ACTs-GC研究 替吉奥 胃癌辅助化疗
奥沙利铂联合替吉奥在胃癌术后辅助化疗中的研究

( De p a r t me n t o f Th o r a c i c S u r g e r y, Du j i a n gy a n Mu n i c i p a l Pe o pl e 5 Ho s pi t a l , Du j i a n g y a n, S i c h u a n 6 1 1 8 3 0, Ch i n a ) Ab s t r a c t : Ob j e c t i v e To i n v e s t i g a t e t h e e f f i c a c y o f o x a l i p l a t i n p l u s S - 1 v e r s u s t h e XEL OX( o x a t i p l a t i n+ c a p e c i t a b i n e )p r o g r a m i n t h e a d j u v a n t c h e mo t h e r a p y i n t h e p a t i e n t s wi t h p o s t o p e r a t i v e g a s t r i c c a n c e r . Me t h o d s A t o t a l o f 8 1 c a s e s wi t h p o s t o p e r a t i v e g a s —
S t u d y o n o x a l i p l a t i n p l u s S - 1 v e r s u s XEL OX p r o g r a m i n a d j u v a n t c h e mo t h e r a p y o f p o s t o p e r a t i v e g a s t r i c c a n c e r
替吉奥联合顺铂新辅助化疗治疗胃癌疗效观察

较差异无统计学 意义 ( P> 0 . 0 5 ) 。观察组行根 治切 除术 例数 明显多 于对 照组 , 行姑 息性切 除术 和探查术 的例数 明显 少于对照组 。比较差异均有统计学 意义 ( P< 0 . 0 5 ) 。结论 替吉奥联 合顺 铂新辅助化疗 治疗 胃癌可 以提高患者行 根 治切除术 的机会 , 从 而提高患者 的长期生存率 。
o t e r a c i l p o t ss a i u m c a p s u l e s nd a c i s p l a t i n i n c o mb i n a t i o n it w h s u r g e y. r T h e s h o r t t e r m e f e c t i v e r a t e nd a o p e r a t i o n r e s e c t i o n s t a ・
w i t h c i s p l a t i n i n t h e t r e a t me n t o f p a i t e n  ̄w 址 h g a s t r i c c a n c e r W A N G
6 7 7 0 0 0, C h i n a
C u r a i t v e e f i f c a c y o b s e r v a i t o n o f n e o ・ a d j u v a n t c h e mo t h e r a p y o f g i me r a c l f a n d o t e r a c i l p o r a s s i u m c a p s u l e s c o mb i n e d
- w u : T h e P e o p l e S H o s p i t a l o fL / n c a n g , Y u n n a n
替吉奥胶囊单药用于老年胃癌患者术后辅助化疗的前瞻性对照研究

t r e a t e d b y S - 1 ( S - 1 4 0 — 6 0 m g p c , t w i c e a d a y , d 1 一 d 2 8 , 6 w e e k s w a s o n e c y c l e )a s t r e a t m e n t ro g u p .T h e a n o t h e r 2 6 c a s e s w e r e t r e a t e d b y
期) , 对照组 ( n= 2 6 ) 给予 F O L F O X 4方 案 辅 助 化 疗 ( 奥沙利铂 8 5 m g / m 静 滴 , d 。 ; 亚 叶酸钙 2 0 0 m g / m 静 滴 2 h , d ~d ; 氟 尿 嘧
啶4 0 0 mg / m 静滴 , d ~ d : ; 后续氟尿嘧啶 6 0 0 mg / m 持续静脉泵人 2 2 h , d ~d , 1 4天 为 1周期 ) 。观察 两组患者 的 1年 、 2年 生存率及无复发生存率 和不 良反应情况 。结果 两组患者均 完成 6个 月 的辅助化疗 , 治疗 组与对 照组 的 1年生存 率分别 为 6 9 . 2 3 %和 6 1 . 5 4 %( P> 0 . 0 5 ) , 1年无复发生存率分别为 5 3 . 8 5 %和 5 7 . 6 9 %( P> 0 . 0 5 ) ; 治疗组与对照组 的 2年生存率分别 为 3 4 . 6 2 %和 3 8 . 4 6 %( P> 0 . 0 5 ) , 2年无复发生存率分别 为 3 0 . 7 7 %和 2 3 . 0 8 %( P> 0 . 0 5 ) 。治 疗组 的恶心呕 吐 、 腹泻 等消化道 反应及周 围神经 毒性 的发生率较对照组明显降低( P< 0 . 0 5 ) 。结论 效不亚 于 F O L F O X 4方案联合 化疗 , 毒副反应低 , 患者依从性好 。 老年 胃癌患者 D 根治术后 接受 S - 1 单 药辅助化疗 的疗
替吉奥联合奥沙利铂新辅助化疗对胃癌的治疗效果观察

· 79 ·
替吉奥联合奥沙利铂新辅助化疗对胃癌的治疗效 果观察
张晓锋(许昌市建安区人民医院,河南 许昌 461000)
摘要:目的 探讨替吉奥联合奥沙利铂辅助化疗对胃癌的应用价值。 方法 回顾性分析治疗的 150 例胃癌患者的临
28.67±3.96 16.33±3.18
21.042 0.000
27.85±5.86 11.95±2.63
28.09±6.15 5.73±1.79
0.245
16.932
0.807
0.000
表 2 两组血管新生因子比较(x±s)
胰岛素样生长因子(μg/L)
行对比分析[J].世界最新医学信息文摘,2016,16(84):137. [10]宋 宇 博.硫 酸 镁 及 硫 酸 镁 与 硝 苯 地 平 联 合 治 疗 妊 高 症 的 临 床 疗 效
对照[J].实用妇科内分泌杂志(电子版),2017,4(5):107,109. 收稿日期:2018 ̄08 ̄17
· 80 ·
Mod Diagn Treat 现代诊断与治疗 2019 Jan 30(1)
表 1 两组肿瘤标志物水平比较(x±s)
糖类抗原 19-9(U/ml)
n
治疗前
治疗后
癌 胚 抗 原 (ng/ml )
治疗前
治疗后
对照组 75 61.78±11.95
观察组 75 62.43±13.35
t
0.314
P
0.754
临床疗效[J].中国医药指南,2017,15(20):132. [8]罗 德 英.硫 酸 镁 与 硫 酸 镁 联 合 硝 苯 地 平 治 疗 妊 高 症 临 床 效 果 探 究
多西他赛联合替吉奥新辅助化疗对进展期胃癌治疗的效果研究

49医学食疗与健康2018年6月临床研究多西他赛联合替吉奥新辅助化疗对进展期胃癌治疗的效果研究张翠朋贵州医科大学第二附属医院 贵州 凯里 556000【摘要】目的:研究多西他赛联合替吉奥新辅助化疗对进展期胃癌治疗的效果。
方法:选择2016年6月~2018年2月我院收治的72例进展期胃癌患者作为研究对象,随机将72例患者分成对照组(n =36)与观察组(n =36),对照组仅应用铂类与氟尿嘧啶进行治疗,观察组应用多西他赛联合替吉奥新辅助化疗治疗,比较2组治疗效果与不良反应。
结果:观察组治疗总有效率明显高于对照组,2组疗效对比差异显著(P <0.05);观察组不良反应发生率明显低于对照组,2组不良反应对比差异显著(P <0.05)。
结论:多西他赛联合替吉奥新辅助化疗对进展期胃癌治疗效果较好,而且不良反应较少,值得推广应用。
【关键词】多西他赛;替吉奥;新辅助化疗;进展期;胃癌;治疗效果[中图分类号]R735.2 [文献标识码]A [文章编号]2096-5249(2018)06-049-02胃癌是消化系统常见的恶性肿瘤,临床根治的唯一方案就是手术切除治疗,可是,在胃癌发病早期并没有明显的症状表现[1],大多患者一经确诊疾病就已进展到中晚期,很难实施手术根治,仅依靠手术治疗生存率仅在20%左右[2]。
本次研究中,选择2016年6月~2018年2月我院收治的72例进展期胃癌患者作为研究对象,随机将72例患者分成2组实施不同的治疗方案,结果对比如下。
1 资料与方法1.1 一般资料 选择2016年6月~2018年2月我院收治的72例进展期胃癌患者作为研究对象,入选对象经胃镜病理活检均确诊胃癌,根据CT 检查结果结果胃镜检查结果均可确诊为进展期胃癌,均未实施抗肿瘤治疗和放化疗治疗,排除了化疗禁忌证患者,所有患者预计生存期均大于3个月,排除了合并严重心脑血管及肝肾功能疾病、精神疾病患者[3]。
所有对象和家属均知情本次研究并签署同意书,经医院伦理委员会批准分组研究,根据手术前后顺序分组,随机将72例患者分成对照组与观察组,各36例。
紫杉醇联合替吉奥与单药替吉奥方案用于胃癌术后辅助化疗的临床研究

