左乙拉西坦 说明书(原研厂家)
左乙拉西坦的功能主治和副作用

左乙拉西坦的功能主治和副作用1. 左乙拉西坦的简介左乙拉西坦是一种抗癫痫药物,它被广泛用于治疗不同类型的癫痫发作。
左乙拉西坦通过调节大脑中的神经递质,从而减少癫痫的发作次数和严重性。
它可作为单药使用,也可与其他抗癫痫药物联合使用。
2. 左乙拉西坦的功能主治左乙拉西坦具有以下主要功能主治:•控制癫痫发作:左乙拉西坦通过抑制神经元兴奋性,从而减少癫痫发作的频率和严重程度。
它对于大脑部分性发作和全面性发作均具有治疗效果。
•减轻癫痫相关的抑郁和焦虑:癫痫患者常常伴有抑郁和焦虑等心理问题,左乙拉西坦可帮助减轻这些症状,提高患者的生活质量。
•改善认知能力:左乙拉西坦对于一些癫痫患者的认知功能有改善作用,可以减少记忆力下降和注意力不集中等问题。
•预防癫痫发作的复发:对于已经控制住癫痫发作的患者,左乙拉西坦能够预防癫痫发作的复发,降低癫痫的复发率。
3. 左乙拉西坦的副作用左乙拉西坦是一种强效的抗癫痫药物,使用过程中可能会出现一些副作用。
以下是一些常见的左乙拉西坦副作用:•疲劳和乏力:左乙拉西坦可能引起患者感到疲劳和乏力,这可能会影响患者的日常生活和工作。
•头晕和失眠:一些患者在使用左乙拉西坦后可能会出现头晕和失眠等问题,这可能会影响他们的日常活动和睡眠质量。
•恶心和胃部不适:左乙拉西坦可能导致一些患者出现恶心、呕吐和胃部不适等消化系统症状。
•皮疹和过敏反应:极少数患者使用左乙拉西坦后可能出现皮疹和过敏反应,如荨麻疹、皮肤瘙痒等,如果出现这些症状应立即停药并就医诊治。
•口干和多尿:一些患者在使用左乙拉西坦后可能会出现口干和多尿的情况,这可能是药物对尿液生成的影响。
4. 注意事项在使用左乙拉西坦之前,患者需注意以下事项:•儿童和青少年患者的使用:左乙拉西坦在12岁以下的儿童患者中的安全性和有效性尚未确定。
•孕妇和哺乳期妇女:左乙拉西坦可能对胎儿产生不良影响,孕妇和哺乳期妇女在使用前需咨询医生的意见。
•其他药物的相互作用:左乙拉西坦可能与其他药物产生相互作用,影响药物的疗效或增加副作用的发生。
左乙拉西坦说明书

上市后不良事件报道-脱发,某些病例中,停药后,自行恢复。
-血液系统和淋巴系统异常变化:
上市不良事件报道:-白细胞减少、嗜中性细胞减少、全血细胞减少、血小板减少,但还没有足够数据,用于估计它们发生率或建立因果关系。
【禁忌】
对左乙拉西坦过敏或者对吡咯烷酮衍生物或者其他任何成分过敏的病人禁用。
其他药物相互作用:
口服避孕药
服用左乙拉西坦(500 mg,每日2次)不影响含有0.03 mg炔雌醇和0.15 mg左炔诺孕酮口服避孕药的药代动力学特性,或促黄体激素和黄体酮含量水平,表明本品不影响避孕药功效。应用口服避孕药并不影响本品的药代学特性。
地高辛
服用左乙拉西坦(1000 mg,每日2次)不影响每日剂量0.25 mg地高辛的药代动力学和药效学(ECG)特性。应用地高辛并不影响本品的药代学特性。
苯妥英
左乙拉西坦(每日3000 mg)对难治性的癫痫病人,苯妥英药代动力学特性不产生作用。苯妥英的应用也不影响本品的药代动力学特性。
丙戊酸钠
左乙拉西坦(1500 mg,每日2次)不改变健康志愿者丙戊酸钠药代动力学特性。丙戊酸钠500 mg,每日2次,不改变左乙拉西坦吸收的速率或程度,或其血浆清除率,或尿液排泄。也不影响主要代谢物ucbL057的暴露水平和排泄。
临床研究中报告有14%服用左乙拉西坦的成人及儿童患者癫痫发作频率增加25%以上,但在服用安慰剂的成人及儿童患者中,也各有26%及21%患者癫痫发作频率增加。对于肝功能损害的病人,参照[用法与用量]。对于严重肝功能损害的病人,应先行检查肾功能,然后进行调整。
对驾驶和应用机器影响:
目前没有研究关于服药后对机器驾驭能力和驾驶车辆能力的影响。
婴儿和小于4岁的儿童患者:
左乙拉西坦注射液EMEA说明书

ANNEX ISUMMARY OF PRODUCT CHARACTERISTICS OF THE MEDICINAL PRODUCTLevetiracetam SUN 100 mg/ml concentrate for solution for infusion2.QUALITATIVE AND QUANTITATIVE COMPOSITIONEach ml contains 100 mg of levetiracetam.Each 5 ml vial contains 500 mg of levetiracetam.Excipients:Each vial contains 57 mg of sodium (11.4 mg/ml).For a full list of excipients, see section 6.1.3.PHARMACEUTICAL FORMConcentrate for solution for infusion (sterile concentrate).Clear, colourless concentrate.4.CLINICAL PARTICULARS4.1Therapeutic indicationsLevetiracetam SUN is indicated as monotherapy in the treatment of partial onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Levetiracetam SUN is indicated as adjunctive therapy-in the treatment of partial onset seizures with or without secondary generalisation in adults and children from 4 years of age with epilepsy-in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy-in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy.Levetiracetam SUN concentrate is an alternative for patients when oral administration is temporarily not feasible.4.2Posology and method of administrationPosologyMonotherapy for adults and adolescents from 16 years of ageThe recommended starting dose is 250 mg twice daily which should be increased to an initial therapeutic dose of 500 mg twice daily after two weeks. The dose can be further increased by 250 mg twice daily every two weeks depending upon the clinical response. The maximum dose is 1500 mg twice daily.Add-on therapy for adults (≥18 years) and adolescents (12 to 17 years) weighing 50 kg or moreThe initial therapeutic dose is 500 mg twice daily. This dose can be started on the first day of treatment.Depending upon the clinical response and tolerability, the daily dose can be increased up to 1,500 mg twice daily. Dose changes can be made in 500 mg twice daily increases or decreases every two to four weeks.Duration of treatmentSpecial populationsElderly (65 years and older)Adjustment of the dose is recommended in elderly patients with compromised renal function (see "Renal impairment" below).Renal impairmentThe daily dose must be individualised according to renal function.For adult patients, refer to the following table and adjust the dose as indicated. To use this dosing table, an estimate of the patient's creatinine clearance (CLcr) in ml/min is needed. The CLcr in ml/min may be estimated from serum creatinine (mg/dl) determination, for adults and adolescents weighting 50 kg or more, the following formula:[140-age (years)] x weight (kg)CLcr (ml/min) = -------------------------------------- (x 0.85 for women)72 x serum creatinine (mg/dl)Then CLcr is adjusted for body surface area (BSA) as follows:CLcr (ml/min)CLcr (ml/min/1.73 m2) = -------------------------------- x 1.73BSA subject (m2)Dosing adjustment for adult and adolescents patients weighing more than 50 kg with impaired renal functionGroup Creatinine clearance Dose and frequency(ml/min/1.73 m2)Normal > 80 500 to 1,500 mg twice dailyMild 50-79 500 to 1,000 mg twice daily Moderate 30-49 250 to 750 mg twice dailySevere < 30 250 to 500 mg twice dailyEnd-stage renal disease patients -- 500 to 1,000 mg once daily (2) undergoing dialysis (1)(1)A 750 mg loading dose is recommended on the first day of treatment with levetiracetam.(2)Following dialysis, a 250 to 500 mg supplemental dose is recommended.For children with renal impairment, levetiracetam dose needs to be adjusted based on the renal function as levetiracetam clearance is related to renal function. This recommendation is based on a study in adult renally impaired patients.The CLcr in ml/min/1.73 m2 may be estimated from serum creatinine (mg/dl) determination using, for young adolescents and children using the following formula (Schwartz formula):Height (cm) x ksCLcr (ml/min/1.73 m2) = -------------------------------------------Serum Creatinine (mg/dl)ks= 0.55 in Children to less than 13 years and in adolescent female; ks= 0.7 in adolescent male Dosing adjustment for children and adolescents patients weighing less than 50 kg with impaired renal functionlevetiracetam. (2) Following dialysis, a 5 to 10 mg/kg (0.05 to 0.10 ml/kg) supplemental dose is recommended. Hepatic impairment No dose adjustment is needed in patients with mild to moderate hepatic impairment. In patients with severe hepatic impairment, the creatinine clearance may underestimate the renal insufficiency. Therefore a 50 % reduction of the daily maintenance dose is recommended when the creatinine clearance is < 60 ml/min/1.73 m 2. Paediatric population The physician should prescribe the most appropriate pharmaceutical form, presentation and strength according to age, weight and dose. Monotherapy The safety and efficacy of Levetiracetam SUN in children below and adolescents 16 years as monotherapy treatment have not been established. There are no data available. Add-on therapy for children aged 4 to 11 years and adolescents (12 to 17 years) weighing less than 50 kg The initial therapeutic dose is 10 mg/kg twice daily. Depending upon the clinical response and tolerability, the dose can be increased up to 30 mg/kg twice daily. Dose changes should not exceed increases or decreases of 10 mg/kg twice daily every two weeks. The lowest effective dose should be used. Dose in children 50 kg or greater is the same as in adults. (2) Dose in children and adolescents 50 kg or more is the same as in adults.Add-on therapy for infants and children less than 4 yearsThe safety and efficacy of Levetiracetam SUN concentrate for solution for infusion in infants and children less than 4 years have not been established.Currently available data are described in sections 4.8, 5.1, and 5.2 but no recommendation on a posology can be made.Method of administrationConversion to or from oral to intravenous administration can be done directly without titration. The total daily dose and frequency of administration should be maintained.Levetiracetam SUN concentrate is for intravenous use only and the recommended dose must be diluted in at least 100 ml of a compatible diluent and administered intravenously as a 15-minute intravenous infusion (see section 6.6).4.3ContraindicationsHypersensitivity to the active substance or other pyrrolidone derivatives or to any of the excipients.4.4Special warnings and precautions for useDiscontinuationIn accordance with current clinical practice, if levetiracetam has to be discontinued it is recommended to withdraw it gradually (e.g. in adults and adolescents weighing more than 50 kg: 500 mg decreases twice daily every two to four weeks; in children and adolescents weighting less than 50 kg: dose decrease should not exceed 10 mg/kg twice daily every two weeks).Renal insufficiencyThe administration of levetiracetam to patients with renal impairment may require dose adjustment. In patients with severely impaired hepatic function, assessment of renal function is recommended before dose selection (see section 4.2).SuicideSuicide, suicide attempt, suicidal ideation and behaviour have been reported in patients treated with anti-epileptic agents (including levetiracetam). A meta-analysis of randomized placebo-controlled trials of anti-epileptic medicinal products has shown a small increased risk of suicidal thoughts and behaviour. The mechanism of this risk is not known.Therefore patients should be monitored for signs of depression and/or suicidal ideation and behaviours and appropriate treatment should be considered. Patients (and caregivers of patients) should be advised to seek medical advice should signs of depression and/or suicidal ideation or behaviour emerge. Paediatric populationon learning, intelligence, growth, endocrine function, puberty and childbearing potential in children remain unknown.Excipientsinto consideration by patients on a controlled sodium diet.4.5Interaction with other medicinal products and other forms of interactionAntiepileptic medicinal productsinfluence the serum concentrations of existing antiepileptic medicinal products (phenytoin,carbamazepine, valproic acid, phenobarbital, lamotrigine, gabapentin and primidone) and that these antiepileptic medicinal products did not influence the pharmacokinetics of levetiracetam.As in adults, there is no evidence of clinically significant medicinal product interactions in paediatric patients receiving up to 60 mg/kg/day levetiracetam.A retrospective assessment of pharmacokinetic interactions in children and adolescents with epilepsy(4 to 17 years) confirmed that adjunctive therapy with orally administered levetiracetam did not influence the steady-state serum concentrations of concomitantly administered carbamazepine and valproate. However, data suggested a 20% higher levetiracetam clearance in children taking enzyme-inducing antiepileptic medicinal products. Dose adjustment is not required.ProbenecidProbenecid (500 mg four times daily), a renal tubular secretion blocking agent, has been shown to inhibit the renal clearance of the primary metabolite but not of levetiracetam. Nevertheless, the concentration of this metabolite remains low. It is expected that other medicinal products excreted by active tubular secretion could also reduce the renal clearance of the metabolite. The effect of levetiracetam on probenecid was not studied and the effect of levetiracetam on other actively secreted medicinal products, e.g. NSAIDs, sulfonamides and methotrexate, is unknown.Oral contraceptives and other pharmacokinetics interactionsLevetiracetam 1,000 mg daily did not influence the pharmacokinetics of oral contraceptives (ethinyl-estradiol and levonorgestrel); endocrine parameters (luteinizing hormone and progesterone) were not modified. Levetiracetam 2,000 mg daily did not influence the pharmacokinetics of digoxin and warfarin; prothrombin times were not modified. Co-administration with digoxin, oral contraceptives and warfarin did not influence the pharmacokinetics of levetiracetam.AlcoholNo data on the interaction of levetiracetam with alcohol are available.4.6Fertility,pregnancy and lactationPregnancyThere are no adequate data available from the use of levetiracetam in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). The potential risk for human is unknown. Levetiracetam is not recommended during pregnancy and in women of childbearing potential not using contraception unless clearly necessary.As with other antiepileptic medicinal products, physiological changes during pregnancy may affect levetiracetam concentration. Decrease in levetiracetam plasma concentrations has been observed during pregnancy. This decrease is more pronounced during the third trimester (up to 60% of baseline concentration before pregnancy). Appropriate clinical management of pregnant women treated with levetiracetam should be ensured. Discontinuation of antiepileptic treatments may result in exacerbation of the disease which could be harmful