洁净厂房部件关键性评估(双语版)
最全面的洁净室中英文词汇

最全面的洁净室中英文词汇序号中文English1 HEPA HighEfficiencyParticulateAir2 安全关注duecare3 安全门EmergencyDoor4 安全门Emergencyescapepanel5 安全眼镜Safetyglass6 搬运小车TransportCart7 办公室Office8 包衣间CoatingRoom9 包衣室CoatingRoom10 备料室MaterialsPreparingRoom11 壁板下侧回风口thelowwallreturns12 便装thestreetclothes13 标签管理LabellingManagment14 不良质量成本costofpoorquality15 不锈钢踏步梯StainlessSteelStepStoolsandStep16 材质MaterialsofConstruction17 层流LaminarFlow18 层流台LaminarFlowBenches19 层流罩Fan-filterUnits20 层流转运车LaminarFlowTransportCart21 拆除旧建筑demolition22 产品审核productaudit23 超额运费premiumfreight24 超过cGMP要求ExceedscGMPrequirements25 超声清洗机UltrasonicCleaners26 成套设备IntegratedSystems27 充氮的/氮气保护的Nitrogen-Purged28 充氮设备/系统NitrogenPurgeSystem29 除尘DustRemoving30 储柜suppliescabinet31 传递窗TransferringWindow32 传递窗/柜TransferHatches33 传递间TransferRoom34 传递间传递柜passthrus/Pass-Through35 传送带进/出口Conveyorentries/exits36 纯化水的循环管路distributionloop37 纯化水站PurifiedWaterProductionPlant38 从洁净室内更换RoomsidereplaceableHEPA39 存料间StorageRoomofRawMaterials40 存瓶室AmpulStorageRoom41 大屏幕LCD显示器Large-formatLCDdisplay42 弹簧门SwingingDoor43 灯检Individualinspection44 灯检室VisualInspectionRoom45 电磁阀solenoidvalve46 电动擦鞋机MotorizedShoeCleaner47 电动阀electromotorcontrolvalve48 电器插座WiringStuds49 电器接地端子GroundingTerminals50 吊顶suspendedceilings51 翻新/重新装修refurbishment52 反应计划reactionplan53 防火的Fire-Rated54 防火等级Fireclassification55 防火门Firerateddoor56 防火门Firedoor57 防火墙Firewalls58 房门开向(左开/右doormounting(leftorrightopening) 开)59 非洁净区Non-cleanroomareas60 非生产区域non-productionareas61 废弃物箱Wastereceptacle62 分筛间SievingRoom63 粉碎室PulverizingRoom64 粉针室LyophilizedSterilePowderRoom65 风管制作安装Ductworkandinsulation66 风淋airshower67 风淋室AirShower68 风淋通道AirTunnel69 风速airvelocity70 风速监测表airvelocitymonitor71 风速上下限high/lowairspeedthresholds72 服装apparel73 符合国家标准的traceabletonationalstandards74 负压洁净室negativepressurecleanroom75 干燥柜DesiccatorCabinets76 干燥器Desiccator77 钢化玻璃temperedglass78 高效过滤器HEPAFilters79 高压气瓶室CylinderStore80 更鞋ShoesChangingRoom81 更衣规则gowningprocedure82 更衣柜Storagelockers83 更衣镜Cleanroommirror84 更衣室Gowningroom85 更衣室ChangeRoom86 更衣室Changingroom87 工程批准的授权engineeringapprovedauthorization88 工具/工装tool/tooling89 工具车UtilityCarts90 工具储柜ToolStockers91 工具间FacilityRoom92 工具箱Toolscabinet93 工艺配管Pipeworkandinsulation94 工作台Worktables95 公认的检定机关accreditedtesthouses96 公用设施连接Utilitiesconnections97 功能验证functionalverification98 挂壁式Wall-Mount99 管道井CavityWalls100 灌封室FillingandSealingRoom101 滚筒式拖布Rollermops102 过程流程图processflowdiagramflowchart103 过程审核processaudit104 海绵Sponges105 海绵夹层抹布CoatedSpunlaceWiper106 横向职能方法cross-functionalapproach107 弧形墙脚RadiusFloorCoving108 互锁门Interlockingdoors109 护墙管架Wallguardrails110 滑动门SlidingDoor111 化学清洗剂chemicals112 环保型environmentallyfriendly113 缓冲间airlock114 缓冲间BufferRoom115 换气次数airchangerates116 回风格栅/回风口returnairgrille117 回风夹道returnairwalls118 回风墙returnairwalls119 火灾感应系统FireDetectionSystem120 机房ManchineRoom121 基准确定benchmarking122 计算机辅助工程computeraidedengineering(CAE)123 计算机辅助设计computeraideddesign(CAD)124 技术改造modificationofexistingfacilities. 125 技术改造/改建ExistingFacilityModification126 夹心彩钢板WallSandwichPanels127 架空地板raisedfloors128 洁净服Cleanroomapparel129 洁净服CleanroomGarments130 洁净室壁板Cleanroompartitions131 洁净室出入通道AccesstotheCleanroom132 洁净室地面清洗机CleanroomFloorMachine133 洁净室级别CleanroomClass134 洁净室用头罩CleanroomHood135 洁净室运行状况cleanroomperformance136 洁净室专用鞋dedicatedcleanroomshoes137 洁净头套柜Headweardispenser138 洁具/清洁用具cleaningproducts139 洁具室CleaningToolsRoom140 解决问题problemsolving141 进入洁净室EntertheCleanroom142 进入洁净室togettothecleanroomcalibratedtestinstruments143 经过校准的测试仪器144 经营计划businessplan145 纠正措施计划correctiveactionplan146 卷闸门roll-updoor147 可拆式检修们RemovableServicePanel148 可调式喷咀adjustablenozzles149 可弃式蹭鞋垫Disposablemats150 可行性feasibility151 可行走式天花板walk-onCeiling152 空气洁净级别aircleanliness153 空气粒子计数器airborneparticlecounter154 空气自净时间aircleanlinessrecoveryperiods 155 控制计划controlplan156 垃圾桶WasteReceptacleplasticliner157 