欧洲药典重金属检测
欧洲药典重金属及溶残方法

01/2005:504005.4.RESIDUAL SOLVENTSLIMITING RESIDUAL SOLVENT LEVELS IN ACTIVE SUBSTANCES,EXCIPIENTS AND MEDICINAL PRODUCTSThe International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use(ICH)has adopted Impurities Guidelines for Residual Solvents which prescribes limits for the content of solvents which may remain in active substances,excipients and medicinal products after processing.This guideline, the text of which is reproduced below,excludes existing marketed products.The European Pharmacopoeia is, however,applying the same principles enshrined in the guideline to existing active substances,excipients and medicinal products whether or not they are the subject of a monograph of the Pharmacopoeia.All substances and products are to be tested for the content of solvents likely to be present in a substance or product.Where the limits to be applied comply with those given below,tests for residual solvents are not generally mentioned in specific monographs since the solvents employedmay vary from one manufacturer to another and the requirements of this general chapter are applied via the general monograph on Substances for Pharmaceutical Use(2034).The competent authority is to be informedof the solvents employed during the production process. This information is also given in the dossier submitted for a certificate of suitability of the monographs of the European Pharmacopoeia and is mentioned on the certificate. Where only Class3solvents are used,a test for loss on drying may be applied or a specific determination of the solvent may be made.If for a Class3solvent a justified and authorised limit higher than0.5per cent is applied,a specific determination of the solvent is required.When Class1residual solvents or Class2residual solvents (or Class3residual solvents which exceed the0.5per cent) are used,the methodology described in the general method (2.4.24)is to be applied wherever possible.Otherwise an appropriate validated method is to be employed.When a quantitative determination of a residual solventis carried out,the result is taken into account for the calculation of the content of the substance except where a test for drying is carried out.IMPURITIES:GUIDELINESFOR RESIDUAL SOLVENTS(CPMP/ICH/283/95)1.INTRODUCTION2.SCOPE OF THE GUIDELINE3.GENERAL PRINCIPLES3.1.CLASSIFICATION OF RESIDUAL SOLVENTS BY RISK ASSESSMENT3.2.METHODS FOR ESTABLISHING EXPOSURE LIMITS 3.3.OPTIONS FOR DESCRIBING LIMITS OF CLASS2 SOLVENTS3.4.ANALYTICAL PROCEDURES3.5.REPORTING LEVELS OF RESIDUAL SOLVENTS4.LIMITS OF RESIDUAL SOLVENTS4.1.SOLVENTS TO BE AVOIDED4.2.SOLVENTS TO BE LIMITED4.3.SOLVENTS WITH LOW TOXIC POTENTIAL4.4.SOLVENTS FOR WHICH NO ADEQUATE TOXICOLOGICAL DATA WAS FOUNDGLOSSARYAPPENDIX1.LIST OF SOLVENTS INCLUDED IN THE GUIDELINEAPPENDIX2.ADDITIONAL BACKGROUNDA2.1:ENVIRONMENTAL REGULATION OF ORGANIC VOLATILE SOLVENTSA2.2:RESIDUAL SOLVENTS IN PHARMACEUTICALS APPENDIX3.METHODS FOR ESTABLISHING EXPOSURE LIMITS1.INTRODUCTIONThe objective of this guideline is to recommend acceptable amounts of residual solvents in pharmaceuticals for the safety of the patient.The guideline recommends the useof less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents. Residual solvents in pharmaceuticals are defined here as organic volatile chemicals that are used or produced in the manufacture of active substances or excipients,or in the preparation of medicinal products.The solvents are not completely removed by practical manufacturing techniques. Appropriate selection of the solvent for the synthesis of active substance may enhance the yield,or determine characteristics such as crystal form,purity,and solubility. Therefore,the solvent may sometimes be a critical parameter in the synthetic process.This guideline does not address solvents deliberately used as excipients nor does it address solvates.However,the content of solvents in such products should be evaluated and justified.Since there is no therapeutic benefit from residual solvents, all residual solvents should be removed to the extent possible to meet product specifications,good manufacturing practices,or other quality-based requirements.Medicinal products should contain no higher levels of residual solvents than can be supported by safety data.Some solvents that are known to cause unacceptable toxicities(Class1,Table1) should be avoided in the production of active substances, excipients,or medicinal products unless their use can be strongly justified in a risk-benefit assessment.Some solvents associated with less severe toxicity(Class2,Table2)should be limited in order to protect patients from potential adverse effects.Ideally,less toxic solvents(Class3,Table3)should be used where practical.The complete list of solvents included in this guideline is given in Appendix1.The lists are not exhaustive and other solvents can be used and later added to the lists.Recommended limits of Class1 and2solvents or classification of solvents may change as new safety data becomes available.Supporting safety data in a marketing application for a new medicinal product containing a new solvent may be based on concepts inthis guideline or the concept of qualification of impurities as expressed in the guideline for active substances(Q3A,Impurities in New Active Substances)or medicinal products (Q3B,Impurities in New Medicinal Products),or all three guidelines.2.SCOPE OF THE GUIDELINEResidual solvents in active substances,excipients,and in medicinal products are within the scope of this guideline. Therefore,testing should be performed for residual solvents when production or purification processes are known to result in the presence of such solvents.It is only necessary to test for solvents that are used or produced in the manufacture or purification of active substances,excipients, or medicinal product.Although manufacturers may choose to test the medicinal product,a cumulative method may be used to calculate the residual solvent levels in the medicinal product from the levels in the ingredients used to produce the medicinal product.If the calculation results in a level equal to or below that recommended in this guideline,no testing of the medicinal product for residual solvents need be considered.If however,the calculated level is above the recommended level,the medicinal product should be tested to ascertain whether the formulation process has reduced the relevant solvent level to within the acceptable amount. Medicinal product should also be tested if a solvent is used during its manufacture.This guideline does not apply to potential new active substances,excipients,or medicinal products used during the clinical research stages of development,nor does it apply to existing marketed medicinal products.The guideline applies to all dosage forms and routes of administration.Higher levels of residual solvents may be acceptable in certain cases such as short term(30days or less)or topical application.Justification for these levels should be made on a case by case basis.See Appendix2for additional background information related to residual solvents.3.GENERAL PRINCIPLES3.1.CLASSIFICATION OF RESIDUAL SOLVENTS BY RISK ASSESSMENTThe term“tolerable daily intake”(TDI)is used by the International Program on Chemical Safety(IPCS)to describe exposure limits of toxic chemicals and“acceptable daily intake”(ADI)is used by the World Health Organisation (WHO)and other national and international health authorities and institutes.The new term“permitted daily exposure”(PDE)is defined in the present guideline as a pharmaceutically acceptable intake of residual solvents to avoid confusion of differing values for ADI’s of the same substance.Residual solvents assessed in this guideline are listed in Appendix1by common names and structures.They were evaluated for their possible risk to human health and placed into one of three classes as follows:Class1solvents:Solvents to be avoidedKnown human carcinogens,strongly suspected human carcinogens,and environmental hazards.Class2solvents:Solvents to be limitedNon-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity.Solvents suspected of other significant but reversibletoxicities.Class3solvents:Solvents with low toxic potential Solvents with low toxic potential to man;no health-based exposure limit is needed.Class3solvents have PDEs of 50mg or more per day.3.2.METHODS FOR ESTABLISHING EXPOSURE LIMITS The method used to establish permitted daily exposures for residual solvents is presented in Appendix3.Summariesof the toxicity data that were used to establish limits are published in Pharmeuropa,Vol.9,No.1,Supplement April 1997.3.3.OPTIONS FOR DESCRIBING LIMITS OF CLASS2 SOLVENTSTwo options are available when setting limits for Class2 solvents.Option1:The concentration limits in ppm stated in Table2 can be used.They were calculated using equation(1)below by assuming a product mass of10g administered daily.