现代分离技术习题(Modern separation technology exercises)
(完整word版)现代分离分析法期末复习题

期末复习题一、最佳选择题,每题2分,共20分。
每题的备选项中只有一个最佳答案。
1. 分离化学中的纯度概念是( )A. 分离过程中目标化合物浓度增加B. 溶液中溶剂蒸发掉,溶液中所有组分浓度同程度增加的过程C. 通过分离操作使产物纯度增加的过程。
D. 表示纯化产物主组分含量高低或杂质多少2. 在常量分离中,当溶液中的金属离子还剩下( )时,即可认为沉淀完全。
A. 10-3mol/LB. 10-4 mol/LC. 10-5 mol/LD. 10-6 mol/L3. 用8-羟基喹啉从水溶液中萃取Al3+,组成的萃取体系是( )A. 简单分子萃取体系B. 中性络合萃取体系C. 螯合萃取体系D. 离子缔合萃取体系4. 下列体系中,形成协萃体系的是( )A. 乙酰基丙酮为萃取剂,萃取Al3+B. 用乙醚萃取FeCl3C. 形成高分子胺盐的萃取体系D. HTTA与2,2-联吡啶为萃取剂,萃取La3+5. 下列属于阴离子交换树脂的是( )A. RSO3HB. ROHC. RNH2(CH3)OHD. RCO2H6.可以作为离子交换树脂合成中的交联剂的是( )A. 苯乙烯B. 丙烯酸C. 甲基丙烯酸D. 二乙烯苯7. 吸附层析分离是利用( )A. 利用吸附剂表面对不同组分吸附性能的差异B. 利用不同组分在流动相和固定相之间的分配系数不同C. 利用不同组分对离子交换剂亲和力的不同D. 利用某些凝胶对于不同分子大小的组分阻滞作用的不同8. 层析用硅胶有不同标号,硅胶GF254代表( )A. 硅胶中不含粘合剂B. 由硅胶和煅石膏混合而成C. 硅胶中既含有煅石膏又含荧光指示剂D. 硅胶中只含有荧光指示剂9. 依据样品组分的分配系数和电泳速度的差别而分离的是( )A. 毛细管区带电泳B. 毛细管凝胶电泳C. 毛细管等点聚焦电泳D. 毛细管电色谱10.过滤粒径由小到大顺序排列正确的是( )A. 反渗透<超滤<微滤<一般过滤B. 反渗透<微滤<超滤<一般过滤C. 微滤<超滤<反渗透<一般过滤D. 超滤<反渗透<微滤<一般过滤11. 分离化学中的纯化是( )A. 分离过程中目标化合物浓度增加B. 溶液中溶剂蒸发掉,溶液中所有组分浓度同程度增加的过程C. 通过分离操作使产物纯度增加的过程。
现代分离技术试题

填空部分:1、我们测定气相色谱仪灵敏度时,如果用102-白色担体,邻苯二甲酸二壬酯固定液,此时按两相所处的状态属于(气—液) 色谱;按固定相性质属于(填充柱) 色谱;按展示方式属于(冲洗) 色谱;按分离过程所依据的物理化学原理属于(分配)色谱。
2、液相色谱分析中常用以低压汞灯为光源,波长固定式的紫外(UV)检测器,它是以低压汞灯的最强发射线(253.8)nm做为测定波长。
3、根据分离原理的不同,液相色谱可分为(液—液);(液—固);(离子交换);(凝胶)色谱法。
4、固定相分为(液体)和(固体)固定相两大类。
固体固定相可分为(吸附剂),(高分子多孔小球),(化学键合)固定相三类。
5、保留值大小反映了(组分)与(固定相)之间作用力的大小,这些作用力包括(定向力),(诱导力),(色散力),(氢键作用力)等。
6、柱温选择主要取决于样品性质。
分析永久性气体,柱温一般控制在(50℃以上);沸点在300℃以下的物质,柱温往往控制在(150℃以下);沸点300℃以上的物质,柱温最好能控制在(200℃以下);高分子物质大多分析其裂解产物。
若分析多组分宽沸程样品,则可采用(程序升温);检测器可采用(FID)。
7、在气相色谱分析中,载气钢瓶内贮存气体都有明显的标记,如氮气,瓶外漆(黑色),用黄色标写“氮”;氢气漆(深绿色),红色标写“氢”。
8、固定液按相对极性可粗分为(五)类,异三十烷是(非极性)固定液,属(0)级;β,β,—氧二丙腈是(强极性)固定液,属(5)级。
9、采用TCD检测器时,要注意先(通载气)后(加桥电流)并且(桥电流)不可过大,否则易烧损铼钨丝。
10、色谱基本参数测量与计算的关键是(控制色谱操作条件的稳定)。
11、气相色谱中,对硫、磷化合物有高选择性和高灵敏度的检测器是火焰光度检测器(FPD)和硫磷检测器(SPD);对大多数有机化合物有很高灵敏度的是氢火焰离子化检测器(FID)。
12、某色谱峰峰底宽为50秒,它的保留时间为50分,在此情况下,该柱子理论板数有(57600)块。
现代分离方法与技术-第2章-沉淀分离法-最终版本

Q—加入沉淀剂瞬间生成沉淀物的浓度;
s— 沉淀物的溶解度;
Q-s — 沉淀物的过饱和度;
K— 比例常数,它与沉淀物的性质、温度、溶液中存在
的其它物质有关。
Q
s
s
— 沉淀物的相对过饱和度;
( 2)哈伯理论
聚集速度
条件
在沉淀的形成过程中,晶核逐渐长大成沉淀微粒,
这些微粒可以聚集成更大的聚集体。这种聚集过程的快慢
CoS:型 Ksp = 4.0×10-20 型 Ksp = 7.9×10-24
2.1.4 沉淀的生成
1). 沉淀的类型
类别 颗粒直径
特性
示例
晶形沉淀
凝乳状沉 淀
无定形沉 淀
0.1~1µm
∠0.02 µm
颗粒大,内部排列规 则,紧密,极易沉于 容器底部
介于两者之间
内部排列杂乱无章, 疏松,絮状沉淀,体 积庞大,含大量水,
溶度积:在微溶化合物的饱和溶液中,组成沉淀的有关
离子浓度的乘积,在一定温度下为一常数,称 为溶度积常数或溶度积。构晶离子
MA型: MA ⇆ M+ + A-
Ksp= [M+ ][A-]
MmAn型: MmAn ⇆ mMn++ nAm-
Ksp= [Mn+ ]m[Am-]n 意义:溶度积是微溶化合物和它的饱和溶液达到平衡
(2)晶核的生长过程
晶核形成后,溶液中的构晶离子向晶核表面扩散,并沉 积在晶核上,使晶核逐渐长大,到一定程度时,成为沉淀微 粒。
结论: 异相成核显著, 易形成大颗粒晶形沉淀; 均相成核显著, 易形成小颗粒非晶形沉淀.
3). 晶形沉淀和无定形沉淀的生成 (1)冯氏经验公式
现代分离专业技术试题

