有关物质方法确认方案
物质纯度检测方法确认方案

物质纯度检测方法验证方案(以药用辅料丙二醇为例)文件编号:XXX公司目录一、验证目的 (1)二、验证职责 (1)1质控 (1)2质保 (1)三、验证概述 (1)1方法原理 (1)2验证内容 (1)2.1标准品与供试品 (2)2.2检验仪器 (2)2.3检验内容 (2)2.3.1专属性 (2)2.3.2精密度 (3)2.3.3准确度 (3)2.3.4线性及范围 (3)2.3.5耐用性 (4)2.3.6样品测定 (4)四、确认人员 (5)五、确认结论 (5)六、偏差与变更记载 (5)一、验证目的:对中国药典2015版中1,2丙二醇含量检测方法进行方法确认,确保此方法适用于检验本公司1,2丙二醇产品含量分析项目的检测。
二、验证职责:1、质控1.1起草确认方案及确认报告,报质量负责人及QA主管审核,质量副总审批。
1.2组织、协调确认方案的实施。
1.3负责对确认实施过程中的任何变更与偏差及时报告QA。
2、质保2.1根据确认对象成立确认人员。
2.2负责确认方案及报告的审核。
三、验证概述:1、方法原理:1,2丙二醇含量采用中国药典2015版的方法,本报告利用气相色谱仪,按外标法测定,验证该方法的准确度、精密度、专属性、线性及范围、耐用性。
2、检验内容:2.1 标准品与供试品标准品供试品2.2 仪器:气相色谱仪(FID检测器、安捷伦7890B),电子天平(梅特勒托利多XS205)2.3 验证步骤2.3.1 专属性a)分别配制一定浓度的环氧丙烷溶液、一缩二乙二醇溶液、一缩二丙二醇溶液、二缩三丙二醇溶液、丙二醇溶液及对照品溶液,按照2015版中国药典药用辅料1,2丙二醇含量检查方法分别进样分析,记录色谱图。
b)可接受标准:①在环氧丙烷溶液、一缩二乙二醇溶液、一缩二丙二醇溶液、二缩三丙二醇溶液、丙二醇溶液谱图中应分别出现环氧丙烷、一缩二乙二醇、一缩二丙二醇、二缩三丙二醇、丙二醇峰。
②在对照品溶液谱图中应依次出现环氧丙烷、一缩二乙二醇、一缩二丙二醇、二缩三丙二醇、丙二醇峰,相邻各色谱峰之间的分离度均应不小于1.5。
紫外分光光度计确认方案精选全文

可编辑修改精选全文完整版UV-1800紫外分光光度计确认方案1 概述1.1紫外-分光光度法是通过被测物质在紫外光区或可见光区的特定波长处或一定波长范围内的吸收度,对该物质进行定性和定量的方法。
本法在药品检验中主要用于药品的鉴别、检查和含量测定。
TU-1901双光束紫外可见分光光度计主要由光源、单色器、样品室、检测器等组成,波长范围为900nm-190nm,光谱带宽分6档(5,2,1,0.5,0.2,0.1),操作简便,测量快速,自动化程度高。
1.2基本情况设备名称:紫外可见分光光度计型号:生产厂家:安装日期:使用部门:质量控制部工作间:质量控制部2 确认目的确认紫外可见分光光度计测定数据准确可靠,适用于预期用途。
3 确认范围3.1 文件的适用范围本文件适用于质量控制部紫外可见分光光度计的确认。
3.2 确认的范围质量控制部紫外可见分光光度计的确认。
4 职责4.1 质量控制部职责✧负责起草确认方案、总结报告;✧负责整个确认方案的实施,并做记录、总结报告;✧负责该确认得出可靠的确认理论,适用于产品检验。
4.2 质量保证部职责✧做好过程监控,确保方案执行过程符合法规要求;4.3 动力部职责负责仪器的正常运行本仪器经实验确认,确认过程是否严格按照仪器操作规程和仪器说明书进行,试验结果稳定是否可靠,是否存在任何实验风险。
7 确认实施7.1相关文件a.仪器、仪表校验情况7.2 安装确认7.3.1 一般检查7.3.2 波长准确度以仪器中氘灯的656.1nm特征谱线检查。
在主菜单中激活光谱扫描窗口,选择【测量】菜单下的【参数设置】子菜单进行设置。
选择能量方式(Es),扫描范围(653nm-659nm),显示范围(0.0000E-50.00E)慢速扫描,采样间隔0.1nm,上述分别确认后,开始扫描。
扫描结束后按读取测得的峰值波长,其与标准波长(656.1nm)之差应不超过±7.3.3 吸光度的准确度取在120℃干燥至恒重的基准重铬酸钾约60mg ,精密称定,置1000ml 量瓶中,用0.005mol/L 硫酸溶液溶解并稀释至1000ml ,用配对的1cm 石英池,以0.005mol/L 硫酸液位空白,在235、257、313、350nm 分别测定吸光度,然后换算成%1E ,测得值应符合下表的允差范围。
有关物质方法计划确认方案

METHOD VERIFICATION PROTOCOL FOR CHROMATOGRAPHIC PURITYOF CYTARABINE阿糖胞苷相关物质检查方法确认方案Area to be Distributed:AD, QA散发部门:剖析部,质量保证部Print Name:Title:Signature:Date:姓名:职位:署名:日期:Author:草拟人:Approver:同意人:Approver:同意人:Reviewer:审察人:Approver:同意人:Contents目录一、Introduction (3)二、Scope. (3)三、Responsibility (3)四、Definitions. (4)五、Project (5)六、References . (26)一、Introduction简介##is in the process of developing Cytarabine Injection, a liquid productfor parenteral administration, for ##. Cytarabine is a chemotherapy agentwith a molecular weight of 