化学专业英语之有机金属化合物——金属配合物
金属有机配合物ppt

✓ -配体:烯烃, 炔烃, 苯, 环戊二烯基, 其 他芳香烃。
• -配合物与-酸配合物有着根本区别:
• -配合物:配体的给予和反馈都通过配体的()轨道 的利用来完成的。
• -酸配合物:配体是利用轨道向金属方面成键, 并且通过-轨道表现出它们的-酸度。
第二节 金属羰基配合物
5-2-1金属羰基配合物的特点
2、按配体分类
(1)、配体的电子表来对金属有机化合物进行分类,如-配合 物,-酸配合物, -配合物
(2)、根据配体的名称加以分类,如金属羰基化合物,烯烃配 合物,炔烃配合物,环戊二烯类配合物(金属茂—夹心化合物) 等。
严格的区分是无意义的,如下例:
4-1-4 金属有机配合物分类
➢-配合物 ➢-酸配合物 ➢-配合物
➢ 金属羰基配合物是由过渡金属与配位体CO所形成的一类配合 物。O之间的化学键很强。如在Ni(CO)4中,Ni-C键能为 147 kJ·mol-1,这个键能值差不多与I-I键能(150 kJ·mol- 1)和C-O单键键能(142 kJ·mol-1)值相差不多。 ②中心原子总是呈现较低的氧化态(通常为O,有时也呈较低的 正氧化态或负氧化态)。氧化态低使得有可能电子占满d-MO, 从而使M→L的电子转移成为可能。 ③大多数配合物都服从有效原子序数规则。
2. 重要转折
1951年T.J.Kealy, P.L. Panson合成了二茂铁Fe(C5H5) 2 1952年 E.D. Fischer, G. Wilkinson同时确定二茂铁-夹心 结构(1973年,Nobel 奖) 1954 年 K. Ziegler , G. Natta 研 究 确 立 了 Ziegler 催 化 剂 (Et3Al-TiCl4)对烯烃立体定向聚合的催化,导致烯烃聚合的工业 化生产,广泛应用烷基铝作烷基化试剂和金属配合物的还原剂。 (1963, Nobel奖) 2000 年 Alan J. Heeger, Alan G. MacDiarmid, Hideki Shirakawa因Ziegler-Natta催化合成导电高分子——聚乙炔而获 得诺贝尔奖。
史上最全——高分子材料与工程专业英语词汇大全
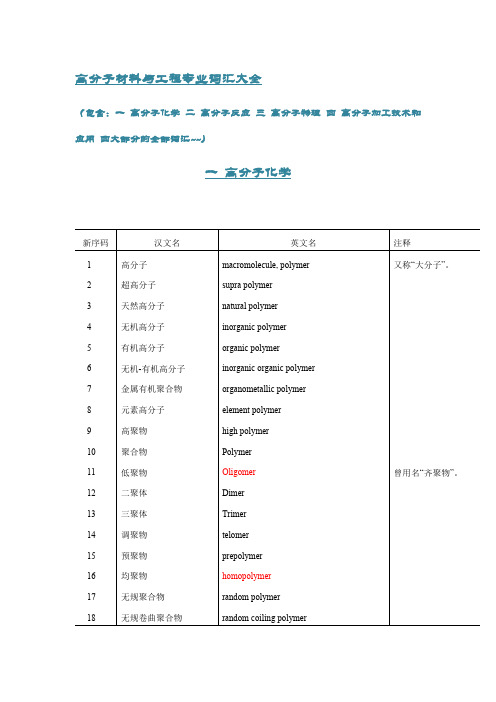
高分子材料与工程专业词汇大全(包含:一高分子化学二高分子反应三高分子物理四高分子加工技术和应用四大部分的全部词汇~~)一高分子化学新序码汉文名英文名注释1高分子macromolecule, polymer又称“大分子”。
2超高分子supra polymer3天然高分子natural polymer4无机高分子inorganic polymer5有机高分子organic polymer6无机-有机高分子inorganic organic polymer7金属有机聚合物organometallic polymer8元素高分子element polymer9高聚物high polymer10聚合物Polymer11低聚物Oligomer曾用名“齐聚物”。
12二聚体Dimer13三聚体Trimer14调聚物telomer15预聚物prepolymer16均聚物homopolymer17无规聚合物random polymer18无规卷曲聚合物random coiling polymer19头-头聚合物head-to-head polymer20头-尾聚合物head-to-tail polymer21尾-尾聚合物tail-to-tail polymer22反式有规聚合物transtactic polymer23顺式有规聚合物cistactic polymer24规整聚合物regular polymer25非规整聚合物irregular polymer26无规立构聚合物atactic polymer27全同立构聚合物isotactic polymer又称“等规聚合物”。
28间同立构聚合物syndiotactic polymer又称“间规聚合物”。
29杂同立构聚合物heterotactic polymer又称“异规聚合物”。
30有规立构聚合物stereoregular polymer, tactic polymer 又称“有规聚合物”。
金属有机配合物