紫杉醇联合替吉奥与单药替吉奥方案用于胃癌术后辅助化疗的临床研究漆文新;卢亚萍;漆丽萍【摘要】目的探析紫杉醇联合替吉奥与单药替吉奥方案用于胃癌根治术后辅助化疗的临床效果.方法选取2014年5月~2016年5月本院收治的行胃癌根治术60例患者为研究资料,按照双盲法将其分两组,每组30例.予以对照组采取单用替吉奥方案,予以研究组采取替吉奥联合紫杉醇方案,观察比较不同治疗方案对胃癌术后辅助化疗的影响.结果通过对比两组患者的治疗失败的时间(TTF)能够看出,研究组的治疗失败的时间明显高于对照组,差异显著具有统计学意义(P<0.05);通过对比两组患者近1年的无复发生存率(RFS)情况能够看出,研究组的复发情况明显低于对照组,差异明显具有统计学意义(P<0.05);在对照组与研究组患者中,肝肾功能受损和骨髓抑制、末梢神经毒性和口腔黏膜炎及胃肠道反应等,均属于常见不良反应现象.对照组胃肠道反应和骨髓抑制反应情况显著低于研究组,组间数据差异存在统计学意义(P<0.05).结论对于胃癌根治术后患者,单药替吉奥方案和紫杉醇联合替吉奥方案都属于有效安全的辅助化疗方案,相比单药替吉奥方案,对有较好依从性患者采取紫杉醇联合替吉奥方案,所取得的效果更佳.【期刊名称】《当代医学》【年(卷),期】2017(023)032【总页数】3页(P44-46)【关键词】紫杉醇;替吉奥;胃癌;辅助化疗【作者】漆文新;卢亚萍;漆丽萍【作者单位】宜丰县人民医院肿瘤科,江西宜春 336300;宜丰县人民医院肿瘤科,江西宜春 336300;宜丰县人民医院肿瘤科,江西宜春 336300【正文语种】中文肿瘤疾病中胃癌属于临床常见且多发病症,具有较高的发病率。
幽门螺杆菌感染、饮食习惯及地域环境等,均易诱发胃癌病症的发生。
针对此病症,临床多采用化疗方法和手术方法治疗,其中,以手术方法最为常见[1-2]。
但由于胃癌病症的发病群体多以中老年患者居多,再加上随着年龄的递增,患者的机体功能会逐渐衰退,因此,为避免术后发生转移或者复发现象,予以相应的辅助化疗尤为重要。
替吉奥的作用机制及临床应用的研究发展

109CH INA FO REIGN MEDIC AL TRE ATMENT 中外医疗药物与临床1980年,5-FU用于胃癌化疗,是被认可的胃肠道肿瘤国际标准的治疗方案。
但5-FU 的静脉输注给药的毒性表现为胃肠道毒性。
人们希望开发出一种同时具有增强疗效和降低不良反应的抗肿瘤药物。
这一理念通过3种成分组成的复方制剂得到了实现,即替加氟(FT),吉美嘧啶(CDHP),奥替拉西钾(OXO)组成的复方制剂替吉奥(S-1)。
S-1是治疗已丧失了手术机会晚期胃癌及复发性胃癌较为显效的化疗药品。
无论是单药应用,还是与其他抗肿瘤药物联合应用,都有较为显著的效果。
1 作用机制在替吉奥这种复方制剂中,替加氟起抗癌作用,吉美嘧啶起增加疗效作用,奥替拉西钾能减低替加氟毒性反应。
替加氟是5-FU前体口服药物。
有优良的生物利用度和缓释能力,其口服后经肝微粒体的细胞色素P450酶系作用而转化为氟尿嘧啶,氟尿嘧啶经过酶转化为5-氟脱氧嘧啶核苷酸而具有抗肿瘤活性。
5-Fu通过抑制胸腺嘧啶核苷酸合成酶而抑制DNA的合成。
另外,5-Fu可整合到R N A 分子,从而破坏R N A 功能。
5-F u 的代谢主要在肝中进行,开环后变α-氟-β-脲基丙酸,再进一步分解为α-氟-β-丙氨酸、尿素及CO 2,大部分由呼吸排出[1]。
5-F U自问世以来,较广泛应用在消化道肿瘤治疗上,但5-F U 的半衰期短,仅10~20min与癌细胞作用时间短,且毒副反应相对较大,影响其抗癌效果[2];替加氟的毒性只有氟尿嘧啶1/4~1/7,化疗指数为氟尿嘧啶的2倍[3]。
替加氟所产生的氟尿嘧啶在体内极不稳定,易被在正常组织和肿瘤中的二氢嘧啶脱氢酶(DPD)快速降解(达85%以上)而失活[4]。
DPD是5-F u降解酶,而CD HP 是一种有效的5-FU 降解酶抑制剂,通过C DH P,能够长时间维持5-FU 血药浓度处于较高水平,肿瘤组织的5-Fu磷酸化产物5-氟核甘酸也可维持较高浓度,增强抗肿瘤疗效。
替吉奥在中晚期胃癌治疗中的研究进展

以上简单介绍了抗肿瘤新药替吉奥在晚期胃癌治疗的一 替吉奥的抗肿瘤活性 些研究成果。作为替加氟的改良制剂 , 谱与氟尿嘧啶相似, 尤其对进行性或晚期胃癌 、 胰腺癌、 结直 肠癌等作用更强。临床试验研究显示, 替吉奥治疗患者的耐 主要副反应仅为胃肠道毒性及骨髓抑制 , 并且他们 受性良好, 的严重程度及发生率相对较低 。替吉奥作为口服药与静脉内 可以门诊给药, 也可以为患者节 连续输注氟尿嘧啶效果相似 , 省开支。 老年胃癌患者往往罹患多种老年病 , 营养状况较差, 免疫 功能低下, 传统静脉化疗虽有一定疗效 , 但在延长生存期、 改 善生活质量方面疗效较差 , 且毒副作用难以耐受, 多采用姑息 [11 ] 治疗或放弃治疗 。对于老年中晚期胃癌患者 , 化疗已成为 如何合理的应用化疗药物 , 使其 积极治疗的主要手段。因此, 发挥最大疗效并尽可能减少其毒副反应是目前老年中晚期胃 癌化疗研究的热点。 目前替吉奥在国内尚处于临床试验阶段 , 从现有的临床 , , 数据看 替吉奥对胃癌疗效较为突出 有望成为胃癌化疗的一 并推广到多种实体瘤的治疗中 。 线用药, 参 考 文 献
·综
述·
《中国老年保健医学》 杂志 2011 年第 9 卷第 5 期
替吉奥在中晚期胃癌治疗中的研究进展
张 西
肿瘤科 610031
作者单位: 成都市第三人民医院
【关键词】 胃癌
晚期
替吉奥
胃癌是临床常见的恶性肿瘤之一 , 居全世界恶性肿瘤死 因第 4 位, 平均每年死亡病例约为 70 万 或直接浸润扩散
个胃癌发病率的 60% ~ 80% , 多伴有淋巴、 血行、 腹膜转移, 。而胃癌对化疗相对敏感 , 化疗是目前中 晚期胃癌的主要治疗手段 。 替吉奥( S - 1 ) 是一种氟尿嘧啶衍生物口服抗癌剂 , 它是 由替加氟、 吉美嘧啶、 奥替拉西钾所组成的复方胶囊制剂 。 替 2001 年被批准用于 吉奥在日本 1999 年被批准用于晚期胃癌 , 2003 年获准用于进行性或转移 进行性或复发性头颈部肿瘤 , 2004 年被批准用于进行性非小细胞肺癌 , 2005 性结直肠癌, 年获准用于转移性乳腺癌及转移性胰腺癌等治疗
多西他赛联合替吉奥对进展期胃癌进行辅助化疗的疗效和安全性分析

多西他赛联合替吉奥对进展期胃癌进行辅助化疗的疗效和安全性分析杨飞【摘要】目的探讨多西他赛联合替吉奥对进展期胃癌进行辅助化疗的疗效和安全性.方法 80例进展期胃癌患者,随机分为试验组和对照组,每组40例.试验组采用多西他赛联合替吉奥进行治疗,对照组采用多西他赛联合顺铂进行治疗.比较两组患者的临床疗效和不良反应发生情况.结果试验组患者的客观有效率(RR)为70.0%,显著高于对照组的47.5%,差异具有统计学意义(P<0.05).试验组患者的不良反应发生率为2.5%,显著低于对照组的15.0%,差异具有统计学意义(P<0.05).结论使用多西他赛联合替吉奥对进展期胃癌患者进行治疗,其临床效果较为显著,不良反应发生率低,值得在临床推广.【期刊名称】《中国现代药物应用》【年(卷),期】2019(013)010【总页数】2页(P71-72)【关键词】多西他赛;替吉奥;进展期胃癌【作者】杨飞【作者单位】116600 中国医科大学附属盛京医院大连医院肿瘤科【正文语种】中文目前, 在临床上进展期的胃癌患者复发率极高, 即使进行了最为标准的根治性切除术和淋巴结清扫术, 但是很难从生物学意义上对肿瘤进行根除[1]。
近几年, 随着对肿瘤生物学行为认识的逐步提高, 对胃癌的治疗手段进入了多学科模式, 根据胃癌的分期对胃癌患者进行治疗。
辅助化疗逐渐受到关注, 成为改善患者肿瘤负荷的主要方式, 替吉奥是一种新型口服氟尿嘧啶类药物, 单独使用有效率为28%~49%[2]。
多国学者均以多西他赛为基础的化疗方案进行了诸多改良, 争取得到不良反应少、治疗效果好的方案。
为了探索出更加理想的治疗方案, 作者从2017 年1 月对患者使用多西他赛联合替吉奥对胃癌进行治疗, 现报告如下。
1 资料与方法1. 1 一般资料选取2017 年1 月~2018 年1 月在中国医科大学附属盛京医院大连医院肿瘤科就诊的80 例进展期胃癌患者, 随机分为试验组和对照组, 每组40 例。
抗代谢类新药替吉奥片治疗复发的晚期胃癌的临床研究