243.2. The API from Zhejiang Hisun PharmaceuticalCo. Ltd, which is approved by FDA, is used for Cytarabine Injection byLummy.The Chromatographic purity method for Cytarabine is the method in thecurrent USP34. The HPLCmethod for Chromatographic purity test of Cytarabineis a quantitative method. The method will be verified according to USP<1226>,including system suitability, specificity, LOD, LOQ, precision, solutionstability and mobile phase stability.阿糖胞苷注射液是一种临床用液体产品,##药业为 ##药业研发该品种。
方法确认与方法转移
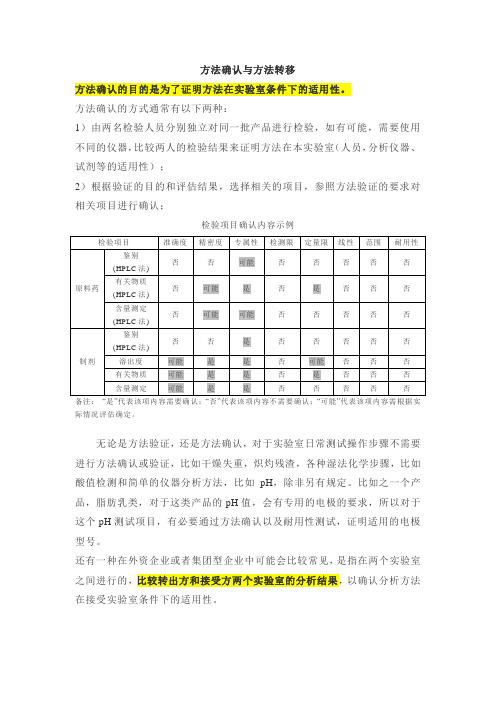
方法转移检验批次示例
类别
检验的批次数
第一类
辅料:中间产品、包装材料
原料和成品(鉴别实验或其他简单实验)
1批
第二类
原料和成品(除鉴别实验或其他简单实验外)
是
否
否
否
否
否
备注:“是”代表该项内容需要确认;“否”代表该项内容不需要确认;“可能”代表该项内容需根据实际情况评估确定。
无论是方法验证,还是方法确认,对于实验室日常测试操作步骤不需要进行方法确认或验证,比如干燥失重,炽灼残渣,各种湿法化学步骤,比如酸值检测和简单的仪器分析方法,比如pH,除非另有规定。比如之一个产品,脂肪乳类,对于这类产品的pH值,会有专用的电极的要求,所以对于这个pH测试项目,有必要通过方法确认以及耐用性测试,证明适用的电极型号。
检验项目确认内容示例
检验项目
准确度
精密度
专属性
检测限
定量限
线性
范围
耐用性
原料药
鉴别
(HPLC法)
否
否
可能
否
否
否
否
否
有关物质(HPLC法)
否
可能
是
否
是
否
否
否
含量测定(HPLC法)
否
可能
可能
否
否
否
否
否
制剂
鉴别
(HPLC法)
否
否
是
否
否
否
否
否
溶出度
可能
是
实验室方法确认

实验室方法确认实验室方法确认,是对实验室所采用的方法进行科学认定的过程。
它包括对方法的准确性、可靠性、稳定性、可重复性、可维护性等方面的评估。
实验室方法确认是实验室质量管理体系的重要组成部分,也是保证实验室检测结果准确可靠的重要环节。
确定需要确认的方法:实验室根据自身的业务范围和检测需求,确定需要确认的检测方法。
这些方法可能包括国家标准、行业标准、客户指定方法等。
制定确认计划:实验室根据方法的特性,制定详细的确认计划。
计划应包括方法的研究设计、实验操作步骤、数据处理和分析方法、不确定度评估等内容。
实施确认实验:实验室按照确认计划进行实验操作,并对实验数据进行详细记录。
在实验过程中,要对实验的重复性、稳定性、可靠性等方面进行评估。
分析确认结果:实验室对实验数据进行处理和分析,评估方法的准确性、可靠性、稳定性等性能指标,并形成确认报告。
审批确认报告:实验室将确认报告提交给相关领导或客户进行审批,以确保报告的有效性和可靠性。
更新确认记录:实验室应定期对确认记录进行更新和维护,以确保方法的持续性和有效性。
提高实验室检测结果的准确性和可靠性:通过实验室方法确认,可以保证实验室所采用的检测方法具有准确性、可靠性、稳定性等性能指标,从而提高实验室检测结果的准确性和可靠性。
满足法规和客户要求:实验室方法确认是实验室质量管理体系的要求之一,也是满足相关法规和客户要求的重要手段。
通过实验室方法确认,可以保证实验室的检测结果符合相关法规和客户的要求。
提高实验室的竞争力:通过实验室方法确认,可以提升实验室的技术实力和管理水平,提高实验室的竞争力,有利于实验室在激烈的市场竞争中脱颖而出。
促进实验室持续改进:通过对方法的确认过程进行总结和分析,可以发现实验室存在的问题和不足之处,为实验室的持续改进提供依据和方向。
实验室方法确认是保证实验室检测结果准确可靠的重要环节,对于提高实验室的质量管理水平和技术实力具有重要意义。
高效液相色谱仪(HPLC)是一种广泛应用于化学、生物和医药等领域的分析仪器,对于实验室中的科研人员来说具有至关重要的地位。
检验方法确认程序【范本模板】

一.目的:适应医学技术的发展,确保检验方法准确可靠,以满足临床需要。
二.适用范围:实验室所有开展的检验方法。
三.工作程序:1.实验室必须使用能保证准确和可靠的检验结果的检验方法、器材、仪器、试剂、质控品和校准品、供应品.2.必须选择能保证检给结果在实验室所确定的方法性能规格内的方法。
在检测标本前,实验室必须对使用方法的下述的性能规格进行确认:准确度、精密度.如有必要,可添加特异性和分析灵敏度,以及检验结果的报告范围、参考值以及其它适合的特性。
2.1.实验室引进我国注册登记的国外方法(试剂盒)或我国取得生产许可证和方法(试剂盒)时,在报告检验结果前,必须得到下列特性的性能规格:准确度、精密度、检验结果的报告范围(或者线性)、参考值。
实验室至少应检查其准确度、精密度。
必要时增加特异性和分析灵敏度,以及报告范围,参考值是否符合本实验室的服务人群等.如与厂商提供的数据进行比较,应取得相符结果。
2.2.实验室自行建立的方法,在报告结果患者前:2.2.1建立每一方法的性能规格,包括:准确度、精密度、特异性、干扰因素的影响、分析灵敏度、检验结果的报告范围(或者线性)、参考范围、其它需要检测的性能.2.2.2.建立该方法的校准程序和ICQ规则。
