磺酰脲类化合物水解综述
降解磺酰脲类化合物微生物资源收集及应用基础研究取得进展

由中科 院微 生 物研究所 承担 的 中科 院知 识创新
磺 酰脲类 除草 剂是 目前使用 最为 广泛 的农 用 除
草剂之 一 .农 田残 留除 草剂对后 茬作 物毒 害作用严 重 。为 了控制 除草 剂残 留造成 的土壤 质量下 降和 消
重 要研究 方 向项 目 “ 降解磺 酰脲类 化合 物微 生物 资
2 8
农 药研 究 与应 用 A R C E C L E E C & A P I A I N G O H MI A S R S AR H P L C TO
第 1卷 5
二 一
日前 ,吉 林 市 绿 胜 农 药 有 限 责 任 公 司 年 产
至 曩
草剂 ,主要适 用 于甜菜 地 除草 。7 %的可 湿性 粉剂 0 可 以防 治 黎龙 葵 、繁 缕 、野 芝麻 、早 熟 禾 等 多 种 杂草 。该项 目投 产后将 面 向 国内外市场 。
10 0吨苯 嗪草 酮原 药扩 建 项 目。主体设 备 已安 装 0
完 毕 ,预计 2 1 0 2年建成 投产 。 苯 嗪草 酮是 由德 国拜 耳公 司开发 的一种 旱地 除
_
’ ’’ ’ ’’ ’’ ’ ’ ’ ’’ ’ ’’ ’ ’ ’’ ’ ’’ ’ ’’ ’ ’’ ’’ ’ ’’ ’’ ’ ’’ ’ ’ ’’ ’ ’ ’ ’’ ’’ ’ ’ ’’ ’ ’’ ’ ’’ ’ ’ ’’ ’ ’ ’ ’ ’ ’ ’ ’ ’ 、
一
定 的成 效 .同时也 填补 了我 国该 领 域 的空 白。 该 项 目 自 20 0 4年 启 动 ,历 时 多年 ,开展 了室
内剂量校 正试 验研究 、田间适应 性试 验研究 和技 术
示 范 推 广 :参 加项 目单 位 还 有 荷 兰 环 境 与 遥 感 公
五种磺酰脲类除草剂在土壤中的环境行为

五种磺酰脲类除草剂在土壤中的环境行为【作者】张伟;【导师】王进军【作者基本信息】西南大学,农药学,2007,博士磺酰脲类除草剂是一类广泛应用于各种作物杂草控制的新型超高效除草剂。
土壤中微量的磺酰脲类除草剂残留就可对当茬及后茬作物造成药害,且对环境带来的污染和生态毒性也十分严重。
因此,对磺酰脲类除草剂在土壤中环境行为进行研究具有重要意义。
本文以常用的甲磺隆、苄嘧磺隆以及近年来开发的新品种-单嘧磺隆、烟嘧磺隆、氯嘧磺隆为对象,较为系统地研究它们在土壤中的环境行为,旨在为该类除草剂的合理使用及生态修复提供理论基础。
主要研究结果如下:1、5种磺酰脲类除草剂在8种土壤中的吸附-解吸特性采用了平衡振荡法和液相色谱检测技术研究了5种磺酰脲类除草剂在8种土壤中的吸附-解吸特性。
结果表明,5种除草剂在土壤中具有较低的吸附容量,其吸附主要以物理吸附为主,且存在不可逆吸附过程。
5种磺酰脲类除草剂在土壤中的吸附-解吸等温线为非线性,符合Frendlich模型。
同时,供试的8种土壤对5种除草剂的吸附容量存在差异,其中在红壤土和黑土中具有较高的吸附性,而砂壤土的吸附能力最差。
进一步分析显示,除草剂在土壤中吸附容量大小与土壤理化性质密切相关,吸附常数和解吸常数均与土壤有机质含量、粘粒含量呈正相关,与土壤pH值呈负相关。
5种磺酰脲类除草剂在土壤中的解吸通常存在滞后现象,滞后现象产生的程度与土壤有机质含量、粘粒含量呈正相关性。
且与溶液中除草剂的浓度有一定相关性。
此外,通过对除草剂在土壤中有机碳吸附常数进行计算发现,5种磺酰脲类除草剂在供试土壤中多具有中等或较高的移动性能。
因此,磺酰脲类除草剂的使用对地下水和地表水等生态环境及食品安全存在一定的风险。
2、5种磺酰脲类除草剂在吸附剂中的吸附特性采用平衡振荡法和液相色谱检测技术研究了5种磺酰脲类除草剂在4种典型吸附剂中的吸附特性。
结果表明,胡敏酸(HA)、蒙脱石、高岭土和硅藻土对磺酰脲类除草剂具有很强的吸附能力,其吸附等温线为非线性,符合Frendlich模型。
浅析磺酰脲类除草剂的发展及未来

是 由于降解代谢 的差异 磺酰脲类除草剂为弱酸性化合物 .在土壤 溶 性随着土壤 p 的增加 而增加 ;在酸性 土壤 H 中 .降解速度快 。在碱性土壤 中降解速度慢 。
影 响等 尤其 突出的是残 留药害问题 .已开始 制约部 分药剂的进一步发展 对不同作物的敏感性差异较大 。如棉花对甲磺 隆最敏感 .玉米次之 。而水稻和油菜耐药性较 强 .因而残留的 甲磺隆对棉花等作物会 产生药 害 。氯磺 隆在 土壤 中的残 留期较 长 .在麦一 稻 连作的地 区 .因使用不 当会对水稻产生药害 。 点单一 .杂草对它们产生抗药性 的速度快 。据 国外报道 .此类 除草剂连续施用 3 5年后 .杂 ~ 草就可能产生抗药性 .有 的产 品上市不久就 产 生抗性 如美 国 Iao 冬小麦 田的杂草刺 莴 dh 州 苣就对氯磺 隆和甲磺 隆混 剂产 生抗性 业 已证
磺 隆在 上个世纪 9 年代初就在我 国推 广使 用 . 0
在 我 国的麦 类 、玉 米 除 草 中去 得 了好 的 效 果 。
3 作 用机 理 特 殊
在木质部和韧皮部传 导 .抑制 乙酰乳酸合成酶 ( L ) 乙酰乳 酸 合 成 酶是 支 链 氨基 酸 缬 氨 AS。
酸 、异亮 氨酸 、亮 氨 酸生 物合 成 的一个 关 键
巴一 基 、 日本 的石 原 产 业 、日产 化 学 、武 田 、 嘉
德 国拜耳 、美 国氰胺等农 药公 司和韩国化学研 究所 、我国南开大学元 素有机化学研究所等也 进行 了该类 除草剂的研制和开发 。甲磺 隆 、甲 嘧磺隆 、氯嘧磺隆 、 苯磺 隆 、噻吩磺隆 、苄嘧
磺 隆 等 一 系 列 产 品随 后 相 继 问世 . 目前 .大 约
滞 作用 .于是将其作为先 导化合物进行结构优 地 。此外 .它们 对 哺乳 动物 和鱼类毒性 较低 . 化 。合 成 了一 系列 该类 化合 物 。发现 由芳 香 基 、磺酰脲桥和杂环 3 部分组成 ,其基本化学 结构式在每一组分上取代基 的微小变化都会导
磺酰脲类除草剂综述

磺酰脲类除草剂综述
邓金保
【期刊名称】《世界农药》
【年(卷),期】2003(025)003
【摘要】@@ 磺酰脲类除草剂是目前世界上最大的一类除草剂.自杜邦公司的G.Levitt首先报告此类化合物具有除草活性,并于1982年首次开发出麦田除草剂氯磺隆(chlorsulfuron),从而使杂草的防除进入超高效时代以来,现已发展成为除草剂中的一大类品种.
【总页数】7页(P24-29,32)
【作者】邓金保
【作者单位】无
【正文语种】中文
【中图分类】TQ45
【相关文献】
1.磺酰脲类除草剂合成综述 [J], 黄世忠
2.超高效液相色谱-串联质谱法测定薏仁米中25种磺酰脲类除草剂残留 [J], 王显贵;吴小毛;杨晓凤;刘炜
3.QuEChERS-高效液相色谱-串联质谱法测定柑橘中13种磺酰脲类除草剂残留[J], 陈其煌
4.拟投资3000万元,内蒙古浩福祥新建磺酰脲类除草剂中间体项目 [J],
5.磺酰脲类除草剂在土壤及植物中行为综述 [J], 仇宏伟;胡继业;周革菲
因版权原因,仅展示原文概要,查看原文内容请购买。
磺酰脲类除草剂与杂草对其抗性的研究进展

