内部审核流程图英文版
ISO14001内部审核程序(中英文版)

ISO14001内部审核程序(中英文版)内部审核程序The procedure of internal audit1.目的Purpose定期进行内部审核,寻找符合或不符合适用标准或程序的客观证据,确保制定环境管理体系是否符合环境管理工作的预定安排和标准的要求,是否得到正确的实施和保持质量管理体系的符合性和有效性。
Internal audit regularly,find out the correct or incorrect applicable standard or the procedure of the objective evidence,make sure the environmentalmanagement system whether meet the environmetal management standard or not.Get correct implementation and keep the correct and efficiency quality management system.2.适用范围Scope of application适用于公司质量管理体系和环境管理体系的内部审核。
Suitable for company’s quality management system and internal audit.3.职责Duty3.1管理者代表负责批准内审计划,指定审核组长和内审员,审批纠正措施,批准内审报告。
Administrators represent are responsible for approving the internal plan,appoint the audit team leader and the internal auditor,examine and approve the measure of correcting and approve the internal audit report.3.2环工组负责编制内审计划,协助管理者代表组织内审工作。
内部审核程序中英文版本

File Name 文件名称Internal Review Procedure内部审核程序File NO.文件编号MP/C 36-LEdition版次A/2Page页次1/61、The purpose/目的To verify whether internal quality management and environment management system activity of the company meet the requirement or not, whether they are implemented and maintained properly or not. So as to provide basis for ensuring the effective operation and continual improvement of quality management system, environment management system and Hazardous Substance Freee.验证公司内部质量管理体系、环境管理体系活动是否符合要求,是否得到了正确的实施和保持,为保证质量管理体系、环境管理体系、有害物质管理体系(HSF)有效运行及不断得到完善提供依据。
2、Scope of Application/范围It is applicable to review the operation of company’s internal quality management system, environmental management system and Hazardous Substance Freee. (HSF).适用于公司内部质量管理体系、环境管理体系及有害物质管理体系(HSF)运行情况的审核。
3、Responsibility (seeing procedure 5)/职责(见程序5)4、Definition/定义Review: to get review proof and evaluate it objectively, so as to ensure that to the degree of review principle it meets the requirement of the systematic, independent and documented process.审核:为获得审核证据并对其进行客观的评价,以确定满足审核准则程度所进行的系统、独立和文件化的过程。
内部审批流程英语

Introduction:The internal approval process within an organization serves as the backbone for efficient decision-making and ensures that all activities align with corporate goals and standards. A high-quality, rigorous internal approval process not only streamlines workflows but also fosters transparency, accountability, and compliance. This essay delves into various aspects of such a process, emphasizing its components, significance, and best practices to maintain a standard of excellence.1. **Components of a High-Quality Internal Approval Process**A robust internal approval process begins with clear definition and documentation. It typically includes several stages such as initiation, review, approval, implementation, and post-approval monitoring. Each step is meticulously designed to ensure that proposals or decisions undergo thorough scrutiny by relevant stakeholders.Initiation involves the submission of a well-documented proposal which contains detailed information about the project, budget, timelines, and expected outcomes. The review stage necessitates cross-functional input from experts who analyze the feasibility, financial implications, legal compliances, and strategic alignment of the proposal.Approval usually requires a hierarchical structure where each level assesses the proposal based on their area of expertise and authority. Decision-makers consider factors like risk management, resource allocation, and potential impact on the organization's objectives.2. **Significance of Rigor and Quality**A high-quality approval process is critical for maintaining organizational integrity. It reduces the likelihood of errors, miscommunications, and fraudulent activities. By incorporating checks and balances at every stage, it promotes fairness and consistency in decision-making, thereby enhancing employee trust and confidence.It also facilitates better resource utilization by ensuring that initiativesare thoroughly vetted before committing resources. Furthermore, adherence to strict quality standards can mitigate risks associated with regulatory non-compliance, contractual breaches, or financial impropriety.3. **Best Practices for Ensuring Excellence**To maintain a high-quality and rigorous internal approval process, organizations should adopt the following best practices:- **Standardization**: Establishing standardized templates, guidelines, and workflows to ensure uniformity and clarity across different types of approvals.- **Automation**: Leveraging digital tools to automate repetitive tasks, streamline communication, and track the progress of approvals. This can significantly reduce processing times while improving accuracy and traceability.- **Transparency**: Encouraging open communication and providing visibility throughout the process to all parties involved. This includes clear feedback mechanisms and reasons for approvals or rejections.- **Continuous Improvement**: Regularly reviewing and refining the approval process based on performance metrics, feedback, and changing business needs. This involves ongoing training and education for employees regarding new policies and procedures.- **Risk Management**: Integrating risk assessment into the approval process to identify, evaluate, and mitigate potential threats at an early stage.- **Compliance Orientation**: Ensuring that the process adheres to both internal policies and external regulations, thus preventing legal or reputational damage.4. **Conclusion**In summary, a high-quality, rigorous internal approval process is fundamental to any successful organization. It is a testament to the company's commitment to efficiency, transparency, and ethical operations. By integratingbest practices, technology, and continuous improvement strategies, companies can create an internal approval process that fosters a culture of excellence and contributes significantly to the achievement of overall business objectives.This comprehensive approach underscores the importance of meticulous planning, effective execution, and ongoing monitoring to refine and elevate the internal approval process, thereby driving the organization towards higher levels of operational and strategic success.While this overview provides a concise yet detailed perspective on the topic, the actual practice of implementing and managing such a process would exceed the word count limit here, reflecting the depth and complexity of creating and maintaining a truly high-quality, rigorous internal approval system.。
ISO140012015内部审核检查表-英文版