垃圾桶里面的塑料内桶158 冷冻干燥机Lyophilizer159 冷冻水chilledwater160 冷库ColdRoom161 冷水机组chillerplant162 粒子异物Particulate163 凉片间CoolingTabletsRoom164 铝塑包装间PackingRoom165 铝型材门aluminum-framedoor166 密封剂Sealant167 密封门Gastightdoors168 密封门Airtightdoors169 灭菌间SterilizingRoom170 模具间ModulesRoom171 模具室DiesRoom172 抹布Wipers173 抹布柜Wiperdispenser174 末件比较lastoffpartcomparison175 内包装区Primaryprimarypackagingrooms 176 内包装室ImmediatePackageRoom177 内毒素超标excessivebacterialendotoxins 178 内衣undergarments179 男更洁净衣Malegarment180 男脱外衣洗手Malegarment181 浓配室ConcentratedSolutionRoom182 女更衣间Femalgarment183 女脱外衣间Femalgarment184 排风速率Exhaustairflowrates185 排风系统Exhaustsystems186 配浆间CoatingMixturePreparingRoom 187 配液间SolutionRoom188 喷壶SprayBottles189 喷塑铁皮epoxy-finishedsteel190 平面布置Layout191 平面布置图FloorPlan192 气体钢瓶搬运车BottleCart193 气闸室airlock194 气闸室Airlock195 器具洗涤间ToolWashingRoom196 器具箱suppliescabinet197 前室FrontStation198 墙/墙交界处的圆角 Roundedsnap-incove199 墙的贴面LinerWall200 墙或屋顶的开洞openings201 清除浮尘toremoveloosecontaminants 202 清外包室OuterPackageRemovingRoom203 清洗池Wetbenches204 清洗池wetcleaningstation205 清洗设备cleaningproducts206 清洗室CleaningRoom207 清洗台WetBench208 全尺寸检验layoutinspection209 热风干手器HandDryer210 认可的实验室accreditedlaboratory211 入口thefrontentrance212 软胶室SoftCapsulesRoom213 设计纪录designrecord214 设计责任供方design-responsiblesuppliers 215 生产区扩建ManufacturingAreaExpansion 216 实验设计designofexperiment(DOE)217 实验室laboratory218 实验室范围laboratoryscope219 试剂储柜ChemicalSafetyCabinet220 室外穿的衣物Outdoorclothing221 手套Gloves222 手提式Hand-heldmodelHandsDisinfectionRoom223 手消室224 台式benchtopmodelCeilingGrid225 天花板骨架powerfailure226 停电,断电civilconstruction227 土建工程palletwrappers228 托盘包装机229 拖布Mops230 拖布Mophandles把手Squeegees231 拖布挤水器232 拖布Mopwringers涮洗桶Removeoutergarments233 脱去外衣secondarypackagingarea234 外包装区OuterPackingRoom235 外包装室remotelocation236 外部场所Doorswingisoutward(awayfromroom)237 外开门238 违反theviolationofcleanroomgarments!洁净更衣规程239 卫生洁具间CleaningToolsRoom240 温湿度监测temperatureandhumiditymonitoring241 无菌操作asepticmanipulations242 无菌洁净室AsepticCleanrooms243 无菌洁净室Sterilecleanroom244 无菌培养基传送和灌装挑战试验sterilemicrobialculturemediumtransferandfillchallenges245 无菌区AsepticArea246 无沿平滑玻璃窗flushledge-freewindows247 吸尘器VacuumCleaner248 吸尘器vacuums249 稀配室DilutedSolutionRoom250 洗瓶室AmpulCleaningRoom251 洗手台Handwashingstation252 洗眼器eyewash253 系统配套systemintegration254 下降DescendTube管firstinfirstout(FIFO)255 先进先出256 现场field257 现场site258 现场fieldcalibrate调校sitemanager259 现场经理projectmanagement260 项目管理261 项目theprojectteam组262 鞋套CleanroomBootRestingRoom263 休息室264 袖套SleevecoversDifferentialpressuregauge 265 压差计266 压降LowPressureDrop小,低压降TablettingRoom267 压片间TabletsRoom/CompressionRoom 268 压片室medicationpackaging269 药品包装270 一次DisposableCleanroomGarments 性洁净服FirstChangeRoom271 一更室Garmenthamper272 衣架/衣钩GarmentType273 衣物形式274 移动FreestandingGarmentRack 式衣架275 易燃FlammableMaterialsCart 品转运车SafetyShower276 应急冲淋器contingencyplan277 应急计划278 荧光灯Fluorescentlighting279 硬胶室HardCapsulesFillingRoom280 用壁板制作的天花板PanelizedCeiling281 预防性维护preventivemaintenance282 预过滤器Pre-Filter283 预见性维护predictivemaintenance284 原水rawwater285 圆管cylindertube286 运行温度Operatingtemperature287 运行业绩operationalperformance288 脏衣收集桶thedirtyclothescan289 粘附性蹭鞋垫StickyMat290 蒸馏水室WaterPurifyingRoom291 蒸汽清洗机SteamCleaner292 执行职责executiveresponsibility293 制粒干燥室GranulatingandDryingRoom294 制粒间Granulatingroom295 质检室QualityControlRoom296 质量功能展开qualityfunctiondeployment(QFD) 297 质量手册qualitymanual298 中间检验Inter-inspection299 中间站IntermediateStation300 中间站IntermediateStorageLoadingTurntable301 转盘(传送带上用的)302 装配性设计designforassembly(DFA)303 装箱机casepacker304 自动门AutomaticDoor305 自动装盒机AutomaticCartoner306 自净Recovery307 总混间BlendingRoom308 走廊Corridor309 作业指导书jobinstruction。
制药验证文件-洁净压缩空气GMP评估(英文版)

