美国药典USP40-左氧氟沙星API
美国药典USP40-左氧片

4834Levofloxacin / Official MonographsUSP 40Acceptance criteriaASSAYIndividual impurities: See Impurity Table 1.•P ROCEDUREDiluent: Acetonitrile and water (20:80)Mobile phase: Transfer 874mg of cupric sulfate,Impurity Table 1918mg of L -isoleucine, and 5.94g of ammonium ace-Relative Relative Acceptance tate to a suitable container. Add 700mL of water, and Retention Response Criteria,mix until dissolved. Add 300mL of methanol.NameTimeFactorNMT (%)Standard stock solution: 2mg/mL of USP Levofloxacin 9-Desfluoro RS in Diluent—*levofloxacin a 0.64 1.0Standard solution: 0.2mg/mL of USP Levofloxacin RS in Mobile phase from the Standard stock solutionDiamine —*Sample stock solution: Nominally 5mg/mL of levoflox-derivative b 0.751.0acin prepared as follows. Transfer intact Tablets (NLT 5)Levofloxacin to a volumetric flask, add 75% of the final volume of relatedDiluent , and allow to stand for 15 min. Shake for 30compound A c 0.910.810.5min, and dilute with Diluent to volume. Pass a portion Levofloxacin 1.0——of the solution through a suitable filter of 0.45-µm pore Levofloxacin size, discarding the first 1–2mL of the filtrate.N -oxide d1.550.930.5Sample solution: Nominally 0.2mg/mL of levofloxacin Any other individual in Mobile phase from the Sample stock solution —impurity1.00.2Chromatographic systemTotal impurities——1.0(See Chromatography 〈621〉, System Suitability .)Mode: LCa(S )-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.Detector: UV 360 nmb (S )-9-Fluoro-2,3-dihydro-3-methyl-10-[2-(methylamino)ethylamino]-7-Column: 4.6-mm × 25-cm; 5-µm packing L1oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.Column temperature: 45°c (S )-9-Fluoro-2,3-dihydro-3-methyl-10-(piperazin-1-yl)-7-oxo-7H -pyrido[1,Flow rate: 0.8mL/min 2,3-de ][1,4]benzoxazine-6-carbocylic acid.Injection volume: 25µLd (S )-4-(6-Carboxy-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H -pyrido-[1,2,3-Run time: 2 times the retention time of levofloxacin de ][1,4]benzoxazine-10-yl)-1-methylpiperazine 1-oxide.System suitability*Disregard this peak because this is a process impurity controlled for the Sample: Standard solution drug substance.Suitability requirements SPECIFIC TESTSTailing factor: NMT 1.8•M ICROBIAL E NUMERATION T ESTS 〈61〉 and T ESTS F OR S PECI-Relative standard deviation: NMT 2.0%FIED M ICROORGANISMS 〈62〉: The total aerobic microbial Analysiscount does not exceed 102 cfu/mL, and the total com-Samples: Standard solution and Sample solution bined molds and yeast count does not exceed 101 cfu/Calculate the percentage of the labeled amount of mL. It also meets the requirement for absence of Escher-levofloxacin (C 18H 20FN 3O 4) in the portion of Tablets ichia coli .taken:•D ELIVERABLE V OLUME 〈698〉: Meets the requirements •P H 〈791〉: 5.0–6.0Result = (r U /r S ) × (C S /C U ) × 100ADDITIONAL REQUIREMENTSr U= peak response of levofloxacin from the Sample•P ACKAGING AND S TORAGE : Store at controlled room tem-solutionperature, and protect from light.r S = peak response of levofloxacin from the•USP R EFERENCE S TANDARDS 〈11〉Standard solutionUSP Levofloxacin RSC S = concentration of USP Levofloxacin RS in the7H -Pyrido[1,2,3-de ]-1,4-benzoxazine-6-carboxylic acid,Standard solution (mg/mL)9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piper-C U = nominal concentration of levofloxacin in theazinyl)-7-oxo-hydrate (2:1), (S )-;Sample solution (mg/mL)(−)-(S )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-Acceptance criteria: 90.0%–110.0%1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ]-1,4-benzox-azine-6-carboxylic acid, hemihydrate.PERFORMANCE TESTS Anhydrous.•D ISSOLUTION 〈711〉C 18H 20FN 3O 4·1/2H 2O 370.38Test 1USP Levofloxacin Related Compound A RSMedium: 0.1 N hydrochloric acid; 900mL (S )-9-Fluoro-2,3-dihydro-3-methyl-10-(piperazin-1-yl)-Apparatus 2: 75 rpm 7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carbo-Time: 30 mincylic acid.Standard solution: 0.56mg/mL of USP Levofloxacin C 17H 18FN 3O 4347.34RS in MediumSample solution: Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size.Instrumental conditions(See Ultraviolet-Visible Spectroscopy 〈857〉.)Mode: UVLevofloxacin TabletsAnalytical wavelength: 294 nm Cell length: 0.1mm DEFINITIONBlank: Medium Levofloxacin Tablets contain NLT 90.0% and NMT 110.0%Analysisof the labeled amount of levofloxacin (C 18H 20FN 3O 4).Samples: Standard solution and Sample solutionIDENTIFICATIONCalculate the percentage (Q ) of the labeled amount of •A . The retention time of the major peak of the Sample levofloxacin (C 18H 20FN 3O 4) dissolved:solution corresponds to that of the Standard solution , as Result = (A U /A S ) × C S × V × D × (1/L ) × 100obtained in the Assay .USP 40Official Monographs / Levofloxacin 4835A U = absorbance of the Sample solution Blank: Medium A S = absorbance of the Standard solution AnalysisC S= concentration of the Standard solutionSamples: Standard solution and Sample solution(mg/mL)Calculate the percentage (Q ) of the labeled amount of V = volume of Medium , 900mL levofloxacin (C 18H 20FN 3O 4) dissolved:D = dilution factor of the Sample solution Result = (A U /A S ) × C S × V × D × (1/L ) × 100L = label claim (mg/Tablet)Tolerances: NLT 80% (Q ) of the labeled amount of A U = absorbance of the Sample solution levofloxacin (C 18H 20FN 3O 4) is dissolved.A S = absorbance of the Standard solution Test 2C S= concentration of the Standard solutionMedium: 0.1 N hydrochloric acid; 900mL (mg/mL)Apparatus 1: 100 rpm V = volume of Medium , 900mL Time: 30 minD = dilution factor of the Sample solution Standard solution: L /900mg/mL of USP Levofloxacin L = label claim (mg/Tablet)RS in Medium , and mix to obtain solutions with known Tolerances: NLT 80% (Q ) of the labeled amount of concentrations as indicated in Table 1, where L is the levofloxacin (C 18H 20FN 3O 4) is bel claim in mg/Tablet.Test 4Sample solution: Pass a portion of the solution under Medium: 0.1 N hydrochloric acid; 900mL test having a concentration similar to that of the Stan-Apparatus 1: 100 rpm dard solution through a suitable filter of 0.45-µm pore Time: 30 minsize.Standard solution: 16µg/mL of USP Levofloxacin RS in MediumTable 1Sample solution: Pass a portion of the solution under test having the same concentration as that of the Stan-Tablet Label ClaimFinal Concentrationdard soluiton through a suitable filter of 0.45-µm pore (mg)(mg/mL)size.2500.27Instrumental conditions5000.55(See Ultraviolet-Visible Spectroscopy 〈857〉.)7500.83Mode: UVAnalytical wavelength: 332 nm Instrumental conditionsCell length: 1cm (See Ultraviolet-Visible Spectroscopy 〈857〉.)Blank: Medium Mode: UVAnalysisAnalytical wavelength: 293 nm Samples: Standard solution and Sample solutionCell length: 0.1mm Calculate the percentage (Q ) of the labeled amount of Blank: Medium levofloxacin (C 18H 20FN 3O 4) dissolved:AnalysisSamples: Standard solution and Sample solutionResult = (A U /A S ) × C S × V × D × (1/L ) × 100Calculate the percentage (Q ) of the labeled amount of levofloxacin (C 18H 20FN 3O 4) dissolved:A U = absorbance of the Sample solution A S = absorbance of the Standard solution Result = (A U /A S ) × C S × V × D × (1/L ) × 100C S= concentration of the Standard solution(mg/mL)A U = absorbance of the Sample solution V = volume of Medium , 900mL A S = absorbance of the Standard solution D = dilution factor of the Sample solution C S= concentration of the Standard solutionL = label claim (mg/Tablet)(mg/mL)Tolerances: NLT 80% (Q ) of the labeled amount of V = volume of Medium , 900mL levofloxacin (C 18H 20FN 3O 4) is dissolved.D = dilution factor of the Sample solution •U NIFORMITY OF D OSAGE U NITS 〈905〉: Meet the L = label claim (mg/Tablet)requirements Tolerances: NLT 80% (Q ) of the labeled amount of levofloxacin (C 18H 20FN 3O 4) is dissolved.IMPURITIESTest 3•O RGANIC I MPURITIESMedium: 0.1 N hydrochloric acid; 900mL Diluent, Mobile phase, Standard stock solution, Sam-Apparatus 1: 100 rpm ple solution, and Chromatographic system: Proceed Time: 30 minas directed in the Assay .Standard solution: L /900mg/mL of USP Levofloxacin Standard solution: 0.2mg/mL of USP Levofloxacin RS RS in Medium , and mix to obtain solutions with known from the Standard stock solution and 1µg/mL of USP concentrations as indicated in Table 1, where L is the Levofloxacin Related Compound A RS in Mobile phase label claim in mg/Tablet.System suitabilitySample solution: Pass a portion of the solution under Sample: Standard solution test having the same concentration as that of the Stan-Suitability requirementsdard solution through a suitable filter of 0.45-µm pore Tailing factor: NMT 1.8 for the levofloxacin peak size.Relative standard deviation: NMT 2.0% for the Instrumental conditionslevofloxacin peak (See Ultraviolet-Visible Spectroscopy 〈857〉.)AnalysisMode: UVSamples: Standard solution and Sample solutionAnalytical wavelength: 326 nmCalculate the percentage of levofloxacin related com-Cell length: 1mm for a 250-mg Tablet, 0.5mm for a pound A in the portion of Tablets taken:500-mg Tablet, and 0.2mm for a 750-mg TabletResult = (r U /r S ) × (C S /C U ) × 100r U= peak response of levofloxacin relatedcompound A from the Sample solution4836Levofloxacin / Official MonographsUSP 40r S= peak response of levofloxacin related•USP R EFERENCE S TANDARDS 〈11〉compound A from the Standard solutionUSP Levofloxacin RSC S = concentration of USP Levofloxacin RelatedUSP Levofloxacin Related Compound A RSCompound A RS in the Standard solution (S )-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-(mg/mL)dihydro-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carbox-C U = nominal concentration of levofloxacin in theylic acid.Sample solution (mg/mL)C 17H 18FN 3O 4347.34Calculate the percentage of any other impurities in the portion of Tablets taken:Result = (r U /r S ) × (C S /C U ) × (1/F ) × 100Levonordefrinr U= peak response of any impurity from theSample solutionr S = peak response of levofloxacin from theStandard solutionC S= concentration of USP Levofloxacin RS in theStandard solution (mg/mL)C U = nominal concentration of levofloxacin in theC 9H 13NO 3183.20Sample solution (mg/mL)1,2-Benzenediol, 4-(2-amino-1-hydroxypropyl)-, [R -(R *,S *)]-.F = relative response factor (see Table 2)(-)-α-(1-Aminoethyl)-3,4-dihydroxybenzyl alcohol Acceptance criteria: See Table 2.[18829-78-2; 829-74-3].Table 2» Levonordefrin, dried in vacuum at 60° for15hours, contains not less than 98.0percent and Relative Relative Acceptance Retention Response Criteria,not more than 102.0percent of C 9H 13NO 3.NameTimeFactor NMT (%)Packaging and storage—Preserve in well-closed contain-Decarboxy ers.levofloxacin a0.380.600.3USP Reference standards 〈11〉—Levofloxacin related —USP Levonordefrin RS compound A b0.470.7Identification—Diamine derivative c 0.520.830.3A: Infrared Absorption 〈197K 〉.Levofloxacin N -oxide d 0.630.680.7B: Ultraviolet Absorption 〈197U 〉—9-Desfluoro Solution: 25µg per mL.——f levofloxacin e 0.73Medium: 0.1 N hydrochloric acid.Levofloxacin 1.00——Specific rotation 〈781S 〉: between −28° and −31°.Dextrofloxacin g 1.23——f Test solution: 50mg, previously dried, per mL, in 0.3 N Levofloxacin hydrochloric acid.9-piperazino ——fLoss on drying 〈731〉—Dry it in vacuum at 60° for isomer h1.6915hours: it loses not more than 1.0% of its weight.Any unspecified —Residue on ignition 〈281〉: not more than 0.2%.impurity1.00.2Chromatographic purity—Total impurities——1Standard solutions—Dissolve an accurately weighed quan-a (S )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine.tity of USP Levonordefrin RS in a mixture of methanol and b (S )-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-dihydro-7H -pyrido[1,glacial acetic acid (96:4) to obtain a Standard stock solution 2,3-de ][1,4]benzoxazine-6-carboxylic acid.having a known concentration of 5mg per mL. Dilute this c (S )-9-Fluoro-2,3-dihydro-3-methyl-10-[2-(methylamino)ethylamino]-7-solution quantitatively with a mixture of methanol and gla-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.cial acetic acid (96:4) to obtain Standard solutions , desig-d (S )-4-(6-Carboxy-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H -pyrido-[1,2,3-nated below by letter, having the following compositions:de ][1,4]benzoxazine-10-yl)-1-methylpiperazine 1-oxide.e (S )-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.Concentra-Percentage (%,f Process impurity, for information only.tion for comparison g (R )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -Standard (µg RS perwith test speci-pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.solutionDilution mL)men)h (S )-10-fluoro-3-methyl-9-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-7H -A (1 in 10)500 1.0pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.B (1 in 20)2500.5ADDITIONAL REQUIREMENTSC (1 in 50)1000.2•P ACKAGING AND S TORAGE : Preserve in tight containers.D(1 in 100)500.1Store at controlled room temperature.•L ABELING : When more than one Dissolution test is given,the labeling states the Dissolution test used only if Test 1Test solution—Dissolve an accurately weighed quantity of is not used.Levonordefrin in a mixture of methanol and glacial acetic acid (96:4) to obtain a solution containing 50mg per mL.Procedure— Apply separately 5µL of the Test solution and 5µL of each Standard solution to a suitable thin-layer chro-matographic plate (see Chromatography 〈621〉) coated with 0.25-mm layer of chromatographic silica gel mixture. Posi-tion the plate in a chromatographic chamber, and develop the chromatograms in a solvent system consisting of a mix-ture of n -butyl alcohol, water, and glacial acetic acid。
左氧氟沙星杂质
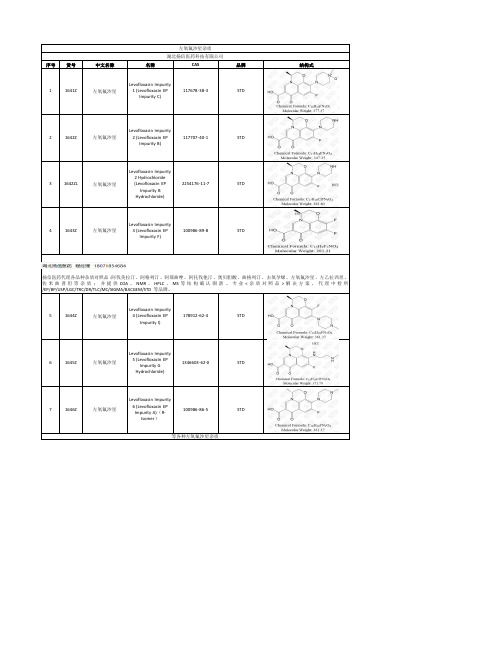
左氧氟沙星杂质湖北扬信医药科技有限公司序号货号中文名称名称CAS品牌结构式11641Z左氧氟沙星Levofloxacin Impurity1 (Levofloxacin EPImpurity C)117678-38-3STD21642Z左氧氟沙星Levofloxacin Impurity2 (Levofloxacin EPImpurity B)117707-40-1STD31642ZL左氧氟沙星Levofloxacin Impurity2 Hydrochloride(Levofloxacin EPImpurity BHydrochloride)2254176-11-7STD41643Z左氧氟沙星Levofloxacin Impurity3 (Levofloxacin EPImpurity F)100986-89-8STD扬信医药代理各品种杂质对照品:阿伐美拉汀、阿格列汀、阿那曲唑、阿托伐他汀、奥贝胆酸、曲格列汀、去氧孕烯、左氧氟沙星、左乙拉西坦、佐米曲普坦等杂质;并提供COA、NMR、HPLC、MS等结构确认图谱。
专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACGEM/STD等品牌。
51644Z左氧氟沙星Levofloxacin Impurity4 (Levofloxacin EPImpurity I)178912-62-4STD61645Z左氧氟沙星Levofloxacin Impurity5 (Levofloxacin EPImpurity GHydrochloride)1346603-62-0STD71646Z左氧氟沙星Levofloxacin Impurity6 (Levofloxacin EPImpurity A)(R-Isomer)100986-86-5STD等各种左氧氟沙星杂质。
美国药典USP40-左氧氟沙星API