(1)Here,PDE is given in terms of mg/day and dose is givenin g/day.These limits are considered acceptable for all substances, excipients,or products.Therefore this option may be applied if the daily dose is not known or fixed.If all excipients and active substances in a formulation meet the limits givenin Option1,then these components may be used in any proportion.No further calculation is necessary provided the daily dose does not exceed10g.Products that are administered in doses greater than10g per day should be considered under Option2.Option2:It is not considered necessary for each component of the medicinal product to comply with the limits givenin Option1.The PDE in terms of mg/day as stated in Table2can be used with the known maximum daily dose and equation(1)above to determine the concentrationof residual solvent allowed in a medicinal product.Such limits are considered acceptable provided that is has been demonstrated that the residual solvent has been reducedto the practical minimum.The limits should be realistic in relation to analytical precision,manufacturing capability, reasonable variation in the manufacturing process,and the limits should reflect contemporary manufacturing standards. Option2may be applied by adding the amounts of a residual solvent present in each of the components of the medicinal product.The sum of the amounts of solvent per day should be less than that given by the PDE.Consider an example of the use of Option l and Option2 applied to acetonitrile in a medicinal product.The permitted daily exposure to acetonitrile is4.1mg per day;thus,the Option1limit is410ppm.The maximum administered daily mass of a medicinal product is5.0g,and the medicinal product contains two excipients.The composition of the medicinal product and the calculated maximum content of residual acetonitrile are given in the following table. Component Amount informulationAcetonitrilecontentDailyexposure Active substance0.3g800ppm0.24mg Excipient10.9g400ppm0.36mg Excipient2 3.8g800ppm 3.04mg Medicinal product 5.0g728ppm 3.64mg Excipient l meets the Option l limit,but the drug substance, excipient2,and medicinal product do not meet the Option l limit.Nevertheless,the product meets the Option2limit of 4.l mg per day and thus conforms to the recommendations in this guideline.Consider another example using acetonitrile as residual solvent.The maximum administered daily mass of a medicinal product is5.0g,and the medicinal product contains two excipients.The composition of the medicinal product and the calculated maximum content of residual acetonitrile is given in the following table.Component Amount informulation AcetonitrilecontentDailyexposureActive substance0.3g800ppm0.24mg Excipient10.9g2000ppm 1.80mg Excipient2 3.8g800ppm 3.04mg Medicinal product 5.0g1016ppm 5.08mgIn this example,the product meets neither the Option1 nor the Option2limit according to this summation.The manufacturer could test the medicinal product to determine if the formulation process reduced the level of acetonitrile.If the level of acetonitrile was not reduced during formulation to the allowed limit,then the manufacturer of the medicinal product should take other steps to reduce the amount of acetonitrile in the medicinal product.If all of these steps fail to reduce the level of residual solvent,in exceptional cases the manufacturer could provide a summary of efforts made to reduce the solvent level to meet the guideline value, and provide a risk-benefit analysis to support allowing the product to be utilised containing residual solvent at a higher level.3.4.ANALYTICAL PROCEDURESResidual solvents are typically determined using chromatographic techniques such as gas chromatography. Any harmonised procedures for determining levels of residual solvents as described in the pharmacopoeias should be used,if feasible.Otherwise,manufacturers would be free to select the most appropriate validated analytical procedure for a particular application.If only Class3solvents are present,a non-specific method such as loss on drying may be used.Validation of methods for residual solvents should conform to ICH guidelines“Text on Validation of Analytical Procedures”and“Extension of the ICH Text on Validation of Analytical Procedures”.3.5.REPORTING LEVELS OF RESIDUAL SOLVENTS Manufacturers of pharmaceutical products need certain information about the content of residual solvents in excipients or active substances in order to meet the criteria of this guideline.The following statements are given as acceptable examples of the information that could be provided from a supplier of excipients or active substances to a pharmaceutical manufacturer.The supplier might choose one of the following as appropriate:—Only Class3solvents are likely to be present.Loss on drying is less than0.5per cent.—Only Class2solvents X,Y,...are likely to be present.All are below the Option1limit(Here the supplier would name the Class2solventsrepresented by X,Y,...)—Only Class2solvents X,Y,...and Class3solvents are likely to be present.Residual Class2solvents are below the Option1limit and residual Class3solvents are below0.5per cent.If Class1solvents are likely to be present,they should be identified and quantified.“Likely to be present”refers to the solvent used in the final manufacturing step and to solvents that are used in earlier manufacturing steps and not removed consistently by a validated process.If solvents of Class2or Class3are present at greater than their Option1limits or0.5per cent,respectively,they should be identified and quantified.4.LIMITS OF RESIDUAL SOLVENTS4.1.SOLVENTS TO BE AVOIDEDSolvents in Class1should not be employed in the manufacture of active substances,excipients,and medicinal products because of their unacceptable toxicity or their deleterious environmental effect.However,if their use is unavoidable in order to produce a medicinal product with a significant therapeutic advance,then their levels should be restricted as shown in Table1,unless otherwise justified. 1,1,1-Trichloroethane is included in Table1because it is an environmental hazard.The stated limit of1500ppm is based on a review of the safety data.Table1.–Class1solvents in pharmaceutical products(solvents that should be avoided)Solvent Concentration limit(ppm)Concern Benzene2CarcinogenCarbon tetrachloride4Toxic and environmental hazard 1,2-Dichloroethane5Toxic1,1-Dichloroethene8Toxic1,1,1-Trichloroethane1500Environmental hazard 4.2.SOLVENTS TO BE LIMITEDSolvents in Table2should be limited in pharmaceutical products because of their inherent toxicity.PDEs are given to the nearest0.1mg/day,and concentrations are given to the nearest10ppm.The stated values do not reflect the necessary analytical precision of determination.Precision should be determined as part of the validation of the method. Table2.–Class2solvents in pharmaceutical products Solvent PDE(mg/day)Concentration limit(ppm) Acetonitrile 4.1410 Chlorobenzene 3.6360 Chloroform0.660 Cyclohexane38.838801,2-Dichloroethene18.71870 Dichloromethane 6.06001,2-Dimethoxyethane 1.0100N,N-Dimethylacetamide10.91090N,N-Dimethylformamide8.88801,4-Dioxane 3.83802-Ethoxyethanol 1.6160 Ethyleneglycol 6.2620 Formamide 2.2220 Hexane 2.9290 Methanol30.030002-Methoxyethanol0.550 Methylbutylketone0.550 Methylcyclohexane11.81180N-Methylpyrrolidone 5.3530 Nitromethane0.550 Pyridine 2.0200Solvent(mg/day)Concentration limit(ppm)Sulfolane 1.6160 Tetrahydrofuran7.2720Tetralin 1.0100 Toluene8.98901,1,2-Trichloroethene0.880Xylene*21.72170*usually60per cent m-xylene,14per cent p-xylene,9per cent o-xylene with17per cent ethyl benzene4.3.SOLVENTS WITH LOW TOXIC POTENTIAL Solvents in Class3(shown in Table3)may be regardedas less toxic and of lower risk to human health.Class3 includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals.However,there are no long-term toxicity or carcinogenicity studies for many of the solvents in Class3.Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies.It is considered that amounts of these residual solvents of50mg per day or less(correspondingto5000ppm or0.5per cent under Option l)would be acceptable without justification.Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice. Table3.–Class3solvents which should be limited by GMP or other quality-based requirementsAcetic acid HeptaneAcetone Isobutyl acetateAnisole Isopropyl acetate1-Butanol Methyl acetate2-Butanol3-Methyl-1-butanolButyl acetate Methylethylketonetert-Butylmethyl ether MethylisobutylketoneCumene2-Methyl-l-propanolDimethyl sulphoxide PentaneEthanol1-PentanolEthyl acetate1-PropanolEthyl ether2-PropanolEthyl formate Propyl acetateFormic acid4.4.SOLVENTS FOR WHICH NO ADEQUATE TOXICOLOGICAL DATA WAS FOUNDThe following solvents(Table4)may also be of interest to manufacturers of excipients,active substances,or medicinal products.However,no adequate toxicological data on which to base a PDE was found.Manufacturers should supply justification for residual levels of these solvents in pharmaceutical products.Table4.