欧盟《食品添加剂标准》
欧盟添加剂限量标准

欧盟议会和理事会指令 95/2/EC
1995 年 2 月 20 除了色素和甜味剂的食品添加剂
欧盟议会和理事会
考虑到欧盟缔结的条约,尤其是第 100a 条, 考虑到委员会的提议, 考虑到经济和社会委员会的提议, 根据条约第 189b 制定的程序进行, 考虑到 1998 年 12 月 21 理事会指令 89/107/EEC,成员国关于为人类消费而在食品中授权使 用的食品添加剂的近似法律,尤其是第 3(2)条, 鉴于国家之间关于防腐剂、抗氧化剂和其它添加剂和它们的使用条件的不同,鉴于这可能造 成不公平的竞争。 鉴于主要考虑这些食品添加剂和它们的使用条件应当必须保护消费者的规定, 鉴于普遍公认未加工的食品和某些其它食品应当远离食品添加剂, 鉴于考虑到最近关于这些物质的科学和毒理学信息,一些仅允许用于某些食品和在某些条件 下使用, 鉴于必须制定严格的制度在婴儿配方、成长配方及断奶食品中使用食品添加剂,象在 1989 年 3 月 3 日理事会指令 89/398/EEC 中提到的,有关成员国用于特殊营养使用的食品的相近 法律,尤其是第 4(1)(e), 鉴于本指令不影响涉及甜味剂和色素的规定, 鉴于依据 1991 年 7 月 15 日理事会指令 91/414/EEC 涉及的植物保护产品投放市场,依据 1990 年 11 月 27 日理事会指令 90/642/EEC 附的在某些植物原始产品中农药残留的最高标准,包 括水果和蔬菜,属于此种类的物质暂时包括在本指令中, 鉴于向食品科学委员会咨询有关那些物质不是联盟规定的科目, 鉴于当决定一个特殊的食品属于食物的某个种类,最好跟随向食品标准委员会咨询的程序, 鉴于除色素和甜味剂外的食品添加剂的现存纯标准和那些没有纯标准存在的的新规范的修 改将采纳指令 89/107/EEC 第 11 条规定的相应程序, 鉴于食品科学委员会还没有提出关于面粉处理剂的的建议,鉴于那些试剂将在一个单独的 指令中规定, 鉴于本指令取代指令 64/54/EEC、70/357/EEC、74/329/EEC 和 83/463/EEC,鉴于那些指令特 此废除,
β-羟基β-甲基丁酸钙欧盟标准

β-羟基β-甲基丁酸钙欧盟标准全文共四篇示例,供读者参考第一篇示例:β-羟基β-甲基丁酸钙是一种在欧盟国家被广泛使用的添加剂,也被称为钙甲基羟丁酸盐。
它主要用作酸度调节剂和抗氧化剂,可以延长食品的保存期限和改善口感。
欧盟针对β-羟基β-甲基丁酸钙的使用制定了严格的标准,以确保食品安全和质量。
欧盟对β-羟基β-甲基丁酸钙的使用制定了严格的标准。
根据欧盟法规,食品添加剂必须符合以下要求:必须是合法的、安全的、有必要的,并且不会欺骗消费者。
对于β-羟基β-甲基丁酸钙,欧盟规定其最大使用量不能超过食品中所有添加剂的最大限值,以确保食品安全。
欧盟还规定了β-羟基β-甲基丁酸钙在食品中的使用范围和限制条件,禁止在某些特定食品中使用,如婴儿食品和特殊医学用途的配方食品。
除了食品安全方面的规定,欧盟还对食品标签中的添加剂信息做出了详细规定。
根据欧盟法律,所有食品标签必须清楚标明添加剂的名称和用量,以便消费者了解食品的成分和安全性。
对于β-羟基β-甲基丁酸钙,其在食品标签上必须以E码的形式标明,如E579,以区别于其他食品成分。
第二篇示例:β-羟基β-甲基丁酸钙,又称为β-hydroxy β-methyl butyrate calcium,简称HMB-Ca。
它是一种具有多种保健功效的营养补充剂,被广泛用于运动员和健身爱好者群体中。
欧盟对于HMB-Ca的标准非常严格,确保产品的质量和安全性,以保护消费者的健康。
为了确保HMB-Ca的质量和安全性,欧盟对其制定了严格的标准。
HMB-Ca的生产必须符合欧盟的生产规范,确保生产过程中严格控制原料的质量和生产工艺。
产品必须通过严格的质量检测,确保每批产品的成分含量准确,不含有害物质,符合欧盟食品安全标准。
欧盟要求HMB-Ca的产品标签必须清晰明了,包括产品成分、使用方法、适用人群、注意事项等信息必须清晰标注,以帮助消费者正确合理地选择和使用产品。
而且,欧盟还规定HMB-Ca的广告宣传必须真实准确,不得夸大功效或虚假宣传,以保护消费者权益。
欧盟与中国食品添加剂法规标准的对比分析

欧盟与中国食品添加剂法规标准的对比分析【论著】Contrast on Food Additive regulations and Standards between EU and China姚斯洁1,代汉慧2,李杏1,邹志飞3YAO Si-jie ,DAI Han-hui ,LI Xing ,ZOU Zhi-fei摘要目的为加强中国食品添加剂的管理及使用提供技术支持。
方法分别从概念、分类、编码、使用品种、范围和限量等方面对欧盟与中国食品添加剂的法规标准进行对比分析。
结果中国食品添加剂在概念、分类、编码、使用品种、范围和限量标准等方面与欧盟仍有差距。
探讨了欧盟与中国食品添加剂标准结构以及色素、甜味剂、防腐保鲜类品种的使用差异,提出了根据中国食品添加剂在生产、使用和检测技术中存在的问题,进一步完善法规标准建设,并探讨我国进出口食品及食品添加剂贸易的对策。
结论中国和欧盟的食品添加剂标准法规存在较大差异,会影响到双方的进出口贸易。
关键词食品添加剂;中国;欧盟;法规;标准;对比中国图书资料分类号:R155.5文献标识码:A文章编号:1004-1257(2011)12-1332-07Subject Contrast on Food Additive regulations and Standards between EU and ChinaAuthorsYAO Si-jie ,DAI Han-hui ,LI Xing ,ZOU Zhi-fei (Guangdong College of Pharmacy ,Guangzhou ,510310,China )Abstract [Objective ]To offer technical support for management and use of food additives in China.[Methods ]The differences in regulations and standards of food additives between EU and China from the definition ,functional categories ,coding ,variety ,service-able range ,dose limits were compared and analyzed.[Results ]There were differences in definition ,functional categories ,coding ,variety ,serviceable range ,dose limits and so on of food additives between EU and China.The paper explored the difference of ad-dictive use between EU and China on standard structure ,and the use of pigment ,sweetener ,preservation class ;put forward the problems which exist in production ,use and detection of addictive ;modified the regulations and standards ;and offered some coun-termeasures on food and food addictive which involved in imports and exports in China.[Conclusion ]There are more differences be-tween China and the EU in food additives standards and regulations ,which will affect the import and export trade.Key wordsFood additives ;China ;EU ;Standard ;Contrast基金项目:国家质检总局科技计划项目(项目编号:2009IK310);中国检科院资助项目(项目编号:2009JK011);广东省科技基础条件建设项目(粤科财字[2010]885)作者简介:姚斯洁,女,食品质量与安全专业在学。
欧盟食品添加剂法规
欧洲议会和欧盟理事会, 考虑到建立欧洲共同体的条约,特别是其第100a条; 考虑到委员会的提案(OJ No C 206,13.8.1992,p.12,和OJ No C 189,13.7.1993, p.11), 考虑到经济与社会委员会的意见(OJ No C 108,19.4.1993,p.26), 按照条约第189b条(1993年5月26日欧洲议会意见(OJ NoCl76,28.6.1993,p.117), 批准于1993年12月2日(OJ NoC 342,20.12.1993),1994年3月10日理事会共同立场(OJ No C 172,24.6.1994,p.4))以及1994年11月16日欧洲议会 决议(OJ NoC 341,5.12.1994) 规定的程序采取措施, 考虑到理事会1988年12月21日关于使各成员国食品添加剂法律趋于一致的指令89/107 /EEC(OJ No L 40,11.2.1989,p,27),特别是其中的第3条第2款,
M5
L 284 1 2003—10—
(EC)No 1882/2003
3l
M6 2003 年 12 月 22 日欧洲议会和理事会指令 2003/ L 24
58 2004—01
114/EC
—29
注:文中凡被修订的条款均加注了M1,M2,M3,M4,M5,M6的上标.修订指令的生效日 期如下:
M1的生效日期为:1997年4月4日。 M2的生效日期为:1998年11月4日。符合该指令的产品将最迟于2000年5月4日获准 销售。不符合该指令 的产品将自2000年11月4日起禁止销售,不过在此日期前已投放市场或已加贴标签 的产品可以继续销售直 至售完为止。 M3的生效日期为:2001年2月24日。 M4的生效日期为:2003年?月l?日。 M5的生效日期为:2003年11月20日。 M6的生效日期为:2004年1月29日。符合该指令的产品将最迟于2005年?月27日获准 销售和使用。不符合该指令的产品将自2006年1月27日起禁止销售和使用,不过在此日 期前已投放市场或已加贴标签的产品可以继续销售直到售完为止。
欧盟食品添加剂标准

欧盟食品添加剂标准欧盟食品添加剂标准是指欧盟对食品中使用的添加剂所制定的一系列规定和要求。
添加剂是指为了改变食品的色、香、味、形态和保质期等特征而向食品中添加的物质。
欧盟对食品添加剂的使用进行了严格的管理和监督,旨在保障消费者的健康和权益,确保食品的安全和质量。
首先,欧盟对食品添加剂的使用进行了明确的限制和规定。
根据欧盟的相关法规,食品添加剂必须经过严格的安全评估和批准程序,才能被允许在食品中使用。
欧盟对食品添加剂的种类、用量和使用范围等方面都做出了详细的规定,以确保食品的安全性和可靠性。
其次,欧盟对食品添加剂的标签和说明也做出了严格的要求。
根据欧盟的法规,食品添加剂必须在食品包装上进行清晰的标注,并提供详细的使用说明和安全警示。
消费者在购买食品时,可以通过查看食品包装上的标签信息,了解食品中是否含有添加剂以及添加剂的种类和用量,从而做出更加明智的消费选择。
此外,欧盟还对食品添加剂的监督和检测进行了严格的规定。
欧盟成员国必须建立健全的食品安全监管体系,对食品添加剂的使用进行全程监督和抽检。
一旦发现食品添加剂使用不当或超过规定的用量,将会采取严厉的处罚措施,以确保食品的安全和合法性。
总的来说,欧盟食品添加剂标准的制定和执行,旨在保障消费者的健康和权益,确保食品的安全和质量。
消费者在购买食品时,可以通过查看食品包装上的标签信息,了解食品中是否含有添加剂以及添加剂的种类和用量,从而做出更加明智的消费选择。
同时,食品生产企业也应当严格遵守欧盟的相关法规,确保食品添加剂的使用符合规定,为消费者提供安全、健康的食品产品。
欧盟食品添加剂标准的不断完善和执行,将为食品安全和消费者权益保驾护航。
聚甘油脂肪酸酯欧盟标准

聚甘油脂肪酸酯欧盟标准全文共四篇示例,供读者参考第一篇示例:聚甘油脂肪酸酯是一种常见的食品添加剂,广泛应用于食品工业中。
欧盟对聚甘油脂肪酸酯的使用做出了一系列标准和规定,以保障消费者的健康和安全。
本文将对欧盟关于聚甘油脂肪酸酯的标准进行详细介绍。
聚甘油脂肪酸酯是由甘油和脂肪酸酯化反应制成的一种混合物。
它能够改善食品的口感、保持食品的形状和稳定性,并延长食品的保质期。
在欧盟,聚甘油脂肪酸酯被广泛用于面包、饼干、糕点、乳制品等各类食品中。
根据欧盟法规,聚甘油脂肪酸酯属于食品添加剂,其使用必须符合《食品添加剂使用标准》(EC)号231/2012的规定。
根据该标准,聚甘油脂肪酸酯的用量应符合《食品添加剂用量指导》(E)号1129/2011的限量,且不得超过最大使用限量。
欧盟还规定了聚甘油脂肪酸酯的质量标准。
根据《食品添加剂质量标准》(EU)号231/2012的规定,聚甘油脂肪酸酯必须符合规定的物理、化学指标,并且不得含有对人体健康有害的金属、病原微生物等有害物质。
欧盟还对聚甘油脂肪酸酯的标签和说明书做出了具体规定。
在食品包装上必须清晰标注聚甘油脂肪酸酯的名称和用量,并且提醒消费者应遵循使用指导。
如果食品中含有聚甘油脂肪酸酯,还必须在产品说明书上明确标注。
除了上述规定外,欧盟还对聚甘油脂肪酸酯的生产企业和检验机构进行了监管。
生产企业必须在符合食品安全生产标准的环境下生产,检验机构必须具备权威的检验技术和设备,并能够准确检测聚甘油脂肪酸酯中有害物质的含量。
欧盟对聚甘油脂肪酸酯的标准和规定旨在确保食品安全和消费者权益。
只有符合规定的聚甘油脂肪酸酯才能在欧盟市场上合法销售和使用。
消费者在购买食品时,应留意食品标签上的成分表,避免食用含有违规聚甘油脂肪酸酯的食品,以保障自身健康与安全。
第二篇示例:聚甘油脂肪酸酯是一种多功能的食品添加剂,被广泛用于食品工业中作为乳化剂、稳定剂和增稠剂等。
在欧盟国家,对于聚甘油脂肪酸酯的使用和标准也有着严格的规定。
中国和欧亚联盟食品添加剂法规标准比较分析

中国和欧亚联盟食品添加剂法规标准比较分析1. 引言1.1 背景介绍随着全球化的发展和食品贸易的增加,食品安全问题变得越发重要。
食品添加剂作为提高食品质量和延长食品保质期的重要手段,受到了广泛关注。
中国和欧亚联盟作为两个重要的食品生产和出口大国,其食品添加剂法规标准对于保障食品安全和贸易畅通具有重要意义。
通过比较中国和欧亚联盟食品添加剂法规标准,可以发现两者之间的差异和共同点,为双方在食品安全领域的合作提供参考和借鉴。
