欧盟食品中微生物限量标准
食品微生物学标准欧盟(ec)no20732005号规章

食品微生物学标准欧盟(ec)no20732005号规章欧盟(EC)No 2073/2005/EC 号规章摘要食品微生物学标准欧盟(EC)No 2073/2005 号规章主要内容1、制定一个用来确定食品加工企业可接受的微生物标准和食品安全微生物限量标准;2、食品加工企业遵照执行、主管当局验证符合性;3、该标准包括依标准设定值(见附录)进行检验、检验方法和执行纠偏措施。
主体框架(总计12条,其中主要相关的7条)第1条范围第2条定义第3条一般要求第4条比对试验第5条特殊试验和取样要求第7条不合格结果第9条趋势分析附录1 食品微生物限量标准第1章食品安全标准第2章加工卫生标准2.1 肉和肉制品2.2 乳及乳制品2.3 蛋制品2.4 水产品2.5 蔬菜及其制品、水果及其制品第3章取样和试样制备准则3.1 取样和试样制备一般准则3.2 屠宰场及肉制品生产车间的细菌取样方法前言测试结果依赖于所使用的分析方法,因此每个微生物标准都应有一个指定的参考方法。
当然,食品生产企业可以使用其他分析方法而非参考方法,特别是一些快速检测方法,只要这些检测方法能达到同等结果。
80/777/EEC(13)的章程关于微生物的标准的有关规定。
第2条定义微生物学的标准是定义产品中微生物的可接受水平。
此可接受水平是基于单位质量、体积、面积或批次产品中的微生物和它们的毒素及代谢物的不存在或存在一定数量。
食品安全标准是对适合在市场上流通的一种食品或一批食品的可接受水平。
即食食品指生产的或经营的可直接食用的食品,此食品不须再经加热或经别的有效杀灭或降低有关微生物使其降到可接受的水平的过程。
过程卫生标准指产品生产过程可接受的标准。
此标准不适用于市场上的产品,标准设定了污染值,超过此值,就应采取措施确保过程的卫生并符合食品法。
“批”指一组或一批特定的产品,其在实际上同一环境条件下的特定过程获得的和在确定生产期内的特定地方生产的一系列可以确认的产品。
欧盟蜡样芽孢杆菌标准

欧盟蜡样芽孢杆菌标准全文共四篇示例,供读者参考第一篇示例:欧盟蜡样芽孢杆菌标准是指欧洲联盟制定的关于蜡样芽孢杆菌(Bacillus cereus)的质量要求和检测方法的标准。
蜡样芽孢杆菌是一种常见的细菌,广泛存在于自然界中的土壤、水源、植物等环境中。
它是一种潜在的食品污染源,能够在一定条件下产生产生毒素,引起食物中毒。
欧盟制定了一系列的标准,旨在控制和监测蜡样芽孢杆菌在食品中的含量,保障食品安全。
欧盟蜡样芽孢杆菌标准主要包括以下几个方面:1. 质量要求:欧盟规定了食品中蜡样芽孢杆菌的最大容许限量。
不同类型的食品具有不同的容许限量,其中以婴幼儿食品和食品中成分含水量高的产品要求最为严格。
这些限量的设定旨在保证人们在食用食品时不会遭受蜡样芽孢杆菌毒素的危害。
2. 检测方法:欧盟规定了蜡样芽孢杆菌的检测方法,包括取样方法、预处理方法、培养方法、毒素检测方法等。
这些方法一般由专业实验室进行操作,确保检测结果的准确性和可靠性。
3. 风险评估:欧盟对食品中蜡样芽孢杆菌的风险进行评估,分析潜在的风险因素和传播途径。
通过风险评估,可以及时采取控制措施,降低食品中蜡样芽孢杆菌的污染风险。
4. 风险管理:欧盟制定了一系列的风险管理措施,包括食品加工中的卫生规范、食品贮存和运输中的控制措施等。
这些措施旨在预防和控制蜡样芽孢杆菌在食品中的传播和繁殖。
欧盟蜡样芽孢杆菌标准的制定和执行,旨在保障食品安全,减少食品中蜡样芽孢杆菌的污染,降低人们因食用受污染食品而引发的食物中毒风险。
对于食品生产企业和相关部门而言,严格执行欧盟蜡样芽孢杆菌标准是确保产品质量和消费者健康的重要措施。
希望通过这些标准的制定和实施,可以有效地保障欧盟市场上食品的安全性,提高消费者对食品的信任度和满意度。
第二篇示例:欧盟蜡样芽孢杆菌标准(European Union Bacillus cereus Standard)是欧洲联盟为保障食品安全所制定的一项重要规范。
食品微生物学标准欧盟(EC)No 20732005 号规章

欧盟(EC)No 2073/2005/EC 号规章摘要食品微生物学标准欧盟(EC)No 2073/2005 号规章主要内容1、制定一个用来确定食品加工企业可接受的微生物标准和食品安全微生物限量标准;2、食品加工企业遵照执行、主管当局验证符合性;3、该标准包括依标准设定值(见附录)进行检验、检验方法和执行纠偏措施。
主体框架(总计12条,其中主要相关的7条)第1条范围第2条定义第3条一般要求第4条比对试验第5条特殊试验和取样要求第7条不合格结果第9条趋势分析附录1 食品微生物限量标准第1章食品安全标准第2章加工卫生标准2.1 肉和肉制品2.2 乳及乳制品2.3 蛋制品2.4 水产品2.5 蔬菜及其制品、水果及其制品第3章取样和试样制备准则3.1 取样和试样制备一般准则3.2 屠宰场及肉制品生产车间的细菌取样方法前言测试结果依赖于所使用的分析方法,因此每个微生物标准都应有一个指定的参考方法。
当然,食品生产企业可以使用其他分析方法而非参考方法,特别是一些快速检测方法,只要这些检测方法能达到同等结果。
80/777/EEC(13)的章程关于微生物的标准的有关规定。
第2条定义微生物学的标准是定义产品中微生物的可接受水平。
此可接受水平是基于单位质量、体积、面积或批次产品中的微生物和它们的毒素及代谢物的不存在或存在一定数量。
食品安全标准是对适合在市场上流通的一种食品或一批食品的可接受水平。
即食食品指生产的或经营的可直接食用的食品,此食品不须再经加热或经别的有效杀灭或降低有关微生物使其降到可接受的水平的过程。
过程卫生标准指产品生产过程可接受的标准。
此标准不适用于市场上的产品,标准设定了污染值,超过此值,就应采取措施确保过程的卫生并符合食品法。
“批”指一组或一批特定的产品,其在实际上同一环境条件下的特定过程获得的和在确定生产期内的特定地方生产的一系列可以确认的产品。
货架期指使用截至日期之前的一段时间或耐受存储的最小日期,同2000/13/EC指令中第9和10条款中的定义。
欧盟食品微生物标准

(强制性法令)欧盟第2005/2073/EC号规章2005年11月15日食品微生物学标准(本文已经EEA批准)欧盟委员会:根据欧共体成立条约,根据欧洲议会、欧盟理事会第2004 / 852 / EC号规章(2004年4月29日)中关于食品卫生的规定(1),特别是第4条第4款和第12条。
鉴于:(1) 如欧洲议会和欧盟理事会第178/2002号规章(2002年1月28日)所指出的,追求高标准地保护公众健康是食品法的基本目的之一。
该规章还确立了制定食品法、成立欧洲食品安全局以及处理食品安全事宜程序的一般原则和要求(2)。
食品中的微生物危害是人类食源性疾病的重要源头。
(2)食品中含有的微生物、微生物毒素或其代谢物的量不应给人类健康带来不能接受的危害。
(3)欧盟第2002/178 /EC号规章制定了食品安全的一般要求,依照此规章,食品若不安全就不能投放市场;万一投放,食品经营者有收回的义务。
为保护公众健康和防止误解,有必要对食品的可接受性制订统一的安全标准,尤其是针对食品中存在的某些致病菌。
(4)微生物学标准也给食品的可接受性以及其生产、处理、销售过程确立了框架。
微生物学标准的使用应作为执行HACCP程序和其他卫生管理措施完整部分中之一。
(5) 食品安全主要通过预防措施来保证,如执行良好卫生规范和HACCP计划。
微生物学标准还可用来验证HACCP计划及其他卫生管理措施的有效性。
因此有必要制定食品微生物学标准以规定食品加工过程是否合适,同时还有必要为保证食品安全制定微生物限值,超出此限值的食品即被认为受微生物污染而不安全。
(6) 依照EC 852/2004规章第4条,食品经营者应遵守食品微生物学标准。
这包括了符合食品法及有关当局规定的测试、分析和纠偏等工作。
因此应该制定有关分析方法的措施,此措施包括应用的地方、不确定度、抽样方法、微生物限制值、和限定值相一致的分析单元的数量等。
此外,应制定执行措施以确保食品及食物链的监控点符合标准,当没有达到食品安全标准时采取的措施。
欧盟食品微生物标准

