化学原料药开发-异构体的分类 ISOMERS
顺反异构体定义

顺反异构体定义
顺反异构体(Stereoisomers)是指具有相同的分子式和相同的原子连接顺序,但由于原子在空间排列不同而导致分子整体构型不同的一对或一组化合物。
顺反异构体是一种立体异构现象,可分为两大类:构象异构体(Conformational Isomers)和构型异构体(Configurational Isomers)。
1. 构象异构体
构象异构体是由于单键周围的原子或基团的旋转而产生的异构现象。
构象异构体之间可以通过旋转单键相互转化,能垒较低,属于动力学异构体。
常见的构象异构体有:
- 烷烃的旋转异构体
- 环丁烷的椅式和船式构象
- 环己烷的椅式和船式构象
2. 构型异构体
构型异构体是由于分子中含有手性中心或手性轴而产生的异构现象。
构型异构体之间不能简单地通过旋转单键相互转化,需要断开化学键并重新连接。
常见的构型异构体有:
- 手性碳原子引起的异构体(对映异构体)
- 手性轴引起的顺反异构体
- 手性平面引起的顺反异构体
顺反异构体是指由于分子中存在手性轴或手性平面而导致整体构型不同的一对立体异构体。
它们是构型异构体的一种,与对映异构体并列,是立体化学的重要内容。
FDA关于开发立体异构体新药的政策简介

FDA关于开发立体异构体新药的政策简介1. 引言与背景立体异构体是原子组成及键接相同而原子在三维空间排列上不同一些分子。
因此,成对的立体异构体即是那些具有一个或多个不对称中心(手性的)且对映结构体(单个对映体)互成镜像的化合物。
它们一般具有相同的物理(除旋光性外)和化学(手性环境下除外)特性。
本文着重讨论法有关单个对映体及外消旋物的研究及药物开发问题。
对这种立体异构体的鉴别、定性、分离及测定一般需要专门的手性技术来进行。
它们通常采用生物技术能容易区分开来,然而,它们具有不同的药代动力学特性(如:吸收,分布,生物转化及排泄)及不同的药理或毒理作用。
当立体异构体在生物学上可区分时,它们似乎为不同的药物,然而,过去往往把它们做成外销旋物(如:两者按50:50的比例组成外消旋物)。
对单个对映体的特性一般研究得不是很深入。
是否能分离对映体很大程度上是个学术问题,因为工业上分离外消旋体较为困难。
既然技术的发展(大规模的手性分离程序及不对称合成方法)已经可容许在工业规模上生产出许多单个对映体,那么,适当地考虑一下FDA对立体异构体混合物的方针是合适的。
开发外消旋体生产过程要考虑的问题包括合成及杂质控制,适当的药理学及毒理学评估,合适的新陈代谢及分布属性,以及适当的临床评估。
“立体异构体”此术语应当解释为所有异构体仅原子在空间的方向不同。
立体异构体不仅包括互成镜像的对映异构体,还包括几何异构体(顺式/反式)和非对映的异构体(若药物含有一个以上的手性中心其异构体相互之间不成镜像的异构体)。
非对映异构体和几何异构体在大多数情况下,化学性质和药理上都存在较大差异(除非它们在体内可以相互转化),一般无需手性技术便可容易地分离。
在个别例外情况即非对映异构体和几何异构体在体内可发生相互转化时,因此,它们就要分别处理和开发。
一般没理由去考虑开发非对映异构体或几何异构体混合物,除非它们之间偶然显示出固定剂量的组合(参见21 CFR 300.50)。
液相色谱 异构体

液相色谱异构体全文共四篇示例,供读者参考第一篇示例:液相色谱(Liquid Chromatography,LC)是一种高效、快速的分离和检测方法,广泛应用于化学、生物化学、药学和环境等领域。
在LC中,液体相相对于固定相以液体形式存在,通过固定相对分析物的吸附、分配和排它作用,使得分离和检测成为可能。
在液相色谱中,异构体(Isomer)是一个常见的概念。
异构体指的是分子结构相同但空间结构不同的化合物。
由于分子的结构和构型的不同,异构体可能在性质上存在差异。
在进行化合物分离和鉴定时,对异构体的分离和检测也是非常重要的。
液相色谱在分析异构体方面具有很大的优势。
液相色谱具有良好的分离能力,可以有效地将不同异构体分离开来。
液相色谱具有高灵敏度和高选择性,可以对微量的异构体进行检测。
液相色谱还可以对复杂样品进行分析,满足实际应用中对异构体监测的需求。
不同类型的异构体在液相色谱中的分离方法也不同。
在立体异构体(Stereoisomers)的分离中,通常采用手性色谱柱进行分离。
手性色谱柱是一种特殊的固定相,具有手性选择性,可以有效地分离手性异构体。
在构象异构体(Conformational isomers)的分离中,通常采用柱温控制、淋洗溶剂的调整等方法进行分离。
对于药物分析中的异构体,液相色谱也发挥着重要作用。
由于药物分子的异构体对药效和毒性有很大影响,因此对药物中异构体的检测和分析至关重要。
通过液相色谱技术,可以对药物中异构体进行高效分离和检测,进而为药物研发和质量控制提供支持。
液相色谱在异构体分离和检测中具有显著的优势,为化学、生物化学、药学和环境等领域的研究提供了重要的技术支持。
随着技术的不断进步和发展,液相色谱在异构体分析领域的应用前景将更加广阔,为科学研究和实际应用带来更多的可能性。
第二篇示例:液相色谱(Liquid Chromatography,LC)是一种高效分离和分析化合物的技术,广泛应用于生物化学、制药、环境监测和食品安全等领域。
有机化学基础知识点整理有机分子的立体异构体分类和性质

有机化学基础知识点整理有机分子的立体异构体分类和性质有机化学基础知识点整理有机分子的立体异构体分类和性质引言:有机化学是研究有机物质的组成、结构、性质、合成、反应与应用的科学。
在有机化学中,立体异构体是一种重要的概念。
立体异构体是指具有相同分子式但空间构型不同的有机分子。
本文将对有机分子的立体异构体进行分类和性质的整理。
一、立体异构体的分类1. 构象异构体(conformational isomers):构象异构体是由于化学键的旋转所产生的异构体。
这种异构体在分子内部的空间构型上有不同的构象,但它们之间的键没有断裂或形成新的键。
常见的构象异构体有转式异构体、扭式异构体和轴式异构体等。
2. 构造异构体(constitutional isomers):构造异构体是由于分子内部原子连接方式的不同而产生的异构体。
这种异构体在原子的连接方式上有所区别,导致它们具有化学性质和物理性质上的差异。
常见的构造异构体有链式异构体、环式异构体和官能团异构体等。
3. 光学异构体(optical isomers):光学异构体是由于分子中手性中心的存在而产生的异构体。
光学异构体的分子拥有相同的构成式,但它们的立体构型是镜像对称的,无法重合。
光学异构体对于旋光性是有影响的,其中左旋异构体为L型,右旋异构体为D型。
二、立体异构体的性质1. 空间构象的影响:构象异构体的不同空间构象对于分子的稳定性、形状、反应性等都有影响。
例如,转式异构体的存在使得分子中的取向限制,并影响其反应性能。
2. 化学性质的差异:构造异构体的存在导致分子之间具有不同的化学性质。
例如,链式异构体由于原子连接方式的不同,其分子之间的键能和键长都会有所差异,从而影响分子的化学性质。
3. 光学活性:光学异构体的存在使得有机分子具有光学活性,能够影响其对极化光的旋光性。
光学异构体的相关性质对于化学和生物学领域具有重要的应用价值。
4. 热力学稳定性:不同立体异构体的热力学稳定性各不相同。
异构体的分类

