国外生物安全法律法规
国外生物遗传资源法律保护及其启示

护法》( 1 9 8 6年 )等 法 律 中 。而 成 为 C B D 的缔 约 国
基金项 目: 河北 省教育厅一般项 目 “ 生物遗传资 源法律规制及 其
域外借鉴”( 编号 :HB 1 5 C F X0 4 2 ) 。
物遗传资源法律保护现状 ,总结出可供借鉴 的经验 , 以期对完善和构建 中国生物遗传资源立法和保护制
1 . 1 印度
年来 , 由于 生物 遗 传 资 源 的 巨大 价 值 及 其 分 布 的 不 均衡 ,生物 遗传 资 源 匮 乏 的发 达 国家 对 生 物 遗 传 资
源 丰 富的发 展 中 国家 的 “ 生 物 剽 窃 ” 现 象 越 来 越 严 重 。中 国的 生 物遗 传 资源 比较 丰 富 ,因 此 ,成 为 发
随着 《 生物 多样 性 公 约 》 的签 订 ,许 多 国家 对 本 国
遗传 资源 。
1 . 1 . 1 相关 立法
在成 为 《 生物多样性公 约》 ( C B D)缔 约 国 之 前 ,印度 有 关 生 物 遗 传 资 源 的法 律 ,比较 分 散 ,主 要分 布 在 《 种子法》 ( 1 9 6 6 年 )《 专 利法 》 ( 2 0 0 2年 修正 本 )《 野生动植物保护法》 ( 1 9 7 2年 ) 《 环 境 保
世孝 壤业 Wo d d A g r i c u h u r e
之后 ,印度加 快 出 台关 于生 物 多样 性 保 护 法 律 的进 程 。2 0 0 2年 印度在 C B D原 则 下制定 了 《 生物 多样 性 法》 ,其 为一 部综 合性 立 法 ,与此 同时制 定 《 生物 多 样性 条 例 》为其 配套 实施 规定 ,使 其具 有可 操作 性 。
实验室生物安全的法律法规课件

PPT学习交流
3
WHO的《实验室生物安全手册》 第三版,2004年
• 在2004年的第3 版中,阐述新千年所面临的生物安全 和生物安全保障问题。在下列几个方面增加了新的内 容:危险度评估、重组DNA 技术的安全利用以及感染 性物质运输。世界上最近发生的事件表明,由于蓄意 滥用和排放微生物因子和毒素,公共卫生正受到新的 威胁。因此,第3 版也介绍了生物安全保障的概念— —保护微生物资源免受盗窃、遗失或转移,以免因微 生物资源的不适当使用而危及公共卫生。第3 版中还 包括了1997 年WHO 出版的《卫生保健实验室安全》 中有关册》 第三版,2004年
• 主要内容有:
• 生物安全柜
• 安全设备
• 实验室技术
• 意外事故应对方案和应急程序
• 消毒和灭菌
• 感染性物质的运输
• 生物安全和重组DNA技术
• 危害性化学
• 生物安全官员和安全委员会
• 后勤保障人员的安全
• 培训规划等
PPT学习交流
6
一、国际组织和国外病原微生物实 验室生物安全相关法规和指南
• 美国的《微生物和生物医学实验室生物安全手册》
1983年第一版,1993年第三版,1999年第四版;
• 美国的《生物危害主要防护:生物安全柜的选择、 安装和使用》
2000年第二版;
• 加拿大的《实验室生物安全指南》
1990年第一版,1996年第二版,2004年第三版
PPT学习交流
7
《微生物和生物医学实验室生物安全 手册》(美国CDC/NIH,1999,第四版)
• 在生物危害委员会的建议下,MRC在1979年和1980年 出版的两版《重组DNA分子、动物病毒和细胞的指南》 的基础上,于1990年出版了第一版《实验室生物安全 指南》,并成立了实验室疾病控制中心联合工作组, 工作组为那些从事研究或开发目的而进行人类病原体 的单位提供相应等级的实验室设计、建设和在其中工 作的人员培训的技术资料,这种技术资料的重点是有 关细菌、病毒、寄生虫、真菌和其他对人类有致病作 用的感染性病原体的实验室生物安全防护措施。
生物安全概述及病原微生物实验室生物安全管理条例解读

