活性炭的介绍(英文)
活性碳危害特性表

活性碳危害特性表中文名称:活性碳英文名称:Active carbon分子式:CCAS号:7440-44-0危规号:UN编号:1362危险性类别:第4.2类自燃物品外观与性状:黑色粉末或颗粒。
主要用途:颗粒活性碳用于有机溶剂蒸汽的回收,有机合成催化剂或载体,去除空气中的不纯物,糖、酒精、食品等溶液的精制,粉末活性碳用于去除砂糖等的色素,乙醇饮料的调味、脱色、脱臭及油脂和医药等脱臭、脱色,并用作药用炭等。
健康危害:属基本无毒的物质,但有时从原料中夹杂无机物,对皮肤、黏膜及呼吸道有一定的刺激。
吸入、食入。
误服者用水漱口。
立即脱去被污染衣着,用大量流动清水冲洗至少15分钟,就医。
立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟,就医。
迅速脱离现场至新鲜空气处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸,就医。
理化特性:相对分子质量为12,易燃,粉尘接触明火有轻度的爆炸性,在空气中易缓慢地发热和自燃。
闪点无意义,燃点为300℃,爆炸下限为(%)300,爆炸上限为(%)无意义,最小点火能为(mJ)。
灭火方法为水、泡沫、二氧化碳、砂土。
储存于干燥、通风的库房,远离火种、热源,防止内外包装袋破裂,不可与氧化剂共储混运,防止受潮,以避免受潮后积热不散可能发生自燃。
严禁与有毒有害气体或易挥发物质混放,存放要远离污染源。
运输与装卸:活性炭在运输过程中,不得用铁钩拖拽,应防止与坚硬物质混装,不可强烈振动、磨擦、踩、砸,严禁抛掷,应轻装轻卸,以减少炭粒破碎,影响使用。
车间卫生标准:3中国MAC(mg/m³),3前苏联MAC (mg/m³)未制定标准,XXX为ACGIH 0.1mg[Hg]/m[皮],美国TLV-STEL未制定标准。
检测方法为工程控制密闭操作,加强通风。
呼吸系统防护作业工人应戴口罩,手防护必要时戴防护手套。
其它工作后,淋浴更衣。
保持良好的卫生惯。
注:文章中删除了明显有问题的段落,并对每段话进行了小幅度的改写,以使文章更加通顺易懂。
活性炭 activated carbon

活性炭activated carbon是一种黑色粉状,粒状或丸状的无定形具有多孔的碳,主要成分为碳,还含少量氧、氢、硫、氮、氯。
也具有石墨那样的精细结构,只是晶粒较小,层层间不规则堆积。
具有较大的表面积(500~1000米2/克),有很强的吸附性能,能在它的表面上吸附气体、液体或胶态固体;对于气体、液体,吸附物质的质量可接近于活性炭本身的质量。
其吸附作用具有选择性,非极性物质比极性物质更易于吸附。
在同一系列物质中,沸点越高的物质越容易被吸附,压强越大温度越低浓度越大,吸附量越大。
反之,减压,升温有利于气体的解吸。
常用于气体的吸附、分离和提纯,溶剂的回收,糖液、油脂、甘油、药物的脱色剂,饮用水及冰箱的除臭剂,防毒面具中的滤毒剂,还可用作催化剂或金属盐催化剂的载体。
早期生产活性炭的原料为木材、硬果壳或兽骨,后来主要采用煤,经干馏、活化处理后得到活性碳生产方法有:①蒸汽、气体活化法。
利用水蒸气或二氧化碳在850~900℃将碳活化。
②化学活化法。
利用活化剂放出的气体,或用活化剂浸渍原料,在高温处理后都可得到活性炭。
活性炭具有微晶结构,微晶排列完全不规则,晶体中有微孔(半径小于20[埃]=10-10米)、过渡孔(半径20~1000)、大孔(半径1000~100000),使它具有很大的内表面,比表面积为500~1700米2/克。
这决定了活性炭具有良好的吸附性,可以吸附废水和废气中的金属离子、有害气体、有机污染物、色素等。
工业上应用活性炭还要求机械强度大、耐磨性能好,它的结构力求稳定,吸附所需能量小,以有利于再生。
活性炭用于油脂、饮料、食品、饮用水的脱色、脱味,气体分离、溶剂回收和空气调节,用作催化剂载体和防毒面具的吸附剂。
物理特性:活性炭是一种多孔径的炭化物,有极丰富的孔隙构造,具有良好的吸附特性,它的吸附作用藉物理及化学的吸咐力而成的,其外观色泽呈黑色。
其成份除了主要的炭以外,还包含了少量的氢、氮、氧,其结构则外形似以一个六边形,由于不规则的六边形结构,确定了其多也体枳及高表面积的特点,每克的活性炭所具的有比表面相当于1000个平方米之多。
活性炭

活性炭一、商品简介活性炭又称活性炭黑。
是黑色粉末状或颗粒状的无定形碳。
活性炭主成分除了碳以外还有氧、氢等元素。
活性炭在结构上由于微晶碳是不规则排列,在交叉连接之间有细孔,在活化时会产生碳组织缺陷,因此它是一种多孔碳,堆积密度低,比表面积大。
英文别名:Charcoal activated,Carbon amorphous,Carbon black,Carbon active,Activated carbon,Activated charcoal,Activated char,Carbon Amorphous。
活性炭是传统而现代的人造材料,又称碳分子筛,化学式:C。
CAS:64365-11-3 EINECS: 264-864-4。
自从问世一百年来,活性炭应用领域日益扩展,应用数量不断递增。
二、商品性质、功能、应用物理性状:黑色无定形粒状物或细微粉末。
无臭。
无味。
无砂性。
不溶于任何溶剂。
对各种气体有选择性的吸附能力,对有机色素和含氮碱有高容量吸附能力。
每g总表面积可达500~1000m2。
相对密度约1.9~2.1。
表观相对密度约0.08~0.45。
密封干燥保存。
吸附特性活性炭是一种很细小的炭粒有很大的表面积,而且炭粒中还有更细小的孔——毛细管。
这种毛细管具有很强的吸附能力,由于炭粒的表面积很大,所以能与气体(杂质)充分接触。
当这些气体(杂质)碰到毛细管被吸附,起净化作用。
活性炭的表面积研究是非常重要的,活性炭的比表面积检测数据只有采用BET方法检测出来的结果才是真实可靠的,国内目前有很多仪器只能做直接对比法的检测,现在国内也被淘汰了。
目前国内外比表面积测试统一采用多点BET法,国内外制定出来的比表面积测定标准都是以BET测试方法为基础的,请参看我国国家标准(GB/T 19587-2004)-气体吸附BET原理测定固态物质比表面积的方法。
比表面积检测其实是比较耗费时间的工作,由于样品吸附能力的不同,有些样品的测试可能需要耗费一整天的时间,如果测试过程没有实现完全自动化,那测试人员就时刻都不能离开,并且要高度集中,观察仪表盘,操控旋钮,稍不留神就会导致测试过程的失败,这会浪费测试人员很多的宝贵时间。
危化品MSDS-活性炭

