分子重排 课件
合集下载
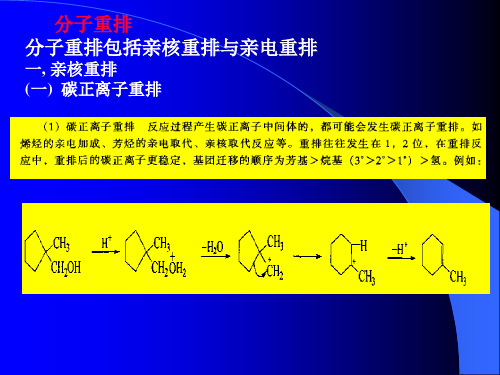
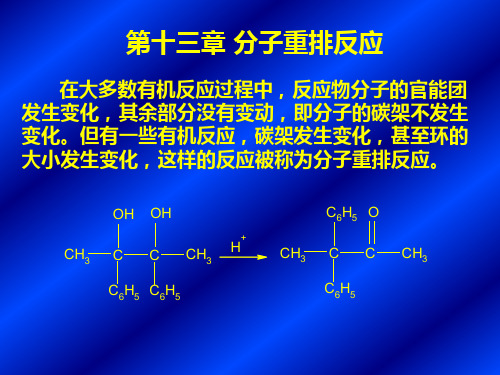
分子重排反应

O R C NH2 Br2 O H R C N Br R NH2 O R C N + CO2 R N C O H2O R N C OH OH
R N C OH H O
• 6、Wolff重排 • 重氮酮在氧化银或光热作用下,与水、醇、胺反应生成酸、 酯、酰胺的反应称为Wolff重排。 O
H2O O + R C CHN2 hv O R C CH R'OH NH3 R C C OH H2 O R C C OR' H2 O R C C NH2 H2 R C C O H
+
CH3 H3C C C OH2 H2
+
- H2 O
CH3 H3C C CH2
+
CH3
CH3
CH3 Cl H3 C C CH2CH3
Cl rearrgement CH3
+
-
H3C
C
CH2 - H+ H 3C
CH3 C CHCH3
CH3
CH3
• 实验证明Wagner-Meerwein重排的历程是生成碳正离子 的SN1历程。重排的趋势一般取决于碳正离子的相对稳定 性。其迁移基团的活性次序如下:
O Ph H C H2 Cl B Ph CH2 O H2O Ph C O OH
H2O
Ph
C O
OH
• 原子上有吸电子基团时,在强碱的作 用下,季铵盐上的取代基重排到具有吸电子的碳原子上, 形成叔胺的反应称为Stevens重排。
R1 Z C H2 N
+
R1 R2 NaNH2 Z C H N
O O C C KOH O C OC OH OH O C C OK
O- O C C OH
R N C OH H O
• 6、Wolff重排 • 重氮酮在氧化银或光热作用下,与水、醇、胺反应生成酸、 酯、酰胺的反应称为Wolff重排。 O
H2O O + R C CHN2 hv O R C CH R'OH NH3 R C C OH H2 O R C C OR' H2 O R C C NH2 H2 R C C O H
+
CH3 H3C C C OH2 H2
+
- H2 O
CH3 H3C C CH2
+
CH3
CH3
CH3 Cl H3 C C CH2CH3
Cl rearrgement CH3
+
-
H3C
C
CH2 - H+ H 3C
CH3 C CHCH3
CH3
CH3
• 实验证明Wagner-Meerwein重排的历程是生成碳正离子 的SN1历程。重排的趋势一般取决于碳正离子的相对稳定 性。其迁移基团的活性次序如下:
O Ph H C H2 Cl B Ph CH2 O H2O Ph C O OH
H2O
Ph
C O
OH
• 原子上有吸电子基团时,在强碱的作 用下,季铵盐上的取代基重排到具有吸电子的碳原子上, 形成叔胺的反应称为Stevens重排。
R1 Z C H2 N
+
R1 R2 NaNH2 Z C H N
O O C C KOH O C OC OH OH O C C OK
O- O C C OH
《分子重排反应》课件

III. 分子重排反应的机理
• 分子重排反应的机理涉及分子内、分子间的结构变化以及化学键的重组。 • 这类反应通常包括断裂原始键、形成新的共价键、转移原子或基团等
步骤。 • 具体反应机理的理解对于控制反应路径和提高反应效率至关重要。
IV. 分子重排反应的影响因素
1 1. 温度
温度对分子重排反应速率和选择性起着重要 作用,通常较高的温度会促进分子重排反应 的进行。
II. 分子重排反应的分类
1. 根据反应类型
分子重排反应可以根据不同 的反应类型进行分类,如醇 酯互变异构、氧杂环化、羰 基互变异构等。
2. 根据反应条件
分子重排反应也可以根据不 同的反应条件进行分类,如 酸催化、碱催化、高温条件 下发生的分子重排反应等。
3. 根据反应底物
分子重排反应还可以根据不 同的反应底物进行分类,如 环状化合物的分子重排反应、 链状化合物的分子重排反应 等。
VII. 分子重排反应与有机合成的关系
分子重排反应是有机合成中的重要环节,可以用于构建复杂分子结构和控制化学反应的选择性。
2
2. 药物合成
分子重排反应在药物合成中发挥着重要作用,可用于合成药物前体和改进药物性 能。
3
3. 新型材料制备
分子重排反应可用于制备新型材料,例如高分子材料、金属配合物等。