to the mother and the foetus.BreastfeedingHowever, if levetiracetam treatment is needed during breastfeeding, the benefit/risk of the treatment should be weighed considering the importance of breastfeeding.Fertilitypotential risk for human is unknown.4.7Effects on ability to drive and use machinesNo studies on the effects on the ability to drive and use machines have been performed.Due to possible different individual sensitivity, some patients might experience somnolence or other central nervous system related symptoms, especially at the beginning of treatment or following a doseincrease. Therefore, caution is recommended in those patients when performing skilled tasks, e.g. driving vehicles or operating machinery. Patients are advised not to drive or use machines until it is established that their ability to perform such activities is not affected.4.8Undesirable effectsSummary of the safety profileThe adverse event profile presented below is based on the analysis of pooled placebo-controlled clinical trials with all indications studied, with a total of 3,416 patients treated with levetiracetam. These data are supplemented with the use of levetiracetam in corresponding open-label extension studies, as well as post-marketing experience. The most frequently reported adverse reactions were nasopharyngitis, somnolence, headache, fatigue and dizziness. The safety profile of levetiracetam is generally similar across age groups (adult and paediatric patients) and across the approved epilepsy indications. Since there was limited exposure for levetiracetam intravenous use and since oral and intravenous formulations are bioequivalent, the safety information of levetiracetam intravenous will rely on levetiracetam oral use.Tabulated list of adverse reactionsAdverse reactions reported in clinical studies (adults, adolescents, children and infants > 1 month) and from post-marketing experience are listed in the following table per System Organ Class and per frequency. The frequency is defined as follows: very common (≥1/10); common (≥ 1/100 to <1/10); uncommon: (≥ 1/1,000 to <1/100); rare (≥ 1/10,000 to <1/1,000) and very rare (<1/10,000).MedDRA SOC Frequency categoryUncommonMedDRA SOC Frequency categoryDescription of selected adverse reactionsThe risk of anorexia is higher when topiramate is coadministered with levetiracetam.In several cases of alopecia, recovery was observed when levetiracetam was discontinued. Paediatric populationIn patients aged 1 month to less than 4 years, a total of 190 patients have been treated with levetiracetam in placebo-controlled and open label extension studies. Sixty (60) of these patients were treated with levetiracetam in placebo-controlled studies. In patients aged 4-16 years, a total of 645 patients have been treated with levetiracetam in placebo-controlled and open label extension studies. 233 of these patients were treated with levetiracetam in placebo-controlled studies. In both these paediatric age ranges, these data are supplemented with the post-marketing experience of the use of levetiracetam.The adverse event profile of levetiracetam is generally similar across age groups and across the approved epilepsy indications. Safety results in paediatric patients in placebo-controlled clinical studies were consistent with the safety profile of levetiracetam in adults except for behavioural and psychiatric adverse reactions which were more common in children than in adults. In children and adolescents aged 4 to 16 years, vomiting (very common, 11.2%), agitation (common, 3.4%), mood swings (common, 2.1%), affect lability (common, 1.7%), aggression (common, 8.2%), abnormal behaviour (common, 5.6%), and lethargy (common, 3.9%) were reported more frequently than in other age ranges or in the overall safety profile. In infants and children aged 1 month to less than 4 years, irritability (very common, 11.7%) and coordination abnormal (common, 3.3%) were reported morefrequently than in other age groups or in the overall safety profile.A double-blind, placebo-controlled paediatric safety study with a non-inferiority design has assessed the cognitive and neuropsychological effects of levetiracetam in children 4 to 16 years of age with partial onset seizures. It was concluded that levetiracetam was not different (non inferior) from placebo with regard to the change from baseline of the Leiter-R Attention and Memory, Memory Screen Composite score in the per-protocol population. Results related to behavioural and emotional functioning indicated a worsening in levetiracetam treated patients on aggressive behaviour as measured in a standardized and systematic way using a validated instrument (CBCL – Achenbach Child Behavior Checklist).However subjects, who took levetiracetam in the long-term open label follow-up study, did not experience a worsening, on average, in their behavioural and emotional functioning; in particular measures of aggressive behavior were not worse than baseline.4.9 OverdoseSymptomsSomnolence, agitation, aggression, depressed level of consciousness, respiratory depression and coma were observed with levetiracetam overdoses.Management of overdoseThere is no specific antidote for levetiracetam. Treatment of an overdose will be symptomatic and may include haemodialysis. The dialyser extraction efficiency is 60 % for levetiracetam and 74 % for the primary metabolite.5. PHARMACOLOGICAL PROPERTIES5.1 Pharmacodynamic propertiesPharmacotherapeutic group: antiepileptics, other antiepileptics, ATC code: N03AX14.The active substance, levetiracetam, is a pyrrolidone derivative (S-enantiomer ofα-ethyl-2-oxo-1-pyrrolidine acetamide), chemically unrelated to existing antiepileptic active substances.Mechanism of actionThe mechanism of action of levetiracetam still remains to be fully elucidated but appears to be different from the mechanisms of current antiepileptic medicinal products. In vitro and in vivo experiments suggest that levetiracetam does not alter basic cell characteristics and normal neurotransmission.In vitro studies show that levetiracetam affects intraneuronal Ca2+ levels by partial inhibition of N-type Ca2+ currents and by reducing the release of Ca2+ from intraneuronal stores. In addition, it partially reverses the reductions in GABA- and glycine-gated currents induced by zinc and -carbolines. Furthermore, levetiracetam has been shown in in vitro studies to bind to a specific site in rodent brain tissue. This binding site is the synaptic vesicle protein 2A, believed to be involved in vesicle fusion and neurotransmitter exocytosis. Levetiracetam and related analogues show a rank order of affinity for binding to the synaptic vesicle protein 2A which correlates with the potency of their anti-seizure protection in the mouse audiogenic model of epilepsy. This finding suggests that the interaction between levetiracetam and the synaptic vesicle protein 2A seems to contribute to the antiepileptic mechanism of action of the medicinal product.Pharmacodynamic effectsLevetiracetam induces seizure protection in a broad range of animal models of partial and primary generalised seizures without having a pro-convulsant effect. The primary metabolite is inactive. In man, an activity in both partial and generalised epilepsy conditions (epileptiform discharge/ photoparoxysmal response) has confirmed the broad spectrum pharmacological profile of levetiracetam.Clinical efficacy and safetyAdjunctive therapy in the treatment of partial onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy:In adults, levetiracetam efficacy has been demonstrated in 3 double-blind, placebo-controlled studies at 1000 mg, 2000 mg, or 3000 mg/day, given in 2 divided doses, with a treatment duration of up to 18 weeks. In a pooled analysis, the percentage of patients who achieved 50% or greater reduction from baseline in the partial onset seizure frequency per week at stable dose (12/14 weeks) was of 27.7%, 31.6% and 41.3% for patients on 1000, 2000 or 3000 mg levetiracetam respectively and of 12.6% for patients on placebo.Paediatric populationIn paediatric patients (4 to 16 years of age), levetiracetam efficacy was established in a double-blind, placebo-controlled study, which included 198 patients and had a treatment duration of 14 weeks. In this study, the patients received levetiracetam as a fixed dose of 60 mg/kg/day (with twice a day dosing).44.6% of the levetiracetam treated patients and 19.6% of the patients on placebo had a 50% or greater reduction from baseline in the partial onset seizure frequency per week. With continued long-term treatment, 11.4% of the patients were seizure-free for at least 6 months and 7.2% were seizure-free for at least 1 year.Monotherapy in the treatment of partial onset seizures with or without secondary generalisation in patients from 16 years of age with newly diagnosed epilepsy.Efficacy of levetiracetam as monotherapy was established in a double-blind, parallel group, non-inferiority comparison to carbamazepine controlled release (CR) in 576 patients 16 years of age or older with newly or recently diagnosed epilepsy. The patients had to present with unprovoked partial seizures or with generalized tonic-clonic seizures only. The patients were randomized to carbamazepine CR 400 – 1200 mg/day or levetiracetam 1000 – 3000 mg/day, the duration of the treatment was up to 121 weeks depending on the response.Six-month seizure freedom was achieved in 73.0% of levetiracetam-treated patients and 72.8% of carbamazepine-CR treated patients; the adjusted absolute difference between treatments was 0.2% (95% CI: -7.8 8.2). More than half of the subjects remained seizure free for 12 months (56.6% and 58.5% of subjects on levetiracetam and on carbamazepine CR respectively).In a study reflecting clinical practice, the concomitant antiepileptic medication could be withdrawn in a limited number of patients who responded to levetiracetam adjunctive therapy (36 adult patients out of 69).Adjunctive therapy in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy.Levetiracetam efficacy was established in a double-blind, placebo-controlled study of 16 weeks duration, in patients 12 years of age and older suffering from idiopathic generalized epilepsy with myoclonic seizures in different syndromes. The majority of patients presented with juvenile myoclonic epilepsy.In this study, levetiracetam, dose was 3000 mg/day given in 2 divided doses.58.3% of the levetiracetam treated patients and 23.3% of the patients on placebo had at least a 50% reduction in myoclonic seizure days per week. With continued long-term treatment, 28.6% of the patients were free of myoclonic seizures for at least 6 months and 21.0% were free of myoclonic seizures for at least 1 year.Adjunctive therapy in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy.Levetiracetam efficacy was established in a 24-week double-blind, placebo-controlled study which included adults, adolescents and a limited number of children suffering from idiopathic generalized epilepsy with primary generalized tonic-clonic (PGTC) seizures in different syndromes (juvenile myoclonic epilepsy, juvenile absence epilepsy, childhood absence epilepsy, or epilepsy with Grand Mal seizures on awakening). In this study, levetiracetam dose was 3000 mg/day for adults and adolescents or 60 mg/kg/day for children, given in 2 divided doses.72.2% of the levetiracetam treated patients and 45.2% of the patients on placebo had a 50% or greater decrease in the frequency of PGTC seizures per week. With continued long-term treatment, 47.4% of the patients were free of tonic-clonic seizures for at least 6 months and 31.5% were free of tonic-clonic seizures for at least 1 year.5.2 Pharmacokinetic propertiesThe pharmacokinetic profile has been characterized following oral administration. A single dose of 1500 mg levetiracetam diluted in 100 ml of a compatible diluent and infused intravenously over 15 minutes is bioequivalent to 1500 mg levetiracetam oral intake, given as three 500 mg tablets.The intravenous administration of doses up to 4000 mg diluted in 100 ml of 0.9% sodium chloride infused over 15 minutes and doses up to 2500 mg diluted in 100 ml of 0.9% sodium chloride infused over 5 minutes was evaluated. The pharmacokinetic and safety profiles did not identify any safety concerns.Levetiracetam is a highly soluble and permeable compound. The pharmacokinetic profile is linear with low intra- and inter-subject variability. There is no modification of the clearance after repeated administration. The