(强制性法令)欧盟第2005/2073/EC号规章2005年11月15日食品微生物学标准(本文已经EEA批准)欧盟委员会:根据欧共体成立条约,根据欧洲议会、欧盟理事会第2004 / 852 / EC号规章(2004年4月29日)中关于食品卫生的规定(1),特别是第4条第4款和第12条。
鉴于:(1) 如欧洲议会和欧盟理事会第178/2002号规章(2002年1月28日)所指出的,追求高标准地保护公众健康是食品法的基本目的之一。
该规章还确立了制定食品法、成立欧洲食品安全局以及处理食品安全事宜程序的一般原则和要求(2)。
食品中的微生物危害是人类食源性疾病的重要源头。
(2)食品中含有的微生物、微生物毒素或其代谢物的量不应给人类健康带来不能接受的危害。
(3)欧盟第2002/178 /EC号规章制定了食品安全的一般要求,依照此规章,食品若不安全就不能投放市场;万一投放,食品经营者有收回的义务。
为保护公众健康和防止误解,有必要对食品的可接受性制订统一的安全标准,尤其是针对食品中存在的某些致病菌。
(4)微生物学标准也给食品的可接受性以及其生产、处理、销售过程确立了框架。
微生物学标准的使用应作为执行HACCP程序和其他卫生管理措施完整部分中之一。
(5) 食品安全主要通过预防措施来保证,如执行良好卫生规范和HACCP计划。
微生物学标准还可用来验证HACCP计划及其他卫生管理措施的有效性。
因此有必要制定食品微生物学标准以规定食品加工过程是否合适,同时还有必要为保证食品安全制定微生物限值,超出此限值的食品即被认为受微生物污染而不安全。
(6) 依照EC 852/2004规章第4条,食品经营者应遵守食品微生物学标准。
这包括了符合食品法及有关当局规定的测试、分析和纠偏等工作。
因此应该制定有关分析方法的措施,此措施包括应用的地方、不确定度、抽样方法、微生物限制值、和限定值相一致的分析单元的数量等。
此外,应制定执行措施以确保食品及食物链的监控点符合标准,当没有达到食品安全标准时采取的措施。
--欧盟20052073EC食品微生物标准中文版

编译说明1、2005年11月15日,欧盟委员会发布了2073/2005/EC《食品微生物标准》,并在2006年1月1日正式实施,该规章规定了严格的食品微生物指标要求,并取代了93/51/EEC规章。
适用产品范围为:肉及可食用肉类内脏、鱼及甲壳类、软体动物及其他水生无脊椎动物(但不包括活鱼)、乳制品、禽蛋、天然蜂蜜、可食用动物源性产品、可食用蔬菜、可食用水果和坚果、人造黄油、鱼类、甲壳类、软体类或其他水生无脊椎动物的肉类半成品、粮谷、面粉、淀粉或乳类半成品、蔬菜、水果、坚果半成品、软体动物可食用半成品。
2、为帮助我检验检疫人员和食品加工企业了解该技术法规,本局应对技术措施小组动植物产品及食品专业组组织编译了本规章,以供参考,但因能力有限,不到之处在所难免,请读者尽可能对照原文使用。
3、编译人员:顿玉慧、李大春、吴海、董晓慧、王忠才、宋宜国审校:顿玉慧、李大春、吴海2007年7月I(强制性法令)欧盟第2005/2073/EC号规章2005年11月15日食品微生物学标准(本文已经EEA批准)欧盟委员会:根据欧共体成立条约,根据欧洲议会、欧盟理事会第2004 / 852 / EC号规章(2004年4月29日)中关于食品卫生的规定(1),特别是第4条第4款和第12条。
鉴于:(1) 如欧洲议会和欧盟理事会第178/2002号规章(2002年1月28日)所指出的,追求高标准地保护公众健康是食品法的基本目的之一。
该规章还确立了制定食品法、成立欧洲食品安全局以及处理食品安全事宜程序的一般原则和要求(2)。
食品中的微生物危害是人类食源性疾病的重要源头。
(2)食品中含有的微生物、微生物毒素或其代谢物的量不应给人类健康带来不能接受的危害。
(3)欧盟第2002/178 /EC号规章制定了食品安全的一般要求,依照此规章,食品若不安全就不能投放市场;万一投放,食品经营者有收回的义务。
为保护公众健康和防止误解,有必要对食品的可接受性制订统一的安全标准,尤其是针对食品中存在的某些致病菌。
北欧食品安全规定(3篇)

第1篇一、概述北欧国家,包括丹麦、挪威、瑞典和芬兰,以其高质量的生活水平和严格的食品安全规定而闻名。
北欧食品安全规定旨在确保消费者能够获得安全、健康的食品,同时保护环境和动物福利。
以下是对北欧食品安全规定的详细阐述。
二、立法框架1. 欧盟法规北欧国家是欧盟成员国,因此必须遵守欧盟的食品安全法规。
欧盟食品安全法规主要包括以下几项:(1)2002/72/EC号法规:《关于食品和饲料中化学物质的限量》(2)2006/88/EC号法规:《关于食品中微生物限量》(3)1169/2011号法规:《关于食品接触材料》(4)2011/65/EU号法规:《关于限制在电子设备中使用的某些有害物质》2. 国家法规北欧国家根据欧盟法规制定本国的食品安全法规,以适应各自国家的实际情况。
以下列举几个北欧国家的食品安全法规:(1)丹麦:《食品安全法》(Fødevareloven)(2)挪威:《食品法》(Fødevaresikkerhetsloven)(3)瑞典:《食品安全法》(Livsmedelslagen)(4)芬兰:《食品安全法》(Elintarvikkeiden turvallisuuslaki)三、食品安全监管机构1. 欧盟层面欧盟食品安全局(EFSA)是欧盟的食品安全监管机构,负责提供科学依据,以支持欧盟食品安全政策的制定和实施。
2. 北欧国家层面北欧国家设有各自的食品安全监管机构,负责执行本国的食品安全法规。
以下列举几个北欧国家的食品安全监管机构:(1)丹麦:丹麦食品安全局(Fødevarestyrelsen)(2)挪威:挪威食品安全局(Fødevaresikkerhetsmyndigheten)(3)瑞典:瑞典食品安全局(Livsmedelsverket)(4)芬兰:芬兰食品安全局(Elintarviketurvallisuusvirasto)四、食品安全管理体系1. 食品生产者责任北欧食品安全规定强调食品生产者的责任,要求其在生产、加工、储存、运输和销售过程中严格遵守食品安全法规。
EC 1441 2007 欧盟微生物限量中文版