–Solvents for which no adequate toxicologicaldata was found1,1-Diethoxypropane Methylisopropylketone1,1-Dimethoxymethane Methyltetrahydrofuran2,2-Dimethoxypropane Petroleum etherIsooctane Trichloroacetic acidIsopropyl ether Trifluoroacetic acidGLOSSARYGenotoxic carcinogens:Carcinogens which produce cancer by affecting genes or chromosomes.LOEL:Abbreviation for lowest-observed effect level.Lowest-observed effect level:The lowest dose of substance in a study or group of studies that produces biologically significant increases in frequency or severity of any effects in the exposed humans or animals.Modifying factor:A factor determined by professional judgement of a toxicologist and applied to bioassay data to relate that data safely to humans.Neurotoxicity:The ability of a substance to cause adverse effects on the nervous system.NOEL:Abbreviation for no-observed-effect level.No-observed-effect level:The highest dose of substanceat which there are no biologically significant increases in frequency or severity of any effects in the exposed humans or animals.PDE:Abbreviation for permitted daily exposure.Permitted daily exposure:The maximum acceptable intake per day of residual solvent in pharmaceutical products.Reversible toxicity:The occurrence of harmful effectsthat are caused by a substance and which disappear after exposure to the substance ends.Strongly suspected human carcinogen:A substance for which there is no epidemiological evidence of carcinogenesis but there are positive genotoxicity data and clear evidence of carcinogenesis in rodents.Teratogenicity:The occurrence of structural malformations in a developing foetus when a substance is administered during pregnancy.APPENDIX 1.LIST OF SOLVENTS INCLUDED IN THE GUIDELINESolvent Other Names Structure Class Acetic acid Ethanoic acid CH 3COOH Class 3Acetone 2-Propanone Propan-2-oneCH 3COCH 3Class 3Acetonitrile CH 3CNClass 2AnisoleMethoxybenzene Class 3BenzeneBenzol Class 11-Butanol n -Butyl alcohol Butan-1-ol CH 3[CH 2]3OH Class 32-Butanol sec -Butyl alcohol Butan-2-olCH 3CH 2CH(OH)CH 3Class 3Butyl acetate Acetic acid butyl ester CH 3COO[CH 2]3CH 3Class 3tert -Butylmethyl ether 2-Methoxy-2-methylpropane (CH 3)3COCH 3Class 3Carbon tetrachloride TetrachloromethaneCCl 4Class 1ChlorobenzeneClass 2Chloroform Trichloromethane CHCl 3Class 2CumeneIsopropylbenzene(1-Methylethyl)benzeneClass 3CyclohexaneHexamethylene Class 21,2-Dichloroethanesym -Dichloroethane Ethylene dichloride Ethylene chloride CH 2ClCH 2ClClass 11,1-Dichloroethene 1,1-Dichloroethylene Vinylidene chloride H 2C=CCl 2Class 11,2-Dichloroethene 1,2-Dichloroethylene Acetylene dichloride ClHC=CHCl Class 2Dichloromethane Methylene chlorideCH 2Cl 2Class 21,2-DimethoxyethaneEthyleneglycol dimethyl ether MonoglymeDimethyl cellosolve H 3COCH 2CH 2OCH 3Class 2N,N -Dimethylacetamide DMA CH 3CON(CH 3)2Class 2N,N -Dimethylformamide DMFHCON(CH 3)2Class 2Dimethyl sulphoxideMethylsulphinylmethane Methyl sulphoxide DMSO (CH 3)2SOClass 31,4-Dioxane p -Dioxane[1,4]DioxaneClass 2Ethanol Ethyl alcohol CH 3CH 2OH Class 32-Ethoxyethanol CellosolveCH 3CH 2OCH 2CH 2OH Class 2Ethyl acetateAcetic acid ethyl esterCH 3COOCH 2CH 3Class 3Ethyleneglycol 1,2-Dihydroxyethane 1,2-Ethanediol HOCH 2CH 2OH Class 2Ethyl etherDiethyl ether Ethoxyethane 1,1′-Oxybisethane CH 3CH 2OCH 2CH 3Class 3Ethyl formate Formic acid ethyl ester HCOOCH 2CH 3Class 3Formamide MethanamideHCONH 2Class 2Formic acid HCOOHClass 3Heptane n -Heptane CH 3[CH 2]5CH 3Class 3Hexane n -HexaneCH 3[CH 2]4CH 3Class 2Isobutyl acetate Acetic acid isobutyl ester CH 3COOCH 2CH(CH 3)2Class 3Isopropyl acetate Acetic acid isopropyl ester CH 3COOCH(CH 3)2Class 3Methanol Methyl alcohol CH 3OH Class 22-Methoxyethanol Methyl cellosolve CH 3OCH 2CH 2OH Class 2Methyl acetate Acetic acid methyl ester CH 3COOCH 3Class 33-Methyl-1-butanolIsoamyl alcohol Isopentyl alcohol 3-Methylbutan-1-ol (CH 3)2CHCH 2CH 2OHClass 3Methylbutylketone 2-Hexanone Hexan-2-one CH 3[CH 2]3COCH 3Class 2MethylcyclohexaneCyclohexylmethaneClass 2Methylethylketone2-Butanone MEKButan-2-one CH 3CH 2COCH 3Class 3Methylisobutylketone4-Methylpentan-2-one 4-Methyl-2-pentanone MIBKCH 3COCH 2CH(CH 3)2Class 32-Methyl-1-propanol Isobutyl alcohol 2-Methylpropan-1-ol (CH 3)2CHCH 2OHClass 3N -Methylpyrrolidone1-Methylpyrrolidin-2-one1-Methyl-2-pyrrolidinoneClass 2Nitromethane CH 3NO 2Class 2Pentane n -Pentane CH 3[CH 2]3CH 3Class 31-PentanolAmyl alcohol Pentan-1-ol Pentyl alcohol CH 3[CH 2]3CH 2OHClass 31-Propanol Propan-1-ol Propyl alcohol CH 3CH 2CH 2OH Class 32-Propanol Propan-2-olIsopropyl alcohol (CH 3)2CHOH Class 3Propyl acetate Acetic acid propyl esterCH 3COOCH 2CH 2CH 3Class 3PyridineClass 2Sulfonane Tetrahydrothiophene1,1-dioxide Class 2TetrahydrofuranTetramethylene oxideOxacyclopentaneClass 2Tetralin1,2,3,4-Tetrahydronaphthalene Class 2TolueneMethylbenzene Class 21,1,1-Trichloroethane Methylchloroform CH 3CCl 3Class 11,1,2-Trichloroethene Trichloroethene HClC=CCl 2Class 2Xylene*Dimethybenzene XylolClass 2*usually 60per cent m -xylene,14per cent p -xylene,9per cent o -xylene with 17per cent ethyl benzene.APPENDIX 2.ADDITIONAL BACKGROUNDA2.1.ENVIRONMENTAL REGULATION OF ORGANIC VOLATILE SOLVENTSSeveral of the residual solvents frequently used in the production of pharmaceuticals are listed as toxic chemicals in Environmental Health Criteria (EHC)monographs and the Integrated Risk Information System (IRIS).The objectives of such groups as the International Programme on Chemical Safety (IPCS),the United States Environmental Protection Agency (USEPA)and the United States Food and Drug Administration (USFDA)include the determination of acceptable exposure levels.The goal is protection of human health and maintenance of environmental integrity against the possible deleterious effects of chemicals resulting from long-term environmental exposure.The methods involved in the estimation of maximum safe exposure limits are usually based on long-term studies.When long-term study data are unavailable,shorter term study data can be used with modification of the approach such as use of larger safety factors.The approach described therein relates primarily to long-term or life-time exposure of the general population in the ambient environment,i.e.ambient air,food,drinking water and other media.A2.2.RESIDUAL SOLVENTS IN PHARMACEUTICALS Exposure limits in this guideline are established by referring to methodologies and toxicity data described in EHC and IRIS monographs.However,some specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits.They are:1)Patients (not the general population)use pharmaceuticalsto treat their diseases or for prophylaxis to prevent infection or disease.2)The assumption of life-time patient exposure is not necessary for most pharmaceutical products but may be appropriate as a working hypothesis to reduce risk to human health.3)Residual solvents are unavoidable components inpharmaceutical production and will often be a part ofmedicinal products.4)Residual solvents should not exceed recommended levels except in exceptional circumstances.5)Data from toxicological studies that are used to determine acceptable levels for residual solvents should have been generated using appropriate protocols such as those described for example,by OECD and the FDA Red Book.APPENDIX 3.METHODS FOR ESTABLISHING EXPOSURE LIMITSThe Gaylor-Kodell method of risk assessment (Gaylor,D.W.and Kodell,R.L.Linear Interpolation algorithm for low dose assessment of toxic substance.J.Environ.Pathology,4,305,1980)is appropriate for Class 1carcinogenic solvents.Only in cases where reliable carcinogenicity data are available should extrapolation by the use of mathematical models be applied to setting exposure limits.Exposure limits for Class 1solvents could be determined with the use of a large safety factor (i.e.,10000to 100000)with respect to the no-observed-effect level (NOEL).Detection and quantification of these solvents should be by state-of-the-art analytical techniques.Acceptable exposure levels in this guideline for Class 2solvents were established by calculation of PDE values according to the procedures for setting exposure limits in pharmaceuticals (Pharmacopeial Forum ,Nov-Dec 1989),and the method adopted by IPCS for Assessing Human Health Risk of Chemicals (Environmental Health Criteria 170,WHO,1994).These methods are similar to those used by the USEPA (IRIS)and the USFDA (Red Book )and others.The method is outlined here to give a better understanding of the origin of the PDE values.It is not necessary to perform these calculations in order to use the PDE valuestabulated in Section 4of this document.PDE is derived from the no-observed-effect level (NOEL),orthe lowest-observed effect level (LOEL),in the most relevant animal study as follows:The PDE is derived preferably from a NOEL.If no NOEL is obtained,the LOEL may be used.Modifying factors proposed here,for relating the data to humans,are the same kind of “uncertainty factors”used in Environmental HealthCriteria (Environmental Health Criteria 170,World Health Organisation,Geneva,1994),and “modifying factors”or。
各国药典中重金属检查方法的比较分析

Co mp a r a t i v e a n a l y s i s o f t e s t Me t h o d s f o r h e a v y me t a l s i n p ha r ma c o p o e i a s
o f t h e e x i s t i n g c h e mi c a l e x a mi n a t i o n me t h o d s a n d t h e c o mp r e h e n s i v e a n a l y s i s o f i n s t u me r n t l a a n a l y s i s p l a y i n g a n i n c r e a s i n g l y i mp o r -
p o e i a( U S P ) , B r i t i s h P h a ma r c o p o e i a( B P )a n d J a p a n e s e P h a r m a c o p o e i a( J P ) w e r e d i s c u s s e d , i n c l u d i n g t h e c o m p a r a t i v e a n a l y s i s
t a nt r o l e i n p h a r ma c o po ei a s . Th i s pa pe r p r o v i d e s a r e f e r e n c e f o r dr u g d e v e l o p e r s t o s e l e c t s ui t a bl e c o nd i t i o ns,me t ho d s a n d l i mi t s or f t h e t e s t o f he a vc a l s, S O a s t o e ns u r e a mo r e r a t i o na l,s a f e a n d e f f e c t i v e qu a l i t y c o nt r o l o f ph a r ma c e ut i — c a l s . K EY W O RD S Ph a m a r c o p o e i a, h ea v y me t a l s , t e s t me t h o d, c h e mi c a l e x a mi n a t i o n, i n s t u me r nt a l a na l y s i s
欧洲药典附录中文版.

