jetPRIME转染试剂说明
RNAi-Mate转染试剂操作手册说明书

目录号 C-01
产品说明 RNAi-Mate 转染试剂
规格 0.1mL
价格 ¥160
交货期限 2 个工作日
操作方法
一、体外转染方法 1. 细胞培养
可广泛应用于多种细胞系的DNA和siRNA转染。已成功地应用于多种代表各种来源的细胞类 型,包括 Hela(人宫颈癌细胞), MCF-7(人乳腺癌细胞), Hep3B(人肝细胞癌细胞), COS-7(猴肾细胞), Neuro-2a(鼠神经 母细胞瘤细胞),NIKS(人角质化细胞),C6(鼠神经胶质瘤细胞), DLD-1(人结肠癌细胞), NIH/3T3(鼠胚胎成纤维细 胞), HT-29(人结肠腺癌细胞), A549(人肺癌细胞), CHO-k1(仓鼠卵巢细胞)和293(腺病毒5 DNA转化的人胚胎 肾细胞), SVRbag4细胞。
3. 细胞出现明显死亡
问题 与细胞生存相关的关键基因被关闭
细胞状态欠佳
siRNA/RNAi-Mate(或DNA/RNAiMate) 复合物浓度过高
重新设计实验
建议
使用传代次数较低的细胞;细胞接种后在24小时内达 到70-90%,并在24小时内完成转染
siRNA/RNAi-Mate(或 DNA/RNAi-Mate)浓度过高 时,有时也会产生毒性
4.悬浮细胞转染程序 本程序适用于使用24孔板悬浮细胞的转染。选用生理状态良好的细胞对提高转染效率很重要。 siRNA(DNA)和DNA的用量和两者的比例可在推荐范围内适当调整。 4.1 转染的当天,收集细胞离心,重悬。 4.2 在50μl的DMEM(或Opti-MEM, 或其他无血清培养基)无血清培养基加入20pmol siRNA(或 0.8μg DNA), 柔和混匀。 4.3 混匀RNAi-Mate试剂,用50μl无血清的DMEM或Opti-MEM,或其他无血清培养基)稀释 1μl RNAi-Mate试剂(DNA转染时,则加入2μl RNAi-Mate试剂),轻轻混匀,室温放置5分钟。 4.4 将稀释好的siRNA和RNAi-Mate试剂混合;轻柔混匀,室温放置20分钟,以便形成siRNA/RNAiMate(或DNA/RNAi-Mate)复合物。 4.5 将100μl siRNA/RNAi-Mate(或DNA/RNAi-Mate)复合物加到含有细胞和培养基的培养板的孔 中,来回轻柔摇晃细胞培养板。 4.6 再加入400μL细胞悬浮液(细胞数量决定于细胞类型和转染后分析测试的时间)。 4.7 细胞在37℃温育24h-48h后进行转染后的其它步骤。如果细胞株比较敏感,孵育4-6小时后,除去 复合物,更换培养基。
罗氏转染试剂说明书

