PEI转染试剂的Protocol教学提纲
质粒转染protocol

1. 细胞分盘:通过胰酶消化收集细胞,用适当的完全培养基以1×105 至4×105 细胞/cm2 的密度平铺细胞于60 mm 组织培养皿上(根据实验需要选择培养皿,使细胞贴壁后所占面积达到培养皿总面积的30%)。
将细胞置于含5%
CO2 的37℃温箱中孵育8-24 h,当细胞贴壁完全后即可开始转染。
转染前2 h 换
液(用4 ml 新鲜的完全培养基置换旧的培养基)。
注:为得到较高的转染效率,尽量使用指数生长的细胞,转染时细胞的密度不超过80%。
2. 制备磷酸钙-DNA 沉淀:以60 mm 组织培养皿用480 μl 反应总体积,6孔板培养皿用240ul反应体系。
在灭菌水中加入质粒DNA(总量1-3 μg 为佳),
3.再加入48ul的10*HBS,vortex 3s左右,再加入24ul的5*CaCl2,vortex 10s
,静置12min,将磷酸钙-DNA悬液逐滴加入上述单层细胞的细胞培养基中,轻轻摇动平皿混匀。
注:可以观察到滴入的部位培养基瞬间会出现浑浊的橘黄色,应尽快将其混匀,避免形成过大的颗粒,影响转染效率。
3.若转染的细胞不用转染促进剂处理,则置于含5%CO2 的37℃温箱孵育。
8 h 后吸去培养基与DNA 沉淀,加入5 ml 37℃预热的完全培养基,继续将细胞放
置温箱孵育,16-40 h 观察其转染效率。
PEI转染技术

PEI法瞬时转染HEK-293F悬浮培养物细胞于37℃,150rpm下,CO2恒温箱中的振荡器平台上悬浮生长。
在进行转染之前,请保持5-7代的培养物,以确保稳定的生长模式。
HEK293悬浮细胞(Freestyle™293-F细胞,Life Technologies,目录号R790-07)于Freestyle™293表达培养基(Life Technologies,货号12338026)中培养。
细胞量应维持在4 x 105和3 x 106细胞/ml之间,体积不超过培养瓶总体积的20%。
最佳旋转速率由每种培养瓶类型和培养量确定。
Life Technologies的Freestyle™293-F细胞在更高的密度和体积下容易结块。
聚乙烯亚胺(PEI)(25 kDa线性PEI,Polysciences,Inc.,目录号23966)在含有25mM HEPES和150mM NaCl(pH 7.5)的缓冲液中以1 mg/ml的浓度制备成母液。
PEI添加到缓冲液,并涡旋振荡,直至完全溶解(这可能需要花费数分钟的涡旋时间)。
一旦完全溶解的PEI可以使用0.22 µM注射器过滤器进行无菌过滤,分装后在-20℃冷冻直至需要。
为了获得最佳的转染效率,细胞在转染时应具有> 95%的活力。
转染前24小时,使细胞分裂增殖至约1 x 106细胞/ml的密度,并在37°C CO2培养箱中振荡过夜。
转染时细胞密度应为约2 x 106细胞/ml。
转染应以2.5 x 106至3.0 x 106细胞/ml的细胞密度进行。
使用血球计数器对细胞计数,并调低足够的体积,以2.5 x 106细胞/ml的密度将细胞重悬在新鲜的293F Freestyle Media中,并以所需的体积进行转染。
转染前将细胞重悬于新鲜培养基至关重要。
条件培养基含有抑制转染的代谢产物。
细胞应在室温下以1200 rpm旋转10分钟。
转染24小时后,将转染产物以1:1稀释,即转染的初始体积是所需最终体积的一半。
liofectamine说明书
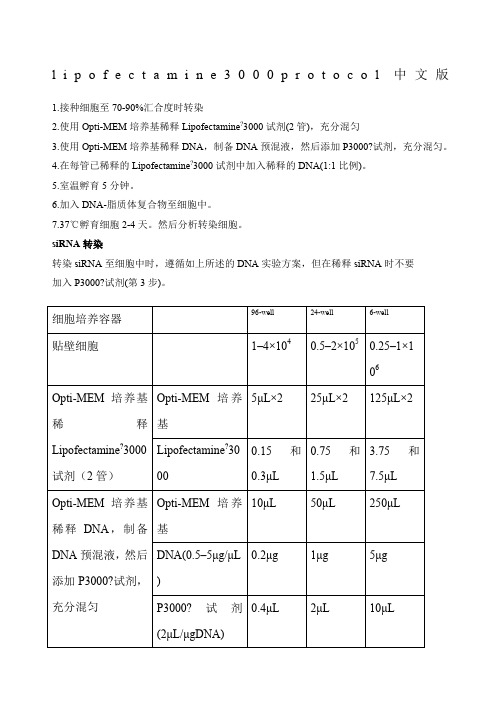
6.加入DNA-脂质体复合物至细胞中。
7.37℃孵育细胞2-4天。然后分析转染,遵循如上所述的DNA实验方案,但在稀释siRNA时不要
加入P3000?试剂(第3步)。
细胞培养容器
96-well
24-well
6-well
贴壁细胞
1–4×104
0.5–2×105
0.25–1×106
Opti-MEM培养基稀释Lipofectamine?3000试剂(2管)
Opti-MEM培养基
5μL×2
25μL×2
125μL×2
Lipofectamine?3000
0.15和0.3μL
0.75和1.5μL
3.75和7.5μL
Opti-MEM培养基稀释DNA,制备DNA预混液,然后添加P3000?试剂,充分混匀
5μL
25μL
125μL
室温孵育5分钟
加入DNA-脂质体复合物至细胞中
96-well
24-well
6-well
DNA-脂质体复合物
10μL
50μL
250μL
37°C孵育细胞2–4天。然后分析转染细胞。
表1:使用lipofectamine?3000转染DNA产品参考用量
Opti-MEM培养基
10μL
50μL
250μL
DNA(0.5–5μg/μL)
0.2μg
1μg
5μg
P3000?试剂(2μL/μgDNA)
0.4μL
2μL
10μL
Lipofectamine?3000试剂中加入稀释的DNA(1:1比例)
稀释的DNA
5μL
25μL
125μL
各种转染试剂的中文转染方法

各种转染试剂的中文转染方法FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2 ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温孵育10-15分钟。
使用之前将FuGENE6颠倒混匀一下。
1. 在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100 ul。
2. 将3-6 ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3. 加入1-2 ug的DNA溶液(0.02-2.0 ug/ul),轻弹管壁混合。
4. 室温孵育20分钟。
5. 将6孔板中的旧营养液吸出,加入约1 ml不含血清和双抗的营养液洗涤一次,再加入2 ml不含血清和双抗的营养液。
6. 将转染复合物加入细胞,混匀使之均匀分布。
7. 3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1. 转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2 ml含血清,不含抗生素的正常生长的培养基中。
2. 对于每孔细胞,使用250 ul无血清培养基(如OPTI-MEM I培养基)稀释4.0 ugDNA,轻轻混匀。
3. 使用前将Lipofectamine 2000转染试剂轻轻混匀,用250 ul无血清培养基(如OPTI-MEM I培养基)稀释10 ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4. 混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5. (optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
lipofectamine3000说明方案
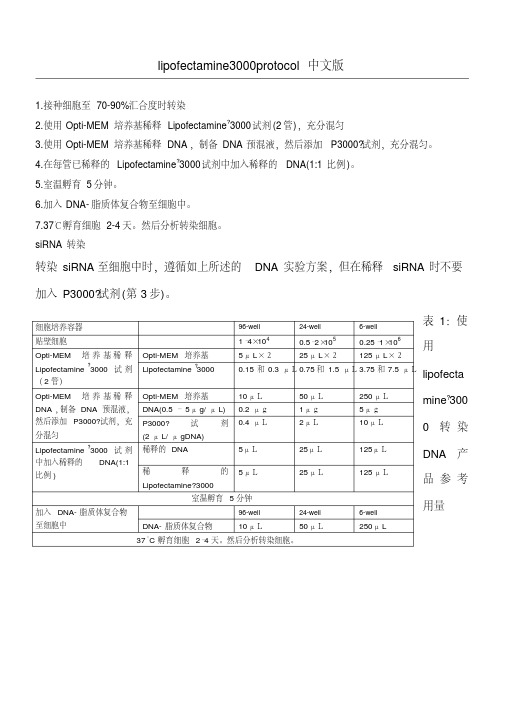
0.2 μg 0.4 μL
5μL 5μL
室温孵育 5 分钟
加入 DNA- 脂质体复合物
96-well
至细胞中
DNA- 脂质体复合物
10 μL
50 μL 1μg 2μL
25μL 25 μL
24-well
50 μL
250 μL 5μg 10 μL
125μL 125 μL
6-well
250μ L
mine?300 0 转染 DNA 产 品参考 用量
37°C 孵育细胞 2–4 天。然后分析转染细胞。
转染 siRNA 至细胞中时,遵循如上所述的 DNA 实验方案,但在稀释 siRNA 时不要
加入 P3000?试剂 (第 3 步)。
细胞培养容器 贴壁细胞
Opti-MEM 培 养 基 稀 释 Lipofectamine ?3000 试 剂 ( 2 管)
Opti-MEM 培养基 Lipofectamine ?3000
10 μL
DNA ,制备 DNA 预混液, 然后添加 P3000?试剂,充 分混匀 Lipofectamine ?3000 试 剂 中加入稀释的 DNA(1:1 比例 )
DNA(0.5 – 5μ g/ μ L)
P3000?
试
剂
(2 μ L/ μ gDNA)
稀释的 DNA
稀
释
的
Lipofectamine?3000
96-well
24-well
6-well
表 1:使
1–4×104 5μ L×2
0.5–2×105 25μ L×2
0.25–1×106
用
125 μ L×2
0.15 和 0.3 μL 0.75 和 1.5 μL 3.75 和 7.5 μL lipofecta
PEI转染细胞方法的标准操作规程

