阿莫西林克拉维酸钾审评文件
阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度测定和有关物质检查

阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度测定和有关物质检查作者:李静玲李瑞明冯鹏胡建楣林小凤范佩冰来源:《中国医药导报》2013年第07期[摘要] 目的对阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度和有关物质进行了研究,用来评价和确定制剂使用中的安全性及药物本身的内在质量。
方法通过对制备得到的阿莫西林克拉维酸钾干混悬剂含量均匀度的测定和有关物质考察,从而确定阿莫西林克拉维酸钾干混悬剂质量的可控性。
结果通过本文中所用方法对阿莫西林克拉维酸钾干混悬剂(14∶1)中阿莫西林、克拉维酸钾的含量均匀度和有关物质测定结果说明制剂质量合格,所以在制剂使用中阿莫西林克拉维酸钾干混悬剂能确保其安全性。
结论阿莫西林克拉维酸钾干混悬剂(14∶1)中阿莫西林、克拉维酸钾的含量均匀度和有关物质的测定均能符合制剂要求。
[关键词] 阿莫西林;克拉维酸钾;含量均匀度;有关物质[中图分类号] R927.11 [文献标识码] A [文章编号] 1673-7210(2013)03(a)-0123-03阿莫西林(Amoxicillin)(C16H19N3O5S)是一种最常用的青霉素类广谱β-内酰胺类抗生素,半衰期约为61.3 min。
阿莫西林杀菌作用强,穿透细胞膜的能力也强,是目前应用较为广泛的口服青霉素之一,其制剂有胶囊、片剂、颗粒剂、分散片等。
单独用阿莫西林时容易产生耐药性而降低药物的疗效;克拉维酸钾(C8H9NO5)仅有微弱的抗菌活性,但可与多数的β-内酰胺酶牢固结合,生成不可逆的结合物,具有强力而广谱的抑制β-内酰胺酶作用。
阿莫西林制剂配合克拉维酸钾使用时能有效稳定阿莫西林,使其降解减少而疗效增强,且对革兰阳性及革兰阴性细菌均有效[1-3]。
有关物质是药物中主要疗效成分在储存中产生或在原料制备中引进的,说明了药物对温度、湿度、光等外在因素的稳定度及药物本身使用中的安全性。
含量均匀度是药物有效成分含量较小和比例较低时,在制剂的制备中可能引起分布不均匀而影响药物的质量。
阿莫西林克拉维酸钾颗粒(4:1)工艺研究及稳定性考察

3 、制备 工艺流程
3 . 1 将阿莫西林、阿莫西林克拉维酸钾混 粉( 2 : 1 ) , 按阿莫西林克拉维酸钾重量 比 ( 4 : 1 )计算投料量 。 3 . 2将辅料庶糖 、微晶纤维素、微粉硅胶 于9 0 ℃ 烘 干 4小 时,庶糖水分 小于 0 . 1 %, 微 晶纤维素、微粉硅胶水分均小于 2 . 0 %,并 过 5号筛备用。 3 _ 3在相对湿度小于 2 5 %, 温度 2 O 。 C以下 的条件下 ,用逐级稀 释法将 阿莫西林 、阿莫 西林 克拉维酸钾 ( 2 :1 ) 、处方量的辅料混合 试脸条件 7 5 ± 5 %R H 考察指 标 性状
1 、处方依据
参照 中国药 典 2 0 1 0版二部标准【 】 及 国家 食 品药品监 督管理局标 准 YB HO 4 4 1 2 0 0 9标准 【 本 品每包含 阿莫西林 0 . 1 2 5克,克拉维酸 0 . 0 3 1 2 5克。本 品为白色至 淡黄色颗粒或 混悬 型颗 粒或细颗 粒 ,气 芳香,味甜 。处方 中大 部分 为庶糖外 ,只加少 量的填充剂 、助流剂 和芳香矫味剂 。
医 学论 坛
阿莫西林克拉维酸钾颗粒 ( 4 :1 ) 工艺研究及稳定性考察
杨启玉
四川子仁制 药有 限公司 6 1 8 3 0 0
摘要 : 1 3 一内酰胺酶抑制剂能有效地抑制 1 3一 内酰胺酶 的活性 ,与 1 3一 内酰胺抗生素联合用 药可扩展抗 生素 的应用 范围。本 阿莫西林 克拉 维酸钾颗 粒 为阿莫西林 与克拉维酸钾 以 4 :1组成 的复方制剂。本 试验 研究阿莫西林 克拉维酸钾颗粒 ( 4 :1 )生产工 艺处方 ,考察温度、湿度、光线及不 同包 装 材料对本制剂 的影响。 关键 词: 1 3 一 内酰胺酶抑制 剂 1 3 一内酰胺 酶 工艺研究
阿莫西林克拉维酸钾片含量测定方法的改进
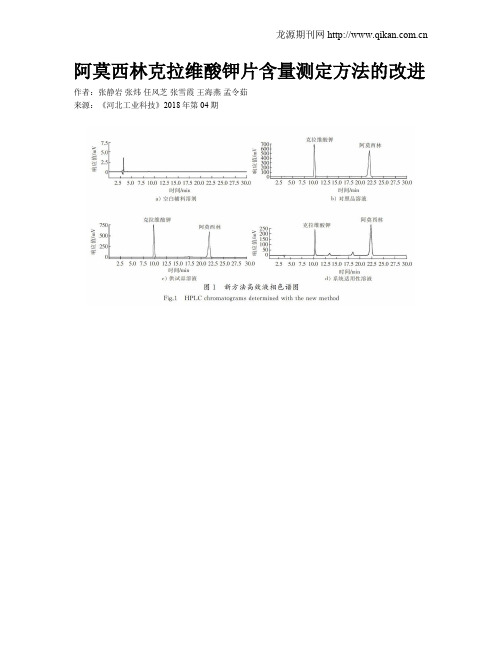
阿莫西林克拉维酸钾片含量测定方法的改进作者:张静岩张炜任风芝张雪霞王海燕孟令茹来源:《河北工业科技》2018年第04期摘要:为解决实际应用中克拉维酸钾峰与后相邻杂质的分离度达不到要求的问题,建立了一种同时测定阿莫西林克拉维酸钾片中阿莫西林和克拉维酸钾含量的新方法,采用反相高效液相色谱法(RP-HPLC), Ultimate AQ-C18色谱柱(4.6 mm×250 mm,5 μm),以pH值为4.4的0.05 mol/L磷酸二氢钠缓冲液为流动相,柱温为35 ℃,流速为1.0 mL/min ,检测波长为220 nm 。
结果表明:阿莫西林和克拉维酸钾两主峰与相邻杂质得到完全基线分离,分离度大于1.5;克拉维酸钾对照品溶液质量浓度为0.050 0~0.500 1 mg/mL时与峰面积的线性关系良好(r=0.999 8),平均回收率(n=9)为100.14%,RSD值为0.24%;阿莫西林对照品溶液质量浓度为0.100 0~1.000 1 mg/mL时与峰面积的线性关系良好(r=0.999 9),平均回收率(n=9)为100.45%,RSD值为017%。
与原有的《中华人民共和国药典》方法相比,新的阿莫西林克拉维酸钾片含量测定方法专属性好,准确度高,操作简便快捷,结果可靠,可作为一种质量控制方法。
关键词:色谱分析;阿莫西林;克拉维酸钾;含量测定;高效液相色谱法中图分类号:R9781+1文献标志码:Adoi: 10.7535/hbgykj.2018yx04013阿莫西林克拉维酸钾片是葛兰素史克(GSK)公司研制的由阿莫西林三水化合物和克拉維酸钾组方的复方制剂,属于国家基本药物[1-2]。
其中阿莫西林为广谱青霉素类抗生素,克拉维酸钾为竞争性广谱β-内酰胺酶抑制剂,两者合用,可保护阿莫西林免受β-内酰胺酶的水解,使对阿莫西林耐药并产生β-内酰胺酶的细菌仍然对阿莫西林敏感,从而不影响阿莫西林的杀菌效果[3-4],临床上广泛应用于敏感菌引起的上呼吸道感染、下呼吸道感染、泌尿系统感染、软组织及皮肤感染以及其他感染。
阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价

阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价急性支气管炎是一种常见的呼吸道疾病,主要表现为咳嗽、咳痰、发热、胸闷等症状。
急性支气管炎通常是由感冒病毒感染引起的,也可以是细菌感染引起的。
临床上常用的治疗方法包括休息、饮食调节、保持室内空气清新、及时采取药物治疗等。
而在药物治疗方面,阿莫西林克拉维酸钾是一种常用的治疗急性支气管炎的药物。
本文将从临床疗效、安全性、耐药性等方面对阿莫西林克拉维酸钾治疗急性支气管炎的疗效进行评价。
一、阿莫西林克拉维酸钾的临床疗效阿莫西林克拉维酸钾是一种广谱抗生素,具有良好的抗感染作用。
它主要通过抑制细菌细胞壁的合成来发挥作用,从而能够有效地杀灭引起急性支气管炎的致病菌,如肺炎链球菌、流感嗜血杆菌等。
多项研究显示,阿莫西林克拉维酸钾治疗急性支气管炎的临床疗效显著。
一项针对500名急性支气管炎患者的临床研究表明,使用阿莫西林克拉维酸钾治疗的患者,在治愈率和症状缓解率上均显著优于对照组。
在使用阿莫西林克拉维酸钾治疗的患者中,症状可以迅速缓解,体温恢复正常,咳嗽和咳痰明显减轻,患者的生活质量明显改善。
在治疗慢性支气管炎合并急性发作的研究中也发现,使用阿莫西林克拉维酸钾联合治疗的患者,治疗效果显著优于单独使用其他抗生素或者其他治疗方法的患者。
这些研究结果表明,阿莫西林克拉维酸钾在治疗急性支气管炎方面具有显著的临床疗效。
在长期治疗中,阿莫西林克拉维酸钾也很少出现耐药性等问题。
研究表明,使用阿莫西林克拉维酸钾治疗的患者,很少出现疗效不佳或者细菌耐药的情况。
这主要是因为阿莫西林克拉维酸钾具有较广的杀菌谱,对大多数引起急性支气管炎的细菌都有良好的杀菌作用,从而能够有效避免耐药性的产生。
阿莫西林克拉维酸钾在临床使用中也很少出现肝肾功能损害等严重不良反应,因此在治疗急性支气管炎时具有较高的安全性。
尽管阿莫西林克拉维酸钾具有较好的疗效和安全性,但在临床使用中仍需注意预防和减少耐药性的发生。
由于抗生素的滥用和不合理使用,导致细菌的耐药性日益增加,这也是目前临床上面临的一个严重问题。
注射用阿莫西林克拉维酸钾稳定性考察报告