罗⽒转染试剂说明书X-tremeGENE HP DNA Transfection ReagentFor transient and stable transfection of eukaryotic cellsVersion 05Content version: May 2011Cat. No. 06 365 752 001Cat. No. 06 366 244 001Cat. No. 06 366 236 001Cat. No. 06 366 546 001Trial-pack 0.4 ml 1 ml 5 ×1 mlStore at –15 to –25°C1.What this Product DoesNumber of TestsUsing the standard procedure, 1 ml of X-tremeGENE HP DNA Trans-fection Reagent can be used to perform up to 10,000 transfections in 96-well plates.FormulationX-tremeGENE HP DNA Transfection Reagent is a proprietary blend of lipids and other components supplied in 80% ethanol, filtered through 0.2 ?m pore size membrane, and packaged in glass vials. It does not contain any ingredients of human or animal origin.Storage and StabilityStore X-tremeGENE HP DNA Transfection Reagent at –15 to –25°C, with the lid tightly closed. The reagent is stable until the expiration date printed on the label when stored under these conditions.L X-tremeGENE HP DNA Transfection Reagent remains fully func-tional even after repeated opening of the vial (at least five times over a two-month period), as long as the vial is tightly recapped and stored at -15 to -25°C.L Note that the shipping temperature of this product is differentfrom the storage temperature. These different temperatures will not affect product performance or product stability.Special HandlingN After removing the amount required, tightly close the vial with thelid immediately after use.N Always bring the vial to +15 to +25°C and mix X-tremeGENE HPDNA Transfection Reagent prior to removing the amount required vortexing for one second.N Do not aliquot X-tremeGENE HP DNA Transfection Reagent; storein the original glass vials.N Minimize the contact of undiluted X-tremeGENE HP DNA Trans-fection Reagent with plastic surfaces.N For use, the minimum amount of X-tremeGENE HP DNA Transfec-tion Reagent: DNA complex is 100 µl. Complex formation at lower volumes can significantly decrease transfection efficiency.N Do not use tubes or microplates made of polystyrene forX-tremeGENE HP Transfection Reagent : DNA complex prepara-tion. When not able to avoid polystyrene materials, make certain to pipet the transfection reagent directly into the serum-free medium (e.g., Opti-Mem).N Do not use siliconized pipette tips or tubes.Additional reagents and equipment required to perform transfection assays using X-tremeGENE HP DNA Transfection Reagent include:?Standard Laboratory Equipment .Standard cell culture equipment (e.g., biohazard hoods, incuba-tors)Standard pipettes and micropipettes Vortex mixerFor Plasmid PreparationPurified plasmid stock (0.1 – 2.0 µg/µl) in sterile TE (10 mM Tris, 1 mM EDTA, pH 8.0) buffer or sterile waterGenopure Plasmid Midi Kit* or Genopure Plasmid Maxi Kit* to prepare plasmidFor Verification of Vector Function Assay appropriately for transfected geneG-418 Solution* or Hygromycin B* (optional for stable transfec-tion experiments)For Transfection-Complex FormationOpti-MEM I Reduced Serum Medium or serum-free medium Sterile polypropylene tubes or round-bottom 96-well plates Growing CellsSelect subconfluent cultures in log phase for preparation of cell culturesQuantify cell number to reproducibly plate the same number of cells ApplicationX-tremeGENE HP DNA Transfection Reagent is a high performance transfection reagent, free of animal-derived components. Benefits of X-tremeGENE HP DNA Transfection Reagent include:Designed to transfect a broad range of eukaryotic cells, including insect cells, many cell lines not transfected well by other reagents, and hard-to-transfect cell lines (e.g., HT-1080, K-562, HepG2).Can be successfully used in a variety of applications, such as gene expression analysis and protein production using transiently trans-fected cells, generation of stable cell lines, expression of shRNA for gene knockdown studies, drug discovery programs, and target evaluation. Samples and detailed transfection protocols are avail-able at/doc/48879f4bad02de80d4d840ed.html .Produces minimal cytotoxicity or changes in morphology when ade-quate numbers of cells are transfected, eliminating the requirement to change media after adding the transfection complex.?Suitable for transient and stable transfection. Functions very well in the presence or absence of serum.For life science research only.Not for use in diagnostic procedures.Print2.How to Use this Product2.1Before You BeginRequired Amount of X-tremeGENE HP DNA Transfection ReagentTo optimize, first transfect a monolayer of cells that is 70 - 90% conflu-ent, using 1:1, 2:1, 3:1 and 4:1 ratios of microliter (?l) X-tremeGENE HP DNA Transfection Reagent to microgram (?g) DNA. A ratio of 3:1 of microliter (?l) X-tremeGENE HP DNA Transfection Reagent to micro-gram (?g) DNA has been shown to be optimal for many cell types.L Lower cell confluencies have also been tested successfully.The recommended starting concentration is a 3:1. For most cell types, these X-tremeGENE HP DNA Transfection Reagent to DNA ratios pro-vide excellent transfection efficiency.L Further optimization may increase transfection efficiency in your particular application. In addition to varying the ratio, other param-eters may also be evaluated, such as the amount of transfection complex added. For additional optimization guidelines, see Section 3, Troubleshooting and visit /doc/48879f4bad02de80d4d840ed.html .For best results, accurately determine the plasmid DNA concentra-tion using 260-nm absorption; estimates of DNA by measuring gel band density are not recommended. Determine DNA purity using a 260 nm/280 nm ratio (the optimal ratio is 1.8).Prepare the plasmid DNA solution using sterile TE (Tris/EDTA) buf-fer or sterile water at a concentration of 0.1 to 2.0 µg/µl. Use high quality DNA preparation kits to obtain endotoxin-free DNA.Cell Culture ConditionsMinimize intra- and inter-experimental variance in transfection effi-ciency using cells that are regularly passaged, proliferating well in a log-growth phase, and plated at a consistent density.For best results, accurately quantify cell concentration using a hematocytometer or automated system.Cells must be healthy and free of Mycoplasma.Cells should have a low passage number to achieve best results.Other Media AdditivesIn some cell types, antimicrobial agents (e.g., antibiotics and fungi-cides) commonly included in cell-culture media may adversely affect the transfection efficiency of X-tremeGENE HP DNA Transfection Reagent. If possible, exclude additives in initial experiments. Once high-efficiency conditions have been established, these components can be added back while monitoring transfection results. Cell growth and/or transfection efficiency may be affected by variations in serum quality and medium formulations.Verification of Vector FunctionOptimize transfection conditions using a known positive-control reporter gene construct before transfecting cells with a new vector construct:Determine transfection efficiency using a reporter gene assay, such as -Gal*, Luciferase*, or SEAP*.Sequence flanking vector insert regions to verify the integrity of your new construct.2.2Preparation of Cells for TransfectionAdherent Cells: Plate cells approximately 24 hours before transfec-tion making sure cells are at the optimal concentration in the appropri-ate cell culture vessel.Suspension Cells: Plate freshly passaged cells at optimal concentra-tion.2.3 Transfection ProcedureAllow X-tremeGENE HP DNA Transfection Reagent, DNA and diluent to equilibrate to +15 to +25°C. Briefly vortex theX-tremeGENE HP DNA Transfection Reagent vial.Dilute DNA with appropriate diluent (e.g., serum-free medium) to a final concentration of 1 µg plasmid DNA /100 µl medium (0.01 µg/µl). Mix gently.Place 100 µl of diluent, containing 1 µg DNA into each of four sterile tubes labeled 1:1, 2:1, 3:1, and 4:1.N Use a minimum of 100 µl of diluent. Lower volumes may significantly decrease transfection efficiency.L Use sterile tubes or tissue culture treated round-bottom, 96-well plates to produce the complex.Pipet the X-tremeGENE HP DNA Transfection Reagent (1, 2, 3, or 4 µl) directly into the medium containing the diluted DNA without coming into contact with the walls of the plastic tubes.Mix gently.N To avoid adversely affecting transfection efficiency, do not allow undiluted X-tremeGENE HP DNA Transfection Reagent to come into contact with plastic surfaces. Do notIncubate the transfection reagent:DNA complex for 15 min-utes at +15 to +25°C.L Some ratios and cell types may required longer incubation (up to 30 min). Determine this for your particular cell lineand the ratio used.Remove the culture vessel from the incubator. Removal of growth medium is not necessary. Add the transfection com-plex to the cells in a dropwise manner.L See Table 1 to determine component amounts corre-sponding to the surface area of the cell culture vesselused.Gently shake or swirl the wells or flasks to ensure even distri-bution over the entire plate surface. If available, use a rotatingplatform shaker for 30 seconds at low speed for mixing96-well plates.Once the transfection reagent: DNA complex has been addedto the cells, there is no need to replace with fresh medium (asmay be necessary with other transfection reagents)Following transfection, incubate cells for 18 – 72 hours before measuring protein expression. The duration of incubation will depend on many factors, including the transfected vector con-struct, the cell type being transfected, the cell medium, celldensity, and the type of protein being expressed. After theincubation period, measure protein expression using an assayappropriate for your system.Notes:L As with any experiment, include appropriate controls. Prepare cul-ture wells with cells that remain untransfected, cells with transfec-tion reagent alone, and cells with DNA alone.L For stable transfection experiments, the complex-containing