国外片剂工艺规程中英文对照 (模版)
阿司匹林片(片剂)工艺规程

阿司匹林片工艺规程1.产品名称及剂型产品名称:阿司匹林片产品剂型:片剂2.产品概述产品名称阿司匹林片汉语拼音名:Asipilin Pian英文名:Aspirin Tablets结构式:阿司匹林本品为2-(乙酰氧基)苯甲酸分子式:C9H8O4 分子量:本品含阿司匹林(C9H8O4)应为标示量的%%产品特点2.2.1性状本品为白色片2.2.2规格0.5g。
2.2.3类别解热镇痛非甾体抗炎药,抗血小板聚集药。
2.2.4用法与用量口服。
成人一次1片,若发热或疼痛持续不缓解,可间隔4-一6个小时重复用药一次。
24小时内不超过4片。
儿童用量请咨询医师或药师。
2.2.5贮藏密封,在干燥处保存。
2.2.6有效期3年。
3.处方和依据批投料处方阿司匹林60kg淀粉5kg枸橼酸滑石粉制成 12万片依据 执行标准:《中国药典》2010年版二部制法 取阿司匹林、淀粉和枸橼酸置高效湿法制粒机中混合均匀,用淀粉浆制粒;干燥;压片;瓶内包装及外包装,制得。
4. 工艺流程图物料工序检验入库中间站注:虚线框内为十万级洁净区。
5.操作过程及工艺条件备料5.1.1领料从库房领取合格原辅料,送入车间称量暂存间。
5.1.2粉碎过筛将以下物料依次粉碎过筛,过筛后再次称量,计算物料平衡,并严格复核。
制粒5.2.1配浆称取纯化水1kg置配浆锅中,加入1kg淀粉,搅拌使均匀,在搅拌下冲入10kg 纯化水加热至糊化,配成10%的淀粉浆作为粘合剂。
5.2.2制粒将60kg阿司匹林粉、4kg淀粉和0.6kg枸橼酸粉投入高速制粒机中,干混4分钟后,加入上述淀粉浆混合5分钟,开机制粒。
5.2.3干燥将上述湿颗粒吸入沸腾制粒机中,将设定好工艺参数(进风温度120±5℃,温度75±5℃,进风温度30±5℃)的冷空气通过初效中高效过滤器进适入后部加热室,经过加热器加热至进风所需温度后进入物料室,在引风拉动下物料呈流化态干燥45分钟至水份为3-4%时,停机出料。
制药工艺流程 英文

制药工艺流程英文《Pharmaceutical Production Process》Pharmaceutical production process, also known as pharmaceutical manufacturing, involves a series of steps and activities to produce a final pharmaceutical product that meets the required quality standards and regulatory requirements. The process begins with the discovery of a new drug molecule or the identification of a potential drug candidate. Once a drug candidate is selected, the pharmaceutical production process begins.The first step in the pharmaceutical production process is the development of the drug formulation. This involves the creation of a stable and effective dosage form for the drug, such as a tablet, capsule, or liquid solution. Formulation development also includes the selection of excipients, which are the inactive ingredients that are used to create the final dosage form.Once the drug formulation is developed, the next step is to manufacture the drug product. This involves the actual production of the dosage form in a pharmaceutical manufacturing facility. The manufacturing process may include activities such as mixing, granulation, compression, coating, and packaging.After the drug product is manufactured, it undergoes a series of quality control tests to ensure that it meets the required quality standards. These tests may include checks for assay, impurities, dissolution, and microbiological contamination. Once the drug product passes all quality control tests, it is ready for distribution and sale to patients.Throughout the pharmaceutical production process, regulatory requirements and good manufacturing practices (GMP) must be followed to ensure that the drug product is safe, effective, and of high quality. This includes maintaining a clean and sanitary manufacturing environment, conducting regular equipment and facility maintenance, and keeping detailed documentation of all production activities.In conclusion, the pharmaceutical production process is a complex and highly regulated series of steps that are essential for the production of safe and effective drug products. From drug formulation development to manufacturing and quality control testing, each step plays a crucial role in ensuring that the final drug product meets the required quality standards and regulatory requirements.。
制药工程专业英语第11单元课文中英文对照

PART 3 INDUSTRIAL PHARMACYUnit 11 Tablets (The Pharmaceutical Tablets Dosage Form)Role in TherapyThe oral route of drug administration is the most important method of administering drugs for systemic effects. Except in cases of Insulin therapy,the parenteral route is not routinely used for self-administration of medications. The topical route of administration has only recently been employed to deliver drugs to the body for systemic effects,with two classes of marketed products: Nitroglycerin硝酸甘油酯for the treatment of angina心绞痛and scopolamine莨菪胺for the treatment of motion sickness晕动病,指晕车、晕船等. Other drugs are certain to follow,but the topical route of administration is limited in its ability to allow effective drug absorption for systemic drug action. The parenteral route of administration is important in treating medical emergencies in which a subject is comatose昏迷的or cannot swallow,and in providing various types of maintenance therapy for hospitalized patients. Nevertheless,it is probable that at least 90 % of all drugs used to produce systemic effects are administered投药,给药by the oral route. When a new drug is discovered,one of the first questions a pharmaceutical company asks is whether or not drug can be effectively administered for its intended effect by the oral route. If it cannot,the drug is primarily relegated to被降级到administration in a hospital setting or physician's office. If patient self- administration cannot be achieved,the sales of the drug constitute only a small fraction of what the market would be otherwise. Of drugs that are administered orally,solid oral dosage forms represent the preferred class of product. The reasons for this preference are as follows. Tablets and capsules represent unit dosage forms in which one usual dose of the drug has been accurately placed. By comparison相比之下,liquid oral dosage forms,such as syrups,suspensions,emulsions,solutions,and elixirs,are usually designed to contain one dose of medication in 5 to 30 ml. The patient is then asked to measure his or her own medication using a teaspoon,第三部分工业药剂学第11单元药片(医药的片剂剂型)在治疗中的作用口服给药途径是通过给药获得全身作用效果中最重要的方法。
FDA-GMP中英文对照标准版

DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OF RAW MATERIALS BY FDATable of Contents 目录1. INTRODUCTION1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
美国良好操作规范(GMP—CFRPart,参考译文)

美国良好操作规范(GMP—21CFR Part 110,参考译文)A分部—总则110.3 定义联邦食品、药物及化妆品条例(以下简称条例)第201节中术语的定义和解释适用于本部分的同类术语。
下列定义亦同样适用:1.酸性食品或酸化食品:是指平衡pH值为4.6或低于4.6的食品。
2.适当的:指为达到良好的公共卫生规范的预期目的所需要满足的要求。
3.面糊:是指一种半流体物质,通常包含面粉和其他辅料,食品的主要成分可浸在其中,或用它涂膜,或直接用来形成烘烤的食品。
4.热烫:除坚果和花生外,指在包装前对食品进行足够时间和充分温度的热处理,以使天然形成的酶部分地或完全失活,并使该食品产生物理或生化方面的变化。
5.关键控制点:是指食品加工过程中的一个点,在这个点上控制不当时,就可能造成或导致危害,或使成品受到杂质污染,或造成成品的腐败。
6.食品:指条例201(F)节所定义的食品.包括各种原料和辅料。
7.食品接触面:指与人类食品的接触表面以及在正常加工过程中因污水滴溅污染的并与食品接触的设备和工器具表面。
“食品接触面”包括与食品接触的工器具及设备的表面。
8.批:指在某一段时间生产的由具体代号标记的食品。
9.微生物:是指酵母菌、霉菌、细菌和病毒,包括(但不仅限于)对公众健康有影响的微生物种类。
“有害微生物”这个术语包括对公众健康有影响的,或使食品发酵分解的,或使食品受到杂质污染的、或使食品成为条例所指的劣质食品的微生物。
有时,美国食品与药物管理局在这些法规中使用“微生物”这个词,而不使用包含“微生物”一词的短语。
10.害虫:指令人厌恶的任何动物或昆虫,包括,但不仅限于鸟、啮齿动物、蝇和幼虫。
11.厂房:指用于人类食品加工、包装、标识或存放,或与人类食品的加工、包装、标识或存放有关的建筑物或设施或其中的某些部分。
12.质量控制操作:是指一种有计划的系统操作,并采取一切必要的行动防止食品成为条例所指的劣质食品。
13.返工品:是指由于一些不卫生因素而从加工过程中检选出的、干净的、未被掺杂的食品,或经过重新加工而再次调制,并适于消费的食品。
欧版GMPC-中文版手工翻译

优质化妆产品生产指导(GMPC)保护消费者健康目录介绍1.术语2.质量体系2.1介绍2.2员工2.3建筑2.4设备2.5程序及流程2.5.1程序和指引2.5.2流程3.采购3.1介绍3.2合同要求3.3采购文件4.生产4.1介绍4.2进场产品接收4.2.1原材,包装材料,散装产品4.2.2水4.2.3仓储4.3生产过程4.3.1准备4.3.2实际生产过程4.3.3待包装产品储存4.4包装4.5成品储存5.转包生产6.质量管理6.1介绍6.2质量控制6.2.1介绍6.2.2设备和试剂6.2.3控制活动6.2.4控制记录6.2.5留样和样品库6.3监控和数据使用6.4文件控制6.4.1跟踪文件6.4.2文件管理6.5不一致产品管理6.6卫生6.6.1工厂卫生6.6.2个人卫生6.7审核参考文献一、专业术语批次:被一个或多个操作所采用的某一原材料,包装材料或产品的指定的量。
他们被认为是一致的。
在线上生产的情况中,一个批次可以是在一段给定时间内一个产品的量。
二、质量体系2.1 介绍对于想达成其质量目标的企业,它应设计,应用和维护适应生产活动,产品特性和由公司高层支持的文件质量体系。
在生产层面,这个体系包括组织结构,职责和涉及质量管理的可用的资源,程序和流程。
组织结构应清晰设定以搞清楚企业的组织和运作。
它应包括企业规模和产品多样化程度。
企业应当在员工,场所,机器,设备和管理上有着合适和充足的资源。
质量体系要求对违规的分析,整改措施的落实,以及确保改进和足够的监管。
2.2 员工组织结构应是的在不同活动领域中有足够的员工层级:员工应具有技术,经验,适应他们呢职责和工作的能力和积极性。
为了这个目的,所有组织内各阶层员工的培训需求应当被识别并制定相应的培训计划。
员工应当:●知道他们在组织结构中的位置●知道他们的职责和任务●能接触到跟他们负责的生产环节相关的指引,信息和数据●持续被鼓励报告可能在各个生产环节出现的违规和其他不一致情况●遵守个人卫生要求,遵守有关工作和操作方法的指引能找到员工可以替代空缺很理想应采取所有必要措施来提高所有员工的技术和积极性:●培训:企业应建立和完成适合涉及执行不同生产操作(称重,执行,维护,行业卫生,生产过程中验证等)的任务和职责的培训活动。
ICH Q a原料药的GM 指南 中英对照