填空部分:1、我们测定气相色谱仪灵敏度时,如果用102-白色担体,邻苯二甲酸二壬酯固定液,此时按两相所处的状态属于(气—液) 色谱;按固定相性质属于(填充柱) 色谱;按展示方式属于(冲洗) 色谱;按分离过程所依据的物理化学原理属于(分配)色谱。
2、液相色谱分析中常用以低压汞灯为光源,波长固定式的紫外(UV)检测器,它是以低压汞灯的最强发射线(253.8)nm做为测定波长。
3、根据分离原理的不同,液相色谱可分为(液—液);(液—固);(离子交换);(凝胶)色谱法。
4、固定相分为(液体)和(固体)固定相两大类。
固体固定相可分为(吸附剂),(高分子多孔小球),(化学键合)固定相三类。
5、保留值大小反映了(组分)与(固定相)之间作用力的大小,这些作用力包括(定向力),(诱导力),(色散力),(氢键作用力)等。
6、柱温选择主要取决于样品性质。
分析永久性气体,柱温一般控制在(50℃以上);沸点在300℃以下的物质,柱温往往控制在(150℃以下);沸点300℃以上的物质,柱温最好能控制在(200℃以下);高分子物质大多分析其裂解产物。
若分析多组分宽沸程样品,则可采用(程序升温);检测器可采用(FID)。
7、在气相色谱分析中,载气钢瓶内贮存气体都有明显的标记,如氮气,瓶外漆(黑色),用黄色标写“氮”;氢气漆(深绿色),红色标写“氢”。
8、固定液按相对极性可粗分为(五)类,异三十烷是(非极性)固定液,属(0)级;β,β,—氧二丙腈是(强极性)固定液,属(5)级。
9、采用TCD检测器时,要注意先(通载气)后(加桥电流)并且(桥电流)不可过大,否则易烧损铼钨丝。
10、色谱基本参数测量与计算的关键是(控制色谱操作条件的稳定)。
11、气相色谱中,对硫、磷化合物有高选择性和高灵敏度的检测器是火焰光度检测器(FP D)和硫磷检测器(SPD);对大多数有机化合物有很高灵敏度的是氢火焰离子化检测器(FI D)。
12、某色谱峰峰底宽为50秒,它的保留时间为50分,在此情况下,该柱子理论板数有(57600)块。
现代生化技术复习题

现代⽣化技术复习题. 第⼀章提取与分离技术⼀、名词解释1、机械破碎法2、物理破碎法3、温度差破碎法4、压⼒差破碎法5、超声波破碎法6、渗透压变化法7、化学破碎法8、酶促(学)破碎法9、⾃溶法10、抽提11、盐溶12、盐析13、沉淀分离法14、分段盐析15、K S分段盐析16、β分段盐析17、等电点沉淀法18、有机溶剂沉淀法19、复合沉淀法20、⾦属盐沉淀法21、选择性变性沉淀法⼆、填空题1、细胞破碎的⽅法有、和。
2、机械破碎法按照使⽤机械的不同可分为、和。
3、常⽤的物理破碎法有、和等。
4、常⽤的压⼒差破碎法有、和等。
5、化学破碎法采⽤的表⾯活性剂有和两种。
其中之⼀按其带电荷性质⼜可分为和两种。
6、根据抽提时所采⽤的溶剂或溶液的不同。
抽提的⽅法主要有、、和等7、常⽤的沉淀分析法有、、、、和等。
8、常⽤的⾦属盐沉淀法有和。
9、蛋⽩质盐析时,带⼊⼤量盐离⼦杂质,可采⽤、和⽅法脱盐。
10、要分离和提纯核酸过程中,常⽤来沉淀DNA和RNA。
11、常⽤的使蛋⽩质沉淀的⽅法有、、、和等。
12、三、是⾮题(对的打√、错的打×)1、渗透压变化法可⽤于⾰兰⽒阳性菌的破碎。
()2、有机溶剂破碎细胞主要是使细胞膜磷脂结构破坏,从⽽使细胞膜的透过性增强。
()3、⽤化学法破碎细胞提取酶时,经常⽤离⼦型表⾯活性剂。
()4、⽤化学法破碎细胞提取酶时,⽤⾮离⼦型表⾯活性剂最好。
()5、对于具有细胞壁结构的细胞采⽤酶法破碎时,应根据细胞壁结构选择不同的酶。
()6、酸性物质易溶于酸性溶剂中,碱性物质易溶于碱性溶液中。
()7、在等电点时两性电解质溶解度最⼩。
()8、抽提两性电解质时应避开其等电点。
()四、选择题1、利⽤突然降压法破碎⾰兰⽒阴性⼤肠杆菌应选择期的细胞破碎效果最佳。
A 调整期B 对数⽣长期C 平衡期D衰退期2、提取膜结合酶采⽤法破碎细胞最佳。
A ⾼压冲击法B 突然降压法C 渗透压变化法3、超声波破碎法最适合于破碎。
现代分离技术习题

第二十章 高效液相色谱法思考题和习题1. 简述高效液相色谱法和气相色谱法的主要异同点。
相同点:均为高效、高速、高选择性的色谱方法,兼具分离和分析功能,均可以在线检测不同点:分析对象及范围 流动相的选择 操作条件GC 能气化、热稳定性好、且沸点较低的样品,占有机物的20% 流动相为有限的几种“惰性”气体,只起运载作用,对组分作用小 加温常压操作HPLC 溶解后能制成溶液的样品,高沸点、高分子量、难气化、离子型的稳定或不稳定化合物,占有机物的80% 流动相为液体或各种液体的混合。
它除了起运载作用外,还可通过溶剂来控制和改进分离。
室温、高压下进行2.何谓化学键合相?常用的化学键合相有哪几种类型?分别用于哪些液相色谱法中?采用化学反应的方法将固定液键合在载体表面上,所形成的填料称为化学键合相。
优点是使用过程不流失,化学性能稳定,热稳定性好,适于作梯度淋洗。
目前常用的Si-O-Si-C型键合相,按极性分为非极性,中等极性与极性三类。
①非极性键合相:常见如ODS键合相,既有分配又有吸附作用,用途非常广泛,用于分析非极性或弱极性化合物;②中等圾性键合相:常见的有醚基键合相,这种键合相可作正相或反相色谱的固定相,视流动相的极性而定:③极性键合相:常用氨基、氰基键合相,用作正相色谱的固定相,氨基键合相还是分离糖类最常用的固定相。
3.什么叫正相色谱?什么叫反相色谱?各适用于分离哪些化合物?正相色谱法:流动相极性小于固定相极性的色谱法。
用于分离溶于有机溶剂的极性及中等极性的分子型物质,用于含有不同官能团物质的分离。
反相色谱法:流动相极性大于固定相极性的色谱法。
用于分离非极性至中等极性的分子型化合物。
4.简述反相键合相色谱法的分离机制。
典型的反相键合色谱法是用非极性固定相和极性流动相组成的色谱体系。
固定相,常用十八烷基(ODS或C18)键合相;流动相常用甲醇-水或乙腈-水。
非典型反相色谱系统,用弱极性或中等极性的键合相和极性大于固定相的流动相组成。
现代分离B卷

现代分离技术试卷(B卷)及答案一、在下列题目中,选出一个你认为正确的答案,并在该答案前的字母上划圈。
若给出的答案均不正确,请在方括号内填上正确的答案。
(30分)1.、用吸收方法对六元混合物进行分离,在采用简捷法进行计算时,应对其中的__(1)___规定分离要求,规定了分离要求的组分称为__(2)__(1) A 1个组分B 2个组分 C 3个组分D 4个组分E【】(2)A 重组分 B 轻关键组分 C 轻组分 D 重关键组分E【关键组分】2.、超临界流体萃取是一种利用________进行分离的方法。
A 被分离物质在超临界状态下的特殊性质B 萃取剂在超临界状态下的特殊性质C 超临界技术队分离装置进行特殊设计D 胶体分子在超临界状态下对萃取剂产生特殊亲和力E 【】3形成恒沸物的数学条件为:EA B C DE []4、在推导芬斯克公式时,用到如下假设:A 回流比无穷大,所需理论板数无限多,全塔a基本不变,恒摩尔流B回流比最小,所需理论板数无限多,全塔a基本不变,恒摩尔流C回流比无穷大,所需理论板数最少,全塔a及摩尔流率取实际数值D回流比无穷大,所需理论板数最少,全塔a基本不变,恒摩尔流5组分X被吸附剂吸附的微观过程依次为:A.外部扩散内部扩散吸附;B. 外部扩散吸附内部扩散C. 吸附外部扩散内部扩散D. 吸附内部扩散外部扩散E.{ }6.在多组精馏简捷法中,塔顶回收率又称为 1 ,其定义为 2 。
1、A.最轻组分回收率;B.轻关键组分回收率;C.最重组分回收率;D.重关键组分回收率E.【】2. A.ψ顶=;B.ψ顶=; C.ψ顶=D.ψ顶=;E.【ψ顶=】7.在多组分吸收塔内,液相从塔顶到塔底流量 1 ,各溶质组分浓度1、A.逐渐变小;B.保持恒定;C.变大或变小由混合物的物性决定;D.2、A.逐渐变小;B.保持恒定;C.变大或变小由混合物的物性决定;D.【】8电渗析过程如图一所示。
1、图中C室称为A、原料室;B、浓缩室;C.淡化室;D、中和室;E、2.A膜称为A.阳膜;B.液膜;C.阴膜;D.反渗透膜;(接上)③ K膜的特性为(C)A.带正电荷,允许阳离子通过; B.中性膜,允许所有离子通过;C.带负电荷,允许阳离子通过; D.带负电荷,允许阴离子通过;9.操作压力对平衡吸收量有很大的影响,一般来说,D可增加平衡吸收量。
最新转-现代分离技术思考题答案