medium should be left unchanged until the cells are passaged. At that time, include appropriate selection antibiotics (e.g., G 418 Solution or Hygromycin B).L To prepare transfection complexes for different-sized containers or parallel experiments, adjust component amounts correspond-ing to the surface area of the cell culture vessel used (see Table 1).L For ease-of-use when transfecting small volumes into 96-well plates containing 0.1 ml culture medium per well, prepare 100 µl of transfection complex, and then add 10 µl to each well (depend-ing on cell type).L The optimal ratio of transfection reagent to DNA, and the optimal total amount of complex, will depend on the cell line, cell density, day of assay, and gene expressed.L After performing the optimization experiment in which several dif-ferent ratios are tested, select a ratio in the middle of the plateau optimum for future experiments.Tab. 1: Guidelines for Preparing X-tremeGENE HP DNA Transfection Reagent: DNA Complex for Various Culture Vessel SizesCulture vessel Surface Areamedium (ml) Suggested amount of100 µl transfection complexto add toeach well (µl) DNA (µg)using 1:1or 4:1 RatioFinal amountof X-tremeGENE HP DNA Transfection Reagent (µl) using 1:1 Ratio Final amountof X-tremeGENE HP DNA Transfection Reagent (µl) using 4:1 Ratio 96-well plate(1 well)0.30.1100.10.10.4 48-well plate(1 well)1.00.3300.30.3 1.2 24-well plate(1 well)1.90.5500.50.52 12-well plate(1 well)(1 well)9.42200228 60-mm dish2155005520 10-cm dish55101000101040 T-25 flask2566006624 T-75 flask75202000202080 2.4TroubleshootingObservation Possible Cause RecommendationLow Transfection Efficiency Suboptimal X-tremeGENE HP DNA Transfec-tion Reagent : DNA ratioTitrate optimal X-tremeGENE HP DNA Transfection Reagent : DNAratio. Refer to the text in Section 2.1 “Before you begin”.Insufficient number of cells Determine optimal cell density for each cell type. For most cell types, 70– 90% confluence at transfection is optimal.X-tremeGENE HP DNA Transfection Reagent :DNA complexes did not form wellPrepare complexes in serum-free medium (e.g., Opti-MEM).Do not use siliconized pipet tips or tubes.Do not aliquot the X-tremeGENE HP DNA Transfection Reagent. Incubation time of transfection Determine the optimal incubation time (18 - 72 h). Optimal for most celltypes and plasmids is 24 – 48h.Inhibition by media components Some media components (e.g., polyanions) may influence the transfec-tion.Low volume of X-tremeGENE HP DNA Trans-fection Reagent : DNA complexThe minimum amount of X-tremeGENE HP DNA Transfection Reagentto DNA complex is 100 µl. Complex formation at lower volumes may sig-nificantly decrease the transfection efficiency; refer to the text in Section1, “Special Handling”.High Cytotoxicity Cell density not optimal For each cell type, the optimal density should be determined. For mostcell types, 70 - 90 % confluence at transfection is recommended, butother confluencies may increase cell viability.Cells are cultured in serum-free medium Transfection using X-tremeGENE HP DNA Transfection Reagent in cells cultured in serum-free medium is possible, however, toxicity may behigher when serum is absent.X-tremeGENE HP DNA Transfection Reagent : DNA complexes and cells not mixed well Add X-tremeGENE HP DNA Transfection Reagent dropwise to the cells. Gently rock the dish/plate back and forth and from side to side to evenly distribute the complexes.Plasmid preparation contaminated with endo-toxinTransfected protein is cytotoxic or is produced at high levels Reduced viability or slow growth rates may be due to high levels of pro-tein expression, with cellular metabolism directed toward production of the heterologous protein. Note that the expressed protein may also be cytotoxic at the expressed levels.Too much transfection complex for number of cells Increase the number of plated cells, and/or decrease the total amount of complex added to the cells.3.Additional Information on This ProductQuality ControlEach lot of X-tremeGENE HP DNA Transfection Reagent is tested using established quality control procedures. Functional AnalysisCells are transfected with a reporter gene vector DNA using X-tremeGENE HP DNA Transfection Reagent (ratio 3:1 µl/µg DNA). Reporter gene activity is monitored by chemiluminescent detection. Using a standard curve analysis method, total amounts of recombinant protein per well are measured to ensure levels that are within specifi-cation.4.ResultsCHO-K1 cells were transfected with a GFP encoding pcDNA3.1 plas-mid containing a CMV promoter with two different transfection reagents. CHO-K1 cells were observed under fluorescence and bright field microscopy at 10× magnification. Pictures were obtained using the Cellavista System 24 hours after transfection.Fig. 1:X-tremeGENE HP DNA Transfection Reagent (1:1 ratio)Fig. 2:Competitor transfection reagent (2:1 ratio)5.Supplementary InformationConventionsIn this document, the following symbols are used to highlight impor-tant information:Symbol DescriptionL Information Note:Additional information about the current topic or proce-dure.N Important Note:Information critical to the success of the procedure or useof the product.Text ConventionsTo make information consistent and understandable, the following textText Convention UseNumbered instructionslabeled ?, ? etc.Steps in a procedure that must be performedin the order listed.Changes to Previous VersionEditorial changesOrdering InformationRoche Applied Science provides a large selection of reagents and sys-tems for life science research. For a complete overview of relatedproducts and manuals, visit and bookmark our home page,/doc/48879f4bad02de80d4d840ed.html , and our Special Interest Site on transfection, /doc/48879f4bad02de80d4d840ed.htmlAsterisk *Denotes a product available from Roche AppliedScience.Product Pack Size Cat. No.Apoptosis and Cell Death ProductsCell ProliferationReagent WST-125 ml (2,500 tests)8 ml (800 tests)11 644 807 001***********Cytotoxicity DetectionKit PLUS (LDH)1 kit 400 tests in 96 wells1 kit 2,000 tests in 96 wells**********************Gene Knockdown ReagentX-tremeGENE siRNATransfection Reagent1 ml (400 transfections in a24-well plate)Mycoplasma Detection ReagentsMycoplasma DetectionKit1 kit (25 tests)11 296 744 001Mycoplasma PCR ELISA1 kit (96 reactions)11 663 925 910 Plasmid Isolation ProductsGenopure Plasmid MidiKit1 kit (for up to 20 prepara-tions)***********Genopure Plasmid MaxiKit1 kit (for up to 10 prepara-tions)***********Protease Inhibitor T ablets and Lysis Reagents cOmplete20 tablets in glass vials3 x 20 tablets in glass vials20 tablets in EASYpacks11 697 498 00111 836 145 001***********cOmplete, EDTA-free20 tablets in a glass vial3 x 20 tablets in glass vials20 tablets in EASYpacks11 873 580 001**********************cOmplete Lysis-M (formammalian cell lysis)1 kit (200 ml lysis reagentand 20 complete ProteaseInhibitor Cocktail Tablets)Roche Diagnostics GmbH Roche Applied Science 68298 Mannheim GermanyContact and SupportTo ask questions, solve problems, suggest enhancements or report new applications, please visit our Online Technical Support Site at:/doc/48879f4bad02de80d4d840ed.html /supportTo call, write, fax, or email us, visit the Roche Applied Science home page,/doc/48879f4bad02de80d4d840ed.html , and select your home country. Country- specific contact information will be displayed. Use the Product Search func-tion to find Pack Inserts and Material Safety Data Sheets. Regulatory DisclaimerFor life science research only.Not for use in diagnostic procedures.TrademarksCOMPLETE, GENOPURE, X-TREMEGENE, XCELLIGENCE, CASY, CEDEX, and CELLAVISTA are trademarks of Roche. E-PLATE and ACEA BIOSCIENCES are registered trademarks of ACEA Biosciences, Inc. in the US.Other brands or product names are trademarks of their respective holders.cOmplete Lysis-M,EDTA-free (for mammalian cell lysis) 1 kit (200 ml lysis reagent and 20 complete, EDTA-free Protease Inhibi-tor Cocktail Tablets)04 719 964 001Reporter Gene Assays CAT ELISA1 kit (192 tests)11 363 727 001-Gal Reporter Gene Assay,chemiluminescent 1 kit (500 assays, micro-plate format, 250 assays, tube format)11 758 241 001?-Gal ELISA 1 kit (192 tests)11 539 426 001hGH ELISA1 kit (192 tests)11 585 878 001Luciferase Reporter Gene Assay, high sensi-tivity200 assays 1,000 assays 11 669 893 00111 814 036 001SEAP Reporter Gene Assay,chemiluminescent 1 kit (500 assays, micro-plate format, or 250 assays, tube format)11 779 842 001Selection Antibiotics G-418 Solution 20 ml 100 ml 04 727 878 00104 727 894 001Hygromycin B1 g (20 ml)10 843 555 001Transfection Reagents X-tremeGENE 9 DNA Transfection Reagent0.4 ml 1 ml 5 x 1 ml 06 365 779 00106 365 787 00106 365 809 001Western Blotting ReagentsLumi-Light PLUS Western Blotting Kit(Mouse/Rabbit)1 kitLumi-Light PLUS Western Blotting Substrate100 ml(1,000 cm 2 membrane)12 015 196 001PVDF Western Blotting Membranes 1 roll(30 cm × 3.00 m)03 010 040 001Western Blocking Reagent, Solution100 ml(10 blots, 100 cm 2)6 × 100 ml(60 blots, 100 cm 2)11 921 673 00111 921 681 001Cellular Analysis RTCA Analyzer 05 228 972 001RTCA SP Station 05 229 057 001RTCA MP Station 05 331 625 001RTCA Control Unit 1.105 454 417 001E-Plate 96 6 Units 6 x 6 Units 05 232 368 001 05 232 376 001E-Plate VIEW 96 6 Units 6 x 6 Units06 472 451 001 06 472 460 001Cellavista BasicMagnification: 4x, 10x Illumination: Brightfield only***********Cellavista Medium Magnification: 4x, 10x, 20xIllumination: Brightfield and Fluorescence, UV, Blue, Green***********Product Pack Size Cat. No.Cellavista High End Magnification: 2x, 4x, 10x, 20x, 40xIllumination: Brightfield and Fluorescence,UV, Blue, Cyan, Green, Amber, Red***********Cedex XS Analyzer with Control Unit 05 926 432 001Cedex Smart Slide package15 x 8measurements05 650 801 001CASY Model TT 45, 60, 150 µm***********ProductPack Size Cat. No.。
上海杰美基因医药 GENMED QuikFusin 非脂质体细胞转染试剂盒产品说明书