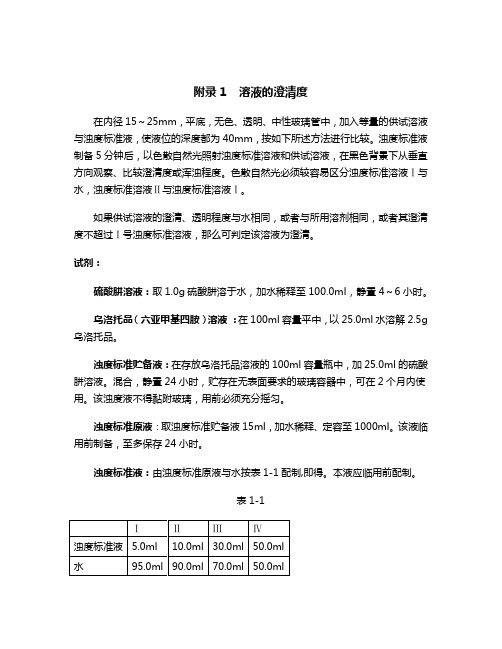
第二部分、附录附录1 溶液的澄清度 (2)附录2 溶液颜色检查 (3)附录3 旋光度 (7)附录4 铵盐检查法 (9)附录5 氯化物检查法 (11)附录6 硫酸盐灰分 (13)附录7 铁 (14)附录8 重金属 (16)附录9 干燥失重 (21)附录10 硫酸盐检查法 (23)附录11 红外吸收分光光度法 (25)附录12 pH测定 (29)附录13 滴定 (34)附录14 氯化物鉴别反应 (37)附录15 指示剂颜色与溶液pH 的关系 (38)附录1 溶液的澄清度在内径15~25mm,平底,无色、透明、中性玻璃管中,加入等量的供试溶液与浊度标准液,使液位的深度都为40mm,按如下所述方法进行比较。
浊度标准液制备5分钟后,以色散自然光照射浊度标准溶液和供试溶液,在黑色背景下从垂直方向观察、比较澄清度或浑浊程度。
色散自然光必须较容易区分浊度标准溶液Ⅰ与水,浊度标准溶液Ⅱ与浊度标准溶液Ⅰ。
如果供试溶液的澄清、透明程度与水相同,或者与所用溶剂相同,或者其澄清度不超过Ⅰ号浊度标准溶液,那么可判定该溶液为澄清。
试剂:硫酸肼溶液:取1.0g硫酸肼溶于水,加水稀释至100.0ml,静置4~6小时。
乌洛托品(六亚甲基四胺)溶液:在100ml容量平中,以25.0ml水溶解2.5g乌洛托品。
浊度标准贮备液:在存放乌洛托品溶液的100ml容量瓶中,加25.0ml的硫酸肼溶液。
混合,静置24小时,贮存在无表面要求的玻璃容器中,可在2个月内使用。
该浊度液不得黏附玻璃,用前必须充分摇匀。
浊度标准原液:取浊度标准贮备液15ml,加水稀释、定容至1000ml。
该液临用前制备,至多保存24小时。
浊度标准液:由浊度标准原液与水按表1-1配制,即得。
本液应临用前配制。
表1-1附录2 溶液颜色检查按本药典规定,用下面两种方法之一可以检出溶液在棕色-黄色-红色范围内的颜色。
如果溶液A的外观与水或所用溶剂相同,或者颜色浅于标准比色液B9,则可判定溶液A为无色。
中、美、英、日和欧洲药典中植物药重金属和农药残留量的限量规定及分析

12. Oriental Ginseng(Asian Ginseng) 东方人参
13. Powdered asian ginseng(人参粉)
14. Peppermint oil (薄荷油)
15. Stronger rose water (浓缩玫瑰水)
Source
参环毛蚓 Pheretima astergillum ( E. Perrier) 等 4 种动物的干 燥体
number of vegetable drugs controlled in China pharmacopoeia are obviously less than that in the United Starmacopoeia ;Vegetable drugs ; Heavy metals ; Pesticide residues
Silybum marianum (Linne) Gaertner 的干燥成熟果实
Commiphora molmol Engler 和同属它种植物茎干渗出的油 胶树脂 ( Fam. Burseraceae) Panax ginseng C. A. Meyer 的干燥根( Fam. Araliaceae)
2. Feverfew(短舌匹菊)
3. Garlic (大蒜) 4. Garlic Fluidextract (大蒜汁) 5. Powdered Garlic (大蒜粉) 6. Ginger (生姜) 7. Ginger Tincture (生姜酊) 8. Powdered Ginger (生姜粉) 9. Ginkgo (银杏) 10. Milk Thistle (乳蓟) 11. Myrrh (没药)
对重金属和农药残留量的要求 ,我们研读了近年出 版的几部药典的相关内容[1~5 ] 。
欧洲药典重金属检查法[1]
![欧洲药典重金属检查法[1]](https://img.taocdn.com/s3/m/bf56e2d088eb172ded630b1c59eef8c75fbf95c2.png)
仪器纳氏比色管应注意选择各管之间的平行性,玻璃色泽一致,内径、刻度标线高度一致。
比色管洗涤时避免划伤内壁。
50ml注射器,应能与滤器上盖入口处紧密联接。
容量瓶试剂硝酸铅储备液:精确称取硝酸铅(II)400.0mg,用水溶解并定量稀释至250ml摇允,备用。
铅标准溶液(2ppm Pb):精密量取1ml储备液,用水稀释至500ml。
铅标准溶液(1ppm Pb):精密量取1ml储备液,用水稀释至1000ml。
硫代乙酰胺试剂:往0.2ml的4%W/V硫代乙酰胺溶液中加入1ml由15ml 1M 氢氧化钠,5ml水和20ml甘油组成的混合物,在水浴中加热20秒钟,冷却,立即使用。
醋酸盐缓冲溶液pH3.5:将25.0g醋酸氨溶解在25ml水中,再加入38.0ml的7M盐酸,并用水稀释至100ml.用2M盐酸或6M氨调整pH至3.5注:对照和空白液应同时制备方法A样品溶液制备:向12ml规定的水溶液中,加入2ml醋酸盐缓冲溶液pH3.5。
混合,加入1.2ml硫代乙酰胺试剂,立即混匀,放置2分钟。
标准溶液制备:按照规定,制备一定量的1 ppm或2 ppm铅标准溶液与2 ml被测溶液的混合物,然后按照同样方法制备。
空白溶液:同样处理由10ml水和2ml被测溶液的混合物。
结果:与空白相比较,标准溶液显浅棕色。
2分钟后样品溶液得颜色不得深于标准溶液。
方法B样品溶液制备:将规定量的样品溶解在一种有机溶剂中,如含有至少15%水的1.4-二氧基己烷或含有至少15%水的丙酮。
向12ml所述的水溶液中,加入2ml醋酸盐缓冲溶液pH3.5。
混合,加入1.2ml硫代乙酰胺试剂,立即混匀,放置2分钟。
标准溶液制备:用样品所用的溶剂来稀释铅标准溶液(100ppm)即可制得1ppm或2ppm铅标准溶液。
按照同样方法制备,由10ml的1ppm或2ppm铅标准溶液与2ml被测溶液的混合物。
空白溶液:用10ml样品所用的溶剂和2ml被测溶液的混合物。
欧洲药典重金属检测

2.4.8 重金属下述方法需要使用硫代乙酰胺试剂。
作为另一种选择,硫化钠溶液(0.1ml)也常常适用。
由于各论中所述测试是使用硫代乙酰胺试剂研发出来的,如需用硫化钠溶液替代,需要包括方法A、方法B和方法H监测溶液,由测试规定的待测物的量进行配制,其已经加入了制备对照溶液规定量的铅标准溶液。
监测溶液至少要与对照溶液一样深,否则测试是无效的。
方法A供试溶液:12ml待测物水溶液。
对照溶液(标准):10ml规定的标准铅溶液(1ppm or 2ppm Pb)和2ml的待测液混合。
空白溶液:10ml的水和2ml的测试溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法B供试溶液:用含最少量水的溶剂(例如含15%水的二氧杂环乙烷或含15%水的丙酮)溶解成12ml待测液。
对照溶液(标准):10ml规定的铅标准溶液(1ppm or 2ppm Pb),加入2ml的待测液。
用待测物所用溶剂稀释100ppm Pb的铅标准溶液至1或2ppm Pb。
空白溶液:10ml待测物所用溶剂和2ml的待测溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法C供试溶液:规定量(不超过2g)的待测物质置于坩埚内,加4ml 250g/l硫酸镁的稀硫酸溶液。
玻璃棒搅拌混和,小心加热。
若混合物仍为液体,则在水浴中蒸发使其干燥。
欧盟草药药品质量标准中杂质检测相关要求

作者:汤依娜,邹文俊,张浩,刘忠荣,刘瑜【关键词】欧盟;草药药品;质量标准;杂质检测2004年4月30日正式颁布的《欧盟传统草药药品注册指令》(directive 2004/24/ec)1不仅明确定义了草药药品、传统草药药品等概念,而且正式承认了传统草药药品作为药品的法律地位,制订了相应的简化注册程序,并明确规定:根据传统应用的情况,可以免做该传统草药药品的临床试验或临床前研究,但并没有因此而降低对产品质量的要求,即欧盟对草药药品、传统草药药品质量控制的基本要求与药品完全一样。