VI. 分子重排反应的实验方法
为了研究和实施分子重排反应,可以使用各种实验方法,如核磁共振(NMR)、质谱(MS)和红外光谱(IR) 等。
2 2. 反应物浓度
反应物浓度越高,分子重排反应的速率通常 会增加。
3 3. 催化剂
催化剂可以显著提高分子重排反应的速率, 并且可以排反应的进行和产物 选择性有重要影响。
第五章 重排反应ppt课件

酯;
30
• (2)环状二酮生成环状羟基酸
31
四、Favorski重排 • 1. 反应通式
32
• 2. 反应机理
33
• 3. 影响因素
催化剂的影响
34
• 4. 应用特点 • 由卤代酮制备羧酸衍生物
35
五、 Wolff重排及Arndt-Eistert反应 • 1. 反应通式 Wolff rearrangement
C H 3O > >C l
> C H 2=C H
> R 3C > R 2C H> C H 3 > H
2020/1/14
17
• (3)胺类化合物的Wagner-Meerwein重排
CH3
CH3 C CH2NH2 CH3
HNO2 - N2
CH3 CH3 C CH2
CH3
CH3 C CH2CH3 CH3
36
• 2. 反应机理
37
38
• 3. 影响因素
Ph CH2 OCH3 PhLi C6H6
PhCHOCH3 Li+
CH3 PhCH
O-Li+
H2O
CH3 Ph CH OH
2020/1/14 8
3. Radical rearrangement
Cl
Cl CCH=CH2 Br Cl
Cl
Cl C CH CH2Br Cl
Cl
C
Cl CH
CH2Br
Br2
Cl
2. 碳原子到杂原子重排
(1)Beckmann重排 (2)Hoffman重排 (3)Curtius重排 (4)Schmidt重排 (5)Bayer-Villiger反应
3
重排反应
30
• (2)环状二酮生成环状羟基酸
31
四、Favorski重排 • 1. 反应通式
32
• 2. 反应机理
33
• 3. 影响因素
催化剂的影响
34
• 4. 应用特点 • 由卤代酮制备羧酸衍生物
35
五、 Wolff重排及Arndt-Eistert反应 • 1. 反应通式 Wolff rearrangement
C H 3O > >C l
> C H 2=C H
> R 3C > R 2C H> C H 3 > H
2020/1/14
17
• (3)胺类化合物的Wagner-Meerwein重排
CH3
CH3 C CH2NH2 CH3
HNO2 - N2
CH3 CH3 C CH2
CH3
CH3 C CH2CH3 CH3
36
• 2. 反应机理
37
38
• 3. 影响因素
Ph CH2 OCH3 PhLi C6H6
PhCHOCH3 Li+
CH3 PhCH
O-Li+
H2O
CH3 Ph CH OH
2020/1/14 8
3. Radical rearrangement
Cl
Cl CCH=CH2 Br Cl
Cl
Cl C CH CH2Br Cl
Cl
C
Cl CH
CH2Br
Br2
Cl
2. 碳原子到杂原子重排
(1)Beckmann重排 (2)Hoffman重排 (3)Curtius重排 (4)Schmidt重排 (5)Bayer-Villiger反应
3
重排反应
第七章 分子重排反应

C O
C R
N
N
Cu2+
H2O R'OH O C CH R NH3 R'NH2
RCH2COOH RCH2COOR' RCH2CONH2 RCH2CONHR'
(5)氮烯重排 )
(1). Hofmann重排 重排 酰胺在次卤酸盐(如Br2/NaOH)的作用下,重派后继而水解 /NaOH)的作用下 的作用下, 酰胺在次卤酸盐( 生成少一个碳原子的伯胺。又叫霍夫曼降级。 生成少一个碳原子的伯胺。又叫霍夫曼降级。
(6) . Beckmann (贝克曼)重排 贝克曼)
醛肟或酮肟在酸性催化剂作用下重排生成取代酰胺的反应
R ' R C N O OH H R C NHR '
机理: 机理:
R R' C N OH2
R
C
N
R'
R
C
N
R'
亲核重排
H2O
RCONHR'
RCOOH + R'NH2
(7) . Baeyer-Villiger (拜耶尔 维利格)重排 拜耶尔-维利格 维利格)
R R
OH-
R R C C O C
R R
OH
R2CHCR2CO2 H
7.3 芳环上的重排