异构体是指以化学结构相似但空间排列不同的分子,它们的分类可以根据它们的结构类型和差异程度进行。
一般而言,异构体可以分为以下几类:
位置异构体(position isomers):这种异构体指的是在分子中化学基团的位置不同,比如2-丙醇和1-丙醇就是位置异构体。
构造异构体(constitutional isomers):这种异构体指的是化学结构不同的分子,因为它们的原子组成或键的连接方式不同。
比如,在分子式为C3H6O的分子中,丙酮和乙醛就属于构造异构体。
立体异构体(stereoisomers):这种异构体指的是有着相同分子式和分子结构的分子,但它们的立体结构不同。
主要分成两种类型,一种是光学异构体(optical isomers)、另一种是对映异构体(enantiomers),常常用手性来描述其特征。
动力学异构体(kinetic isomers):这种异构体指的是在反应条件下化学反应路径和产物不同,在分子的动力学行为引起结构上不同的分子。
例如轴向对称化反应和不对称化反应。
总的来说,异构体是指在分子结构上略有差异但具有相同原子组成的分子形式。
它们之间的分类,通常基于不同的差异类型,例如位置、构造、立体或动力学方面的差异程度进行划分。
新药研究中的异构体分离与制备

新药研究中的异构体分离与制备新药研究是现代医学的重要领域之一,随着科技的进步,人们对新药的要求也越来越高。
在新药的研发过程中,药物的异构体分离与制备显得尤为重要。
那么什么是药物的异构体呢?异构体指的是同一化合物的不同构型,它们在分子结构的排列方式、性质以及药效等方面均存在差异。
在药物研发过程中,通常只有一种异构体拥有所需的药效,而其它异构体则有可能会产生不良反应或者将药效削弱甚至抵消掉。
因此,分离出所需的异构体并制备成药物是非常关键的。
异构体的分离通常需要运用到不同的技术手段,常用的包括黄体萃取、手性分离柱、毛细管电泳、高效液相色谱等等。
其中,手性分离柱是一种较为可靠的异构体分离方法,它可以通过分离手性化合物的手性中心来将不同的异构体分离开来。
手性分离柱的原理是使用特定的手性淀粉酚或者纳米级胶体颗粒配制成手性固定相,并将需要分离的手性化合物通过固定相进行分离。
这种方法简单、可靠,可以广泛应用于药物的股份中。
除了分离异构体,制备药物的合理方法也至关重要。
药物的制备通常需要经历大量的试验、优化和改良,其中制备方法的选择及其配对也对药物的质量和效果产生着极大的影响。
为了达到最佳的研发效果,通常需要背景扎实的实验人员不断进行探索和研究,寻找更好的制备方法并优化提升。
在这个过程中,需要不断试验和验证,通过对反应物、催化剂、溶剂、温度、时间等多个方面的优化,最终制备出符合药品质量标准的药物。
同时,新药研究面临着越来越复杂的挑战,除了需要考虑到药物的异构体分离和制备外,还需要考虑到人体素质、生理状况、对药物的吸收、代谢和排泄等多方面的因素,以充分评估新药的疗效和安全性。
因此,新药研究需要跨学科的团队持续投入大量的时间和精力,来确保药物的质量和效果,同时为疾病治疗作出更大的贡献。
总之,药物的异构体分离与制备是新药研究中的重要环节,分离出所需的异构体并制备成药物可以保证药物的药效和安全性。
然而,单靠这一过程仍然不能保证新药的疗效,还需要充分考虑到人体生理条件等多方面的因素,来进行全面评估。
n,n-亚甲基双丙烯酰胺 同分异构体

n,n-亚甲基双丙烯酰胺同分异构体N,N-亚甲基双丙烯酰胺是一种有机化合物,拥有多个同分异构体。
同分异构体是指化学结构相同但排列不同的化合物。
以下将介绍N,N-亚甲基双丙烯酰胺的几个典型同分异构体。
The compound in question is N,N'-methylenebisacrylamide, which possesses various structural isomers. Isomers refer to compounds with the same chemical formula but different arrangements. We will now explore several distinct isomers of N,N'-methylenebisacrylamide.首先介绍的是顺式同分异构体。
这种顺式异构体中,两个丙烯酰胺官能团在分子结构中彼此相对而位于同一侧。
这导致了分子链的直线排列,形成了一个平面结构。
在这种同分异构体中,两个氨基乙烯基部分与两个丙烯酰胺部分交替进行相互作用。
The first type to be discussed is the cis isomer. In this cis isomer, the two acrylamide functional groups are positioned on the same side of the molecule. This results in a linear arrangement of the molecular chain, forming a planar structure. In this isomer, the aminoethyleneportions alternate with the acrylamide portions, interacting with each other.接下来是反式同分异构体。
miRNA异构体(isomiRs)与靶向识别新机制miRNA专题

