USP 美国药典 甘油标准
国家药品评价抽验质量分析报告-甘油

脂肪酸与酯类
多元线性回归分析 A、B、D 之间正相关
7
A、B、C、D 之间正相关
标准检验—不合格原因分析
不合格项:①丙烯醛、葡萄糖与铵盐(1批) ②二甘醇、乙二醇与其他杂质(同①) ③脂肪酸与酯类(2批)
丙烯醛、葡 萄糖与铵盐
二甘醇、乙二 醇与其他杂质
脂肪酸与 酯类
分装过程 样品受到 二次污染
①分装过程 受到二次污 染;② QA、 QC专业水平 有待提高
26
探索性研究—稳定性
(二)包装材料相容性 选项理由:本品多数包装为塑料材质,该类材料与液体 样品接触时可能有塑化剂迁移的情况
•采用GC-MS法,选择涉及塑料材质包装的样品进行10种塑化剂测试
均未检出塑化剂成分,说明未出现塑化剂迁移现象27源自探索性研究—质量状况及应用
(一)生产工艺对比 选项理由:生产工艺分为水解、皂化、精制和分装4类, 各种工艺对项目的控制能力如何,有待考察
HO O
MW: 31.06
HO
②
O
+
O
OH
C
MW: 31.06
MW: 148.16
O
HO
MW: 117.13
HO
O
③
O
+
O
OH
OH
D
MW: 31.06
MW: 148.16
MW: 117.13
24
探索性研究—有效性
(三)杂质—1,2-丙二醇 选项理由:EP7.0中收录了1,2-丙二醇为杂质C,我国现 行标准没有对其进行要求
• • 选择生产企业的原独立包装样品进行考察 ; 结果样品的细菌内毒素检查均符合规定 ;
• 建议在供注射用时,将标准中增加细菌内毒素检查, 具体方法如下:取本品,按<<中国人民共和国药典(二部) 2010年版>>(附录ⅪE)检查,每1g供试品中内毒素的含量应 小于10EU。
美国药典USP

美国药典USP浓度重量克分子浓度、容量克分子浓度和当量浓度用于本药典内大部分化学含量测定和检测方法中(亦见容量溶液于试剂、指示剂和溶液篇章中)重量克分子浓度用m表示,前面有一个数目字即为该溶质的克分子数(1公斤的所标明的溶液中)容量克分子浓度用M表示,前面的数目字表示在制备1立升溶液中所含的该溶质的克分子数当量浓度用N表示,前面的数目字表示制备1立升溶液中该溶质的克当量数。
百分比计量—百分比浓度如下表示:重量于重量百分比—(w/w)表示一个组分在100克溶液或混合物中的克数重量于容量百分比—(w/v)表示一个组分在100毫升溶液中的毫升数(不管溶剂是水还是其他液体)容量于容量百分比—(v/v)表示一个组分在100毫升溶液中的毫升数用百分比这个名词没有限定意义,对固体和半固体用w/w;对溶液或固体在溶液中的悬浮液用w/v;对液体在液体溶液用v/v;气体在液体内用w/v,例:一个1%的溶液是由溶解1克固体或半固体或1毫升溶液于足够的溶剂使成100毫升溶液。
由于室温的差异,微小的容量计量差异可忽略。
有效数据和允许偏差此处表示的数限是上限和下限并包括此二值以及其间的所有数字,但在限度以外的数值不在内。
在供试品的专篇内,检测中不管数字是以百分比还是绝对数字来表示都是表示最末的数字。
在容量滴定法中相当的叙述——容量滴定法故采用包括相当于标化滴定液的每毫升相当于供试品的重量,在这样的相当陈述,滴定液的浓度中的有效数字被认为相当于供试品的重量有效数字,所有的容量滴定应做空白校正。
允许偏差—药典所述的供试品的专篇内所规定的那些限度的建立是把供试品作为药物来使用或作为营养剂、饮食补充剂使用,除非另有其他指定。
药物的活性成分用分子式来计其强度标明了化学本质,如同已给的供试品的化学全名其绝对纯度为100%。
中略。
一个可写在检测报告上的值通常是几个单独测定值的合计,此结果是按规定所做的一个完整的测定所得而用于可接受的标示值进行比较,当需舍入时,5以下舍去,5(包含5)以上进1,如:标准含量为≥98.0% 实测值舍入前97.95% 舍入后98.0% 判断合格舍入前97.94% 舍入后97.9% 判断不合格标准限度≤0.02% 实测值舍入前0.025% 舍入后0.03% 判断不合格舍入前0.015% 舍入后0.02% 判断合格舍入前0.024% 舍入后0.02% 判断合格检测和含量测定设施——在检测或含量测定中所用的容器或设施的大小型式不过是一种推荐。
甘油(供注射用)质量标准与检验规程

1.目的:建立标准的甘油(供注射用)质量标准与检验规程,保证生产产品质量。
2.范围:适用于甘油(供注射用)的检验。
3.责任人:QA、QC人员。
4.内容:4.1.性状本品为无色、澄明的黏稠液体;味甜;有引湿性,水溶液(1→10) 显中性反应。
本品与水或乙醇能任意混溶,在丙酮中微溶,在氯仿或乙醚中均不溶。
4.1.1.相对密度4.1.1.1.质量指标:在25℃时不小于1.257。
4.1.1.2.检测方法: CP2010版二部附录Ⅵ A相对密度测定法。
4.1.2.折光率4.1.2.1.质量指标:1.470~1.475。
4.1.1.2.检测方法: CP2010版二部附录Ⅵ F折光率测定法4.2.检查4.2.1.酸碱度取本品25.0g,加水稀释成50ml,混匀,加酚酞指示液0.5ml,溶液应无色,加0.1mol/L氢氧化钠溶液0.2ml,溶液应显粉红色。
4.2.2.颜色取本品50ml,置50ml纳氏比色管中,与对照液(取比色用重铬酸钾液0.2ml ,加水稀释至50ml制成)比较,不得更深。
4.2.3.氯化物4.2.3.1.质量指标:≤0.0006%。
4.2.3.2.检测方法:取本品5.0g,依法检查(CP2010版二部附录Ⅷ A),与标准氯化钠溶液3.0ml 制成的对照液比较,不得更浓。
4.2.4.硫酸盐4.2.4.1.质量指标:≤0.002%。
4.2.4.2.检测方法:取本品10g ,依法检查(CP2010版二部附录Ⅷ B),与标准硫酸钾溶液2.0ml 制成的对照液比较,不得更浓。
4.2.5.醛与还原性物质取本品3.75g,置具塞比色管中,加水至15ml,混匀,加脱色副品红溶液[取盐酸副品红(C19H18ClN3)0.1g,置具塞锥形瓶中,加水60ml,加7.5%焦亚硫酸钠溶液10ml,在缓慢搅拌下加稀盐酸4.5ml,加塞摇匀至完全溶解,加水至100ml,放置12小时后使用]1.0ml,密塞放置1小时,溶液的颜色与对照品溶液[每1ml含甲醛(CH2O)5.0µg]7.5ml同法处理后的溶液颜色比较,不得更深。
heparin sodium injection USP 美国药典中肝素溶液要求

