AOAC 2001.03 测定特定食品中的总膳食纤维 包含抗性麦芽糊精 酶重量法和液相色谱法
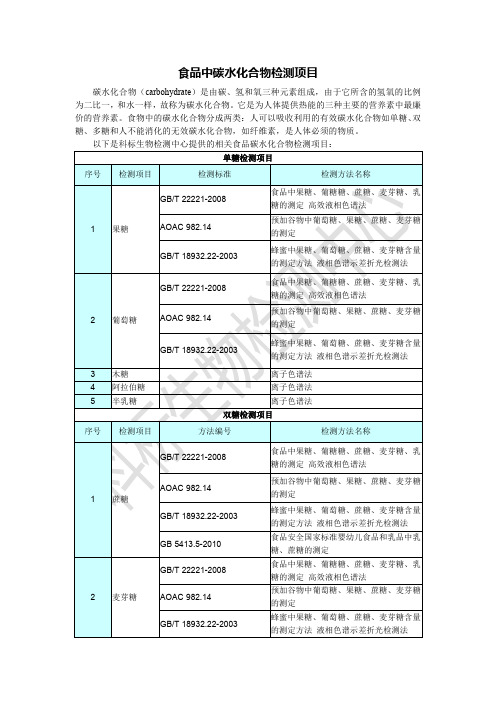
食品中碳水化合物检测项目(科标检测)
GB/T 22221-2008 3 乳糖 GB 5413.5-2010
食品中果糖、葡糖糖、蔗糖、麦芽糖、乳 糖的测定 高效液相色谱法 食品安全国家标准婴幼儿食品和乳品中乳 糖、蔗糖的测定
低聚糖检测项目 序号 检测项目 蔗果三糖 1 低聚果糖 蔗果四糖 蔗果五糖 异麦芽糖 2 低聚异麦芽 异麦芽三糖 糖 潘糖 异麦芽四糖 3 4 5 大豆低聚糖 棉籽糖 水苏糖 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 选定食品产品中反式低聚半乳糖的测定 ——离子色谱法 品中的聚葡萄糖——离子色谱法 检测方法名称
食品中碳水化合物检测项目
碳水化合物(carbohydrate)是由碳、氢和氧三种元素组成,由于它所含的氢氧的比例 为二比一,和水一样,故称为碳水化合物。它是为人体提供热能的三种主要的营养素中最廉 价的营养素。食物中的碳水化合物分成两类:人可以吸收利用的有效碳水化合物如单糖、 双 糖、多糖和人不能消化的无效碳水化合物,如纤维素,是人体必须的物质。 以下是科标生物检测中心提供的相关食品碳水化合物检测项目:
低聚半乳糖 AOAC 2001.02 聚葡萄糖 AOAC 2000.11
膳食纤维检测项目 序号 1 2 3 4 5 检测项目 膳食纤维 总膳食纤维 不溶性膳食纤维 AOAC 991.43 可溶性膳食纤维 膳食纤维 GB/T 22224-2008 方法编号 GB/T 5009.88-2008 检测方法名称 食品中膳食纤维的测定 食品中总膳食纤维、不溶性膳食纤维、可 溶性膳食纤维的测定 酶重量法 MES TRIS 缓冲 食物中的膳食纤维的测定 酶重量法和酶 重量法-液相色谱测定 含有抗性麦芽糊精(简称:RMD)的食物中 6 总膳食纤维 AOAC2001.03 的膳食纤维总量 酶-重量法和液相色谱法 测定 7 8 不溶性膳食纤维 GB 5413.6-2010 不溶性膳食纤维 GB/T 9822-2008 食品安全国家标准婴幼儿食品和乳品中不 溶性膳食纤维的测定 谷物不溶性膳食纤维测定法
aoac标准

aoac标准AOAC标准。
AOAC国际是一个全球性的食品分析标准组织,成立于1884年,总部位于美国马里兰州。
AOAC标准是全球公认的食品分析标准,被广泛应用于食品安全检测、食品质量控制和食品生产过程中。
AOAC标准的制定严格遵循科学、客观、公正、透明的原则,确保标准的可靠性和准确性。
AOAC标准的制定过程经历了多个环节,包括提案、评审、公示、修订等多个步骤。
在提案阶段,任何相关单位和个人都可以向AOAC提出新标准的制定建议,AOAC将对提案进行评估,确定是否具备制定新标准的必要性和可行性。
经过评审通过的提案将进入公示阶段,公示期内,任何单位和个人都可以对提案进行意见反馈。
之后,AOAC将根据公示期内收集的意见对提案进行修订,最终确定新标准的制定方案。
AOAC标准涵盖了食品中的营养成分分析、微生物检测、农药残留检测、重金属检测、食品添加剂检测等多个领域。
在营养成分分析方面,AOAC标准规定了食品中脂肪、蛋白质、碳水化合物、维生素等营养成分的检测方法和标准数值,确保食品标签上的营养成分信息准确无误。
在微生物检测方面,AOAC标准规定了食品中常见的致病菌、霉菌、酵母菌等微生物的检测方法和限量标准,保障食品的微生物安全。
在农药残留检测方面,AOAC标准规定了食品中农药残留的检测方法和安全标准,防止因农药残留导致的食品安全问题。
AOAC标准的制定和应用,对于保障食品安全、促进食品质量提升、维护消费者权益具有重要意义。
通过严格的标准制定和检测方法认证,可以有效防范食品中的各类安全隐患,保障公众健康。
同时,AOAC标准也为食品生产企业提供了科学、规范的质量管理体系,有助于提升企业的竞争力和信誉度。
此外,AOAC标准的国际认可性和权威性,也为全球食品贸易提供了统一的标准基础,促进了国际贸易的顺畅进行。
总之,AOAC标准作为全球食品分析领域的权威标准,对于食品安全、食品质量和国际贸易具有重要意义。
未来,AOAC将继续致力于完善现有标准、制定新的标准,为全球食品行业的可持续发展贡献力量。
【精品】食物中膳食纤维的测定

膳食纤维的测定方法酶-重量法1.原理:样品分别用α-淀粉酶、蛋白酶、葡萄糖苷酶进行酶解消化以去除蛋白质和可消化的淀粉。
总膳食纤维(TDF)是先酶解,然后用乙醇沉淀,再将沉淀物过滤,将TDF残渣用乙醇和丙酮冲洗,干燥称重。
不溶性和可溶性膳食纤维(IDF和SDF)是酶解后将IDF过滤,过滤后的残渣用热水冲洗,经干燥后称重。
SDF是将上述滤出液用4倍量的95%乙醇沉淀,然后再过滤,干燥,称重。
TDF、IDF 和SDF量通过蛋白质、灰分含量进行校正。
2.适用范围AOAC991.43本方法适用于各类植物性食物和保健食品。
3.仪器3.1烧杯:400或600ml高脚型。
3.2过滤用坩埚:玻料滤板,美国试验和材料学会(ASTM)40-60μm,Pyrex60ml(CorningNo.36060buchner,或同等的)。
如下处理:(1)在灰化炉525℃灰化过夜。
炉温降至130℃以下取出坩埚。
(2)用真空装置移出硅藻土和灰质。
(3)室温下用2%清洗溶液浸泡1小时。
(4)用水和去离子水冲洗坩埚;然后用15ml丙酮冲洗然后风干。
(5)在干燥的坩埚中加0.5g硅藻土,在130℃烘干恒重。
(6)在干燥器中冷却1小时,记录坩埚加硅藻土重量,精确至0.1mg。
3.3真空装置:(1)真空泵或抽气机作为控制装置。
(2)1L的厚壁抽滤瓶。
(3)与抽滤瓶相配套的橡皮圈。
3.4振荡水浴箱:(1)自动控温使温度能保持在98±2℃。
(2)恒温控制在60℃。
3.5天平:分析级,精确至±0.1mg。
3.6马福炉:温度控制在525±5℃。
3.7干燥箱:温度控制在105和130±3℃。
3.8干燥器:用二氧化硅或同等的干燥剂。
干燥剂两周一次在130℃烘干过夜。
3.9PH计:注意温控,用pH4.0、7.0和10.0缓冲液标化。
3.10移液管及套头:容量100μl和5ml。
3.11分配器或量筒:(1)15±0.5ml,供分配78%的乙醇,95%的乙醇以及丙酮。
酶重量法-液相色谱法测定食品中总膳食纤维