很高的除草效率 , 用量一般为2— 0 h 比传统 10g m , / 除草剂 的除草效率 高 10~ 0 0 l 0倍 。磺酰脲类 0 】
除草 剂 对 动 物 低 毒 , 非 靶 标 生 物 体 内 几 乎 不 积 在
累【 。随着磺酰脲类除草剂 的开发和广泛应用 , 3 由 磺酰脲类除草剂残 留物引起 的环境问题 、 杂草的抗 药性及药害等问题亦引起 了人们的重视。 12 磺酰脲类除草剂的理化性质 . 磺酰脲类除草剂属非挥发性弱酸, 其蒸气压不 超过 l m g 在 3 5ka 0m H , ~ P 之间。其酸性主要来 自 与磺酰基相连的 N上 H的电离。因而 , 在酸性条件 下, 磺酰脲主要以分子形式存在 , 在弱酸条件下则 以 负离子形式存在。强碱可使脲桥 另一 个 N上的 H 电离。磺酰脲化合物很容易发生水解 , 水解机制 其 随化合物结构的不同和 p H值 的变化而变化 。中 J 性分子易于水解 , 阴离子态分子水解较慢 , 酸可催化 水解 反应 的进 行 。水解 反应 一般遵 循一 级动 力学规
关键词 : 磺酰脲类除草剂 ;混配 ; 抗药性 中图分类号 : 4 1 ¥ 5 文献标识码 : A 文章编号 :10 9 5 20 )4— 0 1— 3 0 3— 3 X{0 6 0 0 0 0
Байду номын сангаас
1 磺 酰脲 类除 草剂 的概 况 11 磺酰脲 类 除草 剂的发展 .
律, 符合 Areu 公式 , r ls hi 活化能为 3 0~15k m l 3 J o / 。 酸性条件下主要以磺 酰脲桥水解形式取代磺酰胺和 氨 基杂 环 。碱性条 件下 主要 以杂 环上烷 氧基 的亲核 取代羟基化的杂环及芳环部分酯键的水解 - 。 6 j
14 磺酰脲 类 除草 剂的应 用情 况 .
磺酰脲类除草剂

磺酰脲类除草剂的开发是除草剂进入“超高效”时代的标志,它最大的特点是高活性,使用剂量通常在5~100g/公顷,以下是几个具体产品:德国艾格福——酰嘧磺隆:防除冬小麦、大麦、燕麦等作物中阔叶杂草,对猪殃殃有特效,对当茬小麦和后茬水稻、玉米安全。
乙氧嘧磺隆:有效防除水稻、小麦和甜菜等作物中的阔叶杂草和莎草科杂草。
汽巴-嘉基——环氧嘧磺隆:大豆苗后除草剂,防除稗草,番薯属、苋属、豚草、苍耳等杂草,有效用量60~90g/公顷,对大豆和后茬作物安全。
氟磺隆:玉米地除阔叶类杂草,主要用于芽后处理,用量10~30g/公顷。
美国杜邦——四唑啶磺隆:稻田苗后除草剂,主要防除稗草、异型莎草、泽泻、眼子菜等杂草,用量为20~25g/公顷。
氟啶嘧磺隆:芽前苗后除草剂,主要防除禾本科和阔叶类杂草,对看麦娘特效,用量10g/公顷。
磺酰脲类除草剂是一种乙酰乳酸合成酶(ALS)抑制剂,即通过抑制植物体内的ALS,阻碍侧链氨基酸如缬氨酸,亮氨酸,异亮氨酸的生物合成,使细胞分裂受抑制,杂草正常生长收到破坏而死亡。
其主要特点有:1、生物活性。
磺酰脲类化合物具有前所未有的超高活性,打破了常规的用药量限制,使除草剂步入了超高效时代。
2、毒性。
磺酰脲类除草剂作用于植物体内的ALS,且再无第二个作用位点。
3、选择性。
磺酰脲类除草剂对许多作物有良好的选择性,一般认为,其选择性是由不同作物和杂草对该类化合物代谢失活能力的差异造成的,而与吸收和传导量的差异及ALS敏感性的差异无关。
4、环境行为。
磺酰脲类除草剂既可做叶面处理也可做土壤处理剂,而且用量少,因其蒸汽压低,进入大气中的量很少,主要被植物吸收和进入土壤,其残留量很小,除少量淋溶进入地下之外,大部分可通过化学水解和微生物分解而降解。
5、残留农药。
磺酰脲类除草剂选择性强,对不同作物的敏感性差异很大。
大多数磺酰脲类除草剂在环境中易分解,但也有一些品种因残效期较长,易对下茬作物产生药害,如甲磺隆、氯磺隆,胺苯磺隆和氯嘧磺隆等,另外新开发的磺酰磺隆和氟啶磺隆也有较长残效的趋向。
磺酰脲类化合物的合成及应用研究进展

a d a p i ain o u f n l r ac mp u d . n p l t fs lo yu e o o n s c o Ke r s u fn l r ac mp u d ; p l ai n ;y t ei t o ; e eo me t y wo d :s l yu e o o n s a p i t s s n h t meh d d v lp n o c o c
s nt t t o n p i ai n r s cs o h u fnyur a c mpo nd t o i h ee e c o y t ss y hei me h ds a d a plc to p o pe t ft e s lo l e o c u s o pr vde t e rf r n e fr s n he i
t n n r ea ii n me ii e a d a rc l r . h sp p rs mma ie h h s a n h mi a r p ris i s a d ma k tb l y i d cn n g iu t e T i a e u o t u r d te p y i la d c e c l o e t , z c p e
药化简答题

药化简答题答:①呈弱酸性,巴比妥类药物因能形成内酰亚氨醇一内酰胺互变异构,故呈弱酸性。
②水解性,巴比妥类药物因含环酰脲结构,其钠盐水溶液,不够稳固,甚至在吸湿情况下,也能水解。
③与银盐的反应,这类药物的碳酸钠的碱性溶液中与硝酸银溶液作用,先生成可溶性的一银盐,继而则生成不溶性的二银盐白色沉淀。
④与铜吡啶试液的反应,这类药物分子中含有-CONHCONHCO-的结构,能与重金属形成不溶性的络合物,可供鉴别。
2-48、为什么巴比妥C5位次甲基上的两个氢原子务必全被取代,才有镇静催眠作用?答:未解离的巴比妥类药物分子较其离子易于透过细胞膜而发挥作用。
巴比妥酸与一取代巴比妥酸的PKa值较小,酸性较强,在生理pH时,几乎全部解离,均无疗效。
如5位上引入两个基团,生成的5,5位双取代物,则酸性大大降低,在生理pH时,未解离的药物分子比例较大,这些分子能透过血脑屏障,进入中枢神经系统而发挥作用。
2-50、合成类镇痛药的按结构能够分成几类?各举一例。
答:合成类镇痛药按结构可分为:哌啶类、氨基酮类与苯吗喃类。
1、吗啡喃类:布托啡诺2、苯并吗喃类:喷他佐辛3、哌啶类:哌替啶4、氨基酮类:美沙酮。
4-42. 简述钙通道阻滞剂的概念及其分类。
答:钙通道阻滞剂是一类能在通道水平上选择性地阻滞Ca2+经细胞膜上的钙离子通道进入细胞内,减少细胞内Ca2+浓度,使心肌收缩力减弱、心率减慢、血管平滑肌松弛的药物。
根据WTO对钙通道阻滞剂的划分,钙通道阻滞剂可分为两大类:一、选择性钙通道阻滞剂,包含:1.苯烷胺类,如维拉帕米。
2、二氢吡啶类,如硝苯地平。
3、苯并硫氮卓类,如地尔硫卓。
二、非选择性钙通道阻滞剂,包含:4、氟桂利嗪类,如桂利嗪。
5、普尼拉明类,如普尼拉明。
5-43、为什么质子泵抑制剂抑制胃酸分泌的作用强,选择性好?答:胃酸分泌的过程有三步。
第一步,组胺、乙酰胆碱或者胃泌素刺激壁细胞底一边膜上相应的受体,引起第二信使cAMP或者钙离子的增加;第二步,经第二信使cAMP或者钙离子的介导,刺激由细胞内向细胞顶端传递;第三步,在刺激下细胞内的管状泡与顶端膜内陷形成的分泌性微管融合,原位于管状泡处的胃质子泵—H/K—ATP酶移至分泌性胃管,将氢离子从胞浆泵向胃腔,与从胃腔进入胞浆的钾离子交换,氢离子与顶膜转运至胃腔的氯离子形成盐酸(即胃酸的要紧成分)分泌。
除草剂微生物降解的研究进展