美国药典USP40-左氧氟沙星APIUSP 40Official Monographs / Levofloxacin 4831Acceptance criteria: See Table 1.Sample solution: 1mg/mL of Levofloxacin in Mobile phase Chromatographic systemTable 1(See Chromatography ?621?, System Suitability .)Relative Relative Acceptance Mode: LCRetention Response Criteria,Detector: UV 360 nmNameTimeFactor NMT (%)Column: 4.6-mm × 25-cm; 5-μm packing L1Levodopa related Column temperature: 45°compound A 0.90.830.1Flow rate:0.8mL/min Injection size: 25μL Levodopa1.0——System suitabilityLevodopa related Sample: Standard solution compound B 2.80.830.5Suitability requirements 5,6-Dihydroxy-in-Tailing factor: 0.5–1.5dole-2-carboxylic Relative standard deviation: NMT 1.0%acid6.0 2.50.1Analysis0.1Samples: Standard solution and Sample solutionindividual Calculate the percentage of levofloxacin (C 18H 20FN 3O 4)—0.3 total in the portion of Levofloxacin taken:Unknown impurities 1.0unknown Total impurities——1.1Result = (r U /r S ) × (C S /C U ) × 100r U= peak response of levofloxacin from the SampleADDITIONAL REQUIREMENTSsolutionP ACKAGING AND S TORAGE : Preserve in tight, light-resistant r S = peak response of levofloxacin from the containers, in a dry place, and prevent exposure to ex-Standard solutioncessive heat.C S = concentration of USP Levofloxacin RS in theUSP R EFERENCE S TANDARDS ?11?Standard solution (mg/mL)USP Levodopa RSC U = concentration of Levofloxacin in the SampleUSP Levodopa Related Compound A RS solution (mg/mL)3-(3,4,6-Trihydroxyphenyl)alanine.Acceptance criteria: 98.0%–102.0% on the anhydrous C 9H 11NO 5213.19basisUSP Levodopa Related Compound B RS 3-Methoxytyrosine.IMPURITIESC 10H 13NO 4211.22R ESIDUE ON I GNITION ?281?: NMT 0.2%. Use a platinum crucible.Delete the following:LevofloxacinH EAVY M ETALS , Method II ?231?: NMT 10ppm ?(Official 1-Jan-2018)O RGANIC I MPURITIES , P ROCEDURE 1[N OTE —Procedure 1 is recommended if levofloxacin N -ox-ide is a potential organic impurity. Procedure 2 and Pro-cedure 3 are recommended if levofloxacin related com-pound B is a potential organic impurity.]Solution A, Mobile phase, Sample solution, and Chro-matographic system: Proceed as directed in the C 18H 20FN 3O 4·1/2H 2O 370.38Assay .7H -Pyrido[1,2,3-de ]-1,4-benzoxazine-6-carboxylic acid,System suitability solution: 1mg/mL of USP Levoflox-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-acin RS in Mobile phase7-oxo-hydrate (2:1), (S )-;Sensitivity solution: 0.3μg/mL of USP Levofloxacin RS (?)-(S )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piper-in Mobile phase azinyl)-7-oxo-7H -pyrido[1,2,3-de ]-1,4-benzoxazine-6-car-System suitabilityboxylic acid, hemihydrate [138199-71-0].Samples: System suitability solution and Sensitivity Anhydrous [100986-85-41].solutionSuitability requirementsDEFINITIONRelative standard deviation: NMT 1.0%, System suit-Levofloxacin contains NLT 98.0% and NMT 102.0% of ability solutionC 18H 20FN 3O 4, calculated on the anhydrous basis.Signal-to-noise ratio: NLT 10, Sensitivity solution IDENTIFICATION AnalysisA . I NFRARED A BSORPTION ?197K ?Sample: Sample solutionB . The retention time of the major peak of the Sample Calculate the percentage of each individual impurity in solution corresponds to that of the Standard solution , as the portion of Levofloxacin taken:obtained in the Assay.Result = (r U /r S ) × (1/F ) × 100ASSAYP ROCEDUREr U = peak response of each impurity Buffer: 8.5g/L of ammonium acetate, 1.25g/L of cu-r S = peak response of levofloxacin pric sulfate, pentahydrate, and 1.3g/L of L -isoleucine in F = relative response factor (see Table 1)waterAcceptance criteria: See Table 1.Mobile phase: Methanol and Buffer (3:7)Standard solution: 1mg/mL of USP Levofloxacin RS in Mobile phaseUSP Monographs4832Levofloxacin / Official MonographsUSP 40Table 1in methanol from Levofloxacin related compound B stock solutionRelative Relative Acceptance Standard solution: 0.4μg/mL of levofloxacin andRetention Response Criteria,0.8μg/mL of levofloxacin relatedcompound B in aceto-NameTimeFactorNMT (%)nitrile and water (1:10) from Levofloxacin standard solu-N -Desmethyl tion and Levofloxacin related compound B standard levofloxacin a0.47 1.00.3solutionDiamine derivative b 0.520.90.3Sample solution: 0.4mg/mL by dissolving the sample Levofloxacin in acetonitrile at about 8% of final volume and diluting N -oxide c0.63 1.10.3with water to volume. [N OTE —Sonicate if necessary.]Chromatographic system9-Desfluoro levoflox-(See Chromatography ?621?, System Suitability .)acin d0.73 1.00.3Mode: LCLevofloxacin 1.0——Detector: 280 nmD -Isomer e1.23 1.00.8Column: 4.0-mm × 15-cm; 3.0-μm packing L1Any unknown Column temperature: 38°—impurity1.00.1Flow rate: 1.0mL/min Total Impurities——0.5*Injection size: 10μL a(S )-9-Fluoro-2,3-dihydro-3-methyl-10-(piperazin-1-yl)-7-oxo-7H -pyrido[1,System suitability2,3-de ][1,4]benzoxazine-6-carboxylic acid.Sample: System suitability solution b (S )-9-Fluoro-2,3-dihydro-3-methyl-10-[2-(methylamino)ethylamino]-7-Suitabilityrequirementsoxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.Relative standard deviation: NMT 2.0% for c (S )-4-(6-Carboxy-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H -pyrido-[1,2,3-levofloxacin de ][1,4]benzoxazine-10-yl)-1-methyl-piperazine-1-oxide.Analysisd (S )-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,Samples: Standard solution and Sample solution2,3-de ][1,4]benzoxazine-6-carboxylic acid.Calculate the percentage of levofloxacin related com-e (R )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.pound B in the portion of Levofloxacin taken:*Do not include the D -isomer in the calculation for total impurities.Result = (r U /r S ) × (C S /C U ) × 100O RGANIC I MPURITIES , P ROCEDURE 2[N OTE —Solutions of levofloxacin are not stable in light;r U = peak response for levofloxacin relateduse amber bottles.]compound B from the Sample solutionBuffer: Dissolve 3.08g/L of ammonium acetate and r S = peak response for levofloxacin related8.43g/L of sodium perchlorate monohydrate in pound B from the Standard solutionAdjust with phosphoric acid to a pH of 2.2.C S = concentration of USP Levofloxacin RelatedSolution A: Acetonitrile and Buffer (16:84)Compound B RS inthe Standard solution Solution B: Acetonitrile, methanol, and Buffer (mg/mL)(30:20:50)C U = concentration of Levofloxacin in the SampleSolution C: 0.4mg/mL of USP Levofloxacin RS by dis-solution (mg/mL)solving in acetonitrile at about 8% of volume and dilut-Calculate the percentage of other impurities in the ing with water to volumeportion of Levofloxacin taken:Solution D: 0.05mg/mL of USP Levofloxacin Related Compound A RS in 0.2% ammonium hydroxide in Result = (r U /r S ) × (C S /C U ) × 100methanolMobile phase: See Table 2.r U= peak response of any other impurity from theSample solutionr S = peak response of levofloxacin from theTable 2Standard solutionTime Solution ASolution BC S = concentration of USP Levofloxacin RS in the(min)(%)(%)standard solution (mg/mL)01000C U = concentration of Levofloxacin in the Samplesolution (mg/mL)51000Acceptance criteria: See Table 3.108218154060Table 330406030.11000Relative Acceptance Retention Criteria,38100NameTimeNMT (%)System suitability solution: 0.1mg/mL of USPLevofloxacin related compound A Levofloxacin RS and 5μg/mL of USP Levofloxacin Re-(N -Desmethyl levofloxacin)a 0.90.20lated Compound A RS in water from Solution C and Levofloxacin1.0—Solution DLevofloxacin related compound B b 2.90.13Levofloxacin stock solution: 0.4mg/mL of USPAny other impurity —0.10Levofloxacin RS. Dissolve USP Levofloxacin RS in aceto-T otal impurities—0.50nitrile at about 8% of final volume, sonicate, and dilute with water to volume.a(S )-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-dihydro-7H -pyrido[1,2,3-de ][1,4-benzoxazine-6-carbocylic acid.Levofloxacin standard solution: 0.02mg/mL of USP b (S )-9,10-Difluoro-3-methyl-7-oxo-2,3-dihydro-7H -pyrido[1,2,3-de ][1,4-Levofloxacin RS in acetonitrile and water (1:10) from benzoxazine-6-carbocylic acid.Levofloxacin stock solutionLevofloxacin related compound B stock solution:?O RGANICI MPURITIES (E NANTIOMERIC P URITY), P ROCEDURE 30.2mg/mL USP Levofloxacin Related Compound B RS Buffer:1.32g/L of D -phenylalanine and 0.75g/L of in methanol. [N OTE—Sonicate if necessary.]copper(II)sulfate pentahydrate in waterLevofloxacin related compound B standard solution:0.04mg/mL USP Levofloxacin Related Compound B RS U S P M o n o g r a p h sUSP 40Official Monographs / Levofloxacin4833Mobile phase: Methanol and Buffer (15:85)ASSAYSystem suitability solution: 0.01mg/mL of USP Oflox-?P ROCEDUREacin RS and 0.01mg/mL of USP Levofloxacin RS in[N OTE—Protect the solutions of levofloxacin from light.] water Diluent: Acetonitrile and water (18:82)Sample solution: 0.08mg/mL in water Mobile phase:Diluent that contains 1mL of trifluoroa-Chromatographic system cetic acid in each 1000mL of solution(See Chromatography ?621?, System Suitability.)Standard solution: 102.5μg/mL of USP LevofloxacinMode: LC RS in DiluentDetector: 294 nm System suitability solution: 102.5μg/mL each of USP Column: 4.6-mm × 15-cm; 3.5-μm pa cking L1Levofloxacin RS and USP Levofloxacin Related Com- Column temperature: 40°pound A RS in DiluentFlow rate: 0.7mL/min Sample solution: 102.5μg/mL of levofloxacin in Dilu-Injection size: 10μL ent based on the label claim. [N OTE—Mix the solution System suitability well after equilibrating the solution for 4 h at roomSample:System suitability solution temperature while protected from light.][N OTE—The relative retention times for D-ofloxacin and Chromatographic systemlevofloxacin are 0.91 and 1.0, respectively.](See Chromatography ?621?, System Suitability.)Suitability requirements Mode: LCResolution: NLT 2.0 between D-ofloxacin (D-isomer)Detector: UV 294 nmand levofloxacin Column: 4.6-mm × 15-cm; 3.5-μm packing L11 Analysis Column temperature: 30°Sample: Sample solution Flow rate: 0.7mL/minCalculate the percentage of D-ofloxacin in the portion Run time: 2.5 times the retention time of the levoflox-of Levofloxacin taken:acin peakInjection size: 20μLResult = (r U/r T) × 100System suitabilitySamples:Standard solution and System suitability r U= peak response for D-ofloxacin solutionr T= sum of responses of all peaks Suitability requirements Acceptance criteria: NMT 1.0%Resolution: NLT 1.9 between levofloxacin relatedcompound A and levofloxacin, System suitability SPECIFIC TESTS solutionO PTICAL R OTATION, Specific Rotation ?781S?Relative standard deviation: NMT 2.0%, Standard Solvent: Methanol solutionSample solution: 5mg/mL in Solvent AnalysisAcceptance criteria:?92° to ?106°, at 20°Sampl es:Standard solution and Sample solutionW ATER D ETERMINATION, Method Ia?921?: 2.0%–3.0%Calculate the percentage of the labeled amount oflevofloxacin (C18H20FN3O4) in the portion of Oral Solu-USP Monographs ADDITIONAL REQUIREMENTStion taken:P ACKAGING AND S TORAGE: Preserve in tight, light-resistantcontainers. Store at room temperature.Result = (rU/r S) × (C S/C U) × 100L ABELING: If a procedure for Organic Impurities other than Procedure 1 is used, then the labeling states with which rU= peak response from the Sample solution Organic Impurities procedure the article complies.rS= peak response from the Standard solutionUSP R EFERENCE S TANDARDS?11?CS= concentration of USP Levofloxacin RS in the USP Levofloxacin RS Standard solution (mg/mL)USP Levofloxacin Related Compound A RS CU= nominal concentration of levofloxacin in the (S)-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-Sample solution (mg/mL)dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carbox-Acceptance criteria: 90.0%–110.0%ylic acid.C17H18FN3O4 347.34IMPURITIESUSP Levofloxacin Related Compound B RS Organic Impurities(S)-9,10-Difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido?P ROCEDURE[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.[N OTE—Protect the solutions of levofloxacin from light.] C13H9F2NO4 281.21Diluent, Mobile phase, Standard solution, System USP Ofloxacin RS suitability solution, Sample solution, Chromato-graphic system, and System suitability: Proceed asdirected in the Assay.AnalysisSamples:Standard solution and Sample solution Levofloxacin Oral Solution Calculate the percentage of each individual impurity inthe portion of Oral Solution taken:DEFINITIONResult = (r U/r S) × (C S/C U) × (1/F) × 100 Levofloxacin Oral Solution contains NLT 90.0% and NMT110.0% of the labeled amount of levofloxacinr U= peak response of each individual impurity(C18H20FN3O4).from the Sample solutionr S= peak response of levofloxacin from the IDENTIFICATION Standard solutionA. The retention time of the major peak of the SampleC S= concentration of USP Levofloxacin RS in thesolution corresponds to that of the Standard solution, asStandard solution (mg/mL) obtained in the Assay.C U= nominal concentration of levofloxacin in theSample solution (mg/mL)F= relative response factor for each impurity (SeeImpurity Table 1)。
左氧氟沙星使用说明书