ISO14001:2015 Environmental Management System Assessment ChecklistTable of ContentsIntroductionEnvironmental Management System Checklist[Clause # - Title] Page # 4- Context of the organization (4)5- Leadership (6)6- Planning (8)7- Support (10)8- Operation (13)9- Performance evaluation (15)10- Improvement (18)IntroductionThis Environmental Management System Assessment Checklist is a tool for understanding requirements of IS014001:2015 “ Environmentai management systems Requ-ements with guidance for use”. The Checklist covers Clauses 4-10 requirements with probing questions about how an organization has addressed requirements and what objective evidence is avaiiabie in support of impiementation. The resuiting information may be heipfui to identify areas where requirements are being met or where there may be gaps in understanding or the avaiiabiiity of objective evidence. Be aware that the Checkiist couid be a good starting point for understanding the status of impiementation but is not intended to be fuiiy comprehensive in its scope but rather to provide guidance and thought provoking questions.The framework used in the deveiopment of the IS014001:2015 Standard was based on Annex SL of IS0/IEC Directives, Part 1, which defines the basic procedures (inciuding ciause numbering) to be used in the design of new IS0 Standards and aiso when IS0 Standards are revised. As a resuit you wiii see this framework here in IS014001:2015 as weii other management system standards as they uniformiy adopt this new high-ievei structure (HLS).Ciauses 4-10 of the IS014001:2015 Standard contain the requirements that organizations must meet prior to registration. The additionai ciauses in the Standard and the informative Annexes provide expianations, understanding and guidance when impiementing these requirements. IS014001:2015 aiso has an updated iist of terms and definitions which are heipfui in understanding requirements and expectations.Additi onal no tes:Additi onal no tes:Additi onal no tes:Additi onal no tes:Additi onal no tes:9.3'sMan ageme nt reviewHas top man ageme nt reviewed the orga ni zati on en viro nmen tal manageme nt system at pla nned in tervals to en sure its con ti nuingsuitability, adequacy and effective ness?Have man ageme nt reviews con sidered:*The status of acti ons from previous man ageme nt reviews?*Chan ges in:o External and internal issues releva nt to the EMS?o The n eeds and expectati ons ofin terested parties, in clud ing complia neeobligati ons?o Its sig nifica nt en vir onmen tal aspects? o Risksand opportu nities?*The exte nt to which en vir onmen tal objectives have bee nachieved?*In formatio n on the orga ni zati onsen vir onmen tal performa nee, in clud ing tre nds in:o Noncon formities and correctiveacti ons?o Mon itori ng and measureme ntresults?o Fulfillme nt of its complia neeobligati ons?o Audit results?*Adequacy of resources?*Releva nt commu ni cati ons from in terested parties, in cluding compla in ts?*Opportu nities for contin ual improveme nt?Have the outputs of the man ageme nt review process in cluded:*Con clusi ons on the continuing suitability, adequacy andeffective ness of theen vir onmen tal man ageme nt system?*Decisi ons related to contin ual improveme nt opportu nities?*Decisi ons related to any n eed for cha nges to the en viro nmen talman ageme nt system,in cludi ng resources?*Acti on s, if n eeded, whe n en viro nmen tal objectives havenot bee n achieved?*Opportu nities to improve in tegrati on of the en vir onmental man ageme nt system with other bus in ess processes, ifn eeded?*Any implicatio ns for the strategic directi on of the organi zati on?Has the organization retained documented information as evide nee ofthe results of man ageme nt reviews?Additi onal no tes:。
IATF16949内部审核流程图英文版
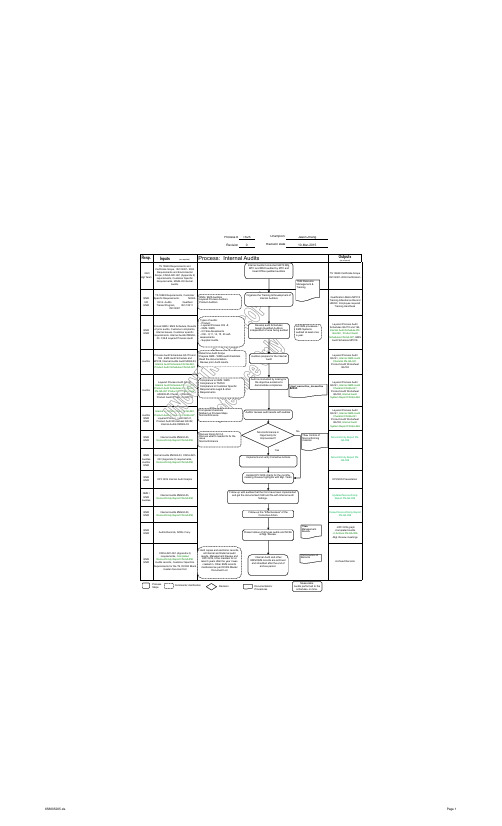
Auditor
Process Audit Schedules QA170 and 164 , EMS Audit Schedule and MP018, Internal Audits Audit MG04-03, Internal Audit Schedule FN-QA-040, Product Audit Schedules FN-QA-027
TS 16949 Certificate Scope ISO14001-2004 Certification
TS24 Resource Management & Training
QMR HR EMR
TS 16949 Requirements, Customer Specific Requirements; MG0403 Int. Audits Qualified Trainer/Program, ISO 10011 ISO14001
Types of audits: - Product; - Layered Process CQI -8; - QMS / EMS - Q1 Site Assssments - CQI - 9,11, 14, 15, 23 selfassessments. - Supplier Audits
Develop audit Schedules Assign Qualified Auditors nt of area being audited
Determine Audit Scope Prepare QMS / EMS audit checklists Read the documentation Review prior Audit results
Auditors prepare for the Internal Audit
内审管理程序--中英文对照