time independent pharmacokinetic profile of levetiracetam was also confirmed following 1500 mg intravenous infusion for 4 days with b.i.d dosing.There is no evidence for any relevant gender, race or circadian variability. The pharmacokinetic profile is comparable in healthy volunteers and in patients with epilepsy.Adults and adolescentsDistributionPeak plasma concentration (C max) observed in 17 subjects following a single intravenous dose of 1500 mg infused over 15 minutes was 51 ± 19 μg/mL (arithmetic average ± standard deviation).No tissue distribution data are available in humans.Neither levetiracetam nor its primary metabolite are significantly bound to plasma proteins (< 10 %). The volume of distribution of levetiracetam is approximately 0.5 to 0.7 l/kg, a value close to the total body water volume.BiotransformationLevetiracetam is not extensively metabolised in humans. The major metabolic pathway (24 % of the dose) is an enzymatic hydrolysis of the acetamide group. Production of the primary metabolite, ucbL057, is not supported by liver cytochrome P450 isoforms. Hydrolysis of the acetamide group was measurable in a large number of tissues including blood cells. The metabolite ucb L057 is pharmacologically inactive.Two minor metabolites were also identified. One was obtained by hydroxylation of the pyrrolidone ring (1.6 % of the dose) and the other one by opening of the pyrrolidone ring (0.9 % of the dose). Other unidentified components accounted only for 0.6 % of the dose.No enantiomeric interconversion was evidenced in vivo for either levetiracetam or its primary metabolite.In vitro, levetiracetam and its primary metabolite have been shown not to inhibit the major human liver cytochrome P450 isoforms (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1 and 1A2), glucuronyl transferase (UGT1A1 and UGT1A6) and epoxide hydroxylase activities. In addition, levetiracetam does not affect the in vitro glucuronidation of valproic acid.In human hepatocytes in culture, levetiracetam had little or no effect on CYP1A2, SULT1E1 or UGT1A1. Levetiracetam caused mild induction of CYP2B6 and CYP3A4. The in vitro data and in vivo interaction data on oral contraceptives, digoxin and warfarin indicate that no significant enzyme induction is expected in vivo. Therefore, the interaction of levetiracetam with other substances, or vice versa, is unlikely.EliminationThe plasma half-life in adults was 7±1 hours and did not vary either with dose, route of administration or repeated administration. The mean total body clearance was 0.96 ml/min/kg.The major route of excretion was via urine, accounting for a mean 95 % of the dose (approximately 93 % of the dose was excreted within 48 hours). Excretion via faeces accounted for only 0.3 % of the dose.The cumulative urinary excretion of levetiracetam and its primary metabolite accounted for 66 % and 24 % of the dose, respectively during the first 48 hours.The renal clearance of levetiracetam and ucb L057 is 0.6 and 4.2 ml/min/kg respectively indicating that levetiracetam is excreted by glomerular filtration with subsequent tubular reabsorption and that the primary metabolite is also excreted by active tubular secretion in addition to glomerular filtration. Levetiracetam elimination is correlated to creatinine clearance.ElderlyIn the elderly, the half-life is increased by about 40 % (10 to 11 hours). This is related to the decrease in renal function in this population (see section 4.2).Renal impairmentThe apparent body clearance of both levetiracetam and of its primary metabolite is correlated to the creatinine clearance. It is therefore recommended to adjust the maintenance daily dose of levetiracetam, based on creatinine clearance in patients with moderate and severe renal impairment (see section 4.2).In anuric end-stage renal disease adult subjects the half-life was approximately 25 and 3.1 hours during interdialytic and intradialytic periods, respectively.The fractional removal of levetiracetam was 51 % during a typical 4-hour dialysis session.Hepatic impairmentIn subjects with mild and moderate hepatic impairment, there was no relevant modification of the clearance of levetiracetam. In most subjects with severe hepatic impairment, the clearance of levetiracetam was reduced by more than 50 % due to a concomitant renal impairment (see section 4.2).Peadiatric populationChildren (4 to 12 years)。
左乙拉西坦-注射液(5ml)说明书中文翻译

左乙拉西坦-打针液(5ml)解释书中文翻译左乙拉西坦打针液解释书参考译文近期重要变动剂量与用法,部分性发生发火(2.6)警告与留意事项(5.1.5.2.5.3.5.4.5.7)顺应症与运用当口服KEPPRA给药临时不成行时,KEPPRA打针液是抗癫痫药物用于成人(≥16岁)以下发生发火类型的帮助治疗:部分性发生发火(1.1)少年肌阵挛癫痫患者的肌阵挛性发生发火(1.2)原发性强直性癫痫发生发火(1.3)剂量与给药办法KEPPRA打针液在运用前用100mL可配伍稀释剂稀释,滴注15min (2.1).初始运用KEPPRA(2.2):部分性发生发火:1000mg/d,天天2次(500mg,天天2次),根据须要和耐受性每2周增长1000mg/d 至最大推举日剂量3000mg/d. 青少年肌阵挛性癫痫患者的肌阵挛性发生发火:初始剂量1000mg/d(500mg,天天2次).每2周增长剂量1000mg/d至推举日剂量为3000mg/d,尚未研讨剂量低于3000mg/d的有用性. 特发性全身强直阵挛发生发火:初始剂量1000mg/d(500mg,天天2次).每2周增长剂量1000mg/d至推举日剂量3000mg/d,尚未研讨剂量低于3000mg/d的有用性.替代治疗(2.3)从口服KEPPRA转向静脉打针剂量:开端静脉打针左乙拉西坦,总日剂量和频率与片剂相当.在静滴治疗期停滞时,患者可转用KEPPRA口服给药,日剂量和频率与静脉打针左乙拉西坦相当.剂量解释(2.5).肾功效伤害成人患者(2.6)和相容性和稳固性(2.7)见全体处方信息.剂型和剂量500mg/5mL,一次性小瓶(3).禁忌无警告与留意事项1精力病反响:行动平常包含精力病症状.自杀意念.易激.进击行动.监测患者的精力病征兆和症状(5.1).嗜睡和疲惫:监测患者的这些症状,在未获得充分的经验前奉劝患者不要驾驶汽车或操纵机械(5.2).撤药痉挛:KEPPRA应逐渐削减(5.5).不良反响最罕有的不良反响(KEPPRA比安慰剂的产生率≥5%)包含:嗜睡.无力.沾染和眩晕(6.1).特别人群运用怀胎:怀胎时代左乙拉西坦的血浆浓度可能降低,需亲密监测.基于动物实验数据可能导致胚胎伤害(5.7.8.1).1 顺应症和运用当口服KEPPRA临时不成行时,打针液可用于成人(16岁及以上)患者.1.1 部分性发生发火KEPPRA用于成人癫痫患者部分性发生发火的帮助治疗.1.2 青少年肌阵挛性癫痫患者的肌阵挛性癫痫发生发火KEPPRA用于成人青少年期肌阵挛性癫痫患者的肌阵挛性癫痫发生发火的帮助治疗.1.3 特发性全身强直阵挛性癫痫发生发火KEPPRA用于成人特发性全身性癫痫患者的原发性全身性强直性阵挛癫痫发生发火的帮助治疗.2 剂量和给药办法2.1 一般信息KEPPRA打针液仅用于静滴,运用前需稀释.KEPPRA打针液(500mg/5mL)用可配伍稀释剂稀释至100mL[见剂量和给药办法(2.7)],静脉静滴15min.消失不溶性微粒或者变色后不克不及运用.KEPPRA打针液小瓶内容物未运用部分应弃掉落.2.2 KEPPRA的运用开端口服或静脉打针KEPPRA无先后次序. 部分性发生发火在口服左乙拉西坦的临床实验中,日剂量分离为1000mg.2000mg和3000mg,天天2次均显示有用性.固然在一些研讨中偏向于高剂量的反响率更高[见临床研讨(14.1)],2尚不清晰反响率的增长是否与剂量增长一致.开端日剂量1000mg/kg,天天2次(500mg,天天2次).增长剂量(每2周增长1000mg/d)至最大推举日剂量3000mg.在一项凋谢性标签研讨中运用剂量>3000mg/d,周期≥6个月,尚无证据标明>3000mg/d有更多的获益. 青少年肌阵挛性癫痫患者的肌阵挛性癫痫发生发火开端治疗剂量1000mg/d,天天2次(500mg,天天2次).每2周增长1000mg/d至推举日剂量3000mg.尚未研讨剂量<3000mg/kg的有用性. 特发性全身强直阵挛性癫痫发生发火开端治疗剂量1000mg/d,天天2次(500mg,天天2次),每2周增长1000mg/d至推举日剂量3000mg.尚未充分研讨剂量<3000mg/d 的有用性.2.3 替代疗法当从口服转为滴注时,开端静滴日剂量和频率与口服相当,100mL 配伍稀释剂稀释后应静滴给药15min.2.4 转为口服剂量在静脉滴注治疗阶段停滞时,患者可运用KEPPRA口服给药,日剂量和频率与打针给药相当.2.5 剂量解释KEPPRA打针剂仅用于静滴,运用前需稀释.每1小瓶KEPPRA打针液含500mg左乙拉西坦(500mg/5mL).剂量500mg.1000mg或1500mg的推举制备和给药办法见表1.表1:KEPPRA打针液制备和给药办法例如:制备剂量1000mg,用可配伍稀释剂100mL稀释10mLKEPPRA 打针液[见剂量和给药办法(2.7)],静脉滴注15min.2.6 成人肾功效伤害患者应当根据患者的肾功效状况共性化给药.推举剂量和调剂计划见表2.为了盘算肾功效患者的推举剂量,根据体概况积调剂盘算肌酐清除率.起首采取以下公式估算患者的肌酐清除率(CLcr,mL/min):3CLcr[140年纪(岁)]体重(kg)0.85)72血清肌酐(mg/dL)CLcr按以下体概况积(BSA)进行调剂:CLcr(mL/min/1.73m2)CLcr(mL/min)2BSA(m)1透析后,推举填补剂量250mg~500mg.2.7 相容性和稳固性KEPPRA打针剂与以下稀释剂及抗癫痫药混杂后贮藏于聚氯乙烯(PVC)袋中,室温15~30℃(59~86˚F)放置达24h,物理相容性和化学性质稳固.稀释剂氯化钠(0.9%)打针液,USP 乳酸钠林格打针液葡萄糖(5%)打针液,USP其他抗癫痫药劳拉西泮地西泮丙戊酸钠除以上所列抗癫痫药物外,尚无KEPPRA打针剂与其他抗癫痫药物之间的物理相容性数据.非口服药物给药前都运用肉眼检讨不溶性微粒和变色现象. 3 剂型和剂量1小瓶KEPPRA打针液含左乙拉西坦500mg(500mg/5mL). 4 禁忌无45 警告与留意事项5.1 精力病反响KEPPRA导致一些患者行动平常.在肌阵挛和原发性全身强直性阵挛发生发火研讨中,行动平常的产生率与成人部分性发生发火研讨中的产生率具有可比性.非精力病行动症状(报导如进击.冲动.恼怒.焦炙.情感淡漠.损掉人道.情感不稳.敌意.易激愤和神经质):KEPPRA治疗的成人患者(13.3%),安慰剂组(6.2%).因行动不良事宜而停滞治疗:KEPPRA治疗的成人患者(1.7%),安慰剂组(0.2%);剂量削减:KEPPRA治疗的成人患者(0.8%),安慰剂组(0.5%).精力病症状:KEPPRA治疗的成人患者(1%),安慰剂组(0.2%).住院:KEPPRA治疗的成人患者,2例患者因精力病住院(0.3%)并停滞治疗.2个事宜,报导为精力病,第1周产生,1~2周内停滞治疗.需监测以上精力病征兆和症状.5.2 嗜睡和疲惫KEPPRA导致一些患者嗜睡和疲惫.以下嗜睡和疲惫的产生率来自于成人部分性发生发火对比研讨.总之,肌阵挛和原发性强直性研讨中嗜睡和疲惫的产生率与成人部分性发生发火研讨中的产生率具有可比性.在部分性发生发火的癫痫成人患者的对比研讨中,报导嗜睡,左乙拉西坦治疗相干的患者14.8%,安慰剂组8.4%.剂量达3000mg/kg 无明显的剂量反响.研讨中无滴定剂量,服用4000mg/kg的患者,约45%报导嗜睡.轻微嗜睡,治疗组0.3%,安慰剂组0%.因嗜睡停滞治疗的患者,左乙拉西坦组约3%,安慰剂组0.7%.因嗜睡剂量削减的患者,治疗组1.4%,安慰剂组0.9%.因嗜睡住院的患者,治疗组0.3%.在部分性发生发火的癫痫成人患者的对比实验中,报导疲惫:治疗组14.7%,安慰剂组9.1%.因疲惫停滞治疗:治疗组0.8%,安慰剂组0.5%.因疲惫削减剂量:治疗组0.5%,安慰剂组0.2%.治疗的前4周最罕有的不良反响是嗜睡和疲惫.需监测患者的这些征兆和症状,在未充分获得关于左乙拉西坦是否会影响患者开车或操纵机械装备的才能的经验前,建议患者不要开车或操纵机械装备.5.3 轻微皮肤病反响已有患者报导运用左乙拉西坦治疗产生轻微皮肤病反响,包含Stevens-Johnson分解征(SJS)和中毒性表皮坏逝世松解症(TEN).开端的中位时光14~17天,均在开端治疗后至少4个月才报导.也有从新运用左乙拉西坦后轻微皮肤反响复发的报导.当消失一个疹时,除非与药物无关,不然停滞运用左乙拉西坦.假如标明为SJS/TEN的征兆或5症状时,不得从新运用该药物并斟酌其他治疗办法.5.4 调和艰苦调和艰苦(报导如共济掉调.平常步态或动作掉调)仅在部分性发生发火的成人患者的研讨中不雅察到,左乙拉西坦组3.4%,安慰剂组1.6%.在对比实验中,因共济掉调处止治疗:左乙拉西坦组0.4%,安慰剂组0%;因调和艰苦削减剂量:治疗组(0.7%)和安慰剂组(0.2%)个中治疗组的一例患者因以往的共济掉调恶化而住院治疗.这些事宜为治疗前4周内最罕有的不良反响.需监测患者的这些征兆和症状,在充分未获得关于左乙拉西坦是否会影响患者开车或操纵机械装备的才能的经验前,建议患者不要开车或操纵机械装备.5.5 撤药痉挛抗癫痫药物,包含KEPPRA,需逐渐削减以削减增长发生发火频率的可能性.5.6 血液学平常部分性发生发火在对比临床研讨中,与安慰剂比,可见左乙拉西坦治疗患者的总平均RBC数(0.03x106/mm3).平均血红蛋白(0.09g/dL)和平均血细胞比容(0.38%)微量削减,但有统计学意义.WBC明显降低(≤2.8 x109/L),左乙拉西坦组(3.2%),安慰剂组(1.8%);中性白细胞数明显降低(≤1.0x109/L),至少有一个可能的意义,左乙拉西坦组(2.4%),安慰剂组(1.4%).左乙拉西坦治疗的患者中性粒细胞数低,中断治疗除1破例其余均升高或达基线值,无患者因继发于中性粒细胞少而停滞治疗.青少年肌阵挛性癫痫固然JME患者无明显的血液学平常,但患者数目有限得出实验性结论.以为起源于部分性发生发火患者的数据与JME患者相干.5.7 怀胎时代掌握发生发火怀胎时代的心理变更可逐渐降低左乙拉西坦的血浆程度,怀胎晚期降低尤为明显.怀胎时代需当心监测,假如在怀胎时代剂量产生转变时,产后期应中断亲密监测. 6 不良反响以下不良反响在其他章节有具体评论辩论:•精力病反响[见警告与留意事项(5.1)]•嗜睡和疲惫[见警告与留意事项(5.2)]•轻微皮肤病反响[见警告与留意事项(5.3)]•调和艰苦[见警告与留意事项(5.4)]6•撤药痉挛[见警告与留意事项(5.5)]•血液学平常[见警告与留意事项(5.6)]•怀胎时代掌握发生发火[见警告与留意事项(5.7)]6.1 临床经验因为临床实验是在一个变更的前提下进行的,药物临床实验中不雅察的不良反响产生率不克不及直接与其他药物临床实验的不良反响产生率比拟较,它可能不克不及反应现实中药物的不良反响产生率.KEPPRA打针剂的不良反响包含KEPPRA片剂和口服液报导的全体不良反响.静脉打针KEPPRA的剂量(滴注15min)与口服KEPPRA的剂量相当,两者的Cmax.Cmin和全身吐露量也相当.处方医师需留意以下表格所列不良反响的产生率,包含KEPPRA作为AED治疗的帮助药物时,在通例医疗实践中,患者特点及其他身分可能都不合于临床实验中罕有的,是以医师不克不及猜测不良反响的产生率.同样地,所引用的频率不克不及直接与其他临床监测到的数据比拟较,因为其他临床监测中涉及到治疗.运用和研讨对象都不合.尽管如斯,在种群研讨中,这些数据仍可以作为处方医师评估药物或非药物与不良反响产生率相干性的一个根据. 部分性发生发火在部分性发生发火的成人患者服用KEPPRA片剂的对比临床研讨中,患者合用KEPPRA与其他AEDs,最罕有且产生率高于安慰剂的不良反响:嗜睡.疲惫.沾染和眩晕.部分性发生发火的成人患者最罕有的不良反响为疲惫.嗜睡和眩晕,重要产生在治疗的前4周内.表4列出了在安慰剂对比研讨中,运用左乙拉西坦片治疗的成人癫痫患者,产生率至少为1%且高于安慰剂组的不良反响.在这些研讨中,左乙拉西坦或安慰剂作为AED治疗的帮助药物,不良反响常为轻中度.表4:在成人部分性发生发火的安慰剂对比.添加研讨中(KEPPRA 组的不良反响产生率至少为1%且高于安慰剂)的不良反响产生率(%)7在服用KEPPRA片剂的成人对比临床研讨中,因不良反响停滞治疗或削减剂量:KEPPRA(15%),安慰剂(12%).停滞或削减剂量最罕有(>1%),且KEPPRA组高于安慰剂组的不良反响见表5.表5:在成人部分性发生发火的安慰剂对比研讨中,导致停滞或削减剂量且KEPPRA组高于安慰剂的不良反响肌阵挛性癫痫发生发火因为本研讨患者数目很小,本研讨的不良反响不合于部分性发生发火患者所见的类型,但估计JME患者的不良反响类型本质上与部分性发生发火的患者雷同.在肌阵挛性发生发火的患者服用KEPPRA片剂的对比临床研讨中,KEPPRA归并其它8AEDs的最罕有且高于安慰剂的不良反响:嗜睡,颈痛和咽炎.表6列出了肌阵挛性癫痫发生发火的少年肌阵挛性癫痫患者运用KEPPRA片治疗的不良反响,产生率至少5%且高于安慰剂组.本研讨中,左乙拉西坦或安慰剂作为AED治疗的帮助药物,不良反响常为轻中度.表6:在肌阵挛性癫痫发生发火的安慰剂对比.添加研讨中(KEPPRA组的不良反响产生率至少为5%且高于安慰剂)的不良反响产生率(%)在服用KEPPRA片剂的安慰剂对比研讨中,因不良反响停滞或削减剂量:KEPPRA(8%),安慰剂(2%).导致停滞或削减剂量且产生率高于安慰剂的不良反响见表7. 表7:在肌阵挛性癫痫发生发火的安慰剂对比研讨中,导致停滞或削减剂量且KEPPRA组高于安慰剂的不良反响特发性全身强直阵挛性癫痫发生发火因为本研讨患者数目很小,不良反响不合于部分性发生发火患者所见的类型.估计PGTC发生发火的患者的不良反响类型本质上与部分性发生发火的患者雷同.9对比临床研讨包含4岁及以上特发性全身性强直阵挛(PGTC)发生发火患者,左乙拉西坦结合其它AEDs,最罕有且高于安慰剂组的不良反响是鼻咽炎.表8列出了左乙拉西坦治疗特发性全身性癫痫发生发火患者的PGTC发生发火的产生率≥5%且高于安慰剂组的不良反响.本研讨中,左乙拉西坦或安慰剂作为AED治疗的帮助药物,不良反响常为轻中度.表8:在4岁及以上PGTC发生发火的安慰剂对比添加研讨中(不良反响产生率至少为5%,且高于安慰剂组)在安慰剂对比研讨中,因不良反响停滞治疗或削减剂量:KEPPRA (5%),安慰剂(8%). 本研讨小而不克不及精确描写可能导致停滞治疗的不良反响的特点,估计导致停滞治疗的不良反响可能与其他癫痫实验类似(见表5和7).别的,以下为其他掌握优越的KEPPRA成人研讨中所见的不良反响:均衡障碍.留意力的干扰.湿疹.记忆缺点.肌痛和视觉隐约.性别.年纪和种族以下为左乙拉西坦上市后的不良反响,这些不良反响来自不肯定人群大小的自觉陈述,是以也不大可能评估其产生频率或肯定与药物的相干性.除上述列出的不良反响 [见不良反响(6.1)]外,以下不良反响均为上市后全球所报导的,依次为:肝功效测试平常.跳舞徐动症.活动障碍.多形性红斑.肝功效虚弱.肝炎.低钠血症.白细胞削减.肌无力.中性粒细胞削减.胰腺炎.全血细胞削减(经判定一些病例为骨髓克制).惊骇发生发火.血小板削减和体重降低,也有报导脱发,大多半不良反响停滞运用左乙拉西坦后恢复. 7药物互相感化左乙拉西坦及其重要代谢物与其它经肝酶P450亚型.环氧物酶.UDP-葡萄苷酸化10酶.pg蛋白或肾小管渗出的药物合用,无明显的药代学互相感化[见临床药理学(12.3)].8特别人群8.1 怀胎怀胎时代KEPPRA程度可能降低[见警告与留意事项(5.7)].怀胎分类C在怀胎妇女中尚无充足且掌握优越的研讨,在动物研讨中,左乙拉西坦具有明显的发育毒性,包含致畸感化,剂量与人体治疗剂量类似或更高.怀胎时代,仅当对胎儿的潜在获益高于潜在风险时运用左乙拉西坦.雌性大鼠怀胎和哺乳时代,赐与左乙拉西坦剂量≥350mg/kg/d(按体概况积比mg/m2推算相当于人体最大推举剂量3000mg[MRHD]),增长幼崽骨骼畸形和子女出生前或出生后的智力发育迟缓的几率,剂量为1800mg/kg/d(按体概况积比mg/m2推算相当于MRHD的6倍)增长幼崽逝世亡率和子女行动转变.剂量为70mg/kg/d(按体概况积比mg/m2推算相当于MRHD的0.2倍)对发育无影响.该研讨所用剂量对母体无明显毒性.怀胎家兔在器官形成期给药,左乙拉西坦的剂量≥600mg/kg/d(按mg/m2推算相当于MRHD的4倍),可见胚胎—胎仔逝世亡率增长和胎仔骨骼平常产生,剂量为1800mg/kg/d(按mg/m2推算相当于MRHD的12倍)时,可见胎仔体重降低,胎仔畸形产生率增长,并可见母体毒性.剂量为200mg/kg/d(按mg/m2推算相当于MRHD)对发育无影响.怀胎大鼠在器官形成期给药,剂量为3600mg/kg/d(按mg/m2推算相当于MRHD的12倍),可见胎仔体重降低和胎仔骨骼变异产生率增长.剂量为1200mg/kg/d(按mg/m2推算相当于MRHD的4倍)对发育无影响,本研讨未见母体毒性.大鼠在怀胎第3期和全部哺乳期给药,剂量达1800mg/kg/d(按mg/m2推算相当于MRHD的6倍)对发育和母体无不良影响.怀胎登记为供给更多关于怀胎妇女子宫吐露于左乙拉西坦的信息,请求医师推举怀胎患者服用KEPPRA入组NAAED怀胎登记体系,德律风(888)233-2334进行登记.为获得更多关于左乙拉西坦对妊妇的安然及其今后健康的影响的材料,UCB公司树立了KEPPRA怀胎登记体系.为确保海外患者可以登录该体系,患者或者其护理人员皆可经由过程拨打免税德律风(888)537-7734进行登记.8.2 阵痛和临蓐尚不清晰左乙拉西坦对阵痛和临蓐的影响.8.3 哺乳左乙拉西坦可渗出到人体乳汁中.因为左乙拉西坦对哺乳婴儿消失潜在轻微不良反11应,需衡量利弊停滞哺乳或停滞用药,特别要斟酌药物对母亲的重要性.8.4 儿童患者尚未肯定左乙拉西坦打针液对16岁以下患者的安然性和有用性.8.5 老年患者左乙拉西坦临床研讨的全体患者,个中347例患者为65岁及以上.老年个别与年青个别之间的安然性无差别.在癫痫对比研讨中,因为老年患者数目少,不克不及充分评估左乙拉西坦对这些患者的有用性.左乙拉西坦经肾脏渗出,该药物对肾功效伤害患者的不良反响风险可能更大.因为老年患者肾功效降低的可能性更大,是以选择剂量时需当心谨严,并细心监测肾功效[见临床药理学(12.3)].8.6 肾功效伤害患者左乙拉西坦在肾功效伤害患者的清除率降低,且与肌酐清除率相干[见临床药理学(12.3)].推举肾功效伤害患者调剂剂量,透析后要填补剂量[见剂量与给药办法(2.6)]. 10 过量10.1 人体急性过量的征兆.症状和实验室检讨成果在临床进展研讨中,已知口服左乙拉西坦最高剂量为6000mg/d,除嗜睡外,无其他不良反响.上市后过量运用左乙拉西坦可见嗜睡.激愤.进击.意识压制.呼吸克制和晕厥的病例.10.2 过量治理过量服用KEPPRA后没有特异性解毒剂,假如有解释,不接收的药物应当经由过程吐逆或者洗胃来清除,一般在服用药物之前都应当浏览留意事项.