Heparin Sodium Injection, USPRx onlyDESCRIPTIONHeparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)>(1)>(4)>(3)>(5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.Structural formula of Heparin Sodium (representative sub-units):Heparin Sodium Injection, USP is a sterile solution of heparin sodium derived from porcine intestinal mucosa, standardized for anticoagulant activity. It is to be administered by intravenous or deep subcutaneous routes. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.Heparin Sodium Injection, USP is available in the following concentrations/mL:Alcohol Heparin Sodium Sodium Chloride Benzyl1000 USP units 8.6 mg 0.01 mL5000 USP units 7 mg 0.01 mL10,000 USP units 5 mg 0.01 mLpH 5.0-7.5; sodium hydroxide and/or hydrochloric acid added, if needed, for pH adjustment.CLINICAL PHARMACOLOGYHeparin inhibits reactions that lead to the clotting of blood and the formation of fibrin clots both in vitro and in vivo. Heparin acts at multiple sites in the normal coagulation system. Small amounts of heparin in combination with antithrombin III (heparin cofactor) can inhibit thrombosis by inactivating activated Factor X and inhibiting the conversion of prothrombin to thrombin. Once active thrombosis has developed, larger amounts of heparin can inhibit further coagulation by inactivating thrombin and preventing the conversion of fibrinogen to fibrin. Heparin also prevents the formation of a stable fibrin clot by inhibiting the activation of the fibrin stabilizing factor.Bleeding time is usually unaffected by heparin. Clotting time is prolonged by full therapeutic doses of heparin; in most cases, it is not measurably affected by low doses of heparin.Patients over 60 years of age, following similar doses of heparin, may have higher plasma levels ofheparin and longer activated partial thromboplastin times (APTTs) compared with patients under 60years of age.Peak plasma levels of heparin are achieved 2 to 4 hours following subcutaneous administration,although there are considerable individual variations. Loglinear plots of heparin plasma concentrationswith time, for a wide range of dose levels, are linear, which suggests the absence of zero orderprocesses. Liver and the reticuloendothelial system are the sites of biotransformation. The biphasic elimination curve, a rapidly declining alpha phase (t1/2=10 min.) and after the age of 40 a slower beta phase, indicates uptake in organs. The absence of a relationship between anticoagulant half-life andconcentration half-life may reflect factors such as protein binding of heparin.Heparin does not have fibrinolytic activity; therefore, it will not lyse existing clots.INDICATIONS AND USAGEHeparin Sodium Injection is indicated for:Anticoagulant therapy in prophylaxis and treatment of venous thrombosis and its extension;Low-dose regimen for prevention of postoperative deep venous thrombosis and pulmonary embolismin patients undergoing major abdominothoracic surgery or who, for other reasons, are at risk ofdeveloping thromboembolic disease (see DOSAGE AND ADMINISTRATION);Prophylaxis and treatment of pulmonary embolism;Atrial fibrillation with embolization;Diagnosis and treatment of acute and chronic consumptive coagulopathies (disseminated intravascularcoagulation);Prevention of clotting in arterial and cardiac surgery;Prophylaxis and treatment of peripheral arterial embolism.Heparin may also be employed as an anticoagulant in blood transfusions, extracorporeal circulation,and dialysis procedures and in blood samples for laboratory purposes.CONTRAINDICATIONSHeparin sodium should NOT be used in patients with the following conditions:Severe thrombocytopenia;When suitable blood coagulation tests, e.g., the whole blood clotting time, partial thromboplastin time,etc., cannot be performed at appropriate intervals (this contraindication refers to full-dose heparin;there is usually no need to monitor coagulation parameters in patients receiving low-dose heparin). An uncontrolled active bleeding state (see WARNINGS), except when this is due to disseminated intravascular coagulation.WARNINGSHeparin is not intended for intramuscular use.HypersensitivityPatients with documented hypersensitivity to heparin should be given the drug only in clearly life-threatening situations. (See ADVERSE REACTIONS, Hypersensitivity.)HemorrhageHemorrhage can occur at virtually any site in patients receiving heparin. An unexplained fall in hematocrit, fall in blood pressure or any other unexplained symptom should lead to serious consideration of a hemorrhagic event.Heparin sodium should be used with extreme caution in disease states in which there is increased danger of hemorrhage. Some of the conditions in which increased danger of hemorrhage exists are: CardiovascularSubacute bacterial endocarditis, severe hypertension.SurgicalDuring and immediately following (a) spinal tap or spinal anesthesia or (b) major surgery, especially involving the brain, spinal cord, or eye.HematologicConditions associated with increased bleeding tendencies, such as hemophilia, thrombocytopenia and some vascular purpuras.GastrointestinalUlcerative lesions and continuous tube drainage of the stomach or small intestine.OtherMenstruation, liver disease with impaired hemostasis.Coagulation TestingWhen heparin sodium is administered in therapeutic amounts, its dosage should be regulated by frequent blood coagulation tests. If the coagulation test is unduly prolonged or if hemorrhage occurs, heparin sodium should be promptly discontinued. (See OVERDOSAGE.)ThrombocytopeniaThrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30% up to 30%. Platelet counts should be obtained at baseline and periodically during heparin administration. Mild thrombocytopenia (count greater than 100,000/mm3) may remain stable or reverse even if heparin is continued. However, thrombocytopenia of any degree should be monitored closely. If the count falls below 100,000/mm3 or if recurrent thrombosis develops (see Heparin-induced Thrombocytopenia and Heparin-induced Thrombocytopenia and Thrombosis),the heparin product should be discontinued, and, if necessary, an alternative anticoagulant administered. Heparin-induced Thrombocytopenia (HIT) and Heparin-induced Thrombocytopenia Thrombosis (HITT)Heparin-induced Thrombocytopenia (HIT) is a serious antibody-mediated reaction resulting from irreversible aggregation of platelets. HIT may progress to the development of venous and arterial thromboses, a condition referred to as Heparin-induced Thrombocytopenia and Thrombosis (HITT). Thrombotic events may also be the initial presentation for HITT. These serious thromboembolic events include deep vein thrombosis, pulmonary embolism, cerebral vein thrombosis, limb ischemia, stroke, myocardial infarction, mesenteric thrombosis, renal arterial thrombosis, skin necrosis, gangrene of the extremities that may lead to amputation, and possibly death. Thrombocytopenia of any degree should be monitored closely. If the platelet count falls below 100,000/mm3 or if recurrent thrombosis develops, the heparin product should be promptly discontinued and alternative anticoagulants considered, if patients require continued anticoagulation.Delayed Onset of HIT and HITTHeparin-induced thrombocytopenia and heparin-induced thrombocytopenia and thrombosis can occur up to several weeks after the discontinuation of heparin therapy. Patients presenting with thrombocytopenia or thrombosis after discontinuation of heparin should be evaluated for HIT and HITT.Use in NeonatesThis product contains the preservative benzyl alcohol and is not recommended for use in neonates. There have been reports of fatal ‘gasping syndrome’ in neonates (children less than one month of age) following the administration of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.PRECAUTIONSGeneralThrombocytopenia, Heparin-induced Thrombocytopenia (HIT) and Heparin-induced Thrombocytopenia and Thrombosis (HITT):see WARNINGS.Heparin ResistanceIncreased resistance to heparin is frequently encountered in fever, thrombosis, thrombophlebitis, infections with thrombosing tendencies, myocardial infarction, cancer and in postsurgical patients. Increased Risk to Older Patients, Especially WomenA higher incidence of bleeding has been reported in patients, particularly women, over 60 years of age. Laboratory TestsPeriodic platelet counts, hematocrits, and tests for occult blood in stool are recommended during the entire course of heparin therapy, regardless of the route of administration. (See DOSAGE AND ADMINISTRATION.)Drug InteractionsOral AnticoagulantsHeparin sodium may prolong the one-stage prothrombin time. Therefore, when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hours after the last intravenous dose or 24 hours after the last subcutaneous dose should elapse before blood is drawn, if a valid prothrombin time is to be obtained.Platelet InhibitorsDrugs such as acetylsalicylic acid, dextran, phenylbutazone, ibuprofen, indomethacin, dipyridamole, hydroxychloroquine and others that interfere with platelet-aggregation reactions (the main hemostatic defense of heparinized patients) may induce bleeding and should be used with caution in patients receiving heparin sodium.Other InteractionsDigitalis, tetracyclines, nicotine or antihistamines may partially counteract the anticoagulant action of heparin sodium. Intravenous nitroglycerin administered to heparinized patients may result in a decrease of the partial thromboplastin time with subsequent rebound effect upon discontinuation ofnitroglycerin. Careful monitoring of partial thromboplastin time and adjustment of heparin dosage are recommended during coadministration of heparin and intravenous nitroglycerin.Drug/Laboratory Tests InteractionsHyperaminotransferasemiaSignificant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin. Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease and pulmonary emboli, increases that might be caused by drugs (like heparin) should be interpreted with caution.Carcinogenesis, Mutagenesis, Impairment of FertilityNo long-term studies in animals have been performed to evaluate carcinogenic potential of heparin. Also, no reproduction studies in animals have been performed concerning mutagenesis or impairment of fertility.PregnancyTeratogenic Effects—Pregnancy Category CAnimal reproduction studies have not been conducted with heparin sodium. It is also not known whether heparin sodium can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Heparin sodium should be given to a pregnant woman only if clearly needed. Nonteratogenic EffectsHeparin does not cross the placental barrier.Nursing MothersHeparin is not excreted in human milk.Pediatric UseSee DOSAGE AND ADMINISTRATION–Pediatric Use.Geriatric UseA higher incidence of bleeding has been reported in patients over 60 years of age, especially women (see PRECAUTIONS, General). Clinical studies indicate that lower doses of heparin may be indicated in these patients (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).ADVERSE REACTIONSHemorrhageHemorrhage is the chief complication that may result from heparin therapy. (See WARNINGS.) An overly prolonged clotting time or minor bleeding during therapy can usually be controlled by withdrawing the drug. (See OVERDOSAGE.) It should be appreciated that gastrointestinal or urinary tract bleeding during anticoagulant therapy may indicate the presence of an underlying occult lesion. Bleeding can occur at any site but certain specific hemorrhagic complications may be difficult to detect:a. Adrenal hemorrhage, with resultant acute adrenal insufficiency, has occurred during anticoagulanttherapy. Therefore, such treatment should be discontinued in patients who develop signs and symptoms of acute adrenal hemorrhage and insufficiency. Initiation of corrective therapy should not depend on laboratory confirmation of the diagnosis, since any delay in an acute situation may result in the patient’s death.b. Ovarian (corpus luteum) hemorrhage developed in a number of women of reproductive agereceiving short- or long-term anticoagulant therapy. This complication, if unrecognized, may be fatal.c. Retroperitoneal hemorrhage.Thrombocytopenia, Heparin-induced thrombocytopenia (HIT) and Heparin-induced Thrombocytopenia and Thrombosis (HITT) and Delayed Onset of HIT and HITT:see WARNINGS.Local IrritationLocal irritation, erythema, mild pain, hematoma or ulceration may follow deep subcutaneous (intrafat) injection of heparin sodium. These complications are much more common after intramuscular use, and such use is not recommended.HypersensitivityGeneralized hypersensitivity reactions have been reported, with chills, fever and urticaria as the most usual manifestations, and asthma, rhinitis, lacrimation, headache, nausea and vomiting, and anaphylactoid reactions, including shock, occurring more rarely. Itching and burning, especially on the plantar side of the feet, may occur.Thrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30%. While often mild and of no obvious clinical significance, such thrombocytopenia can be accompanied by severe thromboembolic complications such as skin necrosis, gangrene of the extremities that may lead to amputation, myocardial infarction, pulmonary embolism, stroke, and possibly death. (See WARNINGS and PRECAUTIONS.)Certain episodes of painful, ischemic and cyanosed limbs have in the past been attributed to allergic vasospastic reactions. Whether these are in fact identical to the thrombocytopenia-associated complications remains to be determined.MiscellaneousOsteoporosis following long-term administration of high doses of heparin, cutaneous necrosis after systemic administration, suppression of aldosterone synthesis, delayed transient alopecia, priapism, and rebound hyperlipemia on discontinuation of heparin sodium have also been reported.Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin. OVERDOSAGESymptomsBleeding is the chief sign of heparin overdosage. Nosebleeds, blood in urine or tarry stools may be noted as the first sign of bleeding. Easy bruising or petechial formations may precede frank bleeding. TreatmentNeutralization of heparin effect.When clinical circumstances (bleeding) require reversal of heparinization, protamine sulfate (1% solution) by slow infusion will neutralize heparin sodium. No more than 50 mg should be administered, very slowly, in any 10-minute period. Each mg of protamine sulfate neutralizes approximately 100 USP heparin units. The amount of protamine required decreases over time as heparin is metabolized. Although the metabolism of heparin is complex, it may, for the purpose of choosing a protamine dose, be assumed to have a half-life of about 1/2 hour after intravenous injection.Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions often resembling anaphylaxis have been reported, the drug should be given only when resuscitation techniques and treatment of anaphylactoid shock are readily available.For additional information consult the labeling of Protamine Sulfate Injection, USP products. DOSAGE AND ADMINISTRATIONParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Slight discoloration does not alter potency.When heparin is added to an infusion solution for continuous intravenous administration, the container should be inverted at least six times to ensure adequate mixing and prevent pooling of the heparin in the solution.Heparin sodium is not effective by oral administration and should be given by intermittent intravenous injection, intravenous infusion, or deep subcutaneous (intrafat, i.e., above the iliac crest or abdominal fat layer) injection. The intramuscular route of administration should be avoided because of the frequent occurrence of hematoma at the injection site.The dosage of heparin sodium should be adjusted according to the patient’s coagulation test results. When heparin is given by continuous intravenous infusion, the coagulation time should be determined approximately every 4 hours in the early stages of treatment. When the drug is administered intermittently by intravenous injection, coagulation tests should be performed before each injection during the early stages of treatment and at appropriate intervals thereafter. Dosage is considered adequate when the activated partial thromboplastin time (APTT) is 1.5 to 2 times normal or when the whole blood clotting time is elevated approximately 2.5 to 3 times the control value. After deep subcutaneous (intrafat) injections, tests for adequacy of dosage are best performed on samples drawn 4 to 6 hours after the injection.Periodic platelet counts, hematocrits and tests for occult blood in stool are recommended during the entire course of heparin therapy, regardless of the route of administration.Converting to Oral AnticoagulantWhen an oral anticoagulant of the coumarin or similar type is to be begun in patients already receiving heparin sodium, baseline and subsequent tests of prothrombin activity must be determined at a time when heparin activity is too low to affect the prothrombin time. This is about 5 hours after the last intravenous bolus and 24 hours after the last subcutaneous dose. If continuous IV heparin infusion is used, prothrombin time can usually be measured at any time.In converting from heparin to an oral anticoagulant, the dose of the oral anticoagulant should be the usual initial amount and thereafter prothrombin time should be determined at the usual intervals. To ensure continuous anticoagulation, it is advisable to continue full heparin therapy for several days after the prothrombin time has reached the therapeutic range. Heparin therapy may then be discontinued without tapering.Therapeutic Anticoagulant Effect With Full-Dose HeparinAlthough dosage must be adjusted for the individual patient according to the results of suitable laboratory tests, the following dosage schedules may be used as guidelines:METHOD OFADMINISTRATION FREQUENCYRECOMMENDED DOSE [based on 150 lb (68 kg) patient]Deep Subcutaneous (Intrafat) Injection Initial dose 5000 units by IV injection, followedby 10,000 to 20,000 units of aconcentrated solution, subcutaneouslyA different site should be used for each injection to prevent the development of massive hematoma Every 8 hoursor8000 to 10,000 units of aconcentrated solutionEvery 12 hours 15,000 to 20,000 units of aconcentrated solutionIntermittent Intravenous Injection Initial dose 10,000 units, either undiluted or in 50to 100 mL of 0.9% Sodium ChlorideInjection, USPEvery 4 to 6 hours 5000 to 10,000 units, either undilutedor in 50 to 100 mL of 0.9% SodiumChloride Injection, USPIntravenous Infusion Initial dose 5000 units by IV injectionContinuous 20,000 to 40,000 units/24 hours in1000 mL of 0.9% Sodium ChlorideInjection, USP (or in any compatiblesolution) for infusionPediatric UseFollow recommendations of appropriate pediatric reference texts. In general, the following dosage schedule may be used as a guideline:Initial Dose50 units/kg (IV, drip)Maintenance Dose100 units/kg (IV, drip) every 4 hours, or 20,000 units/m2/24 hours continuouslyGeriatric UsePatients over 60 years of age may require lower doses of heparin.Surgery of the Heart and Blood VesselsPatients undergoing total body perfusion for open-heart surgery should receive an initial dose of not less than 150 units of heparin sodium per kilogram of body weight. Frequently, a dose of 300 units per kilogram is used for procedures estimated to last less than 60 minutes, or 400 units per kilogram for those estimated to last longer than 60 minutes.Low-Dose Prophylaxis of Postoperative ThromboembolismA number of well-controlled clinical trials have demonstrated that low-dose heparin prophylaxis, given just prior to and after surgery, will reduce the incidence of postoperative deep vein thrombosis in the legs (as measured by the I-125 fibrinogen technique and venography) and of clinical pulmonary embolism. The most widely used dosage has been 5000 units 2 hours before surgery and 5000 units every 8 to 12 hours thereafter for 7 days or until the patient is fully ambulatory, whichever is longer. The heparin is given by deep subcutaneous injection in the arm or abdomen with a fine needle (25- to 26-gauge) to minimize tissue trauma. A concentrated solution of heparin sodium is recommended. Such prophylaxis should be reserved for patients over the age of 40 who are undergoing major surgery. Patients with bleeding disorders and those having neurosurgery, spinal anesthesia, eye surgery or potentially sanguineous operations should be excluded, as should patients receiving oral anticoagulants or platelet-active drugs (see WARNINGS). The value of such prophylaxis in hip surgery has not been established. The possibility of increased bleeding during surgery or postoperatively should be borne in mind. If such bleeding occurs, discontinuance of heparin and neutralization with protamine sulfate are advisable. If clinical evidence of thromboembolism develops despite low-dose prophylaxis, full therapeutic doses of anticoagulants should be given unless contraindicated. All patients should be screened prior to heparinization to rule out bleeding disorders, and monitoring should be performed with appropriate coagulation tests just prior to surgery. Coagulation test values should be normal or only slightly elevated. There is usually no need for daily monitoring of the effect of low-dose heparin in patients with normal coagulation parameters.Extracorporeal DialysisFollow equipment manufacturers’ operating directions carefully.Blood TransfusionAddition of 400 to 600 USP units per 100 mL of whole blood is usually employed to prevent coagulation. Usually, 7500 USP units of heparin sodium are added to 100 mL of 0.9% Sodium Chloride Injection, USP (or 75,000 USP units per 1000 mL of 0.9% Sodium Chloride Injection, USP) and mixed; from this sterile solution, 6 to 8 mL are added per 100 mL of whole blood.Laboratory SamplesAddition of 70 to 150 units of heparin sodium per 10 to 20 mL sample of whole blood is usually employed to prevent coagulation of the sample. Leukocyte counts should be performed on heparinized blood within 2 hours after addition of the heparin. Heparinized blood should not be used for isoagglutinin, complement, or erythrocyte fragility tests or platelet counts.HOW SUPPLIEDHeparin Sodium Injection, USP1000 USP units/mL1 mL DOSETTE vial packaged in 25s (NDC 0641-0391-25)10 mL Multiple Dose vial packaged in 25s (NDC 0641-2440-45)30 mL Multiple Dose vial packaged in 25s (NDC 0641-2450-45)5000 USP units/mL1 mL DOSETTE vial packaged in 25s (NDC 0641-0400-25)10 mL Multiple Dose vial packaged in 25s (NDC 0641-2460-45)10,000 USP units/mL1 mL DOSETTE vial packaged in 25s (NDC 0641-0410-25)4 mL Multiple Dose vial packaged in 25s (NDC 0641-2470-45)Also available from Baxter: HEP-LOCK (Heparin Lock Flush Solution, USP) and HEP-LOCK U/P (Preservative-Free Heparin Lock Flush Solution, USP).StorageStore at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].Baxter, Hep-Lock and Dosette are trademarks of Baxter International, Inc., or its subsidiaries. REFERENCES:1. Tahata T, Shigehito M, Kusuhara K, Ueda Y, et al. Delayed-Onset of Heparin InducedThrombocytopenia – A Case Report – J Jpn Assn Torca Surg. 1992;40(3):110-111.2. Warkentin T, Kelton J. Delayed-Onset Heparin-Induced Thrombocytopenia and Thrombosis.Annals of Internal Medicine. 2001;135:502-506.3. Rice L, Attisha W, Drexler A, Francis J. Delayed-Onset Heparin Induced Thrombocytopenia.Annals of Internal Medicine, 2002;136:210-215.4. Dieck, J., C. Rizo-Patron, et al. (1990). “A New Manifestation and Treatment Alternativefor Heparin-Induced Thrombosis.”. Chest 98(1524-26).5. Smythe M, Stephens J, Mattson. Delayed-Onset Heparin Induced Thrombocytopenia.Annals of Emergency Medicine, 2005;45(4):417-4196. D IVGI A.(R EPRINT),T HUMMA S.,H ARI P.,F RIEDMAN K.,D ELAYED O NSET H EPARIN- I NDUCEDT HROMBOCYTOPENIA (HIT)P RESENTING A FTER U NDOCUMENTED D RUG E XPOSURE AS P OST-A NGIOGRAPHY P ULMONARY EMBOLISM.B LOOD.2003;102(11):127B.Manufactured byBaxter Healthcare CorporationDeerfield, IL 60015 USAFor Product Inquiry 1 800 ANA DRUG (1-800-262-3784)MLT-01119/3.0HEP-LOCK (Heparin Lock Flush Solution, USP)R x onlyDESCRIPTIONHeparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α-L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose 6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2)>(1)>(4)>(3)>(5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.Structural formula of Heparin Sodium (representative sub-units):HEP-LOCK (Heparin Lock Flush Solution, USP) is a sterile solution for intravenous flush only. It is not to be used for anticoagulant therapy. Each mL contains heparin sodium 10 or 100 USP units, derived from porcine intestines and standardized for use as an anticoagulant, sodium chloride 9 mg and benzyl alcohol 0.01 mL in Water for Injection. pH 5.0-7.5; sodium hydroxide and/or hydrochloric acid used, if needed, for pH adjustment. The potency is determined by biological assay using a USP reference standard based on units of heparin activity per milligram.CLINICAL PHARMACOLOGYHeparin inhibits reactions that lead to the clotting of blood and the formation of fibrin clots bothin vitro and in vivo. Heparin acts at multiple sites in the normal coagulation system. Small amounts of heparin in combination with antithrombin III (heparin cofactor) can inhibit thrombosis by inactivating activated Factor X and inhibiting the conversion of prothrombin to thrombin. Once active thrombosis has developed, larger amounts of heparin can inhibit further coagulation by inactivating thrombin and。
甘油 USP34