酶重量法-液相色谱法测定食品中总膳食纤维余枭然;余慧剑【期刊名称】《河南科技》【年(卷),期】2021(40)28【摘要】本研究利用酶重量法-液相色谱法,测定含水溶性膳食纤维食品的总膳食纤维含量。
采用酶重量法测定试样中不溶性膳食纤维(Insoluble Dietary Fiber,IDF)和高分子质量可溶性膳食纤维(Soluble Dietary Fiber,SDF)的含量,采用液相色谱法测定试样的低分子质量抗性麦芽糊精(Resistant Malto Dextrin,RMD)含量。
液相色谱法的方法检出限为0.142%,定量限为0.474%,线性方程为y=1.207x+0.030,相关系数R^(2)为0.999,加标回收率为96.6%~99.7%,相对标准偏差(Relative Standard Deviation,RSD)为1.53%~1.69%(次数n=6)。
精密度试验以酶重量法-液相色谱法测试样品6份,平均含量为65.78%,RSD为0.52%。
实验结果表明,该方法灵敏度、准确率高,适用于添加抗性淀粉、含低分子质量的抗性糊精等水溶性膳食纤维的食品中总膳食纤维的测定。
【总页数】4页(P127-130)【作者】余枭然;余慧剑【作者单位】上海工程技术大学;劲研(上海)生物科技有限公司【正文语种】中文【中图分类】R151.3【相关文献】1.应用酶重量法测定全麦粉的总膳食纤维2.酶——重量法测定食品中的膳食纤维3.应用酶-重量法测定食物中的总膳食纤维4.关于测定粮食中总纤维素的中性净化法和酶重量法的改良5.食品中总的、不溶性及可溶性膳食纤维的酶-重量测定法因版权原因,仅展示原文概要,查看原文内容请购买。
aoac标准

aoac标准
AOAC标准。
AOAC国际是一个专门从事食品分析的非营利性科学组织,成立于1884年,
总部位于美国马里兰州。
AOAC标准是该组织发布的一系列用于食品和环境样品
分析的标准方法,被广泛应用于全球范围内的食品安全监测、质量控制和法规遵从性检验中。
本文将对AOAC标准进行介绍和分析。
首先,AOAC标准的制定是经过严格的科学验证和实践检验的。
这些标准方法
的制定过程需要经过多个实验室的验证和比对,确保其准确性、可重复性和可靠性。
因此,AOAC标准被公认为是行业内的权威标准,被广泛应用于食品和环境样品
的分析检测中。
其次,AOAC标准的内容涵盖了多个方面的分析方法,包括但不限于样品的制备、分离、测定和确认等步骤。
这些方法涵盖了食品中的营养成分、添加剂、农药残留、重金属、微生物污染等多个方面,可以满足不同类型样品的分析需求。
另外,AOAC标准的应用范围非常广泛,不仅可以应用于传统食品,还可以应
用于新型食品、保健食品、农产品和环境样品等多个领域。
这些标准方法的准确性和可靠性为食品行业的质量控制、风险评估和法规遵从性检验提供了有力的支持。
总的来说,AOAC标准是食品分析领域的权威标准,其制定经过严格的科学验
证和实践检验,内容涵盖了多个方面的分析方法,应用范围广泛。
因此,我们可以充分信赖AOAC标准,将其应用于食品和环境样品的分析检测中,以保障食品安
全和环境质量。
食品中膳食纤维的测定

1.1.1.1.1.3食品安全国家标准食品中膳食纤维的测定(征求意见稿)发布实施中华人民共和国卫生部发布前言本标准代替《食品中膳食纤维的测定》。
本标准与相比,主要变化如下:——修改了方法适用范围;——增加了膳食纤维、总膳食纤维、不溶性膳食纤维、可溶性膳食纤维的术语和定义;——修改了试剂顺序和文字格式;——修改了总膳食纤维计算公式;——添加了当食品中含有低分子质量可溶性膳食纤维时总膳食纤维计算方法的注释;——将酶重量法作为第一法,中性洗涤剂法作为第二法。
食品安全国家标准食品中膳食纤维的测定1 范围本标准规定了食品中膳食纤维的测定方法。
本标准酶重量法适用于植物类食品及其制品中总的、可溶性和不溶性膳食纤维的测定;中性洗涤剂法适用于谷物原料中不溶性膳食纤维的测定。
本标准第一法为仲裁法。
2 术语和定义下列术语和定义适用于本标准。
2.1 膳食纤维指植物中天然存在的、提取或合成的、聚合度 的碳水化合物聚合物,不能被人体小肠消化吸收、对人体有健康意义,包括纤维素、半纤维素、木质素、果胶、菊粉及其他一些膳食纤维单体成分等。
2.2 可溶性膳食纤维指能溶于水的膳食纤维部分。
2.3 不溶性膳食纤维指不能溶于水的膳食纤维部分,包括木质素、纤维素、部分半纤维素等。
2.4 总膳食纤维可溶性膳食纤维与不溶性膳食纤维之和。
第一法总的、可溶性和不溶性膳食纤维的测定(酶重量法)3 原理干燥试样经热稳定α淀粉酶、蛋白酶和葡萄糖苷酶酶解消化去除蛋白质和淀粉后,酶解液经乙醇沉淀、过滤,残渣用乙醇和丙酮洗涤,干燥后称重,即为总膳食纤维残渣。
另取同样经酶解的酶解液直接过滤,用热水洗涤残渣,干燥后称重,即得不溶性膳食纤维残渣;滤液用倍体积的乙醇沉淀、过滤、干燥后称重,得可溶性膳食纤维残渣。
扣除残渣中相应的蛋白质、灰分和空白即可计算出试样中总的、不溶性和可溶性膳食纤维的含量。
采用酶重量法测定的总膳食纤维包括不溶性膳食纤维和能被乙醇沉淀的高分子质量可溶性膳食纤维,如纤维素、半纤维素、果胶、其它非淀粉多糖及木质素等;不包括低分子质量的可溶性膳食纤维,如抗性麦芽糊精、果寡糖、低聚半乳糖、多聚葡萄糖等,及部分被加热破坏的抗性淀粉。
AOAC 2001.03 测定特定食品中的总膳食纤维 包含抗性麦芽糊精 酶重量法和液相色谱法