根据该指令新成立的欧盟药品审评局(european medicine agency, emea)下属的草药药品委员会(committee of herbal medicinal product, hmpc)已陆续制订出有关草药药品质量、安全性、有效性等一系列指南(guidelines),在向多方征求意见、讨论后,部分指南已被hmpc采纳并生效。
因此,欧盟对传统草药药品注册的要求日益细化、明确。
中药作为世界传统草药的重要组成部分,从目前和长远来讲,熟悉并深入学习这些新的要求,尤其是对于开展注册相关理论和技术研究的有关政府机构、科研院所以及有意开拓欧盟市场的中药企业来讲都是非常有必要的。
鉴于药品质量对于传统草药药品注册的至关重要性,而且近年来药品中重金属、农药残留(简称“农残”)等不良杂质问题已经引起世界范围内的高度重视,本文将以由hmpc最新制订,并于2006年10月1日生效的2个与质量相关的重要指南——《草药药品/传统草药药品质量指南》(guideline on quality of herbal medicinal products/traditional herbal medicinal products)2和《质量规范指南:药材、草药加工品及草药药品/传统草药药品的检验方法和可接受标准》(guideline on specifications:test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinalproducts/traditional herbal medicinal products,以下简称《质量规范指南》)3,以及涉及到的《欧洲药典》(5.0版)相关标准为依据,重点介绍欧盟对于草药药品和传统草药药品在杂质方面的具体规定与要求,力求为解决中成药质量标准有关杂质问题的研究提供一定的参考。
【推荐下载】欧盟草药药品质量标准中杂质检测相关要求

欧盟草药药品质量标准中杂质检测相关要求 【编者按】医药论文是科技论文的一种是用来进行医药科学研究和描述研究成果的论说性文章。
论文网为您提供医药论文范文参考,以及论文写作指导和格式排版要求,解决您在论文写作中的难题。
欧盟草药药品质量标准中杂质检测相关要求 【关键词】欧盟;草药药品;质量标准;杂质检测 2004年4月30日正式颁布的《欧盟传统草药药品注册指令》(Directive2004/24/EC)[1]不仅明确定义了草药药品、传统草药药品等概念,而且正式承认了传统草药药品作为药品的法律地位,制订了相应的简化注册程序,并明确规定:根据传统应用的情况,可以免做该传统草药药品的临床试验或临床前研究,但并没有因此而降低对产品质量的要求,即欧盟对草药药品、传统草药药品质量控制的基本要求与药品完全一样。
根据该指令新成立的欧盟药品审评局(European Medicine Agency, EMEA)下属的草药药品委员会(Committee of Herbal Medicinal Product, HMPC)已陆续制订出有关草药药品质量、安全性、有效性等一系列指南(Guidelines),在向多方征求意见、讨论后,部分指南已被HMPC采纳并生效。
因此,欧盟对传统草药药品注册的要求日益细化、明确。
中药作为世界传统草药的重要组成部分,从目前和长远来讲,熟悉并深入学习这些新的要求,尤其是对于开展注册相关理论和技术研究的有关政府机构、科研院所以及有意开拓欧盟市场的中药企业来讲都是非常有必要的。
鉴于药品质量对于传统草药药品注册的至关重要性,而且近年来药品中重金属、农药残留(简称农残)等不良杂质问题已经引起世界范围内的高度重视,本文将以由HMPC 最新制订,并于2006年10月1日生效的2个与质量相关的重要指南《草药药品/传统草药药品质量指南》(Guideline On Quality Of Herbal Medicinal Products/Traditional Herbal Medicinal Products)[2]和《质量规范指南:药材、草药加工品及草药药品/传统草药药品的检验方法和可接受标准》(Guideline On Specifications:Test Procedures And Acceptance Criteria For Herbal Substances, Herbal Preparations And Herbal Medicinal Products/Traditional Herbal Medicinal Products,以下简称《质量规范指南》)[3],以及涉及到的《欧洲药典》(5.0版)相关标准为依据,重点介绍欧盟对于草药药品和传统草药药品在杂质方面的具体规定与要求,力求为解决中成药质量标准有关杂质问题的研究提供一定的参考。
USP232-233重金属检测方法-中文

铑
10
40
0.0001
钌
10
40
0.0002
铬
25
100
0.002
钼
25
100
0.0002
镍
25
100
0.002
钒
25
100
0.005
铜
250
1000
0.002
镁
250
1000
0.001
样品制备
范围广泛的各种样品都可以用 USP<232>/<233> 进行分 析,所以提供适合所有样品类型的详细样品处理方法并不现 实。有些药物样品可以直接分析(不用溶解),而其他样品 可以用水性溶剂(如水或稀酸)或适当的有机溶剂(如 2-丁 氧乙醇 : 水(25 : 75)[3],DMSO 或 DGME)简单稀释或 溶解进行制备。用水性或有机溶剂进行简单稀释或溶解的 方法必须考虑样品的化学稳定性,并且对于有机溶剂溶解, 还要考虑样品中组分化合物的不同挥发性。对许多 API 来 说,用有机溶剂稀释是首选方法,这种情况下有必要采取有 助于稳定分析物的方法,以避免因较高或较低挥发性(与校 正标准品相比)成分的存在而造成的回收率波动 [7]。
元素 镉 铅 无机砷 无机汞 铱 锇 钯 铂 铑 钌 铬 钼 镍 钒 铜 镁
每日剂量 PDE(µg/日) 5 10 15 15 100 100 100 100 100 100 250 250 250 250 2500 2500
虽然 USP<232> 中制定的 PDE 限度用任何 USP<233> (ICP-OES 或 ICP-MS)中参考的仪器技术都可以测定 [6], 但许多新药中使用越来越复杂而珍贵的原料药,可能只包 含非常少的量。对这些毫克级样品进行大比例稀释制备, 意味着使用检测限尽可能低的仪器非常重要。低检测限和 宽动态范围线性校正(安捷伦 7700 系列达到 9 个数量级) 是 ICP-MS 最非常可贵的特性。低检测限对于 USP<232> 要求必须以最低限量进行控制的潜在毒性痕量元素(特别是 As、Cd、Hg 和 Pb)尤为重要。
部分国家、地区草药重金属和农药残留限量标准汇总

部分国家、地区草药重金属和农药残留限量标准汇总发布时间:2010-05-24一、中国:(一)中国药典(05版)甘草重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
有机氯农药残留量:六六六(总BHC)不得过千万分之二,滴滴涕(总DDT)不得过千万分之二,五氯硝基苯(PCNB)不得过千万分之一。
黄芪重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
有机氯农药残留量:六六六(总BHC)不得过千万分之二,滴滴涕(总DDT)不得过千万分之二,五氯硝基苯(PCNB)不得过千万分之一。
丹参重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
白芍重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
西洋参重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
