words to the fore. / So really words come from the playground, politics and any area of life / because every area of life is changing from day to day. /)解析:6.(分数:2.00)__________________________________________________________________________________________ 正确答案:(正确答案:Whales Suffering from Shipping Scientists are working to reduce the noise levels / experienced by whales from North Atlantic shipping. / The waters off New England are a home to many species of whale. / Many are now suffering from the noise / which disrupts their communication with each other. / This in turn is affecting their ability to find food and mates. / So a new route has been worked out to reduce the collisions between whales and ships / and increase the transit time of ships. / And a new application has been developed to give real-time information on the location of whales / so that ships can avoid the large gatherings of them. /)解析:。
美国《联邦规章典集》(CFR)目录

美国《联邦规章典集》(CFR)第21篇“食品与药品”总目概述:美国《联邦规章典集》(Code of Federal Regulations,CFR)第21篇“食品与药品”(Title 21—Food and Drugs)共有9卷(Volume)、3章(Chapter)、1499部(Parts)。
其中:第1—8卷第1章第1—1299部,为健康与人类服务部食品与药品管理局(Food and Drug Administration,Department of Health and Human Services)的规章;第9卷第2章第1300—1399部,为司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)的规章;第9卷第3章第1400—1499部,为毒品控制政策办公室(Office of National Drug Control Policy)的规章。
第21篇“食品与药品”(Title 21—Food and Drugs)的概况第21篇“食品与药品”(Title 21—Food and Drugs)的章、部目录部(Part)中译文原英文第Ⅰ章—健康与人类服务部食品与药品管理局(CHAPTER Ⅰ—FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)第A分章—总则(SUBCHAPTER A—GENERAL)INVESTIGATORS56机构审查委员会INSTITUTIONAL REVIEW BOARDS58对非临床实验室研究的良好实验室规范GOOD LABORATORY PRACTICE FOR NONCLINICALLABORATORY STUDIES60专利期恢复PATENT TERM RESTORATION70色素添加剂COLOR ADDITIVES71色素添加剂申请COLOR ADDITIVE PETITIONS73免除认证的色素添加剂的列表LISTING OF COLOR ADDITIVES EXEMPT FROMCERTIFICATION74适用认证的色素添加剂的列表LISTING OF COLOR ADDITIVES SUBJECT TOCERTIFICATION80色素添加剂认证COLOR ADDITIVE CERTIFICATION81用于食品、药品和化妆品的临时性色素添加剂的一般规范和一般限制GENERAL SPECIFICATIONS AND GENERALRESTRICTIONS FOR PROVISIONAL COLOR ADDITIVESFOR USE IN FOODS, DRUGS, AND COSMETICS82经认证的临时性列表的色素和规范的列表LISTING OF CERTIFIED PROVISIONALLY LISTEDCOLORS AND SPECIFICATIONS83-98[预留的][Reserved]99已上市的药品、生物制品和器械的未经批准的/新的用途的信息的发布DISSEMINATION OF INFORMATION ONUNAPPROVED/NEW USES FOR MARKETED DRUGS,BIOLOGICS, AND DEVICES第B分章—用于人类消费的食品(SUBCHAPTER B—FOOD FOR HUMAN CONSUMPTION)100总则GENERAL101食品标识FOOD LABELING102非标准化食品的普通的或者通常的名称COMMON OR USUAL NAME FOR NONSTANDARDIZEDFOODS104食品的营养质量指南NUTRITIONAL QUALITY GUIDELINES FOR FOODS105特殊膳食用途的食品FOODS FOR SPECIAL DIETARY USE106婴儿配方母乳替代食品质量控制程序INFANT FORMULA QUALITY CONTROL PROCEDURES 107婴儿配方母乳替代食品INFANT FORMULA108紧急许可控制EMERGENCY PERMIT CONTROL109在人类食品与食品-包装材料中的不可避免的污染物UNAVOIDABLE CONTAMINANTS IN FOOD FORHUMAN CONSUMPTION AND FOOD-PACKAGINGMATERIAL110在制造、包装或者保存人类食品中的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE INMANUFACTURING, PACKING, OR HOLDING HUMANFOOD113装在密封容器中的热加工低酸食品THERMALLY PROCESSED LOW-ACID FOODSPACKAGED IN HERMETICALLY SEALED CONTAINERS 114酸化食品ACIDIFIED FOODS115带壳蛋SHELL EGGS119存在显著或者不合理风险的膳食补充剂DIETARY SUPPLEMENTS THAT PRESENT ASIGNIFICANT OR UNREASONABLE RISK120危害分析与关键控制点(HACCP)体系HAZARD ANALYSIS AND CRITICAL CONTROL POINT(HACCP) SYSTEMS123鱼与渔业产品FISH AND FISHERY PRODUCTS129饮用水加工与装瓶PROCESSING AND BOTTLING OF BOTTLED DRINKINGWATER130食品标准:总则FOOD STANDARDS: GENERAL131乳与奶油MILK AND CREAM133乳酪与相关乳酪产品CHEESES AND RELATED CHEESE PRODUCTS135冷冻点心FROZEN DESSERTS136烘焙产品BAKERY PRODUCTS137谷物粉与相关产品CEREAL FLOURS AND RELATED PRODUCTS139通心粉与面条产品MACARONI AND NOODLE PRODUCTS145罐装水果CANNED FRUITS146罐装水果汁CANNED FRUIT JUICES150水果黄油、果冻、防腐剂以及相关产品FRUIT