45.4.13AOAC Official Method2001.03Total Dietary Fiber in FoodsContaining Resistant MaltodextrinEnzymatic-Gravimetric Methodand Liquid Chromatography DeterminationFirst Action2001[This method is applicable to resistant maltodextrin(RMD)and to foods containing RMD listed in Table2001.03at 1.4%RMD.] A.PrincipleThis method determines total dietary fiber(TDF)value of pro-cessed foods containing insoluble dietary fiber(IDF)and high mo-lecular weight soluble dietary fiber(HMWSDF),which are precipitated in ethanol and low molecular weight resistant maltodextrin(LMWRMD),which is soluble in ethanol.This method defines dietary fiber(DF)as consisting of nondigestible car-bohydrates having a degree of polymerization with3sugar moieties (DP3)or higher after enzymatic hydrolysis.All the starches con-tained in food are converted to glucose after this enzymatic hydroly-sis.This method to determine TDF content in processed foods containing RMD is a combination of985.29(see45.4.07)for DF and a LC method for LMWRMD.A food is first analyzed for the to-tal quantity of IDF and HMWSDF,precipitated in ethanol,accord-ing to985.29(see45.4.07).Then an LC determination is conducted on the desalted filtrate to obtain the quantity of LMWRMD not pre-cipitated in the78%alcohol preparation.These2values[(IDF+ HMWSDF)+LMWRMD]are summed to obtain the TDF value in the food.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Beakers.—Tall-form,500mL.(c)Water baths.—To maintain a temperature of95–100C and 60C with ability to shake the containers.(d)Filtering crucibles.—Coarse,ASTM,40–60m pore size, Pyrex,50mL.(e)Glass or plastic columns.—To hold ion exchange resins, 75cm15mm id;a shorter(40–75cm15mm id)column can also be used.(f)Liquid chromatograph(LC).—With oven to maintain a col-umn temperature of80C and a20L injection loop.Column oper-ating conditions are:Temperature,80C;mobile phase,distilled water,C(d);flow rate,0.5mL/min.(g)Guard column(or precolumn).—TSK®guard column PWXL,6.0mm id4cm(Tosoh Corp.,distributed by TosoHaas, Montgomeryville,PA,USA;)or equivalent. (h)LC columns.—Two LC columns connected in series, TSK-GEL®G2500PWXL,7.8mm id30cm(Tosoh Corp.),or equivalent.(i)Detector.—Refractive index(RI);maintained at40C. (j)Data integrator or computer.—For peak area measurement. (k)Filters for disposable syringe.—0.2m membrane,13mm. (l)Filters for water.—0.2m,47mm.(m)Filter apparatus.—To hold47mm,0.2m filter,(l);to filter larger volumes of water,C(d).(n)Glass rods.—With fire-polished ends,ca20cm long. (o)Syringes.—10mL,plastic disposable.(p)Pasteur pipet.(q)Volumetric pipet.—10mL.(r)Volumetric flasks.—10,50,250,and1000mL.(s)Top loading balance.—4000g capacity.(t)Tubing.—PVC,2.79mm id(for ion exchange columns). (u)Glass LC syringe.—50L.(v)Teflon scraping rod.—Use in place of glass stirring rod to scrape precipitate from tall-form beaker.(w)Rotary evaporator.—R-3000VW“Student”(Büchi,Swit-zerland;)or equivalent.C.Reagents(a)Ethanol.—95%.Technical grade,used at60C.(b)Ethanol.—78%.Place207mL water in1L volumetric flask and dilute to volume with95%ethanol,(a).(c)Acetone.—Reagent grade.(d)Distilled water.(e)Sodium phosphate dibasic.(f)Sodium phosphate monobasic.(g)Phosphate buffer.—0.08M,pH6.0.Dissolve1.400g Na2HPO4(or1.753g dihydrate)and9.68g NaH2PO4H2O(or10.94g dihydrate)in ca700mL water,(d).Dilute to1L with water,(d),and verify pH with pH meter.(h)Heat stable a-amylase solution(Termamyl).—No.120L(ac-tivity:12units/mg protein;Novo Laboratories,Inc.,59Danbury Rd, Wilton,CT06897,USA),or equivalent(should not contain glycerol).(i)LC retention time standard.—Standard source of the distribu-tion of oligosaccharides(DP$3)in the LMWRMD fraction of RMD, corn syrup solids(DE25;Matsutani Chemical Industry Co.,Ltd.,ItamiTable2001.03Interlaboratory results for the determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatographyFood x,%bs a(b)s r RSD r,%s R RSD R,% Resistant maltodextrin95.368(0) 1.63 1.71 2.37 2.48Hard candy37.997(1)0.58 1.530.68 1.79 Chicken and vegetable soup25.418(0)0.74 2.89 1.18 4.65 Grapefruit juice 1.388(0)0.02 1.330.04 3.20 White bread9.608(0)0.33 3.410.64 6.66 Strawberry Jell-O9.918(0)0.60 6.100.939.39a(b)a=Number of laboratories retained after eliminating outliers;b=number of laboratories removed as outliers.City,Hyogo,Japan;),analyzed by LC(Fig-ure2001.03A)as in D.(j)Protease.—No.P-3910or P-5380(activity:7–15units/mg pro-tein;Sigma Chemical Co.,St.Louis,MO,USA),or equivalent(should not contain glycerol).Prepare protease stock solution just before use by adding100mg protease enzyme to a10mL volumetric flask and bring-ing to volume with water,(d),(amount is sufficient for9test portions in duplicate).(k)Amyloglucosidase.—No.A-9913(activity:400units/mg pro-tein;Sigma Chemical Co.),or equivalent(should not contain glycerol). (l)Celite.—No.C-8656(Sigma Chemical Co.)or No.C-211,acid washed(Fisher Scientific Co.,Fair Lawn,NJ,USA),or equivalent. (m)Mixed-bed ion exchange resins for each test por-tion.—(1)m-1.—25g Amberlite IRA-67(OH-type;Organo Corp., Tokyo,Japan,/organo_corp.htm),or equivalent.(2)m-2.—25g Amberlite200CT(HG)H(H-type;Organo Corp.),or equivalent,are mixed and packed in column for analysis of each test portion.The converted resin should satisfy the following specifications:(a)Total ion exchange capacity:1.74meq/mL (min);(b)Effective ion exchange capacity(R-H exchange capac-ity):1.6meq/mL(min);(c)pH:4–7.Before mixing and packing the 2resins into a column,wash each resin with H2O to obtain a pH value of7–8.8for m-1and4–7for m-2.If Amberlite200CT(HG)H cannot be obtained,Amberlite200(Na-type;Sigma Chemical Co.) or Amberlite200CT(Organo Corp.)can be used by converting it to “H-type”by the following procedure:Fill column(100cm40mm id),B(e),with600mL(500g) Amberlite200“Na-type”resin and determine approximate resin volume.Wash resin with2volumes of water,(d),at the rate of 60mL/min.Pass2volumes of10%HCl(1+3,w/w)through the resin at the rate of60mL/min.Remove HCl with3volumes of water, (d),passed through the resin at the rate of60mL/min.Add3–6vol-umes of additional water,(d),at the rate of120mL/min.The columnis adequately washed of HCl when a pH value of4–7is obtained.(It takes2–3h to charge and rinse these resins.)(n)Sodium hydroxide.—0.275M;reagent grade.Dissolve11.0g NaOH in ca700mL water,(d),in a1L volumetric flask.Dilute to volume with water,(d).(o)Hydrochloric acid.—0.325M;reagent grade.Dilute stock so-lution of known titer,e.g.,325mL1M HCl,to1L with water. (p)Glycerol(LC standard).—10mg/mL.For stock solution: weigh10g glycerol>99.5%purity into a small beaker.Quantita-tively transfer to1L volumetric flask with repeated washes with wa-ter,(d),and dilute to volume.It is important to measure and record the exact weight of the glycerol,weighing as close to10g as possi-ble.Take purity and weight of glycerol into consideration when cal-culating concentration of final glycerol LC standard.(q)Glycerol(for dextrose–glycerol standard).—100mg/mL.Weigh 10g high purity glycerol into a small beaker,transfer to a100mL volu-metric flask with water,(d),and dilute to volume.(r)Ammonium sulfate.—Reagent grade;standard to test mi-cro-Kjeldahl procedure.(s)Dextrose.—LC grade,high purity>99.5%.(t)Silver nitrate solution.—0.1M.Dissolve1.70g AgNO3in ca 70mL water,(d),in a100mL volmetric flask,and dilute to volume with water,(d).D.Determination(a)Enzymatic hydrolysis and filtration.—Weigh1.0g test por-tion(crushed,sieved to10mesh,fat extracted if>10%fat,and dried) into a500mL previously weighed tall-form beaker,B(b).Prepare induplicate with2blank digestion determinations.Disperse in50mL 0.08M phosphate buffer,C(g),and sonicated to ensure complete hydration.Add100L heat stable-amylase,C(h),and cover beaker with aluminum foil.Place beaker in shaker water bath and hold at95C for30min with shaking.Cool to room temperature,and adjust the pH of the solution to pH7.50.1with0.275M NaOH, C(n).Add0.5mL protease solution,C(j),and digest solution for 30min at60C.Cool solution to room temperature(25C),and ad-just pH to4.50.2with0.325M HCl,C(o).Add0.3mL amyloglucosidase,C(k),and digest at60C for30min.Upon com-pletion of the3enzyme digestion sequence,add4volumes of95% ethanol,C(a),by weight,previously heated e the top loading balance to weigh beaker with digestion mixture when add-ing ethanol(obtain tare weight of beaker before adding test portion). Assay the2blank digestions(i.e.,2beakers and2crucibles)in an identical manner.Let solutions stand overnight to form a precipitate.Filter by suc-tion,using a water aspirator or vacuum pump,through1.0g Celite layered on a Pyrex glass crucible filter that previously has been dried to constant weight.Wash the500mL tall-form beaker and the resi-due3times with20mL78%ethanol,C(b),2times with10mL95% ethanol,C(a),and2times with10mL acetone,C(c). Quantitatively transfer filtrate and washings to a1L round bottom flask.Dry residue in an air oven at105C overnight and record weight.This residue weight,minus the protein,ash,and blank resi-due weights represents the weight of the dietary fiber(IDF+ HMWSDF)recovered by the AOAC method.(b)Filtrate recovery,desalting,and LC analysis.—Evaporate with a rotary evaporator to near dryness.Dissolve the residue with a minimum amount of water,C(d),and transfer quantitatively to a 50mL volumetric flask.Add10mL of10mg/mL glycerol LC stan-dard and dilute to volume with water,C(d).Transfer contents of the 50mL volumetric flask to a column(75cm15mm id)containing 25g each,thoroughly mixed,of Amberlite IRA-67(m-1)and Amberlite200CT(HG)H(m-2)prepared just before use.Wash ex-tract through the column with250mL water,C(d),at the rate of 0.8mL/min.Collect250mL eluant from the ion exchange column and quanti-tatively transfer into a500mL round bottom flask.Evaporate to near dryness and quantitatively transfer to a10mL volumetric flask and dilute to volume with water,C(d).Transfer the contents of the 10mL volumetric flask to a10mL disposable syringe,B(o),and fil-ter through a0.2m filter,B(k).Use a50L LC glass syringe,B(u), to fill the20L injection loop on the LC,B(f).(c)Determining the response factor for dextrose;dextrose is equivalent to RMD in LC response.—Each chromatogram must be evaluated or standardized for the RI response of RMD.This is ac-complished using glycerol standard,C(q).The peak areas,represent-ing concentration,obtained by LC analysis of equal amounts of RMD and dextrose are equivalent.Glycerol is used as the internal standard but its peak area compared to the peak area for an equal amount of dextrose or RMD is not equivalent.A glycerol standard curve is there-fore prepared to obtain a“response factor”to calculate the exact amount of RMD in a chromatogram of each test portion.Prepare3solutions in individual100mL volumetric flasks con-taining the same amount of glycerol and3levels of dextrose.It is im-portant to know and use the reported content(i.e.,>99.5%purity)of both glycerol and dextrose standards as reported by suppliers.Accu-rately weigh0.5,1.0,and2.0g dextrose into3separate100mL volu-metric flasks,respectively.To each flask add10mL of the 100mg/mL glycerol standard,C(q).Dilute each flask to volume with water,C(d).These3flasks represent the standard solutions to calculate the“response factor”for dextrose that is used to determine the amount of RMD as displayed in LC chromatograms.Use a50L LC syringe,B(u),to fill the20L injection loop for each standard glycerol–dextrose solution.Obtain the values for the peak areas of dextrose and glycerol from the3chromatograms.The reciprocal of the slope obtained by comparing the ratio of peak area of dextrose/peak area of glycerol(y-axis)to the ratio of the weight of dextrose/weight of glycerol(x-axis)is the“response factor.”The av-erage“response factor”among laboratories is0.82,varying slightly in each laboratory.Response factor=1/(PA-dex/PA-gly)(Wt-gly/Wt-dex) where PA-dex=peak area dextrose;PA-gly=peak area glycerol; Wt-dex=weight of dextrose in standard;Wt-gly=weight of glyc-erol in standard.A flow diagram for a combined enzymatic-gravimetric method and LC determination is shown in Figure2001.03B.E.CalculationAll values used in calculations are in mg,except for percent(%) values.Assay each test portion in duplicate,resulting in2test portion weight values,test portion weight and test portion weight(prime);2cruciblesfor eachblankandtestportion,blankandblank(prime);and test portion and test portion(prime).(a)Calculate average%(IDF+HMWSDF)as fol-lows.—(1)Blank ash(Ab)=(ash+Celite+blank crucible)–(Celite +blank crucible).(2)Blank residue weight(BRW)=((BR+BR)/2)–(Pb+Ab)where Pb=blank protein,determined by micro-Kjeldahl procedure;BR=weight of first blank crucible with residue;BR=weight of second blank cruci-ble with residue;Ab=weight of blank ash from step(a)(1). (3)Test portion residue weight(SR)=(residue+Celite+test portion crucible)–(Celite+test portion crucible).Duplicate test portion residue weight(SR)=(residue+Celite+test portion cru-cible)–(Celite+test portion crucible).(4)Test portion ash weight(As)=(ash+Celite+crucible)–(Celite+crucible).(5)Final test portion residue weight(FSR)=SR–Ps–As–BRW =FSR where Ps=protein,determined by micro-Kjeldahl procedure; SR=final test portion residue weight from step(a)(3);As=test por-tion ash weight from step(a)(4);BRW=blank residue weight from step(a)(2).Repeat this calculation for FSR using SR–Ps–As–BRW(using values from duplicate test portion weights).(6)Percent final test portion residue weight(%FSR)=(FSR/ SW)100=%FSR where FSR=final test portion residue weight from step(a)(5);SW=test portion weight.Repeat this calculation for%FSR using FSR and SW.(7)%(IDF+HMWSDF)=average%FSR=(%FSR+%FSR)/2where %FSR=percent final test portion residue weight;%FSR=percent final duplicate test portion residue weight.(b)C a l c u l a t e a v e r a g e%L M W R M D a s f o l-lows.—(1)LMWRMD=(peak area of LMWRMD/peak area of glycerol)(glycerol standard,mg response factor).(2)%LMWRMD=(LMWRMD/SW)100where LMWRMD =weight of LMWRMD from step(b)(1);SW=test portion weight. Repeat calculations for%LMWRMD using LMWRMD and SW.(3)%ALMWRMD=average%LMWRMD=(%LMWRMD+ %LMWRMD)/2where%LMWRMD=percent LMWRMD for test portion from step(b)(2);%LMWRMD=percent LMWRMD for duplicate test portion from step(b)(2).(c)Calculate average%total dietary fiber(TDF)as fol-lows.—Percent(%)TDF=%(IDF+HMWSDF)+%ALMWRMD where%(IDF+HMWSDF)=average percent IDF+HMWSDF from step(a)(7);%ALMWRMD=average percent LMWRMD from step(b)(3).F.Resistant MaltodextrinThe commercially available U.S.GRAS status RMD is a source of dietary fiber.Resistant maltodextrin is certified as an approved di-etary fiber ingredient for the Program for Foods for Specific Health Use(FOSHU)in Japan.Dietary fiber supplements prepared simply by packaging RMD(or agglomerated RMD)in sachet forms and la-beled as RMD have been on the market.Fibersol®-2,RMD,is manu-factured and was supplied by Matsutani Chemical Industry Co.,Ltd. (Itami City,Hyogo,Japan).The moisture content of the product is 2.7%and DE is10.5.The RMD is produced by the pyrolysis and subsequent enzyme treatment of corn starch.It is an aggregate of glucose polymers with the MW distribution of180(DP-1)to >10000(DP-62)daltons,but the average MW is2000daltons.It contains1–4and1–6glucosidic bonds,which originate from starch and1–2and1–3glucosidic bonds that are created by transglucosidation during pyrolysis.Internal utilization of RMD by in vitro and in vivo tests show that <10%is digested and absorbed in the small intestine.Approximately 50%of the products are fermented in the large intestine and ca40%of the products are excreted into the feces.In order to distinguish this substance from conventional maltodextrin(digestible),the term“re-sistant”is added and used to describe this compound.The sugar,oligosaccharide,and polysaccharide composition of the LMWRMD fraction of the RMD has been determined before and after hydrolytic enzyme treatments and is shown in Fig-ure2001.03A.The distribution of these oligosaccharides is not sig-nificantly changed when RMD is treated with hydrolytic enzymes. To assess the oligosaccharide moieties and their distribution in the LMWRMD of RMD,corn syrup solids were used as a standard source of these oligosaccharides(Figure2001.03A).The nondigestible portions of RMD consists of DP units of3(DP-3)and above(Figure2001.03A).These nondigestible oligosaccharides and polysaccharides constitute>90%of RMD.Approximately60% of RMD consist of polymers having>10DP.References:J.AOAC Int.83,1013(2000);(future issue).。
膳食纤维含量实验报告(3篇)