EN关于食品微生物标准2073/2005/EC的修订条例欧洲共同体委员会条例1441/2007/EC(2007年12月5日)欧洲共同体委员会,根据建立欧洲共同体的条约,根据欧洲经济共同体议会和食品卫生委员会条例852/2004(2004年4月29日),特别是其中的第4条第4款,鉴于:( 1 )委员会条例第2073/2005号(2005年11月15日)食品微生物标准,规定了当食品经营者要补充涉及到委员会条例第852/2004号第4章中总体的和特定的卫生措施时,必须遵守某些微生物的微生物标准和补充条例。
委员会第2073/2005号条例还规定,食品经营者将要确保食品符合有关附件I中制定的微生物标准条例。
( 2 )委员会第2073/2005号条例附件I第1、2章中规定了关于婴儿配方奶粉及干膳食食品和用于特殊医疗目的的6个月以下婴儿(婴儿配方奶粉和干膳食食品)的食品安全标准和操作卫生标准。
附件第2章2.2节中规定,在任何一种抽样单元中,如果婴儿配方奶粉和干膳食食品被检出肠杆菌,将分批进行阪崎肠杆菌和沙门氏菌的测试。
( 3 )在2007年1月24日,欧洲食品安全局(EFSA)关于生物危害( BIOHAZ小组)的科学小组发表了关于肠杆菌作为沙门氏菌和阪崎氏肠杆菌的指标的观点。
结论是,建立肠杆菌和沙门氏菌之间的关联是不可能的,并且阪崎氏肠杆菌的存在与肠杆菌也没有普遍关联。
但是,在工厂个体水平,肠杆菌和阪崎肠杆菌之间可能建立关联。
( 4 )因此,委员会第2073/2005号条例中,在对婴儿配方奶粉和干膳食食品进行沙门氏菌和阪崎肠杆菌的检测中,如果在任何抽样单元中检出肠杆菌,该条例不再适用。
因此,附件I第2章2.2节的条例应作相应的修改。
( 5 )根据欧洲食品安全局生物危害小组2004年9月9日发表的关于婴儿配方和后续配方的微生物风险的观点,干制品后续配方应制定沙门氏菌和肠杆菌的微生物标准。
( 6 )欧洲食品安全局生物危害小组在2005年1月26、27日发表了食品中蜡状芽孢杆菌与其他芽孢杆菌的观点。
欧盟食品微生物标准
欧盟食品微生物标准欧盟第2005/2073/EC号规章2005年11月15日食品微生物学标准欧盟委员会:根据欧共体成立条约,根据欧洲议会、欧盟理事会第2004 / 852 / EC号规章中关于食品卫生的规定(1),特别是第4条第4款和第12条。
鉴于:(1) 如欧洲议会和欧盟理事会第178/2002号规章所指出的,追求高标准地保护公众健康是食品法的基本目的之一。
该规章还确立了制定食品法、成立欧洲食品安全局以及处理食品安全事宜程序的一般原则和要求(2)。
食品中的微生物危害是人类食源性疾病的重要源头。
(2)食品中含有的微生物、微生物毒素或其代谢物的量不应给人类健康带来不能接受的危害。
(3)欧盟第2002/178 /EC号规章制定了食品安全的一般要求,依照此规章,食品若不安全就不能投放市场;万一投放,食品经营者有收回的义务。
为保护公众健康和防止误解,有必要对食品的可接受性制订统一的安全标准,尤其是针对食品中存在的某些致病菌。
(4)微生物学标准也给食品的可接受性以及其生产、处理、销售过程确立了框架。
微生物学标准的使用应作为执行HACCP程序和其他卫生管理措施完整部分中之一。
(5) 食品安全主要通过预防措施来保证,如执行良好卫生规范和HACCP计划。
微生物学标准还可用来验证HACCP计划及其他卫生管理措施的有效性。
因此有必要制定食品微生物学标准以规定食品加工过程是否合适,同时还有必要为保证食品安全制定微生物限值,超出此限值的食品即被认为受微生物污染而不安全。
(6) 依照EC 852/2004规章第4条,食品经营者应遵守食品微生物学标准。
这包括了符合食品法及有关当局规定的测试、分析和纠偏等工作。
因此应该制定有关分析方法的措施,此措施包括应用的地方、不确定度、抽样方法、微生物限制值、和限定值相一致的分析单元的数量等。
此外,应制定执行措施以确保食品及食物链的监控点符合标准,当没有达到食品安全标准时采取的措施。
食品经营者采取措施确保标准中所定义的过程的可接受性,这些措施包括原材料、卫生、温度以及产品保存期的控制。
欧盟食品微生物限量-2007(中文)

3,0 log cfu/cm2 日平均log值
在每个胴体的检测区域不得检出
分析参考方法(3) 标准适用时期
ISO4833
包装后冷却前的胴体
ISO21528-2 EN/ISO 6579
包装后冷却前的胴体 包装后冷却前的胴体
在每个胴体的检测区域不得检出
EN/ISO 6579
2.1.6 肉末、肉馅
2.1.7机械分割肉(9) 2.1.8预制肉
备注:
沙门氏菌
50(5)
2(6)
沙门氏菌
50(5)
5(6)
沙门氏菌
50(5)
5(6)
需氧菌落计数(7)
5
2
大肠杆菌(8) 需氧菌落计数 大肠杆菌(8) 大肠杆菌(8)
5
2
5
2
5
2
5
2
(1) n = 取样数; c =结果数据在m和M之间的样品数
(8)PH≤4.4且AW≤0.92的产品、PH≤5.0且Aw≤0.94的产品、保质期少于5天的产品自动 的证据
(9)机械分割肉制品的检测技术适用于欧洲议会和委员会规则(EC)No 853/2004附录5
(10)排除以下食品:食品生产商能保证食品有最佳的收获时间、合适的水活度及不存在
(11)只指含乳成分的冰激凌 (12)在一批种子开始萌芽之前要进行初步测试或在沙门氏菌达到期望的最高值期间进 (13)参考:凝固酶阳性葡萄球菌共同参考的实验室:欧洲放映方法检测奶及奶制品中 (14)肠杆菌科和坂崎肠杆菌需要做平行试验,除非在个别装置水平上已经建立了这些 (15)在这里大肠杆菌是作为排泄物污染指示菌 (16)采用混样,并应取至少10个动物个体。 (17)尤其是以下这些科目的鱼类:鲭科鱼、鲱科的鱼、鳀科类鱼、 (17)尤其是以下这些科目的鱼类:鲭科鱼、鲱科的鱼、鳀科类鱼、 (18)单个样品可以在零售的范围内取样,在此情况下,(EC) No 178/2002中14(6)款 (19)参考文献
美国欧盟与中国关于出口肉类产品微生物控制标准简介(PPT50页)

(二)2073/2005/EC《食品微生物标准》 将用于生产碎肉和肉制品的细菌学采样
(1)猪、羊、牛、马等牲畜胴体采样规则
在每段采样时间内,应随机采集5头牲畜胴体。采样点的选择根据 屠宰场使用的屠宰技术而定。为检验大肠杆菌和菌落总数进行取样时, 应在牲畜胴体上选择4个采样点。使用破坏性采样方法时,4个采样点组 织样品总面积为20cm2;使用非破坏性采样方法时,每个采样点面积至 少为100cm2,对于小型反刍动物则至少为50cm2。为检验沙门氏菌进行 取样时,应使用海绵磨擦法采样。每个所选择的采样点其面积至少为 100cm2。样品若来自牲畜胴体不同采样点,检验前应先混合。
• 清洗和消毒
清洗是把工厂和加工设备的污物去掉。包括人工和现场清洁。 消毒包括化学剂、加热和辐射。
一、美国肉类微生物控制方法与标准
美国肉类微生物控制方法与标准具有科学化的抽样水 平和合理的判定依据。USDA(美国农业部 )的基本做法是 在全国范围内通过收集对某一种肉类制品中不同病原性微 生物污染情况的调查,运用统计学技术提出该品种某种病 原微生物的抽样检测方案和操作限量标准。 在美国肉类食品安全控制体系的微生物检测与控制中, USDA对原料性肉的重要指标是:细菌总数(判定肉制品的 一般污染状况);大肠菌群(判定加工过程粪源性污染的状 况);病原微生物包括沙门氏菌、单核细胞增生李斯特氏 菌、大肠杆菌O157、耶尔森氏菌和空肠弯曲杆菌。
(ⅲ)对于猪胴体,在后腿、腹部和脸部用海绵擦拭或切割 组织采样。
(c)采样频率
除生产能力很低的企业外,其余企业采样频率 要符合其生产能力,需按照下列要求: (ⅰ)牛、山羊、绵羊、马、骡和其它马类,300 个胴体中采1份。但在生产情况下,每周至少采1份 样品。 (ⅱ)猪每1000个胴体采1份,但在生产情况下,
欧盟蜡样芽孢杆菌标准

欧盟蜡样芽孢杆菌标准全文共四篇示例,供读者参考第一篇示例:欧盟蜡样芽孢杆菌标准是指欧盟针对食品安全领域中蜡样芽孢杆菌的监测、检测和控制制定的相关规定和标准。
蜡样芽孢杆菌是一种常见的细菌,存在于土壤、水源、动植物体表等环境中。
它可以在适宜的条件下繁殖生长,并产生毒素,对人体健康造成危害。
欧盟通过制定相应的标准,以确保食品中不含有危害健康的蜡样芽孢杆菌。
蜡样芽孢杆菌在食品中的检测主要是通过对食品样品进行采样,提取样品中的蜡样芽孢杆菌,然后进行培养、鉴定和计数。
欧盟要求食品生产企业在生产过程中对食品进行严格的监控,确保食品中蜡样芽孢杆菌的数量符合规定的标准。
如果食品中的蜡样芽孢杆菌超过规定的限量,必须立即采取措施加以处理,以避免对消费者造成健康风险。
欧盟蜡样芽孢杆菌的标准主要包括以下几个方面:1. 检测方法:欧盟规定了蜡样芽孢杆菌的检测方法,包括培养、分离、鉴定和计数等步骤。
食品生产企业必须使用认可的检测方法对食品进行检测,确保检测结果的准确性和可靠性。
2. 菌落数量限量:欧盟规定了食品中蜡样芽孢杆菌的菌落数量限量。
不同类型的食品对蜡样芽孢杆菌的容许量也会有所不同。
食品生产企业在生产过程中必须严格按照标准的要求进行控制,保证食品中的蜡样芽孢杆菌数量在安全范围内。
3. 处理措施:如果食品样品中的蜡样芽孢杆菌超过规定的限量,食品生产企业必须立即采取措施,如停产、召回产品等,以避免对消费者造成健康风险。
企业还必须配合相关部门进行溯源调查,查明蜡样芽孢杆菌的来源,并采取措施避免再次发生类似事件。
4. 标签要求:欧盟规定在食品包装上必须标明食品中是否含有蜡样芽孢杆菌,并注明蜡样芽孢杆菌的数量是否符合标准要求。
消费者通过检查食品包装上的标签信息可以了解食品的安全性和质量。
欧盟蜡样芽孢杆菌标准是欧盟为确保食品安全和保护消费者健康而制定的重要规定。
食品生产企业必须严格遵守蜡样芽孢杆菌的检测、限量和处理要求,确保生产的食品符合相关标准,保障消费者的权益和健康。
欧盟20052073EC 食品微生物标准中文版