除草剂微生物降解的研究进展卢美名;尹雯悦;刘传龙;张茗茜;柳文睿;王新【摘要】除草剂施入土壤后会有一部分残留在土壤中,造成严重的环境污染,关于后茬作物的安全性问题一直受到社会的广泛关注.降解土壤中除草剂的主要途径是微生物降解.主要综述了4类常见除草剂(磺酰脲类、咪唑啉酮类、恶啉酮类、三唑并嘧啶磺酰胺类)的残留危害和研究现状,分析了微生物的降解途径以及微生物在降解过程中所面临的问题和解决方案.【期刊名称】《湖北农业科学》【年(卷),期】2019(058)003【总页数】4页(P5-8)【关键词】除草剂;残留;微生物降解【作者】卢美名;尹雯悦;刘传龙;张茗茜;柳文睿;王新【作者单位】沈阳工业大学理学院,沈阳 110870;沈阳工业大学理学院,沈阳110870;沈阳工业大学理学院,沈阳 110870;沈阳工业大学理学院,沈阳 110870;沈阳工业大学理学院,沈阳 110870;沈阳工业大学理学院,沈阳 110870【正文语种】中文【中图分类】X53随着农药的大量使用,土壤中残留农药造成的环境污染已经不容小觑,严重时不但影响土壤肥力和降低农产品品质,甚至可能造成水污染,危害人体健康。
大量研究表明,在自然环境中有大量的微生物能够有效地降解土壤中残留的农药,此外,生物降解的特点是无毒、无二次污染、价格低廉,是理想的降解途径。
微生物具有各种各样的化学功能,如氧化还原、穿梭、脱氨和水解,其能量利用率极高,在降解领域应用极其广泛。
1 4类除草剂残留危害及研究现状1.1 咪唑啉酮类除草剂1.1.1 残留危害咪唑啉酮类除草剂在土壤中既不容易挥发也不容易发生光解,并且残留时间长,施用除草剂金普施特2年之后,甘蓝、甜菜和亚麻等作物仍不能生长[1]。
应用甲氧咪草烟1年后,对油菜、甜菜、卷心菜、亚麻和马铃薯仍有不同程度的毒性,导致这些作物无法正常生长;低剂量对白瓜种子无明显伤害,高剂量对白瓜种子有明显药害[2]。
1.1.2 研究现状咪唑啉酮类化合物是一类ALS抑制剂类除草剂[3]。
磺酰脲类除草剂——砜嘧磺隆

磺酰脲类除草剂——砜嘧磺隆张晓进【摘要】综述了砜嘧磺隆的理化性质、作用方式、毒理学与环境生态安全性,介绍了该除草剂的合成化学及其应用研究与开发进展.【期刊名称】《现代农药》【年(卷),期】2010(009)003【总页数】5页(P44-47,50)【关键词】砜嘧磺隆;除草剂;综述【作者】张晓进【作者单位】江苏省农药研究所股份有限公司,南京,210019【正文语种】中文【中图分类】TQ457.2+3自美国杜邦公司于 1982年发现第一个磺酰脲类除草剂品种氯磺隆以来,磺酰脲类化合物因用量低 (通常不到 100 g/hm2)、对哺乳动物低毒及使用后易降解为无毒化合物等特点,已发展成为世界上最大的一类除草剂。
大量的研究证实,磺酰脲类除草剂主要通过抑制乙酰乳酸合成酶 (ALS)而发挥除草作用,可用于防除禾谷类和其它油料作物田中多种阔叶杂草和禾本科杂草。
目前全球参与该类除草剂品种登记的公司有杜邦、拜耳、先正达、日本武田等多家公司,登记的品种达30种之多[1]。
该类除草剂的共同特点是两个杂环之间以磺酰脲桥连接;化学和热不稳定;具有弱酸性 (pKa 3~5);在酸性溶液中迅速水解和脲桥断裂,在中性和碱性溶液中稳定性和溶解度提高,并易失去一个脲氢原子而以阴离子形式存在;在有机溶剂中一般较稳定,其溶解度大于10 mg/L (但烃类溶剂除外)[2-4]。
砜嘧磺隆是美国杜邦公司开发的新型磺酰脲类除草剂,是乙酰乳酸合成酶 (ALS)抑制剂,通过抑制植物体内的乙酰乳酸合成酶,阻止支链氨基酸的生物合成,从而抑制细胞分裂,使敏感的禾本科和阔叶杂草停止生长,然后褪绿、斑枯直至全株死亡。
1 名称及结构式通用名:砜嘧磺隆,rimsulfuron;其它名称:玉嘧磺隆;IUPAC名:1-(4,6-二甲氧基嘧啶-2-基)-3-(3-乙基磺酰基-2-吡啶磺酰基)脲;CA 主题索引名称:N-{[(4,6-二甲氧基-2-嘧啶基)氨基]羰基}-3-(乙基磺酰基)-2-吡啶磺酰胺;CAS登录号:[122931–48–0];商品名:Matrix (杜邦),Titus (杜邦),Rush (杜邦)。
除草剂草甘膦的性质及环境行为综述

除草剂草甘膦的性质及环境行为综述一、本文概述随着农业现代化的快速发展,除草剂在农业生产中扮演着越来越重要的角色。
其中,草甘膦作为一种广泛使用的除草剂,其性质及环境行为对生态环境和人类健康的影响日益受到关注。
本文旨在对草甘膦的性质及其环境行为进行全面综述,以期为提高除草剂使用的科学性和环保性提供参考。
本文将对草甘膦的基本性质进行介绍,包括其化学结构、理化性质、生物活性等方面。
在此基础上,分析草甘膦在土壤、水体和大气等环境中的行为特征,包括其降解途径、迁移转化规律以及与其他环境因素的相互作用等。
同时,本文还将对草甘膦的环境风险进行评估,探讨其对生态环境和人体健康可能产生的潜在影响。
本文还将对草甘膦的环境行为调控策略进行研究,探讨如何通过合理的使用和管理来降低其环境风险。
这些策略包括改进除草剂使用技术、优化农业生产模式、加强环境监管等方面。
本文旨在通过对草甘膦性质及环境行为的综述,为农业生产中除草剂的合理使用和环境保护提供科学依据。
也希望本文的研究能为相关领域的研究者和实践者提供有益的参考和启示。
二、草甘膦的化学和物理性质草甘膦,化学名称为N-(膦酰基甲基)甘氨酸,是一种非选择性、内吸传导型广谱灭生性除草剂。
其分子式为C3H8NO5P,分子量为07。
草甘膦在常温下为白色结晶粉末,无味,易溶于水、乙醇、丙酮等有机溶剂,微溶于乙醚、氯仿等。
在酸性环境下稳定,而在碱性环境中易分解。
草甘膦的化学性质主要表现为其强烈的除草活性。
作为一种有机磷化合物,草甘膦通过抑制植物体内烯醇丙酮基莽草素磷酸合成酶的活性,从而阻止莽草素向苯丙氨酸、酪氨酸及色氨酸的转化,使蛋白质合成受到干扰,导致植物死亡。
草甘膦还可以通过抑制植物体内5-烯醇丙酮莽草素-3-磷酸酯(EPSP)的合成,破坏植物的光合作用,进而达到除草的效果。
在物理性质方面,草甘膦呈白色或略带浅黄色的无定形粉末,具有吸湿性。
其熔点约为230℃,热稳定性较好。
草甘膦的水溶性较高,易在水中形成透明溶液,这使得它在环境中的迁移和分布能力较强。
色谱质谱联用技术在一起农药污染事故应急监测中的应用