第六条 实验室设立单位及其主管部门的管理职责: 日常管理\建立制度\检查维护设施\控制实验室感染
《病原微生物实验室生物安全管理条例》
• 第二章 病原微生物的分类和管理(七-十七条) 第二章规定了病原微生物的分类和管理的要
•2003年SARS流行期间,我国医院内感染病例占 病人总数的20%左右 •医护人治免疫功能低下患 者(如肿瘤患者等)的病房也需要防止(或尽可 能降低)病原微生物的污染或交叉感染
生物安全实验室的重要意义(四)
3、为加强各实验室对突发公共卫生事件应对能力 提供科学、安全的实验平台
20% 感染的原因是明确的;/// 80%为不明原因感染。
实验室生物安全发展 (六)
在明确的20%中有20% 是由设备故障引起的; 80% 是由工作人员操作失误引起。
导致感染最多的实验室事故
如:溢出和泼洒;针头和注射器锐器;碎玻 璃;动物或动物体外寄生虫的咬伤或抓伤
实验室生物安全发展 (七)
• 最早国内进口少数BSL-3实验室 • 20世纪80年代我国自己研制第一个国产三级生物安
生物安 全水平
Ⅰ
Ⅱ
Ⅲ
实验室类型
基础教学、 研究
诊断、研究 实验室 特殊的诊 断、研究
危险度 等级
实验室操作
1 级 GMT(微生物学操作技
术规范)
2 级 GMT、生物危害 标志
3 级 BSL2增加特殊防护服、进
入制度、定向气流
安全设施
不需要
BSC(生物安全柜) 个人防护装备
BSC 和/或所有实验室工作 需要的基本设备
和寄生虫等生物因子。 • 危害等级Ⅱ(中等个体危害,有限群体危害) 能引起人或动物发病,但一般情况下对健康工作者、
国内外关于病原微生物等级的划分

国内外关于病原微生物危害等级的划分1、《病原微生物实验室生物安全管理条例》第七条规定,国家根据病原微生物的传染性、感染后对个体或群体的危害程度,将病原微生物分为四类:第一类病原微生物,是指能够引起人类或者动物非常严重疾病的微生物,以及我国尚未发现或者已经宣布消灭的微生物。
第二类病原微生物,是指能够引起人类或者动物严重疾病,比较容易直接或间接在人与人、动物与人、动物与动物之间传播的微生物。
第三类病原微生物,是指能够引起人类或者动物疾病,但一般情况下对人、动物或者环境不构成严重危害,传播风险有限,实验室感染后很少引起严重疾病,并且具备有效治疗和预防措施的微生物。
第四类病原微生物,是指在通常情况下不会引起人类或者动物疾病的微生物。
其中,第一类、第二类病原微生物统称为高致病性病原微生物。
2、《实验室生物安全通用要求》(GB 19489-2004根据生物因子对个体和群体的危害程度将其分为4级:危害等级Ⅰ(低个体危害,低群体危害:不会导致健康工作者和动物致病的细菌、真菌、病毒和寄生虫等生物因子。
危害等级Ⅱ(中等个体危害,有限群体危害:能引起人或动物发病,但一般情况下对健康工作者、群体、家畜或环境不会引起严重危害的病原体。
实验室感染不导致严重疾病,具备有效治疗和预防措施,并且传播风险有限。
危害等级Ⅲ( 高个体危害,低群体危害:能引起人或动物严重疾病,或造成严重经济损失,但通常不能因偶然接触而在个体间传播,或能用抗生素抗寄生虫药治疗的病原体。
危害等级Ⅳ(高个体危害,高群体危害:能引起人或动物非常严重的疾病,一般不能治愈,容易直接、间接或因偶然接触在人与人,或动物与人,或人与动物,或动物与动物之间传播的病原体。
3、农业部《兽医实验室生物安全管理规范》中的微生物危害通常分为以下4级:生物危害1级:对个体和群体危害程度低,已知的不能对健康成年人和动物致病的微生物。
生物危害2级:对个体危害程度为中度,对群体危害较低,主要通过皮肤、粘膜、消化道传播。
2020年10月17日生物安全法