PEI转染细胞方法的标准操作规程(编号:031)1、目的及适用范围该SOP用来规范利用PEI为转染试剂进行转染的操作。
2、主要仪器细胞培养箱、细胞培养皿、微量移液器3、试剂及配制方法3.1 PEI (2μg/μL):采用生理盐水配成2μg/μL,60℃烘箱助溶,完全溶解并冷却后,调pH至7.0(不可回调),0.22μm微孔滤器过滤,分装于1.5mL离心管,储存与4℃备用。
3.2生理盐水:0.9g NaCl溶于100mL纯水中,灭菌后微孔滤器过滤分装于1.5mL离心管,储存于4℃备用。
4、操作步骤4.1 细胞铺于细胞培养皿中,培养过夜;4.2 在转染前1-2h,将细胞培养皿中换成预热的培养基;4.3 将要转染的质粒和转染试剂PEI分别用生理盐水稀释,室温处理5min。
质粒和转染试剂的质量比为1:6;一般质粒和PEI稀释后的每管体积为50μL,如转染的质粒量多,则可对应适当增加生理盐水的量;4.4 将处理好的两者混匀,室温放置15-30 min;4.5 将处理好的样品轻轻且均匀滴加到细胞中;4.6 转染后6-8 h换液;4.7 根据实验需要,在转染后的特定时间收集样品,进行分析。
5、注意事项5.1 细胞如何健康而茁壮成长转染前细胞最好经过1~2次传代,以保证细胞生长旺盛,容易转染。
注意,贴壁细胞生长到几乎汇片时就要赶快进行下一次传代,千万不要使细胞保持融合超过24h,一旦长满了,转染效率便会降低。
大多数已建立的细胞系都是非整倍体,细胞培养在实验室中保存数月和数年后会经历突变,总染色体重组或基因调控变化等而演化,这会导致和转染相关的细胞行为的变化。
如果随时间发生61。
ESCORT V 转染剂说明书

ESCORT™V Transfection ReagentCatalog Number E9778Storage Temperature 2-8 °CTECHNICAL BULLETINProduct DescriptionESCORT V Transfection Reagent was developed for highly efficient stable and transient transfections of eukaryotic cells. It is a specially processed polyethyleneimine (PEI) optimized for use in serum-containing media. Transfection in serum-free media has been demonstrated, and Escort V can also be used for transfection experiments with siRNA.The reagent has been extensively tested and found to be highly efficient in many cell types including CHO, BHK, HeLa, HEK293, HEK-293T, Vero, F9, PC12, COS-1, COS-7, NIH 3T3, L929, Human Foreskin Fibroblasts (HFF) primary cells, and Bovine Aorta Endothelial cells (BAEC).ReagentsESCORT V Transfection Reagent,Catalog Number E0654 1.5 ml ESCORT V Transfection Buffer,Catalog Number E0529100 ml ESCORT V Transfection Reagent is provided at a concentration of 1.3 mg/mL. The reagent is sufficient for 700-1000 transfection experiments in 24-well plates.Precautions and DisclaimerThese products are for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Storage/StabilityStore at 2-8 °C.PROCEDUREThis protocol is optimized for use in 24-well plates. All volumes given are for a single reaction (single well) on a 24-well plate. If another format is desired, scale up or down accordingly (Please see Table I). Cell PreparationAdherent cells1.18-24 hours before transfection, seed the plate with5-7.5 x 104 cells/well in 0.5 ml of the appropriatemedium.2.30 minutes to 2 hours before adding thetransfection cocktail to the wells, replace the growth media in the wells with 0.5ml fresh mediacontaining serum (complete media).Cells in suspension1.Before transfection, pellet the cells and dilute to theappropriate concentration (0.5-1X106cells/ml) inthe standard growth medium for the cell line.2.Add 0.5 ml of the cell suspension to each well. Transfection with plasmid DNA in 24-well plateFor optimal transfection, the recommended ratio ofµg DNA/µL ESCORT V Transfection Reagent is 1:3.In some cases, transfection efficiency can be increased by using a different ratio. When changing the amount of ESCORT V Transfection Reagent (step 2), keep the amount of Transfection Buffer constant at 60 µL.1.In a sterile tube, combine 60 µl of ESCORT VTransfection Buffer and 0.7 µg of plasmid DNA.Mix the contents of the tube gently.2.In a separate tube, combine 60 µl of ESCORT VTransfection Buffer and 2.1 µl of Escort VTransfection Reagent. Mix gently.bine the DNA/buffer solution, Step 1, with theESCORT V reagent/buffer solution, Step 2, to make the transfection cocktail. Mix gently.4.Incubate at room temperature for15-20 minutes.The complex is stable for up to 2 hours at roomtemperature.5.Add the total volume of transfection cocktail directlyto the wells. Mix gently by rocking the plate.6.Incubate the transfected cells under standardconditions for 24-72 hours.2Stable Transfection1.Perform cell transfection using the appropriatevector. Follow the protocol above.2.Two days after transfection, replace the media withfresh media containing the appropriate