化学化工专业英语试卷
大学化学化工专业《英语》期末考试试卷含参考答案

大学化学化工专业《英语》期末考试试卷含参考答案1. state-of-the-industry 中文:工业发展水平(1分)2. alkyl ether sulfate中文:烷基醚硫酸盐(酯)(1.5分)3. W/O 英文: water in oil,(oil emulsion) ;中文:油乳胶(油包水)(1.5分)4. 2,6-Dimethy-2,7-octadien-6-ol 画出结构式:(4分)5. The inherent tendency of the whole or a part of a molecule to pass out of or not to penetrate into a water phase.英文: Hydrophoby ;中文:疏水性(亲油性) (1.5分) 6. A substance which, when introduced in a liquid, increases its wetting tendency.英文: Wetting agent ;中文:润湿剂 (1.5分)7. The process by which soil is dislodged from the substrate and bought into a state of solution or dispersion.英文: Detergency ;中文:去污性(力) (1.5分)8. An attribute which is related to benefit not directly but through association or suggestion.英文: Signal attribute ;中文:信号属性 (1.5分) 9. A colorless gas with a characteristic pungent odor, consisting of nitrogen and hydrogen.英文: ammonia ;中文:氨气 (2分)10. A chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom.英文: Carbon dioxide ;中文:二氧化碳 (2分)11. A chemical element with atomic number 9, it is the lightest halogen.英文: Fluorine ;中文:氟 (2分)12. KH2PO4 Potassium dihydrogen phosphate (2分)13. ZnSO4·7H2O Zinc sulfate hept(a)hydrate (2分)14.3-methyl-2-ethyl(-1-)butene (3-methyl-2-ethyl but-1-ene) (3.5分)15.4-(1-ethyl-butayl)-5-hydroxy-2-hexayne-1-al (7.5分) 16. A good example of such a versatile attribute is fragrance. (2分)译文:这样一个多功能属性的好例子就是香味。
04级化学专业《专业英语》试卷

04级化学专业《专业英语》试卷绍兴文理学院 06 学年第二学期化学专业 04 级《专业英语》试卷(答题卷)I. Write the formula for each of the following chemicals: (30 points)1) silver nitrate 2) ferric oxide3) potassium sulfate 4) ammonium chloride5) magnesium hydroxide 6) sodium phosphate7) silicon dioxide 8) zinc sulfide9) lithium bromide 10) calcium carbonate11) lead acetate 12) carbon tetrachloride13) cyclohexane 14) meta-diethyl benzene15) dimethylamine 16) 3-methyl-1-butyne17) meta-nitrotoluene 18) 2-bromo-5-phenyl-3-octene19) 3-hexanone 20) N,N-dimethyl acetamide21) p-phenylbenzamide 22) benzoyl chloride23) ethylene glycol 24) methyl n-propyl ether25) 1,3-pentadiene 26) methyl formate27) o-phthalic anhydride 28) propionic acid29) formaldehyde 30) p-methoxybenzaldehydeII. Give the English name for each of the following compounds (15 points):1) H2SO42) Al2O33) KH2PO44) SO35) CH2=CH-CH36) (CH3)3CCl7) CF3COOH 8) H2NCH2CH2NH29) (CH3)2CHOH 10) p-F-C6H4-OH11) CH3-CH(CH3)-CH2-CO-CH3 12) CH3-CH=CH-CHO13) CH3-CH(OH)-COOH 14) CH3CO-CH2COOC2H515) CH3(CH2)3C≡NIII. For the following descriptions or definitions, determine true or false for each statement based on principles in chemistry (10 points):1) The oxidized and reduced species that appear in an ion-electron equation are may be non-reactants.2) Standard enthalpy of formation is the heat of formation of one mole of a compound by combination of its elements in their standard states at a specified temperature.3) Enantiomers are pairs of molecules with the same formula that rotate plane-polarized light in opposite directions.4) A nucleophile is an electron deficient atom or group that will bond with an atom that has a available electron pair5) Theoretical yield is the maximum amount of a product that can be formed according to a ba lanced chemical equation IV. (5 points) Answer the following questions in ENGLISH:Nitrous oxide, N2O, undergoes decomposition when heated to give N2 and O2.2 N2O (g) → 2 N2 (g) + O2 (g)What is the molar composition of the gaseous mixture produced? Compare this composition to that of air and predict whether the mixture will support combustion or not?V. (5 points) Fill in the blanks in the following paragraph with the appropriate words or phrases listed at the end of paragraph: The rules that govern the naming of chemical compounds are known collectively as chemical ________________. In a simple way, the name of a cation consists of the name of the element, the _____________on the ion as a Roman numeral in parenthesis, and the word “ion”. The name of a monatomic _______________ (e.g., Cl-) consists of the name of the element with the ending“ide”, followed by the word “ion”. A binary compound is one containing atoms or ions of only two ________________. Salts are _________________ formed between cations and anions of acids. For binary molecular compounds, prefixes are used to indicate the number of each element present.anion elements nomenclature ionic compounds chargeVI. (5 points) Fill in the blanks in the following paragraph with the appropriate words or phrases listed as follows:The geometry around the C=C double bond in an __________ plays an important role in the chemistry of these compounds. The presence of the bond restricts rotation around a C=C double bond. There is no way to rotate one end of this bond relative to the other without breaking the bond. Because the _____________ is relatively strong (270 kJ/mol), rotation around the C=C double bond cannot occur at room temperature. Alkenes therefore form stereoisomers that differ in the way substituents are arranged around the C=C double bond. The ____________ in which similar substituents are on the same side of the double bond is called cis; whereas that with similar substituents are across from each other, is called trans. The cis isomer of 2-butene, for example, has both _____________ groups on the same side of the double bond. In the trans isomer the CH3 groups are on the ______________ sides of the double bond.opposite alkene methyl bond energy isomerVII. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraph: So far, we have built a small repertoire of reactions that can be used to convert one functional group to another. We have briefly discussed converting alkenes to alkanes; alkanes to alkyl halides; alkyl halides to alcohols; alcohols to ethers, aldehydes, orketones; and aldehydes to carboxylic acids. We have also shown how carboxylic acids can be converted into esters and amides. We have yet to encounter a reaction, however, that addresses a basic question: How do we make C-C bonds? One answer resulted from the work that Francois A.Grignard started as part of his Ph.D. research at the turn of the last century.Grignard noted that alkyl ____________ react with magnesium metal in diethyl ether to form compounds that contain a metal-carbon bond. Methyl bromide, for example, forms methylmagnesium bromide.Et2OCH3Br + Mg →CH3MgBrBecause carbon is considerably more _______________ than magnesium, the metal-carbon bond in this compound has a significant amount of ionic character. Grignard reagents such as CH3MgBr are best thought of as hybrids of ionic and ___________ Lewis structures.CH3-Mg-Br ?[CH3-] [Mg2+] [Br-]Grignard reagents are our first source of carbanions (literally, "anions of carbon"). The Lewis structure of the CH3- ion suggests that carbanions can be Lewis bases, or electron-pair _____________. Grignard reagents such as methylmagnesium bromide are therefore sources of a nucleophile that can attack the + end of the C=O double bond in aldehydes and ketones. Thus the most important aspect of the chemistry of Grignard reagents is the ease with which this reaction allows us to couple alkyl chains. Isopropylmagnesium bromide, for example, can be used to graft an isopropyl group onto the ____________ chain of an appropriate ketone.electronegative hydrocarbon covalent donors halidesVIII. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraphs: Why do some solids dissolve in water? The sugar we use to sweeten coffee or tea is a molecular solid, in which the individual molecules are held together by relatively weak ________________________. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules are released into ________________. It takes energy to break the bonds between the C12H22O11 molecules in sucrose. It also takes energy to break the hydrogen bonds in water that must be disrupted to insert one of these sucrose molecules into solution. Sugar dissolves in water because energy is give n off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent. In the case of sugar and water, this process works so well that up to 1800 grams of sucrose can dissolve in a liter of water.Ionic solids(or salts) contain positive and negative ions, which are held together by the strong force of __________________ between particles with opposite charges. When one of these solids dissolves in water, the ions that form the solid are released into solution, where they become associated with the ___________________solvent molecules.H2ONaCl(s) →Na+(aq) + Cl-(aq)We can generally assume that salts ________________ into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with watermolecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.dissociate intermolecular forces attraction polar solutionIX. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraphs:A soap bubble is simply a very thin sheet of water sandwiched between two layers of soap molecules, also called _____________________. These molecules are called amphiphilic. This means that part of this molecule is attracted to water, which is hydrophilic, and another part is repelled from water, which is _____________________.When such a molecule is put in water, as many as possible will crowd to the surface, so that the heads can stay in the water, while the tails stick out into the ___________. This is why soap-like molecules are called surfactants, since they mostly affect the _________ of water. It is these molecules that make soap bubbles stable.Some bubbles result from gas being trapped in solution, such as CO2 in soda and champagne, or N2 in the blood of deep sea divers. Much study goes into understanding bubbles dissolved in gases, as well as how the formation of large surfaces from surfactants used to construct bubbles are affected by different factors, e.g., temperature, surface __________ , nucleation sites, etc..air, pressure, hydrophobic, surface, surfactant molecules。
化学化工英语试题及答案