2.3实验室必须有上述活动的记录和文件。
并保存到停止到使用这些方法后半年。
3.实验室必须有与所做检验的专业和工作量相适应的,足够的器材、仪器、试剂、质控品和校准品、供应品。
4.实验室必须确定正确制备、储存和使用上述体外诊断用品的条件。
4.1.这些条件包括:水的质量、温度校准。
保护器材和仪器,不致由于电流的波动各中断引起对检验结果的不利影响.4.2.必须将纠正由于达不到规定所采取的改正措施文件化.5.用商品试剂盒时,对于国家规定应有生产许可证,注册登记证的品种,决不能使用没有生产许可证、注册登记证的商品试剂盒。
尚未规定者,生产厂家应提供该产品性能规格以及质量保证书。
6.试剂、溶液、培养基、校准品、质控品和其它供应品,必须加以标记,标记上应有:6.1.识别名:当有意义时应标明浓度,效价,滴度等6.2.储存要求6.3.制备日期和失效期6.4.其它与正确使用有关的信息7.应根据仪器制造商说明或依据权威机构的要求来选择和使用校准品和质控品.实验室自行选用的校准品或质控品,应有实验依据证明其不影响检验结果的准确性和可靠性。
如何进行实验室方法确认方法验证

2 方法确认的主要内容及注意事项
二、方法确认主要内容
ꢀ依据:《环境监测 分析方法标准制修订技术导则》 (HJ•168-2010)
主要内容: 检出限、精密度、准确度、 校准曲线、实际样品测试、 方法的特定要求等 ★ 可参考方法编制说明中验证方案
检出限—一般确定方法
检出限测定
要求
试验方法 含量/浓度
分析对象 测试步骤 测定方式
含有基体 样品分析的全部过程(包括前处理)
多份样品平行测定
标准溶液 直接进样 单份样品多次进样
检出限-合理性判断1(空白未检出)
判据: 要求
MDL=•t(n-1,0.99)וS, (S:标准偏差)
解决办法
单组分
比值1~10
比值>10或<1
改变样品/加标浓度
多组分
1、50%的目标物比值在3~5之间;
方法的特定要求
. 仪器性能检查-气相色谱 土壤和沉积物 有机氯农药的测定 气相色谱-质谱法土壤和沉积物 (HJ835-2017)
方法的特定要求
. 仪器性能检查-质谱 土壤和沉积物 有机氯农药的测定 气相色谱-质谱法HJ835-2017
方法的特定要求
. 仪器性能检查-质谱 土壤和沉积物 挥发性有机物的测定 吹扫捕集/气相色谱-质变样品/加标浓度;
2、至少90%的目标物比值在1~10之间;
2、相应因子差异大的
3、其余10%的目标物比值<20
组分检出限需分别测定
准确度
满足方法回收率的要求
不满足
查明原因
满足方法要求
不高于方法规定的检出限
高于方法检出限
查明原因
检出限-合理性判断2(空白有检出)
判据: 任意测定值之间允许的差异范围:
(完整版)检验方法验证和确认管理规程
页次:共11 页第1 页文件名称:检验方法验证和确认管理规程编码:03SMP01200起草审核批准颁发部门质量保证部日期日期日期实施日期分发部门及份数:质量管理部1份目的:明确检验方法的验证和确认的管理规程,确保所采用的检验方法科学、合理,符合检验要求并能有效控制药品的内在质量。
范围:仅适用于本公司对物料、产品的理化检验方法的验证和确认;清洁验证方法的验证。
职责:质量管理部QC、QA人员、质量管理部负责人对本规程的实施负责。
内容:1. 方法验证及确认工作职责分工1.1 质量控制部QC负责验证或确认方案的起草、验证或确认工作具体实施以及报告的填写。
1.2质量控制部负责人或其指定人员负责验证或确认方案、报告的审核,组织验证或确认工作的实施,对验证或确认工作中出现的问题及时纠正。
1.3 质量保证部QA负责验证或确认方案、报告的审核,监督确认工作实施,对确认工作中出现的问题提出改进意见并监督落实。
确保检验方法验证或确认程序达到符合性要求,程序被遵照执行,并且方法的预定用途被有效的且以文件记录的数据所支持。
1.4 质量管理部负责人负责验证或确认方案及报告的审核批准。
2 方法验证2.1定义:方法验证就是根据检验项目的要求,预先设置一定的验证内容和验证标准要求,并通过设计合理的实验来验证所采用的分析方法是否符合检验项目的要求。
2.2 目的:方法验证是证明采用的方法适合于相应检测要求。
2.3 适用范围:符合下列情形之一的,应当对检验方法进行验证:(1)采用新的检验方法;(2)检验方法需变更的;(3)采用《中华人民共和国药典》及其他法定标准未收载的检验方法;(4)法规规定的其他需要验证的检验方法。
文件名称:检验方法验证和确认管理规程编码:03SMP012002.3.1 在建立药品质量标准时,应对分析方法中的各检验项目进行完整的验证。
2.3.2 当药品生产工艺变更时,制剂的组分变更、原分析方法修订时,可根据变更的内容决定对分析方法进行部分验证还是完全验证。
化学检测实验室的方法验证与确认

化学检测实验室的方法验证与确认化学检验实验室主要依靠准确掌握检验方法和掌控测试结果的有效性两个方面来保障检测结果的准确性,通常对检测方法的控制要求有两个控制理念,分别为方法验证和方法确认。
一部分实验室工作人员在进行检测时,不区分标准方法和非标准方法,直接使用同一种方式和操作方法进行检测,这就会在实验室检测标准技术方面出现很大的不确定度,影响检测结果的准确性。
标签:化学检测;实验室;方法验证;确认1 方法验证和确认的定义“方法验证”对应于英文单词“Verification”,ISO对其定义为“通过检查并提供客观证据,验证规定要求得以满足”。
通过方法验证可证实方法适用于其预期目的的过程。
确认包括研究性能特征,如:准确度、特异性、检出限、定量限、線性度、范围、耐用性和稳健性。