7-1 金属茂及其催化不对称合成
环戊二烯的负离子, 即 环戊二烯基叫茂, 记作 Cp-。 20 世纪初 ,Wilkinson 等人发现 Cp- 和 Fe2+ 反应生成 Cp2Fe, 叫铁 茂。
这类金属环戊二烯基化合物, 统称为金属茂。
金属茂化学蓬勃发展起来,开创了近代金属有机 化学的新时代。因此 ,Wilkinson 获得了 1973 年的诺贝尔化学奖。
第七章 金属有机配合物
定义:至少含有一个金属-碳键的化合 物称为金属有机配合物
范畴:无机化学和有机化学的交叉学科
金属有机化学研究的主要内容: 过渡金属和稀土元素配合物
金属有机化合物合成和结构多种多样;促进 了基础化学的发展
在工业、精细有机合成、催化剂、新型功 能材料的开发、生命科学等方面具有重要 意义。
➢ 通过围绕键的旋转而产生的分子中原子或基团在 空间的不同排列方式,称为构象。
➢ 其中较稳定的结构,称为该化合物的构象异构体。 ➢ 上下两个茂旋转,形成一系列构象,相对夹角为构象
角α ➢ α=0°为覆盖型 ➢ α =36°为交错型
绝大多数金属茂是交错型构象。
也发现有覆盖型的 , 如 (Me4Cp)2 Ru。
• Al(Me)3+H2O (MeAlO) n (11)
可作为聚合催化剂的金属茂的类型
(M=Ti,Zr,Hf;X=Cl,Br; R=Me)
大多数是柄型夹心化合物 , 是手性分子。这些分子比较刚 性 ,活性空位 ,手性源位置固定且比较接近。
最大的优点是:
结构一致性 , 从而导致催化活性位置的单一 , 这就保证了 催化聚合物的窄分子量分布,这是金属茂聚合物性能上优 于传统催化剂聚合物的原因。
开环夹心化合物又叫开环金属茂,与金属茂比 较 , 其配位体是戊二烯(7)的负离子-戊二烯
金属有机化学

1954年维蒂希(G.Wittig)发现磷叶立德 与羰基化合物反应生成结构确定的烯烃。
1956年布朗(H.C.Brown)发现了烯烃的 硼氢化反应。 1979年布朗与维蒂希分享诺贝尔化学奖。
1958年齐格勒的学生维尔克(Wilke)发 现镍配合物催化丁二烯的环齐聚反应并第 一次通过分离鉴定反应活性物种来确定反 应机理。他还发现了[CpMo(CO)3]2金属之 间存在共价键,为过渡金属原子簇合物奠 定了基础。
➢Ni-CO是π配位 ➢金属羰基配合物及其衍生物在过渡金属有机化合物
的合成和很多催化反应中都有重要的意义
C Ni O
=
5)金属有机化学是研究金属有机化合物和 类金属有机化合物的化学。 无机化学(欧美)
金属有机化学 有机化学(中国)
实际上处于有机化学与无机化学之间的 一门边缘学科。
二、金属有机化学的发展历史
宝库。现在人们称镁nt)。镁有机化合物同有机 化合物的反应称为格林雅反应(Grignard Reaction)。为此,1912年他获得诺贝尔化学 奖。这是第一个获诺贝尔奖的金属有机化学 家。
1922年:T.Midgley T.A. Boyd Pd(C2H5)4作为汽 油中的抗震剂。
RCH 2CH2CHO+RCH 3CCHO
• 这一反应应称之为氢甲酰化反应,但在工业 界常称作Oxo反应,这是起初误以为是氧化 反应,故称为“Oxonation”或Oxo反应。由这 一过程产生的醇,已习惯地称作Oxo醇。这 个反应是第一个均相催化工业应用的例子。
1951年鲍森(Pauson)和米勒(Miller)分别发现了二茂 铁Fe(C5H5)2。 次年威金森(Wilkinson)等确定了它具有夹 心面包式分子结构及新的化学键理论,激起了化 学家对过渡金属有机化合物研究的热情,大大推动 了过渡金属有机化合物的发展。
史上最全——高分子材料与工程专业英语词汇大全 (1)

多嵌段共聚物
segmented copolymer
54
杂聚物
heteropolymer
55
恒[组]分共聚物
azeotropic copolymer
56
多组分共聚物
multicomponent copolymer
57
单分散聚合物
monodisperse polymer, uniform polymer
20
头-尾聚合物
head-to-tail polymer
21
尾-尾聚合物
tail-to-tail polymer
22
反式有规聚合物
transtactic polymer
23
顺式有规聚合物
cistactic polymer
24
规整聚合物
regular polymer
25
非规整聚合物
irregular polymer
单股聚合物
single-strand polymer
63
双股聚合物
double-strand polymer
64
多股聚合物
multi-strand polymer
65
链型聚合物
chain polymer
66
碳链聚合物
carbon chain polymer
67
杂链聚合物
heterochain polymer
79
柔性链聚合物
flexible chain polymer
80
刚棒高分子
rigid rod polymer
81
棒状高分子
rodlike polymer
82
刚-柔嵌段共聚物
有机过渡金属化合物

• 有效原子序数规则(effective atomic number rule) ——18电子规则 – 过渡有机金属化合物
5
4.2 金属烷基化合物
1. 缺电子有机金属化合物 (聚合)
IA、IIA族: Li, Be, Mg IIIA族: B, Al, Ga 例如: Be(CH3)2 的多聚体
Me Be Me Be Me Me Me Be Me Be Me Me Be
18
⑤ 若形成M-M,则每个金属分别提供一个电子。
Mn2(CO)10 Mn 7 5CO 5×2=10 1Mn-Mn 1×1= 1 7+10+1=18
Co2(CO)8 Co 9 4CO 4×2=8 1Co-Co 1×1=1 9+8+1=18
Co2(CO)8 Co 9 3端CO 3×2=6 2桥CO 2×1=2 1Co-Co 1×1=1 9+6+2+1=18
Si (CH3)4
Me
Si Me Si Me
Me
CH3 (CH3 )3Si Si CH3 Si(CH3)3
Sin(CH3)2n+2
Si4(CH3)8
n
9
3.富电子有机金属化合物
: MR3 (Lewis 碱)
As
CH3 CH3 CH3
MR5
Ph Ph As Ph Ph Ph
As(CH 3)2 As(CH 3)2
23
单核羰基化合物
V(CO)6 ( Oh ) Cr(CO)6 Mo(CO)6 W(CO)6 ( Oh ) Fe(CO) 5 Ru(CO)5 ( D3h ) Ni(CO)4 ( Td ) 17e (不符合EAN规则) 18e 18e 18e
CH3Mn(CO)5 ( C4v )
有机金属配合物