精品文档一、名词解释 ............ 1截留率: . (1)水通量: (1)浓差极化: (1)配系数: (1)萃取因素 (1)带溶剂: (1)结晶:. (1)晶核: (1)重结晶: (2)双水相萃取: (2)超临界流体萃取: (2)离子交换技术: (2)膜污染: (2)凝聚值 (2)精馏: (2)最小回流比: (2)萃取精馏: (2)共沸精馏: (2)分离因子: (2)絮凝: (2)错流过滤称切向流过滤: (2)比移值 (2)二、简答题 (2)1.简述进行料液予处理的目的并说明常用的料液预处理方法 (2)2.超临界萃取的特点是什么23.常用的细胞破碎方法有哪些 (2)4.溶剂萃取中萃取剂的选择原则 (2)5.影响溶剂萃取的因素有哪些 (3)6.乳化现象是如何发生的生产中应如何防止乳化现象的发生如何破乳 (3)7.说明双水相萃取的原理影响双水相分配的主要因素有哪些 (3)9.试给出几种常用的工业起晶方法并说明维持晶体生长的条件 (3)10.晶体质量的指标通常包括哪些内容生产中如何获得高质量的晶体产品 (3)11.选择重结晶适宜溶剂的原则是什么 (3)12.离子交换树脂有哪些分类方法 (3)13.离子交换树脂的主要性能指标物理化学性质 (3)14.离子交换树脂为什么要再生活化如何再生 (3)15.微滤超滤纳滤反渗透分离技术的特点及适用范围 (3)16.简要分析膜污染产生的原因和防、治方法 (4)17.简述色谱分离技术的原理及分类 (4)三、实例分析 (4)1.乙醇沉析法提取柑橘皮中的果胶 (4)2.头孢菌素C盐分离工艺剖析 (4)一、名词解释截留率:指溶液经超滤处理后被膜截留的溶质量占溶液中该溶质总量的百分率。
水通量:纯水在一定压力温度0.35MPa25℃下试验透过水的速度L/h×img1815file:///C:/Users/asus/AppData/Local/Temp/ksohtml/wps_clip_image-14539.png2。
现代分析分离技术 复习题

一、简答1.王老师提取分离常用技术、种类、简单原理一、提取方法 (1.2.3为经典法,4,5,6为新提取法)1)溶媒法:利用极性相似相溶浸渍法、渗漉法、煎煮法、回流提取法、连续回流提取法、微波提取法2)水蒸气蒸馏法:将含有挥发性成分的药材与水共蒸馏,使挥发性成分随水蒸气一并馏出,经冷凝分取挥发性成分的浸提方法。
3)升华法: 某些固体物质受热在低于其熔点的温度下,不经过熔化就可直接转化为蒸汽,蒸汽遇冷后又凝结为固体称为升华。
中药中有一些成分具有升华性质,能利用升华的方法直接从中药中提取出来。
4)超/亚临界流体萃取:利用亚临界流体作为萃取剂,在密闭、无氧。
低压的压力容器内,依据有机物相似相溶的原理,通过萃取物料与萃取剂在浸泡过程中的分子扩散过程,达到固体物料中的脂溶性成分转移到液态的萃取剂中,再通过减压蒸发的过程将萃取剂与目的产物分离,最终得到目的产物的一种新型萃取与分离技术。
5)超声萃取:超声波提取中药材是基于超声波的特殊物理性质。
主要是通过压电换能器产生的高频机械振动波来破坏目标萃取物与样品基体之间的作用力,从而实现固--液萃取分离6)微波萃取:利用微波能来提高萃取率的一种技术。
原理是在微波场中,吸收微波能力的差异使得基本物质的某些区域或萃取体系中的某些组分被选择性地加热,从而使得被萃取物质从基体或体系中分离,进入到介电常数较小,微波吸收能力相对差的萃取剂中。
二、分离方法(1~7为经典方法,8~14为现代分离技术)1)沉淀法(铅盐沉淀、溶剂沉淀)利用沉淀反应,将被测组分转化为难溶物,以沉淀形式从溶液中分离出来的方法。
2)溶剂分级抽提法:通过溶剂与溶媒的一系列取代作用,3)两相溶剂萃取法:,是利用混合物中各成分在两种互不相溶的溶剂中分配系数的不同而达到分离的方法。
4)逆流连续萃取法:是一种连续的两相溶剂萃取法。
5)盐析法:在中草药的水提液中、加入无机盐至一定浓度,或达到饱和状态,可使某些成分在水中的溶解度降低沉淀析出,而与水溶性大的杂质分离。
《现代分离技术》练习题

齐鲁工业大学17 / 18 学年第二学期研究生期末考试试卷(A卷)课程名称:现代分离技术得分:年级:2017级姓名:学号:问答题:(每题20分,共100分。
请根据自己的实际情况和理解程度自行独立答题,字数不限,不必长篇大论,脉络清晰就行。
若有雷同,将视情节从严扣分。
答题完成后,暂不要求打印。
)1、透析、渗析、(正)渗透、反渗透、超滤、微滤、纳滤与普通过滤,辨析之。
2、欲对苦咸水进行淡化,根据你所掌握的知识,可以选用哪种(些)分离方法?3、你在此前的科研工作中,接触过哪些分离方法?请尝试对其中的一种方法予以简介。
4、试谈谈分离科学的重要性及你对学习这门课程的体会。
5、计算题:某溶液含Fe3+ 100mg,用某有机溶剂萃取之,分配比D=99。
问用等体积溶剂萃取1次和2次,溶液中剩余的Fe3+量各是多少毫克?若是萃取2次后,将分出的有机层合并,再用等体积的水洗涤1次,有机相中会损失Fe3+多少毫克?答:1、①透析:通过小分子经过半透膜扩散到水(或缓冲液)的原理,将小分子与生物大分子分开的一种分离纯化技术。
②渗析:又称透析。
一种以浓度差为推动力的膜分离操作,利用膜对溶质的选择透过性实现不同性质溶质的分离。
即利用半透膜能透过小分子和离子但不能透过胶体粒子的性质从溶胶中除掉作为杂质的小分子或离子的过程。
③渗透是水分子经半透膜扩散的现象。
它由高水分子区域(即低浓度溶液)渗入低水分子区域(即高浓度溶液),直到半透膜内外浓度平衡为止。
④反渗透又称逆渗透,在膜的两边造成一个压力差,并使其大于渗透压,就会发生溶剂倒流,使浓度较高的溶液进一步浓缩。
是一种以压力差为推动力,从溶液中分离出溶剂的膜分离操作,孔径范围在0.0001~0.001 μm之间;(由于分离的溶剂分子往往很小,不能忽略渗透压的作用,故而成为反渗透)⑤超滤是一种加压膜分离技术,即在一定的压力下,使小分子溶质和溶剂穿过一定孔径的特制的薄膜,而使大分子溶质不能透过,留在膜的一边,从而使大分子物质得到了部分的纯化。
现代分离技术复习题