化学化工英语试题及答案一、选择题(每题2分,共20分)1. Which of the following is a chemical element?A. WaterB. OxygenC. HydrogenD. Carbon答案:B, C, D2. The chemical formula for table salt is:A. NaOHB. NaClC. HClD. NaHCO3答案:B3. What is the process called when a substance changes from a solid to a liquid?A. SublimationB. VaporizationC. MeltingD. Condensation答案:C4. In the periodic table, which group contains alkali metals?A. Group 1B. Group 2C. Group 17D. Group 18答案:A5. What is the name of the process where a substance decomposes into two or more substances due to heat?A. CombustionB. OxidationC. ReductionD. Decomposition答案:D6. Which of the following is a physical property of a substance?A. ColorB. TasteC. SolubilityD. Reactivity答案:A7. What is the term for a compound that releases hydrogen ions (H+) when dissolved in water?A. BaseB. AcidC. SaltD. Neutral答案:B8. The law of conservation of mass states that in a chemical reaction:A. Mass is lostB. Mass is gainedC. Mass remains constantD. Mass can be converted into energy答案:C9. Which of the following is a type of chemical bond?A. Ionic bondB. Covalent bondC. Hydrogen bondD. All of the above答案:D10. What is the name of the process where a substance absorbs energy and changes from a liquid to a gas?A. MeltingB. VaporizationC. SublimationD. Condensation答案:B二、填空题(每题2分,共20分)1. The symbol for the element iron is ________.答案:Fe2. The pH scale ranges from ________ to ________.答案:0 to 143. A compound that produces a basic solution when dissolvedin water is called a ________.答案:base4. The smallest particle of an element that retains its chemical properties is called a ________.答案:atom5. The process of separating a mixture into its individual components is known as ________.答案:separation6. The study of the composition, structure, and properties of matter is called ________.答案:chemistry7. The process of a substance changing from a gas to a liquid is called ________.答案:condensation8. A(n) ________ reaction is a type of chemical reactionwhere two or more substances combine to form a single product. 答案:synthesis9. The volume of a gas at constant temperature and pressureis directly proportional to the number of ________.答案:moles10. The process of converting a solid directly into a gas without passing through the liquid phase is known as ________. 答案:sublimation三、简答题(每题10分,共30分)1. Explain what is meant by the term "stoichiometry" in chemistry.答案:Stoichiometry is the calculation of the relative quantities of reactants and products in a chemical reaction.It is based on the law of conservation of mass and involvesthe use of balanced chemical equations and the molar massesof substances to determine the amounts of reactants needed to produce a certain amount of product or the amounts ofproducts formed from a given amount of reactant.2. Describe the difference between a physical change and a chemical change.答案:A physical change is a change in the state or form of a substance without altering its chemical composition. Examples include melting, freezing, and boiling. A chemical change, on the other hand, involves a change in the chemical composition of a substance, resulting in the formation of new substances. Examples include combustion and rusting.3. What are the three main types of chemical bonds, and givean example of each.答案:The three main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. An ionic bond is formed when electrons are transferred from one atom to another, resulting in the formation of oppositely charged ions. An example is the bond between sodium (Na) and chloride (Cl) in table salt (NaCl). A covalent bond is formed when two atoms share electrons, as seen in water (H2O) where hydrogen atoms share electrons with oxygen. Metallic bonds occur in metals, where a "sea" of delocalized electrons is shared among positively charged metal ions, as in sodium metal。
化工专业外语试卷A答案
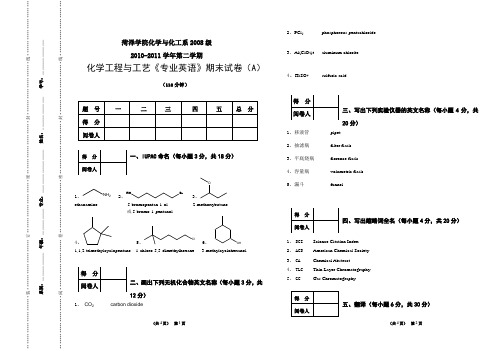
菏泽学院化学与化工系2008级 2010-2011学年第二学期化学工程与工艺《专业英语》期末试卷(A )(110分钟)一、IUPAC 命名(每小题3分,共18分)1、NH 22、HOBr3、Oethanamine 5-bromopentan-1-ol 2-methoxybutane或5-bromo-1-pentanol4、5、Cl6、OH1,1,2-trimethylcyclopentane 1-chloro-5,5-dimethylhexane 3-methylcyclohexanol二、画出下列无机化合物英文名称(每小题3分,共12分)1、 CO 2 carbon dioxide2、PCl 5phosphorous pentachloride3、Al(ClO 2)3 aluminum chlorite4、H 2SO 4sulfuric acid三、写出下列实验仪器的英文名称(每小题4分,共20分)1、移液管pipet2、抽滤瓶 filter flask3、平底烧瓶 florence flask4、容量瓶 volumetric flask5、漏斗funnel四、写出缩略词全名(每小题4分,共20分)1、 SCI Science Citation Index2、 ACS American Chemical Society3、 CA Chemical Abstract4、 TLC Thin Layer Chromatography5、 GCGas Chromatography五、翻译(每小题6分,共30分)························阅·······················卷························密························封························线·························系别:_____________ 年级:____________ 专业:____________________ 姓名:_______________ 学号:························装·······················订························密························封························线·························1、A scientific paper is an organized description of hypotheses, data and conclusions, intended to instruct the reader. Papers are a central part of research. If your research does not generate papers, it might just as well not have been done.科技论文是以向读者论述为目的,对假说、数据和结论所做的系统性描述。
井冈山大学化学化工专业英语题库