8-4-2、插入反应(insertion) 或 迁移反应(migration)
CO R O M C R
M
8-4-3、发生在配体上的亲核进攻
8-4-4、氧化和还原
8-4-5、氧化加成和还原消除
8-4-5-1 氧化加成 (配位不饱和) 一般说来,氧化加成反应形式如下:
LnM
+
X Y
LnM
18e
7-7-1-2 金属羰基化合物成键的表征:
分子振动光谱( 红外, 拉曼)
反馈键强, CO的 轨道的电子云密度增大
则C O间的键级减弱
力常数减小
振动频率减低
结论: 中心M 的电子云密度越大, 或者给电子能力越强, 反馈键越强, CO越小
7-7-1-3 金属羰基化合物中 三种典型的CO配位方式:
Ln M
+
LnM + LnM
+ LnM + E
•
反应物和产物的氧化态都是稳定的
•
反应物中有形成新键的空位置
• 下列化合物能否进行氧化加成反应? TiR4 WR6
(3). 已经是18电子构型的,氧化加成时排除一个配体
例:
Ru(CO)4(PMe3) + CH3I Ru(CH3)(I)(CO)3 (PMe3) + CO 18e ( 0 ) 18e(+2)
+
(+2)
Cl
-
N N
Pt
R Cl
+
R Cl
H消除 ( 氧化数不变, 配位数不变)
agostic H
LnM C C H R C C LnM H
R
LnM H
+
金属有机知识点

金属有机化合物(Metal-Organic Compounds)1.什么是金属有机化合物?金属有机化合物是指含有金属原子与有机基团结合的化合物。
它们具有独特的结构和性质,广泛应用于材料科学、金属催化、药物、电子器件等领域。
2.金属有机化合物的合成方法金属有机化合物的合成方法多种多样,主要包括以下几种:a.直接合成法:将金属与有机配体在适当的条件下反应,生成金属有机化合物。
b.双相法:利用有机溶剂与水或气体形成两相体系,使金属与有机配体在界面上反应生成金属有机化合物。
c.气相法:通过将金属蒸气和有机物蒸气混合,使其在适当的条件下反应生成金属有机化合物。
3.金属有机化合物的性质与应用金属有机化合物具有多样的性质和应用,以下是其中几个重要的知识点:a.具有催化活性:金属有机化合物常用于金属催化反应,如烯烃的氢化、氢气的加氢等。
通过调节金属有机化合物的配体结构和金属中心的性质,可以调控催化反应的活性和选择性。
b.具有光电性能:金属有机化合物在光电器件中具有广泛的应用,如有机光电转换器件、有机发光二极管等。
通过调整有机配体结构和金属中心的性质,可以调节金属有机化合物的光谱性质和电荷转移能力。
c.具有药物活性:金属有机化合物在药物领域中具有重要的应用潜力。
例如,铂类化合物是一类重要的抗癌药物,可与DNA结合形成DNA加成物,从而抑制癌细胞的分裂和生长。
4.金属有机化合物的前景和挑战金属有机化合物的研究在过去几十年取得了很大的进展,但仍面临着一些挑战。
其中一些包括:a.金属有机化合物的合成方法仍然复杂和低效,需要更高效的合成策略。
b.部分金属有机化合物对空气和水敏感,需要找到更稳定的结构和材料。
c.对金属有机化合物的性质和反应机理理解仍不完全,需要进一步的研究和探索。
总结:金属有机化合物是一类具有独特结构和性质的化合物,具有广泛的应用前景。
通过不同合成方法得到的金属有机化合物具有催化、光电和药物活性等重要性质。
化学专业英语(竞赛)