2020年10月17日生物安全法生物安全是国家安全体系的重要组成部分,关系到人民健康、生态安全和国家安全。
2020年10月17日,我国生物安全法正式颁布实施,为生物安全风险防控提供了法律依据,标志着我国生物安全法治建设迈入新阶段。
一、生物安全法的背景与意义近年来,全球生物安全风险不断上升,病毒疫情、生物恐怖主义、生物技术滥用等问题日益突出。
在此背景下,我国生物安全法应运而生,旨在加强生物安全风险防控,保障国家安全和人民生命健康。
生物安全法的制定和实施,既是对国内外生物安全形势的回应,也是我国生物安全法治建设的重要举措。
二、生物安全法的主要内容生物安全法共十章九十四条,涵盖了生物安全风险防控体制、生物安全风险评估与预警机制、生物安全应急措施、生物安全交流合作、生物安全法律责任与处罚等方面内容。
1.生物安全风险防控体制:生物安全法明确了国家生物安全领导机构的职责,各级政府、有关部门和单位的责任,以及公民、法人和其他组织的义务。
2.生物安全风险评估与预警机制:生物安全法规定,国家建立生物安全风险评估制度,对可能导致生物安全风险的活动进行评估。
同时,建立生物安全风险预警制度,及时发布预警信息,指导有关方面采取预防措施。
3.生物安全应急措施:生物安全法明确了生物安全事件的应急处置措施,包括应急预案的制定、应急资源的配置、应急处置的实施等。
4.生物安全交流合作:生物安全法强调,我国积极参与国际生物安全交流合作,加强与其他国家和地区的沟通与合作,共同应对生物安全风险。
5.生物安全法律责任与处罚:生物安全法明确了违法行为的法律责任,对违反生物安全管理规定的行为予以处罚,确保法律的有效实施。
三、我国生物安全法的实施与展望生物安全法的颁布实施,为我国生物安全风险防控提供了有力的法律支撑。
各级政府、有关部门和单位要严格按照法律规定,履行职责,加强生物安全风险防控体系建设,提升生物安全管理水平。
同时,广大人民群众要提高生物安全意识,积极参与生物安全治理,共同维护国家生物安全。
美国国门生物安全法律法规及管理现状

第26卷第1期口岸卫生控制国内外《生物安全法》比较美国国门生物安全法律法规及管理现状王雅丽王利海关总署研究中心(北京,100088)季新成海关总署国际检验检疫标准与技术法规研究中心(北京,100013)摘要重大传染病和生物安全风险是事关国家安全和发展、事关社会大局稳定的重大风险挑战。
国门生物安全法规体系为严防有害生物传入传出国门、保障国门生物安全提供法律保障。
国际上发达国家都很重视国门生物安全防护,并建立了完善的法律体系和管理制度。
本文全面梳理美国国门生物安全方面的法律法规,总结其管理特点,提出完善我国国门生物安全法律法规体系、健全联防管控协同机制、提升能力建设、推进宣传共治等建议,以期通过借鉴来健全我国国门生物安全防控体系和管理制度。
关键词美国国门生物安全管理法律法规中图分类号R185.3 文献标识码B doi 10.3969/j.issn.l008-5777.2021.01.008Laws and Management of the United States Biosafety at Border CrossingsWang Yali Wang Li Research Center of GACC (Beijing,100088)JI Xincheng Research Center of GACC for International Inspection and Quarantine Standards and Technical Regulations (Beijing,100013)Abstract Major infectious diseases and biosafety risks are major risk challenges related to national security and development,and social stability.The laws system of biosafety at border crossings provides legal guarantee for strictly preventing harmful organisms from spreading into and out of the country and ensuring biosafety at border crossings.Developed countries in the world attach great importance to biosafety at border crossings protection, and have established a sound legal system and management system.In order to improve China's laws system of biosafety at border crossings and prevention and control system and management system,the paper comprehensively combs the laws of biosafety at border crossings in the United States,summarizes its management characteristics,and puts forward some suggestions,such as improving China's laws system of biosafety at border crossings, improving the collaborative mechanism of joint prevention and control,enhancing capacity-building,and promoting publicity and co-governance,etc.Key words USA Biosafety at border crossings Laws Management进入21世纪,随着人类活动范围扩大、跨境流作者简介:第一作者:王雅丽(1989-),女,汉族,河南周口人,硕士,助理研究员,研究方向为海关基础理论、农产品贸易,E-mail: 137**************o m。
农业生物安全的法律制度