miRNA异构体(isomiRs)与靶向识别新机制miRNA专题miRNA是长度22nt左右的小RNA,在个体生长发育和疾病发展中发挥重要作用。
传统观点认为,在动物中miRNA靶向mRNA依赖于miRNA的种子区域(seed region,2-7nt)与mRNA 3'非翻译区(Untranslated Regions, UTR)的碱基互补配对,在不减少mRNA 数量的情况下达到抑制mRNA翻译蛋白的功能。
最为常规的研究即是围绕这些miRBase收录的经典miRNA及其调控机制进行的。
然而,近年来的研究结果提出了一些新的看法。
一IsomiRs简介首先,随着高通量测序技术的发展,早期认为的miRNA loci仅产生1条miRNA成熟体序列这一观点被颠覆。
对于某一miRNA来说,它并不是单一的序列,而是由一系列长度/序列及表达不同的异构体(isomiRs)组成。
这些isomiRs表达多样且序列多样,甚至引入多样的5'端及种子区域。
特定miRNA位点在疾病组织中可具有异常的表达模式,现已证实,部分isomiRs具有重要的生物学功能。
1. IsomiRs的产生前人已对miRNA的产生机制有了较为详细地表述(示意图如下),这里不再赘述。
但需要大家了解到Darsha和Dicer酶在其中扮演着重要角色,这也是isomiRs产生的重要因素之一。
图1 动物和植物体内miRNA的形成原理IsomiRs的产生机制主要有:miRNA加工和成熟过程中Darsha 和Dicer酶的不精确或选择性剪切;3'端核苷酸添加;RNA编辑和单核苷酸多态性( single nucleotide polymorphism,SNP)。
主要表现为:5'端修剪;3' 端修剪;3'端核苷酸附加和碱基替换。
其中,5'端修剪和碱基替换可发生在种子区域内部,产生“种子转移”(seed shifting)【1】。
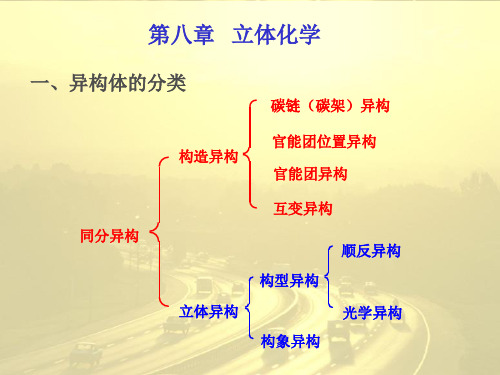
立体化学异构体的分类
任何化合物都有镜像,但多数实物和它的镜像都能 重合。如果实物和它的镜像能重合,它们就是同一 物质,是非手性的,无对映体。
对映异构体的性质
COOH
COOH
一对对映体结构
C
CH3
H
OH
C
H
CH3
HO
差别很小,因此 它们具有相同的 bp , mp , 溶 解 度
(S)-(+)-乳酸 mp 53oC
H
COOH
COOH H
COOH H
(1R,2R)-1,2-环 丙烷二甲酸
H
COOH
(1S,2S)-1,2-环 丙烷二甲酸
COOH COOH
H
H
该顺式化合物与上面的两个反 式异构体互为非对映异构体。
取代环己烷旋光性的情况分析
例一:顺-1,2-二甲基环己烷
旋转120o
(1)和(2)既是构 象转换体,又
COOH
COOH
(1)
(2)
(3) (+)-酒石酸
mp
(+)-酒石酸 170oc
(-)-酒石酸
170oc
()-酒石酸 (dl) 206oc
meso-酒石酸 140oc
[]D(水) +12.0 -12.0 0 0
溶解度(g/100ml) 139 139 20.6 125
COOH HO H (S)
H OH (S)
CH2OH
(iii)
(2S,3R)-(+)-苏阿糖
CHO
H OH R HO H S
CH2OH
(iv)
(2R,3S)-(-)-苏阿糖
2. 含两个相同手性碳原子化合物的对映异构
以酒石酸为例
异构体 英文 标准

异构体英文标准Isomers: The Fascinating World of Molecular DiversityIsomers, a fundamental concept in chemistry, refer to molecules that share the same chemical formula but differ in their arrangement of atoms. This structural variation gives rise to a vast array of unique compounds, each with its own distinct properties and applications. Exploring the world of isomers is akin to unveiling the intricate tapestry of the molecular realm, where the slightest rearrangement can lead to remarkable differences in behavior and function.At the heart of isomerism lies the concept of stereochemistry, which deals with the spatial arrangement of atoms within a molecule. Molecules can be classified into various types of isomers, each with its own set of rules and characteristics. Structural isomers, for instance, arise when the carbon skeleton of a compound is arranged differently, leading to distinct molecular shapes and functionalities. Geometric isomers, on the other hand, occur when double bonds restrict the rotation of atoms, resulting in cis and trans configurations.One particularly intriguing type of isomerism is known as opticalisomerism, where molecules exhibit a property called chirality. Chiral molecules are non-superimposable mirror images of each other, much like our hands. These enantiomers, as they are called, can have vastly different biological and pharmacological properties, with one form often being beneficial while the other may be harmful or even toxic.The significance of isomers extends far beyond the realm of academic interest. In the pharmaceutical industry, the identification and separation of enantiomers have become crucial in the development of safe and effective drugs. Many medications, such as the anti-nausea drug thalidomide, have shown that the different isomeric forms can have drastically different effects on the human body. Understanding and controlling isomerism has, therefore, become a vital aspect of modern drug design and production.Beyond the pharmaceutical realm, isomers play a vital role in various other fields, including materials science, energy storage, and environmental chemistry. Certain isomeric forms of carbon, such as graphene and carbon nanotubes, possess remarkable electronic and mechanical properties that have revolutionized the development of advanced materials and technologies.In the realm of energy storage, isomeric forms of organic molecules have been explored as potential candidates for high-density andsustainable energy storage systems. The ability to tune the molecular structure and the resulting properties of these isomers has opened up new avenues for the development of more efficient and environmentally friendly energy solutions.Furthermore, the study of isomers has implications in environmental chemistry, where the identification and tracking of specific isomeric forms can aid in the detection and remediation of environmental pollutants. Certain isomeric compounds may exhibit different toxicological profiles or undergo distinct degradation pathways, making their identification crucial for effective environmental management.In the realm of analytical chemistry, the separation and characterization of isomers have become essential tools for researchers and industry professionals. Techniques such as chromatography, mass spectrometry, and nuclear magnetic resonance spectroscopy have been extensively employed to identify and quantify the various isomeric forms present in complex mixtures.As the field of chemistry continues to evolve, the understanding and manipulation of isomers will undoubtedly play an increasingly pivotal role in driving scientific and technological advancements. From the development of novel pharmaceuticals to the design of advanced materials and energy storage systems, the versatility and importanceof isomers cannot be overstated.In conclusion, the world of isomers is a fascinating and multifaceted realm that encompasses a wide range of scientific disciplines. By unraveling the intricate relationships between molecular structures and their corresponding properties, researchers and scientists are unlocking new possibilities and pushing the boundaries of what is achievable in the ever-evolving landscape of chemistry and beyond.。
异构化