Glycerin (glis' er in).C 3H 8O 392.101,2,3-Propanetriol; Glycerol [56-81-5].DEFINITIONGlycerin contains NLT 99.0% and NMT 101.0% of C 3H 8O 3, calculated on the anhydrous basis. IDENTIFICATION[N OTE —Compliance is determined by meeting the requirements for Identification tests A, B, and C . ]• A. I NFRARED A BSORPTION 197F• B. L IMIT OF D IETHYLENE G LYCOL AND E THYLENE G LYCOLStandard solution: 2.0 mg/mL of USP Glycerin RS , 0.050 mg/mL of USP Ethylene Glycol RS , 0.050 mg/mL of USP Diethylene Glycol RS , and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanolSample solution: 50 mg/mL of Glycerin and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanol Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and a deactivated split liner with glass wool TemperatureInjector: 220 Detector: 250Column: See the temperature program table.Carrier gas: Helium Injection size: 1.0 µLInitialTemperature ()FinalTemperature ()Flow rate: 4.5 mL/minInjection type: Split ratio, about 10:1 System suitabilitySample: Standard solution[N OTE —The relative retention times for ethylene glycol, 2,2,2-trichloroethanol, diethylene glycol, and glycerin are about 0.3, 0.6, 0.8 and 1.0, respectively. ] Suitability requirementsResolution: NLT 1.5 between diethylene glycol and glycerin AnalysisSample: Sample solutionAcceptance criteria: If a peak at the retention times for the diethylene glycol or ethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is NMT the peak response ratio for diethylene glycol or ethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution; NMT 0.10% each for diethylene glycol and ethylene glycol is found.• C. Examine the chromatograms obtained in Identification test B . The retention time of the glycerinpeak of the Sample solution corresponds to that obtained in the Standard solution . ASSAY• P ROCEDURESodium periodate solution: Dissolve 60 g of sodium metaperiodate in sufficient water containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat to dissolve theperiodate. If the solution is not clear, pass through a sintered-glass filter. Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, and dilute with water to volume. To 550 mg of Glycerin dissolved in 50 mL of water, add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 min, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 min, add 100 mL of water, and titrate with 0.1 N sodium thiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for the glycerin –periodate mixture to that required for the blank should be between 0.750 and 0.765.Analysis: Transfer 400 mg of Glycerin to a 600-mL beaker, dilute with 50 mL of water, addbromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, and neutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with awatch glass, and allow to stand for 30 min at room temperature (not exceeding 35) in the dark or in subdued light. Add 10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to stand for 20 min. Dilute each solution with water to 300 mL, and titrate with 0.1 N sodium hydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C 3H 8O 3.Acceptance criteria: 99.0%–101.0% on the anhydrous basis IMPURITIESInorganic Impurities• C HLORIDE AND S ULFATE , Chloride 221: A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (NMT 10 ppm). • C HLORIDE AND S ULFATE , Sulfate 221: A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (NMT 20 ppm). • H EAVY M ETALS 231Analysis: Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL. Acceptance criteria: NMT 5 ppm • R ESIDUE ON I GNITION 281: Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites,and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%). Organic Impurities• P ROCEDURE 1: R ELATED C OMPOUNDSSystem suitability solution: 0.5 mg/mL each of USP Diethylene Glycol RS and USP Glycerin RSSample solution: 50 mg/mL of Glycerin Chromatographic system(See Chromatography 621, System Suitability .) Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and an inlet liner having an inverted cup or spiral structure TemperatureInjector: 220 Detector: 250Column: See the temperature program table below.Carrier gas: Helium Injection size: 0.5 µL Linear velocity: 38 cm/sInjection type: Split ratio, about 10:1 System suitabilitySample: System suitability solution Suitability requirementsTemperature Ramp (/min)Final Temperature ()Hold Time at FinalTemperature (min)100—100—1007.52204Resolution: NLT 7.0 between diethylene glycol and glycerin AnalysisSample: Sample solutionCalculate the percentage of each impurity, excluding any solvent peaks and diethylene glycol, in the portion of Glycerin taken:Result = (r U /r T) × 100Acceptance criteriaIndividual impurities: NMT 0.1% Total impurities: NMT 1.0%• P ROCEDURE 2: L IMIT OF C HLORINATED C OMPOUNDS Sample: 5 g of GlycerinAnalysis: Transfer the Sample into a dry, round-bottom, 100-mL flask. Add 15 mL ofmorpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 h. Rinse the condenser with 10 mL of water, receiving the washings in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, and dilute with water to 50.0 mL.Acceptance criteria: The turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (NMT 30 ppm of Cl). • P ROCEDURE 3: F ATTY A CIDS AND E STERSSample solution: Mix 50 g of Glycerin with freshly boiled water and 5 mL of 0.5 N sodium hydroxide VS. Boil the mixture for 5 min, cool, and add phenolphthalein TS.Analysis: Titrate the excess alkali with 0.5 N hydrochloric acid VS. Perform a blank determination (see Titrimetry 541, Residual Titrations ).Acceptance criteria: NMT 1 mL of 0.5 N sodium hydroxide is consumed.SPECIFIC TESTS• C OLOR : When viewed downward against a white surface in a 50-mL color-comparison tube, thecolor is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin. • S PECIFIC G RAVITY 841: NLT 1.249• W ATER D ETERMINATION , Method I 921: NMT 5.0% ADDITIONAL REQUIREMENTS• P ACKAGING AND S TORAGE : Preserve in tight containers. • USP R EFERENCE S TANDARDS 11 USP D IETHYLENE G LYCOL RS USP E THYLENE G LYCOL RS USP G LYCERIN RSr U == peak response of each individual impurityfrom the Sample solutionr T== sum of the responses of all the peaks fromthe Sample solution1,2,3-Propanetriol. C 3H 8O 392.10Auxiliary Information — Please check for your question in the FAQs before contacting USP. USP34–NF29 Page 2986Pharmacopeial Forum : Volume No. 28(4) Page 1245 Chromatographic Column — GLYCERINChromatographic columns text is not derived from, and not part of, USP 34 or NF 29.。
甘油