BUTTERS, JELLIES, PRESERVES, AND RELATEDPRODUCTS152水果馅饼FRUIT PIES155罐装蔬菜CANNED VEGETABLES156蔬菜汁VEGETABLE JUICES158冷冻蔬菜FROZEN VEGETABLES160蛋与蛋制品EGGS AND EGG PRODUCTS161鱼与有壳的水生动物FISH AND SHELLFISH163可可制品CACAO PRODUCTS164树坚果与花生制品TREE NUT AND PEANUT PRODUCTS 165饮料BEVERAGES166人造黄油MARGARINE168增甜剂与餐桌糖浆SWEETENERS AND TABLE SIRUPS 169食品敷料与调味料FOOD DRESSINGS AND FLAVORINGS 170食品添加剂FOOD ADDITIVES171食品添加剂申请FOOD ADDITIVE PETITIONS172允许直接加入用于人类消费食品的食品添加剂FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION173在用于人类消费的食品中允许的次直接的食品添加剂SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION174间接食品添加剂:总则INDIRECT FOOD ADDITIVES: GENERAL175间接食品添加剂:胶粘剂与涂层的组分INDIRECT FOOD ADDITIVES: ADHESIVES ANDCOMPONENTS OF COATINGS176间接食品添加剂:纸与纸板组分INDIRECT FOOD ADDITIVES: PAPER ANDPAPERBOARD COMPONENTS177间接食品添加剂:聚合体INDIRECT FOOD ADDITIVES: POLYMERS178间接食品添加剂:辅剂、生产助剂和消毒剂INDIRECT FOOD ADDITIVES: ADJUVANTS,PRODUCTION AIDS, AND SANITIZERS179在食品生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING ANDHANDLING OF FOOD180在额外试验期间临时在食品或者在与食品接触中被允许的食品添加剂FOOD ADDITIVES PERMITTED IN FOOD OR INCONTACT WITH FOOD ON AN INTERIM BASISPENDING ADDITIONAL STUDY181先前核准的食品配料PRIOR-SANCTIONED FOOD INGREDIENTS182一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE184被确认为一般认为安全的直接食品物质DIRECT FOOD SUBSTANCES AFFIRMED ASGENERALLY RECOGNIZED AS SAFE 186被确认为一般认为安全的间接食品物质INDIRECT FOOD SUBSTANCES AFFIRMED ASGENERALLY RECOGNIZED AS SAFE 189禁止用于人类食品的物质SUBSTANCES PROHIBITED FROM USE IN HUMANFOOD190膳食补充剂DIETARY SUPPLEMENTS191-199[预留的][Reserved]第C分章—药品:总则(SUBCHAPTER C—DRUGS: GENERAL)200总则GENERAL201标识LABELING202处方药广告PRESCRIPTION DRUG ADVERTISING203处方药销售PRESCRIPTION DRUG MARKETING205对批发处方药销售商颁发州执照的指南GUIDELINES FOR STATE LICENSING OF WHOLESALEPRESCRIPTION DRUG DISTRIBUTORS 206人用固体口服剂型药品的印码IMPRINTING OF SOLID ORAL DOSAGE FORM DRUGPRODUCTS FOR HUMAN USE207药品生产者的登记与商业销售的药品的列表REGISTRATION OF PRODUCERS OF DRUGS ANDLISTING OF DRUGS IN COMMERCIAL DISTRIBUTION 208处方药的药物治疗指导MEDICATION GUIDES FOR PRESCRIPTION DRUGPRODUCTS210制造、加工、包装或者保存药品的现行良好制造规范;总则CURRENT GOOD MANUFACTURING PRACTICE INMANUFACTURING, PROCESSING, PACKING, ORHOLDING OF DRUGS; GENERAL211药品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FORFINISHED PHARMACEUTICALS216药房配药PHARMACY COMPOUNDING225对含药饲料的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FORMEDICATED FEEDS226对A型含药物品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FORTYPE A MEDICATED ARTICLES250对特殊人用药品的特殊要求SPECIAL REQUIREMENTS FOR SPECIFIC HUMANDRUGS290管制的药品CONTROLLED DRUGS299药品;正式名称与已确定的名称DRUGS; OFFICIAL NAMES AND ESTABLISHED NAMES 第D分章—人用药品(SUBCHAPTER D—DRUGS FOR HUMAN USE)300总则GENERAL310新药NEW DRUGS312试验用新药申请INVESTIGATIONAL NEW DRUG APPLICATION314为FDA批准上市新药的申请APPLICATIONS FOR FDA APPROVAL TO MARKET ANEW DRUG315诊断用放射性药品DIAGNOSTIC RADIOPHARMACEUTICALS316罕见病药ORPHAN DRUGS320生物利用度与生物等效性要求BIOAVAILABILITY AND BIOEQUIVALENCEREQUIREMENTS328含有酒精的预期用于口部摄入的非处方药品OVER-THE-COUNTER DRUG PRODUCTS INTENDEDFOR ORAL INGESTION THAT CONTAIN ALCOHOL330一般认为安全与有效以及不错误标识的非处方人用药品OVER-THE-COUNTER (OTC) HUMAN DRUGS WHICHARE GENERALLY RECOGNIZED AS SAFE ANDEFFECTIVE AND NOT MISBRANDED331用于非处方的人类使用的抗酸产品ANTACID PRODUCTS FOR OVER-THE-COUNTER (OTC)HUMAN USE332用于非处方的人类使用的抗胃肠气胀产品ANTIFLATULENT PRODUCTS FOR OVER-THE-COUNTER HUMAN USE333用于非处方的人类使用的局部抗菌药品TOPICAL ANTIMICROBIAL DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE335用于非处方的人类使用的止泻药品ANTIDIARRHEAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USECOUNTER HUMAN USE338用于非处方的人类使用的帮助夜间睡眠的药品NIGHTTIME SLEEP-AID DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE340用于非处方的人类使用的兴奋药品STIMULANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE341用于非处方的人类使用的感冒、咳嗽、过敏症药、支气管扩张以及平喘药品COLD, COUGH, ALLERGY, BRONCHODILATOR, ANDANTIASTHMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE343用于非处方的人类使用的内服的止痛、退热以及抗风湿药品INTERNAL