异构化中文名称:异构化英文名称:isomerization定义:一种同分异构体与另一种同分异构体相互转化的作用或过程。
改变化合物的结构而不改变其组成和分子量的过程。
一般指有机化合物分子中原子或基团的位置的改变。
常在催化剂的存在下进行。
催化剂主要有下列几类:①弗瑞德-克来福特型催化剂,常用的有三氯化铝-氯化氢、氟化硼-氟化氢等。
这类催化剂活性高,所需反应温度低,用于液相异构化,如正丁烷异构化为异丁烷,二甲苯的异构化等。
②以固体酸为载体的贵金属催化剂,如铂-氧化铝、铂-分子筛、钯-氧化铝等。
这类催化剂属于双功能催化剂,其中金属组分起加氢和脱氢作用,固体酸起异构化作用。
采用这类催化剂时,反应需在氢存在下进行,故也称临氢异构化催化剂,用于气相异构化。
烷烃、烯烃、芳烃、环烷烃的异构化也可采用。
尤其是乙苯异构化为二甲苯和环烷烃的异构化只有这类催化剂有效。
其优点是结焦少,使用寿命长。
③以固体酸为载体的非贵金属催化剂,如镍-分子筛等,一般也需有氢存在,用于气相异构化,但不能使乙苯异构化成二甲苯。
④ZSM-5分子筛催化剂,主要用于二甲苯的气相或液相异构化。
过程条件异构化是可逆反应,反应常常可进行到接近平衡转化率。
由于反应热效应很小,温度对平衡组成影响不甚显著,但低温操作有利于减少副反应。
液相异构化反应温度一般为90~150°C。
气相异构化反应温度则为300~500°C。
气相非临氢异构化可在低压(约0.3MPa)下进行,气相临氢异构化则需较高压力(2.0~2.5Mpa)下进行。
氢烃摩尔比为5~20:1,过量氢气可循环使用。
气相异构化可采用固定床反应器,液相均相异构化可用塔式反应器,非均相异构化则可用涓流床反应器。
7.1-7.3 异构体的分类

1第七章立体化学申宝剑中国石油大学化学科学与工程学院为什么要学习立体化学?¾1957年德国开发了一种用于减轻妇女怀孕时呕吐症状的药物“反映停(沙利度胺)”,结果几年以后,到了1962年发现,使用过这种药物的妇女所产婴儿中出现了9000多名畸形儿,大大高于正常比例。
后来研究发现:原因是所用的沙利度胺是一种由左旋和右旋立体对映异构体组成的称之为“外消旋”的混合物,元凶是药物中对映异构体中的一个….,从这个悲剧故事中我们可以领悟到立体化学的重要性!¾2001年诺贝尔化学奖授予美国科学家威廉·诺尔斯、日本科学家野依良治和美国科学家巴里·夏普雷斯,以表彰他们在不对称合成方面所取得的成绩!分子构造碳骨架异构官能团位置异构7.1 异构体的分类ClBrHFBrClH F HClBrF和¾两个具有相同的分子式、但具有不同原子排列的分子,其构型式不能重叠,两种化合物互为实物和镜像的关系,就像人的左右手,互为对映,不能重合,这种异构称为对映异构(enantiomerism)。
也称为手性(chirality)。
¾具有手性的分子称为手性分子(chiral molecule)。
¾虽然所有的分子都有其镜象,但并不是都是对映异构体¾对映异构体的性质:对映异构好比人的左手和右手的关系,左手和右手互为镜像,它们不能重合,就像左手的手套戴在右手上总是不合适,为此把实物和镜像不能重合的现象称为手性(chirality)。
能够重合(叠起来)不能重合对映异构(手性)的特性?两个对映体结构差别很小,因此它们具有相同的沸点、熔点、溶解度等,化学性质也基本相同,很难用一般的物理及化学方法区分。
对映异构(手性)的特性手性分子的特性:外界手性环境对其性质(作用)有影响z一个人伸出的是右手,另外一个人伸出的也是右手对映异构的特性-旋光性¾不同的对映体对平面偏振光的作用不同:一个可使平面偏振光向右旋(dextrorotation),符号为(+),称为右旋体;另一个可使平面偏振光向左旋(levorotation),符号为(-),称为左旋体。
差向异构体名词解释

差向异构体名词解释异构体是有机化合物的同分异构体,指的是分子式相同、结构不同的有机化合物。
异构体由于结构的不同,具有不同的化学性质和物理性质。
异构体分为以下几种类型:1. 位置异构体(Position isomers):这类异构体的不同在于它们功能团或官能团的位置不同,但分子骨架不变。
例如,对二甲苯和甲基乙基苯是位置异构体,它们都有分子式C8H10,但二甲苯的两个甲基基团在芳香环上的位置不同于甲基乙基苯。
2. 键合异构体(Functional isomers):这类异构体的不同在于它们具有不同的键合方式,但它们的分子式相同。
例如,异丙醇和丙醛是键合异构体,它们的分子式都是C3H8O,但异丙醇的碳原子与氧原子的键合方式不同于丙醛。
3. 构象异构体(Conformational isomers):这类异构体的构象不同,但它们的键合方式和分子骨架相同。
构象异构体通常由于旋转单键而产生。
例如,环丙烯和环丁烯是构象异构体,它们都有相同的分子式C4H6,但环丙烯的碳-碳双键的位置使分子呈现平面结构,而环丁烯的碳-碳双键的位置使分子呈现为非平面结构。
4. 立体异构体(Stereoisomers):这类异构体的分子式和键合方式相同,但它们在空间结构上有差异。
立体异构体分为两大类:同分异构体和对映异构体。
- 同分异构体(Geometric isomers):同分异构体的分子式相同,但它们的立体构型不同,由于取代基的位置不同而引起。
例如,顺-二氯乙烷和反-二氯乙烷是同分异构体,它们的分子式都是C2H4Cl2,但氯原子的位置不同。
- 对映异构体(Enantiomers):对映异构体是具有相同的分子式、结构和化学性质,但它们的立体结构镜像对称,无法重叠。
例如,D-葡萄糖和L-葡萄糖是对映异构体,它们的分子式都是C6H12O6,但它们的空间结构是非重叠的镜像对称。
异构体在生物学、有机合成和药物研究等领域具有重要意义。
它们的存在使得研究人员可以通过合成不同的异构体来探索它们的特定化学性质和药理活性。
含7个碳的烷烃的同分异构体

含7个碳的烷烃的同分异构体英文版Isomers of HeptaneHeptane is a type of alkane with seven carbon atoms. Due to its molecular structure, heptane can exist in different forms known as isomers. Isomers are compounds that have the same molecular formula but different structural arrangements of atoms.There are three main isomers of heptane: n-heptane, isoheptane, and neoheptane. N-heptane, also known as normal heptane, has a straight chain of seven carbon atoms. Isoheptane, on the other hand, has a branched chain structure with six carbon atoms in a straight chain and one carbon atom branching off. Neoheptane has all seven carbon atoms branching off from a central carbon atom.Each isomer of heptane has its own unique physical and chemical properties. For example, n-heptane has a higher boiling point compared to isoheptane and neoheptane due to its straight chain structure. The different isomers of heptane also have varying levels of reactivity in chemical reactions.In conclusion, the isomers of heptane demonstrate the concept of structural isomerism, where compounds with the same molecular formula can have different structural arrangements. Understanding the different isomers of heptane is important in various fields such as organic chemistry and petroleum industry.完整中文翻译含7个碳的烷烃的同分异构体正庚烷是一种含有七个碳原子的烷烃。
液相色谱 异构体