一般支撑疗法包含性命体征的监测以及患者临床状况的不雅察.当过量服用KEPPRA时,要与毒药掌握认证中间接洽并获取药物过量治理的最新信息.在尺度透析进程中清除了左乙拉西坦(4h约50%),是以可以斟酌用于药物过量的病例.固然透析尚未用于过量病例,但它可经由过程患者的临床状况或肾功效轻微伤害的患者标明.11性状KEPPRA打针剂是一种用于静脉打针的无色无菌澄清的抗癫痫液体系体例剂(100mg/mL).左乙拉西坦是单一对映异构体,化学名称:(-)-(S)-α-乙基-2-氧-1-吡咯烷乙酰胺,分子式:C8H14N2O2,分子量:170.21.左乙拉西坦的化学构造与其他抗癫痫药物的构造12无关,构造式如下:左乙拉西坦为白色至类白色结晶性粉末,微臭,苦味.极易溶于水(104.0g/100mL).易溶于氯仿(65.3g/100mL)及甲醇(53.6g/100mL),溶于乙醇(16.5g/100mL),略溶于乙腈(5.7g/100mL),几乎不溶于正己烷.1mLKEPPRA打针液含100mg左乙拉西坦,一次性运用的5mL小瓶中含500mg左乙拉西坦.打针用水.45mg氯化钠.冰醋酸和8.2mg三水合醋酸钠缓冲溶液,pH约为5.5.KEPPRA打针剂静脉滴注前必须稀释[见剂量与给药办法(2.1)].12 临床药理学尚不清晰左乙拉西坦确实的感化机制,在多个癫痫发生发火动物模子中评估了左乙拉西坦的抗癫痫感化.左乙拉西坦对电流或多种化学惊厥剂最大刺激引诱的单纯癫痫发生发火无克制造用,并在亚最大刺激和阈值实验中仅显示微弱的活性.但对毛果芸喷鼻碱和红藻氨基酸引诱的局灶性发生发火继发的全身性发生发火不雅察到呵护感化,这两种化学致惊厥剂能模拟一些人伴继发性全身发生发火的庞杂部分性发生发火的特点.左乙拉西坦对庞杂部分性发生发火的大鼠点燃模子的点燃进程和点燃状况均具有克制造用.这些动物模子对人体特定癫痫的猜测价值尚不明白.体内.体外实验显示:左乙拉西坦克制海马癫痫样突发放电,而对正常的神经元高兴性无影响,提醒左乙拉西坦可能选择性地克制癫痫样突发放电超同步性和癫痫发生发火的传播.左乙拉西坦在浓度高达10μM时,对多种已知受体无亲和力,如苯并二氮卓类.GABA.甘氨酸.NMDA.再摄取位点和第二信使体系.别的,体外实验显示左乙拉西坦对神经源电压门控的钠离子通道或T-型钙电流无影响,左乙拉西坦其实不直接易化GABA能神经传递,但体外研讨显示左乙拉西坦对造就的神经元GABA和甘氨酸门控电流负调控活性有反抗感化,并部分克制神经元N-型钙电流.在大鼠脑组织中发明左乙拉西坦可饱和和立体选择性的神经元结合位点,实验数据标明该结合位点为突触小泡蛋白SV2A,SV2A介入小泡胞吞感化的调控.固然左乙拉西坦结合到突触小泡蛋白SV2A 的分子意义尚不清晰,但左乙拉西坦及其相干类似物对SV2A的亲和力有强弱之分,在听觉性发生发火小鼠中,与其施展抗发生发火效能的才能有相干性,标明左乙拉西坦的抗癫痫感化机制可能与SV2A蛋白的互相感化有关.1312.2 药效动力学对QTc间期的影响在52例健康自愿者进行的一项随机.双盲阳性对比(莫西沙星400mg)和安慰剂对比交叉研讨(1000mg或5000mg)中评估了KEPPRA对QTc延伸的影响.经最大的安慰剂调剂以及基线校订的QTc,90%CI上限低于10ms,是以,没有证据标明本品明显延伸QTc.12.3 药代动力学剂量相当的左乙拉西坦打针液(静滴15min)和口服左乙拉西坦,Cmax.Cmin和全身吐露量相当.左乙拉西坦的药动学研讨包含健康成年个别.成人和儿童癫痫患者.老年患者及肾功效和肝功效伤害患者.概述口服左乙拉西坦后快速几乎完整接收,左乙拉西坦打针液和片剂生物等效.左乙拉西坦呈线性代谢和非时变性,个别内和个别间差别小.左乙拉西坦与血浆蛋白结合率低(<10%),散布容积接近于体液.66%的药物以原型经肾脏渗出,重要代谢门路是经由过程水解酶的乙酰胺化(给药剂量的24%),不经肝脏细胞色素P450代谢.代谢物无药理活性,也是经肾脏渗出.血浆半衰期约为6~8h.在老年(重要与肾脏清除率伤害有关)和肾功效伤害患者中延伸.散布在17例健康自愿者中进行左乙拉西坦打针液和口服的生物等效性研讨,左乙拉西坦1500mg稀释至100mL0.9%灭菌溶液中,滴注时光>15min,滴注速度为滴注停滞时血浆浓度与口服雷同剂量的达峰时光(Tmax)类似,成果显示左乙拉西坦1500mg静脉滴注相当于3 x 500mg口服片剂.左乙拉西坦1500mg静滴,中断4天,BID,药代动力学与时光无关.稳态时,AUC(0-12)相当于单次剂量的AUCinf.左乙拉西坦及其重要代谢物与血浆蛋白结合率低(<10%),与蛋白结合位点竞争性药物不大可能产生具有临床意义的互相感化.代谢人体内左乙拉西坦不完整代谢,重要代谢门路经由过程水解酶的乙酰胺化而不经肝酶P450,生成羧酸代谢物,ucb L057(给药剂量的24%),重要代谢物在动物癫痫发生发火模子中无活性.2种较少的代谢产品经判定是羟化的2-氧代-吡咯烷环(给药剂量的2%)和5位开环的2-氧代-吡咯烷(给药剂量的1%).左乙拉西坦或其重要代谢物均无手性翻转. 清除14成人体中左乙拉西坦血浆半衰期为7±1h,其实不因给药剂量不合,给药门路或反复给药而转变.给药剂量的66%经肾脏以原型渗出,。
左乙拉西坦片说明书

左乙拉西坦片说明书本品大家可能都有模糊印象知道能治疗癫痫病症,但是对于具体怎么用,有什么禁忌就不知道了。
所以,下面一起看看左乙拉西坦片说明书。
左乙拉西坦片说明书1、请仔细阅读说明书并在医师指导下使用2、通用名称:左乙拉西坦片3、批准文号:进口药品注册证号H201104104、汉语拼音:ZuoYiLaXiTanPian5、英文名称:LevetiracetamTablets6、成份:左乙拉西坦。
7、剂型:片剂8、形状:左乙拉西坦片为椭圆形薄膜包衣片(250mg为蓝色片,500mg为黄色片,1000mg为白色9、功能主治:左乙拉西坦片用于成人及4岁以上儿童癫痫患者部分性发作的加用治疗。
10、规格/中西药品:0.5g*30片/盒11、妊娠期妇女及哺乳期妇女用药:目前没有孕妇服用本品的资料,动物试验证明该药有一定的生殖毒性。
对于人类潜在的危险目前尚不明确。
如非必要,孕妇请勿应用左乙拉西坦。
突然中断抗癫痫治疗,可能使病情恶化,对母亲和胎儿同样有害。
动物试验表明左乙拉西坦可以从乳汁中排出,所以,不建议病人在服药同时哺乳。
12、贮藏:密封,阴凉干燥保存。
13、包装:0.5g*30片*10盒14、有效期:36个月左乙拉西坦片什么时间吃效果最好左乙拉西坦片在饭前或饭后服用均可。
但左乙拉西坦片的服用剂量因人而异,患者需根据不同的情况而选择服用的剂量,建议患者在服用左乙拉西坦片进行治疗前需仔细阅读其说明书或向医师详细咨询。
左乙拉西坦片给药途径:口服。
需以适量的水吞服,服用不受进食影响。
给药方法和剂量:成人(>18岁)和青少年(12-17岁)体重≥50kg起始治疗剂量为每次500mg,每日2次。
根据临床效果及耐受性,每日剂量可增加至每次1500mg,每日2次。
其他请详见说明书。
左乙拉西坦片是健康抗癫痫的最好选择吗癫痫治疗要持续数年,故应选择耐受性最好、对生活质量负面影响最小的药物。
研究证实,左乙拉西坦片安全性好,不良反应少,多为轻中度,主要发生在治疗前4周内,导致减量或撤药的绝对比例很低。
左乙拉西坦

左乙拉西坦-注射液(5ml)说明书中文翻译左乙拉西坦注射液说明书参考译文近期主要变更剂量与用法,部分性发作( 2.6 )警告与注意事项( 5.1、5.2 、5.3、5.4、5.7 )适应症与应用当口服KEPPRA给药暂时不可行时,KEPPRA注射液是抗癫痫药物用于成人(》16 岁)以下发作类型的辅助治疗:部分性发作( 1.1 )少年肌阵挛癫痫患者的肌阵挛性发作( 1.2)原发性强直性癫痫发作( 1.3 )剂量与给药方法KEPPRA注射液在使用前用100mL可配伍稀释剂稀释,滴注15min (2.1 )。
初始使用KEPPRA (2.2):部分性发作:1000mg/d ,每天2次(500mg,每天2 次),根据需要和耐受性每 2 周增加1000mg/d 至最大推荐日剂量3000mg/d 。
青少年肌阵挛性癫痫患者的肌阵挛性发作:初始剂量1000mg/d(500mg ,每天2次)。
每2周增加剂量1000mg/d 至推荐日剂量为3000mg/d ,尚未研究剂量低于3000mg/d 的有效性。
特发性全身强直阵挛发作:初始剂量1000mg/d (500mg ,每天 2 次)。
每 2 周增加剂量1000mg/d 至推荐日剂量3000mg/d ,尚未研究剂量低于3000mg/d 的有效性。
替代治疗( 2.3)从口服KEPPRA 转向静脉注射剂量:开始静脉注射左乙拉西坦,总日剂量和频率与片剂相当。
在静滴治疗期结束时,患者可转用KEPPRA 口服给药,日剂量和频率与静脉注射左乙拉西坦相当。
剂量说明( 2.5)、肾功能损害成人患者( 2.6 )和相容性和稳定性( 2.7)见全部处方信息。
剂型和剂量500mg/5mL ,一次性小瓶(3 )。
禁忌禁忌无警告与注意事项1 精神病反应:行为异常包括精神病症状、自杀意念、易激、攻击行为。
监测患者的精神病征兆和症状( 5.1 )。
嗜睡和疲乏:监测患者的这些症状,在未获得充分的经验前劝告患者不要驾驶汽车或操作机械( 5.2)。
左乙拉西坦片的功能主治

左乙拉西坦片的功能主治1. 什么是左乙拉西坦片?左乙拉西坦片是一种抗癫痫药物,属于苯丙酮类化合物。
它被用于治疗不同类型的癫痫发作,包括部分性发作和全面性发作。
2. 左乙拉西坦片的主要功能左乙拉西坦片具有以下功能:•抗癫痫作用:左乙拉西坦片通过调节神经递质的释放,增加抑制性神经递质的效应,从而减少癫痫发作的频率和强度。
•镇静作用:左乙拉西坦片可以减轻焦虑和紧张情绪,使人感到镇静和宁静。
•抗焦虑作用:左乙拉西坦片可以减轻焦虑症状,如恐慌、不安和紧张。
3. 左乙拉西坦片的主治左乙拉西坦片适用于以下疾病和症状的治疗:•部分性发作:左乙拉西坦片对部分性发作具有良好的疗效,可减少或阻止癫痫部分性发作的发生。
•全面性发作:左乙拉西坦片可用于治疗全面性癫痫发作,减少发作的次数和强度。
•癫痫的辅助治疗:左乙拉西坦片可作为其他抗癫痫药物的辅助治疗,增强疗效并减少副作用。
•焦虑症:左乙拉西坦片可用于治疗焦虑症,减轻焦虑和紧张情绪。
•退缩性癫痫:左乙拉西坦片可用于治疗退缩性癫痫,减少发作的频率和强度。
4. 使用左乙拉西坦片的注意事项在使用左乙拉西坦片时,需要注意以下事项:•仅按医生指导使用:左乙拉西坦片是处方药物,必须按医生的指导和处方使用,不可自行调整剂量或停止使用。
•避免突然停药:如果需要停用左乙拉西坦片,应在医生的指导下逐渐减少剂量,以避免出现撤退症状。
•注意副作用:左乙拉西坦片可能会引起一些副作用,如嗜睡、头晕、头痛、肌肉无力等,如果出现严重不适或持续时间较长,应及时向医生报告。
•避免饮酒:饮酒可能会增加左乙拉西坦片的镇静效果,影响工作和驾驶安全,因此在使用期间应避免饮酒。
•注意药物相互作用:左乙拉西坦片可能会与其他药物产生相互作用,包括其他抗癫痫药物和某些麻醉药物,因此在使用期间应告知医生正在使用的其他药物。
5. 总结左乙拉西坦片是一种抗癫痫药物,通过调节神经递质的释放,减少癫痫发作的频率和强度。
它主要用于治疗不同类型的癫痫发作,包括部分性发作和全面性发作,并可用于辅助治疗其他抗癫痫药物的疗效。
左乙拉西坦缓释片FDA说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KEPPRA XR safely and effectively. See full prescribing information for KEPPRA XR.KEPPRA XR (levetiracetam) tablet, film coated, extended release for oral useInitial U.S. Approval: 1999RECENT MAJOR CHANGESWarnings and Precautions (5.1)[04/2009] Patient Counseling Information (17)[04/2009]INDICATIONS AND USAGEKEPPRA XR is an antiepileptic drug indicated for adjunctive therapy in the treatment of partial onset seizures in patients ≥16 years of age with epilepsy (1)DOSAGE AND ADMINISTRATIONTreatment should be initiated with a dose of 1000 mg once daily. Thedaily dosage may be adjusted in increments of 1000 mg every 2 weeks to a maximum recommended daily dose of 3000 mg (2).See full prescribing information for use in patients with impaired renal function (2.1).DOSAGE FORMS AND STRENGTHS•500 mg white, film-coated extended-release tablet (3)•750 mg white, film-coated extended-release tablet (3)CONTRAINDICATIONS•None (4)WARNINGS AND PRECAUTIONS•Suicidal Behavior and Ideation. (5.1)•Neuropsychiatric Adverse Reactions: KEPPRA XR causes somnolence, dizziness, and behavioral abnormalities. The adverse reactions that may be seen in patients receiving KEPPRA XR tablets are expected to be similar to those seen in patients receiving immediate-release KEPPRA tablets. (5.2)•In controlled trials of immediate-release KEPPRA tablets in patients experiencing partial onset seizures, immediate-release KEPPRA causes somnolence and fatigue, coordination difficulties, and behavioral abnormalities (e.g., psychotic symptoms, suicidal ideation, and other abnormalities). (5.2)•Withdrawal Seizures: KEPPRA XR must be gradually withdrawn. (5.3)ADVERSE REACTIONS•Most common adverse reactions (difference in incidence rate is ≥5% between KEPPRA XR-treated patients and placebo-treated patients and occurred more frequently in KEPPRA XR-treated patients) include: somnolence and irritability (6.1).To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 866-822-0068 or FDA at 1-800-FDA-1088 or /medwatchTo report SUSPECTED ADVERSE REACTIONS, contact at or FDA at 1-800-FDA-1088 or /medwatchUSE IN SPECIFIC POPULATIONS•To enroll in the UCB AED Pregnancy Registry call 888-537-7734 (toll free). To enroll in the North American Antiepileptic Drug Pregnancy Registry call (888) 233-2334 (toll free). (8.1)• A dose adjustment is recommended for patients with impaired renal function, based on the patient's estimated creatinine clearance (8.6).See 17 for PATIENT COUNSELING INFORMATION and the FDA-approved Medication GuideRevised: 10/2009FULL PRESCRIBING INFORMATION: CONTENTS *1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION2.1 Adult Patients With Impaired Renal Function3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Suicidal Behavior and Ideation5.2 Neuropsychiatric Adverse Reactions5.3 Withdrawal Seizures5.4 Hematologic Abnormalities5.5 Hepatic Abnormalities5.6 Laboratory Tests6 ADVERSE REACTIONS6.1 Clinical Studies Experience6.2 Postmarketing Experience7 DRUG INTERACTIONS7.1 General Information7.2 Phenytoin7.3 Valproate7.4 Other Antiepileptic Drugs7.5 Oral Contraceptives7.6 Digoxin7.7 Warfarin7.8 Probenecid8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.2 Labor And Delivery8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Use In Patients With Impaired Renal Function9 DRUG ABUSE AND DEPENDENCE10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism Of Action12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility13.2 Animal Toxicology And/Or Pharmacology14 CLINICAL STUDIES16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied16.2 Storage17 PATIENT COUNSELING INFORMATION* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGEKEPPRA XR™ is indicated as adjunctive therapy in the treatment of partial onset seizures in patients ≥16 years of age with epilepsy.2 DOSAGE AND ADMINISTRATIONTreatment should be initiated with a dose of 1000 mg once daily. The daily dosage may be adjusted in increments of 1000 mg every 2 weeks to a maximum recommended daily dose of 3000 mg.2.1 Adult Patients With Impaired Renal FunctionKEPPRA XR dosing must be individualized according to the patient's renal function status. Recommended doses and adjustment for dose for adults are shown in Table 1. To use this dosing table, an estimate of the patient's creatinine clearance (CLcr) in mL/min is needed. CLcr in mL/min may be estimated from serum creatinine (mg/dL) determination using the following formula:[140-age (years)] x weight (kg)CLcr = ---------------------------------------------- x 1 0.8572 x serum creatinine (mg/dL)1. For female patientsThen CLcr is adjusted for body surface area (BSA) as follows:CLcr (mL/min)CLcr (mL/min/1.73m2) = ----------------------------- x 1.73BSA subject (m2)Table 1: Dosing Adjustment Regimen For Adult Patients With Impaired Renal FunctionGroup CreatinineClearance(mL/min/1.73m2) Dosage(mg)FrequencyNormal > 80 1000 to 3000 Every 24 hMild 50 – 80 1000 to 2000 Every 24 h Moderate 30 – 50 500 to 1500 Every 24 h Severe < 30 500 to 1000 Every 24 h3 DOSAGE FORMS AND STRENGTHSKEPPRA XR tablets are white, oblong-shaped, film-coated extended-release tablets imprinted in red with "UCB 500XR" on one side and contain 500 mg levetiracetam.KEPPRA XR tablets are white, oblong-shaped, film-coated extended-release tablets imprinted in red with "UCB 750XR" on one side and contain 750 mg levetiracetam.4 CONTRAINDICATIONSNone5 WARNINGS AND PRECAUTIONS5.1 Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including KEPPRA XR, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.Table 2 Risk by indication for antiepileptic drugs in the pooled analysisIndication Placebo Patients withEvents Per 1000 PatientsDrug Patients withEvents Per 1000 PatientsRelative Risk: Incidenceof Events in DrugPatients/Incidence inPlacebo PatientsRisk DifferenceAdditional DrugPatients with Events Per1000 Patients Epilepsy 1.0 3.4 3.5 2.4Psychiatric 5.7 8.5 1.5 2.9Other 1.0 1.8 1.9 0.9Total 2.4 4.3 1.8 1.9The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.Anyone considering prescribing KEPPRA XR or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.5.2 Neuropsychiatric Adverse ReactionsKEPPRA XR TabletsIn some patients experiencing partial onset seizures, KEPPRA XR causes somnolence, dizziness, and behavioral abnormalities.In the KEPPRA XR double-blind, controlled trial in patients experiencing partial onset seizures, 7.8% of KEPPRA XR-treated patients experienced somnolence compared to 2.5% of placebo-treated patients. Dizziness was reported in 5.2% of KEPPRA XR-treated patients compared to 2.5% of placebo-treated patients.A total of 6.5% of KEPPRA XR-treated patients experienced non-psychotic behavioral disorders (reported as irritability and aggression) compared to 0% of placebo-treated patients. Irritability was reported in 6.5% of KEPPRA XR-treated patients. Aggression was reported in 1.3% of KEPPRA XR-treated patients.No patient discontinued treatment or had a dose reduction as a result of these adverse reactions.The number of patients exposed to KEPPRA XR was considerably smaller than the number of patients exposed to immediate-release KEPPRA tablets in controlled trials. Therefore, certain adverse reactions observed in the immediate-release KEPPRA controlled trials may also occur in patients receiving KEPPRA XR.Immediate-Release KEPPRA TabletsIn controlled trials of immediate-release KEPPRA tablets in patients experiencing partial onset seizures, immediate-release KEPPRA causes the occurrence of central nervous system adverse reactions that can be classified into the following categories: 1) somnolence and fatigue, 2) coordination difficulties, and 3) behavioral