价病灶最大径须≥1.0 cm ;查体的体表病灶最大径 学系对本项研究数据以符合方案集 (per-protocol set,
须≥2.0 cm,经 2 位临床医师确认,经 B 超证实并留有 PPS)进行统计分析。对两组资料的人口学特征、一般情
照片);年龄 18~75 岁,性别不限;体力状态:卡氏 况以及基线情况(疗前)进行可比性分析,计数资料采
>0.05
3(5.45) 1(2.13) <0.05
无伴随疾病 及其治疗史
9(16.36) 9(19.15)
>0.05
·343·
1 35 28 >0.05
病灶数量(个)
2
≥3
14
6
15
4
>0.05
>0.05
2.2 疗效 按 PPS 分析,单药组中位至肿瘤进展时间 示,单药组一线治疗优于顺铂联合 5-FU 方案[7]。
3 讨论 晚期胃癌的二线化疗常常难以兼顾延长患者的中
位生存时间和提高生活质量两方面。替吉奥最早是由 日本大鹏药品工业株式会社研制的一种复方抗癌药, 1999 年上市作为一线用药治疗胃癌,后来被用于治疗 头颈部肿瘤、非小细胞肺癌、转移性乳腺癌、结肠直肠 癌等的治疗[4-5]。替吉奥是由替加氟和其他 2 种生化修 饰剂吉美拉西、奥特拉西钾 3 种成分按照摩尔比 1∶0.4∶1 的比例组成,属第二代氟脲嘧啶类口服抗癌药,替加氟 为 5-FU 的前体药物,进入体内后通过肝脏 P450 酶转化 为 5-FU 后发挥抗癌作用,其他 2 种成分分别起增加 替加氟疗效和降低毒性的作用。吉美拉西为二氢嘧啶 脱氢酶抑制剂,能减缓 5-FU 降解。有报道,单独 5-FU 静脉输注,半衰期仅为 0.22 h,而口服替吉奥片复方制 剂后,5-FU 的半衰期延长至 1.73 h,显著提高了抗癌 活性[6]。奥替拉西钾能特异性抑制肠道黏膜细胞内乳清 酸核糖转移酶,降低 5-FU 在肠道组织内磷酸化,从而 降低 5-FU 引起的胃肠道毒性。吉美拉西及奥替拉西 钾 T1/2 和 Tmax 均与 5-FU 接近,从而能够很好发挥增效 减毒的协同作用。另外,用药过程中应注意定期检查肾 功能。国内 230 例患者参加的替吉奥片的研究结果显
多西他赛周疗法联合替吉奥用于胃癌术后辅助化疗及

多西他赛周疗法联合替吉奥用于胃癌术后辅助化疗及摘要:目的观察多西他赛周疗法联合替吉奥用于术后辅助化疗及晚期胃癌的近期疗效及相关副反应。
方法选取我院2009-2015年胃癌术后及晚期胃癌患者33例,给予多西他赛周疗法联合替吉奥,两疗程后进行效果评价。
结果周疗法多西他赛联合替吉奥近期疗效好,CR 1例(3.8%)。
PR 15例(57%),ORR 60.8%,中位PFS 6.6月。
结论:多西他赛周疗法联合替吉奥用于术后辅助化疗及晚期胃癌的近期疗效好,副反应可以耐受。
关键词:周疗法;多西他赛;替吉奥;胃癌术后;晚期胃癌Abstract:Objective To assess the recent curative efficacy and side reactian of docetaxel weekly plus S-1 in the treatment of postoperative gastric cancer and advanced gastric cancer.Methods From 2009 to 2015,33patients with postoperative gastric cancer and advanced gastric cancer admitted in our hospital wereselected.They were given chemotherapy of docetaxel weekly and S-1.Then we analyzed the adverse reactions and clinical efficacy after 2 weeks of treatment.Result:Docetaxel weekly plus S-1 was more recent curative effective(CR3.8%,PR 57%,ORR 60.8%,PFS 6.6M)and the side effects can be tolerated in the Treatment of postoperative gastric cancer and Advanced Gastric Cancer.Conclusion:Docetaxel weekly plus S-1 was more recent curative effective and the side effects can be tolerated in the Treatment of postoperative gastric cancer and Advanced Gastric Cancer.Key words:Weekly therapy;Docetaxel;S-1;postoperative gastric cancer;Advanced gastric cancer目前,紫杉类化疗药物在胃癌中占重要地位,包括紫杉醇,多西紫杉醇,白蛋白结合型紫杉醇等。
奥沙利铂联合卡培他滨序贯替吉奥方案在Ⅲ期胃癌术后辅助化疗中的疗效观察

奥沙利铂联合卡培他滨序贯替吉奥方案在Ⅲ期胃癌术后辅助化疗中的疗效观察王可武a ,胡琴a ,凌林b ,张涛a ,朱凌燕a ,殷飞a作者单位:芜湖市第二人民医院,a 肿瘤内二科,b普外科,安徽芜湖241000摘要:目的观察奥沙利铂联合卡培他滨(XELOX )序贯替吉奥方案作为Ⅲ期胃癌术后辅助化疗的有效性和安全性。
方法回顾性分析2012年1月至2016年12月芜湖市第二人民医院Ⅲ期胃癌术后辅助化疗病人128例,分为观察组58例和对照组70例,观察组接受XELOX 序贯替吉奥方案,对照组仅接受XELOX 方案。
比较两组肿瘤复发率、总生存时间(OS )、无复发生存时间(RFS )、无局部复发生存时间(LRRFS )和无远处转移生存时间(DMFS ),并比较两组不良反应。
结果观察组复发率低于对照组(53.4%比74.3%),差异有统计学意义(P <0.05)。
两组局部复发率比较,差异无统计学意义(18.9%比20.0%,P >0.05),观察组远处转移的发生率低于对照组(31.3%比48.6%,P <0.05)。
观察组和对照组的中位OS 分别为53.9月和46.2月(P <0.05),观察组和对照组的中位RFS 分别为33.0个月、27.3个月(P <0.05),观察组和对照组的中位LRRFS 分别为30.2个月和30.5个月(P >0.05),观察组和对照组中位DMFS 分别为32.3月和24.8月(P <0.05),观察组的DMFS 高于对照组。
无治疗相关性死亡病例,两组主要血液学不良反应发生率差异无统计学意义;两组色素沉着症发生率观察组比对照组发生率高(P <0.05),两组其余非血液学毒性比较,均差异无统计学意义。
结论XELOX 序贯替吉奥方案作为Ⅲ期胃癌术后辅助化疗方案能降低远处转移的复发率、推迟复发的发生、延长病人的生存时间、不良反应可耐受,值得进一步研究。
替吉奥及希罗达对比

