官能团转化全图解
官能团转化
acidic
C6H13
thioketal: inert to LAH; react with RaNi; smell terrible and stay long; discard shoses
neutral basic O Pd-C Ph HCO2NH4 Ph Ph
(3). Wolff-Kishner reduction:
1-a C-OH
(1). for 1', 2' alcohol:
C-H
O CH3 S Cl O
O RCH2 O S O O CH3 tosylate mesylate triflate toluenesulfonyl chloride (s) ~ $ 30 / Kg methanesulfonyy chloride (l) ~ $ 30 / Kg purification textbook dry pyridine: from CaH2 and distilled
C=O 5- a b c d e f g h i j k C=O C=S C=N-OH, C=N-H C C C N C=C-OR; C=C-SR C(OR)2; C(SR)2 C-OH C-NH2; C-NO2 C-Br C-H
2- a C-OH b C-(OR)2 c C(O)OR d C-H e C=C f C-CN
(1). RCH2NH2
C-H
p-TsCl BuLi LiAlH4 RCH2-H
ArSO2Cl RCH2NH2 Hinsberg's test
O
RCH2NH
SO2Ar
BuLi - BuH N SO2Ar
RCH2N SO2Ar
tosylamide RCH2
第2章 官能团化和官能团转换的基本反应2.

RCH2CH2OH
总结果相当于是在 双键上反马式加一 分子H2O
氧化反应
(1) 空气催化氧化
氧化剂及反应条件不同,氧化产物不同。
O2 , Ag H2C O CH2
CH2=CH2
250℃
CuO
CH3CH=CH2 + O2
O CH3CH=CH2 + CH3C O
370℃
OH
CH2=CHCHO + H2O
-
卤化
H2 C
CHCH2Br
亲电加成
H3 C
C H
CH2
-H反应
氧化
( O)
H2C
CHCHO
② H2O/Zn CH3CHO + HCHO
CH3CH O
CH2
与卤素的加成
Br 2 CCl4 Br
H CH3 C C H CH3 H δ Br Br
δ
Br CH3CHCH2
CH3CH=CH2
Br C H C CH3 CH3
CH3CH=CH2 + HO
Br
该反应也是亲电加成反应,第一步不是质子 的加成,而是卤素正离子的加成。
按照马尔科夫尼科夫规律 ,带正电的X+加到 含有较多氢原子的双键碳上. HO-加到连有较少 氢原子的双键碳上.
CH 3 C CH 3 CH 2 + HOBr CH 3 CH 3 C HO CH 2 Br
(2) 亲核加成—与醇的加成
HC CH CH3OH
KOH 加热,加压
H2C C H OCH3
反应历程:
CH3OH + KOH
HC CH CH3O
CH3O-K+ + H2O
有机物官能团衍变的规律全面版

乙烯
溴乙烷
水解 乙醇 氧化 乙醛 氧化
还原
乙酸
重要知识规律 (1)卤代烃的消去反应规律 (2)醇类的消去反应规律 (3)醇类的催化氧化反应规律
重要知识规律
(1)卤代烃的消去反应规律 例 .既 能 发 生 消 去 反 应 生 成 烯 烃 , 又 能 发 生 水 解 反 应的是
重要知识规律 (1)卤代烃的消去反应规律
酯
关键有机物之间的转化关系 烯
卤代烃
水解 醇 氧化 醛 氧化 羧酸
还原
酯水 化解
酯
主要代表物之间的转化关系 乙烯
溴乙烷
水解 乙醇 氧化 乙醛 氧化
还原
乙酸
酯水 化解
乙酸乙酯
主要代表物之间的转化关系
生成羟基的五种方法
(1)醛 还 原 法 : CH3CHO+H2 Ni CH3CH2OH
(2)卤 代 烃 水 解 法 :
有机物官能团衍变的规律
浙江华维外国语学校
杨兴文
导引: 有机物A能在稀硫酸中发生水解反应,如图 所示
C
X 稀硫酸 △
氧 化
A 氧化 B
X 稀硫酸
△
分析各物质的关系
C
氧 化
A 氧化 B
卤代烃 水解
氧化
醇
醛 氧化 羧酸
还原
酯水 化解
酯
主要有机物之间的转化关系
烷
烯
炔
卤代烃
水解 醇 氧化
还原
氧化
醛
羧酸
酯水 化解
醇 羧酸
X NaOH溶液
△
D 稀硫酸 C
氧 化
A 氧化 B
D为何物?
X NaOH溶液
官能团转换
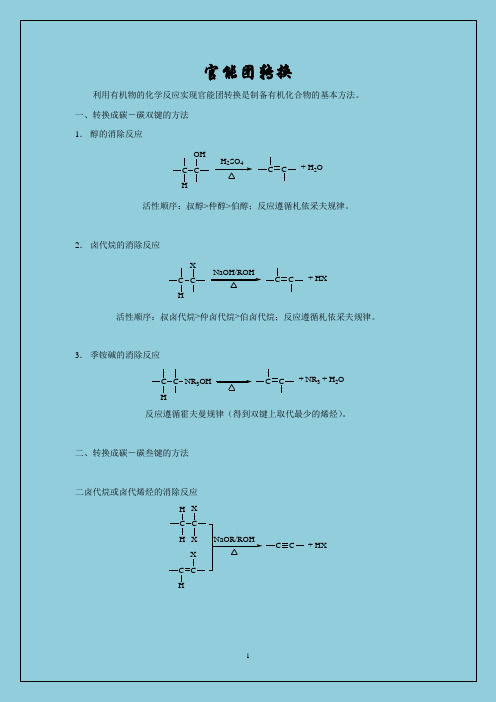
官能团转换利用有机物的化学反应实现官能团转换是制备有机化合物的基本方法。
一、转换成碳-碳双键的方法1.醇的消除反应C H COHC C+ H2OH2SO4活性顺序:叔醇>仲醇>伯醇;反应遵循札依采夫规律。
2.卤代烷的消除反应C H CXC C+ HXNaOH/ROH活性顺序:叔卤代烷>仲卤代烷>伯卤代烷;反应遵循札依采夫规律。
3.季铵碱的消除反应CHC NR3OH C C+ NR3 + H2O反应遵循霍夫曼规律(得到双键上取代最少的烯烃)。
二、转换成碳-碳叁键的方法二卤代烷或卤代烯烃的消除反应C H H CXXC C+ HXNaOR/ROHC H C X三、转换成碳-卤键的方法1. 烷烃的取代反应CH hvX 2CX + HX活性顺序:叔碳H 、烯丙式H 、苄基H > 仲碳H > 伯碳H ; Cl 2>Br 2。
反应条件:光照、高温、过氧化物。
2. 烯烃、炔烃的加成反应C HC XC CHXC XC XC CX 2C CHXC C H H X X C C H X HXC CX 2C C X X X XC CX X X 23. 芳烃的取代反应X 2FeX 3X4. 醇的取代反应C OHHX 或PX 3C X活性顺序:叔醇>仲醇>伯醇;HI>HBr>HCl 。
5. 重氮盐的取代反应N NXCuXX :Cl 、Br ,I 取代时用KI 。
四、转换成羟基的反应1. 卤代烷的水解反应NaOH/H 2OC OHC X活性顺序:碘代烷>溴代烷>氯代烷2. 格氏试剂与醛、酮的加成反应CO (1)RMgX (2)H 2OC OH R由甲醛得到伯醇、由其它醛得到仲醇、由酮得到叔醇。
3. 羰基、酯基、羧基的还原反应CO C OHH H 2/Ni LiAlH 4或 NaBH 4由醛得到伯醇、由酮得到仲醇。
C R'OORCH 2OH R'H 2/Ni LiAlH 4CH 2OH R +C R OOHLiAlH 4CH 2OH R4. 烯烃与水的加成反应C HC OH C CH 2O/H 2SO 4按马氏规则,烯烃在酸催化下加水得不到伯醇(除乙烯外);端基烯可以通过硼氢化反应得到伯醇。
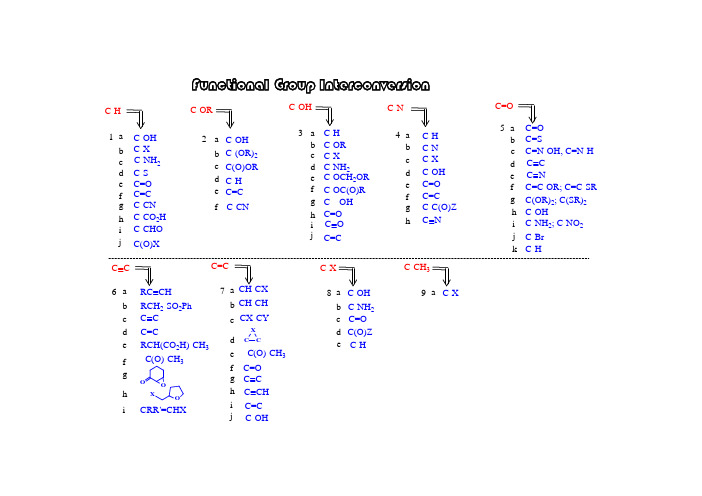
官能团相互转换大全(part1)

i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX F u n c t i o n a l G r o u p I n t e r c o n v e r s i o nC=CC C C=CC C RCH 2-SO 2Ph RC CHC C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Z d c b a e d c b a f C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e dc b a C-CHO C-CO 2HC-CN C=C C=O C-SC-NH 2C-X C-OH C-H CH 3CH3CH 3H n -Bu SnH C S O PhClRCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3PhPhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 2via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.CHCl 2rt, 3 hr1-bBu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)H OJOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOH Zn; Fe; Sn; Mg(3). metal reduction(2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973, 654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney NiBuLi1-d1-c 4RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -2NaH 2(3).Ar-NH 2Ar-H1-e(2). thioketal:(3). Wolff-Kishner reduction:(6). enol derivatives:SHSH/ BFTf2similar:(4). Pd-C / HCO2NH4(7). Et3SiH / CF3COOH1-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me 2N)3P=O 1-g (1). K / Al 2O 3 K / HMPA (2). Na / NH 31-h JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me 2N)3P=O yes for white mouse, uncertain for humanmodified to: NN O1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROO RR OROR RCH 2 OCH 2CH 2OH(1). h ν / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2O RC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t Bu RC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with base t -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl (5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X e d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3RaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-eC-OH C-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2RC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH 232Oi. HOAc: weak acid: good leaving groupii. H 2i. F - : HF, Py-H + F -; n -SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+3-b triphenylmethylorganic base: TMG3-c(1). O H -(2). K O 2 / D M S O 3-d not practically useful: R -O H cheaper than R -XJO C , 1975, 40, 1678.(2). N a 2[F e(C N )5(N O )] / K 2C O 3 / H 2O3-e(1). S ym m etry:ketal: use H 3O +acetal: use H 3O +(2). unsym etry:R O -M O M R O -M E M R O -M T M R O -T H Pi. H 3O +; ii. H C l / M eO H p -T sO H / M eO Hi. H 3O +; ii. Z nB r 2 / C H 2C l 2H gC l 2 / C H 3C N (aq.)actually, a c e t a l e x c h a n g e (3). A g 2O / H 2OT H L , 1975, 3183.JO C , 1986, 51, 3913.R O 2C(C H 2)3H RN H 2R O 2C(C H 2)3H RO H2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversionSynthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOHacetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOH3)266hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.。