Q7a(中英文对照)FDA原料药GMP指南TableofContents 目录1.INTRODUCTION 1.简介1.1Objective 1.1目的1.2RegulatoryApplicability 1.2法规的适用性1.3Scope 1.3范围2.QUALITYMANAGEMENT 2.质量管理2.1Principles 2.1总则2.2质量部门的责任2.2ResponsibilitiesoftheQualityUnit(s)2.3生产作业的职责2.3ResponsibilityforProductionActivities2.4InternalAudits(SelfInspection) 2.4内部审计(自检)2.5ProductQualityReview 2.5产品质量审核3.PERSONNEL 3.人员3.1PersonnelQualifications 3.人员的资质3.2PersonnelHygiene 3.2人员卫生3.3Consultants 3.3顾问4.BUILDINGSANDFACILITIES 4.建筑和设施4.1DesignandConstruction 4.1设计和结构4.2Utilities 4.2公用设施4.3Water 4.3水4.4Containment 4.4限制4.5Lighting 4.5照明4.6SewageandRefuse 4.6排污和垃圾4.7SanitationandMaintenance 4.7卫生和保养5.PROCESSEQUIPMENT 5.工艺设备5.1DesignandConstruction 5.1设计和结构5.2EquipmentMaintenanceandCleaning 5.2设备保养和清洁5.3Calibration 5.3校验5.4ComputerizedSystems 5.4计算机控制系统6.DOCUMENTATIONANDRECORDS 6.文件和记录6.1DocumentationSystemandSpecificatio6.1文件系统和质量标准ns6.2EquipmentcleaningandUseRecord 6.2设备的清洁和使用记录6.3RecordsofRawMaterials,Intermediate s,APILabelingandPackagingMaterials 6.3原料、中间体、原料药的标签和包装材料的记录6.4MasterProductionInstructions(MasterProductionandControlRecords)6.4生产工艺规程(主生产和控制记录)6.5BatchProductionRecords(BatchProductionandControlRecords)6.5批生产记录(批生产和控制记录)6.6LaboratoryControlRecords 6.6实验室控制记录6.7BatchProductionRecordReview 6.7批生产记录审核7.MATERIALSMANAGEMENT7.物料管理7.1GeneralControls7.1控制通则7.2ReceiptandQuarantine7.2接收和待验7.3SamplingandTestingofIncomingProduc7.3进厂物料的取样与测试tionMaterials7.4Storage7.4储存7.5Re-evaluation7.5复验8.PRODUCTIONANDIN-PROCESSCONTROLS8.生产和过程控制8.1ProductionOperations8.1生产操作8.2TimeLimits8.2时限8.3 In-processSamplingandControls8.3工序取样和控制8.4BlendingBatchesofIntermediatesorAP8.4中间体或原料药的混批Is8.5ContaminationControl8.5污染控制9.PACKAGINGANDIDENTIFICATIONLABELINGO9.原料药和中间体的包装和贴签FAPIsANDINTERMEDIATES9.1General9.1总则9.2PackagingMaterials9.2包装材料9.3LabelIssuanceandControl9.3标签发放与控制9.4PackagingandLabelingOperations9.4包装和贴签操作10.STORAGEANDDISTRIBUTION10.储存和分发10.1WarehousingProcedures10.1入库程序10.2DistributionProcedures10.2分发程序BORATORYCONTROLS11.实验室控制11.1GeneralControls11.1控制通则11.2TestingofIntermediatesandAPIs11.2中间体和原料药的测试11.3ValidationofAnalyticalProcedures11.3分析方法的验证11.4CertificatesofAnalysis11.4分析报告单11.5StabilityMonitoringofAPIs11.5原料药的稳定性监测11.6ExpiryandRetestDating11.6有效期和复验期11.7Reserve/RetentionSamples11.7留样12.VALIDATION12.验证12.1ValidationPolicy12.1验证方针12.2ValidationDocumentation12.2验证文件12.3Qualification12.3确认12.4ApproachestoProcessValidation12.4工艺验证的方法12.5ProcessValidationProgram12.5工艺验证的程序12.6PeriodicReviewofValidatedSystems12.6验证系统的定期审核12.7CleaningValidation12.7清洗验证12.8ValidationofAnalyticalMethods12.8分析方法的验证13.CHANGECONTROL13.变更的控制14.REJECTIONANDRE-USEOFMATERIALS14.拒收和物料的再利用14.1Rejection14.1拒收14.2Reprocessing14.2返工14.3Reworking14.3重新加工14.4RecoveryofMaterialsandSolvents14.4物料与溶剂的回收14.5Returns14.5退货PLAINTSANDRECALLS15.投诉与召回16.CONTRACTMANUFACTURERS(INCLUDINGLABORATORIES)16.协议生产商(包括实验室)17.AGENTS,BROKERS,TRADERS,DISTRIBUTOR S,REPACKERS,ANDRELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1Applicability17.1适用性17.2TraceabilityofDistributedAPIsandIntermediates17.2已分发的原料药和中间体的可追溯性17.3QualityManagement17.3质量管理17.4Repackaging,Relabeling,andHolding ofAPIsandIntermediates 17.4原料药和中间体的重新包装、重新贴签和待检17.5Stability17.5稳定性17.6TransferofInformation17.6信息的传达17.7HandlingofComplaintsandRecalls17.7投诉和召回的处理17.8HandlingofReturns17.8退货的处理18.SpecificGuidanceforAPIsManufacture dbyCellCulture/Fermentation 18.用细胞繁殖/发酵生产的原料药的特殊指南18.1General18.1总则18.2CellBankMaintenanceandRecordKeeping18.2细胞库的维护和记录的保存18.3CellCulture/Fermentation18.3细胞繁殖/发酵18.4Harvesting,IsolationandPurification18.4收取、分离和精制18.5ViralRemoval/Inactivationsteps18.5病毒的去除/灭活步骤19.APIsforUseinClinicalTrials19.用于临床研究的原料药19.1General19.1总则19.2Quality19.2质量19.3EquipmentandFacilities19.3设备和设施19.4ControlofRawMaterials19.4原料的控制19.5Production19.5生产19.6Validation19.6验证19.7Changes19.7变更19.8LaboratoryControls19.8实验室控制19.9Documentation19.9文件20.Glossary20.术语Q7aGMPGuidanceforAPIsQ7a原料药的GMP指南1.INTRODUCTION 1.简介1.1Objective 1.1目的Thisdocumentisintendedtoprovideguidan ceregardinggoodmanufacturingpractice( GMP)forthemanufacturingofactivepharma ceuticalingredients(APIs)underanappro priatesystemformanagingquality.Itisal sointendedtohelpensurethatAPIsmeetthe qualityandpuritycharacteristicsthatth eypurport,orarerepresented,topossess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
阿司匹林片(片剂)工艺规程完整