abnormalities.In controlled trials of adult patients with epilepsy experiencing partial onset seizures, 14.8% of immediate-release KEPPRA-treated patients reported somnolence, compared to 8.4% of placebo patients. There was no clear dose response up to 3000 mg/day.In controlled trials of adult patients with epilepsy experiencing partial onset seizures, 14.7% of treated patients reported asthenia, compared to 9.1% of placebo patients.A total of 3.4% of immediate-release KEPPRA-treated patients experienced coordination difficulties, (reported as either ataxia, abnormal gait, or incoordination) compared to 1.6% of placebo patients.Somnolence, asthenia and coordination difficulties occurred most frequently within the first 4 weeks of treatment.In controlled trials of patients with epilepsy experiencing partial onset seizures, 5 (0.7%) immediate-release KEPPRA-treated patients experienced psychotic symptoms compared to 1 (0.2%) placebo patient.A total of 13.3% of immediate-release KEPPRA patients experienced other behavioral symptoms (reported as aggression, agitation, anger, anxiety, apathy, depersonalization, depression, emotional lability, hostility, irritability, etc.) compared to 6.2% of placebo patients.5.3 Withdrawal SeizuresAntiepileptic drugs, including KEPPRA XR, should be withdrawn gradually to minimize the potential of increased seizure frequency.5.4 Hematologic AbnormalitiesAlthough there were no obvious hematologic abnormalities observed in treated patients in the KEPPRA XR controlled study, the limited number of patients makes any conclusion tentative. The data from the partial seizure patients in the immediate-release KEPPRA controlled studies should be considered to be relevant for KEPPRA XR-treated patients.In controlled trials of immediate-release KEPPRA tablets in patients experiencing partial onset seizures, minor, but statistically significant, decreases compared to placebo in total mean RBC count (0.03 x 106/mm3), mean hemoglobin (0.09 g/dL), and mean hematocrit (0.38%), were seen in immediate-release KEPPRA-treated patients. A total of 3.2% of treated and 1.8% of placebo patients had at least one possibly significant (≤2.8 x 109/L) decreased WBC, and 2.4% of treated and 1.4% of placebo patients had at least one possibly significant (≤1.0 x 109/L) decreased neutrophil count. Of the treated patients with a low neutrophil count, all but one rose towards or to baseline with continued treatment. No patient was discontinued secondary to low neutrophil counts.5.5 Hepatic AbnormalitiesThere were no meaningful changes in mean liver function tests (LFT) in the KEPPRA XR controlled trial. No patients were discontinued from the controlled trial for LFT abnormalities.There were no meaningful changes in mean liver function tests (LFT) in controlled trials of immediate-release KEPPRA tablets in adult patients; lesser LFT abnormalities were similar in drug and placebo-treated patients in controlled trials (1.4%). No patients were discontinued from controlled trials for LFT abnormalities except for 1 (0.07%) adult epilepsy patient receiving open treatment.5.6 Laboratory TestsAlthough effects on laboratory tests were not clinically significant with KEPPRA XR treatment, it is expected that the data from immediate-release KEPPRA tablets controlled studies would be considered relevant for KEPPRA XR-treated patients.Although most laboratory tests are not systematically altered with immediate-release KEPPRA treatment, there have been relatively infrequent abnormalities seen in hematologic parameters and liver function tests.6 ADVERSE REACTIONS6.1 Clinical Studies ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.The prescriber should be aware that the adverse reaction incidence figures in the following table, obtained when KEPPRA XR was added to concurrent AED therapy, cannot be used to predict the frequency of adverse experiences in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescriber with one basis to estimate the relative contribution of drug and non-drug factors to the adverse reaction incidences in the population studied.KEPPRA XR TabletsIn the well-controlled clinical study using KEPPRA XR in patients with partial onset seizures, the most frequently reported adverse reactions in patients receiving KEPPRA XR in combination with other AEDs, not seen at an equivalent frequency among placebo-treated patients, were irritability and somnolence.Table 3 lists treatment-emergent adverse reactions that occurred in at least 5% of epilepsy patients treated with KEPPRA XR participating in the placebo-controlled study and were numerically more common than in patients treated with placebo. In this study, either KEPPRA XR or placebo was added to concurrent AED therapy. Adverse reactions were usually mild to moderate in intensity. Table 3: Incidence (%) Of Treatment-Emergent Adverse Reactions In The Placebo-Controlled, Add-On Study By Body System (Adverse Reactions Occurred In At Least 5% Of KEPPRA XR-Treated Patients And Occurred More Frequently Than Placebo-Treated Patients)Body System/ Adverse Reaction KEPPRA XR(N=77)%Placebo(N=79)%Gastrointestinal DisordersNausea 5 3Infections and InfestationsInfluenza 8 4Nasopharyngitis 7 5Nervous System DisordersSomnolence 8 3Dizziness 5 3Psychiatric DisordersIrritability 7 0 Discontinuation Or Dose Reduction In The KEPPRA XR Well-Controlled Clinical StudyIn the well-controlled clinical study using KEPPRA XR, 5.2% of patients receiving KEPPRA XR and 2.5% receiving placebo discontinued as a result of an adverse event. The adverse reactions that resulted in discontinuation and that occurred more frequently in KEPPRA XR-treated patients than in placebo-treated patients were asthenia, epilepsy, mouth ulceration, rash and respiratory failure. Each of these adverse reactions led to discontinuation in a KEPPRA XR-treated patient and no placebo-treated patients. Comparison Of Gender, Age And RaceThere are insufficient data for KEPPRA XR to support a statement regarding the distribution of adverse experience reports by gender, age and race.Table 4 lists the adverse reactions seen in the well-controlled studies of immediate-release KEPPRA tablets in adult patients experiencing partial onset seizures. Although the pattern of adverse reactions in the KEPPRA XR study seems somewhat different from that seen in partial onset seizure well-controlled studies for immediate-release KEPPRA tablets, this is possibly due to the much smaller number of patients in this study compared to the immediate-release tablet studies. The adverse reactions for KEPPRA XR are expected to be similar to those seen with immediate-release KEPPRA tablets.Immediate-Release KEPPRA TabletsIn well-controlled clinical studies of immediate-release KEPPRA tablets as adjunctive therapy to other AEDs in adults with partial onset seizures, the most frequently reported adverse reactions, not seen at an equivalent frequency among placebo-treated patients, were somnolence, asthenia, infection and dizziness.Table 4 lists treatment-emergent adverse reactions that occurred in at least 1% of adult epilepsy patients treated with immediate-release KEPPRA tablets participating in placebo-controlled studies and were numerically more common than in patients treatedwith placebo. In these studies, either immediate-release KEPPRA tablets or placebo was added to concurrent AED therapy. Adverse reactions were usually mild to moderate in intensity.Table 4: Incidence (%) Of Treatment-Emergent Adverse Reactions In Placebo-Controlled, Add-On Studies In Adults Experiencing Partial Onset Seizures By Body System (Adverse Reactions Occurred In At Least 1% Of Immediate-Release KEPPRA-Treated Patients And Occurred More Frequently Than Placebo-Treated Patients)Body System/ Adverse Reaction Immediate-release KEPPRA(N=769)%Placebo(N=439)%Body as a WholeAsthenia 15 9Headache 14 13Infection 13 8Pain 7 6Digestive SystemAnorexia 3 2Nervous SystemSomnolence 15 8Dizziness 9 4Depression 4 2Nervousness 4 2Ataxia 3 1Vertigo 3 1Amnesia 2 1Anxiety 2 1Hostility 2 1Paresthesia 2 1Emotional Lability 2 0Respiratory SystemPharyngitis 6 4Rhinitis 4 3Cough Increased 2 1Sinusitis 2 1Special SensesDiplopia 2 1In addition, the following adverse reactions were seen in other well-controlled studies of immediate-release KEPPRA tablets: balance disorder, disturbance in attention, eczema, hyperkinesia, memory impairment, myalgia, personality disorders, pruritus, and vision blurred.6.2 Postmarketing ExperienceIn addition to the adverse reactions listed above for immediate-release KEPPRA tablets [see Adverse Reactions (6.1)], the following adverse events have been identified during postapproval use of immediate-release KEPPRA tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The listing is alphabetized: abnormal liver function test, hepatic failure, hepatitis, leukopenia, neutropenia, pancreatitis, pancytopenia (with bone marrow suppression identified in some of these cases), thrombocytopenia and weight loss. Alopecia has been reported with immediate-release KEPPRA use; recovery was observed in majority of cases where immediate-release KEPPRA was discontinued.7 DRUG INTERACTIONS7.1 General InformationIn vitro data on metabolic interactions indicate that KEPPRA XR is unlikely to produce, or be subject to, pharmacokinetic interactions. Levetiracetam and its major metabolite, at concentrations well above C max levels achieved within the therapeutic dose range, are neither inhibitors of nor high affinity substrates for human liver cytochrome P450 isoforms, epoxide hydrolase or UDP-glucuronidation enzymes. In addition, levetiracetam does not affect the in vitro glucuronidation of valproic acid.Levetiracetam circulates largely unbound (<10% bound) to plasma proteins; clinically significant interactions with other drugs through competition for protein binding sites are therefore unlikely.Potential pharmacokinetic interactions were assessed in clinical pharmacokinetic studies (phenytoin, valproate, oral contraceptive, digoxin, warfarin, probenecid) and through pharmacokinetic screening with immediate-release KEPPRA tablets in the placebo-controlled clinical studies in epilepsy patients. The following are the results of these studies. The potential for drug interactions for KEPPRA XR is expected to be essentially the same as that with immediate-release KEPPRA tablets.7.2 PhenytoinImmediate-release KEPPRA tablets (3000 mg daily) had no effect on the pharmacokinetic disposition of phenytoin in patients with refractory epilepsy. Pharmacokinetics of levetiracetam were also not affected by phenytoin.7.3 ValproateImmediate-release KEPPRA tablets (1500 mg twice daily) did not alter the pharmacokinetics of valproate in healthy volunteers. Valproate 500 mg twice daily did not modify the rate or extent of levetiracetam absorption or its plasma clearance or urinary excretion. There also was no effect on exposure to and the excretion of the primary metabolite, ucb L057.7.4 Other Antiepileptic DrugsPotential drug interactions between immediate-release KEPPRA tablets and other AEDs (carbamazepine, gabapentin, lamotrigine, phenobarbital, phenytoin, primidone and valproate) were also assessed by evaluating the serum concentrations of levetiracetamand these AEDs during placebo-controlled clinical studies. These data indicate that levetiracetam does not influence the plasma concentration of other AEDs and that these AEDs do not influence the pharmacokinetics of levetiracetam.7.5 Oral ContraceptivesImmediate-release KEPPRA tablets (500 mg twice daily) did not influence the pharmacokinetics of an oral contraceptive containing 0.03 mg ethinyl estradiol and 0.15 mg levonorgestrel, or of the luteinizing hormone and progesterone levels, indicating that impairment of contraceptive efficacy is unlikely. Coadministration of this oral contraceptive did not influence the pharmacokinetics of levetiracetam.7.6 DigoxinImmediate-release KEPPRA tablets (1000 mg twice daily) did not influence the pharmacokinetics and pharmacodynamics (ECG) of digoxin given as a 0.25 mg dose every day. Coadministration of digoxin did not influence the pharmacokinetics of levetiracetam.7.7 WarfarinImmediate-release KEPPRA tablets (1000 mg twice daily) did not influence the pharmacokinetics of R and S warfarin. Prothrombin time was not affected by levetiracetam. Coadministration of warfarin did not affect the pharmacokinetics of levetiracetam.7.8 ProbenecidProbenecid, a renal tubular secretion blocking agent, administered at a dose of 500 mg four times a day, did not change the pharmacokinetics of levetiracetam 1000 mg twice daily. C ss max of the metabolite, ucb L057, was approximately doubled in the presence of probenecid while the fraction of drug excreted unchanged in the urine remained the same. Renal clearance of ucb L057 in the presence of probenecid decreased 60%, probably related to competitive inhibition of tubular secretion of ucb L057. The effect of immediate-release KEPPRA tablets on probenecid was not studied.