ANALGESIC, ANTIPYRETIC, ANDANTIRHEUMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE344用于非处方的人类使用的局部的耳部药品TOPICAL OTIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE346用于非处方的人类使用的肛肠药品ANORECTAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE347用于非处方的人类使用的皮肤保护药品SKIN PROTECTANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE348用于非处方的人类使用的外部的止痛药品EXTERNAL ANALGESIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE349用于非处方的人类使用的眼科药品OPHTHALMIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE350用于非处方的人类使用的止汗药品ANTIPERSPIRANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE352用于非处方的人类使用的遮光药品SUNSCREEN DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE [STAYED INDEFINITELY]355用于非处方的人类使用的防龋药品ANTICARIES DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE357用于非处方的人类使用的其他内服药品MISCELLANEOUS INTERNAL DRUG PRODUCTS FOROVER-THE-COUNTER HUMAN USE564[预留的][Reserved]570食品添加剂FOOD ADDITIVES571食品添加剂申请FOOD ADDITIVE PETITIONS573在动物饲料与饮用水中允许的食品添加剂FOOD ADDITIVES PERMITTED IN FEED AND DRINKINGWATER OF ANIMALS579在动物饲料和宠物食品的生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING, AND HANDLING OF ANIMAL FEED AND PET FOOD582一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE584在动物饲料与饮用水中被确认为一般认为安全的食品物质FOOD SUBSTANCES AFFIRMED AS GENERALLYRECOGNIZED AS SAFE IN FEED AND DRINKINGWATER OF ANIMALS589禁止用于动物食品或者饲料的物质SUBSTANCES PROHIBITED FROM USE IN ANIMALFOOD OR FEED590-599[预留的][Reserved]第F分章—生物制品(SUBCHAPTER F—BIOLOGICS)600生物制品:总则BIOLOGICAL PRODUCTS: GENERAL601颁发执照LICENSING606对血液与血液组分的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FORBLOOD AND BLOOD COMPONENTS607对人类血液与血液制品的制造者的机构登记与产品列表ESTABLISHMENT REGISTRATION AND PRODUCTLISTING FOR MANUFACTURERS OF HUMAN BLOODAND BLOOD PRODUCTS610普通生物制品标准GENERAL BIOLOGICAL PRODUCTS STANDARDS630对血液、血液组分和血液衍生物的一般要求GENERAL REQUIREMENTS FOR BLOOD, BLOODCOMPONENTS, AND BLOOD DERIVATIVES640对人类血液和血液制品的附加标准ADDITIONAL STANDARDS FOR HUMAN BLOOD ANDBLOOD PRODUCTS660对用于实验室检测的诊断物质的附加标准ADDITIONAL STANDARDS FOR DIAGNOSTICSUBSTANCES FOR LABORATORY TESTS680对其他产品的附加标准ADDITIONAL STANDARDS FOR MISCELLANEOUSPRODUCTS第G分章—化妆品(SUBCHAPTER G—COSMETICS)700总则GENERAL701化妆品标识COSMETIC LABELING710化妆品机构的自愿登记VOLUNTARY REGISTRATION OF COSMETIC PRODUCTESTABLISHMENTS720化妆品配料构成声明的自愿存档VOLUNTARY FILING OF COSMETIC PRODUCTINGREDIENT COMPOSITION STATEMENTS 740化妆品警告声明COSMETIC PRODUCT WARNING STATEMENTS741-799[预留的][Reserved]第H分章—医疗器械(SUBCHAPTER H—MEDICAL DEVICES)800总则GENERAL801标识LABELING803医疗器械报告MEDICAL DEVICE REPORTING806医疗器械;改正与移动的报告MEDICAL DEVICES; REPORTS OF CORRECTIONS ANDREMOVALS807对器械的制造者与首次进口者的机构登记与器械列表ESTABLISHMENT REGISTRATION AND DEVICELISTING FOR MANUFACTURERS AND INITIALIMPORTERS OF DEVICES808对州和地方医疗器械要求的联邦优先权的豁免EXEMPTIONS FROM FEDERAL PREEMPTION OF STATE AND LOCAL MEDICAL DEVICE REQUIREMENTS809人用体外诊断产品IN VITRO DIAGNOSTIC PRODUCTS FOR HUMAN USE 810医疗器械召回权MEDICAL DEVICE RECALL AUTHORITY812试验用器械豁免INVESTIGATIONAL DEVICE EXEMPTIONS813[预留的][Reserved]814医疗器械的上市前批准PREMARKET APPROVAL OF MEDICAL DEVICES 820质量体系规章QUALITY SYSTEM REGULATION821医疗器械跟踪要求MEDICAL DEVICE TRACKING REQUIREMENTS822上市后监视POSTMARKET SURVEILLANCE860医疗器械分类程序MEDICAL DEVICE CLASSIFICATION PROCEDURESDEVELOPMENT临床化学与临床毒理学器械CLINICAL CHEMISTRY AND CLINICAL TOXICOLOGY 862DEVICES864血液学与病理学器械HEMATOLOGY AND PATHOLOGY DEVICES866免疫学与微生物学器械IMMUNOLOGY AND MICROBIOLOGY DEVICES868麻醉学器械ANESTHESIOLOGY DEVICES870心血管器械CARDIOVASCULAR DEVICES872牙科器械DENTAL DEVICES874耳、鼻和咽器械EAR, NOSE, AND THROAT DEVICES876胃肠病学-泌尿学器械GASTROENTEROLOGY-UROLOGY DEVICES878普通与整形外科器械GENERAL AND