阿司匹林片工艺规程1.产品名称及剂型1.1产品名称:阿司匹林片1.2产品剂型:片剂2.产品概述2.1产品名称阿司匹林片汉语拼音名:Asipilin Pian英文名:Aspirin Tablets结构式:阿司匹林本品为2-(乙酰氧基)苯甲酸分子式:C9H8O4 分子量:180.16本品含阿司匹林(C9H8O4)应为标示量的95.0%-105.0%2.2产品特点2.2.1性状本品为白色片2.2.2规格0.5g。
2.2.3类别解热镇痛非甾体抗炎药,抗血小板聚集药。
2.2.4用法与用量口服。
成人一次1片,若发热或疼痛持续不缓解,可间隔4-一6个小时重复用药一次。
24小时不超过4片。
儿童用量请咨询医师或药师。
2.2.5贮藏密封,在干燥处保存。
2.2.6有效期3年。
3.处方和依据3.1批投料处方阿司匹林60kg淀粉5kg枸橼酸0.6kg滑石粉 1.25kg制成12万片3.2依据执行标准:《中国药典》2010年版二部3.3制法 取阿司匹林、淀粉和枸橼酸置高效湿法制粒机中混合均匀,用淀粉浆制粒;干燥;压片;瓶包装及外包装,制得。
4. 工艺流程图物料工序检验入库中间站注:虚线框为十万级洁净区。
5.操作过程及工艺条件 5.1备料5.1.1领料从库房领取合格原辅料,送入车间称量暂存间。
5.1.2粉碎过筛将以下物料依次粉碎过筛,过筛后再次称量,计算物料平衡,并严格复核。
5.2制粒5.2.1配浆称取纯化水1kg置配浆锅中,加入1kg淀粉,搅拌使均匀,在搅拌下冲入10kg 纯化水加热至糊化,配成10%的淀粉浆作为粘合剂。
5.2.2制粒将60kg阿司匹林粉、4kg淀粉和0.6kg枸橼酸粉投入高速制粒机中,干混4分钟后,加入上述淀粉浆混合5分钟,开机制粒。
5.2.3干燥将上述湿颗粒吸入沸腾制粒机中,将设定好工艺参数(进风温度120±5℃,温度75±5℃,进风温度30±5℃)的冷空气通过初效中高效过滤器进适入后部加热室,经过加热器加热至进风所需温度后进入物料室,在引风拉动下物料呈流化态干燥45分钟至水份为3-4%时,停机出料。
印刷工艺中英文对照
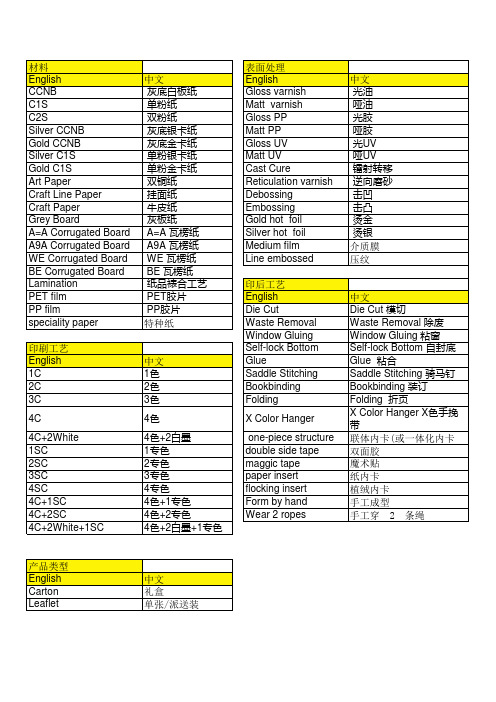
中文 灰底白板纸 单粉纸 双粉纸 灰底银卡纸 灰底金卡纸 单粉银卡纸 单粉金卡纸 双铜纸 挂面纸 牛皮纸 灰板纸 A=A 瓦楞纸 A9A 瓦楞纸 WE 瓦楞纸 BE 瓦楞纸 纸品裱合工艺 PET胶片 PP胶片 特种纸
表面处理 English Gloss varnish Matt varnish Gloss PP Matt PP Gloss UV Matt UV Cast Cure Reticulation varnish Debossing Embossing Gold hot foil Silver hot foil Medium film Line embossed 印后工艺 English Die Cut Waste Removal Window Gluing Self-lock Bottom Glue Saddle Stitching Bookbinding Folding X Color Hanger one-piece structure double side tape maggic tape paper insert flocking insert Form by hand Wear 2 ropes
产品类型 English Carton Leaflet
中文 礼盒 单张/派送装
手挽带
材料 English CCNB C1S C2S Silver CCNB Gold CCNB Silver C1S Gold C1S Art Paper Craft Line Paper Craft Paper Grey Board A=A Corrugated Board A9A Corrugated Board WE Corrugated Board BE Corrugated Board Lamination PET film PP film speciality paper 印刷工艺 English 1C 2C 3C 4C 4C+2White 1SC 2SC 3SC 4SC 4C+1SC 4C+2SC 4C+2White+1SC
英文制药工艺流程