第一章1、分离过程分类?机械分离传质分离(平衡分离、速率控制分离) 反应分离分离装置中,利用机械力简单地将两相混合物相互分离的过程称为机械分离过程。
2、列举几种典型的机械分离过程:过滤、沉降、离心分离、旋风分离、除尘。
3、传质分离的分离过程如何分类?举例说明:平衡分离:蒸发、闪蒸、蒸馏、吸收、萃取、吸附、离子交换、萃取蒸馏结晶速率控制分离:气体渗透、反渗透、渗析、渗透蒸发、泡沫分离、色谱分离、电渗析4、几种典型的反应分离技术?可逆反应:(离子交换、反应萃取)不可逆反应:(反应吸收、反应结晶)生物分解反应:(生物降解)电化学反应:(双极膜水解反应)第二章1、按膜的分离原理及推动力不同,膜分几类?根据分离膜的分离原理和推动力的不同,可将其分为微孔膜、超过滤膜、反渗透膜、纳滤膜、渗析膜、电渗析膜、渗透蒸发膜等。
2、按膜的形态分类?按膜的形状分为平板膜(Flat Membrane)、管式膜(Tubular Membrane)和中空纤维膜(Hollow Fiber)、卷式膜。
3、按膜结构分类?对称膜、非对称膜和复合膜。
4、按膜的孔径大小分类?多孔膜和致密膜。
5、微滤、超滤、纳滤、反渗透,推动力是压力差。
渗析,推动力浓度差。
电渗析,推动力电位差。
气体分离、渗透蒸发推动力是压力差。
液膜分离推动力是浓度差。
6、常用的有机高分子膜材料?聚砜类、聚酰胺类、纤维素脂类。
7、醋酸纤维膜的优缺点?优点:醋酸纤维素性能稳定缺点:在高温和酸、碱存在下易发生水解,易受微生物侵蚀,pH值适应范围较窄,不耐高温和某些有机溶剂或无机溶剂。
8、醋酸纤维膜的结构?是一种非对称的多孔膜。
表皮层、过渡层、支撑层(多孔层)9、固体膜的保存应注意?主要应防止微生物、水解、冷冻对膜的破坏和膜的收缩变形。
微生物的破坏主要发生在醋酸纤维素膜;而水解和冷冻破坏则对任何膜都可能发生。
温度、pH值不适当和水中游离氧的存在均会造成膜的水解。
冷冻会使膜膨胀而破坏膜的结构。
新型分离技术习题解答——第3章

新型分离技术习题解答——第3章第一篇:新型分离技术习题解答——第3章第三章反渗透、纳滤、超滤和微滤(习题解答)3-1试分别求出含NaCl 3.5%的海水和含NaCl 0.1%的苦咸水25℃时的理想渗透压。
若用反渗透法处理这两种水,并要求水的回收率为50%,渗透压各为多少? 哪种水需要的操作压力高?+-解:(1):理想溶液渗透压可用van’t Hoff定律计算。
海水中的NaCl可认为完全解离为Na和Cl,即形成渗透压的离子浓度为NaCl 浓度的两倍,故含3.5%NaCl的海水在298K时的理想渗透压为:π=RT∑ci=RT ⎛CiCj⎫35+⎪=8.314⨯298⨯⨯2=2.97MPa58.45⎝MM⎭含0.1%NaCl的苦咸水在298K时的理想渗透压为:1⨯2=0.0848MPa 58.45(2):水回收率为50%时,被(理想)反渗透浓缩后的海水中,NaCl浓度增为3.5%/0.5=7.0%70π=RT∑ci=8.314⨯298⨯⨯2=5.93MPa58.45(3):水回收率为50%时,被(理想)反渗透浓缩后的苦咸水中,NaCl浓度增为0.1%/0.5=0.2%2π=RT∑ci=8.314⨯298⨯⨯2=0.17MPa58.45π=RT∑ci=8.314⨯298⨯由此可知,因海水中NaCl的浓度远比苦咸水中高,其所需的操作压力也远比苦咸水来得高。
其次,苦咸水的回收率达到50%,则反渗透压力将增高一倍。
3-2 含盐量为10000 mg(NaCl)/L的苦咸水, 采用有效面积为10cm2的醋酸纤维素膜,在压力6.0MPa下进行反渗透试验。
在水温25℃时,水流量Qp为0.01cm2/s时,透过液溶质浓度为400mg/L,试计算水力渗透系数Lp,溶质透过系数ω以及脱盐率。
(溶质渗透压系数Φi与溶质的种类及浓度有关,本题取Φi=2。
)解:苦咸水的渗透压:π=φiCRT=2⨯水力渗透系数:10000⨯0.082⨯298=8.35atm58.5⨯1000JVQP=∆P-∆πA(∆P-∆π)0.01==1.94⨯10-8L/cm2s.atm1000⨯10(60-8.35)Lp=溶质渗透系数:QfCFJSω==∆CSA∆CS=0.01⨯400=4.16⨯10-8L/cm2s.atm1000⨯10(10000-400)脱盐率:R0=CB-CfCB=10000-400=96%10000--3-3 透系数LP等于2×108L/cm2·s·MPa,溶质的透过系数P为4×108L/cm2·s的反渗透膜,在操作压力为4.0MPa、水温为25℃条件下进行实验。
现代分离技术习题

现代分离技术习题1.化工分离过程按分离原理分为机械分离过程与传质分离过程。
2.有相产生或添加得分离过程,就是通过外加能量分离剂产生第二相或直接添加第二相得物质分离剂这两种途径来实现得。
3.料液预处理得目得就是改善料液中非均相组成得分布特征及料液得流动特性,以利于非均相物系得分离,同时还除去杂质。
4.常用得料液预处理方法有:加热、凝聚、絮凝、反映消除、吸附等。
5.当温度升高时,气体物料得粘度增大,液体物料得粘度减小。
6.巴氏杀菌法就是指采用100℃以下得温度与比较短得加热时间来处理物料得灭菌方法。
7.解释凝聚微观机理得模型就是扩散双电层结构模型。
8.凝聚价就是指使胶体粒子发生凝聚作用得最小电解质浓度,凝聚价越大,则凝聚能力越弱。
9.絮凝剂得长链结构上就是具有大量得活性功能团,能与胶体粒子产生吸附作用,另外一个胶体又会同时与多个长链分子发生作用,从而产生架桥作用,形成网状结构得絮团。
10.影响固液悬混物分离过程及效果得主要因素就是粘液粘度、固形物得外姓尺寸以及固相与液相得密度差。
11.通过交替使用低速与高速离心,可以使不同质量得物质在不同强度得离心力作用下分级沉降,这种离心分离方法叫差速离心法,此法使用于混合样品中各沉降速率差别较大组分得分离。
12.过滤就是利用多孔介质对固形颗粒得筛粉截留作用来固液分离得。
常规过滤能够截留10~100 μm 得固型颗粒。
13.Nc就是指描述约束关系得独立方式得数目,这些约束关系包括:①物料平衡式;②能量平衡式;③相平衡关系式;④化学平衡式;⑤内在关系式。
14.不同设备得设计变量数尽管不同,但其中固定设计变量得确定原则就是共同得,既只与进料物流数目与系统内压力等级数有关。
15.任何逆流流动得分离设备得处理能力都受到液泛得限制。
若L/V越小,则液泛气速越大;若液泛气速增大,则说明处理能力越强。
16.雾沫夹带就是气液两相得物理分离不完全得现象。
雾沫夹带随着板间距得减小而增加,随塔负荷得增加而急剧上升。
现代分离技术习题1