化学官能团相互转换大全(part2)

JA C S, 1972, 94, 7159.L A H ------------ alm ost all: ald, ketone, acie, ester, acyl X, anhydrideN aB H4 --------------- not for acid, ester (but L iB H4 w ork for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:L iA lH4; N aB H4; N a / N H3A l (O i Pr)3 / i PrO H ----------- M eerw ein-Pondorf-V erley rxnIrC l4 / i P rO H / P(O M e)3 ------ H enbest rxnL iB H(sec Bu)3 ------------------ H. C. B row nfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). H C H O reagent:M e C H O M eO HH C H OJA C S, 1935, 511, 903.C H3C H O C(C H2O H)42O rg.Syn, 1925, 4, 53.H C H O / K O HH C H O / C a(O H)2S ynthesis, 1994, 1007.PhN O2OPhN O2H O HB H / SM eJO C, 2001, 66, 7514.JO C, 2003, 68, 2030.OB H3 / T H F99.5 % transsolvent: T H F, S M e23-iR3B, H O C H2C H2O H // H2O2 // N aO HJO C, 1986, 51, 4925.C O RRR3BRRRRR3C B OH O C H2C H2O HR3C BOOH2O2O HR3HO BOOR3CH2OR3C O Hp ra c tic e3-k OO HHO HO HOO HO HHO HO HJO C , 1967, 32, 3452.H 2O 2: dangerous,skin w hiten, m etal decom poseH g (O A c)2: toxic, hard to rem ove (3). B 2H 6, H 2O 2 / O H -, H 2O(2). H g(O A c)2, H 2O // N aB H 4(1). H 3O +3-j3-j.13-j.2hydration:(1). K M nO 4 / N aO H (2). O sO 4(3). H 2O 2/H C O 2H (4). N a / E tO HH Hcis tran cis +trancisM e 2NNNC H 3HC lH 3N C H 3H H N C H 3CH H+NC H 3C l N H H C H OCO O HA C H 21. L A H R 3C N H 2RCN R 2R C N H R R 3CO HR 2C O H R C O H R C N H 2tertiarysecondary prim ary C om pare nom enclature class:not a very useful reactionC -NC -H C -N C -X C -O H C =OC =C 4-abc d ef g 4-aSO 2N H 2Ph IO A c O A cS O ON H S OON I P h Fe (T PP )C lS O ON H 2(insertion)T P PN NNNP h2. N aN 3N C O1. SO 2C l 2O 2CCO O h iC N C (O )X C -C (O )XN H 2H 2R C N O 2R C N H 2i ii4-b C F 3C O 3H // F e / H O A c1. m an y red u cin g ag en ts4-b.14-b.21.2.3.4.F e 3(C O )12 / C H 3O HJO C , 1972, 37, 930.N aB H 4 / P d -C N a 2S S n / H C l V o g el's 12.57V o g el's 12.58V o g el's 12.595.H 2 / P t (S )-CJA C S , 1965, 87, 2767.su lfid ed p latiu mn o t affect: aro m atic rin g s, k eto n es, h alid es, n itriles, am id e, eastersJA CS, 1933, 55, 4579.2H CH O N M e 2CO 2EtN H 2CO 2EtRC N CC NRCCRC N H 2iC N R N N+-C N R R'ii 1. H CH O / H CO 2H 1. RBCl 2 / base1. H C(O Et)3 // N aBH 4;2. R 2CO // N aBH 3CN N H 2NCH 3CH 3H CH O H CO O HN 3N HBCl 2N H 2C O O HN CO O HHCH 3H C(O Et)N aBH 4b.3 2. H CH O // H 2 / Pd-CN 3N O 2M eO 2CN aBH 4CoCl 26H 2O (cat)rtN H 2N O 2M eO 2CSynthesis, 1979,537.m ild conditionhigh yieldnot affect:: N O 2, C=C, CN , CO O R, CO O H2. N aBH 4 / CoCl 2-6H 2Ono t g o od , u su ally co n tain p o lyalk ylatio n p ro d u cts2. D elep in e3. N aN 3 / R E D4-d4-c 5. U n p o lu n g4. N aN 3 / R E D3. D elep in e2. G ab riel:1. N H 3N OK N 2H 42Oi. L A H , N aB H 4ii. H 2 / catiii Z n / H C l; A l (H g )i. M g // N H 2C lii. M g // P h S C H 2-N 3co m m ercial av ailab le, tetram er o f M e 3N24. C B r 4, P P h 3, N aN 3, D M F // P P h 3 / T H FJO C , 2000, 65, 7110.u ro tro p in e (乌洛托品)m eth en am in e (六甲烯胺)h ex am eth ylen etetram in e (环六亚甲基四胺)内服后遇酸分解出 H C H O ,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2N O SO 3HB 2H 6 / H 2N OC H 3C N / H 3O +B 2H 6 / N H 2C l C =CC -C -N H C O C H 3C =C C -C -N H 24-f4-e5. P 4S 10 // R aN i4. E t 3O + B F 4- // N aB H 43. B 2H 62. N aB H 3(O C O R )1. L iA lH 46. L aw esson's reagent // R 4-h4-g4-g.a4-g.b RC N H 2R C N H 2R 'formformA lH 3 / T H FB rCNB r N H 2JO C , 2000, 65, 8152.A lH T H FT H , 1989, 30, 5137.JO C , 1987, 52, 3901.R 'L i // N aB H 4R 'M gX // N aB H 4R 'M gX // L i/N H 3(l)R '2C uL i // N aB H 4T H , 1989, 30, 5139.JO C , 1993, 58, 4313.RCNR CN H 2R 'R 'M // H4-iN H 2ON HO C H 3O PhI(O A c)23JO C , 1993, 58, 2478.RCON H 2RCONIPhO A c RNCOR N HCOO C H 3C H 3O HPhI, O A ccPhI(O A c)4-i.2CN H 2R C H 2PhI(O A c)2 // K O H / C H 3O HC(O R)2C(S R)2hC-N H2C-N O2C NC C5-agfdcba5-C=C-O RC=C-S RC-O HC=N-O HC=N-HC=SC=OC=Ov. via: epox ysilan eR COC RR COC H2R242H3O+C O2H3OOO2-4OO3H3O+Z nT sN H N H2M eL i T M S C l M C P B A L A H24C H2ORRC H2ORRaq C H3C N/C H2CORRi. via:α-C O2Hii. via: α-haloketon eiii. via: ald ol p ro cessiv. via: thioen ol etherROC H2Rd raw back: req uire sim ple stru cture, use m an y p ow erful ag ents: M eL i, L A H, M C P B Aeij C-B rk C-Hii. M C P B Ai. h yd ro lysis 5-b5-c C =N -O HC =N -Hi. R aN i ii. T iC l 3iii. K M n O 4 / A l 2O 3H 3O+5-dH g 2+ / H 2OJO C , 1972, 37, 2138.JO C , 1970, 35, 858.H g S O 4 / H 2O / H 2O5-c.15-c.2T H L , 2001, 42, 4775.1. D IB A L / H 3O +5-eS tenphen reductionm ostly forJ.O rg.S yn , 1925, 3, 1874.2. H C l./ S nC l 2 / E t 2O 5-e.1R -CH 2-CN5-e.25-e.3-C H 2-C OHR -C H -C OH R 'R -C H -C OR "R 'R 'X / n -B uL i C H 3I R ''M gB r H 3O+3.O HO HH 3O +O HO H5-fH 3O +H g 2+ / C H 3C N (aq)C =C -O RC =C -SRO C H 3OH 3O +SC H 3OH g 2+3H 3O H g2+3H 3O+OO O SSS S SR SR O O O R O R 5-gH g 2+/ H 3O+H 3O + / solv (aq)H 3O + / solv (aq)H g 2+ / H 3O +O R O RO H O HH 3O +/ solv (aq)acid catalysta very com m on protecting group, deprotect back to ketoneHCO E t O E tO E tR M gX / H 3O +HCO E t O E tO E tR M gXRCHO。