selectionantibiotic.3.Maintain the cells in selective media for 2-3 weeksuntil stably transfected cells are clearly identified. Transfection with siRNA in a 6-well plateThis protocol is optimized for cells plated in a 6-well plate. Volumes indicated in the protocol are for one reaction (one well).1.Prepare 10 nmol siRNA stock solution (dilute withRNAse-free water).2.In a sterile tube, add 60 µL of ESCORT VTransfection Buffer and 9 µL of ESCORT VTransfection Reagent.3.Mix the contents of the tube gently and incubate for5 minutes at room temperature.4.In a second sterile tube, add 60 µL of serum-freemedium and 10 µL of10 nmol siRNA solution.bine the contents of both tubes and gently mix.6.Incubate for 15-20 minutes at room temperature toform the transfection complex.7.During incubation, remove culture media from thewells and add 0.8 ml of complete culture media(media with serum) to each well.8.Carefully add the transfection mix drop-wise to thecells in the well.9.Swirl the plate gently to ensure uniform distributionof the transfection complex.10.Incubate the cells at 37 °C in 5% CO2for 24-72hours before analysis.11.Change the media as required.Transfection with siRNA in a 96-well plateThis protocol is optimized for a 96-well plate. Volumes indicated in the protocol are for one reaction (one well).1.Prepare 100 pmol siRNA stock solution (dilute withRNAse-free water).2.In a sterile tube, add 25 µL of ESCORT VTransfection Buffer and 0.6 µL of ESCORT VTransfection Reagent.3.Mix the contents of the tube gently and incubate for5 minutes at room temperature. 4.In a second sterile tube, add 25 µL of serum-freemedia and 10 µL of the 100 pmol siRNA solution. bine the contents of both tubes and mixcarefully.6.Incubate for 10-15 minutes at room temperature toform the transfection complex.7.During incubation, remove the culture media fromthe wells and add 100 µL of complete culture media (media with serum) to each well.8.Carefully add the transfection mix drop-wise ontothe cells in the well.9.Swirl the plate gently to ensure uniform distributionof the transfection complex.10.Incubate the cells at 37 °C in 5% CO2for 24-72hours before expression analysis.11.Change the media as required.OPTIMIZING TRANSFECTIONFor some cell lines, ESCORT V Transfection Reagent may require optimization. When optimizing conditions, it is important to consider the following optimization factors:Volume of Transfection CocktailIn some cases, a different volume of the transfection mixture may increase transfection rates. For optimization, compare transfection performance when different volumes of transfection mixture are added to the wells (e.g., 75, 100, 120, and 150 µL/well).The Ratio of DNA/ESCORT VThe recommended ratio of µg DNA/ µl ESCORT V Transfection Reagent is 1:3. This ratio produces good results for most cell lines. In some cases, transfection efficiency can be increased by changing the ratio (e.g., 1:2 or 1:4).MediaIt is recommended to change growth media up to 2 hours before transfection. Use complete media (media supplemented with serum). If desired, media containing the transfection mixture can be substituted 4-6 hours after the initial addition without loss of transfection activity.Transfection without ESCORT V Transfection BufferIf use of the ESCORT V Transfection Buffer is not desired, prepare the transfection mixture in any standard media without serum and antibiotics. Follow the same volumes as in transfection protocol.3Cell DensityThe optimal number of cells to be plated depends on the specific cell line. A 40%-70% confluent cell layer at the time of transfection is suggested for best response. When required, cells can be transfected at very low cell densities such as 5-15% confluence.Incubation Time Post-TransfectionIncubation time depends on the