客观分析国产替吉奥、进口替吉奥、希罗达对治疗胃癌单今天看到几个朋友因为家人受病魔折磨而烦恼,但又苦于化疗用药选择。
大家提到最多的就是替吉奥和希罗达(紫杉醇之类的忽略不计了),大家关心的就是替吉奥与希罗达疗效及价格。
现在我为大家做一个专业的分析。
先做价格的对比维康达与TS-1及希罗达比较维康达国产替吉奥胶囊鲁南制药集团规格:20mg*42粒/盒25mg*36粒/盒价格:2500元左右用药疗程:一天两次早晚各一次,一次40mg,14天一个疗程患者日均治疗费用158元TS-1进口替吉奥胶囊日本大鹏工业株式会社规格:20mg*140粒/盒价格:18500元用药疗程:一天两次早晚各一次,一次40mg,14天一个疗程患者日均治疗费用352元希罗达(卡培他滨)上海罗氏规格:500mg*12片/盒用药疗程:同样14天一疗程,一天6片一疗程价格3200患者日均治疗费用228元再做疗效的对比维康达治疗晚期胃癌:维康达单药有效率达44.6%S-1单药治疗晚期胃癌有效率达44.6%在Ⅱ期临床试验中S-1单药治疗晚期胃癌患者101例80mg/m2/d,连服4w,休息2w为一疗程结果平均有效率达44.6%(24%-54%)中位生存期为244d1年生存率36.6%2年生存率16.5%Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003;6 Suppl 1:2-8.S-1单药治疗晚期胃癌有效率达44.6%维康达(替吉奥胶囊)治疗晚期胃癌的临床试验总结报告临床试验批件号:2003L03165研究人员中国人民解放军总医院:主要研究者:焦顺昌杨俊兰军事医学科学院附属307医院主要研究者:徐建明山西省肿瘤医院主要研究者:牛润桂河北医科大学第四医院主要研究者:刘巍大连医科大学附属第二医院主要研究者:张阳中国人民解放军沈阳军区总医院主要研究者:谢晓冬山东省肿瘤医院主要研究者:宋恕平上海第二医科大学附属仁济医院主要研究者:张凤春福建医科大学附属协和医院主要研究者:卢辉山第二军医大学长征医院主要研究者:王杰临床试验统计单位:北京大学第一医院医学统计室姚晨接下来是一些专业的研究(由于我的资料是王金万教授给的幻灯片,在这里不能使用只能这样)ACTS-GC study(替吉奥术后辅助化疗)大型多中心随机临床试验结论:S-1术后辅助化疗对于胃癌是可行有效的,这种方法可以作为Ⅱ/Ⅲ期胃癌患者行D2切除术后的标准治疗!生存率:80.5%S-1胃癌术后辅助化疗与单纯手术的比较1059 例Ⅱ、Ⅲ期胃癌D2手术切除痊愈的患者随访3年S-1单药治疗组529 例生存率:70.1%Randomized phase III trial comparing S-1 monotherapy versus surgery alone for stage II/III gastric cancer patients (pts) after curative D2 gastrectomy (ACTS-GC study). 2007Gastrointestinal cancer symposium, sasako M单纯手术组530例ACTS-GC study JCOG术后3年生存率对比ACTS-GC study JCOG80.5%70.1%3年内无复发生存率对比ACTS-GC study JCOG72.2%60.1%S-1胃癌术后辅助化疗与单纯手术的比较临床结果:术后3年生存率单纯手术组70.1%,试验组80.5%3年内无复发生存率单手术组60.1%,试验组72.2%试验组死亡率比单纯手术组低32%!试验组复发率比单纯手术组低38%!S-1辅助化疗比单纯手术治疗降低胃癌死亡风险38%!ACTS-GC study JCOG维康达治疗AGC:维康达+CDDP 疗效优于单药S-1 + 顺铂vs S1Ⅲ期临床试验,日本38家临床中心,305例晚期胃癌患者,随机分配:联合:S-1 40mg/m2,每日2次,连用21天,DDP 60 mg/m2,第8天,休息2周,5周为一周期S-1单药:40mg/m2,bid,连4周休2周,6周为一周期Koizumi W, ASCO,2008SPIRITS trial转移性胃癌n=305例S-1单药: 40mg/m2, bid,连4周休2周S-1: 40mg/m2, bid, d1-21,DDP: 60mg/m2, iv, d8S-1 + 顺铂vs S1S-1+DDP,n=148S-1,n=150pORR54%(43-65%)31%(23-41%)mPFS6m4m<0.0001mOS13m11m0.04?度粒细胞减少39.9%10.7%?贫血25.7%4.0%?度恶心11.5%1.3%结果:Koizumi W, ASCO,2008SPIRITS trial结论S-1+CDDP 的生存期长于S-1 单药S-1中位生存11.0 MS-1+CDDP 13.0 MS-1+CDDP 耐受性好,两组均无治疗相关性死亡SPIRITS trialS-1+CDDP 方案可以作为AGC 的一线治疗方案S-1+CDDP方案被日本推荐作为治疗胃癌基本方案CAPE VS S-1转移性或复发性老年胃癌患者,随机、多中心II期临床研究65岁以上老年患者,随机分配,主要观察终点为有效率(RR)NCT00278863Journal of Clinical Oncology, 2007,ASCO转移性或复发性胃癌n=91例S-1: 40-60 mg/m2, bid,d 1–28,6 weeksCAPE: 1250 mg/m2,bid, d 1–14,3 weeks?CAPE VS S-1Journal of Clinical Oncology, 2007,ASCO6.8%手足综合症HFS2.3%腹泻9.5%6.8%厌食4.8%6.8%3/4粒细胞减少22.2%(10/45)21.7%(10/46)病情进展PD40.0%(18/45)38.6%(17/46)病情稳定SD26.7%(12/45)29.5%(13/46)部分缓解PR2.2%(1/45)完全缓解CR3.0m4.4m治疗失败时间TTF4.2m4.8m治疗进展时间TTP7.9m(4.1-11.7)10m(8.0-12.0)mOSS-1(S,n=45)卡培他滨(X,n=46)CAPE VS S-1研究结论:无论是卡培他滨还是S-1作为老年患者进展期胃癌的一线治疗都是有效、耐受的RR无统计学差异(P>0.05)不良反应无统计学差异(P>0.05)CAPE与S-1治疗胃癌都有很好的疗效,没有统计学差异,患者都可耐受对于二者的比较尚需大型的临床试验进一步探讨Journal of Clinical Oncology, 2007,ASCO另外根据研究表明替吉奥治疗胃癌在亚洲人种要明显优于希罗达(当然专业人士应该明白亚洲人种与欧美人种的差异,这就是美国学术界打得文字牌,只强调替吉奥对欧美人种有效率没有希罗达好,但避重就轻的不谈替吉奥对于亚洲人种有效率要优于希罗达,并且替吉奥化疗副作用要比希罗达轻)以上是我对三种化疗药物的泛泛浅谈请大家指点替吉奥和希罗达的区别(转载)发表者:单成祥740人已访问替吉奥口服治疗晚期胃癌在日本已经运用了10多年。
胃癌治疗新药替吉奥钾研究进展

胃癌治疗新药替吉奥钾研究进展替吉奥钾即S-1,由日本大鹏药品工业公司开发,1999年在日本首次获得批准,用于治疗胃癌,商品名为TS-1。
替吉奥钾实是抗肿瘤药替加氟(tegafur)的一种改进型制剂,内除替加氟这一活性成分外还含有两种用来调节替加氟生物效应的物质吉美司特(gimestat,亦称gimeracil,化学名为5-氯-2,4-二羟基吡啶,代号CDHP)和奥替托西钾(oteracil potassmm,化学名为1,2,3,4-四氢-2,4-二氧-1,3,5-三嗪6-羧酸钾,代号OXO),三种物质按序以1:0.4:l的摩尔比组成复方制剂。
替吉奥钾为胶囊剂,剂量规格有以替加氟剂量计的20和25mg/胶囊两种,标准给药方案是每6周为一疗程,其中前4wk每日2次、每次口服40~60mg(具体依患者的体表面积决定:<1.25m2服用40mg;1.25~1.5m2服用50mg;≥1.5m2服用60mg),而后停药2wk。
替吉奥钾目前已在日本成为进展期胃癌治疗和早期胃癌术后辅助治疗的最常用药物。
本文就该药的组方依据及临床研究进展作一概述。
1药学特性替吉奥钾所含活性成分是替加氟,后者为抗肿瘤药氟尿嘧啶(fluorouracil)的一种口服前体药物。
氟尿嘧啶最早于1957年在日本上市,由于对胃肠道肿瘤、头颈部肿瘤和乳腺癌等具有良好活性,故至今仍在临床上得到广泛应用。
不过,氟尿嘧啶单药治疗上述肿瘤患者的响应率仅在10%~30%间,同时毒性也较大且呈剂量方案依赖性。
例如,骨髓毒性是氟尿嘧啶快速推注给药的主要副反应,而手一足综合征、口炎和神经、心脏毒性则与氟尿嘧啶连续输注方案相关。
氟尿嘧啶的其它不良反应还有恶心、呕吐、腹泻、脱发和皮炎等。
为提高氟尿嘧啶的抗肿瘤效力并降低其毒、副反应,科学家们随后又相继开发出替加氟等一些氟尿嘧啶相关改进型产品。
其中替加氟属氟尿嘧啶前体药物,口服吸收后可经肝微粒体细胞色素P450酶系作用而转化为氟尿嘧啶。
2022胃癌的术后辅助化疗(全文)
2022胃癌的术后辅助化疗(全文)胃癌是最常见的恶性肿瘤之一,发病率及死亡率一直居高不下,严重危害人类的健康。
目前手术根治切除仍然是胃癌根治的主要手段,D2根治术已成为全球公认的可切除胃癌的标准术式,但中晚期胃癌复发率高达50%以上,规范的术后辅助治疗和定期的复查显得尤为重要。
近年来,通过不断地探索,胃癌的术后辅助治疗方案不断完善,疗效也在不断提升,这给患者带来了更多生存获益。
本文将向大家梳理胃癌术后辅助治疗的研究进展,供诸位读者阅读参考。
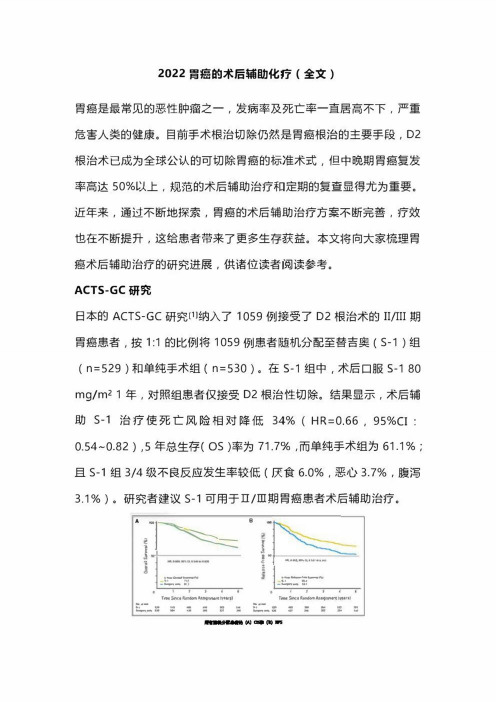
ACTS・GC研究日本的ACTS-GC研究⑴纳入了1059例接受了D2根治术的II/III期胃癌患者,按1:1的比例将1059例患者随机分配至替吉奥(S-1)组(n=529)和单纯手术组(n=530)。
在S・1组中,术后口服S-180 mg/m^1年,对照组患者仅接受D2根治性切除。
结果显示,术后辅助S-1治疗使死亡风险相对降低34%(HR=0.66,95%CI: 0.54~0.82),5年总生存(OS)率为71.7%,而单纯手术组为61.1%;且S-1组3/4级不良反应发生率较低(厌食6.0%,恶心3.7%,腹泻3.1%)。
研究者建议s-1可用于口/ni期胃癌患者术后辅助治疗。
CLASSIC研究⑵是一项随机、开放、平行对照的m期临床研究,纳入了中国大陆、中国台湾地区及薛国共35个中心1035例D2根治术后的II-IIIB期胃癌患者,随机分为单纯手术组(n=515)和术后辅助卡培他滨联合奥沙利钳(XELOX方案)化疗组(n=520)。
结果显示,无论3年无病生存(DFS)率还是5年DFS率,XELOX组较单纯手术组都显著获益(74%vs.59%,HR=0.56,95%CI:0.44-0.72, P<0.0001;68%vs.53%,HR=0.58,95%CI:0.47-0.72,P<0.0001)。
尽管XELOX组表现出较高的3/4级不良反应发生率(中性粒细胞减少22%,恶心8%,血小板减少8%),但基于此研究,XELOX方案也被推荐为胃癌术后辅助化疗的经典方案。
替吉奥联合奥沙利铂新辅助化疗对胃癌的治疗效果分析