液相色谱异构体全文共四篇示例,供读者参考第一篇示例:液相色谱(Liquid Chromatography, LC)是一种广泛应用于分析化学和生物化学领域的技术,其基本原理是利用溶液在固定相(填料)和移动相(溶剂)之间的分配行为来分离混合物中的成分。
在液相色谱中,溶液被注入到柱中,通过填料内部的孔隙和表面吸附分离不同成分,最终实现各组分的分离和定量分析。
异构体(Isomer)是指分子式相同但结构不同的化合物,是化学中常见的现象。
由于异构体分子间的结构差异,其性质和活性也有所不同,因此对异构体进行有效的分离和鉴定具有非常重要的意义。
液相色谱技术可以有效地分离和检测各种类型的异构体,为研究者提供了有力的工具。
在液相色谱分离异构体时,通常会采用不同的分离模式和填料。
手性分离是液相色谱中常见的应用之一,用于分离和鉴定手性异构体(对映体)。
手性异构体具有相同的分子式和结构,但空间构型不同,因此手性分离技术在药物研究和环境分析中具有重要价值。
在液相色谱中还可以利用不同的检测器和柱温控制系统来增强对异构体的分离和鉴定能力。
高效液相色谱-质谱联用(HPLC-MS)技术可以结合高分辨率的分离和灵敏的质谱检测,实现对复杂异构体混合物的快速和准确分析。
液相色谱分离异构体的操作步骤通常包括样品预处理、色谱条件优化、分离和定性分析等。
在进行液相色谱分析时,研究者需要考虑到实验条件的选择、填料的选择和柱温控制等因素,以获得良好的分离效果。
液相色谱技术在异构体分析中发挥着重要作用,为科学研究和实验室分析提供了有效的手段。
随着科学技术的不断发展,液相色谱技术将在异构体分析领域发挥越来越重要的作用,为化学和生物化学领域的研究者提供更多更精确的数据和实验结果。
第二篇示例:液相色谱(HPLC)技术是一种在化学分析领域中广泛应用的高效分离技术,它通过溶液在固定相(填料)上的吸附、分配、离子交换等作用,在液态流动相的作用下,将混合物中的成分进行分离。
regioisomer 顺反异构 光学异构 s r

regioisomer 顺反异构光学异构s r【顺反异构、光学异构及S和R】是有机化学中的重要概念,它们为分析和研究化合物的立体结构提供了关键性的指导。
本文将深入探讨顺反异构、光学异构以及S和R的概念,以及它们在有机化学中的应用。
一、顺反异构在有机化学中,顺反异构指的是同一分子的两种不同排列方式。
具体而言,它们只有在手性中心(具有不对称性的碳原子)处于不同空间方位时才会形成。
顺反异构体即为这两种不同排列方式所构成的异构体。
顺反异构的研究使得我们能够更好地理解化合物的空间结构,并为合成药物、农药等方面的研究提供指导。
常见的例子包括二溴丙烷的顺异构体和反异构体,它们的结构如下所示:顺二溴丙烷:Br-CH2-CH2-Br反二溴丙烷:CH3-CHBr-Br通过比较这两种异构体的结构,我们可以看到在顺异构体中,两个溴原子处于同一侧,而在反异构体中,溴原子处于相对侧。
这种结构上的差异可能会导致它们在化学性质上的差异,例如在反应速率和立体选择性上的不同。
二、光学异构光学异构也是有机化学中的重要概念,它指的是具有手性分子的两种镜像结构。
这两种镜像结构分别称为对映异构体。
对映异构体在物理性质上几乎完全相同,但在与其他手性分子的相互作用中却表现出明显的差异。
光学异构体通常是因为手性中心的存在而产生的。
手性中心是指一个原子或一个原子团,它固定了分子的三维空间结构,并使得分子与其镜像结构不可互相重合。
常见的手性分子包括氨基酸、脱氧核糖核酸(DNA)和脂肪酸等。
光学异构体通过使用一个称为旋光度的参数来描述其光学性质。
旋光度是指光通过样品后发生的偏振方向的改变,它可以是正或负。
根据旋光度的正负,我们可以将光学异构体分为两类:左旋和右旋光学异构体。
左旋光学异构体通常被表示为(-),而右旋光学异构体则被表示为(+)。
这些符号源自于早期研究中,通过光学旋光仪测量样品光学性质时的符号惯例。
三、S和R标志在化学命名中,S和R标志用于确定手性中心的绝对构型。
碳的同系异构体

碳的同系异构体同系异构体(Isomers)是指含有相同分子式但结构不同的化合物。
同系异构体之间的结构差异通常是由于原子在分子中的连接方式或排列顺序不同所引起的。
在化学领域,同系异构体是一个重要的研究方向,对于理解化合物的性质和反应机制有着重要的意义。
本文将介绍同系异构体的定义、分类和研究领域,并讨论同系异构体的重要性。
同系异构体分为结构异构体和空间异构体两大类。
结构异构体是指化合物中原子的连接方式不同,而空间异构体是指原子的构型或排列顺序不同。
结构异构体中最常见的类型是链构异构体和环构异构体。
链构异构体是指分子中的主链或侧链连接方式不同,而环构异构体则是指分子中包含环状结构的不同连接方式。
空间异构体中最常见的类型是立体异构体,包括立体异构体、光学异构体和坐标异构体等。
同系异构体的研究在化学领域有着广泛的应用。
首先,同系异构体的存在对于理解和解释化合物的性质和反应机制非常重要。
同系异构体之间的结构差异可以导致化合物在化学反应中的不同行为和反应速率。
通过研究同系异构体,化学家可以更好地理解和预测化学反应的结果,并优化合成路线或设计新的材料。
其次,同系异构体的研究对于药物发现和设计也具有重要意义。
许多药物分子存在不同的同系异构体,这些异构体可能具有不同的生物活性或药理学特性。
通过研究同系异构体,科学家可以发现更有效或更安全的药物分子,并提高药物疗效。
同系异构体的研究还对于环境保护和能源领域有着重要的意义。
例如,在分子筛材料的研究中,同系异构体的存在和结构差异对材料的吸附性能、分子选择性和传输速率产生重要影响。
通过合理设计同系异构体结构,可以制备高效的分离材料和催化剂,从而提高能源利用效率和减少污染排放。
此外,同系异构体的研究还涉及到生物学、材料科学、配位化学等多个领域。
例如,在生物领域中,同系异构体的存在对于理解生物大分子的结构和功能非常重要。
通过改变同系异构体的结构,可以调控生物分子的功能或活性。
综上所述,同系异构体在化学研究中具有重要的意义。
化学原料药开发-异构体的分类 ISOMERS