活性炭1. 产品标识商品名:活性炭英文名:Charcoal active granular;Carbon active主要用途:吸附剂2. 危险性概述危险性类别:自然物品侵入途径:无资料健康危害:无资料环境危害:无资料燃爆危险:无资料3. 组分信息纯品■混合物□主要成分CAS RN 含量(%) 活性炭7440-44-04. 急救措施吸入:迅速脱离现场至新鲜空气处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
误食:误服者用水漱口。
就医。
皮肤接触:立即脱去被污染衣着,用大量流动清水冲洗,至少15 分钟。
就医。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15 分钟。
就医。
5. 消防措施燃烧性:易燃。
灭火剂:水、泡沫、二氧化碳、砂土。
火场周围可用的灭火介质。
6. 泄漏应急措施泄漏:隔离泄漏污染区,限制出入。
切断火源。
建议应急处理人员戴自给式呼吸器,穿防毒服。
7. 作业与储存操作处置注意事项:注意包装完整密封。
储存于干燥清洁、阴凉、通风的仓间。
防止阳光直射。
搬运时要轻装轻卸,防止包装及容器损坏。
8. 接触控制/个体防护作业场所职业接触限值:中国MAC(mg/m3):未制定标准前苏联MAC(mg/m3):未制定标准美国TVL-TWA:未制定标准美国TVL-STEL:未制定标准工程控制:密闭操作。
提供安全淋浴和洗眼设备。
呼吸系统防护:一般不需要特殊防护,但建议特殊情况下,佩戴自吸过滤式防尘口罩。
眼睛防护:戴化学安全防护眼镜。
身体防护:穿化学防护服。
手防护:戴橡胶手套。
其他防护:工作现场严禁吸烟。
注意个人清洁卫生。
9. 理化特性外观与性状:黑色无定形颗粒或细微粉末,无臭、无味、无砂性。
溶解性:不溶于任何溶剂。
10. 稳定性和反应性稳定性:稳定聚合危害:聚合避免接触的条件:禁忌物:燃烧(分解)产物:二氧化碳。
11. 毒理学信息无资料12. 生态学信息无资料13. 废弃处置废弃方法:处置前应参阅国家和地方有关法规。
活性碳

简介活性碳又称活性炭它是含碳物质经过炭化和活化制成的多孔性产物。
它具有发达的孔隙结构和巨大的比表面积,可用作吸附剂、催化剂和催化剂载体。
目前认为活性炭是由类石墨微晶构成的,为螺层形结构(Turbostratic Structurc)。
活性炭作为人造材料,是在1900年和1901年才发明的,发明者Raphael von Ostrejko,取得英国专利B.P.14224(1900);英国专利B.P.18040(1900)德国专利Ger.P.136792(1901)。
活性碳制作工艺1. 煤的元素组成煤的元素组成以有机质为主体。
煤的工艺用途,主要是由煤中有机质的性质决定的。
煤中有机质主要由碳、氢、氧及少量的氮、硫、磷等元素构成。
2.原煤的破碎在煤质活性碳生产过程中,无论是煤质粉状活性碳还是煤质粒状炭,原料煤首先要经过破碎。
原料煤的破碎是指用机械的方法以达到减小块状料的几何尺寸为目的的操作过程。
韩研活性炭选择以挤压、研磨、撞击、劈裂等。
在破碎过程中往往是一种操作为主,其他方式为铺。
选择破碎方法时主要视破碎物料的物理性质和破碎的要求而定,对于坚硬物料宜采用挤压与撞击;对于韧性物料,则宜采用研磨和剪力;对于脆性物料则以劈裂为宜。
3.煤的磨粉煤的磨粉在大多数煤质粒状活性炭生产中是及为重要的工序。
磨粉的目的是为了将煤进行变性处理,增加其表面积。
成型粒状的密度在一定范围内取决于煤的磨粉程度。
另外,磨粉时煤的性能变化很大,这不仅对成型过程,而且对制得活性炭的质量、强度和孔隙结构均有重大影响。
煤粉颗粒大小一般在0-200um之间。
4.混捏混捏是制造活性炭的关键工序,极大程度上决定了活性炭最终产品的耐磨、耐冲击强度和制造得率。
所谓混捏是指将煤粉颗粒与粘合剂及少量的水在一定温度下混合、捏合成可塑性物料的生产操作。
中国活性炭在应用历史我国活性炭在应用历史简分为三个阶段。
(1)第一阶段使20世纪40年代以前,我国制药工业、化学工业中使用活性炭量大,都用进口货,例如用Carboraffin牌的活性炭。
活性炭activated carbon

pore size texture surface area The factor of AC’S adsorption capacity
surface chemistry
surface functional groups
ash content
PORE STRUCTURE
micropore
<2nm
application range. high carbon loss .
carbon) is Thermal
biology
Easy to operate, low investment and operating costs.
Long reacting time, affected by water temperature, and microorganism treatment of pollutants targeted.
Regeneration method
advantages
disadvantages
• Activated carbon regeneration (degradation / desorption of organic matter adsorbed on activated
also an effective way to reduceefficiency the use cost of activated carbon. Mature process, high regeneration and wide Need additional energy to heat, high cost of investment and
• In order to expand the use of activated carbon, the preparation of cheap activated
活性炭

活性炭安全技术说明第一部分化学品及企业标识化学品中文名称:活性炭英文名:Charcoa activated granular第二部分成分/组成信息化学品名称:活性炭化学品分子式:C组成成分:100%活性炭CAS号:7440-44-0第三部分危险性概述危险性类别:侵入途径:吸入、食入、经皮吸收健康危害:无资料环境危害:爆炸危险:第四部分急救措施皮肤接触:用肥皂水洗掉即可,如有疼痛,及时就医。
眼睛接触:立即提起眼睑,用大量流动清水冲洗至少10分钟。
如有疼痛,就医。
吸入:迅速转移至空气新鲜处,呼吸新鲜空气;如有咳嗽或呼吸不适,及时就医。
食入:让受害者饮足量清水,如胃肠不适感加重,及时就医。
第五部分消防措施危险特性:可燃,高浓度粉尘可引起爆炸。
有害燃烧产物:灭火方法及灭火剂:十粉,泡沫,喷水,二氧化碳。
灭火注意事项:没有配备花谢防护衣和供氧设备请不要呆在危险区。
第六部分泄漏应急处理个人防护:避免产生和吸入其粉尘。
当粉尘浓度过高时,应急处理人员须穿戴安全防护用具进入现场。
环境保护措施:未经处理不允许进入排水系统。
清洁/吸收措施:采用安全的方法将泄漏物收集回收或运至废物处理场所处理,根据化学品性质进一步处置。
第七部分操作处置与储存操作注意事项:无特殊要求储存注意事项:干燥,密封。
常温储存。
第八部分暴露控制/个体防护工程控制:密闭操作,局部排风。
提供安全淋浴和洗眼设备。
呼吸系统防护:当空气中粉尘浓度过高时,建议佩戴过滤式防尘呼吸器。
必要时,佩戴空气呼吸器。
眼睛防护:呼吸系统防护中已作防护。
身体防护:穿方化学品工作服。
手防护:戴防化学品手套。
其他防护:工作毕,洗手。
淋浴更衣。
第九部分理化特性外观与形状:黑色粒状或粉状、无味。
溶解性:水(20℃)不溶颗粒尺寸:≤150mPH值(50g/l 水溶液,20℃): 6升华点:3700℃密度:散装密度:250-350kg/m3闪点:无资料第十部分稳定性和反应活性稳定性:稳定避免接触条件:强加热禁忌物:强氧化剂危险分解产物:碳氧化合物聚合危害:不发生第十一部分毒理学资料活性炭无毒急性毒性:无资料其他资料:小心合理使用产品不会出现有害性。
活性炭