甘油包含不低于99.0%和不超过101.0%的C3H8O3,无水基础上计算。
任何单个杂质的量,不包括二甘醇和乙二醇,如果检测,符合(NMT的0.1%),其他杂质和总杂质,包括二甘醇和乙二醇量的要求,是NMT的1.0%。
包装和储存在密封容器保存。
USP参考标准11 -药典二甘醇RS。
USP乙二醇RS。
美国药典甘油的RS。
颜色---它的颜色,对一个50毫升的色彩对比管的白色表面向下查看时,并不比按标准以50毫升水稀释的0.40 mL三氯化铁颜色暗,同样在看一个大约直径相同的含有甘油色彩对比管,颜色含有甘油。
鉴定的[注意遵守满足为A和B两个鉴定的要求确定]答:红外吸收197F。
A:1转移USP二甘醇的RS准确称量,50毫克的标准储备溶液,100 mL容量瓶,用甲醇定容稀释,拌匀。
标准储备溶液2 - 转移USP乙二醇RS准确称量,50毫克,100 mL容量瓶,用甲醇定容,混匀稀释。
标准储备溶液3 - 转移美国药典甘油准确称量的RS,50毫克,100 mL容量瓶,用甲醇定容,混匀稀释。
决议解决方案转移5.0 mL的标准储备溶液1,标准储备溶液2,3,标准储备溶液100 mL容量瓶,用甲醇定容,混匀稀释。
约5克甘油测试解决方案转移到100 mL容量瓶中,用甲醇稀释至刻度,混合。
色谱系统(见色谱621)- 气相色谱仪配备火焰离子化检测器,1 0.53毫米×30米石英色谱柱涂有3.0微米G43固定相。
进样口温度保持在220和探测器的温度保持在250。
载气,氦气是一个约4.5毫升每分钟的流量。
分流比为10:1左右。
色谱仪进行编程如下:最初,柱温是平衡的,于100 4分钟,然后在上升速度为每分钟50至120,120保持10分钟。
然后在每分钟50至220的速度增加,并保持在220 6分钟。
色谱仪分辨率的解决方案,并记录峰值响应和定向程序的保留时间为:乙二醇的相对保留时间约为0.3,0.8二甘醇和甘油1.0;和重复注射的缩二的相对标准偏差乙二醇是不超过10%。
甘油质量标准及检验操作规程
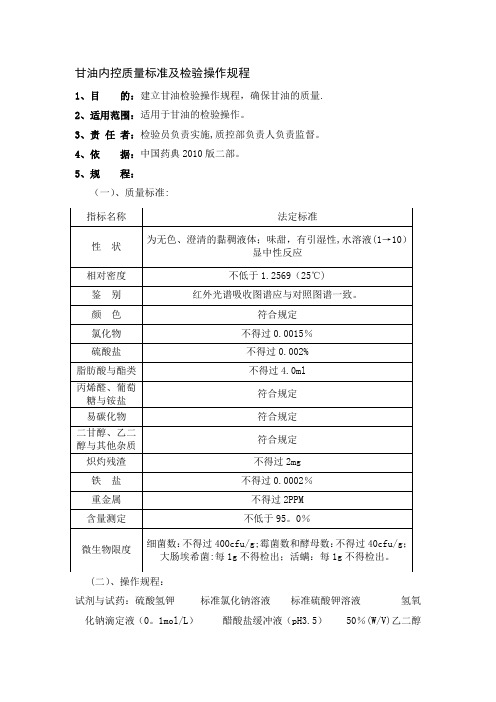
甘油内控质量标准及检验操作规程1、目的:建立甘油检验操作规程,确保甘油的质量.2、适用范围:适用于甘油的检验操作。
3、责任者:检验员负责实施,质控部负责人负责监督。
4、依据:中国药典2010版二部。
5、规程:(一)、质量标准:(二)、操作规程:试剂与试药:硫酸氢钾标准氯化钠溶液标准硫酸钾溶液氢氧化钠滴定液(0。
1mol/L)醋酸盐缓冲液(pH3.5) 50%(W/V)乙二醇溶液 2。
14%(W/V)高碘酸钠溶液仪器与用具:试管5个 50ml烧杯4个 50ml纳氏比色管1个红外光吸收仪1台电子天平1台1.性状1。
1取本品适量,目视观察为无色、澄清的黏稠液体;味甜,感觉;有引湿水溶液(1 →10)显中性反应。
1.2本品与水或乙醇能任意混溶,在丙酮中微溶,在三氯甲烷或乙醚中不溶。
1。
3相对密度本品的相对密度,在25℃时不小于1.2569。
2、鉴别:2.1本品的红外光吸收图谱应与对照的图谱(光谱集77图)一致.3.检查:3.1颜色取本品50ml,置50ml纳氏比色管中,与对照液(取比色用重铬酸钾液0.2ml,加水稀释至50ml制成)比较,不得更深。
3。
2氯化物取本品5。
0g,依法检查(附录Ⅷ A),与标准氯化钠溶液7.5ml 制成的对照液比较,不得更浓(0.0015%)。
3.3硫酸盐取本品10g,依法检查(附录Ⅷ B),与标准硫酸钾溶液2。
0ml 制成的对照液比较,不得更浓(0。
002%)。
3。
4脂肪酸与酯类取本品40g,加新沸过的冷水40ml,再精密加氢氧化钠滴定液(0.1mol/L)10ml,摇匀后,煮沸5分钟,放冷,加酚酞指示液数滴,用盐酸滴定液(0.1mol/L)滴定剩余的氢氧化钠,并将滴定的结果用空白试验校正,消耗的氢氧化钠滴定液(0。
1mol/L)不得过4。
0ml。
3。
5丙烯醛、葡萄糖与铵盐取本品5ml,加10%氢氧化钾溶液5ml,在60℃放置5分钟,不得显黄色或发生氨臭.3.6易炭化物取本品4。
甘油质量标准及检验操作规程