编译说明1、2005年11月15日,欧盟委员会发布了2073/2005/EC《食品微生物标准》,并在2006年1月1日正式实施,该规章规定了严格的食品微生物指标要求,并取代了93/51/EEC规章。
适用产品范围为:肉及可食用肉类内脏、鱼及甲壳类、软体动物及其他水生无脊椎动物(但不包括活鱼)、乳制品、禽蛋、天然蜂蜜、可食用动物源性产品、可食用蔬菜、可食用水果和坚果、人造黄油、鱼类、甲壳类、软体类或其他水生无脊椎动物的肉类半成品、粮谷、面粉、淀粉或乳类半成品、蔬菜、水果、坚果半成品、软体动物可食用半成品。
2、为帮助我检验检疫人员和食品加工企业了解该技术法规,本局应对技术措施小组动植物产品及食品专业组组织编译了本规章,以供参考,但因能力有限,不到之处在所难免,请读者尽可能对照原文使用。
3、编译人员:顿玉慧、李大春、吴海、董晓慧、王忠才、宋宜国审校:顿玉慧、李大春、吴海2007年7月I(强制性法令)欧盟第2005/2073/EC号规章2005年11月15日食品微生物学标准(本文已经EEA批准)欧盟委员会:根据欧共体成立条约,根据欧洲议会、欧盟理事会第2004 / 852 / EC号规章(2004年4月29日)中关于食品卫生的规定(1),特别是第4条第4款和第12条。
鉴于:(1) 如欧洲议会和欧盟理事会第178/2002号规章(2002年1月28日)所指出的,追求高标准地保护公众健康是食品法的基本目的之一。
该规章还确立了制定食品法、成立欧洲食品安全局以及处理食品安全事宜程序的一般原则和要求(2)。
食品中的微生物危害是人类食源性疾病的重要源头。
(2)食品中含有的微生物、微生物毒素或其代谢物的量不应给人类健康带来不能接受的危害。
(3)欧盟第2002/178 /EC号规章制定了食品安全的一般要求,依照此规章,食品若不安全就不能投放市场;万一投放,食品经营者有收回的义务。
为保护公众健康和防止误解,有必要对食品的可接受性制订统一的安全标准,尤其是针对食品中存在的某些致病菌。
欧盟关于食品污染物最高限量的最新法规

欧盟关于食品污染物最高限量的最新法规欧盟关于食品污染物最高限量的最新法规(EC 1881/2006号条例)于2007年3月1日正式生效,同时废止了原EC 466/2001号食品污染物限量法规。
新法规确定的允许食品所含最高限量的污染物包括:第一类:硝酸盐(Nitrate)主要涉及新鲜和冷冻菠菜,生菜等。
第二类:真菌毒素(Mycotoxins),其中2.1黄曲霉毒素(Aflatoxins B1, B2, G1, G2, M1)主要涉及花生,带皮坚果,干果,粮食,玉米,原奶,部分调味品等2.2 赭曲霉毒素A(Ochratoxin A)主要涉及未加工粮食,葡萄干,咖啡豆,葡萄酒,葡萄汁等2.3棒曲霉毒素(Patulin)主要涉及果汁,果酒,苹果食品2.4 脱氧雪腐镰刀菌烯醇(Deoxynivalenol)主要涉及其他未加工粮食如硬麦,燕麦,玉米等,面团,面包等2.5 玉米烯酮(Zearalenone)其他未加工粮食和玉米,直接食用的粮食粉、麸皮、植物胚胎,面包等2.6 伏马霉菌B1、B2(Fumonisins,B1和B2)主要涉及玉米、玉米碴,玉米胚胎,玉米油,玉米食品2.7 T-2 & HT-2毒素主要涉及未加工粮食和粮食制品第三类:重金属,其中3.1 铅(Lead)主要涉及原奶,(牛、羊、猪和禽类)的肉、副产品,鱼的肌肉,虾类,贝类,头足纲动物,豆荚蔬菜和水果,部分蔬菜和水果,莓类水果,脂肪,果汁,葡萄酒等3.2 镉(Cadmium)主要涉及肉,动物的肝、肾,鱼的肌肉,虾类,贝类,头足纲动物,粮食,黄豆,蔬菜和水果等3.3汞(Mercury)主要涉及鱼制品,鱼的肌肉等3.4 无机锡(Tin, inorganic)主要涉及罐头食品等第四类:三氯丙醇(3-MCPD)主要涉及植物蛋白和酱油。
第五类:二恶英及类二恶英多氯联苯(Dioxins & Dioxin-like PCBs)主要涉及牛、羊、猪和禽类的肉、脂肪和肉制品,肝,鱼的肌肉,原奶,鸡蛋和蛋制品,动物和植物脂肪等第六类:苯并(a)吡(Benzo(a)pyrene)主要涉及直接食用的脂肪,熏制肉制品,虾类,贝类等。
食品微生物学标准欧盟(ec)no20732005号规章

欧盟(EC)No 2073/2005/EC 号规章摘要食品微生物学标准欧盟(EC)No 2073/2005 号规章主要内容1、制定一个用来确定食品加工企业可接受的微生物标准和食品安全微生物限量标准;2、食品加工企业遵照执行、主管当局验证符合性;3、该标准包括依标准设定值(见附录)进行检验、检验方法和执行纠偏措施。
主体框架(总计12条,其中主要相关的7条)第1条范围第2条定义第3条一般要求第4条比对试验第5条特殊试验和取样要求第7条不合格结果第9条趋势分析附录1 食品微生物限量标准第1章食品安全标准第2章加工卫生标准2.1 肉和肉制品2.2 乳及乳制品2.3 蛋制品2.4 水产品2.5 蔬菜及其制品、水果及其制品第3章取样和试样制备准则3.1 取样和试样制备一般准则3.2 屠宰场及肉制品生产车间的细菌取样方法前言测试结果依赖于所使用的分析方法,因此每个微生物标准都应有一个指定的参考方法。
当然,食品生产企业可以使用其他分析方法而非参考方法,特别是一些快速检测方法,只要这些检测方法能达到同等结果。
80/777/EEC(13)的章程关于微生物的标准的有关规定。
第2条定义微生物学的标准是定义产品中微生物的可接受水平。
此可接受水平是基于单位质量、体积、面积或批次产品中的微生物和它们的毒素及代谢物的不存在或存在一定数量。
食品安全标准是对适合在市场上流通的一种食品或一批食品的可接受水平。
即食食品指生产的或经营的可直接食用的食品,此食品不须再经加热或经别的有效杀灭或降低有关微生物使其降到可接受的水平的过程。
过程卫生标准指产品生产过程可接受的标准。
此标准不适用于市场上的产品,标准设定了污染值,超过此值,就应采取措施确保过程的卫生并符合食品法。
“批”指一组或一批特定的产品,其在实际上同一环境条件下的特定过程获得的和在确定生产期内的特定地方生产的一系列可以确认的产品。
货架期指使用截至日期之前的一段时间或耐受存储的最小日期,同2000/13/EC指令中第9和10条款中的定义。
欧洲药典微生物限度标准

欧洲药典对微生物限度的标准通常包括以下几项:
1.细菌总数:每克或每毫升药品中含有的细菌总数不得超过10000个。
2.霉菌和酵母菌总数:每克或每毫升药品中含有的霉菌和酵母菌总数不得超过100个。
3.革兰阴性杆菌:每克或每毫升药品中不得检出革兰阴性杆菌。
4.沙门氏菌:每克或每毫升药品中不得检出沙门氏菌。
5.志贺氏菌:每克或每毫升药品中不得检出志贺氏菌。
6.金黄色葡萄球菌:每克或每毫升药品中不得检出金黄色葡萄球菌。
7.支原体和衣原体:每克或每毫升药品中不得检出支原体和衣原体。
8.病毒:每毫升药品中不得检出病毒。
这些标准旨在确保药品的安全性和有效性,防止因微生物污染而对使用者造成危害。
请注意,具体的微生物限度标准可能因不同的药品和用途而有所不同。
在实际操作中,应严格按照相关法规和标准进行药品的微生物限度检查。
欧盟微生物标准