cell line, the protein being expressed, as well as the vector construct.DNA QualityDNA quality is a critical factor for successful transfection. OD260/280should be ~1.8 or greater (1.85 is recommended). DNA should be free of endotoxin and other contaminants. RNA contamination does not prevent transfection; however, RNA substitutes DNA in the complex and may lead to an incorrect DNA concentration estimation. Taken together, these factors may greatly reduce transfection efficiency.DNA VectorThe expression of the transfected gene depends on the cell line, the promoter used, and the nature of the expressed protein.Condition of the CellsCells should be healthy, free of contamination, proliferating well and plated at an appropriate density. The Level of the Expressed GeneHigh level of expression of some proteins can be cytotoxic.Table I Scaling up/down4Troubleshooting GuideESCORT is a trademark of Sigma-Aldrich Biotechnology LPKH,PHC 03/07-1Sigma brand products are sold through Sigma-Aldrich, Inc.Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications.Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply.Please see reverse side of the invoice or packing slip.。
[说明]转染详细步骤大攻略
![[说明]转染详细步骤大攻略](https://img.taocdn.com/s3/m/8d4c02e6bb68a98270fefa98.png)
[说明]转染详细步骤大攻略转染详细步骤大攻略范例----真核重组表达质粒pDsRed-N1-NS1在A549细胞中表达按上海索莱宝生物科技有限公司去内毒素质粒小提试剂盒说明书方法进行质粒抽提,测得质粒浓度为410.32ng/µl。
将培养的A549细胞铺板,待细胞密度长到90%左右时,按lipo2000说明书转染A549细胞,36h后于荧光显微镜下观察DsRed-NS1融合蛋白的表达情况。
具体操作步骤如下: 1)质粒准备按上海索莱宝生物科技有限公司去内毒素质粒小提试剂盒说明书方法进行质粒抽提,具体步骤如下:(1) 取1-5ml细菌培养物,12000rpm离心1 min,尽量吸除上清(菌液较多时可以通过多次离心将菌体沉淀收集到一个离心管中)。
(2) 向留有菌体沉淀的离心管中加入200µl溶液P1(请先检查是否已加入RNaseA),使用移液器或涡旋振荡器彻底悬浮细菌细胞沉淀。
(注:如果菌块未彻底混匀,会影响裂解导致质粒提取量和纯度偏低)(3)向离心管中加入200µl溶液P2,温和地上下翻转6-8次使菌体充分裂解。
(注:混匀一定要温和,以免污染基因组DNA,此时菌液应变得清亮粘稠,所用时间不应超过5min,以免质粒受到破坏)(4)向离心管中加入250µl溶液P3,立即温和地上下翻转6-8次,充分混匀,此时会出现白色絮状沉淀。
12000rpm 离心10min,用移液器小心地将上清转移到另一个干净的离心管中,尽量不要吸出沉淀。
(注:溶液P3加入后应立即混合,避免产生局部沉淀。
如果上清中还有微小白色沉淀,可再次离心后取上清)(5)加入1/5体积冰预冷的去内毒素清除剂,振荡混匀,溶液变浑浊,冰浴2min至溶液变清亮。
(6)37?水浴5 min,不时振荡,溶液又变浑浊。
12000rpm室温离心5min,溶液应分为两相,上层水相含质粒DNA,下层油状相含内毒素。
(7)将质粒DNA上层水相转移至新管,弃下层油状相,注意不要吸入油状相,重复抽提三次,即重复步骤5-7三次。
慢病毒转染PROTOCOL

慢病毒转染PROTOCOL第一天病毒感染前24 小时将细胞置于12 孔板。
加入 1 ml 适当的完全培养基(含有血清和抗生素)孵育过夜。
在传染当天细胞应该达到约50%平铺(Day 2)。
注意:也可用其它型号培养板转导。
这样需要根据孔径或培养板调节细胞量。
第二天准备完全培养基和Polybrene? (sc-134220) 混合物,Polybrene 终浓度为:5 μg/ml。
移去培养基并添加1 ml Polybrene/培养基混合物于每孔中(适用于12 孔板)。
注意:Polybrene 是一种聚阳离子,可以中和电荷以促进假病毒外壳与细胞膜的作用。
Polybrene 最佳浓度因不同细胞株而异并应根据经验而定(通常范围为2-10 μg/ml)。
过长时间暴露于Polybrene (> 12 小时)可对某些细胞产生毒性作用。
在室温下溶化慢病毒颗粒,使用前轻轻混匀。
培养液中加入shRNA 慢病毒颗粒以感染细胞。
轻轻振动培养板使其混匀并孵育过夜。
病毒颗粒使用量因细胞株的特点而有较大不同。
注意:溶化的shRNA 慢病毒颗粒置于冰上。
反复冻溶或长时间将病毒颗粒暴露于常温可使病毒效价降低。
注意:当你是第一次转导shRNA 慢病毒结构进入细胞,我们建议你用几个不同剂量的shRNA 慢病毒颗粒。
此外,我们建议你用一孔shRNA 慢病毒颗粒转导的细胞作对照(sc-108080)。
注意:用copGFP 对照慢病毒颗粒:sc-108084 观察转导效率。
第三天移去培养液并添加1 ml 完全培养基(不含Polybrene)。
孵育细胞过夜。
第四天欲选择稳定表达shRNA 的克隆,根据细胞类型不同将其分成1:3 到1:5 并继续在完全培养基中孵育24-48 小时。
第五-六天及以后用Puromycin dihydrochloriede (sc-108071) 筛选稳定表达shRNA 的克隆。
Puromycin 筛选法:用足够剂量的Puromycin 杀死非转导的细胞。
LipoFiter转染试剂中文说明书
类型 (培养皿/培养板)
96-well 48-well 24-well 12-well 6-well 60mm 100mm
表面积 /cm2
0.3cm2 0.8cm2 2cm2 4cm2 10cm2 20cm2 60cm2
对应细胞培养 液体积 100µl 350µl 500µl 1ml 2ml 4ml 12ml
7
LipoFiterTM 脂质体转染试剂
注意事项 1. 使用高纯度的 DNA(A260/A280 比值越近 1.8 越好)有助于获得 较高的转染效率。对于质粒,推荐使用 Qiagen 公司生产的质粒大量 抽提试剂盒进行高质量无内毒素抽提。 2. 转染前细胞必须处于良好的生长状态。 3. 需自备 DMEM 培养基,其他培养基如 1640、MEM、alpha-MEM, F12,DMEM/F12、M199 也均可以用于转染实验。 4. LipoFiterTM 不能 vortex 或离心,宜缓慢晃动混匀。 5. LipoFiterTM 使用后请立即盖好盖子,避免长时间暴露在空气中,影 响转染效率。 6. 经测试,LipoFiterTM 细胞毒性不明显,非常适用于病毒包装(腺 病毒,慢病毒,逆转录病毒等);请在进行病毒包装时严格按照病毒 安全操作进行。
关于病毒安全操作可参考《汉恒生物-病毒载体操作安全手册》或来电来信和我 们的病毒技术工程师沟通,您还可以扫描 加微信和我们技术工程师就技术上进 一步沟通。
7. 为了您的安全和健康,请在符合洁净度要求的细胞培养室中进行 转染操作,操作时请穿实验服并戴一次性手套、口罩和无菌帽。
8
LipoFiterTM 脂质体转染试剂 汉恒生物 进入汉恒官网观看 LipoFiterTM 转染实验操作视频
5
LipoFiterTM 脂质体转染试剂
PEI纳米颗粒基因转染技术方法.