替吉奥联合奥沙利铂新辅助化疗对胃癌的治疗效果分析【摘要】目的:探讨在胃癌治疗中以新辅助化疗方式治疗的价值。
方法:纳选对象为2022.01至2023.07在院治疗的60例胃癌患者,按数字奇偶法分成对照组与观察组,组内均30例患者,对照组采取常规化疗法,观察组采取新辅助化疗法,比对治疗效果。
结果:2组的治疗效果无明显差异,P>0.05;观察组的不良反应率低于对照组,P<0.05。
结论:以新辅助化疗法治疗有着疗效确切及安全性高特点,患者治疗耐受性较为良好,建议推广。
【关键词】胃癌;治疗效果;不良反应;新辅助化疗;奥沙利铂;替吉奥胃癌属于临床上常见恶性肿瘤之一,其发生率较高,常常会出现上消化道症状,对于中晚期胃癌患者而言,其体内的癌细胞很难以手术方式进行清除,所残留的癌细胞将对患者生命安全造成威胁,因此为了提高手术切除率并延长患者的生存时间,临床上常采用化疗的方式来降低病情严重程度,而不同的化疗方案对患者的影响也会有所不同[1-2]。
此试验将分析新辅助化疗在此类患者中的应用价值,总结如下:1.资料与方法1.1病例资料纳入者均患胃癌,纳入总数60例,分组采取随机信封法,对照组:年龄45岁至75岁(60.41±5.29岁),男18例女12例;观察组:年龄最低/最高40岁和80岁,平均60.62±5.31岁;性别男/女15例和15例。
组间各项资料无差异,可比对,P>0.05。
1.2方法2组入院后均接受常规基础性治疗,包括保护肝脏和胃部、纠正水电解质和酸碱平衡、抗感染等。
对照组接受常规化疗,治疗药物选择奥沙利铂、亚叶酸钙以及5-氟尿嘧啶,其中沙利铂用药量为剂量为85mg/m2,持续用药时间2h,亚叶酸钙用药剂量400mg/m2;5-氟尿嘧啶用药剂量400mg/m2,用药方式均为静脉滴注,持续用药5d,并辅以止吐、抑酸等治疗;观察组则接受新辅助化疗,药物选择替吉和奥沙利铂,其中奥沙利铂的用药量为130mg/m2,将其与5%的250m;葡萄糖溶液混合后以静滴方式用药,于2h内完成治疗;替吉奥胶囊以口服方式用药,剂量为40mg/m2,2次/日,于第1d到14d内持续进行用药,每隔3周用药1次,并辅以止吐、抑酸等治疗,2组至少接受为期2个周期化疗。
替吉奥联合奥沙利铂新辅助化疗治疗胃癌的应用效果分析

替吉奥联合奥沙利铂新辅助化疗治疗胃癌的应用效果分析王军【摘要】目的分析替吉奥联合奥沙利铂新辅助化疗治疗胃癌的应用效果.方法研究时间:2016年4月至2018年2月,研究对象:62例本院收治的胃癌患者,按照随机数字表法将其分为实验组(n=31)、对照组(n=31),给予实验组患者替吉奥+奥沙利铂新辅助化疗,给予对照组患者奥沙利铂+亚叶酸钙+5-氟尿嘧啶辅助治疗,观察两组患者的治疗效果、手术切除率、术后并发症发生率、生存率、生活质量.结果治疗效果对比实验组高于对照组,P<0.05.术后并发症发生率对比显示实验组低于对照组,P<0.05.生存率对比实验组高于对照组,P<0.05.生活质量对比实验组高于对照组,P<0.05.结论替吉奥联合奥沙利铂新辅助化疗治疗胃癌的应用效果较明显,生活质量较好,值得临床推广和应用.【期刊名称】《中国医药指南》【年(卷),期】2019(017)008【总页数】2页(P52-53)【关键词】替吉奥;奥沙利铂;辅助化疗;胃癌;生活质量【作者】王军【作者单位】包头市肿瘤医院,内蒙古包头 014030【正文语种】中文【中图分类】R735.2胃癌是胃黏膜上皮细胞的恶性肿瘤,发病率较高,多发于我国西北地区和东部沿海地区,好发年龄在50岁以上,多由于饮食结构的改变、工作压力增大、感染等原因导致,近年来胃癌的发病率逐渐呈现年龄化趋势,胃癌可发于胃体任何部位,多发于胃窦部、胃大弯、胃小弯及胃体前后壁,临床早期症状不明显,主要表现为疼痛和体质量减轻。
随着病情的加重,疼痛逐渐剧烈,食欲下降、浑身乏力等症状逐渐显现,肿瘤细胞破坏血管后,出现呕血、黑便等症状。
本次旨在研究吉奥联合奥沙利铂新辅助化疗治疗胃癌的应用效果,报道如下。
1 资料与方法1.1 一般资料:我院2016年4月至2018年2月收治的62例胃癌患者按照随机数字表法分为实验组和对照组,每组患者31例。
纳入标准:符合胃癌诊断标准的患者。
替吉奥胶囊联合奥沙利铂新辅助化疗治疗进展期胃癌的有效性和不良