欧盟微生物标准MICROBIOLOGICAL CRITERIA FOR FOODSTUFFS IN COMMUNITY LEGISLATION IN FORCESampling plan Food categoryMicroorganismsLimitN c mMAdditional informationAerobic colony count 5-103,5 log cfu/cm 25,0 log cfu/cm 2Daily log mean Carcasses of cattle, sheep,goat and horse (Decision 2001/471/EC)Enterobacteriaceae (E.coli)5-101,5 log cfu/cm 22,5 log cfu/cm 2Daily log mean Aerobic colony count 5-104,0 log cfu/cm 25,0 log cfu/cm 2Daily log mean Carcasses of pig(Decision 2001/471/EC)Enterobacteriaceae (E.coli)5-102,0 log cfu/cm 23,0 log cfu/cm 2Daily log meanAerobic mesophilic bacteria 525x105 /g 5x106 /g E. coli 5250 /g 500 /g Salmonella Absence in 10 g50 Minced meat(Directive 94/65/EEC)S. aureus 52100 /g 1000 /g E. coli 52500 /g 5000 /g S. aureus 51500 /g5000 /gMeat preparations (Directive 94/65/EEC)SalmonellaAbsence in 1 g 50Plate count at 30°C 105 cfu/ml Raw cow’s milk intended for processing(Directive 92/46/EEC)S. aureus52500 cfu/ml 2000cfu/mlMilk for manufactu-ring of raw products Raw goat’s, sheep’s and buffalo’s milk(Directive 92/46/EEC)Plate count at 30°C5x 105 cfu/mlMilk intended formanufacture of products made from raw milkRaw goat’s, sheep’s and buffalo’s milk(Directive 92/46/EEC)Plate count at 30°C1.5x106 cfu/mlMilk intended for manufacture of heat treated milk based productsSalmonella Absence in 25 g50S. aureus52100 /ml500 /mlPlate count at 30°C5x104 cfu/ml Raw cow’s milkIntended for direct human consumption(Directive 92/46/EEC)Pathogenic microorganisms and their toxinsNot in quantities to affect human healthPathogenic microorganisms Absent in 25 g50Coliforms510 cfu/ml 5 cfu/ml Pasteurised drinking milk (Directive 92/46/EEC)Plate count at 21°C 515x104cfu/g 5x105cfu/gAfter incubation at 6°C for 5 daysUHT milk and strerilized milk (Dir. 92/46/EEC)Plate count at 30°C 10 cfu/0,1 ml After incubation at 30°C for 15 days Listeria monocytogenesAbsence in 1 g (hard cheeses)or in 25 g (other)5Cheeses made from raw milk and from thermized milk(Directive 92/46/EEC)SalmonellaAbsence in 1 g50S. aureus, guideline 1521000 cfu/g 10000cfu/g Cheeses made from raw milk and from thermized milkE. coli, guideline 252104 cfu/g 105 cfu/g1Mandatory if pathogenic or enterotoxinogenic strains found Listeria monocytogenes Absence in 25 g 50SalmonellaAbsence in 1 g50S. aureus, guideline 252100 cfu/g 1000 cfu/g E. coli, guideline 252100 cfu/g 1000 cfu/g Soft cheese (made from heat-treated milk)(Directive 92/46/EEC)Coliforms, guideline 52104 cfu/g 105 cfu/g Listeria monocytogenes Absence in 25 g 50SalmonellaAbsence in 1 g 50Fresh cheese(Directive 92/46/EEC)S. aureus, guideline 5210 cfu/g100 cfu/gListeria monocytogenesAbsence in 1 g (hard cheeses)or in 25 g (other)50Other cheeses than those mentioned above(Directive 92/46/EEC)SalmonellaAbsence in 1 g 50Listeria monocytogenes Absence in 1 g 50SalmonellaAbsence in 1 g 50Butter(Directive 92/46/EEC)Coliforms, guideline 520 cfu/g 10 cfu/g SalmonellaAbsence in 1 g 50Listeria monocytogenes Absence in 1 g50S. aureus, guideline 5210 cfu/g 100 cfu/g Powdered milk Powdered milk and milk based products(Directive 92/46/EEC)Coliforms, guideline 520 cfu/g 10 cfu/gPowdered milk-based productsSalmonellaAbsence in 1 g 50Listeria monocytogenes Absence in 1 g50S. aureus , guideline 5210 cfu/g 100 cfu/g Coliforms, guideline 5210 cfu/g 100 cfu/g Frozen milk-based products (Directive 92/46/EEC)Plate count, guideline52105 cfu/g5x105cfu/gSalmonellaAbsence in 1 g 50Listeria monocytogenes Absence in 1 g50Coliforms 520 cfu/g 5 cfu/g Liquid milk-based products(Directive 92/46/EEC)Plate count 525x104cfu/g105 cfu/gHeat-treated andunfermented products SalmonellaAbsence in 25 g Faecal coliforms, or <300 / 100g < 6000 / 100 g < 60000/ 100g Production area A Production area B Production area C Live bivalve molluscs (Directive 91/492/EEC)E. coli<230 / 100g <4600 / 100 g Production area A Production area BSalmonella Absence in 25 g 50S. aureus 52100 cfu/g1000 cfu/gAny pathogenQuantities to affect human healthCooked crustaceans and molluscan shellfish (Decision 93/51/EEC)Thermotolerant coliforms or 5210 cfu/g 100 cfu/g E. coli , guideline5110 cfu/g 100 cfu/g 52104 cfu/g 105 cfu/g Whole products 525x104 fu/g 5x105cfu/g Shelled and shucked Cooked crustaceans and molluscan shellfish (Decision 93/51/EEC) Mesophilic aerobic bacteria,guideline52105 cfu/g 106 cfu/gCrabmeatSalmonellaAbsence in 25 g Aerobic mesophilic bacteria 105 cfu/g or ml Enterobacteriaceae 100 cfu/g or ml Egg products(Directive 89/437/EEC)S. aureusAbsent in 1 g20 cfu/ml At source, incubated at20-22°C for 72 hours5 cfu/ml At source, incubated at37°C for 24 hours100 cfu/ml After bottling, incubatedat 20-22°C for 72 hours Total colony count20 cfu/ml After bottling, incubatedat 37°C for 24 hoursE.coli, other coliforms, feacal streptocci Absence in 250mlAt source and duringmarketingSporulated sulphite-reducing anaerobes Absence in 50mlAt source and duringmarketingNatural mineral waters (Directive 80/777/EEC)Pseudomonas aeruginosa Absence in 250ml At source and during marketing。
欧盟修订食品中黄曲霉毒素的最大限量值
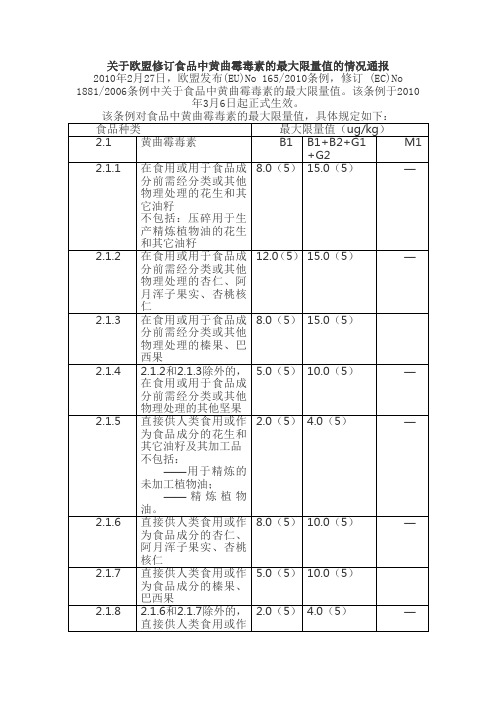
关于欧盟修订食品中黄曲霉毒素的最大限量值的情况通报2010年2月27日,欧盟发布(EU)No 165/2010条例,修订 (EC)No 1881/2006条例中关于食品中黄曲霉毒素的最大限量值。
该条例于2010年3月6日起正式生效。
该条例对食品中黄曲霉毒素的最大限量值,具体规定如下:食品种类最大限量值(ug/kg)2.1黄曲霉毒素B1B1+B2+G1+G2M18.0(5)15.0(5)—2.1.1在食用或用于食品成分前需经分类或其他物理处理的花生和其它油籽不包括:压碎用于生产精炼植物油的花生和其它油籽2.1.2在食用或用于食品成12.0(5)15.0(5)—分前需经分类或其他物理处理的杏仁、阿月浑子果实、杏桃核仁8.0(5)15.0(5)2.1.3在食用或用于食品成分前需经分类或其他物理处理的榛果、巴西果5.0(5)10.0(5)—2.1.4 2.1.2和2.1.3除外的,在食用或用于食品成分前需经分类或其他物理处理的其他坚果2.0(5) 4.0(5)—2.1.5直接供人类食用或作为食品成分的花生和其它油籽及其加工品不包括:——用于精炼的未加工植物油;——精炼植物油。
8.0(5)10.0(5)—2.1.6直接供人类食用或作为食品成分的杏仁、阿月浑子果实、杏桃核仁2.1.7直接供人类食用或作5.0(5)10.0(5)为食品成分的榛果、巴西果2.1.8 2.1.6和2.1.7除外2.0(5) 4.0(5)—的,直接供人类食用或作为食品成分的其他坚果)5.010.0—2.1.9在食用或用于食品成分前需经分类或其他物理处理的干制水果2.0 4.0—2.1.10直接供人类食用或作为食品成分的干制水果及其加工品2.1.11以下2.1.12、2.1.152.0 4.0—和2.1.17除外的所有谷物和所有谷物制品5.010.0—2.1.12在食用或用于食品成分前需经分类或其他物理处理的玉米和大米——0.0502.1.13生牛奶、热加工处理牛奶和用于生产奶制品的牛奶5.010.0—2.1.14如下种类的调味料:辣椒(整的或磨碎的干辣椒,包括辣椒和辣椒粉)、胡椒(包括白胡椒和黑胡椒)、豆蔻、姜、姜黄、包含以上这些调味料中一种或多种的混合调味料0.10——2.1.15加工的谷物制品和婴幼儿食品——0.0252.1.16婴幼儿配方食品,包括婴幼儿配方奶粉0.10—0.0252.1.17用于婴儿的以特殊医用为目的的饮食食品注:(5)表示最大限量值只适用于花生和其它坚果的可食部分。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
L 322/12 EN Official Journal of the European UnionCOMMISSION REGULATION (EC) No 1441/2007of 5 December 20077.12.2007amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs(Text with EEA relevance)THE COMMISSION OF THE EUROPEAN COMMUNITIES,Having regard to the Treaty establishing the European Community,Having regard to Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygieneof foodstuffs (1), and in particular Article 4(4) thereof,Whereas:(1) Commission Regulation (EC) No 2073/2005 of 15November 2005 on microbiological criteria for food-stuffs (2) lays down microbiological criteria for certainmicro-organisms and the implementing rules to becomplied with by food business operators when im-plementing the general and specific hygiene measuresreferred to in Article 4 of Regulation (EC) No852/2004. Regulation (EC) No 2073/2005 alsoprovides that food business operators are to ensurethat foodstuffs comply with the relevant microbiological criteria set out in Annex I to that Regulation.