It should be pointed out that for molecules of contaminants to be adsorbed, they must be smaller than the size of carbon pore opening so that they can pass into the carbon pores and accumulate. Now that you can imagine why we are always using different raw materials and conditions of activation to produce varieties of activated carbon with different pores structure, aiming to make our products suitable for different applications.
PROCESS BLOCK DIAGRAM FOR COAL BASED ACTIVATED CARBONS
How activated carbon works?
The atoms of carbon comprising the large internal surface area of activated carbon present attractive forces outward from the surface. These forces, known as Van der Waals forces, attract the molecules of the surrounding gas or liquid. The combination of these attractive forces and those of molecules in the surrounding medium result in adsorption of molecules at the surface of the activated carbon. Some molecules have structures which make them more easily adsorbed than others and it is due to this that separation of molecules is achieved.
活性炭英文描述

Product Description:our company's product nut shell activated carbon is a nonpolar adsorben,it selects high quality shells and anthracite as the raw material, through the processing, dehydration,carbonization, Our facotry is certified by ISO9001:2000,ISO9001-2008.activation , processed from the use of advanced technology refined.Appearance is black powder, granules, column and so on .Significant advantages:1)high developed porous structure;2)large specifiction surface area;3)adsorption capacity;4)high mechanical intensity;5)renewable etc.Function:widely used in toxic gas purification, waste gas treatment,agriculture environment protection,national defense industrial, industrial and water purification processing the solvent recovery etc.Amount of Use: According to the different water quality, it is depends on production process.Quality Index:(HG3-1290-80)Item Test Data Item Test DataGranularity diameter 0.4-3mm Real specific gravity 2-2.2g/cm3Phenol adsorption rate 450mg/g bulk specific weight 0.45-0.55g/cm3 Hardness ≥80-95% Total porous volume 0.7-1cm3/gIodine number 1000-1100mg/g Specific surface area 590-1500m2/g Methylene blue number 100-150mg/g PH value 8-10Half de-chlorine value ≤5cm Ash ≤8-12%Moisture ≤3%Specific heat -1.00J/g. °CActivated carbonFrom Wikipedia, the free encyclopedia(Redirected from Active carbon)This article may be divided into too many sections considering its overall length. Tohelp improve Wikipedia's quality standards, some of the sections may need to becondensed or merged. Please discuss this issue on the talk page. October 2009Activated carbonActivated carbon, also called activated charcoal or activated coal is a form of carbon that has been processed to make it extremely porous and thus to have a very large surface area available for adsorption or chemical reactions.[1]The word activated in the name is sometimes replaced with active. Due to its high degree of microporosity, just 1 gram of activated carbon has a surface area in excess of 500 m2 (about one tenth the size of a football field), as determined typically by nitrogen gas adsorption. Sufficient activation for useful applications may come solely from the high surface area, though further chemical treatment often enhances the absorbing properties of the material. Activated carbon is usually derived from charcoal.ProductionActivated carbon is carbon produced from carbonaceous source materials like nutshells, peat, wood, coir, lignite, coal and petroleum pitch. It can be produced by one of the following processes:1Physical reactivation: The precursor is developed into activated carbons using gases.This is generally done by using one or a combination of the following processes:▪Carbonization: Material with carbon content is pyrolyzed at temperatures in therange 600–900 °C, in absence of oxygen (usually in inert atmosphere with gases likeargon or nitrogen)▪Activation/Oxidation: Raw material or carbonized material is exposed tooxidizing atmospheres (carbon monoxide, oxygen, or steam) at temperatures above250 °C, usually in the temperature range of 600–1200 °C.2Chemical activation: Prior to carbonization, the raw material is impregnated with certain chemicals. The chemical is typically an acid, strong base, or a salt(phosphoric acid, potassium hydroxide, sodium hydroxide, zinc chloride, respectively). Then, the raw material is carbonized at lower temperatures (450–900 °C). It is believed that the carbonization / activation step proceeds simultaneously with the chemical activation. Chemical activation is preferred over physical activation owing to the lower temperatures and shorter time needed for activating material.ClassificationActivated carbons are complex products which are difficult to classify on the basis of their behaviour, surface characteristics and preparation methods. However, some broad classification is made for general purpose based on their physical characteristics.Powdered activated carbon (PAC)A micrograph of activated charcoal under bright field illumination on a light microscope. Notice the fractal-like shape of the particles hinting at their enormous surface area. Each particle in this image, despite being only around 0.1 mm wide, has a surface area of several square metres. This image of activated charcoal in water is at a scale of 6.236 pixels/μm, the entire image covers a region of approximately 1.1 by 0.7 mm.Traditionally, active carbons are made in particular form as powders or fine granules less than 1.0 mm in size with an average diameter between .15 and .25 mm.[2] Thus they present a large surface to volume ratio with a small diffusion distance. PAC is made up of crushed or ground carbon particles, 95–100% of which will pass through a designated mesh sieve or sieve. Granularactivated carbon is defined as the activated carbon being retained on a 50-mesh sieve (0.297 mm) and PAC material as finer material, while ASTM classifies particle sizes corresponding to an 80-mesh sieve (0.177 mm) and smaller as PAC. PAC is not commonly used in a dedicated vessel, owing to the high head loss that would occur. PAC is generally added directly to other process units, such as raw water intakes, rapid mix basins, clarifiers, and gravity filters.Granular activated carbon (GAC)Granular activated carbon has a relatively larger particle size compared to powdered activated carbon and consequently, presents a smaller external surface. Diffusion of the adsorbate is thus an important factor. These carbons are therefore preferred for all adsorption of gases and vapors as their rate of diffusion are faster. Granulated carbons are used for water treatment, deodorization and separation of components of flow system. GAC can be either in the granular form or extruded. GAC is designated by sizes such as 8×20, 20×40, or 8×30 for liquid phase applications and 4×6, 4×8 or 4×10 for vapor phase applications. A20×40 carbon is made of particles that will pass through a U.S. Standard Mesh Size No. 20 sieve (0.84 mm) (generally specified as 85% passing) but be retained on a U.S. Standard Mesh Size No. 40 sieve (0.42 mm) (generally specified as 95% retained). A WWA(1992) B604 uses the 50-mesh sieve (0.297 mm) as the minimum GAC size. The most popular aqueous phase carbons are the 12×40 and 8×30 sizes because they have a good balance of size, surface area, and head loss characteristics.Extruded activated carbon (EAC)Extruded activated carbon combines powdered activated carbon with a binder, which are fused together and extruded into a cylindrical shaped activated carbon block with diameters from 0.8 to 130 mm. These are mainly used for gas phase applications because of their low pressure drop, high mechanical strength and low dust content.Impregnated carbonPorous carbons containing several types of inorganic impregnant such as iodine, silver, cations such as Al, Mn, Zn, Fe, Li, Ca have also been prepared for specific application in air pollution control especially in museums and galleries. Due to antimicrobial/antiseptic properties, silver loaded activated carbon is used as an adsorbent for purification of domestic water. Drinking water can be obtained from natural water by treating the natural water with a mixture of activated carbon and Al(OH)3, a flocculating agent. Impregnated carbons are also used for the adsorption of H2S and thiols. Adsorption rates for H2S as high as 50% by weight have been reported.Polymer coated carbonThis is a process by which a porous carbon can be coated with a biocompatible polymer to give a smooth and permeable coat without blocking the pores. The resulting carbon is useful for hemoperfusion. Hemoperfusion is a treatment technique in which large volumes of the patient's blood are passed over an adsorbent substance in order to remove toxic substances from the blood.OtherActivated carbon is also available in special forms such as cloths and fibres. The "carbon cloth" for instance is used in personnel protection for the military.PropertiesA gram of activated carbon can have a surface area in excess of 500 m2, with 1500 m2being readily achievable.[3] Carbon aerogels, while more expensive, have even higher surface areas, and are used in special applications.Activated carbon, as viewed by an electron microscopeUnder an electron microscope, the high surface-area structures of activated carbon are revealed. Individual particles are intensely convoluted and display various kinds of porosity; there may be many areas where flat surfaces of graphite-like material run parallel to each other, separated by only a few nanometers or so. These micropores provide superb conditions for adsorption to occur, since adsorbing material can interact with many surfaces simultaneously. Tests of adsorption behaviour are usually done with nitrogen gas at 77 K under high vacuum, but in everyday terms activated carbon is perfectly capable of producing the equivalent, by adsorption from its environment, liquid water from steam at 100 °C and a pressure of 1/10,000 of an atmosphere.James Dewar, the scientist after whom the Dewar (vacuum flask) is named, spent much time studying activated carbon and published a paper regarding its absorption capacity with regard to gases.[4]In this paper, he discovered that cooling the carbon to liquid n itrogen temperatures allowed it to absorb significant quantities of numerous air gases, among others, that could then be recollected by simply allowing the carbon to warm again and that coconut based carbon was superior for the effect. He uses oxygen as an example, wherein the activated carbon would typically absorb the atmospheric concentration (21%) under standard conditions, but release over 80% oxygen if the carbon was first cooled to low temperatures.Physically, activated carbon binds materials by van der Waals force or London dispersion force.Activated carbon does not bind well to certain chemicals, including alcohols, glycols, strong acids and bases, metals and most inorganics, such as lithium, sodium, iron, lead, arsenic, fluorine, and boric acid.Activated carbon does adsorb iodine very well and in fact the iodine number, mg/g, (ASTM D28 Standard Method test) is used as an indication of total surface area.Contrary to a claim repeated[citation needed]throughout the web, activated carbon does not absorb ammonia.Carbon monoxide is not well absorbed by activated carbon. This should be of particular concern to those using the material in filters for respirators, fume hoods or other gas control systems as the gas is undetectable to the human senses, toxic to metabolism and neurotoxic.Substantial lists of the common industrial and agricultural gases absorbed by activated carbon can be found online.[5]Activated carbon can be used as a substrate for the application of various chemicals to improve the adsorptive capacity for some inorganic (and problematic organic) compounds such as hydrogen sulfide(H2S), ammonia (NH3), formaldehyde (HCOH), radioisotopes iodine-131(131I) and mercury (Hg). This property is known as chemisorption.Iodine numberMany carbons preferentially adsorb small molecules. Iodine number is the most fundamental parameter used to characterize activated carbon performance. It is a measure of activity level (higher number indicates higher degree of activation), often reported in mg/g (typical range 500–1200 mg/g). It is a measure of the micropore content of the activated carbon (0 to 20 Å, or up to 2 nm) by adsorption of iodine from solution. It is equivalent to surface area of carbon between 900 m²/g and 1100 m²/g. It is the standard measure for liquid phase applications.Iodine number is defined as the milligrams of iodine adsorbed by one gram of carbon when the iodine concentration in the residual filtrate is 0.02 normal. Basically, iodine number is a measure of the iodine adsorbed in the pores and, as such, is an indication of the pore volume available in the activated carbon of interest. Typically, water treatment carbons have iodine numbers ranging from 600 to 1100. Frequently, this parameter is used to determine the degree of exhaustion of a carbon in use. However, this practice should be viewed with caution as chemical interactions with the adsorbate may affect the iodine uptake giving false results. Thus, the use of iodine number as a measure of the degree of exhaustion of a carbon bed can only be recommended if it has been shown to be free of chemical interactions with adsorbates and if an experimental correlation between iodine number and the degree of exhaustion has been determined for the particular application.MolassesSome carbons are more adept at adsorbing large molecules. Molasses number or molasses efficiency is a measure of the mesopore content of the activated carbon (greater than 20 Å, or larger than 2 nm) by adsorption of molasses from solution. A high molasses number indicates a high adsorption of big molecules (range 95–600). Caramel dp (decolorizing performance) is similar to molasses number. Molasses efficiency is reported as a percentage (range 40%–185%) and parallels molasses number (600 = 185%, 425 = 85%). The European molasses number (range 525–110) is inversely related to the North American molasses number.Molasses Number is a measure of the degree of decolorization of a standard molasses solution that has been diluted and standardized against standardized activated carbon. Due to the size of color bodies, the molasses number represents the potential pore volume available for larger adsorbing species. As all of the pore volume may not be available for adsorption in a particular waste water application, and as some of the adsorbate may enter smaller pores, it is not a good measure of the worth of a particular activated carbon for a specific application. Frequently, this parameter is useful in evaluating a series of active carbons for their rates of adsorption. Given two active carbons with similar pore volumes for adsorption, the one having the higher molasses number will usually have larger feeder pores resulting in more efficient transfer of adsorbate into the adsorption space.T anninTannins