金银花重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
石膏重金属:含重金属不得过百万分之十;含砷量不得过百万分之二。
芍药 0.118) 三泰芬(Triadimefon)芍药 0.0119) 赛福宁(Triforine)芍药 0.120) 赛福唑(Triflumizole)黄芪 0.1 芍药 1.021) 芬瑞莫(Fenarimol)黄芪 0.522) 二甲戊乐灵(Pendimethalin)当归 0.2 麦门冬 0.2 柴胡 0.2芍药 0.2 红花 0.123) 芬普宁(Fenpropathrin)当归 0.224) 福赛绝(Fosthiazate)柴胡 0.0225) 甲基锌乃浦(Propineb)芍药 0.226) 派灭净(Pymetrozine)红花 0.05 黄芪 0.0527) 勿落菌恶(Fludioxonil)芍药 0.1八、日本:重金属及砷盐限量:铅(Pb)≤20PPM砷As2O3 ≤ 2PPM农药残留限量:1、中药材:(生药农药残留量的行业标准)适用范围:黄芪、远志、甘草、桂皮、细辛、山茱萸、苏叶、大枣、陈皮、枇杷叶、牡丹皮BHC总量≤0.2 mg/kgDDT总量≤0.2 mg/kg2、中药制剂:(汉方及生药制剂农药残留量的行业标准)1)有机氯类农药:适用范围:含有黄芪、远志、甘草、桂皮、细辛、山茱萸、苏叶、大枣、陈皮、枇杷叶、牡丹皮、人参、红参、番泻叶的汉方及生药制剂BHC总量≤0.2 mg/kgDDT总量≤0.2 mg/kg2)有机磷类农药:适用范围:含有远志、山茱萸、苏叶及陈皮的汉方制剂对硫磷≤0.5 mg/kg甲基对硫磷≤0.2 mg/kg杀扑磷≤0.2 mg/kg马拉硫磷≤1.0 mg/kg3)菊酯类农药适用范围:含有远志、苏叶、大枣、陈皮及枇杷叶的汉方制剂氰戊菊酯≤1.5 mg/kg氯氰菊酯≤1.0 mg/kg九、德国:重金属限量:铅(Pb)≤5 mg/kg。
USP35(231)重金属检查第二法中英对照

Method II方法ⅡpH 3.5 Acetate Buffer— Prepare as directed under Method I.取醋酸铵25.0g,加水25ml溶解后,加38.0ml6N盐酸,如有必要用6N氨水或6N 盐酸调PH至3.5 ,用水稀释至100ml,摇匀。
Standard Preparation— Prepare as directed under Method I.标准溶液的制备取50ml比色管,用移液管移取2.0ml标准铅溶液(20 gPb),用水稀释到25ml,用1N醋酸或6N氨水溶液调PH至3.0~4.0之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
Test Preparation— Use a quantity, in g, of the substance to be tested as calculated by the formula:2.0 / (1000L),供试品溶液的制备称取一定量的样品(以g计算) ,按公式2.0/1000L计算,in which L is the Heavy metals limit, in percentage. Transfer the weighed quantity of the substance to a suitable crucible, add sufficient sulfuric acid to wet the substance, and carefully ignite at a low temperature until thoroughly charred. (The crucible may be loosely covered with a suitable lid during the charring.)其中L为重金属的限度(%),置适宜的坩埚中,加适量的硫酸预以湿润,在低温小心炽灼,直至全部炭化,(炭化过程坩埚盖可不盖严),Add to the carbonized mass 2 mL of nitric acid and 5 drops of sulfuric acid, and heat cautiously until white fumes no longer are evolved. Ignite, preferably in a muffle furnace, at 500 to 600, until the carbon is completely burned off.炭化物中加2ml硝酸和5滴硫酸,小心加热,直至不再挥发白色烟雾,于500~600℃炽灼, 直至炭完全灰化。
USP 231重金属

USP<231>重金属本检验用以证实供试品中与硫离子作用显色的金属杂质含量,在规定检验条件下,不超过个论中规定的供试品重金属限度,以铅百分含量(重量比)计,与用标准铅溶液配制的对照进行目测比较(参看分光光度法和光散射法<851>中操作步骤部分的视觉比较)测定。
[注意:与本检验起反应的代表性物质为铅、汞、铋、砷、锑、锡、镉、银、铜和钼。
]除个论另有规定外,用方法I测定重金属含量。
方法I用于规定条件下可生成无色的、透明的制品的物质。
方法II适用于在方法I规定条件下无法生成无色透明制品的物质,或由于自身复杂特性,会对硫离子生成的金属沉淀造成干扰的物质,以及不挥发油和挥发油。
方法III湿消化法,仅在方法I和方法II均不适用时使用。
专用试剂硝酸铅贮备液:取硝酸铅159.8 mg,加已加硝酸1 mL的水100 mL,溶解,加水稀释至1000 mL。
于不含可溶铅盐的玻璃容器中配制和贮存该溶液。
标准铅溶液:使用当天配制。
取硝酸铅贮备液10.0 mL,加水稀释至100.0 mL。
每mL标准铅溶液相当于10 µg的铅。
以每g供试品100µL标准铅溶液为基准制备的参比溶液相当于每一百万份供试品中含有一份铅。
方法IpH 3.5醋酸盐缓冲液:取醋酸铵25.0 g,加水25 mL,溶解,加6 mol/L盐酸38.0 mL。
如有需要,用6 mol/L氨水或6 mol/L盐酸调节pH 为3.5,用水稀释至100 mL。
混匀。
标准溶液:用移液管取标准铅溶液2 mL(20 µg的铅),置50 mL比色管中,用水稀释至25 mL。
使用pH计或窄范围pH试纸作外部指示剂,用1mol/L醋酸或6mol/L氨水调节pH至3.0-4.0,用水稀释至40 mL,混匀。
供试溶液:取如个论所述制备的供试液25 mL,置50 mL比色管中;或当个论另有规定时,使用个论中规定体积量的酸溶解,并用水稀释至25 mL。
欧洲药典7.0附录炽灼残渣 熔点 干燥失重 重金属

熔点:毛细管法测定的熔点是由原来的固体颗粒紧列物质转变为液态时的温度。
专注规定,该装置和方法,用于测定其他因素,如液面凹陷或熔化范围,来描述物质的熔化过程。
装置。
该装置由:-一个合适的玻璃容器含有液体浴(例如,水,液体石蜡或硅油)和安装一个合适的加热装置,-一个合适的手段,搅拌,保证了温度的均匀性的浴室内,-一个合适的温度计毕业不超过0.5摄氏°间隔设有浸泡标记。
一系列的温度不超过100摄氏°,-无碱硬玻璃毛细管内径0.9毫米到1.1毫米与0.10毫米至0.15毫米,壁厚和一端封闭除非另有规定,干燥的细粉状物质在真空和无水硅胶为24小时介绍了足够数量的毛细管管给紧凑型柱4毫米到6毫米的高度。
提高浴的温度约10摄氏°以下的假定的熔点和调整加热速度约1°℃/分钟。
当温度为5℃以下的假定°熔点,正确地介绍了毛细管管插入仪器。
对上述设备,使毛细管管,封闭端附近的中心温度计的灯泡,浸泡标记,是一级液体表面。
记录温度在过去的粒子进入液相校准装置。
该仪器可以校准使用熔点参考物质如世界卫生组织或其他适当的物质。
干燥失重干燥失重质量损失表示质量分数的方法。
将一定量的待测物质在干燥至恒重的称量瓶中检测。
干燥待测物质至恒重或按下列步骤干燥,浮动范围为±2°C。
A, 在干燥器中:常温常压下,以五氧化二磷干燥。
B,真空干燥:室温下,在压强为1.5千帕~2.5千帕,放置五氧化二磷的真空干燥箱内干燥。