一、词汇翻译(每题1分,共10分)二、单句翻译(每题2分,共20分)1.The operation of a machine needs some knowledge of its performance.操作机器需要懂得机器的一些性能。
2.The continuous process can ordinarily be handled in the less space.连续过程通常能节操作空间。
3.lens of thousands of foreign friends visit this factory every year.每年有几万人参观这座工厂。
lion billion billion atoms.106*10*109 也就是1024 个原子(美语)。
5.Half of a millionth of a billionth of billionth of a pound.0.5*10-30 磅(10-6*10-12*10-12)(英语)。
6.Other things being equal copper heats up faster than iron.相同条件下,铜比铁热得快。
7.Steel and cast iron also differ in carbon.钢和铸铁的含碳量也不相同。
8.Alloys belongs to a half-way house between mixture and compounds.合金是介于混合物和化合物之间的一种中间结构。
9.Industrialization and environmental degradation seem to go hand in hand.工业化发展似乎伴随着环境的退化。
10.The atom is the smallest particle of an element原子是元素的最小粒子。
11.Although the world is large, man is able to live in only a small part of it.尽管地球很大,可人类只能在其中很小的一部分地方生活。
化工英语试卷

化工专业英语试卷Ⅰ.Put the following into Chinese or English.(20 points)1.polypropylene2.refinery3.extract4.corrosion5.pigment6. complex7. initial state8. hydrogen energy9. branch 10. alkali metal 11.表面活性剂12.热力学13.蒸馏14.高聚物15.溶液16. Seed crystal 17. Litmus paper 18. Evaporating dish 19.Distilling tube20. Simple substance1._____2._____3._____4.____5._____6._____7._____8.____9._____ 10._____ 11._____ 12.____13.____ 14._____ 15._____ 16.____17._____ 18.______ 19._____ 20._____Ⅱ.Translate these sentences into Chinese.(10 points)21. The drive to increased recycling and the ideal of emission-free plants will be a major factor influencing the development of the industry in the next decade.__________________________________________________________ 22. Chemical engineering has a bright future as interfacial discipline, that will bridge science engineering in the multidisciplinary environments where these new technologies will be brought into being.__________________________________________________________ 23. Foaming and cleaning agents are basic constituents of shampoos and cleansers.__________________________________________________________ 24. Surfactants are substances with molecular structures consisting of a hydrophilic and a hydrophobic part.__________________________________________________________ 25. While this is true for the manufacturing any cosmetic, emulsions are particularly delicate: small deviations in the procedure of raw material specification can bring about marked changes in product viscosity and stability.__________________________________________________________Ⅲ.Guess word.(5 points)26.The inherent tendency of the whole or apart of the molecule to pass out of or not to penetrate into a water phase.H_______27. A substance which, when introduced in a liquid, increases it is wetting tendency.W______ ______28. A colorless gas with a characteristic pungent odor, consisting of nitrogen and hydrogen.A______29. A chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom.C______ _______30. A chemical element with atomic number 9, it is the lightest halogen. F______Ⅳ. In this section, there are 5 questions, each question has four options, one of them is correct, choose the answer you think is right.(10 points)31. The main constituents of plants are_____.A. oxygenB. waterC. carbonD. carbon hydrates32.____ is not categorized as high-volume sectors.A. carbon dioxideB. sculptures acidC. chloral-alkaliD. polythene33. Of all soda-ash, 50% is sold to the_____ industry.A building B. paper-making C. glass –making D. transportation34. ____is the chemical that is produced in the largest tonnage.A. carbonB. oxygenC. euphoric acidD. ammonia35. Almost all explosives are ultimately derive from_____.A. ureaB. nitric acidC. euphoric acidD. ammonia36. Light is given off by a sodium vapor streetlight when____A. electrons move from a given energy level to a higher energy levelB. electrons are removed from atoms and captions are formed.C. electrons move from a given energy level to a lower energy levelD. electrons are added to atoms and anions are formed37. Which mixture is most likely to be an ideal solution?A.CH3CH2OH and CH3(CH2)3CH3B. CH3(CH2)3CH3 and CH3(CH2)4CH3C. CH3CH2OH and H2OD. NaOH and H2O38. Electricity is carried through a solution of an electrolyte by____A. electrons onlyB. anions onlyC. cations onlyD. both cations and anions39. Which of the following is electrophilic reagent?A. H2N-NH2B. NaHSO3C. HNO3D. HCN40. Arrange the bases ClCH2COO-, CH3COO- and FCH2COO- in order of increasing strengthA. FCH2COO- < ClCH2COO- < CH3COO-B. CH3COO- < ClCH2COO- < FCH2COO-C. ClCH2COO- < FCH2COO- < CH3COO-D. CH3COO- < FCH2COO- < ClCH2COO-Ⅴ. In this section, there is a passage with 10 blanks. You are required to select one word for each blank from a list of choices given in aword bank following the passage.(10 points)One of the main reasons for the rapid growth of the chemical industry in the developed world has been its great 41____to, and investment in research and development (R&D). A typical figure is 5% of sales income, with this figure being almost doubled for the most research42_____ sector, pharmaceuticals. It is important to 43___ that we are quoting percentages here not of profits but of sales income, i.e. the total money received, which has to pay for 44____materials, overheads, staff salaries, etc. as well. In the past this tremendous investment has 45____well, leading to many useful and valuable products being 46____to the market. Examples include 47____polymers like nylons and polyesters, and drugs and pesticides. Although the number of new products introduced to the market has declined 48____in recent years, and in times of 49____the research department is usually one of the first to suffer50____, the commitment to R&D remains at a very high level.paid off syntheticemphasize cutbacksintensive introducedsignificantly commitmentrecession rawⅥ. There are one passage in this section, passage is followed by somequestions or statements. You should decide on the best choice.(10 points)The accuracy of scientific observations and calculations is always at the mercy of the scientist's timekeeping methods. For this reason, scientists are interested in devices that give promise of more precise timekeeping.In their search for precision, scientists have turned to atomic clocks that depend on various vibrating atoms or molecules to supply their "ticking" .This is possible because each kind of atom or molecule has its own characteristic rate of vibration. The nitrogen atom in ammonia, for example, vibrates or "ticks" 24 billion times a second.One such atomic clock is so accurate that it will probably lose no more than a second in 3000 years. It will be of great importance in fields such as astrological observation and long-range navigation. The heart of this atomic Ron is a cesium atom that vibrates 9.2 billion times a second when heated to the temperature of boiling water.An atomic clock that operates with an ammonia molecule may be used to check the accuracy of predictions based on Einstein's relativity theories, according to which a clock in motion and a clock at rest should keep time differently. Placed in an orbiting satellite moving at a speed of 18000 miles an hour, the clock could broadcast its time readings to a ground station, where they would be compared with the readings on a similarmodel. Whatever differences develop would be checked against the differences predicted.51. Scientists expect that the atomic clocks will be ______A more preciseB absolutely accurateC more durableD indestructible52. The passage says that the accuracy of scientific observation depends on ________.A. methods of measurementB. timekeeping methodsC. basic assumptionsD. earlier experiments53. Which of the following is not mentioned in the passage as to the usage of atomic clock?A. Scientific research.B. Astronomical observation.C. To check Einstein relativity theory.D. Long range navigation.54. Which of the following is implied but not stated?A. Precise timekeeping is essential in science.B. Scientists expect to disprove Einstein relativity theories.C. Atomic clocks will be important in space flight.D. The rate of vibration of an atom never varies.55. An appropriate title for this passage would be.A. A Peacetime Use of the AtomB. Atoms and MoleculesC. The Satellite TimekeepersD. The Role of the ClockⅦ.Translate into Chinese.(15 points)Note, however, that there has been a major change in recent years as academic institutions have increasingly turned to industry for research funding, with the result that much more of their research effort is mow devoted to more applied research. Even so, in academia the emphasis generally is very much on the research rather than the development.ⅧWriting.(20 points)For this part, you should write a short essay entitled The Development of Fine Chemicals. You should write at least 150 words following the outline given below.1.精细化工现状2.精细化工未来发展前景3.我的建议___________________________________________________________ ___________________________________________________________ ___________________________________________________________KEY:1.聚丙烯2.炼油厂3.萃取;提炼4.腐蚀;锈蚀5.颜料;色素6. 络合物 6. surface-active agent7. 始态8. 氢键9. 支键10. 碱金属11.thermodynamics12. still 13.macromer 14.dissolution 15.solution 16. 晶种17. 石蕊试纸18. 蒸发皿19. 整流管20. 简单物质21.增加回收利用的动力和无废物排放工厂的理想在下个十年里是影响工业发展的主要因素.22.作为界面科学,化学工程有美好的未来,它把科学和工程连接在多学科的环境中,这将促使新技术的生成。
内蒙古大学鄂尔多斯学院化工专业英语期末试卷及答案