PEI纳米颗粒基因转染技术方法发布日期:2008-1-8 热门指数:2417ABSTRACTThis protocol describes the preparation of polyethylenimine (PEI)/DNA nanoparticles for targeted gene delivery. This delivery strategy improves the efficiency of gene transfer by enhancing the entry of gene vectors into the desired cells and reducing uptake by nontarget cells. We describe here methods for the conjugation of targeting peptides to PEIs, formation of DNA complexes using the conjugated PEIs or nonconjugated PEIs together with targeting peptides, and cell transfection using these complexes. The conjugation step involves the use of the succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC), a heterobifunctional cross-linker, to form a stable bond between PEI and peptides containingthiol groups.MATERIALSReagents∙∙oo o o o ∙Dc protein assay kitDMEM cell culture medium with 10% fetal bovine serum (FBS)DMEM (Dulbecco's Modified Eagle's Medium)100 units/ml penicillin 100 µg/ml streptomycin2 mM glutamine10% fetal bovine serum (FBS)Exponentially growing mammalian cells∙∙∙∙∙Lithium chlorideLuciferase assay reagentsDimethylsulfoxide (DMSO)OptiMEM serum-free cell culture mediumPEI polymers (MW 600-1000 kDa, Fluka; MW 750 kDa, 25 kDa, 2 kDa, and 800 Da, Sigma-Aldrich; MW 1.2, 10, or 70 kDa, Polysciences)∙ Peptides, prepared using conventional solid-phase, chemical synthesis method∙oPhosphate buffered saline (PBS)137 mM NaClo o o2.7 mM KCl 10 mM Na2HPO4 2 mM KH2PO4To prepare 1 liter of PBS(Phosphate-buffered Saline), dissolve 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4 in 800 ml of distilled H2O. Adjust the pH to 7.4 (or 7.2 if required) with HCl. Add H2O to 1 liter. Dispense the solution into aliquots and sterilize them by autoclaving for 20 minutes at 15 psi (1.05 kg/cm) on liquid cycle or by filter sterilization. Store the buffer at room temperature. If necessary, PBS may be supplemented with 1 mM CaCl2 and 0.5 mM MgCl2. Can bemade as a 10x stock.∙∙∙Plasmid DNA encoding the luciferase reporter geneReporter Lysis Buffer, 5X (Promega), dilute to 1X in PBSSuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC)∙ Water, ultrapure sterilized Equipment∙ Dialysis membranes (molecular weight size exclusion specification for purification of sidereaction and excess products)∙∙∙∙∙Freeze dryerLuminometerMagnetic stirrer and magnetic rodReaction vesselTissue culture vesselsMETHODActivation of PEI with a cross-linker1.Prepare a SMCC stock solution of 50 mM in DMSO. SMCC is moisture sensitive. The stocksolution should be prepared using high-quality anhydrous DMSO in a dry nitrogen atmosphere. When stored at 4°C, the SMCC solution remains stable for ~3 months. Steps 1-10 should be performed in achemical fume hood following chemical safe handling procedures.2.Prepare a 10 mg/ml stock solution of PEI in DMSO. Add 2-5 mg of lithium chloride to increasethe solubility of PEI.3. Using a syringe, slowly add the SMCC solution into the PEI solution. Incubate the reaction for2 hours at room temperature. The amount of SMCC solution added should be based on the desiredmolar ratio between SMCC to PEI.4. Purify the modified PEI by dialysis against ultrapure water for 2 days, changing the water atleast five times a day.5. Collect the solution in a dialysis tube and freeze dry the sample.Conjugation of peptide to activated PEI6.7.8. Prepare a peptide stock solution of 20-50 mM in PBS. Prepare a 10 mg/ml stock solution of the activated PEI (Step 5) in PBS. Slowly add the peptide solution to the activated PEI solution. Incubate the conjugationreaction for 24 hours at room temperature.The amount of the peptide added to the reaction should be based on the desired molar ratio in the finalconjugate.9. Purify the peptide-conjugated PEI by dialysis against ultrapure water for 2 days, changing thewater at least five