现代分离技术习题1.化工分离过程按分离原理分为机械分离过程和传质分离过程。
2.有相产生或添加的分离过程,是通过外加能量分离剂产生第二相或直接添加第二相的物质分离剂这两种途径来实现的。
3.料液预处理的目的是改善料液中非均相组成的分布特征及料液的流动特性,以利于非均相物系的分离,同时还除去杂质。
4.常用的料液预处理方法有:加热、凝聚、絮凝、反映消除、吸附等。
5.当温度升高时,气体物料的粘度增大,液体物料的粘度减小。
6.巴氏杀菌法是指采用100℃以下的温度和比较短的加热时间来处理物料的灭菌方法。
7.解释凝聚微观机理的模型是扩散双电层结构模型。
8.凝聚价是指使胶体粒子发生凝聚作用的最小电解质浓度,凝聚价越大,则凝聚能力越弱。
9.絮凝剂的长链结构上是具有大量的活性功能团,能与胶体粒子产生吸附作用,另外一个胶体又会同时与多个长链分子发生作用,从而产生架桥作用,形成网状结构的絮团。
10.影响固液悬混物分离过程及效果的主要因素是粘液粘度、固形物的外姓尺寸以及固相与液相的密度差。
11.通过交替使用低速和高速离心,可以使不同质量的物质在不同强度的离心力作用下分级沉降,这种离心分离方法叫差速离心法,此法使用于混合样品中各沉降速率差别较大组分的分离。
12.过滤是利用多孔介质对固形颗粒的筛粉截留作用来固液分离的。
常规过滤能够截留10~100 μm 的固型颗粒。
13.Nc是指描述约束关系的独立方式的数目,这些约束关系包括:①物料平衡式;②能量平衡式;③相平衡关系式;④化学平衡式;⑤内在关系式。
14.不同设备的设计变量数尽管不同,但其中固定设计变量的确定原则是共同的,既只与进料物流数目和系统内压力等级数有关。
15.任何逆流流动的分离设备的处理能力都受到液泛的限制。
若L/V越小,则液泛气速越大;若液泛气速增大,则说明处理能力越强。
16.雾沫夹带是气液两相的物理分离不完全的现象。
雾沫夹带随着板间距的减小而增加,随塔负荷的增加而急剧上升。
现代分离技术(PDF)
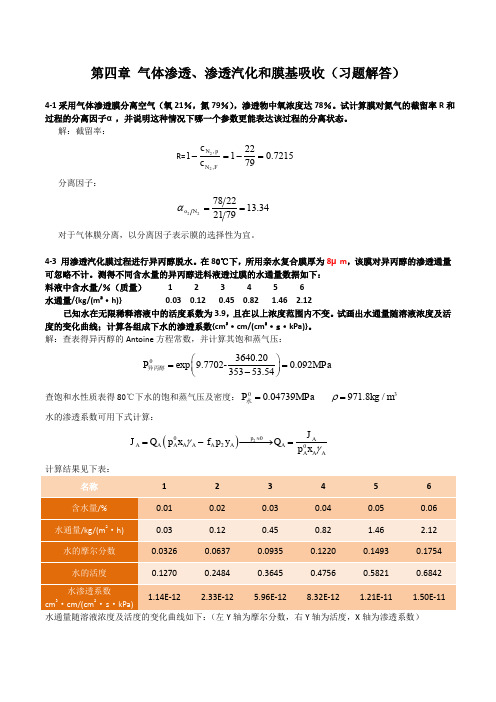
第四章 气体渗透、渗透汽化和膜基吸收(习题解答)4-1采用气体渗透膜分离空气(氧21%,氮79%),渗透物中氧浓度达78%。
试计算膜对氮气的截留率R 和过程的分离因子α,并说明这种情况下哪一个参数更能表达该过程的分离状态。
解:截留率:R=7215.0792211,,22=-=-FN p N c c 分离因子:34.137921227822==N oα 对于气体膜分离,以分离因子表示膜的选择性为宜。
4-3 用渗透汽化膜过程进行异丙醇脱水。
在80℃下,所用亲水复合膜厚为8μm ,该膜对异丙醇的渗透通量可忽略不计。
测得不同含水量的异丙醇进料液透过膜的水通量数据如下: 料液中含水量/%(质量) 1 2 3 4 5 6水通量/{kg/(m 2·h)} 0.03 0.12 0.45 0.82 1.46 2.12已知水在无限稀释溶液中的活度系数为3.9,且在以上浓度范围内不变。
试画出水通量随溶液浓度及活度的变化曲线;计算各组成下水的渗透系数{cm 3·cm/(cm 2·s ·kPa)}。
解:查表得异丙醇的Antoine 方程常数,并计算其饱和蒸气压:3640.20exp 9.7702-0.09235353.54P MPa ⎛⎫== ⎪-⎝⎭异丙醇查饱和水性质表得80℃下水的饱和蒸气压及密度:00.04739P MPa =水 3971.8/kg m ρ=水的渗透系数可用下式计算:()220p AA A A A A A A A A A AJ J Q p x f p y Q p x γγ≈=-−−−→= 计算结果见下表:1 2 3 4 5 60.01 0.02 0.03 0.04 0.05 0.06 20.03 0.12 0.45 0.82 1.46 2.12 0.0326 0.0637 0.0935 0.1220 0.1493 0.17540.12700.24840.36450.47560.58210.6842321.14E-122.33E-12 5.96E-12 8.32E-12 1.21E-11 1.50E-11 水通量随溶液浓度及活度的变化曲线如下:(左Y 轴为摩尔分数,右Y 轴为活度,X 轴为渗透系数)0.000.020.040.060.080.100.120.140.160.18A4-4 蒸汽渗透或气体分离过程中,原料和渗透物压强比一定,且原料液流与渗余液流的浓度近似相等时,渗透物浓度最高。
现代分离技术-3

此时,水溶液中残留的碘量m1为 m1=1mg×(1-0.987)=0.013mg 每次用3mL四氯化碳连续萃取3次后,此时水溶液中 残留的碘量为m2
wn = w0( Vaq DVorg + Vaq )n
10 m 2 = 1mg( )3 = 0.0001mg 85 × 3 + 10
此时的萃取率E2为
(1 − 0.0001) E2 = × 100% = 99.99% 1
(4)分离因子βA,B ) ,
当水相中同时存在两种以上的溶质时,如果他们在给 定的两相中的分配比不相同,经过萃取操作之后,他 们在两相中的相对含量就会发生变化。 如果A和B两种溶质在两相中的分配比分别为DA和DB, 当DA越大,DB越小的时候,则进入有机相的溶质A 就越多,留在水相中的溶质B就越多; 当DA和DB值相差达到一定程度时,A和B就能够分离。
(3)萃取率 )
在萃取分离实验中,通常用萃取率(E)表示在 一定条件下被萃取溶质进入有机相的量,即
E= 溶质在有机相中的量 × 100% 溶质在两相中的总量
对于一次萃取操作,萃取率为
E = C org V org C org V org + C aq V aq × 100 %
Vaq和Vorg分别表示水相和有机相的体积
例
题:
已知碘在四氯化碳和水相中的分配比为85,有 10mL水溶液中含碘1mg,用9mL四氯化碳1次萃取 和每次3mL四氯化碳连续萃取3次,求两种情况下 的萃取率和水溶液中残留的碘的量。 解:当用9mL四氯化碳1次萃取时,萃取率E1为
D 85 E1 = × 100% = × 100% = 98.7% Vorg 10 85 + D+ 9 Vorg
新型分离技术习题解答——第1章