英文制药工艺流程Pharmaceutical Process FlowThe pharmaceutical industry plays a crucial role in the development, production, and distribution of medicines that save lives and improve the quality of life for people around the world. One of the key aspects of the pharmaceutical industry is the manufacturing process, which involves a series of steps to ensure the safety, efficacy, and quality of the final product. In this article, we will discuss the general process flow of pharmaceutical manufacturing.1. Research and Development: The first step in the pharmaceutical process is research and development (R&D), where scientists and researchers identify potential drug candidates and conduct extensive studies to understand their properties, efficacy, and safety. This involves the synthesis of new chemical compounds or the modification of existing compounds to create new drugs. R&D also includes preclinical testing, such as in vitro and animal studies, to assess the safety and effectiveness of the drug candidates.2. Formulation and Pre-Formulation: Once a drug candidate is identified, the next step is formulation and pre-formulation. During this stage, scientists determine the optimal dosage form of the drug, such as tablets, capsules, or injections, and develop a formulation that ensures the drug's stability, bioavailability, and patient acceptability. Pre-formulation studies focus on understanding the physical and chemical properties of the drug and determining the best excipients and manufacturing processes to achieve the desired formulation.3. Manufacturing: After formulation, the drug moves into the manufacturing phase. This involves scaling up the production from laboratory-scale to commercial-scale. The manufacturing process depends on the dosage form and can be divided into several steps. For solid dosage forms, such as tablets, powders or granules are mixed with excipients and compressed into tablets. For liquid dosage forms, such as syrups or suspensions, active pharmaceutical ingredients (APIs) are dissolved or suspended in a suitable vehicle. Sterile dosage forms, like injections or eye drops, require strict aseptic processing to prevent contamination.4. Quality Control: Throughout the manufacturing process, quality control is crucial to ensure that the final product meets the required standards and specifications. This involves the testing of raw materials, intermediates, and final products for chemical, physical, and microbiological parameters. Quality control also includes stability studies to determine the shelf life of the drug and ensure that it maintains its safety and efficacy over time.5. Packaging and Labeling: Once the final product has been manufactured and passed quality control, it is ready for packaging and labeling. The packaging must be designed to protect the drug from degradation, moisture, and tampering while maintaining its stability. The labeling includes important information such as drug name, strength, dosage instructions, and any necessary warnings or precautions.6. Distribution: After packaging, the pharmaceutical product is ready for distribution to pharmacies, hospitals, or other healthcarefacilities. This requires careful handling and storage to maintain the integrity and quality of the drugs. Distribution also involves proper documentation, including batch records, shipping records, and tracking systems, to ensure traceability and accountability.In conclusion, the pharmaceutical manufacturing process involves several stages, from research and development to distribution. Each step is crucial in ensuring the safety, efficacy, and quality of the final product. By following a well-defined process flow, pharmaceutical companies can produce medicines that meet regulatory standards and contribute to the health and well-being of individuals worldwide.。
FDA-GMP中英文对照标准版

DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OF RAW MATERIALS BY FDATable of Contents 目录1. INTRODUCTION1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES 原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess. 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
阿司匹林片剂的制备工艺流程

阿司匹林片剂的制备工艺流程英文回答:Aspirin Tablet Manufacturing Process Flow.Step 1: Raw Material Preparation.Aspirin powder is received from the supplier.Other excipients, such as binders, disintegrants, and lubricants, are weighed and blended together.Step 2: Granulation.The aspirin powder and excipients are mixed with water to form granules.The granules are then dried and sized.Step 3: Compression.The granules are compressed into tablets using atablet press.The tablets are ejected from the press and de-dusted.Step 4: Coating.Some tablets may be coated with a film coating to protect them from moisture and/or to improve their appearance.The coating is applied using a coating pan.Step 5: Packaging.The tablets are packaged into bottles, blisters, or other containers.The containers are labeled and shipped to the end user.中文回答:阿司匹林片剂的制备工艺流程。
ICHQ中英文对照

I C H Q中英文对照 The latest revision on November 22, 2020Q7a(中英文对照)FDA原料药GMP指南Table of Contents 目录1. INTRODUCTION 1. 简介Objective目的Regulatory Applicability法规的适用性Scope范围2. QUALITY MANAGEMENT 2.质量管理Principles总则质量部门的责任Responsibilities of theQuality Unit(s)生产作业的职责Responsibility for ProductionActivities内部审计(自检) Internal Audits (SelfInspection)Product Quality Review产品质量审核3. PERSONNEL 3. 人员Personnel Qualifications 3.人员的资质Personnel Hygiene人员卫生Consultants顾问4. BUILDINGS AND FACILITIES 4. 建筑和设施Design and Construction设计和结构Utilities公用设施Water水Containment限制Lighting照明Sewage and Refuse排污和垃圾Sanitation and Maintenance卫生和保养5. PROCESS EQUIPMENT 5. 工艺设备Design and Construction设计和结构设备保养和清洁 Equipment Maintenance andCleaningCalibration校验Computerized Systems计算机控制系统6. DOCUMENTATION AND RECORDS 6. 文件和记录Documentation System andSpecifications文件系统和质量标准Equipment cleaning and UseRecord设备的清洁和使用记录Records of Raw Materials, Intermediates, API Labeling and Packaging Materials原料、中间体、原料药的标签和包装材料的记录Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)Laboratory Control Records实验室控制记录批生产记录审核Batch Production RecordReview7. MATERIALS MANAGEMENT7. 物料管理General Controls控制通则Receipt and Quarantine接收和待验进厂物料的取样与测试 Sampling and Testing ofIncoming Production MaterialsStorage储存Re-evaluation复验8. 生产和过程控制8. PRODUCTION AND IN-PROCESSCONTROLSProduction Operations生产操作Time Limits时限工序取样和控制In-process Sampling andControls中间体或原料药的混批Blending Batches ofIntermediates or APIsContamination Control污染控制9. 原料药和中间体的包装和贴签9. PACKAGING ANDIDENTIFICATION LABELING OFAPIs AND INTERMEDIATESGeneral总则Packaging Materials包装材料Label Issuance and Control标签发放与控制Packaging and Labeling 包装和贴签操作Operations10. STORAGE AND DISTRIBUTION10.储存和分发Warehousing Procedures入库程序Distribution Procedures分发程序11. LABORATORY CONTROLS11.实验室控制General Controls控制通则中间体和原料药的测试 Testing of Intermediates andAPIs分析方法的验证Validation of AnalyticalProceduresCertificates of Analysis分析报告单Stability Monitoring of APIs原料药的稳定性监测Expiry and Retest Dating有效期和复验期Reserve/Retention Samples留样12. VALIDATION12.验证Validation Policy验证方针Validation Documentation验证文件Qualification确认工艺验证的方法Approaches to ProcessValidationProcess Validation Program工艺验证的程序验证系统的定期审核 Periodic Review of ValidatedSystemsCleaning Validation清洗验证分析方法的验证Validation of AnalyticalMethods13. CHANGE CONTROL13.变更的控制14.拒收和物料的再利用14. REJECTION AND RE-USE OFMATERIALSRejection拒收Reprocessing返工Reworking重新加工物料与溶剂的回收Recovery of Materials andSolventsReturns退货15. COMPLAINTS AND RECALLS15.投诉与召回16. CONTRACT MANUFACTURERS(INCLUDING LABORATORIES)16.协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 17.代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者Applicability适用性Traceability of Distributed APIs and Intermediates 已分发的原料药和中间体的可追溯性Quality Management质量管理Repackaging, Relabeling, and Holding of APIs and Intermediates 原料药和中间体的重新包装、重新贴签和待检Stability稳定性Transfer of Information信息的传达Handling of Complaints andRecalls投诉和召回的处理 Handling of Returns退货的处理18. Specific Guidance for APIs Manufactured by CellCulture/Fermentation 18. 用细胞繁殖/发酵生产的原料药的特殊指南General总则Cell Bank Maintenance andRecord Keeping细胞库的维护和记录的保存 Cell Culture/Fermentation细胞繁殖/发酵Harvesting, Isolation andPurification收取、分离和精制病毒的去除/灭活步骤Viral Removal/Inactivationsteps19.用于临床研究的原料药19. APIs for Use in ClinicalTrialsGeneral总则Quality质量Equipment and Facilities设备和设施Control of Raw Materials原料的控制Production生产Validation验证Changes变更Laboratory Controls实验室控制Documentation文件20. Glossary20. 术语Q7a GMP Guidance for APIsQ7a原料药的GMP指南1. INTRODUCTION 1. 简介Objective目的This document is intended to provide guidance regarding good manufacturing practice (GMP)for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and 本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
工艺流程中英文对照