化学官能团相互转换大全(part3)
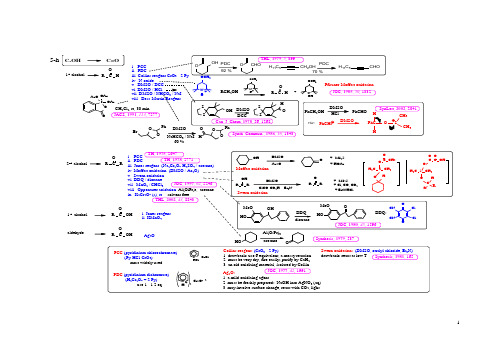
1N H+2C r 2O 7-2N H C l.C rO 3A g 2O :1. a m ild oxidizing agent2. m ust be freshly prepared : N aO H into A gN O 3 (aq)3. m ay involve surface change, react w ith C O 2, lightS w ern oxidation: (D M S O , ox alyl chloride, E t 3N )draw back: react at low TC ollins reagent: (C rO 3 - 2 P y)1. draw back: use 6 equivalent, a m essy reaction2. m ust b e very dry, fire easily; purify by C aH 23. an old ox idizing m aterial, isolated by C ollin. i. P C Cii. P D Civ. M v. S w vi. D D Q vii. M viii. O ix. K 2C RO HOaldehyde1st alcohol2nd alcohol1st alcoholRO HORRORHO 5-hi. P C C ii. P D Ciii. C P D C (pyridinium dicho rm ate) (H 2C r 2O 7 + 2 P y)P C C (pyridinium chlorochrom ate) (P y-H C l-C rO 3)m ost w idely useduse 1 - 1.2 eq.60 %JO C , 1977, 42, 1991.S ynthesis, 1981, 165.O I OO A c O A cO2pH 6: w eak acid buffer, avoid interfere w ith ketal groupM cM urray reactioni. C orey approach: subtituted-quinone // H 3O +ii. W att approacha. PhC H O // M C PB A // H 3O +b. A rPhO // M C PB A // H 3O +c. N B S // K O H // H 3O +5-iOPhPhOPhN H 2Ph PhN H 2NCO H// H 3O +O O5-i.15-i.2i. Et 3N // H 3O+N ef reactionii. TiC l 3 / pH 1 or 6iii. SiO 2 / N aO H // H 3O +JA C S, 1977, 99, 3861.iv. LD A / M oO 5-Py -v. N aO H // C H 3O H OOH 3O +vi. K M nO 4 / K O H C hem. R ev. 1955, 55, 137.35-j5-kIOOO H O (3 eq.)JA C S, 2001, 123, 3183.C H 3C H O2. D D Q / TFA.Synthesis, 1979, 537.JC S, 1932, 1875.Ph-F / D M SO 3.1. SeO 2a select oxidantindrect: change to R C -O H follow ed by oxidation direct:1. D M A PO / D B U / C H 3C Ni. D M SO / A gBF 4RB r RD M SO / A gB F 4- M e 2SB ull Soc. Jpn., 1981, 54, 2221.TH L, 1974, 917.2. N aIO 4 / D M FO B r84 %150 oC , 40 m inN aIO 4 / D M F a new m ethod3. D M SO reagents:ii. D M SO / ZnSR CH B rM eR C (O )M eD M SO B rO H OO HZ nSJA C S, 2003, 68, 2480.RO44T H L , 1975, 4467.C C R C H O H RR C C H C C R R 'R C C HR C C ArR C C HC CRPhR C C Hsteric base, prevent N u attackn -B uL i: not M eL i, or t -B uL i, fire easilyR X : R -B r, R -T O S , R C H O , R C (O )Rn -B uL i / R 'C H O // A c 2O // K O t B uC lO M eN L iiii.ii. (Ph 3P)2PdC l 2, C uI, E t 2N H / PhIi. n-B uL i / R X6-b 6-a b c d e g 6-aCCC CCCsulfonic acid: PhSO 3H ; sulfinic acid: PhSO 2H ; sulfenic acid: P hS O Hiv. C uI, N aI, N a 2C O 3, RC C C H 2C lRCCHC lC H 2C C R 'RCCC H 2CCR 'Synthesis, 2000, 691.R C H 2-SO 2Ph R C C Hh fiR C H (C O 2H )-C H3-C(O )-C H 3OOOXC R R '=C H X5in fact: convert to C =C firstlyii. protect - deprotecti. m ove to term inal 6-c N H 2N H Kuse: K A P Ause: C o (C O )8 // F e(N O 3)3, E tO HJA C S , 1975, 97, 891.6-d use: i. B r 2 / C C l 4 // K O t B u6-euse: P b(O A c)4, L iC l // K O t B u // B r 2/C C l 4 // K O t B F e(N O 3)3: w eak oxidizing agentii. B r 2 // K O HJAC S, 1941, 63, 1180.P hP hP hP h66-fi. N aB H 4, H 3O +, B r 2, K O tB uii. N H 2O H , N aN O 2 / H 2S O 4 // A c 2O / D M A Piii. L D A , C lP O (O E t)2ON NOD M A P :4-N ,N -D i m ethyl a m inop y ridinem ixture ofA c 2O / D M A P :N NC H 3O6-guse: T sN H N H 2 / E tO H , heatT H L , 1967, 3943.3(l)G erm an invention, as acylating agentL D A : L i N (iP r)2, ignored a long tim e, re-introduced by M ichigan S tate U. becam e fam ous, appeared every w eek7H ORLiN H 2 / N H 3 (l)R Xuse: LiN H 2 / N H 3 (l) / R -XO C l6-h6-i.JAC S, 1958, 80, 4599.JAC S, 1955, 77, 3293.M ePhHO SO 2C F 3B uLiM e C C Phvia:M e CPhJO C , 1978, 43, 364.A rA r'HB r A rC C A r'N aO Etvia:A r A r'B ri. N aO E t (w hen X = B r)ii. B uL i (w hen X = -O SO 2C F 3)?8R C H =C H 2:P B uR C H 2C H 2-O -P B u 3R C H 2C H 2-O HP h-S e-P B u 3P h-S e-C Nm echanism :O A c C O 2M eO A cM eOM eO 2CN 2S eNO A cC O 2M eO A c M eO M eO 2Capplied for reactions: w ithout rearrangem ent; no regiosiom erC CHO HD ean-S tark(C O 2H )2 / benzeneO H hP hOC l C lC l C lOC l C lN C N COiii. P d-C ; or N i; P t, R hii. chloronaili. D D Q use base: D B Ni. C H 3I / A g 2O ii. H C H O / H C O 2H use: heatuse: heatuse base: i. R O N b7-i. p-T S O H.H 2O or C S Aii. w eak acid: H O A c; H C O 2H ; H 2C 2O 4iii strong acid: H iv. A rS eC N , P B use:C C H I C C HN H 2C CO C (S )S M e C C A c C C M s C C HO Ha7-i hg CCX C CC C CC HC O C C f e dcb a7--C(O )-C H 3C H -C HC H -C XC -O HjC X -C Y9N aI / Zn (C u)i. Zn / acetone i. C SC l 2/C CO M s O M s C C B rB rC C O H O Hc7-C C O HIii. C SC l 2 / P(O M e)3P NN PO C l 3 / py // Snvia:C C IIapplication: i. protect alkene: via B r 2 // ZnC C C C C 36 o C C C C C =C 31 o C C C C C C ll148 o CSR O R O RC C B rO A cZn / H O A cOA cO A cOA cOB rO A cZn OO A c O A cO A cJO C , 1978, 43, 364.HO A cJC S.C C , 1998, 2113.ii. In / M eO Hii. purify com pound10d7-e7-i. W C l 6 / R L i ii. L iP Ph 2 / C H 3I product retention product inversionN aR C C HC H 2C H 2C H 2O H OC lRiii. N a(special structure):7-d.7-d.SR 1R 2R 1R 2(E tO )3Puse: (E tO )3PSynthesis , 1977, 1134.117-f.2not for W ittig, ylid unstableJO C , 1978, 43, 3253.JA C S, 1974, 96, 4706.C hem. Lett, 1973, 1041.T iC l 3-L iA lH 4 / T H F T iC l 3 / M g T iC l 4 / Z n T iC l 4 / Kii. M cM urry C ouplingi. use: N 2H 4 / H 2S / Pb(O A c)4B A SF, 1973, 2147.via:Z n-C uP(O E t)1. H 2S2. Pb(O A c)43. H 3O 1. H 2S2. Pb(O A c)4OON SN N NOSN NSNONNNSNN NOOSO ONOOO OOT iC l 3N 2H 412via : betaine, oxaphosphetane (N M R )expensivedifficult to prepareOE tC