注射用阿莫西林克拉维酸钾稳定性考察报告一、背景注射用阿莫西林克拉维酸钾为广谱青霉素阿莫西林与酶抑制剂克拉维酸的复合制剂,属于非限制使用级抗菌药物,临床广泛用于治疗儿童及成人社区获得性肺炎、皮肤和软组织感染等。
近期,我院儿科病区反映该药配置过程中易变色,给临床使用造成困惑,不知道是药品稳定性有问题还是配置操作不规范造成。
为考察药物稳定性,摸索最佳配置方法,药剂科与儿科联合,选取注射用阿莫西林克拉维酸钾(1.2g 华北制药生产批号F7025605)进行对照试验。
二、实验过程1.实验器材20ml注射器7支、避光袋1个由儿科二病区提供;1.2g 阿莫西林克拉维酸钾7瓶、0.9%氯化钠注射液100mL 5袋、5%葡萄糖注射液100ml 1袋、室内温度计1个由儿科药房提供。
2.实验目的考察浓度、温度、溶媒、光线、放置时间对阿莫西林克拉维酸钾稳定性的影响。
3.实验设计试验共设7组,见表1。
表中未特别注明者,按照标准条件处理,标准条件为:在25±2℃下,向1.2g药瓶中加入20mL生理盐水溶解,并立即加入到100mL 生理盐水输液袋中。
1、2为高浓度组,3、4为对照组、5号用5%GS做溶媒,6号避光,7号为高温组。
配制操作:付俊拍照及记录:董自然、赵洁试验场地:(1)室温组:儿科二病区配液室;(2)高温组:检验科三、实验结果实验过程中,1号、2号、5号、7号四组药液颜色在4h内发生变化,具体情况见表2。
1.高浓度组(1.2g/10ml)药液颜色变化对比组图0min 20min 60min 120min 240min 2.高温组(37℃)药液颜色变化对比组图0min 60min 120min 180min 240min四、小结1.浓度、温度、溶媒可明显影响阿莫西林克拉维酸钾药液的稳定性。
高浓度组(1.2g/10ml)放置20min,高温组(37℃)放置30min,5%葡萄糖注射液作溶媒组放置120min,药液即变淡黄色,且放置时间越长药液颜色越深。
阿莫西林克拉维酸钾颗粒人体生物利用度试验-中国临床试验注册中心

知情同意书临床研究项目名称:益母草注射液促进人工流产术后子宫复旧作用的随机、对照、多中心临床研究亲爱的患者:医生已经确诊您妊娠,需要做人工流产手术。
我们将邀请您参加一项用益母草注射液促进人工流产术后子宫复旧作用的随机、对照、多中心临床研究临床试验。
在您决定是否参加这项研究之前,请尽可能仔细阅读以下内容。
它可以帮助您了解该项研究以及为何要进行这项研究,研究的程序和期限,参加研究后可能给您带来的益处、风险和不适。
如果您愿意,您也可以和您的亲属、朋友一起讨论,或者请医生给予解释,帮助您做出决定。
一、研究背景和研究目的早期人工流产是避孕失败最常用的补救措施之一,是妇产科最常用的终止早期妊娠的常用方法。
益母草是传统的缩宫调经药物, 在妇产科用药中从古到今都占有重要的位置,其在《神农本草经》《本草纲目》中均有记载。
现代药理作用研究证明其具有调经止血的作用,此外还有保护心肌缺血再灌注损伤、抗血小板聚集、降低血液黏度等作用。
在临床上常用来治疗流产后出血、冠心病、心肌缺血、高黏血症、痛经等疾病。
益母草活血调经、祛瘀生新的作用,有利于流产后子宫内膜创伤的恢复再生。
益母草注射液是上市药品,为我国独有的中药益母草的提取物制成的静脉注射液,临床已经应用40余年,常用于顺产及剖宫产后、流产后等预防产后出血。
本研究的目的是观察其对流产后促进子宫复旧的治疗效果。
本研究方案中的用药方法、剂量和适应症与该上市药品说明书是一致的。
试验药为益母草注射液,规格:20mg/ml/支,生产厂家:成都第一药业有限公司,批文号国药准字Z51021448。
本试验预计将入选400余名受试者。
本研究为四川省科技支撑项目、任务书编号:2014SZ0139。
二、哪些人不宜参加研究过敏性体质者不适宜参加本研究。
三、如果参加研究将需要做什么?1. 在您入选研究前,您将接受以下检查以确定您是否可以参加研究:医生将询问、记录您的病史,并进行体格检查。
您需要做血常规、凝血四项、腹部B超等检查。
国家药品监督管理局关于公布抗生素类抗感染药等药品地方标准品种再评价结果的通知