化学专业英语一、无机化学1. periodic table 元素周期表element 元素metal 金属nonmetal 非金属transition metal 过渡金属group / family 族alkali metal 碱金属alkaline earth metal 碱土金属chalcogen 氮族元素halogen 卤素noble gas 稀有气体period 周期lanthanide 镧系元素actinide 锕系元素block 区s-block s区(H、He、碱金属、碱土金属)p-block p区(IIIA~VIIA族、稀有气体(He除外))d-block d区(过渡金属)f-block f区(镧系元素、锕系元素)2. electron configuration 电子排布,电子构型electron shell 电子层shell (电子)层subshell (电子)亚层atomic orbital 原子轨道structure 结构molecule 分子molecular 分子的atom 原子atomic nucleus 原子核electron 电子electron cloud 电子云ion 离子anion /ˈæn.aɪ.ən/ 阴离子cation /ˈkæt.aɪ.ən/ 阳离子3. quantum number 量子数principal quantum number 主量子数(n)1≤nazimuthal quantum number 角量子数(ℓ)0≤ℓ≤n-1magnetic quantum number 磁量子数(m)- ℓ≤m ≤ℓspin quantum number 自旋量子数(s或m s)±1/2Pauli exclusion principle(泡利不相容原理):Two electrons cannot occupy the same quantum state within a quantum system simultaneously.Hund’s principle / Hund’s rule(洪特规则):If two orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs.4. chemical bond 化学键ionic bond 离子键ionization energy 电离能electron affinity 电子亲和能ionic polarization 离子极化dipole 偶极covalent bond 共价键metallic bond 金属键(=metallic bonding)intermolecular force 分子间作用力van der Waals force 范德华力5. Lewis structure 路易斯结构lone pairs 孤电子对,孤对电子valence electron 价电子single bond 单键multiple bond 多重键(double bond 双键,triple bond 三键)6. chemical reaction 化学反应四种基本反应类型(four basic types):combination reaction 化合反应 C + O2= C O2decomposition reaction 分解反应Cu(OH)2 = CuO + H2Odisplacement reaction (single displacement reaction) 置换反应Fe + CuSO4 = FeSO4 + Cumetathesis reaction (double displacement reaction) 复分解反应AgNO3 + NH4I = NH4NO3+ AgI↓precipitation 沉淀(作用)precipitate 沉淀物其它反应:reduction-oxidation reaction (=redox reaction) 氧化还原反应oxidation 氧化reduction 还原combustion 燃烧(=burning)stoichiometry /ˌstɔɪkiˈɒmɪtri/ 化学计量stoichiometric ration 化学计量比reactivity series of metals / activity series of metals 金属活动性顺序standard electrode potential 标准电极电势(符号Eθ)chemical stability 化学稳定性acid-base reaction 酸碱(中和)反应conjugated acid 共轭酸conjugated base 共轭碱Lewis acid 路易斯酸Lewis base 路易斯碱Brønsted acid Brønsted酸Brønsted base Brønsted碱7. solution 溶液solute 溶质solvent 溶剂concentration 浓度concentrated 浓的dilute 稀的molality 质量摩尔浓度(mol溶质/kg溶剂) mole fraction 摩尔分数mass fraction 质量分数mass concentration 质量浓度(kg/m3)osmotic pressure 渗透压molar concentration 摩尔浓度(mol/L)solubility 溶解度solubility product 溶度积(K sp)soluble 可溶的slightly soluble 微溶的insoluble 难溶的,不溶的solvation 溶剂化作用solvate 溶剂合物(如CaCl2·C2H5OH)hydration 水合作用hydrate 水合物(如CuSO4·5H2O)hemihydrate 半水合物monohydrate 一水合物dihydrate 二水合物(tri- 3, tetra- 4, penta- 5, hexa- 6, hepta- 7, octa- 8, nona- 9, deca- 10, undeca- 11, dodeca- 12)8. compound (=chemical compound) 化合物inorganic compound 无机化合物organic compound 有机化合物nomenclature /nəˈmenklətʃə(r)/ 命名法chemical formula 化学式empirical formula 实验式,简式structural formula 结构式molecular formula 分子式macromolecule 高分子polymer 聚合物coordination complex 配合物,络合物元素名以ium(或um)结尾的,去掉后缀,某化物加ide,如硫化钠sodium sulfide。
高分子专业英语

高分子专业英语词汇英汉对照关键词:英语高分子词汇英汉对照序号中文英文1 高分子 macromolecule, polymer 又称"大分子"。
2 超高分子 supra polymer3 天然高分子 natural polymer4 无机高分子 inorganic polymer5 有机高分子 organic polymer6 无机-有机高分子 inorganic organic polymer7 金属有机聚合物 organometallic polymer8 元素高分子 element polymer9 高聚物 high polymer10 聚合物 polymer11 低聚物 oligomer 曾用名"齐聚物"。
12 二聚体 dimer13 三聚体 trimer14 调聚物 telomer15 预聚物 prepolymer16 均聚物 homopolymer17 无规聚合物 random polymer18 无规卷曲聚合物 random coiling polymer19 头-头聚合物 head-to-head polymer20 头-尾聚合物 head-to-tail polymer21 尾-尾聚合物 tail-to-tail polymer22 反式有规聚合物 transtactic polymer23 顺式有规聚合物 cistactic polymer24 规整聚合物 regular polymer25 非规整聚合物 irregular polymer26 无规立构聚合物 atactic polymer27 全同立构聚合物 isotactic polymer 又称"等规聚合物"。
28 间同立构聚合物 syndiotactic polymer 又称"间规聚合物"。
29 杂同立构聚合物 heterotactic polymer 又称"异规聚合物"。
有机金属化合物

氰(CN-)作为配体的配合物,金属碳化物等虽然也含有M-C键,但与金属有机化合物性质差别较大,因此不 属于金属有机化合物。
根据《IUPAC有机化学命名原则》的规定,通常包含1-12族的金属的有机金属化合物属于无机物;而不包含 这些元素,且可以用《IUPAC有机化学命名原则》命名的有机金属化合物属于有机物。
化合物简介
有机金属化合物在生产和生活中用途广泛。例如,在制备高分子化合物时,常用烷基铝作催化剂,在有机合 成中,有机金属化合物可提供碳负离子、自由基和卡宾等活泼中间体,因此有机金属化合物是一类极为有用的合 成试剂。近年来由于多种有机金属化合物在生物活性研究的推广和应用,使有机金属化合物的生物活性和药理作 用的研究和应用日益深入发展。因此,掌握一些有机金属化合物的基础知识是必要的。
生物体内存在的金属离子常常是利用氮、氧等电负性大的原子,通过配位键与有机分子结合。这些化合物并 不属于有机金属化合物。但是,过渡金属与有机化合物生成的碳一金属配位体的研究使有机金属化合物与有机金 属配合物之间的距离日益缩小。酶分子中的金属离子对维持生物大分子构象,酶催化功能极为重要。例如锌离子 是碳酸酐酶的组成部分,血红蛋白体内运载氧的功能也是通过血红素中铁离子与氧可逆结合来完成的。
有机锌化合物通式为R2Zn,分子中都含有直线型C—Zn—C骨架。 二甲基锌[(CH3)2Zn]常温下为具有挥发性的液体,沸点44℃,其化学性质不如有机锂化合物活泼。 二甲基锌常用作聚合反应的催化剂,如作各种烯烃单体和羰基化聚合时的引发剂,也是齐格勒一纳塔复合催 化剂的组成成分。在有机锌作为引发剂的情况下,聚合速度和聚合物的分子量随复合催化剂物类型
金属有机配合物和mof 关系