C,要求温度范围内真空干燥:在专论规定的温度范围内,压强为1.5千帕~2.5千帕,放置五氧化二磷的真空干燥箱内干燥。
D,在要求温度范围内的干燥箱内干燥:在专论规定的温度范围内干燥E,高真空干燥:在专论规定的温度范围内,压力不超过0.1千帕,放置五氧化二磷的真空干燥箱内干燥。
如有其它要求的条件,根据专论中的具体规定操作。
干燥失重可按下列公式计算:B-C干燥失重(%)= × 100B-AA 称量瓶重量(g)B 干燥前称量瓶与样品的重量(g)C 干燥后称量瓶与样品的重量(g)重金属方法A供试溶液:12ml待测水溶液,2ml pH为3.5的缓冲溶液,混合后加1.2ml 的硫代乙酰胺试液,立即混合。
【标准】各国重金属和农残限量和标准

部分国家、地区草药重金属和农药残留限量标准汇总甘草重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
有机氯农药残留量:六六六(总BHC)不得过千万分之二,滴滴涕(总DDT)不得过千万分之二,五氯硝基苯(PCNB)不得过千万分之一。
黄芪重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
有机氯农药残留量:六六六(总BHC)不得过千万分之二,滴滴涕(总DDT)不得过千万分之二,五氯硝基苯(PCNB)不得过千万分之一。
丹参重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
白芍重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
西洋参重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
金银花重金属及有害元素:铅、镉、砷、汞、铜含量限定如下:铅不得过百万分之五,镉不得过千万分之三,砷不得过百万分之二,汞不得过千万分之二,铜不得过百万分之二十。
石膏重金属:含重金属不得过百万分之十;含砷量不得过百万分之二。
煅石膏重金属:含重金属不得过百万分之十。
白矾重金属:含重金属不得过百万分之二十。
玄明粉重金属:含重金属不得过百万分之二十。
含砷量不得过百万分之二十。
地龙重金属:含重金属不得过百万分之三十。
芒硝重金属:含重金属不得过百万分之十。
含砷量不得过百万分之十。
西瓜霜重金属:含重金属不得过百万分之十。
含砷量不得过百万分之十。
欧洲药典附录中文翻译
附录1溶液的澄清度在内径15~25mm,平底,无色、透明、中性玻璃管中,加入等量的供试溶液与浊度标准液,使液位的深度都为40mm,按如下所述方法进行比较。
浊度标准液制备5分钟后,以色散自然光照射浊度标准溶液和供试溶液,在黑色背景下从垂直方向观察、比较澄清度或浑浊程度。
色散自然光必须较容易区分浊度标准溶液Ⅰ与水,浊度标准溶液Ⅱ与浊度标准溶液Ⅰ。
如果供试溶液的澄清、透明程度与水相同,或者与所用溶剂相同,或者其澄清度不超过Ⅰ号浊度标准溶液,那么可判定该溶液为澄清。
试剂:硫酸肼溶液:取1.0g硫酸肼溶于水,加水稀释至100.0ml,静臵4~6小时。
乌洛托品(六亚甲基四胺)溶液:在100ml容量平中,以25.0ml水溶解2.5g 乌洛托品。
浊度标准贮备液:在存放乌洛托品溶液的100ml容量瓶中,加25.0ml的硫酸肼溶液。
混合,静臵24小时,贮存在无表面要求的玻璃容器中,可在2个月内使用。
该浊度液不得黏附玻璃,用前必须充分摇匀。
浊度标准原液:取浊度标准贮备液15ml,加水稀释、定容至1000ml。
该液临用前制备,至多保存24小时。
浊度标准液:由浊度标准原液与水按表1-1配制,即得。
本液应临用前配制。
表1-1附录2溶液颜色检查按本药典规定,用下面两种方法之一可以检出溶液在棕色-黄色-红色范围内的颜色。
如果溶液A的外观与水或所用溶剂相同,或者颜色浅于标准比色液B9,则可判定溶液A为无色。
方法I用外径为12mm的无色、透明中性玻璃管取2ml的供试溶液,与相同玻璃管中的2ml的水,或2ml本文所规定的标准比色液(见标准比色液表)进行比较。
在散射自然光,白色的背景下,水平观察比较颜色。
方法Ⅱ用同样平底、内径为15~25mm的无色透明中性玻璃管,液位的深度为40mm,将供试溶液与水或溶剂或本文中规定的标准比色液(见标准比色液表)对比。
在散射自然光,白色的背景下,垂直地观察比较颜色。
贮备液黄色液称取46克氯化铁,加大约900ml盐酸溶液(25ml浓盐酸和975ml水混和)溶解,继续添加,并定容1000.0ml。
最全的 关于 药品 炽灼残渣检查方法(中国药典、美国药典、欧洲药典)

药品的炽灼残渣检测方法(欧洲药典、美国药典)1原理:药品(多为有机化合物)经高温加热分解或挥发后遗留下的不挥发无机物(多为金属的氧化物,碳酸盐,磷酸盐,硅酸盐和氯化物等)。
2仪器与用具:高温炉、坩埚、坩埚钳、通风柜3试剂与试液:硫酸分析纯44.1~800℃炽灼约30500~600℃4.24.34.4炉上加热至硫酸蒸气除尽,白烟完全消失(以上操作应在通风柜内进行),将坩埚移置高温炉内,盖子斜盖于坩埚上,在700~800℃炽灼,约60分钟,使供试品完全灰化,(如供试品要做重金属试验,则灰化温度应在500~600℃)。
恒重:按操作方法5.4.4,依法操作炽灼30分钟,直至恒重。
如无特殊情况,在700~800℃(或500~600℃)炽灼二小时即可恒重。
4.5如需将残渣留作重金属检查,则炽灼温度控制在500~600℃。
5欧洲药典检测方法5.1在600±50℃灼烧一个白金、瓷或石英坩埚30分钟,干燥器内冷却后称重。
加入规定量的样品于上述坩埚内,称重。
5.2用1mL 的硫酸湿润样品,在低温上加热直至样品完全炭化。
冷却后,用少量的硫酸湿润残渣,加热直至白烟不再产生。
5.3在600±50℃的高温炉内灼烧,直至残渣完全灰化(在操作过程不应有火焰5.466.1(600℃±6.2再产生。
6.36.4烧(与前面操作相同),并计算残渣的量。
除非另有规定,继续烧烧直至恒重或残渣的量符合规定的限量。
6.5恒重:系指连续两次炽灼后的重量差异在0.3mg (ChP )或0.5mg (EP ,USP )以下的重量。
7计算:炽灼残渣%=样品重量空坩埚重残渣及坩埚重 ×100%。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2.4.8 重金属下述方法需要使用硫代乙酰胺试剂。
作为另一种选择,硫化钠溶液(0.1ml)也常常适用。
由于各论中所述测试是使用硫代乙酰胺试剂研发出来的,如需用硫化钠溶液替代,需要包括方法A、方法B和方法H监测溶液,由测试规定的待测物的量进行配制,其已经加入了制备对照溶液规定量的铅标准溶液。
监测溶液至少要与对照溶液一样深,否则测试是无效的。
方法A供试溶液:12ml待测物水溶液。
对照溶液(标准):10ml规定的标准铅溶液(1ppm or 2ppm Pb)和2ml的待测液混合。
空白溶液:10ml的水和2ml的测试溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法B供试溶液:用含最少量水的溶剂(例如含15%水的二氧杂环乙烷或含15%水的丙酮)溶解成12ml待测液。
对照溶液(标准):10ml规定的铅标准溶液(1ppm or 2ppm Pb),加入2ml的待测液。
用待测物所用溶剂稀释100ppm Pb的铅标准溶液至1或2ppm Pb。
空白溶液:10ml待测物所用溶剂和2ml的待测溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法C供试溶液:规定量(不超过2g)的待测物质置于坩埚内,加4ml 250g/l硫酸镁的稀硫酸溶液。
玻璃棒搅拌混和,小心加热。
若混合物仍为液体,则在水浴中蒸发使其干燥。
连续加热灼烧,灼烧温度不超过800℃,直到获得白色或灰白色的残渣。
取出,冷却后加数滴稀硫酸润湿残渣。
再次蒸发、灼烧并冷却。
灼烧的总时间不能超过2小时。