第一章绪论(习题解答)1-1 厨房、卧室、庭院内有哪些现象可用分离方法来改善?答:厨房、卧室、庭院内的尘埃、油烟、VOCs(VOC是挥发性有机化合物volatile organic compounds的英文缩写,例如装修产生的甲醛),还有空气中的NO x、SO2、CO等有害物质,都可用分离方法来去除,以改善生活环境。
1-2 请设计某些基于分离概念的家用电器设备,以提高人们的日常生活质量。
答:例如可以设计基于“过滤”的水进化器,基于“膜分离”的油烟机、空调(除尘)、生活污水处理器等等,以提高人们的日常生活质量。
1-3 汽车尾气中含有大量的CO,有人正在探索开发一种催化分离膜,在尾气排放时及时将CO转化为CO2而除去,你认为这属于哪一类分离方法,有可能实现吗?请说明理由。
答:这属于反应分离,有可能实现。
因为CO可以在铂、铑和钯等贵金属催化剂或稀土催化剂等的作用下发生氧化反应转化为CO2,所以可以设计一种膜(表面附上催化剂),它可以让CO2直接通过,而CO则只有在膜上催化剂作用下转化为CO2后才能通过,这样就可以去除尾气中的CO。
1-4 学校教室内空气质量比户外好吗?主要有哪些物质导致的?请选择并设计一种较合理的分离技术有效地将这些污染物质去除。
答:学校教师内的空气质量一般比户外要差,因为教室内CO2浓度较高,有粉笔灰等有害易吸入颗粒物,也可能存在因装修而产生的甲醛等有害有机化合物。
要去除这些污染物质,我们可以利用膜分离技术,即设计一种膜,使粉笔灰、甲醛等有害物质不能通过而达到去除效果。
1-5 请指出自来水、饮用水、纯净水、活性水的制备方法,各自采用了哪些分离技术?1-6 不少科学家认为载人航天事业发展推动科技的进步,你赞同这个观点吗?宇航员在空间站内生活与工作需要哪些新型分离技术为基础?答:是否认同观点,因人而异,只要说明理由即可。
宇航员在太空中生活与工作涉及到包括CO2的收集与浓缩、水电接产生氧、生活污水的再生回用等多方面,在这些过程中需要膜分离、渗透、超滤等新型分离技术为基础。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
现代分离技术习题(Modern separation technologyexercises)现代分离技术习题(Modern separation technology exercises)The twentieth chapter, HPLCThinking questions and exercises1. the main similarities and differences between HPLC and gas chromatography are briefly described.The same point: both high efficiency, high speed, high selectivity chromatography method, both separation and analysis function, can be detected onlineDifference:Analysis of objects and ranges, selection of mobile phases,operating conditionsGC has the advantages of good gasification, good thermal stability and low boiling point, and occupies 20% of the machine. The mobile phase is a limited number of "inert" gas, which only acts as a carrier, and has little effect on the composition, and operates at atmospheric pressureHPLC can be made into solution after the dissolution of the sample, high boiling point, high molecular weight, difficult to gasification, ionic stability or unstable compounds, occupy 80% of the machine, the liquid phase for the liquid or a variety of liquid mixture. In additionto its carrying capacity, it can also be controlled and improved by solvent. At room temperature and under high pressure2. what is the chemical bonding phase? What are the common chemical bonding phases? Which liquid chromatography are used?A chemical reaction is used to bond the fixed liquid to the surfaceof the carrier and the resulting packing is called a chemically bonded phase. The utility model has the advantages that the use process is not lost, the chemical property is stable, the thermal stability is good,and the utility model is suitable for gradient elution.The commonly used Si-O-Si-C type bonding phases are classified into three kinds: non-polar, medium polarity and polarity according to polarity. The nonpolar bonded phase: such as the common ODS bonded phase, both the distribution and the adsorption effect, is widely used for the analysis of non-polar or weak polar compounds; the middle of the bonded phase: a common ether bonded phase, the bonded phase can be positive or reversed-phase chromatographic stationary phase, mobile phase polarityas the polarity: the bonded phase: common amino and cyano bonded phase, are used as chromatographic stationary phase, phase separation of sugar or amino bonded stationary phase is the most commonly used.3. what is called normal phase chromatography? What is reverse phase chromatography? Which compounds are applied separately?Normal phase chromatography: a method of chromatography having a polarity of mobile phase less than the polarity of the stationary phase.A molecular substance used to separate polar and moderately polarsubstances dissolved in organic solvents; used in the separation of substances containing differentfunctional groups.Reversed-phase chromatography: a method for chromatography having a polarity of mobile phase greater than the polarity of the stationary phase. A molecular compound used to separate nonpolar to moderatelypolar compounds.4. briefly describe the separation mechanism of reversed-phasebonded phase chromatography.A typical reversed-phase bond chromatography is a system consistingof nonpolar stationary phases and polar mobile phases. Stationary phases are commonly used in the eighteen alkyl (ODS or C18) bonded phase; the mobile phase is usually methanol water or acetonitrile water. Anatypical reversed-phase chromatography system consists of a weak polaror moderately polar bonding phase and a mobile phase having a polarity greater than the stationary phase.The surface of the antiphase bonded phase has nonpolar alkylfunctional groups and an amorphous silanol group. The silanol group has the adsorption properties, and the amount of the remaining silanolgroups depends on the coverage. For the separation mechanism ofreversed-phase chromatographic retention mechanism at present, there is no consensus, there are two views, one that belongs to the distribution of chromatography, another that belongs to the adsorption chromatography. The mechanism of the partition chromatography is that the polar organicsolvent with mixed polar solvent (water, ten organic solvent) is adsorbed on the non-polar alkyl coordination group surface, The component molecules are assigned in the mobile phase with the liquid phase adsorbed by the non-polar alkyl ligand. The mechanism of adsorption chromatography can be explained by the theory of solvent extraction. The theory treats nonpolar alkyl linkage phases as molecular hairs covered with a bound eighteen alkyl group on the surface of silica gel, which has strong hydrophobic properties. When the polar solvent water and organic solvent composition of the mobile phase to separate organic compounds, on the one hand, the non-polar part of the nonpolar component molecules or component molecules, due to hydrophobic interaction, will be "squeeze out from the water, produce association between hydrophobic alkyl stationary phase. As a result, the component molecules are retained in the fixed phase. On the other hand, the polar component of separated material under the action of the polarity of mobile phase, make it from the fixed phase, reduce the retention, obviously this dissociation process, namely, the two force difference, determines the retention behavior of molecules inchromatography. Generally speaking, on a stationary phase with alkyl nonpolar part of the surface area of the large base or isolated molecules in the mobile phase or the surface tension and the dielectric constant is bigger, the stronger association, k'distribution ratio is greater, the greater the value of reserves. It is not difficult tounderstand that in reversed-phase bonded chromatography, the polar components first flow out, and the less polar components flow out.What are the differences between the 5. ion chromatography,Reversed-phase Ion Pair Chromatography and ion suppression chromatography?Ion chromatography (Ion Chromatography): the ion exchange resin is the stationary phase, and the electrolyte solution is the mobile phase.A conductivity detector is used as a universal detector. The reaction principle of the sample component on the separating column and the restraining column is the same as that of the ion exchange chromatography. Ion chromatography is the best method for the analysis of anions in solution. It can also be used for the analysis of cations.Reversed-phase Ion Pair Chromatography (IPC or PIC): in reversed-phase chromatography, an ion pair reagent is added to the polar mobile phase to form a neutral ion pair between the measured component and its counter ion, increasing K and tR to improve separation. Apply to stronger organic acids and bases.Reversed-phase ion suppression chromatography: in reversed phase chromatography, the buffer solution is used to adjust the pH value of the mobile phase, so as to inhibit the dissociation of the group and increase its K and tR, so as to improve the separation. Apply to extremely weak acid and base matter (pH=3~7 weak acid, pH=7~8 weak base, amphoteric compound)What is the separation mechanism of 6. affinity chromatography? What are the characteristics?Conventional wisdom holds that affinity chromatography is based on ligand ligand affinity reaction and uses differential migration theory of chromatography to achieve separation of target molecules, and is only a selective filtration method for component separation. However, this theory is based on ligandligand affinity reactions that are homogeneous reactions and macroscopic equilibrium thermodynamics. However, in fact, the partition coefficients of the target molecules in the two phase are too large, and the adsorption isotherms on the stationary phase are mostly linear. Therefore, the retention mechanism of biological macromolecules in affinity chromatography and the mathematical model of chromatography process have been a relatively weak link, which needs to be further improvedAffinity chromatography has high specificity, the separation, purification, concentration after biological samples have high purity, greatly reduce the subsequent determination (such as HPLC) when the background noise,Thus, the subsequent determination has very high sensitivity.What are the similarities and differences between the 7. rate theoretical equations in HPLC and those in GC? How to guide theselection of HPLC experimental conditions?Solution: the main factors causing chromatographic peaks in liquid chromatography are eddy current diffusion, mobile flow phase mass transfer, retained mobile phase mass transfer, and off column effect.In gas chromatography, radial diffusion is often significant, while the effect of radial diffusion in liquid chromatography is weak and can often be neglected. In addition, liquid retention, mass transfer and off column effects are more prominent in liquid chromatography.In high performance liquid chromatography, the complete expressionfor liquid-liquid partition chromatography, Van, and Deemter equationsisTherefore, the experimental conditions of HPLC should be as follows: 1. Small grain size and uniform spherical chemical bonding phase; the low viscosity mobile phase should not flow too fast; and the column temperature is appropriate.8., try to discuss the factors that affect the separation degree of HPLC, how to improve the separation degree?