LEVAQUIN®(左氧氟沙星)片剂LEVAQUIN®(左氧氟沙星)口服液LEVAQUIN®(左氧氟沙星)注射剂LEVAQUIN®(含5%葡萄糖的左氧氟沙星)注射剂处方信息为减少耐药菌株的发生并维持LEV AQUIN®(左氧氟沙星)与其他抗菌药物的疗效,LEVAQUIN®(左氧氟沙星)只能用于治疗或预防已证实由细菌引起或高度疑似由细菌引起的感染。
描述LEVAQUIN®(左氧氟沙星)是人工合成的口服或静脉滴注用广谱抗菌药。
左氧氟沙星是消旋氧氟沙星的纯-(S)-异构体,化学上来讲它是一种手性氟羧基喹诺酮。
化学名是(S)-(-)-9-氟-2,3-二氢-3-甲基-10-(4-甲基-1-哌嗪基)-7-氧代-7H-吡啶并[1,2,3-de]-[1,4]苯并噁嗪-6-羧酸半水合物。
化学结构是:分子式是C18H20FN3O4·1/2H2O,分子量370.38。
左氧氟沙星是一种白色至淡黄色的晶体或晶状粉末。
在小肠pH条件下呈两性离子。
数据显示在pH0.6-5.8范围内,左氧氟沙星的溶解度恒定(大约为100mg/ml)。
USP命名原则指出在这个pH范围内,左氧氟沙星溶解度是“可溶至易溶”。
超过pH5.8,溶解度迅速增加并在pH6.7时的达最大值(272mg/ml),此范围内是“易溶”。
超过pH6.7,溶解度下降并在pH约为6.9时达到最小值(约50mg/ml)。
左氧氟沙星能与许多金属离子生成稳定的配位化合物。
在体外,金属离子与其螯合的能力顺序为:Al+3>Cu+2>Zn+2>Mg+2>Ca+2。
LEVAQUIN片剂是薄膜衣片剂,含有下列非活性成分:250mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80和合成氧化铁红500mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80和合成氧化铁红750mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80LEVAQUIN口服液(25mg/ml)是多用途,自我防腐的左氧氟沙星溶液,pH范围在5.0至6.0 LEVAQUIN口服液外观澄清,呈黄色至绿黄色。
Ciprofloxacin Tablets(环丙沙星片)USP40

3432Ciprofloxacin / Official MonographsUSP 40IDENTIFICATION•USP R EFERENCE S TANDARDS 〈11〉• The retention time of the major peak of the Sample solu-USP Ciprofloxacin Ethylenediamine Analog RStion corresponds to that of the Standard solution , as ob-1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[(2-ami-tained in the Assay .noethyl)amino]-3-quinolinecarboxylic acid hydrochloride.ASSAYC 15H 16FN 3O 3·HCl 341.77•P ROCEDUREUSP Ciprofloxacin Hydrochloride RSSolution A: 0.005 M tetrabutylammonium phosphate solution. Adjust with phosphoric acid to a pH of 2.0.Mobile phase: Methanol and Solution A (1:3)Standard solution: 0.14mg/mL of USP Ciprofloxacin Hydrochloride RS in waterCiprofloxacin TabletsSystem suitability solution: 0.01mg/mL of USP Ciprofloxacin Ethylenediamine Analog RS in Standard DEFINITIONsolutionCiprofloxacin Tablets contain Ciprofloxacin Hydrochloride Sample solution: Equivalent to 0.12mg/mL of equivalent to NLT 90.0% and NMT 110.0% of the labeled ciprofloxacin from Ophthalmic Solution, in water amount of ciprofloxacin (C 17H 18FN 3O 3).Chromatographic system(See Chromatography 〈621〉, System Suitability .)IDENTIFICATIONMode: LCThe retention time of the major peak of the Sample solution Detector: UV 280 nmcorresponds to that of the Standard solution , as obtained Column: 4.6-mm × 25-cm; packing L1in the Assay .Flow rate: 1.5mL/min Injection size: 20µL ASSAYSystem suitability•P ROCEDURESamples: Standard solution and System suitability Solution A: 0.025 M phosphoric acid. Adjust with tri-solutionethylamine to a pH of 2.0 ± 0.1.[N OTE —The relative retention times for the ciprofloxacin Solution B: Acetonitrile and Solution A (13:87)ethylenediamine analog and ciprofloxacin are 0.8 and Solution C: 0.025 M phosphoric acid. Adjust with tri-1.0, respectively.]ethylamine to a pH of 3.0 ± 0.1.Suitability requirementsMobile phase: Acetonitrile and Solution C (13:87)Resolution: NLT 1.5 between the ciprofloxacin ethyl-Standard solution: 0.2mg/mL of USP Ciprofloxacin enediamine analog and ciprofloxacin, System suitabil-Hydrochloride RS in Solution Bity solutionSystem suitability solution: 0.05mg/mL of USPCapacity factor: 1.5–6 for the ciprofloxacin peak,Ciprofloxacin Ethylenediamine Analog RS in the Stan-Standard solutiondard solutionColumn efficiency: NLT 500 theoretical plates, Stan-Sample solution: Transfer 5 Tablets to a 500-mL volu-dard solutionmetric flask, add 400mL of Solution B , and sonicate for Tailing factor: 0.9–2.0, Standard solutionabout 20 min. Dilute with Solution B to volume, mix,Relative standard deviation: NMT 2%, Standard and pass through a membrane filter of 0.45-µm pore solution size. Prepare the equivalent of 0.20mg/mL of ciproflox-Analysisacin from the filtrate with Solution B .Samples: Standard solution and Sample solution Chromatographic systemCalculate the percentage of the labeled amount of (See Chromatography 〈621〉, System Suitability .)C 17H 18FN 3O 3 in the portion of Ophthalmic Solution Mode: LCtaken:Detector: UV 278 nmColumn: 4.6-mm × 25-cm; packing L1Result = (r U /r S ) × (C S /C U ) × (M r1/M r2) × 100Column temperature: 30±1°Flow rate: 1.5mL/min r U = peak response from the Sample solution Injection size: 10µL r S = peak response from the Standard solution System suitabilityC S= concentration of USP CiprofloxacinSamples: Standard solution and System suitability Hydrochloride RS in the Standard solution solution(mg/mL)[N OTE —The retention time for ciprofloxacin is 6.4–10.8C U = nominal concentration of ciprofloxacin in themin. The relative retention times for ciprofloxacin eth-Sample solution (mg/mL)ylenediamine analog and ciprofloxacin are 0.7 and M r1= molecular weight of ciprofloxacin, 331.34 1.0, respectively.]M r2= molecular weight of anhydrous ciprofloxacinSuitability requirementshydrochloride, 367.81Resolution: NLT 6 between the ciprofloxacin ethyl-Acceptance criteria: 90.0%–110.0%enediamine analog peak and the ciprofloxacin peak,System suitability requirementsSPECIFIC TESTS Column efficiency: NLT 2500 theoretical plates from •P H 〈791〉: 3.5–5.5the ciprofloxacin peak, Standard solution•S TERILITY T ESTS 〈71〉: It meets the requirements when Tailing factor: NMT 2.0 for the ciprofloxacin peak,tested as directed under Test for Sterility of the Product to Standard solutionBe Examined, Membrane Filtration .Relative standard deviation: NMT 1.5%, Standard solution ADDITIONAL REQUIREMENTSAnalysis•P ACKAGING AND S TORAGE : Preserve in tight containers,Samples: Standard solution and Sample solutionprotected from light, at room temperature.Calculate the percentage of C 17H 18FN 3O 3 in the portion of Tablets taken:Result = (r U /r S ) × (C S /C U ) × (M r1/M r2) × 100r U= peak response from the Sample solutionUSP 40Official Monographs / Ciprofloxacin3433r S= peak response from the Standard solution Sample stock solution: Nominally 0.5mg/mL in MobileC S= concentration of USP Ciprofloxacin phase prepared as follows. Transfer an equivalent toHydrochloride RS in the Standard solution250mg of ciprofloxacin from finely powdered Tablets(mg/mL), calculated on the anhydrous basis(NLT 20) to a 500-mL volumetric flask. Add 400mL ofC U= nominal concentration of ciprofloxacin in the Mobile phase, place on a rotary shaker for 15 min, andSample solution (mg/mL)sonicate for 25 min. Allow the solution to cool to room M r1= molecular weight of ciprofloxacin, 331.34temperature, and dilute with Mobile phase to volume.M r2= molecular weight of anhydrous ciprofloxacin Pass a portion of the solution through a suitable filter of hydrochloride, 367.810.45-µm pore size.Acceptance criteria: 90.0%–110.0%Sample solution: Nominally 0.05mg/mL of ciproflox-acin in water from Sample stock solution PERFORMANCE TESTS Chromatographic system•D ISSOLUTION〈711〉(See Chromatography 〈621〉, System Suitability.) Medium: 0.01 N hydrochloric acid; 900mL Mode: LCApparatus 2: 50 rpm Detector: UV 278 nmTime: 30 min Column: 4.6-mm × 25-cm; 5-µm packing L1Sample solution: Pass a portion of the solution under Column temperature: 30°test through a suitable filter. Dilute with Medium, if Flow rate: 1.5mL/minnecessary.Injection volume: 10µLStandard solution: USP Ciprofloxacin Hydrochloride RS System suitabilityin Medium Samples:System suitability solution and Standard Spectrometric conditions solution(See Ultraviolet-Visible Spectroscopy 〈857〉.)Suitability requirementsMode: UV Resolution: NLT 6 between the ciprofloxacin and Analytical wavelength: 276 nm ciprofloxacin ethylenediamine analog peaks, System Analysis suitability solutionSamples:Sample solution and Standard solution Tailing factor: NMT 4.0 for the ciprofloxacin peak, Tolerances: An amount of ciprofloxacin hydrochloride System suitability solution(C17H18FN3O3·HCl) equivalent to NLT 80% (Q) of the Relative standard deviation: NMT 2.0% for the labeled amount of ciprofloxacin (C17H18FN3O3) is ciprofloxacin peak, Standard solutiondissolved.Analysis•U NIFORMITY OF D OSAGE U NITS〈905〉: Meet the Samples:Standard solution and Sample solution requirements Calculate the percentage of the labeled amount ofciprofloxacin (C17H18FN3O3) in the portion of Tablets ADDITIONAL REQUIREMENTS taken:•P ACKAGING AND S TORAGE: Preserve in well-closedcontainers.Result = (rU/r S) × (C S/C U) × (M r1/M r2) × 100•USP R EFERENCE S TANDARDS〈11〉USP Ciprofloxacin Ethylenediamine Analog RS rU= peak response of ciprofloxacin from the 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[(2-ami-Sample solutionnoethyl)amino]-3-quinolinecarboxylic acid rS= peak response of ciprofloxacin from the hydrochloride.Standard solutionC15H16FN3O3·HCl341.77CS= concentration of USP Ciprofloxacin USP Ciprofloxacin Hydrochloride RS Hydrochloride RS in the Standard solution(mg/mL)C U= nominal concentration of ciprofloxacin in theSample solution (mg/mL)M r1= molecular weight of ciprofloxacin, 331.34 Ciprofloxacin Extended-Release Tablets M r2= molecular weight of anhydrous ciprofloxacinhydrochloride, 367.81Acceptance criteria: 90.0%–110.0%DEFINITIONCiprofloxacin Extended-Release Tablets contain NLT 90.0%PERFORMANCE TESTSand NMT 110.0% of the labeled amount of ciprofloxacin•D ISSOLUTION〈711〉(C17H18FN3O3).Test 1Medium: pH 4.5 acetate buffer (transfer 3g of sodium IDENTIFICATIONacetate and 14mL of 2N acetic acid to a 1-L volumet-•A. The retention time of the major peak of the Sampleric flask, and dilute with water to volume); 900mL, solution corresponds to that of the Standard solution, asdeaeratedobtained in the Assay.Apparatus 2: 50 rpmASSAY Times: 30, 60, and 120 min•P ROCEDURE Standard solution: 6.5µg/mL of USP Ciprofloxacin Buffer: Dilute 2.9mL of phosphoric acid in water to Hydrochloride RS in Medium1000mL. Adjust with triethylamine to a pH of 3.0.Sample solution: Pass a portion of the solution under Mobile phase: Acetonitrile and Buffer (135:865)test through a suitable filter of 0.45-µm pore size. For System suitability solution: 0.58mg/mL of USP500-mg Tablets, transfer 2mL of the filtrate to a Ciprofloxacin Hydrochloride RS and 0.5mg/mL of USP200-mL volumetric flask, and dilute with Medium to Ciprofloxacin Ethylenediamine Analog RS in Mobile volume. For 1000-mg Tablets, transfer 1mL of the fil-phase trate to a 200-mL volumetric flask, and dilute with Me-Standard stock solution: 1.16mg/mL of USP dium to volume. Replace the aliquots withdrawn for Ciprofloxacin Hydrochloride RS in Mobile phase analysis with fresh portions of Medium.Standard solution: 0.058mg/mL of USP CiprofloxacinHydrochloride RS in Mobile phase from Standard stocksolution。
氧氟沙星 USP32
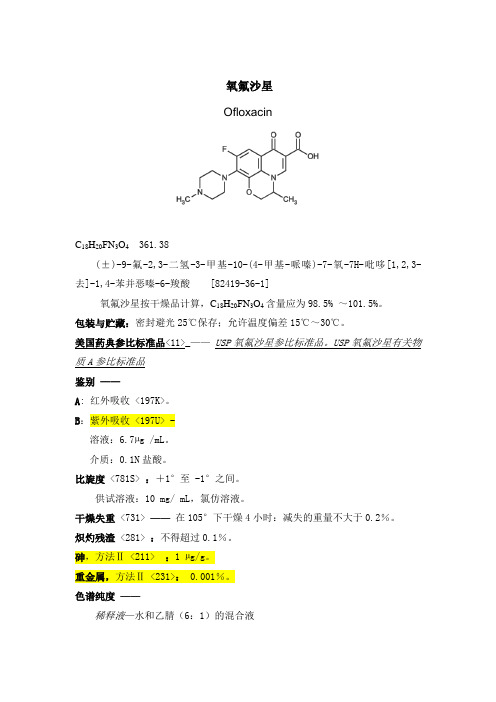
氧氟沙星OfloxacinC18H20FN3O4 361.38(±)-9-氟-2,3-二氢-3-甲基-10-(4-甲基-哌嗪)-7-氧-7H-吡哆[1,2,3- 去]-1,4-苯并恶嗪-6-羧酸 [82419-36-1]氧氟沙星按干燥品计算,C18H20FN3O4含量应为98.5% ~101.5%。
包装与贮藏:密封避光25℃保存;允许温度偏差15℃~30℃。
美国药典参比标准品<11>_——USP氧氟沙星参比标准品。
USP氧氟沙星有关物质A参比标准品鉴别——A: 红外吸收 <197K>。
B:紫外吸收 <197U> -溶液:6.7µg /mL。
介质:0.1N盐酸。
比旋度<781S>:+1°至 -1°之间。
供试溶液:10 mg/ mL,氯仿溶液。
干燥失重<731> ——在105°下干燥4小时:减失的重量不大于0.2%。
炽灼残渣<281> :不得超过0.1%。
砷,方法Ⅱ <211> :1 µg/g。
重金属,方法Ⅱ <231>: 0.001%。
色谱纯度——稀释液—水和乙腈(6:1)的混合液流动相——将4.0g醋酸铵和7.0g高氯酸钠溶解于1300ml水中,用磷酸调节pH值至2.2,混合均匀。
准备过滤,脱气过的此混合液和240ml乙腈。
可根据需要调整(见色谱<621> 中系统适应性)。
系统适应性标准溶液——取10.0mg USP氧氟沙星有关物质A参比标准品和10.0mg USP氧氟沙星对照品到100ml容量瓶, 稀释液溶解稀释,用稀释液稀释10.0ml此溶液到50.0ml,用稀释液稀释1.0ml此溶液到50.0ml。
标准溶液——精确称量USP氧氟沙星对照品,溶解于稀释液中,配制成约0.0004 mg/ ml的溶液。
供试溶液——精确称量本品,溶解于稀释液中,配制成约0.2 mg/ ml的溶液。
左氧氟沙星—搜狗百科