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category CThere are no adequate and well-controlled studies in pregnant women. In animal studies, levetiracetam produced evidence of developmental toxicity, including teratogenic effects, at doses similar to or greater than human therapeutic doses. KEPPRA XR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. As with other antiepileptic drugs, physiological changes during pregnancy may affect levetiracetam concentration. There have been reports of decreased levetiracetam concentration during pregnancy. Discontinuation of antiepileptic treatments may result in disease worsening, which can be harmful to the mother and the fetus.Oral administration of levetiracetam to female rats throughout pregnancy and lactation led to increased incidences of minor fetal skeletal abnormalities and retarded offspring growth pre- and/or postnatally at doses ≥350 mg/kg/day (approximately equivalent to the maximum recommended human dose of 3000 mg [MRHD] on a mg/m2 basis) and with increased pup mortality and offspring behavioral alterations at a dose of 1800 mg/kg/day (6 times the MRHD on a mg/m2 basis). The developmental no effect dose was 70 mg/kg/day (0.2 times the MRHD on a mg/m2 basis). There was no overt maternal toxicity at the doses used in this study.Oral administration of levetiracetam to pregnant rabbits during the period of organogenesis resulted in increased embryofetal mortality and increased incidences of minor fetal skeletal abnormalities at doses ≥600 mg/kg/day (approximately 4 times MRHD on a mg/m2 basis) and in decreased fetal weights and increased incidences of fetal malformations at a dose of 1800 mg/kg/day (12 times the MRHD on a mg/m2 basis). The developmental no effect dose was 200 mg/kg/day (1.3 times the MRHD on a mg/m2 basis). Maternal toxicity was also observed at 1800 mg/kg/day.When levetiracetam was administered orally to pregnant rats during the period of organogenesis, fetal weights were decreased and the incidence of fetal skeletal variations was increased at a dose of 3600 mg/kg/day (12 times the MRHD). 1200 mg/kg/day (4 times the MRHD) was a developmental no effect dose. There was no evidence of maternal toxicity in this study.Treatment of rats with levetiracetam during the last third of gestation and throughout lactation produced no adverse developmental or maternal effects at oral doses of up to 1800 mg/kg/day (6 times the MRHD on a mg/m2 basis).Pregnancy RegistriesTo provide information regarding the effects of in utero exposure to KEPPRA XR, physicians are advised to recommend that pregnant patients taking KEPPRA XR enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by the patients themselves. Information on the registry can also be found at the website /.UCB, Inc. has established the UCB AED Pregnancy Registry to advance scientific knowledge about safety and outcomes in pregnant women being treated with all UCB antiepileptic drugs including KEPPRA XR. To ensure broad program access and reach, either a healthcare provider or the patient can initiate enrollment in the UCB AED Pregnancy Registry by calling (888) 537-7734 (toll free). 8.2 Labor And DeliveryThe effect of KEPPRA XR on labor and delivery in humans is unknown.8.3 Nursing MothersLevetiracetam is excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants from KEPPRA XR, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.8.4 Pediatric UseSafety and effectiveness of KEPPRA XR in patients below the age of 16 years have not been established.8.5 Geriatric UseThere were insufficient numbers of elderly subjects in controlled trials of epilepsy to adequately assess the effectiveness of KEPPRA XR in these patients. It is expected that the safety of KEPPRA XR in elderly patients 65 and over would be comparable to the safety observed in clinical studies of immediate-release KEPPRA tablets.Of the total number of subjects in clinical studies of immediate-release levetiracetam, 347 were 65 and over. No overall differences in safety were observed between these subjects and younger subjects. There were insufficient numbers of elderly subjects in controlled trials of epilepsy to adequately assess the effectiveness of immediate-release KEPPRA in these patients.A study in 16 elderly subjects (age 61-88 years) with oral administration of single dose and multiple twice-daily doses of immediate-release KEPPRA tablets for 10 days showed no pharmacokinetic differences related to age alone.Levetiracetam is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.8.6 Use In Patients With Impaired Renal FunctionThe effect of KEPPRA XR on renally impaired patients was not assessed in the well-controlled study. However, it is expectedthat the effect on KEPPRA XR-treated patients would be similar to the effect seen in well-controlled studies of immediate-release KEPPRA tablets. Caution should be taken in dosing patients with moderate and severe renal impairment and in patients undergoing hemodialysis. The dosage should be reduced in patients with impaired renal function receiving KEPPRA XR [see Clinical Pharmacology (12.3) and Dosage and Administration (2.1)].Clearance of immediate-release levetiracetam is decreased in patients with renal impairment and is correlated with creatinine clearance.9 DRUG ABUSE AND DEPENDENCEThe abuse and dependence potential of KEPPRA XR has not been evaluated in human studies.。
左乙拉西坦注射液FDA说明书
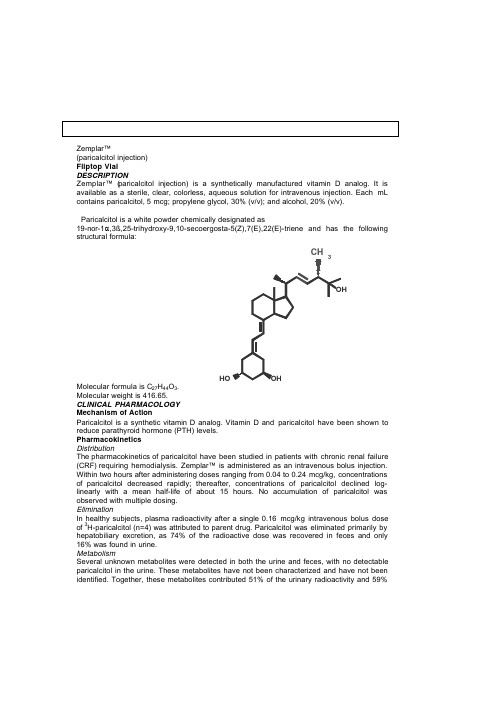
Zemplar™(paricalcitol injection)Fliptop VialDESCRIPTIONZemplar™ (paricalcitol injection) is a synthetically manufactured vitamin D analog. It is available as a sterile, clear, colorless, aqueous solution for intravenous injection. Each mL contains paricalcitol, 5 mcg; propylene glycol, 30% (v/v); and alcohol, 20% (v/v).Paricalcitol is a white powder chemically designated as19-nor-1a,3ß,25-trihydroxy-9,10-secoergosta-5(Z),7(E),22(E)-triene and has the following structural formula:Molecular weight is 416.65.CLINICAL PHARMACOLOGYMechanism of ActionParicalcitol is a synthetic vitamin D analog. Vitamin D and paricalcitol have been shown to reduce parathyroid hormone (PTH) levels.PharmacokineticsDistributionThe pharmacokinetics of paricalcitol have been studied in patients with chronic renal failure (CRF) requiring hemodialysis.Zemplar™ is administered as an intravenous bolus injection. Within two hours after administering doses ranging from 0.04 to 0.24 mcg/kg, concentrations of paricalcitol decreased rapidly; thereafter, concentrations of paricalcitol declined log-linearly with a mean half-life of about 15 hours. No accumulation of paricalcitol was observed with multiple dosing.EliminationIn healthy subjects, plasma radioactivity after a single 0.16 mcg/kg intravenous bolus dose of3H-paricalcitol (n=4) was attributed to parent drug. Paricalcitol was eliminated primarily by hepatobiliary excretion, as 74% of the radioactive dose was recovered in feces and only 16%was found in urine.MetabolismSeveral unknown metabolites were detected in both the urine and feces, with no detectable paricalcitol in the urine. These metabolites have not been characterized and have not been identified. Together, these metabolites contributed 51% of the urinary radioactivity and 59%of the fecal radioactivity.In vitro plasma protein binding of paricalcitol was extensive (>99.9%) and nonsaturable over the concentration range of 1 to 100 ng/mL.Paricalcitol Pharmacokinetic Characteristics in CRF Patients (0.24 mcg/kg dose) Parameter n Values (Mean ± SD) .C max (5 min. after bolus)6 1850 ± 664 (pg/mL)AUC0- 527382 ± 8230 (pg•hr/mL)CL5 0.72 ± 0.24 (L/hr)V ss5 6 ± 2 (L)Laboratory TestsIn placebo-controlled studies, paricalcitol reduced serum total alkaline phosphatase levels. Special PopulationsParicalcitol pharmacokinetics have not been investigated in special populations (geriatric, pediatric, hepatic insufficiency), or for drug-drug interactions. Pharmacokinetics were not gender-dependent.Clinical StudiesIn three 12-week, placebo-controlled, phase 3 studies in chronic renal failure patients on dialysis, the dose of Zemplar™ was started at 0.04 mcg/kg 3 times per week. The dose was increased by 0.04 mcg/kg every 2 weeks until intact parathyroid hormone (iPTH) levels were decreased at least 30% from baseline or a fifth escalation brought the dose to 0.24 mcg/kg, or iPTH fell to less than 100 pg/mL, or the Ca x P product was greater than 75 within any 2 week period, or serum calcium became greater than 11.5 mg/dL at any time.Patients treated with Zemplar™ achieved a mean iPTH reduction of 30% within 6 weeks. In these studies, there was no significant difference in the incidence of hypercalcemia or hyperphosphatemia between Zemplar™ and placebo-treated patients. The results from these studies are as follows:Mean (SE) Change Group Baseline Mean From Baseline to (No. of Pts.) (Range)Final Evaluation PTH (pg/mL)Zemplar™ (n=40)783 (291 – 2076)-379 (43.7) .placebo (n=38)745 (320 – 1671) -69.6 (44.8) . Alkaline Zemplar™ (n=31)150 (40 – 600) -41.5 (10.6) . Phosphatase (U/L)placebo (n=34)169 (56 – 911) +2.6 (10.1) . Calcium (mg/dL)Zemplar™ (n=40) 9.3 (7.2 – 10.4) +0.47 (0.1) .placebo (n=38) 9.1 (7.8 – 10.7) +0.02 (0.1) . Phosphorus (mg/dL)Zemplar™ (n=40) 5.8 (3.7 – 10.2) +0.47 (0.3) .placebo (n=38) 6.0 (2.8 – 8.8) -0.47 (0.3) . Calcium x Zemplar™ (n=40) 54 (32 – 106) +7.9 (2.2) . Phosphorus Product placebo (n=38) 54 (26 – 77) -3.9 (2.3) .A long-term, open-label safety study of 164 CRF patients (mean dose of 7.5 mcg three times per week), demonstrated that mean serum Ca, P, and Ca x P remained within clinically appropriate ranges with PTH reduction (mean decrease of 323 pg/mL at 13 months).INDICATIONS AND USAGEZemplar™ is indicated for the prevention and treatment of secondary hyperparathyroidism associated with chronic renal failure. Studies in patients with chronic renal failure show that Zemplar™ suppresses PTH levels with no significant difference in the incidence of hypercalcemia or hyperphosphatemia when compared to placebo. However, the serum phosphorus, calcium and calcium x phosphorus product (Ca x P) may increase when Zemplar™ is administered.CONTRAINDICATIONSZemplar™ should not be given to patients with evidence of vitamin D toxicity, hypercalcemia, or hypersensitivity to any ingredient in this product (see PRECAUTIONS, General).WARNINGSAcute overdose of Zemplar™ may cause hypercalcemia, and require emergency attention. During dose adjustment, serum calcium and phosphorus levels should be monitored closely (e.g., twice weekly). If clinically significant hypercalcemia develops, the dose should be reduced or interrupted. Chronic administration of Zemplar™ may place patients at risk ofhypercalcemia, elevated Ca x P product, and metastatic calcification. Signs and symptoms of vitamin D intoxication associated with hypercalcemia include:EarlyWeakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, and metallic taste.LateAnorexia, weight loss, conjunctivitis (calcific),pancreatitis, photophobia, rhinorrhea, pruritus,hyperthermia, decreased libido, elevated BUN, hypercholesterolemia, elevated AST and ALT, ectopic calcification, hypertension, cardiac arrhythmias, somnolence, death, and, rarely, overt psychosis.Treatment of patients with clinically significant hypercalcemia consists of immediate dose reduction or interruption of Zemplar™ therapy and includes a low calcium diet, withdrawal of calcium supplements, patient mobilization, attention to fluid and electrolyte imbalances, assessment of electrocardiographic abnormalities (critical in patients receiving digitalis), and hemodialysis or peritoneal dialysis against a calcium-free dialysate, as warranted. Serum calcium levels should be monitored frequently until normocalcemia ensues.Phosphate or vitamin D-related compounds should not be taken concomitantly with Zemplar™.PRECAUTIONSGeneral: Digitalis toxicity is potentiated by hypercalcemia of any cause, so caution should be applied when digitalis compounds are prescribed concomitantly with Zemplar™. Adynamic bone