NPPh 3CNPPh 3HO PPh 3OC O 2M e+notPh 3PC H E tH COC O 2M enotPh 3P C H C O 2M eE tH O ++++stable ylid gives trans (E )unstable ylid gives cis (Z )w ater soluble, rem oved by extraction(com parison: O =PPh 3 highly soluble in organic solvent)use:L i Ph S ONM eCH 2// A l (H g)M e 3SiC H R -L i +Ph 3SiC H 2-L i +=== Ph 3SiC H 2B r + n-B uL i (exchange)M e 3SiC -H -M gB r === M e 3SiC H 2C l + M g (m etal reduction)Ph 3SiC -H C H 2Ph === Ph 3SiC H =C H 2 + PhL M e 3SiC -H C O 2E t === M e 3SiC H 2C O 2E t + L i (m etalation)M e 3SiC H =PPh 3 === M e 3SiC H 2PPh 3+ X - + K HR O = M eO -, E tO -use: (R O )2PO -C H R 'use: Ph 3P-C H R 'vi. Sulfoxim ide (Johnson C.)iii. Silyl W ittig R eaction (Peterson R eaction)ii. Phosphonate W ittig R eaction (H orner-Em m ons M odification)i. W ittig R eaction7-f7-f.Synthesis, 1984, 384.TH L, 1981, 2751.JO C , 1968, 33, 780.iv. C H 2(ZnI)2C hem. Lett, 1995, 259.Synlett, 1988, 12, 1369.H 2C H 2(ZnI)2v. C H 2C H B r 2, Sm , SnI 2 / C rC l 3, TH FR O Rvii. G rignard reagent:1. T M SC H M gC l use: TM SC H 2M gC lTH L, 1973, 3497.TH L, 1988, 4339.2. N aO A c, A cO Hm ethylenationR R 'e 3H advantages over the W ittig:1. by-products are m ore easily rem oved,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discoverg 7-form trans alkene: form cis alkene:i. Li / N H3; or other IA m etalsii. Li / EtN H2iii. L iA lH4 / TH Fi. H2 / N i2B (P-2 catalyst)ii. H2 / Pd-C aC O3 (Lindlar catalyst)iii. H2 / Pd-B aSO4iv. B2H6 / H O A c (D iborane)v. N2H2vi. H C H O / Pd-C / E t3Nnot use H2 / Pt: m ight convert to alkaneh7-all form trans alkene:i. R2B H / B r-C N (hydroboration)C C HRHHii. D IB A L / n-B uLi / C H3I (hydroalum ination) iii. C p2ZrC lH / R X (hydrozirconation)13application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=CC=C C=CC=CC CC CH O Hnot for double bond m ight m oveM nO2 / Ph3P C H3B r- / M TB DNNNC H3M TB Dvia diradicalJAC S, 1998, 100, 877.Ph Ph Ph7-i7-j148-a8-a.28-a.38-a.41. H I3. T sC l / C62. P I3JC S, 1905, 87, 1592.C H O H C H IP I38-a.12. F3S-N E t21.(D A S T)SNSFO OOOC h em. R ev., 1996, 96, 1737.C H C l2FC H3SOOO HO NC H C l2C H31. C F C H F C F N E t2. H O A c / i P rO H$ 65 / 500 g$ 80 / 50 gP B r3P I3$ 35 / 1000 gP B r3$ 500 / 125 gJO C, 1993, 58, 3800.8-C-XC-O H C(O)ZdcbaC-N H2C=O2. P P h3 / I2e C-H15168-d8-d.1R C OO H1. A gN O 3/K O H2. B r 2R B r B er. 1942, 75, 296.8-d.2C lOClR hC l(PPh 3)38-bN aN O 2 / H C l / H B F 4 /for arom C hem R ev., 1956, 56, 219.8-cC F 2B r 2 / ZnFF 58 %JC S.PT I, 1993, 335.178-e8-e.1I86 %I 2 / H N O 3JA C S,1917, 39, 437.8-e.3I 2 / H N O 3PhC H 2C (O )C H 3PhC H C (O )C H 3F N S FOOF +C hem. R ev., 1996, 96, 1737.F-TEDA-B F4 JC S.C C , 1994, 149.F 2-N 2 / C FC l 3-C H C l 3JO C , 1988, 53, 2803.90 %ada m anta ne1. regioselective fluorination at the m ore substituted positions2. electrophilic in natureF -N C FCl 3-C HCl 3OO NXR 1R 3OOR 2HM g(C lO 4)2R 1R 3O OR 2XN B X / M g(C lO 4)2JO C , 2002, 67, 7429.8-e.2X = C l, B r, IX = C l, B r, IN B XN B X :i.ii.iii.iv.RRF R = C H 3C O , C O C F 3, C C l 3, N O 2 H F / electrolysis1.4-1.6 Valready industrilizedN F 3O / TB A H / C H 3C Nv.T B H A : Tetrabutylam m onium hydroxideTH L , 2003, 44, 2799.189-a9-C -C H 3C -Xa (C H 3)3AlM e 3A l98 %O rganom et. C hem. R ev., 1996, 4, 47.C H 2C l 2bridgehead m ethylation。
04第四章 有机官能团的转换34

在SN1反应中,决定速度步骤不含亲核试剂,所以亲核试剂 对SN1反应影响很小。与SN1反应不同,亲核试剂包括在SN2 反应的决定速度步骤内,所以亲核试剂的浓度、性质对 SN2反应是重要的。一般亲核试剂的亲核性越强, SN2反 应的活性越高。 一些亲核试剂,例如NO2-,其分子中的O和N都可作为亲核中 心与中心碳反应,这类试剂称为两可亲核试剂。在不同条 件下,两可亲核试剂可在不同位臵发生反应,生成不同的 产物。例如,在乙醚中,NO2-与伯卤代烷作用生成硝基烷, 与仲或叔卤代烷作用生成亚硝酸酯。 RCH2Br + NO2- RCH2NO2 + Br- (SN2) R2CHBr + NO2- R2CHONO + Br- (SN1)
SN1反应是分步进行的。作用物首先离解为碳正离子,这一步是 慢反应;然后亲电性很强的碳正离子迅速与溶液中的亲核试 剂结合成产物。反应速度由第一步控制,其动力学式符合一 级速度表示式: V = k1[RX]
碳正离子是平面型,亲核试剂可从平面的两边进入,从而使产 物发生消旋化。但在SN1反应中,生成的碳正离子中间体并不 是完全自由的,它很可能与离去基组成离子对,使其溶剂化, 而不完全对称,从而有可能使试剂从离子对背面进攻占优势, 结果使部分产物反转。例如
8
偶极非质子溶剂由于偶极正端包藏于分子内部,对负离子 的溶剂化作用很小,所以亲核试剂一般可不受溶剂层的包围而 呈所谓的“裸露”状态,从而表现出很强的亲核活性,有利于 SN2反应的进行。 溶剂极性对SN反应的影响还与过渡态的电荷状态有关。中 性的作用物发生SN1反应时,过渡态的电荷发生分离,即过渡 态的极性增大,这时极性较高的溶剂比较低的溶剂更能降低过 渡态的能量和增加反应速度。相反,正离子作用物进行SN1反 应时,其过渡态的电荷比基态分散,这时极性较小的溶剂更为 有利。同样的规律也适合SN2反应。因为SN2反应过渡态的电 荷也比基态分散,增加溶剂极性只会使电荷集中的亲核试剂溶 剂化,而不利于SN2过渡态的形成。 至于非极性溶剂,由于较难溶解SN反应中极性的作用物和 试剂,其反应性能较低,所以很少使用。
常见官能团的确定和转化关系

7、请认真阅读下列3个反应: 、请认真阅读下列 个反应 个反应:
利用这些反应,用四步从甲苯合成一种染料中间体 利用这些反应,用四步从甲苯合成一种染料中间体DSD 酸
请用合成反应流程图表示你的合成路线。 请用合成反应流程图表示你的合成路线。 标出反应物和反应条件) (标出反应物和反应条件)
6、A为有机合成中间体,在一定条件下发生消去反应,可能得 、 为有机合成中间体 在一定条件下发生消去反应, 为有机合成中间体, 到两种互为同分异构体的产物,其中的一种B可用于制取合成树 到两种互为同分异构体的产物,其中的一种 可用于制取合成树 染料等多种化工产品。 能发生如下图所示的变化 能发生如下图所示的变化。 脂、染料等多种化工产品。A能发生如下图所示的变化。试回答
一、常见有机官能团的确定
1.根据反应物性质确定官能团 根据反应物性质确定官能团
反应物性质
反应生成CO2、与新制 与NaHCO3反应生成 Cu(OH)2反应得到蓝色溶液
可能官能团 羧基 羧基、 羧基、酚羟基 羧基、酚羟基、 羧基、酚羟基、醇羟反应 能与Na反应 反应 与银氨溶液反应产生银镜或与 新制氢氧化铜产生红色沉淀 能使溴水褪色 加溴水产生白色沉淀、 加溴水产生白色沉淀、遇Fe3+ 显紫色
(3)C→D的反应类型是 的反应类型是____,E→F的反应类型是 的反应类型是_____ 的反应类型是 , 的反应类型是 a.氧化反应 b.还原反应 c.加成反应 d.取代反应 . . . . (4)写出化学方程式:A→B______________________。 写出化学方程式: 写出化学方程式 。 (5)写出 生成高聚物的化学方程式: 写出E生成高聚物的化学方程式: 写出 生成高聚物的化学方程式 ____________________________________________。 。 (6)C的同分异构体 l与C有相同官能团,两分子 l脱去两分 的同分异构体C 有相同官能团, 的同分异构体 有相同官能团 两分子C 子水形成含有六元环的C 写出C 的结构简式: 子水形成含有六元环的 2,写出 2的结构简式: ______________________________
第二章 官能团化和官能团转化的基本反应

2 与卤化磷和亚硫酰氯反应
3ROH + PX3(P + X2) 3R-X + P(OH)3 X = Br 、 I ( 制备溴代或碘代烃) ROH + PCl5 ROH + SOCl2 R-Cl + POCl3 + HCl R-Cl + SO2 + HCl 制氯代烃
此反应产物纯净
3 与酸反应(成酯反应)
为什么简单杂环化合物的亲电取代反应一般发生在α位?