第1篇一、实验目的本次实验旨在测定不同食物中膳食纤维的含量,了解膳食纤维在食物中的分布情况,以及其对人体健康的重要性。
通过实验,我们可以掌握膳食纤维的测定方法,并对富含膳食纤维的食物进行评估。
二、实验材料1. 食物样品:大米、小麦、玉米、燕麦、豆类、蔬菜、水果等。
2. 试剂与仪器:无水乙醇、丙酮、热稳定α-淀粉酶、蛋白酶、葡萄糖苷酶、电子天平、离心机、烘箱、烧杯、漏斗、滤纸等。
三、实验方法1. 样品处理:将各种食物样品分别研磨成粉末,过筛,以去除杂质。
2. 酶解:取一定量的样品粉末,加入适量的热稳定α-淀粉酶、蛋白酶和葡萄糖苷酶,在适宜的温度和pH条件下进行酶解反应。
3. 沉淀与抽滤:酶解后的溶液加入无水乙醇和丙酮,充分混合,静置沉淀,抽滤,得到膳食纤维残渣。
4. 洗涤与干燥:将残渣用无水乙醇和丙酮洗涤,干燥称量,得到总膳食纤维(TDF)含量。
5. 可溶性膳食纤维(SDF)测定:将酶解后的溶液直接抽滤,用热水洗涤残渣,干燥称量,得到不溶性膳食纤维(IDF)含量;滤液用无水乙醇沉淀,抽滤,干燥称量,得到SDF含量。
四、实验结果1. 大米:TDF含量为2.2%,SDF含量为0.6%。
2. 小麦:TDF含量为2.5%,SDF含量为0.8%。
3. 玉米:TDF含量为2.8%,SDF含量为0.9%。
4. 燕麦:TDF含量为5.3%,SDF含量为1.2%。
5. 豆类:TDF含量为6.5%,SDF含量为1.8%。
6. 蔬菜:TDF含量为3.2%,SDF含量为0.9%。
7. 水果:TDF含量为2.7%,SDF含量为0.8%。
五、实验讨论1. 从实验结果可以看出,不同食物中膳食纤维的含量差异较大。
豆类、蔬菜和燕麦的膳食纤维含量较高,适合作为高纤维食物的来源。
2. 燕麦的膳食纤维含量最高,其TDF含量是大米的2倍多,小麦的2倍。
这说明燕麦是一种非常优秀的膳食纤维来源。
3. 豆类、蔬菜和水果中的膳食纤维含量较高,可以促进肠道蠕动,增加粪便体积,有助于缓解便秘症状。
膳食纤维 标准方法

膳食纤维标准方法
膳食纤维是指人体无法消化吸收的碳水化合物类物质。
膳食纤维对人体健康具有重要的作用,包括促进消化系统健康、调节血糖和胆固醇水平、预防便秘以及控制体重等。
为了准确测量食物中的膳食纤维含量,需要进行标准方法的测定。
目前,国际通用的膳食纤维含量测定方法有两种:AOAC (Association of Official Analytical Chemists)方法和ISO (International Organization for Standardization)方法。
1. AOAC方法:AOAC方法是美国官方方法,也是国际上最常用的方法。
根据AOAC 991.43或AOAC 985.29方法,首先将食物样品经过一系列处理,如酶解、水解等,获得可溶性和不可溶性纤维。
然后,借助酶解、滴定、重量等技术手段,可以得到总纤维、不可溶性纤维和可溶性纤维的含量。
2. ISO方法:ISO方法是由国际标准化组织制定的方法,与AOAC方法相似。
ISO 13904和ISO 15954方法是常用的ISO 方法。
这些方法主要利用酶解、水解、甲弹法等技术,将膳食纤维分为不可溶性纤维和可溶性纤维,并使用滴定、重量等手段进行测定。
无论使用AOAC方法还是ISO方法,都需要进行样品的预处理、酶解、滴定等步骤,以获得准确的膳食纤维含量。
这些方法在实验室条件下进行,需要仪器设备和专业操作人员进行操作。
需要注意的是,虽然AOAC和ISO方法都是国际通用的标准方法,但在具体的实验操作过程中,可能会存在一些差异,因此在测定过程中应当依据相应的方法详细操作,并遵循实验室的操作规程。
aoac标准