GENMED SCIENTIFICS INC. U.S.A GMS10028 v.A GENMED QuikFusin非脂质体细胞转染试剂盒产品说明书(中文版)主要用途GENMED QuikFusin非脂质体细胞转染试剂是一种旨在通过高效低毒的非脂质体的脂质型介导载体,不用清除血清,能与目标DNA形成复合物,而转染进细胞的权威而经典的技术方法。
该技术由大师级科学家精心研制、成功实验证明的。
其适用于各种片段包括大片断DNA(12 kb以上)、寡聚核苷酸等的转染,可以转入进700多种类型的贴壁和悬浮培养细胞系(株)。
可以被用于稳定转染或暂态转染。
产品严格无菌,即到即用,无核酶污染,配比优化,操作简捷,性能稳定,转染效率明显。
技术背景QuikFusin为转染效率更高、细胞毒性更低的新型非脂质体的脂质型介导载体,经包装在任何条件下形成高效转染复合物。
无需更换培养基,穿膜效率高,转染速度快。
对细胞的负面影响最低。
产品内容GENMED转染液(Reagent A) 毫升GENMED介导液(Reagent B) 微升产品说明书 1份保存方式保存在4℃冰箱里,有效保证6月用户自备胰蛋白酶乙二胺四乙酸混合液(GMS12024):用于脱离细胞1.5毫升离心管:用于转染操作的容器完全细胞培养液(GMS12052):用于细胞转染后恢复使用的培养基实验步骤转染实验开始前,移取微升GENMED转染液(Reagent A)到无菌的1.5毫升离心管,然后加入微升GENMED介导液(Reagent B),用200微升枪头轻轻上下抽吸混匀,成为GENMED转染工作液。
然后进行下列操作。
1.准备一个25cm2细胞培养瓶的细胞(106细胞)2.在转染前一天,用胰蛋白酶乙二胺四乙酸混合液脱离细胞一次3.次日,准备1个无菌的1.5毫升离心管4.加入5微升用户需转染的DNA(9微克)5.用1毫升枪头移取微升含有GENMED转染液(Reagent A)和GENMED介导液(Reagent B)的转染工作液6.一滴一滴缓慢加入到DNA管中7.室温下孵育15分钟8.用1毫升枪头移取所有内容物9.直接加入到含有70%细胞铺满率的25cm2细胞培养瓶(内有5毫升细胞培养液,无需更换)10.轻轻摇动细胞培养瓶,使其分布均匀11.放进37℃细胞培养箱,孵育3小时12.小心抽掉含有转染液的细胞培养液13.加入5毫升用户自备的完全细胞培养液14.放进37℃细胞培养箱,孵育过夜15.如果稳态表达,小心抽掉培养液,加入用户自备的筛选培养液16.如果暂态表达,可以收集细胞后保存或即刻进行后续实验注意事项1.本产品为10次操作2.整个操作须无菌操作3.本产品转染106细胞4.本产品可以转染各种类型的细胞株5.本产品提供的成分配比优化6.DNA用量可以使用7至9微克7.转染期间不受血清影响8.建议转染时间不要超过3小时,否则可能造成细胞G1期停顿9.建议使用操作说明的进行转染。
jetPRIME 转染siRNA说明书
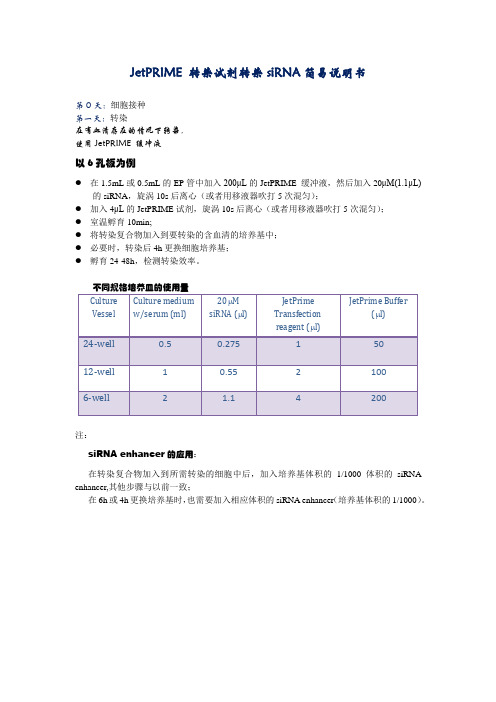
JetPRIME 转染试剂转染siRNA简易说明书
第0天:细胞接种
第一天:转染
在有血清存在的情况下转染,
使用JetPRIME缓冲液
以6孔板为例
●在1.5mL或0.5mL的EP管中加入200μL的JetPRIME 缓冲液,然后加入20μM(1.1μL)
的siRNA,旋涡10s后离心(或者用移液器吹打5次混匀);
●加入4μL的JetPRIME试剂,旋涡10s后离心(或者用移液器吹打5次混匀);
●室温孵育10min;
●将转染复合物加入到要转染的含血清的培养基中;
●必要时,转染后4h更换细胞培养基;
●孵育24-48h,检测转染效率。
不同规格培养皿的使用量
注:
siRNA enhancer的应用:
在转染复合物加入到所需转染的细胞中后,加入培养基体积的1/1000体积的siRNA enhancer,其他步骤与以前一致;
在6h或4h更换培养基时,也需要加入相应体积的siRNA enhancer(培养基体积的1/1000)。
各种转染试剂中文说明

FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温孵育10-15分钟。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100ul。
2.将3-6ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2ug的DNA溶液(0.02-2.0ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1ml不含血清和双抗的营养液洗涤一次,再加入2ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250ul无血清培养基(如OPTI-MEM I培养基)稀释4.0ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250ul无血清培养基(如OPTI-MEM I培养基)稀释10ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
加入2ml无血清配养基。
吉玛重组腺病毒操作手册说明书

如有疑问欢迎垂询上海电话:************-8009苏州电话:*************-8042E-mail:************************** E-mail:************************吉玛重组腺病毒操作手册GenePharma Recombinant Adenovirus Operation Manual2017 年 6 月如有疑问欢迎垂询上海电话:************-8009苏州电话:*************-8042E-mail:**************************E-mail:************************目录一、产品简介 (4)二、腺病毒载体优势 (4)三、生物安全性 (5)3.1安全性 (5)3.2实验室安全措施 (5)四、流程描述 (6)五、材料与仪器 (7)5.1实验材料 (7)5.1.1细胞株 (7)5.1.2质粒 (7)5.1.3试剂 (8)5.2实验耗材及仪器 (8)5.2.1耗材 (8)5.2.2仪器 (9)六、具体步骤 (9)6.1细胞培养 (9)6.1.1活细胞计数 (9)6.1.2细胞株的冻存 (9)6.1.3细胞复苏 (10)6.1.4细胞传代 (10)6.2病毒包装 (10)6.3病毒收集 (11)6.4病毒扩增 (12)6.5病毒纯化 (12)6.6病毒重悬和保存 (13)6.7病毒滴度检测 (13)七、使用方法 (14)如有疑问欢迎垂询上海电话:************-8009苏州电话:*************-8042E-mail:************************** E-mail:************************重组腺病毒在细胞水平使用 (14)7.1病毒滴度复核(有限稀释法测定) (14)7.2靶细胞感染预实验 (16)7.3正式试验 (18)八、运输和储存 (18)九、常见问题及解决方案 (19)十、吉玛案例 (20)十一、附录 (21)如有疑问欢迎垂询上海电话:************-8009苏州电话:*************-8042E-mail:************************** E-mail:************************一、产品简介腺病毒(Adenovirus,Adv)是一种线性双链DNA 病毒。
jetPRIME转染siRNA说明书

JetPRIME转染试剂转染siRNA简易说明书
第0天:细胞接种
第一天:转染
在有血清存在的情况下转染,
使用JetPRIME缓冲液
以6孔板为例
在1.5mL或0.5mL的EP管中加入200 ^L勺JetPRIME缓冲液,然后加入20卩M(1.1卩L)的siRNA,旋涡10s后离心(或者用移液器吹打5次混匀);
加入4^L的JetPRIME试剂,旋涡10s后离心(或者用移液器吹打5次混匀);
室温孵育10mi n;
将转染复合物加入到要转染的含血清的培养基中;
必要时,转染后4h更换细胞培养基;
孵育24-48h,检测转染效率。
注:siRNA enhancer 的应用:
在转染复合物加入到所需转染的细胞中后,加入培养基体积的1/1000体积的siRNA
en ha ncer其他步骤与以前一致;
在6h或4h更换培养基时,也需要加入相应体积的siRNA enhancer(培养基体积的1/1000)。
EndoFectinTM-Max 转染试剂用户手册说明书