+
进攻2位
E X H E
+
E X E H X H
+ X
E H
X
+ E
+
进攻3位
H X
+
2、加成-加H2
X H2/Ni X X= O 、S、NH (THF是良好的溶剂,b.p65℃)
3、呋喃的共轭二烯性质 (吡咯、噻吩较难)呋喃芳香性最差
O
O 30℃
Br
Cl2
N H
-40℃
N H
Cl
Br2 S CH3COOH, 0 ℃ S Br
(2) 硝化
+ HNO3 N H (CH3COO)2O 5℃ N H 83% NO2 + NO2 N H 7%
(3)磺化:吡咯,呋喃对强酸敏感,需要较缓和的磺化剂, SO3-吡啶
SO3 C5H5N O O SO3H
活泼性:吡咯>呋喃>噻吩>苯
构效关系
1 烷烃的官能团化
结构特点: C:SP3杂化 正四面体 σ键
碳氢键极性较小
(特殊条件) 碳氢键断裂: 氢原子被取代
自由基机理:
Cl CH 3 33.5% 22% 28% 16.5% CH 3 CH 3 CH 3CHCH 2CH 3 Cl 2 光 CH 3-C-CH2CH 3 Cl CH 3CH CHCH 3 CH 3 Cl CH 3CHCH 2CH 2 CH 3 Cl CH 2-CHCH 2CH 3
第五章 官能团转换(1)

H SO Me M 2 4 Me Me HO OH OO Me M Et O, rt O O Me Me 2 O O
5.3 由烯合成醇
12
由烯烃生成醇的反应,一般在酸性条件下进行,且符合Markovni kov规则。但在强酸条件下,对很多基团不稳定,所以实验室一 般采用以下方法合成醇。
5.3.1 硼氢化氧化(反马氏规则)
反应实例:Kono, H.; Hooz, J. Org. Synth., Coll. Vol. VI 1988, 919.
13
C H NaOH 6 13 B O H C H H 2 2 6 13 C 6 13 aq
q N a O H OR a B D ROOR
Me
OH
A: 通过一个四元环电子移动进行硼氢化反应。B: 过氧化氢负离
子进攻硼烷形成一个硼酸盐。C: 烷基迁移生成硼酸酯。D: 水解
生成末端醇。
14
5.3.2 羟汞化脱汞(符合马氏规则)
机理?
机理解答:
M e M e OO M e B HO OH M e M e OO M e HO
7
M e M e
H A
+ H
OH
M e M e OO M e O OH
M eO
OM e
M e M e O M e M e O
A: 异丁烯质子化形成一个稳定的叔碳离子。B: 羧酸的孤对电子 进攻碳正离子形成单酯,然后继续反应生成二酯。
官能团转化全现用图解

i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX Functional Group InterconversionC=CC C C=CC C RCH 2-SO 2Ph RC CHC C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Zd c b ae d c b af C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3OOOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e d c b a C-CHO C-CO 2HC-CN C=C C=O C-S C-NH 2C-X C-OH C-HCH 3CH3CH 3H n -Bu 3SnH C S O PhCl RCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3Ph PhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 22via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.CHCl 2rt, 3 hr1-b Bu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)H 2OMg / Et O JOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOHZn; Fe; Sn; Mg(3). metal reduction (2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973,654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney NiBuLi1-d1-c RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2H PO Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -2p-TsClp-TsCl2(3).Ar-NH 2Ar-H1-e(2). thioketal:(3). Wolff-Kishner reduction:(6). enol derivatives:SHSH/ BFTf2similar:(4). Pd-C / HCO2NH4(7). Et3SiH / CF3COOH1-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me2N)3P=O(3). organic electrochemistryβ-CO(1). particular structure:1-g(1). K / Al2O3K / HMPA(2). Na / NH31-h(2). normal structure: SOCl2JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me2N)3P=Oyes for white mouse, uncertain for humanmodified to: N NO1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROORR OROR RCH 2 OCH 2CH 2OH(3). AlCl 3 / LiAlH 4(2). HCl / NaBH 3(CN)(1). h / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2ORC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t BuRC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with baset -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl(5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X application: for protecting groupe d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3t-BuORaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-e 2-e.vi. H 2O 2, t -BuOH, MnSO 4 // NaHCO 3, pH 8JA CS, 2001, 123, 2933.HO 22COnew, cheap,, simple, green chemistryconvenient, inexpensive, powerful.JOC, 1980, 45, 4758.JOC, 1982, 47, 2670.OOHOOBr via:Br 2 / ROH2-f ROH / HClEtCNEt C OEtOEtOEtHClJA CS, 1942, 64, 1825.JOC, 2001, 66, 521.C-OHC-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2Ph = RC-OBZl = RC-OBnRC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH2Ni. TMSIii. BF 3-Et 2O // R-SH (or HS-CH 2CH 2-SH)iii. BBr 3 / CH 2Cl 2, 0-10 C / LiI, heatvi.OCH 3OH+- CH 3Cl i. H 2 / Pd-C ii.CN CN Cl ClO, OH-OH-O COCH 3O O CH 2OCH 3RhCl(PPh 3)3, H 3O +OH - Me 32Oi. TFA (CF 3CO 2H)ii. HBr / HOAc iii. TMS-Ii. HOAc: weak acid: good leaving groupii. H 2need stronger acidi. F - : HF, Py-H + F -; n +--SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+JOC, 1973, 38, 3224.iv. AlCl 3 / RSH THL, 2001, 42, 9207.MeOCO 2Me HOCO 2MeCH 3(CH 2)11SHodorless3v./ heatCl -N H3-b NH Cl -triphenylmethylorganic base: TMG3-c(1). OH -(2). KO 2 / DMSO 3-d not practically useful: R-OH cheaper than R-XJOC, 1975,40, 1678.(2). Na 2[Fe(CN)5(NO)] / K 2CO 3 / H 2O3-e(1). Symmetry:ketal: use H 3O +acetal: use H 3O +(2). unsymetry:RO-MOM RO-MEM RO-MTM RO-THPi. H 3O +p -TsOH / MeOHi. H 3O +; ii. ZnBr 2 / CH 2Cl 2HgCl 2 / CH 3CN (aq.)actually, acetal exchange (3). Ag 2O / H 2OTHL, 1975, 3183.JOC, 1986, 51, 3913.RO 2C (CH 2)3NH 2RO 2C (CH 2)3OHNa [Fe(CN)(NO)]2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversion Synthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH acetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOHNaAlH 2(OCH 2CH 2OCH 3)2C 6H 6, r.t.hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.JACS, 1972, 94, 7159.LAH ------------ almost all: ald, ketone, acie, ester, acyl X, anhydrideNaBH4 --------------- not for acid, ester (but LiBH4 work for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:LiAlH4; NaBH4; Na / NH3Al (O i Pr)3 / i PrOH ----------- Meerwein-Pondorf-Verley rxnIrCl4 / i PrOH / P(OMe)3 ------ Henbest rxnLiBH(sec Bu)3 ------------------ H. C. Brownfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). HCHO reagent:Me CHO MeOHHCHOKOHJACS, 1935, 511, 903.CH3CHO C(CH2OH)42Org.Syn, 1925, 4, 53.HCHO / KOHHCHO / Ca(OH)2Synthesis, 1994, 1007.PhNO2OPhNO2H OHBH / SMeJOC, 2001, 66, 7514.JOC, 2003, 68, 2030.OBH3 / THF99.5 % transsolvent: THF, SMe23-iR3B, HOCH2CH2OH // H2O2 // NaOHJOC, 1986, 51, 4925.C O RRR3BRRRRR3C B OHOCH2CH2OHR3C BOO H2O2R3BOOO HO BOOR3CH2OR3C OHpractice3-k OOHOHOHOHOOHOHOHOHOHJOC, 1967, 32, 3452.H 2O2: dangerous,skin whiten, metal decomposeHg (OAc)2: toxic, hard to remove (3). B 2H 6, H 2O 2 / OH -, H 2O(2). Hg(OAc)2, H 2O // NaBH 4(1). H 3O +3-j3-j.13-j.2hydration:(1). KMnO 4 / NaOH (2). OsO 4(3). H 2O 2/HCO 2H (4). Na / EtOHcis tran cis +trancis3Me2NNN CH3HCl3hνN CH3HHN CH3HH+NCSN CH3ClNHCHC2R3C NH2R C NR2R C NHRR3C OHR2C OHRC OHR C NH2tertiarysecondaryprimaryCompare nomenclature class:not a very useful reactionC-NC-HC-NC-XC-OHC=OC=C4-abcdefg4-a SO2NH2Ph I OAcOAc SOONHSOON I Ph Fe(TPP)Cl SOONH2(insertion)TPPNNNNPh2. NaN3CON N N N C O1. SO2Cl2CO2CCO OhiC NC(O)XC-C(O)XNH 22RC NO 2RC N 3RC N Me RC N CPh RC N CPh 3RC NH 2RC NH 2RC NH 2RC NH 2RC NH 2iiiiiiRC N C O OtBu RC NC O OPhRC NH 2RC NH 24-b CF 3CO 3H // Fe / HOAc1. many reducing agents1. NaBH 4;2. Al (Hg)H 2O 2 // Ac 2O, heat / H 3O +H 2 / Pd-C1. HOAc;2. H 2 / Pd-C1. TFA;2. HCl H 2 / Pd-C4-b.14-b.22. organic electrochemistry1.2.3.4.Fe 3(CO)12 / CH 3OH JOC, 1972, 37, 930.NaBH 4 / Pd-C Na 2S Sn / HCl Vogel's 12.57Vogel's 12.58Vogel's 12.59NO 2OCH 3NH 2OCH 3Eg-Ni rt. 15 hrJOC, 1999, 64, 2301.Eg-Ni: electrogenerated nickel5.H 2 / Pt (S)-CJACS, 1965, 87, 2767.sulfided platium not affect: aromatic rings, ketones, halides, nitriles, amide, eastersJACS, 1933, 55, 4579.2HCHO NMe 2CO 2EtNH 2CO 2EtRC NCC NR C C RC NH 2iC N R N N+-C N R R'ii 1. HCHO / HCO 2H 1. RBCl 2 / base1. HC(OEt)3 // NaBH 4;2. R 2CO // NaBH 3CN NH 2N CH 3CH 3HCHO N 3NHBCl 2NH 2COOHN COOHH CH 3HC(OEt)NaBH 4b.3 2. HCHO // H 2 / Pd-CN 3NO 2MeO 2CNaBH 422rt NH 2NO 2MeO 2CSynthesis, 1979, 537.mild conditionhigh yieldnot affect:: NO 2, C=C, CN, COOR, COOH2. NaBH 4 / CoCl 2-6H 2Onot good, usually contain polyalkylation products2. Delepine3. NaN 3 / RED4-d4-c 5. Unpolung4. NaN 3 / RED3. Delepine2. Gabriel:1. NH 3N OO K N 2H 42Oi. LAH, NaBH 4ii. H 2 / catiii Zn / HCl; Al (Hg)i. Mg // NH 2Clii. Mg // PhSCH 2-N 3commercial available, tetramer of Me 3N24. CBr 4, PPh 3, NaN 3, DMF // PPh 3 / THFJOC, 2000, 65, 7110.