AOAC国际(Association of Official Analytical Chemists International)是一个专注于食品和药物分析的国际性组织,成立于1884年。
该组织发布的方法标准被广泛用于检测和分析食品、药物、化妆品等样品,为确保产品的质量和安全提供了可靠的分析方法。
AOAC标准是一系列详细描述分析方法的指南,确保在全球范围内实验室得到相同结果的一致性。
### AOAC标准的特点:1. **权威性:** AOAC标准是由一群专业的化学分析师和科学家共同制定的,确保了方法的科学性和可靠性。
2. **广泛适用性:** AOAC标准适用于各种类型的食品、饮料、药物和化妆品等产品的检测和分析。
3. **标准化:** AOAC标准是国际上广泛接受的标准,有助于提高实验室间和国际间的数据可比性。
4. **定期更新:** 鉴于科技的不断进步,AOAC标准定期进行修订和更新,以确保其方法与最新科学技术保持同步。
5. **多领域应用:** AOAC标准不仅仅关注于一种分析方法,而是覆盖了多个领域,包括化学、微生物学、生物技术等。
### AOAC标准的制定过程:AOAC标准的制定是一个系统且透明的过程,确保标准的可靠性和有效性。
主要步骤包括:1. **提案:** 任何AOAC成员都可以提出新的标准或修改现有标准的提案。
提案需要包括详细的实验步骤、数据分析和相关文献等。
2. **审查:** 提案将被提交给专门的委员会进行评审。
委员会由专业领域的专家组成,他们会仔细审查提案的科学性和可行性。
3. **公开评论:** 审查通过后,提案将被公开发表,接受来自公众和科学界的评论。
这有助于确保标准的广泛接受和适用性。
4. **修订:** 根据公开评论的反馈,委员会可能需要对提案进行修改。
这确保了标准的科学性和实用性。
5. **最终批准:** 一旦经过多轮的审查和修订,标准最终会被提交给AOAC的理事会,获得最终批准后,成为AOAC标准。
食品中总的、不溶性及可溶性膳食纤维的酶-重量测定法

食品中总的、不溶性及可溶性膳食纤维的酶-重量测定法当前,膳食纤维在预防慢性病中有着广泛的作用,膳食纤维与人体健康关系的研究日益受到重视。
现已知道可溶性膳食纤维的作用主要为调节血脂、血糖及调节益生菌丛。
而不溶性膳食纤维主要的作用为肠道通便。
目前市场上富含膳食纤维的食物、食品添加剂和保健食品越来越多,原有膳食纤维的检测方法已不适应当前需要。
古老的方法只能测定粗纤维[1],该方法所测数值与总纤维含量有较大差异,两者之间也没有一定的换算系数。
现有的洗涤剂法只能测定不溶性膳食纤维[2],但不能测定可溶性膳食纤维,尤其是可溶性膳食纤维已明确具有保健功能,并成为保健功能食品中的功效成分,这就给膳食纤维成分更加细致的分类测定提出了要求。
目前膳食纤维的测定方法可分为两大类:重量法和化学法。
重量法较简单[3],主要测定总膳食纤维、可溶性膳食纤维和不可溶性膳食纤维。
化学法则可定量地测定其中每一种中性糖和总的酸性糖(糖醛酸),还可单独测定木质素[4],但化学法受仪器设备制约,因而不适用于常规的膳食纤维分析。
酶-重量法于20世纪80年代在国外首先发展起来,现已成为AOAC认可的分析方法,已被美国、日本、瑞典及北欧许多国家广泛采用。
1材料和方法1.1原理:分别用热稳定的α-淀粉酶、蛋白酶、葡萄糖苷酶进行酶解消化样品以去除蛋白质和淀粉。
总膳食纤维(TDF)的测定是先酶解,然后用乙醇沉淀,将过滤的TDF残渣用乙醇和丙酮冲洗,干燥后称重。
不溶性和可溶性膳食纤维(IDF和SDF)是在样品酶解后即刻将IDF过滤,过滤后的残渣用热水冲洗,经干燥后称重。
SDF是将上述滤出液用4倍量的95%乙醇沉淀,然后将滤渣干燥、称重。
TDF、IDF和SDF的量通过蛋白质和灰分含量进行校正。
1.2仪器:意大利VELP公司CSF6&GDE型膳食纤维测定仪;天平:精确至±01mg;马福炉:温度控制在(525±5)℃;干燥箱:温度控制在(105±3)℃和(130±3)℃。
总膳食纤维国标测定方法-符合AOAC等

总膳食纤维测定的介绍1、在α-淀粉酶的作用下,PH为6的磷酸盐缓冲溶液,95—100度下加热15分钟。
2、用蛋白酶在PH为7.5时60度培养30分钟。
3、用淀粉葡(萄)糖苷酶在PH为4.0---4.6下60度培养30分钟。
4、4体积的95%的乙醇沉淀。
5、过滤。
6、用78%和95%的乙醇和丙酮清洗沉淀物。
7、烘干称重。
8、干样可以拿去做凯氏定氮,也可以在525度的马弗炉里灰份5个小时,然后去称重。
不溶的膳食纤维的定义为进行烘干前用乙醇进行清洗并用温水洗涤后残留物。
总膳食纤维(TDF)—不溶膳食纤维= 可溶膳食纤维(SDF)标准酶法测定食品和饲料中的总膳食纤维量1、研磨分级样品2、在105度的烘箱烘干并恒重,在干燥箱中冷却到室温。
3、如果样品脂肪含量高于10%,需要用石油醚进行脱脂,在最终结果中再进行校正。
4、称出0.5—1克的样品,并转移到400毫升的烧杯中。
5、用α-淀粉酶在50毫升的PH为6的磷酸盐缓冲溶液中培养15分钟,培养温度为95—100度,温度可以用温度计控制。
6、冷却到室温,并用0.275 N 浓度的氢氧化钠溶液调节PH到7.5。
7、将烧杯和样品一起转移到磁力搅拌培养器中(GDE)。
8、在搅拌的情况下,加入蛋白酶在60度的情况下培养30分钟。
9、冷却到室温,用0.325的盐酸调节PH值为4.0—4.6。
10、在搅拌的情况下,加淀粉葡(萄)糖苷酶,在60度时培养30分钟。
11、通过加4体积的95%的乙醇沉淀可溶性膳食纤维,并且在室温下沉淀大约1个小时。
12、称量已经添加了0.5克的硅藻土(作为助滤剂)玻璃坩埚.13、将坩埚放在CSF6 (或者FIWE6)上,倒入上述操作的沉淀物,并用V ACUUM进行吸液排空,用78%的乙醇溶液进行洗涤转移沉淀物。
14、用20毫升的78%的乙醇溶液洗涤玻璃坩埚中的沉淀物两次,再用10毫升95%的乙醇溶液洗涤两次,10毫升的丙酮溶液洗涤两次并排除废液。
食品营养成分标示准则

食品营养成分标示准则(卫监督发[2007]300号附件1)依据《食品营养标签管理规范》中所涉及的内容要求,制定本准则。
本准则规定了能量和营养成分的定义、折算系数、营养成分分析和标示方法、数值表达、允许误差和推荐的营养标签格式等内容。
一、术语和定义1.预包装食品(prepackaged foods)经预先定量包装,或装入(灌入)容器中,向消费者直接提供的食品。
2.营养成分(nutritional components)指食品中具有的营养素和有益成分。
包括营养素、水分、膳食纤维等。
3. 营养素(nutrients)指食品中具有特定生理作用,能维持机体生长、发育、活动、繁殖以及正常代谢所需的物质,缺少这些物质,将导致机体发生相应的生化或生理学的不良变化。
包括蛋白质、脂肪、碳水化合物、矿物质、维生素五大类。
4. 能量(energy)指食品中的蛋白质、脂肪和碳水化合物等营养素在人体代谢中产生的能量。
推荐以千焦(kJ)或焦耳(J)标示,当以千卡(kcal)标示能量值时,应同时标示千焦(kJ)。
食品中产能营养素的能量折算系数如表1所示:表 1 食物中产能营养素的能量折算系数* 1千卡(kcal)的能量相当于4.184千焦(kJ)。
5. 蛋白质(protein)蛋白质是含氮的有机化合物,以氨基酸为基本单位组成。
食品中蛋白质含量可通过“总氮量”乘以“氮折算系数”,或食品中各氨基酸含量的总和来确定。
在测定出“总氮量”后,食品中蛋白质含量的计算公式如下:蛋白质(g/100g)=总氮量(g/100g)×氮折算系数不同食品的氮折算系数如表2所示,对于原料复杂的加工或配方食品,统一使用折算系数6.25。
表2 不同食品氮折算系数*来源:*《中国食物成分表2002》6. 脂肪和脂肪酸(fat and fatty acid)由于检测方法的不同,脂肪有粗脂肪(crude fat)或总脂肪(total fat)之分,在营养标签上均可标示为“脂肪”。
aoac标准

aoac标准AOAC标准。
AOAC国际(Association of Official Analytical Chemists)是一个专门从事食品和环境分析的国际性组织。
AOAC标准是该组织制定的一系列分析方法和标准,被广泛应用于食品、农产品、环境和药品等领域。
AOAC标准的制定严格依据科学原理和实验数据,具有权威性和可靠性。
本文将对AOAC标准的背景、意义和应用进行介绍。
首先,AOAC标准的制定背景。
AOAC成立于1884年,至今已有百余年的历史。
随着科学技术的不断发展,人们对食品和环境分析的要求也越来越高。
为了保证分析结果的准确性和可比性,AOAC组织陆续制定了大量的分析方法和标准,形成了AOAC标准体系。
这些标准不仅是科学研究和检测实验的重要依据,也是保障食品安全和环境保护的重要手段。
其次,AOAC标准的意义。
AOAC标准的制定严格遵循科学原理和实验数据,经过多次验证和修订,具有权威性和可靠性。
采用AOAC标准进行分析,可以保证结果的准确性和可比性,为食品安全、环境监测、药品质量等提供了可靠的技术支持。
同时,AOAC标准的推广和应用也促进了全球范围内的食品贸易和科学交流,对于推动食品行业的发展和提升国际竞争力具有重要意义。
再次,AOAC标准的应用。
AOAC标准涵盖了食品成分分析、微生物检测、农药残留、重金属检测、环境污染物分析等多个方面。
这些标准不仅适用于实验室科研和食品生产企业,也被广泛应用于政府监管、质量检验、法律诉讼等多个领域。
同时,AOAC标准也得到了国际组织和标准化机构的认可和采纳,成为全球范围内的权威标准。
总之,AOAC标准作为食品和环境分析领域的权威标准,具有重要的科学意义和实际应用价值。
在未来的发展中,AOAC标准将继续发挥其在食品安全、环境保护、质量监管等方面的重要作用,为人类的健康和生活质量提供坚实的保障。
食品标签表示及测定方法