(1)联苯胺重排 )
在强酸催化下,氢化偶氮苯类重排生成 , 二氨基联苯 在强酸催化下,氢化偶氮苯类重排生成4,4`-二氨基联苯 类的反应。 类的反应。
NH NH
H+ ~70%
H2N
,
H2N ~30% NH2 H2N
苯甲基三烷基季铵盐(或锍盐)在PhLi、NaNH2等强碱作用下 苯甲基三烷基季铵盐(或锍盐) 、 发生重排,苯环起亲核烷基化反应,烷基的α-碳原子与苯环的 发生重排,苯环起亲核烷基化反应,烷基的 碳原子与苯环的 邻位碳原子相连成叔胺。 邻位碳原子相连成叔胺。
分子重排
+
HO
CH3COO
O
+
CH3
HO O
+
HO CH3 O CH3
H
+
O O CH3
(四)碳烯的重排 (1)Wolf重排的定义 (2)应用Wolf重排如果是水解可以合成高一级羧酸;如果是醇解可以 合成高一级酯
O R C Cl + CH2N2 O R C CH
H2O
O R C CHN2 O N
+
N
N2
Ag2O
+
R C OO H
O CH3 ph
H+
C O
Ph
O
C
CH3
(3)开链酮氧化成一般酯,环酮氧化生成内酯
O O RCOOOH
HO
O
O
C R
H+
OH
HO
O
H
+
O
+
+
C O
C O
(4)基团亲核性愈大,迁移的倾向性愈大,迁移顺序为: 苯基 >叔烃基>仲烃基>伯烃基>甲基 氢过氧化物的重排 苯基 >叔烃基>仲烃基>伯烃基>甲基
二,芳环上的重排 1 联苯胺重排 联苯胺重排是指氢化偶氮苯重排为联苯胺类的反应
2 H+ +
NH
NH
NH H
+ NH H
NH H
NH H
H
2 H+
NH2
+
NH2
+
H NH2
+
H H
NH2
+
H2N
NH2
HO
CH3COO
O
+
CH3
HO O
+
HO CH3 O CH3
H
+
O O CH3
(四)碳烯的重排 (1)Wolf重排的定义 (2)应用Wolf重排如果是水解可以合成高一级羧酸;如果是醇解可以 合成高一级酯
O R C Cl + CH2N2 O R C CH
H2O
O R C CHN2 O N
+
N
N2
Ag2O
+
R C OO H
O CH3 ph
H+
C O
Ph
O
C
CH3
(3)开链酮氧化成一般酯,环酮氧化生成内酯
O O RCOOOH
HO
O
O
C R
H+
OH
HO
O
H
+
O
+
+
C O
C O
(4)基团亲核性愈大,迁移的倾向性愈大,迁移顺序为: 苯基 >叔烃基>仲烃基>伯烃基>甲基 氢过氧化物的重排 苯基 >叔烃基>仲烃基>伯烃基>甲基
二,芳环上的重排 1 联苯胺重排 联苯胺重排是指氢化偶氮苯重排为联苯胺类的反应
2 H+ +
NH
NH
NH H
+ NH H
NH H
NH H
H
2 H+
NH2
+
NH2
+
H NH2
+
H H
NH2
+
H2N
NH2
《重排反应》PPT课件

P C H3OC 6H5 Ph
H 2S O 4
P C H3OC 6H5 C C Ph
OH OH
P C H3OC 6H5
P C H3OC 6H5 C C Ph Ph O
72%
+ Ph
P C H3OC 6H5 C C Ph
28%
O整理课件C 6 H 5 O C H 3 P
9
Ph Ph C
CH3 C CH3
第四章 重排反应
定义:受试剂或介质的影响,同一有机分子内的一个基团 或原子从一个原子迁移到另一个原子上,使分子构架发生 改变而形成一个新的分子的反应称为重排反应。
重排反应类型(按终点原子电荷分) 缺电子重排 富电子重排 自由基重排
• 从碳原子到碳原子的重排 • 从碳原子到杂原子的重排 • 从杂原子到碳原子的重排 • -键迁移重排
关注1,2重排 烯丙基结构 构型保留
R
碱B R
机理
YC H
BH
YC
R YC
整理课件
51
一、Stevens重排
季铵盐分子中连于氮原子的碳原子上具有吸电子的 基团取代时,在强碱性条件下,可重排生成叔胺的 反应称为Stevens重排反应。
RC H C O H2O RC H2C O O H
COCl
1. C H 2 N 2 2. P h C O O A g /E tO H /T E A
C H2C O O C 2H5
84~92%
整理课件
28
Arndt-Eistert同系列羧酸的合成反应
Arndt-Eistert合成是将一个酸变成它的高一级同系 物或转变成同系列酸的衍生物,(如酯或酰胺)的反 应。该反应可应用于脂肪族酸和芳香族酸的制备。