扫描 ,推 断可能为三嗪类除苹荆—— 甲磺 隆。正、负 E I 离子全扫描后 图谱 与 甲磺 隆标 准物质 图谱 相对 照,完全吻 S子 合。最后,通过 S M模式下对 30O 3. R 8. 一19O的母、子离子选择 反应监测 ,测定了农田灌溉水源和土壤 中甲磺隆的含量。
关
键
词 :质谱技术 ;农药污染 ; 三嗪类 除草剂 ;甲磺隆
f l 8 a fp st e a d n g t e p o u tin n E Imo e t e s cr m f a lswa o itn i esa d r p cr m f ul c n o o i v n e a v r d c s i S d , h p tu o mpe sc n se tw t t tn a d s e tu o i i o e s s hh
H L — / S做 了 四个 实 验 , 别 是 :cn全 扫 描 P C MS M 分 8a 过程 (E IQ M : 0 0 0~60 0 0 ; R 一S 1 S 5 .0 0 . 0 ) S M选 择 反 应监 测 (E IMS : 8.0 @ CD 00 0 1850 ・S 2 3000 I2.0 [ 3.0
a ay i h l tn u l aiey a d q a t aie y i t y a o t g t e tc oo y o / n lsst e p l a tq ai t l n i t l .F r l b d p i h e h lg fGC MS,t e p l tn s i e t e o u t v n u t v sy n n h ol a t u wa d n i d i f a r zn .S c n l t r u h fl c n b PL — / 8t a i e e o d y, h o g ls a y H C MS MS,t ep l t t sd d ce 8 mes l r n,ap s cd f ra i e y i u h u a e u td a t f o o n wa u u e t ie o iz n .B i t
磺酰脲类

磺酰脲类百科名片磺酰脲类药物(sulfonylureas,SU)是应用最早、品种最多、临床应用也最广泛的口服降糖药,SU类药物有第一代和第二代之分,近年研制的格列美脲则以其用药剂量小、具有一定的改善胰岛素抵抗作用、减少胰岛素用量而被称为第三代SU类药物;除草剂品种开发始于70年代末期。
1978年Levitt等报道,绿磺隆(chlorsulfuron)以极低用量进行苗前土壤处理或苗后茎叶处理,可有效地防治麦类与亚麻田大多数杂草。
目录医疗作用除草剂其他磺酰类药品医疗作用除草剂其他磺酰类药品展开编辑本段医疗作用药物分类磺酰脲类药物(sulfonylureas,SU)是应用最早、品种最多、临床应用也最广泛的口服降糖药,SU类药物有第一代和第二代之分,近年研制的格列美脲则以其用药剂量小、具有一定的改善胰岛素抵抗作用、减少胰岛素用量而被称为第三代SU类药物。
磺酰脲类药物是那些并非很肥胖的2型糖尿病病人的一线治疗药物。
就降糖作用强度而言,第二代磺酰脲类药物是第一代的100-200倍,但所有SU类的最大活性是一样的。
虽然SU类的降糖最大活性没有区别,但各个药物又有不同的作用特点,如:格列本脲作用强度大,作用时间长,但低血糖发生率也高;格列喹酮则95%由胆汁经粪便排泄,仅5%从肾脏排泄,故较适于老年或轻度肾功能不全者;格列齐特和格列吡嗪则除了降糖作用外对糖尿病并发症有一定的防治作用。
所以临床应用时应根据患者的具体情况选择用药。
应该强调,所有磺酰脲类药物都能引起低血糖。
对于老年人和肾功能不全者,长效的磺酰脲类药物是特别危险的。
因此,对这些患者建议使用短效的磺酰脲类药物。
对有轻、中度肾功能不全者,格列喹酮更为合适。
作用机制(1)对胰岛β细胞的作用:已知SU在发挥对胰岛β细胞的作用时,必须先与β细胞表面的SU受体相结合,然后与β细胞表面的ATP敏感钾通道藕联,使此通道关闭,细胞膜去极化,从而释放胰岛素。
因此,SU能刺激β细胞释放胰岛素,从而降低血糖。
磺酰脲类除草剂对玉米敏感性及残留研究

望 !! ! : 兰
室皇
垒! / 旦鱼 垒 / ! _ 田
磺 酰脲 类 除草 剂对 玉米敏 感性 及残 留研究
◎ 林 木 森
( 县理 工 学校 ,河南 安 阳 4 6 7 滑 5 4 4)
摘 要 : 采 用 室 内 模 拟 添 加 法 和 栽 法 分 别 测 定 1 同 丘米 品 种 对 磺 酰 脲 类 除 草 剂 敏 感 性 和 在 土 壤 中 的 动 态 残 留 。 r不 结 果表 明 :不 同 的供 试 玉 米 品种 对磺 酰 } 类 除 草 剂 都 非 常 敏感 , 主要 抑 制 根 长 , 其 次 为 叶 面积 ,最 后 为 株 高 。不 同 】 I j { 碟 酰 脲 类 除草 剂 在 f壤 , 留期 不 同 。 {残 关 键 词 : 磺酰 脲 类 除 学剂 : 水 ;敏 感 性 茁期 ; 残 田 . 中 图 分 类 号 :S 8 . 42 4 ¥8 .+ 4 2 4 4 文 献 标 识 码 :A 文 章 编 号 : 1 7 0 9 ( 01 )0 0i 0l 6 9 2 2 3 0 2 卜
磺酰 脲 类化 合物 是 一 类高 选 择性 ,广谱 ,低毒 超 高效 除 草 2 结 果 与 分 析 剂 ,现 已代 表 农药 的主 要 发展 方 向。近 年 来广 泛 被 应 用。 但是 2 1磺 酰脲 类 除 草剂 对 不 同玉 米 品种 根长 抑 制试 验 . 若 使 用不 当 会对 敏感 的 后 茬作 物 造成 伤 害 或抑 制 生长 ,且 其残 不 同 玉米 品 种对 三 种磺 酰 脲 类 除草 剂都 比较敏 感 ,且 差 异 留对敏 感 后 茬作 物产 生 药 害 屡有 报道 。为 了科 学 用 药 ,有 必要 不 大 。供 试 品种 间登 海 9 对苯 磺 隆 、噻 磺 隆 相 对 最 敏 感 ,郑 号 了解 不 同玉 米 品种对 磺 酰脲 类除 草 剂的 敏 感性 差 异 。 因此 ,采 单 9 8 苯磺 隆 相 对 最敏 感 i供 试品 种 间 郑单 9 8 噻磺 隆 相对 5对 5对 用 室 内生物 测 定 法研 究 了不 同玉 米 品种 对磺 酰 脲 类除 草 剂 的敏 有耐 性 ,登 海 9 对苄 嘧磺 隆相 对 有 耐性 。但 其 E 值 均 达 到级 号 D 感 性和 磺酰 脲 类 除草 剂 对玉 米 苗期 影 响 的盆 栽 试 验 , 同时 ,也 水平 ,说 明供 试 品 种均 对 三种 磺 酰脲 类 除 草剂 都相 当敏 感 。 做 了施 药后 不 同时期 的 土壤 残 留试 验 。 22磺 酰脲 类除 草 剂对 玉米 苗期 影 响 的盆 栽 试验 .
【精品】糖尿病治疗药物研究进展文献综述