本文旨在对中国和欧亚联盟食品添加剂法规标准进行比较分析,探讨两者的差异和启示,并展望未来的发展趋势。
1.2 研究目的研究目的:本文旨在通过对中国和欧亚联盟食品添加剂法规标准的比较分析,探讨两者在食品安全监管体系和执行情况方面的异同,为我国食品安全监管体系的完善和提升提供借鉴和启示。
通过对比中国和欧亚联盟食品添加剂法规标准的内容和执行情况,可以发现双方在食品安全监管的经验和措施上的优势和不足之处,为双方在食品安全领域的合作提供参考。
通过分析两者的法规标准差异,可以为我国食品安全监管体系的改进和发展提供建议,促进我国食品安全水平的提高,保障公众健康和权益。
通过本研究的深入分析,可以为未来食品安全监管的发展趋势提供参考,为建立更加完善的食品安全监管体系提供借鉴和指导。
2. 正文2.1 中国食品添加剂法规标准概述中国食品添加剂法规标准旨在保障食品安全,维护消费者权益,促进食品行业健康发展。
中国食品安全法规将食品添加剂分为色素、防腐剂、甜味剂等多个类别,并对其使用范围、最大使用量等进行了详细规定。
中国食品安全法规强调了食品添加剂的安全性和合法性,对生产、储存、运输、销售等各个环节都有严格的监管要求。
中国食品添加剂法规标准在制定过程中充分考虑了国际食品安全标准和国内实际情况,力求达到国际先进水平。
中国食品添加剂法规标准涵盖了众多细则和规范,例如《食品添加剂使用标准》、《食品添加剂安全评价规范》等,这些规定详细规定了不同种类食品添加剂的使用标准和安全评价方法。
欧盟添加剂限量标准

No L61/1Ⅰ欧盟议会和理事会指令95/2/EC1995年2月20除了色素和甜味剂的食品添加剂欧盟议会和理事会考虑到欧盟缔结的条约,尤其是第100a条,考虑到委员会的提议,考虑到经济和社会委员会的提议,根据条约第189b制定的程序进行,考虑到1998年12月21理事会指令89/107/EEC,成员国关于为人类消费而在食品中授权使用的食品添加剂的近似法律,尤其是第3(2)条,鉴于国家之间关于防腐剂、抗氧化剂和其它添加剂和它们的使用条件的不同,鉴于这可能造成不公平的竞争。
鉴于主要考虑这些食品添加剂和它们的使用条件应当必须保护消费者的规定,鉴于普遍公认未加工的食品和某些其它食品应当远离食品添加剂,鉴于考虑到最近关于这些物质的科学和毒理学信息,一些仅允许用于某些食品和在某些条件下使用,鉴于必须制定严格的制度在婴儿配方、成长配方及断奶食品中使用食品添加剂,象在1989年3月3日理事会指令89/398/EEC中提到的,有关成员国用于特殊营养使用的食品的相近法律,尤其是第4(1)(e),鉴于本指令不影响涉及甜味剂和色素的规定,鉴于依据1991年7月15日理事会指令91/414/EEC涉及的植物保护产品投放市场,依据1990年11月27日理事会指令90/642/EEC附的在某些植物原始产品中农药残留的最高标准,包括水果和蔬菜,属于此种类的物质暂时包括在本指令中,鉴于向食品科学委员会咨询有关那些物质不是联盟规定的科目,鉴于当决定一个特殊的食品属于食物的某个种类,最好跟随向食品标准委员会咨询的程序,鉴于除色素和甜味剂外的食品添加剂的现存纯标准和那些没有纯标准存在的的新规范的修改将采纳指令89/107/EEC第11条规定的相应程序,鉴于食品科学委员会还没有提出关于面粉处理剂的的建议,鉴于那些试剂将在一个单独的指令中规定,鉴于本指令取代指令64/54/EEC、70/357/EEC、74/329/EEC和83/463/EEC,鉴于那些指令特此废除,兹采纳如下指令:第1条1.本指令是一个构成综合指令的特定的指令,在89/107/EEC指令的第3条的内涵之内,适用于除色素和甜味剂和面粉处理剂外的添加剂。
欧盟允许使用的食品添加剂

欧盟允许使用的食品添加剂欧洲委员会的立法对食品添加剂有以下3个粗略的分类:(1)甜味剂(94/35/EC法令);(2)着色剂(94/36/EC法令);(3)除甜味剂和着色剂以外的添加剂(95/2/EC法令)——“多种”添加剂法令。
欧盟的食品添加剂分类及定义见下表:欧盟添加剂种类定义酸增加食物酸度的物质以及赋予食物酸味的物质酸度调节剂调节和控制食物酸碱性的物质抗结剂能减少食物颗粒黏附到一起的物质消泡剂能阻止或减少泡沫形成的物质膨松剂能增加食品的体积,而对其能量值没有明显影响的物质载体以及载体溶剂用于溶解、稀释食物或用其他物理手段以改变食物状态的添加剂,以方便操作、应用或使用着色剂增加或恢复食物颜色的物质,包括食物的天然成分和天然来源的物质,这些物质不能作为食物来消费,也不能作为食物的特征成分乳化剂将两种或两种以上互不相容的物质如食物中的油和水形成均匀混合物或能维持混合物均匀状态的物质乳化盐将乳酪中的蛋白质转化为分散的形式,从而使脂肪和其他成分呈均匀分布状态的物质固定剂使水果和蔬菜组织保持新鲜状态的物质或者可与凝胶剂作用形成和增强凝胶的物质增味剂增强食物原有口味或/和风味的物质面粉处理剂(FTA)添加到面粉和面团,改善面团烘焙质量的物质(与乳化剂不同)上光剂涂抹到食物外表面后,可赋予食物光泽外观或能给食物提供保护层的物质水分保持剂能防止食物在低湿度的空气中变干的物质,或促进粉状物在水溶液中溶解的物质改性淀粉将食用淀粉进行一种或多种化学处理,如物理处理、酶处理、酸处理或碱处理后得到的物质填充气体除了空气以外的,在将食物放入容器前、放入时或放入后充入到容器中的气体推进气体除了空气以外的,将食物从容器中排出的气体发泡剂本身或混合后可释放出气体,从而增大生面团或面糊体积的物质发泡剂本身或混合后可释放出气体,从而增大生面团或面糊体积的物质螯合剂可与金属离子形成化合物的物质稳定剂能维持食物物理状态的物质,能使食物中两种或两种以上互不相容的物质呈均匀分散状态的物质,能使食物已有色泽保持稳定的物质甜味剂能赋予食物甜味的物质增稠剂能增加食物黏度的物质欧盟食品添加剂列表酸编码添加剂名称270 乳酸296 苹果酸297 富马酸330 柠檬酸334 酒石酸338 磷酸353 偏酒石酸355 己二酸363 琥珀酸507 盐酸513 硫酸574 葡萄糖575 葡糖酸一δ一内醋酸度调节剂编码添加剂名称327 乳酸钙331 柠檬酸钠:(1)柠檬酸一钠;(2)柠檬酸二钠;(3)柠檬酸三钠332 柠檬酸钾:(1)柠檬酸一钾;(2)柠檬酸三钾333 柠檬酸钙:(1)柠檬酸一钙;(2)柠檬酸二钙;(3)柠檬酸三钙339 磷酸钠:(1)磷酸一钠;(2)磷酸二钠;(3)磷酸三钠340 磷酸钾:(1)磷酸一钾;(2)磷酸二钾;(3)磷酸三钾341 磷酸钙:(1)磷酸一钙;(2)磷酸二钙;(3)磷酸三钙343 磷酸镁:(1)磷酸一镁;(2)磷酸二镁350 苹果酸钠:(1)苹果酸钠;(2)苹果酸氢钠351 苹果酸钾352 苹果酸钙:(1)苹果酸钙;(2)苹果酸氢钙354 酒石酸钙356 己二酸钠357 己二酸钾380 柠檬酸三铵450 二磷酸二钙500 碳酸钠:(1)碳酸钠;(3)碳酸氢三钠501 碳酸钾:(1)碳酸钾;(2)碳酸氢钾503 碳酸铵:(1)碳酸铵;(2)碳酸氢铵504 碳酸镁:(1)碳酸镁;(2)碳酸氢镁514 硫酸氢钠515 磷酸氢钾522 硫酸钾铝523 硫酸饺铝524 氢氧化钠525 氢氧化钾526 氢氯化钙527 氢氧化铵528 氢氧化镁529 氧化钙577 葡糖酸钾578 葡糖酸钙抗结剂编码添加剂名称530 氧化镁535 亚铁氰化钠536 亚铁氰化钾538 亚铁氰化钙551 二氧化硅552 硅酸钙553 硅酸镁:a(1)硅酸镁;(2)1硅酸镁(合成) 、2三硅酸镁b 滑石粉554 硅酸铝钠555 硅酸铝钾556 硅酸铝钙558 膨润土559 硅酸铝(高岭土)消泡剂编码添加剂名称900 甲基聚硅氧烷1521 聚乙二醇6000膨松剂编码添加剂名称1200 聚糊精着色剂编码添加剂名称100 姜黄素101 核黄素:(1)核黄素;(2)核黄素-5’-磷酸102 柠檬黄104 喹啉黄110 日落黄;柑橘黄120 胭脂虫红,胭脂红酸,洋红122 偶氮玉红,酸性红123 苋菜红124 胭脂红,洋红A127 赤藓红128 红色2G129 诱惑红131 专利蓝V132 靛蓝133 亮蓝140 叶绿素和叶绿酸:(1)叶绿素;(2)叶绿酸141 叶绿素和叶绿酸的铜盐:(1)叶绿素铜盐;(2)叶绿酸铜盐142 绿色S150 焦糖色:a 不加氨生产;b 苛性亚硫酸法;c加氨生产; d 亚硫酸铵法151 亮黑.黑色PN153 植物炭154 棕色FK155 棕色HT160 胡萝卜素:a 胡萝卜素(1)复合胡萝卜素;(2)β-胡萝卜素b 胭脂橙红,胭脂树橙,降胭脂树橙c 红辣椒提取物,辣椒红素,辣椒玉红素d 番茄红素e β-阿朴-8’-胡萝卜醛(C30)f β-阿朴-8’-胡萝卜酸(C30)乙酯161 b 叶黄素;g 斑蝥黄质162 甜菜红,甜菜苷163 花青素苷类170 碳酸钙171 二氧化钛172 铁的氧化物和氢氧化物173 铝174 银175 金180 立素玉红BK(Litholrubine BK)乳化剂编码添加剂名称322 卵磷脂431 聚氧乙烯硬脂酸酯432 聚氧乙烯山梨醇酐433 聚氧乙烯山梨醇酐油酸酯434 聚氧乙烯山梨醇酐单棕榈酸酯435 聚氯乙烯山梨醇酐单硬脂酸酯436 聚氧乙烯山梨醇酐三硬脂酸酯442 磷脂酸铵444 酸异丁酸葡糖酯445 木松香甘油酯460 纤维素:(1)微晶纤维素;(2)粉状纤维素470 a 脂肪酸钠、钾、盐;b 脂肪酸镁盐471 脂肪酸单、双甘油酯472 脂肪酸单、双甘油酸酯:a 脂肪酸单、双甘油乙酸酯b 脂肪酸单、双甘油乳酸酯c 脂肪酸单、双甘油柠檬酸酯d 脂肪酸单、双甘油酒石酸酯e 脂肪酸单、双甘油单、双乙酰化酒石酸酯f 脂肪酸单、双甘油混合乙酸和酒石酸酯481 硬脂酰乳酸钠482 硬脂酰乳酸钙491 单硬脂酸山梨醇酐酯492 三硬脂酸山梨醇酐酯493 单月桂酸山梨醇酐酯494 单油酸山梨醇酐酯495 单棕榈酸山梨醇酐酯473 蔗糖脂肪酸酯474 蔗糖甘油酯475 脂肪酸聚甘油酯476 聚甘油聚蓖麻醇酸酯477 脂肪酸丙二醇单、双酯479 b 热氧化大豆油与脂肪酸单、双甘油酯的交联物乳化盐编码添加剂名称450 二磷酸盐:(2)二磷酸三钠;(3)二磷酸四钠;(5)二磷酸四钾(7)二磷酸二氢酐452 聚磷酸盐:(1)2聚磷酸钠(不溶);(2)聚磷酸钾;(3)聚磷酸钠钙;(4)聚磷酸钙固定剂编码添加剂名称。
欧盟食品添加剂法规

成同质分布状态的物质; (k)“固化剂”是指那些使水果或蔬菜的组织保持结实或脆的,或与胶凝剂彼此作用,
产生或加强凝胶体的物质; (1)“增味剂”是指那些增加食品现存味道和(或)气味的物质; (m)“发泡剂”是指在液体或固体食品中可使气态的物质形成同质弥散状态的物质; (n)“胶凝剂”是指那些通过形成凝胶体赋予食品一定质地的物质; (o)“上光剂”(包括润滑剂)是指那些用于食品外表时可产生光亮外表或提供保护膜的
的物质; (b)“抗氧化剂”是指那些防止因氧化引起的食品腐败变质,如脂肪变质以及颜色变化,
从而延长食品保存期的物质; (c)“载体”,包括载体溶剂,是指那些为了方便食品添加剂的处理,应用或使用,用
来溶解、稀释、分散或其他不改变食品添加剂的技术功能只产生物理变化的物质(并且它们 本身未发挥任何技术作用);
以外的气体; (s)“推进气体”是指那些把食品从容器中排出的气体(不是空气); (t)“膨松剂”是指能用来释放气体并借此增加面团或糊状物的体积的物质或某些物质
的混合物; (u)“螯合剂”是指与金属离子一起形成化学复合物的物质; (v)“6“稳定剂”是保持食品物理化学状态的物质;稳定剂包括使食品中两种或多种不
(d)“酸”是指增加食品酸度和(或)使食品具有酸味的物质; (e)“酸度调节剂”是改变或控制食品酸度或碱度的物质; (f)“抗结剂”是指那些减少食品个体微粒彼此粘合倾向的物质; (g)“消泡剂”是指那些预防或减少泡沫的物质; (h)“填充剂”是指那些有助于食品的体积而无益于其可获取的能量价值的物质; “乳化剂”是指那些可能使两种或更多的不可溶合状态的物质,例如食品中的油和水形 成或保持一种同质混合状态的物质; “乳化盐”是指那些使干酪中的蛋白质转化为一种分散状态,从而使脂肪和其他成分形
欧盟关于食品添加剂限量
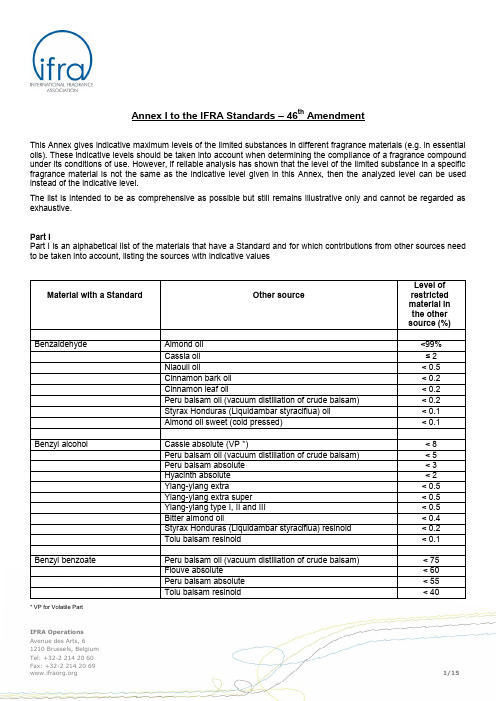
* VP for Volatile PartIFRA Operations Avenue des Arts, 61210 Brussels, Belgium Annex I to the IFRA Standards – 46th AmendmentThis Annex gives indicative maximum levels of the limited substances in different fragrance materials (e.g. in essential oils). These indicative levels should be taken into account when determining the compliance of a fragrance compound under its conditions of use. However, if reliable analysis has shown that the level of the limited substance in a specific fragrance material is not the same as the indicative level given in this Annex, then the analyzed level can be used instead of the indicative level.The list is intended to be as comprehensive as possible but still remains illustrative only and cannot be regarded as exhaustive.Part IPart I is an alphabetical list of the materials that have a Standard and for which contributions from other sources need to be taken into account, listing the sources with indicative values1Please note that this material is prohibited in the 44th Amendment due to insufficient data.Part IIPart II is an alphabetical list of other sources (complex synthetic materials or essential oils) for restricted materials having Standards and which must therefore be taken into account for determination of the maximum level.2Please note that this material is prohibited in the 44th Amendment due to insufficient data.* VP for Volatile Part* VP for Volatile Part* VP for Volatile Part* VP for Volatile Part* VP for Volatile Part。