替吉奥胶囊联合奥沙利铂新辅助化疗治疗进展期胃癌的有效性和不良反应分析发表时间:2016-07-14T16:10:47.763Z 来源:《健康世界》2016年第11期作者:吴志军[导读] 替吉奥胶囊联合奥沙利铂新辅助化疗治疗进展期胃癌疗效好,毒副反应小,安全性高。
湖南省常德市第一人民医院肿瘤科湖南常德 415000 摘要:目的:探讨替吉奥胶囊联合奥沙利铂新辅助化疗治疗进展期胃癌的有效性和不良反应。
方法:采用随即数字表法将70例进展期胃癌患者随机分为两组:研究组和对照组,每组患者各35例。
研究组患者采用替吉奥胶囊联合奥沙利铂新辅助化疗治疗,对照组患者采用FOLFOX治疗方案。
分别治疗三个周期后,观察并比较两组患者的治疗疗效以及不良反应发生情况。
结果:研究组患者的总有效率为71.43%显著高于对照组患者的45.71%(16/35),(P<0.05)。
CD8,CD4/CD8研究组放疗前后比较差异有统计学意义(P<0.05),中性粒细胞比较差异无统计学意义(P>0.05)。
对照组CD8,CD4/CD8治疗前、后比较差异无统计学意义(P>0.05),WBC、中性粒细胞差异有统计学意义(均P<0.05);CD8,CD4/CD8及中性粒细胞治疗后研究组显著高于对照组(P<0.05)。
两组患者的主要不良反应有血液学不良反应(血小板减少、白细胞减少、贫血)、消化道不良反应(恶心呕吐、腹泻)、神经毒性和器官损伤,其中血液学不良反应、神经毒性和肝肾损伤等不良反应的生生率比较两组均无统计学差异(均P>0.05),而对照组消化道不良反应的发生率显著高于研究组患者(均P<0.05)。
研究组患者治疗后1年生存率为85.71%、2年生存率为57.14%,1年复发率为20.00%,2年复发率为40.00%,对照组患者治疗后1年生存率为71.43%、2年生存率为42.86%,1年复发率为22.86%,2年复发率为42.86%,两组比较差异均无统计学意义(P >0.05)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1Versus Surgery Alone in Stage II or III Gastric CancerMitsuru Sasako,Shinichi Sakuramoto,Hitoshi Katai,Taira Kinoshita,Hiroshi Furukawa,Toshiharu Yamaguchi,Atsushi Nashimoto,Masashi Fujii,Toshifusa Nakajima,and Yasuo OhashiSee accompanying editorial on page4348;listen to the podcast by Dr Mayer at www.jco.org/podcastMitsuru Sasako,Hyogo College of Medicine,Nishinomiya;Shinichi Sakuramoto,Kitasato University School of Medicine,Sagamihara;Hitoshi Katai, National Cancer Center Hospital;Toshi-haru Yamaguchi and Toshifusa Naka-jima,Cancer Institute Hospital, Japanese Foundation for Cancer Research;Masashi Fujii,Nihon Univer-sity School of Medicine;Yasuo Ohashi, School of Public Health,The University of Tokyo,Tokyo;Taira Kinoshita, National Cancer Center Hospital East, Kashiwa;Hiroshi Furukawa,Sakai Municipal Hospital,Sakai;and Atsushi Nashimoto,Niigata Cancer Center Hospital,Niigata,Japan.Submitted April19,2011;accepted June30,2011;published online ahead of print at on October17, 2011.Written on behalf of the Adjuvant Chem-otherapy Trial of S-1for Gastric Cancer group.Supported by Taiho Pharmaceutical, Tokyo,Japan.Presented in part at the35th European Society for Medical Oncology Congress,Milan,Italy,October8-12, 2010.Authors’disclosures of potential con-flicts of interest and author contribu-tions are found at the end of this article.Clinical Trials repository link available on .Corresponding author:Mitsuru Sasako, MD,PhD,Department of Surgery, Hyogo College of Medicine,1-1 Mukogawa-cho,Nishinomiya,Hyogo,663-8501,Japan;e-mail: msasako@hyo-med.ac.jp.©2011by American Society of Clinical Oncology0732-183X/11/2933-4387/$20.00DOI:10.1200/JCO.2011.36.5908A B S T R A C TPurposeThefirst planned interim analysis(median follow-up,3years)of the Adjuvant Chemotherapy Trial of S-1for Gastric Cancer confirmed that the oralfluoropyrimidine derivative S-1significantly improved overall survival,the primary end point.The results were therefore opened at the recommendation of an independent data and safety monitoring committee.We report5-year follow-up data on patients enrolled onto the ACTS-GC study.Patients and MethodsPatients with histologically confirmed stage II or III gastric cancer who underwent gastrectomy with D2lymphadenectomy were randomly assigned to receive S-1after surgery or surgery only. S-1(80to120mg per day)was given for4weeks,followed by2weeks of rest.This6-week cycle was repeated for1year.The primary end point was overall survival,and the secondary end points were relapse-free survival and safety.ResultsThe overall survival rate at5years was71.7%in the S-1group and61.1%in the surgery-only group(hazard ratio[HR],0.669;95%CI,0.540to0.828).The relapse-free survival rate at5 years was65.4%in the S-1group and53.1%in the surgery-only group(HR,0.653;95%CI, 0.537to0.793).Subgroup analyses according to principal demographic factors such as sex, age,disease stage,and histologic type showed no interaction between treatment and any characteristic.ConclusionOn the basis of5-year follow-up data,postoperative adjuvant therapy with S-1was confirmed to improve overall survival and relapse-free survival in patients with stage II or III gastric cancer who had undergone D2gastrectomy.J Clin Oncol29:4387-4393.©2011by American Society of Clinical OncologyIn2008,there were737,000deaths from gastric can-cer worldwide.Gastric cancer is the second leadingcause of cancer-related death,with the highest mor-tality rates in East Asia,including Japan,Korea,andChina(28.1per100,000in males;13.0per100,000in females).1Approximately60%of gastric cancersin the world are diagnosed in this area.The mainstayof treatment for gastric cancer is surgery.However,in stages II(excluding T1disease)and III(moder-ately advanced),an appreciable proportion of pa-tients have recurrence,even after curative resection.Consequently,various regimens for adjuvant chem-otherapy have been implemented to prevent post-operative recurrence.Although the results of many randomized,controlled studies conducted to verify the effective-ness of postoperative adjuvant chemotherapy forgastric cancer were negative on an individual studybasis,meta-analyses of these results have suggestedthat postoperative adjuvant chemotherapy is thera-peutically useful in patients with gastric cancer.2-7However,no regimens have been clearly recom-mended for adjuvant chemotherapy after gastrec-tomy with D2lymphadenectomy(D2gastrectomy),established as the standard procedure for advancedgastric cancer in East Asia.J OURNAL OF C LINICAL O NCOLOGYV O L U M E29⅐N U M B E R33⅐N O V E M B E R202011©2011by American Society of Clinical Oncology4387 Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.The Adjuvant Chemotherapy Trial of S-1for Gastric Cancer (ACTS-GC)is a randomized phase III trial to confirm the effectiveness of1-year postoperative treatment with S-1compared with surgery alone in patients with stage II or III gastric cancer who underwent D2 gastrectomy.S-1(TS-1;Taiho Pharmaceutical,Tokyo,Japan)is a dihydropyrimidine dehydrogenase inhibitoryfluoropyrimidine prep-aration combining tegafur,gimeracil,and oteracil potassium in a molar ratio of1:0.4:1.8,9Two phase II studies10,11in patients with advanced or recurrent gastric cancer obtained high response rates exceeding40%.Postoperative adjuvant chemotherapy with S-1was thus expected to be effective.In this phase III trial,1,059patients with histologically confirmed stage II or III gastric cancer who underwent D2gastrectomy were enrolled.A protocol-based interim analysis performed1year after the completion of enrollment(median follow-up,3years)confirmed that S-1was effective.Because statistical analysis indicated that there was minimal probability that the results of this study would turn out to be negative after5years of follow-up,an independent data and safety monitoring committee recommended that the results should be dis-closed at that time.An analysis of the results available at that time showed that the3-year overall survival(OS)was80.1%in the S-1 group compared with70.1%in the surgery-only group.S-1was dem-onstrated to reduce the risk of death by32%(hazard ratio[HR],0.68; 95%CI,0.52to0.87;Pϭ.003).12Although the study results were disclosed early because of these promising results,we considered it important to have5-year follow-up data available.Such data would facilitate a comparison of our results for5-year OS and other out-comes with those of previous trials.Moreover,this analysis may justifyFig1.CONSORT diagram.D1gastrec-tomy;ITT,intent-to-treat.Fig2.Kaplan-Meier estimates of(A)overall survival and(B)relapse-free survival for all randomly assigned patients.HR,hazard ratio.Sasako et al4388©2011by American Society of Clinical Oncology J OURNAL OF C LINICAL O NCOLOGY Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.the present controversial use of3-year relapse-free survival(RFS)as the primary end point in clinical trials of adjuvant chemotherapy for potentially curable gastric cancer.PATIENTS AND METHODSThe trial was conducted in accordance with the World Medical Association Declaration of Helsinki and Japanese Good Clinical Practice guidelines. This protocol was approved by the institutional review board of each participating hospital(see Data Supplement).Written informed consent was obtained from all patients.Tumor stage classification and D classifica-tion were in accordance with the Japanese Classification of Gastric Carci-noma(Second English Edition).13Patients and TreatmentEligibility criteria were as follows:a histopathologically confirmed diag-nosis of stage II(except for T1disease),IIIA,or IIIB gastric cancer;R0resection (with no tumor cells at the margin)with D2or more extensive lymph node dissection;no evidence of hepatic,peritoneal,or distant metastasis;no tumor cells in peritonealfluid on cytologic analysis;age20to80years;no previous treatment for cancer except for the initial gastric resection for the primary lesion;and adequate organ function.Patients were enrolled within6weeks after surgery over the telephone or by means of facsimile.Patients were ran-domly assigned to either the S-1group or the surgery-only group.The assign-ments were made by the minimization method according to disease stage(II, IIIA,or IIIB)at the ACTS-GC data center.Patients assigned to the S-1group received S-1in a daily dose of80,100, or120mg in two divided doses.The dose of S-1was assigned on the basis of body surface area.S-1was given for4weeks,followed by2weeks of rest. Treatment was continued for1year after surgery.Patients assigned to the surgery-only group received no anticancer treatment postoperatively until the confirmation of recurrence.The criteria for dose reduction and toxicity were described previously.12Follow-UpIn the S-1group,the results of blood tests and clinicalfindings were assessed at2-week intervals during treatment with S-1.In the surgery-only group,patients came to the hospital for re-examination at least once every3 months for thefirst year after surgery.From the second year onward,all patients were followed up in the same manner.Relapse was confirmed by imaging studies,including ultrasonography,computed tomography,and GI radiography,as well as endoscopy.Patients underwent at least one imaging study at6-month intervals for thefirst2years after surgery and at1-year intervals until5years after surgery.Individual patients