国家药品监督管理局关于公布抗生素类抗感染药等药品地方标准品种再评价结果的通知文章属性•【制定机关】国家药品监督管理局•【公布日期】2001.06.01•【文号】•【施行日期】2001.06.01•【效力等级】部门规范性文件•【时效性】现行有效•【主题分类】标准化正文国家药品监督管理局关于公布抗生素类抗感染药等药品地方标准品种再评价结果的通知(2001年6月1日)各省、自治区、直辖市药品监督管理局,药检所:根据国家药品监督管理局关于药品地方标准品种再评价工作的要求,我局组织医药学专家对抗生素类抗感染药等药品地方标准品种进行了再评价;对已公布的拟停止使用品种的申诉意见进行了论证;对需补充资料后再议的品种进行了复审。
现将结果予以公布(见附件),并就有关事项通知如下:一、请迅速将本通知传达到辖区内相关品种生产企业,对本通知中拟停止使用品种如有申诉意见,务于2001年7月底前报我局安全监管司,对需补报资料的品种,请于2001年6月底前报国家药典委员会。
二、无论是申诉意见还是补报资料,均应经所在省(区、市)药品监督管理局审核后统一上报。
三、对本通知中拟停止使用品种无申诉意见的相关企业,也必须报所在省(区、市)药品监督管理局备案。
四、对公布结果有疑义的单位,请与我局安全监管司或国家药典委员会联系。
联系电话:(010)68313344-1039或(010)67152761特此通知。
附件:抗生素类抗感染药、抗肿瘤及调节免疫功能药、循环系统用药、激素及调节内分泌功能药、专科用药等药品地方标准品种再评价结果国家药品监督管理局二00一年六月一日附件:抗生素类抗感染药、抗肿瘤及调节免疫功能药、循环系统用药、激素及调节内分泌功能药、专科用药等类药品地方标准品种再评价结果通过品种抗生素类抗感染药注射用氨氯西林钠限用于金葡菌及其它敏感菌的感染阿莫西林颗粒剂(阿莫西林干糖浆、羟氨苄青霉素)阿莫西林口服混悬剂(羟氨苄青霉素)苯唑青霉素胶囊苯唑青霉素钠片(苯唑西林钠片)苯唑青霉素钠胶囊(苯唑西林钠胶囊,安迪灵)注射用肤脲苄青霉素钾(注射用肤苄青霉钾)说明书需注明钾离子对人体的影响硫酸卡那霉素片硫酸卡那霉素胶囊硫酸卡那霉素软膏单硫酸卡那霉素干糖浆(康纳)硫酸卡那霉素B阿米卡星滴眼液(丁胺卡那霉素滴眼液)溃疡药膜(口腔溃疡药膜)胍哌甲基四环素胍哌甲基四环素胶囊取消抗流感病毒的适应症四环素泼尼松眼膏的确当滴眼液新地松眼膏盐酸土霉素软膏盐酸土霉素胶囊盐酸脱氧土霉素干糖浆(多西环素干糖浆,强力霉素干糖浆)红霉素肠溶微丸胶囊红霉素肠溶胶囊(红霉素胶囊)注射用盐酸柔红霉素(注射用盐酸正定霉素)注射用盐酸阿霉素(注射用盐酸多柔米星)麦迪霉素胶囊硫酸庆大霉素片(肠溶)硫酸庆大霉素胶囊注射用硫酸庆大霉素酒石酸柱晶白霉素(酒石酸吉他霉素)注射用柱晶白霉素(注射用酒石酸柱晶霉素)灰黄霉素胶囊复方灰黄霉素癣药水硫酸核糖霉素注射液盐酸林可霉素滴眼液(盐酸洁霉素滴眼液)盐酸林可霉素滴耳液注射用盐酸林可霉素盐酸林可霉素软膏磷霉素钙干糖浆适应用于肠道感染多西环素胶丸(强力霉素胶丸)复方克林霉素搽剂多粘菌素E注射用硫酸多粘菌素B氯霉素耳丸(滴丸)复方制霉素栓(妇科制霉栓)制霉素栓(制霉菌素栓)制霉菌素泡腾阴道片(米可定泡腾阴道片)利福平眼膏(甲哌利福霉素眼膏)利福霉素钠(力复霉素SV钠)限用于抗结核菌利福霉素钠注射液(力复霉素SV钠注射液)限用于抗结核菌复合利福平软胶囊(复合甲哌力复霉素软胶囊)利福定(异甲哌利福霉素)利福定片(异丁基哌嗪利福霉素片)利福定胶囊(异丁基哌嗪利福霉素胶囊)交沙霉素胶囊妥布拉霉素注射液头孢硫脒注射用头孢硫脒克念菌素克念菌素片克念菌素阴道片新福菌素(更新霉素)新福菌素注射液(更新霉素注射液)抗肿瘤及调节免疫功能类氟尿嘧啶乳复方氟脲嘧啶脂质体注射液复方氟脲嘧啶多相脂质体口服液(康宁口服液)复方氟脲嘧啶乳复方替加氟胶囊替加氟胶囊(喃氟啶)替加氟栓(呋喃氟脲嘧啶栓)优福定片羟基脲胶囊喜树碱羟基喜树碱羟基喜树碱注射液注射用羟基喜树碱去甲斑蟊素去甲斑蟊素片去甲斑蟊酸钠注射液(依尔康注射液)斑蟊酸钠斑蟊酸钠片(奇宁片)斑蟊酸钠注射液(奇宁注射液)艾康注射液甲基斑蟊胺甲基斑蟊胺片氮烯咪胺注射用氮烯咪胺适用于黑色素瘤等少见肿瘤靛玉红靛玉红片小檗胺小檗胺片盐酸小檗胺盐酸小檗胺片依托泊甙(足叶乙甙)足叶乙甙注射液三尖杉酯碱三尖杉酯碱注射液六甲密胺肠溶片复方环磷酰胺片谷固醇膜谷固醇软膏限用于慢性皮肤溃疡的辅助治疗薄芝注射液顺铂注射液苦参素苦参素注射液(博尔利康注射液)迈清注射液茜草双酯茜草双酯片循环系统用药甲基橙皮甙橙皮甙地拉卓地拉卓片地拉卓胶囊没食子酸丙酯(通脉酯)适用于预防脑血栓等血栓性疾病的辅助治疗注射用通脉酯(注射用赤芍801)脑复清胶囊脑力隆胶囊脑心通胶囊降压嗪(二氮嗪)降压嗪注射液(二氮嗪注射液)北京降压平片(北京降压0号)人参茎叶总皂甙人参活力胶囊限定适应症为辅助治疗硝酸乙氧烟酸胺(硝烟酯、烟浪丁、尼可地尔)尼可地尔片(硝烟酯片、烟浪丁片)卡托普利胶囊降压灵片塞易通(阿斯达美片,复方双嘧达莫片)柳胺苄心定柳胺苄心定片(盐酸拉贝洛尔片)柳胺苄心定注射液盐酸麻黄苯丙酮(安心酮)盐酸麻黄苯丙酮片(安心酮片)吗导敏吗导敏片(脉导敏片)吗导敏气雾剂羟乙基芦丁(维脑路通、曲克芦丁)羟乙基芦丁片(维脑路通片、曲克芦丁片)羟乙基芦丁口服液(维脑路通口服液、曲克芦丁口服液)羟乙基芦丁胶囊(维脑路通胶囊、曲克芦丁胶囊)羟乙基芦丁氯化钠注射液(奇奥脉通注射液)羟乙基芦丁注射液(维脑路通注射液、曲克芦丁注射液)灯盏花素(脑栓通)月见草油月见草油胶丸心脑三效胶丸阿魏酸哌嗪阿魏酸哌嗪片(保肾康)阿魏酸钠阿魏酸钠片注射用阿魏酸钠盐酸川芎嗪磷酸川芎嗪磷酸川芎嗪片磷酸川芎嗪胶囊磷酸川芎嗪注射液(脑血通注射液)氢溴酸樟柳碱注射液氢溴酸樟柳碱片盐酸苄丙酚胺(脑清)盐酸苄丙酚胺片(脑清片)亚油酸亚油酸胶囊亚油酸丸亚油酸乙酯亚油酸乙酯胶囊(脉通胶囊)亚油酸乙酯胶丸(脉通)烟酸羟丙茶碱(脑脉康)烟酸羟丙茶碱片(脑脉康片)脑脉宁脑脉宁片(托哌酮片)脑脉宁胶囊(托哌酮胶囊)硝酸戊四醇酯片脉舒静片复方催降压片复方降压素胶囊安降片复方罗布麻片三甲氧卞嗪片曲匹地尔片甘露醇烟酸酯甘露醇烟酸酯片复方氨苯喋啶片(双克安片)治疗水肿及腹水的剂量为一次1~2片新捷络软胶囊环轮宁说明书应特别注明安全问题环轮宁注射液说明书应特别注明安全问题杏丁注射液限用于缺血性心脑血管病碟脉灵注射液冠脉苏片(微调1号片)盐酸培他定注射液盐酸培他司汀氯化钠注射液(盐酸培他定氯化钠注射液,美培啶)天麻素天麻素片天麻素胶囊天麻素注射液可乐定贴片盐酸可乐定滴丸激素及调节内分泌功能类三合激素注射液双炔失碳酯双炔失碳酯片(53-探亲抗孕片)炔诺孕酮片避孕反应抑制片地塞米松片通泰精粉(糖脂泰精粉)通泰胶囊(糖脂泰胶囊)适用于便秘和糖尿病、高血脂症的辅助治疗氢化泼尼松注射液催奶片妇复春胶囊木糖醇片木糖醇颗粒专科用药、辅料辣椒痛可贴愈创膏(创伤帖)卡普欣乳膏青光明(催醒安)青光明滴眼液(益明眼药水,催醒安)法可林(治障宁,消白灵,白可明,睛可明,障眼净,四氮戊省磺酸钠)光安眼药限用于角膜溃疡复方盐酸萘甲唑琳眼药水(消疲灵、通明)复方托品酰胺滴眼剂盐酸吗啉胍滴眼液(盐酸吗啉双胍眼药水)复方疱疹性眼膏复方醋酸曲安奈德滴耳液(复方醋酸去炎松滴耳液)盐酸麻黄素滴鼻液呋麻滴鼻液鼻通药膏(鼻灵药膏,鼻通油膏)统一到非抗生素类的鼻通软膏处方富马酸酮替芬气雾剂(酮替芬气雾剂)氯化钙葡萄糖注射液复方电解质葡萄糖R2A注射液氨丁三醇注射液(缓血酸胺注射液、氨基丁三醇注射液)防暑颗粒高渗枸橼酸盐嘌呤液(离体肾保存液)人工肾透析液AD-1人工肾透析液BD-1氯磷定氯磷定注射液解磷注射液二巯基丙磺酸钠注射液依地酸二钠依地酸钙钠片(依地钙片)氧化淀粉玉米朊氢氧化镁氯化镁醋酸钠十二烷基硫酸镁聚糖酐粉(清创愈伤散)3-7-二甲基嘌呤-2-6(3H,1H)-二酮(柯柯豆碱)樟脑酚液氟化钠甘油氢氧化钙糊剂利凡诺溶液复方硼砂溶液新洁尔灭酊甲醛甲酚溶液(牙用煤酚醛液,牙用甲酚醛液)牙用碘液牙痛水寒痛乐热敷袋吉娜舒润剂钡餐乐混悬剂(硫酸钡混悬剂,钡胶浆)硫酸钡混悬液靛胭脂注射液注射用碳酸酰胺过氧化氢(注射用内给氧)复方释氧剂复方产氧剂(氧福康产氧器)复方供氧剂氧利舒帕特药盒麻醉药类阿片片氟哌定注射液盐酸丁氧普鲁卡因盐酸丁氧普鲁卡因滴眼剂盐酸丁氧普鲁卡因胶浆剂注射用盐酸氯胺酮钠石灰(碱石灰)润滑止痛胶(丁卡因)盐酸利多卡因凝胶其它类(是指已公布过的各类药品中未包括的品种和经论证或复审通过的品种)非抗生素类抗感染药复方酮康唑软膏诺氟沙星片(氟哌酸片)苦参碱苦参碱注射液苦参碱栓(妇炎栓)鞣酸苦参碱胶囊小儿鞣酸苦参碱片磺胺嘧啶碳酸氢钠片(复方磺胺嘧啶片)应按实际体重计算用量小儿增效联磺颗粒剂(散)*说明书需注明年龄限制和不良反应复方润喉片熊胆润喉片黑癣油*去掉苯酚和硝酸新脚气药水(癣敌)*减少二甲基桠枫的含量或用乙醇替换女宝栓*去掉雄黄和樟丹解热镇痛类盐酸金刚烷胺糖浆盐酸金刚烷胺颗粒汉防己甲素感冒康胶囊重感片感炎清胶囊阿苯糖丸*(小儿退热糖丸)须按公斤体重计算用量安热静注射液*应注明不良反应,儿童慎用肺宝三效片*去苯妥英钠和扑热息痛,限适应症,注明不良反应和疗程。
安徽省物价局关于阿莫西林克拉维酸钾等药品最高零售价格的通知-皖价医[2012]42号
![安徽省物价局关于阿莫西林克拉维酸钾等药品最高零售价格的通知-皖价医[2012]42号](https://img.taocdn.com/s3/m/40bb34de9fc3d5bbfd0a79563c1ec5da50e2d686.png)
安徽省物价局关于阿莫西林克拉维酸钾等药品最高零售价格的通知正文:---------------------------------------------------------------------------------------------------------------------------------------------------- 安徽省物价局关于阿莫西林克拉维酸钾等药品最高零售价格的通知(皖价医〔2012〕42号)各市、县物价局:根据《国家发展改革委关于印发〈药品差比价规则〉的通知》(发改价格〔2011〕2452号)有关规定,我局对药品生产经营企业2月份申报的政府定价药品最高零售价格进行了审核,现公布阿莫西林克拉维酸钾等药品最高零售价格。
本通知价格自2012年 3月 20日起执行,今后国家有新的价格政策按新政策执行。
附件:阿莫西林克拉维酸钾等药品最高零售价格表二〇一二年三月十四日附件阿莫西林克拉维酸钾等药品最高零售价格表西药部分金额单位:元序号编号药品名称剂型规格单位最高零售价备注1308006387阿莫西林克拉维酸钾片剂阿莫西林875mg/克拉维酸钾125mg×120 盒7212308006388倍氯米松粉雾剂0.2mg×60盒53.33308006389非那雄胺片剂5mg×20盒147杭州默沙东制药有限公司308006390 果糖二磷酸钠片剂250mg×48 盒62.35 308006391 坎地沙坦片剂4mg×24盒48.76 308006392 齐多夫定片剂100mg×120 瓶1797 308006393 齐多夫定片剂300mg×60 瓶8308006394沙丁胺醇粉雾剂0.4mg×60盒34.39308006395舍曲林片剂50mg×28盒15510308006396头孢氨苄异形素片500mg×36盒(瓶)35.7清远华能制药有限公司11308006397头孢羟氨苄片剂500mg×10盒(瓶)36.8清远华能制药有限公司12308006398头孢羟氨苄片剂500mg×12盒(瓶)43.9清远华能制药有限公司13308006399硝苯地平缓释片(II)20mg×48盒3714308006400乙酰半胱氨酸片剂0.6g×12盒6415308006401注射剂250ml:果糖6.25g 塑瓶45.816308006402佐匹克隆片剂7.5mg×7盒15.317308006403佐匹克隆片剂7.5mg×14盒29.8中成药部分金额单位:元序号编号药品名称剂型单位最高零售价备注1308006404复方斑蝥胶囊剂0.25g×72瓶214重庆希尔安药业有限公司2308006405复方南星止痛膏橡胶膏剂10cm×13cm×1盒8.73308006406猴头菌片剂0.25g×60瓶6.24308006407健脾丸浓缩丸3g/8丸×240瓶(盒)9.55308006408健脾益肾颗粒颗粒剂14g×20盒123重庆希尔安药业有限公司6308006409六味地黄胶囊胶囊剂0.3g×80瓶697308006410痛血康胶囊胶囊剂0.2g×24盒(瓶)60.68308006411心脉通片剂60瓶33.29308006412治伤软膏软膏剂30g支54.510308006413牛黄蛇胆川贝胶囊胶囊剂0.25g×30盒16.7——结束——。
阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价

阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价急性支气管炎是一种常见的呼吸道疾病,主要症状包括咳嗽、咳痰、胸闷、气促等。
在临床上,通常采用抗生素治疗急性支气管炎,其中阿莫西林克拉维酸钾是一种常用的抗生素药物。
本文将对阿莫西林克拉维酸钾治疗急性支气管炎的疗效进行评价,以期为临床实践提供一定的参考依据。
一、阿莫西林克拉维酸钾的药理作用阿莫西林克拉维酸钾是一种广谱抗生素,其成分包括阿莫西林和克拉维酸钾。
阿莫西林属于β-内酰胺类抗生素,通过抑制细菌细胞壁合成而起到杀菌作用。
而克拉维酸钾是β-内酰胺酶抑制剂,能够抑制细菌产生β-内酰胺酶,从而增强阿莫西林的抗菌活性。
阿莫西林克拉维酸钾具有广谱、高效、低毒和良好的耐受性,被广泛应用于呼吸道感染等疾病的治疗。
二、阿莫西林克拉维酸钾治疗急性支气管炎的疗效1. 对比疗效研究治疗急性支气管炎的药物有多种选择,如红霉素、头孢类、大环内酯类等,而阿莫西林克拉维酸钾也是一种常用的抗生素药物。
有研究对比分析了不同药物治疗急性支气管炎的疗效,结果显示,阿莫西林克拉维酸钾组在治疗后的症状缓解时间、症状改善程度、临床疗效等方面均优于其他药物组,且安全性较高,不良反应少。
2. 临床观察研究临床上也有一些关于阿莫西林克拉维酸钾治疗急性支气管炎的观察性研究。
这些研究通常采用了大样本、多中心、随机对照的方法,对患者的临床疗效、炎症指标、疾病复发率等进行观察和评价。
结果显示,阿莫西林克拉维酸钾治疗急性支气管炎的总有效率较高,炎症指标如白细胞计数、C反应蛋白水平等也呈现明显的下降趋势,且复发率相对较低。
3. Meta分析结果还有一些对阿莫西林克拉维酸钾治疗急性支气管炎疗效进行Meta分析的研究。
这些Meta分析通常汇总了大量的临床试验数据,对阿莫西林克拉维酸钾在急性支气管炎治疗中的临床疗效、安全性、耐药性等方面进行了综合评价。
结果显示,阿莫西林克拉维酸钾治疗急性支气管炎的总有效率在80%以上,且安全性较高,耐药性相对较低。
集中带量采购阿莫西林与阿莫西林克拉维酸钾根除幽门螺杆菌临床疗效评估

◇医院药学之窗◇摘要目的:评估集中带量采购(集采)阿莫西林与阿莫西林克拉维酸钾根除幽门螺杆菌(helico-bacter pylori ,Hp )的疗效,为临床方案选择提供依据。
方法:利用合理用药管理系统调取2021年5月至2022年5月接受Hp 根除治疗患者的数据,筛选使用阿莫西林方案(集采阿莫西林1.0g bid +枸橼酸铋钾220mg bid +艾司奥美拉唑20mg bid +克拉霉素0.5g bid ,14d )和阿莫西林克拉维酸钾方案(阿莫西林克拉维酸钾0.914g bid +枸橼酸铋钾220mg bid +艾司奥美拉唑20mg bid +克拉霉素0.5g bid ,14d )患者的数据,对两方案的疗效进行比较探讨。
结果:集采阿莫西林组共收集到171例,根除成功率87.8%(150/171)。
阿莫西林克拉维酸钾组共收集到69例,根除成功率76.8%(53/69)。
两组患者基线资料差异无显著性(P >0.05)。
两组临床疗效存在统计学差异(P <0.05)。
阿莫西林方案成本效果比值(C/E )低于阿莫西林克拉维酸钾方案。
结论:集采阿莫西林方案根除Hp 感染临床疗效优于阿莫西林克拉维酸方案,值得临床推广。
关键词幽门螺杆菌;集中带量采购;阿莫西林;阿莫西林克拉维酸钾中图分类号:R969文献标志码:A 文章编号:1009-2501(2023)09-1061-06doi :10.12092/j.issn.1009-2501.2023.09.013幽门螺杆菌(helicobacter pylori ,Hp )感染与消化性溃疡、胃黏膜相关淋巴组织淋巴瘤、胃增生性息肉、早期胃癌、胃炎、不明原因缺铁性贫血、原发免疫性血小板减少、维生素B12缺乏症等多种疾病的发生发展密切相关,对人类健康构成严重的威胁。
1994年WHO 下属国际癌症研究机构将Hp 定义为I 类致癌原,2022年美国卫生和公共服务部将Hp 列为明确致癌物[1]。
阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价

阿莫西林克拉维酸钾治疗急性支气管炎的疗效评价【摘要】本研究旨在评价阿莫西林克拉维酸钾治疗急性支气管炎的疗效。
通过临床试验设计,采用疗效评价方法对治疗效果进行评估,结果分析显示阿莫西林克拉维酸钾能有效缓解急性支气管炎症状。
副作用分析显示其安全性较高,安全性评估结果良好。
结论表明阿莫西林克拉维酸钾在治疗急性支气管炎中具有显著疗效,为治疗该疾病提供了一种有效的药物选择。
未来研究可继续探索其在不同类型支气管炎中的应用,并深入研究其作用机制,以进一步提高治疗效果。
【关键词】阿莫西林克拉维酸钾、急性支气管炎、疗效评价、临床试验、副作用、安全性、有效性、研究方向、药物治疗1. 引言1.1 背景介绍急性支气管炎是一种常见的呼吸道感染病,常见于冬春季节。
患者主要表现为咳嗽、咳痰、胸闷、气促等症状,严重者可出现发热、胸痛等症状。
急性支气管炎主要由病毒或细菌感染引起,其中细菌感染是治疗的重要因素。
阿莫西林克拉维酸钾是一种广泛应用于临床的抗生素药物,常用于治疗呼吸道感染等疾病。
针对阿莫西林克拉维酸钾在治疗急性支气管炎中的疗效,目前还缺乏大规模的临床试验数据支持。
本研究旨在通过临床试验评估阿莫西林克拉维酸钾在治疗急性支气管炎中的疗效,为临床实践提供科学依据和指导。
1.2 研究目的研究目的:本研究旨在评价阿莫西林克拉维酸钾在治疗急性支气管炎中的疗效,探讨其对疾病症状的改善情况,分析其对症状缓解的速度及持续时间。
本研究将评估阿莫西林克拉维酸钾在急性支气管炎患者中的安全性表现,分析其可能存在的副作用及并发症发生率。
通过本研究的结果,旨在为临床医生提供更多关于阿莫西林克拉维酸钾在急性支气管炎治疗中的实际效果和安全性的信息,为临床实践提供科学依据,并为今后的研究和临床实践提供参考和指导。
2. 正文2.1 临床试验设计临床试验设计是本研究的重要部分,其设计合理性直接影响到研究结果的可靠性和科学性。
本研究采用随机对照试验设计,以确保研究结果的客观性和可靠性。
阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度测定和有关物质检查

阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度测定和有关物质检查目的对阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度和有关物质进行了研究,用来评价和确定制剂使用中的安全性及药物本身的内在质量。
方法通过对制备得到的阿莫西林克拉维酸钾干混悬剂含量均匀度的测定和有关物质考察,从而确定阿莫西林克拉维酸钾干混悬剂质量的可控性。
结果通过本文中所用方法对阿莫西林克拉维酸钾干混悬剂(14∶1)中阿莫西林、克拉维酸钾的含量均匀度和有关物质测定结果说明制剂质量合格,所以在制剂使用中阿莫西林克拉维酸钾干混悬剂能确保其安全性。
结论阿莫西林克拉维酸钾干混悬剂(14∶1)中阿莫西林、克拉维酸钾的含量均匀度和有关物质的测定均能符合制剂要求。
标签:阿莫西林;克拉维酸钾;含量均匀度;有关物质阿莫西林(Amoxicillin)(C16H19N3O5S)是一种最常用的青霉素类广谱β-内酰胺类抗生素,半衰期约为61.3 min。
阿莫西林杀菌作用强,穿透细胞膜的能力也强,是目前应用较为广泛的口服青霉素之一,其制剂有胶囊、片剂、颗粒剂、分散片等。
单独用阿莫西林时容易产生耐药性而降低药物的疗效;克拉维酸钾(C8H9NO5)仅有微弱的抗菌活性,但可与多数的β-内酰胺酶牢固结合,生成不可逆的结合物,具有强力而广谱的抑制β-内酰胺酶作用。
阿莫西林制剂配合克拉维酸钾使用时能有效稳定阿莫西林,使其降解减少而疗效增强,且对革兰阳性及革兰阴性细菌均有效[1-3]。
有关物质是药物中主要疗效成分在储存中产生或在原料制备中引进的,说明了药物对温度、湿度、光等外在因素的稳定度及药物本身使用中的安全性。
含量均匀度是药物有效成分含量较小和比例较低时,在制剂的制备中可能引起分布不均匀而影响药物的质量。
本文主要针对制备的三批阿莫西林克拉维酸钾干混悬剂(14∶1)的含量均匀度和有关物质进行测定,根据测定结果从而确定阿莫西林和克拉维酸含量均匀度和有关物质符合制剂的控制指标[4-5],从而说明其安全性和制剂工艺的可靠合理。
阿莫西林克拉维酸钾(71)分散片工艺规程答辩

1.目的:1.1.依据产品质量标准及GMP要求,规范阿莫西林克拉维酸钾分散片的生产,为药品生产车间提供必须遵守的技术准则,以保证生产的批与批之间尽可能与原设计吻合,保证药品在有效期内保持预定的质量。
1.2.提供阿莫西林克拉维酸钾分散片生产所需要的技术、质量标准。
1.3.明确生产过程中环境、设备、安全、人员、定额、消耗等条件要求。
1.4.提供制定岗位SOP的依据。
2.适用范围:适用于阿莫西林克拉维酸钾分散片生产过程的控制及管理。
3.责任者:3.1.贯彻执行本规程是阿莫西林车间、生产部共同责任。
3.2.监督执行本规程是质量保证部的责任。
工艺规程目录产品概述 (3)生产工艺流程 (4)处方和依据 (5)工艺过程及条件 (5)设备一览表及主要设备生产能力 (11)技术安全、工艺卫生及劳动保护 (12)技术经济指标的计算及控制 (13)包装要求及标签、说明书的贮存方法 (14)人员要求 (14)劳动组织定员与批工时消耗 (14)原辅料规格、质量标准和检查方法 (14)半成品质量标准和检查方法 (20)成品质量标准和检查方法 (20)包装材料,包装规格和质量标准 (21)半成品控制和检查方法 (23)原辅材料、包装、消耗定额 (23)附录(常用计量单位制) (24)4.规程:4.1.产品概述:4.1.1.药品名称:品名:阿莫西林克拉维酸钾(7:1)分散片商品名:盛西凯汉语拼音:Amoxilin Kelaweisuanjia FensanPian英文名:Amoxicillin and Clavulanate Potassium DispersibleTablets4.1.2.类别:本品为抗菌素类药。
4.1.3.剂型:片剂4.1.4.规格:228.5mg4.1.5.主要成分:主要成分为阿莫西林(C16H19N3O5S)和克拉维酸(C8H9NO5)。
4.1.6.批量:75万片。
4.1.7.性状:本品为薄膜衣片,除去包衣后显白色或淡黄色。
阿莫西林克拉维酸钾说明书

阿莫西林克拉维酸钾临床试验介绍

对于单药治疗无效但没有最终入选的受试者,研究者应给予其他有效的 的治疗措施。
试验流程
研究全程分为5个阶段: ① 筛选期间(-3天) ② 基线期(第0天) ③ 用药治疗期间(治疗第4天) ④ 治疗结束后(治疗结束后第1天) ⑤ 末次随访(治疗结束后第7天)
2
试验目的
采用已上市不同配比的注射用阿 莫西林钠克拉维酸钾5:1为对照药,对 苏州二叶制药有限公司开发的注射用阿 莫西林钠克拉维酸钾10:1进行临床研究, 客观评价其抗菌活性的临床疗效及安全 性,为注册提供临床依据。
3
试验设计
随机 双盲 阳性药平行对照 多中心临床研究 非劣效性设计
4
入选标准
尿常规 临床评价 细菌学检查 不良事件 伴随的合并用药及治疗 脱落(退出)标准判断
15
试验பைடு நூலகம்程
五、末次访视(治疗结束后第7天),需进行: 体格检查 临床评价 细菌学检查(必要时才做) 伴随的合并用药及治疗
观察, 已 进行“筛选期间(-3天)”阶段患者对注明*项 目不需观察。
13
试验流程
三、用药治疗期间访视(治疗第4天),需进行:
体格检查 血常规 尿常规 临床评价 不良事件 伴随的合并用药及治疗 脱落(退出)标准判断
14
试验流程
四、结束后访视(治疗结束后第1天),需进行:
体格检查 心电图 胸部X片 血常规 肝功能 肾功能 凝血功能
注射用阿莫西林钠克拉维酸钾10:1 临床研究
试验药物简介
研发背景 注射用阿莫西林钠克拉维酸钾10:1,是阿莫西林钠与β-内酰
胺酶抑制剂克拉维酸钾按10:1配比组成的复方制剂,由葛兰素史克 公司(GSK)研制开发,该品已在瑞士、德国等国家上市,但国内尚 未上市。 药物信息
阿莫西林钠克拉维酸钾注射剂说明书修订要求-国家药品不良反应监测中心

附件2阿莫西林钠克拉维酸钾注射剂说明书修订要求一、说明书修订总体要求本次阿莫西林钠克拉维酸钾注射剂说明书修订主要针对【不良反应】、【禁忌】、【注意事项】三部分内容,应遵循以下原则:如本次修订内容较国家药品监督管理局已批准的相关内容更严格、全面的,说明书应按本次修订意见修改。
国家药品监督管理局已批准的相关内容原则上不得删减,如原批准内容较本次修订意见更全面或更严格的,应保留原批准内容。
二、【不良反应】项应包含以下内容皮肤及其附件损害:皮疹、瘙痒、荨麻疹、潮红、多形性红斑、Stevens-Johnson综合征、中毒性表皮坏死松解症、剥脱性皮炎(红皮病)和急性泛发性发疹性脓疱病。
胃肠损害:恶心、呕吐、消化不良、腹胀、腹泻、胃炎、口腔炎、舌炎、黑毛状舌、伪膜性肠炎、出血性结肠炎。
免疫功能紊乱和感染:药物热、变应性血管炎、血管性水肿、皮肤与黏膜的念珠菌病、二重感染、血清病样综合征(荨麻疹并伴随关节炎、关节痛、肌痛和发热)、哮喘、严重过敏样反应、过敏性休克。
神经系统损害:头晕、头痛、眩晕、失眠、激动、焦虑、烦—1 —躁、行为改变、意识混乱、惊厥。
用药部位损害:注射部位疼痛、静脉炎或血栓性静脉炎。
血液系统损害:白细胞减少症(包括中性粒细胞减少症)和血小板减少症、血小板减少性紫癜、嗜酸性粒细胞增多、血小板增多症、凝血酶原时间延长、粒细胞缺乏症和溶血性贫血。
泌尿系统损害:血尿、结晶尿、间质性肾炎、急性肾损伤(包括急性肾功能衰竭、肌酐升高)。
肝胆损害:转氨酶升高、肝炎及胆汁淤积性黄疸。
其他损害:心悸、紫绀、呼吸困难、胸闷、寒战。
三、【禁忌】项下应包含以下内容1.青霉素皮试阳性反应者、对本品及其他青霉素类药物过敏者及传染性单核细胞增多症患者禁用。
2.曾经出现过阿莫西林钠克拉维酸钾相关胆汁淤积或肝功能损伤的患者禁用。
四、【注意事项】应包含以下内容1.对头孢菌素类药物过敏者及有哮喘、变应性鼻炎、荨麻疹等过敏性疾病史者慎用。
阿莫西林克拉维酸钾研究资料