国内外食品添加剂标准对比

国内外食品添加剂标准对比食品添加剂是指在食品生产、加工过程中添加的具有特定技术效果的物质。
食品添加剂在提高食品质量和保持食品特性方面起着重要作用。
不同国家和地区对食品添加剂的标准存在一定的差异,本文将对国内外食品添加剂标准进行对比。
一、国内食品添加剂标准国内食品添加剂标准主要由中国国家标准化管理委员会和中国食品药品监督管理局制定并实施。
1.标准范围:国内食品添加剂标准主要涉及食品安全、质量控制、食品添加剂使用范围、质量指标、添加量等方面。
2.标准分类:国内将食品添加剂分为色素、防腐剂、发酵剂、甜味剂、香精香料等不同类别,并对每个类别的使用范围、添加量、质量指标等进行明确规定。
3.审批程序:国内食品添加剂需经过严格的审批程序才能上市销售。
从理论研发到实际应用,需要通过实验室研究、动物实验、人体试验等多个环节。
通过各项测试合格后,才能获得相关部门的批准。
4.限用范围:国内对食品添加剂的使用范围有较为明确的规定,特定类型的食品添加剂只能在特定食品中使用,如某些防腐剂只能在肉制品中使用,某些色素只能在糕点中使用等。
二、国外食品添加剂标准1.标准范围:不同国家和地区对食品添加剂的标准有所不同,但大多关注食品安全和质量控制。
标准根据不同食品添加剂的类别制定,包括色素、防腐剂、甜味剂、酸味剂、稳定剂等。
2.标准分类:国外食品添加剂标准根据不同食品添加剂的特性和用途进行分类。
其中,欧盟、美国、日本的标准相对较为严格,对食品添加剂的监管较为细致。
3.审批程序:国外食品添加剂的审批程序相对国内来说更加简化,同时也更加注重科学性和实际应用效果,重点关注安全性和效果。
在审批过程中,通常需要提交相关的实验和测试报告,但相对于国内,审批周期较短。
4.限用范围:不同国家和地区对食品添加剂的使用范围也有所不同。
例如,欧盟的食品添加剂标准要求严格,对于某些可能对人体健康产生潜在风险的食品添加剂,有可能被禁止使用或限制使用。
三、国内外食品添加剂标准对比1.标准颁布机构差异:国内食品添加剂标准由中国国家标准化管理委员会和中国食品药品监督管理局制定,而国外标准则由各自的相关部门制定,如欧盟食品安全局、美国食品药品监督管理局等。
欧盟食品添加剂法规04
理事会指令89/107/EEC使各成员国食品添加剂法律趋于一致的指令(OJ L40,11.2.1989,P.27)Ml的生效日期为:1994年9月10日。
M2的生效日期为:2003年11月20日。
欧洲共同体理事会,考虑到建立欧洲经济共同体的条约,特别是其第100a条,考虑到委员会的提案,为与欧洲议会合作(OJ No C 99,13.4.1987,p.65和OJ No C 12,16.1.1989),考虑到经济与社会委员会的意见(OJ No C 328,22.12.1986,p.5),鉴于各国食品添加剂的法律和使用条件的差异阻碍了食品的自由流通;鉴于这些差异可能会为不公平的竞争创造条件,进而会对共同市场的建立或机能产生直接影响;鉴于有必要使这些法律趋于一致;鉴于这些要求应包含在一项全面综合性的指令中,而该指令有必要分阶段起草;鉴于一个指令所涵盖的食品添加剂种类的清单,根据条约第100a条规定的程序,是由理事会做出决定;鉴于属于上述种类的食品添加剂的使用,只有建立在与理事会规定的科学技术标准一致的基础上,才应被批准;鉴于在起草食品添加剂清单和它们的使用条件时,在采用很可能会影响公众健康的规定之前,应与依据委员会74/234/EEC(OJ No L 136,20.5.1974,p.1),决议成立的食品科学委员会磋商;鉴于采用认可的添加剂清单必须就科学技术的发展而言是可行的;有鉴于此,除了条约规定程序的规则外,为了寻求共同体的解决方法,通过采用临时的国家措施来建立允许各成员国发挥作用的体制也可能是适当的;鉴于上述食品添加剂纯度标准的测定以及分析和取样方法的制定都是委托委员会处理的技术问题;鉴于任何情况下理事会都会授权委员会实施有关食品的规则,应制订条款规范个成员国与委员会间就食品常务委员会依据委员会决议69/414/EEC(OJ No L 29l,19.11.1969,p.9)设立)业务范围内紧密合作的程序;兹通过本指令:第1条1.本指令应适用于附录中列出的、在食品加工或制备过程中用作或打算用作配料且在最终产品中仍然存在(尽管改,变了存在的形式)的各种食品添加剂。
欧盟食品添加剂标准法规(食品添加、食品酶制剂和食品用香料的通用许可程序)

I(Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory)REGULATIONSREGULATION(EC)No1331/2008OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof16December2008establishing a common authorisation procedure for food additives,food enzymes and food flavourings(Text with EEA relevance)THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EURO-PEAN UNION,Having regard to the Treaty establishing the European Commu-nity,and in particular Article95thereof,Having regard to the proposal from the Commission,Having regard to the opinion of the European Economic and Social Committee(1),Acting in accordance with the procedure laid down in Article251 of the Treaty(2),Whereas:(1)The free movement of safe and wholesome food is anessential aspect of the internal market and contributes sig-nificantly to the health and well-being of citizens,and totheir social and economic interests.(2)A high level of protection of human life and health shouldbe assured in the pursuit of Community policies.(3)In order to protect human health,the safety of additives,enzymes and flavourings for use in foodstuffs for humanconsumption must be assessed before they are placed onthe Community market.(4)Regulation(EC)No1333/2008of the European Parlia-ment and of the Council of16December2008on foodadditives(3),Regulation(EC)No1332/2008of the Euro-pean Parliament and of the Council of16December2008on food enzymes(4)and Regulation(EC)No1334/2008of the European Parliament and of the Council of16December2008on flavourings and certain food ingre-dients with flavouring properties for use in and on foods(5)(hereinafter referred to as the sectoral food laws)lay downharmonised criteria and requirements concerning theassessment and authorisation of these substances.(5)It is envisaged,in particular,that food additives,foodenzymes and food flavourings,to the extent that the safetyof food flavourings must be assessed in accordance withRegulation(EC)No1334/2008[on flavourings and certainfood ingredients with flavouring properties for use in andon foods],must not be placed on the market or used infoodstuffs for human consumption,in accordance with theconditions laid down in each sectoral food law,unless theyare included on a Community list of authorised substances.(6)Ensuring transparency in the production and handling offood is absolutely crucial in order to maintain consumerconfidence.(7)In this context,it appears appropriate to establish for thesethree categories of substances a common Communityassessment and authorisation procedure that is effective,time-limited and transparent,so as to facilitate their freemovement within the Community market.(1)OJ C168,20.7.2007,p.34.(2)Opinion of the European Parliament of10July2007(OJ C175E,10.7.2008,p.134),Council Common Position of10March2008 (OJ C111E,6.5.2008,p.1),Position of the European Parliament of 8July2008(not yet published in the Official Journal)and Council Decision of18November2008.(3)See page16of this Official Journal.(4)See page7of this Official Journal.(5)See page34of this Official Journal.(8)This common procedure must be founded on the prin-ciples of good administration and legal certainty and mustbe implemented in compliance with those principles.(9)This Regulation will thus complete the regulatory frame-work concerning the authorisation of the substances bylaying down the various stages of the procedure,the dead-lines for those stages,the role of the parties involved andthe principles that apply.Nevertheless,for some aspects ofthe procedure,it is necessary to take the specific character-istics of each sectoral food law into consideration.(10)The deadlines laid down in the procedure take into accountthe time needed to consider the different criteria set in eachsectoral food law,as well as allowing adequate time forconsultation when preparing the draft measures.In par-ticular,the nine-months deadline for the Commission topresent a draft regulation updating the Community listshould not preclude the possibility of this being donewithin a shorter period.