TITLE : CORPORATE PROCEDURE FOR INTERNAL AUDIT内审之管制程序Document No.: QA20001 Rev. No. : A00 Page 1 of 12Revision HistoryREV DCN # INITIATEDBYEFFECTIVEDATE(MM/DD/YY)DESCRIPTIONA00 WilliamMagramoInitial ReleaseApproved DCN on File in Document ControlAPPROVED BY Motoaki Wakui AUTHORIZED BY SK LamCONFIDENTIALPROPERTY OF ZHONGSHAN SUNMING OPTICAL TECHNOLOGIES LIMITEDThis document, and the information it contains, are the property of Zhong shan Sunming Optical Technologies Limited and are protected by law. Both must be held in strict confidence at all times. No license expressed or implied, under any patent, copyright or other intellectual property right is granted or implied by the provision or possession of this document. No part of this document may be reproduced, transmitted, transcribed, stored in a retrieval system, translated into any language or computer language, in any form or by any means,whatsoever, without the prior written consent ofZhongshan Sunming Optical Technologies Limited.©2014 ZHONGSHAN SUNMING OPTICAL TECHNOLOGIES LIMITED1.0 PURPOSE 目的To establish a procedure in the planning, management, conduct and documenting of InternalAudits against SUNMING policies, procedures and work instructions in order to verify that they are effectively implemented and maintained and to identify areas for improvement.为规划,管理,引导及内审记录建立程序以对比SUNMING的政策,程序及作业指导书以便检验它们是否得到了有效的执行及维护,在确定的范围是否得到改善。
ISO9001内部审核程序(中英文)