(2) Chapters 1 and 2 of Annex I to Regulation (EC) No2073/2005 set out food safety criteria and processhygiene criteria regarding dried infant formulae anddried dietary foods for special medical purposesintended for infants below six months of age (driedinfant formulae and dried dietary foods). Part 2.2 ofChapter 2 of that Annex provides that where driedinfant formulae and dried dietary foods are tested andEnterobacteriaceae are detected in any of the sampleunits, the batch is to be tested for Enterobacter sakazakiiand Salmonella.(3) On 24 January 2007, the Scientific Panel on BiologicalHazards (BIOHAZ Panel) of the European Food SafetyAuthority (EFSA) issued an opinion with regard toEnterobacteriaceae as indicators of Salmonella andEnterobacter sakazakii. It concluded that it is not possible(1) OJ L 139, 30.4.2004, p. 1, as corrected by OJ L 226, 25.6.2004,p. 3.(2) OJ L 338, 22.12.2005, p. 1.to establish a correlation between Enterobacteriaceae andSalmonella, and no universal correlation between Entero-bacteriaceae and Enterobacter sakazakii exists. At individualplant level, a correlation between Enterobacteriaceae andEnterobacter sakazakii may however be established.(4) Therefore the requirement laid down in Regulation(EC) No 2073/2005 as regards the testing of driedinfant formulae and dried dietary foods for Salmonellaand Enterobacter sakazakii where Enterobacteriaceae aredetected in any of the sample units should no longerapply. Part 2.2 of Chapter 2 of Annex I to that Regu-lation should therefore be amended accordingly.(5) In line with the opinion on the microbiological risks ininfant formulae and follow-on formulae issued by theBIOHAZ Panel of EFSA on 9 September 2004, micro-biological criteria on Salmonella and Enterobacteriaceaeshould be laid down for dried follow-on formulae.(6) The BIOHAZ Panel of EFSA issued an opinion on Bacilluscereus and other Bacillus spp. in foodstuffs on 26 and 27January 2005. It concluded that one of the major controlmeasures is to control temperature and to establish asystem based on hazard analysis and critical controlpoint principles. Dehydrated foods, in which thepresence of spores of pathogenic Bacillus spp. isfrequent, might permit the growth of Bacillus cereusonce rehydrated in warm water. Some dehydratedfoods, including dried infant formulae and dried dietaryfoods, are consumed by potentially fragile consumers. In line with the EFSA opinion, the numbers of Bacillus cereusspores in dried infant formulae and dried dietary foodsshould be as low as possible during processing and aprocess hygiene criterion should be laid down inaddition to good practices designed to reduce delaybetween preparation and consumption.(7) Chapter 1 of Annex I to Regulation (EC) No 2073/2005provides for the analytical reference method for staphy-lococcal enterotoxins in certain cheeses, milk powder andwhey powder. That method has been revised by theCommunity reference laboratory for coagulase positivestaphylococci. The reference to that analytical referencemethod should therefore be amended. Chapter 1 ofAnnex I to that Regulation should therefore beamended accordingly.7.12.2007 EN Official Journal of the European Union L 322/13(8) Chapter 3 of Annex I to Regulation (EC) No 2073/2005sets out sampling rules for carcasses of cattle, pig, sheep,goats and horses for Salmonella analyses. Pursuant tothose rules the sampling area is to cover a minimumof 100 cm2per site selected. However, neither thenumber of sampling sites nor the minimum total areaof sampling is specified. In order to improve theimplementation of these rules in the Community, it isappropriate to further specify in Regulation (EC) No2073/2005 that the areas most likely to be contaminatedshould be selected for sampling and that the totalsampling area should be increased. Chapter 3 ofAnnex I to that Regulation should therefore beamended accordingly.(9) In the interests of clarity of Community legislation, it isappropriate to replace Annex I to Regulation (EC) No2073/2005 by the text set out in the Annex to thisRegulation. (10) The measures provided for in this Regulation are inaccordance with the opinion of the StandingCommittee on the Food Chain and Animal Health,HAS ADOPTED THIS REGULATION:Article 1Annex I to Regulation (EC) No 2073/2005 is replaced by the text in the Annex to this Regulation.Article 2This Regulation shall enter into force on the 20th day following its publication in the Official Journal of the European Union.This Regulation shall be binding in its entirety and directly applicable in all Member States. Done at Brussels, 5 December 2007.For the CommissionMarkos KYPRIANOUMember of the CommissionL 322/14 ENChapter 1.Chapter 2.Official Journal of the European UnionANNEX‘ANNEX IMicrobiological criteria for foodstuffsFood safety criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15Process hygiene criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 207.12.20072.1 Meat and products thereof . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 202.2 Milk and dairy products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 232.3 Egg products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 262.4 Fishery products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 272.5 Vegetables, fruits and products thereof . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Chapter3. Rules for sampling and preparation of test samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 293.1 General rules for sampling and preparation of test samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 293.2 Bacteriological sampling in slaughterhouses and at premises producing minced meat and meatpreparations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 297.12.2007ENOfficial Journal of the European UnionL 322/15C h a p t e r 1. F o o d s a f e t y c r i t e r i aeeeeeeeeL 322/16 EN Official Journal of the European Union 7.12.2007e e e e e e e e e e7.12.2007 EN Official Journal of the European Union L 322/17e e e e e e e eL 322/18ENOfficial Journal of the European Union7.12.2007e1 (2 ) F o r p o i n t s 1.1-1.25 m = M . (3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . (4 ) R e g u l a r t e s t i n g a g a i n s t t h e c r i t e r i o n i s n o t r e q u i r e d i n n o r m a l c i r c u m s t a n c e s f o r t h e f o l l o w i n g r e a d y -t o -e a t f o o d s : — t h o s e w h i c h h a v e r e c e i v e d h e a t t r e a t m e n t o r o t h e r p r o c e s s i n g e f f e c t i v e t o e l i m i n a t e L . m o n o c y t o g e n e s , w h e n r e c o n t a m i n a t i o n i s n o t p o s s i b l e a f t e r t h i s t r e a t m e n t (f o r e x a m p l e , p r o d u c t s h e a t t r e a t e d i n t h e i r f i n a l p a c k a g e ), — f r e s h , u n c u t a n d u n p r o c e s s e d v e g e t a b l e s a n d f r u i t s , e x c l u d i n g s p r o u t e d s e e d s , — b r e a d , b i s c u i t s a n d s i m i l a r p r o d u c t s , — b o t t l e d o r p a c k e d w a t e r s , s o f t d r i n k s , b e e r , c i d e r , w i n e , s p i r i t s a n d s i m i l a r p r o d u c t s , — s u g a r , h o n e y a n d c o n f e c t i o n e r y , i n c l u d i n g c o c o a a n d c h o c o l a t e p r o d u c t s , — l i v e b i v a l v e m o l l u s c s . (5 ) T h i s c r i t e r i o n s h a l l a p p l y i f t h e m a n u f a c t u r e r i s a b l e t o d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y , t h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e . T h e o p e r a t o r m a y f i x i n t e r m e d i a t e l i m i t s d u r i n g t h e p r o c e s s t h