are a mixture of large and medium size molecules. Carbons with a combination of macropores and mesopores adsorb tannins. The ability of a carbon to adsorb tannins is reported in parts per million concentration (range 200 ppm–362 ppm).Methylene blueSome carbons have a mesopore (20 Å to 50 Å, or 2 to 5 nm) structure which adsorbs medium size molecules, such as the dye methylene blue. Methylene blue adsorption is reported in g/100g(range 11–28 g/100g).DechlorinationSome carbons are evaluated based on the dechlorination half-value length, which measures the chlorine-removal efficiency of activated carbon. The dechlorination half-value length is the depth of carbon required to reduce the chlorine level of a flowing stream from 5 ppm to 3.5 ppm. A lower half-value length indicates superior performance.Apparent densityHigher density provides greater volume activity and normally indicates better quality activated carbon.Hardness/abrasion numberIt is a measure of the activated carbon’s resistance to attrition. It is important indicator of activated carbon to maintain its physical integrity and withstand frictional forces imposed by backwashing, etc. There are large differences in the hardness of activated carbons, depending on the raw material and activity level.Ash contentIt reduces the overall activity of activated carbon. It reduces the efficiency of reactivation. The metal oxides (Fe2O3) can leach out of activated carbon resulting in discoloration. Acid/water soluble ash content is more significant than total ash content. Soluble ash content can be very important for aquarists, as ferric oxide can promote algal growths. A carbon with a low soluble ash content should be used for marine, freshwater fish and reef tanks to avoid heavy metal poisoning and excess plant/algal growth.Carbon tetrachloride activityMeasurement of the porosity of an activated carbon by the adsorption of saturated carbon tetrachloride vapour.Particle size distributionThe finer the particle size of an activated carbon, the better the access to the surface area and the faster the rate of adsorption kinetics. In vapour phase systems this needs to be considered against pressure drop, which will affect energy cost. Careful consideration of particle size distribution can provide significant operating benefits.Examples of adsorptionHeterogeneous catalysisThe most commonly encountered form of chemisorption in industry, occurs when a solid catalyst interacts with a gaseous feedstock, the reactant/s. The adsorption of reactant/s to the catalyst surface creates a chemical bond, altering the electron density around the reactant molecule and allowing it to undergo reactions that would not normally be available to it.Adsorption refrigerationAdsorption refrigeration and heat pump cycles rely on the adsorption of a refrigerant gas into an adsorbent at low pressure and subsequent desorption by heating. The adsorbent acts as a "chemicalcompressor" driven by heat and is, from this point of view, the "pump" of the system. It consists of a solar collector, a condenser or heat-exchanger and an evaporator that is placed in a refrigerator box. The inside of the collector is lined with an adsorption bed packed with activated carbon adsorbed with methanol. The refrigerator box is insulated filled with water. The activated carbon can adsorb a large amount of methanol vapours in ambient temperature and desorb it at a higher temperature (around 100 degrees Celsius). During the daytime, the sunshine irradiates the collector, so the collector is heated up and the methanol is desorbed from the activated carbon. In desorption, the liquid methanol adsorbed in the charcoal heats up and vaporizes. The methanol vapour condenses and is stored in the evaporator.At night, the collector temperature decreases to the ambient temperature, and the charcoal adsorbs the methanol from the evaporator. The liquid methanol in the evaporator vaporizes and absorbs the heat from the water contained in the trays. Since adsorption is a process of releasing heat, the collector must be cooled efficiently at night. As mention ed above, the adsorption refrigeration system operates in an intermittent way to produce the refrigerating effect.Helium gas can also be 'pumped' by thermally cycling activated carbon 'sorption pumps' between 4 kelvins and higher temperatures. An example of this is to provide the cooling power for the Oxford Instruments AST series dilution refrigerators. 3He vapour is pumped from the surface of the dilute phase of a mixture of liquid 4He and its isotope 3He. The 3He is adsorbed onto the surfaces of the carbon at low temperature (typically <4K), the regeneration of the pump between 20 and 40 K returns the 3He to the concentrated phase of the liquid mixture. Cooling occurs at the interface between the two liquid phases as 3He 'evaporates' across the phase boundary. If more than one pump is present in the system a continuous flow of gas and hence constant cooling power can be obtained, by having one sorption pump regenerating while the other is pumping. Systems such as this allow temperatures as low as 10 mK (0.01 kelvin) to be obtained with very few moving parts.ApplicationsActivated carbon is used in gas purification, gold purification, metal extraction, water purification, medicine, sewage treatment, air filters in gas masks and respirators, filters in compressed air and many other applications.One major industrial application involves use of activated carbon in the metal finishing field. It is very widely employed for purification of electroplating solut ions. For example, it is a main purification technique for removing organic impurities from bright nickel plating solutions. A variety of organic chemicals are added to plating solutions for improving their deposit qualities and for enhancing properties like brightness, smoothness, ductility, etc. Due to passage of direct current and electrolytic reactions of anodic oxidation and cathodic reduction, organic additives generate unwanted break down products in solution. Their excessive build up can adversely affect the plating quality and physical properties of deposited metal. Activated carbon treatment removes such impurities and restores plating performance to the desired level.Analytical chemistry applicationsActivated carbon, in 50% w/w combination with celite, is used as stationary phase in low-pressure chromatographic separation of carbohydrates (mono-, di- trisacchardes) using ethanol solutions(5–50%) as mobile phase in analytical or preparative protocols.Environmental applicationsActivated carbon is usually used in water filtration systems. In this illustration, the activated carbon is in the fourth level (counted from bottom).Carbon adsorption has numerous applications in removing pollutants from air or water streams both in the field and in industrial processes such as:▪Spill cleanup▪Groundwaterremediation▪Drinking waterfiltration▪Air purification▪V olatile organic compounds capture from painting, dry cleaning, gasoline dispensing operations, and other processes.In 2007, West-Flanders University (in Belgium) began research in water treatment after festivals.[6] A full scale activated carbon installation was built at the Dranouter music festival in 2008, with plans to utilize the technology to treat water at this festival for the next 20 years.[6]Activated charcoal is also used for the measurement of radon concentration in air.Medical applicationsActivated carbon is used to treat poisonings and overdoses following oral ingestion.It is thought to bind to poison and prevent its absorption by the gastrointestinal tract. In cases of suspected poisoning, medical personnel administer activated charcoal on the scene or at a hospital's emergency department. Dosing is usually empirical at 1 gram/kg of body mass (for adolescents or adults, give 50–100 g), usually given only once, but depending on the drug taken, it may be given more than once. In rare situations activated charcoal is used in Intensive Care to filter out harmful drugs from the blood stream of poisoned patients. Activated charcoal has become the treatment of choice for many poisonings, and other decontamination methods such as ipecac-induced emesis or stomach pumping are now used rarely.Activated charcoal for medical use.While activated carbon is useful in acute poisoning, it has been shown to not be effective in long term accumulation of toxins, such as with the use of toxic herbicides.[7]Mechanisms of action:▪Binding of the toxin to prevent stomach and intestinal absorption. Binding is reversible soa cathartic such as sorbitol may be added as well.▪It interrupts the enterohepatic and enteroenteric circulation of some drugs/toxins and their metabolitesIncorrect application (e.g. into the lungs) results in pulmonary aspiration which can sometimes be fatal if immediate medical treatment is not init iated.[8]The use of activated charcoal is contraindicated when the ingested substance is an acid, an alkali, or a petroleum product.For pre-hospital (paramedic) use, it comes in plastic tubes or bottles, commonly 12.5 or 25 grams, pre-mixed with water. The trade names include InstaChar, SuperChar, Actidose, Charcodote, and Liqui-Char, but it is commonly called activated charcoal.Ingestion of activated charcoal prior to consumption of alcoholic beverages appeared to reduce absorption of ethanol into the blood. 5 to 15 milligrams of charcoal per kilogram of body weight taken at the same time as 170 ml of pure ethanol (which equals to about 10 servings of an alcoholic beverage), over the course of one hour, seemed to reduce potential blood alcohol content.[9] Y et other studies showed that this is not the case, and that ethanol blood concentrations were increased because of activated charcoal use.[10]Charcoal biscuits were sold in England starting in the early 19th century, originally as an antidote to flatulence and stomach trouble.[11]Tablets or capsules of activated charcoal are used in many countries as an over-the-counter drug to treat diarrhea, indigestion, and flatulence.[12]There is some evidence of its effectiveness as a treatment for irritable bowel syndrome (IBS),[13]and to prevent diarrhea in cancer patients who have received irinotecan.[14] It can interfere with the absorbency of some medications, and lead to unreliable readings in medical tests such as the guaiac card test.[15] Activated charcoal is also used for bowel preparation by reducing intestinal gas content before abdominal radiography to visualize bile and pancreatic and renal stones. A type of charcoal biscuit has also been marketed as a pet care product.Fuel storageResearch is being done testing various activated carbons' ability to store natural gas and hydrogen gas. The porous material acts like a sponge for different types of gasses. The gas is attracted to the carbon material via V an der Waals forces. Some carbons have been able to achieve bonding energies of 5–10 kJ per mol. The gas may then be desorbed when subjected to higher temperatures and either combusted to do work or in the case of hydrogen gas extracted for use in a hydrogen fuel cell. Gas storage in activated carbons is an appealing gas storage method because the gas can be stored in a low pressure, low mass, low volume environment that would be much more feasible than bulky on board compression tanks in vehicles. The United States Department of Energy has specified certain goals to be achieved in the area of research and development of nano-porous carbon materials. As of yet all of the goals are yet to be satisfied but numerous institutions, including the Alliance for Collaborative Research in Alternative Fuel Technology (ALL-CRAFT, ) program, are continuing to conduct work in this promising field.Gas purificationFilters with activated carbon are usually used in compressed air and gas purification to remove oil vapors, odors, and other hydrocarbons from the air. The most common designs use a 1 stage or 2 stage filtration principle in which activated carbon is embedded inside the filter media. Activated charcoal is also used in spacesuit Primary Life Support Systems. Activated charcoal filters are used to retain radioactive gases from a nuclear boiling water reactor turbine condenser. The air vacuumed from the condenser contains traces of radioactive gases. The large charcoal beds adsorb these gases and retains them while they rapidly decay to non-radioactive solid species. The solids are trapped in the charcoal particles, while the filtered air passes through.Chemical purificationActivated carbon is commonly used to purify homemade non-dangerous chemicals such as sodium acetate.Distilled alcoholic beverage purificationSee also: Lincoln County ProcessActivated carbon filters can be used to filter vodka and whiskey of organic impurities which can affect color, taste, and odor. Passing an organically impure vodka through an activated carbon filter at the proper flow rate will result in vodka with an identical alcohol content and significantly increased organic purity, as judged by odor and taste.[citation needed]Mercury scrubbingActivated carbon, often impregnated with iodine or sulfur, is widely used to trap mercury emissions from coal-fired power stations, medical incinerators, and from natural gas at the wellhead. This carbon is a specialty product costing more than US$4.00 per kg. However, it is often not recycled.Disposal in the USA after absorbing mercuryThe mercury laden activated carbon presents a disposal dilemma.[citation needed]If the activated carbon contains less than 260 ppm mercury, Federal regulations allow it to be stabilized (for example, trapped in concrete) for landfilling.[citation needed] However, waste containing greater than 260 ppm is considered to be in the high mercury subcategory and is banned from landfilling (Land-Ban Rule).[citation needed] It is this material which is now accumulating in warehouses and in deep abandoned mines at an estimated rate of 1000 tons per year.[citation needed]The problem of disposal of mercury laden activated carbon is not unique to the U.S. In the Netherlands this mercury is largely recovered[16] and the activated carbon is disposed by complete burning.RegenerationThe regeneration of activated carbons involves restoring the adsorptive capacity of saturated activated carbon by desorbing adsorbed contaminants on the activated carbon surface.Thermal regenerationThe most common regeneration technique employed in industrial processes is thermal regeneration.[17] The thermal regeneration process generally follows three steps [18]:▪Adsorbent drying at approximately 105 °C▪High temperature desorption and decomposition (500–900°C) under an inert atmosphere ▪Residual organic gasification by an oxidising gas (steam or carbon dioxide) at elevated temperatures (800°C)The heat treatment stage utilises the exothermic nature of adsorption and results in desorption, partial cracking and polymerization of the adsorbed organics. The final step aims to remove charred organic residue formed in the porous structure in the previous stage and re-expose the。
活性碳文案描述