PLASTIC SURGERY DEVICES880普通医院与个人使用器械GENERAL HOSPITAL AND PERSONAL USE DEVICES 882神经学器械NEUROLOGICAL DEVICES884产科与妇科学器械OBSTETRICAL AND GYNECOLOGICAL DEVICES886眼科器械OPHTHALMIC DEVICES888矫形外科器械ORTHOPEDIC DEVICES890内科学器械PHYSICAL MEDICINE DEVICES892放射学器械RADIOLOGY DEVICES895禁止的器械BANNED DEVICES电极铅线与患者电缆的性能标准PERFORMANCE STANDARD FOR ELECTRODE LEAD 898WIRES AND PATIENT CABLES第I分章—乳房造影质量标准法(SUBCHAPTER I—MAMMOGRAPHY QUALITY STANDARDS ACT)900乳房造影法MAMMOGRAPHY第J分章—放射学的健康(SUBCHAPTER J—RADIOLOGICAL HEALTH)1000总则GENERAL1002记录与报告RECORDS AND REPORTS1003缺陷与未能守法的通报NOTIFICATION OF DEFECTS OR FAILURE TO COMPLYELECTRONIC PRODUCTS1005电子产品的进口IMPORTATION OF ELECTRONIC PRODUCTS1010电子产品的性能标准:总则PERFORMANCE STANDARDS FOR ELECTRONICPRODUCTS: GENERAL1020电离辐射发生产品的性能标准PERFORMANCE STANDARDS FOR IONIZINGRADIATION EMITTING PRODUCTS 1030微波与射电频率发生产品的性能标准PERFORMANCE STANDARDS FOR MICROWAVE ANDRADIO FREQUENCY EMITTING PRODUCTS 1040发光产品的性能标准PERFORMANCE STANDARDS FOR LIGHT-EMITTINGPRODUCTS1050声波、次声波和超声波发生产品的性能标准PERFORMANCE STANDARDS FOR SONIC, INFRASONIC,AND ULTRASONIC RADIATION-EMITTING PRODUCTS 第K分章—[预留的](SUBCHAPTER K—[RESERVED])第L分章—根据由食品与药品管理局行政执行的某些其他法的规章(SUBCHAPTER L—REGULATIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRATION)1210根据《联邦进口乳法》的规章REGULATIONS UNDER THE FEDERAL IMPORT MILKACT1230根据《联邦腐蚀性毒物法》的规章REGULATIONS UNDER THE FEDERAL CAUSTICPOISON ACT1240传染病的控制CONTROL OF COMMUNICABLE DISEASES1250州际运输卫生INTERSTATE CONVEYANCE SANITATION 1251-1269[预留的][Reserved]1270预期用于移植的人体组织HUMAN TISSUE INTENDED FOR TRANSPLANTATION1271人体细胞、组织以及细胞的和基于组织的产品HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS1272-1299[预留的][Reserved]第Ⅱ章—司法部毒品强制执行局(CHAPTER Ⅱ—DRUG ENFORCEMENT ADMINISTRATION, DEPARTMENT OF JUSTICE)1300定义DEFINITIONSAND DISPENSERS OF CONTROLLED SUBSTANCES 1302对管制物质的标识与包装要求LABELING AND PACKAGING REQUIREMENTS FORCONTROLLED SUBSTANCES1303定额QUOTAS1304登记者的记录与报告RECORDS AND REPORTS OF REGISTRANTS1305令的格式ORDER FORMS1306处方PRESCRIPTIONS1307杂项MISCELLANEOUS1308管制物质的表SCHEDULES OF CONTROLLED SUBSTANCES1309表I化学品的制造者、分销者、进口者和出口者的登记REGISTRATION OF MANUFACTURERS, DISTRIBUTORS, IMPORTERS AND EXPORTERS OF LIST I CHEMICALS1310列入表的化学品和某些机器的记录与报告RECORDS AND REPORTS OF LISTED CHEMICALS ANDCERTAIN MACHINES1311[预留的][Reserved]1312管制物质的进口与出口IMPORTATION AND EXPORTATION OF CONTROLLEDSUBSTANCES1313前体与必要化学品的进口与出口IMPORTATION AND EXPORTATION OF PRECURSORSAND ESSENTIAL CHEMICALS 1314-1315[预留的][Reserved]1316行政职能、规范和程序ADMINISTRATIVE FUNCTIONS, PRACTICES, ANDPROCEDURES第Ⅲ章—毒品控制政策办公室(CHAPTER Ⅲ—Office of National Drug Control Policy)1400[预留的][Reserved]1401信息的公众可及性PUBLIC AVAILABILITY OF INFORMATION1402强制性解密审查MANDATORY DECLASSIFICATION REVIEW1403对给予州和地方政府资金和合作协议的统一行政要求UNIFORM ADMINISTRATIVE REQUIREMENTS FORGRANTS AND COOPERATIVE AGREEMENTS TO STATEAND LOCAL GOVERNMENTS1404政府范围的排除与暂停(非获得)GOVERNMENTWIDE DEBARMENT AND SUSPENSION(NONPROCUREMENT)1405对无毒品工作场所的政府范围的要求(财政援助)GOVERNMENTWIDE REQUIREMENTS FOR DRUG-FREE WORKPLACE (FINANCIAL ASSISTANCE)1406-1499[预留的][Reserved]。
AMERICAN GOVERNMENT

We all know that we have a proverb: "A nation is only through the men it produces, but it also honors the people who do not remember it themselves." (John F. Kennedy, U.S. president) has a profound significance and value, not only in our work, and in our study. This means that a country history, a symbol of great significance. As the saying goes you can explain, through a series of examples below.This allows us to understand the historical development of our society has an important role. Looking back at history, the old Enlightenment idea of the importance of liberating liberation against the ignorance doctrine, movement, promoting universal education. Early