糖尿病治疗药物研究进展文献综述【摘要】:【关键词】:糖尿病,治疗药物,研究进展【摘要】:随着对糖尿病基础理论研究的深入,加深了对胰岛B细胞生理学和胰岛素外周作用机制的了解,已研制出具有多种作用机制的新型抗糖尿病药物用于临床评价和治疗。
本文将降血糖药物研究进展按作用机制分别综述如下。
糖尿病是由遗传因素、免疫功能紊乱、微生物感染及其毒素、自由基毒素、精神因素等等各种致病因子作用于机体导致胰岛功能减退、胰岛素抵抗等而引发的糖、蛋白质、脂肪、水和电解质等一系列代谢紊乱综合征,临床上以高血糖为主要特点,典型病例可出现多尿、多饮、多食、消瘦等表现,即“三多一少"症状,糖尿病(高血糖)一旦控制不好会引发并发症,导致肾、眼、足等部位的衰竭病变,且无法治愈。
糖尿病可分为胰岛素依赖型(1型,即IDDM)和非胰岛素依赖型(2型,即NIDDM),其中2型患者占糖尿病病例的80%以上。
目前,对于1型糖尿病的治疗,研究方向是开发给药方便、有效的胰岛素制剂及代用品。
而对于2型糖尿病的治疗,传统的磺酰脲类和双胍类口服降糖药疗效有限,并且无法根本阻止胰岛13细胞的进一步坏死,导致胰岛素依赖。
随着对糖尿病基础理论研究的深入,加深了对胰岛B细胞生理学和胰岛素外周作用机制的了解,已研制出具有多种作用机制的新型抗糖尿病药物用于临床评价和治疗.本文将降血糖药物研究进展按作用机制分别综述如下。
1胰岛素、胰岛素类似物及其制剂胰岛素(insulin)是一种由两条多肽链组成的酸性蛋白质,A链含21个氨基酸残基,B链含30个氨基酸残基,A、B两链通过两个二硫键共价相联。
药用胰岛素多从猪、牛胰腺提取。
目前可通过DNA重组技术人工合成胰岛素,还可将猪胰岛素B链第30位的丙氨酸用苏氨酸替代而获得人胰岛素。
对于1型糖尿病一般采用运动饮食疗法和胰岛素控制血糖水平的联合治疗。
科研人员在开发胰岛素类似物并寻找更方便的输药系统方面做了大量工作,目前已有多种产品面市。
药物降解机理研究e
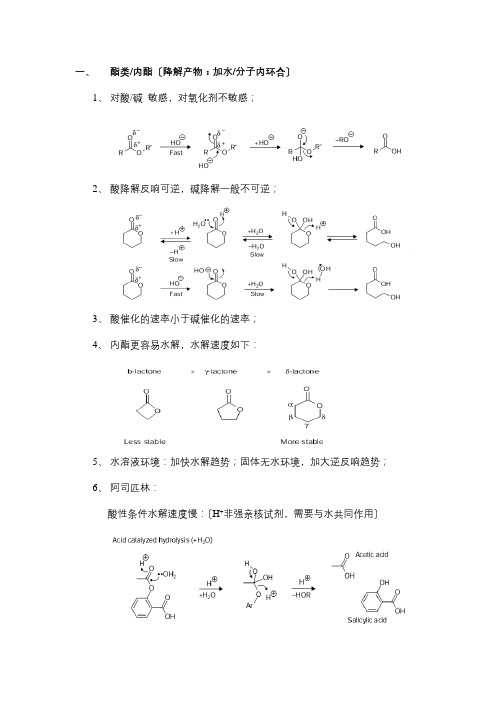
一、酯类/内酯〔降解产物:加水/分子内环合〕1、对酸/碱敏感,对氧化剂不敏感;2、酸降解反响可逆,碱降解一般不可逆;3、酸催化的速率小于碱催化的速率;4、内酯更容易水解,水解速度如下:5、水溶液环境:加快水解趋势;固体无水环境,加大逆反响趋势;6、阿司匹林:酸性条件水解速度慢:〔H+非强亲核试剂,需要与水共同作用〕碱性条件水解速度快:〔OH-强亲核试剂〕7、分子内环合:头孢呋辛钠二、酰胺/内酰胺类〔降解产物:加水〕1、水解速度:酰胺键比酯键稳定;硫代酰胺比酰胺易水解;2、利多卡因酸性条件易于水解,碱性条件不易水解〔空间位阻/静电排斥〕3、β-内酰胺药物〔开环水解/聚合〕⑴青霉素类与头孢类水解⑵氨苄西林的水解聚合反响⑶阿莫西林水解脱羧4、氨基甲酸酯类氯雷他定的水解反响:5、二酰亚胺:两侧都可水解6、内酰亚胺:〔水解+脱氨基+进一步水解〕三、羧酸类〔酯化反响/脱羧反响〕1、亲核进攻生成酯/酰胺/硫酯等;2、羧酸类药物在用甲醇结晶时,易生成酯类杂质;3、局部羧酸类药物可以发生脱羧反响〔β位有羰基〕,例如拉氧头孢:4、羧酸类药物可与辅料〔糖类,环糊精,聚乙烯醇等发生酯化反响〕四、酮类/醛类〔互变/美拉德/氧化/羟醛缩合/光降解〕1、酮类可与烯醇/二醇互相转化;2、醛与胺类发生类似美拉德反响;3、醛易被氧化,生成醇;酮不易被氧化,不饱和酮易发生加成反响;4、羟醛缩合反响5、醛/酮对光敏感,可发生光降解反响五、腈类〔水解/氧化〕1、腈类可与强酸强碱发生水解反响,生成酰胺后可再水解酸;腈类在P下,双氧水中水解生成过氧化物中间体,再生成酰胺,水解成酸;2、西咪替丁的水解:3、腈类可与游离氧反响:六、胺类〔美拉德/氧化降解/脱烷基/辅料/异构化/水解〕1、未质子化时,亲核性强,更易被氧化,更易挥发;2、伯胺、仲胺可与亲电试剂反响,例如醛基;因类似反响造成的事故:36人死亡,1500人患病,其原因就是API L-色氨酸与辅料中甲醛发生反响,进一步生成了二聚体杂质EBT,该杂质有较大毒性!3、氧化降解反响:(1)雷洛昔芬:(2)cope反响(3)更复杂的反响:4、芳胺/脂肪胺:芳胺氧化物可能生成基因毒性杂质;脂肪胺可能生成醇或烯;5、脱烷基反响:6、美拉德反响:糖与胺的反响7、胺类药物与辅料反响:〔1〕诺氟沙星与硬脂酸镁反响〔2〕塞罗西汀:淀粉为辅料,与马来酸发生1,4加成反响;滑石粉为辅料,与马来酸发生1,2加成反响;延胡索酸盐辅料可抑制上述反响;(3)药物与香草醛反响,导致API异构化:〔4〕度罗西汀与HPMCAS〔醋酸羟丙甲纤维素琥珀酸酯〕中的琥珀酸酐反响;8、亚胺:酸碱条件下易水解,因此HPLC检测时流动相中尽可能为中性。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
REVIEWSHydrolysis of Sulfonylurea Herbicides in Soils and AqueousSolutions:a ReviewA JIT K S ARMAH*,†AND J EAN S ABADIE‡School of Civil Engineering,Purdue University,1284Civil Engineering Building,West Lafayette,Indiana47907-1284,and CNRS,UMR5054,Universite´de Perpignan,52Avenue de Villeneuve,66860Perpignan Cedex,FranceSulfonylureas are a unique group of herbicides used for controlling a range of weeds and some grasses in a variety of crops and vegetables.They have been extremely popular worldwide because of their low mammalian toxicity,low use rate,and unprecedented herbicidal activity.Knowledge about the fate and behavior of sulfonylurea herbicides in the soil-water environment appears to be of utmost importance for agronomic systems and environmental protection.Because these herbicides are applied at a very low rate,and their mobility is greatly affected by the chemicals’anionic nature in alkaline soils,a thorough understanding of their degradation/hydrolysis processes and mechanisms under aqueous and soil systems is important.This review brings together published information on the hydrolysis of several sulfonylureas in aqueous and soil solutions that includes the effects of pH, temperature,functional relationship between pH vs hydrolysis rate constants,and hydrolysis behavior of sulfonylureas in the presence of minerals.In addition,the transformations of sulfonylureas in soil, under laboratory and field experiments,have been discussed in connection with the compounds’varied structural features,i.e.,sulfonylueas that are with or without the pyridinic,pyrimidine,and triazinic ring.Keywords:Sulfonylurea;hydrolysis;degradation pathways;pyridinic ring,triazinic ringINTRODUCTIONSulfonylureas are a unique group of herbicides used to control a range of weeds and some grasses in a variety of crops and vegetables including wheat,barley,oats,rice,maize,turf, soybeans,oilseed rap,flax,sugar beets,plantation crops, pastures,forestry,blueberries,potatoes,and tomatoes(1-3). Because of their low application rates(10-40g ha-1),low mammalian toxicity,and unprecedented herbicidal activity they have become very popular worldwide.The subsequent world-wide development effort has led to the commercialization of about25different active ingredients,and additional develop-mental products have been identified.Sulfonylureas are based on a general structure where R1 moiety can be either aliphatic,aromatic,or heterocyclic grouping connected by the sulfonylurea bridge to the R2moiety.This can be either a substituted triazine or pyrimidine system(4,5) as shown in Figure1a,b,and c.The chemistry,biology, biochemistry,degradation,and mode of action of sulfonylurea herbicides have been discussed in several reviews(1,6,7).Knowledge about the fate and behavior of sulfonylurea herbi-cides in the soil-water environment appears to be of utmost importance for agronomic systems and environmental protection. Because the herbicides are applied at a very low rate,and their mobility is greatly affected by their anionic nature in alkaline soils,analysis of sulfonylurea herbicide residues in soil is a challenging task for analytical chemists(8).This has resulted in a number of diverse analytical techniques being developed worldwide involving three main methodologies:chromato-graphic analyses(9-18),bioassays(19,20),and enzyme-linked immunosorbent assays(ELISA;21,22).Sulfonylureas are weak acids and have p K a values generally ranging from3to5.The herbicides in aqueous solution exist primarily in the neutral form at pH values below p K a,and in the anionic form at pH levels above the p K a.Therefore,the herbicides are predominantly anionic in most agricultural soils, and the relative concentrations of the neutral form are greatest in soils of low pH(23).This group of herbicides is subject to pH-dependent hydrolysis of the sulfonylurea linkage.The hydrolysis half-lives(t1/2)for several sulfonylurea herbicides are summarized in Table1.The two primary hydrolytic mechanisms are acid-catalyzed cleavage and base-catalyzed contraction/rearrangement of the sulfonylurea linkage.This bridge or the linkage is susceptible to attack by water on the*To whom correspondence should be addressed at Landcare ResearchNZ Ltd.,Private Bag3127,Hamilton,New Zealand.Tel:+6478583737.Fax:+6478584964.E-mail:sarmahA@†Purdue University.‡Universite´de Perpignan.J.Agric.Food Chem.2002,50,6253−6265625310.1021/jf025575p CCC:$22.00©2002American Chemical SocietyPublished on Web10/01/2002carbonyl carbon of the bridge,and thus produces CO 2and the corresponding aryl sulfonamide and amino-heterocyclic portions of the molecule.The rate of this reaction can often be hundreds of times faster under acidic conditions.For example,the hydrolysis half-lives (t 1/2)for chlorsulfuron at pH 3and pH 7.5at 25°C are reported to be 1day and >500days,respectively (24,25).Likewise,Sabadie (26)reported that bensulfuron-methyl at 30°C hydrolyzes with t 1/2of 7and 460days at pH 5and 8,respectively.Although the recovery of sulfonylurea herbicides from soils does not seem to present any difficulty (8),their degradation pathway in soil often remains complicated to investigate because most of the degradation products are difficult to extract,identify,and quantify.However,concomitant biotransformation and chemical hydrolysis appear to be significant for the breakdown of these herbicides in soil (24,27).To assess the relative importance of these two processes,degradation in sterilized and nonsterilized soil has been extensively studied (15,23,28,29).Observed differences in the rate of breakdown between thebioticFigure 1.