活性碳文案描述时间:2013/7/14工作室:欣悦传媒影视-------------------------------------------------------------活性炭,相信如今很多人都对它深有接触,可以用于清除装修污染,制造口罩和防毒面具、冰箱除味、净水、脱色等。
而且其环保,健康的产品特点,深得消费者青睐,可以这样说,这是一个活性炭的时代。
英文别名:activated carbon活性碳来源:有机材料:如煤、木材、果壳、椰壳、核桃壳等所有富含碳的材料。
安全可靠环保。
什么是活性炭?活性炭有哪些种类?哪些用途?答:活性炭是一种含碳材料制成的外观呈黑色,内部孔隙结构发达,比表面积大、吸附能力强的一类微晶质碳素材料,是一种常用的吸附剂、催化剂或催化剂载体。
活性炭按原料来源可分为:木质活性炭、果壳活性炭、兽骨/血活性炭、矿物原料活性炭、合成树脂活性炭、橡胶/塑料活性炭、再生活性炭等;活性炭按外观形态可分为:粉状、颗粒状、不规则颗粒状、圆柱形、球形和纤维状等。
活性炭的应用极其广泛,其用途几乎涉及所有的国民经济部门和人们日常生活,如水质净化、黄金提取、糖液脱色、药品针剂提炼、血液净化、空气净化、人体安全防护等。
吸附能力很强的炭,是把硬木、果壳、骨头等放在密闭的容器中烧成炭再增加其孔隙后制成的。
防毒面具中用来过滤气体,工业上用来脱色、使溶液纯净,医药上用来吸收胃肠中的毒素、细菌或气体。
二、净化空气用的活性碳究竟吸附多少有毒有害气体才会饱和?答:净化空气用的活性碳也存在品质高低的区分,新空间活性炭(CTC 吸附>100%),选用的是净化空气用活性碳中的上品,能吸附约占自身重量60%的有毒有害气体,才会饱和。
三、净化空气用的活性碳是怎样吸附空气中的有毒有害气体的?答:净化空气用的活性碳内部有发达的空隙结构和丰富的微孔组织,这些微孔组织具有强大的吸附力场,当空气中的有毒有害气体与活性炭接触时,活性炭微孔强大的吸附力场,能将有毒气有害体的分子吸附到微孔内。
活性炭