左氧氟沙星—搜狗百科药理作用本品为氧氟沙星的左旋体,其体外抗菌活性约为氧氟沙星的两倍。
其作用机制是通过抑制细菌DNA解旋酶的活性,阻止细菌DNA的合成和复制而导致细菌死亡。
左氧氟沙星和双黄连注射剂存在严重不良反应国家药品不良反应监测中心发布最新一期《药品不良反应信息通报》,提醒医疗机构医护人员和药品生产经营企业,警惕左氧氟沙星注射剂和双黄连注射剂的严重不良反应。
据介绍,左氧氟沙星注射剂严重不良反应/事件以全身性损害、中枢及外周神经系统损害、皮肤及其附件损害、呼吸系统损害、胃肠系统损害为主,其中过敏反应问题较为典型,临床主要表现为过敏性休克、过敏样反应、呼吸困难、多形性红斑型药疹、喉水肿等。
在国家药品不良反应监测中心数据库中,左氧氟沙星注射剂的严重病例报告分析表明,该产品存在临床不合理使用现象,且部分不合理用药问题已成为引起严重不良事件的主要因素。
如左氧氟沙星注射剂说明书中明确提示18岁以下患者禁用在数据库中不乏18岁以下患者应用该药品且引起严重不良事件的病例报告。
国家药品不良反应监测中心建议临床医生在使用左氧氟沙星注射剂时,应严格按照《抗菌药物临床应用指导原则》和药品说明书规定用药。
避免配伍禁忌,确需联合使用其他抗菌药物时应合理选择。
肾功能不全者、老年患者、神经系统疾病患者等应慎用或在严格监护下使用。
用药过程中医护人员应仔细观测患者的症状和体征,一旦发现异常应立即停药,并尽快明确诊断,及时给予对症治疗。
药代动力学口服后吸收完全,相对生物利用度接近100%。
单剂量空腹口服0.1g和0.2g后,血药峰浓度(Cmax)分别达1.36mg/L和3.06mg/L,达峰时间(Tmax)约为1小时。
血消除半衰期(t1/2β)约为5.1~7.1小时。
蛋白结合率约为30%~40%。
本品吸收后广泛分布至各组织、体液,在扁桃体、前列腺组织、痰液、泪液、妇女生殖道组织、皮肤和唾液等组织和体液中的浓度与血药浓度之比约在1.1~2.1之间。
左氧氟沙星FDA说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVAQUIN® safely and effectively. See full prescribing informationfor LEVAQUIN ® .LEVAQUIN® (levofloxacin) Tablet, Film Coated for Oral use LEVAQUIN® (levofloxacin) Solution for Oral useLEVAQUIN® (levofloxacin) Injection, Solution, Concentrate for Intravenous useLEVAQUIN® (levofloxacin) Injection, Solution for Intravenous useInitial U.S. Approval: 1996WARNING:See full prescribing information for complete boxed warning. Fluoroquinolones, including LEVAQUIN®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants [See Warnings and Precautions (5.1)]. Fluoroquinolones, including LEVAQUIN®, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid LEVAQUIN® in patients with a known history of myasthenia gravis [See Warnings and Precautions (5.2)].effectiveness of LEVAQUIN® and other antibacterial drugs, LEVAQUIN® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.—————————RECENT MAJOR CHANGES——————— Warnings and Precautions• Peripheral Neuropathy (5.8) 07/2013 —————————INDICATIONS AND USAGE———————— LEVAQUIN® is a fluoroquinolone antibacterial indicated in ad ults (≥18 years of age) with infections caused by designated, susceptible bacteria (1, 12.4).• Pneumonia: nosocomial (1.1) and community acquired (1.2, 1.3)• Acute bacterial sinusitis (1.4)• Acute bacterial exacerbation of chronic bronchitis (1.5)• Skin and skin structure infections: complicated (1.6) and uncomplicated (1.7)• Chronic bacterial prostatitis (1.8)• Urinary tract infections: complicated (1.9, 1.10) and uncomplicated(1.12)• Acute pyelonephritis (1.11)• Inhalational anthrax, post-exposure (1.13)• Plague (1.14)———————DOSAGE AND ADMINISTRATION——————— • Dosage in patients with normal renal function (2.1)Type of Infection Dose Every 24hours Duration (days)Nosocomial Pneumonia (1.1) 750 mg 7–14 Community Acquired Pneumonia (1.2) 500 mg 7–14 Community Acquired Pneumonia (1.3) 750 mg 5 Acute Bacterial Sinusitis (1.4) 750 mg 5500 mg 10–14 Acute Bacterial Exacerbation of ChronicBronchitis (1.5)500 mg 7 Complicated Skin and Skin Structure Infections(SSSI) (1.6)750 mg 7–14 Uncomplicated SSSI (1.7) 500 mg 7–10 Chronic Bacterial Prostatitis (1.8) 500 mg 28 Complicated Urinary Tract Infection (1.9) orAcute Pyelonephritis (1.11)750 mg 5 Complicated Urinary Tract Infection (1.10) orAcute Pyelonephritis (1.11)250 mg 10 Uncomplicated Urinary Tract Infection (1.12) 250 mg 3Type of Infection Dose Every 24hoursDuration(days) Inhalational Anthrax (Post-Exposure) (1.13)Adults and Pediatric Patients > 50 kgPediatric Patients < 50 kg and ≥ 6 months ofage500 mg8 mg/kg BID(not to exceed250 mg/dose)6060 Plague (1.14)Adults and Pediatric Patients > 50 kgPediatric Patients < 50 kg and ≥ 6 months ofage500 mg8 mg/kg BID(not to exceed250 mg/dose)10 to 1410 to 14• Adjust dose for creatinine clearance < 50 mL/min (2.3, 8.6, 12.3)• IV Injection, Single-Use or Premix: Slow IV infusion only, over60 or 90 minutes depending on dose. Avoid rapid or bolus IV (2.5) • Dilute single-use vials to 5 mg/mL prior to IV infusion (2.6)• Do not mix with other medications in vial or IV line (2.6) ———————DOSAGE FORMS AND STRENGTHS——————Formulation (3) StrengthTablets 250 mg, 500 mg, and 750 mg Oral Solution 25 mg/mLInjection: single-use vials for dilution 500 mg in 20 mL750 mg in 30 mL Injection: premix single-use flexiblecontainers250 mg in 50 mL500 mg in 100 mL750 mg in 150 mL ——————————CONTRAINDICATIONS————————— Known hypersensitivity to LEVAQUIN® or other quinolones (4, 5.3) ———————WARNINGS AND PRECAUTIONS——————— • Risk of tendinitis and tendon rupture is increased. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroids, and in patients with kidney, heart or lung transplants. Discontinue if pain or inflammation in a tendon occurs(5.1, 8.5)• May exacerbate muscle weakness in persons with myasthenia gravis.Avoid use in patients with a known history of myasthenia gravis (5.2) • Anaphylactic reactions and allergic skin reactions, serious, occasionally fatal, may occur after first dose (4, 5.3)• Hematologic (including agranulocytosis, thrombocytopenia), and renal toxicities may occur after multiple doses (5.4)• Hepatotoxicity: Severe, and sometimes fatal, hepatoxicity has been reported. Discontinue immediately if signs and symptoms of hepatitis occur (5.5)• Central nervous system effects, including convulsions, anxiety, confusion, depression, and insomnia may occur after the first dose. Use with caution in patients with known or suspected disorders that may predispose them to seizures or lower the seizure threshold. Increased intracranial pressure (pseudotumor cerebri) has been reported (5.6)• Clostridium difficile-associated colitis: evaluate if diarrhea occurs (5.7) • Peripheral neuropathy: discontinue immediately if symptoms occur in order to prevent irreversibility (5.8)• Prolongation of the QT interval and isolated cases of torsade de pointes have been reported. Avoid use in patients with known prolongation, those with hypokalemia, and with other drugs that prolong the QT interval (5.9, 8.5)——————————ADVERSE REACTIONS————————— The most common reactions (≥3%) were nausea, headache, diarrhea, insomnia, constipation and dizziness (6.2).To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or /medwatch.——————————DRUG INTERACTIONS—————————Interacting Drug InteractionMultivalent cation-containing products including antacids, metal cations or didanosine Absorption of levofloxacin is decreased when the tablet or oral solution formulation is taken within 2 hours of these products. Do not co-administer the intravenous formulation in the same IV line with a multivalent cation, e.g., magnesium (2.4, 7.1)Warfarin Effect may be enhanced. Monitor prothrombintime, INR, watch for bleeding (7.2)Antidiabetic agents Carefully monitor blood glucose (5.11, 7.3) ———————USE IN SPECIFIC POPULATIONS——————— • Geriatrics: Severe hepatotoxicity has been reported. The majority of reports describe patients 65 years of age or older (5.5, 8.5, 17). Mayhave increased risk of tendinopathy (including rupture), especiallywith concomitant corticosteroid use (5.1, 8.5, 17). May be moresusceptible to prolongation of the QT interval. (5.9, 8.5, 17).FULL PRESCRIBING INFORMATION: CONTENTS*WARNING:1 INDICATIONS AND USAGE1.1 Nosocomial Pneumonia1.2 Community-Acquired Pneumonia: 7–14 dayTreatment Regimen1.3 Community-Acquired Pneumonia: 5-dayTreatment Regimen1.4 Acute Bacterial Sinusitis: 5-day and 10–14 dayTreatment Regimens1.5 Acute Bacterial Exacerbation of ChronicBronchitis1.6 Complicated Skin and Skin Structure Infections1.7 Uncomplicated Skin and Skin StructureInfections1.8 Chronic Bacterial Prostatitis1.9 Complicated Urinary Tract Infections: 5-dayTreatment Regimen1.10 Complicated Urinary Tract Infections: 10-dayTreatment Regimen1.11 Acute Pyelonephritis: 5 or 10-day TreatmentRegimen1.12 Uncomplicated Urinary Tract Infections1.13 Inhalational Anthrax (Post-Exposure)1.14 Plague2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adult Patients with Normal RenalFunction2.2 Dosage in Pediatric Patients2.3 Dosage Adjustment in Adults with RenalImpairment2.4 Drug Interaction With Chelation Agents:Antacids, Sucralfate, Metal Cations,Multivitamins2.5 Administration Instructions2.6 Preparation of Intravenous Product3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Tendinopathy and Tendon Rupture5.2 Exacerbation of Myasthenia Gravis5.3 Hypersensitivity Reactions5.4 Other Serious and Sometimes Fatal Reactions5.5 Hepatotoxicity5.6 Central Nervous System Effects5.7 Clostridium difficile-Associated Diarrhea5.8 Peripheral Neuropathy5.9 Prolongation of the QT Interval5.10 Musculoskeletal Disorders in Pediatric Patientsand Arthropathic Effects in Animals5.11 Blood Glucose Disturbances5.12 Photosensitivity/Phototoxicity5.13 Development of Drug Resistant Bacteria6 ADVERSE REACTIONS6.1 Serious and Otherwise Important AdverseReactions • Pediatrics: Musculoskeletal disorders (arthralgia, arthritis, tendinopathy, and gait abnormality) seen in more LEVAQUIN®treated patients than in comparator. Shown to cause arthropathy andosteochondrosis in juvenile animals (5.10, 8.4, 13.2). Safety in pediatric patients treated for more than 14 days has not been studied.Risk-benefit appropriate only for the treatment of inhalational anthrax(post-exposure) (1.13, 2.2, 8.4, 14.9) and plague (1.14, .2.2, 8.4,14.10)See 17 for PATIENT COUNSELING INFORMATION and the FDA-approved Medication GuideRevised: 05/20146.3 Postmarketing Experience7 DRUG INTERACTIONS7.1 Chelation Agents: Antacids, Sucralfate, MetalCations, Multivitamins7.2 Warfarin7.3 Antidiabetic Agents7.4 Non-Steroidal Anti-Inflammatory Drugs7.5 Theophylline7.6 Cyclosporine7.7 Digoxin7.8 Probenecid and Cimetidine7.9 Interactions with Laboratory or DiagnosticTesting8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Renal Impairment8.7 Hepatic Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.3 Pharmacokinetics12.4 Microbiology13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment ofFertility13.2 Animal Toxicology and/or Pharmacology14 CLINICAL STUDIES14.1 Nosocomial Pneumonia14.2 Community-Acquired Pneumonia: 7–14 dayTreatment Regimen14.3 Community-Acquired Pneumonia: 5-dayTreatment Regimen14.4 Acute Bacterial Sinusitis: 5-day and 10–14 dayTreatment Regimens14.5 Complicated Skin and Skin Structure Infections14.6 Chronic Bacterial Prostatitis14.7 Complicated Urinary Tract Infections and AcutePyelonephritis: 5-day Treatment Regimen14.8 Complicated Urinary Tract Infections and AcutePyelonephritis: 10-day Treatment Regimen14.9 Inhalational Anthrax (Post-Exposure)14.10 Plague15 REFERENCES16 HOW SUPPLIED/STORAGE AND HANDLING16.1 LEVAQUIN® Tablets16.2 LEVAQUIN® Oral Solution16.3 LEVAQUIN® Injection, Single-Use Vials16.4 LEVAQUIN® Injection Pre-Mixed Solution,Single-Use in Flexible Container17 PATIENT COUNSELING INFORMATION17.1 Antibacterial Resistance17.2 Administration with Food, Fluids, andConcomitant Medications17.3 17.4Serious and Potentially Serious AdverseReactionsDrug Interactions with Insulin, OralHypoglycemic Agents, and Warfarin17.517.6Plague and Anthrax StudiesFDA-Approved Medication Guide*Sections or subsections omitted from the full prescribing information arenot listedFULL PRESCRIBING INFORMATIONWARNING:Fluoroquinolones, including LEVAQUIN® , are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants [See Warnings and Precautions (5.1)].Fluoroquinolones, including LEVAQUIN®, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid LEVAQUIN® in patients with a known history of myasthenia gravis [See Warnings and Precautions (5.2)].1 INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of LEVAQUIN® and other antibacterial drugs, LEVAQUIN® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiologyand susceptibility patterns may contribute to the empiric selection of therapy.LEVAQUIN® Tablets/Injection and Oral Solution are indicated for the treatment of adults (≥18 years of age) with mild, moderate, and severe infections caused by susceptible isolates ofthe designated microorganisms in the conditions listed in this section. LEVAQUIN® Injectionis indicated when intravenous administration offers a route of administration advantageous tothe patient (e.g., patient cannot tolerate an oral dosage form).Culture and susceptibility testingAppropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin [see Microbiology (12.4)]. Therapy with LEVAQUIN® may be initiated before results of these tests are known; once results become available, appropriate therapy should be selected.As with other drugs in this class, some isolates of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with LEVAQUIN®. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.1.1 Nosocomial PneumoniaLEVAQUIN® is indicated for the treatment of nosocomial pneumonia due to methicillinsusceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an antipseudomonal β-lactam is recommended [see Clinical Studies (14.1)].1.2 Community-Acquired Pneumonia: 7–14 day Treatment RegimenLEVAQUIN® is indicated for the treatment of community-acquired pneumonia due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multidrug-resistant Streptococcus pneumoniae [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae [see Dosage and Administration (2.1) and Clinical Studies (14.2)].MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.1.3 Community-Acquired Pneumonia: 5-day Treatment RegimenLEVAQUIN® is indicated for the treatment of community-acquired pneumonia due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, or Chlamydophila pneumoniae [see Dosage and Administration (2.1) and Clinical Studies (14.3)].1.4 Acute Bacterial Sinusitis: 5-day and 10–14 day Treatment Regimens LEVAQUIN® is indicated for the treatment of acute bacterial sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis [see Clinical Studies (14.4)].1.5 Acute Bacterial Exacerbation of Chronic BronchitisLEVAQUIN® is indicated for the treatment of acute bacterial exacerbation of chronic bronchitis due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.1.6 Complicated Skin and Skin Structure InfectionsLEVAQUIN® is indicated for the treatment of complicated skin and skin structure infections due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, or Proteus mirabilis [see Clinical Studies (14.5)].1.7 Uncomplicated Skin and Skin Structure InfectionsLEVAQUIN® is indicated for the treatment of uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections, due to methicillin-susceptible Staphylococcus aureus, or Streptococcus pyogenes.1.8 Chronic Bacterial ProstatitisLEVAQUIN® is indicated for the treatment of chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis, or methicillin-susceptible Staphylococcus epidermidis [see Clinical Studies (14.6)].1.9 Complicated Urinary Tract Infections: 5-day Treatment RegimenLEVAQUIN® is indicated for the treatment of complicated urinary tract infections due to Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis [see Clinical Studies (14.7)].1.10 Complicated Urinary Tract Infections: 10-day Treatment Regimen LEVAQUIN® is indicated for the treatment of complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa [see Clinical Studies (14.8)].1.11 Acute Pyelonephritis: 5 or 10-day Treatment RegimenLEVAQUIN® is indicated for the treatment of acute pyelonephritis caused by Escherichia coli, including cases with concurrent bacteremia [see Clinical Studies (14.7, 14.8)].1.12 Uncomplicated Urinary Tract InfectionsLEVAQUIN® is indicated for the treatment of uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.1.13 Inhalational Anthrax (Post-Exposure)LEVAQUIN® is indicated for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis. The effectiveness of LEVAQUIN® is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. LEVAQUIN® has not been tested in humans for the post-exposure prevention of inhalation anthrax. The safety of LEVAQUIN® in adults for durations of therapy beyond 28 days or in pediatric patients for durations of therapy beyond 14 days has not been studied. Prolonged LEVAQUIN® therapy should only be used when the benefit outweighs the risk [see Dosage and Administration (2.1, 2.2) and Clinical Studies (14.9)].1.14 PlagueLEVAQUIN® is indicated for treatment of plague, including pneumonic and septicemic plague, due to Yersinia pestis (Y. pestis) and prophylaxis for plague in adults and pediatric patients, 6 months of age and older. Efficacy studies of LEVAQUIN® could not be conducted in humans with plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals [see Dosage and Administration (2.1, 2.2) and Clinical Studies (14.10)].2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adult Patients with Normal Renal FunctionThe usual dose of LEVAQUIN® Tablets or Oral Solution is 250 mg, 500 mg, or 750 mg administered orally every 24 hours, as indicated by infection and described in Table 1. The usual dose of LEVAQUIN® Injection is 250 mg or 500 mg administered by slow infusion over 60 minutes every 24 hours or 750 mg administered by slow infusion over 90 minutes every 24 hours, as indicated by infection and described in Table 1.These recommendations apply to patients with creatinine clearance ≥ 50 mL/min. For patients with creatinine clearance <50 mL/min, adjustments to the dosing regimen are required [see Dosage and Administration (2.3)].Table 1: Dosag e in Adult Patients with Normal Renal Function (creatinine clearance ≥ 50 mL/min) Type of Infection* Dosed Every 24 hours Duration(days)† Nosocomial Pneumonia 750 mg 7–14 Community Acquired Pneumonia‡ 500 mg 7–14 Community Acquired Pneumonia§ 750 mg 5 Acute Bacterial Sinusitis 750 mg 5500 mg 10–14Table 1: Dosag e in Adult Patients with Normal Renal Function (creatinine clearance ≥ 50 mL/min) Type of Infection* Dosed Every 24 hours Duration(days)† Acute Bacterial Exacerbation of Chronic Bronchitis 500 mg 7 Complicated Skin and Skin Structure Infections (SSSI) 750 mg 7–14 Uncomplicated SSSI 500 mg 7–10 Chronic Bacterial Prostatitis 500 mg 28 Complicated Urinary Tract Infection (cUTI) orAcute Pyelonephritis (AP)¶750 mg 5Complicated Urinary Tract Infection (cUTI) orAcute Pyelonephritis (AP)#250 mg 10 Uncomplicated Urinary Tract Infection 250 mg 3Inhalational Anthrax (Post-Exposure), adult and pediatric patients > 50 kg Þ,ßPediatric patients < 50 kg and ≥ 6 months of age Þ,ß500 mgsee Table 2 below (2.2)60ß60ßPlague, adult and pediatric patients > 50 kg à Pediatric patients < 50 kg and ≥ 6 months of age500 mgsee Table 2 below (2.2)10 to 1410 to 14Due to the designated pathogens [see Indications and Usage (1)].† Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.‡ Due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae [see Indications and Usage (1.2)].§ Due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]), Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, or Chlamydophila pneumoniae [see Indications and Usage (1.3)].¶ This regimen is indicated for cUTI due to Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and AP due to E. coli, including cases with concurrent bacteremia.# This regimen is indicated for cUTI due to Enterococcus faecalis, Enterococcus cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa; and for AP due to E. coli.Þ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized B. anthracis. This indication is based on a surrogate endpoint. Levofloxacin plasma concentrations achieved in humans are reasonably likely to predict clinical benefit [see Clinical Studies (14.9)].ß The safety of LEVAQUIN® in adults for durations of therapy beyond 28 days or in pediatric patients for durations beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients [see Warnings and Precautions (5.10), Use in Specific Populations (8.4), and Clinical Studies (14.9)]. Prolonged LEVAQUIN® therapy should only be used when the benefit outweighs the risk.à Drug administration should begin as soon as possible after suspected or confirmed exposure to Yersinia pestis. Higher doses of LEVAQUIN® typically used for treatment of pneumonia can be used for treatment of plague, if clinically indicated.2.2 Dosage in Pediatric PatientsThe dosage in pediatric patients ≥ 6 months of age is described below in Table 2.Table 2: Dosage in Pediatric Patients ≥ 6 months of ageType of Infection* Dose Freq. Once every Duration† Inhalational Anthrax (post-exposure)‡,§Pediatric patients > 50 kg 500 mg 24 hr 60 days§Pediatric patients < 50 kg and ≥ 6 months of age8 mg/kg(not to exceed 250 mgper dose)12 hr 60 days§Table 2: Dosage in Pediatric Patients ≥ 6 months of agePlague¶Pediatric patients > 50 kg 500 mg 24 hr 10 to 14 daysPediatric patients < 50 kg and ≥ 6 months of age8 mg/kg(not to exceed 250 mgper dose)12 hr 10 to 14 daysDue to Bacillus anthracis [see Indications and Usage (1.13)] and Yersinia pestis [see Indications and Usage (1.14)].† Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.‡ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized B. anthracis. This indication is based on a surrogate endpoint. Levofloxacin plasma concentrations achieved in humans are reasonably likely to predict clinical benefit [see Clinical Studies (14.9)]§ The safety of LEVAQUIN® in pediatric patients for durations of therapy beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients [see Warnings and Precautions (5.10), Use in Specific Populations (8.4), and Clinical Studies (14.9)]. Prolonged LEVAQUIN® therapy should only be used when the benefit outweighs the risk.¶ Drug administration should begin as soon as possible after suspected or confirmed exposure to Yersinia pestis.2.3 Dosage Adjustment in Adults with Renal ImpairmentAdminister LEVAQUIN® with caution in the presence of renal insufficiency. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced.No adjustment is necessary for patients with a creatinine clearance ≥ 50 mL/min.In patients with impaired renal function (creatinine clearance <50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance [see Use in Specific Populations (8.6)].Table 3 shows how to adjust dose based on creatinine clearance.Table 3: Dosage Adjustment in Adult Patients with Renal Impairment (creatinine clearance <50 mL/min)Dosage in Normal Renal Function Every 24 hours Creatinine Clearance20 to 49 mL/minCreatinine Clearance10 to 19 mL/minHemodialysis or ChronicAmbulatory PeritonealDialysis (CAPD)750 mg 750 mg every 48 hours 750 mg initial dose, then500 mg every 48 hours 750 mg initial dose, then 500 mg every 48 hours500 mg 500 mg initial dose, then250 mg every 24 hours 500 mg initial dose, then250 mg every 48 hours500 mg initial dose, then250 mg every 48 hours250 mg No dosage adjustmentrequired 250 mg every 48 hours.If treating uncomplicated UTI,then no dosage adjustment isrequiredNo information on dosingadjustment is available2.4 Drug Interaction With Chelation Agents: Antacids, Sucralfate, MetalCations, MultivitaminsLEVAQUIN® Tablets and Oral SolutionLEVAQUIN® Tablets and Oral Solution should be administered at least two hours before or two hours after antacids containing magnesium, aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc or didanosine chewable/buffered tablets or the pediatric powder for oral solution [see Drug Interactions (7.1) and Patient Counseling Information (17.2)].LEVAQUIN® InjectionLEVAQUIN® Injection should not be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same intravenous line [see Dosage and Administration (2.6)].2.5 Administration InstructionsFood and LEVAQUIN® Tablets and Oral SolutionLEVAQUIN® Tablets can be administered without regard to food. It is recommended that LEVAQUIN® Oral Solution be taken 1 hour before or 2 hours after eating.LEVAQUIN® InjectionCaution: Rapid or bolus intravenous infusion of LEVAQUIN® has been associated with hypotension and must be avoided. LEVAQUIN® Injection should be infused intravenously slowly over a period of not less than 60 or 90 minutes, depending on the dosage. LEVAQUIN® Injection should be administered only by intravenous infusion. It is not for intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.Hydration for Patients Receiving LEVAQUIN® Tablets, Oral Solution, and InjectionAdequate hydration of patients receiving oral or intravenous LEVAQUIN® should be maintained to prevent the formation of highly concentrated urine. Crystalluria and cylindruria have been reported with quinolones [see Adverse Reactions (6.1) and Patient Counseling Information (17.2)].2.6 Preparation of Intravenous ProductParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.Because only limited data are available on the compatibility of LEVAQUIN® Injection with other intravenous substances, additives or other medications should not be added to。
左氧氟沙星注射液使用说明书