摘要:随着生物技术的快速发展,农业生物安全问题日益凸显。
为保障国家粮食安全、生态环境和人民健康,我国已建立了较为完善的农业生物安全法律制度。
本文将从农业生物安全法律制度的概述、主要内容、实施与监管以及存在的问题与展望等方面进行探讨。
一、农业生物安全法律制度的概述农业生物安全法律制度是指国家为预防和控制农业生物灾害,保障国家粮食安全、生态环境和人民健康,依法对农业生物安全进行管理和监督的法律规范体系。
我国农业生物安全法律制度主要包括《中华人民共和国生物安全法》、《中华人民共和国种子法》、《中华人民共和国植物检疫法》等法律法规。
二、农业生物安全法律制度的主要内容1. 确立农业生物安全的基本原则农业生物安全法律制度确立了预防为主、防治结合、科学管理、依法监管的基本原则。
这要求各级政府、相关部门和农业生产经营者,在农业生物安全管理工作中,坚持科学、严谨、规范、高效的原则,确保农业生物安全。
2. 明确农业生物安全的管理主体和职责农业生物安全法律制度明确了各级政府、相关部门和农业生产经营者在农业生物安全管理中的职责。
其中,政府负责制定农业生物安全政策、法规和标准,加强监管;相关部门负责农业生物安全的监测、预警、防控和应急处置;农业生产经营者负责落实农业生物安全措施,确保农业生产安全。
3. 规范农业生物安全管理与监督农业生物安全法律制度对农业生物安全管理与监督进行了规范,包括:(1)农业生物安全风险评估与预警:对农业生物安全风险进行评估,发布预警信息,引导农业生产经营者采取相应措施。
(2)农业生物安全监测与防控:建立健全农业生物安全监测网络,对农业生物灾害进行监测、预警和防控。
(3)农业生物安全应急处置:制定农业生物安全应急预案,明确应急处置程序和责任,确保应急处置工作有序进行。
4. 保障农业生物安全技术研发与应用农业生物安全法律制度鼓励农业生物安全技术研发与应用,支持农业生产经营者采用生物安全新技术、新材料、新工艺,提高农业生物安全管理水平。
国外生物安全法律法规
关于凭借现代生物技术获得的转基因生物的获取、测试、利用、商业化法
Romanian Law on the Obtaining, Testing, Use and Commercialization of the Genetically Modified Organisms Resulting from Modern Biotechnology, as or in Products
转基因生物有意释放条例
Genetically Modified Organisms (Deliberate Release) Regulations (2003)
转基因生物越境转移条例
Genetically Modified Organisms (Transboundary Movement) Regulations (2004)
关于转基因高等植物环境释放结果报告格式的决定〔2003〕
Commission Decision of 29 September 2003 Establishing Pursuant to Directive 2001/18/EC of the European Parliament and of the Council a Format for Presenting the Results of the Deliberate Release into the Environment of Genetically Modified Higher Plants for Purposes other than Placing on the Market
关于建立转基因生物惟一标识系统的条例〔2004〕
COMMISSION REGULATION (EC) No 65/2004 of 14 January 2004 establishing a system for the development and assignment of unique identifiers for genetically modified organisms
国外生物安全法律法规

Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 Concerning the Traceability and Labelling of Genetically Modified Organisms and the Traceability of Food and Feed Products Produced from Genetically Modified Organisms and Amending Directive 2001/18/EC
关于建立转基因生物惟一标识系统的条例(2004)
COMMISSION REGULATION (EC) No 65/2004 of 14 January 2004 establishing a system for the development and assignment of unique identifiers for genetically modified organisms
转基因生物越境转移条例(2003)
Regulation (EC) No 1946/2003 of the European Parliament and of the Council of 15 July 2003 on Transboundary Movements of Genetically Modified Organisms
转基因生物环境释放法令(2001)
Directive 2001/18/EC of theEuropean Parliament and of the Councilof 12 March 2001on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC
浅议国外的生物安全立法

浅议国外的生物安全立法[摘要]随着现代生物技术的迅速发展,生物安全立法越来越引起各国立法者的重视。
目前,人类还不能完全掌握现代生物技术,不能完全否定其对生物安全存在潜在的危害性。
通过制定一定的法律法规,对其潜在的危害性加以预防,是具有现实意义的。
但是,我国当前生物安全方面的立法层次比较低,立法技术比较落后,不能满足现实生活的需要。
因此,文章在阐述了现代生物技术存在的安全性隐患的基础上,分析了国外生物安全的立法状况。
[关键词]生物安全问题;生物技术;生物安全立法一、生物技术引发的生物安全问题关于生物技术,1992年的《生物多样性公约》第2条将其定义为生物技术是指使用生物系统、生物体或其衍生物的任何技术应用,以制作或改变产品或过程以供特定用途。
[1]这一定义一般被认为是广义的生物技术,它包括传统的生物技术和现代生物技术。
而狭义的生物技术仅指现代生物技术。
现代生物技术又称为生物工程技术,是20世纪70年代初在分子生物学、遗传学、细胞学、微生物学及生物化学和计算机学科的基础上发展起来的一门新型的跨学科的应用技术科学,是以生命科学的理论和成就为基础,运用基因重组、细胞融合、固定化酶以及细胞或组织培养和生物传感器等新技术和工程技术原理,加工生物材料或定向地组建具有特定性状的新物种或新产品系,为人类提供所需的产品和服务。
现代生物技术的范围和领域十分广泛,目前主要包括基因工程(又称为转基因技术)、细胞工程、酶工程和微生物工程。
其中,基因工程技术是核心,运用DNA 重组技术,可以按照人类的意志来改造和组建新生物物种,赋予细胞工程、酶工程和微生物工程等以新的内容。
[2]本文所称的生物技术即为狭义的生物技术,即现代生物技术。
生物安全问题主要体现在转基因食品上。
转基因食品指利用转基因技术生产的食品。
我国《转基因食品卫生管理办法》第2条对转基因食品的定义:利用基因工程技术改变基因组构成的动物、植物和微生物生产的食品和食品添加剂。
国内外实验室生物安全标准发展历程与展望