活性炭:英文名为activated carbon,是一种孔隙结构发达,比表面积很大(1500m2/g以上),吸附能力很强的炭,主要以煤、木材和果壳等原料,经炭化、活化和后处理而得,按外观形状可分为粉状活性炭、颗粒活性炭、成型活性炭和活性炭纤维四大类。
主要用途:1、脱色和过滤,使带色液体脱色。
2、吸收各种气体与蒸气。
3、色谱分析用。
4、测甲醇、锡和硅的还原剂。
5、粒状物可用作催化剂的载体。
硬度比表面积总C孔隙价格:活性炭价格根据生产原料、产品性能、应用领域决定:针剂活性炭价格在13500元/吨;糖用粉状活性炭一等品12000元/吨;脱色用粉状活性炭价格在11000元/吨,自来水公司用粉状活性炭价格在7500元/吨。
主要机理:活性炭含有大量微孔,具有巨大的比表面积,能有效地去除色度、臭味,可去除二级出水中大多数有机污染物和某些无机物,包含某些有毒的重金属。
影响活性炭吸附的因素有:活性炭的特性;被吸附物的特性和浓度;废水的PH值;悬浮固体含量等特性;接触系统及运行方式等。
活性炭吸附是城市污水高级处理中最重要最有效的处理技术,得到广泛的应用。
活性炭吸附法与其他处理方法联用,出现了臭氧-活性炭法、混凝-吸附活性炭法、Habberer工艺、活性炭-硅藻土法等,使活性炭的吸附周期明显延长,用量减少,处理效果和范围大幅度提高。
技术指标:主要用途:1、家用活性炭空气净化:用活性炭摆放在室内有效的吸收空气中含有的甲醛\二甲苯等有害物质(特别是新装修的房子),家具去异味:活性炭可适用于新买的家具放于橱柜\抽屉\冰箱中.也可放在鞋子里面除臭味.汽车除味:新车一般都含有很多的有害物质\难闻刺鼻的气味,用活性炭可以有效的去除2、污水处理场排气吸附3、饮料水处理4、电厂水预处理5、废水回收前处理6、生物法污水处理7、有毒废水处理8、石化无碱脱硫醇9、溶剂回收(因为活性炭可吸附有机溶剂)10、化工催化剂载体11、滤毒罐12、黄金提取13、化工品储存排气净化14、制糖、酒类、味精医药、食品精制、脱色15、乙烯脱盐水填料16、汽车尾气净化17、PTA氧化装置净化气体18、印刷油墨的除杂19、气体分离:例如从城市煤气中回收苯;从天然气中回收汽油、丙烷和丁烷;用于处理费托合成中的废气,以回收其中的烃类等。
活性炭MSDS
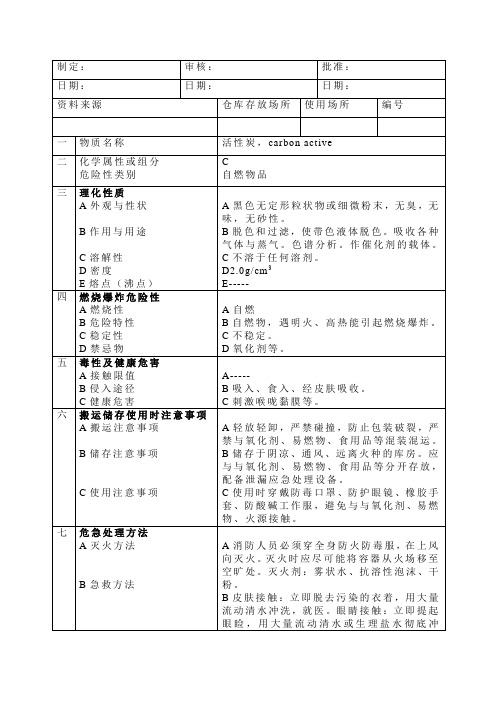
八
废弃处理注意事项
按《废弃物管理规定》执行。
制定:
审核:
批准:
日期:
日期:
日期:
资料来源
仓库存放场所
使用场所
编号
一
物质名称
活性炭,carbon active
二
化学属性或组分
危险性类别
C
自燃物品
三
理化性质
A外观与性状
B作用与用途
C溶解性
D密度
E熔点(沸点)
A黑色无定形粒状物或细微粉末,无臭,无味,无砂性。
B脱色和过滤,使带色液体脱色。吸收各种气体与蒸气。色谱分析。作催化剂的载体。
C不溶于任何溶剂。
D2.0g/cm3
E-----
四
燃烧爆炸危险性
A燃烧性
B危险特性
C稳定性
D禁忌物
A自燃
B自燃物,遇明火、高热能引起燃烧爆炸。C不ຫໍສະໝຸດ 定。D氧化剂等。五
毒性及健康危害
A接触限值
B侵入途径
C健康危害
A-----
B吸入、食入、经皮肤吸收。
C刺激喉咙黏膜等。
六
搬运储存使用时注意事项
A搬运注意事项
B储存注意事项
C使用注意事项
A轻放轻卸,严禁碰撞,防止包装破裂,严禁与氧化剂、易燃物、食用品等混装混运。
B储存于阴凉、通风、远离火种的库房。应与与氧化剂、易燃物、食用品等分开存放,配备泄漏应急处理设备。
C使用时穿戴防毒口罩、防护眼镜、橡胶手套、防酸碱工作服,避免与与氧化剂、易燃物、火源接触。
活性炭危险化学品安全周知卡