EndoFectin TM -Max 转染试剂高效转染核酸到哺乳动物细胞产品编号:EF003 / EF004 / EF003T 包装规格:1 mL / 3 mL / 100 μL储存条件:4℃~8℃密闭保存,可保持稳定至少12个月,常温运输。
产品概述EndoFectin TM -Max 转染试剂是以脂质体转染为原理的转染试剂,它能与核酸形成复合物,并使该复合物进入哺乳动物细胞。
EndoFectin TM -Max 转染试剂广泛适用于常见细胞系,如HEK-293、HEK293T 、CHO-K1、Hep G2、Hela 、MCF-7、NIH/3T3和A549等。
即使在有血清存在的情况下,该试剂仍能高效将核酸导入细胞。
GeneCopoeia 公司提供的EndoFectin TM -Max 转染试剂具有如下优点: y 转染效率更优良 y 细胞毒性低y 适用于多种细胞系的转染操作,操作简便y 与含血清的培养基相兼容,转染前不需去除细胞培养液或血清,转染后不需清洗细胞质量控制每批次EndoFectin TM -Max 转染试剂均经过转染测试。
将eGFP 表达质粒(GeneCopoeia Cat.No. EX-EGFP-M02)用EndoFectin TM -Max 转染试剂转入亚融合状态的HEK-293细胞,转染24小时后,超过95%的细胞表达eGFP 。
注意事项使用高质量的质粒:请务必使用高质量的转染级无内毒素质粒。
可通过260 nm 光吸收测定DNA 浓度,并以260 nm / 280 nm 比值确定DNA 纯度(比值应在1.8~2.0的范围内)。
如有可能,请通过琼脂糖凝胶电泳检测质粒的完整性。
保证细胞状态:请使用适当保存和经常传代的健康细胞,并确保培养基无细菌、真菌或支原体污染。
如果细胞是近期复苏的液氮冻存细胞,请在转染前至少传代两次。
实验材料y EndoFectin TM -Max 转染试剂、含目的基因的DNA 质粒y 无蛋白细胞培养液(如Opti-MEM I TM ,来自Life Technologies . 货号:31985-088) y 培养至70~80%汇合度的目的细胞条件摸索在进行正式转染前,推荐以EndoFectin TM -Max 转染试剂摸索目的细胞的最佳转染条件。
Agilent GeneJammer 转染试剂,货号 204131 - 化学品安全说明书

GeneJammer Transfection Reagent, Part Number 204131*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818GeneJammer Transfection Reagent, Part Number 204131化学品的推荐用途和限制用途204131部件号:安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:GeneJammer 转染试剂,货号 204131推荐用途:有关环境保护措施,请参阅第 12 节。
物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述液体。
无资料。
无资料。
物理状态:颜色:气味:GHS危险性类别警示词:危险危险性说明::防范说明预防措施:P241 - 使用防爆的电气、通风、照明设备。
P242 - 使用不产生火花的工具。
P243 - 采取行动防止静电放电。
P233 - 保持容器密闭。
P264 - 作业后彻底清洗。
标签要素象形图易燃液体 - 类别 2严重眼损伤/眼刺激 - 类别 2AP305 + P351 + P338 - 如进入眼睛: 用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
P337 + P313 - 如仍觉眼刺激: 求医要么就诊。
安全储存:废弃处置:P501 - 处置内装物/容器按照地方/区域/国家/国际规章。
物理和化学危险高度易燃液体和蒸气。
健康危害::与物理,化学和毒理特性有关的症状皮肤接触食入吸入没有具体数据。
没有具体数据。
Polyplus公司转染试剂FAQ(常见问题解答)中文版-朱艳梅2012.8

请 浏 览 Polyplus 网 站 的 细 胞 转 染 数 据 库 , 在 这 里 可 以 找 到 许 多 细 胞 的 具 体 转 染 条 件 (/technical-resources/cell-line-database/) 。 悬浮细胞 jetPRIME®推荐用于悬浮细胞的 DNA 转染吗?
悬浮细胞,例如 T 和 B 淋巴细胞是众所周知的难转染 DNA 的细胞,无论使用哪种化学方法的试剂。 因此,我们通常推荐电转或病毒转染核酸到悬浮细胞。 植物细胞 jetPRIME®是否测试过可以用于植物细胞的转染?
植物细胞富含纤维素的膜阻止了 jetPRIME®/DNA 复合物进入细胞。甚至在脱去纤维素的原生质体阶段, 复合物也无法穿透进入细胞质。 HUVEC(人脐静脉内皮细胞) jetPRIME®对 HUVEC 的 DNA 转染是否有效?
INTERFERin® 仅用于体外转染。对于动物的 siRNA 转染,我们推荐使用 in vivo-jetPEI®,其已成功
用于多种动物的 siRNA 和寡核苷酸的转染。更多信息,请参见 in vivo-jetPEI® web page。
保存 INTERFERin®如何保存? INTERFERin®应该盖紧盖子放在 4°C。 冷冻 我不小心将 INTERFERin®试剂冻存在-20°C。它是否仍有效,可否继续使用?
jetPRIME®可以耐受偶尔的冻融,而不影响转染效率。然而,对于长期保存来说,我们建议分装并储 存在 4°C,以确保试剂长时间有效。 有效期 jetPRIME®试剂可以使用多长时间?
保存适当时,jetPRIME® cat#114-01 (0.1 ml)可以保存 6 个月。其他规格的 jetPRIME®至少可以保存 一年。
转染试剂使用说明书

2. 3).
配制转染工作液: ( 6 孔板或 35 mm 平皿, 2 ml 培养液) 取 5~8μ g DNA (起始用量 5μ g) ,加入稀释液中至总体积为 100μ l,轻轻混匀,室 温放置。
4).
先将 GenFectin TM 涡旋振荡混匀。取 GenFectinTM 1~4μl(起始用量 2μl,) ,加入稀 释液中至总体积为 100μl,轻轻混匀,室温放置 5 分钟。
5).
将稀释的 GenFectinTM 逐滴加入稀释的 DNA 溶液中,轻轻混匀,所得的转染工作 液在室温放置 15 分钟。
6).
将转染工作液轻轻混匀,逐滴加入 2 ml 培养液中,轻轻混匀培养液,置 37℃,5% CO 2 培养。
3. 细胞后续处理: 7). 24~48 小时后,观察或收取细胞。
-4-
8).
稳定转染时,于转染后 24~48 小时消化细胞分至 3~5 个培养皿中,加适当浓度的 相应抗生素(如 G418)筛选。
建议的起始转染条件 : 培养容器
96 孔板 24 孔板 6 孔板 35mm 培养皿 60mm 培养皿
转染前一天 接种细胞数
1-1.510 个 0.5-1 10 个 2-4 10 个 2-4 10 个 4-6 105 个
5 5 5 4
转染时 培养液体积
0.1 ml 0.5 ml 2 ml 2 ml 4 ml
DNA 用量 与稀释后体积
0.25 μg 1.25 μg 5 μg 5 μg 10 μg 5 μl 25 μl 100 μl 100 μl 200 μl
GenFectin 量 与稀释后体积
0.1 μl 0.5 μl 2 μl 2 μl 4 μl 5 μl 25 μl 100 μl 100 μl 200 μl
转染试剂的中文详解

各种转染试剂的中文转染方法2014-05-06丁香园FuGENE6(Roche)转染步骤:转染前一天将细胞分至培养板,转染当天细胞应50-80%融合。
将细胞以1-3×105/2 ml接种于6孔板后孵育过夜将达到如此密度。
将FuGENE6 Reagent在室温静置10-15分钟以平衡到室温。
使用之前将FuGENE6颠倒混匀一下。
1.在PCR管中加入不含血清和双抗的营养液以稀释FuGENE6,直至总体积到100 ul。
2.将3-6 ul FuGENE6 Reagent直接加入营养液,轻弹管壁混合。
3.加入1-2 ug的DNA溶液(0.02-2.0 ug/ul),轻弹管壁混合。
4.室温孵育20分钟。
5.将6孔板中的旧营养液吸出,加入约1 ml不含血清和双抗的营养液洗涤一次,再加入2 ml不含血清和双抗的营养液。
6.将转染复合物加入细胞,混匀使之均匀分布。
7.3-8小时后,加入血清或换成含血清的营养液。
Lipofectamine 2000(Invitrogen)转染试剂转染步骤(6孔板):1.转染前一天,胰酶消化细胞并计数,细胞铺板,使其在转染日密度为90-95%。
细胞铺板在2 ml含血清,不含抗生素的正常生长的培养基中。
2.对于每孔细胞,使用250 ul无血清培养基(如OPTI-MEM I培养基)稀释4.0 ugDNA,轻轻混匀。
3.使用前将Lipofectamine 2000转染试剂轻轻混匀,用250 ul无血清培养基(如OPTI-MEM I培养基)稀释10 ul Lipofectamine 2000转染试剂,轻轻混匀。
Lipofectamine 2000稀释后,在5分钟内同稀释的DNA混合(<30分钟)。
NOTE:若使用DMEM培养基,则需在5分钟内同稀释的DNA混合。
4.混合稀释的DNA(第二步)和稀释的Lipofectamine 2000(第三步)。
室温放置20分钟。
5.(optional)将6孔板中的旧营养液吸出,用无血清培养基清洗两次。
转染试剂使用注意事项