内蒙古大学鄂尔多斯学院《化工专业英语》期末考试试卷(A)(2010——2011学年第一学期)(闭卷 120 分钟)学号姓名专业年级重修标记□I.阅读理解(Reading and Understanding )(i) This might be achieved in a number of ways, for example, improving the profitability(利润) of the process, increasing the capacity (产量) by introduction a new catalyst, or lowering the energy requirement of the process.1. Which one is not mentioned in above section.( )(A) increasing the profitalbility(B) improving the capacity(C) using large amount of catalyst(D) lowing the energy requirement(ii) This often means that there will be close liaison(联系) between the chemical companies’technical sales representatives(代表) and the customer, and the level of technical support for the customer can be a major factor in winning sales.2. We can conclude that ( ) from the above section.(A) there are close liaison between the products and customer.(B) a major factor for winning sales is the level of technical support for customer(C) a major factor in winning sales is the close liaison between the chemical companies’technical sales representatives and the customer.(D) the major factors in winning sales are the the close liaison between the chemical companies’technical sales representatives and the customer and the level of technical support for the customer.(iii) A major advance in polymer chemistry was provided by the work of Karl Ziegler and Giulio Natta, which led in 1955 to the introduction of some revolutionary (革命性的) catalysts which bear their name. During the late 1970s production of linear (线性) low-density polyethylene (聚乙烯) was commercialized.3. The introduction of some revolutionary catalysts is due to the in polymerchemistry.4. This paragraph implies that()(A) During the late 1970s, the linear low-density polythylene are widely produced.(B) In 1955, the linear polyethylene was commercialized.一、阅读与理解(每小题2分,共20分)二、翻译一——英译汉(每小题6分,共30分)三、翻译二——汉译英(每小题6分,共30分)四、翻译三——摘要(20 分)(C) Some revolutionary catalysts were introduced during the late 1970s..(D) The linear low-density polyethylene bears the name of Karl Ziegler and Giulio Natta. (iv) The basic components (构成) of a typical chemical process are shown in Fig. 1, in which each block (方框) represents (代表) a stage in the overall (全部) process for producing a product from the raw materials. Fig. 1 represents a generalized (一般的) process; not all stages will be needed for any particular process and the complexity (复杂程度) of each stage will depend on the nature of the process.5. This paragraph implies that ( )(A)Figure 1 represents particular process.(B)each block in Fig.1 represents a stage of particular process.(C)the complexity of each stage will depend on the cost.(D)not all stages in Fig.1 are included in every chemical process.6. Fig 1 represents a process for producing a product from the. (v) There are over three million known compounds, with no end(没有尽头) in sight as to the number that can and will be prepared(制备,准备) in the future. Each compound is unique(唯一的) and has characteristic(特有的) physical and chemical properties(性质).Let us consider in some detail two compounds—water and mercuric(汞,水银) oxide.Water is a colorless, odorless(无嗅), tasteless (无味) liquid that can be changed to a solid, ice at 0℃and to a gas, steam(蒸汽) at 100℃. It is composed of two atoms of hydrogen(氢) and one atom of oxygen per molecule (分子), which represents(表现) 11.2 percent hydrogen and88.8 percent oxygen by mass (质量). Water reacts chemically with sodium (钠) to producehydrogen gas and sodium hydroxide(氢氧化物), with lime (石灰) to produce calcium (钙) hydroxide, and with sulfur(硫) trioxide(三氧化物) to produce sulfuric(硫的,硫磺的) acid(酸).No other compound has all these exact(精确的) physical and chemical properties; they are characteristic of water alone.Mercuric oxide is a dense(致密的), orange-red powder composed of a ratio of one atom of mercury to one atom of oxygen. Its composition(组成) by mass is 92.6 precent mercury and7.4 percent oxygen. When it is heated to temperatures greater than 360℃, a colorless gas,oxygen, and a silvery(银子似的;有银色光泽的) liquid metal, mercury, are produced. Here again are specific(明确的,特定的) physical and chemical properties belonging to mercuricoxide and to no other substance. Thus, a compound may be identified(区别,识别,鉴定) and distinguished(区分) from all other compounds by its characteristic properties.7. Which one is not correct as to the above passage. ( )(A)There are more than 3 million compounds which are known at present.(B)The number of compounds which can be prepared in the future could be calculated.(C)Every compound is special and unique.(D)There are characteristic physical and chemical properties for each compound.8. There are two compounds are detailed introduced in this passage, they areand , respectively.9. This passage suggested that ( )(A) Water is liquid, but can be changed into solid and gas under cetain conditions.(B) Water is composed of one atom of hydrogen and one atom of oxygen per molecule.(C) Water could react with sodium or lime, but could not react with sulfur trioxide.(D) No other compound has exact physical and chemical properties expect water and mercuricoxide.10. Mercuric oxide has the following properties expect ( )(A)dense (B)orange-red color(C)turn to liquid at more than 360o C(D)the liquid mercury are much darker in color than mercuric oxideII.翻译一(Translating English to Chinese,英译汉)1. Next reaction (反应) was designed based on the obtained experimental (实验的) data.2. The explosive growth in petrochemistry (石油化学) was largely due to the enormous increaseof synthetic polymers (合成聚合物).3. Because of the diversity (多样性) of operations (操作) and close links (联系) in many areas,there is no simple definition (定义) of the chemical industry.4. The purpose of this meeting is to provide information and knowledge in order to reduceuncertainty (不确定性), solve problems and provide better data.5. The basic components (构成) of a typical chemical process are shown in Fig. 1, in which each block (方框) represents (代表) a stage in the overall (全部) process for producing a product from the raw materials.III.翻译二(Translating Chinese to English,汉译英)1. 值得注意的是这些化合物(compounds)的大多数都是无机物(inorganic materials)。
化工专业英语期末