(1) chromatographic filling propertiesThe performance of liquid chromatographic column separation is determined by the three parameters: the size of the stationary phase, the length of the column, the column pressure and the pressure drop determined by the filling condition. The three parameter determines the sample component retention time, K thermodynamic factors not only with the retention time of chromatographic process, and also directly decide on the viscosity parameters and flow performance of column columnefficiency and the degree of separation phase, these parameters are important factors affecting the chromatographic separation process dynamics. But in high-performance liquidchromatography, the preparation of separation column is a very demanding technical work, usually the purchase of commercial products, rarely prepared by itself.(2) the polarity of mobile phase and mobile phaseIn liquid chromatography, changing the composition and polarity of eluent is the most direct factor to improve the separation. Liquid chromatography is unlikely to improve mass transfer by increasing column temperature. Therefore, most of them are constant temperature analysis. The mobile phase selection is especially important in liquid chromatography. The mobile phase can significantly change the separation of components.(3) flow velocityWhen the velocity is greater than 0.5 cm/s, the curve from H to u is a straight line with little slope. The column efficiency is not greatly improved by decreasing the flow velocity. But in actual operation, the flow is still an important optional parameter for adjusting the separation and the peak time.9., discuss the choice of the separation conditions of reversed-phase HPLC.Reverse phase HPLC is a liquid chromatographic separation model with surface non-polar carrier as stationary phase and solvent with strongerpolarity than stationary phase as mobile phase. The retention values of samples in reversed-phase HPLC chromatography are mainly determined by the fixed surface area, the type and concentration of bonding phases,and the retention values usually increase with the increase of chain length or the hydrophobicity of the bonded phase. The solute retention value is directly proportional to the surface area of the fixed surface, and when other conditions are the same, the solute has a short retention value on the low surface area chromatographiccolumn. The retention value of the sample can also be adjusted by changing the composition of the mobile phase or the strength of the solvent. The strength of the solvent depends on the nature of theorganic solvent and its concentration in the mobile phase.10. is the intensity of the mobile phase the same in the positiveand reverse phase HPLC?In normal phase chromatography, because the stationary phase is polar, the stronger the solvent polarity is, the stronger the elution capacity is, i.e., the strong solvent is a strong solvent. In reversed-phase chromatography, as the stationary phase is nonpolar, the strength of the solvent increases with decreasing polarity of the solvent,A solvent with a weak polarity is a strong solvent.11. what is called gradient elution? What are the similarities and differences between it and GC?In an analysis period, according to certain procedures for changing the mobile phase composition or concentration, known as gradient elution.It is an important method to improve the separation of liquid chromatography.Gradient elution and gas chromatography in the temperature programmed is similar, but the former is the continuous change of the mobile phase polarity, pH or ionic strength, and temperature change. Programmed temperature is also an important method to improve gas chromatographic separation.12. what are the principles and characteristics of evaporative light scattering detectors?Evaporative light scattering detector (Evaporative Light-scattering Detector) is a universal detector that can detect organic substances such as ginsenosides and Huang Qijia glycosides that have no UV absorption.One, ELSD principleConstant velocity (high performance liquid chromatograph, countercurrent chromatography and high performance capillary electrophoresis) eluent after entering the detector, first by high-pressure gas atomization, droplet atomization formed into the evaporation chamber (drift tube, drift tube), mobile phase and low boiling components by evaporation, the remaining small droplets of high boiling components divided into the scattering cell, passing through the scattering cell by scattering, light scattering by photoelectric tube receiving form an electrical signal, electric signal through theamplifying circuit, analog-to-digital conversion circuit, a digital computer - chromatogram signal chromatography workstation.Two, characteristics1., the eluent needs to be atomized, so the purity and pressure ofthe atomization will affect the signal-to-noise ratio of the detector.2., the mobile phase to evaporate, so can not use volatilesubstances to regulate the pH value of the mobile phase. The components of the lower boiling point of the material can be vaporizedby adjusting the evaporation temperature. In the absence of evaporationof the material under test, the higher the temperature, the morecomplete the evaporation of the mobile phase, the better the baseline of the chromatogram, and the higher the signal-to-noise ratio. If the measured material is close to or below the boiling point flowevaporation temperature phase, however, is unable to detect; 100% of the water as the mobile phase, temperature is only the evaporation chamberis set to 150 degrees Celsius, organic matter lower boiling point than water can be separated by gas chromatography detection. Since the flow phase and solvent evaporates, the chromatogram collected by the ELSD detector generally has no solvent peak; and the gradient elution has no refractive parallax effect and generally does not exhibit baseline drift.3. to detect light scattering changes, all materials entering the scattering pool can be detected, and the response value is only relatedto the amount of matter.The 4. concentration is not linear with the peak area, and followsthe logarithm of the natural logarithm.13. what is the commonly used method of quantitative analysis of HPLC? Which methods need correction factor to correct peak area? Which methods do not have to correct the factor?The commonly used methods for quantitative analysis of HPLC are:External standard method: external standard work curve method, external standard point method, external standard two point method, etc.Internal standard method: internal standard work curve method,internal standard point method, internal standard two point method, internal standard comparison method, etc.The calibration factor is not necessary when using the standardcurve method of internal standard and external standard, and other methods need correction factor to correct peak area14. point out the elution sequence of benzene, naphthalene and anthracene in reversed-phase chromatography and explain the reason.The order of polarity of the three is from large to small, benzene, naphthalene and anthracene,Therefore, the elution order in reverse phase chromatography is benzene, naphthalene and anthracene, and benzene is the first peak.15. which HPLC method should be used to separate the following substances?(1) ethanol and butanol; (2) Ba2+ and Sr2+; (3) pentanoic acid and butyric acid; (4) high molecular weight glucoside.(1) positive phase bonded phase chromatography(2) ion exchange chromatography(3) ion pair chromatography(4) steric exclusion chromatography;"Chromatography analysis" exercisesChapter 1 Introduction to chromatography analysisJudgment question:1 the following conditions will increase the performance of the chromatographic column:A reduces the velocity of the mobile phase; ()B particles uniformly packed; ()C increases the diameter of the stationary phase particles; ()D increases the column temperature. ()Question 1: Please select the most suitable chromatographic analysis method for the following samples.The thermal instability of the sample. (2) low boiling aromatic hydrocarbons. () the complex, multiple groups of samples. ()A gas chromatography.B liquid chromatography.2. points out which of the following parameter changes will cause an increase in relative retention values?