乌洛托品)methenamine (六甲烯胺)hexamethylenetetramine (环六亚甲基四胺)内服后遇酸分解出 HCHO,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.R C O NH 2R C O NIPh OAcR N C OR NH C OOCH 3CH 3OHPhI, OAcPhI(OAc)24-i.2C O NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2hC-NH2C-NO2C NC C5-agfdcba5-C=C-ORC=C-SRC-OHC=N-OHC=N-HC=SC=OC=Ov. via: epoxysilaneR COCRR COCH2R242H3O+CO23OOBr2-HBr4OONaBH3H3O+ZnTsNHNH2MeLi TMSCl MCPBA LAH24CH2CORRCH2CORR3SCH2CORRPhCHOi. via:α-CO2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherR COCH2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAeij C-Brk C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-C N5-e.25-e.3-CH 2-C O HR -CH -C O HR -CH -C OR"R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3PDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +5-iPh OPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +OO5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-j 5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHhf i RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:MeCPhJOC, 1978, 43, 364.ArAr'HBr Ar C C Ar'NaOEtvia:Ar Ar'Bri. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb7-i. p-TSOH.H 2O or CSAii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C CH OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C CCC CCHC O C Cf e dc b a 7--C(O)-CH 3CH-CHCH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrC C OH OHc 7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C I Iapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcOAcOAcOBrOAcZn OOAc OAcOAc JOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.SR 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CN3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH 2MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)31. H 2S 2. Pb(OAc)431. H 2S 2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metalsii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh 7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNN3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)S N S F O OOOChem. Rev., 1996, 96, 1737.2FCH 3SOO OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 2$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-cCF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SF OOF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N CFCl 3-CHCl 3ON XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFHFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 V already industrilizedNF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.。
官能团转化全图解

官能团转化全图解Lemsay 第 2 页 2021-3-8Lemsay 第 3 页 2021-3-8Lemsay第 4 页 2021-3-8i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX Functional Group InterconversionC=CC C C=CC C RCH 2-SO 2Ph RC CHC C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Z dc b a ed c b a f C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3OOOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=OLemsay第 5 页 2021-3-81-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e d c b a C-CHO C-CO 2HC-CN C=C C=O C-S C-NH 2C-X C-OH C-HCH 3CH3CH 3H n -Bu 3SnH C S O PhCl RCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3Ph PhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 22via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.2rt, 3 hrLemsay第 6 页 2021-3-81-b Bu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)H 2OMg / Et 2O JOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOHZn; Fe; Sn; Mg(3). metal reduction (2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)Lemsay第 7 页 2021-3-8mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973,654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney Ni1-d1-c RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2H PO Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -Ag Op-TsClNaHp-TsCl2(3).Ar-NH 2Ar-H1-e(2). thioketal:(3). Wolff-Kishner reduction:(6). enol derivatives:SHSH/ BFTf2similar:(4). Pd-C / HCO2NH4(7). Et3SiH / CF3COOHLemsay 第 8 页 2021-3-81-fLemsay 第 9 页 2021-3-8Lemsay 第 10 页 2021-3-8b.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me2N)3P=O(3). organic electrochemistryβ(1). particular structure:1-g(1). K / Al2O3K / HMPA(2). Na / NH31-h(2). normal structure: SOCl2JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me2N)3P=Oyes for white mouse, uncertain for humanmodified to: N NO1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROORR OROR RCH 2 OCH 2CH 2OH(3). AlCl 3 / LiAlH 4(2). HCl / NaBH 3(CN)(1). h / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2ORC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t BuRC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with baset -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl(5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X application: for protecting groupe d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3t-BuORaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-e2-e.vi. H 2O 2, t -BuOH, MnSO 4 // NaHCO 3, pH 8JA CS, 2001, 123, 2933.HO 22COnew, cheap,, simple, green chemistryconvenient, inexpensive, powerful.JOC, 1980, 45, 4758.JOC, 1982, 47, 2670.OOHOOBr via:Br 2 / ROH2-f ROH / HClEtCNEt C OEtOEtOEtHClJA CS, 1942, 64, 1825.JOC, 2001, 66, 521.C-OHC-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2Ph = RC-OBZl = RC-OBnRC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH2Ni. TMSIii. BF 3-Et 2O // R-SH (or HS-CH 2CH 2-SH)iii. BBr 3 / CH 2Cl 2, 0-10 C / LiI, heatvi.OCH 3OH+- CH 3Cl i. H 2 / Pd-C ii.CN CN Cl ClOO, OH-OH-O COCH 3O O CH 2OCH 3RhCl(PPh 3)3, H 3O +OH 32Oi. TFA (CF 3CO 2H)ii. HBr / HOAc iii. TMS-Ii. HOAc: weak acid: good leaving groupii. H 2need stronger acidi. F - : HF, Py-H + F -; n +--SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+JOC, 1973, 38, 3224.iv. AlCl 3 / RSH THL, 2001, 42, 9207.MeOCO 2Me HOCO 2MeCH 3(CH 2)11SHodorless3v./ heatCl -N H3-b NH Cl -triphenylmethylorganic base: TMG3-c(1). OH -(2). KO 2 / DMSO 3-d not practically useful: R-OH cheaper than R-XJOC, 1975,40, 1678.