化工专业英语期末测试班级:化工11203 姓名: 王泽州 学号:201202647乳状液 emulsion氨 ammonia胺 amine吸收 absorb吸附 adsorption碳氢化合hydrocarbons 吸热的 endothermic 放热的 exothermic 绝热的 adiabatic 阴离子 anion 阳离子 cation 裂解 cracker 纯化 purification 冷凝 condensing 共聚物 copolymer 腐蚀的 corrosive 平衡 balance 均相的 homogeneous 非均相的Heterogeneous2. Text explanation(A) The revolution in materials science and engineering presents both opportunities and challenges to chemical engineers. With their basic background in chemistry, physics, and mathematics and their understanding of transport phenomena, thermodynamics, reaction engineering, and process design, chemical engineers can bring innovative solutions to the problems of modern materials technologies.Translation:材料科学与工程革命既是机遇和挑战对于化学工程师来说。
凭借其在化学,物理和数学的基本背景和对运输现象,热力学,反应工程和工艺设计的理解,化学工程师可以带来创新的解决方案来解决现代材料技术的问题。
(B) The application of distillation can roughly be divided in four groups: laboratory scale, industrial distillation, distillation of herbs for perfumery and medicinals (herbal distillate), and food processing.The lattertwo are distinctively different from the former two in that in the processing of beverages, the distillation is not used as a true purification method but more to transfer all volatiles from the source materials to the distillate.(C) To understand how matter is classified by its chemical constitution, we must first distinguish between physical and chemical changes and between physical and chemical properties. A physical change is a change in the form of matter but not in its chemical identity. Changes of physical state are examples of physical changes. The process of dissolving one material in another is a further example of a physical change. For instance, you can dissolve sodium chloride (table salt) in water. The result is a clear liquid, like pure water, though many of its other characteristics are different from those of pure water. The water and sodium chloride in this liquid retain their chemical identities and can be separated by some method that depends on physical changes.Distillation is one way to separate the sodium chloride and water components of this liquid. You place the liquid in a flask to which a device called a condenser is attached. The liquid in the flask is heated to bring it to a boil. Water vapor forms and passes from the flask into the cooled condenser, where the vapor changes back to liquid water. The liquid water is collected in another flask, called a receiver. The original flask now contains the solid sodium chloride. Thus, by means of physical changes (the change of liquid water to vapor and back to liquid), you have separated the sodium chloride and water that you had earlier mixed together.A chemical change, or chemical reaction, is a change in which one or more kinds of matter are transformed into a new kind of matter or several new kinds of matter. The rusting of iron, during which iron combines with oxygen in the air to form a new material called rust, is a chemical change. The original materials (iron and oxygen) combine chemically and cannot be separated by any physical means. To recover the iron and oxygen from rust requires a chemical change or a series of chemical changes.(D)Types of Chemical Reactions1. Precipitation reactions. In these reactions, you mix solutions of two ionic substances, and a solid ionic substance (a precipitate) forms.A precipitate is an insoluble solid compound formed during a chemical reaction in solution. To predict whether a precipitate will form when you mix two solutions of ionic compounds, you need to know whether any of the potential products that might form are insoluble.2. Acid–base reactions. An acid substance reacts with a substance called a base. Such reactions involve the transfer of a proton between reactants.An acid is a substance that produces hydrogen ions, H-, when it dissolves in water. A base is a substance that produces hydroxide ions, OH-, when it dissolves in water. An acid-base reaction (neutralization reaction) is a reaction of an acid and a base that results in an ionic compound and possibly water. When a base is added to an acid solution, the acid is said to be neutralized. The ionic compound that is a product of a neutralization reaction is called a salt. Most ionic compounds other than hydroxides and oxides are salts.3. Oxidation-reduction reactions. These involve the transfer of electrons between reactants. Many oxidation–reduction reactions can be described as one of the following:1) Combination reaction: A combination reaction is a reaction in which two substances combine to form a third substance. Note that not all combination reactions are oxidation–reduction reactions. However, the simplest cases are those in which two elements react to form a compound; these are clearly oxidation–reduction reactions.2) Decomposition reaction: A decomposition reaction is a reaction in whicha single compound reacts to give two or more substances. Often these reactions occur when the temperature is raised.3) Displacement reaction: A displacement reaction (also called a single replacement reaction) is a reaction in which an element reacts with a compound, displacing another element from it. Since these reactions involve an element and one of its compounds, these must be oxidation–reduction reactions.4) Combustion reaction: A combustion reaction is a reaction in which a substance reacts with oxygen, usually with the rapid release of heat to produce a flame. The products include one or more oxides.Translation:化学反应的类型:1.沉淀反应:在这些反应中,将两种离子溶液混合得到一种离子(沉淀物)的形式。
化工专业英语(期末练习题)

PRACTICE一,英译汉Hydrolyze —水解 Alkane —烷烃 Evaporation —蒸发 Aluminum —Al Oxidation —氧化反应 Methylamine —甲胺 Halogen —卤素 carbon dioxide 混合物 binary compounds 二元化合物 Cyclohexane —环己烷 monophase 单相的 polyethylene 聚乙烯 stainless steel 不锈钢 aminobenzene 苯胺 1. The Ideal-Gas Equation of State 理想气体状态方程 2. The First Law of Thermodynamics 热力学第一定律 3. Reaction Rates 反应速率 4. Activation Energy 活化能 5. Separatory Funnel 分液漏斗 6. Homogeneous Catalysis 均相催化7. Conjugate Acid-Base Pairs 共轭酸碱对 8. The Common-Ion Effects 同离子效应9. The Solubility-Product Constant 溶度积常数 二,命名 1. 甲烷 methane2. 2-甲基-3-乙基辛烷 3-ethyl- 2-methyloctane3. 2-乙基-1,3-丁二烯 2- ethyl -1, 3-butadiene4. 环己烷 Cyclohexane5. 对二甲苯 paraxylene6. 乙酸甲酯 Methyl acetate7. 醋酸 Acetic acid8. 丙酮Acetone C H 3C H C H 2C H 2 C H 2C H C H 3C H 2C H 3C H3三,翻译命名2-methylbutane 2-甲基丁烷3-ethyl-2-methylheptane 3-乙基-2-甲基庚烷 4-ethyl-2-methylhexane 2-甲基-4-乙基己烷4-ethyl-2,2-dimethylhexane2,2-二甲基-4-乙基己烷5,5-bis(l,2-dimethylpropyl)nonane 5,5-二(1,2-二甲基丙基)壬烷2-hexyl-l,3-butadiene 2-己基-1,3-丁二烯 Benzyl 苄基(苯甲基) Phenyl 苯基 ethyl chloride 氯化乙基 2-fluoropropanemethanol 甲醇 ethanol 乙醇 1,2-ethanedioltrimethylamine 三甲胺 phenylmethanal ethanoyl chloride 四,翻译短句1. Acetylene (乙炔) is hydrocarbon especially high in heat value.乙炔烃特别是高热值2. It is common knowledge that bodies are lighter in water than they are in air.大家都知道,水中的物体比在空中更轻。
化学专业英语化学专业英语课期末考试试卷含答案