金属有机配合物和mof 关系金属有机配合物(Metal-Organic Frameworks,MOFs)是一类由金属离子与有机配体组成的晶态材料。
其独特的结构和性质使其在多个领域具有广泛的应用前景。
本文将探讨金属有机配合物与MOFs 之间的关系,以及它们在材料科学和催化领域的应用。
金属有机配合物是由金属离子与有机配体通过配位键结合而成。
金属离子可以是过渡金属、稀土金属等,而有机配体则通常是含有氮、氧、硫等原子的有机化合物。
这种配位作用使得金属离子与有机配体形成稳定的结构,形成了多孔的晶体结构。
MOFs是一种由金属离子与有机配体组成的晶态材料。
由于金属离子与有机配体之间的配位作用,MOFs具有高度有序的孔道结构和大比表面积。
这使得MOFs在气体吸附、储能、分离等方面具有独特的性能。
同时,MOFs还具有可调控的孔道大小和功能化的特点,使其在催化反应中具有重要的应用价值。
金属有机配合物和MOFs之间存在着密切的关系。
金属有机配合物可以作为MOFs的前体,通过合适的合成方法转化为MOFs。
同时,金属有机配合物的结构和性质也为MOFs的设计和合成提供了重要的参考。
通过合理选择金属离子和有机配体的种类和比例,可以调控MOFs的结构和性能,实现对其物理和化学性质的精确控制。
金属有机配合物和MOFs在材料科学和催化领域具有广泛的应用前景。
MOFs的高度有序的孔道结构和大比表面积使其在气体吸附和储能材料中具有潜在的应用价值。
同时,MOFs还可以作为催化剂的载体,通过调控其结构和功能化,实现对催化反应的高效控制。
此外,金属有机配合物和MOFs还可以应用于分子传感、药物释放等领域。
金属有机配合物和MOFs之间存在着密切的关系,它们在材料科学和催化领域具有广泛的应用前景。
通过合理设计和合成金属有机配合物和MOFs,可以实现对其结构和性能的精确控制,从而实现对材料和催化反应的高效调控。
随着对金属有机配合物和MOFs的深入研究,相信它们将为我们带来更多的惊喜和应用价值。
金属有机催化剂

2,6-二羟基嘌呤(黄嘌呤)
黄 嘌 呤 氧 化 酶 的 作 用 机 理 示 意 图
2,6, -
羟基嘌呤(
)
黄嘌呤氧化酶氧化黄嘌呤后的 产物尿酸结构式以及三维示意图
金属有机化合物又称有机金属化合物(metallo-organic compound),是指烷基(包括甲基、乙基、丙基、丁 基等)和芳香基(苯基等)的烃基与金属原子结合形 成的化合物,以及碳元素与金属原子直接结合的物质 σ π 之总称,是一类至少含有一个金属-碳(σ或π)键 的化合物
常见的有机金属配合物举例:
人们发现,如果用适当的方法把酶的“心”(锌 挖去,X-射线分析发现该酶的三维结构几乎没 有发生变化,然而它的催化活性却几点也没有 了。如果将锌重新植入其中,酶的催化活性又 恢复了,可见酶的活性和金属离子有很大的关 系。以下是公认的碳酐酶的催化机理:
碳酐酶的催化反应机制
2,金属酶--黄嘌呤氧化酶 金属酶--黄嘌呤氧化酶 --
三、金属有机催化剂选讲
1,金属酶——碳酐酶 2 2,金属酶——黄嘌呤氧化酶 ——
1,金属酶——碳酐酶 ,金属酶 碳酐酶
问题:在人体中CO 的运输方式有几种? 问题:在人体中CO2的运输方式有几种? 1、物理溶解。每100ml血液只能运输0.3ml的CO2。 2、碳酸氢盐结合方式 CO2在碳酐酶的催化下 与水反应形成碳酸,解离成碳酸氢根与氢。 3、氨基甲酸Hb结合方式 一部分的CO2与Hb结合 生成氨基甲酸Hb。 理论上,与Hb和其他的血浆蛋白结合运输的CO2可 以达到总运输量的30%,然而CO2与蛋白质的结合的 反应速度比与水结合的反应速度小很多,实际的结 合量不超过总量的20%。可见碳酐酶的重要性。
BuLi PhCH2MgBr (AlMe3)2
金属有机配合物