times a day.10. Collect the solution into a dialysis tube and freeze dry the sample.Preparation of PEI/DNA complexes11.i.ii.iii. Prepare the stock solutions. Prepare a 1 mg/ml plasmid DNA stock solution in ultrapure water. Prepare a stock solution of PEI (Step 5) or peptide-conjugated PEI (Step 10) to contain 10 nmol amino nitrogen per microliter in ultrapure water (pH 7.2). For ternary complexes, prepare a 5-mg/ml stock solution of a targeting peptidelinked with a DNA-binding sequence in ultrapure water.Steps 11-22 should be performed under aseptic conditions using sterile reagents. Manipulation of thecomplexes should be performed at room temperature in a horizontal flow hood.12.i.. Prepare the working solutions. Dilute 1 µg of plasmid DNA (for transfection of cells seeded in a well of a 24-well plate) in 50 µl of OptiMEM serum-free cell culture medium. Dilute the appropriate quantity of PEI or peptide-conjugated PEI in 50 µl of OptiMEMserum-free cell culture medium.ii. For ternary complexes, dilute the appropriate quantity of the targeting peptide in 50 µl of OptiMEM serum-free cell culture medium.13. Add the peptide-conjugated PEI solution dropwise into the DNA solution while vortexing. Or,for ternary complexes, add the targeting peptide dropwise into the DNA solution while vortexing.14. Incubate the mixture for 30 minutes at room temperature. The peptide-conjugated PEI/DNAcomplexes may now be used directly for transfection (Step 16)15. To form ternary complexes, add the PEI solution dropwise into the targeting peptide/DNAcomplexes while vortexing. Incubate the ternary complexes for 30 minutes at room temperature.The ternary complexes may now be used directly for transfection (Step 16) Transfection assay16. One day before transfection, harvest mammalian cells, grown in DMEM complete cell culturemedium with 10% FBS by trypsinization, and replate them into 24-well plates at a density of 50,000cells/well. Incubate the cultures for 24 hours at 37°C in a humidified incubator with 5% CO2.17.18. Remove the medium from the wells and wash the cells twice with PBS (prewarmed to 37°C). Add the following to the cells, and incubate them for 4 hours at 37°C in a humidified incubatorwith 5% CO2. 100-150 µl/well of OptiMEM serum-free cell culture medium 100-150 µl/well of the genetransfection complex containing 1 µg of plasmid DNA (Step 14 or Step 15)19. Remove the transfection solution, wash the cells twice with PBS (prewarmed to 37°C) andadd 1 ml/well of DMEM complete cell culture medium with 10% FBS. Incubate the cells for 24-48hours.20. To assay for luciferase expression, lyse the cells by adding 100 µl/well of 1X Reporter LysisBuffer (dilute 5X stock solution with PBS).21. To detect luciferase activity, add 20 µl of cell lysate to 100 µl of luciferase assay reagent.Measure luciferase activity with a luminometer.22. To normalize the luciferase activity, determine the protein concentrations of the cell lysatesusing the Dc protein assay kit.ACKNOWLEDGMENTSThe work was funded by the Institute of Bioengineering and Nanotechnology, the Agency for Science,Technology and Research (A* STAR) in Singapore.Anyone using the procedures in this protocol does so at their own risk. Cold Spring Harbor Laboratory makes norepresentations or warranties with respect to the material set forth in this protocol and has no liability in connection with the use of these materials. Materials used in this protocol may be considered hazardous and should be used with caution. For a full listing of cautions regarding these material, please consult:Gene Transfer: Delivery and Expression of DNA and RNA, A Laboratory Manual, edited by Theodore Friedmann and JohnRossi, © 2007 by Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, p. 473-478。
细胞转染 Protocol