(a)Sulfonylurea herbicides without pyridinic ring [R 1]and triazinicring [R 2];(b)sulfonylurea herbicides with a triazinic ring [R 2];(c)sulfonylurea herbicides with a pyridinic ring [R 1]and a pyrimidine ring [R 2].6254J.Agric.Food Chem.,Vol.50,No.22,2002Reviewsand abiotic degradation can be used to estimate the contribution of chemical hydrolysis(1),but problems with regard to the extraction and identification of the daughter products have not been fully investigated,and whatever efforts have been made lately(25,30)are mostly directed at laboratory investigations using radio-labeled compounds.Therefore,it is not surprising that many hydrolysis studies have been conducted in the past in an attempt to describe the behavior of sulfonylurea herbicides in aqueous buffer solutions as simplified models of soil solution(31-34).These studies provide useful information allowing a deeper understanding and proper evaluation of the hydrolysis of this group of compounds,as their role is a significant one in terms of evaluating their potential contamination of aquatic bodies such as groundwater and surface water.Besides hydrolysis studies,chemical degra-dation of several sulfonylureas on various dry minerals and on solid humic acid has also been investigated in the past(35,36). The purpose of this study is to bring together published information on hydrolysis of several sulfonylureas and present an overview of major results of chemical hydrolysis of sulfon-ylurea herbicides in aqueous buffer solutions/natural water,soils, and sediments.In addition,transformation of this group of herbicides in soil with regard to their structural composition is briefly discussed.MECHANISMS OF DEGRADATION/HYDROLYSIS IN AQUEOUS ENVIRONMENTA literature search on the degradation and metabolism of sulfonylurea herbicides in soil and water suggests that although there are quantitative differences in the degradation pathways, the most common primary degradation and metabolic pathways are the cleavage of the sulfonylurea bridge,O-and N-dealkylation,aryl and aliphatic hydroxylation,ester hydrolysis, and conjugation reactions with glutathione and carbohydrates (2,7).Cleavage of the sulfonylurea bridge can also occur through the base-catalyzed reaction of the linkage.For instance, pyridine-2-sulfonylureas such as flazasulfuron,rimsulfuron,and flupyrsulfuron methyl have been reported to undergo an interesting base-catalyzed contraction rearrangement of the sulfonylurea bridge,and their hydrolysis rates are faster in alkaline conditions than acidic conditions(37).It has been reported that these three sulfonylureas degrade in water10-1000times faster than many other sulfonylureas via an intramolecular nucleophilic addition and elimination reaction (38,39).The2-position of the pyridine ring is readily attacked by the distal urea nitrogen of the linkage,eliminating SO2and producing a“bridge contracted”product.Under alkaline condi-tions,this product can be further hydrolyzed to a pyridinyl-pyrimidinylamine;however,the products formed upon bridge contraction have been found to be biologically inactive. Furthermore,bridge hydrolysis can occur on the N-methyl group or due to the sterically induced intramolecular ester hydrolysis of the herbicide molecule.An example of N-methyl sulfonylurea bridge hydrolysis is the tribenuron methyl,unique among the commercial sulfonylureas in that it has an N-methyl substituent on the linkage(Figure1b).This linkage is much more susceptible to acid-catalyzed hydrolytic cleavage within the environmental pH regime(5-7),and at25°C the compound hydrolyzes nearly15-110times more rapidly than its N-demethylated product,metsulfuron-methyl(26).In addition, alcoholysis and ethanolyis can be other degradation pathways for these groups of herbicides(26,40).Chemical Hydrolysis of Sulfonylurea Herbicides.Sulfo-nylurea herbicides undergo hydrolysis in aqueous media at a rate which is a function of both temperature and pH(Tables1 and2),and the hydrolysis rate seems to vary according to the structural feature of the molecule.This was well demonstrated in several studies involving a number of sulfonylureas which examined the effect of both pH and temperature in aqueous buffer and soil solutions(1,26,31-34,40-44). Temperature Effect.Hydrolysis showed a marked effect with temperature,and a very acceptable description with first-order kinetics allowed determination of first-order half-lives(1,39, 31).An Arrhenius diagram was described at pH4for chlor-sulfuron(40),metsulfuron methyl(41),primisulfuron and rimsulfuron(38),and prosulfuron,primisulfuron methyl,rim-sulfuron,and thifensulfuron methyl(39).The activation energy values from83to135kJ/mol,obtained from the slope of the line,were compared(43)according to diverse literature data (1,45),but it was impossible to draw any significant conclusion on hydrolysis of these compounds as affected by different temperatures due to other confounding factors such as pH and moisture content.pH Effect.Because the aqueous solubility of sulfonylurea herbicides is pH-dependent,pH is expected to have a direct effect on the hydrolyis of sulfonylureas in aqueous buffer solution.Several studies in the past showed that these com-pounds hydrolyze more rapidly in water at acidic pH,but remained fairly stable in neutral solutions(26,34,40,46-47). This effect is illustrated in Figure2for chlorimuron ethyl hydrolysis at45°C(2).When the available literature data on the observed rate constants for three most commonly usedTable1.Hydrolysis Rate Constants(k,Day-1)and First-Order Half-Lives(t1/2,Days)for Some Sulfonylureas in Aqueous Buffer Solutions at pH4as a Function of Temperature(39)chlorsulfuron prosulfuron primisulfuron rimsulfuron thifensulfuron-methyl metsulfuron-methyl triasulfuron temp(°C)k t1/2k t1/2k t1/2k t1/2k t1/2k t1/2k t1/2 150.088.780.29 2.340.33 2.09 1.050.66200.12 5.590.14 4.970.49 1.410.62 1.11 1.310.530.14 4.980.15 4.62 250.29 2.380.26 2.640.65 1.06 2.480.27 1.610.430.37 1.870.36 1.92 300.46 1.48 1.050.65 3.290.21 1.990.3535 1.190.580.880.79 1.470.47 6.250.11 2.460.28 1.070.640.950.7345 2.760.25 2.520.27 3.200.2121.810.03 3.520.19 3.090.22 3.370.21558.110.08 5.850.12 5.70.1231.220.02 6.480.117.950.087.950.09Table2.Model-Estimated Rate Constants(Day-1)of Triasulfuron,Metsulfuron-methyl,and Chlorsulfuron in Buffer Solutions(pH5.2−11.2)at25°C arate constants(day-1)herbicide k1k2k3triasulfuron0.477(±0.015)0.00479(±0.00109)26.10(±1.96)metsulfuron-methyl 5.471(±0.161)0.00846(±0.00084)43.34(±1.52)chlorsulfuron 3.273(±0.100)0.00853(±0.00103)44.93(±1.87)a Standard errors(±)of regressions are given in parentheses(n)3). Reviews J.Agric.Food Chem.,Vol.50,No.22,20026255sulfonylureas (with distinct triazinic ring [R 2])were plotted against the aqueous solution pH (at 25°C),a similar trend was observed,as shown in Figure 3.Because sulfonylureas are weak acids,they exist as an equilibrium mixture of the neutral form and dissociated form much less subject to hydrolysis (1)as shown by the following equation:In that way,degradation rates were measured at different pH values for diverse sulfonylureas such as sulfometuron methyl (48),triasulfuron (33),chlorsulfuron (40),and metsulfuron methyl (42).In the same experimental conditions,hydrolysis rates were compared for several sulfonylureas (31,38,43,49).Relationship between degradation constants (natural logarithm)and pH was established for chlorsulfuron (40,41),metsulfuron methyl (42),prosulfuron,primisulfuron methyl,rimsulfuron,and thifensulfuron methyl (39).All sulfonylureas showed a linear but discontinuous dependence at different pH levels as illustrated in Figure 4.The slope variation occurred presumably as an effect of a different reactivity with respect to dissociated and neutral forms of these herbicides.Alkaline hydrolysis for sulfonylureas was also reported in the literature,although the rate constants appeared to be discordant (41,27).For example,Berger et al.