有机化学分子重排
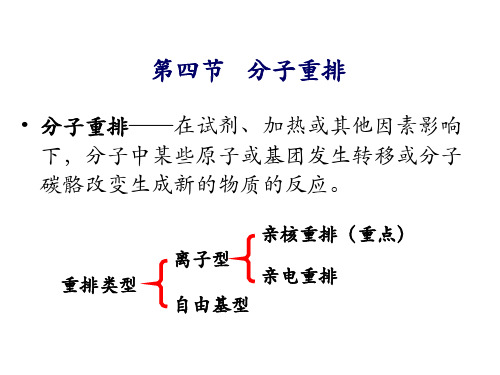
H
+ N2
+
R H H O H
H
反式迁移
H R H H OH NH2 HNO2 R H H
H R C O
H
+ OH
N2
- N2
H R H H
+
C O H
-H+
(二)迁移到缺电子的氮原子
1.贝克曼(Beckmann)重排
OH
O R C R′ NH2OH
OH H R C N OH R′
2
N R
N R C R′
H
+
H3C
CH3 CH3 C C CH3
CH3 C CH3
OH OH
-H2O
OH +OH H
重 排
H3C C
+
+
: OH CH3
-H
+
H3C
CH3 C C CH3 CH3 O
注意:
• R:脂肪烃基、脂环烃基、芳基; 可各不相同,也可部分相同或全同。 问题: 如果R为4个不同的基团时: ① 哪个羟基先被H+进攻,再失水生成C+? ② 与C+相邻的基团中哪个基团先转移? ③ 反应的立体化学要求。
+
+
- N2
HO
CH2
+
OH
+
-H
+
O
3. 重排反应的立体化学 问题:
① 迁移基团迁移前后构型是否变化;
② 迁移起点的碳和迁移终点的碳构型是否变化;
③ 迁移基团与离去基团的空间位置的关系。
在同一反应体系中:
Ar Ar C CH3 C CH3 H
分子重排

亲核重排反应
重排过程,Z带着一对电子从原子C迁移到另一个缺 少一对电子的原子A上。 多数亲核重排是1,2-重排,即 基团的迁移发生于相邻的两个原子间。上式中,A为C、 N、O等原子,而Z为X、O、S、N、C、H等原子。 重排后的分子与反应体系的负性部分结合生成重排取代 产物或失去质子生成重排消除产物。
反应机理
反应实例
Cope(考普)重排反应
1,5二烯类化合物受热时发生类似于 O-烯丙基重排为 C-烯丙基的重排反应 (Claisen 重排)反应称为Cope重排。这个反应30多年来引起人们的广泛注意。1,5二烯在150—200℃单独加热短时间就容易发生重排,并且产率非常好。
反应机理:Cope重排是[3,3]σ-迁移反应,反应过程是经过一个环状过渡 态进行的协同反应. 在立体化学上,表现为经过椅式环状过渡态:
分子重排分类
按分子内及分子间重排分类 分子内重排 分子间重排
按反应历程分类 亲核重排、亲电重排、游离基重排和σ键的迁移重排
按迁移基团的相对位置分类 1,2—迁移重排、1,3—迁移重排、1,5—迁移重排 以及3,3—迁移重排等
按不同元素间的迁移分类 C→C、C→O、O→C、N→O重排
将羧酸制成不稳定的酰基叠氮,后者在惰性溶剂中加热 发生脱氮重排析离出中间体异氰酸酯,再经水解得到伯胺, 此反应称Curtius重排反应,过程:
反应机理:
反应实例
Schmidt(施米特)重排
羧酸、醛或酮分别与等摩尔的叠氮酸(HN3)在强酸(硫酸、 聚磷酸、三氯乙酸等)存在下发生分子内重排分别得到胺、 腈及酰胺
③亲核性强的那个基团优先发生迁移。
④Pinacol (频哪醇)重排,其立体特征是迁移基团在连 接离去基团的键完全断裂之前从离去基团的背面连 接到迁移末端,因此优先迁移的基团应与反应基质 分子的离去基团处于反式共平面,而且迁移终点 (具有手性中心的)构型往往发生反转。
第十二章 分子重排反应
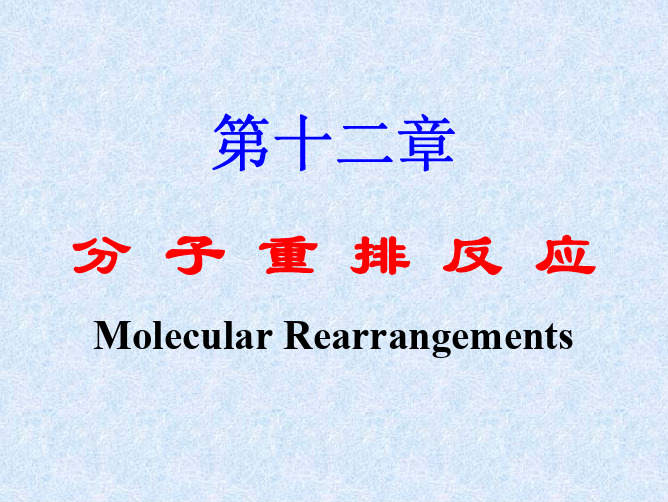
12.1.3 二苯基乙二酮重排(Bezil重排)α-二酮经强碱处理会发生重排,生成α-羟基
乙酸盐。二苯基乙二酮重排,亦称Benzil重排。
本重排是制备二芳基乙醇酸的常用方法,产率一般较 高。α-芳二酮主要是由α-羟基酮氧化而得,而α-羟基酮 是由芳醛通过二苯乙醇缩合(Benzoin缩合)来制备。
12.1.4 Demyanov重排 脂肪族或脂环族伯胺与亚硝酸作用发生的脱氮重 排,称为Demyanov重排。
脂环族化合物的伯胺与HNO2作用重氮化后能引起 环的扩大或缩小。
脂环化合物环上碳原子带正电荷时,通过亲核 1,2-重排环会收缩;相反,如果碳正离子位于环的 α位,通过亲核1,2-重排,环会扩大。降低张力是 重排的动力之一,因此小环的扩环反应产率通常都 较高,而五元环却难以通过亲核1,2-重排收缩成四 元环,这是由于环张力增加的缘故。而在三元环和 四元环之间的转化中,环张力不是主要因素。
12.1 亲核重排 亲核重排系分子在亲电试剂影响下,发生 基团 Z 带着一对电子从一个原子迁移到另一个 原子上去的反应,其中以 1,2- 迁移的重排较为 重要。