国内外实验室生物安全标准发展历程与展望随着生物技术的快速发展,生物研究的范围和深度也得到了极大扩展,与此同时,生物安全问题也越来越受到关注。
实验室生物安全标准的制定和执行是保障生物实验工作者的安全以及生物安全的重要措施。
本篇文章将从发展历程和展望两方面,介绍国内外实验室生物安全标准的发展及其未来。
一、国内实验室生物安全标准发展历程我国生物技术研究与应用快速发展的同时,相对应的生物安全问题也逐渐引起重视。
回顾国内实验室生物安全标准的制定历程,经历了以下几个发展阶段:1. 初期探索阶段(20世纪70年代至80年代)这个时期的我国生物实验室还十分匮乏,多数从事生物研究的单位没有专门的实验室,也没有相关的安全管理制度和技术人员。
针对这种情况,国家科技部于20世纪80年代初开始组织编写生物实验室安全规定,对生物实验室的建设、管理和保护等进行详细规定,这为我国实验室生物安全标准制定奠定了基础。
2. 完善制度阶段(20世纪90年代至21世纪初)在经过初期探索阶段,我国生物实验室的安全管理逐渐完善。
1995年,卫生部发布了《生物安全管理条例》。
2004年,科技部颁布《生物安全管理办法》,出台了一系列生物安全管理制度和规范文件,如《生物实验室安全管理规程》和《高等教育生物学类专业生物实验室安全管理规定》等,有效地保障了实验室生物安全。
3. 质量管控阶段(21世纪中期至今)随着我国生物实验室的不断建设壮大,实验室质量管控成为一个必须要重视的问题。
针对实验室生物与环境交互复杂、生物材料易发生变异、实验室操作人员生物安全意识和实验技能参差不齐等现状,我国生物实验室在标准制定、人员培训等方面的要求越来越严格。
在质量管控的背景下,我国实验室生物安全标准修订和完善工作也得到了很大推进。
二、国际实验室生物安全标准发展历程作为国际标准的制定和推广者,国际生物安全标准的发展历程相对中国要长,也要复杂。
国际实验室生物安全标准主要由两个机构主导,一个是世界卫生组织下的生物安全规定管理局(BSL),另一个是美国国家环境保护局(EPA)。
国外生物安全法律法规

国外生物安全法律法规国外生物安全法律法规引言生物安全法律法规是国家对于生物安全保护进行规范和监管的重要手段。
随着全球生物技术的迅猛发展,各国纷纷制定并完善了相关法律法规,以确保生物资源的合理利用、保护环境、维护公共安全。
本文将对国外生物安全法律法规的发展和特点进行介绍。
1. 美国美国是世界上生物技术研发最活跃的国家之一,其生物安全法规体系较为完善。
美国的生物安全法律法规主要由联邦政府和各州政府共同管理。
生物安全法(Bioterrorism Act):2002年,美国制定了《生物安全法》,旨在加强对生物威胁的预防和应对能力。
该法规主要关注生物恐怖主义、生物安全管理和食品安全等方面,并对涉及生物安全的企业和组织进行注册和监管。
转基因生物法规(Regulation of Genetically Modified Organisms):美国对转基因生物的管理相对较为宽松。
目前,美国没有单独的转基因生物法律法规,但美国农业部和食品药品监管局分别负责转基因作物和转基因食品的监管。
2. 欧盟欧盟是全球最大的转基因生物进口市场之一,其生物安全法律法规相对较为严格。
欧盟对生物安全的管理主要由欧洲委员会和各成员国共同负责。
转基因生物法规(Regulation on Genetically Modified Organisms):欧盟制定了严格的转基因生物法规,对进口、种植和销售转基因作物及其衍生产品进行严格监管。
该法规要求对转基因产品进行严格的安全评估和标识,并设立了专门的机构负责监管和审批。
生物安全法(Biosecurity Law):欧盟各成员国也分别制定了自己的生物安全法律法规。
这些法规主要关注生物恐怖主义、生物安全管理和动植物疫病防控等方面,保护农业、环境和公共健康安全。
3. 澳大利亚澳大利亚是生物安全管理方面经验丰富的国家之一,其生物安全法律法规以灵活性和科学性著称。
澳大利亚的生物安全管理主要由联邦政府和各州政府合作进行。
《实验室 生物安全通用要求》相关内容