lesions may develop if PTH levels are suppressed to abnormal levels. Information for the Patient: The patient should be instructed that, to ensure effectiveness of Zemplar™ therapy, it is important to adhere to a dietary regimen of calcium supplementation and phosphorus restriction. Appropriate types of phosphate-binding compounds may be needed to control serum phosphorus levels in patients with chronic renal failure (CRF), but excessive use of aluminum containing compounds should be avoided. Patients should also be carefully informed about the symptoms of elevated calcium.Essential Laboratory Tests:During the initial phase of medication, serum calcium and phosphorus should be determined frequently (e.g., twice weekly). Once dosage has been established, serum calcium and phosphorus should be measured at least monthly. Measurements of serum or plasma PTH are recommended every 3 months. An intact PTH (iPTH) assay is recommended for reliable detection of biologically active PTH in patients with CRF. During dose adjustment of Zemplar™, laboratory tests may be required more frequently.Drug Interactions:Specific interaction studies were not performed. Digitalis toxicity is potentiated by hypercalcemia of any cause, so caution should be applied when digitalis compounds are prescribed concomitantly with Zemplar™.Carcinogenesis,Mutagenesis, Impairment of Fertility:Long-term studies in animals to evaluate the carcinogenic potential of paricalcitol have not been completed. Paricalcitol did not exhibit genetic toxicity in vitro with or without metabolic activation in the microbial mutagenesis assay (Ames Assay), mouse lymphoma mutagenesis assay (L5178Y), or a human lymphocyte cell chromosomal aberration assay. There was also no evidence of genetic toxicity in an in vivo mouse micronucleus assay. Zemplar™ had no effect on fertility (male or female) in rats at intravenous doses up to 20 mcg/kg/dose [equivalent to 13 times the highest recommended human dose (0.24 mcg/kg) based on surface area, mg/m2]. Pregnancy:Pregnancy Category C.Paricalcitol has been shown to cause minimal decreases in fetal viability (5%) when administered daily to rabbits at a dose 0.5 times the0.24mcg/kg human dose (based on surface area, mg/m2) and when administered to rats ata dose 2 times the 0.24 mcg/kg human dose (based on plasma levels of exposure). At the highest dose tested (20 mcg/kg 3 times per week in rats,13 times the 0.24 mcg/kg human dose based on surface area), there was a significant increase of the mortality of newborn rats at doses that were maternally toxic (hypercalcemia). No other effects on offspring development were observed. Paricalcitol was not teratogenic at the doses tested.There are no adequate and well-controlled studies in pregnant women. Zemplar™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Nursing Mothers: It is not known whether paricalcitol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Zemplar™ is administered to a nursing woman.Pediatric Use:Safety and efficacy of Zemplar™ in pediatric patients have not been established.Geriatric Use:Of the 40 patients receiving Zemplar™ in the three phase 3 placebo-controlled CRF studies, 10 patients were 65 years or over. In these studies, no overall differences in efficacy or safety were observed between patients 65 years or older and younger patients.ADVERSE REACTIONSZemplar™ has been evaluated for safety in clinical studies in 454 CRF patients.In four, placebo-controlled, double-blind, multicenter studies, discontinuation of therapy due to any adverse event occurred in 6.5% of 62 patients treated with Zemplar™ (dosage titrated as tolerated, see CLINICAL PHARMACOLOGY, Clinical Studies) and 2.0% of 51 patients treated with placebo for one to three months. Adverse events occurring with greater frequency in the Zemplar™ group at a frequency of 2% or greater, regardless of causality, are presented in the following table:Adverse Event Incidence Rates for All Treated PatientsIn All Placebo-Controlled StudiesZemplar™ (n=62)Placebo (n=51)Adverse Event number of events, %number of events, % Overall7178 .Body as a WholeChills5 0Feeling unwell3 0Fever5 2Flu5 4Sepsis5 2Cardiovascular SystemPalpitation3 0Digestive SystemDry mouth3 2Gastrointestinal bleeding5 2Nausea 13 8Vomiting8 4Metabolic and Nutritional DisordersEdema7 0Nervous SystemLight-headedness5 2Respiratory SystemPneumonia5 0A patient who reported the same medical term more than once was counted only once for that medical term.Safety parameters (changes in mean Ca, P, Ca x P) in an open-label safety study up to 13 months in duration support the long-term safety of Zemplar™in this patient population.OVERDOSAGEOverdosage of Zemplar™ may lead to hypercalcemia (see WARNINGS).DOSAGE AND ADMINISTRATIONThe currently accepted target range for iPTH levels in CRF patients is no more than 1.5 to 3 times the non-uremic upper limit of normal.The recommended initial dose of Zemplar™ is 0.04 mcg/kg to0.1mcg/kg(2.8 – 7 mcg) administered as a bolus dose no more frequently than every other day at any time during dialysis. Doses as high as 0.24 mcg/kg (16.8 mcg) have been safely administered.If a satisfactory response is not observed, the dose may be increased by 2 to 4 mcg at 2-to 4-week intervals. During any dose adjustment period, serum calcium and phosphorus levels should be monitored more frequently, and if an elevated calcium level or a Ca x P product greater than 75 is noted, the drug dosage should be immediately reduced or interrupted until these parameters are normalized. Then, Zemplar™ should be reinitiated at a lower dose. Doses may need to be decreased as the PTH levels decrease in response to therapy. Thus, incremental dosing must be individualized.The following table is a suggested approach in dose titration:Suggested Dosing GuidelinesPTH Level Zemplar™ Dosethe same or increasing increasedecreasing by <30% increasedecreasing by >30%, <60% maintaindecreasing by >60% decreaseone and one-half to threetimes upper limit of normal maintainParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.Discard unused portion.HOW SUPPLIEDZemplar™ (paricalcitol injection) 5 mcg/mL is supplied as1 and2 mL single-dose Fliptop Vials.List No. Volume/Container Concentration Total Content1658 1 mL/Fliptop Vial 5 mcg/mL 5 mcg1658 2 mL/Fliptop Vial 5 mcg/mL 10 mcgStore at 25°C (77°F). Excursions permitted to 15°– 30°C]onlyU.S. patents: 5,246,925; 5,587,497ÓAbbott 1998 Reference 06-9998-R1-Rev. April, 1998。
临床左乙拉西坦药物适应证、用法用量、严重不良反应和注意事项

临床左乙拉西坦药物适应证、用法用量、严重不良反应和注意事项适应证及用法用量适应证:①口服给药:癫痫患者部分性发作的加用治疗。
②注射给药:癫痫患者部分性发作(伴或不伴继发性全面性发作) 的加用治疗。
用法用量:①片剂、口服液体制剂:成人(≥ 18 岁)体重50 kg 或以上:起始治疗剂量为每次500 mg,每日2 次。
此剂量可以在治疗的第一天开始服用。
根据临床效果及耐受性,剂量可增加至每次1500 mg,每日 2 次。
应每2~4 周做一次剂量的调整,调整幅度500 mg/次(即调整幅度1000 mg/日)。
②注射给药:成人(≥ 18 岁)和青少年(12 岁~17 岁,体重≥ 50 kg):起始治疗剂量为每日2 次,500 mg/次。
根据临床效果及耐受性,每日剂量可每2~4 周增加或减少500 mg,每日2 次;最高剂量为1500 mg/次,每日2 次。
青少年(12~17 岁,体重小于50 kg):初始治疗剂量是10 mg/kg,每日 2 次。
根据临床效果及耐受性,剂量可以增加至 30 mg/kg,每日2 次。
剂量变化应以每2 周增加或减少10 mg/kg,每日2 次。
应尽量使用最低有效剂量。
注意:从口服左乙拉西坦转换时,左乙拉西坦注射液的初始每日总静脉注射剂量应等于口服左乙拉西坦的每日总剂量和频率。
严重不良反应精神症状:行为异常、精神病症状、自杀行为和意念;免疫系统:过敏反应和血管性水肿;皮肤:严重的皮肤病反应;血液系统:血液学异常。
注意事项①停药:建议逐渐停药。
例如:成人和体重50 kg 或以上的青少年每隔 2 到 4 周,每次减少500 mg,每日2 次;6 个月以上婴幼儿、儿童和体重50 kg 以下的青少年:每隔2 周,每次减少不超过10 mg/kg,每日2 次;小于6 个月的婴儿:每隔2 周,减少不超过7 mg/kg,每日2 次。
②肾功能不全对于肾功能损害的患者需要剂量调整;对于严重肝功能损害,需进行肝功能检测,患者的服用剂量需参照用法用量。
左乙拉西坦片说明书

Tonnellier
Manufacturing Site BRA
Artwork Information Fontsize Bodytext 7,5 pt Technical Changes
Linespacing
9 pt
VPT-Pool/Technik/ABV_SOP_Vorgaben/Freigabefeld/Freigabefeld_Aesica_08/2011
总结成人和儿童临床研究结果和上市后经验,评估了每个系统的 不良反应和发生频率:很常见(≥1/10);常见(≥1/100,<1/10); 不常见:(≥1/1000,<1/100); 罕见(≥1/10000,<1/1000); 非 常罕见 (<1/10000),未知(根据现有的资料无法评估)。上市 后临床应用的数据,尚不足以估计在治疗人群中不良反应的发生 率。
坦的清除与肾功能有关。
X
CIA70505E_GI_BRA_Keppra_CN.indd 1
Aesica Pharmaceuticals GmbH Packaging Technology & Artwork Services Alfred-Nobel-Straße 10 40789 Monheim, Germany
- 肝胆管系统异常: 未知:肝功能衰竭,肝炎。
- 代谢和营养异常: 常见:食欲减退、体重增加;当病人同时服用托吡酯时,食欲减退 的危险性增加; 未知:肝功能检查异常,体重减轻。
- 耳及迷路系统异常: 常见剂量,起始治疗应使用口服溶 液。
- 眼部异常: 常见:复视、视物模糊。
1项双盲、安慰剂对照的儿童安全性研究,通过非劣效性设计评 估左乙拉西坦对儿童癫痫部分性发作患者(4-16岁)的认知和 神经心理学的影响。参考符合方案人群Leiter-R注意及记忆力、 记忆筛查综合评分较基线的变化情况,未发现左乙拉西坦和安慰 剂之间存在差异(非劣效分析)。应用CBCL-Achenbach儿童 行为评定量表对行为情感功能的评估提示,服用左乙拉西坦的患 者的攻击行为有所加重。然而,开放性长期随访研究结果显示, 服用左乙拉西坦的患者整体上并未出现行为和情感功能的恶化, 尤其是攻击行为与基线比较无恶化。
左乙拉西坦片说明书

左乙拉西坦片以下内容仅供参考,请以药品包装盒中的说明书为准。
妊娠:禁用哺乳:慎用核准日期:2014年06月06日修改日期:2014年07月01日左乙拉西坦片说明书请仔细阅读说明书并在医师指导下使用。
【药品名称】通用名称:左乙拉西坦片英文名称:Levetiracetam Tablets汉语拼音:Zuoyilaxitan Pian【成份】本品的活性成份为左乙拉西坦【性状】本品为薄膜衣片,除去包衣后显白色或类白色。
【适应症】用于成人及4岁以上儿童癫痫患者部分性发作的加用治疗。
【规格】0.5g【用法用量】(1)给药途径:口服。
需以适量的水吞服,服用不受进食影响。
(2)给药方法:成人(>18岁)和青少年(12~17岁)体重≥50 kg:起始治疗剂量为每次500 mg(即1片),每日2次。
根据临床效果及耐受性,每日剂量可增加至每次1500mg(即3片),每日2次。
剂量的变化应每2~4周增加或减少500 mg(即1片)/次,每日2次。
老年人 (≥65岁) :根据肾功能状况,调整剂量 (详见下文有关肾功能受损病人描述)。
4~11岁的儿童和青少年(12~17岁)体重≤50 kg:起始治疗剂量10 mg/kg,每日2次。
根据临床效果及耐受性,剂量可以增加至30 mg/kg,每日2次。
剂量变化应以每2周增加或减少10 mg/kg,每日2次。
应尽量使用最低有效剂量。
儿童和青少年体重≥50 kg者,剂量和成人一致。
青少年和儿童推荐剂量:起始剂量10 mg/kg,每日2次,最大剂量30 mg/kg,每日2次。
青少年和儿童推荐剂量见表1:表1 青少年和儿童推荐剂量:体重起始剂量最大剂量15kg* 150mg/次,每日2次450mg/次,每日2次20kg 200 mg/次,每日2次600 mg/次,每日2次25kg 250 mg/次,每日2次750 mg/次,每日2次50kg 500 mg/次mg,每日2次1500 mg/次,每日2次*注:20 kg以下的儿童,为精确调整剂量,起始治疗应使用口服溶液。
开浦兰(左乙拉西坦片)说明书

【开浦兰成份】 开浦兰成份】 开浦兰的活性成份为左乙拉西坦, 开浦兰的活性成份为左乙拉西坦,其化学名称为 (S)-α-乙基 氧代 吡咯烷乙酰胺。 乙基-2-氧代 吡咯烷乙酰胺。 乙基 氧代-1-吡咯烷乙酰胺 【开浦兰性状】 开浦兰性状】 开浦兰为椭圆形薄膜包衣片( 为蓝色片, 开浦兰为椭圆形薄膜包衣片(250mg为蓝色片, 为蓝色片 500mg为黄色片,1000mg为白 为黄色片, 为黄色片 为白 色片),除去包衣后均显白色。 ),除去包衣后均显白色 色片),除去包衣后均显白色。
【开浦兰药物过量】 开浦兰药物过量】 据观察有嗜睡、激动、攻击性、 症状 :据观察有嗜睡、激动、攻击性、意识水平下 呼吸抑制及昏迷。 降、呼吸抑制及昏迷。 在急性药物过量后, 药物过量急救措施 :在急性药物过量后,应采取催 吐或洗胃使胃排空。目前尚无左乙拉西坦的解毒剂。 吐或洗胃使胃排空。目前尚无左乙拉西坦的解毒剂。 治疗需对症治疗,也可包括血液透析。 治疗需对症治疗,也可包括血液透析。透析排出的 左乙拉西坦60%,主代谢产 效果 :左乙拉西坦 , 物74%。 。
【百济药师温馨提示】 百济药师温馨提示】
对左乙拉西坦过敏或者对吡咯烷酮衍生物或者其他 任何成分过敏的病人禁用。 任何成分过敏的病人禁用。
【开浦兰注意事项】 开浦兰注意事项】 根据当前的临床实践,如需停止服用开浦兰, 根据当前的临床实践,如需停止服用开浦兰,建议 逐渐停药。 例如 成人每隔2-4周 每次减少500 逐渐停药。(例如 :成人每隔 周,每次减少 mg,每日 次 ;儿童应每隔 周,每次减少 ,每日2次 儿童应每隔2周 每次减少10 mg/kg,每日 次)。 ,每日2次 。 临床研究中, 临床研究中,一些患者对加用左乙拉西坦治疗有效 可以停止原合并应用的抗癫痫药物( 应,可以停止原合并应用的抗癫痫药物(研究中共 位患者其中的36位成人患 有69位患者其中的 位成人患 位患者其中的 者)。
LEVETIRACETAM左乙拉西坦 台湾,用药,说明

LEVETIRACETAM左乙拉西坦<<商品名>>台灣健保藥品-E p i l e n懷特癲寶膜衣錠,K e p p r a優閒膜衣錠,L e t a m p i n力癲平膜衣錠,L e v i m樂閒膜衣錠, N o b e l i n意必寧膜衣錠.美國-K e p p r a X r;K e p p r a加拿大-A p o-L e v e t i r a c e t a m;C o L e v e t i r a c e t a m;D o m-L e v e t i r a c e t a m;K e p p r a;P h l-L e v e t i r a c e t a m;P m s-L e v e t i r a c e t a m;P r o-L e v e t i r a c e t a mK e p p r a(阿根廷,奧地利,澳大利亞,比利時,瑞士,智利,德國,丹麥,西班牙,芬蘭,法國,希臘,香港,印尼,愛爾蘭,以色列,義大利,馬爾他,墨西哥,馬來西亞,荷蘭,挪威,紐西蘭,巴拿馬,秘魯,菲律賓,波蘭,葡萄牙,俄羅斯,瑞典,新加坡,斯洛伐克,薩爾瓦多,泰國,土耳其)<<藥物作用>L e v e t i r a c e t a m為一抗癲癇藥物﹑目前為止其藥理作用的機轉並不明確﹑的但相信與影響神經內鈣離子濃度有關。
由於病患對此藥物的耐受度高﹑並且它藥物作用機轉此與傳統抗癲癇藥物不同﹑病患對藥物的耐受度高,因此常用以癲癇發作控制不佳或無法忍受其它藥品的副作用的輔助治療。
因此可與其它抗癲癇藥物合併治療全身性癲癇(G e n e r a l i z e d S e i z u r e)﹑肌抽躍性發作(M y o c l o n i c s e i z u r e s)﹑強直抽躍性發作(T o n i c-C l o n i cs e i z u r e s)的輔助治療藥物。
开浦兰(左乙拉西坦片)

开浦兰(左乙拉西坦片)【药品名称】商品名称:开浦兰通用名称:左乙拉西坦片英文名称:Levetiracetam Tablets【成份】左乙拉西坦的化学名称为(S)-α-乙基-2-氧代-1-吡咯烷乙酰胺,分子式:C8H14N2O2,分子量:170.21。
【适应症】用于成人及4岁以上儿童癫痫患者部分性发作的加用治疗。
【用法用量】1.给药途径:口服。
需以适量的水吞服,服用不受进食影响。
2.给药方法和剂量:成人(>18岁)和青少年(12-17岁)(体重≥ (greater than or equal to) 50 kg者) :起始治疗剂量为每次500 mg,每日2次。
根据临床效果及耐受性,每日剂量可增加至每次1500 mg,每日2次。
剂量的变化应每2-4周增加或减少500 mg/次,每日2次。
老年人(≥ (greater than or equal to) 65岁) :根据肾功能状况,调整剂量(详见下文有关肾功能受损病人描述)。
4-11岁的儿童和青少年(12-17岁)(体重≤ (smaller than or equal to) 50 kg者) :起始治疗剂量是10 mg/kg,每日2次。
根据临床效果及耐受性,剂量可以增加至30 mg/kg,每日2次。
剂量变化应以每2周增加或减少10 mg/kg,每日2次。
应尽量使用最低有效剂量。
儿童和青少年体重≥ (greater than or equal to) 50 kg者,剂量和成人一致。
青少年和儿童推荐剂量:起始剂量10 mg/kg,每日2次,最大剂量30 mg/kg,每日2次。
体重15 kg:起始剂量每次150 mg,每日2次,最大剂量每次450 mg,每日2次。
体重20 kg:起始剂量每次200 mg,每日2次,最大剂量每次600 mg,每日2次。
体重25 kg:起始剂量每次250 mg,每日2次,最大剂量每次750 mg,每日2次。
左乙拉西坦
2. 左乙拉西坦( levetiracetam, 开浦兰,Keppra)
用于儿童癫癎的药物,已经获得 美国FDA批准。美国FDA批准左乙 拉西坦可以作为4岁或更大一些 儿童癫癎首次发作的辅助治疗药 物。这种药物即将面向儿童患者, 而儿童难治性癫癎占病例的10~ 15%。
3. Keppra® tablets and oral solution
8. efficacy and safety good
LEV is safe in young children and infants, LEV controls epilepsy but does not cure it. 并对认知功能影响小。 LEV单药治疗1年,完全控制率在56.6% ~72.2%, 难治性癫癎完全控制率24.1%~26%。 3年保留率(retention ratio)58%, 撤药率15%。
13. Respective study of the of LEV in children seizure disorders.
52 plts with partial and generalized Sz of mean 8 yrs(8months~10 yrs). Treated by LEV monotherapy(8~35mg/kg/d)for 9 M. Results. >75%Sz reduction-27cases, >50%;<75%3.<50%-8. ADRs were mostly behavioral and cognitive, such as aggression 7.7%,agitation 5.8%. sadation 5.6%.irritability 3.8%. All to be tolerable. Tolerability was surprisingly favorable, even at doses far exceeding 40mg/kg/d..