甘油内控质量标准及检验操作规程1、目的:建立口油检验操作规程,确保甘油的质量2、适用范围:适用于甘油的检验操作。
3、责任者:检验员负责实施,质控部负责人负责监督4、依据:中国药典2010版二部。
5、规程:(一)、质量标准:(二)、操作规程:试剂与试药:硫酸氢钾标准氯化钠溶液标准硫酸钾溶液氢氧仪器与用具:试管5个50ml烧杯4个50ml纳氏比色管1个红外光吸收仪1 台电子天平1台1.性状1・1取本品适量,目视观察为无色、澄清的黏稠液体;味甜,感觉;有引湿水溶液(1 —10)显中性反应。
1・2本品与水或乙醇能任意混溶,在丙酮中微溶,在三氯甲烷或乙醍中不溶。
1・3相对密度本品的相对密度,在25E时不小于1.2569。
2.鉴别:2.1本品的红外光吸收图谱应与对照的图谱(光谱集77图)一致。
3.检查:3. 1颜色取本品50ml,置50ml纳氏比色管中,与对照液(取比色用重銘酸钾液0. 2ml,加水稀释至50ml制成)比较,不得更深。
3.2氯化物取本品5. 0g,依法检查(附录毗A),与标准氯化钠溶液7. 5ml制成的对照液比较,不得更浓(0. 0015%) o3.3硫酸盐取本品10g,依法检查(附录毗B ),与标准硫酸钾溶液2. 0ml制成的对照液比较,不得更浓(0. 002%) o3. 4脂肪酸与酯类取本品40g,加新沸过的冷水40ml,再精密加氢氧化钠滴定液(0・lmol/L )10ml,摇匀后,煮沸5分钟,放冷,加酚瞅指示液数滴,用盐酸滴定液(0・lmol/L )滴定剩余的氢氧化钠,并将滴定的结果用空白试验校正,消耗的氢氧化钠滴定液(0. lmol/L )不得过 4.0ml。
3.5丙烯醛、葡萄糖与鞍盐取本品5ml,加10%K氧化钾溶液5ml,在60C放置5分钟,不得显黄色或发生氨臭。
3.6易炭化物取本品4.0引照易炭化物检查法(附录毗0)项下方法检查,静置时间为1小时,如显色,与对照溶液(取比色用氯化钻溶液0・2ml、比色用重銘酸钾溶液l・6ml与水8・2ml制成)比较,不得更深。
医药级甘油企业标准

医药级甘油企业标准
医药级甘油是一种常用的药用辅料,在医药和个人护理产品中被广泛使用。
医药级甘油必须符合一定的质量标准,根据不同国家或地区的法规和标准,医药级甘油的生产和质量标准可能会有所不同。
以下是一般情况下医药级甘油的一些可能的标准:
1. 纯度要求:医药级甘油的纯度通常要求在99.5%以上,含水量、酸度、残留溶剂等杂质的含量有严格规定。
2. 重金属和有害物质的含量:医药级甘油必须符合严格的重金属和有害物质含量标准,如铅、砷、汞等。
3. 质量控制:生产企业需要建立健全的质量管理体系,并符合相关的GMP(Good Manufacturing Practice)或药典要求,确保产品的质量稳定和可追溯性。
4. 包装标准:医药级甘油的包装必须符合相关的卫生标准,包装材料应当符合药品包装的要求。
5. 生产环境标准:生产企业的生产环境必须符合相关的
卫生和安全标准,保证产品不受污染。
需要注意的是,具体的医药级甘油标准可能会有所不同,取决于国家法规和药典要求。
生产医药级甘油的企业需要遵守当地的法规、标准和规范,确保产品的质量和安全性。
美国USP标准 甘油检测操作规程

1目的1.1 通过对所采购药用辅料甘油各项质量标准的检测,确定其自身安全性。
1.2 通过对所采购药用辅料甘油各项质量标准的检测,确定是否影响产品生产、产品质量、产品的安全性和有效性。
2 适用范围适用于本公司用于生产液体棉签所采购的药用辅料甘油。
3 责任者:质量部经理化验员4引用标准:中华人民共和国药典 2010年版二部美国药典 USP5包装与贮存要求:保存在密闭容器6 操作6.1鉴别(符合红外吸收197 F和6.1.2的鉴别反应)6.1.1 所需仪器、试剂:气象色谱仪、USP二甘醇(对照品)、USP乙二醇(对照品)、USP 甘油(对照品)、甲醇、氦气6.1.26.1.2.1 标准储存溶液1:准确称量50mg的USP二甘醇(对照品),用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.2 标准储存溶液2:准确称量50mg的USP乙二醇(对照品),用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.3标准储存溶液3:准确称量50mg的USP甘油(对照品),用甲醇溶解并稀释到100ml 容量瓶中。
6.1.2.4解决方案---把每种储存溶液中各取5毫升,放入100毫升的容量瓶中,用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.5测试溶液---取5g甘油,加入到100毫升的容量瓶中,用甲醇溶解并稀释到100ml 容量瓶中。
6.1.2.6色谱系统(见色谱621)--- 在气相色谱仪配备一个火焰离子化检测器,一个0.53毫米×30 m熔融石英分析柱涂有3.0 -µm G43固定相。
注射口温度保持在220和检测器温度保持在250。
载气是氦气,流率约为每分钟4.5毫升。
分流比相当于10:1。
色谱程序,如下所示:最初,柱温是在100,保持4分钟,然后温度以50的比率增加到120,保持10分钟。
然后温度再以50的比率增加到220,保持6分钟。
色谱仪拆分溶液,并记录峰值响应和保留时间。
他相对保留时间约为乙二醇0.3 ,二甘醇0.8,甘油1.0;复制注射的二甘醇的相对标准偏差为不会超过10%。
甘油USP36