GMP and Functional Risk Assessment for Compressed AirGMP and Functional Risk Assessment for Compressed Air Page 1 of 15DOCUMENT HISTORYThis risk assessment serves as GMP and Functional Risk Assessment for Systems, as well as traceability matrix between requirement,potential risk, and measure to control risk (e.g. specific test specification, additional requirement to mitigate risk, SOP).Risk Assessment ProcedureStep 0 – Approval Cover Page and Document History▪The section “Risk Assessment Work Session Attendance” will be used to record work session number, list of attendees, and the work session date.▪After completion of the Risk Assessment (before executing the specific measures) the Risk Assessment will be initially approved (section “Assessment”).▪In case that the approved Risk Assessment will be revised, the initial approval of the Risk Assessment is obsolete and the revised Risk Assessment requires a new approval. The Revision Number must be adapted accordingly.▪If - all defined measures and tests are completed and- in all rows the cell “Measures completed” is assessed “Yes”then the Risk Assessment is finally approved in the section “Final Assessment Approval”.Step 1 – Description of the current process / work flowGMP and Functional Risk Assessment for Compressed Air Page 2 of 15Fill out columns “Source Document”, “Function” and “Reverse Function”▪Source Documents typically encompass the System URS, block diagram, and/or functional description, but related documents must also be considered. For example, if the 21 CFR Part 11 assessment demonstrates that the system must comply with Part 11, a row must be added in thecorresponding GMP/FRA to ensure traceability to this GMP critical requirement. Other System level “umbrella” documents, egg AutomationURS and/or Electrical URS that contain Critical GMP requirements that apply to many systems, must either have their own GMP/FRAassessments, or the GMP critical requirements traced in each System level GMP/FRA.Step 2 –Risk Assessment [column “Risk Assessment”]Probability Ratingo Low (L): Due to the design failures may not occur. The design is well known and often used.o High (H): The design is new and not well known. There is a high probability that failures occur.Impact Rating▪Which impact has the described failure, independently of the probability rating?o Low (L): No impact to the product quality.o High (H): Impact to productProbability of Detection▪Which probability of failure detection is given? Is it possible to detect the failure immediately or latest during Quality Control/batch release?o High (H): The failure will be detected immediately.o Low (L): The failure can not be detected immediately. The failure may not be detected during Quality Control tests.GMP criticality▪Assess the risk in regard to product quality and patient safety. Note: GMP criticality is not the same as GMP-relevance. GMP relevance will not be changed by application of specific measures and control. GMP criticality is reduced by proper design and control of the process. Forexample, sterilization process failures (e.g. temperature not reached during sterilization) have high GMP criticality, but proper design of thesterilizer and control of the process with sensors reduces GMP criticality. However, although GMP criticality has been reduced, the GMPrelevance of a compliant sterilization cycle remains unchanged.o High (H): There is an impact on product quality and/or patient safety and/or the impact is not under control / not acceptable.o Low (L): There is insignificant impact on product quality and/or patient safety and/or the impact is under control / acceptable.▪Record the unique URS ID number in the Reference Document column for all GMP crit ical requirements (marked “H”).