“方法确认”对应于英文单词“Validation”,根据ISO,其定义为:“通过考察和提供客观证据,确认是否能满足某个特定预期用途的特殊要求”,即:方法确认需要“确认某个特定的分析方法能满足分析目的。
”换句话说就是“表明一个规定的方法协议,适用于某个特定类型的测试物料,以及待测物特定的浓度水平”。
通过方法确认可证实一个实验室有能力重复已确认的方法,同时方法能够达到可接受的性能水平。
从方法确认和方法验证两者的定义可知,如果一个实验室希望使用某一标准方法(已通过协作研究被全面确认),则应开展方法验证。
而某一实验室需要研发一个新方法或使用非标方法时,则应进行方法确认。
2 方法验证和确认的实施程序2.1 方法验证的实施程序一般来说,进行方法验证有以下几种情况:①采用标准方法前;②对已确认方法进行了细微改变时(例如:使用了不同厂家的相同类型的色谱柱、改变了样品稀释倍数等)。
FDA实验室手册规定,对标准方法的性能验证可分为两种情况:①批量分析情况下的性能验证;②实验室首次使用标准方法前的验证。
而前者更多意义上是属于内部质量控制的范畴。
在方法验证过程中,需要考虑哪些关键参数,取决于方法的性质和可能遇到的样品类型。
物质的鉴别教学设计

物质的鉴别教学设计物质的鉴别教学设计物质的鉴别教学设计一、设计思路《物质的鉴别》一直是初中化学的重要内容,也是历年中考的热点。
由于它涉及多个基本概念,而且知识点分散,相关的题型多变,所以它也是初中化学教学的难点。
本节课主要引导学生利用酸、碱、盐的特征反应,解决一些简单的化学实际问题。
为了培养学生梳理知识的能力,方便学生复习零散的化学知识,笔者将有关内容(根据其内在联系)整合为知识条块,使其薄有纲、厚有目;为了培养学生的表达、交流能力,及时发现练习中存在的问题,笔者鼓励学生主动上台展示学案,共享学习成果、贡献学习困惑。
在实验过程中,笔者相信学生、敢于放手,关注全体、重视指导,倡导民主、鼓励质疑,从而激发学生创新精神和实践能力。
为培养学生的操作技能,提高化学素养,笔者引导学生以化学原理为核心、以实验操作为手段去探究物质的组成和变化。
为了夯实学生的化学基础知识,提高实验设计能力和解题技巧,笔者安排学生适当演练实验习题,而且在解题过程中能够站在学生的思维角度分析解题思路、总结解题方法。
为了体现化学知识的实用性和趣味性,笔者还注重联系生产生活中的一些特征反应。
另外,制作的ppt课件、编写的学案简捷实用,有利于学生的学习,提高了课堂信息量和复习效率。
二、教学目标根据“课程标准”和“教科书”的要求,并结合所带班级学生的知识现状和笔者的教学经历,确定本节课的教学目标如下:①能够根据物质的特征和特征反应,设计鉴别典型酸、碱、盐的实验方案。
②学会药品的取用、胶头滴管的使用、实验废液处理等基本操作。
③初步体验科学家通过实验手段研究物质的方法。
④培养解决化学实际问题的能力。
三、教学准备①编印本节课使用的学案(提前一天发给学生练习)。
②准备有关的实验用品,摆放在实验台上。
③调试实物投影仪等多媒体设备;制作ppt课件。
四、教学过程(一)新课导入[引言]酒驾猛于虎,那么如何检查司机酒驾呢?人们想到了重铬酸钾的特征反应:橙色的重铬酸钾遇到酒精会变为绿色的硫酸铬。
实验室如何进行方法变更、非标方法的确认

实验室如何进行方法变更、非标方法的确认方法发生变更时或颁布新标准时,如何对方法进行确认?非标方法如何进行方法确认?检测方法选择的核心是什么?。
.这类问题是多数实验室都会面临到的实际问题,如何搞定?请看下文!《实验室资质认定评审准则》5。
3.2条款中规定:“实验室应确认能否正确使用所选用的新方法。
如果方法发生了变化,应重新进行确认。
实验室应确保使用标准的最新有效版本。
”在《GB/T 27025-2008 检测和校准实验室能力的通用要求》条款中也有相应的规定.实验室采用的检验检测方法包括样品的抽取、处理、运输、存储和制备等各个环节,确认时应当记录确认所获得的结果、使用确认的程序、确认对方法是否适合于预期的用途等,必要时还应包括不确定度和分析数据的统计学处理技术。
检测方法确认的目的确保实验室所采用的标准方法、非标准方法、实验室自制方法超出其预定范围使用的标准方法及经过扩充和更改的标准方法得到有效确认,保证上述方法适合于预期用途,并满足特定要求.方法发生变更时或颁布新标准时,如何对方法进行确认:1.在首次对外出具数据之前应确认标准方法已被正确的运用.2。
标准方法发生了变化应重新确认。
3。
对标准方法定期清理或者查新,以确保最新有效版本.1检测方法的选择及使用要求实验室资质认定(或认可)现场考核时确定的检测项目的依据是国家标准、行业标准和地方标准。
所以说,当没有国际、国家、行业、地方规定的检验检测方法时,实验室应尽可能选择已经公布或由知名的技术组织或有关科技文献或杂志上公布的方法,但应经实验室技术主管确认。
如是在实验室计量认证或认可批准业务范围内,因客户的特殊要求而发生的情况,其检验检测结果和报告上应有明确的说明.另外需要使用非标准方法时,这些方法应征得委托方同意,并形成有效文件,使出具的报告为委托方和用户所接受。
这是指必须在实验室计量认证或认可批准业务范围内使用,所谓有效文件是指甲乙双方对使用非标准方法检测达成协议,一般来说应有双方签字盖章,也可以在检测委托(协议)书上注明,实验室在检测报告中也必需加以说明。
化学分析方法验证

线性