A=C、N、O Z=X、O、S、N、C、H
在这类重排反应中, Z在迁移过程中带着孤对 电子,故 A一定是一个缺电子的被进攻中心,所以 该重排称为缺电子重排。重排反应一般需要三步完 成,这三步的常见形式是:
基团Z带着一对电子从一个碳原子迁移到另一 个碳原子上去的反应称为C→C迁移重排。
12.1.1 Wagner-Meerwein重排 烷基、芳基或氢从一个碳原子向另一个碳原子迁移的反应称 为Wagner-Meerwein重排。实践表明Wagner-Meerwein重排大多 是按SN1机理进行的,其重排趋势取决于碳正离子的稳定性。 1)甲基迁移
第四节 分子重排
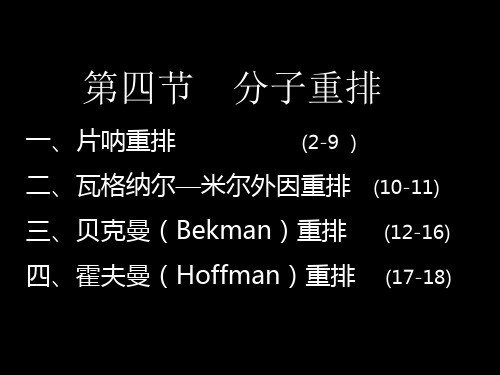
+
C6H5
OH O H 迁移 C6H5 CH NH C CH3 CH N=C CH3 CH3 CH3
四、霍夫曼(Hoffman)重排
酰胺与溴或氯,在碱溶液中作用时, 生成伯胺的反应称为Hoffman重排。 由于重排产物比反应物少一个碳, 所以又叫Hoffman降级。
O R C
+
NH2
-
O=C NR
(2)发生迁移的基团 生成碳正离子之后,由于迁移过程是向碳 正离子转移过程。基团的亲核性决定了迁移能 力的大小;如果迁移基团亲核能力大小不明显, 则空间位阻起作用。 不同基团迁移顺序 : 芳基中:
CH3O
>H3C
>Cl
H> R> rA
>
> OCH3
(3)反应的立体化学要求 在重排过程中迁移基团和离去基团彼此处于 反式,例如:
(Wagner-Meerwein) 瓦米重排在E1消除和SN1历程中提过,它的中 间体是碳正离子,凡是能有碳正离子生成的反 应都可能发生瓦米重排。
例1:写出重排产物
(1) (2)
CH3 CH3 HBr CH2OH Ag+ CH2Br 3C
(1)
H2 + H2
CH3 H+ CH2OH H O 2 i CH2CH3
OHBr Br Br HBr R C N H O OH重排 R C ¨∶ N 氮宾六个电子 R 迁移
烯醇结构
CO2
O
δ-δ + O=C=NR 碱中
水解
HO C=NR OH
O=C NHR OH
RNH2
H R H
亚硝酸
+
向下 H
+
C6H5
OH O H 迁移 C6H5 CH NH C CH3 CH N=C CH3 CH3 CH3
四、霍夫曼(Hoffman)重排
酰胺与溴或氯,在碱溶液中作用时, 生成伯胺的反应称为Hoffman重排。 由于重排产物比反应物少一个碳, 所以又叫Hoffman降级。
O R C
+
NH2
-
O=C NR
(2)发生迁移的基团 生成碳正离子之后,由于迁移过程是向碳 正离子转移过程。基团的亲核性决定了迁移能 力的大小;如果迁移基团亲核能力大小不明显, 则空间位阻起作用。 不同基团迁移顺序 : 芳基中:
CH3O
>H3C
>Cl
H> R> rA
>
> OCH3
(3)反应的立体化学要求 在重排过程中迁移基团和离去基团彼此处于 反式,例如:
(Wagner-Meerwein) 瓦米重排在E1消除和SN1历程中提过,它的中 间体是碳正离子,凡是能有碳正离子生成的反 应都可能发生瓦米重排。
例1:写出重排产物
(1) (2)
CH3 CH3 HBr CH2OH Ag+ CH2Br 3C
(1)
H2 + H2
CH3 H+ CH2OH H O 2 i CH2CH3
OHBr Br Br HBr R C N H O OH重排 R C ¨∶ N 氮宾六个电子 R 迁移
烯醇结构
CO2
O
δ-δ + O=C=NR 碱中
水解
HO C=NR OH
O=C NHR OH
RNH2
H R H
亚硝酸
+
向下 H
+
4.分子重排

瓦-米重排包括:
醇及其酯的消去与取代 过程中
卤代烃的消去与取代
所发生的
烯烃的亲电加成
重排
精选版课件ppt
16
瓦-米重排的一般机理:即通常所说的碳正离子重 排历程
例1:2-莰醇与硫酸作用生成莰烯的机理为:
OH H + -H 2O
-H
精选版课件ppt
17
• 但是底物不同,机理不尽相同。如下述反 应也不排除是同步机理:
-N 2 CH 3CHC 2 H
CH 3CHC3H-H H 2 +O CH 3CHC3H
OH
精选版课件ppt
22
• 特点:
• (1)C3-C8的酯环族伯胺的扩环重排, 其中小环的产率较高.