好好学习社区 更多优惠资料下载: 德信诚培训网 危险化学品安全周知卡
危险性类别 易缓慢发热自燃 品名、英文名及分子式、CC 码及CAS 号 活性炭
acetic carbon C 4H 6O 3 CAS :64365-11-3
危险性标志 危险性理化数据 熔点:3500 ℃以下 沸点:4000 ℃以上 相对密度(水=1):1.48(20℃) 饱和蒸气压(kPa): 危险特性
在空气中易缓慢的发生发热和自燃 接触后表现 活性炭是非腐蚀性物质。
如有意外,处理方法应以一般颗粒 性异物对待,其可能会引起人体轻度疼痛,不会引起皮肤不适,公在颗粒受到摩擦里会造成皮肤轻度痛感。
现场急救措施 皮肤接触:用肥皂清洗即可,如有疼痛,就医。
眼睛接触:用大量流动清水,如有疼痛,就医。
吸入:呼吸新鲜空气,如有咳嗽或呼吸不适,适时就医。
食入:喝一至两杯水如不适感加重,适时就医。
身体防护措施
泄漏应急处理 有泄漏发生,应及时清洁泄漏物以免炭尘混入空气中,操作时应遵守工业卫生相关的条例。
注意眼睛,皮肤,防护服的清洁,收集到的没用过的活性炭应该放入相当容器内,以没有危险的废物对待。
对收集到的用过的活性炭应该根据相关法规来处置。
浓度
MAC (mg/m 3): 未制定标准 当地应急救援单位名称 市消防大队 市人民医院 当地应急救援单位电话 消防队:119
人民医院:120。
环境功能材料4活性炭

和D-A (Dubinin-Astakhor)方程
体积填充理论
吸附剂的孔按规定分为大孔 ( > 5 0 n m )、中孔 ( 2 ~ 5 0nm )和微孔 ( < 2n m )。蒸气在大孔中的吸附 可用 B E T 多分子层吸附理论描述 ;在中孔中,可认 为先进行多分子层吸附再进行毛细冷凝 ;在微孔中 的吸附 ,一般用杜宾宁D ub i n i n等的微孔容积填充 理论 ( T V F M )描述。
与之类似的还有KOH、H3PO4等。
(3)、气体、药剂联合法
不同的原料+不同制备方法的组合可以对活性炭 的孔隙结构进行调控,从而制取许多性能不同的活 性炭。这种联合方法是许多年来及今后相当长时期 内世界各国活性炭工作者非常关注的活性炭制取方 法。
活性炭的外观分类
(1)、粉状活性炭 一般将90%以上通过80目标准筛或粒度小于
颗粒活性炭的应用
较早阶段粉状炭的产量与用量均超过粒状炭。 糖和药品的脱色精制以及早期的水处理都以粉状 炭为主。后来随着应用范围的扩大、使用工艺的 改进,特别是再生方法与再生设备的解决,使粒 状炭的用量不断上升。加上各种煤制粒状炭的开 发,使成本降低,因此粒状活性炭的产量与用量 逐渐超过了粉状活性炭。
六十年代以后,随着环境保护事业的发展,
各国开始采用和建立大型的粒状活性炭净化自来
水的装置和工业废水的净化处理装置。将粒状活
性炭装在吸附塔或吸附池内,水以一定的流速通
过活性炭装填层,以达到净化的目的。可以间隙
或连续操作,失效的粒状炭经再生可多次使用,
明显降低了炭的用量。使用中没有二次淤渣和粉
活性炭安全MSDS技术说明书

活性炭安全技术说明书
一、成分/组成信息:
化学品名称:活性炭分子式:C 英文名称:Carbon activated 有害成分含量 CAS NO C
二、危险性概述:
健康危害:症状有结膜炎,角膜再生不良、湿疹和支气管炎等。
燃爆危险:易燃。
三、急救措施:
皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟;就医。
吸入:迅速脱离现场至空气新鲜处,保持呼吸道通畅,如呼吸困难,给输氧,如呼吸停止,立即进行人工呼吸;就医。
食入:用水漱口,给饮牛奶或蛋清,就医。
四、消防措施:
危险特性:吸入粉尘有中等程度危险,易燃。
燃烧(分解)产物:二氧化碳。
灭火方法:用水、砂土等扑救。
五、接触控制/个体防护:
呼吸系统防护:可能接触其粉尘时,必须佩过滤式防尘口罩。
眼睛防护:戴防护眼镜。
身体防护:穿工作服。
手防护:戴手套。
其它防护:工作场所禁止吸烟、进食和饮水,饭前要洗手,工作完毕,淋浴更衣,注意个人清洁卫生。
六、理化特性:
外观与性状:黑色细微粉末。
无臭,无味,无砂性
分子量 12.011 蒸汽压
沸点 4200℃溶解性不溶于水和有机溶剂
密度相对密度 1.8~2.1 稳定性
七、废弃处置:
废弃处置方法:处置前应参阅国家和地方有关法规。
活性炭

