原辅料相容性研究国外综述性文献
口服固体制剂原辅料相容性研究进展

口服固体制剂原辅料相容性研究进展摘要:口服固体制剂是治疗和预防疾病的常用药物形式。
然而,原辅料之间的相互作用可能会对制剂的质量和有效性产生不利影响。
因此,在口服固体制剂的研发和生产过程中,需要对原辅料之间的相容性进行评估和研究。
本文通过对口服固体制剂原辅料相容性的深入研究,可以为制剂的开发和生产提供指导和依据,并提高制剂的质量和安全性。
关键词:口服固体制剂原辅料相容性研究进展引言在药物制备过程中,需要选择性能良好的辅料,辅料的物理性质、化学性质等的不同都会极大的影响药物成品的制剂参数,例如溶解度、保质期等等。
一、价格评估口服固体制剂原辅料相容性价格评估是一个很重要的研究领域,它涉及到制药企业的财务成本和生产效率。
1. 原辅料成本分析针对制作口服固体制剂的原辅料进行成本分析,将影响成本的因素进行拆解和透析,并探讨各因素对原辅料相容性价格的影响。
这种方法适用于市场前景不确定或其他不确定因素对原料和辅料成本产生影响的情况。
2. 生产效率评估通过研究制药企业的工艺流程和设备管理情况,可以评估口服固体制剂原辅料相容性价格。
生产效率评估需要收集制药企业的各项成本记录,例如人工成本,机器成本,直接材料成本,制备过程评估,以及品质控制记录。
通过全面的数据分析找出生产效率低的环节和成本高的领域,并寻找改进措施。
3. 市场统计分析通过针对口服固体制剂原辅料市场的统计数据分析(如市场增长率、参与方或企业数量、价格变化趋势等),评估价格趋势,预测口服固体制剂原辅料价格的变化,找出价格波动的主因以及寻找应对之策,从而对企业未来的财务预算和保障做好充分的准备。
二、技术性评估1、安全性口服固体制剂原辅料相容性技术评估需要涉及到一定的安全性问题。
因为一些不相容的原辅料可能会导致制剂的不稳定性,进而影响药物的安全性和疗效。
选择符合规定的原辅料生产企业,确保原辅料的质量安全和稳定性。
生产企业要有较为先进的生产设备和技术,完善的生产质量管理体系和规范的操作制度,确保不相容的原辅料不会加入到药品的生产中,保证制剂安全性。
相容性研究4综述

脆碎度 滴(粒) 分布
现代生物医药研究所 90页 Institute of Modern Bioparmaceuticals
重金属检查法---玻璃
重金属系指在实验条件下能与显色的金属杂质。当人 体中重金属元素的浓度达到一定程度时,会使人体器官、 组织发生病变,严重时还会丧失机能。 药物中重金属的检查方法分析,中国药典以前历版药 典均用硫化氢试液为显示剂,但该试液有恶臭和毒性, 污染环境且不够稳定,浓度难予控制,因此后来在1990 年版本以后的修改中改用硫代乙酰胺法试液进行。 此外,中国药典采用微孔滤膜过滤法为检查重金属的 第四法。 《中华人民共和国药典)2005年版对中药材规定:砷 (A8)≤2.0 mg/kg,汞(Hg)≤0.2 mg/kg,镉 (Cd)≤0.30 mg/kg,铅(Pb)≤5.0 mg/kg。
相容性研究中相关考察项 目及方法
现代生物医药研究所 Institute of Modern Bioparmaceuticals
考察项目
现代生物医药研究所 Institute of Modern Bioparmaceuticals
药物:
pH值(粉针考察酸碱度)、澄明度、不溶性微粒、紫外吸 收、无菌、细菌内毒素、装量 颜色、含量、有关物质、重金属、渗透压、旋光度、相对密 度、失重、释放度、熔点、水分、崩解时限、溶出度、连粘、 融变时限、脆碎度、滴(粒)分布
现代生物医药研究所 Institute of Modern Bioparmaceuticals
4.样品前处理 塑料输液管、葡萄糖袋样品: 称取各样品0.5g ,平行5份,剪成碎片放入具塞三角瓶中,用20mL无水醇、 丙酮等有机溶剂浸泡24 小时,超声提取3 次,15min/ 次。再经无水Na2SO4除 水、过滤、水浴蒸发浓缩,浓缩液过0.45μm一次性微孔滤膜,无水乙醇定容 15mL 备用。 塑料袋内葡萄糖液体: (1)样品直接点板展开; (2)将样品经有机溶剂萃取后点板展开: 用移液管准确量取5mL葡萄糖溶液于分 液漏斗,再加入同体积的丙酮剧烈震动并不时放气,静置使溶液分层,取上层清 液进行测定。 用微量进样器分别准确吸取邻苯二甲酸酯混合标准溶液 (1000mg/L)3.0,6.0,9.0,12.0,15.0μL,分别点于硅胶G板上,用上述法进行展 开和测量。 5.用标准混合溶液做加标回收实验,得到各组分加标回收率。
药物与辅料相容性研究进展

Vol.4 No.1Feb. 2018生物化工Biological Chemical Engineering第 4 卷 第 1 期2018 年 2 月药物与辅料相容性研究进展张卉(南京艾德凯腾生物医药有限责任公司,江苏南京211100)摘 要:随着医学的不断发展,药物质量越来越受到人们的重视。
在药物生产过程中,辅料扮演着不可替代的角色。
在辅料的设计过程中,如果不考虑辅料和活性成分的配比禁忌等因素,没有进行实时测定分析,就可能影响药物质量,从而对用药者产生潜在的风险。
本文从辅料和有效成分之间的相互作用进行分析,从而提高药物与辅料的相容性。
关键词:药物-辅料相互作用;相容性;热分析法;光谱法;色谱法中图分类号:R943 文献标志码:AAdvances in Study on Compatibility of Drugs and ExcipientsZhang Hui(Nanjing Kaiteng Ed Biological Medicine Co., Ltd, Jiangsu Nanjing 211100)Abstract: With the continuous development of medicine, people pay more and more attention to the quality of drugs. In the process of drug production, the excipient plays an irreplaceable role. In the process of excipient design, if we do not consider the factors such as the ratio of accessories and active ingredients, taboo and other factors, no real-time determination and analysis will affect the quality of the drugs, and thus have potential risks to the users. In this paper, the interaction between the excipients and the effective components is analyzed to improve the compatibility between the drugs and the excipients.Keywords: Drug excipient interaction; Compatibility; Thermal analysis; Spectrometry; Chromatography在药物研究开发过程中,经常会使用辅料来保证药物的有效性和增加病人的顺应性。
原辅料相容性作用机制及研究方法分析
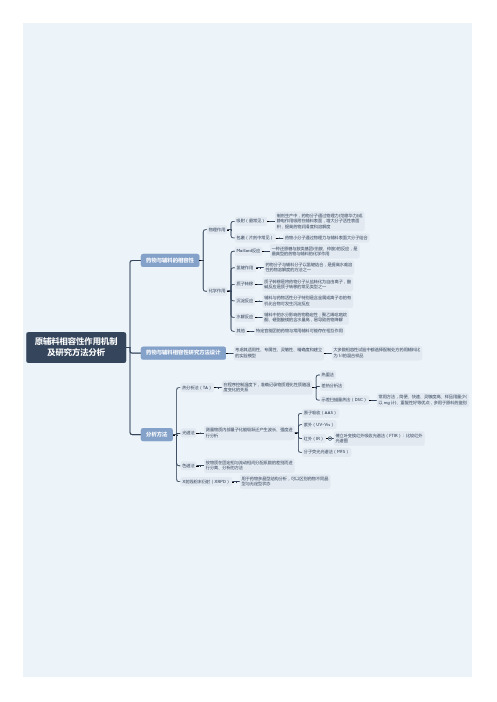
氢键作用
药物分子与辅料分子以氢键结合,是提高水难溶 性药物溶解度的方法之一
质子转移
质子转移是将药物分子从盐转化为自由离子,酸 碱反应是质子转移的常见类型之一
沉淀反应
辅料与药物活性分子特别是含金属或离子态的有 机化合物可发生沉淀反应
水解反应
辅料中的水分影响药物稳定性;聚乙烯吡咯烷 酮、硬脂酸镁的含水量高,易导致药物降解
常用方法,简便、快速、灵敏度高、样品用量少( 以 mg 计)、重复性好等优点,多用于原料药鉴别
原子吸收(AAS)
光谱法
测量物质内部量子化能级跃迁产生波长、强度进 行分析
紫外(UV-Vis)
红外(IR)
傅立叶变换红外吸收光谱法(FTIR):比较红外 光谱图
分子荧光光谱法(MFS)
色谱法
按物质在固定相与流动相间分配系数的差别而进 行分离、分析的方法
X射线粉末衍射(XRPD)其他
特定官能团的药物与常用辅料可能存在相互作用
药物与辅料相容性研究方法设计
考虑其适用性、专属性、灵敏性、精确度和建立 的实验模型
大多数相容性试验中都选择配制处方药用辅料比 为 1:1的混合样品
分析方法
热分析法(TA)
在程序控制温度下,准确记录物质理化性质随温 度变化的关系
热重法 差热分析法 示差扫描量热法(DSC)
原辅料相容性作用机制 及研究方法分析
药物与辅料的相容性
物理作用 化学作用
吸附(最常见)
制剂生产中,药物分子通过物理力(范德华力)或 静电作用吸附在辅料表面,增大分子活性表面 积,提高药物润滑度和溶解度
包裹(片剂中常见)
药物小分子通过物理力与辅料表面大分子结合
Maillard反应
药用辅料聚维酮(PVP)论文Word编辑