能引起人或动物发病,但一般情况下对健康工作者、群体、家畜或环境不会引 起严重危害的病原体。实验室感染不导致严重疾病,具备有效治疗和预防措施, 并且传播风险有限。 3.3 危害等级Ⅲ(高个体危害,低群体危害) 能引起人类或动物严重疾病,或造成严重经济损失,但通常不能因偶然接触而 在个体间传播,或能使用抗生素、抗寄生虫药治疗的病原体。 3.4 危害等级Ⅳ(高个体危害,高群体危害) 能引起人类或动物非常严重的疾病,一般不能治愈,容易直接或间接或因偶然 接触在人与人,或动物与人,或人与动物,或动物与动物间传播的病原体。
第三章 实验室的设立与管理
第十八条 国家根据实验室对病原微生物的生物安全防护水平, 并依照实验室生物安全国家标准的规定,将实验室分为一级、 二级、三级、四级。
GB19489-2008 5.2 生物安全水平分级
根据所操作的生物因子的危害程度和采取的防护措施,将生物 安全防护水平(biosafety level,BSL)分为4级,1级防护水 平最低,4级防护水平最高。以BSL-1、BSL-2、BSL-3、 BSL-4表示实验室的相应生物安全防护水平;以ABSL-1、 ABSL-2、ABSL-3、ABSL-4表示动物实验室的相应生物安全 防护水平。
病原微生物和实验室管理的分类和分级(第4条)
病原微生物
分类管理
实验室
分级管理
病 高原 致微 病生 性物
一类 ………………………… 四级 二类 ………………………… 三级 三类(食品致病菌) ………… 二级 四类 ………………………… 一级
生
物 高安 级全 别实
验
转基因生物安全法

② 申请转基因植物种子、种畜禽、水产苗种生产许可证,首先要取得农业转基因生物安全证
书,并符合有关法律、行政法规规定的条件
基本制度及条例
3、经营许可证制度 1、经营转基因植物种子、种畜禽、水产苗种的单位和个人,应当取得农业部颁发的种子、种 畜禽、水产苗种经营许可证 2、申请经营许可证,除办当符合有关法律、行政法规规定的条件外,还应当符合《条例》规
01
实验安全法规
02
食品安全法规
第一阶段是隔离条件下的试验 第二阶段是开放环境下的栽培试验 从2001年4月起,转基因饲料的安全评价纳入现行的 《饲料安全保障与质量改进法》中执行
04
食品安全法规
1991年发布转基因食品安全评价指南(试 行),2001年4月正式实施
规定转基因产品只有通过环境安全评价和 食品安全评价,或者通过环境安全评价和 饲料安全评价,才能被允许在日本进行商 业化生产和从国外进口
01 02 03
04
。
其他国家法律法规
国外转基因生物安全综述
PART THREE
美国转基因生物安全 欧盟转基因生物安全 日本转基因生物安全
我国转基因安全立法时间一览
1993 年 12 月原国家科委颁布《基因工程安 全管理办法》
2004 年 5 月 24 日,国家质检总局 62 号令发 布并实施《进出口境转基因产品检验检疫 管理办法》
向口岸出入境检验检疫机构报检,经检疫合格后,方可向海关申请办理有关手续
农业转基因生物加工审批制度 1. 在中华人民共和国境内从事以具有活性的农业转基因生物为原料加工活动的单位和个 人,应当取得加工所在地省级人民政府农业行政主管部门颁发的《农业转基因生物加
工许可ቤተ መጻሕፍቲ ባይዱ》
国内外关于病原微生物危害等级的划分

国内外关于病原微生物危害等级的划分一、《病原微生物实验室生物安全管理条例》(国务院令第424号)第七条国家根据病原微生物的传染性、感染后对个体或者群体的危害程度,将病原微生物分为四类:第一类病原微生物,是指能够引起人类或者动物非常严重疾病的微生物,以及我国尚未发现或者已经宣布消灭的微生物。
第二类病原微生物,是指能够引起人类或者动物严重疾病,比较容易直接或者间接在人与人、动物与人、动物与动物间传播的微生物。
第三类病原微生物,是指能够引起人类或者动物疾病,但一般情况下对人、动物或者环境不构成严重危害,传播风险有限,实验室感染后很少引起严重疾病,并且具备有效治疗和预防措施的微生物。
第四类病原微生物,是指在通常情况下不会引起人类或者动物疾病的微生物。
第一类、第二类病原微生物统称为高致病性病原微生物。
二、《实验室生物安全通用要求》GB 19489—2004(2004-10-01实施)3 危害程度分级根据生物因子对个体和群体的危害程度将其分为4级3.1危害等级Ⅰ(低个体危害,低群体危害)不会导致健康工作者和动物致病的细菌、真菌、病毒和寄生虫等生物因子。
3.2 危害等级Ⅱ(中等个体危害,有限群体危害)能引起人或动物发病,但一般情况下对健康工作者、群体、家畜或环境不会引起严重危害的病原体。
实验室感染不导致严重疾病,具备有效治疗和预防措施,并且传播风险有限。
3.3 危害等级Ⅲ( 高个体危害,低群体危害)能引起人或动物严重疾病,或造成严重经济损失,但通常不能因偶然接触而在个体间传播,或能用抗生素抗寄生虫药治疗的病原体。
3.4 危害等级Ⅳ(高个体危害,高群体危害)能引起人或动物非常严重的疾病,一般不能治愈,容易直接、间接或因偶然接触在人与人,或动物与人,或人与动物,或动物与动物之间传播的病原体。
三、农业部《兽医实验室生物安全管理规范》5 微生物危害分级5.1. 微生物危害通常分为以下4级生物危害1级:对个体和群体危害程度低,已知的不能对健康成年人和动物致病的微生物。
实验室生物安全法律、法规及标准