线性是指再设计范围内,测试结果与试样中被 测物浓度直接呈正比例关系的程度。 线性应在分析需要的范围内进行评价 一般以最小二乘法处理数据求的回归曲线的斜 率来表示,必要时可对响应信号进行数字转换。 数据要求:至少需要五个浓度考察线性,需提 供相关系数,Y截距,应列出回归方度,准确度和线性条件下,测 试方法是用的高,低限量浓度或量的区间。 范围应根据分析方法的具体应用和线性,准确度,精 密度结果和要求确定。 原料药和制剂含量测定范围为80%-120% 制剂含量均匀度范围为70%-130% 杂质测定应为被测杂质限度的-20%到+20% 溶出度应为测定范围的-20%到+20%,释放度因规定 了限度范围,应为下限的-20%到上线的+20%,例如 缓释片1h<20%,7h>70%,则验证范围定为0-90%
化学分析方法验证
化学分许方法验证成功的前提
能够研究建立一个有水准的分析方法的能力
有化学分析方法验证的知识和水平
化学分许方法验证成功的前提
有水平,经过培训的实施人员 仪器性能已经得到确认(校准,验证,校准在 有效期内) 可靠的对照品(法定机构提供的或有水平自己 建立的) 质量可靠的实验试剂
SD 100% x
精密度
重复性:在相同条件下,有同一个分析人员测定所得 结果的精密度
在规定的范围内,至少用9次测定结果评价,如制备 不同浓度样 品各测三次 把被测浓度当做100%,至少测6次进行评价
中间精密度:同一实验室,不同时间有不同的分析人 员用不同设备所得结果的精密度(三人三机) 重现性:不同实验室,不同分析人员测定结果的精密 度。以SD,RSD表示。
有关物质分析方法验证方案

8***原料有关物质分析方法验证方案20**年**月验证方案的起草与审批方案实施日期:目录1.验证目的 (4)2.方法简介与确认范围 (4)3.标准品、供试品 (4)4.风险评估 (4)5.验证的可接受标准 (5)6.验证步骤 (6)6.1系统适应性 (6)6.2专属性 (6)6.3检测限与定量限 (8)6.4线性 (9)6.5准确度 (9)6.6精密度 (10)6.6范围 (10)6.7耐用性 (10)6.8样品测定 (11)7.偏差 (11)8.风险的接收与评审 (11)9.再验证 (11)10.确认结果评审和结论 (11)11.更改历史 (12)12. 附录 (12)1.验证目的根据法规的要求,采用非药典或其它法规未收载的分析方法应进行验证,证明采用的方法适合于相应的检测要求。
这个验证方案的目的是为验证提供具体方法参数、可接受标准和研究步骤。
2.方法简介与确认范围***原料有关物质检测方法为自行开发的液相室色谱方法。
为确保方法的准确性和可行性,为日常检测方法提供依据,现对该方法进行验证。
方法验证必须按照验证方案进行,此次验证方案提供***原料含量分析方法验证验证,包括:专属性、精密度、线性、范围、准确度、检测限&定量限、耐用性。
3.标准品、供试品3.1标准品3.2供试品4.风险评估按照《质量风险管理规程》,质量控制部和质量管理部共同对分析方法进行了风险评估,确定了需进行方法确认的项目。
具体见下表:风险评估人:评估日期:5.验证的可接受标准6.验证步骤6.1系统适应性分离度测试液:取***产品分离度标准品5mg,置于一比色管中,加稀释液10ml溶解,在95℃下水浴加热30分钟,放冷。
标准液:取***产品标准品25mg,精密称定,置于一25ml量瓶中,用乙腈5ml溶解,然后用稀释液溶解并稀释至刻度;精密移取1ml,置于一100ml量瓶中,用稀释液稀释至刻度。
取分离度测试液进样,记录色谱图,***产品与***Δ-3异构体之间的分离度应不小于2.0。
聚醚多元醇中钾和钠元素的方法确认

聚醚多元醇中钾和钠元素的方法确认摘要:在相关标准及文献的指导下,对测定聚醚多元醇中钾和钠元素的进行相关测试条件的确认及方法的验证。
关键词:聚醚多元醇;钾和钠元素;方法确认前言:基于聚醚多元醇产品的测试需求以及本检测中心仪器参数提供的钾和钠两个元素优异的检出限(PE仪器的检出限K为1ppb,Na为0.5ppb),提出用ICP仪器测定聚醚多元醇中钾和钠元素的测试要求。
本检测方法预备采用电感耦合等离子体发射光谱仪ICP-OES测试聚醚多元醇中钠和钾的含量。
以GB/T12008.4-2009塑料聚醚多元醇第4部分:钠和钾的测定为参考测试方法。
1仪器相关参数设置1.1仪器1.1.1电感耦合等离子体发射光谱仪ICP-OES(珀金艾尔默,Avio200)。
1.1.2分析天平:精确至0.1mg。
1.1.3聚乙烯瓶:500mL。
1.1.4容量瓶(PP):10mL。
1.1.5移液器:1mL、100μL。
1.1.6离心管:10mL。
1.2试剂1.2.1混和离子标准溶液(含钾钠):100ppm。
1.2.2体积浓度为65~75%的DMSO溶液:DMSO与一级水的混合溶液,贮存在聚乙烯瓶中。
1.3仪器条件设定仪器参数设定如下;Plasma:17L/min,Aux:0.6L/min,Neb:0.6L/min,Power:1200watts,ViewDist.:15,PlasmaView:Axial。
2350G类样品方法确认的测试方案2.1检出限的测定[1]标准溶液配制:用移液器移取2mL混合离子标准溶液于离心管,用体积浓度为65%~75%的DMSO溶液稀释定容,最终配制获得钾钠元素含量为:0.25mg/L、0.5mg/L、1mg/L、2mg/L、5mg/L。
表1标准曲线参数在仪器处于正常工作状态下,吸喷系列标准溶液,制作工作曲线,连续10次测量空白溶液,以10次空白值标准偏差3倍对应的浓度为检出限。
2.2定量限(LOQ)[2]LOQ(定量限)的确定主要是从其可信性考虑,如:测试是否基于法规要求、目标测量不确定度和可接受准则等。
方法确认报告

方法确认报告一、实验目的本实验旨在通过分光光度法对一种未知溶液中其中一种物质进行浓度测定,并通过对比标准曲线进行定量分析。
二、实验原理分光光度法是利用物质对特定波长的光的吸收来测定物质的浓度。