Glycerin(glis' er in).C 3H 8O 392.09 1,2,3-Propanetriol;Glycerol [56-81-5].DEFINITIONGlycerin contains NLT 99.0% and NMT 101.0% of C 3H 8O 3, calculated on the anhydrous basis.IDENTIFICATION[NOTE—Compliance is determined by meeting the requirements for Identification tests A, B, and C . ]• A. I NFRARED A BSORPTION 197F• B. L IMIT OF D IETHYLENE G LYCOL AND E THYLENE G LYCOLStandard solution: 2.0 mg/mL of USP Glycerin RS, 0.050 mg/mL of USP Ethylene Glycol RS, 0.050 mg/mL of USP Diethylene Glycol RS, and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanolSample solution: 50 mg/mL of Glycerin and 0.10 mg/mL of 2,2,2-trichloroethanol (internal standard) in methanolChromatographic system(See Chromatography 621, System Suitability .)Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase, and a deactivated split liner with glass woolTemperatureInjector: 220Detector: 250Column:See the temperature program table.Initial Temperature ()Temperature Ramp (/min)Final Temperature ()Hold Time at Final Temperature (min)100—10041005012010120502206Carrier gas: HeliumInjection size: 1.0 µLFlow rate: 4.5 mL/minInjection type: Split ratio, about 10:1System suitabilitySample: Standard solution[NOTE—The relative retention times for ethylene glycol, 2,2,2-trichloroethanol,diethylene glycol, and glycerin are about 0.3, 0.6, 0.8 and 1.0, respectively. ]Suitability requirementsResolution: NLT 1.5 between diethylene glycol and glycerinAnalysisSample: Sample solutionAcceptance criteria: If a peak at the retention times for the diethylene glycol orethylene glycol is present in the Sample solution, the peak response ratio relative to 2,2,2-trichloroethanol is NMT the peak response ratio for diethylene glycol orethylene glycol relative to 2,2,2-trichloroethanol in the Standard solution; NMT 0.10% each for diethylene glycol and ethylene glycol is found.• C. Examine the chromatograms obtained in Identification test B. The retention time of the glycerin peak of the Sample solution corresponds to that obtained in theStandard solution.ASSAY• P ROCEDURESodium periodate solution: Dissolve 60 g of sodium metaperiodate in sufficientwater containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat todissolve the periodate. If the solution is not clear, pass through a sintered-glass filter.Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, and dilute with water to volume. To 550 mg of Glycerin dissolved in 50 mL of water, add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 min, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 min, add 100 mL of water, and titrate with 0.1 N sodiumthiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for theglycerin–periodate mixture to that required for the blank should be between 0.750 and 0.765.Analysis: Transfer 400 mg of Glycerin to a 600-mL beaker, dilute with 50 mL ofwater, add bromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, andneutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with a watch glass, and allow to stand for30 min at room temperature (not exceeding 35) in the dark or in subdued light. Add10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to standfor 20 min. Dilute each solution with water to 300 mL, and titrate with 0.1 N sodium hydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C3H8O3.Acceptance criteria: 99.0%–101.0% on the anhydrous basisIMPURITIESInorganic Impurities•C HLORIDE AND S ULFATE, Chloride221: A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (NMT 10 ppm).•C HLORIDE AND S ULFATE, Sulfate221: A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (NMT 20 ppm).•H EAVY M ETALS231Analysis: Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL.Acceptance criteria: NMT 5 ppm•R ESIDUE ON I GNITION281: Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites, and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%).Organic Impurities• P ROCEDURE 1: R ELATED C OMPOUNDSSystem suitability solution: 0.5 mg/mL each of USP Diethylene Glycol RS and USP Glycerin RSSample solution: 50 mg/mL of GlycerinChromatographic system(See Chromatography 621, System Suitability.)Mode: GCDetector: Flame ionizationColumn: 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43stationary phase, and an inlet liner having an inverted cup or spiral structureTemperatureInjector: 220Detector: 250Column: See the temperature program table below.Carrier gas: HeliumInjection size: 0.5 µLLinear velocity: 38 cm/sInjection type: Split ratio, about 10:1System suitabilitySample: System suitability solution Suitability requirementsResolution: NLT 7.0 between diethylene glycol and glycerinAnalysisSample: Sample solutionCalculate the percentage of each impurity, excluding any solvent peaks anddiethylene glycol, in the portion of Glycerin taken:Result = (r U /r T) × 100 Acceptance criteriaIndividual impurities: NMT 0.1%Total impurities: NMT 1.0%• P ROCEDURE 2: L IMIT OF C HLORINATED C OMPOUNDSSample: 5 g of GlycerinAnalysis: Transfer the Sample into a dry, round-bottom, 100-mL flask. Add 15 mL of morpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 h. Rinse the condenser with 10 mL of water, receiving the washings in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, and dilute with water to 50.0 mL. Acceptance criteria: The turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (NMT 30 ppm of Cl).• P ROCEDURE 3: F ATTY A CIDS AND E STERSSample solution: Mix 50 g of Glycerin with 50 mL of freshly boiled water and 5 mLInitial Temperature ()Temperature Ramp (/min)Final Temperature ()Hold Time at Final Temperature (min)100—100—1007.52204r U== peak response of each individual impurity from the Sample solution r T == sum of the responses of all the peaks from the Sample solutionof 0.5 N sodium hydroxide VS. Boil the mixture for 5 min, cool, and addphenolphthalein TS.Analysis: Titrate the excess alkali with 0.5 N hydrochloric acid VS. Perform a blank determination (see Titrimetry 541, Residual Titrations ).Acceptance criteria: NMT 1 mL of 0.5 N sodium hydroxide is consumed.SPECIFIC TESTS• C OLOR : When viewed downward against a white surface in a 50-mL color-comparisontube, the color is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin . • S PECIFIC G RAVITY 841: NLT 1.249• W ATER D ETERMINATION , Method I 921: NMT 5.0%ADDITIONAL REQUIREMENTS• P ACKAGING AND S TORAGE : Preserve in tight containers.• USP R EFERENCE S TANDARDS 11USP Diethylene Glycol RSUSP Ethylene Glycol RSUSP Glycerin RS1,2,3-Propanetriol.C 3H 8O 392.10 Auxiliary Information— Please check for your question in the FAQs before contactingUSP. USP36–NF31 Page 3749Pharmacopeial Forum : Volume No. 28(4) Page 1245 Chromatographic Column—GLYCERINChromatographic columns text is not derived from, and not part of, USP 36 or NF 31.Topic/QuestionContact Expert Committee Monograph Kevin T. Moore, Ph.D. Senior Scientific Liaison 1-301-816-8369(EXC2010) Monographs - Excipients Reference Standards RS Technical Services 1-301-816-8129 rstech@。
USP 美国药典 甘油 Glycerin
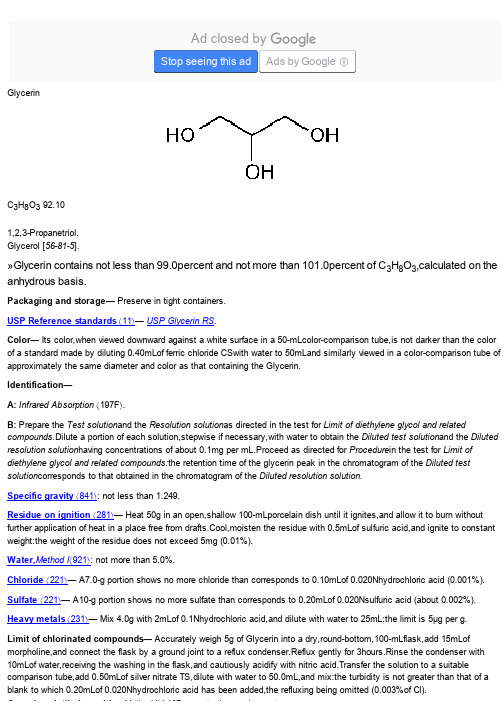
Glycerin C 3H 8O 3 92.101,2,3-Propanetriol.Glycerol [56-81-5].»Glycerin contains not less than 99.0percent and not more than 101.0percent of C 3H 8O 3,calculated on the anhydrous basis.Packaging and storage— Preserve in tight containers.USP Reference standards á11ñ— USP Glycerin RS .Color— Its color,when viewed downward against a white surface in a 50-mLcolor-comparison tube,is not darker than the color of a standard made by diluting 0.40mLof ferric chloride CSwith water to 50mLand similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin.Identification—A: Infrared Absorption á197F ñ.B: Prepare the Test solution and the Resolution solution as directed in the test for Limit of diethylene glycol and relatedcompounds .Dilute a portion of each solution,stepwise if necessary,with water to obtain the Diluted test solution and the Diluted resolution solution having concentrations of about 0.1mg per mL.Proceed as directed for Procedure in the test for Limit of diethylene glycol and related compounds:the retention time of the glycerin peak in the chromatogram of the Diluted test solution corresponds to that obtained in the chromatogram of the Diluted resolution solution.Specific gravity á841ñ: not less than 1.249.Residue on ignition á281ñ— Heat 50g in an open,shallow 100-mLporcelain dish until it ignites,and allow it to burn without further application of heat in a place free from drafts.Cool,moisten the residue with 0.5mLof sulfuric acid,and ignite to constant weight:the weight of the residue does not exceed 5mg (0.01%).Water,Method I á921ñ: not more than 5.0%.Chloride á221ñ— A7.0-g portion shows no more chloride than corresponds to 0.10mLof 0.020Nhydrochloric acid (0.001%).Sulfate á221ñ— A10-g portion shows no more sulfate than corresponds to 0.20mLof 0.020Nsulfuric acid (about 0.002%).Heavy metals á231ñ— Mix 4.0g with 2mLof 0.1Nhydrochloric acid,and dilute with water to 25mL:the limit is 5µg per g.Limit of chlorinated compounds— Accurately weigh 5g of Glycerin into a dry,round-bottom,100-mLflask,add 15mLof morpholine,and connect the flask by a ground joint to a reflux condenser.Reflux gently for 3hours.Rinse the condenser with 10mLof water,receiving the washing in the flask,and cautiously acidify with nitric acid.Transfer the solution to a suitablecomparison tube,add 0.50mLof silver nitrate TS,dilute with water to 50.0mL,and mix:the turbidity is not greater than that of a blank to which 0.20mLof 0.020Nhydrochloric acid has been added,the refluxing being omitted (0.003%of Cl).Organic volatile impurities,Method IV á467ñ: meets the requirements.Ad closed byStop seeing this adAds by GoogleOrganic volatile impurities,Method IVá467ñ: meets the requirements.Fatty acids and esters— Mix 50g of Glycerin with 50mLof freshly boiled water and 5mLof 0.5Nsodium hydroxide VS,boil the mixture for 5minutes,cool,add phenolphthalein TS,and titrate the excess alkali with 0.5Nhydrochloric acid VS.Perform a blank determination (see Residual Titrations under Titrimetry á541ñ):not more than 1mLof 0.5Nsodium hydroxide is consumed.Limit of diethylene glycol and related compounds—Resolution solution— Dissolve accurately weighed quantities of diethylene glycol and USP Glycerin RS in water,and dilute quantitatively,and stepwise if necessary,with water to obtain a solution having a known concentration of about 0.5mg of each per mL.Standard solution— Dissolve an accurately weighed quantity of diethylene glycol in water,and dilute quantitatively,and stepwise if necessary,with water to obtain a solution having a known concentration of about 0.05mg per mL.Test solution— Transfer 5g of Glycerin,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.Chromatographic system (see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector,a 0.53-mm ×30-m fused-silica analytical column coated with 3.0-µm G43stationary phase,and an inlet liner having an invertedcup or spiral structure.The chromatograph is programmed as follows.Initially,the column temperature is equilibrated at 100until the time of injection,is increased at a rate of 7.5per minute to 220,and is maintained at 220for 4minutes.The injection porttemperature is maintained at 220,and the detector temperature is maintained at 250.The carrier gas is helium.The split flow ratio is about 10:1,and the linear flow is maintained at about 38cm per second.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the resolution,R,between diethylene glycol and glycerin is not less than 7.0.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 15%.Procedure— Separately inject equal volumes (about 0.5µL)of the Standard solution and the Test solution into the chromatograph,record the chromatograms,and measure the responses for all the peaks.Calculate the percentage of diethylene glycol in the portion of Glycerin taken by the formula:100(C S/C U)(r U/r S),in which C S is the concentration,in mg per mL,of diethylene glycol in the Standard solution;C U is the concentration,in mg per mL,of Glycerin in the Test solution;and r U and r S are the peak responses for diethylene glycol obtained from the Testsolution and the Standard solution,respectively:not more than 0.1%is found.Calculate the percentage of each otherimpurity,excluding any solvent peaks,in the portion of Glycerin taken by the formula:100(r i/r s),in which r i is the peak response of each individual impurity obtained from the Test solution;and r s is the sum of the responses of all the peaks obtained from the Test solution:not more than 0.1%of any individual impurity,excluding diethylene glycol,is found;and not more than 1.0%of total impurities,including diethylene glycol,is found.Assay—Sodium periodate solution— Dissolve 60g of sodium metaperiodate in sufficient water containing 120mLof 0.1Nsulfuric acid to make 1000mL.Do not heat to dissolve the periodate.If the solution is not clear,pass through a sintered-glass filter.Store the solution in a glass-stoppered,light-resistant container.Test the suitability of this solution as follows.Pipet 10mLinto a 250-mLvolumetric flask,dilute with water to volume,and mix.To about 550mg of Glycerin dissolved in 50mLof water add 50mLof the diluted periodate solution with a pipet.For a blank,pipet 50mLof the solution into a flask containing 50mLof water.Allow the solutions to stand for 30minutes,then to each add 5mLof hydrochloric acid and 10mLof potassium iodide TS,and rotate to mix.Allow to stand for 5minutes,add 100mLof water,and titrate with 0.1Nsodium thiosulfate,shaking continuously and adding3mLof starch TSas the endpoint is approached.The ratio of the volume of 0.1Nsodium thiosulfate required for the glycerin–periodate mixture to that required for the blank should be between 0.750and 0.765.Procedure— Transfer about 400mg of Glycerin,accurately weighed,to a 600-mLbeaker,dilute with 50mLof water,add bromothymol blue TS,and acidify with 0.2Nsulfuric acid to a definite green or greenish yellow color.Neutralize with 0.05Nsodium hydroxide to a definite blue endpoint,free from green color.Prepare a blank containing 50mLof water,and neutralize in the samehydroxide to a definite blue endpoint,free from green color.Prepare a blank containing 50mLof water,and neutralize in the same manner.Pipet 50mLof the Sodium periodate solution into each beaker,mix by swirling gently,cover with a watch glass,and allowto stand for 30minutes at room temperature (not exceeding 35)in the dark or in subdued light.Add 10mLof a mixture of equal volumes of ethylene glycol and water,and allow to stand for 20minutes.Dilute each solution with water to about 300mL,and titrate with 0.1Nsodium hydroxide VSto a pHof 8.1±0.1for the specimen under assay and 6.5±0.1for the blank,using a pHmeter.Each mLof 0.1Nsodium hydroxide,after correction for the blank,is equivalent to 9.210mg of C3H8O3.Auxiliary Information—Staff Liaison:Justin Lane,B.S.,Scientific AssociateExpert Committee:(EMC)Excipients:Monograph ContentUSP28–NF23Page 911Pharmacopeial Forum:Volume No.28(4)Page 1245Phone Number:1-301-816-8323。
注射用甘油药典标准