ISOMERSDefinitionIsomers are compounds that have the same molecular (empirical) formula, but different structures, and demonstrate physico-chemical and pharmacological differencesClassificationStructural IsomersDefinitionCompounds with the same empirical formula, but whose atoms are connected in a different sequenceChain isomers–have different branching patterns of carbon chainsPosition isomers–the functional group is located on different carbons in the chain–tend to have similar chemical propertiesFunctional group isomers–have different types of bonds and hence different functional groups–tend to have very different chemical propertiesTautomers–compounds in which, under differing conditions eg pH, the substituent groupings may alter their position–structural isomers that readily convert from one isomeric form to another and hence exist in equilibriumStereoisomersDefinitionCompounds which have the same empirical formula and whose atoms are attached in the same sequence, but differ in the spatial arrangement of the atomsSignificance–may have marked pharmacokinetic and pharmacodynamic differences eg levo-bupivacaine is less cardiotoxic than dextro-bupivacaine, only levo isomer of morphine has opioid activity –different 3 dimensional arrangement → different ability to interact with receptors, enzymes and non-specific binding sites–isomer-specific ability of a drug to produce a pharmacological effect is evidence supporting the presence of receptorsEnantiomers–stereoisomers that are non-superimposable mirror images (like left and right hands)–contain a chiral centre (an asymmetric carbon with four different groups attached to it) –identical physical properties, except the direction in which they rotate polarised lightClassification Systemsa) Rotation of polarised light to the:left: levorotatory l- (-)right: dextrototatory d- (+)a racemic mixture contains equal amounts of levo and dextro isomers and therefore has no overall rotating effect on polarised lightb) Cahn Ingold Prelog conventionLigands around the chiral carbon are assigned a priority based on their atomic number(higher atomic number = higher priority)Rectus (R-) priorities increase in clockwise directionSinister (S-) priorities increase in anti-clockwise directionNot important to know the exact priority rules. Note that this system has nothing to do with rotation of polarised light and therefore classification in one system does not alwayscorrespond to the same classification in the other. “Coincidentally〞, this seeminglyarbitrary nomenclature (R, S) are the initials of Mr R. S. Cahn.c) Simple sugars and amino acids can be classified as D- or L- according to the clockwise or anticlockwise spatial arrangement of COOH, NH3, H and hydrocarbon chain around the chiral carbon. This is an old classification system, don’t bother learning it, just be aware of it.(D- and L- are not to be confused with d- and l-!!)Diastereoisomers–Stereoisomers with different orientation of substituent groups on either side of a rigid bond (eg double bond or ring structure)–Alternatively, can be thought of as isomers with two chiral carbonsClassification systemsa) cis- and trans-cis- functional groups are on the same side of the double bondtrans- functional groups are on opposite sides of the double bondeg mivacurium is presented as a mixture of isomers:trans-trans 60%cis-trans 30%cis-cis 10%b) other systems include syn-/anti- and Z-/E- (forget about this crap)More detailed info on isomers can be found atClassification of IsomersIsomers:Compounds that have identical chemical and molecular formulas but differ in the nature of sequence of bonding of their atoms or in their arrangement of atoms in space. According to their topology they are classified as either structural or stereoisomers. Isomers are broadly classified into two broad categories:Structural Isomers: Compounds that have the same atoms present but differ in their order of connectivity. They are also called as constitutional isomers. They have the same molecular formula but different structures. It can be distinguished by planar diagrams such as fischer projections.Skeletal Isomers: Compounds that have the same functional groups, but differ in the length of the side chains. They are also called as chain isomers. For example: pentane, 2-methyl butane and 2,2 dimethyl propane.Positional Isomers: Compounds that have the same functional groups, but are present on different positions of the chain. For example: butan-1-ol and butan-2-ol.Functional Isomers: Compounds that have different functional groups. For example: ethanol and methoxy methane.Tautomers:Compounds whose structures differ in arrangement of atoms but which are in dynamic equilibrium with each other..1Keto-Enol tautomerism:.2Ring-Chain isomerism: seen in case of glucoseStereoisomer:Compounds that have the same chemical formula, same atoms, same connectivity and differ only in the arrangement of their atoms in space.Anomers: Stereoisomers where the molecule is cyclized and the difference in configuration is about the anomeric carbon only. In case of aldoses the anomeric carbon is C1 and for ketoses it is C2. e.g. sugar hemiacetal. Glucose in open chain form is not chiral at C1 but in ring form has two optically active stereoisomers: alpha & beta glucose.Rotamers and Conformers: On the basis of spatial arrangement of atoms in the molecule that can be achieved by rotation(or torsion) around one or more single bonds, they are classified as rotamers and conformers. Conformers assume the chair/boat and equitorial/axial forms. Rotamers assume the different newmann projections (staggered/eclipsed/gauche).Configurational Isomers have a chiral (stereogenic center). Chiral center refers to a carbon atom attached to four different groups. The molecule is said to possess chirality and to have a stereogeniccenter..1Enantiomer:Stereoisomers that are non-identical, mirror-symmetric for all atoms, nonsuperimposable, optically active (e.g. levo/dextro-rotatory), inverted only by breaking bonds and remaking them in the reverse sense. e.g.: D-glucose and L-glucose..2Diastereomer: Stereoisomers that are not mirror images, but have identical configuration for at least one asymmetric center and at least one different configuration for the remaining asymmetric centers. e.g. Threonine has 2 chiral centers and therefore 4 diastereomers.Epimers: They are a special case of diastereoisomerism where there is a difference for one and only one asymmetric center. e.g. D-glucose and D-mannose; D-glucose and D-galactose are epimers..3Meso-isomer (Achiral molecules): super-imposable mirror images which have more than one stereogenic center. Meso-isomers have two planes of symmetry; the usual mirror plane of reflection and a second plane perpendicular to it through the molecule (in the ``middle'' of the molecule). The asymmetric centers are distributed around this second place so that they are mirror inverses of each other. Hence optical rotations from the two ``halves'' of the molecule cancel out..4Geometric Isomers: Stereoisomers which are isomeric about double bonds. If the bond is C=C, the terms are cis/trans; if the bond is C=N, the terms are syn (cis-like) and anti (trans-like). For example: cis-2-butene and trans-2-butene.Chiral Center Naming Classification:• +/- Indicates the direction in which plane of polarized light is rotated(clockwise/anticlockwise).• D/L (Dextrorotatory/Levorotatory): Plane of polarized light is rotated to the right or left.• alpha/beta stereochemistry of the anomeric carbon. It is u sed for sugars.• R-S convention: The four groups surrounding the stereocenter are given a priority from a -> d (from highest to lowest). The molecule is then observed from the side with the lowest priority group. If the remaining three groups form a clockwise array (a->b->c), then it is R convention. If it is in anti-clockwise then S convention. Priority is assigned according to atomic number, with the higher the atomic number the higher the priority. This is used for chiral molecules (enantiomers/diastereomers/epimers).• E-Z convention: The two groups attached to the carbon around the double bond are given the priority. If the two highest priority groups are on the same side of the double bond, then the molecule is given the Z convention(similar to cis); and if on the opposite side then E convention(similar to trans). e.g.: 1-chloro-1-bromo-2-iodoethene is differentiated on basis of E-Z convention. Priority is assigned according to the atomic number; with the higher the atomic number, the higher the priority. Hyrdogen always has the lowest priority. If there are two identical atoms attached to the stereocenter (say the carbon of the methyl group and carbon of the ethyl group) then work along the chain of the attached group until a difference occurs.。
异构体类型