一、中国实验室生物安全法律、法规及标准(6)
标准——《实验室 生物安全通用要求》GB19489-2004(续) 主要内容 1.范围 11.安全工作行为 2.术语和定义 12.化学品安全 3.危害程度分析 13.放射安全 4.生物危害评估 14.紫外线和激光光源(包括高强度光源的光线) 5.防护屏障和生物安全水平分级 15.电气设备 6.设施和设备要求 16.防火 7.动物实验室的生物安全 17.水灾和其他自然灾害 8.个人防护装备 18.紧急撤离 9.管理要求 19.样本的运送 10.良好内务行为 20.废弃物处置
开创性 标准
一、中国实验室生物安全法律、法规及标准(9)
认可文件(中国实验室国家认可委员会,CNAL) 准则——《实验室生物安全认可准则》CNAL/AC30:2005 (2005.1.1生效,中国实验室国家认可委员会) 是《实验室 生物安全通用要求》和《病原微生物实验 室生物安全管理条例》的“杂合体” 规则——《实验室生物安全认可程序规则》(试行) CNAL/AR13:2004 (2005.1.1生效,中国实验室国家认可委员会) 信息—— 《实验室生物安全认可申请书》 CNAL/AI08:2003 (2005.1.1生效,中国实验室国家认可委员会)
一、中国实验室生物安全法律、法规及标准(7)
标准——《生物安全实验室建筑技术规范》GB50346-2004 Architectural and technical code for biosafety laboratories (2004.8.3 建设部、国家质量监督检验检疫总局联合发布,2004.9.1实施) 共10章,3个附录。主要内容: 1.规定了生物安全实验室建筑平面布局、装修和结构的技术要求。 2.实验室的基本技术指标要求。 3.对空调、通风和净化部分规定了气流组织、系统构成及系统部件和材料的选 择方案,以及构造和设计要求。 4.规定了实验室的给排水、气体供应、配电、自动控制和消防设施配置的原则。 5.对施工、检测个验收的原则、方法等做出了必要的规定。
病原微生物实验室生物安全

病原微生物实验室标准和指南
《微生物和生物医学实验室生物安全通用准则》WS 233-2019 《生物安全实验室建筑技术规范》GB50346-2019 《人间传染的病原微生物名录〉卫生部 《可感染人类的高致病性病原微生物(毒)种或样本运输管理 规定》卫生部第45号令 《实验室生物安全通用要求》GB19489-2019
菌(毒)种和样本分类
A 类感染性物质 指在运输过程中当人或动物与之接触时,能导致永久性的
残疾构成生命威胁或致死疾病。 UN2814(使人染病或使人和动物都染病) UN2900(仅使动物染病)
B类感染性物质 未达到A级标准的感染性物质,其联合国编号为UN3373。 UN3291(医疗废物) UN3373(临床标本)
II级B1型生物安全柜
30%柜内循环,70%空气排出,工作窗口流速100fpm
II级B2型生物安全柜
100%空气排出,工作窗口流速100fpm
Ⅲ级生物安全柜
箱体完全封闭,将微生物与人完全隔离; 通过手套箱操作。
保护工作人员、产品和环境。 一般无定型产品,需特殊定货。 可将若干III级生物安全柜连在一起使用 全部生物流程的操作均在柜内完成,以确保安全。
适用于要求生物安全等级为(Ⅰ-Ⅲ)的工作
Ⅱ级生物安全柜的分类
Type A A1 A2
Class II
Type B B1 B2
Ⅱ级生物安全柜分类
II级A1型生物安全柜
70%柜内循环,30%空气排出,工作窗口流速75fpm
II级A2型生物安全柜
70%柜内循环,30%空气排至室外,工作窗口流速100fpm
各级生物安全柜之间的差别
生物安全柜 级别
正面气流速 度(m/s)
气流循环量(%) 室内循环 排出室外量
实验室生物安全法律法规