(2). Na 2[Fe(CN)5(NO)] / K 2CO 3 / H 2O3-e(1). Symmetry:ketal: use H 3O +acetal: use H 3O +(2). unsymetry:RO-MOM RO-MEM RO-MTM RO-THPi. H 3O +p -TsOH / MeOHi. H 3O +; ii. ZnBr 2 / CH 2Cl 2HgCl 2 / CH 3CN (aq.)actually, acetal exchange (3). Ag 2O / H 2OTHL, 1975, 3183.JOC, 1986, 51, 3913.RO 2C (CH 2)3CHRNH 2RO 2C (CH 2)3OHNa [Fe(CN)(NO)]2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversion Synthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH acetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOHNaAlH 2(OCH 2CH 2OCH 3)2C 6H 6, r.t.hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.JACS, 1972, 94, 7159.LAH ------------ almost all: ald, ketone, acie, ester, acyl X, anhydrideNaBH4 --------------- not for acid, ester (but LiBH4 work for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:LiAlH4; NaBH4; Na / NH3Al (O i Pr)3 / i PrOH ----------- Meerwein-Pondorf-Verley rxnIrCl4 / i PrOH / P(OMe)3 ------ Henbest rxnLiBH(sec Bu)3 ------------------ H. C. Brownfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). HCHO reagent:Me CHO MeOHHCHOJACS, 1935, 511, 903.CH3CHO C(CH2OH)42Org.Syn, 1925, 4, 53.HCHO / KOHHCHO / Ca(OH)2Synthesis, 1994, 1007.PhNO2OPhNO2H OHBH / SMeJOC, 2001, 66, 7514.JOC, 2003, 68, 2030.OBH3 / THF99.5 % transsolvent: THF, SMe23-iR3B, HOCH2CH2OH // H2O2 // NaOHJOC, 1986, 51, 4925.C O R BRR3BRRRRR3C B OHOCH2CH2OHR3C BOO H2O2R3BOOO HO BOOR3CH2OR3C OHpractice3-k OOHOHOHOHOOHOHOHOHOHJOC, 1967, 32, 3452.H 2O 2: dangerous,skin whiten, metal decomposeHg (OAc)2: toxic, hard to remove (3). B 2H 6, H 2O 2 / OH -, H 2O(2). Hg(OAc)2, H 2O // NaBH 4(1). H 3O +3-j3-j.13-j.2hydration:(1). KMnO 4 / NaOH (2). OsO 4(3). H 2O 2/HCO 2H (4). Na / EtOHcis tran cis +trancis3Me2NNN CH3HCl3hνN CH3HHN CH3HH+NCSN CH3ClNHCHC2R3C NH2R C NR2R C NHRR3C OHR2C OHRC OHR C NH2tertiarysecondaryprimaryCompare nomenclature class:not a very useful reactionC-NC-HC-NC-XC-OHC=OC=C4-abcdefg4-a SO2NH2Ph I OAcOAc SOONHSOON I Ph Fe(TPP)Cl SOONH2(insertion)TPPNNNNPh2. NaN3CON N N N C O1. SO2Cl2CO2CCO OhiC NC(O)XC-C(O)XNH 22RC NO 2RC N 3RC N Me RC N CPh RC N CPh 3RC NH 2RC NH 2RC NH 2RC NH 2RC NH 2iiiiiiRC N C O OtBu RC NC O OPhRC NH 2RC NH 24-b CF 3CO 3H // Fe / HOAc1. many reducing agents1. NaBH 4;2. Al (Hg)H 2O 2 // Ac 2O, heat / H 3O +H 2 / Pd-C1. HOAc;2. H 2 / Pd-C1. TFA;2. HCl H 2 / Pd-C4-b.14-b.22. organic electrochemistry1.2.3.4.Fe 3(CO)12 / CH 3OH JOC, 1972, 37, 930.NaBH 4 / Pd-C Na 2S Sn / HCl Vogel's 12.57Vogel's 12.58Vogel's 12.59NO 2OCH 3NH 2OCH 3Eg-Ni rt. 15 hrJOC, 1999, 64, 2301.Eg-Ni: electrogenerated nickel5.H 2 / Pt (S)-CJACS, 1965, 87, 2767.sulfided platium not affect: aromatic rings, ketones, halides, nitriles, amide, eastersJACS, 1933, 55, 4579.2HCHO NMe 2CO 2EtNH 2CO 2EtRC NCC NR C C RC NH 2iC N R N N+-C N R R'ii 1. HCHO / HCO 2H 1. RBCl 2 / base1. HC(OEt)3 // NaBH 4;2. R 2CO // NaBH 3CN NH 2N CH 3CH 3HCHO N 3NHBCl 2NH 2COOHN COOHH CH 3HC(OEt)NaBH 4b.3 2. HCHO // H 2 / Pd-CN 3NO 2MeO 2CNaBH 422rt NH 2NO 2MeO 2CSynthesis, 1979, 537.mild conditionhigh yieldnot affect:: NO 2, C=C, CN, COOR, COOH2. NaBH 4 / CoCl 2-6H 2Onot good, usually contain polyalkylation products2. Delepine3. NaN 3 / RED4-d4-c 5. Unpolung4. NaN 3 / RED3. Delepine2. Gabriel:1. NH 3N OO K N 2H 42Oi. LAH, NaBH 4ii. H 2 / catiii Zn / HCl; Al (Hg)i. Mg // NH 2Clii. Mg // PhSCH 2-N 3commercial available, tetramer of Me 3N24. CBr 4, PPh 3, NaN 3, DMF // PPh 3 / THFJOC, 2000, 65, 7110.urotropine (乌洛托品)methenamine (六甲烯胺)hexamethylenetetramine (环六亚甲基四胺)内服后遇酸分解出 HCHO,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.R C O NH 2R C O NIPh OAcR N C OR NH C OOCH 3CH 3OHPhI, OAcPhI(OAc)24-i.2C O NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2hC-NH2C-NO2C NC C5-agfdcba5-C=C-ORC=C-SRC-OHC=N-OHC=N-HC=SC=OC=Ov. via: epoxysilaneR COCRR COCH2R242H3O+CO23OOBr2-HBr4OONaBH3H3O+ZnTsNHNH2MeLi TMSCl MCPBA LAH24CH2CORRCH2CORR3SCH2CORRPhCHOi. via:α-CO2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherR COCH2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAeij C-Brk C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-C N5-e.25-e.3-CH 2-C O HR -CH -C OHR -CH -C OR"R'X / n -BuLiCH 3I R''MgBr H 3O +3.OHH 3O +OH OH5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3PDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +5-iPh OPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +OO5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-j5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHhf i RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:MeCPhJOC, 1978, 43, 364.ArAr'HBr Ar C C Ar'NaOEtvia:Ar Ar'Bri. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatMCPBA RCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Capplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 2use: heatuse: heatb7-i. p-TSOH.H 2O or CSAii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C CH OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C CCC CCHC O C Cf e dc b a 7--C(O)-CH 3CH-CHCH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrC C OH OHc 7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C I Iapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAc JOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compound。
有机物衍生关系及官能团的引入和消除ppt课件

性水解;③醛的加氢还原;④酮的加氢还原;⑤酯的酸 性或碱性水解;⑥苯氧离子与酸反应;⑦烯烃与HO– Cl的加成。 (5)醛基或羰基的引入方法:①烯烃的催化氧化;②烯 烃的臭氧氧化分解;③炔烃与水的加成;④醇的催化氧 化。 (6)羧基的引入方法:①羧酸盐酸化;②苯的同系物被 酸性高锰酸钾溶液氧化;③醛的催化氧化;④酯的水解; ⑤-CN的酸性水解;⑥多肽、蛋白质的水解;⑦酰氨 (–CONH2)的水解。
有机合成路线选择的基本要求
1.
原理正确、步骤简单(产率高)
2.
3. 4.
原料丰富、价格低廉(成本低)
条件合适、操作方便(能耗少)
产物纯净、污染物少(易分离)
有机合成题的解题思路
首先确定要合成的有机物含什么官能团,它属于哪
一类有机物。然后对比原料,看是否有官能团的引 入、消除、衍变及碳链增减。 据有机物结构将其划分为“若干部分”,据此确定 合成原料——反应物。 读懂并利用信息,据1、2选择正确的反应及合成步 骤。
(3)芳香化合物合成路线
5、官能团的衍变
(4)改变官能团的位置
消去 加成 CH CH CH Br CH CH CH CH C H CH 3 2 2 3 2 3 3 HBr HBr | Br
(5)官能团数量的转化
C2H5OH→C2H4 → CH2ClCH2Cl→CH2OHCH2OH
R
R
据以上信息,以苯、溴 、石油产品为原料合成
CH CH3 OH
然后由此合成
O C
高考题
P275,2008北京
⑴羟基 ⑵CH2=CH-CH2-OH ⑶
⑷①ac ②ClCH2COOH取代反应③
第二章官能团化及转换

②取代芳环上的取代反应
CH3
H3C
+CO+HCl CH3
AlCl3
CH3CHO
H3C
CH3 80%
H O
O H
H C l
+ C H 3C N Z n C l2 H O
O H C N H 2C l- H 2 OH O C H 3
O H O
C C H 3
70 %
现在学习的是第10页,共22页
③芳环侧链上的反应
.
R C HC HC H 2
NBS
B r
B r
R C HC HC H 2+R C HC H C H 2
现在学习的是第6页,共22页
◆ N-溴代丁二酰亚胺(NBS)在光催化条件下,可使多种甾
烯的亚甲基氧化,具有良好的区域选择性.
H 3C CO O
hv/N BS/CaC O 3 TH F/H 2O
H 3C CO O
现在学习的是第2页,共22页
2.1.1 烷烃的官能团化
◆烷烃对亲电试剂和亲核试剂都不活泼,但在自由基反 应中,特别是在卤化反应里,烷烃却很活泼,由于这 些反应难以控制,其合成应用受到限制。
H3C
CH3 CH CH3
Br2
300 o C
CH3 H3C C Br
CH3
Cl2
300 o C
CH3 H3C C Cl +
CH3
CH2 Cl H3C C H
CH3
CH3
Cl2
CH3
H3CCCH3 紫 外 光 H3CC CH2Cl
CH3
CH3
注意伯、仲、叔氢的活泼性及碳自由基的稳定性.