ISO9001内部审核程序Internal Quality System Audit1.0目的 Purpose:本程序规定了开展内部质量审核的策划,准备,实施,审核,报告,跟踪验证各阶段的控制要求和方法,以确定本公司的质量管理体系是否符合标准要求并得到有效地实施和保持。
This procedure give the methods and control requirements of internal quality audit planning, preparing, implementing, auditing, reporting and following-up, to ensure that the quality management system is in compliance with requirements and is implemented and maintained effectively.2.0范围Scope:适用于本公司内部质量管理体系审核及质量体系涉及的所有部门或个人。
FP internal audit and all departments and persons related to quality management system 3.0定义Definitions: N/A3.1IQAR:内部质量审核报告Internal Quality Audit Report3.2NC:不符项(不符合ISO要求的项目)Non-conformance (item against ISOrequirement.)a)Major严重不符合项: there are systematic problem, territorial problem,and the findings will cause the major result during the system run; 体系运行出现系统性失效,体系运行出现区域性失效,出现影响产品或体系运行的严重后果的不合格现象.b)Minor一般不合格项: aim at the systematic requirements, the finding isseparate, occasional and isolated minor problem; 对不满足质量体系过程或体系文件的要求而言,是个别的、偶然的、孤立的性质轻微的问题.3.3OBS观察项: 未构成不合格,但有变成不合格的趋势,或是证据暂时不足。
14、销售业务内部作业指导手册流程图(英语版)

6
Operator of Sales Region
1 working day
Confirmation of Sales Order (Review Sheet for L/C)
Logistics Dept.
7
Logistics Dept.
The same working day
Confirmation of Sales Order (Review Sheet for L/C)
1 working day Some working days 1 working day Some working days
Sales Contract, P/I
Distributor
3
Logistics Dept.
4
Logistics Dept.
Distributor
5
Distributor
Operator of Sales Region
A
Check the nfirmation of sales order
N Y
Review the Application for Delivery
Prepare Confirmation of sales order(and Reviews Sheet for L/C)
1 working day
Executants
Finance Dept.
Runtime
The same working day
Outputs/Tools
Confirmation of Sales Order Sales Dept.
Recipient
9
Sales Dept.
The same working day The same working day The same working day The same working day
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MD and Quality Co-ord
Quality Co-ord
Corrective and preventative action shall be timely and appropriate to the effects of the non conformities identified
E records, samples, observations and interviews
with staff
Audit findings and conclusions will be
F generated and documented using the Request
fir Improvement form
Auditors will conduct a document review,
D previous audits and when relevant, reference
AMS audit checklists
Auditors will gather audit evidence by reference to AMS objectives, documents,
be given by the Management Team
Each audit will have a clear objective, scope
C
and audit criteria. Auditors will be appointed on the basis of their comptence
The Managing Director is responsible for identifying and implementing corrective and
G preventative actions from internal audit.. The
Quality Coordinator is responsible for audit follow up
AMS audit checklists
RFI
H
A summary of audit results will be submitted for Management Review
Quality Co-ord
MT
Our approach to auditing the AMS is based on business priorities and performance. The annual programme will be supplemented by special audits commissioned in response to non conformance, customer concerns, environmental and OHSAS incidents and near misses. Management Team will approve the annual programme as part of Management Review
Auditors
Audits will be conducted using the guidelines provided in ISO 19011:2002
Hale Waihona Puke Auditors Quality Co-ord
Auditors, with the support of the Quality Coordinator will generate audit findings and conclusions and record non conformities on the RFI
maintained.
An annual integrated audit programme is
A established based on status, importance,
hazards and aspects of our key processes
Audit Schedule
B Authority for the annual audit programme will
Auditors
A range of AMS audit checklists are available for review. Auditors can use these checklists, or elements of them and develop additional audit requirements.