(11)Upon receipt of an application the Commission should ini-tiate the procedure and where necessary seek the opinionof the European Food Safety Authority(hereinafter referredto as the Authority)established by Regulation(EC)No178/2002of the European Parliament and of the Coun-cil of28January2002laying down the general principlesand requirements of food law,establishing the EuropeanFood Safety Authority and laying down procedures in mat-ters of food safety(1)as soon as possible after the validityand applicability of the application have been assessed.(12)In accordance with the framework for risk assessment inmatters of food safety established by Regulation(EC)No178/2002,the authorisation to place substances on themarket must be preceded by an independent scientificassessment,of the highest possible standard,of the risksthat they pose to human health.This assessment,whichmust be carried out under the responsibility of the Author-ity,must be followed by a risk management decision takenby the Commission under a regulatory procedure thatensures close cooperation between the Commission andthe Member States.(13)The authorisation to place substances on the market shouldbe granted pursuant to this Regulation provided that thecriteria for authorisation laid down under the sectoral foodlaws are satisfied.(14)It is recognised that,in some cases,scientific risk assess-ment alone cannot provide all the information on which arisk management decision should be based,and that otherlegitimate factors relevant to the matter under consider-ation may be taken into account,including societal,eco-nomic,traditional,ethical and environmental factors andthe feasibility of controls.(15)In order to ensure that both business operators in the sec-tors concerned and the public are kept informed of theauthorisations in force,the authorised substances shouldbe included on a Community list created,maintained andpublished by the Commission.(16)Where appropriate and under certain circumstances,thespecific sectoral food law may provide for protection ofscientific data and other information submitted by theapplicant for a certain period of time.In this case,the sec-toral food law should lay down the conditions under whichthese data may not be used for the benefit of anotherapplicant.(17)Networking between the Authority and the Member States’organisations operating in the fields within the Authority’smission is one of the basic principles of the Authority’soperation.In consequence,in preparing its opinion,theAuthority may use the network made available to it byArticle36of Regulation(EC)No178/2002and by Com-mission Regulation(EC)No2230/2004(2).(18)The common authorisation procedure for the substancesmust fulfil transparency and public information require-ments while guaranteeing the right of applicants to pre-serve the confidentiality of certain information.(19)Protecting the confidentiality of certain aspects of an appli-cation should be maintained as a consideration in order toprotect the competitive position of an applicant.However,information relating to the safety of a substance,includ-ing,but not limited to,toxicological studies,other safetystudies and raw data as such,should under no circum-stances be confidential.(20)Pursuant to Regulation(EC)No178/2002,Regulation(EC)No1049/2001of the European Parliament and of theCouncil of30May2001regarding public access to Euro-pean Parliament,Council and Commission documents(3)applies to documents held by the Authority.(1)OJ L31,1.2.2002,p.1.(2)Regulation(EC)No2230/2004of23December2004laying downdetailed rules for the implementation of European Parliament and Council Regulation(EC)No178/2002with regard to the network of organisations operating in the fields within the European Food Safety Authority’s mission(OJ L379,24.12.2004,p.64).(3)OJ L145,31.5.2001,p.43.(21)Regulation(EC)No178/2002establishes procedures fortaking emergency measures in relation to foodstuffs ofCommunity origin or imported from third countries.Itauthorises the Commission to adopt such measures in situ-ations where foodstuffs are likely to constitute a seriousrisk to human health,animal health or the environmentand where such risk cannot be contained satisfactorily bymeasures taken by the Member State(s)concerned.(22)In the interests of efficiency and legislative simplification,there should be a medium-term examination of the ques-tion whether to extend the scope of the common proce-dure to other legislation in the area of food.(23)Since the objectives of this Regulation cannot be suffi-ciently achieved by the Member States on account of dif-ferences between national laws and provisions and cantherefore be better achieved at Community level,the Com-munity may adopt measures,in accordance with the prin-ciple of subsidiarity as set out in Article5of the Treaty.Inaccordance with the principle of proportionality,as set outin that Article,this Regulation does not go beyond what isnecessary in order to achieve those objectives.(24)The measures necessary for the implementation of thisRegulation should be adopted in accordance with CouncilDecision1999/468/EC of28June1999laying down theprocedures for the exercise of implementing powers con-ferred on the Commission(1).(25)In particular the Commission should be empowered toupdate the Community lists.Since those measures are ofgeneral scope and are designed to amend non-essential ele-ments of each sectoral food law,inter alia,by supplement-ing it with new non-essential elements,they must beadopted in accordance with the regulatory procedurewith scrutiny provided for in Article5a of Decision1999/468/EC.(26)On grounds of efficiency,the normal time-limits for theregulatory procedure with scrutiny should be curtailed forthe addition of substances to the Community lists and foradding,removing or changing conditions,specifications orrestrictions associated with the presence of a substance onthe Community lists.