转染试剂使用注意事项1.细胞密度:转染DNA(质粒)时,细胞密度为80-100%,转染RNA(siRNA或miRNA)时,细胞密度为60-80%,具体细胞密度必须结合考虑核酸种类、转染试剂种类和细胞生长密度极限;2.DNA用量:0.5-1ug/孔(以24孔板为例),具体用量根据转染效率检测实验确定;3.RNA用量:10-30 pmol/孔(以24孔板为例),具体用量根据转染效率检测实验确定;4.转染试剂的用量:转染试剂说明书推荐了一个使用范围,具体用量根据转染效率检测实验确定;5.根据转染效率检测实验确定核酸的用量及转染试剂的用量(核酸与转染试剂的比例关系),转染效率检测实验一般在24孔板中进行,其他格式的培养器皿请根据底面积的比例(表1)进行计算;6.转染试剂使用前才从4℃中取出,取用前,先短暂离心(转速达到3000RPM时即可停止),然后放在涡旋振荡器上点动混匀三次,每次持续1秒,混匀后短暂离心(转速达到3000RPM时即可停止),上述措施是为了混匀转染试剂,保证使用效果,使用后立即放回4℃保存;7.核酸和转染试剂分别用Opti-MEM(这是专用的转染稀释培养基,如果没有Opti-MEM,可以用细胞对应的基础培养基代替)稀释,稀释的核酸可以通过弹匀,吹打均匀,颠倒混匀或涡旋点动混匀(三次,每次持续1秒),稀释的转染试剂可以通过弹匀,颠倒混匀或涡旋点动混匀(三次,每次持续1秒),不可使用吹打均匀;混匀后均需短暂离心;8.把稀释的核酸和稀释的转染试剂复合混匀时,应该把稀释的核酸加入稀释的转染试剂中(顺序不可调转);加入后立即进行(不要耽搁)弹匀或颠倒混匀(稀释的核酸和稀释的转染试剂一旦复合,转染复合物的形成立即启动,如果不及时混匀,所形成的转染复合物的效率就会很低),先不进行短暂离心,待所有的管子复合完毕后,在一起进行涡旋点动混匀三次,每次持续1秒,然后短暂离心;9.转染复合物孵育形成时尽量避光室温或37℃放置;10.如果进行RNA转染时,尽量使用无酶及灭菌处理的耗材;11.Opti-MEM用量参照转染说明书建议的用量;表1:资料仅供参考!!!。
转染技术要点及转染试剂的选择

[watermark]转染技术要点及转染试剂的选择转染技术是指将外源分子如DNA,RNA等导入真核细胞的技术,它是研究基因表达调控,突变分析等的常规工具。
随着功能研究的兴起,其应用越来越广泛。
以下就向大家介绍一些转染的技术要点及市面上主要的转染试剂类型的选择。
常规转染技术可分为两大类,一类是瞬时转染,一类是稳定转染(永久转染)。
前者外源DNA/RNA不整合到宿主染色体中,因此一个宿主细胞中可存在多个拷贝数,产生高水平的表达,但通常只持续几天,多用于启动子和其它调控元件的分析。
一般来说,超螺旋质粒DNA转染效率较高,在转染后24-96小时内(依赖于各种不同的构建)分析结果,常常用到一些报告系统如荧光蛋白,β半乳糖苷酶等来帮助检测。
后者也称稳定转染,外源DNA既可以整合到宿主染色体中,也可能作为一种游离体(episome)存在。
尽管线性DNA比超螺旋DNA转入量低但整合率高。
外源DNA整合到染色体中概率很小,大约1/104转染细胞能整合,通常需要通过一些选择性标记,如来氨丙基转移酶(APH;新霉素抗性基因),潮霉素B磷酸转移酶(HPH),胸苷激酶(TK)等反复筛选,得到稳定转染的同源细胞系。
转染效率收多种因素影响,主要因素有下面几个:1. 细胞培养物健康的细胞培养物是成功转染的基础。
不同细胞有不同的培养基,血清和添加物。
低的细胞代数(<50)能确保基因型不变。
高的转染效率需要一定的细胞密度,一般的转染试剂都会有专门的说明。
推荐在转染前24小时分细胞,这将提供正常细胞代谢,增加对外源DNA摄入的可能。
一定要避免细菌,支原体或真菌的污染。
2. 血清大多数培养基在使用前需要加血清。
胎牛血清(FCS)经常用到,便宜一点的有马或牛血清。
通常的,血清是一种包含生长因子及其它辅助因子的不确切成分的添加物,对不同细胞的生长作用有很大的差别。
血清质量的变化直接影响转染效率。
因此在转染前建议先测(转右)试出对细胞生长良好的血清批号,转染时用同一批号的血清,并同时做负对照(不加转染试剂及外源DNA)以测试细胞生长是否正常。
jetPRIME 转染siRNA说明书

JetPRIME 转染试剂转染siRNA简易说明书
第0天:细胞接种
第一天:转染
在有血清存在的情况下转染,
使用JetPRIME缓冲液
以6孔板为例
●在1.5mL或0.5mL的EP管中加入200μL的JetPRIME 缓冲液,然后加入20μM(1.1μL)
的siRNA,旋涡10s后离心(或者用移液器吹打5次混匀);
●加入4μL的JetPRIME试剂,旋涡10s后离心(或者用移液器吹打5次混匀);
●室温孵育10min;
●将转染复合物加入到要转染的含血清的培养基中;
●必要时,转染后4h更换细胞培养基;
●孵育24-48h,检测转染效率。
不同规格培养皿的使用量
注:
siRNA enhancer的应用:
在转染复合物加入到所需转染的细胞中后,加入培养基体积的1/1000体积的siRNA enhancer,其他步骤与以前一致;
在6h或4h更换培养基时,也需要加入相应体积的siRNA enhancer(培养基体积的1/1000)。
上海常用转染试剂配方

上海常用转染试剂配方
转染试剂是在细胞培养研究中必不可少的试剂。
它将核酸或蛋白质带入目标细胞中,以达到研究目的。
本文将介绍上海常用的几种转染试剂配方。
1. Lipofectamine 2000转染试剂
Lipofectamine 2000是一种常用的脂质体转染试剂,其用途广泛且转染效率高。
其配方如下:
无血清培养基:500μL
要转染的DNA:2μg(根据实验需要调整)
将Lipofectamine 2000试剂与无血清培养基混合,放置5分钟,再加入要转染的DNA 液体,混合后放置15分钟,滴加在细胞上。
2. PEI转染试剂
聚乙烯亚胺(PEI)是一种阳离子高分子,由于其能够形成与DNA负电荷的缔合物,使其成为一种有效的转染试剂。
PEI转染试剂可用于转染细胞以获得较高的转染效率。
其配方如下:
PEI试剂:4μg/μL,将其与等体积的无血清培养基混合。
3. PolyJet转染试剂
PolyJet是一种高效、无毒、具有低细胞损伤的DNA转染试剂。
其配方如下:
4. FuGENE HD转染试剂
细胞的转染操作应该注意以下几点:
1. 转染时间应该控制在半小时至1小时内,过长或过短都会对转染效率造成影响。
2. 转染的DNA浓度应该适当,过高的DNA浓度会对细胞产生毒性。
3. 转染试剂与DNA的混合比例也应该适当,过多或过少都会导致转染效率降低。
总之,合理选择转染试剂和配方,并严格按照操作步骤进行转染可以提高实验的可靠性和成功率。
三种阳离子转染试剂对小鼠血管瘤内皮细胞转染效率及细胞毒性的影响