药学研究资料综述一、药品名称:通用名:阿莫西林克拉维酸钾(4:1)干混悬剂英文名:Amoxicillin and Clavulanate Potassium for Suspension汉语拼音: Amoxilin Kelaweisuanjia Ganhunxuanji本品为复方制剂,其组分为阿莫西林和克拉维酸钾,两者之比为4:1。
规格:156.25mg(阿莫西林125mg与克拉维酸31.25mg)二、处方工艺:根据《中国药典》2000年版二部及国家药品标准新药转正标准第32册152页“阿莫西林克拉维酸钾(4:1)干混悬剂”项下[WS1-(X-017)-2003Z],本品为阿莫西林钠克拉维酸钾(4:1)均匀混合制成的干混悬剂:1、处方:阿莫西林克拉维酸钾(4:1) 156.25g(阿莫西林125 g与克拉维酸31.25g)微粉硅胶 300g羧甲淀粉钠 100g微晶纤维素 62.5g草莓香精 20g干燥蔗糖粉加至2000 g制成 1000包2、详细制备工艺(1)蔗糖于65℃烘干,备用。
(2)将各原辅料粉碎过80目筛,备用。
(3)按处方量准确称取除蔗糖粉外的各原辅料,混合均匀后,按等量递加法再与处方量干燥蔗糖粉混合均匀。
(4)中间体送检含量,确定装量。
(5)药粉按装量分装于复合膜袋内。
(6)成品全检,包装,入库。
3、工艺处方研究:按《中国药典》2000年版二部及国家药品标准新药转正标准第32册152页阿莫西林克拉维酸钾(4:1)干混悬剂项下[WS1-(X-017)-2003Z],经对辅料的选择;以药粉流动性、装量差异、沉降体积比、粒度、再分散性、含量为目的指标,对本品进行处方筛选,并对含量与溶出度;样品微粉学试验的堆密度、休止角、测装量差异、粒度、沉降体积比、溶化性;临界相对湿度考察进行了考察,结果显示本处方组方合理,适合大生产要求。
影响因素试验:影响因素试验是在较为激烈的条件下进行的。
其目的是探讨药物的固有稳定性、了解影响其稳定性的因素及可能的降解途径与降解产物,为制剂生产工艺、包装、贮存条件以及运输建立质量控制方法提供科学依据。
国家药品监督管理局公告2019年第14号——关于修订阿莫西林(钠)克拉维酸钾制剂说明书的公告