(27)When,on imperative grounds of urgency,the normal time-limits for the regulatory procedure with scrutiny cannot becomplied with,the Commission should be able to applythe urgency procedure provided for in Article5a(6)ofDecision1999/468/EC for the removal of a substancefrom the Community lists and for adding,removing orchanging conditions,specifications or restrictions associ-ated with the presence of a substance on the Communitylists,HAVE ADOPTED THIS REGULATION:CHAPTER IGENERAL PRINCIPLESArticle1Subject matter and scope1.This Regulation lays down a common procedure for the assessment and authorisation(hereinafter referred to as the com-mon procedure)of food additives,food enzymes,food flavour-ings and source materials of food flavourings and of food ingredients with flavouring properties used or intended for use in or on foodstuffs(hereinafter referred to as the substances),which contributes to the free movement of food within the Community and to a high level of protection of human health and to a high level of consumer protection,including the protection of con-sumer interests.This Regulation shall not apply to smoke flavour-ings falling within the scope of Regulation(EC)No2065/2003of the European Parliament and of the Council of10November 2003on smoke flavourings used or intended for use in or on foods(2).2.The common procedure shall lay down the procedural arrangements for updating the lists of substances the marketing of which is authorised in the Community pursuant to Regulation (EC)No1333/2008[on food additives],Regulation(EC) No1332/2008[on food enzymes]and Regulation(EC) No1334/2008[on flavourings and certain food ingredients with flavouring properties for use in and on foods](hereinafter referred to as the sectoral food laws).3.The criteria according to which substances can be included on the Community list provided for in Article2,the content of the regulation referred to in Article7and,where applicable,the transitional provisions concerning ongoing procedures are laid down in each sectoral food law.Article2Community list of substances1.Under each sectoral food law,substances that have been authorised to be placed on the Community market shall be included on a list the content of which is determined by the said law(hereinafter referred to as the Community list).The Commu-nity list shall be updated by the Commission.It shall be published in the Official Journal of the European Union.2.‘Updating the Community list’means:(a)adding a substance to the Community list;(1)OJ L184,17.7.1999,p.23.(2)OJ L309,26.11.2003,p.1.(b)removing a substance from the Community list;(c)adding,removing or changing conditions,specifications orrestrictions associated with the presence of a substance on the Community list.CHAPTER IICOMMON PROCEDUREArticle3Main stages of the common procedure1.The common procedure for updating the Community list may be started either on the initiative of the Commission or fol-lowing an application.Applications may be made by a Member State or by an interested party,who may represent several inter-ested parties,in accordance with the conditions provided for by the implementing measures referred to in Article9(1)(a)(herein-after referred to as the applicant).Applications shall be sent to the Commission.2.The Commission shall seek the opinion of the European Food Safety Authority(hereinafter referred to as the Authority),to be given in accordance with Article5.However,for the updates referred to in Article2(2)(b)and(c),the Commission shall not be required to seek the opinion of the Authority if the updates in question are not liable to have an effect on human health.3.The common procedure shall end with the adoption by the Commission of a regulation implementing the update,in accor-dance with Article7.4.By way of derogation from paragraph3,the Commission may end the common procedure and decide not to proceed with a planned update,at any stage of the procedure,if it judges that such an update is not justified.Where applicable,it shall take account of the opinion of the Authority,the views of Member States,any relevant provisions of Community law and any other legitimate factors relevant to the matter under consideration.In such cases,where applicable,the Commission shall inform the applicant and the Member States directly,indicating in its letter the reasons for not considering the update justified.Article4Initiating the procedure1.On receipt of an application to update the Community list, the Commission:(a)shall acknowledge receipt of the application in writing to theapplicant within14working days of receiving it;(b)where applicable,shall as soon as possible notify the Author-ity of the application and request its opinion in accordance with Article3(2).The application shall be made available to the Member States by the Commission.2.Where it starts the procedure on its own initiative,the Com-mission shall inform the Member States and,where applicable, request the opinion of the Authority.Article5Opinion of the Authority1.The Authority shall give its opinion within nine months of receipt of a valid application.2.The Authority shall forward its opinion to the Commission, the Member States and,where applicable,the applicant.Article6Additional information concerning risk assessment1.In duly justified cases where the Authority requests addi-tional information from applicants,the period referred to in Article5(1)may be extended.After consulting the applicant,the Authority shall lay down a period within which this information can be provided and shall inform the Commission of the addi-tional period needed.If the Commission does not object within eight working days of being informed by the Authority,the period referred to in Article5(1)shall be automatically extended by the additional period.The Commission shall inform the Member States of the extension.2.If the additional information is not sent to the Authority within the additional period referred to in paragraph1,the Authority shall finalise its opinion on the basis of the informa-tion already provided.3.Where applicants submit additional information on their own initiative,they shall send it to the Authority and to the Com-mission.In such cases,the Authority shall give its opinion within the original period without prejudice to Article10.4.The additional information shall be made available to the Member States and the Commission by the Authority.Article7Updating the Community list1.Within nine months of the Authority giving its opinion,the Commission shall submit to the Committee referred to in Article14(1)a draft regulation updating the Community list,tak-ing account of the opinion of the Authority,any relevant provi-sions of Community law and any other legitimate factors relevant to the matter under consideration.In those cases where an opinion of the Authority has not been requested,the nine-month period shall start from the date the Commission receives a valid application.2.In the Regulation updating the Community list,the consid-erations