三种阳离子转染试剂对小鼠血管瘤内皮细胞转染效率及细胞毒性的影响周晓彤;沈振亚;于曙东;余云生;叶文学;黄浩岳;焦鹏;滕小梅【期刊名称】《江苏医药》【年(卷),期】2008(34)3【摘要】目的从三种常用的阳离子转染试剂中筛选出对小鼠血管瘤内皮细胞(EOMA)有较高转染效率和较低细胞毒性的转染试剂.方法以含有增强型绿色荧光蛋白(EGFP)报告基因的真核表达载体pEGFP-N1为报告基因,以阳离子脂质体LipofectamineTM2000、LipofectamineTM PLUS和阳离子聚合物JetPEITM为转染试剂,按照转染试剂盒的说明优化其转染条件,分别转染EOMA细胞,24 h后在荧光显微镜下计数阳性细胞率、MTT法检测各转染条件下对EOMA的细胞毒性.结果经优化转染条件,LipofectamineTM PLUS和JetPEITM转染的细胞中阳性细胞的最高比例分别为45%和47%,LipofectamineTM2000转染的细胞中荧光蛋白表达的比例最高(>80%),Lipofecta-mineTM2000试剂在最高转染效率的剂量下仍然保证了80%的细胞存活率.结论阳离子脂质体LipofectamineTM2000对小鼠EOMA细胞有较高的转染效率和较低的细胞毒作用.【总页数】3页(P254-256)【作者】周晓彤;沈振亚;于曙东;余云生;叶文学;黄浩岳;焦鹏;滕小梅【作者单位】221002,徐州医学院附属医院心胸外科;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室;苏州大学附属第一医院心血管外科器官移植研究室【正文语种】中文【中图分类】R31【相关文献】1.三种转染试剂对PEF细胞与BHK细胞转染效率的比较 [J], 张文智;李亚;王鹏雁;陈创夫2.多聚胺阳离子脂质体转染效率及细胞毒性的评价 [J], 孙瑞琳;金发光;吴道澄;吴红;刘斌;南鹏娟;张颖;温德升3.自制多聚胺阳离子脂质体转染效率及细胞毒性的评价 [J], 孙瑞琳;金发光;吴道澄;吴红;刘彬;温德升4.三种阳离子脂质体介导血管内皮细胞基因转染效率的比较 [J], 童仁联;杨黎;张金明;邹海燕;张璇;梁佩红;戴丽冰;李叶扬5.三种转染试剂对RSC96细胞miRNA转染效率及细胞毒性影响的比较 [J], 刘玉璞;国海东;邵水金因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Polyplus-transfection S.A. - Bioparc - 850 Bd S. Brant - 67400 Illkirch - France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USAjetPRIME®in vitro DNA & siRNA transfection reagentPROTOCOLDESCRIPTIONjetPRIME® is a novel powerful molecule based on a polymer formulation manufactured at Polyplus-transfection®. jetPRIME® ensures effective and reproducible DNA and siRNA transfection into mammalian cells. jetPRIME® is extremely efficient on a wide variety of cell lines. This powerful reagent only requires low amounts of nucleic acid per transfection, hence resulting in very low cytotoxicity .1Transient DNA transfection protocol (2)1.1 Cell seeding .......................................................................................................................................... 2 1.2 DNA transfection protocol ................................................................................................................... 2 1.3 Virus production in adherent cells ....................................................................................................... 5 1.4Optimization guidelines (5)2 siRNA transfection protocol (6)2.1 Cell seeding .......................................................................................................................................... 6 2.2 siRNA transfection protocol .. (7)3 DNA & siRNA cotransfection protocol (7)3.1 Cell seeding .......................................................................................................................................... 7 3.2 DNA & siRNA cotransfection protocol . (8)4 Transfection of CRISPR/Cas9 ..................................................................................... 95 Stable DNA transfection ............................................................................................. 96 Troubleshooting ....................................................................................................... 10 7Product information (11)}CPT114vJ (March 2015)2jetPRIME® DNA & siRNA Transfection Reagent1 TRANSIENT DNA TRANSFECTION PROTOCOL1.1 CELL SEEDINGFor optimal DNA transfection conditions, we recommend using cells which are 60 to 80% confluent at the time of transfection. Typically, for experiments in 6-well plates, 200 000 cells are seeded per well in 2 ml of cell growth medium 24 h prior to transfection. For other culture formats, refer to Table 1.jetPRIME® is stable in presence of serum and antibiotics therefore you may use serum and antibiotic containing medium during the entire experiment.Table 1. Recommended number of cells to seed the day before transfection.1.2 DNA TRANSFECTION PROTOCOLThe following conditions are given per well of a 6-well plate. For other culture format, please refer to Table 2.1. Dilute 2 µg DNA into 200 µl jetPRIME® buffer (supplied). Mix by vortexing.2. Add 4 µl jetPRIME®, vortex for 10 s, spin down briefly.3. Incubate for 10 min at RT.4. Add 200 µl of transfection mix per well drop wise onto the cells in serum containing medium, and distribute evenly.5. Gently rock the plates back and forth and from side to side.6. If needed, replace transfection medium after 4 h by cell growth medium and return the plates to the incubator.7. Analyze after 24 h or later.Polyplus-transfection S.A. - Bioparc - 850 Bd S. Brant 67400 Illkirch France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USA3jetPRIME® DNA & siRNA Transfection ReagentTable 2. DNA transfection guidelines according to the cell culture vessel per well* Prepare a master mix of minimum 50 µl to allow accurate pipetting and homogenous preparation of the complexesNOTE: jetPRIME® buffer must be used for successful transfection.Browse our cell transfection database to find the optimized conditions according to your cell line./technical-resources/cell-line-database/}CPT114vJ (March 2015)4jetPRIME® DNA & siRNA Transfection ReagentDNA Transfection in 6-well platesPolyplus-transfection S.A. - Bioparc - 850 Bd S. Brant 67400 Illkirch France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81 Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USA 5jetPRIME® DNA & siRNA Transfection Reagent 1.3VIRUS PRODUCTION IN ADHERENT CELLSjetPRIME® is ideal for virus production, especially retrovirus, AAV and lentivirus, in adherent cells (ex: HEK-293T). For cotransfection of multiple plasmids, the total DNA amount per well/plate should not exceed the DNA amount indicated in Table 2. The ratio to use for each plasmid depends on the size of the plasmids, the plasmid constructs and the desired expression level of each plasmid. Please adjust the ratios according to your application. Each plasmid should represent at least 10% of the total DNA amount per well/plate.The following conditions are given per 100 mm dish. For other culture format, please refer to Table 2. For optimization, please refer to Table 3.1.Dilute 10 µg total DNA amount into 500 µl jetPRIME®buffer (supplied). Mix by vortexing.2.Add 20 µl jetPRIME®, vortex for 10 s, spin down briefly.3.Incubate for 10 min at RT.4.Add 500 µl of transfection mix per dish drop wise onto the cells in serum containing medium,and distribute evenly.5.Gently rock the dish back and forth and from side to side.6.Eventually replace transfection medium after 4 h by cell growth medium and return the dish tothe incubator.7.Incubate 24 to 72 h and proceed to virus purification and titration.1.4OPTIMIZATION GUIDELINESTransfection conditions should be optimized for each tested cell line according to the conditions detailed below (Table 3). Specific conditions for many cell lines can be found in our cell transfection database, following this link: /technical-resources/cell-line-database/You may adjust the volume of reagent and/or the amount of DNA. The volume of jetPRIME® may range between 2 to 3 µl per µg of DNA depending on the transfected cell line (1:2 and 1:3 DNA to jetPRIME®ratio (w/v)). The amount of DNA may range between 0.5 X and 1.5 X, X being the amount indicated in Table 2.Due to the high performance of jetPRIME® reagent (onto HeLa and HEK-293 cells for example), you may decrease the amount of plasmid DNA down to 0.5 X (Table 3).}CPT114vJ (March 2015)6jetPRIME® DNA & siRNA Transfection ReagentTable 3: Transfection optimization guidelines*Prepare a master mix of minimum 50 µl to allow accurate pipetting and homogenous preparation of thecomplexes.2 siRNA TRANSFECTION PROTOCOL2.1 CELL SEEDINGFor optimal siRNA transfection conditions, we recommend using cells which are 50% confluent at the time of transfection. Typically, for experiments in 6-well plates, 100 000 to 150 000 cells are seeded per well in 2 ml of growth medium 24 h prior to transfection. For other culture formats, refer to Table 4.jetPRIME® is stable in the presence of serum and antibiotics therefore you may use serum and antibiotic containing medium during the entire experiment.Table 4. Recommended number of cells to seed the day before transfection.Polyplus-transfection S.A. - Bioparc - 850 Bd S. Brant 67400 Illkirch France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81 Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USA 7jetPRIME® DNA & siRNA Transfection Reagent 2.2s iRNA TRANSFECTION PROTOCOLFor optimal siRNA-mediated silencing, we recommend using 10 - 50 nM siRNA.The following conditions are given per well in a 6-well plate. For other culture format, please refer to Table 5.1.Dilute 22 to 110 pmoles siRNA (for a final concentration of 10 to 50 nM per well) into 200 µl ofjetPRIME® buffer. Mix by pipeting up and down.2.Add 4 µl jetPRIME® reagent, vortex for 10 s, spin down briefly.3.Incubate for 10 to 15 min at RT.4.Add the transfection mix to the cells in serum containing medium drop wise.5.Gently rock the plate back and forth and return the plate to the incubator.6.If needed, replace transfection medium by cell growth medium 24 h after transfection and analyzeas required.Table 5. siRNA transfection guidelines according to the cell culture vessel.3DNA & siRNA COTRANSFECTION PROTOCOL3.1CELL SEEDINGFor optimal cotransfection conditions, we recommend using cells which are 60 to 80% confluent at the time of transfection. Typically, for experiments in 6-well plates, 150 000 to 250 000 cells are seeded per well 24 h prior to transfection. For other culture formats, refer to Table 1.jetPRIME® is stable in the presence of serum and antibiotics therefore you may use serum and antibiotic containing medium during the entire experiment.}CPT114vJ (March 2015)8jetPRIME® DNA & siRNA Transfection Reagent3.2 DNA & s i RNA COTRANSFECTION PROTOCOLFor DNA/siRNA cotransfection experiments, we recommend using 2 µg DNA and 10 to 50 nM siRNA per well in a 6-well plate.The following conditions are given per well of a 6-well plate. For other culture formats, please refer to Table 6.1. Dilute 2 µg of DNA and 22 to 110 pmoles siRNA (final concentration: 10 to 50 nM) into 200 µl ofjetPRIME® buffer. Mix by pipeting up and down. 2. Add 4 µl jetPRIME® reagent, vortex for 10 s, spin down briefly. 3. Incubate for 10 to 15 min at RT.4. Add the transfection mix to the cells still in serum containing medium drop wise.5. Gently rock the plate back and forth and return the plate to the incubator.6. If needed, replace transfection medium by cell growth medium 24 h after transfection and analyzeas required.NOTE: if you aim to silence the transfected plasmid with the transfected siRNA, then reduce the amount of DNA to 25% of the quantity indicated in Table 6 (for example: reduce to 500 ng DNA per well of a 6-well plate instead of 2 µg DNA)The amount of plasmid DNA and the volume of jetPRIME® may be optimized according to Table 3.Table 6. DNA & siRNA cotransfection guidelines according to the cell culture vessel.Polyplus-transfection S.A. - Bioparc - 850 Bd S. Brant 67400 Illkirch France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81 Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USA 9jetPRIME® DNA & siRNA Transfection Reagent4TRANSFECTION OF CRISPR/Cas9jetPRIME® is well-suited for gene editing using the CRISPR/Cas9 technology which can involve transient transfection of different types of nucleic acids into mammalian cells:-One single DNA plasmid (one expression vector comprising sequences for both Cas9 and gRNA), -Multiple DNA plasmids (separate plasmids coding for Cas9, gRNA and potentially a sequence to be inserted),- A mix of one or two DNA plasmids and a RNA molecule (the gRNA).For cotransfection of multiple nucleic acids, the total DNA or nucleic acid amount per well (or plate) should not exceed the DNA amount indicated in Table 2. The optimal ratio of each nucleic acid in the transfection mix depends on the plasmid size, the constructs and the desired expression level for each nucleic acid. Please adjust the ratios according to your application. Each plasmid or nucleic acid should represent at least 10% of the total nucleic acid amount per well/plate.The following conditions are given per well of a 6-well plate. For other culture format, please refer to Table 2. For optimization, please refer to Table 3.1.Dilute 2 µg total nucleic acid amount into 200 µl jetPRIME®buffer (supplied).Mix by vortexing.2.Add 4 µl jetPRIME®, vortex for 10 s, spin down briefly.3.Incubate for 10 min at RT.4.Add the transfection mix to the well drop wise onto the cells in serum containing medium, anddistribute evenly.5.Gently rock the dish back and forth and from side to side.6.Eventually replace transfection medium after 4 h by cell growth medium and return the plate tothe incubator.5STABLE DNA TRANSFECTIONjetPRIME® is suitable for stable DNA transfection.Perform stable transfection in 6-well plates, 60 mm or 100 mm dishes.1.If needed, linearize plasmid DNA construct encoding for antibiotic selection.2.Perform transfection using the standard protocol described in Section 1.2.3.Start antibiotic selection 24 to 48 h after transfection.4.Maintain antibiotic selection as long as required.5.Check for integration of the plasmid DNA or stable expression of your protein of interest.} CPT114vJ (March 2015)10jetPRIME® DNA & siRNA Transfection Reagent6 TROUBLESHOOTINGPolyplus-transfection S.A. - Bioparc - 850 Bd S. Brant 67400 Illkirch France - Phone: +33 3 90 40 61 80 - Fax: +33 3 90 40 61 81 Polyplus-transfection Inc. - 1251 Ave of the Americas - 34th fl. - New-York - NY 10020 - USA 11jetPRIME® DNA & siRNA Transfection Reagent7PRODUCT INFORMATION7.1ORDERING INFORMATION7.2ADDITIONAL BUFFERjetPRIME®reagent is provided with an optimized sterile buffer (jetPRIME®buffer). This buffer must be used to ensure successful transfection experiments. The 5X concentrated buffer provided with catalogue number 114-75C needs to be diluted 1:5 in sterile ddH2O before use.7.3CONTENT1.5 ml of jetPRIME®transfection reagent is sufficient to perform up to 1500 transfections in 24-well plates or 375 transfections in 6-well plates.7.4REAGENT USE AND LIMITATIONSFor research use only. Not for use in humans.7.5QUALITY CONTROLEvery batch of jetPRIME® reagent is tested by DNA transfection of HeLa cells with a GFP-expressing plasmid.7.6FORMULATION AND STORAGEjetPRIME®and its buffer are shipped at room temperature but should be stored at 4°C upon arrival to ensure long term stability. jetPRIME®, as guaranteed and indicated on the Certificate of Analysis, is stable for 6 months (114-01) to at least one year (other packaging sizes) when stored appropriately.Polyplus-transfection® has been awarded ISO 9001 Quality Management System Certification since 2002, which ensures that the company has established reliable and effective processes for manufacturing, quality control, distribution and customer support.}CPT114vJ (March 2015) 12 jetPRIME® DNA & siRNA Transfection Reagent7.7 TRADEMARKSPolyplus-transfection and jetPRIME are registered trademarks of Polyplus-transfection. 7.8 TECHNICAL ASSISTANCE AND SCIENTIFIC ADVICE Contact the friendly Polyplus technical support via :▪ The Polyplus website: ▪ Email: support@▪ Phone: +33 3 90 40 61 87。