附件3盐酸左氧氟沙星口服和注射剂说明书请仔细阅读说明书并在医师指导下使用【药品名称】根据原审批文件确定通用名称: 商品名称: 英文名称: 汉语拼音: 【成份】本品主要成份为盐酸左氧氟沙星,其化学名称为:(-)-(S )-3-甲基-9-氟-2,3-二氢 -10-(4-甲基-1-哌嗪基)-7氧代-7H -吡啶并[1,2,3-de ]-1,4 苯并口恶 嗪-6-羧酸半 水合物。
其化学结构式:分子式: C 18H 20FN 3O 4·HCl ·H 2O 分子量: 415.85本品其他成份为:根据原审批文件确定。
【性状】根据具体品种确定N H 3CN H ,HCl,H 2O【适应症】为减少耐药菌的产生,保证盐酸左氧氟沙星及其他抗菌药物的有效性,左氧氟沙星只用于治疗或预防已证明或高度怀疑由敏感细菌引起的感染。
在选择或修改抗菌药物治疗方案时,应考虑细菌培养和药敏试验的结果。
如果没有这些试验的数据做参考,则应根据当地流行病学和病原菌敏感性进行经验性治疗。
在治疗前应进行细菌培养和药敏试验以分离并鉴定感染病原菌,确定其对盐酸左氧氟沙星的敏感性。
在获得以上检验结果之前可以先使用左氧氟沙星进行治疗,得到检验结果之后再选择适当的治疗方法。
与此类中的其他药物相同,使用盐酸左氧氟沙星进行治疗时,铜绿假单胞菌的某些菌株可以很快产生耐药性。
在治疗期间应定期进行细菌培养和药敏试验以掌握病原菌是否对抗菌药物持续敏感,并在细菌出现耐药性后能够及时发现。
盐酸左氧氟沙星口服制剂和注射剂可用于治疗成年人(≥ 18岁)由下列细菌的敏感菌株所引起的下列轻、中、重度感染。
如静脉滴注对患者更为有利时(如患者不能耐受口服给药等)可使用盐酸左氧氟沙星注射液。
1.医院获得性肺炎治疗由对甲氧西林敏感的金黄色葡萄球菌、铜绿假单胞菌、粘质沙雷氏菌、大肠埃希菌、肺炎克雷白杆菌、流感嗜血杆菌或肺炎链球菌引起的医院获得性肺炎。
同时应根据临床需要采取其他辅助治疗措施。
左氧氟沙星溶出曲线