on which it is based shall be explained.3.Where the draft regulation is not in accordance with the opinion of the Authority,the Commission shall explain the rea-sons for its decision.4.The measures,designed to amend non-essential elements of each sectoral food law,relating to the removal of a substance from the Community list,shall be adopted in accordance with the regulatory procedure with scrutiny referred to in Article14(3).5.On grounds of efficiency,the measures designed to amend non-essential elements of each sectoral food law,inter alia,by supplementing it,relating to the addition of a substance to the Community list and for adding,removing or changing conditions, specifications or restrictions associated with the presence of the substance on the Community list,shall be adopted in accordance with the regulatory procedure with scrutiny referred to in Article14(4).6.On imperative grounds of urgency,the Commission may use the urgency procedure referred to in Article14(5)for the removal of a substance from the Community list and for adding, removing or changing conditions,specifications or restrictions associated with the presence of a substance on the Community list.Article8Additional information concerning risk management1.Where the Commission requests additional information from applicants on matters concerning risk management,it shall determine,together with the applicant,a period within which that information can be provided.In such cases,the period referred to in Article7may be extended accordingly.The Commission shall inform the Member States of the extension and shall make the additional information available to the Member States once it has been provided.2.If the additional information is not sent within the addi-tional period referred to in paragraph1,the Commission shall act on the basis of the information already provided.CHAPTER IIIMISCELLANEOUS PROVISIONSArticle9Implementing measures1.In accordance with the regulatory procedure referred to in Article14(2),within a period of no longer than24months from the adoption of each sectoral food law,the implementing mea-sures for this Regulation shall be adopted by the Commission,and shall concern in particular:(a)the content,drafting and presentation of the applicationreferred to in Article4(1);(b)the arrangements for checking the validity of applications;(c)the type of information that must be included in the opinionof the Authority referred to in Article5.2.With a view to the adoption of the implementing measures referred to in paragraph1(a),the Commission shall consult the Authority,which,within six months of the date of entry into force of each sectoral food law,shall present it with a proposal concerning the data required for risk assessment of the substances concerned.Article10Extension of time periodsIn exceptional circumstances,the periods referred to in Article5(1)and Article7may be extended by the Commission on its own initiative or,where applicable,at the Authority’s request, if the nature of the matter in question so justifies,without preju-dice to Article6(1)and Article8(1).In such cases the Commis-sion shall,where appropriate,inform the applicant and the Member States of the extension and the reasons for it.Article11TransparencyThe Authority shall ensure the transparency of its activities in accordance with Article38of Regulation(EC)No178/2002.In particular,it shall make its opinions public without delay.It shall also make public any request for its opinion as well as any exten-sion of period pursuant to Article6(1).Article12Confidentiality1.Among the information provided by applicants,confiden-tial treatment may be given to information the disclosure of which might significantly harm their competitive position.Information relating to the following shall not,in any circum-stances,be regarded as confidential:(a)the name and address of the applicant;(b)the name and a clear description of the substance;(c)the justification for the use of the substance in or on specificfoodstuffs or food categories;(d)information that is relevant to the assessment of the safety ofthe substance;(e)where applicable,the analysis method(s).2.For the purposes of implementing paragraph1,applicants shall indicate which of the information provided they wish to be treated as confidential.Verifiable justification must be given in such cases.3.The Commission shall decide after consulting with the applicants which information can remain confidential and shall notify applicants and the Member States accordingly.4.After being made aware of the Commission’s position, applicants shall have three weeks in which to withdraw their application so as to preserve the confidentiality of the informa-tion provided.Confidentiality shall be preserved until this period expires.5.The Commission,the Authority and the Member States shall,in accordance with Regulation(EC)No1049/2001,take the necessary measures to ensure appropriate confidentiality of the information received by them under this Regulation,except for information which must be made public if circumstances so require in order to protect human health,animal health or the environment.6.If an applicant withdraws,or has withdrawn,its application, the Commission,the Authority and the Member States shall not disclose confidential information,including information the con-fidentiality of which is the subject of disagreement between the Commission and the applicant.7.The implementation of paragraphs1to6shall not affect the circulation of information between the Commission,the Authority and the Member States.Article13EmergenciesIn the event of an emergency concerning a substance on the Com-munity list,particularly in the light of an opinion of the Author-ity,measures shall be adopted in accordance with the procedures referred to in Articles53and54of Regulation(EC)No178/2002.Article14Committee1.The Commission shall be assisted by the Standing Commit-tee on the Food Chain and Animal Health established by Article58 of Regulation(EC)No178/2002.2.Where reference is made to this paragraph,Articles5and7 of Decision1999/468/EC shall apply,having regard to the pro-visions of Article8thereof.The period laid down in Article5(6)of Decision1999/468/EC shall be set at three months.3.Where reference is made to this paragraph,Article5a(1) to(4)and Article7of Decision1999/468/EC shall apply,having regard to the provisions