in the 17th to the 19th century against the ignorance of religion in Europe this trend is the rise of Marxism against the feudal autocracy but in its essence, it is the bourgeois political thought, It is the Renaissance bourgeois anti-feudal, anti-abstinence, anti-church struggle to continue and develop, directly to the French Revolution in 1789, laid the ideological foundation.Enlightenment thinkers believed that social progress is not the reason, why the ignorance of people, religious forces, mainly due to the people of the spirit of domination and slavery, in order to change this situation, must be based on rational and scientific authority. Leaders of the 18th century French Enlightenment, Voltaire, his thoughts had a great impact in Europe, so people have to say later: "18. Century is the century of Voltaire," the main advocates. Rousseau is a radicaldemocrat, the essence of his thought and the basic sovereignty of the people ideological principles. This "popular sovereignty" idea, he thought all the rights belong to the people, right on the performance and application must reflect the will of the people. ("Social Contract," On the Origins of Inequality "") their ideas are bad for the liberation of the old ideas played an irreplaceable role.Locke is the property of the people made a great contribution, Locke bourgeois revolution in England during the time in order to meet the needs of the British bourgeoisie. He believes that Hobbes was amending the contract, by the country of registration, protection of private property, so the state should not interfere in private property of citizens. He had a famous saying about "my son into the cottage wind, rain may enter, the king can’t enter." Locke even further pointed out that the human rights of private property, private property, no right to talk about the foundation. Formal political power, he supported the constitutional monarchy, advocating national legislation, administrative and foreign affairs, the right should belong to boards. Later, the Second World War, colonial, semi-colonial upsurge of the national independence movement, a large number of Asian, African nations gained independence, destroying the colonial system of imperialism. eighteenth century 60's and 70's between the 18th century, the British in North America, the tension between the thirteen colonies of Great Britain Kingdom continues to rise, and eventually broke out in 1775.In summary, human and social development is inseparable from the joint efforts of society to move forward,the emancipation is very important, from the Enlightenment to the present federal state, are inseparable from the emancipation of the mind, and ultimately to a democratic republic.。
《红星照耀中国》英语读后感

【导语】作品真实记录了埃德加·斯诺⾃1936年6⽉⾄1936年10⽉在中国西北⾰命根据地进⾏实地采访的所见所闻,报道了中国和中国⼯农红军以及许多红军领袖、红军将领的情况。
从多个⽅⾯展⽰中国共产党为民族解放⽽艰苦奋⽃和牺牲奉献的精神,⽡解了种种歪曲、丑化共产党的谣⾔。
斯诺通过对领导⼈和普通民众的观察和描述,把枯燥的红区党组织、各种⽂件、会议等内容转变为让读者读起来感到亲切⽣动的⽂字以下是©⽆忧考⽹为⼤家精⼼整理的内容,欢迎⼤家阅读。
1.《红星照耀中国》英语读后感 This year is the 100th anniversary of the founding of the party. In my spare time, I read this red historical book red star shines on China. It is a classic documentary literary work, and the author is Edgar s, an American journalist who amazed us. During this period, he conducted a large number of interviews with Mao Zedong, Zhu De, Zhou Enlai, Peng Dehuai, Lin Boqu, Xu Haidong and other red army generals, including ordinary Red Army soldiers, medical personnel, young pioneers and local people who warmly support the Red Army. Snow recorded this miracle created by the Chinese Communists in objective but not passionate language, explained