(1) the column length increased; (2) the ratio increased; (3) the column temperature decreased; (4) the phase velocity of mobile phase decreased.3. it is pointed out that the following conditions can reduce the height of theoretical plate:(1) increasing the column length; (2) decreasing the Dt value; (3) decreasing the speed of the flow line; (4) decreasing the diameter of the fixed phase particles,(5) increasing column temperature. Explain why.Simple answer:1. What is the balance of chromatographic distribution? How do the partition coefficients, the capacity factor, the relative rate, and the distribution temperature line measure the distribution balance of the components in the two phases?2, what factors do the theoretical equations of the plate derive from? What is their significance?3, try to explain the function and shortcomings of the plate theory in the interpretation of chromatographic separation.4, try to explain the theoretical basis of Van Deemter equation and the implications of its expressions.What is the practical significance of the 5 and Van Deemterequations? What is the reason for the deformation of the coupled curve?6. Test the basis and classification of chromatography.7. Briefly describe the types and characteristics of chromatographic columns.8. The separation principle and characteristics of chromatographyare briefly described.9. The characteristics and application scope of gas chromatography (OLC, GSC, CGC), high performance liquid chromatography (HPlC) and supercritical fluid chromatography are briefly described.10. Besides the factors discussed in the Van Deemte equation, what are the factors that affect the broadening of chromatographic peaks? How to overcome it in actual work?11, select the plate height (H) what is the color spectrumseparation index according to the operating conditions?12, try to explain the meaning of A, B and C constants in Van Deemter equation, and the unit and its calculation method.13, a gas chromatography column marked: OV - 101 column, the column efficiency of n is higher than 1200, which marked the correct way? Why?14, compare the chromatography (GC, HPLC) and chemical analysis, mass spectrometry, infrared spectroscopy, the similarities and differences between fluorescence spectroscopy, nuclear magnetic resonance spectroscopy, atomic spectrometry, electrochemical analysis method, analysis method and how to improve the development of chromatography?15. Mark the name of each parameter in the chromatogram (see below) and point out the parameters of the qualitative and quantitative chromatographyFixed film thickness 16, higher than the B A column column, other conditions completely different, is two on the optimal linear velocity (uopt) value of the same?17, there is a lower boiling paraffin homologen samples withnonpolar stationary liquid chromatographic column, low column temperature and low liquid loading ratio, or high high liquid column temperature, load ratio (good to complete the same time analysis)? Why?Calculation problem:1. there is a liquid chromatography column, t, M = 4 points, and now the A and B, C, D, four components of the retention value and peak width as follows:Please calculate the n value and N eff value of each component.2. on a chromatographic column, the peak time of air peak is 0.5 minutes, the sample peak time is 2.44 minutes, and the peak width is 9.7 seconds. Assuming that the chromatographic peaksare normally distributed and the chromatographic column is 1 meters long, the theoretical plate number of the column, the theoretical height of the tray, the number of effective plates and the height of the effective plate are calculated.3. A and B two components are separated at column 100. The partition coefficient of A is 110, and the partition coefficient of B is 120. How long will it take to separate the two from 1.1? The theoretical plate height is 0.1mm.The second chapter gas chromatography analysisJudgment question:1 using polar column (GC) analysis of samples, the first out of the peak component is:A boiling high component; ()B large molecular weight component; ()C polar component; ()Choice question:1 select the most suitable gas chromatography stationary phase (or fixative) according to the sample.The n-pentane, octane. (2) N 2, O 2. ()A zeolite.B methyl silicone rubber SE-30.C polyethylene glycol PEG -20M.Simple answer:By experiment, do you think a newly prepared gas liquid packed column needs aging before use? Why?Please list at least four qualitative methods commonly used in chromatography.What is the quantitative correction factor? Why is correction factor introduced in chromatographic quantitative analysis?4. after the analysis of the experiment, we will estimate what type of fixative or stationary phase should be used.(1) determination of SO2 and H2S in air; (2) determination of moisture in several hundred ppm grades in acetone;(3) determination of trace organic phosphorus andorganochlorine pesticides residues in vegetables and fruits;(4) determination of trace amounts of benzene, toluene and xylene in styrene in the production section of styrene;(5) analysis of C2 - C4 hydrocarbon in petroleum cracking gas; (6) determination of ortho - and para - cresol in cresol wastewater;(7) analysis of C4 - C2 alcohols.5. what are the common carrier gas? How to select and purify carrier gas?6. gas system leakage may occur what? Why?What are the similarities and differences between 7. gas liquid chromatographic stationary phases and gas solidchromatographic stationary phases?8. what are the requirements for chromatographic fixative? How are they classified?What is the relationship between the order of the 9. components and the intermolecular force?10. briefly describe the meanings and characteristics of WCOT, SCOT and PLOT column abbreviations.11. why 0.2mm fine bore capillary column must be split during operation12., why does capillary gas chromatography have to be combined witha tail blowing device, such as no tail blowing operation?13. compare the difference between capillary gaschromatography and packed column gas chromatography.Calculation problem:1. the content of fatty acids in eggs was analyzed by chromatography. The relative correction factor of 0.43 palmitic acid, oleic acid area correction factor is 0.52, the relative correction factor of 0.37stearic acid, linoleic acidrelative correction factor was 0.41; palmitic acid peak area of 80mm 2, the peak area is 100mm oleic acid 2, stearic acid peak area for the50mm 2, the peak area of Asia oleic acid is 70mm 2. Please calculate the percentage of palmitic acid in eggs by normalization method2. there are four chromatographic peaks on the color spectrum. The distance from the introduction to the highest point of each group is as follows:Air 2.50 min; heptane 16.4 min; toluene 19.2 min; octane 31.5 min.Please calculate the retention index of toluene.3. a mixture of ethanol, heptane, benzene and ethyl acetate was analyzed. The measured peak area of them was 5, 9, 4, and 7.0cm 2, by manual check their relative weight correction factor of FW were 0.64,0.70, 0.78 and 0.79 respectively, according to the normalization method for weight percent concentration of them.The third chapter is the analysis of HPLCJudgment question:1, to improve the selection performance of columns, which of the following most effective measures should be adopted?:A uses a highly selective stationary phase; ()B reduces theaffinity of the relative component of the flow; ()C increase column length; ()Choice question:1 if you wish to analyze the following compounds by liquid chromatography, please select the optimum separation type.The determination of halogenated alkane isomers. (2) glutathione S-transferase separation in animal liver. ()Separation and determination of proteins and molecular weight in the serum. () the separation of chicken ovomucoid occurred in the ionization under alkaline conditions. (A) liquid liquid partition chromatography, B affinity chromatography, C, volume exclusion chromatography, D ion exchange chromatographyE liquid solid adsorption chromatographyFill in the:In reversed-phase ion pair chromatography, equilibrium ion concentration increases with retention values (); equilibrium ions increase in hydrophobicity, retention values (); mobile phase The ratio of water to water decreases, the organic solvent increases, and the retention value (); if the component is acid, when the pH value of the mobile phase decreases, the retention value (...).Simple answer:1. point out the elution sequence of n-hexane and acetic acid in reverse liquid liquid partition chromatography and explain the reason.2. what is the chemical bonding phase? What are the characteristics?3., compare the HPLC and GC separation principle, instrument structure and application methods of similarities and differences.4. How does high-performance liquid chromatography achieve efficient and high-speed separations (compared with classical column chromatography)?5. Briefly describe the main influencing factors and improvement methods of chromatographic peak broadening of HPLC.6. What are the classes of HPLC methods? What is their essence?7. Describe the characteristics of positive Reversed-phase Ion Pair Chromatography and the factors affecting the separation of components.8. What are the effects of the following conditions on the separation and detection of components?(1) when using an ultraviolet absorption detector, the mobilephase is hexane containing impurities (Fang Ting)(2) in liquid solid chromatography, by containing a small amount of polar impurities (such as water) of n-hexane as mobile phase(3) in the gradient elution, the non-polar mobile phase with a trace of polar impurities is used to replace the more volatile mobile phase.The fourth chapter is thin layer chromatography analysisBrief answer: 1.. Compared with liquid chromatography analysis,what's special about thin layer chromatography?2. briefly describe the working process of thin layer chromatography analysis.。