化学专业英语试卷学号:姓名:成绩: 一:把下列单词或词组译成英文本题共 30 分,每小题 1 分1. NiClO42 nickel perchlorate3. FeCl2 iron2chloride5. AlNO33 aluminum nitrate7. MnO2 manganese dioxide9. N2O3 dinatrogen trioxide11. NaClO sodium hypochloride13. P2O5 diphosphorous pentaoxide15. KMnO4 patassium permangate17. 盐酸hydrochloric acid19. KCN patassium cyanide21. 5-甲基-4-丙基壬烷5-methyl-4-propylnonaane23. 四氯化碳carbon tetrachloride25. 中和neutralize27. 比热容specific heat capacity29. 酸酐anhytride 2. CuSO4 copper sulfate4. CoCO3 cobalt carbate6. CaC2H3O22 calcium acetate8. H2SO410. 六氰合铁Ⅱ酸钾12. Ag2SO3 sliver sulfite14. 草酸铅 lead cyanate16. ZnOH2 zinc hydroxide18. 磷酸根 phosphate20. 2,3-二甲基戊烷2,3-dimethylpentane22. 2,3,7-三甲基-5-乙基辛烷2,3,7-trimethyl-5-ethyloct ane24. 石蕊试纸litmus paper 26. 滴定titration28. 非电解质electrolyte 30. 配位化合物complex compound三. 把下列短文译成汉语本题共 40 分,每小题 10 分1. Without chemistry our lives would be unrecognisable, for chemistry is at work all around us. Think what life would be like without chemistry - there would be no plastics, no electricity and no protective paints for our homes. There would be no synthetic fibres to clothe us and no fertilisers to help us produce enough food. We wouldn’t be able to travel because there would be no metal, rubber or fuel for cars, ships and aeroplane. Our lives would be changed considerably without telephones, radio, television or computers, all of which depend on chemistry for the manufacture of their parts. Life expectancy would be much lower, too, as there would be no drugs to fight disease.没有化学反应我们的生活将会大变样,化学就在我们周围;没有化学生活会是什么样子——没有塑料,,家里没有电,也没有防护漆;不会给我们合成纤维,没有化肥帮助我们生产足够的食物;我们不能旅行,因为不会有金属、橡胶或燃料汽车、船只和飞机;我们的生活将会大大改变了没有电话、收音机、电视或电脑,所有这些依赖化学生产的部分;没有药物来抵抗疾病,预期寿命将低得多;2. The first and second laws of thermodynamics and the meaning of entropy will be discussed. and expanded upon in this lesson. It will be shown that energy transformations on a macroscopic scale — that is, between large aggregates of atoms and/or molecules —can be understood in terms of a set of logical principles. Thus thermodynamics provides a model of the behavior of matter in bulk. The power of such a model is that it does not depend on atomic or molecular structure. Furthermore, conclusions about a given process .based on this model, do not require details of how the process is carried out.探讨热力学第一和第二定律和熵的意义.和扩展在这个知识;也就是说它将表明能源在宏观上的转换,根据一组逻辑原则可以理解能量在大量的原子或分子内的转换;因此热力学定理提供了一个物质体积变化的模型;这样一个模型的能力在于它不依赖于原子或分子结构;此外,给定进程的结论依托于这种模式,不需要的详细说明过程是如何进行的3.Preparation of Cuen2cdaH2O: H2cda 4-羟基-2,6 吡啶二酸 g, mmol was dissolvedin water 10 mL and the pH value of the solution was adjusted to 7~8 with aqueous NaOH solution molL-1, then adding it dropwise to a methanol solution 10mL ofCuClO42·6H2O , and ethylenediamine mmol under stirring at room temperature.After the resulting small quantity of precipitates was filtered off, dark blue crystals suitable for X-ray structure analysis were obtained by slow evaporation of the filtrate at room temperature.制备CUen2cdaH2O:使克,的4 -羟基2、6吡啶二酸溶解在10ml水中加入氢氧化钠水溶液调整到pH值7 ~ 8,然后将它一滴一滴地添加到CuClO42·6H2O,的乙醇溶液和乙二胺,在室温下搅拌;在室温下,缓慢蒸发滤液,得到深蓝色晶体,用x射线分析它的结构4. Measure 50 ml of vinegar with a pipette and pour into a 250-ml beaker. Add 2 drops of phenolphthalein indicator. Fill a burette with a 1 N solution of sodium hydroxide NaOH and draw out the excess as described above. From the burette add NaOH to the beaker of vinegar until 1 drop of NaOH produces a pale pink color in the solution. Maintain constant stirring. The appearance of pink tells you that the acid has been neutralized by the base and there is now 1 drop of excess base which has turned the indicator. Read the burette and record this reading as the volume of base used to neutralize the acid. One molecule of NaOH neutralizes one molecule of acetic acid, or one gram-molecular weight of NaOH neutralizes one gram-molecular weight of acetic acid. Calculate the amount of acetic acid present in the vinegar. Report this amount as the percentage of acetic acid. 用移液管吸取50ml醋加入到250毫升烧杯,加2滴酚酞指示剂;在滴定管中加入1M的氢氧化钠溶液,去除刻度线以上的溶液,将氢氧化钠溶液加入到醋中,并不断震荡,至到加入一滴氢氧化钠溶液变成粉红色;出现粉红色的颜色,表示酸中和了碱,而且多余的一滴碱使指示剂变色;阅读并纪律中和酸消耗碱的体积;一个分子的氢氧化钠中和一个分子的醋酸,或一个分子重量的氢氧化钠中和一个分子重量的醋酸反应;计算醋酸在醋的量;报告醋酸的百分比;。
化学专业英语
A.HBrO4B.HBrO3C.HBrO2D.HBrO
3、The nonmetal diatomic molecules having low-molecular weight usually are ()at room temperature.
西华师范大学学生试卷
年月日学年第学期考室
题号
一
二
三
四
五
六
七
八
九
十
总分
阅卷教师
得分
化学化工学院化学、应用化学专业XX级《化学专业英语》试题A卷
闭卷考试时间120分钟
注意事项:1.满分:100分。保持卷面整洁,否则扣卷面2分。
2.交卷时请将试题卷与答题卷一起交,否则扣分。
3.学生必须将姓名、班级、学号完整填写在规定的密封栏目内,否则视为废卷。
3、...
4、Water is widely used in various process applications in industry. Other major industrial uses are boiler feed water and cooling water.The kind and degree of treatment of water in these applications depends upon the end use. As examples, cooling water may require only minimal treatment, while water used in food processing must be free ofpathogens(病原体) and toxid substances.(5 points)
化工专业英语练习测验题题目

1)过程指引起一种物质和(多种)物质地混合物发生物理变化或化学变化地操作或一系列操作.进入过程地物料称之为对该过程地输入或进料,而离开地物料称之为输出或产品.A process is any operation or series of operations that causes a physical or chemical change in asubstance or a mixture of substances . The material that enters a process is referred to as input or feed theprocess, and that which leaves is called output or product.2)B.Braun 成立于1839 年,从一个生产医药产品地小生产厂家不断发展,以成为一个跨国公司,现经营医药产品、医疗技术、药物及生物技术Founded in 1839 from a small production firm for pharmaceutical products, B. Braun has grown steadilyinto a multinational company dealing with medical products, medical technology, pharmaceutical andbiotechnology.b5E2R。
1)In the same way that a complex plant can be divided into basic unit operations, so chemical reactions involved in the process industries can be classified into certain groups, or unitprocesses (e.g., polymerizations, etherifications, and nitrations), have common characteristics.复杂地工厂可划分为基本地操作单元一样,过程工业中涉及地化学反应也可划分为一定地单元过程(如聚合、酯化、和硝化),它们具有共同地特性.p1Ean。
201202 化工专业英语练习题 题目 1-8

化学工程与工艺专业英语 习题 东华理工大学化学生物与材料科学学院练习一1 将下列句子或段落翻译成英语1)过程指引起一种物质和(多种)物质的混合物发生物理变化或化学变化的操作或一系列操作。
进入过程的物料称之为对该过程的输入或进料,而离开的物料称之为输出或产品。
2) B.Braun 成立于1839年,从一个生产医药产品的小生产厂家不断发展,以成为一个跨国公司,现经营医药产品、医疗技术、药物及生物技术。
2 将下列句子或段落翻译成汉语1)In the same way that a complex plant can be divided into basic unit operations, so chemical reactions involved in the process industries can be classified into certain groups, or unit processes (e.g., polymerizations, etherifications, and nitrations), have common characteristics. 2)The notion of a processing plant encompassing a number of operations, such as mixing,evaporation, and filtration, and of these operations being essentially similar, whatever the product, led to the concept of unit operations.3)The chemical engineer is often involved in “scaling up” a chemist-developed small-scale reactorand separation system to a very large commercial plant. The chemical engineer must work closely with the chemist in order to understand thoroughly the chemistry involved in the process and to make sure that the chemist gets the reaction kinetic data and the physical property data needed to design, operate, and optimize the process .4)All fluids are compressible - even water - their density will change as pressure changes. Under steady conditions, and provided that the changes in pressure are small, it is usually possible to simplify analysis of the flow by assuming it is incompressible and has constant density.5)In a region such as outer space, which is virtually void of gases, the pressure is essentially zero.Such a condition can be approached very nearly in a laboratory when a vacuum pump is used to evacuate a bottle. The pressure in a vacuum is called absolute zero, and all pressures referenced with respect to this zero pressure are termed absolute pressures.Many pressure-measuring devices like the manometer measure not absolute pressure but only difference in pressure. The reference pressure is actually the atmospheric pressure. Whenever atmospheric pressure is used as a reference, the possibility exists that the pressure thus measured can be either positive or negative. When this difference is positive, this type of pressure reading is called gage pressure.化学工程与工艺专业英语 习题 东华理工大学化学生物与材料科学学院练习二1.将下列句子或段落翻译成英语1) 图7所示的一个基本实例,是石油化工某部分的一个完整设计(主要是单元操作,换热网络和控制回路)。
化学专英试题及答案