聚维酮的应用与发展药理2班 1340924 蔡周坤摘要:聚乙烯吡咯烷酮(polyvinyl pyrrolidone)简称PVP,是一种非离子型高分子化合物,是N-乙烯基酰胺类聚合物中最具特色,且被研究得最深、广泛的精细化学品品种。
PVP 具有许多优良的物理化学性能,如优异的溶解性、低毒性、成膜性、增溶性、络合性、生理相容性、表面活性和化学稳定性等。
目前它被广泛用于医药、化妆品、酿造、饮料、食品和纺织等领域。
关键词:聚维酮药剂辅料应用发展与现状正文:①前言:聚维酮在医药等各方面都发挥着重要的作用,它具有许多优良的物理化学性能,极易溶于水,安全无毒;能与多种高分子、低分子物质互溶或复合;具有优良的吸附性、成膜性、粘接性及生物相容性,而且热稳定性良好。
聚维酮系列药用辅料的优异生理相容性是其固有而独特的产品性质,发展到如今,它已与纤维素类衍生物、丙烯酸类化合物一起成为当今三大主要合成药用辅料。
本文的写作目的主要是综合信息,阐述聚维酮在医药等各方面的作用以及它目前的发展前景,进一步深入了解它的各方面的信息,再一次佐证它所展现出来的价值,并同时让自己得到收获。
②主体:1.聚维酮的性质概述聚维酮具有粘合、增稠、助悬、分散、助溶、络合等特性和作用,自问世以来,由于其安全无毒,性质优良,规格众多,在药剂上有广泛的应用,二战期间曾被用作代血浆。
现常作粘合剂、崩解剂、增稠剂、助悬剂、分散剂、助溶剂、络合剂、前体药物制剂载体剂、成膜剂、包衣材料、控释缓释制剂的基质等。
随着药物制剂工艺的不断发展,聚维酮作为非离子型水溶性高分子化合物药用辅料得到越来越广泛的应用。
2.聚维酮的应用2.1在医药卫生中的应用:聚乙烯吡咯烷酮具有优异的生物相容性,对皮肤、粘膜和眼睛等不形成任何刺激,因此它在医药领域应用广泛。
在制药工业中,聚维酮与纤维素类衍生物、丙烯酸类化合物一样,成为当今合成药中的三大主要辅料之一 ,用它制成的血浆无抗原性,不需交叉配血,还能避免疾病在血液中传播。
药品研发中的原辅料相容性实验

在药品设计过程中,辅料的选择至关重要。
对于辅料及其在处方中的用量选择,不仅基于它们的功能性,更重要的是要考虑药物与辅料之间的相容性。
所谓的不相容性,可以定义为药物与处方中一种或多种辅料发生不良相互作用,从而导致制剂在物理、化学、微生物学或治疗性质方面的改变。
因此,辅料相容性的研究主要是用于预测药物在最终剂型中可能潜在的不相容性,同时为申报注册法规文件所需的处方中辅料及其用量的选择提供合理依据。
辅料相容性的研究通常被认为是很普通并且繁琐的。
但是,这些研究恰恰是药物研发过程中非常重要的工作,原因在于:包括处方的选择、药物稳定性的评估,降解产物的鉴定,以及对于相互作用机制的了解,都可以从辅料相容性研究所获得的知识中得到有益指导。
如果发现药物稳定性不能尽如人意,就要采取对策以提高其稳定性。
因此,在药品开发的后阶段进行系统的,周详计划和执行的相容性研究,可以有效地节省由于稳定性问题而浪费的资源和拖延的时间;同时,如果当药物产品进入后期开发的阶段,辅料相容性的研究对于引起稳定性问题的原因推测也非常有帮助。
而且随时间的推移,监管的期望对此有了显著的增加。
正如一直都在提倡的质量源于设计(QbD)的倡议那样,这种趋势将会一直延续。
在进入申请的开发报告中,需要药物与辅料的相容性数据以证明对处方成分选择的正确性。
基于辅料对药品和生产工艺的影响,相关法规已经越来越多地关注其关键质量特性(critical quality attributesCQA)和控制策略。
从药物开发过程的角度而言,通常是在对药品(药物活性成分,API)液体和固体稳定性有一定的了解之后开展这些研究,但应在处方开发之前。
剂型中不相容导致的变化可以归纳为以下几点①颜色或外观的变化;②机械性能的损失(如片剂硬度);③溶出行为的变化;④物理晶型的转变,⑤升华导致的损失;⑥药效降低;⑦降解产物的增加。
2.药物与辅料之间的化学作用在药物制剂中能观察到最普遍的反应为水解作用、脱水作用、同分异构化作用、消除作用、成环作用、氧化作用,光降解作用,以及与处方成分(辅料及其杂质)之间的特殊反应。
外文文献中文

反应型相容剂的合成及其对PE/PA6合金的增容作用泰盟.比尔德詹斯.怀尔德海恩西蒙. 布伦奇利摘要:用乳液聚合的方法合成了St-MAH-GMA-AA四元反应型相容剂,以红外光谱测试对合成产物进行结构分析,并研究了相容剂制备过程中的影响因素以及相容剂对PE/PA6合金的力学性能和微观形貌的影响。
结果表明,各单体均参加了反应,相容剂的加入可以提高合金的力学性能。
关键词:乳液聚合;St-MAH-GMA-AA相容剂;PE/PA6合金;增容Synthesis of Reaction Compatibilizer and Its Compatibilizationof PE / PA6 AlloyLI Yu-hong1,ZHANG Cheng-you2,WANG Yong-hui1,LIU Jun-long*1(1 College of Textile and Materials,Dalian University of Technology,Dalian 116034,China 2 Shenzhen Prade Chemical Co. LTD,Shenzhen,518049,China)Abstract: Four element reaction compatibilizer St-MAH-GMA-AA is synthesized by emulsion polymerization method. The structure of product was analyzed by using infrared spectroscopy, and the influence factors of compatibility of preparing as well as mechanical properties and microstructure of PE / PA6 alloy . The results show that the monomers are participated in the reaction and compatibilizer could improve the mechanical properties of the alloys.Key words: emulsion polymerization;St-MAH-GMA-AA compatibilizer;PE / PA6 alloy;compatibilization开发聚合物合金的关键问题是解决不同聚合物共混体系的相容性。
SIS热塑丁苯橡胶的生物相容性研究