a t m u s t b e l o w e n o u g h t o g u a r a n t e e t h a t t h e l i m i t o f 100 c f u /g i s n o t e x c e e d e d a t t h e e n d o f s h e l f -l i f e . (6 ) 1 m l o f i n o c u l u m i s p l a t e d o n a P e t r i d i s h o f 140 m m d i a m e t e r o r o n t h r e e P e t r i d i s h e s o f 90 m m d i a m e t e r . (7 ) T h i s c r i t e r i o n s h a l l a p p l y t o p r o d u c t s b e f o r e t h e y h a v e l e f t t h e i m m e d i a t e c o n t r o l o f t h e p r o d u c i n g f o o d b u s i n e s s o p e r a t o r , w h e n h e i s n o t a b l e t o d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y , t h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t o f 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e . (8 ) P r o d u c t s w i t h p H ≤ 4,4 o r a w ≤ 0,92, p r o d u c t s w i t h p H ≤ 5,0 a n d a w≤ 0,94, p r o d u c t s w i t h a s h e l f -l i f e o f l e s s t h a n f i v e d a y s s h a l l b e a u t o m a t i c a l l y c o n s i d e r e d t o b e l o n g t o t h i s c a t e g o r y . O t h e r c a t e g o r i e s o f p r o d u c t s c a n a l s o b e l o n g t o t h i s c a t e g o r y , s u b j e c t t o s c i e n t i f i c j u s t i f i c a t i o n . ( 9 ) T h i s c r i t e r i o n s h a l l a p p l y t o m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ) p r o d u c e d w i t h t h e t e c h n i q u e s r e f e r r e d t o i n p a r a g r a p h 3 o f C h a p t e r I I I o f S e c t i o n V o f A n n e x I I I t o R e g u l a t i o n (E C ) N o 853/2004 o f t h e E u r o p e a n P a r l i a m e n t a n d o f t h e C o u n c i l . ( 10 ) E x c l u d i n g p r o d u c t s w h e n t h e m a n u f a c t u r e r c a n d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t i e s t h a t , d u e t o t h e r i p e n i n g t i m e a n d a wo f t h e p r o d u c t w h e r e a p p r o p r i a t e , t h e r e i s n o s a l m o n e l l a r i s k . ( 11 ) O n l y i c e c r e a m s c o n t a i n i n g m i l k i n g r e d i e n t s . ( 12 ) P r e l i m i n a r y t e s t i n g o f t h e b a t c h o f s e e d s b e f o r e s t a r t i n g t h e s p r o u t i n g p r o c e s s o r t h e s a m p l i n g m u s t b e c a r r i e d o u t a t t h e s t a g e w h e r e t h e h i g h e s t p r o b a b i l i t y o f f i n d i n g S a l m o n e l l a i s e x p e c t e d . ( 13 ) R e f e r e n c e : C o m m u n i t y r e f e r e n c e l a b o r a t o r y f o r c o a g u l a s e p o s i t i v e s t a p h y l o c o c c i . E u r o p e a n s c r e e n i n g m e t h o d f o r t h e d e t e c t i o n o f s t a p h y l o c o c c a l e n t e r o t o x i n s i n m i l k a n d m i l k p r o d u c t s . ( 14 ) P a r a l l e l t e s t i n g f o r E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i s h a l l b e c o n d u c t e d , u n l e s s a c o r r e l a t i o n b e t w e e n t h e s e m i c r o -o r g a n i s m s h a s b e e n e s t a b l i s h e d a t a n i n d i v i d u a l p l a n t l e v e l . I f E n t e r o b a c t e r i a c e a e a r e d e t e c t e d i n a n y o f t h e p r o d u c t s a m p l e s t e s t e d i n s u c h a p l a n t , t h e b a t c h m u s t b e t e s t e d f o r E . s a k a z a k i i . I t s h a l l b e t h e r e s p o n s i b i l i t y o f t h e m a n u f a c t u r e r t o d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y w h e t h e r s u c h a c o r r e l a t i o n e x i s t s b e t w e e n E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i . ( 15 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r o f f a e c a l c o n t a m i n a t i o n . ( 16 ) A p o o l e d s a m p l e c o m p r i s i n g a m i n i m u m o f 10 i n d i v i d u a l a n i m a l s . ( 17 ) P a r t i c u l a r l y f i s h s p e c i e s o f t h e f a m i l i e s : S c o m b r i d a e , C l u p e i d a e , E n g r a u l i d a e , C o r y f e n i d a e , P o m a t o m i d a e , S c o m b r e s o s i d a e . ( 18 ) S i n g l e s a m p l e s m a y b e t a k e n a t r e t a i l l e v e l . I n s u c h a c a s e t h e p r e s u m p t i o n l a i d d o w n i n A r t i c l e 14(6) o f R e g u l a t i o n (E C ) N o 178/2002, a c c o r d i n g t o w h i c h t h e w h o l e b a t c h i s t o b e d e e m e d u n s a f e , s h a l l n o t a p p l y . ( 19 ) R e f e r e n c e s : 1 . M a l l e P ., V a l l e M ., B o u q u e l e t S . A s s a y o f b i o g e n i c a m i n e s i n v o l v e d i n f i s h d e c o m p o s i t i o n . J . A O A C I n t e r n a t . 1996, 79, 43-49. 2. D u f l o s G ., D e r v i n C . , M a l l e P ., B o u q u e l e t S . R e l e v a n c e o f m a t r i x e f f e c t i n d e t e r m i n a t i o n o f b i o g e n i c a m i n e s i n p l a i c e ( P l e u r o n e c t e s p l a t e s s a ) a n d w h i t i n g ( M e r l a n g u s m e r l a n g u s . J . A O A C I n t e r n a t . 1999, 82, 1097-1101.7.12.2007ENOfficial Journal of the European UnionL 322/19I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d , e x c l u d i n g l i v e b i v a l v e m o l l u s c s a n d l i v e e c h i n o d e r m s , t u n i c a t e s a n d g a s t r o p o d s i n r e l a t i o n t o t e s t i n g E . c o l i , w h e r e t h e l i m i t r e f e r s t o a p o o l e d s a m p l e .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e b a t c h t e s t e d ( 1 ).L . m o n o c y t o g e n e s i n r e a d y -t o -e a t f o o d s i n t e n d e d f o r i n f a n t s a n d f o r s p e c i a l m e d i c a l p u r p o s e s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .L . m o n o c y t o g e n e s i n r e a d y -t o -e a t f o o d s a b l e t o s u p p o r t t h e g r o w t h o f L . m o n o c y t o g e n e s b e f o r e t h e f o o d h a s l e f t t h e i m m e d i a t e c o n t r o l o f t h e p r o d u c i n g f o o d b u s i n e s s o p e r a t o r w h e n h e i s n o t a b l e t o d e m o n s t r a t et h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t o f 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .L . m o n o c y t o g e n e s i n o t h e r r e a d y -t o -e a t f o o d s a n d E . c o l i i n l i v e b i v a l v e m o l l u s c s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ t h e l i m i t ,— u n s a t i s f a c t o r y , i f a n y o f t h e v a l u e s a r e > t h e l i m i t .S a l m o n e l l a i n d i f f e r e n t f o o d c a t e g o r i e s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .S t a p h y l o c o c c a l e n t e r o t o x i n s i n d a i r y p r o d u c t s :— s a t i s f a c t o r y , i f i n a l l t h e s a m p l e u n i t s t h e e n t e r o t o x i n s a r e n o t d e t e c t e d ,— u n s a t i s f a c t o r y , i f t h e e n t e r o t o x i n s a r e d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .E n t e r o b a c t e r s a k a z a k i i i n d r i e d i n f a n t f o r m u l a e a n d d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w 6 m o n t h s o f a g e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .H i s t a m i n e i n f i s h e r y p r o d u c t s f r o m f i s h s p e c i e s a s s o c i a t e d w i t h a h i g h a m o u n t o f h i s t i d i n e :— s a t i s f a c t o r y , i f t h e f o l l o w i n g r e q u i r e m e n t s a r e f u l f i l l e d :1. t h e m e a n v a l u e o b s e r v e d i s ≤ m2. a m a x i m u m o f c /n v a l u e s o b s e r v e d a r e b e t w e e n m a n d M3. n o v a l u e s o b s e r v e d e x c e e d t h e l i m i t o f M ,— u n s a t i s f a c t o r y , i f t h e m e a n v a l u e o b s e r v e d e x c e e d s m o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M o r o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M .