光的吸收与物质的浓度成正比关系,根据比例关系,可以通过测量吸收光的强度来推算物质的浓度。
三、实验步骤1.准备工作通过标准曲线法,根据已知浓度的溶液和其吸光度之间的关系,制备出一条标准曲线。
根据已知浓度和吸光度的对应关系,利用线性回归等方法得到拟合曲线方程。
2.实验仪器使用分光光度计进行实验测量。
3.校准分光光度计使用标准溶液进行校准,调节分光光度计的零点和百分比透过率。
4.测量未知溶液将待测溶液装入比色皿,使用样品室吸光度的模式进行测量。
根据测得的吸光度值和标准曲线的关系,计算出溶液中待测物质的浓度。
5.数据处理根据实验得到的数据,通过标准曲线方程计算溶液中待测物质的浓度。
四、实验结果根据实验数据,计算出未知溶液中待测物质的浓度,得出定量分析结果。
五、误差分析实验中可能存在的误差包括仪器误差、操作误差和环境误差等。
为减小误差,可以重复多次实验取平均值,增加实验的精确性。
六、实验结论通过分光光度法对一种未知溶液中其中一种物质进行浓度测定,得出了该物质的浓度。
该方法具有快速、准确、无污染的特点,广泛应用于化学、生物等领域的定量分析。
七、改进措施实验中可以通过增加校准点、提高仪器的精度、减小操作误差等方式进一步提高实验结果的准确性。
同时可以尝试其他分析方法,以验证浓度测定结果的可靠性。
八、实验心得通过本次实验,我对分光光度法的原理和操作流程有了更深入的了解。
实验中需要注意仪器的正确操作和数据的准确记录,以确保实验结果的可靠性。
在以后的实验中,我会进一步加强实验操作的技巧,提高实验结果的精确性。
实验室方法确认

实验室方法确认1.引言实验室方法确认是确保实验室所采用的分析方法能够满足特定要求的过程。
在实验室工作中,正确选择和验证分析方法至关重要,以确保实验结果的准确性和可靠性。
本文将介绍实验室方法确认的基本概念、步骤和要求,以及相关的方法确认文件和记录。
2.方法确认的基本概念方法确认是指通过实验室内部和外部的一系列评估活动,证实所选用的分析方法能够满足特定要求。
这些要求可能包括分析方法的选择、验证和验证等。
方法确认的主要目的是确保实验室所采用的分析方法能够提供准确、可靠和可重复的实验结果。
3.方法确认的步骤和要求3.1方法选择:根据实验目的和样品特性,选择适合的分析方法。
在选择方法时,应考虑方法的灵敏度、准确度、精密度、线性范围、检测限和定量限等因素。
3.2方法验证:对所选用的分析方法进行验证,以证实其能够满足特定要求。
方法验证通常包括对方法的灵敏度、准确度、精密度、线性范围、检测限和定量限等参数的评估。
3.3方法确认文件:制定方法确认文件,明确方法的原理、操作步骤、仪器设备、试剂材料、数据处理和结果报告等内容。
方法确认文件应包括方法的来源、修订历史和批准日期等信息。
3.4方法确认记录:记录方法确认过程中的关键数据和结果,包括实验条件、仪器设备、试剂材料、数据处理和结果报告等。
方法确认记录应包括实验日期、实验人员和审核人员等信息。
4.方法确认的文件和记录方法确认的文件和记录是实验室方法确认的重要组成部分,用于证明实验室所采用的分析方法能够满足特定要求。
方法确认文件和记录应清晰、准确、完整地记录方法确认过程中的关键信息和结果。
4.1.1方法名称和编号:明确方法的名称和编号,以便于识别和追溯。
4.1.2方法原理:简要描述方法的原理和操作步骤,以便于理解和使用。
4.1.3仪器设备:列出所需的仪器设备,包括型号、规格和校准要求等。
4.1.4试剂材料:列出所需的试剂材料,包括名称、规格和纯度等。
4.1.5数据处理和结果报告:明确数据处理和结果报告的要求,包括计算公式、单位和精度等。
药典方法确认规程
目的建立药典方法确认规范,确保药典方法适用于本实验室的实验条件。
定义药典方法的确认:收载于CP、USP、BP、EP等药典的产品分析方法,使用前证明该验证过的产品测试方法、条件确实适用于本实验室的实际使用环境。
职责质量控制室所有人员负责按照本规程执行药典分析方法的确认。
程序药典方法确认需要做的项目原料药:制剂:被检测项目类型的说明:系统适用性实验按药典正文要求的内容进行;正文没有要求的内容,如RSD%,按药典附录的要求进行。
专属性系指在其他成分(如杂质、降解物、辅料等)可能存在下,采用的分析方法能够正确鉴定、检出被分析物质的特性。
通常,鉴别、杂质检查、含量测定方法中均应考察其专属性。
如采用的方法不够专属,应采用多个方法予以补充。
鉴别反应专属性鉴别试验应确证被分析物符合其特征。
专属性试验要求证明能与可能共存的物质或结构相似化合物区分,需确证含被分析物的供试品呈正反应,而不含被测成分的阴性对照呈负反应,结构相似或组分中的有关化合物也应呈负反应。
杂质检测专属性在杂质可获得的情况下,可向供试品中加入一定量的杂质,证明杂质与共存物质能得到分离和检出,并具适当的准确度与精密度。
在杂质或降解产物不能获得的情况下,专属性可通过与另一种已证明合理但分离或检测原理不同、或具较强分辨能力的方法进行结果比较来确定。
或将供试品用强光照射,高温,高湿,酸、碱水解及氧化的方法进行破坏(制剂应考虑辅料的影响),比较破坏前后检出的杂质个数和量。
必要时可采用二极管阵列检测和质谱检测,进行色谱峰纯度检查。
含量检测专属性➢对于API主成分含量测定可在供试品中加入杂质,考察测定结果是否受干扰,并与未加杂质的供试品比较测定结果;在杂质或降解产物不能获得的情况下,可采用另一个经验证了的或药典方法进行比较,对比两种方法测定的结果。
也可采用破坏性试验(强光照射,高温,高湿,酸、碱水解及氧化),得到含有杂质或降解产物的试样,用两种方法进行含量测定,比较测定结果。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