• (2)氨基在侧链的底物以扩环产物为主:
CH 2NH 2HN2O CH 2NN-N2
-HH+OH CH 2OH
HCl
Cl
精选版课件ppt
13
• 形成缺电子体系主要有下面四种方法:
• (a)碳正离子的形成。
• (b)氮烯的生成。
• (c)碳烯的形成。
• (d)缺电子氧原子的形成。
• 其中以形成碳正离子与氮烯的两种方法 最为重要。下面即根据生成缺电子中心的四 种方法、分别介绍碳正离子重排,缺电子氮 原子参加的重排、缺电子氧原子的重排、碳 烯的重排等。
6
HH NN HH NN Me Me
H2N H
H2N
H2N
Me Me Me
NH2 NH2
NH2
精选版课件ppt
7
(二)立体化学的研究
• 如果反应物具有旋光性,可以从重排后是否保 持其旋光性来判断有关历程。如后面讨论的贝 克曼重排,反应物有旋光性,重排后形成99.6% 光学纯的酰胺,即旋光性在重排后保留下来, 说明构型保持不变,所以手性碳原子上的构型 没有变,证明重排过程中迁移基团并没有脱离 原来分子而裂解下来,因为如果裂解下来,则 应得到外消旋化的产物,所以贝克曼重排是分 子内重排。
第十章 分子重排反应

历程:
反应特点:与羟基处于反位的烃基迁移至N 上。
立体化学:迁移基含手性碳时,产物构型保持。
四、由碳原子迁移至氧原子的重排 Baeryer – Villiger (拜依尔-维利格) 重排(氧化) 酮 + 过氧酸 → 酯 常用过氧酸: CF3CO3H , C6H5 CO 3H , C6H5 CH2 CO3H
脂肪族重氮盐分解放氮发生重排生成醇,该 重排叫Demjanov(捷米杨诺夫重排),也可看作 瓦-梅重排的一种。
不同基团的迁移倾向:
CH3O > R3C > R2CH > >CH3 > Cl >H
O C H 3 O C H 3 C H 3 C H 3 C l
> CH2
CH
迁移倾向:500
15.7
1.95
1
0.7
0.3
●芳基中:对位及间位有供电基,迁移倾向增大; 邻位有供电基,迁移倾向降低; 凡有吸电基,则迁移倾向减小
苄基正离子比叔碳正离子稳定,不发生重排
环张力大小也是发生重排的推动力(如: 三、四元环重排为五元环、六元环)
2、频哪醇(Pinacol)重排 三或四取代的(1,2-二醇),频哪醇 邻二叔醇(Pinacol) →
①形成缺电子体系(C +, C : , - N: ….) ②迁移基团带一对电子与缺电中心结合 ③迁移始点与亲核试剂结合 有时只是两步或三步同时进行。
一、碳正离子重排 1、碳正离子的形成 ①碳碳双键的加成:
R2C=C R2 + H+ →R2 CH – C+R2
②SN 1 , E 1 :
R3C- L →R3 C + + LROH + H+ →RO+ H2→ R+ + H2O
反应特点:与羟基处于反位的烃基迁移至N 上。
立体化学:迁移基含手性碳时,产物构型保持。
四、由碳原子迁移至氧原子的重排 Baeryer – Villiger (拜依尔-维利格) 重排(氧化) 酮 + 过氧酸 → 酯 常用过氧酸: CF3CO3H , C6H5 CO 3H , C6H5 CH2 CO3H
脂肪族重氮盐分解放氮发生重排生成醇,该 重排叫Demjanov(捷米杨诺夫重排),也可看作 瓦-梅重排的一种。
不同基团的迁移倾向:
CH3O > R3C > R2CH > >CH3 > Cl >H
O C H 3 O C H 3 C H 3 C H 3 C l
> CH2
CH
迁移倾向:500
15.7
1.95
1
0.7
0.3
●芳基中:对位及间位有供电基,迁移倾向增大; 邻位有供电基,迁移倾向降低; 凡有吸电基,则迁移倾向减小
苄基正离子比叔碳正离子稳定,不发生重排
环张力大小也是发生重排的推动力(如: 三、四元环重排为五元环、六元环)
2、频哪醇(Pinacol)重排 三或四取代的(1,2-二醇),频哪醇 邻二叔醇(Pinacol) →
①形成缺电子体系(C +, C : , - N: ….) ②迁移基团带一对电子与缺电中心结合 ③迁移始点与亲核试剂结合 有时只是两步或三步同时进行。
一、碳正离子重排 1、碳正离子的形成 ①碳碳双键的加成:
R2C=C R2 + H+ →R2 CH – C+R2
②SN 1 , E 1 :
R3C- L →R3 C + + LROH + H+ →RO+ H2→ R+ + H2O
高等有机化学第十章 分子重排反应

CH3 CH3 20%H2SO4 OH OH
CH3 CH3 OH
CH3 O CH3
二、瓦格涅尔—米尔外因重排反应
(CH3)3CCHCH3 OH
无水草酸
100—110 ℃
(CH3)3CCH=CH2 3%
+ (CH3)2CHC=CH2
CH3 31%
+ (CH3)2C=C(CH3)2
61%
反应机理是: 醇首先在酸作用下脱去羟基形成碳正离子
R2 R1 O R3
R2
O R1 O R3
ONa
H3C
CH3
+ CH2=CHCHBr
OCH2CH=CH2
H3C
CH3
OH
H3C
CH3
CH2CH=CH2
二、柯柏重排反应
1,5-己二烯化合物加热时,重排为一种新的1,5-己二烯化合物
2
X3
1
2
X3
1
4
6
5
4
6
5
柯柏反应反应均发生在1、5—二烯键系统,反应中首先生成六员环:
H2N(CH2)5COOH
第二节 亲电重排反应
一、斯蒂文重排反应 季胺盐在强碱作用下重排成叔胺的反应
R N—CH2—R'
CH3 CH3
B
R
[
N—CH—R' ]
CH3
CH3
CH3
N—CH—R' ] CH3
R
R=苄基、烯丙基、烃基等;R‘=RCO、ROCO、C6H5等吸电子基团
O (CH3)2NCHCC6H5
第七章 分子重排反应
重排反应:化学键的断裂和形成发生在同一个有机分子中, 引起组成分子的原子配置方式发生改变,从而成为组成相同、 结构不同的新分子的反应。
第七章-重排反应PPT课件

1、史帝文斯重排(Stevens)
——季铵盐在碱作用下,烃基从氮迁移到邻近 的负碳离子上得到胺。
例如:
CH3 NCH+ C2PHh2CBHr- CH2 CH3
C4H9OK
CH2Ph
+-
CH3 N CH CH CH2
CH3
CH2ph
CH3 N CH CH CH2
CH3
.
形成叔胺 型化合物
22
C H 2R CH3 N+ R'
X
两处的碳负离子谁易形成?
容易
但后者除了进行分子内取代外,另有变化
.
30
✓分子内取代:
O
-OR
R
CH3
O
-
O
R
R
CH3
这个结构 不予成立
以原先与卤素 相连处断开
问题二 如果该结构式成立的话,就有二个分离 可能,但事实上只有一种。
O C OR R CH CH CH3
ROH
.
O C OR R CH CH2 CH3
1
重排反应的分类:
➢(1)以重排范围分类
分子内重排:(以此为主) 分子间重排:(是以后发展的方向)
➢(2)以重排发生的距离分类
1,2-重排:邻位重排(*) 1,3-重排:间位重排 各种位置之间的重排
重排反应的特点:
➢(1)剧烈性:与上述所说的不稳定性有关, 瞬间性
.
2
➢(2)复杂性:不可测性
许多重排的产物复杂; 许多重排的机理尚不十分明了,尚无定论
CH2N2 重氮甲烷
RO O C CH R
可用于生成 酯、酸、酰胺
+-
ROH, H2O, NH3
高等有机化学课件第十三章
CHO KOH CHO
CH2OH COOK
五、碳-氮重排
1、贝克曼重排
R
R'
R
R'
C
+
H
C
N
N
HO
O+H2
2、霍夫曼重排
O R C NH2
O R C NHBr
R
R
O
C+ OH2
C
N R'
NH R'
O RC N
RNC O
六、碳-氧重排 Baeyer-Villiger重排
酮在过氧酸的存在下,氧原子插入到酮和迁移基 团之间,生成酯的重排反应。常用过氧酸是三氟过氧 乙酸。
CH3 CH3
C6H5 C
+
C C6H5 H
OH OH
O CH3 C6H5 C C C6H5
CH3 C6H5
C6H5 C C CH3 O
CH3
3、反式迁移 迁移基团与离去基团处于反式位置时,迁移速率
较快。例如 顺-1,2-二甲基-1,2-环己二醇在稀硫酸作用 下能迅速重排;而反- -1,2-二甲基-1,2-环己二醇在相 同条件下,由于迁移基团与离去基团不处于反式位置, 反应很慢,并导致环缩小反应。
三、按迁移的相对位置分类 按迁移基团的相对位置,可分为[1,2]迁移、[1,3]
迁移等等,大多数的重排反应都是[1,2]迁移。
第二节 亲核重排
亲核重排是迁移基团带着一对电子转移到缺电子 的迁移终点。亲核重排一般包括三步:第一步是缺电 子中心的创建;第二步是迁移基团带着一对电子迁移
到缺电子中心,使迁移起点成为缺电子中心;第三步
C C C6H5
C6H5
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
CH3
CH3 C C CH3 +OH CH3
H+
CH3
CH3 C C CH3 O CH3
The stable cation formed superior
Ph2C CH2 H+ Ph2C+ CH2
OH OH
OH
Ph2CH CH +OH
H+ Ph2CH
CH O
Phenyl group move first
p MeOC6H4C CPh2 28% O C6H4OMe p
Under HNO2, the amino alcohol rearranges as that of pinacol
NH2 HNO2
OH
CH2NH2 HNO2 OH
CHO O
3 α-Ethandione Rearrangement
O O NaOH Ph C C Ph EtOH
CO
OH O-
CO
α-Ethandione with α- hydrogen will proceed condensation
OO
C CH3
C CH3 CH3
CH3 C C
OO
OH-
H2O
OO C C CH3
CH3 CH3 C C
O
OH-
H2O
O
C C CH3
CH3 C C O
If NaOH was replaced by NaOMe or t-BuONa, α-hydroxycarboxyester will be produced
Molecular Rearrangements:it refers those reactions in which the carbon skeleton or the position of functional group changed.