of Article8thereof.4.Where reference is made to this paragraph,Article5a(1) to(4)and(5)(b)and Article7of Decision1999/468/EC shall apply,having regard to the provisions of Article8thereof.The time-limits laid down in Article5a(3)(c)and(4)(b)and(e)of Decision1999/468/EC shall be two months,two months and four months respectively.5.Where reference is made to this paragraph,Article5a(1),(2), (4)and(6)and Article7of Decision1999/468/EC shall apply, having regard to the provisions of Article8thereof.Article15Competent authorities of the Member StatesNot later than six months after the entry into force of each sec-toral food law,Member States shall forward to the Commission and to the Authority,in relation to each sectoral food law,the name and address of the national competent authority for the purposes of the common procedure,as well as a contact point therein.CHAPTER IVFINAL PROVISIONArticle16Entry into forceThis Regulation shall enter into force on the20th day following its publication in the Official Journal of the European Union.For each sectoral food law,it shall apply from the date of appli-cation of the measures referred to in Article9(1).Article9shall apply from20January2009.This Regulation shall be binding in its entirety and directly applicable in all Member States. Done at Strasbourg,16December2008.For the European ParliamentThe PresidentH.-G.PÖTTERING For the Council The President B.LE MAIRE。
欧盟允许使用的食品添加剂

欧盟允许使用的食品添加剂对物质进行分类是一个比较复杂的过程,食品添加剂的分类也不例外,欧盟是从使用功能上对添加剂进行分类的。
从功能方面对食品添加剂进行分类是一种比较好的方法,由于添加剂的功能取决于其加入量的多少,所以它与摄入量是有密切联系的,在评价消费者可能的摄入量时,功能以及添加剂量是需要考虑的两个重要方面。
欧洲委员会的立法对食品添加剂有以下3个粗略的分类:(1)甜味剂(94/35/EC法令);(2)着色剂(94/36/EC法令);(3)除甜味剂和着色剂以外的添加剂(95/2/EC法令)——“多种”添加剂法令。
欧盟的食品添加剂分类及定义见下表:欧盟添加剂种类定义酸增加食物酸度的物质以及赋予食物酸味的物质酸度调节剂调节和控制食物酸碱性的物质抗结剂能减少食物颗粒黏附到一起的物质消泡剂能阻止或减少泡沫形成的物质膨松剂能增加食品的体积,而对其能量值没有明显影响的物质载体以及载体溶剂用于溶解、稀释食物或用其他物理手段以改变食物状态的添加剂,以方便操作、应用或使用着色剂增加或恢复食物颜色的物质,包括食物的天然成分和天然来源的物质,这些物质不能作为食物来消费,也不能作为食物的特征成分乳化剂将两种或两种以上互不相容的物质如食物中的油和水形成均匀混合物或能维持混合物均匀状态的物质乳化盐将乳酪中的蛋白质转化为分散的形式,从而使脂肪和其他成分呈均匀分布状态的物质固定剂使水果和蔬菜组织保持新鲜状态的物质或者可与凝胶剂作用形成和增强凝胶的物质增味剂增强食物原有口味或/和风味的物质面粉处理剂(FTA) 添加到面粉和面团,改善面团烘焙质量的物质(与乳化剂不同)上光剂涂抹到食物外表面后,可赋予食物光泽外观或能给食物提供保护层的物质水分保持剂能防止食物在低湿度的空气中变干的物质,或促进粉状物在水溶液中溶解的物质改性淀粉将食用淀粉进行一种或多种化学处理,如物理处理、酶处理、酸处理或碱处理后得到的物质填充气体除了空气以外的,在将食物放入容器前、放入时或放入后充入到容器中的气体推进气体除了空气以外的,将食物从容器中排出的气体发泡剂本身或混合后可释放出气体,从而增大生面团或面糊体积的物质发泡剂本身或混合后可释放出气体,从而增大生面团或面糊体积的物质螯合剂可与金属离子形成化合物的物质稳定剂能维持食物物理状态的物质,能使食物中两种或两种以上互不相容的物质呈均匀分散状态的物质,能使食物已有色泽保持稳定的物质甜味剂能赋予食物甜味的物质增稠剂能增加食物黏度的物质欧盟食品添加剂列表酸编码添加剂名称270 乳酸296 苹果酸2330 柠檬酸334 酒石酸338 磷酸353 偏酒石酸355 己二酸363 琥珀酸507 盐酸513 硫酸574 葡萄糖575 葡糖酸一δ一内醋酸度调节剂编码添加剂名称327 乳酸钙331 柠檬酸钠:(1)柠檬酸一钠;(2)柠檬酸二钠;(3)柠檬酸三钠332 柠檬酸钾:(1)柠檬酸一钾;(2)柠檬酸三钾333 柠檬酸钙:(1)柠檬酸一钙;(2)柠檬酸二钙;(3)柠檬酸三钙339 磷酸钠:(1)磷酸一钠;(2)磷酸二钠;(3)磷酸三钠340 磷酸钾:(1)磷酸一钾;(2)磷酸二钾;(3)磷酸三钾341 磷酸钙:(1)磷酸一钙;(2)磷酸二钙;(3)磷酸三钙343 磷酸镁:(1)磷酸一镁;(2)磷酸二镁350 苹果酸钠:(1)苹果酸钠;(2)苹果酸氢钠351 苹果酸钾352 苹果酸钙:(1)苹果酸钙;(2)苹果酸氢钙354 酒石酸钙356 己二酸钠357 己二酸钾380 柠檬酸三铵450 二磷酸二钙500 碳酸钠:(1)碳酸钠;(3)碳酸氢三钠501 碳酸钾:(1)碳酸钾;(2)碳酸氢钾503 碳酸铵:(1)碳酸铵;(2)碳酸氢铵504 碳酸镁:(1)碳酸镁;(2)碳酸氢镁514 硫酸氢钠515 磷酸氢钾522 硫酸钾铝523 硫酸饺铝524 氢氧化钠525 氢氧化钾526 氢氯化钙527 氢氧化铵528 氢氧化镁529 氧化钙577 葡糖酸钾3抗结剂编码添加剂名称530 氧化镁535 亚铁氰化钠536 亚铁氰化钾538 亚铁氰化钙551 二氧化硅552 硅酸钙553 硅酸镁:a(1)硅酸镁;(2)1硅酸镁(合成) 、2三硅酸镁b 滑石粉554 硅酸铝钠555 硅酸铝钾556 硅酸铝钙558 膨润土559 硅酸铝(高岭土)消泡剂编码添加剂名称900 甲基聚硅氧烷1521 聚乙二醇6000膨松剂编码添加剂名称1200 聚糊精着色剂编码添加剂名称100 姜黄素101 核黄素:(1)核黄素;(2)核黄素-5’-磷酸102 柠檬黄104 喹啉黄110 日落黄;柑橘黄120 胭脂虫红,胭脂红酸,洋红122 偶氮玉红,酸性红123 苋菜红124 胭脂红,洋红A127 赤藓红128 红色2G129 诱惑红131 专利蓝V132 靛蓝133 亮蓝140 叶绿素和叶绿酸:(1)叶绿素;(2)叶绿酸141 叶绿素和叶绿酸的铜盐:(1)叶绿素铜盐;(2)叶绿酸铜盐142 绿色S150 焦糖色:a 不加氨生产;b 苛性亚硫酸法;c加氨生产;d 亚硫酸铵法151 亮黑.黑色PN153 植物炭4154 棕色FK155 棕色HT160 胡萝卜素:a 胡萝卜素(1)复合胡萝卜素;(2)β-胡萝卜素b 胭脂橙红,胭脂树橙,降胭脂树橙c 红辣椒提取物,辣椒红素,辣椒玉红素d 番茄红素e β-阿朴-8’-胡萝卜醛(C30)f β-阿朴-8’-胡萝卜酸(C30)乙酯161 b 叶黄素;g 斑蝥黄质162 甜菜红,甜菜苷163 花青素苷类170 碳酸钙171 二氧化钛172 铁的氧化物和氢氧化物173 铝174 银175 金180 立素玉红BK(Litholrubine BK)乳化剂编码添加剂名称322 卵磷脂431 聚氧乙烯硬脂酸酯432 聚氧乙烯山梨醇酐433 聚氧乙烯山梨醇酐油酸酯434 聚氧乙烯山梨醇酐单棕榈酸酯435 聚氯乙烯山梨醇酐单硬脂酸酯436 聚氧乙烯山梨醇酐三硬脂酸酯442 磷脂酸铵444 酸异丁酸葡糖酯445 木松香甘油酯460 纤维素:(1)微晶纤维素;(2)粉状纤维素470 a 脂肪酸钠、钾、盐;b 脂肪酸镁盐471 脂肪酸单、双甘油酯472 脂肪酸单、双甘油酸酯:a 脂肪酸单、双甘油乙酸酯b 脂肪酸单、双甘油乳酸酯c 脂肪酸单、双甘油柠檬酸酯d 脂肪酸单、双甘油酒石酸酯e 脂肪酸单、双甘油单、双乙酰化酒石酸酯f 脂肪酸单、双甘油混合乙酸和酒石酸酯481 硬脂酰乳酸钠482 硬脂酰乳酸钙491 单硬脂酸山梨醇酐酯492 三硬脂酸山梨醇酐酯493 单月桂酸山梨醇酐酯494 单油酸山梨醇酐酯5495 单棕榈酸山梨醇酐酯473 蔗糖脂肪酸酯474 蔗糖甘油酯475 脂肪酸聚甘油酯476 聚甘油聚蓖麻醇酸酯477 脂肪酸丙二醇单、双酯479 b 热氧化大豆油与脂肪酸单、双甘油酯的交联物乳化盐编码添加剂名称450 二磷酸盐:(2)二磷酸三钠;(3)二磷酸四钠;(5)二磷酸四钾(7)二磷酸二氢酐452 聚磷酸盐:(1)2聚磷酸钠(不溶);(2)聚磷酸钾;(3)聚磷酸钠钙;(4)聚磷酸钙固定剂编码添加剂名称520 硫酸铝521 硫酸钠铝增味剂编码添加剂名称515 (1)硫酸钾620 谷氨酸621 谷氨酸一钠622 谷氨酸一钾623 谷氨酸钙624 谷氨酸一铵625 谷氨酸镁626 鸟苷酸627 鸟苷酸二钠628 鸟苷酸二钾629 鸟苷酸钙630 肌酐酸631 鸟苷酸二钠632 鸟苷酸二钾633 鸟苷酸钙634 5’一核糖核苷酸钙635 5’一核糖核苷酸二钠640 甘氨酸及其钠盐面粉处理剂编码添加剂名称483 硬脂酰酒石酸酯517 硫酸铵541 酸性磷酸铝钠920 L一半胱氨酸927 b 尿素516 硫酸钙抛光剂6编码添加剂名称469 酶水解的羧甲基纤维素904 虫胶905 结晶石蜡901 蜂蜡,白色和黄色902 小烛树蜡903 巴西棕榈蜡水分保持剂编码添加剂名称422 甘油1518 三乙酸甘油酯(甘油三乙酸酯)1520 丙烷一1,2—二元醇填充气体编码添加剂名称938 氩939 氦948 氧推进气体编码添加剂名称290 二氧化碳941 氮942 一氧化二氮发泡剂编码添加剂名称450 (1)二磷酸二钠500 (2)碳酸氢钠999 皂树皮提取物螯合剂编码添加剂名称350 EDTA钙钠451 (2)三磷酸五钾452 (1)1 聚磷酸钠(可溶)451 (1)三磷酸五钠576 葡糖酸钠稳定剂编码添加剂名称335 酒石酸钠盐:(1)酒石酸一钠;(2)酒石酸二钠336 酒石酸钾盐:(1)酒石酸一钾;(2)酒石酸二钾337 酒石酸钾钠468 交联羧甲基纤维素钠1201 聚乙烯毗咯烷酮1505 柠檬酸三乙酯570 脂肪酸甜味剂编码添加剂名称420 山梨醇:(1)山梨醇;(2)山梨醇糖浆7953 异麦芽糖醇965 麦芽糖醇:(1)麦芽糖醇;(2)麦芽糖醇糖浆966 乳糖醇967 木糖醇950 乙酰磺胺酸钾(安赛蜜)951 天门冬酰苯丙氨酸甲酯(阿斯巴甜)952 环己基氨基磺酸,环己基氨基磺酸钠/钙(用量以环己基氨基磺酸计) 954 糖精,糖精钠/钾/钙(以游离酰亚胺的量表示)957 索马甜959 新橙皮苷防腐剂编码添加剂名称200 山梨酸202 山梨酸钾203 山梨酸钙210 苯甲酸211 苯甲酸钠212 苯甲酸钾213 苯甲酸钙214 对羟基苯甲酸乙酯215 对羟基苯甲酸乙酯钠216 对羟基苯甲酸丙酯217 对羟基苯甲酸丙酯钠218 对羟基苯甲酸甲酯219 对羟基苯甲酸甲酯钠220 二氧化硫221 亚硫酸钠222 亚硫酸氢钠223 偏亚硫酸钠224 偏亚硫酸钾226 亚硫酸钙227 亚硫酸氢钙228 亚硫酸氢钾230 联(二)苯231 邻苯基苯酚232 邻苯基苯酚钠234 乳酸链球菌素235 纳他霉素239 六亚甲基四胺242 焦磷酸二甲酯249 亚硝酸钾250 亚硝酸钠251 硝酸钠252 硝酸钾260 乙酸8262 乙酸钠:(1)乙酸钠;(2)乙酸氢钠263 乙酸钙280 丙酸281 丙酸钠282 丙酸钙283 丙酸钾284 硼酸285 四硼酸钠(硼砂)912 二十九烷酸酯914 氧化聚乙烯蜡1105 溶菌酶助色剂及保色助剂编码添加剂名称514 (1)硫酸钠512 氯化锡579 葡糖酸亚铁585 乳酸亚铁1202 聚乙烯聚吡咯烷酮9。
欧盟防腐剂标准

欧盟防腐剂标准1. 范围本标准规定了欧盟内销售的食品中防腐剂的使用要求,包括食品添加剂和营养强化剂的使用要求、食品标签和营养标签的要求、食品安全性评估程序等。
2. 规范性引用文件本标准引用了以下文件:(1)欧盟委员会(EC)No 1333/2008号条例,关于食品添加剂的使用范围和限量;(2)欧盟委员会(EC)No 1169/2011号条例,关于食品标签和营养标签;(3)欧盟委员会(EC)No 2273/2006号条例,关于食品中微生物限量;(4)欧盟委员会(EC)No 852/2004号条例,关于食品卫生的一般要求。
3. 术语和定义本标准规定了以下术语和定义:(1)防腐剂:用于防止或延缓食品腐败变质的一类物质。
(2)最大使用量:在食品中添加的防腐剂的最大限量。
(3)食品标签:在食品包装上标明食品成分、营养信息、使用方法等信息的说明。
4. 通用要求(1)防腐剂的使用应符合欧盟相关法规的规定。
(2)在食品中添加防腐剂时,应考虑其化学性质、作用机制、使用量等因素,确保其安全性。
(3)防腐剂的使用应结合食品的成分、含量、质量等因素,以确保其有效性。
5. 食品添加剂和营养强化剂的使用要求(1)在食品中添加防腐剂时,应按照规定的种类、浓度和用量使用,不得超过最大使用量。
(2)在食品中添加营养强化剂时,应按照规定的种类、浓度和用量使用,不得影响食品的安全性和质量。
6. 食品标签和营养标签的要求(1)在食品包装上应清晰标注食品的成分、含量、质量等信息,以便消费者了解食品的详细情况。
(2)在食品包装上应标注防腐剂的使用情况,包括种类、浓度和用量等信息,以便消费者了解食品中的防腐剂情况。