China's red revolution to the whole country and the world for the first time, introduced the basic policies of the Communist Party, and let the people all over the world understand the unconquerable fighting spirit of the Red Army and the enthusiasm and power to change the world. Snow also created many lifelike characters in this book, which are vivid, full and natural. Remember when snow called his two children "hello" impolitely at the baijiaping traffic office, but he was ignored? These thirteen or fourteen year old children have added some fresh blood to the battle of our Red Army. People call them "red imps". In snow's humorous and funny narration, these almost one stroke plots make the image of the red imps fresh and lovely. They have a high degree of personal self-esteem that Chinese children generally lacked at that time. They are happy, optimistic and vibrant. They are patient, hardworking, smart and study hard, adding infinite hope and vitality to Red China! The long march is not only a military miracle achieved by the Chinese workers' and peasants' Red Army under the leadership of the Communist Party of China, but also a magnificent heroic epic contributed by China to the world. The unyielding and tenacious of the Red Army soldiers in extreme difficulties, and the happiness and self-confidence they show are invaluable spiritual wealth of the Chinese nation. It can be said that the red star shines on China is a valuable spiritual wealth, just like the bright "Red Star", which always reminds us to look back on history. It is precisely because of this spirit, the spirit of the Red Army. Let's have such a peaceful life now. All that we have gained now is fresh blood. For these, we have to study hard and serve our motherland. And lead us to the future with down-to-earth courage.2.《红星照耀中国》英语读后感 What is the spirit of Red China, a country in civil war after liberation, that allows foreign friends to travel thousands of miles to join the Red Army and join the war? What spirit, who, or what? Edgar Snow, the author of this book, was born in a poor family in the United States. When he was young, he worked as a farmer, railway worker, printing apprentice and so on. Later, when he was teaching in Shanghai, he came into contact with some underground Communist Party members. In 1936, snow risked his life to explore the answers to questions he could not understand about the Chinese Red Army. Snow wrote this book "red star shines on China" while negotiating with the Chinese Red Army. From this book, we can learn about the life of the Red Army from a "zero distance" - when Red Army soldiers are not fighting or on duty, they rest one day a week. They get up at five o'clock and go to bed at nine o'clock. The daily schedule includes: one hour of morning exercises after getting up; Breakfast; Two hours of military training; Two hours of political class and discussion; Lunch; An hour's rest; Two hour literacy class; Two hours of exercise; Dinner; Singing and group meetings“Turn off the light. This is the most true descrip tion of snow, who has personally experienced the life of the Red Army. In the "political lesson" of "Chapter 8 being with the Red Army", snow asked the Red Army soldiers: in what way is the red army better than other Chinese armies? The Red Army soldiers answered countless questions. Some said that the Red Army was revolutionary, some said that the Red Army was Anti Japanese, and some said that the Red Army was helped by farmers... Later, snow asked how everyone knew that farmers loved the Red Army, and the Red Army soldiers gave thousands