CH3CH2CH=CH2 H3PO4 CH3CH=CHCH3
Chapter 12 Molecular Rearrangements
I Classification of Rearrangement Reactions( ) II Nucleophilic Rearrangement ( ) 1 Wagner-Meerwein rearrangement( )
>primary alkyl>methyl
CH3
O C C(CH3)3
CF3CO3H CH2Cl2
CH3
O C OC(CH3)3
Ph
O C
CH2CH3
CF3CO3H CH2Cl2
PhO
O C
OCH2CH3
O Ph C
CF3CO3H CH2Cl2
O PhO C
The cumene hydroperoxide rearrangement is quite resemble the Baeyer-Villiger Rearrangement
Br OH
Br
Br
O2N
Ph
O2N
N OH PCl5
Br
NHCOPh H2O
O2N
Br
NH2 Br
PhCOOH
The shift will keep the stereostructure of
chiral center
CH3 C N
HO
C2H5 C CH2C2H5
H
H2SO4 Et2O
C2H5
C CH2C2H5
PhHC CHPh H+ PhHC CHPh
OH OH
+
OH
Ph2CH CH +OH
H+
Ph2CH
CH O
Phenyl with electron donor group move first
(p MeOC6H4)2C CPh2 H+ OH OH
(p MeOC6H4)2C CPh 72% Ph O
CH3CH=CH2 anhydrous AlCl3 PhCH(CH3)2
PhCH(CH3)2
O2
Na2CO3/H2O 100℃
Ph
CH3 C OOH
H+
PhOH
CH3
CH3COCH3
III Electrophilic Rearrangement
Electrophilic rearrangement is not so common
CH3 PhC O
OH
H+
CH3
H3C
CH3 + C O OH2
_H2O
Ph
CH3
H3C
C
+
O
Ph
H2O
CH3 + H3C C O Ph
CH3+
H3C
CO OHH
Ph
CH3 H3C C O Ph
+OH2
+OH
PhOH + CH3 C CH3 O
CH3 C CH3 + H+
Important industrial reaction for manufacturing phenol and acetone
as nucleophilic rearrangement
Ph
Ph C CH2Cl Ph
Ph
Na Ph C CH2-Na+
Na+ Ph ROH Ph C CH2Ph
Ph
Ph2CHCH2Ph
The transition state is:
Ph C CH2 Ph
1 Favorskii Rearrangement
Nucleophilic rearrangement, Electrophilic rearrangement, Radical rearrangement, etc.
Nucleophilic rearrangement
CH3CH2CH2CH2OH H+
H
CH3CH2CH2CH2OH2 H2O
CH3CH2CHCH2
H+
+
2%
NH2
HNO2 N2
+
H2O
H+
H2O H+
OH 58%
+ H2O H+
CH2=CHCH2OH
2 Pinacolic Rearrangement
CH3 CH3 H+
CH3 CH3
CH3 C C CH3 OH OH
CH3 C C CH3 OH +OH2
H2O
CH3
CH3 C C+ CH3 OH CH3
O
O
H C Cl RO-
CC
ROH
C Cl CC
Cl-
O C CC
RO O-
RO-
C
CC
RO O C
CC
RO O
ROH
C
C CH
For asymmetric cycloprapanone, the stable anion form dominantly
O Ph
RO- RO O-
Ph
PhCH CH2COOR stable carbon anion
O O NaOR Ph C C Ph ROH
Ph2C
COOR
O-
H3O+
COOR Ph2C
OH
Usually, ethyloxy anion acts as a reduction reagent.
H Ph C C Ph CH3CH
OO
O-Ph C CH Ph H3O+ Ph C CH Ph
O O-
RO O-
COOR PhCH CH2
Ph relative unstable carbon anion
Different α- chloroketone form same rearrangement product:
O
PhCH2 C CH2 Cl
O
PhCH C CH3 Cl
O ROROH Ph
PhCH2 CH2COOR
1 favorskii Rearrangement 2 Stevens Rearrangement 3 Wittig Rearrangement 4 Fries Rearrangement
IV Radical Rearrangement
I Classification of Rearrangement Reactions
CH3CONH
H
5 Baeyer-Villiger Rearrangement
O CH3 C Ph
O CF3CO3H CH2Cl2 CH3 C OPh
O
O
CF3CO3H CH3COOEt
O
O CF3COOH
CH3
+OH C Ph
CH2Cl2
OH CH3 C Ph
CF3COO+H
O
OH
CH3 C Ph CF3C O+ O
CH3 Br
CH3
CH3 C CHCH3
CH3 CH3
Stable cation
CH3 CH3 C CHPh
CH3 Br
SN1
CH3
CH3 C CHPh
CH3
Stable cation Product
Product
CH3 C CHPh CH3 CH3
CH3O > R3C
>
> Cl
> CH2=CH
> R2CH >CH3 > H
O C C R KOH