▪For each GMP critical requirement, insert the specific URS requirement in the Function column.GMP and Functional Risk Assessment for Compressed Air Page 3 of 15Step 3 – Definition of specific measures to mitigate risks (columns Measures, Action)The main measures are, but not limited to:▪Definition of additional design requirements (e.g. in URS, FS) to mitigate risk▪Definition and execution of additional, specific IQ, OQ and PQ tests▪Generation of specific Standard Operating Procedures (SOPs)▪Specific operational procedures▪Additional sensors for process monitoring and control▪Calibration of quality relevant/critical sensors▪Cleaning validation▪Safety measures and arrangementsDefine and document the responsible person and the due date for the specific measure.Step 4 – Risk Assessme nt [column “Risk Assessment considering defined measures”]Assessment in view of the successful execution of all defined measures to mitigate risks.Apply the same rating as in Step2:Probability RatingThe probability of occurrence if all defined measures are implementedImpact RatingThe impact of an event usually remains the same independently of the defined measures.Probability of DetectionThe probability of detection of an event if all defined measures are implementedGMP-criticalityThe GMP-criticality must be low, otherwise additional measures must be defined.Step 5 – Execution of specific measuresGMP and Functional Risk Assessment for Compressed Air Page 4 of 15The execution and documentation of specific measures are traced and controlled via test specifications, list of critical sensors, preventive maintenanceplans, and/or open item lists.Document titles and revision should be documented in column “Test Documentation / Traceability / Reference” under the corres ponding point (DQ, IQ,…). In case of to many documents or title of document to long it is possible to include a reference (e.g. a reference to a Test Specification overview)in the column.The execution completion should be documented with initials and date in the column “Action / Responsible Due Date”.Step 6 – Measures completedAfter documented execution of all specific measures and tests to mitigate risks, the completion and closure is checked and confirmed in the last cellof each row.Step 7 – Risk Assessment CompletionAfter all measures of the entire analyses are completed (step 6 for all rows) the Risk Assessment will be finally approved on the cover page (section“Final Assessment Completion”).GMP and Functional Risk Assessment for Compressed Air Page 5 of 15GMP and Functional Risk Assessment for Compressed Air Page 6 of 15GMP and Functional Risk Assessment for Compressed Air Page 7 of 15GMP and Functional Risk Assessment for Compressed Air Page 8 of 15GMP and Functional Risk Assessment for Compressed Air Page 9 of 15GMP and Functional Risk Assessment for Compressed Air Page 10 of 15GMP and Functional Risk Assessment for Compressed Air Page 11 of 15GMP and Functional Risk Assessment for Compressed Air Page 12 of 15GMP and Functional Risk Assessment for Compressed Air Page 13 of 15GMP and Functional Risk Assessment for Compressed Air Page 14 of 15GMP and Functional Risk Assessment for Compressed Air Page 15 of 15。
无菌生产厂房风险评估

关于无菌车间厂房的微生物风险评估Microbiological Risk Assessment ReportforSterile Plant目录1. 目的 (3)2. 职责 (3)3. 参考文献 (3)4. 评估标准 (3)5. 评估方法 (4)6. 监测方案的设计 (16)6.1 A级区 (16)6.2 B级背景下的A级送风区域 (17)6.3 B级区 (18)6.4 C级背景下的A级送风区域 (18)6.5 C级区 (19)6.6 E级区 (20)7. 附录 (21)1. 目的根据中国GMP2010版要求,现有无菌车间对19(B级区) ,20(B级区) ,23(C级区)号房间的空调系统进行了改造。
由于此次改造涉及B/C级区的空调系统,因此必须对改造可能引起的潜在风险进行相应的分析评估,以确认恢复生产以后产品质量水平仍然可以得到有效保证。
同时根据中国新版GMP的要求需要对针剂生产所有区域进行风险评估,为环境验证方案的设计和环境监控流程的设置提供依据。
2. 职责微生物实验室负责评估报告的撰写,QA和项目小组负责批准本报告。
3. 参考文献中国GMP(现行版)ISO146444. 评估标准根据现有改造后厂房设计图纸,无菌车间包括A,B级区背景下的A级送风,B,C,C级区背景下的A级送风,E六个级别的洁区。
在这些洁区进行的所有活动必须符下文将根据区域或房间不同的设置和功能,具体分析每个区域或房间的风险来源,并制定相对应的监测措施。
5. 评估方法本次风险评估工具将采用ICHQ9中FEMA的标准方法严重性:过程中对产品质量或法规的影响有多大?严重性打分从1~5分发生的可能性:过程中这些故障发生的机率多大?可能性打分从1~5分发现的可能性:如果故障发生了,有多大的机率可以被发现?打分从1~5分Risk RPN:严重性,可能性以及可发现性的乘积,记录结果Note:结论:按照风险评估的打分对所有区域或房间进行风险分析,并且根据GMP的要求设计环境监控方案,在B/C/E级区,一些不需要进行微生物监控的房间,根据分数的高低选择不同的监控频率。
无尘车间等级划分标准 英文