化学专英试题及答案一、选择题(每题2分,共10分)1. The term "stoichiometry" refers to the:A. Study of chemical reactionsB. Calculation of amounts of reactants and products in chemical reactionsC. History of chemistryD. Physical properties of substances2. Which of the following is not a state of matter?A. SolidB. LiquidC. GasD. Energy3. The SI unit for the amount of substance is the:A. CoulombB. JouleC. MoleD. Newton4. In the periodic table, elements are arranged in order of increasing:A. Atomic massB. Atomic numberC. ElectronegativityD. Ionization energy5. The process of converting a solid to a liquid is called:A. SublimationB. VaporizationC. MeltingD. Decomposition二、填空题(每空1分,共10分)1. The chemical symbol for the element oxygen is ________.2. The law that states that the volume of a gas is directly proportional to the number of molecules is known as________'s law.3. The process of a substance changing from a liquid to a solid is called ________.4. The pH scale ranges from ________ to ________, with 7 being neutral.5. A compound that releases hydrogen ions when dissolved in water is known as an ________.三、简答题(每题5分,共20分)1. Explain what is meant by the term "valency" in chemistry.2. Describe the difference between a physical change and a chemical change.3. What is the significance of the Avogadro's number in chemistry?4. Discuss the role of catalysts in chemical reactions.四、计算题(每题10分,共20分)1. If 5 moles of a gas occupy 22.4 liters at standard temperature and pressure (STP), calculate the volume occupied by 10 moles of the same gas at STP.2. A 1.5 M solution of hydrochloric acid (HCl) is mixed witha 3.0 M solution of sodium hydroxide (NaOH) in a 1:1 volume ratio. Calculate the molarity of the resulting solution.五、实验题(每题15分,共30分)1. Describe a laboratory procedure to test for the presence of chloride ions in a solution.2. Outline the steps to prepare a standard solution of potassium permanganate (KMnO4) for titration.答案:一、选择题1. B2. D3. C4. B5. C二、填空题1. O2. Boyle3. Solidification4. 0, 145. Acid三、简答题1. Valency refers to the combining power of an element, which is the number of hydrogen atoms it can combine with or replace in a chemical reaction.2. A physical change is a change in the state or form of a substance without altering its chemical composition, while a chemical change involves a transformation that results in theformation of new substances.3. Avogadro's number (6.022 x 10^23) is significant becauseit represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance.4. Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process, thus facilitating the reaction without altering the overall chemical equilibrium.四、计算题1. 44.8 liters2. 0.75 M五、实验题1. To test for chloride ions, add a small amount of silver nitrate solution to the test solution. If a white precipitate forms, it indicates the presence of chloride ions.2. To prepare a standard solution of KMnO4, dissolve a known mass of the compound in a minimal amount of distilled water, then dilute it to a known volume in a volumetric flask. The concentration can be calculated using the mass and volume of the solution.。
化工专业英语试题及答案可编辑.doc

2014〜2015学年秋季学期化工专业英语期末考试一、简单词汇翻译(每题1分,共20分)1、Alkali ( 3、ammonia ( ))2、sulphuric (4、polymer ())5、polyethylene( )6、polyurethane ( )7、cyclohexane ( )8、hydrogen( )9、nitric ( ) 10> profitability( )11、Seale-up ( ) 12、leaching( )13、corriosion ( )14、distillation( )15、gradient ( ) 16> exothermic( )17> polycarbonate( )18> isothermal( )19> cybernetics ( )20 > filtration( )二、句子翻译(每题5分,共30分)1、Once the pilot plant is operational,performance and optimization data can be obtained in order to evaluate the process from an economic point of view.2、By contrast,the chemical engineer typically works with much larger quantities of material and with very large equipment.3、pressure drives the equilibrium forward ,as four molecules of gas are being transformed into two.4、What industry needs to achieve in the process is an acceptable combination of reaction speed and reaction yield.5、The ammonia and air mixture can be oxidized to dinitrogen and water.6> The important point to keep in mind is that all energy of all kinds must be included,although it may be converted to a single equivalent.三、化工专业名词书写(每题一分,共24分)1、加热()2、焙烧()3、吸收()4、冷凝()5、沉降()6、结晶()7、粉碎()8、电解()9、搅动()10、离心()11、平衡()12、体积()13、催化剂()14、一()15、二()16、三()17、四()18、五()19、六()20、七()21、八()22、九、()23、十()24、氮基化合物(四、表达方式运用,用括号里的单词翻译下列句子(每题5分,共20分)1、化学工程师经典的角色是把化学家在实验室里的发现拿来并发展成为能赚钱的、商业规模的化学过程。
化工英语试题及答案

化工英语试题及答案一、选择题(每题2分,共20分)1. Which of the following is not a type of catalyst used in chemical reactions?A. Homogeneous catalystB. Heterogeneous catalystC. Biological catalystD. Inert catalyst答案:D2. The process of converting raw materials into products in a chemical plant is known as:A. SynthesisB. DistillationC. ReactionD. Processing答案:D3. What is the term used to describe the separation of a mixture into its individual components?A. FiltrationB. EvaporationC. DistillationD. Crystallization答案:C4. In chemical engineering, what does the acronym "P&ID" stand for?A. Process and Instrumentation DiagramB. Product and Industry DesignC. Plant and Industrial DevelopmentD. Power and Industrial Devices答案:A5. Which of the following is a common method for measuring the concentration of a solution?A. SpectrophotometryB. ChromatographyC. TitrationD. All of the above答案:D6. What is the main purpose of a heat exchanger in a chemical process?A. To increase the temperature of the reactantsB. To cool down the productsC. To transfer heat between two fluidsD. To separate components of a mixture答案:C7. Which of the following is a unit of measurement for pressure?A. Pascal (Pa)B. Newton (N)C. Joule (J)D. Coulomb (C)答案:A8. What is the term used to describe a chemical reaction that produces energy in the form of heat or light?A. Endothermic reactionB. Exothermic reactionC. Isothermal reactionD. Photochemical reaction答案:B9. In the context of chemical engineering, what does the term "yield" refer to?A. The amount of product produced per unit of timeB. The percentage of theoretical product that is actually producedC. The efficiency of a chemical processD. The amount of raw material used in a process答案:B10. Which of the following is a type of pollution control technology used in chemical plants?A. ScrubbersB. FiltersC. Both A and BD. Neither A nor B答案:C二、填空题(每题2分,共20分)1. The chemical formula for water is __________.答案:H2O2. The SI unit for temperature is __________.答案:Kelvin (K)3. The process of converting a solid into a liquid by heating is called __________.答案:Melting4. A __________ is a device used to control the flow of a fluid in a pipeline.答案:Valve5. The __________ is a type of diagram that shows the relationship between different parts of a chemical process. 答案:Flowchart6. The term __________ refers to the study of the physical and chemical properties of materials.答案:Material Science7. The __________ is a type of equipment used to separate liquid mixtures based on differences in their boiling points. 答案:Distillation Column8. The __________ is a type of chemical reaction where two ormore substances combine to form a new compound.答案:Synthesis Reaction9. __________ is a method used to remove impurities from a substance by passing it through a semipermeable membrane.答案:Dialysis10. The __________ is a unit of measurement for the amount of substance in a system.答案:Mole三、简答题(每题10分,共40分)1. Explain the difference between a homogeneous and a heterogeneous catalyst.答案:A homogeneous catalyst is a catalyst that is in thesame phase as the reactants, usually a liquid or gas. A heterogeneous catalyst is a catalyst that is in a different phase from the reactants, typically a solid.2. Describe the purpose of a control system in a chemical process.答案:A control system in a chemical process is used tomonitor and adjust the process variables to maintain the desired operating conditions. This ensures the process runs efficiently, safely, and produces the desired product quality.3. What are the three main types of distillation processes?答案:The three main types of distillation processes aresimple distillation, fractional distillation, and vacuum distillation.4. Discuss the importance of safety measures in a chemical plant.答案:Safety measures in a chemical plant are crucial to prevent accidents, protect the health of workers, and minimize environmental impact. They include proper equipment design, training of personnel, emergency response plans, and adherence to safety regulations.。
化学化工专业英语试卷