of examples. The red star shines on China is about the Chinese revolution, but the author is a foreigner. What made foreign friends join the Chinese revolution? Is it money? Power? None of them. It is the golden spirit of the Chinese Red Army to sacrifice itself for others and serve the country faithfully! Comparing the Red Army with the White army, we will find how firm and persistent the Red Army is. In the confrontation with the Kuomintang, we won under the situation of being outnumbered. This victory depends not only on the firm belief of the Red Army soldiers, but also on the support of farmers and workers. The success and failure of a team depends on the support or negation of others. If you want to finish the road to success, you must be aware that you may fail at any time and full of faith, so that you can go on with a smile and welcome success. A single thread does not form a line, and a single tree does not form a forest.3.《红星照耀中国》英语读后感 This is a great war, suddenly noisy, suddenly dissipated, as if it sounds sound but invisible, looks tangible but silent, this is a war between the bourgeoisie and the proletariat, this is a war between the helpless people and unreasonable landlords, this is a war········· Red star shines on China was written by Edgar Snow, an American. At that time, everyone did not know the Red Army and the Chinese Soviet. Only under the guise of "red bandits", "anti-government" and "countless killings by the Red Army" released by the Kuomintang government, no one entered the red area and wrote a real red army book, but snow did it. He ignored the raids of "white bandits" Threats and external rumors of the rape of "red bandits" resolutely entered the red zone. He created the book "red star shines on China" with his reputation, sincerity and as a reporter. He is red, he is sincere, and he is worthy of commemoration by the people all over the world. He will never forget the Red China. When he was critically ill, he squeezed out a sentence: "I love China". As Chairman Mao said, "Mr. Snow is a friend of the Chinese people. He has made unremitting efforts and made important contributions to enhancing the friendship between the Chinese and American people all his life. He will always live in the hearts of the Chinese people.". This book describes the Red Army's strength, perseverance, fearlessness, and even many interesting things. The children in the Red Army are also very strong but lovely. They have a military temperament integrated into their bones, but there is a layer of childishness outside, which makes people love. Some people's stories are touching, but there are also some bourgeoisie with great wealth, However, he has been exploiting the proletariat, greedy, and even made "Litong foreigners" like Ma Hongkui in the "four horses", who brazenly asked the Japanese to build an airport in the northwest. How did he do his tax project? There are more than 40 taxes and levies, which can hardly be carried out. Moreover, salt monopoly requires to buy half a pound of salt every month, whether he uses it or not. The red army defeated him, so that the people can live an equal life. They are no longer treated as slaves or in debt, but become completely free. There are many, many things like this····· Thank you, Mr. Snow. Thank you for letting us know about old China after more than 80 years, the Red Army and the Chinese Soviet。
American-Government(美国政府)

• When the constitution was written, the smaller states worried that the larger states would be much more powerful in Washington.
• To calm there worries, the Senate was also created.