SIS热塑丁苯橡胶的生物相容性研究摘要:生物相容性是评价医疗材料适用性的重要指标之一。
本文旨在研究SIS热塑丁苯橡胶的生物相容性,通过实验和文献综述的方式,探讨其在生物医学领域的应用前景。
引言:在生物医学领域,材料的生物相容性是评估其在生物体内应用的重要考虑因素。
生物相容性是指医疗材料与生物体接触后不引起明显的毒性反应,并保持良好的生物相互作用。
SIS热塑丁苯橡胶作为一种新型的材料,具有良好的物理性能和生物相容性。
因此,对其生物相容性的研究具有重要意义。
1. 实验方法为了研究SIS热塑丁苯橡胶的生物相容性,我们采用了以下实验方法:1.1 细胞毒性实验通过细胞毒性实验可以评估材料对细胞的影响。
我们以人体细胞株为研究对象,将SIS热塑丁苯橡胶与细胞共培养一段时间后,观察细胞的生长情况和形态变化。
1.2 肉芽组织形成实验肉芽组织形成实验是评价材料在体内是否能够促进血管新生的重要方法。
我们将SIS热塑丁苯橡胶植入小鼠皮下组织中,并观察材料周围是否形成肉芽组织,并且血管新生情况如何。
1.3 免疫原性实验免疫原性是指材料与机体接触后是否能够引起免疫反应。
我们通过测定SIS热塑丁苯橡胶对小鼠免疫系统的影响,评价其免疫原性。
2. 结果与讨论2.1 细胞毒性实验结果通过细胞毒性实验,我们观察到SIS热塑丁苯橡胶与人体细胞的共培养并没有引起明显的细胞毒性反应。
细胞的形态没有发生明显的变化,细胞数量也没有明显减少,这表明SIS热塑丁苯橡胶在细胞水平上具有较好的生物相容性。
2.2 肉芽组织形成实验结果我们观察到SIS热塑丁苯橡胶植入小鼠皮下组织后,周围形成了肉芽组织,并且血管新生情况良好。
这表明SIS热塑丁苯橡胶具有良好的生物相容性,并能够促进血管新生,有助于组织修复。
2.3 免疫原性实验结果我们的实验结果显示,SIS热塑丁苯橡胶对小鼠免疫系统没有明显的刺激作用,没有引发免疫反应。
这表明SIS热塑丁苯橡胶在免疫系统水平上具有较好的生物相容性。
国外药用辅料的研究进展

国外药用辅料知名企业
美国陶氏化学(DOW)系列产品 美国ISP 国际特品公司 美国诺誉(NOVEON)化工系列产品 法国GATTEFOSSE(佳法赛)公司系列产品 德国罗姆(Rohm) 德国BASF 公司 日本信越化学公司 日本旭化成公司
美国国际特品公司
美国国际特品公司
• 美国国际特品公司(ISP,现在叫领先特品公司) 是全球领先的特殊化学品生产商之一,公司拥 有160多年的历史,公司总部设在美国新泽西州 (New Jersey),生产基地在美国,同时在德国及 苏格兰设有独资厂。ISP除了在美国总部设有开 发及技术服务中心之外,在全球包括英国、巴 西、新加坡、印度及中国均设有技术服务中心。 ISP公司已经通过全球销售和客户服务分公司及 办事处在涂料,化妆品,医药,啤酒,食品, 农业等领域为90多个国家提供约三百多种主要产 品。
5. 动物提取技术,如:甲壳素,甲壳糖等
乳糖 纤维素
蔗糖 糊精 微晶纤维素 微粉硅胶
Ludipress
Cellactose
DiPac
BASF AG
吸湿性低, 流动性好, 片剂硬度与来自压片速度无 关Meggle Gmbh 可压性好, & Co. KG 口感好,成
本低
直接压片用
Prosolv
Penwest
流动性好,减少
Pharmaceuti 湿法制粒的影响,
药用辅料的品种
• Gattefosse公司的系列药用甘油液态辅料产品,其中包括 Gellucire 44/14、Capryol90、CaprylolPGMC、Lauoglycol、 Labrafac PG 等。这些药用甘油系列液态药用辅料能大大提高 难溶药物的溶解度,进而提高药物的体内生物利用度。
中国与欧美药品包装材料相容性研究规范

t h e c o mp a t i b i l i t y s t u d y b e t we e n d ug r a n d p a c k a g i n g ma t e i r a l s .I t h o p e d t o p r o v i d e p h a r ma c e u t i c a l c o mp a n y a n d r e s e a r c h
王学芬 。 ,陈祖坚 ,闫
( 1上 海交 通 大学药 学 院 ,上 海
超
2 0 1 2 0 4 )
2 0 0 2 4 0;2组迪 希 亚制 药 ( 无锡 ) 有 限公 司,上 海
摘 要 :药品包装是药品的重要组成部分 ,近年来由于药品包装选择不当,由药品与包装材料相容性引起的问题不断出现。
文献标 志 码 :B
文章 编号 :1 0 0 1 — 9 6 7 7 ( 2 0 1 4 ) 0 5 — 0 0 3 1 — 0 4
Re g u l a t i o n s a n d Te c h n i c a l Gu i d e l i n e s o f Dr u g Pa c k a g i n g Ma t e r i a l ’ S Co mp a t i b i l i t y S t u d y i n Ch i n a,EU a n d US A
本文综合 收载了中国 、欧盟 以及美 国的药 品监管 机构对药品与包装材料 相容性研 究 的不 同法规要求 , 希 望能为药 品生产企业及 其 研发机构进行 相容性研究提供 了基本 的理论 依据和试验思路 。
关键词 :中国;欧美;药品;包装材料 ; 相容性研究;法规要求
中图分 类 号 :R 9 5 1
原辅料相容性报告范文

原辅料相容性报告范文英文回答:Raw Material and Auxiliary Material Compatibility Report.Introduction.Ensuring the compatibility of raw materials and auxiliary materials is crucial for the successful production of finished goods. This report provides a comprehensive analysis of the compatibility of various raw materials and auxiliary materials used in the manufacturing process.Materials Tested.Raw materials: List of raw materials used, e.g., plastic resins, metals, fabrics.Auxiliary materials: List of auxiliary materials used, e.g., solvents, pigments, additives.Compatibility Assessment.The compatibility of the materials was assessed using a combination of methods, including:Physical testing: Examination of the physical properties of the materials when combined, e.g., adhesion, strength, flexibility.Chemical testing: Analysis of the chemicalinteractions between the materials, e.g., reactivity, corrosion resistance.Performance testing: Evaluation of the performance of the finished goods made with the materials, e.g., durability, efficiency, functionality.Results.The compatibility assessment revealed the following results:Compatible Materials:List of materials that exhibited good compatibility when combined, e.g., resin A with solvent X.Incompatible Materials:List of materials that exhibited poor compatibility when combined, e.g., resin B with solvent Y.Conditional Compatibility:List of materials that exhibited compatibility under certain conditions, e.g., resin C with additive Z at specific ratios.Recommendations.Based on the compatibility assessment, the followingrecommendations are made:Use compatible materials in the manufacturing process to ensure the desired performance and quality of the finished goods.Avoid using incompatible materials to prevent adverse effects on the production process and the final products.Consider the conditions for conditional compatibility when using specific materials.Conclusion.This Raw Material and Auxiliary Material Compatibility Report provides a valuable guide for ensuring the compatibility of materials used in the manufacturing process. By adhering to the recommendations outlined in this report, manufacturers can optimize production, minimize risks, and enhance the quality of their finished goods.中文回答:原材料和辅料相容性报告。
印刷工程毕业设计文献综述—聚乳酸PLA与EMA复合板材的制备