制取2份残渣,分别加入5ml稀盐酸,0.1ml的酚酞试液,然后滴加氨水,直到出现粉红色。
冷却,滴加冰醋酸至颜色消失,再多加0.5ml冰醋酸。
如有需要进行过滤,并冲洗过滤器。
加水稀释至20ml。
对照溶液(标准):按供试溶液的制备方法,用规定量的铅标准溶液(10ppm Pb)代替待测物质。
取10ml的该溶液,加2ml待测液。
监测溶液:按供试溶液的制备方法,向待测物质中加入配制对照溶液规定量的铅标准溶液(10ppm Pb)。
取10ml的该溶液,加2ml待测液。
空白溶液:10ml的水和2ml待测液混合。
向12ml每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:-相较于空白溶液,对照溶液呈浅棕色,-监测溶液至少要同对照溶液颜色深度相同。
结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法D供试溶液:在坩埚内,充分的混合规定量的待测物质和0.5克的氧化镁,灼烧退去暗红色,直至出现同质的白色或灰白色物质。
如果灼烧30分钟后仍有颜色,取出冷却,用玻璃棒混和,重复进行灼烧。
如有必要,重复此项操作。
在800℃加热约1小时。
分别制备两份残渣,各加5mL等体积的盐酸和水的混和溶液。
加0.1ml酚酞试液,然后滴加浓氨水直至出现粉红色。
冷却,加冰醋酸直到溶液褪去颜色,再多加0.5ml 冰醋酸。
如有必要,过滤并冲洗过滤器。
加水稀释至20ml。
对照溶液(标准):按供试溶液的制备方法,用规定量的铅标准溶液(10ppm Pb)代替待测物质并在100-105℃烘箱中干燥。
取10ml的该溶液,加2ml 待测液。
监测溶液:按供试溶液的制备方法,向待测物质中加入配制对照溶液规定量的铅标准溶液(10ppm Pb)并在100-105℃烘箱中干燥。
取10ml的该溶液,加2ml待测液。
空白溶液:10ml的水和2ml待测液混合。
向12ml每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:-相较于空白溶液,对照溶液呈浅棕色,-监测溶液至少要同对照溶液颜色深度相同。
结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法E供试溶液:溶解规定量的待测物质于30ml或规定量的水中。
对照溶液(标准):除有另外的规定,稀释规定量的铅标准溶液(1ppm Pb)至与供试溶液相同的体积。
将供试溶液注入注射器,将注射器放置好,然后均匀用力压活塞,使供试溶液全部通过过滤器。
在打开载体和移开预滤器时,检查膜过滤器是否被杂质污染。
如不是需要替换过滤器的情况,发现杂质,则在相同条件下重复以上操作。
向预过滤或规定量的预过滤溶液中加入2ml pH3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合并静置10分钟。
再按上述方法进行过滤,但是要改变过滤顺序,使过滤液先经过滤膜再经过预滤装置(图2.4.8.-1)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
过滤完成后,打开载体,移除过滤器,干燥滤纸。
用同样的方法处理对照溶液。
结果:供试溶液的铅斑颜色不得比标准铅斑深。
方法F供试溶液:将规定量或体积的待测物放入洁净干燥的100ml长颈烧瓶中(若反应产生过多的泡沫液可选用300ml体积)。
45°角夹住烧瓶。
如果待测物是固体,加入足量8ml硫酸和10ml硝酸的混合物来彻底湿润待测物,如果待测物是液体,加入少量8ml硫酸和10ml硝酸的混合物。
轻微加热直至反应开始,使反应逐渐减弱并加入额外分量的酸混合物,每次加入后进行加热,直到加入了18ml酸混合物。
增加热量使其稍微沸腾,直到溶液颜色变深。
冷却,加入2ml 硝酸,并再次加热直到溶液变深。
继续加热,随后加入硝酸直至颜色不能变得更深,然后剧烈加热直到产生密集的白色烟雾。
冷却,小心加入5ml水,轻微煮沸直到产生密集的白色烟雾且继续加热使其体积减至2-3ml。
冷却,小心加入5ml水,观察溶液的颜色。
如果为黄色,小心加入1ml浓过氧化氢溶液并再次蒸发直到产生密集的白色烟雾且体积减至2-3ml。
如果溶液仍显黄色,重复加入5ml水和1ml浓过氧化氢溶液直到溶液无色。
冷却,小心用水稀释并冲洗至50ml比色管中,保证总体积不超过25ml。
使用浓氨水(如有需要,依照要达到的特定范围,可使用稀氨水)调整溶液的pH至3.0~4.0,使用短距pH 试纸作为外指示剂。
加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
用水稀释至50ml。
对照溶液(标准):使用规定体积的铅标准溶液(10ppm Pb)在同一时间用同一方法进行配制除。
监测溶液:按供试溶液的方法进行配制,加入配制对照溶液所需体积的铅标准溶液(10ppm Pb)。
空白溶液:按供试溶液的方法进行配制,略去待测物。
在白色背景下垂直目测。
2分钟后,供试溶液的棕色不得比对照溶液深。
系统适用性:-相较于空白溶液,对照溶液呈浅棕色,-监测溶液至少要同对照溶液颜色深度相同。
结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法G注意:使用高压消解器时,必须遵循安全注意事项和厂家提供的操作说明书。
消化周期必须详尽取决于微波炉的类型(如能控微波炉、温控微波炉或高压炉)。
周期必须遵循厂家的说明书。
若得到澄明的液体,说明此消解周期适宜。
供试溶液:规定量的待测物(不超过0.5g)放置于合适洁净的烧杯中。
加入过量的2.7ml硫酸,3.3ml 硝酸和2.0ml浓过氧化氢,使用磁力搅拌器。
在加入下一种试剂前,要使物质与溶剂进行反应。
将混合物转移至耐高压消解器(含氟聚合物或石英玻璃)。
对照溶液(标准):按供试溶液的制备方法,用规定量的铅标准溶液(10ppm Pb)代替待测物质。
监测溶液:按供试溶液的制备方法,向待测物质中加入配制对照溶液规定量的铅标准溶液(10ppm Pb)。
空白溶液:按供试溶液的方法进行配制,略去待测物质。
关闭消解器放置在实验室微波炉中。
消解使用两种分离的合适程序序列。
程序设计成几步来控制反应,根据微波炉的不同类型进行压力、温度或能量的监测。
第一程序完成后,等消解器冷却了再打开。
每个容器内加入2.0ml浓过氧化氢溶液,然后开始第二程序。
第二程序完成后,等消解器冷却了再打开。
如有需要,重复添加浓过氧化氢溶液和第二消解程序来得到澄明的溶液。
冷却,小心用水稀释并冲洗至烧瓶中,保证总体积不超过25ml。
使用浓氨水(如有需要,依照要达到的特定范围,可使用稀氨水)调整溶液的pH至3.0~4.0,使用短距pH试纸作为外指示剂。
为了避免过热,使用冰浴和磁力搅拌。
用水稀释至40ml,混合均匀。
加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
用水稀释至50ml,混合并放置2分钟。
进行膜过滤(孔径3μm;见图2.4.8-1,无预滤器)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
系统适用性:-相较于空白溶液,对照溶液产生的斑点呈棕色,-监测溶液至少要同对照溶液斑点颜色深度相同。
结果:供试溶液产生的斑点棕色不深于对照溶液。
方法H供试溶液:将规定量的供试品溶解于20ml溶剂或规定的溶剂混合物中。
对照溶液:稀释规定量的铅标准溶液(10ppm Pb)于20ml溶剂或规定的溶剂混合物中。
空白溶液:20ml溶剂或规定的溶剂混合物。
向每种溶液中加入2ml pH3.5的缓冲溶液(某些情况下会出现沉淀作用,指定的专论会建议以一定量的给定溶剂进行再溶解)。
混合后加1.2ml的硫代乙酰胺试液,立即混合并静置2分钟。
进行膜过滤(孔径0.45μm)。
比较不同溶液在过滤器上产生的斑点。
系统适用性:相较于空白溶液,对照溶液产生的斑点呈棕黑色。
结果:供试溶液产生的棕黑色斑点不深于对照溶液。
图8-1重金属检查装置(尺寸的单位为mm)。