细胞转染是一种常用的生物技术,用于将外源基因或药物引入细胞中。
下面是细胞转染的实验原理、所需试剂和耗材、实验仪器、准备工作、实验方法、注意事项、常见问题及解决方法。
一、实验原理细胞转染是利用细胞膜的通透性,将带有特定基因或药物的载体分子导入细胞内。
常用的载体分子包括病毒载体和非病毒载体。
病毒载体如腺病毒、逆转录病毒等,能将外源基因高效地导入细胞中,但可能对细胞产生一定毒性。
非病毒载体如脂质体、阳离子聚合物等,相对安全,但导入效率较低。
通过细胞转染,可以实现对基因的表达调控,探索基因功能和药物筛选等研究。
二、所需试剂和耗材1.试剂:o培养基:如DMEM、F12等,用于细胞培养。
o血清:如胎牛血清,提供细胞生长所需的营养物质。
o抗生素:如青霉素、链霉素等,用于防止细胞污染。
o转染试剂:如Lipofectamine、Effectene等,用于细胞转染。
o DNA或RNA:携带目的基因的质粒或寡核苷酸。
2.耗材:o细胞培养瓶、板:用于细胞培养。
o离心管:用于细胞转染后洗涤和离心。
o移液器及枪头:用于精确加样。
o过滤器:用于过滤溶液中的杂质。
o无菌水:用于稀释和配制溶液。
三、实验仪器1.实验室搅拌器:用于混合溶液。
2.高速冷冻离心机:用于离心和分离细胞。
3.水浴锅:用于加热溶液。
4.无菌工作台或超净工作台:用于进行无菌操作。
5.分光光度计:用于测量细胞生长状况和转染效率。
6.荧光显微镜:用于观察细胞转染后荧光蛋白的表达情况。
7.CO2培养箱:提供细胞培养所需的气体环境。
四、准备工作1.仔细阅读实验步骤和注意事项,了解所需的试剂和耗材及其使用方法。
2.准备好所需的试剂和耗材,并确保它们处于保质期内。
3.检查实验室内是否具备上述实验仪器,并确保其正常运行。
4.用70%乙醇擦拭实验台面,以确保无菌环境。
5.用高压蒸汽灭菌法灭菌所有的实验器具,包括离心管、移液器等,需在适当的压力和温度下进行灭菌处理,以消除所有潜在的污染源。
PEI瞬时转染方法

匀后,再加入 20µl GenEscortTMⅠ 混匀,室温放置 10-15 min,获得 50 µl 转染复合物。
(3)瘤体局部消毒后,将前述的 50µl 转染复合物直接注射到小鼠肿瘤内。
(4)动物继续饲养,如需要多次给药,则转染方法按 (2), (3) 进行。定期用游标卡尺测量
移植瘤直径,计算肿瘤体积。
南京慧基生物技术有限公司
高通量系统实验步骤 (在 96-孔板上的转染):
转染复合物的制备
• 对每孔, 在 EP 管中将 200 ng DNA 稀释于 15 µl PBS 中, 轻轻混匀。 • 对每孔, 在 EP 管中将 0.4 µl 的 GenEscortTMⅠ 溶液稀释于 15 µl PBS 中, 轻轻混匀。 • 将 15 µl GenEscortTMⅠ 稀释液加到 15 µl DNA 稀释液中,轻轻混匀。 注意: 两种溶液的混合顺序对转染结果非常重要,切勿颠倒滴加顺序。 • 轻轻振荡混匀后,室温下静止 10-15 分钟后,立刻加入到每孔中。
每孔转 染复合 物溶液 的用量
(µl) 30 50 100 100
200
200
500 1000 2000
慧基生物、转染利器
To strengthen your research capabilities and to facilitate innovation 电话:+86 25 84312606;传真:+86 25 84312602
慧基生物、转染利器 To strengthen your research capabilities and to facilitate innovation
电话:+86 25 84312606;传真:+86 25 84312602
PEI转染

Protocol for Transfection with PEIAlthough the transfection efficiency is somewhat lower than commercial kits, this cheap protocol already satisfys for most transfectional purposes on HeLa and 293T cell lines and should be used as daily method.PEI Solutions:1. HEPES buffer (pH7.2~7.4): Store at 4o C2. 2XPEI stock solution: aliquot into 0.5 mL/tube, store in -20℃3. 1XPEI working solution: dilute(稀释的) 2Xstock PEI with HEPES buffer 1:1 (final concentration: 1.7ug/ul PEI), Store at 4o CCell split:A. For 293T cells:the confluent of 293T cells up to 80-90% at the time of transfection.B. For HeLa cells: the confluent of HeLa cells up to 60-70% at the time of transfection.Steps:Example for a 150mm dish of HeLa based cells1. Dilute 30ug DNA in 750ul of HEPES buffer (pH 7.2~7.4), mix well by votex.2. In a separate tube, add 45~90ul 1XPEI to 750ul of HEPES buffer (pH7.2~7.4), mix well by votex.3. Mix the diluted DNA and PEI thoroughly, incubate for 20-30 min at RT.Note: if the white deposition is found, it maybe results by high concentration of DNA in local area. Throw away and do it again.4. Add the mixture to dish by dropwise, shake dish to mix PEI/DNA tomedium (No antibiotic; Optional: No fetal serum and antibiotic medium.after incubation for 6hr at 37℃ in CO2 incubator, add serum directly into medium to 10%).5. After incubation for 24h at 37℃in CO2incubator, change NOMAL freshmedium to remove PEI from medium. (Optional: Split cells on the second day----one dish into two dishes).6. Test transgene expression after 48h.NOTE: The ratio of PEI to DNA is key problem, maybe various to different cell lines. But for most cell line, it is usually around of 1.7:1.Transfection recipes (Typically for HeLa cells):I. For one well of a six-well dish (Sq: 9.6cm2):A. Add 2.0ug DNA to 50ul of HEPES buffer (pH 7.2~7.4), mix well by votex.B. In a separate tube, add 3-6ul 1XPEI to 50ul of HEPES buffer, mix well byvotex.C. Mix the diluted DNA and PEI thoroughly, incubate for 20-30 min at RT.D. Add mixture to cells, mix by gentle shaking.E. Indubate in CO2 incubator for 24hr and then change with fresh medium.II. For 60mm dish (Sq: 19.5cm2):F. Add 8.0ug DNA to 100ul of HEPES buffer (pH 7.2~7.4), mix well by