聚乳酸PLA与EMA复合板材的制备及印刷适性的研究1 聚乳酸PLA在国外的发展历史和现状早在20 世纪30 年代末,美国、日本的科学家就开始进行聚乳酸的合成研究,但由于原料成本高,一直未得到推广。
进入20 世纪80 年代,受石油短缺和环保压力的驱使世界对生物可降解材料的研究和发展再次活跃。
在玉米的深加工技术中努力研制聚乳酸的先驱是日本的钟纺合纤公司和美国的卡吉尔·道(CagillDaw)聚合物公司。
早在20 世纪60~70 年代,日本钟纺合纤公司在生物可降解塑料筛选中发现了聚乳酸的生物可降解性。
在此期间Cargill Daw公司开发了能从玉米产生聚乳酸纤维的工艺。
最后由日本钟纺合纤公司联合岛津制作所于1994 年共同开发出了商品名为Lactron的PLA 纤维,又称作玉米纤维。
1997 年,Dow 聚合物公司看好聚乳酸纤维的后期发展,与Cargill Daw公司以各占50%的股份建造了年产量达14 万吨的生产线,并于2002 年投产,商品名为“Nature Works”。
2005 年,Nature Works 公司从Dow 和Cargill Daw公司中独立出来,更名为英吉尔(Ingeo),至此英吉尔公司成为世界上最大的聚乳酸生产厂家。
目前全世界有100多家使用英吉尔生产的原料和消费品,其中包括了食品包装、餐具、服装、家居用品、个人护理产品以及消费类电子产品。
此外,德国UhdeInventa - Fischer 公司、意大利Snamprogetti公司、荷兰Hycail公司、德国巴斯夫公司等也开发了聚乳酸生产技术。
毫无疑问,日本是最精明的。
日本并没有丰富的玉米资源,不可能像美国、中国等玉米资源大国一样不断地生产聚乳酸,但日本人善于设计和创造。
自从美国卡吉尔陶氏(Cargill-Dow)公司发明聚乳酸后,日本就开始了他们的玉米塑料产品加工的研制和创新,据世界聚乳酸发展势态专利系列分析报告称,在1985-2005 年的20 年间申请专利数目最多的前十位专利拥有权人都是日本人,他们在聚乳酸方面申请的专利占到了在此期间总专利数的50%,日本人在聚乳酸的改性和产品的开发力上可见一斑。
FDA_原辅料相容性

• Guidance for industry - M4: Organization of the CTD
– /downloads/Drugs/GuidanceComplianceRegul atoryInformation/Guidances/ucm073257.pdf
02-Mar-2011
6
3.2.S.2 Manufacture
• 3.2.S.2.4 Controls of Critical Steps and Intermediates
– Critical steps - tests, acceptance criteria and methods performed at critical steps in the synthesis of non-radioactive intermediate – Quality and control of intermediates isolated during the synthesis of non-radioactive intermediate – Critical process parameters – Normal operating ranges and acceptable ranges
3.2.S.4.2 Analytical Procedures
– Descriptions of the analytical procedures that are referenced in section S.4.1
•
3.2.S.2.3 Control of Materials
– Identify starting materials and provide specifications (tests, acceptance criteria, and analytical procedures) – Specifications for reagent, solvents, and auxiliary materials
03.药用辅料相容性研究及安全性评价

2019/3/5
Page 15
China Pharmaceutical University
谢谢!
16
3、生物学相容性:以有效性、毒性(一般/特殊)、过敏性等为指标, 以药品安全性评价为核心(可部分归纳在安全性评价范畴)
2019/3/5
Page 7
China Pharmaceutical University
二、相容性研究案例
1、(物理、化学)相容性研究:
常见过程 辅料+药物→混合均匀→置高温、高湿、强光→5、10天取样测定( 外观、吸湿量/结晶水、有关物质、含量、晶型等)→以有关物质等 变化为评价指标,确认辅料是否可行! 存在主要问题 1)评价指标“单一化”、“人为化”、“比例不当”等; 2)缺少制剂制备工艺过程,预测可能有误(如热压灭菌、湿法制粒干 燥等) ; 3)多晶型药物常因粉碎、溶剂、制剂机械的工作原理(如热熔挤出等 )或工艺参数(如干燥温度、速度等)差异而发生转晶
2)胶塞:添加剂转移(相容性+安全性)
……等等
2019/3/5
Page 14
China Pharmaceutical University
四、展 望
高质量药用辅料是生产高质量药品的基础;
药用辅料与药物是否相容或匹配是高质量药品处 方设计和优化的基本研究内容; 如何采用科学、合理的方法和手段,对药用辅料 进行相容性研究及安全性评价,是保障高质量辅 料和药品研发的关键!
系指生产药品和调配处方时使用的赋形剂和附加剂。 是除活性成分以外,在安全性方面已进行了合理的评估,且包含在 药物制剂中的物质(包括制备过程中)。
2、基本要求
对人体无毒害作用;化学性质稳定,不易受温度、pH值、保存时间 等的影响;与药物成分之间无配伍禁忌;不影响制剂的检验,或可 按允许的方法除去对制剂检验的影响等
蜘蛛丝蛋白聚吡咯复合纤维膜细胞相容性研究【文献综述】

毕业设计文献综述纺织工程蜘蛛丝蛋白/聚吡咯复合纤维膜细胞相容性研究一、前言部分组织工程材料是当前生命科学和材料科学共同的前沿研究热点之一。
目前已经开发应用于组织工程等生物医学领域的生物相容性高分子材料主要有胶原蛋白、聚乳酸、聚乙醇酸及其共聚物等,但大多数这类材料的原创性研究工作属于国外。
我国是一个人口大国,因创伤和疾病造成的组织、器官丧失或功能障碍病例居世界各国之首,每年仅因烧伤需进行皮肤移植的患者就达百万之多。
因此,积极寻找合适的原料,研制具有我国自主知识产权的生物材料,对于减小我国组织工程支架等生物材料对国外的依赖性,培育新的高新技术产业和实现国民经济的可持续发展具有重要意义。
研究者一直在寻找具有良好的生物相容性的支架材料以应用于细胞培养中,以及研究支架材料在细胞培养中的各项性能指标以及实验环境对细胞培养的影响,了解细胞在支架材料上的生长情况,以便更好的应用在临床中[1]。
而导电支架细胞培养技术发展迅速,是近年来研究的热点。
本文主要蜘蛛丝蛋白和聚吡咯复合制得的支架材料,将细胞培养在带电的支架材料上,重点研究培养过程中支架材料的性质、电刺激的电流强度及电刺激的时间长短对细胞再生的影响。
二、主题部分2.1 蜘蛛丝蛋白的概况和研究现状2.1.1蜘蛛丝蛋白的概况蜘蛛丝的主要成份为蛋白质,如所有的蛋白质纤维一样,其组成长链蛋白质的单元为带不同侧链R的酰胺结构,同尼龙2结构相似[2]。
蜘蛛丝的氨基酸的摩尔分数和氨基酸的主链序列与天然聚肽如蚕丝、羊毛和人头发有很大的差异。
这种差异和组成取决于蜘蛛的种类、食物、气候及其它因素。
不同蜘蛛丝所含的氨基酸种类差异不大,为十七种左右,各种氨基酸的含量也因蜘蛛的种类不同而有一定的差异。
它们的共同点为具有小侧链的氨基酸如甘氨酸和丙氨酸的含量丰富,十字圆蛛和大腹圆蛛的这两者含量之和分别达到59.6%和53.2%与蚕丝的含量74.0%相比较就显得较低[3]。
蜘蛛丝是一种特殊的蛋白纤维,它具有很高的强度、弹性、柔韧性、伸长度和抗断裂功能,以及轻盈、较耐紫外线、生物可降解等优点,作为一种新兴的功能材料具有得天独厚的条件,是其他纤维材料无法比拟的,可以作为高性能的生物材料如人造肌腱、人工韧带、人工器官、组织修复等组织工程新材料,正逐步受到生物学、医学、材料科学等学科领域研究者的重视,具有十分诱人的应用前景。
国外药用辅料的研究进展