国家药品监督管理局公告2019年第14号——关于修订阿莫西林(钠)克拉维酸钾制剂说明书的公告文章属性•【制定机关】国家药品监督管理局•【公布日期】2019.02.26•【文号】国家药品监督管理局公告2019年第14号•【施行日期】2019.02.26•【效力等级】部门规范性文件•【时效性】现行有效•【主题分类】药政管理正文国家药品监督管理局公告2019年第14号关于修订阿莫西林(钠)克拉维酸钾制剂说明书的公告为进一步保障公众用药安全,国家药品监督管理局决定对阿莫西林(钠)克拉维酸钾制剂(包括注射剂、片剂、混悬剂、颗粒剂和胶囊剂)说明书【不良反应】、【禁忌】、【注意事项】等项进行修订。
现将有关事项公告如下:一、所有阿莫西林(钠)克拉维酸钾制剂生产企业均应依据《药品注册管理办法》等有关规定,按照阿莫西林克拉维酸钾口服制剂说明书修订要求(见附件1)或阿莫西林钠克拉维酸钾注射剂说明书修订要求(见附件2),提出修订说明书的补充申请,于2019年4月26日前报省级药品监管部门备案。
修订内容涉及药品标签的,应当一并进行修订;说明书及标签其他内容应当与原批准内容一致。
在补充申请备案后6个月内对所有已出厂的药品说明书及标签予以更换。
各阿莫西林(钠)克拉维酸钾制剂生产企业应当对新增不良反应发生机制开展深入研究,采取有效措施做好使用和安全性问题的宣传培训,涉及用药安全的内容变更要立即以适当方式通知药品经营和使用单位,指导医师、药师合理用药。
二、临床医师、药师应当仔细阅读阿莫西林(钠)克拉维酸钾制剂说明书的修订内容,在选择用药时,应当根据新修订说明书进行充分的效益/风险分析。
三、患者应严格遵医嘱用药,用药前应当仔细阅读说明书。
四、各省级药品监管部门应当督促行政区域内的上述药品生产企业按要求做好相应说明书修订和标签、说明书更换工作;对违法违规行为组织依法严厉查处。
特此公告。
附件:1.阿莫西林克拉维酸钾口服制剂说明书修订要求2.阿莫西林钠克拉维酸钾注射剂说明书修订要求国家药监局2019年2月26日。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
PUBLIC ASSESSMENT REPORTof the Medicines Evaluation Boardin the NetherlandsAmoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mgfilm-coated tabletsPfizer B.V., the Netherlandsamoxicillin (as trihydrate potassium clavulanate)This assessment report is published by the MEB pursuant Article 21 (3) and (4) of Directive 2001/83/EC. The report comments on the registration dossier that was submitted to the MEB and its fellow –organisations in all concerned EU member states.It reflects the scientific conclusion reached by the MEB and all concerned member states at the end of the evaluation process and provides a summary of the grounds for approval of a marketing authorisation.This report is intended for all those involved with the safe and proper use of the medicinal product, i.e. healthcare professionals, patients and their family and carers. Some knowledge of medicines and diseases is expected of the latter category as the language in this report may be difficult for laymen to understand.This assessment report shall be updated by a following addendum whenever new information becomes available. General information on the Public Assessment Reports can be found on the website of the MEB.To the best of the MEB’s knowledge, this report does not contain any information that should not have been made available to the public. The MAH has checked this report for the absence of any confidential information.EU-procedure number: NL/H/2241/001- 002/MRRegistration number in the Netherlands: RVG 107460-1Date of first publication: 29 August 2011Last revision: 21 March 2012Pharmacotherapeutic group: combinations of penicillins, incl. beta-lactamase inhibitorsJ01CR02code:ATCRoute of administration: oralTherapeutic indication: acute bacterial sinusitis (adequately diagnosed); acute otitismedia; acute exacerbations of chronic bronchitis (adequatelydiagnosed); community acquired pneumonia; cystitispyelonephritis; skin and soft tissue infections in particularcellulitis, animal bites, severe dental abscess with spreadingcellulitis; bone and joint infections, in particular osteomyelitis. Prescription status: prescription onlyDate of first authorisation in NL: 28 September 2010Concerned Member States: Mutual recognition procedure with AT, BE, CY, CZ, DE, DK, EE,EL, ES, FI, HU, IE, IT, LT, LU, MT (not for 875 mg/125 mgstrength), NO, PL, PT, RO, SE, SI, SK, and UKApplication type/legal basis: Directive 2001/83/EC, Article 10(1).For product information for healthcare professionals and users, including information on pack sizes and presentations, see Summary of Product Characteristics (SPC), package leaflet and labelling.I INTRODUCTIONBased on the review of the quality, safety and efficacy data, the member states have granted a marketing authorisation for Amoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mg film-coated tablets from Pfizer B.V. The date of authorisation was on 28 September 2010 in the Netherlands. The product is indicated for the treatment of the following infections in adults and children :∙Acute bacterial sinusitis (adequately diagnosed)∙Acute otitis media∙Acute exacerbations of chronic bronchitis (adequately diagnosed)∙Community acquired pneumonia∙ Cystitis∙ Pyelonephritis∙Skin and soft tissue infections in particular cellulitis, animal bites, severe dental abscess with spreading cellulitis.∙Bone and joint infections, in particular osteomyelitis.Consideration should be given to official guidance on the appropriate use of antibacterial agents.A comprehensive description of the indications and posology is given in the SPC.AmoxicillinAmoxicillin is a semisynthetic penicillin (beta-lactam antibiotic) that inhibits one or more enzymes (often referred to as penicillin-binding proteins, PBPs) in the biosynthetic pathway of bacterial peptidoglycan, which is an integral structural component of the bacterial cell wall. Inhibition of peptidoglycan synthesis leads to weakening of the cell wall, which is usually followed by cell lysis and death.Amoxicillin is susceptible to degradation by beta-lactamases produced by resistant bacteria and therefore the spectrum of activity of amoxicillin alone does not include organisms which produce these enzymes. Clavulanic acidClavulanic acid is a beta-lactam structurally related to penicillins. It inactivates some beta-lactamase enzymes thereby preventing inactivation of amoxicillin. Clavulanic acid alone does not exert a clinically useful antibacterial effect.This mutual recognition procedure concerns a generic application claiming essential similarity with the innovator products Augmentin 500/125 mg and 875/125 mg filmcoated tablets (NL license RVG 09840 and 18553, respectively) which have been registered in the Netherlands by GlaxoSmithKline BV since 1983 (500 mg/ 125 mg) and 1996 (875 mg/ 125 mg).In addition, reference is made to Augmentin authorisations in the individual member states (reference product).The marketing authorisation is granted based on article 10(1) of Directive 2001/83/EC.This type of application refers to information that is contained in the pharmacological-toxicological and clinical part of the dossier of the authorisation of the reference product. A reference product is a medicinal product authorised and marketed on the basis of a full dossier, i.e. including chemical, biological, pharmaceutical, pharmacological-toxicological and clinical data. This information is not fully available in the public domain. Authorisations for generic products are therefore linked to the ‘original’ authorised medicinal product, which is legally allowed once the data protection time of the dossier of the reference product has expired. For this kind of application, it has to be demonstrated that the pharmacokinetic profile of the product is similar to the pharmacokinetic profile of the reference product. To this end the MAH has submitted two bioequivalence study in which the pharmacokinetic profile of the product is compared with the pharmacokinetic profile of the reference products Augmentin 500 mg/ 125 mg and 875 mg/ 125 mg tablets, registered in the UK and Germany, respectively. A bioequivalence study is the widely accepted means of demonstrating that difference of use of different excipients and different methods of manufacture have no influence on efficacy and safety. This generic product can be used instead of its reference product.No new pre-clinical and clinical studies were conducted, which is acceptable for this abridged application. No scientific advice has been given to the MAH with respect to these products, and no paediatric development programme has been submitted, as this is not required for generic medicinal products.II SCIENTIFIC OVERVIEW AND DISCUSSIONII.1 Quality aspectsCompliance with Good Manufacturing PracticeThe MEB has been assured that acceptable standards of GMP (see Directive 2003/94/EC) are in place for this product type at all sites responsible for the manufacturing of the active substance as well as for the manufacturing and assembly of this product prior to granting its national authorisation.Active substancesAmoxicillin trihydrate is an established active substance which is described in the Ph.Eur.*. Amoxicillin trihydrate is a white or almost white crystalline powder.Potassium clavulanate is an established active substance which is described in the Ph.Eur.*. Potassium clavulanate is a white or almost white hydroscopic crystalline powder.The CEP procedure is used for both active substances. Under the official Certification Procedures of the EDQM of the Council of Europe, manufacturers or suppliers of substances for pharmaceutical use can apply for a certificate of suitablity concerning the control of the chemical purity and microbiological quality of their substance according to the corresponding specific monograph, or the evaluation of reduction of Transmissible Spongiform Encephalopathy (TSE) risk, according to the new general monograph, or both. This procedure is meant to ensure that the quality of substances is guaranteed and that these substances comply with the European Pharmacopoeia, the official handbook in which methods of analysis with specifications for substances are laid down by the authorities of the EU.ManufactureFor both substances this is covered by the CEP.Quality control of drug substancesFor both amoxicillin trihydrate and potassium clavulanate, Ph. Eur. specifications plus additional requirements from the involved CEPs are applicable.Stability of drug substancesAmoxicillin trihydrate: on the CEP a re-test period of 2 years is stated when adequately stored.Potassium clavulanate: on the CEP a re-test period of 48 months is stated when adequately stored. Assessment thereof was part of granting the CEP and has been granted by the EDQM.* Ph.Eur.is an official handbook (pharmacopoeia) in which methods of analysis with specifications for substances are laid down by the authorities of the EU.Medicinal ProductCompositionAmoxicillin/Clavulanic acid Pfizer 500mg/125mg tablets are white, oval, film-coated tablets inscribed with ‘A’ on one side and ‘64’ on the other side.Amoxicillin/Clavulanic acid Pfizer 875mg/125mg tablets are white, capsule shaped, film-coated tablets inscribed with ‘A’ on one side and with a score line in between ‘6’ and ‘5’ on the other side. The score-line is only to facilitate breaking for ease of swallowing and not to divide into equal doses.Both strengths are immediate-release tablets. The tablets can be distinguished by their dimensions.The excipients are:Tablet core: microcrystalline cellulose (E460), colloidal anhydrous silica, magnesium stearate (E470b), sodium starch glycolate (Type A)Film-coating: hypromellose (E464), macrogol 400, titanium dioxide (E171)The film-coated tablets are packed in Alu/Alu (polyamide/aluminium/PVC - aluminium foil) blister packs in a cardboard box.The excipients and packaging are usual for this type of dosage form.Pharmaceutical developmentThe development is mainly based on the qualitative composition of the originator product Augmentan tablets for both strengths (from GlaxoSmithKline, Germany). In the development the requirements of the monograph Co-Amoxiclav Tablets BP (British Pharmacopoeia) have been used as a guiding principle. The MAH concluded that the two originator strengths are not dose weight proportional, therefore this was also not intended for the proposed product. In the bioequivalence study, the batch size of the test bio-batch is of pilot-scale, the mentioned future manufacturing scale size is acceptable. The DE 875+125 mg reference product is acceptable in view of the composition of the NL originator product.Dissolution studiesComparing dissolution studies are performed for two batches of both strengths of the proposed product, including the 875+125 mg test bio-batch, using the DE reference bio-batch (875+125 mg) and UK originator bio-batch (500+125 mg). The proposed product shows slightly faster dissolution results in the 0-20 minutes traject in comparison with the originator products, this is not a problem.Manufacturing processThe manufacturing process has been adequately described in sufficient detail, and adequate in-process controls have been listed. Pilot-scale batches have been validated, 2 batches per strength, and including the 875+125 mg test bio-batch. The finished product manufacturer commits in the dossier to validate a third batch (first commercial batch) at pilot-scale and the first three commercial batches at two specific scale sizes for the two strengths, respectively. This is acceptable.Quality control of drug productAdequate specifications are proposed for the drug product including the Ph. Eur. test on uniformity of dosage units. For two pilot-scale batches of each strength batch results have been provided. It is committed (see above) by the company to validate a third batch (first commercial batch) at pilot-scale and the first three commercial batches for the three strengths, which will render additional batch results. Stability tests on the finished productThe stability results show that the proposed tablets are sufficiently stable. For both components slight to moderate assay decreases are observed, but are within the set requirements. Total impurities increase after 12 months at 30°C/70% RH, but remain within limits. Based on the stability data the claimed shelf-life of 2 years for both strengths without specific storage condition is justified.Specific measures concerning the prevention of the transmission of animal spongiform encephalopathies There are no substances of ruminant animal origin present in the product nor have any been used in the manufacturing of this product, so a theoretical risk of transmitting TSE can be excluded.II.2 Non clinical aspectsThis product is a generic formulation of Augmentin, which is available on the European market. No new preclinical data have been submitted, and therefore the application has not undergone preclinical assessment. This is acceptable for this type of application.Environmental risk assessmentThe product is intended as a substitute for