附件食品营养标签管理规范(再次征求意见稿)第一条为指导和规范食品营养标签管理工作,保证食品符合应有的营养品质,保护消费者健康,根据《中华人民共和国食品卫生法》(下称《食品卫生法》)的有关规定,制定本规范。
第二条凡在中华人民共和国境内销售的预包装食品标签上标注食品营养标签的,必须符合本规范的规定。
国家法律、行政法规和标准另有规定的,按相关规定执行。
第三条国家鼓励食品生产企业按本规范标示营养标签。
卫生部根据本规范的实施情况,制定强制标示营养标签的食品品种和类型名单,确定实施时间。
第四条本规范所称的食品营养标签是指向消费者提供食物营养特性的一种描述,包括营养成分、营养声称、健康声称。
第五条营养成分指食物中含有的具有健康益处的成分。
包括营养素、水分、膳食纤维及其它有效功能成分等。
第六条食品生产企业对食品的某一营养素标示营养含量和健康声称时,必须标示该营养素和以下四种营养成分:(一)能量;(二)蛋白质;(三)脂肪;(四)碳水化合物。
第七条食品生产企业在食品营养标签上可以标示下列营养成分:(一)能量;(二)蛋白质;(三)脂肪(饱和脂肪酸,不饱和脂肪酸,单不饱和脂肪酸,多不饱和脂肪酸,反式脂肪酸);(四)胆固醇;(五)碳水化合物;(六)糖;(七)膳食纤维(可溶性和不可溶性膳食纤维,单体成分);(八)维生素:维生素A(β-胡萝卜素)、维生素D、维生素E、维生素K、维生素B1(硫胺素)、维生素B2(核黄素)、维生素B6、维生素B12、维生素C(抗坏血酸)、烟酸(烟酰胺)、叶酸、泛酸、生物素和胆碱;(九)矿物质:钙、磷、钾、钠、镁、铁、锌、碘、硒、铜、氟、铬、锰和钼。
标示上款营养成分,不得改变名称。
营养强化食品还应标示所强化成分的含量。
第八条营养成分的含量应当以每100毫升(ml)、每100克(g)和/或每份食品中的确定数值标示,可以同时标示所含营养素占营养素参考值(NRV)的百分比。
第九条营养成分的定义、标示和数值的允许误差应符合《食品营养标签标示规范》的规定。
食品中膳食纤维含量的测定与分析

食品中膳食纤维含量的测定与分析随着人们健康意识的提升,越来越多的人开始关注食物中的营养成分,其中膳食纤维作为一种重要的营养物质备受关注。
膳食纤维在维持肠道健康、调节血糖和血脂、预防肥胖等方面起着重要的作用。
那么,如何准确测定食品中的膳食纤维含量呢?一、测定方法目前常用的测定食品中膳食纤维含量的方法包括酶解-重量法(AOAC 985.29)和酶解-HPLC法(AOAC 991.43),其中HPLC法相对较为准确和简便。
在使用HPLC法测定膳食纤维含量时,通常采用两种酶解方法,即使用α-淀粉酶和葡萄糖酸酶进行酶解。
通过比较未酶解样品和酶解后样品中的膳食纤维含量,可以计算出样品中的膳食纤维含量。
二、食品中膳食纤维的分析1.粗纤维含量的分析粗纤维是指食物中不容易被消化吸收的纤维部分,一般包括纤维素、半纤维素和木质素等。
粗纤维含量的分析是衡量食品中纤维素含量的一种方法,一般通过水解和洗涤的方式来进行。
首先,将食品样品经过一定时间的水解,然后用水或酸进行洗涤,最后干燥并称重。
所得的质量差值即为粗纤维的含量。
2.溶解性膳食纤维含量的分析溶解性膳食纤维是指在水中可溶解的膳食纤维,如果胶、树胶等。
溶解性膳食纤维含量的分析主要通过酶解-过滤的方法进行。
首先,将食品样品经过一定时间的酶解,然后用滤液进行过滤,将溶解性膳食纤维从样品中分离出来。
最后,将滤渣干燥并称重,所得的质量差值即为溶解性膳食纤维的含量。
3.不溶性膳食纤维含量的分析不溶性膳食纤维是指在水中不溶解的膳食纤维,如纤维素、半纤维素等。
不溶性膳食纤维含量的分析主要通过酶解-过滤的方法进行。
首先,将食品样品经过一定时间的酶解,然后用滤液进行过滤,将溶解性膳食纤维从样品中分离出来。
将滤渣干燥并称重,所得的质量即为不溶性膳食纤维的含量。
三、膳食纤维含量的参考范围根据世界卫生组织的建议,成年人每天的膳食纤维摄入量应为25-30克。
然而,现代人的饮食结构大部分偏向高脂肪、高糖分的食物,膳食纤维的摄入量普遍不足。
特殊保健食品素材难消化麦芽糖糊精(水溶性膳食纤维)

a 250
200
b 250
200
c 250
200
150 100
*
150
**
100
*
150
**
* 100
250
d
200 150 100
50
50
50
50
0 30 60 90 120 0 30 60 90 120 0 30 60 90 120 0 30 60 90 120
时间(分)
饮用含有Fibersol2的茶饮料对进食后的血糖值变化所造成的影响(平均值)
胰岛素
体内脂肪的蓄积 【肥满】
脂肪
高热量: 9kcal/g
脂肪蛋白酶 (LPL)
炭水化合物易于转化为脂肪. 胰岛素的分泌第一会刺激脂蛋白酶使之活 性化, 第二会阻碍脂肪的分解.
↓ 胰岛素分泌过多, 会促进体内脂肪的蓄积.
摄取Fibersol 2可以抑制进食后血糖值的上升
体内脂肪的减低效果(RATS·1)
95 ± 3 213 ± 34 147 ± 17
226 ± 10 230 ± 25 265 ± 10
47 ± 4 40 ± 4 49 ± 2
242 ± 64 285 ± 60 243 ± 34
4周后
8周后
12周后
97 ± 2 214 ± 33 107 ± 7
95 ± 5 220 ± 38 147 ± 21
Fibersol 2 : 化学结构
葡糖甙结合 麦芽糊精(%) Fibersol 2 (%)
1-4 94.5 62.5
1-6
1-2
5.5
0
23.0
3.3
1-3 0 11.2
麦芽糖糊精的化学结构
高膳食纤维的标准