交联聚维酮
丙烯酸树脂
甘露醇
低取代羟丙基纤维素
国外药用辅料的技术
1. 高分子聚合技术,如:聚乙二醇系列、聚羧 乙烯系列、聚丙交酯系列、聚氧乙烯烷酸酯 系列等 2. 生物合成多糖类技术,如:黄原胶、环糊 精、普鲁蓝等 3. 半合成技术,如:预胶化淀粉、羧甲基淀粉 钠、纤维素系列 4. 植物提取技术,如:海藻酸、红藻酸、卡拉 胶等 5. 动物提取技术,如:甲壳素,甲壳糖等
国外药用辅料开发特点
•生产专业化 •品种系列化 •应用科学化 •服务优质化
国外药用辅料开发重点
1.优良的缓释、控释材料 2.优良的肠溶、胃溶材料 3.高效崩解剂和具有良好流动性、可压性、黏 合性的填充剂和黏合剂 4.具有良好流动性、润滑性的助流剂、润滑剂 5.无毒高效的透皮促进剂 6.适合多种药物制剂需要的复合辅料 7.开发可生物降解的高分子辅料
Tha 药用辅料的安全性问题 辅料是药品生产过程中不可欠缺的材料,因此对 辅料的安全性问题应引起研究人员的注意。然而 药用辅料安全监管体系的相对不完善,所以这是法 律法规和相关标准所面临的一个重要问题。 • 药用辅料的应用研究 1. 研究新辅料的理化性质及其如何适用于制剂的 开发和生产 2. 结合口服生产设备及制剂工艺研究辅料与药物 的配伍特性,得到最佳辅料配方 3. 进行辅料间的配伍研究,结合各国生产实际, 设计最佳复合辅料
日本旭化成公司
日本旭化成公司
• 成立日期 1931年5月21日 • 旭化成总部日本东京都千代田区神田神保 町1-105 • 董事长 伊藤一郎。 • 首次发展是通过扩大工业化学品和衍生品 如苛性钠、氯、化肥、硝化纤维、工业炸 药、铜氨丝、以及粘胶长丝(粘胶长丝的 生产于2001年9月结束)的生产来实现的。 第二次世界大战后的几年,旭化成开始扩 展到更广阔的新领域,并发展成为日本化 工业最前端的企业。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review.Sonali S. Bharate, Sandip B. Bharate and Amrita N. Bajaj a* b cP.E. Society’s Modern College of Pharmacy (For Ladies), Borhadewadi, At/Post- Moshi, Tal-Haweli, Dist- Pune, Maharashtra –a412105, IndiaSTES’s Sinhgad College of Pharmacy, Off Sinhgad Road, Vadgaon (Budruk), Pune-411041, Indiab C.U. Shah College of Pharmacy, S.N.D.T. Women’s University, Juhu Tara Road, Santacruz (E), Mumbai, Maharashtra-400049, IndiacReceived:05 March 2010; Accepted: 03 November 2010ABSTRACTStudies of active drug/excipient compatibility represent an important phase in the preformulation stage of the development of all dosage forms. The potential physical and chemical interactions between drugs and excipients can affect the chemical nature, the stability and bioavailability of drugs and, consequently, their therapeutic efficacy and safety. The present review covers the literature reports of interaction and incompatibilities of commonly used pharmaceutical excipients with different active pharmaceutical ingredients in solid dosage forms. Examples of active drug/excipient interactions, such as transacylation, the Maillard browning reaction, acid base reactions and physical changes are discussed for different active pharmaceutical ingredients belonging to different thera-peutic categories viz antiviral, anti-inflammatory, antidiabetic, antihypertensive, anti-convulsant, an-tibiotic, bronchodialator, antimalarial, antiemetic, antiamoebic, antipsychotic, antidepressant, anti-cancer, anticoagulant and sedative/hypnotic drugs and vitamins. Once the solid-state reactions of a pharmaceutical system are understood, the necessary steps can be taken to avoid reactivity and imp-rove the stability of drug substances and products.KEY WORDS: Incompatibility, interaction, active pharmaceutical ingredient, excipients, lactose, magnesium stearateINTRODUCTIONPreformulation is the first step in the rational formulation of an active pharmaceutical ingredient (API). It is an investigation of the physical-chemical properties of the drug subs-tance, alone and in combination withexcipients. Assessment of possible incom-patibilities between the drug and different excipients is an important part of pre-formulation. The formulation of a drug subs-tance frequently involves it being blended with different excipients to improve manufac-turability, and to maximize the product’s ability to administer the drug dose effectively.Excipients are known to facilitate administration and modulate release of the active component. They can also stabilize it against degradation from theenvironment.Corresponding author: Dr. Sonali S. Bharate, Assistant Professor, P.E.*Society’s Modern College of Pharmacy (For Ladies), Borhadewadi,At/Post- Moshi, Tal- Haweli, Dist- Pune, Maharashtra – 412105, India, Email: sonalibharate@Most excipients have no direct pharmacological action but they can impart useful properties to the formulation. However, they can also give rise to inadvertent and/or unintended effects such as increased degradation of the drug. Physical and chemical interactions between drugs and excipients can affect the chemical nature, the stability and bioavailability of drug products, and consequently, their therapeutic efficacy and safety (1).There have been several approaches proposed that satisfy the requirements of a drug-excipient chemical compatibility screen. The most resource sparing of these approaches is computational, where drug-excipient chemical compatibility can be predicted. This requires a comprehensive database of reactive functional groups for both drugs and excipients, com-bined with an in-depth knowledge of excipients and their potential impurities. Such an app-roach provides a rapid analysis and requires no bulk substance. However, there are inherent risks with using this computational approach as the sole source of information.Binary mixture compatibility testing is another commonly used method. In this approach, binary (1:1 or customized) mixtures of the drug and excipient, with or without, added water and sometimes compacted or prepared as slurries, are stored under stressed conditions (also known as isothermal stress testing (IST)) and analyzed using a stability-indicating method, e.g. high performance liquid chromatography (HPLC). The water slurry approach allows the pH of the drug-excipient blend and the role of moisture to be investigated. Alternatively, binary mixtures can be screened using other thermal methods, such as differential scanning calorimetry (DSC) (2, 3). DSC is currently the leading technique in this field (4). The main benefit of DSC, rather than stressed storage methods, is its ability to quickly screen potential excipients for incompatibilities derived from the appearance, shifts or disappearances of peaks and/or variations in the corresponding ÄH (enthalpy of transition) (5). Other features such as low sample consumption also makes it an attractive method. Although DSC is unquestionably a valuable technique, interpret-nation of the data may not be straightforward. In this method, the sample is exposed to high temperatures (up to 300°C or more), which in reality is not experienced by the dosage form. Thus, DSC results should be interpreted carefully, as the conclusions based on DSC results alone can be often misleading and in-conclusive. Therefore results obtained with DSC should always be confirmed with IST. IST involves storage of drug-excipient blends with, or without moisture, at elevated tem-pertures for a specific period of time (typically 3–4 weeks) to accelerate drug degradation and interaction with excipients. The samples are then visually observed for any changes in color or physical characteristics, and the drug content, along with any degradants, is deter-mined quantitatively (6). Although more useful, the disadvantage with this method is that it is time consuming and requires quantitative analysis using e.g. HPLC. Ideally, both tech-piques, DSC and IST should be used in combination for the selection of excipients. Similarly, isothermal micro calorimetry is a po-pular method for detecting changes in the solid state of drug-excipient mixtures through changes in heat flux (7). The results from using thermal techniques can be indicative of whet-her formulations are stable, but reveal no information concerning the cause or nature of any incompatibility. Techniques such as hot stage microscopy and scanning electron microscopy can be used in conjunction with DSC to determine the nature of an apparent incompatibility (8). These techniques study the morphology of the drug substance and can determine the nature of physical transformations, thus indicating the type of incompatibility that has occurred (4). A more definitive result would be obtained using e.g. HPLC or HPLC-MS/MS. It is well known that the chemical compatibility of an API in a binary mixture may differ from that of a multi-component prototype formulation. An alterna-tive is to test “prototype” formulations. The amount of API in the blend can be modifiedaccording