异构体类型异构体类型是指天然产物中以结构、构造、生物化学性质或含量的不同而形成的若干种不同天然产物,这些天然产物统称为异构体。
它们彼此之间具有某种确定的关系,但由于不完全清楚,所以又叫作未确定型。
它们与同分异构体不同,虽然也具有相同的组成,但它们却有各自的特征。
异构体中由于结构和/或构造上的原因使某些化学成分有所差别的天然产物,称为同分异构体(isomerism)。
1、合成异构体是在化学反应过程中通过物理、化学方法得到的。
不同结构、不同取代基的原子或原子团取代了同一分子的同一原子,使异构体的分子结构发生了变化。
常见的化学合成异构体如下:(1)立体异构体:立体异构体的数目比较少,并且常常与其他结构异构体并存。
(2)旋光异构体:取代基对物质分子所起的影响是很复杂的。
例如,从化学角度看,乙酰水杨酸、谷氨酸等左旋化合物,当左旋体的羟基中氧原子与甲氧基(或者羧基)中碳原子之间,或者甲氧基(或者羧基)与氢原子之间相连时,均可以形成旋光异构体。
从旋光性上看,同一个物质有无数种旋光异构体。
二、异构体的特点(1)具有相同的组成,但不同的物理、化学性质1、光学异构体分为旋光异构和几何异构,光学异构体的每种结构不完全相同,一般旋光性不如几何异构。
2、化学异构体具有相同的化学式,但由于反应条件的不同,不一定都能生成。
(2)化学性质比较稳定在物理变化中,由一种单质分子分解为两种或多种分子,就是分子的化学变化。
因为化学变化中,每一种分子都失去了其原来的化学键,而形成了新的化学键。
同样地,由两种或多种原子结合成分子的化学变化也属于化学变化。
物质中存在着各种化学键,这些化学键可以破坏,也可以形成,这种化学变化,既包括化学键的断裂,又包括化学键的重新形成。
化学变化一般是分子在其分子结构内部进行的,在化学反应中经常伴随着物理变化。
化学变化一般在微观粒子(如分子、原子等)层次上进行。
在物理变化中,由一种单质分子分解为两种或多种分子的变化。
3氨基 环戊烷甲酸甲酯异构体