针对食物中膳食纤维含量的测定,要符合国家的下列标准:1. GB 5009.88-2014 食品安全国家标准食品中膳食纤维的测定本标准规定了食品中膳食纤维的测定方法(酶重量法)。
本标准适用于所有植物性食品及其制品中总的、可溶性和不溶性膳食纤维的测定,但不包括低聚果糖、低聚半乳糖、聚葡萄糖、抗性麦芽糊精、抗性淀粉等膳食纤维组分。
2. GB/T 9822-2008 粮油检验谷物不溶性膳食纤维的测定本标准规定了测定谷物不溶性膳食纤维的术语和定义、原理、试剂和材料、仪器设备、操作步骤、结果计算,以及精密度的要求。
本标准适用于谷物中不溶性膳食纤维的测定。
3. NY/T 1594-2008 水果中总膳食纤维的测定(非酶-重量法)本标准规定了水果中总膳食纤维含量测定的非酶-重量法。
本标准适用于总膳食纤维含量≥10%、淀粉含量≤2%(以干基计)的水果中总膳食纤维含量的测定。
4. GB 5413.6-2010 食品安全国家标准婴幼儿食品和乳品中不溶性膳食纤维的测定本标准规定了婴幼儿食品和乳品中不溶性膳食纤维的测定方法。
本标准适用于婴幼儿食品和乳品中不溶性膳食纤维的测定。
膳食纤维产品的安全标准:1. DBS42/ 007-2015 食品安全地方标准魔芋膳食纤维本标准规定了魔芋膳食纤维的产品分类、技术要求、检验方法、检验规则以及标志、标签、包装、运输、储存和保质期。
本标准适用于湖北省地域范围内生产的供冲调或冲泡饮用的即食型魔芋膳食纤维。
魔芋膳食纤维产品分类:1)原味魔芋膳食纤维:以魔芋为单一原料,经加工、包装而成的供冲调或冲饮的即食型魔芋膳食纤维。
按照葡甘聚糖含量可以分为:特纯魔芋膳食纤维、高纯魔芋膳食纤维、魔芋膳食纤维。
2)复合魔芋膳食纤维:以原味魔芋膳食纤维为主要材料,添加其他食品原辅料和食品添加剂,经加工制成的供冲调或冲饮的即食型魔芋膳食纤维。
2. GB/T 22494-2008 大豆膳食纤维粉本标准适用于商品大豆膳食纤维粉。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
45.4.13AOAC Official Method2001.03Total Dietary Fiber in FoodsContaining Resistant MaltodextrinEnzymatic-Gravimetric Methodand Liquid Chromatography DeterminationFirst Action2001[This method is applicable to resistant maltodextrin(RMD)and to foods containing RMD listed in Table2001.03at 1.4%RMD.] A.PrincipleThis method determines total dietary fiber(TDF)value of pro-cessed foods containing insoluble dietary fiber(IDF)and high mo-lecular weight soluble dietary fiber(HMWSDF),which are precipitated in ethanol and low molecular weight resistant maltodextrin(LMWRMD),which is soluble in ethanol.This method defines dietary fiber(DF)as consisting of nondigestible car-bohydrates having a degree of polymerization with3sugar moieties (DP3)or higher after enzymatic hydrolysis.All the starches con-tained in food are converted to glucose after this enzymatic hydroly-sis.This method to determine TDF content in processed foods containing RMD is a combination of985.29(see45.4.07)for DF and a LC method for LMWRMD.A food is first analyzed for the to-tal quantity of IDF and HMWSDF,precipitated in ethanol,accord-ing to985.29(see45.4.07).Then an LC determination is conducted on the desalted filtrate to obtain the quantity of LMWRMD not pre-cipitated in the78%alcohol preparation.These2values[(IDF+ HMWSDF)+LMWRMD]are summed to obtain the TDF value in the food.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Beakers.—Tall-form,500mL.(c)Water baths.—To maintain a temperature of95–100C and 60C with ability to shake the containers.(d)Filtering crucibles.—Coarse,ASTM,40–60m pore size, Pyrex,50mL.(e)Glass or plastic columns.—To hold ion exchange resins, 75cm15mm id;a shorter(40–75cm15mm id)column can also be used.(f)Liquid chromatograph(LC).—With oven to maintain a col-umn temperature of80C and a20L injection loop.Column oper-ating conditions are:Temperature,80C;mobile phase,distilled water,C(d);flow rate,0.5mL/min.(g)Guard column(or precolumn).—TSK®guard column PWXL,6.0mm id4cm(Tosoh Corp.,distributed by TosoHaas, Montgomeryville,PA,USA;)or equivalent. (h)LC columns.—Two LC columns connected in series, TSK-GEL®G2500PWXL,7.8mm id30cm(Tosoh Corp.),or equivalent.(i)Detector.—Refractive index(RI);maintained at40C. (j)Data integrator or computer.—For peak area measurement. (k)Filters for disposable syringe.—0.2m membrane,13mm. (l)Filters for water.—0.2m,47mm.(m)Filter apparatus.—To hold47mm,0.2m filter,(l);to filter larger volumes of water,C(d).(n)Glass rods.—With fire-polished ends,ca20cm long. (o)Syringes.—10mL,plastic disposable.(p)Pasteur pipet.(q)Volumetric pipet.—10mL.(r)Volumetric flasks.—10,50,250,and1000mL.(s)Top loading balance.—4000g capacity.(t)Tubing.—PVC,2.79mm id(for ion exchange columns). (u)Glass LC syringe.—50L.(v)Teflon scraping rod.—Use in place of glass stirring rod to scrape precipitate from tall-form beaker.(w)Rotary evaporator.—R-3000VW“Student”(Büchi,Swit-zerland;)or equivalent.C.Reagents(a)Ethanol.—95%.Technical grade,used at60C.(b)Ethanol.—78%.Place207mL water in1L volumetric flask and dilute to volume with95%ethanol,(a).(c)Acetone.—Reagent grade.(d)Distilled water.(e)Sodium phosphate dibasic.(f)Sodium phosphate monobasic.(g)Phosphate buffer.—0.08M,pH6.0.Dissolve1.400g Na2HPO4(or1.753g dihydrate)and9.68g NaH2PO4H2O(or10.94g dihydrate)in ca700mL water,(d).Dilute to1L with water,(d),and verify pH with pH meter.(h)Heat stable a-amylase solution(Termamyl).—No.120L(ac-tivity:12units/mg protein;Novo Laboratories,Inc.,59Danbury Rd, Wilton,CT06897,USA),or equivalent(should not contain glycerol).(i)LC retention time standard.—Standard source of the distribu-tion of oligosaccharides(DP$3)in the LMWRMD fraction of RMD, corn syrup solids(DE25;Matsutani Chemical Industry Co.,Ltd.,ItamiTable2001.03Interlaboratory results for the determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatographyFood x,%bs a(b)s r RSD r,%s R RSD R,% Resistant maltodextrin95.368(0) 1.63 1.71 2.37 2.48Hard candy37.997(1)0.58 1.530.68 1.79 Chicken and vegetable soup25.418(0)0.74 2.89 1.18 4.65 Grapefruit juice 1.388(0)0.02 1.330.04 3.20 White bread9.608(0)0.33 3.410.64 6.66 Strawberry Jell-O9.918(0)0.60 6.100.939.39a(b)a=Number of laboratories retained after eliminating outliers;b=number of laboratories removed as outliers.City,Hyogo,Japan;),analyzed by LC(Fig-ure2001.03A)as in D.(j)Protease.—No.P-3910or P-5380(activity:7–15units/mg pro-tein;Sigma Chemical Co.,St.Louis,MO,USA),or equivalent(should not contain glycerol).Prepare protease stock solution just before use by adding100mg protease enzyme to a10mL volumetric flask and bring-ing to volume with water,(d),(amount is sufficient for9test portions in duplicate).(k)Amyloglucosidase.—No.A-9913(activity:400units/mg pro-tein;Sigma Chemical Co.),or equivalent(should not contain glycerol). (l)Celite.—No.C-8656(Sigma Chemical Co.)or No.C-211,acid washed(Fisher Scientific Co.,Fair Lawn,NJ,USA),or equivalent. (m)Mixed-bed ion exchange resins for each test por-tion.—(1)m-1.—25g Amberlite IRA-67(OH-type;Organo Corp., Tokyo,Japan,/organo_corp.htm),or equivalent.(2)m-2.—25g Amberlite200CT(HG)H(H-type;Organo Corp.),or equivalent,are mixed and packed in column for analysis of each test portion.The converted resin should satisfy the following specifications:(a)Total ion exchange capacity:1.74meq/mL (min);(b)Effective ion exchange capacity(R-H exchange capac-ity):1.6meq/mL(min);(c)pH:4–7.Before mixing and packing the 2resins into a column,wash each resin with H2O to obtain a pH value of7–8.8for m-1and4–7for m-2.If Amberlite200CT(HG)H cannot be obtained,Amberlite200(Na-type;Sigma Chemical Co.) or Amberlite200CT(Organo Corp.)can be used by converting it to “H-type”by the following procedure:Fill column(100cm40mm id),B(e),with600mL(500g) Amberlite200“Na-type”resin and determine approximate resin volume.Wash resin with2volumes of water,(d),at the rate of 60mL/min.Pass2volumes of10%HCl(1+3,w/w)through the resin at the rate of60mL/min.Remove HCl with3volumes of water, (d),passed through the resin at the rate of60mL/min.Add3–6vol-umes of additional water,(d),at the rate of120mL/min.The columnis adequately washed of HCl when a pH value of4–7is obtained.(It takes2–3h to charge and rinse these resins.)(n)Sodium hydroxide.—0.275M;reagent grade.Dissolve11.0g NaOH in ca700mL water,(d),in a1L volumetric flask.Dilute to volume with water,(d).(o)Hydrochloric acid.—0.325M;reagent grade.Dilute stock so-lution of known titer,e.g.,325mL1M HCl,to1L with water. (p)Glycerol(LC standard).—10mg/mL.For stock solution: weigh10g glycerol>99.5%purity into a small beaker.Quantita-tively transfer to1L volumetric flask with repeated washes with wa-ter,(d),and dilute to volume.It is important to measure and record the exact weight of the glycerol,weighing as close to10g as possi-ble.Take purity and weight of glycerol into consideration when cal-culating concentration of final glycerol LC standard.(q)Glycerol(for dextrose–glycerol standard).—100mg/mL.Weigh 10g high purity glycerol into a small beaker,transfer to a100mL volu-metric flask with water,(d),and dilute to volume.(r)Ammonium sulfate.—Reagent grade;standard to test mi-cro-Kjeldahl procedure.(s)Dextrose.—LC grade,high purity>99.5%.(t)Silver nitrate solution.—0.1M.Dissolve1.70g AgNO3in ca 70mL water,(d),in a100mL volmetric flask,and dilute to volume with water,(d).D.Determination(a)Enzymatic hydrolysis and filtration.