to the anticipated drug-excipient ratio in the final compression blend (9). There are several examples of formulation instability resulting from solid–solid interac-tions (10, 11). Certain classes of compounds are known to be incompatible with particular excipients (12). Therefore knowledge of the chemistry of the drug substance and excipients can often minimize formulation surprises. Heat and water are the primary catalysts for drug-excipient interactions and play a critical role in the degradation of a drug substance (13). The majority of ‘small molecule’ API instability reactions occur via hydrolysis, oxidation and Maillard reaction. The moisture content of the drug and excipients plays a critical role in their incompatibility. Heat and moisture accelerate most reactions, even if moisture is not involved in the reaction scheme, since moisture brings molecules closer together, and heat always increases the reaction rate. The incompatibility between a drug and an excipient per se, and that which occurs between a drug and moisture/ water activity due to the ability of the excipient to absorb moisture, represents two different kinds of incompatibilities. Excipients such as starch and povidone may possess a high water content (the equilibrium moisture content of povidone is about 28% at 75% relative humi-dity), which can increase drug degradation. The moisture level will affect the stability depending on how strongly it is bound and whether it can come into contact with the drug (14). It is generally recognized that aspirin is incom-patible with magnesium salts. Higher moisture contents and humidity accelerate the degradation even further (14). Many different moisture mediated degradation mechanisms exist, but those mediated by surface moisture appear to be the most common (15). Consequently it is important that stress met-hods incorporate water to encourage the for-mation of all possible impurities. The way in which water facilitates degradation is not fully understood, but work carried out by Kontny et al. (15) has confirmed its importance. Degra-dation problems can, therefore, be difficult to avoid because water often cannot be entirely excluded from drug product formulations. Many excipients are hygroscopic, (16, 17) and absorb water during manufacturing, for example during wet granulation. Depending on the degree of hydrolytic susceptibility, different approaches to tablet granulation can be used to minimize hydrolysis. For compounds such as acetylsalicylic acid that are readily hydrolysable, direct compression or dry granulation is pre-ferable, to wet granulation. However drug-excipient incompatibility may still occur. Chemical interaction between the drug and excipients may lead to increased decom-position. Stearate salts (e.g. magnesium stearate, sodium stearate) should be avoided as tablet lubricants if the API is subject to hydrolysis via ion-catalyzed degradation. Excipients generally contain more free moisture than the drug substance (16), and in an attempt to obtain the most thermodynamically stable state, water is able to equilibrate between the various compo-nents (18). Formulation can, therefore, poten-tially expose the drug substance to higher levels of moisture than normal, which greatly increases the possibility of even stable com-pounds degrading. In selecting excipients, it is probably best to avoid hygroscopic excipients when formulating hydrolytically labile com-pounds, although examples exist where the drug is deliberately formulated with over-dried hygroscopic excipients (e.g. Starch 1500) which act as a moisture scavenger to prevent the drug from coming into contact with water.One of the most effective ways to stabilize a pH sensitive drug is through the adjustment of the microscopic pH of the formulation. Ex-cipients with high pH stability and buffering agents are recommended. Humidity is another major determinant of product stability in solid dosage forms. Elevation of relative humidity usually decreases the stability, particularly for drugs that are highly sensitive to hydrolysis. For example, nitrazepam in the solid state showed a linear relationship between the logarithm of the rate constant for the decomposition of the drug at different relative humidities (19). In addition, increased humidity can also accelerate thedegradation process by facilitating interaction with excipients (20, 21). Molecular mobility is also responsible for solid-solid reactions (22-24). Systems with enhanced mobility have more reactivity. Mechanical stress is also expected to accelerate such reactions by creating a larger surface area, increasing the number of defects, and increasing the amount of amorphous material (10).Oxidation reactions are complex and it can be difficult to understand the reaction mechanism. The best approach is to avoid excipients containing oxidative reactants such as peroxides and fumed metal oxides like fumed silica and fumed titanium dioxide. Excipients such as povidone and polyethylene glycols (PEGs) may contain organic peroxides as synthetic by-products which are typically more reactive than hydrogen peroxide. Although most compendial excipients have limits on metals, free ethylene oxide or other oxidizing agents via the peroxide or iodine value there are still some instances of oxidative degradation of drug due to presence of reactive peroxides in excipients such as povidone. For example, Hartauer et al.(2000) reported oxidative degradation of Raloxifene H.L. occurring via peroxide impurities in povidone (25). Thus, it is important to under-stand the purity and composition of the excipient prior to formulation.Aldehyde-amine addition is another important type of reaction, which is responsible for incompatibility between excipients comprising reducing sugars (e.g. lactose, dextrose) and amine-containing drugs. Aldehyde-amine addi-tion leads to the formation of a Schiff base, which further cyclizes to form a glycosamine followed by an Amadori rearrangement (26, 27). This sequence of reactions is called the Maillard reaction, and is responsible for a large number of incompatibilities between APIs and excipients.Monkhouse (28) listed common solid-state incompatibilities as shown in Table 1.Table 1 List of common solid-state incompatibilities Functional Group(Example of API)Incompatible with Type of Reaction ReferencePrimary amine (e.g.Acyclovir)Mono and disaccharides(e.g. lactose)Maillard reaction(26, 29)Esters (e.g.Moexipril)Basic components (e.g.magnesium hydroxide)Ester hydrolysis(30)Lactone (e.g.Irinotecan HCl)Basic components (e.g.magnesium hydroxide)Ring opening(hydrolysis)(31) Carboxyl Bases Salt formation(28, 32)Alcohol (e.g.Morphine)OxygenOxidation toaldehydes andketones(33, 34)Sulfhydryl (e.g.Captopril)Oxygen Dimerization(33, 34) Phenol Metals, polyplasdone Complexation(28, 32) Gelatin Cationic surfactants Denaturation(28, 32)This present review discusses incompatibilities of ‘small molecule’ API’s associated with different pharmaceutical excipients as sum-marized in Table 2. Incompatibilities of APIs with different pharmaceutical excipients are discussed below in subsequent sections relating to excipient types as follows: Saccharides, Stea-rates, Polyvinylpyrrolidone, Dicalcium phos-phate dihydrate, Eudragit polymers, Celluloses, Polyethylene glycol, Polysorbate 80, Sodium lauryl sulfate, Chitosan, Magnesium oxide, Sili-con dioxide, Carbonates, and Miscellaneous.SaccharidesLactose is perhaps one of the most commonly used excipients in pharmaceutical oral dosage forms (tablets) and, is in its crystalline form, generally considered to be least reactive. Blaug and Huang (99) reported that, crystalline lactose, rather than, amorphous lactose caused a problem in interaction of dextroamphetamine sulfate with spray dried lactose. Lactose is a reducing disaccharide and reactions between it and drugs containing