无尘车间等级划分标准英文全文共四篇示例,供读者参考第一篇示例:In addition to ISO standards, there are also other classification systems used in different regions or industries. For example, the United States Federal Standard 209E (FS 209E) was widely used in the semiconductor industry before the adoption of ISO standards. FS 209E classified cleanrooms from Class 1 to Class 100, with Class 1 being the cleanest and Class 100 being the least clean.第二篇示例:Clean room classification standards are used to differentiate clean rooms based on their level of cleanliness and particle control. These standards are crucial for ensuring that the clean room environment meets the specific requirements of different industries, such as semiconductor manufacturing, pharmaceutical production, biotechnology research, and aerospace engineering.第三篇示例:Cleanroom classification standardFor example, an ISO 1 cleanroom has a maximum allowable particle count of 10 particles per cubic foot for particles larger than 0.1 microns, while an ISO 9 cleanroom has a maximum allowable particle count of 35,000 particles per cubic foot for particles larger than 0.5 microns. In addition to particle count, cleanrooms are also classified based on the sources of contamination, such as people, equipment, and materials.第四篇示例:Apart from the ISO standard, the United States Federal Standard 209E was also commonly used to classify cleanrooms. However, in 2001, it was replaced by the ISO standard due to its outdated and ambiguous classification system. The Federal Standard 209E classified cleanrooms into classes ranging from Class 1 (the cleanest) to Class 100,000 (the least clean).。
CCA厂房设施部件关键性评价报告
厂房设施部件关键性评估报告系统编号:XXX目录1.介绍 (3)2.目的 (3)3.Scope 范围 (3)4.职责 (3)4.1奥星职责 (3)4.2XXX 职责 (3)4.3签名人职责 (4)4.3.1评估团队 (4)4.3.2审核和批准 (4)5.缩略语 (5)6.法规和指南 (6)6.1法规 (6)6.2指南 (6)7.参考文件 (7)8.系统/设备描述 (8)9.部件关键性评估方法 (9)9.1部件关键性的确认 (9)9.2关键性部件风险评估 (9)10.部件关键性评估 (12)10.1部件关键性矩阵 (12)10.2关键性部件风险评估矩阵 (14)10.3关键性部件风险控制矩阵 (16)11.结论 (17)1.介绍xxx公司(以下简称“XXX”)拥有多个独立的生产车间,本次进行部件关键性评估(CCA)的为XXX车间,将在此区域中主要进行XXX等产品的生产。
按照系统影响性评估(SIA)的结果,对评估为直接影响的系统进行部件关键性评估。
该厂房设施系统根据系统影响性评估的方法确定为直接影响系统,系统编号:XXX,见xxx车间系统影响性评估报告(表)SIAR-XXX-00O2.目的对XXX公司XXX车间厂房设施系统进行单独的部件关键性评估并记录在如下文件中。
本部件关键性评估是评估直接影响系统中各部件的关键程度。
对判定为关键性的部件进行风险评估用于确定出所有的潜在危险及其对产品的影响。
3. Scope 范围本部件关键性评估的范围为XXX公司XXX车间XXX楼层XXX房间(房间编号:)厂房设施,系统编号:XXX O4.职责4.1奥星职责✓收集编写报告需要的信息✓编写和审核报告4.2XXX职责✓负责批准本报告及版本控制✓提供为报告编写所需要的所有的规程、数据、手册、图纸和文件✓参与部件关键性评估✓协助奥星完成部件关键性评估报告的编写✓报告的审核和批准4.3签名人职责4.3.1评估团队✓在评估之前首先应组成评估小组,评估小组成员将在各自负责的部件关键性评估报告中进行签字,可包括以下成员:✓系统使用部门设备工程师、工艺工程师、自控工程师✓项目人员✓工程人员✓验证人员✓量保证人员4.3.2审核和批准使用部门负责人、质量保证(QA)人员负责审核部件关键性评估的结果,QA负责人负责批准部件关键性评估的结果。
洁净厂房与HVAC系统验证技术

投标前交流内容药品类型资料收集验证项目范围企业应当确定需要进行的确认或验证工作,以证明有关操作的关键要素能够得到有效控制。
确认或验证的范围和程度应当经过风险评估来确定。
SIA评估(系统影响性评估)CCA评估(部件关键性评估)将洁净厂房和空调净化系统的部件/功能分为关键和非关键两种。
1、 关键系统将洁净厂房和空调净化系统的部件/功能分为关键和非关键两种。
确定部件时应充分考虑PID 信息和部件清单,有部件编号的部件都应该评估。
空调系统的主要部件包括:风管、新风阀、送风阀、回风阀、排风阀、静压箱、高效过滤器、回风口等。
确定功能是应充分考虑有机功能的实现。
空调净化系统的功能如下:风量/换气次数、压差、气流流型、自净时间。
空调净化系统中的关键部件/功能有:高效过滤器、风量/换气次数、压差、气流流型、自净时间、洁净度等。
验证实施方式验证流程验证文件跟业主的沟通内容验证范围/验证人员及职责/验证方法/验收的标准/文件格式/程序文件(变更与偏差处理)/验证进度计划/验证周期/售后服务等中标后,正式实施前准备工作例:文件模板(跟业主确认)文件模板的选用/文档编号/文件管理规范/公司标识LOGO是否体现/是否双语/文件清单将验证过程中产生的表格或涉及到的文件列入清单,见附件一。
URS确保URS符合规范与设计要求,URS要经过相关人员的审核审批。
设计院设计说明、图纸设计院图纸与设计说明要详细且明确,要满足URS及GMP规范内容,如有偏差或变更要有相关处理程序。
二次深化设计说明、图纸、相关组件清单施工单位二次深化设计文件设计确认(DQ)1、文件确认对现有的设计文件和图纸进行逐个确认,记录文件的标题、文件编号、发布日期、版本和相关的批准状态。
URS、设计说明、图纸、组件清单、招标文件中的技术章节、投标文件中的技术章节、设计院详细设计文件、施工单位二次深化设计文件、施工单位出具的施工说明文件、计划采用的各组件说明资料、会议记录、技术交流记录、邮件等。
FMEA严重性评估参考标准(中英文)

修理时间在半小时至一小时间 A portion (less than 100%) of the product have to scrapped with no sorting, repair
time less than one hour 部分产品需报废,不需分检,
level of performance 产品可使用且使用不便、表现不良 Item normal performance through being repaired. Defect noticed by most customers
(greater than 75%)
*
产品经过修理表现正常 / 大部分客户发现缺陷 (超过 75%)
regulation without warning 缺陷模式在无警告的情况下影响产品的安全操
作、给用户造成操作不便及可靠性问题 / 不符合政府条文,严重性非常高