___________( 1 ) T h e t e s t r e s u l t s m a y b e u s e d a l s o f o r d e m o n s t r a t i n g t h e e f f e c t i v e n e s s o f t h e h a z a r d a n a l y s i s a n d c r i t i c a l c o n t r o l p o i n t p r i n c i p l e s o r g o o d h y g i e n e p r o c e d u r e o f t h e p r o c e s s .L 322/20ENOfficial Journal of the European Union7.12.2007C h a p t e r 2. P r o c e s s h y g i e n e c r i t e r i a2.1 M e a t a n d p r o d u c t s t h e r e o fr f r f r f r f r s f r f f f r f f f7.12.2007ENOfficial Journal of the European UnionL 322/21f f f f f ( 2 ) F o r p o i n t s 2.1.3-2.1.5 m = M . ( 3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . ( 4 ) T h e l i m i t s (m a n d M ) s h a l l a p p l y o n l y t o s a m p l e s t a k e n b y t h e d e s t r u c t i v e m e t h o d . T h e d a i l y m e a n l og sh a l l b e c a l c u l a t e d b y fi r s t t a k i n g a l o g v a l u e o f e a c h i n d i v i d u a l t e s t r e s u l t a n d t h e n c a l c u l a t i n g t h e m e a n o f t h e s e l o g v a l u e s . ( 5 ) T h e 50 s a m p l e s s h a l l b e d e r i v e d f r o m 10 c o n s e c u t i v e s a m p l i n g s e s s i o n s i n a c c o r d a n c e w i t h t h e s a m p l i n g r u l e s a n d f r e q u e n c i e s l a i d d o w n i n t h i s R e g u l a t i o n . ( 6 ) T h e n u m b e r o f s a m p l e s w h e r e t h e p r e s e n c e o f s a l m o n e l l a i s d e t e c t e d . T h e c v a l u e i s s u bj e c t t o r e v i e w i n o r d e r t o t ak e i n t o a c c o u n t t h e p r o g r e s s m a d e i n r e d u c i n g t h e s alm on e l l a p r e v a l e n c e . M e m b e r S t a t e so r r e g i o n s h a v i n g l o w s a l m o n e l l ap r e v a l e n c e m a y u s e l o w e r c v a l u e s e v e n b e f o r e t h e r e v i e w . ( 7 ) T h i s c r i t e r i o n s h a l l n o t a p p l y t o m i n c e d m e a t p r o d u c e d a t r e t a i l l e v e l w h e n t h e s h e l f -l i f e o f t h e p r o d u c t i s l e s s t h e n 24 h o u r s . ( 8 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r o f f a e c a l c o n t a m i n a t i o n . ( 9 ) T h e s e c r i t e r i a a p p l y t o m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ) p r o d u c e d w i t h t h e t e c h n iq u e sr e f e r r e d t o i n p a r a g r a p h 3 o f C h a p t e r I I I o f S e c t i o n V o f A n n e x I I I t o R e g u l a t i o n (E C ) N o 853/2004 o f t h e E u r o p e a n P a r l i a m e n t a n d o f t h e C o u n c i l .L 322/22ENOfficial Journal of the European Union7.12.2007I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d , e x c l u d i n g t e s t i n g o f c a r c a s e s w h e r e t h e l i m i t s r e f e r t o p o o l e d s a m p l e s .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e p r o c e s s t e s t e d .E n t e r o b a c t e r i a c e a e a n d a e r o b i c c o l o n y c o u n t i n c a r c a s e s o f c a t t l e , s h e e p , g o a t s , h o r s e s a n d p i g s :— s a t i s f a c t o r y , i f t h e d a i l y m e a n l o g i s ≤ m ,— a c c e p t a b l e , i f t h e d a i l y m e a n l o g i s b e t w e e n m a n d M ,— u n s a t i s f a c t o r y , i f t h e d a i l y m e a n l o g i s > M. S a l m o n e l l a i n c a r c a s e s :— s a t i s f a c t o r y , i f t h e p r e s e n c e o f S a l m o n e l l a i s d e t e c t e d i n a m a x i m u m o f c /n s a m p l e s ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f S a l m o n e l l a i s d e t e c t e d i n m o r e t h a n c /n s a m p l e s .A f t e r e a c h s a m p l i n g s e s s i o n , t h e r e s u l t s o f t h e l a s t t e n s a m p l i n g s e s s i o n s s h a l l b e a s s e s s e d i n o r d e r t o o b t a i n t h e n n u m b e r o f s a m p l e s .E . c o l i a n d a e r o b i c c o l o n y c o u n t i n m i n c e d m e a t , m e a t p r e p a r a t i o n s a n d m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ):— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .7.12.2007ENOfficial Journal of the European UnionL 322/232.2 M i l k a n d d a i r y p r o d u c t sf s f s d --L 322/24ENOfficial Journal of the European Union7.12.2007f l- 1 ( 2 ) F o r p o i n t s 2.2.7, 2.2.9 a n d 2.2.10 m = M . ( 3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . ( 4 ) T h e c r i t e r i o n s h a l l n o t a p p l y t o p r o d u c t s i n t e n d e d f o r f u r t h e r p r o c e s s i n g i n t h e f o o d i n d u s t r y . ( 5 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r f o r t h e l e v e l o f h y g i e n e . ( 6 ) F o r c h e e s e s w h i c h a r e n o t a b l e t o s u p p o r t t h e g r o w t h o f E . c o l i , t h e E . c o l i c o u n t i s u s u a l l y t h e h i g h e s t a t t h e b e g i n n i n g o f t h e r i p e n i n g p e r i o d , a n d f o r c h e e s e s w h i c h a r e a b l e t o s u p p o r t t h e g r o w t h o f E . c o l i , i t i s n o r m a l l y a t t h e e n d o f t h e r i p e n i n g p e r i o d . ( 7 ) E x c l u d i n g c h e e s e s w h e r e t h e m a n u f a c t u r e r c a n d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t i e s , t h a t t h e p r o d u c t d o e s n o t p o s e a r i s k o f s t a p h y l o c o c c a l e n t e r o t o x i n s . ( 8 ) O n l y i c e c r e a m s c o n t a i n i n g m i l k i n g r e d i e n t s . ( 9 ) P a r a l l e l t e s t i n g f o r E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i s h a l l b e c o n d u c t e d , u n l e s s a c o r r e l a t i o n b e t w e e n t h e s e m i c r o -o r g a n i s m s h a s b e e n e s t a b l i s h e d a t a n i n d i v i d u a l p l a n t l e v e l . I f E n t e r o b a c t e r i a c e a e a r e d e t e c t e d i n a n y o f t h e p r o d u c t s a m p l e s t e s t e d i n s u c h a p l a n t , t h e b a t c h h a s t o b e t e s t e d f o r E . s a k a z a k i i . I t s h a l l b e t h e r e s p o n s i b i l i t y o f t h e m a n u f a c t u r e r t o d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y w h e t h e r s u c h a c o r r e l a t i o n e x i s t s b e t w e e n E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i . ( 10 ) 1 m l o f i n o c u l u m i s p l a t e d o n a P e t r i d i s h o f 140 m m d i a m e t e r o r o n t h r e e P e t r i d i s h e s o f 90 m m d i a m e t e r .7.12.2007ENOfficial Journal of the European UnionL 322/25I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e p r o c e s s t e s t e d .E n t e r o b a c t e r i a c e a e i n d r i e d i n f a n t f o r m u l a e , d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w s i x m o n t h s o f a g e a n d d r i e d f o l l o w -o n f o r m u l a e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .E . c o l i , E n t e r o b a c t e r i a c e a e (o t h e r f o o d c a t e g o r i e s ) a n d c o a g u l a s e -p o s i t i v e s t a p h y l o c o c c i :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .P r e s u m p t i v e B a c i l l u s c e r e u s i n d r i e d i n f a n t f o r m u l a e a n d d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w s i x m o n t h s o f a g e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .。