• The legislative branch is called Congress and is made up of elected representatives from all 50 states.
• The Legislative branch has two sections: the House of Representatives and the Senate.
• Each state has two senators, no matter how large or how small the state population.
• All states are represented equally in the Senate.
• Senators are elected to six-year terms.
• If Congress passes a law that the Supreme Court feels goes against the Constitution, the law is called unconstitutional and is no longer a law.
• The Judiciary Branch does not make laws. They only interpret federal law and the Constitution.
American Government(美国政府)

Donkey vs. Elephant
The Democratic Party
(Honest Stubborn Diligent )
The Republican Party
(Steady Pragmatic Conservative)
漫画:驴象4年就来一回合
Two-party System
President Washington / Hamilton vs. Tomas Jefferson Federaliຫໍສະໝຸດ ts Anti-federalists
The Constitution of the United States
Preface • We the people of the United States, in order to form a more perfect Union, establish justice, insure domestic tranquility, provide for the common defense, promote the general welfare, and secure the blessings of liberty to ourselves and our posterity, do ordain and establish this Constitution for the United States of America.
Democratic-Republicans
Republicans
Democrats
Federalists died out in 1816 with the end of the War of 1812. Andrew Jackson Democrats Before the Civil War, Republicans
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The advantage and disadvantage of the political system
• The political system based on these principles is of great benefit for the United States in the way that power is spread and counterbalanced. • The great weakness of the system is that it makes government slow, complicated, and legalistic which is a particular disadvantage in a world.
• the power of the states and the federal government: • The states have, under the Constitution, the primary functions of providing law and order, education, public health and most of the things which concern day-to-day life. • The Federal government at Washington is concerned with foreign affairs and with matters of general concern to all the states including commerce between the states.
Chapter Thirteen American Government
Main Contห้องสมุดไป่ตู้nt
• • • • • • • I. The Constitution II. Congress: The Legislature III. Government: the Executive IV. Federal court V . Political Parties VI. the election of president VII. Federal System
back
Congress
back
• What do you know about the US Congress? • Why sometimes is congress applied incorrectly to the House of Representatives? • How many members are sent to the congress by each state? • What are the duties of the representatives of the Congress? • How do bills become law? • How is the President impeached?
back
How is the president impeached?
• Under the U.S. Constitution, only the House of Representatives may impeach the president or other federal officers and the Senate alone has the authority to try impeachments. • The House of Representatives, upon sufficient evidence, brings a “bill of impeachment” approved by two-thirds of its membership. Then comes a trial in the Senate. • The president is tried for his crimes and improper acts reported by the house, with the Chief Justice of the United States as the judge, and the senators as the jury. If two-thirds of the Senate vote that the president is guilty, he is removed from office.
• 1. passed by each house separately, • 2. signed by the president of the United States within 10 days of their submission, or they become law automatically. • If vetoed by the president, a bill may become law only by its repassage by a two-thirds majority in each house.
Congress
• 1. the bicameral legislature, • 2. consisting of the Senate and the House of Representatives. • 3. direct election. • 4. function: Congress is the legislative branch of the federal government. It is the law-making and the supreme legislative body of the nation. The duty of the congress: 1. Make laws. 2. Levy taxes and 3. appropriate money for government expenditure • The Congress meets in the United States Capitol in Washington, D.C.
• Respect for the Constitution and the rule of law means that decisions should be made by applying the Constitution or laws. And the law is above everyone and it applies to everyone. Whether governor or governed, rulers or ruled, no one is above the law, no one is exempted from the law, and no one can grant exemption to the application of the law.
Congress: The Legislature
Congress: the Legislature
• Organization
Senate (100mumbers) The House of Representatives(435 mumbers)
back
How do bills become law?
Page one of the original copy of the Constitution
The Significance of the Constitution
• 1. The United States Constitution, written in 1787, established the country’s political system and is the basis for its laws. • 2. According to the Constitution, all governments in the United States are “of the people, by the people, and for the people ”. • 3. The whole system of American government is established in the back Constitution and Bill of Rights.
• Federalism refers to the division of political power between two levels of government – the federal government and the state government. Each American is subject to both of them.
The three branches are independent of each other, check and balance against each other. No one of the three branches may dominate the others.
Respect for the Constitution and the rule of law
Federalism
The separation of powers
• The separation of powers means that power is spread between three institutions of the state – the executive, the legislature and the judiciary • – and no one institution has too much power and no individual can be a member of more than one institution. • This principle is also known as ‗checks and balances(制衡) ‘, since each of the three branches of the state has some authority to act on its own, some authority to regulate the other two branches, and has some of its own authority, in turn, regulated by the other branches.