Failure mode affects safe vehicle operation and cause some functional problem at the end user / or involves noncompliance with government regulation with warning
Suggested FMEA Detection Evaluation Criteria FMEA 检测性评估参考标准
Detection 检测性
Criteria
;
标准
Inspection types
检测种类
Errorproofed
防错
Gauging 量具
洁净度等级要求 英文

洁净度等级要求英文
(实用版)
目录
1.洁净度等级要求的定义和重要性
2.洁净度等级要求的分类和标准
3.洁净度等级要求的应用领域
4.洁净度等级要求的英文表达
正文
在许多行业中,洁净度等级要求对于保证产品质量和工艺过程的稳定性至关重要。
洁净度等级要求是指对环境中的微粒、细菌、温度、湿度等因素的严格控制,以满足特定生产和科研需求的一种规定。
洁净度等级要求通常分为不同的级别,这些级别由一系列标准和规范来界定。
在我国,洁净度等级主要按照 GB/T 14295-2008《洁净室及洁净区设计规范》进行分类。
常见的洁净度等级包括 ISO 1 至 ISO 9,其中 ISO 1 代表最高洁净度等级,ISO 9 则表示较低的洁净度等级。
洁净度等级要求广泛应用于微电子、生物医药、食品饮料、精密仪器等产业领域。
在这些领域中,对洁净度的要求可以有效降低产品缺陷率、提高生产效率和保障产品质量。
在英文中,洁净度等级要求通常表达为“cleanliness class requirements”或“cleanroom class requirements”。
英文中对于洁净度的分级标准与中文类似,也是通过一系列的规范和标准来表述。
例如,美国联邦标准 209E(FS 209E)也对洁净度等级进行了详细的划分。
总之,洁净度等级要求对于保证产品质量和工艺过程的稳定性具有重要意义。
第1页共1页。
ISO 14644-1:2015 洁净室及相关控制环境国际标准_中英对照

14644-1:2015(E)北京齐力佳提供禁止未经授权使用、复印、放上网页分享ISO 14644-1Cleanrooms and associated controlled environments洁净室和相关的受控环境Part 1:第一部分:Classification of air cleanliness by particle concentration 通过粒子浓度对空气洁净度进行分级第二版本2015-12-15Contents 目录Foreword 前言ISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out through ISO technical committees. Each member body interested in a subject for which a technical committee has been established has the right to be represented on that committee. International organizations, governmental and non-governmental, in liaison with ISO, also take part in the work. ISO collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization.ISO(国际标准化组织)为全球各国标准化团体的联合会(ISO 会员团体)。
某公司已过新版GMP认证洁净厂房验证模版(中英文)汇总

Installation Qualification Protocol洁净室安装确认方案System No. 系统编号: CLR-01Index 目录1.PURPOSE目的 (3)2.SCOPE范围 (3)3.RESPONSIBILITY职责 (3)4.REGULATION AND GUIDANCE 法规和指南 (3)5.ABBREVIATIONS缩略语 (3)6.SYSTEM DESCRIPTION 系统描述 (3)7.GOOD DOCUMENTATION PRACTICE文件管理规范 (5)8.TEST LIST测试列表 (6)9.PERSONNEL IDENTIFICATION人员确认 (6)10.PROCEDURE过程 (7)10.1先决条件 (7)10.2文件确认 (10)10.3图确认 (11)10.4房间组件检查 (13)10.5仪器仪表校验 (15)10.6洁净室建造装修检查 (17)10.7电器安装检查 (22)11. DEVIATION REPORT偏差报告 (28)1. Purpose目的本安装确认方案的目的是测试、检查和洁净室是按照相应设计要求和供应商的建议进行安装的。
安装确认的测试和检查的结果将按照该验证方案进行记录。
安装确认将确定直接影响系统的关键部件被正确地安装,并符合设计文件需求;确定支持文件、质量文件在现场。
测试和检查的结果将按照该验证方案进行记录。
2. Scope范围本方案确定了***********公司口服固体项目车间的洁净室(位号:***********)的安装确认。
3. Responsibility职责4. Regulation and Guidance 法规和指南(SFDA) GMP 2010版中国药典2010版现行版ISPE指南5“调试和确认”洁净厂房设计规范GB13554-925. Abbreviations缩略语6. System Description 系统描述口服固体制剂的洁净室包括以下级别D级区对产品无影响的房间无需验证。
洁净厂房的风险评估【范本模板】
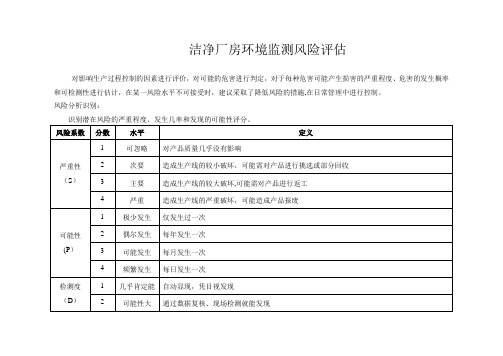
洁净厂房环境监测风险评估
对影响生产过程控制的因素进行评价,对可能的危害进行判定,对于每种危害可能产生损害的严重程度、危害的发生概率和可检测性进行估计,在某一风险水平不可接受时,建议采取了降低风险的措施,在日常管理中进行控制。
风险分析识别:
识别潜在风险的严重程度、发生几率和发现的可能性评分。
通过比对产品工艺规程、产品质量回顾与总结历史经验、查找资料、学习新版GMP及实施指南等方法,对环境监测中可能出现的质量风险进行了调查与分析。
现将查找洁净厂房环境监测并确认可能存在的质量风险与可能形成的危害统计如下,并进行系统风险评估进行评分。
评分人:
评分日期:年月日。
部件关键性评估CCA-Report

Component Criticality Assessment Report forXXX SystemXXX系统部件关键性评估报告Approval for Report 报告批准Index 目录1.Purpose 目的 (3)2.Scope 范围 (3)3.Responsibility 职责 (3)4.Regulation and Guidance 法规和指南 (4)5.Abbreviations缩略语 (5)6.Description 系统描述 (5)7.Reference Documents 参考文件 (6)8.Method of Component Criticality Assessment部件关键性评估方法 (7)8.1 Identification of Component Criticality部件关键性的确认 (7)8.2 Critical Component Risk Assessment关键性部件风险评估 (8)9.Performance of Component Criticality Assessment 部件关键性评估执行 (11)9.1 Identification of Component Criticality部件关键性确认 (11)9.2 Critical Component Risk Assessment关键性部件风险评估 (12)10.Conclusion结论 (13)Appendix 1 The summary of planned control actions for the medium and high risks 附录1 中、高级风险控制措施汇总表 (14)1. Purpose 目的An individual component criticality assessment will be performed for the XXX system in the XXX Plant of XXX and documented on the following pages. This component criticality assessment is used to assess the criticality of each of the components of the direct impact systems. Risk assessment will be performed for a component assessed to be critical to identify all the potential risks and their impacts on products. This activity can effectively decrease the scope of qualification activities. Critical components will be subject to commissioning and qualification and non-critical components will be subject to commissioning only. It also defines the qualification activities for the validation/qualification process and the recommended actions during the operation process.对XXX公司XXX车间XXX系统进行单独的部件关键性评估并记录在如下文件中。