食品添加剂添加标准

食品添加剂添加标准英文回答:Food additives are substances that are added to food products to improve their taste, appearance, texture, and shelf life. These additives can be natural or synthetic and are regulated by food safety authorities to ensure thatthey are safe for consumption.In the United States, the Food and Drug Administration (FDA) establishes and enforces standards for food additives. The FDA conducts extensive research to determine the safety of additives before they are approved for use. They alsoset maximum allowable levels for each additive and require manufacturers to label their products with a list of ingredients.For example, one common food additive is monosodium glutamate (MSG), which is used to enhance the flavor of savory foods. The FDA has determined that MSG is generallyrecognized as safe (GRAS) when consumed in normal amounts. However, some individuals may have a sensitivity to MSG and experience symptoms such as headaches or flushing.Another example is the use of preservatives, such as sodium benzoate, to extend the shelf life of food products. The FDA has established a maximum allowable level for sodium benzoate and requires manufacturers to indicate its presence on the product label. This allows consumers to make informed choices based on their individual dietary needs or preferences.Food additives are also regulated in other countries. For instance, in the European Union, the European Food Safety Authority (EFSA) is responsible for evaluating the safety of food additives. The EFSA sets acceptable daily intake levels for additives and assesses their potential risks to human health.In China, the Ministry of Health is in charge of food safety regulations, including the use of food additives. The Chinese Food Safety Standards (GB) outline therequirements for food additives, including their permitted uses and maximum allowable levels. These standards are regularly updated to ensure the safety of food products in the Chinese market.中文回答:食品添加剂是为了改善食品的口感、外观、质地和保质期而添加的物质。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1995L0002 — EN — 29.01.2004 — 005.001 — 1
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
►B
EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC
of 20 February 1995
on food additives other than colours and sweeteners
(OJ L 61, 18.3.1995, p. 1)
Amended by:
Official Journal
No
page
date
►M1 Directive 96/85/EC of the European Parliament and of the Council of 19 L 86 December 1996
4
28.3.1997
►M2 Directive 98/72/EC of the European Parliament and of the Council of 15 L 295
Whereas the Commission is to adapt Community provisions to accord with the rules laid down in this Directive;
(1) OJ No C 206, 13. 8. 1992, p. 12, and OJ No C 189, 13. 7. 1993, p. 11. (2) OJ No C 108, 19. 4. 1993, p. 26. (3) Opinion of the European Parliament of 26 May 1993 (OJ No C 176, 28. 6.
Whereas differences between national laws relating to preservatives, antioxidants and other additives and their conditions of use hinder the free movement of foodstuffs; whereas this may create conditions of unfair competition;
Having regard to the proposal from the Commission (1),
Having regard to the opinion of the Economic and Social Committee (2),
Acting in accordance with the procedure laid down in Article 189b of the Treaty (3),
Whereas this Directive is not intended to affect rules relating to sweeteners and colours;
Whereas, pending specific provisions pursuant to Council Directive 91/ 414/EEC of 15 July 1991 concerning the placing of plant protection products on the market (6), and pursuant to Council Directive 90/642/ EEC of 27 November 1990 on the fixing of maximum levels for pesticide residues in and on certain products of plant origin, including fruit and vegetables (7), certain substances belonging to this category are provisionally covered by this Directive;
58
29.1.2004
22 ted by: ►C1 Corrigendum, OJ L 248, 14.10.1995, p. 60 (95/2/EC)
1995L0002 — EN — 29.01.2004 — 005.001 — 2
▼B EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/ 2/EC
Whereas it is necessary to include in this Directive specific provisions concerning additives referred to in other Community provisions;
Whereas it is desirable that when a decision is taken on whether a particular foodstuff belongs to a certain category of foods, the consultation of the Standing Committee for Foodstuffs procedure is followed;
1993, p. 117), confirmed on 2 December 1993 (OJ No C 342, 20. 12. 1993), common position of the Council of 10 March 1994 (OJ No C 172, 24. 6. 1994, p. 4) and decision of the European Parliament of 16 November 1994 (OJ No C 341, 5. 12. 1994) (4) OJ No L 40, 11. 2. 1989, p. 27. (5) OJ No L 186, 30. 6. 1989, p. 27. (6) OJ No L 230, 19. 8. 1991, p. 1. Directive as last amended by Commission Regulation (EEC) No 3600/92 (OJ No L 366, 15. 12. 1992, p. 10). (7) OJ No L 350, 14. 12. 1990, p. 71.
23
18 June 2003
17.7.2003
►M5 Regulation (EC) No 1882/2003 of the European Parliament and of the L 284 Council of 29 September 2003
1
31.10.2003
►M6 Directive 2003/114/EC of the European Parliament and of the Council of L 24
Whereas, having regard to the most recent scientific and toxicological information on these substances, some of them are to be permitted only for certain foodstuffs and under certain conditions of use;
18
October 1998
4.11.1998
►M3 Directive 2001/5/EC of the European Parliament and of the Council of L 55
59
24.2.2001
12 February 2001
►M4 Directive 2003/52/EC of the European Parliament and of the Council of L 178
EN
Consolidated TEXT
produced by the CONSLEG system
of the Office for Official Publications of the European Communities
CONSLEG: 1995L0002 — 29/01/2004
<
Number of pages: 53 Office for Official Publications of the European Communities
Whereas modifications of existing purity criteria for food additives other than colours and sweeteners and new specifications for those where no purity criteria exist will be adopted in accordance with the procedure laid down in Article 11 of Directive 89/107/EEC;
Whereas it is necessary to lay down strict rules for the use of food additives in infant formulae, follow-on formulae and weaning foods, as referred to in Council Directive 89/398/EEC of 3 May 1989 on the approximation of the laws of the Member States relating to foodstuffs intended for particular nutritional uses (5), and in particular Article 4 (1) (e) thereof;