METHOD VERIFICATION PROTOCOL FOR CHROMATOGRAPHIC PURITY OF CYTARABINE 阿糖胞苷有关物质检查方法确认方案Contents目录一、In troduct ion (3)二、Scope (3)三、Resp on sibility (3)四、Defin iti ons (4)五、Project (6)六、Refere nces (27)In troduct ion简介## is in the process of developing Cytarabine Injection, a liquid product forpare nteral adm ini strati on, for ##. Cytarab ine is a chemotherapy age nt with a molecular weight of 243.2. The API from Zhejia ng Hisun Pharmaceutical Co. Ltd, which is approvedby FDA, is used for Cytarab ineInjection by Lummy.The Chromatographic purity method for Cytarab ine isthe method in the current USP34. The HPLC method for Chromatographicpurity test of Cytarabine is a quantitative method. The method will be verified according to USP<1226>, including system suitability, specificity,LOD, LOQ, precisi on, soluti on stability and mobile phase stability .阿糖胞苷注射液是一种临床用液体产品,##药业为##药业研发该品种。
阿糖胞苷是一种化学药,分子量为243.2。
莱美研发阿糖胞苷注射液所用原料药来源于浙江海正药业,是获得了FDA认证的原料药。
阿糖胞苷色谱纯度检测方法来自现行USP34, 色谱纯度检查方法,是定量检测方法。
依照USP<1226>,本次确认内容包括系统适用性,专属性,检测限,定量限、精密度、溶液稳定性和流动相稳定性。
Scope范围This protocol applies to the verification for chromatographic purity of cytarab ine.该方案适用于阿糖胞苷色谱纯度的方法确认。
Resp on sibility职责四、Defin iti ons定义1 Limit of Detectio n (LOD)检测限(LOD )The limit of detecti on of an in dividual an alytical procedure is the lowestamount of an alyte in a sample which can be detected but not n ecessarily qua ntitated asan exact value.分析规程中的检测限是指样品中的被分析物在该分析方法中能被检测,但不需要准确定量的最低浓度。
The detection limit is determined by analyzing samples with knownconcentrations of analyte and by establishing the minimum level at which the an alyte canbe reliably detected.检测限是通过分析样品已知浓度的被分析物,从而建立其可稳定检测的最低浓度水平。
2 Limit of Quantitation (LOQ)定量限(LOQ)The qua ntitati on limit of an in dividual an alytical procedure is the lowestamount of analyte in a sample which can be quantitatively determinedwith suitable precisi on and accuracy.分析方法中的定量限是指样品中被分析物能够被定量检测并保证一定精密度和准确度的最低浓度。
The limit of quantitation is a parameter of quantitative assays for low levelsof compounds in sample matrices, and is used particularly for the determ in ati on ofimpurities an d/or degradati on products.定量限是在样品中低浓度化合物被定量检测的参数,该参数经常被用于杂质和/或降解产物的测定。
3 system suitability系统适用性System suitability tests are an in tegral part of gas and liquidchromatographic methods. They are used to verify that resolution andreproducibility of the chromatographic system are adequatefor thean alysis to be done. The tests are based on the con cept that theequipme nt, electr oni cs, an alytical operati ons, and sample to be an alyzed con stitute an in tegral system that can be evaluated as such.系统适用性试验是气相色谱和液相色谱方法的必要组成部。