amino groups have been reported. This interaction is shown in Figure 1. This sequence of such reaction is referred to as the Maillard reaction (26).Table 2 A list of excipients and their incompatibilities with API’s belonging to different therapeutic classesExcipient API and its Therapeutic ClassSaccharides(A)Lactose Acyclovir (29) - Antiviral; Aceclofenac (35) and Ketoprofen (36-38) - Anti-inflammatory; Metformin (29)-Antidiabetic; Amlodipine (39), Ceronapril (40), Lisinopril (41) and Oxprenolol (42) - Antihypertensive; Fluconazole (43) - Antifungal; Primaquine (44) - Antimalarial; Promethazine (45) - Antiemetic; Fluoxetine (46) and Seproxetine Maleate (47) – Antidepressant; Picotamide (48) – Anticoagulant; Etamsylate (43) - Antihemorrhagic; Aminophylline (49) and Clenbuterol (50) – Bronchodialator; Baclofen (51)- CNS Drug; Ranitidine (52) – GI Agent; Doxylamine (53)– Antihistaminic; Thiaminechloride HCL (54) – Vitamin; Pefloxacin (55) - Antibiotic(B)Lactose/meglumine/ Tris Buffer Blend Glipizide (56) - Antidiabetic(C)Mannitol, Pearlitol (80% Mannitol +20% Maize Starch)Quinapril (9)- Antihypertensive; Primaquine (44)- Antimalarial; R-omeprazole (57) – GI Agent; Promethazine (45) –Antiemetic(D)Starch Seproxetine Maleate (47) – Antidepressant; Clenbuterol (50) - Bronchodialator(E)Sodium Starch Glicollate Clenbuterol (50) - Bronchodialator(F)Dextrose Pefloxacin (55) - AntibioticStearates(A)Magnesium Stearate Acyclovir (58) – Antiviral; Aspirin (59-62), Ibuproxam (8), Indomethacin (63, 64) and Ketoprofen (36-38)- Anti-inflammatory; Glipizide (56), Chlorpropamide (65), Glimepiride (66) and Glibenclamide (67) - Antidiabetic; Captopril (68, 69), Fosinopril (40), Moexipril (10, 30, 70), Oxprenolol (42) and Quinapril (9) - Antihypertensive; Cephalexin (71), Erythromycin (72), Nalidixic Acid (73), Oxacillin (74) and Penicillin G (74) - Antibiotic; Primaquine (44)- Antimalarial; Promethazine (45)- Antiemetic; Albendazole (75) - Antiamoebic; â-lapachone (76)- Anticancer; Clopidogrel (77)- Anticoagulant; Doxylamine (53) – Antihistaminic; Temazepam (42) - Hypnotic(B)Stearic Acid Doxylamine (53)- AntihistaminicPolyvinyl Pyrolidone (PVP)Indomethacin (63, 64), Ibuproxam (8) and Ketoprofen (36-38) – Anti-inflammatory; Atenolol (63) and Oxprenolol (42)- Antihypertensive; Sulfathiazole (78) – Antibiotic; Haloperidol (79)- Antipsychotic; Ranitidine (52)- GI Agent; Doxylamine (53)- Antihistaminic; Temazepam (42)- Hypnotic; Clenbuterol (50) – BronchodialatorDicalcium Phosphate Dihydrate (DCPD)Ceronapril (40), Oxprenolol (42) and Quinapril (9)- Antihypertensive; Metronidazole (80) – Antiamoebic; â-lapachone (76) and Parthenolide (81)- Anticancer; Famotidine (82)- GI Agent; Temazepam (42) - HypnoticEudragit Polym ers(A)Eudragit RS and Rl Diflunisal (83, 84), Flurbiprofen (83, 84) and Piroxicam (83, 84) – Anti-inflammatory(B)Eudragit RL100Ibuprofen (85) (86)– Anti-inflammatory(C)Eudragit E100Ranitidine (52) – GI AgentCelluloses(A)Microcrystalline Cellulose (MCC),Avicel PH 101Enalapril (87, 88) – Antihypertensive; Isosorbide Mononitrate (6) – Antiangina; Clenbuterol (50) - Bronchodialator(B)Cellulose Acetate Isosorbide Mononitrate (6)- Antiangina(C)Hypromellose Acetate Succinate(HPMCAS)Dyphylline (89) - Bronchodialator(D)Hydroxypropyl Cellulose Trichlormethiazide (90) - DiureticPEG Ibuprofen (91), Ibuproxam (8) and Ketoprofen (36-38)– Anti-inflammatory; Phosphomycin (92) - Antibiotic; Clopidogrel (77)- AnticoagulantPolysorbate 80Ibuprofen (91) – Anti-inflammatorySodium Lauryl Sulfate Chlorpropamide (65) – Antidiabetic; Clopidogrel (77)– Anticoagulant; Chlordiazepoxide (11, 93) – Hypnotic Chitosan Diclofenac (11, 94) and Piroxicam (95)– Anti-inflammatoryM agnesium O xide Ibuprofen (96) – Anti-inflammatorySilicon Dioxide Enalapril (87, 88) - AntihypertensiveCarbonates(A)Sodium Carbonate Adefovir Dipivoxil (97) - Antiviral(B)Sodium Bicarbonate Ibuprofen (96) – Anti-inflammatoryM iscellaneous(A) Plasdone Glimepiride (66) - antidiabetic(B) Ascorbic acid Atenolol (63) – antihypertensive(C) Citric acid Atenolol (63)- antihypertensive(D) Butylated hydroxyanisole Atenolol (63)- antihypertensive(E) Succinic acid Phosphomycin (92)- antibiotic(F) Na dioctylsulfocuccinate Phosphomycin (92) - antibiotic(G) Ca and Mg salts Tetracyclins (98) - Antibiotic(H) Talc Seproxetine maleate (47) - antidepressant(i). Precirol ATO 5Temazepam (42)- hypnoticThere are a number of reports of the incom-patibility of lactose with amine-containing APIs. DSC and FT-IR studies showed that aminophylline, a bronchodilator drug consisting of a purine ring system coupled with an ethylenediamine moiety was incompatible with lactose. Brown discoloration appeared in samples containing 1:5 (w/w) mixtures of aminophylline and lactose following three weeks of storage at 60°C (100). Another group of researchers also reported similar findings from stability experiments using aminophylline combined with four tablet excipients (cellulose starch, glucose (dextrose) and lactose) stored at 5°C, room temperature (27 ± 3°C), 45°C and in direct sunlight for 30 days. Degradation of the drug increased with increase in temperature and on exposure to sunlight. The degradation was particularly intense in the presence of glucose and lactose and a change in color from white to yellow occurred in such mixtures. At 45°C and in sunlight both pure drug and its mixtures changed color during the storage period (49).Seproxetine, a selective serotonin re-uptake inhibitor, is a primary amine and an active metabolite of fluoxetine. Its maleate hemi-hydrate salt demonstrated an incompatibility with lactose and starch in a gelatin capsule dosage form. Analogous to a Micheal addition,there is a formation of 1-4 addition product between the drug and the maleic acid facilitated by the free moisture associated with the starch.Formation of the 1,4-addition product was noticed in the presence of pregelatinized starch while a Maillard reaction product was formedby interaction of the drug with lactose as shown in Figure 1 (47). The schematic of the interac-tion between saproxetine and maleic acid in the presence of starch is shown in Figure 2. An ACE inhibitor, ceronapril containing a primary amine function, also exhibited incompatibility with lactose due to a Maillard reaction. HPLC studies of mixtures of ceronapril with lactose after 3 weeks storage at 50ºC showed that the ceronapril had degraded by 8.4% (40).Amlodipine is a dihydropyridine calcium chan-nel blocker containing a primary amine group.It was found to be unstable in a multi-component mixture with lactose, magnesium stearate and water. Amlodipine besylate glyco-syl was identified as a major reason for degradation due to a Maillard reaction between the drug and lactose (39). Metformin is a bigua-nide class of antidiabetic drug also containing a primary amine group. Binary mixtures of met-formin with starch and lactose showed interac-tion upon heating, changing the melting temperature of metformin and the accom-panying enthalpy. A change in the melting temperature of metformin was observed for all mixtures tested i.e. metformin: starch ratios of 2:8; 3:7; 1:1; 7:3 and 8:2. The melting peak for metformin was not visible in the presence of lactose suggesting an interaction between them.Displacement of the melting temperature of metformin, for all the metformin–starch mix-tures tested, confirmed the interaction between the two materials. One possible reason for thisinteraction can be a Maillard reaction betweenFigure 1 A general Maillard reaction between lactoseand an amine group-containing APIFigure 2 Interaction between Seproxetine and maleic acid in the presence of starch (47).the lactose carbonyl that can react with an amine group of metformin (101). Acyclovir is a purine class of antiviral drug, again, containing a primary amine group. Results of DSC studies of physical mixtures of