金属有机配合物M0Fs一般都是用溶剂热法合成的,有研究表明,合成的方法不同得到的MOFs的性能有可能不一样。
O.M.Yaghi等采用分步合成和一次性合成制备MOF-500([(Fe304)一(SO4)(BPDC)6(BPE)6]8-[NH2(CH3)2+13H2O·8DMF],BP—DC为4,4二苯二甲酸,BPE为顺式一1,2-二一4一乙烷吡啶)时发现合成的最终产物在吸氢性能上有所差异,这是因为分步合成的中间产物IRMOP-51([(Fe3O4)(SO4)(BPDC)6(PY)12] 8-[NH2一(CH3) 13H2O·8DMF),py为嘧啶]不溶,从而阻碍了MOF- 500的合成。
进一步的研究还发现,制备过程中MOFs暴露在空气或水中的程度不同,其储氢性能也不同。
J.Perles等用溶剂热法合成Sc(BDC)3时用有机酸及其钠盐的混合液为有机配体,并加入O-Phen(邻菲罗啉)来调整溶液的pH值,最后得到了单一相的产物,这可以用来指导我们合成高纯度的MOFs。
目前,已有许多研究者在金属离子调节MOFs孔结构方面做了系统的研究,如日本 Myoudaiji大学分子科学学院的Kumagai等人用2一氨基苯甲酸(2一abaH)分别与MCl2·nH2 0(M=Ca、Sr、Ba)桥联生成配位聚合物[Ca(2-aba)2 (H2O)3]、[{Sr(2-aba)2(H 2O)2}·H2O]和[Ba(2-aba)2(H20)]。
Rogan等人以2,2’-联吡啶 (dipya)作为有机配体。
在对苯二甲酸钠(Na2BDC)溶液中分别与Co(II)、Ni(II)、Cu(Ⅱ)络合,生成立方体的红色晶体Co(BDC)(dipya)·5H2 0、棱镜状的浅蓝色晶体Ni(BDC) (dipya)·5H2O和八面体的绿色晶体Cu(BDC)(dipya)·H2 O。
金属有机化合物的合成化学

Hepto数
• Hepto数(η):用于表示同金属直接结合的原
子数。
2.2 过渡金属有机化合物的合成
碳金属化
• 金属有机化合物 + 烯、炔烃:
M R+ C C
MC C R
• 与M-H对比,M正电性越大,对M-C插入反应越有利。
卡宾插入
• 金属有机化合物 + 卡宾物(Carbene):
PhSiH3 CH 2 N2 hv PhSi(CH3 )H 2 N2 Me2SnCl2 CH2 N2 Me2Sn(CH 2Cl)Cl N2
• 取代反应; • 金属盐 + 烯烃 + 还原剂; • 丁二烯镁转移丁二烯; • 金属原子配体蒸气共凝集; • 炔烃π配合物合成; • 芳烃金属π-配合物Fischer-Hafner合成。
• 环戊二烯(σ,π- 给电子 / 受电子配体)过渡金属配合物:
• 1951年,二茂铁的合成:
2C5H6 Fe 300C Fe(C5H5 )2 H 2
2K 2(C6H5 )3CH 2(C6H5 )3CK H 2
1.4 2族和12族金属有机化合物的 合成
• 铍金属有机化合物:
BeCl2 2(CH3 )3CMgCl [(CH3 )3C]2 Be 2MgCl2 BeCl2 2C5H5Na (C5H5 )2 Be 2NaCl
• B溶e剂R2,化容产易物生。成稳定的溶剂加成物 (Et2O)2BeR2,较难得到非
1.3 碱金属有机化合物的合成
4.1--有机金属化合物

§4-1 金属羰基配合物 §4-2 烯烃、炔烃配合物 烯烃、 §4-3 夹心化合物 §4-4 配位催化
本章要求: 本章要求:
1、了解什么是有机金属化合物,它与金属有机配合物有 、了解什么是有机金属化合物, 何不同; 何不同; 2、熟悉典型化合物的合成方法,能描述典型化合物的主 、熟悉典型化合物的合成方法, 要化学性质; 要化学性质; 3、能熟练运用 规则; 、能熟练运用EAN规则; 规则 4、能准确描述 配合物和 酸配合物成键的特点; 配合物和π–酸配合物成键的特点 、能准确描述π–配合物和 酸配合物成键的特点; 5、能根据分子轨道理论定性解释羰基配合物的成键,并 、能根据分子轨道理论定性解释羰基配合物的成键, 能根据振动光谱数据判断羰基的配位模式; 能根据振动光谱数据判断羰基的配位模式; 6、能正确描述蔡斯盐、二茂铁的立体结构特点; 、能正确描述蔡斯盐、二茂铁的立体结构特点; 7、能根据分子轨道理论定性解释蔡斯盐、二茂铁的成键 、能根据分子轨道理论定性解释蔡斯盐、 特点,并能据此解释其基本特性; 特点,并能据此解释其基本特性; 8、能说出配位催化过程的基本特点。体会 个实例的实际 、能说出配位催化过程的基本特点。体会5个实例的实际 价值,理解已提出的机理。 价值,理解已提出的机理。
第四章 有机金属化合物 Organometallic Compounds
§4-1 金属羰基配合物
金属羰基配合物:CO与金属形成的配合物 金属羰基配合物:CO与金属形成的配合物 简称: 简称:羰合物 1. 概述 最早发现的羰基化合物是Ni(CO) 最早发现的羰基化合物是Ni(CO)4 1890, L. Mond Ni + 4CO
有机金属化合物的应用
第十章 金属配位化合物