甘油(供注射用)G a n y o u (G o n g z h u s h e y o n g)G l y c e r o l f o r I n je c t i on C 3H 8O 392.09[56-81-5]本品为1,2,3-丙三醇㊂按无水物计算,含C 3H 8O 3不得少于98.0%㊂ʌ性状ɔ本品为无色㊁澄清的黏稠液体;味甜;有引湿性;水溶液(1ң10)显中性㊂本品与水或乙醇能任意混溶,在丙酮中微溶,在三氯甲烷或乙醚中均不溶相对密度 本品的相对密度(通则0601)在25ħ时不小于1.257㊂折光率 本品的折光率(通则0622)应为1.470~1.475㊂ʌ鉴别ɔ本品的红外光吸收图谱应与对照的图谱(光谱集77图)一致(通则0402﹏﹏﹏﹏﹏)㊂ʌ检查ɔ酸碱度 取本品25.0g ,加水稀释成50m l ,混匀,加酚酞指示液0.5m l ,溶液应无色,加0.1m o l /L 氢氧化钠溶液0.2m l ,溶液应显粉红色㊂颜色 取本品50m l ,置50m l 纳氏比色管中,与对照液(取比色用重铬酸钾液0.2m l ,加水稀释至50m l 制成)比较,不得更深㊂氯化物 取本品5.0g,依法检查(通则0801),与标准氯化钠溶液3.0m l 制成的对照液比较,不得更浓(0.0006%)㊂硫酸盐 取本品10g,依法检查(通则0802),与标准硫酸钾溶液2.0m l 制成的对照液比较,不得更浓(0.002%)㊂醛与还原性物质 取本品约1g ,置50m l 量瓶中,加水25m l 溶解,加入﹏﹏10%﹏﹏﹏﹏新配制的盐酸甲基苯并噻唑酮腙溶液(1g /L ﹏﹏,用0.02m o l /L 的氢氧化钠溶液调节p H 至4.0㊂﹏﹏临用新制﹏﹏﹏㊂)2m l 静置30分钟㊂加新配制的0.5%三氯化铁溶液5m l ,摇匀,静置5分钟,﹏加﹏用甲醇稀释至刻度,摇匀㊂照紫外-可见分光光度法(通则0401),在655n m 的波长处测定吸光度,供试品溶液的吸光度不得大于对照品溶液[每1m l 含甲醛(C H 2O )5.0μg ]2.0m l 同法处理后的吸光度㊂糖 取本品5.0g ,加水5m l ,混匀,加稀硫酸1m l ,置水浴上加热5分钟,加不含碳酸盐的2m o l /L 氢氯化钠溶液3m l ,滴加硫酸铜试液1m l ,混匀,应为蓝色澄清溶液,继续在水浴上加热5分钟,溶液应仍为蓝色,无沉淀产生㊂㊃594㊃甘油(供注射用)甘油(供注射用)脂肪酸与脂类取本品40g,加新沸的冷水40m l,再精密加氢氧化钠滴定液(0.1m o l/L)10m l,摇匀,煮沸5分钟,放冷,加酚酞指示液数滴,用盐酸滴定液(0.1m o l/L)滴定至红色消失,并将滴定的结果用空白试验校正㊂消耗的氢氧化钠滴定液(0.1m o l/L)不得过2.0m l㊂易炭化物取本品约6.3g,在振摇下逐滴加入硫酸5m l,过程中控制温度不得超过20ħ,静置1小时后,如显色,与同体积对照溶液(取比色用氯化钴溶液0.2m l,比色用重铬酸钾溶液1.6m l与水8.2m l制成)比较,不得更深㊂有关物质取本品约10g,精密称定,置25m l量瓶中,精密加入内标溶液(每1m l中含0.5m g正己醇的甲醇溶液)5m l,用甲醇溶解并稀释至刻度,作为供试品溶液㊂取二甘醇㊁乙二醇㊁1,2-丙二醇适量,精密称定,用甲醇溶解并稀释制成每1m l中含二甘醇㊁乙二醇㊁1,2-丙二醇各0.5m g的溶液,﹏精密置﹏量取5m l,置25m l量瓶中,精密加入内标溶液5m l,用甲醇稀释至刻度,作为对照品溶液㊂另取二甘醇,乙二醇㊁1,2-丙二醇㊁正己醇和甘油适量,精密称定,用甲醇溶解并稀释制成每1m l中含甘油400m g,二甘醇㊁乙二醇㊁1,2-丙二醇㊁正己醇各0.1m g的溶液,﹏﹏作为系统适用性试验溶液㊂照气相色谱法(通则0521),用6%氰丙基苯基-94%二甲基聚硅氧烷为固定液(或极性相近的固定液)的毛细管柱,程序升温,起始温度为100ħ,维持4分钟,以每分钟50ħ的速率升温至120ħ,维持10分钟,再以每分钟50ħ的速率升温至220ħ,维持20分钟;进样口温度为200ħ,检测器温度为250ħ,色谱图记录时间至少为主峰保留时间的两倍㊂取系统适用性试验溶液1μl,注入气相色谱仪,记录色谱图,各组分色谱峰之间的分离度应符合要求㊂取对照品溶液重复进样,二甘醇和乙二醇峰面积与内标峰面积比值的相对标准偏差均不得大于5%㊂﹏﹏依次精密量取供试品溶液和对照品溶液各1μl,注入气相色谱仪,记录色谱图,按内标法以峰面积计算,供试品中含二甘醇㊁乙二醇均不得过0.025%;含1,2-丙二醇不得过0.1%;如有其他杂质峰,扣除内标峰按面积归一化法计算,单个未知杂质不得过0.1%;杂质总量(包含二甘醇㊁乙二醇和1,2-丙二醇)不得过0.5%㊂水分取本品,照水分测定法(通则0832第一法A)测定,含水分不得过2.0%㊂炽灼残渣取本品20.0g,加热至自燃,停止加热,待燃烧完毕,放冷,依法检查(通则0841),遗留残渣不得过2m g㊂铵盐取本品4.0g,加10%氢氧化钾溶液5m l,混匀,在60ħ放置5分钟,不得发生氨臭㊂铁盐取本品20.0g,依法检查(通则0807)与标准铁溶液1.0m l制成的对照液比较,不得更深(0.00005%)㊂重金属取本品5.0g,依法检查(通则0821第一法),含重金属不得过百万分之二㊂砷盐取本品6.65g,加水23m l和盐酸5m l混匀,依㊃694㊃法检查(通则0822第一法),应符合规定(0.00003%)㊂微生物限度 取本品,依法检查(中国药典2010﹏﹏﹏﹏﹏﹏﹏﹏年版二部附录Ⅺ﹏﹏﹏﹏﹏J 通则1105㊁﹏﹏﹏﹏﹏﹏﹏1106),每1g ﹏﹏供试品中细菌﹏﹏﹏需氧菌总数不得过1000﹏个﹏c f u ,﹏﹏霉菌和酵母菌总数不得过100﹏个﹏c f u ,不得检出大肠埃希菌㊂细菌内毒素 取本品,依法检查(通则1143),每1g甘油(供注射用)中含细菌内毒素的量应小于10E U ㊂无菌(﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏供无除菌工艺的无菌制剂用)(﹏﹏﹏﹏﹏﹏﹏适用于非终端灭菌,进行本项目检查则不再检查微生物限度﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏) 取本品,依法检查(通则1101),应符合规定㊂ʌ含量测定ɔ取本品约0.1g ,精密称定,加水45m l ,混匀,精密加入2.14%(g /m l ﹏﹏﹏)高碘酸钠溶液25m l ,摇匀,暗处放置15分钟后,加50%(g /m l )乙二醇溶液5m l ,摇匀,暗处放置20分钟,加酚酞指示液0.5m l ,用氢氧化钠滴定液(0.1m o l /L )滴定至红色,30秒内不褪色,并将滴定的结果用空白试验校正㊂每1m l 氢氧化钠滴定液(0.1m o l /L )相当于9.21m g 的C 3H 8O 3㊂ʌ类别ɔ药用辅料,溶剂和助悬剂等㊂ʌ贮藏ɔ密封,在干燥处保存㊂ʌ注意ɔ本品可与硼酸形成复合物,过热会分解放出有毒的丙烯醛;与强氧化剂共研可能爆炸,受光照或与碱式硝酸铋㊁氧化剂接触会变黑㊂㊃794㊃甘油(供注射用)。
美国USP标准 甘油检测操作规程

1目的1.1 通过对所采购药用辅料甘油各项质量标准的检测,确定其自身安全性。
1.2 通过对所采购药用辅料甘油各项质量标准的检测,确定是否影响产品生产、产品质量、产品的安全性和有效性。
2 适用范围适用于本公司用于生产液体棉签所采购的药用辅料甘油。
3 责任者:质量部经理化验员4引用标准:中华人民共和国药典 2010年版二部美国药典 USP5包装与贮存要求:保存在密闭容器6 操作6.1鉴别(符合红外吸收197 F和6.1.2的鉴别反应)6.1.1 所需仪器、试剂:气象色谱仪、USP二甘醇(对照品)、USP乙二醇(对照品)、USP 甘油(对照品)、甲醇、氦气6.1.26.1.2.1 标准储存溶液1:准确称量50mg的USP二甘醇(对照品),用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.2 标准储存溶液2:准确称量50mg的USP乙二醇(对照品),用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.3标准储存溶液3:准确称量50mg的USP甘油(对照品),用甲醇溶解并稀释到100ml 容量瓶中。
6.1.2.4解决方案---把每种储存溶液中各取5毫升,放入100毫升的容量瓶中,用甲醇溶解并稀释到100ml容量瓶中。
6.1.2.5测试溶液---取5g甘油,加入到100毫升的容量瓶中,用甲醇溶解并稀释到100ml 容量瓶中。
6.1.2.6色谱系统(见色谱621)--- 在气相色谱仪配备一个火焰离子化检测器,一个0.53毫米×30 m熔融石英分析柱涂有3.0 -µm G43固定相。
注射口温度保持在220和检测器温度保持在250。
载气是氦气,流率约为每分钟4.5毫升。
分流比相当于10:1。
色谱程序,如下所示:最初,柱温是在100,保持4分钟,然后温度以50的比率增加到120,保持10分钟。
然后温度再以50的比率增加到220,保持6分钟。
色谱仪拆分溶液,并记录峰值响应和保留时间。
他相对保留时间约为乙二醇0.3 ,二甘醇0.8,甘油1.0;复制注射的二甘醇的相对标准偏差为不会超过10%。
注射用甘油药典标准