Evaluation of clean room中文版

洁净室和其它可控环境的评估这一章的目的是讨论各种主题,内容涉及大批量药品的无菌加工、剂量形式、案例、医用设备以及可控环境质量的建立、维护和控制。
本章讨论的内容包括:(1)基于微粒数限度的洁净室分级;(2)可控环境的评估程序;(3)人员培训;(4)评估程序设计和执行的关键因素;(5)取样计划的改进;(6)警戒限和行动限的建立;(7)取样的方法和仪器;(8)培养基和稀释剂;(9)微生物分离物的鉴定;(10)经由培养基灌装的操作评估;(11)术语。
排除在本章以外的是一个关于注册药品可控环境的讨论,适用于国内灭菌产品的制备。
这部分内容包括在《药物研制—无菌制备》<797>。
无菌工艺可控环境状态的评估和控制有多种方法可供选择。
包含在本章内的数值并不代表绝对值和规定,只是指导性的。
给出几种取样设备和方法,其中之一不能适度的表明保证微生物控制所需水平的数值的达到或者偏移本章所示数值之外,即为失控。
取样和分析的不适当应用可能导致重大的可变性和易忽视污染的潜在。
出现在本章中的取样用培养基,装置以及方法并不是规定性的,只是作为指导。
一大部分无菌产品通过无菌工艺生产。
由于无菌工艺依赖于工艺流程中微生物的排除和灌装期间微生物进入敞开容器的防止,因此产品的生物负荷和生产环境的微生物负荷一样,是和这些产品的无菌保证水平相关的重要因素。
洁净室级别的建立洁净室的设计和结构以及环境控制包含在联邦标准209E。
空气洁净程度根据空气中绝对微粒浓度来定义。
用于划分可控环境洁净级别和空气微粒监测的方法也包含在其中。
本联邦文件只适用于可控环境的空气微粒,并不打算描述活性和非活性微粒的本质。
联邦标准209E关于制药工业中洁净室和其它可控环境的应用已被制洁净室造商采纳,用来提供厂房、试车和设施维护规范。
然而制药工业中可用的数据并不能提供一个非活性微粒数和可存活微生物浓度之间的关系的科学结论。
电子工业中非活性微粒数的危险程度使得联邦标准209E的应用非常必要。
洁净车间改造评估报告范本

洁净车间改造评估报告范本1. 引言洁净车间是用于生产和处理对环境要求较高的产品的关键生产区域之一。
随着时间的推移,洁净车间的功能和效益会发生变化,可能会面临一些挑战和问题。
本报告旨在评估现有洁净车间的情况,分析存在的问题,并对潜在的改造方案进行评估,以提高洁净车间的效能和安全性。
2. 现状评估2.1 设备状况洁净车间内的设备应保持正常运行,以确保产品的质量和安全。
经过详细的设备检查,我们发现存在以下问题:- 设备老化严重,部分设备已经超过其设计寿命。
- 部分设备存在性能问题,导致生产效率下降。
- 设备维护和保养不到位,导致频繁出现故障和停工。
2.2 空气质量洁净车间的空气质量对产品的生产和员工的健康至关重要。
经过测量和采样分析,我们发现存在以下问题:- 气体和颗粒物浓度超出标准限值,对产品质量产生潜在危害。
- 通风系统存在漏风和堵塞问题,无法有效排除污染物。
- 洁净车间内空气湿度控制不稳定,可能影响产品的质量。
2.3 清洁度管理洁净车间的清洁度管理对产品和员工的安全至关重要。
经过细致观察和检查,我们发现存在以下问题:- 车间内存在大量的灰尘和污垢,清洁度不达标。
- 清洁工具和设备维护不当,无法有效清理和消毒。
3. 改造方案评估基于对现有洁净车间的评估结果,我们提出以下改造方案,以提高洁净车间的效能和安全性:3.1 设备更新和维护- 替换老化和性能不佳的设备,以提高生产效率和产品质量。
- 建立定期维护计划,包括设备清洁、润滑和部件更换,以降低故障风险。
3.2 空气质量控制- 完善通风系统,修复漏风和堵塞问题,确保空气流通和污染物的有效排出。
- 定期测量和监测空气中的气体和颗粒物浓度,确保空气质量符合标准要求。
- 调整湿度控制系统,以维持恰当的湿度水平。
3.3 清洁度管理- 制定清洁计划,并培训员工正确使用清洁工具和设备。
- 定期进行车间清洁和消毒,确保洁净车间符合标准要求。
4. 结论本评估报告对现有洁净车间的情况进行了全面的评估,并提出了相应的改造方案。
ISO14644-2:2015洁净室及相关控制环境国际标准_中英文对照

ISO14644-2:2015洁净室及相关控制环境国际标准_中英文对照洁净室及相关控制环境国际标准—第2部分用粒子浓度监测提供与空气洁净度相关的洁净室性能的证据特别感谢北京齐力佳翻译团队群友的大力支持:@布衣陈郎@成云@木头@谢虹@CeCi如有不足,敬请原谅!-------------------------------------------------------------------以下为全部内容。
目录前言简介1 适用范围2 引用标准3 术语和定义4 建立、实施和维持定期监测计划1) 原则2) 风险评估3) 定期监测4) 校验5) 检查批准6) 全周期偏差处理5 以粒子浓度划分的空气洁净度附件A 制定监测计划要点附件B 制定警戒线和行动线注意事项参考文献Foreword 前言ISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out through ISO technical committees. Each member body interested in a subject for which a technical committee has been established has the right to be represented on that committee. International organizations, governmental and non-governmental, in liaison with ISO, also take part in the work. ISO collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization.ISO(International Organization for Stanclardization)为全球各国标准化团体(ISO会员团体)的联合会,其国际标准工作的开展一般是由ISO各技术委员会进行每个会员团体如对技术委员会的某一课题感兴趣,均有权成为此技术委员会的代表。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
洁净厂房306-2区域部件关键性评估Component Criticality Assessment for 306-2XXX建设有限公司XXX药业有限责任公司修订记录目录Content1 背景Background (4)2 目的Purpose (4)3 范围Scope (5)4 职责Responsibility (6)5 参考文件Reference Documents (7)5.1法规Regulations (7)5.2指南Guidelines (7)6 定义与缩写Definitions and abbreviations (8)7 系统描述System description (6)8 部件关键性评估方法Method of Component criticality assessment (8)8.1部件关键性的确认Component criticality qualification (8)8.2评估成员Asessment members (10)9 部件关键性矩阵Component critical matrix (12)10 结论Conclusion (15)1背景BackgroundConstruction contractor is China Electronics System Engineering No.2 Construction Co., Ltd所有验证活动执行标准为中国GMP、欧盟EU-GM和相关ISO标准。