硫酸四氨合铜(Ⅱ) 六异硫氰根合铁(Ⅲ)酸钾
六氯合铂(Ⅳ)酸
氢氧化四氨合铜(Ⅱ) 五氯•氨合铂(Ⅳ)酸钾
硝酸羟基•三水合锌(Ⅱ)
(三)氯化五氨•水合钴(Ⅲ) 五羰(基)合铁
三硝基•三氨合钴(Ⅲ)
乙二胺四乙酸根合钙(Ⅱ) 上页 下页 目录 返回
Question 2
命名下列配合物和配离子: (1)(NH4)3[SbCl6]; (2)[Co(en)3]Cl3 (3)[Cr(H2O)4Br2]Br· 2O 2H
上页 下页 目录 返回
10.2.1 价键理论: 外轨络合物和内轨络 合物
这里把第二章的s-p杂化轨道扩大到d轨道上,形 成s-p-d杂化轨道。
(1) 价键理论的要点
● 形成体(M)有空轨道,配位体(L)有孤对电子,形
成配位键 ML
● 形成体(中心离子)采用杂化轨道成键 ● 杂化方式与空间构型有关
上页 下页 目录 返回
6. 螯合物和金属大环配合物
螯合物(Chelate)是多齿配位体以2个或2个以上 配位原子配位于金属原子而形成的一种环状络合物 (环中包含了金属原子)。能用作多齿配体的试剂叫螯 合剂(Chelating agent)。
1,10-菲咯啉与Fe2+形成的螯 合物,其中存在3个五元环
上页 下页 目录 返回
10.4.1
M
n
稳定常数
(1)稳定常数
xL ML
Cu2++4NH3
( n x ) x
C O ( 羰基 )
N O (硝基) 2
H2 O N H3
N CS (异硫氰根)
● 多齿配体: 一个配体中含有多个配位原子
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化学专业英语之有机金属化合物——金属配合物ORGANOMETALLICS—METAL π COMPLEXESMetal π complexes are characterized by a type of direct carbon-to-metal bonding that is not a classical ionic, σ, or πbond . Numerous molecules and ions, e.g., mono- and diolefins, polyenes, arenes, cyclopentadienyl ions, tropylium ions, andπ-allylic ions, can form metal πcomplexes with transition-metal atoms or ions. These are classified as organ metallic complexes, because of their direct carbon-metal bond, and as coordination complexes, because the nature and characteristics of the TT ligands are similar to those in coordination complexes. In 1827, Zeise reported thatethylene reacts with platinum (II ) chloride to form a salt K (C2H4)PtCl3(l),but it was not until after the elucidation of the structure of ferrocene (2) in 1953 that attention was redirected to Ziese's salt, which was the first reported metal π complex.Generally, metal TT complexes can be classified into three main groups; olefin-, cyclopentadienyl-, and arene-metal π complexes; mixed complexes are categorized according to structural or chemical analogies within these groups. Allyl π complexes are designated as olefin πcomplexes in this review. Study of metal πcomplexes has contributed to the elucidation of the mechanisms of Ziegler-Natta polymerization, the oxo reaction, and catalytic hydrogenation, and to the development of the Wacker process which is used for the oxidation of olefins1.The following nomenclature for metal it complexes is used:(1) Organic πligands precede the metal atom. (2)Organic πligands precede inorganic 7t ligands. (3)Inorganic π ligands, e.g., carbonyl or nitrosyls, generally follow the metal atom; halides also follow the metal but precede carbonyls or nitrosyls. (4)A prefix, e.g., di, is preferred rather than bis in describing sandwich-typeπ complexes, e.g., dibenzenechromium.(5) The symbol π can be used preceding a ligand in order to distinguish π-complex bonding from a, ionic, or other bonding. The symbol η(eta or hapto)precedes a ligand and indicates the number of C—M bonds in the ligand.Monoolefins , dienes, polyolefins, and acetylenes serve as ligands to transition metals and form olefin πcomplexes. Typical examples of olefin πcomplexes are monoolefin ligands, e.g., potassium η2-ethyleneplatinum trichloride (1); and cyclopentadienylium. –η3-cycloheptatrienylium molybdenum dicarbonyl (3); diene ligands, eg, η4-butadieneiron tricarbonyl(4 ).Certain of the delocalized π-electron ring systems of aromaticmolecules overlap with dxy and dy3metal orbitals as do the π electronsof alkenes with metal d orbitals2. The following aromatic rings can form π complexes;The C5H5- ,C6H6,and C8HSarenes are the most common in arene K complexesthat are characterized by π-bonded rings alone or π-bonded rings that are associated with one ring and other ligands, eg, halogens, CO, RNC, and R3P. Typical examples are the di-η5-cyclopentadienyl complexes , ie, metallocenes , eg , di-η5-cyclopentadienyliron (2 ). Indi-η4-5-cyclopentadienyliron ,ie, ferrocene, the 6-π-electron system ofthe C5H5- ion is bonded to the metal. Other aromatic ring systems aremono-η5-cyclopentadienylmetal nitrosyl and carbonyl complexes.PropertiesThe π-Complex Bond.Metal πcomplexes are among those that are least satisfactorily described by crystal-field theory (CFT) or valence-bond theory (VBT). The nature of the bonding can be treated more completely and quantitatively by molecular-orbital theory (MOT) or ligand-field theory (LFT). The ligand-field theory originally was advanced as a corrected CFT. The LFT relies on the use of molecular orbitals and often is used interchangeably with the MOT. The usual approach is to use the linear combination of atomic orbitals (LCAO) method. It is assumed that when an electron in a molecule is near a particular nucleus, the molecular wave function is approximately an atomic orbital that is centered at the nucleus. The molecular orbitals are formed by adding or subtracting the appropriate atomic orbitals. For transition metals .the "3d, 4s, and 4p orbitals are the atomic orbitals of interest. The ligands may have σ-and π-valence orbitals. Once the appropriate atomic orbitals have been selected for the metal and ligands, the proper linear combination of valence atomic orbitals is determined for the molecular orbitals. The determination of orbital overlaps that are possible, ie, meet inherent symmetry requirements, is done by application of the principles of group theory. At this point, the procedure becomes arbitrary in that approximate wave functions must be selected for use in the calculations of the overlap integrals and coulomb integrals3. Finally, an arbitrary charge distribution is chosen and the orbital energies and interaction energies