甘油(供注射用)G a n y o u (G o n g z h u s h e y o n g)G l y c e r o l f o r I n je c t i on C 3H 8O 392.09[56-81-5]本品为1,2,3-丙三醇㊂按无水物计算,含C 3H 8O 3不得少于98.0%㊂ʌ性状ɔ本品为无色㊁澄清的黏稠液体;味甜;有引湿性;水溶液(1ң10)显中性㊂本品与水或乙醇能任意混溶,在丙酮中微溶,在三氯甲烷或乙醚中均不溶相对密度 本品的相对密度(通则0601)在25ħ时不小于1.257㊂折光率 本品的折光率(通则0622)应为1.470~1.475㊂ʌ鉴别ɔ本品的红外光吸收图谱应与对照的图谱(光谱集77图)一致(通则0402﹏﹏﹏﹏﹏)㊂ʌ检查ɔ酸碱度 取本品25.0g ,加水稀释成50m l ,混匀,加酚酞指示液0.5m l ,溶液应无色,加0.1m o l /L 氢氧化钠溶液0.2m l ,溶液应显粉红色㊂颜色 取本品50m l ,置50m l 纳氏比色管中,与对照液(取比色用重铬酸钾液0.2m l ,加水稀释至50m l 制成)比较,不得更深㊂氯化物 取本品5.0g,依法检查(通则0801),与标准氯化钠溶液3.0m l 制成的对照液比较,不得更浓(0.0006%)㊂硫酸盐 取本品10g,依法检查(通则0802),与标准硫酸钾溶液2.0m l 制成的对照液比较,不得更浓(0.002%)㊂醛与还原性物质 取本品约1g ,置50m l 量瓶中,加水25m l 溶解,加入﹏﹏10%﹏﹏﹏﹏新配制的盐酸甲基苯并噻唑酮腙溶液(1g /L ﹏﹏,用0.02m o l /L 的氢氧化钠溶液调节p H 至4.0㊂﹏﹏临用新制﹏﹏﹏㊂)2m l 静置30分钟㊂加新配制的0.5%三氯化铁溶液5m l ,摇匀,静置5分钟,﹏加﹏用甲醇稀释至刻度,摇匀㊂照紫外-可见分光光度法(通则0401),在655n m 的波长处测定吸光度,供试品溶液的吸光度不得大于对照品溶液[每1m l 含甲醛(C H 2O )5.0μg ]2.0m l 同法处理后的吸光度㊂糖 取本品5.0g ,加水5m l ,混匀,加稀硫酸1m l ,置水浴上加热5分钟,加不含碳酸盐的2m o l /L 氢氯化钠溶液3m l ,滴加硫酸铜试液1m l ,混匀,应为蓝色澄清溶液,继续在水浴上加热5分钟,溶液应仍为蓝色,无沉淀产生㊂㊃594㊃甘油(供注射用)甘油(供注射用)脂肪酸与脂类取本品40g,加新沸的冷水40m l,再精密加氢氧化钠滴定液(0.1m o l/L)10m l,摇匀,煮沸5分钟,放冷,加酚酞指示液数滴,用盐酸滴定液(0.1m o l/L)滴定至红色消失,并将滴定的结果用空白试验校正㊂消耗的氢氧化钠滴定液(0.1m o l/L)不得过2.0m l㊂易炭化物取本品约6.3g,在振摇下逐滴加入硫酸5m l,过程中控制温度不得超过20ħ,静置1小时后,如显色,与同体积对照溶液(取比色用氯化钴溶液0.2m l,比色用重铬酸钾溶液1.6m l与水8.2m l制成)比较,不得更深㊂有关物质取本品约10g,精密称定,置25m l量瓶中,精密加入内标溶液(每1m l中含0.5m g正己醇的甲醇溶液)5m l,用甲醇溶解并稀释至刻度,作为供试品溶液㊂取二甘醇㊁乙二醇㊁1,2-丙二醇适量,精密称定,用甲醇溶解并稀释制成每1m l中含二甘醇㊁乙二醇㊁1,2-丙二醇各0.5m g的溶液,﹏精密置﹏量取5m l,置25m l量瓶中,精密加入内标溶液5m l,用甲醇稀释至刻度,作为对照品溶液㊂另取二甘醇,乙二醇㊁1,2-丙二醇㊁正己醇和甘油适量,精密称定,用甲醇溶解并稀释制成每1m l中含甘油400m g,二甘醇㊁乙二醇㊁1,2-丙二醇㊁正己醇各0.1m g的溶液,﹏﹏作为系统适用性试验溶液㊂照气相色谱法(通则0521),用6%氰丙基苯基-94%二甲基聚硅氧烷为固定液(或极性相近的固定液)的毛细管柱,程序升温,起始温度为100ħ,维持4分钟,以每分钟50ħ的速率升温至120ħ,维持10分钟,再以每分钟50ħ的速率升温至220ħ,维持20分钟;进样口温度为200ħ,检测器温度为250ħ,色谱图记录时间至少为主峰保留时间的两倍㊂取系统适用性试验溶液1μl,注入气相色谱仪,记录色谱图,各组分色谱峰之间的分离度应符合要求㊂取对照品溶液重复进样,二甘醇和乙二醇峰面积与内标峰面积比值的相对标准偏差均不得大于5%㊂﹏﹏依次精密量取供试品溶液和对照品溶液各1μl,注入气相色谱仪,记录色谱图,按内标法以峰面积计算,供试品中含二甘醇㊁乙二醇均不得过0.025%;含1,2-丙二醇不得过0.1%;如有其他杂质峰,扣除内标峰按面积归一化法计算,单个未知杂质不得过0.1%;杂质总量(包含二甘醇㊁乙二醇和1,2-丙二醇)不得过0.5%㊂水分取本品,照水分测定法(通则0832第一法A)测定,含水分不得过2.0%㊂炽灼残渣取本品20.0g,加热至自燃,停止加热,待燃烧完毕,放冷,依法检查(通则0841),遗留残渣不得过2m g㊂铵盐取本品4.0g,加10%氢氧化钾溶液5m l,混匀,在60ħ放置5分钟,不得发生氨臭㊂铁盐取本品20.0g,依法检查(通则0807)与标准铁溶液1.0m l制成的对照液比较,不得更深(0.00005%)㊂重金属取本品5.0g,依法检查(通则0821第一法),含重金属不得过百万分之二㊂砷盐取本品6.65g,加水23m l和盐酸5m l混匀,依㊃694㊃法检查(通则0822第一法),应符合规定(0.00003%)㊂微生物限度 取本品,依法检查(中国药典2010﹏﹏﹏﹏﹏﹏﹏﹏年版二部附录Ⅺ﹏﹏﹏﹏﹏J 通则1105㊁﹏﹏﹏﹏﹏﹏﹏1106),每1g ﹏﹏供试品中细菌﹏﹏﹏需氧菌总数不得过1000﹏个﹏c f u ,﹏﹏霉菌和酵母菌总数不得过100﹏个﹏c f u ,不得检出大肠埃希菌㊂细菌内毒素 取本品,依法检查(通则1143),每1g甘油(供注射用)中含细菌内毒素的量应小于10E U ㊂无菌(﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏供无除菌工艺的无菌制剂用)(﹏﹏﹏﹏﹏﹏﹏适用于非终端灭菌,进行本项目检查则不再检查微生物限度﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏﹏) 取本品,依法检查(通则1101),应符合规定㊂ʌ含量测定ɔ取本品约0.1g ,精密称定,加水45m l ,混匀,精密加入2.14%(g /m l ﹏﹏﹏)高碘酸钠溶液25m l ,摇匀,暗处放置15分钟后,加50%(g /m l )乙二醇溶液5m l ,摇匀,暗处放置20分钟,加酚酞指示液0.5m l ,用氢氧化钠滴定液(0.1m o l /L )滴定至红色,30秒内不褪色,并将滴定的结果用空白试验校正㊂每1m l 氢氧化钠滴定液(0.1m o l /L )相当于9.21m g 的C 3H 8O 3㊂ʌ类别ɔ药用辅料,溶剂和助悬剂等㊂ʌ贮藏ɔ密封,在干燥处保存㊂ʌ注意ɔ本品可与硼酸形成复合物,过热会分解放出有毒的丙烯醛;与强氧化剂共研可能爆炸,受光照或与碱式硝酸铋㊁氧化剂接触会变黑㊂㊃794㊃甘油(供注射用)。
甘油(56-81-5)

目标器官
大量摄取,影响中枢神经系统,增加糖分和脂肪水平
Medical Conditions Generally Aggravated by exposure 暴露后将恶化
Eyes 眼部
XII. Ecological Information 生态学资料
Mobility
可动性
Not known 不详
Bioaccumulation Not known 生物积累性 不详
Vapour Density 蒸汽密度
3.1 ( Air = 1 )
Specific Gravity 比重
1.2606 - 1.2612 at 25oC
Flash Point 闪点
Autoignition Temperature 自燃温度
Flammable limit (%) 可燃性极限
Other Properties If Applicable 其它特性
No harmful effects expected 无不良影响
Effects of Overexposure 露置影响
No harmful effects expected 无不良影响
Chronic Effects Eyes, slight irritation
慢性效应
眼部,轻微刺激
Target Organs Ingestion - high doses - CNS effects, increase sugar and fat levels
Wear a positive pressure self contained breathing apparatus. Caution acrolein may be released by combustion 佩戴自给正压式呼吸器,注意燃烧可能释放出丙烯醛。
化妆品用甘油原料要求和编制说明

附件3:化妆品用甘油原料要求为规范化妆品原料技术要求,提高化妆品卫生质量安全,参考国内外化妆品法规的变化,编写《化妆品用甘油原料要求》(以下称《要求》),本《要求》针对性地规定了甘油的安全性要求及检验方法,其他相关要求及检验方法按相应规定执行。
1. 基本信息1.1 名称甘油1.1.1 INCI名称及其ID号GLYCERINID:10771.1.2 INCI标准中文译名甘油1.1.3 化学系统命名法名称或《中国药典》中名称系统命名法:1,2,3-丙三醇(Propane-1,2,3-triol)2010年版《中国药典》(二部)中名称:甘油1.1.4 常见俗名Glycerol (INN,RIFM, EP)Glycerolum (EP)1.2 登记号1.2.1 CAS登记号56-81-51.2.2 EINECS登记号200-289-51.3 分子式、结构式及分子量分子式:C3H8O3结构式:分子量:92.091.4 性状及理化指标无色、澄清的黏稠液体;味甘甜,有引湿性,水溶液(1→10)显中性反应;本品与水或乙醇任意混溶,在丙酮中微溶,在三氯甲烷或乙醚中均不溶;相对密度在25℃时不小于1.2569。
2. 技术要求2.1 使用目的及适用范围可作保湿剂,降粘剂、变性剂等,广泛用于化妆品中。
2.2限量要求2.2.1 甘油纯度要求甘油(%)≥95.02.2.2甘油相关组分要求应对甘油中二甘醇含量进行必要的安全性风险评估分析,以保证产品在正常以及合理的、可预见的使用条件下,不会对人体健康产生安全危害。
3. 检验方法3.1 鉴别试验方法甘油样品的红外光吸收图谱(膜法)应与对照的图谱(2010年版《中国药典》光谱集77图)一致,图谱见下图。
3.2 含量测定方法取本品0.20g,精密称定1,加水90ml,混匀,精密加入2.14%(g/ml)高碘酸钠溶液50ml,摇匀,暗处放置15分钟后,加50%(g/ml)乙二醇溶液10ml,摇匀,暗处放置20分钟,加酚酞指示液20.5ml,用氢氧化钠滴定液(0.1mol/L)滴定至红色,30秒内不褪色,并将滴定的结果用空白试验校正3。