【左氧氟沙星】日文名:レボフロキサシン英文名:Levofloxacin结构式:解离常数(25℃):pKa1= 6.11(针对羧基、采用滴定法测定)pKa2= 8.18(针对哌嗪的4位氮、采用滴定法测定)在各溶出介质中的溶解度(37℃):pH1.2:46.1mg/ml pH4.0:22.3mg/mlpH6.8:13.1mg/ml 水:11.2mg/ml在各溶出介质中的稳定性:水:未测定。
在各pH值溶出介质中:在中性和碱性水溶液中稳定。
光:1.0mg/ml水溶液在荧光灯照射下(光强约30万lx·hr)、降解约44%。
《四条标准溶出曲线》溶出度试验条件:桨板法/75转、溶出介质中不添加表面活性剂。
< 1g:100mg规格细粒剂>溶出度试验条件:桨板法/50转、溶出介质中不添加表面活性剂。
< 100mg规格片剂>《质量标准》1:100mg规格细粒剂取本品,混匀,精密称取适量【相当于左氧氟沙星(C18H20FN3O4·1/2H2O)0.1g】,照溶出度测定法(桨板法),以水900ml为溶剂,转速为每分钟75转,依法操作,经90分钟时,取溶液适量滤过,弃去至少10ml初滤液,精密量取续滤液5ml,置100ml量瓶中,加水稀释至刻度,摇匀,作为供试品溶液。
另精密称取预经卡氏水分测定法测定水分的左氧氟沙星对照品0.028g,置100ml量瓶中,加水溶解并稀释至刻度,摇匀,精密量取2ml,置100ml量瓶中,加水稀释至刻度,摇匀,作为对照品溶液。
取上述两种溶液照紫外-可见分光光度法,分别在289nm波长处测定吸光度,计算每袋溶出量,限度为标示量的70%,应符合规定。
《附左氧氟沙星对照品质量标准》分子式C18H20FN3O4·1/2H2O分子量370.38精制法取本品30g,加乙酸乙酯1200ml,在50~60℃下搅拌1小时。
趁热滤过,滤液于50~60℃蒸发至干。
左氧氟沙星片质量标准

测定法 取本品20片,精密称定,研细,精密称
取适量(约相当于左氧氟沙星50mg),置50ml量 瓶中,加流动相溶解并稀释至刻度,摇匀,滤过, 精密量取续滤液5ml,置50ml量瓶中,用流动相稀 释至刻度(浓度0.1mg/ml)摇匀,精密量取10μl,注 入色相色谱仪,记录色谱图;另取左氧氟沙星对 照品适量,加流动相定量稀释制成每1ml中含 100μg的溶液,同法测定。按外标法以峰面积计算, 即得。
左氧氟沙星片质量标准
左氧氟沙星片 Levofloxacin Tablets
本品含左氧10.0%。 【性状】 本品为薄膜衣片,除去包衣后,显白色至淡黄 色。 【鉴别】
取本品的细粉适量,加0.1mol/L盐酸溶液溶解并稀释制成 每1ml中含左氧氟沙星10μg的溶液,滤过,滤液照紫外-可见 分光光度法(附录IV A)测定,在226nm和294nm的波长处 有最大吸收,在263nm的波长处有最小吸收。
【含量测定】 照高效液相色谱法(附录V D)测定。
色谱条件与系统适用性试验 用十八烷基硅烷键和硅胶为填充剂;以己烷磺酸钠溶液[取己 烷磺酸钠0.98g,加磷酸盐缓冲液(取磷酸二氢钾6.8g,加水溶 解并稀释至1000ml) 1000ml使溶解,摇匀,加0.05mol/L磷酸溶 液约500ml,调节pH值至2.4]—甲醇(3:1)为流动相;柱温 40℃;流速1ml/min;检测波长为293nm。 取左氧氟沙星对照品适量,加水溶解并稀释制成每1ml中含 1mg的溶液,量取10ml置一无色试管中,在日光灯下照射3小 时,取照射后溶液10μl注入液相色谱仪,记录色谱图,以左 氧氟沙星峰为参比,相对保留时间约为1.1和1.2处应均能检测 出色谱峰,且分离度均符合要求。
【检查】
有关物质 取本品细粉适量,按标示量用0.1mol/L盐酸溶液溶解并 定量稀释制成每1ml中约含1.0mg的溶液作为供试品溶液;照 左氧氟沙星项下的方法测定,供试品溶液色谱图中如有杂 质峰,含杂质A(238nm检测)的量,按外标法以峰面积计 算,不得过0.3%。其他单个杂质(294nm检测)峰面积不得 大于对照溶液主峰面积的1.5倍(0.3%),其他各杂质峰面 积的和(294nm检测)不得大于对照溶液主峰面积的3.5倍 (0.7%)。(供试品溶液中任何小于对照溶液主峰面积0.1 倍的峰可忽略不计)。 其他 应符合片剂项下有关的各项规定(附录I A)。
左氧氟沙星杂质整理列表

中文名称
英文名称
CAS
规格
用途
ห้องสมุดไป่ตู้
结构式
左氧氟沙星
Levofloxacin
N/A
10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
左氧氟沙星N-氧化 物
Levofloxacin N-oxide
117678-38-3 10mg-25mg-50mg-100mg
2
103995-33-1 10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
左氧氟沙星杂质
3
Levofloxacin 3
Impurity
N/A
10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
武汉斯坦德供应各种杂质对照品:泊沙康唑杂质、替卡格雷杂质、索拉非尼杂质、索拉菲尼相关杂质、去氧肾上腺素杂质、维生素BI
项目报批 纯度高于98.89%
四氟左氧氟沙星杂 质1
Levofloxacin Tetrafluoro Impurity
1
110548-02-2 10mg-25mg-50mg-100mg
项目报批 纯度高于98.89%
四氟左氧氟沙星杂 质2
Levofloxacin Tetrafluoro Impurity
杂质、马来酸氯苯那敏杂质、瑞格列奈杂质等;并提供COA、NMR、HPLC、MS等图谱。详情请点用户名。
专注各种杂质对照品
代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌
甲磺酸左氧氟沙星质量标准

甲磺酸左氧氟沙星是一种药物,质量标准通常是指其药典标准。
药典是各个国家或组织制定的药物质量和安全性的标准参考手册。
甲磺酸左氧氟沙星的质量标准可以参考以下药典:
美国药典(USP):美国药典是美国药物质量标准的权威参考,其中包含了甲磺酸左氧氟沙星的质量标准。
欧洲药典(EP):欧洲药典是欧洲药物质量标准的权威参考,其中也包含了甲磺酸左氧氟沙星的质量标准。
中国药典(CP):中国药典是中国药物质量标准的权威参考,其中也包含了甲磺酸左氧氟沙星的质量标准。
这些药典中的甲磺酸左氧氟沙星质量标准包括了该药物的物理性质、化学性质、纯度要求、微生物限度、残留溶剂和杂质要求等方面的要求。
具体的质量标准可以在相应的药典中查阅。
APIs_Chn