无资料。
危险性类别:第4.2项易于自燃的物质
燃烧(分解)产物:一氧化碳、二氧化碳
聚合危害:
稳定性:稳定
禁忌物:臭氧、氯、重铬酸盐等强氧化剂
火灾危险性分类:乙
危险特性:粉尘接触明火有轻度的爆炸性。在空气中易缓慢地发热和自燃。属基本无毒的物质。但有时从原料中加杂无机物,对皮肤、黏膜及呼吸道有一定的刺激。
灭火方法:
临界温度(℃):
熔点(℃):3500
沸点(℃):4000
饱和蒸气压(kPa):
溶解性:不溶于水和任何溶剂
临界压力(MPa):
燃烧热(kJ/mol):
密度:1.9~2.1g/cm3
表观密度0.08~0.45g /cm3。
燃烧爆炸危险性
燃烧性:可(自)燃
闪点(℃):无资料
自燃温度(℃):无资料
爆炸极限(V%):无资料
毒性
安全卫生标准:中国MAC:—,PC-TWA:—,PC-STEL—。
侵入途径:
毒性:
LD50:
LC50:
健康危害
无资料
急救
无资料
防护
无资料
泄漏处理
扫起,倒至垃圾箱内。
操作注意事项
无资料
储存注意事项
储存于阴凉、通风的库房。远离火种、热源。不可与氧化剂共储混运,、防止受潮,以避免受潮后积热不散可能发生自燃。如抽查发现有发热现象应及时倒垛散热,防止发生事故。
基本信息
中文名:活性炭
英文名:AcБайду номын сангаасive carbon;Active ted chlarcoal
分子式:C
分子量:12.00
CAS号:
RTECS号:
UN编号:1362
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
New Developments inActivated CarbonsJan van den DikkenbergNorit Activated CarbonThe topics•What is Activated Carbon?•New Developments in Activated Carbons(AC)–Catalytic GAC in water treatment–Anaerobic groundwater treatment–WFD, municipal wastewater–WFD, 1-STEP ©filter–Water reuseWhat is Activated Carbon ?Highly porous carbonaceous adsorbentHigh surface area1 teaspoon of carbon (5 g) hasthe same surface area asa football field (≈1000 m²/g)Raw Materials Used for Norit Activated CarbonsLignitePeatCoalCoconut shellsWoodOlive stonesParticle sizes and shapesPowdered (PAC)D50: 10-50 µmDifferentfiltration rates Granular(GAC)Broken0.25-5 mm(4-50 mesh)Granular(GAC)ExtrudedDiameter0.8-4 mmMicro pores< 1 nmMeso pores1-25 nmMacro pores> 25 nmAdsorption procesDriving force•Target compounds: hydrophobic, dissolved organics, typically not or poorly biodegradable•Examples: (halogenated) aromatics, chlorinated solvents, dissolved oil, PAH, non-biodegradable COD, AOX, colour, DOC•Concentration level: relatively low (polishing)The position of AC in water treatment ApplicationsxAquarium water xPoint of Use water x x Pool water x x Waste water xUltra-Pure water xSteam condensate xBeverage Industry x x Potable water PACGACNew DevelopmentsClick here and type text•First level•First level–Second level–Second levelCatalytic GAC in water treatmentClick•First level•First level–Courtesy PWNCatalytic GAC in water treatment Process outlineHeemskerkPre-treatment Surface water H2O2/UVCatalytic GACNorit ROW 2 CATInfiltrationAnaerobic groundwater treatment–Courtesy Van Der Hoeven KliniekAnaerobic groundwater treatment Process outlineUtrechtAnaerobic extractionSolvents adsorptionNRS CARBON EA 0.5-1.5AnaerobicinfiltrationWFDWFDThe role of AC?•Natural and synthetic hormones•Detergents (Alkyl phenols)•Plasticizers (Phthalates)•PAH•Pesticides and herbicides•Medicine residualsThe Swindon ProjectIntroduction•Demonstration project for advanced wastewater treatment •GAC filters in operation since Feb 2008•Once exhausted the carbon is due for reactivation at our Purton thermal reactivation plantProcess Outline SwindonPost-clarificationCarbon adsorptionNorit GAC 830 TWSDischargeinto river RayActive sludgePre-clarificationThe Swindon ProjectMain compounds monitored •Oestrone(E1)•17β-Oestradiol(E2)•17α-Ethinyloestradiol(EE2)•Treatment objective–E1/3 +E2/1 + EE2/0.1 < 1 ng/l(E2eq.)The Swindon ProjectIndicative GAC influent•E2eq. 1-10 ng/l•COD 20 mg/l•NO3¯40-60 mg/l•Tot P 0.05-0.15 mg/lThe Swindon Project Construction ofthe adsorbersCarbon fillingFull scaleThe Swindon ProjectFull scaleACT Lab PerformanceCarbon performance00.511.522.550001000015000Bedvolumes (n)C o m p u n d l e v e l (µg /l )Influent level 17α-Ethynil Estradiol AtrazineWFD, 1-STEP ©filterAll in one filterEnhanced coagulationOne -filter conceptBiologyAdsorptionWFD, 1-STEP ©filter Process outlineHorstermeerWWTW effluent 1-STEP ©Dischargeinto environmentWFD, 1-STEP ©filterOne Step Total Effluent Polishing = 1-STEP ©•Elimination in one filter of–Nitrogen and Phosphorus–Suspended solids–Organic micro-pollutants–Heavy metals•3 treatment concepts in one filter–Enhanced coagulation, flocculation and filtration–Biological activity–Adsorption on GACWFD, 1-STEP ©filterEffl.Washwater outBackwash in (filtrate)CoagulantC-sourceNTUNO3-NPO4-PNTUNO3-N PO4-PP-totbufferbufferWFD, 1-STEP ©filterPilot UnitWFD, 1-STEP ©filterPhosphorus removal•First level•First level–Second level–Second levelWFD, 1-STEP ©filterNitrate removal•First level•First level–Second level–Second levelWFD, 1-STEP ©filterRemoval of medicine residuals•First level•First level–Second level–Second levelWFD, 1-STEP ©filterProcess outlineWWTW effluent 1-STEP ©Dischargeinto environmentRe-useoptionsWFDSummary of developments and research needs;•Full scale applications and practical experience from pilotstudies•New technology developments;–Combination of PAC and MBR technology–Multi-functional GAC filtration•Modeling of adsorption behavior–Including isotherm databaseRe-use of municipal waste water Process outlineEmmen/SchoonebeekWWTW effluentBACF UF2 stage RO EDIHeat/Power Co-generationBACFGAC surface colonization •First level•First level–Second level–Second levelRe-use of municipal waste waterClick here and type text•First level•First level–Courtesy NieuWaterSummaryNorit Activated Carbon•The multi-purpose adsorbent•Many new developments in water applicationsNo doubts. Norit. Just proof.。