are calculated, and a solution of the secular equation for the energies and coefficients of the atomic wave functions can be determined. A new initial charge distribution is repeated until consistent values are obtained.ReactionsMetal πcomplexes react with a wide range of chemical reagents. However, the reactions of the π-olefin-, π-cyclopentadienyl-, andit-arene-metal complexes are distinctly characteristic of each group, πCyclopentadienyl complexes, ie, metallocenes ,exhibit a high degree of aromaticity and undergo many typical aromatic substitution reactions. However, the π arene complexes do not exhibit a discernible degree of aromaticity.Although most physical properties, particularly the structure of metal TT complexes, are interpreted by use of the basic principles of coordination chemistry, these established principles do not explain suitably some reaction anomalies of the different groups of metal π complexes.Olefin πComplexes. Reactions involving olefin x. complexes similarly are characteristic of uncomplexed and complexed olefinic functions. Generally, reactions involving the former are not very different from those observed for free olefins. However, reactions of the latter are altered significantly by π-complex formation. Among the reactions of interest are addition, elimination, and substitution.Cyclopentadienyl πComplexes. The most significant feature of the reactions of π-cyclopentadienyl complexes in general and ferrocene in particular involves their aromatic nature. The resonance stabilization energy for ferrocene is 210 kj/mol(50 kcal/mol). Ferrocene undergoes a large number of typical ionic aromatic substitution reactions, eg, Friedel-Crafts acylation, alkylation, metalation, sulfonation, and aminomethylation.Friedel-Crafts Acylation. The acylation of metallocenes proceeds easily. The equimolar reaction of ferrocene and acetyl chloride in the presence of aluminum chloride yields monoacetylferrocene almostexclusively. When an excess of acetyl chloride and aluminum chloride is used, a mixture of two isomeric diacetylferrocenes is produced. The heteroannular disubstituted derivative 1,1'-diacetylferrocene and the homoannular isomer 1,2-diacetylferrocene are obtained in a ratio of 60:1. The first acetyl group deactivates the π-cyclopentadienyl ligand toward further electrophilic substitution. Thus, the second acetyl group enters the other ring.Sulfonation. Ferrocene can be sulfonated readily by sulfuric acid or cholrosulfonic acid in acetic anhydride to form ferrocenesulfonic acid and heteroannular disulfonic acid, π-Cyclopentadienylrhenium tricarbonyl can be sulfonated with concentrated sulfuric acid in acetic anhydride; the product is isolated as the p-toluidine salt. Formylation. Ferrocene is formylated with N-methylformanilide in the presence of phosphorus oxychloride. This reaction also is characteristic of highly reactive aromatic rings.Arylation. The most significant radical substitution reaction of ferrocene is its reaction with aryl diazonium salts giving an arylation product.Arene-Metal πComplexes.Generally, arene πcomplexes do not undergo the reactions that are characteristic of benzene and its derivatives. However, arene π complexes do undergo a limited number of substitution .addition .expansion, and condensation reactions.UsesCatalysis Involving Metal 7t-Complex Intermediates. Manymetal-catalyzed reactions proceed by way of a substrate metal π-complex intermediate. Commercially, the most-significant of these include the polymerization of ethylene,the hydroformylation of olefins yieldingaldehydes , ie , the oxo process (qv ), and the air oxidation of ethylene-producing acetaldehyde(qv) ,ie ,the Wacker process. Polymerization of Olefins. Ziegler-Natta Process. During the 1950s, ethylene was polymerized using a Ziegler-Natta catalyst, ie, a mixture of transition metal halides, eg, titanium halides, and trialkylaluminum (triethylaluminum commonly is used). The use of trialkylaluminum stimulated research into the use of organ metallic compounds in general. It has been determined that the Ziegler-Natta process involves a metal π-complex intermediate. A plausible mechanism for the polymerization can be formulated by applying typical organometallic and coordination reactions.Oxidation of Olefins. Wacker Process. The oxidation of ethylene exclusively to ace-taldehyde and of other straight-chain olefins to ketones is achieved by the catalytic reaction of ethylene in an aqueous solution by palladium (II) or by oxygen in the presence of palladium( II ) chloride, copper (II)chloride,or iron(III)chloride. Generally, the oxidation of olefins by other metal ions ,eg ,Hg(II) ,Th(III) ,andPb( IV ) ,yields glycol derivatives as well as carbonyl products. The mechanism for the oxidation is postulated to include n-o rearrangements. Addition of Carbon Monoxide. Oxo Reaction. The oxo process has been developed extensively to produce primary alcohols by the reduction of the aldehydes which are formed in the process.Health and Safety FactorsSome metal π complexes are air-sensitive and, therefore, their preparation requires an air-free reaction system. Their toxicity usually is based on the metal; however, organometallic compounds generally exhibit greater toxicities than their corresponding inorganic salts. The alkyl derivatives tend to be more toxic than the aryl complexes, which exhibit toxicities similar to those of the corresponding inorganic compounds.。