工艺流程中英文对照1. 引言本文档旨在提供一份工艺流程的中英文对照,以帮助读者更好地了解和实施该工艺流程。
2. 工艺流程的定义工艺流程是指将原材料或半成品转化为可使用的最终产品的一系列步骤和操作。
3. 工艺流程的步骤下面是工艺流程的详细步骤和对应的中英文对照:1. 原材料准备(Raw Material Preparation)2. 清洗(Cleaning)3. 切割(Cutting)4. 排列组装(Assembly)5. 焊接(Welding)6. 喷涂(Coating)7. 检验(Inspection)8. 包装(Packaging)9. 质量控制(Quality Control)10. 出厂(Shipping)4. 每个步骤的说明下面是每个步骤的详细说明和对应的中英文对照:4.1 原材料准备(Raw Material Preparation)该步骤包括选择和准备用于生产的原材料。
4.2 清洗(Cleaning)该步骤涉及清洗原材料,以去除表面的污垢和杂质。
4.3 切割(Cutting)该步骤用于将原材料切割成所需的尺寸和形状。
4.4 排列组装(Assembly)该步骤将切割好的部件按照设计要求进行排列组装。
4.5 焊接(Welding)该步骤使用焊接技术将组件连接在一起。
4.6 喷涂(Coating)该步骤涉及给产品表面进行喷涂,以提供保护和美观效果。
4.7 检验(Inspection)该步骤涉及对成品进行检查和测试,以确保其质量符合要求。
4.8 包装(Packaging)该步骤将成品进行包装,以便存储和运输。
4.9 质量控制(Quality Control)该步骤包括对整个工艺流程进行质量控制,以确保产品符合质量标准。
4.10 出厂(Shipping)该步骤将成品运送到客户或销售点。
5. 总结本文档提供了工艺流程的中英文对照,通过了解每个步骤的含义和操作,读者可以更好地理解和实施该工艺流程。
制药行业常用英语(中英文对照版)