—Weigh1.0g test por-tion(crushed,sieved to10mesh,fat extracted if>10%fat,and dried) into a500mL previously weighed tall-form beaker,B(b).Prepare induplicate with2blank digestion determinations.Disperse in50mL 0.08M phosphate buffer,C(g),and sonicated to ensure complete hydration.Add100L heat stable-amylase,C(h),and cover beaker with aluminum foil.Place beaker in shaker water bath and hold at95C for30min with shaking.Cool to room temperature,and adjust the pH of the solution to pH7.50.1with0.275M NaOH, C(n).Add0.5mL protease solution,C(j),and digest solution for 30min at60C.Cool solution to room temperature(25C),and ad-just pH to4.50.2with0.325M HCl,C(o).Add0.3mL amyloglucosidase,C(k),and digest at60C for30min.Upon com-pletion of the3enzyme digestion sequence,add4volumes of95% ethanol,C(a),by weight,previously heated e the top loading balance to weigh beaker with digestion mixture when add-ing ethanol(obtain tare weight of beaker before adding test portion). Assay the2blank digestions(i.e.,2beakers and2crucibles)in an identical manner.Let solutions stand overnight to form a precipitate.Filter by suc-tion,using a water aspirator or vacuum pump,through1.0g Celite layered on a Pyrex glass crucible filter that previously has been dried to constant weight.Wash the500mL tall-form beaker and the resi-due3times with20mL78%ethanol,C(b),2times with10mL95% ethanol,C(a),and2times with10mL acetone,C(c). Quantitatively transfer filtrate and washings to a1L round bottom flask.Dry residue in an air oven at105C overnight and record weight.This residue weight,minus the protein,ash,and blank resi-due weights represents the weight of the dietary fiber(IDF+ HMWSDF)recovered by the AOAC method.(b)Filtrate recovery,desalting,and LC analysis.—Evaporate with a rotary evaporator to near dryness.Dissolve the residue with a minimum amount of water,C(d),and transfer quantitatively to a 50mL volumetric flask.Add10mL of10mg/mL glycerol LC stan-dard and dilute to volume with water,C(d).Transfer contents of the 50mL volumetric flask to a column(75cm15mm id)containing 25g each,thoroughly mixed,of Amberlite IRA-67(m-1)and Amberlite200CT(HG)H(m-2)prepared just before use.Wash ex-tract through the column with250mL water,C(d),at the rate of 0.8mL/min.Collect250mL eluant from the ion exchange column and quanti-tatively transfer into a500mL round bottom flask.Evaporate to near dryness and quantitatively transfer to a10mL volumetric flask and dilute to volume with water,C(d).Transfer the contents of the 10mL volumetric flask to a10mL disposable syringe,B(o),and fil-ter through a0.2m filter,B(k).Use a50L LC glass syringe,B(u), to fill the20L injection loop on the LC,B(f).(c)Determining the response factor for dextrose;dextrose is equivalent to RMD in LC response.—Each chromatogram must be evaluated or standardized for the RI response of RMD.This is ac-complished using glycerol standard,C(q).The peak areas,represent-ing concentration,obtained by LC analysis of equal amounts of RMD and dextrose are equivalent.Glycerol is used as the internal standard but its peak area compared to the peak area for an equal amount of dextrose or RMD is not equivalent.A glycerol standard curve is there-fore prepared to obtain a“response factor”to calculate the exact amount of RMD in a chromatogram of each test portion.Prepare3solutions in individual100mL volumetric flasks con-taining the same amount of glycerol and3levels of dextrose.It is im-portant to know and use the reported content(i.e.,>99.5%purity)of both glycerol and dextrose standards as reported by suppliers.Accu-rately weigh0.5,1.0,and2.0g dextrose into3separate100mL volu-metric flasks,respectively.To each flask add10mL of the 100mg/mL glycerol standard,C(q).Dilute each flask to volume with water,C(d).These3flasks represent the standard solutions to calculate the“response factor”for dextrose that is used to determine the amount of RMD as displayed in LC chromatograms.Use a50L LC syringe,B(u),to fill the20L injection loop for each standard glycerol–dextrose solution.Obtain the values for the peak areas of dextrose and glycerol from the3chromatograms.The reciprocal of the slope obtained by comparing the ratio of peak area of dextrose/peak area of glycerol(y-axis)to the ratio of the weight of dextrose/weight of glycerol(x-axis)is the“response factor.”The av-erage“response factor”among laboratories is0.82,varying slightly in each laboratory.Response factor=1/(PA-dex/PA-gly)(Wt-gly/Wt-dex) where PA-dex=peak area dextrose;PA-gly=peak area glycerol; Wt-dex=weight of dextrose in standard;Wt-gly=weight of glyc-erol in standard.A flow diagram for a combined enzymatic-gravimetric method and LC determination is shown in Figure2001.03B.E.CalculationAll values used in calculations are in mg,except for percent(%) values.Assay each test portion in duplicate,resulting in2test portion weight values,test portion weight and test portion weight(prime);2cruciblesfor eachblankandtestportion,blankandblank(prime);and test portion and test portion(prime).(a)Calculate average%(IDF+HMWSDF)as fol-lows.—(1)Blank ash(Ab)=(ash+Celite+blank crucible)–(Celite +blank crucible).(2)Blank residue weight(BRW)=((BR+BR)/2)–(Pb+Ab)where Pb=blank protein,determined by micro-Kjeldahl procedure;BR=weight of first blank crucible with residue;BR=weight of second blank cruci-ble with residue;Ab=weight of blank ash from step(a)(1). (3)Test portion residue weight(SR)=(residue+Celite+test portion crucible)–(Celite+test portion crucible).Duplicate test portion residue weight(SR)=(residue+Celite+test portion cru-cible)–(Celite+test portion crucible).(4)Test portion ash weight(As)=(ash+Celite+crucible)–(Celite+crucible).(5)Final test portion residue weight(FSR)=SR–Ps–As–BRW =FSR where Ps=protein,determined by micro-Kjeldahl procedure; SR=final test portion residue weight from step(a)(3);As=test por-tion ash weight from step(a)(4);BRW=blank residue weight from step(a)(2).Repeat this calculation for FSR using SR–Ps–As–BRW(using values from duplicate test portion weights).(6)Percent final test portion residue weight(%FSR)=(FSR/ SW)100=%FSR where FSR=final test portion residue weight from step(a)(5);SW=test portion weight.Repeat this calculation for%FSR using FSR and SW.(7)%(IDF+HMWSDF)=average%FSR=(%FSR+%FSR)/2where %FSR=percent final test portion residue weight;%FSR=percent final duplicate test portion residue weight.(b)C a l c u l a t e a v e r a g e%L M W R M D a s f o l-lows.—(1)LMWRMD=(peak area of LMWRMD/peak area of glycerol)(glycerol standard,mg response factor).(2)%LMWRMD=(LMWRMD/SW)100where LMWRMD =weight of LMWRMD from step(b)(1);SW=test portion weight. Repeat calculations for%LMWRMD using LMWRMD and SW.(3)%ALMWRMD=average%LMWRMD=(%LMWRMD+ %LMWRMD)/2where%LMWRMD=percent LMWRMD for test portion from step(b)(2);%LMWRMD=percent LMWRMD for duplicate test portion from step(b)(2).(c)Calculate average%total dietary fiber(TDF)as fol-lows.—Percent(%)TDF=%(IDF+HMWSDF)+%ALMWRMD where%(IDF+HMWSDF)=average percent IDF+HMWSDF from step(a)(7);%ALMWRMD=average percent LMWRMD from step(b)(3).F.Resistant MaltodextrinThe commercially available U.S.GRAS status RMD is a source of dietary fiber.Resistant maltodextrin is certified as an approved di-etary fiber ingredient for the Program for Foods for Specific Health Use(FOSHU)in Japan.Dietary fiber supplements prepared simply by packaging RMD(or agglomerated RMD)in sachet forms and la-beled as RMD have been on the market.Fibersol®-2,RMD,is manu-factured and was supplied by Matsutani Chemical Industry Co.,Ltd. (Itami City,Hyogo,Japan).The moisture content of the product is 2.7%and DE is10.5.The RMD is produced by the pyrolysis and subsequent enzyme treatment of corn starch.It is an aggregate of glucose polymers with the MW distribution of180(DP-1)to >10000(DP-62)daltons,but the average MW is2000daltons.It contains1–4and1–6glucosidic bonds,which originate from starch and1–2and1–3glucosidic bonds that are created by transglucosidation during pyrolysis.Internal utilization of RMD by in vitro and in vivo tests show that <10%is digested and absorbed in the small intestine.Approximately 50%of the products are fermented in the large intestine and ca40%of the products are excreted into the feces.In order to distinguish this substance from conventional maltodextrin(digestible),the term“re-sistant”is added and used to describe this compound.The sugar,oligosaccharide,and polysaccharide composition of the LMWRMD fraction of the RMD has been determined before and after hydrolytic enzyme treatments and is shown in Fig-ure2001.03A.The distribution of these oligosaccharides is not sig-nificantly changed when RMD is treated with hydrolytic enzymes. To assess the oligosaccharide moieties and their distribution in the LMWRMD of RMD,corn syrup solids were used as a standard source of these oligosaccharides(Figure2001.03A).The nondigestible portions of RMD consists of DP units of3(DP-3)and above(Figure2001.03A).These nondigestible oligosaccharides and polysaccharides constitute>90%of RMD.Approximately60% of RMD consist of polymers having>10DP.References:J.AOAC Int.83,1013(2000);(future issue).。