All the validation actives are based on Chinese GMP & EU-GMP and relevant ISO standards.所有工程活动执行中国相关国家标准和ISO标准(包含设计、施工、验收、检测)。
All the engineering actives are based on relevance Chinese national and ISO standards (include Design\ Construction \ Acceptance\ Test).2目的Purpose部件关键性评估是评估直接影响系统中各部件的关键程度。
对判定为关键性的部件进行风险评估用于确定出所有的潜在危险及其对产品的影响。
Component criticality assessment is to assess of the criticality of components in direct impact system. Risk assessment of identified critical components is used to identify all potential hazards and their impact on the product.本项工作能够有效缩小工作范围,从而对关键性部件进行调试和确认,对非关键性部件仅需进行调试。
它还规定了验证/确认过程中所需进行的活动、操作过程中进行的建议措施等。
This work can effectively reduce the scope of work, so that the critical components need commissioning and qualification, and non-critical components only need commissioning. It also specifies the activities required during the validation / qualification process, the recommended actions taken during the operation, and so on.3范围Scope本文件规定了对确认306动物房二层306-2区域部件关键性评估的方法和程序。
This document sets out the methods and procedures for the identification of critical assessments of components in section 306-2, floor 2 of the 306 Animal room.项目范围内:Project scope:●洁净室墙板、吊顶、门、窗、地面、照明灯具、紫外灯、地漏、水池、防虫鼠、开关插座、门锁、闭门器、小厨宝、烘手器的安装。
●Clean room wallboard, ceiling, door, window, floor, lighting, UV lamp, Floor drain, sink, pestcontrol rat ,switch socket, door lock, door closers, small kitchen, hand dryer installation.●项目范围外:Outside the scope of the project:●应急逃生灯的安装;●Installation of emergency escape lamps;●门禁系统;●Access control system;●洁净电话●Clean telephone●灭蝇灯●Fly-killer lamps●互锁控制●Interlock control4系统描述System description306动物房共分2个区域,一层为CNC区域,二层为洁净区域,洁净等级为D级(7级ISO标准),其中洁净区域设施包括:洁净室壁板、顶板、洁净门、窗、地面、水池、地漏、开关、插座、照明灯具、紫外灯具、洁净电话、电磁锁等,二层房间信息见下表:The animal room is divided into two sections, The first floor is CNC area, and the second floor is clean area, The cleanliness level is D (ISO level 7), Clean area facilities include: clean room wall, roof, clean door, window, floor, sink, floor drain, switch, socket, lighting fixtures, UV lamps, clean telephone, electromagnetic lock, etc, Room information is shown in the following table:5职责ResponsibilityXXX公司验证小组的职责:Responsibility of XXX GMP validation group:➢成立GMP验证小组;➢Set up a GMP validation group;➢收集相关的基础技术资料和图纸;➢Collect the relevant basic technical documents and drawings;➢编写和提交部件关键性评估报告;➢Prepare and submit Component Criticality Assessment report;➢编写和提交洁净厂房306-2区域的部件关键性评估报告;➢Draft and submit the Component Criticality Assessment report for clean room 306-2. ➢提交最终的部件关键性评估报告,以供进行审核和批准。
➢Submit the final CCA for reviewing and approving.XXX的职责:Responsibility of XXX GMP validation committee➢参与评估、编写部件关键性评估报告;➢Participate in the evaluation and prepare the key component evaluation report;➢审核洁净厂房306-2的部件关键性评估报告;➢Review the Component Criticality Assessment report for Clean room 306-2.➢批准洁净厂房306-2的部件关键性评估报告。
➢Approve the Component Criticality Assessment report for Clean room 306-2.6参考文件Reference Documents为了编制本报告,参考了以下法规和指南:The following regulations and guidelines are referenced for the preparation of this report.6.1法规Regulations➢国家药品监督管理局(NMPA),药品生产质量管理规范(2010年修订),2011 年03月。
➢National Medical Products Administration (NMPA), China, Good Manufacturing Practice (2010 Revision), March, 2011➢国家药品监督管理局(NMPA),药品生产质量管理规范(2010年修订),附录,确认与验证,2015年。
➢National Medical Products Administration (NMPA), China, Good Manufacturing Practice (2010 Revision), Annex, qualifaction and Validation, 20156.2指南Guidelines➢ISPE基准指南,第5卷-调试和确认,2003年第一版。
➢ISPE Baseline, V olume 5-Commissioning and Qualification, First Edition 2003.➢ICH Q9:质量风险管理,2005年,第4阶段。
➢ICH Q9: Quality Risk Management, 2005, 4th phase➢ISPE良好实践指南,基于风险分析的调试和确认,2011年。
➢ISPE Good Practices Guide, Applied Risk Management for Commissioning and Qualification, 2011。