制药行业常用英语1、药品生产质量管理规范GMP:Good ManufacturingPractice2、国家食品与药品监督管理局State Food andDrug Administration3、总则GeneralProvisions4、《中华人民共和国药品管理法》the DrugAdministration Law of the People's Republic of China 5、制剂Preparation6、原料药API: Active PharmaceuticalIngredient7、成品finished goods8、工序process9、机构与人员organization and personnel10、专业知识professional knowledge11、生产经验production experience12、组织能力organizational skill13、技术人员technical staff14、实施implementation15、药品生产pharmaceutical manufacturing 16、质量管理quality management17、质量检验quality inspection18、专业技术培训professional and technicaltraining 19、基础理论知识basic theoreticalknowledge20、实际操作技能practical operationskills 21、高生物活性highly potent22、高毒性high toxicity23、污染contamination24、考核评估assessment25、厂房与设施buildings and facilities 26、生产环境production environment 27、空气洁净级别clean air level28、昆虫insect29、洁净室(区)clean room(area)30、光滑smooth31、无裂缝no cracks32、无颗粒物脱落no particle shedding 33、耐受endure34、消毒disinfection35、无菌sterile36、交界处junction, joint37、弧形arc38、灰尘积聚dues accumulation 39、储存区store area40、生产规模production scale 41、设备equipment42、物料material43、中间产品intermediate product 44、待验品quarantined material 45、交叉污染cross-contamination 46、管道pipeline, ductwork 47、风口tuber48、公用设施, 公用工程utilities of publicservice 49、照明lighting50、照度illumination51、应急紧急情况emergency52、净化purification, clean53、微生物, 微生物学, 微生物的micro-organism, microbiology,microbiologic 54、监测monitoring55、记录record56、天棚天花板ceiling, roof57、密封seal58、静压差Static DifferentialPressure 59、温度temperature60、相对湿度RH: Relative Humidity 61、低漏地漏floor drainer62、青霉素penicillin63、分装室separating room, fillingroom64、相对负压relative negativepressure65、废气waste gas,exhausted air66、β-内酰胺结构类药品β-Lactasestructure drug, drugs of β-Lactic group67、避孕药品contraceptives68、激素类hormone69、抗肿瘤类anti-tumor, oncology70、放射性药品Radiopharmaceuticals71、包装packing, package72、循环使用recycling73、微粒particles74、辐射radiation, irradiation75、细菌bacteria76、病毒virus77、细胞cell78、脱毒前后pre and post detoxification 79、活疫苗与灭活疫苗active vaccine/inactivatedvaccine 80、人血液制品blood products 81、预防制品prevention products82、灌装filling83、中药Chinesetraditional medicines 84、前处理pretreatment85、提取extraction86、浓缩concentration87、动物脏器viscera of animal,organ of animal 88、蒸、炒、炙、煅steaming, frying,sunburn, testing 89、炮制concocted90、通风ventilation91、除烟smoke removal92、除尘dust removal93、降温设施temperature-reducingestablishment, cooling94、筛选screening, sift95、切片slicing96、粉碎grinding97、压缩空气compressed air98、惰性气体noble gas99、取样Sampling100、称量室weighing room, dispensingroom 101、中药标本Chinese herbal sample,exemplar of TCM 102、检定鉴定verification, identification 103、同位素Isotope104、设备equipment105、选型model/type selection 106、耐腐蚀anticorrosion107、吸附adsorption, absorption 108、润滑剂, 润滑lubricant, lubricate 109、冷却剂coolant110、流向flow direction111、纯化水PW: Purified Water 112、注射用水WFI: Water for Injection 113、滋生breeding114、储罐tank115、死角neglected portion 116、盲管blind pipe117、纤维fiber118、疏水性hydrophobicity119、仪表instrumentation 120、量具measuring tool 121、衡器weighing instrument 122、精密度precision123、维修、保养maintenance 124、不合格disqualified reject 125、物料material126、购买purchasing127、发放releasing128、产地origin129、入库loading130、固体solid131、液体liquid132、挥发性volatile133、净药材net medicine, netTCM 134、麻醉药品narcotics135、精神药品psychotropic drug 136、易燃combustible137、易爆explosive138、验收acceptance139、使用说明书instruction140、标签label 141、卫生, 清洁/消毒sanitation142、车间, 辅房workshop143、间隔时间time interval144、清洁剂detergent145、消毒剂disinfectant146、废弃物wastes147、更衣室changing room148、工作服,work clothes149、颗粒性物质, 颗粒剂granules150、耐药菌株drug-resistant strain151、传染病infectiousdisease152、皮肤病dermatitis153、验证verification, validation 154、确认qualification155、安装installation156、运行running operation 157、性能performance158、原辅料raw material and incipient 159、文件document160、投诉complaint 161、报废reject162、品名product name163、处方preion, formula164、技术参数technicalparameter165、容器container166、半成品semi-finished product,intermediate167、申请application168、稳定性stability169、起草draft170、生产管理production management,manufacturing control.171、事故accident172、混淆mix-up173、喷雾spray174、合格证certificate175、清场clearance176、质量管理quality management 177、内控internal control,on-line test 178、滴定液tartan179、培养基medium180、有效期validity, expiry date,shelf life 181、产品销售与收回product sales andrecovery/recall 182、投诉与不良反应报告complaints and adversereaction 183、自检self-inspection184、附则schedule appendix185、平衡balance186、饮用水drinking water, potablewater 187、蒸馏法distillation188、离子交换法ion exchange189、反渗透法RO: Reverse Osmosis190、附加剂添加剂additives191、滞留stranded resort192、批batch, lot193、组分, 组成component194、无纤维脱落的过滤器non-fiber-releasingfilter195、活性成份Active Ingredient196、非活性成份Inactive ingredient197、中间产品in-process product,intermediate product 198、批号batch number199、药用物料medicated feed200、药用预混合料medicated premix 201、质量控制部门Quality control department 202、理论产量Theoretical yield203、实际产量Actual yield204、比率Percentage, rate205、验收标准可接受标准Acceptance criteria206、代表性样品Representative sample 207、微粒状的particulate208、污染物contaminant209、石棉asbestos210、诊断diagnosis211、缓解mitigation212、化学变化chemical change 213、组分ingredient, component 214、制备fabricate preparation 215、复合compound216、混合blend217、加工processing218、浓度concentration219、单位剂量unit dose220、药品包装容器drug product containers 221、密封件, 封盖closure222、效价Titer223、纯度purity224、规格strength225、监督supervise, monitor226、实验室laboratory227、无菌操作aseptic operation,sterile operation 228、层流laminar flow229、湍流turbulent air flow230、空气过滤air filtration231、空气加热air heating 232、预过滤器profiler233、排气系统exhaust system 234、管件plumbing 235、虹吸倒流back-siphon age 236、污水sewage237、废料refuse238、盥洗设备toilet facilities 239、空气干燥器air drier240、垃圾trash 241、有机废料organic waste242、杀鼠剂rodenticides243、杀昆虫剂insecticides244、杀真菌剂fungicides245、熏蒸剂fumigating reagents246、去垢剂cleaning agents247、消毒剂sanitizing agents248、滂沱剂lubricant249、自动化设备、机械化设备和电子设备automatic, mechanical,or electronic equipment 250、微型胶卷microfilm251、注射剂injection252、灭菌设备sterilization equipment 253、无菌取样技术aseptic sampling techniques 254、显微镜microscope255、热源, 内毒素pyrogen, endotoxin256、偏差deviation257、变更change control258、进料charge-in259、项目代码item code260、鉴别identify 261、片剂tablet262、胶囊capsule263、颗粒剂granule264、溶解时间溶出时间dissolution time265、澄明度clarity266、隔离系统quarantinesystem, isolation system 267、返工reprocessing268、发放issuance, release269、非处方药OTC:over-the-counter270、处方药preed medicine271、皮肤科药、牙粉、胰岛素、喉片dermatological, dentifrice,insulin, or throat lozenge product272、保险包装tamper-resistant package273、明胶硬胶囊hard gelatin capsule274、顺势治疗homeopathic275、入库warehousing276、变质deteriorate277、准确性accuracy278、灵敏性sensitivity279、特异性specificity280、重复性reproducibility, repeatability 281、变应原提取物allergenic extracts282、眼膏ophthalmic ointment283、粗糙或磨蚀物质harsh or abrasivesubstances 284、控释制剂controlled-releasedosage form 285、实验动物laboratory animals286、供应商Supplier287、光谱spectrum288、测量单位units of measure289、换算系数conversion factors290、试剂reagent291、安慰剂placebo292、明确地explicitly293、取代supersede294、溶液solution295、批准approval296、(美国)食品药品监督管理局FDA: Food and DrugAdministration 297、标准操作程序SOP: Standard OperatingProcedure 298、质量保证QA: Quality Assurance299、质量控制QC:Quality Control300、批生产记录BPR: Batch ProductionRecord 301、批检验记录BAR: Batch AnalysisRecord302、工艺规程PP: Process Procedure303、健康,安全,环保EHS: Environment,Health and Safe 304、美国联邦法规CFR: Code of FederalRegulation 305、美国药典USP: The United StatesPharmacopeia 306、欧洲药典EP: European pharmacopeia307、英国药典BP: British pharmacopeia308、药物主文件DMF: Drug Master File309、验证主计划VMP: Validation MasterPlan310、验证方案VP: Validation Protocol311、验证报告VR: Validation Report312、安装确认IQ: Installation Qualification 313、运行确认OQ: Operation Qualification 314、性能确认PQ: Performance Qualification 315、超出标准(限度)OOS: Out of Specification 316、冻干产品freeze-dry product,lyophilizated product317、工厂主述文件SMF: Site Master File。
工艺流程-英文(最新)
Production Dep. Production plan. (JL-7.5.1-1/2)
Quality inspection Dep. Visual and Drift inspection record (JL-8.2.4-3)
Production Dep. Marking record (JL-7.5.1-8)
Quality inspection Dep. 1.Raw material inspection record (JL-7.5.1-4) 2.Physical & Chemical analysis record. (JL-8.2.4-19)
Internal trade & Foreign trade Dep. Production order notification (JL-7.2-7)
Coupling make-up, pipe end drift
Threading
100% inspection.
100% inspection
100% inspection
Quality inspection Dep. 1.Finished product inspection report (JL-8.2.4-1) 2.Material Test Certificate (JL-8.2.4-20)
Quality inspection Dep. 1.First piece inspection record (JL-8.2.4-2) 2.Coupling threading inspection record (JL-8.2.4-11) 3.Coupling threading random inspection record (JL-8.2.4-16)
药品工艺流程英文