现在学习的是第3页,共22页
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX Functional Group InterconversionC=CC C C=CC C RCH 2-SO 2Ph RC CHC C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Zd c b ae d c b af C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3OOOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e d c b a C-CHO C-CO 2HC-CN C=C C=O C-S C-NH 2C-X C-OH C-HCH 3CH3CH 3H n -Bu 3SnH C S O PhCl RCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3Ph PhPhOHPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 22via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.2rt, 3 hr1-b Bu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)H 2OMg / Et 2O JOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOHZn; Fe; Sn; Mg(3). metal reduction (2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973,654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney Ni1-d1-c RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2H PO Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -Ag Op-TsClNaHp-TsCl2(3).Ar-NH 2Ar-H1-e(2). thioketal:(3). Wolff-Kishner reduction:(6). enol derivatives:SHSH/ BFTf2similar:(4). Pd-C / HCO2NH4(7). Et3SiH / CF3COOH1-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me2N)3P=O(3). organic electrochemistryβ-CO(1). particular structure:1-g(1). K / Al2O3K / HMPA(2). Na / NH31-h(2). normal structure: SOCl2JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me2N)3P=Oyes for white mouse, uncertain for humanmodified to: N NO1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROORR OROR RCH 2 OCH 2CH 2OH(3). AlCl 3 / LiAlH 4(2). HCl / NaBH 3(CN)(1). h / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2ORC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t BuRC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with baset -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl(5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X application: for protecting groupe d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3t-BuORaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-e 2-e.vi. H 2O 2, t -BuOH, MnSO 4 // NaHCO 3, pH 8JA CS, 2001, 123, 2933.HO 22COnew, cheap,, simple, green chemistryconvenient, inexpensive, powerful.JOC, 1980, 45, 4758.JOC, 1982, 47, 2670.OOHOOBr via:Br 2 / ROH2-f ROH / HClEtCNEt C OEtOEtOEtHClJA CS, 1942, 64, 1825.JOC, 2001, 66, 521.C-OHC-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2Ph = RC-OBZl = RC-OBnRC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH2Ni. TMSIii. BF 3-Et 2O // R-SH (or HS-CH 2CH 2-SH)iii. BBr 3 / CH 2Cl 2, 0-10 C / LiI, heatvi.OCH 3OH+- CH 3Cl i. H 2 / Pd-C ii.CN CN Cl ClO, OH-OH-O COCH 3O O CH 2OCH 3RhCl(PPh 3)3, H 3O +OH - Me 32Oi. TFA (CF 3CO 2H)ii. HBr / HOAc iii. TMS-Ii. HOAc: weak acid: good leaving groupii. H 2need stronger acidi. F - : HF, Py-H + F -; n +--SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+JOC, 1973, 38, 3224.iv. AlCl 3 / RSH THL, 2001, 42, 9207.MeOCO 2Me HOCO 2MeCH 3(CH 2)11SHodorless3v./ heatCl -N H3-b NH Cl -triphenylmethylorganic base: TMG3-c(1). OH -(2). KO 2 / DMSO 3-d not practically useful: R-OH cheaper than R-XJOC, 1975,40, 1678.(2). Na 2[Fe(CN)5(NO)] / K 2CO 3 / H 2O3-e(1). Symmetry:ketal: use H 3O +acetal: use H 3O +(2). unsymetry:RO-MOM RO-MEM RO-MTM RO-THPi. H 3O +p -TsOH / MeOHi. H 3O +; ii. ZnBr 2 / CH 2Cl 2HgCl 2 / CH 3CN (aq.)actually, acetal exchange (3). Ag 2O / H 2OTHL, 1975, 3183.JOC, 1986, 51, 3913.RO 2C (CH 2)3NH 2RO 2C (CH 2)3OHNa [Fe(CN)(NO)]2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversion Synthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH acetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOHNaAlH 2(OCH 2CH 2OCH 3)2C 6H 6, r.t.hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.JACS, 1972, 94, 7159.LAH ------------ almost all: ald, ketone, acie, ester, acyl X, anhydrideNaBH4 --------------- not for acid, ester (but LiBH4 work for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:LiAlH4; NaBH4; Na / NH3Al (O i Pr)3 / i PrOH ----------- Meerwein-Pondorf-Verley rxnIrCl4 / i PrOH / P(OMe)3 ------ Henbest rxnLiBH(sec Bu)3 ------------------ H. C. Brownfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). HCHO reagent:Me CHO MeOHHCHOJACS, 1935, 511, 903.CH3CHO C(CH2OH)42Org.Syn, 1925, 4, 53.HCHO / KOHHCHO / Ca(OH)2Synthesis, 1994, 1007.PhNO2OPhNO2H OHBH / SMeJOC, 2001, 66, 7514.JOC, 2003, 68, 2030.OBH3 / THF99.5 % transsolvent: THF, SMe23-iR3B, HOCH2CH2OH // H2O2 // NaOHJOC, 1986, 51, 4925.C O R BRR3BRRRRR3C B OHOCH2CH2OHR3C BOO H2O2R3BOOO HO BOOR3CH2OR3C OHpractice3-k OOHOHOHOHOOHOHOHOHOHJOC, 1967, 32, 3452.H 2O 2: dangerous,skin whiten, metal decomposeHg (OAc)2: toxic, hard to remove (3). B 2H 6, H 2O 2 / OH -, H 2O(2). Hg(OAc)2, H 2O // NaBH 4(1). H 3O +3-j3-j.13-j.2hydration:(1). KMnO 4 / NaOH (2). OsO 4(3). H 2O 2/HCO 2H (4). Na / EtOHcis tran cis +trancis3Me2NNN CH3HCl3hνN CH3HHN CH3HH+N CH3ClNHCHC2R3C NH2R C NR2R C NHRR3C OHR2C OHRC OHR C NH2tertiarysecondaryprimaryCompare nomenclature class:not a very useful reactionC-NC-HC-NC-XC-OHC=OC=C4-abcdefg4-a SO2NH2Ph I OAcOAc SOONHSOON I Ph Fe(TPP)Cl SOONH2(insertion)TPPNNNNPh2. NaN3CON N N N C O1. SO2Cl2CO2CCO OhiC NC(O)XC-C(O)XNH 22RC NO 2RC N 3RC N Me RC N CPh RC N CPh 3RC NH 2RC NH 2RC NH 2RC NH 2RC NH 2iiiiiiRC N C O OtBu RC NC O OPhRC NH 2RC NH 24-b CF 3CO 3H // Fe / HOAc1. many reducing agents1. NaBH 4;2. Al (Hg)H 2O 2 // Ac 2O, heat / H 3O +H 2 / Pd-C1. HOAc;2. H 2 / Pd-C1. TFA;2. HCl H 2 / Pd-C4-b.14-b.22. organic electrochemistry1.2.3.4.Fe 3(CO)12 / CH 3OH JOC, 1972, 37, 930.NaBH 4 / Pd-C Na 2S Sn / HCl Vogel's 12.57Vogel's 12.58Vogel's 12.59NO 2OCH 3NH 2OCH 3Eg-Ni rt. 15 hrJOC, 1999, 64, 2301.Eg-Ni: electrogenerated nickel5.H 2 / Pt (S)-CJACS, 1965, 87, 2767.sulfided platium not affect: aromatic rings, ketones, halides, nitriles, amide, eastersJACS, 1933, 55, 4579.2HCHO NMe 2CO 2EtNH 2CO 2EtRC NCC NR C C RC NH 2iC N R N N+-C N R R'ii 1. HCHO / HCO 2H 1. RBCl 2 / base1. HC(OEt)3 // NaBH 4;2. R 2CO // NaBH 3CN NH 2N CH 3CH 3HCHO N 3NHBCl 2NH 2COOHN COOHH CH 3HC(OEt)NaBH 4b.3 2. HCHO // H 2 / Pd-CN 3NO 2MeO 2CNaBH 422rt NH 2NO 2MeO 2CSynthesis, 1979, 537.mild conditionhigh yieldnot affect:: NO 2, C=C, CN, COOR, COOH2. NaBH 4 / CoCl 2-6H 2Onot good, usually contain polyalkylation products2. Delepine3. NaN 3 / RED4-d4-c 5. Unpolung4. NaN 3 / RED3. Delepine2. Gabriel:1. NH 3N OO K N 2H 42Oi. LAH, NaBH 4ii. H 2 / catiii Zn / HCl; Al (Hg)i. Mg // NH 2Clii. Mg // PhSCH 2-N 3commercial available, tetramer of Me 3N24. CBr 4, PPh 3, NaN 3, DMF // PPh 3 / THFJOC, 2000, 65, 7110.乌洛托品)methenamine (六甲烯胺)hexamethylenetetramine (环六亚甲基四胺)内服后遇酸分解出 HCHO,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.R C O NH 2R C O NIPh OAcR N C OR NH C OOCH 3CH 3OHPhI, OAcPhI(OAc)24-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2hC-NH2C-NO2C NC C5-agfdcba5-C=C-ORC=C-SRC-OHC=N-OHC=N-HC=SC=OC=Ov. via: epoxysilaneR COCRR COCH2R242H3O+CO23OOBr2-HBr4OONaBH3H3O+ZnTsNHNH2MeLi TMSCl MCPBA LAH24CH2CORRCH2CORR3SCH2CORRPhCHOi. via:α-CO2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherR COCH2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAeij C-Brk C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-C N5-e.25-e.3-CH 2-C O HR -CH -C O HR -CH -C OR"R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3PDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +5-iPh OPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +OO5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-j 5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHhf i RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:MeCPhJOC, 1978, 43, 364.ArAr'HBr Ar C C Ar'NaOEtvia:Ar Ar'Bri. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb7-i. p-TSOH.H 2O or CSAii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C CH OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C CCC CCHC O C Cf e dc b a 7--C(O)-CH 3CH-CHCH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrC C OH OHc 7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C I Iapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAc JOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.SR 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CN3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)31. H 2S 2. Pb(OAc)431. H 2S 2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metalsii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh 7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNN3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I 38-a.12. F 3S-NEt 21.(DAST)S N S F O OOOChem. Rev., 1996, 96, 1737.2FCH 3SOO OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 2$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-cCF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SF OOF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N CFCl 3-CHCl 3ON XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFHFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 V already industrilizedNF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.。