were followed up for5 years from the date of random assignment.Fig3.Subgroup analysis:overall survival and relapse-free survival for eligible population.In the surgery-only group,cancers in three patients could not be classified as differentiated or undifferentiated.HR,hazard ratio;UICC,International Union Against Cancer(UICC)TNM Classification of Malignant Tumours.5-Year Results of S-1Adjuvant Therapy in Gastric Cancer©2011by American Society of Clinical Oncology4389 Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.Statistical AnalysisThe sample size was calculated as follows.Given that the5-year survival rate would be70%in the surgery-only group,with an HR of0.70,␣ϭ.05 (two-sided),and a statistical power of80%,we estimated that1,000patients would be required.OS and RFS were estimated on the basis of all randomly assigned patients.The results in eligible patients were analyzed according to disease stage.OS was defined as the interval from the date of random assign-ment to the date of death from any cause.RFS was defined as the interval from the dateofrandomassignmenttothedateofconfirmingrecurrenceordeathfromany cause,whichever camefirst.Data for up to5years from the date of random assignment were analyzed.Data obtained after5years were not included in this analysis.The survival rate was estimated by using the Kaplan-Meier method.The Cox proportional hazards model was used to calculate HRs.All statistical analyses were done with SAS,version9.1(SAS Institute,Cary,NC).PatientsFrom October2001through December2004,a total of1,059 patients were enrolled at109centers throughout Japan;529were assigned to the S-1group and530to the surgery-only group (intention-to-treat population;Fig1).In both groups combined, 474patients(44.8%)had stage II disease,409(38.6%)had stage IIIA disease,and175(16.5%)had stage IIIB disease.The numbers of patients with each stage of disease were similar in the two treatment groups.The groups were also well balanced with respect to the type of gastrectomy performed,the combined resection of other organs,and other factors.Details of the patient demograph-ics and baseline characteristics have been reported previously.12 Fourteen patients in the S-1group and11in the surgery-only group were ineligible,as shown in Figure1.In the S-1group,12patients did not receive S-1.In the surgery-only group,four patients received adjuvant treatment at their strong request,violating the protocol.SafetyDetails of the safety analysis have been reported previously.12In brief,except for anorexia(incidence,6%),grade3or4adverse events occurred in less than5%of the patients in the S-1group.OS and RFS in All Randomly Assigned Patients Among1,059patients,145and199died,32and42patients are alive with recurrence,and352and289patients are alive without recurrence in the S-1and the surgery-only groups,respectively.Data on131patients lost to follow-up within5years from the date of random assignment were censored.OS and RFS were analyzed in all1,059randomly assigned patients. The5-yearOSratewas71.7%(95%CI,67.8%to75.7%)intheS-1group and61.1%(95%CI,56.8%to65.3%)in the surgery-only group.The HR for death in the S-1group compared with the surgery-only group was 0.669(95%CI,0.540to0.828),indicating that S-1reduced the risk of death by33.1%(Fig2A).The5-year RFS rate was65.4%(95%CI,61.2% to69.5%)in the S-1group and53.1%(95%CI,48.7%to57.4%)in the surgery-only group.The HR for relapse in the S-1group compared with that in the surgery-only group was0.653(95%CI,0.537to0.793).Treat-ment with S-1thus reduced the risk of relapse by34.7%(Fig2B). Subgroup AnalysisOS and RFS in eligible patients were analyzed according to sex, age,disease stage(Japanese Classification,13th edition),and histo-logic type.There was no interaction between treatment and any of these factors(Fig3).Kaplan-Meier estimates of OS and RFS are shown according to disease stage,which was used as a stratification factor when patients were randomly assigned(Figs4,5,and6).The5-year OS rates of the patients with stage II disease were 84.2%(95%CI,79.5%to89.0%)in the S-1group and71.3%(95%CI, 65.3%to77.2%)in the surgery-only group,with an HR of0.509(95% CI,0.338to0.765;Fig4A).Their5-year RFS rates were79.2%(95% CI,73.8%to84.6%)in the S-1group and64.4%(95%CI,58.1%to 70.7%)in the surgery-only group,with an HR of0.521(95%CI,0.362 to0.750;Fig4B).The5-year OS rates of stage IIIA patients were67.1% (95%CI,60.4%to73.8%)intheS-1groupand57.3%(95%CI,50.3% to64.2%)in the surgery-alone group,with an HR of0.708(95%CI, 0.510to0.983;Fig5A).Their5-year RFS rates were61.4%(95%CI, 54.5%to68.4%)in the S-1group and50.0%(95%CI,42.9%to 57.0%)in the surgery-alone group,with an HR of0.696(95%CI,Fig4.Kaplan-Meier estimates of(A)overall survival and(B)relapse-free survival for eligible patients with stage II gastric cancer.HR,hazard ratio.Sasako et al4390©2011by American Society of Clinical Oncology J OURNAL OF C LINICAL O NCOLOGY Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.0.514to0.941;Fig5B).As for stage IIIB disease,we enrolled90patients in the S-1group and85in the surgery-only group;the5-year OS rates were50.2%(95%CI,39.5%to61.0%)in the S-1group and44.1% (95%CI,33.1%to55.0%)in the surgery-alone group,with an HR of 0.791(95%CI,0.520to1.205;Fig.6A).Their5-year RFS rates were 37.6%(95%CI,27.0%to48.2%)intheS-1groupand34.4%(95%CI, 24.1%to44.7%)in the surgery-alone group,with an HR of0.788 (95%CI,0.539to1.151;Fig6B).Site of First RelapseCommon sites offirst relapse were the peritoneum,hema-togenous sites,and lymph nodes(Table1).Rates of metastasis and relapse were consistently lower in the S-1group than in the surgery-only group for all sites.In particular,the rates of recur-rence in lymph nodes and of peritoneal relapse were markedly lower in the S-1group.DISCUSSIONTo the best of our knowledge,the ACTS-GC study is thefirst large clinical trial of adjuvant chemotherapy enrolling more than1,000 patients who underwent D2gastrectomy for gastric cancer.The results of this follow-up study showed that1-year treatment with S-1im-proved OS and RFS at5years compared with surgery alone,thus reconfirming the conclusions reached on early publication of the study results after a median follow-up of3years.Fig5.Kaplan-Meier estimates of(A)overall survival and(B)relapse-free survival for eligible patients with stage IIIAgastric cancer.HR,hazard ratio.Fig6.Kaplan-Meier estimates of(A)overall survival and(B)relapse-free survival for eligible patients with stage IIIB gastric cancer.HR,hazard ratio.5-Year Results of S-1Adjuvant Therapy in Gastric Cancer©2011by American Society of Clinical Oncology4391 Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.Our present results confirmed that postoperative adjuvant chemotherapy with S-1alone reduced the risk of death by 33.1%,thereby demonstrating that effectiveness was maintained since the previous analysis.This reduction in the risk of mortality is comparable with that obtained with combined regimens for adjuvant chemotherapy intheMedicalResearchCouncilAdjuvantGastricInfusionalChemother-apy (MAGIC)trial 14and the Intergroup 0116(INT-0116)trial.15Whether the results of this study can be extrapolated to countries outside East Asia remains uncertain because of possible differences in pharmacokinetics of S-1between whites and East Asians.If S-1is used as adjuvantchemotherapyinwhites,thedoseshouldbecarefullyadjusted.A second reason is that all patients in this study underwent D2gastrectomy although more limited surgery (D0/1)is commonly performed in the United States and some parts of Europe.In the surgery-only group,OS at 5years was 61.1%,which was much better than that of patients undergo-ingD2gastrectomyinEurope(33%)inaDutchtrial.16Oneofthereasons for this large difference may be the high level and widespread use of diagnostic technology in Japan,potentially leading to stage migration between Japan and Western countries.17Another important reason might be the high quality of D2gastrectomy in Japan,whereas D0or D1gastrectomyremainsthestandardprocedureintheUnitedStatesandwas the standard in Europe until recently.Although a Dutch trial comparing D1with D2gastrectomy reported negative results,16,18a 15-year follow-up study showed that the rate of mortality from gastric cancer was significantly lower in the D2gastrectomy group.19Thus,the most recent European Society for Medical Oncology (ESMO)clinical practice guide-lines recommend D2gastrectomy as the standard procedure for curable advanced gastric cancer.20The primary end point of this study was 5-year OS,although that of an ongoing adjuvant chemotherapy study in Korea and China is 3-year disease-free survival.The latter is designed to evaluate the efficacy of postoperative adjuvant chemotherapy with capecitabine and oxaliplatin compared with surgery alone.To justify the use of RFS or disease-free survival as the primary end point for adjuvant chemotherapy after cura-tive resection of gastric cancer,more evidence is needed,but the results of this study may strongly suggest that RFS can be used as the primary end pointofsuchstudies.