other identical products on the market. The approval of this product will not result in an increase in the total quantity of amoxicillin trihydrate or potassium clavulanate released into the environment. It does not contain any component, which results in an additional hazard to the environment during storage, distribution, use and disposal.II.3 Clinical aspectsAmoxicillin trihydrate and potassium clavulanate are well-known active substance with established efficacy and tolerability.For this generic application, the MAH has submitted two bioequivalence studies in which the pharmacokinetic profile of the test products Amoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mg film-coated tablets Pfizer B.V., the Netherlands) is compared with the pharmacokinetic profile of the reference products Augmentin 500/125 mg (GSK, UK) and 875/125 mg (GSK, Germany) film-coated tablets.The choice of the reference productThe choice of the reference product in the bioequivalence study has been justified by comparison of dissolution results and compositions of reference products (if applicable) in different member states.The formula and preparation of the bioequivalence batch is identical to the formula proposed for marketing.The SPC states that the tablet should be taken with food to prevent gastro-intestinal AEs. There is no pharmacokinetic reason for intake with food. Therefore the study design (fed conditions) is considered acceptable.Bioequivalence study with 875/125 mg tablets under fed conditionsAn open-label, randomized, two-treatment, two sequence , two period, cross-over single-dose comparative oral bioequivalence study was carried out under fed conditions in 48 healthy Indian males, aged 19-40 years. Each subject received a single dose (875 mg amoxicillin trihydrate, 125 mg potassium clavulanate) of one of the 2 formulations in randomized order. Drugs were administered 30 minutes after a high fat meal of 955 KCal (approximately 60% of total calories consisted of fat). Tablets were taken with 240 ml of water. There was a washout interval of 8 days between the 2 dose administrations.Blood samples were collected pre-dose and at 0.25, 0.5, 0.75, 1, 1.33, 1.67, 2, 2.33, 2.67, 3, 4, 5, 6 ,7, 8,10, and 12 hours after administration of the products.The analytical method is adequately validated and considered acceptable for analysis of the plasma samples. The methods used in this study for the pharmacokinetic calculations and statistical evaluation are considered acceptable.ResultsOne subject was dismissed for non-compliance (positive benzodiazepine test). Three subjects did not show up for Period II. Analysis of samples of 46 subjects who completed the study was performed. However the results of analysis for amoxicillin samples of subjects 1, 2, 38, 39 and 49 and those for clavulanic acid of subjects 1, 2 and 39 have not been reported. When these samples came up for analysis, they had already completed 3 freeze-thaw cycles. Stability of analyte beyond 3 freeze thaw samples could not be established during analytical method validation. Therefore, data from only 41 subjects for amoxicillin and 43 for clavulanic acid were reported.Table 1. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, t max (median, range)) of amoxicillin under fed conditions.Treatment N = 41 AUC0-tµg.h/mlAUC0-∞µg.h/mlC maxµg/mlt maxht1/2hTest 50.7 ± 8.9 51.4 ± 9.0 15.7 ± 3.5 2.3(1 - 5)1.5 ± 0.3Reference 48.8 ± 8.6 49.4 ± 8.5 15.3 ± 3.4 2.3(1.3 - 5)1.5 ± 0.3*Ratio (90% CI)1.03(1.00 -1.07)1.03(1.00 -1.07)1.02(0.97 – 1.08)--- ---CV (%) 8.3 8.2 15.1 --- --- AUC0-∞area under the plasma concentration-time curve from time zero to infinityAUC0-t area under the plasma concentration-time curve from time zero to t hoursC max maximum plasma concentrationt max time for maximum concentrationt1/2half-life*ln-transformed valuesTable 2. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, t max (median, range)) of clavulanic acid under fed conditions.Treatment N = 43 AUC0-tµg.h/mlAUC0-∞µg.h/mlC maxµg/mlt maxht1/2hTest 4.0 ± 2.3 4.6 ± 2.5 1.9 ± 1.1 2.3(1.3 - 5)1.0 ± 0.2Reference 3.7 ± 1.6 4.3 ± 1.7 1.7 ± 0.8 2(1.3 - 3)1.0 ± 0.2*Ratio (90% CI)1.01(0.91 -1.13)1.01(0.88 - 1.15)1.04(0.92 -1.16)--- ---CV (%) 30.9 30.4 32.4 --- --- AUC0-∞area under the plasma concentration-time curve from time zero to infinityAUC0-t area under the plasma concentration-time curve from time zero to t hoursC max maximum plasma concentrationt max time for maximum concentrationt1/2half-life*ln-transformed valuesThe 90% confidence intervals calculated for AUC0-t, AUC0-∞and C max are in agreement with those calculated by the MAH and are within the bioequivalence acceptance range of 0.80 – 1.25. Based on the pharmacokinetic parameters of amoxicillin and clavulanic acid under fed conditions, it can be concludedthat Amoxicilline/Clavulaanzuur Pfizer 875 mg/125 mg film-coated tablets and the Augmentin 875/125 mgfilm-coated tablets. are bioequivalent with respect to rate and extent of absorption, and fulfill the bioequivalence requirements outlined in the relevant CHMP Note for Guidance.Bioequivalence study with 500/125 mg tablets under fasted conditionsAn open label, randomized, two-treatment, two-sequence, two-period, crossover, single dose comparativeoral bioequivalence study was carried out under fed conditions in 50 (48+2 alternates) healthy male volunteers, aged 18-39 years. 42 of them were non smokers, and 8 subjects smoked 9 or less than 9cigarettes a day. Each subject received a single dose (500 mg amoxicillin trihydrate, 125 mg potassium clavulanate) of one of the 2 formulations in randomized order after. Tablets were taken with 240 ml of water after an overnight fast. There was a washout interval of 3 days between the 2 dose administrations. Blood samples were collected pre-dose and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.5, 4, 5, 6, 7, 8, 10 and 12 hours after administration of the products.The analytical method is adequately validated and considered acceptable for analysis of the plasma samples. The methods used in this study for the pharmacokinetic calculations and statistical evaluation are considered acceptable.The SPC states that the tablet should be taken with food to prevent gastro-intestinal AEs. There is no pharmacokinetic reason for intake with food. Therefore the study design (fasted conditions) is considered acceptable.ResultsOne subject was withdrawn from study due to adverse events (fever) in the second period. Forty-nine subjects completed the study entirely, and as per protocol the first 48 subject were included in the analysis.Table 3. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, t max (median, range)) of amoxicillin under fasted conditions.Treatment N = 48 AUC0-tµg.h/mlAUC0-∞µg.h/mlC maxµg/mlt maxht1/2hTest 24.80 ± 5.36 25.37 ± 5.43 8.21 ± 2.29 2.0 (0.75 – 4.0) 1.16 ± 0.19 Reference 26.44 ± 6.04 26.92 ± 6.06 8.78 ± 2.68 2.0 (1.0 – 5.0) 1.15 ± 0.18*Ratio (90% CI)0.95(0.92 – 0.98)0.94(0.92 – 0.97)0.94(0.90 – 0.99)--- ---CV (%) 8.1 7.9 14.9 --- --- AUC0-∞area under the plasma concentration-time curve from time zero to infinityAUC0-t area under the plasma concentration-time curve from time zero to t hoursC max maximum plasma concentrationt max time for maximum concentrationt1/2half-life*ln-transformed valuesTable 4. Pharmacokinetic parameters (non-transformed values; arithmetic mean ± SD, t max (median, range)) of clavulananic acid under fasted conditions.Treatment N = 48 AUC0-tµg.h/mlAUC0-∞µg.h/mlC maxµg/mlt maxht1/2hTest 6.40 ± 2.52 6.62 ± 2.52 2.63 ± 0.98 1.25 (0.75 –3.0)1.10 ± 0.15 Reference 6.72 ±2.41 6.96 ± 2.41 2.71 ± 0.96 1.5 (1.0 –3.0) 1.13 ± 0.19*Ratio (90% CI)0.93(0.87 – 1.01)0.93(0.87 – 1.00)0.96(0.89 – 1.03)--- ---CV (%) 22.1 20.8 22.8 --- --- AUC0-∞area under the plasma concentration-time curve from time zero to infinityAUC0-t area under the plasma concentration-time curve from time zero to t hoursC max maximum plasma concentrationt max time for maximum concentrationt1/2half-life*ln-transformed valuesThe 90% confidence intervals calculated for AUC0-t, AUC0-∞and C max are in agreement with those calculated by the MAH and are within the bioequivalence acceptance range of 0.80 – 1.25. Based on the pharmacokinetic parameters of amoxicillin and clavulanic acid under fasted conditions, it can be concluded that Amoxicilline/Clavulaanzuur Pfizer 500 mg/125 mg film-coated tablets and the Augmentin500/125 mg film-coated tablets. are bioequivalent with respect to rate and extent of absorption, and fulfillthe bioequivalence requirements outlined in the relevant CHMP Note for Guidance.The MEB has been assured that the bioequivalence study has been conducted in accordance with acceptable standards of Good Clinical Practice (GCP, see Directive 2005/28/EC) and Good Laboratory Practice (GLP, see Directives 2004/9/EC and 2004/10/EC).Risk management planThe combination of amoxicillin trihydrate and potassium clavulanate was first approved in 1983, and thereis now more than 10 years post-authorisation experience with the active substance. The safety profile of amoxicillin trihydrate and potassium clavulanate can be considered to be well established and no product specific pharmacovigilance issues were identified pre- or postauthorisation which are not adequately covered by the current SPC. Additional risk minimisation activities have not been identified for the reference medicinal product. The MAH has a pharmacovigilance system at their disposal, which is basedon the current European legislation. Routine pharmacovigilance activities are sufficient to identify actual or potential risks and a detailed European Risk Management Plan is not necessary for this product.Product informationSPCThe SPC of Amoxicillin/Clavulanic acid Pfizer is compared with the Dutch approved SPC of Augmentin, filmcoated tablets 500/125 mg and 875/125 mg. This text results from the article 30 procedure, which is published on the website of the EU Commission in October 2009.Readability testThe package leaflet has been evaluated via a user consultation study in accordance with the requirementsof Articles 59(3) and 61(1) of Directive 2001/83/EC. Testing was performed with in total 20 participants. This cohort of 20 participants was recruited in the Geleen area, NL in the period October 2007. The way of recruitment and individual demographic and sociologic details were provided in the final report.A total of fifteen questions have been evaluated with regard to the use of finding, ease of understanding and subjective impression of the PIL by the participants. The responses were recorded satisfactory.The user test showed that the leaflet enabled more than of the 90% of participants to find the information and more than 90% of those understood the information good or in detail. The overall impression of the methodology and the overall impression of the leaflet structure are positive.III OVERALL CONCLUSION AND BENEFIT-RISK ASSESSMENTAmoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mg film-coated tablets have a proven chemical-pharmaceutical quality and are generic forms of Augmentin 500/125 mg and 875/125 mg filmcoated tablets. Augmentin is a well-known medicinal product with an established favourable efficacy and safety profile.Bioequivalence has been shown to be in compliance with the requirements of European guidance documents.The MAH has provided written confirmation that systems and services are in place to ensure compliance with their pharmacovigilance obligations.The SPC, package leaflet and labelling are in the agreed templates.The Board followed the advice of the assessors. Amoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mg film-coated tablets are authorised in the Netherlands on 28 September 2010.There was no discussion in the CMD(h). Agreement between member states was reached during a written procedure. The concerned member states, on the basis of the data submitted, considered that essential similarity has been demonstrated for Amoxicilline/Clavulaanzuur Pfizer 500/125 and 875 mg/125 mg film-coated tablets with the reference product, and have therefore granted a marketing authorisation. The mutual recognition procedure was finished on 24 May 2011.The PSUR submission cycle will follow the Harmonized Birth Date Amoxicillin/Clavulanic Acid. The EU harmonised birth date for Amoxicillin and clavulanate is 7 March 1972. The next Data Lock Point will be 31 March 2012, the company commits to submit PSUR in May 2012 to harmonise with the birthdate of Amoxicillin and clavulanate.The date for the first renewal will be November 2012.The following post-approval commitments have been made during the procedure:Quality - medicinal product- The MAH has committed to validate a third batch (first commercial batch) at pilot-scale and the first three commercial batches for the three strengths, which will render additional batch results.List of abbreviationsASMF Active Substance Master FileATC Anatomical Therapeutic Chemical classificationAUC Area Under the CurvePharmacopoeiaBritishBPCEP Certificate of Suitability to the monographs of the European Pharmacopoeia CHMP Committee for Medicinal Products for Human UseCI ConfidenceIntervalC max MaximumconcentrationplasmaCMD(h) Coordination group for Mutual recognition and Decentralised procedure for human medicinal productsVariationofCoefficientCVFileMasterDrugEDMFEuropeanEDQM European Directorate for the Quality of MedicinesUnionEUEuropeanPracticeClinicalGoodGCPLaboratoryPracticeGoodGLPPracticeManufacturingGMPGoodICH International Conference of HarmonisationHolderAuthorisationMarketingMAHMEB Medicines Evaluation Board in the NetherlandsOTC Over The Counter (to be supplied without prescription)ReportAssessmentPARPublicPharmacopoeiaPh.Eur.EuropeanLeafletPackagePILPSUR Periodic Safety Update ReportStandardDeviationSDSPC Summary of Product Characteristicst½ Half-lifet max Time for maximum concentrationEncephalopathySpongiformTSETransmissibleUSP Pharmacopoeia in the United States。