(27)investigated 12sulfonylurea herbicides and did not observe (except for thifensulfuron)an increase in degradation rates when pH values were increased from 7to 10.However,an increase in degrada-tion rates in alkaline buffers was described for some of the same herbicides such as rimsulfuron (37),chlorimuron ethyl (44),bensulfuron methyl (26),and more recently for triasulfuron,metsulfuron methyl,and chlorsulfuron (34).It is conceivable that a buffer effect could explain these opposed results reported in these studies.In another study,thifensulfuron,which was hydrolyzed at the ester moiety,appears as a special case (32).Hydrolysis Pathways .Until the 1990s,it was believed that the predominant hydrolysis reaction of all sulfonylureas (under mildly acidic conditions)was the cleavage of the sulfonylureabridge (1,49)as depicted by the equation below.Hydrolysis proceeds through the attack of the neutral bridge carbonyl carbon by water,releasing carbon dioxide and the herbicidal-inactive sulfonamide and heterocyclic amine,which may sometimes undergo further hydrolytic degradation.How-ever,Dinelli et al.(11)who evaluated the potential of capillary electrophoresis for the hydrolysis studies of nine sulfonylurea herbicides,observed an unexpected number of degradation products.If simple sulfonylurea bridge cleavage can explain the four degradation compounds obtained with chlorimuron or bensulfuron (hydrolysis of the formed sulfonamide then cy-clization),the six compounds observed for chlorsulfuron or metsulfuron methyl (triazinic ring as R 2)probably implicated a more complex pathway.However,rimsulfuron led to only one (pyridinic ring as R 1).The number of daughter products that were produced during hydrolysis appeared to be related to the structure of the sulfonylurea.On the basis of their mechanisms of hydrolysis,three classes of sulfonylureas can be grouped as follows.Sulfonylurea Herbicides Without Pyridinic (R 1)and Triazinic Ring (R 2).This class involves most of these herbicides in which chemical structures do not include triazinic (R 2)and a pyridinic ring (R 1)as shown in Figure 1a .The simple hydrolyticcleavageFigure 2.Chlorimuron-ethyl hydrolysis rate constants versus pH at 45°C (2).R 1-SO 2-NH -CO -NH -R 2S[R 1-SO 2-N -CO -NH -R 2]-+H+Figure parison of rate constant (day -1)for three sulfonylureas inaqueous buffer solutions against pH at 25°C.Data are from b (46);O (47);2(33);and 4(34).R 1-SO 2-NH -CO -NH -R 2+H 2O wR 1-SO 2-NH 2+NH 2-R 2+CO 26256J.Agric.Food Chem.,Vol.50,No.22,2002Reviewsof sulfonylurea bridge is observed.The stable heteocyclic amine (R 2-NH 2)was identified as a major hydrolysis product of amidosulfuon in aqueous solutions (43),whereas the sulfona-mide moiety (R 1-SO 2-NH 2)was detected when sulfometuron methyl was hydrolyzed at pH 5,7,and 9(48,50).Further cyclization into saccharin was also observed.The two moieties of sulfonylurea bridge cleavage were simultaneously observed with chlorimuron ethyl (51,52),and an extensive hydrolysis study of this herbicide was described (44).The proposed degradation pathway suggested the break-down of the urea bridge only at pH e 8,however,under alkaline conditions (pH g 10)the way of the saponification of ethyl ester appeared preponderant (Figure 5).Similar results were obtained for another sulfonylurea,bensulfuron methyl;however,significant saponification appeared to occur above pH 8(26).Sulfonylurea Herbicides With a Triazinic Ring (R 2).An unexpected degradation pathway for this class of sulfonylureas (Figure 1b )with R 2as a methoxy-triazinic ring was first described for metsulfuron methyl (42).Most of the hydrolysis studies with regard to this class of herbicide were involved with chlorsulfuron (25),which was,incidently,the first sulfonylurea herbicide to be discovered and commercialized (53).For this class of sulfonylureas,the major degradation products are the stable chlorobenzene sulfonamide and triazine amine resulting from the hydrolytic cleavage of the sulfonylurea bridge (49,54),and it appears to be the major hydrolytic pathway especially at elevated temperature (Figure 6).However,a second concomitant hydrolysis pathway was described under acidic pH (10,40).The product of the O -demethylation of the methoxy substitute fixed to the triazine ring was first formed,followed by hydrolytic cleavage of the triazine ring (Figure 6).Further studies confirmed these observations (31).Structure of the triazine ring cleavage compound has been a subjectofFigure 4.Hydrolysis of chlorsulfuron (25°C);Ln of rate constant versuspH (40).Figure 5.Simplified pathway of chemical hydrolysis of chlorimuron ethyl (44,71).Figure 6.Simplified hydrolysis pathway of chlorsulfuron at pH 5(25,78).Reviews J.Agric.Food Chem.,Vol.50,No.22,20026257considerable debate.From NMR studies,two possible structures were proposed(55).The acetyl triuret structure,initially suggested by Reiser et al.(10)seems more acceptable,and later a detailed hydrolytic degradation pathway for chlorsulfuron at pH5was proposed by Strek(25).Other methoxy-triazine substituted sulfonylurea herbicides also presented a similar hydrolysis pathway with both sulfon-ylurea bridge breakdown and O-demethylation observed whenmetsulfuron methyl was hydrolyzed(8).The proposed degrada-tion pathways with the structure of the acetyl triuret(triazine ring cleavage compound)appeared to be inconclusive(42),or incorrect(31).Others reported an unexpected cyclization of the sulfonamide moiety(56).More recently,under highly alkaline conditions(pH11.2)the hydrolysis of the methyl ester group fixed to the aromatic ring and the O-demethylation were simultaneously observed by Sarmah et al.(34)with the correspondent acetyl triuret compound being formed upon hydrolysis.Braschi et al.(33)described a complete hydrolysis pathway for triasulfuron,another methoxy-triazine substituted sulfonyl-urea,in aqueous buffer solutions at pH values ranging from from2to9.The primary path of degradation was the cleavage of the sulfonylurea bridge,and a minor degradation path was also observed such as O-demethylation and opening of the methoxy-triazine ring.Similar pathways were described for hydrolysis of prosulfuron at pH5(57).The work of Braschi et al.(33)and Bray et al.(57)were analogous,with both groups proposing a two-step mechanism for the hydrolytic opening of the triazine ring.Therefore,the type of reactions that are discussed above seem to be common to the new methoxy-triazine-substituted sulfon-ylurea herbicides such as tribenuron methyl,ethametsulfuron methyl,or triflusulfuron methyl(7).However,when their hydrolysis rates were measured in aqueous solutions under controlled laboratory conditions(31),their degradation products still remained unidentified(56).Thifensulfuron methyl is also a member of this same class of herbicide,and the thiofene part of the molecule seems to induce a particular reactivity(24,1,32).The proposed acidic hydrolysis pathways for this compound in aqueous buffer solutions(pH4and5)showed a concomitant cleavage of the sulfonylurea bridge and O-demethylation of the methoxy group according to the general scheme.However,hydrolysis in alkaline condition was observed to be specific to this herbicide leading to the saponification of the methyl ester substitute and formation of the metabolite,thifensulfuron(32).This metabolite was, however,also found to be rapidly produced in soil by biological degradation as reported earlier by Smith et al.(58)and later by Brown et al.(59)through laboratory studies.Sulfonylurea Herbicides With a Pyridine Ring(R1).The replacement of the aromatic ring(R1)by a pyridine one affords a new class of sulfonylurea herbicide(R2)pyrimidine ring), rimsulfuron.This compound has been found to degrade rapidly in soil and water through a specific path attested by hydrolysis of rimsulfuron in water at25°C at pH4,7,or9(60),and gave only one product at55°C and pH4(61).For rimsulfuron,the breakdown of sulfonylurea bridge appeared as a minor degrada-tion path as shown below,whereas sulfonylurea bridge contrac-tion was preponderant(37,62-64):The primary hydrolysis product at pH5was compound1, resulting from the contraction of the sulfonylurea bridge, whereas amine2was the major hydrolysis product at pH7and 9.A reaction mechanism proceeding through a five-member transition state was suggested(65).Studies conducted by Teaney et al.(66)and Rouchaud et al.