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KEPPRA® safely and effectively. See full prescribing information for KEPPRA.KEPPRA (levetiracetam) tablets, for oral useKEPPRA (levetiracetam) oral solutionInitial U.S. Approval: 1999----------------------------INDICATIONS AND USAGE--------------------------KEPPRA is an antiepileptic drug indicated for adjunctive therapy in the treatment of:• Partial onset seizures in patients one month of age and older with epilepsy (1.1)• Myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy (1.2)• Primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy (1.3)----------------------DOSAGE AND ADMINISTRATION----------------------• Use the oral solution for pediatric patients with body weight ≤ 20 kg(2.1).• For pediatric patients, use weight-based dosing for the oral solution witha calibrated measuring device (not a household teaspoon or tablespoon)(2.1)Partial Onset Seizures• 1 Month to < 6 Months: 7 mg/kg twice daily, increase in increments of 7 mg/kg twice daily every 2 weeks to recommended dose of 21 mg/kgtwice daily (2.2)• 6 Months to < 4 Years: 10 mg/kg twice daily, increase in increments of10 mg/kg twice daily every 2 weeks to recommended dose of 25 mg/kgtwice daily (2.2)• 4 Years to < 16 Years: 10 mg/kg twice daily, increase in increments of10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kgtwice daily (2.2)• Adults 16 Years and Older: 500 mg twice daily, increase as needed and tolerated in increments of 500 mg twice daily every 2 weeks to a maximum recommended dose of 1500 mg twice daily (2.2)Myoclonic Seizures in Adults and Pediatric Patients 12 Years and Older• 500 mg twice daily, increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily (2.3) Primary Generalized Tonic-Clonic Seizures• 6 Years to < 16 Years: 10 mg/kg twice daily, increase in increments of 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily (2.4)• Adults 16 Years and Older: 500 mg twice daily, increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily (2.4) Adult Patients with Impaired Renal Function• Dose adjustment is recommended, based on the patient’s estimated creatinine clearance (2.5, 8.6)---------------------DOSAGE FORMS AND STRENGTHS---------------------• 250 mg, 500 mg, 750 mg, and 1000 mg film-coated, scored tablets (3) • 100 mg/mL solution (3)-------------------------------CONTRAINDICATIONS-----------------------------• None (4)-----------------------WARNINGS AND PRECAUTIONS-----------------------• Psychiatric Symptoms: Behavioral abnormalities including psychotic symptoms, suicidal ideation, irritability, and aggressive behavior have been observed. Monitor patients for psychiatric signs and symptoms (5.1) • Suicidal Behavior and Ideation: Monitor patients for new or worsening depression, suicidal thoughts/behavior, and/or unusual changes in mood or behavior (5.2)• Somnolence and Fatigue: Monitor patients for these symptoms and advise patients not to drive or operate machinery until they have gained sufficient experience on KEPPRA (5.3)• Withdrawal Seizures: KEPPRA must be gradually withdrawn (5.6)------------------------------ADVERSE REACTIONS------------------------------Most common adverse reactions (incidence in KEPPRA-treated patients is ≥ 5% more than in placebo-treated patients) include:• Adult patients: somnolence, asthenia, infection and dizziness (6.1)• Pediatric patients: fatigue, aggression, nasal congestion, decreased appetite, and irritability (6.1)To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 866-822-0068 or FDA at 1-800-FDA-1088 or /medwatch.-----------------------USE IN SPECIFIC POPULATIONS-----------------------• Pregnancy: Plasma levels of levetiracetam may be decreased and therefore need to be monitored closely during pregnancy. Based on animal data, may cause fetal harm (5.9, 8.1)See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.Revised: 08/2014FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE1.1 Partial Onset Seizures1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy1.3 Primary Generalized Tonic-Clonic Seizures2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions2.2 Partial Onset Seizures2.3 Myoclonic Seizures in Patients 12 Years of Age and Older WithJuvenile Myoclonic Epilepsy2.4 Primary Generalized Tonic-Clonic Seizures2.5 Adult Patients with Impaired Renal Function3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Psychiatric Reactions5.2 Suicidal Behavior and Ideation5.3 Somnolence and Fatigue5.4 Serious Dermatological Reactions5.5 Coordination Difficulties5.6 Withdrawal Seizures5.7 Hematologic Abnormalities5.8 Blood Pressure Increases5.9 Seizure Control During Pregnancy6 ADVERSE REACTIONS6.1 Clinical Trials Experience6.2 Postmarketing Experience7 DRUG INTERACTIONS8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.2 Labor and Delivery8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Use in Patients with Impaired Renal Function10 OVERDOSAGE10.1 Signs, Symptoms and Laboratory Findings of AcuteOverdosage in Humans10.2 Management of Overdose10.3 Hemodialysis11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES14.1 Partial Onset Seizures14.2 Myoclonic Seizures in Patients with Juvenile MyoclonicEpilepsy14.3 Primary Generalized Tonic-Clonic Seizures16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied16.2 Storage17 PATIENT COUNSELING INFORMATION*Sections or subsections omitted from the Full Prescribing Information are not listed.1 INDICATIONS AND USAGE1.1 Partial Onset SeizuresKEPPRA is indicated as adjunctive therapy in the treatment of partial onset seizures in adults and children 1 month of age and older with epilepsy.1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic EpilepsyKEPPRA is indicated as adjunctive therapy in the treatment of myoclonic seizures in adults and adolescents 12 years of age and older with juvenile myoclonic epilepsy.1.3 Primary Generalized Tonic-Clonic SeizuresKEPPRA is indicated as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures in adults and children 6 years of age and older with idiopathic generalized epilepsy.2 DOSAGE AND ADMINISTRATION2.1 Important Administration InstructionsKEPPRA is given orally with or without food. The KEPPRA dosing regimen depends on the indication, age group, dosage form (tablets or oral solution), and renal function.Prescribe the oral solution for pediatric p atients with body weight ≤ 20 kg. Prescribe the oral solution or tablets for pediatric patients with body weight above 20 kg.When using the oral solution in pediatric patients, dosing is weight-based (mg per kg) using a calibrated measuring device (not a household teaspoon or tablespoon).KEPPRA tablets should be swallowed whole. KEPPRA tablets should not be chewed or crushed.2.2 Partial Onset SeizuresAdults 16 Years and OlderIn clinical trials, daily doses of 1000 mg, 2000 mg, and 3000 mg, given as twice-daily dosing were shown to be effective. Although in some studies there was a tendency toward greater response with higher dose [see Clinical Studies (14.1)], a consistent increase in response with increased dose has not been shown.Treatment should be initiated with a daily dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Additional dosing increments may be given (1000 mg/day additional every 2 weeks) to a maximum recommended daily dose of 3000 mg. Doses greater than 3000 mg/day have been used in open-label studies for periods of 6 months and longer. There is no evidence that doses greater than 3000 mg/day confer additional benefit.Pediatric Patients1 Month to < 6 MonthsTreatment should be initiated with a daily dose of 14 mg/kg in 2 divided doses (7 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 14 mg/kg to the recommended daily dose of 42 mg/kg(21 mg/kg twice daily). In the clinical trial, the mean daily dose was 35 mg/kg in this age group. Theeffectiveness of lower doses has not been studied.6 Months to <4 Years:Treatment should be initiated with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). The daily dose should be increased in 2 weeks by an increment of 20 mg/kg to the recommended daily dose of 50 mg/kg (25 mg/kg twice daily). If a patient cannot tolerate a daily dose of 50 mg/kg, the daily dose may be reduced. In the clinical trial, the mean daily dose was 47 mg/kg in this age group.4 Years to < 16 YearsTreatment should be initiated with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). If a patient cannot tolerate a daily dose of 60 mg/kg, the daily dose may be reduced. In the clinical efficacy trial, the mean daily dose was 44 mg/kg. The maximum daily dose was 3000 mg/day.For KEPPRA tablet dosing in pediatric patients weighing 20 to 40 kg, treatment should be initiated with a daily dose of 500 mg given as twice daily dosing (250 mg twice daily). The daily dose should be increased every 2 weeks by increments of 500 mg to a maximum recommended daily dose of 1500 mg (750 mg twice daily).For KEPPRA tablet dosing in pediatric patients weighing more than 40 kg, treatment should be initiated with a daily dose of 1000 mg/day given as twice daily dosing (500 mg twice daily). The daily dose should be increased every 2 weeks by increments of 1000 mg/day to a maximum recommended daily dose of 3000 mg (1500 mg twice daily).KEPPRA Oral Solution Weight-Based Dosing Calculation For Pediatric PatientsThe following calculation should be used to determine the appropriate daily dose of oral solution for pediatric patients:Daily dose (mg/kg/day) x patient weight (kg)Total daily dose (mL/day) = ----------------------------------------100 mg/mL2.3 Myoclonic Seizures in Patients 12 Years of Age and Older with Juvenile Myoclonic EpilepsyTreatment should be initiated with a dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Dosage should be increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of doses lower than 3000 mg/day has not been studied.2.4 Primary Generalized Tonic-Clonic SeizuresAdults 16 Years and OlderTreatment should be initiated with a dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Dosage should be increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of doses lower than 3000 mg/day has not been adequately studied.Pediatric Patients Ages 6 to <16 YearsTreatment should be initiated with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). The effectiveness of doses lower than 60 mg/kg/day has not been adequately studied. Patients with bo dy weight ≤20 kg should be dosed with oral solution. Patients with body weight above 20 kg can be dosed with either tablets or oral solution [see Dosage and Administration (2.1)]. Only whole tablets should be administered.2.5 Adult Patients with Impaired Renal FunctionKEPPRA dosing must be individualized according to the patient’s renal function status. Recommended doses and adjustment for dose for adults are shown in Table 1. In order to calculate the dose recommended forpatients with renal impairment, creatinine clearance adjusted for body surface area must be calculated. To do this an estimate of the patient’s creatinine clearance (CLcr) in mL/min must first be calculated using the following formula:[140-age (years)] x weight (kg) (x 0.85-----------------------------------------for femaleCLcr=72 x serum creatinine (mg/dL) patients)Then CLcr is adjusted for body surface area (BSA) as follows:CLcr (mL/min)CLcr (mL/min/1.73m2)= ----------------------------x 1.73BSA subject (m2)Table 1: Dosing Adjustment Regimen For Adult Patients With Impaired Renal FunctionGroup Creatinine Clearance(mL/min/1.73m2)Dosage (mg) FrequencyNormal > 80 500 to 1,500 Every 12 hoursMild 50 – 80 500 to 1,000 Every 12 hoursModerate 30 – 50 250 to 750 Every 12 hoursSevere ESRD patients < 30---250 to 500500 to 1,0001Every 12 hoursEvery 24 hours1using dialysis1 Following dialysis, a 250 to 500 mg supplemental dose is recommended.3 DOSAGE FORMS AND STRENGTHSKEPPRA 250 mg tablets are blue, oblong-shaped, scored, film-coated, and debossed with "ucb 250" on one side.KEPPRA 500 mg tablets are yellow, oblong-shaped, scored, film-coated, and debossed with "ucb 500" on one side.KEPPRA 750 mg tablets are orange, oblong-shaped, scored, film-coated, and debossed with "ucb 750" on one side.KEPPRA 1000 mg tablets are white, oblong-shaped, scored, film-coated, and debossed with “ucb 1000” on one side.KEPPRA 100 mg/mL oral solution is a clear, colorless, grape-flavored liquid.4 CONTRAINDICATIONSNone.5 WARNINGS AND PRECAUTIONS5.1 Psychiatric ReactionsIn some patients KEPPRA causes behavioral abnormalities. The incidences of behavioral abnormalities in the myoclonic and primary generalized tonic-clonic seizure studies were comparable to those of the adult and pediatric partial onset seizure studies.A total of 13.3% of adult KEPPRA-treated patients and 37.6% of pediatric KEPPRA-treated patients (4 to 16years of age) compared to 6.2% and 18.6% of adult and pediatric placebo patients respectively, experienced non-psychotic behavioral symptoms (reported as aggression, agitation, anger, anxiety, apathy, depersonalization, depression, emotional lability, hostility, hyperkinesias, irritability, nervousness, neurosis, and personalitydisorder). A randomized double-blind, placebo-controlled study was performed to assess the neurocognitive and behavioral effects of KEPPRA as adjunctive therapy in pediatric patients (4 to 16 years of age). The results from an exploratory analysis indicated a worsening in KEPPRA-treated patients on aggressive behavior (one of eight behavior dimensions) as measured in a standardized and systematic way using a validated instrument, the Achenbach Child Behavior Checklist (CBCL/6-18).In pediatric patients 1 month to < 4 years of age, irritability was reported in 11.7% of the KEPPRA-treated patients compared to 0% of placebo patients.A total of 1.7% of adult KEPPRA-treated patients discontinued treatment due to behavioral adverse events, compared to 0.2% of placebo patients. The treatment dose was reduced in 0.8% of adult KEPPRA-treated patients and in 0.5% of placebo patients. Overall, 10.9% of KEPPRA-treated pediatric patients experienced behavioral symptoms associated with discontinuation or dose reduction, compared to 6.2% of placebo patients. One percent of adult KEPPRA-treated patients, 2% of children 4 to 16 years of age, and 17% of children 1 month to <4 years of age experienced psychotic symptoms, compared to 0.2%, 2%, and 5% respectively, in the placebo patients. In the controlled study that assessed the neurocognitive and behavioral effects of KEPPRA in pediatric patients 4 to 16 years of age, 1 (1.6%) KEPPRA-treated patient experienced paranoia compared to no placebo patients. There were 2 (3.1%) KEPPRA-treated patients that experienced confusional state compared to no placebo patients [see Use in Specific Populations (8.4)].Two (0.3%) adult KEPPRA-treated patients were hospitalized and their treatment was discontinued due to psychosis. Both events, reported as psychosis, developed within the first week of treatment and resolved within 1 to 2 weeks following treatment discontinuation. There was no difference between drug and placebo-treated patients in the incidence of the pediatric patients who discontinued treatment due to psychotic and non-psychotic adverse reactions.The above psychiatric signs and symptoms should be monitored.5.2 Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including KEPPRA, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.Pooled analyses of 199 placebo-controlled clinical trials (mono-and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.Table 2: Risk by indication for antiepileptic drugs in the pooled analysisIndication PlaceboPatients withEvents Per1000Patients DrugPatients withEvents Per1000PatientsRelative Risk:Incidence of Eventsin DrugPatients/Incidencein Placebo PatientsRisk Difference:Additional DrugPatients withEvents Per 1000PatientsEpilepsy 1.0 3.4 3.5 2.4Psychiatric 5.7 8.5 1.5 2.9Other 1.0 1.8 1.9 0.9Total 2.4 4.3 1.8 1.9The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.Anyone considering prescribing KEPPRA or any other AED must balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.5.3 Somnolence and FatigueIn some patients, KEPPRA causes somnolence and fatigue. The incidences of somnolence and fatigue provided below are from controlled adult partial onset seizure studies. In general, the incidences of somnolence and fatigue in the pediatric partial onset seizure studies, and in pediatric and adult myoclonic and primary generalized tonic-clonic seizure studies were comparable to those of the adult partial onset seizure studies.In controlled trials of adult patients with epilepsy experiencing partial onset seizures, 14.8% of KEPPRA-treated patients reported somnolence, compared to 8.4% of placebo patients. There was no clear dose response up to 3000 mg/day. In a study where there was no titration, about 45% of patients receiving 4000 mg/day reported somnolence. The somnolence was considered serious in 0.3% of the treated patients, compared to 0% in the placebo group. About 3% of KEPPRA-treated patients discontinued treatment due to somnolence, compared to 0.7% of placebo patients. In 1.4% of treated patients and in 0.9% of placebo patients the dose was reduced, while 0.3% of the treated patients were hospitalized due to somnolence.In controlled trials of adult patients with epilepsy experiencing partial onset seizures, 14.7% of KEPPRA-treated patients reported asthenia, compared to 9.1% of placebo patients. Treatment was discontinued due to asthenia in 0.8% of treated patients as compared to 0.5% of placebo patients. In 0.5% of treated patients and in 0.2% of placebo patients the dose was reduced due to asthenia.Somnolence and asthenia occurred most frequently within the first 4 weeks of treatment.Patients should be monitored for these signs and symptoms and advised not to drive or operate machinery until they have gained sufficient experience on KEPPRA to gauge whether it adversely affects their ability to drive or operate machinery.5.4 Serious Dermatological ReactionsSerious dermatological reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in both children and adults treated with levetiracetam. The median time of onset is reported to be 14 to 17 days, but cases have been reported at least four months after initiation of treatment. Recurrence of the serious skin reactions following rechallenge with levetiracetam has also been reported. Keppra should be discontinued at the first sign of a rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered.5.5 Coordination DifficultiesCoordination difficulties were only observed in the adult partial onset seizure studies. A total of 3.4% of adult KEPPRA-treated patients experienced coordination difficulties, (reported as either ataxia, abnormal gait, or incoordination) compared to 1.6% of placebo patients. A total of 0.4% of patients in controlled trials discontinued KEPPRA treatment due to ataxia, compared to 0% of placebo patients. In 0.7% of treated patients and in 0.2% of placebo patients the dose was reduced due to coordination difficulties, while one of the treated patients was hospitalized due to worsening of pre-existing ataxia. These events occurred most frequently within the first 4 weeks of treatment.Patients should be monitored for these signs and symptoms and advised not to drive or operate machinery until they have gained sufficient experience on KEPPRA to gauge whether it could adversely affect their ability to drive or operate machinery.5.6 Withdrawal SeizuresAntiepileptic drugs, including KEPPRA, should be withdrawn gradually to minimize the potential of increased seizure frequency.5.7 Hematologic AbnormalitiesPartial Onset SeizuresAdultsMinor, but statistically significant, decreases compared to placebo in total mean RBC count (0.03 x 106/mm3), mean hemoglobin (0.09 g/dL), and mean hematocrit (0.38%), were seen in KEPPRA-treated patients in controlled trials.A total of 3.2% of treated and 1.8% of placebo patients had at least one possibly significant (≤2.8 x 109/L) decreased WBC, and 2.4% of treated and 1.4% of placebo patients had at least one possibly significant (≤1.0 x 109/L) decreased neutrophil count. Of the treated patients with a low neutrophil count, all but one rose towards or to baseline with continued treatment. No patient was discontinued secondary to low neutrophil counts. Pediatric Patients 4 Years to < 16 YearsStatistically significant decreases in WBC and neutrophil counts were seen in KEPPRA-treated patients as compared to placebo. The mean decreases from baseline in the KEPPRA-treated group were -0.4 × 109/L and 0.3 × 109/L, respectively, whereas there were small increases in the placebo group. Mean relative lymphocyte counts increased by 1.7% in KEPPRA-treated patients, compared to a decrease of 4% in placebo patients (statistically significant).In the controlled trial, more KEPPRA-treated patients had a possibly clinically significant abnormally low WBC value (3.0% KEPPRA-treated versus 0% placebo), however, there was no apparent difference between treatment groups with respect to neutrophil count (5.0% KEPPRA-treated versus 4.2% placebo). No patient was discontinued secondary to low WBC or neutrophil counts.In the controlled cognitive and neuropsychological safety study, two subjects (6.1%) in the placebo group and 5 subjects (8.6%) in the KEPPRA-treated group had high eosinophil count values that were possibly clinically significant (≥10% or ≥0.7X109/L).Juvenile Myoclonic EpilepsyAlthough there were no obvious hematologic abnormalities observed in patients with JME, the limited number of patients makes any conclusion tentative. The data from the partial seizure patients should be considered to be relevant for JME patients.5.8 Blood Pressure IncreasesIn a randomized, placebo-controlled study in patients aged 1 month to <4 years of age, a significantly higher risk of at least one measured increase in diastolic blood pressure was observed in the KEPPRA-treated patients (17%) compared to the placebo-treated patients (2%). There was no overall difference in mean diastolic blood pressure between the treatment groups. This disparity between the KEPPRA and placebo treatment groups was not observed in the studies of older children or in adults.5.9 Seizure Control During PregnancyPhysiological changes may gradually decrease plasma levels of levetiracetam throughout pregnancy. This decrease is more pronounced during the third trimester. It is recommended that patients be monitored carefully during pregnancy. Close monitoring should continue through the postpartum period especially if the dose was changed during pregnancy.6 ADVERSE REACTIONSThe following adverse reactions are discussed in more details in other sections of labeling:•Psychiatric Symptoms [see Warnings and Precautions (5.1)]•Suicidal Behavior and Ideation [see Warnings and Precautions (5.2)]•Somnolence and Fatigue [see Warnings and Precautions (5.3)]•Serious Dermatological Reactions [see Warnings and Precautions (5.4)]•Coordination Difficulties [see Warnings and Precautions (5.5)]•Withdrawal Seizures [see Warnings and Precautions (5.6)]•Hematologic Abnormalities [see Warnings and Precautions (5.7)]•Blood Pressure Increases [see Warnings and Precautions (5.8)]•Seizure Control During Pregnancy [see Warnings and Precautions (5.9)]6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.The prescriber should be aware that the adverse reaction incidence figures in the following tables, obtained when KEPPRA was added to concurrent AED therapy, cannot be used to predict the frequency of adverse reactions in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during clinical trials. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescriber with one basis to estimate the relative contribution of drug and non-drug factors to the adverse reaction incidences in the population studied.Partial Onset SeizuresAdults。