来自资料和结构的信息 3-甲酰-利福霉素
醛基为活性的
13 |
Theo Dekker -- Jiaxing, China --September 2007
来自资料和结构的信息 利福平(讨论-1)
氧化
氢醌基
– API主要分解(为利福平苯醌) – 增强在碱性介质中的溶解性
叔胺
– 中度易氧化(为N-氧化物)prone towards oxidation (to N-oxide) – 增强在酸性介质的溶解性
6|
Theo Dekker -- Jiaxing, China --September 2007
哪里/如何寻找信息
1. 标准著作 /丛书 /书籍 – 例如:
– – – – 药物物质和赋形剂(分析)概述 [版本:Florey / Brittain – 32卷] Merck 索引 (结构、性质) 药学原理 (第12版) (“旧” API)
左氧氟沙星 (S-氧氟沙星) 莫西沙星
丙硫异烟胺 p-氨基水杨酸 (和钠盐) ✓ ✓ ✓
✓ ✓ ✓
✓ ✓ ✓
10 |
Theo Dekker -- Jiaxing, China --September 2007
来自资料和结构的信息
API为有机物质,其具有独特的化学结构,包括立体化学 这些结构,和固/液态条件,大体上决定了API的化学和物 理性质
– –
公开部分 (申请人) 保密部分(WHO 或 DRA) 稳定性文献证据 专著中控制合成中的杂质和分解物 不包括1类溶剂;控制2类/3类溶剂
药典专著
– – –
FPP 在ICH地区注册 (DRA)
24 |
Theo Dekker -- Jiaxing, China --September 2007
左氧氟沙星的质量标准
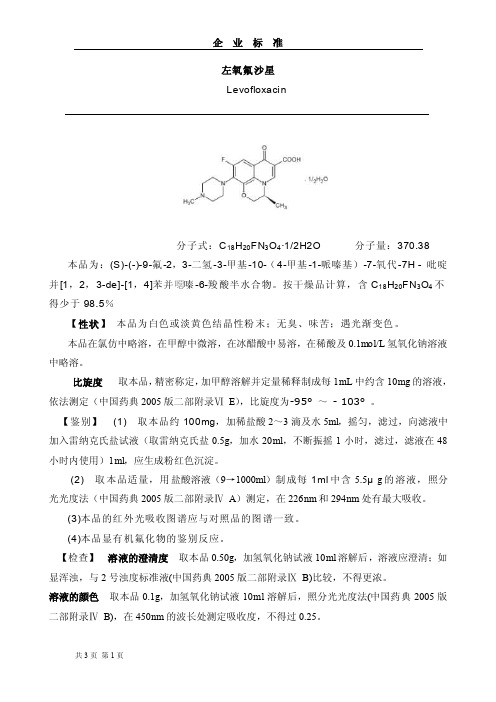
左氧氟沙星Levofloxacin分子式:C18H20FN3O4·1/2H2O 分子量:370.38 本品为:(S)-(-)-9-氟-2,3-二氢-3-甲基-10-(4-甲基-1-哌嗪基)-7-氧代-7H - 吡啶并[1,2,3-de]-[1,4]苯并噁嗪-6-羧酸半水合物。
按干燥品计算,含C18H20FN3O4不得少于98.5%【性状】本品为白色或淡黄色结晶性粉末;无臭、味苦;遇光渐变色。
本品在氯仿中略溶,在甲醇中微溶,在冰醋酸中易溶,在稀酸及0.1mol/L氢氧化钠溶液中略溶。
比旋度取本品,精密称定,加甲醇溶解并定量稀释制成每1mL中约含10mg的溶液,依法测定(中国药典2005版二部附录ⅥE),比旋度为-95°~- 103°。
【鉴别】(1) 取本品约100mg,加稀盐酸2~3滴及水5ml,摇匀,滤过,向滤液中加入雷纳克氏盐试液(取雷纳克氏盐0.5g,加水20ml,不断振摇1小时,滤过,滤液在48小时内使用)1ml,应生成粉红色沉淀。
(2) 取本品适量,用盐酸溶液(9→1000ml)制成每1ml中含5.5μg的溶液,照分光光度法(中国药典2005版二部附录ⅣA)测定,在226nm和294nm处有最大吸收。
(3)本品的红外光吸收图谱应与对照品的图谱一致。
(4)本品显有机氟化物的鉴别反应。
【检查】溶液的澄清度取本品0.50g,加氢氧化钠试液10ml溶解后,溶液应澄清;如显浑浊,与2号浊度标准液(中国药典2005版二部附录ⅨB)比较,不得更浓。
溶液的颜色取本品0.1g,加氢氧化钠试液10ml溶解后,照分光光度法(中国药典2005版二部附录ⅣB),在450nm的波长处测定吸收度,不得过0.25。
氟取本品约15mg,精密称定,照氟检查法(中国药典2005版二部附录ⅧE)测定,含氟量应为5.0%~5.8%。
右旋异构体取本品,加0.1mol/L盐酸溶液溶解并稀释制成每1ml中含左氧氟沙星1.0mg 的溶液,作为供试品溶液;另取氧氟沙星对照品,用0.1mol/L盐酸溶液制成每1ml中含10μg 的溶液,作为预试溶液。
左氧氟沙星使用说明书

左氧氟沙星使用说明书一、药品名称通用名称:左氧氟沙星英文名称:Levofloxacin二、成分本品主要成分为左氧氟沙星,其化学名称为:(S)()-9-氟-2,3-二氢-3-甲基-10-(4-甲基-1-哌嗪基)-7-氧代-7H 吡啶并1,2,3-de1,4苯并噁嗪-6-羧酸。
三、性状本品为类白色至淡黄色结晶性粉末。
四、适应症适用于敏感细菌所引起的下列轻、中度感染:1、呼吸系统感染:急性窦炎、慢性支气管炎急性发作、社区获得性肺炎。
2、泌尿系统感染:急性肾盂肾炎、复杂性尿路感染等。
3、生殖系统感染:前列腺炎、附睾炎、宫腔感染、子宫附件炎等。
4、皮肤软组织感染:传染性脓疱病、蜂窝组织炎、淋巴管(结)炎、皮下脓肿、肛周脓肿等。
5、肠道感染:细菌性痢疾、感染性肠炎、沙门菌属肠炎、伤寒及副伤寒等。
6、其他感染:外伤、烧伤及手术后伤口感染、腹腔感染(必要时合用甲硝唑)、乳腺炎、胆囊炎、胆管炎、骨与关节感染以及五官科感染等。
五、规格以左氧氟沙星计,常见规格有:01g、02g、05g 等。
六、用法用量1、口服成人常用量:一次 01g(1 片),一日 2~3 次。
病情较重者,最大剂量可增至一日 06g(6 片),分 3 次口服。
对于感染较重或感染病原体敏感性较低者,可增至一次 02g(2 片),一日 2 次。
可根据感染的种类及症状适当增减。
服用时,应整片吞服,不要掰开、嚼碎或溶解后服用。
2、静脉滴注成人每日 04g(以左氧氟沙星计),分 2 次静滴。
重度感染患者及病原菌对本品敏感性较差者(如铜绿假单胞菌),每日最大剂量可增至 06g,分 2 次静滴。
七、不良反应1、胃肠道反应:腹部不适或疼痛、腹泻、恶心或呕吐等。
2、中枢神经系统反应:头昏、头痛、嗜睡或失眠。
3、过敏反应:皮疹、皮肤瘙痒,偶可发生渗出性多形性红斑及血管神经性水肿。
光敏反应较少见。
4、关节疼痛。
5、少数患者可发生血清氨基转移酶升高、血尿素氮增高及周围血象白细胞降低,多属轻度,并呈一过性。
左氧氟沙星使用说明书

LEVAQUIN®(左氧氟沙星)片剂LEVAQUIN®(左氧氟沙星)口服液LEVAQUIN®(左氧氟沙星)注射剂LEVAQUIN®(含5%葡萄糖的左氧氟沙星)注射剂处方信息为减少耐药菌株的发生并维持LEV AQUIN®(左氧氟沙星)与其他抗菌药物的疗效,LEVAQUIN®(左氧氟沙星)只能用于治疗或预防已证实由细菌引起或高度疑似由细菌引起的感染。
描述LEVAQUIN®(左氧氟沙星)是人工合成的口服或静脉滴注用广谱抗菌药。
左氧氟沙星是消旋氧氟沙星的纯-(S)-异构体,化学上来讲它是一种手性氟羧基喹诺酮。
化学名是(S)-(-)-9-氟-2,3-二氢-3-甲基-10-(4-甲基-1-哌嗪基)-7-氧代-7H-吡啶并[1,2,3-de]-[1,4]苯并噁嗪-6-羧酸半水合物。
化学结构是:分子式是C18H20FN3O4·1/2H2O,分子量370.38。
左氧氟沙星是一种白色至淡黄色的晶体或晶状粉末。
在小肠pH条件下呈两性离子。
数据显示在pH0.6-5.8范围内,左氧氟沙星的溶解度恒定(大约为100mg/ml)。
USP命名原则指出在这个pH范围内,左氧氟沙星溶解度是“可溶至易溶”。
超过pH5.8,溶解度迅速增加并在pH6.7时的达最大值(272mg/ml),此范围内是“易溶”。
超过pH6.7,溶解度下降并在pH约为6.9时达到最小值(约50mg/ml)。
左氧氟沙星能与许多金属离子生成稳定的配位化合物。
在体外,金属离子与其螯合的能力顺序为:Al+3>Cu+2>Zn+2>Mg+2>Ca+2。
LEVAQUIN片剂是薄膜衣片剂,含有下列非活性成分:250mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80和合成氧化铁红500mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80和合成氧化铁红750mg(以无水形式):羟丙甲纤维素,交联聚维酮,维晶纤维素,硬脂酸镁,聚乙二醇,钛白粉,聚山梨酯80LEVAQUIN口服液(25mg/ml)是多用途,自我防腐的左氧氟沙星溶液,pH范围在5.0至6.0 LEVAQUIN口服液外观澄清,呈黄色至绿黄色。
左氧氟沙星专利到期,首批通用名药获准上市

左氧氟沙星专利到期,首批通用名药获准上市
夏训明
【期刊名称】《广东药学院学报》
【年(卷),期】2011(27)4
【摘要】美国FDA于2011年6月20日批准了首批12家公司提交的左氧氟沙星(levofloxacin)通用名药上市的申请,主要用于18岁以上人群治疗各种细菌感染。
左氧氟沙星是一种应用广泛的氟喹诺酮抗生素,
【总页数】1页(P403-403)
【关键词】左氧氟沙星;通用名药;上市;氟喹诺酮抗生素;专利;美国FDA;细菌感染【作者】夏训明
【作者单位】
【正文语种】中文
【中图分类】R978.19
【相关文献】
1.抗抑郁药欣百达(Cymbalta)专利到期,首批通用名药获准上市 [J], 夏训明
2.精神病药物Abilify专利到期,首批通用名药获准上市 [J], 夏训明
3.多发性硬化症药物Copaxone专利到期,首批通用名药获准上市 [J], 夏训明
4.降血脂药Crestor专利到期,首个通用名药获准上市 [J], 夏训明
5.辉瑞公司镇痛药西乐葆(Celebrex)专利到期,首批通用名药获批上市 [J], 夏训明因版权原因,仅展示原文概要,查看原文内容请购买。
不同厂家盐酸左氧氟沙星片的质量考察_王丽荣