(67)revealed that the hydrolysis pathway of flupyrsulfuron methyl seems comparable with that of other pyridylsulfonylurea herbicides,however,the formed product1′was allowed to cyclize into3′as shown below:At pH7or9this latter product3′appeared in good yields, whereas at pH5there were four major degradation products: compound1′and its internal cyclization product3′,as well as the two compounds which resulted from the simple breakdown of the sulfonylurea bridge(68).Nicosulfuron,which belongs to the substitute pyridine-sulfonylurea herbicides,exhibits a pyridyl-sulfonylurea bridge in place of the usual phenyl-sulfonylurea group(Figure1c). From a recent work on hydrolysis of nicosulfuron,it was concluded that the main degradation pathway for this compound was the hydrolysis of the sulfonylurea bridge(69),whereas the bridge contraction was more prevalent for other compounds within this category such as rimsulfuron(37,63-65)and flupyrsulfuron-methyl(67,68).Degradation of Sulfonylurea Herbicides in the Presence of Minerals.Degradation of several sulfonylurea herbicides in the presence of minerals was investigated with a view to realize a simplified model of the soil,and obtained results were compared to the conclusions derived from hydrolysis studies (31,40,42).For instance,the observed degradation rates of12 sulfonylureas in two water-sediment systems could be ex-plained by both biotic and chemical process(33).In native sediments at neutral pH,microbial degradation was found to be prevalent,however at lower pH,chemical hydrolysis became more important.The results of Berger and Wolfe(31)allowed a direct comparison of the degradation rates of the herbicides in water and in sediments.Influence of clay mineral on the adsorption and degradation of sulfonylurea herbicides has been investigated by few researchers in the past.For example,Pantani et al.(70)reported a partial decomposition of cinosulfuron to several products after undergoing adsorption from the organic solvent on Al-montmorillonite.One of these was identified as the correspondent hydroxy-s-triazine(R2-OH).Others have investigated the behavior of sulfonylurea deposition on various dry minerals such as kaolinite,silica gel,alumina,and bentonite, and suggested degradation pathways for bensulfuron methyl (36),chlorimuron ethyl(71),chlorsulfuron(72),or metsulfuron methyl(73).These pathways were found to be similar to those previously proposed for chemical hydrolysis.Degradation of rimsulfuron adsorbed on Al-hectorite was investigated by Pantani et al.(74)and they proposed a degradation mechanism that involved the similar bridge contrac-tion products(1)and(2)as previously formed during hydrolysis (37).In contrast,Ukrainczyk and Rashid(75)described therapid6258J.Agric.Food Chem.,Vol.50,No.22,2002Reviewsdisappearance of nicosulfuron deposited on clay minerals,however,the identification of the degradation product was not reported.Elsewhere,degradation on solid humic acid was investigated for chlorsulfuron,metsulfuron methyl (35),ben-sulfuron-methyl,and chlorimuron ethyl (36,44),and the observed pathways were similar to those described for hydrolysis studies for the same compounds.Unlike most sulfonylurea herbicides that are weak acids and exhibit only a limited affinity for various minerals (76,77),nicosulfuron (p K a ) 4.3)showed an unexpectedly strong interaction with clay minerals (75).However,in contrast to the findings of Ukrainczyk and Rashid (75),Sabadie (69)reported slower degradation of this compound at 30°C after its deposition on various oven-dried minerals that included kaolinite,silica gel,H +-bentonite,montmorillonite K10,and alumina.Although these minerals did not seem to provoke the expected rapid degradation,catalyzed by clay surface as reported earlier (75),the measured half-lives appeared to be in close approximation to those described for bensulfuron-methyl (36)and were higher than chlorimuron ethyl (44)under similar experimental condi-tions.Model for pH vs Hydrolysis Rate.It has been well established that for weak acids,such as the sulfonylureas,there exists an equilibrium between the undissociated and the negatively charged dissociated molecules in aqueous solution (1).Hence,depending upon the pH of the system,the molecules of the herbicide can be present as anions or as the conjugate acid.On the basis of the ionization constants (p K a )of these molecules,the anions can thus experience either slightly acidic to neutral or alkaline environments.The most important and the first step in the hydrolysis process involves the cleavage of the sulfonylurea linkage via attack by water,or at higher pH,by the hydroxyl ions.On the basis of this,Sarmah et al.(34)has developed a model to describe the pH-dependent hydrolysis rate (in aqueous buffer)for three sulfonylurea herbicides:chlorsulfuron,metsulfuron-methyl,and triasulfuron.The main assumptions of the model were that,over the pH range of 4-12,the observed rate of sulfonylurea hydrolysis is the sum of the following three simultaneous reactions:(a)hydrolysis of the undissociated sulfonylurea molecules;(b)hydrolysis of the anionic species of sulfonylurea molecules at near neutral pH;and (c)hydrolysis of the anionic species of sulfonylurea molecules by hydroxide ion.The three reactions can be represented as follows:The equation is based on the concept of the Henderson -Hasselbach equation,which can be written as follows:where [SU ]is the concentration of sulfonylurea herbicides,HAis acid,and A -is the conjugate base.The above three processescan be thus represented as follows:whereIn equation 3,k 1,k 2,and k 3are the rate constants for each of the hydrolysis reactions,X is the pH of the buffer solutions,and p K a is the dissociation constant of the sulfonylurea herbicide.Each of the individual rate constants exhibits a temperature dependence that conforms to the Arrhenius equa-tion,and under constant temperature these rate constants will be constant.When the pH is held constant,then the right-hand side of the eq 3is constant and the rate of loss of sulfonylurea herbicide follows the pseudo-first-order kinetics (34).Figure 7shows the functional relationship between the hydrolysis rate constants and pH for three sulfonylurea herbicides at a constant temperature of 25°C.A nonlinear regression was used to estimate the values for the rate constants k 1,k 2,and k 3for each of the three compounds,and the relative magnitude of the model-estimated rate constants was in the order k 3>k 1.k 2for each compound investigated (Table 2).This study confirmed similar findings reported in the past (1,31)that sulfonylurea hydrolysis occurs most readily via hydroxyl ion attack of the bridge carbonyl (alkaline conditions)and that hydrolysis involv-ing attack by neutral water is at least 1000-times faster when the molecule is undissociated (acidic conditions)than when it is present as the anion at near neutral pH.Transformation of Sulfonylurea Herbicides in Soil.Many persistence evaluation experiments of sulfonyurea herbicides in soil can be found in the literature (e.g.,1,6,25,29,58,76-78).Although these studies presented some results of undeniable agronomic interest,an extensive knowledge of sulfonylurea behavior in soil seemed to be lacking in regard to their implication in the environment:phytotoxicity of residues,remnants of degradation products,presence of “bound residues”,and groundwater pollution.HA 98k 1H 2Osulfonamide +heterocycle +CO 2(undissociated)vA -98k 2H 2Osulfonamide +heterocycle +CO 2(anion,acidic -neutral pH)vA -98k 3OH -sulfonamide +heterocycle +CO 2(anion,alkaline pH)v[SU ])[HA ]+[A -](1)Figure 7.Hydrolysis rate constants (day -1)of triasulfuron (b ),metsulfuron-methyl (O ),and chlorsulfuron (4)in aqueous buffer solution as a function of pH.Symbols represent measured data,whereas the solid lines represent the fit of eq 3.-d (ln[SU ])dt)Y (2)Y )[k 1+[k 210(X -pK a )]+[k 310(2X -14-pK a )]][1+10(X -pK a )](3)ReviewsJ.Agric.Food Chem.,Vol.50,No.22,20026259。