the drug with lactose suggested an incompatibility, confirmed by li-quid chromatography coupled with mass spect-rometry (LC-MS) which identified the for-mation of a Maillard reaction as shown in Figure 1 (29).Baclofen is a primary amine used in treatment of spastic movement disorders. Using LC-MS studies showed that it was incompatible with lactose as indicated by the formation of a Mail-lard reaction product. Fourier transform infra-red spectroscopic analysis (FTIR) showed the formation of the imine bond, which indicated that baclofen, undergoes a Maillard-type reac-tion with lactose (51).Lactose monohydrate also interacted with moisture sensitive drugs and affected the stabi-lity of the drug (102). A mixture of thiamine hydrochloride and lactose underwent the Mail-lard reaction forming the N-glycosylamine, which was further converted to the amino ketone via an Amadori rearrangement. The configuration of an á-(spray-dried) lactose and â-anhydrous lactose was not relevant for the Maillard reaction because the reaction of lactose is believed to occur through an open-chain form. Spray dried lactose also contains amorphous lactose which is important for reactivity (99). Similar changes in spectra were noticed for binary mixtures of both forms of lactose with thiamine hydrochloride. A change of tablet color due to the degradation of drug under accelerated conditions was observed (54). Although primary amines are the most commonly reported substrates for the Maillard reaction, there are also reports of Maillard reactions with secondary amines. Oxprenolol hydrochloride (42), fluoxetine hydrochloride (46), etamsylate (43), ranitidine (52) and aceclo-fenac (35) are examples.Etamsylate is 2,5-di-hydroxybenzene sulfonic acid containing an N-ethylethanamine group. The DSC thermograms of the mixtures of etamsylate and lactose sho-wed additional peaks, in addition to those recorded for the individual components. In the case of the etamsylate–lactose mixture an additional prominent endotherm appeared at 118.5°C. The main peak at 136.7°C in the original etamsylate thermogram shifted to a lower temperature of 131.4°C. This was pro-bably due to solid solubility of lactose in etamsylate (43).Ranitidine HCl is a drug used for the treatment of gastric ulcer. The drug is a secondary amine. An incompatibility was reported in a 1:1 binary mixture of the drug and lactose. The DSC thermogram of lactose monohydrate showed a dehydration peak at 150°C followed by an endothermic melting peak at 222°C. However, the thermogram of the binary mixture showed a broader peak at 150°C and the disappearance of the melting peak of lactose at 222°C, indicating a possible interaction between ranitidine and lactose (52). Aceclofenac is a Non-Steroidal Anti-inflammatory drug (NSAID) with a secondary amine group. It showed an incompatibility with spray-dried lactose (SDL). In the binary mixture of aceclofenac and SDL (7:3), an IR peak at 1848.6 cm using pure aceclofenac was-1observed. This peak disap-peared and another peak at 1771.5 cm shifted to 1762.8 cm. In-1-1 the mixture of aceclofenac and SDL at a ratio of 6:4, peaks at 1848.6 and 1771.5 cm-1 disappeared completely using pure aceclofenac.A further increase in the SDL concentration gave similar results (35).There are also some reports of lactose interaction with drugs not having a primary or secondary amine group such as Fluconazole and Pefloxacin. The mixture of fluconazole and lactose showed additional peaks by DSC at 86.1°C and 136.4°C respectively, in addition to the peak corresponding to the melting of pure drug at 140.2°C. This indicates an interaction between the drug and the excipient (43).The antibacterial drug pefloxacin mesylate also does not contain a primary or secondary aminegroup but is reported to interact with anhydrous dextrose, Pearlitol® (mannitol), Lactopress SD (directly compressible lactose), Lactochem FP [Natural L (+) Lactic Acid], and Lycatab® (starch/calcium carbonate), as indica-ted by the loss of the characteristic melting endothermic peak of perfloxacin mesylate in the DSC thermogram. The enthalpy changes in admixtures were within a 10% limit of the calculated enthalpy.However, the results of isothermal stability studies did not support the conclusions from the DSC results (55). Since elevated temperatures in DSC study are not necessarily relevant to ambient conditions, isothermal stress results are more reliable. Further, additional stability studies of prototype formulation at accelerated conditions can be carried out to discover any potential incom-patibility. Another tertiary amine drug, prome-thazine hydrochloride used as an antiemetic, antihistamine and sedative, resulted in a brown color in stressed binary mixture studies (stored at 55°C for 3 weeks) indicating a potential in-teraction with lactose monohydrate (45). Meglumine is an amino sugar derived from sorbitol. The incompatibility of the antidiabetic drug glipizide with the blend of meglumine/ TRIS buffer has been reported. An interaction was observed between meglumine and glipizide but the mechanism has not been established. A yellow coloration caused by the interaction of the amine group of meglumine/TRIS buffer and lactose was observed. Because the solubility modifier (meglumine/TRIS buffer) was requi-red to increase the solubility of glipizide in the formulation, the presence of lactose in the for-mulation was considered detrimental to long-term stability. In addition, a melting endo-thermic peak of glipizide was found to be mis-sing in a glipizide–lactose mixture, which suggested an interaction between the two com-ponents. A sharp endothermic peak at 130.32°C was observed in the DSC thermo-gram of meglumine, which broadened and shifted to lower temperature (122.81°C) for a glipizide and meglumine mixture. The drug peak was completely absent in the DSC trace of the mixture, which suggested a possible interac-tion.The DSC trace of TRIS buffer showed two endothermic peaks at 139.14ºC (probably due to evaporation of adsorbed moisture) and 172.73°C (melting point of TRIS buffer), followed by an endotherm at 300°C, probably due to the decomposition of TRIS buffer. In the thermogram of glipizide and TRIS buffer mixture, the TRIS buffer peak at 139.14°C shifted to a lower temperature (135.60°C). In addition, the drug peak was missing and a broad endothermic peak was observed at 296.16°C (56). The DSC results were indicative of an interaction between glipizide and TRIS buffer. However, the IR spectrum of glipizide–TRIS buffer mixture showed the presence of characteristic bands corresponding to glipizide. There was no appearance of new bands. Hence, there was strong evidence of unchanged drug structure and lack of chemical interaction bet-ween the two. Thus, it was concluded that there was no chemical incompatibility between glipi-zide and TRIS buffer.The results confirmed that DSC could be used as a rapid method to evaluate the compatibility between a drug and excipients. However, caution needs to be exercised while interpreting the DSC results alone. Wherever possible, results from other techniques such as IR and quantitative analysis after storage under stressed conditions, should be taken in conjunction with DSC results to reach any definite conclusion. In the present example, the results from the DSC along with IR and/or HPLC were successfully employed to assess the compatibility of glipizi-de with the excipients used in the development of extended release formulations. Based on the results of IR and/or HPLC analysis, any pos-sible pharmaceutical incompatibility between glipizide and TRIS buffer and magnesium stearate was ruled out. Other APIs which show possible incompatibilities in binary mixtures with lactose include ketoprofen (36, 37), picotamide (48), primaquine (44), clenbuterol (50), sennosides A and B (103) and lisinopril (41).。