(Inthisfollow-upanalysis,the3-yearRFSrateswere 72.4%and 61.1%,and the 5-year OS rates were 71.7%and 61.1%in the S-1group and surgery-only group,respectively.)To compare our results with those of other foreign studies,we also report the stage-specific 3-and 5-year OS and RFS according to the International Union Against Cancer (UICC)TNM Classification of Ma-lignant Tumours,Sixth Edition.Three-year OS rates according to UICCand surgery-only groups were 91.1%and 80.9%(stage (stage IIIA),66.6%and 56.8%(stage IIIB),and and 56.7%(stage IIIA),44.8%and 28.9%(stage IIIB),(stage IV).Five-year OS rates were 83.4%and 68.9%and 56.2%(stage IIIA),43.7%and 40.1%(stage and 42.7%(stage IV).Five-year RFS rates were 77.9%II),64.3%and 48.7%(stage IIIA),35.9%and 28.9%and 25.0%(stage IV).for adjuvant chemotherapy differs among East including Japan,in which D2gastrectomy has long procedure,and Western countries,in which D0or used to be or currently is standard.As Cunningham and Chua 21stated,“surgery alone”is no longer standard treatment anywhere in the world for advanced gastric cancer.Some type of adjuvant chemotherapy,including the use of radiotherapy after D0/1resection,can thus be considered standard treatment at present.Ameta-analysisbytheGlobalAdvanced/AdjuvantStomachTumor Research International Collaboration (GASTRIC)group 7showed that some form of postoperative chemotherapy is associated with a higher survival rate than surgery alone;moreover,the use of monotherapy for postoperative adjuvant treatment resulted in good outcomes.The ACTS-GC trial demonstrated that S-1monotherapy improved OS and RFS.In patients with early-stage (II and IIIA)tumors,the benefits of treatment with S-1were considerable.However,the 5-year OS rate in patients with stage IIIB disease was 50.2%in the S-1group and 44.1%in the surgery-only group,suggesting that there remains some room for improvement.Future studies should evaluate the effectiveness of inten-sive preoperative and/or postoperative chemotherapy with multiple agents in patients at high risk for relapse.The results of the S-1plus cisplatin versus S-1in randomized con-trolled trial in the treatment for stomach cancer (SPIRITS)trial,22dem-onstrating that S-1plus cisplatin is superior to S-1alone with respect to survival in patients with unresectable or recurrent gastric cancer,and the V325study[arandomized,multinationalphaseII/IIItrialofpatientswith untreated advanced gastric cancer],23,24showing that the addition of do-cetaxel to cisplatin plus fluorouracil prolongs survival,indicated that S-1plus cisplatin and S-1plus docetaxel are candidate regimens for postop-erative adjuvant chemotherapy.These regimens were confirmed to be feasible in a postoperative setting,25,26and further studies should be per-formed to examine whether such regimens are superior to S-1alone.The Japan Clinical Oncology Group (JCOG)is now performing the JCOG 0501study to compare S-1plus cisplatin as neoadjuvant chemo-therapy with surgery followed by S-1monotherapy in patients with clini-cally resectable Borrmann type 4(linitis plastica)and large type 3gastric cancer.This trial is expected to be a landmark study,determining the future direction for preoperative chemotherapy in Japan.The use of molecular targeted agents for gastric cancer has been studied extensively.In the Trastuzumab in Combination with Chemo-therapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Esophageal Junction Cancer (ToGA)study,trastuzumab combined with cisplatin and either fluorouracil or capecit-abine significantly prolonged OS in patients with HER2-positive gastric cancer.27The effectiveness of adjuvant chemotherapy with molecular targeted agents such as trastuzumab also needs to be assessed in patients with HER2-positive gastric cancer.In conclusion,this 5-year follow-up study confirmed that adju-vant chemotherapy with S-1given for 1year after surgery improvedSasako et al4392©2011by American Society of Clinical OncologyJ OURNAL OF C LINICAL O NCOLOGYDownloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.OS and RFS at5years in patients with stage II or III gastric cancer who underwent D2gastrectomy.Postoperative chemotherapy with S-1 can be recommended for patients with stage II or III gastric cancer who undergo D2gastrectomy,at least in Asian populations. Although all authors completed the disclosure declaration,the following author(s)indicated afinancial or other interest that is relevant to the subject matter under consideration in this article.Certain relationships marked with a“U”are those for which no compensation was received;those relationships marked with a“C”were compensated.For a detailed description of the disclosure categories,or for more information about ASCO’s conflict of interest policy,please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.Employment or Leadership Position:None Consultant or Advisory Role:Mitsuru Sasako,sanofi-aventis K.K.(C),Taiho Pharmaceutical (C),Chugai Pharmaceutical(C);Atsushi Nashimoto,Taiho Pharmaceutical(C);Masashi Fujii,Taiho Pharmaceutical(C);Toshifusa Nakajima,Taiho Pharmaceutical(C);Yasuo Ohashi,Taiho Pharmaceutical(C)Stock Ownership:Masashi Fujii,Otsuka Holdings Honoraria:Mitsuru Sasako,sanofi-aventis K.K.,Bayer Yakuhin,Genzyme Japan K.K.,Novartis Pharma K.K.,Taiho Pharmaceutical, Bristol-Myers Squibb,Yakult Pharmaceutical Industry;Shinichi Sakuramoto,Taiho Pharmaceutical;Taira Kinoshita,Taiho Pharmaceutical;Hiroshi Furukawa,Taiho Pharmaceutical;Toshiharu Yamaguchi,Taiho Pharmaceutical;Atsushi Nashimoto,Taiho Pharmaceutical;Masashi Fujii,Taiho Pharmaceutical;Toshifusa Nakajima,Taiho Pharmaceutical;Yasuo Ohashi,Taiho Pharmaceutical Research Funding:Mitsuru Sasako,Taiho Pharmaceutical,Bristol-Myers Squibb,Chugai Pharmaceutical,sanofi-aventis K.K.; Shinichi Sakuramoto,Taiho Pharmaceutical Expert Testimony:None Other Remuneration:NoneConception and design:Mitsuru Sasako,Taira Kinoshita,Hiroshi Furukawa,Toshiharu Yamaguchi,Atsushi Nashimoto,Masashi Fujii, Toshifusa Nakajima,Yasuo OhashiCollection and assembly of data:Mitsuru Sasako,Shinichi Sakuramoto, Hitoshi Katai,Taira Kinoshita,Hiroshi Furukawa,Toshiharu Yamaguchi,Atsushi Nashimoto,Masashi FujiiData analysis and interpretation:Mitsuru Sasako,Toshifusa Nakajima, Yasuo OhashiManuscript writing:All authorsFinal approval of manuscript:All authors1.Ferlay J,Shin HR,Bray F,et al:Cancer Inci-dence and Mortality Worldwide:IARC CancerBase No.10,2010.http://www.iarc.fr/en/publications/ eresources/cancerbases/index.php2.Hermans J,Bonenkamp JJ,Boon MC,et al: Adjuvant therapy after curative resection for gastric cancer:Meta-analysis of randomized trials.J Clin Oncol11:1441-1447,19933.Piedbois P,Buyse M:Meta-analyses need time, collaboration,and funding.J Clin Oncol12:878-880,19944.Earle CC,Maroun JA:Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients:Revisiting a meta-analysis of ran-domised trials.Eur J Cancer35:1059-1064,19995.Mari E,Floriani I,Tinazzi A,et al:Efficacy of adjuvant chemotherapy after curative resection for gastric cancer:A meta-analysis of published ran-domised trials—A study of the GISCAD(Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente).Ann Oncol11:837-843,20006.Panzini I,Gianni L,Fattori PP,et al:Adjuvant chemotherapy in gastric cancer:A meta-analysis of randomized trials and a comparison with previous meta-analyses.Tumori88:21-27,20027.GASTRIC(Global Advanced/Adjuvant Stom-ach Tumor Research International Collaboration) Group,Paoletti X,Oba K,et al:Benefit of adjuvant chemotherapy for resectable gastric cancer:A meta-analysis.JAMA303:1729-1737,20108.Shirasaka T,Shimamato Y,Ohshimo H,et al: Development of a novel form of an oral5-fluorouracil derivative(S-1)directed to the potentiation of the tumor selective cytotoxicity of5-fluorouracil by two biochemical modulators.Anticancer Drugs7:548-557,19969.Diasio RB:Clinical implications of dihydropy-rimidine dehydrogenase inhibition.Oncology(Willi-ston Park)13:17-21,199910.Sakata Y,Ohtsu A,Horikoshi N,et al:Latephase II study of novel oralfluoropyrimidine antican-cer drug S-1(1M tegafur-0.4M gimestat-1Motastat potassium)in advanced gastric cancer pa-tients.Eur J Cancer34:1715-1720,199811.Koizumi W,Kurihara M,Nakano S,et al:Phase IIstudy of S-1,a novel oral derivative of5-fluorouracil,inadvanced gastric cancer:For the S-1Cooperative GastricCancer Study Group.Oncology58:191-197,200012.Sakuramoto S,Sasako M,Yamaguchi T,et al:Adjuvant chemotherapy for gastric cancer with S-1,anoralfluoropyrimidine.N Engl J Med357:1810-1820,200713.Japanese Gastric Cancer Association:Japa-nese Classification of Gastric Carcinoma-2nd Eng-lish Edition.Gastric Cancer1:10-24,199814.Cunningham D,Allum WH,Stenning SP,et al:Perioperative chemotherapy versus surgery alonefor resectable gastroesophageal cancer.N EnglJ Med355:11-20,200615.Macdonald JS,Smalley SR,Benedetti J,et al:Chemoradiotherapy after surgery compared withsurgery alone for adenocarcinoma of the stomach orgastroesophageal junction.N Engl J Med345:725-730,200116.Bonenkamp JJ,Hermans J,Sasako M,et al:Extended lymph-node dissection for gastric cancer.N Engl J Med340:908-914,199917.Bunt AM,Hermans J,Smit VT,et al:Surgical/pathologic-stage migration confounds comparisonsof gastric cancer survival rates between Japanand Western countries.J Clin Oncol13:19-25,199518.Bonenkamp JJ,Songun I,Hermans J,et al:Randomised comparison of morbidity after D1andD2dissection for gastric cancer in996Dutch pa-ncet345:745-748,199519.Songun I,Putter H,Kranenbarg EM,et al:Surgical treatment of gastric cancer:15-yearfollow-up results of the randomised nationwideDutch ncet Oncol11:439-449,201020.Okines A,Verheij M,Allum W,et al:Gastriccancer:ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up.Ann Oncol21:v50-v54,201021.Cunningham D,Chua YJ:East meets West inthe treatment of gastric cancer.N Engl J Med357:1863-1865,200722.Koizumi W,Narahara H,Hara T,et al:S-1pluscisplatin versus S-1alone forfirst-line treatment ofadvanced gastric cancer(SPIRITS trial):A phase IIIncet Oncol9:215-221,200823.Van Cutsem E,Moiseyenko VM,Tjulandin S,et al:Phase III study of docetaxel and cisplatin plusfluorouracilcompared with cisplatin andfluorouracil asfirst-line ther-apy for advanced gastric cancer:A report of the V325Study Group.J Clin Oncol24:4991-4997,200624.Ajani JA,Moiseyenko VM,Tjulandin S,et al:Quality of life with docetaxel plus cisplatin andfluoroura-cil compared with cisplatin andfluorouracil from a phaseIII trial for advanced gastric or gastroesophageal adeno-carcinoma:The V-325Study Group.J Clin Oncol25:3210-3216,200725.Fujitani K,Tamura S,Kimura Y,et al:Phase IIfeasibility study of adjuvant S-1plus docetaxel forstage III gastric cancer patients after curative D2gastrectomy(OGSG0604).J Clin Oncol27,2009(suppl;abstr e15567)26.Takahari D,Hamaguchi T,Yoshimura K,et al:Feasibility study of adjuvant chemothe rapy with S-1plus cisplatin for gastric cancer.Cancer ChemotherPharmacol67:1423-1428,201127.Bang YJ,Van Cutsem E,Feyereislova A,et al:Trastuzumab in combination with chemotherapy ver-sus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophagealjunction cancer(ToGA):A phase3,open-label,ran-domised controlled ncet376:687-697,2010■■■5-Year Results of S-1Adjuvant Therapy in Gastric Cancer©2011by American Society of Clinical Oncology4393 Downloaded from on March 3, 2014. For personal use only. No other uses without permission.Copyright © 2011 American Society of Clinical Oncology. All rights reserved.。