药品工艺流程英文《药品工艺流程》The pharmaceutical manufacturing process, also known as drug manufacturing or drug production, is a complex and highly regulated process that involves the synthesis of active pharmaceutical ingredients (APIs) and the formulation of these ingredients into medications. The process begins with the discovery or identification of a potential drug molecule and continues through several stages of development, including preclinical and clinical testing, regulatory approval, and manufacturing scale-up.The pharmaceutical manufacturing process typically involves the following key steps:1. Discovery and development: This stage focuses on identifying potential drug candidates and testing their efficacy and safety in laboratory and animal models.2. Preclinical testing: Once a promising drug candidate has been identified, it undergoes extensive preclinical testing to evaluate its pharmacokinetics, toxicity, and efficacy.3. Clinical trials: If a drug candidate passes preclinical testing, it moves on to clinical trials, which involve testing the drug in human subjects to evaluate its safety and effectiveness.4. Regulatory approval: After successful completion of clinical trials, the drug candidate can be submitted for regulatory approvalby agencies such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in Europe.5. Manufacturing scale-up: Once a drug has been approved, the next step is to scale up the manufacturing process to produce the drug in large quantities for commercial distribution. This involves optimizing the production process, establishing quality control measures, and ensuring compliance with regulatory requirements.6. Formulation: The active pharmaceutical ingredient (API) is combined with other substances to create the final dosage form, such as tablets, capsules, or injections.7. Packaging and labeling: The finished product is then packaged and labeled according to regulatory requirements and industry standards.Throughout the entire pharmaceutical manufacturing process, strict quality control measures are implemented to ensure the safety, efficacy, and purity of the final product. This includes testing the raw materials, in-process samples, and finished products for identity, potency, and purity, as well as ensuring compliance with Good Manufacturing Practices (GMP).In conclusion, the pharmaceutical manufacturing process is a highly complex and regulated process that involves multiple stages of development, testing, and production. By following strict quality control measures and adhering to regulatory requirements,pharmaceutical companies can ensure the safety and efficacy of their products for patients around the world.。
工艺流程材料设备生产常用中英文标准名称模板

刻蚀后关键尺寸测量
AEI
刻蚀后自动光学检查
Micro/Macro Inspection
宏微观检查
Laser Repair
激光修补
Active层
Clean before depo
成膜前清洗
Active film depo
Active成膜
AOI
自动光学检查
Macro Inspection
旋转/冷却单元
Turn Align Unit
旋转/对位单元
Turn Over Unit
翻转单元
After Rubbing Cleaner
摩擦后清洗
Spacer Spray
衬垫球散布
Spacer Counter
衬垫球计数
Spacer rework
衬垫球返工
Spacer Cure
衬垫球附着固化
Short Dispense
线性感应马达传送载具
OHS (Overhead Handling System)
天车搬送系统
Stocker (clean depot)
工装篮存放架
Battery
电池
Bay
作业区
Inter-bay
作业区和作业区之间
Intra-bay
作业区之内
Bumper
减震缓冲器
Charger
充电器
Controller
镜检
Peeling strength test
拉力测试
ET test1
电测1
IC or FPC Repair
修补
UV glue sealing
封胶
FPC reinforcement