用以确认该分析方法色 谱系统的分离度和重现性是适用的。
该试验把分析设备、电子仪器、分析操作和分 析样品作为一个整体来进行评估。
Specificity 专属性Specificity is the ability to assess the an alyte un equivocally in the prese nee of componentswhich may be expected to be present. Typically thesemight in clude process impurities, degradates, matrix, etc.专属性是指在一些预期组分的存在下,被分析物被明确检测评估的能力。
通常这些 预期组分是指工艺杂质,降解产物,基质等。
Precisi on 精密度The precision of an analyticalprocedureexpresses the closeness ofagreeme nt (degree of scatter) betwee na seriesof measureme ntsobta ined from multiple sampli ng of the same homoge neous sample un der the prescribedcon diti ons.分析过程的精密度是指同一样品在指定的条件下所做的一系列测试值的相近程度 (离散度)。
Repeatability 重复性Repeatability expresses the precisi on un der the same operati ng con diti ons over a short in terval of time.重复性是指同一个实验室相同操作条件下短期内测定实验结果的精密度。
In termediate precisi on 中间精密度 In termediateprecisi onexpresses withi n-laboratoriesvariati ons:differe ntdays, differe nt an alysts, differe nt equipme nt, etc.中间精密度是指在同一实验室内改变其他条件,包括不同的日期,不同的人,不同 的仪器5.15.2等的精密度。
五、Project方案内容1 Accepta nee criteria2 Reage nts, sample, refere nee sta ndards, and in strume nts试剂、样品、标准品、仪器2.1 Reage nts试剂Mono basic sodium phosphate (HPLC)磷酸二氢钠(HPLC)Dibasic sodium phosphate (HPLC)磷酸氢二钠(HPLC)Sodium hydroxide (AR)氢氧化钠(AR)Phosphoric acid (AR)磷酸(AR)Metha nol (HPLC)甲醇(HPLC)Uridi ne (AR)尿苷(AR)Uracil (AR)尿嘧啶(AR)Water(purified, fresh daily)水(纯化水,每天新制)2.2 Sample样品Cytarab ine (Zhejia ng His un Pharmaceutical Co. Ltd, 1 batch)阿糖胞苷(浙江海正药业股份有限公司,1批)2.3 Refere nee sta ndards标准品USP Cytarabi ne RS(curre nt lot)阿糖胞苷USP标准品(现行批)USP Uracil arabi noside RS(curre nt lot)阿糖尿苷USP标准品(现行批)2.4 In strume nts仪器HPLC in strume nt 液相色谱仪Electr onic bala nee电子天平Waters e2695 XS205 Dual rang Metrohm 827pH 计3 Test method 测试方法USP 34 Cytarabi ne(in the appe ndix)3.1 Chromatographic con diti on 色谱条件Supelcosil LC-18-DB, 250 x 4.6mm , 5 m See gradie nt 见梯度 1.0 mL/min254 nm Column 20 °C temperature:柱温:Injection volume: 20 pl进样体积:Phosphate buffer: 磷酸盐缓冲液:Prepare a soluti on containing 0.01M mono basic sodium phosphate and0.01M dibasic sodium phosphate in a suitable contain er. Adjust with 0.1M sodium hydroxide or 0.1M phosphoric acid to a pH of 7.0.配制浓度为0.01M 的磷酸二氢钠和0.01M 的磷酸氢二钠溶液,用0.1M 氢氧化钠或 0.1M 磷酸调pH 至7.0。