votex.G. In a separate tube, add 12-24ul 1XPEI to 100ul of HEPES buffer, mix wellby votex.H. Mix the diluted DNA and PEI thoroughly, incubate for 20-30 min at RT.I. Add mixture to cells, mix by gentle shaking.J. Indubate in CO2 incubator for 24hr and then change with fresh medium. III. For 100mm dish (Sq: 59cm2):K. Add 20.0 ug DNA to 250ul of HEPES buffer (pH 7.2~7.4), mix well by votex.L. In a separate tube, add 30-60ul 1XPEI to 250ul of HEPES buffer, mix well by votex.M. Mix the diluted DNA and PEI thoroughly, incubate for 20-30 min at RT. N. Add mixture to cells, mix by gentle shaking.O. Indubate in CO2 incubator for 24hr and then change with fresh medium. IV. For 150mm dish (Sq: 156cm2):P. Add 40.0ug DNA to 750ul of HEPES buffer (pH 7.2~7.4), mix well by votex.Q. In a separate tube, add 60-120ul 1XPEI to 750ul of HEPES buffer, mix well by votex.R. Mix the diluted DNA and PEI thoroughly, incubate for 20-30 min at RT. S. Add mixture to cells, mix by gentle shaking.T. Indubate in CO2 incubator for 24hr and then change with fresh medium. Note: if the amount of DNA you want to transfect is less than the standard amount as above, you should add empty vector DNA to make total DNA to the standard amount.The ratio 3 to 1 of PEI to cDNA is only applied for HeLa cells. Regarding for other cell lines, you have to test the best ratio before beginning your experiments. You can use any plasmid expression of EGFP protein for your testing. Pay specific attention on cell viable and transfection efficiency during testing.。
最新整理lipo转染操作步骤知识讲解

最新整理lipo转染操作步骤知识讲解Lipo2000 瞬时转染细胞步骤Stealth? RNAi or siRNA Transfection以24孔板为例,其余规格的转染见表11 中板,细胞密度为30-50%适宜。
注意:根据转染后细胞检测时间长短决定细胞中板密度,如果转染后需要长时间后检测,则细胞中板密度适当降低,已避免细胞过度生长导致存活降低。
2 第二天(24-36小时后)每个孔转染方式如下:A 将20pmol siRNA溶于50ul Opti-mem无血清培养基中。
B 将1ul lipo2000溶于50ul Opti-mem无血清培养基中,混匀室温放置5min。
C 将A B两管混合,放置20min。
3 转染期间,将24孔板培养基换成无血清培养基,每孔400ul。
将C管mix加入24孔板对应孔中,4-6小时候换成有血清培养基。
Plasmid DNA TransfectionDNA(ug):lipo 2000(ul)=1:2-3转染时细胞密度越高,转染效率,表达效率也越高,并且可以降低细胞毒性。
1 中板。
贴壁细胞:0.5-2X105 cells/well,第二天待细胞密度达到70-80%时转染悬浮细胞:4-8X105 cells/well,中板后随即转染。
2 转染。
A 将0.8ug DNA溶于50ul Opti-mem无血清培养基中。
B 将2ul lipo2000溶于50ul Opti-mem无血清培养基中,混匀室温放置5min。
C 将A B两管混合,放置20min。
转染期间,将24孔板培养基换成无血清培养基,每孔400ul。
将C管mix加入24孔板对应孔中,4-6小时候换成有血清培养基。
Table 1. Culture Shared reagents DNA transfection RNAi transfection*:中板密度根据不同细胞不同实验有所不同,这里仅提的数据仅供参考**:6孔板细胞质粒转染量1-2ug足以。
最新整理lipo转染操作步骤知识讲解

Lipo2000 瞬时转染细胞步骤Stealth™ RNAi or siRNA Transfection以24孔板为例,其余规格的转染见表11 中板,细胞密度为30-50%适宜。
注意:根据转染后细胞检测时间长短决定细胞中板密度,如果转染后需要长时间后检测,则细胞中板密度适当降低,已避免细胞过度生长导致存活降低。
2 第二天(24-36小时后)每个孔转染方式如下:A 将20pmol siRNA溶于50ul Opti-mem无血清培养基中。
B 将1ul lipo2000溶于50ul Opti-mem无血清培养基中,混匀室温放置5min。
C 将A B两管混合,放置20min。
3 转染期间,将24孔板培养基换成无血清培养基,每孔400ul。
将C管mix加入24孔板对应孔中,4-6小时候换成有血清培养基。
Plasmid DNA TransfectionDNA(ug):lipo 2000(ul)=1:2-3转染时细胞密度越高,转染效率,表达效率也越高,并且可以降低细胞毒性。
1 中板。
贴壁细胞:0.5-2X105 cells/well,第二天待细胞密度达到70-80%时转染悬浮细胞:4-8X105 cells/well,中板后随即转染。
2 转染。
A 将0.8ug DNA溶于50ul Opti-mem无血清培养基中。
B 将2ul lipo2000溶于50ul Opti-mem无血清培养基中,混匀室温放置5min。
C 将A B两管混合,放置20min。
转染期间,将24孔板培养基换成无血清培养基,每孔400ul。
将C管mix加入24孔板对应孔中,4-6小时候换成有血清培养基。
Table 1. Culture Shared reagents DNA transfection RNAi transfection*:中板密度根据不同细胞不同实验有所不同,这里仅提的数据仅供参考**:6孔板细胞质粒转染量1-2ug足以。
***:6cm dish细胞质粒转染量4-6ug足以。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
50
12孔板
3.5
1.6
100
6孔板
9.6
4
250
6cm平板
21.5
8
500
10cm平板
56.7
16
1000
15cm平板
147
32
2000
4.将B液缓慢加到A液中并混匀,最好使用旋涡振荡器边加边震荡。然后室温放置20min。
5.将DNA-PEI混合液缓慢加入到细胞培养基中,轻轻混匀。
6.转染4h后更换为完全培养基。
培养皿面积(cm2)
转染DNA量(ug)
稀释DNA/PEI的培养基体积(ul)
96孔板
0.32
0.2
25
48孔板
1.1
0.5
PEI转染试剂的Protocol
PEI转染Protocol
1.转染前一天(24h左右)胰酶消化细胞并计数,细胞铺板,使其在转染时汇合度为70~90%。
2.转染前1h,将细胞培养基更换为Opti-MEM或者DMEM基础培养基(若使用Opti-MEM,PEI转染效果更佳)。
3.用一定体积的Opti-MEM稀释质粒,小心混匀,记为A液;用相等体积的Opti-MEM稀释PEI,小心混匀,记为B液(质粒:PEI=1:2,W/W),室温静止5min。