201307
2005/04/27
1
AMMS
概 述
实验室生物安全防护的发展
实验室生物安全法律法规和标准的发展
2005/04/27
2
AMMS
概 述
实验室生物安全防护技术的发展
二十世纪五十年代,美国在开展生物武器、
化学武器的实验室研究中,为了防止生物 因子(病原微生物)和化学毒剂泄漏逃逸 到实验室外环境,研制了一系列的防护装 备和设备,如高效粒子空气过滤器、生物 安全柜等;建设了BSL-3实验室和BSL -4实验室等。
概 述
遗传修饰微生物体的生物安全问题 重组DNA技术涉及到组合不同来源 的遗传信息,从而创造自然界以前可能从 未 存 在 过 的 遗 传 修 饰 生 物 体 (genetically modified microorganisms,GMMs)。最初,在分子 生物学家中有人担心这些生物体可能具有 不可预测的不良性状,一旦从实验室逸出 将带来生物学危害。这种担心在1975年美 国加利福尼亚州阿西洛马市召开的科学会 2005/04/27 11 议上成为焦点,会议上讨论了重组 DNA 技
2005/04/27 17
AMMS
《微生物和生物医学实验室生物安全手 册》(美国CDC/NIH,1999,第四版)
在 1983 年第一版的《微生物学和生物医学实验 室的生物安全》 (BMBL) 中,最早提出了把病原 微生物和实验室活动分为四级的概念。 1993 年 由CDC/NIH有关专家编写的《微生物和生物医学 实验室的生物安全》的第三版着重描述了微生 物实验室标准操作、实验室设计和安全设备的 不同组合,形成 1 ~ 4 级的实验室生物安全防护 等级,并依据微生物对人的危险程度分为四级 危险组,在实验室实际操作中加以应用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
盛年不重来,一日难再晨。
及时宜自勉,岁月不待人。
国外生物安全法律法规
转基因生物法
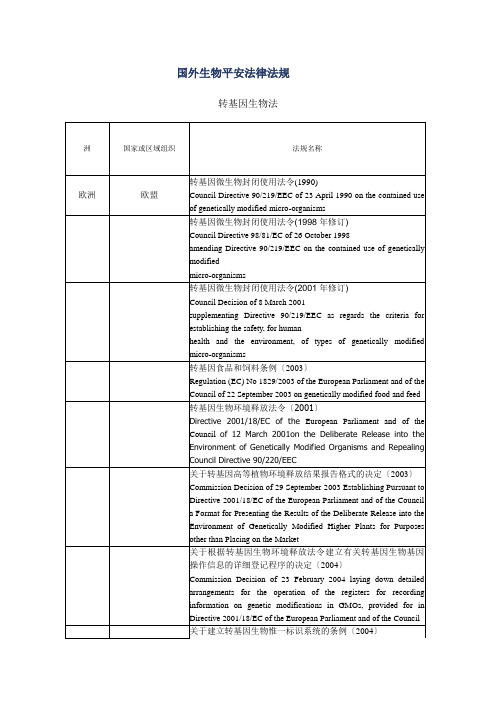
洲国家或区域组织法规名称
欧洲欧盟转基因微生物封闭使用法令(1990)
Council Directive 90/219/EEC of 23 April 1990 on the contained use of genetically modified micro-organisms
转基因微生物封闭使用法令(1998年修订)
Council Directive 98/81/EC of 26 October 1998
amending Directive 90/219/EEC on the contained use of genetically modified
micro-organisms
转基因微生物封闭使用法令(2001年修订)
Council Decision of 8 March 2001
supplementing Directive 90/219/EEC as regards the criteria for establishing the safety, for human
health and the environment, of types of genetically modified micro-organisms
转基因食品和饲料条例(2003)
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed 转基因生物环境释放法令(2001)
Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC
关于转基因高等植物环境释放结果报告格式的决定(2003)Commission Decision of 29 September 2003 Establishing Pursuant to Directive 2001/18/EC of the European Parliament and of the Council a Format for Presenting the Results of the Deliberate Release into the Environment of Genetically Modified Higher Plants for Purposes other than Placing on the Market
关于根据转基因生物环境释放法令建立有关转基因生物基因操作信息的详细登记程序的决定(2004)
Commission Decision of 23 February 2004 laying down detailed arrangements for the operation of the registers for recording information on genetic modifications in GMOs, provided for in Directive 2001/18/EC of the European Parliament and of the Council。