element:11. orbital electron::2.In contrast to inorganic compounds, the molecularattraction of organiccompounds is weak, soorganic compounds areusually volatile andpossess low meltingpoints.3.Benzene can undergo the typical substitutionreactions ofhalogenation,nitration,sulphonation andFriedel-Craftsreaction. 4.Evaporation is conducted by vaporizing a portion ofthe solvent to produce aconcentrated solution orthick liquor.5.The presence of a substituent group inbenzene exerts aprofound control overboth orientation and theease of introduction ofthe enteringsubstituent.6.The functional group of a ketone consists of acarbon atom connected by adouble bond to an oxygenatom.7.At equilibrium, these two rate are equal; cupricion is still reactingwith ammonia moleculesto form the complex, andthe complex is stilldecomposing, but just asmuch cupric ammoniacomplex is beingdecomposed in unit timeas is being formed. 8.The reaction of an acid chloride with an amine isused commercially in themanufacture of the veryimportant range ofsemi-syntheticpenicilings,firstproduced by the BeechanGroup in 1959. 9.Thus satisfactory binding propertise are essentialfor trouble-freecompression and theproduction of goodquality cakes over longmanufacturing periods. 10.The synthesis of organic compounds involvesconversion ofavailable substancesof known structure,through a sequence ofparticular,controlled chemicalreactions, into othercompounds bearing adesired molecularstructure.The active ingredients were identified in the unsaponifiable fraction of this vegetable product. After solvent extraction and drying, the pure unsaponifiables are obtained in the form of a waxy solid. This waxy solid is then redissolved in untreated shea butter toincrease the unsaponifiable content and thus lead to the unsaponifiable shea butter concentrate. Used incosmetics at levels of up to 2%,it provides excellent protection against sunlight and skin dryness.Another example is the extract of the kola nut, known for its anti-irritant properties. As available in the market, it has an objectionable color and odor . At Estee Lauder, we analyzed and separated its constituents, identified the individual components with anti-irritantproperties, and recombined them in the most effective ratio. In the process , objectionable color and odor were removed and possible allergens 过敏原 eliminated. All this indicates that cosmetics formulated with plantextracts today can be more effective and , at the same time, more elegant than 10 or 20 years ago. 采用一种简单、可靠并且有效的气相色谱法,来同时测定草药鱼腥草和鱼腥草注射液中8种活性组分的含量;在研究的浓度范围内,发现鱼腥草中草药和注射液中8种活性组分的R 2的值高于,都有良好的线性行为,其日内和日间的精度都很高,其RSD 小于2%,且在三种不同浓度下获得的8种组分的平均回收率范围为%–%,RSD 为~%;该方法已成功应用于鱼腥草中草药和注射液的这8种活性成分的同时测定,包括不同厂家、不同批次生产注射液过程中的中间产物;这表明四、 Translate the following paragraph intoChinese本大题共1个小题,共25分五、 Translate the following paragraph intoEnglish 共15分本文提出的方法特别适合注射液的常规分析和在生产过程中的质量控制;。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
8. oxidation reaction:
9. organic chemistry: element:
enough area to merit
another separate aritcle. 2.In contrast to inorganic
compounds, the molecular
attraction of organic
compounds is weak, so
organic compounds are
usually volatile and
possess low melting
points.
3.Benzene can undergo the typical substitution
reactions of
halogenation,
nitration,
sulphonation and
Friedel-Crafts
reaction. 4.Evaporation is conducted by vaporizing a portion of
the solvent to produce a
concentrated solution or
thick liquor.
5.The presence of a substituent group in
benzene exerts a
profound control over
both orientation and the
ease of introduction of
the entering
substituent.
6.The functional group of a ketone consists of a
carbon atom connected by a
double bond to an oxygen
atom.
7.At equilibrium, these two rate are equal; cupric
ion is still reacting
with ammonia molecules
to form the complex, and
the complex is still
decomposing, but just as
much cupric ammonia
complex is being
decomposed in unit time
as is being formed. 8.The reaction of an acid chloride with an amine is
used commercially in the
manufacture of the very
important range of
semi-synthetic
penicilings,first
produced by the Beechan
Group in 1959. 9.Thus satisfactory binding propertise are essential
for trouble-free
compression and the
production of good
quality cakes over long
manufacturing periods. 10.The synthesis of organic compounds involves
conversion of
available substances
of known structure,
through a sequence of
particular,
controlled chemical
reactions, into other
compounds bearing a
desired molecular
structure.
The active ingredients were identified in the unsaponifiable fraction of this vegetable product. After solvent extraction and drying, the pure unsaponifiables are obtained in the form of a waxy solid. This waxy solid is then redissolved in untreated shea butter to
increase the unsaponifiable content and thus lead to the unsaponifiable shea butter concentrate. Used in cosmetics at levels of up to 2%,it provides excellent protection against sunlight and skin dryness.
Another example is the extract of the kola nut, known for its anti-irritant properties. As available in the market, it has an objectionable color and odor . At Estee Lauder, we analyzed and separated its
constituents, identified the individual components
with anti-irritant properties, and recombined them in the most effective ratio. In the process , objectionable color and odor were removed and possible allergens(过敏
原) eliminated. All this indicates that cosmetics formulated with plant
extracts today can be more effective and , at the same time, more elegant than 10 or 20 years ago.
采用一种简单、可靠并且有效的气相色谱法,来同时测定草药鱼腥草和鱼腥草注射液中8种活性组分的含量。
在研究的浓度范围内,发现鱼腥草中草药和注射液中8种活性组分的R2的值高于,都有良好的线性行为,其日内和日间的精度都很高,其RSD 小于2%,且在三种不同浓度下获得的8种组分的平均回收率范围为%–%,RSD为~%。
该方法已成功应用于鱼腥草中草药和注射
四、Translate the following paragraph into Chinese
(本大题共1个小题,共25分)
五、Translate the following paragraph into
English
(共15分)
液的这8种活性成分的同时测定,包括不同厂家、不同批次生产注射液过程中的中间产物。
这表明本文提出的方法特别适合注射液的常规分析和在生产过程中的质量控制。