54China Pharmaceuticals2013年3月20日第22卷第6期Vol.22,No.6,March 20,2013左氧氟沙星为第3代氟喹诺酮类药物[1],是氧氟沙星的左旋体,抗菌效力是右旋体的8 128倍,是氧氟沙星的2倍,且不良反应明显小于右旋体,临床应用广泛[2]。
盐酸左氧氟沙星片剂是目前临床常用药品,已被2010年版《中国药典(一部)》收载[3],在国内、外有多个厂家生产。
为考察其质量,现选取国内、外4个厂家共12批片剂样品,对其有关物质、溶出度、异构体及含量等进行考察并比较。
1仪器与试药岛津LC -10A 型及LC -20A 型高效液相色谱仪(日本岛津公司);溶出仪(Hanson 公司)。
左氧氟沙星对照品(批号为130455-200905,含量为97.3%)、氧氟沙星对照品(批号为130454-200905,含量为98.8%)均由中国药品生物制品检定所提供;左氧氟沙星片(规格为0.1g )、盐酸左氧氟沙星片(规格为0.1g )分别来自4个厂家(A ,B ,C ,D ),共12个批号(每个厂家3个批号,分别以A1,A2,A3,B1,B2,B3,C1,C2,C3,D1,D2,D3表示);甲醇、乙腈均为色谱纯,其余试剂均为分析纯。
2方法与结果2.1有关物质检测取本品片剂10片,研细,取适量,精密称定,用流动相溶解并稀释成每1mL 中约含1.0mg 的溶液,摇匀,作为供试品溶液;精密量取适量,加流动相稀释成每1mL 中含左氧氟沙星5μg 的溶液,作为对照溶液。
照含量测定项下的色谱条件,取对照溶液10μL 注入液相色谱仪,调节仪器的灵敏度,使主成分色谱峰的峰高约为满量程的50%,再精密量取对照溶液和供试品溶液各10μL ,分别注入液相色谱仪,记录色谱图至主成分峰保留时间的3倍。
按照外标法,以峰面积计算供试品溶液中杂质A ,B ,C (相对于保留时间约为0.74,1.08,2.57处)和其他各杂质的量,结果见表1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
USP 40Official Monographs / Levofloxacin 4831Acceptance criteria: See Table 1.Sample solution: 1mg/mL of Levofloxacin in Mobile phaseChromatographic systemTable 1(See Chromatography 〈621〉, System Suitability .)Relative Relative Acceptance Mode: LCRetention Response Criteria,Detector: UV 360 nmNameTimeFactor NMT (%)Column: 4.6-mm × 25-cm; 5-µm packing L1Levodopa related Column temperature: 45°compound A 0.90.830.1Flow rate: 0.8mL/min Injection size: 25µL Levodopa1.0——System suitabilityLevodopa related Sample: Standard solution compound B 2.80.830.5Suitability requirements 5,6-Dihydroxy-in-Tailing factor: 0.5–1.5dole-2-carboxylic Relative standard deviation: NMT 1.0%acid6.0 2.50.1Analysis0.1Samples: Standard solution and Sample solutionindividual Calculate the percentage of levofloxacin (C 18H 20FN 3O 4)—0.3 total in the portion of Levofloxacin taken:Unknown impurities 1.0unknown Total impurities——1.1Result = (r U /r S ) × (C S /C U ) × 100r U= peak response of levofloxacin from the SampleADDITIONAL REQUIREMENTSsolution•P ACKAGING AND S TORAGE : Preserve in tight, light-resistant r S = peak response of levofloxacin from thecontainers, in a dry place, and prevent exposure to ex-Standard solutioncessive heat.C S = concentration of USP Levofloxacin RS in the•USP R EFERENCE S TANDARDS 〈11〉Standard solution (mg/mL)USP Levodopa RSC U = concentration of Levofloxacin in the SampleUSP Levodopa Related Compound A RS solution (mg/mL)3-(3,4,6-Trihydroxyphenyl)alanine.Acceptance criteria: 98.0%–102.0% on the anhydrous C 9H 11NO 5213.19basisUSP Levodopa Related Compound B RS 3-Methoxytyrosine.IMPURITIESC 10H 13NO 4211.22•R ESIDUE ON I GNITION 〈281〉: NMT 0.2%. Use a platinum crucible.Delete the following:Levofloxacin••H EAVY M ETALS , Method II 〈231〉: NMT 10ppm •(Official 1-Jan-2018)•O RGANIC I MPURITIES , P ROCEDURE 1[N OTE —Procedure 1 is recommended if levofloxacin N -ox-ide is a potential organic impurity. Procedure 2 and Pro-cedure 3 are recommended if levofloxacin related com-pound B is a potential organic impurity.]Solution A, Mobile phase, Sample solution, and Chro-matographic system: Proceed as directed in the C 18H 20FN 3O 4·1/2H 2O 370.38Assay .7H -Pyrido[1,2,3-de ]-1,4-benzoxazine-6-carboxylic acid,System suitability solution: 1mg/mL of USP Levoflox-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-acin RS in Mobile phase7-oxo-hydrate (2:1), (S )-;Sensitivity solution: 0.3µg/mL of USP Levofloxacin RS (−)-(S )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piper-in Mobile phase azinyl)-7-oxo-7H -pyrido[1,2,3-de ]-1,4-benzoxazine-6-car-System suitabilityboxylic acid, hemihydrate [138199-71-0].Samples: System suitability solution and Sensitivity Anhydrous [100986-85-41].solutionSuitability requirementsDEFINITIONRelative standard deviation: NMT 1.0%, System suit-Levofloxacin contains NLT 98.0% and NMT 102.0% of ability solutionC 18H 20FN 3O 4, calculated on the anhydrous basis.Signal-to-noise ratio: NLT 10, Sensitivity solution IDENTIFICATIONAnalysis•A . I NFRARED A BSORPTION 〈197K 〉Sample: Sample solution•B . The retention time of the major peak of the Sample Calculate the percentage of each individual impurity in solution corresponds to that of the Standard solution , as the portion of Levofloxacin taken:obtained in the Assay.Result = (r U /r S ) × (1/F ) × 100ASSAY•P ROCEDUREr U = peak response of each impurity Buffer: 8.5g/L of ammonium acetate, 1.25g/L of cu-r S = peak response of levofloxacin pric sulfate, pentahydrate, and 1.3g/L of L -isoleucine in F = relative response factor (see Table 1)waterAcceptance criteria: See Table 1.Mobile phase: Methanol and Buffer (3:7)Standard solution: 1mg/mL of USP Levofloxacin RS in Mobile phase4832Levofloxacin / Official MonographsUSP 40Table 1in methanol from Levofloxacin related compound B stock solutionRelative Relative Acceptance Standard solution: 0.4µg/mL of levofloxacin andRetention Response Criteria,0.8µg/mL of levofloxacin related compound B in aceto-NameTimeFactorNMT (%)nitrile and water (1:10) from Levofloxacin standard solu-N -Desmethyl tion and Levofloxacin related compound B standard levofloxacin a0.47 1.00.3solutionDiamine derivative b 0.520.90.3Sample solution: 0.4mg/mL by dissolving the sample Levofloxacin in acetonitrile at about 8% of final volume and diluting N -oxide c0.63 1.10.3with water to volume. [N OTE —Sonicate if necessary.]Chromatographic system9-Desfluoro levoflox-(See Chromatography 〈621〉, System Suitability .)acin d0.73 1.00.3Mode: LCLevofloxacin 1.0——Detector: 280 nmD -Isomer e1.23 1.00.8Column: 4.0-mm × 15-cm; 3.0-µm packing L1Any unknown Column temperature: 38°—impurity1.00.1Flow rate: 1.0mL/min Total Impurities——0.5*Injection size: 10µL a(S )-9-Fluoro-2,3-dihydro-3-methyl-10-(piperazin-1-yl)-7-oxo-7H -pyrido[1,System suitability2,3-de ][1,4]benzoxazine-6-carboxylic acid.Sample: System suitability solution b (S )-9-Fluoro-2,3-dihydro-3-methyl-10-[2-(methylamino)ethylamino]-7-Suitability requirementsoxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.Relative standard deviation: NMT 2.0% for c (S )-4-(6-Carboxy-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H -pyrido-[1,2,3-levofloxacin de ][1,4]benzoxazine-10-yl)-1-methyl-piperazine-1-oxide.Analysisd (S )-2,3-Dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,Samples: Standard solution and Sample solution2,3-de ][1,4]benzoxazine-6-carboxylic acid.Calculate the percentage of levofloxacin related com-e (R )-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido[1,2,3-de ][1,4]benzoxazine-6-carboxylic acid.pound B in the portion of Levofloxacin taken:*Do not include the D -isomer in the calculation for total impurities.Result = (r U /r S ) × (C S /C U ) × 100•O RGANIC I MPURITIES , P ROCEDURE 2[N OTE —Solutions of levofloxacin are not stable in light;r U= peak response for levofloxacin relateduse amber bottles.]compound B from the Sample solutionBuffer: Dissolve 3.08g/L of ammonium acetate and r S = peak response for levofloxacin related8.43g/L of sodium perchlorate monohydrate in pound B from the Standard solutionAdjust with phosphoric acid to a pH of 2.2.C S = concentration of USP Levofloxacin RelatedSolution A: Acetonitrile and Buffer (16:84)Compound B RS in the Standard solution Solution B: Acetonitrile, methanol, and Buffer (mg/mL)(30:20:50)C U = concentration of Levofloxacin in the SampleSolution C: 0.4mg/mL of USP Levofloxacin RS by dis-solution (mg/mL)solving in acetonitrile at about 8% of volume and dilut-Calculate the percentage of other impurities in the ing with water to volumeportion of Levofloxacin taken:Solution D: 0.05mg/mL of USP Levofloxacin Related Compound A RS in 0.2% ammonium hydroxide in Result = (r U /r S ) × (C S /C U ) × 100methanolMobile phase: See Table 2.r U= peak response of any other impurity from theSample solutionr S = peak response of levofloxacin from theTable 2Standard solutionTime Solution ASolution BC S = concentration of USP Levofloxacin RS in the(min)(%)(%)standard solution (mg/mL)01000C U = concentration of Levofloxacin in the Samplesolution (mg/mL)51000Acceptance criteria: See Table 3.108218154060Table 330406030.11000Relative Acceptance Retention Criteria,38100NameTimeNMT (%)System suitability solution: 0.1mg/mL of USPLevofloxacin related compound A Levofloxacin RS and 5µg/mL of USP Levofloxacin Re-(N -Desmethyl levofloxacin)a 0.90.20lated Compound A RS in water from Solution C and Levofloxacin1.0—Solution DLevofloxacin related compound B b 2.90.13Levofloxacin stock solution: 0.4mg/mL of USPAny other impurity —0.10Levofloxacin RS. Dissolve USP Levofloxacin RS in aceto-Total impurities—0.50nitrile at about 8% of final volume, sonicate, and dilute with water to volume.a(S )-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-dihydro-7H -pyrido[1,2,3-de ][1,4-benzoxazine-6-carbocylic acid.Levofloxacin standard solution: 0.02mg/mL of USP b (S )-9,10-Difluoro-3-methyl-7-oxo-2,3-dihydro-7H -pyrido[1,2,3-de ][1,4-Levofloxacin RS in acetonitrile and water (1:10) from benzoxazine-6-carbocylic acid.Levofloxacin stock solutionLevofloxacin related compound B stock solution:•O RGANIC I MPURITIES (E NANTIOMERIC P URITY), P ROCEDURE 30.2mg/mL USP Levofloxacin Related Compound B RS Buffer: 1.32g/L of D -phenylalanine and 0.75g/L of in methanol. [N OTE —Sonicate if necessary.]copper(II)sulfate pentahydrate in waterLevofloxacin related compound B standard solution:0.04mg/mL USP Levofloxacin Related Compound B RSUSP 40Official Monographs / Levofloxacin4833Mobile phase: Methanol and Buffer (15:85)ASSAYSystem suitability solution: 0.01mg/mL of USP Oflox-•P ROCEDUREacin RS and 0.01mg/mL of USP Levofloxacin RS in[N OTE—Protect the solutions of levofloxacin from light.] water Diluent: Acetonitrile and water (18:82)Sample solution: 0.08mg/mL in water Mobile phase:Diluent that contains 1mL of trifluoroa-Chromatographic system cetic acid in each 1000mL of solution(See Chromatography 〈621〉, System Suitability.)Standard solution: 102.5µg/mL of USP Levofloxacin Mode: LC RS in DiluentDetector: 294 nm System suitability solution: 102.5µg/mL each of USP Column: 4.6-mm × 15-cm; 3.5-µm packing L1Levofloxacin RS and USP Levofloxacin Related Com-Column temperature: 40°pound A RS in DiluentFlow rate: 0.7mL/min Sample solution: 102.5µg/mL of levofloxacin in Dilu-Injection size: 10µL ent based on the label claim. [N OTE—Mix the solution System suitability well after equilibrating the solution for 4 h at room Sample:System suitability solution temperature while protected from light.][N OTE—The relative retention times for D-ofloxacin and Chromatographic systemlevofloxacin are 0.91 and 1.0, respectively.](See Chromatography 〈621〉, System Suitability.)Suitability requirements Mode: LCResolution: NLT 2.0 between D-ofloxacin (D-isomer)Detector: UV 294 nmand levofloxacin Column: 4.6-mm × 15-cm; 3.5-µm packing L11 Analysis Column temperature: 30°Sample: Sample solution Flow rate: 0.7mL/minCalculate the percentage of D-ofloxacin in the portion Run time: 2.5 times the retention time of the levoflox-of Levofloxacin taken:acin peakInjection size: 20µLResult = (r U/r T) × 100System suitabilitySamples:Standard solution and System suitability r U= peak response for D-ofloxacin solutionr T= sum of responses of all peaks Suitability requirementsAcceptance criteria: NMT 1.0%Resolution: NLT 1.9 between levofloxacin relatedcompound A and levofloxacin, System suitability SPECIFIC TESTS solution•O PTICAL R OTATION, Specific Rotation 〈781S〉Relative standard deviation: NMT 2.0%, Standard Solvent: Methanol solutionSample solution: 5mg/mL in Solvent AnalysisAcceptance criteria:−92° to −106°, at 20°Samples:Standard solution and Sample solution•W ATER D ETERMINATION, Method Ia〈921〉: 2.0%–3.0%Calculate the percentage of the labeled amount oflevofloxacin (C18H20FN3O4) in the portion of Oral Solu-ADDITIONAL REQUIREMENTStion taken:•P ACKAGING AND S TORAGE: Preserve in tight, light-resistantcontainers. Store at room temperature.Result = (rU/r S) × (C S/C U) × 100•L ABELING: If a procedure for Organic Impurities other thanProcedure 1 is used, then the labeling states with which rU= peak response from the Sample solution Organic Impurities procedure the article complies.rS= peak response from the Standard solution •USP R EFERENCE S TANDARDS〈11〉CS= concentration of USP Levofloxacin RS in the USP Levofloxacin RS Standard solution (mg/mL)USP Levofloxacin Related Compound A RS CU= nominal concentration of levofloxacin in the (S)-9-Fluoro-3-methyl-10-(piperazin-1-yl)-7-oxo-2,3-Sample solution (mg/mL)dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carbox-Acceptance criteria: 90.0%–110.0%ylic acid.C17H18FN3O4 347.34IMPURITIESUSP Levofloxacin Related Compound B RS Organic Impurities(S)-9,10-Difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido•P ROCEDURE[1,2,3-de][1,4]benzoxazine-6-carboxylic acid.[N OTE—Protect the solutions of levofloxacin from light.] C13H9F2NO4 281.21Diluent, Mobile phase, Standard solution, System USP Ofloxacin RS suitability solution, Sample solution, Chromato-graphic system, and System suitability: Proceed asdirected in the Assay.AnalysisSamples:Standard solution and Sample solution Levofloxacin Oral Solution Calculate the percentage of each individual impurity inthe portion of Oral Solution taken:DEFINITIONResult = (r U/r S) × (C S/C U) × (1/F) × 100 Levofloxacin Oral Solution contains NLT 90.0% and NMT110.0% of the labeled amount of levofloxacinr U= peak response of each individual impurity(C18H20FN3O4).from the Sample solutionr S= peak response of levofloxacin from the IDENTIFICATIONStandard solution•A. The retention time of the major peak of the SampleC S= concentration of USP Levofloxacin RS in thesolution corresponds to that of the Standard solution, asStandard solution (mg/mL) obtained in the Assay.C U= nominal concentration of levofloxacin in theSample solution (mg/mL)F= relative response factor for each impurity (SeeImpurity Table 1)。