3氨基环戊烷甲酸甲酯异构体引言3氨基环戊烷甲酸甲酯是一种有机化合物,其分子结构中包含一个氨基基团和一个甲酸甲酯基团。
由于分子中的环戊烷基团可以在不同位置连接到甲酸甲酯基团上,导致存在多个异构体。
本文将对3氨基环戊烷甲酸甲酯异构体的结构、性质、应用等方面进行详细介绍。
1. 异构体的结构3氨基环戊烷甲酸甲酯的异构体主要是由于环戊烷基团连接到甲酸甲酯基团的不同位置所导致的。
根据连接位置的不同,可以得到以下三个异构体:1.1 异构体一异构体一是指环戊烷基团连接到甲酸甲酯基团的第一碳上。
其分子结构如下所示:1.2 异构体二异构体二是指环戊烷基团连接到甲酸甲酯基团的第二碳上。
其分子结构如下所示:1.3 异构体三异构体三是指环戊烷基团连接到甲酸甲酯基团的第三碳上。
其分子结构如下所示:2. 物理性质不同异构体的物理性质可能会有所差异,下面将对各个异构体的物理性质进行简要介绍:2.1 异构体一•分子式:C6H13NO2•分子量:131.17 g/mol•外观:无色液体•熔点:-10 °C•沸点:185 °C•密度:0.96 g/cm³2.2 异构体二•分子式:C6H13NO2•分子量:131.17 g/mol•外观:无色液体•熔点:-5 °C•沸点:190 °C•密度:0.97 g/cm³2.3 异构体三•分子式:C6H13NO2•分子量:131.17 g/mol•外观:无色液体•熔点:-8 °C•沸点:180 °C•密度:0.95 g/cm³3. 化学性质3氨基环戊烷甲酸甲酯异构体在化学性质上可能会有一些差异,下面将对其进行简要介绍:3.1 反应性3氨基环戊烷甲酸甲酯异构体可以与酸、碱等发生反应。
异构体一、二、三在一定条件下可以发生酯水解反应,将甲酸甲酯基团水解为甲酸和甲醇。
3.2 稳定性3氨基环戊烷甲酸甲酯异构体在常温下相对稳定,但在高温、光照等条件下可能会发生分解。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ISOMERSDefinitionIsomers are compounds that have the same molecular (empirical) formula, but different structures, and demonstrate physico-chemical and pharmacological differencesClassificationStructural IsomersDefinitionCompounds with the same empirical formula, but whose atoms are connected in a different sequenceChain isomers–have different branching patterns of carbon chainsPosition isomers–the functional group is located on different carbons in the chain–tend to have similar chemical propertiesFunctional group isomers–have different types of bonds and hence different functional groups–tend to have very different chemical propertiesTautomers–compounds in which, under differing conditions eg pH, the substituent groupings may alter their position–structural isomers that readily convert from one isomeric form to another and hence exist in equilibriumStereoisomersDefinitionCompounds which have the same empirical formula and whose atoms are attached in the same sequence, but differ in the spatial arrangement of the atomsSignificance–may have marked pharmacokinetic and pharmacodynamic differences eg levo-bupivacaine is less cardiotoxic than dextro-bupivacaine, only levo isomer of morphine has opioid activity –different 3 dimensional arrangement → different ability to interact with receptors, enzymes and non-specific binding sites–isomer-specific ability of a drug to produce a pharmacological effect is evidence supporting the presence of receptorsEnantiomers–stereoisomers that are non-superimposable mirror images (like left and right hands)–contain a chiral centre (an asymmetric carbon with four different groups attached to it) –identical physical properties, except the direction in which they rotate polarised lightClassification Systemsa) Rotation of polarised light to the:left: levorotatory l- (-)right: dextrototatory d- (+)a racemic mixture contains equal amounts of levo and dextro isomers and therefore has no overall rotating effect on polarised lightb) Cahn Ingold Prelog conventionLigands around the chiral carbon are assigned a priority based on their atomic number(higher atomic number = higher priority)Rectus (R-) priorities increase in clockwise directionSinister (S-) priorities increase in anti-clockwise directionNot important to know the exact priority rules. Note that this system has nothing to do with rotation of polarised light and therefore classification in one system does not alwayscorrespond to the same classification in the other. “Coincidentally”, this seemingly arbitrary nomenclature (R, S) are the initials of Mr R. S. Cahn.c) Simple sugars and amino acids can be classified as D- or L- according to the clockwise or anticlockwise spatial arrangement of COOH, NH3, H and hydrocarbon chain around the chiral carbon. This is an old classification system, don’t bother learning it, just be aware of it.(D- and L- are not to be confused with d- and l-!!)Diastereoisomers–Stereoisomers with different orientation of substituent groups on either side of a rigid bond (eg double bond or ring structure)–Alternatively, can be thought of as isomers with two chiral carbonsClassification systemsa) cis- and trans-cis- functional groups are on the same side of the double bondtrans- functional groups are on opposite sides of the double bondeg mivacurium is presented as a mixture of isomers:trans-trans 60%cis-trans 30%cis-cis 10%b) other systems include syn-/anti- and Z-/E- (forget about this crap)More detailed info on isomers can be found at Classification of IsomersIsomers:Compounds that have identical chem ical and molecular formulas but differ in the nature of sequence of bonding of their atom s or in their arra ngement of atom s in space. According to their topology they are classified as either structural or stereoisom ers. Isomers are broadly classified into two broad categories:1.1Structural Isomers: Compounds that have the sam e atom s present but differ in their order of connectivity. They are also called as constitutional isomers. They have the sam e molecular formula but different structures. It can be distinguished by planar diagrams such as fischer projections.1.1.1Skeletal Isomers: Compounds that have the sam e functional groups, but differ in the length of the side chains. They are also called as chain isom ers. For example: pentane, 2-m ethyl butane and 2,2 dimethyl propane.1.1.2Positional Isomers:Compounds that have the same functional groups, but are present on different positions of the chain. For example: butan-1-ol and butan-2-ol.1.1.3Functional Isomers: Compounds that have different functional groups. For example: ethanol and m ethoxy methane.1.1.4Tautomers:Compounds whose structures differ in arrangem ent of atom s but which are in dynamic equilibrium with each other.1.1.4.1Keto-Enol tautom erism:1.1.4.2Ring-Chain isomerism: seen in case of glucose1.2Stereoisomer:Compounds that have the sam e chemical formula, sam e atom s, sam e connectivity and differ only in the arrangement of their atom s in space.1.2.1Anomers: Stereoisomers where the m olecule is cyclized and the difference in configuration is about the anom eric carbon only. In case of aldoses the anomeric carbon is C1 and for ke toses it is C2. e.g. sugar hemiacetal. Glucose in open chain form is not chiral at C1 but in ring form has two optically active stereoisom ers: alpha & beta glucose.1.2.2Rotamers and Conformers:On the basis of spatial arrangement of atom s in the molecule that can be achieved by rotation(or torsion) around one or m ore single bonds, they are classified as rotam ers and conform ers. Conformers assum e the chair/boat and equitorial/axial form s. Rotamers assume the different newm ann projections (staggered/eclipsed/gauche).1.2.3Configurational Isomers have a chiral (stereogenic center). Chiral center refers to a carbon atom attached to four different groups. The m olecule is said to possess chirality and to have a stereogeniccenter.1.2.3.1Enantiomer:Stereoisom ers that are non-identical, mirror-symmetric for all atom s, nonsuperimposable, optically active (e.g. levo/dextro-rotatory), inverted only by breaking bonds and remaking them in the reverse sense. e.g.: D-glucose and L-glucose.1.2.3.2Diastereomer: Stereoisomers that are not mirror images, but have identical configuration for at least one asymmetric center and at least one different configuration for the remaining asymmetric centers. e.g. Threonine has 2 chiral centers and therefore 4 diastereomers.1.2.3.2.1Epimers:They are a special case of diastereoisom erism where there is a difference for one and only one asymmetric center. e.g. D-glucose and D-m annose; D-glucose and D-galactose are epimers.1.2.3.3Meso-isomer (Achiral molecules):super-imposable m irror im ages which have m ore than one stereogenic center. Meso-isom ers have two planes of symmetry; the usual mirror plane of reflection and a second plane perpendicular to it through the m olecule (in the ``middle'' of the m olecule). The asymmetric centers are distributed around this second place so that they are mirror inverses of each other. Hence opti cal rotations from the two ``halves'' of the molecule cancel out.1.2.3.4Geometric Isomers: Stereoisomers which are isomeric about double bonds. If the bond is C=C, the term s are cis/trans; if the bond is C=N, the terms are syn (cis-like) and anti (trans-like). For example: cis-2-butene and trans-2-butene.Chiral Center Naming Classification:• +/- Indicates the direction in which plane of polarized light is rotated(clockwise/anticlockwise).• D/L (Dextrorotatory/Levorotatory): Plane of polarized light is rotated to the right or left.• alpha/beta stereochemistry of the anom eric carbon. It is used for sugars.• R-S convention: The four groups surrounding the stereocenter are given a priority from a -> d (from highest to lowest). The m olecule is then observed from the side with the lowest priority group. If the rem aining three groups form a clockwise array (a->b->c), then it is R convention. If it is in anti-clockwise then S convention. Priority is assigned according to atom ic number, with the higher the atomic number the higher the priority. This is used for chiral molecules (enantiomers/diastereomers/epimers).• E-Z convention: The two groups attached to the carbon around the double bond are given the priority. If the two highest priority groups are on the sam e side of the double bond, then the m olecule is given the Z convention(similar to cis); and if on the opposite side then E convention(similar to trans). e.g.: 1-chloro-1-brom o-2-iodoethene is differentiated on basis of E-Z convention. Priority is assigned according to the atomic number; with the higher the atomic number, the higher the priority. Hyrdogen always has the lowest priority. If there are two identical atom s attached to the stereocenter (say the carbon of the m ethyl group and carbon of the ethyl group) then work along the chain of the attached group until a difference occurs.。
